Abstract
5-HT receptors expressed throughout the human body are targets for established therapeutics and various drugs in development. Their diversity of structure and function reflects the important role 5-HT receptors play in physiologic and pathophysiological processes. The present review offers a framework for the official receptor nomenclature and a detailed understanding of each of the 14 5-HT receptor subtypes, their roles in the systems of the body, and, where appropriate, the (potential) utility of therapeutics targeting these receptors.
Significance Statement
This review provides a comprehensive account of the classification and function of 5-hydroxytryptamine receptors, including how they are targeted for therapeutic benefit.
I. Introduction
Classification of 5-HT receptors extends back to the middle of the last century when Gaddum and Picarelli (1957) suggested that the 5-HT–induced contraction of guinea pig ileum was mediated by two different receptors: a neurotropic “M” receptor located on parasympathetic ganglia (effect blocked by morphine and atropine; now known to be the 5-HT3 receptor) and a musculotropic “D” receptor located on smooth muscles (effect blocked by dibenzyline, lysergide, 2-bromolysergide, and dihydroergotamine; now known to be the 5-HT2A receptor). This original classification served well for around two decades, although, from time to time, it was reported that some 5-HT–induced effects (e.g., vasoconstriction in the canine carotid arterial bed) were not mediated by “M” or “D” but instead by “special” receptors (Saxena, 1974). Then, Bennett and Aghajanian (1974) reported the first successful radioligand binding study of 5-HT receptors using [3H]lysergide, and subsequent studies using [3H]5-HT, [3H]spiperone, and [3H]lysergide enabled Peroutka and Snyder (1979) to identify two 5-HT “receptors” named 5-HT1 (nanomolar affinity for 5-HT) and 5-HT2 (micromolar affinity for 5-HT). Subsequently, 5-HT1 “receptors” were subdivided pharmacologically into 5-HT1A and 5-HT1B receptors (Pedigo et al., 1981), and 8-OH-DPAT was designated as a selective 5-HT1A ligand (Gozlan et al., 1983; Middlemiss and Fozard, 1983). However, at these times, 5-HT receptors were being classified by various names (e.g., “D,” “M,” 5-HT1, 5-HT2, S1, S2), hence the clear need for uniform terminology. This effort culminated in the Bradley et al. (1986) publication, classifying 5-HT receptors into “5-HT1-like” (equivalent to some “D” or 5-HT1), 5-HT2 (equivalent to most “D” or 5-HT2), and 5-HT3 (equivalent to “M”) receptors. The authors emphasized that this classification was a “general framework,” which would be regularly updated with new findings. Indeed, with the explosion in new findings around the time, it was clear a new classification was required that gave rise to the 5-HT receptor IUPHAR subcommittee–sanctioned classification of 5-HT receptors into 5-HT1 (“5-HT1-like,” 5-HT1A, 5-HT1B, 5-HT1D, 5-ht1e, and 5-ht1f), 5-HT2 (5-HT2A, 5-HT2B, and 5-HT2C), 5-HT3, 5-HT4, recombinant (5-ht5a/5b, 5-ht6, 5-ht7), and “orphan” receptors (Hoyer et al., 1994). This new classification scheme was based on the conjunction of structural (molecular structure), transductional (intracellular transduction mechanisms), and operational (selective agonists and antagonists and ligand binding affinities) criteria. This first IUPHAR review on 5-HT receptors (Hoyer et al., 1994) was a landmark for the then rather complex 5-HT receptor field and the associated diversity of nomenclature used by operators in the field. In the 1994 review, we noted that the authors had a cumulated 100 years of active 5-HT research to share. A number of our colleagues have, in the meantime, retired from active research or have moved to other professional priorities. The present review provides a comprehensive overview of each of the recognized 5-HT receptors (Table 1) as well as reviewing the roles of 5-HT receptors in the major organs. There is a lot of new “blood” on board to reflect the growing diversity of the research, which is currently performed in many different academic and industrial centers; the combined years in 5-HT research of the present authors has increased considerably, partly because of the expansion of authors to ensure a comprehensive review of 5-HT receptors guided by the IUPHAR subcommittee on 5-HT receptors, which is chaired by Nicholas Barnes and Danny Hoyer.
TABLE 1.
Nomenclature for 5-HT receptors
| 5-HT Receptor Groups | Nomenclature for 5-HT Receptors in the Group | Comments |
|---|---|---|
| 5-HT1 receptors | ||
| 5-HT1A receptor | ||
| 5-HT1B receptor | ||
| 5-HT1D receptor | ||
| 5-ht1e receptor | Lowercase appellation used by convention because a functional response in native cells or tissues has not been identified. | |
| 5-HT1F receptor | ||
| 5-HT2 receptors | ||
| 5-HT2A receptor | ||
| 5-HT2B receptor | ||
| 5-HT2C receptor | ||
| 5-HT3 receptors | Native receptors of unknown stoichiometry: | Five known subunits, 5-HT3A, 5-HT3B, 5-HT3C, 5-HT3D, and 5-HT3E 5-HT3 receptors, are pentameric complexes with the presence of 5-HT3A subunits a prerequisite for function, i.e., only the homomeric 5-HT3A receptor is functional. Heteromeric 5-HT3 receptors are likely to require at least two 5-HT3A subunits. |
| 5-HT3 receptor | ||
| Heterologous expression of known subunits such as | ||
| Homomeric receptor: | ||
| 5-HT3A receptor | ||
| Heteromeric receptor: | ||
| 5-HT3AB receptor | ||
| 5-HT3AC receptor | ||
| 5-HT4 receptor | 5-HT4 receptor | |
| 5-HT5 receptors | 5-HT5A receptor | |
| 5-ht5b receptor | Lowercase appellation is used by convention because a functional response in native cells or tissues has not been identified. | |
| 5-HT6 receptor | ||
| 5-HT7 receptor |
In the present review, we address each receptor separately, as was performed previously, and then have sections that deal with specific aspects in more detail, such as the structures of 5-HT receptors, their functions in the major systems, and translational/clinical outcomes arising from 5-HT research. Readers are also directed to a website (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=1) and the Concise Guide to Pharmacology (Alexander et al., 2019).
II. 5-HT1A Receptor
A. Introduction
5-HT1A receptors have attracted particular interest because of their negative feedback on 5-HT neurons, thus inhibiting 5-HT release and having broad influence on 5-HT tone. Additionally, 5-HT1A receptors are widely distributed in terminal areas of the brain, where they are expressed as postsynaptic heteroceptors in a variety of different brain regions, influencing a range of neuropsychopharmacological sequalae (Albert and Fiori, 2014). After outlining the molecular structure, tissue expression, and the tools that can aid in the delineation of 5-HT1A receptor function, the focus will be on the diverse therapeutic fields in which 5-HT1A receptors have become a target. Accordingly, substantial efforts have focused on targeting 5-HT1A receptors for pharmacotherapy of a variety of neurologic and psychiatric disorders, including major depressive disorder, anxiety, and schizophrenia. In addition, activation or blockade of 5-HT1A receptors has been implicated in control of diverse other effects, including cognition, pain, fear, substance use disorder, and Parkinson disease (PD), and, more recently, in emerging clinical opportunities such as female sexual dysfunction and the treatment of respiratory deficits. The complexity of the effects of 5-HT1A receptors presents both a challenge and a considerable opportunity for investigation of 5-HT function and for the potential identification of novel and improved therapeutic drugs.
B. 5-HT1A Receptor Identification and Expression
The introduction of tritiated [3H] receptor–binding techniques revealed the existence of 5-HT1 (and 5-HT2) receptor families in the prefrontal cortex (PFC) of the brain (Peroutka and Snyder, 1979), and extended studies indicated the existence of different 5-HT1 receptor populations, designated, for the first time, 5-HT1A and 5-HT1B receptors (Pedigo et al., 1981; Middlemiss and Fozard, 1983), leading to a much greater understanding of the pharmacological and functional role of the 5-HT1A receptor in health and disease.
The cloning of the 5-HT1A receptor from various species confirmed the existence of 5-HT1A receptors as distinct gene products that correlated with pharmacologically defined receptor responses (Table 2).
TABLE 2.
Overview of the amino acid structure, gene loci, and symbols and gene name of the human, mouse, rat, dog, and rhesus macaque monkey for 5-HT1A receptors
High receptor homology, but homology in binding receptor domains is higher. Homologs described for other nonhuman primates, mosquito, Gallus gallus domesticus, Danio rerio, Caenorhabditis elegans, Drosophila melanogaster, Bos taurus, Xenopus laevis, Cavia porcellus, Equus caballus, etc. A total of 160 species have orthologs of the human receptor (https://www.ncbi.nlm.nih.gov/gene/?Term=ortholog_gene_3350[group]; http://www.ncbi.nlm.nih.gov/probe/?term=479890[unistsid]).
| Species | Bp | AA | AA Homology with Human (%) | Chromosome Location | Gene Symbol |
|---|---|---|---|---|---|
| Humana,b | 1269 | 422 | 100% | 5q11.2-q13 | HTR1A |
| Mousec | 1266 | 421 | 88% | 13 D2.1 | Htr1a |
| Ratd,e | 1269 | 422 | 90% | 2q16 | Htr1a |
| Dogf | 1272 | 423 | 92% | ? | HTR1A |
| Monkeyg | 1266 | 421 | 98% | 6.122.4cr | HTR1A |
AA, number of amino acids; Bp, coding base pairs.
Partly adapted from Andrade et al. (2019).
Macaca mulatta.
The 5-HT1A receptor has been located in a wide variety of peripheral and central targets. In the periphery, immunohistochemical studies have demonstrated that the receptor is located in human and rat kidney, including medulla and cortical ascending limbs, the convoluted tubules, connecting tubule cells, and the principal cells of the initial collecting tubule (Raymond et al., 1993), and murine peritoneal macrophages (Freire-Garabal et al., 2003). However, other techniques have revealed a wider distribution: Western blotting found the receptor in human benign and malignant prostate tissue (Dizeyi et al., 2004), whereas reverse transcriptase polymerase chain reaction (RT-PCR) demonstrated the presence of 5-HT1A receptors in rat taste buds (Kaya et al., 2004). However, the receptor is relatively poorly expressed in human coronary arteries, heart atrium, heart ventricles, and epicardium (Nilsson et al., 1999a,b). The brain and spinal cord have particularly dense populations of 5-HT1A receptors, consistent with the role of this receptor in neuropsychiatric disease. The use of 5-HT1A receptor agonists has been linked with the management of pain; accordingly, radioligand-binding and in situ hybridization studies have indicated the presence in the human and rat dorsal and ventral horns (Pompeiano et al., 1992; Laporte et al., 1996) and rat superior cervical ganglia (Pierce et al., 1996). In the brain, a wide distribution of the receptor has been described in both terminal regions as postsynaptic sites and in the raphe nuclei, where it has a somatodendritic autoreceptor function (Jacobs and Azmitia, 1992; Fornal et al., 1994). Generally, there is much conservation in regional expression across species, although rat-human cortical and hippocampal differences in laminar organization were reported (Burnet et al., 1995; Barnes and Sharp, 1999). Within the brain, different techniques, including receptor binding, RT-PCR, in situ hybridization (Fig. 1), Western and Northern blotting, and immunohistochemistry, have localized the receptor to the septum, thalamus, hippocampus, entorhinal cortex, interpeduncular nucleus, olfactory bulb, amygdala, hypothalamic subnuclei, and subareas of the cortex and raphe nuclei (Gozlan et al., 1983; Hall et al., 1985; Pazos and Palacios, 1985; Weissmann-Nanopoulos et al., 1985; Dourish et al., 1986; Hoyer et al., 1986a; Vergé et al., 1986; Daval et al., 1987; Hamon et al., 1988; Albert et al., 1990; Hirose et al., 1990; Chalmers and Watson, 1991; Radja et al., 1991; Francis et al., 1992; Miquel et al., 1992; Pompeiano et al., 1992; Khawaja, 1995; Kung et al., 1995; Pike et al., 1995; Lemoine et al., 2010, 2012). More particularly, 5-HT1A receptors are located on septal cholinergic neurons, cortical and hippocampal glutamatergic pyramidal neurons and granule cells (Francis et al., 1992; Pompeiano et al., 1992; Burnet et al., 1995), and calbindin- and parvalbumin-positive neurons (Aznar et al., 2003).
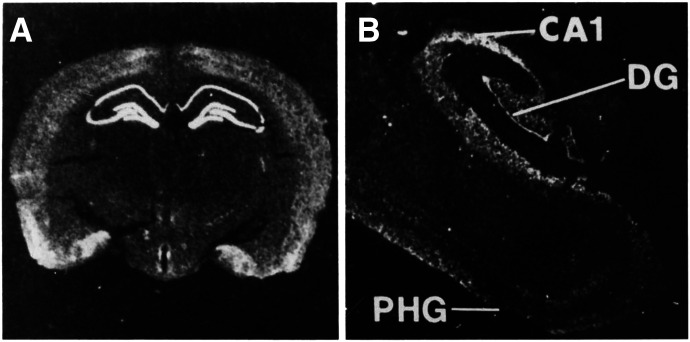
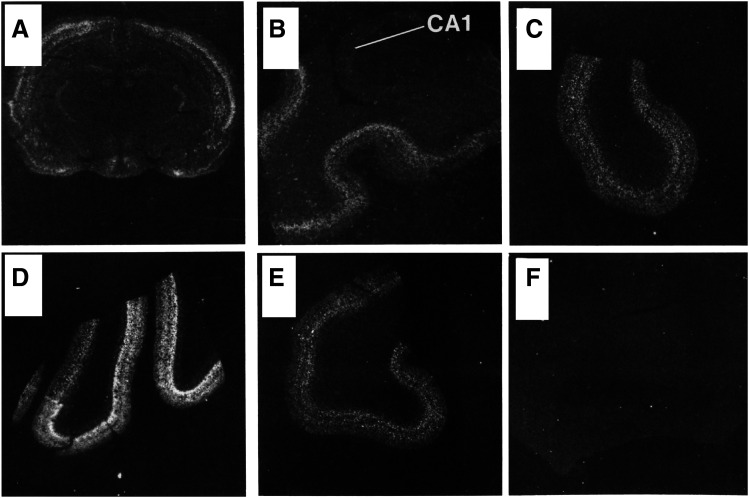
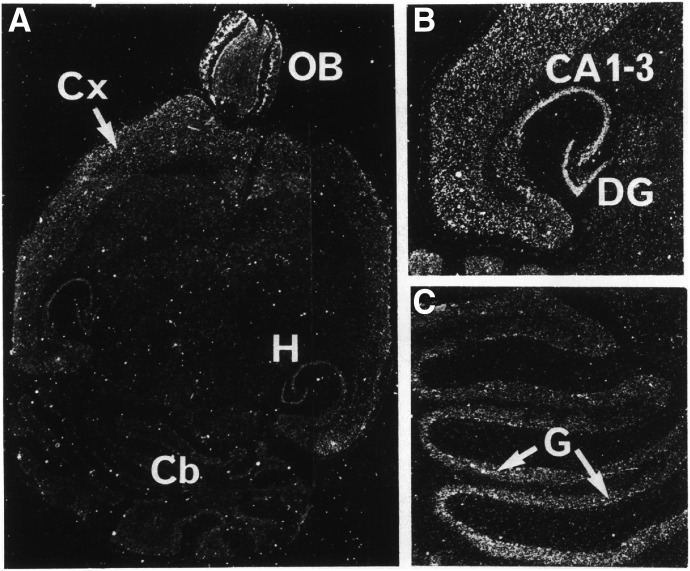
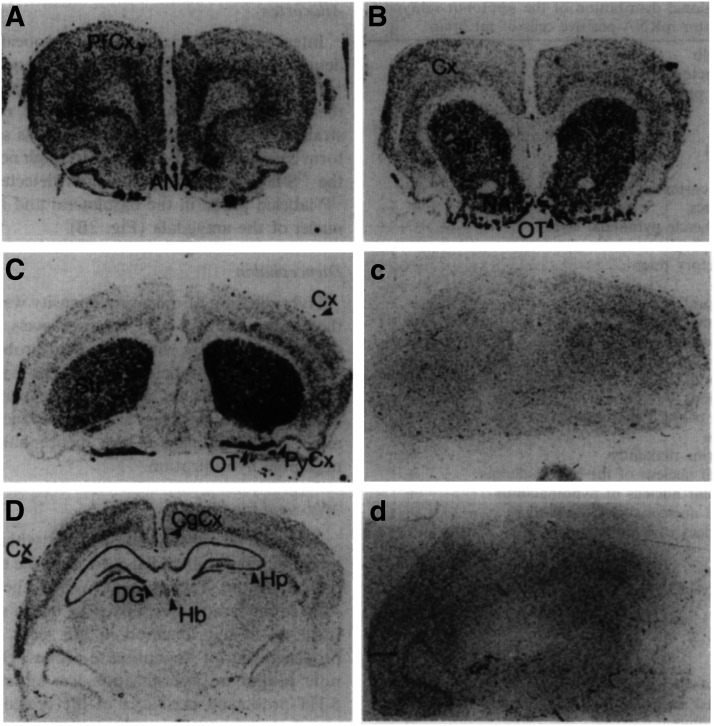
Fig. 1.
In situ hybridization detection of 5-HT1A receptor mRNA expression in rat (A) and human brain (B) at the level of the hippocampus. CA1, dentate gyrus (DG) of the hippocampus, and parahippocampal gyrus (PHG) are shown. Adapted from Burnet et al. (1995) (with permission).
C. Pharmacology
In view of the involvement of 5-HT1A receptors in a wide variety of physiologic responses, the pharmacological profile of these receptors has been investigated extensively using an impressive variety of ligands, with varying degrees of selectivity. These range from drugs preferentially targeting 5-HT1A receptors to nonselective compounds that have broad pharmacological activities. Examples of the latter are atypical antipsychotic drugs such as clozapine, ziprasidone, or aripiprazole, which interact with many receptor subtypes. Notably, there are currently no selective 5-HT1A receptor drugs approved for therapeutic use. This is somewhat surprising in view of the broad therapeutic interest of 5-HT1A receptors but likely reflects the difficulty of identifying chemical scaffolds that selectively engage this target. For example, the anxiolytic agent, buspirone, and its chemical analogs such as ipsapirone and gepirone lack selectivity over some other receptors (for example, buspirone displays submicromolar affinity for dopamine D2, D3, and D4 receptors; 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptors; and α1 adrenoceptors). Similarly, several antagonist ligands have been proposed, but few have proved to be selective “silent antagonists.” Nevertheless, some recent “full agonists” (notably befiradol) have been identified that exhibit good selectivity for 5-HT1A receptors and, as such, may constitute first-in-class therapeutic agents.
Tables 3 and 4 summarize the receptor-binding properties of many 5-HT1A receptor ligands that have been described over the last decades. It is also worth noting that even though certain compounds do display measurable receptor-binding affinity, this may be too low to induce functional responses at the 5-HT1A receptor. Such an example is olanzapine, fails to elicit electrophysiological actions at the level of somatodendritic autoreceptors in contrast to ziprasidone and clozapine (Sprouse et al., 1999). Many of the ligands have been decisive in the operational definition of biochemical and pharmacological function at a basic science level and in key disease models. In addition to the receptor agonists and antagonists, there is some evidence for the existence of allosteric modulators, such as zinc, Galphimine-B, and RS-30199 (Spedding et al., 1998; Barrondo and Sallés, 2009; Jimenez-Ferrer et al., 2011).
TABLE 3.
Receptor-binding characteristics of 5-HT1A receptor agonists
Data are extracted and adapted from Colpaert et al. (2002), Glennon et al. (2006), McCreary et al. (2007), Andrade et al. (2019), and McCreary and Newman-Tancredi (2019).
| Agonist | Agonist Action | Affinity | Units | Clinical Utility |
|---|---|---|---|---|
| 1-naphthylpiperazine | Full | 8 | pKi | |
| 5-CT | Full | 9.4–10.3 | pKi | |
| 5-hydroxytryptamine | Full | 9.1–9.7 | pKi | |
| 7-methoxy-1-naphthylpiperazine | Full | 8.6 | pKi | |
| 8-OH-DPAT | Full | 8.4–9.4 | pKi | |
| (R)-UH 301 | Partial | 8.6 | pKi | |
| Adoprazine (SLV313) | Full/Partial | 8.64–9.1 | pKi | Schizophrenia |
| Apomorphine | Partial | 6.9 | pKi | PD, erectile dysfunction |
| Aripiprazole | Full | 8.2 | pKi | Schizophrenia |
| Asenapine | Full | 8.0–8.3 | pKi | Schizophrenia |
| BMY-7378 | Partial | 6.8–8.0 | pIC50 | |
| BMY-1480 | Full | 7.2 | pKi | |
| Befiradol | Full | 9.1 | pKi | PD dyskinesia |
| Bifeprunox | Partial | 7.19–8.95 | pKi | Schizophrenia |
| Brexpiprazole | Partial | 9.92 | pKi | Schizophrenia |
| BRL-15572 | Partial | 7.7 | pKi | |
| Bromocriptine | Partial | 7.9 | pKi | PD |
| Buspirone | Partial | 7.7–8.0 | pKi | Anxiety |
| Cabergoline | Full | 7.7 | pKi | PD |
| Capeserod | Partial | 6 | pKi | |
| Cariprazine | Partial | 8.59 | pKi | Schizophrenia |
| Clozapine | Full | 6.8–6.9 | pKi | Schizophrenia |
| CP 93129 | Full | 6.1 | pKi | |
| Donitriptan | Full | 7.6 | pKi | Migraine |
| Eletriptan | Full | 7.4 | pKi | |
| Eltoprazine | Partial | 8.03 | pKi | |
| EMDT | Full | 6.8 | pKi | |
| F13714 | Full | 10.1 | pKi | |
| F15063 | Partial | 8.24 | pKi | |
| F15599 | Full | 8.6 | pKi | Rett syndrome |
| FG-5893 | Full | 8.7 | pKi | |
| Flesinoxan | Full | 9.3 | pKi | Major depression |
| Flibanserin | Agonist | 9 | pKi | Female hypoactive sexual desire |
| Fluparoxan | Partial | 6.8 | pKi | |
| Frovatriptan | Agonist | 7.2 | pKi | |
| GR127935 | Partial | 7.1–7.2 | pKi | |
| Ipsapirone | Partial | 8.6–8.8 | pKi | |
| L-694,247 | Full | 9.3 | pKi | |
| L-772,405 | Full | 7.2 | pIC50 | |
| Lisuride | Full | 9.7–9.8 | pKi | Migraine |
| LP-12 | Agonist | 7.2 | pKi | |
| LP-44 | Agonist | 7.3 | pKi | |
| LP-211 | Agonist | 6.7 | pKi | |
| LSD | Full | 9 | pKi | |
| Lurasidone | Full | 8.17 | pKi | Schizophrenia |
| LY293284 | Full | 10.1 | pKi | |
| LY334370 | Full | 7.8 | pKi | |
| LY344864 | Full | 6.3 | pKi | |
| LY 165,163 | Full | 8.9 | pKi | |
| Nafadotride | Full | 7.3 | pKi | |
| Naratriptan | Full | 7.1–7.6 | pKi | Migraine |
| Nemonapride | Partial | 8.35 | pKi | Schizophrenia |
| Ocaperidone | Full | 8 | pKi | |
| Olanzapine | Full | 5.6–5.8 | pKi | Schizophrenia |
| Pardoprunox (SLV308) | Full | 8.5 | pKi | PD |
| Pergolide | Partial | 8.7 | pKi | PD |
| Piribedil | Partial | 6.4 | pKi | PD |
| Quetiapine | Full | 6.5–6.6 | pKi | Schizophrenia |
| Quinpirole | Full | 5.8 | pKi | |
| Repinotan | Full | 9.4 | pKi | |
| Rizatriptan | Full | 6.4 | pKi | Migraine |
| Roxindole | Partial | 9.4–9.9 | pKi | |
| RU 24969 | Full | 9 | pKi | |
| S 16924 | Partial | 8.4 | pKi | |
| S-14506 | Full | 9.6–9.7 | pKi | |
| S-14671 | Full | 10.2–10.5 | pKi | |
| S-15535 | Partial | 9.2 | pKi | |
| Sarizotan | Partial | 8.65 | pKi | PD dyskinesia |
| SB 216641 | Partial | 6.3 | pKi | |
| Spiroxatrine | Full | 8.8 | pKi | |
| SSR181507 | Partial | 8.53 | pKi | |
| Sumatriptan | Full | 6 | pKi | Migraine |
| Tandospirone | Partial | 8.2 | pKi | Anxiety |
| Terguride | Partial | 8.5 | pKi | PD |
| U92016A | Full | 9.7 | pKi | |
| Vilazodone | Partial | 9.7 | pKi | Depression |
| Vilazodone | Partial | 9.5 | pIC50 | Depression |
| Vortioxetine | Partial | 7.8 | pKi | Depression |
| WAY-100135 | Partial | 8 | pKi | |
| Xanomeline | Full | 7.2 | pKi | |
| Zalospirone | Full | 8.1 | pKi | |
| Ziprasidone | Partial | 7.9–8.9 | pKi | Schizophrenia |
| Zolmitriptan | Full | 6.6 | pKi | Migraine |
EMDT, 2-Ethyl-5-methoxy-N,N-dimethyltryptamine.
TABLE 4.
Receptor-binding characteristics of 5-HT1A receptor antagonists
Table adapted from Andrade et al. (2019).
| Antagonist | Affinity | Units |
|---|---|---|
| (+)-butaclamol | 6.4 | pKi |
| (-)-propranolol | 7.5 | pKi |
| (-)-tertatolol | 8.2 | pKi |
| (R)-flurocarazolol | 6.5 | pKi |
| (S)-flurocarazolol | 7.5 | pKi |
| (S)-UH 301 | 7.9 | pKi |
| [3H]p-MPPF | 8.4 | pKd |
| [3H]robalzotan | 9.8 | pKd |
| [3H]WAY100635 | 9.5 | pKd |
| [11C]WAY100635 | — | — |
| Chlorpromazine | 6.2 | pKi |
| Cyamemazine | 6.3 | pKi |
| Fluspirilene | 7.2 | pKi |
| GR 125,743 | 7.3 | pKi |
| GR 218,231 | 6.8 | pKi |
| Haloperidol | 5.7–5.8 | pKi |
| Iloperidone | 6.8–7 | pKi |
| Ketanserin | 5 | pKi |
| Mesoridazine | 7 | pKi |
| Methiothepin | 7.8–8.1 | pKi |
| MPDT | 5.8 | pKi |
| NAN 190 | 9.4 | pKi |
| p-[18F]MPPF | — | — |
| p-MPPI | 8.4 | pKi |
| Pimozide | 6.8 | pKi |
| Pindolol | 8.1 | pKi |
| Pipamperone | 5.6 | pKi |
| Pizotifen | 7.4 | pKi |
| Raclopride | 5.2 | pKi |
| Rec 15/3079 | 9.7 | pKi |
| Risperidone | 6.2–6.5 | pKi |
| 9-OH-risperidone | 6.2 | pKi |
| ritanserin | 5.2–5.5 | pIC50 |
| robalzotan | 9.2 | pKi |
| SB 272183 | 8 | pKi |
| SB 649915 | 8.6 | pKi |
| SB 714786 | 6.5 | pKi |
| SDZ-216525 | 7.8–8.2 | pIC50 |
| Sertindole | 6.4–6.6 | pKi |
| Spiperone | 6.7–8.8 | pKi |
| Thioridazine | 7.1 | pKi |
| Tiospirone | 8.3 | pKi |
| WAY-100635 | 7.9–9.2 | pKi |
| Yohimbine | 7.3 | pKi |
| Zotepine | 6.5 | pKi |
MPPF, 2'-methoxyphenyl–p-fluoro-benzamidoethyipiperazine.
The use of [35S]GTPγS binding, a nonhydrolysable analog of GTP that binds to agonist-activated G proteins, has proved useful for investigating 5-HT1A receptor signaling and pharmacology (Newman-Tancredi et al., 1996b, 1997b, 1998; Barr and Manning, 1997; Pauwels et al., 1997; Sim et al., 1997; Stanton and Beer, 1997; Dupuis et al., 1999a,b; Cosi and Koek, 2000; Gonzalez-Maeso et al., 2000; McLoughlin and Strange, 2000; Shen et al., 2002; Odagaki and Toyoshima, 2005a,b, 2007). Notably, the use of [35S]GTPγS binding enabled the investigation of both positive and negative efficacy ligands at 5-HT1A receptors. Thus, whereas a range of ligands efficaciously stimulated G proteins, other drugs, such as spiperone and methiothepin, markedly inhibited the [35S]GTPγS basal binding in both membranes prepared from 5-HT1A receptor–transfected Chinese Hamster Ovary (CHO) cells and native tissue, confirming the capacity of 5-HT1A receptors to elicit constitutive activation of G proteins in vitro (Newman-Tancredi et al., 1997a; Stanton and Beer, 1997; McLoughlin and Strange, 2000; Corradetti et al., 2005; Martel et al., 2007). In contrast to spiperone, WAY1000635 exhibited neither positive nor negative efficacy yet blocked the actions of both agonists and inverse agonists, consistent with “neutral antagonist” properties (Fletcher et al., 1996; Martel et al., 2007) also evident in vivo using electrophysiological procedures (e.g., Fornal et al., 1996). This was important because other compounds claimed as antagonists at 5-HT1A receptors, such as NAN190, BMY7378, SDZ216,525, and even WAY100135, were found to display partial agonist properties when tested in systems that exhibit high degrees of receptor reserve (Greuel and Glaser, 1992; Routledge, 1996); changes in receptor expression level can markedly affect functional responses, and this is important when considering the nature of ligand engagement and the notion that different brain areas exert distinct physiologic influence (Newman-Tancredi et al., 1997c). A threefold increase in receptor:G protein ratio almost doubled relative efficacy of the partial agonist eltoprazine (53%–93%), without a change in potency, whereas 5-HT exhibited a twofold increase in potency (decrease in EC50 value) (Newman-Tancredi et al., 1997c). In addition to these changes, the increase in 5-HT1A receptor:G protein ratio roughly doubled the negative efficacy of spiperone. These data therefore lead to the supposition that the targeting of agonist efficacy in vivo at different receptor populations is possible, which may offer therapeutic benefits.
D. Biased Agonism: Differential Activation of 5-HT1A Receptor Subpopulations
The term “biased agonism” (“functional selectivity” or “agonist-directed signaling”) (Berg and Clarke, 2006; Evans et al., 2010; Kenakin, 2010; Tzingounis et al., 2010) was coined to denote a pattern of agonist signaling that was distinct from the concept of “intrinsic activity.” Whereas the latter posits that receptor activation is an outcome of the “intrinsic” properties of the agonist, the concept of “biased agonism” is based on the capacity of agonists to preferentially mediate receptor signaling via specific pathways while not affecting, or even blocking, other secondary messenger pathways coupled to the same receptor. If the different signaling cascades mediate distinct functionality (e.g., therapeutic vs. side effects), then biased agonism will offer a strategy to potentially target different mechanisms with the opportunity to potentially develop more effective, better-tolerated drugs.
An early study of 5-HT1A receptors suggested that different agonists displayed differential Gαi2 and Gαi3 activation, determined using a photoreactive GTP analog (4-azidoanilido-[α-32P]GTP) (Gettys et al., 1994). Rauwolscine displayed similar EC50 values for activation of the two G protein subtypes; ipsapirone showed a nearly fourfold lower EC50 for Gαi3 activation. 5-HT and 8-OH-DPAT had intermediate EC50 values (Gettys et al., 1994). In another study, the presence of anti-Gαi3 antibodies almost completely suppressed G protein activation by pindolol, a 5-HT1A receptor partial agonist that preferentially elicits activation of Gαi3, a property that may underlie its preferential occupancy of midbrain 5-HT1A autoreceptors (Hirani et al., 2000; Martinez et al., 2001; Newman-Tancredi et al., 2002). Drug differences were also seen in transduction experiments on native rat raphe; buspirone elicited Gαi2-, Gαi3-, and Gαo-mediated responses as well as inhibition of adenylyl cyclase (AC), whereas 8-OH-DPAT only elicited coupling to Gαi3 and did not elicit the other responses (Valdizán et al., 2010).
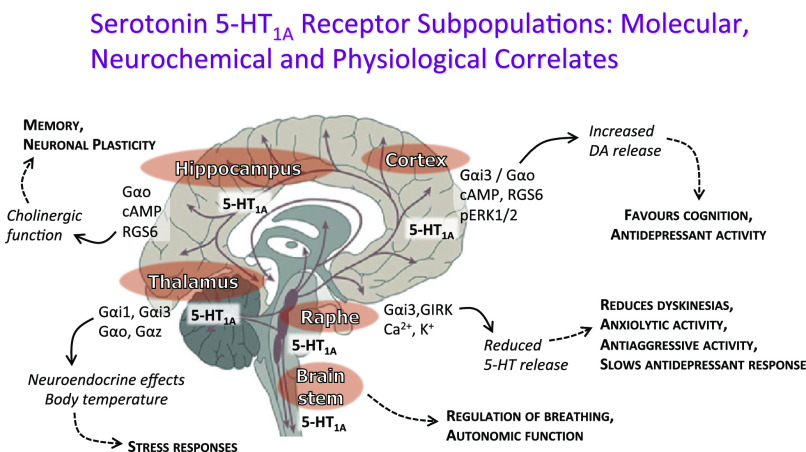
Together, these data support that different 5-HT1A receptor agonists possess different G protein activation “fingerprints,” backing the biased agonist concept and hence suggesting that 5-HT1A receptor subpopulation targeting is possible (Fig. 2). Compounds such as the biased 5-HT1A receptor agonists, F15599 and F13714, reversed immobility in the rat forced swim test via actions at presumed postsynaptic receptors. Similarly, anxiolytic-like actions were seen in the rat ultrasonic vocalization test (De Vry et al., 1993; Assié et al., 2010). However, in animal tests related to side effects, F15599 exhibited a better profile compared with F13714 (Gaggi et al., 1997; Prinssen et al., 2000; Assié et al., 2010), further supporting the potential for improved therapeutics utilizing biased agonists to target the appropriate 5-HT1A receptor subpopulation (Table 5), which includes potential to improve the cognitive state of patients with schizophrenia (Depoortère et al., 2010; Horiguchi and Meltzer, 2012).
Fig. 2.
Biased agonism at the 5-HT1A receptor offers the potential to target subpopulations of 5-HT1A receptors.
TABLE 5.
Comparison of properties of 5-HT1A receptor “biased agonists” F15599, F13714, and befiradol, and the reference agonists 8-OH-DPAT and 5-HT
Based on the publications indicated below. Target brain regions are those identified in microPET imaging and neurochemical experiments. In vivo readouts are nonexhaustive and focus on the primary activities of the biased agonists.
| Agonist | In Vitro Affinity/Selectivitya | Cellular Transduction Pathwaysb | Target Brain Regionsc | Readout In Vivod | Relevant Therapeutic Indications |
|---|---|---|---|---|---|
| F15599 | Nanomolar/highly selective | Preferential pERK activation | Cortex, brain stem | Reverses PCP-induced cognitive deficits, active in antidepressant and anxiolytic tests. Normalizes breathing in MeCP2+/− mice | Cognitive deficits, mood disorders, respiratory difficulties (Rett syndrome) |
| F13714 | Subnanomolar/highly selective | Multiple (pERK, receptor internalization, G protein, cAMP, Ca2+ release) | Mid-brain, thalamus, hippocampus, | Potently eliminates L-DOPA–induced AIMs, active in antidepressant and anxiolytic tests | Not clinically tested (research tool) |
| Befiradol | Nanomolar/highly selective | Multiple (pERK, receptor internalization, G protein, cAMP, Ca2+ release) | Mid-brain, cortex, thalamus, hippocampus | Potently eliminates L-DOPA–induced AIMs, active in antidepressant and anxiolytic tests | Dyskinesias in Parkinson disease, mood deficits, chronic pain |
| 8-OH-DPAT | Nanomolar/binds 5-HT7 | Preferential pERK activation | Hippocampus, mid-brain, cortex, thalamus, brain stem | Disparate effects on cognition tests, active in antidepressant and anxiolytic tests, reduces L-DOPA–induced AIMs | Not clinically tested (research tool) |
| 5-HT | Nanomolar/ nonselective | Multiple (pERK, receptor internalization, G protein, cAMP, Ca2+ release) | All 5-HT projection areas | N/A |
N/A, not applicable.
Colpaert et al., 2002; Pauwels and Colpaert, 2003; Buritova et al., 2009; Newman-Tancredi et al., 2009b.
Lemoine et al., 2010, 2012; Lladó-Pelfort et al., 2010, 2012; Vidal et al., 2014.
Assié et al., 2010; Depoortere et al., 2010; Levitt et al., 2013; Iderberg et al., 2015; van Goethem et al., 2015.
E. 5-HT1A Receptor Intracellular Signal Transduction
The transfection (Fargin et al., 1988) and heterologous expression of 5-HT1A receptors in various different cellular environments (including COS7, HeLa, CHO, NIH3T3, Sf9, and Escherichia coli cells) enabled the study of their G protein coupling to secondary messenger systems (Raymond et al., 1999). A well characterized intracellular functional response is the inhibition of AC activity and has been extensively used to differentiate ligands for this receptor, their agonist and partial agonist actions, or their degree of antagonism (De Vivo and Maayani, 1986; Markstein et al., 1986; Bockaert et al., 1987; Shenker et al., 1987; Dumuis et al., 1988b; Fargin et al., 1989; Varrault and Bockaert, 1992; Raymond et al., 2006). 5-HT1A receptors can also activate G protein inward rectifying potassium channels (GIRK), high-conductance anion channels to inhibit calcium conductance modulating intracellular calcium mobilization, and stimulate nitric oxide synthase (NOS) and an NADP oxidase-like enzyme (Adayev et al., 2003; Hsiung et al., 2005; Polter and Li, 2010). The receptor can affect metabolism and arachidonic acid (AA) production (Raymond et al., 1999); activate protein kinase C production, Src kinase, and mitogen-activated protein kinases (MAPKs); and activate or inhibit phosphoinositol hydrolysis and stimulate reactive oxygen species (ROS) production (superoxide and peroxide) (Raymond et al., 1999). Together, the elucidation of this diverse pattern has led to important developments in establishing test systems to probe receptor and drug function.
F. Function
1. Differential Function of 5-HT1A Receptors at Cellular, Tissue, and In Vivo Levels
The functional properties of 5-HT1A receptors have been extensively investigated. The overall conclusion from these studies is that subpopulations of 5-HT1A receptors expressed in different brain regions exhibit specific patterns of receptor signaling, with differing impact on central function. These diverse properties indicate that separate subpopulations of 5-HT1A receptors mediate particular responses and may constitute therapeutic targets in their own right (see also Fig. 2). For example, agonist activation of somatodendritic 5-HT1A autoreceptors expressed on serotonergic neurons in the raphe elicits inhibition of 5-HT release in terminal regions such as the hippocampus and cortex. In contrast, activation of postsynaptic cortical 5-HT1A heteroreceptors expressed on glutamatergic pyramidal cells and/or GABAergic interneurons elicits different neurochemical responses, including stimulation of dopamine release in the frontal cortex (Santana et al., 2004; Bortolozzi et al., 2010).
Activation of 5-HT1A autoreceptors induces anxiolytic activity in rodent behavioral tests (De Vry et al., 2004; Akimova et al., 2009), whereas antidepressant-like responses are seen upon activation of 5-HT1A heteroceptors (De Vry et al., 2004). These data obtained in rat behavioral experiments are consistent with observations in transgenic mice overexpressing raphe 5-HT1A autoreceptors; accentuated depressive-like behavior was observed and diminished response to antidepressant treatment (Richardson-Jones et al., 2010). These data support the interpretation that desensitization of presynaptic 5-HT1A receptors is necessary before antidepressant efficacy may be achieved (Artigas et al., 2006; Millan, 2006), consistent with the relatively long latency (typically 3 to 4 weeks) to clinical responsivity in patients with depression treated with 5-HT reuptake inhibitors.
Diverse responses to 5-HT1A receptor agonists are also observed in tests of cognition/memory function relevant to numerous neuropsychiatric diseases, including major depressive disorder, schizophrenia, Parkinson disease, and Alzheimer disease. Interestingly, the prototypical 5-HT1A receptor agonist, 8-OH-DPAT, facilitated rat passive avoidance at low doses, whereas higher doses impaired performance (Lüttgen et al., 2005; Madjid et al., 2006). This suggests that opposite responses are mediated by 5-HT1A receptor subpopulations (i.e., improved performance is elicited by 5-HT1A autoreceptors, whereas impairment is due to activation of hippocampal 5-HT1A heteroreceptors) (Ogren et al., 2008). This interpretation is supported by local administration experiments in which the 5-HT1A receptor weak partial agonist/antagonist S15535 was microinjected into the hippocampus. The compound reversed the memory deficit elicited by systemic injection of 8-OH-DPAT in a spatial discrimination task (Millan et al., 2004), indicating that activation of postsynaptic receptors in this brain region was detrimental to mnesic performance.
Given that only a single 5-HT1A receptor gene has been identified in human and rat, and that it is intronless and hence without splice variants (Fargin et al., 1988; Albert et al., 1990; Kobilka et al., 1987), the variety of responses described above are likely attributable to regional “receptor interactome” differences, including coupling to distinct G protein subtypes (see below) (Mannoury la Cour et al., 2006), regulators of G protein signaling (RGS) (Talbot et al., 2010), or transcriptional regulation.
At a molecular level, 5-HT1A receptor inactivation studies using N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline demonstrated the existence of receptor reserve in the raphe for inhibition of 5-HT synthesis (Meller et al., 1990). In contrast, receptor reserve was not evident in the hippocampus for the inhibition of adenylyl cyclase or for control of hypothermia (Meller et al., 1992; Yocca et al., 1992).
The agonist radioligand, [3H]8-OH-DPAT, which preferentially recognizes 5-HT1A receptors when coupled to G proteins, displayed fivefold higher affinity for hippocampal compared with raphe binding sites (Johnson et al., 1997b), supporting differing receptor–G protein coupling state between the two brain regions. Furthermore, whereas 5-HT1A receptors are coupled to inhibition of adenylyl cyclase in hippocampus, such coupling was not detected in raphe homogenates (Clarke et al., 1996). Further support that 5-HT1A receptors couple to different G protein subtypes depending on brain region arises from immunoprecipitation studies in which raphe 5-HT1A receptors couple preferentially to Gαi3 subtypes, whereas they couple preferentially to Gαo in hippocampus and to a combination of G proteins in cortex and hypothalamus (Mannoury la Cour et al., 2006).
Functional ([35S]GTPγS) autoradiography experiments also support the contention that regional variations exist in native brain activation of G proteins by 5-HT1A receptors. Indeed, whereas 5-HT1A receptor density is similar in the raphe and hippocampus, agonist-induced [35S]GTPγS labeling was markedly lower in the former [Hensler, 2003; see also Newman-Tancredi et al. (2003) for relevant evidence].
An additional level of complexity of 5-HT1A receptor signaling has been reported (i.e., the existence of receptor homo-, hetero-, and potential trimers with a variety of targets). First, 5-HT1A homodimers may be formed constitutively (Łukasiewicz et al., 2007; Renner et al., 2012) and are affected by the presence of selective ligands such as 8-OH-DPAT, which enhanced dimerization, whereas methysergide reduced dimer formation potentially via a mechanism modulated by Gαs subunits. The 5-HT7 receptor, like the 5-HT1A receptor, has been reported to play a role in depression and form homodimers; but it has also heterodimerized with the 5-HT1A receptor and may have functional consequences insofar as 5-HT1A–5-HT7 heterodimerization reduces GIRK currents in a heterologous cell system, potentially affecting 5-HT1A receptor internalization in the hippocampus (Renner et al., 2012; Naumenko et al., 2014). Heterodimerization with a novel negative response element of 5-HT1A receptors has been suggested with glucocorticoid and mineralocorticoid receptors, which may also be key players in depression (Ou et al., 2001). Galanin receptors form heteromers with a variety of targets, including galanin receptor–5-HT1A heteromers and trimers (Fuxe et al., 2012). With potential relevance to the influence of 5-HT1A receptors in ascending and central pain perception, heterodimerization has also been demonstrated with 5-HT1A receptors and µ-opioid receptors in vitro, and further data suggested that both receptors could exert effects on extracellular signal–regulated kinase (ERK)1/2 phosphorylation (Cussac et al., 2012).
a. 5-HT1A receptors in depression and anxiety
The key role of 5-HT1A receptors in major depression and anxiety has been recognized for nearly four decades [see Barnes and Sharp (1999); Albert et al. (2014)]. Accordingly, animal behavioral models of fear, anxiety, depression, and cognition have been used to identify potential antidepressant and clinically active anxiolytics, such as the partial agonists buspirone, gepirone, ipsapirone, and tandospirone (Peroutka, 1985; Taylor et al., 1985; Gilbert and Dourish, 1987). Buspirone and tandospirone were both clinically developed and received marketing approval for treating anxiety. However, their azapirone chemical structures are associated with only limited selectivity (e.g., vs. α1 adrenoceptors and D2 dopamine receptors), and they also exhibit relatively poor metabolic stability and generate pharmacologically active metabolites, such as 1-(2-pyrimidinyl)-piperazine, which is an α2 adrenoceptor antagonist (Garattini et al., 1982; Cao and Rodgers, 1997; Zuideveld et al., 2002; Sugimoto et al., 2005; Wong et al., 2007). Consequently, the therapeutic usefulness of selective 5-HT1A receptor agonists still remains to be determined.
Indeed, data suggest that the phenotypic expression of normal behavior, anxiety, or depression may be influenced by the differential 5-HT1A receptor–sensitive circuitry at the level of the PFC; the balance between 5-HT1A receptor stimulation of glutamatergic pyramidal cells and GABAergic interneurons may impact the expression of anxiety (Goodfellow et al., 2009; Albert et al., 2014), although juvenile development processes play a key role in determining vulnerability to mood disorders (Leonardo and Hen, 2008; Donaldson et al., 2014; Garcia-Garcia et al., 2014). In depression, the neurobiology appears different. Therefore, activation of pyramidal neurons by stimulation of 5-HT1A receptors expressed on GABAergic interneurons disinhibits the “antidepressive” pyramidal neurons (Albert et al., 2014). It is interesting to note that the rapid antidepressant activity of ketamine appears to be partly mediated via 5-HT1A receptor activation. Indeed, ketamine inhibits 5-HT reuptake in vivo (Martin et al., 1982; Martin and Smith, 1982) and elicits its prolonged antidepressant-like effects in rodents via a 5-HT–dependent mechanism (Gigliucci et al., 2013). This is likely to involve indirect activation of 5-HT1A receptors, as exemplified by the fact that the effects of ketamine in the novelty-suppressed feeding test are blocked by a 5-HT1A receptor antagonist (Fukumoto et al., 2014).
Additional evidence that 5-HT1A receptors are involved in affective disorders comes from genetic studies. The expression of 5-HT1A receptors is differentially regulated by a single-nucleotide polymorphism (SNP) in the promoter region of the 5-HT1A receptor gene (C-1019G substitution) (Lesch and Gutknecht, 2004; Albert and Francois, 2010). This SNP impairs repression of the 5-HT1A promoter by the nuclear DEAF-1-related/drosophila deformed epidermal autoregulatory factor-1 transcription factors in raphe cells, consistent with overexpression of presynaptic 5-HT1A receptors (Lemonde et al., 2004; Parsey et al., 2006). Thus, C-1019G polymorphism is associated with higher levels of symptom remission failure and suicidal behavior in patients with depression (Lemonde et al., 2003), consistent with impaired antidepressant efficacy caused by excessive feedback inhibition by presynaptic 5-HT1A receptors.
Taken together, the above considerations indicate that 5-HT1A receptors remain promising targets for the pharmacotherapy of affective disorders, both as a somatodendritic and postsynaptic receptor target in the brain. Accordingly, various efforts have been made to incorporate 5-HT1A receptor activity in antidepressant/anxiolytic drug candidates For example, SB-649915-B is a 5-HT reuptake inhibitor (SSRI) that also acts as a 5-HT1A receptor antagonist (Hughes et al., 2007; Starr et al., 2007) based on the rationale that accelerated antidepressant response may be achieved by avoiding feedback inhibition of terminal 5-HT release by blocking the activation of 5-HT1A autoreceptors (Gartside et al., 1999; Artigas et al., 2006; Portella et al., 2011). However, though antidepressant efficacy may be enhanced by 5-HT1A autoreceptor antagonism, the blockade of postsynaptic 5-HT1A receptors likely opposes antidepressant activity (De Vry et al., 2004; Berrocoso and Mico, 2009). Accordingly, a clinical trial in which a selective 5-HT1A receptor antagonist was administered as adjunct to fluoxetine did not show any acceleration of antidepressant onset of efficacy (Scorza et al., 2012), likely because of its concurrent blockade of both pre- and postsynaptic 5-HT1A receptors. In contrast, adjunct treatment with pindolol, which preferentially occupies 5-HT1A autoreceptors (Martinez et al., 2001), appears to reliably elicit acceleration of antidepressant efficacy (Artigas et al., 1996, 2006; Portella et al., 2011). Compounds such vilazodone, vortioxetine, and VN2222 are SRIs possessing partial agonist actions at 5-HT1A receptors (Romero et al., 2003; Dawson and Watson, 2009; Mork et al., 2009; Alvarez et al., 2012) that might assist in engaging diverse frontal circuitry, leading to better treatment of the disease.
b. 5-HT1A receptor activation for improved antipsychotic action
A noteworthy development in the study of 5-HT1A receptors has been the increasing therapeutic interest for this target in psychotic disorders. This has stemmed from extensive clinical and preclinical observations [see McCreary and Newman-Tancredi (2015) for review].
Schizophrenia, which shares some symptoms with other neuropsychiatric diseases, includes positive symptoms (auditory and visual hallucinations, delusions, conceptual disorganization, thought disorders, and some motor disturbances); negative symptoms (affective blunting, social withdrawal, anhedonia, avolition, and poverty of thought and speech); and cognitive impairments, such as working-memory abnormalities, deficits of cognitive processing, and attention and affective disorders (depression and anxiety) (Meltzer, 1999). 5-HT1A receptors appear involved both in the pathophysiology and in functionality of potential novel treatments. Thus, the newer generation antipsychotics clozapine, ziprasidone, quetiapine, aripiprazole, lurasidone, and cariprazine possess (partial) agonist effects at 5-HT1A receptors; however, interestingly, risperidone and olanzapine do not (McCreary and Newman-Tancredi, 2015; Newman-Tancredi et al., 1996a, 2005). In patients, changes in 5-HT1A receptor binding or functional activity have been identified (Burnet et al., 1996; Kasper et al., 2002; Yasuno et al., 2003; Bantick et al., 2004; Frankle et al., 2006; Lerond et al., 2013; Billard et al., 2014) along with SNPs at loci ss212928868 and rs6294, which are associated with the clinical outcome in women with paranoid schizophrenia (Zhou et al., 2013). Polymorphisms were also associated with much of the depression and negative treatment outcomes (Reynolds et al., 2006; Newman-Tancredi and Albert, 2012). Preliminary studies assessing cytosine methylation at a site close to this rs6295 polymorphism suggested that this was associated with a lower incidence of negative symptoms (Reynolds et al., 2006; Tang et al., 2014b), reinforcing the importance of this site in the negative symptoms of schizophrenia. Taken together, these accumulated data support the assertion that there is involvement of 5-HT1A receptors in the pathophysiology and treatment-related facets of the disease, particularly negative symptomatology.
A net hypofunctionality of the PFC, a brain area key in working memory, decision, and attentional processing, has been proposed in schizophrenia (Weinberger and Lipska, 1995; McCreary et al., 2007). It is therefore interesting that many atypical antipsychotic drugs may impact this deficit (McCreary and Newman-Tancredi, 2015). It may therefore be relevant that the 5-HT1A receptor agonist agents possessing antipsychotic properties (SSR181507, adoprazine, and lurasidone) augment extracellular microdialysate dopamine and acetylcholine levels in the PFC to “normalize” hypofrontal tone (Claustre et al., 2003; McCreary et al., 2007; Huang et al., 2014b) and promote potential therapeutic outcomes. This is supported by preclinical evidence (Depoortère et al., 2007) and clinical evidence with the partial agonist, tandospirone, which improved cognitive symptoms in patients with schizophrenia treated with neuroleptics (Sumiyoshi et al., 2001a,b, 2007; Meltzer and Sumiyoshi, 2008). Additionally, blonanserin, tandospirone, lurasidone, and buspirone reduced MK-801–induced novel object recognition deficits (Horiguchi and Meltzer, 2012; Horiguchi and Meltzer, 2013), and PCP-induced reversal learning was attenuated by 5-HT1A receptor activation (McLean et al., 2009b). In the social interaction test, a model for negative symptoms, aripiprazole, SSR181507, and F-15063 induced a 5-HT1A receptor–dependent performance improvement (Boulay et al., 2004; Bruins Slot et al., 2005; Depoortère et al., 2007; Snigdha and Neill, 2008). In addition, administration of 5-HT1A receptor (partial) agonists reversed PCP-induced decreases of tickling-induced 50-kHz ultrasound vocalization in juvenile rats, a model for negative symptoms, and improved attentional processing in a five-choice serial reaction time task (Winstanley et al., 2003; Boulay et al., 2013). In conclusion, data from preclinical and clinical findings support that 5-HT1A receptor activation will benefit the treatment of cognitive, attentional, and negative symptom domains.
An additional complication of antipsychotic treatment is so-called extrapyramidal side effects induced by the typical antipsychotics, such as haloperidol, which can reduce striatal output and lead to a parkinsonian phenotype. Such symptoms in preclinical models can be reduced by 5-HT1A receptor agonists (McCreary et al., 2007).Compounds such as adoprazine, bifeprunox, and F-15063 elicit less catalepsy than neuroleptics such as haloperidol. However, treatment, with WAY-100635 unmasked this blockade of catalepsy, indicating a key role of 5-HT1A receptors (Kleven et al., 2005; Bardin et al., 2006). Consistently, mesolimbic selectivity, and therefore the ability to treat the positive symptoms, was supported with electrophysiological studies demonstrating that depolarization block of VTA, but not substantia nigra pars compacta, dopaminergic neurons was mediated by 5-HT1A receptor agonists (Nakamura et al., 2006; McCreary et al., 2007) and that PFC 5-HT1A receptors influenced VTA cell firing by indirectly affecting pyramidal cell afferents to the VTA, thereby increasing dopamine cell firing (Lladó-Pelfort et al., 2012; Santana et al., 2013). Such mechanisms may indirectly influence mesoaccumbal dopaminergic output and impact positive symptoms. Some clinical meta-analytical studies support this assertion and suggest a trend for improved cognitive symptoms following the addition of 5-HT1A receptor partial agonists, together with a trend for improved positive symptoms (Kishi et al., 2013), but more extensive clinical studies are warranted. It is interesting to speculate that fully efficacious agents might offer added benefit. Moreover, benefit in other symptom domains might be expected, particularly mood. Accordingly, bifeprunox, SSR181507, and adoprazine (SLV313) all demonstrated anxiolytic-like and antidepressive-like properties (Depoortere et al., 2003), and 5-HT1A receptors appear to mediate the antidepressant effects of ketamine and metabotropic glutamate (2/3) receptor antagonists (Fukumoto et al., 2014). Moreover, 5-HT1A gene loci polymorphism linkage studies support this in schizophrenic patients with depression (Albert, 2012).
Taken together, these data support a role for the 5-HT1A receptor in schizophrenia. This is particularly interesting in light of the clinical development and marketing approval of lurasidone and cariprazine, which possess dopamine D2 and 5-HT1A receptor agonist action (Ishibashi et al., 2010; Kiss et al., 2010). Indeed, pharmacodynamic studies support the described 5-HT1A receptor–mediated mechanisms in the actions of lurasidone on augmented PFC dopamine and acetylcholine levels and cognitive actions (Horiguchi and Meltzer, 2012; Huang et al., 2012, 2014). Consistently, clinical benefit in a variety of symptom domains was evident (Veselinović et al., 2013; Citrome et al., 2014; Durgam et al., 2014; Loebel et al., 2014a,b).
G. 5-HT1A Receptors and Some Emerging Treatment Areas
1. Parkinson Disease
Parkinson disease is characterized by a loss of nigrostriatal dopaminergic neurons, resulting in the cardinal motor symptoms (Schapira et al., 2006). Symptomatic treatment ultimately relies on the gold-standard medication and dopamine precursor levodopa (L-DOPA) (Jenner et al., 2011). However, over time, the effects of L-DOPA are prone to wearing off (i.e., there is a tolerance to the actions of L-DOPA), and patients develop dose-limiting dyskinesia (Jenner et al., 2011). The treatment of L-DOPA–induced dyskinesia (LID) has been hampered by a lack of approved medications. Recently, the 5-HT system has emerged as a key player in the induction of LID. 5-HT neurons possess the enzymes necessary to convert exogenous L-DOPA to dopamine (DA) and mediate its vesicular storage and “false neurotransmitter” release. However, 5-HT neurons lack appropriate control mechanisms to regulate synaptic DA levels (e.g., via presynaptic D2 receptors or dopamine transporters), resulting in excessive DA release and pulsatile (over) stimulation of postsynaptic dopamine receptors that generate dyskinesia. Theoretically, it might be possible to mitigate dopamine release from serotonergic neurons by suppressing serotonergic tone by the application of 5-HT1A (or 5-HT1B) receptor agonists, which suppress neurotransmission by influencing the negative feedback somatodendritic (or terminal autoreceptors). Indeed 5-HT1A receptor agonist treatment does reduce LID in both rat and nonhuman primate models (Bibbiani et al., 2001; Eskow et al., 2007, 2009; Munoz et al., 2009; Huot, 2015; Iderberg et al., 2015) and appears to translate in clinical studies using the partial agonists buspirone and the mixed 5-HT1A/5-HT1B agonist eltoprazine (Svenningsson et al., 2015). However, other clinical attempts to target the 5-HT1A receptor have been disappointing, with compounds such as sarizotan and tandospirone also impairing the antiparkinsonian activity (Bonifati et al., 1994; Kannari et al., 2002; Olanow et al., 2004; Goetz et al., 2007), whereas eltoprazine showed only modest effects (Svenningsson et al., 2015). Together, this suggests that although 5-HT1A receptors can reduce dyskinesia, compounds tested to date may be less than optimal (Hamik et al., 1990; Newman-Tancredi et al., 1997c, 1998, 2003). Interestingly, only full agonists succeed in completely reversing haloperidol-induced catalepsy, whereas partial agonists failed to do so (Prinssen et al., 2002), suggesting that maximal efficacy may be required. The selective 5-HT1A receptor “biased agonist” F13714, which preferentially targets raphe 5-HT1A autoreceptors (Assié et al., 2006), completely abolished abnormal involuntary movements (AIMs) along with inhibiting 5-HT release (Iderberg et al., 2015). Comparable findings were evident with Befiradol (McCreary and Newman-Tancredi, 2015).
In addition, “full agonist” activity at 5-HT1A receptors may also provide beneficial influence on nonmotor symptoms of PD, such as the mood deficits likely elicited by deficient 5-HT neurotransmission (Eskow Jaunarajs et al., 2010; Politis, 2010). Indeed, whereas treatment of depressive symptoms in PD using 5-HT reuptake inhibitors is poorly effective, direct activation of postsynaptic (cortical) 5-HT1A receptors is associated with potent antidepressant actions (Celada et al., 2004). In restless legs syndrome, another movement disorder typically managed with low doses of dopamine receptor agonists or L-DOPA, 5-HT1A receptor agonists may also display clinical benefit (Shioda et al., 2006).
2. Pain
There is good evidence for the involvement of the 5-HT system in chronic pain (Millan, 2002), which is not surprising given their expression by descending pathways of the dorsal horn and other relevant structures. The receptors of the dorsal horn appear pivotally involved in the pronociceptive effects (Fasmer et al., 1986; Millan, 1994, 2002; Millan et al., 1996; You et al., 2005; Colpaert, 2006; Avila-Rojas et al., 2015; Sagalajev et al., 2015) and may also influence antinociception (Millan et al., 1996). Recent evidence suggests that the newer generation antipsychotic agent (e.g., aripiprazole), which possesses 5-HT1A receptor partial agonist actions, displays antinociceptive effects (Fei et al., 2012; Almeida-Santos et al., 2015). Moreover, the ability of 5-HT1A receptors to form heterodimers with µ-opioid receptors (Cussac et al., 2012) suggests 5-HT1A receptor targeting as an adjunct to opioid strategies may be useful.
3. Attention Deficiency Hyperactivity Disorder
In animal models of impulse control, 5-HT1A receptor stimulation reduced the impulsivity, suggesting potential benefit in diseases such as attention deficiency hyperactivity disorder (ADHD; Winstanley et al., 2003). Furthermore, in an isolation rearing model, which models some components of ADHD, 5-HT1A receptor binding sites were altered in a region-specific manner (Preece et al., 2004). Pharmacological study using the agonists SSR181507 (Terranova et al., 2005) and sarizotan (Danysz et al., 2015) suggest efficacy in animal models of ADHD. It is also relevant that a HTR1A rs10042486 polymorphism is associated with ADHD (Park et al., 2013). Indeed, buspirone may benefit ADHD management (Levin, 2015), though to a lesser extent than methylphenidate (Mohammadi et al., 2012).
4. Autism Spectrum Disorder
Preclinical studies reveal altered central 5-HT1A receptor activity, in a rat valproate model of autism (Wang et al., 2013b) and BTBR mice(BTBR T+Itpr3tf/J mouse), which have a phenotype paralleling that of autism spectrum disorder, elevated [35S]GTPγS binding is evident, corresponding to enhanced 5-HT1A receptor functional activity that potentially contributes to poor social behavior (Gould et al., 2011). Clinical data are limited, but anti–5-HT1A receptor antibodies have been identified in the blood of an autistic boy (Todd and Ciaranello, 1985). Furthermore, a HTR1A C-1019G polymorphism in autism may influence clinical outcomes (Egawa et al., 2012).
5. Respiratory Control
5-HT1A receptor agonists increased respiration in rats and cats (Edwards et al., 1990; Rose et al., 1995), and morphine-induced ventilatory depression was reduced by the 5-HT1A receptor agonist repinotan (Guenther et al., 2010). Electrophysiological studies support a modulatory role of the 5-HT1A receptor in the bursting activity of respiratory neurons (Onimaru et al., 1998), and 5-HT1A receptors activate bronchioconstrictor vagal preganglionic neurons and phrenic nerve neurons (Bootle et al., 1998; Valic et al., 2008). These and other data have led to the suggestion that 5-HT1A receptor agonists display potential to treat sleep apnea (Futuro-Neto et al., 1993; Khater-Boidin et al., 1996, 1999; Dando et al., 1998; Sahibzada et al., 2000) that may translate to the clinic given an evident reduction in apnea evoked by buspirone (Wilken et al., 1997). In addition, activation of 5-HT1A receptors may be beneficial to reverse compromised respiration; for instance, in a transgenic mouse model of Rett syndrome that also models disordered breathing, (+)8-OH-DPAT and sarizotan reduced the apneic frequency to restore the respiratory pattern (Abdala et al., 2010, 2014a,b; Levitt et al., 2013). Furthermore, the 5-HT1A receptor–biased agonist, F15599, impacts apnea and respiration frequency in MECP2-null male and heterozygous female mice (Levitt et al., 2013). Clinical experiences investigating the 5-HT1A receptor role in Rett syndrome are limited, but buspirone administered with fluoxetine reduced the frequency of hyperventilation and apneic attacks (Gokben et al., 2012).
6. Sexual Dysfunction
5-HT1A receptors may be a promising target in the treatment of sexual dysfunction. The 5-HT1A receptor agonist flibanserin (which also possesses 5-HT2A receptor antagonist and dopamine D4 receptor partial agonist properties; Mendelson and Gorzalka, 1986; Borsini et al., 2002; Heusler et al., 2009; Stahl, 2015) is a treatment of female hypoactive sexual desire disorder (Clayton et al., 2010; Jayne et al., 2012; Thorp et al., 2012; Katz et al., 2013) and is the culmination of research indicating a role for 5-HT1A receptors in sexual function (e.g., Mendelson and Gorzalka, 1986; Olivier et al., 2011; Aubert et al., 2012; Gelez et al., 2013; Snoeren et al., 2014a,b), although its clinical effects are likely not exclusively related to actions at 5-HT1A receptors (Allers et al., 2010; Stahl et al., 2011; Stahl, 2015). However, inclusion of 5-HT1A agonist actions in the profile of activity of psychotropic drugs has been reasoned to potentially alleviate the sexual dysfunction seen in some patients treated with antidepressant or antipsychotic agents.
7. Food Intake and Eating Disorders
The role of 5-HT in modulating food intake and satiety has been investigated extensively (Blundell et al., 1995; Halford et al., 2007). Early studies demonstrated 5-HT1A receptor activation induces hyperphagia, suggesting agonists may help treat patients with eating disorders such as bulimia and/or anorexia nervosa (Dourish et al., 1987). In vivo imaging studies suggest 5-HT1A receptor binding increases in cortical and limbic structures of the brain of patients with anorexia and/or bulimia, consistent with a potential role in anxiety, behavioral inhibition, and body ideation (Kaye et al., 2005; Bailer et al., 2007, 2011; Galusca et al., 2008; Bailer and Kaye, 2011). Although clinical pharmacology studies are limited, and restricted to case studies, the partial agonist tandospirone improved the weight gain of patients with anorexia nervosa (restricting and binge-eating/purging subtypes) and also improved scores on the Eating Disorder Examination Questionnaire following treatment of up to 6 months (Okita et al., 2013). The mechanistic basis for this may involve control of mood: the anxiolytic effects of 5-HT1A receptor agonists are likely to be beneficial (Crow and Mitchell, 1994) and potentially contribute to treatment outcome.
8. Aggressive Behavior
5-HT1A receptor activation appears to reduce aggressive behavior in preclinical and clinical (buspirone) settings (Olivier and Mos, 1992; Bell and Hobson, 1994; Takahashi et al., 2012) with animal models, indicating impact at the level of the dorsal raphe, and hence a reduction in 5-HT neurotransmission, may underlie the response (Mos et al., 1993). This is supported by results generated with S15535, a preferential autoreceptor agonist and, possibly, via blockade of hypersensitive postsynaptic 5-HT1A heteroreceptors (Millan et al., 1997; de Boer et al., 2000). Indeed, elevated postsynaptic 5-HT1A heteroceptors in the forebrain are associated with aggressive behavior (Korte et al., 1996), although direct administration of F15599 into ventral orbital PFC reduces aggression in male mice (Stein et al., 2013).
9. Neuroplasticity and Neuroprotection
5-HT1A receptor agonists evoke neurogenesis and synaptogenesis in the adult hippocampus, thereby improving cognitive performance in this structure that is important for mnemonic function (Mogha et al., 2012; Vines et al., 2012; Schreiber and Newman-Tancredi, 2014). Moreover, 5-HT1A receptor stimulation can lead to long-term potentiation or depression (Meunier et al., 2013) with consequent elevated BDNF expression to influence neurogenesis (Luoni et al., 2013; Quesseveur et al., 2013).
In addition to the effects of 5-HT1A receptor agonists on neuroplasticity, targeting this receptor may also have a beneficial role in neuroprotection. Indeed, there is considerable data supporting this assertion: repinotan reduced staurosporine-induced apoptosis (Suchanek et al., 1998), and 8-OH-DPAT reduced the impact of excitotoxic doses of NMDA in vivo (Oosterink et al., 1998) and, further, may protect neurons via protective effects of astrocytes; conversely, 5-HT1A receptor antagonism by WAY100635 increased damage (Ramos et al., 2004). Similarly, the selective 5-HT1A receptor agonist F13714 and the antipsychotic drugs clozapine, ziprasidone, and aripiprazole attenuated kainic acid–induced lesion volume in the striatum—effects that were reversed by WAY100635 (Cosi et al., 2005).
In models of Parkinson disease, 5-HT1A receptor agonists may slow neuronal damage (Bezard et al., 2006) and limit astrogliosis (Miyazaki et al., 2013). In the experimental autoimmune encephalopathy model of multiple sclerosis and in vitro cell-based models, the efficacy of a novel arylpiperazine D2/5-HT1A receptor ligand suggested this was due to combined action of the compound to limit inflammation and neuroprotective actions (Popovic et al., 2015), and buspirone appears to exert some efficacy against apneusis in multiple sclerosis (O’Sullivan et al., 2008). Interestingly, repinotan was developed for activity in ischemic stroke and traumatic brain injury (Lutsep, 2002; Berends et al., 2005; Mauler and Horváth, 2005; Guenther et al., 2010), therapeutic areas that are historically very difficult for drug development. However, repinotan failed to show efficacy in acute ischemic stroke, and its development was discontinued (Teal et al., 2009).
III. 5-HT1B Receptors
A. Introduction
The 5-HT1B receptor and its counterpart the 5-HT1D receptor have experienced a complex and debated history (Fig. 3) that is explained here. The two receptors are clearly closely related and result probably from gene duplication, which explains that in most species, their pharmacological profiles are almost indistinguishable (however, this is less evident in some species such as rat, mouse, hamster, or opossum; see below). In addition, 1) expression levels of the 5-HT1D receptor are very low compared with those of the 5-HT1B receptor, 2) the two receptors tend to be expressed together in many brain regions (although not in the periphery; Fig. 4), and 3) 5-HT1B and 5-HT1D receptors are coexpressed and may form heterodimers in certain brain cells. In essence, the 5-HT1B receptor is predominant, and, in the absence of selective compounds, it is very challenging to identify a separate population of 5-HT1D receptors in the brain. Except in rodents, hamster, and opossum, in which both receptors display somewhat different pharmacological profiles, the 5-HT1B receptor is still largely predominant in terms of expression and function.
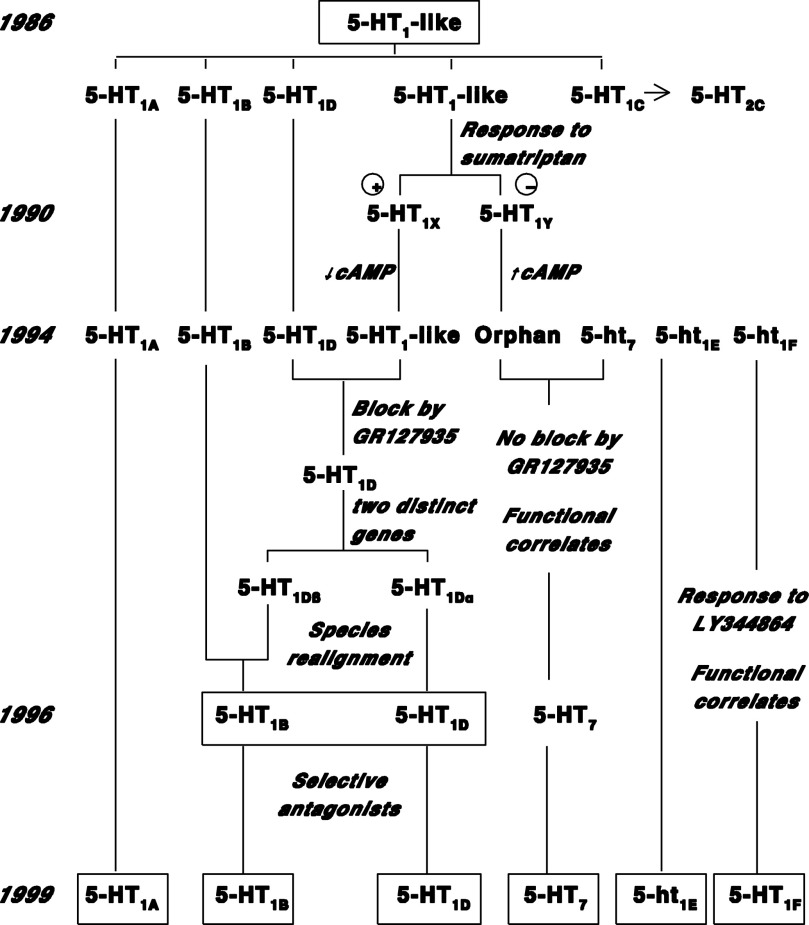
Fig. 3.
The evolution of “5-HT1–like” receptors into the different sumatriptan-sensitive 5-HT1 receptor subtypes and the sumatriptan-insensitive 5-HT7 receptor. Modified from Saxena et al. (1998). For references, see Hartig et al. (1996), Hoyer and Martin (1997), Villalón et al. (1997a), and Villalón and Centurión (2007).
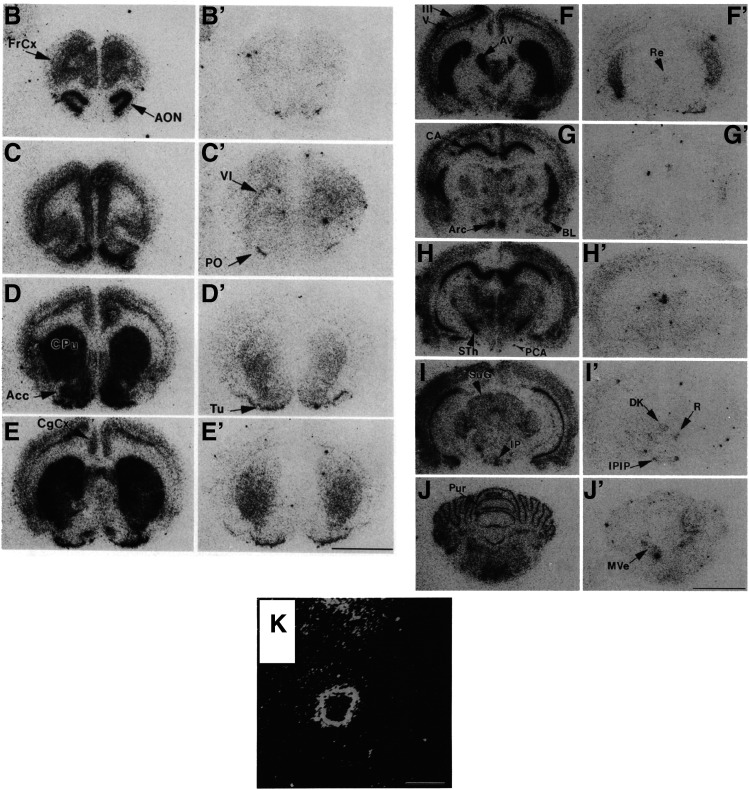
Fig. 4.
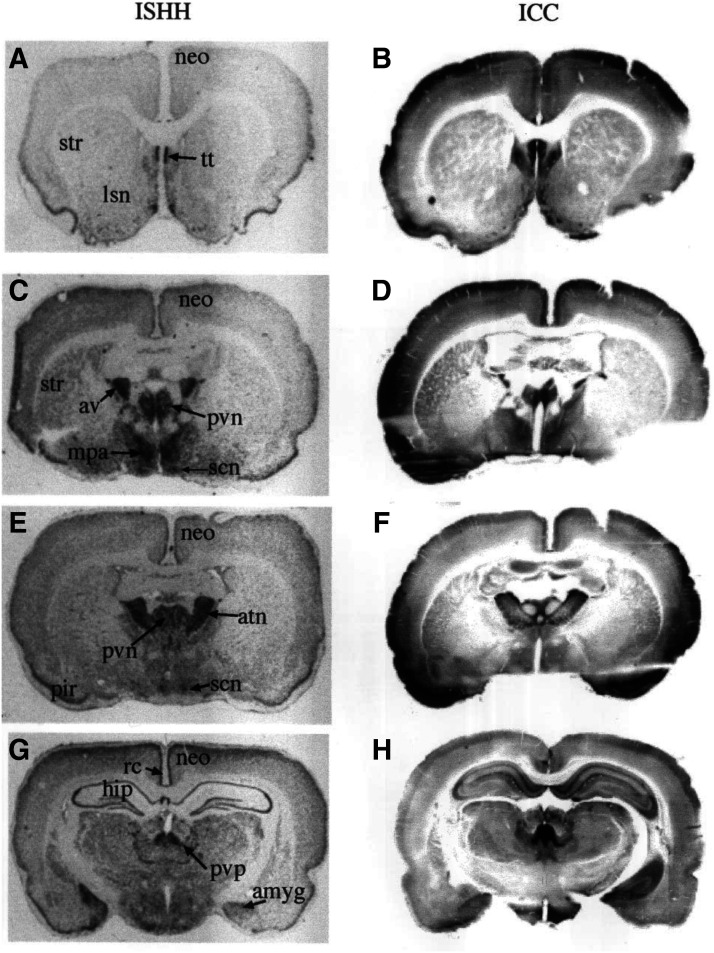
In situ hybridization detection of 5-HT1B and 5-HT1D receptor mRNA in rat brain (and 5-HT1B receptor mRNA in the posterior communicating artery [reverse autoradiogram; K]). 5-HT1B (B–K) and 5-HT1D (B’–J’) receptor mRNA. Ace, nucleus accumbens; AON, anterior olfactory nucleus; Arc, arcuate hypothalamic nucleus; AV, anteroventral thalamic nucleus; BL, basolateral amygdaloid nucleus; CA1, CA1 region of the hippocampus; CgCx, cingulate cortex; CPu, caudate putamen; DK, nucleus of Darkschewitsch; FrCx [layer VI], frontal cortex; IP, interpeduncular nucleus; IPIP, inner posterior subnucleus of the interpeduncular nucleus; layers III and V, parietal motor cortex; MVe, medial vestibular nucleus; PCA, posterior communicating artery; PO, primary olfactory cortex; Pur, Purkinje cells of the cerebellum; R, red nucleus; Re, reuniens nucleus; STh, subthalamic nucleus; SuG, superficial gray layer of the superior colliculus; Tu, olfactory tubercle. Scale bar, 5 mm (except K, where it is 0.5 mm). Adapted from Bruinvels et al. (1994a) (with permission).
The 5-HT1B receptor was originally defined according to operational and transductional criteria, and it was initially thought to be a rodent-specific receptor [for references, see Hoyer et al. (1994)]. In the 1970s, Peroutka and Snyder (1979) and others postulated that whereas [3H]5-HT labeled 5-HT1 binding sites, [3H]spiperone (and later [3H]ketanserin) labeled 5-HT2 binding sites, and [3H]LSD labeled both 5-HT1 and 5-HT2 binding sites. In 1981, Nelson and colleagues (Pedigo et al., 1981) proposed that 5-HT1 binding sites were a heterogeneous population, as [3H]5-HT was displaced biphasically by spiperone; accordingly, the high affinity site for spiperone was called 5-HT1A, and the low affinity was 5-HT1B. Middlemiss et al. (1977) had reported earlier that certain indole β-blockers displayed high affinity for some 5-HT receptors. In 1982/1983, a breakthrough was reached when Hjorth et al. (1982) and Middlemiss and Fozard (1983) described 8-OH-DPAT as a selective 5-HT1A ligand. Furthermore, Gozlan et al. (1983) reported the selective labeling of 5-HT1A sites using [3H]8-OH-DPAT. This allowed a clear definition of the 5-HT1A pharmacological profile and, by extension, of the features of non–5-HT1A sites [see Pazos et al. (1984a,b); Hoyer et al. (1985a,b)]. Thus, Palacios and Hoyer and colleagues (Hoyer et al, 1985b) at Sandoz in Basel characterized [3H]mesulergine binding in the choroid plexus (Pazos et al., 1984a), which 5-HT competed for with high affinity, but the relatively low affinity of ketanserin and spiperone suggested a 5-HT1 receptor pharmacology. The features of [3H]mesulergine-labeled sites were different from classic 5-HT2 binding sites labeled with, for example, [3H]ketanserin. The novel [3H]mesulergine-labeled binding site was named 5-HT1C (now 5-HT2C). Indeed, [3H]mesulergine binding was also markedly different from 5-HT1B binding as evidenced in radioligand binding and autoradiographic studies (Hoyer et al., 1985a,b, 1986a,b; Pazos and Palacios, 1985; Pazos et al., 1985, 1987a,b). More specifically in rodents, 5-HT1B binding sites were characterized extensively with the iodinated version of cyanopindolol, [125]ICYP (Engel et al., 1981), a potent β-blocker with high affinity for 5-HT1B binding sites. These sites displayed high affinity for 5-HT, 5-carboxamidotryptamine (5-CT), some β-blockers, some ergolines, lysergic acid diethylamide (LSD), and RU24969 (Hoyer et al., 1985a, 1986a; Engel et al., 1986). Species differences in receptor pharmacology soon became evident with [3H]mesulergine, which had different binding profiles in rodents, pigs, and humans; this pattern would repeat itself with a number of 5-HT receptors, most prominently with the 5-HT1B receptor (Hoyer et al., 1988; Waeber et al., 1988a,b). The Sandoz group used rat, mouse, hamster, rabbit, guinea pig, cat, dog, bovine, human, and more atypical for research species such as pigeons, opossum, and trout (e.g., Waeber et al., 1988a,b, 1989a,b) to investigate the pharmacology of 5-HT1A, 1B, 1C, and 5-HT2 receptor–binding sites. In addition, the pharmacology, transduction, and distribution of non–5-HT1A/1B/1C receptor binding sites, identified initially in calf and human brain and then most other species investigated, was termed 5-HT1D receptor binding sites (Hoyer and Schoeffter, 1988; Schoeffter et al., 1988; Waeber et al., 1988a,b; Hoyer et al., 1988). Although the pharmacology of 5-HT1B and 5-HT1D binding sites/receptors displayed some distinct differences, their distribution pattern in brain was similar (if not overlapping), and they shared transductional and functional responses (Hoyer and Schoeffter, 1988; Schoeffter and Hoyer, 1989a, 1990). Therefore, rodent “5-HT1B” and nonrodent “5-HT1D” receptors were proposed initially to represent species homologs (Hoyer and Middlemiss, 1989), a view that was unequivocally confirmed when genetic and structural information became available with the cloning of these receptors (Voigt et al., 1991; Adham et al., 1992; Hamblin et al., 1992a,b; Hartig et al., 1992; Levy et al., 1992b; Maroteaux et al., 1992; Mochizuki et al., 1992).
However, matters were further complicated when Weinshank et al. (1992) identified two structurally distinct genes encoding human 5-HT1 receptors with, at the time, almost overlapping pharmacological profiles, both resembling the 5-HT1D receptor. Earlier on, a canine “orphan” clone called RDC4 (later named 5-HT1Dα) had been reported to display a 5-HT1D–like pharmacological profile (Libert et al., 1990; Hamblin and Metcalf, 1991; Maenhaut et al., 1991; Zgombick et al., 1991). A human receptor, initially called S12, was cloned independently and differed in sequence from the canine RDC4, yet it displayed 5-HT1D–like pharmacology (Levy et al., 1992b). Since the operational profiles of these two new receptors were mostly indistinguishable, they were called 5-HT1Dα (canine RDC4 and species homologs) and 5-HT1Dβ receptors (human S12 and species homologs). It soon became evident, however, that in spite of some fundamental differences in their pharmacological profiles (see below), the 5-HT1Dβ receptor was a human homolog of the rodent 5-HT1B receptor (displaying 96% overall sequence homology; Adham et al., 1992). The subsequent identification of the 5-HT1Dα gene in rats confirmed that 5-HT1B and 5-HT1D receptors represent just two different receptor classes (Hartig et al., 1992), which prompted a realignment of 5-HT receptor nomenclature to recognize primacy (preeminence) of the human genome (Hartig et al., 1996). As a result, the 5-HT1Dβ receptor was renamed 5-HT1B (subsuming the rodent 5-HT1B receptor), whereas the 5-HT1Dα nomenclature was abandoned for 5-HT1D in recognition of the fact that this gene product encodes the 5-HT1D receptor (see Fig. 3; Hartig et al., 1996). This nomenclature for 5-HT1B and 5-HT1D receptors has been used since 1996 and remains to date.
B. Pharmacology
The 5-HT1–like receptor mediating smooth muscle contraction and inhibition of noradrenaline release showed close similarities to the 5-HT1B and/or 5-HT1D receptors; however, the lack of selective ligands at these receptors made it difficult to distinguish these receptors with confidence, hampering research for quite some time (Hoyer, 1988a; Hoyer et al., 1994). Clitherow et al. (1994) reported the properties of several compounds, including a piperazinylbenzanilide derivative, GR127935, which shows a high affinity for and selective antagonist activity at 5-HT1B/1D receptors. But more importantly, the subsequent identification of potent and relatively selective antagonists at either the 5-HT1B (SB224289; Hagan et al., 1997; Gaster et al., 1998) or 5-HT1D (BRL15572; Price et al., 1997) receptors allowed responses to be attributed to either 5-HT1B or 5-HT1D receptors; for example, the sumatriptan-induced contraction of vascular smooth muscle was mediated via the 5-HT1B receptor (e.g., De Vries et al., 1998, 1999; Verheggen et al., 1998, 2004).
Despite the 96% amino acid sequence homology in the transmembrane regions (Adham et al., 1992), the rodent 5-HT1B receptor displays a distinct pharmacology compared with the 5-HT1B receptor in other species (Hartig et al., 1996). The differences in the pharmacology of these species homologs are largely attributed to the mutation of a single amino acid in the transmembrane spanning region Asp123 to Arg123 (Adham et al., 1994a). Thus, CP93129 is a selective agonist at the rodent 5-HT1B receptor, whereas some β-adrenoceptor antagonists, such as cyanopindolol, (−)pindolol, and (−)propranolol, are selective antagonists at the rodent 5-HT1B receptor but not in other species. Unfortunately, no selective agonist is thus far available for the nonrodent 5-HT1B receptor.
C. Receptor Structure and Transduction
The 5-HT1B receptor gene is intronless, encoding for a 386-amino-acid protein in rat and mouse and 390-amino-acid protein in humans that displays the typical structure of a seven-transmembrane–spanning GPCR. The human, mouse, and rat 5-HT1B receptor genes are located on chromosomes 6q13, 9E1, and 8q31, respectively. The rat receptor has 96% homology in the TMR with the human receptor, but the rat and mouse receptor (Voigt et al., 1991; Adham et al., 1992; Maroteaux et al., 1992) exhibit the typical 5-HT1B receptor operational profile in contrast to the human receptor, which is close to the 5-HT1D receptor operational profile (Levy et al., 1992b; Weinshank et al., 1992).
The 5-HT1B receptor couples negatively to adenylyl cyclase (Bouhelal et al., 1988; Hoyer and Schoeffter, 1988, 1991; Adham et al., 1992; Levy et al., 1992b; Maroteaux et al., 1992). Native 5-HT1B receptors expressed in opossum kidney cells also mediate elevation of intracellular calcium (Zgombick and Branchek, 1998).
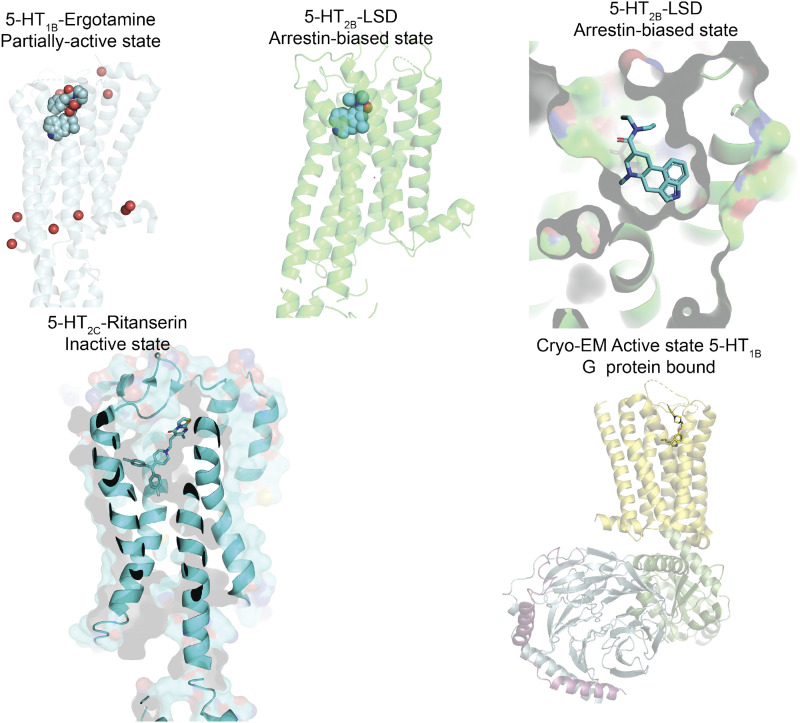
It is noteworthy that 5-HT1B (and 5-HT2B) receptors have been crystallized (Wang et al., 2013; Wacker et al., 2013; McCorvy and Roth, 2015; see section XVI. A. 5-HT GPCRs), which greatly increases knowledge of the structure pharmacology of the receptor. Indeed, the conformation of a number of agonists is different when bound to 5-HT1B or 5-HT2B receptors, in spite of very similar orthosteric binding sites (Wacker et al., 2013; Wang et al., 2013; McCorvy and Roth, 2015). Sumatriptan and a range of other triptans fit well into the orthosteric pocket of the human 5-HT1B receptor (in contrast to the 5-HT2B receptor), thus confirming the high affinity and potency reported for the triptans at 5-HT1B (and 5-HT1D) receptors. Some ergolines [LSD, metergoline, dihydroergotamine (DHE), ergotamine] bind to an accessory, possibly allosteric, site, which is located outside of the orthosteric pocket. It has been proposed that a short peptide, 5-HT-moduline, is a negative allosteric modulator of both 5-HT1B and 5-HT1D receptors (Rousselle et al., 1998). Research concerning this peptide appears to have waned in recent years; the interested reader is directed to previous reviews on the subject (Fillion, 2000; Moret et al., 2003).
D. Distribution and Function
Autoradiographic studies performed in various species showed that both 5-HT1A and 5-HT1C (now named 5-HT2C) receptor binding was evident, in addition to 5-HT2 receptor binding. However, what was then called 5-HT1B binding site was apparently absent in pig, calf, and human brain in contrast to rodent brain. This observation was extended to the guinea pig and then to an increasing number of other species (Hoyer at al., 1988; Waeber et al., 1988a,b; Hoyer and Middlemiss, 1989). Eventually, it was found that only rat, mouse, hamster, and opossum had a 5-HT1 receptor with a classic 5-HT1B profile [see Hoyer et al. (1985a,b)]. By contrast, other species expressed what was called 5-HT1D receptors in the brain (e.g., guinea pig, bovine, dog, rabbit, monkey, and humans) (see Waeber et al., 1988a, 1989a,b; Hoyer and Schoeffter, 1991; Hoyer et al., 1992). It was subsequently shown that [3H]sumatriptan and a number of other triptans label both 5-HT1B and 5-HT1D sites. However, they may also label 5-HT1F sites (Waeber and Moskowitz, 1995b). It also became evident when using selective antagonists that both 5-HT1B and 5-HT1D receptors could be detected in a single species (Bruinvels et al., 1993a,b, 1994a; Doménech et al., 1997; Bonaventure et al., 1997; Napier et al., 1999; Varnäs et al., 2001), but 5-HT1D receptor levels were minor when compared with the 5-HT1B receptor.
An elegant study demonstrated the rat brain autoreceptors mediating inhibition of 5-HT release displayed the pharmacology of the 5-HT1B receptor (Engel et al., 1986). In various other species, including humans, inhibitory autoreceptors displayed 5-HT1D receptor pharmacology (Schlicker et al., 1989). 5-HT1B receptors were also reported to mediate inhibition of GABA, cholinergic, and glutamatergic neurotransmission (Maura and Raiteri, 1986; Johnson et al., 1992; Singer et al., 1996; Chadha et al., 2000; Morikawa et al., 2000). The 5-HT1B receptor is highly concentrated in the substantia nigra (SN) and was shown to be negatively coupled to adenylyl cyclase activity (Bouhelal et al., 1988; Hoyer and Schoeffter, 1988, 1991).
Both 5-HT1B and 5-HT1D receptors have a neuronal localization (Waeber et al., 1990a,b; Bruinvels et al., 1991, 1992a,b, 1993a,b, 1994a,b; Sari et al., 1999), including in the trigeminal ganglia (Bruinvels et al., 1992a, 1994a,b; Hou et al., 2001; Ma, 2001; Potrebic et al., 2003). There is also evidence that both receptors colocalize and may form heterodimers (Xie et al., 1999; Ma, 2001).
Evidence from radioligand binding experiments using 5-HT neuronal lesions is equivocal regarding the location of the rat 5-HT1B receptor, with some studies finding that the lesion causes an upregulation of 5-HT1B binding sites and others finding a downregulation in the same areas. However, it is now clear that, like the 5-HT1A receptor, the 5-HT1B receptor functions as a presynaptic autoreceptor (see also section XVIII. 5-HT Receptors and the Brain). In situ hybridization studies have located mRNA encoding the 5-HT1B receptor in the dorsal and median raphe nuclei (Bruinvels et al., 1994a). Furthermore, 5-HT1B receptor mRNA in the raphe nuclei is markedly reduced by a 5-HT neuronal lesion. Together, these data suggest that 5-HT1B receptors are located both presynaptically (inhibitory autoreceptor) and postsynaptically (heteroreceptor) relative to 5-HT neurons [see Waeber et al. (1990b)]; as an example of the latter, 5-HT1B heteroreceptors inhibit CGRP release from sensory perivascular nerves in the rat systemic vasculature (González-Hernández et al., 2010).
5-HT1B receptors are also located on cerebral arteries and other vascular tissues mediating direct vasoconstriction [see Villalón et al. (2003) and Villalón and Centurión (2007)]. Furthermore, it seems that the receptor may be “silent” in a number of vascular preparations, becoming responsive in conditions such as atherosclerosis or when costimulated with “priming” factors (Sahin Erdemli et al., 1991; Kaumann et al., 1993, 1994). Other peripheral effects have also been described, such as 1) inhibition of noradrenaline release from sympathetic nerves in vena cava (Göthert et al., 1986) and systemic vasculature (Villalón et al., 1998) and 2) inhibition of plasma extravasation produced by trigeminal ganglion stimulation (Buzzi and Moskowitz., 1991). 5-HT1B receptors also mediate vasoconstriction in the rat caudal arteries (Craig and Martin, 1993) and the canine external carotid circulation (De Vries et al., 1998) or guinea pig iliac artery (Sahin Erdemli et al., 1991), although endothelium-mediated relaxation has also been reported (Schoeffter and Hoyer, 1989, 1990). Interestingly 5-HT1B receptor mRNA is more abundant within vascular smooth muscle cells compared with 5-HT1D receptor mRNA (Bouchelet et al., 1996; Sgard et al., 1996). The latter was reinforced by evident 5-HT1B but not 5-HT1D receptor immunoreactivity in cranial blood vessels (Longmore et al., 1997). Consistent with these findings, the subsequent advent of the potent and relatively selective antagonists at either the 5-HT1B (SB224289; Hagan et al., 1997; Gaster et al., 1998) or 5-HT1D (BRL15572; Price et al., 1997) receptors made it possible to demonstrate that the 5-HT1B, but not the 5-HT1D, receptor mediates the sumatriptan-induced contraction of vascular smooth muscle (e.g., De Vries et al., 1998, 1999; Verheggen et al., 1998), 2004.
E. Radioligand Binding
Autoradiographic studies using [3H]-5-HT (in the presence of 8-OH-DPAT), [125I]ICYP (in the presence of isoprenaline), or [125I]–carboxymethylglycyl iodotyrosinamide demonstrated a high density of 5-HT1B sites in the rat basal ganglia (particularly the substantia nigra, globus pallidus, ventral pallidum, and entopeduncular nucleus) but also in many other regions (Hoyer, 1988; Palacios et al, 1992; Hoyer et al., 1994; Mengod et al., 2010). The discrimination of 5-HT1B and 5-HT1D receptors in both rodent and nonrodent species has become more straightforward with the availability of a new 5-HT1B/1D radioligand, namely, [3H]-GR-125743 (Doménech et al., 1997; Varnäs et al., 2001), or various triptans (Leysen et al., 1996; Bonaventure et al., 1997; Napier et al., 1999) as well as cold ligands, which discriminate 5-HT1B and 5-HT1D receptors (Price et al., 1997; Middlemiss et al., 1999).
IV. 5-HT1D Receptors
A. Introduction
To recap, following the cloning of 5-HT1Dα and 5-HT1Dβ receptor genes in various species, 5-HT1Dα was renamed the 5-HT1D, and 5-HT1Dβ became the 5-HT1B receptor, keeping in mind that 5-HT1D expression levels are generally low compared with the 5-HT1B receptor (see section III. 5-HT1B Receptors for more detail).
B. Pharmacology
As noted in III. 5-HT1B Receptors, the pharmacological distinction of 5-HT1B from 5-HT1D receptors was a challenge until the advent of selective and silent antagonists (devoid of intrinsic activity) for 5-HT1B and 5-HT1D receptors (Hagan et al., 1997).
A series of isochroman-6-carboxamide derivatives, including PNU109291 (Ennis et al., 1998), PNU142633 (McCall, 1997; McCall et al., 2002), and L775606 (MacLeod et al., 1997), have been reported to be selective 5-HT1D receptor agonists, although they display low intrinsic efficacy at primate 5-HT1D receptors in GTPγ35S binding assays (Pregenzer et al., 1999).
The 5-HT1D receptor is potently antagonized by the 5-HT1B/1D receptor antagonist GR127935 (Clitherow et al., 1994; Skingle et al., 1996) and by the selective 5-HT1D receptor antagonist BRL15572 (Price et al., 1997). Additionally, some 5-HT2 receptor antagonists (e.g., ketanserin and ritanserin) can discriminate the 5-HT1D receptor from 5-HT1B and 5-HT1F receptors (Hoyer et al., 1994), although this is highly species-dependent (see Branchek et al., 1995; Zgombick et al., 1995, 1997). Sumatriptan and the second-generation triptans are potent agonists at the 5-HT1D receptor (but also interact with 5-HT1B and 5-HT1F receptors; Villalón et al., 2003; Table 6). It has been demonstrated that the 5-HT1D receptor is located preferentially on neuronal, rather than vascular, tissues (Ullmer et al., 1995; Sgard et al., 1996; Longmore et al., 1997).
TABLE 6.
pKi values of sumatriptan and second-generation triptans at human (except when stated otherwise) 5-HT receptors
| Receptor | Sumatriptan | Zolmitriptan | Naratriptan | Rizatriptan | Eletriptan | Almotriptan | Frovatriptan | F11356 |
|---|---|---|---|---|---|---|---|---|
| 5-HT1A | 6.4a | 6.6b | 7.6c | 6.4b | 7.4b | 6.3d | 7.3e | 7.6f |
| 6.9c | 6.5g | 7.1b | ||||||
| 6.0b | 7.1 (rat)h | |||||||
| 5-HT1B | 7.8a | 7.7b | 8.1b | 6.9b | 8.0b | 8.0d | 8.6e | 9.4f |
| 7.4b | 8.3g | 8.7h | 7.7i | |||||
| 8.3j | 8.1k | |||||||
| 5-HT1D | 8.5a | 8.9b | 8.4b | 7.9b | 8.9b | 8.0d | 8.4e | 9.3f |
| 8.0b | 9.2g | 8.3h | 8.6k | |||||
| 5-ht1e | 5.8a | 7.7b | 7.7b | 6.8b | 7.3b | <6.0e | 5.9f | |
| 5.8b | 8.0k | |||||||
| 5.6l | ||||||||
| 5-HT1F | 7.9a | 7.5b | 8.2b | 6.8b | 8.0b | 7.0e | 5.5f | |
| 7.9b | 7.2g | |||||||
| 7.6l | ||||||||
| 5-HT2A | <5.5b | <5.5b | <5.5b | <5.5b | <5.5b | <5.3e | 6.7f | |
| 5-HT2B | 6.9m | 7.2m | 6.6m | |||||
| 5-HT2C | <5.5b | <5.5b | <5.5b | <5.5b | <5.5b | <5.3e | ||
| m5-HT3 | <5.5b | <5.5b | <5.5b | <5.5b | <5.5b | <6.0e | <5.0f | |
| gp5-HT4 | <5.5b | <5.5b | <5.5b | <5.5b | <5.5b | 5.7f | ||
| 5-HT5A | <5.5 (rat)b | 6.4 (rat)b | 5.5 (rat)b | 5.3 (rat)b | 5.8 (rat)b | 6.1f | ||
| 5-HT6 | <5.5b | <5.5b | <5.5b | <5.5b | 6.3b | 5.6f | ||
| 5-HT7 | 5.9b | 7.0b | <5.5b | 5.7b | 6.7b | <6.5d | 6.7e | 6.4f |
gp, guinea pig; m, Mouse.
Data taken from Leysen et al. (1996).
Data taken from Napier et al. (1999).
Data taken from Newman-Tancredi et al. (1997b).
Data taken from Bou et al. (1997).
Data taken from Brown et al. (1998c).
Data taken from John et al. (1999).
Data taken from Martin (1997).
Data taken from Connor et al. (1997).
Data taken from Wurch et al. (1997).
Data taken from Beer et al. (1998).
Data taken from P. J. Pauwels, personal communication.
Data taken from Adham et al. (1993).
Data taken from P. Gupta, personal communication.
Given the cardiovascular liabilities of triptans potentially via 5-HT1B receptors expressed by vasculature (Nilsson et al., 1999a,b), which is not the case for 5-HT1D receptors (Nilsson et al., 1999b), it was hypothesized that selective 5-HT1D receptor agonists may treat migraine, with reduced cardiovascular side effects. Unfortunately, this has not translated to the clinic; the 5-HT1D receptor agonist PNU-142633 was ineffective in the acute treatment of migraine (Gómez- Mancilla et al., 2001), although the intrinsic activity of this compound may complicate interpretation.
C. Receptor Structure and Transduction
The 5-HT1D receptor gene, like the 5-HT1B receptor gene, is intronless. The human 5-HT1D receptor gene is located on chromosome 1p34.3-p36.3, codes for a 377-amino-acid protein, and possesses 63% overall structural homology with the 5-HT1B receptor; the mouse and rat receptor genes are located on chromosomes 4D3 and 5q36 and code for 374-amino-acid proteins. These receptors are made of a single polypeptide chain that spans the membrane seven times, with the amino terminus being extracellular and the carboxyl terminus intracellular in the manner typical of GPCRs (Hamblin and Metcalf, 1991; Hamblin et al., 1992; Weinshank et al., 1992; Weydert et al., 1992). The receptor is negatively coupled to adenylyl cyclase activity (Weinshank et al., 1992).
D. Distribution and Function
The distribution of 5-HT1D receptors is known but understood with less confidence because protein levels are low along with the relative difficulty of radioligands to discriminate this receptor from the 5-HT1B receptor. Receptor autoradiographic studies in rat (CP93129-insensitive [125I]–carboxymethylglycyl iodotyrosinamide binding) or human (ketanserin-insensitive [3H]-sumatriptan binding) brain clearly indicate 5-HT1D receptor site is expressed in the basal ganglia (globus pallidus, substantia nigra, and caudate putamen) and also the hippocampus and cortex (Pineyro et al., 1995; Hou et al., 2001; Potrebic et al., 2003; Mengod et al., 2010).
In situ hybridization experiments allow a greater confidence of gene expression, albeit not at the level of protein. Thus, 5-HT1D mRNA is present in rat brain, including the caudate putamen, nucleus accumbens (NAc), olfactory cortex, dorsal raphe nucleus, and locus coeruleus (e.g., Bruinvels et al., 1994a; Mengod et al., 2010; Fig. 4). The mRNA shows low abundance in all regions and was undetectable in the globus pallidus, ventral pallidum, and substantia nigra where, as noted above, 5-HT1D receptor sites appear to be present, which is perhaps indicative of the 5-HT1D receptor being located predominantly on axon terminals of both 5-HT and non–5-HT neurons.
In the periphery, the presence of 5-HT1D receptors is rather limited with evidence of presence in autonomic and trigeminal nerve terminals/ganglia (Molderings et al., 1996; Villalón et al., 1998).
The function of the 5-HT1D receptor still remains, to some extent, enigmatic. There is little evidence supporting the role of the 5-HT1D receptor in any pathology. The availability of suitable tools for investigation in vivo has limited the investigations into the importance of 5-HT1D receptors; they have been identified as autoreceptors in the dorsal raphe (Pineyro et al., 1995) or terminal brain regions. Thus, given their autoreceptor activity, 5-HT1D receptor antagonists may have antidepressant potential, and to maximize 5-HT release in terminal brain regions, 5-HT1D, 5-HT1B, and 5-HT1A receptors must be blocked simultaneously.
Operationally, 5-HT1D receptors mediate inhibition of noradrenaline release in human atrium. Additionally, the 5-HT1D receptor seems to be involved in the inhibition of guinea pig dural plasma protein extravasation (Ennis et al., 1998) and the central trigeminal inhibitory effects by some antimigraine compounds (Mills and Martin, 1995; Cumberbatch et al., 1998; De Vries et al., 1999a,b; Villalón et al., 2003).
It has been proposed that the 5-HT1D receptor modulates growth hormone release (Mota et al., 1995; Whale et al., 1999), although this requires clearer pharmacological verification.
V. 5-ht1e Receptors
A. Introduction
There has been relatively limited research on the 5-ht1e receptor, with an apparent lack of expression in rodents complicating preclinical studies. The lack of functional data concerning natively expressed 5-ht1e receptors means by convention lower case appellation is still used for nomenclature.
With hindsight, the 5-ht1e receptor was likely discovered by virtue of an atypical pharmacology of a [3H]5-HT binding site in human frontal cortex (Leonhardt et al., 1989), which was sensitive to guanyl nucleotides, suggesting association with the GPCR family. The high affinity [3H]5-HT displayed for the binding sites and the low affinity of drugs displaying affinity for the 5-HT2 receptor (e.g., mesulergine) supported membership of the 5-HT1 receptor family. However, the low affinity of 5-CT, the prototypical 5-HT1 receptor agonist, and detailed pharmacological characterization of the new [3H]5-HT binding site in human and bovine cortical homogenates highlighted that this site likely represented a further member of the 5-HT1 family, and hence it was given that next available name: 5-HT1E (Leonhardt et al., 1989; now reclassified as 5-ht1e until a functional response in native tissue/cell preparation can be attributed).
B. Cloning and Distribution of 5-ht1e Receptors
Soon after the 5-ht1e receptor binding site was pharmacologically characterized by radioligand binding in human and bovine brain tissue, a human GPCR gene, termed S31, was cloned (Levy et al., 1992a; see h5-ht1e in Fig. 5 for sequence) and assigned to human chromosome 6q14-q15 (Levy et al., 1994). When S31 was expressed in cell lines, the gene product was found to have pharmacological properties similar to the tissue-expressed 5-ht1e receptor binding site, and the conclusion was that this gene encodes the protein for the receptor binding site discovered by Leonhardt et al. (1989) (McAllister et al., 1992; Zgombick et al., 1992; Gudermann et al., 1993). However, the 5-HT1F receptor, discovered subsequent to these early reports on the 5-ht1e receptor, shares a high degree of sequence homology with the 5-ht1e receptor compared with other 5-HT receptors (see Fig. 5 for h5-ht1e and h5-HT1F amino acid sequence alignment) and bears a pharmacological profile very similar to the 5-ht1e receptor (Adham et al., 1993a,b; Lovenberg et al., 1993b). A careful examination of the binding data presented in the original report on the 5-ht1e receptor (Leonhardt et al., 1989) suggests the binding site identified in this report is likely a composite of both 5-ht1e and 5-HT1F receptor binding sites. Drugs that can discriminate between these receptor subtypes were not identified until after a number of studies were published that attempted to identify the distribution of 5-ht1e receptors via radioligand binding and autoradiography methodologies (Miller and Teitler, 1992; Beer et al., 1993; Barone et al., 1994; Stanton et al., 1996; Fugelli et al., 1997). This resulted in reports that incorrectly attributed [3H]5-HT radioligand binding to the 5-ht1e receptor in both rat and mouse brain tissue, species that were later identified to lack the 5-ht1e receptor gene (Bai et al., 2004). Even those reports that used tissue from species that do express a 5-ht1e receptor gene (e.g., humans, monkeys, guinea pigs, and bovine) were, in hindsight, confounded by the labeling of 5-HT1F receptors and thus need to be viewed as data that reflects a mixture of 5-ht1e and 5-HT1F receptor populations. Subsequent pharmacological isolation of 5-ht1e receptors (Klein and Teitler, 2012) has shown the following pattern of expression of the 5-ht1e receptor binding sites: olfactory bulb = hippocampus > frontal cortex > hypothalamus = cerebellum > brainstem-thalamus = striatum (Fig. 6). Issues impacting the ability to define with confidence the distribution of the 5-ht1e receptor were to some extent overcome with the development of an antibody recognizing the 5-ht1e receptor protein (Klein and Teitler, 2012), allowing protein expression to be revealed in native tissue. Such immunohistochemical studies revealed that the 5-ht1e receptor immunoreactivity was expressed in the olfactory bulb (glomerula cells), whereas in the hippocampus, expression is limited to the dentate gyrus (Klein and Teitler, 2012). Interestingly, 5-ht1e receptor immunoreactivity was also expressed in cerebral arteries (guinea pig; Klein and Teitler, 2012).
Fig. 5.
Alignment of human 5-ht1e and 5-HT1F receptor amino acid sequences. Sequence homology is assessed by Basic Local Alignment Search Tool (BLAST, copyright National Library of Medicine) for protein sequences. Transmembrane domains (TMD 1–7) were determined previously (Bai et al., 2004) and highlighted by yellow rectangles. Nonpolar, uncharged polar, acidic polar, and basic polar amino acids are labeled by corresponding color.
Fig. 6.
5-ht1e receptor expression in guinea pig and rat brain. The top image shows immunohistochemical staining of the 5-ht1e receptor in the hippocampus. (A) Coronal section of guinea pig hippocampus at the septal pole of the DG. (B) Coronal section of guinea pig hippocampus near the temporal pole of the DG. (C) Coronal section of rostral guinea pig hippocampus shows a lack of 5-ht1e receptor staining in the CA3-CA2 region. (D) Rat hippocampus (coronal section of DG septal pole) does not stain for 5-ht1e receptors. GCL, granular cell layer; ML, molecular layer; PML, polymorphic layer. Scale bars, 100 µm. The bottom image shows histogram of radioligand 5-ht1e receptor binding sites in homogenates of guinea pig brain. High levels of 5-ht1e receptor binding sites are detected in the hippocampus and olfactory bulb. *P < 0.05 compared with “whole-brain,” one-way ANOVA with Dunnett’s post-test. Data are the means ± S.E.M. of three independent experiments performed in triplicate. Adapted from Klein and Teitler (2012) (with permission).
C. Pharmacology
Bai et al. (2004) demonstrated that the rhesus monkey, pig, rabbit, and guinea pig express a homolog of the human 5-ht1e receptor gene. Because of the relative utility in preclinical models, the guinea pig 5-ht1e receptor (gp5-ht1e) sequence was cloned for further study. The guinea pig homolog shares 88% nucleic acid and 95% amino acid sequence homology with the human 5-ht1e receptor. The pharmacological properties of the guinea pig recombinant 5-ht1e receptor correlate well with the human counterpart in terms of affinity (R2 = 0.99) and potency (R2 = 0.96), indicating a high degree of evolutionary conservation for the receptor.
Quantitative RT-PCR of guinea pig brain regions revealed high levels of gp5-ht1e receptor mRNA in the cortex, hippocampus, and olfactory bulb and moderate expression in some other regions, similar to the expression pattern in the human brain (Bai et al., 2004). Thus, the structural and pharmacological similarities of the human and guinea pig receptors, along with comparable patterns of expression in gross brain regions, lend a great deal of support to the guinea pig as a valid model to study the functionality of the h5-ht1e receptor.
Some attempts have been made to develop selective pharmacological tools for the h5-ht1e receptor (Dukat et al., 2004) but failed to identify 5-ht1e receptor ligands with affinities substantially higher than 5-HT. A relatively selective high-affinity 5-ht1e receptor ligand has been identified, BRL54443 (Brown et al., 1998); this drug displays similar affinities for the h5-ht1e and h5-HT1F receptors but, more usefully, at least 60-fold lower affinities for other 5-HT, dopamine, and adrenergic receptors. Few published reports exist regarding the pharmacology of this compound, and the reports of BRL54443 action in vivo have used species that do not express the 5-ht1e receptor (mice and rats; Adham et al., 1994; Brown et al., 1998; McKune and Watts, 2001; Watts et al., 2001; Hisadome et al., 2009; Granados-Soto et al., 2010).
A high throughput screening study conducted at the Scripps Research Institute’s Molecular Screening Center in collaboration with Milt Teitler’s laboratory, with the aim of identifying highly potent, selective agonists or antagonists for the 5-ht1e receptor, has been performed [PubChem BioAssay Database, AID (accession #): 567; 574; 613; 718; 726; 730]. Nearly 65,000 compounds from a broad range of structural classes were screened for agonist and antagonist properties at the h5-ht1e receptor and counter-screened at the 5-HT1A receptor as an assessment of selectivity. Though none of the compounds were highly selective for the h5-ht1e receptor, a number of high-potency agonists (EC50 low nanomolars) were identified that displayed some structural similarity to BRL54443. In a more recent study comparing 51 tryptamine-based compounds for affinities at the human 5-ht1e and 5-HT1F receptors, no drugs were identified that showed a significant preference for the 5-ht1e receptor over the 5-HT1F receptor, again demonstrating the difficulties in attempting to identify 5-ht1e receptor–selective drugs (Klein et al., 2011).
D. Functions
Recombinant expression of the 5-ht1e receptor in cell lines demonstrates the coupling to Gi/o signaling pathways (Levy et al., 1992a; Gudermann et al., 1993; Adham et al., 1994) although no signaling pathways have been identified in native tissues, and in the absence of a recognized functional response, the lower case appellation nomenclature is retained.
VI. 5-HT1F Receptors
A. Introduction
Although the first published reports for the 5-HT1F receptor occurred in 1992 and 1993 (Amlaiky et al., 1992; Adham et al., 1993b; Lovenberg et al., 1993), there is still only limited information about this receptor. Much of the current literature centers on possible roles for the 5-HT1F receptor in the treatment of migraine despite the 5-HT1F receptor displaying a broad distribution within the central nervous system (CNS), and it also appears to be expressed in the periphery.
B. Cloning and Structure
The 5-HT1F receptor was discovered as the result of homology cloning strategies. The first report of the cloning of the human 5-HT1F receptor was in a patent application by Synaptic Pharmaceuticals, Inc., (Weinshank et al. 1994, U.S. patent number 5,360,735, filed in 1992, issued in 1994). Amlaiky et al. (1992) reported the cloning of the mouse receptor (initially called 5-HT1Eβ) that same year, followed by a peer-reviewed report on the human receptor (Adham et al., 1993b) and the cloning of both the rat and human versions (initially called 5-HT1E-like) of the receptor in 1993 (Lovenberg et al., 1993) (Table 7).
TABLE 7.
Chromosomal location of the 5-HT1F receptor gene
The chromosomal location of 5-HT1F receptor genes can be found in the following databases: http://www.ncbi.nlm.nih.gov/gene/?term=HTR1F%5Bsym%5D and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=5.
| Species | Locus | Name |
|---|---|---|
| Humana | 3p12 | HTR1F |
| Rat | 11p12 | Htr1f |
| Mouse | 16 C1.3 | Htr1f |
Maassen Vandenbrink et al. (1998).
The 5-HT1F receptor gene is intronless, coding for a GPCR of 366 amino acids that conform to the classic GPCR structure, and has been sequenced in a number of species (mouse, rat, guinea pig, pig, human; Amlaiky et al., 1992; Adham et al., 1993b, 1997; Lovenberg et al., 1993; Bhalla et al., 2002b). In mouse and rat, it appears that the 5-HT1F receptor is encoded by three different mRNA transcripts (Guptan et al., 1997), which differ in their 3′ untranslated regions.
C. Distribution
1. mRNA
The initial studies of 5-HT1F receptor distribution located 5-HT1F receptor mRNA, either by RT-PCR or in situ hybridization (Fig. 7; Table 8). 5-HT1F receptor mRNA has a rather broad distribution within the brain; the cerebral cortex shows a relatively dense band of expression within the internal layers (approximately layers IV–VI), and hippocampal areas CA1–CA3 display relatively high expression, as do the thalamus and striatum.
Fig. 7.
In situ hybridization detection of 5-HT1F receptor mRNA expression in guinea pig brain. (A) Frontal cortex (FRCX), anterior olfactory nucleus (AON). (B) Cingulate cortex (CGCX), septo-hippocampal nucleus (SHI), olfactory tubercle (TU), primary olfactory cortex (PO). (C) Claustrum (CL), medial amygdaloid nucleus (ME), supraoptic hypothalamic nucleus (SO). (D) Layer IV of the parietal motor cortex (IV), dentate gyrus (DG), and CA1-3 field (CA1-3) of the hippocampus are shown. Adapted from Bruinvels et al. (1994) (with permission).
TABLE 8.
Localization of 5-HT1F receptor mRNA
The most intensely labeled regions or areas of unique interest from the different references are listed in this table. See the original references for a more complete expression profile.
| Tissue or Cells | Species | Method | Reference |
|---|---|---|---|
| Forebrain, hindbrain, spinal cord, cerebellum | Mouse | RT-PCR | Amlaiky et al., 1992 |
| CA1, CA2, CA3 of the hippocampus | Mouse | ISHH | Amlaiky et al., 1992 |
| Brain, uterus, mesentery | Human | RT-PCR | Adham et al., 1993a |
| Layer V of cerebral cortex (pyramidal cells), layer VI nonpyramidal cells, CA1-CA3 of the hippocampus (pyramidal cells), Dorsal raphe (large neurons), Dentate gyrus (granule cells) | Guinea pig | ISHH | Adham et al., 1993a |
| Cerebral cortex, striatum, hippocampus, thalamus, pons, hypothalamus, cerebellum | Rat | RT-PCR | Lovenberg et al., 1993 |
| Olfactory, cingulate, frontal, and entorhinal cortex (especially layer V), hippocampus (CA1, CA2, CA3 and dentate gyrus) | Guinea Pig | ISHH | Bruinvels et al., 1994 |
| Claustrum, cerebral cortex, thalamus, striatum | Guinea Pig | ISHH | Mengod et al., 1996 |
| Trigeminal ganglion | Human | RT-PCR | Bouchelet et al., 1996 |
| Cerebral blood vessels | Human | RT-PCR | Bouchelet et al., 1996 |
| Trigeminal ganglion | Guinea pig | ISHH | Johnson et al., 1997 |
| Dorsal root ganglia, trigeminal ganglion | Guinea pig | ISHH | Adham et al., 1997 |
| Dorsal root ganglia | Human | RT-PCR | Pierce et al., 1997 |
| Cerebral cortex, hippocampus, cerebellum, brain stem | Mouse | Northern blot | Guptan et al., 1997 |
| Hippocampus, cerebral cortex | Rat | Northern blot | Guptan et al., 1997 |
| Dorsal root ganglia (cultured) | Rat | RT-PCR | Chen et al., 1998 |
| Neonatal and cultured astrocytes | Rat | RT-PCR | Hirst et al., 1998 |
| Coronary arteries, atrium, ventricle, and epicardium | Human | RT-PCR | Nilsson et al., 1999b |
| Brain cortical microvessels and capillaries | Human | RT-PCR | Cohen et al., 1999 |
| Astrocytes (cultured) | Human | RT-PCR | Cohen et al., 1999 |
| Immune tissue: spleen, thymus, mitogen-activated spleen cells, and peripheral blood lymphocytes | Rat | RT-PCR | Stefulj et al., 2000 |
| Lumbar dorsal root ganglia | Rat | RT-PCR | Wu et al., 2001 |
| Cerebral cortex, trigeminal ganglion | Pig | RT-PCR | Bhalla et al., 2002 |
| Coronary artery, pulmonary artery, saphenous vein | Pig | RT-PCR | Bhalla et al., 2002 |
| Lumbar dorsal root ganglia | Rat | RT-PCR | Liu et al., 2005 |
| Intestine (four separate regions) | Bovine | RT-PCR | Engel et al., 2006 |
| Various forebrain areas of embryonic brain, including cerebral cortex, amygdala, globus pallidus, caudate putamen, hippocampus, dentate gyrus | Mouse | ISHH | Bonnin et al., 2006 |
| Midbrain periaqueductal gray | Rat | RT-PCR | Jeong et al., 2008 |
| Neonatal rat: cerebellum, cortex, cortical astrocytes; adult rat: cerebellum, cortex | Rat | RT-PCR | Osredkar and Kržan, 2009 |
| Trigeminal ganglion, trigeminal nucleus caudalis | Rat | RT-PCR | Amrutkar et al., 2012 |
| Renal proximal tubule cells | Rabbit | RT-PCR | Garrett et al., 2014 |
| Heart, liver | Mouse | RT-PCR | Garrett et al., 2014 |
Documentation of the presence of 5-HT1F receptor mRNA in peripheral tissues is limited to a few single reports in different species, including human, bovine, pig, rat, and rabbit (Table 8). Overall, there is still a need for a systematic mapping of the peripheral distribution of 5-HT1F receptors. Peripheral blood vessels such as the coronary artery have been reported to express 5-HT1F message, although the findings in human coronary appear variable, with Ishida et al. (1999) finding none, Nilsson et al. (1999b) reporting a strong signal, and Bouchelet et al. (2000) reporting a weak signal in about 40% of patients. Bhalla et al. (2002b) found a 5-HT1F receptor mRNA signal in porcine coronary artery. Regardless, the 5-HT1F receptor agonist LY334370 did not elicit contractions in human coronary artery (Nilsson et al., 1999b). 5-HT1F receptor transcripts are also present in vascular preparations from the CNS (Table 8). However, when the brain microvessel preparations were treated to yield cultures of either smooth muscle or endothelial cells, no 5-HT1F receptor mRNA was detected (Cohen et al., 1999).
In contrast to peripheral tissues, evidence for the presence of 5-HT1F receptor mRNA in the peripheral nervous system is clear; thus, multiple studies in trigeminal ganglia and dorsal root ganglia (fresh or cultured) have identified 5-HT1F receptor mRNA (Table 8).
2. Radioligand Binding
The first localization studies of 5-HT1F receptor binding sites used [3H]sumatriptan, which displays high affinity for 5-HT1F receptors as well as 5-HT1B/1D receptors. This radioligand has been used to map the 5-HT1F receptor in a variety of species (including “cold” competing ligands such as 5-CT or methiothepin prevents radiolabeling of 5-HT1B and 5-HT1D receptors; Waeber and Moskowitz, 1995b; Mengod et al., 1996; Scarr et al., 2004; Dean et al., 2006). Although [3H]5-HT can be used to label heterologous expression of 5-HT1F receptors in cultured cells (Adham et al., 1993b), lack of selectivity makes 5-HT1F receptor localization studies with [3H]5-HT challenging (Fugelli et al., 1997). A second useful radioligand to label the 5-HT1F receptor is [3H]LY334370, albeit in the presence of 5-HT1A receptor ligands such as 8-OH-DPAT to prevent labeling of the latter receptor (Wainscott et al., 2005). It is probably noteworthy that with all 5-HT1F receptor–labeling studies, the lack of a selective radioligand necessitating the use of “cold” blocking drugs to better isolate radioligand binding to the 5-HT1F receptor may underestimate the reported levels of 5-HT1F receptors, or conversely, the specific radioligand binding signal consists of a heterogenous population of sites that includes the 5-HT1F receptor; either way, interpretation should be made with caution.
3. Immunoreactivity
Relatively few studies have used antibodies to localize 5-HT1F receptors (Table 9). The antibody studies of Ma (2001) and Classey et al. (2010) are consistent with investigations showing 5-HT1F receptor mRNA in trigeminal ganglia and dorsal root ganglia (Table 8). Classey et al. (2010) describe 5-HT1F receptor–like immunoreactivity in dorsal root ganglia from all regions examined, including cervical, thoracic, and lumbar. Ma (2001) reported colocalization of 5-HT1F receptor with glutamate-containing neurons of the trigeminal ganglion, and Ahn et al. (2009) showed colocalization of 5-HT1F receptors with CGRP in neurons of rat vestibular nuclei. Garrett et al. (2014) provide a unique perspective on the 5-HT1F receptor, showing the presence of both mRNA (Table 8) and protein (Table 9) in the rabbit renal proximal tubule.
TABLE 9.
Localization of 5-HT1F receptor protein using antibodies
| Tissue or Cells | Species | Method | Reference |
|---|---|---|---|
| Trigeminal ganglion neurons | Rat | Immunohistochemistry | Ma, 2001 |
| Superior, lateral, spinal, and medial vestibular nuclei | Rat | Immunohistochemistry | Ahn et al., 2009 |
| Neurons of trigeminal ganglia and dorsal root ganglia (C2, C5, T5, and L5 levels) | Rat | Immunohistochemistry | Classey et al., 2010 |
| Renal proximal tubule cells | Rabbit | Immunoblot | Garrett et al., 2014 |
D. Pharmacology
1. Agonists
The 5-HT1F receptor, like the other members of the 5-HT1 family, has high affinity for 5-HT itself. This characteristic has driven much of the structural work devoted to developing high-affinity, selective 5-HT1F receptor agonists.
Compared with 5-HT1A, 5-HT1B, and 5-HT1D receptors, the pharmacology described for 5-HT1F receptors is rather sparse. Because of a potential link between 5-HT1F receptor activation and the treatment of migraine (see below), most of the effort to develop selective compounds has focused on the development of orthosteric agonists. No selective orthosteric 5-HT1F receptor antagonists have been reported. Likewise, there are no literature descriptions of allosteric 5-HT1F receptor ligands.
Almost all of the structure activity relationship (SAR) work has come from the laboratories of Eli Lilly and Company, which had exclusive rights to the 5-HT1F receptor through a collaborative agreement with the patent holder, Synaptic Pharmaceutical Corporation. Table 10 summarizes published named compounds showing selectivity for the 5-HT1F receptor. Lasmiditan (COL-144, LY573144) and LY344864 display very good selectivity for the 5-HT1F receptor relative to all other 5-HT receptors, as does LY334370, except that it is only about eight- to ninefold selective over the 5-HT1A receptor. LY302148 was an early molecule that showed selectivity for the 5-HT1F receptor relative to other 5-HT1 subtypes. However, it also has high affinity for 5-HT2A and 5-HT2B receptors (Table 10) and is illustrative of the difficulty in identifying 5-HT receptor subtype selectivity in simple indole-based compounds.
TABLE 10.
Affinities of selective 5-HT1F receptor agonists at cloned human 5-HT receptors
Binding values are expressed as Ki in nanomolar (± S.E.M. where available) from studies using cloned human receptors. Values in parentheses are the number of individual determinations from the cited references.
| LY302148 | LY306258 | LY334370a | LY344864b | LY397584 | Lasmiditana (COL-144, LY573144) | |
|---|---|---|---|---|---|---|
| Receptor | Ki, nM | Ki, nM | Ki ± S.E.M., nM | Ki, nM | Ki, nM | Ki ± S.E.M., nM |
| 5-HT1A | 12.0c | 5370c | 16.4 ± 2.7 (9) | 530 | 1046d | 1053 ± 134 (8) |
| 5-HT1B | 53.7e | 1698e | 189 ± 25 (10) | 549 | 723d | 1043 ± 124 (8) |
| 5-HT1D | 21.9e | 794.3e | 281 ± 58 (10) | 575 | 724d | 1357 ± 156 (8) |
| 5-ht1e | 50.1c | 73.6f | 176 ± 34 (8) | 1415 | 776c | 594 ± 59.1 (6) |
| 5-HT1F | 2.5e | 10.2e | 1.87 ± 0.34 (10) | 6 | 5.5d | 2.21 ± 0.22 (8) |
| 5-HT2A | 5.89c | 1072c | 1530 ± 200 (3) | 3935 | 3890c | >5 µM (6) |
| 5-HT2B | 6.17c | 1072c | 1280 ± 90 (3) | 1695 | 6166c | >2 µM (6) |
| 5-HT2C | 13.5c | 813c | 3250 ± 930 (3) | 3499 | 309c | >3 µM (6) |
| 5-HT6 | >3 µM (2) | >4 µM (6) | ||||
| 5-HT7 | 1550 ± 260 (5) | 4851 | >3 µM (6) |
Taken from Nelson et al. (2010).
Taken from Phebus et al. (1997).
Taken from Johnson (2005).
Taken from Ramadan et al. (2003) (structure not reported).
Taken from Johnson et al. (1997).
Taken from Flaugh et al. (1998).
Lasmiditan (COL-144, LY573144) is a departure from the other structures in that it contains no indole nucleus. The progression from a bicyclic aromatic nucleus (indole) to the monocyclic nucleus of lasmiditan apparently involved some serendipity, as Zhang et al. (2015) describe how, in the process of producing a homolog of LY334370, a monocyclic intermediate was formed that had moderately good affinity for the 5-HT1F receptor. Expanding an SAR around this finding, they discovered several compounds that had high affinity and good selectivity for the 5-HT1F receptor (Zhang et al., 2015). Replacing the indole to eventually generate lasmiditan resulted in a highly selective 5-HT1F receptor agonist.
Several additional studies have generated significant SAR for 5-HT1F receptor agonists (Xu et al., 2001; Filla et al., 2003; Mathes et al., 2004; Zhang et al., 2004). The most potent and selective compounds are included in Table 11. These molecules represent riffs on the indoleethylamine core of 5-HT. For example, compound A (Table 11) employs N,N-dimethyltryptamine as its core, resulting in a molecule that has slightly lower affinity for the 5-HT1F receptor compared with LY334370 but overall greater selectivity. Compounds B, C, D, and E illustrate that the indole nucleus can be replaced with other bicycles (e.g., azaindole, indazole, and indoline), resulting in compounds with very good 5-HT1F receptor affinity and selectivity.
TABLE 11.
Affinities of selective 5-HT1F receptor agonists from SAR studies at cloned human 5-HT receptors
Binding values are expressed as Ki in nanomolar from studies using cloned human receptors.
| Compound Aa | Compound Bb | Compound Cc | Compound Dd | Compound Ee | |
|---|---|---|---|---|---|
| Receptor | Ki ± S.E.M., nM | Ki ± S.E.M., nM | Ki ± S.E.M., nM | Ki, nM | Ki, nM |
| 5-HT1A | 265 ± 99 | 620 ± 37 | 1000 ± 270 | 870 | 240 |
| 5-HT1B | 1060 ± 204 | 270 ± 47 | 720 ± 220 | 2300 | >10 µM |
| 5-HT1D | 1620 ± 100 | 250 ± 38 | 720 ± 55 | 3100 | 2300 |
| 5-ht1e | >4830 | 660 ± 110 | 770 ± 80 | ||
| 5-HT1F | 8.2 ± 1.2 | 5.0 ± 0.5 | 5.5 ± 0.6 | 3.9 | 9 |
| 5-HT2A | 1000 ± 85 | 4800 ± 2000 | 3400 ± 1600 | ||
| 5-HT2B | 676 ± 118 | >10,000 | >10,000 | ||
| 5-HT2C | 2200 ± 390 | 1900 ± 1600 | 310 ± 41 | ||
| 5-HT4 | 4000 ± 350 | 2400 ± 740 | |||
| 5-HT6 | 380 ± 40 | >10,000 | 1120 ± 203 | ||
| 5-HT7 | 1770 ± 130 | >5000 | Not determined |
N-[3-(2-(dimethylamino)-ethyl)-2-methyl-1H-indol-5-yl]-4-fluorobenzamide, from Xu et al. (2001).
N-(3-(1-methyl-4-piperidinyl)-1H-pyrrolo(3,2-b)pyridin-5-yl)acetamide, from Filla et al. (2003).
N-[3-(1-Methyl-4-piperidinyl)-1H-pyrrolo[3,2-b]pyridin-5-yl]propanamide, from Filla et al. (2003).
4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indazol-5-yl]benzamide, from Zhang et al. (2004).
4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-2,3-dihydro-1H-indol-5-yl]benzamide, from Zhang et al. (2004).
2. Partial Agonists and Antagonists
There are no published SAR studies for 5-HT1F receptor antagonists. Methiothepin has been used in in vitro functional studies, but it is nonselective and of only moderate affinity for the 5-HT1F receptor (Adham et al., 1993b). 1-naphthylpiperazine and metergoline were described as partial agonists to inhibit adenylyl cyclase activity (Adham et al., 1993a). Other compounds have been reported as partial agonists at 5-HT1F receptor–stimulated [35S]GTPγS binding, such as dihydroergotamine (68% of 5-HT; Wainscott et al., 1998), LY302148 (17% of 5-HT; Wainscott et al., 1998), frovatriptan (46% of 5-HT; Nelson et al., 2010), 2-thienyl-LYX (61% of 5-HT; Filla et al., 2003), and 2-pyridyl-LYX (63% of 5-HT; Filla et al., 2003).
The prototypical triptan, sumatriptan, has relatively high affinity for the 5-HT1F receptor, and for comparison with the selective 5-HT1F compounds, Table 6 lists the affinities of a number of triptans across the 5-HT receptor subtypes. In efficacy studies using a [35S]GTPγS binding system to measure functional activity at the 5-HT1 family of receptors, naratriptan, rizatriptan, sumatriptan, and zolmitriptan were found to act as full agonists at all the 5-HT1 receptors that they stimulated. Frovatriptan, the only carbazole (i.e., a three-ring system containing indole), was a full agonist at 5-HT1B and 5-HT1D receptors but only a partial agonist at 5-HT1A and 5-HT1F receptors (Nelson et al., 2010). Hence, several triptans have high affinity for the 5-HT1F receptor and may activate 5-HT1F receptors in vivo at doses sufficient to also activate 5-HT1B/1D receptors (as well as other receptors; Table 6).
E. Signal Transduction
The 5-HT1F receptor inhibits forskolin-stimulated adenylyl cyclase activity in recombinant cell systems (Amlaiky et al., 1992; Adham et al., 1993b), with later studies suggesting coupling through Gi/Go proteins based on pertussis toxin sensitivity (Adham et al., 1993a). The human 5-HT1F receptor can also mediate inositol phosphate production and calcium flux through a pertussis toxin–sensitive mechanism in a recombinant system (Adham et al., 1993a). There are no reports on the transduction mechanisms affected by the 5-HT1F receptor in native tissues.
F. Function
As noted above, signal transduction studies with heterologously expressed 5-HT1F receptor readily identify responses, such as inhibition of forskolin-stimulated adenylyl cyclase activity (Amlaiky et al., 1992; Adham et al., 1993b), stimulation of inositol phosphate production and cellular calcium flux (Adham et al., 1993a), and stimulation of [35S]GTPγS binding (Wainscott et al., 1998).
Neurogenic dural inflammation has been used as a model for the development of antimigraine drugs, in which the triptans display efficacy. Early dogma considered the mechanism of action of the triptans to be exclusively through the activation of 5-HT1B/1D receptors (Buzzi and Moskowitz., 1991; Humphrey, 2007). Yet other studies (Johnson et al., 1997; Wainscott et al., 1998) demonstrate that the pharmacologic profile for the inhibition of neurogenic dural inflammation in the guinea pig correlated better with the pharmacology of the 5-HT1F receptor. Several additional studies support that 5-HT1F receptor–selective agonists inhibit neurogenic dural inflammation; LY344864 and lasmiditan inhibit neurogenic dural inflammation in the rat (Phebus et al., 1997; Nelson et al., 2010), and “Compound A, B, and C” likewise in the guinea pig (Xu et al., 2001; Filla et al., 2003).
Several studies have examined the effects of 5-HT1F receptor agonists on c-fos levels in the trigeminal system. Intracisternal administration of capsaicin in rats stimulates c-fos production in the trigeminal nucleus caudalis, an effect that is inhibited by pretreatment with either sumatriptan or LY344864 (Mitsikostas et al., 1999). The effect of sumatriptan, but importantly not that of LY344864, was blocked by a rat 5-HT1B receptor antagonist. The inhibition of capsaicin-induced c-fos production by LY344864 and sumatriptan was also reproduced in the mouse (Mitsikostas et al., 2002). Electrical stimulation of the trigeminal ganglion in the rat induces c-fos production in the nucleus caudalis, which was blocked by lasmiditan in a dose-dependent fashion (Nelson et al., 2010). LY334370 was also active in this model.
Other models of neuronal stimulation have shown that 5-HT1F receptor agonists produce inhibitory effects. For example, LY344864 inhibited a CGRP-mediated vasodepressor response produced by electrical stimulation of primary sensory nerves in the rat (González-Hernández et al., 2011). It did not affect vasodepressor responses induced by exogenously administered CGRP, consistent with the concept that prejunctional 5-HT1F receptors inhibit CGRP release. LY344864 inhibited potassium-stimulated release of CGRP in the dura mater of the rat but not in the trigeminal ganglion or trigeminal nucleus caudalis (Amrutkar et al., 2012). Additionally, LY344864 inhibited activation of second-order neurons in the trigeminal nucleus caudalis elicited by electrical stimulation of the dura mater in rats (Shepheard et al., 1999).
Little work has been devoted to potential peripheral actions of 5-HT1F receptors. Granados-Soto et al. (2010) have suggested that 5-HT1F receptors are involved in peripheral pain mechanisms, as LY344864 blocked nociception induced by formalin injection into the rat paw. In a more direct examination of peripheral tissues LY334370 and LY344864 stimulated the production of markers of mitochondrial biogenesis in isolated rabbit renal proximal tubules, which was diminished when the tubule preparation was subject to 5-HT1F receptor knockdown by siRNA transfection (Garrett et al., 2014). Administration of LY344864 in vivo to mice also led to an increase in a panel of markers for mitochondrial biogenesis in renal cortex, heart, and liver (Garrett et al., 2014).
Extensive study has failed to demonstrate that 5-HT1F receptors contract blood vessels (e.g., Johnson et al., 1997; Cohen and Schenck, 1999, 2000; Razzaque et al., 1999; Shepheard et al., 1999; Bouchelet et al., 2000; Nelson et al., 2010). As detailed in III. 5-HT1B Receptors, this contrasts functions associated with the 5-HT1B receptor, through which triptans act to contract certain vascular tissues, including coronary artery, that can present serious adverse effects for patients, hence the optimism for 5-HT1F receptor agonists as treatments for migraine with a reduced side-effect profile (see below).
G. Clinical Relevance and Therapeutics
As discussed above, because 5-HT1F receptor agonism correlates with the pharmacology of the inhibition of a model of neurogenic dural inflammation—combined with the apparent absence of the 5-HT1F receptor to contract vasculature—much of the translatable work concerning the 5-HT1F receptor has centered on the treatment of migraine. This led, ultimately, to the development of LY334370, the first selective 5-HT1F receptor agonist examined clinically for which efficacy to treat the pain of acute migraine attacks was noted (Goldstein et al., 2001). However, development of LY334370 was terminated because of safety concerns identified in animal toxicology studies (Ramadan et al., 2003; Ramadan and Buchanan, 2006). Further efforts to search for a more selective 5-HT1F receptor agonist with preclincal toxicology issues identified LY573144. This molecule was subsequently out-licensed to CoLucid Pharmaceuticals (becoming COL-144; lasmiditan; Table 10), although the acquisition of CoLucid in 2017 by Eli Lilly returned the molecule to the parent company.
Lasmitidan is efficacious in alleviating symptoms of acute migraine in clinical trials. The first peer-reviewed published trial was a proof-of-concept investigation that demonstrated the efficacy of lasmiditan given intravenously (Ferrari et al., 2010). The primary efficacy measure was headache relief 2 hours after administration; a significant dose-response effect on efficacy separated lasmitidan from placebo. A trial using oral lasmiditan used the same primary efficacy measure, and all doses significantly improved headache response at 2 hours compared with placebo (Färkkilä et al., 2012). The most common side effects were dizziness, fatigue, vertigo, and paresthesia. Positive results from two pivotal phase III trials of lasmitidan (Kuca et al., 2018; Loo et al., 2019) led to subsequent marketing approval in 2019.
VII. 5-HT2A Receptors
A. Introduction
The 5-HT2A receptor (formerly 5-HT2) was first identified as a binding site in rat brain with high (nanomolar) affinity for [3H]spiperone and [3H]ketanserin and low (micromolar) affinity for 5-HT (Peroutka and Snyder, 1979; Leysen et al., 1981). Soon after its discovery, the 5-HT2A receptor was found to mediate several effects of 5-HT in the periphery, including platelet aggregation (De Clerck et al., 1982) and smooth muscle contraction (Cohen et al., 1981; Maayani et al., 1984; Engel et al., 1985). The peripheral 5-HT2A receptors were originally classified as “D-type” 5-HT receptors based on pharmacological evidence (Bradley et al., 1986). The 5-HT2A receptor was also the first 5-HT receptor found to couple to stimulate phosphatidyl inositol hydrolysis (Conn and Sanders-Bush, 1984).
B. Cloning of the Gene
The first 5-HT2A receptor clone was isolated from rat brain cDNA libraries by homology screening based on the sequence of structurally related 5-HT2C receptor (Pritchett et al., 1988; Julius et al., 1990). Functional expression of the cloned receptor confirmed coupling to phosphoinositide hydrolysis and Ca2+ mobilization. The human 5-HT2A receptor was subsequently cloned by Saltzman et al. (1991) and displayed 87% homology with the rat receptor. The receptor contains 471 amino acids, with five potential glycosylation sites in the N-terminal extracellular domain and 11 potential phosphorylation sites in the C-terminal intracellular domain. The HTR2A gene encoding the human 5-HT2A receptor has been mapped to chromosome 13q14–q21 (Sparkes et al., 1991). Analysis of the genomic structure of the human 5-HT2A receptor revealed that it contains three exons separated by two introns, spanning more than 20 kb (Chen et al., 1992; Stam et al., 1992). Other species from which the 5-HT2A receptor has been cloned include hamster (Van Obberghen-Schilling et al., 1991), mouse (Yang et al., 1992), and pig and rhesus monkey (Johnson et al., 1995) (Table 12). Sequence alignments for the 5-HT2A receptor from eight species are shown in Fig. 8.
TABLE 12.
5-HT2A receptor genes, transcripts, and proteins
| Gene | mRNA Transcript | Receptor Protein | ||||
|---|---|---|---|---|---|---|
| Organism | Location | Ensembl Gene ID | NCBI RefSeq ID | Base Pairs | NCBI RefSeq ID | Amino Acids (aa) |
| Bos taurus (cow) | Ch 12: 16.82–16.88 Mb | ENSBTAG00000013498 | NM_001001157 | 3562 bp | NP_001001157 | 470 aa |
| Equus caballus (horse) | Ch 17: 23.89–23.95 Mb | ENSECAG00000024282 | NM_001081784 | 1413 bp | NP_001075253 | 470 aa |
| Homo sapiens (human) | Ch 13: 46.83–46.89 Mb (13q14–q21) | ENSG00000102468 | NM_000621 | 5429 bp | NP_000612 | 471 aa |
| Macaca mulatta (rhesus macaque) | Ch 17: 25.98–26.05 Mb | ENSMMUG00000004210 | NM_001032966 | 1438 bp | NP_001028138 | 471 aa |
| Mus musculus (mouse) | Ch 14: 74.64–74.70 Mb | ENSMUSG00000034997 | NM_172812 | 2971 bp | NP_766400 | 471 aa |
| Rattus norvegicus (rat) | Ch 15: 56.66–56.73 Mb (15q11) | ENSRNOG00000010063 | NM_017254 | 1566 bp | NP_058950 | 471 aa |
| Sus scrofa (pig) | Ch 11: 20.89–20.95 Mb | ENSSSCG00000009406 | NM_214217 | 1432 bp | NP_999382 | 470 aa |
Fig. 8.
Primary structure of 5-HT2A receptors from various species.
1. Regulation of 5-HT2A Receptor Gene Expression
The structure of the 5-HT2A promoter region has been characterized in humans, rats, and mice; the promoters lack canonical TATA or CAAT boxes. Fragments of a 1.6-kb segment from the 5′ flanking region of the human gene showed promoter activity when transfected into receptor-expressing human cell lines (Zhu et al., 1995). The human promoter sequence contains multiple transcription initiation sites, along with several binding sites for transcription factors, including simian virus 40 promoter factor 1, polyomavirus enhancer activator 3, cAMP response element, and E-box binding proteins. There was also evidence that the 5′ flanking sequence contains an alternative promoter as well as a silencing element upstream from the translation start codon. Falkenberg et al. (2011) subsequently demonstrated that the human promoter contains a glucocorticoid receptor (GR) binding site at position −1420. Furthermore, the A-allele of the −1438G/A (rs6311) polymorphism is believed to create a binding site for the transcription factor Th1/E47, which reportedly increases promoter activity (Smith et al., 2008).
Multiple cis elements in the mouse 5-HT2A promoter act in a dynamic manner to regulate transcription. The 5′ flanking region of the mouse 5-HT2A receptor gene contains a basal promoter (located −0.6 to −2.3 kb from the translational start site), which includes 11 transcription initiation sites, and binding sites for AP-2 (activating protein 2), polyomavirus enhancer activator 3, and simian virus 40 promoter factor 1 transcription factors (Ding et al., 1993; Toth et al., 1994). The activity of the basal promoter is attenuated in nonneuronal cells by two upstream repressor elements (extending from −2.3 to −4.2 kb), domains that presumably contain binding sites for transcription-inhibiting factors present in nonneuronal but not in neuronal cell types. Repressed genes can be reactivated in particular cell types via cell-specific activators, which may be responsible for 5-HT2A receptor expression in certain nonneuronal cells. For example, Ding et al. (1993) identified a domain located upstream (−4.2 to −5.6 kb) from the repressor elements that reactivates transcription of the 5-HT2A gene in C6 glioma cells.
The organization of the rat 5-HT2A promoter is similar to that of mice, containing multiple negative and positive regulatory elements. Garlow et al. (1994) identified a primary transcription initiation site −1173 from the translational start site and a minimal promoter sequence in the 0.2-kb sequence immediately upstream from the primary initiation site. The activity of the minimal promoter is enhanced by proximal positive transitional elements 0.2–1.1 kb from the initiation site and attenuated by two distal negative domains located further upstream (1.1–2.2 and 2.3–2.5 kb from the initiation site, respectively). Analysis of the promoter and enhancer sequences revealed the presence of binding sites for the transcription factors nuclear factor 1, AP-1, AP-2, and Egr 1 as well as a GR element (Garlow et al., 1994; Garlow and Ciaranello, 1995). Experiments with transfected promoter-reporter plasmids showed that dexamethasone and AP-1 affected transcription of the promoter (Garlow and Ciaranello, 1995). The GR element appears to regulate 5-HT2A receptor transcription in rat brain, as evidenced by the significant increase in 5-HT2A mRNA expression induced by GR knockdown (Islam et al., 2004). AP-1 may play a role in agonist-induced upregulation of the 5-HT2A receptor in rat cerebellar granule cells (Chalecka-Franaszek et al., 1999). In contrast, Du et al. (1994, 1995) reported that the primary 5-HT2A transcriptional start site in rat myometrial smooth muscle cells is located at position −1120 from the translational start site. They also identified a basal promoter and two upstream repressor domains, but no enhancer region was detected. These discrepant findings may reflect cell type–specific differences in the function of the rat 5-HT2A promoter.
C. Distribution
Many cell types in peripheral tissues express 5-HT2A receptors, including platelets, fibroblasts, lymphocytes, and myocytes. In the CNS, neurons are the main site of localization, although the presence of 5-HT2A receptors on nonneuronal cells types (glia, astrocytes) has also been reported (see below). The localization of 5-HT2A receptors in the brain has been mapped by a combination of receptor autoradiography, in situ hybridization, immunocytochemistry, and, more recently, PET neuroimaging. Receptor autoradiography studies using [3H]spiperone, [3H]ketanserin, [125I]DOI, and [3H]MDL 100907 as radioligands have revealed high levels of 5-HT2A receptor binding sites in many forebrain regions, including cortical and hippocampal areas, the basal ganglia, and olfactory tubercle, and the pattern is similar across species (e.g., Pazos et al., 1987b and López-Giménez et al., 1997). The distribution of 5-HT2A receptor binding sites agrees well with that of 5-HT2A mRNA (Mengod et al., 1990b; Morilak et al., 1994; Burnet et al., 1995), suggesting that 5-HT2A receptors are largely expressed in the region of the somatodendritic and not trafficked along axons; however, there are some conflicting immunohistochemical data (for review, see Weber and Andrade, 2010; Nocjar et al., 2015). Moreover, much 5-HT2A receptor immunoreactivity in rat neocortex has been detected in the cytoplasmic rather than membrane-bound compartments (Cornea-Hébert et al., 1999, 2002), which might reflect a high intracellular reserve of the 5-HT2A receptors and could be useful for the dynamic insertion of these receptors into the membrane.
A combination of immunocytochemical and in situ hybridization studies have investigated the cell types expressing the 5-HT2A receptor in cerebral cortex (Fig. 9). Early data demonstrated the presence of 5-HT2A receptors in cortical glutamatergic pyramidal (projection) neurons (Burnet et al., 1995), which have subsequently been mapped to specific cortical pathways (Vázquez-Borsetti et al., 2009; Mocci et al., 2014). Most such studies indicate that these cortical 5-HT2A receptors are predominantly postsynaptic and localized to either the apical dendrites or soma of pyramidal neurons. However, 5-HT2A receptors have also been detected in GABAergic interneurons in the cortex (Morilak et al., 1994; Burnet et al., 1995; Mengod et al., 2015) and amygdala. There has also been an immunohistochemical analysis of 5-HT2A receptor localization in the ventral tegmental area, and the majority of immunolabeling was colocalized with tyrosine hydroxlyase, suggesting that the receptors are expressed on dopaminergic neurons; however, there is also evidence for localization on VTA GABA neurons (Doherty and Pickel, 2000; Nocjar et al., 2002).
Fig. 9.
In situ hybridization detection of 5-HT2A receptor mRNA expression in rat and human brain. Reverse autoradiograms of the rat (A) and human brain (B–F). Human section: hippocampus and surrounding cortex (B), orbitofrontal cortex (Brodmann area 11) (C), striate cortex (Brodmann area 17) (D), superior temporal gyrus (Brodmann area 22) (E), and brainstem at the level of the raphe nucleus (F); no lack of 5-HT2A receptor mRNA was evident. Adapted from Burnet et al. (1995) (with permission).
More recently, 5-HT2A receptor localization has been mapped using bacterial artificial chromosome (BAC) transgenic mice engineered to express a fluorescent reporter (enhanced green fluorescent protein) under the control of the 5-HT2A receptor promoter, thus revealing 5-HT2A expression (Weber and Andrade, 2010). These data show a striking pattern of 5-HT2A receptor distribution at the regional and cellular levels. Mapping within the cortical microcircuitry revealed 5-HT2A receptor expression in specific lamina and in both pyramidal and interneurons. Interestingly, and in agreement with previous observations (Puig et al., 2010), expression was marked in cortical parvalbumin-positive interneurons, which underpin the formation of certain network oscillations (gamma frequency) thought critical for sensory information processing. The BAC transgenic mouse study and previous immunocytochemical studies (Stein et al., 2000; Weber and Andrade, 2010) also found 5-HT2A receptors are located on parvalbumin-containing interneurons in the basolateral nucleus of the amygdala. This finding is consistent with data from electrophysiological studies showing that in the amygdala, 5-HT acts on 5-HT2A receptors to potentiate GABAergic inhibition, including the GABA input to pyramidal neurons in this region (Jiang et al., 2009; Bocchio et al., 2015).
The study of BAC transgenic mice with enhanced green fluorescent protein under the control of the 5-HT2A receptor promoter (Weber and Andrade, 2010) did not report the presence of 5-HT2A receptors in nonneuronal cells, as suggested in earlier immunocytochemical studies (Xu and Pandey, 2000); however, further confirmation is awaited. Colocalization of 5-HT2A receptors with other 5-HT receptor subtypes has been reported (5-HT1A, 5-HT2C; e.g., Puig et al., 2010; Stephens et al., 2014; Mengod et al., 2015; Nocjar et al., 2015; Tian et al., 2016), providing further evidence of potential crosstalk in 5-HT signaling at the receptor level.
The development of a number of 5-HT2A receptor–selective radioligands has been useful for research tools, such as the imaging of 5-HT2A receptors in humans, with the most successful including the single-photon emission computerized tomography radioligand [123I]R91150 and the PET radioligands [18F]setoperone, [18F]altanserin, and [11C]MDL 100907 (Paterson et al., 2013; Herth and Knudsen, 2015). The first 5-HT2A receptor agonist PET ligand, [11C]N-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine ([11C]Cimbi-36), has recently been reported (Ettrup et al., 2014) and raised the interesting possibility that this may be displaceable by endogenous 5-HT and therefore provide an index of 5-HT release. [18F]Altanserin PET has also been used to quantify 5-HT release (Quednow et al., 2012). A potential confound for the development of 5-HT2A receptor PET ligands is the reported high levels of 5-HT2A receptors in the intracellular compartment (see above). If the significant levels of PET binding are intracellular, then it is less likely to be in a position to be displaced by endogenous 5-HT. However, collectively, these imaging studies confirm the cross-species localization of 5-HT2A receptor and, more importantly, have opened the way for investigations of 5-HT2A receptors in disease states.
D. Post-translational Modifications and Impact
N-Glycosylation is known to regulate the intracellular sorting, surface expression, ligand binding, and signal transduction of GPCRs (Couvineau et al., 1996; Michineau et al., 2004). The extracellular N-terminus of the 5-HT2A receptor contains five potential N-glycosylation sites; glycosylation is apparently required for the 5-HT2A receptor to be targeted to the cell surface (Maginnis et al., 2010). Multiple proteins have been shown to interact with the 5-HT2A receptor (Table 13; see also XVII. 5-HT GPCRs and their Interacting Proteins).
TABLE 13.
Proteins reported to interact with the 5-HT2A receptor
| Interacting Protein | Region of the 5-HT2A Receptor | Reference |
|---|---|---|
| ADP-ribosylation factor 1 (Arf1) | C-terminus (NPxxY motif) | Robertson et al., 2003; Johnson et al., 2006 |
| β-arrestin | ICL3, C-terminus (ASK motifa) | Gelber et al., 1999; Bhattacharya et al., 2010 |
| Calmodulin (CaM) | ICL2, C-terminus | Turner and Raymond, 2005 |
| Caveolin-1 (Cav-1) | ND | Bhatnagar et al., 2004 |
| Glutamine synthetase | ICL3 | Sheffler et al., 2006 |
| Jak2 kinase | ND | Guillet-Deniau et al., 1997 |
| Microtubule-associated protein 1A (MAP1A) | ICL3 | Sheffler et al., 2006 |
| Multi-PDZ domain protein 1 (MUPP1) | C-terminus (PDZ domain) | Jones et al., 2009 |
| Na+/H+ exchange regulatory factor 3 (NHERF3) | ND | Walther et al., 2015 |
| Nucleoside-diphosphate kinase 3 (NME3) | ICL3 | Sheffler et al., 2006 |
| Paraoxonase 2 (PON2) | ICL3 | Sheffler et al., 2006 |
| Postsynaptic density protein 95 kDa (PSD-95) | C-terminus (PDZ domain) | Xia et al., 2003 |
| Protein phosphatase 5 (PP-5) | ICL3 | Sheffler et al., 2006 |
| Ribosomal S6 kinase 2 (RSK2) | ICL3 | Sheffler et al., 2006 |
| Synapse-associated protein 97 (SAP97) | C-terminus (PDZ domain) | Dunn et al., 2014 |
C-terminus, carboxyl-terminus; ICL2, second intracellular loop; ICL3, third intracellular loop; ND, not determined.
Specific to the human and monkey 5-HT2A receptor.
E. Pharmacology
The three members of the 5-HT2 receptor family share significant sequence homology (Fig. 10). Depending on the species examined, the seven transmembrane domains of 5-HT2A and 5-HT2C receptors display 79%–80% amino acid sequence conservation. Because of the high degree of structural homology, not surprisingly, 5-HT2A and 5-HT2C receptor binding affinities are highly correlated (Glennon et al., 1992a,b, 1994; Nelson et al., 1999). It is now recognized that most of the antagonists that have traditionally been used to block 5-HT2A receptors, including N-alkylpiperidines (e.g., ketanserin, ritanserin, pirenperone, and altanserin), ergolines (e.g., methysergide, metergoline, and LY53857), and tricyclic benzocycloheptenes (e.g., cyproheptadine and pizotifen), are also active at 5-HT2C receptor (Newton et al., 1996; Hoyer, 1988a,b). For example, altanserin is only 20-fold selective for 5-HT2A versus 5-HT2C receptor sites (Table 14). Ketanserin has been used extensively for reported pharmacological definition of 5-HT2A receptor responses and does show some selectivity for 5-HT2A receptor (pKi = 8.7) compared with 5-HT2B (pKi = 6.4) and 5-HT2C (pKi = 6.8) receptors (Wainscott et al., 1996). However, ketanserin also has moderate affinity for adrenergic (α1) and histaminergic (H1) receptors as well as 5-HT1D receptors and the vesicular monoamine transporter (Erickson et al., 1996; Leysen et al., 1996; Bucholtz et al., 1999; Yoshio et al., 2001), which can complicate interpretation of arising data. Ritanserin is even less selective for 5-HT2A versus 5-HT2C receptors and also interacts with 5-HT1D, 5-HT6, 5-HT7, D2, D3, D4, H1, and α1 sites (Bard et al., 1993; Monsma et al., 1993; Shen et al., 1993; Leysen et al., 1996; Seeman and Tallerico, 1998; Yoshio et al., 2001). The butyrophenone neuroleptic spiperone displays 500- to 2000-fold selectivity for 5-HT2A versus 5-HT2C and has often been used to discriminate those receptors, but it binds to numerous other receptors, including dopaminergic D2, D3, and D4; adrenergic α1 and α2; and 5-HT1A and 5-HT7 receptors (Ruat et al., 1993b; Tang et al., 1994; Metwally et al., 1998; Corradetti et al., 2005). The spiperone derivative AMI-193 (8-[3-(4-fluorophenoxy)propyl]-1-phenyl-1,3,8-triazaspiro[4,5]decan-4-one) is twice as selective as spiperone for 5-HT2A versus 5-HT2C but retains nanomolar affinity for D2 and 5-HT1A (Ismaiel et al., 1993). Atypical antipsychotics such as risperidone, olanzapine, and clozapine block 5-HT2A receptors with high affinity but generally have limited selectivity versus 5-HT2C and dopamine receptors. By contrast, haloperidol and other typical antipsychotics have higher affinity for dopamine D2 receptors than for 5-HT2A receptors.
Fig. 10.
Primary structure of human 5-HT2A, 5-HT2B, and 5-HT2C receptors.
TABLE 14.
Affinities of 5-HT2A receptor antagonists
| Ligand | h5-HT2A Ki (nM) | r5-HT2A Ki (nM) | h5-HT2C Ki (nM) | r5-HT2C Ki (nM) | hα1A Ki (nM) | hα1B Ki (nM) | References |
|---|---|---|---|---|---|---|---|
| Spiperone | 1.4 | 0.43 | 661 | 871 | 5.0 | 0.6 | Bonhaus et al., 1997; Yoshio et al., 2001 |
| Ketanserin | 3.2 | 1.3 | 200 | 63 | 6.3 | 6.3 | Yoshio et al., 2001; Bonhaus et al., 1995 |
| Ritanserin | 0.25 | 0.30 | 0.25 | 2.7 | 4.0 | 10 | Bonhaus et al., 1997; Yoshio et al., 2001 |
| Pirenpirone | 1.08 | 77 | Wainscott et al., 1996 | ||||
| Risperidone | 1.1 | 0.16 | 12 | 32 | 4.0 | 10 | Yoshio et al., 2001; Roth et al., 1992; Kongsamut et al., 1996; Schotte et al., 1996 |
| Altanserin | 0.51 | 0.3 | 6.0 | Smith et al., 1998; Tan et al., 1999 | |||
| MDL 11,939 | 6.06 | 2.893 | 1020 | 853.6 | 1876 | 1579 | Wainscott et al., 1996; Pehek et al., 2006 |
| M100,907 | 1.50 | 1.92 | 88 | 128 | 424.7 | Pehek et al., 2006; Kehne et al., 1996 |
h, human; r, rat.
The 4-carbinolpiperidines volinaserin (MDL 100907, M100907) and glemaserin (MDL 11939) were the first truly selective 5-HT2A receptor antagonists. Volinaserin has subnanomolar affinity for 5-HT2A and 50- to 100-fold lower affinity for 5-HT2C and α1 receptors, with negligible affinity for other investigated sites (Palfreyman et al., 1993; Kehne et al., 1996). In contrast to most 5-HT2A receptor antagonists, the selectivity of M100907 for 5-HT2A versus 5-HT2C receptors has been verified in mice (Canal et al., 2013). Compared with volinaserin, glemaserin displays even greater selectivity for 5-HT2A receptor (Ki = 2.893 nM) versus 5-HT2B (Ki = 1419 nM), 5-HT2C (Ki = 853.6 nM), and α1 (Ki = 588 nM) receptor sites (Dudley et al., 1988; Pehek et al., 2006). Like most drugs that block 5-HT2A receptor responses, volinaserin and glemaserin were initially thought to be neutral antagonists but are now known to act as inverse agonists (Weiner et al., 2001; Aloyo et al., 2009). Although it was previously difficult to conclusively discriminate responses mediated by individual 5-HT2 receptor subtypes in vitro and in vivo, volinaserin or glemaserin at appropriate concentrations in combination with selective 5-HT2B and 5-HT2C receptor antagonists, such as RS-127445 and SB-242084, respectively, allow such pharmacological investigations.
In contrast to initial reports, it is now recognized that the 5-HT2A receptor has high affinity for 5-HT. For example, 5-HT competes for the agonist radioligand [3H]DOB with a Ki of 6.13 nM (Titeler et al., 1985), and [3H]5-HT reportedly radiolabels the 5-HT2A receptor with Kd = 1.3 nM (Sleight et al., 1996). In contrast, competition binding experiments with antagonist radioligands tend to underestimate the affinity of 5-HT2A receptor agonists; 5-HT2A receptors exist in low-affinity and high-affinity agonist binding conformations depending on whether they are coupled to G proteins, and only a small fraction of 5-HT2A receptors are in the G protein–coupled, agonist high-affinity conformation at any given time. 5-HT2A receptor antagonists bind to both conformations with equal affinity (Lyon et al., 1987; Glennon et al., 1988). Therefore, the apparent affinity of 5-HT2A receptor agonists varies depending on the intrinsic activity of the radioligand used to label the receptor, with agonists displaying 10- to 100-fold higher affinity for agonist-labeled receptors versus antagonist-labeled receptors (Titeler et al., 1987; Glennon et al., 1994; Sleight et al., 1996).
