Abstract
Background
The glutamatergic modulator ketamine has created a blueprint for studying novel pharmaceuticals in the field. Recent studies suggest that “classic” serotonergic psychedelics (SPs) may also have antidepressant efficacy. Both ketamine and SPs appear to produce rapid, sustained antidepressant effects after a transient psychoactive period.
Methods
This review summarizes areas of overlap between SP and ketamine research and considers the possibility of a common, downstream mechanism of action. The therapeutic relevance of the psychoactive state, overlapping cellular and molecular effects, and overlapping electrophysiological and neuroimaging observations are all reviewed.
Results
Taken together, the evidence suggests a potentially shared mechanism wherein both ketamine and SPs may engender rapid neuroplastic effects in a glutamatergic activity-dependent manner. It is postulated that, though distinct, both ketamine and SPs appear to produce acute alterations in cortical network activity that may initially produce psychoactive effects and later produce milder, sustained changes in network efficiency associated with therapeutic response. However, despite some commonalities between the psychoactive component of these pharmacologically distinct therapies—such as engagement of the downstream glutamatergic pathway—the connection between psychoactive impact and antidepressant efficacy remains unclear and requires more rigorous research.
Conclusions
Rapid-acting antidepressants currently under investigation may share some downstream pharmacological effects, suggesting that their antidepressant effects may come about via related mechanisms. Given the prototypic nature of ketamine research and recent progress in this area, this platform could be used to investigate entirely new classes of antidepressants with rapid and robust actions.
Keywords: Ketamine, serotonergic psychedelics, depression, glutamate, mechanism
Introduction
Our understanding of antidepressant pharmacotherapy dramatically changed with the discovery that subanesthetic doses of the glutamatergic modulator ketamine exert antidepressant effects in a matter of minutes and that these effects persist long after drug excretion (Hashimoto, 2019). For nearly half a century, the antidepressant effects of most conventional monoaminergic antidepressants took weeks to months to manifest, a considerable lag time between regimen initiation and realization of therapeutic effects (Drewniany et al., 2015).
In the past 2 decades, multiple randomized, placebo-controlled trials have shown that i.v. subanesthetic-dose ketamine has rapid, robust, and relatively sustained antidepressant effects in individuals with major depressive disorder (MDD) (Berman et al., 2000; Zarate et al., 2006; Murrough et al., 2013; Fava et al., 2020) or bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012). Ketamine also offers hope to the roughly 33% of individuals with treatment-resistant depression (TRD) who do not respond to conventional antidepressants (Gaynes et al., 2009). A meta-analysis of 9 randomized, placebo-controlled trials found that a single ketamine infusion exerted antidepressant effects that typically began 40 minutes post-infusion, peaked 24 hours later, and lost superiority to placebo 10–12 days post-infusion (Kishimoto et al., 2016). Despite this encouraging antidepressant profile, ketamine is associated with psychoactive effects that peak at about 40 minutes post-infusion, requiring medical supervision during administration (Kraus et al., 2017). For the purposes of this paper, psychoactive states are defined as profound alterations in consciousness, including domains of perception, mood, thought, and self-awareness; these effects are also alternatively referred to as hallucinogenic, psychotomimetic, or psychedelic (Nichols, 2016).
Building on these findings, investigators have scrutinized ketamine’s pharmacological profile to not only understand its antidepressant mechanism of action but also to develop similar, glutamatergic-based agents that lack ketamine’s psychoactive effects (Sanacora et al., 2012). More broadly, ketamine trials have created a blueprint for studying novel pharmaceuticals in the field (Kraus et al., 2019). In particular, thoughtfully designed ketamine studies have shown that agents with abuse potential in non-medical settings can nevertheless be administered in a safe and scientifically rigorous fashion. The paradigm shift triggered by ketamine’s therapeutic success has also created a model whereby investigators can methodically explore the potential therapeutic value of new or existing (but repurposed) substances that carry abuse liability risk. At the forefront of such work, several recent studies have suggested that “classic” serotonergic psychedelics (SPs) may also have antidepressant efficacy; in particular, they appear to produce rapid, sustained antidepressant effects after a transient psychoactive period (Muttoni et al., 2019). Indeed, prior to being banned as Schedule I drugs in 1967, SPs showed promise for treating a number of psychiatric disorders, including depression, anxiety, obsessive-compulsive disorder, and alcoholism (Rucker et al., 2018). However, by today’s standards, these studies had serious methodological flaws, including lack of control groups, lack of adverse events reporting, lack of blinding, and unvalidated outcome measures.
From the outset, it should be noted that modern research into the antidepressant effects of SPs is in its infancy, and the rigor of these studies remains questionable. Broadly, recreational use of SPs in the 1960s resulted in wide mistrust in the medical community. These agents exert powerful and potentially life-altering effects. Adverse risks associated with SP therapy include distressing experiences, prolonged psychosis, flashbacks, or persisting perceptual disturbances (Branchi, 2011; Johnson et al., 2019; Lascelles et al., 2019). The characteristics that might predict unacceptable risks, such as personal or family history of psychosis (Johnson et al., 2008), also remain unknown. Furthermore, because SPs acutely increase serotonin levels—an effect that alters neural plasticity—these powerful agents can have both positive (e.g., improved capacity to recover) and negative (increased vulnerability to depression) effects (Branchi, 2011). Nevertheless, their safety in clinical and research settings (Dos Santos et al., 2018) as well as abuse/addiction liability (Heal et al., 2018; Johnson et al., 2018) are considered to be within acceptable limits, although more research is clearly needed to definitively establish their therapeutic safety profile (Reiff et al., 2020).
Pharmacologically, SPs are defined as drugs that induce subjectively similar psychoactive states via agonism at the 5-hydroxytryptamine receptor 2A (5-HT2A) receptor and binding at other serotonergic receptors (Reiff et al., 2020). Specific SPs currently under investigation for the treatment of mood and anxiety disorders include psilocybin (the active ingredient in “magic mushrooms”), lysergic acid diethylamide-25 (LSD), 2,5-Dimethoxy-4-iodoamphetamine (DOI), Ayahuasca (a plant brew whose active component is N,N-dimethyltryptamine [DMT]), and 5-methoxy-DMT (5-MeO-DMT from the Bufo alvarius toad). These compounds have been used for centuries in traditional rituals and ethno-medicinal settings by indigenous peoples, particularly in the Americas (Carhart-Harris and Goodwin, 2017; McKenna and Riba, 2018).
Two randomized, placebo-controlled, crossover trials, one using low-dose psilocybin (1 or 3 mg/70 kg) as an active placebo (n = 51) and the other using niacin (n = 29), found that psilocybin (22 or 30 mg/70 kg) decreased Hospital Anxiety & Depression Scale (HADS) scores in terminally ill cancer patients with mood disorders; in 1 trial, more than 80% of participants met remission criteria (>50% HADS reduction to <7 overall score) the day after administration. Furthermore, in both trials, 60%–80% of participants met response criteria (>50% HADS reduction) at or beyond 6 months (Griffiths et al., 2016; Ross et al., 2016). A third randomized, placebo-controlled, crossover trial that examined psilocybin (0.2 mg/kg) vs a niacin placebo (n = 12) reported significant improvement in Beck Depression Inventory scores in individuals with advanced-stage cancer and anxiety disorders, but only after 6 months (Grob et al., 2011). Finally, an open-label trial of 20 TRD participants reported large group reductions (Cohen’s d = 1.4) in Self-Reported Quick Inventory of Depressive Symptoms (QIDS-SR) scores 6 months after 2 psilocybin sessions (Carhart-Harris et al., 2018). In light of these findings, the FDA granted psilocybin breakthrough therapy designation for 2 multi-site, phase 2 clinical trials for TRD, and in 2019 the European Medicines Agency approved psilocybin for a phase 3 clinical trial for TRD (Compass Pathways, 2019; Haridy, 2019).
