Abstract
Objectives:
To determine the association between comorbidities and the severity of the disease among COVID-19 patients.
Methods:
We searched the Cochrane, Medline, Trip, and EMBASE databases from 2019. The review included all available studies of COVID-19 patients published in the English language and studied the clinical characteristics, comorbidities, and disease outcomes from the beginning of the pandemic. Two authors extracted studies characteristics and the risk of bias. Odds ratio (OR) was used to analyze the data with 95% confidence interval (CI).
Results:
The review included 1,885 COVID-19 patients from 7 observational studies with some degree of bias risk and substantial heterogeneity. A significant association was recorded between COVID-19 severity and the following variables: male (OR= 1.60, 95%CI= 1.05 - 2.43); current smoker (OR=2.06, 95%CI= 1.08 - 3.94); and the presence of comorbidities including hypertension (OR=2.05, 95%CI= 1.56 - 2.70), diabetes (OR=2.46, 95%CI= 1.53 - 3.96), coronary heart disease (OR=4.10, 95%CI= 2.36 - 7.12), chronic kidney disease (OR=4.06, 95%CI= 1.45 - 11.35), and cancer (OR=2.28, 95%CI= 1.08 - 4.81).
Conclusions:
Comorbidities among COVID-19 patients may contribute to increasing their susceptibility to severe illness. The identification of these potential risk factors could help reduce mortality by identifying patients with poor prognosis at an early stage.
Keywords: Comorbidities, coronavirus, COVID-19, patients, the severity of illness
COVID-19 is a public health emergency as about 20 million laboratory-confirmed cases and about million deaths had been reported on August 2020.1-6 The disease covers wide clinical pictures including asymptomatic infection, mild upper respiratory tract illness, severe pneumonia, respiratory failure, and even death.7 Patients’ clinical manifestations include fever, non-productive cough, dyspnea, myalgia, fatigue, normal or decreased leukocyte counts, and radiographic evidence of pneumonia.8 However, as the pandemic continues, available data regarding the association between pre-existing comorbidities and the severity of COVID-19 illness is limited. Thus, investigation of the possible factors affecting the prognosis of patients with COVID-19 is needed to help clinicians identify highly susceptible individuals and those with poor prognosis at an early stage, in order to reduce mortality.
This review targeted to investigate the association between comorbidities and the severity of illness among COVID-19 patients.
Methods
The review included all the available studies, which investigated clinical characteristics, underlying comorbidities, and the severity of illness among COVID-19 patients from the beginning of the pandemic.
The inclusion criteria comprised hospitalized patients diagnosed with COVID-19 according to WHO guidance (severe acute respiratory syndrome-coronavirus 2 [SARS-CoV-2] detection in respiratory specimens by next-generation sequencing or real-time reverse transcription-polymerase chain reaction [RT-PCR] methods).9 The exclusion criteria comprised patients diagnosed with severe pneumonia without confirmation of COVID-19.
The outcome measure is the severity of COVID-19-related illness namely, intensive care unit (ICU) admission, mechanical ventilation and death. COVID-19 severity was defined based on the criteria of China’s National Health Commission as mild, moderate, severe, and critical.10
We searched Cochrane Library, EMBASE, TRIP, and MEDLINE databases. We also reviewed the primary references for additional studies. The following terms were used: Clinical features of COVID-19, OR Comorbidities associated with COVID-19 OR CORONA virus disease, OR NOVEL CORONA, OR Severe pneumonia, OR Patients infected with CORONA, and Outcome of COVID-19, OR adverse outcome, OR fatality of Covid-19, OR survivors and non-survivors of COVID-19, OR Intensive care unit for COVID-19.
Data collection and analysis
Two authors independently checked the titles and abstracts of potential articles for inclusion criteria. Then, they obtained and read all the relevant articles.
