Abstract
Tigecycline is unique glycylcycline class of semisynthetic antimicrobial agents developed for the treatment of polymicrobial infections caused by multidrug-resistant Gram-positive and Gram-negative pathogens. Tigecycline evades the main tetracycline resistance genetic mechanisms, such as tetracycline-specific efflux pump acquisition and ribosomal protection, via the addition of a glycyclamide moiety to the 9-position of minocycline. The use of the parenteral form of tigecycline is approved for complicated skin and skin structure infections (excluding diabetes foot infection), complicated intra-abdominal infections, and community-acquired bacterial pneumonia in adults. New evidence also suggests the effectiveness of tigecycline for the treatment of severe Clostridioides difficile infections. Tigecycline showed in vitro susceptibility to Coxiella spp., Rickettsia spp., and multidrug-resistant Neisseria gonnorrhoeae strains which indicate the possible use of tigecycline in the treatment of infections caused by these pathogens. Except for intrinsic, or often reported resistance in some Gram-negatives, tigecycline is effective against a wide range of multidrug-resistant nosocomial pathogens. Herein, we summarize the currently available data on tigecycline pharmacokinetics and pharmacodynamics, its mechanism of action, the epidemiology of tigecycline resistance, and its clinical effectiveness.
Keywords: Tigecycline resistance, Tigecycline antibacterial activity, Tigecycline clinical Effectiveness
Introduction
The increasing incidence of multidrug-resistant (MDR) or extensively drug-resistant (XDR) bacterial pathogens is a major public health concern that poses an economic burden to healthcare system due to prolonged hospital stays and higher morbidity and mortality [1]. Tigecycline is a tetracycline-class antibacterial agent developed for the treatment of polymicrobial MDR infections [2] including both Gram-negative and Gram-positive bacteria. Tigecycline, known as GAR-936, or Tygacil, is the first, unique glycylcycline class of semisynthetic agents which is administered in a parenteral form [3] and was approved by the Food and Drugs Administration (FDA) in 2005 [4]. Later, in 2010, the FDA issued an alert that use of tigecycline in the treatment of severe infections and sepsis was significantly associated with an increased risk for all-cause mortality [5]. Currently, tigecycline has been approved as a monotherapy in adults for three indications including complicated skin and skin structures infections (cSSTI) with the exclusion of diabetes foot infection, complicated intra-abdominal infections (cIAI), and community-acquired bacterial pneumonia (CAP) [6, 7], and recent evidence suggests that tigecycline may be effective in the treatment of severe Clostridioides difficile infection [8]. The resistance to tigecycline includes chromosomally or accessory gene-encoded mechanisms. Herein, we summarize the currently available data on tigecycline pharmacokinetics and pharmacodynamics, its mechanism of action, the epidemiology of tigecycline resistance, and its clinical effectiveness.
Structural characterization
Tigecycline is chemically (4 S, 4 aS,5 aR,12aS)- 9- [2-(tert-butylamino) acetami do]- 4,7-bis(dimethylamino)-l,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-l, ll-dioxo-2-naphthacene-carboxamide [6, 9]. Its chemical formula is C29H39N508 with molecular weight of 585.65 Da [10]. Tigecycline is a chemically modified minocycline (9-t-butylglycylamido derivative of minocycline) [6, 9]. Compared with other tetracyclines, tigecycline’s extended, wide-range antibiotic activity is due to a main backbone of minocycline with an N-alkyl-glycylamido side chain addition to the C9 carbon of the “D” tetracycline ring [6, 9].
Pharmacokinetics and pharmacodynamics
Due to insufficient absorption from the gut, tigecycline administration is intravenous; ~ 30–60 min every 12 h [6]. The in vitro plasma protein binding of tigecycline at 0.1, 1, and 15 ug/mL was reported as 71, 89, and 96, respectively, and showed nonlinear plasma-protein-binding behavior since the unbound fraction of tigecycline decreased with an increase in the total concentration of tigecycline [11]. Tigecycline has a systemic clearance (from 0.2 to 0.3 L/h/kg), a large volume of distribution (7–10 L/kg), and extensive distribution into various tissues [10]. The recommended standard dosage regimen for tigecycline is an initial dose of 100 mg followed by 50 mg every 12 hrs [12]. The recommended duration of treatment with tigecycline for cSSTI or cIAI and CAP is 5–14 and 7–14 days, respectively [13].
Tigecycline is excreted mainly unchanged in the bile [12] and has a very long half-life (t1/2) in humans (~ 27–42 h) [12]. Tigecycline achieves therapeutic concentrations by effectively and extensively penetrating body fluids and tissues, such as the lungs, skin, liver, heart, bone, and kidneys [14–16]. Tigecycline has relatively low mean steady-state serum concentrations of 0.403 mg/L and 0.633 mg/L in patients with cSSTI in the standard dosing [17]. The data on tigecycline pharmacokinetics showed that the ratio of tissue to serum tigecycline concentrations was 38-fold, 8.6-fold, 2.1-fold, 0.35-fold, and 0.58-fold higher in the gall bladder, lungs, colon, bone, and synovial fluid, measured at 4 h after administration of a single 100 mg dose [18]; a higher ratio of tissue to serum of tigecycline in skin and soft tissue was also found after 1–6 days of standard treatment [15]. The penetration of tigecycline into bones was reported by Bhattacharya et al. (bone: serum ratio; 4.77-fold) [19]. Data from several pharmacokinetic-pharmacodynamic (PK/PD) analyses and clinical trials showed that the ratio for the area under the concentration time curve and minimal inhibitory concentration (AUC/MIC) for serum tigecycline concentrations is a predictor of therapeutic response [20, 21]. Tigecycline does not readily cross the blood-brain barrier.
The experimental data suggested that tigecycline exhibits a time-dependent bactericidal activity and has a prolonged postantibiotic effect (PAE) against Gram-positive and Gram-negative pathogens following a 3 mg/kg dose [22–24]. In comparison to minocycline, tigecycline has a uniformly longer PAE for tested pathogens (3.4–4 h for Staphylococcus aureus and 1.8–2.9 h for Escherichia coli) [22, 23].
Tigecycline is eliminated from the body through biliary excretion in the feces (59%) and urine (22%). Age, sex, and renal function do not appear to interfere with the pharmacokinetics of tigecycline, and no dose adjustment is required for patients with renal impairment (including hemodialysis) [25–27]. However, clinical caution in the use of tigecycline is needed in patients who have severe hepatic dysfunction (Child Pugh C); an initial dose of 100 mg of tigecycline should be followed by reduced maintenance doses of 25 mg every 12 h [27–29].
Mechanism of Action
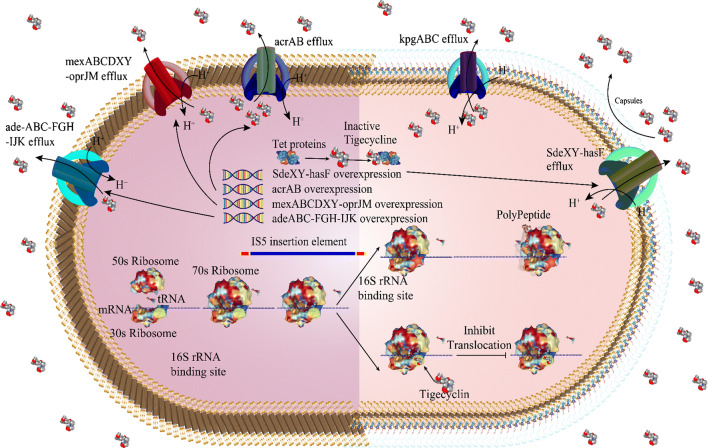
Tigecycline is a bacteriostatic, parenteral glycylcycline antibiotic with a stronger (5-fold) binding affinity and structural similarities to the tetracyclines [4, 14, 27]. The main mechanism of action of tigecycline is similar to other tetracyclines in that it acts an inhibitor of bacterial protein translation (i.e., elongation of the peptide chain) via reversible binding to a helical region (H34) on the 30S subunit of bacterial ribosomes. The binding of tigecycline prevents the incorporation of amino acid residues into the elongation of peptide chains and results in the loss of peptide formation and bacterial growth [4, 14, 27] (Fig. 1). Tigecycline was developed to overcome the main molecular mechanisms of tetracycline resistance, such as tetracycline-specific efflux pump acquisition [e.g., tet(A)] and ribosomal protection [e.g., tet(M)], through the addition of a glycyclamide moiety to the 9-position of minocycline.
Fig. 1.
Tigecycline mechanisms of action and resistance
Antimicrobial susceptibility testing to tigecycline
Currently, several laboratory methods, including broth microdilution and disk diffusion, have been used for the determination of in vitro susceptibility to tigecycline [30, 31]. Broth microdilution is the reference method for the testing of in vitro susceptibility to tigecycline, though, according to the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [30, 31], the medium must be prepared fresh on the day of use and must be not more than 12 h old at the time the panels are made.
For other Enterobacterales, except for E. coli, the activity of tigecycline varies from insufficient in Proteus spp., Morganella morganii, and Providencia spp. to variable in other species [31]. The interpretative minimal inhibitory concentration breakpoints to tigecycline recommended by EUCAST [31], the Food and Drug Administration (FDA) [32], and the British Society for Antimicrobial Chemotherapy (BSAC) [33] to various bacteria are indicated in Table 1. The CLSI interpretative minimal inhibitory concentration breakpoints to tigecycline are not available.
Table 1.
Tigecycline international in vitro susceptibility breakpoints.
| Bacterial family/species | International breakpoints standard | Broth microdilution (mg/L) | Disk diffusion (mm) |
|---|---|---|---|
| Enterobacteriaceae | EUCAST | S ≤ 0.5, R > 0.5 | S ≥ 18, R < 18 |
| FDA | S ≤ 2, R ≥ 8 | S ≥ 19, R ≤ 14 | |
| BSAC | S ≤ 1, R > 2 | S ≥ 24, R ≤ 19 | |
| Staphylococcus spp. | EUCAST | S ≤ 0.5, R > 0.5 | S ≥ 18, R < 18 |
| FDA | S ≤ 0.5 | S ≥ 19 | |
| BSAC | S ≤ 0.5, R > 0.5 | S ≥ 26, R ≤ 25 | |
| Enterococcus spp. | EUCAST | S ≤ 0.25, R > 0.25 | S ≥ 18, R < 18 |
| FDA | S ≤ 0.25 | S ≥ 19 | |
| BSAC | S ≤ 0.25, R > 0.5 | S ≥ 21, R < 20 | |
| Streptococcus groups A, B, C and G | EUCAST | S ≤ 0.125, R > 125 | S ≥ 19, R < 19 |
| FDA | S ≤ 0.25 | S ≥ 19 | |
| BSAC | S ≤ 0.25, R > 0.5 | S ≥ 25, R < 19 | |
| Streptococcus pneumoniae | EUCAST | - | - |
| FDA | S ≤ 0.06 | S ≥ 19 | |
| BSAC | - | - | |
| Clostridioides difficile | EUCAST | S ≤ 0.25, R > 0.25 | - |
| FDA | S ≤ 4, R > 16 | - | |
| BSAC | S ≤ 0.25 | - | |
| Acinetobacter spp. | EUCAST | - | - |
| FDA | S ≤ 2, R ≥ 8 | - | |
| BSAC | S ≤ 1, R > 2 | S ≥ 20, R < 20 | |
| Pseudomonas spp. | EUCAST | - | - |
| FDA | S ≤ 2, R ≥ 8 | - | |
| BSAC | - | - |
EUCAST European Committee on Antimicrobial Susceptibility Testing, FDA Food and Drug Administration, BSAC British Society for Antimicrobial Chemotherapy, S sensitive, R:Resistance
Antibacterial activity
Alterations to the tetracycline structure resulted in an expansion of tigecycline’s spectrum of an antibacterial activity against a wide spectrum of Gram-positive and Gram-negative pathogens [34]. Currently, due to its effectiveness, tigecycline is the last-line treatment option against MDR bacterial pathogens, especially carbapenem-resistant Enterobacteriaceae [35–40]. Tigecycline showed good activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and penicillin-resistant Streptococcus pneumoniae [41].
In addition, tigecycline was highly active against Stenotrophomonas maltophilia, Moraxella catarrhalis, Haemophilus influenzae, and Neisseria gonorrhoeae [42–44]. Blanton et al. [45] have indicated that tigecycline is effective against Rickettsia rickettsii [45].
Antibacterial activity was also observed against Coxiella burnetii derived from patients with acute Q fever [46]. The flow cytometry assay data suggest that tigecycline has antibacterial activity [(IC50) 0.71 × 10-3 μg/mL] against Orientia tsutsugamushi and that it may be a therapeutic option for the treatment of scrub typhus [47]. The susceptibility of Clostridioides difficile isolates was proved during pan-European, longitudinal surveillance [48]. In addition, clinical data on the use of tigecycline administered alone, or as a part of combination therapy of oral vancomycin and intravenous metronidazole, showed its efficiency in patients with a severe course of Clostridium difficile infection (CDI) [49]; however, randomized controlled trials are necessary before tigecycline can be recommended for routine use in the treatment of CDI [50].
Some pathogens, such as P. aeruginosa, Proteus spp. Providencia spp., and Morganella spp., are intrinsically resistant to tigecycline [51–53] and the development of acquired resistance to tigecycline has been described in several bacterial species such as Acinetobacter baumannii, Klebsiella pneumonia, Enterobacter spp., and Bacteroides fragilis [49].
Mechanisms of tigecycline resistance
In the last decades, the emergence of tigecycline resistance has been reported worldwide [49, 54, 55] though there are relatively few data available regarding the molecular basis for resistance to tigecycline. As shown in vitro, the Tet proteins [e.g., Tet(X), Tet(A), Tet(K) and Tet(M)] have the potential to acquire mutations leading to a reduced susceptibility (i.e,. increased MICs) to tigecycline [56] possibly through the horizontal transfer of mobile genetic elements carrying several resistance genes. In addition, the mobile tigecycline-resistance tet(X) gene variants are newly emerging tigecycline resistance mechanisms in humans and animals [57]. The Tet(X) is a flavin-dependent monooxygenase that originated from Bacteroides spp. and was detected in Enterobacteriaceae and some Acinetobacter spp. isolates [58–60].