Though psilocybin is the most studied agent, antidepressant effects have also been reported for other SPs. A randomized trial of Ayahuasca using a zinc-oxide brew that mimicked Ayahuasca’s appearance and nausea-inducing side effects as an active placebo found that 64% of 29 Ayahuasca-naive TRD participants had a 50% reduction in depressive symptoms from baseline 7 days later (Palhano-Fontes et al., 2019). In this study, as well as in 2 open-label trials of 6 (Osório et al., 2015) and 14 (Sanches et al., 2016) depressed participants given Ayahuasca, significant reductions in depressive symptoms were observed the following day. In addition, a placebo-controlled pilot study found that LSD produced trend-level reductions in HADS scores 2 months post-administration in 12 participants experiencing end-of-life anxiety (Gasser et al., 2014). Finally, a recent observational study found that 43 participants who inhaled 5-MeO-DMT had significant reductions in depressive, anxiety, and stress symptoms within 24 hours and that these effects persisted for 4 weeks (Uthaug et al., 2019); approximately three-quarters of the participants were healthy volunteers (HVs), and the remaining participants suffered from a variety of psychiatric disorders.
Although this evidence is still preliminary, future studies may well suggest that SPs should be added to the armamentarium of rapid-acting antidepressant drugs (RAADs). This, of course, begs the question: Why do pharmacotherapies such as ketamine and SPs—which are mechanistically distinct and do not share the same pharmacodynamic profile—nevertheless share such similar fundamental characteristics with regard to their ability to rapidly alter mood? Uncovering how serotonergic RAADs work would bring the field closer to being able to optimize their application, develop novel drugs, and generally improve our understanding of the molecular, neural, and pathophysiological underpinnings of depression. Rather than exhaustively review the clinical evidence for these pharmacotherapies, this paper seeks to summarize areas of overlap between SP and ketamine research—including converging cellular and molecular mechanisms as well as physiological, imaging, and behavioral findings—and considers the possibility of a common, downstream mechanism of action.
Converging Evidence for Shared Characteristics Between Ketamine and Serotonergic Psychedelics
Rapid and Transient Psychoactive Effects
Arguably the most salient feature of these pharmacotherapies is their rapid and transient induction of psychoactive symptoms. With regard to ketamine, a major goal of current research is to produce a widely distributable antidepressant agent that lacks psychoactive side effects. Thus, ketamine research has largely sought to understand the mechanisms underlying its antidepressant efficacy rather than study its psychoactive profile. Furthermore, ketamine’s antidepressant effects occur at subanesthetic doses (0.5 mg/kg) that appear to be relatively well-tolerated and create mild, transient psychoactive effects. For instance, a recent study assessing side effects associated with a 0.5-mg/kg ketamine infusion in 163 participants with TRD across 4 clinical trials reported that only 50% experienced SP-like psychoactive effects (e.g., the sensation of floating), and 80% reported “feeling strange, weird, or bizarre” (Acevedo-Diaz et al., 2020). This is not to say that ketamine’s psychoactive effects are not well-documented (Reiff et al., 2020). In fact, the earliest descriptions of subanesthetic-dose ketamine (0.5 mg/kg) administration in HVs reported effects such as altered perception of time and space, loss of a sense of self, and visual hallucinations (Krystal et al., 1994). Since then, multiple studies have confirmed that psychoactive effects occur at or below this standard antidepressant dose (Höflich et al., 2015; Schartner et al., 2017; Vlisides et al., 2018; Li and Mashour, 2019).
Nevertheless, due to the dosing disparity between ketamine and SPs, the prevalence of any kind of psychoactive effect is far more common in clinical trials of SPs than of ketamine. While the lowest dose of various SPs needed to produce antidepressant effects is presently unknown, the high dose of psilocybin (~30 mg/70 kg) used in antidepressant trials causes strong psychoactive effects in virtually all participants (Griffiths et al., 2006), and therapeutic Ayahuasca doses are typically the same as those used to elicit “visions” in religious ceremonies (~1.67 mg/kg DMT) (Sanches et al., 2016).
Furthermore, far more research has examined the therapeutic relevance of psychoactive effects with regard to SPs than ketamine. In particular, growing evidence suggests that particular experiences during the psychoactive period are uniquely predictive of therapeutic outcome with SPs (Kyzar et al., 2017). The most consistent finding thus far with regard to SPs is that “mystical experiences” increase the likelihood and magnitude of depressive symptom reductions (Barrett and Griffiths, 2018). Mystical experiences—reportedly stable across time periods and cultures—are broadly defined as psychological phenomena where individuals report experiences of bliss, sacredness, transcendence of space and time, and encounters with greater truths (Johnson et al., 2019). With SP therapy, such experiences appear to increase the likelihood and magnitude of depressive symptom reductions (Barrett and Griffiths, 2018). In 1 of the 3 randomized, double-blind, placebo-controlled psilocybin trials discussed above, scores on the 30-item Mystical Experience Questionnaire (MEQ30) mediated the sustained reductions in HADS scores observed 5 weeks later (n = 51) (Griffiths et al., 2016). In another such study, sustained reductions in HADS and Beck Depression Inventory scores 6.5 months after treatment were again mediated by MEQ30 scores (n = 29) (Ross et al., 2016). In addition, in the open-label study of 20 TRD participants treated with psilocybin on 2 occasions, sustained QIDS-SR reductions at 5 weeks were predicted by scores on a 5-Dimensional Altered States of Consciousness Rating Scale (5D-ASC) subscale approximating the MEQ30 (Roseman et al., 2018). Therapeutic insights are not reported in qualitative studies of ketamine treatment and may help explain the heightened duration of antidepressant effects associated with SPs (van Schalkwyk et al., 2018; Carhart-Harris and Friston, 2019; Lascelles et al., 2019). However, the necessity of such insights for the therapeutic efficacy of both SPs and ketamine remains to be systematically investigated.
It should also be noted that the effects of subjective experiences on overall drug action have not been detected in every study. For instance, in the randomized, double-blind, placebo-controlled study of Ayahuasca in 29 TRD participants, researchers reported a negative correlation between an MEQ30 subscale (“transcendence of time and space”) and reduction in Montgomery-Asberg Depression Rating Scale (MADRS) scores at day 7 (Palhano-Fontes et al., 2019). In addition, the follow-up to Ross and colleagues’ (Ross et al., 2016) clinical trial found that persisting antidepressant effects 3.2–4.5 years later were no longer mediated by individuals’ acute MEQ30 scores, though the sample size had been reduced by approximately 50% in this analysis (Agin-Liebes et al., 2020).
