Abstract
Smart contact lenses attract extensive interests due to their capability of directly monitoring physiological and ambient information. However, previous demonstrations usually lacked efficient sensor modalities, facile fabrication process, mechanical stability, or biocompatibility. Here, we demonstrate a flexible approach for fabrication of multifunctional smart contact lenses with an ultrathin MoS2 transistors-based serpentine mesh sensor system. The integrated sensor systems contain a photodetector for receiving optical information, a glucose sensor for monitoring glucose level directly from tear fluid, and a temperature sensor for diagnosing potential corneal disease. Unlike traditional sensors and circuit chips sandwiched in the lens substrate, this serpentine mesh sensor system can be directly mounted onto the lenses and maintain direct contact with tears, delivering high detection sensitivity, while being mechanically robust and not interfering with either blinking or vision. Furthermore, the in vitro cytotoxicity tests reveal good biocompatibility, thus holding promise as next-generation soft electronics for healthcare and medical applications.
Keywords: soft bioelectronics, 2D semiconductor materials, smart contact lens, integrated sensor systems, molybdenum disulphide, flexible and wearable devices, photodetection, glucose sensing, temperature monitoring
Graphical abstract
Progress and potential
Wearable smart contact lenses have attracted extensive interests because of their ability to monitor physiological information and ambient information directly from eyeball and body fluids. However, conventional smart contact lens systems lack efficient sensor modalities, facile fabrication process, mechanical stability, or biocompatibility. This study develops a multifunctional, high-transparency and easy-access smart contact lens system. Specifically, the serpentine mesh sensor system can be directly mounted onto the lens substrate and maintain direct contact with tears, delivering high detection sensitivity, while being mechanically robust and not interfering with either blinking or vision. This integrated contact lens sensor system and fabrication strategy allows for easy incorporation of other functional components, such as an electrode array for electroretinography, antennas for wireless communication, and power modules for future in vivo exploration.
A multifunctional smart contact lens with an ultrathin MoS2 transistors-based serpentine mesh sensor system was developed, featuring easy assembly, good detection sensitivity, strong robustness, high stretchability, transparency, and full biocompatibility. The integrated sensor systems contain a photodetector for receiving optical information, imaging and vision assistance, a temperature sensor for diagnosing potential corneal disease, and a glucose sensor for monitoring glucose levels directly from the tear fluid.
Introduction
The enormous impact of the coronavirus disease 2019 (COVID-19) pandemic, together with other diseases or chronic health risks has significantly prompted the development and application of bioelectronics and medical devices for real-time monitoring and diagnosing health status.1, 2, 3, 4, 5 Stretchable and flexible devices worn on or implanted in the human body with embedded multifunctional biosensors should enable real-time sensing of physiological signals (e.g., heart rate, body temperature, oxygen saturation) and/or ambient conditions (e.g., the surrounding temperature, humidity, and light and audio levels) without affecting the normal body movements.6, 7, 8, 9, 10, 11, 12, 13 By integrating and analyzing a large amount of live data, these devices could dramatically enhance the efficiency of healthcare delivery and carry out delivery of medicines more timely, personalized, and precise.14, 15, 16, 17, 18 Among all these devices, contact lenses are one of the most popular wearable devices, designed for vision correction, and aesthetic and therapeutic purposes.19, 20, 21, 22, 23 They are in direct contact with both internal chemistry from tear fluid and eyeball and external air for hours without serious irritation, which can provide a unique platform for continuous interrogation of both physiological information and ambient conditions. Taking the diabetic diagnostics as an example, to collect accurate data with non- or minimally invasive techniques, the glucose level tends to be tested from the "open" body fluids rather than blood.23 Multipatient clinical trials have demonstrated clearly that tear fluid can be a valuable marker for measurements of systemic glucose.24 Smart contact lenses equipped with high sensitivity glucose sensors could open the possibility of a non-invasive method to continuously detect biomarkers in tears.25, 26, 27, 28, 29 Owing to the abundant proteins or biomarkers from tear fluid, smart contact lenses are expected to provide higher accuracy/reliability of the resulting data in comparison with the analyses from skin or sweat by other conventional wrist-worn watches or smart patches. Although controversy may remain, recent studies have shown that the blood glucose concentrations estimated with the tear glucose concentrations obtained by glucose sensors exhibit a high correlation with those actually measured with a clinically available glucometer (R2 = 0.9617).30 As complications of diabetes, stroke, and heart disease are also closely related to disorders of blood glucose regulation; these disorders could also increase the risk of high blood pressure, lower limb nerve damage, kidney diseases, and retina injury.31, 32, 33 Meanwhile, monitoring the intraocular and extraocular environment helps to provide a database to diagnose and treat ocular diseases. For example, embedded photodetectors on the lenses are capable of potentially monitoring the light intensity, testing and utilizing eye blinking, receiving optical data, and assisting vision.27 Embedded temperature sensors could be used for monitoring corneal temperature, tracking of intraocular pressure,34 and providing information for ocular disease, such as diagnosing dry eye disease35 and inflammation.36 Monitoring ocular temperature after ophthalmic surgery also has clinical significance in case of postoperative infection.35 The smart contact lenses could also be equipped with application-specific integrated circuit chips,26 multiple electrode arrays,37 , 38 or antenna25 , 39 to further enrich their functionality to obtain, process, and transmit physiological properties, manage illnesses and health risks, and finally promote health and wellbeing.
Despite significant efforts, use of smart contact lenses remains limited because of their mechanical biocompatibility, detection sensitivity, and challenging integration process.20 , 40 Many characteristics are required for the successful development of smart contact lenses. Firstly, they should not only have a high degree of transparency in visual field but also be comfortable to wear. To minimize the potential for inflammation and irritation, the embedded sensors or circuit chips should be fully stable, biocompatible, and have negligible mechanical stiffness relative to the eyeball. Flexible and transparent ultrathin electronic materials, e.g., mono- to few-layered 2D transition metal dichalcogenide (TMDC),41, 42, 43, 44, 45 could be the ideal building blocks for this purpose, although the difficulty remains in the choice of the specific materials and manufacturing process. Also, unlike traditional rigid or bulk sensors and circuit chips that are sandwiched in the lens substrate and contacted with tear fluid via microfluidic sensing channels,46, 47, 48 the ultrathin sensors could directly stick onto the lenses and contact the tear fluid, yielding a better detection sensitivity and easier assembly without interfering with blinking.
To achieve the above goals, in this work, soft contact lenses with multifunctional sensor systems were fabricated using ultrathin (few-to-monolayer) TMDC semiconductors, elastic interconnect electrodes, polyimide (PI) passivation layers, and a polydimethylsiloxane (PDMS) lens substrate. Here, we chose MoS2 transistors as the core sensing material because of their large surface-to-volume ratio, layered dangling-bond-free surface, tunable bandgap and charge transfer properties, and good biocompatibility.13 , 49, 50, 51, 52, 53, 54, 55, 56, 57 Unlike traditional top-down fabrication58 , 59 and bottom-up synthesis methods,60, 61, 62 we applied a gold-mediated exfoliation63 and assembly strategy (Figure 1 A) to pattern mono-to-few-layered MoS2 flakes with large lateral size (~200 × 100 μm). The MoS2 transistors were mechanically reinforced with a thin layer of photo-patternable PI, while the elastic parts were formed using a serpentine gold (Au) and PI mesh structure. The sensor system is placed around the outer ring of the cornea to avoid blocking vision (Figure 1B). Such a design can effectively protect electronics from destructive mechanical deformations while maintaining high flexibility/stretchability and can easily convert functional components into various shapes of curvatures, which was verified by mechanical analysis. The integrated sensor system allows the collection of optical, glucose, and temperature information from the contact lens. The ultrathin MoS2 transistors exhibit a high sensitivity (<0.1 mM) for glucose monitoring, a fast response (<1 s), high current on/off ratio (>103 with UV illumination), and high responsivity (4.8 A/W) for photodetection; while the temperature sensor made of Au wires yields a precise measurement of temperature even when highly stretched. In addition, we examined the light absorption of the entire contact lens to demonstrate its transparency and conducted in vitro cytotoxicity tests to confirm its biocompatibility. Taken together, our material and device characteristics demonstrate that the requirements for practical applications have been well fulfilled by such a contact lens, providing a novel and facile approach for multifunctional contact lens sensor systems.
Figure 1.
