Abstract
Background:
Evaluation of broad-spectrum micronutrient (vitamins and minerals) treatment for childhood ADHD has been limited to open-label studies that highlight beneficial effects across many aspects of psychological functioning.
Method:
This is the first fully blinded randomized controlled trial of medication-free children (n = 93) with ADHD (7–12 years) assigned to either micronutrients (n = 47) or placebo (n = 46) in a 1:1 ratio, for 10 weeks. All children received standardized ADHD assessments. Data were collected from clinicians, parents, participants and teachers across a range of measures assessing ADHD symptoms, general functioning and impairment, mood, aggression and emotional regulation.
Results:
Intent-to-treat analyses showed significant between-group differences favouring micronutrient treatment on the Clinical Global Impression-Improvement (ES = 0.46), with 47% of those on micronutrients identified as ‘much’ to ‘very much’ improved versus 28% on placebo. No group differences were identified on clinician, parent and teacher ratings of overall ADHD symptoms (ES ranged 0.03–0.17). However, according to clinicians, 32% of those on micronutrients versus 9% of those on placebo showed a clinically meaningful improvement on inattentive (OR = 4.9; 95% CI: 1.5–16.3), but no group differences on improvement in hyperactive-impulsive symptoms (OR = 1.0; 95% CI: 0.4–2.5). Based on clinician, parent and teacher report, those on micronutrients showed greater improvements in emotional regulation, aggression and general functioning compared to placebo (ES ranged 0.35–0.66). There were two dropouts per group, no group differences in adverse events and no serious adverse events identified. Blinding was successful with guessing no better than chance.
Conclusions:
Micronutrients improved overall function, reduced impairment and improved inattention, emotional regulation and aggression, but not hyperactive/impulsive symptoms, in this sample of children with ADHD. Although direct benefit for core ADHD symptoms was modest, with mixed findings across raters, the low rate of adverse effects and the benefits reported across multiple areas of functioning indicate micronutrients may be a favourable option for some children, particularly those with both ADHD and emotional dysregulation. Trial registered with the Australian New Zealand Clinical Trials Registry ACTRN12613000896774.
Keywords: ADHD, micronutrient, vitamin, mineral, Treatment, Mood, aggression
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a chronic neurodevelopmental disorder affecting approximately 5% of children (American Psychiatric Association, 2013). The disorder conveys a risk for school failure, occupational problems, substance addiction, incarceration and ongoing psychiatric problems in adulthood, despite receiving treatments (Hechtman et al., 2016; Klein et al., 2012; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993; Molina et al., 2009). Pharmacological treatments can reduce symptoms, but are often unsatisfactory due to side effects, failure to prevent or alter long-term course and discontinuance due to patient and family preferences (Storebø et al., 2015; Swanson et al., 2017).
Research has highlighted the importance of nutrition for brain health, including its influence on emotions, behaviour and ADHD symptoms (Nigg, Lewis, Edinger, & Falk, 2012). Processed foods, food dyes and low consumption of fruit and vegetables have shown an association with ADHD symptom severity (Howard et al., 2011; Pelsser et al., 2011; Rios-Hernandez, Alda, Farran-Codina, Ferreira-Garcia, & Izquierdo-Pulido, 2017). One treatment that logically stems from these studies is providing children with ADHD the nutrients required for optimal brain functioning. A strong theoretical basis exists for supplementing children with ADHD with a broad-spectrum of micronutrients (vitamins and minerals) ranging from: correcting inborn errors of metabolism that slow metabolic reactions (Ames, Elson-Schwab, & Silver, 2002), addressing identified vitamin deficiencies present in people with ADHD (Landaas et al., 2016), improving the microbiome (Kaplan, Rucklidge, McLeod, & Romijn, 2015), correcting deficiencies present in western diets (Davis, 2009) and/or increasing the production of adenosine triphosphate (ATP), the energy source produced by mitochondria (Gardner & Boles, 2005). All of these hypothesized mechanisms of action support a broad-spectrum of micronutrients as an intervention rather than any one nutrient (e.g. zinc, iron), the more typical approach used in research on ADHD, but a method that has yielded small and often inconsistent findings (Hariri & Azadbakht, 2015). While a multinutrient approach challenges conventional psychiatric research practices that favour manipulating one variable at a time, a single nutrient strategy is at odds with human physiology, as optimal functioning requires the presence of a wide range of nutrients consumed in balance, rather than one nutrient provided in high doses (Mertz, 1994).
Inclusion of the micronutrients in the formula chosen for this study (Daily Essential Nutrients or DEN) was based on several factors. Initial interest in micronutrients followed research showing reduced aggressive behaviour in farm animals administered a broad-spectrum of dietary minerals (Fraser, 1987). Subsequently, the considerable human research has suggested that broad-spectrum micronutrient treatment approaches have been more beneficial for the improvement of mood and behaviour than single nutrient studies (for reviews see: (Kaplan, Crawford, Field, & Simpson, 2007; Popper, Kaplan, & Rucklidge, 2017; Rucklidge, Johnstone, & Kaplan, 2009; Rucklidge & Kaplan, 2013). There are now a number of double-blind randomized controlled trials (RCT) supporting the positive effect of broad-spectrum micronutrients for the treatment of symptoms associated with clinical conditions, including autism (Adams et al., 2011), ADHD (Rucklidge, Frampton, Gorman, & Boggis, 2014), conduct disorder (Schoenthaler & Bier, 2000) and depression (Mech & Farah, 2016).
DEN was selected as an appropriate micronutrient preparation for investigation as it contains a comprehensive range of micronutrients (13 vitamins, 17 minerals, and four amino acids) at doses likely to be sufficient to elicit a possible response without being likely to elicit adverse effects in the majority of participants. There are suggestions that the therapeutic level of micronutrients required for optimal brain functioning is often higher than the recommended dietary allowance (RDA), but lower than the Upper Level (UL; Benton, 2013). Most of the micronutrient levels in DEN fall within this range. The few nutrient levels above the UL are supported by physiologically logical reasoning (e.g. the UL for zinc was set at a level that would not interfere with copper absorption; however, if taken with copper, zinc can safely be consumed over the UL).
This paper presents the first exploratory study using a fully blinded, parallel-group RCT design to assess the symptom control and the efficacy and safety of a broad-spectrum micronutrient formula, DEN, compared to a placebo in children with ADHD. Open-label pilot research in children with ADHD has demonstrated on-off-on-off control of symptoms using a similar micronutrient formula (EMPowerplus) (Gordon, Rucklidge, Blampied, & Johnstone, 2015), and an RCT in adults with ADHD also using EMPowerplus confirmed preliminary efficacy and safety in the short term (Rucklidge et al., 2014) as well as at 1-year follow-up (Rucklidge, Frampton, Gorman, & Boggis, 2017). Although this combination of micronutrients has shown evidence of both short- and long-term effectiveness and safety for a variety of mental health conditions (e.g. mood disorders, anxiety disorders, autism), as reported in more than 30 papers (Popper, 2014; Simpson et al., 2011), as yet, no randomized, fully blinded trials have been conducted with children with ADHD. Given the exploratory nature of the study, we examined symptoms across a broad range of problem behaviours including emotional dysregulation, aggression and general impairment because these problems contribute to the overall clinical presentation for children with ADHD.
Methods
Participants
Ninety-three children (aged 7–12 years) were recruited in Canterbury, New Zealand, from September 2013 to October 2016, via referrals from public services (n = 16), private clinicians (n = 12), social media and paper advertising (n = 48) and word of mouth (n = 17).
Informed consent and assent
Written informed consent was obtained from all of the participants’ parents or legal guardians and assent was obtained from the participants. The exploratory nature of the study, as well as other treatment options for ADHD, was explained to participants and their parents prior to enrolling. This study was approved by the university and national institutional review boards. The trial was prospectively registered with the Australia and New Zealand Clinical Trial Registry ACTRN12613000896774.
Inclusion criteria:
(a) between the ages of 7–12 years; (b) met criteria for ADHD based on the Kiddie Schedule for Affective Disorders and Schizophrenia Lifetime Version (K-SADS-PL) (Kaufman et al., 1997), as well as parent and teacher Conners Rating Scales (CRS-R:L; T score > 65 on parent form and >60 on teacher form) (Conners, 1997); (c) medication-free (psychiatric) for ≥4 weeks; and (d) able to ingest up to 15 capsules/ day with food.
The K-SADS-PL, a semistructured diagnostic interview to assess for ADHD and comorbid disorders according to DSM-IV criteria, was administered to the participant’s parent or guardian by a clinical psychologist or senior graduate clinical psychology student. To ensure compatibility with DSM-5, additional clinical questions covered Autistic Spectrum Disorders (ASD) and Disruptive Mood Dysregulation Disorder (DMDD), and other questions were adjusted to ensure DSM-5 diagnoses were adequately covered. All cases were reviewed with the PI (a registered clinical psychologist) and diagnoses discussed, including other factors that may better explain the symptoms being presented (e.g. presence of trauma). Participants were also seen by our study psychiatrist. Based on the assessment, all children were assigned a clinician-rated Clinical Global Impression rating of severity of illness (CGI-S) from 1 (not at all ill) to 7 (among the most extremely ill patients) (Guy, 1976). Forty-nine (53%) of the participants had previously received a diagnosis of ADHD by other mental health professionals. We purposefully included participants with other co-occurring disorders (except ASD), appreciating that the clinical utility of the results would be more meaningful if the sample was representative of children affected by ADHD.
