Abstract
Study Objectives
To investigate the dose-dependent impact of moderate alcohol intake on sleep-related cardiovascular (CV) function, in adult men and women.
Methods
A total of 26 healthy adults (30–60 years; 11 women) underwent 3 nights of laboratory polysomnographic (PSG) recordings in which different doses of alcohol (low: 1 standard drink for women and 2 drinks for men; high: 3 standard drinks for women and 4 drinks for men; placebo: no alcohol) were administered in counterbalanced order before bedtime. These led to bedtime average breath alcohol levels of up to 0.02% for the low doses and around 0.05% for the high doses. Autonomic and CV function were evaluated using electrocardiography, impedance cardiography, and beat-to-beat blood pressure monitoring.
Results
Presleep alcohol ingestion resulted in an overall increase in nocturnal heart rate (HR), suppressed total and high-frequency (vagal) HR variability, reduced baroreflex sensitivity, and increased sympathetic activity, with effects pronounced after high-dose alcohol ingestion (p’s < 0.05); these changes followed different dose- and measure-dependent nocturnal patterns in men and women. Systolic blood pressure showed greater increases during the morning hours of the high-alcohol dose night compared to the low-alcohol dose night and placebo, in women only (p’s < 0.05).
Conclusions
Acute evening alcohol consumption, even at moderate doses, has marked dose- and time-dependent effects on sleep CV regulation in adult men and women. Further studies are needed to evaluate the potential CV risk of repeated alcohol-related alterations in nighttime CV restoration in healthy individuals and in those at high risk for CV diseases, considering sex and alcohol dose and time effects.
Keywords: alcohol, sleep, cardiovascular, autonomic, polysomnography
Statement of Significance.
Excessive chronic alcohol consumption has detrimental effects on cardiovascular (CV) health. Mechanisms underlying this association are not fully elucidated. Our findings highlight that acute evening alcohol consumption, even at levels below those considered indicative of heavy drinking, leads to pronounced sex-, time-, and dose-dependent alterations in autonomic and CV restoration during sleep in adult men and women. Results suggest a potential mechanism by which alcohol may adversely impact CV functioning.
Introduction
The 2015–2020 U.S. Dietary Guidelines for Americans [1] states that “if alcohol is consumed, it should be in moderation,” operationalized as up to one drink per day for women and up to two drinks per day for men of legal drinking age. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), excessive alcohol consumption includes binge drinking (>3 drinks for women and >4 drinks for men within about 2 h) and heavy drinking (>7 drinks/week for women and >14 drinks/week for men, or >3 drinks for women and >4 drinks for men on any day), reflecting daily and weekly recommended drinking limits.
Alcohol is among the most widely used psychoactive substances. Substantial scientific and public consensus exists in considering habitual heavy alcohol consumption and irregular heavy drinking patterns (e.g. binge drinking) as a health-related and economic burden on society [2], being associated with severe negative health outcomes including risks for cardiovascular (CV) disease (CVD) [3–5]. Less clear is the evidence evaluating the impact of drinking below heavy drinking levels on an individual’s health and mortality. There is epidemiological evidence suggesting no negative associations [6, 7] or even benefits of light or moderate habitual alcohol use [3, 4, 8].
However, controlled laboratory investigations, mainly conducted in small samples of awake young men, have demonstrated that alcohol has complex transient effects on autonomic nervous system (ANS) and CV function, which depend on several factors including the observed outcome measures, the amount and time of alcohol consumption, the timing of the assessments (mostly within a few hours of intake), and the sample characteristics and genetic factors [9]. Alcohol ingestion, even at low or moderate doses (approximately up to four standard drinks), resulted in transient alterations across several indices of ANS and CV function, lasting for several hours after intake. Multiple effects have been documented [10–17], including increases in heart rate (HR) and sympathetic ANS activity (e.g. muscle sympathetic nerve activity), and alterations in CV control mechanisms, that is decreases in baroreflex sensitivity (BRS), and reductions in heart rate variability (HRV) particularly in its high-frequency (vagal) component, which is an important ANS indicator of system flexibility and biomarker of health [18]. The effects of alcohol on BP are more complex. BP appears to be reduced or unchanged after low doses, possibly due to direct vasodilation effects dominating over sympathetic vasoconstriction [10], and increasing BP after high doses, possibly through sympathetic vasoconstriction activation [9, 12].
There is a lack of data about the impact of presleep alcohol consumption on sleep-dependent CV restoration. This is surprising given that: (1) due to social and cultural factors, alcohol consumption is prevalent in the evening [19]; (2) due to its sedative effects, alcohol is frequently erroneously used as an “over the counter” self-medication to promote sleep in those having trouble sleeping [20], a pathway that could lead to dependence; (3) sleep is known to be crucial for CV homeostasis (“sleep as a CV holiday”) [21, 22], and the alcohol-stimulating effects may disrupt sleep CV regulation. Understanding the acute impact of alcohol on the dynamics of sleep ANS and CV regulatory processes is a fundamental step in elucidating the relationship between repeated alcohol exposure, chronic alteration in CV regulation, and CVD risks.
In a controlled laboratory polysomnographic (PSG) study, the acute effect of different doses of presleep alcohol intake on sleep ANS function was assessed from the analysis of HRV in 10 healthy male students [23]. Following high-dose (1.0 g/kg) alcohol consumption, HR was increased, low- and high-frequency components of HRV were decreased, and HRV low-to-high-frequency ratio was increased across the first and last 3 h of the night (indicating a reduction in vagal dominance after alcohol intake); a significant reduction in high-frequency HRV at night was also evident after low-dose (0.5 g/kg) alcohol consumption. Similar results were found by Pietila and colleagues [24], who investigated the acute effect of alcohol intake on HRV during sleep using a wearable beat-to-beat HR tracker in over 4,000 men and women in a noncontrolled ecological setting. While it can be argued that most alcohol consumption probably occurred in the evening, no information was collected about timing and patterns of drinking. Results indicated that alcohol intake at all doses tested (lowest ≤ 0.25 g/kg; highest >0.75 g/kg) altered sleep ANS function (toward faster HR and suppressed HRV) in a dose-dependent manner. Most of the effects of alcohol on ANS functioning were similar in men and women, with alcohol affecting sleep ANS to a greater extent in younger participants.
Here, we aimed to investigate the acute effects of moderate doses of alcohol in the evening on sleep-related ANS and CV function, in a laboratory-based, single-blind, placebo-controlled study of healthy adult men and women. To address limitations of prior research, we considered potential sex differences in alcohol impact, evaluated the effects of two different doses of alcohol, and we used state-of-the-art noninvasive assessments of ANS and CV functioning across the whole night. The experimental manipulations included: evening alcohol intake within the thresholds for light to moderate alcohol amounts (one standard drink for women and two drinks for men; low-alcohol dose night), below the thresholds for heavy drinking (three standard drinks for women and four drinks for men; high-alcohol dose night), and placebo (no alcohol intake; placebo night). Standard PSG assessment was performed in combination with electrocardiography (ECG), impedance cardiography (ICG), photoplethysmography, and beat-to-beat blood pressure monitoring across the night.
Method
Participants
A total of 26 healthy adults (age range: 30–60 years; 11 women; see Table 1) who were recruited from the San Francisco Bay area community participated. All participants completed the informed consent process, and the study was approved by the SRI International’s Institutional Review Board.
Table 1.