Data extraction and management
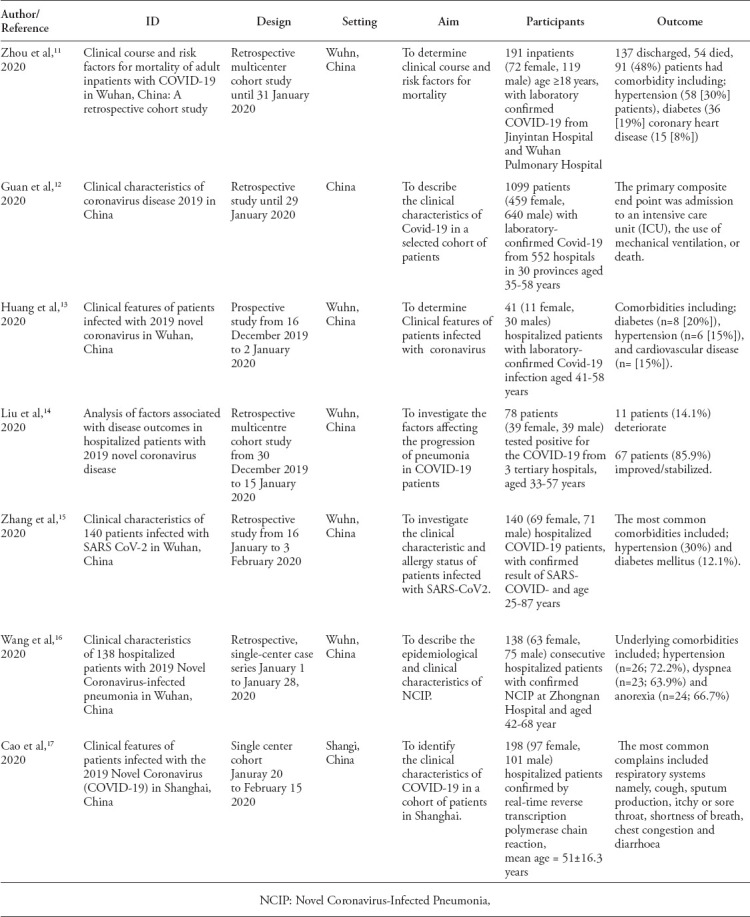
The following characteristics were extracted from the included studies11-17 (Table 1): study design, setting, duration, number and age of patients, and outcome measures including ICU admission, the need for mechanical ventilation and death. One author entered the data into the Review Manager (RevMan) 5.3.18
Table 1.
Characteristics of included studies.
Assessment of the risk of bias
The Newcastle-Ottawa Quality Assessment Scale for Case-Control/Cohort Studies was used to assess the risk of bias for the included studies.19 Each risk of bias was graded high, low, or unclear based on the following domains: i) adequate case definition (selection bias), ii) consecutive representativeness of cases (selection bias), iii) selection of community controls (selection bias), iv) Adequate control definition (selection bias), v) ascertainment of exposure/independent blind assessment of outcome (selection bias), vi) cases and controls comparability based on the design /independent blind assessment of outcome (comparability bias), vii) method for the ascertainment of cases and controls/Adequacy of the follow-up period (exposure/outcome bias), viii) all subjects complete follow up period/ Same response rate for both groups (exposure/outcome bias).
Assessment of the quality of evidence
The quality of evidence for each outcome measure was judged as high, moderate, low, or very low according to the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation).20
Measures of treatment effect
A random effect model of Review Manager 5.311 was used to analyze the data. Dichotomous variables were reported as odds ratios (ORs) with 95% confidence intervals (CIs).
I2 statistic was used to assess heterogeneity among the studies included in each analysis.21
Results
Out of 219 potentially relevant articles, 104 remained after removing duplicates. Sixteen full-text articles were assessed for eligibility; of these, 7 met the inclusion criteria. The Prisma flow diagram shows the details of the search method (Figure 1).
Figure 1.