Participants were allowed to continue psychological therapies (n = 3) and supplements such as essential fatty acids (n = 0) or melatonin (n = 15) if frequency or dose did not change throughout the duration of the study. Participants were not encouraged to come off of medication in order to participate in the trial. Medications for physical conditions were considered individually, but generally were allowed; e.g. medications for asthma (n = 8).
Participants who identified as having trouble swallowing pills completed the pill swallowing program (http://tinyurl.com/y7tqj8mg) developed by Kaplan et al. (2010). Thirty-eight individuals assessed for eligibility were unable to swallow pills at the consent meeting. Of those, 18 successfully learned to swallow pills using the pill swallowing program and proceeded with the trial.
Exclusion criteria:
(a) estimated IQ < 75, as assessed by two subtests (vocabulary and block design) of the WISC-IV (Wechsler, 2004), or previous educational assessments; (b) ASD; (c) epilepsy; (d) any major psychiatric condition likely to require hospitalization (e.g. Psychotic Disorders; Bipolar Disorders); (e) any serious medical condition; and (f) allergy to ingredients of the intervention or any known abnormality of mineral metabolism (e.g. Wilson’s disease, haemochromatosis).
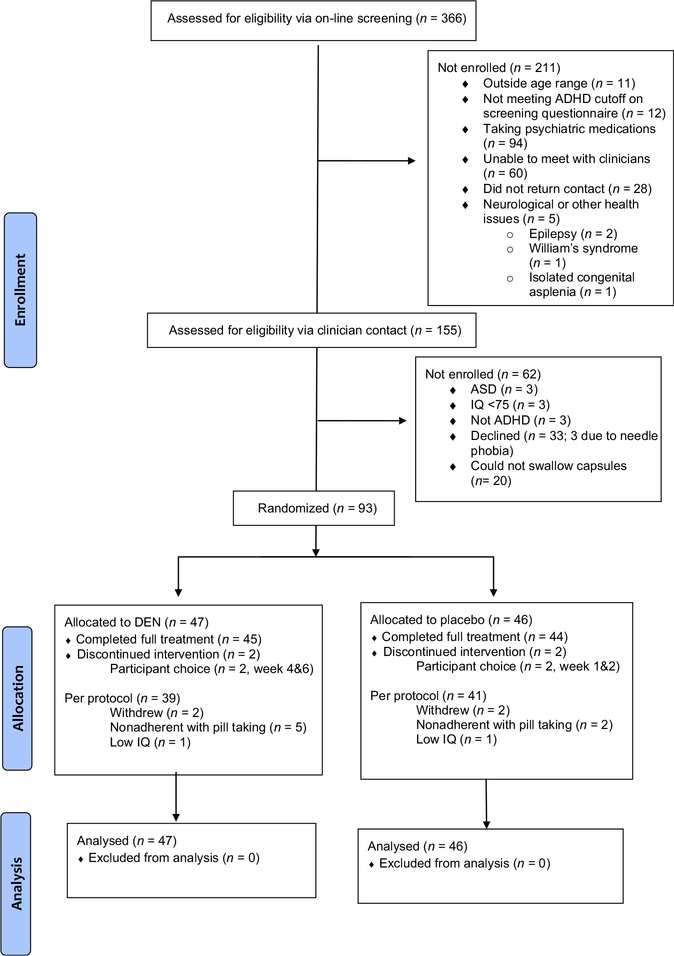
These criteria resulted in six participants being excluded, three due to low IQ and three due to meeting criteria for ASD. Two children were enrolled with an IQ > 70, but less than 75, as ADHD behaviours appeared to compromise accurate estimation of IQ. See Figure 1: CONSORT Diagram.
Figure 1.
CONSORT flow diagram
Efficacy and safety assessments
Clinician-rated measures.
All participants were monitored by a clinical psychologist or psychology graduate student under a psychologist’s supervision with face-to-face meetings or phone contact at the screening visit, baseline, and weeks 2, 4, 6, 8 and 10 (or end of study). At each visit, the following measures were completed: (a) The Clinical Global Impressions-Improvement (CGI-I) Scale (Guy, 1976) was adapted to produce three scores: CGI-I-Overall (capturing overall change in functioning from baseline), as well as a CGI-I-ADHD and CGI-I-Mood. These scores took into account parent verbal reports of functioning and impairment, change in ratings on questionnaires, and information from others (e.g. teachers), as well as behaviour in the clinic. The baseline assessment was used as the comparison for change. The scale spans from 1 (very much improved) to 7 (very much worse). (b) The Children’s Global Assessment Scale (C-GAS) (Shaffer et al., 1983) was used by the clinician to assess the overall level of children’s functioning based on all the information gathered since last visit. It is a single numerical scale from 1 to 100 with a higher score indicative of better functioning.
At baseline and 10 weeks, the clinician also completed the ADHD Rating Scale IV – (ADHD-RS-IV) – clinician version (Faries, Yalcin, Harder, & Heiligenstein, 2001; Zhang, Faries, Vowles, & Michelson, 2005). The ADHD-RS-IV contains 18 items directly linked to DSM-IV diagnostic criteria for ADHD and provides a total score and two subscale scores for inattention and hyperactivity/impulsivity, assessing ADHD symptoms based on frequency (0 ‘never or rarely’ to 3 ‘very often’). The clinician took into account observations from visits and formal cognitive testing, information from others, as well as parent report in determining ratings. However, frequency of behaviours was the main focus of the rating, considering how often the behaviours were present.
Parent-rated measures.
At every visit, parents completed the Child Mania Rating Scale, Parent Version (CMRS-P), a 21-item rating scale based on DSM-IV criteria for mania (Pavuluri, Henry, Devineni, Carbray, & Birmaher, 2006). Items are rated from 0 (never/rarely) to 3 (very often) and cover symptoms such as feeling irritable, racing thoughts, rage attacks and rapid mood swings. Total scores range from 0 to 63. A cut-off score of 20 is used to identify children at risk for severe mood dysregulation and a score under 20 indicates a child in remission (West, Celio, Henry, & Pavuluri, 2011). This measure was chosen to capture change in emotional dysregulation, given that these behaviour challenges are increasingly recognized to be a core feature of ADHD (Van Stralen, 2016).
At baseline and 10 weeks (or end of treatment), the parent completed the CPRS-R:L (Conners, 1997), considering the child’s behaviour over the previous month, utilizing a 4-point Likert scale with 0 = ‘not very true at all,’ to 3 = ‘very much true.’ This scale includes three DSM-IV subscales for inattention, hyperactivity/impulsivity and combined inattention and hyperactivity/impulsivity. Parents also completed the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 2001). The SDQ uses a 3-point scale (not true, somewhat true and certainly true) to assess psychological behaviours that are both positive (prosocial) and negative (emotional, conduct problems, hyperactivity and peer problems).
Other areas assessed at baseline and end of treatment included a brief diet intake questionnaire (modified from Baker, Little, and Brownell (2003)) to assess the child’s dietary patterns, including consumption of fruit and vegetables, breakfast and fast foods). Demographic variables, including participant’s ethnicity and parents’ occupation were also collected at baseline. The SES of each participant’s family was estimated using the New Zealand Socioeconomic Index of Occupational Status (NZSEI) (Milne, Byun, & Lee, 2013).
Teacher-rated measures.
Prior to trial commencement and 2 weeks before the end of the RCT, parents were asked to provide the child’s teacher with: (a) CTRS-R, (b) SDQ-teacher and (c) the Behaviour Rating Inventory of Executive Function (BRIEF-Teacher Form) (Gioia, Isquith, Guy, & Kenworthy, 2000) to mail back to the research laboratory. The BRIEF is a behavioural rating measure that was specifically designed to assess child and adolescent executive skills in natural, everyday environments. For purposes of this paper, we focused on the Behavioural Regulation Index (BRI) given its relationship to other measures administered. The BRI is composed of the Inhibit, Shift and Emotional Control subscales. Items are rated from 0 (never a problem) to 2 (often a problem). Seventy-two (77%) teachers completed both pre and postassessments.
Child-rated measures.
At every visit, the child was asked to rate his or her symptoms (e.g. attention, mood, sleep) using the Measure Yourself Medical Outcome Profile [MYMOP (Paterson, 1996)]. Scores range from 0 (no problems) to 5 (lots of problems). Emoticons were used to assist children in matching their severity rating with a numerical score.
Safety measures.
At every visit, the child and his or her parent/guardian were asked about adverse events. Specific potential side effects were also reviewed (e.g. rash, dry mouth, insomnia, nausea, change of appetite). Any concerning adverse events were discussed with the study child psychologist and/ or psychiatrist.
At baseline and study completion, laboratory tests for haematological and biochemical variables, thyroid function, prolactin, fasting glucose, homocysteine, iron, zinc, vitamin D, vitamin B12 and copper levels were conducted. Test results were provided to the participant’s family physician with consent. Blood pressure, height and weight were recorded at each visit. Laboratory results were reviewed and cleared by the study child psychiatrist prior to beginning the trial and discussed with families.
Procedures
Participants were randomly assigned in a 1:1 ratio to 10 weeks of treatment with either micronutrients or placebo using a computer-generated randomization sequence from the website www.randomization.com, with the randomization sequence arranged in permuted blocks of 4. Medication kits containing all required study medication for the 10-week intervention were prepared in advance by the pharmacist. The kit contained six bottles, one for every 2 weeks and one extra one with additional pills to cover unexpected illness resulting in a missed appointment or extra pills in case the dose needed to be increased. Only the pharmacist had knowledge of the randomization list until all study data had been adjudicated and the study database was completed and locked. This process ensured participants, parents/guardians, investigators and clinicians were unaware of any participant’s treatment allocation.