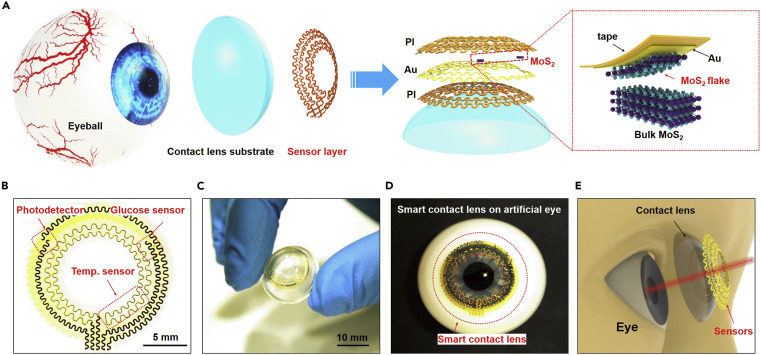
Structural design of a smart contact lens with ultrathin MoS2 transistors-based serpentine mesh sensor system
(A) Schematic illustration of the different layers of smart contact lens structure attached to an eyeball. The dashed region highlights the gold-mediated mechanical exfoliation of monolayer MoS2.
(B) Optical image of the serpentine electrode and sensor structure.
(C) Photograph of the sensor layer transferred onto a dome-shaped PDMS substrate.
(D) Photograph of the system placed on an artificial eye.
(E) Illustration of the smart contact lens and the corresponding dimensions with human eyes.
Results and discussion
Structural design of the smart contact lens
Figure 1A illustrates the layout and the overall structure of the smart contact lens, comprising a donut-shaped sensor layer and a PDMS lens substrate attached to the eyeball. In the sensor layer, hybrid sensors with a serpentine interconnect of Au electrodes (150 nm) are passivated or supported in a single-layer manner by the thin PI layer(s) (2.5 μm per layer) (Figure 1A, right). This serpentine mesh structure provides not only good flexibility and stretchability but also maintains a neutral mechanical plane for keeping the uniaxial strain close to zero when the structure bends slightly.64, 65, 66 The sensor layer is composed of a MoS2 photodetector, a MoS2 glucose sensor, and a Au temperature sensor, each interconnected by a pair of serpentine metal electrodes (Figure 1B). The photograph in Figure 1C displays the sensor layer on top of a dome-shaped PDMS substrate (30 μm), featuring good adhesion and bonding to the surface. As the most widely used silicon-based organic polymer, PDMS has been broadly applied as the substrate in diverse biomedical fields, including contact lenses.67 , 68 Compared with other commercial materials used in contact lenses (e.g., polymethyl methacrylate, polyvinyl acetate, rigid gas-permeable materials, and hydrogels), PDMS is inexpensive, easily manufactured, durable, comfortable to wear, and exhibits high oxygen permeability;69 , 70 the main disadvantage of low hydrophilicity could be mitigated by plasma treatment, hydrophilic polymer grafting, or surface modification with embedded surfactants71 (Table S1). Figure 1D shows a photograph of the smart lens on an artificial eye, indicating that the pupil is not covered by the sensors and therefore that the electrode will not block vision (Figure 1E).
Fabrication and structural characterization of the smart contact lens
In our design, the large-area ultrathin MoS2 flake transistors are used for photodetection and glucose sensing. To obtain the key materials, traditional mechanical exfoliation has been widely applied (Figure S1), but often limited by the small size (<1,000 μm2), low yield with uncontrollable thickness, and electrical performance. To overcome this limitation, a gold-mediated exfoliation method63 was used. As shown in Figure 2 A, a Au film (120 nm) was electron-beam evaporated on top of the MoS2 bulk materials to help detach the topmost layer from the bulk. The topmost layer of MoS2 was peeled off using a thermal release tape, which was later released with heat after transferring the MoS2 to a target substrate. After Au etching, large-area MoS2 flakes were obtained for further assembly. Figure 2B shows a representative optical image of a gold-mediated exfoliated MoS2 flake with a lateral size of ~200 × 100 μm. A Raman spectrum of the obtained MoS2 flakes presents the A1g mode at 408 cm−1 and the E1 2g mode at 385 cm−1 (Figure 2C), in agreement with the typical vibration modes of MoS2.72 A high-resolution transmission electron microscopy (TEM) image shows the ultrathin layered structure with a lattice spacing of 0.27 nm, corresponding to the (101) interplanar distance of MoS2 (Figure 2D). The MoS2 flakes exfoliated by this method are mostly monolayers, except for some that are “few-layers” (<5 layers). A representative atomic force microscope (AFM) image in Figure S2 shows the typical thickness of 1.8 nm for few-layers. To demonstrate the large area size of the gold-mediated exfoliation over the traditional mechanical exfoliation method, we counted 100 flakes of both types and calculated their area sizes. The histograms show that the area size of MoS2 flakes obtained using this method (Figure 2E, bottom) is two orders of magnitude larger than that using the traditional method (Figure 2E, upper).
Figure 2.
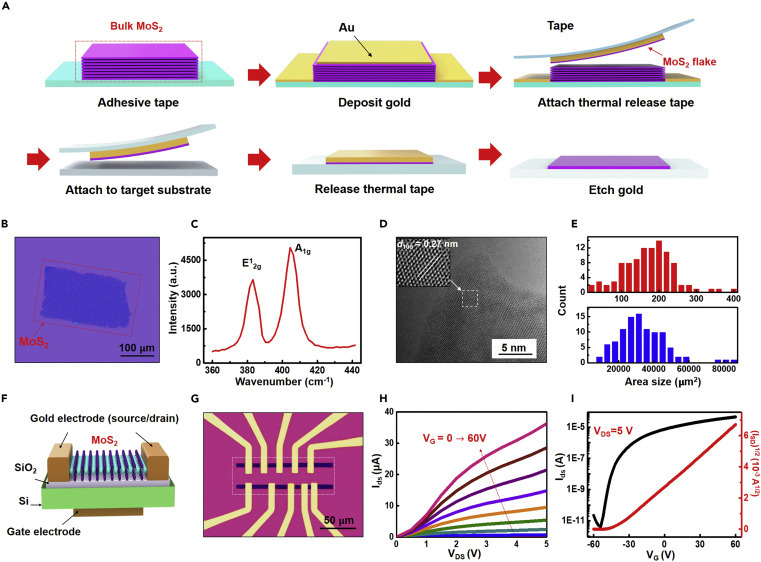
Transfer process and characterization of an exfoliated large-area MoS2 flake transistor
(A) Schematic illustration of the gold-mediated exfoliation process.
(B) Optical image of an exfoliated MoS2 flake with a lateral size of ~200 × 100 μm on a SiO2/Si substrate.
(C) Room-temperature Raman spectrum of MoS2 showing the A1g mode at 408 cm−1 and the E12g mode at 385 cm−1.
(D) High-resolution TEM image of the atomic layer of MoS2. Inset is the zoom-in view exhibiting a lattice d100 of 0.27 nm.
(E) Histogram comparing flake area size between traditional mechanical exfoliation (in red) and the gold-assisted exfoliation method (in blue).
(F) 3D schematics of the back-gated FET device on the SiO2/Si substrate.
(G) Optical image of the MoS2 FET device from the top. Different channel lengths were fabricated for optimization of the design.
(H) Output curve (source-drain current IDS versus source-drain voltage VDS) with the back-gate voltage VG sweep from 0 to 60 V.
(I) Transfer curve (IDS as a function of VG) with VDS = 5 V, shows n-type behavior with an on/off ratio of 107.
To quickly optimize the device configuration, MoS2 field-effect transistors (FETs) with a basic back-gate configuration were fabricated directly on a SiO2/Si substrate using a standard nanofabrication process without any other surface functionalization or dielectric coating (Figures 2F, 2G, and S3). The Ti/Au contacts (5 nm/150 nm) on top of MoS2 work as the source and drain electrodes, while the SiO2 (285 nm) underneath serves as the gate dielectric (Figure 2F). The highly p-doped silicon substrate, serving as the back-gate electrode, is connected with silver paste. As displayed in the optical image (Figure 2G), the exfoliated MoS2 flake is capable of hosting several FETs with different channel lengths, providing a platform for optimizing device configuration. Typical output characteristics (IDS versus VDS) (Figure 2H) illustrate the MoS2 FETs with an increasing VG from 0 to 60 V in steps of 6 V. The transfer curves (IDS versus VG, black for log scale, red for linear scale) at VDS = 5 V (Figure 2I) exhibit the n-type property with an ON/OFF ratio exceeding 107. The field-effect mobility can be extracted with the equation: , where L and W are channel length and channel width. The device displays ON currents of 10 μA μm−1 at VDS = 5 V with a field-effect mobility of 9.18 cm2/Vs, which agree with the reported parameters of monolayer MoS2 FETs using same or similar device configuration,50 , 73 , 74 while use of a top-gate configuration, or with additional surface functionalization or coating, could further exhibit superior performance.75 In our experiment, pure gold with high biocompatibility was deposited on the surface of MoS2 and used as the transfer layer, which can eliminate biologically toxic components or other obvious surface contaminants.76 Overall, the gold-mediated exfoliated MoS2 exhibits suitably high performance for application as transistors.