Sample characterization
| Men | Women | t- or z-values, P values | |
|---|---|---|---|
| Sample, No. | 15 | 11 | – |
| Age, years | 42.3 (9.7) | 49.7 (7.2) | t = 2.12, p = 0.043 |
| Race | |||
| Asian, No. | 7 | 2 | – |
| White, No. | 7 | 8 | – |
| More than one race, No. | 1 | 1 | – |
| Ethnicity | |||
| Hispanic or Latino, No. | 1 | 2 | – |
| Habitual mood and sleep | |||
| Depression, BDI-II total score | 0.4 (0.9) | 1.3 (2.7) | z = 0.39, p = 0.697 |
| Anxiety, STAI-Y2 total score | 25.9 (4.8) | 28.3 (6.0) | t = 1.13, p = 0.268 |
| Sleep quality, PSQI total score | 1.6 (1.6) | 1.9 (1.6) | z = 0.88, p = 0.378 |
| Resting BP* | |||
| SBP, mmHg | 116.4 (11.3) | 102.5 (8.7) | t = −3.40, p = 0.002 |
| DBP, mmHg | 74.1 (6.2) | 72.1 (5.6) | t = −0.87, p = 0.395 |
| Body composition | |||
| BMI, kg × m−2 | 24.8 (3.0) | 24.1 (4.3) | t = −0.47, p = 0.640 |
| Normal (BMI: 18.5 to < 25), No. | 11 | 7 | |
| Overweight (BMI: 25 to < 30), No. | 2 | 3 | |
| Obese (BMI: 30 or higher), No. | 2 | 1 | |
| Body fat, % | 19.1 (4.4) | 30.6 (7.0) | t = 5.13, p < 0.001 |
| Body water, % | 56.0 (3.0) | 48.1 (4.3) | t = −5.47, p < 0.001 |
| Waist circumference, inches | 35.7 (2.9) | 31.9 (4.1) | t = −2.70, p = 0.012 |
| Health-Related Quality of Life (RAND-36)† | |||
| Physical functioning, score | 98.7 (2.3) | 99.0 (2.0) | z = 0.34, p = 0.736 |
| Role limitations due to physical health, score | 100.0 (0.0) | 100.0 (0.0) | – |
| Role limitations due to emotional problems, score | 100.0 (0.0) | 97.0 (10.0) | z = −0.36, p = 0.716 |
| Energy/fatigue, score | 78.0 (12.9) | 77.3 (11.9) | z = −0.08, p = 0.938 |
| Emotional well-being, score | 88.3 (5.1) | 89.8 (5.5) | z = 0.83, p = 0.406 |
| Social functioning, score | 100.0 (0.0) | 97.7 (5.1) | z = −0.75, p = 0.452 |
| Pain, score | 98.0 (4.1) | 92.3 (11.0) | z = −1.22, p = 0.222 |
| General health, score | 87.7 (11.5) | 85.0 (9.2) | z = −0.60, p = 0.551 |
| Physical activity | |||
| IPAQ Low activity level, No. | 0 | 1 | – |
| IPAQ Moderate activity level, No. | 8 | 3 | – |
| IPAQ High activity level, No. | 7 | 7 | – |
| Habitual alcohol consumption, No. standard drinks/week over the past month | 5.0 (3.2) | 3.5 (2.4) | z = −1.06, p = 0.287 |
| Current smokers, No. | 2 | 0 | – |
Mean and standard deviation (SD), t- or z- (for not-normally distributed variables) values and associated probability, or frequency of observations are reported, comparing men and women. BDI, Beck Depression Inventory; BMI, body mass index (calculated as weight[kg]/height[m2]; BMI categories were defined according to the definition of the Centers for Disease Control and Prevention: https://www.cdc.gov/obesity/adult/defining.html); IPAQ, Physical Activity Questionnaires; PSQI, Pittsburgh Sleep Quality Index; STAI, State-Trait Anxiety Inventory.
*Automatic upper arm sphygmomanometer BP (SBP, DBP) measurements were taken three consecutive times after a 5 min resting period, while participants were sitting in a chair. The first reading was discharged, and the final value was obtained by averaging the other two measurements.
†RAND-36 outcomes are expressed in scores ranging from 0 to 100 across the 8 health domains, with lower scores indicating more disability.
Potential participants completed a brief phone screen to evaluate major eligibility criteria, followed by an in-person visit which included a structured clinical interview for DSM-IV axis I disorders and questionnaires about demographics, trait mood (Beck Depression Inventory [BDI-II] [25], and State-Trait Anxiety Inventory [STAI-Y2] [26]) habitual sleep (Pittsburgh Sleep Quality Index [PSQI]) [27], habitual alcohol consumption, current smoking, and reproductive history (for women). If still eligible, participants underwent a clinical PSG overnight to evaluate the presence of potential sleep disorders. As part of the initial assessment, measures were made of height, waist circumference, body composition (weight, percentage of body fat and body mass measured via Tanita SC-240 bioelectrical impedance body composition analyzer [Tanita, Arlington Heights, IL, USA]), resting blood pressure (after 10 min resting, three measurements separated by 1 min were taken in a sitting position, using an upper arm automatic sphygmomanometer [Omron Healthcare, Inc.]), physical activity (International Physical Activity Questionnaires [IPAQ] [28], long form with a reference period of last 7 days), and health-related quality of life (RAND-36) [29].
All participants were free from major medical (e.g. hypertension and diabetes) and mental (e.g. major depressive disorder) conditions, insomnia and other major sleep disorders (e.g. obstructive sleep apnea syndrome), as confirmed by clinical PSG. None of them was currently using medications potentially impacting sleep, the CV system, or potentially interacting with alcohol administration (e.g. hypnotics and antihypertensives). None of the participants were shift workers and none of them had traveled across time-zones within the past 3 months. None of the participants were total alcohol abstainers and none of them were heavy drinkers (consuming 15 drinks or more per week for men and consuming 8 drinks or more per week for women).
Four women were premenopausal (one was currently using hormonal birth control pills), four women were perimenopausal, and three were postmenopausal (one was currently using hormone replacement therapy), according to the Stages of Reproductive Aging Workshop (STRAW) criteria [30]. None of the women were pregnant.
Laboratory procedures
All participants slept in temperature-controlled and sound-attenuated bedrooms in the SRI Human Sleep Research Laboratory; they self-selected their bedtimes and wake-up times, based on their usual routines.
All participants had a clinical/adaptation PSG night to exclude the presence of sleep disorders (e.g. breathing and leg movement disorders) and to adapt them to the sleep lab environment and staff. This was followed by three counterbalanced experimental PSG nights in which different doses of alcohol (wine) were administered in the evening before bedtime: placebo (no alcohol: dealcoholized wine), low-alcohol dose (two standard drinks for men and one for women), and high-alcohol dose (four standard drinks for men and three for women) nights. A total of 19 participants completed the whole protocol, while seven participants had one (N = 4) or two (N = 2) missed nights, resulting in a total of 21 high-alcohol dose, 23 low-alcohol dose, and 23 placebo PSG nights completed. Women had their nights scheduled irrespective of menstrual cycle phase.
On each of the experimental nights, participants arrived at the lab about 4 h before their desired usual bedtime. A breath alcohol test (S75 Pro, BACtrack Breathalyzers, San Francisco, CA, USA) and urine drug test (10 Panel iCup drug test kit, Instant Technologies, Inc.) were performed to confirm the absence of recent alcohol or drug use. After that, participants received their dinner (~400–500 kcals) and sensors for sleep and CV assessment were attached. All participants had 0.0 breath alcohol content (BrAC) and negative urine drug test at the lab entry.
