Abstract
Rationale
N-acylethanolamine acid amidase (NAAA) is an intracellular cysteine hydrolase that terminates the biological actions of oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), two endogenous lipid-derived agonists of the nuclear receptor, peroxisome proliferator-activated receptor-α. OEA and PEA are important regulators of energy balance, pain and inflammation, but recent evidence suggests that they might also contribute to the control of reward-related behaviors.
Objectives and Methods
In the present study, we investigated the effects of systemic and intracerebral NAAA inhibition in the two-bottle choice model of voluntary alcohol drinking and on operant alcohol self-administration.
Results
Intraperitoneal injections of the systemically active NAAA inhibitor ARN19702 (3 and 10 mg/kg) lowered voluntary alcohol intake in a dose-dependent manner, achieving ≈ 47% reduction at the 10 mg/kg dose (p<0.001). Water, food or saccharin consumption was not affected by the inhibitor. Similarly, ARN19702 dose-dependently attenuated alcohol self-administration under both fixed-ratio 1 (FR-1) and progressive ratio schedules of reinforcement. Furthermore, microinjection of ARN19702 (1, 3 and 10 μg/μl) or of two chemically different NAAA inhibitors, ARN077 and ARN726 (both at 3 and 10 μg/μl), into the midbrain ventral tegmental area produced dose-dependent decreases in alcohol self-administration under FR-1 schedule. Microinjection of ARN19702 into the nucleus accumbens had no such effect.
Conclusion
Collectively, the results point to NAAA as a possible molecular target for the treatment of alcohol use disorder.
Introduction
The amides of long-chain fatty acids with ethanolamine (fatty acid ethanolamides, FAEs) are a family of lipid-derived signaling molecules that participate in the control of various physiological and pathological processes, including energy balance, pain initiation and inflammation (Calignano et al. 1998; Fotio et al. 2019; Piomelli 2003; Piomelli and Sasso 2014; Rodriguez de Fonseca et al. 2001). Two members of this family, palmitoylethanolamide (PEA) and oleoylethanolamide (OEA), are produced in various cell types by the action of the zinc-containing hydrolase, N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) (Okamoto et al. 2004), and exert antinociceptive and anti-inflammatory effects in animal models and in humans (Artukoglu et al. 2017; Gabrielsson et al. 2016; LoVerme et al. 2006; Suardiaz et al. 2007). Such effects are primarily due to the ability of PEA and OEA to engage the ligand-operated transcription factor, peroxisome proliferator-activated receptor-α (PPARα) (Fu et al. 2003; LoVerme et al. 2005).
A growing body of evidence suggests that OEA and PEA might also participate in the regulation of reward-related behaviors (Coppola and Mondola 2013; Grosshans et al. 2014; Melis et al. 2008). For example, habit-forming substances such as alcohol and nicotine may alter OEA and PEA content in brain regions involved in reward (Bilbao et al. 2016; Buczynski et al. 2013; Melis et al. 2008), while exogenous administration of these compounds has been shown to reduce the intake of alcohol and nicotine (Bilbao et al. 2016; Melis et al. 2008). In addition, OEA and PEA may prevent the activation of dopaminergic neurons in the ventral tegmental area (VTA), a key node in the brain reward circuitry (Gilpin and Koob 2008; Ikemoto 2007; 2010), through a PPARα-dependent mechanism (Melis et al. 2008).
The actions of OEA and PEA are terminated via enzyme-mediated hydrolysis, which can be catalyzed by either of two distinct intracellular enzymes: N-acylethanolamine acid amidase (NAAA) and fatty acid amide hydrolase (FAAH) (Piomelli et al. 2020a). Even though NAAA and FAAH share the ability to cleave lipid-amide bonds, they differ in primary structure, substrate selectivity and cellular localization. NAAA is an N-terminal nucleophile cysteine amidase that displays a strong preference for PEA and, to a lesser extent, OEA (Tsuboi et al. 2005). FAAH, on the other hand, is a member of the serine amidase signature family of enzymes and preferentially hydrolyzes polyunsaturated FAEs such as the endocannabinoid anandamide (Cravatt et al. 1996; Desarnaud et al. 1995). Moreover, NAAA is primarily localized to the endosomal/lysosomal compartment of macrophages and other immune cells (Tsuboi et al. 2007), whereas FAAH is found on the outer face of mitochondria and endoplasmic reticulum of most mammalian cells (Gulyas et al. 2004; McKinney and Cravatt 2005).
While there is a growing appreciation for the role of FAAH in the control of the motivational and rewarding properties of alcohol (Best et al. 2020; Cippitelli et al. 2008; Stopponi et al. 2018; Zhou et al. 2017), it is still unknown whether inhibition of NAAA activity might also regulate these properties. To address this question, in the present study we utilized the benzothiazole derivative ARN19702, a selective and reversible NAAA inhibitor that is able to access the brain (Migliore et al. 2016; Piomelli et al. 2020a). Our results show that systemic administration of ARN19702 decreased voluntary alcohol drinking and alcohol self-administration in male alcohol-preferring Marchigian Sardinian rats. We further found that microinjection into the VTA of three chemically distinct NAAA inhibitors – ARN19702, ARN077 or ARN726 (Piomelli et al. 2020) – reduced alcohol self-administration, whereas microinjection of ARN19702 into the nucleus accumbens (NAc) had no such effect. The results point to NAAA as a possible molecular target for the treatment of alcohol use disorder (AUD).
Materials and Methods
Animals
Ten to eleven-week-old male Marchigian Sardinian alcohol-preferring (msP) rats (Ntotal = 98) weighing 250–280g were used for this study. This strain is characterized by spontaneous alcohol binge drinking and is highly vulnerable to reinstatement from stress or presentation of environmental stimuli that predict alcohol availability (Ciccocioppo et al. 2006). MsP rats were bred and housed in a reverse 12h light/dark cycle at the vivarium of the University of Camerino, at controlled temperature (22°C) and humidity (55%). Food (4RF18, Mucedola, Settimo Milanese, Italy) and water were available ad libitum. Before starting the experiments, animals were pair housed in conventional clear plastic cage and standard bedding. The experiments were conducted during the dark phase of the light/dark cycle and procedures were carried out in accordance with directives on care and use of laboratory animals of the European Community Council and the National Institutes of Health. Formal approval was obtained from the Italian Ministry of Health and the Internal Ethical Committee for the Laboratory Animal Protection and Use of the University of Camerino. All efforts were made to minimize animals suffering and distress.
Chemicals and treatments
Alcohol (95%, FL Carsetti SNC, Italy) and saccharin (Sigma, Italy) were dissolved in tap water to obtain final solutions at 10% (v/v) and 0.2% (w/v) respectively. The NAAA inhibitors ARN19702, ARN726 and ARN077 were synthesized as described (Migliore et al. 2016). ARN077 and ARN726 are defined by the presence of a chemical warhead – β-lactone (ARN077) or β-lactam (ARN726)– that can react covalently with NAAA’s catalytic cysteine to form a thioester bond. While these compounds are potent and selective for NAAA, the reactive warhead lowers their metabolic stability and limit their use as oral drugs. ARN19702 instead is a benzothiazole-piperazine derivatives that inhibits NAAA in a potent and selective manner via a non-covalent mechanism. Moreover, it displays high oral bioavailability and crosses the blood-brain barrier (Bandiera et al. 2014; Piomelli et al. 2020). For these reasons we used ARN19702 as our inhibitor of choice. The later was dissolved in a mixture of polyethylene glycol-Tween 80-distilled water (15%−15%−70%, by volume) and was administered at the doses of 3 and 10 mg/kg intraperitoneally (i.p.) 1 h prior to behavioral testing. For intracranial treatments, ARN726 and ARN077 were dissolved in a mixture of dimethyl sulfoxide-Tween 80-distilled water (5%−5%−90%, by volume) and delivered into the ventral tegmental area (VTA) and the nucleus accumbens (NAc) through a stainless-steel injector that was 1.5 mm longer than the guide cannula. A volume of 0.5 μl of solution per site was injected bilaterally into these brain areas, using a 10 μl injection syringe (Hamilton® 1700) mounted on an automated micropump (Harvard Apparatus®, Pump 11 Elite).
