Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating adult-onset neurodegenerative disease characterized by the progressive degeneration of upper and lower motoneurons. Most ALS cases are sporadic but approximately 10% of ALS cases are due to inherited mutations in identified genes. ALS-causing mutations were identified in over 30 genes with superoxide dismutase-1 (SOD1), chromosome 9 open reading frame 72 (C9orf72), fused in sarcoma (FUS), and TAR DNA-binding protein (TARDBP, encoding TDP-43) being the most frequent. In the last few decades, Drosophila melanogaster emerged as a versatile model for studying neurodegenerative diseases, including ALS. In this review, we describe the different Drosophila ALS models that have been successfully used to decipher the cellular and molecular pathways associated with SOD1, C9orf72, FUS, and TDP-43. The study of the known fruit fly orthologs of these ALS-related genes yielded significant insights into cellular mechanisms and physiological functions. Moreover, genetic screening in tissue-specific gain-of-function mutants that mimic ALS-associated phenotypes identified disease-modifying genes. Here, we propose a comprehensive review on the Drosophila research focused on four ALS-linked genes that has revealed novel pathogenic mechanisms and identified potential therapeutic targets for future therapy.
Keywords: amyotrophic lateral sclerosis, Drosophila melanogaster, SOD1, C9orf72, FUS, TDP-43
1. Introduction
ALS (amyotrophic lateral sclerosis) also known as Charcot’s disease or Lou Gehrig’s disease is a fatal adult-onset neurodegenerative disease affecting the motor system [1,2,3,4]. ALS is the most common motoneuron disorder with an incidence of two per 100,000 individuals, which varies according to geographical differences, and a mean onset at 65 [5,6]. ALS is characterized by the progressive dismantling of the neuromuscular junctions and degeneration of motoneurons in the brain and spinal cord [7,8]. Motoneuron loss leads to progressive paralysis and death due to respiratory failure within 3 to 5 years of onset disease [9]. In addition to motoneuron degeneration, ALS is clearly a non-cell-autonomous disease as astrocytes, oligodendrocytes, microglial cells, and blood-derived immune cells also contribute to the selective degeneration of motoneurons [10]. Moreover, ALS forms a broad neurodegenerative disease continuum with frontotemporal dementia (FTD) disease, and up to 50% of ALS patients concomitantly develop cognitive impairment or behavioral changes [11,12,13]. Despite recent promising gene therapy approaches, no effective cure is currently available for ALS patients [14]. Most cases of ALS are sporadic (sALS), and up to 10% have been classified as familial ALS (fALS) [15]. Currently, fALS-associated mutations have been found in approximately 50 genes, and more than 30 are thought to be causatives [16,17]. The most commonly mutated ALS-linked genes are superoxide dismutase-1 (SOD1), chromosome 9 open reading frame 72 (C9orf72), fused in sarcoma (FUS), and TAR DNA-binding protein (TARDBP) [18].
The antioxidant enzyme SOD1 was the first gene linked to fALS in 1993 [19]. This gene encodes a Cu/Zn superoxide dismutase, whose function is to catalyze the conversion of the superoxide ion, a toxic reactive oxygen species (ROS) produced during cellular respiration, to dioxides [20]. In 2011, abnormal GGGGCC hexanucleotide repeat expansion (HRE) within the C9orf72 gene was identified as a new cause of ALS and frontotemporal dementia (FTD) [21,22]. Currently, intronic HRE in the C9orf72 gene represents the most common genetic cause of ALS [23]. C9orf72 is part of a guanine nucleotide exchange factor complex [24], whose precise function remains unclear, but which was shown to be an important regulator of membrane trafficking and autophagy [25]. Lastly, FUS and TARDBP encode two DNA/RNA-binding proteins, which play distinct roles in transcription, as well as numerous roles in RNA metabolism, including splicing, stability, and transport [26]. FUS is a protein belonging to the heterogenous nuclear ribonucleoproteins (hnRNPs) (also known as hnRNP P2) [27], belonging to the FET protein family that includes two other RNA-binding proteins (RBPs) EWS and TAF15 [28]. In 2009, FUS was identified to be involved in fALS cases [29,30]. TARDBP encodes the TDP-43 protein, which is mainly nuclear and shuttles between the nucleus and the cytoplasm. Nuclear depletion and cytoplasmic aggregation of TDP-43 are found in most if not all ALS patients independently of the mutated status of TDP-43, making it a hallmark of the disease [31]. However, it is still debated whether TDP-43 cytoplasmic aggregation is deleterious or protective for ALS disease [32].
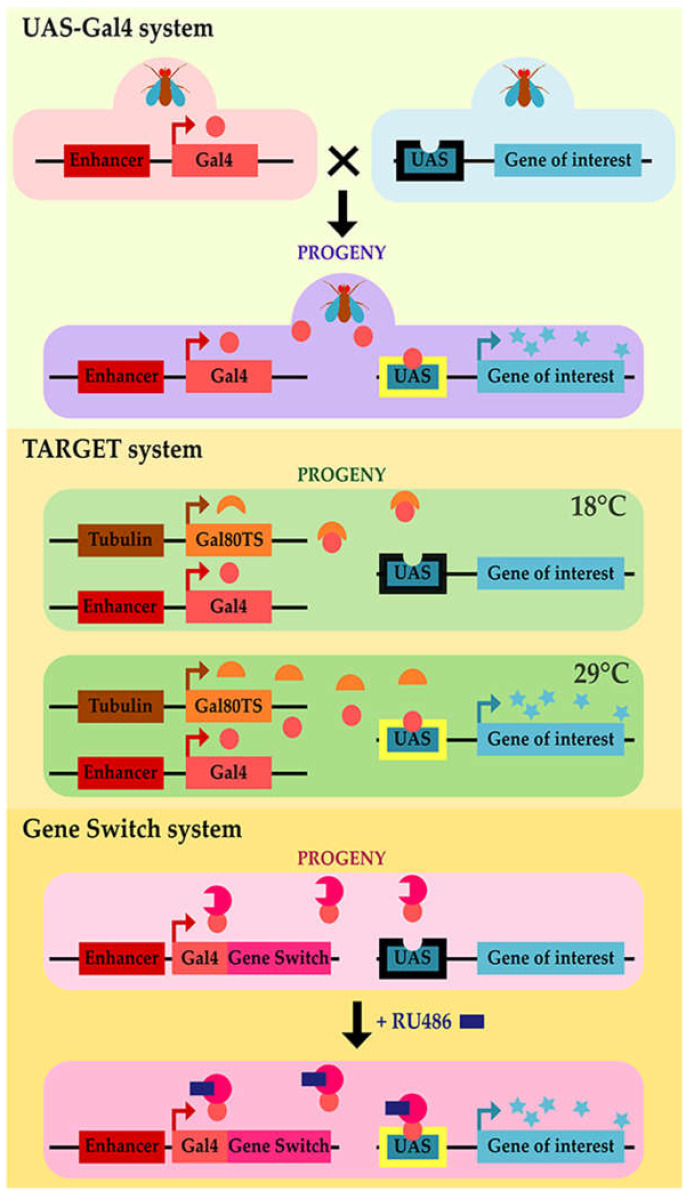
Drosophila melanogaster is a model easy to handle, cost-effective, with a short lifespan and a fully sequenced genome since 2000 [33,34]. In addition, Drosophila is a powerful genetic model with several genetic tools, such as the upstream activating sequence (UAS)/Gal4 system (Figure 1) [35], which is extensively used to overexpress Drosophila or disease-associated human genes in a tissue/cell-specific manner. Combined with the temporal and regional gene expression targeting (TARGET) or gene-switch systems [36] (Figure 1), gene expression can be controlled temporally allowing to investigate behavioral studies, avoiding developmental alterations. Furthermore, pan-genomic screenings, using RNA interference (RNAi)-induced gene knockdown for example, have been successfully used to identify genetic modifiers of human disease-associated phenotypes. It is estimated that as many as 77% of the human disease-associated genes have fly orthologs [37]. Furthermore, 76% of human proteins involved in synaptic vesicle trafficking have a Drosophila ortholog [38], indicating that synaptic transmission machinery is well conserved in flies. For all these reasons, Drosophila has emerged as a powerful genetic model for studying several neurodegenerative diseases (for reviews, see [39,40,41]) (Figure 2). Genetic studies in Drosophila have provided novel insights into the cellular and molecular mechanisms of ALS-linked neurodegeneration. Here, we review the Drosophila models that have been developed to better understand the function and decipher the pathological consequences associated with SOD1, C9orf72, FUS, and TARDBP genes.
Figure 1.
Drosophila genetic tools. The Gal4 system introduced in Drosophila allows transgene ectopic expression with spatiotemporal control. The upstream activating sequence (UAS)/Gal4 is a bipartite system. One transgenic line expresses the Gal4 transcription factor from yeast in a tissue-specific manner using an endogenous promoter or an enhancer sequence. The other line carries the transgene under the control of the UAS (upstream activating sequence). In the progeny of the cross, the UAS is bound by Gal4 protein and the transcription of the gene of interest starts. The temporal and regional gene expression targeting (TARGET) method allows a more flexible temporal control of the Gal4 system. A temperature-sensitive version of Gal80 protein (Gal80TS) is expressed ubiquitously under the control of the Tubulin promoter. At permissive temperature (18 °C), Gal80TS bound to Gal4 and prevents the starting of the transcription. A temperature shift to 29 °C relieves the transcriptional repression and permits the transcription of the gene of interest. The GeneSwitch system is based on hormone-inducible Gal4 and allows a temporal regulation of transgene expression. Gal4 is fused to a progesterone receptor, and the addition of hormone or synthetic ligand (RU486) in the feeding of the fly (adults or larvae) activates the transcription of the gene of interest. This system is reversible as the removal of ligand silent the system. All these genetic tools are routinely used in laboratory to precisely control transgenes expression not only in a specific tissue but also in a precise temporal manner.
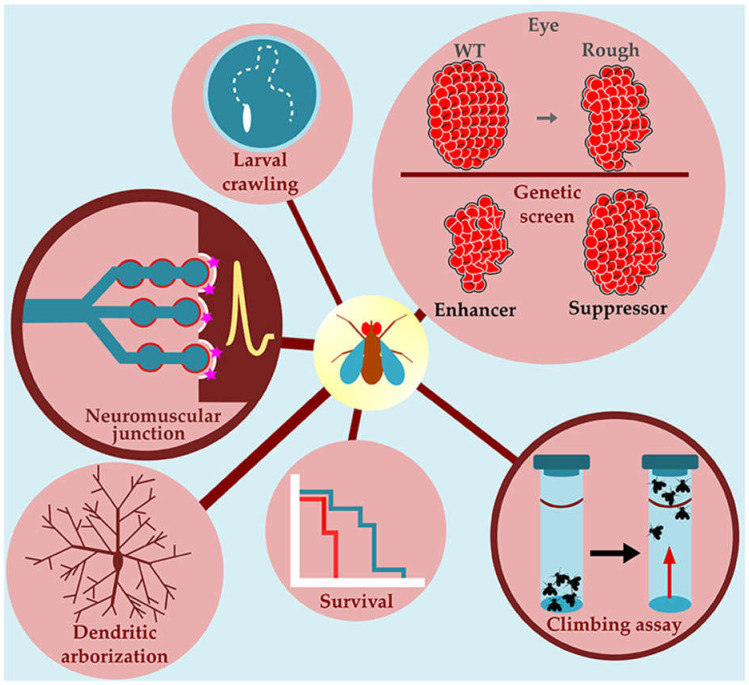
Figure 2.