With the confirmation of the use of high-performance large-area ultrathin MoS2 transistors, we fabricated a hybrid, soft, smart contact lens with the key steps of “pattern-release-transfer” (Figure 3 A). The entire procedure started with spin-coating PI (~2.5 μm) onto a clean glass substrate as a bottom supporting layer, which was further cured at 150°C for 10 min followed by 250°C for 1 h. The exfoliated MoS2 flakes were transferred onto the PI layer through a standard printing process with a PDMS stamp77 (see the equipment in Figure S4), followed by patterning with a thin Cr mask (5 nm) and SF6/O2 reactive-ion etching (RIE). A thin Au layer (150 nm) was electron-beam evaporated and patterned on the PI to form a functional Au mesh with a serpentine structure. A top passivation layer of PI was further spin coated with the same thickness (~2.5 μm), cured, and patterned with oxygen plasma. The patterned geometries for two layers of PI match with those of the metal traces, which places the metal in a mechanically neutral plane while keeping sensors exposed. Finally, the entire sensor layer was cleaned and chemically released with buffered oxide etchant. This process can readily implement flexible and stretchable electronics on elastomeric substrates or curved surfaces. A PDMS contact lens substrate was baked and cured into a dome shape with two enclosed stainless-steel hollow hemispheres. To form a functional contact lens, we developed a physically adhesive strategy that allows the sensor layer to be accurately and firmly attached to the contact lens substrate. The sensor layer was first floated in deionized (DI) water and picked up by a lens substrate from the water (Figure S5). The width and radius of the serpentine mesh structure were rationally designed to match the curvature of the lens substrate perfectly for precise transfer. To ensure good adhesion between the sensor layer and the substrate, the entire lens was baked in a vacuum oven at 60°C for 30 min for dehydration and bubble release. To avoid the enzyme losing its efficacy, the device was kept dry, and finally treated with the glucose-sensing enzyme solution before use. Details of all other dimensions of the entire system are shown in Figure S6.
Figure 3.
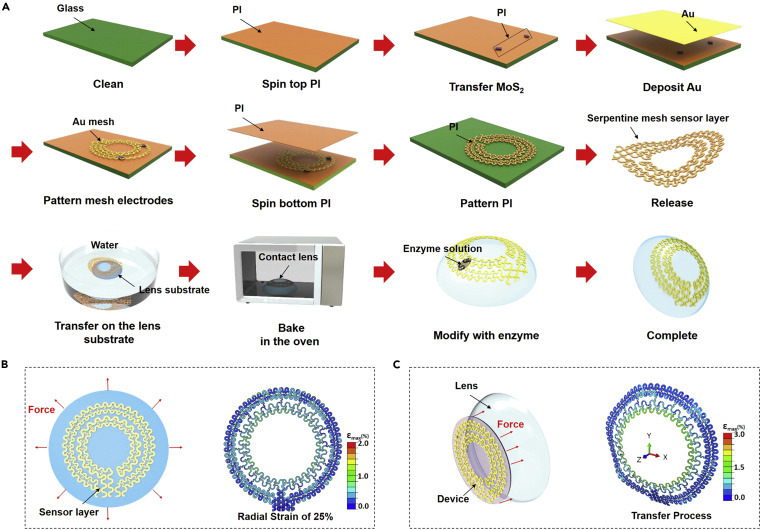
The “pattern-release-transfer” process and mechanical analysis of a smart contact lens
(A) Illustration of the fabrication process of a smart contact lens. We began by coating the bottom PI layer on a glass substrate. The MoS2 and gold mesh electrodes were consecutively placed and patterned to form the sensor and the interconnect layer. Subsequently, the top PI layer was coated and patterned, followed by release from the glass substrate. Finally, the device was picked up by the PDMS contact lens, oven baked, and enzyme modified to complete the process.
(B) Illustration for the sensor layer with the radial strain (x, y axis) (left) and the FEA strain map under a radial strain of 25% (right).
(C) Illustration of the sensor layer with the longitudinal strain on the perpendicular plane (z axis) (left) and the FEA strain map with 4 mm of z axis displacement (right).
In the process of transferring or wearing the contact lenses, curvature-induced stress and deformation may occur due to external forces or a geometric mismatch between the lens and the eyeball. Therefore, strain in the radial and longitudinal direction is possible, which may cause local buckling if the stress is larger than the critical buckling load. To understand the mechanical properties and verify the robustness of our design, we performed a finite element analysis (FEA) that showed the strain distribution of the sensor layer on the contact lens under different forces or deformations (Figures 3B and 3C) (see detailed FEA models in the Experimental procedures). Here, all the initial parameters were selected after consideration of the design and practical applications; the maximum tensile and deformation set here has far exceeded the normal operating range of contact lenses. When the sensor layer is placed under symmetrical radial strain from 5% to 25% (Figures 3B and S7) in a plane surface (x, y axes) or uniaxial tensile (x axis, Figure S8; y axis, Figure S9), the maximum principal strains are below 2.0%, lower than the fracture strain of gold film (~7%). The calculated strains in the outer electrode region, where MoS2 is situated, are below 0.5%, significantly lower than the fracture strain of MoS2 (~20%). Because the stretching is mainly absorbed by the deformations of serpentine interconnects, no mechanical fracture is expected on the device. When the sensor layer is placed under a longitudinal strain on the perpendicular plane (z axis) with a displacement from 0 to 4 mm (Figures 3C and S10), the maximum principal strains remain <3.0% and the strain in the regions with MoS2 remains below 0.5%.
We also investigated the mechanical properties of the smart contact lens when attached to an artificial eyeball (Figures S11A and S11B) to simulate operational use. Simulating the movement of the eyeball resulted in only up to 0.5 mm Z displacement with less than 1% strain in the serpentine interconnects, and <0.3% strain in the key sensing region (MoS2 and Au) (Figure S11C). These results show that all of the architecture of the serpentine mesh interconnects;78 the PI support layers dominate the mechanical performance of the devices, resulting in a low strain at key functional regions of devices when the substrate is under extreme deformation.,64 , 79, 80, 81 indicating the strong robustness of the device.
Characterization of the multifunctional sensor system
The multifunctional sensor system is composed of a photodetector, a glucose sensor, a temperature sensor, and with five interconnects where the MoS2-based photodetector and glucose sensor share one common source electrode and record signals independently by separated drain electrodes (Figure 4 A). The sensor system was tested after being connected with an external print circuit board through an anisotropic conductive film cable (Figure S12). Figure 4B gives a 3D schematic view of the photodetector with a large-top PI open window, and with 180 μm channel length for higher photosensitivity. The corresponding optical image is shown in Figure 4C. The photodetector was first characterized under pulsed illumination using a 365 nm wavelength UV light with an intensity of 4 nW under different VDS values (1 V, 2 V, and 3 V, Figure 4D). When the device is illuminated, it exhibits an increase of two orders of magnitude in photocurrent Iph (Iph = Ion − Ioff, where Ion and Ioff are the current under illumination and in the dark, respectively) and fast rise time (~50 ms) and fall time (~50 ms) (see magnified plot in Figure S13), indicating excellent photosensitivity and fast response to light illumination. This is enabled by the mechanism illustrated in Figure S14: the illumination can excite electrons from the valence to the conduction band and thus generate photocurrent under source-drain bias. The Iph increases with higher VDS values due to the faster carrier drift velocity. Research and studies claim that light or electromagnetic radiation can result in eye damage through various mechanisms, including photothermal, photomechanical, and photochemical mechanisms.82 Due to the demand of monitoring light harmful to the retina and of preventing light-induced retinal diseases caused by UV light and short high energy visible light (λ = 320–450 nm),83 , 84 we tested our device under different light wavelengths and illumination intensities (Figures 4E–4G). Figure 4E illustrates the IDS versus VDS curve under red laser light (λ = 650 nm), green laser light (λ = 532 nm), and UV light (λ = 365 nm) with the same intensity (5 nW). The Iph at the wavelength of 650 nm is smaller than that at 532 nm and further than 365 nm, indicating a higher transition probability with a shorter excitation wavelength. The optical response and photoresponsivity were further investigated under different UV illumination intensities (20–80 nW) at VDS = 5 V (Figures 4F and 4G). Here, the photoresponsivity R is defined as the ratio of the photocurrent Iph and the incident power P0:.85 The Iph is increased from 96 to 172 nA, with the intensity increasing from 20 to 80 nW, and R exhibiting very high responsivity for photodetection from 4.8 to 2.15 A/W. The decrease in R with intensity increase is due to the loss of photoexcited carriers by recombination effects, which is commonly observed in TMDC,86 and the saturation of R is due to the screening effects of space charge.87 Overall, this ultrathin MoS2 photodetector exhibits a fast response, high current on/off ratio, and high responsivity for photodetection. The higher responsivity under harmful wavelengths makes it useful for hazard warning, thereby avoiding potential damage to the eyes.