Intracranial surgeries
Animals were anesthetized by intramuscular injection (100–150 μl) of a solution containing tiletamine (58.17 mg/ml) and zolazepam (7.5 mg/ml). Bilateral guide cannulae (0.65 mm outside diameter) aimed at the VTA and NAc were implanted and cemented onto the skull. Stereotaxic coordinates (from bregma) of the VTA (anterior/posterior: −5.8 mm; medial/lateral: +/− 2.2 mm; dorsal/ventral: −7.4 mm; Angle 12°) and NAc (anterior/posterior: +1.2 mm; medial/lateral: +/−1 mm; dorsal/ventral: −6.1 mm) were chosen according to recent reports (Borruto et al. 2020; Stopponi et al. 2018). Following surgeries, animals received a single injection of ketoprofen (2.5 mg/kg, subcutaneous, s.c.) and were allowed to recover for 1 week in their home cage. During this period, rats were handled daily and habituated to the injection procedure, which consisted of inserting a stainless-steel injector unit into the guide cannulas, for at least 3 days prior to the beginning of tests. The injector was left in place for 20 s after injection to allow diffusion of the solution to occur. After completion of the experiments, animals were anesthetized with isoflurane and black India ink (0.5 μl) was injected into the brain region of interest. The animals were then immediately euthanized and brains were collected for histological analysis of cannula placement.
Two-bottle choice paradigm
The two-bottle choice (2-BC) paradigm (free choice between water and 10% alcohol) was used to measure voluntary alcohol drinking and preference (Cannella et al. 2019; Leeman et al. 2010). Animals were single housed in experimental chambers (30 cm long × 30 cm wide × 30 cm high) for a week of habituation before the beginning of the test. They were given free access to water and alcohol (10% by volume) for the following 15 days to establish a stable baseline and preference for alcohol (80–90% preference vs water). Fluids were offered through graduated drinking tubes equipped with metallic spouts and intake was measured by reading the volume consumed at specific time points (2, 8 and 24 h) following initiation of the active phase. The drinking tubes were switched daily to avoid the development of side preference. Animals had also free access to food and its consumption was measured by weighing the food container and considering spillage weight. Alcohol, water, and food intakes were calculated as absolute values of consumption at each time interval and were expressed as g/kg body weight (Devgun and Dunbar 1990).
Operant Alcohol and Saccharin Self-Administration
Operant chambers were used on a daily based 30-min session to establish alcohol and saccharin self-administration under fixed-ratio one (FR-1) schedule of reinforcement (Domi et al. 2019; Fotio et al. 2020; Stopponi et al. 2018). Each chamber was equipped with an active and an inactive lever symmetrically centered on the side panel. Responding at the active lever activated the infusion pump (Razel Sci., Stamford, CT) and released 0.1 ml of alcohol (10 % v/v) or saccharin (0.2 % w/v) in a liquid drop receptacle located between the two levers. Presses at the inactive control lever were recorded, but did not activate the infusion pump. During the infusion a stimulus light located above the active lever was turned on for 5 s, considered as the time-out period. Lever pressing during the time-out was scored, but did not lead to further infusions. When rats achieved a stable baseline of self-administration for both alcohol and saccharin over the last 3 days of training, we evaluated the effects of intracranial microinfusions of NAAA inhibitors every 4 days using a counterbalance Latin squared design.
Progressive ratio schedule of reinforcement
In this experiment, msP rats (n=11) were tested under a progressive ratio (PR) schedule of reinforcement to measure their motivation for alcohol intake. Animals were first trained to self-administer 10% alcohol solution under an FR-1 schedule of reinforcement. After acquisition of a stable baseline of alcohol responding, the animals went through the PR schedule in which the number of lever responses or the ratio required to receive one infusion of alcohol was increased according to the following progression: for the first 4 alcohol deliveries the ratio was increased by 1; for the next 4 deliveries, the ratio increased by 2 and for the all the following deliveries the ratio was increased by 4. Hence, the arithmetic progression of the ratio was as follows: 1, 1, 1, 1, 2, 2, 2, 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52, 56, 60 etc. (Economidou et al. 2006b; Fotio et al. 2020). Sessions were automatically terminated 30 min after the last reinforced response. The point in the series at which responding ceases is termed ‘break point’ and presumably reflects the maximum effort that an animal is willing to perform to earn a reward (Arnold and Roberts 1997; Hodos 1961).
Statistical analyses
Data analysis was planned as part of the experimental protocol (Michel et al. 2020). Results are expressed as mean ± S.D and analysis of variance (ANOVA) was followed by a Dunnett’s multiple comparisons test when appropriate. All experiments were conducted in a counterbalanced Latin-squared design. Effect of NAAA inhibition on voluntary alcohol drinking was assessed by two-way repeated-measures ANOVA with “time” and “treatment” as within-subject factors. Effects of systemic or intracranial NAAA inhibition on alcohol and saccharin self-administration were analyzed by 1-way repeated measures ANOVA with “treatment” as a within-subject factor. During intracranial treatments, only data collected from rats with cannulas correctly placed were included in statistical analyses. Statistical significance was set at p < 0.05 vs vehicle. Analyses were performed using GraphPad prism version 8.0 (GraphPad Software Inc., San Diego, CA).
Results
Effects of systemic NAAA inhibition on two-bottle choice alcohol drinking
To investigate the impact of NAAA blockade on voluntary alcohol drinking, we used the two bottle-free choice (2-BC) paradigm, in which msP rats (n=10) had free choice between water and alcohol (10% by volume). As shown in Fig. 1A, alcohol drinking was decreased in a dose- and time-dependent manner by administration of the systemically active NAAA inhibitor ARN19702 (3 and 10 mg/kg, i.p.). The magnitude of this effect was not correlated with the baseline alcohol drinking (data not shown). ANOVA revealed a significant effect of time [F (2, 18) = 174.2; ***p < 0.0001], treatment [F (2, 18) = 12.76; ***p < 0.001] and their interaction [F (4, 36) = 9.752; ***p < 0.0001]. A Dunnett’s post hoc test demonstrated that the effect of ARN19702 on voluntary alcohol drinking was statistically detectable 24 h after treatment. Moreover, the effect could not be attributed to a non-selective reduction in activity, since the drug did not alter water intake [Fig. 1B; time: F (2, 18) = 8.983; **p < 0.01, treatment: F (2, 18) = 0.3532; p = 0.7072 (ns) and their interaction: F (4, 36) = 0.7396; p = 0.5712 (ns)] or food intake [Fig. 1C; time: F (2, 18) = 103.5; ***p < 0.0001, treatment :F (2, 18) = 0.4184; p = 0.6643 (ns) and their interaction: F (4, 36) = 0.3814; p = 0.8204 (ns)].