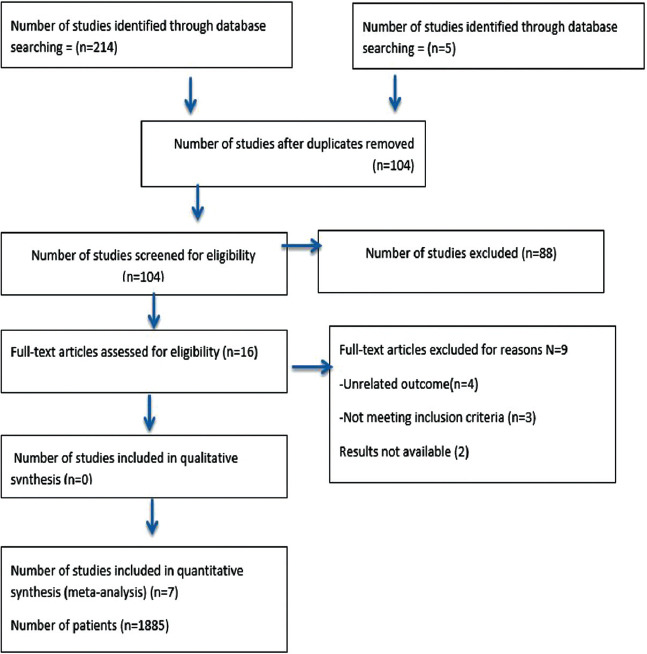
PRISMA flow diagram.
The review included 1,885 patients (810 females and 1,075 males) who were hospitalized and diagnosed with COVID-19. The median patient ages in years were reported as 56 (18-87) by Zhou et al,11 47.0 (35–58) by Guan et al,12 57 (25-87) by Zhang et al,15 and 56 (22-92) by Wang et al.16 Cao et al17 reported mean age of 50.1±16.3 years and Huang et al13 reported 49.0±41.58 years.
Risk of bias in the surveyed studies
Overall, the included studies in this review had some risk of bias. All the studies recorded a high risk of bias in the selection of community controls. Regarding the adequate definition of controls, 5 studies,11,13-15,17 reported unclear bias while 2 studies12,16 reported high risk. Only the Zhang et al15 study had a high risk of bias for the same response rate for both groups (Figure 2).
Figure 2.

Risk of bias among included trials.
Figure 3 presents the association between background risk factors and the severity of COVID-19 illness. Male gender (OR=1.60, 95%CI=1.05-2.43) and smoking are significant risk factors for severe COVID-19 illness (OR=2.06, 95%CI=1.08-3.94). Low (I2=48%) and insignificant heterogeneity was reported in the analysis (I2=25% (p>0.05). On the other hand, no significant association was observed between patients aged >50 years and disease severity (OR=2.08, CI=0.79-5.49), with considerable significant heterogeneity (I2=81%, p<0.05).
Figure 3.
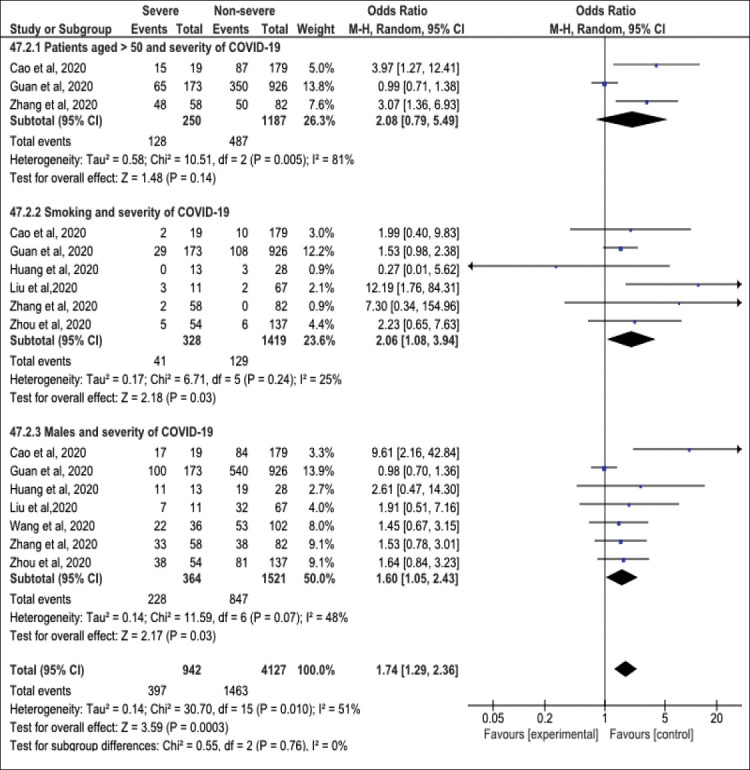
Forest plot of background risk factors and severity of COVID-19 among studied participants.