Numerous 5-HT2A receptor agonists are available (Table 15). Tryptamines such as α-methyl-5-HT and 5-methoxy-N,N-dimethyltryptamine are widely used as 5-HT2A receptor agonists, but they tend to activate 5-HT receptors nonselectively and can inhibit the 5-HT transporter at micromolar concentrations (Ismaiel et al., 1990; Nagai et al., 2007; Blough et al., 2014). 2,5-Dimethoxy-4-iodoamphetamine (DOI) and its structural analogs 2,5-dimethoxy-4-methylphenyl)-2-aminopropane, DOB, and (4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine have high affinity and selectivity for 5-HT2 receptors but do not discriminate between the three 5-HT2 receptor subtypes. In contrast to most phenylalkylamines, which are nonselective for 5-HT2A versus 5-HT2C receptors, 25CN-NBOH (N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenethylamine) is reportedly 100-fold selective for 5-HT2A (Ki = 1.3 nM) versus 5-HT2C receptors (Ki = 132 nM) (Hansen et al., 2014). The conformationally restricted phenethylamine (+)-(2S,6S)-trans-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine displays even greater selectivity for 5-HT2A receptors, binding to 5-HT2C receptors with 124-fold lower affinity (Juncosa et al., 2013). Racemic trans-DMBMPP is less selective but still shows 98-fold higher affinity for 5-HT2A over 5-HT2C receptors.
TABLE 15.
Affinities of 5-HT2A receptor agonists
| Liganda | h5-HT2A Ki (nM) [3H]ketanserin | h5-HT2A Ki (nM) [3H]mesulergine | Reference |
|---|---|---|---|
| (±)-DOI | 3.2 | 19.1 | Canal et al., 2013 |
| Cimbi-5 (25I-NBOMe) | 0.52 | 0.69 | Nichols et al., 2015 |
| Cimbi-36 (25B-NBOMe) | 0.19 | 4.0 | Juncosa et al., 2013 |
| (+)-(2S,6S)-DMBMPP | 2.5 | 310 | Juncosa et al., 2013 |
| 25CN-NBOH (NBOH-2C-CN) | 2.2 | 49.8 | Halberstadt et al., 2016 |
Alternative names are shown in parentheses.
F. Function
1. Signaling
The Gαq–PLCβ cascade is the canonical signaling pathway coupled to 5-HT2A receptor activation (Conn and Sanders-Bush, 1984; Kendall and Nahorski, 1985), resulting in the hydrolysis of membrane phospholipids to inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). In turn, IP3 elevates the concentration of Ca2+ in the cytosol by releasing it from the endoplasmic reticulum (ER), whereas DAG activates protein kinase C (PKC) and facilitates its translocation from the cytosol to the membrane. Both PKC and Ca2+ are known to have widespread and far-reaching influences on intracellular signaling; PKC phosphorylates various target proteins, whereas Ca2+ is known to modulate the activity of enzymes (e.g., Ca2+/calmodulin-dependent kinases) and ion channels.
In addition to the classic signaling mediated by the PLC-IP3 cascade, the 5-HT2A receptor can activate a variety of other effector mechanisms. For example, the 5-HT2A receptor has been shown to stimulate phospholipase A2 (PLA2), resulting in increased production of the second messenger AA (Felder et al., 1990; Berg et al., 1996). The 5-HT2A receptor also increases release of the endocannabinoid 2-arachidolylglycerol (Parrish and Nichols, 2006). The 5-HT2A receptor can also couple to an MAPK such as ERK1/2 (Hershenson et al., 1995; Watts, 1996; Greene et al., 2000). Other signaling molecules that have been linked to 5-HT2A activation in native tissues and in cell lines expressing the receptor, including Akt (Johnson-Farley et al., 2005), phospholipase D1 (PLD) (Barclay et al., 2011), JAK/STAT (Guillet-Deniau et al., 1997; Banes et al., 2005), NOS (Miller et al., 1997), cAMP response element binding (CREB) (Chalecka-Franaszek et al., 1999), calmodulin (Turner and Raymond, 2005), and glycogen synthase kinase-3β (Li et al., 2004).
Some of the aforementioned signaling pathways are coupled to the 5-HT2A receptor in a Gαq-independent manner. The ability of the 5-HT2A receptor to stimulate PLD is mediated by the monomeric G protein ADP-ribosylation factor-1 (ARF1), which interacts directly with the receptor (Barclay et al., 2011). The coupling of the 5-HT2A receptor to PLA2 in NIH3T3 cells appears to be mediated by two independent signaling cascades. In the first case, activation of Src by Gαi/o-associated Gβγ subunits results the formation of a ternary complex between SHC/GRB/SOS, which in turn activates the Ras–Raf–MEK1/2–ERK1/2 cascade. In the second, activation of RhoA by Gα12/13 stimulates p38 MAPK (Kurrasch-Orbaugh et al., 2003a). Reports also indicate PLC and PKC are not involved in the ERK activation produced by 5-HT2A receptor agonists in PC12 and vascular smooth muscle cells (Florian and Watts, 1998; Banes et al., 1999; Quinn et al., 2002). Nevertheless, in certain cell types, the coupling of ERK1/2 to 5-HT2A receptors is dependent on Gαq. Activation of the Ras–Raf–MEK–ERK1/2 cascade by 5-HT2A receptors in tracheal smooth muscle cells and mesangial cells is downstream from Gαq and dependent on PKC (Hershenson et al., 1995; Watts, 1996; Greene et al., 2000). The coupling of the 5-HT2A receptor to MAPK may actually be ligand-specific. In mouse embryonic fibroblasts transfected with the 5-HT2A receptor, the stimulation of ERK1/2 by DOI is dependent on PLC, whereas the stimulation by 5-HT is PLC-independent and requires β-arrestin2 (Schmid et al., 2008).
The 5-HT2A receptor was one of the first receptors shown to display functional selectivity. According to Berg et al. (1998a,b), 5-HT2A receptors activate PLC and PLA2 independently in CHO cells, and the relative efficacies of agonists differ depending on which response is measured. Subsequent studies confirmed the PLC-IP3 and PLA2-AA pathways coupled to 5-HT2A receptor in NIH3T3 cells have different receptor reserves, indicating they are activated independently (Kurrasch-Orbaugh et al., 2003b). It has been proposed that 5-HT2A receptor functional selectivity (biased signaling) may explain why certain 5-HT2A receptor agonists produce hallucinogenic effects, whereas other agonists such as lisuride are nonhallucinogenic (González-Maeso et al., 2007). Specifically, although both hallucinogenic and nonhallucinogenic 5-HT2A receptor agonists increase the expression of the immediate-early gene c-fos by activating Gαq, only agonists with hallucinogenic effects increase the cortical expression of the immediate-early gene (e.g., r-2) by activating Gαi/o and Src. Another group has reported that the activation of the 5-HT2A receptor by 5-HT and hallucinogens results in vastly different downstream signaling responses (Schmid and Bohn, 2010). The behavioral response to 5-HT in mice requires Akt phosphorylation and formation of a complex between Akt, Src, and β-arrestin2, whereas the response to hallucinogens is independent of Akt and β-arrestin2.
It is now apparent that many 5-HT2A receptor antagonists act in a functionally selective manner. Like most GPCRs, the 5-HT2A receptor is downregulated by exposure to agonists. Somewhat paradoxically, however, prolonged exposure to certain 5-HT2A receptor antagonists also induces 5-HT2A receptor downregulation (Leysen et al., 1986; Eison et al., 1989; Pranzatelli, 1991; Moreno et al., 2013). The anomalous downregulation of 5-HT2A receptors induced by antagonists is not a consequence of altered gene transcription (Roth and Ciaranello, 1991) but is likely caused by redistribution of the receptor from the cell surface to intracellular compartments (Willins et al., 1998, 1999; Bhatnagar et al., 2001). Interestingly, although the 5-HT2A receptor antagonists ketanserin, clozapine, and olanzapine reduce 5-HT2A immunoreactivity and [3H]ketanserin binding in mouse frontal cortex, M100907, MDL 11939, and altanserin do not alter receptor expression (Yadav et al., 2011). These findings indicate that certain 5-HT2A receptor antagonists have agonist-like effects on the signaling pathways responsible for promoting receptor internalization. For example, although ketanserin and risperidone act as inverse agonists on the 5-HT2A–PLC and 5-HT2A–ERK pathways, they also act as 5-HT2A receptor agonists by stimulating β-arrestin translocation (Clarke et al., 2013). Nevertheless, it appears that 5-HT2A receptor antagonists can promote 5-HT2A receptor internalization through multiple mechanisms because clozapine does not recruit β-arrestin (Schmid et al., 2014). Clozapine does act in a functionally selective manner—it has been shown to induce Akt phosphorylation in cortical neuronal cultures via the 5-HT2A receptor (Schmid et al., 2014); but the mechanism by which clozapine promotes receptor downregulation remains to be determined.
Adding to the complexity of 5-HT2A receptor signaling is the discovery that it can oligomerize with other GPCRs, potentially allowing the 5-HT2A receptor to couple to an even wider range of effector mechanisms. Gonzalez-Maseo et al. (2008) have reported that 5-HT2A and mGlu2 receptors form heterocomplexes, providing a mechanism whereby 5-HT2A receptors can modulate Gαi/o signaling. Heteromers between 5-HT2A and D2 receptors (Borroto-Escuela et al., 2010; Lukasiewicz et al., 2010) and 5-HT2A and CB1 receptors (Viñals et al., 2015) have also been detected in transfected cells, but the functional significance of these interactions and the extent to which they occur in native tissues is unclear.
2. Action in Cells, Tissues, and In Vivo
5-HT2A receptor activation produces a variety of physiologic effects in peripheral tissues and in the CNS. There is evidence for 5-HT2A receptor involvement in the proliferation, differentiation, and contraction of vascular and extravascular smooth muscle (Maayani et al., 1984; Watts and Cohen, 1992; Fanburg and Lee, 1997; Shum et al., 2002; Itoh and Kajikuri, 2011) as well as increased contractility of cardiac muscle, where it is expressed in neonatal and reexpressed in failing and hypertrophic heart (Qvigstad et al., 2005c; Birkeland et al., 2007b; Brattelid et al., 2007a,b, 2012; Levy et al., 2008). Similarly, the 5-HT2A receptor increases the proliferation and synthesis of extracellular matrix proteins by glomerular mesangial cells (Kasho et al., 1998; Göoz et al., 2006). 5-HT2A receptor antagonists inhibit the platelet aggregation and shape change induced by 5-HT, DOB, and DOI (de Clerck et al., 1982, 1984; Seggel et al., 1987). The neuroendocrine effects of DOI in rats, including increases in the release of adrenocorticotropic hormone, corticosterone, oxytocin, renin, and prolactin, appear to be mediated by 5-HT2A (Calogero et al., 1990; van de Kar et al., 2001; Zhang et al., 2002). 5-HT2A receptor activation produces sympathoexcitatory effects and increases arterial pressure through a combination of central and peripheral effects (Tadepalli et al., 1975; McCall et al., 1987; Alper, 1990; Dedeoğlu and Fisher, 1991; Chaouche-Teyara et al., 1994).
5-HT2A receptor activation produces long-lasting increases in the excitability and firing rate of glutamatergic and GABAergic neurons, resulting in increased excitatory and inhibitory network activity (McCormick and Wang, 1991; Sheldon and Aghajanian, 1991; Cumming-Hood et al., 1993; Pessia et al., 1994; Marek and Aghajanian, 1996; Aghajanian and Marek, 1997; Shen and Andrade, 1998; Zhou and Hablitz, 1999; Lambe and Aghajanian, 2006, 2007; Béïque et al., 2007; Benekareddy et al., 2010; Avesar and Gulledge, 2012; but see also Carr et al., 2002; Tian et al., 2016). These electrophysiological effects are absent in Htr2A-knockout mice and are restored upon conditional cortical rescue of 5-HT2A receptors (Weisstaub et al., 2006). Reductions in resting K+-conductances are thought to contribute to many of the excitatory effects of 5-HT2A receptor activation. For example, 5-HT2A receptor activation induces membrane depolarization, reduces afterhyperpolarization, and evokes a slow afterdepolarization in layer V pyramidal neurons in the PFC, effects thought to be mediated by inhibition of the Ca2+-activated K+-current IK(Ca) and activation of the Ca2+-dependent nonselective cation current ICAN (Araneda and Andrade, 1991; Tanaka and North, 1993; Villalobos et al., 2005, 2011; Zhang and Arsenault, 2005). The ability of 5-HT2A receptor activation to enhance motoneuron excitability is mediated by inhibition of a leak K+-current [IK(leak)] and enhancement of the hyperpolarizing-activated nonselective cation current (Garratt et al., 1993; Hsiao et al., 1997; Larkman and Kelly, 1998; Xu et al., 2009). TASK-1 and TASK-3 two-pore domain K+-channels are believed to be responsible for IK(leak) in motoneurons (Talley et al., 2000; Larkman and Perkins, 2005). Likewise, the 5-HT2A receptor–mediated depolarization produced by 5-HT in entorhinal cortex interneurons occurs as a consequence of TASK-3 channel inhibition (Deng and Lei, 2008). 5-HT2A has been shown to depolarize nucleus accumbens medium spiny neurons by inhibiting an inwardly rectifying K+-current (North and Uchimura, 1989). There is also evidence that 5-HT2A receptor–induced arterial constriction is mediated by inhibition of Kv channels (Cogolludo et al., 2006; Sung et al., 2013). Other ion channels known to couple to the 5-HT2A receptor include rapidly inactivating and persistent voltage-dependent Na+ channels (Carr et al., 2002), CaV 1.2 L-type Ca2+ channels (Day et al., 2002), Kv1.2 K+ channels (Lambe and Aghajanian, 2001), and voltage-independent Ca2+ channels (Hagberg et al., 1998).
G. Clinical Relevance
5-HT2A receptors are clinically relevant to numerous CNS disorders, ranging from mood disorder and schizophrenia to drug dependence. The links between the 5-HT2A receptor and the causes of such CNS disorders are not well understood. Early life stress has been shown trigger strong upregulation of the functional effects of 5-HT2A receptors on electrophysiology, signaling, and gene expression in prefrontal cortex (Benekareddy et al., 2010). Emerging findings regarding 5-HT2A gene polymorphisms and epigenetic regulation of 5-HT2A receptor expression are also of great interest. The association between the 5-HT2A receptor and successful treatment of certain CNS disorders is strong, and there is an ongoing active research interest in the therapeutic potential of 5-HT2A receptor ligands. The majority of these studies have had a particular focus on schizophrenia and depression and the actions of antipsychotic and antidepressant drugs.
In schizophrenia, there are reports of reduced 5-HT2A receptor binding in frontal cortex of postmortem brains, and there is supporting evidence from PET imaging studies for similar changes, as recently reviewed (Selvaraj et al., 2014). However, given that not all the PET studies used 5-HT2A receptor–selective radiotracers and that there are inconsistencies in the postmortem studies, the extent to which impaired cortical 5-HT2A receptor function contributes to the symptoms of schizophrenia is unclear.
The psychotomimetic effects of hallucinogens such as LSD and psilocybin are undoubtedly linked to activation of 5-HT2A receptors (Halberstadt, 2015). Furthermore, commonly used second-generation antipsychotics such as clozapine, risperidone, and sertindole are potent 5-HT2A receptor antagonists in addition to having affinity at other 5-HT receptor subtypes and receptors for other neurotransmitters (Meltzer, 2012). 5-HT2A receptor blockade has been linked to the antipsychotic efficacy of second-generation antipsychotics as well as a reduced side effect profile (Meltzer and Massey, 2011). This provided support for the idea that selective 5-HT2A receptor antagonists might be useful as a monotherapy for some psychoses and as antipsychotic augmenting agents, although schizophrenia trials with the selective 5-HT2A receptor antagonist MDL 100907 were disappointing. Nevertheless, the subsequent discovery that 5-HT2A receptor inverse agonists have antipsychotic actions in preclinical models (Weiner et al., 2001) suggests an alternative way to treat psychosis. This thinking advanced to the clinical development of pimavanserin (Vanover et al., 2006) for the treatment of psychosis in patients with Parkinson disease (Meltzer and Roth, 2013). In 2016, pimavanserin was approved by the U.S. FDA as a treatment for hallucinations and delusions associated with Parkinson disease. Although the degree of inverse agonism required for an antipsychotic effect is uncertain, 5-HT2A receptor inverse agonists are currently considered as a potential new generation of antipsychotic agents.
In postmortem studies of suicide victims, there is evidence of an upregulation in 5-HT2A receptors in cortex [for review, see Meyer (2013)]. This finding parallels results from PET imaging studies that report increased 5-HT2A receptor binding in patients with depression, particularly in association with severe pessimism (Meyer et al., 2003; Bhagwagar et al., 2006). The latter observations fit with findings in healthy subjects, in that individuals with high scores for pessimistic personality had higher cortical 5-HT2A receptor binding (Frokjaer et al., 2008). It has been hypothesized that the increase in cortical 5-HT2A receptor binding in depression is associated with severe pessimism and arises as an adaptive response to chronic 5-HT deficiency (Meyer, 2013).
The above findings in patients with depression link to evidence that certain antidepressants, and particularly tricyclics, have high affinity for 5-HT2A receptors (e.g., Millan, 2006). Though tricyclic antidepressants have high affinity for multiple receptors, it can be argued that evidence of the superior therapeutic efficacy of tricyclic antidepressant drugs over other antidepressant drug classes, particularly in the treatment of severe melancholic depression, is linked in part to their ability to block 5-HT2A receptors. Thus, 5-HT2A receptor antagonists augment the effect of 5-HT uptake inhibitors in preclinical models (Marek et al., 2005; Boothman et al., 2006), and drugs with 5-HT2A receptor antagonist properties are advocated as an add-on to antidepressant therapy in treatment-resistant cases (Marek et al., 2003); all are a possible link to evidence of an inhibitory 5-HT2A receptor–mediated feedback on 5-HT neurons (Sharp et al., 2007).
Aside from schizophrenia and depression, 5-HT2A receptors are relevant to the pathophysiology of many other CNS disorders. Currently, there is large and ongoing research effort to understand the importance of polymorphic variation in the 5-HT2A receptor gene to a large variety of psychiatric disorders (e.g., Smith et al., 2013; Paquette and Marsit, 2014). Despite the many reports of associations between 5-HT2A gene polymorphisms and neuropsychiatric disorders, some of which are subject to careful meta-analysis, effect sizes are at best modest, and the role of genetic 5-HT2A variability in mental health continues to be uncertain. This work is expanding to incorporate recent findings of epigenetic variation of the 5-HT2A receptor gene and specifically methylation sites near the gene promoter region [for review, see Paquette and Marsit (2014)].
Ligands for 5-HT2A receptors have a current untapped potential for the management of cognitive dysfunction. There are well established links between the receptor and the formation of different memory types, including recognition (Morici et al., 2015) and fear memories (Bombardi and Di Giovanni, 2013; Zhang and Stackman, 2015). The latter connects with the considerations of ecstasy and other similar psychotomimetics for the management of post-traumatic stress disorder (Smith et al., 2014; Sessa and Nutt, 2015). There are also very interesting links between the 5-HT2A receptor and impulse control; in animal models, 5-HT2A receptor antagonists consistently reduce measures of impulsivity (Higgins et al., 2003; Winstanley et al., 2004; Fletcher et al., 2007; Winstanley, 2011) and attenuate abuse-related effects of cocaine (Howell and Cunningham, 2015). This raises the possibility that 5-HT2A antagonists/inverse agonists may have utility in the control of addictions and disorders of impulse control more generally.
5-HT2A receptors are required for entry of JC polyomavirus (JCV) into cells (Elphick et al., 2004). JCV causes progressive multifocal leukoencephalopathy, a fatal demyelinating disease. JCV attaches to the sialic acid receptor motif α2,6-linked lactoseries tetrasaccharide c on the cell surface and then undergoes endocytosis with the 5-HT2A receptor (Assetta et al., 2013). 5-HT2A antagonists have been found to inhibit infection of cell by JCV (O’Hara and Atwood, 2008). Furthermore, treatment with mirtazepine, an antidepressant that acts as a 5-HT2A receptor antagonist, has proven beneficial in patients infected with JCV (Verma et al., 2007; O’Hara and Atwood, 2008; Cettomai and McArthur, 2009; Park et al., 2011a).
5-HT2A receptor agonists have ocular hypotensive effects. R-(–)-DOI, 5-methoxy-N,N-dimethyltryptamine, and α-methyl-5-HT have been shown to lower intraocular pressure in a cynomolgus monkey model of ocular hypertension (May et al., 2003). 5-HT2A receptors have been identified in tissues involved in the regulation of aqueous humor dynamics, including ciliary muscle (Sharif et al., 2006a), ciliary epithelium (Inoue-Matsuhisa et al., 2003), and trabecular meshwork (Sharif and Senchyna, 2006; Sharif et al., 2006b). Studies have not completely elucidated the mechanism for the reduction of intraocular pressure by 5-HT2A receptor agonists, but increased uveoscleral outflow may play a role (Gabelt et al., 2005). This effect led to the evaluation of 5-HT2A receptor agonists as potential treatments for glaucoma. To reduce the potential for psychotropic side effects, much of the work to develop 5-HT2A receptor agonists as ocular hypotensive agents has focused on compounds with limited blood-brain barrier permeability. Several 5-HT2A receptor agonists with limited blood-brain barrier permeability have been shown to lower intraocular pressure following topical ocular administration to monkeys, including AL-34662 (1-[(2S)-2-aminopropyl]-1H-indazol-6-ol) (Sharif et al., 2007), (8R)-1-[(2S)-2-aminopropyl]-8,9-dihydro-7H-pyrano[2,3-g]indazol-8-ol (May et al., 2015), phenylisopropylamines incorporating α-hydroxy or α-methoxy substituents (Glennon et al., 2004), and benzodifuran derivatives (Feng et al., 2007).
Ketanserin can reportedly lower interocular pressure in patients with glaucoma (Costagliola et al., 1993; Mastropasqua et al., 1997). However, the ocular hypotensive effect of ketanserin is attenuated in unilaterally sympathectomized rabbits and therefore is thought to be mediated by a blockade of α1-adrenoceptors (Chang et al., 1985).
VIII. 5-HT2B Receptor
A. Introduction
5-HT–induced contractions of rat stomach fundus strips were used as a sensitive bioassay for 5-HT long before more specific analytical assays became available (Vane, 1957); however, the 5-HT receptor was only defined more than three decades later as the 5-HT2B receptor. Early pharmacological studies suggested a strong similarity to the 5-HT2C receptor, in line with the high potency of 5-HT and blockade by 5-HT2 receptor antagonists. However, 5-HT2C receptor mRNA was absent, and molecular cloning identified the new receptor, first in rat and mouse (Foguet et al., 1992a,b; Kursar et al., 1992; Loric et al., 1992; Wainscott et al., 1993) and then in humans (Choi et al., 1994; Kursar et al., 1994; Schmuck et al., 1994; Wainscott et al., 1996). Initially, given the tissue associated primarily with the functional receptor, the receptor was named the 5-HT2F receptor, but it was reclassified subsequently to the 5-HT2B receptor to better fit 5-HT receptor nomenclature. The pharmacological characterization of this receptor in various species confirmed its close relationship to both 5-HT2C and 5-HT2A receptors, as expected from their closely related structural and transductional features. The physiological and pathophysiological functions of the 5-HT2B receptor both in the peripheral and central nervous systems are rather unique.
B. Expression Profile
1. Peripheral Expression
5-HT2B receptor mRNA and/or protein are detected in the stomach fundus, intestine, liver, kidney, pancreas, spleen, lung, and heart in rats, mice, and humans (Kursar et al., 1992; Choi et al., 1994; Kursar et al., 1994; Choi and Maroteaux, 1996). Functionally active 5-HT2B receptors are present in human uterine smooth muscle (Kelly and Sharif, 2006). The 5-HT2B receptor is also expressed in the vasculature, in various smooth muscle cells (Ullmer et al., 1995), endothelial cells of pig pulmonary arteries (Glusa and Pertz, 2000), human meningeal blood vessels (Schmuck et al., 1996), and rat jugular vein (Ellis et al., 1995). The 5-HT2B receptor is expressed in rat spleen, thymus, and peripheral blood lymphocytes (Stefulj et al., 2000) and rat osteocytes, osteoblasts, and periosteal fibroblasts containing osteoblast precursor cells (Bliziotes et al., 2001; Westbroek et al., 2001). The 5-HT2B receptor gene is expressed in human and rodent anagen, but not telogen skin, in various melanomas and Mel A melanocytes (Slominski et al., 2003, 2004). Finally, the 5-HT2B receptor is expressed in c-kit+ bone marrow cells (Launay et al., 2012). Together, the very heterogeneous expression of the 5-HT2B receptor suggests very diverse physiological and pathophysiological roles.
2. Central Expression
The 5-HT2B receptor is expressed in human cerebral cortex, cerebellar nuclei and their projection areas, lateral septum, dorsal hypothalamus, and medial amygdala (Kursar et al., 1992; Choi et al., 1994; Kursar et al., 1994; Choi and Maroteaux, 1996). The presence of 5-HT2B receptor mRNA was reported in several mammalian brain nuclei, including the dorsal raphe (Bonaventure et al., 2002a). 5-HT2B receptor expression (determined by qPCR) was confirmed in the human frontal, temporal, parietal, and occipital lobes and the olfactory region, cerebellum, diencephalon, hippocampus, thalamus, pituitary gland, pons, medulla oblongata, and nucleus accumbens (Bevilacqua et al., 2010). 5-HT2B receptor mRNA was reported in rat cultured astrocytes (Sanden et al., 2000; Osredkar and Kržan, 2009). Microglial expression of 5-HT2B receptors was documented in primary cultured and acutely isolated adult microglia, using two-photon microscopy on brain slices (Kolodziejczak et al., 2015) and patch-clamp studies in cultured microglia (Krabbe et al., 2012), suggesting 5-HT2B receptor-may mediate modulation of microglial functions such as phagocytosis and migration, synaptogenesis, and neuronal death.
The 5-HT2B receptor modulates the release of rat growth hormone (GH) in the pituitary (Papageorgiou and Denef, 2007). The 5-HT2B receptor is expressed in the spinal cord (Helton et al., 1994; Holohean and Hackman, 2004), the rat organ of Corti lateral wall, and spiral ganglion subfractions (Oh et al., 1999) and is upregulated in ageing mice cochlea (Tadros et al., 2007). 5-HT2B receptor mRNA is expressed predominantly in the human retina, ciliary body, ciliary epithelium, choroid, conjunctiva, and iris. 5-HT2B receptor mRNA was also documented in optic nerve tissue of human donor trabecular meshwork cells (Sharif and Senchyna, 2006).
C. Post-translational Modifications and Impact
1. Gene Structure
The 5-HT2B receptor gene is composed of four Exons, including one 5′ noncoding exon and three coding exons in all vertebrates that have been sequenced.
2. Gene Regulation
The transcriptional regulation of the 5-HT2B receptor gene is not well understood. In breast tumors, the c-Myc transformation induces an increased 5-HT2B receptor expression (Pai et al., 2009). In human umbilical endothelial cells, Wnt2 downstream targets the 5-HT2B receptor gene (Klein et al., 2009). The 5-HT2B receptor expression is downregulated via nuclear factor-κB (NF-κB) (RelAp65-p52) in C-reactive protein–stimulated pulmonary arterial endothelial cells (Wynants et al., 2013). In pulmonary artery smooth muscle cells, 5-HT induces 5-HT2B receptor mRNA expression, which is inhibited by peroxisome proliferator–activated receptor (PPAR)γ activation and suggests the suppression of AP-1 activity (Liu et al., 2012b; Maroteaux, 2013). The presence of retinoic acid response elements in the 5-HT2B receptor promoter suggests a negative regulatory relationship between retinoic acid and 5-HT signaling at sites of epitheliomesenchymal interaction (Bhasin et al., 2004).
3. Receptor Isoforms
Only a few studies have investigated putative splice variants in the 5-HT2B receptor. In puffer fish, 5-HT2B receptor splicing variants have been identified (De Lucchini et al., 2001). Splice variant 1 contains a 136-bp deletion that eliminates a portion of exon 2 by alternative splice sites located within transmembrane regions I and II, which results in a premature stop codon to produce a very short truncated 31-amino-acid protein. Splice variant 2 contains a 201-bp deletion because of an exon-skipping mechanism that eliminates exon 3 (which is also found in human and mice). The resulting RNA retains the same open reading frame. Splice variant 3 contains a 19-bp deletion, probably by an alternative 5′ splice site upstream of the canonical 5′ splice site of intron C; this leads to a frame shift and a premature termination codon, which was also found in human and mice. This short variant results in a putative 177-amino-acid protein, with 28 specific residues at the carboxyl terminus. The functions of these splice variants remain to be defined. Finally, an exon-skipping mechanism that eliminates exon 3 was found in fish, humans, and rodents; this leads to a truncated receptor containing only the first transmembrane domain.
D. Protein Structure
The 5-HT2B receptor displays the characteristic structure of a GPCR receptor, with a relatively long N-terminus of about 55 amino acids. The human 5-HT2B receptor has 481 amino acids (479 amino acids in rat or mouse), with 79% and 82% homology for human versus rat and mouse, respectively. The 5-HT2B receptor N-terminus may act as a negative modulator, affecting both constitutive and agonist-stimulated activity (Belmer et al., 2014).
The ergotamine-bound 5-HT2B receptor crystal structure exhibits some conformational features of both the active and inactive states: an active-like state in the helix VII conformation but only partial changes in helix VI, which mirror the strong β-arrestin bias of ergotamine seen in functional assays (Wacker et al., 2013; Wang et al., 2013). A structural explanation for the distinct conformational features and the biased pharmacology of ergotamine can be seen in the extracellular loop 2 (ECL2) junction with helix V, E212-R213-F214 forming an additional helical turn stabilized by a structured water molecule at the extracellular tip of helix V. The segment of ECL2 connecting helices III and V via the conserved disulfide bond is, therefore, shortened and creates a conformational constraint on the extracellular tip of helix V (Martí-Solano et al., 2014). However, this structured water molecule involved in ECL2 junction with helix V has been challenged, as differential interactions of ergotamine with the top of helices V and VI could determine the rotational freedom of helix VI (Liu et al., 2013a). For more discussion of the crystal structure, see XVI. A. 5-HT GPCRs.
E. Heteromeric Receptor Associations
In cardiac fibroblasts, angiotensin AT1 receptors and 5-HT2B receptors, which share common signaling pathways, could exist in heterodimeric complexes as shown by coimmunolocalization and a pull-down assay (Jaffre et al., 2009), but experimental confirmation is lacking.
F. Pharmacology
1. Agonists
Biased agonism is evident with a range of drugs that impact the 5-HT2B receptor, and the influence of this phenomenon on the pharmacological profile of agonists can be profound (see, for example, Huang et al., 2009). Hence, when defining the action of agonists at the 5-HT2B receptor—at least in terms of their potency and efficacy—the particular readout (e.g., [Ca2+]i, arrestin, ERK, IP3) and the nature of the receptor preparation needs to be taken into account. Against this background, the pharmacology of agonists will be described.
BW723C86, 1-methyl-2-[5-(2-thienylmethoxy)-1H-indole-3-yl] ethylamine hydrochloride, has 10- and 100-fold selectivity for the human 5-HT2B receptor over the human 5-HT2C and 5-HT2A receptors, respectively. (recommended use: ≤100 nM concentration or ≤3 mg/kg i.p. in rodents; Porter et al., 1999; Jerman et al., 2001; Knight et al., 2004; Cussac et al., 2008). α-Methyl-5-HT is a full agonist with high potency for the 5-HT2B receptor (pEC50 = 8.4) and lower potency for both 5-HT2C and 5-HT2A receptors. 5-Methoxytryptamine is also 25- and 400-fold selective over the 5-HT2A and 5-HT2C receptors, respectively. Nordexfenfluramine (metabolite of dexfenfluramine), methylergonovine (metabolite of methysergide), and Ro 60-0175 (2(S)-1-(6-chloro-5-fluoro-1H-indol-1-yl)-2-propanamine fumarate) are all somewhat preferential 5-HT2B receptor agonists with about 10-fold selectivity over 5-HT2C receptor.
The 5-HT2B receptor displays high affinity to 5-HT (Kd ∼10 nM) and many nonselective 5-HT2 receptor active compounds, including some metabolites of therapeutics and drugs of abuse. Such agonists include MDA (3,4-methylene dioxyamphetamine-MDA, a metabolite of 3,4-methylenedioxy methamphetamine-MDMA) (Setola et al., 2003), MDMA (“ecstasy”) itself, tryptamine, and LSD. DOI is a nearly full agonist at 5-HT2B receptors but with similar affinity to 5-HT2A and 5-HT2B receptors (Porter et al., 1999; Jerman et al., 2001; Knight et al., 2004; Cussac et al., 2008).
Many substances known as “legal highs” display notable affinity for 5-HT2B receptors, including 5-APB, commonly “marketed” as “benzofury” (Ki = 14 nM) and 6-APB (Ki = 3.7 nM), and 5-iodo-aminoindane (Ki = 70 nM). 5-APB and 6-APB act as potent (i.e., nanomolar EC50 values) full agonists at 5-HT2B receptors (Iversen et al., 2013; Rickli et al., 2015). 5-APB contracts the rat stomach fundus and is antagonized by the 5-HT2B receptor antagonist, RS127445 (Dawson et al., 2014). Other such drugs show submicromolar affinities for the 5-HT2B receptor (mephedrone, naphyrone, 1-naphyrone, and methylenedioxy-aminotetralin). Indeed, there is a correlation in a series of phenyliso-propylamines between hallucinogenic activity and affinity for the 5-HT2B receptor (Nelson et al., 1999), although 5-HT2A receptor agonism is still also considered to play a major role in “psychedelics,” such as LSD or psyloscibine. Activation of the 5-HT2B receptor appears to play a key role in the behavioral stimulant and 5-HT releasing effects of MDMA (Doly et al., 2008) and in the reinforcing effects of MDMA in mice (Doly et al., 2009).
2. Antagonists
The first highly 5-HT2B selective antagonist is LY266097, 1-(2-chloro-3,4- dimethoxybenzyl)-6-methyl-1,2,3,4-tetrahydro-9Hpyrido [3,4-b]indole hydrochloride, with a pKi of 9.7 for the human cloned 5-HT2B receptor and a 100-fold greater selectivity over human 5-HT2C and 5-HT2A receptor binding sites (recommended use: 20 nM concentration in vitro or 0.5 mg/kg i.p. in rodents; Audia et al., 1996). SB204741, N-(1-methyl-5-indolyl)-N'-(3-methyl-5-isothiazolyl)urea, is another selective 5-HT2B receptor antagonist with approximately 100-fold selectivity over the 5-HT2C and 5-HT2A sites but with rather low potency (Ki around 100 nM) (recommended use: 500 nM concentration in vitro or 10 mg/kg i.p. in rodents; Bonhaus et al., 1995). The tetrahydro-β-carboline, LY272015 (6-chloro-5-methyl-N-(5-quinolinyl)-2,3-dihydro-1H-indole-1-carboxamide) is also a fairly selective and highly potent antagonist (recommended use: 50 nM concentration in vitro or 1.0 mg/kg i.p. in rodents; Cohen et al., 1996). RS127445, 2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine, has subnanomolar affinity for the 5-HT2B receptor (pKi = 9.5) and 1000-fold selectivity compared with numerous other receptors and ion channels, and it appears as the most selective, high-affinity 5-HT2B receptor antagonist suitable now (Bonhaus et al., 1999; recommended use: 20 nM concentration or 0.25 mg/kg i.p. in rodents). The methoxythioxanthene BF-1 is a highly selective and potent 5-HT2B receptor antagonist lacking high affinities for 5-HT1A, 5-HT2A, 5-HT2C, histamine H1, dopamine D1, and D2 as well as muscarinic M1 and M2 receptors (Schmitz et al., 2015). S33526, 6-chloro-2,3,4,9-tetrahydro-1H-b-carbolin-1-yl)-phenyl-acetic acid ethyl ester, is a high-affinity and relatively selective antagonist at 5-HT2B receptors (Cussac et al., 2002). SB215505, 6-chloro-5-methyl-N-(5-quinolinyl)-2,3-dihydro-1H-indole-1-carboxamide, behaves as a high-affinity and preferential inverse agonist at 5-HT2B receptors. SB206553, 5-methyl-N-(3-pyridyl)-1,2,3,5-tetrahydrobenzo[1,2-b:4,5-b']dipyrrole-1-carboxamide, is a mixed 5-HT2C/5-HT2B receptor inverse agonist with 50- to 100-fold lower affinity for the 5-HT2A and other sites.
Nonselective 5-HT2 receptor antagonists such as ritanserin and metergoline antagonize 5-HT2B receptor–mediated effects. Furthermore, the α2 adrenergic receptor antagonists yohimbine and rauwolscine are potent 5-HT2B antagonists, with low affinity for the 5-HT2C and 5-HT2A receptors. Atypical antipsychotics have also fairly high affinity for 5-HT2B receptors, including clozapine, asenapine, or cariprazine (Wainscott et al., 1996; Millan et al., 2003; Shahid et al., 2009; Kiss et al., 2010). Aripiprazole (OPC-14597) is a novel atypical antipsychotic, with high antagonist affinity (IC50 = 11 nM) for the human 5-HT2B receptor (Shapiro et al., 2003).
3. Allosteric Modulators
No selective 5-HT2B allosteric modulator has been definitely identified. In the crystal, ergotamine binds to two distinct sites at the 5-HT2B receptor, the orthosteric site, where the indole nucleus of ergotamine resides, and the “extended” binding site, to which the tripeptide portion of the ergoline binds. This potential allosteric site is also present in the muscarinic M2 receptor at the same extracellular region. The similarities in both the M2 and 5-HT2B receptors suggest that the location of the extracellular allosteric site for class A GPCRs is rather similar and conserved; these common features suggest that ergotamine and other ergolines may function as “bitopic” ligands, acting at both the orthosteric and the putative extracellular allosteric site in the 5-HT2B receptor. It is thought that a sodium ion allosterically modulates the binding pocket to reduce G protein signaling, thus favoring β-arrestin recruitment (McCorvy and Roth, 2015).
G. Transduction System(s)
1. Transfected Cells
5-HT2B receptors expressed in mouse fibroblast L-cells stimulate GTPase activity and inositol 1,4,5-triphosphate production upon agonist stimulation. This GTPase activation is mediated by Gαq/11 but not by Gαs or Gαi. The GTPase activation was also blocked by anti–β1-4 or anti-γ2 subunit antibodies. The 5-HT2B receptor couples to phospholipase A2 (PLA2)-mediated release of arachidonic acid (Tournois et al., 1998). In addition, stimulation of the 5-HT2B receptor triggers intracellular cyclic guanosine monophosphate (cGMP) production through dual activation of constitutive nitric-oxide synthase (cNOS) and inducible NOS. The group I PDZ motif at the carboxy terminus of the 5-HT2B receptor is required for cNOS transduction pathways, whereas inducible NOS stimulation is Gα13-dependent (Manivet et al., 2000). The 5-HT2B receptor shares the C-terminal E-X-V/I-S-X-V sequence with the 5-HT2C receptors and binds MUPP1-PDZ domains in vitro (Becamel et al., 2001). Agonist-induced stimulation of the 5-HT2B receptor promotes rapid and transient activation of the proto-oncogene product p21ras, as measured by an increase in GTP-bound Ras (Launay et al., 1996). 5-HT2B receptor stimulation activates the MAPKs p42mapk/p44mapk as well as ERK2/ERK1. It results in the formation of foci and to the formation of tumors from these foci in nude mice (Launay et al., 1996). The 5-HT2B receptor–dependent cell-cycle progression happens through retinoblastoma protein hyperphosphorylation and the activation of both cyclin D1/cdk4 and cyclin E/cdk2 kinases. The induction of cyclin D1, but not that of cyclin E expression, is under MAPK control, indicating an independent regulation of these two cyclins in 5-HT2B receptor–induced mitogenesis. Similarly, platelet-derived growth factor receptor (PDGFR) kinase activity is essential for 5-HT2B-triggered MAPK/cyclin D1, but not cyclin E, signaling pathways. 5-HT2B receptor activation also increases activity of the Src family kinases c-Src, Fyn, and c-Yes. Strikingly, c-Src, but not Fyn or c-Yes, is the crucial link between the Gq protein–coupled 5-HT2B receptor and the cell-cycle regulators (Nebigil et al., 2000b). Inhibition of c-Src activity is sufficient to abolish 5-HT–induced PDGFR tyrosine kinase phosphorylation and MAPK activation, cyclin D1 and cyclin E expression levels, and thymidine incorporation. Thus, c-Src activation by the 5-HT2B receptor controls cyclin E induction and, in concert with PDGFR, also induces cyclin D1 expression via the MAPK/ERK pathway (Nebigil et al., 2000b). The 5-HT2B signal transduction pathways are thus quite diverse and are similar to those of 5-HT2A/2C receptors. On the other hand, the NOS pathway seems to be 5-HT2B receptor–specific.
2. Primary Cell Cultures
In cultivated cardiac fibroblasts, angiotensin II (AngII)- or 5-HT–dependent cytokine release is critical for the expression of HB-EGF and Src activity via endogenous AT1 and 5-HT2B receptors (Jaffre et al., 2009). Matrix metalloproteinases (MMPs) are responsible for HB-EGF shedding and subsequent EGF-receptor transactivation induced by AngII or 5-HT. Tumor necrosis factor-α (TNF-α)–converting enzyme controls HB-EGF shedding in fibroblasts and is directly regulated by 5-HT2B receptors (Pietri et al., 2005). Blockade of one of the two receptors prevents cytokine release induced by the other receptor (Jaffre et al., 2009). These findings also indicate that AT1 and 5-HT2B receptors share common EGF receptor–dependent signaling pathways in adult cardiac fibroblasts, and the two receptors were shown to interact in a common cell compartment. Together, the data support AT1 and 5-HT2B receptors exist as heterodimers that may play a key role in receptor maturation and trafficking to the plasma membrane and/or signaling (Bulenger et al., 2005) to drive common signaling regulating hypertrophic factors in the heart (Jaffre et al., 2009).
5-HT2B receptor stimulation in hepatic stellate cells (HSC) activates the expression of TGFβ1 (a powerful suppressor of hepatocyte proliferation) via ERK/JunD signaling. 5-HT2B receptor antagonists decrease the mRNA levels of TGFβ1, connective growth factor, plasminogen activator inhibitor-1, Smad-3, and JunD in lung and skin fibroblasts (Dees et al., 2011). 5-HT2B receptor activation leads to sustained phosphorylation of two downstream targets of mTOR, p70S6K and 4E-BP1, thereby facilitating survival and inhibiting autophagy of hepatocellular carcinomas (Soll et al., 2010). The 5-HT2B receptor protects newborn postmitotic cardiomyocytes against serum deprivation–induced apoptosis as manifested by DNA fragmentation, nuclear chromatin condensation, and terminal deoxynucleotidyl transferase dUTP nick end labeling. 5-HT prevents cytochrome c release and caspase-9 and -3 activation after serum deprivation via crosstalk between phosphatidylinositol-3 kinase (PI3K)/Akt and ERK1/2 signaling pathways. 5-HT2B receptor–activated ERK kinases inhibit Bax expression induced by serum deprivation. 5-HT activates NF-κB via PI3K/Akt required for the regulation of the mitochondrial adenine nucleotide translocator and mitochondrial permeability. Thus, 5-HT via the 5-HT2B receptor is a novel survival factor targeting mitochondria (Nebigil et al., 2003). Interestingly, NF-κB regulation by 5-HT2B receptors is confirmed in a large screen for genes regulating NF-κB and the MAPK pathways (Matsuda et al., 2003).
Primary osteoblasts from mutant 5-HT2B receptor KO mice show reduced proliferation and delayed differentiation; calcium incorporation is markedly reduced in osteoblasts after 5-HT2B receptor inactivation (by genetic invalidation or by pharmacological inhibition; Collet et al., 2008). A functional link between the 5-HT2B receptor and the activity of the tissue-nonspecific alkaline phosphatase (TNAP) was established in an osteoprogenitor C1 cell line (Baudry et al., 2010a). During osteogenic differentiation, both 5-HT2B receptor and TNAP mRNA translations are delayed with respect to extracellular matrix deposition. Once the receptor is expressed, it constitutively controls TNAP activity at a post-translational level along the entire period of mineral deposition. The lack of 5-HT2B receptors is associated with a 10-fold overproduction of prostacyclin in osteoblast primary cultures. A specific prostacyclin synthase (CYP8A1) inhibitor (U51605) totally rescued osteoblast aggregation and matrix mineralization in 5-HT2B receptor KO osteoblasts without any effect on WT osteoblasts. Prostacyclin is the endogenous ligand of the nuclear receptor PPAR-β/δ, and its inhibition in 5-HT2B KO cells totally rescued the alkaline phosphatase TNAP and osteopontin SPP1 mRNA levels, cell-cell adhesion, and matrix mineralization. The absence of 5-HT2B receptors leads to the overproduction of prostacyclin, inhibiting osteoblast differentiation because of PPAR-β/δ–dependent target regulation and defective cell-cell adhesion and matrix mineralization (Chabbi-Achengli et al., 2013), supporting a physiologic negative control of prostacyclin synthase by 5-HT2B receptors. Thus, endogenously expressed 5-HT2B receptors can modulate various transduction pathways, including Src, MMPs, and PLA2 activities in a cell type–dependent manner.
H. Regulatory Mechanisms
1. Internalization
In transfected cells, prior exposure to 5-HT results in a rapid and considerable (up to 80%) 5-HT2B receptor desensitization (Porter et al., 2001). Internalization of 5-HT2B receptors is caveolin1-independent and clathrin- and β-arrestin2–dependent (Janoshazi et al., 2007).
Some ergot derivatives are “slow” 5-HT2B receptor binders, with very slow association and dissociation rates. The compounds have apparent lower potency to increase intracellular concentrations of calcium ions relative to inositol phosphate accumulation assays. Similarly, the potency of ergolines to activate ERK1/2 is highly time-dependent. In addition, a number of ergot derivatives produce “wash-resistant” 5-HT2B receptor signaling that persists for hours without appreciable loss of potency, which is not explained simply by slow receptor-dissociation kinetics. Thus, this persistent signaling has been proposed to originate from internalized or sequestered receptors (Unett et al., 2013). The 5-HT2B receptor crystal structure (Huang et al., 2009) reveals an intermediate state of activation stabilized by the extracellular-facing tripeptide portion of ergotamine, which likely drives β-arrestin bias and is not seen in unbiased ligands such as 5-HT itself. Thus, the long duration of action of some ergolines may be explained by a combination of very slow kinetics at the receptor, coupled with persistent intracellular signaling.
2. Interacting Proteins
Proteins known to interact with the 5-HT2B receptor include constitutive and inducible NOS; Gαq, Gα11, and Gα13, involved in signaling of the receptor; and MUPP1, a multivalent PDZ scaffolding protein. (Becamel et al., 2001).
For more details on proteins that interact with the 5-HT2B receptor, see XVII. B. 4. 5-HT2B Receptor.
I. Function at Cellular, Tissue, and In Vivo Level
1. Hematopoiesis
5-HT promotes megakaryocyte (MK) proliferation and reduces cell apoptosis via activation of the 5-HT2B receptor and Akt pathway (Liu and Yang, 2006). 5-HT increases proplatelet-bearing MKs and polymerizes actin via ERK1/2 (Ye et al., 2014). Tph1−/− mice are deficient in peripheral 5-HT and display features of ineffective erythropoiesis. The central event starts in the bone marrow where the absence of 5-HT inhibits the terminal differentiation of erythroid precursors expressing 5-HT2A and 5-HT2B receptors. In addition, red blood cells from 5-HT–deficient mice are more sensitive to macrophage phagocytosis and have a shortened in vivo half-life (Amireault et al., 2011). In addition, the 5-HT2B receptor is expressed in c-kit+ bone marrow cells (Launay et al., 2012). The 5-HT2B receptor antagonist RS127445 decreases colony-forming capacity, with inhibition of both early stem/progenitors and erythroid burst-forming unit formation attributed to a reduction of cell proliferation and/or an apoptotic effect. By contrast, 5-HT significantly enhances the expansion of CD34+ cells to early stem/progenitors and committed progenitors (erythroid burst-forming units) (Yang et al., 2007).
In human macrophages, 5-HT inhibits the LPS-induced release of proinflammatory cytokines to upregulate the expression of M2 polarization–associated genes and to reduce the expression of M1-associated genes. 5-HT2B receptors mediate the pro-M2 skewing effect of 5-HT. Blockade of this receptor during in vitro monocyte-to-macrophage differentiation preferentially modulates the acquisition of M2 polarization markers. 5-HT2B receptor mRNA is preferentially expressed by anti-inflammatory M2 (macrophage colony-stimulating factor) macrophages and is detected in vivo in liver Kupffer cells and in tumor-associated macrophages (de Las Casas-Engel et al., 2013). 5-HT2B receptor expression is found in postnatal microglia, suggesting that 5-HT participates in microglial functions (Kolodziejczak et al., 2015). 5-HT2B receptor mRNA expression is evident in spleen, thymus, and peripheral blood lymphocytes (Stefulj et al., 2000). Immature dendritic cells express 5-HT2B receptor mRNA, and 5-HT2B receptor stimulation induces intracellular Ca2+ mobilization in immature, but not mature, dendritic cells. Thus, 5-HT stimulates, in a maturation-dependent manner, different signaling pathways in dendritic cells (Idzko et al., 2004).
A proper balance between different T-helper (Th) cell subsets is necessary for normal functioning of the adaptive immune system. Th cells (from human umbilical cord blood) differentiated in vitro into Th1 or Th2 cells reveal the latter express 5-HT2B receptor mRNA (Aijö et al., 2012). In gene expression profiles during human CD41 T-cell differentiation, 5-HT2B receptor mRNA was found to be SP4-specific (∼10-fold) among the 16 transcripts expressed in SP4 thymocytes at levels threefold or higher than in any other isolated T-cell subpopulation (Lee et al., 2004b).
Treatment with aggregated (1–40 or 1–42) and oligomeric (1–42) amyloid β (Aβ, found in Alzheimer disease) promoted differentiation of bone marrow–derived mesenchymal stem cells without toxic effects. The effect of Aβ was shown to be mediated by the neuropeptide Y1 receptor and the 5-HT2B receptor via PI3K-dependent activation of the MAPK/ERK1/2 pathway (Jin et al., 2009). Thus, the 5-HT2B receptor, among others, mediates the balance among various hematopoietic lineages.
2. Pancreas
A strong lactogen-dependent upregulation of 5-HT biosynthesis takes place in a subpopulation of mouse islet β-cells during pregnancy (Schraenen et al., 2010). Pancreatic islet cells express the genes encoding all of the products necessary for synthesizing, packaging, and secreting 5-HT, including both isoforms of the 5-HT synthetic enzyme tryptophan hydroxylase (TPH) and the archetypal 5-HT transcription factor Pet1. In β-cells, Pet1 can bind to the 5-HT–relevant genes but also to a conserved insulin gene regulatory element. Mice lacking Pet1 display reduced insulin production and secretion and impaired glucose tolerance (Ohta et al., 2011). Inhibition of 5-HT synthesis blocks β-cell expansion and induced glucose intolerance in pregnant mice without affecting insulin sensitivity. Expression of the 5-HT2B receptor in maternal islets has been reported to increase during pregnancy and to normalize just before parturition. Blocking 5-HT2B receptor signaling in pregnant mice may also block β-cell expansion and cause glucose intolerance (Kim et al., 2010).
3. Adipocytes
By inhibiting 5-HT2B receptor signaling during adipogenesis using RS127445, an increased fat accumulation was observed similar to the knockdown phenotype (Söhle et al., 2012). In adipocytes, 5-HT2B receptors favor lipolysis by increasing phosphorylation and activity of hormone-sensitive lipase (Sumara et al., 2012).
4. Cardiovascular and Pulmonary Systems
5-HT2B receptor inactivation in mice leads to partial embryonic lethality caused by major defects in heart development (Monassier et al., 2010). Neonates exhibit a second wave of partial lethality because of cardiac dilation resulting from contractility deficits and structural deficits at the intercellular junctions between cardiomyocytes. Echocardiography and electrocardiography studies in animals that live past the first week and survive until adulthood confirm the presence of left-ventricular dilation and decreased systolic function. 5-HT, via the 5-HT2B receptor, regulates heart differentiation and proliferation during development as well as cardiac structure and function in adults (Nebigil et al., 2000a). The 5-HT2B receptor is functionally coupled to ROS synthesis through NADPH oxidase (NOX) stimulation in 1C11 cells (Schneider et al., 2006) and in angiotensin II and isoproterenol-induced cardiac hypertrophy (Monassier et al., 2008). In human atrial myocytes, 5-HT reduces the amplitude of L-type calcium currents and affects the strength of gap junctional intercellular communication, which is markedly reduced by blocking receptors, showing that activation of 5-HT2B receptors inhibit gap junctional intercellular communication (Derangeon et al., 2010). Upon pulmonary artery banding, the 5-HT2B receptor antagonist SB204741 reduces right-ventricular fibrosis and improves heart function in mice (Janssen et al., 2015).
A model in which 5-HT2B receptor signaling promotes cardiac hypertrophy by stimulating calcineurin/nuclear factor of activated T cells signaling suggests the recruitment of histone acetyl transferases to regulatory regions of nuclear factor of activated T-cells target genes. 5-HT2B receptor agonist–induced hypertrophy of cardiac muscle cells results from a signaling pathway involving calcineurin and a kinase-dependent mechanism that inactivates class II histone deacetylases (HDAC), which act as repressors of cardiac growth (Bush et al., 2004). Because it also stimulates nuclear export of class II HDACs, myocyte enhancer factor-2 protein may play a role in the mechanism by which 5-HT2B receptor signaling triggers cardiac remodeling (McKinsey and Olson, 2005). A cDNA encoding the 5-HT2B receptor was found in a screen for genes encoding HDAC5 modulators, and the ability of 5-HT2B receptors to promote HDAC5 phosphorylation and cardiomyocyte hypertrophy was confirmed (McKinsey and Olson, 2005). The 5-HT2B receptor–triggered intracellular calcium ion release and PKC activation accounts, at least in part, for the overexpressed receptor-induced HDAC5 phosphorylation (Chang et al., 2005).
5. Endothelial Cells
In human pulmonary artery endothelial cells, 5-HT2B receptors stimulate calcium ion release from intracellular stores (Ullmer et al., 1996a). 5-HT2B receptors mediate the endothelium-dependent relaxation of rat jugular vein (Ellis et al., 1995) and pig pulmonary artery (Glusa and Pertz, 2000). Activation of 5-HT2B/5-HT1B receptors stimulates NO production in human coronary artery endothelial cells (Ishida et al., 1998). Compared with nonstimulated pulmonary artery endothelial cells, C-reactive protein–stimulated cells show downregulation of 5-HT2B receptor by 25%, of inhibitor of NF-κB kinase subunit epsilon by 30%, and of toll-like receptor-4 and -6 by 18% and 39%, respectively (Wynants et al., 2013). A cardioprotective function of the 5-HT2B receptor in an integrated model of heart failure with preserved ejection fraction can be explained by a contribution of the endothelial 5-HT2B receptors to coronary vasodilatation (Ayme-Dietrich et al., 2015).
6. Aorta
In normotensive rats, 5-HT–induced contraction of the aorta is primarily 5-HT2A receptor–dependent; however, in hypertensive rats, it is mediated by both 5-HT2A and 5-HT2B receptors. The endothelium-denuded isolated superior mesenteric artery of hypertensive (DOCA-salt) rat displays a marked increase in maximum arterial contraction to 5-HT2B receptor agonists when compared with control rats, confirming that the 5-HT2B receptor plays a greater role in 5-HT–induced contraction in arteries from hypertensive rats (Watts and Fink, 1999; Banes and Watts, 2003).
7. Liver
5-HT is a potent growth factor for liver development and regeneration. The expression of both 5-HT2A and 5-HT2B receptors in the liver increases following hepatectomy. 5-HT2 receptor inhibition by ketanserin blocks liver regeneration when administrated close to the G1/S transition point, suggesting 5-HT as a cofactor for DNA synthesis (Papadimas et al., 2006). In Tph1−/− mice, the failure of liver regeneration is rescued by reloading 5-HT–free platelets with a 5-HT precursor molecule (Lesurtel et al., 2006). Elderly mice have decreased ability of the liver to restore normal volume after partial hepatectomy. The 5-HT2 receptor agonist DOI reverses the age-related pseudocapillarization of old liver and improves hepatosinusoidal blood flow (Furrer et al., 2011); it also enhances hepatocyte proliferation after liver transplantation in mice. 5-HT2B receptor activation significantly improves survival in recipients of a small, otherwise nonviable graft by enhancing liver regeneration and hepatic microcirculation, thereby reducing ischemia/reperfusion injury (Tian et al., 2011). 5-HT protects the liver in an IL-6–independent manner. The protective effects of DOI is lost in animals treated with SB206553, a 5-HT2B/2C receptor antagonist. DOI may thus preserve microcirculation and accelerate liver regeneration via 5-HT2B receptors, thus preventing the liver parenchyma from further injury (Tian et al., 2011). However, in a pathophysiological setting, the regenerative influence of 5-HT acting through 5-HT2A receptors on hepatocytes may be subjected to opposite antiregenerative effects arising from 5-HT acting through 5-HT2B receptors in hepatic stellate cells (Ebrahimkhani et al., 2011). In hepatocytes, signaling through 5-HT2B receptors also promotes gluconeogenesis (Sumara et al., 2012).
8. Gut
The 5-HT2B receptor was initially characterized as the receptor contracting the rat stomach fundus (Vane, 1957). The 5-HT2B receptor is expressed by smooth muscles in the small intestine, the stomach, and by enteric neurons. 5-HT, stimulating 5-HT2B receptors, affects the fate of the large subset of enteric neurons that arises after the development of endogenous sources of 5-HT (Fiorica-Howells et al., 2000). High levels of both 5-HT2B receptor mRNA and protein are found predominantly in the muscle layers and in the myenteric nerve plexus throughout the colon, where they cause neuronally mediated contractions of longitudinal muscle (Borman et al., 2002) and tonically regulate colonic motility (Bassil et al., 2009).
Ghrelin, an orexigenic peptide present in the stomach, has gastroprokinetic properties. In vivo, the ghrelin receptor antagonist D-Lys(3)-GHRP-6 reduces food intake and delays gastric emptying in rodents. D-Lys(3)-GHRP-6 contracts stomach strips, an effects blocked by methysergide and yohimbine, suggesting an interaction with 5-HT2B receptors (Depoortere et al., 2006).
Normal gastrointestinal (GI) motility requires functional interstitial cells of Cajal (ICC) that proliferate in adult mice. 5-HT2B receptor activation increases the proliferation of ICC in vivo. On the other hand, lack of 5-HT2B receptor signaling reduces the density of ICC networks in mature mice. Targeting 5-HT2B receptors may protect ICC networks from injury and/or help repair them after injury (Tharayil et al., 2010).
9. Central Autonomic Nervous System
5-HT2B and 5-HT2A receptors are expressed in pontomedullary respiratory motor centers. They are colocalized in the Kolliker-Fuse and parabrachial regions of the pons and in the Bötzinger and pre-Bötzinger complex of the ventral medulla with a stronger expression of 5-HT2A receptors in all regions. BW723C86 applied in the pre-Bötzinger complex increases respiratory frequency and is blocked by the 5-HT2B receptor–selective antagonist, LY272015. Hence, endogenous 5-HT appears to support tonic action on respiratory rhythm generation via 5-HT2B receptors. However, respiratory activity is unaffected in 5-HT2B receptor–deficient mice; BW723C86 and LY272015 had no effects, whereas ketanserin blocked rhythmic activity. Thus, endogenous 5-HT2B receptor activation has a stimulatory role at the pre-Bötzinger complex and hypoglossal motoneurons that can be substituted by 5-HT2A receptors in the absence of 5-HT2B receptors. The presence of functional 5-HT2B receptors in the neonatal medullary breathing center suggests a potential convergent regulatory role of 5-HT2B and 5-HT2A receptors on the central respiratory network (Günther et al., 2006). When 5-HT2A and 5-HT2B receptor agonists are applied concurrently, there is a frequency increase that exceeds the effects of either agonist alone. This is an expected outcome if frequency-controlling neurons are preferred targets for 5-HT2B receptor modulation in the pontomedullary respiratory compartment (Niebert et al., 2011).
Phrenic motor neurons express 5-HT2B receptors, and acute intermittent hypoxia induces phrenic long-term facilitation via spinal 5-HT2 receptor activation and NOX activity. Pretreatment with NOX inhibitors blocks 5-HT2B receptor–induced phrenic motor facilitation (Macfarlane et al., 2011). Episodic spinal 5-HT receptor activation alone, without changes in oxygenation, is sufficient to elicit NOX-dependent phrenic motor facilitation. Intrathecal BW723C86 elicits progressive and sustained phrenic motor facilitation, blocked by the 5-HT2B receptor antagonist SB206553. Spinal neuronal NOS (nNOS) activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation (Macfarlane et al., 2014). Thus, episodic spinal NO elicits phrenic long-term facilitation via 5-HT2B receptor activation and NOX-derived ROS formation.
10. Peripheral Autonomic Nervous System
Heart rate control originates in parasympathetic preganglionic cardiac vagal neurons in the nucleus ambiguus. 5-HT2 receptors modulate spontaneous and respiratory-evoked GABAergic neurotransmission to cardioinhibitory vagal neurons within the nucleus ambiguus as well as rhythmic fictive inspiratory-related activity. Neurons in the rat nucleus tractus solitarius are affected differently by 5-HT2 receptor ligands regarding their vagal postsynaptic location, the type of cardiopulmonary afferent they receive, and the nature of the 5-HT2 receptor. 5-HT2B receptors are excitatory, whereas 5-HT2C receptors are inhibitory (Jordan, 2005). In neurons of the nucleus tractus solitarius receiving vagal afferent input, the selective 5-HT2B receptor antagonist LY202715 significantly reduced the excitatory actions of BW723C86 on “intermediate” and “polysynaptic” cells (Sévoz-Couche et al., 2000).
Multiple, as opposed to single, applications of α-methyl-5-HT cause a long-lasting inhibition of both spontaneous and fictive inspiratory-related GABAergic neurotransmission to cardioinhibitory vagal neurons, which are prevented by the 5-HT2B receptor antagonist SB204741 (Dergacheva et al., 2008). BW723C86 reversibly increases both the frequency and amplitude of miniature excitatory postsynaptic currents in cardiac vagal neurons. The facilitation evoked by α-methyl-5-HT is blocked by the 5-HT2B/2C receptor antagonist SB206553. Interestingly, the blockade of both NMDA and non-NMDA glutamatergic receptors did not prevent α-methyl-5-HT–evoked facilitation of miniature excitatory postsynaptic currents; however, the responses were blocked by P2 receptor antagonists (Dergacheva et al., 2008). These results indicate that activation of 5-HT2 receptors facilitates excitatory purinergic, but not glutamatergic, neurotransmission to cardiac vagal neurons.
11. Amygdala and Anxiety
Animals administered the 5-HT2B receptor agonist BW723C86 exhibit anxiolytic-like behavior in both the rat social interaction test and two conflict models of anxiety, the rat Geller-Seifter and marmoset conflict test (Kennett et al., 1995). Adult rat neurons in the medial amygdolid nucleus express 5-HT2B receptors protein (Fig. 11), where local application of a 5-HT2B receptor agonist displays anxiolytic activity in the social-interaction model but has little effect on behavior in a punished conflict model of anxiety (Kennett et al., 1996a,b). This 5-HT2B receptor agonist also increased the time spent in feeding behavior of freely fed rats in observation cages over 15 minutes. The effect was also likely to be 5-HT2B receptor–mediated, as no response to BW723C86 was evident in freely fed rats pretreated with the 5-HT2C/2B receptor antagonist SB206553. BW723C86 also reduced the frequency of grooming bouts of rats in observation cages (Kennett et al., 1997a). Finally, BW723C86 increased the number of punishments accepted in a rat Vogel drinking conflict paradigm over 3 minutes, as do anxiolytic benzodiazepine drugs. The antipunishment effect of BW723C86 was blocked by the 5-HT2B/2C receptor antagonists SB206553 or SB215505 but not by the selective 5-HT2C receptor antagonist SB242084. Thus, the antipunishment action of BW723C86 is likely to be 5-HT2B receptor–mediated (Kennett et al., 1998).
Fig. 11.
5-HT2B receptor immunoreactivity in rat brain. Immunohistochemical detection of 5-HT2B receptor immunoreactivity within Purkinje cells in the cerebellum (A), multipolar neurons in the lateral septum (B), multipolar and bipolar neurons in the medial amygdala (C), and cells in the dorsal hypothalamic nucleus (D). In each case, the staining was abolished in adjacent sections by coincubation with synthetic 5-HT2B receptor peptide (data not shown). Adapted from Duxon et al. (1997) (with permission).
12. Sleep
5-HT2A and 5-HT2C receptors modulate deep [slow wave (SW)] sleep and low-frequency EEG power in humans and rodents. Antagonists of 5-HT2A and/or 5-HT2C receptors have a well known slow-wave sleep–enhancing effect. In contrast, blockade of 5-HT2B receptors increases motor activity and wakefulness along with decreased theta activity during wakefulness and REM sleep. The 5-HT2B receptor antagonist SB215505 dose-dependently increases wakefulness at the expense of the intermediate stage of sleep, REM sleep, and slow-wave sleep and reduces low-frequency (<8 Hz) EEG power. In REM sleep, the 5-HT2B receptor antagonist SB215505 dose-dependently decreases EEG power solely in the theta (6–9 Hz) band, primarily affecting the peak power value (7 Hz) (Kantor et al., 2004). 5-HT exerts a 5-HT2B receptor–mediated facilitation of NREM sleep and an influence that is, respectively, inhibitory on NREM sleep and facilitatory on sleep apnea generation via 5-HT2A receptors (Popa et al., 2005). Htr2B−/− mice exhibited significantly increased wakefulness, with less NREM sleep, whereas REM sleep is not affected. Chronic oral intake of haloperidol restored the balance between wakefulness and NREM sleep in Htr2B−/− mice (Pitychoutis et al., 2015). Activation of 5-HT2B receptors may thus contribute to initiation of sleep and to theta generation during wakefulness and REM sleep under physiologic conditions. However, it should be kept in mind that, clinically, there have been no successes in insomnia/sleep disorders with 5-HT2 receptor antagonists in spite of multiple attempts, at least with 5-HT2A receptor antagonists.
J. Clinical Relevance
1. Feeding and Anorexigens
The hypophagic response to the anorexigen and 5-HT releaser, dexfenfluramine, observed in wild-type mice is absent in Htr2B−/− mice or in wild-type mice treated with RS127445. The dexfenfluramine-induced hypothalamic peak of 5-HT release is strongly reduced in Htr2B−/− awake mice compared with control mice. Dexfenfluramine-induced 5-HT release is observed in synaptosomal preparation from wild-type mice but absent in Htr2B−/− mice (Banas et al., 2011). 5-HT2B receptor–induced NO production phosphorylates SERT and maximizes 5-HT uptake in raphe neuron primary culture. 5-HT2B receptor–PKC coupling promotes additional phosphorylation of both SERT and Na+, K+-ATPase α-subunit, impairing the electrochemical gradient necessary for 5-HT uptake. Such 5-HT2B receptor–mediated control of SERT activity operated in primary neurons from the raphe nuclei (Launay et al., 2006). Thus, activation of 5-HT2B receptors is a limiting step in the SERT-dependent–releasing effect of dexfenfluramine, whereas other 5-HT receptors may act downstream with respect to feeding behavior.
2. Raphe and Antidepressant Activity
5-HT neurotransmission is tightly regulated by autoreceptors that fine-tune 5-HT neurotransmission through negative feedback inhibition at the cell bodies (predominantly 5-HT1A) or at the axon terminals (predominantly 5-HT1B), although 5-HT2B receptors may play different roles (McDevitt and Neumaier, 2011). The therapeutic effects induced by 5-HT–selective reuptake inhibitor (SSRI) antidepressants are initially triggered by blocking the SERT and rely on long-term adaptations of pre- and postsynaptic receptors. Long-term behavioral and neurogenic SSRI effects were abolished after either genetic or pharmacologic inactivation of 5-HT2B receptors (Diaz et al., 2012). Conversely, direct agonist stimulation of 5-HT2B receptors induced an SSRI-like response in behavioral and neurogenic assays. The 5-HT2B receptor is expressed in raphe serotonergic neurons. The SSRI-induced increase in hippocampal extracellular 5-HT concentration is strongly reduced in the absence of functional 5-HT2B receptors; hence, selective 5-HT2B activation mimics SSRI effects (Diaz et al., 2012). Thus, the 5-HT2B receptor modulates 5-HT neuron activity and may be required for the therapeutic actions of SSRIs.
3. Dopamine and Antidepressant Activity
Agomelatine, a potent melatonin MT1/MT2 receptor agonist, is an effective antidepressant and a potent 5-HT2B/2C receptor antagonist (Millan et al., 2003). Twice daily melatonin increases the number of spontaneously active dopamine neurons without affecting noradrenaline neurons. Long-term administration of either melatonin or the 5-HT2C receptor antagonist SB242084 has no effect on the firing rate and burst parameters of 5-HT and dopamine neurons, whereas their combination enhances the number of spontaneously active dopamine neurons while leaving the firing of 5-HT neurons unchanged. The addition of the 5-HT2B receptor antagonist LY266097 to the previous regimen, which by itself is devoid of effect, increases the activity of dopamine neurons (Chenu et al., 2014). Hence, the combination of melatonin receptor activation, 5-HT2C, and 5-HT2B receptor blockade results in a disinhibition of dopamine neurons, reproducing the antidepressant effect of agomelatine.
4. Serotonin (5-HT) Syndrome
The 5-HT syndrome occurs in humans after a combination of drugs inducing a massive increase in extracellular 5-HT or direct targeting of several 5-HT receptor subtypes [see Scotton et al. (2019)]. Htr2B−/− mice are more prone to develop the 5-HT syndrome symptoms after administration of high dose of SSRI or the 5-HT precursor, 5-hydroxytryptophan, although increases in 5-HT plasma levels are similar in both genotypes (Diaz and Maroteaux, 2011).
5. Drug of Abuse
The “club drug” MDMA (“ecstasy”) inhibits SERT activity, releasing 5-HT stores from nerve terminals. The subsequent activation of postsynaptic 5-HT receptors by released 5-HT is critical for the unique psychostimulatory effects of MDMA. Acute pharmacological inhibition or genetic ablation of the 5-HT2B receptor in mice completely abolishes MDMA-induced hyperlocomotion and 5-HT release in nucleus accumbens and ventral tegmental area. The MDMA-stimulated release of endogenous 5-HT from superfused midbrain synaptosomes is 5-HT2B receptor–dependent (Doly et al., 2008). Htr2B−/− mice show no behavioral sensitization or conditioned place preference following MDMA. In addition, MDMA-induced reinstatement of conditioned place preference and locomotor sensitization are both abolished by RS127445 in mice, whereas MDMA-induced dopamine D1 receptor–dependent phosphorylation of extracellular regulated kinase in nucleus accumbens is abolished in mice lacking functional 5-HT2B receptors. These results underpin the importance of 5-HT2B receptors in the reinforcing properties of MDMA (Doly et al., 2009).
The selective 5-HT2B receptor antagonist LY266097 reduces dopamine outflow in the ventral striatum/nucleus accumbens but not in the dorsal striatum (Auclair et al., 2010). The locomotor response of Htr2B−/− mice to both dizocilpine and amphetamine is significantly enhanced compared with control mice (Pitychoutis et al., 2015). 5-HT2B receptor antagonists reduce cocaine-induced hyperlocomotion independently of changes of subcortical dopamine outflow, supporting a regulatory control exerted by this receptor on ascending dopamine pathways (Devroye et al., 2015). Thus, 5-HT2B receptors may also exert, in addition to 5-HT neurons, a facilitatory control on mesoaccumbens dopamine pathway activity.
6. Impulsivity
A functional stop codon in the human 5-HT2B receptor gene enhances impulsive behavior (Bevilacqua et al., 2010). Especially under conditions in which control is impaired, the carriers of the stop codon are more vulnerable to alcohol and more impulsive upon drinking. Similarly, Htr2B−/− mice display more impulsive choice in delayed discounting tasks, sought novelty, and are more active after receiving a D1 dopamine receptor agonist (Bevilacqua et al., 2010). Interestingly, early onset schizophrenia is more prevalent in human 5-HT2B receptor gene Q*20 carriers. Domains related to the positive, negative, and cognitive symptom clusters of schizophrenia are affected in Htr2B−/− mice, with deficits in sensorimotor gating, selective attention, social interactions, learning, and memory processes. Htr2B−/− mice show enhanced locomotor response to dizocilpine and amphetamine and alterations in sleep architecture. 5-HT2B receptor ablation induces a region-selective decrease of dopamine and glutamate concentrations in the dorsal striatum. Importantly, selected schizophrenic-like phenotypes and endophenotypes are rescued by chronic haloperidol treatment (Pitychoutis et al., 2015). The phenotypes of Htr2B−/− mice result from a combination of both the direct absence of 5-HT2B receptor signaling and the resultant neural adaptations.
7. Fragile X Syndrome
Fragile X syndrome, caused by the loss of Fmr1 gene function, is the most common form of inherited mental retardation, with no current effective treatment. 5-HT2B receptor activation moderately enhances Ras-PI3K/PKB signaling input, GluA1-dependent synaptic plasticity, and learning in Fmr1 knockout mice without causing anxiety-related side effects (Lim et al., 2014).
8. Cardiovascular Diseases
5-HT2B receptors are overexpressed in heart tissue from patients with congestive heart failure, in parallel to increased cytokine and norepinephrine plasma levels (Jaffre et al., 2009). 5-HT plasma levels are also increased in patients with heart failure and in rodents with cardiac hypertrophy induced by aortic constriction. Thus, 5-HT–induced cardiac hypertrophy and/or heart failure may be 5-HT2B receptor–dependent or at least have responses likely exacerbated via the lack of 5-HT2B receptor. In support of which, 5-HT2B receptor KO mice do not develop isoproterenol-induced left-ventricular hypertrophy (Jaffré et al., 2004). Mice expressing the 5-HT2B receptor exclusively in cardiomyocytes, similarly to global 5-HT2B receptor–null mice, are resistant to isoproterenol-induced cardiac hypertrophy and dysfunction as well as to isoproterenol-induced increases in plasma cytokine levels (Jaffre et al., 2009). In primary culture of cardiac fibroblasts, angiotensin II– and isoproterenol-stimulated NOX activity is prevented by the selective 5-HT2B receptor antagonist (SB215505). SB215505 prevents the increase in cardiac superoxide generation and hypertrophy in two models of cardiac hypertrophy (i.e., angiotensin II and isoproterenol infusions in mice) (Monassier et al., 2008). A functional interaction between AT1 and 5-HT2B receptors via a transinhibition mechanism may involve heterodimeric receptor complexes and trigger cytokine release in cardiac fibroblasts (Jaffre et al., 2009).
The 5-HT2B receptor is involved in cardiac hypertrophy by acting directly on cardiac myocytes. After 2 weeks of aortic banding surgery, 5-HT2B receptor mRNA and protein expression are increased. SB215505 significantly reduces the arising increase in heart weight, heart wall thickness, left-ventricular mass, and expression of the brain natriuretic peptide (BNP) but does not attenuate the upregulation of 5-HT2B receptor protein expression in rats after aortic banding. Following in vitro mechanical stretch of cardiomyocytes and incubation with 5-HT, the level of 5-HT2B receptors and BNP protein increases time-dependently. 5-HT2B receptor siRNA applied to cardiomyocytes reversed both the increase of NF-κB translocation and BNP protein induced by 5-HT incubation plus mechanical stretch (Liang et al., 2006). 5-HT2B receptors are involved in the generation of apoptotic events associated with cardiac remodeling during increased adrenergic stimulation (Bai et al., 2010). Thus, there is a dual role for 5-HT2B receptors on both cardiomyocytes and cardiac fibroblasts in regulating cardiac hypertrophy in vivo.
9. Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a progressive and often fatal disorder that results from increased pulmonary blood pressure associated with abnormal vascular proliferation. 5-HT is associated with the pathogenesis of PAH (Chan and Loscalzo, 2008). Therapeutics with PAH as a side effect (e.g., dexfenfluramine) are potent 5-HT releasers acting at SERT and/or 5-HT2B receptor agonists (Weir et al., 2008). The blockade of 5-HT2B receptors, either by genetic (Htr2B−/−) or pharmacologic inactivation (5-HT2B receptor antagonist RS127445), completely prevents the development of hypoxia-induced pulmonary hypertension in mice and lung remodeling including the increase in vascular proliferation, elastase activity, and TGFβ1 levels (Launay et al., 2002). In the monocrotaline-induced pulmonary hypertension model, a number of 5-HT2B receptor antagonists (terguride, PRX-08066, or C-122) reduce pulmonary pressure, arterial wall thickening, and lumen occlusion but maintained cardiac function (Porvasnik et al., 2010; Dumitrascu et al., 2011; Zopf et al., 2011). Pulmonary hypertension is associated with a substantial increase in 5-HT2B receptor expression in the pulmonary arteries of both rodents and humans (Launay et al., 2002; Dumitrascu et al., 2011). Activation of 5-HT2B receptors appears to be, therefore, a limiting step in the development of pulmonary hypertension. However, the restricted expression of 5-HT2B receptors to bone marrow cells is necessary and sufficient for pulmonary hypertension to develop via an action at hematopoietic stem cell differentiation (Launay et al., 2012). There appears to be a limiting role of 5-HT2B receptors in PAH development; thus, the contribution of 5-HT to PAH may be an extrapulmonary, and specifically hematopoietic, event.
10. Vascular Hypertension
Mesenteric arteries from DOCA-salt hypertensive rodents predominantly contract via 5-HT2B receptors; they display an increase in 5-HT2B receptor mRNA and receptors (Watts et al., 1996). The isolated endothelium-denuded superior mesenteric artery of DOCA-salt rats displays a marked increase in the maximal contraction to the 5-HT2B receptor agonist BW723C86. In chronically instrumented rats, the 5-HT2B receptor antagonist LY272015 significantly reduces mean blood pressure (Watts and Fink, 1999). LY272015 also inhibited 5-HT–induced contractions in aorta from rats made hypertensive by exposure to the nitric-oxide synthase inhibitor N(omega)-nitro-L-arginine, whereas ketanserin was inactive (Russell et al., 2002). Thus, the 5-HT2B receptor appears to play an important role in 5-HT–induced contraction in hypertensive rodent arteries.
11. Fibrosis
5-HT increases proliferation and collagen synthesis of lung fibroblasts. 5-HT concentrations in lung homogenates increase significantly following bleomycin-induced fibrosis, paralleled with an increased expression of 5-HT2A and 5-HT2B receptors (Königshoff et al., 2010). Blockade of 5-HT2B receptors by SB215505 reduces bleomycin-induced lung fibrosis, with reduced lung collagen levels and procollagen 1 and 3 mRNA expression. 5-HT2B receptor antagonists decrease levels of lung TGFβ1 mRNA, connective tissue growth factor and plasminogen activator inhibitor-1, and JunD mRNA, consistent with their antifibrotic activity. Interestingly, the 5-HT2B receptor is strongly overexpressed in fibroblastic foci in human idiopathic pulmonary fibrosis (Fabre et al., 2008). Thus, it is likely that 5-HT–induced lung fibrosis is controlled by 5-HT2B receptors regulating TGFβ1 levels.
In the liver, fibrogenic HSC, which are negative regulators of hepatocyte regeneration, are known to express both 5-HT2A and 5-HT2B receptors, which may regulate TGFβ1 and Smad signaling (Li et al., 2006). HSCs play a key role in hepatic wound healing and fibrosis. After HSC activation, expression of 5-HT2A and 5-HT2B receptors is around 100- and 50-fold that of quiescent cells, respectively. The 5-HT2B receptor expression is strongly associated with fibrotic tissue in diseased liver. 5-HT2 receptor antagonist–treated HSCs display suppressed proliferation and increased apoptosis. 5-HT synergizes with platelet-derived growth factor to stimulate HSC proliferation (Ruddell et al., 2006). In contrast to quiescent cells, activated HSCs exhibit [Ca2+]i transients following treatment with 5-HT, which is inhibited by ritanserin. Expression of type 1 inositol-5′-triphosphate receptor and type 2 sarcoplasmic/endoplasmic reticulum Ca2+ ATPase is also increased during activation of HSCs and serves as the major isotype for ER Ca2+ storage and release in activated HSCs. ER Ca2+–binding chaperone proteins, including calreticulin, calnexin, and calsequestrin, are upregulated following activation of HSCs (Park et al., 2011). 5-HT2B receptor stimulation of HSCs increases the expression of TGFβ1 (a powerful suppressor of hepatocyte proliferation) via ERK/JunD signaling. Similar effects are evident in mice lacking 5-HT2B receptor or JunD or when HSCs have been selectively depleted. 5-HT2B receptor blockade attenuates fibrogenesis and improves liver function in disease models in which fibrosis is pre-established and progressive (Ebrahimkhani et al., 2011). Therefore, the hepatic 5-HT2B receptor appears to have a dual role, promoting regeneration in physiologic conditions and fibrosis in pathologic conditions.
Dermal fibrosis is reduced in Htr2B−/− mice using both inducible and genetic models of fibrosis. Pharmacologic inactivation of the 5-HT2B receptor effectively prevents the onset of experimental fibrosis and ameliorates established fibrosis by decreasing mRNA levels of TGFβ1, connective growth factor, plasminogen activator inhibitor-1, and Smad-3 (Dees et al., 2011). Moreover, inhibition of platelet activation prevents fibrosis in models of skin fibrosis. In TPH1-deficient mice, the rate-limiting enzyme for 5-HT production outside the central nervous system shows reduced experimental skin fibrosis (Dees et al., 2011). Skin fibrosis is thus controlled by 5-HT2B receptors via regulation of TGFβ1 levels.
Treatment of neonatal rat cardiac fibroblasts with 5-HT increases the expression of smooth muscle α-actin, a marker of fibroblast differentiation into myofibroblasts and stimulated cardiac fibroblast migration. 5-HT enhances secretion of TGFβ1 and expression of MMPs in cardiac fibroblasts (Yabanoglu et al., 2009). 5-HT– or AngII-stimulated cytokine release and secretion of TGFβ1 in adult cardiac fibroblasts is sensitive to 5-HT2B receptor blockade. Treatments with epidermal growth factor receptor (ErbB1/4)-selective inhibitors or with selective inhibitors of MMPs also abolished AngII- and 5-HT–induced cytokine release. Finally, the use of HB-EGF−/− cardiac fibroblasts confirmed that epidermal growth factor receptor stimulation is absolutely required for AngII- and 5-HT–dependent cytokine release (Jaffre et al., 2009). Collectively, these results highlight that a convergent action of norepinephrine, AngII, and 5-HT via interactions between AT1 and 5-HT2B receptors coexpressed by noncardiomyocytes is limiting key events in cardiac hypertrophy.
12. Valvular Heart Disease
Valvular heart disease occurs in over 65% of patients with carcinoid syndrome and is characterized by fibrous thickening of cardiac valves, leading to heart failure [for review, see Roth (2007)]. High plasma 5-HT levels correlate with valvular abnormalities detected by cardiac catheterization and echocardiography (Robiolio et al., 1995). The similarity of lesions in carcinoid heart disease and in methysergide-associated valvular disease suggested a direct stimulation of myofibroblast growth by an unknown 5-HT receptor agonism (Hendrikx et al., 1996). The increase in fenfluramine-associated valvular heart disease raised concerns that other 5-HT–relevant medications might also increase the risk of valvular heart disease (Connolly et al., 1997). Dexfenfluramine had been approved in the United States for long-term use as an appetite suppressant until it was associated with valvular heart disease. The valvular changes (myofibroblast proliferation) are histopathologically indistinguishable from those observed in carcinoid disease or following long-term exposure to 5-HT2 receptor–preferring ergot drugs (ergotamine, methysergide). The amphetamine derivative, MDMA (“ecstasy”), and its N-demethylated metabolite, 3,4-methylenedioxyamphetamine (MDA), both preferentially bind to and activate human recombinant 5-HT2B receptors. Like fenfluramine and norfenfluramine, they elicit mitogenic responses in human valvular interstitial cells via likely activation of 5-HT2B receptors (Setola et al., 2003). Based on these strikingly similar echocardiographic and histopathologic features, it is now considered that ergot-derived dopamine agonists (e.g., pergolide and cabergoline) cause a valvular heart disease nearly identical to that seen in patients with carcinoid syndrome (Horvath et al., 2004). Population studies of patients with Parkinson disease compared with nonparkinsonian controls show that pergolide and cabergoline have a similar risk of inducing fibrotic changes in cardiac valve leaflets. Pergolide and cabergoline have high affinity for the 5-HT2B receptors. The frequency of moderate-to-severe regurgitation in at least one heart valve was higher in patients receiving cabergoline or pergolide compared with patients taking nonergot agonists or controls, and the incidence of new-onset valvulopathy was relatively high in patients taking the ergot-derived drugs (Antonini and Poewe, 2007; Roth, 2007). A simultaneous mitral bioprosthesis hypertrophic scaring and native aortic valve fibrosis was recently reported following benfluorex therapy. The bioprosthesis and aortic valves exhibit similar histopathological lesions. Thickening and plaque deposits made by smooth muscle α-actin– and vimentin-positive cells in a glycosaminoglycan matrix were observed, supporting that activation of the 5-HT2B receptor by norfenfluramine may trigger the development of drug-induced heart disease (Ayme-Dietrich et al., 2012).
5-HT2B and 5-HT2A receptor transcripts are reported to be present in heart valves, whereas no 5-HT2C receptor transcript is detectable. Preferential stimulation of valvular 5-HT2B receptors (with or without accompanying 5-HT2A receptor activation) may contribute to valvular fibroplasia in humans (Fitzgerald et al., 2000). Mitral valve regurgitation has been associated with increased mRNA expression of valvular 5-HT2B receptors and SERT in pigs (Cremer et al., 2015b). Canine myxomatous mitral valve disease was associated with higher expression of 5-HT2B receptors in mitral valve (Cremer et al., 2015a).
These findings suggest that 5-HT2B receptor signaling links vascular damage and platelet activation to tissue remodeling and identify the 5-HT2B receptor as a novel potential therapeutic target to treat valvular heart diseases. As a result of these investigations, the development of 5-HT2B receptor agonists have been banned by the FDA.
13. Acute Pain
5-HT released from mast cells or platelets in peripheral tissues is an important inflammatory mediator of pain. The involvement of 5-HT in pain is complex since it can inhibit or facilitate nociceptive transmission because of the presence of multiple functionally opposing 5-HT receptors on peripheral and central nociceptors. An acute injection of 5-HT or the 5-HT2 receptor, agonist α-methyl-5-HT, into hindpaws of mice induces significant hyperalgesia to mechanical stimuli, which can be inhibited by the 5-HT2B/2C receptor antagonist SB206553, which also blocks 5-HT–induced transient [Ca2+] signaling in DRG neurons. 5-HT2B receptors are involved in mechanical hyperalgesia in mice (Lin et al., 2011). 5-HT released from descending pain modulation pathways to the dorsal horn is crucial in spinal nociception processing. Local peripheral ipsilateral, but not contralateral, administration of RS127445 significantly prevents formalin-induced flinching behavior. Moreover, local peripheral ipsilateral, but not contralateral, administration of the 5-HT2 receptor agonist DOI augmented formalin-induced nociception. The local pronociceptive effect of DOI is prevented by local RS127445. Moreover, intrathecal RS127445 also prevented formalin-induced nociception. By contrast, spinal DOI increases flinching behavior induced by formalin. The spinal pronociceptive effect of DOI is prevented by intrathecal RS127445 (Cervantes-Durán et al., 2012). It is therefore evident that 5-HT2B receptors play a pronociceptive role in peripheral as well as spinal sites in the rat formalin test.
14. Chronic Pain
In rats subjected to spinal nerve ligation, the 5-HT2B receptor agonist BW723C86 enhances evoked potentials, whereas the 5-HT2B receptor antagonist SB204741 depresses them. Spinal hyperexcitation promoted by 5-HT2B receptors has been proposed as a pathogenic pathway contributing to pain (Aira et al., 2010). Plasticity of spinal serotonergic neurotransmission selectively reduces spinal µ-opioid receptor mechanisms via increased expression of 5-HT2B receptors in dorsal horn neurons also expressing the µ-opioid receptor (Aira et al., 2012). The involvement of glutamate receptors in dorsal neuron hyperexcitation can also be promoted by 5-HT2B receptor after spinal nerve ligation. Augmentation of C-fiber–evoked potentials by spinal superfusion with the 5-HT2B receptor agonist BW723C86 in nerve-ligated rats is impeded by coadministration of NMDA receptor antagonist. Evoked potentials are increased by NMDA receptor agonist in nerve-injured rats, irrespective of simultaneous 5-HT2B receptor blockade by SB204741. In uninjured rats, NMDA receptor agonist enhances evoked potentials in the presence of BW723C86 but not if administered alone. Blockade of 5-HT2B receptors with the selective antagonist SB204741 after spinal nerve ligation bilaterally decreases phosphorylation of the NMDA receptor subunit NR1 (pNR1), in the synaptic fraction, and colocalization of both PKCγ and pNR1 with postsynaptic marker PSD-95. Delivery of SB204741 bilaterally attenuates thermal and mechanical allodynia occurring after spinal nerve ligation, at early time periods, day 2 postinjury. The transient activation of the PKCγ/NMDA receptor pathway is critically involved in 5-HT2B receptor–mediated facilitation in the spinal nerve ligation model (Aira et al., 2013). The adaptor protein NADH dehydrogenase subunit 2 (ND2) is involved in NR1 phosphorylation and spinal hyperexcitability secondary to peripheral nerve injury. Spinal nerve ligation is followed by increased colocalization of ND2 with pNR1. C-fiber–evoked dorsal horn field potentials are increased after spinal nerve ligation by superfusion with an NMDA receptor agonist. This increased postsynaptic upregulation of ND2/pNR1 can be prevented by prior administration of selective 5-HT2B antagonist SB204741 (Aira et al., 2014). Thus, NMDA receptor phosphorylation is instrumental in coupling 5-HT2B receptor–mediated input to NMDA receptor expressing synapses in spinal hyperexcitation involved in pain.
15. Neuropathic Pain
5-HT was also implicated in a rat model of neuropathic pain evoked by chronic constriction injury (CCI) of the sciatic nerve. 5-HT2B receptor activation has been reported to both prevent and reduce CCI-induced allodynia at 3 weeks postinjury. Intrathecal administration of the 5-HT2B receptor agonist BW723C86 attenuated established mechanical and cold allodynia, an effect prevented by coinjection of RS127445. A single application of BW723C86 on the sciatic nerve concomitantly to CCI dose-dependently prevented mechanical allodynia and reduced cold allodynia 17 days after CCI. This behavioral effect is accompanied with a marked decrease in macrophage infiltration into the sciatic nerve and, in the DRG, with an attenuated abnormal expression of several markers associated with local neuroinflammation and neuropathic pain. CCI results in a marked upregulation of 5-HT2B receptor expression in sciatic nerve and DRG. In the latter structure, it is biphasic, consisting of a transient early increase 2 days after surgery (around 23-fold) before neuropathic pain emergence, followed by a steady (around fivefold) increase that remains relatively constant until the pain disappeared. In DRG and sciatic nerve, 5-HT2B receptors are immunolocalized on sensory neurons and infiltrating macrophages (Urtikova et al., 2012).
It thus appears that 5-HT2B receptor involvement in pain takes place at various sites and time periods; early events are more pronociceptive, whereas at later stages, this receptor contribution may be more antinociceptive, although this varies according to the animal models.
16. Spasticity in Amyotrophic Lateral Sclerosis
Spinal cord injury leads to an initial phase of hyporeflexia followed by hyperreflexia, often referred to as spasticity. Spasticity is a common and disabling symptom also observed in patients with amyotrophic lateral sclerosis, a disease that can affect both upper and lower motor neurons. A rat tail spasticity model with a caudal spinal transection demonstrates 5-HT2B receptor downregulation at 21 days postinjury (Wienecke et al., 2010). Motoneurons, which recover from denervation, function autonomously, exhibiting large persistent calcium currents that help with functional recovery and contribute to uncontrolled muscle spasms. Application of agonists relatively selective to 5-HT2B receptors (including BW723C86) increase persistent calcium currents. 5-HT2B receptors on motoneurons ultimately contribute to recovery of motoneuron function and emergence of spasms (Murray et al., 2011). In amyotrophic lateral sclerosis, spasticity is traditionally thought to be the result of degeneration of the upper motor neurons in the cerebral cortex, although degeneration of other neuronal types, particularly 5-HT neurons, might also underlie the spasticity. In superoxide dismutase 1 (G86R) mice, a transgenic model of amyotrophic lateral sclerosis, 5-HT levels are decreased in brainstem and spinal cord before onset of motor symptoms. Furthermore, there is noticeable atrophy of 5-HT neuronal cell bodies along with neuritic degeneration at disease onset. In superoxide dismutase 1 (G86R) mice, tail muscle spastic-like contractions occur at end-stage. Importantly, they are abolished by the 5-HT2B/2C receptors inverse agonist, SB206553. In keeping with this, 5-HT2B receptor expression is strongly increased at disease onset (Dentel et al., 2013). In summary, 5-HT2B receptors on motoneurons can become constitutively active after injury and ultimately contribute to some recovery of motoneuron function and emergence of spasms.
17. Migraine
A role for 5-HT in migraine is supported by changes in circulating levels of 5-HT and its metabolites during the phases of a migraine attack. A migraine headache is thought to be transmitted by the trigeminal nerve from the meninges and their associated blood vessels. Correlation of the receptor affinities with the potencies of drugs used in migraine prophylaxis demonstrates correlations with the 5-HT2B receptor, and various human meningeal tissues express 5-HT2B mRNAs (Schmuck et al., 1996). The 5-HT2B receptor can activate the release of the smooth muscle relaxant NO and induce relaxation of the cerebral arteries and the jugular vein. 5-HT2B receptors expressed by endothelial cells of meningeal blood vessels may trigger migraine headache through the formation of NO, which results in the dilation of cerebral blood vessels and the concomitant activation of sensory trigeminovascular afferents, thus initiating the manifestation of head pain (Johnson et al., 2003). In addition, a genetic study identified 5-HT2B receptors as a susceptibility gene to migraine (Corominas et al., 2010). Endothelial 5-HT2B receptors may thus trigger dilation of meningeal blood vessels, which by activating sensory trigeminovascular afferents, induces head pain.
18. Visceral Pain
5-HT2B receptors are involved in signaling from the colon in rats, in which there is visceral hypersensitivity. Oral administration of RS127445 inhibits visceral hypersensitivity provoked by restraint stress without significant effect on the visceral nociceptive threshold of naive rats (Ohashi-Doi et al., 2010). Moreover, when administered intracerebroventricularly, RS127445 also decreases the number of pain behaviors during noxious colorectal distension. A selective 5-HT2B receptor antagonist has thus been proposed to have therapeutic potential for the treatment of gut disorders characterized by visceral hypersensitivity (O’Mahony et al., 2010). The 5-HT2B receptor appears thus to be involved in regulating sensory pathways but only under hyperalgesic conditions, suggesting the possible utility of 5-HT2B receptor antagonism in reducing visceral hypersensitivity in patients with irritable bowel syndrome and other hypersensitivity conditions.
19. Bones
5-HT2B receptor mRNA, which is undetectable in anaplastic osteoblasts, appears in differentiated and matured osteoblasts (Bliziotes et al., 2001; Westbroek et al., 2001). The differentiation and maturation of osteoblasts might thus be regulated by 5-HT2B receptor activation (Hirai et al., 2009). Of interest, Htr2B−/− female mice display reduced bone density from the age of 4 months that intensifies by 12 and 18 months. This histomorphometrically confirms that osteopenia is due to reduced bone formation (Collet et al., 2008). Using the osteoprogenitor cell line C1, blockade of 5-HT2B receptor intrinsic activity affects the efficiency of mineralization by decreasing calcium incorporation. Optimal bone matrix mineralization involves both NO and PLA2 signaling pathways, and the 5-HT2B receptor promotes prostaglandin E2 production through cyclooxygenase activation. When C1 osteoblasts undergo conversion into osteocyte-like cells, cyclooxygenase activity is quenched. The 5-HT2B receptor contributes in an autocrine manner to osteogenic differentiation (Locker et al., 2006). There is a functional link between the 5-HT2B receptor and the activity of the tissue nonspecific alkaline phosphatase (TNAP). Agonist stimulation of the receptor increases TNAP activity during the initial mineralization phase, whereas inhibition of 5-HT2B receptor intrinsic activity prevents TNAP activation. In contrast, agonist stimulation of the receptor further increased TNAP activity during the initial mineralization phase. The 5-HT2B receptor couples to PLA2 pathway and prostaglandin production at the beginning of mineral deposition. The 5-HT2B receptor also controls leukotriene synthesis via PLA2 at the terminal stages of differentiation. These two 5-HT2B receptor–dependent eicosanoid productions delineate distinct time windows of TNAP regulation during the osteogenic program. Finally, prostaglandins or leukotrienes relay the post-translational activation of TNAP via stimulation of the phosphatidylinositol-specific phospholipase C. In agreement with this, primary calvarial osteoblasts from Htr2B−/− mice exhibit defects in TNAP activity (Baudry et al., 2010). Brain 5-HT may indirectly favor bone mass accrual following activation of 5-HT2C receptors on ventromedial hypothalamic neurons and 5-HT2B receptors on arcuate neurons (Yadav et al., 2009). Compared with control osteoblasts, the lack of 5-HT2B receptors is associated with a 10-fold overproduction of prostacyclin, and the specific prostacyclin synthase inhibitor (U51605) totally rescues osteoblast aggregation and matrix mineralization in Htr2B−/− osteoblasts without effect on WT osteoblasts. Prostacyclin is the endogenous ligand of PPAR-β/δ, and its inhibition in Htr2B−/− cells totally rescues the alkaline phosphatase and osteopontin mRNA levels, cell-cell adhesion, and matrix mineralization. The absence of 5-HT2B receptors leads to the overproduction of prostacyclin, inducing reduced osteoblast differentiation because of PPAR-β/δ –dependent target regulation and defective cell-cell adhesion and matrix mineralization (Chabbi-Achengli et al., 2013). Of relevance, the 5-HT2A receptor is expressed only in osteoblasts, whereas 5-HT2B receptor expression increases from precursor to mature osteoclasts (Hodge et al., 2013b). The 5-HT2B receptor therefore appears to contribute in an autocrine manner to osteogenic differentiation.
20. Teeth Development
Periodontal diseases occur in patients treated with antidepressants such as SSRIs (e.g., fluoxetine). In the molar teeth of Htr2B−/− mice, rod curvatures and twisting are altered compared with WT mice, suggesting involvement of the 5-HT2B receptor at early stages of enamel formation. The volume of the enamel layer in Htr2B−/− mice is also reduced, with smaller crystallite thickness. The outer aprismatic enamel border is 1.5- to twofold larger in Htr2B−/− compared with WT mice. Finally, although no noticeable difference is observed in dentin, the micro-CT three-dimensional pulp reconstruction reveals a decrease in both length and width of dentin formation in the root canals of the Htr2B−/− mice (Dimitrova-Nakov et al., 2014). Therefore, 5-HT2B receptors may mediate some harmful effects of long-term use of SSRIs on bone and teeth regeneration.
21. Cancers
a. Carcinoid tumors
5-HT2B receptor expression is observed in spontaneous human carcinoid tumors, along with coupling to p21ras activation (Launay et al., 1996). The tumor proliferative activity of small intestinal neuroendocrine tumors (including cell growth and the development of desmoplasia) is associated with the particular microenvironment in the peritoneum, and tumor cells support this necessary milieu through the secretion of profibrotic/angiogenetic factors (Svejda et al., 2010).
b. Breast tumors
Increased 5-HT biosynthetic capacity accompanied by multiple changes in 5-HT receptor expression and signaling favor malignant progression of human breast cancer cells. Among them, expression of 5-HT2B receptors is increased (Pai et al., 2009). 5-HT2B receptor mRNA expression is lower in basal estrogen receptor–negative tumors compared with luminal tumors, which are most commonly estrogen receptor–positive. 5-HT2B receptor mRNA is elevated in carcinomas, increased with tumor stage, and higher in lymph node–positive tumors compared with node-negative tumors. c-Myc transformation induces an increase in 5-HT2B receptor expression (Pai et al., 2009). In human breast cancer, there is a significant correlation of 5-HT2B receptor expression with estrogen receptor-α expression (Kopparapu et al., 2013).
c. Melanoma
Uveal (ocular) melanoma is an aggressive cancer that often forms undetectable micrometastases before diagnosis of the primary tumor. High increases in 5-HT2B receptor mRNA are evident in all uveal melanomas with monosomy 3 compared with low expression in all tumors with disomy 3. As monosomy 3 is associated with metastatic disease, 5-HT2B receptor expression has been proposed as a marker to identify patients with poor prognosis (Tschentscher et al., 2003). The 5-HT2B receptor gene is among the genes showing the highest overexpression in class 2 uveal melanoma (van Gils et al., 2008). A PCR-based 15-gene assay comprising 12 discriminating genes, including the 5-HT2B receptor gene, is now part of a prognostic assay, which provides an important addition to help manage patients with uveal melanoma (Onken et al., 2010) by distinguishing whether uveal melanomas contain liver metastases and thus aid in the diagnosis and prevention of uveal melanoma liver metastases based on their different features (Zhang et al., 2014a).
d. Prostate cancer
Prostate cancer is the most commonly diagnosed noncutaneous cancer in men. Despite this fact, many of the genetic changes that coincide with prostate cancer progression remain enigmatic. The 5-HT2B receptor has been shown to be upregulated in tumors (Magee et al., 2001). Overexpression of receptors to neuroendocrine cell products may contribute to development of hormone-refractory prostate cancer. Immunostaining for the 5-HT2B receptor is evident in low-grade and high-grade tumors, prostatic intraepithelial neoplastic and benign prostatic hyperplasia cells, and vascular endothelial cells. Antagonists for the 5-HT2B receptor inhibit proliferation of prostate cancer cells in a dose-dependent manner (Dizeyi et al., 2005).
e. Adrenocortical carcinoma
Gene expression profiles of adrenocortical tumors demonstrate underexpression of 5-HT2B receptor mRNA as a marker of malignant adrenocortical carcinoma (Fernandez-Ranvier et al., 2008). Analysis of biomarkers of malignancy of adrenocortical cancers in a meta-analysis suggests the combination of overexpressed anillin and underexpressed 5-HT2B receptor mRNA to be the best predictor of malignancy (Zsippai et al., 2011). However, in adrenocorticotropin-dependent adrenal hyperplasia, the mechanisms responsible for the ectopic adrenal expression of glucose-dependent insulinotropic peptide (GDIP) receptor in GDIP-dependent Cushing’s syndrome are unknown. Chronic adrenal stimulation by GDIP in GDIP-dependent adrenocorticotropic hormone–independent macronodular adrenal hyperplasia leads to the significant induction of genes for the GPR54, 5-HT2B, GPR4, and endothelial differentiation sphingolipid EDG8 receptor (Lampron et al., 2006).
f. Hepatocellular carcinoma
Among 64 genes for which mRNA expression differed between non–hepatitis B and non–hepatitis C compared with hepatitis C–type hepatocellular carcinoma (HCC), the most affected is the gene for the 5-HT2B receptor (Iizuka et al., 2004). The function of 5-HT as a survival factor of HCC cells has been demonstrated; activation of the 5-HT2B receptor leads to sustained phosphorylation of two downstream targets of mTOR, p70S6K and 4E-BP1, thereby facilitating survival and inhibiting autophagy. Inhibiting the 5-HT2B receptor reduces cancer cell growth in vitro and in vivo. The presence of 5-HT2B receptors in HCC and the activation of autophagy-related mechanisms provide novel insights of 5-HT in cancer biology and propose 5-HT–mediated signaling as a therapeutic target (Soll et al., 2010). 5-HT1B and 5-HT2B receptors are expressed, respectively, in around 32% and 35% of the patients with hepatocellular cancer. Both receptors are associated with an increased proliferation index (Soll et al., 2012). The 5-HT2B receptor mediates 5-HT–induced proliferation in the serum-deprived HCC Huh7 cells. Additionally, selective 5-HT2B receptor antagonism using SB204741 in Huh7 cells decreases the expression of FOXO3a (Liang et al., 2013).
g. T-cell leukemia
A proteasome inhibitor, bortezomib, is a potential therapeutic agent to treat adult T-cell leukemia. A network including the 5-HT2B receptor was identified that converges upon the secreted protein acidic and rich in cysteine gene, a tumor-invasiveness–related gene, which may act as a modulator of bortezomib-induced cell death in adult T-cell leukemia cells (Ohyashiki et al., 2008).
h. Myosarcoma
In the pathogenesis of uterine leiomyosarcoma, there is a fourfold overexpression of the 5-HT2B receptor gene, and it is one of the most overexpressed genes (Arslan et al., 2005; Matsumura et al., 2006).
i. Tumor angiogenesis
5-HT does not enhance colon cancer tumor cell proliferation but may act as a regulator of angiogenesis by reducing the expression of MMP-12 and lower levels of angiostatin—an endogenous inhibitor of angiogenesis (Nocito et al., 2008). 5-HT stimulates the phosphorylation of ERK1/2 in bovine endothelial cells, and the 5-HT2B receptor plays a role in the activation of eNOS in human endothelial cells. In SB204741-treated mice, the selective blockade of the 5-HT2B receptor results in the reduction of tumor angiogenesis and growth through the inhibition effect of ERK1/2 and eNOS (Asada et al., 2009). Therefore, the 5-HT2B receptor may participate in tumor angiogenesis.
22. Clinically Relevant Knowledge Gained from the Gene
a. The human 5-HT2B receptor gene
The human 5-HT2B receptor gene (MIM 601122) is located on chromosome 2q37.1 (Le Coniat et al., 1996). The human 5-HT2B receptor gene rate of evolution displays high conservation in primates (Andrés et al., 2007). Within the 23 sites that are highly conserved among primates and that have changed on the modern human lineage after separation from Denisovan ancestors, one (D216N) is found in ECL2 of the 5-HT2B receptor, suggesting that crucial aspects of synaptic transmission involving the receptor may have changed in modern humans (Meyer et al., 2012). There is evidence of the contribution of 5-HT2B receptor gene variants to intelligence quotation, intellectual disability, and language onset delay in patients with autism spectrum disorders (ASD) (Hervas et al., 2014).
The 5-HT2B receptor gene was identified as a susceptibility gene for impulsivity disorders; one single-nucleotide polymorphism (SNP) introducing a stop codon after amino acid 21 was found more frequently in severely impulsive individuals presenting suicidal behavior (Bevilacqua et al., 2010). In the same cohort, early-onset schizophrenia was more prevalent in 5-HT2B receptor gene Q*20 carriers. Other work testing SNPs within the 5-HT2B receptor gene for potential associations with the behavioral inhibition system and the three components of the behavioral approach system (fun-seeking, drive, and reward responsiveness) in a Han Chinese sample found four 5-HT2B receptor gene SNPs significantly associated with behavioral approach system fun-seeking (Zhu et al., 2012a).
Several SNPs confer a double-mutant R6G/E42G of the receptor protein associated with drug abuse, suggesting that 5-HT2B receptor contributes to pathways that are involved in drug dependence (Lin et al., 2004). Peripheral blood DNA methylation levels of CpGs in the promoter regions were examined in African Americans and European Americans. In European Americans, six CpGs in the 5-HT2B receptor gene promoter are significantly hypermethylated in alcohol-dependent cases (Zhang et al., 2013a).
An association study on susceptibility to migraine in a Spanish population supports the involvement of the 5-HT2B receptor gene and the monoamine oxidase A gene in the genetic predisposition to migraine without aura (Corominas et al., 2010). Finally, by investigating the 5-HT2B receptor gene in patients who developed pulmonary hypertension after use of fenfluramine, a heterozygous mutation was identified in one female patient who, 5 years earlier, had followed a 9-month anorexigen treatment (Blanpain et al., 2003). This heterozygous mutation R393X in the 5-HT2B receptor generates a carboxy terminus-truncated receptor characterized by a switch of coupling from Gαq/11 to Gα13, reduced NOS activation, and an increase in cell proliferation, modifications relevant to pathophysiological vasoconstriction (Deraet et al., 2005).
IX. 5-HT2C Receptor
A. Introduction
The 5-HT2C receptor is a G protein–coupled receptor (GPCR) with the characteristic seven-transmembrane domain structure with an extracellular N terminus and intracellular C terminus. Binding of 5-HT to the 5-HT2C receptor results in a conformational change that catalyzes the diffusion of multiple second messenger effectors. The canonical G protein–dependent signaling through the 5-HT2C receptor is engendered by 5-HT–stimulated coupling predominantly to Gαq/11 to activate the enzyme phospholipase C (PLC), which generates phosphoinositide hydrolysis and intracellular calcium (Cai2+) mobilization (Conn and Sanders-Bush, 1986a; Hoyer et al., 1989c; Chang et al., 2000).The 5-HT2C receptor also signals through other second messengers, which can include phospholipase D (PLD) and phospholipase A2 (PLA2), cyclic nucleotides, and extracellular signal–regulated kinases (Berg et al., 1994, 1998b; Kaufman et al., 1995; Werry et al., 2005). Landmarks in the progress of 5-HT2C receptor research are illustrated in Fig. 12 [see also Palacios et al. (2017)].
Fig. 12.
Landmarks in 5-HT2C receptor research and development. Identification and characterization of the 5-HT2C receptor and the development of 5-HT2C receptor–directed ligands has evolved over the last 40 years (black text). The 5-HT1C receptor was reclassified as the 5-HT2C receptor in the mid-1990s (purple text). Examples of novel pharmacological tools (blue text) as well as the first-in-class selective 5-HT2C receptor agonist approved for obesity (red text) are illustrated in the timeline. Refer to the text for details; superscripted numbers indicate literature citations: 1) Peroutka and Snyder, 1979; 2) Pazos et al., 1984b; Yagaloff and Hartig, 1985; 3) Closse, 1983; 4) Pazos et al., 1984a; Pazos and Palacios, 1985; Pazos et al., 1985; Hoyer et al., 1986; 5) Conn et al., 1986; Conn and Sanders-Bush, 1986a; Hoyer et al., 1989; Chang et al., 2000; 6) (rat) Lübbert et al., 1987; Julius et al., 1988; (human) Saltzman et al., 1991; Milatovich et al., 1992; Stam et al., 1994; Xie et al., 1996; (mouse) Yu et al., 1991; Foguet et al., 1992a,b; 7) Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990; 8) Millar et al., 2007; 9) Hoyer, 1988a,b; Humphrey et al., 1993; Hoyer et al., 1994; 10) Tecott et al., 1995; 11) Berg et al., 1994, 1998a; 12) Burns et al., 1997; 13) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm180078.htm; 14) Bromidge et al., 1997; Kennett et al., 1997; 15) Herrick-Davis et al., 1999; 16) Di Giovanni et al., 1999; Di Matteo et al., 1999; Gobert et al., 2000; 17) Bécamel et al., 2002, 2004; 18) Basile et al., 2002; Tsai et al., 2002; Theisen et al., 2004; Templeman et al., 2005; 19) Herrick-Davis et al., 2006; Herrick-Davis et al., 2007; 20) Rosenzweig-Lipson et al., 2007a; Tong et al., 2010; Dunlop et al., 2011; Dunlop et al., 2005; 21) Xu et al., 2008 22) Leggio et al., 2009b; 23) Kawahara et al., 2008; Morabito et al., 2010a; 24) Kishore and Stamm, 2006; Doe et al., 2009; Kishore et al., 2010; 25) Ji et al., 2006; Anastasio et al., 2013; 26) Im et al., 2003; Ding et al., 2012; Wild et al., 2019; 27) http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309993.htm; Thomsen et al., 2008; Smith et al., 2009; Smith et al., 2010; 28) http://www.eisai.com/news/news201465.html; Cunningham and Anastasio, 2014; Rezvani et al., 2014; Higgs et al., 2015; Howell and Cunningham, 2015; Harvey-Lewis et al., 2016; 29) Nocjar et al., 2015; Anastasio et al., 2015; 30) Herrick-Davis et al., 2015; 31) Schellekens et al., 2015; Kamal et al., 2015; 32) Di Giovanni and De Deurwaerdere, 2016; Venzi et al., 2016; 33) Xu et al., 2017.
The gene for the human 5-HT2C receptor (HTR2C) was cloned and localized to the X chromosome (Xq24) in the early 1990s (Yu et al., 1991; Milatovich et al., 1992; Stam et al., 1994; Xie et al., 1996). The genomic DNA for the 5-HT2C receptor is divided into six exons with five introns (Xie et al., 1996), whereas the coding region contains four exons with three introns (Stam et al., 1994). The gene encodes a 458-amino-acid protein in humans and 460-amino-acid protein in rats, which share 90% amino acid homology (Saltzman et al., 1991). The 5-HT2C receptor is the only GPCR known to undergo RNA editing, resulting in the functional expression of multiple isoforms of the receptor that differ in their distribution, pharmacology, and signaling capabilities.
The 5-HT2C receptor was first identified in choroid plexus tissue by receptor autoradiography as a highly expressed binding site with high affinity for radioligands that bind to the 5-HT1 receptor family (Pazos et al., 1984; Yagaloff and Hartig, 1985). This binding site displayed a different pharmacological profile from the known 5-HT1A receptor and 5-HT1B receptor and thus was originally named 5-HT1C receptor. It was cloned by functional expression of choroid plexus RNA injected into Xenopus oocytes (Lübbert et al., 1987; Julius et al., 1988). The genomic organization of the 5-HT1C receptor (with the presence of introns), a 50% overall homology to the 5-HT2A receptor and similar signal transduction mechanisms as 5-HT2A receptor and 5-HT2B receptor, indicated that the 5-HT1C receptor was more similar to members of the 5-HT2 receptor family than the intronless, adenylyl cyclase–coupled members of the 5-HT1 receptor family. Therefore, the 5-HT1C receptor was reclassified as a member of the 5-HT2 receptor family and renamed 5-HT2C receptor (Hoyer et al., 1994).
Early autoradiography studies reported a predominant localization of this receptor in choroid plexus tissue, whereas subsequent in situ hybridization studies revealed a widespread distribution throughout the basal ganglia, limbic system, and prefrontal cortex (Hoyer et al., 1986; Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990; Fig. 13). In fact, 5-HT2C receptor mRNA has been reported to be more abundant and widespread throughout the CNS than mRNA of the closely related 5-HT2A receptor (Pompeiano et al., 1994; Wright et al., 1995). Thus, the 5-HT2C receptor, largely thought of as “the choroid plexus receptor,” is actually well positioned throughout the CNS to mediate many of the central actions of 5-HT, including regulation of appetite, cognition, mood, movement, and sleep [for reviews, see Berg et al. (2008a) and Di Giovanni et al., 2010)]. The 5-HT2C receptor has been implicated in addiction, anxiety, depression, epilepsy, schizophrenia, and obesity. Therefore, the 5-HT2C receptor is a therapeutic target of great interest [for reviews, see Bubar and Cunningham (2006); Howell and Cunningham (2015); Sullivan et al. (2015); Di Giovanni and De Deurwaerdere (2016)].
Fig. 13.
5-HT2C receptor expression in rat brain. In situ hybridization detection of 5-HT2C receptor mRNA (A–D) and 5-HT2C receptor autoradiography (A’–D’) from adjacent sections. Acb, nucleus accumbens; AON, anterior olfactory nucleus; BST, bed nucleus stria terminalis; CA1, hippocampus CA1 field; CG, central gray; ChP, choroid plexus; CPu, caudate-putamen; DG, dentate gyrus; GP, globus pallidus; Lhb, lateral habenular nucleus; MG, medial geniculate nucleus; PCg, posterior cingulate cortex; PO, primary olfactory cortex; S, subiculum; SN, substantia nigra; STh, subthalamic nucleus. Scale bar, 1 mm. Adapted from Mengod et al. (1990) (with permission).
B. Expression Profile
The 5-HT2C receptor mRNA is differentially overexpressed in neurons relative to astrocytes and oligodendrocytes in the postnatal mouse forebrain (Cahoy et al., 2008). Regional analyses indicate that the 5-HT2C receptor mRNA is present in high levels in the choroid plexus, hippocampus, and the subthalamic and lateral habenular nuclei (Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990). Brain regions with moderate to high levels of 5-HT2C receptor mRNA include the amygdala, nuclei of the basal ganglia (NAc, striatum, VTA, SN, and internal globus pallidus), cortex, hypothalamus, dorsal raphe, brain stem, and spinal cord (Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990). The 5-HT2C receptor mRNA is found in regions containing the major dopaminergic cell bodies, including the SN and the VTA (Mengod et al., 1990; Pompeiano et al., 1994; Eberle-Wang et al., 1997; Bubar and Cunningham, 2007; Bubar et al., 2011); cholinergic cell bodies (Lopez-Gimenez et al., 2001); encephalin-, substance P–, and dynorphin-containing neuropeptidergic neurons in the dorsal and ventral striatum (Ward and Dorsa, 1996); and neuropeptide Y–containing neurons in the lateral and basolateral amygdala (Bonn et al., 2013). The 5-HT2C receptor mRNA is not present in neurons expressing 5-HT transporter (SERT) mRNA in the raphe nuclei but is localized to GABA interneurons in this region (Serrats et al., 2005).
There is good agreement between mRNA and protein distribution in the majority of brain regions, suggesting that the 5-HT2C receptor is predominantly localized in somatodendritic compartments (Mengod et al., 1990; Abramowski et al., 1995; Abramowski and Staufenbiel, 1995; Eberle-Wang et al., 1997; Clemett et al., 2000; Anastasio et al., 2010). The exceptions are the subthalamic nucleus, in which 5-HT2C receptor mRNA expression is high while its protein levels are low, and the external globus pallidus where the 5-HT2C receptor is present but 5-HT2C receptor mRNA is absent.
Early radioligand binding studies suggested that the 5-HT2C receptor is located on the apical surface of epithelial cells in the choroid plexus (Hartig et al., 1990). More recently, confocal microscopy and fluorescence correlation spectroscopy were used to directly visualize native 5-HT2C receptors and reveal their expression as homodimers on the apical surface of choroid epithelial cells (Herrick-Davis et al., 2015).
The 5-HT2C receptor is widely distributed throughout the basal ganglia and limbic-corticostriatal circuit. The 5-HT2C receptor is located postsynaptic to serotonergic neurons on GABAergic, glutamatergic, dopaminergic, neuropeptidergic, and cholinergic neurons. For example, the 5-HT2C receptor is expressed on GABAergic interneurons in the raphe nuclei (Serrats et al., 2005) and on GABAergic projection neurons in the NAc and striatum (Alex and Pehek, 2007). They are also found on GABAergic neurons in the substantia nigra (Eberle-Wang et al., 1997; Invernizzi et al., 2007) and VTA (Di Giovanni et al., 2001; Bubar and Cunningham, 2007; Bubar et al., 2011). Because the 5-HT2C receptor stimulates IP turnover (Conn and Sanders-Bush, 1986a; Hoyer et al., 1989; Chang et al., 2000) and increases Cai2+ levels resulting in membrane depolarization and neuronal firing (Stanford et al., 2005), activation of the 5-HT2C receptor on GABAergic neurons would be expected to increase the firing of these neurons, which have an inhibitory influence in that region. For example, stimulation of the 5-HT2C receptor in the VTA increases the firing rate of GABAergic interneurons, resulting in a decreased firing rate of dopaminergic neurons (Prisco et al., 1994; Di Giovanni et al., 2001). Conversely, 5-HT2C receptor antagonism has been reported to increase dopaminergic neurotransmission and dopamine levels in the NAc and prefrontal cortex (Di Giovanni et al., 1999; Di Matteo et al., 1999; Gobert et al., 2000). However, the story is likely to be more complicated, as the 5-HT2C receptor has also been shown to be expressed on a subset of dopaminergic neurons in the VTA, with higher expression in the middle relative to the rostral and caudal regions (Bubar and Cunningham, 2007; Bubar et al., 2011). These findings suggest the possibility that activation of the 5-HT2C receptor may directly enhance dopamine neurotransmission within specific subnuclei of the VTA in a region-specific manner.
5-HT neurons from the raphe terminate in layers V and VI of the medial prefrontal cortex, adjacent to GABAergic interneurons that express the 5-HT2C receptor (Liu et al., 2007; Nocjar et al., 2015). In turn, the GABAergic interneurons terminate on pyramidal efferents (likely glutamatergic), which may also express the 5-HT2C receptor (Vysokanov et al., 1998; Carr et al., 2002; Liu et al., 2007; Nocjar et al., 2015). In the prefrontal cortex, the 5-HT2C receptor associates with PSD-95 in postsynaptic densities (Anastasio et al., 2010) and colocalizes with the 5-HT2A receptor on GABAergic interneurons (Nocjar et al., 2015). In the hypothalamus, the 5-HT2C receptor is expressed on pro-opiomelanocortin (POMC) neurons in the arcuate nucleus, where they stimulate the release of the anorectic peptide α-melanocyte–stimulating hormone (Heisler et al., 2002).
Previous studies provide little evidence that the 5-HT2C receptor is expressed outside the CNS. However, portions of the HTR2C gene are expressed outside the CNS. Exons I and II of the HTR2C gene are located in the 5′ untranslated region, and the translational start site for the 5-HT2C receptor protein is located in the middle of exon III (Xie et al., 1996). Exons I–III of the HTR2C gene have been reported to be expressed in non-neuronal cells, along with four different microRNAs (miRNA) generated from intron II (Zhang et al., 2013c). Expression of these miRNAs is regulated by the small nucleolar RNAs (snoRNA) SNORD 115/MBII-52 and SNORD 116/MBII-85. Patients with Prader-Willi syndrome lack MBII-52 and MBII-85 and express different levels of the 5-HT2C receptor miRNAs than control subjects (Zhang et al., 2013c). Although the function of these miRNAs is not fully understood, it is possible that their dysregulation may contribute to the Prader-Willi syndrome phenotype as explored in 5-HT2C receptor transgenic mouse models.
Because the 5-HT2C receptor mRNA in human brain includes exons I–VI, and the mRNA found outside the CNS contains exons I–III, it was hypothesized that a transcriptional termination signal prior to exon IV may be employed in non-neuronal cells outside the CNS (Zhang et al., 2013c). In contrast, a 185-base-pair fragment of 5-HT2C receptor mRNA was identified by quantitative RT-PCR from rat adipocyte visceral tissue (Stunes et al., 2011). The expression level of the 5-HT2C receptor mRNA was only 5% of the expression level of the β-actin housekeeping gene control, and Western blots revealed that 5-HT2C receptor expression in undifferentiated adipocytes was negligible compared with expression in rat brain (Stunes et al., 2011). However, expression of 5-HT2C receptor mRNA and protein expression could be induced in cultured adipocytes. In addition, low levels of 5-HT2C receptor mRNA and protein have been reported in rat pancreatic islet cells (Zhang et al., 2013b). Further research is required to elucidate the physiologic role of the 5-HT2C receptor in the function of adipocytes (Stunes et al., 2011) and potentially pancreatic β-cells (Zhang et al., 2013b).
C. Post-transcriptional and Post-translational Modifications
1. RNA Editing
The 5-HT2C receptor is the only GPCR reported to undergo RNA editing. The 5-HT2C receptor pre-mRNA is unique in containing a region of exonic and intronic sequence complementarity between the distal half of exon V and the beginning of intron V (Burns et al., 1997). This results in base pairing between the exonic and intronic sequences, giving the pre-mRNA a double-stranded, stem-loop structure. The secondary structure of the 5-HT2C receptor pre-mRNA influences the pattern of editing (Fukuda et al., 2015) as well as splicing (Shen et al., 2013). The double-stranded RNA is a substrate for adenosine deaminases that act on RNA and RNA-specific adenosine deaminase (ADAR) 1 and ADAR2 (Burns et al., 1997; Liu et al., 1999). These enzymes catalyze the deamination of adenosine to inosine, which is read as guanosine when the RNA is translated into protein. Adenosine to inosine RNA editing can occur at up to five different locations (termed A–E) within exon V (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999; Wang et al., 2000a) and one site located in intron V (Flomen et al., 2004). The editing sites in exon V are located in the second intracellular loop of the receptor in close proximity to the highly conserved “DRY” motif at the base of transmembrane domain III. The resulting adenosine to guanosine conversions change the coding potential of amino acids I156, N158, and I160 from INI (isoleucine, asparagine, isoleucine) in the unedited isoform to VSV (valine, serine, valine) or VGV (valine, glycine, valine) in the fully edited isoforms.
The RNA editing of the HTR2C gene can produce up to 32 different 5-HT2C receptor pre-mRNAs encoding 24 different proteins. There are seven predominant 5-HT2C receptor isoforms that are expressed in a region-specific manner in human and rodent brain (Burns et al., 1997; Fitzgerald et al., 1999; Niswender et al., 1999; Wang et al., 2000; Dracheva et al., 2009; Abbas et al., 2010; Morabito et al., 2010b). The more highly edited isoforms are the predominant isoforms expressed in whole brain, with VSV and VNV being the most prominent. Regions such as the hypothalamus, hippocampus, striatum, and cortex predominantly express the edited VNV, VSV, and VSI isoforms, whereas the choroid plexus and cerebellum express significantly higher levels of the unedited INI and partially edited INV and ISV isoforms.
Because RNA editing occurs in the second intracellular loop of the receptor, a region known to play an important role in G protein activation, it has profound effects on the pharmacology and signaling capabilities of the 5-HT2C receptor. RNA editing decreases agonist binding affinity and G protein coupling efficiency (Burns et al., 1997; Fitzgerald et al., 1999; Herrick-Davis et al., 1999; Niswender et al., 1999; Wang et al., 2000; Berg et al., 2001). In these studies, the fully edited VSV and VGV isoforms displayed a 5- to 40-fold decrease in 5-HT binding affinity and potency for stimulating phosphoinositide production compared with the unedited INI isoform, and they displayed decreased affinity and potency for a variety of different agonists. When expressed in COS-7 cells at levels similar to native 5-HT2C receptor in choroid epithelial cells, the unedited INI isoform displayed high constitutive activity that was successively diminished by RNA editing to very low levels of constitutive activity in the fully edited VSV and VGV isoforms (Herrick-Davis et al., 1999; Niswender et al., 1999). In addition to regulating the level of basal activity, RNA editing alters the specificity of G protein coupling and second messenger activation. For example, the unedited INI isoform can signal through Gq, G13, and G15, but the fully edited VSV and VGV isoforms predominantly couple to and signal through Gq (Price et al., 2001; McGrew et al., 2002, 2004). The 5-HT2C receptor has been shown to signal in an agonist-specific manner to differentially regulate phosphoinositide and arachadonic acid production (Berg et al., 1998b) and the phosphorylation of ERK1/2 (Werry et al., 2005), effects which are diminished following RNA editing (Berg et al., 2001, 2008b; Werry et al., 2008). In addition to impacting signaling, RNA editing also influences receptor desensitization and trafficking (Marion et al., 2004).
The 5-HT2C receptor pre-mRNA editing is regulated by ADAR1 and ADAR2 (Burns et al., 1997). Both in vitro and in vivo studies have reported that RNA editing at amino acid 156 of the 5-HT2C receptor is predominantly accomplished by ADAR1, editing at amino acid 160 requires ADAR2, and both enzymes participate in editing at amino acid 158 (Liu et al., 1999; Wang et al., 2000, 2004; Hartner et al., 2004). ADAR1 and ADAR2 may act in a concerted manner whereby ADAR1 editing at amino acid 156 enhances ADAR2 pre-mRNA binding and subsequent editing at amino acids 160 and 158 (Carmel et al., 2012). In addition, factors that regulate the alternative splicing of the ADAR1 and ADAR2 pre-mRNAs have been reported to influence 5-HT2C receptor RNA editing (Liu et al., 1999; Schmauss et al., 2010). Alterations in ADAR activity or pattern of expression can result in altered 5-HT2C receptor isoform expression. Studies using neuronal cell cultures showed that factors that increase ADAR1 expression, such as treatment with interferon, increase 5-HT2C receptor editing at amino acid 156 (Yang et al., 2004) and that reduced ADAR1 expression abolished editing at amino acid 156 (Sukma et al., 2005). In vivo evidence supporting this hypothesis is provided by a recent study reporting a downregulation in ADAR2 expression following spinal cord injury in rats, which resulted in decreased 5-HT2C receptor RNA editing at amino acid 160 (Di Narzo et al., 2015). Conversely, RNA editing has been reported to be increased in transgenic mice overexpressing ADAR2, which resulted in an increase in the expression of the more fully edited, and a decrease in expression of the unedited, 5-HT2C receptor isoforms (Singh et al., 2011). The results of these studies indicate that alterations in ADAR activity and expression patterns alter the degree and pattern of 5-HT2C receptor editing, resulting in changes in 5-HT2C receptor isoform expression and signal transduction.
2. RNA Splicing
Three different variants of the 5-HT2C receptor are produced by RNA splicing, only one of which is expressed on the plasma membrane and is fully functional. The full-length functional 5-HT2C receptor (2Cfl) is generated following splicing at the traditional exon/intron boundaries to remove introns III–V (Xie et al., 1996). There are two alternative donor splice sites flanking the traditional donor splice site located at the exon V/intron V boundary: one located in the middle of exon V (Canton et al., 1996; Xie et al., 1996) and an infrequently used site located within intron V (Wang et al., 2000; Flomen et al., 2004). The splice sites are conserved across rat, mouse, and human species. Splicing at either one of the alternative splice sites produces an RNA that encodes a prematurely truncated protein. Use of the donor splice site located in the middle of exon V results in the deletion of the last 95 nucleotides of exon V, including the RNA editing sites, and causes a frame shift mutation with stop codon. The result is a truncated protein (2Ctr) containing the N terminus and first three transmembrane domains followed by 96 unique amino acids (Canton et al., 1996; Xie et al., 1996; Wang et al., 2000). In human brain tissue, 2Ctr mRNA is found in all brain regions containing 2Cfl mRNA. Though Western blots of membrane extracts from 2Ctr-transfected NIH3T3 cells revealed immunoreactive bands the predicted size of 2Ctr protein, radioligand binding and phosphoinositide production were not observed in the transfected cells (Canton et al., 1996; Wang et al., 2000). The 2Ctr protein is not expressed on the plasma membrane but is retained within the endoplasmic reticulum where the 2Ctr can form heterodimers with 2Cfl (Herrick-Davis and Farrington, 2011; Martin et al., 2013). Thus, one possible function of 2Ctr is to regulate 5-HT2C receptor signaling by forming heterodimers with 2Cfl and trapping 2Cfl in the endoplasmic reticulum (Herrick-Davis and Farrington, 2011; Martin et al., 2013).
Factors that influence splice site selection will impact 5-HT2C receptor function and signaling by altering the relative balance between 2Cfl and 2Ctr within a given brain region. RNA editing and factors that influence RNA editing, such as ADAR1 and ADAR2 activity, have been shown to influence splice site selection (Rueter et al., 1999; Maas et al., 2001; Flomen et al., 2004; Tohda et al., 2004; Dracheva et al., 2008a). Increased editing of the 5-HT2C receptor pre-mRNA, generating the more fully edited and less active isoforms, promotes the use of the traditional donor splice site at the exon V/intron V boundary and favors the generation of 2Cfl over 2Ctr (Flomen et al., 2004). On the other hand, the unedited and highly active INI isoform is associated with increased use of the alternative donor splice site in the middle of exon V, increasing the production of 2Ctr. The preferred splicing of the INI isoform into 2Ctr (Flomen et al., 2004) may explain the higher plasma membrane expression levels of 2Cfl observed in transgenic mice expressing the VGV isoform compared with mice expressing the INI isoform of the 5-HT2R (Kawahara et al., 2008). These results are consistent with the original studies in which 2Ctr was first identified and was reported to be most abundant in the choroid plexus (Canton et al., 1996; Xie et al., 1996), the same brain region that was subsequently reported to express the highest levels of unedited and partially edited isoforms of the 5-HT2C receptor (Burns et al., 1997; Niswender et al., 1999; Wang et al., 2000).
Several studies have provided evidence for a link between 5-HT2C receptor RNA editing and splicing in vivo. In postmortem brain samples from patients who committed suicide, RNA editing was increased along with the ratio of 2Cfl to 2Ctr, with increased expression of the less-active 5-HT2C receptor isoforms (Dracheva et al., 2008a). In malignant gliomas from human brain, decreased ADAR2 activity (predicted to reduce RNA editing) was positively correlated with increased alternative splicing and the production of 2Ctr (Maas et al., 2001). In several of the gliomas examined and in glioma-derived cells lines, 2Ctr was predominant and 2Cfl was mostly absent (Maas et al., 2001; Tohda et al., 2004). In light of these findings, it is interesting to note that patients with glioblastoma have an increased incidence of seizures, as do 5-HT2C receptor knockout mice.
noRNAs also regulate 5-HT2C receptor RNA splicing. The snoRNA MBII-52 binds to exon V of the HTR2C gene in the region containing the alternative splice site and the RNA editing sites (Kishore and Stamm, 2006; Kishore et al., 2010). MBII-52 reduces RNA editing and the use of the alternative splice site, thereby favoring the production of the full-length, unedited, and more active 5-HT2C receptor isoforms. In mice lacking MBII-52, RNA editing is increased, leading to increased expression of the edited and less-active 5-HT2C receptor isoforms (Doe et al., 2009). These mice display phenotypic and behavioral changes similar to those observed in Prader-Willi syndrome (Doe et al., 2009). Consistent with these findings, transgenic mice expressing only the fully edited VGV isoform display characteristics of Prader-Willi syndrome (Morabito et al., 2010a). The human homolog HBII-52 has been shown to be absent in patients with Prader-Willi syndrome, and 5-HT2C receptor RNA editing is increased in patients with Prader-Willi syndrome (Kishore and Stamm, 2006). These studies highlight the important roles that RNA editing and splicing play in the regulation of 5-HT2C receptor activity and demonstrate how deregulation of this system can have profound phenotypic and behavioral consequences.
3. Single-Nucleotide Polymorphisms
The 5-HT2C receptor not only achieves diversity through RNA editing and splicing but also through the incorporation of SNPs, substitutions of a novel nucleotide for a wild-type nucleotide, a common type of genetic variation (Wang et al., 1998). These SNPs can produce an unstable conformation that alters the protein structure of a GPCR and affects its ultimate functional properties (Wenkert et al., 1996). A number of HTR2C SNPs have been reported in the literature: three polymorphisms as well as a GT nucleotide repeat variation have been identified in the promoter (Xie et al., 1996); three polymorphisms have been reported within intronic regions (Gibson et al., 2004); one polymorphism has been reported in the coding region, resulting in the replacement of cysteine with serine at amino acid 23 (C23S) in the amino-terminus of the receptor (Lappalainen et al., 1995); and there is one polymorphism in the 3′ untranslated region (Song et al., 1999). Few studies are reported that have ascertained the impact of these HTR2C SNPs on function at either the cellular or whole organism level. The impact of the S23 variant, when expressed in insect cells, was reported to have slightly higher affinity for 5-HT than the C23 variant and to alter 5-HT2C receptor desensitization/resensitization mechanisms (Okada et al., 2004; Walstab et al., 2011). However, this finding remains controversial, as other studies have reported no difference between the C23 and S23 variants with respect to agonist-binding affinity or potency, G protein coupling, constitutive activity, and homodimerization (Lappalainen et al., 1995; Fentress et al., 2005). Given suggestions that the C23 variant may have clinical implications (Lappalainen et al., 1995; Okada et al., 2004; Piva et al., 2011; Walstab et al., 2011), further studies of this and other HTR2C SNPs are required.
4. Glycosylation
The rat 5-HT2C receptor is reported to be glycosylated, and bands with different molecular weights were observed on Western blot of brain and cells expressing the receptor (Abramowski and Staufenbiel, 1995). Antibodies raised against the third and fourth cytoplasmic domain of the 5-HT2C receptor identified an N-glycosylated polypeptide with a 60-kDa apparent molecular mass. Polypeptides were detected in immunoprecipitates from extracts of pig choroid plexus upon Western blot and binding assays with [3H]-mesulergine. A signal sequence was cleaved during membrane insertion, resulting in a 38-kDa polypeptide. During further maturation, the receptor was N-glycosylated at two sites via a 48-kDa intermediate, which was more abundant in choroid plexus than in hippocampus. However, there may be more glycosylated species, as following transfection of 5-HT2C receptor cDNAs into cultured cells, polypeptides were observed that differed from the ones found in the brain. Similarly, the N-glycosylated 5-HT2C receptor was identified in solubilized extracts from cell lines and rat brain (Backstrom et al., 1995). Extracts from NIH3T3 fibroblasts stably expressing rat 5-HT2C receptor contained immunoreactive proteins of 51 to 52 kDa and 58 to 68 kDa. In the brain, immunoreactive proteins were identified from choroid plexus extracts with masses of 51 kDa and 58–62 kDa. On the other hand, the major 58- to 62-kDa and minor 51-kDa proteins were not detected in extracts prepared from the hippocampus, striatum, or frontal cortex prepared under the same conditions. The association of asparagine-linked (N-linked) oligosaccharides with the receptors was also examined. Cells grown in the presence of tunicamycin to inhibit N-linked glycosylation resulted in proteins with masses of 40 and 41 kDa. Extracts prepared from NIH3T3 cells and choroid plexus incubated with N-glycosidase F showed proteins of 41 and 42 kDa from NIH3T3 cells and 41 kDa from choroid plexus. Neuraminidase treatment, to cleave sialic acid, reduced the mass of the 51-kDa and 58- to 62-kDa proteins from the choroid plexus to 50 kDa and 54–58 kDa, whereas the proteins from NIH3T3 cells were not affected by neuraminidase. Altogether, the 5-HT2C receptor contains N-linked sugars, and it is suggested that sialic acid residues associate with the receptor from the choroid plexus but not from other brain regions. These oligosaccharide moieties contribute up to approximately 30% of the relative mass of the receptor and may affect the functional properties of the 5-HT2C receptor.
5. Phosphorylation
The constitutively active 5-HT2C receptor is phosphorylated under basal conditions, and phosphorylation is increased by agonist treatment (Westphal et al., 1995). Pretreatment of cells with 5-HT resulted in 5-HT2C receptor desensitization, which was blocked by calcineurin (presumably by its phosphatase activity) (Boddeke et al., 1993). It was subsequently shown that S458 and S459 are phosphorylated (Backstrom et al., 2000). Phosphorylation of a mutant 5-HT2C receptor that lacks the carboxyl-terminal PDZ recognition motif [Ser(458)-Ser-Val-COOH; δPDZ] was not detectable, although these cells produced similar amounts of phosphoinositide and Cai2+ with similar kinetics as wild-type cells. Alanine mutations S458A or S459A decreased phosphorylation to 50% of wild-type receptor levels. Subsequent Cai2+ responses of S459A receptors were diminished relative to S458A and wild-type receptors. Thus, desensitization may occur in the absence of 5-HT2C receptor phosphorylation, suggesting that receptor phosphorylation at S459 enhances resensitization of 5-HT2C receptor responses. Agonist-induced phosphorylation of the 5-HT2C receptor was later established to regulate the receptor interaction with multiple PDZ protein 1 (Parker et al., 2003), also known as MUPP1 (Ullmer et al., 1998). MUPP1 is a putative scaffolding protein containing 13 PSD-95, Dlg, ZO-1 (PDZ) domains, identified by a yeast two-hybrid screen as one of a series of 5-HT2C receptor–interacting proteins. The MUPP1 PDZ domain 10 (PDZ 10) associates with Ser458-Ser-Val of the 5-HT2C receptor. An Asp mutation at Ser458 significantly decreased receptor interaction with PDZ 10. Also, 5-HT treatment of 5-HT2C receptor–NIH3T3 cells reduced receptor interaction with PDZ 10, an effect that was blocked by a 5-HT2C receptor antagonist.
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) interacts within several amino acids of the third intracellular loop (termed 3L4F) of the 5-HT2C receptor (Ji et al., 2006). The tumor suppressor PTEN is widely distributed in the brain (Lachyankar et al., 2000). The PTEN and 5-HT2C receptor proteins coimmunoprecipitate in fractions of the VTA (Ji et al., 2006; Anastasio et al., 2013). Employing a proximity ligation assay, the assembly of the 5-HT2C receptor:PTEN complex, and the ability of 3L4F to disrupt the complex, was validated under native conditions within intact live cells (Anastasio et al., 2013). A peptide fragment of the 5-HT2C receptor third intracellular loop (3L4F), labeled with the cell-penetrating peptide TAT, disrupts the 5-HT2C receptor:PTEN complex and PTEN-mediated dephosphorylation of the 5-HT2C receptor in PC12 cells (Ji et al., 2006) as well as enhances 5-HT–mediated i Cai2+ release in CHO cells stably transfected with the human 5-HT2C-INI receptor (but not the 5-HT2A receptor) (Anastasio et al., 2013). Interestingly, the cell-adhesion molecule close homology of L1 protein also binds to the third intracellular loop of the 5-HT2C receptor (amino acids 292–304) and may regulate the 5-HT2C receptor association with PTEN and β-arrestin2 to control its phosphorylation (Kleene et al., 2015).
Systemic administration of the TAT-3L4F peptide suppresses the Δ9-tetrahydrocannabinol (THC)-induced increase in firing rate of VTA dopaminergic neurons in the rat (Ji et al., 2006) and suppresses the place association conditioned to Δ9-THC and nicotine in rats (Ji et al., 2006). In more recent studies, whereas TAT-3L4F had no effect alone, the combination of ineffective doses of TAT-3L4F plus WAY163909 synergize to suppress motor impulsivity, a primary symptomatic element of multiple neuropsychiatric disorders (American Psychiatric Association, 2013) that is consistently suppressed by pretreatment with a selective 5-HT2C receptor agonist (Winstanley et al., 2004; Fletcher et al., 2007; Fletcher et al., 2011; Anastasio et al., 2013; Cunningham et al., 2013). In addition, TAT-3L4F augments 5-HT2C receptor agonist–mediated suppression of spontaneous locomotor activity, effects that are consistent with positive allosteric modulation of 5-HT2C receptor function (Anastasio et al., 2013; Wild et al., 2014, 2019). Together, coupled with the findings that Tat-3L4F did not affect spatial learning and memory (Maillet et al., 2008) or generate the total behavioral profile of 5-HT2C receptor agonists (Ji et al., 2006; Anastasio et al., 2013), inhibition of the PTEN:5-HT2C receptor interface and, thus, 5-HT2C receptor dephosphorylation is a novel pharmacological approach with therapeutic potential in substance use disorders (Ji et al., 2006; Muller and Carey, 2006; Maillet et al., 2008; Anastasio et al., 2013; Cunningham and Anastasio, 2014).
6. Dimerization
Homodimerization for many GPCRs is thought to be a post-translational event that occurs within the endoplasmic reticulum as a prerequisite for the transport and expression of functional receptors on the plasma membrane [for review, see Milligan (2010)]. Many members of the 5-HT receptor family, including the 5-HT2C receptor, have been reported to form homodimers [for review, see Herrick-Davis (2013)]. Using a confocal microscopy-based resonance energy-transfer technique, 5-HT2C receptor homodimer formation was visualized within the endoplasmic reticulum during receptor biosynthesis (Herrick-Davis et al., 2006). The 5-HT2C receptor is transported through the Golgi complex to the plasma membrane as a homodimer.
The 5-HT2C receptor forms detergent-sensitive homodimers (Herrick-Davis et al., 2004) that do not dissociate or associate to form higher-order complexes following agonist or inverse agonist treatment (Herrick-Davis et al., 2007). Coexpression of wild-type receptors with inactive, mutant receptors provided evidence that the 5-HT2C receptor homodimer interacts with a single G protein, that both protomers participate in signaling, and that both protomers must be functional in order for signaling to occur (Herrick-Davis et al., 2005). Time-lapse fluorescence confocal microscopy provided direct visualization of β-arrestin2 recruitment to the plasma membrane following 5-HT binding to the homodimer (Herrick-Davis et al., 2007; Herrick-Davis et al., 2012). Homodimerization was observed for both the unedited INI and the fully edited (VSV and VGV) isoforms (Herrick-Davis et al., 2007; Herrick-Davis et al., 2012).
Advanced imaging techniques with near-single-molecule sensitivity have been employed more recently in an attempt to distinguish between dimers and higher-order oligomers. Fluorescence correlation spectroscopy studies report that the 5-HT2C receptor expressed in HEK293 cells exists as homodimers, with no evidence for monomers, tetramers, or higher-order oligomers (Herrick-Davis et al., 2012). Spatial intensity distribution analysis was used to monitor the oligomer status of the 5-HT2C receptor over a wide range of receptor-expression levels (Ward et al., 2015). In this study, the 5-HT2C receptor was expressed as a mixture of monomers, dimers, and tetramers; treatment with a 5-HT2C receptor antagonist for 90 minutes converted the majority of dimers and tetramers to monomers. However, it should be noted that tetramers were prominent only when 5-HT2C receptor expression exceeded 100 receptors/μm2 (Ward et al., 2015), greatly in excess of physiologic expression levels for native GPCRs (Hegener et al., 2004; Herrick-Davis et al., 2015). Similarly, the homodimer was the predominant species observed for biogenic amine receptors when expressed within their normal physiologic range in HEK293 cells (Herrick-Davis et al., 2013).
Only one study to date has examined the 5-HT2C receptor endogenously expressed in its native cellular environment. The native 5-HT2C receptor, endogenous to the choroid plexus, is expressed as homodimers on the apical surface of the epithelial cells at a density of 32 receptors/μm2 (Herrick-Davis et al., 2015). Though this is similar to a reported density of 20 receptors/μm2 for native β2-adrenergic receptors in alveolar epithelial cells, expression levels in neurons were much lower at 4.5 receptors/μm2 (Hegener et al., 2004). The study by Herrick-Davis et al. (2015) found no evidence for monomers or tetramers of the native 5-HT2C receptor in choroid epithelial cells or when the 5-HT2C receptor was expressed at normal physiologic levels in HEK293 cells. In this study, the signaling properties of the 5-HT2C receptor homodimer were investigated using agonists that bind in a wash-resistant manner to one or both protomers of the 5-HT2C receptor homodimer. Agonist binding to one protomer stimulated a half-maximal phosphoinositide response, whereas binding to both protomers was required to produce a maximal response (Herrick-Davis et al., 2015). These experiments provide pharmacological evidence supporting the hypothesis that the 5-HT2C receptor functions as a homodimer.
Heterodimers can form between the different isoforms of the 5-HT2C receptor generated by RNA editing (Herrick-Davis and Farrington, 2011). In HEK293 cells, positive resonance energy transfer was observed between the INI/VSV, INI/VGV, and VSV/VGV isoform pairs. However, the influence of heteromer pairs of edited 5-HT2C receptor isoforms on receptor signaling and neural function in vivo are yet to be demonstrated. In terms of heterodimers between the 5-HT2C receptor and other members of the 5-HT receptor family, the 5-HT2A receptor would be the most likely candidate given the similarity in structure (for review, see Hannon and Hoyer, 2008a,b). Immunohistochemical analyses indicate that the 5-HT2A receptor and 5-HT2C receptor protein colocalize in the same GABAergic neurons as well as in a population of pyramidal projection neurons in the rat medial prefrontal cortex (mPFC) (Nocjar et al., 2015). Coimmunoprecipitation studies suggest that the 5-HT2A receptor and 5-HT2C receptor are found in the same protein complex in the rat mPFC (Anastasio et al., 2015). Further analyses of the structural and biologic significance of a possible heteromeric protein complex incorporating both the 5-HT2A receptor and 5-HT2C receptor are required to understand its potential role in behavior and neuropsychiatric disorders.
Selective 5-HT2A receptor antagonists and selective 5-HT2C receptor agonists have been noted to suppress a wide range of behaviors that are particularly well studied in preclinical models of addictive disorders (Bubar and Cunningham, 2008; Cunningham and Anastasio, 2014; Howell and Cunningham, 2015). The combination of low doses of the selective 5-HT2A receptor antagonist M100907 plus the preferential 5-HT2C receptor agonist MK212 evoked modest effects but resulted in an approximately additive suppression of cocaine-evoked hyperlocomotion and Fos expression in the caudate putamen (Pockros et al., 2012). In a second study, the combination of subthreshold doses of M100907 plus the selective 5-HT2C receptor agonist WAY163909 synergistically suppressed inherent and cocaine-evoked motor impulsivity as well as cocaine-induced hyperactivity and cocaine-seeking behavior (Cunningham et al., 2013). These data raise the possibility that the 5-HT2A receptor and 5-HT2C receptor may act in concert to regulate the neural bases for behavior. Further analyses of the structural and functional interactions between the 5-HT2A receptor and 5-HT2C receptor are necessary to disentangle the manner in which these GPCRs interact at neuronal and circuit levels.
Microinfusion of the preferential 5-HT2A receptor agonist DOI into the mPFC enhances (Wischhof et al., 2011), whereas intra-mPFC infusion of M100907 (Winstanley et al., 2003) suppresses, motor impulsivity. The density of 5-HT2A receptor (Fink et al., 2015) as well as 5-HT2C receptor protein expression in the mPFC (Anastasio et al., 2014b) predicts the level of motor impulsivity in outbred rats. Highly impulsive rats exhibit a greater 5-HT2A receptor–mediated head twitch response and are more sensitive to the suppressive effects of the selective 5-HT2A receptor antagonist M100907 on motor impulsivity (Fink et al., 2015). The levels of 5-HT2A receptor and 5-HT2C receptor protein predicted the intensity of motor impulsivity, and the ratio of the 5-HT2A receptor to 5-HT2C receptor protein in mPFC positively correlated with levels of motor impulsivity in individual outbred rats (Anastasio et al., 2015). High phenotypic motor impulsivity was associated with a diminished mPFC synaptosomal 5-HT2AR:5-HT2C receptor protein:protein interaction assessed by coimmunoprecipitation (Anastasio et al., 2015). Knockdown of 5-HT2C receptor in the mPFC resulted in increased motor impulsivity and triggered a compensatory upregulation of 5-HT2A receptor protein expression in mPFC and a leftward shift in the potency of M100907 to suppress impulsive behavior (Anastasio et al., 2015). These data further support the concept that an interactive relationship between the mPFC 5-HT2A receptor and 5-HT2C receptor is behaviorally relevant. The manner in which a potential 5-HT2A receptor and 5-HT2C receptor heteromeric protein complex in the mPFC contributes to high levels of inherent motor impulsivity remains to be uncovered.
The 5-HT2C receptor has also been reported to form heterodimers with ghrelin GHS-R1a receptor when overexpressed in HEK293 cells and to colocalize with the GHS-R1a in cultured primary hypothalamic and hippocampal neurons from the rat (Schellekens et al., 2015). In this study, activation and blockade of 5-HT2C receptor in vivo attenuated and potentiated, respectively, the orexigenic effects of ghrelin. Heterodimers between 5-HT2C receptor and melatonin MT2 receptors have also been reported in transfected cells as well as human cortex and hippocampus (Kamal et al., 2015). Interestingly, the novel antidepressant agomelatine demonstrates biased signaling and displays 5-HT2C receptor and MT2 receptor agonist properties, suggesting the heterodimer as a potential target for the development of a novel class of therapeutics for the treatment of psychiatric disorders as well as eating disorders and obesity.
Immunohistochemical studies demonstrated that the NMDA receptor GluN2A colocalizes with the 5-HT2C receptor in rat spinal cord neurons, whereas coimmunoprecipitation analysis of synaptosomal fractions suggests formation of a 5-HT2C receptor and GluN2A protein complex (Bigford et al., 2012). Stimulation of the 5-HT2C receptor enhanced NMDA-evoked motoneuron depolarization through involvement of Src tyrosine kinase (Bigford et al., 2012). These results support the assembly of a functionally relevant 5-HT2C receptor and NMDA receptor complex in the spinal cord, although the presence of this complex in the brain and its involvement in higher-order neural function is unexplored.
D. Pharmacology
For the 5-HT2C receptor, the identification of selective orthosteric ligands relative to the close family members 5-HT2A receptor and 5-HT2B receptor is challenged by their ∼50% overall homology, which rises to ∼80% in the transmembrane domains, comprising the orthosteric receptor–binding pocket (Hoyer et al., 2002). Because of this challenge, the earliest preclinical and clinical research employed “preferential” 5-HT2C receptor ligands, which frequently displayed affinity (agonists, antagonists) and/or efficacy (agonists) at the 5-HT2A receptor and 5-HT2B receptor. Because of the lack of selectivity of available agonists [e.g., MK212 and m-chlorophenylpiperazine (mCPP)] and antagonists (e.g., ketanserin), experimental outcomes with such compounds initially led to ambiguous conclusions concerning the biologic roles for the 5-HT2C receptor, particularly in vivo. Furthermore, with the understanding that 5-HT2A receptor or 5-HT2B receptor agonists may evoke hallucinations (Nichols, 2004) or cardiac valvulopathy (Fitzgerald et al., 2000; Roth, 2007), respectively, the need for 5-HT2C receptor orthosteric agonists that lack demonstrable efficacy at 5-HT2A receptor or 5-HT2B receptor is recognized. In 1997, the chemists at SmithKline Beecham synthesized and characterized SB242084 as the first selective 5-HT2C receptor antagonist (Bromidge et al., 1997; Kennett et al., 1997). Because the 5-HT2C receptor exhibits constitutive activity dependent on the edited isoform and the in vitro and in vivo conditions employed for analyses, SB242084 and other compounds in this series (e.g., SB206553 and SB243213) (Bromidge et al., 1997; Kennett et al., 1997) have been noted to act as inverse agonists to attenuate constitutive 5-HT2C receptor activity (for reviews, see Aloyo et al., 2009; Sullivan et al., 2015). Atypical antipsychotic drugs are also reported to act as inverse agonists with the ability to inhibit 5-HT2C receptor constitutive activity (Herrick-Davis et al., 2000; Rauser et al., 2001) and Spampinato and colleagues have identified the role of 5-HT2C receptor constitutive activity in the control of dopamine corticoaccumbens function (Aloyo et al., 2009; Leggio et al., 2009b).
A body of knowledge has been developed around several useful 5-HT2C receptor agonists that have been available to scientists for the last 10 years. The compound RO60-0175 (Knight et al., 2004) has generated a great deal of information concerning the biologic role of the 5-HT2C receptor (Millan et al., 1998; Di Matteo et al., 2000; Grottick et al., 2000; Filip and Cunningham, 2002; Tomkins et al., 2002; Leggio et al., 2009a; Fletcher et al., 2012). RO60-0175 exhibits affinity and efficacy for all three 5-HT2 receptor subtypes, although many of its effects in vivo are blocked by SB242084 (Martin et al., 1998; Porter et al., 1999; Knight et al., 2004). Lorcaserin is a selective, high-efficacy 5-HT2C receptor agonist that was marketed as Belviq for weight reduction in patients with a body mass index >30 or with a body mass index >27 comorbid with type 2 diabetes, hypertension, or dyslipidemia (www.us.eisai.com/). The availability of lorcaserin for clinical research has prompted a growing number of studies, particularly focused on addictive disorders (Rezvani et al., 2014; Higgs et al., 2016; Harvey-Lewis et al., 2016; for review, see Higgins et al., 2020). The preliminary results of a clinical trial (http://www.eisai.com/news/news201465.html) demonstrated the efficacy of lorcaserin to increase abstinence from nicotine, a highly abused psychostimulant, whereas preclinical studies continue to promote the prospects of lorcaserin as a tool to reduce substance use disorders (Levin et al., 2011; Cunningham and Anastasio, 2014; Rezvani et al., 2014; Howell and Cunningham, 2015; Harvey-Lewis et al., 2016; Higgins et al., 2012; for review, see Higgins et al., 2020). Strikingly, Di Giovanni’s group has also recently shown that lorcaserin is very effective as an anticonvulsant in both animal models of generalized nonconvulsive absence epilepsy and temporal lobe epilepsy (Orban et al., 2014; Venzi et al., 2016).
A compound series developed at Wyeth Research includes vabicaserin (SCA-136), which acts as a selective 5-HT2C receptor full agonist (Ki = 3 nM; efficacy 100% relative to 5-HT), a 5-HT2B receptor antagonist (IC50 = 29 nM), and a weak 5-HT2A receptor antagonist (IC50 = 1650 nM) (Rosenzweig-Lipson et al., 2007a; Tong et al., 2010; Dunlop et al., 2011). A randomized, double-blind, placebo-controlled study suggested the efficacy, safety, and tolerability of vabicaserin in the treatment of acute schizophrenia (Shen et al., 2014), although few preclinical analyses of this compound are published. WAY163909 is chemically similar to vabicaserin with high affinity (Ki = 10.5 nM) and full efficacy (90% relative to 5-HT) at the 5-HT2C receptor. WAY163909 exhibits a lower affinity (Ki = 212 nM) and no efficacy at the 5-HT2A receptor and is a weak partial agonist at the 5-HT2B receptor (Dunlop et al., 2005). WAY163909 has been employed as a tool compound to test hypotheses related to the involvement of the 5-HT2C receptor in animal models of addiction, depression, impulsivity, and schizophrenia (Dunlop et al., 2006; Marquis et al., 2007; Rosenzweig-Lipson et al., 2007b; Navarra et al., 2008; Cunningham et al., 2011, 2013; Anastasio et al., 2013; Navailles et al., 2013b; Anastasio et al., 2014a). More recently, rational medicinal chemistry approaches have been employed to craft novel, highly selective 5-HT2C receptor agonists (Storer et al., 2014; Rouquet et al., 2015; Cheng et al., 2016). Some of these newer 5-HT2C receptor agonists have been made commercially available (e.g., PF-3246799 and PF-4479745 from Pfizer) (Storer et al., 2014), which will allow their increasing employment in in vitro and in vivo studies and provide greater breakthroughs in our understanding of 5-HT2C receptor biology.
The orthosteric site of a GPCR at which the endogenous agonist binds has been the traditional target for ligand discovery, but the chemical space for GPCR neuroprobes and therapeutics has greatly expanded with the discovery of allosteric ligands for many GPCR subfamilies. An allosteric modulator is a ligand that binds to a spatially distinct allosteric site and alters the receptor conformation to modulate its interaction with other ligands and/or signal transduction molecules [for reviews, see Conn et al. (2009) and Christopoulos et al. (2014)]. Allosteric sites are expected to exhibit higher sequence divergence across receptor subtypes relative to the highly conserved orthosteric domain (Kenakin, 2009; Kenakin and Miller, 2010). For example, a positive allosteric modulator (PAM) can increase the affinity and/or efficacy of the orthosteric ligand (Conn et al., 2009) and, thus, has the potential to improve its therapeutic index and diminish negative side effects. Such allosteric modulation is saturable (comes to a finite magnitude when the allosteric site is fully occupied) and probe-dependent (varies dependent on the orthosteric ligand) with the prospects for separate control of affinity and efficacy, making allosteric ligands intriguing therapeutic chemical targets (Kenakin, 2010). At present, allosteric modulators are defined operationally as positive (PAM), negative allosteric modulators, or neutral allosteric ligands, with the possibility of the additional property of allosteric agonism (agonist effects consequent to binding to allosteric sites; Christopoulos et al., 2014).
Adron Harris and colleagues were the first to identify the effects of the fatty acid amide oleamide to positively modulate 5-HT2C receptor–mediated activity in Xenopus oocytes (Huidobro-Toro et al., 1996a), and further analyses have identified oleamide as a member of a family of amphipathic lipid metabolites that allosterically promote 5-HT receptor signaling through other receptors (e.g., 5-HT2A receptor and 5-HT7 receptor; Thomas et al., 1997; Thomas et al., 1998; Alberts et al., 2001) but also exhibit a myriad of additional effects on receptor signaling (Leggett et al., 2004). In 2003, chemical library screening at Pharmacia (now Pfizer) resulted in the discovery and characterization of PNU-69176E as a PAM highly selective for the 5-HT2C receptor over the 5-HT2A receptor, 5-HT2B receptor, 5-HT7 receptor, and dopamine receptors (Im et al., 2003). In 2012, Zhou and colleagues optimized the synthetic route to generate PNU-69176E and its diastereomer (Ding et al., 2012). A series of new molecules based on the 4-alkylpiperidine-2-carboxamide scaffold were designed, synthesized, and pharmacologically evaluated as 5-HT2C receptor PAMs (Wild et al., 2019). Several analogs, potentiated 5-HT–evoked Cai2+ in 5-HT2C receptor CHO cells but not in 5-HT2A receptor CHO cells; one compound was further evaluated in vivo and exhibited a favorable overall pharmacokinetic and behavioral profile in rats (Wild et al., 2018). In addition, two predicted allosteric sites were identified by molecular docking to a 5-HT2C receptor homology model (Wild et al., 2018). Taken together, these data provide proof of concept that allosteric modulation of 5-HT2C receptor may be a viable strategy toward the discovery of novel neurotherapeutics. Recent preclinical indications of the efficacy of allosteric modulators in disease models, coupled with the launch of cinacalcet and maraviroc as the first marketed GPCR allosteric modulators, provide strong validation of the potential clinical utility of allosteric modulators (Conn et al., 2009). Ultimately, further analyses of novel allosteric modulators will improve understanding of 5-HT2C receptor function and how allosteric modulators may provide gain (or loss) of function in this system.
E. Signal Transduction
Intracellular signaling cascades stimulated by the 5-HT2C receptor have been assessed predominantly in recombinant cellular models and to a lesser extent within natural cellular environments (e.g., choroid plexus epithelial cells). Multiple G proteins (e.g., Gαq/11, Gα12/13, and Gαi/o) and activation of second messengers such as phospholipases, cyclic nucleotides, and ERK1/2 are essential mediators of 5-HT2C receptor actions in cells. By the late 1990s, structurally diverse 5-HT2C receptor agonists (Berg et al., 1994, 1998a; Moya et al., 2007) were noted to differentially activate intracellular signaling pathways, variably referred to as “agonist-directed trafficking of receptor stimulus,” biased agonism (signaling), stimulus trafficking, collateral efficacy, and functional selectivity (Berg and Clarke, 2009; Whalen et al., 2011; Kenakin and Christopoulos, 2013). Recent modeling studies suggest that 5-HT2C receptor ligands with fewer docking poses may stabilize a structural conformation contributory to a specific signaling pathway (Canal et al., 2011). Thus, distinct conformational states may differentially modulate the receptor interaction with immediate effectors (e.g., G proteins vs. β-arrestins), resulting in biased intracellular signal transduction patterns depending on the recruited effector (Gesty-Palmer et al., 2006; Whalen et al., 2011; Kenakin and Christopoulos, 2013). For example, G protein–dependent signaling pathways result in activation of specific downstream signaling effectors (e.g., pERK1/2), whereas β-arrestin2–dependent signaling can result in a different subset of downstream effectors as well as subcellular distribution of shared effectors (e.g., cytoplasmic vs. nuclear pERK1/2). These signaling profiles can then lead to distinct overall effects of GPCR activation (Gesty-Palmer et al., 2006). Targeting G protein– versus β-arrestin2–dependent mechanisms can allow for selectively inducing certain outcomes of receptor activation and perhaps not only reducing undesired side effects but also providing new therapeutic possibilities (Luttrell et al., 2015), a hypothesis that is supported for the 5-HT2C receptor system (Berg and Clarke, 2009) as well as for other GPCR signaling systems (Gesty-Palmer et al., 2006; Masri et al., 2008; Allen et al., 2011; Lovell et al., 2015; Marti-Solano et al., 2015).