Figure 1:
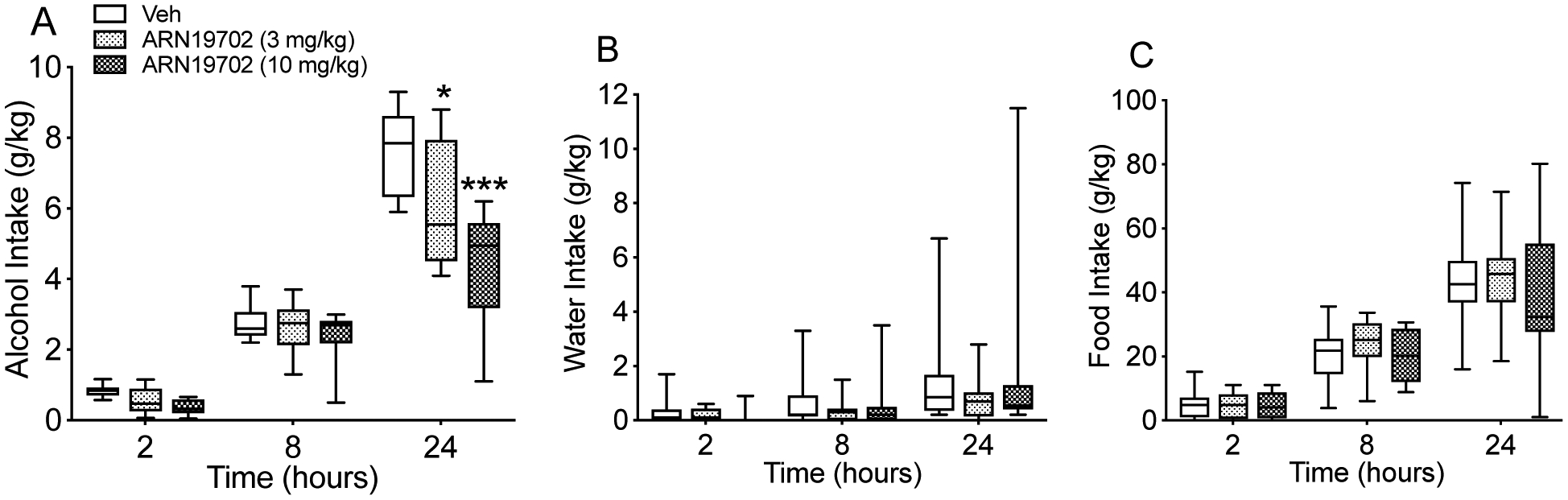
Effects of systemic NAAA inhibitor ARN19702 (3 and 10 mg/kg, i.p.) on voluntary alcohol drinking. MsP (n=10) rats received the drug 1 h before testing. Voluntary intake of (A) alcohol, (B) water and (C) food were monitored at various time points after treatment (2, 8 and 24 h). Results are expressed in g/kg of body weight and represented as Box and Whiskers (5–95 percentile). *p < 0.05 and ***p < 0.001 vs vehicle controls (Veh).
Effects of systemic NAAA inhibition on fixed ratio alcohol and saccharin self-administration
To further assess the impact of NAAA inhibition on alcohol intake, we treated msP rats (n=13) with ARN19702 (3 and 10 mg/kg, i.p.) or its vehicle in a counterbalanced Latin squared design, and then let the rats undergo the alcohol self-administration protocol. Fig. 2A shows that the animals acquired robust alcohol self-administration under an FR-1 schedule of reinforcement, which was significantly attenuated by ARN19702 [F (2, 24) = 14.89; ***p < 0.0001]. A Dunnett’s post hoc test revealed that the effect of ARN19702 on FR-1 alcohol self-administration was dose-dependent. Responses at the inactive lever were negligible and were not affected by NAAA blockade [F (2, 24) = 0.7347; p = 0.4901; Fig. 2B].
Figure 2:
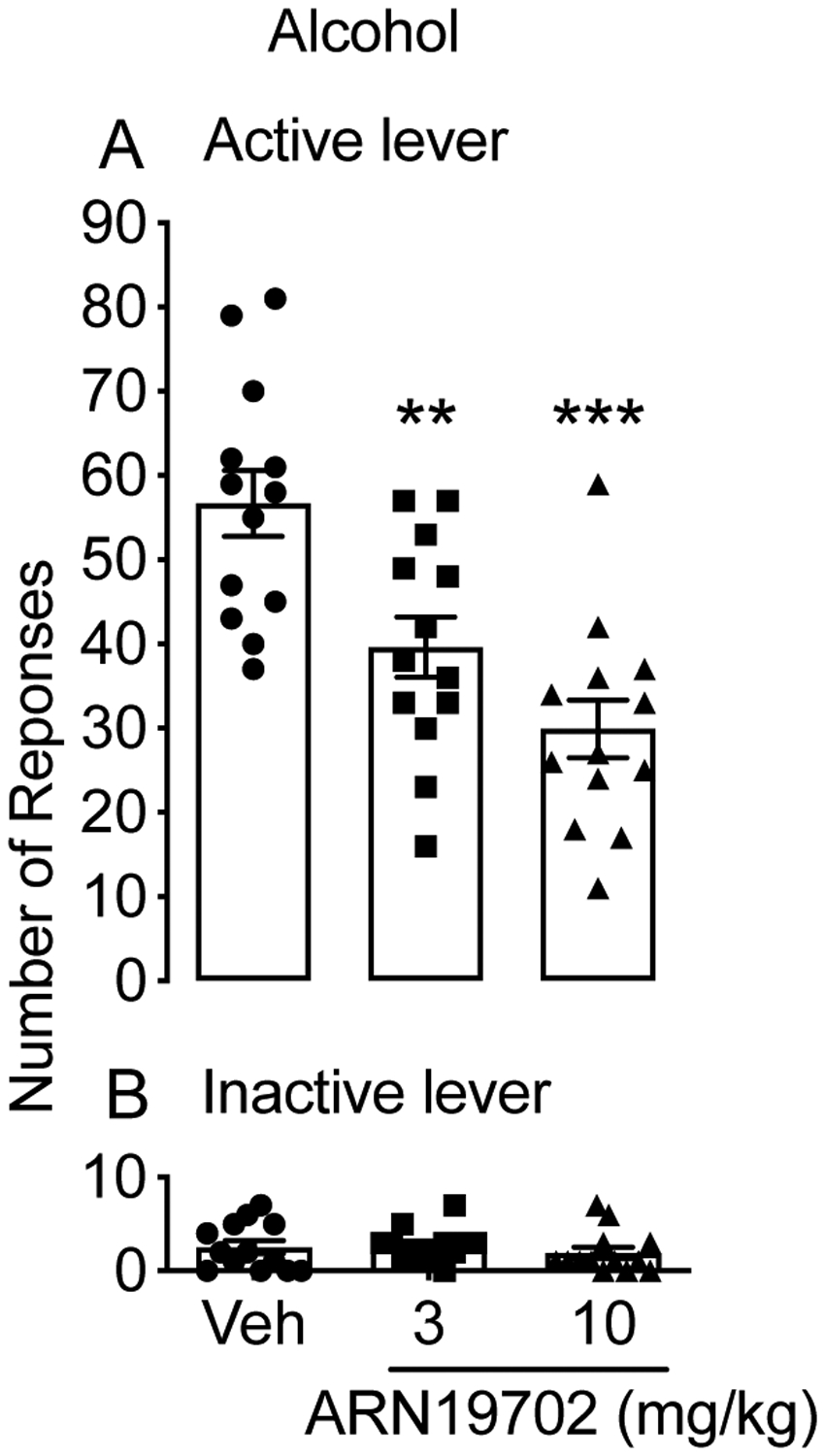
Effects of systemic NAAA inhibitor ARN19702 on alcohol self-administration. MsP rats (n=13) were treated in a counterbalanced Latin squared design with ARN19702 (3 and 10 mg/kg, i.p.) and were subjected to alcohol (10%, by volume) self-administration under an FR-1 schedule of reinforcement. Results are expressed as mean (±SD) of the number of rewards earned at the active lever (A) or responses at the nonreinforced inactive lever (B). **p < 0.01 and ***p < 0.001 vs vehicle controls (Veh).
Another group of rats (n=10) was trained to self-administer a saccharin solution (0.2% weight/volume) under an FR-1 schedule, and subsequently received ARN19702 (3 and 10 mg/kg, i.p.) or its vehicle in a counterbalanced Latin squared design. ANOVA showed that there was no effect of the drug on either the number of saccharin infusions earned [F (2, 18) = 1.85; p = 0.186; Fig. 3A] or responding at the inactive lever [F (2, 18) = 0.1368; p = 0.873; Fig. 3B], confirming the selectivity of ARN19702 in reducing alcohol intake.
