Abstract
αKlotho (called Klotho here) is a membrane protein that serves as the coreceptor for the circulating hormone fibroblast growth factor 23 (FGF23). Klotho is also cleaved and released as a circulating substance originating primarily from the kidney and exerts a myriad of housekeeping functions in just about every organ. The vital role of Klotho is shown by the multiorgan failure with genetic deletion in rodents, with certain features reminiscent of human disease. The most common causes of systemic Klotho deficiency are AKI and CKD. Preclinical data on Klotho biology have advanced considerably and demonstrated its potential diagnostic and therapeutic value; however, multiple knowledge gaps exist in the regulation of Klotho expression, release, and metabolism; its target organs; and mechanisms of action. In the translational and clinical fronts, progress has been more modest. Nonetheless, Klotho has potential clinical applications in the diagnosis of AKI and CKD, in prognosis of progression and extrarenal complications, and finally, as replacement therapy for systemic Klotho deficiency. The overall effect of Klotho in clinical nephrology requires further technical advances and additional large prospective human studies.
Keywords: Klotho;, nephrology;, kidney;, diagnosis;, therapy
Introduction
αKlotho (referred here as Klotho) was serendipitously discovered in 1997 by Kuro-o et al. (1) as a gene linked to aging. This gene was named Klotho after the goddess who spins the thread of life to govern human lifespan, working with two other goddesses of fate, Lachesis who measures the spun thread from Klotho’s spindle and Atropos who ends the life by cutting the thread (2). Kuro-o et al. (1) observed that Klotho hypomorphic mice exhibited a multiorgan failure syndrome resembling premature aging, including short lifespan, disturbed mineral metabolism, and multiple organ degeneration or failure, such as infertility, arteriosclerosis, cardiomyopathy, ectopic calcification, skin atrophy, osteoporosis, and emphysema. Later, both human (3) and rat (4) Klotho genes were cloned with high homology in amino acid sequences with the mouse. Subsequently, two paralogous genes were discovered and termed βKlotho (5) and γKlotho (6) to distinguish them from the first Klotho gene, which was renamed αKlotho. The α- and βKlotho proteins have known biologic roles, but the function of γKlotho is still elusive (7,8). In this manuscript, we only focus on αKlotho (Klotho).
Klotho is mostly expressed in the kidney as a transmembrane protein. In membrane-bound form, Klotho serves as a coreceptor for fibroblast growth factor 23 (FGF23) in conjunction with fibroblast growth factor receptors (FGFRs) (9) (Figure 1). The extracellular domain of transmembrane Klotho is also cleaved by proteases and released from the kidney into circulation, both as a full-length protein or as Kl1 and Kl2 fragments (10,11). These circulating forms of Klotho protein are collectively called “soluble” Klotho (Figure 1). Soluble Klotho protein is also detected in cerebrospinal fluid and urine.
Figure 1.
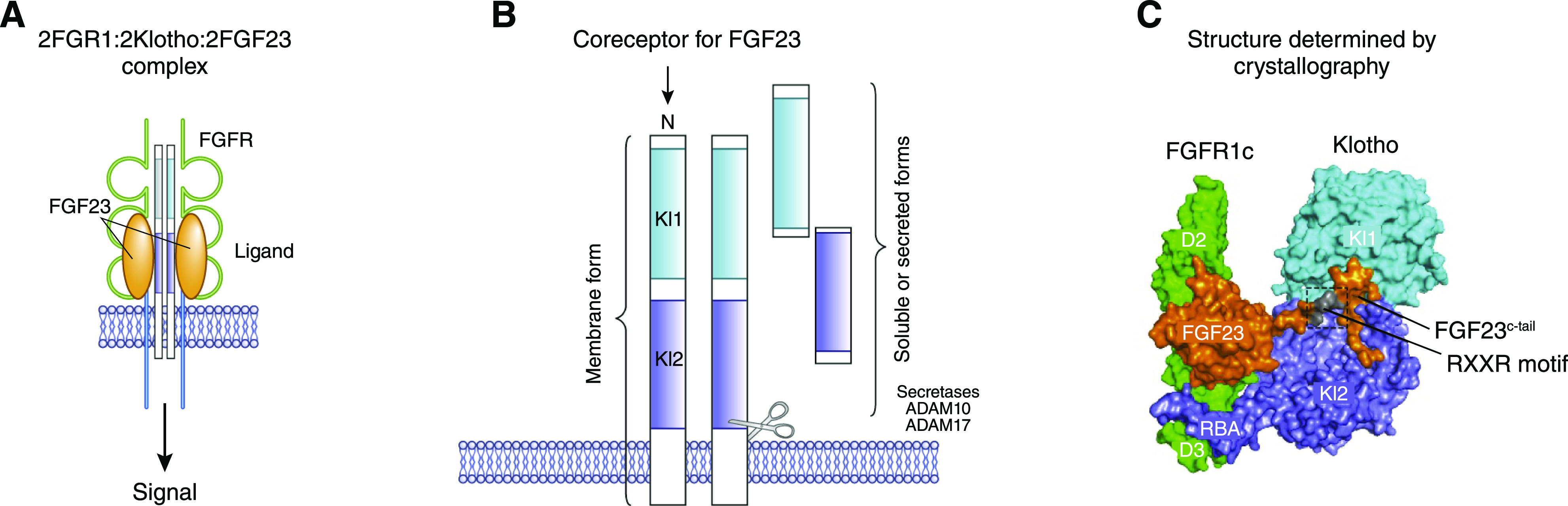
Overview of Klotho protein. (A) Fibroblast growth factor 23 (FGF23) engages the fibroblast growth factor receptor (FGFR)-Klotho coreceptor complex that triggers cellular signaling. (B) Transmembrane Klotho with its Kl1 and Kl2 domains and the generation of soluble circulating Klotho by secretases (ADAM10 and ADAM17). (C) Structure of FGFR1c, Klotho, and FGF23 (one molecule each) as determined by Chen et al. (12). RXXR motif, proteolytic cleavage motif.
Klotho is a pleiotropic protein with multifaceted functions relevant to distinct biology and pathobiology in multiple organs and tissues. Klotho is an inhibitor of apoptosis, fibrosis, and cell senescence and is an inducer of autophagy (13–16). In this review, we discuss its potential clinical application in nephrology, with emphasis on diagnostic and therapeutic utility.
Physiology of Klotho
Klotho Expression and Metabolism
Klotho is highly expressed in the kidney, specifically in the distal tubules and, to a lesser extent, in the proximal tubules (17). Klotho is expressed in other organs, such as the brain, pancreas, and parathyroid glands (1). The Klotho gene encodes a single-pass 130-kD transmembrane protein that consists of two extracellular domains (Kl1 and Kl2), a transmembrane domain, and a short cytoplasmic tail (Figure 1). The extracellular domain of transmembrane Klotho is cleaved at the juxtamembrane region and between Kl1 and Kl2 by proteases (18–20). A secreted form of Klotho from an alternatively spliced transcript has been proposed, but the data are not conclusive (3,21). The spliced Klotho transcript possibly undergoes nonsense-mediated mRNA decay and is not translated to protein (22). The cleaved Klotho is released into the circulation and is known as soluble Klotho (17,22). Soluble Klotho acts as an endocrine or paracrine factor affecting multiple organs, such as the kidney, bone, brain, heart, lungs, and endothelium (23–29). Soluble Klotho suppresses FGF23 production in physiologic concentrations (Figure 2), but it can increase FGF23 expression in the bone (28) or act as a nonenzymatic scaffold protein that enhances FGF23 signaling on the basis of in vitro data in very high, nonphysiologic concentrations (12). Soluble Klotho is not filtered at the glomerulus, but it translocates across the kidney tubules, from the basolateral to the luminal side, and is excreted in the urine (17). Klotho expression is downregulated during acute kidney disease and CKD.
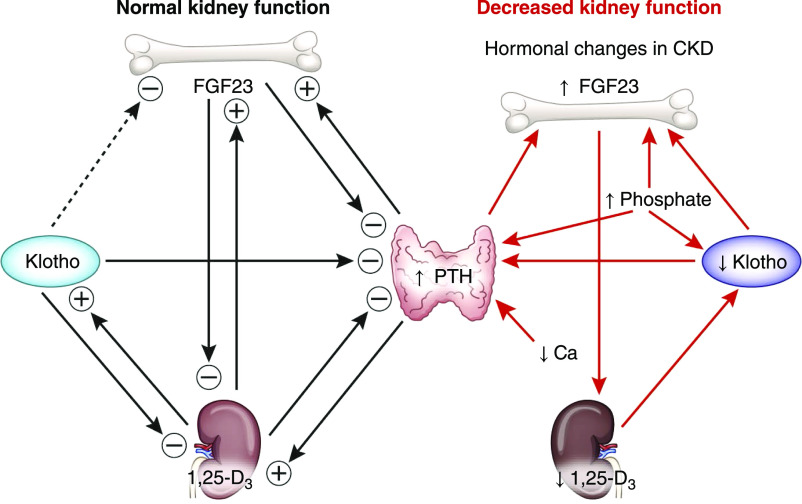
Figure 2.