1. Phospholipase C
The 5-HT2C receptor is characterized to stimulate phospholipase C (PLC) signaling through pharmacological analyses in heterologous expression systems (Westphal and Sanders-Bush, 1996; Briddon et al., 1998; Herrick-Davis et al., 1999; Rosendorff et al., 2000; Devlin et al., 2004), choroid plexus cells (Conn and Sanders-Bush, 1986b; Conn et al., 1986; Sanders-Bush and Conn, 1986), and corticostriatal regions of the brain (Wolf and Schutz, 1997). In this regard, the 5-HT2C receptor is largely thought to act through pertussis toxin–insensitive Gαq/11 proteins (Conn et al., 1986; Berg et al., 1994; Chang et al., 2000), although there is evidence that pertussis toxin–sensitive Gαi/o G proteins may couple the 5-HT2C receptor to PLC in Xenopus laevis oocytes (Chen et al., 1994) and HEK293 cells (Alberts et al., 1999). Thus, the canonical G protein–dependent signaling through the 5-HT2C receptor is engendered by 5-HT–stimulated coupling to Gαq/11 to activate the enzyme phospholipase Cβ(PLCβ), which generates the intracellular second messenger inositol-1,4,5-trisphosphate (IP3), accumulation of the downstream IP3 metabolite inositol monophosphate (IP1), and DAG. IP3 interacts with the IP3 receptor, leading to increased i Cai2+ into the cytoplasm; Cai2+ mobilization, measured with calcium-binding fluorescent dyes, and IP1 levels, assessed with [3H]-inositol, are well characterized to be elevated following activation of the 5-HT2C receptor [for reviews, see Raymond et al. (2001) and Millan et al. (2008)]. Utilizing cell-permeable small peptide disruptors mimicking the C terminal of Gαq, Sanders-Bush and colleagues demonstrated that a Gαq, but not a Gs, disruptor was able to block 5-HT2C receptor–mediated phosphoinositide hydrolysis in choroid plexus endothelial cells (Chang et al., 2000). Furthermore, a PLCβ1, but not a PLCβ2, peptide blocked 5-HT2C receptor activation, suggesting that 5-HT2C receptor–evoked phosphoinositide hydrolysis is mediated through a Gαq- and PLCβ1-dependent mechanism (Chang et al., 2000).
Hydrolysis of phosphoinositides generates the signaling lipid DAG, leading to activation of PKC and downstream stimulation of the MAPK cascade, resulting in phosphorylation of ERK1/2 (Werry et al., 2005). In fact, 5-HT2C receptor–transfected CHO cells were shown to couple ERK1/2 via a PLD- and PKC-dependent pathway likely through Gα12/13 proteins (Werry et al., 2005). The PLD and PKC involvement in ERK1/2 phosphorylation evoked by 5-HT2C receptor stimulation was recently validated in a hypothalamic cell line (mHypoA-2/10) derived from the periventricular nucleus of an adult male mouse (Lauffer et al., 2016). These further analyses indicated that the native 5-HT2C receptor activates the cellular transcription factor CREB via PKC-induced ERK1/2 activation in this cellular model (Lauffer et al., 2016).
Desensitization and resensitization processes regulate the functional activity of 5-HT2C receptor. Agonist-dependent desensitization is associated with 5-HT2C receptor phosphorylation involving G protein–coupled receptor kinase (GRK) 2 (Berg et al., 2001), binding of β-arrestins, and uncoupling of the receptor from the G protein to result in receptor internalization into endosomes; resensitization and recycling to the plasma membrane occurs with dephosphorylation (Marion et al., 2004; Schlag et al., 2004). The interaction of the 5-HT2C receptor with the C-terminal domain of PSD-95/Disc large/Zonula occludens (PDZ) domain–containing proteins (Bécamel et al., 2002, 2004; Anastasio et al., 2010; Anastasio et al., 2014b) is known to play an important role in 5-HT2C receptor desensitization/resensitization processes and trafficking (Gavarini et al., 2006). Intracellular Cai2+ release in mouse cortical neurons in primary culture is regulated by the PDZ proteins postsynaptic density 95 (PSD-95) and MAGUK p55 subfamily member 3 (MPP3) (Gavarini et al., 2006; Møller et al., 2013). Although PSD-95 and MPP3 do not modify the efficacy of 5-HT2C receptor signaling triggered by a single 5-HT exposure, PSD-95 increases signal desensitization and trafficking upon repeated agonist exposure, an effect that is blocked by a peptidyl mimetic of the 5-HT2C receptor C-terminus, which disrupts the interaction between the 5-HT2C receptor and PSD-95 (Gavarini et al., 2006). On the other hand, MPP3 stabilizes the 5-HT2C receptor at the plasma membrane and prevents desensitization of the 5-HT2C receptor–mediated Cai2+ release (Gavarini et al., 2006). This regulation correlates with surface expression of the receptor and indicates that 5-HT2C receptor signaling is highly regulated by PDZ proteins.
2. Phospholipase D
The 5-HT2C receptor also activates PLD, an enzyme that catalyzes the conversion of phosphatidylcholine to choline and phosphatidic acid; phosphatidic acid transduces most of PLD-evoked activity, whereas soluble choline diffuses into the cytosol, but has little second messenger activity [for reviews, see Frohman (2015) and Nelson and Frohman (2015)]. The activation of PLD can occur through the canonical receptor/G protein/effector signal transduction cascade via Gα12/13. In rat choroid plexus epithelial cells, 5-HT–evoked PLD activation occurs at levels similar to PLC activation; however, PLD activation is not downstream to G protein–linked PLC activation (McGrew et al., 2002). The 5-HT2C receptor antagonist SB206553 and a peptide targeting the Gα13, but not the Gαq, subunit blocked 5-HT–evoked PLD activation (McGrew et al., 2002). Inactivation of RhoA GTPase in NIH3T3 cells stably expressing the 5-HT2C receptor by the C3 exoenzyme from Clostridia botulinum blocks 5-HT2C receptor–evoked PLD, but not PLC, signaling (McGrew et al., 2002). Also, in a NIH3T3 cell line derived from Gαq/11-deficient mice, 5-HT2C receptor–dependent stress fiber formation is dependent on Gα13 and Rho signaling (Gohla et al., 1999). The PLD signaling was not seen in cells transfected with the 5-HT2C-VGV receptor; this highly edited isoform has a diminished ability to couple to Gα13 and is unable to promote Rho GTPase activity (McGrew et al., 2004). Together, these studies suggest that 5-HT2C receptor–mediated PLD signaling is dependent on Gα13 activation of Rho, a property of this receptor that is affected by pre-RNA editing.
3. Phospholipase A2
Stimulation of the 5-HT2C receptor is thought to activate cytosolic PLA2, which hydrolyzes arachidonic acid–containing phospholipids to produce free arachidonic acid and a host of its metabolites (for reviews, see Burke and Dennis, 2009a,b). In an early study, 5-HT was demonstrated to stimulate the release of arachidonic acid through activation of PLA2, but not phosphoinositide turnover, in hippocampal neurons cocultured with glial cells, but not in glial cultures alone; the studies supported the involvement of the 5-HT2 receptor subtype in the PLA2 activation (Felder et al., 1990). In 5-HT2C receptor–transfected CHO cells, 5-HT increases the release of arachidonic acid, an effect that is blocked by the PLA2 inhibitor mepacrine, which had no effect on 5-HT2C receptor–mediated phosphoinositide hydrolysis (Berg et al., 1996). The G protein effector is sensitive to pertussis toxin inactivation, but the exact G proteins involved are as of yet unknown (Felder et al., 1990). This research was extended to demonstrate that the relative efficacy of agonists is distinct for the PLA2–arachidonic acid versus PLC-phosphoinositide pathways. For example, DOI acts as a full agonist to stimulate arachidonic acid release (equivalent to 5-HT), whereas 3-trifluoromethylphenylpiperazine and d-LSD preferentially activate the PLC-phosphoinositide and PLA2–arachidonic acid pathways, respectively (Berg et al., 1998b). In addition, arachidonic acid release, though sensitive to pretreatment with 5-HT, is not as sensitive as phosphoinositide hydrolysis, suggesting that arachidonic acid release may be more difficult to demonstrate in vivo as a 5-HT2C receptor–mediated output when compared with phosphoinositide hydrolysis (Berg et al., 1998b). The involvement of arachidonic acid stimulation in 5-HT2 receptor signaling in vivo is supported by the observation that systemic administration of DOI resulted in increased incorporation of labeled arachidonic acid into brain membranes (Qu et al., 2005). The increases were seen in brain regions with the highest densities of 5-HT2A receptor (e.g., cerebral cortex) but not in the choroid plexus, which expresses the highest 5-HT2C receptor density (choroid plexus). Future studies are necessary to clarify the relative roles of the 5-HT2A receptor and 5-HT2C receptor in the control of arachidonic acid incorporation in vivo (Basselin et al., 2012).
4. Cyclic Nucleotides
Signaling through cyclic nucleotides, such as cAMP and cGMP, are also reported to be engaged by the 5-HT2C receptor. Activation of the 5-HT2C receptor inhibits forskolin-stimulated cAMP production in AV12 fibroblast cells that stably express the receptor at high density (∼12 pmol/mg membrane protein); at low 5-HT2C receptor density (∼150 fmol/mg of membrane protein), 5-HT couples to the PLC-IP pathway but evokes a stimulation rather than an inhibition of cAMP production (Lucaites et al., 1996). Upon pertussis toxin treatment, a modest 5-HT2C receptor stimulatory effect on cAMP accumulation as well as its potential dependence on the Gi/o family of G proteins was observed (Lucaites et al., 1996). In Xenopus oocytes transfected with the 5-HT2C receptor and G proteins, the 5-HT2C receptor was shown to couple to Go in addition to Gq (Quick et al., 1994). Because there is limited evidence that the 5-HT2C receptor links to cAMP in cells natively expressing the receptor (porcine choroid plexus) (Palacios et al., 1986), further analyses as to its biologic significance in vitro and in vivo are required.
The antimigraine medication dihydroergotamine, which inhibits [3H]-mesulergine binding to the 5-HT2C receptor and is a full 5-HT2C receptor agonist in porcine choroid plexus (Brown et al., 1991), elevates cGMP levels in LMTK− fibroblasts stably expressing the 5-HT2C receptor (Schaerlinger et al., 2003). 5-HT rapidly elevates cGMP production in the 5-HT2C receptor–enriched porcine choroid plexus tissue slices (Kaufman et al., 1995) with an efficacy similar to phosphatidyl inositol turnover (Conn et al., 1986); the potencies of antagonists that suppress 5-HT–mediated cGMP formation align with their affinity for the 5-HT2C receptor (Kaufman et al., 1995). The pertussis toxin–insensitive 5-HT2C receptor–mediated cGMP formation is dependent on calcium and PLA2-arachidonate release as well as lipoxygenase (Kaufman et al., 1995). Thus, the 5-HT2C receptor appears to exhibit efficacy to evoke cGMP formation in a native tissue in a PLA2-dependent manner (Kaufman et al., 1995) as well as regulate NMDA-mediated production of nitric oxide and elevation of cGMP (Marcoli et al., 1997).
5. Extracellular Signal-Regulated Kinases
The activation of the 5-HT2C receptor can diverge to influence several G protein–dependent downstream cascades and may also converge on others, such as members of the MAPK class, P42 and 44 (p44/p42-MAPK), also known as ERK1/2. In fact, there is evidence to suggest that phosphorylation of ERK1/2 is an important integrator of the multiple upstream signaling events for the 5-HT2C receptor. In CHO cells stably expressing the 5-HT2C receptor, 5-HT stimulated phosphorylation of ERK1/2, which is inhibited by the 5-HT2C receptor antagonist mianserin (Werry et al., 2005). Though PKC and PLD inhibitors suppress 5-HT–mediated phosphorylation of ERK1/2, PLC and PLA2 inhibitors are ineffective, suggesting the 5-HT2C receptor–mediated pERK1/2 is dependent on PKC and PLD signaling (Werry et al., 2005). Activation of ERK1/2 by 5-HT2C receptor ligands is not solely mediated by coupling of the 5-HT2C receptor to G proteins and can, in fact, be mediated by coupling to other protein transducers. The C terminus of the 5-HT2C receptor contains a calmodulin domain that is critical for β-arrestin recruitment and ERK1/2 signaling (Labasque et al., 2008). A calmodulin mutant prevented phosphorylation of ERK1/2 in cortical neurons and choroid plexus epithelial cells, suggesting that the calmodulin interaction is critical for G protein–independent signaling (Labasque et al., 2008). In HEK293 cells transiently expressing the 5-HT2C receptor, pERK1/2 levels were increased over nontransfected cells, suggesting the expression of constitutive activity of the 5-HT2C receptor (Labasque et al., 2008). This elevated basal pERK1/2 was inhibited by a 5-HT2C receptor inverse agonist toward PLC (Labasque et al., 2008), again suggesting that pERK1/2 activity is PLC-mediated. Interestingly, this activity was unaffected by depletion of Gα12 or Gα13 proteins, whereas in cells lacking β-arrestin or calmodulin, basal pERK1/2 levels were decreased, providing the first evidence of constitutive activity of a G protein–coupled receptor toward a G protein–independent, β-arrestin–dependent signaling mechanism (Labasque et al., 2008).
6. Ion Channels
The impact of 5-HT2C receptor on the function of ion channels has been addressed extensively in the choroid plexus, which controls the composition and secretion of cerebrospinal fluid, a function in which chloride (Cl−) and potassium (K+) channels play a key role (Millar et al., 2007). 5-HT acting through the 5-HT2C receptor has been noted to activate Cl− and inhibit K+ channels in mouse and rat choroid plexus epithelium (Hung et al., 1993; Speake et al., 2004), and the 5-HT2C receptor has been shown to inhibit K+ channels through a PKC-dependent pathway in rat choroid plexus epithelial cells (Speake et al., 2004). Activation of the 5-HT2C receptor leads to G protein–dependent PLC activation, IP3 and DAG production, and the release of Cai2+ from the endoplasmic reticulum, which triggers the opening of calcium-activated Cl− channels in Xenopus oocytes (Julius et al., 1988; Panicker et al., 1991; Woodward et al., 1992; DiMagno et al., 1996). The Gα and Gq subunits may be involved in the generation of this calcium-activated Cl− current, which also involves PLCβ production (Quick et al., 1994; DiMagno et al., 1996). Upon coexpression of the 5-HT2C receptor and a brain-derived K+ channel in Xenopus oocytes, 5-HT suppresses K+ conductance through a calcium/calmodulin-activated phosphatase thought to dephosphorylate the K+ channel in a kinase-dependent manner, evoking its closure (Hoger et al., 1991).
The 5-HT2C receptor also modulates the Kv1.5 channel through a PLC-dependent pathway in Xenopus oocytes (Panicker et al., 1991), which contrasts the observation that the 5-HT2C receptor inhibits K+ channels through a PKC-dependent pathway in rat choroid plexus epithelial cells (Speake et al., 2004). The 5-HT2C receptor also inhibits the GABAA receptor in Xenopus oocytes through a calcium-dependent, phosphorylation-independent mechanism (Huidobro-Toro et al., 1996b) and by suppressing an inwardly rectifying K+ current in striatal cholinergic interneurons and rat brain slices (Blomeley and Bracci, 2005; Blomeley and Bracci, 2009). The 5-HT2C receptor was Gαq-coupled to PLC activation and phosphoinositide hydrolysis to directly inhibit GIRK channels in POMC neurons of the arcuate (Qiu et al., 2007) as well as in lateral (but not basolateral) amygdala neurons (Yamamoto et al., 2014), which are known to stabilize resting membrane potential and therefore polarization of these neurons (Delmas and Brown, 2005). Hence, though stimulation of 5-HT2C receptor significantly modulates ion channel function, the involvement of specific signal transduction molecules in the processes is variable and underexplored at present.
F. The 5-HT2C Receptor as an Oncogene
5-HT is known to act as a mitogen to regulate the proliferation and differentiation of a variety of cells through its binding to several 5-HT receptors and control of downstream signaling (for review, see Fanburg and Lee, 1997). The NIH3T3 mouse fibroblast cells are often used to identify oncogenes (genes that can transform a cell to a tumor cell) because they display several well characterized phenotypes that are indicative of cellular transformation, including the formation of foci (regions of dense cell growth within an otherwise confluent monolayer) (Land et al., 1983). Stimulation of NIH3T3 cells transfected with the 5-HT2C receptor leads to the generation of transformed foci, maintenance of which requires the activation of the 5-HT2C receptor as a 5-HT2C receptor antagonist blocks foci formation (Julius et al., 1988; Julius et al., 1989). Upon injection of these cells into nude mice, tumors form, a finding that led to the initial conclusion that the 5-HT2C receptor is a proto-oncogene (Julius et al., 1989). The constitutive activity of the 5-HT2C receptor also stimulated cell division in the transfected NIH3T3 cells, and these data further suggested that multiple G proteins and signaling pathways were engaged in the cell division generated by agonist-evoked and constitutively active receptor signaling (Westphal and Sanders-Bush, 1996).
G. Clinical Relevance of the 5-HT2C Receptor
The 5-HT2C receptor is widely distributed throughout the basal ganglia, limbic system, and prefrontal cortex (Hoyer et al., 1986; Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990) and is well poised to mediate 5-HT–dependent appetite, cognition, mood, movement, and sleep, whereas dysfunctional 5-HT2C receptor signaling has been implicated in neuropsychiatric (e.g., addiction, anxiety, and depression) and neuropathological conditions (e.g., schizophrenia) as well as obesity and metabolic disorders (Berg et al., 2008a; Di Giovanni et al., 2010; Fig. 14). Therefore, the 5-HT2C receptor is a therapeutic target of great interest (for other reviews, see Cunningham and Anastasio, 2014; Howell and Cunningham, 2015; Sullivan et al., 2015; Di Giovanni and De Deurwaerdere, 2016; Fig. 12). The behavioral pharmacology of 5-HT2C receptor ligands as well as the clinical relevance of a dysfunctional 5-HT2C receptor system has recently been reviewed in detail (Cunningham and Anastasio, 2014; Howell and Cunningham, 2015; Sullivan et al., 2015; Di Giovanni and De Deurwaerdere, 2016), and an overview of clinical implications of the 5-HT2C receptor system is provided here.
Fig. 14.
Regional CNS localization 5-HT2C receptors and functional correlates. The full-length 5-HT2C receptor is localized exclusively in the central nervous system with good agreement between mRNA and protein distribution in the majority of brain regions. The postulated signaling components (shown in normal text), neurochemical and/or neurophysiological correlates (shown in italic text), and in vivo effects (shown in capital text) are illustrated.
1. Substance Use Disorders (Addiction)
The pharmacological and molecular mechanisms underlying the effects of abused drugs have been the subject of extensive study. As early work unfolded, a central concept emerged that the dopamine pathway projection from the VTA to the nucleus accumbens plays a mechanistic role in the rewarding and incentive-salience value of abused drugs (for reviews, see Koob and Volkow, 2010; Volkow et al., 2010). As the research progressed, the field began to recognize that the transition to problematic drug abuse and substance use disorder (addiction and dependence) involves an “expanding cycle of dysfunction” (Koob and Volkow, 2010), engaging multiple neurotransmitter substrates within limbic-corticostriatal circuitry, including 5-HT (for reviews, see Kalivas and Volkow, 2005; Koob and Volkow, 2010; Volkow et al., 2010; Cunningham and Anastasio, 2014; Muller and Homberg, 2015; Di Giovanni and De Deurwaerdere, 2016; Wolf, 2016). Much of the research has focused on abused drugs as rewarding substances and the evoked, long-lasting dysregulation of the dopamine mesoaccumbens pathway (for reviews, see Koob and Volkow, 2010; Volkow et al., 2010). However, a second focus has evolved toward identifying genotypic and phenotypic drivers of individual differences in vulnerability to substance use disorders and relapse, as drug abuse culminates in addiction in only a subset of users (SAMHSA, 2015). Impulsivity, a predisposition toward rapid unplanned reactions to stimuli without regard to the negative consequences, is one such phenotype that contributes to initial drug use and is perpetrated by continued use of the abused drug (for reviews, see Moeller et al., 2001a,b; Cunningham and Anastasio, 2014). The impact of impulsivity in psychostimulant addiction is best described with roles for 5-HT and dopamine prominently identified (for reviews, see Moeller et al., 2001a; Dalley and Roiser, 2012; Bari and Robbins, 2013; Cunningham and Anastasio, 2014; Logue and Gould, 2014). Cue reactivity, the sensitivity to cues previously linked with the drug-taking experience, is a second such phenotype that plays a prominent role in craving and relapse in humans (Carter and Tiffany, 1999; O’Brien et al., 1998; Drummond, 2001). The extended limbic-corticostriatal circuitry underlies both impulsivity and cue reactivity with multiple neurotransmitters involved (Childress et al., 1999; Bechara, 2005; Goldstein et al., 2007; Liu et al., 2012). Thus, addiction involves the generation of drug use within a background of vulnerability (e.g., propensity for impulsive behavior) and progresses with repeated drug exposure, neuronal plasticity, and the entrainment of addictive behaviors. In particular, the role of the 5-HT2C receptor in various aspects of these addictive processes has been well studied for the class of psychostimulants; several studies have reported the efficacy of 5-HT2C receptor agonists to suppress nicotine intake and nicotine seeking (Grottick et al., 2001; Levin et al., 2011; Higgins et al., 2012). Relevant to this are the extensive studies of the role of the 5-HT2C receptor in the rewarding and incentive-salience value of cocaine as well as factors involved in vulnerability to addiction and relapse, especially impulsivity and cue reactivity.
Cocaine is a psychomotor stimulant that inhibits 5-HT reuptake (Koe, 1976). Employing the self-administration assay, the preclinical model with the best validity for human drug taking, studies demonstrated that voluntary cocaine administration elevates 5-HT efflux in the nucleus accumbens (Parsons and Justice, 1993; Parsons et al., 1996; Howes et al., 2000). Depletion of forebrain 5-HT induces compulsive cocaine seeking, which is reversed by a 5-HT2C receptor antagonist (Pelloux et al., 2012). Constitutive knockout of the 5-HT2C receptor increases the motivation to take cocaine and enhances cocaine-induced elevation in dopamine in the nucleus accumbens (but not the dorsal striatum) of mice (Rocha et al., 2002). Pretreatment with a 5-HT2C receptor agonist, systemically or into the VTA, enhances, whereas systemic administration of a 5-HT2C receptor antagonist inhibits, the elevated dopamine efflux in the NAc evoked by nonresponse contingent cocaine administration (Navailles et al., 2004, 2008; Cathala et al., 2015). The 5-HT2C receptor control over dopamine function is thought to mediate, in large part, the efficacy of a selective 5-HT2C receptor agonist (e.g., RO60-0175 or WAY163909) to suppress the voluntary intake of cocaine (Grottick et al., 2000; Fletcher et al., 2002a; Fletcher et al., 2004; Neisewander and Acosta, 2007; Cunningham et al., 2011).
Stimulation of the 5-HT2C receptor also dose-dependently suppresses reinstatement induced by cocaine and cocaine-associated cues as measures of cue reactivity (Grottick et al., 2000; Neisewander and Acosta, 2007; Burbassi and Cervo, 2008; Fletcher et al., 2008; Cunningham et al., 2011; Swinford-Jackson et al., 2016). Conversely, systemic administration of 5-HT2C receptor antagonists have been shown to exert effects opposite to those following agonist administration, thus enhancing cocaine self-administration (Fletcher et al., 2002a) and cue reactivity (Fletcher et al., 2002a; Pelloux et al., 2012). In nonhuman primates, a 5-HT2C receptor agonist attenuated the stimulant, reinforcing, and reinstatement effects of cocaine, effects reversed by the selective 5-HT2C receptor antagonist SB242084 (Manvich et al., 2012a,b; Ruedi-Bettschen et al., 2015). Interestingly, SB242084 induced modest stimulant effects and exhibited reinforcing effects in primates (Manvich et al., 2012a,b) but had contrasting results (Ruedi-Bettschen et al., 2015).
The efficacy of 5-HT2C receptor agonists to suppress cue reactivity upon systemic administration is mirrored following intracranial microinjection of a 5-HT2C receptor agonist into the mPFC, which attenuates both cocaine- and cue-induced reinstatement (Pentkowski et al., 2010). These data highlight the importance of the 5-HT2C receptor in regulation of cortical substrates underlying cocaine-associated cue reactivity specifically, as 5-HT2C receptor agonist microinfusions did not alter cocaine intake (Pentkowski et al., 2010). Thus, the 5-HT2C receptor provides inhibitory tone over cocaine reward and cue reactivity as well as the neurochemical effects of cocaine (for reviews, see Cunningham and Anastasio, 2014; Howell and Cunningham, 2015; Di Giovanni and De Deurwaerdere, 2016), and mPFC-localized 5-HT2C receptors underlie, in part, the generation of cue reactivity.
Cocaine administered nonresponse contingently has been reported to result in 5-HT2C receptor neuroadaptations (Zayara et al., 2011; Craige et al., 2015) as well as regulation of the brain-specific snoRNA MBII-52, which is involved in the regulation of the 5-HT2C receptor pre-mRNA (Chen et al., 2014). Abstinent cocaine users exhibit lower sensitivity to the effects of a 5-HT2C receptor agonist (Lee and Meltzer, 1994; Buydens-Branchey et al., 1997; Patkar et al., 2006), whereas the highest cue reactivity was observed in those cocaine-dependent subjects carrying the C23S SNP in the 5-HT2C receptor gene (Anastasio et al., 2014a), which may be associated with diminished 5-HT2C receptor signal transduction (Lappalainen et al., 1995; Okada et al., 2004; Piva et al., 2011; Walstab et al., 2011). Further evidence that the functional status of the 5-HT2C receptor in the mPFC (Lopez-Gimenez et al., 2001; Liu et al., 2007; Nocjar et al., 2015) influences the incentive-motivational effects of cocaine and cocaine-associated cues has accumulated in rats (Anastasio et al., 2014a,b; Swinford-Jackson et al., 2016). Cunningham and colleagues found that the highest levels of cocaine cue reactivity correlated with the lowest levels of mPFC 5-HT2C receptor protein and a blunted sensitivity to the suppressive effects of the selective 5-HT2C receptor agonist WAY163909 (Anastasio et al., 2014a; Swinford-Jackson et al., 2016). The efficacy of WAY163909 to suppress high levels of cue reactivity associated with extended forced abstinence (“incubation”) was also reduced at a time point (30 days) at which lower synaptosomal expression of 5-HT2C receptor protein was observed in the mPFC (Swinford-Jackson et al., 2016), a key site involved in incubation phenomena (Koya et al., 2009; Whitfield et al., 2011; Ma et al., 2014). Furthermore, a greater proportion of the expressed 5-HT2C receptor protein was sequestered in the cytoplasmic (vs. membrane) compartment of the mPFC at prolonged versus early forced abstinence, and there was an inverse correlation of the membrane to cytoplasmic 5-HT2C receptor ratio in the mPFC with levels of cocaine cue reactivity. Collectively, these outcomes indicate that the functional status of the 5-HT2C receptor system in the mPFC is a key contributor to cocaine cue reactivity and its incubation.
Cocaine-dependent subjects who express high cocaine cue reactivity express high impulsivity (Liu et al., 2011b), and a similar relationship has been observed in cigarette smokers (Doran et al., 2007, 2008). In outbred rats, lower mPFC 5-HT2C receptor membrane protein levels and an increase in edited 5-HT2C receptor mRNA variants with reduced 5-HT2C receptor signaling capacity distinguish high impulsive rats from low impulsive rats (Anastasio et al., 2014b) as well high and low responders to novelty, another model of addiction vulnerability (Dracheva et al., 2009). The virally mediated knockdown of the 5-HT2C receptor localized to the mPFC also results in elevated impulsivity and cue reactivity relative to controls (Anastasio et al., 2014b), suggesting that reduced 5-HT2C receptor tone in the mPFC confers vulnerability to these interlocked behaviors (Anastasio et al., 2014b). The status of 5-HT2C receptor function in the orbitofrontal cortex may also be a contributor to the vulnerability of impulsive rats to cocaine reward and cue reactivity (Besson et al., 2013). Together, these data suggest that the functional status of the cortical 5-HT2C receptor system may be a mechanistic driver in the generation of cocaine use disorder and relapse phenomena.
The body of knowledge in support of a role of the 5-HT2C receptor in regulating the rewarding properties and voluntary intake of other abused substances is less well developed than that of cocaine and nicotine. However, stimulation of the 5-HT2C receptor has been noted to suppress ethanol self-administration (Maurel et al., 1999; Tomkins et al., 2002; Kasper et al., 2013; Rezvani et al., 2014) and reinstatement in rodents (Kasper et al., 2013). Exposure to ethanol vapor for several days is associated with increased expression of the 5-HT2C receptor transcript in several corticostriatal and hypothalamic nodes (Yoshimoto et al., 2012), higher levels of the 5-HT2C receptor protein in the NAc (Yoshimoto et al., 2012), and enhanced pre-mRNA editing of the 5-HT2C receptor (Watanabe et al., 2014), suggesting that neuroadaptations in the 5-HT2C receptor function are mechanistically involved in ethanol preference behavior.
Pretreatment with 5-HT2C receptor ligands have been shown to impact various behavioral sequela associated with d-amphetamine (O’Neill et al., 1999; Rippberger et al., 2015; Wöhr et al., 2015), MDMA (Bankson and Cunningham, 2002; Fletcher et al., 2002b), methamphetamine (Steed et al., 2011; Graves and Napier, 2012), and the marijuana alkaloid Δ9-THC (Ji et al., 2006), suggesting that rich prospects to explore the potential therapeutic value of selective 5-HT2C receptor agonists in addictive processes engaged by these abused drugs remain.
5-HT is involved in the pharmacology of opioid abused drugs (including heroin and prescription opioids) (Tao and Auerbach, 1994; Tao et al., 1998; Singh et al., 2003), and systemic administration of a 5-HT releaser (e.g., dexfenfluramine) was shown to suppress heroin self-administration in rats (Wang et al., 1995). Pretreatment with a selective 5-HT2C receptor agonist also reduced opioid-induced behavioral sensitization (Wu et al., 2015a; Zhang et al., 2016). Recent studies have further demonstrated that lorcaserin significantly decreases the reinforcing effects of oxycodone and shifts the oxycodone dose-effect curve downward at doses that do not alter motor activity (Neelakantan et al., 2017). Thus, 5-HT2C receptor agonists may prove therapeutically useful to promote recovery and extend abstinence from several classes of abused drugs.
2. Appetite, Satiety, and Obesity
Hunger is the physiologic need for food. Appetite (the desire for food), satiation (the end of the desire for food during a meal), and satiety (the feeling of “fullness” that prevents further eating before the return of hunger) include both internal (e.g., glucose homeostasis) and conditioned factors (e.g., hedonics) (Blundell, 1999). 5-HT in the CNS has long been implicated in the control of the processes involved in satiation and satiety (Lucki, 1998; Halford and Blundell, 2000; Saper et al., 2002; Voigt and Fink, 2015). The 5-HT releaser d-fenfluramine, employed clinically for weight loss (until withdrawn in 1997), its metabolite d-norfenfluramine, and preferential 5-HT2C receptor agonists (e.g., mCPP) evoke hypophagia in rodents, which is associated with increased satiety, an effect blocked by 5-HT2C receptor antagonists or constitutive knockdown of the 5-HT2C receptor (Kennett and Curzon, 1988; Tecott et al., 1995; Halford et al., 1997; Vickers et al., 1999; Dalton et al., 2006; Nonogaki et al., 2008). Selective 5-HT2C receptor agonists (e.g., lorcaserin, RO60-0175) have been consistently demonstrated to suppress food intake (Clifton et al., 2000; Somerville et al., 2007; Thomsen et al., 2008; Grottick et al., 2015; Higgs et al., 2015; for review, see Higgins et al., 2020). In fact, the selective 5-HT2C receptor agonist WAY163909 dose-dependently decreases food intake in normal Sprague-Dawley rats, obese Zuker rats, and mice with diet-induced obesity (Dunlop et al., 2005) without the anxiogenic profile of mCPP (Dunlop et al., 2006). Tecott et al. (1995) reported that the constitutive 5-HT2C receptor knockout mouse exhibited hypophagia and increased body mass in the context of both insulin resistance and late-onset obesity (Nonogaki et al., 2008), whereas weight gain as well as a greater relative risk of metabolic dysfunction and diabetes develops with the chronic treatment of atypical antipsychotics with 5-HT2C receptor antagonist properties (e.g., olanzapine) in humans and animals (Wirshing et al., 1999; Kirk et al., 2009). Interestingly, a selective 5-HT2C receptor antagonist has been reported to variably increase (Bonhaus et al., 1997) or decrease food intake depending on the preclinical model employed (Kennett et al., 1997; Murotani et al., 2011).
The behavioral satiety sequence (BSS) describes the orderly process through which eating transitions to other behaviors (e.g., grooming and resting) and is a well validated model for analyzing satiation (meal termination) and satiety (postingestive inhibition of food intake) in rodents and humans (for review, see Rodgers et al., 2010). Consistent with the proposed role of 5-HT to promote satiety, d-fenfluramine, its metabolite analogs, and preferential and selective 5-HT2C receptor agonists (Halford et al., 1998; Clifton et al., 2000; Hewitt et al., 2002; Dalton et al., 2006; Somerville et al., 2007) accelerate the BSS without disruption of its integrity (for review, see Rodgers et al., 2010). For example, d-fenfluramine and RO60-0175 reduced the rate of feeding and meal size as well as increased the latency to feed consistent with enhanced satiety (Clifton et al., 2000). The effects of d-fenfluramine on the BSS was markedly reduced in mice constitutively lacking the 5-HT2C receptor (Vickers et al., 1999). In contrast, appetite-enhancing drugs (e.g., cyproheptadine) (Chinuck et al., 2007) disrupt the satiety sequence (Bergen, 1964; Ishii et al., 2003). A recent microstructural analysis of ingestive behavior found that lorcaserin reduced the number of bouts of licking behavior (Higgs et al., 2016) indicative of the promotion of satiety (Davis et al., 2001). Thus, the 5-HT2C receptor is an important mediator of food intake through the control of satiety mechanisms (for reviews, see Lucki, 1998; Halford and Blundell, 2000; Voigt and Fink, 2015).
Investigations of 5-HT involvement in the mechanisms underlying satiety have focused predominantly on neural loci in the hypothalamus and midbrain/hindbrain circuits, which synchronize energy balance and glucose homeostasis in concert with peripheral systems (for reviews, see Saper et al., 2002; Gautron et al., 2015; Voigt and Fink, 2015). The 5-HT neurons in the dorsal and median raphe innervate multiple hypothalamic subnuclei (van de Kar and Lorens, 1979; Peyron et al., 1998), which richly express 5-HT2C receptor mRNA and protein (Hoffman and Mezey, 1989; Molineaux et al., 1989; Mengod et al., 1990). A subpopulation of arcuate POMC neurons express the 5-HT2C receptor and are activated by d-fenfluramine and mCPP (Heisler et al., 2002; Lam et al., 2008). Elmquist and colleagues elegantly demonstrated that the activation of the 5-HT2C receptor localized to POMC neurons stimulates POMC synthesis and its cleavage into α-melanocyte–stimulating hormone, which acts on melanocortin 4 receptors in the paraventricular nucleus of the hypothalamus to promote satiety, weight loss, and glucose regulation (Heisler et al., 2007a; Zhou et al., 2007; Xu et al., 2008; Berglund et al., 2013). Stimulation of the 5-HT2C receptor following application of mCPP depolarized a subpopulation of POMC neurons potentially via PLC-dependent activation of transient receptor potential channels, independent of GIRK channel activity (Sohn et al., 2011). In mice that selectively lack the 5-HT2C receptor in POMC neurons, body weight was normal; however, these mice were insensitive to d-fenfluramine– or mCPP-evoked hypophagia and developed metabolic dysfunction, including hyperinsulinemia, hyperglucagonemia, hyperglycemia, and insulin resistance (Berglund et al., 2013). Rescue of 5-HT2C receptor in POMC neurons of 5-HT2C receptor–null mice normalized food intake, adiposity, and body weight as well as the anorexigenic effects of d-fenfluramine and mCPP (Xu et al., 2008). Interestingly, POMC expression within 5-HT2C receptor–expressing neurons in the arcuate regulates whole body energy balance, body weight, and adiposity in male, but not female, mice; these authors proposed that this molecular mechanism may explain, in part, sex differences in the prevalence of obesity (Burke et al., 2016). This provocative idea requires further investigation; however, the observations corroborate the conclusion that the 5-HT2C receptor in POMC neurons is a required component of the neural mechanisms that control energy and glucose homeostasis.
The adipocyte-derived ob gene product leptin (Halaas et al., 1995) acts via the leptin receptor (Tartaglia et al., 1995) to regulate long-term energy stores and homeostasis (Friedman, 2014). This landmark discovery marshalled in an era of advanced understanding of the CNS circuits involved in obesity [for review, see Friedman (2014)]. It was not long before genetic deletion of the leptin receptor in neurons was shown to result in hyperphagia and obesity (Cohen et al., 2001), whereas the rescue of central leptin receptor signaling corrects the obesity and diabetes phenotype of db/db mice, which possess mutations in the leptin receptor (de Luca et al., 2005). Mice deficient for both leptin and the 5-HT2C receptor exhibited a synergistic disruption of glucose homeostasis and a profound diabetes phenotype (Wade et al., 2008). On the other hand, transgenic mice that overexpress leptin but lack the 5-HT2C receptor exhibited a lean phenotype on a chow diet; however, on a high-fat diet, these mice developed diet-induced obesity (Wang and Chehab, 2006). In POMC neurons, pharmacological stimulation of the 5-HT2C receptor was shown to activate the same POMC neurons activated by leptin (Qiu et al., 2010). Lastly, coadministration of the preferential 5-HT2C receptor agonist mCPP plus leptin was recently shown to have an additive effect on reducing body weight in diet-induced obese mice (Yan et al., 2015). The growing knowledge of the interface between leptin and 5-HT regulatory systems prompted Halford and Blundell to propose that these systems function independently, but coordinate within the hypothalamus, to control satiety and energy reserves (Halford and Blundell, 2000).
The drive to eat palatable foods, composed essentially of high fat and/or sugars, involves hedonic mechanisms, which are also important for maintaining the homeostatic nutritional requirements for energy balance [for reviews, see Saper et al. (2002) and Volkow et al. (2012)]. These foods activate limbic-corticostriatal systems, which mediate reward and motivation [for reviews, see Gautron et al. (2015) and Voigt and Fink (2015)], and hedonic eating has been identified is a contributor to the obesity epidemic (Saper et al., 2002; Volkow et al., 2012). Preclinical analysis of the rewarding (reinforcing) and motivational effects of food self-administration can be evaluated on a fixed ratio and a progressive ratio (PR) schedule, respectively, in either freely fed or food-restricted animals. Performance under PR schedules is thought to reflect the motivational “efficacy” of the food given that deprivation level as well as reinforcer magnitude can increase breakpoints on the PR schedule (Hodos and Kalman, 1963). The preferential (mCPP, MK212) and selective 5-HT2C receptor agonists (R0 60-0175) decrease the breakpoint for a grain reinforcer on a PR schedule in food-restricted pigeons (Wolff and Leander, 2000). WAY163909 dose-dependently reduces self-administration of sucrose on a fixed ratio schedule in freely fed rats, an effect blocked by pretreatment with SB242084 (Cunningham et al., 2011), whereas mCPP decreases the breakpoint for Ensure in freely fed mice (Ward et al., 2008). Lorcaserin efficaciously suppresses the rewarding effects of food in food-restricted rats based on its 5-HT2C receptor agonist actions [Higgins et al., 2012; for review, see Higgins et al. (2020)]. Interestingly, though 5-HT2C receptor agonists effectively suppress cocaine seeking [for reviews, see Cunningham and Anastasio (2014) and Howell and Cunningham (2015)], WAY163909 failed to affect sucrose seeking (Cunningham et al., 2011), suggesting that the 5-HT2C receptor differentially regulates the incentive-salience value of cocaine- versus sucrose-associated cues. Thus, there is evidence that the 5-HT2C receptor system controls the hedonic, rewarding aspects of palatable food; the brain locus of action for the 5-HT2C receptor to control these behaviors requires further evaluation (Pratt et al., 2009; Pratt et al., 2012; Clissold et al., 2013).
Multiple lines of investigation suggest a relationship between 5-HT2C receptor SNPs and obesity, antipsychotic-induced weight gain, and transcriptional activity of the HTR2C gene. Eight HTR2C gene polymorphisms have been reported in the literature: three polymorphisms, as well as a GT nucleotide repeat variation, have been identified in the promoter (Xie et al., 1996); three polymorphisms have been reported within intronic regions (Gibson et al., 2004); one polymorphism has been reported in the coding region, resulting in the replacement of cysteine with serine at amino acid 23 (C23S) in the amino-terminus of the receptor (Lappalainen et al., 1995); and there is one polymorphism in the 3′ untranslated region (Song et al., 1999).
The C23S SNP occurs with a frequency of approximately 10%–15% in the human population (Lappalainen et al., 1995). However, there is no evidence for an association of this SNP with obesity (Lentes et al., 1997; Gibson et al., 2004). The promoter haplotype −995A/−759T/−697C has been reported to be associated with obesity in a Japanese population (Yuan et al., 2000) and the −759C allele to be more common in obese than nonobese Caucasian women (Pooley et al., 2004). Several studies suggest that the −759T allele may be associated with less weight gain following antipsychotic drug treatment (Reynolds et al., 2003; Miller, 2005; Templeman et al., 2005). Although additional research has reported no association between antipsychotic-induced weight gain (AIWG) and HTR2C promoter polymorphisms (Basile et al., 2002; Tsai et al., 2002; Theisen et al., 2004; Templeman et al., 2005), others have reported an association of the −759C allele with AIWG (Wallace et al., 2011). It is interesting to note that greater promoter activity, resulting in increased HTR2C transcription, has been reported to be associated with the −759T allele (Yuan et al., 2000). Also, in a luciferase-based reporter assay, HTR2C promoter haplotypes containing the −759C allele showed lower transcriptional activity than those containing the −759T haplotype (Buckland et al., 2005). Although these studies suggest that the −759C/T polymorphism may regulate gene transcription in vitro, a subsequent study reported that 5-HT2C receptor mRNA levels in the frontal cortex of 43 subjects are unaffected by −759C/T status (Pooley et al., 2004).
3. Schizophrenia
Both selective 5-HT2C receptor agonists and 5-HT2C receptor antagonists have been suggested for the treatment of schizophrenia. Antipsychotic medications with a profile as a 5-HT2C receptor antagonist seem to be effective to suppress positive symptoms, whereas 5-HT2C receptor agonists appear useful for inhibition of the negative symptoms and cognitive impairments in schizophrenia (Wood et al., 2001; Rosenzweig-Lipson et al., 2007a, 2012) with fewer motor side effects (Di Giovanni et al., 2006; Di Giovanni and De Deurwaerdere, 2016). The therapeutic potential of a specific antipsychotic would depend indirectly on the opposite modulation that these receptors exert on dopaminergic systems and the preferential 5-HT2C receptor modulation of the mesocortical and limbic system versus the nigrostriatal system (Di Giovanni et al., 2006; Di Giovanni and De Deurwaerdere, 2016). Moreover, the analysis of the pharmacological profile of some atypical antipsychotics led to 5-HT2C receptor blockade as a valuable strategy to improve the efficacy of dopamine antagonists in long-term treatments (Meltzer, 1999). Vabicaserin is a novel antipsychotic and anorectic agent with high agonist efficacy at the 5-HT2C receptor (Dunlop et al., 2011) and has been shown to be effective in treating schizophrenia, improving positive symptoms (Shen et al., 2014). Unfortunately, the clinical development of vabicaserin by Pfizer was terminated because the drug failed to meet the primary efficacy end point in clinical trials (https://clinicaltrials.gov/ct2/show/results/NCT00563706?term=vabicaserin&rank=2).
Sertindole, a newer antipsychotic (Juruena et al., 2011), alternatively exerts a potent inverse agonist activity at the 5-HT2C receptor (Herrick-Davis et al., 2000), along with dopamine D2, α1-adrenergic receptor and 5-HT2A receptor blockade (Hietala et al., 2001). Sertindole is effective in reducing anxiety and improving cognition/memory and brain plasticity, most probably by reducing 5-HT2C receptor tonic activation (Hietala et al., 2001).
Several lines of evidence have identified the 5-HT2C receptor in the origin of some side effects, both motor and metabolic, associated with the chronic use of antipsychotic drugs. For instance, 5-HT2C receptor blockade seems to contribute to AIWG, one of the most common and debilitating side effects induced by chronic treatment with these medications (Reynolds et al., 2005; Shams and Muller, 2014). Therefore, 5-HT2C receptor agonists may have an antipsychotic activity without inducing AIWG and alterations of glucose homeostasis caused by atypical antipsychotics. However, agomelatine as a 5-HT2C receptor antagonist has a more favorable profile and does not influence body weight in depressed patients (Pompili et al., 2013).
Other important side effects induced by the chronic use of antipsychotics that may be related to the activation of the 5-HT2C receptor are the movement disorders, such as dystonia, acute Parkinsonism, and tardive dyskinesia, referred to globally as antipsychotic-induced extrapyramidal side effects (EPS) (Tarsy and Baldessarini, 1984; Tarsy et al., 2002; Janno et al., 2004). The inverse agonism at the 5-HT2C receptor evoked by some atypical antipsychotics might indeed explain their fewer EPS effects (Herrick-Davis et al., 2000). Consistently, ritanserin, a nonselective 5-HT2 antagonist, limits the occurrence of EPS in patients treated with typical neuroleptic drugs (Bersani et al., 1990). Moreover, the affinity of typical antipsychotics toward the 5-HT2C receptor inversely correlates to the EPS severity (Richtand et al., 2007; Richtand et al., 2008). Nevertheless, experimental evidence suggests that concurrent 5-HT2C receptor agonism might increase the efficacy of typical and atypical antipsychotics, allowing dose-sparing with a reduction of side effects (Grauer et al., 2009).
4. Mood Disorders and Anxiety
Depression and anxiety are complex illnesses that have in common an altered central 5-HT tone. Compelling evidence accumulating over more than four decades has indicated the 5-HT2C receptor is critically involved in the serotonergic regulation of these pathologic states. Indeed, mCPP and MK-212 induce anxiogenic-like behaviors in animals (Kennett et al., 1989; Benjamin et al., 1990; Shepherd et al., 1994; Bilkei-Gorzo et al., 1998; Millan, 2005; Martin et al., 2013) and induce anxiety and panic when administered in humans (Lowy and Meltzer, 1988; Kahn and Wetzler, 1991; Sevy et al., 1994; Southwick et al., 1997; Gatch, 2003). These anxiogenic effects are likely due to the activation of the 5-HT2C receptor. In agreement, Tecott and colleagues showed that 5-HT2C receptor knockout mice exhibit an anxiolytic-like phenotype not attributable to locomotor alterations (Heisler et al., 2007b). Moreover, desensitization of the 5-HT2C receptor in SERT knockout mice has been shown to contribute to the moderation of the anxiety phenotype (Martin et al., 2014b5) and to the antidepressant effects (Prisco and Esposito, 1995; Di Giovanni et al., 2006). On the other hand, mCPP induced anxiolytic effects in mice (Nic Dhonnchadha et al., 2003) but showed antidepressant-like properties in the anhedonia model in rats (Moreau et al., 1996) and was recently observed to be anorexigenic without inducing anxiety/depression in humans (Thomas et al., 2014). Moreover, RO60-0175 presented an antidepressant profile or an anxiolytic/anticompulsive profile in some tests (Cryan and Lucki, 2000; Nic Dhonnchadha et al., 2003). Interestingly, anxiogenic responses induced by RO60-0175 (Martin et al., 2013, 2014a) are sometimes related to its sedative properties (Kennett et al., 2000). CP809101 is ineffective in some models (Siuciak et al., 2007) and anxiogenic in others (Strong et al., 2009, 2011; Christianson et al., 2010).
Consistent with proposed anxiogenic effects of 5-HT2C receptor activation, 5-HT2C receptor antagonists display anxiolytic/antidepressant properties in numerous tests (Kennett et al., 1994, 1996, 1997; Wood et al., 2001; Millan, 2005; Harada et al., 2006). Interestingly, there are models in which both 5-HT2C receptor agonists and antagonists display anxiolytic/antidepressant properties, including the chronic mild stress–induced anhedonia model and the activity consequent to olfactory bulbectomy. The tendency is that selective 5-HT2C receptor agonists would be more appropriate in the treatment of depression, obsessive-compulsive disorder (OCD), or panic attacks, whereas the antagonists would be better suited for generalized anxiety and obsessive-compulsive disorder (Jenck et al., 1998; Millan, 2003, 2005). On the contrary, the atypical antidepressants mirtazapine and mianserin (Hayasaka et al., 2015) and a recently developed new antidepressant agomelatine (Millan et al., 2003, 2011; Millan, 2005) have clear antagonistic 5-HT2C receptor profiles. The lack of selectivity of the pharmacological 5-HT2C receptor tools employed to date as well as the limited appreciation of 5-HT2C receptor function and its unique molecular mechanisms (e.g., edited and spliced 5-HT2C receptor isoforms, constitutive activity, and biased signaling) complicate final conclusions at present.
The paradoxical efficacy of both 5-HT2C receptor agonists and antagonists is likely due to the complex neurobiological basis of depression/anxiety and the fact that different behavioral responses involve different areas expressing the 5-HT2C receptor, of which activation produces opposite effects (Millan et al., 2005). Indeed, local activation of the 5-HT2C receptor in the basolateral part of the amygdala induces anxiety (Campbell and Merchant, 2003), whereas the activation of those in the dorsal periaqueducal gray triggers anxiolytic responses (Yamashita et al., 2011). Thus, the long-term antidepressant effect of compounds such as SSRIs may be related to a region-dependent desensitization of the 5-HT2C receptor in determined regions (Prisco and Esposito, 1995; Di Giovanni et al., 2006; Martin et al., 2014a).
In summary, selective 5-HT2C receptor antagonists may present promising molecules for developing new antidepressant/anxiolytic drugs. These could be considered as monotherapy or for augmentation strategies to improve other antidepressant responses (Cremers et al., 2004, 2007) with the ability to reverse several SSRI-induced side effects.
5. Epilepsy
Early evidence implicated a 5-HT involvement in the pathophysiology of epilepsy (Bonnycastle et al., 1957). Since then, evidence has accumulated clearly showing that there is a direct link between 5-HT levels and epilepsy. Increasing 5-HT levels in the CNS is generally antiepileptic, whereas a decrease favors epileptogenesis and seizure generation [for reviews, see Bagdy et al. (2007) and Guiard and Di Giovanni (2015)]. Moreover, a common 5-HT dysfunction might underlie both epilepsy and comorbid depression seen in patients with epilepsy (Kanner et al., 2012; Guiard and Di Giovanni, 2015).
The 5-HT2C receptor is thought to be involved in seizure generation and cell excitability (Jakus et al., 2003; Isaac, 2005). Tecott and colleagues showed that 5-HT2C receptor knockout mice display spontaneous convulsive generalized seizures, which cause their high mortality rate (Tecott et al., 1995) and a reduced threshold for various convulsing stimuli (Applegate and Tecott, 1998; Heisler et al., 1998b). Conversely, 5-HT2C receptor activation increased the threshold of general convulsion induced by pentylenetetrazole and electroshock in mice (Upton et al., 1998). The 5-HT2C receptor also negatively controls nonconvulsive generalized seizures. Di Giovanni and colleagues showed that, in the polygenic animal model of absence epilepsy, the Genetic Absence Epilepsy Rat from Strasbourg (Danober et al., 1998), RO60-0175 (unpublished data), lorcaserin, and CP809101 were capable of blocking spike and wave discharges (Venzi et al., 2016). Interestingly, as expected, SB242084 blocked the effect of lorcaserin and CP809101 but also showed some antiabsence effects. One possible mechanism by which 5-HT2C receptor activation exerts antiepileptic effects is via the normalization of the aberrant GABAA receptor tonic inhibition in the ventrobasal thalamus seen in different animal models of absence epilepsy (Cope et al., 2009; Crunelli and Di Giovanni, 2014, 2015). Findings with the Genetic Absence Epilepsy Rat from Strasbourg animal model of absence epilepsy are in agreement with those obtained in another model of absence epilepsy, the Wistar Albino Glaxo/Rij-rat, in which mCPP decreases the cumulative duration of spike and wave discharges via the activation of the 5-HT2C receptor (Jakus et al., 2003; Jakus and Bagdy, 2011).
The 5-HT2C receptor system seems devoid of any modulatory role in partial seizures or, paradoxically, has a proepileptic role in this type of epilepsy. Indeed, observations from Di Giovanni’s group show that mCPP and lorcaserin, but not RO60-0175, were able to halt hippocampal after discharges in a rat model of temporal lobe epilepsy, an effect potentiated and insensitive to SB242084 pretreatment (Orban et al., 2014). These data indicate that other 5-HT receptors are involved in the antiepileptic effect of mCPP and lorcaserin, probably the 5-HT1A/7 receptor (Orban et al., 2013) or unknown targets, confirming previous findings (Damjanoska et al., 2003; Navailles et al., 2013a; Orban et al., 2014).
In summary, 5-HT2C receptor agonists may have new therapeutic utility in epilepsy. In particular, the FDA-approved lorcaserin may be useful for the treatment of human generalized convulsive and nonconvulsive epilepsy, which is very important in consideration of the fact that the epileptic drug pipeline is limited. Moreover, activation of the 5-HT2C receptor may also be useful for treating comorbid neuropsychiatric comorbidity commonly seen in patients with epilepsy (Di Giovanni and De Deurwaerdere, 2016; Venzi et al., 2016).
6. Sleep
A role for 5-HT in the sleep-wake cycle is well documented, as 5-HT has been shown to promote wakefulness and reduce REM sleep [for review, see Monti (2011)]. In general, nonselective 5-HT2A/2C receptor agonists and selective 5-HT2C receptor agonists increase wakefulness and decrease SW and/or REM sleep following systemic administration in rats, whereas nonselective 5-HT2A/2C receptor antagonists and selective 5-HT2C receptor antagonists tend to decrease wakefulness and promote SW with reduced REM sleep [for review, see Monti (2011)]. In contrast to what may be anticipated, 5-HT2C receptor knockout mice display increased wakefulness with reduced SW and non-REM sleep (Frank et al., 2002). The increased wakefulness and reduced SW sleep have been attributed to compensatory mechanisms, possibly including the enhanced dopaminergic and adrenergic neurotransmission that occurs as a result of the constitutive knockout of the 5-HT2C receptor (Frank et al., 2002).
The nonselective 5-HT2C/2A receptor antagonists ritanserin and ketanserin enhanced SW sleep in a dose-dependent manner in human subjects with normal sleep patterns (Sharpley et al., 1990; Idzikowski et al., 1991). Similar results were observed following treatment with seganserin, ICI169369, and SR46349B (Dijk et al., 1989; Landolt et al., 1999). In addition, ritanserin has been reported to improve SW sleep in subjects with insomnia (Adam and Oswald, 1989), major depression (Staner et al., 1992), and generalized anxiety disorder (da Roza Davis et al., 1992). In patients with schizophrenia, typical and atypical antipsychotic drugs with nonselective 5-HT2C/2A receptor antagonist properties tend to increase total sleep time and efficiency but reduce the latency and duration of REM sleep (Taylor et al., 1991; Nofzinger et al., 1993; Wetter et al., 1996; Sharpley et al., 2000; Muller et al., 2004). Taken together, these results indicate an involvement of the 5-HT2C receptor in the maintenance of normal sleep architecture and suggest possible avenues for future research and medication development for the treatment of insomnia.
7. Clinical Impact of RNA Editing of the 5-HT2C Receptor
Many studies have attempted to analyze 5-HT2C receptor RNA editing profiles in postmortem samples from patients with psychiatric disorders. The majority of these reported that 5-HT2C receptor RNA editing in postmortem prefrontal cortex samples from patients with schizophrenia or bipolar disorder is not altered (Niswender et al., 2001; Dracheva et al., 2003, 2008b; Iwamoto and Kato, 2003; Zhu et al., 2012). In contrast, mixed results were obtained from postmortem prefrontal cortex samples from patients with major depression, with some studies reporting no change (Niswender et al., 2001; Zhu et al., 2012; Lyddon et al., 2013) and other studies reporting a decrease (Gurevich et al., 2002b) or increase in 5-HT2C receptor RNA editing (Iwamoto and Kato, 2003). Several studies have reported that psychiatric patients who committed suicide had increased 5-HT2C receptor RNA editing with increased levels of the less-active 5-HT2C receptor isoforms (Niswender et al., 2001; Gurevich et al., 2002b; Iwamoto and Kato, 2003; Dracheva et al., 2008b; Lyddon et al., 2013; Di Narzo et al., 2014) as well as increased expression of ADAR1 (Simmons et al., 2010). However, there were notable differences in the patterns of 5-HT2C receptor RNA editing that were reported in these studies. In a similar fashion, there is great variation in the reported effects of antipsychotic and antidepressant medications on RNA editing (Sodhi et al., 2005; Abbas et al., 2010; Iwamoto et al., 2011; Martin et al., 2014a) and the effect of altered synaptic 5-HT levels on 5-HT2C receptor RNA editing (Gurevich et al., 2002a; Abbas et al., 2010; Lyddon et al., 2010; Moya et al., 2011). These variations in results highlight the requirement of a large sample size and the need to analyze hundreds of sequences per sample to obtain an accurate profile of all possible RNA editing events. In some of the more recent studies, the statistical power of the results has been increased by employing high-throughput sequencing methods that allow larger numbers of samples to be processed, in a more timely and less expensive manner, along with the ability to analyze hundreds or even thousands of sequences from a single sample (Lanfranco et al., 2009; Abbas et al., 2010; Morabito et al., 2010b; O’Neil and Emeson, 2012; Zhu et al., 2012; Lyddon et al., 2013; Anastasio et al., 2014b; Di Narzo et al., 2014). Although these studies have reported changes in 5-HT2C receptor RNA editing patterns following chronic drug treatment in rodent models or changes in editing in patients with psychiatric disorders who committed suicide, the magnitude of the observed change is small, typically less than 10%. This raises several important questions. Could such a small change in 5-HT2C receptor editing be physiologically relevant? Could there be larger changes in 5-HT2C receptor RNA editing occurring within discrete neuronal populations, and could these changes be underestimated or masked when sampling an entire brain region?
A few of the studies described in the preceding paragraph examined RNA editing in both cortical and subcortical regions, whereas many studies examined RNA editing only in the prefrontal cortex of patients with psychiatric illnesses. Because the 5-HT2C receptor is located within mesolimbic and mesostriatal regions that have been shown to regulate the activity of ascending dopamine, it still remains possible that changes in RNA editing occurring within discrete populations of neurons in cortical and/or subcortical regions could play a role in the etiology of psychiatric disorders. Future studies capable of examining RNA editing profiles of individual neuronal populations within localized subregions will provide a more accurate picture of the relationship between 5-HT2C receptor RNA editing, 5-HT2C receptor isoform expression, and the functional regulation of 5-HT and dopamine neurotransmission in psychiatric disorders.
X. 5-HT3 Receptor
A. Introduction
The 5-HT3 receptor is a Cys-loop ligand-gated ion channel, which is structurally and functionally distinct from the other six classes of 5-HT receptors whose metabotropic actions are mediated via G proteins. 5-HT3 receptors are pentameric assemblies of five identical or nonidentical subunits that pseudosymmetrically surround the ion pore (Boess et al., 1992; Green et al., 1995). Each subunit has a large extracellular domain (ECD), four transmembrane domains (TMD I–IV), and an intracellular domain (ICD) between M3 and M4.
The ECD contains the agonist binding site, which is located at the interface of two adjacent subunits and is formed by three loops (A–C) from one (the principal) subunit and three β-strands (referred to as loops D–F) from the adjacent or complementary subunit; key residues that contribute to the binding pocket in these loops have been identified from structural data (Hassaine et al., 2014), and these are supported by a range of functional studies [for reviews, see Barnes et al. (2009) and Thompson et al. (2010)]. The binding site contains the aromatic box, which is found in all Cys-loop receptors, and in the homomeric 5-HT3A receptor, is constituted of W183 (loop B), W90 (loop D), Y153 (loop E), F226 (loop C), and Y234 (loop C).
The transmembrane domain of each 5-HT3 receptor subunit is composed of four (M1–M4) transmembrane α-helices, with short loops between M1 and M2 (intracellular) and M2 and M3 (extracellular). The M2 α-helices line the ion pore, and pore-facing residues contribute to ion flux and selectivity. M1, M3, and M4 all protect M2 from the surrounding membrane lipids and play a role in receptor function. The conserved proline in M1, for example, is essential for activation, and the receptor is expressed but cannot function when this proline is replaced by alanine, glycine, or leucine (Dang et al., 2000). However, substitution with noncanonical amino acids that lack hydrogen bond donor activity yields active channels similar to wild-type receptors. These suggest flexibility in secondary structure in this region of M1 is a key element in channel gating.
The ICD is formed primarily by the large M3–M4 intracellular loop; this region is responsible for receptor modulation and also plays a role in trafficking. Deletion studies reveal the ICD is not essential, as the mouse 5-HT3A receptor subunit ICD can be replaced by the heptapeptide M3–M4 linker of GLIC without loss of function (Jansen et al., 2008). Further evidence that the ICD can function as a separate domain comes from studies in which it was added to the GLIC linker peptide, resulting in modification of function by the intracellular protein RIC-3 (Goyal et al., 2011).
ICD structural details are sparse, but each subunit is known to possess an α-helix, which contributes to openings, known as portals, just below the level of the membrane. The residues that line these portals are important for ion conductance; when the 5-HT3A subunit residues are replaced with those found in the 5-HT3B subunit, the single channel conductance, which is very low in the homomeric 5-HT3A receptor, is increased to that of the heteromeric 5-HT3AB receptor (Kelley et al., 2003b).
Only 5-HT3A subunits can form functional homomeric 5-HT3 receptors. These subunits have been cloned from a range of species, including human hippocampus, amygdala, and colon (Belelli et al., 1995; Miyake et al., 1995); guinea pig small intestine (Lankiewicz et al., 1998); ferret colon (Mochizuki et al., 2000); and dog brain (Jensen et al., 2006); however, homologs are absent from invertebrates (although there is a related 5-HT–gated anion selective receptor). Multiple isoform of 5-HT3A subunits are known. Alternative splicing of the transcript encoding guinea pig, mouse, and rat, (but not dog, ferret, or human) 5-HT3A subunits results in “long” [5-HT3A(a)] and short [5-HT3A(b)] isoforms, where the 5-HT3A(b) isoform lacks five or six amino acid residues within the M3–M4 intracellular loop, which results in some subtle differences in receptor properties (Hope et al., 1993; Lankiewicz et al., 1998). In the human 5-HT3A subunit, three different splice variants have been described. Two of these (5-HT3AL and HT3Rext) would result in larger proteins, whereas the other (5-HT3AT) codes for a truncated subunit containing only a single transmembrane domain. 5-HT3Rext has not yet been functionally evaluated, but 5-HT3AT and 5-HT3AL, while not functional when expressed alone, form receptors with modified functional properties when coexpressed with canonical 5-HT3A subunits (Brüss et al., 2000).
A range of other 5-HT3 receptor subunits have been identified (B–E), of which only the 5-HT3B subunit has been extensively investigated (Davies et al., 1999; Dubin et al., 1999; Niesler et al., 2003). Coexpression of this subunit with the 5-HT3A subunit to yield hetermeric 5-HT3AB receptors resulted in functional receptors with properties that more closely represented those found in some native neuronal receptors, including relatively high single-channel conductance and relatively low Ca2+ permeability compared with homomeric 5-HT3A receptors.
The stoichiometry of heteromeric receptors is still not clear, although the presence of at least one 5-HT3A subunit appears to be obligatory in heteromeric receptors (Niesler et al., 2008; Holbrook et al., 2009). 5-HT3AB receptors were originally suggested to possess a 3B:2A subunit ratio, and using atomic force microscopy with tagged subunits indicated a BABBA arrangement (Barrera et al., 2005). The physiologic relevance of this, however, has been questioned in more recent studies, in which the presence of an AA interface was clearly demonstrated (Lochner and Lummis, 2010; Thompson et al., 2011b), and fluorescently tagged subunits indicate a 3A:2B arrangement at the plasma membrane (Miles et al., 2013). This is also consistent with the near identical orthosteric binding site pharmacology when comparing h5-HT3A and h5-HT3AB receptors (Brady et al., 2001). The arrangement and number of 5-HT3C, 5-HT3D, and 5-HT3E subunits in functional receptors has not yet been determined, although there are many potential 5-HT3 receptor isoforms arising from the utilization of multiple subunits in different combinations.
For more information of the structure of the 5-HT3 receptor, see XVI. B. 5-HT Ligand-Gated Ion Channels.
B. Expression
5-HT3 receptors are located in many brain areas, including the hippocampus (Fig. 15), entorhinal cortex, frontal cortex, cingulated cortex, dorsal horn ganglia, amygdala, nucleus accumbens, substantia nigra, and ventral tegmental area (Parker et al., 1996a; Barnes et al., 2009). The dorsal vagal complex in the brainstem, which is key to the vomiting reflex and contains the area postrema and nucleus tractus solitarius, has the highest levels, consistent with the potent antiemetic properties of 5-HT3 receptor antagonists (Pratt et al., 1990; Parker et al., 1996; Fig. 16).
Fig. 15.
Immunohistochemical detection of 5-HT3A and 5-HT3B receptor subunit expression in human hippocampus. 5-HT3A and 5-HT3B subunit immunoreactivity brown immunoreaction product in whole hippocampal section (large plates) is shown with fields of the hippocampus CA1-CA3, hilus (CA4), and dentate gyrus (DG), which are also shown at higher magnification (small plates; Scale bar, 50 µm). Immunoreactivity was not detected when either primary antibody was replaced by preimmune serum (No 1°) or following preabsorption with the immunizing peptide (peptide block). Subsequent to the detection of immunoreactivity, sections were histologically stained with hematoxylin to aid identification of hippocampal fields. Adapted from Brady et al. (2007) (with permission).
Fig. 16.
5-HT3 receptor autoradiography in the human brainstem. Left: [3H]-(S)-zacopride binding to the 5-HT3 receptor in coronal section of human brainstem at the level of the chemoreceptor trigger zone (AP, area postrema; NTS, nucleus tractus solitarius; X, dorsal motor nucleus of the vagus nerve). Right: Adjacent section stained for acetylcholinesterase (hypoglossal nerve nucleus). [3H]-(S)-zacopride binding in the presence of ondansetron (1.0 µM) was absent (data not shown).
The functional studies are supported by expression studies, with 5-HT3A receptor mRNA and protein being observed in regions of the CNS known to have 5-HT3 receptors. Such studies also indicate 5-HT3 receptor expression in a wide range of other tissues, including peripheral and sensory ganglia and the gastrointestinal tract (e.g., Michel et al., 2005; Barnes et al., 2009; Fig. 17). In addition, expression of the 5-HT3A subunit has been reported in immune cells such as monocytes, chondrocytes, T cells, synovial tissue, and platelets (Fiebich et al., 2004; Stratz et al., 2008).
Fig. 17.
5-HT3 receptor protein expression (5-HT3A and 5-HT3B subunits) in human gut. (A) Immunolabeling of a ganglion in the human submucous plexus with an antibody against the 5-HT3A subunit. (B) The same ganglion as in (A) stained with an antibody against the human neuronal protein HuC/HuD, which labels all neurons. All anti-HuC/HuD–positive neurons are immunoreactive for the 5-HT3A antibody. (C) Immunolabeling of another human submucous ganglion with an antibody against the 5-HT3B subunit. (D) The same ganglion as in (C) stained with anti-HuC/HuD. All anti-HuC/HuD–positive neurons were immunoreactive for the 5-HT3B antibody. Scale bar, 25 μm (A–D). (F) Detection of h5-HT3B immunoreactivity by SDS polyacrylamide gel electrophoresis/Western blot. Lane 1, HEK293 cells stably expressing with h5-HT3A subunit; lane 2, HEK293 cells stably expressing both the h5-HT3A and h5-HT3B subunits; lane 3, human large intestine; lane 4, human small intestine. Numbers are the positions of the molecular weight markers (kilodalton). Adapted from Michel et al. (2005) (with permission).
There was initial controversy as to the presence of 5-HT3B subunits in brain, but later studies show it is expressed here (e.g., Brady et al., 2007), with a preference for distinct “brain-type” isoforms. The longer canonical 5-HT3B subunit is broadly expressed in many tissues, including kidney, liver, and the gastrointestinal tract, with relatively high levels in spleen, colon, small intestine, and kidney (Tzvetkov et al., 2007; Holbrook et al., 2009).
5-HT3C, 5-HT3D, and 5-HT3E receptor subunits were first identified in humans, and genes for these proteins have now been shown to exist in a range of species, although not in rodents (Niesler et al., 2008; Holbrook et al., 2009). Initial studies suggested that the 5-HT3D and 5-HT3E subunits had a very restricted expression in the GI tract, but more recent data suggests all these subunits have a relatively widespread distribution. Studies examining protein levels have lagged behind the genetic work, but expression of 5-HT3C, 5-HT3D, and 5-HT3E subunits at the protein level in the GI tract has recently been confirmed (Kapeller et al., 2011).
C. Post-translational Modifications
The human 5-HT3A subunit has four consensus sequence N-glycosylation sites in the N-terminal ECD domain and all can be N-glycosylated (Monk et al., 2004). N-glycosylation is essential for export from the ER, cell surface expression, and radioligand binding, although it is not necessary to preserve a ligand binding site once the receptor has matured (Green et al., 1995; Boyd et al., 2003; Quirk et al., 2004). Three of the four N-glycosylation sites are conserved between a range of species (N104, N170, and N186) and appear to be critical, whereas the N-glycosylation site at residue 28 is less important and indeed absent in rodents (Monk et al., 2004).
The other subunits have been less well studied, but it has been shown that 5-HT3B receptor subunits, when expressed alone, fail to exit the ER, providing a possible explanation as to why these subunits cannot form functional homomeric receptors. ER retention is due, at least in part, to a CRAR retention motif, which forms part of the M1–M2 intracellular loop (Boyd et al., 2003). Coexpression with the 5-HT3A subunit may shield this ER retention motif, allowing heteromeric 5-HT3AB receptors to reach the cell surface. There is some evidence that the 5-HT3B subunit forces a preference for expression of the heteromeric 5-HT3 receptor, as coexpression of the 5-HT3A and 5-HT3B subunits in tsA-201 cells did not indicate the presence of homomeric 5-HT3A receptors (Barrera et al., 2005), although the physiologic relevance of this finding is not yet clear.
BiP, calnexin, and RIC-3 have been identified as ER chaperone proteins that associate with the 5-HT3 receptor and are likely to promote correct folding, oligomerisation, post-translational modification, and/or export from the ER (Boyd et al., 2003). RIC-3 has been the most widely studied but has different effects depending on the subunits, the species they originated from, and the expression system (Castillo et al., 2005; Cheng et al., 2005, 2007). Expression of human homomeric 5-HT3A receptors in transfected mammalian cells, for example, is enhanced by RIC-3, but it causes inhibition of heteromeric (5-HT3AB) receptor expression, and mouse 5-HT3A receptor expression in oocytes is completely abolished (Cheng et al., 2005). This apparent discrepancy may be due to other proteins that could influence 5-HT3 receptor expression. Cyclophillin A, for example, promotes 5-HT3A receptor expression in the cell membrane via an integral peptidyl prolyl isomerase activity (Helekar and Patrick, 1997), and there may be a range of other proteins yet to be identified that can modify 5-HT3 receptor expression.
Similar to the human 5-HT3A subunit, the human 5-HT3B subunit has a number of consensus sequences for N-glycosylation sites in the N-terminal ECD domain (five; Massoura et al., 2011), and all can be N-glycosylated (Massoura et al., 2011). N-glycosylation of the human 5-HT3B subunit at each of the five consensus sites facilitates export from the ER and efficient cell surface expression when the 5-HT3B subunit is coexpressed with the human 5-HT3A subunit to generate hetermeric 5-HT3AB receptors (Massoura et al., 2011).
D. Pharmacology
There are many selective and potent compounds that act at 5-HT3 receptors. Many 5-HT3 receptor agonists have in common a basic amine, an aromatic ring, a hydrophobic group, and two hydrogen bond acceptors; potent agonists include 2-methyl-5-HT, phenylbiguanide, and m-chlorophenylbiguanide (Kilpatrick et al., 1990; Cockcroft et al., 1995). Data from AChBP suggest that agonists tend to be relatively small compounds, as they need to allow the C loop to close over the binding site, initiating the gating process; the structure of 5-HT binding protein (5-HTBP), a modified version of AChBP, which binds 5-HT and granisetron, supports a similar mechanism of action for the 5-HT3 receptor (Tsetlin and Hucho, 2009; Kesters et al., 2013).
A range of highly selective 5-HT3 receptor partial agonists with a range of intrinsic activities are available (e.g., Manning et al., 2011, 2014; Roberts et al., 2020) that have been proposed as therapeutics to treat the symptoms of, for example, irritable bowel syndrome (IBS) with diarrhea (IBS-D) and carcinoid syndrome (Roberts et al., 2020).
5-HT3 receptor competitive antagonists, which bind at the orthosteric (agonist) binding site, are usually larger than agonists; they require an aromatic part, a basic moiety, and an intervening hydrogen bond acceptor. For most antagonists, these are a rigid aromatic or heteroaromatic ring system, a basic amine, and a carbonyl group (or isosteric equivalent) that is coplanar to the aromatic system (Evans et al., 1991), and there are slightly longer distances between the aromatic and amine group when compared with the agonist pharmacophore. Only small substituents, such as a methyl group, can be accommodated on the charged amine (Schmidt and Peroutka, 1989). Many potent antagonists of 5-HT3 receptors have 6.5 heterocyclic rings, and the most potent compounds contain an aromatic six-membered ring. Morphine and cocaine (Gaddum and Picarelli, 1957) were the first antagonists used to characterize the 5-HT3 receptor, with more selective 5-HT3 receptor antagonists being developed in the 1980s, including MDL72222 or bemestron (Fozard, 1984) and ICS 205-930 or tropisetron (Donatsch et al., 1985). Other compounds that were developed include ondansetron, granisetron, and zacopride, which act at nanomolar concentrations, and there is now a wide range of similarly potent compounds, with many containing bicyclic heteroaromatic structures, that is, quinoxalines, quinazolines, or quinolines (Verheij et al., 2012). Data indicate that these compounds, and therefore possibly all ligands that bind to the orthosteric site, are stabilized in the binding pocket by interaction with water molecules.
Some 5-HT3 receptor inhibitors act by blocking the channel; picrotoxin, which was originally considered to be relatively selective for the GABAA receptor, also blocks the 5-HT3 receptor channel; the binding of picrotoxinin (the active component of picrotoxin) has been localized to the 6’ position of M2 (Das and Dillon, 2003; Thompson et al., 2011a). Compounds structurally similar to picrotoxin, such as the gingkolides and bilobalide, act similarly (Thompson et al., 2011a). Diltiazem, which blocks voltage-gated calcium channels, is also known to block the 5-HT3 receptor pore and acts close to the 7’ and/or 12’ residues in homomeric receptors (Gunthorpe and Lummis, 1999; Thompson et al., 2011a). Morphine and its analog methadone as well as the antimalarial compounds quinine and mefloquine may also exert their inhibitory effects via binding to the pore, highlighting the common mechanisms that many of these drugs share and also the promiscuity that many of these compounds display (Brady et al., 2001; Deeb et al., 2009; Baptista-Hon et al., 2012)
There are a number of allosteric modulators that effect 5-HT3 receptor function, including n-alcohols, anesthetics, antidepressants, cannabinoids, opioids, steroids, and natural compounds; these can inhibit or enhance receptor activity, and many also modulate other Cys-loop receptors, although not always in the same direction [see reviews by Parker et al. (1996), Walstab et al. (2010), Davies (2011), and Machu (2011)]. Specific binding sites for these compounds have mostly not yet been confirmed, although some may bind in an intersubunit binding cavity at the top of the TMD (Trattnig et al., 2012). The effects of these compounds have mostly been studied on 5-HT3A receptors [see Downie et al. (1995) and Parker et al. (1996), but see Bentley and Barnes (1998) for impact on native 5-HT3 receptors], although alcohols and inhaled anesthetics have been shown to have reduced sensitivity at 5-HT3AB receptors, whereas the effects of etomidate, propofol, and pentobarbital are similar at 5-HT3A and 5-HT3AB receptors (Solt et al., 2005; Stevens et al., 2005; Rusch et al., 2007).
A further pharmacological phenomenon termed cryptic orthosteric modulation was identified using the human 5-HT3A receptor as a model (Powell et al., 2016); this pharmacological mechanism may convey therapeutic benefits when targeting ligand-gated ion channels (Powell et al., 2016).
E. Function
The 5-HT3 receptor pore is a relatively nonselective cation channel, constructed of five pseudosymmetrically arranged M2 α-helices (one from each of the five subunits). The residues that line the ion-accessible face of M2 are predominantly nonpolar and are the major controlling influence on ion flux (Reeves et al., 2001; Panicker et al., 2002; McKinnon et al., 2011). Currents are primarily carried by Na+ and K+ ions, although divalent and small organic cations are also permeable (Derkach et al., 1989; Yang, 1990; Maricq et al., 1991).
Ionic selectively is predominantly mediated via residues in M2. A triple mutant receptor with a proline insertion at −1’ and the substitutions E−1’A and V13’T resulted in an anion-permeable mouse 5-HT3A receptor (Gunthorpe and Lummis, 2001), although subsequent studies showed that the replacement of only two residues (E–1’ and S19’R) was needed to invert ion selectivity (Thompson and Lummis, 2003). Changing −1’E alone resulted in nonselective channels, indicating that the rings of charge at either end of M2 charge make the most critical contribution (Thompson and Lummis, 2003). Equivalent residues participate in the selectivity filters of other Cys-loops (Thompson et al., 2010).
5-HT3A receptors are almost equally permeable to monovalent and divalent cations (PCa/PCs = 1.0–1.4) (Brown et al., 1998; Davies et al., 1999; Livesey et al., 2011). However, human 5-HT3AB receptors have lower Ca2+ permeability [PCa/PCs = 0.6 (Davies et al., 1999)], possibly the consequence of the 20’ residue being neutral (Asn and not Asp) in human 5-HT3B subunits. Consistent with this, a D20’A substitution in 5-HT3A subunits reduces Ca2+-relative permeability [PCa/PCs = 0.4 (Livesey et al., 2008)]. Recent studies suggest that the ICD may also play a role in Ca2+ permeability, as substitutions of charged residues in this region can have a major effect on Ca2+ permeability (Livesey et al., 2011).
The single-channel conductance of the homomeric receptor is low; values of 0.4–0.76 pS have been reported (Davies et al., 1999; Gunthorpe et al., 2000; Kelley et al., 2003). The presence of Lys in the M2 regions was originally considered a possible explanation, but substitutions here revealed this was not the case (Gunthorpe et al., 2000). Subsequent work revealed that the low conductance was due to Arg residues located in the amphipathic helix of the M3–M4 loop, which forms holes, known as portals, through which ions can cross between the intracellular vestibule of the receptor and the cell interior (Kelley et al., 2003).
Heteromeric 5-HT3AB receptors show a range of distinct biophysical characteristics when compared with 5-HT3A receptors: their desensitization is more rapid, concentration-response curves reveal lower EC50 values and Hill slopes, their voltage dependence is linear, and their divalent cation permeability is much reduced; most noticeable, however, is the large single-channel conductance (13–16 pS) (Peters et al., 2005). This has been shown to be due to the substitution of Arg residues in the M3–M4 helical region of the 5-HT3A subunit by neutral or negative-charged residues in the 5-HT3B subunit (Kelley et al., 2003).
Other heteromeric receptors have not yet been extensively investigated, but studies to date indicate that they are more similar to homomeric 5-HT3A receptors.
The gate of the 5-HT3A receptor channel is located centrally within M2 (Panicker et al., 2002), consistent with the “hydrophobic girdle” model of channel gating. The hydrophobic girdle is a region in the center of M2 that is less than 3.5 Å diameter over a distance of ∼8 Å; the residues that face the pore here are hydrophobic (13’Val and 9’Leu), making it effectively impermeable to ions in the closed conformation (Miyazawa et al., 2003). Data consistent with this hypothesis are substitution of Val13’ residues in the 5-HT3A receptor by threonine or serine, which caused an increase in agonist potency (Dang et al., 2000) or spontaneous channel openings (Bhattacharya et al., 2004a), and substitutions of Leu9’ by a range of amino acids, which affected agonist potency and desensitization rates (Yakel et al., 1993).
F. Clinical Relevance
Studies implicate the malfunction of 5-HT3 receptors in a range of neurologic and gastrointestinal disorders (Walstab et al., 2010; Niesler, 2011). Selective 5-HT3 receptor antagonists (e.g., first-generation selective antagonists such as ondansetron and granisetron and second-generation antagonists such as palonosetron) have revolutionized the control of emesis, particularly in patients receiving high aggressive cytotoxic anticancer chemotherapy (e.g., cisplatin) and radiotherapy. Thus, the release of substantial amounts of 5-HT from enterochromaffin cell stores in the gastrointestinal tract as a consequence of antimitotic anticancer therapy may activate local (vagal) and central (chemoreceptor trigger zone) 5-HT3 receptors to evoke nausea and vomiting. Simple antagonism of these 5-HT3 receptors offers substantial relief to patients. See XX. 5-HT Receptors and the Gastrointestinal Tract for further discussion of the 5-HT3 receptor and emesis.
The identification of 5-HT3 receptors in immune cells also suggests a possible role of 5-HT3 receptors in immunologic processes and inflammation and suggests that they may plausibly be involved in diseases such as atherosclerosis, tendomyopathies, and fibromyalgia (Fiebich et al., 2004; Stratz et al., 2008).
Some SNPs have been identified in patients with bipolar affective disorder (BPAD) or schizophrenia, disorders that segregate with cytogenetic abnormalities involving a region on chromosome 11 that harbors the HTR3A gene (Weiss et al., 1995). Further studies are needed with two SNPs found in schizophrenic patients (R344H and P391R) to determine if they contribute to disease in these patients, but a significant association was found in BPAD with a P16S mutation in the 5-HT3A subunit, with reporter constructs indicating this mutant could modulate expression levels. (Niesler et al., 2001; Thompson et al., 2006; Krzywkowski et al., 2007). Additional SNPs in the HTR3A gene result in the 5-HT3A(A33T) and 5-HT3A(M257I) subunit variants, both of which are associated with reduced levels of cell surface expression, and the 5-HT3A(S253N) variant, which does not appear to compromise plasma membrane expression (Krzywkowski et al., 2007). The significance of these is yet to be determined.
In the 5-HT3B subunit, there has been an extensive investigation into a very common SNP, Y129S, which is linked both to BPAD and major depression in women (Krzywkowski, 2006; Hammer et al., 2012). Unusually, the Y129S variant is a gain of function mutation, as 5-HT3AB(Y129S) receptors have an increased maximal response to 5-HT, decreased desensitization and deactivation kinetics, and a sevenfold increase in mean channel open time in comparison with heteromeric receptors containing the wild-type 5-HT3B subunit (Krzywkowski et al., 2008). An intermediate effect is apparent for receptors assembled from a mixture of wild-type 5-HT3A, wild-type 5-HT3B, and 5-HT3B(Y129S) subunits, suggesting that signaling via the 5-HT3AB receptor in heterozygous as well as homozygous individuals is altered by this SNP (Krzywkowski et al., 2008).
Studies in the more recently discovered HTR3C, HTR3D, and HTR3E genes also indicate possible involvement in disease. An SNP in the 5-HT3C gene (N163K) has been correlated with IBS, and expression studies suggest it causes an increase in receptor density (Kapeller et al., 2011). Increased expression has also been associated with an SNP in the 3′untranslated region of the HTR3E gene, also associated with IBS, which inhibits the binding of a microRNA, as described above (Kapeller et al., 2008).
IBS-D is another disorder in which 5-HT3 receptor antagonists can be effective therapeutic agents, although side effects that may be associated with a high level of 5-HT3 receptor inhibition supports the development of 5-HT3 receptor partial agonists (e.g., Roberts et al., 2020). See XX. 5-HT Receptors and the Gastrointestinal Tract for discussion of the role of the 5-HT3 receptor and IBS.
Additional studies suggest that a wide range of other diseases have the potential to be treated with 5-HT3 receptor–selective drugs, including addiction, pruritis, migraine, chronic heart pain, bulimia, and neurologic phenomena such as anxiety, psychosis, nociception, and cognitive function (Thompson and Lummis, 2007; Walstab et al., 2010).
See XVIII. 5-HT Receptors and the Brain, XIX. 5-HT Receptors and the Cardiovascular System, XX. 5-HT Receptors and the Gastrointestinal Tract, and XXI. 5-HT Receptors and the Immune System for further discussion of the clinical relevance of the 5-HT3 receptor.
XI. 5-HT4 Receptors
A. Introduction
An atypical 5-HT receptor was initially identified to be positively coupled to adenylyl cyclase in colliculi neurons (Dumuis et al., 1988), guinea pig hippocampus (Bockaert et al., 1990), and human and porcine cardiac atria (Kaumann, 1990; Kaumann et al., 1990; Villalon et al., 1990). This receptor was insensitive to 5-HT1 receptor and 5-HT2 receptor antagonists and was antagonized (but with a relatively low affinity) by tropisetron (at the time thought to be a relatively selective 5-HT3 receptor antagonist) but not by another 5-HT3 receptor antagonist, MDL 72222. This new 5-HT receptor was named the “5-HT4” receptor.
B. Gene and Primary Structure
The cloning of the rat cDNA by Gerald et al. (1995) revealed the first primary sequence of 5-HT4 receptor. It is a classic GPCR with seven transmembrane domains (TMD). Two splice variant sequences were found initially [5-HT4S and 5-HT4L, later renamed 5-HT4(a) and 5-HT4(b)] coding for proteins of 387 and 388 amino acids, respectively. The splicing occurs within the C-terminal domain following the splicing site coding for the leucine 358 (L358). This splicing site at position L358 was subsequently found in the majority of the numerous splice variant transcripts sequenced in rodents, pig, and human (Blondel et al., 1997, 1998; Claeysen et al., 1999; Bach et al., 2001; Brattelid et al., 2004a; Bockaert et al., 2006; Coupar et al., 2007). Subsequently in human, additional splice variants differing in their C-terminal sequences following L358 have been identified [a, b, c (a shorter nonrelevant form of the c variant has been reported that is due to a sequencing error), d, e, f, g, i, and n; Bockaert et al., 2006; Coupar et al., 2007; Fig. 18]. For a complete analysis of intron/exon junctions of the human gene, see Bockaert et al. (2004a). Another splice variant (h) occurs in the second extracellular loop (Bender et al., 2000). In pig, at least nine other different splice variants, not described in humans, have been reported, including a functional homofusion variant (De Maeyer et al., 2008a). The coding sequence of the 5-HT4 receptor gene (HTR4) extends over more than 200 kb (Genes, Ensembl release 82) and is localized on chromosome 5 (5q31-q-33) (Claeysen et al., 1997). It contains at least 14 exons. Each of the C-terminal variant sequences contains one of the exons 8–13 (Bockaert et al., 2006). The variant h is the only form that results from expression of exon 6. The presence or not of exon 1 in the 5-HT4(a) transcript is debated (Hodge et al., 2013), as the ATG starting site is localized in exon 2. Recently, a novel transcript has been found in the human brain in which exon 1 and 2 are substituted for a novel exon (N) containing an expected ATG starting site, the C-terminal sequence being identical to the 5-HT4(b) receptor splice variant (Hodge et al., 2013). The predicted protein sequence for this 5-HT4 receptor variant should have a different N terminus sequence, as it is longer and the N-linked glycosylation site is at N2 instead of N7 (Hodge et al., 2013). Similar additional N-terminal exons have been identified in mouse brain and heart (Azim et al., 2012). However, further studies to clone the complete cDNA sequences and express and functionally characterize them are required before concluding on the physiologic importance of this splice variant. As some exons (such as h or i) do not present any in-frame stop codons, many additional combinations probably remain to be discovered (Brattelid et al., 2004a). SNPs within the noncoding region of HTR4 gene have been associated with chronic obstructive pulmonary disease and asthma/airways obstruction (Repapi et al., 2010; Hodge et al., 2013). The locus presents features consistent with a regulatory region and involves SNPs that significantly alter the binding motifs of a transcription factor regulating lung development (Foxp1) (Hodge et al., 2013). Consistent with this finding, mice devoid of the 5-HT4 receptor have an altered baseline lung function (House et al., 2015).
Fig. 18.
Primary structure of human 5-HT4 receptors. The amino acid sequences of the identified splice variants are depicted. 5-HT4 receptors have identical sequences up to L358 and differ by the length and composition of their C-terminal domain. The 5-HT4(h) variant, which presents an insertion of 14 residues in the second extracellular loop, has been isolated in combination with the b isoform, and it is called 5-HT4(hb). N-glycosylation sites on N7 and N180 are indicated. Palmytoylation sites on C328, C329 (common to all splice variants), and C386 (in the a isoform) are schematized. This figure has been established in accordance with the last human genome assembly (Genes, Ensembl release 82) and modified from Padayatti et al. (2013).
C. Expression Profile
1. Central Nervous System
Brain distribution of 5-HT4 receptors has been studied using radiolabeled antagonists [3H]GR 113808, [125I]SB 207710, and [3H]R116712 in many species, including mouse, rat, guinea pig, monkey, and human (Eglen et al., 1995b; Bockaert et al., 1997; Bonaventure et al., 2000; Fig. 19). Generally, a heterogeneous and comparable distribution is found in the adult brain of these various species with some interspecies differences in the globus pallidus, the substantia nigra, and the interpeduncular nucleus. The highest receptor densities are found in several regions of the limbic system (islands of Calleja, olfactory bulbs, striatum, ventral pallidum, septum, hippocampus, and amygdala) or in areas belonging to several pathways such as hippocampo-habenulo-interpeduncular and striato-nigro-tectal pathways (Waeber et al., 1993, 1994, 1996; Jakeman et al., 1994). Abundant localization of 5-HT4 receptor mRNA in the rat brain was reported in the olfactory system, striatum, medial habenula, and the hippocampus (Ullmer et al., 1996). In the rat striatum, lesion studies showed the presence of 5-HT4 receptor on the cell bodies of neurons that project to the substantia nigra and/or globus pallidus (Compan et al., 1996), whereas in situ hybridization experiments performed in guinea pig reported a localization of 5-HT4 receptors on the terminals of the striatopallidal and striatonigral projections (Mengod et al., 1996). In the rat brain, comparison of mRNA distribution with [125I]SB 207710 binding sites confirmed that 5-HT4 receptors are localized both somatodendritically (caudate putamen) and on axon terminals (substantia nigra and globus pallidus) (Vilaró et al., 1996). Bonaventure et al. (2000) performed a mapping analysis of both 5-HT4 receptor mRNA and binding sites in postmortem human brains and reported combined receptor detection in caudate nucleus, putamen, nucleus accumbens, and hippocampus with moderate to low densities in the cortex. Mismatches between absence of 5-HT4 receptor mRNA with high densities of receptor-binding sites in the globus pallidus and the substantia nigra suggested that the receptors may be localized on axonal projections originating from the striatum (Bonaventure et al., 2000).
Fig. 19.
5-HT4 receptor expression in the human brain. In situ hybridization detection of 5-HT4 receptor mRNA (B–D) and 5-HT4 receptor autoradiography of radioligand binding sites [[3H]R116712 total binding (G and H) and nonspecific binding (J and K)]. Acc, nucleus accumbens; CA1-3, hippocampal fields; Cd, caudate nucleus; DG, dentate gyrus; Ent, Cx entorhinal cortex; Ic, internal capsule; Pu, putamen; S, subiculum; SN; no mRNA evident, substantia nigra; TCd, tail of caudate nucleus. Adapted from Bonaventure et al. (2000) (with permission).
In the cortex, hippocampus, and amygdala, 5-HT4 receptors were described on cholinergic neurons, where they stimulate the release of acetylcholine, as well as on the glutamatergic neurons (King et al., 2008). In the striatum and nucleus accumbens, the receptors have been found on the intrinsic GABA neurons (spiny neurons) but also on glutamatergic neurons (King et al., 2008). Work using dual-label in situ hybridization found precise localization of 5-HT4 receptors in relation with the cholinergic system. In the basal forebrain, 5-HT4 receptor mRNA was not detected in the cholinergic cell population but in parvalbumin synthesizing and glutamatergic cells (Penas-Cazorla and Vilaro, 2015). Thus, noncholinergic cell populations within the basal forebrain, hippocampal and cortical areas that express 5-HT4 receptors (likely glutamatergic cells) would mediate the 5-HT4 receptor–mediated enhancement of acetylcholine (Penas-Cazorla and Vilaro, 2015) and other neurotransmitters, such as the increase in 5-HT release in the hippocampus (Ge and Barnes, 1996).
Localization of 5-HT4 receptor splice variants in rodents appears to be similar within brain areas and concordant to the binding studies, with a low level of 5-HT4 receptor mRNA in the cerebellum, where no receptor-binding site is detected (Claeysen et al., 1999; Vilaro et al., 1996). A complex pattern of human 5-HT4 receptor isoforms, which depends not only on the region studied but also on each individual brain, requires analysis of a larger cohort to be conclusive (Bender et al., 2000). However, the variations in C-terminal domain of the 5-HT4 receptor influence its constitutive activity (Claeysen et al., 1999), internalization properties (Mnie-Filali et al., 2010), and subcellular localization via interacting partners (Joubert et al., 2004) (see 2.). The distribution of 5-HT4(a) receptors in the juvenile rat brain and spinal cord recently achieved using a polyclonal antibody have produced global data in accordance with the literature. However, striking staining of cerebellum cells raises a doubt on the specificity of the antibody used, which has not been tested on animals devoid of 5-HT4 receptor (KO) (Suwa et al., 2014).
5-HT4 receptors are also located on the respiratory Pre-Boetzinger complex in the ventrolateral medulla of the brainstem, where they modulate spontaneous respiratory activity (Manzke et al., 2003; Richter et al., 2003).
2. Periphery
a. Heart
In porcine and human heart, 5-HT4 receptors are mainly expressed in the atrial myocytes, and their expression was long believed to be atria-selective (Kaumann et al., 1991; Ouadid et al., 1992; Kaumann and Levy, 2006). However, expression was later also found in human and porcine ventricle (Bach et al., 2001; Brattelid et al., 2004a,b), and during heart failure and cardiac hypertrophy, their mRNA level and expression increased in the porcine, rat, and human ventricles (Brattelid et al., 2004b; Qvigstad et al., 2005a,b,c), possibly reflecting reactivation of a fetal gene expression pattern (Brattelid et al., 2012).
b. Gastrointestinal tract
In rodents, 5-HT4 receptors are expressed in the smooth muscle of the esophagus. In many species, they are present on the intrinsic afferent neurons, motor neurons, and enterocytes of the gastrointestinal tract (Hegde and Eglen, 1996). The expression of 5-HT4 receptors on excitatory motor neurons in the gut facilitates acetylcholine release, which stimulates gastrointestinal motility (Liu et al., 2005a; Gershon and Tack, 2007; Ren et al., 2008). In the human colon, 5-HT4 receptors are also expressed on circular smooth muscle cells, where they induce relaxation (McLean et al., 1995). The 5-HT4(h) receptor variants appear to be predominantly expressed in porcine GI mucosa, suggesting their contribution to the 5-HT4 receptor–mediated mucosal effects of 5-HT (Priem et al., 2013). In human colon, RT-PCR analysis shows that 5-HT4(d) and 5-HT4(g) receptor splice variants are significantly less likely to be detected in mucosa and longitudinal muscle compared with the 5-HT4(a), 4(b), 4(c), 4(n) splice variants (Yaakob et al., 2015).
c. Adrenal gland
5-HT4 receptors are present in glomerulosa and zona fasciculata of the adrenal cortex (Lefebvre et al., 1992, 1998a). An RT-PCR study demonstrated that 5-HT4 receptors are overexpressed in adrenocortical aldosterone-producing adenoma tissues in comparison with normal adrenal cortex (Cartier et al., 2005). Moreover, a different splicing mechanism seems to occur in these tumors, where the 5-HT4(a) and 5-HT4(b) receptor splice variants, usually present in normal adrenal cortex, are absent, and the 5-HT4(d), a rare isoform, is over-represented (Cartier et al., 2005).
d. Salivary glands
In the rat submandibular gland, expression of 5-HT4(b) but not 5-HT4(a) receptors was demonstrated and linked to cyclic AMP formation and regulation of volume and protein content of saliva, together with 5-HT7 receptors (Turner et al., 1996; Bourdon et al., 2000).
e. Urinary bladder
In the bladder, 5-HT4 receptors are present on cholinergic/purinergic neurons that innervate the smooth muscle (detrusor) (Candura et al., 1996). Their stimulation with cisapride enhances human bladder contraction without affecting urethral contractions or relaxations (Kullmann et al., 2013).
f. Lung
5-HT4 receptor mRNA has been detected at low density in human alveolar epithelial cells type II, airway epithelial cell lines, and human airway smooth muscle (Bayer et al., 2007; Einstein et al., 2008; Hodge et al., 2013).
D. Post-translational Modifications
Post-translational modifications of 5-HT4 receptors and their related functional consequences have been studied on receptors expressed in heterologous cells rather than native tissues.
1. N-Glycosylation
There are two putative N-linked glycosylation sites in the extracellular side of 5-HT4 receptors that conform to the consensus sequence N-X-S/T, where X can be any amino acid except a Pro residue (N-terminal N7 and second extracellular loop N180; Fig. 18). The pattern of glycosylation is dependent on the nature of the cell in which 5-HT4 receptors are expressed. Salom et al. (2012) report that the glycosylation pattern of 5-HT4 receptors ectopically expressed in mouse rod cells in vivo is more homogeneous than the glycosylation pattern of 5-HT4 receptors expressed artificially in HEK293 cells. The h5-HT4(b) receptors expressed in mouse rod cell display high-mannose–type and complex-type sugars at both N7 and N180 sites. Most of the oligosaccharides had the core structure of three mannoses (Man) and two N-acetylglucosamine residues (23 species for N7 and eight species for N180). This N-glycosylation pattern is much more complex than for other GPCRs expressed in mouse rod cells.
2. Palmitoylation
The first crystal structure of rhodopsin reveals that after the TM7, there is a fourth intracellular loop (I-4) with α-helical structure. Thus, this I-4 loop is also called helix 8 and terminates with two palmitoyled cysteines that are well conserved in many GPCRs as in the 5-HT4 receptors (C328 and C329). The palmitoylation/depalmitoylation of those cysteines are associated with the wide spectrum of GPCRs biologic activities, from coupling to G proteins and regulated endocytosis to receptor phosphorylation and desensitization. Ponimaskin et al. (2002a) were the first to show that, in insect Sf9 cells, palmitoylation of the 5-HT4(a) is a reversible process and that agonist increases the turnover rate for receptor-bound palmitate. They identified C328/C329 as potential acylation sites in the 5-HT4(a) receptor, but, in contrast to most other palmitoyled GPCRs, an additional cysteine residue C386, located in the very distal portion of the C-terminal domain, was also identified as a palmitoylation site. Therefore, “complete” palmitoylation of the 5-HT4(a) may result in the formation of a putative additional intracellular loop (I-5). Mutation of the proximal palmitoylation sites (C328S and C329S) increased the capacity of the receptor to convert from the inactive (R) to the active (R*) form in the absence of agonists, whereas the mutation of C386S had no effect on this parameter. 5-HT4 receptors expressed in rod cells in vivo are palmitoylated either on C328 or C329 (Salom et al., 2012).
3. Phosphorylation
The 5-HT4 receptor C-terminal sequence, common to all splice variants (upstream of L358), contains a remarkable cluster of serine and threonine residues (S347TTTINGSTHVL358), which are potential phosphorylated sites for GRK. This cluster is absolutely required for β-arrestin/dynamin–dependent receptor endocytosis but not for receptor uncoupling (Barthet et al., 2005). This cluster is also required for β-arrestin binding to the receptor (Barthet et al., 2005). Like many GPCRs, 5-HT4 receptors stimulate ERK. However, this stimulation is Gs/Gq/Gi/Go/cAMP/PKA-independent as well as β-arrestin–independent. In contrast, this stimulation requires activation of Src tyrosine kinase (Barthet et al., 2007). This Gs-independent Src-mediated ERK activation is inhibited by GRK5, which is physically associated with the proximal region of the C terminus (upstream of the S/T cluster) (Barthet et al., 2009). Tandem mass spectra of the stimulated 5-HT4 receptors in HEK293 cells expressing GRK5 revealed a peptide (R336-R359) found under a nonphosphorylated and three phosphorylated forms with one, two, and three phosphates attached, respectively (Barthet et al., 2009). Loss of phosphate on fragmentation indicated phosphorylation of S354 in the monophosphorylated peptide and the presence of an additional phosphorylated residue within the S347TTT350 motif. The tri-phosphorylated peptide incorporated an additional phosphate within the S347TTT350 motif. This indicates that GRK5 sequentially phosphorylates the peptide, first on S354 and then in the S347TTT350 motif. The same 5-HT4 receptor peptide was found to be phosphorylated in receptors expressed in mouse rods (0–5 phosphorylated residues) (Salom et al., 2012). In the same model, another peptide was found to be phosphorylated within the I-3 loop (A232 to R250). S235, S236, S238, and S242 residues were the targets for phosphorylation as well as S247 or T248 (Salom et al., 2012).
E. Pharmacology
1. Agonists
Very early, benzamides substituted by 2-methoxy-4-amino-5-chloro such as metoclopramide, renzapride, cisapride, or zacopride were identified as potent agonists of the 5-HT4 receptors [for reviews, see Bockaert et al. (1997, 2004a, 2008) and Langlois and Fischmeister (2003)]. This was an important step in the pharmacological characterization of 5-HT4 receptors (Clarke et al., 1989) and prompted a number of pharmaceutical companies to develop selective ligands for this receptor.
The first class of 5-HT4 receptor to be discussed includes indole derivatives such as tryptamines (including 5-HT) and indole carbazimidamines derivatives. Tegaserod, prescribed to treat IBS in women suffering from constipation, belongs to this class. This compound was withdrawn from the market in 2007 because of cardiovascular side effects but has made a recent return to the market, albeit with limitations on use.
The second class includes benzamide derivatives such as metoclopramide, which was commercialized as an antiemetic (action via dopamine D2 receptors and 5-HT3 receptors at higher doses) and stimulating gastrointestinal transit (action via 5-HT4 receptors). Mosapride, which also acts as a 5-HT4 receptor agonist, is used to treat gastrointestinal disorders, as it accelerates gastric emptying (Curran and Robinson, 2008; Tack et al., 2012), but a main metabolite is pharmacologically active and antagonizes the 5-HT3 receptor.
The benzofurane carboxamide prucalopride is selective for the 5-HT4 receptor (Briejer et al., 1999) and has been approved for the treatment of chronic constipation, in case of laxative resistance, in Europe (2009) and in Canada (2011) (Tack et al., 2013).
Lead of the benzoates class is ML10302, which presents a high affinity for 5-HT4 receptor and weak affinity for 5-HT3 receptors (Yang et al., 1997). ML10302 was described as the first potent and selective 5-HT4 receptor agonist, but further investigation in vivo was limited by its susceptibility to hydrolysis.
Benzodioxane class is represented by only one product, the SL65.0155, particularly proactive as a mnemonic in several species. This compound has reached Phase IIb for the treatment of Alzheimer disease (Moser et al., 2002). It was abandoned for undisclosed reasons that may be related to its low efficacy and very partial agonist profile on 5-HT4 receptors.
The sixth class includes aryl ketones, which were developed to overcome the metabolic lability related to the related 5-HT4 receptor ester ligands. These compounds have a high bioavailability and easily pass the blood-brain barrier. RS 67333 (Eglen et al., 1995a), containing a large alkyl group, was widely used in vivo in rodent and particularly for behavioral studies demonstrating the procognitive effects of 5-HT4 receptor agonists (Fontana et al., 1997; Marchetti et al., 2000; Lamirault and Simon, 2001; Lelong et al., 2001; Kulla and Manahan-Vaughan, 2002).
The seventh class is represented by a carboxamide pyridine, PRX-03140 (also known in the literature as VRX-03011), another partial 5-HT4 receptor agonist and promnemonic substance that reached Phase IIb for the treatment of Alzheimer disease (Mohler et al., 2007) in 2008, although development of the drug was then stopped.
Benzimidazolone derivatives constitute the eighth class with BIMU1 and BIMU8 as examples, which are potent and efficacious 5-HT4 receptor agonists with good brain-penetrating properties (Dumuis et al., 1991; Rizzi et al., 1992). However, their affinity for 5-HT3 and σ2 receptors limits their utility (Bonhaus et al., 1993).
The last two classes include naphthalimide derivatives (Eglen et al., 1994) (RS 56532 and RS 66332) and the quinoline derivative velusetrag (Beattie et al., 2008). Velusetrag displays promising efficacy in patients with constipation (e.g., Nelson et al., 2017) and may also be useful in Alzheimer disease.
Other 5-HT4 receptor agonists are listed in Table 16, indicating key pharmacological parameters.
TABLE 16.
Affinities and efficacies of several 5-HT4 receptor ligands in radioligand binding (pKi) and functional (pEC50) studies
| Compounds | Affinity | Efficacy | References | |
|---|---|---|---|---|
| pKi | pEC50 or pIC50 | Emax or Imax | ||
| Agonists | ||||
| PF-00885706 | 8.4 | 8.2–8.4 | 78%–84% | Komada et al., 2009 |
| PF-01354082 | 8.7 | 8.1–8.5 | 74%–66% | Mikami et al., 2009 |
| Compound 26 | 7.9 | 9.4 | 63% | McKinnell et al., 2009 |
| ATI-75025 (Naronapride) | 8.9 | Camilleri et al., 2007; Bowersox et al., 2011 | ||
| Compound 2d | 9.3–9.8 | 9.2–9.6 | 7%–33% | Brodney et al., 2012 |
| Compound 3 | 8.1–8.6 | 8.7–9.1 | 21%–63% | Brodney et al., 2012 |
| TD-2749 | 8.0 | 8.6 | 85% | Long et al., 2012 |
| PF-4995274 (TBPT) | 9.5 | 9.0 | 19% | Sawant-Basak et al., 2013 |
| SSP-002392 | 9.1–9.2 | 10.4–10.8 | ∼100% | Tesseur et al., 2013 |
| TD-8954 | 9.4 | 8.6 | 55% | McKinnell et al., 2013 |
| Dual ligands | ||||
| 5-HT4 agonist/5-HT3 antagonist Cpd 17 | 6.7 (5-HT4R) 7.4 (H3R) | 7.3 (H3R) | Lepailleur et al., 2014 | |
| Donecopride 5-HT4 agonist/AChE inhibitor | 8.1 (5-HT4) | 9.0 (5-HT4) 7.8 (pIC50 AChE) | 48% (5-HT4) | Lecoutey et al., 2014; Rochais et al., 2015 |
| Antagonists | ||||
| Compound 34d | 8.8 | 9.2 | 80%–100% | Lemaître et al., 2009 |
| Compound 30 | 8.8 | 7.9 (pKb) | Brudeli et al., 2010 | |
| Compound 12g | 8.7 | 8.6 | 100% | Furlotti et al., 2012 |
| Compound 20 | 11.9 | 10.6 (pKb) | Brudeli et al., 2013a | |
| Compound 9 | 9.9 | 8.6 (pKb) | Brudeli et al., 2013b | |
| Compound 25 | 10.1 | 9.1 (pKb) | Brudeli et al., 2014 | |
2. Antagonists
GR 113808, belonging to the indole class, was the first selective 5-HT4 receptor antagonist presenting low affinity for 5-HT3 receptors (Gale et al., 1994) and the first tritiated radioligand commercially available. This molecule enabled the accurate definition of pharmacological characteristics and localization of 5-HT4 receptors, especially in the brain (Grossman et al., 1993). Other antagonists are benzoate derivatives (such as SDZ 205557) or benzoate dioxane derivatives such as SB 204070, which by substitution of the chlorine by a radioactive iodine, lead to [125I]SB 207710, another excellent radioligand (Kaumann et al., 1995). Other antagonist classes include benzimidazolones, imidazolpyridines, and aryl ketones.
5-HT4 receptor antagonists have been mainly developed for the purpose of binding studies and in situ labeling using radioligands. Recent work includes the development of imaging probes for positron emission tomography (PET) (Dubost et al., 2012; Buiter et al., 2013; Tavares et al., 2014). However, because of the presence of 5-HT4 in the failing heart, some antagonists have been designed for cardioprotective purposes, trying to avoid CNS penetration and improving oral administration route (Brudeli et al., 2010, 2013a,b, 2014).
Some 5-HT4 receptor antagonists are listed in Table 16, indicating key pharmacological parameters.
3. Inverse Agonists
Inverse agonists of the 5-HT4 receptor (Claeysen et al., 2000; Joubert et al., 2002) have been generated by Roche in collaboration with Bockaert’s group (RO 116-0086, RO 116-1148, and RO 116-2617; Fig. 20). SB 207266, which is a carboxylate indole derivative, is also an inverse agonist at the 5-HT4 receptor (Fig. 20).
Fig. 20.
Structure and activities of 5-HT4 receptor inverse agonists. The inverse agonist activity was studied according to the ability of the compounds to inhibit the constitutive activity (activity in the absence of agonists). 5-HT4(a) receptors were expressed in COS-7 cells (1500 ± 130 fmol/mg). The constitutive cAMP production in presence of the receptor is equal to 720% ± 50% of the activity obtained in the absence of receptor. From Joubert et al. (2002).
4. Multifunctional Ligand
Dallemagne and colleagues reported the synthesis and characterization of donecopride, a multitarget directed molecule combining 5-HT4 receptor agonist properties with acetylcholinesterase (AChE) inhibition (Lecoutey et al., 2014); the drug may display disease-modifying actions by increasing soluble amyloid precursor protein (sAPPα) release as well as procognitive effects (Lecoutey et al., 2014; Rochais et al., 2015).
5. 5-HT4 Receptor Radioligands
The first tritiated or iodinated 5-HT4 receptor compounds, mainly antagonists, were particularly useful to determine brain regional distribution of the receptor (Grossman et al., 1993; Waeber et al., 1993, 1994, 1996), [3H]GR 113808 and [125I]SB 207710 being the lead compounds (Kaumann et al., 1995). Then, several [11C]-labeled compounds have been developed for use in PET (Gee et al., 2008; Xu et al., 2010; Buiter et al., 2013), enabling live imaging studies in animals (Kornum et al., 2009) and in humans (Marner et al., 2009; Madsen et al., 2011). New [125I]-labeled antagonists have also been developed for use as PET tracers (Dubost et al., 2012). More recently, promising fluoride radioligands have been described: [18F]MNI-698 and [18F]MNI-699 (Caille et al., 2013; Tavares et al., 2014).
F. Function
1. Physiologic Function
The 5-HT4 receptors have many physiologic functions. They are implicated in learning and memory and in the stimulation of the nonamyloidogenic cleavage of APP (Bockaert et al., 2008, 2011; Claeysen et al., 2015). Thus, they are potential targets to treat Alzheimer disease.
A role of 5-HT4 receptors in feeding behavior is now clear. Agonists and antagonists have hypo- and hyperphagic properties, respectively (Jean et al., 2007; Bockaert et al., 2011).
5-HT4 receptor stimulation has rapid antidepressant action and may contribute to the actions of SSRI (Lucas, 2009; Samuels et al., 2016).
In the periphery, the role of 5-HT4 receptors in gastrointestinal tract is well established. 5-HT4 receptors evoke gastric emptying, peristaltic reflex, intestinal motility, inhibition of hypersensibility, short free fatty acid–stimulated HCO3− secretion, and, importantly, enteric nervous system development and adult neurogenesis of enteric plexus (Matsuyoshi et al., 2010; Gershon, 2011, 2013; Hoffman et al., 2012; Akiba et al., 2015). For further discussion, see XX. 5-HT Receptors and the Gastrointestinal Tract.
Although it was initially believed that 5-HT4 receptors were confined to the atria of porcine and human heart (Kaumann and Levy, 2006), functional 5-HT4 receptors mediating increased cardiac contractility (inotropic effect), as well as hastened relaxation (lusitropic effect), were later found in porcine and human ventricle (Brattelid et al., 2004b). In human, and also rat ventricle, 5-HT4 receptor mRNA expression and function is increased or even induced in pathologies such as heart failure and hypertrophy, and following cardiac infarction (Brattelid et al., 2004b; Qvigstad et al., 2005a,c), maybe reflecting reactivation of foetal gene expression (Brattelid et al., 2012). 5-HT4 receptor antagonists may be useful in those cardiac pathologies (Birkeland et al., 2007a; Kjekshus et al., 2009).
Although 5-HT4 receptors are weakly expressed in whole lung, airways epithelial cells, and airways smooth muscle cells, meta-analyses of genome-wide association studies indicate that intronic SNPs in HTR4 gene are associated with some pulmonary diseases (Hancock et al., 2010; Repapi et al., 2010; Soler Artigas et al., 2011; Hodge et al., 2013).
5-HT4 receptors are present in corticoadrenal gland where they stimulate aldosterone production, in salivary glands where they modify the volume and protein content of saliva (Turner et al., 1996; Bourdon et al., 2000), and in urinary bladder (Hegde and Eglen, 1996; Lefebvre et al., 2015).
2. Cellular Function
a. G protein coupling of 5-HT4 receptors and primary signaling events
5-HT4 receptors stimulate adenylyl cyclase in colliculi neurons as well as in hippocampus (Dumuis et al., 1988). This coupling has also been found in nucleus accumbens (Jean et al., 2007) and in heart atrium (Kaumann et al., 1990; Ouadid et al., 1992) and ventricle (Afzal et al., 2008). Thus, in native tissues, the 5-HT4 receptor–mediated Gs/cAMP/PKA signaling pathway is well established. In primary cortical neurons and HEK293 cells, cAMP produced by 5-HT4 receptors also activates the exchange factor Epac. Epac, via an Epac/Rap1/Ras pathway, activates an α-secretase and the release of sAPPα (Lezoualc’h and Robert, 2003; Cochet et al., 2013).
In transfected cell lines, several signaling pathways have been found, including coupling to Gi/o, Gq, and G13 (Ponimaskin et al., 2002b; Bockaert et al., 2006; Woehler and Ponimaskin, 2009). Expression of different 5-HT4 receptor splices variants in heterologous cells (COS and HEK293 cells, rodent cardiac myocytes) have identified putative differences in their signaling coupling. One clear difference is their constitutive activities measured on Gs coupling. Generally, the shorter the C terminus, the higher the constitutive activity (Claeysen et al., 1999). In addition, it has been reported that, in HEK293 cells and adult cardiac myocytes, 5-HT4(b) but not 5-HT4(a) or 5-HT4(d) are coupled to both Gs and Gi/o (Pindon et al., 2002). In similar heterologous systems, activation of transiently expressed 5-HT4(a) receptors by 5-HT and other agonists induce both cAMP and inositol phosphate accumulation (Chang et al., 2007; Gaven et al., 2013).
Following PKA activation, a series of ionic currents are modulated in colliculi and CA1 hippocampal neurons (Andrade and Chaput, 1991; Ansanay et al., 1995). These include a long-lasting inhibition of K+ currents, including Ca2+-activated K+ channels (mediated by a PKA-dependent inhibition of phosphatases), which generates neuronal excitability and a decrease in spike accommodation (Ansanay et al., 1995) as well as activation of the hyperpolarization-activated current known to adjust repetitive discharge behavior in CA1 neurons (Andrade and Chaput, 1991). In prefrontal cortex pyramidal neurons, GABAA current and GABAergic transmission are either stimulated or inhibited by 5-HT4 receptors. A role of PKA and A kinase anchoring proteins has been reported (Cai et al., 2002). 5-HT4 receptors also activate L-type Ca2+ channels in human and porcine atrial myocytes via a cAMP/PKA pathway (Ouadid et al., 1992; Kaumann and Levy, 2006).
The receptor-Gs uncoupling phase of 5-HT4 receptor desensitization is very rapid and efficient in colliculi neurons (Ansanay et al., 1992) and rat esophagus (Ronde et al., 1995) and is specifically dependent on the presence of a high expression of GRK2 but not of its catalytic activity (Barthet et al., 2005). In contrast, the β-arrestin/dynamin–dependent endocytosis of 5-HT4 receptors in HEK293 cells does not require a high expression of GRK2 and is dependent of the S/T cluster upstream of L258 (Barthet et al., 2005).
b. G protein–independent coupling of 5-HT4 receptors
5-HT4 receptors activate ERK in a G protein– and β-arrestin–independent manner, both in colliculi neurons and HEK293 cells (Barthet et al., 2007). The first step in 5-HT4 receptor–ERK activation is the phosphorylation-activation of the tyrosine kinase Src bound to the receptor (Barthet et al., 2007). This G protein–independent activation of Src by 5-HT4 receptors has also been described in epithelial cell lines (Gill et al., 2005). Indeed, in the enterocyte cell line, Caco 2, 5-HT4 receptor–induced Src activation was required to activate the PLCγ1 pathway and to inhibit Na+/H+ exchanger (Gill et al., 2005).
5-HT4 receptor–operated Src/ERK pathway, but not the Gs pathway, was negatively regulated by GRK5 physically preassociated with the proximal C-terminal region of the receptor in both human HEK293 cells and mouse colliculi neurons (Barthet et al., 2009). This desensitization requires two sequences of events: the association of β-arrestin1 to the phosphorylated S/T cluster already described and the phosphorylation, by GRK5, of β-arrestin1 (at S412) bound to the receptor. Phosphorylated β-arrestin1, in turn, prevented the activation of Src constitutively bound to 5-HT4 receptors (Barthet et al., 2009).
3. Gastrointestinal Tract
5-HT4 receptor localization and functions in GI tract have been extensively studied in many species, including guinea pig, pig, dog, mice, rat, and human (Craig and Clarke, 1990; McLean et al., 1995; Hegde and Eglen, 1996; Poole et al., 2006; Gershon and Tack, 2007; Sanger, 2008; Priem et al., 2013).
Activation of 5-HT4 receptors is prokinetic on GI and stimulate guinea pig ileum contractions is concomitant to discovery of 5-HT4 receptors (Craig and Clarke, 1990). Subsequently, 5-HT4 receptor facilitation of the peristaltic reflex has been identified (Hegde and Eglen, 1996; Grider et al., 1998). The mechanism by which 5-HT4 receptors stimulate peristaltic reflex is debated. Two proposals have been provided. 1) Within circular muscles of colon (human), 5-HT4 receptors stimulate the release the inhibitory (descending relaxation) and excitatory (ascending contractions) neurotransmitters (nitric oxide and acetylcholine respectively) (Tonini et al., 1989; Liu et al., 2005; Cellek et al., 2006; Ren et al., 2008). 2) 5-HT4 receptors localized on the luminal surface of epithelial cells, including enterochromaffin cells and goblet cells, stimulate the peristaltic effect via secreted mucus, 5-HT, and fluid secretion (Hegde and Eglen, 1996). Luminal HCO3− secretion is an important component of normal colonic fluid and electrolyte movement and is a major fraction of the fluid secreted in several diarrheal diseases. It has been recently found that short-chain fatty acids (SCFAs) stimulate HCO3− secretion. This effect involves several steps, including the following ones. SCFA via the stimulation of a GPCR receptor localized on enterochromaffin cells (free fatty acid 2) increases 5-HT release, and 5-HT4 receptors localized on epithelial cells and afferent neurons stimulate HCO3− secretion (Akiba et al., 2015).
4. Visceral Pain
Tegaserod, a 5-HT4 receptor agonist, administered orally in human reduces abdominal pain and discomfort (De Maeyer et al., 2008b; Sanger, 2008). Because tegaserod is also a 5-HT2B receptor antagonist, its exact mechanism of action is not clear. Hoffman et al. (2012) found that on a colorectal distension model of visceral pain, a more selective 5-HT4 receptor agonist (naronapride) is also active and that both tegaserod and naronapride effects were blocked by a 5-HT4 receptor antagonist. The mechanism of this antivisceral pain action of 5-HT4 receptor agonists is unknown.
5. Enteric Nervous System Development and Enteric Nervous System Adult Neurogenesis
5-HT4 receptors are important in the early development of enteric neurons, whose number increases through 4 months of age (Gershon, 2013). In mice lacking 5-HT4 receptors (KO), the early increase fails, and the later decline is more severe. 5-HT4 receptors specifically protect cultured enteric neurons from apoptosis and also activate CREB (Liu et al., 2009). In adults, 5-HT4 receptors are also able to stimulate neurogenesis in mice (Gershon, 2011). Adult neurogenesis appears to only start when ENS is injured, either following rectal transection and end-to-end anastomosis or chemical ablation with, for example, benzalkonium chloride (Matsuyoshi et al., 2010; Laranjeira et al., 2011). In the former model, mosapride, a 5-HT4 receptor agonist, promoted the regeneration of the neural circuit in the impaired myenteric plexus and the recovery of the defecation reflex in the distal gut (Matsuyoshi et al., 2010; Kawahara et al., 2012).
6. Central Nervous System
a. Learning and memory
One of the first roles of 5-HT4 receptors in vivo was their promnesic effect in rat, mouse, monkey, and human (Eglen et al., 1995b; Bockaert et al., 2008, 2011; Haahr et al., 2013; Meneses, 2015). Procognitive effects of 5-HT4 receptor agonists have been described, both on short-term memory (such as social olfactory memory) and on long-term olfactory memory (such as olfactory associative memory). Acute treatments with 5-HT4 receptor agonists induced an increase in memory acquisition in autoshaping task, object and social recognition, Morris water-maze, task with long intertrial intervals (2 hours), delayed matching performance, and impeded spontaneous alteration scores (King et al., 2008; Bockaert et al., 2011). Chronic treatments also improve memory performance (Quiedeville et al., 2015) and increase working memory. Old rats have poor memory relative to adult rats that can be upgraded with 5-HT4 receptor agonists, which are also reported to antagonize atropine- or scopolamine-induced learning and memory deficits in a variety of tasks, such as spatial navigation in Morris water maze, spontaneous alternation, and olfaction (Lamirault and Simon, 2001; Lelong et al., 2003; King et al., 2008; Marchetti et al., 2011). In 5-HT4 receptor KO mice, the loss of learning and memory appears to be circumvented by adaptative changes in cholinergic systems (Buhot et al., 2003).
Hippocampal 5-HT4 receptor expression correlates inversely with human memory (Haahr et al., 2013). The cellular basis of such learning and memory effects may be an increase in acetylcholine release found in frontal cortex and hippocampus, a complex modulation of synaptic plasticity (long-term potentiation and LTD) and a potentiation of learning-induced spine growth in the hippocampus [see Kemp and Manahan-Vaughan (2004), King et al. (2008), and Bockaert et al. (2011)]. Interestingly, the 5-HT4 receptor agonist SL65.0155 enhances simultaneous olfactory discrimination performance and potentiates learning-induced dendritic spine growth in the mouse hippocampus (Restivo et al., 2008). Finally, an association between 5-HT4 receptor mRNA and protein expression in cortical areas, hippocampus, olfactory tubercles on one hand, and memory consolidation on the other hand has been reported (Manuel-Apolinar et al., 2005).
b. Control of mood and role of 5-HT4 receptor agonists
SSRIs are common drugs to treat depression that eventually increase tonic 5-HT release in relevant limbic structures such as the hippocampus. It has been established that stimulation of 5-HT4 receptors localized on pyramidal mPFC stimulate the activity of a fraction of DRN 5-HT neurons (responder neurons with high-frequency discharges), an effect expected to result in a positive effect on mood behavior (Lucas and Debonnel, 2002; Lucas, 2009). Thus, 5-HT4 receptor agonists may have relatively fast antidepressant actions or may be used in combination to reduce the delay of action of SSRIs when given alone (Samuels et al., 2016).
5-HT4 receptors interact with p11, a protein that is downregulated in brain of depressed patients. This interaction is particularly clear in brain regions important for anxiety/depression and cognition such as the hippocampus (Warner-Schmidt et al., 2009; Egeland et al., 2011). The antidepressant-like activity of RS 67333, a 5-HT4 receptor agonist, was abolished in p11-KO mice, which are known to have a depressive-like phenotype (Warner-Schmidt et al., 2009).
Adult hippocampal neurogenesis in the subgranular zone has gained considerable attention in relation to mood and depression. The hypothesis is that a decrease in newborn dentate granule cell production leads to depression, whereas enhanced neurogenesis (proliferation, survival, and maturation) is required for treatment of depression (Duman and Monteggia, 2006; Samuels et al., 2016). Chronic antidepressant treatments, including SSRIs, stimulate adult hippocampal neurogenesis (proliferation of newborn cells as well as the survival and maturation of the young neurons). 5-HT4 receptor agonists can also induce a rapid neurogenesis in the hippocampus in adult rodents (Duman and Monteggia, 2006; Pascual-Brazo et al., 2012; Ishizuka et al., 2014) and the synthesis of BDNF, a key growth factor implicated in antidepressant actions and neurogenesis. Interestingly, the 5-HT4 receptor antagonist GR 125487 partially blocks the neurogenic effects of chronic fluoxetine treatment and its antidepressant and anxiolytic effects (Mendez-David et al., 2014), which may relate to a reduced hippocampal 5-HT release evident with 5-HT4 receptor antagonists (Ge and Barnes, 1996). In addition to neurogenesis, SSRIs can reverse neuronal maturation in the adult hippocampal granular cell neurons via 5-HT4 receptor–mediated signaling (Kobayashi et al., 2010).
c. Feeding behavior
In 5-HT4 receptor KO mice, the stress-induced hypophagia and novelty-induced exploratory activity were reduced (Compan et al., 2004). This suggests that 5-HT4 receptors may be involved in stress-induced anorexia. Anorexia and bulimia are motivation disorders, which may implicate a reward structure such as nucleus accumbens that displays relatively high expression of 5-HT4 receptors. Direct stimulation of 5-HT4 receptors in the nucleus accumbens reduced the physiologic drive to eat and increased the mRNA of the anorectic peptide cocaine- and amphetamine-regulated transcript in WT but not KO mice (Jean et al., 2007, 2012). 5-HT4 receptors control cocaine- and amphetamine-regulated transcript mRNA expression via a cAMP/PKA signaling pathway. Finally, intra-accumbal injection of 5-HT4 receptor antagonists or siRNA-mediated 5-HT4 receptor KO decrease satiety (Jean et al., 2007).
d. Cardiac system
5-HT via 5-HT4 receptors induces cardioexcitation in atrial but not in ventricles of healthy humans and pig (Kaumann, 1990; Kaumann et al., 1991; Jahnel et al., 1992). However, an inotropic effect can also be found in human and porcine cardiac ventricle under phosphodiesterase inhibition (Brattelid et al., 2004b). In the atrium, 5-HT4 receptors activate and phosphorylate L-type Ca2+ channels via a cAMP/PKA signaling pathway (Ouadid et al., 1992; Kaumann and Levy, 2006). It has been proposed that atrial fibrillation, mediated by 5-HT4 receptors, may occur in case of altered circulation during, for example, ageing (Kaumann and Levy, 2006). 5-HT4 receptors expressed in sinoatrial node in piglets, and possibly in humans, may be responsible for some 5-HT–mediated arrhythmias (Sanders and Kaumann, 1992). In addition, in failing human heart (Brattelid et al., 2004b), there is an upregulation of 5-HT4 receptor mRNA level in ventricles accompanied by a 5-HT4 receptor–mediated positive inotropic response to 5-HT. Similar observations have been reported in infarcted, failing, and hypertrophic rat hearts (Qvigstad et al., 2005a,c; Brattelid et al., 2007a). Note that 5-HT4 receptors are not expressed in healthy rat ventricles. Because in those pathologies there is an increase in plasma 5-HT concentration, likely released from platelets, this may result in cardiotoxic effects mediated via 5-HT4 receptors.
Cardiac remodeling in heart failure is characterized by activation of a fetal gene program. Indeed, Brattelid et al. (2012) found, in rat, that 5-HT4 receptor mRNA expression and 5-HT4 receptor–mediated inotropic response are augmented not only in heart failure but also in ventricles during late fetal development. In rodent cardiomyocytes, prucalopride responses, characterized by a high propensity to trigger diastolic Ca2+ events, absent in controls, can be achieved following BDNF and imipramine treatments (Meschin et al., 2015). Because both treatments increase expression of p11, the authors are logically proposing that it is p11 that upregulates 5-HT4 receptor responses as seen in the brain. This is likely but not formally demonstrated in the report. The difference in p11 expression in human and rodent cardiomyocytes could explain their different responsiveness to 5-HT4 receptor agonists. This remains to be demonstrated.
For further discussion, see XIX. 5-HT Receptors and the Cardiovascular System.
e. Respiratory system
At the level of the brainstem central respiratory center, the Pre-Boetzinger complex, 5-HT4(a) receptors via a cAMP signaling pathway, control respiration (Manzke et al., 2003). They stimulate phrenic nerve activity and respiratory minute volume. Thus, 5-HT4 receptors are able to avert opioid-induced breathing depression, likely by having an opposing effect on the cAMP decrease induced by those drugs in the respiratory center (Manzke et al., 2003). Although noncoding variant SNPs in HTR4 are associated with pulmonary diseases in human genome?wide association studies (Hancock et al., 2010; Repapi et al., 2010; Soler Artigas et al., 2011; Hodge et al., 2013), the relatively low expression of 5-HT4 receptors in lungs cast some doubt on the functional impact of 5-HT4 receptors in pulmonary functions (Hodge et al., 2013). However, 5-HT4 receptor KO mice display an altered baseline lung function (lung resistance, tissue resistance, and tissue elastance) and an increase in methacholine and 5-HT–induced airway hyper-responsiveness (House et al., 2015).
f. Adrenal gland
In the human adrenal gland, 5-HT4 receptors are mainly localized on zona glomerulosa (Lefebvre et al., 2015), which is consistent with 5-HT stimulating preferentially aldosterone secretion rather than cortisol in vitro. Similarly, 5-HT4 receptor agonists administered to healthy individuals increases plasma aldosterone levels without any change in plasma cortisol concentrations (Lefebvre et al., 2015).
g. Urinary bladder
Neuromuscular cholinergic transmission in human isolated detrusor muscle is facilitated by neural 5-HT4 receptors (Tonini et al., 1994). In contrast, in urinary bladder strips from rhesus and Cynomolgus monkeys, experiments using direct electrical stimulation of bladder smooth muscle indicate that the 5-HT4 receptors are located postjunctionally (Waikar et al., 1994).
XII. 5-HT5A Receptors
A. Introduction
In the early 1990s, the genes encoding the human and rodent 5-HT5A receptor genes were cloned and localized. Subsequent work in the mid-1990s revealed the primary protein structure, expression pattern, and cellular function of the receptor using in vitro preparations (Plassat et al., 1992; Erlander et al., 1993; Matthes et al., 1993; Rees et al., 1994). Yet, the function of the receptor in vivo remained elusive until the early 2000s. By generating the Htr5A receptor knockout mouse, Grailhe et al. (1999, 2001) were the first to provide evidence of 5-HT5A receptor function in vivo. Subsequently, an ex vivo approach was used to characterize the electrophysiological effects of 5-HT5A receptors in native mouse and rat cortical brain tissue and the consequences of its deletion (Goodfellow et al., 2012), which led to the Receptor Nomenclature Committee promoting to receptor status (i.e., 5-ht5a to 5-HT5A receptor). Though further investigations have been pursued in vivo (Curtin et al., 2013; Muñoz-Islas et al., 2014; Yamazaki et al., 2014, 2015), clinical examination has been severely limited by the lack of selective ligands.
B. Gene and Primary Structure
The human HTR5A is located on chromosome 7 at position 7q36 (Matthes et al., 1993). Partial sequencing revealed an intron-exon boundary located in the middle of the third cytoplasmic loop at exactly the same position as in the mouse and rat 5-HT5A genes (Rees et al., 1994; Grailhe et al., 2001). The homologous mouse Htr5A is located on chromosome 5 at position 5B (Matthes et al., 1993). Partial sequence analysis revealed that the 5-HT5A receptor gene contains an intron in the third cytoplasmic loop (Matthes et al., 1993), approximately 8 kb in length (Matthes et al., 1993). The human 5-HT5A receptor, composed of a 1071-bp open reading frame, displays approximately 82% nucleotide homology with the mouse 5-HT5A gene (Rees et al., 1994; Grailhe et al., 2001).
Polymorphisms in the 5-HT5A receptor have been reported, including two nucleotide substitutions in the 5′ untranslated region and two synonymous substitutions in the coding region as well as a Pro15Ser amino acid substitution near the N terminus (Shimron-Abarbanell et al., 1997; Iwata et al., 1998; Birkett et al., 2000; Iwata et al., 2001; Arias et al., 2001; Dubertret et al., 2004). The latter polymorphism is proximal to a phosphorylation site and is located in a region likely involved in agonist-induced downregulation (Iwata et al., 1998; Thomas, 2006). Additional polymorphisms in the promoter region of the 5-HT5A receptor have been identified (Zhang et al., 2010).
Hydropathy analysis revealed that the 5-HT5A receptor contains seven hydrophobic domains (numbered I–VII), a classic feature of GPCRs (Plassat et al., 1992; Erlander et al., 1993; Matthes et al., 1993; Rees et al., 1994; Grailhe et al., 2001; Thomas et al., 2004). Sequencing analysis of the 5-HT5A receptor protein uncovered a single long open reading frame and a poly A tail (Plassat et al., 1992; Matthes et al., 1993; Hurley et al., 1998; Grailhe et al., 2001; Thomas et al., 2004). The length of the 5-HT5A receptor protein is 357 amino acids for the human, mouse, and rat proteins (Plassat et al., 1992; Erlander et al., 1993; Matthes et al., 1993; Rees et al., 1994; Hurley et al., 1998; Grailhe et al., 2001) but 356 amino acids in length for the guinea pig receptor (Thomas et al., 2004). In all species, the 5-HT5A receptors display N-linked glycosylation sites on the amino terminals as well as consensus phosphorylation sites for protein kinase C and protein kinase A on the presumed cytoplasmic domains (Plassat et al., 1992; Erlander et al., 1993; Grailhe et al., 2001).
C. Expression Profile
The 5-HT5A receptor is expressed widely in the central nervous system (Plassat et al., 1992; Rees et al., 1994; Pasqualetti et al., 1998a; Grailhe et al., 2001; Kinsey et al., 2001; Tanaka et al., 2012; Fig. 21) but seemingly not in peripheral organs (Plassat et al., 1992; Rees et al., 1994; Grailhe et al., 2001). There is some controversy over its expression in the peripheral nervous system (Pierce et al., 1996; Chen et al., 1998a; Wang et al., 2000; Nicholson et al., 2003; Avila-Rojas et al., 2015).
Fig. 21.
In situ hybridization detection of 5-HT5A receptor mRNA expression in mouse brain. (A) Dark field of the emulsion autoradiograph of a horizontal section through an adult mouse brain, with images at higher magnification showing (B) hippocampus and (C) cerebellum. CA1-3, CA fields of the hippocampus CA; Cb, cerebellum; Cx, cerebral cortex; DG, dentate gyrus; G, granule cell layer of the cerebellum; H, hippocampus; OB, olfactory bulb. Adapted from Plassat et al. (1992) (with permission).
In the human brain, the 5-HT5A receptor mRNA expression has been reported in many regions of the cerebral cortex, including prefrontal, parietal, temporal, occipital, and entorhinal cortices (Pasqualetti et al., 1998b; Grailhe et al., 2001; Lambe et al., 2011; Fig. 22). The 5-HT5A mRNA was also detected in the hippocampus, the amygdala, the cerebellum, the striatum, the caudate nucleus, and the substantia nigra (Rees et al., 1994; Pasqualetti et al., 1998a; Grailhe et al., 2001; Fig. 22). Similar expression patterns have been reported in the mouse (Plassat et al., 1992; Tanaka et al., 2012) and the rat (Erlander et al., 1993; Kinsey et al., 2001; García-Alcocer et al., 2010; Lopez-Esparza et al., 2015). Expression was also noted in the suprachiasmatic nucleus of the hypothalamus, raphe nuclei, the ventral tegmental area, and the locus coeruleus (Duncan et al., 2000; Oliver et al., 2000). Because the 5-HT5A receptor mRNA has not been detected in astrocytes (Hirst et al., 1998), expression of this receptor in the central nervous system is likely restricted to neuronal cells, consistent with the morphology of cells expressing 5-HT5A receptor immunoreactivity (Oliver et al., 2000).
Fig. 22.
In situ hybridization detection of 5-HT5A receptor mRNA expression in human brain. (i and ii) Dark-field autoradiographs of coronal sections of human hippocampus and surrounding regions. CA1, CA3 fields and the dentate gyrus (DG) of hippocampus, entorhinal cortex (EC), and subiculum (S). Scale bars, 0.2 cm. (A) Dark-field autoradiograph of a coronal section of the cerebellar cortex: the Purkinje cells are heavily labeled [high magnification of Purkinje cells in bright-field (B) and dark-field (C)]. Scale bars, 600 µm (A), 500 µm (B), and 900 µm (C). Adapted from Pasqualetti et al. (1998b) (with permission).
D. Post-translational Modifications and Impact
Treatment with the N-glycosylation inhibitor tunicamycin fails to alter [3H]5-HT binding to the 5-HT5A receptor yet results in redistribution of the 5-HT5A receptor from the cell membrane to the intracellular compartment, suggesting that the N-glycosylation sites may play a role in targeting the 5-HT5A receptor to the cell membrane (Dutton et al., 2008). Although both the N6 and N21 residues appear N-glycosylated within the mature human receptor protein, amino acid mutagenesis demonstrates that N-glycosylation of only the N6 residue results in protein insertion into the cell membrane (Dutton et al., 2008), a presumed prerequisite for receptor function.
E. Pharmacology
The psychedelic hallucinogen LSD binds to and stimulates the 5-HT5A receptor with high affinity, as does another nonselective 5-HT receptor agonist, 5-CT. However, the quest to characterize the functions of the 5-HT5A receptor in native tissue has been limited by a lack of selective agonists. Such characterization has relied on nonselective agonists in the presence of antagonists for other 5-HT receptors (Goodfellow et al., 2012).
Among selective antagonists for 5-HT5A receptors, SB699551 (Corbett et al., 2005) has been most widely used in the literature (Table 17). It is important to note, however, that although SB-699551 possesses high affinity for the human and guinea pig 5-HT5A receptor (∼pKi 8.3), it displays a 100-fold lower affinity for the rat and mouse 5-HT5A receptor (pKi 6.3; Thomas et al., 2006), limiting its utility in preclinical studies.
TABLE 17.
Affinities of selective antagonists at 5-HT5A and other 5-HT receptors
Adapted from data reported in Corbett et al. (2005) (for SB699551; compound 11a), Thomas (2006) (for SB699551), and Yamazaki et al. (2015) (for ASP5736, AS2030680, and AS2674723). Values are binding IC50 or Ki (where indicated). Note Ki values are given for the human, mouse, and rat cloned 5-HT5A receptors, whereas the other values have been reported mainly for the human receptor (also see Thomas et al., 2004 for guinea pig values).
| Receptor | Species | SB699551 | ASP5736 | AS2030680 | AS2674723 |
|---|---|---|---|---|---|
| 5-HT1A | Human | Ki = 501 nM | >1000 nM | Ki = 21 nMa | Ki = 84 nM |
| 5-HT1B | Human | Ki = 316 nM | NR | NR | NR |
| 5-HT1D | Human | Ki = 398 nM | NR | NR | NR |
| 5-ht1e | Human | Ki > 1000 nM | NR | NR | NR |
| 5-HT1F | Human | Ki > 1000 nM | NR | NR | NR |
| 5-HT2A | Human | Ki = 794 nM | >1000 nM | >300 nM | >300 nM |
| 5-HT2B | Human | Ki = 1000 nM | >1000 nM | Ki = 22 nMa | >300 nM |
| 5-HT2C | Human | Ki = 398 nM | Ki = 287 nM | >300 nM | >300 nM |
| 5-HT3 | Human | NR | >1000 nM | >100 nM | >300 nM |
| 5-HT4 | Guinea pig | NR | >1000 nM | >100 nM | >300 nM |
| 5-HT5A | Human | Ki = 3 - 6 nMa | Ki = 4 nMa | Ki = 1 nMa | Ki = 1 nMa |
| 5-HT5A | Rat | Ki = 501 nM | Ki = 2 nMa | Ki = 1 nMa | Ki = 1 nMa |
| 5-HT5A | Mouse | Ki = 501 nM | Ki = 4 nMa | Ki = 3 nMa | Ki = 2 nMa |
| 5-HT6 | Human | Ki > 1000 nM | >1000 nM | Ki = 39 nM | >300 nM |
| 5-HT7 | Human | Ki > 1000 nM | Ki = 123 nM | Ki = 10 nMa | Ki = 7 nMa |
NR, not reported.
High affinity binding at 5-HT5A receptors or medium- to high-affinity nonselective binding.
Other 5-HT5A receptor antagonists have been proposed, including A-842377 (Garcia-Ladona et al., 2006) and 2-aminodihydroquinazolines (Peters et al., 2008). However, only SB699551 and a series of compounds, ASP5736 (Yamazaki et al., 2014, 2015), AS2030680, and AS2674723 (Yamazaki et al., 2015) have extensive published pharmacological validation. Nonselective antagonists for the 5-HT5A receptor include methiothepin and ritanserin (Thomas et al., 2004).
Based on rank order of affinity alone, the following in vitro pharmacological profile can be suggested for the human and mouse 5-HT5A receptors: LSD (human pKi 8.40–8.70; mouse pKi 9.10) > 5-CT (human pKi 7.6–8.28; mouse pKi 7.8) > 5-HT (human pKi 6.7–7.40; mouse pKi 6.6–7.12) > 8-OH-DPAT (human pKi 5.6–6.07; mouse pKi 6.13–5.9) (Plassat et al., 1992; Rees et al., 1994; Francken et al., 1998; Grailhe et al., 2001; Thomas et al., 2004). By contrast, the human and mouse 5-HT5A receptors display low affinity for ketanserin, norepinephrine, dopamine, or spiperone (pKi < 5) (Plassat et al., 1992; Rees et al., 1994; Francken et al., 1998).
F. Function
1. Cellular Function
Functional effects of 5-HT5A receptors have been examined in cell systems, in native tissue, and in vivo. Broadly, these data suggest that the 5-HT5A receptor can activate pertussis toxin–sensitive G proteins (Francken et al., 1998; Hurley et al., 1998; Thomas et al., 2004) and suppress adenylyl cyclase and production of cAMP (Francken et al., 1998; Hurley et al., 1998; Thomas et al., 2000; Noda et al., 2003). Electrophysiological examination in native rodent cerebral cortex is consistent with coupling to Gαi/o proteins that activate Kir3 channels (Goodfellow et al., 2012). Studies in vivo indicate 5-HT5A receptors may influence cognitive function and memory (Gonzalez et al., 2013; Yamazaki et al., 2014, 2015; Nikiforuk et al., 2016) and participate in acoustic startle circuitry (Curtin et al., 2013) and potentially antinociception (Muñoz-Islas et al., 2014).
2. G Protein Coupling of the 5-HT5A Receptor In Vitro
To elaborate on the functional work in cell systems, treatment of cell lines transfected with the 5-HT5A receptor with either 5-HT or 5-CT increases labeled GTPγS binding (Francken et al., 1998; Hurley et al., 1998; Thomas et al., 2004), an effect that could be abolished by pretreatment with pertussis toxin (Francken et al., 1998; Thomas et al., 2004). Conversely, pretreatment of the nonhydrolysable GTP analog, Gpp(NH)p, reduced the high affinity binding of [3H]5-CT in transfected HEK293 cells (Francken et al., 1998). Moreover, stimulation of the 5-HT5A receptor with 5-HT can reduce the basal activity of adenylyl cyclase (Noda et al., 2003) and inhibit forskolin-induced formation of cAMP in both C6 glioma and HEK293 cell lines (Francken et al., 1998; Hurley et al., 1998; Thomas et al., 2000; Noda et al., 2003). Moreover, pretreatment with pertussis toxin prevented this 5-HT–elicited inhibition of basal adenylyl cyclase activity (Noda et al., 2003). These findings are also consistent with the 5-HT5A receptor coupling to a Gαi/o protein pathway. However, this inhibitory effect of the 5-HT5A receptor on cAMP could not be replicated in transfected HeLa or COS-M6 cells (Erlander et al., 1993). Similarly, in both HEK and NIH-3T3 cell lines, stimulation of the transfected 5-HT5A receptors had no detectable effect in basal cAMP levels, forskolin-induced cAMP levels, or accumulation of inositol phosphates (Grailhe et al., 2001). In C6 glioma cells, 5-HT5A receptor has been shown to inhibit ADP-ribosyl cylase activity in response to 5-HT, an effect that was abolished following pretreatment with pertussis toxin (Noda et al., 2003).
3. G Protein Coupling of the 5-HT5A Receptor Ex Vivo
Research in native tissue has shown that cerebral cortical 5-HT5A receptors couple to G proteins and has assessed the electrophysiological consequences of such coupling. In the presence of clozapine and spiperone, cortical slices from wild-type mice display a rightward shift of the [3H]5-CT binding following pretreatment with Gpp(NH)p, suggesting that in the native system, the 5-HT5A receptor is coupled to G proteins (Waeber et al., 1998). The 5-HT5A receptor–activated ion current has been characterized in native prefrontal cortical tissue of both rats and mice (Goodfellow et al., 2012). The native 5-HT5A receptor produces a small outward current that has a potent inhibitory effect on excitability of layer V pyramidal neurons of the prefrontal cortex. 5-HT has submicromolar potency at the native 5-HT5A receptor and activates inwardly rectifying potassium channels (Goodfellow et al., 2012).
4. 5-HT5A Receptor Function in the Spinal Cord
Spinal 5-HT5A receptors are upregulated by painful stimuli (Muñoz-Islas et al., 2014) and have been suggested to participate in antinociception and pain (Doly et a., 2004; Muñoz-Islas et al., 2014; Avila-Rojas et al., 2015). Their spinal localization suggests possible motor, somatosensory, and autonomic functions (Doly et al., 2004).
5. Function In Vivo
Grailhe et al. (1999) generated 5-HT5A receptor knockout mice. In terms of its biologic phenotype, the central nervous system of the 5-HT5A receptor knockout mice appeared normal. There were no major differences in the cytoarchitectonic divisions, neuronal morphology, or distribution of glia (Grailhe et al., 1999). Moreover, 5-HT5A receptor knockout mice display no differences in the distribution of neuronal markers for the monoaminergic system, calcium binding protein, neuropeptides, nitric oxide synathase, or amino acid receptor subunits (Grailhe et al., 1999).
There appears to be the potential for significant cross talk between 5-HT5A receptors and 5-HT1A receptors in cerebral cortex given their electrophysiological function (Goodfellow et al., 2012, 2014). In mice constitutively deleted for Htr5A, however, there were striking, supracompensatory changes in the magnitude of the prefrontal 5-HT1A receptor currents, with 5-HT5A receptor knockout mice displaying nearly a doubling of the 5-HT1A receptor outward current in layer V neurons from the prefrontal cortex (Goodfellow et al., 2012). The mechanism underlying this homeostatic plasticity is unknown. Further electrophysiological examination of 5-HT5A receptor knockout mice revealed no difference in GABAB receptor–mediated outward current, 5-HT1A receptor protein levels, or cell intrinsic properties in the adult prefrontal cortical neurons (Goodfellow et al., 2012).
Mice deleted for Htr5A display an increase of explorative-like behaviors, rather than anxiety-like behaviors, on the open field, the elevated plus maze, and the marble burying task (Grailhe et al., 1999). Moreover, 5-HT5A receptor knockout mice display greater increase in the number of entries into the central region after the introduction of the novel object into the center of the open field, suggesting an increase in “inspective” explorative-like behaviors (Grailhe et al., 1999). In addition, 5-HT5A receptor knockout mice displayed a reduction on LSD-elicited increase in locomotion in the open field (Grailhe et al., 1999). These changes occurred in the absence of any difference in motor activity (Grailhe et al., 1999). Given the evident considerable homeostatic plasticity detected in the cerebral cortex of the 5-HT5A receptor knockout mice (strong upregulation of 5-HT1A receptor–mediated effects; Goodfellow et al., 2012), the behavioral characterization of the 5-HT5A receptor knockout may underestimate its behavioral effects.
G. Clinical Relevance
The 5-HT5A receptors are positioned neuroanatomically to play a role in emotional regulation, cognition, antinociception, and control of circadian rhythms and metabolism (Plassat et al., 1992; Pasqualetti et al., 1998a,b; Oliver et al., 2000; Duncan et al., 2000; Grailhe et al., 2001; Doly et al., 2004; Lambe et al., 2011; Tanaka et al., 2012). Unfortunately, clinical examination of 5-HT5A receptors has been restricted by the lack of selective ligands.
1. Psychosis and Depression
A nonsynonomous SNP (Pro15Ser) in the HTR5A gene has been linked to schizophrenia (Iwata et al., 2001; Dubertret et al., 2004) and depression (Birkett et al., 2000), and polymorphisms in the promoter region of the 5-HT5A receptor link to elevated triglyceride levels (Zhang et al., 2010). The high affinity of the psychedelic hallucinogen LSD for the 5-HT5A receptor also underscores a potential link to psychosis (Thomas, 2006), keeping in mind that LSD, like many other ergolines, has affinity for multiple 5-HT receptors, of which only the 5-HT2A receptor has been shown to play a significant role in hallucinations or psychosis. Yet in additional relevance to schizophrenia, 5-HT5A receptors have been suggested to participate in prepulse inhibition of the acoustic startle response (Curtin et al., 2013). This effect was demonstrated in goldfish with SB-699551 and with A-843277. The latter compound has been reported to have antipsychotic and antidepressant properties in rodent models, in line with the distribution of the receptor in higher cortical and limbic regions (Garcia-Ladona et al., 2006; Jongen-Relo et al., 2006; but see Kassai et al., 2012). Recent work suggests that blocking 5-HT5A receptors may ameliorate positive symptoms in a rodent model of schizophrenia and will potentially enhance cognition and social behavior in such models (Yamazaki et al., 2014; Nikiforuk et al., 2016). It may be relevant that the atypical antipsychotic drug asenapine has high affinity for h5-HT5A receptors (along with a number of other receptors; Shahid et al., 2009).
2. Memory and Circadian Rhythm
Yamazaki et al. (2014, 2015) demonstrated the 5-HT5A receptor antagonists (ASP5736, AS2030680, and AS2674723; Table 17) benefit memory, supporting a role for 5-HT5A receptors in memory consolidation (Gonzalez et al., 2013). Additional preclinical work focuses on the localization of 5-HT5A receptors in the hypothalamus, where it is densely expressed in the suprachiasmatic nucleus (Oliver et al., 2000; Duncan et al., 2000), suggesting potential roles in circadian rhythm and metabolism.
XIII. 5-ht5b Receptors
A. Introduction
The genes encoding the human and rodent 5-ht5b receptor were identified in 1993. The human 5-ht5b receptor gene HTR5B is located on chromosome 2 at position 1q11–13 (Matthes et al., 1993). However, this gene contains stop codons in exon I, resulting in a transcriptional product likely yielding a presumed nonfunctional truncated protein (Grailhe et al., 2001). The mouse 5-ht5b receptor gene Htr5B is located on chromosome 1 at position 1F (Matthes et al., 1993) and forms a functional protein upon heterologous expression. Partial sequence analysis revealed that the 5-ht5b receptor gene contained an intron in the third cytoplasmic loop (Matthes et al., 1993; Grailhe et al., 2001).
B. Receptor Structure
Using hydropathy analysis, the predicted 5-ht5b receptor protein has been shown to contain seven hydrophobic domains (numbered I–VII), a classic feature of GPCRs (Plassat et al., 1992, 1992; Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993; Rees et al., 1994; Grailhe et al., 2001). Sequencing analysis of the 5-ht5b receptor protein revealed a single long open reading frame and a poly A tail (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993). The length of the 5-ht5b receptor is 370 amino acids in both the rat and mouse protein (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993).
C. Expression Profile
The 5-ht5b receptor has a restricted expression profile within the central nervous system. In the rodent, expression of the 5-ht5b receptor is exclusively found in the CA1 field of the hippocampus, the habenula, the inferior olivary nucleus, and the raphe nuclei (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993; Kinsey et al., 2001; Serrats et al., 2004, 2004; Tanaka et al., 2012; Fig. 23). Interestingly, the 5-ht5b receptor mRNA was strongly expressed in the medial portion of the raphe nuclei and was found to have high coexpression with 5-HT transporter, suggesting that this receptor may be localized on 5-HT–producing neurons (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993; Serrats et al., 2004; Fig. 24). Expression of the 5-ht5b receptor has not been detected in any peripheral organs, including the heart, kidney, lung, liver, or intestine (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993). Evidence for the 5-ht5b receptor being expressed primarily in endosomes rather than the cell membrane raises potentially novel mechanisms of the functional relevance of this protein (Niebert et al., 2017) by impacting cell membrane expression of the 5-HT1A receptor via intracellular direct protein-protein interaction.
Fig. 23.
In situ hybridization detection of 5-ht5b receptor mRNA expression in rat brain. Coronal (A) and horizontal (E) rat brain sections. (C) Nissl stain of the section used to generate the autoradiogram in (A). (F) Radioactive oligonucleotide probe in the presence of excess unlabeled probe. CA1 and CA3, CA fields of the hippocampus; Cb, cerebellum; CPu, caudate-putamen; Ctx, cortex; DG, dentate granule cells; Ent, entorhinal cortex; Hy, hypothalamus; MHb, medial habenula. Adapted from Wisden et al. (1993) (with permission).
Fig. 24.
Evidence for 5-HT raphe neurons expressing the 5-ht5b receptor. Top row: Visualization of 5-HTT and 5-ht5b receptor mRNAs in the dorsal raphe. (A) A consecutive section to (B and C), stained with cresyl violet for anatomic reference. The approximate limits of the dorsal raphe (DR) and its subdivisions—lateral wings and medial portion—are depicted. (B) a bright-field photomicrograph where numerous cell profiles express 5-HTT mRNA are visualized using digoxigenin-labeled oligonucleotide probes. (C) A dark-field photomicrograph from an emulsion-dipped tissue section displaying 5-ht5b receptor mRNA signal in the DR. mlf, medial longitudinal fasciculus; PAG, periaqueductal gray. Scale bar, 0.5 mm. Bottom row: Schematic representations of the rat lower midbrain and upper pons showing the subregional location of cells coexpressing 5-ht5b receptor mRNA and 5-HTT mRNA (filled circles) and cells expressing only 5-HTT mRNA (empty circles) in the DR and central superior nucleus (CS or median raphe). AQ, cerebral aqueduct; AT, anterior tegmental nucleus; dscp, decussation of the superior cerebellar peduncle; IPN, interpeduncular nucleus; tsp, tectospinal pathway; VTA, ventral tegmental area; VTN, ventral tegmental nucleus. Adapted from Serrats et al. (2004) (with permission).
D. Regulatory Mechanisms and Post-translational Modifications
The 5-ht5b gene expression is regulated by the transcription factor ATF-7, which can directly bind to the 5-ht5b promoter region, resulting in histone methylation and, ultimately, silencing of the receptor gene transcription (Maekawa et al., 2010). The 5-ht5b receptor displays N-linked glycosylation sites on the amino-terminal end as well as consensus phosphorylation sites for protein kinase C and protein kinase A on the presumed cytoplasmic domains (Erlander et al., 1993; Matthes et al., 1993; Wisden et al., 1993).
E. Pharmacology
To date, selective ligands for the 5-ht5b receptor have not been reported. Nonselective agonists include LSD and 5-CT, and a nonselective antagonist is methiothepin. Based on affinity alone, the following in vitro pharmacological profile can be suggested for rodent 5-ht5b receptors: LSD (rat pKi 7.49), 5-CT (mouse pKi 7.4; rat pKi 6.26–8.88), and methiothepin (mouse pKi 7.8; rat pKi 7.35–8.87) but low affinity for ketanserin, dopamine, and norepinephrine (Matthes et al., 1993).
F. Function at Cellular, Tissue, and In Vivo Levels
The transduction pathway of the 5-ht5b receptor has not been well examined. Pretreatment with Gpp(NH)p reduced [3H]5-CT binding in 5-ht5b–transfected COS1 cells, suggesting that the 5-ht5b receptor readily couples to G protein (Wisden et al., 1993). However, this receptor had no effect on basal cAMP levels in either HeLa or COS-M6 cell lines (Erlander et al., 1993), although high levels of 5-ht5b expression in mouse brain are associated with low cAMP levels (Vogelgesang et al., 2017).
It is speculated that the 5-ht5b receptor acts as an inhibitory autoreceptor on 5-HT neurons in the raphe in rodents. However, neither an electrophysiological nor pharmacological profile has been described for the 5-ht5b receptor in native tissue.
In a mouse model of Rett syndrome, there is a relatively high level of 5-ht5b receptor expression in the brainstem because of a failure to downregulate receptor expression (Vogelgesang et al., 2017), with further studies suggesting a role for the 5-ht5b receptor in the modulation of the complex breathing phenotype of the mouse model of Rett syndrome (Vogelgesang et al., 2018) as well a potential link to the native receptor inhibiting generation of cAMP.
G. Clinical Relevance
Functional 5-ht5b receptors are likely not expressed in humans (Grailhe et al., 2001), which limits interest in this receptor particularly from the pharmaceutical industry.
XIV. 5-HT6 Receptors
A. Introduction
The 5-HT6 receptor has moderately high affinity for 5-HT, with an apparent binding affinity of approximately 30 nM. The gene for the 5-HT6 receptor was originally cloned from rat in the early 1990s (Monsma et al., 1993; Ruat et al., 1993a) and later from human (Kohen et al., 1996) and mouse (Kohen et al., 2001) cDNA libraries; the human gene was located on chromosome 1 (Kohen et al., 1996). For each known species, the complete gene contains three exons and has a total length of around 14 kb with a coding sequence of approximately 1.4 kb. The receptor protein is composed of 440 amino acids in humans and mice and 436 amino acids in rats; a sequencing error in the originally published rat sequence was later corrected (Kohen et al., 1996). In humans, there is one described polymorphism (a C267T variant; Masellis et al., 2001) as well as a truncated, nonfunctional splice variant (Olsen et al., 1999). No replicated polymorphisms in the human gene have been associated with human diseases, including schizophrenia, methamphetamine-associated psychosis, tardive dyskinesia, Alzheimer disease, obesity, or antidepressant drug responsiveness.
The protein structure of the 5-HT6 receptor conforms to the typical motif of GPCRs with the typical seven transmembrane domain configuration. There have been no detailed investigations of whether this receptor exists in dimers or higher-order oligomers.
B. Expression
5-HT6 receptor mRNA is expressed primarily in the brain (Fig. 25), being detectable in rat brain by day 12 of embryogenesis (Grimaldi et al., 1998). Originally, Northern blot analyses detected RNA transcripts in brain tissue with the following regional density profile: hypothalamus > hippocampus = mesencephalon > cerebral cortex = olfactory bulb > olfactory tubercle (Monsma et al., 1993). Ruat et al. (1993a) also reported a very similar distribution, noting highest expression in dorsal and ventral striatum, olfactory tubercle, and hippocampus, with lower expression in stomach and very low to no expression detected in a variety of other peripheral tissues. Radioligand binding and immunohistochemical localization supported a similar distribution in rat and human brain (Gerard et al., 1997; Marazziti et al., 2013b). Using RT-PCR, Hirst et al. (2003) detected a similar distribution of 5-HT6 receptor mRNA in various regions of rat and human brain, whereas mouse brain had much lower apparent levels of 5-HT6 receptor mRNA and, unlike in other species, no enrichment in the basal ganglia. Specific binding of [125I]-SB258585, a highly selective 5-HT6 receptor radioligand, displayed the same patterns of regional localization, with substantially higher levels of 5-HT6 receptors in striatum than in other regions in rats and humans but fairly homogenous levels of expression in all of the mouse brain regions that expressed 5-HT6 receptors. Differences in 5-HT6 receptor pharmacology between these species were evident but did not explain the disparate distributions of receptor expression, highlighting important species differences in 5-HT6 receptor distribution (and pharmacology).
Fig. 25.
In situ hybridization detection of 5-HT6 receptor mRNA expression in rat brain. Autoradiographic visualization of 5-HT6 receptor mRNA in rat brain (A–D) and relative absence of signal when adjacent sections probed with the sense control (c and d). ANA, anterior nucleus accumbens; CgCx, cingulate cortex; Cx, cortex; DG, dentate gyrus; Hb, habenula; Hp, hippocampus; OT, olfactory tubercle; PfCx, prefrontal cortex; PyCx, pyriform cortex. Adapted from Ward et al. (1995) (with permission).
The cellular profile of expression has been examined in some detail in several brain regions. In the hippocampus and cortex, 5-HT6 receptor mRNA is present in glutamatergic neurons containing vGluT1 mRNA as well as a subset of GABAergic interneurons that coexpress the 5-HT3A receptor; 5-HT6 receptor mRNA is also detected in other classes of interneurons at a lower frequency (Helboe et al., 2015). 5-HT6 receptor immunoreactive glial cells have also been reported in human cortex (Lorke et al., 2006; Marazziti et al., 2013b). In striatum, 5-HT6 receptor mRNA is expressed in both direct and indirect pathway medium spiny neurons (Ward and Dorsa, 1996; Helboe et al., 2015) and occasionally in cholinergic interneurons (Bonsi et al., 2007; Helboe et al., 2015).