Drosophila is a model to study neurodegenerative diseases. Several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), have been modeled in Drosophila via transgenic expression of wildtype or mutated human proteins. The toolkit available at Drosophila allows an in-depth study of the neurodegenerative mechanisms associated with these diseases. Several behavioral tasks, such as larval crawling and climbing assays, allow monitoring the locomotor activity during Drosophila life. Drosophila lifespan assays are useful to follow the time course of neurodegeneration and might be used as a readout for genetic screens. When expressed in the eye, toxic proteins disrupt the stereotyped organization of ommatidia and bristles, leading to a rough eye phenotype. This easily observable readout allows to perform genetic screens aimed at identifying modifiers (enhancers or suppressors) of the rough eye phenotype. Dendritic arborization neurons have a complex dendritic branching pattern and are widely used to identify genes involved in dendrite morphogenesis processes. The larval neuromuscular junctions (NMJs) are glutamatergic synapses that use ionotropic glutamate receptors and post-synaptic scaffolding proteins sharing similarities with mammalian brain synapses. These NMJs are easy to visualize and relatively simple with few axonal branches composed of synaptic boutons, which contain the active zones (sites of neurotransmitter release). Morphological and electrophysiological analyses on larval NMJs are frequently used to study how gene loss or gain of function might influence synapse development and function.
2. SOD1 Gene
2.1. dSod1 and the Aging Theory
In the 1980s, the concept of the aging process emerged with the assumption that aging reflects over time an accumulation of changes associated with an increasing susceptibility to develop diseases and, finally, death [42]. The free-radical theory assumed that the basic cause of aging lies on the deleterious effects produced by free-radical reactions [43,44,45,46]. This theory was supported by the observation that caloric restriction or lowering the metabolic rate decreases free-radical production, increasing the average lifespan in different species including Drosophila [47]. The prediction was to extend lifespan by increasing antioxidant levels to overcome the damages caused by free-radical reactions. As the main source of free radicals in aerobic eukaryotes is generated by oxygen metabolism, in that context, many studies have focused on the dSod1 gene. At that time, the link between ALS disease and the mutations in hSOD1 was not known, and the idea was to genetically increase lifespan in animal models especially in Drosophila using dSod1.
In Drosophila, the gene dSod1 was cloned in 1989 [48]. Many studies have shown that different genetic conditions leading to dSod1-null mutants, such as deletions or missense mutations inactivating the enzymatic activity of the protein, exhibited several phenotypes. The lifespan was drastically reduced by 85–90% and the locomotor activity was also impaired. The resistance to oxidative stress conditions was lowered in dSod1-null animals, whereas abnormal wing morphology and infertility were also observed [49,50,51]. The dSod1 loss-of-function (LOF) phenotypes were unsurprising for a ubiquitous housekeeping enzyme involved in detoxification and remained in the line with predictions and aging model. More unexpected were the consequences of dSod1 gain of function (GOF). Transgenic Drosophila lines carrying an additional copy of dSod1 were constructed using random P element insertion [52]. These flies showed 30–40% increase in the dismutase activity but with a minor effect on both lifespan and oxidative stress resistance (Table 1). One explanation was that the increase in dSod1 activity level was too low to markedly increase the maximal lifespan of these flies. On the other hand, a previous study using transgenic flies expressing the bovine form of SOD1 under the control of actin5C promoter showed that ubiquitously increasing bovine SOD1 expression to high levels led to a deleterious effect with flies that did not emerge from their pupal cases [53].
Table 1.
Phenotypes observed in dSod1 (superoxide dismutase-1) mutant backgrounds.
| Mutant Line | Phenotype | Reference |
|---|---|---|
| dSod1n108 | Homozygous lethality with rare eclosing adults, sterile, and early dying within 2–3 days No detectable superoxide dismutase activity Hypersensitivity to paraquat |
[49] |
| Necrotic lesions throughout retina | [50] | |
| dSod1x16 | No detectable superoxide dismutase activity | [50] |
| Partially lethal | [51] | |
| dSod1x139 | No detectable superoxide dismutase activity | [50] |
| Partially lethal | [51] | |
| Reduced eclosion rate, eclosing adults with shorter lifespan | [54,55] | |
| dSod1x16/dSod1x139 | Very reduced lifespan Impaired adult locomotion |
[56] |
| dSod1G37R | No lethality, adult eclosion as wildtype, no adult locomotion defect. | [57] |
| dSod1G51S | Impaired larval crawling Reduced adult viability, adult escapers with locomotor defect |
[57] |
| dSod1G85R | Impaired larval crawling Reduced adult viability, mostly lethal at pharate stage Adult escapers with muscle atrophy and denervation |
[57] |
| Reduction in NMJ bouton number and electrophysiological defect | [58] | |
| dSod1H48R | Reduced adult viability, mostly lethal at pharate stage | [57] |
| dSod1H71Y | Impaired larval crawling Reduced adult viability, mostly lethal at pharate stage, adult escapers with locomotor defect, muscle atrophy, and denervation |
[57] |
This table describes the phenotypes associated with different dSod1 mutant lines. Note that the experiments could be done at different temperatures.
2.2. Drosophila as a Modeling Tool to Understand hSOD1-Induced ALS
2.2.1. hSOD1 Was the First Gene Linked to ALS Disease
In 1993, the discovery of the genetic linkage between hSOD1 and the fALS [19] changed not only the vision of the disease but also the way the pathogenicity of SOD1 gene was considered. Indeed, to date, about 200 ALS-associated mutations in hSOD1 have been described [59]; most of them are missense point mutations (Figure 3). The first lines of research considered to explain the death of motoneurons characteristic in ALS were oriented toward an LOF hypothesis. The mutated form of hSOD1 protein could lead to a decrease in the superoxide dismutase enzymatic activity, implying an impairment of free-radical elimination, leading to an LOF phenotype. Nevertheless, hSOD1 mutants showed different degrees of alteration in superoxide dismutase activity. For example, hSOD1G93A (at position 93, a glycine is substituted to an alanine) showed an identical enzymatic activity compared to wildtype protein [60]. In mice models expressing the G93A or G37R mutation in hSOD1, the level of dismutase activity was not altered but motoneuron degeneration still took place [8,61]. In addition, the lack of phenotype observed in mSOD1 knockout mice [62] strengthened the idea that motoneuron death in ALS is not only from a loss in dismutase activity. Indeed, it was shown that the mutations found in hSOD1 induce the misfolding of the protein and confer new noxious properties. This toxic GOF is worsened by the ability of misfolded hSOD1 to spread from cell to cell causing the propagation of the disease [63,64,65,66,67,68,69,70,71,72,73].
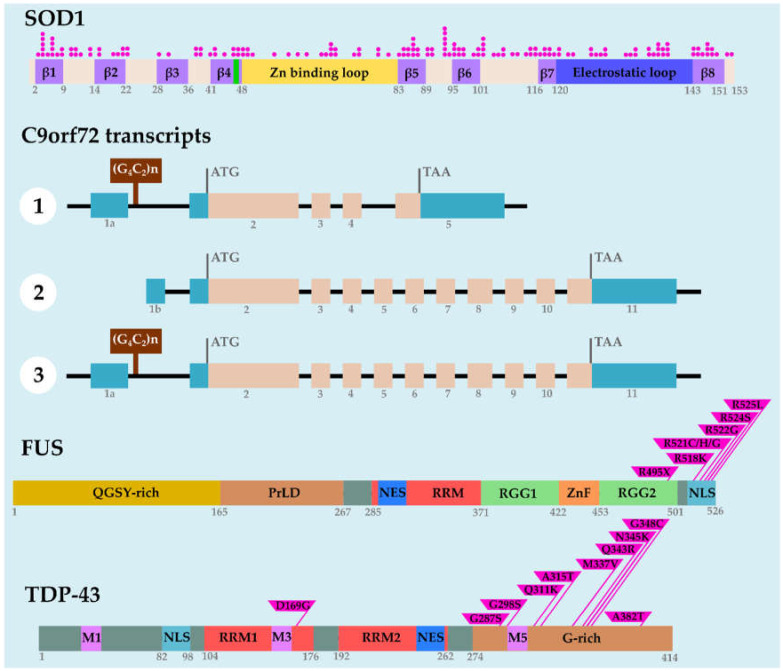
Figure 3.
Structure of chromosome 9 open reading frame 72 (C9orf72) transcripts and SOD1, fused in sarcoma (FUS), and TAR DNA-binding protein (TDP)-43 proteins. The human SOD1 protein is composed of eight beta-strands (in purple) and connecting loops. The Cu-binding region, the Zn-binding loop, and the electrostatic loop are represented in green, yellow, and blue, respectively. ALS-associated missense mutations are located throughout the protein and are listed vertically in pink dots. The human C9orf72 gene produces three variants. The (G4C2) hexanucleotide repeat expansion (HRE) is located in the first intron of variants 1 and 3, and in the promoter region of variant 2. The human FUS protein constitutes a N-terminal glutamine/glycine/serine/tyrosine-rich region (QGSY-rich in dark yellow), a prion-like domain (PrLD in brown), a nuclear export signal (NES in blue), a RNA recognition motif (RRM in red), two arginine/glycine-rich regions (RGG1 and RGG2 in green), a zinc finger domain (ZnF in orange), and a C-terminal non classical nuclear localization signal (NLS in cyan). Most ALS-related mutations are found in the C-terminal NLS region of FUS protein. The human TDP-43 protein carries three mitochondrial localization domains (M1, M3, and M5 in pink), a nuclear localization signal (NLS), two RNA recognition motifs (RRM1 and RRM2 in red), a nuclear export signal (NES), and a glycine-rich domain (G-rich in brown). The ALS-associated mutations described in the review are indicated.
2.2.2. Gain-of-Function Drosophila Models
As we mentioned above, dSod1-null mutants showed a severe reduction in lifespan. In a dSod1-null background (dSod1x16/sodx39), expression of hSOD1WT under the control of endogenous cis-regulatory dSod1 sequences fully rescued the lifespan reduction as dSod1 expression did. In this genetic background, the expression of different fALS-related hSOD1 mutant alleles (hSOD1A4V, hSOD1G37R, hSOD1G93C, hSOD1G41D, hSOD1I113T) showed a partial rescue of the lifespan compared to dSod1x16/sodx39 flies. However, the lifespan of fALS-related hSOD1 flies was shortened compared to flies expressing hSOD1WT allele. This lifespan reduction was coupled with an early drop of negative geotaxis performance in line with pathological phenotypes [56]. This first study did not allow the targeting of hSOD1 expression specifically in motoneurons, and the level of expression was limited to the endogenous level of Drosophila Sod1. Drosophila models were, therefore, generated using the UAS/Gal4 system [35] to overexpress different hSOD1 transgenes (Figure 1). Targeting wildtype hSOD1 or different mutated forms found in patients directly in motoneurons allowed analyzing the consequences of this expression at the whole animal level. Using a motoneuron Gal4 driver (D42-Gal4) to express either wildtype or ALS-related forms of hSOD1 (A4V or G85R), Nancy Bonini’s team showed that these different forms did not alter Drosophila lifespan [74] (Table 2).
Table 2.