In Gram-negative bacteria, the chromosomally encoded, overexpression of resistance-nodulation division (RND) efflux pumps such as AdeABC, AdeFGH, AdeIJK, MexXY, and AcrAB are important molecular mechanisms in the resistance of bacteria to tigecycline [61–63].
Acinetobacter baumannii
The occurrence of increased MICs and resistance to tigecycline among Acinetobacter spp. was associated with the upregulation of AdeABC, AdeFGH, AdeIJK, AbeM, and AdeDE pumps and also the presence of the tetX gene [64, 65] although some studies noted that additional efflux pumps or different molecular mechanisms might contribute to tigecycline resistance [58, 66, 67]. The nucleotide and amino acid alterations in the AdeRS two-component system may lead to adeABC overexpression and tigecycline resistance [68]. Besides it was found that the BaeSR system positively regulates the expression of adeA and adeB and stimulated tigecycline-resistant strains [69].
Additional mechanisms of decreased susceptibility to tigecycline, such as a novel RND pump, the presence of tet(X1) or tetA genes, a mutation in the trm gene encoding S-adenosyl-L-methionine (SAM)-dependent methyltransferase, and a frameshift mutation in the plsC gene that encodes for 1-acyl-sn-glycerol-3-phosphate acyltransferase, have been detected in the clinical A. baumannii isolates[69–71].
Enterobacteriaceae
The intrinsic resistance to tigecycline in Enterobacteriaceae has been described in Morganella morganii and Proteus mirabilis and was attributed to the constitutive upregulation of the multidrug AcrAB efflux pump [50]. The AcrAB efflux pumps and their regulatory genes also play a role in the decreased susceptibility to tigecycline in E. coli and Klebsiella spp. [55, 62, 72–74].
Currently, SoxS, MarA, RamA, and Rob have been characterized as global regulators of the AcrAB pump in Enterobacteriaceae [75] although the exact mechanism of AcrAB pump overexpression has not been clarified [76, 77].
Escherichia coli
Tigecycline is a possible substrate for the AcrAB and AcrEF pumps in E. coli [78]. The physiological role of the AcrAB pump in E. coli is critical, and it excretes a diversity of lipophilic and amphiphilic antibiotics as substrates [79]. MarA, SoxS, and Rob have been suggested as regulators involved in the MDR phenotype in E. coli [80]. One of the major mechanisms involved in the E. coli MDR phenotype is mediated by the mar regulon that stimulates the downregulation of the OmpF outer membrane porin and also stimulates the upregulation of the AcrAB efflux pump [81, 82]. In E. coli, MarA (controlled by the local repressor MarR) acts as a positive regulator of the AcrAB–TolC efflux pump [83]. Additionally, in some E. coli strains that have high tigecycline MICs, a frameshift mutation (insertion of a cytosine at position 355) has been described in marR (one of the targets for reduced susceptibility to tigecycline) that led to the overexpression of MarA and AcrAB pumps [83]. Linkevicius et al. [84] selected tigecycline-resistant E. coli mutants in vitro and evaluated their biological fitness and cross-resistance.
A relatively low-level resistance and a high fitness cost were identified in isolates with mutations of efflux regulatory network genes (lon, acrR, and marR) and related lipopolysaccharide core biosynthesis pathway genes (lpcA, rfaE, rfaD, rfaC, and rfaF). Remarkably, the fitness cost of mutations in E. coli under tigecycline exposure may decrease the ability of mutants to trigger a successful infection [84]. The reduced fitness and virulence in clinical isolates when acrA and tolC were inactivated have already been described, implying that the AcrAB pump may also play a role in adaptation and host virulence [85]. However, more in vivo research is needed to determine how these different mutation types are involved in bacterial virulence.
Klebsiella pneumoniae
In K. pneumoniae, tigecycline resistance is related extensively to the overexpression of RamA [86, 87]. There is a positive association between the upregulation of ramA with an overexpression of AcrAB [75, 87–89]. Nevertheless, no association between the upregulation of ramA and AcrA expression has been described [90]. RarA is a new AraC-type global regulator that acts via the control of AcrAB and OqxAB efflux pump expression and is mediated by the MDR phenotype in K. pneumoniae [62, 88, 91]. However, He et al. have reported no marked correlation between OqxAB and tigecycline resistance [73]. Sheng et al. [92] have also proposed that RamA may be a positive regulator of the OqxAB pump since variants in ramR have been suggested as a mechanism of acrAB downregulation and tigecycline resistance [77, 92, 93]. IS5 element integration in the new efflux pump operon kpgABC is correlated with a novel mechanism for the rapid in vivo development of tigecycline non-susceptibility [94]. Villa et al. [77] highlighted the role of the ribosomal S10 protein mutation (a mutation in the rpsJ gene that has already been reported to reduce tigecycline susceptibility in both Gram-negative and positive bacteria) in conferring tigecycline resistance. In addition, an alternative pathway involved in K. pneumoniae resistance to tigecycline is the overexpression of marA that is associated with AcrAB upregulation overexpression [62, 88]. The failure of tigecycline treatment in patients with carbapenem-resistant K. pneumoniae (CRKP) strains that harbor the tetA gene has been reported [95]. Additional tigecycline resistance mechanisms conferred by Tet proteins (mainly Tet(X)) have been published, [96]. In a recent study conducted in China [97], mutations in the ramR and tet(A) efflux genes were found to be the major tigecycline resistance mechanisms among the studied tigecycline- and carbapenem-resistant K. pneumoniae isolates.
Serratia marcescens
The upregulation of the SdeXY–HasF efflux pump (a part of the RND efflux pump family) has been associated with tigecycline resistance in S. marcescens [98]. The upregulation of the SdeXY–HasF efflux system that confers resistance to tigecycline is also active against ciprofloxacin and cefpirome. On the other hand, in an experimental mutant strain, the insertional independent inactivation of the sdeY and hasF genes also led to a reduced sensitivity to ciprofloxacin, cefpirome, and tetracycline [98].
Enterobacter spp.
In Enterobacter spp., the ramA-mediated mechanisms involving AcrAB efflux pump regulation are the primary mechanisms of tigecycline resistance [62, 99]. In E. aerogenes and E. cloacae, the nucleotide mutations include frameshifts, deletions, and amino acid variations in ramR (mainly in the ligand-binding domain) that lead to the overexpression of ramA and tigecycline resistance [62]. However, the other probable alternative mechanisms of tigecycline resistance that have been reported in E. cloacae include ramA overexpression without any ramR alterations; rarA overexpression and upregulation of the OqxAB pump; and upregulation of the AcrAB through SoxS, RobA, and RamA [62, 85]. Further in vivo and in vitro investigations are needed to characterize fully the probable other efflux pumps and/or regulators involved in tigecycline resistance mechanisms in Enterobacteriaceae [73, 75, 90, 100].
Salmonella spp.
In S. enterica, a positive correlation between the upregulation of ramA (via an inactivating mutation in ramR) and the consecutive overexpression of AcrAB with tigecycline resistance have been reported [101–103], although how ramA is controlled in bacteria other than Salmonella spp. is currently unknown. Similar to tigecycline resistance in carbapenem-resistant K. pneumoniae isolates, the combination of mutations in ramR and tet(A) genes was also reported in tigecycline-resistant S. enterica [61, 97, 104].
Pseudomonas aeruginosa
Currently, several Resistance-Nodulation- Division (RND) efflux pumps including MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM have been suggested as mechanisms for drug resistance in P. aeruginosa [105–110]. Dean et al. suggested the overexpression of MexXY-OprM as a drug efflux-mediated tigecycline resistance mechanism [110, 111]. In addition, the overexpression of the SdeXY pump frequently underlies tigecycline intrinsic resistance in P. aeruginosa [110]. In addition, the expression of other efflux pumps in MDR P. aeruginosa isolates has also been reported [112, 113].
Gram-positive bacteria
Relatively few data on tigecycline resistance in gram-positive bacteria are available. Overexpression of the multi-antimicrobial extrusion protein (MATE) family efflux pump MepA has been suggested as mechanism of decreased susceptibility to tigecycline in Staphylococcus aureus but does not confer resistance [52, 114, 115]. More recently, Fiedler et al. confirmed that overexpression of two tetracycline-resistance determinants, a tet(L)-encoded Major facilitator superfamily (MFS) pump and a tet(M)-encoded ribosomal protection protein, confer tigecycline resistance in Enterococci spp. [116]. The mechanisms of resistance to tigecycline are shown in Fig. 1.
Effectiveness of tigecycline in clinical settings
In September 2010, the FDA Adverse Event Reporting System (FAERS) reported [117] an increased risk of mortality with tigecycline (4%; 150/3788) compared with other antibiotics (3%; 110/3646) used to treat similar infections. However, data from a prospective, multicenter, non-interventional study demonstrated the efficacy and safety of tigecycline in a population of severely ill patients with complicated infections [118]. In a retrospective observational study, Kwon et al. [119] evaluated the efficacy and safety profile of tigecycline in comparison with colistin in XDR A. baumannii-positive patients. No difference was observed between both antibiotic groups in terms of treatment success and mortality rates. Serum creatinine elevation and nephrotoxic prevalence cases were observed more commonly in the colistin group (p = 0.028). On the other hand, the excess mortality of 16.7% (60.7 vs. 44%, 95% confidence interval 0.9–32.4%, p = 0.04) was reported in 294 of subjects treated with tigecycline versus colistin for the treatment of pneumonia caused by the multidrug-resistant A. baumannii [120].
In September 2013, FAERS analyzed the data from 10 clinical trials conducted only for FDA-approved uses (cSSSI, cIAI, CABP) [121]. This analysis showed a higher risk of mortality among subjects treated with tigecycline compared with comparators: 2.5 (66/2640) vs. 1.8% (48/2628), respectively. In general, the deaths resulted from worsening infections, complications of infection, or other underlying medical conditions.
In a meta-analysis [122] of 5 trials, comparing tigecycline monotherapy versus combination therapy for the treatment of patients with hospital-acquired pneumonia, no significant difference was observed in the development of the mortality rate from two prospective cohort studies (OR = 2.22, 95% CI 0.79–6.20 p = 0.13).
In a systematic review and meta-analysis [123], including 24 controlled studies, tigecycline-induced secondary bacteremia was found in 4.6% (91/1961) of patients with bloodstream infections. All-cause mortality and clinical cure rates for tigecycline were relatively similar to control antibiotic agents. Tigecycline, in combination with other antimicrobial agents, was suggested as a suitable choice for at-risk patients with BSI. However, tigecycline is not superior to comparator agents for the treatment of serious infections [2].
Due to the rise of multidrug-resistant infections, tigecycline has been used for non-approved indications. In a Spanish university hospital, one-third of tigecycline prescriptions were non-approved mainly as a rescue therapy and concomitantly with other antibiotics in patients with nosocomial pneumonia [124]; and in an Argentinean hospital, it was 79%, especially in ventilator-associated pneumonia due to MDR Acinetobacter spp. [125]. In a Taipei Veterans’ General Hospital, tigecycline was used for non-Food and Drug Administration-approved indications, to treat healthcare-associated pneumonia (38, 57.6%), bacteremia (3, 4.5%), catheter-related infections (3, 4.5%), urinary tract infection (4, 6.1%), osteomyelitis (4, 6.1%), and others (2, 3%) [126]. In a Turkish university hospital, tigecycline was used in the intensive care unit for patients infected with carbapenem-resistant Acinetobacter baumannii [127]. A study carried out in a Lebanese tertiary-care hospital reported 81% of tigecycline non-approved indications in critically ill patients with non-inferior outcome to that of FDA-approved indications [128].
In a pediatric population, tigecycline is not recommended in children and adolescents below 18 years of age. However, clinical studies reported the efficacy of a tigecycline therapy combined with other antimicrobial agents in the treatment of multidrug-resistant infection, i.e., nosocomial infections in newborn infants [129–131] and carbapenem-resistant gram-negative bacteria infections in liver transplant recipients [132]. Recently, tigecycline was used as a treatment in a case of ventriculoperitoneal shunt-related meningitis in a 5-month-old infant [133].
Adverse effects
Available evidence from 15 randomized controlled trials (RCTs), including a recent meta-analysis [134], assessed the available data with regard to the effectiveness and safety of tigecycline in comparison to other antimicrobials in the treatment of 7689 adult patients with infectious diseases. Adverse events and all-cause mortality were frequent in the tigecycline group. Twelve of the 15 RCTs (6292/7689) described various adverse events with tigecycline use. The adverse events rate was considerably higher in the tigecycline group compared with the comparator drug group (OR = 1.49, 95% CI = 1.23 to 1.80, p < 0.0001).
Based on the results from the preclinical animal safety studies, tigecycline was not thought to be teratogenic [27]; however, in rats and dogs a decrease of white and red blood cells, bone marrow hypocellularity, reductions in fetal weights, and an increased incidence of fetal loss and minor skeletal abnormalities were reported [27, 135]. Now, tigecycline is categorized as teratogenic effect class D and should be used with caution in specific populations, including nursing mothers, pregnant women, pediatrics, and patients with severe hepatic impairment [4, 13, 27, 135, 136]. In addition, the use of tigecycline may affect tooth development particularly if used during the last half of pregnancy and in children under the age of 8 as it can cause permanent tooth discoloration [137].
The human clinical trial studies and the FAERS [138] reported that the most common side effects following tigecycline administration, especially in adults aged between 18 and 50 years, and which were more likely in women, are gastrointestinal (GI) symptoms, i.e., nausea, vomiting, and diarrhea. Further reported side effects relevant to tigecycline administration were pancreatitis, acute generalized exanthematous pustulosis, local reaction at the i.v. site, increased hepatic function, thrombophlebitis, pruritus, fever, mitochondrial dysfunction-associated acute metabolic acidosis abdominal pain, headache, cholestatic, jaundice, and Steven-Johnson syndrome [2, 139–144].