Physiologic and pathophysiologic role of Klotho in mineral metabolism with preserved and decreased kidney function. The dashed line indicates putative action on the basis of experimental or clinical data; no evidence supports direct effect yet. PTH, parathyroid hormone.
Klotho and Mineral Metabolism
Klotho decreases kidney phosphate reabsorption by acting as a coreceptor for FGF23 binding to FGFR1 (30) (Figure 1). Soluble Klotho also directly promotes the internalization and degradation of the NaPi2a cotransporter in the kidney proximal tubules contributing to phosphate excretion (31). Klotho may also be a suppressor of vitamin D and FGF23 production (32) because Klotho-deficient mice exhibit higher CYP27B1 gene (33) and FGF23 production (1) (Figure 2).
Klotho in Disease States
CKD
Klotho expression in the kidney and soluble Klotho in the blood and urine are decreased in animals and humans with CKD from a variety of etiologies, including glomerular and tubulointerstitial diseases (32). Because the kidneys are the primary source of soluble Klotho, circulating soluble Klotho expectedly declines as CKD progresses (Figure 3). Decreased Klotho expression in CKD is not simply due to loss of viable tissue but can be attributable to hyperphosphatemia (34) and hypermethylation or deacetylation of the Klotho gene promoter by inflammatory cytokines or uremic toxins, such as indoxyl sulfate (35,36). Similarities of clinical features, including short lifespan, cardiac remodeling, vascular calcification, bone disease, muscle wasting, and hyperphosphatemia, exist in the context of Klotho deficiency and in CKD (37).
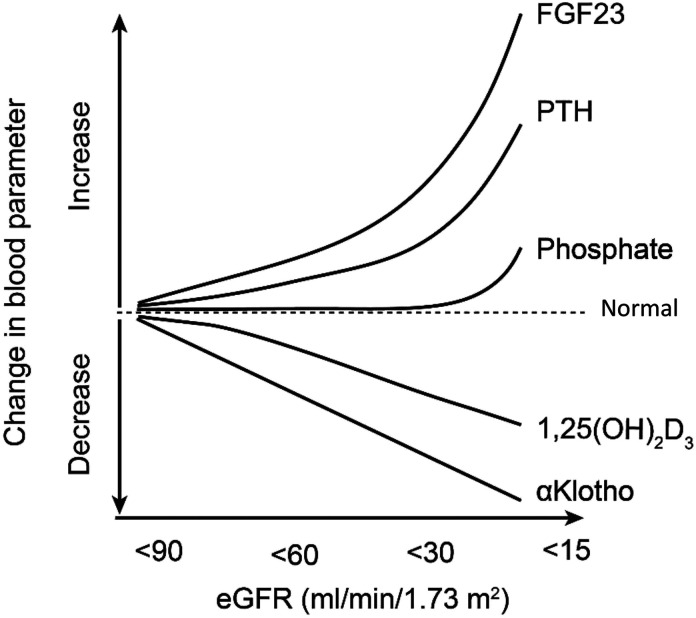
Figure 3.
Changes in circulating soluble Klotho according to eGFR decline and its relationship to other mineral metabolism parameters. 1,25(OH)2D3, 1,25-dihydroxyvitamin D.
Klotho is also expressed in the choroid plexus of the brain (1). Klotho-deficient mice display central nervous system lesions including hypomyelination, synaptic loss, and behavioral impairments such as dementia and cognitive deficits (38). Klotho deficiency in the brain impairs blood barrier and promotes immune-mediated central nervous system disorders (39). CKD is associated with depression and cognitive impairment (40). Low Klotho in cerebrospinal fluid was confirmed in Alzheimer disease (41). Low soluble Klotho in CKD may conceivably contribute to central nervous system dysfunction, although there is no evidence to date of Klotho deficiency in cerebrospinal fluid or brain in CKD.
Cardiovascular Disease
A novel risk factor of cardiovascular disease in CKD is soluble Klotho deficiency (42,43). Hyperphosphatemia and low soluble Klotho associate with more severe cardiac hypertrophy and fibrosis. Interestingly, elevated levels of FGF23 were associated with pathologic cardiac remodeling only if Klotho deficiency was also present (34). However, exogenous FGF23, independent of Klotho, induced cardiac hypertrophy through FGFR4-mediated PLC-γ signaling activation in cardiac myocytes (43,44). Klotho, independent of FGF23, was postulated to protect the heart against cardiac hypertrophy through inhibition of transient receptor potential channel-6 activity in cardiomyocytes (45). Klotho supplementation also protects against indoxyl sulfate–mediated cardiac hypertrophy in mice (46).
AKI
AKI is a syndrome with a myriad of inflammatory cytokines such as TNF-α and TNF-like weak inducer of apoptosis, which downregulate Klotho expression in the kidney through NF-kB activation in murine AKI (47). Similarly, in other inflammatory conditions such as inflammatory bowel disease, TNF-α and IFN-γ reduced Klotho expression in murine kidney (48). The reduced kidney Klotho is partly mediated by an increase in inducible nitric oxide synthase, nitric oxide production, and oxidative stress, but the precise mechanisms of how inflammation downregulates Klotho in AKI are unknown (48,49). A differential transcriptional splicing resulting in decay of Klotho mRNA has been postulated as a potential mechanism of Klotho downregulation in settings of acute illness (22).
Current experimental data support that low Klotho is not just a biomarker but pathogenic. Klotho-deficient mice have lower kidney and circulatory soluble Klotho, and they have more severe kidney damage and fibrosis and higher risk of AKI-to-CKD transition compared with wild-type mice after exposure to distinct kidney insults (14,16,23,50,51), indicating that Klotho deficiency renders the kidney more susceptible to acute insults.
Potential Mechanisms of Klotho-Mediated Kidney and Cardiovascular Protection
Preclinical data strongly suggest a pathogenic role of Klotho acting through multiple mechanisms in contributing to AKI development, AKI-to-CKD transition, progression of CKD, and development of extrarenal complications of CKD (Figure 4, Table 1).
Figure 4.
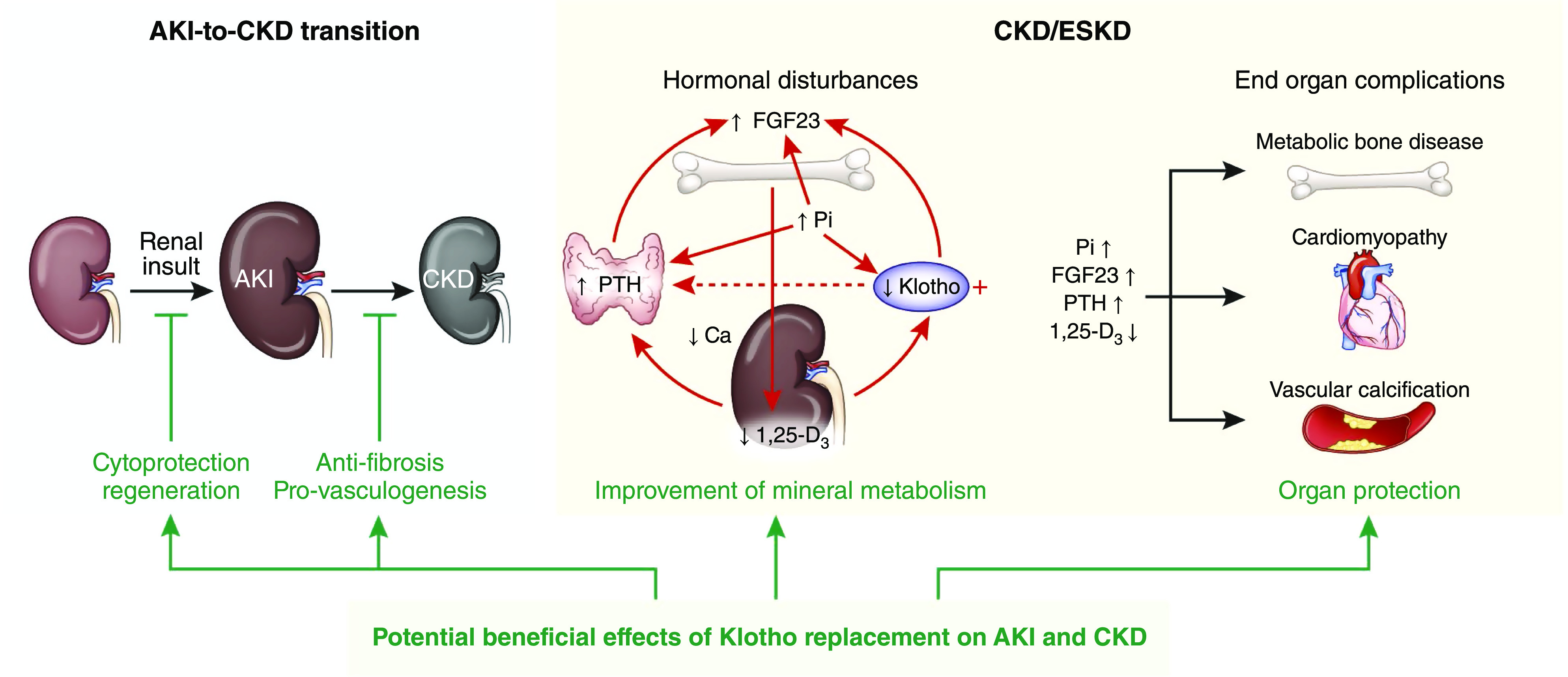
Role of Klotho in kidney and cardiovascular protection. AKI occurs after exposure to kidney insults. If the insult is strong or long enough, kidney recovery is impaired, and AKI progresses to CKD. With CKD progression, kidney Klotho is decreased followed by increase in circulating FGF23 levels, low 1,25-dihydroxyvitamin D3, high blood phosphate, and increase in PTH. Abnormal mineral hormones individually and synergistically exacerbate each other in the manner of a vortex of downhill spiral (dashed line: putative action) and contribute to end organ complications, including CKD-MBD, uremic cardiomyopathy, and vascular calcification. Klotho replacement provides beneficial effects from protection of kidney against acute injury, promotion of kidney regeneration, retardation of CKD progression, and amelioration of extrarenal complications. Klotho also improves mineral metabolism disturbances and directly or indirectly lessens end organ dysfunction or abnormalities of advanced CKD. CKD-MBD, CKD-mineral and bone disorder; Ca, calcium; 1,25-D3, 1,25-dihydroxyvitamin D; Pi, phosphate.