The alcohol manipulation protocol began about 3 h before the desired bedtime. Participants received four 150 mL glasses of alcoholic and/or nonalcoholic wine according to the alcohol manipulation protocol at 30 min intervals (see Table 2), while sitting in the sleep lab common area. All participants were blinded to the experimental condition and they were told that they would receive four standard glasses of wine containing different alcohol contents. After that, participants went to their bedroom and their BrAC was measured via breathalyzer (S75 Pro, BACtrack Breathalyzers). Then, lights were turned off and participants could sleep. BrAC was measured again upon awakening.
Table 2.
A total of four standard 150 mL glasses of alcoholic and/or nonalcoholic wine were consumed within 2 h before bedtime
| Alcohol condition* | Sex | Alcohol manipulation start time (hh:mm) | 1st 150 mL drink (0–30 min) | 2nd 150 mL drink (30–60 min) | 3rd 150 mL drink (60–90 min) | 4th 150 mL drink (90–120 min) | Presleep BrAC (%) | Bedtime (hh:mm) |
|---|---|---|---|---|---|---|---|---|
| Placebo | Women | 20:21 (00:44) | Dealcoholized wine | Dealcoholized wine | Dealcoholized wine | Dealcoholized wine | 0.000 (0.000) | 23:14 (00:42) |
| Men | 20:41 (00:41) | Dealcoholized wine | Dealcoholized wine | Dealcoholized wine | Dealcoholized wine | 0.000 (0.000) | 23:35 (00:40) | |
| Low-alcohol dose | Women | 20:23 (00:37) | Dealcoholized wine | Dealcoholized wine | Dealcoholized wine | Wine | 0.010 (0.009) | 23:03 (00:35) |
| Men | 20:39 (00:51) | Dealcoholized wine | Dealcoholized wine | Wine | Wine | 0.024 (0.010) | 23:33 (00:48) | |
| High-alcohol dose | Women | 20:11 (00:39) | Dealcoholized wine | Wine | Wine | Wine | 0.057 (0.018) | 23:04 (00:36) |
| Men | 20:35 (01:13) | Wine | Wine | Wine | Wine | 0.056 (0.015) | 23:24 (01:15) |
Alcoholic wine had ~12% alcohol concentration (~14 g of pure alcohol), while dealcoholized wine had less than 0.5% alcohol concentration (see http://www.arielvineyards.com/). Mean and standard deviation (SD) for the timing of alcoholic and dealcoholized wine consumption, presleep BrAC, and bedtime for the placebo, low-alcohol dose, and high-alcohol dose nights, in both women and men, are provided.
*On the low-alcohol dose night, instead of 150 mL of alcoholic wine, one woman was unable to consume the full dose and consumed 75 mL of alcoholic wine. Two other women on the high-alcohol dose nights consumed, respectively, 350 and 310 mL, instead of 450 mL of alcoholic wine, while a man instead of 600 mL of alcoholic wine, consumed 525 mL of alcoholic wine. In these cases, the missed amount of liquid was replaced with water.
PSG sleep assessment
Sleep recordings were performed via standard PSG assessment using the Compumedics Grael HD-PSG system (Compumedics, Abbotsford, Victoria, Australia). EEG (F3/4, C3/4, O1/2, referred to the contralateral mastoids; 256 Hz sampled, 0.3–35 Hz filtered), submental electromyogram, and bilateral electrooculogram were recorded according to American Academy of Sleep Medicine (AASM) guidelines [31]. PSG records were scored in 30-s epochs (wake, N1, N2, N3, and REM sleep) according to AASM criteria. For each participant and hour of the night, the proportion of time spent in each stage of sleep was calculated.
Nighttime autonomic and CV assessment
Electrocardiographic (ECG) was recorded using 1 cm diameter Ag/AgCl surface spot electrodes placed in a modified Lead II Einthoven configuration. The ICG basal impedance signal (Z0; Ω) and the and rate of change in the impedance waveform on a given beat (dZ/dt; Ω) was acquired using HIC-4000/HIC-2500 Bioelectric Impedance Cardiographs (Bio-Impedance Technology, Inc., Chapel Hill, NC), using a Dual-Spot arrangement (8-leads), more suitable for sleep. The measurement of systolic time intervals is unaltered by spot electrode configurations [32]. The inner (voltage) electrodes were positioned around the lateral base of the neck and around the lateral thorax, at the level of the xiphisternal junction. Two outer (current) electrodes were placed to encompass the neck and the thorax, at least 3 cm above each of the recording electrodes. The outer electrodes transmit a 4-mA AC at 100 kHz, and Z0 and dZ/dt signals were registered from the inner electrodes (see Sherwood et al. [33], for a full description of the ICG technique). Beat-to-beat blood pressure (BP) recordings were made using Portapres Model-2 (TNO TPD Biomedical Instrumentation, Amsterdam, The Netherlands), a validated technique based on the volume-clamp method [34, 35], allowing noninvasive prolonged BP recordings. Photoplethysmography cuffs, which inflate and deflate continuously to keep finger blood volume constant (switching between fingers every 30 min) were applied to the participants’ index and middle fingers of the nondominant hand. Between-cuffs BP reading discrepancies of below 5 mmHg was required before starting the recording (cuffs were re-positioned until this requirement was met).
Raw ECG and BP (sampled at 512 Hz), ICG Z0 and dZ/dt signals (sampled at 1,024 Hz) were acquired via dedicated channels in the Compumedics Grael HD-PSG system. All preprocessing and processing of CV indices were performed in MATLAB R2018a (MathWorks, Inc., Natick, MA) via customized algorithms. All CV signals were digitally filtered with a 4th-order Butterworth bandpass filter with lower and upper cutoff frequencies of 0.5 and 35 Hz for ECG, and 0.5 and 25 Hz for ICG, respectively. The filter was applied in both forward and backward directions to avoid any phase shift.
Customized algorithms were applied to compute normal-to-normal inter-beat-intervals via automatic detection of ECG R peaks (see [36]). Those ECG cycles that happened to have an out-of-range (10 standard deviations away from the mean) ECG signal level or R-R value were identified as invalid beats (corrupted by noise and artifacts or ectopic beats) and were excluded from the analysis. Q wave onset (beginning of the electrical systole) was determined by a fixed interval (35 ms) backward from the ECG R-wave peak [37, 38]. ICG cycles were identified based on the ECG R peak locations and automatic algorithms were used to detect the B points (opening of the aortic valve and onset of left-ventricular ejection) and X points (closure of the aortic valve and end of left-ventricular ejection; see [39, 40]). ICG corrupted cycles were identified according to a similarity index [41], and were excluded from the study. The continuous BP signal was processed using customized algorithms to identify and exclude data corresponding to calibration and cuff switching, and to detect peaks and troughs [42].
Time-domain HRV analysis was performed on consecutive 5-min windows across the night (from lights-off to lights-on), and the standard deviation of normal-to-normal R-R intervals (SDNN, ms; an index reflecting total HRV) and the root mean square of the successive differences in normal to normal R-R intervals (RMSSD, ms; an index of high-frequency HRV reflecting vagal activity) were calculated according to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology guidelines [43]. The pre-ejection period (PEP; ms), a validated index inversely related to cardiac sympathetic activity [44], was calculated as the time interval between the Q point on the ECG signal and the B point on the ICG dZ/dt signal. From the systolic blood pressure (SBP) and diastolic blood pressure (DBP) peaks analysis of the raw BP waveform, SBP (mmHg) and DBP (mmHg) were derived.