Gain-of-function phenotypes induced by hSOD1. WT, wildtype.
| Gal4 Line | UAS Line | Phenotype/Reference |
|---|---|---|
| D42-Gal4 (motoneurons) |
hSOD1 WT | No effect on lifespan, progressive motor dysfunction [74,75] Abnormal synaptic transmission [74] |
| hSOD1 A4V | No effect on lifespan, progressive motor dysfunction [74] | |
| hSOD1 G85R | No effect on lifespan, progressive motor dysfunction [74,75] Abnormal synaptic transmission [74] Induction of stress response in glial cells [74] |
|
| D42-Gal4 (motoneurons) 3 mM BMAA (β-N-methylamino-l-alanine) |
hSOD1 WT | Increased lifespan compared to control flies [76] |
| hSOD1 A4V | Increased lifespan compared to control flies [76] | |
| hSOD1 G85R | Increased lifespan compared to control flies [76] | |
| M1B-Gal4 (glial cells) 3 mM BMAA (β-N-methylamino-l-alanine) |
hSOD1 WT | Accelerated death compared to control flies [76] |
| hSOD1 A4V | Accelerated death compared to control flies [76] | |
| hSOD1 G85R | Accelerated death compared to control flies [76] | |
| 24B-Gal4 (muscles) |
hSOD1 WT | No phenotype in thoracic muscle fibers, Slight motor behavior defect and normal lifespan [77] |
| hSOD1 G93A | Upheld wings, swollen mitochondria, Impaired motor behavior and reduced lifespan [77] |
This table describes the phenotypes associated with different UAS lines. Note that the experiments could be done at different temperatures.
These flies displayed progressive motor function deterioration over time. Contrary to what is observed in vertebrates, no neuronal cell death has been demonstrated in the adult ventral nerve cord where the motoneuron cell bodies are located. Therefore, ALS Drosophila and mouse models showed different phenotypes and specificity. In mice, directing mutant hSOD1 to all neurons did not cause motoneuron disease [78,79,80], while motoneuron death is contingent on the ubiquitous expression of mutant hSOD1. In the same manner, the presence of mutant hSOD1 aggregates was not obvious in Drosophila nervous system compared to mouse models of ALS [68,70,81,82]. The insoluble hSOD1 inclusions cannot explain the observed motoneuron dysfunction. However, misfolded hSOD1 mutant proteins could be detected in flies using conformation-specific antibodies developed in mice [83,84]. Indeed, a study using the hSOD1G93A transgene expressed under the control of the muscle specific driver (24B-Gal4) showed that misfolded hSOD1 protein was produced in muscles. This expression led to shortened lifespan, drop in motor activity, and mitochondrial impairment [77] (Table 2). Unfortunately, this study did not investigate the effects of the hSOD1G93A transgene expression in the nervous system. Interestingly, when hSOD1 mutant forms were cell-autonomously expressed by motoneurons, glial cells exhibited a stress response as evidenced by the expression of the heat-shock protein 70 (HSP70) [74]. The role of heat-shock protein in endoplasmic reticulum stress and, more particularly, the activation of the unfolded protein response (UPR) have been extensively studied in ALS disorder [85,86,87,88] (for a review, see [89]). Consistent with the important cross-talk between neurons and glial cells, recent studies suggested the idea of a non-cell-autonomous role of the UPR to modulate ALS progression [90,91,92]. The stress response induced in glial cells could, thus, help to provide support to motoneurons in the first stages of the disease. For example, HSP70 upregulation was protective during the disease progression in mouse models of ALS helping to maintain motoneuron innervation [93]. In a more general way, although the motoneurons are the main cells affected, ALS-related hSOD1 mutants in a non-cell-autonomous manner and the glial cells also play a part in ALS pathogenesis. The importance of glial cells was highlighted in BMAA (β-N-methylamino-l-alanine) resistance study. BMAA is a neurotoxin found in cycad seeds that causes Guam disease, an ALS–Parkinsonism dementia complex [94]. The expression of hSOD1 mutant (A4V, G85R) transgenes in either Drosophila motoneurons or glial cells led to different results. Expressed selectively in glial cells, the sensibility to BMAA was increased with a loss of motor performance over time. When hSOD1 constructs were expressed in motoneurons or in both motoneurons and glial cells, the resistance to the neurotoxin was enhanced, as well as the motor functions [76]. The mechanism leading to these different phenotypes according to the targeted cell type remains unknown, but it shows that glial cells are involved in the course of the disease as documented in rodent models of ALS [63,95,96].
2.2.3. Knock-In hSOD1 in Drosophila Led to Unexpected Results
The overall literature on the study of hSOD1 ALS-associated forms has shown that the phenotypes observed were not only dependent of the transgene expression level but were also related to the cellular type targeted. To overcome these difficulties and increase the understanding of the mechanisms leading to the toxicity of the SOD protein in ALS, recent studies chose to express hSOD1 at an endogenous level. For this, they used the ends-out homologous recombination strategy to replace wildtype dSod1 and introduce ALS-related mutations at conserved residues in dSod1, thereby creating dSod1G85R, dSod1H71Y, and dSod1H48R mutants [57]. In homozygous conditions, these mutants die throughout development, with escaper adult flies showing shortened lifespan and severe locomotion defects. Although these phenotypic traits are characteristic of ALS, the different mutants showed developmental defects, as dSod1G85R homozygous died at the pharate stage and did not emerge properly. Surprisingly, despite the severe locomotor defects observed, no motoneuron death was detected in larvae or adult flies. Interestingly, all mutants produced significant amounts of dSod1 protein, and expression of wildtype dSod1 partially saved the dSod1G85R/G85R phenotypes, leading to the hypothesis that both toxic GOF and LOF are combined to explain the observed ALS-associated phenotypes. Further study of dSod1G85R mutants at the neuromuscular junction (NMJ) level showed that both the number of boutons and the neurotransmission were reduced in the mutant, at the pharate stage [58]. Earlier in development, larvae dSod1G85R displayed a clear crawling impairment that was not associated with a disturbing phenotype at the NMJ level. Electrophysiological recordings of NMJs and motoneurons did not show any difference in post-synaptic excitatory events between dSod1G85R and wildtype flies, although motoneurons exhibited a slightly reduced excitability. The crawling behavior is generated by the central pattern generator network, an innate neural circuit composed of interneurons and motoneurons that generate rhythmic motor output. Sensory inputs play a role in adapting locomotor activity to external cues. Extracellular recordings and deafferentiation experiments showed that defective sensory feedback lead to reduced locomotor activity in dSod1G85R larvae [58] (Table 1). These findings highlight previous existing hypotheses on non-cell-autonomous factors involved in ALS degeneration [95].
2.3. ALS Drosophila Model to Test Neuroprotective Drug Candidates
Drosophila studies also allowed, in the ALS context with ectopic hSOD1 expression, to easily test different compounds for their protective or negative actions, on lifespan or motor activity for example. Thus, α-lipoic acid (LA) produced from plants and known for its various properties including antioxidant potential [97] has been tested in Drosophila expressing hSOD1G85R in motoneurons. LA was shown to moderate the neurotoxicity, extending lifespan and improving motor activity, in hSOD1G85R flies [98]. In the same technical manner, γ-oryzanol (Orz), a component of rice bran oil known for its antioxidative activity was also tested [99]. In the Drosophila ALS context, Orz increased HSP70 expression and alleviated oxidative damage [75]. These results open the door to other studies to investigate the role of new drugs potentially neuroprotective for ALS disease.
Until now, studies concerning hSOD1 have focused on major phenotypes (longevity, motor deficit), but a more detailed analysis at the cellular level of the hSOD1 actions could provide answers on the pathways leading to the pathology. Moreover, future studies using Drosophila could help to understand the mode of propagation of misfolded hSOD1, as well as the relationships between different cell types (motoneurons, glial cells, muscles).
3. C9orf72 Repeat Expansions
In 2011, intronic GGGGCC (G4C2) HRE in the C9orf72 gene was identified as a new cause of ALS [21,22]. In healthy individuals, the number of repeats is below 30, while it may reach thousands in ALS patients [100]. Actually, it is clearly established that abnormal HRE is the most common genetic cause of ALS [23], which accounts for around 40–50% of fALS and 5–10% of sporadic cases [23]. The human C9orf72 gene produces three alternative spliced transcripts [21] and, depending on the splice variant, the (G4C2) repeat is present in the promoter region (for transcript variant 2) or within the first intron (for transcript variants 1 and 3) (Figure 3). To date, the underlying mechanisms of neurodegeneration associated with C9orf72 HRE have been classified into three nonexclusive categories: (1) loss of C9orf72 function through haploinsufficiency, (2) sequestration of proteins by HRE-induced RNA foci, and (3) toxic GOF induced by dipeptide repeat proteins.
3.1. Loss of C9orf72 Function
Several studies have shown that the HRE in C9orf72 gene decreases the levels of messenger RNA (mRNA) [21,101] and protein [102,103] in patient tissues, suggesting that reduced C9orf72 protein function may play a role in the disease. C9orf72 LOF in Zebrafish [104] and Caenorhabditis.elegans [105] induced motor deficits, but degeneration of motoneurons was not observed in mice lacking C9orf72 gene [106]. However, a recent study showed that reduced C9orf72 function may exacerbate the repeat-dependent gain of toxicity [107], suggesting that the loss of C9orf72 function may be involved in the disease. The Drosophila genome does not contain a C9orf72 ortholog gene [108], making it impossible to determine the consequence of C9orf72 LOF in this organism.
3.2. Sequestration of Proteins by Expanded Repeat in RNA Foci
A hallmark found in different tissues, including motoneurons, of C9orf72-linked ALS patients is the presence of RNA foci [109]. These RNA foci are the result of bidirectional transcription of the HRE, leading to the accumulation of repeat-containing RNA aggregates, generally localized in the nucleus but also found in the cytoplasm. In Drosophila, it was found that components of the Drosophila DRB sensitivity-inducing factor (dDSIF) and Drosophila polymerase-associated factor 1 (dPAF1) complexes, two regulators of RNA polymerase II, are selectively required for the transcription of the expanded (G4C2) repeats [110,111]. Indeed, RNAi-induced silencing of these components decreased the RNA production only from long expanded repeats, leading to reduced toxicity. One possible toxic effect of these RNA foci is that they may sequester RBPs, interfering with their function and altering general RNA metabolism. For example, Xu et al. incubated biotinylated (G4C2)10 repeat RNA with mouse spinal cord lysates and identified Purα as a protein able to specifically bind to the repeat [112]. Purα is an evolutionarily conserved RBP that modulates transcription and translation, and it is also a component of ribonucleoprotein granules [113]. The authors used a UAS construct carrying a (G4C2)30 repeat cloned upstream of the translation start of GFP to express it in the developing eye. This induced a rough eye phenotype, revealing the toxicity of the expanded repeat that was not observed when the same construct carrying only 3 repeats was used. Overexpression of Purα with the (G4C2)30-GFP construct suppressed the neurodegeneration induced by the (G4C2)30-GFP transgene, supporting the idea that Purα function was attenuated in the presence of the expanded (G4C2) repeat. When the (G4C2)30-GFP construct was expressed in motoneurons using the OK371-Gal4 driver, around 50% adult eclosion failure and locomotion defect in 28 day old flies were observed. In addition, larval NMJs showed a reduction in active zone number. These phenotypes were suppressed by the overexpression of Zfp106, a zinc finger protein that binds specifically to a (G4C2)8 repeat construct [114]. Interestingly, Zfp106 interacts with other RBPs including TDP-43 and FUS, suggesting that its sequestration by the expanded repeat may alter the function of ALS-related RBPs.