Figure 3:
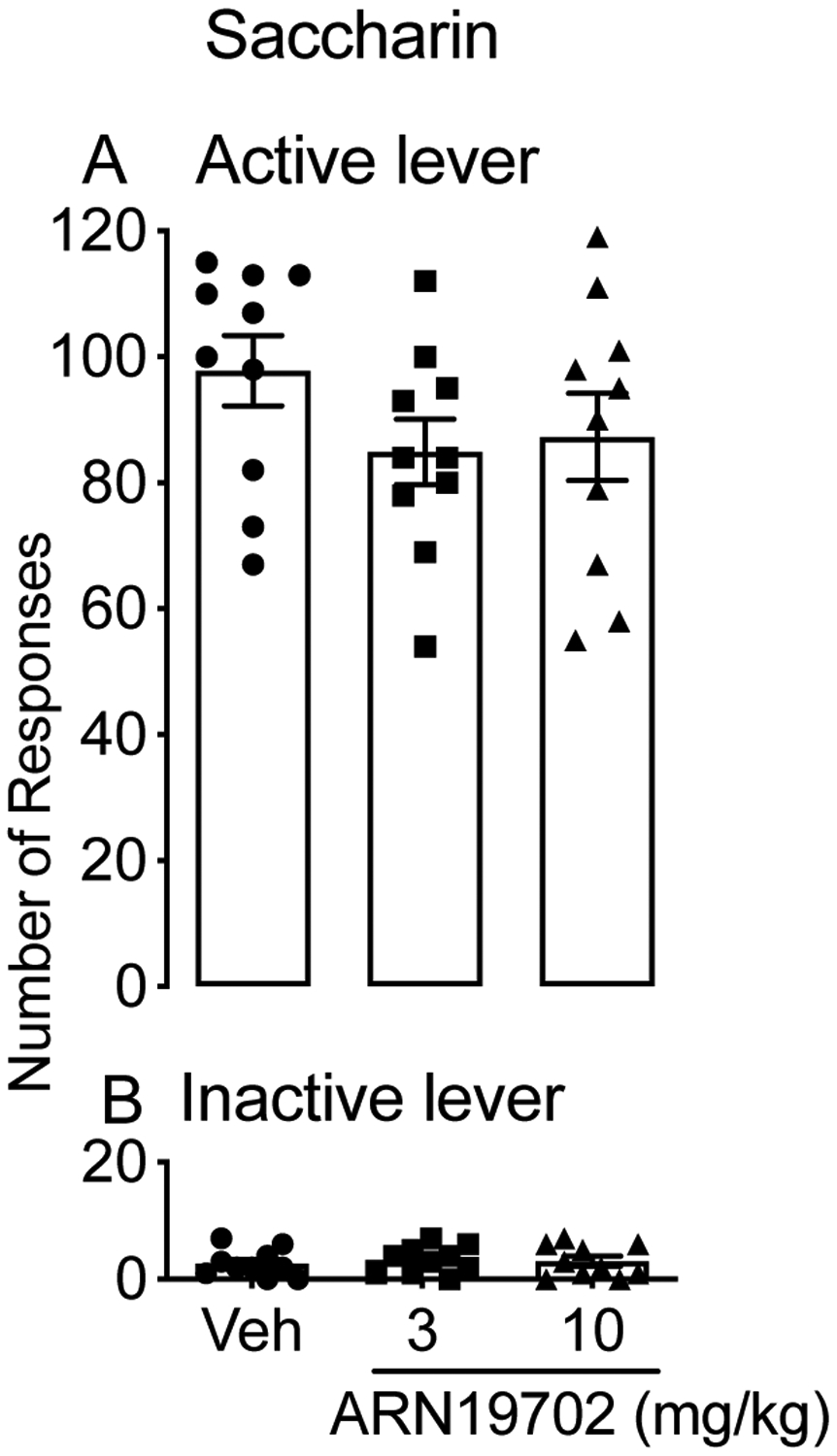
Effects of systemic NAAA inhibitor ARN19702 on saccharin self-administration. MsP rats (n=10) were treated in a counterbalanced Latin squared design with ARN19702 (3 and 10 mg/kg, i.p.) and subjected to saccharin (0.2%, weight/volume) self-administration under an FR-1 schedule. Results are expressed as mean (±SD) of the number of infusion (A) or responses at the inactive lever (B).
Effects of systemic NAAA inhibition on progressive ratio alcohol self-administration
We used a progressive ratio (PR) schedule to explore the impact of systemic NAAA blockade on the motivation of msP (n= 11) rats to obtain alcohol. Animals underwent an operant self-administration task in which they were required to progressively increase the number of active lever presses between 2 successive alcohol rewards until they reached a break point. As shown in Fig. 4, ARN19702 dose-dependently reduced the break point, compared to vehicle-treated animals [F (2, 20) = 11.06; ***p < 0.0001]. A Dunnett’s multiple comparison analysis showed that the effect of ARN19702 alcohol self-administration under a PR schedule was more pronounced at 10 mg/kg.
Figure 4:
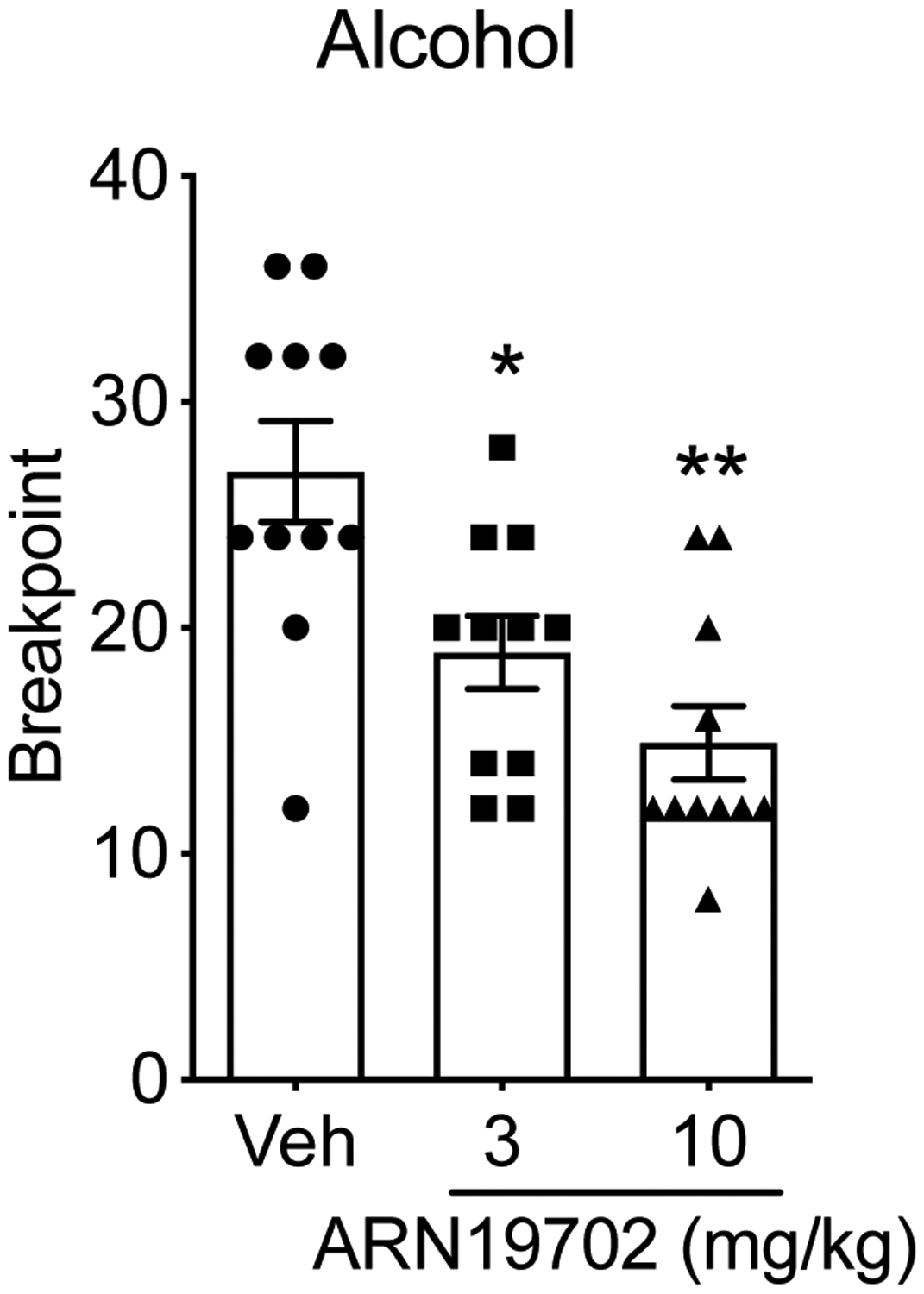
Effect of systemic NAAA inhibitor ARN19702 on motivation for alcohol seeking. MsP rats (n=11) were treated in a counterbalanced Latin squared design with ARN19702 (3 and 10 mg/kg, i.p.) and subjected to alcohol (10%, v/v) self-administration under a progressive ratio (PR) schedule. Results are expressed as the mean (±SD) of the last ratio completed by animals for alcohol (break point). *p < 0.05 and **p < 0.01 vs vehicle controls (Veh).