Once eligibility was confirmed and baseline assessment completed, participants were allocated to the next sequentially numbered kit. The participants were instructed to titrate the dose up over a week, starting with three capsules/ day in divided doses, and increasing up to 12 capsules/day, in three divided doses, taken with food and water. There is considerable clinical feedback indicating that optimal dose can vary; our target dose was based on the response rates from our open-label pilot study on children with ADHD, which varied dose across time (Gordon et al., 2015). All participants were provided with a 3-slot pill box, one for each day of the week, to assist with adherence. Those participants who showed no evidence of substantial improvement based on the CGI-I (a score of three or greater) after 4 weeks, increased their dose to 15 capsules a day (29 on micronutrients, 34 on placebo). Depending on initial response, some participants had the dose increased more slowly. The placebo and DEN (see Appendix S1 for ingredients) were identical in appearance. The placebo included a small amount of riboflavin to mimic the smell and urine colour associated with taking vitamins. Unused pills were collected to obtain an estimate of adherence. At each visit, participants received a NZ$10 petrol voucher to cover travel costs. No other compensation was provided.
Participants were monitored at in-person visits every 2 weeks, for a total of six visits. Families received a new bottle of capsules at each assessment and participants, together with their parent/guardian, reported adherence to the pill-taking protocol, which was measured by recording the number of doses missed in the previous 2 weeks.
Sample size
An open-label single arm pilot study using EMPowerplus (a formula similar in composition to Daily Essential Nutrients) provided the basis for sample size estimation (Gordon et al., 2015). Based on within-group effect sizes from this pilot study, which ranged between 0.80 and 1.20, a sample size of 36 participants per group was required to detect statistically significant (2-tailed α = .05) between-group effect sizes of 0.67 or greater, with 80% power. To allow for an anticipated 10%-15% attrition rate, it was intended to recruit approximately 50 participants per group.
Statistical analyses
Three primary outcome measures were defined a priori: (a) CGI-I-Overall (clinician-rated); (b) ADHD-RS-IV scale (clinician-rated) and (c) CPRS-R:L (parent-rated). End-of-treatment response was defined a priori in two ways: (a) a final CGI-I-Global Impression of either ‘much’ or ‘very much’ improved based on global improvement across all areas of functioning; and (b) ≥ 30% decrease on the ADHD-RS-IV, a percentage change frequently used in the ADHD literature to identify a clinically meaningful response (Sprich, Safren, Finkelstein, Remmert, & Hammerness, 2016; Stein et al., 2015).
Change scores from baseline to end of treatment were compared between randomized groups using ANCOVA, with baseline level as the covariate, for all continuous outcome measures. Change measures (CGI-I ratings) assessed at the end of treatment were compared using one-way ANOVA. Treatment effects were summarized as mean differences with 95% confidence intervals generated from ANCOVA/ANOVA models. Cohen’s d was used to estimate effect sizes between groups. Categorical outcomes were compared between groups using Chi-square tests, and summarized using odds ratios and 95% confidence intervals. All analyses were undertaken on an intention-to-treat (ITT) basis, with all randomized participants included in the analysis. For those not completing 10 weeks of treatment, data from their final assessment were used. Secondary analyses were undertaken on all outcomes using the per-protocol analysis set (see Figure 1), which included participants completing 10 weeks of treatment without any major protocol violations. All tests were two-tailed, and p-values <.05 were considered statistically significant. Whilst acknowledging the increased potential for type 1 errors, as this was an exploratory study, we did not make adjustments for multiple comparisons in order to guard against false negatives.
Results
Sample description
Ninety-three participants gave informed consent and were randomized: 47 to micronutrients and 46 to placebo. The two groups were generally well matched (Table 1) although there was a higher prevalence of Generalized Anxiety Disorder among participants in the micronutrient group (30%) compared with the placebo group (7%). About 30% of the sample had a past history of psychiatric medication use, of which 20 (22%) had been prescribed a stimulant only (e.g. methylphenidate), two (2%) had been prescribed a medication other than a stimulant (e.g. antidepressant, nonstimulant) and seven (8%) had been prescribed both a stimulant and another class of psychiatric medication. Two participants in each group did not complete the study (Figure 1). Participant and clinician blinding appears to have been successful as there were no between-group differences regarding what intervention parents/children and clinicians thought the child was receiving. Clinicians’ guesses were correct 47.5% of the time, and parent’s/children’s 52%.
Table 1.
Baseline demographic and clinical characteristics of study participants
| Characteristic | Micronutrients (n = 47) | Placebo (n = 46) |
|---|---|---|
| Demographics | Mean ± SD or n (%) | Mean ± SD or n (%) |
| Age | 10.06 ± 1.56 | 9.43 ± 1.53 |
| Male | 35 (74) | 36 (78) |
| Estimated IQa | 95.85 ± 14.72 | 98.12 ± 13.97 |
| Socioeconomic statusb | 51.2 ± 14.3 | 54.2 ± 17.0 |
| Body Mass Index | 18.1 ± 2.6 | 17.9 ± 2.8 |
| Ethnic origin | ||
| New Zealanders of European descent | 39 (83) | 34 (74) |
| Mäori (indigenous people of New Zealand) or Tongan | 8 (17) | 12 (26) |
| Clinical Characteristics | ||
| CGI-Severity | 5.1 ± 0.9 | 4.9 ± 0.8 |
| ADHD type | ||
| Inattentive | 14 (30) | 12 (26) |
| Hyperactive/Impulsive | 2 (4) | 3 (7) |
| Combined | 31 (66) | 31 (67) |
| Parent CPRS-R:L (T scores) | ||
| DSM-IV Inattention | 76.6 ± 8.1 | 76.2 ± 7.9 |
| DSM-IV H/I | 83.5 ± 8.0 | 81.7 ± 8.9 |
| DSM-IV Combined | 81.8 ± 6.4 | 80.6 ± 7.3 |
| Teacher CTRS-R:L (T scores) | ||
| DSM-IV Inattention | 68.1 ± 8.7 | 67.4 ± 8.5 |
| DSM-IV H/I | 67.9 ± 12.4 | 69.2 ± 12.4 |
| DSM-IV Combined | 69.8 ± 9.5 | 69.7 ± 9.5 |
| Major Depressive Disorder | 0 (0) | 0 (0) |
| Disruptive Mood Dysregulation Disorder | 4 (9) | 6 (13) |
| Specific Phobia | 6 (13) | 4 (9) |
| Social Anxiety Disorder | 0 | 2 (4) |
| Generalized Anxiety Disorder | 14 (30) | 3 (7) |
| Obsessive Compulsive Disorder | 2 (4) | 1 (2) |
| Separation Anxiety Disorder | 5 (11) | 2 (4) |
| Oppositional Defiant Disorder | 29 (62) | 22 (48) |
| Conduct Disorder | 4 (9) | 4 (9) |
| Learning Disabilityc | 22 (47) | 19 (41) |
| Enuresis | 3 (6) | 7 (15) |
| Encopresis | 5 (11) | 2 (4) |
| Tics | 3 (6) | 3 (7) |
| Any co-occurring disorder | 41 (87) | 36 (78) |
| Previous contact with health services | 36 (77) | 36 (78) |
| History of past use of psychiatric medications | 18 (38) | 10 (22) |
Assessed using Block Design and Vocabulary subtests of the WISC-IV (Wechsler, 2004).
Based on the New Zealand Socio economic Index (NZSEI 2006).
Defined as having at least one standard score below 100 on either reading or spelling of the WRAT3 or previous assessment confirming an LD (Wilkinson, 1993).
H/I, hyperactivity/impulsivity; CGI, Clinical Global Impression; CPRS-R:L, Conners Parent Rating Scale-Revised:Long version; CTRS-R:L, Conners Teacher Rating Scale-Revised:Long version.
Primary efficacy outcomes
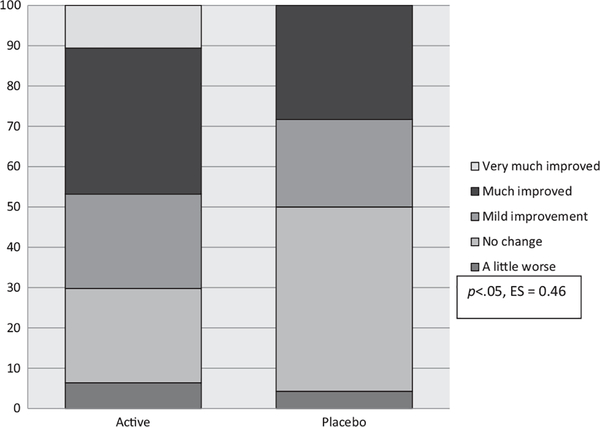
Intention-to-treat analyses of the CGI-I-Overall showed that clinicians rated participants on micronutrients as having shown more improvement across all areas of functioning (p = .029, ES = 0.46) as compared with those on placebo (Table 2, Figure 2). No between-group differences were observed for ADHD ratings based on either clinician (ADHD-RS-IV) (p = .415, ES = 0.17) or parent ratings (CPRS-R:L) (p = .540, ES = 0.13).
Table 2.