Clinical studies showed a significant higher (~ > 4-fold) incidence of nausea and vomiting induced by tigecycline in patients treated for cSSSI compared with patients treated with vancomycin/aztreonam. However, in patients with cIAI, the incidence of nausea and vomiting occurred equally often in patients treated with imipenem/cilastatin as it did in patients treated with tigecycline (25%/20% for tigecycline and 21%/15% for imipenem/cilastatin group, respectively). In community-acquired bacterial pneumonia, the occurrence of GI symptoms was higher in the group of patients treated with tigecycline than the group treated with levofloxacin [138].
The mechanism of action of tigecycline-associated nausea and vomiting remains uncertain and their incidence is dose-related [145]. Whether it is preventable by the pre-emptive use of antiemetics as concomitant drugs (metoclopramide. ondasetron, prochlorperazine, sucralfate, and trimethobenzamide) is unclear [146, 147] . From 2514 patients, the total discontinuation rate was 7% during tigecycline treatment and discontinuation was most frequently associated with nausea (1%) and vomiting (1%) [138].
The phase III clinical trials evaluated tigecycline tolerability and efficacy in patients receiving tigecycline (i.e., 100-mg IV loading dose followed by 50 mg IV q12h) [2, 148–151]. The difference in the incidence of nausea and vomiting between tigecycline and the comparators (vancomycin+aztreonam or imipenem/cilastatin) was statistically significant (p < 0.05) in ≥ 2 of the 4 Phase III trials.
Clinical and pharmacokinetic literature outcomes stated that co-administration of tigecycline with food led to an improvement in the gastrointestinal adverse events; however it did not change the drug’s pharmacokinetics [152].
In pancreatitis, the data from all phase 3 and 4 clinical trials found no significant difference in the incidence of pancreatitis between patients treated with tigecycline and patients treated with comparators [153]. On the other hand, a significantly higher rate of pancreatitis of 20% (cases = 10) was observed in a French study [154]. The exact mechanism of tigecycline-induced pancreatitis is unclear; however, some suggested mechanisms are hypertriglyceridemia and toxic metabolite formation that might be involved in the development of tigecycline-induced pancreatitis [153–155].
Several studies also reported tigecycline-induced coagulopathy [156, 157]. The impact of a recommended dose of tigecycline, 50 mg q12h and/or a higher dose of 100 mg q12h, on coagulation parameters in 50 patients with severe infection was evaluated in a Chinese retrospective analysis [158]. A considerable decrease in the levels of plasma fibrinogen (p < 0.001) and a significant increase in the mean values of prothrombin time (PT) and activated partial thromboplastin time (aPTT) (p ≤ 0.002) were observed. In another study, non-anion gap acute metabolic acidosis (NAGAMA), developed through mitochondrial toxicity, was observed after an unusually high dose of 100 mg, twice daily following a single 200 mg loading dose of tigecycline administration; however, the mechanism of NAGAMA is unclear [34]. The routine monitoring of pancreatitis, NAGAMA, and coagulation parameters may be a necessity when administering tigecycline to critically ill patients.
Interaction
The coadministration of tigecycline and warfarin (25 mg single dose) to healthy volunteers resulted in a 40 and 23% decrease in the clearance of R-warfarin and S-warfarin and their AUC, from time zero extrapolated to infinity, was increased by 68 and 29%, respectively [159]. The prothrombin time, or any other suitable anticoagulation test, should be used if tigecycline is administered with warfarin.
The prevalence of tigecycline resistance by continent
A summary of tigecycline resistance studies according to the individual countries worldwide are shown in Table 2 and Table 3.
Table 2.
The prevalence of tigecycline resistance by continents and pathogens.
| Asian Countries | Pathogen | Area | No. (%) of Resistant rate | Type of study | First author, year |
| Klebsiella pneumoniae | Saudi Arabia | 1 | Case report | Al-Qadheeb et al., 2010 [160] | |
| Acinetobacter spp. | India | 224/32 (14.2) | Original research | Taneja et al., 2011 [161] | |
| Acinetobacter spp. | Kuwait | 250/34 (13.6) | Original research | Al-Sweih et al., 2011 [162] | |
| E. coli | India | 166/0 (0) | Original research | Manoharan et al., 2010 [163] | |
| Acinetobacter spp. | 50/6 (12) | ||||
| Pseudomonas aeruginosa | 50/47 (94) | ||||
| S. aureus | 125/0 (0) | ||||
| S. pneumoniae | 102/0 (0) | ||||
| Enterococcus spp. | 100/0 (0) | ||||
| Enterobacteriaceae | Taiwan | 412/10 (2.4) | Original research | Hsu et al., 2011 [164] | |
| Stenotrophomonas maltophilia | Taiwan | 377/66 (17.5) | Original research | Wu et al., 2012 [165] | |
| Stenotrophomonas maltophilia | China | 442/71 (16.1) | Original research | Zhang et al., 2012 [166] | |
| Enterobacteriaceae (MBL-producing) | Taiwan | 95/36 (37.9) (resistant or intermediately susceptible) | Original research | Liao et al., 2011 [167] | |
| Enterobacteriaceae (NDM-1-producing) | Pakistan | 64/7 (11) | Original research | Perry et al., 2011 [168] | |
| E. coli | Lebanon | 150/0 (0) | Original research | Araj and Ibrahim, 2008 [169] | |
| K. pneumoniae | 100/3 (3) | ||||
| Acinetobacter spp. | 64/0 (0) | ||||
| Acinetobacter baumannii | Taiwan | 393/27 (6.9) (resistant or intermediately susceptible) | Original research | Liu et al., 2008 [170]. | |
| Acinetobacter baumannii (MDR) | India | 26/15 (57.7) | Original research | Behera et al., 2009 [171] | |
| Acinetobacter baumannii (imipenem-non-susceptible) | Taiwan | 114/21 (18) | Original research | Lee et al., 2009 [172] | |
| Acinetobacter baumannii (MDR) | Thailand | 148/4 (2.7) (resistant or intermediately susceptible) | Original research | Tiengrim et al., 2006 [173] | |
| Acinetobacter baumannii (MDR) | Israel | 82/54 (66) | Original research | Navon-Venezia et al., 2007 [174] | |
| Acinetobacter baumannii (MDR) | Taiwan | 134/61 (45.5) | Original research | Chang et al., 2012 [175] | |
| Colistin-resistant Acinetobacter spp. | South Korea | 145/14 (9.7) (non-susceptible) | Original research | Park et al., 2009 [176] | |
| OXA carbapenemase-producing Acinetobacter baumannii | South Korea | 47/11 (23.4) | Original research | Kim et al., 2010 [177] | |
| Acinetobacter baumannii (MDR) | Turkey | 82/21 (25.8) | Original research | Dizbay et al., 2008 [178] | |
| S. aureus | India | 127/68 (53.5) | Original research | Swati Sharma.,2017 [179] | |
| Acinetobacter baumannii | Taiwan | 393/27 (6.9) | Original research | Liao CH.,2008[180] | |
| Stenotrophomonas maltophilia | China | 450/61 (13.56) | Original research | Jin Zhao.,2018[181] | |
| Carbapenemase-producing Klebsiella pneumoniae | Saudi Arabia | 1 case | Case report | Nada S. Al-Qadheeb.,2010[160] | |
| Carbapenem-resistant Klebsiella pneumoniae | Taiwan | 16/16 (100) | Original research | Sheng-Kang Chiu., 2017[182] | |
| Enterobacter spp. | Asia | 516/4 (0.8) | Original research | Harald Seifert., 2018[183] | |
| Serratia marcescens | 204/1 (0.5) | ||||
| E. coli | 314/1 (0.3) | ||||
| K. pneumoniae | 541/7 (1.3) | ||||
| European Countries | Bacteroides fragilis | Europe | 824/14 (1.7) | Original research | Nagy et al., 2011 [184] |
| Acinetobacter baumannii | Spain | 142/17 (12) | Original research | Insa et al., 2007 [185] | |
| S. maltophilia | 120/2 (2) | ||||
| E. coli | Spain | 220/0 (0) | Original research | Tubau et al., 2010 [186] | |
| K. pneumoniae | 28/0 (0) | ||||
| K. oxytoca | 14/0 (0) | ||||
| Enterococcus faecalis | 53/1 (1.9) | ||||
| Enterococcus faecium | 39/0 (0) | ||||
| Enterobacter cloacae | 23/1 (4.3) | ||||
| M. morganii | 14/0 (0) | ||||
| P. mirabilis | 12/4 (33.3) | ||||
| P. vulgaris | 7/1 (14.3) | ||||
| Citrobacter spp. | 9/0 (0) | ||||
| S. aureus | 18/0 (0) | ||||
| viridans group streptococcus | 23/1 (4.3) | ||||
| E. coli (ESBL-producing) | Italy | 430/7 (1.6) | Original research | Grandesso et al., 2010 [187] | |
| Klebsiella spp. | Poland | 108/7 (7.5) | Original research | Sekowska and Gospodarek, 2010 [188] | |
| KPC-producing Klebsiella pneumoniae | Spain | 215/24 (11.2) | Original research | Vázquez et al., 2008 [73] | |
| ESBL producing E. coli | Belgium |
Nonsusceptibility rates 26/9 (35) |
Original research | Naesens et al., 2009 [189] | |
| ESBL-producing Klebsiella spp. | 10/10 (100) | ||||
| Enterobacter spp. | 27/26 (96) | ||||
| Enterobacteriaceae | France | 1070/52 (4.9) | Original research | Froment Gomis P et al., [190] | |
| Acinetobacter baumannii | 47/25 (53) | ||||
| Bacteroides fragilis | 645/102 (15.8) | ||||
| MDR-producing Enterobacteriaceae | Greece | 152/12 (7.9) (Intermediate) | Original research | Falagas ME et al., [191] | |
| Enterobacteriaceae spp. (carbapenem-resistant) | Europe | 280/32 (11.4) | Original research | Sader HS et al., [192] | |
| Enterobacteriaceae (imipenem resistant) | Greece | 110/1 (1) | Original research | Papaparaskevas J et al., [193] | |
| Enterococcus spp. (vancomycin resistant) | 151/0 (0) | ||||
| Methicillin-resistant S. aureus | 338/3 (<1) | ||||
| ESBL-positive E. coli | Eastern Europe | 337/5 (1.5) | Original research | Balode A et al., [194] | |
| Vancomycin-resistant Enterococci | France | 18/0 (0) | Original research | Cattoir V et al., [195] | |
| Methicillin-resistant S. aureus | 631/0 (0) | ||||
| ESBL-positive E. coli | 275/3 (1.1) | ||||
| ESBL-positive K. pneumoniae | 274/60 (21.9) | ||||
| Enterobacter hormaechei | France | 1 case | Case report | Daurel et al., 2009 [196] | |
| Enterococcus faecalis | Germany | 1 case | Case report | Werner et al., 2008 [197] | |
| African Countries | carbapenem resistant A. baumannii complex | South Africa | 232/17 (7.6) | Original research | Nahid H Ahmed et al., 2012 [198] |
| Acinetobacter baumannii | South Africa |
(Non-susceptible) 705/53 (7.5) |
Original research | Olga Perovic et al., 2015 to 2016 [199, 200] | |
| E. coli | Africa | 199/0 (0) | Original research | Harald Seifert et al., 2018[183] | |
| Klebsiella pneumoniae | 185/0 (0) | ||||
| Enterobacter spp. | 188/2 (1.1) | ||||
| Serratia marcescens | 79/1 (1.3) | ||||
| carbapenem resistant A. baumannii complex | South Africa | 232/17 (7.6) | Original research | Ahmed et al., 2010 [198] | |
| American Countries | Acinetobacter baumannii | USA | 1 | Case series |
Anthony et al., 2008 [148] |
| Bacteroides fragilis | USA | 1 | Case report |
Sherwood et al., 2011 [201] |
|
| E. coli | USA | 131/0 (0) | Original research | DiPersio and Dowzicky, 2007 [202] | |
| Klebsiella pneumoniae | 174/16 (9.2) | ||||
| E. aerogenes | 24/5 (20.8) | ||||
| E. cloacae | 126/32 (25.4) | ||||
| S. marcescens | 20/4 20 | ||||
| Bacteroides fragilis | USA | 1 | Case report |
Sherwood et al., 2011 [201] |
|
| E. faecium | Latin America | 106/0 (0) | Original research | Rossi F et al., [203] | |
| Enterobacter spp. | 766/2 (0.3) | ||||
| K. pneumoniae | 763/10 (1.3) | ||||
| E. coli | 932/0 (0) | ||||
| S. marcescens | 328/2 (0.6) | ||||
| E. coli | USA | 6643/0 (0) | Original research | Denys GA et al., [204] | |
| K. pneumoniae | 4951/208 (4.2) | ||||
| Klebsiella oxytoca | 1170/13 (1.1) | ||||
| Serratia marcescens | 2421/99 (4.1) | ||||
| Enterobacter spp. | 6065/285 (4.7) | ||||
| ESBL-E. coli | Latin America | 870/0 (0) | Original research | Fernández-Canigia L et al., [205] | |
| ESBL-K. pneumoniae | 1045/15 (1.4) | ||||
| K. oxytoca | 311/0 (0) | ||||
| Enterobacter spp. | 2804/14 (0.5) | ||||
| S. marcescens | 1126/9 (0.8) | ||||
| ESBL-producing K. pneumoniae | USA | 337/7 (2) | Original research | Dowzicky MJ et al., [206] | |
| K. oxytoca | 801/2 (0.2) | ||||
| E. coli | 4861/0 (0) | ||||
| E. aerogenes | 1095/11 (0.01) | ||||
| E. cloacae | 2866/56 (0.02) | ||||
| S. marcescens | 1698/11 (<0.01) | ||||
| S. aureus | Mexico | 250/23 (9) | Original research | Garza-González et al., 2010 [207] | |
| Klebsiella pneumoniae | 150/5 (3) | ||||
| E. coli | 150/6 (4) | ||||
| A. baumannii | 550/6 (1) | ||||
| Enterobacter cloacae | 100/7 (7) | ||||
| Serratia | 100/0 (0) | ||||
| E. coli | Canada | 3789/4 (0.1) | Original research | Lagacé-Wiens et al., 2011 [208] | |
| CTX-M-producing Enterobacteriaceae | USA | 67/0 (0) | Original research | Castanheira et al., 2010 [209] |
ESBL, extended-spectrum b-lactamase; MDR, multidrug-resistant. MBL, Metallo-β-lactamase. NDM; New Delhi Metallo-beta lactamase.