Figure 4.
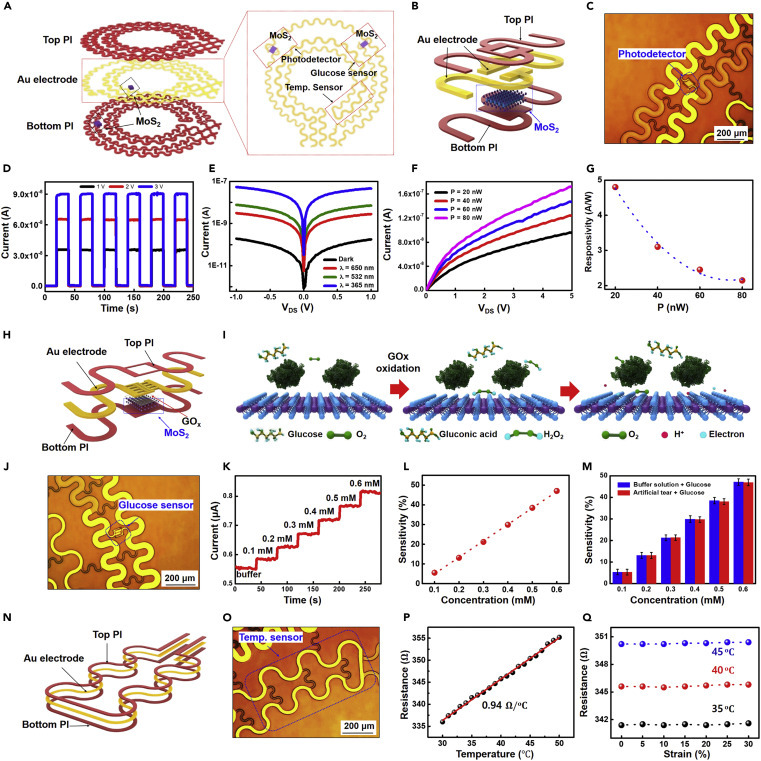
The characteristics of the multifunctional sensor system
(A) Schematic illustration of the multifunctional sensor structure.
(B and C) 3D schematic illustration (B) and optical image (C) of the two-terminal MoS2 photodetector.
(D) Photoswitching behavior under pulsed illumination by 365 nm wavelength UV light with different VDS values.
(E) Drain-source characteristics in the dark and under illumination with different wavelengths of light.
(F) Drain-source characteristics of the photodetector under different illuminating light intensities.
(G) The photoresponsivity of the MoS2 phototransistor, exhibiting a high photoresponsivity of 4.8 A/W for a UV power of 20 nW.
(H) Schematic illustration of ac MoS2 glucose sensor.
(I) Illustration of the sensing mechanism of the device with oxidation of glucose.
(J) Optical image of the MoS2 glucose sensor.
(K) Time versus current curve based on changes in glucose levels.
(L) The sensitivity (|R|/R0) of the glucose sensor with a buffer solution, where R0 is the initial resistance at zero glucose concentration.
(M) Sensitivity of the sensors with the PBS buffer solution (blue) and the artificial tear solution (red). Five devices with each solution were tested and the standard deviation is represented by error bars.
(N and O) Schematic illustration (N) and optical image (O) of the temperature sensor.
(P) The temperature-dependent resistance curve for the Au thermal resistor.
(Q) The resistance of the temperature sensor versus strain under different temperatures.
In addition to photodetection, we also characterized the glucose-sensing property. Figures 4H and 4J give a 3D schematic view and an optical image of the MoS2-based glucose sensor, showing a large open window on the top of the PI layer and interdigital source/drain electrodes with an optimized channel length of 30 μm. This structure is designed to increase sensitivity by using a large area to absorb and immobilize the enzyme and to allow contact with the tear fluid for better glucose recognition and enhanced conductance. Here, we chose immobilized glucose oxidase (GOD) as the bio-enzyme for glucose sensing. As the standard enzyme for biosensors, GOD has a high selectivity for glucose with fully or quasi-reversible glucose conversion, is easy to obtain, and can withstand great extremes of pH, ionic strength, and temperature; it shows good durability and stability in the long term and has reversible implantable biosensors.88, 89, 90 As shown in Figure 4I, the glucose sensing begins with the immobilization of GOD (β-d-glucose from Aspergillus niger) on the MoS2 surface.91 , 92 The charge transfer process is attributed to the reaction described below, where the oxidation of glucose with GOD forms H2O2, which then reacts with oxygen and produces hydrogen ions (H+) and electrons (e−):
The free electrons generated by this reaction contribute to an increase of device current owing to the n-type FET behavior. Figure 4K plots the real-time response of glucose concentration versus current with a fixed bias of 5 V. Testing started with a pure phosphate-buffered saline (PBS) solution, increasing the concentration from 0.1 to 0.6 mM, which is within the same level of glucose concentration typically found in human tear fluid (mean values: 0.16 ± 0.03 mM) and diabetic tear fluid (mean values: 0.35 ± 0.04 mM).93 , 94 The significant current changes indicate high sensitivity (|R|/R0, e.g., 48% at 0.6 mM) proportional to the glucose levels (Figure 4L). The glucose buffer solution and artificial tears from other species were then used to test the selectivity of the glucose sensor. As shown in Figure 4M, there is no significant variation in the sensitivity of the device between these two solutions, indicating a good selectivity of for detecting glucose in the tear fluid. A variety of enzyme-based and enzyme-free glucose biosensors with different sensing materials have been reported, including metal oxides, nanoparticles, and low-dimensional materials. Table S2 summarizes and lists some of the most recent materials studied. Our results show relatively high sensitivity in the desired range of detection, while the selectivity is guaranteed by selection of the enzyme. The sensor was also tested with various concentrations of glucose in artificial tears for up to 24 h. These experiments showed no visible change in sensitivity of the sensor, indicating the high stability of the sensor (Figure S15).
Another key function of our smart contact lens is temperature sensing. Although the accurate and continuous monitoring of temperature variation of the cornea remains a challenge due to the tenderness of eyes, it can be a powerful tool to provide additional information about ocular physiology and pathology.95Therefore, there is high demand for smart contact lenses equipped with a low-cost, lightweight, and flexible temperature sensor. In our design, a serpentine, thin Au wire-based temperature sensor is integrated into the lens (Figures 4N and 4O). To establish the relationship between the temperature and thermal resistance of the sensor, the device was first calibrated in boiled DI water with a platinum (Pt)-based resistance temperature detector (RTD thermometer, Center 376) as a standard temperature reference. The calibration temperature spans from 30°C to 50°C, covering the full human body range and ocular surface temperature. Figure 4P displays the temperature-dependent resistance of the Au wire sensor, whose slope represents a high sensitivity of 0.94 Ω/°C and shows good linearity of the device to temperature.
The sensing properties of the device under a mechanical strain of up to 30% and Z displacement of up to 4 mm were further studied to ensure reliable operation on soft contact lenses. When the devices are stretched up to 30% of uniaxial tensile strain on the x/y axes (Figure S16) or with large displacement in the z axis (Figure S18A), the performance of the MoS2 transistor-based photodetector and glucose sensor remains largely unchanged (i.e., relative changes of less than 8%) (Figures S17, S18C, and S18D). The resistance of the Au wire-based temperature sensor also shows negligible change due to mechanical strain (Figures 4K and S18B). These results, plus the previous mechanical simulation, further indicate the strong robustness of the integrated contact lens sensor system with good sensing stability.
Characterization of the transparency and cytotoxicity of the smart contact lens
To check the transparency of the smart contact lens, we measured the optical transmittance and haze using a visible light spectrum from 400 to 800 nm (Figures 5A–5C). The contact lens exhibits high optical transmittance of ~93% and low haze of ~3% in the entire wavelength range (Figure 5B) and remains stable under a tensile strain from 0% to 30% (Figure 5C).
Figure 5.