Effects of intra-VTA NAAA inhibition on alcohol self-administration
Next, we sought to identify potential brain areas involved in the alcohol-suppressing effect of ARN19702. We microinfused ARN19702 (1, 3 and 10 μg/μl) or its vehicle into the VTA of msP rats. Twelve rats were used in this experiment, but 3 had incorrect cannula placements, thus only 9 animals were included in the analyses. ANOVA showed that intra-VTA administration of ARN19702 reduced alcohol self-administration in a dose-dependent manner [F (3, 24) = 7.814; ***p < 0.0001; Fig. 5A]. Responding at the inactive lever was small and unchanged by the treatment [F (3, 24) = 1.443; p = 0.2612; Fig. 5B].
Figure 5:
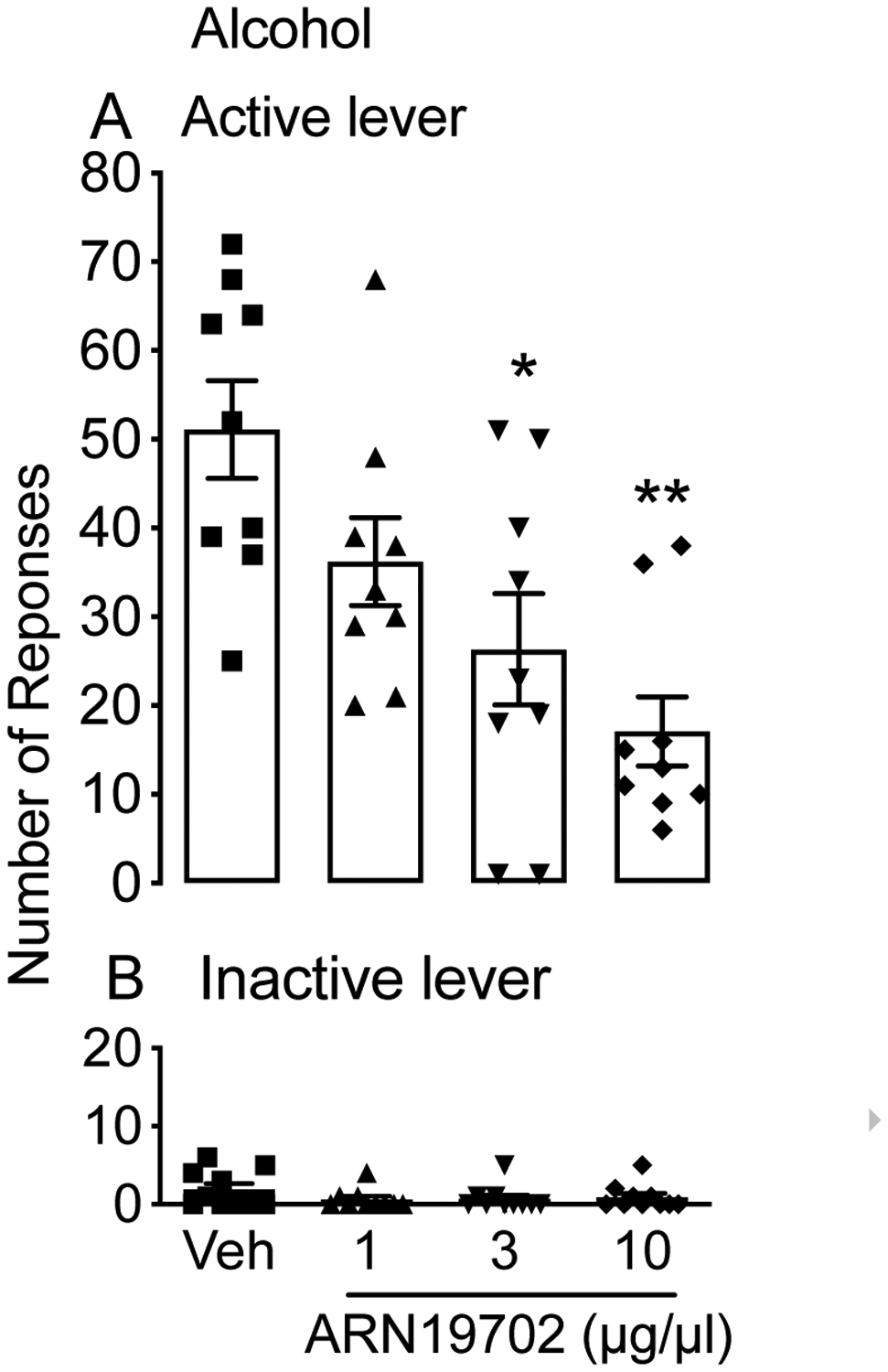
Effect of microinjection of NAAA inhibitor ARN19702 into the VTA on alcohol self-administration. Bilateral cannulas aimed at the VTA were implanted in MsP rats (n=9), which were then subjected to alcohol self-administration. Following microinjection of ARN19702 (1, 3 and 10 μg/μl), the number of rewards earned at the active lever (A) and responses at the inactive lever (B) were recorded. Results are expressed as the mean (±SD). **p < 0.01 and ***p < 0.001 vs vehicle controls (Veh).
To confirm that the effect of intra-VTA injections of ARN19702 was mediated by NAAA blockade, we microinjected 2 additional NAAA inhibitors, ARN726 (3 and 10 μg/μl) and ARN077 (3 and 10 μg/μl), which are chemically distinct from ARN19702 (Piomelli et al. 2020a). Twelve rats were used to test each compound, but only 10 (for ARN726) and 9 (for ARN077) had cannulas correctly positioned and were included in the analyses. The number of rewards earned was significantly reduced in a dose-dependent manner by both ARN726 [F (2, 18) = 11.17; ***p < 0.001; Fig. 6A] and ARN077 [F (2, 16) = 9.74; **p < 0.01; Fig. 6C]. A Dunnett’s post hoc test showed that all injected doses of NAAA inhibitors lowered alcohol self-administration. By contrast, inactive lever responding was not changed [ARN726: F (2, 18) = 0.2635; p = 0.7094; Fig. 6B and ARN077: F (2, 16) = 1.905; p = 0.1846; Fig. 6D].