Table 3.
Worldwide reports of tigecycline resistance in gram negative and positive-bacteria.
| First author, year | Type of study | Area | Pathogen | Resistant rate (%) |
|---|---|---|---|---|
| Anna Giammanco et al., 2014[210] | Original research | Worldwide | E. coli | 0.2 |
| Klebsiella spp. | 6 | |||
| Enterobacter aerogenes | 12 | |||
| Klebsiella oxytoca | 5.9 | |||
| K. pneumoniae | 5.7 | |||
| Sue C. Kehl et al., 2004 - 2012[211] | Original research | Worldwide | E. coli | < 0.1 |
| K. pneumoniae | 3.5 | |||
| Klebsiella oxytoca | 0.6 | |||
| Enterobacter spp. | 2.6 | |||
| Serratia marcescens | 3.8 | |||
| Mendes et al et al., 2010 [212] | Original research | Worldwide | Acinetobacter spp. | 3 |
| Garrison MW et al., 2009 [213] | Original research | Worldwide | E. cloacae | 1.5 |
| E. coli | 0.01 | |||
| K. oxytoca | 0.2 | |||
| K. pneumoniae | 1.1 | |||
| S. marcescens | 0.6 | |||
| Hoban DJ et al., 2015 [214] | Original research | Worldwide | Enterobacter spp | 1.1 |
| E. coli | < 0.1 | |||
| Klebsiella oxytoca | 0.2 | |||
| Klebsiella pneumoniae | 0.8 | |||
| Serratia marcescens | 0.7 | |||
| Sader HS et al., 2013 [215] | Original research | Worldwide | S. aureus | 0 |
| Enterococcus spp. | 0.2 | |||
| Streptococcus pneumoniae | 0.2 | |||
| E. coli | 0 | |||
| Klebsiella spp. | 1.4 | |||
| Bertrand X et al., 2012 [216] | Original research | Worldwide | Klebsiella pneumoniae | 5.1 |
| Enterobacter cloacae | 4.3 | |||
| E. coli | < 0.1 | |||
| Serratia marcescens | 4.5 | |||
| Harald Seifert et al., 2018 [183] | Original research | Worldwide | E. coli | < 0.1 |
| Klebsiella pneumoniae | 0.6 | |||
| Enterobacter spp. | 0.8 | |||
| Serratia marcescens | 0.4 |
Asia
In Asia, the occurrence of tigecycline resistance was reported in different bacterial species ranging from 0.% to 66% with a different distribution within the individual Asian countries (Table 2). The most frequently reported species, regarding tigecycline resistance, was A. baumannii [174] with a high resistance rate of 66% revealed in Israel [150].
In Enterobacteriaceae, a tigecycline resistance of 11% was reported for NDM-1-positive isolates from Pakistan and, a resistance of 37.9% was reported for tigecycline non-susceptible Metallobeta-lactamases producing isolates from Taiwan [167]; the prevalence of tigecycline-resistant K. pneumoniae was found to be 1.3% [183]. The reports of tigecycline-resistant K. pneumoniae came from Saudi Arabia [160, 169, 173], Taiwan[144], and Lebanon[169]; further tigecycline resistance was reported for Escherichia coli, Enterobacter cloacae, and S. marcescens [194, 217].
In other gram-negatives, tigecycline resistance was reported in S. maltophilia from Taiwan and China [165, 166, 181] and in 90% of Pseudomonas aeruginosa isolates from India [163].
For gram-positive pathogens, a tigecycline resistance rate of 3% in MRSA isolates [49, 218] was reported from India by Veeraraghavan et al. and in the study of Sharma et al.; 53.5% (n = 68) of S. aureus isolates showed non-susceptibility to tigecycline [179]. In recent years, the trend of increasing minimal inhibitory concentrations to tigecycline and linezolid was observed in Taiwan; however, strains with resistance to these agents were rare [219]. Interestingly, a 2% tigecycline resistance rate was reported in S. pneumoniae isolates gathered between 2004 and 2010 from the Asia-Pacific region, while in 2015, all S. pneumoniae isolates investigated were susceptible to tigecycline [220].
Europe
Tigecycline resistance is frequently studied in Enterobacteriaceae in Europe (Table 2). In ESBL producing Enterobacteriaceae, tigecycline resistance was reported in Italy, Belgium, Turkey, and France [187, 194, 195, 207]. Sader et al. reported that 11.4% of European carbapenem-resistant Enterobacteriaceae are not susceptible to tigecycline [192]. In France, cephalosporin-resistant Enterobacteriaceae were shown to be not susceptible to tigecycline in 23.8% of isolates [190].
For other gram-negative pathogens, resistance to tigecycline was reported in Acinetobacter baumannii [185, 221–224], as well as S. marcescens [211] and H. influenzae [211]. In gram-positive pathogens, tigecycline resistance was reported in two and three MRSA isolates from the Netherlands [225]. In Spain, tigecycline resistance was identified in E. faecium, E. faecalis and viridans streptococci [186] and in Germany, in E. faecalis [197]. In anaerobes, tigecycline resistance was investigated in the B. fragilis group in a Europe-wide study involving 13 countries, and a resistance rate of 1.7% was detected [226].
America
In the USA, high resistance rates to tigecycline were reported in K. pneumoniae (9.2%), E. aerogenes (20.8%), K. oxytoca (38.5%), E. cloacae (25.4%), and S. marcescens (20.0%) [202]. Sporadic cases were detected in A. baumannii [148, 150, 227–229] and B. fragilis [201]. ESBL-producing Enterobacteriaceae were shown to be tigecycline-resistant in the USA and Latin America [206]. In gram-positive pathogens, tigecycline resistance was reported in 9% of S. aureus in Mexico [207].
Africa
The tigecycline resistance rates in isolates collected between 2004–2016 in Africa were 5.8% ( 37/642) lower than in Europe (37.4%; 240/642) and North America (36.8%; 236/642) [49]. In the study of Seifert et al., 1.1% of Enterobacter spp. and 1.3% of S. marcescens isolates were tigecycline-resistant [183]. In the South of the continent, resistance to tigecycline was reported in A. baumannii, K. pneumoniae, Enterobacter spp., C. freundii, P. aeruginosa, and S. marcescens [198, 230–234].
Conclusion
Tigecycline is a unique glycylcycline class of semisynthetic agents designed to overcome the main tetracycline resistance mechanisms. Although tigecycline was approved for cSSTI, cIAI, and CAP in adults, its therapeutic potential is undoubtedly wider. Its antimicrobial activity against anaerobes and its greater penetration into tissues is advantageous for the treatment of inflammatory lesions and granulomas. Recently available clinical data support the use of tigecycline in severe C. difficile infections. In vitro antimicrobial susceptibility testing showed the susceptibility of a number of pathogens to tigecycline including those MDR pathogens associated with healthcare infections. However, the bacteriostatic activity of tigecycline is probably associated with a higher mortality risk in patients with sepsis or severe infection.
Acknowledgments
We thank the Dr. Abazar Pournajaf for scientific reviewing and kind support.
Availability of data and material
All the data in this review are included in the manuscript.
Abbreviations
- MDR
Multidrug-Resistant
- XDR
Extensively Drug-Resistant
- FDA
Food and Drugs Administration
- cSSTI
Complicated Skin and Skin Structures Infections
- cIAI
Complicated Intra-Abdominal Infections
- CAP
Community-Acquired Bacterial Pneumonia
- PK/PD
Pharmacokinetic-Pharmacodynamic
- AUC: MIC
Concentration-Time Curve and Minimal Inhibitory Concentration
- PAE
Post-Antibiotic Effect
- CLSI
Clinical and Laboratory Standards Institute
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- BSAC
British Society for Antimicrobial Chemotherapy
- MRSA
Methicillin-Resistant Staphylococcus aureus
- VRE
Vancomycin-Resistant enterococci
- ESBL
Extended-Spectrum β-lactamase
- CDI
Clostridium difficile infection
- RND
Resistance-Nodulation Division
- SAM
S-adenosyl-L-methionine
- CRKP
Carbapenem-Resistant K. pneumoniae
- MATE
Multi-Antimicrobial Extrusion Protein
- MFS
Major Facilitator Superfamily
- FAERS
FDA Adverse Event Reporting System
- RCTs
Randomized Controlled Trials
- GI
Gastrointestinal
- aPTT
Activated Partial Thromboplastin Time
- NAGAMA
Non-Anion Gap Acute Metabolic Acidosis
Authors’ contribution
Sajad Yaghoubi, Angelina Olegovna Zekiy, Marcela Krutova, Mehrdad Gholami, Mohammad Sholeh, and Zahra Ghafouri contributed to the conception, design, and drafting of the work. Angelina Olegovna Zekiy, Farajolah Maleki, and Ebrahim Kouhsari contributed in revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no competing interests.
Ethical approval
Not applicable in this section.
Informed consent
Not applicable in this section.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ebrahim Kouhsari, Email: Ekouhsari1987@gmail.com.
Farajolah Maleki, Email: fmaleki88@yahoo.com.
References
- 1.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharmacy and therapeutics. 2015;40(4):277. [PMC free article] [PubMed] [Google Scholar]
- 2.Tasina E, Haidich A-B, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 3.Guay DR. Oritavancin and tigecycline: investigational antimicrobials for multidrug-resistant bacteria. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2004;24(1):58–68. doi: 10.1592/phco.24.1.58.34808. [DOI] [PubMed] [Google Scholar]
- 4.Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–336. doi: 10.1016/j.diagmicrobio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.FDA U FDA drug safety communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections (1 September 2010).