It was shown that G4C2 HRE RNA forms hairpin and G-quadruplex structures that have the potential to recruit and sequester proteins. One such example is RanGAP (Ras-related nuclear GTPase-activating protein) that binds preferentially the sense RNA quadruplex of the HRE [115]. RanGAP stimulates the hydrolysis of GTP to GDP carried out by Ran GTPase, which is involved in nucleocytoplasmic transport. The overexpression of RanGAP suppressed the neurodegeneration induced by (G4C2)30-GFP expression in the developing eye. This was also the case when eye-expressing (G4C2)30-GFP flies were fed with 5,10,15,20-tetrakis-(N-methyl-4-pyridyl)porphine (TMPyP4), a porphyrin compound that destabilizes RNA G-quadruplex tertiary structures. This indicates that the G-quadruplex structure was required for the recruitment of RanGAP on (G4C2) HRE RNA [115].
Burguette et al. found that (G4C2) expanded repeat RNAs, which generally aggregate as nuclear RNA foci, also localize to neuritic granules that were actively transported along neuronal processes [116]. The presence of such hexanucleotidic repeat RNAs containing granules in neurites was associated with defects in neuronal branching, suggesting that they may confer local toxicity [116]. The class IV epidermal sensory dendritic arborization neurons of Drosophila have a complex dendritic branching pattern that was altered by the expression of a (G4C2)48 RNA repeat. The overexpression of dFMR1 or Orb2, two transport granule components that regulate local translation, enhanced the dendrite branching defects, while their RNAi-induced knockdown rescued the phenotype [116]. This suggests that local translation may be affected by the presence of (G4C2) HRE RNAs in neuronal processes.
Despite the putative sequestration of RBPs by HRE RNAs, several studies have demonstrated that RNA foci per se were not toxic. Tran et al. used a C9orf72 minigene containing (G4C2)160 repeat flanked by human intronic and exonic sequences [117]. When ubiquitously expressed using the actin-Gal4 driver, they did not detect antisense RNA foci but they observed an average of 42 sense nuclear RNA foci in glutamatergic neurons and 49 in glial cells. However, adult brain transcriptome analysis revealed that transcription was quite normal, strongly suggesting that sense RNA foci were not sufficient to alter global mRNA expression.
Depending on C9orf72 splice variant, the (G4C2) repeat is present in the promoter region or within the first intron (Figure 3). To answer whether the genomic location of the repeat could influence its toxicity, Moens et al. used two types of “RNA-only” constructs containing 100 copies of the repeat. One type of construct contained the sense or antisense repeats upstream of a polyA (sense/antisense polyA) and the other one contained the repeat within an artificial intron introduced into the GFP coding sequence (sense/antisense intronic) [118]. When expressed in adult Drosophila neurons, sense and antisense repeat-polyA constructs induced RNA foci within the cytoplasm, while RNA foci were mostly nuclear with both the sense and antisense intronic constructs. Despite the presence of these RNA foci, no effect on survival or climbing ability was observed indicating that they were not the primary cause of neurotoxicity [118]. However, this study did not investigate whether the co-expression of sense and antisense repeats could cause any detrimental effect.
3.3. Dipeptide Repeats Protein Toxicity
Despite its intronic localization, the (G4C2) expansion undergoes repeat-associated non-AUG (RAN) translation from both sense and antisense transcripts to produce five dipeptide proteins (DPRs) [109,119,120]. The sense transcript gives rise to poly-glycine/alanine (poly-GA) and poly-glycine/arginine (poly-GR) while the antisense transcript produces poly-proline/arginine (poly-PR) and poly-proline/alanine (poly-PA). Both sense and antisense transcripts produce poly-glycine/proline (poly-GP). To address whether these DPRs were toxic, Mizielinska et al. generated flies carrying transgene encoding “protein-only” by modifying the (G4C2) expansion with alternative codons [121]. Upon eye expression, they showed that (PR)36 and (GR)36 were toxic but not (GA)36 or (PA)36, indicating that only arginine-rich DPRs were toxic. When 100-copy DPRs were expressed, the toxic effect of arginine-rich DPRs was exacerbated but the other DPRs did not show toxicity.
The toxicity of arginine-rich DPRs was also demonstrated by several other studies. For example, Drosophila expressing poly(PR)50 in glutamatergic neurons, including motoneurons, developed normally but were unable to escape out of the pupal case due to the absence of movement [122]. In contrast, flies expressing poly(GA)50 or poly(PA)50 developed normally [122]. The expression of an ATG-driven GFP-tagged poly(GR)50 induced eye degeneration that was not observed with ATG-driven GFP-tagged poly(GA)50 or poly(GP)47 constructs [123,124]. At larval NMJs, the expression of poly(GR)100 but not poly(GA)100 induced a decrease of presynaptic area, as well as a reduction in active zone number [125].
Several DPRs are found in ALS patients; however, it remains to be determined whether some DPRs can interact with others to modulate toxicity. Indeed, one study has shown that (GR)80 had a diffuse cytoplasmic localization in Drosophila salivary gland cells, while (GA)80 formed cytoplasmic inclusions [126]. Interestingly, when both (GR)80 and (GA)80 were co-expressed, part of (GR)80 formed cytoplasmic inclusions, suggesting that (GA)80 recruited (GR)80 into these inclusions. Of note, the same observation was found in induced pluripotent stem cell (iPSC)-derived human neurons [126]. The expression of (GR)80 induced cell loss at the wing margin that was partially suppressed by the co-expression of (GA)80. However, this protective effect of (GA)80 was not observed in the eye [126]. Thus, it is still unclear whether DPRs may interact with each other and modulate the toxicity of arginine-rich DPRs.
The transcellular spreading of misfolded proteins is thought to participate in the clinical progression of several neurodegenerative diseases. To answer whether DPRs have the ability to spread, mCherry-tagged DPRs were expressed in olfactory receptor neurons (ORNs) that target their axonal projections in fly brain. When 36 copies of (GA), (GR), or (PR) were used, no spreading was observed. In contrast, (GA)100 but not (GR)100 or (PR)100 was detected in fly brains after 3 days of expression in ORNs [127]. Accordingly, only (GA)100 was detected in axons and synaptic terminals of ORNs. Interestingly, the spreading was increased when a longer construct was used. It would be interesting to combine several DPRs to determine whether poly-GA may recruit other DPRs allowing them to spread between neurons.
In a way to understand how arginine-rich DPRs may cause toxicity, Lee et al. analyzed the interactome of GFP-tagged DPRs [124]. Among the identified interactors, 81 proteins were common to GFP-(PR)50 and GFP-(GR)50 and included some ALS-related RBPs, such as TDP-43 and FUS, for example, suggesting that arginine-rich DPRs may alter RNA metabolism. Moreover, these common interactors showed enrichment in proteins containing low-complexity sequence domains (LCDs), which mediate the assembly of membrane-less organelles. By using an RNAi genetic screen targeting the orthologous genes in Drosophila, the authors found that most of the genetic modifiers of GFP-(PR)50-induced toxicity were components of membraneless organelles such as nucleoli, the nuclear pore complex, and stress granules [124]. This strongly suggested that arginine-rich DPRs disturb the function of these organelles. In agreement with this, nucleocytoplasmic transport defects were also reported by other studies. A LOF genetic screen based on the modification of (G4C2)58-induced eye degeneration identified 18 modifiers that play a role in nucleocytoplasmic transport, suggesting that nuclear retention of mRNA may be a cause of toxicity [123]. By performing a targeted RNAi genetic screen to identify modifiers of the rough eye phenotype induced by the expression of (PR)25, Boeynaems et al. showed that nucleocytoplasmic transport was implicated in the pathogenic mechanism of C9orf72 HRE [128]. Another DPR interactome study was performed on flies expressing DPRs in adult brain neurons [129]. This study identified mostly ribosomal proteins as interactors of arginine-rich DPRs. A genetic screen based on the overexpression of ribosomal proteins or translation initiation factors revealed that the eukaryotic translation initiation factor 1A (eIF1A) mitigated the lifespan defects induced by (G4C2)36 repeat or (GR)100 pan-neuronal expression. As eIF1A plays an important role in translation initiation, these data suggested that translation machinery might be affected by arginine-rich DPRs.
A recent study showed that poly(GR)80 expression in Drosophila muscles induced alteration of indirect flight muscles leading to defects in wing posture [130]. These alterations were the consequence of the entry of poly(GR)80 within mitochondria where they interacted with components of the mitochondrial contact site and cristae organizing system, leading to mitochondrial defects. Thus, it appears that poly(GR) may cause toxicity by interfering with mitochondrial functions, at least in muscle cells.
Altogether these studies have identified several pathways of C9orf72-associated toxicity that may highlight novel therapeutic targets. For example, reducing the function of components of the specific machinery required for transcription of expanded repeat RNA, such as dDSIF and dPAF1 complexes, should reduce levels of toxic expanded RNA, as well as the production of toxic DPRs [110,111]. Another strategy would be to target the G-quadruplex structures of the expanded repeat RNAs. Indeed, the destabilization of the G-quadruplex structure by TMPyP4 treatments seems to be efficient as it suppressed HRE-induced neurodegeneration in the eye [115]. Furthermore, Simone et al. identified small molecules that specifically stabilize the G-quadruplex structures [131]. When administrated in the food, these small molecules decreased DPR production and improved survival of Drosophila expressing (G4C2)36 repeats [131], confirming the therapeutic potential of this approach. Lastly, the mitochondrial alterations induced by poly(GR) expression in muscles was rescued by treatment with nigericin, a K+/H+ antiporter that rebalances mitochondrial matrix ion levels, opening the way to future potential therapeutic strategies [130].
4. FUS, an RNA-Binding Protein Associated with ALS
Fused in sarcoma, also known as translocated in liposarcoma (TLS), is a DNA/RNA-binding protein ubiquitously expressed. ALS-causative mutations in this RBP were discovered in 2009 [29,30]. In the nervous system, FUS is predominantly located in the nucleus and able to shuttle between the nucleus and the cytoplasm [132]. In patients, FUS is nuclear but FUS mutant forms are also found aggregated in the cytoplasm of neurons [29,30]. In ALS, the formation of protein aggregates is one hallmark of pathogenic mechanisms leading to motoneuron death, and the role of FUS in this process is important [65,133,134,135].
As an RBP, FUS has a pivotal role in many aspects of RNA metabolism and processing, including RNA splicing [136,137], transcription [138,139], nucleocytoplasmic transport [140], and translation (for a review, see [141]). FUS is a 526 amino acid protein encoded by 15 exons. The protein contains seven domains: an N-terminal region rich in glutamine, glycine, serine, and tyrosine residues (QGSY-rich domain), a prion-like domain, an RNA recognition motif (RRM), a nuclear export sequence (NES), two regions rich in arginine and glycine (RGG1 and RGG2), a zinc finger (ZnF) domain, and a C-terminal part containing a nonclassical PY-nuclear localization signal (NLS) motif [142] (Figure 3). ALS-related mutations are located preferentially in the C-terminal part affecting the NLS domain of the protein. The pathogenic FUS mutations are missense changes, and the R521H and R521C mutations are the most commonly found in patients. As a result, in patient postmortem studies, a strong labeling of FUS was observed in neuron and glial cell nuclei but also as aggregates in the cytoplasm. Only few point mutations have been identified in the sequence encoding the N-terminal part or prion-like domain of the FUS protein [29,30,143,144]. More recently, mutations in the 3′ untranslated transcribed region (UTR) of FUS have been described. Interestingly, these mutations affect the expression level of FUS rather than the cellular localization of the protein [145,146].