While the connection between ketamine’s psychoactive and therapeutic effects has received far less attention, it appears to be considerably weaker. For instance, a systematic review of 8 studies found that the relationship between dissociation and antidepressant effect was mixed, and only 3 of the 8 analyses found a relationship between antidepressant response to ketamine and psychosis scores assessed via the Clinician-Administered Dissociative States Scale (CADSS) (Mathai et al., 2020). Furthermore, for those studies that did observe a significant relationship, the explained variance of dissociative experiences for antidepressant response was 12%–21% (Mathai et al., 2020). Most clinical studies investigating ketamine’s psychoactive effects in TRD trials have used the CADSS, but the only psychometric evaluation of the CADSS for this purpose found that it failed to capture much of ketamine’s psychoactive profile (van Schalkwyk et al., 2018). One observational study of 31 participants undergoing repeated infusions at a community ketamine clinic used the 5D-ASC, a questionnaire capable of assessing the full spectrum of ketamine’s psychoactive effects, and found that participants’ subjective experience (e.g., drug-induced anxiety) during the first in a series of ketamine infusions negatively predicted MADRS score reductions at the end of approximately 2 weeks of infusions (Aust et al., 2019). Unlike results observed with psilocybin, the 5D-ASC subscale score—which approximates the MEQ30—was not associated with long-term antidepressant effects.
It is also important to note that differences in therapeutic setting constitute a major confound for comparing subjective experiences and efficacy associated with these compounds (Carhart-Harris et al., 2018). In accordance with guidelines established by Johnson and colleagues, SPs are typically administered after extensive psychological preparation in soothing surroundings that may include attractive furnishings and supportive music (Johnson et al., 2008); research suggests that such measures may reduce the chance of distressing reactions. In an open-label trial of psilocybin for TRD (n = 19), the manner in which music was experienced during psilocybin administration predicted outcome at 1 week, while participants’ subjective ratings of peak drug effect intensity did not (Kaelen et al., 2018). In contrast, virtually nothing is known about the relationship between the setting in which ketamine infusions take place, subjective experience, and outcome; ketamine is typically administered in more clinical settings such as hospitals or clinics. While some clinicians have argued that the context in which ketamine is administered heavily influences subjective experience and efficacy (Kolp et al., 2014), this claim requires further investigation.
Thus, despite some commonalities between the psychoactive component of these pharmacologically distinct therapies, the connection between psychoactive impact and antidepressant efficacy remains unclear and requires more rigorous research. The evidence linking ketamine’s antidepressant effects to its psychoactive effects is weak and, although early evidence suggests that psychoactive effects observed in SP trials may be linked to antidepressant effects, it is difficult to draw firm conclusions given small sample sizes, often improper trial designs, and functional unblinding (Reiff et al., 2020). Indeed, though some evidence suggests that type of psychoactive experience influences outcome, the hypothesis that the psychoactive effects of RAADs correlate with target engagement or are byproducts of various brain states marked by increased neuroplasticity requires systematic investigation. Moreover, much remains unknown regarding the extent of antidepressant and psychoactive effects with SPs and the lowest dose needed to produce such effects. Comparison studies optimized to compare antidepressant doses of SPs vs ketamine are needed to fully evaluate this question.
Cellular and Molecular Effects
Studies suggest that both ketamine and SPs rapidly facilitate changes in neuroplasticity such as neurite growth, synapse formation, and strengthened synaptic connections (Table 1). In particular, evidence suggests that both ketamine and SPs increase the expression of neuroplasticity-regulating genes. In rodents, i.p. ketamine injections resulted in an antidepressant response observed concurrently with increased brain-derived neutrotrophic factor (Bdnf) gene expression and translation in the hippocampus and across the cortex (Garcia et al., 2008; Autry et al., 2011; Silva Pereira et al., 2017). BDNF is known to promote neuronal growth and plasticity and has often been implicated in the pathophysiology of depression (Groves, 2007). Other studies have shown that ketamine also induces glutamate signaling-related neuroplasticity genes such as Activity Related Cytoskeletal protein (Arc) and Homer1a (de Bartolomeis et al., 2013; Bagot et al., 2017). With regard to SPs, DOI injections have been found to induce Bdnf expression in the rat neocortex (Vaidya et al., 1997), and both LSD and DOI administration in rodent models increased expression of Bdnf, Arc, Nor1, egr-1, sgk, Ania3, C/EBP-β, and Iκβ-α (Nichols and Sanders-Bush, 2002; Nichols et al., 2003; González-Maeso et al., 2007; Martin et al., 2014; Martin and,Nichols, 2016). All of these genes have in some way been linked to synaptic strength or neuronal growth and can be induced through G-protein-coupled receptor pathways linked primarily to stimulation of 5-HT2A receptors (Martin and Nichols, 2017).
Table 1.
Representative Publications Showing Common Cellular and Molecular Effects of Ketamine and Serotonergic Psychedelics in Rodent Models
Abbreviations: + + +, strong evidence (3 or more studies); + +, moderate evidence (2 studies); +, weak evidence (1 study); 5-MeO-DMT, 5-methoxy-DMT; AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; DMT, N,N-dimethyltryptamine; DOI, 2,5-Dimethoxy-4-iodoamphetamine; LSD, lysergic acid diethylamide-25; mTOR, mechanistic target of rapamycin; NEE, no existing evidence; TrkB, tropomyosin-related kinase B.
This gene induction may promote neuroplasticity and some of the key downstream effects associated with RAADs. Ketamine’s neuroplastic effects are well established (for review, see Zanos and Gould, 2018). Briefly, in rats, ketamine administration increased spine synapse number and synaptic strength in the prefrontal cortex (PFC); both this effect and ketamine’s antidepressant-like effects were abolished by pharmacological blockade of mechanistic target of rapamycin (mTOR) or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Li et al., 2010). In addition, in the Flinders Sensitive Line rat model of depression, ketamine rapidly restored apical dendritic spine deficits in pyramidal neurons of the hippocampal CA1 region within 60 minutes of infusion (Treccani et al., 2019). Other studies have reported that BDNF is required for ketamine’s antidepressant and synaptogenic effects (Autry et al., 2011; Liu et al., 2012; Lepack et al., 2014). In addition, preclinical studies have suggested that these neuroplastic effects may lead to antidepressant effects by altering cortical connectivity and subsequent functionality. For instance, a recent mouse model study of depression-like phenotypes found that ketamine infusion selectively reversed stress-induced loss of dendritic spines and coordinated the multicellular ensemble activity of PFC neurons 2 days post-treatment, an effect associated with a sustained, but not immediate, antidepressant response (Moda-Sava et al., 2019).
Evidence suggests that SPs similarly promote neuroplasticity (Nichols, 2016; Ly et al., 2018). In a recent study, DMT, LSD, and DOI all led to increased spine density, dendritic branching, and synapse formation in cultured rat cortices as measured by super-resolution structured illumination microscopy (Ly et al., 2018). In vivo treatment with LSD and DOI caused the same effects in Drosophila brains, and ex vivo slice recordings also revealed synaptic potentiation in the form of increased spike amplitudes and frequencies (Ly et al., 2018). In addition, i.p. administration of DMT at antidepressant-like concentrations led to spinogenesis in rat cortices examined hours later. This effect was abolished by mTOR blockade or antagonism of either the 5-HT2A receptor or tropomyosin-related kinase B (TrkB), BDNF’s primary target and an upstream activator of mTOR. When researchers performed the same assays with ketamine, they found that SPs were significantly more potent and effective than ketamine in promoting neuritogenesis (Ly et al., 2018), suggesting that the longer duration of antidepressant effects associated with SP administration may result from more efficacious neurotrophic induction. Similar results were obtained via intracerebroventricular injections of 5-MeO-DMT in adult mice; researchers reported increased neuronal proliferation in the dentate gyrus as measured by a significant increase in bromodeoxyuridine cells as well as increased dendritic tree complexity in granule cells (Lima da Cruz et al., 2018).