5-HT6 receptors are not presynaptic autoreceptors on 5-HT neurons, as 5-HT6 receptor mRNA in rat is not altered by ablation of the DRN with 5,7-dihydroxtryptamine (Gérard et al., 1996) and is not colocalized with SERT mRNA (Helboe et al., 2015). Nonetheless, their abundant expression on forebrain cortical neurons thought to project to DRN 5-HT neurons may explain how 5-HT6 receptor ligands can modify 5-HT neuronal firing (see Electrophysiology below).
The localization of 5-HT6 receptors within neurons is still somewhat controversial, perhaps because of differences in antibody specificity. Initial studies described 5-HT6 receptors in the neuropil and electron microscopy suggested that immunolabeling was predominantly associated with dendrites (Gerard et al., 1997). A subsequent electron microscopy study revealed that 5-HT6 receptor immunolabeling is not only associated with dendrites but also with primary neuronal cilia (Hamon et al., 1999; Brailov et al., 2000). This is a surprising result because there are few GPCRs that localize to primary neuronal cilia (e.g., somatostatin sst3 receptor), and the 5-HT6 receptor is the only 5-HT receptor that may do so (Berbari et al., 2008). The ciliary localization may require the presence of key amino acids in the third intracellular loop of the receptor, which is the case for 5-HT6 receptors in mice, rats, and humans (Berbari et al., 2008). This subcellular distribution may have implications for interactions with other signaling proteins, such as adenylyl cyclase 3, which is localized exclusively in primary cilia (Baker et al., 1998), and resultant functional consequences of 5-HT6 receptor signaling. However, the distribution and function of 5-HT6 receptors that are heterologously expressed may be strongly dependent on a number of factors, including the differentiation state of the neuron and the amount of receptor expressed. As an example, exogenous overexpression of transfected 5-HT6 plasmid DNA has a strong effect on cilia localization, with heavy expression leading to increased rates of nonciliary localization.
C. Pharmacology
There are important species differences between mammalian 5-HT6 receptors. The mouse 5-HT6 receptor has similar sequence homology to the homologous receptor of human, pig, or rat, but the pharmacological profile is much closer between human and rat than mouse (Setola and Roth, 2003). Hirst et al. (2003) detected marked differences in the affinity of a number of ligands for the 5-HT6 receptor in these three species. For example, the antagonist compound Ro 04-6790 binds to recombinant rat or human 5-HT6 receptors with similar affinity (pKi ∼7.4) but has relatively very low affinity for mouse 5-HT6 receptor (pKi < 4); several other antagonists (including SB-258585 and mianserin) have lower affinities (5- and 12-fold, respectively) at mouse compared with rat or human receptors (Hirst et al., 2003); these differences are explained to some degree by variances in the binding pocket as demonstrated by site-directed mutagenesis. On the other hand, several highly selective agonists have similar affinities for mouse, human, and rat 5-HT6 receptors.
There are relatively far lower levels of 5-HT6 receptor radioligand binding sites in mouse brain, with lesser density variation across regions, than is evident in rat and human brain. Importantly, this reduced level of receptor binding and rather homogenous density across the mouse brain is method-independent, as it is equally observed using a variety of techniques to localize the receptors. Differences in residues 188 in TM5 and 290 in TM6 contribute to these species differences.
A null mutation (knockout) for the mouse 5-HT6 receptor results in only subtle changes to the physiologic and behavioral phenotype (Bonasera et al., 2006), consistent with a limited contribution of the 5-HT6 receptor to baseline mouse behavior. Thus, rats may be a more useful model for studying the pharmacological and (patho)physiogical roles of 5-HT6 receptors in relation to humans, whereas the availability of a knockout strain makes mice very useful for studying other aspects of the biology of this receptor.
Several strategies for radiolabeling 5-HT6 receptor have been described. [125I]-SB258585 (Hirst et al., 2006) is the most selective, commercially available option; other radioligands require masking of non–5-HT6 receptor sites and include [3H]-LSD (Sleight et al., 1998), [3H]-clozapine (Glatt et al., 1995), and [11C]-GSK215083 (Parker et al., 2012, 2015).
Several selective 5-HT6 receptor agonists are available. 2-Ethyl-5-methoxy-N,N-dimethyltryptamine was the first moderately selective agonist developed (Glennon et al., 2000); it is brain penetrant and has >10-fold selectivity for 5-HT6 compared with other 5-HT receptors. More selective agonists include WAY181187, WAY208466 (Schechter et al., 2008), and ST1936 (Borsini et al., 2015). Some of these ligands display partial agonism under certain circumstances, such as EMD386088 (Jastrzebska-Wiesek et al., 2013) and E6801 (Romero et al., 2006); many nonselective 5-HT receptor agonists are either full or partial agonists at 5-HT6 receptors, which can be useful tools in specific cases, but care must be given to rule out actions at other 5-HT receptors. For instance, EMD386088 has moderate affinity (IC50 = 34 nM) for the 5-HT3 receptor (Mattson et al., 2005), and ST1936 has moderate affinity for 5-HT7, 5-HT2B, and α2-adrenoceptors (Ki = 168, 245, and 300 nM, respectively; Riccioni et al., 2011). However, the contribution of partial agonism to the behavioral or physiologic effects of 5-HT6 receptor ligands has not been thoroughly characterized.
A number of clinically effective drugs have affinity for 5-HT6 receptors but also for other receptor targets. Several antidepressants and antipsychotics have high affinity for 5-HT6 receptors (Monsma et al., 1993; Ruat et al., 1993a), but none of these are selective for the receptors. After the discovery of the 5-HT6 receptor, a number of modestly selective antagonists were reported based on high-throughput screening of chemical libraries, although these often shared affinity for other 5-HT or dopamine receptors (Upton et al., 2008). The first CNS penetrant selective 5-HT6 receptor antagonists were produced by Roche (e.g., Ro 04-6790; Sleight et al., 1998) and GlaxoSmithKline (e.g., SB-271046; Bromidge et al., 1999). Subsequently, more highly selective 5-HT6 receptor antagonists were developed, which are useful for both in vitro and in vivo experimentation. Among these are SB258585, SB399885 (Hirst et al., 2003), and Ro4368554 (Lieben et al., 2005).
In heterologous systems expressing wild-type or constitutively active 5-HT6 receptors, a number of the selective agonists may show partial agonist activity, whereas numerous antagonists display inverse agonist properties (Purohit et al., 2003; Romero et al., 2006).
The first 5-HT6 receptor somewhat selective PET ligand, [11C]-GSK215083 (with approximately fivefold-lower affinity for the 5HT2A receptor), demonstrates 5-HT6 receptor occupancy in the human striatum (Parker et al., 2012, 2015) and will no doubt help to investigate the role of this receptor in human pathology and in therapeutic response/target engagement of existing or new therapeutics or drugs.
D. Post-translational Modifications and Protein Interactions
There is a predicted glycosylation site in the amino terminal of the 5-HT6 receptor and a number of predicted phosphorylation sites (Monsma et al., 1993; Ruat et al., 1993a; Kohen et al., 1996, 2001), although none of these have been examined systematically. However, phosphorylation of serine 350 appears to be mediated by cyclin-dependent kinase 5 (Cdk5); treatment of NG108 cells with a 5-HT6 receptor–selective agonist leads to coimmunoprecipitation of 5-HT6 receptor and Cdk5, whereas the 5-HT6 receptor–selective antagonist SB258585 reduces the receptor phosphorylation and coimmunoprecipitation with Cdk5 (Duhr et al., 2014). Furthermore, bioluminescence energy transfer experiments confirm the direct interaction of this receptor and Cdk5 in transfected NG108 cells, which is also inhibited by the 5-HT6 receptor antagonist SB258585. Duhr et al. (2014) demonstrated that Cdk5-dependent phosphorylation of the 5-HT6 receptor leads to activation of Cdc42 (a Rho GTPase), which can in turn activate further downstream mechanisms. The role of serine 350 phosphorylation was confirmed by site-directed mutagenesis, which may result in constitutive activity of the receptor in the absence of agonist and inhibition of phosphorylation and downstream events by the 5-HT6 receptor–selective antagonist SB258585. These signaling events may enhance NG108 cell differentiation and neurite outgrowth both in this cell line and in striatal and cultured hippocampal and striatal neurons.
Using HEK293 cells transfected with epitope-tagged 5-HT6 receptors, Marin’s group also detected direct interactions of this receptor with mTOR and several related proteins in the mTOR complex 1 (Meffre et al., 2012). 5-HT6 receptor–selective agonists induce mTOR activation, social cognition impairments in mice, and object recognition impairment in rats that could be blocked with the mTOR antagonist rapamycin. In summary, these data suggest that direct protein-protein interactions involving the 5-HT6 receptor may lead to activation of multiple signaling cascades in addition to the canonical Gs-mediated activation of adenylyl cyclase (see XVII. C. 5-HT6 Receptor Receptosome: Toward New Signaling Mechanisms Underlying Its Control of Cognition and Neurodevelopmental Processes for further discussion of protein-protein interactions with the 5-HT6 receptor).
Though not examined in detail, dimerization of 5-HT6 receptors can be inferred from the immunoprecipitation and Western blot analysis (Meffre et al., 2012; Duhr et al., 2014).
E. Signaling Pathways
There is growing appreciation for the complexity of the pharmacological and signaling properties of the 5-HT6 receptor. The ability of the 5-HT6 receptor to activate adenylyl cyclase in a Gs-dependent manner was first described when the receptor was originally cloned and expressed heterologously as well as in native tissues (Monsma et al., 1993; Ruat et al., 1993a; Sebben et al., 1994; Unsworth and Molinoff, 1994; Choi et al., 2007; Kim et al., 2014b). 5-HT6 receptors can activate several adenylyl cyclase isoforms, including AC3, AC5, and AC8, but not AC1 or AC8 (Baker et al., 1998). Because 5-HT6 receptors activate adenylyl cyclase, thereby increasing cAMP and therefore protein kinase A activity, a number of additional downstream signaling consequences can be presumed, although these have not been systematically examined. Svenningsson et al. (2002) examined the potential synergistic interactions between 5-HT6 and D1 dopamine receptors; 5-HT6 receptor activation in striatum regulates DARPP32, an enzyme previously associated mainly with dopaminergic neurotransmission. Selective 5-HT6 receptor agonists modulate the DARPP32 phosphorylation state in a manner consistent with increasing this enzyme’s activity; phosphorylation of DARPP32 is blocked by selective 5-HT6 receptor antagonists; 5-HT–mediated c-Fos activation and motor activity is blunted in DARPP32 knockout mice (Svenningsson et al., 2002). However, 5-HT regulation of DARPP32 is unlikely to involve exclusively 5-HT6 receptors, as 5-HT4 and 5-HT7 receptors can also activate DARPP32. This pathway is an interesting regulatory node that potentially integrates 5-HT and dopamine signaling in neurons that coexpress 5-HT6 and D1 receptors, such as striatonigral (direct pathway) medium spiny neurons.
The 5-HT6 receptor induces fyn kinase activation. (Yun et al., 2007; Riccioni et al., 2011). Fyn is an src tyrosine kinase family member, present in both cilia and neuronal soma. Fyn is expressed in the same brain regions as 5-HT6 receptor. Phosphorylated-Fyn is thought to activate Erk1/Erk2 kinases via the Ras-Raf-MEK pathway. Direct protein interactions with Cdk5 also predict that 5-HT6 receptors signal through this kinase pathway, which was shown to regulate neurite outgrowth in NG108 cells and primary neurons (Duhr et al., 2014). This pathway regulates neuronal migration in both slice cultures and in vivo (Jacobshagen et al., 2014). 5-HT6 receptor activation phosphorylates doublecortin and focal adhesion kinase, two mediators of neuronal migration, and is Cdk5-dependent, although additional or alternative mediators may be at play. Similarly, mTOR interactions were predicted based on studies of protein interactions, and 5-HT6 receptor agonists induce phosphorylation of mTOR into the active form, which is blocked by selective 5-HT6 receptor antagonists both in vitro and in vivo (Meffre et al., 2012).
The constitutive activity of 5-HT6 receptors represents an intriguing issue. Constitutive activity implies that the receptor couples to and activates downstream effectors in the absence of the agonist; the clearest evidence for this with 5-HT6 receptors comes from heterologous expression systems and with activation of Cdk5, Fyn, and mTOR signaling events (Meffre et al., 2012; Duhr et al., 2014; Jacobshagen et al., 2014; see XVII. C. 5-HT6 Receptor Receptosome: Toward New Signaling Mechanisms Underlying Its Control of Cognition and Neurodevelopmental Processes). However, it has been more difficult to confirm this in systems that are dependent on activation of 5-HT6 receptors expressed in native cells or tissues. For example, Sebben et al. (1994) described 5-HT6 receptor–mediated cAMP accumulation in primary cultured mouse striatal neurons, and several full agonists, such as 5-HT and LSD, caused cAMP production, but no constitutive activity was apparent. Because the cell type, signal transduction pathways, and level of receptor expression may all affect the extent of constitutive activity, its contribution to 5-HT6 function likely varies a great deal depending on the cellular context; hence, it still remains to be demonstrated with natural expression levels in vivo. However, the 5-HT6 receptor clearly has a propensity to display constitutive activity, and many of the available antagonists are in fact inverse agonists, meaning they will reduce 5-HT6 signaling if constitutive activity is present (Purohit et al., 2003).
F. Function
Prior to the availability of selective 5-HT6 receptor antagonists, antisense oligonucleotides were used to reduce expression/function of the 5-HT6 receptor; yawning and stretching behavior (Bourson et al., 1995), weight loss, and reduced retention of spatial learning in the Morris water maze were reported following receptor knockdown (Woolley et al., 2001), later replicated by the use of selective antagonists. Interestingly, the extent of 5-HT6 receptor knockdown was limited (20%–30% using [3H]LSD binding), and it is only more recently that knockout mice have been available. Tecott and Brennan (2000) produced the first 5-HT6 receptor knockout mouse and reported weight loss in a patent, which triggered the exploration of 5-HT6 receptor antagonists as appetite suppressants (Heal et al., 2008; Higgs et al., 2016). More recently, germ-line knockout of 5-HT6 receptors produced no changes in viability, health, weight, or longevity of the null mutant mice (Bonasera et al., 2006) and no deficits in fertility or maternal behavior. Furthermore, no overt changes in circadian activity, emotional behavior, sensorimotor gating, or cognition were detected. The only behavioral change evident was a reduced sensitivity to the sedative and ataxic effects of acute ethanol administration. However, mouse 5-HT6 receptor KO may underestimate the potential roles of 5-HT6 receptors in any of these effects, as mice have much lower 5-HT6 receptor expression than many other mammalian species (Hirst et al., 2003), as discussed above.
Though 5-HT6 null mutant mice seem to develop normally, there are very interesting effects of acute modulation of 5-HT6 receptors during cortical development. Using both in vivo and explant culture models, Dayer and colleagues have shown that modulating 5-HT6 receptor signaling produces deficits in neuronal migration (Jacobshagen et al., 2014). The 5-HT6 receptor partial agonist EMD386088 delays the rate of pyramidal neuron and interneuron migration (Riccio et al., 2009, 2001), which can be blocked by the antagonist SB 258585, although the concentrations of drugs used are rather high, questioning receptor classification.
The effect of activation versus inhibition of 5-HT6 receptor activity is complex, though, as short hairpin RNA knockdown of endogenous 5-HT6 receptors produces deficits in neuronal speed and extent of neuronal migration (Jacobshagen et al., 2014). This deficit is partially rescued by coexpressing 5-HT6 receptors or overexpressing Cdk5 but not by coexpressing 5-HT6 receptor mutants that disrupt Gs-coupling and thus adenylyl cyclase activation. Furthermore, 5-HT6 receptor–mediated Cdk5 signaling facilitates dendritic outgrowth. There may be additional mechanisms of 5-HT6 receptor–mediated developmental effects, as the selective agonist WAY181187 activates mTOR in mouse cortex, and this is blocked by SB258585 (Meffre et al., 2012). The psychomimetic drug phencyclidine, which models aspects of the development and symptoms of schizophrenia, induces mTOR activation in neonatal rat cortex and impairs social cognition in the adult mice, both of which are blocked by the mTOR inhibitor rapamycin or the 5-HT6 receptor–selective antagonist SB258585. Interestingly, both drugs also reverse visual recognition impairments associated with isolation rearing in rats (Marsden et al., 2011; Meffre et al., 2012). Though adenylyl cyclase, mTOR, Cdk5, and fyn kinase signaling are complex and impacted by many parallel pathways, the potential that multiple signaling pathways emanating from 5-HT6 receptors play a crucial role in establishing normal cortical circuitry has far-reaching implications given that drugs that modulate 5-HT dynamics (such as SSRIs and other antidepressants) or that directly interact with 5-HT6 receptors (whether exclusively so or not) may produce alterations in cortical development. These issues need to be examined more fully to understand the consequences of 5-HT drug treatments during gestation or in adolescence.
It is reasonable to conclude that 5-HT6 receptors may increase the excitability of neurons, as this is the expected result following activation of adenylyl cyclase. The 5-HT6 receptor agonist WAY181187 increases fos-like immunoreactivity in cortex (Burnham et al., 2010), and the 5-HT6 receptor antagonist Ro4368554 reduces scopolamine-induced increase of fos-like immunoreactivity in lateral amygdala (Mitchell et al., 2009b); however, both of these manipulations produce complex behavioral and pharmacological changes that make it difficult to conclude that the effects are directly related to 5-HT6 receptors or are more indirect consequences of circuitry level events.
5-HT6 receptors modulate many different neurotransmitter systems (Mitchell and Neumaier, 2005; Dawson, 2011). The impacts on extracellular levels of acetylcholine, glutamate, GABA, dopamine, and norepinephrine have been reported using microdialysis. Selective 5-HT6 receptor ligands alter neuronal firing in a variety of brain regions, but it is difficult to conclude whether these effects are direct or indirect. In any event, it appears that 5-HT6 receptor ligands modulate numerous brain regions and neurotransmitter systems.
There is a rich literature addressing the potential cognitive effects of 5-HT6 receptor modulation (Woolley et al., 2004; Mitchell and Neumaier, 2005; King et al., 2008; Codony et al., 2011; Marsden et al., 2011; Meneses, 2015). In most cases, 5-HT6 receptor antagonists display procognitive effects in a variety of rodent memory tasks, such as spatial learning, novel object recognition, social discrimination, and autoshaping (Mitchell and Neumaier, 2005). Generally, these experiments used parenteral drug administration either just before or just after a memory “training” session that was then assessed after a short interval. The procognitive effects of 5-HT6 receptor antagonists have often been shown to be associated with changes in other neurotransmitter systems, especially acetylcholine, glutamate, or GABA release. However, it is difficult to reach mechanistic conclusions based on the correlative nature of these observations. At this time, it is not possible to attribute the memory-enhancing effects of 5-HT6 receptor antagonists to a single brain region or neurotransmitter system, and it seems likely that multiple mechanisms are responsible.
Although there are numerous reports that inhibiting 5-HT6 receptors promotes (or restores) memory, there is some apparent inconsistency in the cognitive role of 5-HT6 receptor agonists. For example, WAY117187 (an agonist) impaired social recognition in an antagonist-sensitive manner whether given systemically or locally into frontal cortex (Loiseau et al., 2008); EMD386088 or WAY466 impaired memory in the autoshaping or social recognition tasks, respectively (Meneses et al., 2008; Schechter et al., 2008). However, other reports have observed procognitive effects of 5-HT6 receptor agonists (Kendall et al., 2011; Woods et al., 2012; Pereira et al., 2015). There are several potential explanations for these discrepancies. One explanation is that 5-HT6 receptor activation in some brain regions is procognitive, whereas inhibition of 5-HT6 receptors in other brain regions is also procognitive. Another possible explanation is that the effect of increased or decreased 5-HT6 receptor activity is entirely dependent on the specific cognitive domain being tested. A third possibility is that either high or low levels of 5-HT6 receptor activity enhance cognition in specific regions, albeit by different neurochemical mechanisms (e.g., facilitating glutamatergic function in states with a relative deficit or facilitating GABAergic function in states of excessive glutamatergic activity). Even when the same cognitive task has been used, each laboratory likely performs these tests in at least slightly different ways—with or without amnestic maneuvers (such as using scopolamine to disrupt memory function), in young or old animals, or with short- versus long-interval testing; thus, it seems unlikely that agonists or antagonists will “win” this battle, but instead there may be a role for both 5-HT6 receptor agonists and antagonists for different cognitive problems in different pathologic states.
The effects of striatal 5-HT6 receptors on autoshaping and related instrumental learning tasks have been investigated based on the early observations that 5-HT6 receptor antagonists facilitate cholinergic function (Mitchell and Neumaier, 2005); it was thought that these drugs facilitate consolidation of memory by cortical or hippocampal mechanisms. However, because 5-HT6 receptors are most heavily expressed in medium spiny neurons in striatum, these neurons might also be an important site of 5-HT6 receptor action in cognition. Because the autoshaping task used extensively by Meneses’ group was clearly sensitive to 5-HT6 receptor antagonism, this task was studied in mice with striatum-specific increased 5-HT6 receptor expression (Meneses 2015; Mitchell et al., 2007). High 5-HT6 receptor density in dorsomedial striatum impaired the acquisition but not the expression of previously learned instrumental learning. This effect is unlikely to involve memory consolidation, as the deficit is only reversed by giving a 5-HT6 receptor antagonist before but not after the training session. The operant conditioning parameters were altered to allow single-session acquisition; increased 5-HT6 receptor expression interfered with learning under these conditions (Eskenazi and Neumaier, 2011b). Furthermore, the type of learning affected by increasing the local expression of 5-HT6 receptors depended entirely on the subregion of striatum targeted (Mitchell et al., 2007; Ferguson et al., 2008; Eskenazi and Neumaier, 2011a,b). These studies do not exclude a role of 5-HT6 receptors in cholinergic interneurons in striatum, as the focus was on the direct and indirect striatal output pathways, which have generally opposing effects on behavior (Yager et al., 2015). Increased 5-HT6 receptor expression in indirect pathway medium spiny neurons is sufficient to disrupt instrumental learning in dorsomedial striatum or to facilitate learning of new behavior in overtrained animals when the dorsolateral striatum was targeted (Eskenazi et al., 2015). Indeed, 5-HT6 receptors tend to oppose the effects of dopamine on striatal-based behaviors including learning, as they are expressed in both output pathways, whereas D1 and D2 dopamine receptors are differentially expressed in these pathways (Gerfen and Surmeier, 2011). These subtleties illustrate how 5-HT6 receptors can have diverse effects on behavior depending on the cells that express these receptors. Thus, a full understanding of how 5-HT6 receptors modulate learning and memory in a specific cognitive disorder may depend, at least in part, on how that disorder changes 5-HT receptor signaling.
In addition to effects on cognition, 5-HT6 receptors have been proposed to promote satiety and reduce feeding and body weight (Woolley et al., 2001; Fisas et al., 2006; Voigt and Fink, 2015; Higgs et al., 2016); however, not all studies report effects of chronic 5-HT6 receptor antagonism on body weight in rats or humans (Mitchell et al., 2009; Wilkinson et al., 2014; Quiedeville et al., 2015). Most of these studies used animals with normal body weight, whereas the 5-HT6 receptor antagonist idalopirdine reduced feeding, peritoneal fat, and body weight when rats were fed a high-fat diet (Dudek et al., 2015). This metabolic profile might be advantageous clinically, as reducing feeding only in obese individuals would mitigate the risks of the drug when used for treating individuals with dementia. Furthermore, as several atypical antipsychotics that have high affinity for 5-HT6 receptors (along with many other sites) are associated with increased appetite, weight gain, and the development of the metabolic syndrome, the preclinical and limited clinical data available suggest that 5-HT6 receptor antagonism is unlikely to be responsible for these adverse effects, which are more likely due, at least in part, to 5-HT2C receptor antagonism.
The 5-HT6 receptor has also been examined in relation to drug dependence given their dense expression in the striatum, a key mediator of reward seeking and habit formation. However, some reports have found no effect of systemic 5-HT6 receptor ligands on cocaine self-administration (Frantz et al., 2002; Fijał et al., 2010; Valentini et al., 2013), whereas others suggest that cocaine reinforcement or reinstatement is regulated by 5-HT6 receptor activity (van Gaalen et al., 2010; Valentini et al., 2013). Increased striatal 5-HT6 receptors modulate learning of drug-associated behaviors as well as the stability of habitual responding (Ferguson et al., 2008; Eskenazi and Neumaier, 2011a; Eskenazi et al., 2015), but selectively increasing 5-HT6 receptors in the indirect pathway medium spiny neurons in ventral striatum increases the sensitivity to the reinforcing properties of self-administered cocaine (Brodsky et al., 2016).
5-HT6 receptors may also play a role in the development and treatment of epilepsy (Hirst et al., 2006). Increased 5-HT6 receptor levels were detected in brain tissue surgically removed from humans with epilepsy and in the pilocarpine model of epilepsy in rats (Wang et al., 2015). The selective antagonist SB399885 reduces mTOR activation and epileptic activity in this animal model. Furthermore, the colocalization of 5-HT3A and 5-HT6 receptors in a subset of cortical interneurons (Helboe et al., 2015) is intriguing given the potential role of these interneurons in cortical excitability for both normal cognition as well as pathologic states.
1. Electrophysiology
Much of the electrophysiological data concerning 5-HT6 receptors primarily concerns in vivo recording; the cellular mechanisms are difficult to infer. The selective 5-HT6 receptor agonist WAY181187 increases excitability and spiking by rat medium spiny neurons in acutely prepared striatal slices in a SB258585-reversible manner; but this has not been sufficiently explored to identify whether this is a direct or indirect phenomenon. In whole-cell patch clamp mode, the 5-HT6 receptor agonist ST1936 reduces spontaneous excitatory postsynaptic currents in striatal medium spiny neurons and cortical pyramidal neurons, which is prevented by SB258585 (Tassone et al., 2011). Thus, the agonist-induced reduction in glutamatergic neuronal activity (consistent with microdialysis reports of enhanced glutamate overflow following microinfusion of 5-HT6 receptor antagonists; Upton et al., 2008; Dawson, 2011) might be indirect by modulation of GABAergic interneurons in these brain areas. Although systemic administration of 5-HT6 receptor agonists to anesthetized rats produced both excitation and inhibition of ventral tegmental area (VTA) dopamine neurons, only excitation is observed following microiontophoretic application (Borsini et al., 2015), suggesting a direct excitatory activation, which may be relevant for cognitive and addictive properties of 5-HT6 receptor ligands.
Moderate levels of 5-HT6 receptors are also expressed in the DRN, and local injection of the agonist WAY-208466 decreases REM sleep and increases wakefulness (Monti et al., 2013). Single-unit recording in the anesthetized rat shows that systemic application of the full agonist WAY-181187 increases and the antagonist SB-399885 decreases firing of putative DRN 5-HT neurons; further studies are needed to establish if this involves cortical feedback loops, interneurons, and/or a direct effect on 5-HT neurons as proposed by the authors (Brouard et al., 2015). Consistent with this idea of indirect modulation of 5-HT release in the raphe, the 5-HT6 receptor antagonist SB399885 inhibits spontaneous firing of GABAergic interneurons (expressing 5-HT6 receptor mRNA) in the DRN in mouse brain slices (Asaoka et al., 2015). This mechanism may be relevant to the action of antipsychotic drugs, as the effect is mirrored by olanzapine.
G. Clinical Relevance
Treatment of cognitive impairment is a leading potential application of 5-HT6 receptor ligands in humans. There is ample evidence that manipulation of 5-HT6 receptors can improve cognitive function based on preclinical animal models. Though schizophrenia is associated with positive and negative symptoms that can respond to available antipsychotic drugs, cognitive impairment is an often disabling and persistent problem that typically does not respond well to antipsychotic medications. Although there are some preliminary clinical trials in schizophrenia, these trials had technical limitations because the 5-HT6 receptor ligands investigated were as add-ons to treatments already underway, and in some cases, the ongoing treatments were atypical antipsychotic drugs that possess potent antagonist properties at 5-HT6 receptors, making it unlikely that additional benefits from 5-HT6 receptor blockade could be readily detected. Early clinical studies with 5-HT6 receptor ligands are reviewed elsewhere (Heal et al., 2008; Codony et al., 2011). A double-blind, placebo-controlled phase II trial for treatment of dementia associated with Alzheimer Disease (Wilkinson et al., 2014) investigated whether the addition of Lundbeck’s idalopirdine, a selective 5-HT6 receptor antagonist, to an established treatment (donepezil, a cholinesterase inhibitor) delays the progression of dementia. This study was successful in two important ways: the addition of idalopirdine was well tolerated and led to improved cognitive function as compared with addition of placebo. However, disappointingly, idalopirdine suffered a late-stage failure in a pivotal phase III trial. Along the same lines, intepirdine, previously known as RVT-101, a 5-HT6 receptor antagonist originally developed by GSK, displayed positive effects in patients with AD in a phase II clinical trial. A subsequent phase III trial of the drug after it was acquired by Axovant failed to achieve any of its main efficacy targets in mild to moderate AD patients. Intepirdine did not improve symptoms compared with placebo on two widely used AD symptom measures—the ADAS-Cog (the Alzheimer’s Disease Assessment Scale–Cognitive Subscale) and ADCSADL (Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale) scales. Subsequently, intepirdine was evaluated in a clinical trial of patients with dementia with Lewy bodies also without success, leading to a halt in the development of this drug. Hence, although the availability of new effective treatments for dementia is a crucial health imperative, it would appear that 5-HT6 receptor antagonism may not transform the preclinical promises into clinical practice; understanding the mechanisms that underlie this apparent failure is important and may allow opportunity for patient stratification to better identify patients that may respond favorably to 5-HT6 receptor ligands.
Several other pathologies might also be amenable to treatment with 5-HT6 receptor ligands, as preclinical data suggests that other progressive dementing disorders, depression, obesity, and epilepsy have potential to become indications for 5-HT6 receptor ligands. As now appreciated, the multiple signal transduction pathways engaged by 5-HT6 receptors and various 5-HT6 receptor ligands with differing degrees of intrinsic efficacy; the role of agonism partial agonism, antagonism, and inverse agonism; and biased signaling need to be considered in future development of 5-HT6 receptor therapeutic agents. Such pharmacological complexity may also account for the apparent opposing actions of 5-HT6 receptor ligands in preclinical behavioral models as discussed above.
XV. 5-HT7 Receptors
A. Introduction
The 5-HT7 receptor was the last 5-HT receptor to be discovered. In 1993, several research groups, almost at the same time, identified the 5-HT7 receptor from the screening of cDNA libraries from various species, including humans. 5-HT7 receptor mRNA is localized in discrete areas of the mammalian brain, including thalamus, hippocampus, and cortex, and matched with the expression of 5-HT7 receptor protein. Based on its distribution in the CNS, the 5-HT7 receptor is proposed to be involved in thermoregulation, circadian rhythm, learning and memory, hippocampal signaling, and sleep. Various drugs (clozapine, cyproheptadine, and amitryptiline) and pharmacological tools (5-CT and 8-OH-DPAT) binding to 5-HT7 receptor poses the question of whether some of the effects of these compounds are mediated, at least in part, by 5-HT7 receptor. Initially, the lack of selective 5-HT7 receptor agonists and antagonists slowed down the elucidation of the (patho)physiologic role of the receptor, especially because of the high sequence homology in the transmembrane domains of 5-HT7 and 5-HT1A receptors. Moreover, the two receptors are distributed in the same areas of the CNS and, at the cellular level, they may have opposite effects: the 5-HT7 receptor is positively coupled to adenylyl cyclase whereas the 5-HT1A receptor is negatively coupled. After 2000, various selective antagonists (SB-258719 and SB-269970) and agonists (AS-19, LP-211, and E-55888) became available, providing the scientific community with powerful pharmacological tools to get deeper insights on the role of 5-HT7 receptors in health and disease.
B. Cloning of the Gene
The 5-HT7 receptor is a class A GPCR, cloned by screening of cDNA libraries from mouse (Plassat et al., 1993), rat (Lovenberg et al., 1993a, Meyerhof et al., 1993, Ruat et al., 1993b; Shen et al., 1993), human (Bard et al., 1993), guinea pig (Tsou et al., 1994), Xenopus laevis (Nelson et al., 1995), pig (Bhalla et al., 2002a), Caenorhabditis elegans (Hobson et al., 2003), and honeybee (Schlenstedt et al., 2006).
The open reading frame of the human cDNA codes for a protein of 445 amino acids with 57% sequence identity within the transmembrane regions in comparison with the Drosophila melanogaster 5-HTdro1 receptor and 39%–53% homology with human 5-HT1, 5-HT2, 5-HT5, and 5-HT6 receptors (Bard et al., 1993). The gene encoding the human 5-HT7 receptor is located on chromosome 10 (q21-q24) (Gelernter et al., 1995) and contains several introns in the coding region (Ruat et al., 1993b, Erdmann et al., 1996; Heidmann et al., 1997). The guinea pig 5 HT7 receptor has 466 amino acids, with an amino acid homology within the transmembrane regions with other 5-HT receptors of 34% and 48% (Tsou et al., 1994). The mouse brain 5-HT7 receptor cDNA has one long open reading frame for 448 amino acids. The homology with other 5-HT receptors is low, with the best score with the 5-HTdro1 receptor (42%) and the next closest homology being with 5-HT1B, 5-HT1D, and 5-ht1e receptors (Plassat et al., 1993). The 5-HT7 receptor was cloned from rat by four groups, which reported different amino acid lengths ranging from 404 to 448. Shen et al. (1993) reported the sequencing of a full-length clone isolated from a rat hippocampal cDNA library, revealing a 404-amino-acid protein with seven hydrophobic regions. Within these regions, rat 5-HT7 receptor is 44%–50% identical with members of the 5-HT1, 5-HT5, and 5-HT6 subfamilies, with lower homology to the 5-HT2 receptor subtypes (37%–40%). Lovenberg et al. (1993a) isolated and determined the nucleotide sequence of a clone showing an open reading frame that encoded a 435-amino-acid protein. Within the conserved transmembrane domains of known 5-HT receptors, the rat 5-HT7 receptor exhibited the greatest identity with the 5-HTdro1 receptor (54%). Instead, the entire coding sequence showed low identity (33%–39%) with 5-HTdro1, 5-HT1A, 5-HT1D, 5-ht1e, 5-HT5 receptor subtypes. Ruat et al. (1993b) reported the characterization of a nucleotide sequence containing an open reading frame encoding a 448-amino-acid protein. This rat 5-HT7 receptor showed the highest sequence homology in the hydrophobic regions with the 5 HTdro1 receptor (60%). In the transmembrane domains, the homologies with other 5-HT receptors were as follows: 5-HT1A, 51%; 5-HT1B, 55%; 5-HT1D, 52%; 5-ht1e, 53%; 5-HT1F, 52%; 5-HT2A, 43%; 5-HT2B, 40%; 5-HT2C, 42%; 5-HT5, 48%; and 5-HT6, 45%. The rat 5-HT7 receptor reported by Meyerhof et al. (1993) has an open reading frame encoding a 448-amino-acid protein, showing the highest sequence homology with 5-HTdro1 receptor (36% identity). The 5-HT7 receptor from pig (Bhalla et al., 2002a) encoded an open reading frame of a 447-amino-acid protein that showed high homology (92%–96%) with the 5-HT7 receptor protein cloned from the other species.
The presence of introns in the 5-HT7 receptor gene results in a number of functional splice variants. Although no alternative splicing has been reported for the first intron, located in the sequence encoding the second intracellular loop of the receptor, alternative splicing at the second intron, which is located in the sequence encoding the C-terminal end, generates a number of splice variants, namely 5-HT7(a), (b), (c), (e) receptors in rat and 5-HT7(a), (b), (d) receptors in man (Heidmann et al., 1998; Krobert et al., 2001; Liu et al., 2001). Splice variants in dog, marmoset, and zebrafish are to be found in the www.ensembl.org database, but no related reports are available in the literature. In the mouse, two additional splice variants have been described, named 5-HT7(b) and 5-HT7(c) in analogy with the rat (Gellynck et al., 2008). In guinea pig and pig, the 5-HT7 receptor is homologous to the human 5-HT7(a) variant. Although the splice variants differ in the lengths of their carboxy terminal ends, they do not show major differences in their membrane localization nor significant differences in their respective pharmacology and signal transduction properties or functional coupling to Gs protein (see below).
A transcribed human 5-HT7 receptor pseudogene has been identified by a degenerate PCR approach. The original clone (S771) has homology greater than 90% to the 5-HT7 receptor sequence; expression of the pseudogene transcript is detected throughout the brain and peripheral tissues, in general agreement with 5-HT7 receptor mRNA localization. However, the transcript was also detected in tissues not known to express the 5-HT7 receptor (i.e., liver and kidney) (Olsen et al., 1999).
There is as yet no crystal structure of the 5-HT7 receptor, but molecular modeling and site-directed mutagenesis has identified essential residues for ligand binding and activation of the human receptor (Impellizzeri et al., 2015).
C. Expression
1. mRNA
The distribution of mRNA encoding the 5-HT7 receptor protein has been studied in several species using various techniques, as summarized in Tables 18 and 19. In all species, high levels of 5-HT7 receptor mRNA are expressed in the CNS (hypothalamus, thalamus, and hippocampus; Fig. 26). In peripheral tissues, 5-HT7 receptor mRNA is present in the ileum, spleen, endocrine glands, and arteries. In blood vessels and the gastrointestinal tract, 5-HT7 receptor mRNA expression is generally present in smooth muscle cells.
TABLE 18.
Localization and relative abundance of 5-HT7 receptor mRNA in the rat
| Technique | Localization (Relative Abundance) |
|---|---|
| In situ hybridizationa | Hippocampus (+++), thalamus (+), hypothalamus (+) |
| In situ hybridizationb | Hippocampus, thalamus, enthorinal and piriform cortices, tenia tecta |
| In situ hybridizatiocc | Thalamus (+++), hippocampus (++), retrosplenial cortex (++), neocortex (++), hypothalamus (+) (not detected in suprachiasmatic nucleus) |
| In situ hybridizationd | Outer layer of the cortex, thalamus, hippocampus |
| In situ hybridizatione | Thalamus (+++), hippocampus (+++), retrosplenial cortex (+++), mammillary region (+++), posterior thalamic nucleus (+), suprachiasmatic nucleus (+) |
| In situ hybridizationf | Forebrain (+++), olfactory complex (+++), thalamus (+++), hippocampal formation (+++), hypothalamus (+++), suprachiasmatic nucleus (+++), septal region(++), amygdala (++), parvicellular (++) |
| In situ hybridizationg | Retrosplenial cortex (+++), hippocampus (+++), tenia tecta (+++), indusium griseus (+++), posterior hypothalamus (+++), medial amygdala nucleus (+++), thalamus (+++), cerebellum-Purkinije cell layer- (+++), pontine nuclei (+++), superior colliculus (+), raphe nucleus (+) |
| Northern Bloth | Hypothalamus (+++), hippocampus (++), mesencephalon (++), cortex (++), olfactory bulb (+), olfactory tubercle (+), spleen (+) (not detected in retina, pituitary, testis, stomach, prostate, ovary, skeletal muscle, lung, liver, kidney, gut) |
| Northern Blotg | Hypothalamus (+++), brainstem (+++), hippocampus (+++), stomach (+), ileum (+) |
| Northern Blota | Hypothalamus (+++), thalamus (+++), hippocampus (+), cortex (+) (not detected in urinary bladder, testis, liver, spleen, adrenal gland, kidney, lung, heart, pituitary) |
| Northern Blotc | Hypothalamus (+++), thalamus (+++), hippocampus (++), cortex (++), medulla (++) (not detected in cerebellum, striatum, heart, liver, kidney, adrenal glands, testis, ovaries, spleen) |
| RT-PCRi | Lumbar dorsal root ganglia, superior cervical ganglia, lumbar synpathetic ganglia |
| RT-PCRj | Vena cava (+++), femoral vein (++), aorta (+), renal artery (+), portal vein (+) (not detected in jugular vein) |
| RT-PCRk | Frontocortical astrocytes, hypothalamus |
| RT-PCRl | Adrenal gland |
| RT-PCRm | Submandibular gland |
| RT-PCRn | Thymus, peripheral blood lymphocytes, spleen, mitogen activate spleen cells |
| RT-PCRo | Adrenal cortex |
| RT-PCRp | Jejunum, ileum, stomach fundus, esophagus, colon |
Lovenberg et al. (1993).
Lenglet et al. (2000).
TABLE 19.
Localization and abundance of 5-HT7 receptor mRNA in guinea pig, human, mouse, and pig
| Species | Technique | Localization (Relative Abundance) |
|---|---|---|
| Guinea piga | In situ hybridization | Hippocampus (+++), periventricular thalamus (+++), superficial cortex (+++), cerebellar granule cells (+++) |
| Guinea pigb | In situ hybridization | Medial thalamic nucleus (+++), hippocampal formation (+++), superficial layer cortex (++), medial geniculate nucleus (++), amygdala (++), hypothalamus (++), midbrain (+), hindbrain (+) |
| Guinea pigc | In situ hybridization | Outer layer of the cortex, thalamus, hippocampus |
| Guinea piga | Northern Blot | Parietal cortex (+++), hippocampus (++), frontal cortex (++), cerebellum (+), ileum (+), spleen (+) |
| Guinea pigd | Northern Blotb | Thalamus (+++), brainstem (+++), hypothalamus (+++), substantia nigra (+++), olfactory bulb (+++), olfactory tubercle (+++) (not detected in peripheral organs) |
| Hamstere | In situ hybridization | Thalamus (+++), cortex (++), hypothalamus (++), amygdala (++), midbrain (+), basal forebrain (+) |
| Humanf | RT-PCR | Artery smooth cells (+++), pulmonay artery smooth cells (++) (not detected in coronary artery, pulmonary artery, aortic endothelial) |
| Humang | RT-PCR | Dorsal root ganglia |
| Humanh | RT-PCR | Granulosa-lutein cells |
| Humani | RT-PCR | Brain (+++), kidney (+), liver (+), pancreas (+), spleen (+), coronary artery (++), stomach (++), descending colon (++), ileum (++) |
| Humanj | RT-PCR | Trigeminal ganglia |
| Humank | qPCR | Colonic circular muscle |
| Mousel | Northern Blot | Not detected in brain, heart, kidney, lung, liver |
| Mousel | RT-PCR | Forebrain (+++), brain stem (+++), cerebellum (++), colliculi (++), intestine (+), heart (+), not detected in spleen, kidney, lung, liver |
| Mousem | RT-PCR | Substantia gelatinosa of the trigeminal subnucleus caudalis |
| Pigf | RT-PCR | Pulmonary artery (++), coronary artery (++), cerebral artery (++), cerebral vein (++) |
| Pign | RT-PCR | Myometrium |
| Pigo | RT-PCR | Brain cortex, trigeminal ganglion, cerebellum, pulmonary artery, coronary artery, superior vena cava, saphenous vein, not detected in heart |
Ruat et al. (1993).
Irwing et al. (2007).
Bhalla et al. (2002).
Fig. 26.
5-HT7 receptor mRNA and protein expression in rat brain. Comparative in situ hybridization localization of 5-HT7 receptor mRNA (ISHH) (A, C, E, and G) and immunocytochemical location of 5-HT7 receptor protein (ICC) (B, D, F, and H), respectively. amyg, amygdala; atn, anterior thalamic nuclei; av, anteroventral thalamic nucleus; hip, hippocampus; lsn, lateral septal nuclei; mpa, medial preoptic area; neo, neocortex; pir, piriform cortex; pvp, posterior paraventricular nucleus; pvn, paraventricular thalamic nuclei; rc, retrosplenial cortex; scn, suprachiasmatic nuclei, str, striatum; tt, tenia tecta. Adapted from Neumaier et al. (2001) (with permission).
The relative abundance of the three human 5-HT7 isoforms 5-HT7(a), (b), and (d) within brain (fetal brain, caudate, hippocampus) and peripheral tissues (uterus, trachea, small intestine, stomach, saphenous vein) has been examined (Krobert et al., 2001; Guthrie et al., 2005). These tissues expressed all three isoforms. Although the 5-HT7(b) isoform is most prevalent, the relative amounts of 5-HT7(a) and 5-HT7(d) differed by tissue type, with the 5-HT7(d) isoform being most abundant in smooth muscle and least common in brain tissues (Krobert et al., 2001; Guthrie et al., 2005). In the rat, the 5-HT7(a) receptor isoform is most prevalent in both the CNS (cerebellum, cortex, hippocampus, hindbrain, thalamus) and the periphery (heart, kidney, spleen) (Heidmann et al., 1997, 1998).
2. Radioligand Binding
Radioligand binding assays have been used to study the distribution of 5-HT7 receptor protein in native tissues. A first study to define 5-HT7 receptor binding sites in rat hypothalamic membranes was performed using [3H]5-HT in the presence of 100 nM pindolol that blocks the binding of the radioligand to 5-HT1A and 5-HT1B receptors. The pharmacology of the identified binding sites correlated well with that of rat recombinant 5-HT7 receptors (Sleight et al., 1995). A subsequent study showed that 100 nM of pindolol does not completely mask 5-HT1A and 5-HT1B receptors and that the population of pindolol-insensitive receptors labeled by [3H]5-HT in rat hypothalamus appeared to be heterogeneous (Gobbi et al., 1996). Also, [3H]5-CT failed to define a homogeneous population of 5-HT7 binding sites in rat hypothalamus homogenates even in the presence of various masking agents (pindolol, sumatriptan, DOI) (Stowe and Barnes, 1998). [3H]5-CT has been used to label 5-HT7 receptors in guinea pig brain cortex membranes in the presence of sumatriptan and cyanopindolol (To et al., 1995). The pharmacology of these binding sites was well correlated to that of the guinea pig cloned 5-HT7 receptor. Because affinity values of reference compounds were consistently lower in the binding assay performed in native tissue, the authors hypothesized that sumatriptan and cyanopindolol were likely to occupy, at least in part, the 5-HT7 receptors. [3H]Mesulergine labels 5-HT7 receptors in guinea pig ileal longitudinal muscle in the presence of several masking agents (cinanserin, prazosin, raclopride, RS 102221, and yohimbine). However, under these same conditions, no binding was detected in the rat jejunum (Hemedah et al., 1999).
[3H]SB-269970 is the first selective radioligand to label with high affinity 5-HT7 receptors in rat, mouse, guinea pig, pig, marmoset, and human brain homogenates. Guinea pig brain homogenate displays markedly higher 5-HT7 receptor expression in comparison with rat, mouse, and pig brain homogenates (Thomas et al., 2002).
Autoradiographic studies on the localization of 5-HT7 receptors have been performed by using various radioligands. [3H]5-CT has been used in the presence of various masking agents (To et al., 1995; Waeber and Moskowitz, 1995a; Gustafson et al., 1996; Mengod et al., 1996). In guinea pig and rat brain, the distribution of the 5-HT7 receptor binding sites was largely consistent with that reported for 5-HT7 receptor mRNA. The highest densities were in the medial thalamic nuclei and related limbic and cortical regions. However, as the detected densities were very low, it was hypothesized that the masking compounds might have sufficient affinity for 5-HT7 receptor sufficient to compete with [3H]5-CT to some degree for the 5-HT7 receptor. In a subsequent study, the localization of 5-HT7 receptor was studied in 5-HT1A receptor knockout and 5-HT1A/B receptor double-knockout mice by using [3H]5-CT (5-HT1A, 5-HT1B, and 5-HT7 agonist) and [3H]8-OH-DPAT (5-HT1A and 5-HT7 agonist) (Bonaventure et al., 2002b). [3H]8-OH-DPAT was better than [3H]5-CT for measuring 5-HT7 receptor binding sites, even if it displays lower 5-HT7 receptor affinity than [3H]5-CT in tissue homogenates. The anatomic distribution of the [3H]8-OH-DPAT–labeled sites observed in these knockout mice was in agreement with the distribution of 5-HT7 receptor mRNA and immunoreactivity reported previously. Within the hippocampal formation, strong labeling was found in the CA3 region, whereas low binding was found in CA1 region. 5-HT7 receptors were also found within the dorsal raphe and the hypothalamus, including the suprachiasmatic nucleus.
The selective high-affinity radioligand [3H]SB-269970 was used to localize the 5-HT7 receptors in human brain (Varnäs et al., 2004; Fig. 27). The distribution of the 5-HT7 receptors was largely similar to that shown by the autoradiographic studies in rat (Gustafson et al., 1996), guinea pig (To et al., 1995), and mouse (Martín-Cora and Pazos, 2004). High receptor density was detected in thalamus, hypothalamus, and hippocampus. However, unlike in rodents, human brain 5-HT7 receptors were also found in high levels in caudate nucleus, putamen, and substantia nigra (Varnäs et al., 2004). Eventually, using [3H]SB-269970, Horisawa et al. (2013) reported that 5-HT7 receptor distribution in rat brain was similar to that in human (Varnäs et al., 2004), with small differences in regions with an intermediate to low density of 5-HT7 receptors.
Fig. 27.
5-HT7 receptor binding sites in human brain. Autoradiographic detection of [3H]SB-269970 binding to 5-HT7 receptors in the human brain. Autoradiograms showing the distribution of 5-HT7 receptors at the level of the thalamus (left column) and dorsal striatum (right column). (A) Plates; line drawings of brain hemisphere contours with square showing location of sections for the autoradiograms below. (B) Plates; total binding. (C) Plates; nonspecific binding (inclusion of 5-HT, 10 µM). ACG, anterior cingulate gyrus; Amg, amygdala; Ath, anterior thalamus; Ca, caudate nucleus; DG, dentate gyrus; DR, dorsal raphe; Hi, hippocampus; PCG, posterior cingulate gyrus; Pu, putamen; SN, substantia nigra; Th, thalamus. Adapted from Varnäs et al. (2004) (with permission).
3. Immunoreactivity
Rabbit polyclonal antibodies raised against amino acid sequence 8–23 of the rat 5-HT7 receptor are commercially available. Immunocytochemistry has been used to localize the distribution of 5-HT7 receptors in rat forebrain (Neumaier et al., 2001), which were detected in the cortex, hippocampal formation, tenia tecta, thalamus, and hypothalamus, in agreement with the localization reported for the 5-HT7 receptor mRNA. In particular, in the suprachiasmatic nucleus, both cell bodies and proximal fibers were strongly stained, suggesting a somatodentric subcellular distribution of the receptor. The presence of 5-HT7 receptors was also reported in both pre- and postsynaptic GABA, vasoactive intestinal polypeptide, and vasopressin processes in the suprachiasmatic nucleus in mouse (Belenky and Pickard, 2001). 5-HT7 receptors are detected in Purkinje cells of rat cerebellum but not in the cerebellar cortex or in deep nuclei (Geurts et al., 2002).
5-HT7 receptor immunolabeling was detected mainly in the two superficial laminae of the dorsal horn and in small- and medium-sized dorsal root ganglion cells in the rat spinal cord (Doly et al., 2005), consistent with a predominant role of 5-HT7 receptor in nociception. In addition, moderate labeling was found in the lumbar dorsolateral nucleus (Onuf’s nucleus), suggesting an involvement of the receptor in the control of pelvic floor muscles (Doly et al., 2005). Electron microscopic examination of the dorsal horn shows three main localizations of the receptor: 1) a postsynaptic localization on peptidergic cell bodies in laminae I–III and in numerous dendrites, 2) a presynaptic localization on unmyelinated and thin myelinated peptidergic fibers, and 3) in lamina I and II in astrocytes (Doly et al., 2005). 5-HT7 receptors have been localized to numerous myenteric neurons, some submucosal neurons, and a few smooth muscle cells of guinea pig ileum (Tonini et al., 2005) and in human corneal epithelium and endothelium (Grueb et al., 2006). An upregulated expression of 5-HT7 receptors was detected during maturation of bone marrow–derived dendritic cells, suggesting a critical role of 5-HT7 receptors in the regulation of immune cell polarization and, thus, in the peripheral inflammatory processes (Holst et al., 2015).
D. Pharmacology
1. Agonists
Various selective 5-HT7 receptor agonists have been identified (Table 20). The aminotetraline derivative AS-19 has a Kd of 0.6 nM at human cloned 5-HT7 receptors, with high selectivity over all the other 5-HT receptor subtypes (>100-fold) except 5-HT1D receptors (11-fold). However, AS-19 behaves as a potent partial 5-HT7 receptor agonist (EC50 = 9 nM) with a maximal effect reaching 77% of that of 5-HT in a functional assay measuring cAMP stimulation in HEK293F cells overexpressing human 5-HT7 receptors (Brenchat et al., 2009), which can complicate interpretation of arising data using tissue and in vivo preparations.
TABLE 20.
Binding profile of the selective 5-HT7 receptor agonists and antagonists
Ki >1 µM or percent inhibition at 1.0 µM lower than 50%.
| Receptor | Affinity (Ki [nM]) | |||||
|---|---|---|---|---|---|---|
| Agonists/Partial Agonists | Antagonists | |||||
| E-55888a | AS-19a | LP-211b | SB-258719c | SB-269970d | SB-656104e | |
| h5-HT7 | 0.6 | 2.5 | 15 | 31.6 | 1.3 | 2.0 |
| h5-HT1A | 89.7 | 700 | 379 | >7900 | >10,000 | 562 |
| h5-HT1B | ND | ND | 215 | >5000 | >1000 | 630 |
| r5-HT1B | 490 | n.s. | ND | ND | ND | ND |
| h5-HT1D | 6.6 | n.s. | 394 | 3162 | 1584 | 25 |
| b5-HT1D | n.s. | n.s. | ND | ND | ND | ND |
| h5-ht1e | ND | ND | >10,000 | >10,000 | >6000 | >5000 |
| h5-HT2A | n.s. | n.s. | 626 | >10,000 | >10,000 | 63 |
| h5-HT2B | n.s. | n.s. | 67 | >5000 | 10,000 | 91 |
| h5-HT2C | n.s. | n.s. | ND | >10,000 | 10,000 | 269 |
| r5-HT2C | ND | ND | 91 | ND | ND | ND |
| h5-HT3 | n.s. | n.s. | >10,000 | ND | ND | ND |
| h5-HT4 | ND | n.s. | ND | >10,000 | 1259 | 1900 |
| h5-HT5A | 98.5 | n.s. | 178 | ND | 63 | 182 |
| h5-HT6 | n.s. | n.s. | 1571 | >10,000 | 6300 | 851 |
| h5-HT transporter | n.s. | n.s. | 812 | ND | ND | ND |
ND, not determined; n.s., not significant.
Data taken from Brenchat et al. (2009).
Data taken from Hedlund et al. (2010).
Data taken from Forbes et al. (1998).
Data taken from Lovell et al. (2000).
Data taken from Thomas et al. (2003).
E-55888 is another potent and selective 5-HT7 receptor agonist with high efficacy, displaying high affinity for 5-HT7 receptors (Kd = 2.5 nM), low affinity for 5-HT1A (Kd = 700 nM), and no significant affinity for the other 5-HT receptors. E-55888 behaves as a full agonist (EC50 = 16 nM) and increases cAMP levels in HEK293F-expressing human 5-HT7 receptors (Brenchat et al., 2009). Another selective 5-HT7 receptor agonist is LP-211, with high but different affinities for rat and human recombinant 5-HT7 receptors (Kd = 0.58 and 15.0 nM, respectively) and moderate to low affinity for other 5-HT receptors, including 5-HT1A (Leopoldo et al., 2008; Hedlund et al., 2010). LP-211 induces 5-HT7–mediated relaxation of substance P-stimulated guinea pig ileum contracture (82% of the maximal effect elicited by 5-CT). However, in HEK293 cells stably expressing human 5-HT7 receptor (Atanes et al., 2013), LP-211 displayed insurmountable antagonism of 5-CT–stimulated cAMP signaling. These results were unexpected, as LP-211 have been extensively characterized as a 5-HT7 receptor agonist in several ex vivo and in vivo studies [for review, see Di Pilato et al. (2014)]. The authors suggested that the inhibitory effects of LP-211 are due to an irreversible stabilization of an inactive conformational state of the receptor that is tightly associated with Gs protein, independent of agonist binding (Bruheim et al., 2003; Andressen et al., 2006).
2. Antagonists
Various selective 5-HT7 receptor antagonists have been identified (Table 20). The first was SB-258719, which displays useful 5-HT7 receptor affinity (Ki = 31.6 nM) with 100-fold selectivity against all the other 5-HT receptors (Forbes et al., 1998). In HEK293 cells stably expressing the human 5-HT7 receptor, SB-258719 did not stimulate basal adenylyl cyclase activity, suggesting a lack of agonist activity, but produced a surmountable antagonism of the 5-CT response with a pKB of 7.0. Further chemical optimization of SB-258719 led to SB-269970, which is the prototype 5-HT7 receptor antagonist (Hagan et al., 2000). SB-269970 displays high affinity at both human cloned 5-HT7 receptors (pKi = 8.9) and in guinea pig cortex (pKi = 8.3). The compound is at least 100-fold selective against a wide range of receptors, except for the human 5-HT5A receptor (about 50-fold). SB-269970 displays potent antagonism in both HEK293 cells stably expressing the 5-HT7 receptor (pA2 = 8.5) and in the guinea pig hippocampal membranes (pKB = 8.3). SB-269970 also produces a small inhibition of basal adenylyl cyclase activity in the absence of added 5-CT, consistent with inverse agonism (Hagan et al., 2000). Subsequent studies led to the identification of the antagonist SB-656104, which is as potent as SB-269970 (Ki = 2 nM; pA2 = 8.4) but displays only 10-fold selectivity over the 5-HT1D receptor. Both SB-269970 and SB-656104 are brain-penetrant, and the latter is more stable metabolically (Thomas et al., 2003).
3. Allosteric Modulators
Oleamide, an amidated fatty acid, is an allosteric modulator of the 5-HT7 receptor (Thomas et al., 1997a, 1999,b; Hedlund et al., 1999; Alberts et al., 2001). In cells expressing the 5-HT7 receptor, oleamide increases cAMP accumulation in a concentration-dependent manner but with a lower efficacy than 5-HT. In the presence of 5-HT, oleamide displayed the opposite effect on cAMP, causing insurmountable antagonism of 5-HT, suggesting that oleamide acts at an allosteric site on the 5-HT7 receptor. In receptor-binding studies, oleamide modulated the binding properties of 5-HT7 receptors expressed in vitro (Hedlund et al., 1999). In addition, the binding of both agonists and antagonists at human recombinant 5-HT7 receptors stably expressed in HEK293 cells was allosterically inhibited by zinc (Satała et al., 2018).
E. Signaling
The 5-HT7 receptor is positively coupled to adenylyl cyclase through activation of Gs, resulting in intracellular increase in cAMP (Bard et al., 1993; Lovenberg et al., 1993a; Ruat et al., 1993b). The human, rat, and mouse splice variants all activate adenylyl cyclase (Heidmann et al., 1998; Krobert et al., 2001; Krobert and Levy, 2002; Gellynck et al., 2008) and do not show evident differences in their respective pharmacology and signal transduction properties or functional coupling to Gs protein (Heidmann et al., 1998; Krobert et al., 2001). The only reported functional difference is that the human 5-HT7(d) receptor displays a differential pattern of receptor internalization (Guthrie et al., 2005). Furthermore, variation in the length of the C-terminal tails and in the number of consensus sites for phosphorylation by PKA and PKC raises the possibility that the splice variants can display different desensitization or trafficking properties (Heidmann et al., 1997). Moreover, the human 5-HT7(b) and the rat 5-HT7(b) and 5-HT7(c) receptor splice variants contain recognition sites for PDZ domain–containing proteins (Hamblin et al., 1998), suggesting that these splice variants may be targeted to active zones within cells and may couple to alternative signaling pathways.
Stimulation of the human 5-HT7(a) receptor in HEK293 cells coexpressed with various adenylyl cyclase isoforms revealed that this receptor not only activates the Gs-sensitive AC5 isoform but also the Gs-insensitive and Ca2+/calmodulin-stimulated AC1 and AC8 isoforms (Nielsen et al., 1996; Xia and Storm, 1997; Baker et al., 1998).
5-HT7 receptor expression density does not appear to influence agonist or partial agonist potency (or efficacy), suggesting the absence of receptor reserve effects (Bruheim et al., 2003; Andressen et al., 2006) and implying independence from receptor-Gs stoichiometry. This may also suggest the 5-HT7 receptor is preassociated with Gs (Adham et al., 1998; Alberts et al., 2001; Krobert et al., 2001), which was supported by a more recent study investigating receptor-immobilized fluorescence recovery after photobleaching and fluorescence resonance energy transfer (Andressen et al., 2018) to compare the Gs coupling of the 5-HT7 and 5-HT4 receptors.
Prolonged stimulation by 5-HT induces both homo- and heterologous desensitization of Gs signaling in HEK293 cells. A similar effect is seen on prolonged incubation with the inverse agonists SB-269970 and methiothepin, implying that desensitization is independent of Gs activation (Krobert et al., 2006).
Clozapine and olanzapine, which are 5-HT7 receptor inverse agonists [see Krobert and Levy (2002)], display functional selectivity at the human 5-HT7 receptor (i.e., different ligands at the same receptor elicit different functional effects) (Andressen et al., 2015). Thus, clozapine and olanzapine downregulate 5-HT7 receptors expressed in HEK293 cells, an effect normally evident for agonists but not for antagonists or inverse agonists. This inverse agonist–mediated receptor downregulation may be mediated by interaction with the G protein–associated sorting protein GASP-1 (Manfra et al., 2015).
In rat hippocampal neurons, 5-HT stimulation leads to the activation of serine/threonine kinases ERK1 and ERK2, and most of this activity is mediated by 5-HT7 receptors (Errico et al., 2001). A PKA-dependent pathway for 5-HT7 receptor–mediated ERK1 and ERK2 activation has been proposed (Norum et al., 2003, 2005). 5-HT7 receptor activation increases intracellular cAMP levels to activate PKA, which phosphorylates the guanine nucleotide exchange factor (GEF) Ras-GRF1 that controls the activity of Ras, an upstream activator of c-Raf (or Raf-1; a member of the Raf kinase family; Norum et al., 2003). Consequently, c-Raf phosphorylates and activates various kinases, including MEK1 and, further downstream, ERK1/2. An alternative pathway could involve the activation of GEF proteins Epac1 and Epac2 by cAMP, which in turn activate the Rap1 proteins through GDP-GTP exchange. GTP-bound Rap1 proteins can bind and activate different members of the Raf kinase family (i.e., B-Raf, A-Raf, and c-Raf), leading to ERK1/2 activation (Norum et al., 2005). The contribution of these GEFs in the ERK1/2 activation pathway mediated by 5-HT7 receptor has been demonstrated in the pheochromocytoma PC12 cells stably overexpressing 5-HT7 receptor and in rat hippocampal neurons (Lin et al., 2003; Johnson-Farley et al., 2005). Moreover, ERK1/2 seems to be required for normal neuronal function, such as neurotrophin-stimulated neuronal differentiation and neuroprotection as well as regulation of neurite outgrowth and formation of neuronal networks (Hetman et al., 1999; Speranza et al., 2013, 2015).
Stimulation of 5-HT7 receptor expressed in HEK293 cells increases intracellular Ca2+ levels (Baker et al., 1998). However, the exact mechanism for this increase is not known. In rat adrenal glomerulosa cells, this 5-HT7 receptor–mediated increase of intracellular Ca2+ levels is mediated by T-type Ca2+ channels in a cAMP∕PKA-dependent manner (Lenglet et al., 2002a,b), which is likely to contribute to ERK1/2 activation (Lin et al., 2003; Norum et al., 2003; Johnson-Farley et al., 2005).
The 5-HT7 receptor interacts with the G12 member of the G protein family (Kvachnina et al., 2005; Kobe et al., 2012), which can activate multiple signaling pathways. The prominent downstream effectors are members of the Rho family of small GTPases (Rho, Rac, and Cdc42). 5-HT7 receptor–mediated stimulation of G12 protein results in Rho-dependent activation of the transcription factor serum response factor, which binds to the serum response element (SRE). The stimulation of 5-HT7 receptor increases SRE-driven gene expression even in the presence of a PKA inhibitor or pertussis toxin, suggesting that the receptor-mediated SRE activation is not PKA-dependent (Kvachnina et al., 2005). Stimulation of 5-HT7 receptor/G12 signaling pathway selectively activates both RhoA and Cdc42 (Kvachnina et al., 2005), suggesting a cross talk between Cdc42 and RhoA pathways. The 5-HT7 receptor/G12 signaling pathway in cultured hippocampal neurons promotes formation of dendritic spines and accelerates synaptogenesis, leading to enhanced spontaneous synaptic activity (Kobe et al., 2012). A morphogenic action of the 5-HT7 receptor is confirmed in neuronal primary cultures from the cortex, hippocampus, and striatal complex of embryonic rat or mouse brain (Speranza et al., 2013, 2015) and postnatal cortical and striatal neurons (Speranza et al., 2017). The involvement of mTOR, Cdc42, Cdk5, and ERK in this process suggests these proteins to be downstream targets of G12 (Speranza et al., 2013, 2015). In the hippocampus, the 5-HT7 receptor/G12 signaling pathway undergoes strong developmental regulation. In organotypic hippocampal cultures from juvenile mice, G12 signaling potentiates formation of dendritic spines, increases the basal neuronal excitability, and modulates synaptic plasticity. In contrast, in preparations from older mice, 5-HT7 receptor stimulation had no effect on neuronal morphology, synaptogenesis, and synaptic plasticity (Kobe et al., 2012). Consistently, the expression level of both 5-HT7 receptor and G12 proteins in the hippocampus progressively decreases during postnatal development (Kobe et al., 2012).
F. Post-translational Modifications
1. Regulatory Mechanisms
The 5-HT7 receptor appears to undergo N-glycosylation and palmitoylation. The receptor is N-glycosylated at the asparagine residues N5 and N66 (Gellynck et al., 2012), but this does not appear to influence agonist binding, potency, or efficacy. Furthermore, immunocytochemical studies revealed the presence of the N-glycosylation mutants at the cell surface.
The mouse 5-HT7 receptor expressed in Sf9 insect cells undergoes dynamic palmitoylation in an agonist-dependent manner (Kvachnina et al., 2009). Mutation analysis shows that cysteines located in the C-terminal receptor domain at positions 404, 438, and 441 represent the main potential palmitoylation sites. Palmitoylation-deficient mutants reveal that agonist-induced activation of Gs and G12 proteins is unaffected. Instead, mutation of the Cys404 alone or in combination with Cys438/Cys441 increases Gs-mediated constitutive activity of the 5-HT7 receptor (agonist-independent), whereas the activation of G12 protein is not affected. Thus, palmitoylation of 5-HT7 receptors might be directly involved in the isomerization of the receptor from the inactive to the active form in the absence of agonists. Considering that the 5-HT7 receptor is coupled to both Gs and G12 proteins, dynamic palmitoylation may represent a molecular mechanism responsible for selective Gs- or G12-mediated signaling.
2. Interacting Proteins
Apart from G proteins, only a few 5-HT7 receptor–interacting proteins have been described. Interacting proteins mostly act as adaptor or scaffolding proteins and help in trafficking of the receptor, not only to the plasma membrane but also in receptor internalization, recycling, and degradation. Among the many regulatory proteins that are involved in GPCR desensitization and downregulation, GPCR-associated sorting proteins (GASPs) participate in the sorting of several receptors toward the degradation pathway (Bornert et al., 2013). GASP-1 and GASP-2 interact with several GPCRs, including 5-HT7 receptors, through interaction of C-terminal tails containing two critical amino acid residues within the sequence that corresponds to helix 8 in the three-dimensional structure of rhodopsin (Simonin et al., 2004).
PLAC-24 (protein that localizes at cell-cell contacts), also known as the eukaryotic initiation of translation factor 3, subunit k, is another intracellular interaction partner for all three splice variants of the human 5-HT7 receptor (De Martelaere et al., 2007). PLAC-24 might be involved in the transport of newly synthesized 5-HT7 receptors toward the plasma membrane, as this transport is hampered by the overexpression of certain domain constructs of PLAC-24. PLAC-24 has been proposed to be additionally involved in the stabilization of the receptor at the cell surface by anchoring it at the actin cytoskeleton, as PLAC-24 might be part of a multisubunit complex that links the actin and microtubule cytoskeleton and causes an increase in the expression of the receptor.
Periplakin (an actin- and intermediate filament–binding protein) and the neurite-outgrowth promoting protein, neurochondrin, strongly interact with the C-terminal tail of 5-HT7 receptor. The functional consequences of these specific interactions are not yet known (Ward et al., 2009).
RhoBTB3, a member of the Ras superfamily of small GTPases, is expressed in several brain regions where the 5-HT7 receptor is also localized, including hippocampus and nucleus accumbens. The physical interaction between the 5-HT7a receptor and RhoBTB3 has been demonstrated through yeast two hybrid, GST pull-down, and coimmunoprecipitation assays (Matthys et al., 2012). Not only the C-terminal tail but also the third intracellular loop of the 5-HT7a receptor seem to be involved in the interaction. The 5-HT7a receptor may be targeted for proteasomal degradation following endocytosis at the plasma membrane, and RhoBTB3 appears to strongly inhibit proteasomal degradation of the receptor.
S100B is a Ca2+-regulatory protein that controls Ca2+ homeostasis in various cell types, including astrocytes. S100B also regulates the activity of adenylyl cyclase in preparations from rat brain and skeletal muscle (Donato et al., 2009). S100B physically interacts with 5-HT7 receptors; it negatively regulates cAMP accumulation in 5-HT7–transfected HeLa cells and mouse cortical astrocytes. Overexpression of S100B causes brain region–specific dysregulation of cAMP pathways in vivo that may relate to depressive-like behavior, which can be normalized by 5-HT7 receptor blockade by SB-269970 (Stroth and Svenningsson, 2015).
For further discussion of interacting proteins, see XVII. B. A Survey of 5-HT Receptor GIPs.
3. Homo- and Heteromeric Receptor Associations
GPCRs can form oligomers, and it is now widely accepted that homo- and heterodimerization provides an additional mechanism for regulating cellular processes through the fine tuning of receptor-mediated signaling (Devi, 2001; Bulenger et al., 2005). The 5-HT7 receptor forms homodimers in both intact HEK293 cells and neuroblastoma N1E-115 cells transfected with 5-HT7 receptor and rat cortical astrocytes (Teitler et al., 2010; Smith et al., 2011; Renner et al., 2012; Teitler and Klein, 2012).
Heterodimerization between 5-HT1A and 5-HT7 receptors has been demonstrated by coimmunoprecipitation and by fluorescence resonance energy transfer approaches (Renner et al., 2012); the coimmunoprecipitation studies in mouse brain provide direct evidence that 5-HT1A and 5-HT7 receptors can form heterodimers in vivo. Such heterodimerization alters the signaling properties of the 5-HT1A receptor by attenuating the ability of 5-HT1A receptor to activate Gi protein, in contrast to 5-HT7 receptor–mediated activation of Gs protein, which is not affected. In addition, heterodimerization reduces the ability of 5-HT1A receptors to activate GIRK channels, an effect mediated through the Gβγ subunits of Gi proteins (Reuveny et al., 1994; Kofuji et al., 1995). The inhibitory effect of heterodimerization on GIRK currents is also evident in mouse hippocampal neurons, suggesting a physiologic relevance in vivo.
Heterodimerization between the 5-HT1A and 5-HT7 receptors appears to promote agonist-mediated internalization of the 5-HT1A receptor (5-HT1A receptors expressed alone are relatively resistant to the agonist-induced internalization). The pharmacological blockade of 5-HT7 receptors, but not of 5-HT1A receptors, abolishes internalization of both 5-HT7 homo- and heterodimers, suggesting that 5-HT7 receptor–mediated signaling is an initial step responsible for 5-HT1A receptor cointernalization. Once internalized, 5-HT1A receptors can activate G protein–independent signaling pathways such as a β-arrestin–mediated coupling to MAPK. Thus, depending on the relative amount of 5-HT1A receptors participating in dimers, stimulation by 5-HT can activate distinct ERK-mediated pathways (i.e., G protein–dependent or β-arrestin–dependent), further implicating the physiologic relevance of heterodimerization.
G. Function
Hedlund et al. (2003) first reported on the generation of 5-HT7 KO mice by targeted disruption within exon II of the 5-HT7 receptor gene, thus inactivating all known splice variants of the receptor. 5-HT7 KO mice (Roberts et al., 2004a; Guscott et al., 2005) grow and reproduce, normally suggesting that the receptor does not play an essential role during development. These mice also have normal body weight and basal rectal temperature and appear to be in good health (Guscott et al., 2005). No differences are detected between 5-HT7 KO mice and the wild-type littermates in general motor ability, visual acuity, pain sensitivity, anxiety-like behavior, or the capacity to show freezing behavior in the habituation, conditioning, or cued components of the cued and contextual conditioning procedure (Roberts et al., 2004a). Also, in the prepulse inhibition paradigm, no difference is observed in the 5-HT7 receptor KO mice (Guscott et al., 2005; Semenova et al., 2008).
1. Thermoregulation
5-HT7 agonists induce a considerable hypothermic response in vivo in various species (Yamada et al., 1988; Won and Lin, 1988; Sugimoto et al., 1991; Guscott et al., 2003; Hedlund et al., 2003, 2004; Naumenko et al., 2011). Initially, the 5-HT1A receptor was thought responsible, largely on the basis of data obtained using the 5-HT1A receptor agonist 8-OH-DPAT (Hjorth, 1985) before this drug was also recognized as a 5-HT7 receptor agonist (Guscott et al., 2003; Hedlund et al., 2003, 2004). It appears the 5-HT7 receptor is more important at lower 5-HT concentrations, playing a role in the fine tuning of temperature homeostasis, whereas the 5-HT1A receptor is activated at higher 5-HT concentrations, possibly as a defense against hyperthermia (Hedlund et al., 2004), such that both 5-HT1A and 5-HT7 receptors are important (Brenchat et al., 2012a).
2. Learning and Memory
5-HT7 receptor KO mice under certain types of examination can display specific impairments in contextual learning. Two forms of place learning, spatial (Barnes maze) and contextual (fear conditioning), in addition to three hippocampus-dependent learning tests have been studied (motor, cued conditioning, and operant conditioning) to demonstrate effects of 5-HT7 receptor KO mice in contextual fear conditioning, whereas there was no evident effect in the other learning tests. Of potential relevance, electrophysiological studies on hippocampal slices from 5-HT7 receptor KO mice demonstrate deficits in long-term potentiation (Roberts et al., 2004).
Further behavioral studies demonstrate hippocampus-associated spatial memory deficits in 5-HT7 receptor KO mice (impairments in memory compilation required for resolving spatial tasks), which result in impaired hippocampus-dependent allocentric memory (Sarkisyan and Hedlund, 2009), whereas no effect was evident in the novel object recognition test. Egocentric spatial memory, which is striatum-dependent, remains intact. On the other hand, 5-HT7 receptor KO mice do not exhibit learning impairments and/or dysfunctions in short-term memory if the environment remains static, such as in the Barnes maze test. In the same test, 5-HT7 receptor KO mice have no impairment in long-term memory or memory consolidation.
3. Antipsychotic Potential
5-HT7 receptor antagonists have been evaluated in animal models used to test antipsychotic-like activity. The selective 5-HT7 receptor antagonist SB-258741 reverses hyperactivity induced by PCP in rats (Pouzet et al., 2002). SB-269970 partially but significantly blocks hyperactivity induced by ketamine in mice (Galici et al., 2008). Furthermore, 5-HT7 receptor KO mice display less pronounced deficits in the PCP-induced prepulse inhibition compared with WT mice (Semenova et al., 2008), although this effect was not replicated by SB-269970 treatment (Semenova et al., 2008). Collectively, the available data suggest that the antipsychotic-like activity elicited by selective 5-HT7 receptor blockade is weaker than that obtained with clinically proven antipsychotic drugs (Thomas and Hagan, 2004).
Current antipsychotic drugs are not very effective to reverse the cognitive deficits associated with schizophrenia. Cognitive impairments induced by subchronic PCP administration in rats are believed to mimic cognitive deficits in schizophrenia (Javitt and Zukin, 1991; Jentsch and Roth, 1999). PCP selectively impairs performance in reversal learning test (Abdul-Monim et al., 2006, 2007; McLean et al., 2009a), attentional set-shifting test (McLean et al., 2008), and novel object recognition test (a paradigm for studying visual episodic memory) (Grayson et al., 2007). The acute administration of SB-269970 reverses subchronic PCP-induced deficits in a reversal learning task in rats (McLean et al., 2009b) and in the novel object recognition test in rats (Horiguchi et al., 2011). Pretreatment with SB-269970 or lurasidone reverses the subchronic PCP-induced deficit in reversal learning in mice (Rajagopal et al., 2016). This effect was not elicited by the agonist AS-19, confirming that antagonism, but not agonism, at 5-HT7 receptors restores function in principal cortical neurons impaired by NMDA receptor blockade.
Cognitive deficits in mice induced by dizocilpine are also used to model impaired working memory in schizophrenia. SB-269970 reverts dizocilpine-induced cognitive deficits in a translational behavioral model of working memory, the delayed nonmatching to position task. At a neurochemical level, SB-269970 normalizes the dizocilpine-induced glutamate efflux but not dizocilpine-induced dopamine extracellular levels in the cortex of freely moving rats (Bonaventure et al., 2011). SB-269970 also reverses dizocilpine-induced memory deficits in an autoshaping Pavlovian instrumental learning task in rats (Meneses, 2004). Moreover, 5-HT7 receptor blockade by another antagonist, SB-656104, reverses dizocilpine-induced learning and memory impairments in the passive avoidance and Morris water maze tests in rats (Horisawa et al., 2011).
Ketamine-based animal models represent a valuable tool in preclinical research because ketamine is commonly used in the clinic to model the transient neurocognitive impairments in healthy volunteers (Krystal et al., 1994). Acute administration of SB-269970 in rats ameliorates ketamine-induced cognitive deficits in the attentional set-shifting task (a measure of cognitive flexibility) and the novel object recognition test (Nikiforuk et al., 2013). SB-269970 reverses memory deficits in an autoshaping Pavlovian instrumental learning task in rats after an intraprefrontal infusion of ketamine (Liy-Salmeron and Meneses, 2008).
Experimental evidences suggest a role of 5-HT7 receptor blockade in the procognitive actions of the atypical antipsychotics amisulpride and lurasidone. Both drugs ameliorate the PCP-induced deficits in the novel object recognition task in rats (Horiguchi et al., 2011). This effect is reversed by coadministration of the selective 5-HT7 agonist AS-19. Lurasidone attenuates the dizocilpine-induced deficits in the passive avoidance test in rats. Also, in this case, AS-19 abolishes the effect of lurasidone (Horisawa et al., 2013).
The pharmacological blockade of 5-HT7 receptors may also have therapeutic implications for the treatment of negative symptoms in schizophrenia because SB-269970 ameliorates ketamine-induced social withdrawal in rats (Nikiforuk and Popik, 2013). Consistently, acute administration of amisulpride reverses ketamine-induced social withdrawal. The prosocial efficacy of amisulpride is abolished by the agonist AS-19. Finally, coadministration of subactive doses of SB-269970 and amisulpride results in prosocial effects in rats (Hołuj et al., 2015).
4. Antidepressant-Like Behavior
5-HT7 receptor KO mice have an antidepressant-like behavior, with reduced immobility in commonly used preclinical animal models of depression, such as the tail suspension test (TST) and the forced swim test (Guscott et al., 2005; Hedlund et al., 2005; Sarkisyan et al., 2010). The decreased immobility in both models is most likely not due to a general increase in motor activity, as no genotype difference in locomotor activity or motor learning were evident between 5-HT7 receptor KO and WT mice (Roberts et al., 2004).
Because 5-HT7 receptor KO mice display an antidepressant-like behavior, the potential role of the 5-HT7 receptor was also investigated in OCD models: it is recognized OCD involves the 5-HT system and patients may benefit from antidepressant therapy. Thus, 5-HT7 receptor KO mice have been tested in three models believed to mimic some of the stereotypic aspects of OCD (Hedlund and Sutcliffe, 2007). Inactivation of the 5-HT7 receptor leads to decreased marble burying, a model linked to OCD and anxiety. However, there is no difference between 5-HT7 receptor KO and WT mice in the two other models (i.e., head dipping and plastic-mesh screen chewing models). Thus, a possible role of the receptor in OCD remains an open question.
5. Sleep
Overall, a normal sleep pattern is observed in 5-HT7 receptor KO mice, although during rest, 5-HT7 receptor KO mice spend less time in REM sleep (with less frequent but longer REM episodes) compared with WT mice (Hedlund et al., 2005). There is no difference between the genotypes in time spent awake or in slow wave sleep, and the frequency of slow wave sleep episodes is not altered. These sleep patterns of 5-HT7 receptor KO mice are in agreement with the antidepressant-like profile observed in this genotype.
A variety of circadian parameters in 5-HT7 receptor KO mice, including rate of entrainment and photic responsiveness, have been investigated. There are no evident differences in the average number of days that 5-HT7 receptor KO mice need to reach entrainment to an advance of 6 hours in the light/dark cycle compared with WT mice (Gardani and Biello, 2008). Both groups of mice display minimal effects to light stimulation during the subjective day, whereas, during the early night, light induces phase delays and, later in the subjective night, results in advances of the circadian phase.
Similarly, administration of the 5-HT7 receptor antagonists SB-269970 and SB-656104 to rats reduces the total amount of REM sleep, whereas wake and slow wave sleep are not affected (Hagan et al., 2000; Thomas et al., 2003; Monti and Jantos, 2006). Furthermore, both genetic inactivation and pharmacological blockade of 5-HT7 receptor augmented the effects of SSRIs on REM sleep suppression (Bonaventure et al., 2007; Shelton et al., 2009).
As for the effect of 5-HT7 receptor agonists on sleep, systemic administration of LP-211 during the light phase increases wakefulness in rats and reduces REM sleep duration and periods. To assess the potential neural sites that mediate the changes in REM and wake in the rat, LP-211 was microinjected into the brain regions involved in sleep-wake regulation. Local administration of LP-211 into the DRN, locus coeruleus, lateral basal forebrain, and laterodorsal tegmental nucleus suppressed REM sleep, and microinjection of LP-211 into the basal forebrain augmented wake (Monti et al., 2014). The authors proposed that activation of 5-HT7 receptors expressed by GABAergic interneurons decreases the activity of REM sleep–promoting cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei and reduces REM sleep. Suppression of REM sleep was observed also when LP-44, another 5-HT7 receptor agonist, was microinjected into the DRN (Monti et al., 2008).
A possible explanation for these apparently contradictory findings is that 5-HT7 receptors can stimulate GABAergic neurons only in the absence and/or at low concentrations of 5-HT, whereas at high 5-HT concentrations, 5-HT7 receptors inhibit these GABAergic neurons. Therefore, a concentration-dependent switch in 5-HT7 receptor signaling could explain why inhibition of 5-HT7 receptor activity, elevated 5-HT concentrations, or administration of 5-HT7 receptor agonist at high concentrations prevent 5-HT7 receptor–mediated stimulation of the REM stimulatory GABAergic neurons.
5-HT7 receptors have also been implicated in the regulation of the mammalian circadian clock located in the suprachiasmatic nucleus. Studies have demonstrated that 8-OH-DPAT induces nonphotic phase resetting through activation of 5-HT7 receptors in vitro and in vivo. This effect is reversed by genetic inactivation or pharmacological blockade by the antagonists SB-269970, DR-4004, and JNJ-18038683 (Sprouse et al., 2004; Guscott et al., 2005; Shelton et al., 2015). Consistently, the agonist LP-211 induced a phase advancement of the circadian rhythm in mice (Adriani et al., 2012). Moreover, activation of 5-HT7 receptors by the partial agonist AS19 shortens the period length of oscillation of clock gene period circadian protein homolog 2 expression in the suprachiasmatic nucleus. Period circadian protein homolog 2 expression is used to monitor changes in circadian period length and amplitude (Westrich et al., 2013).
6. Autism Spectrum Disorders
Clinical studies suggest a deficient brain 5-HT system as a causal mechanism in autism spectrum disorders (McDougle et al., 1996; Boccuto et al., 2013). Moreover, the lack of 5-HT during early stages of development may contribute to disrupt the wiring architecture of the brain (Azmitia et al., 2011).
5-HT7 receptor activation corrects molecular, electrophysiological, and behavioral manifestations in mice models of Fragile X syndrome (FXS) and Rett syndrome (RTT), both genetic forms of intellectual disabilities associated with autistic behavior (Costa et al., 2012, 2015; De Filippis et al., 2014, 2015). FXS is the most common inherited intellectual disability; it is caused by silencing of the Fmr1 gene coding for the Fragile X Mental Retardation Protein (Pieretti et al., 1991).
Activation of 5-HT7 receptors by the agonists 8-OH-DPAT and LP-211 rescues mGluR-LTD in Fmr1 knockout mice and restores LTD levels to those of WT mice (Costa et al., 2012, 2015). This might have important functional consequences, as long-term synaptic plasticity plays a fundamental role in shaping the structure and function of brain circuits. LTD is crucially involved in learning and memory, in novelty detection, and in the extinction of previously acquired memories, and it is believed to underlie behavioral flexibility (Collingridge et al., 2010). On that basis, Costa et al. (2012, 2015) suggested that selective activation of 5-HT7 receptors, by restoring mGluR-mediated synaptic plasticity to normal levels, might also rescue cognitive functions and behavioral flexibility in the mouse model of FXS.
De Filippis et al. (2014) have demonstrated that the selective 5-HT7 agonist LP-211 rescues the behavioral impairments in MeCP2-308 male mice, a mouse model of RTT. RTT is a rare neurodevelopmental disorder characterized by severe behavioral symptoms, including autistic-like behaviors, anxiety, motor disturbances, stereotypic hand movements, and severe cognitive dysfunction (Hagberg, 2002; Ricceri et al., 2013). Mutations in the methyl-CpG–binding protein 2 (MeCP2) gene have been identified as the main genetic cause of RTT. MeCP2 encodes a multifunctional protein that binds to methylated DNA and mainly acts as a key transcriptional regulator. Systemic treatment with LP-211 rescues RTT-related defective performance: anxiety-related profiles in a light/dark test, motor abilities in a dowel test, the exploratory behavior in the marble burying test, and memory in the novelty preference task. At a molecular level, LP-211 administration in MeCP2-308 mice restores levels of the Rho GTPases effector molecules p21 activated kinases and cofilin, which both are key regulators of actin cytoskeleton dynamics and, thus, crucially involved in neuronal plasticity. A follow-up study reported similar effects in MeCP2-308 heterozygous female mice; the genetic and hormonal milieus of these mice more closely resemble those of RTT patients (De Filippis et al., 2015). In addition, targeting 5-HT7 receptors can rescue brain mitochondrial dysfunction in heterozygous female MeCP2-308 and MeCP2-Bird mice (a more severely affected model). Moreover, LP-211 treatment completely restores the radical species overproduction by brain mitochondria in the MeCP2-308 model and partially recovers the oxidative imbalance in MeCP2-Bird mice (Valenti et al., 2017).
A core symptom of autism spectrum disorder is repetitive and stereotypic behavior. The dual 5-HT1A/5-HT7 partial agonist (+)-5-(2'-fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine reduces or eliminates stereotypy in three different mouse models of stereotypy: idiopathic jumping, repetitive body rotations after treatment with the NMDA antagonist dizocilpine, and repetitive head-twitching after treatment with the 5-HT2 receptor agonist DOI (Canal et al., 2015) without altering locomotor behavior.
Finally, it has been proposed that the reduced behavioral inflexibility elicited by 5-HT7 receptor antagonists (Nikiforuk, 2012; Nikiforuk and Popik, 2013) might be of relevance in autism spectrum disorder, as reduced behavioral flexibility (i.e., a reduced ability to replace a previously acquired rule with a new one in adaptation to a new environmental context) is a typical feature of this pathology (Ciranna and Catania, 2014).
There is a suggestive link between a HTR7 genetic abnormality, which encodes the 5-HT7 receptor, and neurodevelopmental disorders (Helsmoortel et al., 2016). The whole-genome sequencing of a severely affected dizygotic twin with an autism spectrum disorder and intellectual disability revealed a compound heterozygous mutation in the HTR7 gene as the only variation.
7. Epilepsy
The involvement of 5-HT7 receptors in epilepsy has been actively investigated. The 5-HT7 receptor antagonist SB-258719 reduces spontaneous epileptic activity in the WAG/Rij rat model of absence epilepsy (Graf et al., 2004). In pilocarpine-induced rat models of temporal lobe epilepsy, SB-269970 also reduces the number of seizures (Yang et al., 2012). Consistently, in epileptic rats, the presumed activation of 5-HT7 receptors by AS-19 increases the number of seizures (Yang et al., 2012). In contrast to the above data, inactivation of the 5-HT7 receptor gene caused a generalized threshold reduction in electrical- or chemical-induced seizures (Witkin et al., 2007).
It has been suggested that the evident seizure-prone phenotype of 5-HT7 receptor KO mice may be due to adaptive changes to the loss of perinatal 5-HT7 receptor–induced depolarization. In line with these data, the 5-HT7/5-HT1A receptor agonist 5-CT increased the seizure threshold for picrotoxin in stressed mice (Pericic and Svob Strac, 2007), which is abolished by SB-269970 but not by the 5-HT1A receptor antagonist WAY-100635 (Pericic and Svob Strac, 2007).
8. Drug Abuse
The link between the 5-HT7 receptor and substance abuse has been under explored. Hauser et al. (2015) suggest that 5-HT7 receptors may play a key role in addiction on the basis of neuroanatomical, biochemical, physiologic, and behavioral observations.
Interestingly, genomic studies in humans suggest a link between variants in the gene encoding the 5-HT7 receptor and alcoholism (Zlojutro et al., 2011; Kim et al., 2014). In this respect, mice exposed to alcohol vapors present increased expression of 5-HT7 receptors in brain areas specifically involved in dependence (Yoshimoto et al., 2012). However, blockade of 5-HT7 receptors with SB-258719 does not alter alcohol drinking behavior in the mice exposed to alcohol vapor (Yoshimoto et al., 2012).
9. Pain
Much effort has been made to investigate the potential (patho)physiologic role of 5-HT7 receptors in nociception and chronic pain. Early studies suggested a peripheral pronociceptive action of 5-HT through 5-HT7 receptor activation (Meuser et al., 2002). The pain-promoting effect of 5-HT or 5-CT injection into a hindpaw on formalin-induced local nociceptive responses is blocked by SB-269970 (Rocha-González et al., 2005).
In rat models of neuropathic pain (i.e., chronic constriction injury to the sciatic nerve or spinal nerve ligation) systemic administration of SB-269970 reduces hyperalgesia and tactile allodynia (Amaya-Castellanos et al., 2011; Viguier et al., 2012). Intrathecal administration of SB-269970 also reduces tactile allodynia in spinal nerve–ligated rats, suggesting the involvement of 5-HT7 receptors in pronociceptive mechanisms at the spinal level (Amaya-Castellanos et al., 2011). A pronociceptive effect of 5-HT7 receptor stimulation has been reported at the trigeminal level. SB-656104 significantly decreases the c-Fos immunostaining in the spinal nucleus of the trigeminal nerve in response to intracisternal injection of capsaicin (Martínez-García et al., 2011).
However, such findings appear in contradiction with the data from Brenchat et al. (2009, 2010), in which systemic administration of SB-269970 or SB-258719 enhance mechanical hypersensitivity associated with capsaicin-induced hyperalgesia or nerve injury in mice. Interestingly, local injection of SB-269970 or SB-258719 in control mice does not promote hypersensitivity, suggesting that 5-HT7 receptors might be involved in some pronociceptive modulatory mechanisms only under neuronal sensitization conditions.
In addition to possible species differences, the selected model of neuropathic pain might also have relevance with respect to the aforementioned contradictions.
The antinociceptive potential of 5-HT7 receptor antagonists would suggest a pronociceptive effect of 5-HT7 receptor agonists for which there is some support (Brenchat et al., 2010; Martínez-García et al., 2011). However, other studies report 5-HT7 receptors mediate antinociceptive effects. Thus, blockade of spinal 5-HT7 receptors by intrathecal injection of SB-269970 prevents the antinociceptive effects of systemic administration of morphine, tramadol, or cannabinoids in the tail flick test (Dogrul and Seyrek, 2006; Dogrul et al., 2009; Yanarates et al., 2010), and intrathecal administration of E-57431 and E-55888 inhibit mechanical hypersensitivity caused by capsaicin injection or nerve injury–induced mechanical hypersensitivity in both mice and rats (Brenchat et al., 2011; Viguier et al., 2012). Furthermore, systemic treatment with 5-HT7 receptor agonists produce marked reductions in mechanical and thermal hypersensitivity in various chronic pain models with central and/or peripheral sensitization (Brenchat et al., 2009, 2010, 2012a,b; Ulugol et al., 2012; Viguier et al., 2012, 2013).
H. Clinical Relevance
1. Depression
Multiple experimental approaches tend to support the hypothesis that 5-HT7 receptor blockade or genetic inactivation displays an antidepressant-like activity (Guscott et al., 2005; Hedlund et al., 2005; Sarkisyan et al., 2010). In agreement with mouse KO data, the pharmacological blockade of 5-HT7 receptors results in antidepressant-like effects in the TST (Hedlund et al., 2005; Wesołowska et al., 2006a; Bonaventure et al., 2007) and in the FST in both mice (Hedlund et al., 2005; Wesołowska et al., 2006b) and rats (Wesołowska and Kowalska et al., 2008; Mnie-Filali et al., 2011).
The 5-HT7 receptor antagonist SB-269970 was assessed in the olfactory bulbectomy paradigm, which is considered as a “chronic” behavioral model of depression, in which classic antidepressants require the administration for 2 to 3 weeks before any antidepressant-like effects can be observed (Song and Leonard, 2005). SB-269970 induced a faster antidepressant-like response when compared with 1 week of treatment with the SSRI fluoxetine (Mnie-Filali et al., 2011). 5-HT7 receptor blockade may also augment the effects of antidepressant drugs; thus, the combination of an ineffective dose of SB-269970 with an ineffective dose of one of several antidepressants, results in a synergistic reduction in immobility in both FST and TST (Wesołowska et al., 2006b; Bonaventure et al., 2007; Wesołowska and Kowalska, 2008; Sarkisyan et al., 2010) that correlates with increases in 5-HT release in the frontal cortex (Bonaventure et al., 2007; Wesołowska and Kowalska, 2008). Besides the prefrontal cortex, the hippocampus has also been implicated in the effects of SB-269970 and imipramine, as intrahippocampal SB-269970 administration reduces immobility in the rat FST (Wesołowska et al., 2006b).
Preclinical tests in mice suggest that the clinically established antidepressant effect of the atypical antipsychotic drugs amisulpride, aripiprazole, or lurasidone may be due to 5-HT7 receptor blockade. Thus, amisulpride, aripiprazole, and lurasidone are potent but nonselective 5-HT7 receptor antagonists that have clear antidepressant actions (Lecrubier et al., 1997; Smeraldi, 1998; Lawler et al., 1999; Shapiro et al., 2003; Nakamura et al., 2009; Ishibashi et al., 2010; Citrome, 2011) that reduce immobility in the TST and the FST in WT but not in 5-HT7 KO mice (Abbas et al., 2009a; Sarkisyan et al., 2010; Cates et al., 2013), strongly suggesting a role for the 5-HT7 receptor.
The 5-HT7 receptor antagonist JNJ-18038683 (Bonaventure et al., 2012) reduces immobility in mice in the TST. Coadministration of subeffective doses of citalopram and JNJ-18038683 elicits an antidepressant effect. However, JNJ-18038683, when tested in patients with major depressive disorder, produced no statistically significant improvement over placebo on the Montgomery-Åsberg Depression Rating Scale, although escitalopram in that same study was also inactive, complicating the interpretation.
2. Sleep
The potent and selective 5-HT7 receptor antagonist JNJ-18038683 prolonged REM latency and decreased REM sleep duration in healthy volunteers (Bonaventure et al., 2012). Furthermore, JNJ-18038683 appeared to enhance REM sleep suppression induced by citalopram.
XVI. High-Resolution Structure of 5-HT Receptors
A. 5-HT GPCRs
Since the initial cloning of a 5-HT receptor in 1988 (Julius et al., 1988), it has been appreciated that the G protein–coupled 5-HT receptors would have a topology similar to other members of the GPCR superfamily (Kroeze et al., 2003). These features included a predicted seven-transmembrane helical arrangement with an orthosteric binding pocket near the upper one-third of the helical array (Choudhary et al., 1992, 1993, 1995). Indeed, early molecular models—bolstered by site-directed mutagenesis studies—predicted that 5-HT and serotonergic drugs such as ergolines and ergopeptines would be anchored by a highly conserved aspartic acid in helix III and aromatic residues in helix VI (Choudhary et al., 1993, 1995; Wang et al., 1993; Sealfon et al., 1995).
These predictions were confirmed in 2013 with the publication of the first high-resolution structures of the human 5-HT1B (Wang et al., 2013) and 5-HT2B (Wacker et al., 2013) receptors—both in complex with the ergopeptine ergotamine (Fig. 28). Additionally, the 5-HT1B receptor was also solved in complex with dihydroergotamine (Wang et al., 2013), revealing an essentially identical ligand orientation. Perhaps not surprisingly, and as predicted many years ago (Choudhary et al., 1995), ergotamine was anchored by the highly conserved TMIII aspartic acid residue and stabilized by hydrophobic and edge-on-face interactions with aromatic residues in helix VI (Wacker et al., 2013; Wang et al., 2013).
Fig. 28.
Structures of 5-HT1B, 5-HT2B, and 5-HT2C receptors. PyMol renderings of the 5-HT1B receptor in complex with ergotamine (Left: Wang et al., 2013; Wacker et al., 2013) and the 5-HT2B receptor in complex with LSD (Wacker et al., 2017) demonstrating different binding pockets for ergotamine and LSD [see Wacker et al. (2017) for details]. The figure also shows inactive-state structure of the 5-HT2C receptor (Peng et al., 2018) and the G protein–coupled state of the 5-HT1B receptor (Garcia-Nafria et al., 2018)
Given the large differences between 5-HT1B and 5-HT2B receptors in terms of pharmacology, signal transduction, and amino acid sequence, the high-resolution structures of both receptors allowed the investigators to identify both important structural commonalities and dissimilarities between these two distinct 5-HT receptor families (Wacker et al., 2013; Wang et al., 2013); the orthosteric binding pockets of both receptors are nearly identical, with only two amino acids in the conserved orthosteric binding site differing (Wang et al., 2013). This high degree of conservation readily explains why many serotonergic drugs are promiscuous. Indeed, LSD was found to be a potent agonist at every G protein–coupled 5-HT receptor except the 5-HT7 (Wacker et al., 2013).
Subtle differences in the general region of the binding pocket were able to explain heretofore puzzling aspects of 5-HT receptor pharmacology. For instance, the apparent preference of rodent versions of the 5-HT1B, 5-HT1D, 5-ht1e, and 5-HT1F receptors for β-adrenergic antagonists (Hoyer and Middlemiss, 1989; Adham et al., 1994) was now apparent based on structural, mutagenesis, and modeling studies (Wang et al., 2013). Thus, a Thr7.39Asn7.39 to mutation, changing the human to a rodent version, led to the formation of a polar interaction network now favoring the binding of the propanolamine moiety of adrenergic antagonists. Additionally, the high affinity of norfenfluramine for 5-HT2B receptors, which may lead to valvular heart disease (Rothman et al., 2000), was explained by the orientation of a nonconserved methionine residue (M5.39) that was oriented in the binding pocket in the 5-HT2B receptor and absent in all other 5-HT receptors (Wang et al., 2013). Finally, distinctly different patterns of signaling by ergotamine at 5-HT1B receptors, where it is unbiased, and 5-HT2B receptors, where it shows arrestin bias, led to explication of the structural features responsible for functional selectivity (Wacker et al., 2013). Thus, the apparent arrestin-biased signaling by ergotamine at 5-HT2B receptors is due to a preferential stabilization of inactive states of particular G protein conformational microswitches (which are essential for mediating canonical signaling) and the preferential allowance of conformations responsible for arrestin-dependent signaling (Wacker et al., 2013).
More recent studies have clarified the mechanisms responsible for the differential activation of G protein versus arrestin signaling (Wacker et al., 2017a) at 5-HT2A (Wacker et al., 2017b) and 5-HT2B receptors (Wacker et al., 2017b; McCorvy et al., 2018). In particular, a detailed study of several 5-HT2B receptor structures (Wacker et al., 2017b; McCorvy et al., 2018) disclosed that key interactions with A5.46 and T3.37 are essential for canonical G protein signaling, whereas interactions with extracellular loop residue L7.35 are essential for arrestin-ergic signaling. Additionally, other structures of an intermediate-active state of the 5-HT2C receptor (Peng et al., 2018; Fig. 28) and the G protein heterotrimer-stabilized active state of the 5-HT1B receptor (Garcia-Nafria et al., 2018) implicated a key “trigger motif” P-I-F as being essential for the active-inactive switch and biased signaling (Wacker et al., 2017a; McCorvy et al., 2018).
B. 5-HT Ligand-Gated Ion Channels
5-HT3 receptors are ion channels of the Cys-loop receptor family (Thompson et al., 2010; Corringer et al., 2012). Thus, they share a common architecture with other members, such as nicotinic acetylcholine, ionotropic γ-aminobutyric, and glycine receptors. All of them are composed of five subunits, symmetrically disposed around a central ionic pore axis, forming an urn-like architecture (Fig. 29). Each subunit has three functional domains (Fig. 29): a large extracellular domain harboring the neurotransmitter binding pocket, a four-helix transmembrane domain establishing the ion channel, and an intracellular domain that mediates the receptor interaction with intracellular proteins.
Fig. 29.
Architecture and ligand binding sites of 5-HT3 receptors. (A) Cartoon representation of a single subunit viewed parallel to the plane of the membrane. (B) Cartoon representation of the entire pentameric receptor, in the same orientation [the subunit of (A) is equivalent to the yellow subunit]. One out of the five stabilizing VHH15 single-chain llama antibodies is shown in pale green and labeled VHH15. (C) Surface representation of the receptor highlighting binding clefts in blue (neurotransmitter site), yellow (anesthetics intrasubunit site), orange (PU-02 and anesthetics intersubunit site), purple (extracellular allosteric pocket), and olive (pore blockers site). The subunit equivalent to the one of (A) appears in darker gray.
Recent years have seen the emergence of a solid structural framework to help interpret functional and pharmacological studies. First, 5-HT, granisetron, and palonosetron have been crystallized in complex with a soluble model protein (termed 5-HTBP; Kesters et al., 2013; Price et al., 2016), providing possible orientations of ligands and adding up to the huge variety of nicotinic receptor ligands cocrystallized with the same type of model protein. Second, the initial mouse 5-HT3A receptor X-ray structure (Hassaïne et al., 2014) has provided an almost complete picture (∼60 unstructured residues missing in the intracellular domain) of a 5-HT3 receptor but with empty neurotransmitter sites capped by stabilizing llama antibodies instrumental to crystallization. Third, a series of distinct conformations of the full-length murine receptor imaged by Cryo-Electron Microscopy (Basak et al., 2018a,b; Polovinkin et al., 2018) has shed light on its gating mechanism and revealed how 5-HT and antiemetic drugs such as tropisetron bind in the neurotransmitter site. Specialized reviews discuss Cys-loop receptor structure at length (daCosta and Baenziger, 2013; Nys et al., 2013; Sauguet et al., 2015; Wu et al., 2015; Nemecz et al., 2016).
The five equivalent neurotransmitter binding pockets of the homopentameric 5-HT3A receptor are located in electronegative clefts at interfaces between two adjacent subunits (Fig. 30). Three loops of the principal subunit and four portions of beta strands of the complementary subunit contribute to the pocket (Fig. 30). Therefore, the shape of the binding pocket arises from the combination of the quaternary arrangement (how one subunit is oriented relative to its neighbor) and of the local conformation of loops and side chains. During activation of Cys-loop receptors and gating of the channel, both global subunit/subunit orientation and more local conformation changes take place (Sauguet et al., 2013; Du et al., 2015; Basak et al., 2018a; Polovinkin et al., 2018). The 5-HTBP and other model proteins present a stiff quaternary structure in which the subunit/subunit orientation is fixed, and in that respect, they are imperfect models for ligand binding.
Fig. 30.
The neurotransmitter binding site of the 5-HT3 receptor. (A) Global view of the site, at the interface between two subunits represented as cartoons, viewed from parallel to the place of the membrane. Binding elements of the principal subunit (A–C) and of the complementary subunit (D–G) are color-coded. (B) Surface view representing the electrostatic potential in the same orientation. The surface has been removed around to yellow loop (C) that would cover it, for clarity. The inset illustrates motions of loop (C) associated with binding of an agonist (blue, contracted conformation, PDB 2BYQ), an antagonist (gray, extended conformation, PDB 2C9T), or the stabilizing llama antibody VHH15 (salmon). (C) Close-up views of the binding site with essential residues, including those of the aromatic box. On the right panel, the 5-HT3A receptor structure is superimposed with the 5-HTBP structure cocrystallized with granisetron (in yellow). The principal subunits are superimposed, and, as a consequence of different subunit/subunit orientation, the strands of the complementary subunit are shifted. This superimposition illustrates the diversity and complementarity of structural templates.
The binding cleft of the 5-HT3A receptor is surrounded by aromatic residues forming a 10-Å wide box, including W156 (loop B), W63 (loop D), Y126 (loop E), F199, and Y207 (loop C). W156 (W145 in 5-HTBP) lies at the bottom of the cleft and has cation-Pi interaction with 5-HT (Beene et al., 2002). This part of the site looks rigid with many intra- and intersubunit interactions. In contrast, the mouth of the cleft seems prone to reorganization. The C loop adopts different conformations in the apo, the antagonist-bound, and 5-HT-bound (Fig. 30). Other important residues lining the site include N101 (loop A), T152 and T154 (loop B), R65 (loop D), D177, S179, I180 (loop F), D42, and I44 (loop G).
A picture of the molecular mechanism of 5-HT3 receptors starts to emerge from the accumulated structural data. The closed apo conformation resembles those of the receptor inhibited by small molecules (antiemetics such as tropisetron) or by the inhibitory VHH15. Upon 5-HT binding, the receptors undergo a transition to the open state characterized by 1) a compaction of the neurotransmitter pocket because of quaternary reorganization of the extracellular domain and 2) an opening of the transmembrane pore linked to the pivot of each subunit transmembrane domain. Two independent cryo-EM studies have captured a second 5-HT–bound state featuring a closed pore similar to that of the resting state (Basak et al., 2018a; Polovinkin et al., 2018). Based on functional experiments, this state was tentatively described as preactive rather than desensitized (Polovinkin et al., 2018).
There is no direct structural data on allosteric modulation sites of 5-HT3 receptors. Nevertheless, allosteric sites identified on homologous Cys-loop receptors are relevant to the 5-HT3 pharmacology. For instance, PU-02, a selective inhibitor of the 5-HT3 receptor acting in the micromolar range, binds to a transmembrane intersubunit site (orange in Fig. 29) that correlates to the ivermectin site of the C. elegans glutamate-gated chloride channels receptor (Hibbs and Gouaux, 2011) and to the ethanol and bromoform sites of the model bacterial GLIC receptor (Sauguet et al., 2013). In the 5-HT3A receptor structure, a deep cleft between subunits is indeed accessible from the lipid bilayer and lined by residues determining PU-02 activity [16]: L266, S270, G282, and V288 on the (+) side and S226, I268, and T272 on the (−) side (Fig. 29). A second membrane-accessible site, this time located within a single subunit (Fig. 29), has been identified as the site of action of the positive allosteric modulator TMPPAA (Gasiorek et al., 2016; Polovinkin et al., 2018) and might be involved in anesthetics inhibition (Lopreato et al., 2003). Some compounds acting as physical blockers of the ion flux bind directly in the channel lumen. A variety of sites have been identified at different depths in the pore, spanning almost the whole transmembrane part, from divalent cations binding close to the intracellular mouth to TEA and lidocaine in the middle (Hilf et al., 2010) and mementine that binds close to the extracellular mouth (between 13’ and 16’) (Rammes et al., 2001; Ulens et al., 2014).
The 5-HT3 receptor structures also shed light on the neglected intracellular domain. Only part of it is seen in the structures: a short helical stretch after the M3 transmembrane helix and a long helix MA continuous with the M4 transmembrane helix (Figs. 29 and 31). At their N-terminal side, MA helices form a tight pentameric bundle stabilized by hydrophobic interactions (Fig. 31). Further up, and closer to the mouth of the transmembrane pore, they carry charged residues whose mutations were shown to profoundly affect the channel conductance. At this level, the spacing between MA helices results in lateral fenestrations, plugged in the closed-pore structures by the loop linking M3 and MA (Fig. 31). Recent cryo-EM structures have shown that this intracellular region is drastically reorganized in the open-pore state, with wide portals, and displays flexibility. The ion exit pathway and the way in which the ion flux depends on the flexibility and the local electrostatic environment remain to be fully described (Di Maio et al., 2015).
Fig. 31.
The intracellular domain of the 5-HT3 receptor. (A) Surface representation of the intracellular domain viewed parallel to the membrane plane. The left part shows the external surface, whereas the right part shows a cut-through and thus depicts the intracellular vestibule, the lateral obstructed portal, and the constriction along the pore axis. The arrow indicates the plausible exit for ions. (B) Backbone representation of MA-M4 helices (gray, two subunits shown) with the numerous charged residues (blue and red for positively and negatively charged residues) depicted as sticks. The green cartoon shows for one subunit the M3 and MX helices and their connecting loop that plug the portal (yellow oval). The hydrophobic residues that create the tight bundle of MA helices are in yellow, and the triplet of arginine determinant for channel conductance are labeled in bold font.
Molecular dynamic simulations established the existence of a dewetted zone in the transmembrane pore in the closed-pore conformations and its conversion to a fully hydrated pore permeant to cations in the open conformations (Yuan et al., 2016; Polovinkin et al., 2018).
XVII. 5-HT GPCRs and Their Interacting Proteins
A. Introduction
The history of cell signaling started more than 60 years ago with the seminal discovery of cAMP by Earl Sutherland (Nobel prize in 1971). The mechanisms by which some receptors activate the production of second messengers has been revealed by Martin Rodbell (Nobel Prize 1994) in the 1970s when he showed that the receptors do not directly stimulate second messenger synthetizing enzymes but indirectly, via an allosteric activation of G proteins. The GPCR ternary complex consisting of a receptor, a G protein, and an effector, enzyme, or channel was born. Surprisingly, five decades later, the nature and functions of the huge protein complexes comprising GPCRs and GPCR-interacting proteins (GIPs) still remain poorly characterized. During their cellular journey, GPCRs are assisted by GIPs for their proper folding, targeting proper subcellular compartments, trafficking to and out of the plasma membrane, and select several alternative signaling pathways, including G protein–independent pathways. In addition, GIPs are able to allosterically modify the pharmacology of associated GPCR and even activate some GPCRs in the absence of ligands. This section of the review is dedicated to GIPs of 5-HT receptors, which are among the GPCRs for which those proteins are the most extensively characterized, mainly thanks to proteomics screens. Given the large number of 5-HT receptor GIPs identified, it provides a wide overview of their role in GPCR physiology. Recent advances in the role of GIPs in fine-tuning 5-HT6 receptor signaling and associated physiologic functions, including neurodevelopment and cognition, are particularly highlighted.
The evolution of multicellular organisms has been closely linked to their capacity to communicate with their environment and to develop sophisticated communications between their own cells. Most of these communications involve chemical messengers (e.g., hormones, neurotransmitters, growth factors) that interact with one or several transmembrane receptors. Among those receptors, GPCRs are the most numerous ones (Bockaert and Pin, 1999). Around 1000 genes encode such receptors in the human genome (representing 3% to 4% of the genome), of which ∼300 are nonolfactory receptors (Fredriksson et al., 2003). 5-HT receptors are particularly well represented in this important receptor family. The diversity of 5-HT GPCRs is certainly due to molecular tinkering from one ancestral gene and has been instrumental for the implication of 5-HT in a large number of physiologic and pathophysiological functions both in the CNS and peripheral tissues. Because of their structural diversity, 5-HT receptors are able to trigger a large panel of signaling events (Marin et al., 2012). Many of them are transduced by different G proteins. In addition, signaling events elicited by 5-HT receptor activation as well as by many GPCRs are triggered or finely modulated through their interaction with multiple intracellular proteins, “GIPs,” and are assembled into functional complexes designated as “receptosomes” (Bockaert et al., 2003, 2004b, 2010b; Maurice et al., 2011; Marin et al., 2012). GIPs are not only involved in GPCR signaling but also in their targeting to subcellular compartments, including axonal and dendritic compartments, in their trafficking in and out of the plasma membrane through the endoplasmic reticulum, Golgi apparatus, endosomal, and lysosomal compartments (Bockaert et al., 2003, 2004b, 2010; Maurice et al., 2011; Marin et al., 2012). Several reviews have been published on GIPs, including two dedicated to 5-HT receptor GIPs (Allen et al., 2008; Marin et al., 2012). Accordingly, only a brief description of the nature, cellular, and physiologic functions of 5-HT receptor GIPs will be provided here (see Table 21), focusing on intracellular GIPs and not considering 5-HT receptor association with other GPCRs (homodimer/heteromer formation). A more detailed account of proteins interacting with the 5-HT6 receptor is presented to highlight the interest of a comprehensive description of GIPs for a given receptor to unravel novel mechanisms underlying GPCR activation and physiologic functions controlled by those receptors.
TABLE 21.
5-HT receptor–interacting proteins and their cellular and physiologic functions
For each receptor, the nature of identified GIPs, their site of interaction, the function of their interaction with the receptor (if known) at the cellular (receptor targeting, trafficking, and signal transduction) and physiologic levels as well as their role in pathologies are indicated. The question marks indicate that the site of interaction of the GIP in the receptor sequence or the physiologic/pathologic functions of its interaction with the receptor remain unknown.
| Receptor | GIP | Site of Interaction | Cellular Functions | Physiologic/Pathologic Functions | References |
|---|---|---|---|---|---|
| 5-HT1A | Calmodulin | i3/Ct | Endocytosis | ? | Della Rocca et al., 1999; Turner et al., 2004, 2007 |
| PKC-dependent receptor phosphorylation | |||||
| Erk1,2 and Jun kinase activation | |||||
| NHE-1 activation | |||||
| Yif1B | Ct | Anterograde trafficking | ? | Al Awabdh et al., 2012; Alterio et al., 2015 | |
| Dendritic targeting | |||||
| 5-HT1B/D | p11 | i3 | ➚Cell surface density and signaling | ➘Depression in rodent models | Svenningsson et al., 2006; Eriksson et al., 2013 |
| ➘Emotional memory in rodents | |||||
| 5-HT2A | β-arrestin 2 | ? | ➚PI3K/Src/Akt signaling | Hallucinogenic effects induced by high 5-HT concentration | Schmid et al., 2008; Bhattacharya et al., 2010; Schmid and Bohn, 2010 |
| Delayed recycling (human receptor) | |||||
| MAP1A | ? | Trafficking in apical dendrites | Allen et al., 2008 | ||
| Arf1-6 | i3/Ct | Coupling to phospholipase D | Robertson et al., 2003 | ||
| Jak/STAT3 | ? | ➚Transcription | Guillet-Deniau et al., 1997 | ||
| RSK2 | i3 | Receptor phosphorylation and desensitization | Putative implication in psychiatric disorders (Coffin-Lowry syndrome) | Sheffler et al., 2006; Allen et al., 2008; Strachan et al., 2009 | |
| 5-HT2A | Calmodulin | i2/Ct | ➘Gq coupling | Turner and Raymond, 2005 | |
| Caveolins | ? | Targeting to lipid rafts Endocytosis | Bhatnagar et al., 2004 | ||
| PSD-95 | Ct (PDZ ligand-SCV) | Dendritic targeting | Hallucinogen effects in mice | Xia et al., 2003; Becamel et al., 2004; Abbas et al., 2009; Pichon et al., 2010; Vogrig et al., 2013; Wattiez et al., 2013 | |
| Postsynaptic localization | Antipsychotic drug effects | ||||
| ➚Phospholipase C | ➘Inflammatory and neuropathic pain | ||||
| ➚Endocytosis | |||||
| Cell surface density | |||||
| SAP97 | Ct (PDZ ligand-SCV) | ➚Gq signaling | ? | Allen et al., 2008 | |
| ➘Endocytosis | |||||
| MUPP1 | Ct (PDZ ligand-SCV) | Postsynaptic localization | Drug dependence | Jones et al., 200; Becamel et al., 2001; Shirley et al., 2004 | |
| ➚Cell surface density | |||||
| MAGI2 | Ct (PDZ ligand-SCV) | Postsynaptic localization | ? | Bécamel et al., 2004 | |
| CIPP | Ct (PDZ ligand-SCV) | Postsynaptic localization | ? | Bécamel et al., 2004 | |
| NHERF3 | ? | ➚Gq signaling | ? | Walther et al., 2015 | |
| ➘Endocytosis | |||||
| 5-HT2B | MUPP1 | Ct (PDZ ligand-SYV/I) | ? | ? | Becamel et al., 2001 |
| PDZ protein | Ct (PDZ ligand-SYV/I) | NO synthesis | ? | Manivet et al., 2000 | |
| 5-HT2C | Calmodulin | Ct | ➚Erk1/2 signaling | ? | Bécamel et al., 2002; Labasque et al., 2008 |
| β-arrestins | i2/? | ➚Erk1/2 signaling | ? | Labasque et al., 2008, 2010; Marion et al., 2004 | |
| Constitutive and agonist-dependent endocytosis | |||||
| MUPP1 | Ct (PDZ ligand-SSV) | ? | ? | Becamel et al., 2001; Parker et al., 2003 | |
| PSD-95 | Ct (PDZ ligand-SSV) | ➚Endocytosis | ? | Bécamel et al., 2002; Gavarini et al., 2006 | |
| ➘Signaling | |||||
| MPP3 | Ct (PDZ ligand-SSV) | ➘Endocytosis | ? | Bécamel et al., 2002; Gavarini et al., 2006 | |
| Veli3/CASK/MintMAGI2, SAP102, PSD93 | Ct (PDZ ligand-SSV) | ? | ? | Bécamel et al., 2002 | |
| 5-HT2C | PICOT | Ct | ? | ? | Bécamel et al., 2002 |
| PTEN | i3 | ? | Rewarding effects of THC and nicotine | Ji et al., 2006 | |
| 5-HT4 | Src | ? | ➚Erk1/2 signaling | ? | Gill et al., 2005; Barthet et al., 2007; Barthet et al., 2009 |
| ➚PLCγ1 | |||||
| p11 | i3 | ➚Cell surface localization | ➘Depression in rodent models | Warner-Schmidt et al., 2009 | |
| ➚Signaling | |||||
| ADAM10 | ? | ➚sAPPα release | ➘β-amyloid plaques | Cochet et al., 2013 | |
| ➘Neuroinflammation | |||||
| GRK5 | Ct | ➘Src activation | ? | Barthet et al., 2009 | |
| ➘Erk1/2 signaling | |||||
| 5-HT4(a) | SNX27a | Ct (PDZ ligand-SCF) | Targeting to endosomes | ? | Joubert et al., 2004 |
| Receptor recycling? | |||||
| NHERF | Ct (PDZ ligand-SCF) | Targeting to microvilli | ? | Joubert et al., 2004 | |
| - interaction with Ezrin | |||||
| CRMP2 | Ct | Neuronal architecture | ? | Joubert et al., 2004 | |
| 5-HT4(b) | PDE3A1, PDE4D3 | ? | Regulation of cAMP signaling | ? | Weninger et al., 2014 |
| 5-HT4(e) | CIPP | Ct (PDZ ligand-VPV) | ? | ? | Joubert et al., 2004 |
| 5-HT4(e) | nNOS | Ct (PDZ ligand-VPV) | ? | ? | Joubert et al., 2004 |
| 5-HT6 | Fyn | Ct (PXHPXR) | ➚Gs signaling | ? | Yun et al., 2007 |
| ➚Erk1/2 signaling | |||||
| ➚Cell surface expression | |||||
| Jab1 | i3/Ct | ➚Gs signaling | ➘Cell death | Yun et al., 2010 | |
| ➚Cell surface expression | |||||
| ➚c-Jun phosphorylation | |||||
| Map1B-LC1 | Ct | ➚Cell surface expression | ? | Kim et al., 2014 | |
| ➘Endocytosis | |||||
| ➚Erk1/2 signaling | |||||
| SNX14 | i3 | ➚Endocytosis | ? | Ha et al., 2015 | |
| ➘Gs signaling | |||||
| mTORC1 | Ct/unknown | ➚mTOR signaling | Cognitive impairment in neurodevelopmental models of schizophrenia | Meffre et al., 2012 | |
| Cdk5, p35 | Ct | Receptor phosphorylation Cdk5 signaling | Neurite growth migration of pyramidal neurons | Duhr et al., 2014; Jacobshagen et al., 2014; Dayer et al., 2015 | |
| 5-HT7 | Neurochondrin | ? | ? | Ward et al., 2009 | |
| Periplakin | ? | ? | Ward et al., 2009 | ||
| S100B | i3 | ➘cAMP | Behavioral despair | Stroth and Svenningsson, 2015 |
PICO, protein kinase C interacting cousin of thioredoxin.
B. A Survey of 5-HT Receptor GIPs
1. 5-HT1A Receptor
The 5-HT1A receptor recruits and activates both Gi3 and Go (Bockaert et al., 2006; Marin et al., 2012). Coupling to Gi3 is predominant in the soma of 5-HT raphe neurons and mainly leads to adenylyl cyclase (AC) inhibition, whereas coupling to Go prevails in hippocampus and leads to inhibition of GIRK channels (Mannoury la Cour et al., 2006); see also Marin et al. (2012) for a detailed review of Gi/Go-mediated signaling events elicited by 5-HT1A receptor and their physiologic impact. The molecular basis of the difference in G protein coupling is unknown. Only two GIPs are known to interact with 5-HT1A receptors, namely calmodulin (Della Rocca et al., 1999; Turner et al., 2004) and Yif1B (Carrel et al., 2008; Al Awabdh et al., 2012; Alterio et al., 2015). The Ca2+-calmodulin complex binds to two distinct sites located in the third intracellular loop (i3) of the receptor (Turner et al., 2004), whereas Yif1B (Yip1 interacting factor homolog B) associates with its short C-terminal domain (Ct). Ca2+-calmodulin is implicated in receptor internalization, Erk1/2 activation, and modulation of PKC-dependent receptor phosphorylation (Della Rocca et al., 1999; Turner et al., 2004). In addition, Ca2+-calmodulin contributes to receptor-operated Janus kinase 2 (Jak2) and type 1 sodium-proton exchanger (NHE-1) activation (Turner et al., 2007). 5-HT1A receptor activation leads to the assembly of a ternary signaling complex that includes activated Jak2, Ca2+-calmodulin, and NHE-1, in which calmodulin is activated by tyrosine phosphorylation instead of cellular Ca2+ elevation. This results in tighter interaction of calmodulin with NHE-1, NHE-1 conformational change, and increased NHE-1 transport activity (Turner et al., 2007). Yif1B is a protein implicated in the traffic of proteins from the endoplasmic reticulum (ER) to the Golgi (Alterio et al., 2015) that plays a key role in the selective targeting of 5-HT1A receptors to dendrites (Carrel et al., 2008). It has been proposed that Yif1B serves as a scaffold allowing the recruitment of 5-HT1A receptor to a complex comprising Rab6, kinesin family member 5B, and dynein that coordinates its anterograde traffic to terminal dendrites (Al Awabdh et al., 2012).
2. 5-HT1B Receptor
In addition to its coupling to Gi/Go (Bockaert et al., 2006; Marin et al., 2012), the 5-HT1B receptor interacts via its i3 loop with a particularly interesting GIP, named p11 (Svenningsson et al., 2006; Svenningsson and Greengard, 2007). p11 (also known as S100A10) is a member of the S100 EF-hand protein family. S100 proteins are small acidic proteins (10–12 kDa) characterized by two calcium-binding sites that have helix-loop-helix (“EF-hand type”). p11 increases cell surface density of 5-HT1B receptor and, thus, receptor-operated signaling (Svenningsson et al., 2006). Reduction in p11 level has been found in postmortem human tissue from depressed individuals and suicide victims as well as in a rodent model of depression, whereas its level in rodent brain is increased by antidepressants or electroconvulsive therapy (Svenningsson et al., 2006; Svenningsson and Greengard, 2007). Furthermore, invalidation of the gene encoding p11 induces a depression-like phenotype, reduces response to antidepressants and emotional memory, and enhances dependence to cocaine (Svenningsson and Greengard, 2007; Eriksson et al., 2013; Svenningsson et al., 2013; Svenningsson, 2014).
3. 5-HT2A Receptor
The 5-HT2A receptor is mainly coupled to Gq/G11 proteins. 5-HT2A receptors also engage Gi/Go-mediated signaling upon stimulation by hallucinogenic agonists, including LSD and synthetic agonists such as DOI (Bockaert et al., 2006; Gonzalez-Maeso et al., 2007; Marin et al., 2012; Karaki et al., 2014). Like many GPCRs, the 5-HT2A receptor also recruits β-arrestins following agonist stimulation (Bhattacharya et al., 2010). Intriguingly, it has been found that β-arrestin 2 is specifically involved in the hallucinogenic-like effects elicited by high concentrations of 5-HT (or its metabolites) but not in those induced by DOI (Schmid et al., 2008). These hallucinogenic-like effects are mediated by the activation, via β-arrestin 2, of a PI3K/Src/Akt signaling cascade (Schmid and Bohn, 2010). Likewise, β-arrestins are required for Erk1/2 activation by 5-HT but not by DOI (Schmid et al., 2008).
The 5-HT2A receptor (like several other 5-HT receptors, including 5-HT2B and 5-HT2C receptors; see below) expresses a canonical recognition motif for PDZ domain–containing proteins at its C-terminal extremity. PDZ domain–containing proteins (or PDZ proteins) are scaffolding proteins containing one or several PDZ domains, in addition to other protein-protein interaction domains. PDZ domains have been classified according to their specificity for PDZ motifs (or PDZ ligands): class I PDZ domains preferentially bind to -S/Txφ, class II to -φxφ, and class III to -E/Dxφ motifs (where φ represents a hydrophobic residue and X any residue), respectively. The 5-HT2A receptor PDZ ligand (-SCV) clearly belongs to class I. Several PDZ proteins have been found to interact with the 5-HT2A receptor. The majority of them have been identified thanks to affinity purification coupled to mass spectrometry (AP-MS) proteomic strategies or two-hybrid screens. These include Multi-PDZ protein1 (MUPP1), PSD-95, membrane-associated guanylate kinase with inverted domain structure2 (MAGI2), synapse-associated protein (SAP)97, channel interacting PDZ protein (CIPP), and MAGUK p55 subfamily member 2 (MPP2) and 3 (MPP3) (Becamel et al., 2001; Bécamel et al., 2004; Pichon et al., 2010). Among the 5-HT2A receptor GIPs identified, PSD-95 is certainly the most extensively studied with respect to the functional impact of its association with the receptor. PSD-95, which is located in postsynaptic spine membranes together with other receptor PDZ partners, is required for dendritic targeting of the receptor (Xia et al., 2003; Allen et al., 2008). In addition, microtubule-associated protein (MAP)1A may scaffold the receptor to the microtubule cytoskeleton and facilitate its trafficking to apical dendrites (Allen et al., 2008). PSD-95, like SAP97 and MUPP1, increases receptor cell surface density likely by inhibiting receptor endocytosis (Allen et al., 2008; Jones et al., 2009). The impact of the 5-HT2A receptor/PSD-95 complex upon receptor-operated signaling is less clear. On the one hand, 5-HT2A receptor–mediated downstream signaling is impaired in PSD-95–deficient mice (Abbas et al., 2009), suggesting that the interaction promotes receptor-operated signal transduction. On the other hand, acute disruption of 5-HT2A receptor/PSD-95 association with an interfering peptide increases receptor-mediated cytosolic Ca2+ increase and its antihyperalgesic effects in models of neuropathic and inflammatory pain in the rat (Pichon et al., 2010; Wattiez et al., 2013), suggesting that this interaction inhibits receptor-operated signaling. These opposite effects of sustained (PSD-95 knockout) and acute (interfering peptide) disruption of the 5-HT2A receptor/PSD-95 interaction may reflect long-term adaptive changes in PSD-95 knockout mice. In line with the antihyperalgesic effects produced by disruption of 5-HT2A receptor/PSD-95 interaction with a peptide, docking simulation studies identified a series of substituted indoles as potential small-molecule inhibitors of this interaction. One of them efficiently inhibited mechanical hyperalgesia in an experimental model of traumatic neuropathic pain in the rat (Vogrig et al., 2013). Collectively, these studies identify the 5-HT2A receptor/PSD-95 complex as a new therapeutic target for the treatment of neuropathic pain, which remains poorly controlled by currently available analgesics. Consistent with the inhibition of 5-HT2A receptor–operated signaling in PSD-95 deficient mice, the hallucinogenic-like effects of drugs are impaired in these mice (Abbas et al., 2009). Likewise, deletion of PSD-95 renders ineffective atypical antipsychotics such as clozapine, which act as 5-HT2A receptor inverse agonists, against PCP-induced disruption of prepulse inhibition, a widely used pharmacological model of schizophrenia (Abbas et al., 2009). Na+/H+ exchanger regulatory factor (NHERF) 3 is another PDZ protein that interacts with the 5-HT2A receptor but, surprisingly, in a PDZ-independent manner (Walther et al., 2015). NHERF3 negatively regulates 5-HT2A receptor endocytosis and positively influences 5-HT2A receptor–stimulated inositol phosphate formation (Walther et al., 2015).
Several 5-HT2A receptor–interacting proteins have been identified in addition to PDZ proteins. Targeting of 5-HT2A receptor to caveolae microdomains is ensured by its interaction with the multifunctional proteins caveolins (Allen et al., 2008). Moreover, association of the receptor with caveolins has a profound impact on receptor-operated signal transduction and functions; interaction of 5-HT2A receptor with caveolin-1 within lipid raft/caveolae membranes enhances receptor-mediated signal transduction by facilitating its coupling to Gαq protein in C6 glioma cells (Bhatnagar et al., 2004), whereas its interaction with caveolin-3, the predominant caveolin isoform in heart, upon 5-HT stimulation, negatively regulates receptor-mediated hypertrophy in cardiomyoblasts and neonatal cardiomyocytes (Mialet-Perez et al., 2012).
ARF1 and, to a lesser extent, ARF6, associate with the i3 loop and Ct of the 5-HT2A receptor (Robertson et al., 2003). This association is enhanced by GTP loading and is essential for 5-HT2A receptor–mediated PLD activation, which seems to be independent of Gq/11 (Robertson et al., 2003). 5-HT2A receptors also bind to the tyrosine kinase Jak2 in fetal myoblasts (Guillet-Deniau et al., 1997). This is accompanied by Jak2 autophosphorylation, the recruitment of the transcription factor signal transducer and activator of transcription (STAT)3, and its translocation to the nucleus (Guillet-Deniau et al., 1997). P90 ribosomal S6 kinase 2 (RSK2) interacts with 5-HT2A receptor i3 loop and exerts a tonic break on receptor-operated signaling (Sheffler et al., 2006; Allen et al., 2008). RSK2 phosphorylates 5-HT2A receptor at Ser314 located in the i3 loop. Ser314 phosphorylation underlies heterologous desensitization of the receptor elicited by growth factors (Allen et al., 2008; Strachan et al., 2009). A role of 5-HT2A receptors in symptoms associated with Coffin-Lowry syndrome (because of null mutations in RSK2 gene), such as moderate to severe mental retardation, movement disorders, and schizophrenia-like psychosis, in heterozygote females has been proposed (Allen et al., 2008). Calmodulin interacts in a Ca2+-dependent manner with consensus binding motifs located in 5-HT2A receptor i2 loop and Ct. This association inhibits receptor coupling to Gq (Turner and Raymond, 2005).
4. 5-HT2B Receptor
The 5-HT2B receptor is mainly coupled to Gq [see also Bockaert et al. (2006), Marin et al. (2012) for detailed review of Gi/Go-mediated signaling events elicited by this receptor].
A number of recognized signaling proteins interact with the 5-HT2B receptor, including constitutive and inducible NOS and the G proteins Gαq, Gα11, and Gα13. The 5-HT2B receptor also binds to the multivalent PDZ scaffold protein, MUPP1; this is in common with the other 5-HT2 receptors 5-HT2A and 5-HT2C. 5-HT2A and 5-HT2B receptors also bind MUPP1-PDZ domains in vitro and share the C-terminal -E-X-V/I-S-X-V sequence (Becamel et al., 2001). This PDZ motif is also required for the recruitment of the constitutive NO synthase cNOS (Manivet et al., 2000).
Ubiquitin E3 ligases (E3s) confer specificity to ubiquitination by recognizing target substrates. The ligand of numb protein X (LNX) family of E3s is a group of PDZ domain–containing RING-type E3 ubiquitin ligases. The substrate recognition mechanism of LNX E3s involves their specific PDZ domains by binding to the C termini of the target proteins. The human C-terminal LNX1 PDZ3-binding motifs of the 5-HT2B receptor promotes ubiquitination by LNX1ΔPDZ4 (Guo et al., 2012).
5. 5-HT2C Receptor
The 5-HT2C receptor activates phospholipase C (PLC) via Gq/11, PLD via G13, phospholipase A2 (PLA2), and Akt/glycogen synthase kinase (GSK)3β via Gi3 (Bockaert et al., 2006). 5-HT2C receptors also activate Erk1/2 signaling via a mechanism entirely independent of G proteins but dependent of their interaction with both Ca2+/calmodulin and β-arrestin 2 (Labasque et al., 2008; Marin et al., 2012). As already mentioned, the receptor expresses a canonical class I PDZ ligand at its C-terminal extremity. MUPP1 was the first PDZ protein identified as a partner of the 5-HT2C receptor in 1998, using the yeast two hybrid system (Ullmer et al., 1998). MUPP1 contains 13 PDZ domains (it is the PDZ protein that contains the largest number of PDZ domains identified) and selectively interacts with the receptor via its 10th PDZ domain (Becamel et al., 2001). This interaction is dynamically regulated by agonist-dependent receptor phosphorylation at Ser458 located in the PDZ binding motif (Parker et al., 2003). The gene encoding MUPP1 (Mpdz) has been identified as a quantitative trait underlying physical dependence to drugs of abuse (Shirley et al., 2004). Additional PDZ partners of 5-HT2C receptor have been identified by means of AP-MS proteomic strategies (Bécamel et al., 2002, 2004). These include PSD-95, SAP102, MPP3, and the ternary complex Veli3/CASK/Mint1. These studies revealed that in spite of their similar PDZ binding motifs, 5-HT2A and 5-HT2C receptors bind to different sets of PDZ proteins that differ in their synaptic localizations: 5-HT2A receptors preferentially interact with PDZ proteins exclusively located at the postsynapse (e.g., PSD-95, SAP97, and CIPP), whereas 5-HT2C receptors associate with PDZ proteins exhibiting both presynaptic and postsynaptic localizations such as the ternary complex Veli3/CASK/Mint1, consistent with their different distribution at the synapse (Bécamel et al., 2004). 5-HT2C receptor PDZ partners differentially regulate receptor endocytosis and desensitization: PSD-95 increases both constitutive and agonist-induced receptor endocytosis and desensitization, whereas MPP3 stabilizes the receptor at the plasma membrane and prevents its desensitization (Gavarini et al., 2006). Note that PSD-95 has an opposite effect on 5-HT2A and 5-HT2C receptor endocytosis (Xia et al., 2003; Gavarini et al., 2006).
5-HT2C receptors also interact via their i3 loop with PTEN, a phosphatase that negatively controls the Akt/PkB signaling pathway and thus is a tumor suppressor (Ji et al., 2006). The 5-HT2C receptor/PTEN interaction was established in the VTA, the site of inhibition of the rewarding effects of many drugs of abuse by 5-HT2C receptor agonists. This inhibition is linked to the ability of receptors to prevent the increase in the firing rate of VTA dopaminergic neurons projecting to the nucleus accumbens elicited by drugs. Disrupting the 5-HT2C receptor-PTEN interaction with a cell-penetrating interfering peptide reduced the firing rate of VTA dopaminergic neurons and prevented rewarding effects of drugs of abuse such as THC and nicotine, suggesting that this interaction might be a relevant target for the treatment of drug addiction (Ji et al., 2006). 5-HT2C receptors also interact with protein kinase C interacting cousin of thioredoxin, a PKC theta-interacting protein with a thioredoxin homology domain involved in the regulation of the thioredoxin system (Bécamel et al., 2002). The functional consequence of this interaction remains to be established.
6. 5-HT4 Receptors
5-HT4 receptors engage signaling pathways through multiple G proteins, especially GS and in some cell lines G13, Gq, and Gi [for reviews, see Coupar et al. (2007), Bockaert et al. (2008)]. In addition, 5-HT4 receptors stimulate Erk1/2 in a G protein– and β-arrestin–independent mechanism that requires activation of the tyrosine kinase Src constitutively associated with 5-HT4 receptor (Gill et al., 2005; Barthet et al., 2007; Bockaert et al., 2011). This pathway is also implicated in the activation of PLCγ1-mediated inhibition of NHE-1 in intestinal epithelial cells (Gill et al., 2005). Furthermore, GRK5 interacts with the proximal Ct of 5-HT4 receptors and phosphorylates β-arrestin 1. This prevents Src activation and underlies desensitization of Src-dependent Erk1/2 activation by 5-HT4 receptors (Bockaert et al., 2008; Barthet et al., 2009).
Like 5-HT1B receptors, 5-HT4 receptors interact with p11 via their i3 loop (Warner-Schmidt et al., 2009). p11 enhances receptor cell surface localization and signal transduction (Warner-Schmidt et al., 2009). Given the extensive data showing that p11 is downregulated in depressive states (Svenningsson and Greengard, 2007) and that activation of 5-HT4 receptors has antidepressant effects (Ge and Barnes, 1996; Bockaert et al., 2011), this interaction might be of particular interest for the treatment of depression.
The 5-HT4 receptor interacts, directly or indirectly, with the α-secretase ADAM10. This interaction might be an important step in the constitutive (agonist-independent) activation of nonamyloidogenic cleavage of amyloid precursor protein (APP) and release of soluble APPα (sAPPα) fragment elicited by the receptor (Cochet et al., 2013). sAPPα has neurotrophic and neuroprotective properties, and its release on constitutive (and agonist-dependent) receptor activation might contribute to receptor-mediated reduction of amyloid pathology observed in several mouse models of Alzheimer disease (Cochet et al., 2013; Tesseur et al., 2013; Pimenova et al., 2014; Claeysen et al., 2015).
The 5-HT4 receptor is one of the GPCRs for which alternative mRNA splicing generates the most variants that differ in their Ct, with some variants (a, e, and f) expressing canonical PDZ ligands, suggesting that these receptors may recruit specific GIPs, including different sets of PDZ partners. Ten proteins have been shown to interact with the Ct of the 5-HT4(a) receptor. Most of them are PDZ proteins that associate with its canonical class I PDZ motif (-SCF) (Joubert et al., 2004). These include SNX27a, a member of the sorting nexin family, which targets 5-HT4(a) receptors to early endosomes in cell lines (Joubert et al., 2004). A more recent study showed that SNX27 associates with β2-adrenergic receptor PDZ ligand and that this interaction is essential for PDZ-directed receptor recycling (Temkin et al., 2011). It also showed that SNX27 serves as an essential adaptor protein linking PDZ motif containing cargo to the retromer. SNX27 also directly interacts with endosomes through its lipid-binding phox homology (PX) domain. A similar role of SNX27a in endocytic 5-HT4 receptor trafficking is likely. The interaction of the 5-HT4(a) receptor with the PDZ protein NHERF promotes its recruitment to microvilli, where it localizes with activated ezrin, consistent with a role of 5-HT4 receptors in cytoskeleton remodeling (Joubert et al., 2004). The Ct of the 5-HT4(a) receptor also recruits CRMP2, a member of the collapsing response mediator protein (CRMP) family through a PDZ-independent mechanism (Joubert et al., 2004). 5-HT4 receptors cause G13- and RhoA-dependent neurite retraction and cell rounding in neuroblastoma cells (Ponimaskin et al., 2002b). Their association with CRMP2 might also contribute to regulation of neuronal architecture.
Three GIPs associate with the class II PDZ ligand (-VPV) of the 5-HT4(e) receptor. These include two PDZ partners, namely CIPP and nNOS, the only NOS isoform expressing a PDZ domain. The third protein that binds to the Ct of 5-HT4(e) receptor is Sec23A, a protein of the COPII complex, which is likely involved in its trafficking from the ER to the Golgi (Joubert et al., 2004). The 5-HT4(b) receptor interacts with the phosphodiesterases PDE3A1 and PDE4D3, which may thereby regulate receptor-operated cAMP signaling (Weninger et al., 2014).
7. 5-HT7 Receptor
The 5-HT7 receptor is coupled to Gs and G12 (Bard et al., 1993; Ruat et al., 1993b). 5-HT7 receptor stimulation promotes neurite outgrowth and SRE-mediated gene transcription through Gα12-dependent activation of RhoA and Cdc42 GTPases (Kvachnina et al., 2005; Guseva et al., 2014b). The 5-HT7 receptor Ct interacts with neurochondrin, a protein expressed in bone and brain that promotes neurite growth. Neurochondrin interacts with several GPCRs, including melanin-concentrating hormone receptor 1, orexin-1 and thromboxane A2 receptors, and the mGlu5 metabotropic glutamate receptor (Ward et al., 2009; Marin et al., 2012). Generally, neurochondrin inhibits GPCR coupling to G proteins. The 5-HT7 receptor Ct also recruits the intermediate filament periplakin, which, like neurochondrin, inhibits receptor-operated Ca2+ signaling (Ward et al., 2009). The role of neurochondrin in neurite outgrowth elicited by 5-HT7 receptor stimulation remains unexplored.
5-HT7 receptors, via their i3 loop, interact with S100B, a member of the S100 family, and S100B negatively regulates 5-HT7 receptor–induced cAMP production (Stroth and Svenningsson, 2015). S100B seems to play a role in the antidepressant effects of fluoxetine (Baudry et al., 2010), and its serum concentration may serve as a biomarker predicting response to antidepressant treatment (Arolt et al., 2003). Notably, 5-HT7 receptor antagonists have been reported to display rapid antidepressant action, making the receptor a promising target for antidepressants with improved onset of clinical efficacy compared with most of the currently available treatments (Mnie-Filali et al., 2011). Suggestive of a possible influence of 5-HT7 receptor/S100B interaction in the pathogenesis of mood disorders and their treatment, transgenic female mice overexpressing S100B show depressive-like behavior that is normalized by administration of a 5-HT7 receptor antagonist (Stroth and Svenningsson, 2015).
The human and rat genes coding for the 5-HT7 receptors generate different splice variants (Heidmann et al., 1997). One of them (5-HT7(b) receptor) is common to rat and human and expresses at its extreme Ct a canonical class II PDZ ligand (-FVL). To date, no PDZ partner of 5-HT7(b) receptor has been identified.
C. 5-HT6 Receptor Receptosome: Toward New Signaling Mechanisms Underlying Its Control of Cognition and Neurodevelopmental Processes
1. Introduction
The 5-HT6 receptor has early been considered as a promising target for psychiatric diseases in line with its near exclusive expression in the central nervous system (Hirst et al., 2003). Although initial studies suggested that 5-HT6 receptors are almost exclusively localized on GABA interneurons, more recent studies, including some in human brain, indicate that they are also present on pyramidal glutamatergic neurons in prefrontal cortex and hippocampus (Woolley et al., 2004; Marazziti et al., 2013b). A more detailed study of 5-HT6 receptor mRNA distribution showed highest expression in both D1 and D2 receptor–containing medium-size spiny neurons of caudate putamen and nucleus accumbens and confirmed consistent expression in glutamatergic neurons of hippocampus and cerebral cortex expressing vGluT1 (Helboe et al., 2015). It also showed the presence of 5-HT6 receptor mRNA in a minor fraction of GABAergic cortical neurons, including mainly neurons coexpressing 5-HT3A receptor and a subset of calbindin- and calretinin-positive neurons (Helboe et al., 2015). 5-HT6 receptors thus display an ideal distribution to regulate the balance between excitatory and inhibitory signaling in brain regions implicated in cognition, which is altered in schizophrenia (Uhlhaas and Singer, 2010). The pharmacology of 5-HT6 receptors includes a great number of ligands exhibiting agonist or antagonist activities. Some of them have shown putative interest for treating cognitive deficits, depression, anxiety, sleep, and feeding disorders as well as pain (Mitchell and Neumaier, 2005; Svenningsson et al., 2007; Fone, 2008; Ly et al., 2013; Wilkinson et al., 2014; Claeysen et al., 2015; Karila et al., 2015; Pereira et al., 2015). Unfortunately, phase III clinical trial results for treating cognitive impairment have been disappointing. An increase in our understanding of how the cellular and molecular events associated with the 5-HT6 receptor translate to behavioral responses may inform and support stratified medicine approaches.
The 5-HT6 receptor is positively coupled to Gs and, like many GPCRs, activates Erk1/2 (Ruat et al., 1993a; Sebben et al., 1994) (Fig. 32). As Gs/cAMP and Erk1/2 pathways can have a positive influence on cognition, it was unlikely that their inhibition would mediate the procognitive effects of 5-HT6 receptor antagonists. This suggested that alternative coupling mechanisms might be involved. Likewise, the cellular mechanisms controlling 5-HT6 receptor functional activity remained largely unexplored until the use of unbiased strategies to identify 5-HT6 receptor–interacting proteins. These included two-hybrid screens (Yun et al., 2007, 2010; Kim et al., 2014) and AP-MS proteomic strategies based on different methods to purify receptor partners, such as coimmunoprecipitation (Meffre et al., 2012) or pull-down assays using particular receptor sequences as baits (Duhr et al., 2014; Ha et al., 2015).
Fig. 32.
Role of Fyn, Jab1, and SNX 1 recruitment by 5-HT6 receptor in receptor-operated signaling and functions. Association of Fyn with 5-HT6 receptor Ct increases receptor cell surface localization and, consequently, receptor-operated G protein signaling. Fyn is also involved in Erk1/2 activation by 5-HT6 receptors. Fyn phosphorylates Tau to control its association with microtubules and is involved in neuronal migration and Aβ-induced synaptic deficits and neurotoxicity. Association of 5-HT6 receptor with Jab1 stabilizes surface expression of the receptor and is essential for its activity. 5-HT6 receptor stimulation increases nuclear translocation of Jab1, a process that might reduce cell death induced by hypoxia. The 5-HT6 receptor also interacts with SNX14, which inhibits receptor functions by sequestering Gαs and promoting receptor endocytosis and degradation.
2. Fine-Tuning of 5-HT6 Receptor Trafficking and Signal Transduction by GIPs
The nonreceptor tyrosine kinase Fyn was the first GIP discovered for 5-HT6 receptor, thanks to a two-hybrid screen using the receptor Ct as bait (Fig. 32). Fyn is a member of the Src family of tyrosine kinases that are highly expressed in brain and involved in synaptic plasticity. Several lines of evidence suggest that Fyn might be implicated in Alzheimer disease pathogenesis: Fyn phosphorylates Tau at Tyr18 and controls its association with microtubules (Lee et al., 2004); it has also been involved in Aβ-induced synaptic deficits and neurotoxicity (Yang et al., 2011). In addition, Fyn plays a key role in neurodevelopmental processes: in combination with Src, Fyn controls cortical lamination, the formation of the Purkinje cell plate, and, thus, neuronal migration (Kuo et al., 2005). It is involved in netrin-1–mediated axon attraction of cortical neurons via the phosphorylation of Trio, a Rho/Rac-GEF (DeGeer et al., 2013), and in the maturation of GABAergic synapses elicited by neural cell adhesion molecule (Chattopadhyaya et al., 2013). Fyn binds to 5-HT6 receptor Ct (likely to the conserved polyproline sequence PXhPXR, where X represents any amino acid and h any hydrophobic amino acid) via its SH3 domain (Yun et al., 2007). Functional studies showed that Fyn increases 5-HT6 receptor cell surface localization and, consequently, receptor-operated G protein signaling (Yun et al., 2007). Conversely, 5-HT6 receptor activation triggers Fyn phosphorylation, as assessed by an increase in Tyr420 phosphorylation, an event contributing to 5-HT6 receptor–induced activation of the Ras/Erk1/2 pathway (Fig. 32) (Yun et al., 2007). Whether 5-HT6 receptor–mediated Fyn activation is involved in neuronal migration, attraction, and maturation of GABAergic synapses remains to be elucidated.
Further two-hybrid screens identified two other 5-HT6 receptor–interacting proteins that control receptor trafficking. The first one is Jun activation domain–binding protein (Jab)1, a protein initially isolated as a c-Jun and Jun D binding partner that stabilizes their binding to activator protein 1 (AP-1) sites and potentiates them as transcription factors (Claret et al., 1996; Yun et al., 2010). Jab1 interacts with both i3 loop and Ct of the receptor (Yun et al., 2010). Jab1 stabilizes surface expression of 5-HT6 receptor and is necessary to maintain activity of endogenous receptors. In turn, 5-HT6 receptor stimulation increases nuclear translocation of Jab1, suggesting that they may play a role in the regulation of gene expression via Jab1 (Fig. 32) (Yun et al., 2010). Furthermore, 5-HT6 receptors and Jab1 are upregulated following middle cerebral artery occlusion–induced focal cerebral ischemia in rats. Likewise, exposure of cultured cells to hypoxic insults increased expression of both protein partners, which in turn reduce cell death induced by hypoxia (Fig. 32) (Yun et al., 2010). The second protein identified is light chain 1 (LC1) subunit of MAP1B protein (MAP1B-LC1), a microtubule-associated protein highly expressed in the brain (Kim et al., 2014). MAP1B-LC1 interacts with the Ct of the receptor and reduces receptor endocytosis, thereby increasing its cell surface expression and signal transduction activity (Gs coupling and Erk1/2 activation) (Kim et al., 2014).
The 5-HT6 receptor i3 loop recruits several proteins of the endocytic machinery, such as dynamin, AP-2, amphiphysin, and epsin as well as SNX14 (Ha et al., 2015). Like SNX27, SNX14 belongs to the sorting nexin family, predicted to have a role in protein sorting and vesicular trafficking (Carlton et al., 2005). It contains a putative RGS domain and a phox homology (PX) domain, an N-terminal hydrophobic region, and a PX-associated domain of unknown function. SNX14 is expressed at high levels in the nervous system (Carroll et al., 2001) and plays a critical role in both excitatory and inhibitory transmissions (Huang et al., 2014). SNX14 was found to inhibit 5-HT6 receptor–dependent signaling via two different mechanisms: 1) it specifically binds to and sequesters Gαs, thus inhibiting the Gs-cAMP pathway; and 2) it promotes 5-HT6 receptor endocytosis and degradation (Fig. 32) (Ha et al., 2015). Furthermore, PKA phosphorylates SNX14 at two serine residues located in the RGS domain. SNX14 phosphorylation strongly reduces its affinity for Gαs and thereby prevents Gαs sequestration. Accordingly, phosphorylated SNX14 preferentially associates with 5-HT6 receptor and can thereby enhance receptor internalization (Ha et al., 2015). Therefore, SNX14 has been considered as a dual, sequential, negative regulator of 5-HT6 receptor–operated signaling, first by sequestering Gαs and second by inducing receptor endocytosis (Ha et al., 2015). Collectively, these findings indicate that 5-HT6 receptor functional activity is finely modulated by its association with GIPs that exert contrasting effects on receptor trafficking and receptor-mediated signal transduction.
3. Recruitment of mTOR Complex 1 by 5-HT6 Receptor: Potential Role in Cognitive Deficits Associated with Schizophrenia
Using a proteomics strategy combining coimmunoprecipitation of full-length receptor and tandem mass spectrometry, Meffre et al. (2012) identified 28 proteins that specifically associate with the 5-HT6 receptor. This “receptosome” showed a remarkable enrichment in proteins of the mTOR pathway (Meffre et al., 2012). These include mTOR itself; Raptor (regulatory associated protein of TOR), an activator of mTOR that together with mTOR and proline-rich Akt substrate of 40 kDa constitutes the mTOR complex 1 (mTORC1); the small GTPase Rheb (Ras homolog enriched in brain), which directly activates mTOR when bound to GTP; the Tti1/Tel2 complex, required for assembly and stability of mTOR complexes; and the Ras GTPase activating protein neurofibromin, an upstream negative regulator of the pathway leading to mTOR activation (Fig. 33) (Meffre et al., 2012). This “receptosome” also includes Vps34, a protein of the class III PI3K family implicated in autophagosome formation (Bockaert and Marin, 2015). In line with this remarkable enrichment of the 5-HT6 receptor complex in proteins of the mTOR pathway, agonist stimulation of the receptor results in the activation of the mTOR pathway both in a transfected cell line and in rodent brain, particularly in prefrontal cortex and striatum (Meffre et al., 2012). Notably, 5-HT6 receptor–elicited mTOR activation depends on both its physical association with mTOR and the canonical PI3K/Akt/TSC/Rheb pathway involved in activation of mTORC1 by tyrosine kinase receptors (Fig. 33) (Meffre et al., 2012).
Fig. 33.
Engagement of Cdk5 and mTOR signaling pathways by 5-HT6 receptor and their role in neurodevelopmental processes and cognition. Left panel: The 5-HT6 receptor constitutively interacts with Cdk5 (and its activator p35), which phosphorylates the receptor at Ser350. This enables 5-HT6 receptor to promote neurite growth via the activation of the Rho GTPase Cdc42. Cdk5 activity, under the control of 5-HT6 receptor, also enables migration of pyramidal cortical neurons, likely via the phosphorylation of doublecortin (DCX) and focal adhesion kinase (FAK). These effects are agonist-independent and prevented by inverse agonists. Right panel: The 5-HT6 receptor recruits several proteins of the mTOR pathway, including mTOR itself and Raptor, two protein components of the mTORC1 complex, neurofibromin (NF1), Vps34, and Rheb. Prefrontal 5-HT6 receptors engage mTOR signaling upon agonist stimulation and in rat neurodevelopmental models of schizophrenia to compromise social cognition and episodic memory, whereas 5-HT6 receptor blockade by antagonists or direct mTOR inhibition by rapamycin rescue cognitive deficits in these models.
An overactivation of mTOR is observed in many genetic diseases in which mental retardation or cognitive deficits are observed [for a review, see Bockaert and Marin (2015)]. One classic example is tuberous sclerosis (TSC) caused by mutations in the TSC1 or TSC2 genes, which encode proteins of the TSC complex, namely, hamartin and tuberin, respectively. The TSC complex is the main GTPase activating protein for Rheb. Mutations in TSC1 or TSC2 result in inactivation of TSC, a process leading to nonphysiologic mTOR activation. About 50% of TSC patients show intellectual disabilities as well as deficits in memory, attention, and executive functions, and 20%–60% display ASD (Bockaert and Marin, 2015). Moreover, the mTORC1 inhibitor rapamycin rescues cognitive deficits in a mouse model of TSC (Tsc2+/− mice). Likewise, systemic administration of rapamycin prevents deficits in social cognition and novel object recognition induced by a 5-HT6 receptor agonist in the rat (Meffre et al., 2012). In two neurodevelopmental rodent models of schizophrenia, the neonatal PCP model and the social isolation model (isolation after weaning), a sustained 5-HT6 receptor–mediated mTOR activation that persists at the adult stage occurs specifically in prefrontal cortex (Meffre et al., 2012). Again, cognitive impairment observed in both models is reversed by an acute injection of rapamycin at the adult stage (Meffre et al., 2012). These findings suggest a critical role of mTOR activation not only in rare autism-related genetic disorders, such as TSC, but also in schizophrenia, a more frequent, multifactorial, and debilitating disorder. The mechanisms by which nonphysiologic mTORC1 activation induces cognitive deficits remain largely unknown. A recent report has shown that enhanced activity of mTORC1 in postmortem brain of ASD patients is associated with a decrease in autophagy, an increase in spine density, and a reduction of developmental spine pruning in layer V pyramidal neurons (Tang et al., 2014). Spine defects are likewise observed in Tsc2+/− mice (Bockaert and Marin, 2015). Whether inhibition of mTOR pathway contributes to the procognitive effects of 5-HT6 receptor antagonists in patients with Alzheimer disease remains to be explored. Nonphysiologic mTOR activation, under the control of 5-HT6 receptor, might also be involved in epilepsy. Indeed, an increase in 5-HT6 receptor expression was found both in human epileptic tissue and the brain of rats treated with pilocarpine, a model of temporal lobe epilepsy (Wang et al., 2014). mTOR is also overactivated in the pilocarpine model, and a 5-HT6 receptor antagonist prevents mTOR activation, increases latency of seizures, and reduces their severity (Wang et al., 2014). This suggests that blocking the 5-HT6/mTOR pathway might be a valuable therapeutic strategy for epilepsy treatment.
4. Recruitment of Cyclin-Dependent Kinase 5 by 5-HT6 Receptor: An Essential Step in Its Control of Neuronal Migration and Differentiation
The 5-HT6 receptor is implicated in early steps of brain development, including neurulation and neuronal migration within the cortex (Jacobshagen et al., 2014; Dayer et al., 2015). Although the cAMP pathway contributes to the control of neuronal migration by 5-HT6 receptors, a PKA inhibitor only partially reversed the effect of its stimulation upon neuronal migration (Riccio et al., 2009), suggesting that other signaling pathways might be involved. Cyclin-dependent kinase (Cdk)5, which has been identified as a 5-HT6 receptor partner by different AP-MS proteomics strategies (Fig. 33; Meffre et al., 2012; Duhr et al., 2014), was an obvious candidate. Indeed, Cdk5 is known to control actin cytoskeleton dynamics and various neurodevelopmental processes, such as neuronal migration (including migration of cortical pyramidal neurons), neurite growth, and synapse morphogenesis (Jessberger et al., 2009; Lalioti et al., 2010). Moreover, the 5-HT6 receptor recruits, via its Ct, not only Cdk5 and its activator p35 but also a network of proteins functionally connected with Cdk5, including Wiskott-Aldrich syndrome protein–family verprolin homologous protein 1 (WAVE-1) and G protein inducer of neurite growth 1, two Cdk5 substrates; phosphatase 2A, which dephosphorylates and activates WAVE-1; and the Arp2/3 complex, which is also known to be activated by WAVE-1 (Duhr et al., 2014). Several lines of evidence indicate a role of Cdk5, under the control of the 5-HT6 receptor, in the migration of pyramidal neurons: in utero electroporation of a 5-HT6 receptor short hairpin RNA at E14.5 induces a mispositioning of these neurons, which can be rescued by electroporation of plasmids encoding wild-type 5-HT6 receptor or 5-HT6 receptors unable to couple to Gs or to bind to 5-HT (Jacobshagen et al., 2014). This indicates that the effect of 5-HT6 receptors on pyramidal neuron migration is agonist-independent and is not mediated by the Gs-adenylyl cyclase pathway. Defect in migration elicited by silencing 5-HT6 receptor expression is rescued by electroporation of plasmids expressing Cdk5 and its activator p35 (Jacobshagen et al., 2014). Moreover, 5-HT6 receptor knockdown significantly reduces phosphorylation of doublecortin (at Ser297) and focal adhesion kinase (at Ser732) in primary cortical neurons, two Cdk5 substrates known to control migration of pyramidal neurons (Xie et al., 2003; Tanaka et al., 2004). Collectively, these findings indicate that 5-HT6 receptors control migration of cortical pyramidal neurons through an agonist-independent, Cdk5-dependent mechanism (Jacobshagen et al., 2014; Dayer et al., 2015).
Beyond neuronal migration, the 5-HT6/Cdk5 complex also controls neurite outgrowth and neuronal differentiation (Fig. 33). Its role in neurite growth was not only established in neuroblastoma-glioma NG108-15 cells, a commonly used cellular mode for investigating mechanisms underlying neuronal development, but also in primary neurons and brain explants (Duhr et al., 2014; Seo and Tsai, 2014). Reminiscent of its control of neuronal migration, the growth-promoting effects of the receptor is Gs- and agonist-independent. They are reversed by the selective 5-HT6 receptor antagonist SB258585, which thus behaves as an inverse agonist in this model (Duhr et al., 2014). Likewise, SB258585 inhibits Cdk5 association with the 5-HT6 receptor, indicating a specific recruitment of Cdk5 by a constitutively active receptor conformation. 5-HT6 receptor–elicited neurite growth also depends on receptor phosphorylation at Ser350 by associated Cdk5. This suggests a reciprocal interplay between 5-HT6 receptor and Cdk5, whereby the receptor stimulates Cdk5 activity and is itself a Cdk5 substrate. The signaling events downstream of the 5-HT6/Cdk5 complex contributing to neurite growth have been partially characterized and involve Cdk5-dependent activation of the Rho GTPase cell division cycle (Cdc)42, a key regulator of actin cytoskeleton dynamics (Fig. 33) (Duhr et al., 2014). The precise mechanisms that control 5-HT6 receptor/Cdk5 interaction, including the potential influence of other receptor partners, and the cellular events downstream of the 5-HT6/Cdk5/Cdc42 pathway remain to be explored. As already mentioned, the regulatory role of Cdk5 during brain development extends to synapse formation. Whether 5-HT6 receptor–dependent activation of Cdk5 controls synaptogenesis in addition to neuron migration and shaping also remains to be investigated.
XVIII. 5-HT Receptors and the Brain
A. Introduction
The diversity of 5-HT receptor signaling pathways and associated functional complexity in the brain is greater than any other tissue or organ. For historical perspectives on the contribution of neurophysiology, pharmacology, and molecular biology to the discovery of 5-HT receptor subtypes in the brain, see reviews in Bradley et al. (1986), Barnes and Sharp (1999), and Bockaert et al. (2010).
B. 5-HT Receptor Signaling in Neurons
All 5-HT receptor subtypes found in the brain are also found in the periphery except 5-ht1e, 5-HT2C, and 5-HT6 receptors, for which there is little evidence for functional expression outside the CNS. The signaling properties of 5-HT receptors in the brain are identical to those in the periphery. Thus, the metabotropic 5-HT receptors couple to the three canonical signaling pathways, namely Gi, Gs, and Gq/11, which elicit the expected second messenger cascades, and this is proven to occur in neurons in most cases.
The Gi-coupled 5-HT receptors that inhibit adenylyl cyclase and cause a fall in cAMP encompass the 5-HT1 receptor family, comprising 5-HT1A, 5-HT1B, 5-HT1D, 5-ht1E, and 5-HT1F subtypes. Evidence suggests that the 5-HT5 receptor family, 5-HT5A and 5-ht5b, is also Gi-coupled, although, to date, this has only been demonstrated in cultured cells and not native neurons. Excellent reviews on the signaling (Hannon and Hoyer, 2008; Bockaert et al., 2010; McCorvy and Roth, 2015) and consequent electrophysiological (Lamb and Aghajanian, 2006; Andrade and Beck, 2010; Marek, 2010) properties of 5-HT1 receptors can be found elsewhere.