Figure 6:
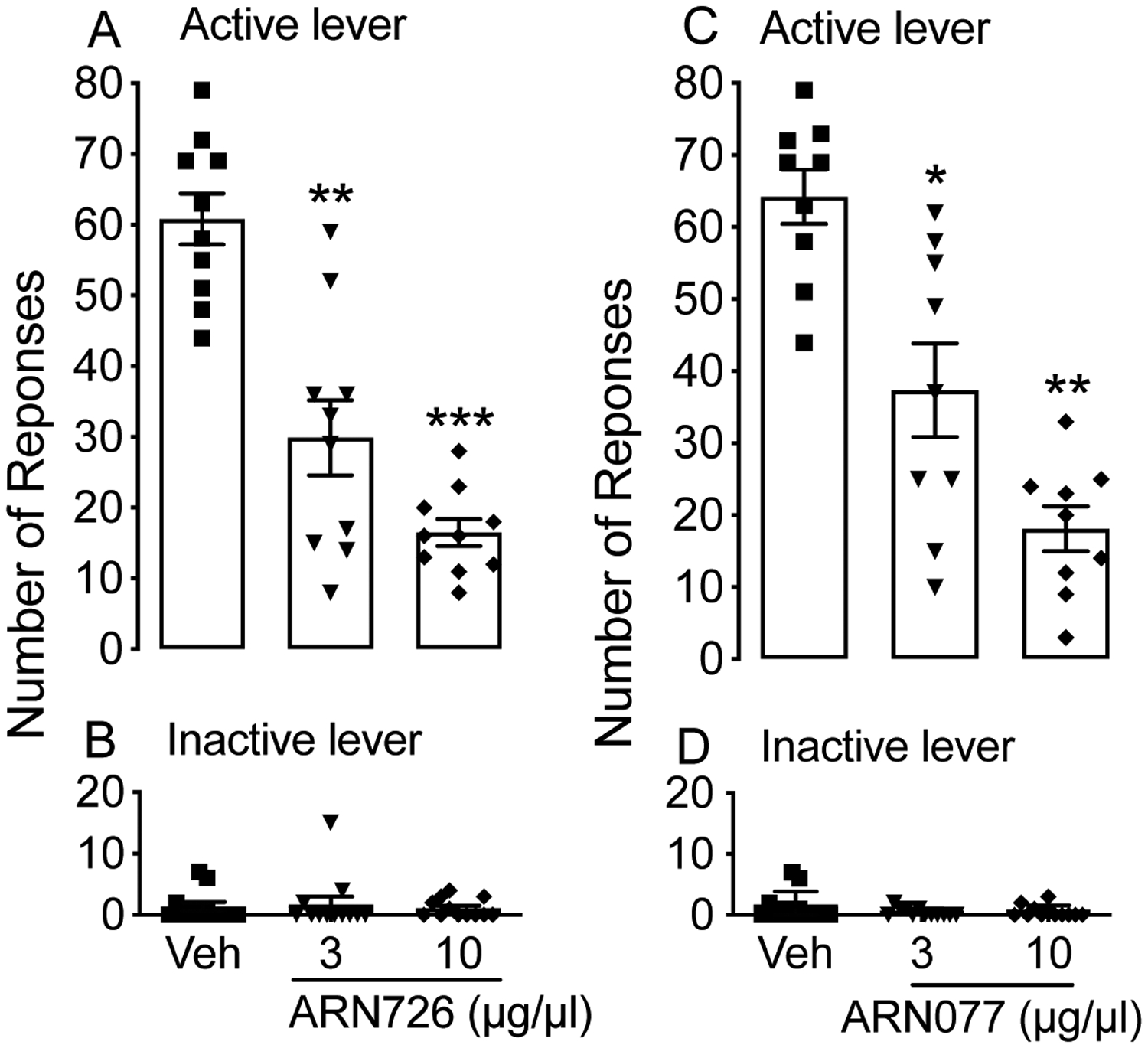
Effect of NAAA inhibitors ARN726 and ARN077 on alcohol self-administration. ARN726 (3 and 10 μg/μl) and ARN077 (3 and 10 μg/μl) were injected into the VTA of msP rats (n=10 for ARN726 and n=9 for ARN077) through guide cannulas. Results are expressed as the mean (±SD). (A) and (C): number of rewards earned by animals at the active lever. (B) and (D): responses at the inactive lever. **p < 0.01 and ***p < 0.001 vs vehicle controls (Veh).
Effects of intra-NAc NAAA inhibition on alcohol self-administration
An additional group of animals (n=12) received intra-NAc injections of ARN19702 (1, 3 and 10 μg/μl) or its vehicle in a counterbalanced Latin squared design. Four rats were excluded because of incorrect cannula placement. ANOVA did not detect significant changes in alcohol intake following intra-NAc administration of ARN19702 [Fig. 7A; F (3, 21) = 1.818, p = 0.1747 (ns)] or alteration of the responses at the inactive lever [Fig. 7B; F (3, 21) = 1.745; p = 0.1885 (ns)].
Figure 7:
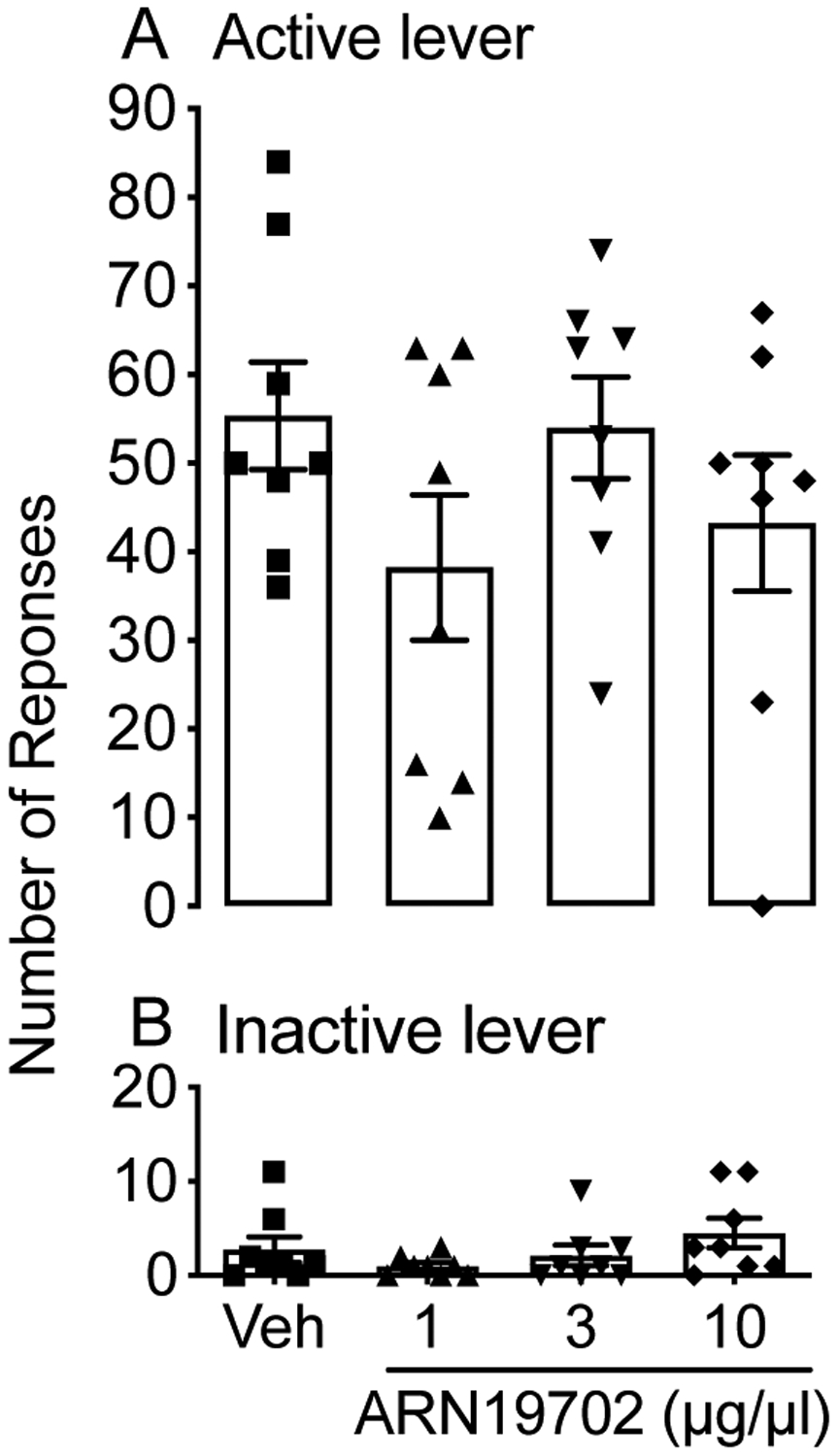
Effect of intra-NAc microinjection of NAAA inhibitor ARN19702 on alcohol self-administration. ARN19702 (2.5, 5 and 10 μg/μl) was injected into the NAc and msP rats (n=8) were subjected to alcohol self-administration. The number of rewards earned at the active lever (A) and responses at the inactive lever (B) were recorded. Results are expressed as mean (±SD); no statistically detectable differences were noted.