Table 1.
Potential mechanisms of beneficial effects of Klotho in kidney disease
| AKI | AKI to CKD | CKD |
|---|---|---|
| Protect kidney | Promote recovery | Inhibit fibrosis |
| Antiapoptosis | Preserve stem cells | ↓ TGF-β1 signal |
| ↓ Cell senescence | ↑ Angiogenesis | ↓ Wnt signal |
| ↑ Autophagy | ↑ Vasculogenesis | Mineral metabolism |
| Antioxidation | Inhibit fibrosis | ↓ Blood phosphate |
| ↓ TGF-β1 signaling | Modulate FGF23 activity | |
| ↓ Wnt signaling | Control 1,25-(OH)2D3 production | |
| Control PTH production | ||
| Control BP |
FGF23, fibroblast growth factor 23; 1,25-(OH)2D3, 1,25-dihydroxyvitamin D; PTH, parathyroid hormone.
Klotho Renders the Kidney More Resistant to Injury
Inhibition of Cell Senescence.
Cell senescence is a complex process in normal aging (52) and in kidney disease (53). Klotho deficiency induces and Klotho overexpression suppresses cell senescence through increased Wnt signaling activity (54,55). Intracellular Klotho also suppresses cell senescence by inhibiting expression of IL-6 and -8 (56,57).
Inhibition of Cell Apoptosis.
Klotho deficiency increases apoptosis in cultured cells (58,59) and in the kidney (23,49,50,60). Increasing Klotho decreases apoptotic cell number and improves kidney function and morphology after acute and chronic kidney damage (50,60,61).
Antioxidation.
The kl/kl mice (low Klotho expression) have higher levels and Tg-Kl mice (Klotho overexpressing) have lower levels of oxidative markers and oxidation-induced cell damage than normal mice (62,63). Klotho exerts its antioxidation effect through stimulating the FOXO family, manganese SOD (55,62), and phosphatidylinositol 3-kinase/AKT/Nrf2/heme oxygenase-1 pathways (64).
Induction of Autophagy.
Autophagy is a conserved process to degrade defective and unwanted cytoplasmic and organelle components and reuse the constituents (65). Either extremely high or low autophagy activity is associated with kidney damage (16,66). Klotho upregulates autophagy activity, protects kidney against ischemic injury, and, consequently, mitigates the progression of AKI to CKD (16).
Klotho Promotes Kidney Recovery
When recombinant Klotho protein is injected right after ischemic injury, mice had similar acute kidney damage but recovered much faster than vehicle injection (16). Intravenous administration of urinary extracellular vesicles carrying Klotho restores kidney Klotho levels in injured kidney and promotes kidney function recovery after glycerol injection (67).
Preservation of Stem Cells.
Klotho deficiency is associated with stem cell depletion (15,68) via augmentation of Wnt signaling and more cell senescence, which were rescued by Klotho protein overexpression (54).
Maintenance of Endothelial Function and Angiogenesis.
Swift and complete recovery of endothelium structure and function promotes kidney regeneration after ischemic injury (69). Klotho exerts proangiogenic actions (70) and maintains endothelial integrity (71).
Klotho Suppresses Kidney Fibrosis
Klotho suppresses kidney fibrosis in several animal models (14,72,73) via suppression of TGF-β1 activity, TGF-receptor II (14,73), and Wnt signaling activity (72). Supplementation of Klotho in rodents inhibits kidney fibrosis and retards CKD progression.
Klotho Ameliorates Disordered Mineral Metabolism in CKD
CKD is a state of Klotho deficiency, hyperphosphatemia, high FGF23 levels, and low 1,25-dihydroxyvitamin D3 (Figure 3). Klotho deficiency participates in CKD development and progression and contributes to extrarenal complications of CKD directly and indirectly through disturbed mineral metabolism (Figures 2 and 4) (29,34,74,–76).
Reduction of Serum Phosphate.
Klotho deficiency impairs phosphaturia (31,77) and, consequently, accelerates phosphate accumulation in CKD. Administration of Klotho protein or enhancement of native Klotho is an effective strategy for the correction of hyperphosphatemia in experimental CKD (75).
Control of Fibroblast Growth Factor 23 Activity.
FGF23 participates in systemic phosphate balance by its triple action on intestine, bone, and kidneys through interplay with Klotho (8), parathyroid hormone (PTH), and 1,25-dihydroxyvitamin D3 (8,12,31,77,–79) (Figures 2 and 4). High serum FGF23 may serve as a diagnostic biomarker and pathogenic intermediate for higher risk of cardiovascular disease and mortality in both patients with AKI (80) and patients with CKD (81). The fact that high FGF23 correlates with cardiac adverse effect only with concomitant low Klotho in animals suggests that Klotho may modulate the toxic effects of FGF23 (34).
Modulation of 1,25-Dihydroxyvitamin D3 Production.
Low 1,25-dihydroxyvitamin D3 is attributed to bone mineralization defects and secondary hyperparathyroidism in CKD (82). Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy (83) and vascular calcification in uremic rats (84) in part through upregulation of circulating Klotho.
Control of Parathyroid Hormone Production.
Secondary hyperparathyroidism is associated with cardiovascular morbidity and mortality in CKD (85,86). In CKD, parathyroid gland loses its response to inhibitory signaling from FGF23 possibly due to lower Klotho and FGFR1 in the parathyroid gland (Figure 2) (87–89). Correction of Klotho to improve mineral homeostasis does not only benefit bone and mineral metabolism but also may attenuate cardiovascular disease and improve the quality of life of patients with CKD (75).
Klotho Reduces Blood Pressure
Hypertension is a complication of CKD and contributes to progression of CKD and cardiovascular disease. Klotho-deficient mice develop salt-sensitive hypertension after high sodium challenge (90) and increased arterial stiffness (91). Klotho single-nucleotide polymorphism (KL rs9536314) associates with salt-sensitive hypertension in adults with newly diagnosed hypertension (92).
Biomarker Candidacy in Human Kidney Disease
Klotho in Human CKD
Kidney Klotho mRNA was significantly lower in patients with CKD versus healthy controls and positively correlated with eGFR (93,94). In CKD stages 1–5D with available kidney biopsies, Klotho mRNA levels positively correlated with eGFR, after adjustment for age and other mineral parameters (95). To date, many studies have shown a positive correlation between Klotho levels (serum and urine) and eGFR in adults with CKD (74,96–99). Soluble Klotho levels correlate with markers of oxidative stress (8-isoprostane) and inflammation (IL-6), although some of these associations dissipated after multivariable analyses (100). In a recent larger study, serum Klotho levels associated with kidney function decline in a cohort of well-functioning older adults aged 70–79 with mean eGFR of 73 ml/min per 1.73 m2 (101). With each doubling of serum Klotho, there was an associated 20% decreased odds of significant decline in kidney function over 10 years, which persisted after adjustment for demographics, cardiovascular risk factors, and mineral metabolism parameters, including FGF23 (101). The decline of soluble Klotho levels in adults with mild CKD (e.g., stages ≤2) antedates elevation in FGF23, PTH, and phosphate, and therefore, it may represent an early marker of CKD and CKD-MBD (Figure 3, Table 2) (96,102–104). A recent meta-analysis concluded that there is a significant positive correlation between soluble Klotho and eGFR in patients with CKD (105).
Table 2.