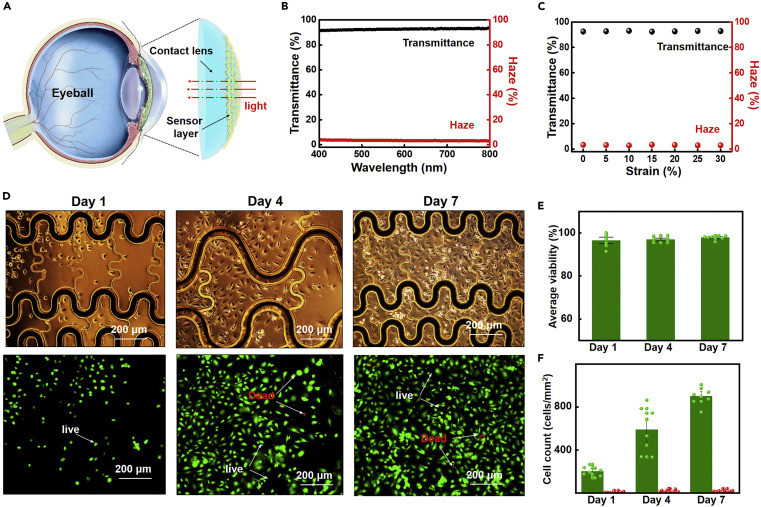
The optical transmittance/haze test and in vitro cytotoxicity test of the smart contact lens
(A) Schematic illustration of optical transmittance testing.
(B) Optical transmittance (black) and haze (red) spectra from 400 to 800 nm.
(C) Optical properties at 55 nm as a function of tensile strain up to 30%.
(D) The optical images (upper) and fluorescent images (lower) of the HUVECs at different times. In a live/dead assay, green and red fluorescence indicate living and dead cells, respectively.
(E) The average viability (percent of living cells) at days 1, 4, and 7. The percentage of living cells remained the same during the 7 days.
(F) Cell count (number of cells per area) at different times. The increasing number of living cells demonstrates cell culture reliability.
To confirm the biocompatibility of the lenses, in vitro cytotoxicity tests were performed using human umbilical vein endothelial cells (HUVECs) that were seeded and cultured for 7 days (see details in the Experimental procedures). Here HUVECs were chosen as the test model system because of their low cost, the simple technique used for isolating them, and because they are closely related to cytotoxicity in the eye system.96 The endothelial cells inside the blood vessels are a critical component in angiogenesis where new blood vessels are formed and are similar to the blood vessels grown from retinal blood cell in retinal neovascularization.97 Tissue engineering has proved that it is a good candidate for prediction of ocular toxicology.98 , 99 Figure 5D illustrates the liveness and mitotic behaviors of micro cells with an optical image with live cells on the substrate (upper) and corresponding fluorescence images (lower) on days 1, 4, and 7. Two fluorescence dyes—calcein AM and propidium iodide—are used in the cell assay, which stains live and dead cells to green and red colors, respectively. Figure 5E presents the average viability of over 95% at different time points, consistent with the regular cell culture results. The numbers of live (green) and dead (red) cells were obtained via the fluorescence experiments in Figure 5F. The number of live cells grows significantly throughout the duration of the experiment, while the number of dead cells remains low. These results thereby provide clear evidence that the smart contact lens is not cytotoxic to human endothelial cells, indicating good biocompatibility.
Conclusions
In summary, we have demonstrated a facile approach for fabricating multifunctional ultrathin MoS2 transistors and a Au wire-based sensor system that could be directly incorporated onto soft contact lenses using an easy assembly method that leaves sensors exposed to directly contact the tear fluid, providing high detection sensitivity. The thin donut serpentine mesh architecture ensures strong mechanical robustness, high stretchability, and eye comfort, while maintaining high transparency. In vitro studies have shown that this system is fully biocompatible and non-toxic to human cells. The capabilities of the devices include detection of optical signals, glucose levels, and corneal temperature, which are essential in ocular-related research. These results provide not only an alternative solution for manufacturing advanced smart contact lenses but also a novel insight into designing other multifunctional implantable bioelectronics. In addition, our integrated contact lens sensor system and fabrication strategy allows for incorporation of other functional components, such as electrode arrays for electroretinograms,36 , 37 antennas for wireless communication25, 26, 27 and power modules (e.g., thin-film batteries/supercapacitors),100, 101, 102 biofuel cells,103 moisture-based energy harvesting,104 or wireless radiofrequency105 for future in vivo research.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yunlong Zhao (yunlong.zhao@surrey.ac.uk).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze any datasets/code.
Device fabrication
Gold-mediated exfoliation
A 2D bulk MoS2 was first obtained by being peeled from a large crystal using adhesive tape (3M Blue Vinyl 471, 3M), followed by e-beam evaporation of a thin layer of Au film (120 nm) on top of the MoS2 bulk. A thermal release tape (3195M, Semiconductor Equipment) was then attached to the top of the Au film, transferred to a target substrate, and kept on a hot plate (120°C) until released. The target substrate was cleaned with acetone, isopropanol, and O2 plasma. The Au layer was wet etched using a gold etchant (KI/I2). The substrate with the MoS2 flake can be further cleaned by immersing and rinsing in acetone at 60°C for 20 min to remove organic residues from the thermal tape.
Fabrication of devices on a rigid substrate
MoS2 flakes were exfoliated and transferred to a SiO2/Si substrate. The active channel was then patterned and etched by RIE (O2/SF6). An e-beam evaporator was used to deposit the metal layer (10 nm Ti/150 nm Au), followed by the patterning of the source and drain electrodes with photolithography.
PDMS lens mold
The elastomer and curing agent were mixed at a ratio of 10:1, followed by stirring and de-bubbling. The mixture was then poured into the gap between two concentric stainless-steel half-sphere molds and cured in a vacuum oven (StableTemp oven, Cole-Parmer) at 60°C for 30 min.
The fabrication of the smart contact lens with a “pattern-release-transfer” process is detailed in the main text.
Structure and performance characterization
Material characterization
Optical microscopy (AmScope) was used for imaging the materials and devices. Raman spectroscopy (Laser Quantum DPSS, 532 nm) was used for material verification by identification peaks associated with single-layer MoS2. A Bruker AFM was used for measurements of the height of the MoS2 flakes, and TEM (FEI Titan 80-300) was used to obtain a high-resolution crystal structure.
Electrical and optical measurements
The electrical measurements were conducted using a parameter analyzer (Agilent B1500A) under ambient conditions. A Panasonic NUJ6170/NU6420 light source (365 nm) was used for intensity-tunable UV light illumination. Laser light (Meade Instruments) for green and red illumination was applied for characterization of various wavelengths. The optical transmittance measurements were conducted with the transmittance of the substrate subtracted. Optical haze was calculated as the ratio of diffuse transmittance (Tdiffuse) to total transmittance (Ttotal): (Tdiffuse/Ttotal × 100%).
Strain test
The device was transferred onto a highly elastic PDMS film to perform the strain test with a tensile strain from 0% to 30%.
Preparation of the glucose solution
The glucose solution, with different concentrations, was prepared using 100 mL of PBS solution (0.1 mol/L) or artificial tear solution (Refresh Optive Advanced preservative-free lubricant eye drops) as the solvent. Different quantities of the glucose of were dissolved in the solvent to obtain glucose solution concentrations of 0.1–0.6 mM.
Mechanical FEA models
FEA simulations based on a commercial software package (Abaqus 6.14) allowed examination of the strain distribution of stretchable electronic devices with serpentine interconnect structures. These structures, in both 3D (contact lens with a ball radius of 10 mm, thickness of 50 μm, and crown height of 4 mm) and 2D (contact lens with a thickness of 50 μm and diameter of 20 mm), were modeled by the composite shell element (S4R). The eight-node solid element modeled the eyeball, with the eyeball being regarded as a rigid body. The sensor device and contact lens were tied together using the tie constraints in Abaqus. The friction between the contact lens and the eyeball was neglected. Radial displacement and uniaxial tensile displacement (x axis and y axis tensile state) were applied to the boundary of the contact lens to explore the PI strain distributions of the serpentine interconnects, respectively. The stretchable electronic device attached to the contact lens was adhered to the eyeball by applying Z direction displacements at the boundary of the contact lens, with the eyeball fixed. The eyeball was then moved 0.5 mm along the z axis, while the boundary of the coronal contact lens was fixed. Young's modulus (E) and Poisson's ratio (v) of the materials were set as follows: PI, E PI = 2.5 GPa and v PI = 0.34; gold, E Au = 79 GPa and v Au = 0.4; MoS2, E MoS2 = 330 GPa and v MoS2 = 0.3; PDMS contact lens, E c = 0.5 MPa and v c = 0.33.