Discussion
AUD is a chronic disease of the brain that urgently needs safe and effective new therapies (Hasin et al. 2013; Weiss and Porrino 2002). In the present study, we showed that systemic administration of the brain-permeant NAAA inhibitor, ARN19702 (Migliore et al. 2016), lowered alcohol intake in male alcohol-preferring msP rats. Using the two-bottle choice model, we found that pretreatment with ARN19702, attenuated voluntary alcohol drinking but not water or food consumption. To investigate this further, we administered ARN19702 to two groups of rats trained to self-administer alcohol or saccharin under an FR-1 schedule of reinforcement. We found that ARN19702 substantially reduced alcohol but not saccharin intake. We interpret these results as suggesting that NAAA inhibition attenuates the motivation of msP rats to obtain alcohol. This possibility was further supported by findings showing that ARN19702 reduced in a dose-dependent manner the break point in animals undergoing an operant self-administration task under a progressive ratio schedule of reinforcement. Finally, we found that microinjection into the VTA of ARN19702 or two additional NAAA inhibitors – ARN077 and ARN726 (Piomelli et al. 2020a) – decreased alcohol self-administration, whereas microinjection of ARN19702 into the NAc had no such effect. The results support a role for NAAA in the control of alcohol intake and point to this intracellular cysteine amidase as a possible molecular target for the treatment of AUD.
Several lines of evidence suggest that the present findings might be relevant to AUD. Human studies have shown that the plasma concentrations of PEA and OEA are higher in alcohol binge drinkers than in non-drinkers (Anton et al. 2018; Garcia-Marchena et al. 2017). Similarly, experiments in mice suggested that acute or chronic alcohol consumption increases levels of OEA in plasma and in the NAc (Bilbao et al. 2016), a central locus of the brain reward circuit (Gardner 2011; Wise 2002). Based on these findings, it has been suggested that endogenous PEA and OEA may serve a protective function in the response to alcohol intake. If this is the case, then pharmacological strategies aimed at enhancing the intrinsic actions of these lipid messengers might provide a novel approach for the treatment of AUD. Consistent with this possibility, administration of OEA decreased alcohol intake and negative alcohol withdrawal symptoms in rats (Anton et al. 2018; Bilbao et al. 2016). However, the low bioavailability, rapid degradation and lack of brain penetration of OEA and PEA limit their therapeutic use. A possible alternative might be to magnify endogenous OEA and PEA signaling by protecting these lipid amides from NAAA-mediated degradation. The present findings demonstrate that systemic or intracerebral NAAA blockade reduces alcohol intake, provisionally validating this approach. Unlike OEA, whose suppressive effects extend to feeding and the motivation for high caloric foods (Bilbao et al. 2016), the NAAA inhibitor ARN19702 did not affect water intake, food intake or operant saccharin self-administration, arguing against non-specific actions (e.g. on malaise or motor activity) and highlighting its selective impact on alcohol consumption (June and Gilpin 2010; Nadal et al. 2002).
The mesocorticolimbic system consists of dopamine neurons that from the VTA project to various neural structures involved in reward processing, including the NAc and the medial prefrontal cortex (Ikemoto 2010; Wise 2002). Dopamine neurons in the VTA encode behaviors associated with alcohol consumption via changes in burst firing (Brodie et al. 1990; Di Chiara and Imperato 1985), indicating that this region may be a key node to control AUD. To test whether the VTA could be involved in the alcohol suppressive effects of NAAA blockade, we microinjected in this structure three chemically distinct NAAA inhibitors – ARN19702, ARN077 and ARN726 (Piomelli et al. 2020). The results show that each of these agents dose-dependently decreased alcohol self-administration, whereas microinjection of ARN19702 into the NAc had no such effect. These findings implicate the VTA as a potential neural substrate for the effects of NAAA inhibitors on alcohol drinking. Of note, Sagheddu and collaborators have recently proposed a similar role for the VTA in the suppressive effects of NAAA blockade on nicotine reward (Sagheddu et al. 2019). These investigators found that NAAA blockade inhibits the formation of nicotine-induced conditioned place preference, an effect that was linked to the ability of NAAA inhibition to magnify endogenous PEA and OEA activity at PPAR-α. A similar mechanism might be implicated in the alcohol-suppressing effects of ARN19702.
The present study has several limitations. First, we used a strain of rats that has a genetically determined preference for alcohol. Though widely used in alcohol research (Borruto et al. 2020; Domi et al. 2019; Economidou et al. 2006a; Fotio et al. 2020; Kirson et al. 2018; Logrip et al. 2018; Stopponi et al. 2013; Stopponi et al. 2018), this strain suffers from the inherent limitations of a genetic model. Thus, studies in other strains should be carried out to confirm the external validity of our findings. Second, we did not measure PEA and OEA content in brain tissue following administration of NAAA inhibitors. Mitigating this weakness, however, we showed that three chemically distinct NAAA inhibitors produce a similar inhibition of alcohol intake, making it unlikely that the observed reduction of alcohol consumption may be due to a shared off-target effect. Moreover, previous work from our lab demonstrated that these same agents effectively inhibit NAAA activity and elevate PEA and OEA levels in mouse and rat tissues, including the brain (Bonezzi et al. 2016; Migliore et al. 2016; Ribeiro et al. 2015; Sasso et al. 2013). Finally, we did not address the molecular mechanism(s) engaged by NAAA blockade in reducing alcohol intake. While PEA- or OEA-mediated activation of PPAR-α is a likely possibility, NAAA inhibitors might engage also other mechanisms such as TRPV1 channels and GPR-119 (Ahern 2003; Godlewski et al. 2009) or reduced formation of palmitic acid (Piomelli et al. 2020). Additional experimentation is needed to parse out these different possibilities.
Despite these limitations, our findings do suggest that NAAA inhibition attenuates the rewarding and motivational properties of alcohol through a mechanism that involves the VTA, and identify NAAA as a potential new molecular target for the treatment of AUD.
Funding:
This work was partially supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant AA017447 to R. Ciccocioppo [with M. Roberto].
List of non-conventional abbreviations:
- AUD
Alcohol Use Disorder
- FAAH
Fatty Acid Amide Hydrolase
- VTA
Ventral Tegmental Area
- OEA
Oleoylethanolamide
- PEA
Palmitoylethanolamide
- NAAA
N-Acylethanolamine Acid Amidase
- PPARα
Peroxisome Proliferator-Activated Receptor-alpha
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: DP is an inventor in patent applications owned by the University of California, Irvine, which describe NAAA inhibitors. YF and RC have no conflict of interest.