The association between comorbidities and COVID-19 severity is plotted in Figure 4. The risk for COVID-19 severity increased from two to four folds among patients with hypertension (OR=2.05, 95% CI=1.56 - 2.70), chronic kidney disease (CKD) (OR=4.06, 95%CI=1.45-11.35), chronic obstructive pulmonary disease (OR=6.09, 95%CI=2.77-13.3), and cancer (OR=2.28, 95%CI=1.08 - 4.81). No heterogeneity was detected between the surveyed studies (I2=0%). Also, a statistically significant association was detected between diabetic patients, Coronary Heart Disease (CHD) patients, and COVID-19 severity (OR=2.46, 95%CI=1.53-3.96, and OR=4.10, 95%CI=2.36-7.12, respectively). Moderate insignificant heterogeneity was detected in the analysis (I2=31%, p=0.19 and I2=26%, p>0.05).
Figure 4.
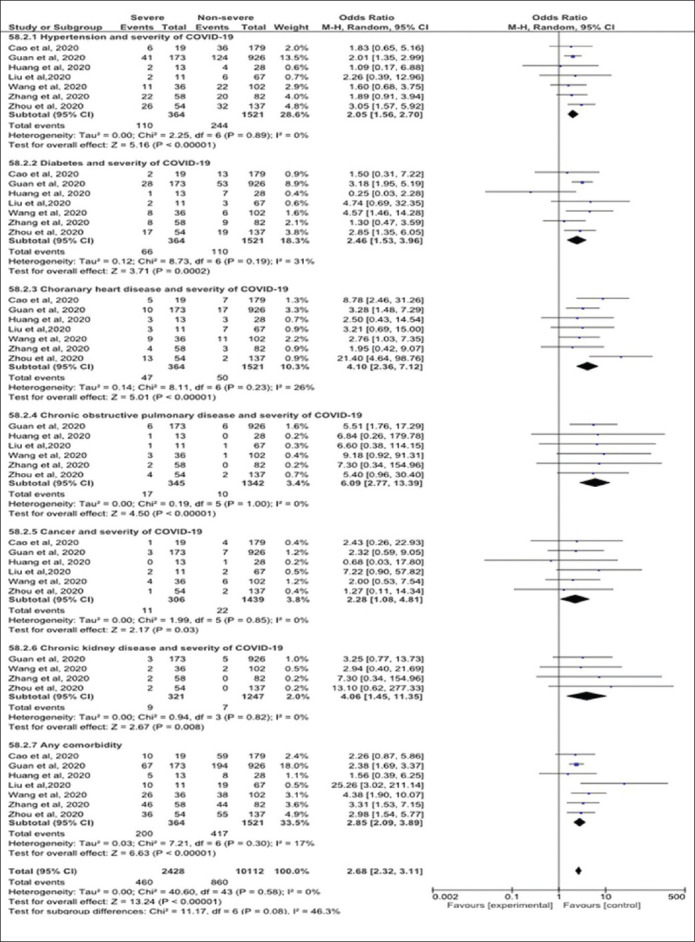
Forest plot of underlying comorbidities and the severity of COVID-19.
Figure 5 explains the forest plot of the pooled effects of the significant risk factors for the severity of illness among COVID-19 patients.
Figure 5.
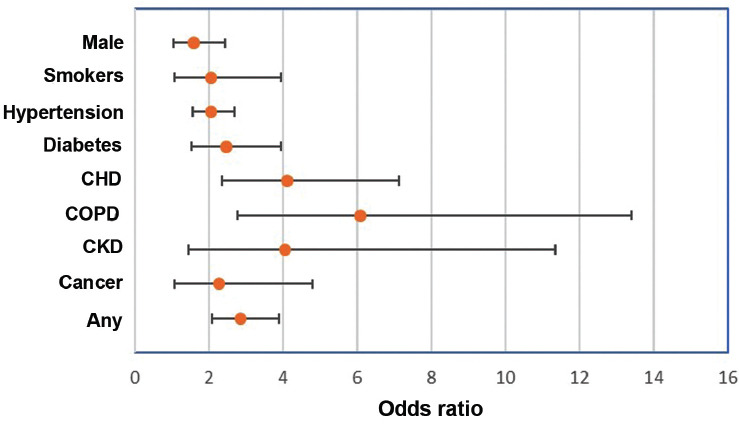
Odds ratio and confidence interval of the pooled effect of risk factors for the severity of COVID-19.