The mechanisms underlying the cause of neurodegeneration in FUS-ALS patients are still unknown even if numerous hypotheses have been made. Hence, mislocalization of FUS mutant forms in the cytoplasm leads to a disruption of FUS nuclear functions, such as transcription regulation or mRNA processing, and produces a LOF phenotype [134,147,148,149,150,151]. Conversely, the presence of FUS mutant forms in cytoplasmic aggregates creates a new toxic function of FUS in this compartment [144,152,153,154]. In an attempt to test these two hypotheses (LOF or new toxic GOF) to better understand the mode of action via which FUS mediates neurotoxicity, animal models for FUS-ALS have been generated. In vertebrate models, axonal degeneration and neuromuscular damages with protein aggregation in motoneuron were described as main phenotypes [148,155,156,157].
4.1. Drosophila Models of FUS-Related Neurodegeneration
In Drosophila, the first studies showed that eye-overexpression of wildtype FUS or different ALS-related mutated forms led to progressive neurodegeneration of the photoreceptors [147,158,159]. When expressed in the eyes, the phenotypes induced by the expression of wildtype FUS were a mild rough eye surface and a reduction in red pigment. The expression of different FUS mutants (R521C, 521H, 518K, R524S, or P525L) generated a more severe rough eye phenotype and even total depigmentation of the eyes (Table 3).
Table 3.
Gain-of-function phenotypes induced by FUS. CNS, central nervous system.
| Gal4 Line | UAS Line | Phenotype/Reference |
|---|---|---|
| Act5C-Gal4 (ubiquitous) |
FUS WT | Lethal, no eclosion [160] |
| FUS R521G | Lethal, no eclosion [160,161] | |
| FUS R521H | Lethal, no offspring [161] | |
| FUS P525L | Lethal, no offspring [161] | |
| FUS Δ32 | No effect on viability [160] | |
| Tubulin-Gal4 (ubiquitous) |
FUS WT | Lethal, no offspring [161] |
| FUS R521G | Lethal, no offspring [161] | |
| FUS R521H | Lethal, no offspring [161] | |
| FUS P525L | Lethal, no offspring [161] | |
| Tubulin-Gal4 Tubulin-Gal80TS (expression induced at adult stage) |
FUS WT | Severe reduction of lifespan [161] |
| FUS R521G | Severe reduction of lifespan [161] | |
| FUS R521H | Severe reduction of lifespan [161] | |
| FUS P525L | Severe reduction of lifespan [161] | |
| Appl-Gal4 (pan-neuronal) |
FUS WT | Normal eclosion [158] |
| FUS R518K | Pupal lethality [158] | |
| FUS R521C | Pupal lethality [158] | |
| FUS R521H | Pupal lethality [158] | |
| Elav-Gal4 (pan-neuronal) |
FUS WT | Rescued eclosion and locomotion in caz1 mutants [159] Lethal pupal [162] Reduced viability at 25 °C, improved at 19 °C [160] Lethal, no offspring [161] |
| FUS R521G | Reduced viability at 25 °C, improved at 19 °C [160] Lethal, no offspring [161] |
|
| FUS R521H | Lethal, no offspring [161] | |
| FUS P525L | Rescued eclosion in caz1 mutants [159] Lethal, no offspring [161] |
|
| FUSΔ32 | No effect on viability [160] | |
| ElavGS (pan-neuronal, inducible) |
FUS WT | RU486 treatment at eclosion, decline in lifespan, 50% lethality at 28 days [158] Decreased lifespan, no degeneration in brain [162] |
| FUS R521C | RU486 treatment at eclosion, 50% lethality at 10 days, impairment in climbing [158] | |
| D42-Gal4 (motoneuron) |
FUS WT | Reduced viability at 25 °C, improved at 19 °C, defect in adult climbing [160] Mitochondria defect [163] Defect in adult eclosion [164] Eclosion defect and escapers with immature phenotype and reduced lifespan [161] |
| FUS R495X | No effect on adult eclosion [164] | |
| FUS R521G | Reduced viability at 25 °C, improved at 19 °C, defect in adult climbing [160] Eclosion defect and escapers with immature phenotype and reduced lifespan [161] |
|
| FUS R521H | Eclosion defect and escapers with immature phenotype and reduced lifespan [161] | |
| FUS P525L | Mitochondria defect [163] Eclosion defect and escapers with immature phenotype and reduced lifespan [161] |
|
| FUS Δ32 | No effect on viability and adult climbing [160] | |
| D42-Gal4 Tubulin-Gal80TS (expression induced at adult stage) |
FUS WT | Reduced lifespan and impaired flight ability [161] |
| FUS R521G | Reduced lifespan and impaired flight ability [161] | |
| FUS R521H | Reduced lifespan and impaired flight ability [161] | |
| FUS P525L | Reduced lifespan and impaired flight ability [161] | |
| OK371-Gal4 (glutamatergic neurons) |
FUS WT | Mild larval crawling defect, no effect on synaptic boutons [158] Disruption in motoneuron cluster, decreased synaptic bouton number, reduction in mobility [147] Larval locomotion activity impaired, decreased synaptic bouton number, pupal lethality [160] Reduced larval crawling, no effect on larval CNS size, 70% of adult eclosion [165] Larval locomotion impaired, pupal lethality [164] |
| FUS R495X | Normal larval locomotion, no defect in adult eclosion, no defect in climbing activity, no effect on adult viability [164] | |
| FUS R518K | Impaired larval locomotion, no effect on synaptic boutons, pupal lethality [158] Drastic larval crawling reduction, reduction in larval CNS size, no adult eclosion [165] |
|
| FUS R521C | Impaired larval locomotion, no effect on synaptic boutons, pupal lethality [158] Drastic larval crawling reduction, reduction in larval CNS size, no adult eclosion [165] |
|
| FUS R521G | Larval locomotion activity impaired, decreased synaptic bouton number, pupal lethality [160] | |
| FUS R521H | Impaired larval locomotion, no effect on synaptic boutons, pupal lethality [158] | |
| FUS R524S | Disruption in MNs cluster, decreased synaptic bouton number, reduction in mobility, tail lifted [147] | |
| FUS P525L | Disruption in MNs cluster, decreased synaptic bouton number, reduction in mobility, tail lifted [147] Larval locomotion impaired, pupal lethality [164] |
|
| FUS 4F-L (RRM Mutant) | No effect on larval crawling, no effect on the larval CNS size, no effect on adult eclosion [165] | |
| FUSΔ32 | No effect on larval locomotion, no effect on synaptic bouton number [160] | |
| OK6-Gal4 (motoneurons) |
FUS WT | Increased synaptic bouton number [159] Adult eclosion defect with immature escapers [166] |
| FUS R521G | Adult eclosion defect with immature escapers [166] | |
| FUS R521H | Adult eclosion defect with immature escapers [166] | |
| FUS R522G | No effect on synaptic bouton number [159] | |
| FUS P525L | No effect on synaptic bouton number [159] | |
| GMR-Gal4 (eye) |
FUS WT | Very mild rough eye [158,165] Reduction in red pigment, rough surface [147] Malformed interommatidial bristles [162] Severe rough eye phenotype [160] Rough eye with pigment loss [164] Reduced and rough eye [167] |
| FUS R495X | Mild rough eye with slight pigmentation defect [164] | |
| FUS R518K | Rough eye [158,165,167] | |
| FUS R521C | Rough eye [158,160,165,167] | |
| FUS R521G | Rough eye [160] | |
| FUS R521H | Rough eye [158] | |
| FUS R524S | Severe rough eye [147] | |
| FUS P525L | Severe rough eye, depigmentation [147] Rough eye with pigment loss [164] |
|
| FUSΔ32Cter | No phenotype [160] | |
| FUS 4F-L (RRM Mutant) |
No phenotype [165] | |
| CCAP-Gal4 (bursicon neurons) |
FUS WT | Adult eclosion impairment and escapers immature phenotype [166] |
| FUS R521G | Adult eclosion impairment and escapers immature phenotype [166] | |
| FUS R521H | Adult eclosion impairment and escapers immature phenotype [166] | |
| OK107-Gal4 (mushroom bodies) |
FUS WT | Thin mushroom body lobes [147] |
| FUS R524S | Drastic decreased size of MB neurons, axonal degeneration [147] | |
| FUS P525L | Drastic decreased size of MB neurons, axonal degeneration [147] | |
| MS1096-Gal4 (wing pouch) |
FUS WT | Defect in wing formation [160] |
| FUS R521G | Defect in wing formation [160] | |
| FUS Δ32 | No effect [160] |
This table describes the phenotypes associated with different UAS FUS lines. Note that the experiments could be done at different temperatures and the UAS lines were generated using different genetic strategies (site-specific or random insertion) and tags.
As ALS is characterized by the degeneration of motoneurons, the action of FUS expression in this specific neural population was considered. When expressed in motoneurons, all forms of FUS (wildtype and ALS-related) led to a deficit in locomotion at the larval stage followed by a lethality occurring at late pupal stage ([147,158] and Table 3). At the NMJ level, the synaptic endings were altered. No consensus has emerged concerning a clear morphological change in the bouton numbers following FUS expression in either wildtype or mutants [147,158,159,160,168,169]. However, the synaptic boutons exhibited less and aberrantly organized active zones [168,169]. The post-synaptic compartment of the NMJs, like the clustering of the glutamate receptors, was altered when FUS was expressed in motoneurons [168]. As a result, the synaptic transmission was severely impaired at the NMJs. Electrophysiological studies showed that the amplitude of the excitatory junctional potentials was decreased in FUS mutant expression conditions, strengthening the idea that synaptic defects appear earlier than MN degeneration [168,169].
Adult eclosion defect and late pupal lethality were also observed using a general neuronal driver as Elav-Gal4. Nevertheless, use of inducible Elav-Gal4-GS line has allowed showing that FUS mutant forms induced a drastic climbing decline associated with a reduced lifespan, more severe than wildtype FUS did [158]. Thus, the FUS fly models recapitulate several characteristics found in ALS patients. Nevertheless, depending on the studies, some discrepancies can be noted concerning the severity of different phenotypes. It appears that the expression level of the different forms of FUS is a critical element that must be taken into consideration for the analysis and comparison of the observed phenotypes. Thus, the viability and the adult eclosion rate when FUS is expressed in motoneurons can vary, as well as the severity of the induced rough eye phenotype [160,168].
4.2. From FUS Endogenous Functions to Toxicity
4.2.1. Nuclear and Cytoplasmic Localization of a Shuttle Protein
The cellular localization of FUS has also been studied. As in ALS patients, mutated forms of FUS were both found in the nucleus and mislocalized in the cytoplasm, whereas wildtype FUS was always detected in the nucleus [29,30]. ALS-related FUS mutations are predominantly found in the C-terminal part of the protein containing the NLS domain. These mutations led to a redistribution of the FUS protein in the cytoplasm in all system models used. Nuclear import of FUS is mediated by the nuclear transport receptor transportin (also known as karyopherin-β2) and impairment of this interaction led to FUS mislocalization in the cytoplasm [144,152,164]. This observation gave rise to the hypothesis that loss of physiological function of FUS in the nucleus contributes to the ALS pathology [170,171,172].
To decipher FUS functions, studies have focused on the role of the cabeza (caz) gene. caz is the only ortholog of FUS in Drosophila [173]. The caz1 mutants appeared morphologically normal but displayed an adult eclosion defect, and the resulting adult escapers showed reduced lifespan and deficit in locomotion ([159] and Table 4). Overexpression of FUS in the nervous system of caz1 Drosophila rescued the adult eclosion defect and restored both lifespan and locomotor deficits. By contrast, mutated forms of FUS (P525L and R522G) acted on the survival to adulthood of caz1 Drosophila with no effect on the lifespan and locomotion of adults [159].
Table 4.