- 6.Food Administration D (2017) FDA drug safety communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. US Food and Drug Administration, Silver Spring, MD
- 7.Lauf L, Ozsvár Z, Mitha I, Regöly-Mérei J, Embil JM, Cooper A, Sabol MB, Castaing N, Dartois N, Yan JJDM, disease i (2014) Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. 78(4):469–480 [DOI] [PubMed]
- 8.Kechagias KS, Chorepsima S, Triarides NA, Falagas ME (2020) Tigecycline for the treatment of patients with Clostridium difficile infection: an update of the clinical evidence. Eur J Clin Microbiol Infect Dis:1–6 [DOI] [PubMed]
- 9.Marot J-C, Jonckheere S, Munyentwali H, Belkhir L, Vandercam B, Yombi JC. Tigecycline-induced acute pancreatitis: about two cases and review of the literature. Acta Clin Belg. 2012;67(3):229–232. doi: 10.2143/ACB.67.3.2062663. [DOI] [PubMed] [Google Scholar]
- 10.Finch RG, Greenwood D, Whitley RJ, Norrby SR. Antibiotic and chemotherapy e-book. 2010. [Google Scholar]
- 11.Mukker JK, Singh RP, Derendorf HJJOPS (2014) Determination of atypical nonlinear plasma–protein-binding behavior of tigecycline using an in vitro microdialysis technique. 103 (3):1013-1019 [DOI] [PubMed]
- 12.Bennett JE, Dolin R, Blaser MJ (2014) Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases: 2-Volume Set. Elsevier Health Sciences
- 13.DE ROSA FG, Corcione S, Di Perri G, Scaglione F (2015) Re-defining tigecycline therapy. [PubMed]
- 14.Cai Y, Bai N, Liu X, Liang B, Wang J, Wang R. Tigecycline: Alone or in combination? Infectious Diseases. 2016;48(7):491–502. doi: 10.3109/23744235.2016.1155735. [DOI] [PubMed] [Google Scholar]
- 15.Stein GE, Smith CL, Missavage A, Saunders JP, Nicolau DP, Battjes SM, Kepros JP. Tigecycline penetration into skin and soft tissue. Surg Infect. 2011;12(6):465–467. doi: 10.1089/sur.2011.022. [DOI] [PubMed] [Google Scholar]
- 16.Tombs N (1999) Tissue distribution of GAR-936, a broad spectrum antibiotic in male rats. Program and abstracts of the Thirty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, pp 26-29
- 17.Postier RG, Green SL, Klein SR, Ellis-Grosse E, Loh E, Group TS Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin Ther. 2004;26(5):704–714. doi: 10.1016/S0149-2918(04)90070-7. [DOI] [PubMed] [Google Scholar]
- 18.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58(6):1221–1229. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya I, Gotfried MH, Ji AJ, Saunders JP, Gourley I, Diehl A, Korth-Bradley JM. Reassessment of tigecycline bone concentrations in volunteers undergoing elective orthopedic procedures. J Clin Pharmacol. 2014;54(1):70–74. doi: 10.1002/jcph.201. [DOI] [PubMed] [Google Scholar]
- 20.Meagher A, Passarell J, Cirincione B, Van Wart S, Liolios K, Babinchak T, Ellis-Grosse E, Ambrose P. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother. 2007;51(6):1939–1945. doi: 10.1128/AAC.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passarell J, Meagher A, Liolios K, Cirincione B, Van Wart S, Babinchak T, Ellis-Grosse E, Ambrose P. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2008;52(1):204–210. doi: 10.1128/AAC.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend ML, Pound MW, Drew RH. Tigecycline in the treatment of complicated intra-abdominal and complicated skin and skin structure infections. Ther Clin Risk Manag. 2007;3(6):1059. [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen PJ, Bradford PA, Weiss WJ, Murphy TM, Sum P, Projan SJ. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob Agents Chemother. 2002;46(8):2595–2601. doi: 10.1128/AAC.46.8.2595-2601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Ogtrop M, Andes D, Stamstad T, Conklin B, Weiss W, Craig W, Vesga OJAA, chemotherapy (2000) In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria 44 (4):943-949 [DOI] [PMC free article] [PubMed]
- 25.Rello J (2005) Pharmacokinetics, pharmacodynamics, safety and tolerability of tigecycline. Journal of chemotherapy 17 (sup1):12-22 [DOI] [PubMed]
- 26.Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2005;49(1):220–229. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaewpoowat Q, Ostrosky-Zeichner L. Tigecycline: a critical safety review. Expert Opin Drug Saf. 2015;14(2):335–342. doi: 10.1517/14740338.2015.997206. [DOI] [PubMed] [Google Scholar]
- 28.Korth-Bradley JM, Baird-Bellaire SJ, Patat AA, Troy SM, Böhmer GM, Gleiter CH, Buecheler R, Morgan MY. Pharmacokinetics and safety of a single intravenous dose of the antibiotic tigecycline in patients with cirrhosis. J Clin Pharmacol. 2011;51(1):93–101. doi: 10.1177/0091270010363477. [DOI] [PubMed] [Google Scholar]
- 29.Greer ND (2006) Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics. Baylor University Medical Center Proceedings. Taylor & Francis, pp 155-161 [DOI] [PMC free article] [PubMed]
- 30.CLSI C (2019) Performance standards for antimicrobial susceptibility testing. Clinical Lab Standards Institute
- 31.Testing ECOAS. European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. Sweden: Växjö; 2019. [Google Scholar]
- 32.Wyeth P. Tygacil (tigecycline) for injection. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2010. [Google Scholar]
- 33.Nathwani D (2018) British Society for Antimicrobial Chemotherapy. Antimicrobial stewardship: from principles to practice
- 34.Hawkey P, Finch R. Tigecycline: in-vitro performance as a predictor of clinical efficacy. Clin Microbiol Infect. 2007;13(4):354–362. doi: 10.1111/j.1469-0691.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 35.Seiffert SN, Marschall J, Perreten V, Carattoli A, Furrer H, Endimiani A. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int J Antimicrob Agents. 2014;44(3):260–262. doi: 10.1016/j.ijantimicag.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo L, Hopkins KL, Gutierrez B, Ovejero CM, Shukla S, Douthwaite S, Prasad KN, Woodford N, Gonzalez-Zorn B. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J Antimicrob Chemother. 2013;68(7):1543–1550. doi: 10.1093/jac/dkt078. [DOI] [PubMed] [Google Scholar]
- 38.Du X, He F, Shi Q, Zhao F, Xu J, Fu Y, Yu Y. The Rapid Emergence of Tigecycline Resistance in blaKPC–2 Harboring Klebsiella pneumoniae, as Mediated in Vivo by Mutation in tetA During Tigecycline Treatment. Front Microbiol. 2018;9:648. doi: 10.3389/fmicb.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhashem F, Tiren-Verbeet NL, Alp E, Doganay M. Treatment of sepsis: What is the antibiotic choice in bacteremia due to carbapenem resistant Enterobacteriaceae? World J Clin Cases. 2017;5(8):324. doi: 10.12998/wjcc.v5.i8.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iovleva A, Doi Y. Carbapenem-resistant enterobacteriaceae. Clin Lab Med. 2017;37(2):303–315. doi: 10.1016/j.cll.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horiyama T, Nikaido E, Yamaguchi A, Nishino K. Roles of Salmonella multidrug efflux pumps in tigecycline resistance. J Antimicrob Chemother. 2010;66(1):105–110. doi: 10.1093/jac/dkq421. [DOI] [PubMed] [Google Scholar]
- 42.Gales AC, Jones RN, Andrade SS, Pereira AS, Sader HS. In vitro activity of tigecycline, a new glycylcycline, tested against 1,326 clinical bacterial strains isolated from Latin America. Braz J Infect Dis. 2005;9(5):348–356. doi: 10.1590/S1413-86702005000500001. [DOI] [PubMed] [Google Scholar]
- 43.Y-y Z, Zhou L, Zhu D-m, Wu P-c, Hu F-p, Wu W-h, Wang F. In vitro activities of tigecycline against clinical isolates from Shanghai, China. Diagn Microbiol Infect Dis. 2004;50(4):267–281. doi: 10.1016/j.diagmicrobio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Kim H, Seo YH, Yong D, Jeong SH, Lee K, Chong Y. In vitro activity of tigecycline alone and antimicrobial combinations against clinical Neisseria gonorrhoeae isolates. Diagn Microbiol Infect Dis. 2017;87(2):160–162. doi: 10.1016/j.diagmicrobio.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Blanton LS, Wilson NM, Quade BR, Walker DH. Susceptibility of Rickettsia rickettsii to Tigecycline in a Cell Culture Assay and Animal Model for Rocky Mountain Spotted Fever. The American Journal of Tropical Medicine and Hygiene. 2019;101(5):1091–1095. doi: 10.4269/ajtmh.19-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spyridaki I, Psaroulaki A, Vranakis I, Tselentis Y, Gikas A. Bacteriostatic and bactericidal activities of tigecycline against Coxiella burnetii and comparison with those of six other antibiotics. Antimicrob Agents Chemother. 2009;53(6):2690–2692. doi: 10.1128/AAC.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S-M, Kwon H-Y, Im J-H, Baek JH, Hwang S-S, Kang J-S, Chung M-H, Lee J-S. In vitro activity of tigecycline against Orientia tsutsugamushi. Yonsei Med J. 2016;57(4):1034–1037. doi: 10.3349/ymj.2016.57.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman J, Vernon J, Pilling S, Morris K, Nicolson S, Shearman S, Clark E, Palacios-Fabrega JA, Wilcox MJEJoCM, Diseases I (2020) Five-year Pan-European, longitudinal surveillance of Clostridium difficile ribotype prevalence and antimicrobial resistance: the extended ClosER study 39 (1):169-177 [DOI] [PMC free article] [PubMed]
- 49.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kechagias KS, Chorepsima S, Triarides NA, Falagas MEJEJoCM, Diseases I (2020) Tigecycline for the treatment of patients with Clostridium difficile infection: an update of the clinical evidence.1-6 [DOI] [PubMed]
- 51.Bauer G, Berens C, Projan SJ, Hillen W. Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA. J Antimicrob Chemother. 2004;53(4):592–599. doi: 10.1093/jac/dkh125. [DOI] [PubMed] [Google Scholar]
- 52.Jenner L, Starosta AL, Terry DS, Mikolajka A, Filonava L, Yusupov M, Blanchard SC, Wilson DN, Yusupova G. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc Natl Acad Sci. 2013;110(10):3812–3816. doi: 10.1073/pnas.1216691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson MW, Ruzin A, Feyfant E, Rush TS, O'Connell J, Bradford PA. Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob Agents Chemother. 2006;50(6):2156–2166. doi: 10.1128/AAC.01499-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pournaras S, Koumaki V, Gennimata V, Kouskouni E, Tsakris A (2015) In vitro activity of tigecycline against Acinetobacter baumannii: global epidemiology and resistance mechanisms. Advances in Microbiology, Infectious Diseases and Public Health. Springer, pp 1-14 [DOI] [PubMed]
- 55.Pournaras S, Koumaki V, Spanakis N, Gennimata V, Tsakris A. Current perspectives on tigecycline resistance in Enterobacteriaceae: susceptibility testing issues and mechanisms of resistance. Int J Antimicrob Agents. 2016;48(1):11–18. doi: 10.1016/j.ijantimicag.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Gordon N, Wareham D. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother. 2009;63(4):775–780. doi: 10.1093/jac/dkn555. [DOI] [PubMed] [Google Scholar]
- 57.Linkevicius M, Sandegren L, Andersson DI. Potential of tetracycline resistance proteins to evolve tigecycline resistance. Antimicrob Agents Chemother. 2016;60(2):789–796. doi: 10.1128/AAC.02465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng M, Zhu M-H, Li J-J, Bi S, Sheng Z-K, Hu F-S, Zhang J-J, Chen W, Xue X-W, Sheng J-F. Molecular epidemiology and mechanisms of tigecycline resistance in clinical isolates of Acinetobacter baumannii from a Chinese university hospital. Antimicrob Agents Chemother. 2014;58(1):297–303. doi: 10.1128/AAC.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore IF, Hughes DW, Wright GD. Tigecycline is modified by the flavin-dependent monooxygenase TetX. Biochemistry. 2005;44(35):11829–11835. doi: 10.1021/bi0506066. [DOI] [PubMed] [Google Scholar]
- 60.Leski TA, Bangura U, Jimmy DH, Ansumana R, Lizewski SE, Stenger DA, Taitt CR, Vora GJ. Multidrug-resistant tet (X)-containing hospital isolates in Sierra Leone. Int J Antimicrob Agents. 2013;42(1):83–86. doi: 10.1016/j.ijantimicag.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Hentschke M, Christner M, Sobottka I, Aepfelbacher M, Rohde H. Combined ramR mutation and presence of a Tn1721-associated tet (A) variant in a clinical isolate of Salmonella enterica serovar Hadar resistant to tigecycline. Antimicrob Agents Chemother. 2010;54(3):1319–1322. doi: 10.1128/AAC.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veleba M, De Majumdar S, Hornsey M, Woodford N, Schneiders T. Genetic characterization of tigecycline resistance in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J Antimicrob Chemother. 2013;68(5):1011–1018. doi: 10.1093/jac/dks530. [DOI] [PubMed] [Google Scholar]
- 63.Keeney D, Ruzin A, Bradford PA. RamA, a transcriptional regulator, and AcrAB, an RND-type efflux pump, are associated with decreased susceptibility to tigecycline in Enterobacter cloacae. Microb Drug Resist. 2007;13(1):1–6. doi: 10.1089/mdr.2006.9990. [DOI] [PubMed] [Google Scholar]
- 64.Ni W, Cai X, Liang B, Cai Y, Cui J, Wang R. Effect of proton pump inhibitors on in vitro activity of tigecycline against several common clinical pathogens. PloS one. 2014;9(1):e86715. doi: 10.1371/journal.pone.0086715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montana S, Vilacoba E, Traglia GM, Almuzara M, Pennini M, Fernandez A, Sucari A, Centrón D, Ramirez MS. Genetic variability of AdeRS two-component system associated with tigecycline resistance in XDR-Acinetobacter baumannii isolates. Curr Microbiol. 2015;71(1):76–82. doi: 10.1007/s00284-015-0829-3. [DOI] [PubMed] [Google Scholar]
- 66.Sun J-R, Perng C-L, Lin J-C, Yang Y-S, Chan M-C, Chang T-Y, Lin F-M, Chiueh T-S. AdeRS combination codes differentiate the response to efflux pump inhibitors in tigecycline-resistant isolates of extensively drug-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2014;33(12):2141–2147. doi: 10.1007/s10096-014-2179-7. [DOI] [PubMed] [Google Scholar]