Cell culture and biocompatibility studies
HUVECs cultured at 37°C and 5% CO2 with endothelial cell basal medium-2 (EBM-2, Lonza) and an EGM-2 SingleQuots growth supplement kit (Lonza) were trypsinized using 0.25% trypsin-EDTA solution for 4 min, centrifuged at 200 rpm for 5 min, resuspended in the cell culture medium, and counted using a blood counting chamber. Then the cell suspension (containing ~5 × 104 cells) was seeded in a 35 mm Petri dish containing the electronic device. After adding 5 mL of fresh medium, the Petri dish was placed in an incubator at 37°C and 5% CO2 (Forma 3131, Thermo Scientific). The cell viability of the HUVECs and the cytotoxicity of the device were identified on days 1, 4, and 7 by staining the cells with two fluorescence dyes: calcein AM and propidium iodide from Aladdin Industrial (Shanghai, China). In the experiments, the working concentrations of calcein AM and propidium iodide were 2 μM and 10 μg/mL, respectively. Cells were incubated at 37°C for ~30 min after the two dyes were added and imaged in an inverted fluorescence microscope (Olympus IX 53). The green-stained cells were living while the red-stained cells were dead. Cell viability (%) was expressed by the ratio of the number of living cells to the total number of cells, quantified using ImageJ software. The experiments were repeated at least three times.
Acknowledgments
We would like to thank GW Nanofabrication and Imaging Center for providing the nanofabrication facilities. This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication. We also appreciate the support from the UK National Measurement System and ISCF Measurement Fellowship.
Author contributions
D.Y. conceived the concept and designed the experiments. S.G. carried out the main experiments. K.W. performed the mechanism analysis. Y.Z. supervised the research process. S.G., D.Y., and Y.Z. analyzed the data and wrote the manuscript. C.L., H.W., W.D., and C.W. carried out the biological experiments. Z.S. and S.Z collected the temperature sensor and glucose sensor data. D.X. performed the optical transmittance test. M.E.Z. and F.A.C. provided advice on the concept. All authors contributed to the discussion and provided feedback on the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: December 30, 2020
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.matt.2020.12.002.
Supplemental information
References
- 1.Morales-Narváez E., Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020:112274. doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Da Silva Neves M.M.P., González-García M.B., Hernandez-Santos D., Fanjul-Bolado P. Future trends in the market for electrochemical biosensing. Curr. Opin. Electrochem. 2018;10:107–111. [Google Scholar]
- 3.Mück J.E., Ünal B., Butt H., Yetisen A.K. Market and patent analyses of wearables in medicine. Trends Biotechnol. 2019;37:563–566. doi: 10.1016/j.tibtech.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Campbell A.S., de Ávila B.E.-F., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birmingham K., Gradinaru V., Anikeeva P., Grill W.M., Pikov V., McLaughlin B., Pasricha P., Weber D., Ludwig K., Famm K. Bioelectronic medicines: a research roadmap. Nat. Rev. Drug Discov. 2014;13:399–400. doi: 10.1038/nrd4351. [DOI] [PubMed] [Google Scholar]
- 6.An B.W., Shin J.H., Kim S.Y., Kim J., Ji S., Park J., Lee Y., Jang J., Park Y.G., Cho E., et al. Smart sensor systems for wearable electronic devices. Polymers (Basel) 2017;9:303. doi: 10.3390/polym9080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y., Tian B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 2018;3:473–490. doi: 10.1038/s41578-018-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers J.A., Ghaffari R., Kim D.-H. Springer; 2016. Stretchable Bioelectronics for Medical Devices and Systems. [Google Scholar]
- 9.Someya T., Bao Z., Malliaras G.G. The rise of plastic bioelectronics. Nature. 2016;540:379–385. doi: 10.1038/nature21004. [DOI] [PubMed] [Google Scholar]
- 10.Yetisen A.K., Martinez-Hurtado J.L., Ünal B., Khademhosseini A., Butt H. Wearables in medicine. Adv. Mater. 2018;30:1706910. doi: 10.1002/adma.201706910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y., Nyein H.Y.Y., Gao W., Javey A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 2020;32:1902083. doi: 10.1002/adma.201902083. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A., Lieber C.M. Nano-bioelectronics. Chem. Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo S., Wu S., Xu J., Zhang X., Zou L., Yao R., et al. Osteogenic differentiation of BMSCs on MoS2 composite nanofibers with different cell seeding densities. Appl. Nanosci. 2020;10:3703–3716. doi: 10.1007/s13204-020-01473-0. [DOI] [Google Scholar]
- 14.Bhavnani S.P., Narula J., Sengupta P.P. Mobile technology and the digitization of healthcare. Eur. Heart J. 2016;37:1428–1438. doi: 10.1093/eurheartj/ehv770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S.R., Lieber C.M. Precision electronic medicine in the brain. Nat. Biotechnol. 2019;37:1007–1012. doi: 10.1038/s41587-019-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J.C., Mun J., Kwon S.Y., Park S., Bao Z., Park S. Electronic skin: recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 2019;31:1904765. doi: 10.1002/adma.201904765. [DOI] [PubMed] [Google Scholar]
- 17.Yu K.-H., Beam A.L., Kohane I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X. Nanowires pin neurons: “a nano Moon Landing”. Matter. 2019;1:560–562. [Google Scholar]
- 19.Kim J., Cha E., Park J.U. Recent advances in smart contact lenses. Adv. Mater. Tech. 2020;5:1900728. [Google Scholar]
- 20.Bennett E.S., Henry V.A. Lippincott Williams & Wilkins; 2019. Clinical Manual of Contact Lenses. [Google Scholar]
- 21.Farandos N.M., Yetisen A.K., Monteiro M.J., Lowe C.R., Yun S.H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 2015;4:792–810. doi: 10.1002/adhm.201400504. [DOI] [PubMed] [Google Scholar]
- 22.Moreddu R., Vigolo D., Yetisen A.K. Contact lens technology: from fundamentals to applications. Adv. Healthc. Mater. 2019;8:1900368. doi: 10.1002/adhm.201900368. [DOI] [PubMed] [Google Scholar]
- 23.Tseng R.C., Chen C.-C., Hsu S.-M., Chuang H.-S. Contact-lens biosensors. Sensors. 2018;18:2651. doi: 10.3390/s18082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kownacka A.E., Vegelyte D., Joosse M., Anton N., Toebes B.J., Lauko J., Buzzacchera I., Lipinska K., Wilson D.A., Geelhoed-Duijvestijn N., Wilson C.J. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing a biopolymer coating. Biomacromolecules. 2018;19:4504–4511. doi: 10.1021/acs.biomac.8b01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu M., Iguchi S., Miyajima K., Arakawa T., Kudo H., Mitsubayashi K. In: 5th European Conference of the International Federation for Medical and Biological Engineering. Jobbágy A., editor. Springer; 2011. Development of a soft contact-lens biosensor for in-vivo tear glucose monitoring; pp. 1007–1010. [Google Scholar]
- 26.Kim J., Kim M., Lee M.-S., Kim K., Ji S., Kim Y.-T., Park J., Na K., Bae K.-H., Kim H.K. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017;8:1–8. doi: 10.1038/ncomms14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.-K., Koo J., Lee G.-H., Jeon C., Mok J.W., Mun B.H., Lee K.J., Kamrani E., Joo C.-K., Shin S. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020;6:eaba3252. doi: 10.1126/sciadv.aba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J., Kim J., Kim S.-Y., Cheong W.H., Jang J., Park Y.-G., Na K., Kim Y.-T., Heo J.H., Lee C.Y. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018;4:eaap9841. doi: 10.1126/sciadv.aap9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu M.X., Miyajima K., Takahashi D., Arakawa T., Sano K., Sawada S.-i., Kudo H., Iwasaki Y., Akiyoshi K., Mochizuki M. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta. 2011;83:960–965. doi: 10.1016/j.talanta.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.H., Cho Y.C., Choy Y.B. Noninvasive self-diagnostic device for tear collection and glucose measurement. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-41066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matz K., Keresztes K., Tatschl C., Nowotny M., Dachenhausen A., Brainin M., Tuomilehto J. Disorders of glucose metabolism in acute stroke patients: an underrecognized problem. Diabetes Care. 2006;29:792–797. doi: 10.2337/diacare.29.04.06.dc05-1818. [DOI] [PubMed] [Google Scholar]