Although many of these neuroplastic effects still need to be verified in humans, clinical evidence supports BDNF’s role in the rapid-acting properties of RAADs. However, the evidence is mixed for ketamine, with some studies supporting these findings (Laje et al., 2012; Duncan et al., 2013; Haile et al., 2014) and other studies finding no such evidence (Machado-Vieira et al., 2009). With regard to SPs, 1 clinical trial of Ayahuasca for TRD (n = 28) found that BDNF levels increased 48 hours post-treatment and that this increase correlated with MADRS score reductions (de Almeida et al., 2019). However, another study found no changes in plasma BDNF levels in 28 HVs given LSD (Holze et al., 2020). Regardless of the therapeutic agent examined, a key limitation for all of these studies is the inability to directly assess BDNF activity in the brain.
In terms of immediate drug effects, both ketamine and SPs appear to trigger a “glutamate surge” (Razoux et al., 2007; Vollenweider and Kometer, 2010). For ketamine, this effect is well-documented and widely believed to be relevant to its antidepressant effects (Duman et al., 2016, 2019; Chowdhury et al., 2017; Kadriu et al., 2019). Studies similarly suggest that SPs cause a glutamate surge, primarily in layer V pyramidal neurons expressing 5-HT2A receptors (Lambe and Aghajanian, 2006; De Gregorio et al., 2018). For instance, systemic LSD or DOI administration increased glutamate concentrations as measured by in vivo microdialysis in rat prefrontal and somatosensory cortices (Scruggs et al., 2003; Muschamp et al., 2004). In both cases, 5-HT2A receptor antagonists abolished the glutamate surge. Another rat study found that DOI injection increased expression of the early-activation gene cFos, a marker of neuronal activity, in a subset of 5-HT2A receptor-expressing neurons (Martin and,Nichols, 2016). This active population was localized primarily to the medial prefrontal cortex (mPFC), somatosensory cortex, orbital cortex, and claustrum—regions overwhelmingly composed of glutamate-releasing pyramidal neurons (Martin and,Nichols, 2016).
The increases in cortical glutamate noted in preclinical ketamine studies have been confirmed via carbon-13 magnetic resonance spectroscopy in both HVs and depressed human participants (Abdallah et al., 2018b). Such proof-of-concept studies are sparse for SPs. One PET study reported that LSD administration in HVs increased metabolism in the primarily glutamatergic frontal cortices, suggesting increased glutamate signaling (Carhart-Harris et al., 2016). A recent double-blind, placebo-controlled trial using ultra-high field 7T MRS found that psilocybin (0.17 mg/kg) acutely induced region-dependent alterations in PFC glutamate levels that correlated with behavioral changes during the psychoactive state (Mason et al., 2020).
With regard to ketamine, the glutamate surge associated with its administration translates into increased AMPAR throughput, which likely triggers BDNF release and activates mTOR (Duman, 2018; Olson, 2018). This AMPA activity appears to be critical, as rodent studies found that AMPA antagonists abolished ketamine’s behavioral, antidepressant, and mTOR-stimulating effects (Moghaddam et al., 1997; Maeng et al., 2008; Li et al., 2010). To date, few studies have directly examined the relevance of AMPAR throughput in SPs, although AMPAR activation is known to be necessary for the behavioral effects and sustained glutamatergic activity of DOI in rodents (Zhang and Marek, 2008; Marek, 2018).
Despite the evidence suggesting that SPs may exhibit similar molecular and cellular effects as ketamine (see Table 1; Figure 1), it should be noted that this evidence exists only for a few SPs and primarily for DOI, which has not been clinically investigated. Hence, there are far more evidence-based data stemming from preclinical and translational research for ketamine’s cellular and molecular mechanisms than for that of SPs (see Table 1). Moreover, no studies investigating SPs have yet established a link between these molecular/cellular effects and antidepressant efficacy in rodent models of depression, a gap that should be addressed in future preclinical studies. However, studies have shown that DMT (Cameron et al., 2018), LSD, and psilocybin (Hibicke et al., 2020) all exert antidepressant-like effects in rats, as assessed via the forced swim test. Interestingly, and consistent with the clinical literature, one such study reported that LSD and psilocybin—but not ketamine—produced antidepressant-like effects that persisted 5 weeks later (Hibicke et al., 2020).
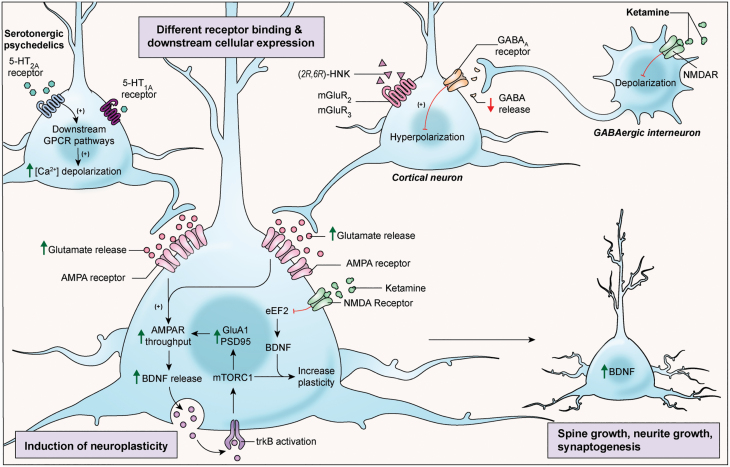
Figure 1.
The cellular mechanisms underlying ketamine and serotonergic psychedelics (SPs) may converge at glutamatergic synapses. The mechanism of action of SPs primarily begins with 5-hydroxytryptamine receptor 2A (5-HT2A) receptor agonism and activation (although 5-hydroxytryptamine receptor 1A [5-HT1A] agonism is also present) that, in turn, leads to glutamate release and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) potentiation, with subsequent stimulation of tropomyosin-related kinase B (TrkB), the primary target of brain-derived neurotrophic factor (BDNF), and an upstream activator of mechanistic target of rapamycin complex 1 (mTORC1). In contrast, ketamine selectively blocks a subset of N-methyl-D-aspartate receptors (NMDARs) localized to gamma aminobutyric acid (GABA)ergic interneurons, though the mechanisms underlying this action are not fully elucidated. This causes disinhibition of their glutamatergic (pyramidal) target neurons, triggering a surge in extracellular glutamate and correspondingly elevated non-cell autonomous glutamatergic synaptic transmission coupled with AMPA potentiation. Ketamine may also induce rapid BDNF translation in brain areas, in part through reduced phosphorylation and activation of eukaryotic elongation factor (eEF2). Growing evidence suggests that the (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] metabolite acts independently of ketamine at presynaptic sites to promote glutamate signaling. As with SPs, the enhanced neural activity triggers BDNF release and is followed by transient activation of the mTOR pathway and increased expression of diverse proteins acting at glutamatergic synapses, along with functional strengthening of glutamatergic synapses. GPCR, G protein-coupled receptor; mGluR, metabotropic glutamate receptor; PSD95, post-synaptic density protein 95.