Quality of evidence
We judged the quality of evidence for each outcome measure based on the four domains recommended by the GRADE approach 13 for the evaluation of study limitations; namely, the risk of bias in each study, the directness of the evidence, consistency across studies, and precision of the pooled estimate. Overall, the surveyed studies were primarily observational with a considerable risk of bias, which resulted in downgrading the evidence by one level for all outcome measures. However, directness was not an issue, as all studies recorded the same outcome measures. Regarding the pooled estimate for the outcome measures (male gender, current smoking, any comorbidity, hypertension, diabetes, CHD, cancer), the quality of evidence was recorded as moderate. We downgraded the evidence by one level only due to the observational designs of the included studies. Imprecision, directness, and heterogeneity were not significant issues. Low and moderate heterogeneity (30-60%) was found between the outcome measures (I2=48% for the male gender, 25% for current smoking, 17% for any comorbidity, 0% for hypertension, 31% for diabetes, 26% CHD, and 0% for cancer. We judged the quality of evidence for the association between COPD and CKD was judged as low. We lowered the evidence by two levels because of the observational design and the degree of imprecision indicated by the wide confidence intervals due to the limited number of events included in the analysis. The heterogeneity between the surveyed studies could be due to differences in participants regarding geographical location, clinical features, method of diagnosis and duration of comorbidities, and treatment strategies (namely, dose, duration, route of administration, and follow-up).
Discussion
Summary of the evidence: This review investigated the relationship between comorbidities and the severity of illness among COVID-19 patients. We included 1,885 COVID-19 patients from seven studies. We found that male gender, smoking, and pre-existing comorbidities (including hypertension, diabetes, CHD, COPD, CKD, and cancer) were significant risk factors for disease progression.
Similarly, Huang et al13 and Chen et al7 reported that most COVID-19 infected patients were men (73% and 68% respectively), and a predominance of males was recorded by Li et al22 in their meta-analysis (60%, 95%CI=0.54, 0.65).
Liu et al14 found that old age was a risk factor for disease severity, which is inconsistent with our findings. Wang et al16 and Cao et al17 reported that ICU admitted patients had a higher median age than non-admitted (median age, 66 versus 51 years and 63.7± versus 48.6 ± 15.6 years) and were more likely to have underlying comorbidities (72% vs. 37%). In addition, Zhou et al11 found that the mortality rate was higher among older COVID-19 patients (OR 1.10, 95% CI 1.03, 1.17, per year increase; p=0.0043. However, in the present review, the pooled estimate of patients aged >50 years was not associated with disease severity. This discrepancy could be attributed to the older age group of the previous studies compared to our review.
The present review indicated that current smokers were twice as likely to have severe COVID-19-related illness as non-smokers (OR=2.06, 95%CI=1.08, 3.94). Our findings are consistent with those of Vardavas and Nikitara23 and Liu et al14 who investigated the pooled effect of smoking, and reported a significant association with the adverse outcome of COVID-19. Similarly, Wu et al24 and Guan et al12 reported that smokers were twice as likely as non-smokers to develop severe symptoms and higher mortality of the Middle East Respiratory Syndrome (MARS). The adverse effects of smoking on the immune system have been documented by previous studies.25-28
The present review demonstrated that the presence of comorbidities among COVID-19 patients, increases the severity of the disease by approximately 3-fold (OR=2.85, 95%CI=2.09, 3.89). This finding is consistent with the results of Liu et al,14 Zhang et al,15 and Guan et al12 who recorded comorbidities among severe cases of COVID-19 patients (25.6%, 79.3%, and 23.7%, respectively). Huang et al13 and Zhang et al15 recorded these comorbidities including mainly hypertension, diabetes, and CVD. Also, a higher fatality rate was recorded among the patients compared to those with non-underlying comorbidities.29 It is unclear whether hypertension is primarily a predictor of COVID-19 severity or a contributor to the deterioration of the disease.30 Our results showed that hypertension in COVID-19 patients was significantly associated with a 2-fold increase in the risk of disease severity (OR=2.05, 95%CI=1.56, 2.70, I2=0%). Similarly, a pooled analysis of hypertension in COVID-19 patients by Lippi et al31 showed that hypertensive patients were at a 2.5-fold risk of severe COVID-19 and mortality (OR: 2.49, 95%CI 1.98, 3.12, I2=24% and OR: 2.42, 95% CI 1.51-3.90, I2=0%). In contrast, Wan et al21 and Guan et al12 failed to find an association between hypertension and COVID-19 severity.