Cardiac BRS was calculated using the sequence method (see [45]). Baroreflex sequences were selected across the night as follows: ≥3 adjacent heartbeats containing progressive increases (up-sequences) or decreases (down-sequences) in both SBP (≥1 mmHg change in successive SBPs) and R-R intervals (≥5 ms change in successive R-R intervals); ≥0.8 coefficient of correlation between changes in SBPs and RR intervals. BRS for up- (BRS+) and down- (BRS−) sequences were estimated from the slope of the regression line between SBP and RR intervals and expressed as ms × mmHg−1. Sequences of three heartbeats characterized most of the BRS+ and BRS− sequences across the three alcohol manipulation nights, and hours of the night (>86% of the total sequences), as previously observed [45, 46].
ANS and CV outcomes were averaged and analyzed across the first 6 h of the night. DBP and RMSSD were log-transformed before analysis to improve normality. Two nights from one participant were excluded from the analyses because of less than 5 h of time in bed, limiting the amount of data available.
Statistical analysis
Dependent variables (DVs) for demographic and sample characterization (see Table 1) were analyzed using independent t-tests, using sex (women and men) as the grouping variable. Mann–Whitney U-tests were used when DVs were non-normally distributed. t- or z-values and associated p-values are reported. PSG, ANS, and CV DVs were analyzed using mixed models with robust errors. Independent factors were sex (women and men) × alcohol condition (placebo, low-alcohol dose, and high-alcohol dose) × time (h1, h2, h3, h4, h5, and h6). The models included a random effect for an individual with alcohol condition. Robust errors were obtained using Huber–White estimates of variance. Wald’s test was conducted to calculate the statistical significance of regression coefficients. Marginal (expected) values were calculated for all combinations of independent factors. Wald’s test was also applied to determine the statistical significance of the marginal effects for each factor, each two-way interaction, and the three-way interaction. The same model was used to analyze presleep BrAC with the exceptions that there was no time factor and the alcohol condition factor had only two levels (low-alcohol dose and high-alcohol dose). Chi-squared and associated p values were reported for the significant models. The effects were considered significant at p < 0.05. Analyses were performed using Stata/SE 14.1 for Windows by a senior biostatistician (H.J.).
Results
Sample demographics, body composition, and resting-state blood pressure and HR
Four men and four women fell within the “overweight or obesity” categories, according to their BMI, and all but one reported “moderate or high activity levels,” as determined by IPAQ. All participants fell within the BDI-II cutoff scores (0–13) for minimal depression [25], and all but one fell below the PSQI threshold (score of 5 and above) for poor sleepers [27]. Women had a greater percentage of body fat, a reduced percentage of body water, and smaller waist circumference (p < 0.05) but similar BMI, compared with men. Overall, women were older than men (p = 0.043) and had significantly lower resting SBP (p = 0.002). However, all participants had SBP ≤ 140 mmHg (cut-off for “high blood pressure”) [47]. No sex differences were detected in the participants’ habitual alcohol consumption. See Table 1 for details.
Alcohol manipulation protocol and BrAC
There were no alcohol condition or sex effects, or sex × alcohol condition interactions in the timing that participants received their drinks (p > 0.05). The average alcohol intake in relation to body weight resulted in 0.71 ± 0.33 g/kg in men and 0.64 ± 0.24 g/kg in women in the high-alcohol dose condition, and 0.38 ± 0.14 g/kg in men and 0.21 ± 0.08 g/kg in women in the low-alcohol dose condition. All participants had a BrAC of 0.000, after receiving the placebo-dose. All but one man (BrAC: 0.008%) had a BrAC of 0.000 upon awakening.
Presleep BrAC displayed a significant sex × alcohol condition (chi2 = 6.53, p = 0.011) interaction effect. As expected, BrAC was greater in the high-alcohol dose condition compared to the low-alcohol dose condition in both men and women (p < 0.001). After low-dose alcohol consumption, men reached a significantly higher presleep BrAC than women (BrAC men: 0.024 ± 0.010%; BrAC women: 0.010 ± 0.009%; p < 0.001). No sex differences in presleep BrAC were detected after high-dose alcohol consumption (BrAC men: 0.056 ± 0.015%; BrAC women: 0.057 ± 0.018%; p = 0.945).
Nocturnal HR, total and high-frequency (reflecting cardiac vagal activity) HRV
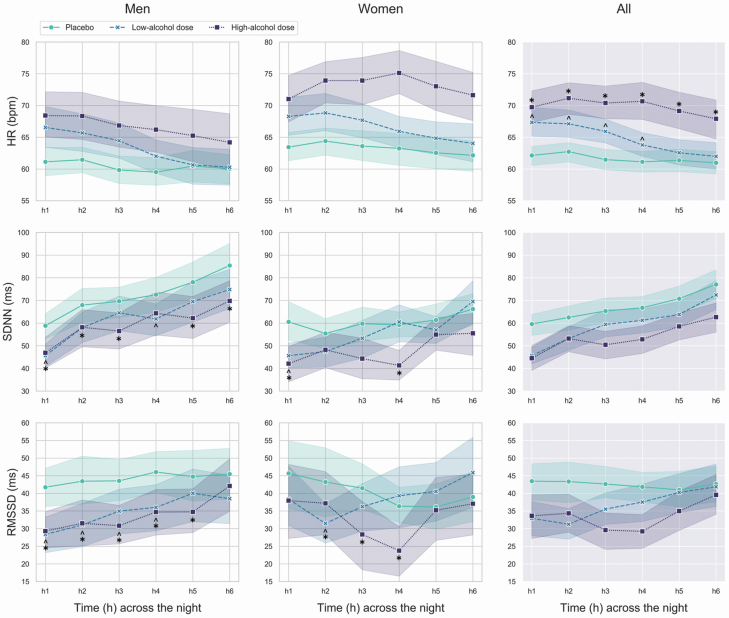
Nocturnal patterns for HR, SDNN, and RMSSD are shown in Figure 1.
Figure 1.
Mean and standard errors of HR, SDNN (indicating total HRV), and root mean square of successive differences between normal heartbeats (RMSSD; indicating high-frequency [vagal] HRV), across the first 6 h of the placebo, low-alcohol dose, and high-alcohol dose nights in men and women. In the graphs, the significant differences between alcohol conditions are marked by “caret” (low-alcohol dose night vs. placebo night), and “asterisks” (high-alcohol dose night vs. placebo night).
HR displayed a significant alcohol condition × time (chi2 = 102.01, p < 0.001) interaction effect. As expected, on the placebo night, HR slowed across the night, with a lower HR in h3–6 compared to h2 (p < 0.05). HR was faster during the first 4 h of the low-alcohol dose night and across the first 6 h of the high-alcohol dose night, compared to the placebo night (p < 0.01), indicating a dose-dependent positive chronotropic action of alcohol. Despite being particularly elevated at the beginning of the night, HR recovered across hours of the low-alcohol dose night (h4–6 < h1–3, h6 < h4) and, to a lesser extent, across hours of the high-alcohol dose night (after an initial acceleration [h2 > h1], HR reduced [h6 < h2–4]). Sex and sex × alcohol condition factors were not significant.