Baseline and post 10-week data on primary and secondary outcome measures
| Micronutrients (n = 47) |
Placebo (n = 46) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Post |
Change from baselinea | Baseline |
Post |
Change from baselinea | Difference (confidence interval) | p | ESb | |||||
| Variable | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||||
| Primary outcomes | |||||||||||||
| CGI-I-Overallc | 2.8 | 0.2 | 2.83 | 3.3 | 0.1 | 3.26 | −0.47 (−0.05 to −0.90) | 0.029d | 0.46 | ||||
| Clinician ADHD-RS-IV Symptoms Total | 44.8 | 1.0 | 37.1 | 1.6 | 7.75 | 45.1 | 0.8 | 38.7 | 1.4 | 6.32 | −1.43 (−4.91 to 2.05) | 0.415 | 0.17 |
| Parent CPRS-R:L DSM-IV ADHD Symptoms Total | 42.5 | 1.0 | 33.4 | 1.6 | 9.08 | 42.4 | 1.1 | 34.6 | 1.6 | 7.79 | −1.29 (−5.45 to 2.88) | 0.540 | 0.13 |
| Additional measures | |||||||||||||
| CGI-I-ADHDc | 2.9 | 0.2 | 2.87 | 3.4 | 0.1 | 3.37 | −0.50 (−0.88 to −0.11) | 0.012d | 0.53 | ||||
| CGI-I-Moodc | 2.9 | 0.2 | 2.92 | 3.4 | 0.1 | 3.43 | −0.52 (−0.10 to −0.95) | 0.017d | 0.51 | ||||
| C-GAS | 48.1 | 0.9 | 54.2 | 1.4 | 6.07 | 48.8 | 0.9 | 51.8 | 1.3 | 2.97 | 3.10 (0.45 to 5.75) | 0.022d | 0.48 |
| CMRS-P | 25.0 | 1.7 | 15.2 | 1.5 | 9.46 | 23.4 | 1.6 | 17.3 | 1.7 | 6.45 | −3.00 (−6.64 to 0.62) | 0.100 | 0.35 |
| Clinician ADHD-RS-IV | |||||||||||||
| DSM-IV Inattention | 24.1 | 0.5 | 20.0 | 0.8 | 4.05 | 23.7 | 0.4 | 21.6 | 0.7 | 2.10 | −1.95 (−3.94 to 0.04) | 0.055d | 0.41 |
| DSM-IV H/I | 20.7 | 0.8 | 17.1 | 1.0 | 3.67 | 21.4 | 0.7 | 17.2 | 1.0 | 4.24 | 0.54 (−1.46 to 2.55) | 0.591 | 0.11 |
| Teacher CTRS-R:L DSM-IV Totale | 34.7 | 1.8 | 32.8 | 1.7 | 1.95 | 34.4 | 1.7 | 33.2 | 2.1 | 1.23 | −0.33 (−5.08 to 4.42) | 0.889 | 0.03 |
| Parent SDQ – total problem score | 23.0 | 0.7 | 18.1 | 0.9 | 5.09 | 21.9 | 0.8 | 19.3 | 0.9 | 2.79 | −1.95 (−4.0 to 0.10) | 0.062 | 0.41 |
| Parent SDQ - Conduct problems score | 5.3 | 0.3 | 4.2 | 0.3 | 1.10 | 5.0 | 0.4 | 4.8 | 0.4 | 0.14 | −0.87 (−1.57 to −0.17) | 0.015d | 0.52 |
| Teacher SDQ – total problem score | 18.6 | 1.0 | 16.0 | 1.1 | 3.36 | 17.7 | 0.9 | 17.1 | 0.9 | 0.33 | −1.78 (−3.88 to 0.32) | 0.064 | 0.45 |
| Teacher SDQ - Conduct problems score | 4.0 | 0.4 | 2.9 | 0.4 | 1.13 | 3.8 | 0.4 | 3.6 | 0.5 | 0.27 | −0.86 (−1.74 to 0.18) | 0.055d | 0.47 |
| Teacher BRIEF – Behavioural Regulation Index | 62.6 | 1.0 | 58.6 | 1.0 | 4.01 | 60.8 | 2.6 | 61.5 | 2.3 | −0.30 | −4.31 (−8.68 to −0.07) | 0.053 | 0.48 |
| Teacher BRIEF – Emotional Control Subscale | 18.5 | 2.6 | 16.6 | 2.4 | 1.91 | 18.2 | 0.9 | 18.5 | 1.0 | −0.24 | −2.15 (−3.74 to −0.60) | 0.009d | 0.66 |
Adjusted for baseline.
Cohen’s d (effect size) measured as the mean difference in the change divided by the within-group SD of the difference in the change.
Assesses change so not measured at baseline.
p < .05 based on per-protocol.
Based on completed questions (n = 72).
Results in bold are significant.
H/I, hyperactivity/impulsivity; C-GAS, Child Global Assessment Scale; CGI-I, Clinical Global Impression-Improvement; SDQ, Strengths and Difficulties Questionnaire; CMRS-P, Child Mania Rating Scale – Parent; CPRS-R, Conners Parent Rating Scale-Revised:Long version; CTRS, Conners Teacher Rating Scale-Revised:Long version; BRIEF, Behaviour Rating Inventory of Executive Function.
Figure 2.
Per cent within each group falling in the different categories identifying extent of change pre to post for active versus placebo based on CGI-I-Overall scores
Other treatment-related outcomes
Consistent with the CGI-I-Overall, the micronutrient group showed significantly greater improvement based on C-GAS, CGI-I-ADHD and CGI-I-Mood (Table 2) as compared to the placebo group (ES ranged 0.48–0.53). ITT analyses revealed no significant group differences separately for either attention [based on parent report (ES = 0.13)] or hyperactivity/ impulsivity(H/I) [based on parent report (ES = 0.08)]. The significant improvement in the clinician-rated CGI-I-ADHD reported for the micronutrient group largely reflected a greater improvement in the inattentive symptoms (ES = 0.41), rather than the H/I symptoms (ES = 0.11). Teacher ratings of ADHD symptoms showed no group differences across either dimension (ES ranged from 0.03 to 0.13).
There were significant group differences on the SDQ-Parent Conduct Problems subscale and the BRIEF-Teacher Emotional Control subscale (Table 2), with those on micronutrients showing greater improvement in aggression and emotional dysregulation compared with those on placebo (ES were 0.52 and 0.66 respectively). There were trends identifying greater change in parent ratings of dysregulated mood based on the CMRS-P (ES = 0.35) as well as greater improvement in conduct problems based on SDQ-Teacher (ES = 0.48) and problem behaviours based on both the SDQ parents (ES = 0.41) and teacher (ES = 0.45) in the micronutrient group as compared with the placebo group; however, they were not significant. There were no significant group differences on the other SDQ subscales (ES ranged from 0.07 to 0.31) or BRIEF subscales [inhibition (ES 0.32) and shifting (ES 0.42)].
In the per-protocol analyses, when excluding participants who: (a) withdrew before 10 weeks, or, (b) were nonadherent with study protocol, or, (c) did not meet full inclusion criteria (e.g. IQ < 75) (n = 13: eight micronutrients, five placebo), results favoured the micronutrient group more than with the ITT analyses. For example, the SDQ ratings showed significant group differences favouring the micronutrient group for the Conduct Problems subscale for teachers (ES = 0.63) and the clinician rating of inattention on the ADHD-RS-IV also showed a significant group difference with those on micronutrients improving more on inattentive symptoms as compared with placebo (ES = 0.49).
Treatment response
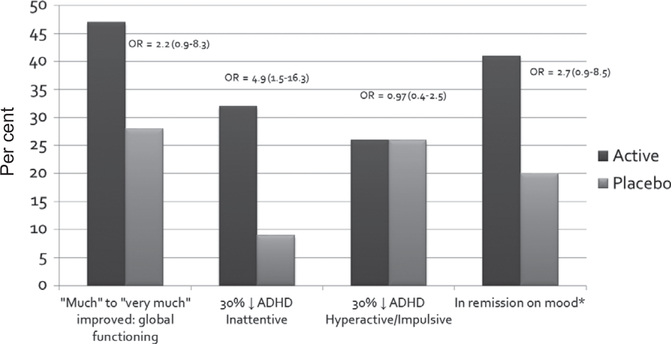
Twenty-two (47%) of those on micronutrients versus 13 (28%) on placebo (, p = .065; OR = 2.2; 95% CI: 0.9–5.3) were ‘much’ to ‘very much’ improved on the CGI-I-Overall, with 11% of those on micronutrients and none on placebo being identified as ‘very much’ improved. Based on per-protocol analyses, the number of responders was 20 (51%) on micronutrients versus 11 (27%) on placebo (, p = .03; OR = 2.9; 95% CI: 1.1–7.3).
A nonsignificant trend showed that more children were identified as responders in the micronutrient group as compared with the placebo group based on a 30 per cent reduction from baseline on the ADHD-RS-IV, with 13 (28%) of those on micronutrients versus 6 (13%) of those on placebo showing a clinically meaningful change in ADHD scores (, p = .08; OR = 2.5; 95% CI: 0.9–7.4).
Post hoc analyses of the ADHD-RS-IV subscales revealed that 15 (32%) of those on micronutrients versus 4 (9%) of those on placebo showed a substantial change (30% drop) in inattentive symptoms (, p = .005; OR = 4.9; 95% CI: 1.5–16.3). In contrast, there was no difference between groups for those showing a 30 per cent drop in hyperactivity/ impulsivity (, p = .951; OR = 1.0; 95% CI: 0.4–2.5).