- 32.Koponen H., Kautiainen H., Leppänen E., Mäntyselkä P., Vanhala M. Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiatry. 2015;15:163. doi: 10.1186/s12888-015-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen M.C., Vatner D.F., Shulman G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017;13:572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabiani C., Li Voti R., Rusciano D., Mutolo M.G., Pescosolido N. Relationship between corneal temperature and intraocular pressure in healthy individuals: a clinical thermographic analysis. J. Ophthalmol. 2016;2016:3076031. doi: 10.1155/2016/3076031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan L.L., Sanjay S., Morgan P.B. Static and dynamic measurement of ocular surface temperature in dry eyes. J. Ophthalmol. 2016;2016:7285132. doi: 10.1155/2016/7285132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawali A.A. Thermography in ocular inflammation. Indian J. Radiol. Imaging. 2016;23:281–283. [PMC free article] [PubMed] [Google Scholar]
- 37.Hong G., Fu T.-M., Qiao M., Viveros R.D., Yang X., Zhou T., Lee J.M., Park H.-G., Sanes J.R., Lieber C.M. A method for single-neuron chronic recording from the retina in awake mice. Science. 2018;360:1447–1451. doi: 10.1126/science.aas9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin R., Xu Z., Mei M., Chen Z., Wang K., Liu Y., Tang T., Priydarshi M.K., Meng X., Zhao S. Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-04781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vásquez Quintero A., Verplancke R., De Smet H., Vanfleteren J. Stretchable electronic platform for soft and smart contact lens applications. Adv. Mater. Tech. 2017;2:1700073. [Google Scholar]
- 40.Alipour F., Khaheshi S., Soleimanzadeh M., Heidarzadeh S., Heydarzadeh S. Contact lens-related complications: a review. J. Ophthalmic Vis. Res. 2017;12:193. doi: 10.4103/jovr.jovr_159_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jariwala D., Sangwan V.K., Lauhon L.J., Marks T.J., Hersam M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano. 2014;8:1102–1120. doi: 10.1021/nn500064s. [DOI] [PubMed] [Google Scholar]
- 42.Chhowalla M., Shin H.S., Eda G., Li L.-J., Loh K.P., Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013;5:263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 43.Tan C., Zhang H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015;44:2713–2731. doi: 10.1039/c4cs00182f. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q.H., Kalantar-Zadeh K., Kis A., Coleman J.N., Strano M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 45.Choi C., Choi M.K., Liu S., Kim M.S., Park O.K., Im C., Kim J., Qin X., Lee G.J., Cho K.W. Human eye-inspired soft optoelectronic device using high-density MoS2-graphene curved image sensor array. Nat. Commun. 2017;8:1–11. doi: 10.1038/s41467-017-01824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang N., Montelongo Y., Butt H., Yetisen A.K. Microfluidic contact lenses. Small. 2018;14:1704363. doi: 10.1002/smll.201704363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filipe H., Bozukova D., Pimenta A., Vieira A., Alves P., Coimbra P., Guiomar A., Gil M., Mata J., Serro A. Surface modification of intraocular lenses towards controlled drug delivery. Acta Ophthalmol. 2015;93 doi: 10.1111/j.1755-3768.2015.0561. [DOI] [Google Scholar]
- 48.Kamei T., Wada T. Contact-lens type of micromachined hydrogenated amorphous Si fluorescence detector coupled with microfluidic electrophoresis devices. Appl. Phys. Lett. 2006;89:114101. [Google Scholar]
- 49.Radisavljevic B., Radenovic A., Brivio J., Giacometti V., Kis A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011;6:147. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Sanchez O., Lembke D., Kayci M., Radenovic A., Kis A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013;8:497–501. doi: 10.1038/nnano.2013.100. [DOI] [PubMed] [Google Scholar]
- 51.Shokri A., Salami N. Gas sensor based on MoS2 monolayer. Sens. Actuat. B Chem. 2016;236:378–385. [Google Scholar]
- 52.Kim T.H., Kim Y.H., Park S.Y., Kim S.Y., Jang H.W. Two-dimensional transition metal disulfides for chemoresistive gas sensing: perspective and challenges. Chemosensors. 2017;5:15. [Google Scholar]
- 53.Jariwala D., Sangwan V.K., Late D.J., Johns J.E., Dravid V.P., Marks T.J., Lauhon L.J., Hersam M.C. Band-like transport in high mobility unencapsulated single-layer MoS2 transistors. Appl. Phys. Lett. 2013;102:173107. [Google Scholar]
- 54.Cho B., Hahm M.G., Choi M., Yoon J., Kim A.R., Lee Y.-J., Park S.-G., Kwon J.-D., Kim C.S., Song M. Charge-transfer-based gas sensing using atomic-layer MoS2. Sci. Rep. 2015;5:8052. doi: 10.1038/srep08052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee G.-H., Yu Y.-J., Cui X., Petrone N., Lee C.-H., Choi M.S., Lee D.-Y., Lee C., Yoo W.J., Watanabe K. Flexible and transparent MoS2 field-effect transistors on hexagonal boron nitride-graphene heterostructures. ACS Nano. 2013;7:7931–7936. doi: 10.1021/nn402954e. [DOI] [PubMed] [Google Scholar]
- 56.Velusamy D.B., Kim R.H., Cha S., Huh J., Khazaeinezhad R., Kassani S.H., Song G., Cho S.M., Cho S.H., Hwang I. Flexible transition metal dichalcogenide nanosheets for band-selective photodetection. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo S., Yang D., Zhang S., Dong Q., Li B., Tran N., Li Z., Xiong Y., Zaghloul M.E. Development of a cloud-based epidermal MoSe2 device for hazardous gas sensing. Adv. Funct. Mater. 2019;29:1900138. [Google Scholar]
- 58.Splendiani A., Sun L., Zhang Y., Li T., Kim J., Chim C.-Y., Galli G., Wang F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010;10:1271–1275. doi: 10.1021/nl903868w. [DOI] [PubMed] [Google Scholar]
- 59.Bertolazzi S., Brivio J., Kis A. Stretching and breaking of ultrathin MoS2. ACS Nano. 2011;5:9703–9709. doi: 10.1021/nn203879f. [DOI] [PubMed] [Google Scholar]
- 60.Plechinger G., Mann J., Preciado E., Barroso D., Nguyen A., Eroms J., Schueller C., Bartels L., Korn T. A direct comparison of CVD-grown and exfoliated MoS2 using optical spectroscopy. Semiconductor Sci. Technol. 2014;29:064008. [Google Scholar]
- 61.Chen X., Park Y.J., Kang M., Kang S.-K., Koo J., Shinde S.M., Shin J., Jeon S., Park G., Yan Y. CVD-grown monolayer MoS2 in bioabsorbable electronics and biosensors. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-03956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim T.-Y., Song Y., Cho K., Amani M., Ahn G.H., Kim J.-K., Pak J., Chung S., Javey A., Lee T. Analysis of the interface characteristics of CVD-grown monolayer MoS2 by noise measurements. Nanotechnology. 2017;28:145702. doi: 10.1088/1361-6528/aa60f9. [DOI] [PubMed] [Google Scholar]
- 63.Desai S.B., Madhvapathy S.R., Amani M., Kiriya D., Hettick M., Tosun M., Zhou Y., Dubey M., Ager J.W., III, Chrzan D. Gold-mediated exfoliation of ultralarge optoelectronically-perfect monolayers. Adv. Mater. 2016;28:4053–4058. doi: 10.1002/adma.201506171. [DOI] [PubMed] [Google Scholar]
- 64.Kim D.-H., Lu N., Ma R., Kim Y.-S., Kim R.-H., Wang S., Wu J., Won S.M., Tao H., Islam A. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 65.Su Y., Ping X., Yu K.J., Lee J.W., Fan J.A., Wang B., et al. In-plane deformation mechanics for highly stretchable electronics. Adv. Mater. 2017;29:1604989. doi: 10.1002/adma.201604989. [DOI] [PubMed] [Google Scholar]
- 66.Hwang S.-W., Lee C.H., Cheng H., Jeong J.-W., Kang S.-K., Kim J.-H., Shin J., Yang J., Liu Z., Ameer G.A. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015;15:2801–2808. doi: 10.1021/nl503997m. [DOI] [PubMed] [Google Scholar]
- 67.An H.B., Chen L.Z., Liu X.J., Zhao B., Ma D.L., Wu Z.G. A method of manufacturing microfluidic contact lenses by using irreversible bonding and thermoforming. J. Micromech. Microeng. 2018;28:105008. [Google Scholar]