It should also be noted that, although similar, important differences remain with regard to the precise mechanisms underlying these drugs’ actions in the brain. For example, ketamine disinhibits thalamo-cortical communication specifically via upstream cortical and subcortical somatostatin and parvalbumin gamma aminobutyric acid (GABA)-ergic neurons (Gerhard et al., 2020), whereas SPs seem to directly activate pyramidal neurons (Puig et al., 2003) as well as cause extrasynaptic glutamate spillover (Lambe and Aghajanian, 2006). Such differences may explain the varied psychoactive phenomenology and/or duration of antidepressant efficacy between ketamine and SPs and warrant additional study (Carhart-Harris and Friston, 2019).
Electrophysiological and Neuroimaging Observations
The psychoactive effects of both ketamine and SPs are accompanied by acute (during administration) and delayed (measured hours after dosing) electrophysiological and hemodynamic changes in brain activity (see Table 2). A recent study in HVs reported that subanesthetic-dose ketamine, LSD, and psilocybin all similarly increased spontaneous magnetoencephalography (MEG) signal complexity as measured by Lempel-Ziv complexity (Schartner et al., 2017); notably, Lempel-Ziv complexity measures the number of different electrophysiological timeseries patterns and their rate of occurrence—here, a canonical measure for “level of consciousness.” In that study, the psychoactive effects of both SPs and ketamine led to greater signal complexity than normal waking consciousness, reflecting an elevated level of consciousness following drug administration (Schartner et al., 2017).
Table 2.
Common Electrophysiological and Neuroimaging Effects of Ketamine and Serotonergic Psychedelics
Abbreviations: + + +, strong evidence (3 or more studies); + +, moderate evidence (2 studies), +, weak evidence (1 study); DMN, default mode network; HV, healthy volunteer; PFC, prefrontal cortex.
One MEG study that directly compared ketamine, LSD, and psilocybin found that all 3 drugs induced altered states of consciousness characterized by decreased spectral power and lower source-level functional connectivity (Pallavicini et al., 2019). Indeed, power reductions in low-frequency signals as measured by resting-state MEG/EEG are among the most consistently reported observations following ketamine and SP administration, with reduced alpha band power most closely tracking psychoactive/hallucinatory effects (Muthukumaraswamy et al., 2013; Tagliazucchi et al., 2014; de la Salle et al., 2016; Atasoy et al., 2018; Vlisides et al., 2018). Similar alterations in alpha band power have also been reported in response to illusory stimuli following psilocybin administration in HVs (Kometer et al., 2013). Such reductions in low-frequency oscillations may also indicate a general trend toward decreased long-range communication and increased modularity during psychoactive states (Buzsaki, 2011).
Interestingly, while ketamine has consistently been reported to acutely increase gamma band power in HVs (Gilbert and Zarate, 2020)—a phenomenon linked to pyramidal cell disinhibition downstream of NMDAR antagonism (Homayoun and Moghaddam, 2007; Widman and McMahon, 2018)—studies assessing the effects of SPs on gamma power have yielded mixed results. For example, in HVs, decreased gamma power was observed in frontal and motor regions following psilocybin administration during a visuomotor task (Muthukumaraswamy et al., 2013), and increased resting-state gamma power was noted 1 hour after Ayahuasca ingestion (Schenberg et al., 2015). Gamma power may be of particular interest because delayed gamma power increases following ketamine’s psychoactive period may be a putative biomarker of antidepressant response in TRD (Gilbert and Zarate, 2020), potentially reflecting increased synaptic efficiency and synaptogenesis mediated by AMPAR glutamatergic throughput (Cornwell et al., 2012; Nugent et al., 2019). To our knowledge, no study has yet examined the delayed effects of SPs on gamma power in TRD.
These acute electrophysiological findings are complemented by functional magnetic resonance imaging (fMRI) studies measuring resting-state functional connectivity (RSFC) changes following drug administration (see Table 2). However, little of this work has examined SPs. Three studies exploring ketamine’s effects on connectivity measured via global brain connectivity (GBC)—a graph-based measure of intrinsic whole-brain network connectivity (Cole et al., 2012)—suggested it may be a biomarker of antidepressant response; individuals who responded to ketamine had higher delta GBCr values in the PFC, caudate, and insula (Abdallah et al., 2017, 2018a, 2018b). Nevertheless, a recent randomized, placebo-controlled trial did not replicate this finding 48 hours post-ketamine (Kraus et al., 2020). Although only 1 study examined the effect of SPs on GBC, it found that, unlike ketamine, LSD acutely decreased GBC in the PFC in HVs (Preller et al., 2018).
Another area of particular interest is alterations in default mode network (DMN) functional connectivity, consistent with a growing body of literature reporting the relevance of DMN activity to the pathophysiology of depression (Whitfield-Gabrieli and Ford, 2012; Barrett and Griffiths, 2018; Li et al., 2018). A double-blind, placebo-controlled, crossover study of 33 individuals with MDD and 25 HVs who received ketamine found that the MDD group had increased connectivity between the DMN and other nodes at 2 days, but not 10 days, post-treatment (Evans et al., 2018). RSFC between the DMN and the insula (part of the salience network), which was reduced in MDD participants compared with HVs prior to treatment, normalized in MDD participants following ketamine and returned to baseline at the time point when most participants relapsed (Evans et al., 2018). With regard to SPs, a recent study looked at changes in RSFC in 19 TRD participants 1 day after the second of 2 psilocybin administrations (8 days after the first) and found increased DMN connectivity post-treatment (Carhart-Harris and Goodwin, 2017); stronger RSFC between particular nodes predicted sustained response 5 weeks later.
One consistent fMRI finding is that the effects of RAADs correlate with increased functional connectivity between resting-state functional networks and decreased within-network functional connectivity, sometimes referred to as “network disintegration” (Bonhomme et al., 2016; Atasoy et al., 2018; Mueller et al., 2018; Fleming et al., 2019). As regards the DMN in particular, 1 randomized, double-blind, placebo-controlled, crossover study of 24 HVs given subanesthetic-dose ketamine recently replicated findings from an earlier study of 8 HVs reporting acute decreases in functional connectivity to the mPFC node of the DMN (Bonhomme et al., 2016; Zacharias et al., 2020). The earlier study also noted between-network disintegration; the strength of the typical anti-correlations between the DMN and other functional networks was significantly weaker post-ketamine administration (Bonhomme et al., 2016). Similarly, an uncontrolled study of 15 HVs who received psilocybin found decreased mPFC activity and functional connectivity with the posterior cingulate cortex node of the DMN (Carhart-Harris et al., 2012). In another study of 10 HVs, Ayahuasca decreased activity in all DMN nodes and decreased functional connectivity between the posterior cingulate cortex and precuneus nodes (Palhano-Fontes et al., 2015). Finally, an LSD study of 20 HVs also observed decreased RSFC within the DMN coupled with increased connectivity between the DMN and other large-scale brain networks (Carhart-Harris et al., 2016).