Given the clinical challenges associated with emotional dysregulation in children with ADHD, we explored how many children showed a clinically meaningful improvement in mood by identifying those children (n = 62) who entered the trial with severe mood dysregulation (≥20 on the CMRS-P). It is important to note that most of these children did not meet the full criteria for DMDD, which involves severe impairment requiring clinical attention. Responders were defined as those whose mood symptoms went into remission (post-RCT score <20) and who received ratings of 1 or 2 on the CGI-I-Mood scale, to reflect that the clinician had noted an improvement in mood regulation as well. Thirteen (41%) of those on micronutrients and six (20%) of those on placebo showed a clinically meaningful improvement in the ratings of emotional dysregulation (, p = .08; OR = 2.74; 95% CI: 0.9–8.5). See Figure 3 for a summary of response rates.
Figure 3.
Per cent of responders per group across different symptoms *based on children entering trial with severe mood dysregulation (n = 62). OR, odds ratio
Safety and adherence
Change in eosinophil levels differed significantly between groups (p = .003). Mean levels for the micronutrient group decreased from 0.43 to 0.33 whereas mean levels for the placebo group increased from 0.42 to 0.47; however, all these values fell within the normal reference range. Homocysteine levels decreased significantly in the micronutrient, but not the placebo group (ES = 1.54). Two children on micronutrients and two children on placebo had elevated aspartate transaminase (AST) and alanine transaminase (ALT) scores at post-RCT assessment. No other group differences were found in the biochemistry and haematology safety screens and no adverse events were identified from these measurements (Table 3). There were no group differences in blood pressure, pulse, height and weight; a nonsignificant trend identified that those on micronutrients grew slightly more than those on placebo (p = .06; ES = 0.40).
Table 3.
Baseline and post 10-week data on blood results, biometrics and nutrient levels
| Micronutrients (n = 44) |
Placebo (n = 41) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Change |
Baseline |
Change |
||||||||
| Variable | Reference Ranges | Mean | SE | Mean | SE | Mean | SE | Mean | SE | p-value | Effect size |
| Safety markers | |||||||||||
| Prolactin (mIU/L) | Male 50–350 | 172.00 | 14.22 | −3.58 | 15.98 | 149.51 | 10.46 | −5.70 | 7.99 | .906 | 0.14 |
| Female 50–550 | |||||||||||
| Creatinine (μmol/L) | 40–80 | 60.25 | 1.08 | 0.91 | 0.79 | 61.17 | 0.66 | −0.49 | 0.66 | .181 | 0.35 |
| Fasting glucose (mmol/L) | 3.9–5.8 | 4.96 | 0.58 | 0.03 | 0.09 | 5.06 | 0.05 | −0.02 | 0.05 | .667 | 0.22 |
| Eosinophils | 0.0–0.9 | 0.43 | 0.06 | −0.10* | 0.04 | 0.42 | 0.04 | 0.05 | 0.03 | .003 | 0.62 |
| Platelets (×10(9)/L) | 150–425 | 301.86 | 7.80 | −9.37 | 6.81 | 303.07 | 9.74 | 51.32 | 51.16 | .227 | 0.23 |
| Haemoglobin | 115–145 | 132.93 | 0.98 | −0.91 | 0.83 | 131.83 | 1.19 | 1.32 | 1.06 | .099 | 0.36 |
| Haematocrit | 0.35–0.43 | 0.40 | 0.00 | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 | .418 | 0.12 |
| MCV | 75–90 | 82.30 | 0.45 | 0.16 | 0.27 | 82.34 | 0.59 | −0.37 | 0.28 | .180 | 0.27 |
| MCH | 24–30 | 27.68 | 0.18 | 0.00 | 0.07 | 27.49 | 0.20 | −0.05 | 0.11 | .709 | 0.23 |
| WBC (×10(9)/L) | 5.0–14.5 | 6.10 | 0.34 | −0.25 | 0.27 | 6.08 | 0.26 | −0.10 | −0.30 | .704 | 0.02 |
| Lymphocytes (×10(9)/L) | 1.4–4.5 | 2.41 | 0.11 | −0.06 | 0.09 | 2.38 | 0.09 | 0.05 | 0.07 | .342 | 0.13 |
| Neutrophils (×10(9)/L) | 1.5–7.0 | 2.77 | 0.26 | −0.08 | 0.24 | 2.81 | 0.24 | −0.20 | 0.28 | .760 | 0.09 |
| Monocytes | 0.3–0.9 | 0.47 | 0.02 | −0.01 | 0.02 | 0.45 | 0.03 | 0.00 | 0.03 | .722 | 0.01 |
| GGT (U/L) | <30.00 | 13.23 | 0.40 | −0.28 | 0.31 | 13.17 | 3.02 | −0.51 | 0.43 | .659 | 0.19 |
| AST (U/L) | 15–40 | 25.64 | 0.84 | 3.09* | 0.91 | 27.39 | 1.04 | 2.02 | 1.23 | .484 | 0.24 |
| ALT (U/L) | 10–35 | 17.86 | 1.16 | 3.39* | 1.05 | 18.27 | 1.86 | 1.71 | 1.75 | .405 | 0.21 |
| Urea | 1.4–5.7 | 4.61 | 0.15 | 0.26 | 0.19 | 4.72 | 0.16 | −0.21 | 0.20 | .092 | 0.42 |
| TSH (mlU/L) | 0.32–5.00 | 1.81 | 0.11 | 0.13 | 0.28 | 1.88 | 0.11 | −0.03 | 0.08 | .162 | 0.36 |
| T4 | 10–24 | 13.23 | 0.23 | −0.09 | −0.27 | 13.45 | 0.23 | 0.15 | 0.20 | .366 | 0.02 |
| T3 | 3.8–8.6 | 5.60 | 0.86 | −0.01 | −0.20 | 5.51 | 0.08 | 0.07 | 0.09 | .547 | 0.06 |
| Homocysteine | 5.0–15.0 | 5.42 | 0.25 | −1.62* | 0.24 | 4.71 | 0.16 | 0.32* | 0.14 | <.001 | 1.54 |
| Physiological markers | |||||||||||
| Height (cm) | N/A | 139.81 | 1.74 | 1.51 | 0.13 | 135.90 | 1.49 | 1.15 | 0.13 | .060 | 0.40 |
| Weight (kg) | 35.95 | 1.29 | 1.06 | 0.22 | 33.44 | 1.30 | 0.92 | 0.15 | .608 | 0.11 | |
| Blood pressure – systolic | 109.20 | 1.77 | 2.15 | 1.92 | 108.14 | 2.12 | 2.30 | 1.98 | .957 | 0.01 | |
| Blood pressure – diastolic | 61.02 | 1.58 | 0.52 | 2.50 | 65.19 | 2.77 | 6.88 | 2.60 | .081 | 0.37 | |
| Pulse | 81.02 | 1.85 | −1.41 | 1.70 | 78.12 | 2.31 | −0.56 | 1.76 | .732 | 0.07 | |
| Nutrient levels | |||||||||||
| Potassium | 3.5–5.2 | 4.07 | 0.04 | 0.04 | 0.05 | 4.14 | 0.04 | 0.01 | 0.05 | .705 | 0.23 |
| Vitamin D (nmol/L) | 50–150 | 72.68 | 3.35 | 9.09* | 2.96 | 73.48 | 3.39 | −1.73 | 3.20 | .015 | 0.59 |
| Vitamin B12 (pmol/L) | 130–650 | 457.42 | 27.15 | 433.44* | 31.81 | 461.36 | 24.97 | −8.26 | 18.05 | <.001 | 2.51 |
| Folate (nmol/L) | >8 | 25.95 | 1.25 | 25.19* | 3.32 | 28.74 | 1.08 | −3.72* | 0.90 | <.001 | 1.77 |
| Lithium | Undetectable in all samples | ||||||||||
| Magnesium (mmol/L) | 1.6–2.3 | 0.83 | 0.01 | 0.01 | 0.01 | 0.82 | 0.01 | 0.01 | 0.01 | .736 | 0.22 |
| Ferritin (ug/L) | 15–200 | 49.58 | 7.94 | −4.28 | 3.61 | 40.55 | 2.71 | −5.15 | 3.18 | .858 | 0.06 |
| Iron (umol/L) | 6–25 | 15.61 | 0.74 | 0.33 | −2.10 | 16.56 | 0.89 | −1.28 | 1.10 | .256 | 0.32 |
| Calcium (mmol/L) | 2.2–2.6 | 2.46 | 0.01 | 0.02 | 0.01 | 2.47 | 0.01 | −0.01 | 0.01 | .137 | 0.30 |
| Zinc (umol/L) | 10–17 | 12.50 | 0.29 | 0.15 | 0.27 | 12.52 | 0.22 | 0.09 | 0.24 | .865 | 0.16 |
| Copper (umol/L) | 13.2–21.4 | 15.99 | 0.51 | −0.45 | 0.43 | 15.94 | 0.39 | 0.32 | 0.44 | .213 | 0.14 |
Results in bold are significant.
TSH, thyroid-stimulating hormone; APTT, Activated Partial Thromboplastin Time; GGT, Gamma-glutamyl transpeptidase; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; WBC, White Blood Cells; MCV, Mean corpuscular volume; MCH, Mean Corpuscular Haemoglobin; T4, thyroxine; T3, triiodothyronine;
p < 0.05 pre-post within group t-test.
Nutrient blood assays showed significant group differences in changes for vitamin D (p = .015; ES = 0.59), B12 (p < .001; ES = 2.51) and folate (p < .001; ES = 1.77) levels, with those taking micronutrients having higher levels at endpoint (Table 3). Only B12 levels increased beyond the reference range in the micronutrient group. Three children on micronutrients and three on placebo showed elevated copper levels at end of treatment.