- 67.Yoon E-J, Courvalin P, Grillot-Courvalin C. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother. 2013;57(7):2989–2995. doi: 10.1128/AAC.02556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun J-R, Perng C-L, Chan M-C, Morita Y, Lin J-C, Su C-M, Wang W-Y, Chang T-Y, Chiueh T-S. A truncated AdeS kinase protein generated by ISAba1 insertion correlates with tigecycline resistance in Acinetobacter baumannii. PLoS One. 2012;7(11):e49534. doi: 10.1371/journal.pone.0049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin M-F, Lin Y-Y, Yeh H-W, Lan C-Y. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 2014;14(1):119. doi: 10.1186/1471-2180-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Q, Li X, Zhou H, Jiang Y, Chen Y, Hua X, Yu Y. Decreased susceptibility to tigecycline in Acinetobacter baumannii mediated by a mutation in trm encoding SAM-dependent methyltransferase. J Antimicrob Chemother. 2013;69(1):72–76. doi: 10.1093/jac/dkt319. [DOI] [PubMed] [Google Scholar]
- 71.Rumbo C, Gato E, López M, de Alegría CR, Fernández-Cuenca F, Martínez-Martínez L, Vila J, Pachón J, Cisneros JM, Rodríguez-Baño J. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57(11):5247–5257. doi: 10.1128/AAC.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruzin A, Keeney D, Bradford PA. AcrAB efflux pump plays a role in decreased susceptibility to tigecycline in Morganella morganii. Antimicrob Agents Chemother. 2005;49(2):791–793. doi: 10.1128/AAC.49.2.791-793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He F, Fu Y, Chen Q, Ruan Z, Hua X, Zhou H, Yu Y. Tigecycline susceptibility and the role of efflux pumps in tigecycline resistance in KPC-producing Klebsiella pneumoniae. PLoS One. 2015;10(3):e0119064. doi: 10.1371/journal.pone.0119064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pérez A, Poza M, Aranda J, Latasa C, Medrano FJ, Tomás M, Romero A, Lasa I, Bou G. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56(12):6256–6266. doi: 10.1128/AAC.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Chen H, Zhang Y, Wang Q, Zhao C, Li H, He W, Zhang F, Wang Z, Li S. Genetic characterisation of clinical Klebsiella pneumoniae isolates with reduced susceptibility to tigecycline: Role of the global regulator RamA and its local repressor RamR. Int J Antimicrob Agents. 2015;45(6):635–640. doi: 10.1016/j.ijantimicag.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 76.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villa L, Feudi C, Fortini D, García-Fernández A, Carattoli A. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob Agents Chemother. 2014;58(3):1707–1712. doi: 10.1128/AAC.01803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirata T, Saito A, Nishino K, Tamura N, Yamaguchi A. Effects of efflux transporter genes on susceptibility of Escherichia coli to tigecycline (GAR-936) Antimicrob Agents Chemother. 2004;48(6):2179–2184. doi: 10.1128/AAC.48.6.2179-2184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elkins CA, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominately by two large periplasmic loops. J Bacteriol. 2002;184(23):6490–6498. doi: 10.1128/JB.184.23.6490-6499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chollet R, Chevalier J, Bollet C, Pages J-M, Davin-Regli A. RamA is an alternate activator of the multidrug resistance cascade in Enterobacter aerogenes. Antimicrob Agents Chemother. 2004;48(7):2518–2523. doi: 10.1128/AAC.48.7.2518-2523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alekshun MN, Levy SB. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41(10):2067. doi: 10.1128/AAC.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbosa TM, Levy SB. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol. 2000;182(12):3467–3474. doi: 10.1128/JB.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keeney D, Ruzin A, McAleese F, Murphy E, Bradford PA. MarA-mediated overexpression of the AcrAB efflux pump results in decreased susceptibility to tigecycline in Escherichia coli. J Antimicrob Chemother. 2007;61(1):46–53. doi: 10.1093/jac/dkm397. [DOI] [PubMed] [Google Scholar]
- 84.Linkevicius M, Sandegren L, Andersson DI. Mechanisms and fitness costs of tigecycline resistance in Escherichia coli. J Antimicrob Chemother. 2013;68(12):2809–2819. doi: 10.1093/jac/dkt263. [DOI] [PubMed] [Google Scholar]
- 85.Pérez A, Poza M, Fernández A, del Carmen FM, Mallo S, Merino M, Rumbo-Feal S, Cabral MP, Bou G. Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56(4):2084–2090. doi: 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruzin A, Immermann FW, Bradford PA. Real-time PCR and statistical analyses of acrAB and ramA expression in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2008;52(9):3430–3432. doi: 10.1128/AAC.00591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruzin A, Visalli MA, Keeney D, Bradford PA. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2005;49(3):1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong X, Xu H, Chen D, Zhou H, Hu X, Cheng G. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One. 2014;9(12):e115185. doi: 10.1371/journal.pone.0115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roy S, Datta S, Viswanathan R, Singh AK, Basu S. Tigecycline susceptibility in Klebsiella pneumoniae and Escherichia coli causing neonatal septicaemia (2007–10) and role of an efflux pump in tigecycline non-susceptibility. J Antimicrob Chemother. 2013;68(5):1036–1042. doi: 10.1093/jac/dks535. [DOI] [PubMed] [Google Scholar]
- 90.Rosenblum R, Khan E, Gonzalez G, Hasan R, Schneiders T. Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. Int J Antimicrob Agents. 2011;38(1):39–45. doi: 10.1016/j.ijantimicag.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Majumdar S, Veleba M, Finn S, Fanning S, Schneiders T. Elucidating the regulon of multidrug resistance regulator RarA in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(4):1603–1609. doi: 10.1128/AAC.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheng Z-K, Hu F, Wang W, Guo Q, Chen Z, Xu X, Zhu D, Wang M. Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother. 2014;58(11):6982–6985. doi: 10.1128/AAC.03808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hentschke M, Wolters M, Sobottka I, Rohde H, Aepfelbacher M. ramR mutations in clinical isolates of Klebsiella pneumoniae with reduced susceptibility to tigecycline. Antimicrob Agents Chemother. 2010;54(6):2720–2723. doi: 10.1128/AAC.00085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nielsen LE, Snesrud EC, Onmus-Leone F, Kwak YI, Avilés R, Steele ED, Sutter DE, Waterman PE, Lesho EP. IS5 element integration, a novel mechanism for rapid in vivo emergence of tigecycline nonsusceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58(10):6151–6156. doi: 10.1128/AAC.03053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lat A, Clock SA, Wu F, Whittier S, Della-Latta P, Fauntleroy K, Jenkins SG, Saiman L, Kubin CJ. Comparison of polymyxin B, tigecycline, cefepime, and meropenem MICs for KPC-producing Klebsiella pneumoniae by broth microdilution, Vitek 2, and Etest. J Clin Microbiol. 2011;49(5):1795–1798. doi: 10.1128/JCM.02534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pharmaceutics W. Tygacil®(package insert) Philadelphia, PA: Wyeth Pharmaceutics; 2009. [Google Scholar]
- 97.Chiu S-K, Huang L-Y, Chen H, Tsai Y-K, Liou C-H, Lin J-C, Siu LK, Chang F-Y, Yeh K-M. Roles of ramR and tet (A) mutations in conferring tigecycline resistance in carbapenem-resistant Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother. 2017;61(8):e00391–e00317. doi: 10.1128/AAC.00391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hornsey M, Ellington MJ, Doumith M, Hudson S, Livermore DM, Woodford N. Tigecycline resistance in Serratia marcescens associated with up-regulation of the SdeXY-HasF efflux system also active against ciprofloxacin and cefpirome. J Antimicrob Chemother. 2010;65(3):479–482. doi: 10.1093/jac/dkp475. [DOI] [PubMed] [Google Scholar]
- 99.Hornsey M, Ellington MJ, Doumith M, Scott G, Livermore DM, Woodford N. Emergence of AcrAB-mediated tigecycline resistance in a clinical isolate of Enterobacter cloacae during ciprofloxacin treatment. Int J Antimicrob Agents. 2010;35(5):478–481. doi: 10.1016/j.ijantimicag.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Zheng B, Li A, Jiang X, Hu X, Yao J, Zhao L, Ji J, Ye M, Xiao Y, Li L. Genome sequencing and genomic characterization of a tigecycline-resistant Klebsiella pneumoniae strain isolated from the bile samples of a cholangiocarcinoma patient. Gut Pathogens. 2014;6(1):40. doi: 10.1186/s13099-014-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abouzeed YM, Baucheron S, Cloeckaert A. ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother. 2008;52(7):2428–2434. doi: 10.1128/AAC.00084-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kehrenberg C, Cloeckaert A, Klein G, Schwarz S. Decreased fluoroquinolone susceptibility in mutants of Salmonella serovars other than Typhimurium: detection of novel mutations involved in modulated expression of ramA and soxS. J Antimicrob Chemother. 2009;64(6):1175–1180. doi: 10.1093/jac/dkp347. [DOI] [PubMed] [Google Scholar]
- 103.Ricci V, Piddock LJ. Ciprofloxacin selects for multidrug resistance in Salmonella enterica serovar Typhimurium mediated by at least two different pathways. J Antimicrob Chemother. 2009;63(5):909–916. doi: 10.1093/jac/dkp054. [DOI] [PubMed] [Google Scholar]
- 104.Akiyama T, Presedo J, Khan AA. The tetA gene decreases tigecycline sensitivity of Salmonella enterica isolates. Int J Antimicrob Agents. 2013;42(2):133–140. doi: 10.1016/j.ijantimicag.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 105.Aires JR, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43(11):2624–2628. doi: 10.1128/AAC.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ko¨ hler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Kocjancic Curty L, Pechère JC (1997) Characterization of MexE–MexF–OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol 23 (2):345-354 [DOI] [PubMed]
- 107.Li X-Z, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39(9):1948–1953. doi: 10.1128/AAC.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43(2):415–417. doi: 10.1128/AAC.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Ji Y, Li XZ, Nishino T. Overexpression of the mexC–mexD–oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21(4):713–725. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 110.Dean CR, Visalli MA, Projan SJ, Sum P-E, Bradford PA. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother. 2003;47(3):972–978. doi: 10.1128/AAC.47.3.972-978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noskin GA (2005) Tigecycline: a new glycylcycline for treatment of serious infections. Clinical infectious diseases 41 (Supplement_5):S303-S314 [DOI] [PubMed]
- 112.Pumbwe L, Piddock LJ. Two efflux systems expressed simultaneously in multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44(10):2861–2864. doi: 10.1128/AAC.44.10.2861-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beinlich KL, Chuanchuen R, Schweizer HP. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol Lett. 2001;198(2):129–134. doi: 10.1111/j.1574-6968.2001.tb10631.x. [DOI] [PubMed] [Google Scholar]
- 114.McAleese F, Petersen P, Ruzin A, Dunman PM, Murphy E, Projan SJ, Bradford PA. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob Agents Chemother. 2005;49(5):1865–1871. doi: 10.1128/AAC.49.5.1865-1871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dönhöfer A, Franckenberg S, Wickles S, Berninghausen O, Beckmann R, Wilson DN. Structural basis for TetM-mediated tetracycline resistance. Proc Natl Acad Sci. 2012;109(42):16900–16905. doi: 10.1073/pnas.1208037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fiedler S, Bender J, Klare I, Halbedel S, Grohmann E, Szewzyk U, Werner G. Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet (L) and tet (M) J Antimicrob Chemother. 2015;71(4):871–881. doi: 10.1093/jac/dkv420. [DOI] [PubMed] [Google Scholar]
- 117.Food U, Administration D (2011) FDA drug safety communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. Drugs
- 118.Eckmann C, Heizmann WR, Leitner E, Von Eiff C, Bodmann K-F. Prospective, non-interventional, multi-centre trial of tigecycline in the treatment of severely ill patients with complicated infections–new insights into clinical results and treatment practice. Chemotherapy. 2011;57(4):275–284. doi: 10.1159/000329406. [DOI] [PubMed] [Google Scholar]
- 119.Kwon SH, Ahn HL, Han OY, La HO. Efficacy and safety profile comparison of colistin and tigecycline on the extensively drug resistant Acinetobacter baumannii. Biol Pharm Bull. 2014;37(3):340–346. doi: 10.1248/bpb.b13-00109. [DOI] [PubMed] [Google Scholar]
- 120.Chuang Y-C, Cheng C-Y, Sheng W-H, Sun H-Y, Wang J-T, Chen Y-C, Chang S-C. Effectiveness of tigecycline-based versus colistin-based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumanniiin a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14(1):102. doi: 10.1186/1471-2334-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Food Administration D (2015) Investigational New Drug Applications (INDs)—Determining Whether Human Research Studies Can Be Conducted Without an IND.
- 122.Bai X-R, Liu J-M, Jiang D-C, Yan S-Y. Efficacy and safety of tigecycline monotherapy versus combination therapy for the treatment of hospital-acquired pneumonia (HAP): a meta-analysis of cohort studies. J Chemother. 2018;30(3):172–178. doi: 10.1080/1120009X.2018.1425279. [DOI] [PubMed] [Google Scholar]
- 123.Wang J, Pan Y, Shen J, Xu Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16(1):24. doi: 10.1186/s12941-017-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Conde-Estévez D, Grau S, Horcajada JP, Luque SJIJOAA (2010) Off-label prescription of tigecycline: clinical and microbiological characteristics and outcomes. 36 (5):471-472 [DOI] [PubMed]
- 125.Curcio D, Fernández F, Vergara J, Vazquez W, Luna CJJOC (2009) Late onset ventilator-associated pneumonia due to multidrug-resistant Acinetobacter spp.: experience with tigecycline. 21 (1):58-62 [DOI] [PubMed]
- 126.Kuo S-C, Wang F-D, Fung C-P, Chen L-Y, Chen S-J, Chiang M-C, Hsu S-F, Liu C-YJJoM, Immunology, Infection (2011) Clinical experience with tigecycline as treatment for serious infections in elderly and critically ill patients. 44 (1):45-51 [DOI] [PubMed]
- 127.Guner R, Hasanoglu I, Keske S, Kalem A, Tasyaran MJI. Outcomes in patients infected with carbapenem-resistant. Acinetobacter baumannii and treated with tigecycline alone or in combination therapy. 2011;39(6):515–518. doi: 10.1007/s15010-011-0161-1. [DOI] [PubMed] [Google Scholar]