One final area of research overlap concerns drug-induced changes in emotional face processing in the amygdala. Separate analyses of fMRI data collected from TRD participants in an open-label psilocybin trial found that increased day 1 amygdala activation in response to fearful vs neutral face stimuli predicted QIDS-SR score reductions at 1 week (Roseman et al., 2018); in addition, reduced ventromedial PFC-amygdala functional connectivity correlated with Ruminative Response Scale scores at day 7 (Mertens et al., 2020). In contrast, near-opposite findings have been reported in response to ketamine. For example, reductions in MADRS scores correlated with reduced amygdala responsivity to angry face stimuli and increased responsivity to happy face stimuli 2 days post-ketamine in 33 TRD participants (Reed et al., 2018). In another study of 27 MDD participants, repeated doses of ketamine decreased amygdala responsivity to both fearful and happy face stimuli 1 to 3 days after 4 serial ketamine infusions (Loureiro et al., 2020). These intriguing preliminary findings suggest that the antidepressant effects of both ketamine and SPs involve changes in brain network connectivity and functionality; nevertheless, the small sample sizes and lack of controls in SP studies, as well as the large variety of ketamine studies, suggest that few converging findings can confidently be concluded at this time. Additional research is needed to better illuminate these links.
Activity-Induced Neuroplasticity: an Exploration of Common Cellular and Circuit-Based Mechanisms
Taken together, the evidence reviewed above suggests a potentially shared mechanism wherein both ketamine and SPs may engender rapid neuroplastic effects in a glutamatergic activity-dependent manner. These different RAADs, though seemingly distinct, both appear to produce acute alterations in cortical network activity that may initially produce psychoactive effects and later produce milder, sustained changes in network efficiency associated with therapeutic response.
In this context, the first step in serotonergic RAAD pharmacotherapy is molecular binding (see Figure 1). Notably, serotonergic RAADs primarily exert their actions by stimulating 5-HT2A receptors, which, in turn, leads to glutamate-dependent increases in pyramidal neuron activity in the PFC (Puig et al., 2003; Béïque et al., 2007), thus modulating prefrontal network activity (Vollenweider and Kometer, 2010). This extracellular glutamate surge also triggers the activation of AMPARs located in the same neurons throughout the cortex. Given the central and ubiquitous role that glutamate-AMPA signaling plays in the cortex and in conscious experience, this increase in cortical excitatory signaling may be the origin of the entropic brain state underlying these agents’ psychoactive effects (Buzsaki, 2011). In this model, high AMPA throughput would lead to BDNF release and mTOR signaling (Cavalleri et al., 2018), triggering upregulation of plasticity genes associated with neural growth, strengthening certain synapses, and causing new synapses to form. In this manner, the effects of SPs and of ketamine would be accompanied by a highly plastic brain state capable of “rewiring” functional brain circuits. At present, however, the preliminary nature of many of the SP studies discussed above means that a common mechanism between SPs and ketamine remains largely speculative; further investigation is warranted.
Directions for Future Research
As reviewed above, RAADs currently under investigation may share downstream pharmacological effects, suggesting that their antidepressant effects may come about via related mechanisms. Given the prototypic nature of ketamine research and recent progress in this area, this platform could be applied to the investigation of entirely new classes of antidepressants with rapid and robust actions. In this context, research examining the therapeutic mechanisms of SPs should assess the “glutamate surge,” potentiation of AMPAR throughput, and plasticity properties in SPs as rigorously as in ketamine studies, connecting them to preclinical models of depression. A more complete understanding of the cellular and molecular mechanisms of SPs could lead to convergent drug targets with ketamine research, accelerating antidepressant drug development. As noted throughout this article, research into the mechanisms of action of RAADs remains in its infancy, and multiple research avenues of likely relevance exist. As one notable example, multiple preclinical (Réus et al., 2015) and clinical (Chen et al., 2018) studies suggest that ketamine may exert its antidepressant effects at least in part by reducing inflammatory tone, and similar evidence is emerging for SPs (Flanagan and Nichols, 2018; Galvão-Coelho et al., 2020).
Another key area of research is whether SPs would exert similar pharmacological efficacy in the absence of concomitant psychological support. Another avenue for further research includes understanding the disparity in symptom relief duration for ketamine vs SPs; in particular, understanding how SPs confer sustained symptom relief may be key to characterizing treatments that best reduce risk of relapse. An important caveat, however, is that, to date, the quantity and robustness of the preclinical and clinical data for ketamine far supersede those currently available for SPs. An additional caveat is that many SP studies have been open-label—a significant limitation that needs to be addressed (Carhart-Harris et al., 2018)—and often conducted in participants with subsyndromal illness or mild depressive or anxious symptoms (Griffiths et al., 2016) who are not representative of the TRD population studied in ketamine trials (Schatzberg, 2020).
In the short term, rigorously controlled studies using active placebos that minimize issues of functional unblinding (an issue that also plagues ketamine research) should examine SPs to investigate the most successful ways to elicit antidepressant effects. Given the powerful and potentially life-altering effects of these agents, further research is particularly needed to determine clinical efficacy, optimal dosing, and administration characteristics. Ultimately, identifying common downstream mechanisms of action for these rapid-acting—but pharmacologically distinct —antidepressants has the potential to improve treatments for depression and other stress-related brain disorders.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. They also thank Sydney Shuster and Claire Punturieri for editing and consultation early in the drafting of this review as well as Erina He of the NIH Medical Arts program for producing the figure.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857); by a NARSAD Independent Investigator Award to Dr Zarate; and by a Brain and Behavior Mood Disorders Research Award to Dr Zarate.
Submitted to International Journal of Neuropsychopharmacology as an invited Review Article, July 2020. Revised October 2020.
Statement of Interest
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW (2017) Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Dutta A, Averill CL, McKie S, Akiki TJ, Averill LA, Deakin JFW (2018a) Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks, Calif) 2: 2470547018796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, de Graaf RA, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G, Mason GF (2018b) The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43:2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA, Park LT (2020) Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord 263:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, Bossis AP, Grigsby J, Fischer S, Ross S (2020) Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol 34:155–166. [DOI] [PubMed] [Google Scholar]
- Atasoy S, Vohryzek J, Deco G, Carhart-Harris RL, Kringelbach ML (2018) Common neural signatures of psychedelics: frequency-specific energy changes and repertoire expansion revealed using connectome-harmonic decomposition. Prog Brain Res 242:97–120. [DOI] [PubMed] [Google Scholar]
- Aust S, Gärtner M, Basso L, Otte C, Wingenfeld K, Chae WR, Heuser-Collier I, Regen F, Cosma NC, van Hall F, Grimm S, Bajbouj M (2019) Anxiety during ketamine infusions is associated with negative treatment responses in major depressive disorder. Eur Neuropsychopharmacol 29:529–538. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, LaBonté B, Peña CJ, Shen L, Wittenberg GM, Nestler EJ (2017) Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry 81:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Griffiths RR (2018) Classic hallucinogens and mystical experiences: phenomenology and neural correlates. In: Behavioral neurobiology of psychedelic drugs (Halberstadt AL, Vollenweider FX, Nichols DE, eds), pp 393–430. Berlin, Heidelberg: Springer Berlin Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R (2007) Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A 104:9870–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno MA, Jaquet O, Bahri MA, Plenevaux A, Boly M, Boveroux P, Soddu A, Brichant JF, Maquet P, Laureys S (2016) Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology 125:873–888. [DOI] [PubMed] [Google Scholar]
- Branchi I. (2011) The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36:339–351. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. (2011) Rhythms of the brain. 1st ed Oxford: Oxford University Press. [Google Scholar]
- Cameron LP, Benson CJ, Dunlap LE, Olson DE (2018) Effects of N, N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci 9:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. (2016) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113:4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker J, Watts R, Erritzoe DE, Kaelen M, Giribaldi B, Bloomfield M, Pilling S, Rickard JA, Forbes B, Feilding A, Taylor D, Curran HV, Nutt DJ (2018) Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl) 235:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109:2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ (2019) REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev 71:316–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Goodwin GM (2017) The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 42:2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G (2018) Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23:812–823. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, Cheng CM, Su TP (2018) Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res 269:207–211. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G (2017) Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS (2012) Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci 32:8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compass Pathways (2019) COMPASS pathways receives FDA breakthrough therapy designation for psilocybin therapy for treatment-resistant depression https://www.prnewswire.com/news-releases/compass-pathways-receives-fda-breakthrough-therapy-designation-for-psilocybin-therapy-for-treatment-resistant-depression-834088100.html. Accessed 24 January 2020.