References
- Ahern GP (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278: 30429–34. [DOI] [PubMed] [Google Scholar]
- Anton M, Rodriguez-Gonzalez A, Rodriguez-Rojo IC, Pastor A, Correas A, Serrano A, Ballesta A, Alen F, Gomez de Heras R, de la Torre R, Rodriguez de Fonseca F, Orio L (2018) Increased plasma oleoylethanolamide and palmitoleoylethanolamide levels correlate with inflammatory changes in alcohol binge drinkers: the case of HMGB1 in women. Addict Biol 23: 1242–1250. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57: 441–7. [DOI] [PubMed] [Google Scholar]
- Artukoglu BB, Beyer C, Zuloff-Shani A, Brener E, Bloch MH (2017) Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 20: 353–362. [PubMed] [Google Scholar]
- Bandiera T, Ponzano S, Piomelli D (2014) Advances in the discovery of N-acylethanolamine acid amidase inhibitors. Pharmacol Res 86: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best LM, Williams B, Le Foll B, Mansouri E, Bazinet RP, Lin L, De Luca V, Lagzdins D, Rusjan P, Tyndale RF, Wilson AA, Hendershot CS, Heilig M, Houle S, Tong J, Kish SJ, Boileau I (2020) Lower brain fatty acid amide hydrolase in treatment-seeking patients with alcohol use disorder: a positron emission tomography study with [C-11]CURB. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Serrano A, Cippitelli A, Pavon FJ, Giuffrida A, Suarez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Orio L, Alen F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2016) Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol 21: 859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonezzi FT, Sasso O, Pontis S, Realini N, Romeo E, Ponzano S, Nuzzi A, Fiasella A, Bertozzi F, Piomelli D (2016) An Important Role for N-Acylethanolamine Acid Amidase in the Complete Freund’s Adjuvant Rat Model of Arthritis. J Pharmacol Exp Ther 356: 656–63. [DOI] [PubMed] [Google Scholar]
- Borruto AM, Fotio Y, Stopponi S, Brunori G, Petrella M, Caputi FF, Romualdi P, Candeletti S, Narendran R, Rorick-Kehn LM, Ubaldi M, Weiss F, Ciccocioppo R (2020) NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharmacol 177: 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508: 65–9. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Polis IY, Parsons LH (2013) The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology 38: 574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D (1998) Control of pain initiation by endogenous cannabinoids. Nature 394: 277–81. [DOI] [PubMed] [Google Scholar]
- Cannella N, Ubaldi M, Masi A, Bramucci M, Roberto M, Bifone A, Ciccocioppo R (2019) Building better strategies to develop new medications in Alcohol Use Disorder: Learning from past success and failure to shape a brighter future. Neurosci Biobehav Rev 103: 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M (2006) Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol 11: 339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R (2008) Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 198: 449–60. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R (2013) Palmitoylethanolamide: from endogenous cannabimimetic substance to innovative medicine for the treatment of cannabis dependence. Med Hypotheses 81: 619–22. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–7. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D (1995) Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem 270: 6030–5. [DOI] [PubMed] [Google Scholar]
- Devgun MS, Dunbar JA (1990) Alcohol consumption, blood alcohol level and the relevance of body weight in experimental design and analysis. J Stud Alcohol 51: 24–8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1985) Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol 115: 131–2. [DOI] [PubMed] [Google Scholar]
- Domi A, Stopponi S, Domi E, Ciccocioppo R, Cannella N (2019) Sub-dimensions of Alcohol Use Disorder in Alcohol Preferring and Non-preferring Rats, a Comparative Study. Front Behav Neurosci 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R (2006a) Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides 27: 3299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R (2006b) Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 183: 394–403. [DOI] [PubMed] [Google Scholar]
- Fotio Y, Aboufares El Alaoui A, Borruto AM, Acciarini S, Giordano A, Ciccocioppo R (2019) Efficacy of a Combination of N-Palmitoylethanolamide, Beta-Caryophyllene, Carnosic Acid, and Myrrh Extract on Chronic Neuropathic Pain: A Preclinical Study. Front Pharmacol 10: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotio Y, Borruto AM, Benvenuti F, Demopulos G, Gaitanaris G, Roberto M, Ciccocioppo R (2020) Activation of peroxisome proliferator-activated receptor gamma reduces alcohol drinking and seeking by modulating multiple mesocorticolimbic regions in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425: 90–3. [DOI] [PubMed] [Google Scholar]
- Gabrielsson L, Mattsson S, Fowler CJ (2016) Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br J Clin Pharmacol 82: 932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marchena N, Pavon FJ, Pastor A, Araos P, Pedraz M, Romero-Sanchiz P, Calado M, Suarez J, Castilla-Ortega E, Orio L, Boronat A, Torrens M, Rubio G, de la Torre R, Rodriguez de Fonseca F, Serrano A (2017) Plasma concentrations of oleoylethanolamide and other acylethanolamides are altered in alcohol-dependent patients: effect of length of abstinence. Addict Biol 22: 1366–1377. [DOI] [PubMed] [Google Scholar]
- Gardner EL (2011) Addiction and brain reward and antireward pathways. Adv Psychosom Med 30: 22–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF (2008) Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health 31: 185–95. [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G (2009) Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans M, Schwarz E, Bumb JM, Schaefer C, Rohleder C, Vollmert C, Vollstadt-Klein S, Tost H, Meyer-Lindenberg A, Kiefer F, Leweke FM (2014) Oleoylethanolamide and human neural responses to food stimuli in obesity. JAMA Psychiatry 71: 1254–61. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20: 441–58. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Auriacombe M, Borges G, Bucholz K, Budney A, Crowley T, Grant BF, O’Brien C, Petry NM, Schuckit M, Wall MM (2013) The DSM-5 field trials and reliability of alcohol use disorder. Am J Psychiatry 170: 442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134: 943–4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S (2010) Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev 35: 129–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Gilpin NW (2010) Operant self-administration models for testing the neuropharmacological basis of ethanol consumption in rats. Curr Protoc Neurosci Chapter 9: Unit 9 12 1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M (2018) CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addict Biol 23: 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS (2010) Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol 15: 109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Walker JR, Ayanwuyi LO, Sabino V, Ciccocioppo R, Koob GF, Zorrilla EP (2018) Evaluation of Alcohol Preference and Drinking in msP Rats Bearing a Crhr1 Promoter Polymorphism. Front Psychiatry 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D (2005) The search for the palmitoylethanolamide receptor. Life Sci 77: 1685–98. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D (2006) Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther 319: 1051–61. [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF (2005) Structure and function of fatty acid amide hydrolase. Annu Rev Biochem 74: 411–32. [DOI] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M (2008) Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci 28: 13985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Murphy TJ, Motulsky HJ (2020) New Author Guidelines for Displaying Data and Reporting Data Analysis and Statistical Methods in Experimental Biology. J Pharmacol Exp Ther 372: 136–147. [DOI] [PubMed] [Google Scholar]
- Migliore MD, Pontis SD, Fuentes de Arriba AL, Realini N, Torrente E, Armirotti A, Romeo E, Di Martino S, Russo D, Pizzirani D, Summa M, Lanfranco M, Ottonello G, Busquet P, Jung KM, Garcia-Guzman M, Heim R, Scarpelli R, Piomelli D (2016) Second-Generation Non-Covalent NAAA Inhibitors are Protective in a Model of Multiple Sclerosis. Angew Chem Int Ed Engl 55: 11193–11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH (2002) Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 162: 333–8. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279: 5298–305. [DOI] [PubMed] [Google Scholar]
- Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–84. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Sasso O (2014) Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci 17: 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Scalvini L, Fotio Y, Lodola A, Spadoni G, Tarzia G, Mor M (2020) N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition. J Med Chem 63: 7475–7490. [DOI] [PubMed] [Google Scholar]
- Ribeiro A, Pontis S, Mengatto L, Armirotti A, Chiurchiu V, Capurro V, Fiasella A, Nuzzi A, Romeo E, Moreno-Sanz G, Maccarrone M, Reggiani A, Tarzia G, Mor M, Bertozzi F, Bandiera T, Piomelli D (2015) A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation. ACS Chem Biol 10: 1838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D (2001) An anorexic lipid mediator regulated by feeding. Nature 414: 209–12. [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Scherma M, Congiu M, Fadda P, Carta G, Banni S, Wood JT, Makriyannis A, Malamas MS, Pistis M (2019) Inhibition of N-acylethanolamine acid amidase reduces nicotine-induced dopamine activation and reward. Neuropharmacology 144: 327–336. [DOI] [PubMed] [Google Scholar]
- Sasso O, Moreno-Sanz G, Martucci C, Realini N, Dionisi M, Mengatto L, Duranti A, Tarozzo G, Tarzia G, Mor M, Bertorelli R, Reggiani A, Piomelli D (2013) Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain 154: 350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R (2013) Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res 37: 1351–60. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Fotio Y, Domi A, Borruto AM, Natividad L, Roberto M, Ciccocioppo R, Cannella N (2018) Inhibition of fatty acid amide hydrolase in the central amygdala alleviates co-morbid expression of innate anxiety and excessive alcohol intake. Addict Biol 23: 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suardiaz M, Estivill-Torrus G, Goicoechea C, Bilbao A, Rodriguez de Fonseca F (2007) Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 133: 99–110. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N (2005) Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem 280: 11082–92. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Zhao LY, Okamoto Y, Araki N, Ueno M, Sakamoto H, Ueda N (2007) Predominant expression of lysosomal N-acylethanolamine-hydrolyzing acid amidase in macrophages revealed by immunochemical studies. Biochim Biophys Acta 1771: 623–32. [DOI] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ (2002) Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci 22: 3332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36: 229–40. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schwartz BI, Giza J, Gross SS, Lee FS, Kreek MJ (2017) Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology (Berl) 234: 2955–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]