SDNN displayed a significant sex × alcohol condition × time (chi2 = 23.91, p = 0.008) interaction effect. SDNN was reduced during the low- (men: significantly in h1,4; women: significantly in h1) and high-alcohol dose (men: significantly in h1–3,5,6; women: significantly in h1,4) nights, compared to placebo (p < 0.05), indicating a suppressing effect of alcohol on nighttime total HRV. No significant differences in SDNN were detected between low- and high-alcohol doses. Within-night increases in SDNN were observed across all conditions in both men and women: placebo (men: h3–6 > h1, h6 > h2–4; women: h6 > h2), low-alcohol dose (men: h2–6 > h1, h6 > h2–4, h5 > h2; women: h4–6 > h1, h6 > h2–5, h4 > h2), and high-alcohol dose (men: h2–6 > h1, h6 > h2,3, h4 > h2,3; women: h5,6 > h1,3,4; p < 0.05). Despite an overall tendency for greater SDNN in men compared to women, significant sex differences were only evident in h4 of the high-alcohol dose night (p = 0.031).
RMSSD displayed a significant sex × alcohol condition × time (chi2 = 44.53, p = 0.002) interaction effect. RMSSD was reduced during both the low- (men: significantly in h1–4; women: significantly in h2) and, to a greater extent, the high-alcohol dose (men: significantly in h1–5; women: significantly in h2–4) nights, compared to placebo (p < 0.05), indicating a suppressing effect of alcohol on nighttime high-frequency (vagal) HRV. No significant differences were detected between low- and high-alcohol doses. Within-night changes in RMSSD followed different pattern between sexes, and across conditions (see Figure 1): placebo (men: h4 > h3; women: ns.), low-alcohol dose (men: h3–6 > h1, h6 > h4,5, h5 > h2–4, h4 > h2; women: h6 > h1–3, 5, h5 > h2), and high-alcohol dose (men: h6 > h1–5, h4,5 > h3; women: h4 < h1,2, h3 < h2, h5,6 > h3,4; p < 0.05).
Nocturnal cardiac sympathetic nervous system activity
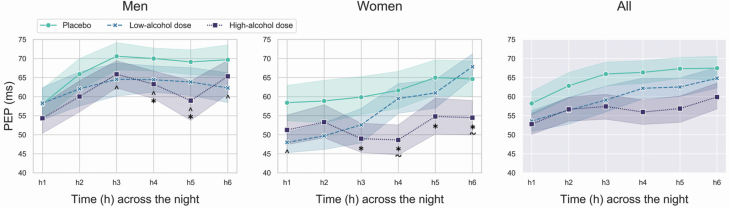
Nocturnal patterns for PEP are presented in Figure 2.
Figure 2.
Mean and standard errors of PEP (an index inversely related to cardiac sympathetic activity) across the first 6 h of the placebo, low-alcohol dose, and high-alcohol dose nights in men and women. In the graphs, the significant differences between alcohol conditions are marked by “caret” (low-alcohol dose night vs. placebo night), “asterisks” (high-alcohol dose night vs. placebo night), and “tilde” (high-alcohol dose night vs. low-alcohol dose night).
PEP displayed a significant sex × alcohol condition × time (chi2 = 83.27, p < 0.001) interaction effect. PEP was faster during the low- (men: significantly in h3–6; women: significantly in h1) and high-alcohol dose nights (men: significantly in h4,5; women significantly in h3–6; p < 0.05) compared to placebo, indicating elevated cardiac sympathetic activity following alcohol consumption (positive inotropic effect of alcohol). No significant differences were detected between low- and high-alcohol doses in men, while in women, PEP was significantly faster in h4,6 of the high-alcohol dose night compared to the low-alcohol dose night (p < 0.05). Within-night changes in PEP followed different patterns between sexes, and across conditions towards an overall reduction in cardiac sympathetic activity with the progression of the night (see Figure 2): placebo (men: h2–6 > h1, h3 > h2; women: h6 > h1, h5 > h3,4), low-alcohol dose (men: h2,3 > h1, h3 > h1; women: h4–6 > h1–3, h6 > h4,5), and high-alcohol dose (men: h2–4,6 > h1, h3 > h2; women: h5–6 > h4) (p < 0.05). PEP was faster (greater sympathetic activity) in h3 of the placebo night and in h3,4of the high-alcohol dose night, in women compared to men (p < 0.05).
Nocturnal SBP and DBP
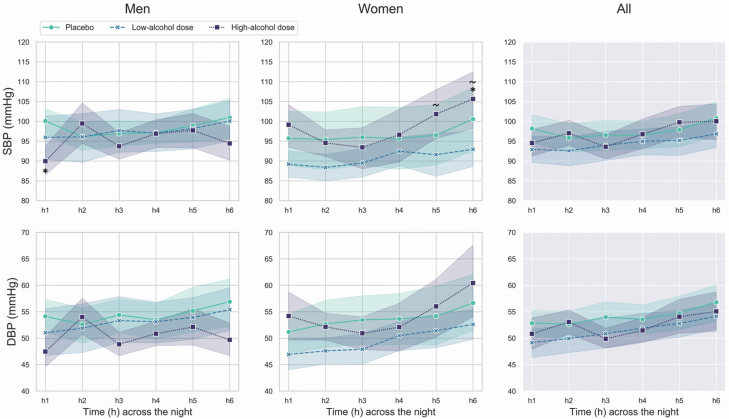
Nocturnal patterns for SBP and DBP are shown in Figure 3.
Figure 3.
Mean and standard errors of SBP and DBP across the first 6 h of the placebo, low-alcohol dose, and high-alcohol dose nights in men and women. In the graphs, the significant differences between alcohol conditions are marked by “asterisks” (high-alcohol dose night vs. placebo night) and “tilde” (high-alcohol dose night vs. low-alcohol dose night).
SBP displayed a significant sex × alcohol condition × time (chi2 = 29.20, p = 0.001) interaction effect. SBP was lower in h1 of the high-alcohol dose night in men (p = 0.042) and higher in h6 of the high-alcohol dose night in women (p = 0.034), compared to placebo. No significant differences were detected between low- and high-alcohol doses in men, while in women, SBP was higher during the high-alcohol dose night compared to the low-alcohol dose night in h5,6 (p < 0.05). Within-night changes in SBP followed different patterns between sexes and across conditions: placebo (men: ns.; women: h6 > h4,5), low-alcohol dose (men: ns.; women: h6 > h2,3), and high-alcohol dose (men: h2–5 > h1, h4 > h3; women: h5,6 > h2–4; p < 0.05).
Similarly, DBP displayed a significant sex × alcohol condition × time (chi2 = 37.43, p < 0.001) interaction effect, with both alcohol condition- and sex-specific within-night patterns for DBP: placebo (men: ns.; women: h6 > h5), low-alcohol dose (men: h3,6 > h1, h6 > h2; women: h4–6 > h1,2, h6 > h3), and high-alcohol dose (men: h2,5 > h1, h6 < h2; women: h6 > h2–4, h5 > h4; p < 0.05).