- 68.Chen J.S., Liu T.Y., Tsou H.M., Ting Y.S., Tseng Y.Q., Wang C.H. Biopolymer brushes grown on PDMS contact lenses by in situ atmospheric plasma-induced polymerization. J. Polym. Res. 2017;24:69. [Google Scholar]
- 69.Musgrave C.S.A., Fang F. Contact lens materials: a materials science perspective. Materials (Basel) 2019;12:261. doi: 10.3390/ma12020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marc P., Wolf G.B.S.-B., Hunziker P. PDMS with designer functionalities—properties, modifications strategies, and applications. Prog. Polym. Sci. 2018;83:97–134. [Google Scholar]
- 71.Quintana-Puebla A., Paranjape M. Systematic characterization of hydrophilized polydimethylsiloxane. J. Microelectromechanical Syst. 2020;29:1216–1224. [Google Scholar]
- 72.Jeong S., Shin H.-Y., Shin R., Jo W., Yoon S., Rübhausen M. Raman scattering studies of the lattice dynamics in layered MoS2. J. Korean Phys. Soc. 2015;66:1575–1580. [Google Scholar]
- 73.Bao W., Cai X., Kim D., Sridhara K., Fuhrer M.S. High mobility ambipolar MoS2 field-effect transistors: substrate and dielectric effects. Appl. Phys. Lett. 2013;102:042104. [Google Scholar]
- 74.Radisavljevic B., Kis A. Mobility engineering and a metal–insulator transition in monolayer MoS2. Nat. Mater. 2013;12:815–820. doi: 10.1038/nmat3687. [DOI] [PubMed] [Google Scholar]
- 75.Yu Z., Ong Z., Li S., Xu J., Zhang G., Zhang Y., Shi Y., Wang X. Analyzing the carrier mobility in transition-metal dichalcogenide MoS2 field-effect transistors. Adv. Funct. Mater. 2017;18:1604093. [Google Scholar]
- 76.Wang H.H., Su C.H., Wu Y.J., Lin C.A.J., Lee C.H., Shen J.L., Chan W.H., Chang W.H., Yeh H.I. Application of gold in biomedicine: past, present and future. Int. J. Gerontol. 2012;6:1–4. [Google Scholar]
- 77.Menard E., Lee K., Khang D.-Y., Nuzzo R.G., Rogers J.A. A printable form of silicon for high performance thin film transistors on plastic substrates. Appl. Phys. Lett. 2004;84:5398–5400. [Google Scholar]
- 78.Verplancke R., Bossuyt F., Cuypers D., Vanfleteren J. Thin-film stretchable electronics technology based on meandering interconnections: fabrication and mechanical performance. J. Micromechanics Microengineering. 2011;22:015002. [Google Scholar]
- 79.Huang X., Liu Y., Kong G.W., Seo J.H., Ma Y., Jang K.-I., Fan J.A., Mao S., Chen Q., Li D. Epidermal radio frequency electronics for wireless power transfer. Microsystems Nanoengineering. 2016;2:1–9. doi: 10.1038/micronano.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu S., Zhang Y., Cho J., Lee J., Huang X., Jia L., Fan J.A., Su Y., Su J., Zhang H. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013;4:1–8. doi: 10.1038/ncomms2553. [DOI] [PubMed] [Google Scholar]
- 81.Jang K.-I., Li K., Chung H.U., Xu S., Jung H.N., Yang Y., Kwak J.W., Jung H.H., Song J., Yang C. Self-assembled three dimensional network designs for soft electronics. Nat. Commun. 2017;8:1–10. doi: 10.1038/ncomms15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Youssef P.N., Sheibani N., Albert D.M. Retinal light toxicity. Eye (London, England) 2011;25:1–14. doi: 10.1038/eye.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young R.W. The family of sunlight-related eye diseases. Optom. Vis. Sci. 1994;71:125–144. doi: 10.1097/00006324-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Rozanowska M.B. Light-induced damage to the retina: current understanding of the mechanisms and unresolved questions: a symposium-in-print. Photochem. Photobiol. 2012;88:1303–1308. doi: 10.1111/j.1751-1097.2012.01240.x. [DOI] [PubMed] [Google Scholar]
- 85.De Fazio D., Goykhman I., Yoon D., Bruna M., Eiden A., Milana S., Sassi U., Barbone M., Dumcenco D., Marinov K. High responsivity, large-area graphene/MoS2 flexible photodetectors. ACS Nano. 2016;10:8252–8262. doi: 10.1021/acsnano.6b05109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saenz G.A., Karapetrov G., Curtis J., Kaul A.B. Ultra-high photoresponsivity in suspended metal-semiconductor-metal mesoscopic multilayer MoS2 broadband detector from UV-to-IR with low Schottky barrier contacts. Sci. Rep. 2018;8:1276. doi: 10.1038/s41598-018-19367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mueller T., Xia F., Avouris P. Graphene photodetectors for high-speed optical communications. Nat. Photon. 2010;4:297. [Google Scholar]
- 88.Bankar S.B., Bule M.V., Singhal R.S., Ananthanarayan L. Glucose oxidase—an overview. Biotechnol. Adv. 2009;27:489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 89.Rinky Sha T.K.B. MoS2-based nanosensors in biomedical and environmental monitoring applications. Electrochimica Acta. 2020;349:136370. [Google Scholar]
- 90.Tim Bobrowski W.S. Long-term implantable glucose biosensors. Curr. Opin. Electrochemistry. 2018;10:112–119. [Google Scholar]
- 91.Shan J., Li J., Chu X., Xu M., Jin F., Wang X., Ma L., Fang X., Wei Z., Wang X. High sensitivity glucose detection at extremely low concentrations using a MoS2-based field-effect transistor. RSC Adv. 2018;8:7942–7948. doi: 10.1039/c7ra13614e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu S., Huang H., Shang M., Du C., Wu Y., Song W. High visible light sensitive MoS2 ultrathin nanosheets for photoelectrochemical biosensing. Biosens. Bioelectron. 2017;92:646–653. doi: 10.1016/j.bios.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 93.Lane J.D., Krumholz D.M., Sack R.A., Morris C. Tear glucose dynamics in diabetes mellitus. Curr. Eye Res. 2006;31:895–901. doi: 10.1080/02713680600976552. [DOI] [PubMed] [Google Scholar]
- 94.Sen D., Sarin G. Tear glucose levels in normal people and in diabetic patients. Br. J. Ophthalmol. 1980;64:693–695. doi: 10.1136/bjo.64.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Purslow C., Wolffsohn J.S. Ocular surface temperature: a review. Eye Contact Lens. 2005;31:117–123. doi: 10.1097/01.icl.0000141921.80061.17. [DOI] [PubMed] [Google Scholar]
- 96.Cao Y., Gong Y., Liu L., Zhou Y., Fang X., Zhang C., Li Y., Li J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J. Appl. Toxicol. 2017;37:1359–1369. doi: 10.1002/jat.3470. [DOI] [PubMed] [Google Scholar]
- 97.Medina-Leyte D.J., Domínguez-Pérez M., Mercado I., Villarreal-Molina M.T., Jacobo-Albavera L. Use of human umbilical vein endothelial cells (HUVEC) as a model to study cardiovascular disease: a review. Appl. Sci. 2020;10:938. [Google Scholar]
- 98.Kumar R., Harris-Hooker S., Kumar R., Sanford G. Co-culture of retinal and endothelial cells results in the modulation of genes critical to retinal neovascularization. Vasc. Cell. 2011;3:27. doi: 10.1186/2045-824X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronkko S., Vellonen K.S., Jarvinen K., Toropainen E., Urtti A. Human corneal cell culture models for drug toxicity studies. Drug Deliv. Transl Res. 2016;6:660–675. doi: 10.1007/s13346-016-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H., Kim S., Kim K.-B., Choi J.-W. Scalable fabrication of flexible thin-film batteries for smart lens applications. Nano Energy. 2018;53:225–231. [Google Scholar]
- 101.Park J., Ahn D.B., Kim J., Cha E., Bae B.-S., Lee S.-Y., Park J.-U. Printing of wirelessly rechargeable solid-state supercapacitors for soft, smart contact lenses with continuous operations. Sci. Adv. 2019;5:eaay0764. doi: 10.1126/sciadv.aay0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nasreldin M., Delattre R., Ramuz M., Lahuec C., Djenizian T., de Bougrenet de la Tocnaye J.-L. Flexible micro-battery for powering smart contact lens. Sensors. 2019;19:2062. doi: 10.3390/s19092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reid R.C., Minteer S.D., Gale B.K. Contact lens biofuel cell tested in a synthetic tear solution. Biosens. Bioelectron. 2015;68:142–148. doi: 10.1016/j.bios.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 104.Liu X., Gao H., Ward J.E., Liu X., Yin B., Fu T., Chen J., Lovley D.R., Yao J. Power generation from ambient humidity using protein nanowires. Nature. 2020;578:550–554. doi: 10.1038/s41586-020-2010-9. [DOI] [PubMed] [Google Scholar]
- 105.Borges L.M., Chávez-Santiago R., Barroca N., Velez F.J., Balasingham I. Radio-frequency energy harvesting for wearable sensors. Healthc. Technol. Lett. 2015;2:22–27. doi: 10.1049/htl.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze any datasets/code.