Independent of the involvement of a decrease in cAMP, 5-HT1 receptors also open GIRKs to hyperpolarize neurons and inhibit the opening of voltage-gated calcium channels; this is best understood for 5-HT1A receptors (e.g., Montalbano et al., 2015). 5-HT1 receptors are also likely to elicit other (noncanonical) G protein–dependent signals, including via ERK and Akt kinase, again as exemplified by studies of the 5-HT1A receptor. Adding further complexity, it is well established that 5-HT1A receptors have different properties depending on their pre- or postsynaptic localization. In particular, agonists tend to have greater efficacy at presynaptic 5-HT1A receptors, and the latter have greater tendency to desensitize than postsynaptic 5-HT1A receptors. The reasons for these differences are uncertain but, as discussed elsewhere (Clarke et al., 1996; Barnes and Sharp, 1999; Mannoury la Cour et al., 2006; Garcia-Garcia et al., 2014), could relate to presynaptic versus postsynaptic differences in 5-HT1A receptor reserve, efficiency of G protein coupling, non–G protein–dependent signaling, or even biased agonism—that is, the generation of signals that are ligand-dependent. Regarding the latter, emerging data strongly support the view that selective 5-HT1A receptor agonists can preferentially direct receptor signaling to particular intracellular pathways in a brain region–specific manner (Becker et al., 2016).
The 5-HT2 receptor family, 5-HT2A, 5-HT2B, and 5-HT2C, comprise Gq/11-coupled receptors that activate phospholipase C, leading to increased formation of inositol trisphosphate and diacylglycerol and then mobilization of intracellular calcium and PKC activation. 5-HT2 receptor stimulation also results in neuronal excitation in a variety of brain regions, likely involving the closure of potassium channels. A 5-HT2 receptor–mediated increase in excitatory postsynaptic currents is also often observed, which is thought to be mediated either directly or indirectly through increased release of glutamate (Lambe and Aghajanian, 2006; Marek, 2010).
It is now recognized that 5-HT2 receptor signaling goes well beyond activation of the phospholipase C/PKC pathway. For instance, noncanonical signaling pathways associated with 5-HT2A receptor activation include phospholipase A2, the ERK pathway, and signaling via small G proteins (e.g., Rho A and Rab4), and there are similar such findings for 5-HT2B and 5-HT2C receptors [for review, see Bockaert et al. (2010), Halberstadt (2015), McCorvy and Roth (2015), Maroteaux et al. (2017)]. Interesting findings are emerging regarding noncanonical signaling by 5-HT2 receptors. Specifically, the 5-HT2C receptor is able to activate ERK via a G protein–independent mechanism through recruitment of calmodulin and β-arrestin (Labasque et al., 2008). Furthermore, recent studies on noncanonical signaling by the 5-HT2B receptor found evidence of ligand-dependent signals and then used the crystal structure of the receptor to identify high-resolution, structural basis for the biased signaling (Wacker et al., 2013, 2017a,b). This evidence of ligand-dependent signaling through 5-HT2 receptors has relevance to the psychotropic effects of 5-HT2A receptor agonists, some of which are hallucinogenic but not all (Gonzalez-Maeso et al., 2007), and may also explain the long-lasting effects of LSD that are difficult to explain on the basis of pharmacokinetics alone (Wacker et al., 2017). However, the signaling pathways mediating the characteristic effects of hallucinogens have not been identified conclusively. Biased agonism at 5-HT2 receptors offers intriguing possibilities for therapeutic potential (e.g., avoidance of hallucinogenic properties), although this is yet to be explored, keeping in mind that 5-HT2A receptor agonists have profound effects on vascular and other smooth muscle in addition to their psychotropic effects. Also, findings of the existence of constitutive activity at 5-HT2A and 5-HT2C receptors, and 5-HT2 receptor ligands with inverse agonist properties, provides further avenues for 5-HT2 receptor drug development (e.g., Aloyo et al., 2009).
The remaining metabotropic 5-HT receptors to be considered, specifically 5-HT4, 5-HT6, and 5-HT7 receptors, are Gs coupled and thus activate adenylyl cyclase to increase cAMP. At the electrophysiological level, 5-HT4 receptor activation on neurons is associated with a slow membrane depolarization mediated by a reduction of a slow after-hyperpolarization potential and facilitation of L-type calcium channels through protein kinase A activation (Andrade and Chaput, 1991; Birnstiel and Beck, 1995). Similarly, the neuronal 5-HT7 receptor is also frequently associated with a direct depolarizing or excitatory effect, also likely mediated by actions on slow after-hyperpolarization and L-type calcium channels (Bacon and Beck, 2000; Andrade, 2006).
As with the other metabotropic 5-HT receptors, the 5-HT4 receptor generates a diversity of signals independent of the second messenger. In addition, both the 5-HT4 and 5-HT6 receptors provide further examples of metabotropic 5-HT receptors that are capable of noncanonical signaling. For example, 5-HT4 receptor stimulation activated ERK in cultured cells and neurons in a manner that was G protein–independent (Gq, Gi, Go) but dependent on Src tyrosine kinase (Barthet et al., 2007; Bockaert et al., 2010), and there are analogous observations for 5-HT6 receptors (Yun et al., 2007).
As a complement to the slow signaling elicited by metabotropic 5-HT receptors, the ionotropic 5-HT3 receptor mediates rapid synaptic transmission. All five human 5-HT3 receptor genes (5-HT3A-3E) are homologous with other members of the Cys-Cys loop ligand-gated channel superfamily (e.g., nicotinic, GABAA, glutamate, or glycine receptors) (Barnes et al., 2009). Although there is uncertainty regarding the composition of the native 5-HT3 receptors in the brain, pentameric coassemblies of 5-HT3A and 5-HT3B subunits is a likely common occurrence [for reviews, see Niesler (2011) and Thompson and Lummis (2013)]. Allosteric modulators of the 5-HT3 receptor are emerging to add to the possibilities for 5-HT3 receptor manipulation for therapeutic benefit (e.g., Trattnig et al., 2012 and Newman et al., 2013).
Overall, the complexity of neuronal signaling events elicited by 5-HT receptors is yet to be fully revealed. The metabotropic 5-HT receptors are clearly capable of both G protein–dependent and G protein–independent signaling, and it is also clear that the diverse signals generated by these receptors can be biased according to the agonist used. Moreover, as covered elsewhere, further complexity arises from the large number of protein partners influencing cellular localization and trafficking as well as signal tuning of 5-HT receptors, their homo- or heterodimerization with other GPCRs, and the alternative RNA splicing and editing of certain 5-HT receptors (especially 5-HT2C, 5-HT4, and 5-HT7 receptors) to generate variability in function (Hannon and Hoyer, 2008; Bockaert et al., 2010; McCorvy and Roth, 2015).
C. Expression of 5-HT Receptors in the Brain
Application of a combination of techniques such as receptor autoradiography, in situ hybridization, and immunocytochemistry has revealed maps of 5-HT receptor subtype distribution in the brain that are largely similar across a range of vertebrate species (although of note, the 5-ht1e receptor is not expressed in rodents, forebrain 5-HT3 receptors are differentially expressed across species, and the full-length 5-ht5b receptor is not expressed in man). The use of BAC transgenic mice engineered to express a fluorescent or colored reporter genes under the control of specific 5-HT receptor promoters, such as in the case of 5-HT2A receptor (Weber and Andrade, 2010), reveals further anatomic detail of 5-HT receptor distribution. Although the 5-HT receptor maps are largely based on data collected from the rodent brain, a high-resolution PET atlas of four 5-HT receptors (5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4 receptors) and the 5-HT transporter was recently described for the human brain (Beliveau et al., 2017). It is now clear from these mapping studies that each 5-HT receptor subtype has a unique distribution pattern in the brain but one that often overlaps with that of other 5-HT receptor subtypes. These differential distribution patterns suggest that different 5-HT receptor subtypes are likely associated with distinct CNS functions amenable to manipulation using 5-HT receptor subtype selective pharmacological agents.
D. Presynaptic 5-HT Receptors
Two of the 14 5-HT receptors are highly expressed presynaptically (i.e., expressed by 5-HT neurons themselves). Thus, 5-HT1A receptors are located on the soma and dendrites of 5-HT neurons, whereas 5-HT1B receptors are expressed at the soma but then trafficked down the axons to the terminals (Riad et al., 2000). It is firmly established from both in vitro and in vivo models that 5-HT1A and 5-HT1B receptors function as autoreceptors and exert direct inhibitory control over the firing of 5-HT neurons and terminal 5-HT release, respectively. Thus, studies over 40 years ago finding that 5-HT itself, or nonselective 5-HT agonists such as LSD, inhibited the efflux of preloaded radiolabeled 5-HT from rodent brain slice preparations were followed up by extensive pharmacological analysis that identified that this effect was mediated by 5-HT1B receptors located on 5-HT nerve terminals (Gothert and Weinheimer, 1979; Mounsey et al., 1982; Engel et al., 1986; Fink and Gothert, 2007). Similarly detailed pharmacological studies using electrophysiological recordings of 5-HT neurons and in vivo microdialysis measurements of 5-HT release characterized the autoreceptor function of somatodendritic 5-HT1A receptors (e.g., Vandermaelen and Aghajanian, 1983; Sharp et al., 1989; and Adell and Artigas, 1998).
There are more recent reports of the presence of other 5-HT receptor subtypes in the midbrain raphe nuclei, particularly 5-HT1D, 5-HT1F, 5-HT2B, 5-HT2C, and 5-HT5A. However, with the exception of 5-HT2C (located on raphe GABAergic interneurons and not 5-HT neurons), expression levels of these receptors in the midbrain raphe are low, and their functional significance in terms of 5-HT neuron control is not certain. Nevertheless, recent findings demonstrate that feedback control of 5-HT neurons is not likely limited to 5-HT1 autoreceptors; instead, it includes 5-HT receptors located on postsynaptic targets that have the physiologic effects of 5-HT autoreceptors but use additional 5-HT receptor subtypes and operate via neural inputs to 5-HT neurons. For example, evidence supports a role for postsynaptic 5 HT1A, 5-HT2A, and 5-HT2C receptors in the inhibitory control of 5-HT neurons, whereas 5-HT4 and 5-HT6 receptors are excitatory in this regard (Ge and Barnes, 1996; Sharp et al., 2007; Sharp, 2010; Brouard et al., 2015). Many of these feedback pathways appear to be localized on cortical pathways projecting back to the midbrain raphe nuclei (5-HT1A, 5-HT2A, 5-HT4, and 5-HT6 receptors), although contributions from non–5-HT neurons such as habenula inputs to the raphe (5-HT2C receptors) further emphasize the complexity of 5-HT neuron control.
E. Postsynaptic 5-HT Receptors
5-HT receptor mapping studies demonstrate that the majority of 5-HT receptor subtypes are located postsynaptically (i.e., expressed by non–5-HT neurons, sometimes referred to as heteroceptors, including the receptor subtypes involved in feedback control of 5-HT neurons) and that each 5-HT receptor subtype has an expression pattern that is distinct but often overlaps with that of other 5-HT receptors. Even 5-HT receptors from the same family have different CNS distributions (e.g., 5-HT2A vs. 5-HT2C and 5-HT1A vs. 5-HT1B). Some 5-HT receptors are expressed at higher levels than others; in particular, 5-HT1A and 5-HT2A receptors are among the most abundant, whereas in comparison, levels of 5-HT1D, 5-ht1E, 5-HT1F, 5-HT2B, and 5-HT5A receptors are much less abundant. Detailed maps of 5-HT receptor binding sites and protein and mRNA distribution in the brain are reported in a number of review articles (Mengod et al., 2006, 2010; Palacios, 2016).
At the macrolevel, and in keeping with the widespread 5-HT innervation of the brain, 5-HT receptors of different types are expressed in many brain regions that play key roles in numerous CNS functions. For example, regions rich in 5-HT receptors include cortical and limbic areas (5-HT1A, 5-HT2A/2C, 5-HT3, 5-HT4, and 5-HT6 receptors), the basal ganglia (5-HT1B, 5-HT4, and 5-HT6 receptors), mesolimbic pathways (5-HT1B, 5-HT2A/2C, and 5-HT4 receptors), hypothalamus (e.g., paraventricular and arcuate nuclei; 5-HT2C receptor), suprachiasmatic nucleus (5-HT7 receptor), trigeminal nucleus (5-HT1B/1D receptors), dorsal vagal complex (encompassing the area postrema and nucleus tractus solitarus; 5-HT3 receptor), and the spinal cord (dorsal root ganglia; 5-HT2B/2C and 5-HT3 receptors). This list depicting 5-HT receptor distribution is of course not exclusive and does not take into account the relative density of receptors in the different regions [e.g., in many regions, 5-HT2C receptors are present at much lower densities than 5-HT2A receptors (with the remarkable exception of the choroid plexus), and this is also the case for 5-HT1D compared with 5-HT1B receptors].
F. Cellular Localization of Central Nervous System 5-HT Receptors
The principal cellular location of CNS 5-HT receptors is neurons; indeed, evidence for the expression of 5-HT receptors by adult native nonneural cells such as glial cells is, at best, inconsistent. For example, reports of glial cell expression of 5-HT1A and 5-HT2A receptors appear to be dependent on the antibody used. However, emerging evidence suggests that activated microglia may express 5-HT2B and other 5-HT receptors (Krabbe et al., 2012; Kolodziejczak et al., 2015). Nevertheless, 5-HT receptors are undoubtedly present in cells of the cerebrovasculature, as most evident for 5-HT1B receptors (Riad et al., 1998).
On the whole, the expression of specific 5-HT receptors is not restricted to particular neuron types. For example, among the complex microcircuitry of the cerebral cortex, hippocampus, and amygdala, 5-HT1A and 5-HT2A receptors are expressed both on pyramidal neurons (glutamatergic) and certain classes of GABAergic interneurons (particularly those expressing parvalbumin). Elsewhere in the brain, 5-HT1A receptors are localized on 5-HT neurons in the raphe nuclei (see above) and cholinergic neurons in the septum, whereas 5-HT2A receptors are expressed by midbrain dopamine neurons. On the other hand, 5-HT3 receptors in rodent cortex and hippocampus mark a specific population of GABA interneurons with distinct chemical, morphologic, and anatomic properties (Lee et al., 2010), and these receptors are also present in pyramidal neurons, particularly in humans (Brady et al., 2007). In comparison, cortical 5-HT6 receptors are preferentially (although not exclusively) expressed by pyramidal cells (Helboe et al., 2015).
Not surprisingly, there are multiple interactions between 5-HT and other neurotransmitter systems, and particular attention has been paid to 5-HT interactions with the other monoamines, noradrenaline and dopamine. These interactions are highly complex, involving multiple 5-HT receptor subtypes located at pre- and postsynaptic sites within complicated, overlapping circuitry, such that prediction of the overall effect of 5-HT is often not possible. However, at the level of individual receptors, the rules can be more straightforward. For example, the distribution of 5-HT2A and 5-HT2C receptors within the mesolimbic dopamine system has allowed the general consensus that 5-HT2A receptors stimulate and 5-HT2C receptors inhibit dopamine transmission (Howell and Cunningham, 2015).
Given the differential expression patterns of the 5-HT receptors, it is highly unlikely that all 14 5-HT receptors are expressed by a single neuron. Evidence from earlier double- and triple-labeling in situ hybridization studies support the idea that a single postsynaptic synapse may express a combination of two to three 5-HT receptors (Mengod et al., 2010). Studies combining whole-cell patch clamp recordings with single-cell reverse transcriptase polymerase chain reaction (RT-PCR) to characterize 5-HT receptor expression are in keeping with this idea. Thus, analysis of the 5-HT receptor mRNA content of neurons of the bed nucleus of the stria terminalis revealed that individual neurons could be subdivided according to the prominent expression of two to three distinct 5-HT receptor transcripts in largely excitatory/inhibitory combinations (e.g., 5-HT1A/5-HT7, 5-HT3/5-HT7, 5-HT1B/5-HT4, 5-HT1A/5-HT1B/5-HT2A, and 5-HT1A/5-HT2A receptors) that matched electrophysiological observations (Hazra et al., 2012). A similar analysis of the 5-HT receptor mRNA content of single neurons of the preoptic nucleus revealed two types of neurons that predominantly expressed a combination of inhibitory (5-HT1A) and excitatory (5-HT2C, 5-HT4, 5-HT7) 5-HT receptors, whereas others expressed excitatory 5-HT receptors alone, again in keeping with electrophysiological findings (Sangare et al., 2016). This evidence of 5-HT receptor expression conferring both excitatory and inhibitory signaling effects of 5-HT at the single-cell level is born out more generally in electrophysiological experiments recording the effects of either bath application of 5-HT or electrical and optogenetic activation of 5-HT neurons (Andrade, 2006; Andrade and Beck, 2010; Sengupta et al., 2017).
Generally speaking, there is a very good match between 5-HT receptor mRNA and protein (e.g., Mengod et al., 2010), suggesting that the majority of 5-HT receptor proteins are not trafficked significantly along axons and reside largely at the levels of the neuronal soma and dendrites. Notable exceptions to this rule are 5-HT1B and 5-HT3 receptors. The former are trafficked either to nerve terminals of 5-HT neurons where they function as autoreceptors or to nerve terminals of other neurons and especially GABA neurons where they modulate GABA release. On the other hand, it is estimated that about 70%–80% of the 5-HT3 receptors are located on nerve endings, where their role is also to modulate neurotransmitter release (e.g., dopamine, cholecystokinin, glutamate, acetylcholine, and GABA) (Hannon and Hoyer, 2008; Walstab et al., 2010). A final point of note is that, unusually, 5-HT6 receptors are expressed on neuronal primary cilia (Hamon et al., 1999), the functions of which remain obscure.
At the ultrastructural level, electron microscopy studies show that all metabotropic 5-HT receptors visualized thus far are located extrasynaptically, which has reinforced the idea that 5-HT principally signals via volume transmission (Descarries et al., 2006). However, synaptic localization of 5-HT receptors cannot be excluded, as these are challenging studies that are often limited by the properties and specificity of available antibodies. Moreover, it is beyond doubt that 5-HT is present in vesicles that are synaptic as well as nonsynaptic. An additional complexity in signaling at 5-HT synapses is cotransmission, with coreleased glutamate emerging as a key player (e.g., Sengupta et al., 2017).
G. Behavioral Roles of 5-HT Receptors in the Brain
1. Introduction
Knowledge of the regional and cellular location of 5-HT receptors in the brain has been immensely helpful in understanding a vast literature on the behavioral effects of pharmacological and genetic manipulation of 5-HT receptors. Collectively, these developments are casting light on the likely behavioral functions of 5-HT receptors. So far, no behavioral response can be confidently ascribed to activation of the CNS 5-HT1D, 5-ht1e, 5-HT1F, or 5-HT5 receptors.
2. 5-HT1 Receptor Family
a. 5-HT1A receptors
The behavioral functions of 5-HT1A receptors have been the focus of much research given the widespread and high CNS expression of these receptors, combined with the availability of a large number of selective 5-HT1A receptor ligands. It has long been known that in rodents, administration of 8-OH-DPAT and other 5 HT1A receptor agonists causes a wide range of behavioral and physiologic effects, including changes in motor function (especially induction of the 5-HT behavioral syndrome), hyperphagia, hypothermia, altered sexual behavior, and changes in pain threshold. Also, there is a large literature demonstrating that 5-HT1A receptor agonists have antidepressant and anxiolytic activity and influence a range of cognitive domains relevant to symptoms of schizophrenia (for reviews, see Traber and Glaser, 1987; Handley, 1995; and Newman-Tancredi, 2010). These findings accord with preclinical and clinical evidence implicating changes in 5-HT1A receptors in the pathophysiology of a variety of psychiatric illnesses, including depression, anxiety and other stress-related disorders, and schizophrenia. Appropriate 5-HT1A receptor function also appears critical for antidepressant drug efficacy (Richardson-Jones et al., 2010; Samuels et al., 2016). The diverse behavioral effects of 5-HT1A receptor agonists are likely to involve an action at 5-HT1A receptors in multiple forebrain and midbrain sites; the contribution of presynaptic and postsynaptic 5-HT1A receptors to specific behavioral effects of 5-HT1A receptor agonists is not always certain, and indirect effects on other transmitter systems, particularly noradrenaline and dopamine, may often be involved.
Despite these complexities, a significant body of evidence links the decrease in 5-HT transmission evoked by 5-HT1A autoreceptor activation to anxiolytic effects, whereas an increase in 5-HT transmission evoked by activation of postsynaptic 5-HT1A receptors is associated with antidepressant effects [see Barnes and Sharp (1999) and II. 5-HT1A Receptor in the present review]. Much of the evidence for this has come from psychopharmacological studies in animals and humans, and it is being increasingly reinforced by studies using advanced genetic mouse models that have the power to make targeted manipulation of pre- versus postsynaptic 5-HT1A receptors. In particular, genetic mouse constructs with selective knockdown of 5-HT1A autoreceptor expression generate an increase in anxiety phenotypes (Richardson-Jones et al., 2011). In contrast, selective knockdown of postsynaptic 5-HT1A receptors is associated with depressive-like phenotype, suggesting a differential impact of pre- versus postsynaptic 5-HT1A receptors on anxiety and depression mechanisms. Paradoxically, although these phenotypes associated with genetic manipulation can be reproduced by pharmacological means, they are only observed when 5-HT1A receptor suppression is initiated in early life and not during adulthood. Thus, these data point to a developmental mechanism, that is, altered pre- and postsynaptic 5-HT1A receptor signaling during development, causing an adult behavioral phenotype through impacting the normal formation of anxiety/depression circuitry (Richardson-Jones et al., 2011; Garcia-Garcia et al., 2014, 2016). An analogous theory has been posited to explain the behavioral phenotype of 5-HT transporter mutant mice (Ansorge et al., 2004). Consideration of developmental mechanisms may therefore be appropriate when interpreting the behavioral phenotype of other mouse models with altered 5-HT receptor expression (Gingrich et al., 2003; Berger and Tecott, 2006; O’Leary and Cryan, 2010).
Aside from the interesting picture emerging through genetic models, there is increasing evidence that behavioral effects of 5-HT1A agonist administration are dependent on the agonist used. Divergent effects of certain 5-HT1A agonists have been reported across a range of models of cognition and emotional behavior as well as in relevant neurochemical and neurophysiological paradigms. These findings raise the possibility that different behaviors are being evoked by different signals because of biased agonism, and differential sensitivity of pre- and postsynaptic 5-HT1A receptors to the agonists may be a contributing factor. Thus, functionally and anatomically distinct subpopulations of 5-HT1A receptors, combined with an emerging diversity of biased 5-HT1A receptor agonists, forecasts new categories of 5-HT1A drugs for selective behavioral manipulation and thereby potential multiple therapeutic applications.
b. 5-HT1B receptors
Preclinical studies on the behavioral effects of the 5-HT1B receptor ligands have been hampered by the lack of drug tools with sufficient selectivity or brain penetration, and this continues to be somewhat problematic in the case of 5-HT1B receptor agonists. Early studies on the behavioral effects of 5-HT1B receptor agonists and antagonists in rodents have been extensively reviewed (Lucki, 1992; Middlemiss and Tricklebank, 1992; Barnes and Sharp, 1999). These earlier findings, combined with more recent preclinical and clinical studies, emphasize a role for 5-HT1B receptors in depression and anxiety behaviors on the one hand [for review, see Ruf and Bhagwagar (2009) and Fakhoury (2016)] and aggression and impulse control on the other [for review, see Nautiyal et al. (2015)]. Moreover, the role of pre- versus postsynaptic 5-HT1B receptors in these behaviors is beginning to be addressed using tissue-specific and time-dependent conditional 5-HT1B receptor mutant mice.
Evidence from earlier neuropharmacological studies suggests on the whole that 5-HT1B receptor agonists have antidepressant effects in animal models, whereas 5-HT1B receptor antagonists are anxiolytic (Fakhoury, 2016). The findings with 5-HT1B receptor antagonists are to some extent consistent with evidence that mice with a global 5-HT1B receptor knockout demonstrate anxiolytic and antidepressant-like phenotypes (Mayorga et al., 2001; Jones and Lucki, 2005; Bechtholt et al., 2008). Moreover, it is reported these phenotypic effects can be recapitulated in genetic mice with selective loss of 5-HT1B autoreceptors (Nautiyal et al., 2015). The latter group argue that relative to 5-HT1B autoreceptors, postsynaptic 5-HT1B receptors may have an opposing effect on anxiety and depressive behaviors, thereby potentially accounting for the divergent actions of 5-HT1B receptor agonists and antagonists noted above. Importantly, an anxiolytic and antidepressant-like phenotype was generated by knockout of 5-HT1B autoreceptors in adulthood and not during early postnatal life, thereby making a developmental mechanism unlikely (Nautiyal et al., 2015). Collectively, these data suggest that drugs that selectively block 5-HT1B autoreceptors may be useful for the treatment of anxiety and depression. Currently, there are no drugs with this property, although developments with biased 5-HT1A receptor agonists (see above) offer the potential for such agents in the future.
Another interesting phenotype of mice with a global 5-HT1B receptor knockout is increased aggression and impulsivity. This finding fits in with earlier evidence that certain 5-HT1B receptor agonists (“serenics”) have antiaggressive properties (Olivier et al., 1995). Moreover, these data fit with a consistent line of evidence associating reduced brain 5-HT transmission with high levels of impulsivity and aggression. The recently available conditional 5-HT1B receptor knockout mouse has allowed for further examination of the role of pre- versus postsynaptic 5-HT1B receptors in aggression and impulsivity (Nautiyal et al., 2015). It was found that heightened aggression and impulsivity was linked to loss postsynaptic 5-HT1B receptors and not 5-HT1B autoreceptors. However, although the increase in aggression involved a development mechanism (i.e., recapitulated by early life knockout), the increase in impulsivity was separable and was linked to loss of postsynaptic 5-HT1B receptors in adulthood. These data raise the possibility that pharmacologic agents targeting 5-HT1B receptors may be therapeutically effective in disorders associated with loss of impulse control. At least in the case of 5-HT1B receptor agonists, these agents have been widely used in the clinic since the first development of sumatriptan for the acute treatment of migraine (Humphrey, 2008). It may be argued at length whether the effects of triptans in migraine involve a neuronal component versus vascular/inflammatory mechanisms (Humphrey and Goadsby, 1994) and whether triptans are considered safe in terms of CNS adverse effects.
3. 5-HT2 Receptor Family
The behavioral effects of 5-HT2 receptor agonists in rodents are many, ranging from changes in both unconditioned (e.g., head-twitches, increased motor activity, hypophagia, and hyperthermia) and conditioned responses (e.g., punished responding and drug discrimination) [for review, see Koek et al. (1992), Barnes and Sharp (1999), and Halberstadt (2015)]. It has become possible to identify the role of the different 5-HT2 receptor subtypes in these behaviors with some degree of confidence through the availability of highly selective antagonists (for each of 5-HT2A, 5-HT2B, and 5-HT2C receptors) and agonists (especially 5-HT2C receptor agonists) as well as mutant mice with the different receptors selectively knocked out [for review, see Berger and Tecott (2006), O’Leary and Cryan (2010), Halberstadt (2015), and Di Giovanni and De Deurwaerdere (2016)].
a. 5-HT2A receptors
It is clear from detailed pharmacological analysis that the 5-HT2A receptor mediates the effects of serotonergic hallucinogens such as LSD and psilocybin in various behavioral models in animals, including drug discrimination, the head-twitch response, and locomotion (Halberstadt, 2015). The evidence is equally strong that the 5-HT2A receptor mediates the hallucinogenic effects of these drugs in humans. Prior to their prohibition in the late 1960s, psychedelic drugs such as LSD and psilocybin were used extensively in the treatment of major depression as well as anxiety-related disorders and addictions, and results were generally encouraging. Despite the restrictions that remain in place around these agents, there has been renewed interest in their use for experimental medicine studies and therapeutic purposes. Psilocybin in particular has been subject to investigation in a range of human psychopharmacological and brain imaging studies, which have contributed proof-of-principle and dose/safety data for clinical trials (Nutt, 2016). Moreover, recent clinical data demonstrate that acute administration of psilocybin (combined with psychologic support) causes a sustained lowering of depression and anxiety ratings in cancer patients (Griffiths et al., 2016; Ross et al., 2016) and treatment resistant-depression (Carhart-Harris et al., 2016), which is likely to encourage further studies in these and other patient groups.
The potent 5-HT2A receptor antagonist properties of many second-generation antipsychotics has been linked to the reported superior antipsychotic efficacy and reduced side-effect profile of these agents compared with early drugs (Meltzer and Massey, 2011). Although selective 5-HT2A receptor blockade is unlikely to be sufficient to generate a useful antipsychotic effect per se, evidence that the 5-HT2A receptor inverse agonist pimavanserin has antipsychotic actions in relevant animal models (Vanover et al., 2006) and in certain patient populations (Fox, 2014) suggests a novel alternative way to treat psychosis.
Recent reports that 5-HT2A receptor antagonists augment the effect of 5-HT uptake inhibitors in preclinical models (Marek et al., 2003, 2005; Boothman et al., 2006) may link to evidence that drugs with 5-HT2A receptor antagonist properties are helpful as augmenting agents in treatment-resistant depression (Marek et al., 2003). Although the mechanism behind this effect of 5-HT2A receptor blockade is not certain, it may link to evidence of an inhibitory 5-HT2A receptor–mediated feedback on 5-HT neurons (Sharp et al., 2007). 5-HT2A receptor blockade has also been associated with impulse control; thus, selective 5-HT2A receptor antagonists decrease impulsivity in animal models (Winstanley et al., 2004; Winstanley, 2011), and there is support for this action from psychopharmacological studies in humans (Rock et al., 2013). Interestingly, both lithium and the organoselenium compound, ebselen, which like lithium blocks signaling through phosphoinositide pathway, inhibit 5-HT2A receptor function, and both agents reduce impulsivity in animal models and humans (Singh et al., 2013; Masaki et al., 2016). This raises the possibility that 5-HT2A receptor antagonists/inverse agonists as well as novel lithium mimetics may have utility in the control of disorders of impulse control.
b. 5-HT2B receptors
The combination of low 5-HT2B receptor expression in the brain and the lack of selective 5-HT2B receptor ligands has hampered progress in establishing whether these receptors are able to elicit robust behavioral effects. This situation has moved forward with the development of 5-HT2B knockout mice, which have striking phenotypes across a range of modalities, including deficits in sensorimotor gating, social interaction, attention, learning, and memory as well as elevated impulsivity and altered sleep patterns (Pitychoutis et al., 2015). Furthermore, some of these effects (sleep and sensorimotor gating) can be phenocopied by administration of a selective 5-HT2B receptor antagonist. However, 5-HT2B knockout is also associated with severe cardiac abnormalities and embryonic and postnatal lethality, which might lead to neurodevelopment issues in surviving animals that could confound the interpretation of these adult CNS phenotypes (as noted above). Although studies involving conditional 5-HT2B knockout strategies and further pharmacological phenocopying are awaited, these data raise interesting possibilities regarding the functional role of CNS 5-HT2B receptors. As noted elsewhere (Hutcheson et al., 2011), 5-HT2B receptor agonism as a therapeutic approach is fraught by clinical evidence that such agents may induce valvulopathies, pulmonary hypertension that can have lethal consequences; thus, many therapeutic agents with 5-HT2B receptor agonist properties (e.g., fenfluramine, pergolide, and cabergoline) have now been withdrawn from the market (Hutcheson et al., 2011). Nevertheless, recent data showing biased agonism at the 5-HT2B receptor (Wacker et al., 2013) offers the potential for future 5-HT2B receptor agonists that may be devoid of these systemic adverse effects.
c. 5-HT2C receptors
Early studies recognized that several behavioral responses were likely associated with activation of central 5-HT2C receptors, including hypolocomotion, hypophagia, anxiety, penile erection, and hyperthermia [for review, see Barnes and Sharp (1999)]. Initially, these associations were largely based on observations using nonselective 5-HT2C receptor ligands. However, with increased availability of 5-HT2C receptor–selective compounds and the creation of 5-HT2C knockout mice, evidence for the involvement of the 5-HT2C receptor in many of these responses is now compelling [for review, see Di Giovanni and De Deurwaerdere (2016)]. Moreover, these developments expanded the CNS processes likely to be under 5-HT2C receptor control to include various behaviors and cognitions linked to compulsive drug- and food-seeking as well as the central control of energy homeostasis, oral dyskinesia, wakefulness, and even control seizure threshold.
In the last decade or so, there has been considerable research interest in the link between 5-HT2C receptors and addictive behaviors associated with food and psychostimulant drugs (Higgins and Fletcher, 2015; Howell and Cunningham, 2015). It is now clear that 5-HT2C receptor agonists reduce palatable food consumption and other effects associated with obesity (increased body mass and fat content; Heisler et al., 2006, 2007a,b; Lam et al., 2008; Higgs et al., 2011, 2016). These agents can also disrupt various steps in the sequence of events leading up to compulsive use of addictive drugs such as cocaine and nicotine (including stimulant action, positive reinforcement, behavioral sensitization, and reinstatement). The mechanism is, in part, likely to involve a 5-HT2C receptor–mediated decrease in mesolimbic dopamine function, but interactions with other transmitter systems (especially cortical glutamate) are likely to play a role. In addition, the lowering of food intake and metabolism by 5-HT2C receptor agonists is likely to involve an action on hypothalamic nuclei that promotes satiety and regulates energy balance pathways (Heisler et al., 2006, 2007; Lam et al., 2008).
Interestingly, separate studies have revealed a link between 5-HT2C receptors and impulse control; thus, 5-HT2C receptor agonists reduce premature responses, whereas 5-HT2C receptor antagonists have the opposite effect (Higgins et al., 2003; Winstanley et al., 2004; Fletcher et al., 2007). Given that impulsivity may influence many aspects of addictive behavior, 5-HT2C receptors may modulate addiction indirectly via its effects on impulsive behavior.
Many of these experiments detect opposing interactions between 5-HT2C receptors and 5-HT2A receptors (e.g., 5-HT2A receptor antagonists also lower impulsivity) that were detected in earlier neuropharmacological studies (Berendsen and Broekkamp, 1990). This opposing interaction between 5-HT2C and 5-HT2A receptors could explain the poor efficacy in drug addiction models of drugs that elevate 5-HT itself (SSRIs, fenfluramine). Consequently, it is proposed that an optimal way to control addictive behavior (and also avoid potential adverse effects associated with 5-HT2C receptor agonists that have off-target effects at 5-HT2A and 5-HT2B receptors) may be a drug that combines in the same molecule agonist activity at the 5-HT2C receptor and antagonist activity at the 5-HT2A receptor (Anastasio et al., 2015; Higgins and Fletcher, 2015). Additional approaches for future investigation could include 5-HT2C receptor agonists with biased agonist properties or positive allosteric modulators of the 5-HT2C receptor.
4. 5-HT3 Receptors
Outside of the well established role of central (as well as peripheral) 5-HT3 receptors in the control of emesis, 5-HT3 receptors have been linked to multiple behavioral effects, ranging from changes in anxiety and cognition to altered pain processing and sensitivity to addictive drugs. Most of the original evidence for this comes from reports of the behavioral effects of 5-HT3 receptor ligands (mostly antagonists) in animal models; these and more recent findings are extensively reviewed elsewhere (Costall and Naylor, 1992; Bentley and Barnes, 1995; Barnes and Sharp, 1999; Walstab et al., 2010; Gupta et al., 2016). However, many of the behavioral effects observed in preclinical investigations are not confirmed by studies of selective 5-HT3 receptor antagonists in clinical populations (Bentley and Barnes, 1995; Walstab et al., 2010). The apparent failure for the translation of these preclinical findings could, in part, be explained by suboptimal clinical dosing, as bell-shaped dose-response curves are often observed for 5-HT3 receptor antagonists, particularly in relation to CNS effects. Nevertheless, the involvement of 5-HT3 receptors in some of these behaviors receives support from more recent studies of mutant mice with altered 5-HT3A receptor expression. In particular, support for a role for 5-HT3 receptors in depression/anxiety-related behaviors, learning and memory, and pain processing comes from the phenotypic analysis of transgenic mice with 5-HT3A receptor knockout or overexpression (Harrell and Allan, 2003; Kelley et al., 2003; Berger and Tecott, 2006). However, interpretation of the phenotypes of these mice is complicated by findings that 1) they are not always phenocopied by pharmacological agents, 2) they are dependent on the mouse background strain, and 3) overexpression and deletion of the 5-HT3A receptor sometimes produced similar effects [for review, see Berger and Tecott (2006) and O’Leary and Cryan (2010)].
More recent evidence associating 5-HT3 receptors with emotional behaviors and cognition comes from findings with vortioxetine, which has a complex pharmacology leading to potent inhibition of 5-HT3 receptors, although the drug is also a 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist, and 5-HT transporter inhibitor. Vortioxetine has significant antidepressant and procognitive activity in both rodent models and clinical trials (Mørk et al., 2012; Sanchez et al., 2015) and is currently marketed as an antidepressant with cognition-enhancing properties. Despite vortioxetine’s polymodal pharmacology, inhibition of 5-HT3 receptors is thought to play a prominent role in its mechanism of action. Thus, in rodents, vortioxetine preferentially occupies 5HT3 receptors and the 5-HT transporter at low doses, and either 5-HT3 receptor blockade alone produces similar effects to vortioxetine or effects of vortioxetine can be replicated by coadministration of an SSRI and a 5-HT3 receptor antagonist (Mørk et al., 2012; Sanchez et al., 2015). One current mechanistic explanation of these findings is that blockade of 5-HT3 receptors on specific populations of GABA interneurons in the cerebral cortex contributes to vortioxetine’s action (Riga et al., 2016).
5. 5-HT4 Receptors
Early preclinical studies detected positive effects of 5-HT4 receptor agonists on cognitive performance as well as a reduction in anxiety-related behaviors [for review, see Barnes and Sharp (1999)], and these observations have proven to be consistent in later work as recently reviewed by others (Bockaert et al., 2011; Claeysen et al., 2015; Hagena and Manahan-Vaughan, 2017). Stemming in part from studies of 5-HT4 knockout mice (Compan et al., 2004), a role of 5-HT4 receptors in feeding behavior also seems clear, with 5-HT4 receptor agonists and antagonists having hypo- and hyperphagic properties, respectively (Jean et al., 2007; Bockaert et al., 2011).
Procognitive effects of 5-HT4 receptor agonists have been described across a range of species and in a variety of experimental paradigms that model different aspects of short- and long-term memory, many of them dependent on the hippocampus where 5-HT4 receptors are reasonably abundant (Hagena and Manahan-Vaughan, 2017). These agents also reverse the cognitive deficits induced by factors such as ageing, pharmacological interventions (e.g., muscarinic antagonists), and Alzheimer disease–like pathology. The mechanisms underlying the procognitive effects of 5-HT4 receptor agonists are not certain but may be mediated by one or more of increased release of acetylcholine, induction of synaptic plasticity, increased synaptic spine formation, and altered hippocampal network properties [see Boddeke and Kalkman (1990), Claeysen et al. (2015), and Hagena and Manahan-Vaughan (2017)]. Administration of 5-HT4 receptor agonists is also associated with increased amyloid precursor protein cleavage, which has led to speculation that these agents may be useful in the management of Alzheimer disease (Claeysen et al., 2015). A contribution from 5-HT4 receptors in hippocampus seems likely as noted above, but a role for other circuits such as corticostriatal connectivity, which is abundant in 5-HT4 receptors, seems likely.
Administration of 5-HT4 receptor agonists is also associated with rapid antidepressant effects in animal models (Lucas et al., 2007), and there is preclinical evidence that 5-HT4 receptor activation plays an important role in the action of SSRIs (Mendez-David et al., 2014). Thus, 5-HT4 receptor agonists induce a number of responses in common with repeated antidepressant treatment (e.g., efficacy in models of depression or increased expression of neural plasticity markers) but with a rapid onset of action (Vidal et al., 2014b; Samuels et al., 2016). Moreover, antidepressant administration causes adaptive changes in 5-HT4 receptor expression and function (Licht et al., 2010a). Part of the mechanism involved in the antidepressant effects of 5-HT4 receptor agonists may be the activation of a positive feedback control of midbrain 5-HT neurons via 5-HT4 receptor located in the frontal cortex (Lucas et al., 2005; Licht et al., 2010b).
The full interpretation of this literature on the behavioral effects of 5-HT4 receptor ligands is somewhat hampered by the current lack of reports concerning the effect of these agents on mood or cognitive performance in humans, although there is early evidence that a 5-HT4 receptor agonist improved the cognitive performance in nonhuman primates (Terry et al., 1998). Concerns about the potential for 5-HT4 receptor agonists to elicit adverse gastrointestinal and cardiac effects are likely to have held back the transition to clinical studies, but these do not appear to be effects common to all 5-HT4 receptor agonists (Claeysen et al., 2015), which is encouraging for future clinical studies.
6. 5-HT5 Receptors
To date, there are few studies on the CNS effects of 5-HT5 receptor ligands, and the main indications regarding the functions of this receptor currently come from studies on the phenotype of 5-HT5A receptor knockout mice (Grailhe et al., 1999). It is reported that these mice display increased exploratory activity in the open field and various other tests without evidence of altered anxiety levels. Without data from phenocopying experiments using 5-HT5 receptor antagonists or other controls for developmental origins of the phenotype, it remains uncertain whether these behaviors are driven by the absence of 5-HT5 receptor in the adult brain. Intriguingly, in 5-HT5A receptor knockout mice the locomotor activation induced by LSD was blunted, suggesting that 5-HT5A receptor activation might contribute to the psychotropic effects of psychedelic agents.
7. 5-HT6 Receptors
Despite the identification of the 5-HT6 receptor over 20 years ago, the functions of this receptor have been somewhat obscure until recently. Largely through studies on the effects of 5-HT6 receptor ligands (agonists and antagonists) in animal behavioral models, it has been discovered that the receptor likely plays an important role in cognition.
The evidence for procognitive effects of 5-HT6 receptor ligands is in keeping with the predominant localization of 5-HT6 receptors in corticostriatal circuitry and is reviewed elsewhere (King et al., 2008; Codony et al., 2011; Meneses, 2015; see also XIV. 5-HT6 Receptors). There are consistent findings that 5-HT6 receptor antagonists enhance learning and memory mechanisms in a variety of preclinical models ranging from novel object and social recognition to spatial memory tasks. These effects are observed in naive animals and in those with memory deficits induced by, for example, pharmacological means such as cholinergic antagonists.
The mechanism underlying this effect of 5-HT6 receptor blockade is uncertain. An interaction with the cholinergic system was proposed in early studies, and this is supported by more recent data. In particular, treatment with a 5-HT6 receptor antagonist augments the effects of an acetylcholinesterase inhibitor on cognitive measures in both animal models and patients with Alzheimer disease (Wilkinson et al., 2014; Kucinski et al., 2017; although 5-HT6 receptor antagonists have failed in larger phase III clinical trial of patients with pathologic cognitive deficits), and there is neurophysiological and neurochemical evidence that this drug combination has a synergistic effect on cholinergic function (Herrik et al., 2016). However, recent data show that 5-HT6 receptors are not expressed by cholinergic neurons (Helboe et al., 2015), which would invoke an indirect mechanism. A mechanism involving increased information processing through corticostriatal circuits was recently proposed (Kucinski et al., 2017).
A further complication in understanding the procognitive effects of 5-HT6 receptor antagonists is the paradoxical observation that 5-HT6 receptor agonists are also reported to have precognitive effects in some, albeit not all, studies (e.g., Schechter et al., 2008; Burnham et al., 2010; Kendall et al., 2011; Meneses et al., 2011). It is difficult to see how this paradox can be reconciled by inferring subtleties in subpopulations of 5-HT6 receptor at the neural circuit level. Part of the explanation could lie in the cognitive domain being measured, and to date, few studies have systematically compared 5-HT6 receptor agonists and antagonists in the same model. There also remains the intriguing possibility that the full answer lies in the pharmacology of 5-HT6 receptor ligands and that their categorization in terms of partial agonist, inverse agonist, and even biased agonist is not yet complete (e.g., Romero et al., 2006).
8. 5-HT7 Receptors
As with the 5-HT6 receptor, the functions of the 5-HT7 receptor are only now beginning to be revealed. This has largely come about through developments in 5-HT7 receptor pharmacology and genetic mouse models. Preclinical data link the 5-HT7 receptor to a variety of CNS processes, including regulation of circadian rhythms, body temperature, mood, cognitions, seizure threshold, and pain processing as well as mechanisms of addiction, and this is extensively reviewed elsewhere and will only be briefly discussed here (Hedlund, 2009; Hauser et al., 2015; see also XV. 5-HT7 Receptors).
The link between the 5-HT7 receptor and circadian rhythms was established in early studies, which recognized that the circadian phase shift of neurons of the suprachiasmatic nucleus evoked by the 5-HT1A receptor agonist 8-OH-DPAT was mediated by 5-HT7 and not 5-HT1A receptors (Lovenberg et al., 1993). Indeed, in this light, re-evaluation of the hypothermic effect of 8-OH-DPAT using 5-HT7 receptor knockout mice and selective antagonists revealed the involvement of 5-HT7 receptors, although 5-HT1A receptors appear to mediate the hypothermic effect of higher doses of the drug (Hedlund et al., 2004). This hypothermic effect of 5-HT7 receptor activation is sufficiently robust to make it a useful test of 5-HT7 receptor agonist activity in vivo (Di Pilato et al., 2014).
The discovery that 5-HT7 receptor knockout mice have an antidepressant-like phenotype contributed to evidence that 5-HT7 receptors have a role in emotional control. This feature of the mice is phenocopied by selective 5-HT7 receptor antagonists that were shown to have antidepressant effects in a range of models in rats and mice. These agents also augment the effect of SSRI and other antidepressants in behavioral and neurochemical models. These findings have translated into clinical trials of the 5-HT7 receptor antagonist JNJ-18038683 (Bonaventure et al., 2012), although a clear conclusion regarding efficacy of this agent has not yet been reached. It is interesting that in animal models, the antidepressant efficacy of amisulpiride is 5-HT7 receptor–dependent. Many other antidepressant and antipsychotic drugs (tricyclics, lurasidone, aripiprazole, etc.) in clinical use have 5-HT7 receptor antagonist properties, though the contribution of this receptor to their clinical effects is not known.
As a final point of interest, selective brain penetrant 5-HT7 receptor agonists are becoming available as pharmacological tools (Di Pilato et al., 2014) to help further define the function of the 5-HT7 receptor and open new therapeutic avenues.
XIX. 5-HT Receptors and the Cardiovascular System
A. Introduction
Cardiovascular effects of 5-HT are complex: when infused into the rat circulation, 5-HT produces a triphasic response with a short-lasting hypotensive phase, followed by a hypertensive phase and then a long-lasting hypotensive phase mediated by, respectively, 5-HT3, 5-HT2, and 5-HT1 receptors (Kalkman et al., 1984). The cardiovascular effects of 5-HT have been known since the middle ages when ergots produced devastating peripheral vasoconstriction, which resulted in gangrene and limb loss because of consumption of rye bread polluted with fungi that produced ergots. For a long time, the general view was that 5-HT2 receptors are largely responsible for vasoconstrictor effects, which would be blocked by 5-HT2 receptor antagonists, such as ketanserin. However, the situation was much more complex, and it was known that depending on vessel and species, compounds such as the ergolines (ergotamine, DHE, and metergoline) would produce very complex responses: often vasoconstriction, but not infrequently vasodilation, could be observed as well. Even before the full repertoire of 5-HT receptors were known, it was suspected that “5-HT1-like” receptors would at least mediate some of these effects. One of the most important tools for probing “5-HT1-like receptor”–mediated effects was 5-carboxamidotryptamine (5-CT), which became available in the early 1980s. 5-CT 1) potently contracted the dog saphenous vein (Feniuk et al., 1981); 2) inhibited the release of noradrenaline and 5-HT from sympathetic and central 5-HT neurons, respectively (Feniuk et al., 1981; Engel et al., 1983); and 3) displayed nanolomar affinity for the 5-HT1 receptor recognition sites (Engel et al., 1983). Furthermore, several 5-CT–induced responses (associated with “5-HT1–like” receptor recognition sites) were blocked by methiothepin and/or methysergide, but not by ketanserin, including relaxation of smooth muscle, contraction of dog saphenous vein, long-lasting hypotension in the rat, and tachycardia in the cat [see Kalkman et al. (1984), Bradley et al. (1986), Saxena and Villalón (1990)]. The migraine program led by Pat Humphrey at Glaxo used 5-CT as a template to study such effects with the idea to develop a new migraine medication that would be selective for the craniovascular bed (Humphrey et al., 1991). This research culminated with the discovery and development of sumatriptan (and other “triptans” that followed). It was then realized that sumatriptan had high affinity and potency at 5-HT1D receptor sites before the actual receptors were cloned (Peroutka et al., 1989; Schoeffter and Hoyer, 1989a,b; Hoyer et al., 1990). The high affinity of sumatriptan for 5-HT1D receptor recognition sites suggested that the sumatriptan-induced vasoconstriction was mediated by 5-HT1–like receptors resembling the 5-HT1D receptor subtype (Martin, 1994). However, the concept of 5-HT1–like receptor remained alive for some time (Hoyer et al., 1994). One of the main reasons for this uncertainty was that metergoline, a potent 5-HT1D receptor ligand, was less active as an antagonist both in vitro (e.g., canine, human, and rabbit saphenous vein and rabbit renal and cerebral arteries; Deckert et al., 1994; Hoyer et al., 1994) and in vivo (porcine and canine carotid vascular beds; Den Boer et al., 1992; Villalón et al., 1995) than methiothepin, as may have been anticipated from metergoline’s affinity at the then defined 5-HT1D receptor recognition sites. However, the experimental conditions used to detect 5-HT1D receptor binding (Waeber et al., 1988) allowed binding to, at the time unknown, 5-ht1e, 5-HT1F, and 5-HT7 receptors, which clearly complicated interpretation. The cloning of 5-HT1Dα and 5-HT1Dβ receptors combined with their relatively high affinity for sumatriptan, and the eventual distinction between 5-HT1B and 5-HT1D receptors, including the species differences, allowed some order to the complexity (Weinshank et al., 1992) along with an appreciation of a need for subtype-selective 5-HT1 receptor agonists and antagonists. Eventually it was realized that the loosely defined “5-HT1–like receptor” covered at least three structurally distinct receptors, namely, the 5-HT1B, 5-HT1D, and 5-HT7 receptors. Here, basic and more recent knowledge as to the involvement of each 5-HT receptor in the cardiovascular system in normal physiological and pathophysiological conditions will be discussed (the cardiovascular system to include the heart, blood, vasculature, kidneys, and adrenals as well as the peripheral and central nervous systems involved in cardiovascular system regulation).
B. 5-HT in Cardiovascular Tissues
The majority of 5-HT in the body is made by 1) the gastrointestinal system and 2) the neurons of the raphe complexes in the brainstem. 5-HT makes its way to all body organs by storage in blood platelets following SERT uptake and through free 5-HT being released from the enterochromaffin cells of the intestine. Free 5-HT—external to the platelet—is measurable in the plasma of all species examined (Watts et al., 2012). 5-HT has been detected in all rat tissues examined (Linder et al., 2009) and cannot be accounted for by the presence of platelets given that electron microscopic images show no platelet adhesion to the tissues used for measuring 5-HT. Moreover, 5-hydroxyindole acetic acid (5-HIAA) can be detected in these same tissues. 5-HIAA is a metabolite of 5-HT, the product of monoamine oxidase, a mitochondrial enzyme (i.e., 5-HT has to be inside the cell to be converted to 5-HIAA). Interestingly, many cardiovascular tissues express SERT, and, at least in the rat, SERT-dependent uptake of 5-HT takes place in peripheral tissues that include arteries, heart, and adrenals (Linder et al., 2009). The SERT KO rat, created by Edwin Cuppen (Homberg et al., 2007), has been particularly useful in these studies. Moreover, certain tissues of the cardiovascular system synthesize 5-HT, independent of the gastrointestinal system and brain, using tryptophan hydroxyalase (TPH), of which two forms exist, TPH1 and TPH2, the latter being neuron-specific. This includes vascular smooth muscle (Ni et al., 2004, 2008), endothelial cells (Morecroft et al., 2007), kidney (Hafdi et al., 1996; Sole et al., 1986; Stier and Itskovitz, 1985; Stier et al, 1985), cardiomyocytes (Ikeda et al., 2005; Pönicke et al., 2012) and potentially adrenal glands (Brownfield et al., 1985; Csaba and Sudar, 1978; Delarue et al., 1992; Holzwarth and Brownfield, 1985; Holzwarth et al., 1984; Pönicke et al., 2012; Verhofstad and Jonsson, 1983).
C. Cardiovascular Effects Mediated by 5-HT Receptors
1. 5-HT1A Receptors
One of the best-known peripheral actions of 5-HT1A receptor stimulation in rodents is inhibition of stress-evoked cardiovascular responses, reducing the tachycardia and renal sympathoexcitation that accompany stress (Horiuchi et al., 2005, 2011; Nalivaiko and Sgoifo, 2009). Stress models investigated include restraint stress, fear-conditioned stress, cold exposure, and elevated plus maze (anxiety-producing). Interestingly, 5-HT1A receptor agonists can be given systemically to produce their anxiolytic effects independent of sympathoinhibition (Vianna and Carrive, 2009). 5-HT1A receptors also mediate increased vagal drive (Ramage, 1990).
5-HT1A receptor agonists lower blood pressure in the normal (nonhemorrhaged, nonstressed) rat. 8-OH-DPAT given in the ventral medulla of the normal rat causes hypotension and bradycardia (Helke et al., 1993). Similarly, 8-OH-DPAT, flesinoxan, and other 5-HT1A receptor agonists reduce blood pressure in both the spontaneously hypertensive rat and Wistar Kyoto rat (see, e.g., Doods et al., 1988). The 5-HT1A receptor agonist flesinoxan has been suggested to inhibit sympathetic nerve activity, at least in part, through renal nerves and to lower blood pressure in rats (Chamienia and Johns, 1994) and in cats (Wouters et al., 1988; Ramage et al., 1992). Collectively, these studies point to the central role of 5-HT1A receptors modifying autonomic responses as one of the greatest contributions made by this receptor subtype. Interestingly, peripheral 5-HT1A receptors can also mediate inhibition of the sympathetic vasopressor outflow in pithed rats (Villalón et al., 1998; Morán et al., 1998).
In contrast to the decrease in blood pressure caused in a normal rat, 8-OH-DPAT increases whole-body venous tone to protect against hemorrhagic shock (Tiniakov and Scrogin, 2009), described as a sympathetic medicated venoconstriction. 5-HT1A receptors are not highly expressed in the vasculature, and direct effects within the vasculature are uncommon (Villalón and Centurión, 2007; Ramage and Villalón, 2008). Flesinoxan and other 5-HT1A receptor agonists have been developed as blood pressure–lowering agents, but no clinical success has been reported, and these programs were abandoned.
2. 5-HT1B Receptors
5-HT1B receptors are found on cerebral arteries and other vascular tissues mediating direct vasoconstriction [see Villalón et al. (2003) and Villalón and Centurión (2007)]. Furthermore, it seems that the receptor may be silent and may become responsive in conditions such as atherosclerosis (Geerts et al., 2000; Ishida et al., 2001). Peripheral effects in rats have been described, such as 1) inhibition of noradrenaline release from sympathetic nerves in vena cava (Göthert et al., 1986) and systemic vasculature (Villalón et al., 1998) and 2) inhibition of plasma extravasation produced by trigeminal ganglion stimulation (Buzzi and Moskowitz, 1991). 5-HT1B receptors also mediate vasoconstriction in the rat caudal arteries (Craig and Martin, 1993) and the canine external carotid circulation (De Vries et al., 1998).
5-HT1B receptors are expressed in vascular smooth muscle of several different arteries, including rat aorta (Banes and Watts, 2003), aortic vasavasorum (Cohen et al., 2002), tail artery (Craig and Martin, 1993), and middle cerebral artery (Kovács et al., 2012); human arteries and veins, including central, pulmonary, and coronary arteries (Verheggen et al., 1998, 2004; Morecroft et al., 1999; Nilsson et al., 1999a,b; van den Broek et al., 2002; Tanaka et al., 2008b) as well as coronary endothelial cells (Ishida et al., 1998); canine internal (Centurión et al., 2001) and external (Centurión et al., 2001, Valdivia et al., 2004) carotid artery beds; rabbit saphenous vein, basilar artery (Bhattacharya et al., 2004), and renal artery (Hill et al., 2000); porcine coronary artery (Schoeffter and Hoyer, 1989, 1990); bovine pulmonary artery (McKenzie et al., 2010); and guinea pig isolated iliac artery (Sahin Erdemli et al., 1991; Jahnichen et al., 2004). Next to the 5-HT2A receptor, the 5-HT1B receptor is the best studied and most frequently reported in the vasculature. Expression in cerebral arteries has been a significant focus given the potential involvement of these receptors in the pathophysiology of migraine (e.g., Villalón et al., 2003; Villalón and Centurión, 2007).
The 5-HT1B receptor mediates vasoconstriction in a variety of blood vessels, although some 5-HT1B receptors have been described as “silent,” meaning that a vessel needs to be primed with a depolarizing stimulus such as high K+ before a functional receptor is observed (“unmasking”) (Movahedi and Purdy, 1998; Froldi et al., 2008). By contrast, functional 5-HT1B receptors, coupled to nitric oxide synthase, have been reported in cultured bovine aortic endothelial cells (McDuffie et al., 1999). There is thus the potential for 5-HT1B receptors to serve opposing actions in the blood vessel: contraction through smooth muscle and relaxation through the endothelium (Schoeffter and Hoyer, 1989, 1990; Sahin Erdemli et al., 1991).
Functional 5-HT1B receptors are also found in the trigeminocervical complex of cats and dogs, where activation of the receptor inhibits 1) the “nociceptive traffic” within this complex in the former (Goadsby and Classey, 2003) and 2) capsaicin-sensitive trigeminal sensory nerves innervating the external carotid bed in the latter (Muñoz-Islas et al., 2006, 2009). 5-HT1B receptor agonists inhibit the release of sensory neuropeptides (particularly calcitonin gene–related peptide) in migraine, providing the basis for an effective treatment in addition to the craniovascular vasoconstrictor effects (Ma et al., 2001; Villalón and Olesen, 2009; Gupta and Villalón, 2010). Finally, prejunctional 5-HT1B receptors inhibit (by peripheral mechanisms) 1) norepinephrine release in blood vessels (Molderings et al., 1990, 1996; Villalón et al., 2001), 2) the vasopressor (Villalón et al., 1998) and cardioaccelerator (Sánchez-López et al., 2004) sympathetic outflow, and 3) the vasodepressor sensory CGRPergic outflow in rats (González-Hernández et al., 2011). Thus, the expression and function of 5-HT1B receptors is complex and at multiple levels, even within just the vasculature.
In the vasculature, the 5-HT1B receptor is best known for its upregulation and elevated function in pulmonary hypertension (Keegan et al., 2001). The 5-HT1B receptor contractile function is also enhanced in rabbit atherosclerotic coronary arteries (Ishida et al., 2001) and rabbit carotid arteries from animals subjected to a carotid collar (Geerts et al., 2000). Also well established is the use of 5-HT1B/1D/1F receptor agonists (the triptans) for the abortive treatment of migraine attacks (e.g., Villalón et al., 2003). Though useful in causing a cerebral vasoconstriction that is thought to be associated with improved symptoms, the triptans can also cause a coronary artery constriction that limits their use in the treatment of migraine in patients with a cardiac condition; however, long-term surveillance shows a remarkably low incidence of serious side effects (Chan et al., 2011). Exploration for antimigraine mechanisms have expanded beyond the 5-HT1B receptor (there are a multitude of triptans available) with interest on the role of the 5-HT1F receptor playing a potentially nonvascular role (Chan et al., 2011; Rizzoli, 2014) with compounds such as lasmiditan.
3. 5-HT1D Receptors
The external carotid vasoconstrictor responses to 5-HT and sumatriptan are antagonized by the selective 5-HT1B receptor antagonist SB224289 but not the selective 5-HT1D receptor antagonist BRL15572 (De Vries et al., 1998), indicating that 5-HT1B receptors mediate this vasoconstrictor response. It must be noted that sumatriptan and second-generation triptans do not distinguish between 5-HT1B and 5-HT1D receptors (Villalón et al., 2003; Villalón and Centurión, 2007).
A series of isochroman-6-carboxamide derivatives, including PNU-109291 and PNU-142633, have been described as highly selective 5-HT1D receptor agonists (Ennis et al., 1998; McCall et al., 2002). These 5-HT1D receptor agonists do not produce vasoconstriction in in vivo (Centurión et al., 2001) or in vitro preparations (cerebral arteries; Bouchelet et al., 2000).
At the peripheral level, the presence of 5-HT1D receptors seems to be rather limited to autonomic and trigeminal nerve terminals/ganglia (Jones et al., 1995; Molderings et al., 1996; Shepheard et al., 1997; Villalón et al., 1998).
There were attempts to develop 5-HT1D receptor agonists for migraine therapy [for references, see Villalón et al. (2003) and Chan et al. (2011)]; these compounds are active in the trigeminal inflammation/plasma extravasation models yet would presumably be less prone to side effects, as the cardiovascular effects are largely negligible (Villalón et al., 2003; Chan et al., 2011). However, when tested in the clinic to treat migraine, there was little success, and development has been abandoned.
4. 5-ht1e Receptors
The receptor has been immunohistochemically localized to the cerebral vasculature of humans, but mice and rats do not express a functional 5-ht1e receptor (Klein and Teitler, 2012). There is no evidence for a physiologic role for 5-ht1e receptors (Hoyer et al., 1994; Hoyer and Martin, 1997; Villalón and Centurión, 2007; Ramage and Villalón, 2008).
5. 5-HT1F Receptors
5-HT1F receptor mRNA and the corresponding protein is preferentially expressed in neuronal tissues rather than vascular smooth muscle (Ullmer et al., 1995; Bouchelet et al., 1996). Accordingly, 5-HT1F receptor agonists appear devoid of direct vasoconstrictor properties (Johnson et al., 1998; Cohen et al., 1999; Villalón et al., 1999).
Most recently, lasmiditan was developed as a 5-HT1F receptor agonist (Rizzoli, 2014); it did not contract rabbit saphenous vein, which is frequently used as a surrogate for the human coronary artery for which crossover effects of migraine drugs (triptans) can be identified (Nelson et al., 2010). This is consistent with a lack of contractile response via the 5-HT1F receptor generally in this preparation (Cohen and Schenck, 2000) as well as with human cerebral and meningeal arteries (Razzaque et al., 1999; Bouchelet et al., 2000).
6. 5-HT2A Receptors
The 5-HT2A receptor is the original “D” receptor of Gaddum and Picarreli (1957). It is expressed in vascular smooth muscle, cardiac muscle, and platelets in multiple species, including humans (Ullmer et al., 1995; Derangeon et al., 2010; Watts et al., 2012).
The 5-HT2A receptor mediates contraction in arteries and veins from most species (Villalón and Centurión, 2007), including rat (Sung et al., 2013), both directly and indirectly through regulation of sympathetic nerves (Blessing and Seaman, 2003). It promotes platelet aggregation and gap junctional coupling in heart myocytes (Derangeon et al., 2010), induces the activation of cardiac fibroblasts (Yabanoglu et al., 2009), and, within the nucleus tractus solitarius, stimulates a depressor and bradycardic response (Comet et al., 2007). Moreover, the direct component of 5-HT–induced tachycardia in reserpinized pithed rats is mediated by activation of 5-HT2A receptors (Centurión et al., 2002). The receptor mediates increases in contractility in rat cardiac atrium (Läer et al., 1998) and ventricle but the latter only in heart failure and cardiac hypertrophy (Läer et al., 1998; Qvigstad et al., 2005c; Birkeland et al., 2007b; Brattelid et al., 2007a,b).
In most vascular diseases, isolated blood vessels are hyper-responsive to 5-HT via the 5-HT2A receptor. Similar to the 5-HT1B receptor, upregulation of the 5-HT2A receptor has been implicated in elevated vascular tone in pulmonary hypertension (Delaney et al., 2013). The 5-HT2A receptor antagonist sarpogrelate blocked the development of pulmonary hypertension induced by monocrotaline and increased survival rate for this highly fatal disease (Hironaka et al., 2003).
The 5-HT2A receptor has been targeted for the treatment of hypertension; thus, ketanserin had been developed for this indication, but further research revealed that the antihypertensive effects of ketanserin were actually mediated by α1-adrenoceptor blockade. Interestingly, relatively selective 5-HT2A receptor antagonists (e.g., ritanserin) are devoid of antihypertensive potential in humans.
7. 5-HT2B Receptors
The 5-HT2B receptor, originally described in the stomach fundus, is expressed throughout the cardiovascular system, including vascular smooth muscle (many beds), endothelial cells, cardiac myocytes, fibroblast, and valves (Choi and Maroteaux, 1996; Jaffré et a., 2009).
In systemic arteries from normotensive rats, the 5-HT2B receptor is expressed but is apparently not functional (Banes and Watts, 2002, 2003). The endothelial 5-HT2B receptor mediates a nitric oxide–mediated relaxation in normal vessels (Ellis et al., 1995; Glusa and Pertz, 2000; Jahnichen et al., 2005). Strong support for the interaction of the 5-HT2B receptor with NOS was provided by Manivet et al., (2000). In endothelial cells isolated from human coronary artery, 5-HT elevates nitric oxide production through the 5-HT2B receptor (Ishida et al., 1998). The receptor also mediates calcium release in human pulmonary arterial endothelial cells (Ullmer et al., 1996). The 5-HT2B receptor would appear necessary for cardiomyocyte survival (Nebigil et al., 2003a,b); receptor ablation leads to a cardiomyopathy without hypertrophy (Nebigil et al., 2000, 2001).
The 5-HT2B receptor is critical to development of hypoxia-induced pulmonary hypertension (Launay et al., 2002; Esteve et al., 2007) and monocrotaline-induced pulmonary hypertension (Zopf et al., 2011) and becomes functional in a number of models of experimental hypertension (Watts et al., 1995, 1996; Watts and Fink, 1999; Banes and Watts, 2002, 2003; Russell et al., 2002). Inhibition of the 5-HT2B receptor appears to block valvular myofibroblast differentiation, a process involved in calcification of the aortic valves (Hutcheson et al., 2012). The question remains whether this role of the 5-HT2B receptor also applies to pulmonary hypertension in humans, in which the 5-HT1B receptor seems to have a central role (Maclean and Dempsie, 2010). However, the use of the anorectic combination fenfluramine-phentermine precipitated pulmonary hypertension and valvular heart disease, with evidence of mediation via the 5-HT2B receptor (Fitzgerald et al., 2000; Rothman et al., 2000). Valvulopathy is also observed with other 5-HT2B receptor agonists (e.g., norfenfluramine, benfluorex, pergolide, cabergoline, and ergotamine, most of which have been withdrawn from a majority of markets because of concerns around these adverse side-effects). As a consequence of these findings, preclinical safety screens for 5-HT2B receptor agonism have become mandatory in an attempt to avoid the potential risk of valvulopathy (Hutcheson et al., 2011; Reid et al., 2013). Valvulopathy induced by serotonergic compounds did not begin with fenfluramine-phentermine; it has been observed with ergots over the centuries in central and eastern Europe and even in ancient Egypt (Hauck et al., 1990; Eadie, 2003) as well as in patients with carcinoid tumors (Druce et al., 2009). Finally, 5-HT2B receptors appear essential for isoproterenol-induced cardiac hypertrophy in the mouse (Jaffre et al., 2004), and overexpression of the 5-HT2B receptor led to cardiac hypertrophy in the mouse (Nebigil et al., 2003b). It should also be kept in mind that MDMA (ecstasy) and its metabolite MDA, as well as a number of MDMA analogs, act as 5-HT2B receptor agonists and thus carry the risk of valvulopathies if consumed (Setola et al., 2003).
8. 5-HT2C Receptors
This receptor has not been localized to peripheral tissues with confidence, though a few reports suggest this may be so. The 5-HT2C receptor is, however, expressed in cardiovascular-controlling areas of the central nervous system (e.g., nucleus tractus solitarius) and may elevate blood pressure (Ferreira et al., 2005; Austgen et al., 2012).
9. 5-HT3A and 5-HT3AB Receptors
An important action of 5-HT3 receptors in the cardiovascular system is the ability to elicit the neuronally mediated transient von Bezold-Jarisch reflex [for references, see Kalkman et al. (1984), Saxena and Villalón (1990), and Villalón and Centurión (2007)].
Alternatively, activation of some peripheral 5-HT3 receptors evokes tachycardic responses that may involve 1) noradrenaline release from postganglionic cardiac sympathetic neurons (Saxena and Villalón, 1990), 2) a direct action on the cardiac pacemaker (Wilson et al., 1990), and 3) CGRP release from cardiac sensory nerves (Nishio et al., 2002).
5-HT3 receptors in sympathetic ganglia may sustain chronic stress–induced hypertension in the rat (Nalivaiko and Sgoifo, 2009).
10. 5-HT4 Receptors
Cardiac expression is well established for the 5-HT4 receptor, with earliest papers of 5-HT4–like receptors in human atria dating back to 1989 (Kaumann and Levy, 2006). The 5-HT4 receptor is functional in fetal hearts (Brattelid et al., 2012), normally expressed in human and porcine atrium and ventricle (Bach et al., 2001; Brattelid et al., 2004a,b; Weninger et al., 2012), and splice variants of the receptor exist in the human heart (Bach et al., 2001; Brattelid et al., 2004a; Krobert et al., 2005; Kaumann and Levy, 2006). Endothelial cells also express 5-HT4 receptors (Nishikawa et al., 2010; Machida et al., 2013), as does the adrenal gland (Vilaró et al., 2002).
5-HT4 receptors mediate positive inotropic, chronotropic, and lusitropic effects in human and porcine atrium (Kaumann, 1990; Kaumann et al., 1990, 1993; Villalón et al., 1990, 1991; Krobert et al., 2005; De Maeyer et al., 2006; Kaumann and Levy, 2006; Gergs et al., 2009; Chai et al., 2012; Weninger et al., 2012) and positive inotropic and lusitropic effects in human and porcine ventricular myocardium (Brattelid et al., 2004b; Afzal et al., 2008).
5-HT4 receptors also mediate arrhythmogenesis in human atria (Kaumann, 1994; Pino et al., 1998), modulation of angiogenesis in cultured human umbilical vein endothelial cells (Nishikawa et al., 2010; Profirovic et al., 2013), and aldosterone secretion from the adrenal gland (Lefebvre et al., 1998, 2000). Interestingly, a model of cardiac overexpression of the human 5-HT4 receptor in the mouse heart has been developed to test for arrhythmogenesis as a cardiac side effect (Gergs et al., 2010).
As pointed out earlier, only 5-HT2A receptors mediate 5-HT–induced cardiostimulation in healthy rats (Centurión et al., 2002), but both 5-HT4 and 5-HT2A receptors mediate this effect after development of congestive heart failure (Qvigstad et al., 2005a,c). Accordingly, 5-HT4 receptors 1) become functional in ventricles during heart failure (Brattelid et al., 2004b, 2007a, 2012; Qvigstad et al., 2005a,c; Birkeland et al., 2007b) and 2) may contribute to human atrial fibrillation (Kaumann et al., 1994; Lezoualc’h et al., 2007). Thus, 5-HT4 receptor antagonists may have potential therapeutic usefulness for improvement of cardiac function. In line with a proposed arrhythmogenic effect of stimulation of atrial 5-HT4 receptors (Kaumann, 1994), antiarrhythmic effects of a 5-HT4 antagonist were demonstrated in a porcine model of atrial fibrillation (Rahme et al., 1999). However, although atrial 5-HT4 expression levels increase in human chronic atrial fibrillation (Lezoualc’h et al., 2007), the arrhythmic potential of 5-HT4 receptors may be reduced in established human atrial fibrillation (Christ et al., 2014).
By analogy with the use of betablockers to improve prognosis in heart failure (Lohse et al., 2003), the use of 5-HT4 receptor antagonists was proposed (Qvigstad et al., 2005b; Levy et al., 2008) and delivered improvement in a rat model (Birkeland et al., 2007a). 5-HT4 receptors are functional in human failing ventricle (Brattelid et al., 2004b; Afzal et al., 2008), and apparent clinical benefits of 5-HT4 receptor antagonism have been detected (Kjekshus et al., 2009).
11. 5-HT5 Receptors
The knowledge about cardiovascular responses mediated by 5-HT5 receptors is very limited [see Ramage and Villalón (2008)]. It is suggested that putative 5-HT5 receptors can mediate 1) the 5-HT–induced cardiac sympathoinhibition (together with 5-HT1B/1D) receptors in pithed rats (Sánchez-López et al., 2003) and 2) the GR-127935–sensitive mechanism mediating hypotension in anesthetized rats (Sánchez-Maldonado et al., 2015).
12. 5-HT6 Receptors
These receptors have not been localized or found to be functionally relevant within the cardiovascular system (Villalón and Centurión, 2007; Ramage and Villalón, 2008).
13. 5-HT7 Receptors
The 5-HT7 receptor most probably is encompassed with the originally designated “5-HT1–like” receptor that mediates direct (endothelium-independent) vasorelaxation, the late vasodepressor response (i.e., the tertiary component of the triphasic response) following intravenous administration of 5-HT (Kalkman et al., 1984; Saxena and Villalón, 1990, 1991).
5-HT7 receptors are primarily located in vascular smooth muscle of most species (Ullmer et al., 1995; Villalón and Centurión, 2007; Ramage and Villalón, 2008). The 5-HT7 receptor mediates direct vasorelaxation in multiple vascular beds (e.g., Cushing et al., 1996; Terrón, 1996; Villalón et al., 1997a, 2001; Jahnichen et al., 2005; Seto et al., 2009; Watts et al., 2015).
14. Receptor-Independent Actions of 5-HT
It is recognized that 5-HT can exert a (patho)physiologic role that is independent of cell surface 5-HT receptors.
For instance, various groups have reported that animals lacking SERT, or those in which SERT was pharmacologically inhibited, are protected from pulmonary hypertension (Fanburg and Lee, 2000; Marcos et al., 2003; Guignabert et al., 2005; Elangbam et al., 2008; Wang et al., 2012). Similarly, rodents overexpressing SERT develop pulmonary hypertension (Guignabert et al., 2006). It is known that intracellular 5-HT is able to modify proteins, a phenomenon known as “serotonylation” of proteins (Lin et al., 2014). Such protein modification may mediate mitogenic and profibrotic effects of 5-HT independent of receptor activation (Guilluy et al., 2009; Liu et al., 2011; Wei et al., 2012; Penumatsa and Fanburg, 2014). This covalent modification of target proteins by 5-HT is mediated by the enzyme transglutaminase, of which the tissue transglutaminase isoform (TG II, tTG) is abundant in vascular tissue. Targets for serotonylation include Akt (Penumatsa et al., 2014); small GTPases such as Ras, Rab 4, and Rho (Ahmed et al., 2008; Mercado et al., 2011; Walther et al., 2003b, 2011; Lin et al., 2013); fibronectin (Liu et al., 2011; Hummerich et al., 2012); and smooth muscle α-actin (Watts et al., 2009).
XX. 5-HT Receptors and the Gastrointestinal Tract
A. Introduction
The GI tracts of mammals contain a huge store of 5-HT (Gershon and Tack, 2007), melatonin (a derivative of 5-HT; Stone and Darlington, 2002), and kynurenine derivatives of tryptophan, which can interact with 5-HT receptors on GI muscle (Pomfret et al., 1987). This 5-HT is released to act within the GI tract (all known 5-HT receptors are expressed within the GI tract; see below) and externally into the blood, playing various roles in metabolism, osteogenesis, immunity, neurogenesis, and neuroprotection (Gershon, 2012, 2013). Indeed, about 95% of the 5-HT in the human body the GI tract, with 90% being in enterochromaffin (EC) cells in the mucosal epithelium and 5% in the neural structures intrinsic to the bowel wall; in common with the rest of the epithelium, EC cells are continually shed and replaced. Large amounts of 5-HT are also present in mast cells of rats and mice, but human intestinal mast cells usually contain no 5-HT (Buhner and Schemann, 2012); a subset of mast cells in patients with colon carcinoma and ulcerative colitis has been reported to contain 5-HT (Stoyanova and Gulubova, 2002). In the human GI tract, the highest amounts of 5-HT are present in the duodenum and rectum, whereas the lowest is found in the esophagus and ileum (Spiller, 2008a). In rats, the greatest amount of 5-HT is in the cecum (Hansen and Witte, 2008). 5-HT is also present in a limited (2% to 3%) number of myenteric descending interneurons (with variation among species; Gershon, 2003; Gershon and Tack, 2007), potentially regulating secretion and circular muscle movements in guinea pig jejunum (Neal and Bornstein, 2007), and in enteric sensory and motor neurons of mouse colon (Okamoto et al., 2014).
There are two GI 5-HT pools, each dependent on a different isoform of TPH (Li et al., 2011b). The larger pool is present in EC cells and is TPH1-dependent. The smaller pool, present in neurons, is TPH2-dependent, as is CNS 5-HT. These two pools can thus be selectively depleted. Thus, TPH1 KO depletes EC cells, whereas TPH2 KO depletes neuronal 5-HT.
5-HT is released from EC cells by mechanical and chemical stimuli (e.g., pH, bile acids, and nutrients such as glucose and short-chain fatty acids) applied to the luminal surface of the bowel. 5-HT release is modulated by neuronal and hormonal inputs acting at numerous receptors (including 5-HT receptors; see below) expressed by EC cells (Gershon, 2003; Hansen and Witte, 2008). The junctions between EC cells and intrinsic and extrinsic sensory nerve terminals in the mucosa are not morphologically like traditional synapses (Wade and Westfall, 1985). Indeed, 5-HT secreted by EC cells acts locally in a paracrine fashion, although the nerves it activates may be situated far from the EC cells (Wade and Westfall, 1985). In addition, EC cells are in constant motion from the crypts to the surface; they turn over and are replaced by stem cells both in the small and large intestines. As a result of the movement and transient nature of EC cells, traditional synapses are not found (nerves are not good at innervating moving targets) and EC cells do not focus the 5-HT released as precisely as do neurons [see Gershon and Tack (2007)]. Similarly, EC cells are located in relatively close proximity to mucosal lymphocytes (Yang and Lackner, 2004).
The 5-HT released into the mucosa and/or lumen does not normally penetrate to the muscle of the GI tract because of two reasons: first, 5-HT has poor ability to cross lipid layers of cell membranes, and second, 5-HT is readily taken up by neurons and enterocytes via either SERT or organic cation transporters (Wade et al., 1996; Chen et al., 1998). 5-HT is also removed by the vasculature where it is taken up into blood platelets, which lack TPH and thus cannot synthesize 5-HT. Mucosal 5-HT thus does not gain access to the muscle layers of the bowel, except in pathologic conditions in which high plasma concentrations are reached (Sanger, 2008).
The released 5-HT can potentially act at multiple 5-HT receptors [see Costa et al. (2003), Beattie and Smith (2008), Hansen and Witte (2008), Sanger (2008), Chetty et al. (2009), Li et al. (2011b), Hoffman et al. (2012), and Alexander et al. (2015a,c)], which are expressed by several elements of the GI tract:
Isolated enteric crest-derived cells (mRNA for all known 5-HT receptors)
EC cells (5-HT2C, 5-HT3, 5-HT4)
Goblet cells (5-HT4)
Enterocytes (5-HT1A, 5-HT2A, 5-HT3, 5-HT4)
Muscle (5-HT1B/1D, 5-HT2A, 5-HT2B, 5-HT4, 5-HT7)
Interstitial cells of Cajal (5-HT3, 5-HT4)
Motor (5-HT1A, 5-HT1B/1D, 5-HT2A, 5-HT2B, 5-HT3, 5-HT4) and sensory (5-HT3, 5-HT4, 5-HT7) neurons of the myenteric and submucosal plexus
Terminals of GI extrinsic sympathetic and/or parasympathetic nerves (5-HT1A, 5-HT3, 5-HT4 in rodents)
The genes encoding the 5-HT3A and 5-HT3B subunits of the 5-HT3 receptor are widely expressed by different mammalian tissues. In humans, expression of 5-HT3C and 5-HT3E mRNA is greatest within the GI tract (5-HT3C is also present elsewhere), whereas 5-HT3D mRNA is largely restricted to the kidney, colon, and liver (Niesler et al., 2003; Holbrook et al., 2009; Yaakob et al., 2015). Notably, genes encoding 5-HT3C, 5-HT3D, and 5-HT3E are found in humans and other mammals but not in rodents (Holbrook et al., 2009).
5-HT3A mRNA has been found in the mucosa and circular and longitudinal (taenia) muscle of human colon, whereas 5-HT3B mRNA may be more common in muscle and 5-HT3E mRNA in the mucosa (Chetty et al., 2009; Yaakob et al., 2015). Importantly, 5-HT3A and 5-HT3B mRNA and immunoreactivity are coexpressed with neuronal cell markers in the submucosal plexus of human intestine and therefore potentially form heteromeric 5-HT3AB receptors in these nerves (Michel et al., 2005). Others report that in cell bodies of human colon myenteric neurons, 5-HT3C, 5-HT3D, and 5-HT3E mRNA coexpress with 5-HT3A, whereas mRNA for 5-HT3A and 5-HT3D coexpress in the submucosal plexus (Kapeller et al., 2011). The physiologic significance of these different expression patterns is not clear. However, the absence of genes encoding 5-HT3C, 5-HT3D, and 5-HT3E in rodents has been linked to the specific divergence of rodents away from the primate evolutionary line and the peculiar lack of an emetic reflex in rodents (a function strongly linked in humans with 5-HT3 receptors; see below and Holbrook et al., 2009). Here it may be of interest to note that selective 5-HT3 receptor antagonists do not necessarily affect GI functions in the same way (e.g., Banner and Sanger, 1995), but whether such anomalies are explained by an ability to distinguish between different 5-HT3 receptor subunits [see Thompson and Lummis (2013) for examples] is still unknown.
Among the 5-HT GPCRs, splice variants exist in human colon for 5-HT2B, 5-HT7, and 5-HT4 receptors (Coupar et al., 2007; Chetty et al., 2009; Yaakob et al., 2015), although their roles are poorly understood. It has been speculated that the efficiency of 5-HT4 receptor intracellular coupling and/or the desensitization liability and affinity of this receptor for particular ligands depends on which COOH-terminal splice variant is expressed by a particular cell (Coupar et al., 2007; Beattie and Smith, 2008; Sanger, 2009). This would explain why 5-HT4 receptor agonists facilitate GI cholinergic functions with high intrinsic activity (leading to increased GI motility), whereas in cardiac muscle and other tissues, the intrinsic activity of the same agonist is low (De Maeyer et al., 2006). Notably, the 5-HT4(d) isoform is specifically expressed within human intestine (in addition to 5-HT4(b) and 5-HT4(a), which are widely expressed), and the GI prokinetic renzapride (a nonselective 5-HT4 receptor agonist; Sanger, 1987a) acts as a full agonist at this isoform but only as a partial agonist at the 5-HT4(g) isoform (Mialet et al., 2000).
Certain 5-HT GPCRs may modulate ion channel functions within the GI tract. Sugiuar et al. (2004) showed that in mouse colon, the functions of transient receptor potential cation channel subfamily V member 1 channels were facilitated by both 5-HT2A and 5-HT4 receptor activation. In rat colon, a synergistic link between 5-HT4 and 5-HT3 receptors has been suggested (Smith et al., 1999). A proposed intracellular “cross talk” between 5-HT3 and NK1 receptors may also provide a pathway via which certain selective 5-HT3 receptor antagonists can influence the receptor in an allosteric manner (e.g., palonsetron) to inhibit substance P-mediated responses and thereby exert greater antiemetic activity (Rojas et al., 2014; see later).
Finally, it should be noted that 5-hydroxyindalpine, an agonist mimicking certain atypical 5-HT–mediated responses [said to be mediated by a putative 5-HT1P receptor, as yet undefined as a molecular entity despite considerable research; see Galligan (2007)], facilitated peristalsis in mouse colon but had no meaningful affinity for human 5-HT1A, 1B, 1D, 2A, 2B, 2C, 3, 4, 6, and 7 receptors or for other monoamine (adrenoceptor, dopamine, and histamine) receptors (Mitchell et al., 2009). It has been speculated that at least some “atypical 5-HT responses” may be the result of ligands acting at allosteric binding sites and/or at GPCR heterodimers, such as a 5-HT1B/1D and dopamine D2 receptor heterodimer (Galligan, 2007).
B. Functions of 5-HT
Of the many proposed roles for endogenous 5-HT in GI physiology, the most widely studied relate to its involvement in GI movements, secretion, reflex functions, and sensations. Several of these functions are mediated via 5-HT3 and 5-HT4 receptors; other receptors may also have important roles, although the available data are often limited. It is important to appreciate that GI functions can differ markedly between species, especially between rodents and humans (Sanger et al., 2011b, 2013b), and this can greatly change the functions of 5-HT and thereby complicate the translational value of certain animal models.
Additional developmental roles for endogenous 5-HT have also been reported, acting as an enteric neurotrophic or neuroprotective agent via 5-HT2B and 5-HT4 receptors or when released from enteric neurons, to promote epithelial growth via 5-HT2A receptors on submucosal cholinergic neurons (Fiorica-Howells et al., 2000; Liu et al., 2009; Li et al., 2011b; Gross et al., 2012; Gershon, 2013; Mawe and Hoffman, 2013; Takaki et al., 2014). The absence of neuronal 5-HT during development in mice lacking TPH2, for example, is associated with a profound ENS hypoplasia and slow GI transit (Li et al., 2011b). Neurons that are born (become postmitotic) after enteric serotonergic neurons, in the sequence of ENS neurogenesis, are 5-HT–dependent and are particularly deficient in these animals. Thus, 5-HT is a growth factor that is required for enteric neuronal development. The mucosa was also defective in TPH2KO mice, suggesting that neuronal 5-HT promotes division of transit-amplifying cells in intestinal crypts (Gross et al., 2012). Furthermore, the normal postnatal accretion of enteric neurons and growth of mice during the first 4 months of life also does not occur in animals in which 5-HT4 receptors have been deleted (Liu et al., 2009).
One possibility is that neuroprotective functions of neuronal 5-HT might be integrated with a proinflammatory role of mucosal 5-HT. For example, mucosal 5-HT enhances and triggers inflammation (Bischoff et al., 2009; Ghia et al., 2009; Haub et al., 2010; Li et al., 2011a; Margolis et al., 2014), probably by stimulating dendritic cells (Li et al., 2011a). Inflammation is potentially toxic to enteric neurons (Gulbransen et al., 2012), but it is also important in protecting the bowel from microbial invasion. Mucosal 5-HT may therefore enhance the strength of the innate immune response to danger, while at the same time, neuronal 5-HT may protect enteric neurons from being damaged by the response; 5-HT can thus serve both as a sword and a shield of the gut (Gershon, 2012).
1. Movements of the Hungry Stomach
During hunger, the release of 5-HT from the upper GI tract, together with the hormone motilin, has a potential role in initiating a repeating pattern of upper GI movements known as the migrating motor complex, also associated with changes in blood flow, gall-bladder emptying, and gastric and pancreatic secretions.
The migrating motor complex, which in humans repeats every 80–120 minutes, is characterized by a relatively long period of quiescence (phase I), irregular nonpropulsive movements (phase II), and then a short burst (5–8 minutes) of high-amplitude propulsive contractions (phase III) initiated by the vagus nerve in stomach, duodenum, and jejunum but rapidly recovering (phase IV) while migrating to the terminal ileum, where the movement is terminated. Phase III removes undigested material and prevents bacterial overgrowth; it can be disrupted by disease and may help develop feelings of hunger (Sanger and Lee, 2008; Sanger et al., 2011a; Deloose et al., 2012; Tack et al., 2014). In dogs (Nakajima et al., 2010), a gradual release of 5-HT from duodenal enterochromaffin cells during phase I eventually activates 5-HT4 receptors within the myenteric plexus to increase GI motility (phase II), releasing more 5-HT from enterochromaffin cells to initiate phase III via 5-HT3 receptors in the stomach (also Morita et al., 2013) and in humans (Wilmer et al., 1993; Luiking et al., 2002) and 5-HT4 receptors in the intestine (dogs: Davidson et al., 1990; Nakajima et al., 2010). The vagus also increases motilin release from human mucosal enteroendocrine cells (Wilmer et al., 1993) to re-enforce gastric phase III activity by greatly facilitating cholinergic motor nerve activity in a short-lasting manner (Broad et al., 2012).
2. Movements and Sensations of the Stomach and Duodenum after Meals
In healthy volunteers, little or no influence of endogenous 5-HT has been detected on gastric accommodation, compliance, sensation and motility, or on the rate of gastric emptying after ingestion of a meal, at least by acting at 5-HT3 (e.g., Gore et al., 1990; Kuo et al., 2002; Netzer et al., 2002; Janssen et al., 2011b; Kusakabe et al., 2014) or 5-HT4 receptors (e.g., Bharucha et al., 2000). In contrast, 5-HT3 receptor antagonism can increase gastric emptying in rodents (e.g., Costall et al., 1987; Miyata et al., 1995), although an ability of ondansetron, a racemate, to facilitate cholinergic activity in guinea pig ileum via an unknown, non–5-HT3–mediated mechanism should also be noted (Miyata et al., 1995; González and Puig, 1997). However, in the mouse, elimination of neuronal 5-HT as a result of the knockout of TPH2 accelerates gastric emptying (Li et al., 2011b), and 5-HT deletion or antagonism impairs vagal relaxation of the guinea pig stomach (Bülbring and Gershon, 1967).
If a meal is rich in glucose, amino acids, and/or lipids, high concentrations of these nutrients and/or gastric acid within the lumen of the duodenum can release 5-HT from enterochromaffin cells (and other mediators such as cholecystokinin, potentially acting synergistically together; Hayes and Covasa, 2005) to activate 5-HT3 receptors upon abdominal vagal nerve terminals to induce satiety (Feinle and Read, 1996; Savastano and Covasa, 2007) and, if necessary, nausea (see below). In rodents, a clear reduction in gastric emptying has also been observed, but in humans, intraduodenal infusion of glucose or a high-fat meal caused only a small 5-HT3 receptor–mediated reduction in gastric antrum movements and gastric emptying (Stacher et al., 1990; Raybould et al., 2003; Savoye et al., 2007). In rats, 5-HT4 receptors on intrinsic cholinergic neurons may be activated to increase duodenal bicarbonate secretion for a neutralizing action against gastric acid (Akiba et al., 2015).
3. Movements of the Small Intestine
It has long been known that 5-HT is released into the intestinal lumen by mechanical stimuli to the mucosa, leading to facilitation of the peristaltic reflex (e.g., Bülbring and Lin, 1958; Foxx-Orenstein et al., 1996; (Bertrand et al., 2000, 2008; Pan and Gershon, 2000; Patel et al., 2007). However, it seems unlikely that this mechanism has a major physiologic influence on human small-intestinal movements, at least via 5-HT3 (Gore et al., 1990; Houghton et al., 2000) and 5-HT4 receptors (Bharucha et al., 2000). Nevertheless, this does not rule out the possibility that under certain nonphysiologic conditions (e.g., prevention of 5-HT reuptake by use of an SSRI), small-intestinal movements can be stimulated by the released 5-HT (Grover and Camilleri, 2013; Bundeff and Woodis, 2014; see XX. C. 5-HT in GI Pathology for discussion). Furthermore, it should be noted that 5-HT is probably released from EC cells into the lamina propria underlying these cells. Nerve fibers from intrinsic primary afferent neurons are found in this location, as are sensory nerves derived from the vagus and dorsal root ganglia. Because the entire enteroendocrine system, including EC cells, secretes basolaterally, not apically, the luminal appearance of 5-HT is likely to result from spillover (5-HT is a relatively small molecule so it can diffuse into the lumen after its release into the lamina propria). It might thus be expected that endogenous 5-HT release in the small intestine would not affect motility.
4. Movements of the Colon
Endogenous 5-HT acting at 5-HT3 receptors plays a physiologic role in controlling normal movements of the colon via extrinsic and/or intrinsic nerve pathways, depending on the species of mammal. Additionally, and in certain species, 5-HT2B and 5-HT7 receptors may play similar roles (see below). However, the exact role of 5-HT on movements of the colon remain uncertain due, in part, to the high complexity of both the 5-HT system and the movements of colon in different species. This uncertainty has been discussed in a series of “cross talk” articles (Heredia et al., 2013; Smith and Gershon, 2015a,b; Spencer et al., 2015a,b).
a. 5-HT2A receptors
5-HT2A receptor knockout mice, from which 5-HT can no longer cause contraction of the isolated colon, display apparently normal GI transit and colorectal motility patterns (Fiorica-Howells et al., 2002).
b. 5-HT2B receptors
Propulsive movements of the colon and fecal output in vivo are reduced by 5-HT2B receptor antagonism in rodents (Bassil et al., 2009) but not in dogs (Morita et al., 2013). The receptor has been implicated in enteric nerve and in ICC development (Fiorica-Howells et al., 2000; Tharayil et al., 2010). Although exogenously applied 5-HT can cause contraction of human colon via 5-HT2B receptor activation (Borman et al., 2002), a role for endogenous 5-HT acting at the 5-HT2B receptor to affect human colonic functions has yet to be established.
c. 5-HT3 receptors
5-HT3 receptor antagonists slow colonic motility and induce mild constipation in several species, but the mechanisms of action may vary. In humans, the ability to slow colonic motility (e.g., Gore et al., 1990; Talley et al., 1990; Houghton et al., 2000) is at least partly due to inhibition of the gastrocolic reflex, thought to be a vagus nerve–mediated colonic contractile response associated with eating but specifically evoked by distension of the gastric antrum or inclusion of lipids within the lumen of the duodenum (Prior and Read, 1993; von der Ohe et al., 1994; Björnsson et al., 1998, 2002). In these studies, the ascending and descending components of the peristaltic reflex were unaffected by 5-HT3 receptor antagonism. The latter is consistent with an inability to detect 5-HT–mediated fast synaptic potentials in the myenteric plexus of human colon (Brookes et al., 1987) but seems at variance with the ability of local application of 5-HT to excite submucosal neurons (but not chloride ion secretion) in human small and large intestine via 5-HT3 receptors (where 5-HT3A and 5-HT3B receptor subunits are expressed; Michel et al., 2005).
In rodents, 5-HT3 receptors appear to influence colonic movements via more “local” mechanisms. Thus, the ability of 5-HT3 receptor antagonists (granisetron and tropisetron but not ondansetron) to dose-dependently inhibit fecal pellet excretion by guinea pigs could be at least partly mimicked in guinea pig mid-to-distal isolated colon, in which granisetron and tropisetron prevented movement and expulsion of endogenous fecal pellets (recovered by application of naloxone; Sanger and Wardle, 1994). Similar data were reported by Jin et al. (1999) using artificial fecal pellets and approximately the same region of colon after application of different 5-HT3 and 5-HT4 receptor antagonists. However, in guinea pig distal colon, movements of artificially inserted fecal pellets were unaffected or only transiently reduced when 5-HT4 or 5-HT3 receptor antagonists (including granisetron and ondansetron) were applied individually but were inhibited, albeit for only a short period of time, when the antagonists were given together (Kadowaki et al., 1996). In rats, proximal colon transit (measured via an indwelling cannula) and 5-HT–induced diarrhea in mice were unaffected by 5-HT4 or 5-HT3 receptor antagonists (ondansetron) applied separately but were inhibited when applied together (Nagakura et al., 1997). In contrast, Yu et al. (2015) demonstrated an ability of 5-HT3 receptor antagonism to abolish propulsive movements of rat isolated colon, again supporting a local role for 5-HT in the control of rodent colonic movements.
More recent studies confirmed a local release of 5-HT from mouse colon in response to a maintained presence of a fecal pellet, an ability of 5-HT3 receptor activation to promote pacemaker activity generated in mouse ileum by the interstitial cells of Cajal (Liu et al., 2011), and an ability of 5-HT3 receptor antagonism to prevent colonic migrating motor contractions (e.g., Bush et al., 2001; Heredia et al., 2009, 2013; Dickson et al., 2010). The question of whether the 5-HT comes from interneurons or from enterochromaffin cells to evoke these movements—or even if endogenous 5-HT is required at all—is the subject of debate, raising interesting questions about methods, the role of mucosal versus stretch-induced reflexes, putative constitutive expression of receptors (Smith et al., 2010, 2014; Heredia et al., 2013; Sia et al., 2013), and mouse strain differences (Neal et al., 2009). Despite the controversy surrounding the function of colonic 5-HT, myenteric 5-HT neurons project so extensively in the colon that these cells have been called “the central processing unit in the colon” (Okamoto et al., 2014).
d. 5-HT4 receptors
5-HT4 receptor antagonists have little (Morita et al., 2013) or no ability to inhibit colon movements in dogs (Nagakura et al., 1996) or rodents (Kadowaki et al., 1996; Nagakura et al., 1997; Sanger et al., 1998, 2000), although reduced GI activity was observed in 5-HT4 receptor knockout mice, arguably related to loss of 5-HT4 receptor–mediated promotion of survival of enteric neurons (Gershon and Liu, 2007). In human volunteers, there were no changes in colonic transit (a trend toward delayed transit was not statistically significant), fasting or postprandial motor activity, compliance, or sensations evoked by transverse and sigmoid colon distension after 10–12 days administration with pharmacologically effective doses of a 5-HT4 receptor antagonist (Bharucha et al., 2000).
e. 5-HT7 receptors
Activation of 5-HT7 receptors expressed by human intestinal muscle causes muscle relaxation (Prins et al., 1999; Coupar et al., 2007; Irving et al., 2007). In guinea pig ileum, 5-HT7 receptors are localized both to muscle and to myenteric and submucosal neurons (Tonini et al., 2005). In this tissue, the release of 5-HT from enterochromaffin cells is thought to activate 5-HT7 receptors on intrinsic sensory neurons (defined as Dogiel type II neurons) to evoke slow depolarization (Monro et al., 2005) and perhaps also to facilitate a descending inhibitory motor pathway to relax the muscle, increasing its ability to accommodate and thereby reducing the likelihood of inducing peristalsis by intraluminal distension (Tuladhar et al., 2003; Tonini et al., 2005). By a similar process, endogenous 5-HT may activate 5-HT7 receptors in mouse colon to promote descending inhibitory interneurons and contribute to the generation of spontaneous colonic migrating motor complex (Dickson et al., 2010).
C. 5-HT in Gastrointestinal Pathology
The large amount of 5-HT in the GI tract and the expression of all 5-HT receptors on several, functionally different types of GI cells (see above) creates the interesting situation of being able to use selective 5-HT receptor antagonists to treat disease caused by release of endogenous 5-HT and also use 5-HT receptor agonists (“exogenous 5-HT”) to treat other diseases. This section discusses the involvement of endogenous 5-HT in the mechanisms of disease.
Increased release or availability of 5-HT from EC cells is associated with various GI disorders, including diarrhea (e.g., induced by cholera and other bacterial toxins and also by carcinoid tumors), inflammatory bowel disease, and functional disorders such as IBS [Gershon and Tack, 2007; Spiller, 2008b, 2011; Bertrand and Bertrand, 2010; for discussion on expression of 5-HT receptors by human dendritic and immune cells, see Idzko et al. (2004) and Shajib and Khan (2015)]. More recently, the release of 5-HT from enterochromaffin cells of the rat duodenum has been shown to increase following exposure to short-chain fatty acids (Akiba et al., 2015), potentially caused by increased TPH1 transcription (Reigstad et al., 2015). 5-HT release is also increased by cytotoxic anticancer treatments following generation of free radicals in enterochromaffin cells (Minami et al., 2003) and acute stress via agents such as corticotropin-releasing factor (Sanger et al., 2000; Von Mentzer et al., 2007). Increases in 5-HT availability in 5-HT transporter knockout mice can change the level of expression and sensitivity of enteric 5-HT3 receptors (Gershon, 2003).
In GI disease, an increased availability of 5-HT has marked effects on certain autonomic functions (e.g., emesis), GI movements (e.g., diarrhea), and, perhaps, conscious perceptions of discomfort and/or pain. Several studies have investigated the role of 5-HT release into the circulation, 5-HT in tissue (typically by immunohistochemistry), and mRNA expression of 5-HTTLPR gene (which determines SERT levels) in rectal mucosa and platelets and DNA polymorphisms in 5-HTTLPR gene. In summary, the most consistent findings are elevated postprandial plasma 5-HT in IBS-D and reduced levels in IBS with constipation (IBS-C) (Spiller, 2008a,b; El-Salhy et al., 2012; Mawe and Hoffman, 2013; Camilleri, 2014; Zhang et al., 2014).
1. Emesis
There are no “universal” antiemetic drugs, and multiple stimuli evoke emesis via different pathways, not all of which involve the release of 5-HT. The stimuli that involve the release of 5-HT and that have been studied most often are discussed below. In this discussion, the term “emesis” is taken to represent the combined act of vomiting (and dry retching) as well as nausea. It is, however, increasingly appreciated that the sensation of nausea is not fully explained by the pathways that induce vomiting. For example, 5-HT3 and NK1 receptor antagonists are both more effective against vomiting than they are against nausea, implying that different mechanisms are involved (Andrews and Sanger, 2014) and, hence, different approaches to treatment are required (Sanger et al., 2013).
Since the pioneering studies with animals (Costall et al., 1986; Miner and Sanger, 1986; Miner et al., 1987), the use of selective 5-HT3 receptor antagonists as antiemetic drugs has revolutionized treatment of cancer by making chemo- and radiotherapeutic treatments more tolerable (emesis is now viewed as something that can be treated rather than needs to be tolerated, and anticancer drugs can now be given in family-orientated outpatient clinics) by enabling the delivery of more aggressive treatments and by actually reducing health care costs (Currow et al., 1997; Warr and DeAngelis, 2009). Following identification of this role for the 5-HT3 receptor, it rapidly became standard practice to coadminister a 5-HT3 receptor antagonist with the corticosteroid dexamethasone to achieve even better relief from emesis evoked by moderate to severe emetogenic treatments. Later, the “triple-therapy” of 5-HT3 receptor antagonism, dexamethasone, and NK1 receptor antagonism achieved a further benefit in patients receiving treatments classified as “highly emetogenic,” not only controlling the appearance of “acute” emesis (during the first 24 hours after initiation of treatment) but also, even more importantly, now controlling the “delayed emesis,” which in these patients can occur 24–48 hours after the start of treatment (Warr, 2012).
5-HT3 receptor antagonists prevent cytotoxic-associated vomiting by blocking the ability of released 5-HT (Barnes et al., 1990; Cubeddu et al., 1990), likely from EC cells, to activate 5-HT3 receptors on abdominal vagal nerve terminals and thereby effectively “desensitize” the vagus to the proemetic stimulatory actions of other substances (e.g., prostanoids) released during the cytotoxic treatment (see Sanger and Andrews, 2006). Most recently, evidence is emerging that a long-lasting 5-HT3 receptor antagonist (palonosetron) may provide further improvements in emesis control by a mechanism not yet clearly understood but argued to involve inhibition of substance P–mediated responses via a unique interaction with the 5-HT3 receptor (Rojas et al., 2014).
Depending partly on the population studied, a minority of patients treated with moderate to highly emetogenic chemotherapy do not respond well to treatment with a 5-HT3 receptor antagonist. This may be due to mutations in a P-glycoprotein efflux transporter in intestinal epithelia and capillaries of the blood-brain barrier, affecting the availability and target engagement of these and other drugs (Perwitasari et al., 2011; Tsuji et al., 2013; He et al., 2014; Zoto et al., 2015), and/or differences in rates of metabolism of ondansetron and tropisetron associated with polymorphisms of the gene encoding CYP2D6 (Kaiser et al., 2002). Genetic variants of the 5-HT3B, but not the 5-HT3A subunit, are also reported to alter antiemetic efficacy in a small number of patients (Tremblay et al., 2003; Kaiser et al., 2004; de Wit et al., 2005).
In patients at the end of life, perhaps with far-advanced cancer for which chemo- or radiotherapy is no longer provided, emesis can remain a severe problem for reasons associated with the use of drugs; cranial, electrolytic, or metabolic causes; and bowel obstruction, uremia, and/or sepsis. In these patients, 5-HT3 receptor antagonists have often provided effective control of emesis (e.g., Currow et al., 1997; Mystakidou et al., 1998; Buchanan and Muirhead, 2007). Exactly where the 5-HT comes from is not always clear, and 5-HT3 receptors expressed both peripherally (vagus nerve terminals) and centrally within the brainstem are likely to be involved (Sanger and Andrews, 2006).
SSRIs can induce nausea and vomiting that is reduced by 5-HT3 receptor antagonism and associated with polymorphisms of the HTR3B gene but not with genes encoding the 5-HT transporter, the 5-HT2A receptor, or the 5-HT3A receptor subunit (Sugai et al., 2006; Tanaka et al., 2008). Such drugs also stimulate small but not large bowel motility and have been evaluated as potential treatments of IBS, improving general well-being (Grover and Camilleri, 2013; Bundeff and Woodis, 2014).
Notably, there are many drugs and experimental tools other than the SSRIs that increase (e.g., 5-hydroxytryptophan, nonselective SSRIs, and monoamine oxidase inhibitors) or decrease 5-HT availability (e.g., fenfluramine; depletion following the initial increase), sometimes with additional abilities to antagonize certain 5-HT receptors, and these can induce or reduce emesis. The receptors include 5-HT1A, 5-HT2A, and 5-HT3, but which receptors are involved is not always clear, and studies with more selective ligands are required to understand mechanisms of action (Johnston et al., 2014).
Postoperative vomiting (POV) is caused by multiple stimuli but can be reduced by 5-HT3 receptor antagonism (e.g., Chun et al., 2014). The exact mechanism is not clear (Horn et al., 2014). Genetic variations in the HTR3A and HTR3B genes may be associated with the risk of developing POV, but given the multifactorial causes of POV, larger studies are required to determine the true clinical significance of these observations (Rueffert et al., 2009; Ma et al., 2013).
2. Eating Disorders
The 5-HT3 receptor antagonist ondansetron reduces binge-eating, vomiting, and depressive symptoms in patients with severe bulimia nervosa, leading to a return of normal eating, possibly by modulating cyclic increases in vagal nerve activity (Faris et al., 2006). Arguably, an ability of 5-HT3 receptor antagonism to increase the threshold before satiation is reached (Janssen et al., 2011b) could also play a role. Variants of the HTR3B gene have been associated with the restrictive subtype of anorexia nervosa (Hammer et al., 2009). It may also be possible to achieve long-term regulation of body weight by modulating different aspects of gastric motility (Janssen et al., 2011), perhaps with drugs that act at different 5-HT receptors (see below).
3. Carcinoid Diarrhea
Carcinoid tumors in the colon can generate high levels of circulating 5-HT, causing diarrhea by stimulating colonic motor functions (von der Ohe et al., 1993) via 5-HT3 receptors (see above) and chloride ion secretion in human colon via 5-HT2A and 5-HT4 (ascending colon) and 5-HT2A (descending colon) receptors (Borman and Burleigh, 1996). This is primarily treated with loperamide or a somatostatin analog, such as octreotide, but 5-HT3 receptor antagonists have helped, at least when given acutely, as have 5-HT2A (ketanserin) receptor antagonists and methysergide, a nonselective 5-HT1/5-HT2 receptor agonist/antagonist (Camilleri and von der Ohe, 1994; Schwörer et al., 1995; Spiller, 2008a). Given that TPH1 is responsible for 5-HT production, perhaps its inhibition may represent a new treatment paradigm in carcinoid tumors (see Camilleri, 2011).
4. Functional Gastrointestinal Disorders
These are a grouping of GI disorders that cannot be explained by structural or tissue abnormalities and as such are defined by symptoms. They include functional dyspepsia, IBS, and several others (Longstreth et al., 2006).
5. Functional Dyspepsia
The rationale for treatment of functional dyspepsia with a 5-HT receptor antagonist has not been established. Single doses of a 5-HT4 receptor antagonist did not affect symptoms (Van Lelyveld et al., 2006), although an ability of 5-HT4 receptor antagonism to prevent HCO3− release in rat duodenum by SCFAs has suggested a role when high levels of SCFAs occur in the upper GI tract during bacterial overgrowth (Akiba et al., 2015). More promisingly, a pilot study with patients dosed for 12 weeks with a 5-HT3 receptor antagonist showed some improvement in “adequate relief of pain or discomfort” (Talley et al., 2001), and an association between symptoms and 5-HT3A receptor gene polymorphism has been suggested (Mujakovic et al., 2011). Arguably, these data find some consistency with an ability of 5-HT3 receptor antagonism to prevent the experience of nausea in healthy volunteers induced by intraduodenal infusion of lipids [see Gershon and Tack (2007)], as early satiety and nausea are common symptoms in this group of patients (Vanheel et al., 2013) but are at variance with the lack of ability of 5-HT3 receptor antagonism to change sensitivity to gastric distension [see Gershon and Tack (2007)].
6. Irritable Bowel Syndrome
In some pilot studies, 5-HT3 receptor antagonism reduced sensations caused by bowel distension in patients with IBS [e.g., Prior and Read (1993) and Goldberg et al. (1996)], perhaps as a consequence of increased compliance to distension (Delvaux et al., 1998). These studies, together with the known ability to reduce colonic movements, prompted the evaluation and initial success of the 5-HT3 receptor antagonist alosetron as a treatment for patients with IBS with symptoms of diarrhea as well as abdominal discomfort and/or pain. The subsequent occurrence of ischemic colitis in a small number of treated patients restricted use of alosetron and almost entirely stopped research into this area. It has since been concluded that ischemic colitis is two- to four-times more likely to occur in patients with IBS regardless of treatment (Lewis, 2011), suggesting that any future treatments of this form of IBS should not induce severe constipation to potentially exacerbate such a liability. Furthermore, it should be noted that an ability of 5-HT3 receptor antagonists to reduce sensations evoked by bowel distension has not been consistently observed [e.g., Hammer et al. (1993) and Zighelboim et al. (1995) in patients without “psychologic disorders”), perhaps arguing for greater emphasis of future research on colonic movement disorders. Any association between IBS and HTR3 subunit gene mutations remains uncertain (Niesler, 2011). Nevertheless, in patients with diarrhea-predominant IBS, the ability of alosetron to reduce colonic transit may be associated with long (LL) polymorphisms of the 5-HT transporter gene 5-HTTLPR, which is associated with increased synthesis of SERT (SLC6A4) and inactivation of endogenous 5-HT (Camilleri et al., 2002). Also, in this group of patients with IBS, symptoms were improved in a pilot study using the 5-HT3 receptor antagonist ramosetron, an activity correlating with increased expression of TPH1 and with TPH1 gene polymorphism (Shiotani et al., 2015).
The 5-HT4 receptor antagonist SB-207266 reduced stress-induced defecation in mice (Sanger et al., 2000), and in a pilot study with diarrhea-predominant IBS patients, it tended to reduce rectal sensitivity and reduced small-intestinal transit (Houghton et al., 1999). However, lack of significant efficacy in larger studies stopped further development for this indication (De Ponti, 2004).
In some countries, the 5-HT4 receptor agonist tegaserod was registered for treatment of IBS and chronic constipation (see below for discussion on the potential use of 5-HT4 receptor agonists in the treatment of IBS) but was then withdrawn because of potential cardiovascular liability and poor overall efficacy (Schiller and Johnson, 2008). Tegaserod has since been shown to act as a potent 5-HT2B receptor antagonist (Beattie et al., 2004), reducing colonic motility (in rodents, not dogs; see above) and exerting a visceral antinociceptive activity in rodents (Ohashi-Doi et al., 2010; O’Mahony et al., 2010). The extent to which each of these different activities translates to humans and/or patients with IBS is not clear. In parallel with the evidence that LL polymorphisms of the 5-HT transporter gene 5-HTTLPR result in greater changes in colonic transit with alosetron, a clinical trial has shown that LL polymorphism is associated with reduced clinical efficacy of tegaserod in patients with constipation (Li et al., 2007).
LX1031, a locally acting TPH inhibitor, that does not cross the blood-brain barrier, has been found to be safe and well tolerated in an exploratory 4-week phase 2 study in patients with symptomatic, nonconstipating IBS (Brown et al., 2011). Thus, reduction of mucosa-derived 5-HT may positively influence symptoms common to nonconstipating IBS. A relationship was observed between symptom improvement and a reduction in 24-hour urinary 5-HIAA; thus, 5-HIAA can serve as a biomarker to estimate the rate of 5-HT synthesis and target engagement by the TPH inhibitor LX1031. However, data from any further clinical development of this drug is lacking, and the company Lexicon have confirmed development has been terminated.
Any involvement of the 5-HT7 receptor in the mechanisms of visceral pain, following a proposed role in somatic pain (Andrews and O’Neill, 2011), is as yet unknown. Similarly, any involvement of 5-HT in the mechanisms of mucosal inflammation (see earlier) in patients with IBS has yet to be demonstrated.
7. Other Gastrointestinal Disorders
In colon from patients with diverticular disease, 5-HT4 mRNA expression was reduced in the muscle but increased in the mucosa; expression of 5-HT2B and 5-HT3A mRNA was unchanged. The authors speculate that these changes could influence the intestinal motor disturbances associated with this disease (Böttner et al., 2013).
D. Therapeutic Benefits of 5-HT Receptor Agonists and Antagonists
This section discusses the use of 5-HT receptor agonists (“exogenous 5-HT”) to treat diseases where any involvement of endogenous 5-HT in the mechanism of the disease is absent or unclear. The class of 5-HT3 receptor antagonists (e.g., alosetron or ondansetron) for IBS-D and 5-HT4 receptor agonists for chronic constipation or IBS-C (e.g., prucalopride) are extensively used in clinical practice and constitute first- or second-line therapeutic agents for these conditions. Efficacy and safety are documented by systematic reviews and meta-analyses (Andresen et al., 2008; Ford et al., 2009; Shin et al., 2014). The risk of ischemic colitis with alosetron (not observed with ondansetron) is estimated at about 1 in 1000 patients; however, there is also evidence that IBS itself is a risk factor for the development of ischemic colitis (Huerta et al., 2011).
1. 5-HT4 Receptor Agonists
At present, 5-HT4 receptors appear to have little or no major roles in disorders of GI hypomotility; for example, in idiopathic gastroparesis, there were no overall changes in 5-HT4 receptor expression apart from a reduced expression of the 5-HT4(c) splice variant (van Lelyveld et al., 2008), yet 5-HT4 receptor agonists have long been used to treat such disorders. This began with metoclopramide, a derivative of procaine found to have surprising antiemetic and gastric prokinetic properties. Understanding the mechanisms of action of metoclopramide directly led to the discovery of the antiemetic role of the 5-HT3 receptor (Miner and Sanger, 1986) and in the description of a novel “5-HT–like” receptor affecting GI motility (Sanger, 1987b), later named as the 5-HT4 receptor by Bockaert and colleagues who used similar ligands in CNS studies (Dumuis et al., 1988). Subsequently, a number of different 5-HT4 receptor agonists were launched as prokinetic agents, but none of the early examples are selective in their action, leading to cardiovascular complications (Sanger, 2009). Nevertheless, the selective 5-HT4 receptor agonist prucalopride is now marketed as a treatment of chronic idiopathic constipation; others are in development, and the potential use of such agents in the treatment of upper GI disorders associated with gastric and/or esophageal hypomotility is still being explored (Sanger, 2009; Broad et al., 2014a,b; Kessing et al., 2014). Interestingly, a low dose of the 5-HT4 receptor agonist mosapride has been shown to increase human gastric accommodation in healthy volunteers (Amano et al., 2015), perhaps reflecting the ability of 5-HT4 receptor agonists to increase nitrergic as well as cholinergic activity (Cellek et al., 2006), the former potentially increasing gastric fundus accommodation and the latter, gastric antrum motility and emptying. Because impaired gastric accommodation may contribute to the etiology of symptoms in patients with postprandial functional dyspepsia (e.g., early satiety and/or nausea; Talley, 2015), these data support the argument that such symptoms can be relieved by selective 5-HT4 receptor agonists (Janssen et al., 2011a; Sanger et al., 2013).
In human colon, 5-HT4 receptor activation is an effective prokinetic principle, as it facilitates excitatory cholinergic and inhibitory nitrergic motor nerve activities (representing the ascending excitatory and descending inhibitory components of a peristaltic reflex), decreases muscle tension, and increases chloride secretion from the mucosa into the lumen (Borman and Burleigh, 1996; Prins et al., 2000; Cellek et al., 2006; Broad et al., 2013). Evidence for facilitation of enteric sensory nerve activity remains uncertain (Gershon and Tack, 2007). More recent attention has been drawn to the possibility that 5-HT4 receptor activation might stimulate bicarbonate secretion in rat proximal colon (Kaji et al., 2015) and might also reduce nociception in rats exposed to colorectal distension (Hoffman et al., 2012; Gilet et al., 2014). The latter activity could depend on a synergistic interaction with 5-HT3 receptors in the mechanisms of allodynia (Smith et al., 1999) but also contrasts with the findings of Sugiuar et al. (2004), who showed that the nociceptive functions of transient receptor potential cation channel subfamily V member 1 channels were facilitated by 5-HT4 receptor activation in mouse colon. Similar responses have not yet been confirmed to occur in human colon, but if successfully translated, 5-HT4 agonists may be useful as suppositories or enemas for treating IBS (Kale-Pradhan and Wilhelm, 2007; Mawe and Hoffman, 2013). To date, only the 5-HT4 receptor agonist tegaserod has been fully tested as a potential treatment of IBS, but poor efficacy at the 5-HT4 receptor, potential confounding cardiovascular complications, and the existence of additional ability to antagonist at the 5-HT2B receptor (see above for discussion) greatly complicate the use of such data to argue for or against the use of 5-HT4 receptor agonists as a treatment of any form of IBS.
Finally, the possibility that 5-HT4 receptor activation might promote neurogenesis in adults has received support from studies using mice (increased bromodeoxyuridine incorporation into neurons, neural precursors, or stem cells; Liu et al., 2011), guinea pigs, and rats (regeneration of neural circuitry or recovery of reflex activity after rectal transection and anastomosis, accompanied by increased neurofilament and neural stem cell markers; Takaki et al., 2014), suggesting potential use of 5-HT4 receptor agonists in treatments of disorders associated with intestinal aganglia.
2. Other 5-HT Receptor Agonists
Different 5-HT1A receptor agonists, including buspirone, may reduce emesis induced by different stimuli in animals, including cisplatin and motion, although species differences in actions complicates the interpretation of data (Johnston et al., 2014). In patients with functional dyspepsia, repeat-dosing with the 5-HT1A receptor agonist R137696 did not reduce dyspeptic symptoms or gastric accommodation (Tack et al., 2009).
When given acutely after meals to patients with functional dyspepsia, the 5-HT1B/1D receptor agonist sumatriptan delayed gastric emptying, improved gastric accommodation, and reduced the perception of gastric distension and early satiety (Tack et al., 2004). The suggestion that this receptor may be involved in the mechanisms of vomiting has not yet been resolved (Johnston et al., 2014), especially as sumatriptan may act on the yet to be defined 5-HT1P receptor.
Partial 5-HT3 receptor agonists (e.g., pumosetrag; CSTI-300) have been identified for treatment of patients with diarrhea-predominant IBS (e.g., Moore et al., 2013; Roberts et al., 2020). CSTI-300 displays comparable efficacy to alosetron in a rat model of colon distension (Roberts et al., 2020). Pumosetrag has been evaluated in patients with gastroesophageal reflux disease (Choung et al., 2014), reducing the rate of acid reflux events but not symptoms.
A summary of some 5-HT drugs in development for GI therapeutics is summarized in Table 22 and reviewed elsewhere (Valentin et al., 2015).
TABLE 22.
Some novel 5-HT agents for GI indications
| Drug Class | Example | Putative Action in Humans | Pharmacodynamic in Humans | Clinical Efficacy: Phase IIB or III | Safety Issues, Approval, Other |
|---|---|---|---|---|---|
| TPH1 blocker | LX-1031 | Inhibits synthesis of 5-HT by blocking TPH1 | Inhibition of urine 5-HIAA excretion; no studies of PD efficacy | Phase IIB trial in non–C-IBS: 1000-mg dose, improved adequate relief, stool consistency | Reported to be discontinued |
| 5-HT3 receptor antagonist | Ramosetron | Inhibits secretion, motility, nociception | ND | Phase IIB 5- and 10-µg dose studies in IBS-D: benefit on global relief and bowel function | Approved in Asia, under investigation elsewhere; ischemic colitis with same drug class |
| 5-HT4 receptor agonist | Prucalopride | Selective 5-HT4 receptor agonist; stimulates colonic motility | Accelerated CT in health and CC | Multiple phase II/III trials completed; open label experience of ∼1000 cumulative patient-years; efficacy in CC (males and females in phase III and IV clinical trials), with consistent benefits shown by meta-analyses | No clinical cardiac AEs in clinical trials of >4000 humans; approved in virtually all countries except United States |
| 5-HT4 receptor agonist | Velusetrag | Selective 5-HT4 receptor agonist; stimulates colonic motility | Accelerated CT in health in dose-related fashion | Phase IIB efficacy; no effect on QT in health or 400 patients with constipation | Under investigation (although last report 2017) |
| 5-HT4 receptor agonist | Naronapride | Selective 5-HT4 receptor agonist; stimulates colonic motility | Accelerated CT in health | Under investigation | Under investigation (although last report 2018) |
| 5-HT4 receptor agonist | Relenopride | Selective 5-HT4 receptor agonist; stimulates colonic motility | Accelerated CT in functional constipation | Phase II studies ongoing in IBS-C patients (ClinicalTrials.gov trial NCT02082457). | May be discontinued (last active report 2015) |
AEs, adverse events; CC, chronic constipation; CT, colonic transit; ND, not determined.
XXI. 5-HT Receptors and the Immune System
A. Introduction
The defense against pathogens is mediated by innate and adaptive immune mechanisms that act in the periphery and the CNS. 5-HT regulates inflammation and immunity by acting on 5-HT receptors that are differentially expressed on immune cells, both in rodents and humans. 5-HT acts as a potent chemoattractant, recruiting innate immune cells to sites of inflammation. 5-HT also alters the production and release of cytokines and cell activation/proliferation. Some immune cells, including mast cells and T lymphocytes, have the capacity to synthesize and release 5-HT, expanding the range of tissues for 5-HT signaling.
B. How Do Immune Cells Encounter 5-HT?
Although 5-HT is largely studied as a neurotransmitter, enterochromaffin cells of the gut produce most of the body’s 5-HT that functions as a local hormone. These cells express tryptophan hydroxylase TPH1, a rate-limiting enzyme for 5-HT production (Walther et al., 2003). A second TPH isoform, TPH2, synthesizes 5-HT in the CNS and gut enteric nerves (Walther et al., 2003). 5-HT concentrations in blood and tissues are normally kept relatively low. Immune cells, however, may encounter 5-HT released in the gut mucosa or from platelets that sequestered 5-HT via the 5-HT transporter SERT (SLC6A4). In turn, platelets can release accumulated 5-HT at sites of injury and inflammation. Platelet-derived 5-HT is important for attracting innate immune cells such as neutrophils to inflamed tissue (Duerschmied et al., 2013). In addition to platelets, dendritic cells (professional antigen-presenting cells) and B lymphocytes express SERT and, thus, accumulate and release 5-HT. Interestingly, recent studies indicate that some immune cells are capable of 5-HT biosynthesis. Mast cells (tissue-resident cells) in rodents and humans express TPH1, and levels of 5-HT are elevated in patients with mastocytosis, who have greatly elevated mast cell numbers (Kushnir-Sukhov et al. 2007; Nowak et al., 2012). Furthermore, T lymphocytes (O’Connell et al., 2006; León-Ponte et al. 2007; Urbina et al., 2014) also express TPH1 upon mitogen or T-cell receptor activation and can synthesize 5-HT. Interestingly, expression of TPH1 and 5-HT production is greater in CD8+ compared with CD4+ T cells (Chen et al., 2015).
C. 5-HT and Hematopoiesis
It has been proposed that 5-HT acts at hematopoietic stem cell progenitors directly or via modulation of the bone marrow microenvironment (Yang et al., 2007). Mice deficient in peripheral 5-HT (Tph1−/−) display morphologic and cellular features reminiscent of ineffective erythropoiesis (Amireault et al., 2011). Other data show that the bone marrow composition of Htr2B−/− mice displays a significant increase in Cd11b+/Gr+ cells that represents granulocyte precursors. This is associated with a significant reduction in Cd11b-/Cd31+ population that corresponds to immature endothelial progenitor cells in 5-HT2B−/− mice (Launay et al., 2012). These observations support the hypothesis that 5-HT signaling controls the differentiation of myeloid precursor cells, particularly in the monocyte/macrophage lineages.
D. 5-HT and the Immune Tolerance
One well documented way to control immunity and tolerance is through the regulation of nutrients in the microenvironment of immune cells. Best described is tryptophan deficiency mediated by the catabolic enzyme indoleamine 2,3-dioxygenase (IDO), which locally depletes tryptophan and liberates immunoregulatory metabolites known as kynurenines. T-cell activation is exquisitely sensitive to local tryptophan catabolism, and thus this enzyme exerts profound protective effects in allo-fetal rejection, autoimmunity, and inflammation (Munn and Mellor, 2013). Although IDO is thought to be the major tryptophan-catabolizing enzyme outside of the liver, TPH1 shares a similar KM to IDO (∼20 µM) (Mckinney et al., 2005) and can also potentially exhaust tryptophan to regulate immune tolerance. Indeed, in models of skin allograft tolerance, tumor growth, and experimental autoimmune encephalomyelitis (multiple sclerosis), Tph1 deficiency was shown to break allograft tolerance, to induce tumor remission, and to intensify neuroinflammation independent of the downstream product 5-HT (Nowak et al., 2012).
E. 5-HT and the Innate Immune Response
Innate immune system function involves monocytes, macrophages, dendritic cells, neutrophils, eosinophils, mast cells, and natural killer cells that act immediately in the area of infection, leading to the destruction of pathogens. Innate immunity is primarily responsible for recognizing and eradicating “nonself” molecules presented by pathogens and is therefore confined to recognizing extracellular pathogens (bacteria vs. viruses). This response is nonspecific with respect to particular invaders but provides immediate host defense against pathogens via pattern recognition by toll-like receptors (TLRs). Pathogen-associated molecular patterns (e.g., peptidoglycans, bacterial LPS, and double-stranded viral RNAs) bind TLRs on antigen-presenting cells, namely, dendritic cells and macrophages. Antigen-presenting cells then phagocytose pathogens and display pathogen-derived peptides via the major histocompatibility complex on their cell surface for recognition by leukocytes of the “adaptive” immune system. Antigen-presenting cells also secrete proinflammatory cytokines (e.g., IL1β, IL-6, and TNFα), prostaglandins, and histamine, which further activate physiologic responses, alerting the body to infection/invasion. In addition to cellular protective mechanisms, innate immunity also includes the complement system, activated by foreign substances, antigen-antibody complexes (classic pathway), and Gram-negative bacteria (alternative pathway). This system leads to cell lysis, increased vascular permeability (allowing antibodies, innate immune cells, and fluid to enter tissues), and chemotaxis. The complement system also helps to activate antigen-presenting cells, namely, dendritic cells and B cells, during specific immune responses. Innate immunity also functions to communicate the presence of pathogens to cells involved in adaptive immune responses (Baganz and Blakely, 2013). The local environment and the presence of stimulatory signals determine whether monocytes acquire dendritic cell or macrophage characteristics and functions. 5-HT receptors are expressed by a broad range of inflammatory cell types, including monocytes, macrophages, and dendritic cells.
Neutrophils are the most abundant white blood cell and serve an essential role in innate immunity, particularly against bacteria. Duerschmied et al. (2013) reported that Tph1−/− mice show mild leukocytosis (e.g., elevated white blood cells) numbers compared with WT mice, primarily driven by an elevated neutrophil count. Despite this, 50% fewer leukocytes rolled on unstimulated mesenteric venous endothelium of Tph1−/− mice. Diminished rolling in Tph1−/− mice resulted in reduced firm adhesion of leukocytes after LPS treatment, and neutrophil extravasation into lung, peritoneum, and skin wounds was reduced in Tph1−/− mice. 5-HT alone did not induce neutrophil migration in vitro, suggesting that endothelial adhesion was the primary deficit. Consequently, survival from LPS-induced endotoxic shock was improved in Tph1−/− mice. In conclusion, platelet 5-HT promotes the recruitment of neutrophils in acute inflammation (Duerschmied et al., 2013); however, the nature of the 5-HT receptors underlying these effects is unknown.
In human CD14 monocytes, mRNA expression of 5-HT1E, 5-HT2A, 5-HT3, 5-HT4, and 5-HT7 receptors has been revealed (Dürk et al., 2005). 5-HT modulates the release of IL-1β, IL-6, IL-8/CXCL8, IL-12p40, and TNF-α, whereas it has no effect on the production of IL-18 and IFN-γ in LPS-stimulated human blood monocytes. Moreover, 5-HT modulates mRNA levels of IL-6 and IL-8/CXCL8 but not of IL-1β and TNF-α. Pharmacologic experiments suggested that signaling through the 5-HT3 receptor upregulates the LPS-induced production of IL-1β, IL-6, and IL-8/CXCL8 but not that of TNF-α and IL-12p40. Furthermore, activation of the Gs-coupled 5-HT4 and 5-HT7 receptors increases secretion of IL-1β, IL-6, IL-12p40, and IL-8/CXCL8, but in contrast, it inhibits LPS-induced TNF-α release. Interestingly, 5-HT1E and 5-HT2A receptor agonists do not modulate the LPS-induced cytokine production in human monocytes (Dürk et al., 2005). Instead, 5-HT modulates cytokine production via activation of 5-HT3, 5-HT4, and 5-HT7 receptors.
5-HT has been shown to upregulate the activity of peritoneal macrophages and to increase the in vitro activity of phagocytosis in a concentration-dependent manner via 5-HT1A/7 receptors and NF-κB (Freire-Garabal et al., 2003). Gene expression profiling of proinflammatory M1 (granulate-macrophage colony-stimulating factor) and anti-inflammatory M2 (macrophage colony-stimulating factor) macrophages revealed that 5-HT2B and 5-HT7 receptor mRNAs are preferentially expressed by M2 macrophages, whereas the 5-HT7 receptor is the only 5-HT receptor expressed in M1 macrophages (de Las Casas-Engel et al., 2013). The 5-HT2B receptor is preferentially expressed by anti-inflammatory M2 macrophages and is also detected in vivo in liver Kupffer cells and in tumor-associated macrophages. Expression of 5-HT2C receptors was also reported in alveolar macrophages, where 5-HT induces a rise in intracellular Ca2+ concentration and an increased expression of CCL2 (monocyte chemoattractant protein-1) mRNA (Mikulski et al., 2010). LPS, the archetypal macrophage-activating stimulus that signals via TLR4, was shown to regulate the expression of 5-HT2B receptors in mouse macrophages. 5-HT2B receptor mRNA is increased 20-fold in murine thioglycollate-elicited peritoneal macrophages (Lattin et al., 2008). 5-HT was shown to inhibit the LPS-induced release of proinflammatory cytokines, to upregulate expression of macrophage M2 polarization–associated genes, and to reduce the expression of M1-associated genes. Only 5-HT7 receptors mediate the inhibitory action of 5-HT on the release of proinflammatory cytokines. Both 5-HT2B and 5-HT7 receptors mediate the pro-M2 skewing effect of 5-HT. Blockade of both receptors during in vitro monocyte-to-macrophage differentiation preferentially modulates the acquisition of M2 polarization markers (de Las Casas-Engel et al., 2013).
In mice, it has been established that 5-HT is an important regulator of microglia, the brain resident macrophages, which derive from yolk sac hematopoietic stem cell precursors. In the presence of 5-HT, the microglial processes moved more rapidly toward a lesion, which is considered a chemotactic response. Similarly, the chemotactic response of cultured microglia to ATP is enhanced by 5-HT. Phagocytic activity determined by the uptake of microspheres reveals that 5-HT application decreases phagocytic activity of amoeboid microglia. The presence of microglial 5-HT2B, 5-HT5A, and 5-HT7 receptors was confirmed by patch-clamp experiments in culture and amoeboid microglia and by qPCR analysis of RNA isolated from primary cultured and acutely isolated adult microglia (Krabbe et al., 2012). This was recently confirmed by two-photon microscopy, showing that microglial processes moved rapidly toward the source of 5-HT via activation of the 5-HT2B receptor (Kolodziejczak et al., 2015). Modulation of microglial functions such as phagocytosis and migration is fundamental for the CNS, as microglia can influence the balance of synaptogenesis and neuronal death during development and in pathology.
Platelet activation was reported in patients with various allergic disorders. Platelet-derived factors may influence monocytic differentiation into dendritic cells. Indeed, 5-HT alters differentiation of monocytes into dendritic cells (triggered by granulocyte-macrophage colony-stimulating factor and IL-4), leading to dendritic cells with reduced expression of costimulatory molecules and CD1a and higher expression of CD14. These 5-HT–triggered dendritic cells exhibit significantly reduced stimulatory activity toward allogeneic T cells. However, they show enhanced cytokine-producing capacity, including for IL-10 but not IL-12. 5-HT–induced alteration of the dendritic cells phenotype and the reduction in antigen-presenting capacity are mediated via 5-HT1E/5-HT7 receptors (Katoh et al., 2006).
Immature dendritic cells preferentially express mRNA for 5-HT1B, 5-HT1E, and 5-HT2B receptors, whereas mature dendritic cells mostly express 5-HT4 and 5-HT7 receptors. The mRNA expression level of the ligand-gated cation channel 5-HT3 and the GPCR 5-HT2A receptors are not modified during maturation. 5-HT stimulates 5-HT3–dependent Ca2+ influx in both immature and mature dendritic cells. The 5-HT1B/1E and 5-HT2A/2B receptor stimulation induces intracellular Ca2+ mobilization via Gi/Gq proteins in immature, but not mature, dendritic cells. Activation of 5-HT4/7 receptors induces cAMP elevation in mature dendritic cells. Functional studies indicate that activation of 5-HT4 and 5-HT7 receptors enhances the release of the cytokines IL-1β and IL-8 while reducing the secretion of IL-12 and TNF-α in mature dendritic cells (Idzko et al., 2004).
5-HT is able to induce oriented migration in immature but not in LPS-matured dendritic cells via activation of 5-HT1B/1E and 5-HT2A/2B receptors. Accordingly, 5-HT also increases migration of pulmonary dendritic cells to draining lymph nodes in vivo. By binding to 5-HT3, 5-HT4, and 5-HT7 receptors, 5-HT upregulates the production of the proinflammatory cytokine IL-6. Additionally, 5-HT influenced chemokine release by human monocyte–derived dendritic cells: production of the potent T-helper cells Th1 chemoattractant IP-10/CXCL10 was inhibited in mature dendritic cells, whereas CCL22/ macrophage-derived chemokine secretion was upregulated in both immature and mature dendritic cells. Furthermore, dendritic cells matured in the presence of 5-HT switched to a high IL-10– and low IL-12p70–secreting phenotype. Consistently, 5-HT favored the outcome of a Th2 immune response both in vitro and in vivo (Müller et al., 2009). A recent study using Htr7−/− mice confirmed 5-HT7 receptor expression in CD103+CD11c+ dendritic cells found in colon (and spleen) and its importance in immune activation and gut inflammation (Kim et al., 2013).
Interestingly, like platelets, dendritic cells can take up 5-HT from the microenvironment, and the antidepressant fluoxetine inhibits this uptake. Expression of 5-HT transporters (SERTs) is regulated by dendritic cell maturation, exposure to microbial stimuli, and physical interactions with T cells. Significantly, 5-HT sequestered by dendritic cells is stored within LAMP-1+ vesicles and subsequently released via Ca2+-dependent exocytosis, as confirmed by amperometric recordings (O’Connell et al., 2006).
5-HT is chemotactic for eosinophils. Notably, allergic asthma is characterized by infiltration of eosinophils, and plasma levels of 5-HT are elevated in symptomatic asthma patients. There is solid evidence that 5-HT contributes to this eosinophil recruitment. Indeed, 5-HT alone can stimulate in vitro migration of murine and human eosinophils (Boehme et al., 2004; Kang et al., 2013). Although several 5-HT receptor subtypes are expressed, 5-HT2A is the most prominent, and 5-HT2A receptor antagonists inhibit 5-HT–induced but not eotaxin-induced migration. Furthermore, eosinophils roll in response to 5-HT in venules under conditions of physiologic shear stress (Boehme et al., 2004; Kang et al., 2013). Signaling via 5-HT2A receptors is associated with changes in cell shape/morphology via activation of specific intracellular signaling molecules (ROCK, MAPK, PI3K, and the PKC-calmodulin pathway) (Kang et al., 2013).
Mast cells have the capacity to synthesize and accumulate 5-HT (Kushnir-Sukhov et al., 2007). In turn, this stored 5-HT can be released upon IgE crosslinking. Furthermore, mast cells express mRNA for multiple 5-HT receptors, including 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT6, and 5-HT7 receptors (Kushnir-Sukhov et al., 2006). 5-HT can induce mast cell adherence to fibronectin and stimulate cell migration. However, there is no evidence that 5-HT degranulates mast cells or modulates their activation by IgE. Mast cells from the 5-HT1A receptor knockout mouse (Htr1A−/−) do not respond to 5-HT, indicating a principal role for this receptor. Importantly, 5-HT attracts mast cells to sites of inflammation; injection of 5-HT into the skin enhances the accumulation of mast cells in wild-type but not in 5-HT1A receptor–null mice.
Natural killer cells are large lymphocytes with innate killing capacity. Addition of 5-HT to mixtures of target cells and CD56+ natural killer–enriched human mononuclear cells strongly augmented natural killer cell cytotoxicity via 5-HT1A receptors. This effect was indirect and involved 5-HT signaling at accessory monocytes. The cytotoxicity-enhancing effect of 5-HT was additive to that induced by IFN-α, IFN-γ, or IL-2 but not to histamine (Hellstrand and Hermodsson, 1987).
F. 5-HT and Adaptive Immunity
The response of a second immune system division, termed the adaptive, or specific, immune system, occurs within hours of an infection and involves antigen-specific recognition and destruction of pathogens by T and B lymphocytes. The two components of the adaptive immune system involve cell-mediated and humoral immunity. Cell-mediated immunity is carried out by T cells located in the thymus, lymph nodes, and circulation. Antigen-presenting cells that migrate to lymph nodes will prime and educate T cells as to the nature of the pathogen. T cells then proliferate and differentiate into, for example, CD4+ T-helper inflammatory cells (Th1) that activate macrophages, CD4+ Th2 cells that aid antibody responses, or CD8+ cytotoxic cells that target cells infected with intracellular microbes. The second component of adaptive immunity involves the contributions of B cells, located in lymph tissue, spleen, and in the circulation. Upon stimulation, B cells become plasma cells (with or without the help of Th2) that produce and secrete antibodies (immunoglobulins). Memory T and B cells recognize specific antigens and respond quickly. Thus, the adaptive immune system is distinguished from the innate immune system by its ability to identify, remember, and eliminate pathogens that have been designated as nonself. Adaptive immunity is triggered at the immune synapse, where peptide major histocompatibility complexes and costimulatory molecules expressed by dendritic cells are physically presented to T cells (Baganz and Blakely, 2013).
The mRNA expression of 5-HT receptors in lymphoid tissues of the rat, ex vivo isolated spleen, thymus, and peripheral blood lymphocytes include 5-HT1B, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT6, and 5-HT7 receptor mRNAs. Mitogen-stimulated spleen cells additionally expressed mRNA corresponding to the 5-HT3 receptor (Stefulj et al., 2000). In the rhesus macaque, SERT-positive cells were found among CD4+, CD3+, and CD3+CD4+ lymphocytes, respectively (Yang et al., 2007). Fluoxetine significantly increases the number of lymphocytes expressing SERT and stimulates an enrichment of CD8+ T cells, decreasing the CD4+/CD8+ ratio. Fluoxetine administration elevates the levels of IL-4 at 1, 2, and 3 weeks and of IL-2 at 2 and 3 weeks. The IL-4/IL-2 ratio is significantly increased in fluoxetine group compared with the controls and is similar during the 3 weeks of treatment (Fazzino et al., 2009).
There is long-standing evidence that 5-HT can influence T-cell activation. Notably, mice treated with a selective, irreversible inhibitor of TPH1, parachlorophenylalanine, exhibit a reduction in the number of CD25-positive T cells (Young et al., 1993; León-Ponte et al., 2007), suggesting that 5-HT contributes physiologically to T-cell activation. A screen for 5-HT receptor subtypes in murine T cells revealed expression of three subtypes; naive T cells selectively express 5-HT7 receptors, whereas following T-cell activation, there is a strong upregulation of 5-HT1B and 5-HT2A receptors (León-Ponte et al. 2007). Significantly, exogenous 5-HT induces rapid phosphorylation of ERK1/2 and IκBα in naive T cells that is inhibited by preincubation with a selective 5-HT7 receptor antagonist. Thus, 5-HT signaling via the 5-HT7 receptor may contribute to early T-cell activation. Yin et al. (2006) showed that 5-HT1B receptor antagonists impaired the proliferation of helper CD4+ T cells in mouse and human. Inoue et al. (2011) showed that a 5-HT2A receptor agonist enhanced Concavalin-A–induced activation of murine CD4+ and CD8+ T cells, whereas a 5-HT2A receptor antagonist blocked T-cell receptor–mediated IL-2 and interferon-γ production. Consistent with these data, Akiyoshi et al. (2006) showed that treatment with a 5-HT2A receptor antagonist enhanced the survival of cardiac allograft in mice. Thus, these mouse data strongly support involvement of 5-HT receptors (5-HT7, 5-HT1B, and 5-HT2A) during early- and late-stage T-cell activation.
Interestingly, although not detected in mouse, the 5-HT2B receptor is found in human T cells. Gene expression profiles during human CD4+ T-cell differentiation identified the 5-HT2B receptor with 10-fold greater expression in CD3highCD4+CD8− SP4 thymocytes over intrathymic T progenitor cells, CD3−CD4+CD8+ “double positive” thymocytes, CD3+CD4+CD8−CD45RA+CD62L+ “naive” T cells from cord blood, and CD3+CD4+CD8−CD45RA+CD62L+ “naive” T cells from adult blood (Lee et al., 2004). Furthermore, 5-HT2B receptors are differentially expressed among Th subsets. In human umbilical cord blood, Th cells cultured in the presence of cytokines promoting Th2 differentiation were found to increase 5-HT2B receptor expression along with 50 Th2 differentially expressed genes (Aijö et al., 2012).
5-HT may also modulate migration of human T cells. Human but not mouse T cells express functional 5-HT3 receptors. 5-HT3 receptor agonists selectively decrease T-cell migration toward gradients of the chemokine CXCL12 but not to other chemokines such as CCL2 and CCL5. Interestingly, CXCL12 is highly expressed on vascular endothelium and inhibits T-cell migration across endothelium and extravasation. In transmigration experiments, 5-HT3 receptor stimulation reverses this effect of endothelial-bound CXCL12 on T-cell migration (Magrini et al., 2011). These data suggest that 5-HT can stimulate trafficking of T cells from blood to tissues.
T cells have the capacity to synthesize 5-HT, and levels of TPH1 expression increase following T-cell activation (O’Connell et al., 2006; León-Ponte et al. 2007; Urbina et al., 2014; Chen et al., 2015). The precise signaling role for T-cell–produced 5-HT is uncertain. Conceivably, TPH1 activity in T cells could act to exhaust tryptophan, as has been proposed for mast cells (Nowak et al., 2012). On the other hand, 5-HT produced by T cells might act in an autocrine or paracrine manner. Indeed, T cells express the type 1 vesicular monoamine transporter responsible for vesicular storage of 5-HT, and type 1 vesicular monoamine transporter expression increases following T-cell activation concomitant with TPH1. Furthermore, Ca2+ elevations in T cells can trigger secretion of 5-HT. Interestingly, levels of TPH1 and monoamine oxidase A, the principal catabolic enzyme for 5-HT, are greater in CD8+ compared with CD4+ T cells, suggesting a specific biologic role for 5-HT synthesis in this T-cell subset (Chen et al., 2015). B lymphocytes have the capacity to sense and sequester 5-HT via SERT. 5-HT increases mitogen-stimulated CD19+ B lymphocyte proliferation in a concentration- and time-dependent manner. These effects are reproduced by a 5-HT1A receptor agonist. 5-HT–induced increases in proliferation are blocked by 5-HT1A receptor antagonists. Moreover, LPS-activated mouse spleen cells express specific binding sites for 5-HT1A receptor, suggesting that 5-HT upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors (Iken et al., 1995). Furthermore, mitogen-activated B lymphocytes express higher levels of 5-HT1A receptor mRNA and protein than resting cells. This upregulation is seemingly dependent on NF-κB transcription factors, as selective inhibitors of this pathway prevent the increase in mRNA expression for the 5-HT1A receptor (Abdouh et al., 2001).
B lymphocytes express SERT, and uptake of 5-HT leads to apoptosis of Burkitt lymphoma cells (Serafeim et al., 2002). 5-HT may induce apoptosis via the intracellular serotonylation signaling pathway. Furthermore, long-term treatment with SSRIs in humans leads to enhanced (∼30%) numbers of B lymphocytes (Hernandez et al., 2010). Interestingly, higher doses of SSRIs directly promote apoptosis of Burkitt lymphoma cells by inhibiting DNA synthesis, whereas normal peripheral and tonsilar B cells are relatively resistant to SSRI-induced apoptosis (Serafeim et al., 2003). SERT has been detected in a variety of B-cell lines (Meredith et al., 2005), revealing SERT as a potential target for a broad range of B-cell malignancies.
XXII. General Summary and Conclusions
The first official IUPHAR review on 5-HT receptors (Hoyer et al., 1994) was a landmark for the then rather complex 5-HT receptor field: it has come of age and has been cited well over 3600 times (Google Scholar). It followed a number of initiatives and meetings in the late 1980s, when the 5-HT receptor nomenclature committee was established (by our esteemed colleague and friend Paul Vanhoutte in 1987, who sadly died in 2019). The committee was constituted and met formally for the first time in 1990 at the occasion of the 5-HT meeting in Basel (a satellite to the 1990 IUPHAR main meeting); a number of recommendations were made adapting to the new findings in transduction mechanisms and molecular biology of the receptors over the subsequent decade (Humphrey et al., 1993a; Hoyer et al., 1994, 2002; Hartig et al., 1996; Hoyer and Martin, 1997; Martin et al., 1998). It is remarkable that the recommendations made at the time have been largely accepted by a community that was used to very different nomenclatures or even definitions of receptors and that very little needed to be changed or added to these recommendations (Alexander et al., 2015a,b,c; 2019). In the 1994 review, it was noted that the authors had a cumulated 100 years of active 5-HT research to share. A number of our colleagues have in the meantime retired from active research or have moved to other professional priorities. There is a lot of new “blood” now on board to reflect the growing diversity of the research, which is currently performed in many different academic and SME (small or medium enterprise) pharmaceutical centers; the combined years in 5-HT research accumulated by the authorship has increased because of the considerably greater number of authors on the present paper to address a more diverse range of complex issues.
What has clearly changed, though, is the relative representation from the industry, which compared with the 1994 version, is very much reduced. In 1994, there were six out of the eight authors who worked in “Big Pharma,” whereas there are none for the present review. This is explained by the lesser interest for exploratory 5-HT research in the industry on the one hand, with less emphasis on 5-HT translational research and a shift of research toward small or medium enterprise/Biotech; on the other hand, there is an increased interest in very basic aspects of 5-HT research, such as structural biology or the more recent advances in the immunologic aspects, which were hardly addressed in the previous review of 1994. The shift in emphasis and thus authorship was very much needed, as the 5-HT receptor field has become more complex and possibly less the subject of “classical” pharmacologists as it was in the latter decades of the last century.
The apparent good news is that no new receptors have been identified, except for some additional 5-HT3 receptor subunits whose function still remain to be defined clearly. On the other hand, there have been major advances with respect to 5-HT1B/1D receptors; as far as can be told, all triptans act as 5-HT1B/1D receptor agonists, and in the meantime, many different triptans have reached the market primarily for the acute treatment of migraine. Some of them, such as sumatriptan, may also act as 5-HT1F receptor agonists. Thus, the 5-HT1B receptor is most probably the main and sole target for triptans in the treatment of migraine, whereas the 5-HT1D receptor, which is comparatively less abundant, may only play a minor role. Indeed, a study in migraine with a selective 5-HT1D receptor agonist (PNU142633) was not conclusive; it can be argued that the compound was only a partial agonist and that target engagement may not have been optimal. Thus, the jury is still out to define a role for the 5-HT1D receptor in physiology and disease. Because of the triptans, of which many starting with sumatriptan have affinity, the 5-HT1F receptor has attracted quite some attention, and there is clinical evidence (with lasmiditan) that this constitutes another target for the treatment of migraine. This is especially true for patients who want to avoid vascular side effects, as in contrast to the 5-HT1B receptor, the 5-HT1F receptor is not expressed in vascular tissues. The 5-HT1A receptor is still actively investigated, especially because newer highly selective 5-HT1A receptor ligands show very different patterns of pathway selectivity and thus may have clinical application in diseases as different as chronic pain, Parkinson disease, or Rett Syndrome and other forms of autistic disorders/mental retardation, as illustrated by the relatively recent FDA approval for compounds such as flibanserin, cariprazine, vortiotexine for the treatment of female sexual desire deficit, schizophrenia, and depression, respectively. Although these compounds have other activities, they all share 5-HT1A receptor agonism with possible differences in receptor engagement in different brain regions. An interesting aspect with some of the newer 5-HT1A receptorligands is their variety of activities at different transduction systems (i.e., these compounds have various degrees of biased agonism or signaling). This is not unique to 5-HT1A receptors, as probably most 5-HT GPCR agonists show different levels of biased signaling depending on their preferential activation of one or the other multiple combinations of receptor/G protein and accessory proteins. This has become even more evident now that crystal structures exist for both 5-HT1B and 5-HT2B receptors, which show that various ligands can occupy different conformation in the same orthosteric pocket, which may explain why certain 5-HT2A receptor agonists produce hallucinations when others do not. In addition, it has become evident that the orthosteric binding pocket is not the unique target for ligands, since a number of ergolines (e.g., ergotamine or DHE) may bind an accessory (allosteric site?), which has been well described for some metabotropic GPCRs (e.g., mGlu or GABAB receptors), opening the possibility for allosteric 5-HT receptor modulators (some of which have already been proposed for, for example, 5-HT1B/1D or 5-HT3 receptors).
The aspect of species differences is a recurrent theme, as illustrated by the amply documented and well accepted differences in the pharmacology of 5-HT1B receptors (explained by a single amino acid change in the core structure of the ligand-binding site) or the multiple differences observed with splice variants of the 5-HT4 or 5-HT7 receptors. A potential function for the 5-ht1e receptor in native tissue or cells is still to be identified; absence from rodents hinders the research and has made 5-ht1e receptor drug discovery relatively unattractive in the absence of a link to disease. A similar point can be made about the 5-HT5 receptors, in which both a relative lack of tools and the absence of a link to disease also hinder progress, although recent advances in knowledge concerning the 5-HT5A receptor may promote further research. However, as the 5-ht5B receptor appears to be nonfunctional in humans, this likely prevents interest as a therapeutic target.
Significant progress has been made in the 5-HT2 receptor family. The potential role played by 5-HT2B receptors in valvulopathies led to many of the drugs acting as agonists at these receptors being withdrawn from the market since the early 2000s (fenfluramine, norfenfluramine, benfluorex, and pergolide), keeping in mind that MDMA (“ecstasy”) and its active metabolites act as 5-HT2B receptor agonists, as may also be the case for a number of “designer” drugs. As a consequence, there is significant emphasis on screening for 5-HT2B receptor agonism in preclinical safety studies and the “red flag” on further clinical development of such compounds. It has been established that the 5-HT2C receptor comes in a variety of edited forms (up to 24 possible variants in the human brain) that may be linked to disease (e.g., depression, suicide, impulsivity, addiction, spinal cord injury, and obesity as induced by some antipsychotic drugs). The edited forms of the receptor can display different coupling ability, lower affinity for agonists, and lower constitutive activity compared with the nonedited isoform that can couple to multiple G proteins. In addition, both agonists (lorcaserin) and antagonists (agomelatine) have been/are being developed to treat obesity/schizophrenia/addiction and depression/generalized anxiety disorder, respectively. Interestingly, in spinal cord injury, the 5-HT2C receptor is less edited and expression is increased, resulting in constitutive activity and muscle spasms in the absence of any endogenous 5-HT, which can be reversed by inverse 5-HT2C receptor antagonists. The 5-HT2A receptor may be less investigated, as early claims that linked 5-HT2A to hypertension or sleep did not result in clinical success. However, it should be kept in mind that all three, 5-HT2A, 5-HT2B, and 5-HT1B, receptors play a role in pulmonary hypertension, a disease with unmet need for treatment, as neither endothelin antagonists nor PDE inhibitors are highly effective, although commonly prescribed. On another note, 5-HT2B receptors appear to play a significant role in fibrosis, be it in the lung, liver, and especially the heart. 5-HT2A receptor antagonism is a major component of second-generation antipsychotics (in combination with dopamine D2 receptor antagonism and often many other pharmacological activities, e.g., blonanserin), and there is evidence that a 5-HT2A receptor antagonist may show efficacy in certain subpopulations of schizophrenic patients. It may also be relevant that the 5-HT–glutamate link in schizophrenia may be related to the existence of heterodimers between 5-HT2A and mGlu2 receptors, although in vivo proof of concept remains to be established. Along these lines, there is evidence that 5-HT2A receptors are able to form homodimers, similarly to 5-HT1B or 5-HT1D receptors; however, the latter two may also form heterodimers, which may explain the codistribution that has been consistently observed for these two receptors. The 5-HT2C receptor is also capable of dimerization in vitro; it may even form tetra or octamers, and it may form heteromers with the ghrelin or the melatonin 2 receptor.
Clearly, receptor homo- and heterodimerization is an ongoing subject of interest with respect to 5-HT receptors. In addition to information incorporated within relevant sections of this review, the reader is also directed to specialized papers on this subject (e.g., Lee et al., 2000; Herrick-Davis, 2013; Moreno et al., 2016; Moutkine et al., 2017; Maroteaux et al., 2019).
In the 5-HT3 receptor field, similarly to what has been long known and accepted for other members of the Cys-loop ligand-gated ion channel superfamily (e.g., nicotine acetylcholine receptors), homomeric and heteromeric 5-HT3 receptors are evident, and new receptor molecular isoforms are still being investigated. Clinically, alosetron has been developed for the treatment of IBS with diarrhea, in contrast to 5-HT4 receptor agonists, for which the primary indication remains IBS with constipation or primary constipation in spite of the withdrawal of tegaserod from most markets. Obviously, the antiemetic effects of 5-HT3 receptor antagonists, whether in the short term or in the longer term following chemotherapy or possibly surgically induced, are still a very salient feature of these drugs, even if, in the meantime, combination therapy (with, e.g., NK1 receptor antagonists) becomes the accepted treatment. There has been a lot of research dealing with 5-HT6 receptor antagonists in memory/dementia (e.g., idalopirdine), although the phase III clinical data has been disappointing, whereas the 5-HT7 receptor is somewhat less actively investigated as a clinical target. The mystery of the putative 5-HT1P receptor in the gastrointestinal tract remains, although heterodimerization remains a favored potential explanation, albeit without clear direct evidence.
The more troubling news is that whereas receptors used to be defined based on structural, operational, and transductional features, it becomes clear that some refinements are needed:
In terms of structural knowledge, progress has been relatively slow. X-ray diffraction data from crystals exist for the 5-HT3 receptor and also, for example, the 5-HT1B and 5-HT2B receptors. One of the limitations of this approach for GPCRs is that the conformation of the ligand-receptor complex is very much dependent on the G protein heterotrimer associated with the receptor. In more recent years, we have learned that many receptors are able to couple to various pathways and even are able to signal in the absence of G proteins. In addition, there are multiple GPCR-interacting proteins that may affect both signal transduction and receptor-ligand conformation, thus the pharmacological signature.
The operational definition of a receptor (pharmacological profile based on rank orders of affinity of agonists and antagonists), which was the primary feature of receptors up to the 1990s, is now recognized as increasingly complex because of the dependence on the G protein associated with the receptor under study and other GPCR-interacting proteins. Indeed, a number of drugs will have different affinities and potencies depending on the transduction system studied. Such variations may seem artificial and linked to the expression of receptors and transduction components in engineered cells, but we know that an endogenously expressed receptor can react very differently to a given ligand in a cell- and system-dependent manner (e.g., 5-HT4 receptors in the GIT). Thus, the concept of functional selectivity or biased agonism/antagonism is a reality (see so-called β-blockers, which have rather different clinical features depending on their signaling characteristics, e.g., carvedilol compared with propranolol, pindolol, and nadolol). In contrast to other receptors, such as muscarinic or GABA receptors, there is little research addressing allosteric modulation for 5-HT receptors, with the possible exception of the 5-HT3 receptor. However, it becomes clear from X-ray structural studies performed with some ergolines that in addition to the classic orthosteric binding pocket, some 5-HT receptors have an extended binding “site,” which is very reminiscent of that described for muscarinic allosteric ligands. Such molecular targets may offer attractive strategies for novel therapeutics.
The relatively recently recognized importance of the microbiome continues to grow (Gilbert et al., 2018), and one aspect of this relevant to the present paper is the regulation of 5-HT receptors by bacteria-derived metabolites (e.g., Yano et al., 2015; Bhattarai et al., 2017; Cohen et al., 2017); understanding the “physiologic” and “pathologic” consequences of such modulation will no doubt provide impetus for developing therapeutic strategies harnessing these mechanisms.
In conclusion, it is a clear understatement to say a lot has been learned since 1994, but still some old questions remain unanswered, and many new questions will keep 5-HT researchers busy for years to come. Although 5-HT / serotonin / enteramine was only discovered and characterized a little more than 70-80 years ago (Viali and Erspamer, 1933; Rapport et al., 1947, 1948; Erspamer and Asero, 1952; Twarog and Page, 1953), 5-HT, its receptors, enzymes, transporters and multiple accessory proteins, constitute one of the oldest transmitter systems, estimated to have originated about 800 million years ago; hence it had time to develop a certain level of complexity. The appreciated complexity of the 5-HT system relates less to the number of 5-HT receptor classes, families, and subtypes, which was, per se, a conceptual challenge for some researchers in the 1980s and 1990s, than to the actual complex nature of the ligand/receptor/G protein/arrestin and/or other interacting protein complexes that vary according to species, organ, cells, gender, and disease state. This intricacy renders the pharmacological definition of a given receptor, or a ligand, challenging, as it may all depend on the nature and environment of the supramolecular complex, which is being specifically addressed.
Acknowledgments
We thank Dr. Michael Klein for contributing to the section concerning the 5-ht1e receptor. We thank Gail Green, personal assistant to N.M.B., for help and support. R.M.H. and K.A.C. extend thanks to Marcy Bubar Jordan for her editorial assistance.