Cardiovascular disease is the most common underlying condition among COVID-19 patients, as reported by Zhang et al15 and Huang et al13 (OR=4.10, 95%CI=2.36-7.12). Our findings are consistent with those of Zheng et al33 and Cao et al,17 who reported increased risk for COVID-19 severity and mortality among CVD patients. The latter showed that CVDs were significantly more common in ICU COVID-19 cases compared to non-ICU cases (26.3% versus 3.9%, p<0.01). Likewise, COVID-19 diabetic patients recorded higher disease severity and mortality than non-diabetic ones.34-37 The same was documented by the current review (OR=2.46, 95%CI=1.53-3.96). The adverse outcome of COVID-19 among diabetic patients could be attributed to many factors, which include comorbidities such as hypertension and cardiovascular disease, obesity, a pro-inflammatory, glucose-lowering agents, and anti-viral treatments.38 The immune system of diabetic patients is compromised, making it harder to fight the virus and elongating the recovery period. Also, the environment with elevated blood sugar may aid the survival of the virus.37
Regarding COPD, earlier studies reported no significant increased risk for COVID-19 among COPD patients.12,39,40 A more recent study found a significant association and over a 5-fold increase in the risk of severe COVID-19 infection in COPD patients.41 Zhao et al42 recorded the pooled OR to be 4.38 (95% CI: 2.34-8.20). Nearly the same risk was observed in our review (OR=6.09, 95%CI=2.77-13.39, p<0.05). The predisposition of COPD patients to severe COVID-19 could beexplained by the increased levels of Angiotensin-Converting Enzyme 2 (ACE2) in these patients, by which SAR-COV-2 enters human cells and causes COVID-19.43,44
Consistent with our findings, a study assessed the association of COVID-19 and chronic kidney disease in over 700 patients and a higher percentage for acute kidney injury (AKI) and mortality was reported.45 Similarly, Lippi and Henry41 found a significant association between CKD and severe COVID-19 illness (OR=3.03 (95%CI=1.09-8.47). People with CKD and other severe chronic medical conditions tend to have weaker immune systems and are at a higher risk for more severe COVID-19 illness. Moreover, the anti-rejection medication given to patients with kidney transplantation suppresses their immune system, which can make it harder for the body to fight infections.46
In general, patients with cancer are more vulnerable to infection. A recent nationwide analysis in China47 reported increased risks of adverse outcomes in COVID-19 cancer patients, which is consistent with this review (OR=2.28, 95%CI=1.08-4.81). Several strategies have been proposed to offset the risks, such as delaying of required chemotherapy or surgery, and the immunocompromised status.48
Study limitations
The results of this review should be interpreted with caution as the evidence is derived from observational studies that have some risk of bias and substantial heterogeneity.
In conclusion, male gender, smoking, and underlying comorbidities were found to be significantly associated with COVID-19 severity. These pre-existing conditions could increase the susceptibility of such individuals to COVID-19. Recognizing these risk factors could help clinicians reduce mortality by identifying patients with poor prognosis at an early stage.
A systematic review of clinical trials with a lower risk of bias and limited heterogeneity is recommended to support the association of comorbidities and COVID-19 severity.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing. “Also, we would like to thank Miss Mona Ahmed Almusawi for the Arabic translation of the abstract.
Footnotes
References
- 1.Khot WY, Nadkar MY. The 2019 novel coronavirus outbreak-a Global threat. J Assoc Physicians India. 2020;68:67–71. [PubMed] [Google Scholar]
- 2.Callaway E, Cyranoski D. China coronavirus:six questions scientists are asking. Nature. 2020;577:605–607. doi: 10.1038/d41586-020-00166-6. [DOI] [PubMed] [Google Scholar]
- 3.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Coronavirus disease (COVID-2019) situation reports-94. [Accessed: 2020 April]. [Updte 2020] Available from URL: https://www.who.int/emergencies/diseases/novel .