Phenotypes observed in cabeza mutants.
| Mutant Line | Phenotype | Reference |
|---|---|---|
| caz1 | No effect on NMJ morphology Adult eclosion impairment Adult locomotion affected Reduced lifespan |
[159] |
| Loss of ommatidia in the eyes | [174] | |
| caz2 | Developmental delay and pupal lethality | [175] |
| cazKO | Developmental delay and pupal lethality | [175] |
| cazlox, elav-Gal4, UAS Cre (pan-neuronal) |
Reduced adult offspring Adult motor deficit Reduced lifespan |
[175] |
| cazlox, Mef2-Gal4, UAS Cre (muscles) |
Reduced muscle width | [175] |
| CazFRT, elav-Gal4, UAS FLP (pan-neuronal) |
Reduced adult offspring Reduced lifespan |
[175] |
| CazFRT, Mef2-Gal4, UAS FLP (muscles) |
No effect | [175] |
This table describes the phenotypes associated with different cabeza mutant lines. Note that the experiments could be done at different temperatures.
The use of RNAi lines to knockdown caz gene expression in neurons showed that caz silencing did not affect lifespan but altered climbing performances ([150] and Table 5). caz-knockdown in the eye induced a rough phenotype due to apoptotic cells in the pupal retina. Indeed, this phenotype could be rescued by the antiapoptotic p35 expression [176]. The engineering of new caz null mutant and conditional alleles using homologous recombination confirmed the previous phenotypes of pupal lethality and locomotor defects [175]. These results corroborated the fundamental role of caz in neural development and argue in favor of the LOF hypothesis to explain the FUS-induced neurodegeneration in ALS. caz GOF produced the same phenotypes as FUS overexpression (Table 5). The larval locomotion was impaired with a reduced number of synaptic boutons and severe eye degeneration [160]. In addition, both Caz and FUS GOF induced apoptosis when expressed in motoneurons [150].
Table 5.
Cabeza gain of function and RNAi-induced cabeza silencing phenotypes.
| Gal4 Line | Line | Phenotype/Reference |
|---|---|---|
| Act5C-Gal4 (ubiquitous) |
UAS-caz | Lethal, no adult eclosion [160] |
| UAS-RNAi caz (363–399) VDRC 100291 |
Low adult eclosion [160] No effect at 28 °C [150] |
|
| UAS-RNAi caz (1–167) |
Lethal at 28 °C, no effect at 25 °C [150] Late pupal lethality [176] |
|
| UAS-RNAi caz (180–346) |
No effect at 28 °C [150] | |
| Elav-Gal4 (pan neuronal) |
UAS-caz | Almost lethal at pupal stage, few escapers [160] |
| UAS-RNAi caz (363–399) VDRC 100291 |
Adult eclosion defect [160] No effect on lifespan, reduced mobility from young adult, decreased in total axonal branch length [150] and synaptic bouton number [177] Adult climbing defect, number of synaptic bouton and total branch length reduced [178,179] |
|
| UAS-RNAi caz (1–167) |
No effect on lifespan, reduced mobility from young adult. In larvae, decreased synaptic bouton number [150,177] and decreased total axonal branch length [150] | |
| UAS-RNAi caz (180–346) |
No effect on lifespan [150] | |
| D42-Gal4 (motoneurons) |
UAS-Caz | Pupal lethality, no adult eclosion [160] |
| UAS-RNAi caz (363–399) VDRC 100291 |
Low adult eclosion [160] | |
| OK6-Gal4 (motoneurons) |
UAS-caz | Increased synaptic bouton number [159] Rescued the caz1 phenotypes [159] |
| OK371-Gal4 (glutamatergic neurons) |
UAS-caz | Severe larval crawling defect, reduction of synaptic bouton number [160] |
| GMR-Gal4 (eye) |
UAS-caz | Rough eye [160,177] |
| UAS-RNAi caz (1–167) |
Rough eye [176,178] | |
| UAS-RNAi caz (363–399) VDRC 100291 |
Severe rough eye [179,180] apoptosis [176] | |
| nsyb-Gal4 (pan neuronal) | UAS-RNAi caz (363–399) VDRC 100291 |
No significant lethality, slight motor defect [175] |
| UAS-RNAi HMS00790 | No significant lethality, slight motor defect [175] | |
| UAS-RNAi HMS00156 | No significant lethality, slight motor defect [175] | |
| Ppk-Gal4 (da neuron) |
UAS-caz | Reduced synaptic projections [181] |
| UAS-caz P398L | Reduced synaptic projections [181] |
This table describes the phenotypes associated with different UAS-cabeza or UAS RNAi cabeza lines. Note that the experiments could be done at different temperatures and the UAS lines were generated using different genetic strategies (site-specific or random insertion).
Both Caz and FUS are found in the nucleus in the nervous system. To address the role of FUS localization in toxicity, FUS constructs deleted for the NES domain were engineered in both wildtype FUS and ALS-related mutants. While FUSWT, FUSΔNES, and FUSΔNES R518K localized in the nucleus, FUSR518K was also found in the cytoplasm [158]. The double-mutant FUSΔNES R518K became nontoxic, suggesting that FUS mutant cytoplasmic localization is important for the toxicity to arise [158]. However, wildtype FUS deleted for its last 32 amino acids containing NLS domain (FUSΔ32) localized outside of the nucleus but did not exhibit any phenotype when expressed in the eye or in motoneurons. Moreover, addition of an NLS sequence in the FUSΔ32 construct led to a nuclear localization of the protein associated with locomotor defect and eye degeneration phenotypes [160]. These contradictory results do not allow deciphering clearly the location (nucleus or cytoplasm, or both) where FUS induces its toxicity.
Interestingly, the observation that the overexpression of wildtype FUS or mutant forms in the motoneurons led to a drastic downregulation of cabeza points out that FUS could autoregulate its own expression in Drosophila [164,168]. Moreover, mutations affecting the 3′-UTR region cause FUS overexpression and lead to ALS pathology [146], meaning that disruption of FUS autoregulation conducts to overexpression of wildtype FUS, which is sufficient to cause ALS.
These results strengthen (i) the notion of conservation between caz and FUS and (ii) the idea that the expression level of FUS is critical to trigger the neurodegeneration process [159,164,168]. Thus, elucidating the physiological functions of FUS with its different partners is necessary to better understand the mechanisms involved in the pathology.
4.2.2. FUS Alters Mitochondrial Physiology
Mitochondria continually undergo fission and fusion processes; the breakdown of this balance is decisive in neurodegenerative diseases. Mitochondria disruption has been extensively reported from ALS patient studies [182,183] (for a review, see [184]). In motoneurons, expression of FUSWT or ALS mutant FUSP525L led to mitochondrial damages in transgenic flies. Compared to control condition, mitochondria were smaller, and the number of larger ones decreased. With FUS mutant overexpression conditions, the phenotype was more pronounced [163,185]. In addition, the mobility and the mitochondrial transport were reduced. Both anterograde and retrograde transports were affected; the frequency and duration of the transport interruption were increased, while the motile phase of mitochondrial transport was reduced [186]. Similar results were found using another neural model, the class IV dendritic arborization neurons (da neurons). Expression of FUS and Caz wildtype or mutant in da neurons altered the dendritic branching. FUSP525L or CazP398L mutants localized in the cytoplasm and were found at the synaptic projections of the da neurons, which were altered. All forms of FUS and Caz (wildtype or mutant) impaired axonal transport of the synaptic vesicles. A decrease in the number of synaptic mitochondria in da neurons was also observed. The expression of all forms of FUS and Caz induced an increase in the frequency of calcium transients in da neurons [181]. FUS was also found associated with mitochondria, and it interacts directly with HSP60, an ATPase dependent mitochondrial chaperone [187], which is involved in the translocation of FUS to the mitochondria. Knocking down HSP60 expression via RNAi expression in motoneurons was sufficient to rescue the mitochondrial phenotypes induced by FUS overexpression [163]. The physiological role of FUS in mitochondria is still unknown but it seems that, in excess of FUS, the interaction of FUS with HSP60 promotes mitochondrial damage and toxicity.
4.2.3. FUS in the Nucleus Is Associated with Nuclear Bodies
The nucleus is compartmentalized in membraneless intranuclear compartments collectively named nuclear bodies (NBs). NBs include Cajal bodies, nucleoli, nuclear speckles, and paraspeckles that are dynamic structures responding to stress and controlling gene expression. NBs are the location of RNA biogenesis and maturation, and they are involved in the assembling of ribonucleoprotein complexes or in the retention of proteins [188,189,190,191]. NBs are composed of various proteins and RNAs, including the architectural RNAs (arcRNAs) that are long noncoding RNAs (lncRNAs) used as scaffolds [191,192,193]. Interestingly, FUS was found associated with paraspeckles, and both LOF and GOF of FUS caused disruption of NBs [194]. In Drosophila, arcRNAs were found associated with hnRNPs. Notably, arcRNA hsrω is crucial for the formation of specific ω-speckles NBs. It also regulates the intranuclear trafficking and availability of different hnRNPs [195,196,197,198,199]. The loss of hsrω in neurons, using an RNAi-specific line, resulted in phenotypes closely related to the ones of caz LOF. Adults exhibited a shortened lifespan accompanied by locomotor deficit and a reduction in the number of synaptic boutons at the NMJs [179]. In addition, the subcellular localization of Caz changed and became cytoplasmic, leading to the conclusion that ω-speckles are involved in Caz compartmentalization. caz and hsrω genetically interact as the overexpression of hsrω enhanced the rough eye phenotype induced by caz expression [179]. The same interaction occurred between hsrω and FUS. Expressed in eye, hsrω RNAi rescued the toxicity induced by FUS. In this genetic condition, FUS was cytoplasmic as observed in the control condition, but punctate forms were detectable in the cytoplasm [179]. In this hsrω-knockdown background, FUS insoluble aggregates were not toxic and were found associated with Lysosome-associated membrane protein 1 (LAMP1), a marker of lysosomes that was upregulated [179,200]. Thus, misregulation of lncRNA could rescue the FUS-induced toxicity via the formation of nontoxic FUS aggregates through a mechanism that remains to be explored.
4.2.4. FUS Is a Multidomain Protein: Structure and Function
As mentioned above, FUS is composed of different domains whose functions have been revisited in recent years. Previously, the C-terminal domain was the focus of most studies because most ALS mutations cluster in the NLS sequence and were known to disrupt the nuclear localization of FUS. Revisiting the FUS amino-acid sequence showed that the N-terminal part contains a prion-like domain followed by a glycine-rich and an arginine–glycine–glycine repeat sequence (RGG) (Figure 3). These three domains, which are composed of few different amino acids, are considered an LCD sequence. It was shown that the LCD of FUS is necessary for the formation of phase-separated liquid droplets or hydrogel [201,202,203,204]. To better understand the role of each FUS domain in the neurodegeneration process in vivo, systematic deletions or mutations of these domains have been generated in transgenic flies.
In motoneurons, expression of wildtype FUS led to pupal death. Flies cannot emerge from the pupal case and the few escapers observed display a soft cuticle and unexpanded wings that are characteristics of an immature phenotype (Table 3). These phenotypes have been used as readout to determine the toxicity of the different domains of FUS. Bogaert et al. expressed in motoneurons different forms of FUS, deleted for distinct domains [161]. They analyzed the phenotypes obtained, comparing with the toxicity induced by wildtype FUS. In this screen, deletion of the Gly-rich domain, RGG1 domain, or zinc finger domain did not change the pupal death phenotype observed with wildtype FUS. This was also the case for the RRM domain, contrary to previous study reports ([165] and Table 3). The conclusion was that the alteration of all these domains by themselves is not sufficient to drive FUS toxicity.