Abbreviations
- AA
arachidonic acid
- AC
adenylyl cyclase
- AChE
Acetylcholinesterase
- AD
Alzheimer disease
- ADAR
RNA-specific adenosine deaminase
- ADHD
attention deficiency hyperactivity disorder
- AIM
abnormal involuntary movement
- AIWG
antipsychotic-induced weight gain
- AngII
Angiotensin II
- AP-1
activator protein 1
- AP-2
activator protein 2
- AP-MS
affinity purification-mass spectrometry
- APP
amyloid precursor protein
- ARF1
ADP-ribosylation factor 1
- ASD
autism spectrum disorder
- AT1
angiotensin II receptor type 1
- Aβ
amyloid-β
- BAC
bacterial artificial chromosome
- BDNF
brain-derived neutrophic factor
- BNP
brain natriuretic peptide
- BPAD
bipolar affective disorder
- BSS
behavioral satiety sequence
- CCI
chronic constriction injury
- CD
cluster of differentiation
- CDK5
cyclin-dependent kinase 5
- cGMP
cyclic guanosine monophosphate
- CGRP
calcitonin gene-related peptide
- CHO
Chinese Hamster Ovary
- CIPP
channel-interacting PDZ protein
- cNOS
constitutive nitric-oxide synthase
- CNS
central nervous system
- CpG
methyl-cytosine-phosphate-guanine
- CRMP
collapsing response mediator protein
- 5-CT
5-carboxamidotryptamine
- CXCL
chemokine ligand
- CYP
cyanopindolol
- DA
dopamine
- DAG
diacylglycerol
- DARPP32
dopamine and cAMP regulated phosphoprotein
- DHE
dihydroergotamine
- DOB
2,5-Dimethoxy-4-bromoamphetamine
- DOCA
deoxycorticosterone
- DOI
2,5-Dimethoxy-4-iodoamphetamine
- DRG
dorsal root ganglion
- DRN
dorsal raphe nucleus
- EB
embryoid body
- EC
enterochromaffin cells
- ECD
extracellular domain
- ECL2
extracellular loop 2
- EEG
electroencephalography
- ENS
enteric nervous system
- EPS
extrapyramidal side effects
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- E3
ubiquitine E3 ligase
- FDA
Food and Drug Administration
- Fmr1
fragile X mental retardation 1
- FST
forced swim test
- FXS
fragile X syndrome
- GASPs
GPCR-associated sorting proteins
- GEF
guanine nucleotide exchange factor
- GH
growth hormone
- GI
gastrointestinal
- GIP
glucose-dependent insulinotropic peptide
- GIP
GPCR interacting protein
- GIRK
G protein–coupled inwardly rectifying potassium channel
- GLIC
gloeobacter ligand-gated ion channel
- gp5-ht1e
guinea pig 5-ht1e receptor
- GPCR
G protein–coupled receptor
- GR
glucocorticoid receptor
- GRK
G protein–coupled receptor kinase
- GSK3
glycogen synthase kinase-3
- HB-EGF
heparin-binding EGF-like growth factor
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- 5-HIAA
5-hydroxyindole acetic acid
- HSC
hepatic stellate cells
- 5-HT
5-hydroxytryptamine
- 5-HTBP
5-HT binding protein
- IBS
irritable bowel syndrome
- IBS-C
IBS with constipation
- IBS-D
IBS with diarrhea
- ICC
interstitial cells of Cajal
- ICD
intracellular domain
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- IP3
inositol-1,4,5-triphosphate
- ISHH
in situ hybridization histochemistry
- IUPHAR
International Union of Basic and Clinical Pharmacology
- Jab
Jun activation domain–binding protein
- JAK
Janus kinase
- JCV
John Cunningham virus/polyomavirus
- KO
knockout
- L-DOPA
levodopa
- LID
L-DOPA–induced dyskinesia
- LNX
ligand of numb protein X
- LPS
lipopolysaccharide
- LSD
lysergic acid diethylamide
- LTD
long-term depression
- M1
proinflammatory macrophgase
- M2
anti-inflammatory macrophage
- MAGI2
membrane-associated guanylate kinase with inverted domain structure 2
- Man
mannoses
- MAP
microtubule-associated protein
- MAP1B-LC1
light chain 1 subunit of MAP1B protein
- MAPK
mitogen-activated protein kinase
- mCPP
M-chlorophenylpiperazine
- MDA
3,4-methylene dioxyamphetamine
- MDMA
3,4-methylenedioxy methamphetamine
- MeCP2
methyl-CpG-binding protein 2
- miRNA
microRNA
- MK
megakaryocyte
- MMP
matrix metalloproteinase
- mPFC
medial prefrontal cortex
- MPP
MAGUK p55 subfamily member
- MPP3
MAGUK p55 subfamily member 3
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR complex 1
- MUPP1
multi-PDZ domain protein 1
- NAc
nucleus accumbens
- ND2
adaptor protein NADH dehydrogenase subunit 2
- NF-κB
nuclear factor-κB
- NHE-1
type 1 sodium-proton exchanger
- NHERF
Na+/H+ exchanger regulatory factor
- NK
neurokinin
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- NOX
NADPH oxidase
- NR1
NMDA receptor subunit 1
- NREM
non–rapid eye movement sleep
- OCD
obsessive-compulsive disorder
- 8-OH DPAT
(±)-8-Hydroxy-2-dipropylaminotetralin
- P2
purinergic 2 receptors
- PAH
pulmonary arterial hypertension
- PAM
positive allosteric modulator
- PCP
phencyclidine
- PCR
polymerase chain reaction
- PD
Parkinson disease
- PDE
phosphodiesterase
- PDGFR
platelet-derived growth factor receptor
- PDZ 10
MUPP1 PDZ domain 10
- PDZ
PSD-95/Disc large/Zonula occludens
- pEC50
negative log of 50% effective concentration
- PET
positron emission tomography
- PFC
prefrontal cortex
- PI3K
phosphatidylinositol-3 kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLA2
phospholipase A2
- PLAC-24
protein that localizes at cell-cell contacts
- PLC
phospholipase C
- PLD
phospholipase D
- POMC
pro-opiomelanocortin
- POV
postoperative vomiting
- PPAR
peroxisome proliferator–activated receptors
- PPI, PR
progressive ratio
- PSD
postsynaptic marker
- PSD-95
postsynaptic density-95
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PX
phox homology
- qPCR
quantitative polymerase chain reaction
- REM
rapid eye movement sleep
- Rheb
Ras homolog enriched in brain
- RGS
regulator of G protein signaling
- ROS
reactive oxygen species
- RSK2
P90 ribosomal S6 kinase 2
- RT-PCR
reverse transcriptase polymerase chain reaction
- RTT
Rett syndrome
- SAP97
synapse-associated protein 97
- sAPP
soluble amyloid precursor protein
- SAR
structure activity relationship
- SCFAs
short-chain fatty acids
- SERT
serotonin transporter
- Sf9
spodoptera frugiperda 9 cells
- siRNA
small-interfering RNA
- SN
substantia nigra
- snoRNA
small nucleolar RNA
- SNP
single-nucleotide polymorphism
- SNX
sorting nexin family member
- SRE
serum response element
- SRI
serotonin reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- STAT
signal transducer and activator of transcription
- SW
slow wave
- TASK-1
acid-sensitive potassium channel protein-1
- TG
tissue transglutaminase
- TGF-β1
transforming growth factor beta 1
- Th
T-helper cells
- THC
Δ9-tetrahydrocannabinol
- TLR
Toll-like receptors
- TMD
transmembrane domain
- TNAP
tissue nonspecific alkaline phosphatase
- TNF
tumor necrosis factor
- TPH
tryptophan hydroxylase
- TSC
tuberous sclerosis
- TST
tail suspension test
- VTA
ventral tegmental area
- WAVE-1
Wiskott-Aldrich syndrome protein family verprolin homologous protein 1
- WT
wild type
- Yif1B
Yip1 interacting factor homolog B
- 2Cfl
full length 5-HT2C receptor
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Barnes, Ahern, Becamel, Bockaert, Camilleri, Chaumont-Dubel, Claeysen, Cunningham, Fone, Gershon, Di Giovanni, Goodfellow, Halberstadt, Hartley, Hassaine, Herrick-Davis, Hovius, Lacivita, Lambe, Leopoldo, Levy, Lummis, Marin, Maroteaux, McCreary, Nelson, Neumaier, Newman-Tancredi, Nury, Roberts, Roth, Roumier, Sanger, Teitler, Sharp, Villalón, Vogel, Watts, Hoyer.
Footnotes
This work was supported by Ministry of Defence [JFC7a/00018], the University of Birmingham and Celentyx Ltd. Centre national de la recherche scientifique (France), the Institut national de la santé et de la recherche médicale (France), University of Montpellier, Fondation pour la Recherche Médicale [Grant DPA20140629800] and l’Agence nationale de la recherche (France) [Grants ANR-11-BSV4-008-01 “SERO6COGNET,” ANR-12-BSV4-0008-01 “ADAMGUARD” and ANR-17-CE16-0013-01 “StopSero6TOR,” ANR-17-CE16-0010 “Sero6Dev”], France Alzheimer, Languedoc Roussillon region, Fondation Plan Alzheimer and Fondation Vaincre Alzheimer [Grant 12721 and LECMA FR-15072], National Institutes of Health National Institute on Drug Abuse [Grants T32 DA007287, P50 DA033935, and K05 DA020087], Banting and Best Canada Graduate Scholarship from the Canadian Institutes of Health Research, South-Eastern Norway Regional Health Authority, The Norwegian Council on Cardiovascular Diseases, The Research Council of Norway, The Kristian Gerhard Jebsen Foundation, Anders Jahre’s Foundation for the Promotion of Science, The Family Blix foundation, The Simon Fougner Hartmann Family Foundation and the University of Oslo, the European Research Council [Grant ERC-2014-StG PentaBrain], Consejo Nacional de Ciencia y Tecnología [Grant CONACyT, No. 219707], University of Melbourne, Hallmark initiative, National Health and Medical Research Council (Australia) [Grants APP1105284 and APP1105332], Alzheimer’s Association, and Royal Australian College of General Practitioners Ltd foundation (Australia).
References
- Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. (2009a) Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl) 205:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AI, Urban DJ, Jensen NH, Farrell MS, Kroeze WK, Mieczkowski P, Wang Z, Roth BL. (2010) Assessing serotonin receptor mRNA editing frequency by a novel ultra high-throughput sequencing method. Nucleic Acids Res 38:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. (2009b) PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci 29:7124–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Bissonnette JM, Newman-Tancredi A. (2014a) Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: therapeutic perspectives for 5-HT1A agonists. Front Physiol 5:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. (2010) Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 107:18208–18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Lioy DT, Garg SK, Knopp SJ, Paton JF, Bissonnette JM. (2014b) Effect of Sarizotan, a 5-HT1a and D2-like receptor agonist, on respiration in three mouse models of Rett syndrome. Am J Respir Cell Mol Biol 50:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdouh M, Storring JM, Riad M, Paquette Y, Albert PR, Drobetsky E, Kouassi E. (2001) Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem 276:4382–4388. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Neill JC, Reynolds GP. (2007) Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol 21:198–205. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Reynolds GP, Neill JC. (2006) The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res 169:263–273. [DOI] [PubMed] [Google Scholar]
- Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. (1995) Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology 34:1635–1645. [DOI] [PubMed] [Google Scholar]
- Abramowski D, Staufenbiel M. (1995) Identification of the 5-hydroxytryptamine2C receptor as a 60-kDa N-glycosylated protein in choroid plexus and hippocampus. J Neurochem 65:782–790. [DOI] [PubMed] [Google Scholar]
- Adam K, Oswald I. (1989) Effects of repeated ritanserin on middle-aged poor sleepers. Psychopharmacology (Berl) 99:219–221. [DOI] [PubMed] [Google Scholar]
- Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. (2003) The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Calpha. Biochim Biophys Acta 1640:85–96. [DOI] [PubMed] [Google Scholar]
- Adell A, Artigas F. (1998) A microdialysis study of the in vivo release of 5-HT in the median raphe nucleus of the rat. Br J Pharmacol 125:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham N, Bard JA, Zgombick JM, Durkin MM, Kucharewicz S, Weinshank RL, Branchek TA. (1997) Cloning and characterization of the guinea pig 5-HT1F receptor subtype: a comparison of the pharmacological profile to the human species homolog. Neuropharmacology 36:569–576. [DOI] [PubMed] [Google Scholar]
- Adham N, Borden LA, Schechter LE, Gustafson EL, Cochran TL, Vaysse PJ, Weinshank RL, Branchek TA. (1993a) Cell-specific coupling of the cloned human 5-HT1F receptor to multiple signal transduction pathways. Naunyn Schmiedebergs Arch Pharmacol 348:566–575. [DOI] [PubMed] [Google Scholar]
- Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, Durkin M, Hartig PR, Weinshank RL, Branchek TA. (1993b) Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci USA 90:408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham N, Romanienko P, Hartig P, Weinshank RL, Branchek T. (1992) The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol 41:1–7. [PubMed] [Google Scholar]
- Adham N, Tamm JA, Salon JA, Vaysse PJ, Weinshank RL, Branchek TA. (1994a) A single point mutation increases the affinity of serotonin 5-HT1D alpha, 5-HT1D beta, 5-HT1E and 5-HT1F receptors for beta-adrenergic antagonists. Neuropharmacology 33:387–391. [DOI] [PubMed] [Google Scholar]
- Adham N, Vaysse PJ, Weinshank RL, Branchek TA. (1994b) The cloned human 5-HT1E receptor couples to inhibition and activation of adenylyl cyclase via two distinct pathways in transfected BS-C-1 cells. Neuropharmacology 33:403–410. [DOI] [PubMed] [Google Scholar]
- Adham N, Zgombick JM, Bard J, Branchek TA. (1998) Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J Pharmacol Exp Ther 287:508–514. [PubMed] [Google Scholar]
- Adriani W, Travaglini D, Lacivita E, Saso L, Leopoldo M, Laviola G. (2012) Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology 62:833–842. [DOI] [PubMed] [Google Scholar]
- Afzal F, Andressen KW, Mørk HK, Aronsen JM, Sjaastad I, Dahl CP, Skomedal T, Levy FO, Osnes JB, Qvigstad E. (2008) 5-HT4-elicited positive inotropic response is mediated by cAMP and regulated by PDE3 in failing rat and human cardiac ventricles. Br J Pharmacol 155:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. (1997) Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36:589–599. [DOI] [PubMed] [Google Scholar]
- Ahn SK, Khalmuratova R, Jeon SY, Kim JP, Park JJ, Hur DG, Balaban CD. (2009) Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci Lett 465:151–156. [DOI] [PubMed] [Google Scholar]
- Aijö T, Edelman SM, Lönnberg T, Larjo A, Kallionpää H, Tuomela S, Engström E, Lahesmaa R, Lähdesmäki H. (2012) An integrative computational systems biology approach identifies differentially regulated dynamic transcriptome signatures which drive the initiation of human T helper cell differentiation. BMC Genomics 13:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aira Z, Buesa I, García del Caño G, Bilbao J, Doñate F, Zimmermann M, Azkue JJ. (2013) Transient, 5-HT2B receptor-mediated facilitation in neuropathic pain: up-regulation of PKCγ and engagement of the NMDA receptor in dorsal horn neurons. Pain 154:1865–1877. [DOI] [PubMed] [Google Scholar]
- Aira Z, Buesa I, García del Caño G, Salgueiro M, Mendiable N, Mingo J, Aguilera L, Bilbao J, Azkue JJ. (2012) Selective impairment of spinal mu-opioid receptor mechanism by plasticity of serotonergic facilitation mediated by 5-HT2A and 5-HT2B receptors. Pain 153:1418–1425. [DOI] [PubMed] [Google Scholar]
- Aira Z, Buesa I, Rada D, Gómez-Esteban JC, Azkue JJ. (2014) Coupling of serotonergic input to NMDA receptor-phosphorylation following peripheral nerve injury via rapid, synaptic up-regulation of ND2. Exp Neurol 255:86–95. [DOI] [PubMed] [Google Scholar]
- Aira Z, Buesa I, Salgueiro M, Bilbao J, Aguilera L, Zimmermann M, Azkue JJ. (2010) Subtype-specific changes in 5-HT receptor-mediated modulation of C fibre-evoked spinal field potentials are triggered by peripheral nerve injury. Neuroscience 168:831–841. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, et al. (2015) Short-chain fatty acid sensing in rat duodenum. J Physiol 593:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. (2009) The serotonin-1A receptor in anxiety disorders. Biol Psychiatry 66:627–635. [DOI] [PubMed] [Google Scholar]
- Akiyoshi T, Zhang Q, Inoue F, Aramaki O, Hatano M, Shimazu M, Kitajima M, Shirasugi N, Niimi M. (2006) Induction of indefinite survival of fully mismatched cardiac allografts and generation of regulatory cells by sarpogrelate hydrochloride. Transplantation 82:1051–1059. [DOI] [PubMed] [Google Scholar]
- Al Awabdh S, Miserey-Lenkei S, Bouceba T, Masson J, Kano F, Marinach-Patrice C, Hamon M, Emerit MB, Darmon M. (2012) A new vesicular scaffolding complex mediates the G-protein-coupled 5-HT1A receptor targeting to neuronal dendrites. J Neurosci 32:14227–14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR. (2012) Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci 367:2402–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Fiori LM. (2014) Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr Pharm Des 20:3738–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, François BL. (2010) Modifying 5-HT1A receptor gene expression as a new target for antidepressant therapy. Front Neurosci 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Vahid-Ansari F, Luckhart C. (2014) Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. (1990) Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem 265:5825–5832. [PubMed] [Google Scholar]
- Alberts GL, Chio CL, Im WB. (2001) Allosteric modulation of the human 5-HT(7A) receptor by lipidic amphipathic compounds. Mol Pharmacol 60:1349–1355. [DOI] [PubMed] [Google Scholar]
- Alberts GL, Pregenzer JF, Im WB, Zaworski PG, Gill GS. (1999) Agonist-induced GTPgamma35S binding mediated by human 5-HT(2C) receptors expressed in human embryonic kidney 293 cells. Eur J Pharmacol 383:311–319. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Pawson AJ, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators (2019) he Concise Guide To Pharmacology 2019/20: G protein-coupled receptors. Br J Pharmacol 176 (Suppl 1):S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, et al. CGTP Collaborators (2015a) The concise guide to pharmacology 2015/16: G protein-coupled receptors. Br J Pharmacol 172:5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, Buneman OP, et al. CGTP Collaborators (2015b) The concise guide to pharmacology 2015/16: overview. Br J Pharmacol 172:5729–5743.26650438 [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators (2015c) The concise guide to pharmacology 2015/16: ligand-gated ion channels. Br J Pharmacol 172:5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yadav PN, Roth BL. (2008) Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology 55:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, et al. (2011) Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA 108:18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Dremencov E, Ceci A, Flik G, Ferger B, Cremers TI, Ittrich C, Sommer B. (2010) Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: a microdialysis study. J Sex Med 7:1757–1767. [DOI] [PubMed] [Google Scholar]
- Almeida-Santos AF, Ferreira RC, Duarte ID, Aguiar DC, Romero TR, Moreira FA. (2015) The antipsychotic aripiprazole induces antinociceptive effects: possible role of peripheral dopamine D2 and serotonin 5-HT1A receptors. Eur J Pharmacol 765:300–306. [DOI] [PubMed] [Google Scholar]
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. (2009) Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121:160–173. [DOI] [PubMed] [Google Scholar]
- Alper RH. (1990) Hemodynamic and renin responses to (+-)-DOI, a selective 5-HT2 receptor agonist, in conscious rats. Eur J Pharmacol 175:323–332. [DOI] [PubMed] [Google Scholar]
- Alterio J, Masson J, Diaz J, Chachlaki K, Salman H, Areias J, Al Awabdh S, Emerit MB, Darmon M. (2015) Yif1B is involved in the anterograde traffic pathway and the golgi architecture. Traffic 16:978–993. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15:589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Ariga H, Kurematsu A, Yamato S, Morioka S, Masaka A, Kanazawa M, Fukudo S. (2015) Effect of 5-hydroxytryptamine receptor 4 agonist mosapride on human gastric accommodation. Neurogastroenterol Motil 27:1303–1309. [DOI] [PubMed] [Google Scholar]
- Amaya-Castellanos E, Pineda-Farias JB, Castañeda-Corral G, Vidal-Cantú GC, Murbartián J, Rocha-González HI, Granados-Soto V. (2011) Blockade of 5-HT7 receptors reduces tactile allodynia in the rat. Pharmacol Biochem Behav 99:591–597. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association. [Google Scholar]
- Amireault P, Hatia S, Bayard E, Bernex F, Collet C, Callebert J, Launay J-M, Hermine O, Schneider E, Mallet J, et al. (2011) Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci USA 108:13141–13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlaiky N, Ramboz S, Boschert U, Plassat JL, Hen R. (1992) Isolation of a mouse “5HT1E-like” serotonin receptor expressed predominantly in hippocampus. J Biol Chem 267:19761–19764. [PubMed] [Google Scholar]
- Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. (2012) mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 153:830–838. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng Y, Bremer NM, Smith TD, Fox RG, Swinford SE, et al. (2013) Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci 33:1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. (2010) Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem 113:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG. (2014a) Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry 4:e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fink LH, Swinford-Jackson SE, Sears RM, DiLeone RJ, Rice KC, Moeller FG, Cunningham KA. (2015) Serotonin (5-HT) 5-HT2A receptor (5-HT2AR):5-HT2CR imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem Neurosci 6:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O’Neil RT, Fink LH, Li D, Green TA, et al. (2014b) Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology 39:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R. (2006) Electrophysiological properties of gαs-coupled 5-HT receptors (5-HT4, 5-HT6, 5-HT7), in The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics (Roth BL, ed) pp 481–494, Humana press, Totowa, NJ. [Google Scholar]
- Andrade R, Barnes NM, Baxter G, et al. (2019) 5-Hydroxytryptamine receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE. 2019: doi: 10.2218/gtopdb/F1/2019.4. [Google Scholar]
- Andrade R, Beck SG. (2010) Cellular effects of serotonin in the CNS, in Handbook of the Behavioral Neurobiology of Serotonin (Muller CP, Jacobs B. eds) pp 219–232, Elsevier Academic Press. [Google Scholar]
- Andrade R, Chaput Y. (1991) 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257:930–937. [PubMed] [Google Scholar]
- Andrés AM, de Hemptinne C, Bertranpetit J. (2007) Heterogeneous rate of protein evolution in serotonin genes. Mol Biol Evol 24:2707–2715. [DOI] [PubMed] [Google Scholar]
- Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. (2008) Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 6:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andressen KW, Manfra O, Brevik CH, Ulsund AH, Vanhoenacker P, Levy FO, Krobert KA. (2015) The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-HT7 receptors. Br J Pharmacol 172:3846–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andressen KW, Norum JH, Levy FO, Krobert KA. (2006) Activation of adenylyl cyclase by endogenous Gs-coupled receptors in human embryonic kidney 293 cells is attenuated by 5-HT7 receptor expression. Mol Pharmacol 69:207–215. [DOI] [PubMed] [Google Scholar]
- Andressen KW, Ulsund AH, Krobert KA, Lohse MJ, Bünemann M, Levy FO. (2018) Related GPCRs couple differently to Gs: preassociation between G protein and 5-HT7 serotonin receptor reveals movement of Gαs upon receptor activation. FASEB J 32:1059–1069. [DOI] [PubMed] [Google Scholar]
- Andrews N, O’Neill MF. (2011) It might be a big family but the pain remains-last chance saloon for selective 5-HT receptor ligands? Curr Opin Pharmacol 11:39–44. [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Sanger GJ. (2014) Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol 722:108–121. [DOI] [PubMed] [Google Scholar]
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L. (1995) cAMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci USA 92:6635–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansanay H, Sebben M, Bockaert J, Dumuis A. (1992) Characterization of homologous 5-hydroxytryptamine4 receptor desensitization in colliculi neurons. Mol Pharmacol 42:808–816. [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306:879–881. [DOI] [PubMed] [Google Scholar]
- Antonini A, Poewe W. (2007) Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson’s disease. Lancet Neurol 6:826–829. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Tecott LH. (1998) Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol 154:522–530. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40:399–412. [DOI] [PubMed] [Google Scholar]
- Arias B, Collier DA, Gastó C, Pintor L, Gutiérrez B, Vallès V, Fañanás L. (2001) Genetic variation in the 5-HT5A receptor gene in patients with bipolar disorder and major depression. Neurosci Lett 303:111–114. [DOI] [PubMed] [Google Scholar]
- Arolt V, Peters M, Erfurth A, Wiesmann M, Missler U, Rudolf S, Kirchner H, Rothermundt M. (2003) S100B and response to treatment in major depression: a pilot study. Eur Neuropsychopharmacol 13:235–239. [DOI] [PubMed] [Google Scholar]
- Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. (2005) Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod 20:852–863. [DOI] [PubMed] [Google Scholar]
- Artigas F, Adell A, Celada P. (2006) Pindolol augmentation of antidepressant response. Curr Drug Targets 7:139–147. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. (1996) Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 19:378–383. [DOI] [PubMed] [Google Scholar]
- Asada M, Ebihara S, Yamanda S, Niu K, Okazaki T, Sora I, Arai H. (2009) Depletion of serotonin and selective inhibition of 2B receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and extracellular signal-regulated kinase 1/2 phosphorylation. Neoplasia 11:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka N, Nagayasu K, Nishitani N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S. (2015) Olanzapine augments the effect of selective serotonin reuptake inhibitors by suppressing GABAergic inhibition via antagonism of 5-HT(6) receptors in the dorsal raphe nucleus. Neuropharmacology 95:261–268. [DOI] [PubMed] [Google Scholar]
- Assetta B, Maginnis MS, Gracia Ahufinger I, Haley SA, Gee GV, Nelson CD, O’Hara BA, Allen Ramdial SA, Atwood WJ. (2013) 5-HT2 receptors facilitate JC polyomavirus entry. J Virol 87:13490–13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. (2010) F15599, a highly selective post-synaptic 5-HT(1A) receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol 13:1285–1298. [DOI] [PubMed] [Google Scholar]
- Assié MB, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. (2006) Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol 149:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanes P, Lacivita E, Rodríguez J, Brea J, Burgueño J, Vela JM, Cadavid MI, Loza MI, Leopoldo M, Castro M. (2013) The arylpiperazine derivatives N-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide and N-benzyl-4-(2-diphenyl)-1-piperazinehexanamide exert a long-lasting inhibition of human serotonin 5-HT7 receptor binding and cAMP signaling. Pharmacol Res Perspect 1:e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert Y, Gustison ML, Gardner LA, Bohl MA, Lange JR, Allers KA, Sommer B, Datson NA, Abbott DH. (2012) Flibanserin and 8-OH-DPAT implicate serotonin in association between female marmoset monkey sexual behavior and changes in pair-bond quality. J Sex Med 9:694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair AL, Cathala A, Sarrazin F, Depoortère R, Piazza PV, Newman-Tancredi A, Spampinato U. (2010) The central serotonin 2B receptor: a new pharmacological target to modulate the mesoaccumbens dopaminergic pathway activity. J Neurochem 114:1323–1332. [DOI] [PubMed] [Google Scholar]
- Audia JE, Evrard DA, Murdoch GR, Droste JJ, Nissen JS, Schenck KW, Fludzinski P, Lucaites VL, Nelson DL, Cohen ML. (1996) Potent, selective tetrahydro-beta-carboline antagonists of the serotonin 2B (5HT2B) contractile receptor in the rat stomach fundus. J Med Chem 39:2773–2780. [DOI] [PubMed] [Google Scholar]
- Austgen JR, Dantzler HA, Barger BK, Kline DD. (2012) 5-hydroxytryptamine 2C receptors tonically augment synaptic currents in the nucleus tractus solitarii. J Neurophysiol 108:2292–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avesar D, Gulledge AT. (2012) Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Rojas SH, Velázquez-Lagunas I, Salinas-Abarca AB, Barragán-Iglesias P, Pineda-Farias JB, Granados-Soto V. (2015) Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res 1622:377–385. [DOI] [PubMed] [Google Scholar]
- Ayme-Dietrich E, Lawson R, Gasser B, Dallemand R, Bischoff N, Monassier L. (2012) Mitral bioprosthesis hypertrophic scaring and native aortic valve fibrosis during benfluorex therapy. Fundam Clin Pharmacol 26:215–218. [DOI] [PubMed] [Google Scholar]
- Ayme-Dietrich E, Marzak H, Lawson R, Mokni W, Wendling O, Combe R, Becker J, El Fertak L, Champy MF, Matz R, et al. (2015) Contribution of serotonin to cardiac remodeling associated with hypertensive diastolic ventricular dysfunction in rats. J Hypertens 33:2310–2321. [DOI] [PubMed] [Google Scholar]
- Azim S, Banday AR, Tabish M. (2012) Identification of alternatively spliced multiple transcripts of 5-hydroxytryptamine receptor in mouse. Brain Res Bull 87:250–258. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, Whitaker-Azmitia PM. (2011) Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology 60:1347–1354. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. (2003) The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res 959:58–67. [DOI] [PubMed] [Google Scholar]
- Bach T, Syversveen T, Kvingedal AM, Krobert KA, Brattelid T, Kaumann AJ, Levy FO. (2001) 5-HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch Pharmacol 363:146–160. [DOI] [PubMed] [Google Scholar]
- Backstrom JR, Price RD, Reasoner DT, Sanders-Bush E. (2000) Deletion of the serotonin 5-HT2C receptor PDZ recognition motif prevents receptor phosphorylation and delays resensitization of receptor responses. J Biol Chem 275:23620–23626. [DOI] [PubMed] [Google Scholar]
- Backstrom JR, Westphal RS, Canton H, Sanders-Bush E. (1995) Identification of rat serotonin 5-HT2C receptors as glycoproteins containing N-linked oligosaccharides. Brain Res Mol Brain Res 33:311–318. [DOI] [PubMed] [Google Scholar]
- Bacon WL, Beck SG. (2000) 5-Hydroxytryptamine(7) receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther 294:672–679. [PubMed] [Google Scholar]
- Baganz NL, Blakely RD. (2013) A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci 4:48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Kecskemeti V, Riba P, Jakus R. (2007) Serotonin and epilepsy. J Neurochem 100:857–873. [DOI] [PubMed] [Google Scholar]
- Bai C-F, Liu J-C, Zhao R, Cao W, Liu S-B, Zhang X-N, Guo H-J, Yang Q, Yi D-H, Zhao M-G. (2010) Role of 5-HT 2B receptors in cardiomyocyte apoptosis in noradrenaline-induced cardiomyopathy in rats. Clin Exp Pharmacol Physiol 37:e145–e151. [DOI] [PubMed] [Google Scholar]
- Bai F, Yin T, Johnstone EM, Su C, Varga G, Little SP, Nelson DL. (2004) Molecular cloning and pharmacological characterization of the guinea pig 5-HT1E receptor. Eur J Pharmacol 484:127–139. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Bloss CS, Frank GK, Price JC, Meltzer CC, Mathis CA, Geyer MA, Wagner A, Becker CR, Schork NJ, et al. (2011) 5-HT1A receptor binding is increased after recovery from bulimia nervosa compared to control women and is associated with behavioral inhibition in both groups. Int J Eat Disord 44:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, et al. (2007) Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry 61:1090–1099. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Kaye WH. (2011) Serotonin: imaging findings in eating disorders. Curr Top Behav Neurosci 6:59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LP, Nielsen MD, Impey S, Metcalf MA, Poser SW, Chan G, Obrietan K, Hamblin MW, Storm DR. (1998) Stimulation of type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases by the Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J Biol Chem 273:17469–17476. [DOI] [PubMed] [Google Scholar]
- Banas SM, Doly S, Boutourlinsky K, Diaz SL, Belmer A, Callebert J, Collet C, Launay J-M, Maroteaux L. (2011) Deconstructing antiobesity compound action: requirement of serotonin 5-HT2B receptors for dexfenfluramine anorectic effects. Neuropsychopharmacology 36:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes A, Florian JA, Watts SW. (1999) Mechanisms of 5-hydroxytryptamine(2A) receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J Pharmacol Exp Ther 291:1179–1187. [PubMed] [Google Scholar]
- Banes AB, Watts SW. (2002) Upregulation of arterial serotonin 1B and 2B receptors in deoxycorticosterone acetate-salt hypertension. Hypertension 39:394–398. [DOI] [PubMed] [Google Scholar]
- Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D, Marrero MB. (2005) Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin. Am J Physiol Cell Physiol 288:C805–C812. [DOI] [PubMed] [Google Scholar]
- Banes AKL, Watts SW. (2003) Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension. BMC Pharmacol 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. (2002) Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology 26:40–52. [DOI] [PubMed] [Google Scholar]
- Banner SE, Sanger GJ. (1995) Differences between 5-HT3 receptor antagonists in modulation of visceral hypersensitivity. Br J Pharmacol 114:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick RA, Montgomery AJ, Bench CJ, Choudhry T, Malek N, McKenna PJ, Quested DJ, Deakin JF, Grasby PM. (2004) A positron emission tomography study of the 5-HT1A receptor in schizophrenia and during clozapine treatment. J Psychopharmacol 18:346–354. [DOI] [PubMed] [Google Scholar]
- Baptista-Hon DT, Deeb TZ, Othman NA, Sharp D, Hales TG. (2012) The 5-HT3B subunit affects high-potency inhibition of 5-HT3 receptors by morphine. Br J Pharmacol 165:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay Z, Dickson L, Robertson DN, Johnson MS, Holland PJ, Rosie R, Sun L, Fleetwood-Walker S, Lutz EM, Mitchell R. (2011) 5-HT2A receptor signalling through phospholipase D1 associated with its C-terminal tail. Biochem J 436:651–660. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268:23422–23426. [PubMed] [Google Scholar]
- Bardin L, Kleven MS, Barret-Grévoz C, Depoortère R, Newman-Tancredi A. (2006) Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology 31:1869–1879. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Ge J, Jones WG, Naylor RJ, Rudd JA. (1990) Cisplatin induced emesis: preliminary results indicative of changes in plasma levels of 5-hydroxytryptamine. Br J Cancer 62:862–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, Peters JA. (2009) The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology 56:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152. [DOI] [PubMed] [Google Scholar]
- Barone P, Jordan D, Atger F, Kopp N, Fillion G. (1994) Quantitative autoradiography of 5-HT1D and 5-HT1E binding sites labelled by [3H]5-HT, in frontal cortex and the hippocampal region of the human brain. Brain Res 638:85–94. [DOI] [PubMed] [Google Scholar]
- Barr AJ, Manning DR. (1997) Agonist-independent activation of Gz by the 5-hydroxytryptamine1A receptor co-expressed in Spodoptera frugiperda cells. Distinguishing inverse agonists from neutral antagonists. J Biol Chem 272:32979–32987. [DOI] [PubMed] [Google Scholar]
- Barrera NP, Herbert P, Henderson RM, Martin IL, Edwardson JM. (2005) Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc Natl Acad Sci USA 102:12595–12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrondo S, Sallés J. (2009) Allosteric modulation of 5-HT(1A) receptors by zinc: binding studies. Neuropharmacology 56:455–462. [DOI] [PubMed] [Google Scholar]
- Barthet G, Carrat G, Cassier E, Barker B, Gaven F, Pillot M, Framery B, Pellissier LP, Augier J, Kang DS, et al. (2009) Beta-arrestin1 phosphorylation by GRK5 regulates G protein-independent 5-HT4 receptor signalling. EMBO J 28:2706–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Framery B, Gaven F, Pellissier L, Reiter E, Claeysen S, Bockaert J, Dumuis A. (2007) 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell 18:1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, Bockaert J, Dumuis A. (2005) Uncoupling and endocytosis of 5-hydroxytryptamine 4 receptors. Distinct molecular events with different GRK2 requirements. J Biol Chem 280:27924–27934. [DOI] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Rao S, Sansom MSP, Chakrapani S. (2018a) Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Samanta A, Molugu SK, Huang W, Fuente M, Hughes T, Taylor DJ, Nieman MT, Moiseenkova-Bell V, et al. (2018b) Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat Commun 9:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile VS, Masellis M, De Luca V, Meltzer HY, Kennedy JL. (2002) 759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet 360:1790–1791. [DOI] [PubMed] [Google Scholar]
- Basselin M, Ramadan E, Rapoport SI. (2012) Imaging brain signal transduction and metabolism via arachidonic and docosahexaenoic acid in animals and humans. Brain Res Bull 87:154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil AK, Taylor CM, Bolton VJ, Gray KM, Brown JD, Cutler L, Summerfield SG, Bruton G, Winchester WJ, Lee K, et al. (2009) Inhibition of colonic motility and defecation by RS-127445 suggests an involvement of the 5-HT2B receptor in rodent large bowel physiology. Br J Pharmacol 158:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Bitard J, Mouillet-Richard S, Locker M, Poliard A, Launay J-M, Kellermann O. (2010a) Serotonergic 5-HT(2B) receptor controls tissue-nonspecific alkaline phosphatase activity in osteoblasts via eicosanoids and phosphatidylinositol-specific phospholipase C. J Biol Chem 285:26066–26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. (2010b) miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329:1537–1541. [DOI] [PubMed] [Google Scholar]
- Bayer H, Müller T, Myrtek D, Sorichter S, Ziegenhagen M, Norgauer J, Zissel G, Idzko M. (2007) Serotoninergic receptors on human airway epithelial cells. Am J Respir Cell Mol Biol 36:85–93. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Armstrong SR, Shaw JP, Marquess D, Sandlund C, Smith JA, Taylor JA, Humphrey PP. (2008) The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol 378:139–147. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA. (2008) Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol 377:181–203. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA, Marquess D, Vickery RG, Armstrong SR, Pulido-Rios T, McCullough JL, Sandlund C, Richardson C, Mai N, et al. (2004) The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol 143:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécamel C, Alonso G, Galéotti N, Demey E, Jouin P, Ullmer C, Dumuis A, Bockaert J, Marin P. (2002) Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J 21:2332–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. (2001) Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem 276:12974–12982. [DOI] [PubMed] [Google Scholar]
- Bécamel C, Gavarini S, Chanrion B, Alonso G, Galéotti N, Dumuis A, Bockaert J, Marin P. (2004) The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem 279:20257–20266. [DOI] [PubMed] [Google Scholar]
- Bechara A. (2005) Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8:1458–1463. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Smith K, Gaughan S, Lucki I. (2008) Sucrose intake and fasting glucose levels in 5-HT(1A) and 5-HT(1B) receptor mutant mice. Physiol Behav 93:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Bolbos R, Costes N, Redouté J, Newman-Tancredi A, Zimmer L. (2016) Selective serotonin 5-HT1A receptor biased agonists elicitdistinct brain activation patterns: a pharmacoMRI study. Sci Rep 6:26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene DL, Brandt GS, Zhong W, Zacharias NM, Lester HA, Dougherty DA. (2002) Cation-pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochemistry 41:10262–10269. [DOI] [PubMed] [Google Scholar]
- Beer MS, Heald MA, McAllister G, Stanton JA. (1998) Pharmacological characterisation of a cloned dog 5-HT1B receptor cell line. Eur J Pharmacol 360: 117–121. [DOI] [PubMed] [Google Scholar]
- Beer MS, Stanton JA, Hawkins LM, Middlemiss DN. (1993) 5-Carboxamidotryptamine-insensitive 5-HT1-like receptors are concentrated in guinea pig but not rat, claustrum. Eur J Pharmacol 236:167–169. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. (2007) Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA 104:9870–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. (1995) Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. Mol Pharmacol 48:1054–1062. [PubMed] [Google Scholar]
- Belenky MA, Pickard GE. (2001) Subcellular distribution of 5-HT(1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J Comp Neurol 423:371–388. [DOI] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, Svarer C, Greve DN, Knudsen GM. (2017) A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci 37:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Hobson H. (1994) 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci Biobehav Rev 18:325–338. [DOI] [PubMed] [Google Scholar]
- Belmer A, Doly S, Setola V, Banas SM, Moutkine I, Boutourlinsky K, Kenakin T, Maroteaux L. (2014) Role of the N-terminal region in G protein-coupled receptor functions: negative modulation revealed by 5-HT2B receptor polymorphisms. Mol Pharmacol 85:127–138. [DOI] [PubMed] [Google Scholar]
- Bender E, Pindon A, van Oers I, Zhang YB, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W. (2000) Structure of the human serotonin 5-HT4 receptor gene and cloning of a novel 5-HT4 splice variant. J Neurochem 74:478–489. [DOI] [PubMed] [Google Scholar]
- Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. (2010) Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci 30:12138–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Lal H, Meyerson LR. (1990) The effects of 5-HT1B characterizing agents in the mouse elevated plus-maze. Life Sci 47:195–203. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Aghajanian GK. (1974) d-LSD binding to brain homogenates: possible relationship to serotonin receptors. Life Sci 15:1935–1944. [DOI] [PubMed] [Google Scholar]
- Bentley KR, Barnes JM. (1995) Therapeutic potential of 5-HT3 receptor antagonists in neuropsychiatric disorders. CNS Drugs 3:363–392. [Google Scholar]
- Bentley KR, Barnes NM. (1998) 5-hydroxytryptamine3 (5-HT3) receptor-mediated depolarisation of the rat isolated vagus nerve: modulation by trichloroethanol and related alcohols. Eur J Pharmacol 354:25–31. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. (2008) Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell 19:1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends AC, Luiten PG, Nyakas C. (2005) A review of the neuroprotective properties of the 5-HT1A receptor agonist repinotan HCl (BAYx3702) in ischemic stroke. CNS Drug Rev 11:379–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. (1990) Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br J Pharmacol 101:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP. (2006) Development of functionally selective agonists as novel therapeutic agents. Drug Discov Today Ther Strateg 3:421–428. [Google Scholar]
- Berg KA, Clarke WP. (2009) Functional selectivity at serotonin receptors, in Functional Selectivity of G Protein-Coupled Receptor Ligands (Neve KA. ed) pp 155–176, Humana Press, New York. [Google Scholar]
- Berg KA, Clarke WP, Cunningham KA, Spampinato U. (2008a) Fine-tuning serotonin2c receptor function in the brain: molecular and functional implications. Neuropharmacology 55:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. (1994) Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol 46:477–484. [PubMed] [Google Scholar]
- Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. (2001) RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br J Pharmacol 134:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Dunlop J, Sanchez T, Silva M, Clarke WP. (2008b) A conservative, single-amino acid substitution in the second cytoplasmic domain of the human Serotonin2C receptor alters both ligand-dependent and -independent receptor signaling. J Pharmacol Exp Ther 324:1084–1092. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Clarke WP. (1996) 5-hydroxytryptamine2C receptor activation inhibits 5-hydroxytryptamine1B-like receptor function via arachidonic acid metabolism. Mol Pharmacol 50:1017–1023. [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Clarke WP. (1998a) Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci 861:104–110. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. (1998b) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54:94–104. [PubMed] [Google Scholar]
- Bergen SS., Jr (1964) Appetite stimulating properties of cyproheptadine. Am J Dis Child 108:270–273. [DOI] [PubMed] [Google Scholar]
- Berger M, Tecott LH. (2006) Serotonin system gene knockouts: a story of mice with implications for man, in The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics (Roth BL. ed) pp 537–575, Humana Press, Totowa, NJ. [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK. (2013) Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis [published correction appears in J Clin Invest (2014) 124:1868]. J Clin Invest 123:5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. (2009) Role of serotonin 5-HT1A receptors in the antidepressant-like effect and the antinociceptive effect of venlafaxine in mice. Int J Neuropsychopharmacol 12:61–71. [DOI] [PubMed] [Google Scholar]
- Bersani G, Grispini A, Marini S, Pasini A, Valducci M, Ciani N. (1990) 5-HT2 antagonist ritanserin in neuroleptic-induced parkinsonism: a double-blind comparison with orphenadrine and placebo. Clin Neuropharmacol 13:500–506. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bertrand RL. (2010) Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci 153:47–57. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Hu X, Mach J, Bertrand RL. (2008) Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol 295:G1228–G1236. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WAA, Furness JB, Bornstein JC. (2000) The terminals of myenteric intrinsic primary afferent neurons of the Guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101:459–469. [DOI] [PubMed] [Google Scholar]
- Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, Robbins TW, Dalley JW, Everitt BJ, Belin D. (2013) Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology 38:1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, et al. (2010) A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature 468:1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Gerlach I, Moratalla R, Gross CE, Jork R. (2006) 5-HT1A receptor agonist-mediated protection from MPTP toxicity in mouse and macaque models of Parkinson’s disease. Neurobiol Dis 23:77–86. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. (2006) Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry 163:1580–1587. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Saxena PR, Sharma HS. (2002a) Molecular cloning and tissue distribution of mRNA encoding porcine 5-HT7 receptor and its comparison with the structure of other species. Mol Cell Biochem 238:81–88. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Sharma HS, Wurch T, Pauwels PJ, Saxena PR. (2002b) Molecular cloning and expression of the porcine trigeminal ganglion cDNA encoding a 5-ht(1F) receptor. Eur J Pharmacol 436:23–33. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. (2000) Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut 47:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin N, LaMantia AS, Lauder JM. (2004) Opposing regulation of cell proliferation by retinoic acid and the serotonin2B receptor in the mouse frontonasal mass. Anat Embryol (Berl) 208:135–143. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. (2004) Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279:34614–34623. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. (2001) The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276:8269–8277. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Dang H, Zhu QM, Schnegelsberg B, Rozengurt N, Cain G, Prantil R, Vorp DA, Guy N, Julius D, et al. (2004a) Uropathic observations in mice expressing a constitutively active point mutation in the 5-HT3A receptor subunit. J Neurosci 24:5537–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Sankar S, Panicker MM. (2010) Differences in the C-terminus contribute to variations in trafficking between rat and human 5-HT(2A) receptor isoforms: identification of a primate-specific tripeptide ASK motif that confers GRK-2 and beta arrestin-2 interactions. J Neurochem 112:723–732. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Schenck KW, Xu YC, Nisenbaum L, Galbreath E, Cohen ML. (2004b) 5-Hydroxytryptamine1B receptor-mediated contraction of rabbit saphenous vein and basilar artery: role of vascular endothelium. J Pharmacol Exp Ther 309:825–832. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. (2017) Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol 313:G80–G87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. (2001) Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology 57:1829–1834. [DOI] [PubMed] [Google Scholar]
- Bigford GE, Chaundry NS, Keane RW, Holohean AM. (2012) 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-D-Aspartate GLUN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J Biol Chem 287:11049–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Gyertyan I, Levay G. (1998) mCPP-induced anxiety in the light-dark box in rats--a new method for screening anxiolytic activity. Psychopharmacology (Berl) 136:291–298. [DOI] [PubMed] [Google Scholar]
- Billard T, Le Bars D, Zimmer L. (2014) PET radiotracers for molecular imaging of serotonin 5-HT1A receptors. Curr Med Chem 21:70–81. [DOI] [PubMed] [Google Scholar]
- Birkeland JA, Sjaastad I, Brattelid T, Qvigstad E, Moberg ER, Krobert KA, Bjørnerheim R, Skomedal T, Sejersted OM, Osnes JB, et al. (2007a) Effects of treatment with a 5-HT4 receptor antagonist in heart failure. Br J Pharmacol 150:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland JA, Swift F, Tovsrud N, Enger U, Lunde PK, Qvigstad E, Levy FO, Sejersted OM, Sjaastad I. (2007b) Serotonin increases L-type Ca2+ current and SR Ca2+ content through 5-HT4 receptors in failing rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 293:H2367–H2376. [DOI] [PubMed] [Google Scholar]
- Birkett JT, Arranz MJ, Munro J, Osbourn S, Kerwin RW, Collier DA. (2000) Association analysis of the 5-HT5A gene in depression, psychosis and antipsychotic response. Neuroreport 11:2017–2020. [DOI] [PubMed] [Google Scholar]
- Birnstiel S, Beck SG. (1995) Modulation of the 5-hydroxytryptamine4 receptor-mediated response by short-term and long-term administration of corticosterone in rat CA1 hippocampal pyramidal neurons. J Pharmacol Exp Ther 273:1132–1138. [PubMed] [Google Scholar]
- Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. (2009) Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 296:G685–G695. [DOI] [PubMed] [Google Scholar]
- Björnsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL. (2002) Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT(3) pathways. Am J Physiol Gastrointest Liver Physiol 283:G400–G407. [DOI] [PubMed] [Google Scholar]
- Björnsson ES, Chey WD, Ladabaum U, Woods ML, Hooper FG, Owyang C, Hasler WL. (1998) Differential 5-HT3 mediation of human gastrocolonic response and colonic peristaltic reflex. Am J Physiol 275:G498–G505. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Le Poul E, Parma J, Knoop C, Detheux M, Parmentier M, Vassart G, Abramowicz MJ. (2003) Serotonin 5-HT(2B) receptor loss of function mutation in a patient with fenfluramine-associated primary pulmonary hypertension. Cardiovasc Res 60:518–528. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Seaman B. (2003) 5-hydroxytryptamine(2A) receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 117:939–948. [DOI] [PubMed] [Google Scholar]
- Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. (2001) Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone 29:477–486. [DOI] [PubMed] [Google Scholar]
- Blomeley C, Bracci E. (2005) Excitatory effects of serotonin on rat striatal cholinergic interneurones. J Physiol 569:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeley CP, Bracci E. (2009) Serotonin excites fast-spiking interneurons in the striatum. Eur J Neurosci 29:1604–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O, Gastineau M, Dahmoune Y, Langlois M, Fischmeister R. (1998) Cloning, expression, and pharmacology of four human 5-hydroxytryptamine 4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J Neurochem 70:2252–2261. [DOI] [PubMed] [Google Scholar]
- Blondel O, Vandecasteele G, Gastineau M, Leclerc S, Dahmoune Y, Langlois M, Fischmeister R. (1997) Molecular and functional characterization of a 5-HT4 receptor cloned from human atrium. FEBS Lett 412:465–474. [DOI] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB. (2014) Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology (Berl) 231:4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell JE. (1999) The control of appetite: basic concepts and practical implications. Schweiz Med Wochenschr 129:182–188. [PubMed] [Google Scholar]
- Blundell JE, Lawton CL, Halford JC. (1995) Serotonin, eating behavior, and fat intake. Obes Res 3 (Suppl 4):471S–476S. [DOI] [PubMed] [Google Scholar]
- Bocchio M, Fucsina G, Oikonomidis L, McHugh SB, Bannerman DM, Sharp T, Capogna M. (2015) Increased serotonin transporter expression reduces fear and recruitment of parvalbumin interneurons of the amygdala. Neuropsychopharmacology 40:3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Chen CF, Pittman AR, Skinner CD, McCartney HJ, Jones K, Bochner BR, Stevenson RE, Schwartz CE. (2013) Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. (2006) Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326:553–572. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. (2004a) 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord 3:39–51. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. (2008) 5-HT(4) receptors: history, molecular pharmacology and brain functions. Neuropharmacology 55:922–931. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. (2011) 5-HT(4) receptors, a place in the sun: act two. Curr Opin Pharmacol 11:87–93. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Dumuis A, Marin P. (2010a) Classification and signaling characteristics of 5-HT receptors, in Handbook of the Behavioural Neurobiology of Serotonin (Muller CP, Jacobs B. eds) pp 103–122, Elesevier, London. [Google Scholar]
- Bockaert J, Dumuis A, Bouhelal R, Sebben M, Cory RN. (1987) Piperazine derivatives including the putative anxiolytic drugs, buspirone and ipsapirone, are agonists at 5-HT1A receptors negatively coupled with adenylate cyclase in hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol 335:588–592. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Dumuis A, Fagni L, Marin P. (2004b) GPCR-GIP networks: a first step in the discovery of new therapeutic drugs? Curr Opin Drug Discov Devel 7:649–657. [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A. (1997) 5-HT4 Receptors: An update, in Serotoninergic Neurons and 5-HT Receptors in the CNS, Handbook of Experimental Pharmacology, (Baumgarten HG, Göthert M. eds) pp 439–465, Spinger-Verlag, New York. [Google Scholar]
- Bockaert J, Marin P. (2015) mTOR in brain physiology and pathologies. Physiol Rev 95:1157–1187. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L. (2003) The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546:65–72. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, Bécamel C, Marin P, Fagni L. (2010b) GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu Rev Pharmacol Toxicol 50:89–109. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Sebben M, Dumuis A. (1990) Pharmacological characterization of 5-hydroxytryptamine4(5-HT4) receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: effect of substituted benzamide derivatives. Mol Pharmacol 37:408–411. [PubMed] [Google Scholar]
- Boddeke HWGM, Hoffman BJ, Palacios JM, Hoyer D. (1993) Calcineurin inhibits desensitization of cloned rat 5-HT1C receptors. Naunyn Schmiedebergs Arch Pharmacol 348:221–224. [DOI] [PubMed] [Google Scholar]
- Boddeke HW, Kalkman HO. (1990) Zacopride and BRL 24924 induce an increase in EEG-energy in rats. Br J Pharmacol 101:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Sikora L, Pandit TS, Lavrador K, Rao SP, Sriramarao P. (2004) Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol 173:3599–3603. [DOI] [PubMed] [Google Scholar]
- Boess FG, Lummis SC, Martin IL. (1992) Molecular properties of 5-hydroxytryptamine3 receptor-type binding sites purified from NG108-15 cells. J Neurochem 59:1692–1701. [DOI] [PubMed] [Google Scholar]
- Bombardi C, Di Giovanni G. (2013) Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: relevance to memory functions. Exp Brain Res 230:427–439. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Chu HM, Brennan TJ, Tecott LH. (2006) A null mutation of the serotonin 6 receptor alters acute responses to ethanol. Neuropsychopharmacology 31:1801–1813. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Aluisio L, Shoblock J, Boggs JD, Fraser IC, Lord B, Lovenberg TW, Galici R. (2011) Pharmacological blockade of serotonin 5-HT7 receptor reverses working memory deficits in rats by normalizing cortical glutamate neurotransmission. PLoS One 6:e20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Dugovic C, Kramer M, De Boer P, Singh J, Wilson S, Bertelsen K, Di J, Shelton J, Aluisio L, et al. (2012) Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther 342:429–440. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Guo H, Tian B, Liu X, Bittner A, Roland B, Salunga R, Ma XJ, Kamme F, Meurers B, et al. (2002a) Nuclei and subnuclei gene expression profiling in mammalian brain. Brain Res 943:38–47. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, Leysen JE. (2000) Mapping of serotonin 5-HT(4) receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse 36:35–46. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, Miller K, Atack J, Lovenberg TW, Dugovic C. (2007) Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther 321:690–698. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Kwok A, Chai W, Langlois X, Hen R, Stark K, Carruthers N, Lovenberg TW. (2002b) Reconsideration of 5-hydroxytryptamine (5-HT)(7) receptor distribution using [(3)H]5-carboxamidotryptamine and [(3)H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT(1A) knockout and 5-HT(1A/1B) double-knockout mice. J Pharmacol Exp Ther 302:240–248. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Schotte A, Cras P, Leysen JE. (1997) Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels 5:225–230. [PubMed] [Google Scholar]
- Bonhaus DW, Bach C, DeSouza A, Salazar FHR, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. (1995) The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol 115:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus DW, Flippin LA, Greenhouse RJ, Jaime S, Rocha C, Dawson M, Van Natta K, Chang LK, Pulido-Rios T, Webber A, et al. (1999) RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br J Pharmacol 127:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus DW, Loury DN, Jakeman LB, To Z, DeSouza A, Eglen RM, Wong EH. (1993) [3H]BIMU-1, a 5-hydroxytryptamine3 receptor ligand in NG-108 cells, selectively labels sigma-2 binding sites in guinea pig hippocampus. J Pharmacol Exp Ther 267:961–970. [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, et al. (1997) RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36:621–629. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. (1994) Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol 17:73–82. [DOI] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, Asan E. (2013) Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct 218:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Peng W, Hewlett W, Levitt P. (2006) Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 141:781–794. [DOI] [PubMed] [Google Scholar]
- Bonnycastle DD, Giarman NJ, Paasonen MK. (1957) Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br J Pharmacol Chemother 12:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. (2007) Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32:1840–1854. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Mitchell SN, Sharp T. (2006) Investigation of the SSRI augmentation properties of 5-HT(2) receptor antagonists using in vivo microdialysis. Neuropharmacology 50:726–732. [DOI] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. (1998) The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetised guinea-pigs. Neuropharmacology 37:243–250. [DOI] [PubMed] [Google Scholar]
- Borman RA, Burleigh DE. (1996) Human colonic mucosa possesses a mixed population of 5-HT receptors. Eur J Pharmacol 309:271–274. [DOI] [PubMed] [Google Scholar]
- Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. (2002) 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol 135:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornert O, Møller TC, Boeuf J, Candusso MP, Wagner R, Martinez KL, Simonin F. (2013) Identification of a novel protein-protein interaction motif mediating interaction of GPCR-associated sorting proteins with G protein-coupled receptors. PLoS One 8:e56336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, Fuxe K. (2010) Dopamine D2 and 5-hydroxytryptamine 5-HT(2A) receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun 401:605–610. [DOI] [PubMed] [Google Scholar]
- Borsini F, Bordi F, Poggi A, Di Matteo V. (2015) Effects of ST1936, a selective serotonin-6 agonist, on electrical activity of putative mesencephalic dopaminergic neurons in the rat brain. J Psychopharmacol 29:802–811. [DOI] [PubMed] [Google Scholar]
- Borsini F, Evans K, Jason K, Rohde F, Alexander B, Pollentier S. (2002) Pharmacology of flibanserin. CNS Drug Rev 8:117–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Masana M, Díaz-Mataix L, Cortés R, Scorza MC, Gingrich JA, Toth M, Artigas F. (2010) Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. Int J Neuropsychopharmacol 13:1299–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttner M, Barrenschee M, Hellwig I, Harde J, Egberts JH, Becker T, Zorenkov D, Wedel T. (2013) The enteric serotonergic system is altered in patients with diverticular disease. Gut 62:1753–1762. [DOI] [PubMed] [Google Scholar]
- Bou J, Domènech T, Gras J, Beleta J, Llenas J. (1997) Pharmacological profile of almotriptan, a novel antimigraine agent. Cephalalgia 17:421–422. [Google Scholar]
- Bouchelet I, Case B, Olivier A, Hamel E. (2000) No contractile effect for 5-HT1D and 5-HT1F receptor agonists in human and bovine cerebral arteries: similarity with human coronary artery. Br J Pharmacol 129:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchelet I, Cohen Z, Case B, Séguéla P, Hamel E. (1996) Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol Pharmacol 50:219–223. [PubMed] [Google Scholar]
- Bouhelal R, Smounya L, Bockaert J. (1988) 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra. Eur J Pharmacol 151:189–196. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortère R, Louis C, Perrault G, Griebel G, Soubrie P. (2004) SSR181507, a putative atypical antipsychotic with dopamine D2 antagonist and 5-HT1A agonist activities: improvement of social interaction deficits induced by phencyclidine in rats. Neuropharmacology 46:1121–1129. [DOI] [PubMed] [Google Scholar]
- Boulay D, Ho-Van S, Bergis O, Avenet P, Griebel G. (2013) Phencyclidine decreases tickling-induced 50-kHz ultrasound vocalizations in juvenile rats: a putative model of the negative symptoms of schizophrenia? Behav Pharmacol 24:543–551. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Segu L, Chauveau J, Morel A, Lanoir J, Delaage M. (1992) Biochemical and pharmacological characterization of serotonin-O-carboxymethylglycyl[125I]iodotyrosinamide, a new radioiodinated probe for 5-HT1B and 5-HT1D binding sites. J Neurochem 58:951–959. [DOI] [PubMed] [Google Scholar]
- Bourdon DM, Camden JM, Landon LA, Levy FO, Turner JT. (2000) Identification of the adenylyl cyclase-activating 5-hydroxytryptamine receptor subtypes expressed in the rat submandibular gland. Br J Pharmacol 130:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourson A, Borroni E, Austin RH, Monsma FJ, Jr, Sleight AJ. (1995) Determination of the role of the 5-ht6 receptor in the rat brain: a study using antisense oligonucleotides. J Pharmacol Exp Ther 274:173–180. [PubMed] [Google Scholar]
- Boyd GW, Doward AI, Kirkness EF, Millar NS, Connolly CN. (2003) Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem 278:27681–27687. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Lightning LK, Rao S, Palme M, Ellis D, Coleman R, Davies AM, Kumaraswamy P, Druzgala P. (2011) Metabolism and pharmacokinetics of naronapride (ATI-7505), a serotonin 5-HT(4) receptor agonist for gastrointestinal motility disorders. Drug Metab Dispos 39:1170–1180. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PPA, Middlemiss DN, Mylecharane EJ, Richardson BP, Saxena PR. (1986) Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology 25:563–576. [DOI] [PubMed] [Google Scholar]
- Brady CA, Dover TJ, Massoura AN, Princivalle AP, Hope AG, Barnes NM. (2007) Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharmacology 52:1284–1290. [DOI] [PubMed] [Google Scholar]
- Brady CA, Stanford IM, Ali I, Lin L, Williams JM, Dubin AE, Hope AG, Barnes NM. (2001) Pharmacological comparison of human homomeric 5-HT3A receptors versus heteromeric 5-HT3A/3B receptors. Neuropharmacology 41:282–284. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Vergé D. (2000) Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872:271–275. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Kvingedal AM, Krobert KA, Andressen KW, Bach T, Hystad ME, Kaumann AJ, Levy FO. (2004a) Cloning, pharmacological characterisation and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i). Naunyn Schmiedebergs Arch Pharmacol 369:616–628. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Qvigstad E, Birkeland JA, Swift F, Bekkevold SV, Krobert KA, Sejersted OM, Skomedal T, Osnes JB, Levy FO, et al. (2007a) Serotonin responsiveness through 5-HT2A and 5-HT4 receptors is differentially regulated in hypertrophic and failing rat cardiac ventricle. J Mol Cell Cardiol 43:767–779. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Qvigstad E, Lynham JA, Molenaar P, Aass H, Geiran O, Skomedal T, Osnes JB, Levy FO, Kaumann AJ. (2004b) Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn Schmiedebergs Arch Pharmacol 370:157–166. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Qvigstad E, Moltzau LR, Bekkevold SV, Sandnes DL, Birkeland JA, Skomedal T, Osnes JB, Sjaastad I, Levy FO. (2012) The cardiac ventricular 5-HT4 receptor is functional in late foetal development and is reactivated in heart failure. PLoS One 7:e45489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattelid T, Tveit K, Birkeland JA, Sjaastad I, Qvigstad E, Krobert KA, Hussain RI, Skomedal T, Osnes JB, Levy FO. (2007b) Expression of mRNA encoding G protein-coupled receptors involved in congestive heart failure--a quantitative RT-PCR study and the question of normalisation. Basic Res Cardiol 102:198–208. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Ejarque M, Zamanillo D, Vela JM, Romero L. (2011) Potentiation of morphine analgesia by adjuvant activation of 5-HT7 receptors. J Pharmacol Sci 116:388–391. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sánchez-Arroyos R, Portillo-Salido E, Pujol M, Montero A, Codony X, et al. (2010) Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 149:483–494. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Rocasalbas M, Zamanillo D, Hamon M, Vela JM, Romero L. (2012a) Assessment of 5-HT(7) receptor agonists selectivity using nociceptive and thermoregulation tests in knockout versus wild-type mice. Adv Pharmacol Sci 2012:312041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, et al. (2009) 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 141:239–247. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Zamanillo D, Hamon M, Romero L, Vela JM. (2012b) Role of peripheral versus spinal 5-HT(7) receptors in the modulation of pain undersensitizing conditions. Eur J Pain 16:72–81. [DOI] [PubMed] [Google Scholar]
- Briddon SJ, Leslie RA, Elliott JM. (1998) Comparative desensitization of the human 5-HT2A and 5-HT2C receptors expressed in the human neuroblastoma cell line SH-SY5Y. Br J Pharmacol 125:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briejer MR, Schuurkes JA, Sarna SK. (1999) Idiopathic constipation: too few stools and too little knowledge. Trends Pharmacol Sci 20:1–3. [DOI] [PubMed] [Google Scholar]
- Broad J, Góralczyk A, Mannur K, Dukes GE, Sanger GJ. (2014a) Drugs acting at 5-HT4, D2, motilin, and ghrelin receptors differ markedly in how they affect neuromuscular functions in human isolated stomach. Neurogastroenterol Motil 26:851–861. [DOI] [PubMed] [Google Scholar]
- Broad J, Hughes F, Chin-Aleong J, Sifrim D, Sanger GJ. (2014b) Regionally dependent neuromuscular functions of motilin and 5-HT4 receptors in human isolated esophageal body and gastric fundus. Neurogastroenterol Motil 26:1311–1322. [DOI] [PubMed] [Google Scholar]
- Broad J, Kung VWS, Boundouki G, Aziz Q, De Maeyer JH, Knowles CH, Sanger GJ. (2013) Cholinergic interactions between donepezil and prucalopride in human colon: potential to treat severe intestinal dysmotility. Br J Pharmacol 170:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad J, Mukherjee S, Samadi M, Martin JE, Dukes GE, Sanger GJ. (2012) Regional- and agonist-dependent facilitation of human neurogastrointestinal functions by motilin receptor agonists. Br J Pharmacol 167:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodney MA, Johnson DE, Sawant-Basak A, Coffman KJ, Drummond EM, Hudson EL, Fisher KE, Noguchi H, Waizumi N, McDowell LL, et al. (2012) Identification of multiple 5-HT4 partial agonist clinical candidates for the treatment of Alzheimer’s disease. J Med Chem 55:9240–9254. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Gibson AW, Smirnov D, Nair SG, Neumaier JF. (2016) Striatal 5-HT6 receptors regulate cocaine reinforcement in a pathway-selective manner. Neuropsychopharmacology 41:2377–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromidge SM, Brown AM, Clarke SE, Dodgson K, Gager T, Grassam HL, Jeffrey PM, Joiner GF, King FD, Middlemiss DN, et al. (1999) 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem 42:202–205. [DOI] [PubMed] [Google Scholar]
- Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM, Blaney FE, Naylor CB, Blackburn TP, Kennett GA, et al. (1997) 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem 40:3494–3496. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR, Wingate DL. (1987) Intracellular recordings from myenteric neurones in the human colon. J Physiol 390:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard JT, Schweimer JV, Houlton R, Burnham KE, Quérée P, Sharp T. (2015) Pharmacological evidence for 5-HT6 receptor modulation of 5-HT neuron firing in vivo. ACS Chem Neurosci 6:1241–1247. [DOI] [PubMed] [Google Scholar]
- Brown AM, Avenell K, Young TJ, Ho M, Porter RA, Vimal M. (1998a) BRL 54443, a potent agonist with selectivity for human cloned 5-Ht1E and 5-Ht1F receptors. Br J Pharmacol 123:233. [Google Scholar]
- Brown AM, Ho M, Thomas DR, Parsons AA. (1998c) Functional effects of frovatriptan (VML 251), sumatriptan and naratriptan on human recombinant 5-HT1 and 5-HT7 receptors. Headache 38:c376. [Google Scholar]
- Brown AM, Hope AG, Lambert JJ, Peters JA. (1998b) Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A). J Physiol 507:653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Patch TL, Kaumann AJ. (1991) The antimigraine drugs ergotamine and dihydroergotamine are potent 5-HT1C receptor agonists in piglet choroid plexus. Br J Pharmacol 104:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PM, Drossman DA, Wood AJ, Cline GA, Frazier KS, Jackson JI, Bronner J, Freiman J, Zambrowicz B, Sands A, et al. (2011) The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology 141:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield MS, Poff BC, Holzwarth MA. (1985) Ultrastructural immunocytochemical co-localization of serotonin and PNMT in adrenal medullary vesicles. Histochemistry 83:41–46. [DOI] [PubMed] [Google Scholar]
- Brudeli B, Andressen KW, Moltzau LR, Nilsen NO, Levy FO, Klaveness J. (2013a) Acidic biphenyl derivatives: synthesis and biological activity of a new series of potent 5-HT4 receptor antagonists. Bioorg Med Chem 21:7134–7145. [DOI] [PubMed] [Google Scholar]
- Brudeli B, Moltzau LR, Andressen KW, Krobert KA, Klaveness J, Levy FO. (2010) Synthesis and pharmacological properties of novel hydrophilic 5-HT4 receptor antagonists. Bioorg Med Chem 18:8600–8613. [DOI] [PubMed] [Google Scholar]
- Brudeli B, Moltzau LR, Nguyen CH, Andressen KW, Nilsen NO, Levy FO, Klaveness J. (2013b) Synthesis and pharmacological properties of a new hydrophilic and orally bioavailable 5-HT4 antagonist. Eur J Med Chem 64:629–637. [DOI] [PubMed] [Google Scholar]
- Brudeli B, Navaratnarajah M, Andressen KW, Manfra O, Moltzau LR, Nilsen NO, Levy FO, Klaveness J. (2014) Discovery and pharmacological profile of new hydrophilic 5-HT(4) receptor antagonists. Bioorg Med Chem Lett 24:4598–4602. [DOI] [PubMed] [Google Scholar]
- Bruheim S, Krobert KA, Andressen KW, Levy FO. (2003) Unaltered agonist potency upon inducible 5-HT7(a) but not 5-HT4(b) receptor expression indicates agonist-independent association of 5-HT7(a) receptor and Gs. Receptors Channels 9:107–116. [PubMed] [Google Scholar]
- Bruins Slot LA, Kleven MS, Newman-Tancredi A. (2005) Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology 49:996–1006. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. (1994a) Localization of 5-HT1B, 5-HT1D α, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33:367–386. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Moskowitz MA, Hoyer D. (1992a) Evidence for the presence of 5-HT1B receptor messenger RNA in neurons of the rat trigeminal ganglia. Eur J Pharmacol 227:357–359. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Probst A, Palacios JM, Hoyer D. (1994b) A comparative autoradiographic study of 5-HT1D binding sites in human and guinea-pig brain using different radioligands. Brain Res Mol Brain Res 21:19–29. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Waeber C, Palacios JM, Hoyer D. (1991) Homogeneous 5-HT1D recognition sites in the human substantia nigra identified with a new iodinated radioligand. Eur J Pharmacol 202:89–91. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Lery H, Nozulak J, Palacios JM, Hoyer D. (1992b) 5-HT1D binding sites in various species: similar pharmacological profile in dog, monkey, calf, guinea-pig and human brain membranes. Naunyn Schmiedebergs Arch Pharmacol 346:243–248. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. (1993a) Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol 347:569–582. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. (1993b) 5-hydroxytryptamine1 recognition sites in rat brain: heterogeneity of non-5-hydroxytryptamine1A/1C binding sites revealed by quantitative receptor autoradiography. Neuroscience 53:465–473. [DOI] [PubMed] [Google Scholar]
- Brüss M, Barann M, Hayer-Zillgen M, Eucker T, Göthert M, Bönisch H. (2000) Modified 5-HT3A receptor function by co-expression of alternatively spliced human 5-HT3A receptor isoforms. Naunyn Schmiedebergs Arch Pharmacol 362:392–401. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2006) Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem 6:1971–1985. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2007) Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res 172:319–346. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA. (2011) 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One 6:e20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan D, Muirhead K. (2007) Intractable nausea and vomiting successfully related with granisetron 5-hydroxytryptamine type 3 receptor antagonists in Palliative Medicine. Palliat Med 21:725–726. [DOI] [PubMed] [Google Scholar]
- Bucholtz EC, Brown RL, Tropsha A, Booth RG, Wyrick SD. (1999) Synthesis, evaluation, and comparative molecular field analysis of 1-phenyl-3-amino-1,2,3,4-tetrahydronaphthalenes as ligands for histamine H(1) receptors. J Med Chem 42:3041–3054. [DOI] [PubMed] [Google Scholar]
- Buckland PR, Hoogendoorn B, Guy CA, Smith SK, Coleman SL, O’Donovan MC. (2005) Low gene expression conferred by association of an allele of the 5-HT2C receptor gene with antipsychotic-induced weight gain. Am J Psychiatry 162:613–615. [DOI] [PubMed] [Google Scholar]
- Buhner S, Schemann M. (2012) Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta 1822:85–92. [DOI] [PubMed] [Google Scholar]
- Buhot MC, Wolff M, Savova M, Malleret G, Hen R, Segu L. (2003) Protective effect of 5-HT1B receptor gene deletion on the age-related decline in spatial learning abilities in mice. Behav Brain Res 142:135–142. [DOI] [PubMed] [Google Scholar]
- Buiter HJ, Windhorst AD, Huisman MC, De Maeyer JH, Schuurkes JA, Lammertsma AA, Leysen JE. (2013) Radiosynthesis and preclinical evaluation of [11C]prucalopride as a potential agonist PET ligand for the 5-HT4 receptor. EJNMMI Res 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Gershon MD. (1967) 5-hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J Physiol 192:823–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Lin RC. (1958) The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140:381–407. [PMC free article] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. (2005) Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 26:131–137. [DOI] [PubMed] [Google Scholar]
- Bundeff AW, Woodis CB. (2014) Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother 48:777–784. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. (2008) Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 196:15–27. [DOI] [PubMed] [Google Scholar]
- Buritova J, Berrichon G, Cathala C, Colpaert F, Cussac D. (2009) Region-specific changes in 5-HT1A agonist-induced extracellular signal-regulated kinases 1/2 phosphorylation in rat brain: a quantitative ELISA study. Neuropharmacology 56:350–361. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. (2009a) Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. (2009b) Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50 (Suppl):S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LK, Doslikova B, D’Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, et al. (2016) Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab 5:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet PW, Chen CP, McGowan S, Franklin M, Harrison PJ. (1996) The effects of clozapine and haloperidol on serotonin-1A, -2A and -2C receptor gene expression and serotonin metabolism in the rat forebrain. Neuroscience 73:531–540. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Lacey K, Harrison PJ. (1995) The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res 676:157–168. [DOI] [PubMed] [Google Scholar]
- Burnham KE, Baxter MG, Bainton JR, Southam E, Dawson LA, Bannerman DM, Sharp T. (2010) Activation of 5-HT(6) receptors facilitates attentional set shifting. Psychopharmacology (Berl) 208:13–21. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387:303–308. [DOI] [PubMed] [Google Scholar]
- Bush E, Fielitz J, Melvin L, Martinez-Arnold M, McKinsey TA, Plichta R, Olson EN. (2004) A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc Natl Acad Sci USA 101:2870–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. (2001) Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am J Physiol Gastrointest Liver Physiol 281:G974–G983. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Fergeson P, Hudson J, McKernin C. (1997) The meta-chlorophenylpiperazine challenge test in cocaine addicts: hormonal and psychological responses. Biol Psychiatry 41:1071–1086. [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Moskowitz MA. (1991) Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia 11:165–168. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Flores-Hernandez J, Feng J, Yan Z. (2002) Activity-dependent bidirectional regulation of GABA(A) receptor channels by the 5-HT(4) receptor-mediated signalling in rat prefrontal cortical pyramidal neurons. J Physiol 540:743–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé F, Morley TJ, Tavares AA, Papin C, Twardy NM, Alagille D, Lee HS, Baldwin RM, Seibyl JP, Barret O, et al. (2013) Synthesis and biological evaluation of positron emission tomography radiotracers targeting serotonin 4 receptors in brain: [18F]MNI-698 and [18F]MNI-699. Bioorg Med Chem Lett 23:6243–6247. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Szemeredi K, Tartaglia ME, Gold PW, Chrousos GP. (1990) Mechanisms of serotonin receptor agonist-induced activation of the hypothalamic-pituitary-adrenal axis in the rat. Endocrinology 126:1888–1894. [DOI] [PubMed] [Google Scholar]
- Camilleri M. (2011) LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil 23:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. (2014) Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 592:2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. (2002) Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology 123:425–432. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Vazquez-Roque MI, Burton D, Ford T, McKinzie S, Zinsmeister AR, Druzgala P. (2007) Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil 19:30–38. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Von der Ohe MR. (1994) Drugs affecting serotonin receptors. Baillieres Clin Gastroenterol 8:301–319. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. (2003) Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res 993:1–9. [DOI] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. (2013) Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Cordova-Sintjago TC, Villa NY, Fang LJ, Booth RG. (2011) Drug discovery targeting human 5-HT(2C) receptors: residues S3.36 and Y7.43 impact ligand-binding pocket structure via hydrogen bond formation. Eur J Pharmacol 673:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Felsing DE, Liu Y, Zhu W, Wood JT, Perry CK, Vemula R, Booth RG. (2015) An orally active phenylaminotetralin-chemotype serotonin 5-HT7 and 5-HT1A receptor partial agonist that corrects motor stereotypy in mouse models. ACS Chem Neurosci 6:1259–1270. [DOI] [PubMed] [Google Scholar]
- Candura SM, Messori E, Franceschetti GP, D’Agostino G, Vicini D, Tagliani M, Tonini M. (1996) Neural 5-HT4 receptors in the human isolated detrusor muscle: effects of indole, benzimidazolone and substituted benzamide agonists and antagonists. Br J Pharmacol 118:1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton H, Emeson RB, Barker EL, Backstrom JR, Lu JT, Chang MS, Sanders-Bush E. (1996) Identification, molecular cloning, and distribution of a short variant of the 5-hydroxytryptamine2C receptor produced by alternative splicing. Mol Pharmacol 50:799–807. [PubMed] [Google Scholar]
- Cao BJ, Rodgers RJ. (1997) Comparative behavioural profiles of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine (1-PP) in the murine elevated plus-maze. Neuropharmacology 36:1089–1097. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3:619–627. [DOI] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Rutherford A, Cullen P. (2005) Sorting nexins--unifying trends and new perspectives. Traffic 6:75–82. [DOI] [PubMed] [Google Scholar]
- Carmel L, Koonin EV, Dracheva S. (2012) Dependencies among editing sites in serotonin 2C receptor mRNA. PLOS Comput Biol 8:e1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. (2002) Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci 22:6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel D, Masson J, Al Awabdh S, Capra CB, Lenkei Z, Hamon M, Emerit MB, Darmon M. (2008) Targeting of the 5-HT1A serotonin receptor to neuronal dendrites is mediated by Yif1B. J Neurosci 28:8063–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Renoncourt Y, Gayet O, De Bovis B, Alonso S. (2001) Sorting nexin-14, a gene expressed in motoneurons trapped by an in vitro preselection method. Dev Dyn 221:431–442. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. (1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340. [PubMed] [Google Scholar]
- Cartier D, Jégou S, Parmentier F, Lihrmann I, Louiset E, Kuhn JM, Bastard C, Plouin PF, Godin M, Vaudry H, et al. (2005) Expression profile of serotonin4 (5-HT4) receptors in adrenocortical aldosterone-producing adenomas. Eur J Endocrinol 153:939–947. [DOI] [PubMed] [Google Scholar]
- Castillo M, Mulet J, Gutiérrez LM, Ortiz JA, Castelán F, Gerber S, Sala S, Sala F, Criado M. (2005) Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J Biol Chem 280:27062–27068. [DOI] [PubMed] [Google Scholar]
- Cates LN, Roberts AJ, Huitron-Resendiz S, Hedlund PB. (2013) Effects of lurasidone in behavioral models of depression. Role of the 5-HT7 receptor subtype. Neuropharmacology 70:211–217. [DOI] [PubMed] [Google Scholar]
- Cathala A, Devroye C, Maitre M, Piazza PV, Abrous DN, Revest JM, Spampinato U. (2015) Serotonin2C receptors modulate dopamine transmission in the nucleus accumbens independently of dopamine release: behavioral, neurochemical and molecular studies with cocaine. Addict Biol 20:445–457. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Cellek S, John AK, Thangiah R, Dass NB, Bassil AK, Jarvie EM, Lalude O, Vivekanandan S, Sanger GJ. (2006) 5-HT4 receptor agonists enhance both cholinergic and nitrergic activities in human isolated colon circular muscle. Neurogastroenterol Motil 18:853–861. [DOI] [PubMed] [Google Scholar]
- Centurión D, Ortiz MI, Saxena PR, Villalón CM. (2002) The atypical 5-HT2 receptor mediating tachycardia in pithed rats: pharmacological correlation with the 5-HT2A receptor subtype. Br J Pharmacol 135:1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurión D, Sánchez-López A, De Vries P, Saxena PR, Villalón CM. (2001) The GR127935 sensitive 5-HT(1) receptors mediating canine internal carotid vasoconstriction: resemblance of the 5-HT(1B) but not to the 5-HT (1D) or 5-ht(1F), receptor subtypes. Br. J Pharmacol 132:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Durán C, Vidal-Cantú GC, Barragán-Iglesias P, Pineda-Farias JB, Bravo-Hernández M, Murbartián J, Granados-Soto V. (2012) Role of peripheral and spinal 5-HT2B receptors in formalin-induced nociception. Pharmacol Biochem Behav 102:30–35. [DOI] [PubMed] [Google Scholar]
- Cettomai D, McArthur JC. (2009) Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol 66:255–258. [DOI] [PubMed] [Google Scholar]
- Chabbi-Achengli Y, Launay J-M, Maroteaux L, de Vernejoul MC, Collet C. (2013) Serotonin 2B receptor (5-HT2B R) signals through prostacyclin and PPAR-ß/δ in osteoblasts. PLoS One 8:e75783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Chan KY, de Vries R, van den Bogeardt AJ, de Maeyer JH, Schuurkes JA, Villalón CM, Saxena PR, Danser AH, MaassenVanDenBrink A. (2012) Inotropic effects of prokinetic agents with 5-HT(4) receptor agonist actions on human isolated myocardial trabeculae. Life Sci 90:538–544. [DOI] [PubMed] [Google Scholar]
- Chadha A, Sur C, Atack J, Duty S. (2000) The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br J Pharmacol 130:1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chen H, Chuang DM. (1999) 5-Hydroxytryptamine2A receptor stimulation induces activator protein-1 and cyclic AMP-responsive element binding with cyclic AMP-responsive element-binding protein and Jun D as common components in cerebellar neurons. Neuroscience 88:885–898. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. (1991) Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res 561:51–60. [DOI] [PubMed] [Google Scholar]
- Chamienia AL, Johns EJ. (1994) The renal functional responses to 5-HT1A receptor agonist, flesinoxan, in anaesthetized, normotensive rat. Br J Pharmacol 112:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Vermeersch S, de Hoon J, Villalón CM, Maassenvandenbrink A. (2011) Potential mechanisms of prospective antimigraine drugs: a focus on vascular (side) effects. Pharmacol Ther 129:332–351. [DOI] [PubMed] [Google Scholar]
- Chan SY, Loscalzo J. (2008) Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FW, Burke JA, Potter DE. (1985) Mechanism of the ocular hypotensive action of ketanserin. J Ocul Pharmacol 1:137–147. [DOI] [PubMed] [Google Scholar]
- Chang M, Zhang L, Tam JP, Sanders-Bush E. (2000) Dissecting G protein-coupled receptor signaling pathways with membrane-permeable blocking peptides. Endogenous 5-HT(2C) receptors in choroid plexus epithelial cells. J Biol Chem 275:7021–7029. [DOI] [PubMed] [Google Scholar]
- Chang S, Bezprozvannaya S, Li S, Olson EN. (2005) An expression screen reveals modulators of class II histone deacetylase phosphorylation. Proc Natl Acad Sci USA 102:8120–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Ng JK, Nguyen T, Pellissier L, Claeysen S, Hsiao EC, Conklin BR. (2007) Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PLoS One 2:e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouche-Teyara K, Fournier B, Safar M, Dabiré H. (1994) Systemic and regional haemodynamic effects of 1-(2,5-dimethoxy-4-iodo-phenyl)-2-aminopropane (DOI) and alpha-methyl-5-HT, in the anaesthetised rat. Clin Exp Hypertens 16:779–798. [DOI] [PubMed] [Google Scholar]
- Charest A, Wainer BH, Albert PR. (1993) Cloning and differentiation-induced expression of a murine serotonin1A receptor in a septal cell line. J Neurosci 13:5164–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Baho E, Huang ZJ, Schachner M, Di Cristo G. (2013) Neural cell adhesion molecule-mediated Fyn activation promotes GABAergic synapse maturation in postnatal mouse cortex. J Neurosci 33:5957–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Qiang H, Fan K, Wang S, Zheng Z. (2014) The snoRNA MBII-52 regulates cocaine-induced conditioned place preference and locomotion in mice. PLoS One 9:e99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Vasko MR, Wu X, Staeva TP, Baez M, Zgombick JM, Nelson DL. (1998a) Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther 287:1119–1127. [PubMed] [Google Scholar]
- Chen J-X, Pan H, Rothman TP, Wade PR, Gershon MD. (1998b) Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol 275:G433–G448. [DOI] [PubMed] [Google Scholar]
- Chen K, Yang W, Grimsby J, Shih JC. (1992) The human 5-HT2 receptor is encoded by a multiple intron-exon gene. Brain Res Mol Brain Res 14:20–26. [DOI] [PubMed] [Google Scholar]
- Chen Y, Baez M, Yu L. (1994) Functional coupling of the 5-HT2C serotonin receptor to G proteins in Xenopus oocytes. Neurosci Lett 179:100–102. [DOI] [PubMed] [Google Scholar]
- Chen Y, Leon-Ponte M, Pingle SC, O’Connell PJ, Ahern GP. (2015) T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol (Oxf) 213:860–867. [DOI] [PubMed] [Google Scholar]
- Cheng A, Bollan KA, Greenwood SM, Irving AJ, Connolly CN. (2007) Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J Biol Chem 282:26158–26166. [DOI] [PubMed] [Google Scholar]
- Cheng A, McDonald NA, Connolly CN. (2005) Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J Biol Chem 280:22502–22507. [DOI] [PubMed] [Google Scholar]
- Cheng J, Giguere PM, Schmerberg CM, Pogorelov VM, Rodriguiz RM, Huang XP, Zhu H, McCorvy JD, Wetsel WC, Roth BL, et al. (2016) Further advances in optimizing (2-phenylcyclopropyl)methylamines as novel serotonin 2C agonists: effects on hyperlocomotion, prepulse inhibition, and cognition models. J Med Chem 59:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu F, Shim S, El Mansari M, Blier P. (2014) Role of melatonin, serotonin 2B, and serotonin 2C receptors in modulating the firing activity of rat dopamine neurons. J Psychopharmacol 28:162–167. [DOI] [PubMed] [Google Scholar]
- Chetty N, Coupar IM, Tan YY, Desmond PV, Irving HR. (2009) Distribution of serotonin receptors and interacting proteins in the human sigmoid colon. Neurogastroenterol Motil 21:551–558, e14–e15. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinuck RS, Fortnum H, Baldwin DR. (2007) Appetite stimulants in cystic fibrosis: a systematic review. J Hum Nutr Diet 20:526–537. [DOI] [PubMed] [Google Scholar]
- Choi D-S, Birraux G, Launay J-M, Maroteaux L. (1994) The human serotonin 5-HT2B receptor: pharmacological link between 5-HT2 and 5-HT1D receptors. FEBS Lett 352:393–399. [DOI] [PubMed] [Google Scholar]
- Choi D-S, Maroteaux L. (1996) Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett 391:45–51. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kang H, Lee WK, Kim T, Rhim H, Yu YG. (2007) An inhibitory compound against the interaction between G alpha(s) and the third intracellular loop region of serotonin receptor subtype 6 (5-HT6) disrupts the signaling pathway of 5-HT6. Exp Mol Med 39:335–342. [DOI] [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, Roth BL. (1992) Identification of receptor domains that modify ligand binding to 5-hydroxytryptamine2 and 5-hydroxytryptamine1c serotonin receptors. Mol Pharmacol 42:627–633. [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, Roth BL. (1993) A single point mutation (Phe340-->Leu340) of a conserved phenylalanine abolishes 4-[125I]iodo-(2,5-dimethoxy)phenylisopropylamine and [3H]mesulergine but not [3H]ketanserin binding to 5-hydroxytryptamine2 receptors. Mol Pharmacol 43:755–761. [PubMed] [Google Scholar]
- Choudhary MS, Sachs N, Uluer A, Glennon RA, Westkaemper RB, Roth BL. (1995) Differential ergoline and ergopeptine binding to 5-hydroxytryptamine2A receptors: ergolines require an aromatic residue at position 340 for high affinity binding [published erratum appears in Mol Pharmacol (1995) 48:568]. Mol Pharmacol 47:450–457. [PubMed] [Google Scholar]
- Choung RS, Locke GR, III, Francis DD, Katzka D, Winkle PJ, Orr WC, Crowell MD, Devault K, Harmsen WS, Zinsmeister AR, et al. (2014) Novel partial 5HT3 agonist pumosetrag reduces acid reflux events in uninvestigated GERD patients after a standard refluxogenic meal: a randomized, double-blind, placebo-controlled pharmacodynamic study. Neurogastroenterol Motil 26:13–20. [DOI] [PubMed] [Google Scholar]
- Christ T, Rozmaritsa N, Engel A, Berk E, Knaut M, Metzner K, Canteras M, Ravens U, Kaumann A. (2014) Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc Natl Acad Sci USA 111:11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. (2010) 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry 67:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA, Olsen RW, Peters JA, Neubig RR, Pin JP, et al. (2014) International Union of Basic and Clinical Pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev 66:918–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HR, Jeon IS, Park SY, Lee SJ, Kang SH, Kim SI. (2014) Efficacy of palonosetron for the prevention of postoperative nausea and vomiting: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth 112:485–490. [DOI] [PubMed] [Google Scholar]
- Ciranna L, Catania MV. (2014) 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front Cell Neurosci 8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L. (2011) Iloperidone, asenapine, and lurasidone: a brief overview of 3 new second-generation antipsychotics. Postgrad Med 123:153–162. [DOI] [PubMed] [Google Scholar]
- Citrome L, Weiden PJ, McEvoy JP, Correll CU, Cucchiaro J, Hsu J, Loebel A. (2014) Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr 19:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeysen S, Bockaert J, Giannoni P. (2015) Serotonin: a new hope in Alzheimer’s disease? ACS Chem Neurosci 6:940–943. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Faye P, Sebben M, Lemaire S, Bockaert J, Dumuis A, Taviaux S. (1997) Assignment of 5-hydroxytryptamine receptor (HTR4) to human chromosome 5 bands q31-->q33 by in situ hybridization. Cytogenet Cell Genet 78:133–134. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. (1999) Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol Pharmacol 55:910–920. [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Bécamel C, Eglen RM, Clark RD, Bockaert J, Dumuis A. (2000) Pharmacological properties of 5-Hydroxytryptamine(4) receptor antagonists on constitutively active wild-type and mutated receptors. Mol Pharmacol 58:136–144. [DOI] [PubMed] [Google Scholar]
- Claret FX, Hibi M, Dhut S, Toda T, Karin M. (1996) A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature 383:453–457. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Craig DA, Fozard JR. (1989) The 5-HT4 receptor: naughty, but nice. Trends Pharmacol Sci 10:385–386. [DOI] [PubMed] [Google Scholar]
- Clarke WP, Yocca FD, Maayani S. (1996) Lack of 5-hydroxytryptamine1A-mediated inhibition of adenylyl cyclase in dorsal raphe of male and female rats. J Pharmacol Exp Ther 277:1259–1266. [PubMed] [Google Scholar]
- Classey JD, Bartsch T, Goadsby PJ. (2010) Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res 1361:76–85. [DOI] [PubMed] [Google Scholar]
- Claustre Y, Peretti DD, Brun P, Gueudet C, Allouard N, Alonso R, Lourdelet J, Oblin A, Damoiseau G, Francon D, et al. (2003) SSR181507, a dopamine D(2) receptor antagonist and 5-HT(1A) receptor agonist. I: neurochemical and electrophysiological profile. Neuropsychopharmacology 28:2064–2076. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Dennerstein L, Pyke R, Sand M. (2010) Flibanserin: a potential treatment for Hypoactive Sexual Desire Disorder in premenopausal women. Womens Health (Lond) 6:639–653. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. (2000) Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39:123–132. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT. (2000) Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 152:256–267. [DOI] [PubMed] [Google Scholar]
- Clissold KA, Choi E, Pratt WE. (2013) Serotonin 1A, 1B, and 7 receptors of the rat medial nucleus accumbens differentially regulate feeding, water intake, and locomotor activity. Pharmacol Biochem Behav 112:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clitherow JW, Scopes DI, Skingle M, Jordan CC, Feniuk W, Campbell IB, Carter MC, Collington EW, Connor HE, Higgins GA, et al. (1994) Evolution of a novel series of [(N,N-dimethylamino)propyl]- and piperazinylbenzanilides as the first selective 5-HT1D antagonists. J Med Chem 37:2253–2257. [DOI] [PubMed] [Google Scholar]
- Closse A. (1983) [3H]Mesulergine, a selective ligand for serotonin-2 receptors. Life Sci 32:2485–2495. [DOI] [PubMed] [Google Scholar]
- Cochet M, Donneger R, Cassier E, Gaven F, Lichtenthaler SF, Marin P, Bockaert J, Dumuis A, Claeysen S. (2013) 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci 4:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft V, Ortells M, Lunt G. (1995) Ligands, receptor models, and evolution. Ann N Y Acad Sci 757:40–47. [DOI] [PubMed] [Google Scholar]
- Codony X, Vela JM, Ramírez MJ. (2011) 5-HT(6) receptor and cognition. Curr Opin Pharmacol 11:94–100. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeño L, Tamargo J, Perez-Vizcaino F. (2006) Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res 98:931–938. [DOI] [PubMed] [Google Scholar]
- Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ, Cross JR, Emiliano AB, Han SM, et al. (2017) Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Fuller RW, Wiley KS. (1981) Evidence for 5-HT2 receptors mediating contraction in vascular smooth muscle. J Pharmacol Exp Ther 218:421–425. [PubMed] [Google Scholar]
- Cohen ML, Galbreath EJ, Schenck KW, Li D, Hoffman BJ, Bhattacharya A. (2002) Lack of sumatriptan-induced aortic contraction or relaxation: 5-HT1B receptor protein detected in endothelium and smooth muscle of vasa vasorum but not aorta. Receptors Channels 8:71–78. [PubMed] [Google Scholar]
- Cohen ML, Schenck K. (1999) 5-Hydroxytryptamine(1F) receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin(1F) receptor agonist in rabbit saphenous vein. J Pharmacol Exp Ther 290:935–939. [PubMed] [Google Scholar]
- Cohen ML, Schenck K. (2000) Contractile responses to sumatriptan and ergotamine in the rabbit saphenous vein: effect of selective 5-HT(1F) receptor agonists and PGF(2α). Br J Pharmacol 131:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Schenck KW, Mabry TE, Nelson DL, Audia JE. (1996) LY272015, a potent, selective and orally active 5-HT2B receptor antagonist. J Serv Res 3:131–144. [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. (2001) Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Z, Bouchelet I, Olivier A, Villemure JG, Ball R, Stanimirovic DB, Hamel E. (1999) Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J Cereb Blood Flow Metab 19:908–917. [DOI] [PubMed] [Google Scholar]
- Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay J-M, de Vernejoul M-C. (2008) The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J 22:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. (2010) Long-term depression in the CNS. Nat Rev Neurosci 11:459–473. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. (2006) 5-HT(1A) receptor activation: new molecular and neuroadaptive mechanisms of pain relief. Curr Opin Investig Drugs 7:40–47. [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Koek W, Pauwels PJ, Bardin L, Xu XJ, Wiesenfeld-Hallin Z, Cosi C, Carilla-Durand E, Assié MB, et al. (2002) Large-amplitude 5-HT1A receptor activation: a new mechanism of profound, central analgesia. Neuropharmacology 43:945–958. [DOI] [PubMed] [Google Scholar]
- Comet MA, Bernard JF, Hamon M, Laguzzi R, Sévoz-Couche C. (2007) Activation of nucleus tractus solitarius 5-HT2A but not other 5-HT2 receptor subtypes inhibits the sympathetic activity in rats. Eur J Neurosci 26:345–354. [DOI] [PubMed] [Google Scholar]
- Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A. (1996) Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci 8:2591–2598. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. (2004) Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci 24:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. (1984) Selective 5HT-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology 23:993–996. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. (1986a) Agonist-induced phosphoinositide hydrolysis in choroid plexus. J Neurochem 47:1754–1760. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. (1986b) Biochemical characterization of serotonin stimulated phosphoinositide turnover. Life Sci 38:663–669. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E, Hoffman BJ, Hartig PR. (1986) A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc Natl Acad Sci USA 83:4086–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337:581–588. [DOI] [PubMed] [Google Scholar]
- Connor HE, Feniuk W, Beattie DT, North PC, Oxford AW, Saynor DA, Humphrey PP. (1997) Naratriptan: biological profile in animal models relevant to migraine. Cephalagia 17:145-152. [DOI] [PubMed] [Google Scholar]
- Contesse V, Lenglet S, Grumolato L, Anouar Y, Lihrmann I, Lefebvre H, Delarue C, Vaudry H. (1999) Pharmacological and molecular characterization of 5-hydroxytryptamine(7) receptors in the rat adrenal gland. Mol Pharmacol 56:552–561. [DOI] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. (2009) Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med 15:1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett DF, Heightman TD, Moss SF, Bromidge SM, Coggon SA, Longley MJ, Roa AM, Williams JA, Thomas DR. (2005) Discovery of a potent and selective 5-ht5A receptor antagonist by high-throughput chemistry. Bioorg Med Chem Lett 15:4014–4018. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. (1999) Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409:187–209. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N, Descarries L. (2002) Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience 113:23–35. [DOI] [PubMed] [Google Scholar]
- Corominas R, Sobrido MJ, Ribasés M, Cuenca-León E, Blanco-Arias P, Narberhaus B, Roig M, Leira R, López-González J, Macaya A, et al. (2010) Association study of the serotoninergic system in migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet 153B:177–184. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Mlinar B, Falsini C, Pugliese AM, Cilia A, Destefani C, Testa R. (2005) Differential effects of the 5-hydroxytryptamine (5-HT)1A receptor inverse agonists Rec 27/0224 and Rec 27/0074 on electrophysiological responses to 5-HT1A receptor activation in rat dorsal raphe nucleus and hippocampus in vitro. J Pharmacol Exp Ther 315:109–117. [DOI] [PubMed] [Google Scholar]
- Corringer P-J, Poitevin F, Prevost MS, Sauguet L, Delarue M, Changeux JP. (2012) Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure 20:941–956. [DOI] [PubMed] [Google Scholar]
- Cosi C, Koek W. (2000) The putative <silent> 5-HT(1A) receptor antagonist, WAY 100635, has inverse agonist properties at cloned human 5-HT(1A) receptors. Eur J Pharmacol 401:9–15. [DOI] [PubMed] [Google Scholar]
- Cosi C, Waget A, Rollet K, Tesori V, Newman-Tancredi A. (2005) Clozapine, ziprasidone and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: role of 5-HT1A receptor activation. Brain Res 1043:32–41. [DOI] [PubMed] [Google Scholar]
- Costa L, Sardone LM, Lacivita E, Leopoldo M, Ciranna L. (2015) Novel agonists for serotonin 5-HT7 receptors reverse metabotropic glutamate receptor-mediated long-term depression in the hippocampus of wild-type and Fmr1 KO mice, a model of Fragile X Syndrome. Front Behav Neurosci 9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L, Spatuzza M, D’Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, Leopoldo M, Lacivita E, Catania MV, Ciranna L. (2012) Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry 72:924–933. [DOI] [PubMed] [Google Scholar]
- Costa SK, Brain SD, Antunes E, De Nucci G, Docherty RJ. (2003) Phoneutria nigriventer spider venom activates 5-HT4 receptors in rat-isolated vagus nerve. Br J Pharmacol 139:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola C, Iuliano G, Rinaldi M, Russo V, Scibelli G, Mastropasqua L. (1993) Effect of topical ketanserin administration on intraocular pressure. Br J Ophthalmol 77:344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ, Tattersall FD. (1986) 5-Hydroxytryptamine M-receptor antagonism to prevent cisplatin-induced emesis. Neuropharmacology 25:959–961. [DOI] [PubMed] [Google Scholar]
- Costall B, Gunning SJ, Naylor RJ, Tyers MB. (1987) The effect of GR38032F, novel 5-HT3-receptor antagonist on gastric emptying in the guinea-pig. Br J Pharmacol 91:263–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. (1992) Anxiolytic potential of 5-HT3 receptor antagonists. Pharmacol Toxicol 70:157–162. [DOI] [PubMed] [Google Scholar]
- Coupar IM, Desmond PV, Irving HR. (2007) Human 5-HT(4) and 5-HT(7) receptor splice variants: are they important? Curr Neuropharmacol 5:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvineau A, Fabre C, Gaudin P, Maoret JJ, Laburthe M. (1996) Mutagenesis of N-glycosylation sites in the human vasoactive intestinal peptide 1 receptor. Evidence that asparagine 58 or 69 is crucial for correct delivery of the receptor to plasma membrane. Biochemistry 35:1745–1752. [DOI] [PubMed] [Google Scholar]
- Craig DA, Clarke DE. (1990) Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine receptor. J Pharmacol Exp Ther 252:1378–1386. [PubMed] [Google Scholar]
- Craig DA, Martin GR. (1993) 5-HT1B receptors mediate potent contractile responses to 5-HT in rat caudal artery. Br J Pharmacol 109:609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige CP, Lewandowski S, Kirby LG, Unterwald EM. (2015) Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology 93:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer SE, Moesgaard SG, Rasmussen CE, Zois NE, Falk T, Reimann MJ, Cirera S, Aupperle H, Oyama MA, Olsen LH. (2015a) Alpha-smooth muscle actin and serotonin receptors 2A and 2B in dogs with myxomatous mitral valve disease. Res Vet Sci 100:197–206. [DOI] [PubMed] [Google Scholar]
- Cremer SE, Zois NE, Moesgaard SG, Ravn N, Cirera S, Honge JL, Smerup MH, Hasenkam JM, Sloth E, Leifsson PS, et al. (2015b) Serotonin markers show altered transcription levels in an experimental pig model of mitral regurgitation. Vet J 203:192–198. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mørk A, Honig G, Bøgesø KP, Westerink BH, den Boer H, et al. (2004) Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology 29:1782–1789. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Rea K, Bosker FJ, Wikström HV, Hogg S, Mørk A, Westerink BH. (2007) Augmentation of SSRI effects on serotonin by 5-HT2C antagonists: mechanistic studies. Neuropsychopharmacology 32:1550–1557. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Mitchell JE. (1994) Rational therapy of eating disorders. Drugs 48:372–379. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Di Giovanni G. (2014) Monoamine modulation of tonic GABAA inhibition. Rev Neurosci 25:195–206. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Di Giovanni G. (2015) Differential control by 5-HT and 5-HT1A, 2A, 2C receptors of phasic and tonic GABAA inhibition in the visual thalamus. CNS Neurosci Ther 21:967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. (2000) Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther 295:1120–1126. [PubMed] [Google Scholar]
- Csaba G, Sudár F. (1978) Localization of radioactively labelled serotonin in the nucleus of adrenal medulla cells. Acta Anat (Basel) 100:237–240. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS, Fuenmayor NT, Finn AL. (1990) Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med 322:810–816. [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Hill RG, Hargreaves RJ. (1998) The effects of 5-HT1A, 5-HT1B and 5-HT1D receptor agonists on trigeminal nociceptive neurotransmission in anaesthetized rats. Eur J Pharmacol 362:43–46. [DOI] [PubMed] [Google Scholar]
- Cumming-Hood PA, Strahlendorf HK, Strahlendorf JC. (1993) Effects of serotonin and the 5-HT2/1C receptor agonist DOI on neurons of the cerebellar dentate/interpositus nuclei: possible involvement of a GABAergic interneuron. Eur J Pharmacol 236:457–465. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. (2014) Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76:460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, et al. (2013) Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. (2011) Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MP, Robinson DM. (2008) Mosapride in gastrointestinal disorders. Drugs 68:981–991. [DOI] [PubMed] [Google Scholar]
- Currow DC, Coughlan M, Fardell B, Cooney NJ. (1997) Use of ondansetron in palliative medicine. J Pain Symptom Manage 13:302–307. [DOI] [PubMed] [Google Scholar]
- Curtin PCP, Medan V, Neumeister H, Bronson DR, Preuss T. (2013) The 5-HT5A receptor regulates excitability in the auditory startle circuit: functional implications for sensorimotor gating. J Neurosci 33:10011–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing DJ, Zgombick JM, Nelson DL, Cohen ML. (1996) LY215840, a high-affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery. J Pharmacol Exp Ther 277:1560–1566. [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. (2008) Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol 594:32–38. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Quentric Y, Carpentier N, Poissonnet G, Parmentier JG, Goldstein S, Millan MJ. (2002) Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn Schmiedebergs Arch Pharmacol 365:242–252. [DOI] [PubMed] [Google Scholar]
- Cussac D, Rauly-Lestienne I, Heusler P, Finana F, Cathala C, Bernois S, De Vries L. (2012) μ-Opioid and 5-HT1A receptors heterodimerize and show signalling crosstalk via G protein and MAP-kinase pathways. Cell Signal 24:1648–1657. [DOI] [PubMed] [Google Scholar]
- daCosta CJB, Baenziger JE. (2013) Gating of pentameric ligand-gated ion channels: structural insights and ambiguities. Structure 21:1271–1283. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. (2012) Dopamine, serotonin and impulsivity. Neuroscience 215:42–58. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG. (2006) Serotonin 1B and 2C receptor interactions in the modulation of feeding behaviour in the mouse. Psychopharmacology (Berl) 185:45–57. [DOI] [PubMed] [Google Scholar]
- Damjanoska KJ, Muma NA, Zhang Y, D’Souza DN, Garcia F, Carrasco GA, Kindel GH, Haskins KA, Shankaran M, Petersen BR, et al. (2003) Neuroendocrine evidence that (S)-2-(chloro-5-fluoro-indol- l-yl)-1-methylethylamine fumarate (Ro 60-0175) is not a selective 5-hydroxytryptamine(2C) receptor agonist. J Pharmacol Exp Ther 304:1209–1216. [DOI] [PubMed] [Google Scholar]
- Dando SB, Skinner MR, Jordan D, Ramage AG. (1998) Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br J Pharmacol 125:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, England PM, Farivar SS, Dougherty DA, Lester HA. (2000) Probing the role of a conserved M1 proline residue in 5-hydroxytryptamine(3) receptor gating. Mol Pharmacol 57:1114–1122. [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. (1998) Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol 55:27–57. [DOI] [PubMed] [Google Scholar]
- Danysz W, Flik G, McCreary A, Tober C, Dimpfel W, Bizot JC, Kostrzewa R, Brown RW, Jatzke CC, Greco S, et al. (2015) Effects of sarizotan in animal models of ADHD: challenging pharmacokinetic-pharmacodynamic relationships. J Neural Transm (Vienna) 122:1221–1238. [DOI] [PubMed] [Google Scholar]
- da Roza Davis JM, Sharpley AL, Cowen PJ. (1992) Slow wave sleep and 5-HT2 receptor sensitivity in generalised anxiety disorder: a pilot study with ritanserin. Psychopharmacology (Berl) 108:387–389. [DOI] [PubMed] [Google Scholar]
- Das P, Dillon GH. (2003) The 5-HT3B subunit confers reduced sensitivity to picrotoxin when co-expressed with the 5-HT3A receptor. Brain Res Mol Brain Res 119:207–212. [DOI] [PubMed] [Google Scholar]
- Daval G, Vergé D, Basbaum AI, Bourgoin S, Hamon M. (1987) Autoradiographic evidence of serotonin1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci Lett 83:71–76. [DOI] [PubMed] [Google Scholar]
- Davidson HI, Pilot M-A, Thompson HH. (1990) Involvement of 5-hydroxytryptamine in canine intestinal motility patterns. J Gastrointest Motil 2:31–39. [Google Scholar]
- Davis JD, Smith GP, Singh B, McCann DL. (2001) The impact of sucrose-derived unconditioned and conditioned negative feedback on the microstructure of ingestive behavior. Physiol Behav 72:393–402. [DOI] [PubMed] [Google Scholar]
- Davies PA. (2011) Allosteric modulation of the 5-HT(3) receptor. Curr Opin Pharmacol 11:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397:359–363. [DOI] [PubMed] [Google Scholar]
- Dawson LA. (2011) The central role of 5-HT6 receptors in modulating brain neurochemistry. Int Rev Neurobiol 96:1–26. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Watson JM. (2009) Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders. CNS Neurosci Ther 15:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P, Opacka-Juffry J, Moffatt JD, Daniju Y, Dutta N, Ramsey J, Davidson C. (2014) The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog Neuropsychopharmacol Biol Psychiatry 48:57–63. [DOI] [PubMed] [Google Scholar]
- Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. (2002) Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a PLCbeta/IP3/calcineurin signaling cascade. J Neurophysiol 87:2490–2504. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Jacobshagen M, Chaumont-Dubel S, Marin P. (2015) 5-HT6 receptor: a new player controlling the development of neural circuits. ACS Chem Neurosci 6:951–960. [DOI] [PubMed] [Google Scholar]
- Dean B, Pavey G, Thomas D, Scarr E. (2006) Cortical serotonin7, 1D and 1F receptors: effects of schizophrenia, suicide and antipsychotic drug treatment. Schizophr Res 88:265–274. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM. (2000) Somatodendritic 5-HT(1A) autoreceptors mediate the anti-aggressive actions of 5-HT(1A) receptor agonists in rats: an ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology 23:20–33. [DOI] [PubMed] [Google Scholar]
- Deckert V, Pruneau D, Elghazi JL. (1994) Mediation by 5-HT1D receptors of 5-hydoxytryptamine-induced contractions of rabbit middle and posterior cerebral arteries. Br J Pharmacol 112:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clerck F, David JL, Janssen PAJ. (1982) Inhibition of 5-hydroxytryptamine-induced and -amplified human platelet aggregation by ketanserin (R 41 468), a selective 5-HT2-receptor antagonist. Agents Actions 12:388–397. [DOI] [PubMed] [Google Scholar]
- De Clerck F, Xhonneux B, Leysen J, Janssen PAJ. (1984) The involvement of 5-HT2-receptor sites in the activation of cat platelets. Thromb Res 33:305–321. [DOI] [PubMed] [Google Scholar]
- Dedeoğlu A, Fisher LA. (1991) Central and peripheral injections of the 5-HT2 agonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, modify cardiovascular function through different mechanisms. J Pharmacol Exp Ther 259:1027–1034. [PubMed] [Google Scholar]
- Deeb TZ, Sharp D, Hales TG. (2009) Direct subunit-dependent multimodal 5-hydroxytryptamine3 receptor antagonism by methadone. Mol Pharmacol 75:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees C, Akhmetshina A, Zerr P, Reich N, Palumbo K, Horn A, Jüngel A, Beyer C, Krönke G, Zwerina J, et al. (2011) Platelet-derived serotonin links vascular disease and tissue fibrosis. J Exp Med 208:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Chiodi V, Adriani W, Lacivita E, Mallozzi C, Leopoldo M, Domenici MR, Fuso A, Laviola G. (2015) Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Front Behav Neurosci 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Nativio P, Fabbri A, Ricceri L, Adriani W, Lacivita E, Leopoldo M, Passarelli F, Fuso A, Laviola G. (2014) Pharmacological stimulation of the brain serotonin receptor 7 as a novel therapeutic approach for Rett syndrome. Neuropsychopharmacology 39:2506–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGeer J, Boudeau J, Schmidt S, Bedford F, Lamarche-Vane N, Debant A. (2013) Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol Cell Biol 33:739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney C, Gien J, Roe G, Isenberg N, Kailey J, Abman SH. (2013) Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 304:L894–L901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue C, Becquet D, Idres S, Hery F, Vaudry H. (1992) Serotonin synthesis in adrenochromaffin cells. Neuroscience 46:495–500. [DOI] [PubMed] [Google Scholar]
- de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A, Samaniego R, Loza M, Corcuera MT, Gómez-Aguado F, et al. (2013) Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol 190:2301–2310. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. (1999) Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem 274:4749–4753. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. (2005) Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6:850–862. [DOI] [PubMed] [Google Scholar]
- Deloose E, Janssen P, Depoortere I, Tack J. (2012) The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol 9:271–285. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr (2005) Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucchini S, Marracci S, Nardi I. (2001) The serotonin 5-HT2B receptor from the puffer fish Tetraodon fluviatilis: cDNA cloning, genomic organization and alternatively spliced variants. Brain Res Mol Brain Res 97:89–93. [DOI] [PubMed] [Google Scholar]
- De Lucchini S, Ori M, Cremisi F, Nardini M, Nardi I. (2005) 5-HT2B-mediated serotonin signaling is required for eye morphogenesis in Xenopus. Mol Cell Neurosci 29:299–312. [DOI] [PubMed] [Google Scholar]
- Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. (1998) Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 12:849–855. [DOI] [PubMed] [Google Scholar]
- De Maeyer JH, Aerssens J, Verhasselt P, Lefebvre RA. (2008a) Alternative splicing and exon duplication generates 10 unique porcine 5-HT 4 receptor splice variants including a functional homofusion variant. Physiol Genomics 34:22–33. [DOI] [PubMed] [Google Scholar]
- De Maeyer JH, Lefebvre RA, Schuurkes JA. (2008b) 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil 20:99–112. [DOI] [PubMed] [Google Scholar]
- De Maeyer JH, Prins NH, Schuurkes JAJ, Lefebvre RA. (2006) Differential effects of 5-hydroxytryptamine4 receptor agonists at gastric versus cardiac receptors: an operational framework to explain and quantify organ-specific behavior. J Pharmacol Exp Ther 317:955–964. [DOI] [PubMed] [Google Scholar]
- De Martelaere K, Lintermans B, Haegeman G, Vanhoenacker P. (2007) Novel interaction between the human 5-HT7 receptor isoforms and PLAC-24/eIF3k. Cell Signal 19:278–288. [DOI] [PubMed] [Google Scholar]
- De Vries P, Villalón CM, Saxena PR. (1999a) Pharmacological aspects of experimental headache models in relation to acute antimigraine therapy. Eur J Pharmacol 375:61–74. [DOI] [PubMed] [Google Scholar]
- De Vries P, Villalón CM, Saxena PR. (1999b) Pharmacology of triptans. Emerging Drugs 4:107–125. [Google Scholar]
- Den Boer MO, Villalón CM, Saxena PR. (1992) 5?HT1?like receptor?mediated changes in porcine carotid haemodynamics: are 5?HT1D receptors involved?. Naunyn?Schmiedeberg’s Arch. Pharmacol 345:509–515. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. (2008) Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci 39:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentel C, Palamiuc L, Henriques A, Lannes B, Spreux-Varoquaux O, Gutknecht L, René F, Echaniz-Laguna A, Gonzalez de Aguilar J-L, Lesch KP, et al. (2013) Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain 136:483–493. [DOI] [PubMed] [Google Scholar]
- De Ponti F. (2004) Pharmacology of serotonin: what a clinician should know. Gut 53:1520–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere I, Thijs T, Peeters T. (2006) The contractile effect of the ghrelin receptor antagonist, D-Lys3-GHRP-6, in rat fundic strips is mediated through 5-HT receptors. Eur J Pharmacol 537:160–165. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Bruins Slot L, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. (2007) F15063, a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. III. Activity in models of cognition and negative symptoms. Br J Pharmacol 151:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A. (2010) F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol 20:641–654. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Boulay D, Perrault G, Bergis O, Decobert M, Françon D, Jung M, Simiand J, Soubrié P, Scatton B. (2003) SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. II: behavioral profile predictive of an atypical antipsychotic activity. Neuropsychopharmacology 28:1889–1902. [DOI] [PubMed] [Google Scholar]
- Deraet M, Manivet P, Janoshazi A, Callebert J, Guenther S, Drouet L, Launay JM, Maroteaux L. (2005) The natural mutation encoding a C terminus-truncated 5-hydroxytryptamine 2B receptor is a gain of proliferative functions. Mol Pharmacol 67:983–991. [DOI] [PubMed] [Google Scholar]
- Derangeon M, Bozon V, Defamie N, Peineau N, Bourmeyster N, Sarrouilhe D, Argibay JA, Hervé J-C. (2010) 5-HT4 and 5-HT2 receptors antagonistically influence gap junctional coupling between rat auricular myocytes. J Mol Cell Cardiol 48:220–229. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Kamendi H, Cheng Q, Pinol RM, Jameson H, Gorini C, Mendelowitz D. (2008) 5HT2 receptor activation facilitates P2X receptor mediated excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuropharmacology 54:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA. (1989) 5-HT3 receptors are membrane ion channels. Nature 339:706–709. [DOI] [PubMed] [Google Scholar]
- Descarries L, Cornea-Herbert V, Riad M. (2006) Cellular and subcellular localization of serotonin receptors in the central nervous system, in The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics (Roth BL. ed) pp 277–317, Humana Press, Totowa, NJ. [Google Scholar]
- Devi LA. (2001) Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci 22:532–537. [DOI] [PubMed] [Google Scholar]
- De Vivo M, Maayani S. (1986) Characterization of the 5-hydroxytryptamine1a receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J Pharmacol Exp Ther 238:248–253. [PubMed] [Google Scholar]
- Devlin MG, Smith NJ, Ryan OM, Guida E, Sexton PM, Christopoulos A. (2004) Regulation of serotonin 5-HT2C receptors by chronic ligand exposure. Eur J Pharmacol 498:59–69. [DOI] [PubMed] [Google Scholar]
- De Vries P, Apaydin S, Villalón CM, Heiligers JP, Saxena PR. (1997) Interactions of GR127935, a 5-HT(1B/D) receptor ligand, with functional 5-HT receptors. Naunyn Schmiedebergs Arch Pharmacol 355:423–430. [DOI] [PubMed] [Google Scholar]
- De Vries P, De Visser PA, Heiligers JP, Villalón CM, Saxena PR. (1999) Changes in systemic and regional haemodynamics during 5-HT7 receptor-mediated depressor responses in rats. Naunyn Schmiedebergs Arch Pharmacol 359:331–338. [DOI] [PubMed] [Google Scholar]
- De Vries P, Sánchez-López A, Centurión D, Heiligers JP, Saxena PR, Villalón CM. (1998) The canine external carotid vasoconstrictor 5-HT1 receptor: blockade by 5-HT1B (SB224289), but not by 5-HT1D (BRL15572) receptor antagonists. Eur J Pharmacol 362:69–72. [DOI] [PubMed] [Google Scholar]
- Devroye C, Cathala A, Di Marco B, Caraci F, Drago F, Piazza PV, Spampinato U. (2015) Central serotonin(2B) receptor blockade inhibits cocaine-induced hyperlocomotion independently of changes of subcortical dopamine outflow. Neuropharmacology 97:329–337. [DOI] [PubMed] [Google Scholar]
- De Vry J, Benz U, Schreiber R, Traber J. (1993) Shock-induced ultrasonic vocalization in young adult rats: a model for testing putative anti-anxiety drugs. Eur J Pharmacol 249:331–339. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Melon C, Dalmus M, Jentzsch KR. (2004) 5-HT1A receptors are differentially involved in the anxiolytic- and antidepressant-like effects of 8-OH-DPAT and fluoxetine in the rat. Eur Neuropsychopharmacol 14:487–495. [DOI] [PubMed] [Google Scholar]
- de Wit R, Aapro M, Blower PR. (2005) Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients? Cancer Chemother Pharmacol 56:231–238. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Doly S, Narboux-Nême N, Fernández S, Mazot P, Banas SM, Boutourlinsky K, Moutkine I, Belmer A, Roumier A, et al. (2012) 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol Psychiatry 17:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SL, Maroteaux L. (2011) Implication of 5-HT(2B) receptors in the serotonin syndrome. Neuropharmacology 61:495–502. [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Smith TK. (2010) Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299:G144–G157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdère P. (2016) New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol Ther 157:125–162. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdére P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. (1999) Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience 91:587–597. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. (2001) m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience 103:111–116. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E. (2006) Central serotonin2C receptor: from physiology to pathology. Curr Top Med Chem 6:1909–1925. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Esposito E, Di Matteo V. (2010) 5-HT2C Receptors in the Pathophysiology of CNS Disease, Springer Science & Business Media, New York. [Google Scholar]
- Dijk DJ, Beersma DG, Daan S, van den Hoofdakker RH. (1989) Effects of seganserin, a 5-HT2 antagonist, and temazepam on human sleep stages and EEG power spectra. Eur J Pharmacol 171:207–218. [DOI] [PubMed] [Google Scholar]
- DiMagno L, Dascal N, Davidson N, Lester HA, Schreibmayer W. (1996) Serotonin and protein kinase C modulation of a rat brain inwardly rectifying K+ channel expressed in xenopus oocytes. Pflugers Arch 431:335–340. [DOI] [PubMed] [Google Scholar]
- Di Maio D, Chandramouli B, Brancato G. (2015) Pathways and barriers for ion translocation through the 5-HT3A receptor channel. PLoS One 10:e0140258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. (1999) SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38:1195–1205. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. (2000) Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res 865:85–90. [DOI] [PubMed] [Google Scholar]
- Dimitrova-Nakov S, Baudry A, Harichane Y, Collet C, Marchadier A, Kellermann O, Goldberg M. (2014) Deletion of serotonin 2B receptor provokes structural alterations of mouse dental tissues. Calcif Tissue Int 94:293–300. [DOI] [PubMed] [Google Scholar]
- Di Narzo AF, Kozlenkov A, Ge Y, Zhang B, Sanelli L, May Z, Li Y, Fouad K, Cardozo C, Koonin EV, et al. (2015) Decrease of mRNA editing after spinal cord injury is caused by down-regulation of ADAR2 that is triggered by inflammatory response. Sci Rep 5:12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Narzo AF, Kozlenkov A, Roussos P, Hao K, Hurd Y, Lewis DA, Sibille E, Siever LJ, Koonin E, Dracheva S. (2014) A unique gene expression signature associated with serotonin 2C receptor RNA editing in the prefrontal cortex and altered in suicide. Hum Mol Genet 23:4801–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Bremer NM, Smith TD, Seitz PK, Anastasio NC, Cunningham KA, Zhou J. (2012) Exploration of synthetic approaches and pharmacological evaluation of PNU-69176E and its stereoisomer as 5-HT2C receptor allosteric modulators. ACS Chem Neurosci 3:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Toth M, Zhou Y, Parks C, Hoffman BJ, Shenk T. (1993) Glial cell-specific expression of the serotonin 2 receptor gene: selective reactivation of a repressed promoter. Brain Res Mol Brain Res 20:181–191. [DOI] [PubMed] [Google Scholar]
- Di Pilato P, Niso M, Adriani W, Romano E, Travaglini D, Berardi F, Colabufo NA, Perrone R, Laviola G, Lacivita E, et al. (2014) Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: an overview. Rev Neurosci 25:401–415. [DOI] [PubMed] [Google Scholar]
- Dizeyi N, Bjartell A, Hedlund P, Taskén KA, Gadaleanu V, Abrahamsson PA. (2005) Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur Urol 47:895–900. [DOI] [PubMed] [Google Scholar]
- Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. (2004) Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate 59:328–336. [DOI] [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DEH, Humby T, Wilkinson LS, Isles AR. (2009) Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum Mol Genet 18:2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Porreca F. (2009) Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res 1280:52–59. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. (2006) Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br J Pharmacol 149:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. (2000) Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res 864:176–185. [DOI] [PubMed] [Google Scholar]
- Doly S, Bertran-Gonzalez J, Callebert J, Bruneau A, Banas SM, Belmer A, Boutourlinsky K, Hervé D, Launay JM, Maroteaux L. (2009) Role of serotonin via 5-HT2B receptors in the reinforcing effects of MDMA in mice. PLoS One 4:e7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil M-J, Vergé D, Conrath M. (2004) 5-HT5A receptor localization in the rat spinal cord suggests a role in nociception and control of pelvic floor musculature. J Comp Neurol 476:316–329. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil M-J, Vergé D, Conrath M. (2005) Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol 490:256–269. [DOI] [PubMed] [Google Scholar]
- Doly S, Valjent E, Setola V, Callebert J, Hervé D, Launay JM, Maroteaux L. (2008) Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J Neurosci 28:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doménech T, Beleta J, Palacios JM. (1997) Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol 356:328–334. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG, Champagne FA, Hen R. (2014) Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology 39:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793:1008–1022. [DOI] [PubMed] [Google Scholar]
- Donatsch P, Engel G, Richardson BP, Stadler PA. (1985) ICS 205-930: a hihgly selective adn potent antagonist at peripheral neuronal 5-hydroxytryptamine (5-HT) receptors. Br J Pharmacol 81:34P. [Google Scholar]
- Doods HN, Boddeke HW, Kalkman HO, Hoyer D, Mathy MJ, van Zwieten PA. (1988) Central 5-HT1A receptors and the mechanism of the central hypotensive effect of (+)8-OH-DPAT, DP-5-CT, R28935, and urapidil. J Cardiovasc Pharmacol 11:432–437. [DOI] [PubMed] [Google Scholar]
- Doran N, McChargue D, Spring B. (2008) Effect of impulsivity on cardiovascular and subjective reactivity to smoking cues. Addict Behav 33:167–172. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. (2007) Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology (Berl) 194:279–288. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Hutson PH, Kennett GA, Curzon G. (1986) 8-OH-DPAT-induced hyperphagia: its neural basis and possible therapeutic relevance. Appetite. 7 (Suppl):127–140. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Kennett GA, Curzon G. (1987) The 5-HT1A agonists 8-OH-DPAT, buspirone and ipsapirone attenuate stress-induced anorexia in rats. J Psychopharmacol 1:23–30. [DOI] [PubMed] [Google Scholar]
- Downie DL, Hope AG, Belelli D, Lambert JJ, Peters JA, Bentley KR, Steward LJ, Chen CY, Barnes NM. (1995) The interaction of trichloroethanol with murine recombinant 5-HT3 receptors. Br J Pharmacol 114:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Chin B, Haroutunian V. (2008a) Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport 19:379–382. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, Marcus SM, Siever LJ, McGurk SR, Haroutunian V. (2003) RNA editing and alternative splicing of human serotonin 2C receptor in schizophrenia. J Neurochem 87:1402–1412. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Lyddon R, Barley K, Marcus SM, Hurd YL, Byne WM. (2009) Editing of serotonin 2C receptor mRNA in the prefrontal cortex characterizes high-novelty locomotor response behavioral trait. Neuropsychopharmacology 34:2237–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Patel N, Woo DA, Marcus SM, Siever LJ, Haroutunian V. (2008b) Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol Psychiatry 13:1001–1010. [DOI] [PubMed] [Google Scholar]
- Druce M, Rockall A, Grossman AB. (2009) Fibrosis and carcinoid syndrome: from causation to future therapy. Nat Rev Endocrinol 5:276–283. [DOI] [PubMed] [Google Scholar]
- Drummond DC. (2001) Theories of drug craving, ancient and modern. Addiction 96:33–46. [DOI] [PubMed] [Google Scholar]
- Du J, Lü W, Wu S, Cheng Y, Gouaux E. (2015) Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526:24–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YL, Wilcox BD, Jeffrey JJ. (1995) Regulation of rat 5-hydroxytryptamine type 2 receptor gene activity: identification of cis elements that mediate basal and 5-hydroxytryptamine-dependent gene activation. Mol Pharmacol 47:915–922. [PubMed] [Google Scholar]
- Du YL, Wilcox BD, Teitler M, Jeffrey JJ. (1994) Isolation and characterization of the rat 5-hydroxytryptamine type 2 receptor promoter: constitutive and inducible activity in myometrial smooth muscle cells. Mol Pharmacol 45:1125–1131. [PubMed] [Google Scholar]
- Dubertret C, Hanoun N, Adès J, Hamon M, Gorwood P. (2004) Family-based association studies between 5-HT5A receptor gene and schizophrenia. J Psychiatr Res 38:371–376. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Huvar R, D’Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, et al. (1999) The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J Biol Chem 274:30799–30810. [DOI] [PubMed] [Google Scholar]
- Dubost E, Dumas N, Fossey C, Magnelli R, Butt-Gueulle S, Ballandonne C, Caignard DH, Dulin F, Sopkova de-Oliveira Santos J, Millet P, et al. (2012) Synthesis and structure-affinity relationships of selective high-affinity 5-HT(4) receptor antagonists: application to the design of new potential single photon emission computed tomography tracers. J Med Chem 55:9693–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Marcinkowska M, Bucki A, Olczyk A, Kolaczkowski M. (2015) Idalopirdine - a small molecule antagonist of 5-HT6 with therapeutic potential against obesity. Metab Brain Dis 30:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley MW, Weich NL, Miller FP, Carr AA, Cheng HC, Roebel LE, Doherty NS, Yamamura HI, Ursillo RC, Palfreyman MG. (1988) Pharmacological effects of MDL 11,939: a selective, centrally acting antagonist of 5-HT2 receptors. Drug Dev Res 13:29–43. [Google Scholar]
- Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, et al. (2013) Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F, Déléris P, Raynaud F, Séveno M, Morisset-Lopez S, Mannoury la Cour C, Millan MJ, Bockaert J, Marin P, Chaumont-Dubel S. (2014) Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol 10:590–597. [DOI] [PubMed] [Google Scholar]
- Dukat M, Smith C, Herrick-Davis K, Teitler M, Glennon RA. (2004) Binding of tryptamine analogs at h5-HT1E receptors: a structure-affinity investigation. Bioorg Med Chem 12:2545–2552. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dumitrascu R, Kulcke C, Königshoff M, Kouri F, Yang X, Morrell N, Ghofrani HA, Weissmann N, Reiter R, Seeger W, et al. (2011) Terguride ameliorates monocrotaline-induced pulmonary hypertension in rats. Eur Respir J 37:1104–1118. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Bouhelal R, Sebben M, Cory R, Bockaert J. (1988a) A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol 34:880–887. [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Bockaert J. (1988b) Pharmacology of 5-hydroxytryptamine-1A receptors which inhibit cAMP production in hippocampal and cortical neurons in primary culture. Mol Pharmacol 33:178–186. [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Monferini E, Nicola M, Turconi M, Ladinsky H, Bockaert J. (1991) Azabicycloalkyl benzimidazolone derivatives as a novel class of potent agonists at the 5-HT4 receptor positively coupled to adenylate cyclase in brain. Naunyn Schmiedebergs Arch Pharmacol 343:245–251. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Franklin KM. (2007) Expression of 5-HT7 receptor mRNA in the hamster brain: effect of aging and association with calbindin-D28K expression. Brain Res 1143:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Jennes L, Jefferson JB, Brownfield MS. (2000) Localization of serotonin(5A) receptors in discrete regions of the circadian timing system in the Syrian hamster. Brain Res 869:178–185. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Marquis KL, Lim HK, Leung L, Kao J, Cheesman C, Rosenzweig-Lipson S. (2006) Pharmacological profile of the 5-HT(2C) receptor agonist WAY-163909; therapeutic potential in multiple indications. CNS Drug Rev 12:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel RL, Stack G, Schechter L, et al. (2005) WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther 313:862–869. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Watts SW, Barrett JE, Coupet J, Harrison B, Mazandarani H, Nawoschik S, Pangalos MN, Ramamoorthy S, Schechter L, et al. (2011) Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J Pharmacol Exp Ther 337:673–680. [DOI] [PubMed] [Google Scholar]
- Dunn HA, Walther C, Yuan GY, Caetano FA, Godin CM, Ferguson SS. (2014) Role of SAP97 in the regulation of 5-HT2AR endocytosis and signaling. Mol Pharmacol 86:275–283. [DOI] [PubMed] [Google Scholar]
- Dupuis DS, Pauwels PJ, Radu D, Hall H. (1999a) Autoradiographic studies of 5-HT1A-receptor-stimulated [35S]GTPgammaS-binding responses in the human and monkey brain. Eur J Neurosci 11:1809–1817. [DOI] [PubMed] [Google Scholar]
- Dupuis DS, Perez M, Halazy S, Colpaert FC, Pauwels PJ. (1999b) Magnitude of 5-HT1B and 5-HT1A receptor activation in guinea-pig and rat brain: evidence from sumatriptan dimer-mediated [35S]GTPgammaS binding responses. Brain Res Mol Brain Res 67:107–123. [DOI] [PubMed] [Google Scholar]
- Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. (2014) An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res 152:450–457. [DOI] [PubMed] [Google Scholar]
- Dürk T, Panther E, Müller T, Sorichter S, Ferrari D, Pizzirani C, Di Virgilio F, Myrtek D, Norgauer J, Idzko M. (2005) 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol 17:599–606. [DOI] [PubMed] [Google Scholar]
- Dutton AC, Massoura AN, Dover TJ, Andrews NA, Barnes NM. (2008) Identification and functional significance of N-glycosylation of the 5-ht5A receptor. Neurochem Int 52:419–425. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KCF. (1997) Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience 76:323–329. [DOI] [PubMed] [Google Scholar]
- Eadie MJ. (2003) Convulsive ergotism: epidemics of the serotonin syndrome? Lancet Neurol 2:429–434. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. (1997) Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol 384:233–247. [PubMed] [Google Scholar]
- Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, Ellis E, Lakey AF, Burt AD, Douglass A, et al. (2011) Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med 17:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Whitaker-Azmitia PM, Harkins K. (1990) 5-HT1A and 5-HT1B agonists play a differential role on the respiratory frequency in rats. Neuropsychopharmacology 3:129–136. [PubMed] [Google Scholar]
- Egawa J, Endo T, Tamura R, Masuzawa N, Fukui N, Sugai T, Someya T. (2012) Influence of the 5-HTR1A C-1019G polymorphism on clinical phenotypes of autism spectrum disorders. Psychiatry Res 198:336–337. [DOI] [PubMed] [Google Scholar]
- Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. (2011) Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology 61:442–450. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Bonhaus DW, Clark RD, Johnson LG, Lee CH, Leung E, Smith WL, Wong EH, Whiting RL. (1994) (R) and (S) RS 56532: mixed 5-HT3 and 5-HT4 receptor ligands with opposing enantiomeric selectivity. Neuropharmacology 33:515–526. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Bonhaus DW, Johnson LG, Leung E, Clark RD. (1995a) Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506, in vitro and in vivo. Br J Pharmacol 115:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Wong EH, Dumuis A, Bockaert J. (1995b) Central 5-HT4 receptors. Trends Pharmacol Sci 16:391–398. [DOI] [PubMed] [Google Scholar]
- Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. (2008) Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA 105:5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Yocca FD, Gianutsos G. (1989) Effects of imipramine and serotonin-2 agonists and antagonists on serotonin-2 and beta-adrenergic receptors following noradrenergic or serotonergic denervation. Life Sci 44:1419–1427. [DOI] [PubMed] [Google Scholar]
- Elangbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, Slocum N. (2008) 5-hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol 60:253–262. [DOI] [PubMed] [Google Scholar]
- Ellis ES, Byrne C, Murphy OE, Tilford NS, Baxter GS. (1995) Mediation by 5-hydroxytryptamine2B receptors of endothelium-dependent relaxation in rat jugular vein. Br J Pharmacol 114:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, et al. (2004) The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306:1380–1383. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. (2012) Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci 57:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. (1986) Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol 332:1–7. [DOI] [PubMed] [Google Scholar]
- Engel G, Gothert M, Muller-Schweinitzer E, Schlicker E, Sistonen L, Stadler PA. (1983) Evidence for common pharmacological properties of [3H]5-hydroxytryptamine autoreceptors in CNS and inhibitor presynaptic 5-hydroxytryptamine receptors on sympathetic nerves. Naunyn Schmiedebergs Arch Pharmacol 324:116–124. [DOI] [PubMed] [Google Scholar]
- Engel G, Hoyer D, Berthold R, Wagner H. (1981) (+/-)[125Iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol 317:277–285. [DOI] [PubMed] [Google Scholar]
- Engel G, Hoyer D, Kalkman H, Wick MB. (1985) Pharmacological similarity between the 5HT-D-receptor on the guinea pig ileum and the 5-HT2, binding site. Br J Pharmacol 84:106P. [Google Scholar]
- Engel L, Kobel B, Ontsouka EC, Graber HU, Blum JW, Steiner A, Meylan M. (2006) Distribution of mRNA coding for 5-hydroxytryptamine receptor subtypes in the intestines of healthy dairy cows and dairy cows with cecal dilatation-dislocation. Am J Vet Res 67:95–101. [DOI] [PubMed] [Google Scholar]
- Ennis MD, Ghazal NB, Hoffman RL, Smith MW, Schlachter SK, Lawson CF, Im WB, Pregenzer JF, Svensson KA, Lewis RA, et al. (1998) Isochroman-6-carboxamides as highly selective 5-HT1D agonists: potential new treatment for migraine without cardiovascular side effects. J Med Chem. 41:2180–2183. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Nöthen MM, Shimron-Abarbanell D, Rietschel M, Albus M, Borrmann M, Maier W, Franzek E, Körner J, Weigelt B, et al. (1996) The human serotonin 7 (5-HT7) receptor gene: genomic organization and systematic mutation screening in schizophrenia and bipolar affective disorder. Mol Psychiatry 1:392–397. [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. (1996) Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA 93:5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, et al. (2013) Bidirectional regulation of emotional memory by 5-HT1B receptors involves hippocampal p11. Mol Psychiatry 18:1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Lovenberg TW, Baron BM, de Lecea L, Danielson PE, Racke M, Slone AL, Siegel BW, Foye PE, Cannon K, et al. (1993) Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain. Proc Natl Acad Sci USA 90:3452–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico M, Crozier RA, Plummer MR, Cowen DS. (2001) 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience 102:361–367. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Asero B. (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169:800–801. [DOI] [PubMed] [Google Scholar]
- Eskenazi D, Brodsky M, Neumaier JF. (2015) Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience 299:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi D, Neumaier JF. (2011a) Increased expression of 5-HT6 receptors in dorsolateral striatum decreases habitual lever pressing, but does not affect learning acquisition of simple operant tasks in rats. Eur J Neurosci 34:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi D, Neumaier JF. (2011b) Increased expression of the 5-HT6 receptor by viral mediated gene transfer into posterior but not anterior dorsomedial striatum interferes with acquisition of a discrete action-outcome task. J Psychopharmacol 25:944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. (2009) The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse 63:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. (2007) The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav 87:306–314. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Dupre KB, Ostock CY, Button T, Deak T, Bishop C. (2010) Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav Pharmacol 21:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve JM, Launay JM, Kellermann O, Maroteaux L. (2007) Functions of serotonin in hypoxic pulmonary vascular remodeling. Cell Biochem Biophys 47:33–44. [DOI] [PubMed] [Google Scholar]
- Ettrup A, da Cunha-Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, Jørgensen LM, Hansen M, Baandrup AO, Bache S, et al. (2014) Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36. J Cereb Blood Flow Metab 34:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Sato M, Sarwar M, Hutchinson DS, Summers RJ. (2010) Ligand-directed signalling at beta-adrenoceptors. Br J Pharmacol 159:1022–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Galdes A, Gall M. (1991) Molecular modeling of 5-HT3 receptor ligands. Pharmacol Biochem Behav 40:1033–1040. [DOI] [PubMed] [Google Scholar]
- Fabre A, Marchal-Sommé J, Marchand-Adam S, Quesnel C, Borie R, Dehoux M, Ruffié C, Callebert J, Launay J-M, Hénin D, et al. (2008) Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur Respir J 32:426–436. [DOI] [PubMed] [Google Scholar]
- Fakhoury M. (2016) Revisiting the serotonin hypothesis: implications for major depressive disorders. Mol Neurobiol 53:2778–2786. [DOI] [PubMed] [Google Scholar]
- Falkenberg VR, Gurbaxani BM, Unger ER, Rajeevan MS. (2011) Functional genomics of serotonin receptor 2A (HTR2A): interaction of polymorphism, methylation, expression and disease association. Neuromolecular Med 13:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanburg BL, Lee SL. (1997) A new role for an old molecule: serotonin as a mitogen. Am J Physiol 272:L795–L806. [DOI] [PubMed] [Google Scholar]
- Fanburg BL, Lee SL. (2000) A role for the serotonin transporter in hypoxia-induced pulmonary hypertension. J Clin Invest 105:1521–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. (1988) The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 335:358–360. [DOI] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Regan JW, Cotecchia S, Lefkowitz RJ, Caron MG. (1989) Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem 264:14848–14852. [PubMed] [Google Scholar]
- Faris PL, Eckert ED, Kim SW, Meller WH, Pardo JV, Goodale RL, Hartman BK. (2006) Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. J Affect Disord 92:79–90. [DOI] [PubMed] [Google Scholar]
- Färkkilä M, Diener HC, Géraud G, Láinez M, Schoenen J, Harner N, Pilgrim A, Reuter U, COL MIG-202 study group (2012) Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 11:405–413. [DOI] [PubMed] [Google Scholar]
- Fasmer OB, Berge OG, Post C, Hole K. (1986) Effects of the putative 5-HT1A receptor agonist 8-OH-2-(di-n-propylamino)tetralin on nociceptive sensitivity in mice. Pharmacol Biochem Behav 25:883–888. [DOI] [PubMed] [Google Scholar]
- Fazzino F, Urbina M, Cedeño N, Lima L. (2009) Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. Int Immunopharmacol 9:463–467. [DOI] [PubMed] [Google Scholar]
- Fei L, Abrardi L, Mediati RD. (2012) Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol 2:211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinle C, Read NW. (1996) Ondansetron reduces nausea induced by gastroduodenal stimulation without changing gastric motility. Am J Physiol 271:G591–G597. [DOI] [PubMed] [Google Scholar]
- Felder CC, Kanterman RY, Ma AL, Axelrod J. (1990) Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA 87:2187–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Mohapatra S, Klimko PG, Hellberg MR, May JA, Kelly C, Williams G, McLaughlin MA, Klimko PG, Sharif NA. (2007) Novel benzodifuran analogs as potent 5-HT2A receptor agonists with ocular hypotensive activity. Bioorg Med Chem Lett 17:2998–3002. [DOI] [PubMed] [Google Scholar]
- Feniuk W, Humphrey PP, Watts AD. (1981) Modification of the vasomotor actions of methysergide in the femoral arterial bed of the anaesthetized dog by changes in sympathetic nerve activity. J Auton Pharmacol 1:127–132. [DOI] [PubMed] [Google Scholar]
- Fentress HM, Grinde E, Mazurkiewicz JE, Backstrom JR, Herrick-Davis K, Sanders-Bush E. (2005) Pharmacological properties of the Cys23Ser single nucleotide polymorphism in human 5-HT2C receptor isoforms. Pharmacogenomics J 5:244–254. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. (2008) Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry 63:207–213. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ranvier GG, Weng J, Yeh RF, Khanafshar E, Suh I, Barker C, Duh QY, Clark OH, Kebebew E. (2008) Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg 143:841–846, discussion 846. [DOI] [PubMed] [Google Scholar]
- Ferreira HS, Oliveira E, Faustino TN, Silva EdeC, Fregoneze JB. (2005) Effect of the activation of central 5-HT2C receptors by the 5-HT2C agonist mCPP on blood pressure and heart rate in rats. Brain Res 1040:64–72. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Färkkilä M, Reuter U, Pilgrim A, Davis C, Krauss M, Diener HC, European COL-144 Investigators (2010) Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan--a randomised proof-of-concept trial. Cephalalgia 30:1170–1178. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Müller W, Stratz T, Candelario-Jalil E. (2004) Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl 119:9–11. [PubMed] [Google Scholar]
- Fijał K, Pachuta A, McCreary AC, Wydra K, Nowak E, Papp M, Bieńkowski p, Kotlińska J, Filip M. (2010) Effects of serotonin (5-HT)6 receptor ligands on responding for cocaine reward and seeking in rats. Pharmacol Rep 62:1005–1014. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. (2002) Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacol Biochem Behav 71:745–756. [DOI] [PubMed] [Google Scholar]
- Filla SA, Mathes BM, Johnson KW, Phebus LA, Cohen ML, Nelson DL, Zgombick JM, Erickson JA, Schenck KW, Wainscott DB, et al. (2003) Novel potent 5-HT(1F) receptor agonists: structure-activity studies of a series of substituted N-[3-(1-methyl-4-piperidinyl)-1H-pyrrolo[3,2-b]pyridin-5-yl]amides. J Med Chem 46:3060–3071. [DOI] [PubMed] [Google Scholar]
- Fillion G. (2000) Potential of 5-HT-moduline as a drug target for affective disorders. Curr Opin Investig Drugs 1:104–109. [PubMed] [Google Scholar]
- Fink KB, Göthert M. (2007) 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 59:360–417. [DOI] [PubMed] [Google Scholar]
- Fink LH, Anastasio NC, Fox RG, Rice KC, Moeller FG, Cunningham KA. (2015) Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacology 40:1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. (2002) 5-HT(2A) receptors: location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am J Physiol Gastrointest Liver Physiol 282:G877–G893. [DOI] [PubMed] [Google Scholar]
- Fiorica-Howells E, Maroteaux L, Gershon MD. (2000) Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci 20:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Mercé R, Holenz J, Vrang N, Sørensen RV, Heal D, et al. (2006) Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats [published correction appears in Br J Pharmacol (2007) 151:564]. Br J Pharmacol 148:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, et al. (2000) Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 57:75–81. [PubMed] [Google Scholar]
- Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. (1999) Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology 21(2 Suppl):82S–90S. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, et al. (1996) Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res 73:337–353. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. (2004) Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology 29:308–318. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. (2002a) Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27:576–586. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Robinson SR, Baker GB. (2002b) Multiple 5-HT receptors are involved in the effects of acute MDMA treatment: studies on locomotor activity and responding for conditioned reinforcement. Psychopharmacology (Berl) 162:282–291. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. (2011) Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology 61:468–477. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Lê AD, Higgins GA. (2012) Effects of the 5-HT2C receptor agonist Ro60-0175 and the 5-HT2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology 62:2288–2298. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. (2008) The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology 33:1402–1412. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195:223–234. [DOI] [PubMed] [Google Scholar]
- Flomen R, Knight J, Sham P, Kerwin R, Makoff A. (2004) Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res 32:2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian JA, Watts SW. (1998) Integration of mitogen-activated protein kinase kinase activation in vascular 5-hydroxytryptamine2A receptor signal transduction. J Pharmacol Exp Ther 284:346–355. [PubMed] [Google Scholar]
- Foguet M, Hoyer D, Pardo LA, Parekh A, Kluxen FW, Kalkman HO, Stühmer W, Lübbert H. (1992a) Cloning and functional characterization of the rat stomach fundus serotonin receptor. EMBO J 11:3481–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foguet M, Nguyen H, Le H, Lübbert H. (1992b) Structure of the mouse 5-HT1C, 5-HT2 and stomach fundus serotonin receptor genes. Neuroreport 3:345–348. [DOI] [PubMed] [Google Scholar]
- Fone KC. (2008) An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology 55:1015–1022. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Daniels SE, Wong EH, Clark RD, Eglen RM. (1997) The effects of novel, selective 5-hydroxytryptamine (5-HT)4 receptor ligands in rat spatial navigation. Neuropharmacology 36:689–696. [DOI] [PubMed] [Google Scholar]
- Forbes IT, Dabbs S, Duckworth DM, Jennings AJ, King FD, Lovell PJ, Brown AM, Collin L, Hagan JJ, Middlemiss DN, et al. (1998) (R)-3,N-dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl) propyl]benzenesulfonamide: the first selective 5-HT7 receptor antagonist. J Med Chem 41:655–657. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Litto WJ, Metzler CW, Marrosu F, Tada K, Jacobs BL. (1994) Single-unit responses of serotonergic dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. J Pharmacol Exp Ther 270:1345–1358. [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, Jacobs BL. (1996) WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther 278:752–762. [PubMed] [Google Scholar]
- Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. (2009) Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 104:1831–1843; quiz 1844. [DOI] [PubMed] [Google Scholar]
- Fox SH. (2014) Pimavanserin as treatment for Parkinson’s disease psychosis. Lancet 383:494–496. [DOI] [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Kuemmerle JF, Grider JR. (1996) Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology 111:1281–1290. [DOI] [PubMed] [Google Scholar]
- Fozard JR. (1984) MDL 72222: a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn Schmiedebergs Arch Pharmacol 326:36–44. [DOI] [PubMed] [Google Scholar]
- Francis PT, Pangalos MN, Pearson RC, Middlemiss DN, Stratmann GC, Bowen DM. (1992) 5-Hydroxytryptamine1A but not 5-hydroxytryptamine2 receptors are enriched on neocortical pyramidal neurones destroyed by intrastriatal volkensin. J Pharmacol Exp Ther 261:1273–1281. [PubMed] [Google Scholar]
- Francken BJ, Jurzak M, Vanhauwe JF, Luyten WH, Leysen JE. (1998) The human 5-ht5A receptor couples to Gi/Go proteins and inhibits adenylate cyclase in HEK 293 cells. Eur J Pharmacol 361:299–309. [DOI] [PubMed] [Google Scholar]
- Frank MG, Stryker MP, Tecott LH. (2002) Sleep and sleep homeostasis in mice lacking the 5-HT2c receptor. Neuropsychopharmacology 27:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, Hwang DR, Reich E, Cangiano C, Gil R, et al. (2006) Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology (Berl) 189:155–164. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, Hansson KJ, Stouffer DG, Parsons LH. (2002) 5-HT(6) receptor antagonism potentiates the behavioral and neurochemical effects of amphetamine but not cocaine. Neuropharmacol 42:170–180. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272. [DOI] [PubMed] [Google Scholar]
- Freire-Garabal M, Núñez MJ, Balboa J, López-Delgado P, Gallego R, García-Caballero T, Fernández-Roel MD, Brenlla J, Rey-Méndez M. (2003) Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br J Pharmacol 139:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. (2014) 20 years of leptin: leptin at 20: an overview. J Endocrinol 223:T1–T8. [DOI] [PubMed] [Google Scholar]
- Frohman MA. (2015) The phospholipase D superfamily as therapeutic targets. Trends Pharmacol Sci 36:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, et al. (2008) Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry 63:569–576. [DOI] [PubMed] [Google Scholar]
- Froldi G, Montopoli M, Zanetti M, Dorigo P, Caparrotta L. (2008) 5-HT1B receptor subtype and aging in rat resistance vessels. Pharmacology 81:70–78. [DOI] [PubMed] [Google Scholar]
- Fugelli A, Moret C, Fillion G. (1997) Autoradiographic localization of 5-HT1E and 5-HT1F binding sites in rat brain: effect of serotonergic lesioning. J Recept Signal Transduct Res 17:631–645. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Nelson DL, Kashihara K, Varga E, Roeske WR, Yamamura HI. (1990) The cloning and sequence analysis of the rat serotonin-1A receptor gene. Life Sci 47:PL127–PL132. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Oyama Y, Nishitarumizu A, Omura M, Nose K, Deshimaru M. (2015) Identification of an RNA element for specific coordination of A-to-I RNA editing on HTR2C pre-mRNA. Genes Cells 20:834–846. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S. (2014) Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231:2291–2298. [DOI] [PubMed] [Google Scholar]
- Furlotti G, Alisi MA, Apicella C, Capezzone de Joannon A, Cazzolla N, Costi R, Cuzzucoli Crucitti G, Garrone B, Iacovo A, Magarò G, et al. (2012) Discovery and pharmacological profile of new 1H-indazole-3-carboxamide and 2H-pyrrolo[3,4-c]quinoline derivatives as selective serotonin 4 receptor ligands. J Med Chem 55:9446–9466. [DOI] [PubMed] [Google Scholar]
- Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Käch A, Humar B, Graf R, Moritz W, Clavien P-A. (2011) Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci USA 108:2945–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futuro-Neto HA, Pires JG, Gilbey MP, Ramage AG. (1993) Evidence for the ability of central 5-HT1A receptors to modulate the vagal bradycardia induced by stimulating the upper airways of anesthetized rabbits with smoke. Brain Res 629:349–354. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Calvo F, Garriga P, Tena M, Narvaez M, Millón C, Parrado C, et al. (2012) On the existence and function of galanin receptor heteromers in the central nervous system. Front Endocrinol (Lausanne) 3:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelt BT, Okka M, Dean TR, Kaufman PL, et al. (2005) Aqueous humor dynamics in monkeys after topical R-DOI. Invest Ophthalmol Vis Sci 46:4691–4696. [DOI] [PubMed] [Google Scholar]
- Gaddum JH, Picarelli ZP. (1957) Two kinds of tryptamine receptor. Br J Pharmacol 120(4 Suppl):134–139; discussion 132–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggi R, Dall’Olio R, Roncada P. (1997) Effect of the selective 5-HT receptor agonists 8-OHePAT and DOI on behavior and brain biogenic amines of rats. Gen Pharmacol 28:583–587. [DOI] [PubMed] [Google Scholar]
- Gale JD, Grossman CJ, Whitehead JW, Oxford AW, Bunce KT, Humphrey PP. (1994) GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br J Pharmacol 111:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Boggs JD, Miller KL, Bonaventure P, Atack JR. (2008) Effects of SB-269970, a 5-HT7 receptor antagonist, in mouse models predictive of antipsychotic-like activity. Behav Pharmacol 19:153–159. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. (2007) Actions of sumatriptan on myenteric neurones: relief from an old headache in the enteric nervous system? Neurogastroenterol Motil 19:1–3. [DOI] [PubMed] [Google Scholar]
- Galusca B, Costes N, Zito NG, Peyron R, Bossu C, Lang F, Le Bars D, Estour B. (2008) Organic background of restrictive-type anorexia nervosa suggested by increased serotonin 1A receptor binding in right frontotemporal cortex of both lean and recovered patients: [18F]MPPF PET scan study. Biol Psychiatry 64:1009–1013. [DOI] [PubMed] [Google Scholar]
- Garattini S, Caccia S, Mennini T. (1982) Notes on buspirone’s mechanisms of action. J Clin Psychiatry 43:19–24. [PubMed] [Google Scholar]
- García-Alcocer G, Rodríguez A, Moreno-Layseca P, Berumen LC, Escobar J, Miledi R. (2010) Serotonin receptor 5-HT5A in rat hippocampus decrease by leptin treatment. Neurosci Lett 486:171–173. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Meng Q, Richardson-Jones J, Dranovsky A, Leonardo ED. (2016) Disruption of 5-HT1A function in adolescence but not early adulthood leads to sustained increases of anxiety. Neuroscience 321:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. (2014) 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl) 231:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ladona F, Amberg W, Kling A, Lange UEW, Hornberger W, Wernet W, Netz A, Ochse M, Mezler M, Meyer AH, et al. (2006) First potent and highly selective antagonists for the human 5-HT5A receptor. Program number 33.1/B73, in 2006 Neuroscience Meeting Planner. Society for Neuroscience, Atlanta, GA. [Google Scholar]
- García-Nafría J, Nehmé R, Edwards PC, Tate CG. (2018) Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature 558:620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardani M, Biello SM. (2008) The effects of photic and nonphotic stimuli in the 5-HT7 receptor knockout mouse. Neuroscience 152:245–253. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Chin AC, Marinovich AM, Heller MR, Ciaranello RD. (1994) Cloning and functional promoter mapping of the rat serotonin-2 receptor gene. Mol Cell Neurosci 5:291–300. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Ciaranello RD. (1995) Transcriptional control of the rat serotonin-2 receptor gene. Brain Res Mol Brain Res 31:201–209. [DOI] [PubMed] [Google Scholar]
- Garratt JC, Alreja M, Aghajanian GK. (1993) LSD has high efficacy relative to serotonin in enhancing the cationic current Ih: intracellular studies in rat facial motoneurons. Synapse 13:123–134. [DOI] [PubMed] [Google Scholar]
- Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG. (2014) Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther 350:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Clifford EM, Cowen PJ, Sharp T. (1999) Effects of (-)-tertatolol, (-)-penbutolol and (+/-)-pindolol in combination with paroxetine on presynaptic 5-HT function: an in vivo microdialysis and electrophysiological study. Br J Pharmacol 127:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiorek A, Trattnig SM, Ahring PK, Kristiansen U, Frølund B, Frederiksen K, Jensen AA. (2016) Delineation of the functional properties and the mechanism of action of TMPPAA, an allosteric agonist and positive allosteric modulator of 5-HT3 receptors. Biochem Pharmacol 110-111:92–108. [DOI] [PubMed] [Google Scholar]
- Gaster LM, Ham P, Joiner GF, King FD, Mulholland KR, Wyman PA, Hagan JJ, Price GW, Roberts C, Routledge C, et al. (1998) The selective 5-HT1B receptor inverse agonist SB-224289, potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. Ann N Y Acad Sci 861:270–271. [DOI] [PubMed] [Google Scholar]
- Gatch MB. (2003) Discriminative stimulus effects of m-chlorophenylpiperazine as a model of the role of serotonin receptors in anxiety. Life Sci 73:1347–1367. [DOI] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK, Williams KW. (2015) Neural control of energy balance: translating circuits to therapies. Cell 161:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavarini S, Bécamel C, Altier C, Lory P, Poncet J, Wijnholds J, Bockaert J, Marin P. (2006) Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell 17:4619–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaven F, Pellissier LP, Queffeulou E, Cochet M, Bockaert J, Dumuis A, Claeysen S. (2013) Pharmacological profile of engineered 5-HT4 receptors and identification of 5-HT4 receptor-biased ligands. Brain Res 1511:65–72. [DOI] [PubMed] [Google Scholar]
- Ge J, Barnes NM. (1996) 5-HT4 receptor-mediated modulation of 5-HT release in the rat hippocampus in vivo. Br J Pharmacol 117:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee AD, Martarello L, Passchier J, Wishart M, Parker C, Matthews J, Comley R, Hopper R, Gunn R. (2008) Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5-HT4 receptor radioligand for PET imaging in man. Curr Radiopharm 1:110–114. [Google Scholar]
- Geerts IS, De Meyer GR, Bult H. (2000) Collar-induced elevation of mRNA and functional activity of 5-HT(1B) receptor in the rabbit carotid artery. Br J Pharmacol 131:1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber EI, Kroeze WK, Willins DL, Gray JA, Sinar CA, Hyde EG, Gurevich V, Benovic J, Roth BL. (1999) Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J Neurochem 72:2206–2214. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Rao PA, Pauls DL, Hamblin MW, Sibley DR, Kidd KK. (1995) Assignment of the 5HT7 receptor gene (HTR7) to chromosome 10q and exclusion of genetic linkage with Tourette syndrome. Genomics 26:207–209. [DOI] [PubMed] [Google Scholar]
- Gelez H, Greggain-Mohr J, Pfaus JG, Allers KA, Giuliano F. (2013) Flibanserin treatment increases appetitive sexual motivation in the female rat. J Sex Med 10:1231–1239. [DOI] [PubMed] [Google Scholar]
- Gellynck E, Andressen KW, Lintermans B, Haegeman G, Levy FO, Vanhoenacker P, Van Craenenbroeck K. (2012) Biochemical and pharmacological study of N-linked glycosylation of the human serotonin 5-HT7(a) receptor. FEBS J 279:1994–2003. [DOI] [PubMed] [Google Scholar]
- Gellynck E, Laenen K, Andressen KW, Lintermans B, De Martelaere K, Matthys A, Levy FO, Haegeman G, Vanhoenacker P, Van Craenenbroeck K. (2008) Cloning, genomic organization and functionality of 5-HT7 receptor splice variants from mouse brain. Gene 426:23–31 [DOI] [PubMed] [Google Scholar]
- Gerald C, Adham N, Kao HT, Olsen MA, Laz TM, Schechter LE, Bard JA, Vaysse PJ, Hartig PR, Branchek TA, et al. (1995) The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J 14:2806–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C, Martres MP, Lefèvre K, Miquel MC, Vergé D, Lanfumey L, Doucet E, Hamon M, el Mestikawy S. (1997) Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res 746:207–219. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergs U, Baumann M, Böckler A, Buchwalow IB, Ebelt H, Fabritz L, Hauptmann S, Keller N, Kirchhof P, Klöckner U, et al. (2010) Cardiac overexpression of the human 5-HT4 receptor in mice. Am J Physiol Heart Circ Physiol 299:H788–H798. [DOI] [PubMed] [Google Scholar]
- Gergs U, Neumann J, Simm A, Silber RE, Remmers FO, Läer S. (2009) Phosphorylation of phospholamban and troponin I through 5-HT4 receptors in the isolated human atrium. Naunyn Schmiedebergs Arch Pharmacol 379:349–359. [DOI] [PubMed] [Google Scholar]
- Gershon MD. (2003) Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol 3:600–607. [DOI] [PubMed] [Google Scholar]
- Gershon MD. (2011) Behind an enteric neuron there may lie a glial cell. J Clin Invest 121:3386–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. (2012) Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc 123:268–280; discussion 280. [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Liu MT. (2007) Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil 19 (Suppl 2):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132:397–414. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281:10856–10864. [DOI] [PubMed] [Google Scholar]
- Gettys TW, Fields TA, Raymond JR. (1994) Selective activation of inhibitory G-protein alpha-subunits by partial agonists of the human 5-HT1A receptor. Biochemistry 33:4283–4290. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Timmermans JP. (2002) Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum. J Chem Neuroanat 24:65–74. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. (2009) Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137:1649–1660. [DOI] [PubMed] [Google Scholar]
- Gibson WT, Ebersole BJ, Bhattacharyya S, Clayton P, Farooqi IS, Sealfon SC, O’Rahilly S. (2004) Mutational analysis of the serotonin receptor 5HT2c in severe early-onset human obesity. Can J Physiol Pharmacol 82:426–429. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A. (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. (2018) Current understanding of the human microbiome. Nat Med 24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F, Dourish CT. (1987) Effects of the novel anxiolytics gepirone, buspirone and ipsapirone on free feeding and on feeding induced by 8-OH-DPAT. Psychopharmacology (Berl) 93:349–352. [DOI] [PubMed] [Google Scholar]
- Gilet M, Eutamene H, Han H, Kim HW, Bueno L. (2014) Influence of a new 5-HT4 receptor partial agonist, YKP10811, on visceral hypersensitivity in rats triggered by stress and inflammation. Neurogastroenterol Motil 26:1761–1770. [DOI] [PubMed] [Google Scholar]
- Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. (2005) Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128:962–974. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. (2003) New lessons from knockout mice: the role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. CNS Spectr 8:572–577. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Snowman AM, Sibley DR, Snyder SH. (1995) Clozapine: selective labeling of sites resembling 5HT6 serotonin receptors may reflect psychoactive profile. Mol Med 1:398–406. [PMC free article] [PubMed] [Google Scholar]
- Glennon JC, Van Scharrenburg G, Ronken E, Hesselink MB, Reinders JH, Van Der Neut M, Long SK, Feenstra RW, McCreary AC. (2006) In vitro characterization of SLV308 (7-[4-methyl-1-piperazinyl]-2(3H)-benzoxazolone, monohydrochloride): a novel partial dopamine D2 and D3 receptor agonist and serotonin 5-HT1A receptor agonist. Synapse 60:599–608. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K. (1994) Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem 37:1929–1935. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, McLaughlin MA, Sharif NA. (2004) β-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-Bromo-2,5-dimethoxyphenyl)-2-aminopropane. J Med Chem 47:6034–6041. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Lee M, Rangisetty JB, Dukat M, Roth BL, Savage JE, McBride A, Rauser L, Hufeisen S, Lee DK. (2000) 2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors. J Med Chem 43:1011–1018. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Raghupathi R, Bartyzel P, Teitler M, Leonhardt S. (1992a) Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J Med Chem 35:734–740. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Seggel MR, Soine WH, Herrick-Davis K, Lyon RA, Titeler M. (1988) [125I]-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane: an iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem 31:5–7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Teitler M, Sanders-Bush E. (1992b) Hallucinogens and serotonergic mechanisms. NIDA Res Monogr 119:131–135. [PubMed] [Google Scholar]
- Glusa E, Pertz HH. (2000) Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT(2B) receptors. Br J Pharmacol 130:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi M, Parotti L, Mennini T. (1996) Are 5-hydroxytryptamine7 receptors involved in [3H]5-hydroxytryptamine binding to 5-hydroxytryptamine 1nonA-nonB receptors in rat hypothalamus? Mol Pharmacol 49:556–559. [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. (2000) Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse 36:205–221. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H. (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord 22:179–186. [DOI] [PubMed] [Google Scholar]
- Gohla A, Offermanns S, Wilkie TM, Schultz G. (1999) Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J Biol Chem 274:17901–17907. [DOI] [PubMed] [Google Scholar]
- Gökben S, Ardıç UA, Serdaroğlu G. (2012) Use of buspirone and fluoxetine for breathing problems in Rett syndrome. Pediatr Neurol 46:192–194. [DOI] [PubMed] [Google Scholar]
- Goldberg PA, Kamm MA, Setti-Carraro P, van der Sijp JRM, Roth C. (1996) Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron). Digestion 57:478–483. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Roon KI, Offen WW, Ramadan NM, Phebus LA, Johnson KW, Schaus JM, Ferrari MD. (2001) Selective seratonin 1F (5-HT(1F)) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet 358:1230–1234. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. (2007) Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 144:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Cutler NR, Leibowitz MT, Spierings ELH, Klapper JA, Diamond S, Goldstein J, Smith T, Couch JR, Fleishaker J, et al. (2001) Safety and efficacy of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine. Cephalalgia 21:727–732. [DOI] [PubMed] [Google Scholar]
- González AA, Puig MM. (1997) Ondansetron facilitates neuromuscular transmission in the guinea-pig ileum. Eur J Pharmacol 328:201–206. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Chávez-Pascacio K, Meneses A. (2013) Role of 5-HT5A receptors in the consolidation of memory. Behav Brain Res 252:246–251. [DOI] [PubMed] [Google Scholar]
- González-Hernández A, Manrique-Maldonado G, Lozano-Cuenca J, Muñoz-Islas E, Centurión D, Maassen VanDenBrink A, Villalón CM. (2011) The 5-HT(1) receptors inhibiting the rat vasodepressor sensory CGRPergic outflow: further involvement of 5-HT(1F), but not 5-HT(1A) or 5-HT(1D), subtypes. Eur J Pharmacol 659:233–243. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, et al. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Rodríguez-Puertas R, Gabilondo AM, Meana JJ. (2000) Characterization of receptor-mediated [35S]GTPgammaS binding to cortical membranes from postmortem human brain. Eur J Pharmacol 390:25–36. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. [DOI] [PubMed] [Google Scholar]