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA Jr (2012) Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 72:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RN, Galvão ACM, da Silva FS, Silva EADS, Palhano-Fontes F, Maia-de-Oliveira JP, de Araújo LB, Lobão-Soares B, Galvão-Coelho NL (2019) Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol 10:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Sarappa C, Buonaguro EF, Marmo F, Eramo A, Tomasetti C, Iasevoli F (2013) Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry 46:1–12. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Enns JP, Nuñez NA, Posa L, Gobbi G (2018) d-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: mechanism of action and potential therapeutic applications in mood disorders. Prog Brain Res 242: 69–96. [DOI] [PubMed] [Google Scholar]
- de la Salle S, Choueiry J, Shah D, Bowers H, McIntosh J, Ilivitsky V, Knott V (2016) Effects of ketamine on resting-state EEG activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front Pharmacol 7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos RG, Bouso JC, Alcázar-Córcoles MÁ, Hallak JEC (2018) Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: a systematic review of systematic reviews. Expert Rev Clin Pharmacol 11:889–902. [DOI] [PubMed] [Google Scholar]
- Drewniany E, Han J, Hancock C, Jones RL, Lim J, Nemat Gorgani N, Sperry JK 3rd, Yu HJ, Raffa RB (2015) Rapid-onset antidepressant action of ketamine: potential revolution in understanding and future pharmacologic treatment of depression. J Clin Pharm Ther 40:125–130. [DOI] [PubMed] [Google Scholar]
- Duman RS. (2018) Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res 7:F1000 Faculty Rev-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, Krystal JH (2019) Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA (2013) Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 16:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA Jr (2018) Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry 84:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25:1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan TW, Nichols CD (2018) Psychedelics as anti-inflammatory agents. Int Rev Psychiatry 30:363–375. [DOI] [PubMed] [Google Scholar]
- Fleming LM, Javitt DC, Carter CS, Kantrowitz JT, Girgis RR, Kegeles LS, Ragland JD, Maddock RJ, Lesh TA, Tanase C, Robinson J, Potter WZ, Carlson M, Wall MM, Choo TH, Grinband J, Lieberman J, Krystal JH, Corlett PR (2019) A multicenter study of ketamine effects on functional connectivity: large scale network relationships, hubs and symptom mechanisms. Neuroimage Clin 22:101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, Chaki S (2017) Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaça MV, Liu R-J, Duman CH, Li X-Y, Chaki S, Duman RS (2020) Medial PFC AMPA receptor and BDNF signaling are required for the rapid and sustained antidepressant-like effects of 5-HT 1A receptor stimulation. Neuropsychopharmacology 45:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão-Coelho NL, de Menezes Galvão AC, de Almeida RN, Palhano-Fontes F, Campos Braga I, Lobão Soares B, Maia-de-Oliveira JP, Perkins D, Sarris J, de Araujo DB (2020) Changes in inflammatory biomarkers are related to the antidepressant effects of Ayahuasca. J Psychopharmacol 34:1125–1133. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32:140–144. [DOI] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, Brenneisen R (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 202:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ (2009) What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60:1439–1445. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, Taylor SR, Duman CH, Delpire E, Picciotto M, Wohleb ES, Duman RS (2020) GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest 130:1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Zarate CA Jr (2020) Electrophysiological biomarkers of antidepressant response to ketamine in treatment-resistant depression: gamma power and long-term potentiation. Pharmacol Biochem Behav 189:172856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–83; discussion 284. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 30:1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68:71–78. [DOI] [PubMed] [Google Scholar]
- Groves JO. (2007) Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry 12:1079–1088. [DOI] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ 3rd, De La Garza R 2nd, Charney DS, Newton TF, Mathew SJ (2014) Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridy R. (2019) Psilocybin for major depression granted breakthrough therapy by FDA https://newatlas.com/science/psilocybin-major-depression-mdd-usona-breakthrough-therapy-fda/. Accessed 18 April 2020.
- Hashimoto K. (2019) Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci 73:613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Gosden J, Smith SL (2018) Evaluating the abuse potential of psychedelic drugs as part of the safety pharmacology assessment for medical use in humans. Neuropharmacology 142:89–115. [DOI] [PubMed] [Google Scholar]
- Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD (2020) Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem Neurosci 11:864–871. [DOI] [PubMed] [Google Scholar]
- Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Windischberger C, Saria A, Kasper S, Winkler D, Lanzenberger R (2015) Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. Int J Neuropsychopharmacol 18:pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holze F, Vizeli P, Müller F, Ley L, Duerig R, Varghese N, Eckert A, Borgwardt S, Liechti ME (2020) Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22:603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142:143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Hendricks PS, Barrett FS, Griffiths RR (2019) Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther 197:83–102. [DOI] [PubMed] [Google Scholar]
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr (2019) Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 22:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Giribaldi B, Raine J, Evans L, Timmerman C, Rodriguez N, Roseman L, Feilding A, Nutt D, Carhart-Harris R (2018) The hidden therapist: evidence for a central role of music in psychedelic therapy. Psychopharmacology (Berl) 235:505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU (2016) Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolp E, Friedman H, Krupitsky E, Jansen K, Sylvester M, Young MS, Kolp A (2014) Ketamine psychedelic psychotherapy: focus on its pharmacology, phenomenology, and clinical applications. Int J Transpers Stud 33:84–140. [Google Scholar]
- Kometer M, Schmidt A, Jäncke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S (2017) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Kraus C, Wasserman D, Henter ID, Acevedo-Diaz E, Kadriu B, Zarate CA Jr (2019) The influence of ketamine on drug discovery in depression. Drug Discov Today 24:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Mkrtchian A, Kadriu B, Nugent AC, Zarate CA Jr, Evans JW (2020) Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology 45:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- Kyzar EJ, Nichols CD, Gainetdinov RR, Nichols DE, Kalueff AV (2017) Psychedelic drugs in biomedicine. Trends Pharmacol Sci 38:992–1005. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C Jr (2012) Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 72:e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK (2006) Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31:1682–1689. [DOI] [PubMed] [Google Scholar]
- Lascelles K, Marzano L, Brand F, Trueman H, McShane R, Hawton K (2019) Effects of ketamine treatment on suicidal ideation: a qualitative study of patients’ accounts following treatment for depression in a UK ketamine clinic. BMJ Open 9:e029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS (2014) BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18:pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BJ, Friston K, Mody M, Wang HN, Lu HB, Hu DW (2018) A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci Ther 24:1004–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mashour GA (2019) Cortical dynamics during psychedelic and anesthetized states induced by ketamine. Neuroimage 196:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima da Cruz RV, Moulin TC, Petiz LL, Leão RN (2018) A single dose of 5-MeO-DMT stimulates cell proliferation, neuronal survivability, morphological and functional changes in adult mice ventral dentate gyrus. Front Mol Neurosci 11:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro JRA, Leaver A, Vasavada M, Sahib AK, Kubicki A, Joshi S, Woods RP, Wade B, Congdon E, Espinoza R, Narr KL (2020) Modulation of amygdala reactivity following rapidly acting interventions for major depression. Hum Brain Mapp 41:1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23:3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA Jr (2009) Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 70:1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- Marek GJ. (2018) Interactions of hallucinogens with the glutamatergic system: permissive network effects mediated through cortical layer V pyramidal neurons. Curr Top Behav Neurosci 36:107–135. [DOI] [PubMed] [Google Scholar]