- 128.Moghnieh RA, Abdallah DI, Fawaz IA, Hamandi T, Kassem M, El-Rajab N, Jisr T, Mugharbil A, Droubi N, Al Tabah SJFIM (2017) Prescription patterns for tigecycline in severely ill patients for non-FDA approved indications in a developing country: A compromised outcome. 8:497 [DOI] [PMC free article] [PubMed]
- 129.Ipek M. Colistin/tigecycline. Reactions. 2019;1749:143–120. [Google Scholar]
- 130.İpek MS, Gunel ME, Ozbek E. Tigecycline Use in Neonates: 5-Year Experience of a Tertiary Center. J Pediatr Infect Dis. 2019;14(03):103–107. doi: 10.1055/s-0038-1675582. [DOI] [Google Scholar]
- 131.Sharland M, Rodvold KA, Tucker HR, Baillon-Plot N, Tawadrous M, Hickman MA, Raber S, Korth-Bradley JM, Díaz-Ponce H, Wible MJTPIDJ (2019) Safety and efficacy of tigecycline to treat multidrug-resistant infections in pediatrics: an evidence synthesis. 38 (7):710-715 [DOI] [PubMed]
- 132.Chen F, Shen C, Pang X, Zhang Z, Deng Y, Han L, Chen X, Zhang J, Xia Q, Qian YJTID (2020) Effectiveness of tigecycline in the treatment of infections caused by carbapenem-resistant gram-negative bacteria in pediatric liver transplant recipients: A retrospective study. 22 (1):e13199 [DOI] [PubMed]
- 133.Emiroglu M, Alkan G, Dagi HTJP (2017) Tigecycline therapy in an infant for ventriculoperitoneal shunt meningitis. 139 (1):e20160963 [DOI] [PubMed]
- 134.Shen F, Han Q, Xie D, Fang M, Zeng H, Deng Y. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis. 2015;39:25–33. doi: 10.1016/j.ijid.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Insert TP (2005) Philadelphia (PA): Wyeth Pharmaceuticals Inc.(June, 2005). Organism (no tested)/% susceptible/antimicrobial agent MIC50 MIC90 Range resistant a
- 136.Bassetti M, Poulakou G, Giamarellou H (2014) Is there a future for tigecycline? Springer [DOI] [PubMed]
- 137.Song Y, Hu L, Shu Q, Ye J, Liang J, Chen X, Tan LJIJOID (2018) Tigecycline salvage therapy for critically ill children with multidrug-resistant/extensively drug-resistant infections after surgery. 75:82-88 [DOI] [PubMed]
- 138.Edwards BJ, Bunta AD, Lane J, Odvina C, Rao DS, Raisch DW, McKoy JM, Omar I, Belknap SM, Garg V. Bisphosphonates and nonhealing femoral fractures: analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: a systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. J Bone Joint Surg Am. 2013;95(4):297. doi: 10.2106/JBJS.K.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ozturk S, Ustun C, Pehlivan S, Ucak H. Acute generalized exanthematous pustulosis associated with tigecycline. Ann Dermatol. 2014;26(2):246–249. doi: 10.5021/ad.2014.26.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011;55(3):1162–1172. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolids (telithromycin) and glycylcyclines (tigecycline) Med Clin. 2011;95(4):761–791. doi: 10.1016/j.mcna.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 142.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(9):1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 143.Hasan M, Rabbani R, Bachar S, Huq S. High Dose Tigecycline-Induced Mitochondrial Dysfunction-Associated Acute Metabolic Acidosis: A Retrospective Study. J Mol Genet Med. 2019;13(407):1747–0862.1000407. [Google Scholar]
- 144.Lin J, Wang R, Chen J. Tigecycline-induced acute pancreatitis in a renal transplant patient: a case report and literature review. BMC Infect Dis. 2018;18(1):201. doi: 10.1186/s12879-018-3103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rose WE, Rybak MJ. Tigecycline: first of a new class of antimicrobial agents. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2006;26(8):1099–1110. doi: 10.1592/phco.26.8.1099. [DOI] [PubMed] [Google Scholar]
- 146.Brink AJ, Bizos D, Boffard KD, Feldman C, Grolman D, Pretorius J, Richards GA, Senekal M, Steyn E, Welkovic N. Guideline: appropriate use of tigecycline. SAMJ: South Afr Med J. 2010;100(6):388–394. doi: 10.7196/SAMJ.4109. [DOI] [PubMed] [Google Scholar]
- 147.Dryden M (2013) Tigecycline: an antibiotic for the twenty-first century. Journal of Antimicrobial Chemotherapy 68 (suppl_2):ii3-ii4 [DOI] [PubMed]
- 148.Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46(4):567–570. doi: 10.1086/526775. [DOI] [PubMed] [Google Scholar]
- 149.Schafer JJ, Goff DA, Stevenson KB, Mangino JE. Early experience with tigecycline for ventilator-associated pneumonia and bacteremia caused by multidrug-resistant Acinetobacter baumannii. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2007;27(7):980–987. doi: 10.1592/phco.27.7.980. [DOI] [PubMed] [Google Scholar]
- 150.Reid GE, Grim SA, Aldeza CA, Janda WM, Clark NM. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2007;27(8):1198–1201. doi: 10.1592/phco.27.8.1198. [DOI] [PubMed] [Google Scholar]
- 151.Vasilev K, Reshedko G, Orasan R, Sanchez M, Teras J, Babinchak T, Dukart G, Cooper A, Dartois N, Gandjini H. A Phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy. 2008;62(suppl_1):i29–i40. doi: 10.1093/jac/dkn249. [DOI] [PubMed] [Google Scholar]
- 152.Muralidharan G, Getsy J, Mayer P, Paty I, Micalizzi M, Speth J, Wester B, Mojaverian P (1999) Pharmacokinetics (PK), safety and tolerability of GAR-936, a novel glycylcycline antibiotic, in healthy subjects. 39th Interscience Conference on. Antimicrobial Agents and Chemotherapy, p 303
- 153.Xu Z, Yan Y, Li Z, Qian L, Gong Z. The antibiotic drug tigecycline: a focus on its promising anticancer properties. Front Pharmacol. 2016;7:473. doi: 10.3389/fphar.2016.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davido B, Shourick J, Makhloufi S, Dinh A, Salomon J. True incidence of tigecycline-induced pancreatitis: how many cases are we missing? J Antimicrob Chemother. 2016;71(10):2994–2995. doi: 10.1093/jac/dkw255. [DOI] [PubMed] [Google Scholar]
- 155.Eckmann C, Heizmann W, Bodmann K-F, von Eiff C, Petrik C, Loeschmann P-A. Tigecycline in the treatment of patients with necrotizing skin and soft tissue infections due to multiresistant bacteria. Surg Infect. 2015;16(5):618–625. doi: 10.1089/sur.2014.089. [DOI] [PubMed] [Google Scholar]
- 156.Sabanis N, Paschou E, Gavriilaki E, Kalaitzoglou A, Vasileiou S. Hypofibrinogenemia induced by tigecycline: a potentially life-threatening coagulation disorder. Infect Dis. 2015;47(10):743–746. doi: 10.3109/23744235.2015.1043942. [DOI] [PubMed] [Google Scholar]
- 157.McMahan J, Moenster RP. Tigecycline-induced coagulopathy. Am J Health Syst Pharm. 2017;74(3):130–134. doi: 10.2146/ajhp150894. [DOI] [PubMed] [Google Scholar]
- 158.Leng B, Xue YC, Zhang W, tian Gao T, quan Yan G, Tang H (2019) A Retrospective Analysis of the Effect of Tigecycline on Coagulation Function. Chem Pharm Bull 67 (3):258-264 [DOI] [PubMed]
- 159.Zimmerman JJ, Raible DG, Harper DM, Matschke K, Speth JLJPTJOHP, Therapy D (2008) Evaluation of a potential tigecycline-warfarin drug interaction. 28 (7):895-905 [DOI] [PubMed]
- 160.Al-Qadheeb NS, Althawadi S, Alkhalaf A, Hosaini S, Alrajhi AA. Evolution of tigecycline resistance in Klebsiella pneumoniae in a single patient. Ann Saudi Med. 2010;30(5):404–407. doi: 10.4103/0256-4947.67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Taneja N, Singh G, Singh M, Sharma M. Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res. 2011;133(6):681. [PMC free article] [PubMed] [Google Scholar]
- 162.Al-Sweih N, Al-Hubail M, Rotimi V. Emergence of tigecycline and colistin resistance in Acinetobacter species isolated from patients in Kuwait hospitals. J Chemother. 2011;23(1):13–16. doi: 10.1179/joc.2011.23.1.13. [DOI] [PubMed] [Google Scholar]
- 163.Manoharan A, Chatterjee S, Madhan S, Mathai D. Evaluation of tigecycline activity in clinical isolates among Indian medical centers. Indian J Pathol Microbiol. 2010;53(4):734. doi: 10.4103/0377-4929.72061. [DOI] [PubMed] [Google Scholar]
- 164.Hsu M-S, Liao C-H, Liu C-Y, Yang C-J, Huang Y-T, Hsueh P-R. In vitro susceptibilities of clinical isolates of ertapenem-non-susceptible Enterobacteriaceae to nemonoxacin, tigecycline, fosfomycin and other antimicrobial agents. Int J Antimicrob Agents. 2011;37(3):276–278. doi: 10.1016/j.ijantimicag.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 165.Wu H, Wang J-T, Shiau Y-R, Wang H-Y, Lauderdale T-LY, Chang S-C. A multicenter surveillance of antimicrobial resistance on Stenotrophomonas maltophilia in Taiwan. J Microbiol Immunol Infect. 2012;45(2):120–126. doi: 10.1016/j.jmii.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 166.Zhang R, Sun Q, Hu Y-J, Yu H, Li Y, Shen Q, Li G-X, Cao J-M, Yang W, Wang Q. Detection of the Smqnr quinolone protection gene and its prevalence in clinical isolates of Stenotrophomonas maltophilia in China. J Med Microbiol. 2012;61(4):535–539. doi: 10.1099/jmm.0.037309-0. [DOI] [PubMed] [Google Scholar]
- 167.Liao IC, Chen HM, Wu JJ, Tsai PF, Wang LR, Yan JJ. Metallo-β-lactamase-producing Enterobacteriaceae isolates at a Taiwanese hospital: lack of distinctive phenotypes for screening. Apmis. 2011;119(8):543–550. doi: 10.1111/j.1600-0463.2011.02772.x. [DOI] [PubMed] [Google Scholar]
- 168.Perry JD, Naqvi SH, Mirza IA, Alizai SA, Hussain A, Ghirardi S, Orenga S, Wilkinson K, Woodford N, Zhang J. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J Antimicrob Chemother. 2011;66(10):2288–2294. doi: 10.1093/jac/dkr299. [DOI] [PubMed] [Google Scholar]
- 169.Araj GF, Ibrahim GY. Tigecycline in vitro activity against commonly encountered multidrug-resistant Gram-negative pathogens in a Middle Eastern country. Diagn Microbiol Infect Dis. 2008;62(4):411–415. doi: 10.1016/j.diagmicrobio.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 170.Liu J-W, Wang L-S, Cheng Y-J, Hsu G-J, Lu P-L, Liu Y-C, Chen C-M, Lee C-M, Sun W, Jang T-N. In-vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii in Taiwan. Int J Antimicrob Agents. 2008;32:S188–S191. doi: 10.1016/S0924-8579(08)70026-8. [DOI] [PubMed] [Google Scholar]
- 171.Behera B, Das A, Mathur P, Kapil A, Gadepalli R, Dhawan B. Tigecycline susceptibility report from an Indian tertiary care hospital. Indian J Med Res. 2009;129(4):446. [PubMed] [Google Scholar]
- 172.Lee Y-T, Huang L-Y, Chiang D-H, Chen C-P, Chen T-L, Wang F-D, Fung C-P, Siu L-K, Cho W-L. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int J Antimicrob Agents. 2009;34(6):580–584. doi: 10.1016/j.ijantimicag.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 173.Tribuddharat C, Thamlikitkul V. In vitro activity of tigecycline against clinical isolates of multidrug-resistant Acinetobacter baumannii in Siriraj Hospital, Thailand. J Med Assoc Thail. 2006;89(5):S102–S105. [PubMed] [Google Scholar]
- 174.Navon-Venezia S, Leavitt A, Carmeli Y. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;59(4):772–774. doi: 10.1093/jac/dkm018. [DOI] [PubMed] [Google Scholar]
- 175.Chang K-C, Lin M-F, Lin N-T, Wu W-J, Kuo H-Y, Lin T-Y, Yang T-L, Chen Y-C, Liou M-L. Clonal spread of multidrug-resistant Acinetobacter baumannii in eastern Taiwan. J Microbiol Immunol Infect. 2012;45(1):37–42. doi: 10.1016/j.jmii.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 176.Park Y, Choi J, Song J, Ko K. In vitro activity of tigecycline against colistin-resistant Acinetobacter spp. isolates from Korea. Int J Antimicrob Agents. 2009;33(3):289–290. doi: 10.1016/j.ijantimicag.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 177.Kim C-K, Lee Y, Lee H, Woo G-J, Song W, Kim M-N, Lee W-G, Jeong SH, Lee K, Chong Y. Prevalence and diversity of carbapenemases among imipenem-nonsusceptible Acinetobacter isolates in Korea: emergence of a novel OXA-182. Diagn Microbiol Infect Dis. 2010;68(4):432–438. doi: 10.1016/j.diagmicrobio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 178.Dizbay M, Altuncekic A, Sezer BE, Ozdemir K, Arman D. Colistin and tigecycline susceptibility among multidrug-resistant Acinetobacter baumannii isolated from ventilator-associated pneumonia. Int J Antimicrob Agents. 2008;32(1):29–32. doi: 10.1016/j.ijantimicag.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 179.Sharma S, Bhowmik D, Bhattacharjee A. Tigecycline Resistance among Clinical Isolates of Staphylococcus aureus from North-east India. J Microbiol Infect Dis. 2017;7(04):173–177. [Google Scholar]
- 180.Liao C-H, Kung H-C, Hsu G-J, Lu P-L, Liu Y-C, Chen C-M, Lee C-M, Sun W, Jang T-N, Chiang P-C. In-vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii in Taiwan determined by the broth microdilution and disk diffusion methods. Int J Antimicrob Agents. 2008;32:S192–S196. doi: 10.1016/S0924-8579(08)70027-X. [DOI] [PubMed] [Google Scholar]
- 181.Zhao J, Liu Y, Liu Y, Wang D, Ni W, Wang R, Liu Y, Zhang B. Frequency and genetic determinants of tigecycline resistance in clinically isolated stenotrophomonas maltophilia in Beijing, China. Front Microbiol. 2018;9:549. doi: 10.3389/fmicb.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chiu S-K, Chan M-C, Huang L-Y, Lin Y-T, Lin J-C, Lu P-L, Siu LK, Chang F-Y, Yeh K-M. Tigecycline resistance among carbapenem-resistant Klebsiella pneumoniae: clinical characteristics and expression levels of efflux pump genes. PloS one. 2017;12(4):e0175140. doi: 10.1371/journal.pone.0175140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seifert H, Blondeau J, Dowzicky MJ. In vitro activity of tigecycline and comparators (2014–2016) among key WHO ‘priority pathogens’ and longitudinal assessment (2004–2016) of antimicrobial resistance: a report from the TEST study. Int J Antimicrob Agents. 2018;52(4):474–484. doi: 10.1016/j.ijantimicag.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 184.Nagy E. ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17:371–379. doi: 10.1111/j.1469-0691.2010.03256.x. [DOI] [PubMed] [Google Scholar]
- 185.Insa R, Cercenado E, Goyanes M, Morente A, Bouza E. In vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. J Antimicrob Chemother. 2007;59(3):583–585. doi: 10.1093/jac/dkl496. [DOI] [PubMed] [Google Scholar]
- 186.Tubau F, Liñares J, Rodríguez M-D, Cercenado E, Aldea M-J, González-Romo F, Torroba L, Berdonces P, Plazas J, Aguilar L. Susceptibility to tigecycline of isolates from samples collected in hospitalized patients with secondary peritonitis undergoing surgery. Diagn Microbiol Infect Dis. 2010;66(3):308–313. doi: 10.1016/j.diagmicrobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 187.Grandesso S, Sapino B, Mazzucato S, Alessandrini R, Solinas M, Gion M. Study on in-vitro susceptibility of ESBL-positive Escherichia coli isolated from urine specimens. Le infezioni in medicina: rivista periodica di eziologia, epidemiologia, diagnostica, clinica e terapia delle patologie infettive. 2010;18(3):162–168. [PubMed] [Google Scholar]