- Goodfellow NM, Bailey CDC, Lambe EK. (2012) The native serotonin 5-HT(5A) receptor: electrophysiological characterization in rodent cortex and 5-HT(1A)-mediated compensatory plasticity in the knock-out mouse. J Neurosci 32:5804–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. (2009) Layer II/III of the prefrontal cortex: inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci 29:10094–10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow NM, Sargin D, Ansorge MS, Gingrich JA, Lambe EK. (2014) Mice with compromised 5-HTT function lack phosphotyrosine-mediated inhibitory control over prefrontal 5-HT responses. J Neurosci 34:6107–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göoz M, Göoz P, Luttrell LM, Raymond JR. (2006) 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem 281:21004–21012. [DOI] [PubMed] [Google Scholar]
- Gore S, Gilmore IT, Haigh CG, Brownless SM, Stockdale H, Morris AI. (1990) Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther 4:139–144. [DOI] [PubMed] [Google Scholar]
- Göthert M, Schlicker E, Kollecker P. (1986) Receptor-mediated effects of serotonin and 5-methoxytryptamine on noradrenaline release in the rat vena cava and in the heart of the pithed rat. Naunyn Schmiedebergs Arch Pharmacol 332:124–130. [DOI] [PubMed] [Google Scholar]
- Göthert M, Weinheimer G. (1979) Extracellular 5-hydroxytryptamine inhibits 5-hydroxytryptamine release from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol 310:93–96. [DOI] [PubMed] [Google Scholar]
- Goto K, Kawahara I, Kuniyasu H, Takaki M. (2015) A protein tyrosine kinase receptor, c-RET signaling pathway contributes to the enteric neurogenesis induced by a 5-HT4 receptor agonist at an anastomosis after transection of the gut in rodents. J Physiol Sci 65:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. (2011) Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem 116:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R, Salahudeen AA, Jansen M. (2011) Engineering a prokaryotic Cys-loop receptor with a third functional domain. J Biol Chem 286:34635–34642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozlan H, El Mestikawy S, Pichat L, Glowinski J, Hamon M. (1983) Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature 305:140–142. [DOI] [PubMed] [Google Scholar]
- Graf M, Jakus R, Kantor S, Levay G, Bagdy G. (2004) Selective 5-HT1A and 5-HT7 antagonists decrease epileptic activity in the WAG/Rij rat model of absence epilepsy. Neurosci Lett 359:45–48. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Grabtree GW, Hen R. (2001) Human 5-HT(5) receptors: the 5-HT(5A) receptor is functional but the 5-HT(5B) receptor was lost during mammalian evolution. Eur J Pharmacol 418:157–167. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. (1999) Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22:581–591. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Argüelles CF, Rocha-González HI, Godínez-Chaparro B, Flores-Murrieta FJ, Villalón CM. (2010) The role of peripheral 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E and 5-HT1F serotonergic receptors in the reduction of nociception in rats. Neuroscience 165:561–568. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Graf R, Navarra R, Sung A, Logue SF, Stack G, Huselton C, Liu Z, Comery TA, Marquis KL, et al. (2009) WAY-163909, a 5-HT2C agonist, enhances the preclinical potency of current antipsychotics. Psychopharmacology (Berl) 204:37–48. [DOI] [PubMed] [Google Scholar]
- Graveleau C, Paust H-J, Schmidt-Grimminger D, Mukhopadhyay AK. (2000) Presence of a 5-HT7 receptor positively coupled to adenylate cyclase activation in human granulosa-lutein cells. J Clin Endocrinol Metab 85:1277–1286. [DOI] [PubMed] [Google Scholar]
- Graves SM, Napier TC. (2012) SB 206553, a putative 5-HT2C inverse agonist, attenuates methamphetamine-seeking in rats. BMC Neurosci 13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. (2007) Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 184:31–38. [DOI] [PubMed] [Google Scholar]
- Green T, Stauffer KA, Lummis SC. (1995) Expression of recombinant homo-oligomeric 5-hydroxytryptamine3 receptors provides new insights into their maturation and structure. J Biol Chem 270:6056–6061. [DOI] [PubMed] [Google Scholar]
- Greene EL, Houghton O, Collinsworth G, Garnovskaya MN, Nagai T, Sajjad T, Bheemanathini V, Grewal JS, Paul RV, Raymond JR. (2000) 5-HT(2A) receptors stimulate mitogen-activated protein kinase via H(2)O(2) generation in rat renal mesangial cells. Am J Physiol Renal Physiol 278:F650–F658. [DOI] [PubMed] [Google Scholar]
- Greuel JM, Glaser T. (1992) The putative 5-HT1A receptor antagonists NAN-190 and BMY 7378 are partial agonists in the rat dorsal raphe nucleus in vitro. Eur J Pharmacol 211:211–219. [DOI] [PubMed] [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. (1998) 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 115:370–380. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 30:1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bonnin A, Fillion MP, Ruat M, Traiffort E, Fillion G. (1998) Characterization of 5-ht6 receptor and expression of 5-ht6 mRNA in the rat brain during ontogenetic development. Naunyn Schmiedebergs Arch Pharmacol 357:393–400. [DOI] [PubMed] [Google Scholar]
- Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. (2012) Neuronal serotonin regulates growth of the intestinal mucosa in mice [published correction appears in Gastroenterology (2013) 144:249]. Gastroenterology 143:408–417.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman CJ, Kilpatrick GJ, Bunce KT. (1993) Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br J Pharmacol 109:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Corrigall WA, Higgins GA. (2001) Activation of 5-HT(2C) receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology (Berl) 157:292–298. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. (2000) Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295:1183–1191. [PubMed] [Google Scholar]
- Grottick AJ, Whelan K, Sanabria EK, Behan DP, Morgan M, Sage C. (2015) Investigating interactions between phentermine, dexfenfluramine, and 5-HT2C agonists, on food intake in the rat. Psychopharmacology (Berl) 232:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover M, Camilleri M. (2013) Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J Gastroenterol 48:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueb M, Wallenfels-Thilo B, Denk O, Mielke J, Reinthal E, Rohrbach JM, Bartz-Schmidt KU. (2006) Monoamine receptors in human corneal epithelium and endothelium. Acta Ophthalmol Scand 84:110–115. [DOI] [PubMed] [Google Scholar]
- Gudermann T, Levy FO, Birnbaumer M, Birnbaumer L, Kaumann AJ. (1993) Human S31 serotonin receptor clone encodes a 5-hydroxytryptamine1E-like serotonin receptor. Mol Pharmacol 43:412–418. [PubMed] [Google Scholar]
- Guenther U, Wrigge H, Theuerkauf N, Boettcher MF, Wensing G, Zinserling J, Putensen C, Hoeft A. (2010) Repinotan, a selective 5-HT1A-R-agonist, antagonizes morphine-induced ventilatory depression in anesthetized rats. Anesth Analg 111:901–907. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Di Giovanni G. (2015) Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol 6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, et al. (2006) Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98:1323–1330. [DOI] [PubMed] [Google Scholar]
- Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. (2005) Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 111:2812–2819. [DOI] [PubMed] [Google Scholar]
- Guillet-Deniau I, Burnol AF, Girard J. (1997) Identification and localization of a skeletal muscle secrotonin 5-HT2A receptor coupled to the Jak/STAT pathway. J Biol Chem 272:14825–14829. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, et al. (2009) RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 179:1151–1158. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, et al. (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther S, Maroteaux L, Schwarzacher SW. (2006) Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol 66:949–961. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Lummis SC. (1999) Diltiazem causes open channel block of recombinant 5-HT3 receptors. J Physiol 519:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Lummis SC. (2001) Conversion of the ion selectivity of the 5-HT(3a) receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J Biol Chem 276:10977–10983. [PubMed] [Google Scholar]
- Gunthorpe MJ, Peters JA, Gill CH, Lambert JJ, Lummis SC. (2000) The 4'lysine in the putative channel lining domain affects desensitization but not the single-channel conductance of recombinant homomeric 5-HT3A receptors. J Physiol 522:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Song E, Ma S, Wang X, Gao S, Shao C, Hu S, Jia L, Tian R, Xu T, et al. (2012) Proteomics strategy to identify substrates of LNX, a PDZ domain-containing E3 ubiquitin ligase. J Proteome Res 11:4847–4862. [DOI] [PubMed] [Google Scholar]
- Gupta D, Prabhakar V, Radhakrishnan M. (2016) 5HT3 receptors: target for new antidepressant drugs. Neurosci Biobehav Rev 64:311–325. [DOI] [PubMed] [Google Scholar]
- Gupta S, Villalón CM. (2010) The relevance of preclinical research models for the development of antimigraine drugs: focus on 5-HT(1B/1D) and CGRP receptors. Pharmacol Ther 128:170–190. [DOI] [PubMed] [Google Scholar]
- Guptan P, Dhingra A, Panicker MM. (1997) Multiple transcripts encode the 5-HT1F receptor in rodent brain. Neuroreport 8:3317–3321. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. (2002a) Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci 22:10529–10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. (2002b) Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 34:349–356. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, et al. (2005) Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 48:492–502. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Egan E, Cook GP, Stanton JA, Beer MS, Rosahl TW, Hartmann S, Kulagowski J, McAllister G, Fone KC, et al. (2003) The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology 44:1031–1037. [DOI] [PubMed] [Google Scholar]
- Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, Buettner M, Bleich A, Pabst O, Bachmann O, et al. (2014a) Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis 20:1516–1529. [DOI] [PubMed] [Google Scholar]
- Guseva D, Wirth A, Ponimaskin E. (2014b) Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front Behav Neurosci 8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. (1996) A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol 117:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie CR, Murray AT, Franklin AA, Hamblin MW. (2005) Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J Pharmacol Exp Ther 313:1003–1010. [DOI] [PubMed] [Google Scholar]
- Ha CM, Park D, Kim Y, Na M, Panda S, Won S, Kim H, Ryu H, Park ZY, Rasenick MM, et al. (2015) SNX14 is a bifunctional negative regulator for neuronal 5-HT6 receptor signaling. J Cell Sci 128:1848–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr ME, Fisher P, Holst K, Madsen K, Jensen CG, Marner L, Lehel S, Baaré W, Knudsen G, Hasselbalch S. (2013) The 5-HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum Brain Mapp 34:3066–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafdi Z, Couette S, Comoy E, Prie D, Amiel C, Friedlander G. (1996) Locally formed 5-hydroxytryptamine stimulates phosphate transport in cultured opossum kidney cells and in rat kidney. Biochem J 320:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, et al. (2000) Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Slade PD, Gaster L, Jeffrey P, Hatcher JP, Middlemiss DN. (1997) Stimulation of 5-HT1B receptors causes hypothermia in the guinea pig. Eur J Pharmacol 331:169–174. [DOI] [PubMed] [Google Scholar]
- Hagberg B. (2002) Clinical manifestations and stages of Rett syndrome. Ment Retard Dev Disabil Res Rev 8:61–65. [DOI] [PubMed] [Google Scholar]
- Hagberg GB, Blomstrand F, Nilsson M, Tamir H, Hansson E. (1998) Stimulation of 5-HT2A receptors on astrocytes in primary culture opens voltage-independent Ca2+ channels. Neurochem Int 32:153–162. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. (2017) The serotonergic 5-HT4 receptor: a unique modulator of hippocampal synaptic information processing and cognition. Neurobiol Learn Mem 138:145–153. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 277:99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. (2000) Separate systems for serotonin and leptin in appetite control. Ann Med 32:222–232. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW. (2016) Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology 107:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. (2007) Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs 67:27–55. [DOI] [PubMed] [Google Scholar]
- Halford JC, Lawton CL, Blundell JE. (1997) The 5-HT2 receptor agonist MK-212 reduces food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol Biochem Behav 56:41–46. [DOI] [PubMed] [Google Scholar]
- Halford JC, Wanninayake SC, Blundell JE. (1998) Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav 61:159–168. [DOI] [PubMed] [Google Scholar]
- Hall MD, el Mestikawy S, Emerit MB, Pichat L, Hamon M, Gozlan H. (1985) [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding to pre- and postsynaptic 5-hydroxytryptamine sites in various regions of the rat brain. J Neurochem 44:1685–1696. [DOI] [PubMed] [Google Scholar]
- Hamblin MW, Guthrie CR, Kohen R, Heidmann DE. (1998) Gs protein-coupled serotonin receptors: receptor isoforms and functional differences. Ann N Y Acad Sci 861:31–37. [DOI] [PubMed] [Google Scholar]
- Hamblin MW, Metcalf MA. (1991) Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol 40:143–148. [PubMed] [Google Scholar]
- Hamblin MW, Metcalf MA, McGuffin RW, Karpells S. (1992a) Molecular cloning and functional characterization of a human 5-HT1B serotonin receptor: a homologue of the rat 5-HT1B receptor with 5-HT1D-like pharmacological specificity. Biochem Biophys Res Commun 184:752–759. [DOI] [PubMed] [Google Scholar]
- Hamblin MW, McGuffin RW, Metcalf MA, Dorsa DM, Merchant KM. (1992b) Distinct 5-HT(1B) and 5-HT(1D) serotonin receptors in rat: structural and pharmacological comparison of the two cloned receptors. Mol Cell Neurosci 3:578–587. [DOI] [PubMed] [Google Scholar]
- Hamik A, Oksenberg D, Fischette C, Peroutka SJ. (1990) Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol Psychiatry 28:99–109. [DOI] [PubMed] [Google Scholar]
- Hammer C, Cichon S, Mühleisen TW, Haenisch B, Degenhardt F, Mattheisen M, Breuer R, Witt SH, Strohmaier J, Oruc L, et al. (2012) Replication of functional serotonin receptor type 3A and B variants in bipolar affective disorder: a European multicenter study. Transl Psychiatry 2:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C, Kapeller J, Endele M, Fischer C, Hebebrand J, Hinney A, Friedel S, Gratacòs M, Estivill X, Fichter M, et al. (2009) Functional variants of the serotonin receptor type 3A and B gene are associated with eating disorders. Pharmacogenet Genomics 19:790–799. [DOI] [PubMed] [Google Scholar]
- Hammer J, Phillips SF, Talley NJ, Camilleri M. (1993) Effect of a 5HT3-antagonist (ondansetron) on rectal sensitivity and compliance in health and the irritable bowel syndrome. Aliment Pharmacol Ther 7:543–551. [DOI] [PubMed] [Google Scholar]
- Hamon M, Bourgoin S, Le Bars D, Cesselin F. (1988) In vivo and in vitro release of central neurotransmitters in relation to pain and analgesia. Prog Brain Res 77:431–444. [DOI] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefèvre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Vergé D. (1999) Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21(2 Suppl):68S–76S. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, et al. (2010) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL. (1995) 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther 66:103–148. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. (2008a) Molecular biology of 5-HT receptors. Behav Brain Res 195:198–213. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. (2008b) Molecular biology of 5-HT receptors, in Serotonin and Sleep: Molecular, Functional and Clinical Aspects (Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ. eds). pp 155–182. [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. (2014) Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci 5:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MB, Witte A-B. (2008) The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol (Oxf) 193:311–323. [DOI] [PubMed] [Google Scholar]
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, Ishibashi K, Matsuoka N. (2006) Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol 553:171–184. [DOI] [PubMed] [Google Scholar]
- Harrell AV, Allan AM. (2003) Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn Mem 10:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig PR, Branchek TA, Weinshank RL. (1992) A subfamily of 5-HT1D receptor genes. Trends Pharmacol Sci 13:152–159. [DOI] [PubMed] [Google Scholar]
- Hartig PR, Hoffman BJ, Kaufman MJ, Hirata F. (1990) The 5-HT1C receptor. Ann N Y Acad Sci 600:149–166; discussion 166–167. [DOI] [PubMed] [Google Scholar]
- Hartig PR, Hoyer D, Humphrey PPA, Martin GR. (1996) Alignment of receptor nomenclature with the human genome: classification of 5-HT1B and 5-HT1D receptor subtypes. Trends Pharmacol Sci 17:103–105. [DOI] [PubMed] [Google Scholar]
- Hartner JC, Schmittwolf C, Kispert A, Müller AM, Higuchi M, Seeburg PH. (2004) Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem 279:4894–4902. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. (2016) The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101:237–245. [DOI] [PubMed] [Google Scholar]
- Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, et al. (2014) X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512:276–281. [DOI] [PubMed] [Google Scholar]
- Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. (2010) Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil 22:826–834, e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck AJ, Edwards WD, Danielson GK, Mullany CJ, Bresnahan DR. (1990) Mitral and aortic valve disease associated with ergotamine therapy for migraine. Report of two cases and review of literature. Arch Pathol Lab Med 114:62–64. [PubMed] [Google Scholar]
- Hauser SR, Hedlund PB, Roberts AJ, Sari Y, Bell RL, Engleman EA. (2015) The 5-HT7 receptor as a potential target for treating drug and alcohol abuse. Front Neurosci 8:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA. (2015) Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179–184. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Covasa M. (2005) CCK and 5-HT act synergistically to suppress food intake through simultaneous activation of CCK-1 and 5-HT3 receptors. Peptides 26:2322–2330. [DOI] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Dabrowska J, Rainnie DG. (2012) Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience 225:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Yin JY, Xu YJ, Li X, Zhang Y, Liu ZG, Zhou F, Zhai M, Li Y, Li XP, et al. (2014) Association of ABCB1 polymorphisms with the efficacy of ondansetron in chemotherapy-induced nausea and vomiting. Clin Ther 36:1242–1252.e2. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H. (2008) Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacology & therapeutics 117:207–231. [DOI] [PubMed] [Google Scholar]
- Hedlund PB. (2009) The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 206:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Carson MJ, Sutcliffe JG, Thomas EA. (1999) Allosteric regulation by oleamide of the binding properties of 5-hydroxytryptamine7 receptors. Biochem Pharmacol 58:1807–1813. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. (2003) No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA 100:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. (2005) 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry 58:831–837. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. (2004) 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol 487:125–132. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Leopoldo M, Caccia S, Sarkisyan G, Fracasso C, Martelli G, Lacivita E, Berardi F, Perrone R. (2010) LP-211 is a brain penetrant selective agonist for the serotonin 5-HT(7) receptor. Neurosci Lett 481:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. (2007) The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett 414:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Eglen RM. (1996) Peripheral 5-HT4 receptors. FASEB J 10:1398–1407. [DOI] [PubMed] [Google Scholar]
- Hegener O, Prenner L, Runkel F, Baader SL, Kappler J, Häberlein H. (2004) Dynamics of beta2-adrenergic receptor-ligand complexes on living cells. Biochemistry 43:6190–6199. [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. (1997) Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization. J Neurochem 68:1372–1381. [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Szot P, Kohen R, Hamblin MW. (1998) Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 37:1621–1632. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. (1998a) Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA 95:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Tecott LH. (1998b) Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann N Y Acad Sci 861:74–78. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. (2002) Activation of central melanocortin pathways by fenfluramine. Science 297:609–611. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. (2006) Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51:239–249. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O’Rahilly S, Colmers WF, Elmquist JK, et al. (2007a) Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27:6956–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. (2007b) Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav 6:491–496. [DOI] [PubMed] [Google Scholar]
- Helboe L, Egebjerg J, de Jong IE. (2015) Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: a double in situ hybridization study. Neuroscience 310:442–454. [DOI] [PubMed] [Google Scholar]
- Helekar SA, Patrick J. (1997) Peptidyl prolyl cis-trans isomerase activity of cyclophilin A in functional homo-oligomeric receptor expression. Proc Natl Acad Sci USA 94:5432–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helke CJ, McDonald CH, Phillips ET. (1993) Hypotensive effects of 5-HT1A receptor activation: ventral medullary sites and mechanisms of action in the rat. J Auton Nerv Syst 42:177–188. [DOI] [PubMed] [Google Scholar]
- Hellstrand K, Hermodsson S. (1987) Role of serotonin in the regulation of human natural killer cell cytotoxicity. J Immunol 139:869–875. [PubMed] [Google Scholar]
- Helsmoortel C, Swagemakers SM, Vandeweyer G, Stubbs AP, Palli I, Mortier G, Kooy RF, van der Spek PJ. (2016) Whole genome sequencing of a dizygotic twin suggests a role for the serotonin receptor HTR7 in autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet 171:1049–1056. [DOI] [PubMed] [Google Scholar]
- Helton LA, Thor KB, Baez M. (1994) 5-hydroxytryptamine2A, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. Neuroreport 5:2617–2620. [DOI] [PubMed] [Google Scholar]
- Hemedah M, Coupar IM, Mitchelson FJ. (1999) [3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum. Br J Pharmacol 126:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikx M, Van Dorpe J, Flameng W, Daenen W. (1996) Aortic and mitral valve disease induced by ergotamine therapy for migraine: a case report and review of the literature. J Heart Valve Dis 5:235–237. [PubMed] [Google Scholar]
- Hensler JG. (2003) Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci 72:1665–1682. [DOI] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. (2009) Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136:1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. (2013) Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591:5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ME, Martinez-Fong D, Perez-Tapia M, Estrada-Garcia I, Estrada-Parra S, Pavón L. (2010) Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur Neuropsychopharmacol 20:88–95. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K. (2013) Functional significance of serotonin receptor dimerization. Exp Brain Res 230:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Farrington DT. (2011) 5-HT2C Receptor Dimerization, in 5-HT2C Receptors in the Pathophysiology of CNS Disease, pp 129–155, Springer. [Google Scholar]
- Herrick-Davis K, Grinde E, Cowan A, Mazurkiewicz JE. (2013) Fluorescence correlation spectroscopy analysis of serotonin, adrenergic, muscarinic, and dopamine receptor dimerization: the oligomer number puzzle. Mol Pharmacol 84:630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. (2005) Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J Biol Chem 280:40144–40151. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Cowan A, Mazurkiewicz JE. (2012) Oligomer size of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor revealed by fluorescence correlation spectroscopy with photon counting histogram analysis: evidence for homodimers without monomers or tetramers. J Biol Chem 287:23604–23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Teitler M, Mancia F, Cowan A, Mazurkiewicz JE. (2015) Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells. Mol Pharmacol 87:660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Mazurkiewicz JE. (2004) Biochemical and biophysical characterization of serotonin 5-HT2C receptor homodimers on the plasma membrane of living cells. Biochemistry 43:13963–13971. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Niswender CM. (1999) Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem 73:1711–1717. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Teitler M. (2000) Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 295:226–232. [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Weaver BA. (2007) Serotonin 5-HT(2C) receptor homodimerization is not regulated by agonist or inverse agonist treatment. Eur J Pharmacol 568:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. (2006) Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem 281:27109–27116. [DOI] [PubMed] [Google Scholar]
- Herrik KF, Mørk A, Richard N, Bundgaard C, Bastlund JF, de Jong IEM. (2016) The 5-HT6 receptor antagonist idalopirdine potentiates the effects of acetylcholinesterase inhibition on neuronal network oscillations and extracellular acetylcholine levels in the rat dorsal hippocampus. Neuropharmacology 107:351–363. [DOI] [PubMed] [Google Scholar]
- Hershenson MB, Chao TS, Abe MK, Gomes I, Kelleher MD, Solway J, Rosner MR. (1995) Histamine antagonizes serotonin and growth factor-induced mitogen-activated protein kinase activation in bovine tracheal smooth muscle cells. J Biol Chem 270:19908–19913. [DOI] [PubMed] [Google Scholar]
- Herth MM, Knudsen GM. (2015) Current radiosynthesis strategies for 5-HT2A receptor PET tracers. J Labelled Comp Radiopharm 58:265–273. [DOI] [PubMed] [Google Scholar]
- Hervás A, Toma C, Romarís P, Ribasés M, Salgado M, Bayes M, Balmaña N, Cormand B, Maristany M, Guijarro S, et al. (2014) The involvement of serotonin polymorphisms in autistic spectrum symptomatology. Psychiatr Genet 24:158–163. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. (1999) Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 274:22569–22580. [DOI] [PubMed] [Google Scholar]
- Heusler P, Bruins Slot L, Rauly-Lestienne I, Palmier C, Tardif S, Tourette A, Ailhaud MC, Cussac D. (2009) Activation of G proteins and extracellular signal-regulated kinase 1/2 phosphorylation via human dopamine D4.4 receptors: differential pathway-dependent potencies of receptor agonists. Naunyn Schmiedebergs Arch Pharmacol 379:87–99. [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Lee MD, Dourish CT, Clifton PG. (2002) Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav 71:691–700. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietala J, Kuonnamäki M, Pälvimäki EP, Laakso A, Majasuo H, Syvälahti E. (2001) Sertindole is a serotonin 5-HT2c inverse agonist and decreases agonist but not antagonist binding to 5-HT2c receptors after chronic treatment. Psychopharmacology (Berl) 157:180–187. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. (2003) The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 170:309–319. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. (2015) Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem Neurosci 6:1071–1088. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ, Shanahan WR. (2020) Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol Ther 205:107417. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S, Cooper AJ, Barnes NM. (2011) Reversal of sibutramine-induced anorexia with a selective 5-HT(2C) receptor antagonist. Psychopharmacology (Berl) 214:941–947. [DOI] [PubMed] [Google Scholar]
- Higgs S, Cooper AJ, Barnes NM. (2016) The 5-HT2C receptor agonist, lorcaserin, and the 5-HT6 receptor antagonist, SB-742457, promote satiety; a microstructural analysis of feeding behaviour. Psychopharmacology (Berl) 233:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJC, Bertozzi C, Zimmermann I, Reiter A, Trauner D, Dutzler R. (2010) Structural basis of open channel block in a prokaryotic pentameric ligand-gated ion channel. Nat Struct Mol Biol 17:1330–1336. [DOI] [PubMed] [Google Scholar]
- Hill PB, Dora KA, Hughes AD, Garland CJ. (2000) The involvement of intracellular Ca(2+) in 5-HT(1B/1D) receptor-mediated contraction of the rabbit isolated renal artery. Br J Pharmacol 130:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Tokumo K, Tsuchiya D, Nishio H. (2009) Expression of mRNA for 5-HT2 receptors and proteins related to inactivation of 5-HT in mouse osteoblasts. J Pharmacol Sci 109:319–323. [DOI] [PubMed] [Google Scholar]
- Hirani E, Opacka-Juffry J, Gunn R, Khan I, Sharp T, Hume S. (2000) Pindolol occupancy of 5-HT(1A) receptors measured in vivo using small animal positron emission tomography with carbon-11 labeled WAY 100635. Synapse 36:330–341. [DOI] [PubMed] [Google Scholar]
- Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa F, Okumura N, Yamazaki A, Ushiyama Y, Yazaki Y, Kinoshita O. (2003) Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res 60:692–699. [DOI] [PubMed] [Google Scholar]
- Hirose A, Sasa M, Akaike A, Takaori S. (1990) Inhibition of hippocampal CA1 neurons by 5-hydroxytryptamine, derived from the dorsal raphe nucleus and the 5-hydroxytryptamine1A agonist SM-3997. Neuropharmacology 29:93–101. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. (2003) Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 64:1295–1308. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Cheung NY, Rattray M, Price GW, Wilkin GP. (1998) Cultured astrocytes express messenger RNA for multiple serotonin receptor subtypes, without functional coupling of 5-HT1 receptor subtypes to adenylyl cyclase. Brain Res Mol Brain Res 61:90–99. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, et al. (2006) SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol 553:109–119. [DOI] [PubMed] [Google Scholar]
- Hisadome K, Smith MA, Choudhury AI, Claret M, Withers DJ, Ashford ML. (2009) 5-HT inhibition of rat insulin 2 promoter Cre recombinase transgene and proopiomelanocortin neuron excitability in the mouse arcuate nucleus. Neuroscience 159:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S. (1985) Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm (Vienna) 61:131–135. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A, Lindberg P, et al. (1982) 8-Hydroxy-2-(di- n-propylamino)tetralin, 8-OH-DPAT, a potent and selective sim- plifies ergot congener with central 5-HT receptor stimulating activity. J Neural Transm 55:169–188. [Google Scholar]
- Hobson RJ, Geng J, Gray AD, Komuniecki RW. (2003) Ser-7b, a constitutively active Gαs coupled 5-HT7–like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motor neuron. J Neurochem 87:22–29. [DOI] [PubMed] [Google Scholar]
- Hodge E, Nelson CP, Miller S, Billington CK, Stewart CE, Swan C, Malarstig A, Henry AP, Gowland C, Melén E, et al. (2013a) HTR4 gene structure and altered expression in the developing lung. Respir Res 14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, Pasco JA, Dodd S, Nicholson GC, Kennedy RL, et al. (2013b) Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry 74:32–39. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. (1963) Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav 6:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E. (1989) Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett 247:453–462. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, et al. (2012) Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142:844–854.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoger JH, Walter AE, Vance D, Yu L, Lester HA, Davidson N. (1991) Modulation of a cloned mouse brain potassium channel. Neuron 6:227–236. [DOI] [PubMed] [Google Scholar]
- Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, Rance KH, Trinh H, Balmer G, Kelly FM, Yusaf SP, et al. (2009) Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem 108:384–396. [DOI] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC. (2004) Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br J Pharmacol 143:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst K, Guseva D, Schindler S, Sixt M, Braun A, Chopra H, Pabst O, Ponimaskin E. (2015) Serotonin receptor 5-HT₇R regulates the morphology and migratory properties of dendritic cells. J Cell Sci 128:2866–2880. [DOI] [PubMed] [Google Scholar]
- Hołuj M, Popik P, Nikiforuk A. (2015) Improvement of ketamine-induced social withdrawal in rats: the role of 5-HT7 receptors. Behav Pharmacol 26:766–775. [DOI] [PubMed] [Google Scholar]
- Holzwarth MA, Brownfield MS. (1985) Serotonin coexists with epinephrine in rat adrenal medullary cells. Neuroendocrinology 41:230–236. [DOI] [PubMed] [Google Scholar]
- Holzwarth MA, Sawetawan C, Brownfield MS. (1984) Serotonin-immunoreactivity in the adrenal medulla: distribution and response to pharmacological manipulation. Brain Res Bull 13:299–308. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JDA, Smits BMG, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OFM, Cools AR, Ronken E, Cremers T, et al. (2007) Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience 146:1662–1676. [DOI] [PubMed] [Google Scholar]
- Hope AG, Downie DL, Sutherland L, Lambert JJ, Peters JA, Burchell B. (1993) Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor A subunit. Eur J Pharmacol 245:187–192. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. (2011) The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther 338:605–614. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Meltzer HY. (2012) The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl) 221:205–215. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Meltzer HY. (2013) Blonanserin reverses the phencyclidine (PCP)-induced impairment in novel object recognition (NOR) in rats: role of indirect 5-HT(1A) partial agonism. Behav Brain Res 247:158–164. [DOI] [PubMed] [Google Scholar]
- Horisawa T, Ishibashi T, Nishikawa H, Enomoto T, Toma S, Ishiyama T, Taiji M. (2011) The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res 220:83–90. [DOI] [PubMed] [Google Scholar]
- Horisawa T, Nishikawa H, Toma S, Ikeda A, Horiguchi M, Ono M, Ishiyama T, Taiji M. (2013) The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav Brain Res 244:66–69. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Atik A, Iigaya K, McDowall LM, Killinger S, Dampney RA. (2011) Activation of 5-hydroxytryptamine-1A receptors suppresses cardiovascular responses evoked from the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 301:R1088–R1097. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Wakabayashi S, Dampney RA. (2005) Activation of 5-hydroxytryptamine 1A receptors suppresses the cardiovascular response evoked from the dorsomedial hypothalamic nucleus. Hypertension 46:173–179. [DOI] [PubMed] [Google Scholar]
- Horn CC, Wallisch WJ, Homanics GE, Williams JP. (2014) Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol 722:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J, Fross RD, Kleiner-Fisman G, Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski H, Pache JC, et al. (2004) Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov Disord 19:656–662. [DOI] [PubMed] [Google Scholar]
- Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. (2001) 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 909:112–120. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Foster JM, Whorwell PJ. (2000) Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther 14:775–782. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Jackson NA, Whorwell PJ, Cooper SM. (1999) 5-HT4 receptor antagonism in irritable bowel syndrome: effect of SB-207266-A on rectal sensitivity and small bowel transit. Aliment Pharmacol Ther 13:1437–1444. [DOI] [PubMed] [Google Scholar]
- House JS, Li H, DeGraff LM, Flake G, Zeldin DC, London SJ. (2015) Genetic variation in HTR4 and lung function: GWAS follow-up in mouse. FASEB J 29:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 151:55–63. [DOI] [PubMed] [Google Scholar]
- Hoyer D. (1988a) Functional correlates of serotonin 5-HT1 recognition sites. J Recept Res 8:59–81. [DOI] [PubMed] [Google Scholar]
- Hoyer D. (1988b) Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci 9:89–94. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. (1985a) Characterization of the 5-HT1B recognition site in rat brain: binding studies with (-)[125I]iodocyanopindolol. Eur J Pharmacol 118:1–12. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. (1985b) Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (-)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol 118:13–23. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Lery H, Waeber C, Bruinvels AT, Nozulak J, Palacios JM. (1992) “5-HT1R” or 5-HT1D sites? Evidence for 5-HT1D binding sites in rabbit brain. Naunyn Schmiedebergs Arch Pharmacol 346:249–254. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Martin GR. (1996) Classification and nomenclature of 5-HT receptors: a comment on current issues. Behav Brain Res 73:263–268. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Martin G. (1997) 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology 36:419–428. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Middlemiss DN. (1989) Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci 10:130–132. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Pazos A, Probst A, Palacios JM. (1986a) Serotonin receptors in the human brain. I. Characterization and autoradiographic localization of 5-HT1A recognition sites. Apparent absence of 5-HT1B recognition sites. Brain Res 376:85–96. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Pazos A, Probst A, Palacios JM. (1986b) Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res 376:97–107. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Schoeffter P. (1988) 5-HT1D receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in calf substantia nigra. Eur J Pharmacol 147:145–147. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Schoeffter P. (1991) 5-HT receptors: subtypes and second messengers. J Recept Res 11:197–214. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Schoeffter P, Gray JA. (1989a) A comparison of the interactions of dihydroergotamine, ergotamine and GR 43175 with 5-HT1 receptor subtypes. Cephalalgia 9:340–341. [Google Scholar]
- Hoyer D, Waeber C, Karpf A, Neijt H, Palacios JM. (1989b) [3H]ICS 205-930 labels 5-HT3 recognition sites in membranes of cat and rabbit vagus nerve and superior cervical ganglion. Naunyn Schmiedebergs Arch Pharmacol 340:396–402. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Schoeffter P, Waeber C, Palacios JM. (1990) Serotonin 5-HT1D receptors. Ann NY Acad Sci 600:168–181. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Waeber C, Pazos A, Probst A, Palacios JM. (1988) Identification of a 5-HT1 recognition site in human brain membranes different from 5-HT1A, 5-HT1B and 5-HT1C sites. Neurosci Lett 85:357–362. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Waeber C, Schoeffter P, Palacios JM, Dravid A. (1989c) 5-HT1C receptor-mediated stimulation of inositol phosphate production in pig choroid plexus. A pharmacological characterization. Naunyn Schmiedebergs Arch Pharmacol 339:252–258. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. (1997) Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol 77:2910–2924. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Tamir H, Franke TF, Liu KP. (2005) Roles of extracellular signal-regulated kinase and Akt signaling in coordinating nuclear transcription factor-kappaB-dependent cell survival after serotonin 1A receptor activation. J Neurochem 95:1653–1666. [DOI] [PubMed] [Google Scholar]
- Huang HS, Yoon BJ, Brooks S, Bakal R, Berrios J, Larsen RS, Wallace ML, Han JE, Chung EH, Zylka MJ, et al. (2014a) Snx14 regulates neuronal excitability, promotes synaptic transmission, and is imprinted in the brain of mice. PLoS One 9:e98383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Horiguchi M, Felix AR, Meltzer HY. (2012) 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. Neuroreport 23:436–440. [DOI] [PubMed] [Google Scholar]
- Huang M, Panos JJ, Kwon S, Oyamada Y, Rajagopal L, Meltzer HY. (2014b) Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J Neurochem 128:938–949. [DOI] [PubMed] [Google Scholar]
- Huang XP, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, Revankar C, Robers M, Doucette C, Roth BL. (2009) Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment. Mol Pharmacol 76:710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta C, Rivero E, Montoro MA, García-Rodriguez LA. (2011) Risk factors for intestinal ischaemia among patients registered in a UK primary care database: a nested case-control study. Aliment Pharmacol Ther 33:969–978. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Starr KR, Scott CM, Newson MJ, Sharp T, Watson JM, Hagan JJ, Dawson LA. (2007) Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649915-B. Psychopharmacology (Berl) 192:121–133. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Harris RA. (1996a) Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc Natl Acad Sci 93:8078–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Valenzuela CF, Harris RA. (1996b) Modulation of GABAA receptor function by G protein-coupled 5-HT2C receptors. Neuropharmacology 35:1355–1363. [DOI] [PubMed] [Google Scholar]
- Hummerich R, Thumfart JO, Findeisen P, Bartsch D, Schloss P. (2012) Transglutaminase-mediated transamidation of serotonin, dopamine and noradrenaline to fibronectin: evidence for a general mechanism of monoaminylation. FEBS Lett 586:3421–3428. [DOI] [PubMed] [Google Scholar]
- Humphrey PP. (2008) The discovery and development of the triptans, a major therapeutic breakthrough. Headache 48:685–687. [DOI] [PubMed] [Google Scholar]
- Humphrey PPA. (2007) The discovery of a new drug class for the acute treatment of migraine. Headache 47 (Suppl 1):S10–S19. [DOI] [PubMed] [Google Scholar]
- Humphrey PP, Goadsby PJ. (1994) The mode of action of sumatriptan is vascular? A debate. Cephalalgia 14:401–410, discussion 393. [DOI] [PubMed] [Google Scholar]
- Humphrey PPA, Hartig P, Hoyer D. (1993a) A proposed new nomenclature for 5-HT receptors. Trends Pharmacol Sci 14:233–236. [DOI] [PubMed] [Google Scholar]
- Humphrey PPA, Hartig P, Hoyer D. (1993b) A reappraisal of 5-HT receptor classification in Serotonin: From Cell Biology to Pharmacology and Therapeutics Vanhoutte PM.Saxena PR, Paoletti R. eds) vol 5, pp 41–47. [Google Scholar]
- Hung BC, Loo DD, Wright EM. (1993) Regulation of mouse choroid plexus apical Cl- and K+ channels by serotonin. Brain Res 617:285–295. [DOI] [PubMed] [Google Scholar]
- Huot P. (2015) L-DOPA-induced dyskinesia, is striatal dopamine depletion a requisite? J Neurol Sci 351:9–12. [DOI] [PubMed] [Google Scholar]
- Hurley PT, McMahon RA, Fanning P, O’Boyle KM, Rogers M, Martin F. (1998) Functional coupling of a recombinant human 5-HT5A receptor to G-proteins in HEK-293 cells. Br J Pharmacol 124:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. (2012) 5-HT(2B) antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol 53:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson JD, Setola V, Roth BL, Merryman WD. (2011) Serotonin receptors and heart valve disease--it was meant 2B. Pharmacol Ther 132:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iderberg H, McCreary AC, Varney MA, Cenci MA, Newman-Tancredi A. (2015) Activity of serotonin 5-HT(1A) receptor ‘biased agonists’ in rat models of Parkinson’s disease and L-DOPA-induced dyskinesia. Neuropharmacology 93:52–67. [DOI] [PubMed] [Google Scholar]
- Idzikowski C, Mills FJ, James RJ. (1991) A dose-response study examining the effects of ritanserin on human slow wave sleep. Br J Clin Pharmacol 31:193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M, et al. (2004) The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 172:6011–6019. [DOI] [PubMed] [Google Scholar]
- Iizuka N, Oka M, Yamada-Okabe H, Hamada K, Nakayama H, Mori N, Tamesa T, Okada T, Takemoto N, Matoba K, et al. (2004) Molecular signature in three types of hepatocellular carcinoma with different viral origin by oligonucleotide microarray. Int J Oncol 24:565–574. [PubMed] [Google Scholar]
- Ikeda K, Tojo K, Otsubo C, Udagawa T, Kumazawa K, Ishikawa M, Tokudome G, Hosoya T, Tajima N, Claycomb WC, et al. (2005) 5-hydroxytryptamine synthesis in HL-1 cells and neonatal rat cardiocytes. Biochem Biophys Res Commun 328:522–525. [DOI] [PubMed] [Google Scholar]
- Iken K, Chheng S, Fargin A, Goulet AC, Kouassi E. (1995) Serotonin upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell Immunol 163:1–9. [DOI] [PubMed] [Google Scholar]
- Im WB, Chio CL, Alberts GL, Dinh DM. (2003) Positive allosteric modulator of the human 5-HT2C receptor. Mol Pharmacol 64:78–84. [DOI] [PubMed] [Google Scholar]
- Impellizzeri AA, Pappalardo M, Basile L, Manfra O, Andressen KW, Krobert KA, Messina A, Levy FO, Guccione S. (2015) Identification of essential residues for binding and activation in the human 5-HT7(a) serotonin receptor by molecular modeling and site-directed mutagenesis. Front Behav Neurosci 9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Matsuhisa E, Moroi SE, Takenaka H, Sogo S, Mano T. (2003) 5-HT(2) receptor-mediated phosphoinositide hydrolysis in bovine ciliary epithelium. J Ocul Pharmacol Ther 19:55–62. [DOI] [PubMed] [Google Scholar]
- Inoue M, Okazaki T, Kitazono T, Mizushima M, Omata M, Ozaki S. (2011) Regulation of antigen-specific CTL and Th1 cell activation through 5-Hydroxytryptamine 2A receptor. Int Immunopharmacol 11:67–73. [DOI] [PubMed] [Google Scholar]
- Invernizzi RW, Pierucci M, Calcagno E, Di Giovanni G, Di Matteo V, Benigno A, Esposito E. (2007) Selective activation of 5-HT(2C) receptors stimulates GABA-ergic function in the rat substantia nigra pars reticulata: a combined in vivo electrophysiological and neurochemical study. Neuroscience 144:1523–1535. [DOI] [PubMed] [Google Scholar]
- Irving HR, Tan YY, Tochon-Danguy N, Liu H, Chetty N, Desmond PV, Pouton CW, Coupar IM. (2007) Comparison of 5-HT4 and 5-HT7 receptor expression and function in the circular muscle of the human colon. Life Sci 80:1198–1205. [DOI] [PubMed] [Google Scholar]
- Isaac M. (2005) Serotonergic 5-HT2C receptors as a potential therapeutic target for the design antiepileptic drugs. Curr Top Med Chem 5:59–67. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, et al. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181. [DOI] [PubMed] [Google Scholar]
- Ishida T, Hirata K, Sakoda T, Kawashima S, Akita H, Yokoyama M. (1999) Identification of mRNA for 5-HT1 and 5-HT2 receptor subtypes in human coronary arteries. Cardiovasc Res 41:267–274. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kawashima S, Hirata Ki, Sakoda T, Shimokawa Y, Miwa Y, Inoue N, Ueyama T, Shiomi M, Akita H, et al. (2001) Serotonin-induced hypercontraction through 5-hydroxytryptamine 1B receptors in atherosclerotic rabbit coronary arteries. Circulation 103:1289–1295. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kawashima S, Hirata K, Yokoyama M. (1998) Nitric oxide is produced via 5-HT1B and 5-HT2B receptor activation in human coronary artery endothelial cells. Kobe J Med Sci 44:51–63. [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Rodgers RJ. (2003) Palatability, food intake and the behavioural satiety sequence in male rats. Physiol Behav 80:37–47. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Goshima H, Ozawa A, Watanabe Y. (2014) Stimulation of 5-HT4 receptor enhances differentiation of mouse induced pluripotent stem cells into neural progenitor cells. Clin Exp Pharmacol Physiol 41:345–350. [DOI] [PubMed] [Google Scholar]
- Islam A, Thompson KSJ, Akhtar S, Handley SL. (2004) Increased 5-HT2A receptor expression and function following central glucocorticoid receptor knockdown in vivo. Eur J Pharmacol 502:213–220. [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, De Los Angeles J, Teitler M, Ingher S, Glennon RA. (1993) Antagonism of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane stimulus with a newly identified 5-HT2- versus 5-HT1C-selective antagonist. J Med Chem 36:2519–2525. [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. (1990) 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem 33:755–758. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kajikuri J. (2011) Characteristics of the actions by which 5-HT affects electrical and mechanical activities in rabbit jugular vein. Br J Pharmacol 164:979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang X-P, Roth BL. (2013) Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. (2011) RNA editing of 5-HT2C receptor and neuropsychiatric diseases, in 5-HT2C receptors in the pathophysiology of CNS disease (Di Giovanni G, Esposito E, Di Matteo V eds) pp 157–167, Humana Press. [Google Scholar]
- Iwamoto K, Kato T. (2003) RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neurosci Lett 346:169–172. [DOI] [PubMed] [Google Scholar]
- Iwata N, Ozaki N, Inada T, Goldman D. (2001) Association of a 5-HT(5A) receptor polymorphism, Pro15Ser, to schizophrenia. Mol Psychiatry 6:217–219. [DOI] [PubMed] [Google Scholar]
- Iwata N, Virkkunen M, Linnoila M, Goldman D. (1998) Identification of a naturally occurring Pro15-Ser15 substitution in the serotonin5A receptor gene in alcoholics and healthy volunteers. Brain Res Mol Brain Res 58:217–220. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–229. [DOI] [PubMed] [Google Scholar]
- Jacobshagen M, Niquille M, Chaumont-Dubel S, Marin P, Dayer A. (2014) The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development 141:3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffré F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, et al. (2009) Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res 104:113–123. [DOI] [PubMed] [Google Scholar]
- Jaffré F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay JM, Maroteaux L, Monassier L. (2004) Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation 110:969–974. [DOI] [PubMed] [Google Scholar]
- Jahnel U, Rupp J, Ertl R, Nawrath H. (1992) Positive inotropic response to 5-HT in human atrial but not in ventricular heart muscle. Naunyn Schmiedebergs Arch Pharmacol 346:482–485. [DOI] [PubMed] [Google Scholar]
- Jähnichen S, Glusa E, Pertz HH. (2005) Evidence for 5-HT2B and 5-HT7 receptor-mediated relaxation in pulmonary arteries of weaned pigs. Naunyn Schmiedebergs Arch Pharmacol 371:89–98. [DOI] [PubMed] [Google Scholar]
- Jähnichen S, Radtke OA, Pertz HH. (2004) Involvement of 5-HT1B receptors in triptan-induced contractile responses in guinea-pig isolated iliac artery. Naunyn Schmiedebergs Arch Pharmacol 370:54–63. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, To ZP, Eglen RM, Wong EH, Bonhaus DW. (1994) Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacology 33:1027–1038. [DOI] [PubMed] [Google Scholar]
- Jakus R, Bagdy G. (2011) The Role of 5-HT2C Receptor in Epilepsy. In: 5-HT2C Receptors in the Pathophysiology of CNS Disease, in 5-HT2C Receptors in the Pathophysiology of CNS Disease (Di Giovanni G, Esposito E, Di Matteo V. eds) pp 429–444, Springer-Verlag, Wien, Austria. [Google Scholar]
- Jakus R, Graf M, Juhasz G, Gerber K, Levay G, Halasz P, Bagdy G. (2003) 5-HT2C receptors inhibit and 5-HT1A receptors activate the generation of spike-wave discharges in a genetic rat model of absence epilepsy. Exp Neurol 184:964–972. [DOI] [PubMed] [Google Scholar]
- Janno S, Holi M, Tuisku K, Wahlbeck K. (2004) Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am J Psychiatry 161:160–163. [DOI] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay JM, Maroteaux L. (2007) Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol 71:1463–1474. [DOI] [PubMed] [Google Scholar]
- Jansen M, Bali M, Akabas MH. (2008) Modular design of Cys-loop ligand-gated ion channels: functional 5-HT3 and GABA rho1 receptors lacking the large cytoplasmic M3M4 loop. J Gen Physiol 131:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. (2011a) Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 33:880–894. [DOI] [PubMed] [Google Scholar]
- Janssen P, Vos R, Van Oudenhove L, Tack J. (2011b) Influence of the 5-HT3 receptor antagonist ondansetron on gastric sensorimotor function and nutrient tolerance in healthy volunteers. Neurogastroenterol Motil 23:444–449, e175. [DOI] [PubMed] [Google Scholar]
- Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, Luitel H, Murmann K, Krompiec DR, Tretyn A, et al. (2015) 5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure. BioMed Res Int 2015:438403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska-Wiesek M, Siwek A, Kazek G, Nawiesniak B, Partyka A, Marcinkowska M, Kolaczkowski M, Wesolowska A. (2013) Partial agonist efficacy of EMD386088, a 5-HT6 receptor ligand, in functional in vitro assays. Pharmacol Rep 65:998–1005. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308. [DOI] [PubMed] [Google Scholar]
- Jayne C, Simon JA, Taylor LV, Kimura T, Lesko LM, SUNFLOWER study investigators (2012) Open-label extension study of flibanserin in women with hypoactive sexual desire disorder. J Sex Med 9:3180–3188. [DOI] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. (2007) Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA 104:16335–16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A, Laurent L, Bockaert J, Charnay Y, Dusticier N, Nieoullon A, Barrot M, Neve R, Compan V. (2012) The nucleus accumbens 5-HTR4-CART pathway ties anorexia to hyperactivity. Transl Psychiatry 2:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Bös M, Wichmann J, Stadler H, Martin JR, Moreau JL. (1998) The role of 5-HT2C receptors in affective disorders. Expert Opin Investig Drugs 7:1587–1599. [DOI] [PubMed] [Google Scholar]
- Jenner P, McCreary AC, Scheller DK. (2011) Continuous drug delivery in early- and late-stage Parkinson’s disease as a strategy for avoiding dyskinesia induction and expression. J Neural Transm (Vienna) 118:1691–1702. [DOI] [PubMed] [Google Scholar]
- Jensen TN, Nielsen J, Frederiksen K, Ebert B. (2006) Molecular cloning and pharmacological characterization of serotonin 5-HT(3A) receptor subtype in dog. Eur J Pharmacol 538:23–31. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Chenu D, Johnson EE, Connor M, Vaughan CW. (2008) Sumatriptan inhibits synaptic transmission in the rat midbrain periaqueductal grey. Mol Pain 4:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman JC, Brough SJ, Gager T, Wood M, Coldwell MC, Smart D, Middlemiss DN. (2001) Pharmacological characterisation of human 5-HT2 receptor subtypes. Eur J Pharmacol 414:23–30. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Gage FH, Eisch AJ, Lagace DC. (2009) Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci 32:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji SP, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L, Wan Q, Backstrom JR, Zhang X. (2006) Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med 12:324–329. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. (2009) Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology 34:410–423. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ferrer E, Herrera-Ruiz M, Ramírez-García R, Herrera-Arellano A, Tortoriello J. (2011) Interaction of the natural anxiolytic Galphimine-B with serotonergic drugs on dorsal hippocampus in rats. J Ethnopharmacol 137:724–729. [DOI] [PubMed] [Google Scholar]
- Jin HK, Bae JS, Furuya S, Carter JE. (2009) Amyloid beta-derived neuroplasticity in bone marrow-derived mesenchymal stem cells is mediated by NPY and 5-HT2B receptors via ERK1/2 signalling pathways. Cell Prolif 42:571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JG, Foxx-Orenstein AE, Grider JR. (1999) Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288:93–97. [PubMed] [Google Scholar]
- Johns GW, Pauwels PJ, Perez M, et al. (1999) F 11356, a novel 5-hydroxytryptamine (5-HT) derivative with potent, selective, and unique high intrinsic activity at 5-HT1B/1D receptors in models relevant to migraine. J Pharmacol Exp Ther. 290:83–95. [PubMed] [Google Scholar]
- Johnson KW, Nelson DL, Dieckman DK, Wainscott DB, Lucaites VL, Audia JE, Owton WM, Phebus LA. (2003) Neurogenic dural protein extravasation induced by meta-chlorophenylpiperazine (mCPP) involves nitric oxide and 5-HT2B receptor activation. Cephalalgia 23:117–123. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Phebus LA, Cohen ML. (1998) Serotonin in migraine: theories, animal models and emerging therapies. Prog Drug Res 51:219–244. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Schaus JM, Durkin MM, Audia JE, Kaldor SW, Flaugh ME, Adham N, Zgombick JM, Cohen ML, Branchek TA, et al. (1997a) 5-HT1F receptor agonists inhibit neurogenic dural inflammation in guinea pigs. Neuroreport 8:2237–2240. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Kursar JD, Nelson DL. (1995) Species differences in 5-HT2A receptors: cloned pig and rhesus monkey 5-HT2A receptors reveal conserved transmembrane homology to the human rather than rat sequence. Biochim Biophys Acta 1236:201–206. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Roberston DN, Holland PJ, Lutz EM, Mitchell R. (2006) Role of the conserved NPxxY motif of the 5-HT2A receptor in determining selective interaction with isoforms of ADP-ribosylation factor (ARF). Cell Signal 18:1793–1800. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Fiorella D, Winter JC, Rabin RA. (1997b) [3H]8-OH-DPAT labels a 5-HT site coupled to inhibition of phosphoinositide hydrolysis in the dorsal raphe. Eur J Pharmacol 329:99–106. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Mercuri NB, North RA. (1992) 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci 12:2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley NN, Kertesy SB, Dubyak GR, Cowen DS. (2005) Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J Neurochem 92:72–82. [DOI] [PubMed] [Google Scholar]
- Johnston KD, Lu Z, Rudd JA. (2014) Looking beyond 5-HT(3) receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol 722:13–25. [DOI] [PubMed] [Google Scholar]
- Jones JF, Martin GR, Ramage AG. (1995) Evidence that 5-HT1D receptors mediate inhibition of sympathetic ganglionic transmission in anaesthetized cats. Br J Pharmacol 116:1715–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA 106:19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MD, Lucki I. (2005) Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 30:1039–1047. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Bespalov AY, Rueter LE, et al. (2006) Behavioral pharmacological characterization of 5-HT5A receptor antagonists in antipsychotic drug tests, in 36th Annu. Meet. Soc. Neurosci.; 2006 Oct 14-18; Atlanta, GA. Abst.529.26. [Google Scholar]
- Jordan D. (2005) Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 90:175–181. [DOI] [PubMed] [Google Scholar]
- Joubert L, Claeysen S, Sebben M, Bessis AS, Clark RD, Martin RS, Bockaert J, Dumuis A. (2002) A 5-HT4 receptor transmembrane network implicated in the activity of inverse agonists but not agonists. J Biol Chem 277:25502–25511. [DOI] [PubMed] [Google Scholar]
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J. (2004) New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci 117:5367–5379. [DOI] [PubMed] [Google Scholar]
- Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. (1990) The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci USA 87:928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Livelli TJ, Jessell TM, Axel R. (1989) Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science 244:1057–1062. [DOI] [PubMed] [Google Scholar]
- Julius D, MacDermott AB, Axel R, Jessell TM. (1988) Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science 241:558–564. [DOI] [PubMed] [Google Scholar]
- Juncosa JI, Jr, Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE. (2013) Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci 4:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena MF, de Sena EP, de Oliveira IR. (2011) Sertindole in the management of schizophrenia. J Cent Nerv Syst Dis 3:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Wade PR, Gershon MD. (1996) Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol 271:G849–G857. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Wetzler S. (1991) m-Chlorophenylpiperazine as a probe of serotonin function. Biol Psychiatry 30:1139–1166. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay PB, Possinger K, Roots I, Brockmöller J. (2002) Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol 20:2805–2811. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Tremblay PB, Sezer O, Possinger K, Roots I, Brockmöller J. (2004) Investigation of the association between 5-HT3A receptor gene polymorphisms and efficiency of antiemetic treatment with 5-HT3 receptor antagonists. Pharmacogenetics 14:271–278. [DOI] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. (2015) Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172:4655–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale-Pradhan PB, Wilhelm SM. (2007) Tegaserod for constipation-predominant irritable bowel syndrome. Pharmacotherapy 27:267–277. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalkman HO, Engel G, Hoyer D. (1984) Three distinct subtypes of serotonergic receptors mediate the triphasic blood pressure response to 5-HT in rats. J Hypertens 2:143–145. [PubMed] [Google Scholar]
- Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi-Chaachoua A, Luka M, Chen P, Kalbasi Anaraki D, Baroncini M, Mannoury la Cour C, et al. (2015) Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem 290:11537–11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BN, Ha SG, Bahaie NS, Hosseinkhani MR, Ge XN, Blumenthal MN, Rao SP, Sriramarao P. (2013) Regulation of serotonin-induced trafficking and migration of eosinophils. PLoS One 8:e54840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannari K, Kurahashi K, Tomiyama M, Maeda T, Arai A, Baba M, Suda T, Matsunaga M. (2002) [Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease]. No To Shinkei 54:133–137. [PubMed] [Google Scholar]
- Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, Caplan R, et al. (2012) Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav 24:156–168. [DOI] [PubMed] [Google Scholar]
- Kantor S, Jakus R, Balogh B, Benko A, Bagdy G. (2004) Increased wakefulness, motor activity and decreased theta activity after blockade of the 5-HT2B receptor by the subtype-selective antagonist SB-215505. Br J Pharmacol 142:1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, et al. (2008) First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet 17:2967–2977. [DOI] [PubMed] [Google Scholar]
- Kapeller J, Möller D, Lasitschka F, Autschbach F, Hovius R, Rappold G, Brüss M, Gershon MD, Niesler B. (2011) Serotonin receptor diversity in the human colon: expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J Comp Neurol 519:420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prézeau L, Bockaert J, Marin P, Vandermoere F. (2014) Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics 13:1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila D, Freret T, Bouet V, Boulouard M, Dallemagne P, Rochais C. (2015) Therapeutic Potential of 5-HT6 Receptor Agonists. J Med Chem 58:7901–7912. [DOI] [PubMed] [Google Scholar]
- Kasho M, Sakai M, Sasahara T, Anami Y, Matsumura T, Takemura T, Matsuda H, Kobori S, Shichiri M. (1998) Serotonin enhances the production of type IV collagen by human mesangial cells. Kidney Int 54:1083–1092. [DOI] [PubMed] [Google Scholar]
- Kasper J, Tikamdas R, Kim MS, Macfadyen K, Aramini R, Ladd J, Bisceglia S, Booth R, Peris J. (2013) The serotonin-2 receptor modulator, (-)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol 718:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Küfferle B, Barnas C, Stastny J, Praschak-Rieder N, Pezawas L, et al. (2002) Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder--implications for psychopharmacology. World J Biol Psychiatry 3:133–146. [DOI] [PubMed] [Google Scholar]
- Kassai F, Schlumberger C, Kedves R, Pietraszek M, Jatzke C, Lendvai B, Gyertyán I, Danysz W. (2012) Effect of 5-HT5A antagonists in animal models of schizophrenia, anxiety and depression. Behav Pharmacol 23:397–406. [DOI] [PubMed] [Google Scholar]
- Katoh N, Soga F, Nara T, Tamagawa-Mineoka R, Nin M, Kotani H, Masuda K, Kishimoto S. (2006) Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin Exp Immunol 146:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, DeRogatis LR, Ackerman R, Hedges P, Lesko L, Garcia M, Jr, Sand M, BEGONIA trial investigators (2013) Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med 10:1807–1815. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Hartig PR, Hoffman BJ. (1995) Serotonin 5-HT2C receptor stimulates cyclic GMP formation in choroid plexus. J Neurochem 64:199–205. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ. (1990) Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn Schmiedebergs Arch Pharmacol 342:619–622. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ. (1994) Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharmacol Sci 15:451–455. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Frenken M, Posival H, Brown AM. (1994) Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries. 5-HT1-like receptors resemble cloned 5-HT1D beta receptors. Circulation 90:1141–1153. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. (2006) 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther 111:674–706. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Lynham JA, Brown AM. (1995) Labelling with [125I]-SB 207710 of a small 5-HT4 receptor population in piglet right atrium: functional relevance. Br J Pharmacol 115:933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Parsons AA, Brown AM. (1993) Human arterial constrictor serotonin receptors. Cardiovasc Res 27:2094–2103. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ. (1990) A 5-hydroxytryptamine receptor in human atrium. Br J Pharmacol 100:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ. (1991) A 5-HT4-like receptor in human right atrium. Naunyn Schmiedebergs Arch Pharmacol 344:150–159. [DOI] [PubMed] [Google Scholar]
- Kawahara I, Kuniyasu H, Matsuyoshi H, Goto K, Obata K, Misawa H, Fujii H, Takaki M. (2012) Comparison of effects of a selective 5-HT reuptake inhibitor versus a 5-HT4 receptor agonist on in vivo neurogenesis at the rectal anastomosis in rats. Am J Physiol Gastrointest Liver Physiol 302:G588–G597. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, Bale TL, Blendy JA, Nishikura K. (2008) Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci 28:12834–12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. (2004) A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol 286:R649–R658. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, Mathis CA, Wagner A. (2005) Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav 85:73–81. [DOI] [PubMed] [Google Scholar]
- Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. (2001) Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ Res 89:1231–1239. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, et al. (1996) Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277:968–981. [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW. (2003a) Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol 461:19–25. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. (2003b) A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424:321–324. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Sharif NA. (2006) Pharmacological evidence for a functional serotonin-2B receptor in a human uterine smooth muscle cell line. J Pharmacol Exp Ther 317:1254–1261. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. (2004) Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA 101:8192–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. (2009) '7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol Sci 30:460–469. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2010) G protein coupled receptors as allosteric proteins and the role of allosteric modulators. J Recept Signal Transduct Res 30:313–321. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. (2013) Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov 12:205–216. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Miller LJ. (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, Nahorski SR. (1985) 5-Hydroxytryptamine-stimulated inositol phospholipid hydrolysis in rat cerebral cortex slices: pharmacological characterization and effects of antidepressants. J Pharmacol Exp Ther 233:473–479. [PubMed] [Google Scholar]
- Kendall I, Slotten HA, Codony X, Burgueño J, Pauwels PJ, Vela JM, Fone KC. (2011) E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology (Berl) 213:413–430. [DOI] [PubMed] [Google Scholar]
- Kennett G, Lightowler S, Trail B, Bright F, Bromidge S. (2000) Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur J Pharmacol 387:197–204. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Ainsworth K, Trail B, Blackburn TP. (1997a) BW 723C86, a 5-HT2B receptor agonist, causes hyperphagia and reduced grooming in rats. Neuropharmacology 36:233–239. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Bailey F, Piper DC, Blackburn TP. (1995) Effect of SB 200646A, a 5-HT2C/5-HT2B receptor antagonist, in two conflict models of anxiety. Psychopharmacology (Berl) 118:178–182. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Bright F, Trail B, Baxter GS, Blackburn TP. (1996a) Effects of the 5-HT2B receptor agonist, BW 723C86, on three rat models of anxiety. Br J Pharmacol 117:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. (1988) Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 96:93–100. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Pittaway K, Blackburn TP. (1994) Evidence that 5-HT2c receptor antagonists are anxiolytic in the rat Geller-Seifter model of anxiety. Psychopharmacology (Berl) 114:90–96. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Trail B, Bright F. (1998) Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology 37:1603–1610. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Whitton P, Shah K, Curzon G. (1989) Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists. Eur J Pharmacol 164:445–454. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, et al. (1996b) In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol 117:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, et al. (1997b) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36:609–620. [DOI] [PubMed] [Google Scholar]
- Kessing BF, Smout AJPM, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. (2014) Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil 26:1079–1086. [DOI] [PubMed] [Google Scholar]
- Kesters D, Thompson AD, Brams M, van Elk R, Spurny R, Geitmann M, Villalgordo JM, Guskov A, Danielson H, Lummis SCR, et al. (2013) Structural basis of ligand recognition in 5-HT3 receptors. EMBO Rep 14:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Duron B. (1996) Central effects of 5-HT on activity of respiratory and hypoglossally innervated muscles in newborn kittens. J Physiol 495:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Glérant JC, Duron B. (1999) Central effects of 5-HT on respiratory rhythm in newborn rats in vivo. Eur J Neurosci 11:3433–3440. [DOI] [PubMed] [Google Scholar]
- Khawaja X. (1995) Quantitative autoradiographic characterisation of the binding of [3H]WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res 673:217–225. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Butler A, Burridge J, Oxford AW. (1990) 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eur J Pharmacol 182:193–197. [DOI] [PubMed] [Google Scholar]
- Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, et al. (2010) Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 16:804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park BL, Cheong HS, Bae JS, Kim LH, Kim JW, Lee BC, Seo CH, Kang TC, Park SH, et al. (2014a) Association between HTR7 genetic polymorphisms and alcohol dependence, using the alcohol use disorders identification test (AUDIT). Alcohol Clin Exp Res 38:2354–2361. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB, et al. (2013) Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol 190:4795–4804. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim DH, Lee KH, Im SK, Hur EM, Chung KC, Rhim H. (2014b) Direct interaction and functional coupling between human 5-HT6 receptor and the light chain 1 subunit of the microtubule-associated protein 1B (MAP1B-LC1). PLoS One 9:e91402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KC. (2008) A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci 29:482–492. [DOI] [PubMed] [Google Scholar]
- Kinsey AM, Wainwright A, Heavens R, Sirinathsinghji DJ, Oliver KR. (2001) Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mRNAs in the rat brain. Brain Res Mol Brain Res 88:194–198. [DOI] [PubMed] [Google Scholar]
- Kirk SL, Glazebrook J, Grayson B, Neill JC, Reynolds GP. (2009) Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl) 207:119–125. [DOI] [PubMed] [Google Scholar]
- Kishi T, Meltzer HY, Iwata N. (2013) Augmentation of antipsychotic drug action by azapirone 5-HT1A receptor partial agonists: a meta-analysis. Int J Neuropsychopharmacol 16:1259–1266. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsunoka T, Ikeda M, Kitajima T, Kawashima K, Okochi T, Okumura T, Yamanouchi Y, Kinoshita Y, Ujike H, et al. (2010) Serotonin 1A receptor gene is associated with Japanese methamphetamine-induced psychosis patients. Neuropharmacology 58:452–456. [DOI] [PubMed] [Google Scholar]
- Kishore S, Khanna A, Zhang Z, Hui J, Balwierz PJ, Stefan M, Beach C, Nicholls RD, Zavolan M, Stamm S. (2010) The snoRNA MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Hum Mol Genet 19:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Stamm S. (2006) The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science 311:230–232. [DOI] [PubMed] [Google Scholar]
- Kiss B, Horváth A, Némethy Z, Schmidt E, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyán I, et al. (2010) Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333:328–340. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Nakagoshi K, Teraoka H, Taneike T. (2001) 5-HT(7) receptor and beta(2)-adrenoceptor share in the inhibition of porcine uterine contractility in a muscle layer-dependent manner. Eur J Pharmacol 433:187–197. [DOI] [PubMed] [Google Scholar]
- Kjekshus JK, Torp-Pedersen C, Gullestad L, Køber L, Edvardsen T, Olsen IC, Sjaastad I, Qvigstad E, Skomedal T, Osnes JB, et al. (2009) Effect of piboserod, a 5-HT4 serotonin receptor antagonist, on left ventricular function in patients with symptomatic heart failure. Eur J Heart Fail 11:771–778. [DOI] [PubMed] [Google Scholar]
- Kleene R, Chaudhary H, Karl N, Katic J, Kotarska A, Guitart K, Loers G, Schachner M. (2015) Interaction between CHL1 and serotonin receptor 2c regulates signal transduction and behavior in mice. J Cell Sci 128:4642–4652. [DOI] [PubMed] [Google Scholar]
- Klein D, Demory A, Peyre F, Kroll J, Géraud C, Ohnesorge N, Schledzewski K, Arnold B, Goerdt S. (2009) Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis 12:251–265. [DOI] [PubMed] [Google Scholar]
- Klein MT, Dukat M, Glennon RA, Teitler M. (2011) Toward selective drug development for the human 5-hydroxytryptamine 1E receptor: a comparison of 5-hydroxytryptamine 1E and 1F receptor structure-affinity relationships. J Pharmacol Exp Ther 337:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MT, Teitler M. (2012) Distribution of 5-ht(1E) receptors in the mammalian brain and cerebral vasculature: an immunohistochemical and pharmacological study. Br J Pharmacol 166:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Barret-Grévoz C, Bruins Slot L, Newman-Tancredi A. (2005) Novel antipsychotic agents with 5-HT(1A) agonist properties: role of 5-HT(1A) receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology 49:135–143. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. (2004) Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370:114–123. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H. (2010) Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA 107:8434–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe F, Guseva D, Jensen TP, Wirth A, Renner U, Hess D, Müller M, Medrihan L, Zhang W, Zhang M, et al. (2012) 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J Neurosci 32:2915–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK, Frielle T, Collins S, Yang-Feng T, Kobilka TS, Francke U, Lefkowitz RJ, Caron MG. (1987) An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature 329:75–79. [DOI] [PubMed] [Google Scholar]
- Koe BK. (1976) Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther 199:649–661. [PubMed] [Google Scholar]
- Koek W, Jackson A, Colpaert FC. (1992) Behavioral pharmacology of antagonists at 5-HT2/5-HT1C receptors. Neurosci Biobehav Rev 16:95–105. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA. (1995) Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci USA 92:6542–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Fashingbauer LA, Heidmann DE, Guthrie CR, Hamblin MW. (2001) Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Brain Res Mol Brain Res 90:110–117. [DOI] [PubMed] [Google Scholar]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW. (1996) Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem 66:47–56. [DOI] [PubMed] [Google Scholar]
- Kolodziejczak M, Béchade C, Gervasi N, Irinopoulou T, Banas SM, Cordier C, Rebsam A, Roumier A, Maroteaux L. (2015) Serotonin modulates developmental microglia via 5-HT2B receptors: potential implication during synaptic refinement of retinogeniculate projections. ACS Chem Neurosci 6:1219–1230. [DOI] [PubMed] [Google Scholar]
- Komada T, Matsuura T, Mikami T, Suzuki K, Sugimoto H, Kimura N, Ohmi T, Toga T, Nakai Y, Eda H, et al. (2009) Pharmacological characterization and determination of pharmacokinetic and pharmacodynamic relationship of PF-00885706, a novel partial agonist selective for the 5-HT(4) receptor. Pharmacol Res 60:237–246. [DOI] [PubMed] [Google Scholar]
- Königshoff M, Dumitrascu R, Udalov S, Amarie OV, Reiter R, Grimminger F, Seeger W, Schermuly RT, Eickelberg O. (2010) Increased expression of 5-hydroxytryptamine2A/B receptors in idiopathic pulmonary fibrosis: a rationale for therapeutic intervention. Thorax 65:949–955. [DOI] [PubMed] [Google Scholar]
- Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A. (1996) Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol 317:417–4236. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. (2013) Expression and localization of serotonin receptors in human breast cancer. Anticancer Res 33:363–370. [PubMed] [Google Scholar]
- Kornum BR, Lind NM, Gillings N, Marner L, Andersen F, Knudsen GM. (2009) Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Göttingen minipig. J Cereb Blood Flow Metab 29:186–196. [DOI] [PubMed] [Google Scholar]
- Korte SM, Meijer OC, de Kloet ER, Buwalda B, Keijser J, Sluyter F, van Oortmerssen G, Bohus B. (1996) Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res 736:338–343. [DOI] [PubMed] [Google Scholar]
- Kovács A, Hársing LG, Jr, Szénási G. (2012) Vasoconstrictor 5-HT receptors in the smooth muscle of the rat middle cerebral artery. Eur J Pharmacol 689:160–164. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. (2009) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 (Suppl 1):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HWGM, Kettenmann H. (2012) Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun 26:419–428. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Andressen KW, Levy FO. (2006) Heterologous desensitization is evoked by both agonist and antagonist stimulation of the human 5-HT7 serotonin receptor. Eur J Pharmacol 532:1–10. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. (2001) The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol 363:620–632. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Brattelid T, Levy FO, Kaumann AJ. (2005) Prucalopride is a partial agonist through human and porcine atrial 5-HT4 receptors: comparison with recombinant human 5-HT4 splice variants. Naunyn Schmiedebergs Arch Pharmacol 371:473–479. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Levy FO. (2002) The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol 135:1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze WK, Sheffler DJ, Roth BL. (2003) G-protein-coupled receptors at a glance. J Cell Sci 116:4867–4869. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- Krzywkowski K. (2006) Do polymorphisms in the human 5-HT3 genes contribute to pathological phenotypes? Biochem Soc Trans 34:872–876. [DOI] [PubMed] [Google Scholar]
- Krzywkowski K, Davies PA, Feinberg-Zadek PL, Bräuner-Osborne H, Jensen AA. (2008) High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc Natl Acad Sci USA 105:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywkowski K, Jensen AA, Connolly CN, Bräuner-Osborne H. (2007) Naturally occurring variations in the human 5-HT3A gene profoundly impact 5-HT3 receptor function and expression. Pharmacogenet Genomics 17:255–266. [DOI] [PubMed] [Google Scholar]
- Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB, COL MIG-301 Study Group (2018) Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology 91:e2222–e2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, de Jong IE, Sarter M. (2017) Reducing falls in Parkinson’s disease: interactions between donepezil and the 5-HT6 receptor antagonist idalopirdine on falls in a rat model of impaired cognitive control of complex movements. Eur J Neurosci 45:217–231. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D. (2002) Modulation by serotonin 5-HT(4) receptors of long-term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb Cortex 12:150–162. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Kurihara R, Ye L, Wells GI, McKenna DG, Burgard EC, Thor KB. (2013) Effects of the 5-HT4 receptor agonist, cisapride, on neuronally evoked responses in human bladder, urethra, and ileum. Auton Neurosci 176:70–77. [DOI] [PubMed] [Google Scholar]
- Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. (1995) 4-(2′-Methoxy-phenyl)-1-[2′-(n-2"-pyridinyl)-p-iodobenzamido]-ethyl- piperazine ([125I]p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: in vitro binding and autoradiographic studies. J Pharmacol Exp Ther 272:429–437. [PubMed] [Google Scholar]
- Kuo B, Camilleri M, Burton D, Viramontes B, McKinzie S, Thomforde G, O’Connor MK, Brinkmann BH. (2002) Effects of 5-HT(3) antagonism on postprandial gastric volume and symptoms in humans. Aliment Pharmacol Ther 16:225–233. [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. (2005) Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci 25:8578–8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. (2003a) A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem 86:980–991. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. (2003b) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304:229–237. [DOI] [PubMed] [Google Scholar]
- Kursar JD, Nelson DL, Wainscott DB, Baez M. (1994) Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol Pharmacol 46:227–234. [PubMed] [Google Scholar]
- Kursar JD, Nelson DL, Wainscott DB, Cohen ML, Baez M. (1992) Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Mol Pharmacol 42:549–557. [PubMed] [Google Scholar]
- Kusakabe A, Nonaka T, Sekino Y, Iida H, Endo H, Koide T, Takahashi H, Fujita K, Yoneda M, Goto A, et al. (2014) Effects of ramosetron oral disintegrating tablets on gastric emptying: crossover study using the 13C-acetic acid breath test. Hepatogastroenterology 61:1279–1282. [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. (2007) Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol 119:498–499. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. (2006) 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol 177:6422–6432. [DOI] [PubMed] [Google Scholar]
- Kvachnina E, Dumuis A, Wlodarczyk J, Renner U, Cochet M, Richter DW, Ponimaskin E. (2009) Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7(a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochim Biophys Acta 1793:1646–1655. [DOI] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. (2005) 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci 25:7821–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasque M, Meffre J, Carrat G, Becamel C, Bockaert J, Marin P. (2010) Constitutive activity of serotonin 2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants. Mol Pharmacol 78:818–826. [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. (2008) Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell 19:4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, et al. (2000) A role for nuclear PTEN in neuronal differentiation. J Neurosci 20:1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacivita E, Leopoldo M. (2012) Editorial: “fluorescent probes: from bench to bedside”. Curr Med Chem 19:4721. [DOI] [PubMed] [Google Scholar]
- Läer S, Remmers F, Scholz H, Stein B, Müller FU, Neumann J. (1998) Receptor mechanisms involved in the 5-HT-induced inotropic action in the rat isolated atrium. Br J Pharmacol 123:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalioti V, Pulido D, Sandoval IV. (2010) Cdk5, the multifunctional surveyor. Cell Cycle 9:284–311. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GSH, Rochford JJ, O’Rahilly S, Heisler LK. (2008) Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb EK, Aghajanian GK. (2006) Electrophysiology of 5-HT2A receptors and relevance for hallucinogen and atypical antipsychotic drug actions, in The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics (Roth BL. ed) pp 403–418, Humana Press, Totowa, NJ. [Google Scholar]
- Lambe EK, Aghajanian GK. (2001) The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci 21:9955–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. (2006) Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31:1682–1689. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. (2007) Prefrontal cortical network activity: opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience 145:900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, Shannon Weickert C. (2011) Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One 6:e22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamirault L, Simon H. (2001) Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5-HT4 receptors. Neuropharmacology 41:844–853. [DOI] [PubMed] [Google Scholar]
- Lampron A, Bourdeau I, Hamet P, Tremblay J, Lacroix A. (2006) Whole genome expression profiling of glucose-dependent insulinotropic peptide (GIP)- and adrenocorticotropin-dependent adrenal hyperplasias reveals novel targets for the study of GIP-dependent Cushing’s syndrome. J Clin Endocrinol Metab 91:3611–3618. [DOI] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA. (1983) Tumorigenic conversion of primary embryo fibroblasts requires at least 2 cooperating oncogenes. Nature 304:596–602. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Meier V, Burgess HJ, Finelli LA, Cattelin F, Achermann P, Borbély AA. (1999) Serotonin-2 receptors and human sleep: effect of a selective antagonist on EEG power spectra. Neuropsychopharmacology 21:455–466. [DOI] [PubMed] [Google Scholar]
- Lanfranco MF, Seitz PK, Morabito MV, Emeson RB, Sanders-Bush E, Cunningham KA. (2009) An innovative real-time PCR method to measure changes in RNA editing of the serotonin 2C receptor (5-HT(2C)R) in brain. J Neurosci Methods 179:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois M, Fischmeister R. (2003) 5-HT4 receptor ligands: applications and new prospects. J Med Chem 46:319–344. [DOI] [PubMed] [Google Scholar]
- Lankiewicz S, Lobitz N, Wetzel CH, Rupprecht R, Gisselmann G, Hatt H. (1998) Molecular cloning, functional expression, and pharmacological characterization of 5-hydroxytryptamine3 receptor cDNA and its splice variants from guinea pig. Mol Pharmacol 53:202–212. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Doyen C, Nevo IT, Chauveau J, Hauw JJ, Hamon M. (1996) Autoradiographic mapping of serotonin 5-HT1A, 5-HT1D, 5-HT2A and 5-HT3 receptors in the aged human spinal cord. J Chem Neuroanat 11:67–75. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, Virkkunen M, Weight F, Linnoila M, Goldman D. (1995) Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C). Genomics 27:274–279. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. (2011) Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121:3412–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. (1998) Characterization of 5-HT-sensitive potassium conductances in neonatal rat facial motoneurones in vitro. J Physiol 508:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Perkins EM. (2005) A TASK-like pH- and amine-sensitive ‘leak’ K+ conductance regulates neonatal rat facial motoneuron excitability in vitro. Eur J Neurosci 21:679–691. [DOI] [PubMed] [Google Scholar]
- Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, et al. (2008) Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer L, Glas E, Gudermann T, Breit A. (2016) Endogenous 5-HT2C receptors phosphorylate the cAMP response element binding protein via protein kinase C-promoted activation of extracellular-regulated kinases-1/2 in hypothalamic mHypoA-2/10 cells. J Pharmacol Exp Ther 358:39–49. [DOI] [PubMed] [Google Scholar]
- Launay J-M, Birraux G, Bondoux D, Callebert J, Choi D-S, Loric S, Maroteaux L. (1996) Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem 271:3141–3147. [DOI] [PubMed] [Google Scholar]
- Launay J-M, Hervé P, Callebert J, Mallat Z, Collet C, Doly S, Belmer A, Diaz SL, Hatia S, Côté F, et al. (2012) Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood 119:1772–1780. [DOI] [PubMed] [Google Scholar]
- Launay JM, Hervé P, Peoc’h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, et al. (2002) Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8:1129–1135. [DOI] [PubMed] [Google Scholar]
- Launay JM, Schneider B, Loric S, Da Prada M, Kellermann O. (2006) Serotonin transport and serotonin transporter-mediated antidepressant recognition are controlled by 5-HT2B receptor signaling in serotonergic neuronal cells. FASEB J 20:1843–1854. [DOI] [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20:612–627. [DOI] [PubMed] [Google Scholar]
- Le Coniat M, Choi DS, Maroteaux L, Launay JM, Berger R. (1996) The 5-HT2B receptor gene maps to 2q36.3-2q37.1. Genomics 32:172–173. [DOI] [PubMed] [Google Scholar]
- Lecoutey C, Hedou D, Freret T, Giannoni P, Gaven F, Since M, Bouet V, Ballandonne C, Corvaisier S, Malzert Fréon A, et al. (2014) Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc Natl Acad Sci USA 111:E3825–E3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Boyer P, Turjanski S, Rein W, Amisulpride Study Group (1997) Amisulpride versus imipramine and placebo in dysthymia and major depression. J Affect Disord 43:95–103. [DOI] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. (2004a) Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci 24:2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Meltzer HY. (1994) Blunted oral body temperature response to MK-212 in cocaine addicts. Drug Alcohol Depend 35:217–222. [DOI] [PubMed] [Google Scholar]
- Lee MS, Hanspers K, Barker CS, Korn AP, McCune JM. (2004b) Gene expression profiles during human CD4+ T cell differentiation. Int Immunol 16:1109–1124. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30:16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Xie Z, Varghese G, Nguyen T, O’Dowd BF, George SR. (2000) Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology 23 (4 Suppl):S32–S40. [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Cartier D, Duparc C, Contesse V, Lihrmann I, Delarue C, Vaudry H, Fischmeister R, Kuhn JM. (2000) Effect of serotonin4 (5-HT4) receptor agonists on aldosterone secretion in idiopathic hyperaldosteronism. Endocr Res 26:583–587. [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Contesse V, Delarue C, Feuilloley M, Hery F, Grise P, Raynaud G, Verhofstad AA, Wolf LM, Vaudry H. (1992) Serotonin-induced stimulation of cortisol secretion from human adrenocortical tissue is mediated through activation of a serotonin4 receptor subtype. Neuroscience 47:999–1007. [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Contesse V, Delarue C, Vaudry H, Kuhn JM. (1998a) Serotonergic regulation of adrenocortical function. Horm Metab Res 30:398–403. [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Duparc C, Prévost G, Zennaro MC, Bertherat J, Louiset E. (2015) Paracrine control of steroidogenesis by serotonin in adrenocortical neoplasms. Mol Cell Endocrinol 408:198–204. [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Gonzalez KN, Contesse V, Delarue C, Vaudry H, Kuhnl JM. (1998b) Effect of prolonged administration of the serotonin4 (5-HT4) receptor agonist cisapride on aldosterone secretion in healthy volunteers. Endocr Res 24:749–752. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA, Kendall DA. (2004) Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol 141:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Moison D, Cunningham KA, Piazza PV, Spampinato U. (2009a) Serotonin2C receptors in the medial prefrontal cortex facilitate cocaine-induced dopamine release in the rat nucleus accumbens. Neuropharmacology 56:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, Spampinato U. (2009b) In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem 111:614–623. [DOI] [PubMed] [Google Scholar]
- Lelong V, Dauphin F, Boulouard M. (2001) RS 67333 and D-cycloserine accelerate learning acquisition in the rat. Neuropharmacology 41:517–522. [DOI] [PubMed] [Google Scholar]
- Lelong V, Lhonneur L, Dauphin F, Boulouard M. (2003) BIMU 1 and RS 67333, two 5-HT4 receptor agonists, modulate spontaneous alternation deficits induced by scopolamine in the mouse. Naunyn Schmiedebergs Arch Pharmacol 367:621–628. [DOI] [PubMed] [Google Scholar]
- Lemaître S, Lepailleur A, Bureau R, Butt-Gueulle S, Lelong-Boulouard V, Duchatelle P, Boulouard M, Dumuis A, Daveu C, Lezoualc’h F, et al. (2009) Novel antagonists of serotonin-4 receptors: synthesis and biological evaluation of pyrrolothienopyrazines. Bioorg Med Chem 17:2607–2622. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Becker G, Vacher B, Billard T, Lancelot S, Newman-Tancredi A, Zimmer L. (2012) Radiosynthesis and preclinical evaluation of 18F-F13714 as a fluorinated 5-HT1A receptor agonist radioligand for PET neuroimaging. J Nucl Med 53:969–976. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, Zimmer L. (2010) [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging 37:594–605. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. (2004) Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 7:501–506. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, et al. (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23:8788–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet S, Delarue C, Lefebvre H, Vaudry H, Contesse V. Rat glomerulosa cells express functional 5-HT7 receptors. Endocr Res 26:597–602. [DOI] [PubMed] [Google Scholar]
- Lenglet S, Louiset E, Delarue C, Vaudry H, Contesse V. (2002a) Activation of 5-HT(7) receptor in rat glomerulosa cells is associated with an increase in adenylyl cyclase activity and calcium influx through T-type calcium channels. Endocrinology 143:1748–1760. [DOI] [PubMed] [Google Scholar]
- Lenglet S, Louiset E, Delarue C, Vaudry H, Contesse V. (2002b) Involvement of T-type calcium channels in the mechanism of action of 5-HT in rat glomerulosa cells: a novel signaling pathway for the 5-HT7 receptor. Endocr Res 28:651–655. [DOI] [PubMed] [Google Scholar]
- Lentes KU, Hinney A, Ziegler A, Rosenkranz K, Wurmser H, Barth N, Jacob K, Coners H, Mayer H, Grzeschik KH, et al. (1997) Evaluation of a Cys23Ser mutation within the human 5-HT2C receptor gene: no evidence for an association of the mutant allele with obesity or underweight in children, adolescents and young adults. Life Sci 61:PL9–PL16. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hen R. (2008) Anxiety as a developmental disorder. Neuropsychopharmacology 33:134–140. [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Herrick-Davis K, Titeler M. (1989) Detection of a novel serotonin receptor subtype (5-HT1E) in human brain: interaction with a GTP-binding protein. J Neurochem 53:465–471. [DOI] [PubMed] [Google Scholar]
- León-Ponte M, Ahern GP, O’Connell PJ. (2007) Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109:3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, De Giorgio P, Fracasso C, Guzzetti S, Caccia S, Contino M, Colabufo NA, Berardi F, Perrone R. (2008) Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J Med Chem 51:5813–5822. [DOI] [PubMed] [Google Scholar]
- Lepailleur A, Freret T, Lemaître S, Boulouard M, Dauphin F, Hinschberger A, Dulin F, Lesnard A, Bureau R, Rault S. (2014) Dual histamine H3R/serotonin 5-HT4R ligands with antiamnesic properties: pharmacophore-based virtual screening and polypharmacology. J Chem Inf Model 54:1773–1784. [DOI] [PubMed] [Google Scholar]
- Lerond J, Lothe A, Ryvlin P, Bouvard S, d’Amato T, Ciumas C, Daléry J, Poulet E, Saoud M. (2013) Effects of aripiprazole, risperidone, and olanzapine on 5-HT1A receptors in patients with schizophrenia. J Clin Psychopharmacol 33:84–89. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. (2004) Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol 7:381–385. [DOI] [PubMed] [Google Scholar]
- Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. (2006) Platelet-derived serotonin mediates liver regeneration. Science 312:104–107. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. (2011) Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther 338:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin OS. (2015) [The use of buspirone in clinical practice]. Zh Nevrol Psikhiatr Im S S Korsakova 115:83–87. [DOI] [PubMed] [Google Scholar]
- Levitt ES, Hunnicutt BJ, Knopp SJ, Williams JT, Bissonnette JM. (2013) A selective 5-HT1a receptor agonist improves respiration in a mouse model of Rett syndrome. J Appl Physiol (1985) 115:1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy FO, Gudermann T, Birnbaumer M, Kaumann AJ, Birnbaumer L. (1992a) Molecular cloning of a human gene (S31) encoding a novel serotonin receptor mediating inhibition of adenylyl cyclase. FEBS Lett 296:201–206. [DOI] [PubMed] [Google Scholar]
- Levy FO, Gudermann T, Perez-Reyes E, Birnbaumer M, Kaumann AJ, Birnbaumer L. (1992b) Molecular cloning of a human serotonin receptor (S12) with a pharmacological profile resembling that of the 5-HT1D subtype. J Biol Chem 267:7553–7562. [PubMed] [Google Scholar]
- Levy FO, Holtgreve-Grez H, Taskén K, Solberg R, Ried T, Gudermann T. (1994) Assignment of the gene encoding the 5-HT1E serotonin receptor (S31) (locus HTR1E) to human chromosome 6q14-q15. Genomics 22:637–640. [DOI] [PubMed] [Google Scholar]
- Levy FO, Qvigstad E, Krobert KA, Skomedal T, Osnes JB. (2008) Effects of serotonin in failing cardiac ventricle: signalling mechanisms and potential therapeutic implications. Neuropharmacology 55:1066–1071. [DOI] [PubMed] [Google Scholar]
- Lewis JH. (2011) The risk of ischaemic colitis in irritable bowel syndrome patients treated with serotonergic therapies. Drug Saf 34:545–565. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Awouters F, Kennis L, Laduron PM, Vandenberk J, Janssen PA. (1981) Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci 28:1015–1022. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Heylen L, Luyten WH, Van de Weyer I, Vanhoenacker P, Haegeman G, Schotte A, Van Gompel P, Wouters R, et al. (1996) Alniditan, a new 5-hydroxytryptamine1D agonist and migraine-abortive agent: ligand-binding properties of human 5-hydroxytryptamine1D α, human 5-hydroxytryptamine1D β, and calf 5-hydroxytryptamine1D receptors investigated with [3H]5-hydroxytryptamine and [3H]alniditan. Mol Pharmacol 50:1567–1580. [PubMed] [Google Scholar]
- Leysen JE, Van Gompel P, Gommeren W, Woestenborghs R, Janssen PA. (1986) Down regulation of serotonin-S2 receptor sites in rat brain by chronic treatment with the serotonin-S2 antagonists: ritanserin and setoperone. Psychopharmacology (Berl) 88:434–444. [DOI] [PubMed] [Google Scholar]
- Lezoualc’h F, Robert SJ. (2003) The serotonin 5-HT4 receptor and the amyloid precursor protein processing. Exp Gerontol 38:159–166. [DOI] [PubMed] [Google Scholar]
- Lezoualc’h F, Steplewski K, Sartiani L, Mugelli A, Fischmeister R, Bril A. (2007) Quantitative mRNA analysis of serotonin 5-HT4 receptor isoforms, calcium handling proteins and ion channels in human atrial fibrillation. Biochem Biophys Res Commun 357:218–224. [DOI] [PubMed] [Google Scholar]
- Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, Mallet J, Khan WI. (2011a) Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol 178:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Weng S-G, Leng X-S, Peng J-R, Wei Y-H, Mou D-C, Wang W-X. (2006) Effects of 5-hydroxytamine and its antagonists on hepatic stellate cells. Hepatobiliary Pancreat Dis Int 5:96–100. [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. (2004) In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 29:1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Nie Y, Xie J, Tang W, Liang P, Sha W, Yang H, Zhou Y. (2007) The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci 52:2942–2949. [DOI] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. (2011b) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Chen W, Zhi X, Ma T, Xia X, Liu H, Zhang Q, Hu Q, Zhang Y, Bai X, et al. (2013) Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YJ, Lai LP, Wang BW, Juang SJ, Chang CM, Leu JG, Shyu KG. (2006) Mechanical stress enhances serotonin 2B receptor modulating brain natriuretic peptide through nuclear factor-kappaB in cardiomyocytes. Cardiovasc Res 72:303–312. [DOI] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, Dumont JE, Vassart G. (1990) Complete nucleotide sequence of a putative G protein coupled receptor: RDC4. Nucleic Acids Res 18:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CL, Kirkegaard L, Zueger M, Chourbaji S, Gass P, Aznar S, Knudsen GM. (2010a) Changes in 5-HT4 receptor and 5-HT transporter binding in olfactory bulbectomized and glucocorticoid receptor heterozygous mice. Neurochem Int 56:603–610. [DOI] [PubMed] [Google Scholar]
- Licht CL, Knudsen GM, Sharp T. (2010b) Effects of the 5-HT(4) receptor agonist RS67333 and paroxetine on hippocampal extracellular 5-HT levels. Neurosci Lett 476:58–61. [DOI] [PubMed] [Google Scholar]
- Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. (2005) The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology 30:2169–2179. [DOI] [PubMed] [Google Scholar]
- Lim C-S, Hoang ET, Viar KE, Stornetta RL, Scott MM, Zhu JJ. (2014) Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev 28:273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Chou CC, Gao S, Wu SC, Khoo KH, Lin CH. (2013) An in vivo tagging method reveals that Ras undergoes sustained activation upon transglutaminase-mediated protein serotonylation. ChemBioChem 14:813–817. [DOI] [PubMed] [Google Scholar]
- Lin JC, Chou CC, Tu Z, Yeh LF, Wu SC, Khoo KH, Lin CH. (2014) Characterization of protein serotonylation via bioorthogonal labeling and enrichment. J Proteome Res 13:3523–3529. [DOI] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. (2003) Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem 87:1076–1085. [DOI] [PubMed] [Google Scholar]
- Lin S-Y, Chang W-J, Lin C-S, Huang C-Y, Wang H-F, Sun W-H. (2011) Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci 31:1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Walther D, Yu X-Y, Drgon T, Uhl GR. (2004) The human serotonin receptor 2B: coding region polymorphisms and association with vulnerability to illegal drug abuse. Pharmacogenetics 14:805–811. [DOI] [PubMed] [Google Scholar]
- Linder AE, Beggs KM, Burnett RJ, Watts SW. (2009) Body distribution of infused serotonin in rats. Clin Exp Pharmacol Physiol 36:599–601. [DOI] [PubMed] [Google Scholar]
- Liu H, Irving HR, Coupar IM. (2001) Expression patterns of 5-HT7 receptor isoforms in the rat digestive tract. Life Sci 69:2467–2475. [DOI] [PubMed] [Google Scholar]
- Liu H-N, Ohya S, Nishizawa Y, Sawamura K, Iino S, Syed MM, Goto K, Imaizumi Y, Nakayama S. (2011a) Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS One 6:e24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. (2005a) Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol 289:G1148–G1163. [DOI] [PubMed] [Google Scholar]
- Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. (2009) 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29:9683–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. (2007) Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 146:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Green CE, Cunningham KA, Moeller FG. (2012a) Increased intra-individual reaction time variability in cocaine-dependent subjects: role of cocaine-related cues. Addict Behav 37:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. (2011b) Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse 37:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wacker D, Gati C, Han GW, James D, Wang D, Nelson G, Weierstall U, Katritch V, Barty A, et al. (2013a) Serial femtosecond crystallography of G protein-coupled receptors. Science 342:1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wu SX, Wang YY, Wang W, Zhou L, Li YQ. (2005b) Changes of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by bee venom-induced inflammatory pain. Neurosci Lett 375:42–46. [DOI] [PubMed] [Google Scholar]
- Liu Y, Emeson RB, Samuel CE. (1999) Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem 274:18351–18358. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tian H, Yan X, Fan F, Wang W, Han J. (2013b) Serotonin inhibits apoptosis of pulmonary artery smooth muscle cells through 5-HT2A receptors involved in the pulmonary artery remodeling of pulmonary artery hypertension. Exp Lung Res 39:70–79. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tian XY, Mao G, Fang X, Fung ML, Shyy JY, Huang Y, Wang N. (2012b) Peroxisome proliferator-activated receptor-γ ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension 60:1471–1478. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei L, Laskin DL, Fanburg BL. (2011c) Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YS, Yang M. (2006) The effect of 5-hydroxtryptamine on the regulation of megakaryocytopoiesis. Hematology 11:53–56. [DOI] [PubMed] [Google Scholar]
- Livesey MR, Cooper MA, Deeb TZ, Carland JE, Kozuska J, Hales TG, Lambert JJ, Peters JA. (2008) Structural determinants of Ca2+ permeability and conduction in the human 5-hydroxytryptamine type 3A receptor. J Biol Chem 283:19301–19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Cooper MA, Lambert JJ, Peters JA. (2011) Rings of charge within the extracellular vestibule influence ion permeation of the 5-HT3A receptor. J Biol Chem 286:16008–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liy-Salmeron G, Meneses A. (2008) Effects of 5-HT drugs in prefrontal cortex during memory formation and the ketamine amnesia-model. Hippocampus 18:965–974. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Assié MB, Newman-Tancredi A, Artigas F, Celada P. (2010) Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br J Pharmacol 160:1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Assié MB, Newman-Tancredi A, Artigas F, Celada P. (2012) In vivo electrophysiological and neurochemical effects of the selective 5-HT1A receptor agonist, F13640, at pre- and postsynaptic 5-HT1A receptors in the rat. Psychopharmacology (Berl) 221:261–272. [DOI] [PubMed] [Google Scholar]
- Lochner M, Lummis SC. (2010) Agonists and antagonists bind to an A-A interface in the heteromeric 5-HT3AB receptor. Biophys J 98:1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker M, Bitard J, Collet C, Poliard A, Mutel V, Launay JM, Kellermann O. (2006) Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal 18:628–639. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, Sachs G. (2014a) Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 171:160–168. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Silva R, Kroger H, Sarma K, Xu J, Calabrese JR. (2014b) Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 171:169–177. [DOI] [PubMed] [Google Scholar]
- Logue SF, Gould TJ. (2014) The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav 123:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:896–906. [DOI] [PubMed] [Google Scholar]
- Loiseau F, Dekeyne A, Millan MJ. (2008) Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology (Berl) 196:93–104. [DOI] [PubMed] [Google Scholar]
- Long DD, Armstrong SR, Beattie DT, Choi SK, Fatheree PR, Gendron RA, Goldblum AA, Humphrey PP, Marquess DG, Shaw JP, et al. (2012) Discovery, oral pharmacokinetics and in vivo efficacy of a highly selective 5-HT4 receptor agonist: clinical compound TD-2749. Bioorg Med Chem Lett 22:4849–4853. [DOI] [PubMed] [Google Scholar]
- Longmore J, Shaw D, Smith D, Hopkins R, McAllister G, Pickard JD, Sirinathsinghji DJ, Butler AJ, Hill RG. (1997) Differental distribution of 5-HT1B- and 5-HT1D immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia 17:833–842. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. (2006) Functional bowel disorders [published correction appears in Gastroenterology (2006) 131:688]. Gastroenterology 130:1480–1491. [DOI] [PubMed] [Google Scholar]
- Loo LS, Ailani J, Schim J, Baygani S, Hundemer H-P, Port M, Krege JH. (2019) Efficacy and safety of lasmiditan in patients using concomitant migraine preventive medications: findings from SAMURAI and SPARTAN, two randomized phase 3 trials. J Headache Pain 20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Esparza S, Berumen LC, Padilla K, Miledi R, García-Alcocer G. (2015) Expression of hippocampal serotonin receptors 5-HT2C and 5-HT5A in a rat model of diet-induced obesity supplemented with tryptophan. Int J Dev Neurosci 42:80–85. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. (1997) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol 356:446–454. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. (2001) Regional distribution and cellular localization of 5-HT2C receptor mRNA in monkey brain: comparison with [3H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse 42:12–26. [DOI] [PubMed] [Google Scholar]
- Lopreato GF, Banerjee P, Mihic SJ. (2003) Amino acids in transmembrane domain two influence anesthetic enhancement of serotonin-3A receptor function. Brain Res Mol Brain Res 118:45–51. [DOI] [PubMed] [Google Scholar]
- Loric S, Launay J-M, Colas J-F, Maroteaux L. (1992) New mouse 5-HT2-like receptor. Expression in brain, heart and intestine. FEBS Lett 312:203–207. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Lu G, Cho E, Yew DT. (2006) Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, Aube J, Bohn LM. (2015) Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK 1/2 phosphorylation while preserving G protein over βArrestin2 signaling bias. ACS Chem Neurosci 6:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, et al. (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43:342–345. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, et al. (1993a) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11:449–458. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Erlander MG, Baron BM, Racke M, Slone AL, Siegel BW, Craft CM, Burns JE, Danielson PE, Sutcliffe JG. (1993b) Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci USA 90:2184–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy MT, Meltzer HY. (1988) Stimulation of serum cortisol and prolactin secretion in humans by MK-212, a centrally active serotonin agonist. Biol Psychiatry 23:818–828. [DOI] [PubMed] [Google Scholar]
- Lübbert H, Hoffman BJ, Snutch TP, van Dyke T, Levine AJ, Hartig PR, Lester HA, Davidson N. (1987) cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci USA 84:4332–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucaites VL, Nelson DL, Wainscott DB, Baez M. (1996) Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci 59:1081–1095. [DOI] [PubMed] [Google Scholar]
- Lucas G. (2009) Serotonin receptors, type 4: a new hope? Curr Drug Targets 10:1085–1095. [DOI] [PubMed] [Google Scholar]
- Lucas G, Compan V, Charnay Y, Neve RL, Nestler EJ, Bockaert J, Barrot M, Debonnel G. (2005) Frontocortical 5-HT4 receptors exert positive feedback on serotonergic activity: viral transfections, subacute and chronic treatments with 5-HT4 agonists. Biol Psychiatry 57:918–925. [DOI] [PubMed] [Google Scholar]
- Lucas G, Debonnel G. (2002) 5-HT4 receptors exert a frequency-related facilitatory control on dorsal raphé nucleus 5-HT neuronal activity. Eur J Neurosci 16:817–822. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Piñeyro G, et al. (2007) Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55:712–725. [DOI] [PubMed] [Google Scholar]
- Lucki I. (1992) 5-HT1 receptors and behavior. Neurosci Biobehav Rev 16:83–93. [DOI] [PubMed] [Google Scholar]
- Lucki I. (1998) The spectrum of behaviors influenced by serotonin. Biol Psychiatry 44:151–162. [DOI] [PubMed] [Google Scholar]
- Luiking YC, Akkermans LMA, Peeters TL, Cnossen PJ, Nieuwenhuijs VB, Vanberge -Henegouwen GP. (2002) Effects of motilin on human interdigestive gastrointestinal and gallbladder motility, and involvement of 5HT3 receptors. Neurogastroenterol Motil 14:151–159. [DOI] [PubMed] [Google Scholar]
- Łukasiewicz S, Błasiak E, Faron-Górecka A, Polit A, Tworzydło M, Górecki A, Wasylewski Z, Dziedzicka-Wasylewska M. (2007) Fluorescence studies of homooligomerization of adenosine A2A and serotonin 5-HT1A receptors reveal the specificity of receptor interactions in the plasma membrane. Pharmacol Rep 59:379–392. [PubMed] [Google Scholar]
- Lukasiewicz S, Polit A, Kędracka-Krok S, Wędzony K, Maćkowiak M, Dziedzicka-Wasylewska M. (2010) Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim Biophys Acta 1803:1347–1358. [DOI] [PubMed] [Google Scholar]
- Luoni A, Hulsken S, Cazzaniga G, Racagni G, Homberg JR, Riva MA. (2013) Behavioural and neuroplastic properties of chronic lurasidone treatment in serotonin transporter knockout rats. Int J Neuropsychopharmacol 16:1319–1330. [DOI] [PubMed] [Google Scholar]
- Lutsep HL. (2002) Repinotan Bayer. Curr Opin Investig Drugs 3:924–927. [PubMed] [Google Scholar]
- Lüttgen M, Elvander E, Madjid N, Ogren SO. (2005) Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology 48:830–852. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Maudsley S, Bohn LM. (2015) Fulfilling the promise of “biased” G protein-coupled receptor agonism. Mol Pharmacol 88:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly S, Pishdari B, Lok LL, Hajos M, Kocsis B. (2013) Activation of 5-HT6 receptors modulates sleep-wake activity and hippocampal theta oscillation. ACS Chem Neurosci 4:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyddon R, Cuppen E, Haroutunian V, Siever LJ, Dracheva S. (2010) No link of serotonin 2C receptor editing to serotonin transporter genotype. Neuroreport 21:1080–1084. [DOI] [PubMed] [Google Scholar]
- Lyddon R, Dwork AJ, Keddache M, Siever LJ, Dracheva S. (2013) Serotonin 2c receptor RNA editing in major depression and suicide. World J Biol Psychiatry 14:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RA, Davis KH, Titeler M. (1987) 3H-DOB (4-bromo-2,5-dimethoxyphenylisopropylamine) labels a guanyl nucleotide-sensitive state of cortical 5-HT2 receptors. Mol Pharmacol 31:194–199. [PubMed] [Google Scholar]
- Ma QP. (2001) Co-localization of 5-HT(1B/1D/1F) receptors and glutamate in trigeminal ganglia in rats. Neuroreport 12:1589–1591. [DOI] [PubMed] [Google Scholar]
- Ma QP, Hill R, Sirinathsinghji D. (2001) Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci 13:2099–2104. [DOI] [PubMed] [Google Scholar]
- Ma XX, Chen QX, Wu SJ, Hu Y, Fang XM. (2013) Polymorphisms of the HTR3B gene are associated with post-surgery emesis in a Chinese Han population. J Clin Pharm Ther 38:150–155. [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, et al. (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Patt S, Schrey M, Rich A. (2001) Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci USA 98:14687–14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen VanDenBrink A, Vergouwe MN, Ophoff RA, Naylor SL, Dauwerse HG, Saxena PR, Ferrari MD, Frants RR. (1998) Chromosomal localization of the 5-HT1F receptor gene: no evidence for involvement in response to sumatriptan in migraine patients. Am J Med Genet 77:415–420. [PubMed] [Google Scholar]
- Maayani S, Wilkinson CW, Stollak JS. (1984) 5-Hydroxytryptamine receptor in rabbit aorta: characterization by butyrophenone analogs. J Pharmacol Exp Ther 229:346–350. [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. (2011) Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. (2014) Spinal nNOS regulates phrenic motor facilitation by a 5-HT2B receptor- and NADPH oxidase-dependent mechanism. Neuroscience 269:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida T, Iizuka K, Hirafuji M. (2013) 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol Pharm Bull 36:1416–1419. [DOI] [PubMed] [Google Scholar]
- Machu TK. (2011) Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther 130:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean MR, Dempsie Y. (2010) The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol 661:309–322. [DOI] [PubMed] [Google Scholar]
- MacLeod AM, Street LJ, Reeve AJ, Jelley RA, Sternfeld F, Beer MS, Stanton JA, Watt AP, Rathbone D, Matassa VG. (1997) Selective, orally active 5-HT1D receptor agonists as potential antimigraine agents. J Med Chem 40:3501–3503. [DOI] [PubMed] [Google Scholar]
- Madjid N, Tottie EE, Lüttgen M, Meister B, Sandin J, Kuzmin A, Stiedl O, Ogren SO. (2006) 5-Hydroxytryptamine 1A receptor blockade facilitates aversive learning in mice: interactions with cholinergic and glutamatergic mechanisms. J Pharmacol Exp Ther 316:581–591. [DOI] [PubMed] [Google Scholar]
- Madsen K, Neumann WJ, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SG. (2011) Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer’s disease. J Alzheimers Dis 26:457–466. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Kim S, Nakai D, Makino C, Takagi T, Ogura H, Yamada K, Chatton B, Ishii S. (2010) Social isolation stress induces ATF-7 phosphorylation and impairs silencing of the 5-HT 5B receptor gene. EMBO J 29:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut C, Vansande J, Massart C, Dinsart C, Libert F, Monferini E, Giraldo E, Ladinsky H, Vassart G, Dumont JE. (1991) The orphan receptor cDNA RDC4 encodes a 5-HT1D serotonin receptor. Biochem Biophys Res Commun 80:1460–1468. [DOI] [PubMed] [Google Scholar]
- Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. (2001) Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res 61:5692–5696. [PubMed] [Google Scholar]
- Maginnis MS, Haley SA, Gee GV, Atwood WJ. (2010) Role of N-linked glycosylation of the 5-HT2A receptor in JC virus infection. J Virol 84:9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrini E, Szabò I, Doni A, Cibella J, Viola A. (2011) Serotonin-mediated tuning of human helper T cell responsiveness to the chemokine CXCL12. PLoS One 6:e22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet JC, Zhang Y, Li X, Zhang X. (2008) PTEN-5-HT2C coupling: a new target for treating drug addiction. Prog Brain Res 172:407–420. [DOI] [PubMed] [Google Scholar]
- Manfra O, Van Craenenbroeck K, Skieterska K, Frimurer T, Schwartz TW, Levy FO, Andressen KW. (2015) Downregulation of 5-HT7 serotonin receptors by the atypical antipsychotics clozapine and olanzapine. Role of motifs in the C-terminal domain and interaction with GASP-1. ACS Chem Neurosci 6:1206–1218. [DOI] [PubMed] [Google Scholar]
- Manivet P, Mouillet-Richard S, Callebert J, Nebigil CG, Maroteaux L, Hosoda S, Kellermann O, Launay J-M. (2000) PDZ-dependent activation of nitric-oxide synthases by the serotonin 2B receptor. J Biol Chem 275:9324–9331. [DOI] [PubMed] [Google Scholar]
- Manning DD, Cioffi CL, Usyatinsky A, Fitzpatrick K, Masih L, Guo C, Zhang Z, Choo SH, Sikkander MI, Ryan KN, et al. (2011) Novel serotonin type 3 receptor partial agonists for the potential treatment of irritable bowel syndrome. Bioorg Med Chem Lett 21:58–61. [DOI] [PubMed] [Google Scholar]
- Manning DD, Guo C, Zhang Z, Ryan KN, Naginskaya J, Choo SH, Masih L, Earley WG, Wierschke JD, Newman AS, et al. (2014) The discovery of diazepinone-based 5-HT3 receptor partial agonists. Bioorg Med Chem Lett 24:2578–2581. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. (2006) Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol 70:1013–1021. [DOI] [PubMed] [Google Scholar]
- Manuel-Apolinar L, Rocha L, Pascoe D, Castillo E, Castillo C, Meneses A. (2005) Modifications of 5-HT4 receptor expression in rat brain during memory consolidation. Brain Res 1042:73–81. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL. (2012a) The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther 342:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL. (2012b) Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 341:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. (2003) 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301:226–229. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Borsini F, Picchetti M, Vatteroni E, Falaschi V, Catena-Dell’Osso M. (2013a) Serotonin receptors of type 6 (5-HT6): from neuroscience to clinical pharmacology. Curr Med Chem 20:371–377. [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Pirone A, Giannaccini G, Betti L, Testa G, Schmid L, Palego L, Borsini F, Bordi F, et al. (2013b) Serotonin receptor of type 6 (5-HT6) in human prefrontal cortex and hippocampus post-mortem: an immunohistochemical and immunofluorescence study. Neurochem Int 62:182–188. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS. (2000) Differential modulation of the 5-HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39:2017–2027. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Jacquet M, Escoffier G, Miglioratti M, Dumuis A, Bockaert J, Roman FS. (2011) Enhancement of reference memory in aged rats by specific activation of 5-HT(4) receptors using an olfactory associative discrimination task. Brain Res 1405:49–56. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Maura G, Tortarolo M, Raiteri M. (1997) Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in rat cerebellum: involvement of 5-hydroxytryptamine2C receptors. J Neurochem 69:427–430. [DOI] [PubMed] [Google Scholar]
- Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S. (2003) Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168:487–493. [DOI] [PubMed] [Google Scholar]
- Marek GJ. (2010) Electrophysiology of serotonin receptors, in Handbook of the Behavioral Neurobiology of Serotonin (Muller CP, Jacobs B. eds) pp. 163–182, Elsevier Academic Press. [Google Scholar]
- Marek GJ, Aghajanian GK. (1996) LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther 278:1373–1382. [PubMed] [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price LH. (2003) Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology 28:402–412. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. (2005) The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology 30:2205–2215. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. (2014) Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. (1991) Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254:432–437. [DOI] [PubMed] [Google Scholar]
- Marin P, Becamel C, Dumuis A, Bockaert J. (2012) 5-HT receptor-associated protein networks: new targets for drug discovery in psychiatric disorders? Curr Drug Targets 13:28–52. [DOI] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. (2004) RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem 279:2945–2954. [DOI] [PubMed] [Google Scholar]
- Markstein R, Hoyer D, Engel G. (1986) 5-HT1A-receptors mediate stimulation of adenylate cyclase in rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol 333:335–341. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Comley RA, Baaré WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, et al. (2009) Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med 50:900–908. [DOI] [PubMed] [Google Scholar]
- Maroteaux L. (2013) Overexpression of 5-hydroxytryptamine 2B receptor gene in pulmonary hypertension: still a long way to understand its transcriptional regulation. Hypertension 61:e28–e29. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Ayme-Dietrich E, Aubertin-Kirch G, Banas S, Quentin E, Lawson R, Monassier L. (2017) New therapeutic opportunities for 5-HT2 receptor ligands. Pharmacol Ther 170:14–36. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Béchade C, Roumier A. (2019) Dimers of serotonin receptors: impact on ligand affinity and signaling. Biochimie 161:23–33. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. (1992) Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA 89:3020–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, et al. (2007) WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486–496. [DOI] [PubMed] [Google Scholar]
- Marsden CA, King MV, Fone KC. (2011) Influence of social isolation in the rat on serotonergic function and memory--relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology 61:400–407. [DOI] [PubMed] [Google Scholar]
- Martel JC, Ormière AM, Leduc N, Assié MB, Cussac D, Newman-Tancredi A. (2007) Native rat hippocampal 5-HT1A receptors show constitutive activity. Mol Pharmacol 71:638–643. [DOI] [PubMed] [Google Scholar]
- Martin CB, Hamon M, Lanfumey L, Mongeau R. (2014a) Controversies on the role of 5-HT(2C) receptors in the mechanisms of action of antidepressant drugs. Neurosci Biobehav Rev 42:208–223. [DOI] [PubMed] [Google Scholar]
- Martin CB, Martin VS, Trigo JM, Chevarin C, Maldonado R, Fink LH, Cunningham KA, Hamon M, Lanfumey L, Mongeau R. (2014b) 5-HT2C receptor desensitization moderates anxiety in 5-HTT deficient mice: from behavioral to cellular evidence. Int J Neuropsychopharmacol 18:pyu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CB, Ramond F, Farrington DT, Aguiar AS, Jr, Chevarin C, Berthiau AS, Caussanel S, Lanfumey L, Herrick-Davis K, Hamon M, et al. (2013) RNA splicing and editing modulation of 5-HT(2C) receptor function: relevance to anxiety and aggression in VGV mice. Mol Psychiatry 18:656–665. [DOI] [PubMed] [Google Scholar]
- Martin G. (1997) Pre-clinical pharmacology of zolmitriptan (ZomigTM; formerly 311C90), a centrally and peripherally acting 5HT1B/1D agonist for migraine. Cephalalgia. 17:4–14. [DOI] [PubMed] [Google Scholar]
- Martin GR. (1994) Vascular receptors for 5—hydroxytryptamine: distribution, function and classification. Pharmacol Ther 62:283–324. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bös M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HHG, Broekkamp CLE, et al. (1998) 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther 286:913–924. [PubMed] [Google Scholar]
- Martin LL, Bouchal RL, Smith DJ. (1982) Ketamine inhibits serotonin uptake in vivo. Neuropharmacology 21:113–118. [DOI] [PubMed] [Google Scholar]
- Martin LL, Smith DJ. (1982) Ketamine inhibits serotonin synthesis and metabolism in vivo. Neuropharmacology 21:119–125. [DOI] [PubMed] [Google Scholar]
- Martín-Cora FJ, Pazos A. (2004) Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br J Pharmacol 141:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, Parsey RV, Hashimoto T, Huang Y, Shinn A, et al. (2001) Differential occupancy of somatodendritic and postsynaptic 5HT(1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology 24:209–229. [DOI] [PubMed] [Google Scholar]
- Martínez-García E, Leopoldo M, Lacivita E, Terrón JA. (2011) Increase of capsaicin-induced trigeminal Fos-like immunoreactivity by 5-HT(7) receptors. Headache 51:1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Solano M, Iglesias A, de Fabritiis G, Sanz F, Brea J, Loza MI, Pastor M, Selent J. (2015) Detection of new biased agonists for the serotonin 5-HT2A receptor: modeling and experimental validation. Mol Pharmacol 87:740–746. [DOI] [PubMed] [Google Scholar]
- Martí-Solano M, Sanz F, Pastor M, Selent J. (2014) A dynamic view of molecular switch behavior at serotonin receptors: implications for functional selectivity. PLoS One 9:e109312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki C, Sharpley AL, Cooper CM, Godlewska BR, Singh N, Vasudevan SR, Harmer CJ, Churchill GC, Sharp T, Rogers RD, et al. (2016) Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology (Berl) 233:2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masellis M, Basile VS, Meltzer HY, Lieberman JA, Sevy S, Goldman DA, Hamblin MW, Macciardi FM, Kennedy JL. (2001) Lack of association between the T-->C 267 serotonin 5-HT6 receptor gene (HTR6) polymorphism and prediction of response to clozapine in schizophrenia. Schizophr Res 47:49–58. [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. (2008) Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA 105:13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoura AN, Dover TJ, Newman AS, Barnes NM. (2011) The identification of N-glycosylated residues of the human 5-HT3B receptor subunit: importance for cell membrane expression. J Neurochem 116:975–983. [DOI] [PubMed] [Google Scholar]
- Mastropasqua L, Ciancaglini M, Carpineto P, Costagliola C. (1997) Ocular hypotensive effect of topical ketanserin in timolol users. Graefes Arch Clin Exp Ophthalmol 235:130–135. [DOI] [PubMed] [Google Scholar]
- Mathes BM, Hudziak KJ, Schaus JM, Xu YC, Nelson DL, Wainscott DB, Nutter SE, Gough WH, Branchek TA, Zgombick JM, et al. (2004) Substituted furo[3,2-b]pyridines: novel bioisosteres of 5-HT 1F receptor agonists. Bioorg Med Chem Lett 14:167–170. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, et al. (2003) Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 22:3307–3318. [DOI] [PubMed] [Google Scholar]
- Matsumura N, Mandai M, Miyanishi M, Fukuhara K, Baba T, Higuchi T, Kariya M, Takakura K, Fujii S. (2006) Oncogenic property of acrogranin in human uterine leiomyosarcoma: direct evidence of genetic contribution in in vivo tumorigenesis. Clin Cancer Res 12:1402–1411. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi H, Kuniyasu H, Okumura M, Misawa H, Katsui R, Zhang GX, Obata K, Takaki M. (2010) A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil 22:806–813, e226. [DOI] [PubMed] [Google Scholar]
- Matthes H, Boschert U, Amlaiky N, Grailhe R, Plassat JL, Muscatelli F, Mattei MG, Hen R. (1993) Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: cloning, functional expression, and chromosomal localization. Mol Pharmacol 43:313–319. [PubMed] [Google Scholar]
- Matthys A, Van Craenenbroeck K, Lintermans B, Haegeman G, Vanhoenacker P. (2012) RhoBTB3 interacts with the 5-HT7a receptor and inhibits its proteasomal degradation. Cell Signal 24:1053–1063. [DOI] [PubMed] [Google Scholar]
- Mattsson C, Sonesson C, Sandahl A, Greiner HE, Gassen M, Plaschke J, Leibrock J, Bottcher H. (2005) 2-Alkyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as novel 5-HT6 receptor agonists. Bioorganic & medicinal chemistry letters 15:4230–4234. [DOI] [PubMed] [Google Scholar]
- Mauler F, Horváth E. (2005) Neuroprotective efficacy of repinotan HCl, a 5-HT1A receptor agonist, in animal models of stroke and traumatic brain injury. J Cereb Blood Flow Metab 25:451–459. [DOI] [PubMed] [Google Scholar]
- Maura G, Raiteri M. (1986) Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol 129:333–337. [DOI] [PubMed] [Google Scholar]
- Maurel S, De Vry J, Schreiber R. (1999) 5-HT receptor ligands differentially affect operant oral self-administration of ethanol in the rat. Eur J Pharmacol 370:217–223. [DOI] [PubMed] [Google Scholar]
- Maurice P, Guillaume JL, Benleulmi-Chaachoua A, Daulat AM, Kamal M, Jockers R. (2011) GPCR-interacting proteins, major players of GPCR function. Adv Pharmacol 62:349–380. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. (2013) Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JA, McLaughlin MA, Sharif NA, Hellberg MR, Dean TR. (2003) Evaluation of the ocular hypotensive response of serotonin 5-HT1A and 5-HT2 receptor ligands in conscious ocular hypertensive cynomolgus monkeys. J Pharmacol Exp Ther 306:301–309. [DOI] [PubMed] [Google Scholar]
- May JA, Sharif NA, McLaughlin MA, Chen H-H, Severns BS, Kelly CR, Holt WF, Young R, Glennon RA, Hellberg MR, Dean TR. (2015) Ocular hypotensive response in nonhuman primates of (8R)-1-[(2S)-2-Aminopropyl]-8,9-dihydro-7H-pyrano[2,3-g]indazol-8-ol a Selective 5-HT2 receptor agonist. J Med Chem 58:8818–8833. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. (2001) Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther 298:1101–1107. [PubMed] [Google Scholar]
- McAllister G, Charlesworth A, Snodin C, Beer MS, Noble AJ, Middlemiss DN, Iversen LL, Whiting P. (1992) Molecular cloning of a serotonin receptor from human brain (5HT1E): a fifth 5HT1-like subtype. Proc Natl Acad Sci USA 89:5517–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB. (1997) Trigeminal ganglion elicited increases in nucleus trigeminal caudalis blood flow: a novel migraine model. Brain Res 775:189–192. [DOI] [PubMed] [Google Scholar]
- McCall RB, Huff R, Chio CL, TenBrink R, Bergh CL, Ennis MD, Ghazal NB, Hoffman RL, Meisheri K, Higdon NR, et al. (2002) Preclinical studies characterizing the anti-migraine and cardiovascular effects of the selective 5-HT1D receptor agonist PNU-142633. Cephalalgia 22:799–806. [DOI] [PubMed] [Google Scholar]
- McCall RB, Patel BN, Harris LT. (1987) Effects of serotonin1 and serotonin2 receptor agonists and antagonists on blood pressure, heart rate and sympathetic nerve activity. J Pharmacol Exp Ther 242:1152–1159. [PubMed] [Google Scholar]
- McCormick DA, Wang Z. (1991) Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol 442:235–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorvy JD, Roth BL. (2015) Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 150:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorvy JD, Wacker D, Wang S, Agegnehu B, Liu J, Lansu K, Tribo AR, Olsen RHJ, Che T, Jin J, et al. (2018) Structural determinants of 5-HT2B receptor activation and biased agonism. Nat Struct Mol Biol 25:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary AC, Glennon JC, Ashby CR, Jr, Meltzer HY, Li Z, Reinders JH, Hesselink MB, Long SK, Herremans AH, van Stuivenberg H, et al. (2007) SLV313 (1-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-4- [5-(4-fluoro-phenyl)-pyridin-3-ylmethyl]-piperazine monohydrochloride): a novel dopamine D2 receptor antagonist and 5-HT1A receptor agonist potential antipsychotic drug. Neuropsychopharmacology 32:78–94. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Newman-Tancredi A. (2015) Serotonin 5-HT1A receptors and antipsychotics - an update in light of new concepts and drugs. Curr Pharm Des 21:3725–3731. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Neumaier JF. (2011) Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat 41:234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. (1996) Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry 53:993–1000. [DOI] [PubMed] [Google Scholar]
- McDuffie JE, Coaxum SD, Maleque MA. (1999) 5-hydroxytryptamine evokes endothelial nitric oxide synthase activation in bovine aortic endothelial cell cultures. Proc Soc Exp Biol Med 221:386–390. [DOI] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E. (2002) Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol 62:1339–1343. [DOI] [PubMed] [Google Scholar]
- McGrew L, Price RD, Hackler E, Chang MS, Sanders-Bush E. (2004) RNA editing of the human serotonin 5-HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol Pharmacol 65:252–256. [DOI] [PubMed] [Google Scholar]
- McKenzie C, Alapati VR, MacDonald A, Shaw AM. (2010) Mechanisms involved in the regulation of bovine pulmonary vascular tone by the 5-HT1B receptor. Br J Pharmacol 159:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell RM, Armstrong SR, Beattie DT, Choi SK, Fatheree PR, Gendron RA, Goldblum A, Humphrey PP, Long DD, Marquess DG, et al. (2009) A multivalent approach to the design and discovery of orally efficacious 5-HT4 receptor agonists. J Med Chem 52:5330–5343. [DOI] [PubMed] [Google Scholar]
- McKinnell RM, Armstrong SR, Beattie DT, Fatheree PR, Long DD, Marquess DG, Shaw JP, Vickery RG. (2013) Discovery of TD-8954, a clinical stage 5-HT(4) receptor agonist with gastrointestinal prokinetic properties. Bioorg Med Chem Lett 23:4210–4215. [DOI] [PubMed] [Google Scholar]
- McKinney J, Knappskog PM, Haavik J. (2005) Different properties of the central and peripheral forms of human tryptophan hydroxylase. J Neurochem 92:311–320. [DOI] [PubMed] [Google Scholar]
- McLean PG, Coupar IM, Molenaar P. (1995) A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br J Pharmacol 115:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Beck JP, Woolley ML, Neill JC. (2008) A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res 189:152–158. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Woolley ML, Neill JC. (2009a) D(1)-like receptor activation improves PCP-induced cognitive deficits in animal models: implications for mechanisms of improved cognitive function in schizophrenia. Eur Neuropsychopharmacol 19:440–450. [DOI] [PubMed] [Google Scholar]
- McLean SL, Woolley ML, Thomas D, Neill JC. (2009b) Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology (Berl) 206:403–414. [DOI] [PubMed] [Google Scholar]
- McLoughlin DJ, Strange PG. (2000) Mechanisms of agonism and inverse agonism at serotonin 5-HT1A receptors. J Neurochem 74:347–357. [DOI] [PubMed] [Google Scholar]
- McKinnon NK, Reeves DC, Akabas MH. (2011) 5-HT3 receptor ion size selectivity is a property of the transmembrane channel, not the cytoplasmic vestibule portals. J Gen Physiol 138:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Olson EN. (2005) Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest 115:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKune CM, Watts SW. (2001) Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling. J Pharmacol Exp Ther 297:88–95. [PubMed] [Google Scholar]
- Meffre J, Chaumont-Dubel S, Mannoury la Cour C, Loiseau F, Watson DJ, Dekeyne A, Séveno M, Rivet JM, Gaven F, Déléris P, et al. (2012) 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol Med 4:1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller E, Chalfin M, Bohmaker K. (1992) Serotonin 5-HT1A receptor-mediated hypothermia in mice: absence of spare receptors and rapid induction of tolerance. Pharmacol Biochem Behav 43:405–411. [DOI] [PubMed] [Google Scholar]
- Meller E, Goldstein M, Bohmaker K. (1990) Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol 37:231–237. [PubMed] [Google Scholar]
- Meltzer HY. (1999) The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21:106S–115S. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. (2012) Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol 212:87–124. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW. (2011) The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol 11:59–67. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Roth BL. (2013) Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest 123:4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Sumiyoshi T. (2008) Does stimulation of 5-HT(1A) receptors improve cognition in schizophrenia? Behav Brain Res 195:98–102. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB. (1986) 5-HT1A receptors: differential involvement in female and male sexual behavior in the rat. Physiol Behav 37:345–351. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Römer S, Gardier AM, Hen R. (2014) Rapid anxiolytic effects of a 5-HT4 receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology 39:1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A. (2004) Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshaping Pavlovian/instrumental learning task. Behav Brain Res 155:275–282. [DOI] [PubMed] [Google Scholar]
- Meneses A. (2015) Serotonin, neural markers, and memory. Front Pharmacol 6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Liy-Salmeron G, Flores-Galvez D, Castillo C, Castillo E. (2008) The effects of the 5-HT(6) receptor agonist EMD and the 5-HT(7) receptor agonist AS19 on memory formation. Behav Brain Res 195:112–119. [DOI] [PubMed] [Google Scholar]
- Meneses A, Pérez-García G, Ponce-Lopez T, Castillo C. (2011) 5-HT6 receptor memory and amnesia: behavioral pharmacology--learning and memory processes. Int Rev Neurobiol 96:27–47. [DOI] [PubMed] [Google Scholar]
- Mengod G, Cortés R, Vilaró MT, Hoyer D. (2010) Distribution of 5-HT receptors in the central nervous system, in Handbook of the Behavioral Neurobiology of Serotonin (Muller CP, Jacobs B. eds) pp. 123–138, Elsevier Academic Press. [Google Scholar]
- Mengod G, Nguyen H, Le H, Waeber C, Lübbert H, Palacios JM. (1990a) The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience 35:577–591. [DOI] [PubMed] [Google Scholar]
- Mengod G, Palacios JM, Cortés R. (2015) Cartography of 5-HT1A and 5-HT2A receptor subtypes in prefrontal cortex and its projections. ACS Chem Neurosci 6:1089–1098. [DOI] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Martínez-Mir MI, Palacios JM. (1990b) Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res 524:139–143. [DOI] [PubMed] [Google Scholar]
- Mengod G, Vilaro MT, Cortes R, Lopez-Gimenez JF, Raurich A, Palacios JM. (2006) Chemical neuroanatomy of 5-HT receptor subtypes in the mammalian brain, in The Serotonin Receptors; From Molecular Pharmacology to Human Therapeutics (Roth BL. ed) pp 319–364, Humana Press, Totowa, NJ. [Google Scholar]
- Mengod G, Vilaró MT, Raurich A, López-Giménez JF, Cortés R, Palacios JM. (1996) 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem J 28:747–758. [DOI] [PubMed] [Google Scholar]
- Mercado CP, Ziu E, Kilic F. (2011) Communication between 5-HT and small GTPases. Curr Opin Pharmacol 11:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith EJ, Holder MJ, Chamba A, Challa A, Drake-Lee A, Bunce CM, Drayson MT, Pilkington G, Blakely RD, Dyer MJ, et al. (2005) The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential anti-tumor target for psychotropics. FASEB J 19:1187–1189. [DOI] [PubMed] [Google Scholar]
- Meschin P, Demion M, Cazorla O, Finan A, Thireau J, Richard S, Lacampagne A. (2015) p11 modulates calcium handling through 5-HT4R pathway in rat ventricular cardiomyocytes. Cell Calcium 58:549–557. [DOI] [PubMed] [Google Scholar]
- Metwally KA, Dukat M, Egan CT, Smith C, DuPre A, Gauthier CB, Herrick-Davis K, Teitler M, Glennon RA. (1998) Spiperone: influence of spiro ring substituents on 5-HT2A serotonin receptor binding. J Med Chem 41:5084–5093. [DOI] [PubMed] [Google Scholar]
- Meunier CN, Amar M, Lanfumey L, Hamon M, Fossier P. (2013) 5-HT(1A) receptors direct the orientation of plasticity in layer 5 pyramidal neurons of the mouse prefrontal cortex. Neuropharmacology 71:37–45. [DOI] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Gabriel A, Xie GX, Lim KJ, Pierce Palmer P. (2002) 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci 71:2279–2289. [DOI] [PubMed] [Google Scholar]
- Meyer JH. (2013) Neurochemical imaging and depressive behaviours. Curr Top Behav Neurosci 14:101–134. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, Wilson AA, Blak T, Eynan-Harvey R, Goulding VS, et al. (2003) Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry 160:90–99. [DOI] [PubMed] [Google Scholar]
- Meyer M, Kircher M, Gansauge M-T, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prüfer K, de Filippo C, et al. (2012) A high-coverage genome sequence from an archaic Denisovan individual. Science 338:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Obermüller F, Fehr S, Richter D. (1993) A novel rat serotonin receptor: primary structure, pharmacology, and expression pattern in distinct brain regions. DNA Cell Biol 12:401–409. [DOI] [PubMed] [Google Scholar]
- Mialet J, Berque-Bestel I, Sicsic S, Langlois M, Fischmeister R, Lezoualc’h F. (2000) Pharmacological characterization of the human 5-HT(4(d)) receptor splice variant stably expressed in Chinese hamster ovary cells. Br J Pharmacol 131:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mialet-Perez J, D’Angelo R, Villeneuve C, Ordener C, Nègre-Salvayre A, Parini A, Vindis C. (2012) Serotonin 5-HT2A receptor-mediated hypertrophy is negatively regulated by caveolin-3 in cardiomyoblasts and neonatal cardiomyocytes. J Mol Cell Cardiol 52:502–510. [DOI] [PubMed] [Google Scholar]
- Michel K, Zeller F, Langer R, Nekarda H, Kruger D, Dover TJ, Brady CA, Barnes NM, Schemann M. (2005) Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology 128:1317–1326. [DOI] [PubMed] [Google Scholar]
- Michineau S, Muller L, Pizard A, Alhenc-Gélas F, Rajerison RM. (2004) N-linked glycosylation of the human bradykinin B2 receptor is required for optimal cell-surface expression and coupling. Biol Chem 385:49–57. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Blakeborough L, Leather SR. (1977) Direct evidence for an interaction of beta-adrenergic blockers with the 5-HT receptor. Nature 267:289–290. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. (1983) 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol 90:151–153. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Göthert M, Schlicker E, Scott CM, Selkirk JV, Watson J, Gaster LM, Wyman P, Riley G, Price GW. (1999) SB-236057, a selective 5-HT1B receptor inverse agonist, blocks the 5-HT human terminal autoreceptor. Eur J Pharmacol 375:359–365. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Tricklebank MD. (1992) Centrally active 5-HT receptor agonists and antagonists. Neurosci Biobehav Rev 16:75–82. [DOI] [PubMed] [Google Scholar]
- Milatovich A, Hsieh CL, Bonaminio G, Tecott L, Julius D, Francke U. (1992) Serotonin receptor 1c gene assigned to X chromosome in human (band q24) and mouse (bands D-F4). Hum Mol Genet 1:681–684. [DOI] [PubMed] [Google Scholar]
- Miles TF, Dougherty DA, Lester HA. (2013) The 5-HT3AB receptor shows an stoichiometry at the plasma membrane. Biophys J 105:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T, Komada T, Sugimoto H, Suzuki K, Ohmi T, Kimura N, Naganeo R, Nakata E, Nakatani K, Toga T, et al. (2009) In vitro and in vivo pharmacological characterization of PF-01354082, a novel partial agonist selective for the 5-HT(4) receptor. Eur J Pharmacol 609:5–12. [DOI] [PubMed] [Google Scholar]
- Mikulski Z, Zaslona Z, Cakarova L, Hartmann P, Wilhelm J, Tecott LH, Lohmeyer J, Kummer W. (2010) Serotonin activates murine alveolar macrophages through 5-HT2C receptors. Am J Physiol Lung Cell Mol Physiol 299:L272–L280. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (1994) Serotonin and pain: evidence that activation of 5-HT1A receptors does not elicit antinociception against noxious thermal, mechanical and chemical stimuli in mice. Pain 58:45–61. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2002) Descending control of pain. Prog Neurobiol 66:355–474. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2003) The neurobiology and control of anxious states. Prog Neurobiol 70:83–244. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2005) Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie 60:441–460. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2006) Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 110:135–370. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Dekeyne A. (2005) Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology (Berl) 177:448–458. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Veiga S, Cistarelli L, Melon C, Gobert A. (1998) WAY 100,635 enhances both the ‘antidepressant’ actions of duloxetine and its influence on dialysate levels of serotonin in frontal cortex. Eur J Pharmacol 341:165–167. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet J-M, Cussac D. (2003) The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306:954–964. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Roux S, Porsolt R, Meneses A, Carli M, Di Cara B, Jaffard R, Rivet JM, Lestage P, et al. (2004) The serotonin1A receptor partial agonist S15535 [4-(benzodioxan-5-yl)1-(indan-2-yl)piperazine] enhances cholinergic transmission and cognitive function in rodents: a combined neurochemical and behavioral analysis. J Pharmacol Exp Ther 311:190–203. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Hjorth S, Samanin R, Schreiber R, Jaffard R, De Ladonchamps B, Veiga S, Goument B, Peglion JL, Spedding M, et al. (1997) S 15535, a novel benzodioxopiperazine ligand of serotonin (5-HT)1A receptors: II. Modulation of hippocampal serotonin release in relation to potential anxiolytic properties. J Pharmacol Exp Ther 282:148–161. [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. (2008) Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci 29:454–464. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Kamal M, Jockers R, Chanrion B, Labasque M, Bockaert J, Mannoury la Cour C. (2011) The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT(2C) receptors. Int J Neuropsychopharmacol 14:768–783. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Honoré P, Girardon S, Bervoets K. (1996) Pro- and antinociceptive actions of serotonin (5-HT)1A agonists and antagonists in rodents: relationship to algesiometric paradigm. Behav Brain Res 73:69–77. [DOI] [PubMed] [Google Scholar]
- Millar ID, Bruce JIe, Brown PD. (2007) Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ. (2005) Serotonin 5-ht2c receptor agonists: potential for the treatment of obesity. Mol Interv 5:282–291. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Mariano CL, Cruz WR. (1997) Serotonin 5HT2A receptor activation inhibits inducible nitric oxide synthase activity in C6 glioma cells. Life Sci 61:1819–1827. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Teitler M. (1992) Quantitative autoradiography of 5-CT-sensitive (5-HT1D) and 5-CT-insensitive (5-HT1E) serotonin receptors in human brain. Neurosci Lett 136:223–226. [DOI] [PubMed] [Google Scholar]
- Milligan G. (2010) The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr Opin Pharmacol 10:23–29. [DOI] [PubMed] [Google Scholar]
- Mills A, Martin GR. (1995) Autoradiographic mapping of [3H]sumatriptan binding in cat brain stem and spinal cord. Eur J Pharmacol 280:175–178. [DOI] [PubMed] [Google Scholar]
- Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. (2003) Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 99:149–165. [DOI] [PubMed] [Google Scholar]
- Miner WD, Sanger GJ. (1986) Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol 88:497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner WD, Sanger GJ, Turner DH. (1987) Evidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesis. Br J Cancer 56:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel MC, Doucet E, Riad M, Adrien J, Vergé D, Hamon M. (1992) Effect of the selective lesion of serotoninergic neurons on the regional distribution of 5-HT1A receptor mRNA in the rat brain. Brain Res Mol Brain Res 14:357–362. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, McDevitt RA, Neumaier JF. (2009a) Adaptations in 5-HT receptor expression and function: implications for treatment of cognitive impairment in aging. J Neurosci Res 87:2803–2811. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. (2005) 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther 108:320–333. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. (2007) Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology 32:1520–1530. [DOI] [PubMed] [Google Scholar]
- Mitchell NA, Pepperell E, Ociepka S, Brown JD, Witherington J, Tuladhar B, Sanger GJ, Lee K, Cellek S. (2009b) 5-hydroxyindalpine, an agonist at the putative 5-HT receptor, has no activity on human recombinant monoamine receptors but accelerates distension-induced peristalsis in mouse isolated colon. Neurogastroenterol Motil 21:760–768. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Moskowitz MA, Waeber C. (1999) Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur J Pharmacol 369:271–277. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Waeber C. (2002) 5-Hydroxytryptamine(1B/1D) and 5-hydroxytryptamine1F receptors inhibit capsaicin-induced c-fos immunoreactivity within mouse trigeminal nucleus caudalis. Cephalalgia 22:384–394. [DOI] [PubMed] [Google Scholar]
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. (1995) Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol 48:407–416. [PubMed] [Google Scholar]
- Miyata K, Yamano M, Kamato T, Akuzawa S. (1995) Effect of serotonin (5-HT)3-receptor antagonists YM060, YM114 (KAE-393), ondansetron and granisetron on 5-HT4 receptors and gastric emptying in rodents. Jpn J Pharmacol 69:205–214. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Murakami S, Takeshima M, Torigoe N, Kitamura Y, Miyoshi K. (2013) Targeting 5-HT(1A) receptors in astrocytes to protect dopaminergic neurons in Parkinsonian models. Neurobiol Dis 59:244–256. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Amraei MG, Benmbarek S, Archer-Lahlou E, Peñas-Cazorla R, Vilaró MT, Boye SM, Piñeyro G. (2010) Serotonin 4 receptor (5-HT4R) internalization is isoform-specific: effects of 5-HT and RS67333 on isoforms A and B. Cell Signal 22:501–509. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Faure C, Lambás-Señas L, El Mansari M, Belblidia H, Gondard E, Etiévant A, Scarna H, Didier A, Berod A, et al. (2011) Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36:1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocci G, Jiménez-Sánchez L, Adell A, Cortés R, Artigas F. (2014) Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology 79:49–58. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Watanabe T, Miyake A, Saito M, Furuichi K. (2000) Cloning, expression, and characterization of ferret 5-HT(3) receptor subunit. Eur J Pharmacol 399:97–106. [DOI] [PubMed] [Google Scholar]
- Mochizuki D, Yuyama Y, Tsujita R, Komaki H, Sagai H. (1992) Cloning and expression of the human 5-HT1B-type receptor gene. Biochem Biophys Res Commun 185:517–523. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. (2001a) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. (2001b) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198. [DOI] [PubMed] [Google Scholar]
- Mogha A, Guariglia SR, Debata PR, Wen GY, Banerjee P. (2012) Serotonin 1A receptor-mediated signaling through ERK and PKCα is essential for normal synaptogenesis in neonatal mouse hippocampus. Transl Psychiatry 2:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi MR, Hafezi P, Galeiha A, Hajiaghaee R, Akhondzadeh S. (2012) Buspirone versus methylphenidate in the treatment of children with attention- deficit/ hyperactivity disorder: randomized double-blind study. Acta Med Iran 50:723–728. [PubMed] [Google Scholar]
- Mohler EG, Shacham S, Noiman S, Lezoualc’h F, Robert S, Gastineau M, Rutkowski J, Marantz Y, Dumuis A, Bockaert J, et al. (2007) VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 53:563–573. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Frölich D, Likungu J, Göthert M. (1996) Inhibition of noradrenaline release via presynaptic 5-HT1D alpha receptors in human atrium. Naunyn Schmiedebergs Arch Pharmacol 353:272–280. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Werner K, Likungu J, Göthert M. (1990) Inhibition of noradrenaline release from the sympathetic nerves of the human saphenous vein via presynaptic 5-HT receptors similar to the 5-HT 1D subtype. Naunyn Schmiedebergs Arch Pharmacol 342:371–377. [DOI] [PubMed] [Google Scholar]
- Molineaux SM, Jessell TM, Axel R, Julius D. (1989) 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA 86:6793–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller TC, Wirth VF, Roberts NI, Bender J, Bach A, Jacky BP, Strømgaard K, Deussing JM, Schwartz TW, Martinez KL. (2013) PDZ domain-mediated interactions of G protein-coupled receptors with postsynaptic density protein 95: quantitative characterization of interactions. PLoS One 8:e63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monassier L, Laplante MA, Ayadi T, Doly S, Maroteaux L. (2010) Contribution of gene-modified mice and rats to our understanding of the cardiovascular pharmacology of serotonin. Pharmacol Ther 128:559–567. [DOI] [PubMed] [Google Scholar]
- Monassier L, Laplante MA, Jaffré F, Bousquet P, Maroteaux L, de Champlain J. (2008) Serotonin 5-HT(2B) receptor blockade prevents reactive oxygen species-induced cardiac hypertrophy in mice. Hypertension 52:301–307. [DOI] [PubMed] [Google Scholar]
- Monk SA, Williams JM, Hope AG, Barnes NM. (2004) Identification and importance of N-glycosylation of the human 5-hydroxytryptamine3A receptor subunit. Biochem Pharmacol 68:1787–1796. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bornstein JC, Bertrand PP. (2005) Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience 134:975–986. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. (1993) Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43:320–327. [PubMed] [Google Scholar]
- Montalbano A, Corradetti R, Mlinar B. (2015) Pharmacological characterization of 5-HT1A autoreceptor-coupled GIRK channels in rat dorsal raphe 5-HT neurons. PLoS One 10:e0140369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. (2011) Serotonin control of sleep-wake behavior. Sleep Med Rev 15:269–281. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. (2006) Effects of the 5-HT(7) receptor antagonist SB-269970 microinjected into the dorsal raphe nucleus on REM sleep in the rat. Behav Brain Res 167:245–250. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H, Schechter LE. (2013) The effects of systemic and local microinjection into the central nervous system of the selective serotonin 5-HT6 receptor agonist WAY-208466 on sleep and wakefulness in the rat. Behav Brain Res 249:65–74. [DOI] [PubMed] [Google Scholar]
- Monti JM, Leopoldo M, Jantos H. (2008) The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav Brain Res 191:184–189. [DOI] [PubMed] [Google Scholar]
- Monti JM, Leopoldo M, Jantos H. (2014) Systemic administration and local microinjection into the central nervous system of the 5-HT(7) receptor agonist LP-211 modify the sleep-wake cycle in the rat. Behav Brain Res 259:321–329. [DOI] [PubMed] [Google Scholar]
- Moore NA, Sargent BJ, Manning DD, Guzzo PR. (2013) Partial agonism of 5-HT3 receptors: a novel approach to the symptomatic treatment of IBS-D. ACS Chem Neurosci 4:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutkine I, Quentin E, Guiard BP, Maroteaux L, Doly S. (2017) Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J Biol Chem 292:6352–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. (2010a) Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis 39:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MV, Ulbricht RJ, O’Neil RT, Airey DC, Lu P, Zhang B, Wang L, Emeson RB. (2010b) High-throughput multiplexed transcript analysis yields enhanced resolution of 5-hydroxytryptamine 2C receptor mRNA editing profiles. Mol Pharmacol 77:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán A, Fernandez MM, Velasco C, Martin ML, San Roman L. (1998) Characterization of prejunctional 5-HT1 receptors that mediate the inhibition of pressor effects elicited by sympathetic stimulation in the pithed rat. Br J Pharmacol 123:1205–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán A, Ortiz de Urbina AV, Martín ML, García M, Rodriguez-Barbero A, Dorado F, San Román L. (2008) Characterization of contractile 5-hydroxytryptamine receptor subtypes in the in situ autoperfused kidney in the anaesthetized rat. Eur J Pharmacol 592:133–137. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Bos M, Jenck F, Martin JR, Mortas P, Wichmann J. (1996) 5HT2C receptor agonists exhibit antidepressant-like properties in the anhedonia model of depression in rats. Eur Neuropsychopharmacol 6:169–175. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. (2007) Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension 49:232–236. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Heeley RP, Prentice HM, Kirk A, MacLean MR. (1999) 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol 128:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Umali A, Rayannavar V, Sealfon SC, González-Maeso J. (2013) Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice. Psychopharmacology (Berl) 225:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, García-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, et al. (2016) Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal 9:ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret C, Grimaldi B, Massot O, Fillion G. (2003) The role and therapeutic potential of 5-HT-moduline in psychiatry. Semin Clin Neuropsychiatry 8:137–146. [DOI] [PubMed] [Google Scholar]
- Morici JF, Ciccia L, Malleret G, Gingrich JA, Bekinschtein P, Weisstaub NV. (2015) Serotonin 2a receptor and serotonin 1a receptor interact within the medial prefrontal cortex during recognition memory in mice. Front Pharmacol 6:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Somogyi P, Lujan-Miras R, Ciaranello RD. (1994) Neurons expressing 5-HT2 receptors in the rat brain: neurochemical identification of cell types by immunocytochemistry. Neuropsychopharmacology 11:157–166. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. (2000) Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol 58:1271–1278. [DOI] [PubMed] [Google Scholar]
- Morita H, Mochiki E, Takahashi N, Kawamura K, Watanabe A, Sutou T, Ogawa A, Yanai M, Ogata K, Fujii T, et al. (2013) Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J Gastroenterol 19:6604–6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork A, Brennum LT, Fallon SM, Bisulco S, Frederiksen K, Bang-Andersen B, Lassen AB, Zhong H, Patel JG, Poon P, et al. (2009) Pharmacological profile of Lu AA21004, a novel multitarget drug for the treatment of mood disorders, in Society for Neuroscience - 39th Annual Meeting; 2009 Oct 17-21; Chicago, IL, pp. Poster 549.10. [Google Scholar]
- Mørk A, Pehrson A, Brennum LT, Nielsen SM, Zhong H, Lassen AB, Miller S, Westrich L, Boyle NJ, Sánchez C, et al. (2012) Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340:666–675. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. (1993) The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol 238:411–415. [DOI] [PubMed] [Google Scholar]
- Moser PC, Bergis OE, Jegham S, Lochead A, Duconseille E, Terranova JP, Caille D, Berque-Bestel I, Lezoualc’h F, Fischmeister R, et al. (2002) SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther 302:731–741. [DOI] [PubMed] [Google Scholar]
- Mota A, Bento A, Peñalva A, Pombo M, Dieguez C. (1995) Role of the serotonin receptor subtype 5-HT1D on basal and stimulated growth hormone secretion. J Clin Endocrinol Metab 80:1973–1977. [DOI] [PubMed] [Google Scholar]
- Mounsey I, Brady KA, Carroll J, Fisher R, Middlemiss DN. (1982) K+-evoked [3H]-5-HT release from rat frontal cortex slices: the effect of 5-HT agonists and antagonists. Biochem Pharmacol 31:49–53. [DOI] [PubMed] [Google Scholar]
- Movahedi H, Purdy RE. (1998) Pharmacological characterization of the “silent” 5-HT1B-like receptors of rabbit ear artery. Ann N Y Acad Sci 861:258. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. (2007) Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321:1054–1061. [DOI] [PubMed] [Google Scholar]
- Moya PR, Fox MA, Jensen CL, Laporte JL, French HT, Wendland JR, Murphy DL. (2011) Altered 5-HT2C receptor agonist-induced responses and 5-HT2C receptor RNA editing in the amygdala of serotonin transporter knockout mice. BMC Pharmacol 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujakovic S, ter Linde JJ, de Wit NJ, van Marrewijk CJ, Fransen GA, Onland-Moret NC, Laheij RJ, Muris JW, Grobbee DE, Samsom M, et al. (2011) Serotonin receptor 3A polymorphism c.-42C > T is associated with severe dyspepsia. BMC Med Genet 12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Carey RJ. (2006) Intracellular 5-HT 2C-receptor dephosphorylation: a new target for treating drug addiction. Trends Pharmacol Sci 27:455–458. [DOI] [PubMed] [Google Scholar]
- Müller CP, Homberg JR. (2015) The role of serotonin in drug use and addiction. Behav Brain Res 277:146–192. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Rossbach W, Mann K, Röschke J, Müller-Siecheneder F, Blümler M, Wetzel H, Russ H, Dittmann RW, Benkert O. (2004) Subchronic effects of olanzapine on sleep EEG in schizophrenic patients with predominantly negative symptoms. Pharmacopsychiatry 37:157–162. [DOI] [PubMed] [Google Scholar]
- Müller T, Dürk T, Blumenthal B, Grimm M, Cicko S, Panther E, Sorichter S, Herouy Y, Di Virgilio F, Ferrari D, et al. (2009) 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One 4:e6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz A, Carlsson T, Tronci E, Kirik D, Björklund A, Carta M. (2009) Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp Neurol 219:298–307. [DOI] [PubMed] [Google Scholar]
- Muñoz-Islas E, Gupta L, Jimenez-Mena LR, Lozano-Cuenca J, Sanchez-Lopez A, Centurion D, Mehrotra S, MaassenvVanDenBrink A, Villalón CM. (2006) Donitriptan, but not sumatriptan, inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Br J Pharmacol 149:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Islas E, Lozano-Cuenca J, González-Hernández A, Ramirez-Rosas MB, Sanchez-Lopez A, Centurion D, Maassenvandenbrink A, Villalón CM. (2009) Spinal sumatriptan inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Eur J Pharmacol 615:133–138. [DOI] [PubMed] [Google Scholar]
- Muñoz-Islas E, Vidal-Cantú GC, Bravo-Hernández M, Cervantes-Durán C, Quiñonez-Bastidas GN, Pineda-Farias JB, Barragán-Iglesias P, Granados-Soto V. (2014) Spinal 5-HT5A receptors mediate 5-HT-induced antinociception in several pain models in rats. Pharmacol Biochem Behav 120:25–32. [DOI] [PubMed] [Google Scholar]
- Murotani T, Ishizuka T, Isogawa Y, Karashima M, Yamatodani A. (2011) Possible involvement of serotonin 5-HT2 receptor in the regulation of feeding behavior through the histaminergic system. Neuropharmacology 61:228–233. [DOI] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. (2011) Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystakidou K, Befon S, Liossi C, Vlachos L. (1998) Comparison of the efficacy and safety of tropisetron, metoclopramide, and chlorpromazine in the treatment of emesis associated with far advanced cancer. Cancer 83:1214–1223. [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. (2007) The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol 559:132–137. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Kamato T, Nishida A, Ito H, Yamano M, Miyata K. (1996) Characterization of 5-hydroxytryptamine (5-HT) receptor subtypes influencing colonic motility in conscious dogs. Naunyn Schmiedebergs Arch Pharmacol 353:489–498. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Kontoh A, Tokita K, Tomoi M, Shimomura K, Kadowaki M. (1997) Combined blockade of 5-HT3- and 5-HT4-serotonin receptors inhibits colonic functions in conscious rats and mice. J Pharmacol Exp Ther 281:284–290. [PubMed] [Google Scholar]
- Nakajima H, Mochiki E, Zietlow A, Ludwig K, Takahashi T. (2010) Mechanism of interdigestive migrating motor complex in conscious dogs. J Gastroenterol 45:506–514. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Suzuki K, McCreary AC, Ashby CR., Jr (2006) The acute and chronic administration of (+/-)-8-hydroxy-2-(Di-n-propylamino)tetralin significantly alters the activity of spontaneously active midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse 59:359–367. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, Loebel A. (2009) Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 70:829–836. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. (2009) Central 5-HT receptors in cardiovascular control during stress. Neurosci Biobehav Rev 33:95–106. [DOI] [PubMed] [Google Scholar]
- Napier C, Stewart M, Melrose H, Hopkins B, McHarg A, Wallis R. (1999) Characterisation of the 5-HT receptor binding profile of eletriptan and kinetics of [3H]eletriptan binding at human 5-HT1B and 5-HT1D receptors. Eur J Pharmacol 368:259–268. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK. (2011) On the role of brain 5-HT7 receptor in the mechanism of hypothermia: comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology 61:1360–1365. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Popova NK, Lacivita E, Leopoldo M, Ponimaskin EG. (2014) Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci Ther 20:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ, Gardier AM, Blanco C, Hen R, Ahmari SE. (2015) Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdère P, Porras G, Spampinato U. (2004) In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology 29:319–326. [DOI] [PubMed] [Google Scholar]
- Navailles S, Lagière M, Le Moine C, De Deurwaerdère P. (2013a) Role of 5-HT2C receptors in the enhancement of c-Fos expression induced by a 5-HT2B/2C inverse agonist and 5-HT 2 agonists in the rat basal ganglia. Exp Brain Res 230:525–535. [DOI] [PubMed] [Google Scholar]
- Navailles S, Lagiere M, Roumegous A, Polito M, Boujema MB, Cador M, Dunlop J, Chesselet MF, Millan MJ, De Deurwaerdere P. (2013b) Serotonin2C ligands exhibiting full negative and positive intrinsic activity elicit purposeless oral movements in rats: distinct effects of agonists and inverse agonists in a rat model of Parkinson’s disease. Int J Neuropsychopharmacol 16:593–606. [DOI] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. (2008) Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology 33:237–246. [DOI] [PubMed] [Google Scholar]
- Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. (2008) The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav Brain Res 188:412–415. [DOI] [PubMed] [Google Scholar]
- Neal KB, Bornstein JC. (2007) Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience 145:556–567. [DOI] [PubMed] [Google Scholar]
- Neal KB, Parry LJ, Bornstein JC. (2009) Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587:567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. (2000a) Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA 97:9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. (2003a) Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J 17:1373–1375. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Hickel P, Messaddeq N, Vonesch JL, Douchet MP, Monassier L, György K, Matz R, Andriantsitohaina R, Manivet P, et al. (2001) Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation 103:2973–2979. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Jaffré F, Messaddeq N, Hickel P, Monassier L, Launay JM, Maroteaux L. (2003b) Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation 107:3223–3229. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Launay J-M, Hickel P, Tournois C, Maroteaux L. (2000b) 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA 97:2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA. (2017) Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci 8:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. (2007) Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol 18:791–800. [DOI] [PubMed] [Google Scholar]
- Nelson AD, Camilleri M, Chirapongsathorn S, Vijayvargiya P, Valentin N, Shin A, Erwin PJ, Wang Z, Murad MH. (2017) Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut 66:1611–1622. [DOI] [PubMed] [Google Scholar]
- Nelson CS, Cone RD, Robbins LS, Allen CN, Adelman JP. (1995) Cloning and expression of a 5HT7 receptor from Xenopus laevis. Receptors Channels 3:61–70. [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. (1999) Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 359:1–6. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, Xu YC. (2010) Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 30:1159–1169. [DOI] [PubMed] [Google Scholar]
- Nelson RK, Frohman MA. (2015) Physiological and pathophysiological roles for phospholipase D. J Lipid Res 56:2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz Á, Prevost MS, Menny A, Corringer P-J. (2016) Emerging molecular mechanisms of signal transduction in pentameric ligand-gated ion channels. Neuron 90:452–470. [DOI] [PubMed] [Google Scholar]
- Netzer P, Gaia C, Lourens ST, Reber P, Wildi S, Noelpp U, Ritter EP, Ledermann H, Lüscher D, Varga L, et al. (2002) Does intravenous ondansetron affect gastric emptying of a solid meal, gastric electrical activity or blood hormone levels in healthy volunteers? Aliment Pharmacol Ther 16:119–127. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M. (2001) Localization of 5-HT(7) receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J Chem Neuroanat 21:63–73. [DOI] [PubMed] [Google Scholar]
- Newman AS, Batis N, Grafton G, Caputo F, Brady CA, Lambert JJ, Peters JA, Gordon J, Brain KL, Powell AD, et al. (2013) 5-Chloroindole: a potent allosteric modulator of the 5-HT3 receptor. Br J Pharmacol 169:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A. (2010) The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Investig Drugs 11:802–812. [PubMed] [Google Scholar]
- Newman-Tancredi A, Albert PR. (2012) Gene polymorphism at serotonin 5-HT1A receptors: moving towards personalized medicine for psychosis and mood deficits?, in Schizophrenia Research: Recent Advances (Sumiyoshi T. ed) pp. 339–360, Nova Science Publishers Inc., New York. [Google Scholar]
- Newman-Tancredi A, Assié MB, Leduc N, Ormière AM, Danty N, Cosi C. (2005) Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int J Neuropsychopharmacol 8:341–356. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Auclair A, Barret-Grévoz C, Galinier A, Barreto M, Depoortère R. (2009a) F15599, a selective 5-HT1A agonist: frontal cortex and dorsal Raphe microinjections reveal low- and high-dose forced swim test effects, in Society for Neuroscience - 39th Annual Meeting, Chicago, IL, Program number 516.2. [Google Scholar]
- Newman-Tancredi A, Chaput C, Verriele L, Millan MJ. (1996a) Clozapine is a partial agonist at cloned, human serotonin 5-HT1A receptors. Neuropharmacology 35:119–121. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Chaput C, Verrièle L, Millan MJ. (1996b) S 15535 and WAY 100,635 antagonise 5-HT-stimulated [35S]GTP gamma S binding at cloned human 5-HT1A receptors. Eur J Pharmacol 307:107–111. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Conte C, Chaput C, Spedding M, Millan MJ. (1997a) Inhibition of the constitutive activity of human 5-HT1A receptors by the inverse agonist, spiperone but not the neutral antagonist, WAY 100,635. Br J Pharmacol 120:737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Conte C, Chaput C, Verrièle L, Audinot-Bouchez V, Lochon S, Lavielle G, Millan MJ. (1997b) Agonist activity of antimigraine drugs at recombinant human 5-HT1A receptors: potential implications for prophylactic and acute therapy. Naunyn Schmiedebergs Arch Pharmacol 355:682–688. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Conte C, Chaput C, Verrièle L, Millan MJ. (1997c) Agonist and inverse agonist efficacy at human recombinant serotonin 5-HT1A receptors as a function of receptor:G-protein stoichiometry. Neuropharmacology 36:451–459. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Marini L, Millan MJ. (2002) Antibody capture assay reveals bell-shaped concentration-response isotherms for h5-HT(1A) receptor-mediated Galpha(i3) activation: conformational selection by high-efficacy agonists, and relationship to trafficking of receptor signaling. Mol Pharmacol 62:590–601. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verrièle L, Audinot V, Millan MJ. (1998) Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur J Pharmacol 355:245–256. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, et al. (2009b) Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol 156:338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Rivet JM, Cussac D, Touzard M, Chaput C, Marini L, Millan MJ. (2003) Comparison of hippocampal G protein activation by 5-HT(1A) receptor agonists and the atypical antipsychotics clozapine and S16924. Naunyn Schmiedebergs Arch Pharmacol 368:188–199. [DOI] [PubMed] [Google Scholar]