Nocturnal BRS
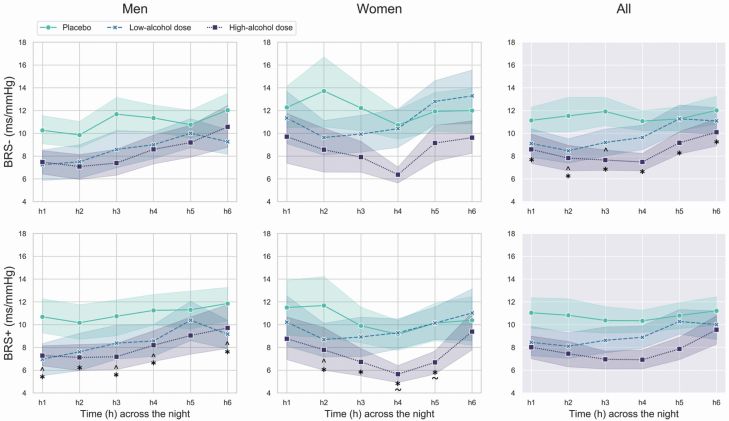
The nocturnal patterns for BRS− and BRS+ are shown in Figure 4.
Figure 4.
Mean and standard errors of BRS down (BRS−) and up (BRS+) sequences, across the first 6 h of the placebo, low-alcohol dose, and high-alcohol dose nights in men and women. In the graphs, the significant differences between alcohol conditions are marked by “caret” (low-alcohol dose night vs. placebo night), “asterisk” (high-alcohol dose night vs. placebo night), and “tilde” (high-alcohol dose night vs. low-alcohol dose night).
There was a significant sex × alcohol condition × time (chi2 = 35.60, p < 0.001) interaction effect for BRS+. In both men and women, BRS+ was suppressed during the low-alcohol dose (significantly in h1,3–4,6 in men, and in h2 in women) and the high-alcohol dose (significantly in h1–4,6 in men and in h2–5 in women) nights, compared to placebo. In addition, in women only, BRS+ was lower during the high-alcohol dose night compared to the low-alcohol dose nights in h4–5. Alcohol condition- and sex-specific within-night patterns for BRS+ were apparent: placebo (no significant within-night changes), low-alcohol dose (men: h3–6 > h1, h5–6 > h2, h5 > h3–4, h6 < h5; women: h2 < h1, h5 > h2, h6 > h3), and high-alcohol dose (men: h6 > h1–3, h5 > h3; women: h4 < h1, h6 > h2–5; p < 0.05).
There was a significant alcohol condition × time (chi2 = 45.73, p < 0.001) interaction effect for BRS−. BRS− was suppressed during both the low-alcohol dose (significantly in h2–3) and high-alcohol dose (significantly in h1–6) nights, compared to placebo, in both men and women (p < 0.05). Alcohol condition-specific within-night patterns for BRS− were detected: placebo (no significant within-night changes), low-alcohol dose (h5–6 > h1–4, h4 > h2), and high-alcohol dose (men: h6 > h2–5, h5 > h3–4; p < 0.05).
PSG sleep stages distribution across hours of the low-alcohol dose, high-alcohol dose, and placebo nights
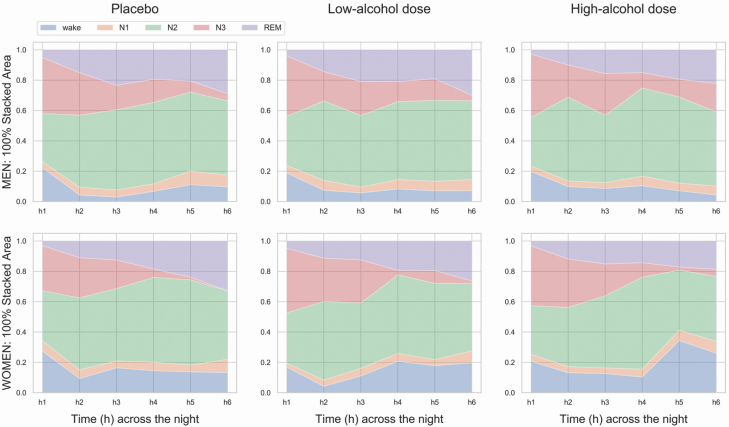
PSG sleep composition across hours of the night is shown in Figure 5.
Figure 5.
Hourly sleep stage composition for the first 6 h of the placebo, low-alcohol dose, and high-alcohol dose nights, separately for men (top panel) and women (bottom panel) (100% stacked area charts).
Wake displayed a significant sex × alcohol condition × time (chi2 = 27.47, p = 0.002) interaction effect. In h5 for women and in h3 for men, the percentage of wake was higher in the high-alcohol dose night compared to placebo (p < 0.05). The percentage of wake was elevated in women compared to men in h5 and h6 of the high-alcohol dose night (p < 0.05). Few differences in alcohol condition- and sex-specific within-night patterns for wake were detected: placebo (men: h2–3 < h1; women: h2,6 < h1), low-alcohol dose (men: h2–3,6 < h1; women: h2 < h1), and high-alcohol dose (men: h5–6 > h1; women: h5 > h4; p < 0.05).
N3 displayed a significant alcohol condition × time (chi2 = 26.14, p = 0.004) interaction effect. The percentage of N3 was greater in h6 of the high-alcohol dose night compare to that of both placebo and low-alcohol dose nights (p < 0.05). As expected, N3 percentage progressively reduced across the placebo (h3–6 < h1, h4–6< h2, h5–6 < h3, h6< h4), low-alcohol dose (h2–6< h1, h4–6< h2–3, h6< h4–5) and high-alcohol dose (h3–6< h1, h4–6< h2, h4–5< h3) nights (p < 0.05). Similarly, there was a main effect of time for N2 (chi2 = 25.93, p < 0.001) and REM (chi2 = 73.71, p < 0.001) sleep. N2 (h2–6> h1, h4> h2–3, h6 < h4–5) and REM (h2–6> h1–2, h4> h2–3, h6 > h3–4) percentages progressively increased across hours of the placebo, low-alcohol dose, and high-alcohol dose nights in both sexes (p < 0.05).
Discussion
Our results show that acute evening alcohol consumption at levels below those considered indicative of heavy drinking, has marked dose- and time-dependent effects on sleep ANS and CV regulation in healthy adult men and women, impacting nocturnal CV homeostasis. Presleep alcohol ingestion resulted in elevated nighttime HR (~4% faster during the low-alcohol dose night and ~14% faster during the high-alcohol dose night, compared to placebo). The alcohol-related cardiac acceleration was particularly evident at the beginning of the night. As the night progressed, HR returned to the placebo level in the morning hours, in the low-alcohol dose, but remained elevated throughout the night in the high-alcohol condition. In association with increased HR, nocturnal total HRV, an index reflecting ANS flexibility, and high-frequency HRV, indicating vagal activity, and BRS, indicating baroreflex control of acute BP changes via ANS modulation, were suppressed and cardiac sympathetic activity was increased following alcohol consumption, particularly during the high-alcohol dose night. In addition, in women only, SBP was increased during the morning hours of the high-alcohol dose night.
Our findings are similar to, and extend those of, Sagawa et al. [23] who found increases in HR, and to a greater extent, a suppressed cardiac vagal HRV activity in the first and last 3 h of the night in response to presleep alcohol intake at similar doses and administration time, in 10 healthy young men. We found that HR took longer to recover after a high-alcohol dose, similar to the findings of Sagawa et al. [23], who found a greater impact of the high-alcohol dose on nighttime cardiac ANS function and less within-night ANS recovery. In addition, they found that low-to-high HRV (an ANS index thought to reflect cardiac sympathovagal balance; see [21]) was increased in response to alcohol intake [23]. Our study confirmed that alcohol-related cardiac vagal ANS suppression was paired by a cardiac sympathetic ANS activation, measured by PEP via ICG, a validated index reflecting cardiac sympathetic beta-adrenergic ANS activity (see [21]). Further studies employing a direct measure of nighttime sympathetic drive (e.g. via microneurography) [21] may help confirm the presence of specific nighttime sympathetic ANS alterations following alcohol ingestion.