No group differences in adverse events were identified. Rates of the more common adverse events (AEs) (Table 4) were generally low. Eighty-three per cent of reported AEs resolved on their own. No serious or severe adverse events were reported and only 3% of the AEs were rated as moderate. Sleep initiation problems that emerged were addressed by ensuring pills were taken at least two hours prior to bedtime, as B vitamins can be quite activating.
Table 4.
Treatment-emergent adverse events
| Micronutrients (n = 47) n (%) |
Placebo (n = 46) n (%) |
|
|---|---|---|
| Headache | 6 (13) | 9 (20) |
| Dry mouth | 2 (4) | 5 (11) |
| Sleep disruptions | 13 (28) | 8 (17) |
| Gastrointestinal disturbances | 9 (19) | 7 (15) |
| Nausea | 6 (13) | 6 (13) |
| Irritability | 8 (17) | 6 (13) |
| Fatigue | 3 (6) | 5 (11) |
| Anxiety | 7 (15) | 10 (22) |
| Eating problems | 4 (9) | 8 (17) |
| Rash | 4 (9) | 6 (13) |
| Bedwetting | 3 (6) | 2 (4) |
| Suicidal thoughts | 2 (4) | 2 (4) |
| Nose bleed | 2 (4) | 1 (2) |
| Migraine | 1 (2) | 0 (0) |
Overall adherence rates, measured by the number of required doses taken, based on self-report were comparable for the two groups (95.4%, SD = 3.8, micronutrient group; 93.0%, SD = 6.0, placebo group). Rates were similar based on pill counts (90.5%, SD = 12.1, micronutrient group; 91.5%, SD = 8.3, placebo group). Five were identified as nonadherent (taking less than 75% of pills) in the micronutrient group versus two in the placebo group. The average number of pills taken per day was 11.8 (SD = 1.0) in the micronutrient group and 11.4 (SD = 1.1) in the placebo group.
Other variables
There were no baseline group differences in dietary habits, nor were changes identified over the 10-week period.
Children were asked to rate their symptoms over the course of the 10-week trial. While both groups identified significant improvement over time, based on the MYMOP, children on micronutrients rated greater improvement in their concentration compared with children on placebo, although the group difference was not significant (p = .07, ES = 0.38). This finding is consistent with clinician ratings of improvement on inattentive symptoms. The lack of a significant difference may have been affected by a floor effect; 51 (55%) of children did not identify that they had any problems with concentration at baseline. This lack of awareness was also observed across the other child ratings.
Discussion
This exploratory study is the first fully blinded RCT to investigate efficacy and safety of micronutrients in children with ADHD. Intent-to-treat analyses of one of the primary outcome variables, the CGI-I, showed significant between-group differences favouring micronutrient treatment (ES = 0.46), with 47% of those on micronutrients identified as ‘much’ to ‘very much’ improved versus 28% on placebo. None of the participants in the placebo group were identified as ‘very much’ improved versus 11% of those on micronutrients. However, no group differences were detected on the other two primary measures, the clinician ADHD-RS-IV and the parent CPRS-R:L (total ADHD). Despite the lack of significant change detected in these measures, clinicians rated children receiving micronutrients as showing greater improvement in ADHD impairment on the CGI-I-ADHD. Inattentive symptoms appeared to be improved more than hyperactive-impulsive symptoms, with 32% of those receiving micronutrients showing 30% reduction in the ADHD-RS-IV inattentive subscale versus 9% receiving placebo (OR = 4.9; 95% CI: 1.5–16.3). The children appeared to have limited insight into their ADHD symptoms at baseline, an observation consistent with literature identifying that children with ADHD can often have a positive illusory bias (Owens, Goldfine, Evangelista, Hoza, & Kaiser, 2007). Nevertheless, consistent with the clinician observations, there was greater improvement in their ratings of inattentive symptoms if they had been taking the micronutrients than if they were taking placebo, although the difference was not significant (p = .07; ES = 0.38).
Micronutrients improved aggression and dysregulated mood, with effect sizes ranging from 0.35 to 0.66. These measures tapped into behaviours including hot tempers, fights with other children, explosive angry outbursts, and moods changing rapidly for no reason. These improvements on emotional control were consistent across the three raters (clinician, parent and teacher). Twice as many of the children who entered the trial with severe mood dysregulation, and were randomized to micronutrients, showed a clinically significant improvement in emotional dysregulation compared with placebo (41% vs. 20%). While poor self-control and emotional dysregulation are not considered core ADHD symptoms according to the DSM-5, these symptoms are often more impairing than those identified as ‘core’ ADHD symptoms (Barkley & Fischer, 2010; Van Stralen, 2016). Management of emotional dysregulation presents a considerable therapeutic challenge for parents, teachers and clinicians (Shaw, Stringaris, Nigg, & Leibenluft, 2014) and children with poor emotional control tend to have poorer long-term outcomes (Slutske, Moffitt, Poulton, & Caspi, 2012), including placing them at risk for future alcohol use (Harty, Gnagy, Pelham, & Molina, 2017). A growing body of literature points to the clinical relevance of a temperament- or personality-based irritable subtype (Gomez & Corr, 2014; Karalunas et al., 2014; Sullivan et al., 2015), characterized by the negative emotionality and anger that improved in the participants who were randomized to micronutrients in this study. The improvement in negative emotionality in children taking micronutrients may underscore the validity of temperament subtypes in ADHD and suggest a targeted treatment for children displaying these challenging behaviours. Improvements in emotional regulation likely contributed to both the significant improvement in the CGI-I-Mood (ES = 0.51), and in global functioning as measured by the C-GAS (ES = 0.48). These improvements bode well for improving life outcomes.
Clinicians, but not parents or teachers, observed some benefit directly on core ADHD symptoms. This difference may be understood by the context in which the clinician decides on a rating. Clinician ratings involve observations of neurocognitive testing, behavioural observations during study visits, children’s reports, and verbal and written reports from parents. In addition to this wide source of data, clinicians’ training may have equipped them to detect more subtle changes in ADHD presentations, particularly improvement in attentional capacity when children were in standardized testing settings. Further, the CGI-I-ADHD captures change in symptoms, both frequency and intensity, alongside impairment, whereas the ADHD symptom scales only assess frequency. Many parents commented that while symptoms may have still been present (frequency rating), their child was calmer, more able to be reasoned with, and happier (impairment/ intensity rating). Obtaining reliable teacher ratings was sometimes difficult due to short school terms, changes in teachers, illness and other logistical issues.
This study partially replicates a double-blind RCT of micronutrients to treat ADHD in adults (Rucklidge et al., 2014), showing improvements in both samples in general overall functioning on micronutrients, with very similar effect sizes and overall response rates to micronutrients (47% in the child sample and 48% in the adult sample). Improvements in core ADHD symptoms were less consistent in children than in the adults, although self-reported ratings of inattention improved more across both age groups on micronutrients as compared with placebo. Further, these results were consistent with other micronutrient studies documenting improved emotional regulation and reduced aggression in children (Adams et al., 2011; Frazier, Fristad, & Arnold, 2012; Kaplan, Hilbert, & Tsatsko, 2015; Mehl-Madrona, Leung, Kennedy, Paul, & Kaplan, 2010; Rucklidge, Gately, & Kaplan, 2010).
Retention of the sample was excellent, withonly two children in both arms prematurely discontinuing the study, none of which were due to adverse events.
There were no group differences in reported adverse events and there were no serious adverse events in either arm. Blood tests demonstrated that micronutrients exerted no negative effect on tests of liver, kidney and thyroid function and haematology. As anticipated, levels of folate and vitamins B12 and D increased significantly for those taking the micronutrients, but these were not associated with adverse effects. Measures of blood pressure, pulse, weight and height further confirmed no adverse effects of taking the micronutrients. Those on micronutrients nominally grew more during the 10-week trial than those on placebo, although the group difference was not significant (p = .06, ES = 0.40). These results are relevant in view of the adverse effects that stimulants can have on height and blood pressure, both in the short term and long term (Hennissen et al., 2017; Swanson et al., 2017; Thapar & Cooper, 2016). Further, there were few reports among participants ofloss of appetite, increased irritability and nausea, side effects frequently associated with stimulant medication (Thapar & Cooper, 2016). A significant decrease in eosinophils in those taking micronutrients was found, but this was not considered to be clinically significant because all means stayed within the normal reference range. Among the children, there was no increase in prolactin, contrasting the significant finding from the adult ADHD micronutrient study (Rucklidge et al., 2014). Overall, safety of consuming the micronutrients is consistent with all previous published reports (Frazier et al., 2013; Gordon et al., 2015; Rucklidge et al., 2014; Simpson et al., 2011).
In thinking about mechanisms of action, micronutrient treatment likely involves different mechanisms than medications. The reduction in homocysteine levels seen in the participants, a finding consistent with other studies using micronutrients (mainly folate, B6 and B12) as a treatment for psychological symptoms (White, Cox, Peters, Pipingas, & Scholey, 2015), suggests that micronutrients may have an impact on the methylation/methionine cycle, responsible for generating the one-carbon units required for the synthesis of DNA/RNA (Kennedy, 2016). High levels of homocysteine have been implicated in increasing oxidative stress, inhibiting methylation reactions, and increasing DNA damage (Kennedy, 2016). Reduction of homocysteine levels has been associated with improvements in mood (Mech & Farah, 2016) and cognitive function (Smith et al., 2010). Micronutrients also assist with the citric acid cycle and electron transport chain, acting as co-enzymes in mitochondrial aerobic respiration and energy production (Kennedy, 2016).