- Newton RA, Phipps SL, Flanigan TP, Newberry NR, Carey JE, Kumar C, McDonald B, Chen C, Elliott JM. (1996) Characterisation of human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors expressed in the human neuroblastoma cell line SH-SY5Y: comparative stimulation by hallucinogenic drugs. J Neurochem 67:2521–2531. [DOI] [PubMed] [Google Scholar]
- Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. (2008) The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol 154:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Thompson JM, Northcott CA, Lookingland K, Watts SW. (2004) The serotonin transporter is present and functional in peripheral arterial smooth muscle. J Cardiovasc Pharmacol 43:770–781. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Bourin M, Hascoët M. (2003) Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res 140:203–214. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ. (2015) N-Benzyl-5-methoxytryptamines as potent serotonin 5-HT2 receptor family agonists and comparison with a series of phenethylamine analogues. ACS Chem Neurosci 6:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Small J, Dixon AK, Spanswick D, Lee K. (2003) Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett 337:119–122. [DOI] [PubMed] [Google Scholar]
- Niebert M, Vogelgesang S, Koch UR, Bischoff A-M, Kron M, Bock N, Manzke T. (2011) Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One 6:e21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebert S, van Belle GJ, Vogelgesang S, Manzke T, Niebert M. (2017) The serotonin receptor subtype 5b specifically interacts with serotonin receptor subtype 1A. Front Mol Neurosci 10:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MD, Chan GC, Poser SW, Storm DR. (1996) Differential regulation of type I and type VIII Ca2+-stimulated adenylyl cyclases by Gi-coupled receptors in vivo. J Biol Chem 271:33308–33316. [DOI] [PubMed] [Google Scholar]
- Niesler B. (2011) 5-HT(3) receptors: potential of individual isoforms for personalised therapy. Curr Opin Pharmacol 11:81–86. [DOI] [PubMed] [Google Scholar]
- Niesler B, Flohr T, Nöthen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. (2001) Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 11:471–475. [DOI] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. (2003) Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 310:101–111. [DOI] [PubMed] [Google Scholar]
- Niesler B, Kapeller J, Hammer C, Rappold G. (2008) Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics 9:501–504. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A. (2012) Selective blockade of 5-HT7 receptors facilitates attentional set-shifting in stressed and control rats. Behav Brain Res 226:118–123. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Hołuj M, Kos T, Popik P. (2016) The effects of a 5-HT5A receptor antagonist in a ketamine-based rat model of cognitive dysfunction and the negative symptoms of schizophrenia. Neuropharmacology 105:351–360. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Fijał K, Hołuj M, Rafa D, Popik P. (2013) Effects of the selective 5-HT7 receptor antagonist SB-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS One 8:e66695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. (2013) Amisulpride promotes cognitive flexibility in rats: the role of 5-HT7 receptors. Behav Brain Res 248:136–140. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Longmore J, Shaw D, Olesen IJ, Edvinsson L. (1999a) Contractile 5-HT1B receptors in human cerebral arteries: pharmacological characterization and localization with immunocytochemistry. Br J Pharmacol 128:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Longmore J, Shaw D, Pantev E, Bard JA, Branchek T, Edvinsson L. (1999b) Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol 372:49–56. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Tsuno NH, Shuno Y, Sasaki K, Hongo K, Okaji Y, Sunami E, Kitayama J, Takahashi K, Nagawa H. (2010) Antiangiogenic effect of a selective 5-HT4 receptor agonist. J Surg Res 159:696–704. [DOI] [PubMed] [Google Scholar]
- Nishio H, Morimoto Y, Hisaoka K, Nakata Y, Watanabe T. (2002) 5-HT-induced, 5-HT3 receptor-mediated, and ruthenium red- and capsaicin-sensitive positive chronotropic effects in the isolated guinea pig atrium. Jpn J Pharmacol 89:242–248. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. (1999) RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem 274:9472–9478. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. (2001) RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 24:478–491. [DOI] [PubMed] [Google Scholar]
- Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, Graf R, Clavien PA. (2008) Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res 68:5152–5158. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA. (2015) Serotonin-2C and -2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience 297:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. (2002) Localization of 5-HT2A receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience 111:163–176. [DOI] [PubMed] [Google Scholar]
- Noda M, Yasuda S, Okada M, Higashida H, Shimada A, Iwata N, Ozaki N, Nishikawa K, Shirasawa S, Uchida M, et al. (2003) Recombinant human serotonin 5A receptors stably expressed in C6 glioma cells couple to multiple signal transduction pathways. J Neurochem 84:222–232. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, van Kammen DP, Gilbertson MW, Gurklis JA, Peters JL. (1993) Electroencephalographic sleep in clinically stable schizophrenic patients: two-weeks versus six-weeks neuroleptic-free. Biol Psychiatry 33:829–835. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Ohba Y, Sumii M, Oka Y. (2008) Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem Biophys Res Commun 372:186–190. [DOI] [PubMed] [Google Scholar]
- North RA, Uchimura N. (1989) 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol 417:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum JH, Hart K, Levy FO. (2003) Ras-dependent ERK activation by the human Gs-coupled serotonin receptors 5-HT4(b) and 5-HT7(a). J Biol Chem 278:3098–3104. [DOI] [PubMed] [Google Scholar]
- Norum JH, Méthi T, Mattingly RR, Levy FO. (2005) Endogenous expression and protein kinase A-dependent phosphorylation of the guanine nucleotide exchange factor Ras-GRF1 in human embryonic kidney 293 cells. FEBS J 272:2304–2316. [DOI] [PubMed] [Google Scholar]
- Nowak EC, de Vries VC, Wasiuk A, Ahonen C, Bennett KA, Le Mercier I, Ha D-G, Noelle RJ. (2012) Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med 209:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D. (2016) Psilocybin for anxiety and depression in cancer care? Lessons from the past and prospects for the future. J Psychopharmacol 30:1163–1164. [DOI] [PubMed] [Google Scholar]
- Nys M, Kesters D, Ulens C. (2013) Structural insights into Cys-loop receptor function and ligand recognition. Biochem Pharmacol 86:1042–1053. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22. [DOI] [PubMed] [Google Scholar]
- O’Connell MT, Curzon G. (1996) A comparison of the effects of 8-OH-DPAT pretreatment of different behavioural responses to 8-OH-DPAT. Eur J Pharmacol 312:137–143. [DOI] [PubMed] [Google Scholar]
- O’Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. (2006) A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 107:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odagaki Y, Toyoshima R. (2005a) 5-HT1A receptor-mediated G protein activation assessed by [35S]GTPgammaS binding in rat cerebral cortex. Eur J Pharmacol 521:49–58. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Toyoshima R. (2005b) Detailed pharmacological characterization of 5-HT1A-receptor-mediated [35S]GTP gamma S binding in rat hippocampal membranes. J Pharmacol Sci 98:66–76. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Toyoshima R. (2007) 5-HT1A receptor agonist properties of antipsychotics determined by [35S]GTPgammaS binding in rat hippocampal membranes. Clin Exp Pharmacol Physiol 34:462–466. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. (2008) The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res 195:54–77. [DOI] [PubMed] [Google Scholar]
- Oh CK, Drescher MJ, Hatfield JS, Drescher DG. (1999) Selective expression of serotonin receptor transcripts in the mammalian cochlea and its subdivisions. Brain Res Mol Brain Res 70:135–140. [DOI] [PubMed] [Google Scholar]
- O’Hara BA, Atwood WJ. (2008) Interferon beta1-a and selective anti-5HT(2a) receptor antagonists inhibit infection of human glial cells by JC virus. Virus Res 132:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Doi K, Himaki D, Nagao K, Kawai M, Gale JD, Furness JB, Kurebayashi Y. (2010) A selective, high affinity 5-HT 2B receptor antagonist inhibits visceral hypersensitivity in rats. Neurogastroenterol Motil 22:e69–e76. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Kosaka Y, Kishimoto N, Wang J, Smith SB, Honig G, Kim H, Gasa RM, Neubauer N, Liou A, et al. (2011) Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 60:3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyashiki JH, Hamamura R, Kobayashi C, Zhang Y, Ohyashiki K. (2008) A network biology approach evaluating the anticancer effects of bortezomib identifies SPARC as a therapeutic target in adult T-cell leukemia cells. Adv Appl Bioinform Chem 1:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D. (2004) Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Mol Psychiatry 9:55–64. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Barton MJ, Hennig GW, Birch GC, Grainger N, Corrigan RD, Koh SD, Sanders KM, Smith TK. (2014) Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil 26:556–570. [DOI] [PubMed] [Google Scholar]
- Okita K, Shiina A, Nakazato M, Iyo M. (2013) Tandospirone, a 5-HT1A partial agonist is effective in treating anorexia nervosa: a case series. Ann Gen Psychiatry 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, Cryan JF. (2010) The Behavioral Genetics of Serotonin: Relevance to Anxiety and Depression, n Handbook of the Behavioral Neurobiology of Serotonin (Muller CP and Jacobs B eds) pp. 139-154, Elsevier Academic Press. [Google Scholar]
- Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H. (2004) Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study). Clin Neuropharmacol 27:58–62. [DOI] [PubMed] [Google Scholar]
- Olivier B, Chan JS, Snoeren EM, Olivier JD, Veening JG, Vinkers CH, Waldinger MD, Oosting RS. (2011) Differences in sexual behaviour in male and female rodents: role of serotonin. Curr Top Behav Neurosci 8:15–36. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J. (1992) Rodent models of aggressive behavior and serotonergic drugs. Prog Neuropsychopharmacol Biol Psychiatry 16:847–870. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. (1995) Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 28 (Suppl 2):80–90. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ. (2000) Localization of 5-ht(5A) receptor-like immunoreactivity in the rat brain. Brain Res 867:131–142. [DOI] [PubMed] [Google Scholar]
- Olsen MA, Nawoschik SP, Schurman BR, Schmitt HL, Burno M, Smith DL, Schechter LE. (1999) Identification of a human 5-HT6 receptor variant produced by alternative splicing. Brain Res Mol Brain Res 64:255–263. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Bulmer DC, Coelho A-M, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG, Cryan JF. (2010) 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil 22:573–578, e124. [DOI] [PubMed] [Google Scholar]
- O’Neil RT, Emeson RB. (2012) Quantitative analysis of 5HT(2C) receptor RNA editing patterns in psychiatric disorders. Neurobiol Dis 45:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MF, Heron-Maxwell CL, Shaw G. (1999) 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol Biochem Behav 63:237–243. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. (1998) Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch 435:485–494. [DOI] [PubMed] [Google Scholar]
- Onken MD, Worley LA, Tuscan MD, Harbour JW. (2010) An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn 12:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterink BJ, Korte SM, Nyakas C, Korf J, Luiten PG. (1998) Neuroprotection against N-methyl-D-aspartate-induced excitotoxicity in rat magnocellular nucleus basalis by the 5-HT1A receptor agonist 8-OH-DPAT. Eur J Pharmacol 358:147–152. [DOI] [PubMed] [Google Scholar]
- Orban G, Bombardi C, Marino Gammazza A, Colangeli R, Pierucci M, Pomara C, Pessia M, Bucchieri F, Benigno A, Smolders I, et al. (2014) Role(s) of the 5-HT2C receptor in the development of maximal dentate activation in the hippocampus of anesthetized rats [published correction appears in CNS Neurosci Ther (2014) 20:950]. CNS Neurosci Ther 20:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban G, Pierucci M, Benigno A, Pessia M, Galati S, Valentino M, Muscat R, Di Giovanni G. (2013) High dose of 8-OH-DPAT decreases maximal dentate gyrus activation and facilitates granular cell plasticity in vivo. Exp Brain Res 230:441–451. [DOI] [PubMed] [Google Scholar]
- Osredkar D, Kržan M. (2009) Expression of serotonin receptor subtypes in rat brain and astrocyte cell cultures: an age and tissue-dependent process. Period Biol 111:129–135. [Google Scholar]
- O’Sullivan RJ, Brown IG, Pender MP. (2008) Apneusis responding to buspirone in multiple sclerosis. Mult Scler 14:705–707. [DOI] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. (2001) Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem 276:14299–14307. [DOI] [PubMed] [Google Scholar]
- Ouadid H, Seguin J, Dumuis A, Bockaert J, Nargeot J. (1992) Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol Pharmacol 41:346–351. [PubMed] [Google Scholar]
- Padayatti PS, Wang L, Gupta S, Orban T, Sun W, Salom D, Jordan SR, Palczewski K, Chance MR. (2013) A hybrid structural approach to analyze ligand binding by the serotonin type 4 receptor (5-HT4). Mol Cell Proteomics 12:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. (2009) Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res 11:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM. (2016) Serotonin receptors in brain revisited. Brain Res 1645:46–49. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Markstein R, Pazos A. (1986) Serotonin-1C sites in the choroid plexus are not linked in a stimulatory or inhibitory way to adenylate cyclase. Brain Res 380:151–154. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Pazos A, Hoyer D. (2017) A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl) 234:1395–1418. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Waeber C, Bruinvels AT, Hoyer D. (1992) Direct visualization of serotonin1D receptors in the human brain using a new iodinated radioligand. Brain Res Mol Brain Res 13:175–178. [DOI] [PubMed] [Google Scholar]
- Palfreyman MG, Schmidt CJ, Sorensen SM, Dudley MW, Kehne JH, Moser P, Gittos MW, Carr AA. (1993) Electrophysiological, biochemical and behavioral evidence for 5-HT2 and 5-HT3 mediated control of dopaminergic function. Psychopharmacology (Berl) 112 (1 Suppl):S60–S67. [DOI] [PubMed] [Google Scholar]
- Pan H, Gershon MD. (2000) Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci 20:3295–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker MM, Parker I, Miledi R. (1991) Receptors of the serotonin 1C subtype expressed from cloned DNA mediate the closing of K+ membrane channels encoded by brain mRNA. Proc Natl Acad Sci USA 88:2560–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker S, Cruz H, Arrabit C, Slesinger PA. (2002) Evidence for a centrally located gate in the pore of a serotonin-gated ion channel. J Neurosci 22:1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, Papadopoulou-Daifoti Z, Mykoniatis MG. (2006) Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int 26:352–361. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A, Denef C. (2007) Stimulation of growth hormone release by 5-hydroxytryptamine (5-HT) in cultured rat anterior pituitary cell aggregates: evidence for mediation by 5-HT2B, 5-HT7, 5-HT1B, and ketanserin-sensitive receptors. Endocrinology 148:4509–4522. [DOI] [PubMed] [Google Scholar]
- Paquette AG, Marsit CJ. (2014) The developmental basis of epigenetic regulation of HTR2A and psychiatric outcomes. J Cell Biochem 115:2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Ryoo S, Noh HJ, Seo JM, Kang HH, Shin JS, Seo SW, Na DL. (2011a) Dual therapy with cidofovir and mirtazapine for progressive multifocal leukoencephalopathy in a sarcoidosis patient. Case Rep Neurol 3:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-S, Sin P-J, Lee DH, Cha S-K, Kim M-J, Kim N-H, Baik S-K, Jeong S-W, Kong ID. (2011b) Switching-on of serotonergic calcium signaling in activated hepatic stellate cells. World J Gastroenterol 17:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Lee KK, Kwon HJ, Ha M, Kim EJ, Yoo SJ, Paik KC, Lim MH. (2013) Association between HTR1A gene polymorphisms and attention deficit hyperactivity disorder in Korean children. Genet Test Mol Biomarkers 17:178–182. [DOI] [PubMed] [Google Scholar]
- Parker CA, Gunn RN, Rabiner EA, Slifstein M, Comley R, Salinas C, Johnson CN, Jakobsen S, Houle S, Laruelle M, et al. (2012) Radiosynthesis and characterization of 11C-GSK215083 as a PET radioligand for the 5-HT6 receptor. J Nucl Med 53:295–303. [DOI] [PubMed] [Google Scholar]
- Parker CA, Rabiner EI, Gunn R, Searle G, Martarello L, Comley R, Davy M, Wilson AA, Houle S, Mizrahi R, et al. (2015) Human kinetic modeling of the 5HT6 PET radioligand 11C-GSK215083 and its utility for determining occupancy at both 5HT6 and 5HT2A receptors by SB742457 as a potential therapeutic mechanism of action in Alzheimer disease. J Nucl Med 56:1901–1909. [DOI] [PubMed] [Google Scholar]
- Parker LL, Backstrom JR, Sanders-Bush E, Shieh BH. (2003) Agonist-induced phosphorylation of the serotonin 5-HT2C receptor regulates its interaction with multiple PDZ protein 1. J Biol Chem 278:21576–21583. [DOI] [PubMed] [Google Scholar]
- Parker RM, Barnes JM, Ge J, Barber PC, Barnes NM. (1996a) Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J Neurol Sci 144:119–127. [DOI] [PubMed] [Google Scholar]
- Parker RM, Bentley KR, Barnes NM. (1996b) Allosteric modulation of 5-HT3 receptors: focus on alcohols and anaesthetic agents. Trends Pharmacol Sci 17:95–99. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. (2006) Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem 99:1164–1175. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. (2006) Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology 31:1745–1749. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr (1993) Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res 606:195–199. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. (1996) Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res 73:225–228. [DOI] [PubMed] [Google Scholar]
- Pascual-Brazo J, Castro E, Díaz A, Valdizán EM, Pilar-Cuéllar F, Vidal R, Treceño B, Pazos A. (2012) Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT4 receptor agonist RS67333. Int J Neuropsychopharmacol 15:631–643. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Marazziti D, Castagna M, Nardi I. (1998a) Distribution of 5-HT2c and 5-ht5a receptor mRNA in human brain. Ann N Y Acad Sci 861:245. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, Castagna M, Cassano GB, Marazziti D. (1998b) Distribution of the 5-HT5A serotonin receptor mRNA in the human brain. Brain Res Mol Brain Res 56:1–8. [DOI] [PubMed] [Google Scholar]
- Patel BA, Bian X, Quaiserová-Mocko V, Galligan JJ, Swain GM. (2007) In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst (Lond) 132:41–47. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Kornum BR, Nutt DJ, Pike VW, Knudsen GM. (2013) 5-HT radioligands for human brain imaging with PET and SPECT. Med Res Rev 33:54–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Hill KP, Gopalakrishnan R, Berrettini WH. (2006) Relationship of disinhibition and aggression to blunted prolactin response to meta-chlorophenylpiperazine in cocaine-dependent patients. Psychopharmacology (Berl) 185:123–132. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Colpaert FC. (2003) Ca2+ responses in Chinese hamster ovary-K1 cells demonstrate an atypical pattern of ligand-induced 5-HT1A receptor activation. J Pharmacol Exp Ther 307:608–614. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Tardif S, Wurch T, Colpaert FC. (1997) Stimulated [35S]GTP gamma S binding by 5-HT1A receptor agonists in recombinant cell lines. Modulation of apparent efficacy by G-protein activation state. Naunyn Schmiedebergs Arch Pharmacol 356:551–561. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM. (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346:231–249. [DOI] [PubMed] [Google Scholar]
- Pazos A, Hoyer D, Palacios JM. (1984a) Mesulergine, a selective serotonin-2 ligand in the rat cortex, does not label these receptors in porcine and human cortex: evidence for species differences in brain serotonin-2 receptors. Eur J Pharmacol 106:531–538. [DOI] [PubMed] [Google Scholar]
- Pazos A, Hoyer D, Palacios JM. (1984b) The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur J Pharmacol 106:539–546. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346:205–230. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. (1987a) Serotonin receptors in the human brain--III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21:97–122. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. (1987b) Serotonin receptors in the human brain--IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience 21:123–139. [DOI] [PubMed] [Google Scholar]
- Pedigo NW, Yamamura HI, Nelson DL. (1981) Discrimination of multiple [3H]5-hydroxytryptamine binding sites by the neuroleptic spiperone in rat brain. J Neurochem 36:220–226. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. (2006) Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology 31:265–277. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. (2012) Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacology 37:2505–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñas-Cazorla R, Vilaró MT. (2015) Serotonin 5-HT4 receptors and forebrain cholinergic system: receptor expression in identified cell populations. Brain Struct Funct 220:3413–3434. [DOI] [PubMed] [Google Scholar]
- Peng Y, McCorvy JD, Harpsøe K, Lansu K, Yuan S, Popov P, Qu L, Pu M, Che T, Nikolajsen LF, et al. (2018) 5-HT2C receptor structures reveal the structural basis of GPCR polypharmacology. Cell 172:719–730.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. (2010) Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology 35:2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL, Toksoz D. (2014) Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal 26:2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumatsa KC, Fanburg BL. (2014) Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306:L309–L315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Martynhak BJ, Andreatini R, Svenningsson P. (2015) 5-HT6 receptor agonism facilitates emotional learning. Front Pharmacol 6:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericić D, Svob Strac D. (2007) The role of 5-HT(7) receptors in the control of seizures. Brain Res 1141:48–55. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. (1985) Selective interaction of novel anxiolytics with 5-hydroxytryptamine1A receptors. Biol Psychiatry 20:971–979. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. (1979) Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol 16:687–699. [PubMed] [Google Scholar]
- Peroutka SJ, Switzer JA, Hamik A. (1989) Identification of 5-hydroxytryptamine1D binding sites in human brain membranes. Synapse 3:61–66. [DOI] [PubMed] [Google Scholar]
- Perwitasari DA, Wessels JA, van der Straaten RJ, Baak-Pablo RF, Mustofa M, Hakimi M, Nortier JW, Gelderblom H, Guchelaar HJ. (2011) Association of ABCB1, 5-HT3B receptor and CYP2D6 genetic polymorphisms with ondansetron and metoclopramide antiemetic response in Indonesian cancer patients treated with highly emetogenic chemotherapy. Jpn J Clin Oncol 41:1168–1176. [DOI] [PubMed] [Google Scholar]
- Pessia M, Jiang ZG, North RA, Johnson SW. (1994) Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res 654:324–330. [DOI] [PubMed] [Google Scholar]
- Peters JA, Hales TG, Lambert JJ. (2005) Molecular determinants of single-channel conductance and ion selectivity in the Cys-loop family: insights from the 5-HT3 receptor. Trends Pharmacol Sci 26:587–594. [DOI] [PubMed] [Google Scholar]
- Peters J-U, Lübbers T, Alanine A, Kolczewski S, Blasco F, Steward L. (2008) Cyclic guanidines as dual 5-HT5A/5-HT7 receptor ligands: optimising brain penetration. Bioorg Med Chem Lett 18:262–266. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82:443–468. [DOI] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer AD, Jr, Branchek TA, et al. (1997) Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61:2117–2126. [DOI] [PubMed] [Google Scholar]
- Pichon X, Wattiez AS, Becamel C, Ehrlich I, Bockaert J, Eschalier A, Marin P, Courteix C. (2010) Disrupting 5-HT(2A) receptor/PDZ protein interactions reduces hyperalgesia and enhances SSRI efficacy in neuropathic pain. Mol Ther 18:1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. (1996) 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience 70:553–559. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Meuser T, Peroutka SJ. (1997) 5-Hydroxytryptamine receptor subtype messenger RNAs in human dorsal root ganglia: a polymerase chain reaction study. Neuroscience 81:813–819. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. (1991) Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66:817–822. [DOI] [PubMed] [Google Scholar]
- Pike VW, McCarron JA, Lammerstma AA, Hume SP, Poole K, Grasby PM, Malizia A, Cliffe IA, Fletcher A, Bench CJ. (1995) First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635. Eur J Pharmacol 283:R1–R3. [DOI] [PubMed] [Google Scholar]
- Pimenova AA, Thathiah A, De Strooper B, Tesseur I. (2014) Regulation of amyloid precursor protein processing by serotonin signaling. PLoS One 9:e87014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindon A, van Hecke G, van Gompel P, Lesage AS, Leysen JE, Jurzak M. (2002) Differences in signal transduction of two 5-HT4 receptor splice variants: compound specificity and dual coupling with Galphas- and Galphai/o-proteins. Mol Pharmacol 61:85–96. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, de Montigny C, Blier P. (1995) 5-HT1D receptors regulate 5-HT release in the rat raphe nuclei. In vivo voltammetry and in vitro superfusion studies. Neuropsychopharmacology 13:249–260. [DOI] [PubMed] [Google Scholar]
- Pino R, Cerbai E, Calamai G, Alajmo F, Borgioli A, Braconi L, Cassai M, Montesi GF, Mugelli A. (1998) Effect of 5-HT4 receptor stimulation on the pacemaker current I(f) in human isolated atrial myocytes. Cardiovasc Res 40:516–522. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Belmer A, Moutkine I, Adrien J, Maroteaux L. (2015) Mice lacking the serotonin Htr2B receptor gene present an antipsychotic-sensitive schizophrenic-like phenotype. Neuropsychopharmacology 40:2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva F, Giulietti M, Baldelli L, Nardi B, Bellantuono C, Armeni T, Saccucci F, Principato G. (2011) Bioinformatic analyses to select phenotype affecting polymorphisms in HTR2C gene. Hum Psychopharmacol 26:365–372. [DOI] [PubMed] [Google Scholar]
- Plassat JL, Amlaiky N, Hen R. (1993) Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol Pharmacol 44:229–236. [PubMed] [Google Scholar]
- Plassat JL, Boschert U, Amlaiky N, Hen R. (1992) The mouse 5HT5 receptor reveals a remarkable heterogeneity within the 5HT1D receptor family. EMBO J 11:4779–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Conway SM, Ullman TE, Zwick KR, Neisewander JL. (2012) 5-HT(2A) receptor blockade and 5-HT(2C) receptor activation interact to reduce cocaine hyperlocomotion and Fos protein expression in the caudate-putamen. Synapse 66:989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M. (2010) Dyskinesias after neural transplantation in Parkinson’s disease: what do we know and what is next? BMC Med 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polovinkin L, Hassaine G, Perot J, Neumann E, Jensen AA, Lefebvre SN, Corringer P-J, Neyton J, Chipot C, Dehez F, et al. (2018) Conformational transitions of the serotonin 5-HT3 receptor. Nature 563:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Li X. (2010) 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal 22:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomfret DW, Schenck KW, Fludzinski P, Cohen ML. (1987) Interaction of 5-hydroxykynurenamine, L-kynurenine and kynuramine with multiple serotonin receptors in smooth muscle. J Pharmacol Exp Ther 241:465–471. [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. (1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12:440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23:163–178. [DOI] [PubMed] [Google Scholar]
- Pompili M, Serafini G, Innamorati M, Venturini P, Fusar-Poli P, Sher L, Amore M, Girardi P. (2013) Agomelatine, a novel intriguing antidepressant option enhancing neuroplasticity: a critical review. World J Biol Psychiatry 14:412–431. [DOI] [PubMed] [Google Scholar]
- Pönicke K, Gergs U, Buchwalow IB, Hauptmann S, Neumann J. (2012) On the presence of serotonin in mammalian cardiomyocytes. Mol Cell Biochem 365:301–312. [DOI] [PubMed] [Google Scholar]
- Ponimaskin EG, Heine M, Joubert L, Sebben M, Bickmeyer U, Richter DW, Dumuis A. (2002a) The 5-hydroxytryptamine(4a) receptor is palmitoylated at two different sites, and acylation is critically involved in regulation of receptor constitutive activity. J Biol Chem 277:2534–2546. [DOI] [PubMed] [Google Scholar]
- Ponimaskin EG, Profirovic J, Vaiskunaite R, Richter DW, Voyno-Yasenetskaya TA. (2002b) 5-Hydroxytryptamine 4(a) receptor is coupled to the Galpha subunit of heterotrimeric G13 protein. J Biol Chem 277:20812–20819. [DOI] [PubMed] [Google Scholar]
- Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. (2006) Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res 325:413–422. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Fairburn CG, Cooper Z, Sodhi MS, Cowen PJ, Harrison PJ. (2004) A 5-HT2C receptor promoter polymorphism (HTR2C - 759C/T) is associated with obesity in women, and with resistance to weight loss in heterozygotes. Am J Med Genet B Neuropsychiatr Genet 126B:124–127. [DOI] [PubMed] [Google Scholar]
- Popa D, Léna C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. (2005) Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci 25:11231–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Stanojevic Z, Tosic J, Isakovic A, Paunovic V, Petricevic S, Martinovic T, Ciric D, Kravic-Stevovic T, Soskic V, et al. (2015) Neuroprotective arylpiperazine dopaminergic/serotonergic ligands suppress experimental autoimmune encephalomyelitis in rats. J Neurochem 135:125–138. [DOI] [PubMed] [Google Scholar]
- Portella MJ, de Diego-Adeliño J, Ballesteros J, Puigdemont D, Oller S, Santos B, Álvarez E, Artigas F, Pérez V. (2011) Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry 72:962–969. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. (1999) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Malcolm CS, Allen NH, Lamb H, Revell DF, Sheardown MJ. (2001) Agonist-induced functional desensitization of recombinant human 5-HT2 receptors expressed in CHO-K1 cells. Biochem Pharmacol 62:431–438. [DOI] [PubMed] [Google Scholar]
- Porvasnik SL, Germain S, Embury J, Gannon KS, Jacques V, Murray J, Byrne BJ, Shacham S, Al-Mousily F. (2010) PRX-08066, a novel 5-hydroxytryptamine receptor 2B antagonist, reduces monocrotaline-induced pulmonary arterial hypertension and right ventricular hypertrophy in rats. J Pharmacol Exp Ther 334:364–372. [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. (2003) Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci 23:10988–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzet B, Didriksen M, Arnt J. (2002) Effects of the 5-HT(7) receptor antagonist SB-258741 in animal models for schizophrenia. Pharmacol Biochem Behav 71:655–665. [DOI] [PubMed] [Google Scholar]
- Powell AD, Grafton G, Roberts A, Larkin S, O’Neill N, Palandri J, Otvos R, Cooper AJ, Ulens C, Barnes NM. (2016) Novel mechanism of modulation at a ligand-gated ion channel; action of 5-Cl-indole at the 5-HT 3 A receptor. Br J Pharmacol 173:3467–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR. (1991) Regulation of 5-HT2 receptors in rat cortex. Studies with a putative selective agonist and an antagonist. Biochem Pharmacol 42:1099–1105. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG, Kilpatrick GJ, Leslie RA, Barnes NM, Naylor RJ, Jones BJ, Nelson DR, Palacids JM, Slater P, et al. (1990) Consensus meeting agrees distribution of 5-HT3 receptors in mammalian hindbrain. Trends Pharmacol Sci 11:135–137. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Blackstone K, Connolly ME, Skelly MJ. (2009) Selective serotonin receptor stimulation of the medial nucleus accumbens causes differential effects on food intake and locomotion. Behav Neurosci 123:1046–1057. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Schall MA, Choi E. (2012) Selective serotonin receptor stimulation of the medial nucleus accumbens differentially affects appetitive motivation for food on a progressive ratio schedule of reinforcement. Neurosci Lett 511:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. (2004) Region specific changes in forebrain 5-hydroxytryptamine1A and 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience 123:725–732. [DOI] [PubMed] [Google Scholar]
- Pregenzer JF, Alberts GL, Im WB, Slightom JL, Ennis MD, Hoffman RL, Ghazal NB, Tenbrink RE. (1999) Differential pharmacology between the guinea-pig and the gorilla 5-HT1D receptor as probed with isochromans (5-HT1D-selective ligands). Br J Pharmacol 127:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. (1997) SB-216641 and BRL-15572--compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol 356:312–320. [DOI] [PubMed] [Google Scholar]
- Price KL, Lillestol RK, Ulens C, Lummis SCR. (2016) Palonosetron-5-HT3 receptor interactions as shown by a binding protein cocrystal structure. ACS Chem Neurosci 7:1641–1646. [DOI] [PubMed] [Google Scholar]
- Price RD, Weiner DM, Chang MS, Sanders-Bush E. (2001) RNA editing of the human serotonin 5-HT2C receptor alters receptor-mediated activation of G13 protein. J Biol Chem 276:44663–44668. [DOI] [PubMed] [Google Scholar]
- Priem EK, De Maeyer JH, Vandewoestyne M, Deforce D, Lefebvre RA. (2013) Predominant mucosal expression of 5-HT4(+h) receptor splice variants in pig stomach and colon. World J Gastroenterol 19:3747–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NH, Briejer MR, Van Bergen PJ, Akkermans LM, Schuurkes JA. (1999) Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol 128:849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NH, Shankley NP, Welsh NJ, Briejer MR, Lefebvre RA, Akkermans LMA, Schuurkes JA. (2000) An improved in vitro bioassay for the study of 5-HT(4) receptors in the human isolated large intestinal circular muscle. Br J Pharmacol 129:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinssen EP, Colpaert FC, Koek W. (2002) 5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur J Pharmacol 453:217–221. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Koek W, Colpaert FC, Kleven MS. (2000) Repeated treatment with 8-OH-DPAT induces tolerance to its ability to produce the 5-HT1A behavioural syndrome, but not to its ability to attenuate haloperidol-induced catalepsy. Behav Pharmacol 11:299–305. [DOI] [PubMed] [Google Scholar]
- Prior A, Read NW. (1993) Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther 7:175–180. [DOI] [PubMed] [Google Scholar]
- Prisco S, Esposito E. (1995) Differential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br J Pharmacol 116:1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco S, Pagannone S, Esposito E. (1994) Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J Pharmacol Exp Ther 271:83–90. [PubMed] [Google Scholar]
- Pritchett DB, Bach AW, Wozny M, Taleb O, Dal Toso R, Shih JC, Seeburg PH. (1988) Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J 7:4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profirovic J, Strekalova E, Urao N, Krbanjevic A, Andreeva AV, Varadarajan S, Fukai T, Hen R, Ushio-Fukai M, Voyno-Yasenetskaya TA. (2013) A novel regulator of angiogenesis in endothelial cells: 5-hydroxytriptamine 4 receptor. Angiogenesis 16:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. (2010) Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci 30:2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Herrick-Davis K, Teitler M. (2003) Creation, expression, and characterization of a constitutively active mutant of the human serotonin 5-HT6 receptor. Synapse 47:218–224. [DOI] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. (2010) Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30:1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. (2007) Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharmacol 72:885–896. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. (2005) 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 180:12–20. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Treyer V, Hasler F, Dörig N, Wyss MT, Burger C, Rentsch KM, Westera G, Schubiger PA, Buck A, Vollenweider FX. (2012) Assessment of serotonin release capacity in the human brain using dexfenfluramine challenge and [18F]altanserin positron emission tomography. Neuroimage 59:3922–3932. [DOI] [PubMed] [Google Scholar]
- Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G, David I, Dranovsky A, et al. (2013) BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry 3:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Simon MI, Davidson N, Lester HA, Aragay AM. (1994) Differential coupling of G protein alpha subunits to seven-helix receptors expressed in Xenopus oocytes. J Biol Chem 269:30164–30172. [PubMed] [Google Scholar]
- Quiedeville A, Boulouard M, Hamidouche K, Da Silva Costa-Aze V, Nee G, Rochais C, Dallemagne P, Fabis F, Freret T, Bouet V. (2015) Chronic activation of 5-HT4 receptors or blockade of 5-HT6 receptors improve memory performances. Behav Brain Res 293:10–17. [DOI] [PubMed] [Google Scholar]
- Quinn JC, Johnson-Farley NN, Yoon J, Cowen DS. (2002) Activation of extracellular-regulated kinase by 5-hydroxytryptamine(2A) receptors in PC12 cells is protein kinase C-independent and requires calmodulin and tyrosine kinases. J Pharmacol Exp Ther 303:746–752. [DOI] [PubMed] [Google Scholar]
- Quirk PL, Rao S, Roth BL, Siegel RE. (2004) Three putative N-glycosylation sites within the murine 5-HT3A receptor sequence affect plasma membrane targeting, ligand binding, and calcium influx in heterologous mammalian cells. J Neurosci Res 77:498–506. [DOI] [PubMed] [Google Scholar]
- Qvigstad E, Brattelid T, Sjaastad I, Andressen KW, Krobert KA, Birkeland JA, Sejersted OM, Kaumann AJ, Skomedal T, Osnes JB, et al. (2005a) Appearance of a ventricular 5-HT4 receptor-mediated inotropic response to serotonin in heart failure. Cardiovasc Res 65:869–878. [DOI] [PubMed] [Google Scholar]
- Qvigstad E, Brattelid T, Sjaastad I, Molenaar P, Birkeland JA, Andressen KW, Krobert KA, Sejersted OM, Skomedal T, Osnes J-B, et al. (2005b) Rationale for treatment of heart failure by blockade of ventricular serotonin receptors appearing in heart failure, in Proceedings of the 25th European Section Meeting, International Society for Heart Research; June 21–25; Tromso, Norway. pp 7–12, Medimond International Proceedings, Bologna, Italy. [Google Scholar]
- Qvigstad E, Sjaastad I, Brattelid T, Nunn C, Swift F, Birkeland JA, Krobert KA, Andersen GØ, Sejersted OM, Osnes JB, et al. (2005c) Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res 97:268–276. [DOI] [PubMed] [Google Scholar]
- Radja F, Laporte AM, Daval G, Vergé D, Gozlan H, Hamon M. (1991) Autoradiography of serotonin receptor subtypes in the central nervous system. Neurochem Int 18:1–15. [DOI] [PubMed] [Google Scholar]
- Rahme MM, Cotter B, Leistad E, Wadhwa MK, Mohabir R, Ford AP, Eglen RM, Feld GK. (1999) Electrophysiological and antiarrhythmic effects of the atrial selective 5-HT(4) receptor antagonist RS-100302 in experimental atrial flutter and fibrillation. Circulation 100:2010–2017. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Massey BW, Michael E, Meltzer HY. (2016) Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology (Berl) 233:649–660. [DOI] [PubMed] [Google Scholar]
- Ramadan NM, Buchanan TM. (2006) New and future migraine therapy. Pharmacol Ther 112:199–212. [DOI] [PubMed] [Google Scholar]
- Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. (2003) 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia 23:776–785. [DOI] [PubMed] [Google Scholar]
- Ramage AG. (1990) Influence of 5-HT1A receptor agonists on sympathetic and parasympathetic nerve activity. J Cardiovasc Pharmcol 15 (Suppl 7):S75–S85. [PubMed] [Google Scholar]
- Ramage AG, Clement ME, McCall RB. (1992) 8-OH-DPAT-induced inhibition of renal sympathetic nerve activity and serotonin neuronal firing. Eur J Pharmacol 219:165–167. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Villalón CM. (2008) 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci 29:472–481. [DOI] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. (2001) The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett 306:81–84. [DOI] [PubMed] [Google Scholar]
- Ramos AJ, Rubio MD, Defagot C, Hischberg L, Villar MJ, Brusco A. (2004) The 5HT1A receptor agonist, 8-OH-DPAT, protects neurons and reduces astroglial reaction after ischemic damage caused by cortical devascularization. Brain Res 1030:201–220. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. (1947) Purification of the substance which is responsible for the vasoconstrictor activity of serum. Fed Proc 6:184. [PubMed] [Google Scholar]
- Rauser L, Savage JE, Meltzer HY, Roth BL. (2001) Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor. J Pharmacol Exp Ther 299:83–89. [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. (2003) Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284:G367–G372. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Kim J, Beach RE, Tisher CC. (1993) Immunohistochemical mapping of cellular and subcellular distribution of 5-HT1A receptors in rat and human kidneys. Am J Physiol 264:F9–F19. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. (2001) Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 92:179–212. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. (1999) The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol 127:1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Turner JH, Gelasco AK, Ayiku HB, Coaxum SD, Arthur JM, Garnovskaya MN. (2006) 5-HT receptor signal transduction pathways, in The Serotonin Receptors ( Roth B. ed) Humana Press, Toyota, NJ. [Google Scholar]
- Razzaque Z, Heald MA, Pickard JD, Maskell L, Beer MS, Hill RG, Longmore J. (1999) Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-HT1B- and 5-HT1F-receptor activation. Br J Clin Pharmacol 47:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, den Daas I, Foord S, Goodson S, Bull D, Kilpatrick G, Lee M. (1994) Cloning and characterisation of the human 5-HT5A serotonin receptor. FEBS Lett 355:242–246. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Goren EN, Akabas MH, Lummis SC. (2001) Structural and electrostatic properties of the 5-HT3 receptor pore revealed by substituted cysteine accessibility mutagenesis. J Biol Chem 276:42035–42042. [DOI] [PubMed] [Google Scholar]
- Reid TE, Kumar K, Wang XS. (2013) Predictive in silico studies of human 5-hydroxytryptamine receptor subtype 2B (5-HT2B) and valvular heart disease. Curr Top Med Chem 13:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisoli E, De Lucchini S, Nardi I, Ori M. (2010) Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development 137:2927–2937. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhou X, Galligan JJ. (2008) 5-HT4 receptor activation facilitates recovery from synaptic rundown and increases transmitter release from single varicosities of myenteric neurons. Am J Physiol Gastrointest Liver Physiol 294:G1376–G1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, Gorinski N, Guseva D, Abdel-Galil D, Fröhlich M, et al. (2012) Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J Cell Sci 125:2486–2499. [DOI] [PubMed] [Google Scholar]
- Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, et al. Wellcome Trust Case Control Consortium; NSHD Respiratory Study Team (2010) Genome-wide association study identifies five loci associated with lung function. Nat Genet 42:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Roman F, Dumuis A, Bockaert J, Marchetti E, Ammassari-Teule M. (2008) The promnesic effect of G-protein-coupled 5-HT4 receptors activation is mediated by a potentiation of learning-induced spine growth in the mouse hippocampus. Neuropsychopharmacology 33:2427–2434. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. (1994) Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature 370:143–146. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Arranz B, Templeman LA, Fertuzinhos S, San L. (2006) Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry 163:1826–1829. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Templeman LA, Zhang ZJ. (2005) The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 29:1021–1028. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Zhang Z, Zhang X. (2003) Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 160:677–679. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED. (2014) Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav 125:8–14. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417:181–194. [PubMed] [Google Scholar]
- Riad M, Tong XK, el Mestikawy S, Hamon M, Hamel E, Descarries L. (1998) Endothelial expression of the 5-hydroxytryptamine1B antimigraine drug receptor in rat and human brain microvessels. Neuroscience 86:1031–1035. [DOI] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. (2013) Rett syndrome treatment in mouse models: searching for effective targets and strategies. Neuropharmacology 68:106–115. [DOI] [PubMed] [Google Scholar]
- Riccio O, Jacobshagen M, Golding B, Vutskits L, Jabaudon D, Hornung JP, Dayer AG. (2011) Excess of serotonin affects neocortical pyramidal neuron migration. Transl Psychiatry 1:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabó G, Vutskits L, Kiss JZ, Dayer AG. (2009) Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry 14:280–290. [DOI] [PubMed] [Google Scholar]
- Riccioni T, Bordi F, Minetti P, Spadoni G, Yun HM, Im BH, Tarzia G, Rhim H, Borsini F. (2011) ST1936 stimulates cAMP, Ca2+, ERK1/2 and Fyn kinase through a full activation of cloned human 5-HT6 receptors. Eur J Pharmacol 661:8–14. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, et al. (2010) 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, et al. (2011) Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci 31:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. (2007) Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology 32:1715–1726. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. (2008) Role of serotonin and dopamine receptor binding in antipsychotic efficacy. Prog Brain Res 172:155–175. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. (2003) Serotonin receptors: guardians of stable breathing. Trends Mol Med 9:542–548. [DOI] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, Liechti ME. (2015) Pharmacological profile of novel psychoactive benzofurans. Br J Pharmacol 172:3412–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga MS, Sánchez C, Celada P, Artigas F. (2016) Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology 108:73–81. [DOI] [PubMed] [Google Scholar]
- Rippberger H, van Gaalen MM, Schwarting RK, Wohr M. (2015) Environmental and pharmacological modulation of amphetamine- induced 50-kHz ultrasonic vocalizations in rats. Curr Neuropharmacol 13:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi CA, Coccini T, Onori L, Manzo L, Tonini M. (1992) Benzimidazolone derivatives: a new class of 5-hydroxytryptamine4 receptor agonists with prokinetic and acetylcholine releasing properties in the guinea pig ileum. J Pharmacol Exp Ther 261:412–419. [PubMed] [Google Scholar]
- Rizzoli PB. (2014) Emerging therapeutic options for acute migraine: focus on the potential of lasmiditan. Neuropsychiatr Dis Treat 10:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Grafton G, Powell AD, Brock K, Chen C, Xie D, Huang J, Liu S, Cooper AJ, Brady CA, et al. (2020) CSTI-300 (SMP-100); a novel 5-HT3 receptor partial agonist with potential to treat patients with irritable bowel syndrome or carcinoid syndrome. J Pharmacol Exp Ther 373:122–134. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. (2004a) Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur J Neurosci 19:1913–1922. [DOI] [PubMed] [Google Scholar]
- Roberts C, Thomas DR, Bate ST, Kew JN. (2004b) GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology 46:935–941. [DOI] [PubMed] [Google Scholar]
- Robertson DN, Johnson MS, Moggach LO, Holland PJ, Lutz EM, Mitchell R. (2003) Selective interaction of ARF1 with the carboxy-terminal tail domain of the 5-HT2A receptor. Mol Pharmacol 64:1239–1250. [DOI] [PubMed] [Google Scholar]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM. (1995) Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 92:790–795. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O’Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. (2002) Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22:10039–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-González HI, Meneses A, Carlton SM, Granados-Soto V. (2005) Pronociceptive role of peripheral and spinal 5-HT7 receptors in the formalin test. Pain 117:182–192. [DOI] [PubMed] [Google Scholar]
- Rochais C, Lecoutey C, Gaven F, Giannoni P, Hamidouche K, Hedou D, Dubost E, Genest D, Yahiaoui S, Freret T, et al. (2015) Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: the design of donecopride. J Med Chem 58:3172–3187. [DOI] [PubMed] [Google Scholar]
- Rock PL, Harmer CJ, McTavish SF, Goodwin GM, Rogers RD. (2013) Short-term quetiapine treatment alters the use of reinforcement signals during risky decision-making and promotes the choice of negative expected values in healthy adult males. J Neurosci 33:15588–15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Holch P, Tallett AJ. (2010) Behavioural satiety sequence (BSS): separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol Biochem Behav 97:3–14. [DOI] [PubMed] [Google Scholar]
- Rojas C, Raje M, Tsukamoto T, Slusher BS. (2014) Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 722:26–37. [DOI] [PubMed] [Google Scholar]
- Romero L, Celada P, Martín-Ruiz R, Díaz-Mataix L, Mourelle M, Delgadillo J, Hervás I, Artigas F. (2003) Modulation of serotonergic function in rat brain by VN2222, a serotonin reuptake inhibitor and 5-HT1A receptor agonist. Neuropsychopharmacology 28:445–456. [DOI] [PubMed] [Google Scholar]
- Romero G, Sánchez E, Pujol M, Pérez P, Codony X, Holenz J, Buschmann H, Pauwels PJ. (2006) Efficacy of selective 5-HT6 receptor ligands determined by monitoring 5-HT6 receptor-mediated cAMP signaling pathways. Br J Pharmacol 148:1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondé P, Ansanay H, Dumuis A, Miller R, Bockaert J. (1995) Homologous desensitization of 5-hydroxytryptamine4 receptors in rat esophagus: functional and second messenger studies. J Pharmacol Exp Ther 272:977–983. [PubMed] [Google Scholar]
- Rose D, Khater-Boidin J, Toussaint P, Duron B. (1995) Central effects of 5-HT on respiratory and hypoglossal activities in the adult cat. Respir Physiol 101:59–69. [DOI] [PubMed] [Google Scholar]
- Rosendorff A, Ebersole BJ, Sealfon SC. (2000) Conserved helix 7 tyrosine functions as an activation relay in the serotonin 5HT(2C) receptor. Brain Res Mol Brain Res 84:90–96. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Dunlop J, Marquis KL. (2007a) 5-HT2C receptor agonists as an innovative approach for psychiatric disorders. Drug News Perspect 20:565–571. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, Grauer S, Brennan J, Cryan JF, Sukoff Rizzo SJ, et al. (2007b) Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 192:159–170. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30:1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. (2007) Drugs and valvular heart disease. N Engl J Med 356:6–9. [DOI] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD. (1991) Chronic mianserin treatment decreases 5-HT2 receptor binding without altering 5-HT2 receptor mRNA levels. Eur J Pharmacol 207:169–172. [DOI] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD, Meltzer HY. (1992) Binding of typical and atypical antipsychotic agents with transiently expressed 5-HT1C receptors. J Pharmacol Exp Ther 260:1361–1365. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. (2000) Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102:2836–2841. [DOI] [PubMed] [Google Scholar]
- Rouquet G, Moore DE, Spain M, Allwood DM, Battilocchio C, Blakemore DC, Fish PV, Jenkinson S, Jessiman AS, Ley SV, et al. (2015) Design, synthesis, and evaluation of tetrasubstituted pyridines as potent 5-HT2C receptor agonists. ACS Med Chem Lett 6:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle JC, Plantefol M, Fillion MP, Massot O, Pauwels PJ, Fillion G. (1998) Specific interaction of 5-HT-moduline with human 5-HT1b as well as 5-HT1d receptors expressed in transfected cultured cells. Naunyn Schmiedebergs Arch Pharmacol 358:279–286. [DOI] [PubMed] [Google Scholar]
- Routledge C. (1996) Development of 5-HT1A receptor antagonists. Behav Brain Res 73:153–156. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. (1993a) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193:268–276. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. (1993b) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90:8547–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. (2006) A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol 169:861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Spealman RD, Platt DM. (2015) Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys by direct and indirect activation of 5-HT2C receptors. Psychopharmacology (Berl) 232:2959–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffert H, Thieme V, Wallenborn J, Lemnitz N, Bergmann A, Rudlof K, Wehner M, Olthoff D, Kaisers UX. (2009) Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting? Anesth Analg 109:1442–1447. [DOI] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. (1999) Regulation of alternative splicing by RNA editing. Nature 399:75–80. [DOI] [PubMed] [Google Scholar]
- Ruf BM, Bhagwagar Z. (2009) The 5-HT1B receptor: a novel target for the pathophysiology of depression. Curr Drug Targets 10:1118–1138. [DOI] [PubMed] [Google Scholar]
- Rüsch D, Braun HA, Wulf H, Schuster A, Raines DE. (2007) Inhibition of human 5-HT(3A) and 5-HT(3AB) receptors by etomidate, propofol and pentobarbital. Eur J Pharmacol 573:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A, Banes A, Berlin H, Fink GD, Watts SW. (2002) 5-Hydroxytryptamine(2B) receptor function is enhanced in the N(omega)-nitro-L-arginine hypertensive rat. J Pharmacol Exp Ther 303:179–187. [DOI] [PubMed] [Google Scholar]
- Sagalajev B, Bourbia N, Beloushko E, Wei H, Pertovaara A. (2015) Bidirectional amygdaloid control of neuropathic hypersensitivity mediated by descending serotonergic pathways acting on spinal 5-HT3 and 5-HT1A receptors. Behav Brain Res 282:14–24. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA. (2000) Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther 292:704–713. [PubMed] [Google Scholar]
- Sahin-Erdemli I, Hoyer D, Stoll A, Seiler MP, Schoeffter P. (1991) 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of guinea-pig isolated iliac artery. Br J Pharmacol 102:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salom D, Wang B, Dong Z, Sun W, Padayatti P, Jordan S, Salon JA, Palczewski K. (2012) Post-translational modifications of the serotonin type 4 receptor heterologously expressed in mouse rod cells. Biochemistry 51:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman AG, Morse B, Whitman MM, Ivanshchenko Y, Jaye M, Felder S. (1991) Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem Biophys Res Commun 181:1469–1478. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2015) Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. Rockville, MD. September 8, 2016.
- Samuels BA, Mendez-David I, Faye C, David SA, Pierz KA, Gardier AM, Hen R, David DJ. (2016) Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 22:26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. [DOI] [PubMed] [Google Scholar]
- Sánchez-Lopez A, Centurión D, Vázquez E, Arulmani U, Saxena PR, Villalón CM. (2003) Pharmacological profile of the 5-HT-induced inhibition of cardioaccelerator sympathetic outflow in pithed rats: correlation with 5-HT1 and putative 5-ht5A/5B receptors. Br J Pharmacol 140:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López A, Centurión D, Vázquez E, Arulmani U, Saxena PR, Villalón CM. (2004) Further characterization of the 5-HT1 receptors mediating cardiac sympatho-inhibition in pithed rats: pharmacological correlation with the 5-HT1B and 5-HT1D subtypes. Naunyn Schmiedebergs Arch Pharmacol 369:220–227. [DOI] [PubMed] [Google Scholar]
- Sánchez-Maldonado C, López-Sánchez P, Anguiano-Robledo L, Leopoldo M, Lacivita E, Terrón JA. (2015) GR-127935-sensitive mechanism mediating hypotension in anesthetized rats: are 5-HT5B receptors involved? J Cardiovasc Pharmacol 65:335–341. [DOI] [PubMed] [Google Scholar]
- Sandén N, Thorlin T, Blomstrand F, Persson PA, Hansson E. (2000) 5-Hydroxytryptamine2B receptors stimulate Ca2+ increases in cultured astrocytes from three different brain regions. Neurochem Int 36:427–434. [DOI] [PubMed] [Google Scholar]
- Sanders L, Kaumann AJ. (1992) A 5-HT4-like receptor in human left atrium. Naunyn Schmiedebergs Arch Pharmacol 345:382–386. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Conn PJ. (1986) Effector systems coupled to serotonin receptors in brain: serotonin stimulated phosphoinositide hydrolysis. Psychopharmacol Bull 22:829–836. [PubMed] [Google Scholar]
- Sangare A, Dubourget R, Geoffroy H, Gallopin T, Rancillac A. (2016) Serotonin differentially modulates excitatory and inhibitory synaptic inputs to putative sleep-promoting neurons of the ventrolateral preoptic nucleus. Neuropharmacology 109:29–40. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. (1987a) Increased gut cholinergic activity and antagonism of 5-hydroxytryptamine M-receptors by BRL 24924: potential clinical importance of BRL 24924. Br J Pharmacol 91:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ. (1987b) Activation of a myenteric 5-hydroxytryptamine-like receptor by metoclopramide. J Pharm Pharmacol 39:449–453. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. (2008) 5-hydroxytryptamine and the gastrointestinal tract: where next? Trends Pharmacol Sci 29:465–471. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. (2009) Translating 5-HT receptor pharmacology. Neurogastroenterol Motil 21:1235–1238. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Andrews PLR. (2006) Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129:3–16. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Banner SE, Smith MI, Wardle KA. (1998) SB-207266: 5-HT4 receptor antagonism in human isolated gut and prevention of 5-HT-evoked sensitization of peristalsis and increased defaecation in animal models. Neurogastroenterol Motil 10:271–279. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Broad J, Andrews PLR. (2013a) The relationship between gastric motility and nausea: gastric prokinetic agents as treatments. Eur J Pharmacol 715:10–14. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Broad J, Kung V, Knowles CH. (2013b) Translational neuropharmacology: the use of human isolated gastrointestinal tissues. Br J Pharmacol 168:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ, Hellström PM, Näslund E. (2011a) The hungry stomach: physiology, disease, and drug development opportunities. Front Pharmacol 1:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ, Holbrook JD, Andrews PLR. (2011b) The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci 32:402–409. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Lee K. (2008) Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov 7:241–254. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Wardle KA. (1994) Constipation evoked by 5-HT3-receptor antagonism: evidence for heterogeneous efficacy among different antagonists in guinea-pigs. J Pharm Pharmacol 46:666–670. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Yoshida M, Yahyah M, Kitazumi K. (2000) Increased defecation during stress or after 5-hydroxytryptophan: selective inhibition by the 5-HT(4) receptor antagonist, SB-207266. Br J Pharmacol 130:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. (2004) Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 14:1100–1109. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. (2013) Expression of α(1)-adrenergic receptors in rat prefrontal cortex: cellular co-localization with 5-HT(2A) receptors. Int J Neuropsychopharmacol 16:1139–1151. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. (1999) Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88:899–915. [DOI] [PubMed] [Google Scholar]
- Sarkisyan G, Hedlund PB. (2009) The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res 202:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, Hedlund PB. (2010) The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res 209:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satała G, Duszyńska B, Lenda T, Nowak G, Bojarski AJ. (2018) Allosteric inhibition of serotonin 5-HT7 receptors by zinc ions. Mol Neurobiol 55:2897–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer P-J, Harris RA, Delarue M. (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Shahsavar A, Delarue M. (2015) Crystallographic studies of pharmacological sites in pentameric ligand-gated ion channels. Biochim Biophys Acta 1850:511–523. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Covasa M. (2007) Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors. Physiol Behav 92:434–442. [DOI] [PubMed] [Google Scholar]
- Savoye G, Brung-Lefebvre M, Bouin M, Maillot C, Denis P, Ducrotté P. (2007) Effects of ondansetron on gastric tone and motility changes induced by a prolonged intraduodenal infusion of nutrients. Results of a placebo-controlled study. Dig Dis Sci 52:2676–2683. [DOI] [PubMed] [Google Scholar]
- Sawant-Basak A, Coffman KJ, Walker GS, Ryder TF, Tseng E, Miller E, Lee C, Vanase-Frawley MA, Wong JW, Brodney MA, et al. (2013) Metabolism of a serotonin-4 receptor partial agonist 4-{4-[4-tetrahydrofuran-3-yloxy)-benzo[d]isoxazol-3-yloxymethyl]-piperidin-1-ylmethyl}-tetrahydropyran-4-ol (TBPT): identification of an unusual pharmacologically active cyclized oxazolidine metabolite in human. J Pharm Sci 102:3277–3293. [DOI] [PubMed] [Google Scholar]
- Saxena PR. (1974) Selective vasoconstriction in carotid vascular bed by methysergide: possible relevance to its antimigraine effect. Eur J Pharmacol 27:99–105. [DOI] [PubMed] [Google Scholar]
- Saxena PR, De Vries P, Heiligers JP, Bax WA, Maassen VanDenBrink A, Yocca FD. (1998) BMS-181885, a 5-HT1B/1D receptor ligand, in experimental models predictive of antimigraine activity and coronary side-effect potential. Eur J Pharmacol 351:329–339. [DOI] [PubMed] [Google Scholar]
- Saxena PR, Villalón CM. (1990) Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol 15 (Suppl 7):S17–S34. [PubMed] [Google Scholar]
- Saxena PR, Villalón CM. (1991) 5-Hydroxytryptamine: a chameleon in the heart. Trends Pharmacol Sci 12:223–227. [DOI] [PubMed] [Google Scholar]
- Scarr E, Pavey G, Copolov D, Dean B. (2004) Hippocampal 5-hydroxytryptamine receptors: abnormalities in postmortem brain from schizophrenic subjects. Schizophr Res 71:383–392. [DOI] [PubMed] [Google Scholar]
- Schaerlinger B, Hickel P, Etienne N, Guesnier L, Maroteaux L. (2003) Agonist actions of dihydroergotamine at 5-HT2B and 5-HT2C receptors and their possible relevance to antimigraine efficacy. Br J Pharmacol 140:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novère N, et al. (2006) Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov 5:845–854. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, Brandt MR, Dawson LA, Cole D, Bernotas R, et al. (2008) Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology 33:1323–1335. [DOI] [PubMed] [Google Scholar]
- Schellekens H, De Francesco PN, Kandil D, Theeuwes WF, McCarthy T, van Oeffelen WE, Perelló M, Giblin L, Dinan TG, Cryan JF. (2015) Ghrelin’s orexigenic effect is modulated via a serotonin 2C receptor interaction. ACS Chem Neurosci 6:1186–1197. [DOI] [PubMed] [Google Scholar]
- Schiller LR, Johnson DA. (2008) Balancing drug risk and benefit: toward refining the process of FDA decisions affecting patient care. Am J Gastroenterol 103:815–819. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Lou Z, Fennell M, Dunlop J. (2004) Ligand dependency of 5-hydroxytryptamine 2C receptor internalization. J Pharmacol Exp Ther 310:865–870. [DOI] [PubMed] [Google Scholar]
- Schlenstedt J, Balfanz S, Baumann A, Blenau W. (2006) Am5-HT7: molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera). J Neurochem 98:1985–1998. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Fink K, Göthert M, Hoyer D, Molderings G, Roschke I, Schoeffter P. (1989) The pharmacological properties of the presynaptic serotonin autoreceptor in the pig brain cortex conform to the 5-HT1D receptor subtype. Naunyn Schmiedebergs Arch Pharmacol 340:45–51. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Zimnisky R, Mehta M, Shapiro LP. (2010) The roles of phospholipase C activation and alternative ADAR1 and ADAR2 pre-mRNA splicing in modulating serotonin 2C-receptor editing in vivo. RNA 16:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. (2010) Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J Neurosci 30:13513–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA 105:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Meltzer HY, Bohn LM. (2014) Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a βarrestin2-independent activation of Akt. Neuropsychopharmacology 39:1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AW, Peroutka SJ. (1989) Three-dimensional steric molecular modeling of the 5-hydroxytryptamine3 receptor pharmacophore. Mol Pharmacol 36:505–511. [PubMed] [Google Scholar]
- Schmitz B, Ullmer C, Segelcke D, Gwarek M, Zhu X-R, Lübbert H. (2015) BF-1--a novel selective 5-HT2B receptor antagonist blocking neurogenic dural plasma protein extravasation in guinea pigs. Eur J Pharmacol 751:73–80. [DOI] [PubMed] [Google Scholar]
- Schmuck K, Ullmer C, Engels P, Lübbert H. (1994) Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett 342:85–90. [DOI] [PubMed] [Google Scholar]
- Schmuck K, Ullmer C, Kalkman HO, Probst A, Lübbert H. (1996) Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache? Eur J Neurosci 8:959–967. [DOI] [PubMed] [Google Scholar]
- Schneider B, Pietri M, Mouillet-Richard S, Ermonval M, Mutel V, Launay JM, Kellermann O. (2006) Control of bioamine metabolism by 5-HT2B and alpha 1D autoreceptors through reactive oxygen species and tumor necrosis factor-alpha signaling in neuronal cells. Ann N Y Acad Sci 1091:123–141. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. (1989a) 5-Hydroxytryptamine 5-HT1B and 5-HT1D receptors mediating inhibition of adenylate cyclase activity. Pharmacological comparison with special reference to the effects of yohimbine, rauwolscine and some beta-adrenoceptor antagonists. Naunyn Schmiedebergs Arch Pharmacol 340:285–292. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. (1989b) How selective is GR 43175? interactions with functional 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol 340:135–138. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. (1989c) Is the sumatriptan (GR 43175)-induced endothelium-dependent relaxation of pig coronary arteries mediated by 5-HT1D receptors? Eur J Pharmacol 166:117–119. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. (1990) 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J Pharmacol Exp Ther 252:387–395. [PubMed] [Google Scholar]
- Schoeffter P, Waeber C, Palacios JM, Hoyer D. (1988) The serotonin 5—HT1D receptor subtype is negatively coupled to adenylate cyclase in calf substantia nigra. Naunyn Schmiedeberg’s Arch Pharmacol 337:602–608. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, Van Gompel P, Lesage AS, De Loore K, Leysen JE. (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 124:57–73. [DOI] [PubMed] [Google Scholar]
- Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, Van Lommel L, Mallet J, Vodjdani G, Gilon P, Binart N, et al. (2010) Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 53:2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Newman-Tancredi A. (2014) Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT(1A) receptor activation. Neurobiol Learn Mem 110:72–80. [DOI] [PubMed] [Google Scholar]
- Schwörer H, Münke H, Stöckmann F, Ramadori G. (1995) Treatment of diarrhea in carcinoid syndrome with ondansetron, tropisetron, and clonidine. Am J Gastroenterol 90:645–648. [PubMed] [Google Scholar]
- Scorza MC, Lladó-Pelfort L, Oller S, Cortés R, Puigdemont D, Portella MJ, Pérez-Egea R, Alvarez E, Celada P, Pérez V, et al. (2012) Preclinical and clinical characterization of the selective 5-HT(1A) receptor antagonist DU-125530 for antidepressant treatment. Br J Pharmacol 167:1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton WJ, Hill LJ, Williams AC, Barnes NM. (2019) Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res 12:1178646919873925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealfon SC, Chi L, Ebersole BJ, Rodic V, Zhang D, Ballesteros JA, Weinstein H. (1995) Related contribution of specific helix 2 and 7 residues to conformational activation of the serotonin 5-HT2A receptor. J Biol Chem 270:16683–16688. [DOI] [PubMed] [Google Scholar]
- Sebben M, Ansanay H, Bockaert J, Dumuis A. (1994) 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport 5:2553–2557. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T. (1998) Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry 3:123–134. [DOI] [PubMed] [Google Scholar]
- Seggel MR, Qureshi GD, Glennon RA. (1987) Effect of 5-HT2-selective agonists on cat platelet aggregation. Life Sci 41:1077–1081. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Cappai A, Howes O. (2014) Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev 45:233–245. [DOI] [PubMed] [Google Scholar]
- Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. (2008) Inactivation of the 5-HT(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry 63:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Bocchio M, Bannerman DM, Sharp T, Capogna M. (2017) Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J Neurosci 37:1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Tsai LH. (2014) Neuronal differentiation: 5-HT6R can do it alone. Nat Chem Biol 10:488–489. [DOI] [PubMed] [Google Scholar]
- Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG, Barnes NM, Gordon J. (2002) 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood 99:2545–2553. [DOI] [PubMed] [Google Scholar]
- Serafeim A, Holder MJ, Grafton G, Chamba A, Drayson MT, Luong QT, Bunce CM, Gregory CD, Barnes NM, Gordon J. (2003) Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood 101:3212–3219. [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortés R. (2005) Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat 29:83–91. [DOI] [PubMed] [Google Scholar]
- Serrats J, Raurich A, Vilaró MT, Mengod G, Cortés R. (2004) 5-ht5B receptor mRNA in the raphe nuclei: coexpression with serotonin transporter. Synapse 51:102–111. [DOI] [PubMed] [Google Scholar]
- Sessa B, Nutt D. (2015) Making a medicine out of MDMA. Br J Psychiatry 206:4–6. [DOI] [PubMed] [Google Scholar]
- Seto SW, Lam HY, Lau WS, Au AL, Lam TY, Chim SS, Ngai SM, Chan SW, Leung TY, Yeung JH, et al. (2009) Role of monoamine oxidases in the exaggerated 5-hydroxytryptamine-induced tension development of human isolated preeclamptic umbilical artery. Eur J Pharmacol 605:129–137 [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. (2003) 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 63:1223–1229. [DOI] [PubMed] [Google Scholar]
- Setola V, Roth BL. (2003) Why mice are neither miniature humans nor small rats: a cautionary tale involving 5-hydroxytryptamine-6 serotonin receptor species variants. Mol Pharmacol 64:1277–1278. [DOI] [PubMed] [Google Scholar]
- Sévoz-Couche C, Spyer KM, Jordan D. (2000) In vivo modulation of vagal-identified dorsal medullary neurones by activation of different 5-Hydroxytryptamine(2) receptors in rats. Br J Pharmacol 131:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Brown S-L, Wetzler S, Kotler M, Molcho A, Plutchik R, van Praag HM. (1994) Effects of alprazolam on increases in hormonal and anxiety levels induced by meta-chlorophenylpiperazine. Psychiatry Res 53:219–229. [DOI] [PubMed] [Google Scholar]
- Sgard F, Faure C, Graham D. (1996) Evidence for 5-HT1D beta but not 5-HT1D alpha receptor subtype expression in canine large coronary arteries and saphenous vein. Cardiovasc Res 31:793–799. [DOI] [PubMed] [Google Scholar]
- Shahid M, Walker GB, Zorn SH, Wong EHF. (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 23:65–73. [DOI] [PubMed] [Google Scholar]
- Shajib MS, Khan WI. (2015) The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 213:561–574. [DOI] [PubMed] [Google Scholar]
- Shams TA, Müller DJ. (2014) Antipsychotic induced weight gain: genetics, epigenetics, and biomarkers reviewed. Curr Psychiatry Rep 16:473. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28:1400–1411. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JR, Davis TL. (2006a) Serotonin-2 (5-HT2) receptor-mediated signal transduction in human ciliary muscle cells: role in ocular hypotension. J Ocul Pharmacol Ther 22:389–401. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, McLaughlin M. (2006b) Human trabecular meshwork cells express functional serotonin-2A (5HT2A) receptors: role in IOP reduction. Investigative Ophthalmology & Visual Science 47:4001–4010. [DOI] [PubMed] [Google Scholar]
- Sharif NA, McLaughlin MA, Kelly CR. (2007) AL-34662: a potent, selective, and efficacious ocular hypotensive serotonin-2 receptor agonist. J Ocul Pharmacol Ther 23:1–13. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Senchyna M. (2006) Serotonin receptor subtype mRNA expression in human ocular tissues, determined by RT-PCR. Mol Vis 12:1040–1047. [PubMed] [Google Scholar]
- Sharp T. (2010) Serotonergic feedback control, in Handbook of the Behavioural Neurobiology of Serotonin (Muller CP, Jacobs B. eds) pp 233–248, Elesevier, London. [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. (2007) Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci 28:629–636. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Grahame-Smith DG. (1989) 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol 96:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley AL, Solomon RA, Fernando AI, da Roza Davis JM, Cowen PJ. (1990) Dose-related effects of selective 5-HT2 receptor antagonists on slow wave sleep in humans. Psychopharmacology (Berl) 101:568–569. [DOI] [PubMed] [Google Scholar]
- Sharpley AL, Vassallo CM, Cowen PJ. (2000) Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry 47:468–470. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, Brüning JC, Roth BL. (2006) p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci USA 103:4717–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon PW, Aghajanian GK. (1991) Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse 9:208–218. [DOI] [PubMed] [Google Scholar]
- Shelton J, Bonaventure P, Li X, Yun S, Lovenberg T, Dugovic C. (2009) 5-HT7 receptor deletion enhances REM sleep suppression induced by selective serotonin reuptake inhibitors, but not by direct stimulation of 5-HT1A receptor. Neuropharmacology 56:448–454. [DOI] [PubMed] [Google Scholar]
- Shelton J, Yun S, Losee Olson S, Turek F, Bonaventure P, Dvorak C, Lovenberg T, Dugovic C. (2015) Selective pharmacological blockade of the 5-HT7 receptor attenuates light and 8-OH-DPAT induced phase shifts of mouse circadian wheel running activity. Front Behav Neurosci 8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Li H, Meller E. (2002) Repeated treatment with antidepressants differentially alters 5-HT1A agonist-stimulated [35S]GTP gamma S binding in rat brain regions. Neuropharmacology 42:1031–1038. [DOI] [PubMed] [Google Scholar]
- Shen JH, Zhao Y, Rosenzweig-Lipson S, Popp D, Williams JB, Giller E, Detke MJ, Kane JM. (2014) A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res 53:14–22. [DOI] [PubMed] [Google Scholar]
- Shen M, Bellaousov S, Hiller M, de La Grange P, Creamer TP, Malina O, Sperling R, Mathews DH, Stoilov P, Stamm S. (2013) Pyrvinium pamoate changes alternative splicing of the serotonin receptor 2C by influencing its RNA structure. Nucleic Acids Res 41:3819–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Andrade R. (1998) 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther 285:805–812. [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268:18200–18204. [PubMed] [Google Scholar]
- Shenker A, Maayani S, Weinstein H, Green JP. (1987) Pharmacological characterization of two 5-hydroxytryptamine receptors coupled to adenylate cyclase in guinea pig hippocampal membranes. Mol Pharmacol 31:357–367. [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. (1994) Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 116:56–64. [DOI] [PubMed] [Google Scholar]
- Shepherd SL, Williamson DJ, Beer MS, Hill RG, Hargreaves RJ. (1997) Differential effects of 5-HT1B/1D receptor agonists on neurogenic dural plasma extravasation and vasodilation in anaesthetized rats. Neuropharmacol 36:525–533. [DOI] [PubMed] [Google Scholar]
- Shepheard S, Edvinsson L, Cumberbatch M, Williamson D, Mason G, Webb J, Boyce S, Hill R, Hargreaves R. (1999) Possible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370. Cephalalgia 19:851–858. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Zensho H, Yamawaki S. (1996) Chronic antidepressant exposure enhances 5-hydroxytryptamine7 receptor-mediated cyclic adenosine monophosphate accumulation in rat frontocortical astrocytes. J Pharmacol Exp Ther 279:1551–1558. [PubMed] [Google Scholar]
- Shimron-Abarbanell D, Erdmann J, Vogt IR, Bryant SP, Spurr NK, Knapp M, Propping P, Nöthen MM. (1997) Human 5-HT5A receptor gene: systematic screening for DNA sequence variation and linkage mapping on chromosome 7q34-q36 using a polymorphism in the 5′ untranslated region. Biochem Biophys Res Commun 233:6–9. [DOI] [PubMed] [Google Scholar]
- Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. (2014) Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther 39:239–253. [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yamauchi Y, Ohtuka K, Kato S. (2006) Use of a serotonin 1A receptor agonist to treat restless legs syndrome. J Clin Psychopharmacol 26:673–675. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Kusunoki H, Ishii M, Imamura H, Manabe N, Kamada T, Hata J, Merchant JL, Haruma K. (2015) Pilot study of Biomarkers for predicting effectiveness of ramosetron in diarrhea-predominant irritable bowel syndrome: expression of S100A10 and polymorphisms of TPH1. Neurogastroenterol Motil 27:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. (2004) Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci 7:699–700. [DOI] [PubMed] [Google Scholar]
- Shum JK, Melendez JA, Jeffrey JJ. (2002) Serotonin-induced MMP-13 production is mediated via phospholipase C, protein kinase C, and ERK1/2 in rat uterine smooth muscle cells. J Biol Chem 277:42830–42840. [DOI] [PubMed] [Google Scholar]
- Sia TC, Whiting M, Kyloh M, Nicholas SJ, Oliver J, Brookes SJ, Dinning PG, Wattchow DA, Spencer NJ. (2013) 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci 7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Xiao R, Childers SR. (1997) In vitro autoradiographic localization of 5-HT1A receptor-activated G-proteins in the rat brain. Brain Res Bull 44:39–45. [DOI] [PubMed] [Google Scholar]
- Simmons M, Meador-Woodruff JH, Sodhi MS. (2010) Increased cortical expression of an RNA editing enzyme occurs in major depressive suicide victims. Neuroreport 21:993–997. [DOI] [PubMed] [Google Scholar]
- Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. (2004) Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem 89:766–775. [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. (1996) Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol 76:799–807. [DOI] [PubMed] [Google Scholar]
- Singh M, Singh MM, Na E, Agassandian K, Zimmerman MB, Johnson AK. (2011) Altered ADAR 2 equilibrium and 5HT(2C) R editing in the prefrontal cortex of ADAR 2 transgenic mice. Genes Brain Behav 10:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, Aley PK, Antoniadou I, Sharp T, Vasudevan SR, et al. (2013) A safe lithium mimetic for bipolar disorder. Nat Commun 4:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Jain NK, Kulkarni SK. (2003) Fluoxetine suppresses morphine tolerance and dependence: modulation of NO-cGMP/DA/serotoninergic pathways. Methods Find Exp Clin Pharmacol 25:273–280. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, et al. (2007) CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52:279–290. [DOI] [PubMed] [Google Scholar]
- Skingle M, Beattie DT, Scopes DI, Starkey SJ, Connor HE, Feniuk W, Tyers MB. (1996) GR127935: a potent and selective 5-HT1D receptor antagonist. Behav Brain Res 73:157–161. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Boess FG, Bös M, Levet-Trafit B, Riemer C, Bourson A. (1998) Characterization of Ro 04-6790 and Ro 63-0563: potent and selective antagonists at human and rat 5-HT6 receptors. Br J Pharmacol 124:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingelstein C, Bourson A. (1995) Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. Mol Pharmacol 47:99–103. [PubMed] [Google Scholar]
- Sleight AJ, Stam NJ, Mutel V, Vanderheyden PM. (1996) Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol 51:71–76. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Wortsman J. (2004) Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim Biophys Acta 1680:67–70. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. (2003) Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol 196:144–153. [DOI] [PubMed] [Google Scholar]
- Smeraldi E. (1998) Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: a double-blind, comparative study. J Affect Disord 48:47–56. [DOI] [PubMed] [Google Scholar]
- Smith AK, Dimulescu I, Falkenberg VR, Narasimhan S, Heim C, Vernon SD, Rajeevan MS. (2008) Genetic evaluation of the serotonergic system in chronic fatigue syndrome. Psychoneuroendocrinology 33:188–197. [DOI] [PubMed] [Google Scholar]
- Smith C, Toohey N, Knight JA, Klein MT, Teitler M. (2011) Risperidone-induced inactivation and clozapine-induced reactivation of rat cortical astrocyte 5-hydroxytryptamine7 receptors: evidence for in situ G protein-coupled receptor homodimer protomer cross-talk. Mol Pharmacol 79:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Raswyck GE, Davidson LD. (2014a) From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethlyamide. J Psychoactive Drugs 46:3–10. [DOI] [PubMed] [Google Scholar]
- Smith MI, Banner SE, Sanger GJ. (1999) 5-HT4 receptor antagonism potentiates inhibition of intestinal allodynia by 5-HT3 receptor antagonism in conscious rats. Neurosci Lett 271:61–64. [DOI] [PubMed] [Google Scholar]
- Smith RM, Papp AC, Webb A, Ruble CL, Munsie LM, Nisenbaum LK, Kleinman JE, Lipska BK, Sadee W. (2013) Multiple regulatory variants modulate expression of 5-hydroxytryptamine 2A receptors in human cortex. Biol Psychiatry 73:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan Me, Anderson CM, Shanahan WR, Group S. (2009) Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity(SilverSpring) 17:494–503. [DOI] [PubMed] [Google Scholar]
- Smith TK, Dickson EJ, Heredia DJ, Hennig GW, Bayguinov PO. (2010) Controversies involving the role of 5-hydroxytryptamine in generating colonic migrating motor complexes: what is spontaneous? Gastroenterology 138:1213–1214, author reply 1214–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Gershon MD. (2015a) CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 593:3225–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Gershon MD. (2015b) Rebuttal from Terence K. Smith and Michael D. Gershon. J Physiol 593:3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Park KJ, Hennig GW. (2014b) Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil 20:423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Neill JC. (2008) Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav Brain Res 191:26–31. [DOI] [PubMed] [Google Scholar]
- Snoeren EM, Veening JG, Olivier B, Oosting RS. (2014a) Serotonin 1A receptors and sexual behavior in male rats: a review. Pharmacol Biochem Behav 121:102–114. [DOI] [PubMed] [Google Scholar]
- Snoeren EM, Veening JG, Olivier B, Oosting RS. (2014b) Serotonin 1A receptors and sexual behavior in female rats: a review. Pharmacol Biochem Behav 121:43–52. [DOI] [PubMed] [Google Scholar]
- Sodhi MSK, Airey DC, Lambert W, Burnet PWJ, Harrison PJ, Sanders-Bush E. (2005) A rapid new assay to detect RNA editing reveals antipsychotic-induced changes in serotonin-2C transcripts. Mol Pharmacol 68:711–719. [DOI] [PubMed] [Google Scholar]
- Söhle J, Machuy N, Smailbegovic E, Holtzmann U, Grönniger E, Wenck H, Stäb F, Winnefeld M. (2012) Identification of new genes involved in human adipogenesis and fat storage. PLoS One 7:e31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. (2011) Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole MJ, Madapallimattam A, Baines, AD (1986) An active pathway for serotonin synthesis by renal proximal tubules. Kidney Int 29:689–694. [DOI] [PubMed] [Google Scholar]
- Soler Artigas, M, Loth, DW, Wain, LV, Gharib, SA, Obeidat, M, Tang, W, Zhai, G, Zhao, JH, Smith, AV, Huffman, JE, et al. International Lung Cancer Consortium; GIANT consortium (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll C, Jang JH, Riener M-O, Moritz W, Wild PJ, Graf R, Clavien P-A. (2010) Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 51:1244–1254. [DOI] [PubMed] [Google Scholar]
- Soll C, Riener M-O, Oberkofler CE, Hellerbrand C, Wild PJ, DeOliveira ML, Clavien P-A. (2012) Expression of serotonin receptors in human hepatocellular cancer. Clin Cancer Res 18:5902–5910. [DOI] [PubMed] [Google Scholar]
- Solt K, Stevens RJ, Davies PA, Raines DE. (2005) General anesthetic-induced channel gating enhancement of 5-hydroxytryptamine type 3 receptors depends on receptor subunit composition. J Pharmacol Exp Ther 315:771–776. [DOI] [PubMed] [Google Scholar]
- Somerville EM, Horwood JM, Lee MD, Kennett GA, Clifton PG. (2007) 5-HT(2C) receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice. Eur J Neurosci 25:3115–3124. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647. [DOI] [PubMed] [Google Scholar]
- Song HR, Gu A, Schanen NC. (1999) Identification of a new polymorphism in the 3′-untranslated region of the human serotonin receptor 2C (5-HT2C) gene. Mol Genet Metab 66:224–227. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, III, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. (1997) Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry 54:749–758. [DOI] [PubMed] [Google Scholar]
- Sparkes RS, Lan N, Klisak I, Mohandas T, Diep A, Kojis T, Heinzmann C, Shih JC. (1991) Assignment of a serotonin 5HT-2 receptor gene (HTR2) to human chromosome 13q14-q21 and mouse chromosome 14. Genomics 9:461–465. [DOI] [PubMed] [Google Scholar]
- Speake T, Kibble JD, Brown PD. (2004) Kv1.1 and Kv1.3 channels contribute to the delayed-rectifying K+ conductance in rat choroid plexus epithelial cells. Am J Physiol Cell Physiol 286:C611–C620. [DOI] [PubMed] [Google Scholar]
- Spedding M, Newman-Tancredi A, Millan MJ, Dacquet C, Michel AN, Jacoby E, Vickery B, Tallentire D. (1998) Interaction of the anxiogenic agent, RS-30199, with 5-HT1A receptors: modulation of sexual activity in the male rat. Neuropharmacology 37:769–780. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. (2015a) CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 593:3229–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. (2015b) Rebuttal from Nick J. Spencer, Tiong Cheng Sia, Simon J Brookes, Marcello Costa and Damien J. Keating. J Physiol 593:3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L, Chambery A, Di Domenico M, Crispino M, Severino V, Volpicelli F, Leopoldo M, Bellenchi GC, di Porzio U, Perrone-Capano C. (2013) The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology 67:155–167. [DOI] [PubMed] [Google Scholar]
- Speranza L, Giuliano T, Volpicelli F, De Stefano ME, Lombardi L, Chambery A, Lacivita E, Leopoldo M, Bellenchi GC, di Porzio U, et al. (2015) Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci 9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L, Labus J, Volpicelli F, Guseva D, Lacivita E, Leopoldo M, Bellenchi GC, di Porzio U, Bijata M, Perrone-Capano C, et al. (2017) Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J Neurochem 141:647–661. [DOI] [PubMed] [Google Scholar]
- Spiller R. (2008a) Serotonin and GI clinical disorders. Neuropharmacology 55:1072–1080. [DOI] [PubMed] [Google Scholar]
- Spiller R. (2008b) Serotonergic agents and the irritable bowel syndrome: what goes wrong? Curr Opin Pharmacol 8:709–714. [DOI] [PubMed] [Google Scholar]
- Spiller RC. (2011) Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol 11:68–74. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Reynolds LS, Braselton JP, Rollema H, Zorn SH. (1999) Comparison of the novel antipsychotic ziprasidone with clozapine and olanzapine: inhibition of dorsal raphe cell firing and the role of 5-HT1A receptor activation. Neuropsychopharmacology 21:622–631. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. (2004) 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 46:52–62. [DOI] [PubMed] [Google Scholar]
- Stacher G, Bergmann H, Schneider C, Steiner-Mittelbach G, Gaupmann G, Steinringer H, Abatzi TA, Stacher-Janotta G. (1990) Effects of the 5-HT3 receptor antagonist ICS 205-930 on fat-delayed gastric emptying and antral motor activity. Br J Clin Pharmacol 30:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. (2015) Mechanism of action of flibanserin, a multifunctional serotonin agonist and antagonist (MSAA), in hypoactive sexual desire disorder. CNS Spectr 20:1–6. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Sommer B, Allers KA. (2011) Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med 8:15–27. [DOI] [PubMed] [Google Scholar]
- Stam NJ, Vanderheyden P, van Alebeek C, Klomp J, de Boer T, van Delft AML, Olijve W. (1994) Genomic organisation and functional expression of the gene encoding the human serotonin 5-HT2C receptor. Eur J Pharmacol 269:339–348. [DOI] [PubMed] [Google Scholar]
- Stam NJ, Van Huizen F, Van Alebeek C, Brands J, Dijkema R, Tonnaer JA, Olijve W. (1992) Genomic organization, coding sequence and functional expression of human 5-HT2 and 5-HT1A receptor genes. Eur J Pharmacol 227:153–162. [DOI] [PubMed] [Google Scholar]
- Staner L, Kempenaers C, Simonnet MP, Fransolet L, Mendlewicz J. (1992) 5-HT2 receptor antagonism and slow-wave sleep in major depression. Acta Psychiatr Scand 86:133–137. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. (2005) 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT(2C), 5-HT(4) and 5-HT(1A) receptors. Neuropharmacology 49:1228–1234. [DOI] [PubMed] [Google Scholar]
- Stanton JA, Beer MS. (1997) Characterisation of a cloned human 5-HT1A receptor cell line using [35S]GTP gamma S binding. Eur J Pharmacol 320:267–275. [DOI] [PubMed] [Google Scholar]
- Stanton JA, Middlemiss DN, Beer MS. (1996) Autoradiographic localization of 5-CT-insensitive 5-HT1-like recognition sites in guinea pig and rat brain. Neuropharmacology 35:223–229. [DOI] [PubMed] [Google Scholar]
- Starr KR, Price GW, Watson JM, Atkinson PJ, Arban R, Melotto S, Dawson LA, Hagan JJ, Upton N, Duxon MS. (2007) SB-649915-B, a novel 5-HT1A/B autoreceptor antagonist and serotonin reuptake inhibitor, is anxiolytic and displays fast onset activity in the rat high light social interaction test. Neuropsychopharmacology 32:2163–2172. [DOI] [PubMed] [Google Scholar]
- Steed E, Jones CA, McCreary AC. (2011) Serotonergic involvement in methamphetamine-induced locomotor activity: a detailed pharmacological study. Behav Brain Res 220:9–19. [DOI] [PubMed] [Google Scholar]
- Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. (2000) mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun 14:219–224. [DOI] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. (2000) 5-HT1A receptor-mediated inhibition and 5-HT2 as well as 5-HT3 receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse 38:328–337. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Miczek KA, Lucion AB, de Almeida RM. (2013) Aggression-reducing effects of F15599, a novel selective 5-HT1A receptor agonist, after microinjection into the ventral orbital prefrontal cortex, but not in infralimbic cortex in male mice. Psychopharmacology (Berl) 230:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens EK, Avesar D, Gulledge AT. (2014) Activity-dependent serotonergic excitation of callosal projection neurons in the mouse prefrontal cortex. Front Neural Circuits 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Rüsch D, Solt K, Raines DE, Davies PA. (2005) Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther 314:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier CT, Jr, Itskovitz HD. (1985) Formation of serotonin by rat kidneys in vivo. Proc Soc Exp Biol Med 180:550–557. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. (2002) Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 1:609–620. [DOI] [PubMed] [Google Scholar]
- Storer RI, Brennan PE, Brown AD, Bungay PJ, Conlon KM, Corbett MS, DePianta RP, Fish PV, Heifetz A, Ho DK, et al. (2014) Multiparameter optimization in CNS drug discovery: design of pyrimido[4,5-d]azepines as potent 5-hydroxytryptamine 2C (5-HT2C) receptor agonists with exquisite functional selectivity over 5-HT2A and 5-HT2B receptors. J Med Chem 57:5258–5269. [DOI] [PubMed] [Google Scholar]
- Stowe RL, Barnes NM. (1998) Selective labelling of 5-HT7 receptor recognition sites in rat brain using [3H]5-carboxamidotryptamine. Neuropharmacology 37:1611–1619. [DOI] [PubMed] [Google Scholar]
- Stoyanova II, Gulubova MV. (2002) Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem 104:185–192. [DOI] [PubMed] [Google Scholar]
- Strachan RT, Sheffler DJ, Willard B, Kinter M, Kiselar JG, Roth BL. (2009) Ribosomal S6 kinase 2 directly phosphorylates the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor, thereby modulating 5-HT2A signaling. J Biol Chem 284:5557–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratz C, Trenk D, Bhatia HS, Valina C, Neumann FJ, Fiebich BL. (2008) Identification of 5-HT3 receptors on human platelets: increased surface immunoreactivity after activation with adenosine diphosphate (ADP) and thrombin receptor-activating peptide (TRAP). Thromb Haemost 99:784–786. [DOI] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN. (2011) 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience 197:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong PV, Greenwood BN, Fleshner M. (2009) The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 203:665–675. [DOI] [PubMed] [Google Scholar]
- Stroth N, Svenningsson P. (2015) S100B interacts with the serotonin 5-HT7 receptor to regulate a depressive-like behavior. Eur Neuropsychopharmacol 25:2372–2380. [DOI] [PubMed] [Google Scholar]
- Stunes AK, Reseland JE, Hauso O, Kidd M, Tømmerås K, Waldum HL, Syversen U, Gustafsson BI. (2011) Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes Metab 13:551–558. [DOI] [PubMed] [Google Scholar]
- Suchanek B, Struppeck H, Fahrig T. (1998) The 5-HT1A receptor agonist BAY x 3702 prevents staurosporine-induced apoptosis. Eur J Pharmacol 355:95–101. [DOI] [PubMed] [Google Scholar]
- Sugai T, Suzuki Y, Sawamura K, Fukui N, Inoue Y, Someya T. (2006) The effect of 5-hydroxytryptamine 3A and 3B receptor genes on nausea induced by paroxetine. Pharmacogenomics J 6:351–356. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takashima N, Noma T, Yamada J. (2005) Inhibitory effects of the 5-HT(1A) receptor agonist buspirone on stress-induced hyperglycemia in mice: involvement of insulin and a buspirone metabolite, 1-(2-pyrimidinyl)piperazine (1-PP). Biol Pharm Bull 28:733–735. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamada J, Horisaka K. (1991) Activation of peripheral serotonin2 receptors induces hypothermia in mice. Life Sci 48:419–423. [DOI] [PubMed] [Google Scholar]
- Sugiuar T, Bielefeldt K, Gebhart GF. (2004) TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci 24:9521–9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukma M, Tohda M, Watanabe H, Matsumoto K. (2005) The mRNA expression differences of RNA editing enzymes in differentiated and undifferentiated NG108-15 cells. J Pharmacol Sci 98:467–470. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Clarke WP, Berg KA. (2015) Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des 21:3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara G, Sumara O, Kim JK, Karsenty G. (2012) Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab 16:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T, Higuchi Y, Matsui M, Arai H, Takamiya C, Meltzer HY, Kurachi M. (2007) Effective adjunctive use of tandospirone with perospirone for enhancing verbal memory and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 31:965–967. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, Jayathilake K, Meltzer HY. (2001a) Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry 158:1722–1725. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, Sumiyoshi S, Sumiyoshi C, Meltzer HY. (2001b) The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol Psychiatry 49:861–868. [DOI] [PubMed] [Google Scholar]
- Sung DJ, Noh HJ, Kim JG, Park SW, Kim B, Cho H, Bae YM. (2013) Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp Mol Med 45:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa B, Bock N, Preusse S, Rothenberger A, Manzke T. (2014) Distribution of serotonin 4(a) receptors in the juvenile rat brain and spinal cord. J Chem Neuroanat 55:67–77. [DOI] [PubMed] [Google Scholar]
- Svejda B, Kidd M, Giovinazzo F, Eltawil K, Gustafsson BI, Pfragner R, Modlin IM. (2010) The 5-HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer 116:2902–2912. [DOI] [PubMed] [Google Scholar]
- Svenningsson P. (2014) Reductions of p11 and 5-HT1B receptor availability in limbic brain regions in cocaine dependence. Biol Psychiatry 76:763–764. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. (2006) Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311:77–80. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Greengard P. (2007) p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol 7:27–32. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. (2013) p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci 14:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, Wictorin K, Keywood C, Shankar B, Lowe DA, Björklund A, Widner H. (2015) Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain 138:963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P. (2002) DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA 99:3188–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P. (2007) Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci 27:4201–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA. (2016) Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience 324:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Caenepeel P, Corsetti M, Janssens J. (2004) Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology 127:1058–1066. [DOI] [PubMed] [Google Scholar]
- Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH, et al. (2012) Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther 35:745–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Quigley E, Camilleri M, Vandeplassche L, Kerstens R. (2013) Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis. United European Gastroenterol J 1:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. (2014) Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil 26:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Van Den Elzen B, Tytgat G, Wajs E, Van Nueten L, De Ridder F, Boeckxstaens G. (2009) A placebo-controlled trial of the 5-HT1A agonist R-137696 on symptoms, visceral hypersensitivity and on impaired accommodation in functional dyspepsia. Neurogastroenterol Motil 21:619–626, e23–e24. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu X, Lynch-Erhardt M, Frisina RD. (2007) Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol Aging 28:1112–1123. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, Miczek KA. (2012) Behavioral and pharmacogenetics of aggressive behavior. Curr Top Behav Neurosci 12:73–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Goto K, Kawahara I. (2014) The 5-hydroxytryptamine 4 receptor agonist-induced actions and enteric neurogenesis in the gut. J Neurogastroenterol Motil 20:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JN, Jutkiewicz EM, Graves SM, Clemans CF, Nicol MR, Mortensen RM, Huang X, Neubig RR, Traynor JR. (2010) RGS inhibition at G(alpha)i2 selectively potentiates 5-HT1A-mediated antidepressant effects. Proc Natl Acad Sci USA 107:11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25:399–410. [DOI] [PubMed] [Google Scholar]
- Talley NJ. (2015) Functional dyspepsia and the Rome criteria: a success story. Neurogastroenterol Motil 27:1052–1056. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Phillips SF, Haddad A, Miller LJ, Twomey C, Zinsmeister AR, MacCarty RL, Ciociola A. (1990) GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci 35:477–480. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Van Zanten SV, Saez LR, Dukes G, Perschy T, Heath M, Kleoudis C, Mangel AW. (2001) A dose-ranging, placebo-controlled, randomized trial of alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther 15:525–537. [DOI] [PubMed] [Google Scholar]
- Tan PZ, Baldwin RM, van Dyck CH, Al Tikriti M, Roth B, Khan N, Charney DS, Innis RB. (1999) Characterization of radioactive metabolites of 5-HT2A receptor PET ligand [18F]altanserin in human and rodent. Nucl Med Biol 26: 601–608. [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA. (1993) Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol 69:1749–1757. [DOI] [PubMed] [Google Scholar]
- Tanaka KF, Samuels BA, Hen R. (2012) Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Philos Trans R Soc Lond B Biol Sci 367:2395–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kobayashi D, Murakami Y, Ozaki N, Suzuki T, Iwata N, Haraguchi K, Ieiri I, Kinukawa N, Hosoi M, et al. (2008a) Genetic polymorphisms in the 5-hydroxytryptamine type 3B receptor gene and paroxetine-induced nausea. Int J Neuropsychopharmacol 11:261–267. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Nakamura E, Ohkura M, Kuwabara M, Yamashita A, Onitsuka T, Asada Y, Hisa H, Yamamoto R. (2008b) Both 5-hydroxytryptamine 5-HT2A and 5-HT1B receptors are involved in the vasoconstrictor response to 5-HT in the human isolated internal thoracic artery. Clin Exp Pharmacol Physiol 35:836–840. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. (2004) Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron 41:215–227. [DOI] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, et al. (2014a) Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Dalton CF, Srisawat U, Zhang ZJ, Reynolds GP. (2014b) Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia. Int J Neuropsychopharmacol 17:645–649. [DOI] [PubMed] [Google Scholar]
- Tang L, Todd RD, Heller A, O’Malley KL. (1994) Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J Pharmacol Exp Ther 268:495–502. [PubMed] [Google Scholar]
- Tao R, Auerbach SB. (1994) Increased extracellular serotonin in rat brain after systemic or intraraphe administration of morphine. J Neurochem 63:517–524. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. (1998) Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther 286:481–488. [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ. (1984) Tardive dyskinesia. Annu Rev Med 35:605–623. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ, Tarazi FI. (2002) Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 16:23–45. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271. [DOI] [PubMed] [Google Scholar]
- Tassone A, Madeo G, Schirinzi T, Vita D, Puglisi F, Ponterio G, Borsini F, Pisani A, Bonsi P. (2011) Activation of 5-HT6 receptors inhibits corticostriatal glutamatergic transmission. Neuropharmacology 61:632–637. [DOI] [PubMed] [Google Scholar]
- Tavares AA, Caillé F, Barret O, Papin C, Lee H, Morley TJ, Fowles K, Holden D, Seibyl JP, Alagille D, et al. (2014) In vivo evaluation of 18F-MNI698: an 18F-labeled radiotracer for imaging of serotonin 4 receptors in brain. J Nucl Med 55:858–864. [DOI] [PubMed] [Google Scholar]
- Taylor DP, Eison MS, Riblet LA, Vandermaelen CP. (1985) Pharmacological and clinical effects of buspirone. Pharmacol Biochem Behav 23:687–694. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Tandon R, Shipley JE, Eiser AS. (1991) Effect of neuroleptic treatment on polysomnographic measures in schizophrenia. Biol Psychiatry 30:904–912. [DOI] [PubMed] [Google Scholar]
- Teal P, Davis S, Hacke W, Kaste M, Lyden PD, Fierus M, Modified Randomized Exposure Controlled Trial Study Investigators; Bayer HealthCare AG (2009) A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, tolerability, and pharmacokinetic/pharmacodynamic effects of a targeted exposure of intravenous repinotan in patients with acute ischemic stroke: modified Randomized Exposure Controlled Trial (mRECT). Stroke 40:3518–3525. [DOI] [PubMed] [Google Scholar]
- Tecott L, Brennan TJ. (2000) Serotonin 5-HT6 receptor knockout mouse. The Regents of the University of California (Oakland, CA), United States, 6060642. [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. (1995) Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542–546. [DOI] [PubMed] [Google Scholar]
- Teitler M, Klein MT. (2012) A new approach for studying GPCR dimers: drug-induced inactivation and reactivation to reveal GPCR dimer function in vitro, in primary culture, and in vivo. Pharmacol Ther 133:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitler M, Toohey N, Knight JA, Klein MT, Smith C. (2010) Clozapine and other competitive antagonists reactivate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl) 212:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. (2011) SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman LA, Reynolds GP, Arranz B, San L. (2005) Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics 15:195–200. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Chabot C, Barnouin MC, Perrault G, Depoortere R, Griebel G, Scatton B. (2005) SSR181507, a dopamine D(2) receptor antagonist and 5-HT(1A) receptor agonist, alleviates disturbances of novelty discrimination in a social context in rats, a putative model of selective attention deficit. Psychopharmacology (Berl) 181:134–144. [DOI] [PubMed] [Google Scholar]
- Terrón JA. (1996) The relaxant 5-HT receptor in the dog coronary artery smooth muscle: pharmacological resemblance to the cloned 5-ht7 receptor subtype. Br J Pharmacol 118:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrón JA, Bouchelet I, Hamel E. (2001) 5-HT7 receptor mRNA expression in human trigeminal ganglia. Neurosci Lett 302:9–12. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Jackson WJ, Prendergast MA, Fontana DJ, Wong EH, Bonhaus DW, Weller P, Eglen RM. (1998) Enhanced delayed matching performance in younger and older macaques administered the 5-HT4 receptor agonist, RS 17017. Psychopharmacology (Berl) 135:407–415. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Pimenova AA, Lo AC, Ciesielska M, Lichtenthaler SF, De Maeyer JH, Schuurkes JA, D’Hooge R, De Strooper B. (2013) Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol Aging 34:1779–1789. [DOI] [PubMed] [Google Scholar]
- Tian MK, Schmidt EF, Lambe EK. (2016) Serotonergic suppression of mouse prefrontal circuits implicated in task attention. eNeuro 3:ENEURO.0269-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharayil VS, Wouters MM, Stanich JE, Roeder JL, Lei S, Beyder A, Gomez-Pinilla PJ, Gershon MD, Maroteaux L, Gibbons SJ, et al. (2010) Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil 22:462–469, e109–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen FM, Hinney A, Brömel T, Heinzel-Gutenbrunner M, Martin M, Krieg JC, Remschmidt H, Hebebrand J. (2004) Lack of association between the -759C/T polymorphism of the 5-HT2C receptor gene and clozapine-induced weight gain among German schizophrenic individuals. Psychiatr Genet 14:139–142. [DOI] [PubMed] [Google Scholar]
- Thomas DR. (2006) 5-ht5A receptors as a therapeutic target. Pharmacol Ther 111:707–714. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Atkinson PJ, Hastie PG, Roberts JC, Middlemiss DN, Price GW. (2002) [3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology 42:74–81. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Hagan JJ. (2004) 5-HT7 receptors. Curr Drug Targets CNS Neurol Disord 3:81–90. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Larminie CG, Lyons HR, Fosberry A, Hill MJ, Hayes PD. (2004) Cloning and pharmacological characterisation of the guinea pig 5-ht5A receptor. Eur J Pharmacol 494:91–99. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, Deeks NJ, Eddershaw PJ, Fenwick SH, Riley G, et al. (2003) SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol 139:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Soffin EM, Roberts C, Kew JNC, de la Flor RM, Dawson LA, Fry VA, Coggon SA, Faedo S, Hayes PD, et al. (2006) SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4′-[(2-phenylethyl)amino]methyl-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology 51:566–577. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Carson MJ, Neal MJ, Sutcliffe JG. (1997a) Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc Natl Acad Sci USA 94:14115–14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Carson MJ, Sutcliffe JG. (1998) Oleamide-induced modulation of 5-hydroxytryptamine receptor-mediated signaling. Ann N Y Acad Sci 861:183–189. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Cravatt BF, Danielson PE, Gilula NB, Sutcliffe JG. (1997b) Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. J Neurosci Res 50:1047–1052. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Cravatt BF, Sutcliffe JG. (1999) The endogenous lipid oleamide activates serotonin 5-HT7 neurons in mouse thalamus and hypothalamus. J Neurochem 72:2370–2378. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Matli JR, Hu JL, Carson MJ, Sutcliffe JG. (2000) Pertussis toxin treatment prevents 5-HT(5a) receptor-mediated inhibition of cyclic AMP accumulation in rat C6 glioma cells. J Neurosci Res 61:75–81. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Dourish CT, Tomlinson JW, Hassan-Smith Z, Higgs S. (2014) Effects of the 5-HT2C receptor agonist meta-chlorophenylpiperazine on appetite, food intake and emotional processing in healthy volunteers. Psychopharmacology (Berl) 231:2449–2459. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Duke RK, Lummis SC. (2011a) Binding sites for bilobalide, diltiazem, ginkgolide, and picrotoxinin at the 5-HT3 receptor. Mol Pharmacol 80:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SC. (2010) The structural basis of function in Cys-loop receptors. Q Rev Biophys 43:449–499. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. (2003) A single ring of charged amino acids at one end of the pore can control ion selectivity in the 5-HT3 receptor. Br J Pharmacol 140:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. (2007) The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets 11:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SCR. (2013) Discriminating between 5-HT3A and 5-HT3AB receptors. Br J Pharmacol 169:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Price KL, Lummis SC. (2011b) Cysteine modification reveals which subunits form the ligand binding site in human heteromeric 5-HT3AB receptors. J Physiol 589:4243–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Sullivan NL, Lummis SC. (2006) Characterization of 5-HT3 receptor mutations identified in schizophrenic patients. J Mol Neurosci 30:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587. [DOI] [PubMed] [Google Scholar]
- Thorp J, Simon J, Dattani D, Taylor L, Kimura T, Garcia M, Jr, Lesko L, Pyke R, DAISY trial investigators (2012) Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med 9:793–804. [DOI] [PubMed] [Google Scholar]
- Tian Y, Graf R, El-Badry AM, Lesurtel M, Furrer K, Moritz W, Clavien P-A. (2011) Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology 53:253–262. [DOI] [PubMed] [Google Scholar]
- Tiniakov R, Scrogin KE. (2009) The spleen is required for 5-HT1A receptor agonist-mediated increases in mean circulatory filling pressure during hemorrhagic shock in the rat. Am J Physiol Regul Integr Comp Physiol 296:R1392–R1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeler M, Herrick K, Lyon RA, McKenney JD, Glennon RA. (1985) [3H]DOB: a specific agonist radioligand for 5-HT2 serotonin receptors. Eur J Pharmacol 117:145–146. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Davis KH, Glennon RA. (1987) Selectivity of serotonergic drugs for multiple brain serotonin receptors. Role of [3H]-4-bromo-2,5-dimethoxyphenylisopropylamine ([3H]DOB), a 5-HT2 agonist radioligand. Biochem Pharmacol 36:3265–3271. [DOI] [PubMed] [Google Scholar]
- To ZP, Bonhaus DW, Eglen RM, Jakeman LB. (1995) Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br J Pharmacol 115:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Ciaranello RD. (1985) Demonstration of inter- and intraspecies differences in serotonin binding sites by antibodies from an autistic child. Proc Natl Acad Sci USA 82:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohda M, Sukma M, Watanabe H. (2004) RNA editing and short variant of serotonin 2C receptor mRNA in neuronally differentiated NG108-15 cells. J Pharmacol Sci 96:164–169. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. (2002) An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav 71:735–744. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chandrasekaran A, DeMaio W, Jordan R, Li H, Moore R, Poola N, Burghart P, Hultin T, Scatina J. (2010) Species differences in the formation of vabicaserin carbamoyl glucuronide. Drug Metab Dispos 38:581–590. [DOI] [PubMed] [Google Scholar]
- Tonini M, Galligan JJ, North RA. (1989) Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology 96:1257–1264. [DOI] [PubMed] [Google Scholar]
- Tonini M, Messori E, Franceschetti GP, Rizzi CA, Castoldi AF, Coccini T, Candura SM. (1994) Characterization of the 5-HT receptor potentiating neuromuscular cholinergic transmission in strips of human isolated detrusor muscle. Br J Pharmacol 113:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini M, Vicini R, Cervio E, De Ponti F, De Giorgio R, Barbara G, Stanghellini V, Dellabianca A, Sternini C. (2005) 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology 129:1557–1566. [DOI] [PubMed] [Google Scholar]
- Torrecilla I, Tobin AB. (2006) Co-ordinated covalent modification of G-protein coupled receptors. Curr Pharm Des 12:1797–1808. [DOI] [PubMed] [Google Scholar]
- Toth M, Ding D, Shenk T. (1994) The 5′ flanking region of the serotonin 2 receptor gene directs brain specific expression in transgenic animals. Brain Res Mol Brain Res 27:315–319. [DOI] [PubMed] [Google Scholar]
- Tournois C, Mutel V, Manivet P, Launay JM, Kellermann O. (1998) Cross-talk between 5-hydroxytryptamine receptors in a serotonergic cell line. Involvement of arachidonic acid metabolism. J Biol Chem 273:17498–17503. [DOI] [PubMed] [Google Scholar]
- Traber J, Glaser T. (1987) 5-HT1A receptor-related anxiolytics. Trends Pharmacol Sci 8:432–437. [Google Scholar]
- Trattnig SM, Harpsøe K, Thygesen SB, Rahr LM, Ahring PK, Balle T, Jensen AA. (2012) Discovery of a novel allosteric modulator of 5-HT3 receptors: inhibition and potentiation of Cys-loop receptor signaling through a conserved transmembrane intersubunit site. J Biol Chem 287:25241–25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay PB, Kaiser R, Sezer O, Rosler N, Schelenz C, Possinger K, Roots I, Brockmoller J. (2003) Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol 21:2147–2155. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Yu YW, Lin CH. (2002) -759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet 360:1790. [DOI] [PubMed] [Google Scholar]
- Tschentscher F, Hüsing J, Hölter T, Kruse E, Dresen IG, Jöckel KH, Anastassiou G, Schilling H, Bornfeld N, Horsthemke B, et al. (2003) Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res 63:2578–2584. [PubMed] [Google Scholar]
- Tsetlin V, Hucho F. (2009) Nicotinic acetylcholine receptors at atomic resolution. Curr Opin Pharmacol 9:306–310. [DOI] [PubMed] [Google Scholar]
- Tsou AP, Kosaka A, Bach C, Zuppan P, Yee C, Tom L, Alvarez R, Ramsey S, Bonhaus DW, Stefanich E, et al. (1994) Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylyl cyclase. J Neurochem 63:456–464. [DOI] [PubMed] [Google Scholar]
- Tsuji D, Kim YI, Nakamichi H, Daimon T, Suwa K, Iwabe Y, Hayashi H, Inoue K, Yoshida M, Itoh K. (2013) Association of ABCB1 polymorphisms with the antiemetic efficacy of granisetron plus dexamethasone in breast cancer patients. Drug Metab Pharmacokinet 28:299–304. [DOI] [PubMed] [Google Scholar]
- Tuladhar BR, Ge L, Naylor RJ. (2003) 5-HT7 receptors mediate the inhibitory effect of 5-HT on peristalsis in the isolated guinea-pig ileum. Br J Pharmacol 138:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JH, Garnovskaya MN, Coaxum SD, Vlasova TM, Yakutovich M, Lefler DM, Raymond JR. (2007) Ca2+-calmodulin and janus kinase 2 are required for activation of sodium-proton exchange by the Gi-coupled 5-hydroxytryptamine 1a receptor. J Pharmacol Exp Ther 320:314–322. [DOI] [PubMed] [Google Scholar]
- Turner JH, Gelasco AK, Raymond JR. (2004) Calmodulin interacts with the third intracellular loop of the serotonin 5-hydroxytryptamine1A receptor at two distinct sites: putative role in receptor phosphorylation by protein kinase C. J Biol Chem 279:17027–17037. [DOI] [PubMed] [Google Scholar]
- Turner JH, Raymond JR. (2005) Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem 280:30741–30750. [DOI] [PubMed] [Google Scholar]
- Turner JT, Sullivan DM, Rovira I, Camden JM. (1996) A regulatory role in mammalian salivary glands for 5-hydroxytryptamine receptors coupled to increased cyclic AMP production. J Dent Res 75:935–941. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Page IH. (1953) Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol 175:157–161. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, von Zastrow M, Yudowski GA. (2010) Beta-blocker drugs mediate calcium signaling in native central nervous system neurons by beta-arrestin-biased agonism. Proc Natl Acad Sci USA 107:21028–21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvetkov MV, Meineke C, Oetjen E, Hirsch-Ernst K, Brockmöller J. (2007) Tissue-specific alternative promoters of the serotonin receptor gene HTR3B in human brain and intestine. Gene 386:52–62. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. [DOI] [PubMed] [Google Scholar]
- Ulens C, Spurny R, Thompson AJ, Alqazzaz M, Debaveye S, Han L, Price K, Villalgordo JM, Tresadern G, Lynch JW, et al. (2014) The prokaryote ligand-gated ion channel ELIC captured in a pore blocker-bound conformation by the Alzheimer’s disease drug memantine. Structure 22:1399–1407. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Boddeke HG, Schmuck K, Lübbert H. (1996a) 5-HT2B receptor-mediated calcium release from ryanodine-sensitive intracellular stores in human pulmonary artery endothelial cells. Br J Pharmacol 117:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C, Engels P, Abdel’Al S, Lübbert H. (1996b) Distribution of 5-HT4 receptor mRNA in the rat brain. Naunyn Schmiedebergs Arch Pharmacol 354:210–212. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Figge A, Lübbert H. (1998) Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett 424:63–68. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Kalkman HO, Lübbert H. (1995) Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett 370:215–221. [DOI] [PubMed] [Google Scholar]
- Ulugol A, Oltulu C, Gunduz O, Citak C, Carrara R, Shaqaqi MR, Sanchez AM, Dogrul A. (2012) 5-HT7 receptor activation attenuates thermal hyperalgesia in streptozocin-induced diabetic mice. Pharmacol Biochem Behav 102:344–348. [DOI] [PubMed] [Google Scholar]
- Unett DJ, Gatlin J, Anthony TL, Buzard DJ, Chang S, Chen C, Chen X, Dang HT-M, Frazer J, Le MK, et al. (2013) Kinetics of 5-HT2B receptor signaling: profound agonist-dependent effects on signaling onset and duration. J Pharmacol Exp Ther 347:645–659. [DOI] [PubMed] [Google Scholar]
- Unsworth CD, Molinoff PB. (1994) Characterization of a 5-hydroxytryptamine receptor in mouse neuroblastoma N18TG2 cells. J Pharmacol Exp Ther 269:246–255. [PubMed] [Google Scholar]
- Upton N, Chuang TT, Hunter AJ, Virley DJ. (2008) 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics 5:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton N, Stean T, Middlemiss D, Blackburn T, Kennett G. (1998) Studies on the role of 5-HT2C and 5-HT2B receptors in regulating generalised seizure threshold in rodents. Eur J Pharmacol 359:33–40. [DOI] [PubMed] [Google Scholar]
- Urbina M, Arroyo R, Lima L. (2014) 5-HT7 receptors and tryptophan hydroxylase in lymphocytes of rats: mitogen activation, physical restraint or treatment with reserpine. Neuroimmunomodulation 21:240–249. [DOI] [PubMed] [Google Scholar]
- Urtikova N, Berson N, Van Steenwinckel J, Doly S, Truchetto J, Maroteaux L, Pohl M, Conrath M. (2012) Antinociceptive effect of peripheral serotonin 5-HT2B receptor activation on neuropathic pain. Pain 153:1320–1331. [DOI] [PubMed] [Google Scholar]
- Valdivia LF, Centurión D, Arulmani U, Saxena PR, Villalón CM. (2004) 5-HT1B receptors, alpha2A/2C- and, to a lesser extent, alpha1-adrenoceptors mediate the external carotid vasoconstriction to ergotamine in vagosympathectomised dogs. Naunyn Schmiedebergs Arch Pharmacol 370:46–53. [DOI] [PubMed] [Google Scholar]
- Valdizán EM, Castro E, Pazos A. (2010) Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuropsychopharmacol 13:835–843. [DOI] [PubMed] [Google Scholar]
- Valenti D, de Bari L, Vigli D, Lacivita E, Leopoldo M, Laviola G, Vacca RA, De Filippis B. (2017) Stimulation of the brain serotonin receptor 7 rescues mitochondrial dysfunction in female mice from two models of Rett syndrome. Neuropharmacology 121:79–88. [DOI] [PubMed] [Google Scholar]
- Valentin N, Acosta A, Camilleri M. (2015) Early investigational therapeutics for gastrointestinal motility disorders: from animal studies to Phase II trials. Expert Opin Investig Drugs 24:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini V, Piras G, De Luca MA, Perra V, Bordi F, Borsini F, Frau R, Di Chiara G. (2013) Evidence for a role of a dopamine/5-HT6 receptor interaction in cocaine reinforcement. Neuropharmacology 65:58–64. [DOI] [PubMed] [Google Scholar]
- Valic M, Pecotic R, Dogas Z. (2008) Phrenic nerve activity is enhanced by 5-HT1A receptor agonist 8-OH-DPAT in spontaneously breathing anesthetized rats. J Physiol Pharmacol 59:17–25. [PubMed] [Google Scholar]
- van de Kar LD, Lorens SA. (1979) Differential serotonergic innervation of individual hypothalamic nuclei and other forebrain regions by the dorsal and median midbrain raphe nuclei. Brain Res 162:45–54. [DOI] [PubMed] [Google Scholar]
- van den Broek RW, Bhalla P, VanDenBrink AM, de Vries R, Sharma HS, Saxena PR. (2002) Characterization of sumatriptan-induced contractions in human isolated blood vessels using selective 5-HT(1B) and 5-HT(1D) receptor antagonists and in situ hybridization. Cephalalgia 22:83–93. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. (1983) Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res 289:109–119. [DOI] [PubMed] [Google Scholar]
- Vane JR. (1957) A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother 12:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Schetters D, Schoffelmeer AN, De Vries TJ. (2010) 5-HT6 antagonism attenuates cue-induced relapse to cocaine seeking without affecting cocaine reinforcement. Int J Neuropsychopharmacol 13:961–965. [DOI] [PubMed] [Google Scholar]
- van Gils W, Lodder EM, Mensink HW, Kiliç E, Naus NC, Brüggenwirth HT, van Ijcken W, Paridaens D, Luyten GP, de Klein A. (2008) Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis. Invest Ophthalmol Vis Sci 49:4254–4262. [DOI] [PubMed] [Google Scholar]
- van Goethem NP, Schreiber R, Newman-Tancredi A, Varney M, Prickaerts J. (2015) Divergent effects of the ‘biased’ 5-HT1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br J Pharmacol 172:2532–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel H, Vanuytsel T, Van Oudenhove L, Farré R, Verbeke K, Tack J. (2013) Postprandial symptoms originating from the stomach in functional dyspepsia. Neurogastroenterol Motil 25:911-e703. [DOI] [PubMed] [Google Scholar]
- Van Lelyveld N, Ter Linde J, Baron A, Mundt M, Wajs E, Samsom M. (2006) The 5-HT4 antagonist R216073 does not affect gastric motor and sensory function in patients with functional dyspepsia. Aliment Pharmacol Ther 24:669–677. [DOI] [PubMed] [Google Scholar]
- van Lelyveld N, Ter Linde J, Schipper M, Samsom M. (2008) Serotonergic signalling in the stomach and duodenum of patients with gastroparesis. Neurogastroenterol Motil 20:448–455. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E, Vouret-Craviari V, Haslam RJ, Chambard JC, Pouysségur J. (1991) Cloning, functional expression and role in cell growth regulation of a hamster 5-HT2 receptor subtype. Mol Endocrinol 5:881–889. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, et al. (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910–918. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Hall H, Bonaventure P, Sedvall G. (2001) Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [(3)H]GR 125743. Brain Res 915:47–57. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Thomas DR, Tupala E, Tiihonen J, Hall H. (2004) Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett 367:313–316. [DOI] [PubMed] [Google Scholar]
- Varrault A, Bockaert J. (1992) Differential coupling of 5-HT1A receptors occupied by 5-HT or 8-OH-DPAT to adenylyl cyclase. Naunyn Schmiedebergs Arch Pharmacol 346:367–374. [DOI] [PubMed] [Google Scholar]
- Vázquez-Borsetti P, Cortés R, Artigas F. (2009) Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex 19:1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero JL, Vizuete ML, Machado A, Cano J. (1997) Developmental expression of 5-HT7 receptor mRNA in rat brain visual structures after neonatal enucleation. Neuroreport 8:1531–1535. [DOI] [PubMed] [Google Scholar]
- Venzi M, David F, Bellet J, Cavaccini A, Bombardi C, Crunelli V, Di Giovanni G. (2016) Role for serotonin2A (5-HT2A) and 2C (5-HT2C) receptors in experimental absence seizures. Neuropharmacology 108:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergé D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. (1986) Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci 6:3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen R, Hundeshagen AG, Brown AM, Schindler M, Kaumann AJ. (1998) 5-HT1B receptor-mediated contractions in human temporal artery: evidence from selective antagonists and 5-HT receptor mRNA expression. Br J Pharmacol 124:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen R, Meier A, Werner I, Wienekamp A, Kruschat T, Brattelid T, Levy FO, Kaumann A. (2004) Functional 5-HT receptors in human occipital artery. Naunyn Schmiedebergs Arch Pharmacol 369:391–401. [DOI] [PubMed] [Google Scholar]
- Verheij MH, Thompson AJ, van Muijlwijk-Koezen JE, Lummis SC, Leurs R, de Esch IJ. (2012) Design, synthesis, and structure-activity relationships of highly potent 5-HT3 receptor ligands. J Med Chem 55:8603–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstad AA, Jonsson G. (1983) Immunohistochemical and neurochemical evidence for the presence of serotonin in the adrenal medulla of the rat. Neuroscience 10:1443–1453. [DOI] [PubMed] [Google Scholar]
- Verma S, Cikurel K, Koralnik IJ, Morgello S, Cunningham-Rundles C, Weinstein ZR, Bergmann C, Simpson DM. (2007) Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis 196:709–711. [DOI] [PubMed] [Google Scholar]
- Veselinović T, Paulzen M, Gründer G. (2013) Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother 13:1141–1159. [DOI] [PubMed] [Google Scholar]
- Vialli M, Erspamer V. (1933) Cellule enterocromaffine e cellule basigranulose acidofile nei vertebrati. Z Zellforsch Mikroskop Anat 19:743–749. [Google Scholar]
- Vianna DM, Carrive P. (2009) Inhibition of the cardiovascular response to stress by systemic 5-HT1A activation: sympathoinhibition or anxiolysis? Am J Physiol Regul Integr Comp Physiol 297:R495–R501. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. (1999) Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 143:309–314. [DOI] [PubMed] [Google Scholar]
- Vidal BFS, Billard T, Newman-Tancredi A, Zimmer L. (2014a) Radiopharmacological evaluation of [18F]F13640, a novel 5-HT1A receptor agonist. J Nucl Med 55:1100. [Google Scholar]
- Vidal R, Castro E, Pilar-Cuéllar F, Pascual-Brazo J, Díaz A, Rojo ML, Linge R, Martín A, Valdizán EM, Pazos A. (2014b) Serotonin 5-HT4 receptors: a new strategy for developing fast acting antidepressants? Curr Pharm Des 20:3751–3762. [DOI] [PubMed] [Google Scholar]
- Viguier F, Michot B, Hamon M, Bourgoin S. (2013) Multiple roles of serotonin in pain control mechanisms--implications of 5-HT7 and other 5-HT receptor types. Eur J Pharmacol 716:8–16. [DOI] [PubMed] [Google Scholar]
- Viguier F, Michot B, Kayser V, Bernard JF, Vela JM, Hamon M, Bourgoin S. (2012) GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology 63:1093–1106. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Cortés R, Gerald C, Branchek TA, Palacios JM, Mengod G. (1996) Localization of 5-HT4 receptor mRNA in rat brain by in situ hybridization histochemistry. Brain Res Mol Brain Res 43:356–360. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Doménech T, Palacios JM, Mengod G. (2002) Cloning and characterization of a novel human 5-HT4 receptor variant that lacks the alternatively spliced carboxy terminal exon. RT-PCR distribution in human brain and periphery of multiple 5-HT4 receptor variants. Neuropharmacology 42:60–73. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. (2005) Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci 22:1120–1126. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Foehring RC, Lee JC, Andrade R. (2011) Essential role for phosphatidylinositol 4,5-bisphosphate in the expression, regulation, and gating of the slow afterhyperpolarization current in the cerebral cortex. J Neurosci 31:18303–18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Centurión D. (2007) Cardiovascular responses produced by 5-hydroxytryptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn—Schmiedeberg’s Arch Pharmacol 376:45–63. [DOI] [PubMed] [Google Scholar]
- Villalón CM, Centurión D, Luján-Estrada M, Terrón JA, Sánchez-López A. (1997a) Mediation of 5-HT-induced external carotid vasodilatation in GR 127935-pretreated vagosympathectomized dogs by the putative 5-HT7 receptor. Br J Pharmacol 120:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Centurión D, Sánchez-López A, De Vries P, Saxena PR. (1999) 5—HT receptors mediating external carotid vasoconstriction in vagosympathectomized dogs. Acta Pharmacol. Sin 20:1057–1067. [PubMed] [Google Scholar]
- Villalón CM, Centurión D, Rabelo G, De Vries P, Saxena PR, Sánchez—López A. (1998) The 5—HT1—like receptors mediating inhibition of sympathetic vasopressor outflow in the pithed rat: operational correlation with the 5—HT1A, 5—HT1B and 5—HT1D subtypes. Brit J Pharmacol 124:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Centurión D, Valdivia LF, de Vries P, Saxena PR. (2003) Migraine: an updated review on pathophysiology, pharmacology, therapy and future trends. Curr Vasc Pharmacol 1:71–84. [DOI] [PubMed] [Google Scholar]
- Villalón CM, den Boer MO, Heiligers JP, Saxena PR. (1990) Mediation of 5-hydroxytryptamine-induced tachycardia in the pig by the putative 5-HT4 receptor. Br J Pharmacol 100:665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, den Boer MO, Heiligers JP, Saxena PR. (1991) Further characterization, by use of tryptamine and benzamide derivatives, of the putative 5-HT4 receptor mediating tachycardia in the pig. Br J Pharmacol 102:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Heiligers JP, Centurión D, De Vries P, Saxena PR. (1997b) Characterization of putative 5-HT7 receptors mediating tachycardia in the cat. Br J Pharmacol 121:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Olesen J. (2009) The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther 124:309–323. [DOI] [PubMed] [Google Scholar]
- Villalón CM, Ramírez—San Juan E, Castillo C, López—Muñoz FJ, Terrón JA. (1995) Pharmacological profile of the receptors that mediate external carotid vasoconstriction by 5—HT in vagosympathectomized dogs. Br J Pharmacol 116:2778–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón CM, Sánchez-López A, Centurión D, Saxena PR. (2001) Unravelling the pharmacological profile of the canine external carotid vasodilator ‘5-HT1-like’ receptors: coexistence of sympatho-inhibitory 5-HT1B and postjunctional 5-HT7 receptors. Naunyn Schmiedebergs Arch Pharmacol 363:73–80. [DOI] [PubMed] [Google Scholar]
- Viñals X, Moreno E, Lanfumey L, Cordomí A, Pastor A, de La Torre R, Gasperini P, Navarro G, Howell LA, Pardo L, et al. (2015) Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol 13:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, Tufik S, Pereira SI, Zanata SM, Ferraz AC. (2012) The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology 62:184–191. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Niebert M, Bischoff AM, Hülsmann S, Manzke T. (2018) Persistent expression of serotonin receptor 5b alters breathing behavior in male MeCP2 knockout mice. Front Mol Neurosci 11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang S, Niebert S, Renner U, Möbius W, Hülsmann S, Manzke T, Niebert M. (2017) Analysis of the serotonergic system in a mouse model of Rett syndrome reveals unusual upregulation of serotonin receptor 5b. Front Mol Neurosci 10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogrig A, Dorr L, Bouzidi N, Boucherle B, Wattiez AS, Cassier E, Vallon G, Ripoche I, Abrunhosa-Thomas I, Marin P, et al. (2013) Structure-based design of PDZ ligands as inhibitors of 5-HT(2A) receptor/PSD-95 PDZ1 domain interaction possessing anti-hyperalgesic activity. ACS Chem Biol 8:2209–2216. [DOI] [PubMed] [Google Scholar]
- Voigt JP, Fink H. (2015) Serotonin controlling feeding and satiety. Behav Brain Res 277:14–31. [DOI] [PubMed] [Google Scholar]
- Voigt MM, Laurie DJ, Seeburg PH, Bach A. (1991) Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J 10:4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. (2012) Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci 11:1–24. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. (2010) Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays 32:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. (1993) Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med 329:1073–1078. [DOI] [PubMed] [Google Scholar]
- von der Ohe MR, Hanson RB, Camilleri M. (1994) Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut 35:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mentzer B, Murata Y, Ahlstedt I, Lindström E, Martínez V. (2007) Functional CRF receptors in BON cells stimulate serotonin release. Biochem Pharmacol 73:805–813. [DOI] [PubMed] [Google Scholar]
- Vysokanov A, Flores-Hernandez J, Surmeier DJ. (1998) mRNAs for clozapine-sensitive receptors co-localize in rat prefrontal cortex neurons. Neurosci Lett 258:179–182. [DOI] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, Roth BL. (2017a) How ligands illuminate GPCR molecular pharmacology. Cell 170:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, et al. (2013) Structural features for functional selectivity at serotonin receptors. Science 340:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, et al. (2017b) Crystal structure of an LSD-bound human serotonin receptor. Cell 168:377–389.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JM, Juneja P, MacKay AW, Graham J, Havel PJ, Tecott LH, Goulding EH. (2008) Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology 149:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. (1996) Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16:2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR, Westfall JA. (1985) Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res 241:557–563. [DOI] [PubMed] [Google Scholar]
- Waeber C, Dietl MM, Hoyer D, Probst A, Palacios JM. (1988a) Visualization of a novel serotonin recognition site (5-HT1D) in the human brain by autoradiography. Neurosci Lett 88:11–16. [DOI] [PubMed] [Google Scholar]
- Waeber C, Grailhe R, Yu XJ, Hen R, Moskowitz MA. (1998) Putative 5-ht5 receptors: localization in the mouse CNS and lack of effect in the inhibition of dural protein extravasation. Ann N Y Acad Sci 861:85–90. [DOI] [PubMed] [Google Scholar]
- Waeber C, Hoyer D, Palacios JM. (1989a) GR 43175: a preferential 5-HT1D agent in monkey and human 1rains as shown by autoradiography. Synapse 4:168–170. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. (1995a) Autoradiographic visualisation of [3H]5-carboxamidotryptamine binding sites in the guinea pig and rat brain. Eur J Pharmacol 283:31–46. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. (1995b) [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn Schmiedebergs Arch Pharmacol 352:263–275. [DOI] [PubMed] [Google Scholar]
- Waeber C, Schoeffter P, Hoyer D, Palacios JM. (1990a) The serotonin 5-HT1D receptor: a progress review. Neurochem Res 15:567–582. [DOI] [PubMed] [Google Scholar]
- Waeber C, Schoeffter P, Palacios JM, Hoyer D. (1988b) Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes. Naunyn Schmiedebergs Arch Pharmacol 337:595–601. [DOI] [PubMed] [Google Scholar]
- Waeber C, Schoeffter P, Palacios JM, Hoyer D. (1989b) 5-HT1D receptors in guinea-pig and pigeon brain. Radioligand binding and biochemical studies. Naunyn Schmiedebergs Arch Pharmacol 340:479–485. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Bockaert J, Dumuis A. (1996) Regional distribution and ontogeny of 5-HT4 binding sites in rat brain. Behav Brain Res 73:259–262. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Grossman C, Javoy-Agid F, Bockaert J, Dumuis A. (1993) [3H]-GR113808 labels 5-HT4 receptors in the human and guinea-pig brain. Neuroreport 4:1239–1242. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. (1994) Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 33:527–541. [DOI] [PubMed] [Google Scholar]
- Waeber C, Zhang LA, Palacios JM. (1990b) 5-HT1D receptors in the guinea pig brain: pre- and postsynaptic localizations in the striatonigral pathway. Brain Res 528:197–206. [DOI] [PubMed] [Google Scholar]
- Waikar MV, Ford AP, Clarke DE. (1994) Evidence for an inhibitory 5-HT4 receptor in urinary bladder of rhesus and Cynomolgus monkeys. Br J Pharmacol 111:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainscott DB, Cohen ML, Schenck KW, Audia JE, Nissen JS, Baez M, Kursar JD, Lucaites VL, Nelson DL. (1993) Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Mol Pharmacol 43:419–426. [PubMed] [Google Scholar]
- Wainscott DB, Johnson KW, Phebus LA, Schaus JM, Nelson DL. (1998) Human 5-HT1F receptor-stimulated [35S]GTPgammaS binding: correlation with inhibition of guinea pig dural plasma protein extravasation. Eur J Pharmacol 352:117–124. [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Krushinski JH, Jr, Audia JE, Schaus JM, Zgombick JM, Lucaites VL, Nelson DL. (2005) [3H]LY334370, a novel radioligand for the 5-HT1F receptor. I. In vitro characterization of binding properties. Naunyn Schmiedebergs Arch Pharmacol 371:169–177. [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL. (1996) Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther 276:720–727. [PubMed] [Google Scholar]
- Wallace TJ, Zai CC, Brandl EJ, Müller DJ. (2011) Role of 5-HT(2C) receptor gene variants in antipsychotic-induced weight gain. Pharm Genomics Pers Med 4:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. (2010) 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther 128:146–169. [DOI] [PubMed] [Google Scholar]
- Walstab J, Steinhagen F, Brüss M, Göthert M, Bönisch H. (2011) Differences between human wild-type and C23S variant 5-HT2C receptors in inverse agonist-induced resensitization. Pharmacol Rep 63:45–53. [DOI] [PubMed] [Google Scholar]
- Walther C, Caetano FA, Dunn HA, Ferguson SS. (2015) PDZK1/NHERF3 differentially regulates corticotropin-releasing factor receptor 1 and serotonin 2A receptor signaling and endocytosis. Cell Signal 27:519–531. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter J-U, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. (2003a) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter J-U, Winter S, Höltje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, et al. (2003b) Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115:851–862. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Stahlberg S, Vowinckel J. (2011) Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J 278:4740–4755. [DOI] [PubMed] [Google Scholar]
- Wang B, Chehab FF. (2006) Deletion of the serotonin 2c receptor from transgenic mice overexpressing leptin does not affect their lipodystrophy but exacerbates their diet-induced obesity. Biochem Biophys Res Commun 351:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W, Huang XP, Vardy E, et al. (2013a) Structural basis for molecular recognition at serotonin receptors. Science 340:610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Lin HC, Chan YH, Gean PW, Yang YK, Chen PS. (2013b) 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int J Neuropsychopharmacol 16:2027–2039. [DOI] [PubMed] [Google Scholar]
- Wang CD, Gallaher TK, Shih JC. (1993) Site-directed mutagenesis of the serotonin 5-hydroxytrypamine2 receptor: identification of amino acids necessary for ligand binding and receptor activation. Mol Pharmacol 43:931–940. [PubMed] [Google Scholar]
- Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J, et al. (1998) Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science 280:1077–1082. [DOI] [PubMed] [Google Scholar]
- Wang HM, Wang Y, Liu M, Bai Y, Zhang XH, Sun YX, Wang HL. (2012) Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats. Can J Physiol Pharmacol 90:1506–1515. [DOI] [PubMed] [Google Scholar]
- Wang L, Lv Y, Deng W, Peng X, Xiao Z, Xi Z, Chen G, Wang X. (2015) 5-HT6 receptor recruitment of mTOR modulates seizure activity in epilepsy. Mol Neurobiol 51:1292–1299. [DOI] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. (2004) Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem 279:4952–4961. [DOI] [PubMed] [Google Scholar]
- Wang Q, O’Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. (2000a) Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem 74:1290–1300. [DOI] [PubMed] [Google Scholar]
- Wang X, Fang Y, Liang J, Yan M, Hu R, Pan X. (2014) 5-HT7 receptors are involved in neurogenic dural vasodilatation in an experimental model of migraine. J Mol Neurosci 54:164–170. [DOI] [PubMed] [Google Scholar]
- Wang Y, Joharchi N, Fletcher PJ, Sellers EM, Higgins GA. (1995) Further studies to examine the nature of dexfenfluramine-induced suppression of heroin self-administration. Psychopharmacology (Berl) 120:134–141. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Keith IM, Beckman MJ, Brownfield MS, Vidruk EH, Bisgard GE. (2000b) 5-HT5a receptors in the carotid body chemoreception pathway of rat. Neurosci Lett 278:9–12. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Jenkins L, Milligan G. (2009) Selectivity and functional consequences of interactions of family A G protein-coupled receptors with neurochondrin and periplakin. J Neurochem 109:182–192. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Pediani JD, Godin AG, Milligan G. (2015) Regulation of oligomeric organization of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor observed by spatial intensity distribution analysis. J Biol Chem 290:12844–12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. (1996) Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol 370:405–414. [DOI] [PubMed] [Google Scholar]
- Ward RP, Hamblin MW, Lachowicz JE, Hoffman BJ, Sibley DR, Dorsa DM. (1995) Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience 64:1105–1111. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Jackson C, Tallarida RJ, Walker EA. (2008) Effects of a Cannabinoid1 receptor antagonist and Serotonin2C receptor agonist alone and in combination on motivation for palatable food: a dose-addition analysis study in mice. J Pharmacol Exp Ther 325:567–576. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. (2009) Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci 29:1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr D. (2012) Management of highly emetogenic chemotherapy. Curr Opin Oncol 24:371–375. [DOI] [PubMed] [Google Scholar]
- Warr D, DeAngelis C. (2009) Controlling nausea and vomiting in patients undergoing Chemotherapy. Toward more effective clinical practice. Oncology Exchange 8:23–27. [Google Scholar]
- Watanabe Y, Yoshimoto K, Tatebe H, Kita M, Nishikura K, Kimura M, Tanaka M. (2014) Enhancement of alcohol drinking in mice depends on alterations in RNA editing of serotonin 2C receptors. Int J Neuropsychopharmacol 17:739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiez AS, Pichon X, Dupuis A, Hernández A, Privat AM, Aissouni Y, Chalus M, Pelissier T, Eschalier A, Marin P, et al. (2013) Disruption of 5-HT2A receptor-PDZ protein interactions alleviates mechanical hypersensitivity in carrageenan-induced inflammation in rats. PLoS One 8:e74661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW. (1996) Serotonin activates the mitogen-activated protein kinase pathway in vascular smooth muscle: use of the mitogen-activated protein kinase kinase inhibitor PD098059. J Pharmacol Exp Ther 279:1541–1550. [PubMed] [Google Scholar]
- Watts SW, Baez M, Webb RC. (1996) The 5-hydroxytryptamine2B receptor and 5-HT receptor signal transduction in mesenteric arteries from deoxycorticosterone acetate-salt hypertensive rats. J Pharmacol Exp Ther 277:1103–1113. [PubMed] [Google Scholar]
- Watts SW, Cohen ML. (1992) Characterization of the contractile serotonergic receptor in guinea pig trachea with agonists and antagonists. J Pharmacol Exp Ther 260:1101–1106. [PubMed] [Google Scholar]
- Watts SW, Darios ES, Seitz BM, Thompson JM. (2015) 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3:e00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW, Fink GD. (1999) 5-HT2B-receptor antagonist LY-272015 is antihypertensive in DOCA-salt-hypertensive rats. Am J Physiol 276:H944–H952. [DOI] [PubMed] [Google Scholar]
- Watts SW, Gilbert L, Webb RC. (1995) 5-Hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension 26:1056–1059. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. (2012) Serotonin and blood pressure regulation. Pharmacol Rev 64:359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW, Priestley JR, Thompson JM. (2009) Serotonylation of vascular proteins important to contraction. PLoS One 4:e5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW, Yang P, Banes AK, Baez M. (2001) Activation of Erk mitogen-activated protein kinase proteins by vascular serotonin receptors. J Cardiovasc Pharmacol 38:539–551. [DOI] [PubMed] [Google Scholar]
- Weber ET, Andrade R. (2010) Htr2a gene and 5-HT(2A) receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL. (2012) Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302:L1273–L1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. (1995) Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res 16:87–110. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. (2001) 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther 299:268–276. [PubMed] [Google Scholar]
- Weinshank RL, Branchek T, Hartig PR. (1994) inventors. DNA encoding a human 5-HT1F receptor, vectors, and host cells. U.S. patent 5,360,735.
- Weinshank RL, Zgombick JM, Macchi MJ, Branchek TA, Hartig PR. (1992) Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D α and 5-HT1D β. Proc Natl Acad Sci USA 89:3630–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Obreztchikova M, Hong Z. (2008) Fenfluramine: riddle or Rosetta stone? Eur Respir J 31:232–235. [DOI] [PubMed] [Google Scholar]
- Weiss B, Mertz A, Schröck E, Koenen M, Rappold G. (1995) Assignment of a human homolog of the mouse Htr3 receptor gene to chromosome 11q23.1-q23.2. Genomics 29:304–305. [DOI] [PubMed] [Google Scholar]
- Weissmann-Nanopoulos D, Mach E, Magre J, Demassey Y, Pujol JF. (1985) Evidence for the localization of 5HT1A binding sites on serotonin containing neurons in the raphe dorsalis and raphe centralis nuclei of the rat brain. Neurochem Int 7:1061–1072. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, et al. (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313:536–540. [DOI] [PubMed] [Google Scholar]
- Weninger S, De Maeyer JH, Lefebvre RA. (2012) Study of the regulation of the inotropic response to 5-HT4 receptor activation via phosphodiesterases and its cross-talk with C-type natriuretic peptide in porcine left atrium. Naunyn Schmiedebergs Arch Pharmacol 385:565–577. [DOI] [PubMed] [Google Scholar]
- Weninger S, Van Craenenbroeck K, Cameron RT, Vandeput F, Movsesian MA, Baillie GS, Lefebvre RA. (2014) Phosphodiesterase 4 interacts with the 5-HT4(b) receptor to regulate cAMP signaling. Cell Signal 26:2573–2582. [DOI] [PubMed] [Google Scholar]
- Wenkert D, Schoneberg T, Merendino JJ, Jr, Rodriguez Pena MS, Vinitsky R, Goldsmith PK, Wess J, Spiegel AM. (1996) Functional characterization of five V2 vasopressin receptor gene mutations. Mol Cell Endocrinol 124:43–50. [DOI] [PubMed] [Google Scholar]
- Werry TD, Gregory KJ, Sexton PM, Christopoulos A. (2005) Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J Neurochem 93:1603–1615. [DOI] [PubMed] [Google Scholar]
- Werry TD, Stewart GD, Crouch MF, Watts A, Sexton PM, Christopoulos A. (2008) Pharmacology of 5HT(2C) receptor-mediated ERK1/2 phosphorylation: agonist-specific activation pathways and the impact of RNA editing. Biochem Pharmacol 76:1276–1287. [DOI] [PubMed] [Google Scholar]
- Wesołowska A, Kowalska M. (2008) Influence of serotonin 5-HT(7) receptor blockade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol Rep 60:464–474. [PubMed] [Google Scholar]
- Wesołowska A, Nikiforuk A, Stachowicz K. (2006a) Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol 553:185–190. [DOI] [PubMed] [Google Scholar]
- Wesołowska A, Nikiforuk A, Stachowicz K, Tatarczyńska E. (2006b) Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 51:578–586. [DOI] [PubMed] [Google Scholar]
- Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. (2001) Expression of serotonin receptors in bone. J Biol Chem 276:28961–28968. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Backstrom JR, Sanders-Bush E. (1995) Increased basal phosphorylation of the constitutively active serotonin 2C receptor accompanies agonist-mediated desensitization. Mol Pharmacol 48:200–205. [PubMed] [Google Scholar]
- Westphal RS, Sanders-Bush E. (1996) Differences in agonist-independent and -dependent 5-hydroxytryptamine2C receptor-mediated cell division. Mol Pharmacol 49:474–480. [PubMed] [Google Scholar]
- Westrich L, Sprouse J, Sánchez C. (2013) The effects of combining serotonin reuptake inhibition and 5-HT7 receptor blockade on circadian rhythm regulation in rodents. Physiol Behav 110–111:42–50. [DOI] [PubMed] [Google Scholar]
- Wetter TC, Lauer CJ, Gillich G, Pollmächer T. (1996) The electroencephalographic sleep pattern in schizophrenic patients treated with clozapine or classical antipsychotic drugs. J Psychiatr Res 30:411–419. [DOI] [PubMed] [Google Scholar]
- Weydert A, Cloez-Tayarani I, Fillion MP, Simon-Chazottes D, Guenet JL, Fillion G. (1992) Molecular cloning of two partial serotonin 5-HT1D receptor sequences in mouse and one in guinea pig. C R Acad Sci III 314:429–435. [PubMed] [Google Scholar]
- Whale R, Bhagwagar Z, Cowen PJ. (1999) Zolmitriptan-induced growth hormone release in humans: mediation by 5-HT1D receptors? Psychopharmacology (Berl) 145:223–226. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. (2011) Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med 17:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. (2011) The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci 31:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke J, Westerdahl A-C, Hultborn H, Kiehn O, Ryge J. (2010) Global gene expression analysis of rodent motor neurons following spinal cord injury associates molecular mechanisms with development of postinjury spasticity. J Neurophysiol 103:761–778. [DOI] [PubMed] [Google Scholar]
- Wild C, Cunningham KA, Zhou J. (2014) Allosteric modulation of G protein-coupled receptors: an emerging approach of drug discovery. Austin J Pharmacol Ther 2:1101. [PMC free article] [PubMed] [Google Scholar]
- Wild CT, Miszkiel JM, Wold EA, Soto CA, Ding C, Hartley RM, White MA, Anastasio NC, Cunningham KA, Zhou J. (2019) Design, synthesis, and characterization of 4-undecylpiperidine-2-carboxamides as positive allosteric modulators of the serotonin (5-HT) 5-HT2C receptor. J Med Chem 62:288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. (1997) Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr 130:89–94. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Windfeld K, Colding-Jørgensen E. (2014) Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 13:1092–1099. [DOI] [PubMed] [Google Scholar]
- Willins DL, Alsayegh L, Berry SA, Backstrom JR, Sanders-Bush E, Friedman L, Khan N, Roth BL. (1998) Serotonergic antagonist effects on trafficking of serotonin 5-HT2A receptors in vitro and in vivo. Ann N Y Acad Sci 861:121–127. [DOI] [PubMed] [Google Scholar]
- Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. (1999) Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience 91:599–606. [DOI] [PubMed] [Google Scholar]
- Wilmer A, Tack J, Coremans G, Janssens J, Peeters T, Vantrappen G. (1993) 5-hydroxytryptamine-3 receptors are involved in the initiation of gastric phase-3 motor activity in humans. Gastroenterology 105:773–780. [DOI] [PubMed] [Google Scholar]
- Wilson H, Coffman WJ, Cohen ML. (1990) 5-hydroxytryptamine3 receptors mediate tachycardia in conscious instrumented dogs. J Pharmacol Exp Ther 252:683–688. [PubMed] [Google Scholar]
- Winstanley CA. (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164:1301–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167:304–314. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176:376–385. [DOI] [PubMed] [Google Scholar]
- Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR. (1999) Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 60:358–363. [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. (2011) Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol 22:805–813. [DOI] [PubMed] [Google Scholar]
- Wisden W, Parker EM, Mahle CD, Grisel DA, Nowak HP, Yocca FD, Felder CC, Seeburg PH, Voigt MM. (1993) Cloning and characterization of the rat 5-HT5B receptor. Evidence that the 5-HT5B receptor couples to a G protein in mammalian cell membranes. FEBS Lett 333:25–31. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Baez M, Yu J, Barton ME, Shannon HE. (2007) Constitutive deletion of the serotonin-7 (5-HT(7)) receptor decreases electrical and chemical seizure thresholds. Epilepsy Res 75:39–45. [DOI] [PubMed] [Google Scholar]
- Woehler A, Ponimaskin EG. (2009) G protein--mediated signaling: same receptor, multiple effectors. Curr Mol Pharmacol 2:237–248. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Rippberger H, Schwarting RK, van Gaalen MM. (2015) Critical involvement of 5-HT2C receptor function in amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Psychopharmacology (Berl) 232:1817–1829. [DOI] [PubMed] [Google Scholar]
- Wolf ME. (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf WA, Schutz LJ. (1997) The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J Neurochem 69:1449–1458. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. (2000) A comparison of the behavioural effects of 5-HT2A and 5-HT2C receptor agonists in the pigeon. Behav Pharmacol 11:355–364. [DOI] [PubMed] [Google Scholar]
- Wood MD, Reavill C, Trail B, Wilson A, Stean T, Kennett GA, Lightowler S, Blackburn TP, Thomas D, Gager TL, et al. (2001) SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety. Neuropharmacology 41:186–199. [DOI] [PubMed] [Google Scholar]
- Woods S, Clarke NN, Layfield R, Fone KC. (2012) 5-HT(6) receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol 167:436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward RM, Panicker MM, Miledi R. (1992) Actions of dopamine and dopaminergic drugs on cloned serotonin receptors expressed in Xenopus oocytes. Proc Natl Acad Sci USA 89:4708–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC. (2001) A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology 41:210–219. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KC. (2004) 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3:59–79. [DOI] [PubMed] [Google Scholar]
- Won SJ, Lin MT. (1988) 5-Hydroxytryptamine receptors in the hypothalamus mediate thermoregulatory responses in rabbits. Naunyn Schmiedebergs Arch Pharmacol 338:256–261. [DOI] [PubMed] [Google Scholar]
- Wong H, Dockens RC, Pajor L, Yeola S, Grace JE, Jr, Stark AD, Taub RA, Yocca FD, Zaczek RC, Li YW. (2007) 6-Hydroxybuspirone is a major active metabolite of buspirone: assessment of pharmacokinetics and 5-hydroxytryptamine1A receptor occupancy in rats. Drug Metab Dispos 35:1387–1392. [DOI] [PubMed] [Google Scholar]
- Wouters W, Tulp MT, Bevan P. (1988) Flesinoxan lowers blood pressure and heart rate in cats via 5-HT1A receptors. Eur J Pharmacol 149:213–223. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. (1995) Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol 351:357–373. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhu M, Wang W, Wang Y, Li Y, Yew DT. (2001) Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund’s adjuvant-induced inflammation. Neurosci Lett 307:183–186. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW, Jr, Zhang G. (2015a) Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett 607:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZS, Cheng H, Jiang Y, Melcher K, Xu HE. (2015b) Ion channels gated by acetylcholine and serotonin: structures, biology, and drug discovery. Acta Pharmacol Sin 36:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurch T, Palmier C, Colpaert FC, Pauwels PJ. (1997) Sequence and functional analysis of cloned guinea-pig and rat 5-HT1D receptors: common pharmacological features within the 5-HT1D receptor family. J. Neurochem. 68:410-418. [DOI] [PubMed] [Google Scholar]
- Wynants M, Vengethasamy L, Ronisz A, Meyns B, Delcroix M, Quarck R. (2013) NF-κB pathway is involved in CRP-induced effects on pulmonary arterial endothelial cells in chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 305:L934–L942. [DOI] [PubMed] [Google Scholar]
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. (2003) The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience 122:907–920. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. (1997) Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol 7:391–396. [DOI] [PubMed] [Google Scholar]
- Xie E, Zhu L, Zhao L, Chang LS. (1996) The human serotonin 5-HT2C receptor: complete cDNA, genomic structure, and alternatively spliced variant. Genomics 35:551–561. [DOI] [PubMed] [Google Scholar]
- Xie Z, Lee SP, O’Dowd BF, George SR. (1999) Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett 456:63–67. [DOI] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. (2003) Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114:469–482. [DOI] [PubMed] [Google Scholar]
- Xu P, He Y, Cao X, et al. (2017) Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol Psychiatry 81:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Hong J, Morse CL, Pike VW. (2010) Synthesis, structure-affinity relationships, and radiolabeling of selective high-affinity 5-HT4 receptor ligands as prospective imaging probes for positron emission tomography. J Med Chem 53:7035–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Pandey SC. (2000) Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull 51:499–505. [DOI] [PubMed] [Google Scholar]
- Xu XF, Tsai HJ, Li L, Chen YF, Zhang C, Wang GF. (2009) Modulation of leak K(+) channel in hypoglossal motoneurons of rats by serotonin and/or variation of pH value. Sheng Li Xue Bao 61:305–316. [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. (2008) 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YC, Johnson KW, Phebus LA, Cohen M, Nelson DL, Schenck K, Walker CD, Fritz JE, Kaldor SW, LeTourneau ME, et al. (2001) N-[3-(2-Dimethylaminoethyl)-2-methyl-1H- indol-5-yl]-4-fluorobenzamide: a potent, selective, and orally active 5-HT(1F) receptor agonist potentially useful for migraine therapy. J Med Chem 44:4031–4034. [DOI] [PubMed] [Google Scholar]
- Yaakob NS, Chinkwo KA, Chetty N, Coupar IM, Irving HR. (2015) Distribution of 5-HT3, 5-HT4, and 5-HT7 receptors along the human colon. J Neurogastroenterol Motil 21:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N. (2009) Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol 46:518–525. [DOI] [PubMed] [Google Scholar]
- Yadav PN, Kroeze WK, Farrell MS, Roth BL. (2011) Antagonist functional selectivity: 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J Pharmacol Exp Ther 339:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu Z-W, Gao X-B, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. (2009) A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagaloff KA, Hartig PR. (1985) 125I-lysergic acid diethylamide binds to a novel serotonergic site on rat choroid plexus epithelial cells. J Neurosci 5:3178–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. (2015) The ins and outs of the striatum: role in drug addiction. Neuroscience 301:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL, Lagrutta A, Adelman JP, North RA. (1993) Single amino acid substitution affects desensitization of the 5-hydroxytryptamine type 3 receptor expressed in Xenopus oocytes. Proc Natl Acad Sci USA 90:5030–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Wakita H, Horisaka K. (1988) The involvement of serotonergic and dopaminergic systems in hypothermia induced in mice by intracerebroventricular injection of serotonin. Jpn J Pharmacol 48:145–148. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Hatano N, Sugai T, Kato N. (2014) Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology 82:49–58. [DOI] [PubMed] [Google Scholar]
- Yamashita PS, de Bortoli VC, Zangrossi H., Jr (2011) 5-HT2C receptor regulation of defensive responses in the rat dorsal periaqueductal gray. Neuropharmacology 60:216–222. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Harada K, Yamamoto N, Yarimizu J, Okabe M, Shimada T, Ni K, Matsuoka N. (2014) ASP5736, a novel 5-HT5A receptor antagonist, ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia. Eur Neuropsychopharmacol 24:1698–1708. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Okabe M, Yamamoto N, Yarimizu J, Harada K. (2015) Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J Pharmacol Sci 127:362–369. [DOI] [PubMed] [Google Scholar]
- Yanarates O, Dogrul A, Yildirim V, Sahin A, Sizlan A, Seyrek M, Akgül O, Kozak O, Kurt E, Aypar U. (2010) Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-desmethyltramadol, via activation of descending serotonergic pathways. Anesthesiology 112:696–710. [DOI] [PubMed] [Google Scholar]
- Yan C, Yang Y, Saito K, Xu P, Wang C, Hinton AO, Jr, Yan X, Wu Q, Tong Q, Elmquist JK, et al. (2015) Meta-chlorophenylpiperazine enhances leptin sensitivity in diet-induced obese mice. Br J Pharmacol 172:3510–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Soulier JL, Sicsic S, Mathé-Allainmat M, Brémont B, Croci T, Cardamone R, Aureggi G, Langlois M. (1997) New esters of 4-amino-5-chloro-2-methoxybenzoic acid as potent agonists and antagonists for 5-HT4 receptors. J Med Chem 40:608–621. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Han SK, Park SJ. (2014) Functional expression of 5-HT7 receptor on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Brain Res 1543:73–82. [PubMed] [Google Scholar]
- Yang G-B, Lackner AA. (2004) Proximity between 5-HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol 146:46–49. [DOI] [PubMed] [Google Scholar]
- Yang J. (1990) Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J Gen Physiol 96:1177–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Belrose J, Trepanier CH, Lei G, Jackson MF, MacDonald JF. (2011) Fyn, a potential target for Alzheimer’s disease. J Alzheimers Dis 27:243–252. [DOI] [PubMed] [Google Scholar]
- Yang M, Li K, Ng PC, Chuen CK, Lau TK, Cheng YS, Liu YS, Li CK, Yuen PM, James AE, et al. (2007) Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells 25:1800–1806. [DOI] [PubMed] [Google Scholar]
- Yang W, Chen K, Lan NC, Gallaher TK, Shih JC. (1992) Gene structure and expression of the mouse 5-HT2 receptor. J Neurosci Res 33:196–204. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. (2004) Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res 124:70–78. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu X, Yin Y, Sun S, Deng X. (2012) Involvement of 5-HT7 receptors in the pathogenesis of temporal lobe epilepsy. Eur J Pharmacol 685:52–58. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, Ando T, Inoue M, Maeda J, Suzuki K. (2003) Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry 160:334–340. [DOI] [PubMed] [Google Scholar]
- Ye JY, Liang EY, Cheng YS, Chan GCF, Ding Y, Meng F, Ng MHL, Chong BH, Lian Q, Yang M. (2014) Serotonin enhances megakaryopoiesis and proplatelet formation via p-Erk1/2 and F-actin reorganization. Stem Cells 32:2973–2982. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Smith JR, Purdy RE. (1998) Serotonin and vasoconstrictor synergism. Life Sci 62:1723–1732. [DOI] [PubMed] [Google Scholar]
- Yin J, Albert RH, Tretiakova AP, Jameson BA. (2006) 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J Neuroimmunol 181:68–81. [DOI] [PubMed] [Google Scholar]
- Yocca FD, Iben L, Meller E. (1992) Lack of apparent receptor reserve at postsynaptic 5-hydroxytryptamine1A receptors negatively coupled to adenylyl cyclase activity in rat hippocampal membranes. Mol Pharmacol 41:1066–1072. [PubMed] [Google Scholar]
- Yoshimoto K, Watanabe Y, Tanaka M, Kimura M. (2012) Serotonin2C receptors in the nucleus accumbens are involved in enhanced alcohol-drinking behavior. Eur J Neurosci 35:1368–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. (2001) Affinity of serotonin receptor antagonists and agonists to recombinant and native α1-adrenoceptor subtypes. Jpn J Pharmacol 86:189–195. [DOI] [PubMed] [Google Scholar]
- You HJ, Colpaert FC, Arendt-Nielsen L. (2005) The novel analgesic and high-efficacy 5-HT1A receptor agonist F 13640 inhibits nociceptive responses, wind-up, and after-discharges in spinal neurons and withdrawal reflexes. Exp Neurol 191:174–183. [DOI] [PubMed] [Google Scholar]
- Young MR, Kut JL, Coogan MP, Wright MA, Young ME, Matthews J. (1993) Stimulation of splenic T-lymphocyte function by endogenous serotonin and by low-dose exogenous serotonin. Immunology 80:395–400. [PMC free article] [PubMed] [Google Scholar]
- Yu L, Nguyen H, Le H, Bloem LJ, Kozak CA, Hoffman BJ, Snutch TP, Lester HA, Davidson N, Lübbert H. (1991) The mouse 5-HT1C receptor contains eight hydrophobic domains and is X-linked. Brain Res Mol Brain Res 11:143–149. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chen J-H, Li H, Yang Z, Du X, Hong L, Liao H, Jiang L, Shi J, Zhao L, et al. (2015) Involvement of 5-HT3 and 5-HT4 receptors in colonic motor patterns in rats. Neurogastroenterol Motil 27:914–928. [DOI] [PubMed] [Google Scholar]
- Yuan S, Filipek S, Vogel H. (2016) A gating mechanism of the serotonin 5-HT3 receptor. Structure 24:816–825. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K. (2000) Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes. Diabetologia 43:373–376. [DOI] [PubMed] [Google Scholar]
- Yun HM, Baik JH, Kang I, Jin C, Rhim H. (2010) Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem 285:10016–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim HJ, Kostenis E, Kim JI, Seong JY, Baik JH, Rhim H. (2007) The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem 282:5496–5505. [DOI] [PubMed] [Google Scholar]
- Zayara AE, McIver G, Valdivia PN, Lominac KD, McCreary AC, Szumlinski KK. (2011) Blockade of nucleus accumbens 5-HT2A and 5-HT2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology (Berl) 213:321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgombick JM, Bard JA, Kucharewicz SA, Urquhart DA, Weinshank RL, Branchek TA. (1997) Molecular cloning and pharmacological characterization of guinea pig 5-HT1B and 5-HT1D receptors. Neuropharmacology 36:513–524. [DOI] [PubMed] [Google Scholar]
- Zgombick JM, Branchek TA. (1998) Native 5-HT1B receptors expressed in OK cells display dual coupling to elevation of intracellular calcium concentrations and inhibition of adenylate cyclase. Naunyn Schmiedebergs Arch Pharmacol 358:503–508. [DOI] [PubMed] [Google Scholar]
- Zgombick JM, Schechter LE, Kucharewicz SA, Weinshank RL, Branchek TA. (1995) Ketanserin and ritanserin discriminate between recombinant human 5-HT1D alpha and 5-HT1D beta receptor subtypes. Eur J Pharmacol 291:9–15. [DOI] [PubMed] [Google Scholar]
- Zgombick JM, Schechter LE, Macchi M, Hartig PR, Branchek TA, Weinshank RL. (1992) Human gene S31 encodes the pharmacologically defined serotonin 5-hydroxytryptamine1E receptor. Mol Pharmacol 42:180–185. [PubMed] [Google Scholar]
- Zgombick JM, Weinshank RL, Macchi M, Schechter LE, Branchek TA, Hartig PR. (1991) Expression and pharmacological characterization of a canine 5-hydroxytryptamine1D receptor subtype. Mol Pharmacol 40:1036–1042. [PubMed] [Google Scholar]
- Zhang D, Blanco MJ, Ying BP, Kohlman D, Liang SX, Victor F, Chen Q, Krushinski J, Filla SA, Hudziak KJ, et al. (2015) Discovery of selective N-[3-(1-methyl-piperidine-4-carbonyl)-phenyl]-benzamide-based 5-HT1F receptor agonists: evolution from bicyclic to monocyclic cores. Bioorg Med Chem Lett 25:4337–4341. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kohlman D, Krushinski J, Liang S, Ying BP, Reilly JE, Dinn SR, Wainscott DB, Nutter S, Gough W, et al. (2004) Design, synthesis and evaluation of bicyclic benzamides as novel 5-HT1F receptor agonists. Bioorg Med Chem Lett 14:6011–6016. [DOI] [PubMed] [Google Scholar]
- Zhang G, Stackman RW., Jr (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol 6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW., Jr (2016) Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology 101:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Herman AI, Kranzler HR, Anton RF, Zhao H, Zheng W, Gelernter J. (2013a) Array-based profiling of DNA methylation changes associated with alcohol dependence. Alcohol Clin Exp Res 37 (Suppl 1):E108–E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhu Y, Zhou W, Gao L, Yuan L, Han X. (2013b) Serotonin receptor 2C and insulin secretion. PLoS One 8:e54250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D’Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, et al. (2002) Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci 22:9635–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Smith EM, Baye TM, Eckert JV, Abraham LJ, Moses EK, Kissebah AH, Martin LJ, Olivier M. (2010) Serotonin (5-HT) receptor 5A sequence variants affect human plasma triglyceride levels. Physiol Genomics 42:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Chen L, Zhang J. (2014a) Expression analysis of genes and pathways associated with liver metastases of the uveal melanoma. BMC Med Genet 15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Falaleeva M, Agranat-Tamir L, Pages A, Eyras E, Sperling J, Sperling R, Stamm S. (2013c) The 5′ untranslated region of the serotonin receptor 2C pre-mRNA generates miRNAs and is expressed in non-neuronal cells. Exp Brain Res 230:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZF, Duan ZJ, Wang LX, Yang D, Zhao G, Zhang L. (2014b) The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: a meta-analysis of 25 studies. BMC Gastroenterol 14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Wang D, Man SC, Ng R, McAlonan GM, Wong HK, Wong W, Lee J, Tan QR. (2014c) Platelet 5-HT(1A) receptor correlates with major depressive disorder in drug-free patients. Prog Neuropsychopharmacol Biol Psychiatry 53:74–79. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Arsenault D. (2005) Gain modulation by serotonin in pyramidal neurones of the rat prefrontal cortex. J Physiol 566:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. (1999) Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol 82:2989–2999. [DOI] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, et al. (2007) Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 6:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ding M, Ding C, Yao J, Pang H, Xing J, Xuan J, Wang B. (2013) Relationship between genetic polymorphisms in the HTR1A gene and paranoid schizophrenia in a Northern Han Chinese population. J Mol Neurosci 49:625–631. [DOI] [PubMed] [Google Scholar]
- Zhu B, Chen C, Moyzis RK, Dong Q, Chen C, He Q, Li J, Lei X, Lin C. (2012a) Association between the HTR2B gene and the personality trait of fun seeking. Pers Individ Dif 53:1029–1033. [Google Scholar]
- Zhu H, Urban DJ, Blashka J, McPheeters MT, Kroeze WK, Mieczkowski P, Overholser JC, Jurjus GJ, Dieter L, Mahajan GJ, et al. (2012b) Quantitative analysis of focused a-to-I RNA editing sites by ultra-high-throughput sequencing in psychiatric disorders. PLoS One 7:e43227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Chen K, Shih JC. (1995) Characterization of the human 5-HT2A receptor gene promoter. J Neurosci 15:4885–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. (1995) Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci 40:819–827. [DOI] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, et al. (2011) Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 156B:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf DA, das Neves LA, Nikula KJ, Huang J, Senese PB, Gralinski MR. (2011) C-122, a novel antagonist of serotonin receptor 5-HT2B, prevents monocrotaline-induced pulmonary arterial hypertension in rats. Eur J Pharmacol 670:195–203. [DOI] [PubMed] [Google Scholar]
- Zoto T, Kilickap S, Yasar U, Celik I, Bozkurt A, Babaoglu MO. (2015) Improved anti-emetic efficacy of 5-HT3 receptor antagonists in cancer patients with genetic polymorphisms of ABCB1 (MDR1) drug transporter. Basic Clin Pharmacol Toxicol 116:354–360. [DOI] [PubMed] [Google Scholar]
- Zsippai A, Szabó DR, Szabó PM, Tömböl Z, Bendes MR, Nagy Z, Rácz K, Igaz P. (2011) mRNA and microRNA expression patterns in adrenocortical cancer. Am J Cancer Res 1:618–628. [PMC free article] [PubMed] [Google Scholar]
- Zuideveld KP, Rusiç-Pavletiç J, Maas HJ, Peletier LA, Van der Graaf PH, Danhof M. (2002) Pharmacokinetic-pharmacodynamic modeling of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine in rats. J Pharmacol Exp Ther 303:1130–1137. [DOI] [PubMed] [Google Scholar]