Human studies of soluble Klotho in CKD
| Study | Clinical Setting | Methods | Results/Observations |
|---|---|---|---|
| Pavik et al. (106) | 87 adults with CKD (stages 1–5) and 21 controls | Serum IBL ELISA | Adjusted mean Klotho decrease was 3.2 pg/ml for each 1-ml/min decrease in eGFR |
| Age and eGFR were independently associated with Klotho | |||
| Patients with PKD and kidney transplant recipients were excluded | |||
| Wan et al. (107) | 154 children with CKD (stages 1–5, 28 on KRT, 44 post-transplant) | Plasma IBL ELISA | Klotho levels decreased with decreasing eGFR but no independent association between Klotho and eGFR was found |
| Decreased Klotho was associated with increased FGF23 and PTH levels | |||
| Kitagawa et al. (97) | 114 adults with CKD | Serum IBL ELISA | Klotho was a significant determinant of arterial stiffness determined by ankle-brachial pulse wave velocity (adjusted OR per 100-pg/ml increase, 0.60; 95% CI, 0.39 to 0.98; P=0.008) |
| Positive correlation between Klotho levels and eGFR | |||
| Patients with established atherosclerotic complications (CAD, CHF, PVOD) or those treated with vitamin D or phosphate binders were excluded | |||
| Kim et al. (96) | 243 adults with CKD (stages 1–5) | Serum IBL ELISA | Klotho levels independently predicted the composite outcome of doubling SCr, ESKD, or death at median follow-up of 30 mo: adjusted HR per 10-pg/ml increase, 0.96; 95% CI, 0.94 to 0.98; P<0.001 |
| If serum Klotho was ≤396.3 pg/ml, 35.2% reached the composite outcome versus 15.7% if >396.3 pg/ml (P=0.03) | |||
| Klotho levels were lower at more advanced CKD stages (P value for trend <0.001) and correlated positively with eGFR and negatively with FGF23 and phosphate levels | |||
| Klotho was independently associated with eGFR (P<0.001) | |||
| Exclusion criteria consisted of KRT, organ transplantation, heart failure, cirrhosis, malignancy, pregnancy, acute coronary syndrome or ischemic stroke within 3 mo prior to the study, progressive CKD within 3 mo prior to the study | |||
| Akimoto et al. (99) | 131 adults with CKD (stages 1–5) | Serum and urine IBL ELISA | Positive correlation between serum and urine Klotho and eGFR |
| 24-h urine Klotho levels were independently associated with eGFR | |||
| Patients on long-term KRT were excluded | |||
| Seiler et al. (108) | 312 adults with CKD (stages 2–4) | Serum IBL ELISA | Klotho levels were significantly associated with age but not with eGFR |
| Klotho levels were not associated with the composite outcome of death or KRT initiation at mean follow-up of 2.2 yr | |||
| Seiler et al. (109) | 444 adults with CKD (stages 2–4) | Plasma IBL ELISA | Klotho levels (highest versus lowest tertile) did not predict atherosclerotic events/death (HR, 0.75; 95% CI, 0.43 to 1.30; P=0.30) or ADHF/death (HR, 0.81; 95% CI, 0.39 to 1.66; P=0.56) in adults with CKD at median follow-up of 2.6 yr |
| Events were adjudicated by two independent nephrologists | |||
| Ozeki et al. (98) | 185 adults with CKD (stages 1–5) | Serum IBL ELISA | eGFR was an independent predictor of Klotho levels, specifically when eGFR was <60 ml/min |
| Klotho levels were not significantly correlated with serum calcium or phosphate | |||
| Patients on long-term KRT were excluded | |||
| Cano et al. (110) | 31 children on PD and 45 healthy controls | Serum ELISA | Baseline Klotho levels were lower in children on PD versus controls (132±58 versus 320±119 pg/ml; P<0.001) and remained virtually unchanged throughout the observation period of 1 yr |
| Klotho levels did not correlate with FGF23 and phosphate levels | |||
| Park et al. (111) | 24 adults with HTN and CKD (12 with RVH and 12 EH) and 12 HV controls | Serum IBL ELISA | Klotho levels were significantly reduced in patients with HTN (mean eGFR =74.7 and 48.8 ml/min in EH and RVH, respectively) versus controls (mean eGFR =73 ml/min) after adjustment by eGFR |
| Klotho levels directly correlated with eGFR | |||
| Exclusion criteria consisted of eGFR<30 ml/min, uncontrolled BP, diabetes, recent cardiovascular event (within 6 mo), pregnancy, kidney transplant | |||
| Sawires et al. (112) | 40 children with CKD (stages 2–5), 44 patients with ESKD on HD, 40 kidney transplant recipients, and 40 HV controls | Serum ELISA | Serum calcium was an independent predictor of Klotho levels in all groups of kidney disease |
| Serum calcium inversely correlated with Klotho levels | |||
| Exclusion criteria consisted of marked hypocalcemia, dysfunctional HD access, combined organ transplantation, surgical parathyroidectomy, and sarcoidosis | |||
| Oh et al. (100) | 78 adults on PD and 30 HV controls | Serum IBL ELISA | Klotho levels were significantly lower in adults on PD versus HV controls: 329.6 versus 717.8; P<0.001 |
| 8-Isoprostane and IL-6 levels were inversely correlated with Klotho levels | |||
| 8-Isoprostane levels were independently associated with Klotho levels (P=0.04) | |||
| Shroff et al. (113) | ESCAPE cohort post hoc analysis, 167 children with CKD on ACEI | Serum IBL ELISA | ACEI therapy significantly increased Klotho levels without any associated changes in serum calcium or phosphate |
| Klotho levels did not correlate with eGFR at baseline (r=0.02; P=0.82) or at 8-mo follow-up (r=0.06; P=0.45) | |||
| Drew et al. (101) | 2496 adults within the Health ABC study (mean eGFR =73 ml/min) | Serum IBL ELISA | Klotho levels independently associated with 30% kidney function decline, with each doubling of Klotho associated with a 20% decreased odds of significant decline in kidney function over 10 yr |
| This association persisted after adjustment for demographics, cardiovascular disease risk factors, and mineral metabolism measures, including FGF23 | |||
| Memmos et al. (114) | 79 adults on HD | Plasma IBL ELISA | Lower Klotho levels (≤745 versus >745 pg/ml) independently associated with the composite of death/nonfatal MI or stroke at median follow-up of 5.5 yr (HR, 2.76; 95% CI, 1.22 to 6.22; P=0.01) after adjustment for cardiovascular disease risk factors and mineral disease parameters |
IBL, Immuno-Biologic Laboratories Co., Ltd.; PKD, polycystic kidney disease; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; OR, odds ratio; 95% CI, 95% confidence interval; CAD, coronary artery disease; CHF, congestive heart failure; PVOD, peripheral vascular occlusive disease; SCr, serum creatinine; HR, hazard ratio; ADHF, acutely decompensated heart failure; PD, peritoneal dialysis; HTN, hypertension; RVH, renovascular hypertension; EH, essential hypertension; HV, healthy volunteer; HD, hemodialysis; ESCAPE, Effect of Strict BP Control and ACE Inhibition on the Progression of CRF in Pediatric Patients; ACEI, angiotensin-converting enzyme inhibitor; ABC, Aging and Body Composition; MI, myocardial infarction.
In a cohort study of adult patients with CKD (stages 1–5), serum Klotho levels independently associated with the composite outcome of doubling serum creatinine, kidney failure, or death, after adjustment for age, diabetes, mean arterial pressure, eGFR, proteinuria, and PTH (96). Note that high-risk patients such as those with acute coronary syndrome, ischemic stroke, or progressive CKD within 3 months prior to the study enrollment were excluded.
Klotho and Cardiovascular Disease in Human CKD
Serum Klotho is associated with arterial stiffness measured by pulse wave velocity in patients with early CKD (97). In contrast, a different study in patients with CKD stages 2–4 showed that plasma Klotho levels were not associated with atherosclerosis, acute heart failure, or death at 2.6 years (109). Serum Klotho levels were lower in patients with primary hypertension compared with healthy volunteers, adjusted for eGFR (111). A post hoc analysis of the Effect of Strict BP Control and ACE Inhibition on the Progression of CRF in Pediatric Patients trial in children with CKD showed that ramipril therapy significantly increased serum Klotho levels without any changes in serum calcium or phosphate (113). In another study including adults with acute cerebral infarction, plasma Klotho levels were negatively associated with the presence, burden, and progression of small vessel disease (115). A recent study showed that low plasma Klotho is associated with mortality and cardiovascular events in patients on hemodialysis (114). The association between Klotho levels and cardiovascular disease has been observed only in small studies in humans mostly using surrogate markers of cardiovascular health rather than hard outcomes, such as cardiovascular events or death. Larger prospective studies with well-defined outcomes are needed to examine if Klotho is a prognostic marker of cardiovascular disease in CKD.
Klotho in AKI
In critically ill patients, urinary Klotho levels were lower in patients with AKI (Kidney Disease Improving Global Outcomes stage ≥2; within 48 hours of diagnosis) compared with well-matched intensive care unit controls (by age, sex, and baseline eGFR; within 48 hours of intensive care unit admission) (116). In a study of patients with acute tubular necrosis or acute tubulointerstitial nephritis by kidney biopsy, lower kidney Klotho protein levels associated with higher peak serum creatinine and more frequent KRT (117). In the kidney transplant population, donor kidney Klotho protein levels decreased following transplantation in those with delayed graft function (118). In postmortem kidney biopsies of critically ill patients who died of sepsis and AKI, there was lower kidney Klotho mRNA and protein in sepsis-associated AKI biopsies when compared with biopsies from control subjects undergoing nephrectomy for kidney cancer (119). Not all human studies in AKI have shown an inverse relationship between Klotho measurements and kidney function (Table 3).