As already reported, mutations in the PY-NLS domain lead to mislocalization of FUS in the cytoplasm and induce a strong eye degeneration phenotype compared to wildtype FUS (Table 3). In motoneurons, expression of FUS lacking its NLS domain partially allowed flies to eclose, contrary to wildtype FUS that induced pharate lethality. These emerging flies stayed immature, indicating that FUS lacking its NLS domain still confers some toxicity [161]. Unexpectedly, FUS lacking its NLS had a cytoplasmic localization but did not induced significant phenotype when expressed in the eye. However, the co-expression of this mutant with wildtype FUS enhanced the rough eye phenotype. In these flies, wildtype FUS was localized in the cytoplasm of retinal cells as aggregates, leading to the idea that expression of the C-terminally truncated FUS could interact with wildtype FUS to form toxic aggregates in the cytoplasm [205]. Moreover, PY-NLS was recently shown to have a function in disaggregation via the nuclear import receptor (NIR) Kapβ2. Indeed, NIRs act as chaperones to mediate nuclear import but also have an additional function in disaggregation activity and preventing fibrillization of RBPs in in vitro experiments [206]. Kapβ2 and FUS genetically interact, as Kapβ2-knockdown in the eye enhanced the rough eye phenotype induced by FUS overexpression [206].
An in vitro study showed that the LCD of FUS is involved in the self-assembly process [203]. In motoneurons, deletion of the QSGY domain (the most N-terminal part of the LCD) led to a partial rescue of the phenotypes induced by wildtype FUS expression. The adult eclosion rate of these flies was recovered, but the emerging adults showed an immature phenotype like the FUS-expressing escapers [161]. When expressed in the eye, an LCD-mutated form of FUS displayed no neurodegeneration phenotype and abolished the phenotype generated by mutations located in the NLS domain. Indeed, the self-assembly of FUS in the cytoplasm, via the LCD, seems indispensable to induce the neurodegeneration process in ALS [205].
The lack of phenotype observed with the C-terminally truncated FUS mutant could be explained by some differences in the LCD of Caz and FUS proteins. Indeed, the QGSY domain is absent in Caz protein [173]. Thus, NLS-mutated Caz did not display any phenotype when expressed in motoneurons, but addition of a QGSY domain to this NLS-mutant form generated a toxic protein and severe eclosion reduction, as observed for FUS mutants [161]. Indeed, Caz protein is not sequestered in the cytoplasm because of the lack of interaction with LCD of the truncated forms of overexpressed FUS [164,205].
4.2.5. FUS Is an RBP Found in Stress Granules
FUS is a protein prone to aggregate and, in patients, FUS is found in cytosolic aggregates. In fact, the LCD of FUS shares similarity with the yeast prion protein [207,208]. How FUS self-assembles in cytoplasmic aggregates in vivo to induce neurodegeneration is still an interesting question. The PrLD and RGG2 domains are required and act in cis to mediate FUS toxicity [161]. In vitro experiments showed that the FUS PrLD domain is necessary and sufficient for FUS fibrillization [154,209] and the LCD to undergo phase separation or sol–gel transition [149,201,203,210,211,212]. These domains are prone to aggregation and are involved in the biogenesis of membraneless organelles such as stress granules (SGs) [213]. In the cytoplasm, SGs are membraneless ribonucleoprotein compartments that have a dynamic nature. They assemble and increase in number through stress conditions, disassemble when stress is removed, or persist under chronic stress. SGs play a crucial role in RNA metabolism, notably in translation inhibition through phosphorylation of eukaryotic initiation factor 2α (eIF2α). Originally protective, under chronic stress, the presence of SGs could lead to pathological conditions (for reviews, see [214,215,216]). Thus, SGs could act as a seeding mechanism that result in accumulation of RBPs.
In ALS patients, FUS co-localizes with SG markers [152], where it may induce a phase transition (liquid to solid) that reduces the SG dynamic [212,217]. In Drosophila, motoneuron expression of a FUS protein containing a RGG2 domain mutated in all arginines rescued the FUS-induced toxicity like the deletion of the entire RGG2 domain. Hence, RGG2 and LCD regions play a crucial role in FUS aggregation.
4.2.6. FUS and Its Post-Translational Modifications (PTMs)
PTMs, which are known to regulate protein structure and function [218], influence protein aggregation in neurodegenerative diseases [219,220,221,222]. FUS can be post-translationally modified at various positions, leading to modification of its cellular localization, aggregation, and self-assembly tendency. Phosphorylation, acetylation, glycosylation, mono- and di-methylation, and ubiquitination have been described to occur at different positions along FUS protein (for extensive reviews, see [223,224]). Interestingly, FUS phosphorylation seems very labile in Drosophila, and hypophosphorylated forms of the protein alter FUS solubility properties, causing toxicity. Indeed, insoluble FUS corresponds to hypophosphorylated forms of the protein that mediate toxicity independently of inclusion formation [162]. Moreover, a detailed observation showed that the solubility profile of FUS depends on the cell type in which it is expressed. Neurons of the brain or photoreceptors of the retina could perform different FUS post-translational processes resulting in different phosphorylation patterns [162]. This suggests that the regulation of the PTMs may also be specific to each cell type and should be taken into account. Recently, arginine methylation has been shown to be a key regulator of FUS solubility and homeostasis [225,226,227]. Arginine methylation (notably on RGG2 neighboring the NLS) is also suspected to interfere with the binding affinity of NIR on the PY-NLS sequence [144,206]. The regulation of FUS methylation is mediated by the Drosophila arginine methyltransferase proteins (DARTs) [228]. More precisely, DART5 is involved in this process [164,229,230]. Overexpression of DART5 and FUS simultaneously rescued the degeneration induced by FUS in the eye [231]. Conversely, DART5 knockdown alone resulted in eye damage, and this phenotype was prevented by the depletion of hsrω. This result with others indicated that the lncRNA hsrω transcriptionally regulates DART5, which in turn regulates the arginine methylation of FUS protein. Interestingly, methylated FUS protein is eliminated via the proteasome that is known to act on protein in soluble phase. It is conceivable to speculate that FUS methylation is involved in the regulation of FUS solubility [231]. According to these results, PTMs could influence FUS-induced pathology and, in the future, could serve as therapeutic orientations.
4.3. Search for Suppressors of FUS-Induced Neurodegeneration
Drosophila has always been a system model used to search for toxicity-modifying genes. Several genetic screens have been done to understand the mechanisms leading to motoneuron neurodegeneration using either Caz or FUS genetic background. This approach has enabled finding different interacting proteins involved in distinct cellular processes, all implied in FUS-induced neurodegeneration.
4.3.1. Nucleocytoplasmic Localization
In an attempt to understand the mechanisms that lead to FUS toxicity and neurodegeneration, the use of caz-knockdown induced phenotypes (rough eye and locomotor defect) has allowed the identification of ter94 (ortholog of human Valosin-containing protein, VCP) [178]. Ter94 is an AAA ATPase. This protein family is implied in various cellular processes such as ubiquitin-dependent protein degradation, vesicle transfer, and nucleocytoplasmic transport [232,233,234]. Loss of ter94 function enhanced the caz-knockdown phenotypes, and conversely the expression of ter94 in eye or neurons can rescue the neurodegenerative phenotype observed in caz-knockdown flies [178]. Interestingly, VCP mutations were identified in fALS patients [235]. Recently, FUS was found mislocalized in the cytoplasm of human induced pluripotent stem cells (iPSCs) derived from VCP-mutant motoneurons [236,237]. Indeed, FUS cytoplasmic mislocalization under a diffuse form could be generalized in different ALS models, suggesting a broader role of FUS in the pathology.
4.3.2. Transcriptional Regulation
More recently, the same strategy enabled highlighting the link of caz with Xrp1, a DNA-binding protein involved in gene expression regulation, chromatin remodeling, and DNA repair [238,239]. In neurons, the knockdown of Xrp1 rescued the caz mutant phenotypes [240]. Xrp1 expression is upregulated in a caz mutant background, and this genetic interaction is dependent on the Xrp1 DNA-binding domain. These observations led to the hypothesis that, in caz mutants, increased Xrp1 expression could induce gene expression dysregulation causing neurodegeneration [240]. No Xrp1 ortholog has yet been found in mammals. Nevertheless, in the context of mutant FUS expression, Xrp1 downregulation rescues the ALS-induced phenotype, somehow suggesting conservation in the gene expression dysregulation mechanism leading to ALS pathology.
4.3.3. Piwi-Interacting RNA (piRNA) Biogenesis
caz genetically interacts with different genes involved in the piRNA biogenesis such as Piwi, Aubergine, and Argonaute3 [180]. piRNAs are small noncoding RNAs that regulate the chromatin structure [241,242,243]. They have been found in Drosophila brain, and Aubergine (Aub) is involved in piRNA biogenesis in neurons [244,245]. In caz knockdown many abnormal pre-piRNAs were found. Aub overexpression enhanced the neuronal defects induced in this genetic background and increased the cytoplasmic localization of Caz [180]. Indeed, Caz seems to have an action on piRNA processing. These results suggest that the creation of complexes containing pre-piRNAs, Aub, and Caz in the cytoplasm could contribute to neuronal degeneration and disorder.
4.3.4. Cytoplasmic Mislocalization and SGs
Pupal lethality and reduced adult eclosion rate phenotypes induced by FUS expression in neurons secreting the bursicon neuropeptide (also known as CCAP neurons) are particularly suitable for the design of genetic screens [166]. This approach was validated using Pink and Parkin, two genes already described to modify FUS toxicity. These genes act in a common pathway to target damaged mitochondria toward an autophagic pathway to control mitochondrial quality [246]. Downregulation of these genes in motoneurons improved the larval locomotion defect and rescued the CCAP phenotypes induced by FUS expression [166,186]. Candidate gene approaches to explore the role of genes involved in the nucleocytoplasmic transport have shown that nucleoporin (Nup154, a nuclear pore protein) and Exportin1 (XPO1, a nuclear transport protein) can suppress the neurodegeneration associated with FUS toxicity. Interestingly, these experiments highlighted the role of XPO1 for FUS localization into SGs even though the mechanism remains unclear [166].
4.3.5. Hippo and c-Jun N-Terminal Kinase (JNK) Signaling Pathways
The rough eye phenotype induced by FUS expression was also used as a readout for genetic screens. Recently, this paradigm led to the identification of the Hippo pathway as a modifier of FUS-induced neurodegeneration [167]. Hippo signaling is a growth regulatory pathway and its LOF leads to cell proliferation. On the contrary, gain of Hippo signaling results in apoptosis or cell death through the activation of JNK signaling [247,248] (for reviews, see [249,250,251]). Downregulation of the Hippo pathway allowed the rescue of the FUS toxic effect in the eye. Indeed, FUS expression triggered the activation of Hippo and JNK signaling, resulting in neurodegeneration [167]. Moreover, the Hippo signaling pathway was also a modifier of the cabeza knockdown-induced phenotypes [177], strengthening the important role of this pathway in the pathology.
Drosophila has allowed gaining insight into the understanding of the mechanisms implied in FUS-induced neurodegeneration. However, many data seem contradictory and deserve to be investigated de novo in light of new data. For example, very recently, FUS was found to be a bicistronic gene coding for two proteins [252]. The FUS coding sequence contains a second protein-coding sequence in a shifted frame in the prion-like domain. The new protein of 170 amino acids (altFUS, which has its coding sequence in the 5′ region of the mRNA) seems to be involved in mitochondrial defects when overexpressed. Even if the primary results did not lead to an obvious action of altFUS in Drosophila [252], a more precise study to understand the physiological functions of the two proteins (FUS and altFUS) is required.