Strengths, limitations and future directions
A strength of this study was that participants had clinically significant levels of ADHD symptomatology (the sample was identified on average to be ‘markedly ill’ on the CGI-S at baseline) and high levels of co-occurring disorder (83%), typical of children seen in clinical practice (Joelsson et al., 2016). Another strength was the somewhat higher percentage of Māori and Pacific peoples in the study compared to the NZ census figures (http://www.stats.govt.nz/Census/2013-census/), suggesting that this treatment approach was appealing and beneficial to people of minority ethnic groups. Sample retention (96%) and adherence (91%–95%) were also excellent. It is also notable that blinding was effective, with accuracy no better than chance, adding to the validity of the CGI-I as a primary outcome measure. This decreases the likelihood that results are due to expectancy effects. The groups were well balanced at baseline on most variables, and although there were more children with GAD in the micronutrient group (30%) compared with the placebo group (7%), we confirmed that the response rate for those with GAD was similar with the response rate for the whole group, suggesting that preferential response to micronutrients due to the presence of anxiety was not driving the results observed. Unlike medications, where improvements can wear off towards the end of the day, benefits observed with micronutrients are sustained.
A limitation of the present study is that this is a single-centre trial with a moderate sample size, sufficient to detect moderate to large effect sizes, and likely underpowered to detect some of the more subtle changes observed in this study. Generalizability of findings is limited by the geographical setting and the potential for cohort effects. Some children (13%) who were assessed for eligibility were unable to participate because they could not swallow pills; however, a powder version of the formula is available. We used a less stringent cut-off for the teacher questionnaires as many children had previously been diagnosed with ADHD and previous use of medication may have influenced teacher ratings. The lower cut-off was viewed as a concession of cross-context ADHD symptoms without unduly compromising recruitment.
It is possible that the participants’ improvement over time was related to diet; however, dietary patterns are unlikely to have altered the relative effect of the micronutrients, compared with the placebo, given that dietary habits were balanced between the two groups at baseline and did not change over the course of the treatment. Future research may focus on comparing dietary manipulation with supplementation to determine whether some people may need additional nutrients beyond what they can obtain or absorb from regular dietary intake.
Using multiple measures increased the chances of making a Type 1 error; however, the fact that all the results were in the same direction, favouring micronutrient treatment over placebo, indicates that it is unlikely that our findings were compromised by false positives. The presentation of effect sizes, confidence intervals and clinical and practical significance (how many responded to the treatment), provides assurance in the clinical interpretation and follows an approach that is increasingly being encouraged (Cumming, 2013; Mark, Lee, & Harrell, 2016). However, given that many of the observed effect sizes in this trial were small to medium, results should be considered encouraging, but preliminary, and replication is required.
In terms of future directions, controlled studies investigating longer term exposure to the micronutrients are recommended, as the full effect of nutrients likely requires more than 10 weeks, based on a naturalistic follow-up study of adults (Rucklidge et al., 2017) and a 6-month study in children (Gordon et al., 2015). Based on the observed improvement in negative emotionality, future studies might consider utilizing a more specific and sensitive emotional dysregulation scale as a primary outcome variable and using temperament-based ADHD subtypes for categorical comparisons.
Conclusions
While improvements on core ADHD symptoms were modest and mixed across raters, results of this trial indicate that a number of children with ADHD derived significant benefit from micronutrients across a range of outcomes, most notably in the areas of inattention, emotional regulation and aggression. Given the findings, it is plausible that children with ADHD who have high levels of emotional dysregulation may respond preferentially to micronutrients. Future research is required to confirm these results, to elucidate which children may derive most benefit and why and whether effects can be enhanced in combination with medications. An important next step is identifying the biological mechanisms associated with symptom improvement (Stevens, Rucklidge, & Kennedy, 2017). Effect sizes in this study appear comparable with other commonly utilized nonpharmacological treatments of ADHD, such as behavioural treatments, omega 3s and food restriction diets (Sonuga-Barke et al., 2013). In addition to conferring the symptom improvements, the micronutrients were safe and well-tolerated over the course of the 10-week trial and as such, they may have an important role in the treatment of childhood ADHD, particularly in cases where conventional stimulant medication is not viable, either due to ineffectiveness, poor tolerability or parental preference.
Supplementary Material
Appendix S1. Ingredients of Daily Essential Nutrients™ and Placebo with recommended dietary allowances (RDA) for children given in the same unit.
Appendix S2. CONSORT Checklist.
Key points.
Nutrition is known to impact mental health as evidenced by epidemiological studies correlating inadequate nutrient intake with poor mental health, and supplementation with micronutrients showing improvement in mental health.
This RCT demonstrated micronutrients were safe and well-tolerated by 7–12 year old children with ADHD.
Inattention and overall functioning, as rated by clinicians, improved more in children who were randomized to micronutrients as compared with placebo.
Emotional dysregulation and aggression, symptoms often associated with ADHD, improved more with micronutrients as compared with placebo.
Micronutrients offered symptom improvement across a range of functional domains in children with ADHD (ES range = 0.35–0.66), with minimal side effects, making it a favourable option for some children.
Acknowledgements
The authors thank the Vic Davis Memorial Trust (E5672), UC Foundation, the Department of Psychology, University of Canterbury for ongoing research support, the GAMA Foundation, Canterbury Medical Research Foundation, Foundation for Excellence in Mental Health Care, National Institutes of Health (NIH), National Center for Complementary and Integrative Health (NCCIH) T32 AT002688 to support JJ and a PhD Scholarship awarded through Gravida to support KD. They thank also David Pugh-Williams for assistance with randomization; Leona Manna for providing cultural consultation; Lucy Kioa, Kate Harris, Anna Lee, Joanna Lothian, Hannah Retallick-Brown, Dr Brigette Gorman, Dr Heather Gordon and Molly Harvie for assistance with data collection and entry; the Canterbury District Health Board, Whakatata House and other private referrers and all the families who participated. They thank Hardy Nutritionals for providing the micronutrient formula and matching placebo for free. The 48-ingredient formula has been modified slightly on several occasions, with each change resulting in a new name. Sold variously as Daily Essential Nutrients, EMPowerplus Advanced and Q96. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
References
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, … & Lee W (2011). Effect of a vitamin/ mineral supplement on children and adults with autism. BMC Pediatrics, 11, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th edn). Arlington, VA: Author. [Google Scholar]
- Ames BN, Elson-Schwab I, & Silver E (2002). High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): Relevance to genetic disease and polymorphisms. American Journal of Clinical Nutrition, 75, 616–658. [DOI] [PubMed] [Google Scholar]
- Baker CW, Little TD, & Brownell KD (2003). Predicting adolescent eating and activity behaviors. The role of social norms and personal agency. Health Psychology, 22, 189–198. [PubMed] [Google Scholar]
- Barkley RA, & Fischer M (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 503–513. [DOI] [PubMed] [Google Scholar]
- Benton D (2013). To establish the parameters of optimal nutrition do we need to consider psychological in addition to physiological parameters? Molecular Nutrition and Food Research, 57, 6–19. [DOI] [PubMed] [Google Scholar]
- Conners CK (1997). Conners rating scales-revised: Technical manual. New York: Multi-Health Systems. [Google Scholar]
- Cumming G (2013). The New Statistics. Psychological Science, 25, 7–29. [DOI] [PubMed] [Google Scholar]
- Davis DR (2009). Declining fruit and vegetable nutrient composition: What is the evidence? HortScience, 44, 15–19. [Google Scholar]
- Faries DE, Yalcin I, Harder D, & Heiligenstein JH (2001). Validation of the ADHD Rating Scale as a clinician administered and scored instrument. Journal of Attention Disorders, 5, 107–115. [Google Scholar]
- Fraser D (1987). Mineral-deficient diets and the pig’s attraction to blood: Implications for tail-biting. Canadian Journal of Animal Science, 67, 909–918. [Google Scholar]
- Frazier EA, Fristad MA, & Arnold LE (2012). Feasibility of a nutritional supplement as treatment for pediatric bipolar spectrum disorders. Journal of Alternative and Complementary Medicine, 18, 678–685. [DOI] [PubMed] [Google Scholar]
- Frazier EA, Gracious B, Arnold LE, Failla M, Chitchumroonchokchai C, Habash D, & Fristad MA (2013). Nutritional and safety outcomes from an open-label micronutrient intervention for pediatric bipolar spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 23, 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, & Boles RG (2005). Is a “Mitochondrial Psychiatry” in the future? A review. Current Psychiatry Reviews, 1, 255–271. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2000). Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Gomez R, & Corr PJ (2014). ADHD and personality: A meta-analytic review. Clinical Psychology Review, 34, 376–388. [DOI] [PubMed] [Google Scholar]
- Goodman R (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Gordon HA, Rucklidge JJ, Blampied NM, & Johnstone JM (2015). Clinically significant symptom reduction in children with attention-deficit/hyperactivity disorder treated with micronutrients: An open-label reversal design study. Journal of Child and Adolescent Psychopharmacology, 25, 783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W (Ed.) (1976). ECDEU assessment manual for psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare. [Google Scholar]