Table 3.
Human studies of soluble Klotho in AKI
| Study | Clinical Setting | AKI Definition | Methods | Results/Observations |
|---|---|---|---|---|
| Hu et al. (50) | 17 adults with AKI (inpatient consults) and 14 HV | ↑ SCr≥50% or ≥2 mg/dl | Urine immunoblot | Mean urine Klotho/Cr levels were significantly lower in AKI versus HV (4.85±1.69 versus 25.38±4.08 fmol/mg of Cr; P=0.01) |
| Liu et al. (120) | 35 adults undergoing cardiac surgery (19 AKI) | AKIN ≥ stage 1 (SCr only) | Serum ELISA | Serum Klotho levels were lower in patients with AKI versus patients without AKI immediately after CS: mean ± SD, 102±17 versus 124±21 U/L; P=0.05. Klotho levels increased as early as day 1 post-CS such that levels were no longer different in patients with AKI versus patients without AKI |
| Torregrosa et al. (121) | 60 adults undergoing cardiac surgery or coronary angiography (30 AKI) | ↑ SCr≥50% | Urine IBL and SSBT ELISA | Urine samples were collected 12 h post-CS or post-CA (single time point). Urine Klotho/Cr was not different in patients with AKI versus no AKI (mean ± SD, 2.45±0.26 versus 2.04±0.20 ng/mg of Cr; P=>0.05 by SSBT and 1.60±0.30 versus 1.24±0.30; P>0.05 by IBL). No correlation between the two Klotho assays was found |
| Kim et al. (122) | 61 adults in the ICU or floor (42 prerenal and 19 intrinsic AKI) | AKIN ≥ stage 1 | Serum and urine IBL ELISA | Patients with prerenal and intrinsic AKI had similar SCr levels. Urine Klotho/Cr levels were lower in prerenal versus intrinsic AKI (mean ± SD, 174±292 versus 381±630 ng/g of Cr; P=0.001). There was no difference between groups in serum Klotho levels |
| Castellano et al. (118) | 30 adults undergoing kidney transplant (15 developed DGF) | DGF | Serum IBL ELISA | Patients with versus without DGF had lower levels of serum Klotho at 2 yr post-transplantation |
| Seibert et al. (123) | 46 hospitalized adults (30 AKI; 16 controls) | AKIN ≥ stage 1 | Serum IBL ELISA | Serum Klotho levels were higher in AKI (assessed at the time of nephrology consultation) versus control patients (mean ± SD, 567.6±294.4 versus 403.5±152.5 pg/ml; P=0.01). AKI and controls were not matched |
| Neyra et al. (116) | 106 adults in the ICU (54 AKI; 52 controls) | KDIGO ≥ stage 2 | Urine immunoblot | Urine Klotho/Cr levels assessed at a single time point (at time of AKI diagnosis [patients] or within 24 h of ICU admission [controls]) were significantly lower in AKI versus controls: median (IQR), 9.2 (3.0–33.6) versus 25.0 (6.0–92.8) fmol/mg of Cr; P<0.001. Each one-fold higher urine Klotho/Cr level was associated with an 83% (95% CI, 60% to 93%) lower risk of major adverse kidney events at 90 d (composite of death, KRT dependence, or decrease in eGFR≥50% from baseline). ICU controls were matched to patients by age, sex, and baseline eGFR |
HV, healthy volunteer; SCr, serum creatinine; Cr, creatinine; AKIN, Acute Kidney Injury Network; CS, cardiac surgery; IBL, Immuno-Biologic Laboratories Co., Ltd.; SSBT, Shangai Sunred Biologic Technology Co., Ltd.; CA, coronary angiography; ICU, intensive care unit; DGF, delayed graft function (defined as need for KRT within 7 days post-transplantation); KDIGO, Kidney Disease Improving Global Outcomes; IQR, interquartile range; 95% CI, 95% confidence interval.
In a cohort of 35 adults undergoing cardiac surgery, there was no difference in preoperative serum Klotho levels in those with (n=19) versus without (n=16) postoperative AKI. However, postoperative serum Klotho levels obtained immediately and 4 hours following cardiac surgery were both lower in patients with versus without AKI (120). Klotho levels increased soon in patients with AKI such that they were no longer different between groups by postoperative day 1 (120). In another study of 60 adults undergoing cardiac surgery or coronary angiography (50% developed postprocedure AKI), urinary Klotho levels were not different in patients with versus without AKI when measured 12 hours after the procedure (121). Neyra et al. (116) found significantly lower levels of urinary Klotho in critically ill patients who developed major adverse kidney events (death, dependence on KRT, or decrease in eGFR≥50% from baseline) within 90 days of enrollment than in those who did not. Each one-fold higher urinary Klotho level was associated with an adjusted 83% lower risk of developing the outcome (Table 3).
Collectively, current clinical data show great promise for soluble Klotho levels as not merely a diagnostic marker but as part of novel risk prediction tools of kidney-related outcomes in high-risk patients with CKD or AKI.
Measurement of Soluble Klotho in Humans
Human studies of measuring circulating soluble Klotho in serum, plasma, or urine have shown that acute or chronic kidney dysfunction is accompanied by lower soluble Klotho (102,103,116), but some studies have reported no change or even higher soluble Klotho depending on level of kidney function (16,97,99,106,109,123–125). These discordant findings may largely be due to problems with performance and reproducibility of current commercially available assays (126–128). An unfortunate fact is that variance in sample type, quality, collection and processing methods, vintage and conditions of storage, and number of freeze-thaw cycles can all drastically affect assay performance and yield widely disparate results (Table 4); however, precautions were not usually made to account for these confounders.
Table 4.
Considerations for soluble Klotho measurements in human samples
| Sample Processing | Klotho Assay |
|---|---|
| Sample type and quality | Methods of measurement (ELISA versus other) |
| Sample vintage (from sample collection to processing) | Reagents |
| Sample vintage (from sample storage to measurement) | Specificity of antibodies |
| Methods of collection | Additives (e.g., protease inhibitors) |
| Methods of storage | Interassay variability |
| Freeze-thaw cycles | Intra-assay variability |
| High-throughput capacity |
Barker et al. (102) showed that a commercial ELISA yielded different results in fresh versus stored serum samples and experienced a drop in capture of exogenous soluble Klotho in patients with advanced CKD as well as when samples have undergone freeze-thaw cycles. Heijboer et al. (126) showed poor interassay and intra-assay agreement between three different commercial Klotho ELISAs, suggesting that problems exist beyond just one ELISA. Measurement of soluble Klotho using commercial ELISA was highly unstable in human urine even when stored at –80°C (129). An alternative method of Klotho assay using immunoprecipitation-immunoblot uses a synthetic anti-Klotho antibody (high affinity for Kl2 domain of Klotho) for pull down and an anti-Kl1 rat mAb (now commercially available KM2076; TransGenic Inc., Kobe, Japan) (10) for detection by immunoblot (102). This assay showed progressive decline of serum Klotho levels with increasing stages of CKD in a small single-center study using fresh samples (102).
A recent study evaluated two methods of measuring serum Klotho in patients with different strata of kidney disease (130). The authors concluded that immunoprecipitation-immunoblot, compared with ELISA, exhibited a stronger direct correlation with eGFR, better recovery (capture) of added exogenous Klotho, less susceptibility to variability from sample additives (protease inhibitors), and much better differentiation across different kidney disease groups, including AKI and CKD, in reference to healthy volunteers. The immunoprecipitation-immunoblot assay performance also declined after multiple freeze-thaw cycles, favoring the use of never-thawed samples when measuring soluble Klotho.
Development of high-throughput Klotho assays and standardized processing and storage conditions will greatly benefit the field because suboptimal assays are populating the human database, continuously rendering the unification of data interpretation very difficult.
Klotho-Focused Therapies
Counteracting the decrease in Klotho that occurs in kidney disease represents a promising strategy to prevent, ameliorate, and reverse kidney disease and its extrarenal complications (Figure 4).
One successful strategy in the laboratory is the use of viral delivery of Klotho cDNA in rodent AKI (60). In murine models, Klotho cDNA plasmids can be directly injected or delivered via a viral carrier (nonpathogenic adeno-associated virus) to rescue many phenotypes of Klotho deficiency (131–134), although its safety in humans is not established. Experimental data revealed attenuation of kidney disease in hypertensive rats (131,135), improvement of kidney function in AKI (60), amelioration of angiotensin II–induced kidney injury (132), improvement of endothelial function (134), and protection from uremic cardiomyopathy in CKD models (45). Another method is by the minicircle DNA vectors, which allows sustained in vivo Klotho gene expression without risk of immunogenicity and confers kidney protection in models of ischemia reperfusion injury and unilateral ureteral obstruction (136).