The changes in CV and ANS measures that we found after presleep alcohol consumption could reflect a variety of central and/or peripheral actions of alcohol and its metabolites as well as compensatory actions of the body against the effects of alcohol. For example, while ethanol exerts a direct depressor effect on the heart (e.g. decreases in contractility), there is a compensatory increase in catecholamines [48, 49], which would increase HR. Activation of compensatory factors against the initial vasodilatory effect of alcohol, such as presso-receptor mediated reflex mechanisms [9, 16, 48], could also explain why we found no change in SBP (women) or only a transient decrease in SBP (men) in the first hour.
ANS and CV system activities are modulated by sleep stage dynamics (sleep stage composition) and by transient desynchronized (e.g. arousals) and synchronized (e.g. K-complex) sleep events, and by circadian rhythms [21, 22, 50]. However, only small hourly differences in PSG sleep composition between placebo, low- and high-alcohol dose nights were detected in our study, suggesting that the impact of evening alcohol consumption on the dynamics of sleep ANS and CV regulation, at the levels investigated here, is largely independent of alterations in sleep macrostructure. Higher amounts of presleep alcohol have a more substantial impact on sleep macrostructure (see [51, 52]), which could contribute to CV changes across the night; further work is required to investigate this possibility. Alcohol also alters sleep microstructure (e.g. enhanced sleep EEG Delta and Alpha activity; see [52]), and given the tight coupling between cortical and cardiac activity during sleep [21], the analytics of continuous CNS-ANS dynamics (e.g. strength, time-course, and directionality) of cortical and cardiac relationships in response to alcohol intake warrants further investigation. Finally, alcohol exerts direct effects on the circadian pacemaker as well as downstream of the pacemaker [53], which could contribute to the alcohol-induced changes in the time-course of ANS and CV measures across the night that we found, although studies are required in individuals kept under constant conditions to determine true circadian effects. Given the multiple mechanisms of action of alcohol and metabolites, as well as compensatory responses, it is challenging in human studies to isolate the specific mechanism of action of alcohol on CV and ANS regulation.
Several factors need to be accounted for in the interpretation of dose-dependent and sex differences in the effect of alcohol on sleep ANS and CV regulation, including alcohol metabolic processes (individual differences in the rate of alcohol absorption, alcohol distribution in the body, rate of elimination, etc.) and sensitivity of the ANS and CV system to alcohol [54]. In the current study design, after receiving a standardized meal, all participants received a fixed amount of alcohol (oral administration) within a 2-h drinking time and fixed 30 min between-drinks intervals before bedtime. The total amount of evening liquid intake was 600 mL (see Table 2 for details), and the amount of alcohol administration was independent of individuals’ body weight and body composition, factors that are known to affect both interindividual variation and sex differences in alcohol metabolism [55], reflecting the complexity of alcohol pharmacokinetics [54].
We found some sex differences in the effects of alcohol consumption on CV and ANS measures, mostly in the patterns of responses across the night. These findings should be interpreted cautiously given the relatively small sample size of men and women, however, given that alcohol use, including binge drinking, is increasing in adult women [56], further study of sex differences in ANS and CV responses to alcohol are warranted. No sex differences were detected in the BrAC after high-dose alcohol consumption (four drinks for men and three drinks for women), but in the low-alcohol dose condition (two drinks for men and one drink for women) men had a higher amount of alcohol intake per body weight and greater presleep BrAC than women. This may have resulted in an underestimation of the potential sex differences in the impact of alcohol on ANS and CV function in the low-alcohol dose condition. Furthermore, as expected, women had greater body fat and lower body water percentages than men, possibly resulting in differences in BAC peak and elimination rate [54, 57] that may have affected sleep ANS and CV patterns between sexes. Other factors like lower levels of alcohol dehydrogenase (the major alcohol-metabolizing enzyme) in women [54] and sex differences in alcohol sensitivity may also be important. For example, Cofresi and Bartholw [58] showed greater sensitivity in daytime acute alcohol-induced HR increases in young women compared to men, even after accounting for between-person differences in BMI, BrAC (the target BrAC peak was 0.08%, obtained by adjusting the alcohol dose based on age, sex, weight, and height), recent alcohol use, perceived stimulating effect of alcohol, and contextual social drinking factors.
In our study, we did not control for menstrual cycle phase or reproductive stage, factors than may have influenced the patterns of sleep ANS and CV activity. Women’s reproductive hormones fluctuations (e.g. menstrual cycle and reproductive stage) may affect alcohol pharmacokinetics [54, 59], and sleep and sleep ANS and CV regulation [60–62]. Further larger studies are needed to evaluate the interaction between women’ s reproductive hormones, and ANS and CV nighttime profiles following alcohol ingestion, as well as the interaction between alcohol metabolism, BAC curves, and nighttime ANS and CV dynamics in men and women.
There is a need for empirical evidence to address the lack of scientific support for how much people could drink within safe (or beneficial) ranges, particularly when considering the complex relationship between alcohol consumption and CV risk. The results of this study highlight a dose-dependent acute alteration in sleep ANS and CV regulation following evening alcohol consumption within “low-risk drinking levels” (below five drinks for men and four drinks for women) in healthy adults. Further studies are needed to investigate central and peripheral mechanisms underlying the impact of repeated evening alcohol consumption on sleep CV homeostasis, accounting for biological factors like sex, pharmacokinetics of alcohol effects on CV function, alcohol metabolism and individuals’ sensitivity to alcohol, within the context of chronic CV alteration and risk profiles.
Disclosure statement
Financial disclosure. This study was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) [grant R21-AA024841 to I.M.C. and M.d.Z.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Non-financial disclosure. The authors declared no conflict of interest related to the current work. M.d.Z., F.C.B., and I.C. have received research funding unrelated to this work from Ebb Therapeutics Inc., Fitbit Inc., International Flavors & Fragrances Inc., and Noctrix Health, Inc.