- 188.Sekowska A, Gospodarek E. Susceptibility of Klebsiella spp. to tigecycline and other selected antibiotics. Med Sci Monit. 2010;16(6):BR193–BR196. [PubMed] [Google Scholar]
- 189.Naesens R, Ursi J, Van Schaeren J, Jeurissen A. In vitro activity of tigecycline against multidrug-resistant Enterobacteriaceae isolates from a Belgian hospital. Eur J Clin Microbiol Infect Dis. 2009;28(4):381–384. doi: 10.1007/s10096-008-0629-9. [DOI] [PubMed] [Google Scholar]
- 190.Gomis PF, Jean-Pierre H, Rousseau-Didelot M, Compan B, Michon A, Godreuil S. Tigecycline: CMI 50/90 towards 1766 Gram-negative bacilli (3rd generation cephalosporins resistant Enterobacteriaceae), Acinetobacter baumannii and Bacteroides fragilis group, University Hospital-Montpellier, 2008-2011. Pathol Biol (Paris) 2013;61:282–285. doi: 10.1016/j.patbio.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 191.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35(3):240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 192.Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: results from the SENTRY surveillance program (2010–2013) Diagn Microbiol Infect Dis. 2015;83(2):183–186. doi: 10.1016/j.diagmicrobio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 193.Papaparaskevas J, Tzouvelekis LS, Tsakris A, Pittaras TE, Legakis NJ, Group HTS In vitro activity of tigecycline against 2423 clinical isolates and comparison of the available interpretation breakpoints. Diagn Microbiol Infect Dis. 2010;66(2):187–194. doi: 10.1016/j.diagmicrobio.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 194.Balode A, Punda-Polić V, Dowzicky MJ. Antimicrobial susceptibility of gram-negative and gram-positive bacteria collected from countries in Eastern Europe: results from the Tigecycline Evaluation and Surveillance Trial (TEST) 2004–2010. Int J Antimicrob Agents. 2013;41(6):527–535. doi: 10.1016/j.ijantimicag.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 195.Cattoir V, Dowzicky MJ. A longitudinal assessment of antimicrobial susceptibility among important pathogens collected as part of the Tigecycline Evaluation and Surveillance Trial (TEST) in France between 2004 and 2012. Antimicrob Resist Infect Control. 2014;3(1):36. doi: 10.1186/2047-2994-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Daurel C, Fiant A-L, Brémont S, Courvalin P, Leclercq R. Emergence of an Enterobacter hormaechei strain with reduced susceptibility to tigecycline under tigecycline therapy. Antimicrob Agents Chemother. 2009;53(11):4953–4954. doi: 10.1128/AAC.01592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Werner G, Gfrörer S, Fleige C, Witte W, Klare I. Tigecycline-resistant Enterococcus faecalis strain isolated from a German intensive care unit patient. J Antimicrob Chemother. 2008;61(5):1182–1183. doi: 10.1093/jac/dkn065. [DOI] [PubMed] [Google Scholar]
- 198.Ahmed NH, Baba K, Clay C, Lekalakala R, Hoosen AA. In vitro activity of tigecycline against clinical isolates of carbapenem resistant Acinetobacter baumannii complex in Pretoria, South Africa. BMC Res Notes. 2012;5(1):215. doi: 10.1186/1756-0500-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Perovic O, Ismail H, Schalkwyk EV. Antimicrobial resistance surveillance in the South African public sector. South Afr J Infect Dis. 2018;33(4):118–129. [Google Scholar]
- 200.Perovic O, Ismail H, Van Schalkwyk E, Lowman W, Prentice E, Senekal M, Govind CN. Antimicrobial resistance surveillance in the South African private sector report for 2016. South Afr J Infect Dis. 2018;33(4):114–117. [Google Scholar]
- 201.Sherwood JE, Fraser S, Citron DM, Wexler H, Blakely G, Jobling K, Patrick S. Multi-drug resistant Bacteroides fragilis recovered from blood and severe leg wounds caused by an improvised explosive device (IED) in Afghanistan. Anaerobe. 2011;17(4):152–155. doi: 10.1016/j.anaerobe.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 202.DiPersio JR, Dowzicky MJ. Regional variations in multidrug resistance among Enterobacteriaceae in the USA and comparative activity of tigecycline, a new glycylcycline antimicrobial. Int J Antimicrob Agents. 2007;29(5):518–527. doi: 10.1016/j.ijantimicag.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 203.Rossi F, García P, Ronzon B, Curcio D, Dowzicky MJ. Rates of antimicrobial resistance in Latin America (2004-2007) and in vitro activity of the glycylcycline tigecycline and of other antibiotics. Braz J Infect Dis. 2008;12(5):405–415. doi: 10.1590/S1413-86702008000500012. [DOI] [PubMed] [Google Scholar]
- 204.Denys GA, Callister SM, Dowzicky MJ. Antimicrobial susceptibility among gram-negative isolates collected in the USA between 2005 and 2011 as part of the Tigecycline Evaluation and Surveillance Trial (TEST) Ann Clin Microbiol Antimicrob. 2013;12(1):24. doi: 10.1186/1476-0711-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fernández-Canigia L, Dowzicky MJ. Susceptibility of important Gram-negative pathogens to tigecycline and other antibiotics in Latin America between 2004 and 2010. Ann Clin Microbiol Antimicrob. 2012;11(1):29. doi: 10.1186/1476-0711-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dowzicky MJ, Park CH. Update on antimicrobial susceptibility rates among gram-negative and gram-positive organisms in the United States: results from the Tigecycline Evaluation and Surveillance Trial (TEST) 2005 to 2007. Clin Ther. 2008;30(11):2040–2050. doi: 10.1016/j.clinthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 207.Garza-González E, Llaca-Díaz JM, Bosques-Padilla FJ, Gonzalez GM. Prevalence of multidrug-resistant bacteria at a tertiary-care teaching hospital in Mexico: special focus on Acinetobacter baumannii. Chemotherapy. 2010;56(4):275–279. doi: 10.1159/000319903. [DOI] [PubMed] [Google Scholar]
- 208.Lagacé-Wiens PR, Simner PJ, Forward KR, Tailor F, Adam HJ, DeCorby M, Karlowsky J, Hoban DJ, Zhanel GG, Alliance CAR. Analysis of 3789 in-and outpatient Escherichia coli isolates from across Canada—results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69(3):314–319. doi: 10.1016/j.diagmicrobio.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 209.Castanheira M, Sader HS, Jones RN. Antimicrobial susceptibility patterns of KPC-producing or CTX-M-producing Enterobacteriaceae. Microb Drug Resist. 2010;16(1):61–65. doi: 10.1089/mdr.2009.0031. [DOI] [PubMed] [Google Scholar]
- 210.Giammanco A, Calà C, Fasciana T, Dowzicky MJ. Global assessment of the activity of tigecycline against multidrug-resistant Gram-negative pathogens between 2004 and 2014 as part of the Tigecycline Evaluation and Surveillance Trial. Msphere. 2017;2(1):e00310–e00316. doi: 10.1128/mSphere.00310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kehl SC, Dowzicky MJ. Global assessment of antimicrobial susceptibility among Gram-negative organisms collected from pediatric patients between 2004 and 2012: results from the Tigecycline Evaluation and Surveillance Trial. J Clin Microbiol. 2015;53(4):1286–1293. doi: 10.1128/JCM.03184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mendes RE, Farrell DJ, Sader HS, Jones RN. Comprehensive assessment of tigecycline activity tested against a worldwide collection of Acinetobacter spp.(2005–2009) Diagn Microbiol Infect Dis. 2010;68(3):307–311. doi: 10.1016/j.diagmicrobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 213.Garrison MW, Mutters R, Dowzicky MJ. In vitro activity of tigecycline and comparator agents against a global collection of Gram-negative and Gram-positive organisms: tigecycline Evaluation and Surveillance Trial 2004 to 2007. Diagn Microbiol Infect Dis. 2009;65(3):288–299. doi: 10.1016/j.diagmicrobio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 214.Hoban DJ, Reinert RR, Bouchillon SK, Dowzicky MJ. Global in vitro activity of tigecycline and comparator agents: Tigecycline Evaluation and Surveillance Trial 2004–2013. Ann Clin Microbiol Antimicrob. 2015;14(1):27. doi: 10.1186/s12941-015-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sader HS, Flamm RK, Jones RN. Tigecycline activity tested against antimicrobial resistant surveillance subsets of clinical bacteria collected worldwide (2011) Diagn Microbiol Infect Dis. 2013;76(2):217–221. doi: 10.1016/j.diagmicrobio.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 216.Bertrand X, Dowzicky MJJCt (2012) Antimicrobial susceptibility among gram-negative isolates collected from intensive care units in North America, Europe, the Asia-Pacific Rim, Latin America, the Middle East, and Africa between 2004 and 2009 as part of the Tigecycline Evaluation and Surveillance Trial 34 (1):124-137 [DOI] [PubMed]
- 217.Hsu M-S, Liao C-H, Liu C-Y, Yang C-J, Huang Y-T. In vitro susceptibilities of clinical isolates of ertapenem-non-susceptible Enterobacteriaceae to nemonoxacin, tigecycline, fosfomycin and other antimicrobial agents. Int J Antimicrob Agents (Print) 2011;37(3):276–278. doi: 10.1016/j.ijantimicag.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 218.Veeraraghavan B, Poojary A, Shankar C, Bari AK, Kukreja S, Thukkaram B, Neethimohan RG, Bakhtavachalam YD, Kamat S. In-vitro activity of tigecycline and comparator agents against common pathogens: Indian experience. The Journal of Infection in Developing Countries. 2019;13(03):245–250. doi: 10.3855/jidc.10376. [DOI] [PubMed] [Google Scholar]
- 219.Chen Y-H, Liu C-Y, Ko W-C, Liao C-H, Lu P-L, Huang C-H, Lu C-T, Chuang Y-C, Tsao S-M, Chen Y-S. Trends in the susceptibility of methicillin-resistant Staphylococcus aureus to nine antimicrobial agents, including ceftobiprole, nemonoxacin, and tyrothricin: results from the Tigecycline In Vitro Surveillance in Taiwan (TIST) study, 2006–2010. Eur J Clin Microbiol Infect Dis. 2014;33(2):233–239. doi: 10.1007/s10096-013-1949-y. [DOI] [PubMed] [Google Scholar]
- 220.Yang Q, Xu Y-C, Kiratisin P, Dowzicky MJ. Antimicrobial activity among gram-positive and gram-negative organisms collected from the Asia-Pacific region as part of the Tigecycline Evaluation and Surveillance Trial: Comparison of 2015 results with previous years. Diagn Microbiol Infect Dis. 2017;89(4):314–323. doi: 10.1016/j.diagmicrobio.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 221.Ricciardi R, Ricciardi A, Danzi G. In vitro activity of tigecycline against multidrug-resistant Acinetobacter baumannii clinical isolates. Le infezioni in medicina: rivista periodica di eziologia, epidemiologia, diagnostica, clinica e terapia delle patologie infettive. 2009;17(4):236–239. [PubMed] [Google Scholar]
- 222.Capone A, D’Arezzo S, Visca P, Petrosillo N. In vitro activity of tigecycline against multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2008;62(2):422–423. doi: 10.1093/jac/dkn172. [DOI] [PubMed] [Google Scholar]
- 223.Kopterides P, Papageorgiou C, Antoniadou A, Papadomichelakis E, Tsangaris I, Dimopoulou I, Armaganidis A. Failure of tigecycline to treat severe Clostridium difficile infection. Anaesth Intensive Care. 2010;38(3):755–758. doi: 10.1177/0310057X1003800339. [DOI] [PubMed] [Google Scholar]
- 224.Caneiras C, Calisto F, Jorge da Silva G, Lito L, Melo-Cristino J, Duarte A. First description of colistin and tigecycline-resistant Acinetobacter baumannii producing KPC-3 carbapenemase in Portugal. Antibiotics. 2018;7(4):96. doi: 10.3390/antibiotics7040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Verkade E, Verhulst C, Huijsdens X, Kluytmans J. In vitro activity of tigecycline against methicillin-resistant Staphylococcus aureus, including livestock-associated strains. Eur J Clin Microbiol Infect Dis. 2010;29(5):503–507. doi: 10.1007/s10096-010-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nagy E, Urbán E, Nord CE, Bacteria ESGoARiA (2011) Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect 17 (3):371-379 [DOI] [PubMed]
- 227.Gallagher JC, Rouse HM. Tigecycline for the treatment of Acinetobacter infections: a case series. Ann Pharmacother. 2008;42(9):1188–1194. doi: 10.1345/aph.1L171. [DOI] [PubMed] [Google Scholar]
- 228.Peleg AY, Potoski BA, Rea R, Adams J, Sethi J, Capitano B, Husain S, Kwak EJ, Bhat SV, Paterson DL. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2006;59(1):128–131. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- 229.Hoban DJ, Bouchillon SK, Dowzicky MJ. Antimicrobial susceptibility of extended-spectrum β-lactamase producers and multidrug-resistant Acinetobacter baumannii throughout the United States and comparative in vitro activity of tigecycline, a new glycylcycline antimicrobial. Diagn Microbiol Infect Dis. 2007;57(4):423–428. doi: 10.1016/j.diagmicrobio.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 230.Sekyere JO, Govinden U, Essack S. The molecular epidemiology and genetic environment of carbapenemases detected in Africa. Microb Drug Resist. 2016;22(1):59–68. doi: 10.1089/mdr.2015.0053. [DOI] [PubMed] [Google Scholar]
- 231.Osei Sekyere J. Current state of resistance to antibiotics of last-resort in South Africa: a review from a public health perspective. Front Public Health. 2016;4:209. doi: 10.3389/fpubh.2016.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Govind C, Moodley K, Peer A, Pillay N, Maske C, Wallis C, Viana R, Chetty A, Perovic O. NDM-1 imported from India–first reported case in South Africa. S Afr Med J. 2013;103(7):476–478. doi: 10.7196/SAMJ.6593. [DOI] [PubMed] [Google Scholar]
- 233.Osei Sekyere J, Govinden U, Bester L, Essack S. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: emerging resistance mechanisms and detection methods. J Appl Microbiol. 2016;121(3):601–617. doi: 10.1111/jam.13169. [DOI] [PubMed] [Google Scholar]
- 234.Sekyerea JO, Pedersenb T, Sivertsenb A, Govindena U, Essacka SY, Moodleyc K, Samuelsenb O, Sundsfjordb A. Molecular epidemiology of carbapenem, colistin and tigecycline resistant Enterobacteriaceae in Durban. South: Africa; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this review are included in the manuscript.