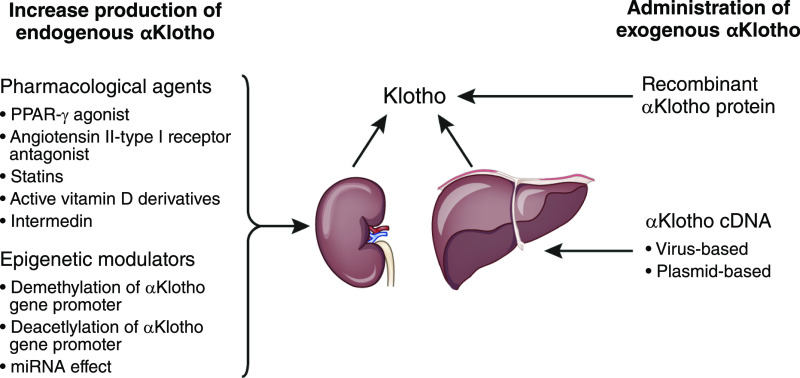
Another approach is to increase endogenous Klotho production by overcoming the mechanisms that suppress Klotho expression and/or release in kidney disease. In a CKD model, the vitamin D receptor agonist, paricalcitol, increased serum Klotho and reduced serum phosphate and FGF23. The source of increased soluble Klotho levels was not determined to be from the kidneys (84). Experimental upregulation of Klotho expression by 1,25-dihydroxyvitamin D3 was also described in other studies (137,138). One recent study showed that sevelamer carbonate increased serum Klotho in CKD (139). Other agents that can potentially increase endogenous Klotho expression are angiotensin II receptor antagonists, PPAR-γ agonists, androgen, and statins (Figure 5) (140–145). Thus far, the efficacy of these agents has only been demonstrated in preclinical studies, except for the use of valsartan, which was shown to increase plasma Klotho levels in patients with diabetic nephropathy following 24 weeks of therapy (143). Off-label use of already approved drugs to pharmacologically manipulate endogenous Klotho remains a viable option. It is important to recognize that pharmacologic interventions have not been tested in clinical trials of kidney disease as the primary outcome. A major hurdle is that the mechanism of how Klotho expression is upregulated needs to be defined. Whether these drugs can stimulate endogenous Klotho production/release in CKD where the Klotho-generating cells are extensively destroyed is unknown.
Figure 5.
Klotho-centric therapies in kidney disease, including increase in endogenous production or administration of exogenous Klotho protein or cDNA. miRNA, microRNA; PPAR-r, peroxisome proliferator-activated receptor gamma.
Another strategy is to target epigenetic modulation of endogenous Klotho expression. Although theoretically feasible, modulation of methylation and acetylation of the Klotho gene promoter needs further confirmation for its application in the management of human kidney disease.
Alternatively, administration of exogenous recombinant Klotho protein has dual effects: restoring soluble Klotho levels and promoting endogenous production of kidney Klotho. This dual action is particularly important in kidney failure, a status in which endogenous capacity is amputated. In a murine model, soluble Klotho injected 30 or 60 minutes after ischemic injury attenuated increase in serum creatinine postinjury and improved kidney histology (50). Further, repeated administration of Klotho starting 1 day after ischemic injury led to reduced fibrosis and improved recovery from AKI (16). Klotho replacement therapy remains the only practical method to control precise levels. Although recombinant Klotho replacement has been successful in many animal models, its application in humans still faces many hurdles.
Summary and Call for Action
Preclinical, translational, and clinical studies have advanced our understanding of Klotho biology, pathobiology, and potential clinical applications. Despite this accomplishment, the translation to clinical application has been slow. Klotho is an evolutionarily conserved housekeeping protein with a myriad of functions on many organs, which can serve as a biomarker for early diagnosis and/or risk stratification of patients with acute kidney disease or CKD. Because of its universal presence and panoply of function, specificity remains an issue (e.g., circulating Klotho levels may change in many diseases with or without kidney involvement).
Despite the emergence of promising human biomarker data of Klotho, additional large-scale and multicenter validation is needed. One difficulty to surpass before one can generate large reliable human databases is the standardization of methodology for soluble Klotho measurements. Given the imperfection of assays, one should exercise caution in interpretation of multiple small studies where kidney disease occurs under diverse clinical conditions and conclusions are made on the basis of variable sample conditions using different commercial reagents, most of which lack vigorous validation. Further, other factors known to influence soluble Klotho measurements, such as age, sex, inflammation, high-phosphate diet, FGF23, vitamin D supplementation, and certain medications, need to be carefully addressed in future prospective studies testing the biomarker candidacy of Klotho. Finally, there is a critical need to distinguish and characterize the subtypes of circulating Klotho protein because current assays do not differentiate between full-length soluble Klotho formed from cleavage of the transmembrane form (18–20), subsequent cleaved Kl1 and Kl2 Klotho fragments, and Klotho complexes with other circulating proteins. Therefore, with lack of knowledge as to which form of Klotho is most biologically relevant and/or captured with current assays, associations drawn between measured soluble Klotho levels and clinical outcomes should be circumspectly interpreted.
To date, no studies of Klotho protein administration in humans have been reported. However, ample animal studies have provided proof of concept that systemic Klotho protein administration is effective with seemingly good safety profiles. Because Klotho deficiency, regardless of etiology, could cause or accelerate dysfunction or degeneration in multiple systems or organs, Klotho is a valuable treatment modality of not just kidney disease but also its complications, in particular cardiovascular disease (Figure 4). The evolution of precision medicine leveraging clinical informatics along with genomic, proteomic, and metabolomic data will help the identification of high-risk patients to be targeted in Klotho-based therapeutic trials. The ongoing development of Klotho therapies and the evolution of more accurate and precise Klotho assays make these clinical trials foreseeable and no longer utopic in the near future. Clearly, bench and bedside interactive collaborative research is critically needed to develop and validate novel Klotho assays, examine its biomarker potential in diagnosis and prognosis, and identify effective therapeutic windows for Klotho administration in acute kidney disease and CKD. Pragmatic therapeutic trials in selected populations that are most likely to benefit from Klotho administration or modulation should be designed (Figure 6).
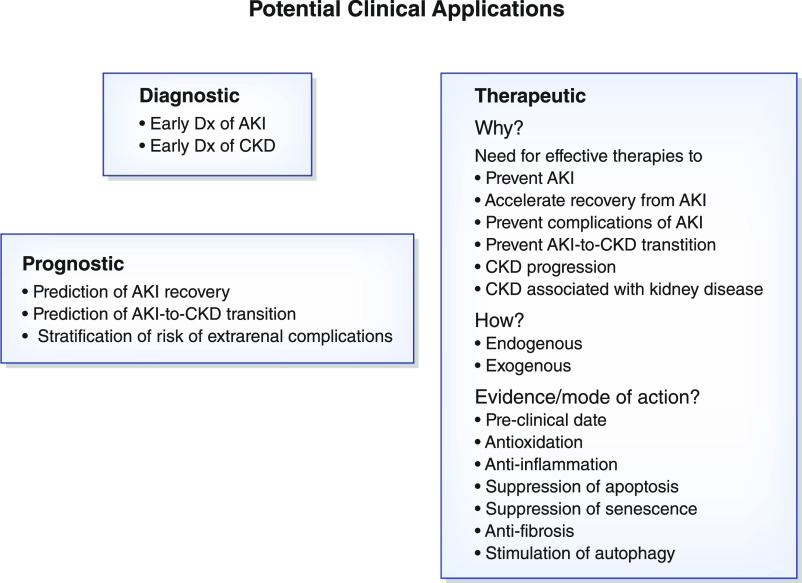
Figure 6.
Potential diagnostic, prognostic, and therapeutic applications of Klotho in clinical nephrology. Dx, diagnosis.
Disclosures
All authors have nothing to disclose.