References
- 1. U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. 2015. http://health.gov/dietaryguidelines/2015/guidelines/. Accessed May 22, 2020. [Google Scholar]
- 2. Sacks JJ, et al. . 2010 National and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73–e79. [DOI] [PubMed] [Google Scholar]
- 3. Di Castelnuovo A, et al. . Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445. [DOI] [PubMed] [Google Scholar]
- 4. O’Keefe JH, et al. . Alcohol and cardiovascular health: the dose makes the poison… or the remedy. Mayo Clin Proc. 2014;89(3):382–393. [DOI] [PubMed] [Google Scholar]
- 5. Mostofsky E, et al. . Alcohol and immediate risk of cardiovascular events: a systematic review and dose-response meta-analysis. Circulation. 2016;133(10):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stockwell T, et al. . Do “Moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs. 2016;77(2):185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knott CS, et al. . All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ. 2015;350:h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brien SE, et al. . Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawano Y. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 2010;33(3):181–191. [DOI] [PubMed] [Google Scholar]
- 10. Spaak J, et al. . Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol. 2008;294(2):H605–H612. [DOI] [PubMed] [Google Scholar]
- 11. Spaak J, et al. . Dose-related effects of red wine and alcohol on heart rate variability. Am J Physiol Heart Circ Physiol. 2010;298(6):H2226–H2231. [DOI] [PubMed] [Google Scholar]
- 12. Romanowicz M, et al. . Changes in heart rate variability associated with acute alcohol consumption: current knowledge and implications for practice and research. Alcohol Clin Exp Res. 2011;35(6):1092–1105. [DOI] [PubMed] [Google Scholar]
- 13. Bau PF, et al. . Acute ingestion of alcohol and cardiac autonomic modulation in healthy volunteers. Alcohol. 2011;45(2):123–129. [DOI] [PubMed] [Google Scholar]
- 14. Fazio M, et al. . Mechanics of the carotid artery wall and baroreflex sensitivity after acute ethanol administration in young healthy volunteers. Clin Sci (Lond). 2001;101(3):253–260. [PubMed] [Google Scholar]
- 15. Brunelle C, et al. . Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Hum Psychopharmacol. 2007;22(7):437–443. [DOI] [PubMed] [Google Scholar]
- 16. Buckman JF, et al. . Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcohol Clin Exp Res. 2015;39(12):2334–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwase S, et al. . Effect of oral ethanol intake on muscle sympathetic nerve activity and cardiovascular functions in humans. J Auton Nerv Syst. 1995;54(3):206–214. [DOI] [PubMed] [Google Scholar]
- 18. Thayer JF, et al. . The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. [DOI] [PubMed] [Google Scholar]
- 19. Dawson DA. Temporal drinking patterns and variation in social consequences. Addiction. 1996;91(11):1623–1635. [DOI] [PubMed] [Google Scholar]
- 20. Johnson EO, et al. . Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21(2):178–186. [DOI] [PubMed] [Google Scholar]
- 21. de Zambotti M, et al. . Dynamic coupling between the central and autonomic nervous systems during sleep: a review. Neurosci Biobehav Rev. 2018;90:84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trinder J, et al. . Sleep and cardiovascular regulation. Pflugers Arch. 2012;463(1):161–168. [DOI] [PubMed] [Google Scholar]
- 23. Sagawa Y, et al. . Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35(11):2093–2100. [DOI] [PubMed] [Google Scholar]
- 24. Pietilä J, et al. . Acute effect of alcohol intake on cardiovascular autonomic regulation during the first hours of sleep in a large real-world sample of Finnish employees: observational study. JMIR Ment Health. 2018;5(1):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beck A, et al. . Beck Depression Inventory-II (BDI-II). San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 26. Spielberger C, et al. . Manual for the State-Trait Anxiety Inventory: STAI (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 27. Buysse DJ, et al. . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 28. Craig CL, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 29. Hays RD, et al. . The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. [DOI] [PubMed] [Google Scholar]
- 30. Soules MR, et al. . Executive summary: stages of reproductive aging workshop (STRAW). Climacteric. 2001;4(4):267–272. [PubMed] [Google Scholar]
- 31. Iber C, et al. . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 32. Sherwood A, et al. . Comparison of impedance cardiographic measurements using band and spot electrodes. Psychophysiology. 1992;29(6):734–741. [DOI] [PubMed] [Google Scholar]
- 33. Sherwood A, et al. . Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. [DOI] [PubMed] [Google Scholar]
- 34. Castiglioni P, et al. . Broad-band spectral analysis of 24 h continuous finger blood pressure: comparison with intra-arterial recordings. Clin Sci (Lond). 1999;97(2):129–139. [PubMed] [Google Scholar]
- 35. Eckert S, et al. . Comparison of portapres non-invasive blood pressure measurement in the finger with intra-aortic pressure measurement during incremental bicycle exercise. Blood Press Monit. 2002;7(3):179–183. [DOI] [PubMed] [Google Scholar]
- 36. de Zambotti M, et al. . The falling asleep process in adolescents. Sleep. 2020;43(6):zsz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seery MD, et al. . Preejection period can be calculated using R peak instead of Q. Psychophysiology. 2016;53(8):1232–1240. [DOI] [PubMed] [Google Scholar]
- 38. Berntson GG, et al. . Where to Q in PEP. Psychophysiology. 2004;41(2):333–337. [DOI] [PubMed] [Google Scholar]
- 39. Forouzanfar M, et al. . Automatic analysis of pre-ejection period during sleep using impedance cardiogram. Psychophysiology. 2019;56(7):e13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forouzanfar M, et al. . Toward a better noninvasive assessment of preejection period: a novel automatic algorithm for B-point detection and correction on thoracic impedance cardiogram. Psychophysiology. 2018;55(8):e13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forouzanfar M, et al. . Automatic artifact detection in impedance cardiogram using pulse similarity index. In: proceedings from the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) July 23–27, 2019; Berlin, Germany; 2019. [DOI] [PMC free article] [PubMed]
- 42. Forouzanfar M, et al. . Electroencephalographic slow-wave activity during sleep in different phases of blood pressure and respiration oscillations. In: proceedings from the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); July 23–27, 2019; Berlin, German; 2019. [DOI] [PMC free article] [PubMed]
- 43. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 44. Schächinger H, et al. . Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63(5):788–796. [DOI] [PubMed] [Google Scholar]
- 45. Parati G, et al. . Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12(2):214–222. [DOI] [PubMed] [Google Scholar]
- 46. Silvani A, et al. . Sleep-dependent changes in the coupling between heart period and blood pressure in human subjects. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1686–R1692. [DOI] [PubMed] [Google Scholar]
- 47. Chobanian AV, et al. . The seventh report of The Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 48. Child JS, et al. . Cardiac effects of acute ethanol ingestion unmasked by autonomic blockade. Circulation. 1979;59(1):120–125. [DOI] [PubMed] [Google Scholar]
- 49. Weise F, et al. . Acute alcohol ingestion reduces heart rate variability. Drug Alcohol Depend. 1986;17(1):89–91. [DOI] [PubMed] [Google Scholar]
- 50. Burgess HJ, et al. . Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273(4):H1761–H1768. [DOI] [PubMed] [Google Scholar]
- 51. Ebrahim IO, et al. . Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. [DOI] [PubMed] [Google Scholar]
- 52. Koob GF, et al. . Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology. 2020;45(1):141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms: human and animal models. Alcohol Res Health. 2001;25(2):126–135. [PMC free article] [PubMed] [Google Scholar]
- 54. Mumenthaler MS, et al. . Gender differences in moderate drinking effects. Alcohol Res Health. 1999;23(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- 55.Thomasson HR. Gender differences in alcohol metabolism. In: Galanter M., et al. eds. Recent Developments in Alcoholism. Recent Developments in Alcoholism. Vol 12 Boston, MA: Springer; 2002: 163–179. [DOI] [PubMed] [Google Scholar]
- 56. Hasin DS, et al. . Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug Alcohol Depend. 2019;205:107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marshall AW, et al. . Ethanol elimination in males and females: relationship to menstrual cycle and body composition. Hepatology. 1983;3(5):701–706. [DOI] [PubMed] [Google Scholar]
- 58. Cofresi RU, et al. . Female drinkers are more sensitive than male drinkers to alcohol-induced heart rate increase [published onlien ahead of print December 2, 2019]. Exp Clin Psychopharmacol. 2019. doi: 10.1037/pha0000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Inkelis S, et al. . Sleep and alcohol use in women. Alcohol Res Curr Rev. 2020;40(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Zambotti M, et al. . Menstrual cycle-related variation in autonomic nervous system functioning in women in the early menopausal transition with and without insomnia disorder. Psychoneuroendocrinology. 2017;75:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Zambotti M, et al. . Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J Clin Endocrinol Metab. 2015;100(8):2918–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Zambotti M, et al. . Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]