- 6.National Health Commission of the People's Republic of China. 2020. [Accessed: 2020 March]. Updte 2020. Available from URL: http://www.nhc.gov.cn . [DOI] [PMC free article] [PubMed]
- 7.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui-Cui L, Xiao-Jia W, Hwa-Chain RW. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discovery Today. 2019;24:726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emery SL, Erdman DD, Bowen MD, Newton Br, Winchell JM, Meyer RF, Tong S, et al. Real-Time Reverse Transcription–Polymerase Chain Reaction Assay for SARS-associated Coronavirus. Emerg Infect Dis. 2004;10:311–316. doi: 10.3201/eid1002.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of China:Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 5) [Accessed: 2020 February]. Update 2020. Available from URL: https://www.chinalawtranslate.com/en/13986-2 .
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Ni ZY, Hu Y, Liang W, Ou C, Heet J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Tao Z, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JJ, Dong X, Cao Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, Xue H, Lin Y, Zhang M, Sun Y, Yang Z. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. MedRxiv. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 19.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Ottawa (CN): Ottawa Hospital Research Institute; 2019. [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE:an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients'clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardavas C, Nikitara K. COVID-19 and smoking:A systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China:a modeling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonnesen P, Marott JL, Nordestgaard B, Bojesen SE, Lange P. Secular trends in smoking in relation to prevalent and incident smoking-related disease:A prospective population-based study. Tob Induc Dis. 2019;17:72–80. doi: 10.18332/tid/112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Chen P, Peng H. Are healthy smokers really healthy? Tob Induc Dis. 2016;14:35–47. doi: 10.1186/s12971-016-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladha KS, Zhao K, Quraishi SA, Kurth T, Eikermann M, Kaafarani HM, et al. The Deyo-Charlson and Elixhauser-van Walraven comorbidity indices as predictors of mortality in critically ill patients. BMJ Open. 2015;5:9–17. doi: 10.1136/bmjopen-2015-008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Accessed: 2020 May]. Update 2020 February 14-20. Available from URL: https://www.ncbi.nlm.nih.gov/pubmed/32267833 .
- 30.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:21–23. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippi G, Wong J, Henry BM. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19):a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 32.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes Association. (homepage on the Internet) Diabetes and Coronavirus. How COVID-19 Impactes People with Diabetes. Arlington: American Diabetes Association; Update 2020; Accessed: 2020 May. Available from URL: https://www.diabetes.org/coronavirus-covid-19/how-coronavirus-impacts-people-with-diabetes . [Google Scholar]
- 35.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China:summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 36.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 37.International Diabetes Federation. Diabetes Voice;COVID-19 and diabetes. [Accessed: 2020 March]. Update 2020. available from URL: https://diabetesvoice.org/en/news/covid-19-and-diabetes .
- 38.Apocella M, Campopiano M, Mantuano M, Mazoni L, Coppelli A. COVID-19 in people with diabetes:understanding the reasons for worse outcome. The Lancet. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhutani M, Hernandez P, Bourbeau J, Dechman G, Penz E, Aceron Raymond, et al. Addressing therapeutic questions to help Canadian health care professionals optimize COPD management for their patients during the COVID-19 pandemic. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2020;4:77–80. [Google Scholar]
- 40.Kim ES, Bum SI, Chin BS, Kang CK, Kim NJ, Kang YM, et al. Clinical course and outcomes of patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection:a Preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020;35:142–154. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. The impact of COPD and smoking history on the severity of COVID-19:A systemic review and meta-analysis. J Med Virol. 2020;4:1–7. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan:An analysis based on decade-long structural studies of SARS. J Med Virol. 2020;94:120–127. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28:346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19 Yichun. Kidney Int. 2020;97:829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Vivekanand JH. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection:a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.William K. COVID-19 infection in cancer patients:early observations and unanswered questions. Ann Oncol. 2020;31:838–839. doi: 10.1016/j.annonc.2020.03.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