Funding
M.C. Hu and O.W. Moe are supported by National Institutes of Health grants R01-DK091392 and R01-DK092461, the Charles and Jane Pak Center Innovative Research Support, and Endowed Professor Collaborative Research Support. O.W. Moe is supported by George O’Brien Kidney Research Center grant P30-DK-07938. J.A. Neyra is a recipient of National Center for Advancing Translational Sciences Early Career Pilot Grant UL1TR001998.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Ellidag HY, Yilmaz N, Kurtulus F, Aydin O, Eren E, Inci A, Dolu S, Ince FDA, Giray Ö, Yaman A: The three sisters of fate in multiple sclerosis: Klotho (Clotho), fibroblast growth factor-23 (Lachesis), and vitamin D (Atropos). Ann Neurosci 23: 155–161, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R: Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun 251: 920–925, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI: Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y: Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576: 341–345, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J: Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553: 501–505, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M: α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553: 461–466, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, Tsuchiya K: Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 302: F1252–F1264, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian A, Neyra JA, Zhan M, Hu MC: Klotho, stem cells, and aging. Clin Interv Aging 10: 1233–1243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC: αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 27: 2331–2345, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW: Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C: Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104: 19796–19801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR: Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 53: 5579–5587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y: Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424: 6–10, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Mencke R, Harms G, Moser J, van Meurs M, Diepstra A, Leuvenink HG, Hillebrands JL: Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight 2: e94375, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC: Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng MF, Chen LJ, Niu HS, Yang TT, Lin KC, Cheng JT: Signals mediating Klotho-induced neuroprotection in hippocampal neuronal cells. Acta Neurobiol Exp (Warsz) 75: 60–71, 2015. [PubMed] [Google Scholar]
- 25.Sun S, Cheng B, Sun PG, Wu XH, Wu QQ, He P: RTEF-1 protects against oxidative damage induced by H2O2 in human umbilical vein endothelial cells through Klotho activation. Exp Biol Med (Maywood) 240: 1606–1613, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW: α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307: L566–L575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, Wu S, Zhao W, Wu J: Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One 8: e57391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE: Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest 122: 4710–4715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW: Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91: 1104–1114, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neyra JA, Hu MC: αKlotho and chronic kidney disease. Vitam Horm 101: 257–310, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi M, Nakatani T, Lanske B, Razzaque MS: Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75: 1166–1172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-O M, Hill JA, Moe OW: Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 26: 1290–1302, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young GH, Wu VC: KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int 81: 611–612, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Sun CY, Chang SC, Wu MS: Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81: 640–650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P: Chronic kidney disease and premature ageing. Nat Rev Nephrol 10: 732–742, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, Hinman JD, Dedeoglu A, Rosene DL, Bansal R, Luebke JI, Kuro-o M, Abraham CR: The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci 33: 1927–1939, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Stein LR, Kim D, Ho K, Yu GQ, Zhan L, Larsson TE, Mucke L: Klotho controls the brain-immune system interface in the choroid plexus. Proc Natl Acad Sci U S A 115: E11388–E11396, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka S, Okusa MD: Crosstalk between the nervous system and the kidney. Kidney Int 97: 466–476, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, O’Brien R: Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett 558: 37–40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C: Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22: 1020–1032, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han X, Cai C, Xiao Z, Quarles LD: FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol 138: 66–74, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J: Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26: 2434–2446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB: The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 22: 1315–1325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D, Vandewalle A, Besselsen DG, Muhlbauer M, Jobin C, Kiela PR, Ghishan FK: Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology 138: 1384–1394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H: Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron, Exp Nephrol 101: e67–e74, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jorge LB, Coelho FO, Sanches TR, Malheiros DMAC, Ezaquiel de Souza L, Dos Santos F, de Sá Lima L, Scavone C, Irigoyen M, Kuro-O M, Andrade L: Klotho deficiency aggravates sepsis-related multiple organ dysfunction. Am J Physiol Renal Physiol 316: F438–F448, 2019. [DOI] [PubMed] [Google Scholar]
- 52.Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, Salimi S, Sierra F, de Cabo R: Measuring biological aging in humans: A quest. Aging Cell 19: e13080, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knoppert SN, Valentijn FA, Nguyen TQ, Goldschmeding R, Falke LL: Cellular senescence and the kidney: Potential therapeutic targets and tools. Front Pharmacol 10: 770, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T: Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Kuro-o M: Klotho as a regulator of oxidative stress and senescence. Biol Chem 389: 233–241, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Wu S, Ren H, Gu J: Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13: 254–262, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Zeng Y, Wang PH, Zhang M, Du JR: Aging-related renal injury and inflammation are associated with downregulation of Klotho and induction of RIG-I/NF-κB signaling pathway in senescence-accelerated mice. Aging Clin Exp Res 28: 69–76, 2016. [DOI] [PubMed] [Google Scholar]
- 58.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T: Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 339: 827–832, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H: Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11: 510–516, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M: Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T: Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine 31: 82–87, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Cui W, Leng B, Wang G: Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol 97: 370–376, 2019. [DOI] [PubMed] [Google Scholar]
- 65.Feng Y, He D, Yao Z, Klionsky DJ: The machinery of macroautophagy. Cell Res 24: 24–41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li P, Shi M, Maique J, Shaffer J, Yan S, Moe OW, Hu MC: Beclin 1/Bcl-2 complex-dependent autophagy activity modulates renal susceptibility to ischemia-reperfusion injury and mediates renoprotection by Klotho. Am J Physiol Renal Physiol 318: F772–F792, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grange C, Papadimitriou E, Dimuccio V, Pastorino C, Molina J, O’Kelly R, Niedernhofer LJ, Robbins PD, Camussi G, Bussolati B: Urinary extracellular vesicles carrying Klotho improve the recovery of renal function in an acute tubular injury model. Mol Ther 28: 490–502, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato S, Kawamata Y, Takahashi A, Imai Y, Hanyu A, Okuma A, Takasugi M, Yamakoshi K, Sorimachi H, Kanda H, Ishikawa Y, Sone S, Nishioka Y, Ohtani N, Hara E: Ablation of the p16(INK4a) tumour suppressor reverses ageing phenotypes of klotho mice. Nat Commun 6: 7035, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polichnowski AJ: Microvascular rarefaction and hypertension in the impaired recovery and progression of kidney disease following AKI in preexisting CKD states. Am J Physiol Renal Physiol 315: F1513–F1518, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazzotta C, Manetti M, Rosa I, Romano E, Blagojevic J, Bellando-Randone S, Bruni C, Lepri G, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M: Proangiogenic effects of soluble α-Klotho on systemic sclerosis dermal microvascular endothelial cells. Arthritis Res Ther 19: 27, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H: Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A 107: 19308–19313, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li S, Yu L, He A, Liu Q: Klotho inhibits unilateral ureteral obstruction-induced endothelial-to-mesenchymal transition via TGF-β1/Smad2/Snail1 signaling in mice. Front Pharmacol 10: 348, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neyra JA, Hu MC: Potential application of klotho in human chronic kidney disease. Bone 100: 41–49, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu X, Hu MC: Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis 3: 15–23, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu MC, Shi M, Moe OW: Role of αKlotho and FGF23 in regulation of type II Na-dependent phosphate co-transporters. Pflugers Arch 471: 99–108, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ide N, Ye R, Courbebaisse M, Olauson H, Densmore MJ, Larsson TE, Hanai JI, Lanske B: In vivo evidence for an interplay of FGF23/Klotho/PTH axis on the phosphate handling in renal proximal tubules. Am J Physiol Renal Physiol 315: F1261–F1270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leaf DE, Siew ED, Eisenga MF, Singh K, Mc Causland FR, Srivastava A, Ikizler TA, Ware LB, Ginde AA, Kellum JA, Palevsky PM, Wolf M, Waikar SS: Fibroblast growth factor 23 associates with death in critically ill patients. Clin J Am Soc Nephrol 13: 531–541, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodelo-Haad C, Santamaria R, Muñoz-Castañeda JR, Pendón-Ruiz de Mier MV, Martin-Malo A, Rodriguez M: FGF23, biomarker or target? Toxins (Basel) 11: 175, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gutiérrez OM: Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clin J Am Soc Nephrol 5: 1710–1716, 2010. [DOI] [PubMed] [Google Scholar]
- 83.Leifheit-Nestler M, Grabner A, Hermann L, Richter B, Schmitz K, Fischer DC, Yanucil C, Faul C, Haffner D: Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant 32: 1493–1503, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82: 1261–1270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carrillo-López N, Panizo S, Alonso-Montes C, Martínez-Arias L, Avello N, Sosa P, Dusso AS, Cannata-Andía JB, Naves-Díaz M: High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol Dial Transplant 34: 934–941, 2019. [DOI] [PubMed] [Google Scholar]