- Hariri M, & Azadbakht L (2015). Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: A systematic review on the recent literature. International Journal of Preventive Medicine, 6, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty SC, Gnagy EM, Pelham WE, & Molina BSG (2017). Anger-irritability as a mediator of attention deficit hyperactivity disorder risk for adolescent alcohol use and the contribution of coping skills. Journal of Child Psychology and Psychiatry, 58, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman L, Swanson JM, Sibley MH, Stehli A, Owens EB, Mitchell JT, … & Nichols JQ (2016). Functional adult outcomes 16 years after childhood diagnosis of attention-deficit/hyperactivity disorder: MTA results. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 945–952. e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennissen L, Bakker MJ, Banaschewski T, Carucci S, Coghill D, Danckaerts M, … & Buitelaar JK (2017). Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: A systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs, 31, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AL, Robinson M, Smith GJ, Ambrosini GL, Piek JP, & Oddy WH (2011). ADHD is associated with a ‘Western’ dietary pattern in adolescents. Journal of Attention Disorders, 15, 403–411. [DOI] [PubMed] [Google Scholar]
- Joelsson P, Chudal R, Gyllenberg D, Kesti AK, Hinkka-Yli-Salomaki S, Virtanen JP, … & Sourander A (2016). Demographic characteristics and psychiatric comorbidity of children and adolescents diagnosed with ADHD in specialized healthcare. Child Psychiatry and Human Development, 47, 574–582. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Crawford SG, Field CJ, & Simpson JSA (2007). Vitamins, minerals, and mood. Psychological Bulletin, 133, 747–760. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Hilbert P, & Tsatsko E (2015). Micronutrient treatment for children with emotional and behavioral dysregulation:A case series. Journal of Medical Case Reports, 9, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BJ, Rucklidge JJ, McLeod K, & Romijn A (2015). The emerging field of nutritional mental health: inflammation, the microbiome, oxidative stress, and mitochondrial function. Clinical Psychological Science, 3, 964–980. [Google Scholar]
- Kaplan BJ, Steiger RA, Pope J, Marsh A, Sharp M, & Crawford SG (2010). Successful treatment of pill-swallowing difficulties with head posture practice. Paediatric Child Health, 15, e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, & Nigg JT (2014). Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry, 71, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–987. [DOI] [PubMed] [Google Scholar]
- Kennedy D (2016). B Vitamins and the brain: Mechanisms, dose and efficacy—a review. Nutrients, 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Roizen E, Hutchison JA, Lashau EC, & Castellanos FX (2012). Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Archives of General Psychiatry, 69, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaas ET, Aarsland TIM, Ulvik A, Halmøy A, Ueland PM, & Haavik J (2016). Vitamin levels in adults with ADHD. BJPsych Open, 2, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, & LaPadula M (1993). Adult outcome of hyperactive boys - educational-achievement, occupational rank, and psychiatric status. Archives of General Psychiatry, 50, 565–576. [DOI] [PubMed] [Google Scholar]
- Mark DB, Lee KL, & Harrell FE Jr (2016). Understanding the role of p values and hypothesis tests in clinical research. JAMA Cardiology, 1, 1048–1054. [DOI] [PubMed] [Google Scholar]
- Mech AW, & Farah A (2016). Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: A randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry, 77, 668–671. [DOI] [PubMed] [Google Scholar]
- Mehl-Madrona L, Leung B, Kennedy C, Paul S, & Kaplan BJ (2010). A naturalistic case-control study of micronutrients versus standard medication management in autism. Journal of Child and Adolescent Psychopharmacology, 20, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz W (1994). A balanced approach to nutrition for health: The need for biologically essential minerals and vitamins. Journal of the American Dietetic Association, 94, 1259–1262. [DOI] [PubMed] [Google Scholar]
- Milne BJ, Byun U, & Lee A (2013). New Zealand socioeconomic index 2006. Wellington: Statistics New Zealand. [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, … & MTA Cooperative Group (2009). The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Lewis K, Edinger T, & Falk M (2012). Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JS, Goldfine ME, Evangelista NM, Hoza B, & Kaiser NM (2007). A critical review of self-perceptions and the positive illusory bias in children with ADHD. Clinical Child and Family Psychology Review, 10, 335–351. [DOI] [PubMed] [Google Scholar]
- Paterson C (1996). Measuring outcomes in primary care: A patient generated measure, MYMOP, compared with the SF-36 health survey. British Medical Journal, 312, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Devineni B, Carbray JA, & Birmaher B (2006). Child mania rating scale: Development, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 550–560. [DOI] [PubMed] [Google Scholar]
- Pelsser LM, Frankena K, Toorman J, Savelkoul HF, Dubois AE, Pereira RR, … & Buitelaar JK (2011). Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): A randomised controlled trial. Lancet, 377, 494–503. [DOI] [PubMed] [Google Scholar]
- Popper CW (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23, 591–672. [DOI] [PubMed] [Google Scholar]
- Popper CW, Kaplan BJ, & Rucklidge JJ (2017). Single and Broad-Spectrum Micronutrient Treatment in Psychiatric Practice In Gerbarg PL, Muskin PR & Brown RP (Eds.), Complementary and Integrative Treatments in Psychiatric Practice (pp. 75–101). Washington, DC: American Psychiatric Association. [Google Scholar]
- Rios-Hernandez A, Alda JA, Farran-Codina A, Ferreira-Garcia E, & Izquierdo-Pulido M (2017). The mediterranean diet and ADHD in children and adolescents. Pediatrics, 139, e20162027. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Frampton CM, Gorman B, & Boggis A (2014). Vitamin-mineral treatment of ADHD in adults: A double-blind, randomized, placebo controlled trial. British Journal of Psychiatry, 204, 306–315. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Frampton CM, Gorman B, & Boggis A (2017). Vitamin-mineral treatment of ADHD in adults: A 1-year naturalistic follow-up of a randomized controlled trial. Journal of Attention Disorders, 21, 522–532. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Gately D, & Kaplan BJ (2010). Database analysis of children and adolescents with bipolar disorder consuming suming a multinutrient formula. BMC Psychiatry, 10, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ, Johnstone J, & Kaplan BJ (2009). Nutrient supplementation approaches in the treatment of ADHD. Expert Review of Neurotherapeutics, 9, 461–476. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, & Kaplan BJ (2013). Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: A systematic review. Expert Review of Neurotherapeutics, 13, 49–73. [DOI] [PubMed] [Google Scholar]
- Schoenthaler SJ, & Bier ID (2000). The effect of vitamin-mineral supplementation on juvenile delinquency among American schoolchildren: A randomized, double-blind placebo-controlled trial. Journal of Alternative and Complementary Medicine, 6, 7–17. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, & Aluwahlia S (1983). A children’s global assessment scale (CGAS). Archives of General Psychiatry, 40, 1228–1231. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, & Leibenluft E (2014). Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry, 171, 276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JSA, Crawford SG, Goldstein ET, Field C, Burgess E, & Kaplan BJ (2011). Systematic review of safety and tolerability of a complex micronutrient formula used in mental health. BMC Psychiatry, 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Moffitt TE, Poulton R, & Caspi A (2012). Undercontrolled temperament at age 3 predicts disordered gambling at age 32: A longitudinal study of a complete birth cohort. Psychological Science, 23, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, … & Refsum H (2010). Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE, 5, e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, … & Sergeant J (2013). Non-pharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. American Journal of Psychiatry, 170, 279–289. [DOI] [PubMed] [Google Scholar]
- Sprich SE, Safren SA, Finkelstein D, Remmert JE, & Hammerness P (2016). A randomized controlled trial of cognitive behavioral therapy for ADHD in medication-treated adolescents. Journal of Child Psychology and Psychiatry, 57, 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sikirica V, Weiss MD, Robertson B, Lyne A, & Newcorn JH (2015). Does guanfacine extended release impact functional impairment in children with attention-deficit/hyperactivity disorder? Results from a randomized controlled trial. CNS Drugs, 29, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AJ, Rucklidge JJ, & Kennedy MA (2017). Epigenetics, nutrition and mental health. Is there a relationship? Nutritional Neuroscience, 1–12. Advanced online publication. [DOI] [PubMed] [Google Scholar]
- Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, … & Gillies D (2015). Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. British Medical Journal, 351, h5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, & Nigg JT (2015). Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry, 56, 949–957. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, … & MTA Cooperative Group (2017). Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: Symptom persistence, source discrepancy, and height suppression. Journal of Child Psychology and Psychiatry, 58, 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, & Cooper M (2016). Attention deficit hyperactivity disorder. Lancet, 387, 1240–1250. [DOI] [PubMed] [Google Scholar]
- van Stralen J (2016). Emotional dysregulation in children with attention-deficit/hyperactivity disorder. Attention Deficit and Hyperactivity Disorders, 8, 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2004). The Wechsler intelligence scale for children (4th edn). London: Pearson Assessment. [Google Scholar]
- West AE, Celio CI, Henry DB, & Pavuluri MN (2011). Child Mania Rating Scale-Parent Version: A valid measure of symptom change due to pharmacotherapy. Journal of Affective Disorders, 128, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D, Cox K, Peters R, Pipingas A, & Scholey A (2015). Effects of four-week supplementation with a multivitamin/mineral preparation on mood and blood biomarkers in young adults: A randomised, double-blind, Placebo-Controlled Trial. Nutrients, 7, 5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS (1993). WRAT3 administration manual. Wilmington, DL: Wide Range. [Google Scholar]
- Zhang S, Faries DE, Vowles M, & Michelson D (2005). ADHD Rating Scale IV: Psychometric properties from a multinational study as a clinician-administered instrument. International Journal of Methods in Psychiatry Research, 14, 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Ingredients of Daily Essential Nutrients™ and Placebo with recommended dietary allowances (RDA) for children given in the same unit.
Appendix S2. CONSORT Checklist.