- Martin DA, Marona-Lewicka D, Nichols DE, Nichols CD (2014) Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology 83:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Nichols CD (2016) Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. Ebiomedicine 11:262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Nichols CD (2017) The effects of hallucinogens on gene expression. In: Behavioral neurobiology of psychedelic drugs. current topics in behavioral neurosciences, vol 36 (Halberstadt AL, Vollenweider FX, Nichols DE, eds), pp 137–158. Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW, Hutten NRPW, Jansen JFA, Stiers P, Feilding A, Ramaekers JG (2020) Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 45:2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai DS, Meyer MJ, Storch EA, Kosten TR (2020) The relationship between subjective effects induced by a single dose of ketamine and treatment response in patients with major depressive disorder: a systematic review. J Affect Disord 264:123–129. [DOI] [PubMed] [Google Scholar]
- McKenna D, Riba J (2018) New world tryptamine hallucinogens and the neuroscience of ayahuasca. In: Behavioral Neurobiology of Psychedelic Drugs (Halberstadt AL, Vollenweider FX, Nichols DE, eds), pp 283–311. Berlin, Heidelberg: Springer. [Google Scholar]
- Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL (2020) Therapeutic mechanisms of psilocybin: changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol 34:167–180. [DOI] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364:eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F, Musso F, London M, de Boer P, Zacharias N, Winterer G (2018) Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. Neuroimage Clin 19:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA (2004) Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res 1023:134–140. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD, Feilding A, Friston KJ, Nutt DJ (2013) Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33:15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttoni S, Ardissino M, John C (2019) Classical psychedelics for the treatment of depression and anxiety: a systematic review. J Affect Disord 258:11–24. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Garcia EE, Sanders-Bush E (2003) Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res 111:182–188. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E (2002) A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26:634–642. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2016) Psychedelics. Pharmacol Rev 68:264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Wills KE, Gilbert JR, Zarate CA Jr (2019) Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: a replication study. Psychiatry Res Neuroimaging 283:64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DE. (2018) Psychoplastogens: a promising class of plasticity-promoting neurotherapeutics. J Exp Neurosci 12:1179069518800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osório FdL, Sanches RF, Macedo LR, dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE, Osório FdL, Sanches RF, Macedo LR, dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatr 37:13–20. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F, Andrade KC, Tofoli LF, Santos AC, Crippa JA, Hallak JE, Ribeiro S, de Araujo DB (2015) The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. Plos One 10:e0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palhano-Fontes F, et al. (2019) Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med 49:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavicini C, Vilas MG, Villarreal M, Zamberlan F, Muthukumaraswamy S, Nutt D, Carhart-Harris R, Tagliazucchi E (2019) Spectral signatures of serotonergic psychedelics and glutamatergic dissociatives. Neuroimage 200:281–291. [DOI] [PubMed] [Google Scholar]
- Preller KH, Burt JB, Ji JL, Schleifer CH, Adkinson BD, Stämpfli P, Seifritz E, Repovs G, Krystal JH, Murray JD, Vollenweider FX, Anticevic A (2018) Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 7:e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Celada P, Díaz-Mataix L, Artigas F (2003) In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex 13:870–882. [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Léna I (2007) Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology 32:719–727. [DOI] [PubMed] [Google Scholar]
- Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA Jr (2018) Ketamine normalizes brain activity during emotionally valenced attentional processing in depression. Neuroimage Clin 20:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, Kalin NH, McDonald WM; the Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research (2020) Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 177:391–410. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, da Luz JR, Gonçalves RC, Vuolo F, Dal-Pizzol F, Carvalho AF, Quevedo J (2015) Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci Lett 584:83–87. [DOI] [PubMed] [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL (2018) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 8:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su Z, Corby P, Schmidt BL (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30:1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJH, Iliff J, Nutt DJ (2018) Psychiatry and the psychedelic drugs. Past, present and future. Neuropharmacology 142:200–218. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches RF, de Lima Osório F, Dos Santos RG, Macedo LR, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT Study. J Clin Psychopharmacol 36:77–81. [DOI] [PubMed] [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD (2017) Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7:46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF. (2020) Some comments on psychedelic research. Am J Psychiatry 177:368–369. [DOI] [PubMed] [Google Scholar]
- Schenberg EE, Alexandre JF, Filev R, Cravo AM, Sato JR, Muthukumaraswamy SD, Yonamine M, Waguespack M, Lomnicka I, Barker SA, da Silveira DX (2015) Acute biphasic effects of ayahuasca. Plos One 10:e0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY (2003) The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 346:137–140. [DOI] [PubMed] [Google Scholar]
- Silva Pereira V, Elfving B, Joca SRL, Wegener G (2017) Ketamine and aminoguanidine differentially affect Bdnf and Mtor gene expression in the prefrontal cortex of adult male rats. Eur J Pharmacol 815:304–311. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR (2014) Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 35:5442–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treccani G, Ardalan M, Chen F, Musazzi L, Popoli M, Wegener G, Nyengaard JR, Müller HK (2019) S-Ketamine reverses hippocampal dendritic spine deficits in flinders sensitive line rats within 1 h of administration. Mol Neurobiol 56:7368–7379. [DOI] [PubMed] [Google Scholar]
- Uthaug MV, Lancelotta R, van Oorsouw K, Kuypers KPC, Mason N, Rak J, Šuláková A, Jurok R, Maryška M, Kuchař M, Páleníček T, Riba J, Ramaekers JG (2019) A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology (Berl) 236:2653–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodríguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mañanas MÀ, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26:1161–1175. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk GI, Wilkinson ST, Davidson L, Silverman WK, Sanacora G (2018) Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: analysis of the Clinician Administered Dissociative State Scale. J Affect Disord 227:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlisides PE, Bel-Bahar T, Nelson A, Chilton K, Smith E, Janke E, Tarnal V, Picton P, Harris RE, Mashour GA (2018) Subanaesthetic ketamine and altered states of consciousness in humans. Br J Anaesth 121:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Widman AJ, McMahon LL (2018) Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115:E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias N, Musso F, Müller F, Lammers F, Saleh A, London M, de Boer P, Winterer G (2020) Ketamine effects on default mode network activity and vigilance: a randomized, placebo-controlled crossover simultaneous fMRI/EEG study. Hum Brain Mapp 41:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, Morris PJ, Stewart BW, Thomas CJ, Thompson SM, Moaddel R, Gould TD (2019) (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol 176:2573–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Marek GJ (2008) AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry 32:62–71. [DOI] [PubMed] [Google Scholar]