- 86.Isakova T, Cai X, Lee J, Mehta R, Zhang X, Yang W, Nessel L, Anderson AH, Lo J, Porter A, Nunes JW, Negrea L, Hamm L, Horwitz E, Chen J, Scialla JJ, de Boer IH, Leonard MB, Feldman HI, Wolf M; CRIC Study Investigators : Longitudinal evolution of markers of mineral metabolism in patients with CKD: The chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 75: 235–244, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T: Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77: 211–218, 2010. [DOI] [PubMed] [Google Scholar]
- 88.Kuro-O M: Klotho and endocrine fibroblast growth factors: Markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant 34: 15–21, 2019. [DOI] [PubMed] [Google Scholar]
- 89.Patel S, Barron JL, Mirzazedeh M, Gallagher H, Hyer S, Cantor T, Fraser WD: Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab 29: 71–79, 2011. [DOI] [PubMed] [Google Scholar]
- 90.Zhou X, Chen K, Lei H, Sun Z: Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z: Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension 68: 1191–1199, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Citterio L, Delli Carpini S, Lupoli S, Brioni E, Simonini M, Fontana S, Zagato L, Messaggio E, Barlassina C, Cusi D, Manunta P, Lanzani C: Klotho gene in human salt-sensitive hypertension. Clin J Am Soc Nephrol 15: 375–383, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y: Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539–547, 2012. [DOI] [PubMed] [Google Scholar]
- 94.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y: Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 9: e86301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo TH, Kang SW, Han DS, Han SH: Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis 61: 899–909, 2013. [DOI] [PubMed] [Google Scholar]
- 97.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H: A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8: e56695, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ozeki M, Fujita S, Kizawa S, Morita H, Sohmiya K, Hoshiga M, Ishizaka N: Association of serum levels of FGF23 and α-Klotho with glomerular filtration rate and proteinuria among cardiac patients. BMC Nephrol 15: 147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, Ito C, Shiizaki K, Ando Y, Muto S, Kuro-o M, Kusano E: Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 13: 155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oh HJ, Nam BY, Lee MJ, Kim CH, Koo HM, Doh FM, Han JH, Kim EJ, Han JS, Park JT, Yoo TH, Kang SW, Han DS, Han SH: Decreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Perit Dial Int 35: 43–51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drew DA, Katz R, Kritchevsky S, Ix J, Shlipak M, Gutiérrez OM, Newman A, Hoofnagle A, Fried L, Semba RD, Sarnak M: Association between soluble klotho and change in kidney function: The health aging and body composition study. J Am Soc Nephrol 28: 1859–1866, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS: The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012. [DOI] [PubMed] [Google Scholar]
- 104.Rotondi S, Pasquali M, Tartaglione L, Muci ML, Mandanici G, Leonangeli C, Sales S, Farcomeni A, Mazzaferro S: Soluble α -klotho serum levels in chronic kidney disease. Int J Endocrinol 2015: 872193, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Q, Su W, Shen Z, Wang R: Correlation between soluble α-Klotho and renal function in patients with chronic kidney disease: A review and meta-analysis. BioMed Res Int 2018: 9481475, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted klotho and FGF23 in chronic kidney disease stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013. [DOI] [PubMed] [Google Scholar]
- 107.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R: Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 28: 153–161, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, Weihrauch A, Fliser D, Heine GH: Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 83: 121–128, 2013. [DOI] [PubMed] [Google Scholar]
- 109.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, Floege J, Fliser D, Heine GH: Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol 9: 1049–1058, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cano FJ, Freundlich M, Ceballos ML, Rojo AP, Azocar MA, Delgado IO, Ibacache MJ, Delucchi MA, Lillo AM, Irarrázabal CE, Ugarte MF: Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J 7: 457–463, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park MY, Herrmann SM, Saad A, Eirin A, Tang H, Lerman A, Textor SC, Lerman LO: Biomarkers of kidney injury and klotho in patients with atherosclerotic renovascular disease. Clin J Am Soc Nephrol 10: 443–451, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sawires HK, Essam RM, Morgan MF, Mahmoud RA: Serum klotho: Relation to fibroblast growth factor-23 and other regulators of phosphate metabolism in children with chronic kidney disease. Nephron 129: 293–299, 2015. [DOI] [PubMed] [Google Scholar]
- 113.Shroff R, Aitkenhead H, Costa N, Trivelli A, Litwin M, Picca S, Anarat A, Sallay P, Ozaltin F, Zurowska A, Jankauskiene A, Montini G, Charbit M, Schaefer F, Wühl E; ESCAPE Trial Group : Normal 25-hydroxyvitamin D levels are associated with less proteinuria and attenuate renal failure progression in children with CKD. J Am Soc Nephrol 27: 314–322, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, Vasilikos V, Papagianni A: Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol 20: 217, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woo HG, Chang Y, Ryu DR, Song TJ: Plasma Klotho concentration is associated with the presence, burden and progression of cerebral small vessel disease in patients with acute ischaemic stroke. PLoS One 14: e0220796, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neyra JA, Li X, Mescia F, Ortiz-Soriano V, Adams-Huet B, Pastor J, Hu M-C, Toto RD, Moe OW: Urine klotho is lower in critically ill patients with versus without acute kidney injury and associates with major adverse kidney events. Crit Care Explor 1: e0016, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seo MY, Yang J, Lee JY, Kim K, Kim SC, Chang H, Won NH, Kim MG, Jo SK, Cho W, Kim HK: Renal Klotho expression in patients with acute kidney injury is associated with the severity of the injury. Korean J Intern Med (Korean Assoc Intern Med) 30: 489–495, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, Franzin R, Accetturo M, Zito A, Fiorentino M, Montinaro V, Lucarelli G, Ditonno P, Battaglia M, Crovace A, Staffieri F, Oortwijn B, van Amersfoort E, Pertosa G, Grandaliano G, Gesualdo L: Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant 16: 325–333, 2016. [DOI] [PubMed] [Google Scholar]
- 119.Jou-Valencia D, Molema G, Popa E, Aslan A, van Dijk F, Mencke R, Hillebrands JL, Heeringa P, Hoenderop JG, Zijlstra JG, van Meurs M, Moser J: Renal klotho is reduced in septic patients and pretreatment with recombinant klotho attenuates organ injury in lipopolysaccharide-challenged mice. Crit Care Med 46: e1196–e1203, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu YJ, Sun HD, Chen J, Chen MY, Ouyang B, Guan XD: Klotho: A novel and early biomarker of acute kidney injury after cardiac valve replacement surgery in adults. Int J Clin Exp Med 8: 7351–7358, 2015. [PMC free article] [PubMed] [Google Scholar]
- 121.Torregrosa I, Montoliu C, Urios A, Giménez-Garzó C, Tomás P, Solís MA, Ramos C, Juan I, Puchades MJ, Saez G, Blasco ML, Miguel A: Urinary Klotho measured by ELISA as an early biomarker of acute kidney injury in patients after cardiac surgery or coronary angiography. Nefrologia 35: 172–178, 2015. [DOI] [PubMed] [Google Scholar]
- 122.Kim AJ, Ro H, Kim H, Chang JH, Lee HH, Chung W, Jung JY: Klotho and S100a8/A9 as discriminative markers between pre-renal and intrinsic acute kidney injury. PLoS One 11: e0147255, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seibert E, Radler D, Ulrich C, Hanika S, Fiedler R, Girndt M: Serum klotho levels in acute kidney injury. Clin Nephrol 87: 173–179, 2017 [DOI] [PubMed] [Google Scholar]
- 124.Devaraj S, Syed B, Chien A, Jialal I: Validation of an immunoassay for soluble klotho protein: Decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol 137: 479–485, 2012. [DOI] [PubMed] [Google Scholar]
- 125.Kim Y, Kim JH, Nam YJ, Kong M, Kim YJ, Yu KH, Lee BC, Lee C: Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett 407: 189–194, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG; NIGRAM consortium : Laboratory aspects of circulating α-Klotho. Nephrol Dial Transplant 28: 2283–2287, 2013. [DOI] [PubMed] [Google Scholar]
- 127.Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM: Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46: 1079–1083, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y: Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adema AY, Vervloet MG, Blankenstein MA, Heijboer AC: α-Klotho is unstable in human urine. Kidney Int 88: 1442–1444, 2015. [DOI] [PubMed] [Google Scholar]
- 130.Neyra JA, Moe OW, Pastor J, Gianella F, Sidhu SS, Sarnak MJ, Ix JH, Drew DA: Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J 13: 235–244, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002. [DOI] [PubMed] [Google Scholar]
- 133.Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, Noda M, Kuro-o M, Nabeshima Y: Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med 2: 233–242, 2000. [DOI] [PubMed] [Google Scholar]
- 134.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R: In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 276: 767–772, 2000. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Sun Z: Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens 32: 1629–1636, 2014. [DOI] [PubMed] [Google Scholar]
- 136.Shin YJ, Luo K, Quan Y, Ko EJ, Chung BH, Lim SW, Yang CW: Therapeutic challenge of minicircle vector encoding klotho in animal model. Am J Nephrol 49: 413–424, 2019. [DOI] [PubMed] [Google Scholar]
- 137.Muñoz-Castañeda JR, Herencia C, Pendón-Ruiz de Mier MV, Rodriguez-Ortiz ME, Diaz-Tocados JM, Vergara N, Martínez-Moreno JM, Salmerón MD, Richards WG, Felsenfeld A, Kuro-O M, Almadén Y, Rodríguez M: Differential regulation of renal Klotho and FGFR1 in normal and uremic rats. FASEB J 31: 3858–3867, 2017. [DOI] [PubMed] [Google Scholar]
- 138.Takenaka T, Inoue T, Ohno Y, Miyazaki T, Nishiyama A, Ishii N, Suzuki H: Calcitriol supplementation improves endothelium-dependent vasodilation in rat hypertensive renal injury. Kidney Blood Press Res 39: 17–27, 2014. [DOI] [PubMed] [Google Scholar]
- 139.Liabeuf S, Ryckelynck JP, El Esper N, Ureña P, Combe C, Dussol B, Fouque D, Vanhille P, Frimat L, Thervet E, Mentaverri R, Prié D, Choukroun G; FRENCH Study collaborators : Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol 12: 1930–1940, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N: Klotho is a target gene of PPAR-gamma. Kidney Int 74: 732–739, 2008. [DOI] [PubMed] [Google Scholar]
- 141.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB: The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20: 2380–2388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narumiya H, Sasaki S, Kuwahara N, Irie H, Kusaba T, Kameyama H, Tamagaki K, Hatta T, Takeda K, Matsubara H: HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc Res 64: 331–336, 2004. [DOI] [PubMed] [Google Scholar]
- 143.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L: Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 8: 1899–1905, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M, Yang CW: Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant 26: 800–813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hsu SC, Huang SM, Lin SH, Ka SM, Chen A, Shih MF, Hsu YJ: Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J 464: 221–229, 2014. [DOI] [PubMed] [Google Scholar]