5. TDP-43 Proteinopathy in the Fruit Fly
In 2006, TDP-43, which is encoded by the TARDBP gene, was identified as the major component of the ubiquitin-positive cytoplasmic inclusions found in ALS patients [31,253]. In 2008, mutations in TARDBP were linked to sporadic and familial ALS [254,255,256,257,258,259]. Currently, over 50 TARDBP mutations have been identified [260], which account for 4–5% of fALS and nearly 1% of sALS [261]. Importantly, in most if not all ALS patients, hyperphosphorylated, ubiquitinated, and truncated forms of TDP-43 are present in cytoplasmic insoluble aggregates, making TDP-43 proteinopathy a neuropathological hallmark of ALS [31].
TDP-43 is a member of the hnRNP family that is ubiquitously expressed. TDP-43, which acts as a transcriptional repressor, has been implicated in a wide variety of RNA metabolic processes including splicing, stability, and transport [262]. TDP-43 protein contains two RNA-recognition motifs (RRM1 and RRM2) and a C-terminal glycine-rich domain (GRD), as well as an NLS, an NES, and three mitochondrial localization motifs (Figure 3). In the physiological state, TDP-43 protein is mainly nuclear but shuttles between the nucleus and the cytoplasm, while, in ALS patients, TDP-43 exits the nucleus and forms cytoplasmic inclusions [31]. For this reason, it is thought that two nonexclusive mechanisms may account for the toxicity linked to TDP-43: an LOF due to the absence of TDP-43 in the nucleus and a GOF mechanism induced by TDP-43-containing aggregates [263]. TDP-43 is highly conserved during evolution [264], making Drosophila an ideal organism to decipher the function of TDP-43. The Drosophila ortholog of TARDBP is TBPH, and both LOF and GOF approaches have been modeled in Drosophila to gain insights into TDP-43 toxicity.
5.1. TBPH Is the Drosophila Ortholog of Human TARDBP
5.1.1. TBPH Loss of Function
TBPH, which is expressed throughout development [265], was detected in neurons [266,267,268,269], glial cells [270,271], and muscles [265,266,270,272,273]. In neurons and glial cells, TBPH is mainly nuclear but was also found in the cytoplasm [266,270]. To better understand the role of TBPH in vivo, several groups have created TBPH-null mutant flies (Table 6).
Table 6.
TDP-43 homolog (TBPH) mutants cited in this review. mRNA, messenger RNA.
| Mutant Name | Description | TBPH mRNA/Protein | Phenotype |
|---|---|---|---|
|
TBPH Δ23 [267] |
1.6 kb deletion (promoter region + exon 1 and part of exon 2) | Absent protein (WB adult head + Immuno on larval and adult brain) Ab against AAs 1–268 |
Lethality throughout development; adult escapers with strongly reduced lifespan, incapacity of walking and climbing |
|
TBPH Δ142 [267] |
0.8 kb deletion (promoter region) | Absent protein (WB adult head) Ab against AAs 1–268 |
Lethality throughout development; adult escapers with strongly reduced lifespan, incapacity of walking and climbing |
|
TBPH Q367X [274] |
Single nucleotide change introducing STOP codon at amino acid 367 | Absent protein (WB adult head) Ab against AAs 179–192 |
Semi-lethal with adult escapers |
|
TBPH KO [268] |
Deletion of entire coding sequence | Absent mRNA (qRT-PCR on first instar larval CNS) | Lethal at 2nd instar larval stage |
|
TBPH ex26 [265] |
932 bp deletion (promoter region) | Absent mRNA (RT-PCR all developmental stages) Absent protein (Immuno on larval brain and muscle) Ab against AAs 307–531 |
Semi-lethal with adult escapers having severe movement defects |
|
TBPH G2 [275] |
Deletion (promoter region + part of exon 1) | Absent protein (WB third instar larval CNS) Ab against full length protein |
100% pupal lethal |
|
TBPH DD100 [266] |
Deletion (promoter region + part of exon 1) | Absent protein (WB adult head + Immuno on adult brain) Ab against AAs 291–305 or 517–531 |
Lethality throughout development, adult escapers with strongly reduced lifespan and impaired climbing |
|
TBPH DD96 [266] |
Deletion (promoter region + exons 1–3 + part of exon 4) |
Absent protein (WB adult head) Ab against AAs 291–305 or 517–531 |
Lethality throughout development, adult escapers with strongly reduced lifespan and impaired climbing |
This table describes the name and the associated reference of each mutant, the type of mutation, the detection of TBPH RNA and/or protein, and the major phenotypes of each mutant (AAs: amino acids; Ab: antibody; Immuno: immunohistochemistry; WB: Western blot).
Depending on the TBPH mutant, lethality was observed at the second instar larval stage [268], at the pupal stage [275], or throughout development with few adult progeny eclosing [265,267,270,274]. One study reported that defective ecdysteroid receptor signaling may be the cause of late pupal death [276]. Altogether, these studies showed that TBPH is an essential gene. Adult flies lacking TBPH are morphologically normal [159,267], and they show a strongly reduced lifespan (3 to 10 days) and very poor climbing performance [159,266,267]. Larval locomotion is also affected by the absence of TBPH. The number of peristaltic waves [266,267] and the traveled distance [265,275,277,278] are severely reduced in mutant larvae compared to control. Larval locomotion impairment was not due to motoneuron loss, since their viability was not affected at this stage [279]. When TBPH was specifically expressed in motoneurons of TBPH mutant, larval locomotion was partially rescued [265,275,277]. Full rescue was obtained with a genomic fragment transgene carrying the TBPH gene [266]. Altogether, these data indicate that larval locomotion relies on the presence of TBPH not only in motoneurons but also in other tissues. However, no muscle alteration was detected in TBPH mutants [266,270,279].
Analyses at the larval NMJs of TBPH mutant have revealed contradictory results (Table 7).
Table 7.
Phenotypes at larval neuromuscular junction of TBPH mutant.
| Mutant | Synaptic Boutons Number | Axonal Branch Number | Axonal Branch Length | Active Zone Number | Larval Locomotion Defect |
|---|---|---|---|---|---|
|
TBPH Δ23/Δ23 |
Reduced [267,280] Normal [281] |
Reduced [267,280] | Normal [281] | nd | Yes [267] |
| TBPHΔ23/Df[2R]BSC610 | Normal [281] | nd | Normal [281] | nd | nd |
|
TBPH Δ23/Df[2R]BSC660 |
Normal [159] | Normal [159] | Normal [159] | nd | Yes [159] |
|
TBPH Δ142 Δ142 |
Reduced [267] | Reduced [267] | nd | nd | Yes [267] |
|
TBPH ex26/ex26 |
Increased [265] | Increased [265] | nd | nd | Yes [265] |
| TBPH G2/G2 | Normal [277] | Normal [277] | Normal [277] | nd | Yes [277] |
|
TBPH DD100/DD100 |
Normal [266] | nd | nd | Normal [266] | Yes [266] |
|
TBPH DD96/DD96 |
Normal [266] | nd | nd | Normal [266] | Yes [266] |
This table describes the TBPH mutant conditions and the parameters used for larval neuromuscular junction analyzes (nd: not determined).
Two groups found that the number of synaptic boutons and axonal branches was reduced [267,280], while another group found that both increased [265]. Four other studies did not detect any alteration in bouton and axonal branch numbers at the NMJs [159,266,277,281]. Thus, it is not clear whether larval locomotion impairment is due to morphology abnormalities at NMJs. Electrophysiological recordings performed by two groups revealed no difference in evoked excitatory junction potential (EJP) amplitude at TBPH mutant larval NMJs [266,278], while another group found a reduced amplitude of EJPs [282]. Nevertheless, both the amplitude and the frequency of miniEJPs were reduced, resulting in an increase of the quantal content [266,278]. These data strongly suggest that lack of TBPH may alter neurotransmission at NMJs, which may participate to the impaired larval locomotion. This idea is consistent with RNA-sequencing data showing that putative TBPH target genes are enriched for synaptic transmission, neurotransmitter secretion and endocytosis [275].
The presence of TDP-43 aggregates in glial cells of ALS patients [283,284,285] and the evidences that non-cell-autonomous mechanisms are at play in ALS disease [10] led to a better understanding of the role of TBPH in glial cells. In TBPH mutant, the number of glial cells does not seem to be affected in larval and adult central nervous system (CNS) [270,271]. However, peripheral glia failed to correctly wrap the motoneuron terminals at larval NMJs of TBPH mutants [271]. In addition, abnormal GluRIIA glutamate receptor clustering was observed at TBPH mutant larval NMJs [271]. Importantly, both mutant phenotypes were fully rescued by pan-glial expression of TBPH [271], confirming that TBPH is required to maintain glial integrity and glutamate receptor distribution. Interestingly, pan-glial expression of the glutamate transporter dEAAT1 in TBPH mutant restored the normal distribution of glutamate receptors but not the glial wrapping defects, suggesting that TBPH acts on different pathways in glial cells [271].
It was also described that TBPH mutants display an upregulation of retrotransposable elements in neural tissues [286]. By using an activity reporter of the small interfering RNA (siRNA) silencing machinery specifically expressed in motoneurons, the authors showed that TBPH mutants have a defective retrotransposon silencing. This was likely due to the reduced level of Dicer-2 observed in TBPH mutant as pan-neuronal expression of Dicer-2 prevented the upregulation of retrotransposable elements [286].
Another phenotype described in TBPH mutants was the decrease in dendritic branching of larval sensory neurons [274]. The same phenotype was observed in larvae expressing TBPH RNAi specifically in sensory neurons (Table 8), indicating that TBPH modulates dendritic branching cell-autonomously, as confirmed by mosaic analysis with a repressible cell marker (MARCM) analysis [274].
Table 8.
Major phenotypes associated with RNA interference (RNAi)-induced TPBH silencing.
| Gal4 Line | RNAi Line | Phenotype/Reference |
|---|---|---|
| Act5C-Gal4 (ubiquitous) |
104401 (VDRC) | Lethal [269] |
| 38377 (VDRC) | Lethal [269] Adult flies with ectopic bristles [287] |
|
| Tubulin-Gal4 (ubiquitous) |
38377 (VDRC) | Lethal pupal with adult escapers [274] |
| 38379 (VDRC) | Lethal pupal with adult escapers [274] | |
| Homemade RNAi targeting exon 5 | Larval/pupal lethality with adult escapers, impaired adult motor behavior [266] | |
| Tubulin-Gal4, Tubulin-Gal80TS (ubiquitous, induced at adult stage) |
38379 (VDRC) | Climbing defect, reduced lifespan [288] |
| Elav-Gal4 (pan-neuronal) |
38377 (VDRC) | Impaired larval locomotion [265] Age progressive climbing deficit [267,269] Reduced lifespan [269] |
| 38379 (VDRC) | Age progressive climbing deficit [267] | |
| 104401 (VDRC) | Age progressive climbing deficit, reduced lifespan [269] | |
| 29517 (BDSC) | Age progressive climbing deficit [269] | |
| 39014 (BDSC) | Age progressive climbing deficit [269] | |
| Homemade RNAi targeting exon 5 | Impaired synaptic transmission (increase quantal content) at larval NMJ and abnormal adult motor behavior [266] | |
| Elav-Gal4, Tubulin-Gal80TS (pan-neuronal, induced at larval stage) |
38379 (VDRC) | Abnormal distribution of Dlg and GluRIIA at larval NMJs [288] |
| 1407-Gal4 (pan-neuronal) | 38377 (VDRC) | Strong climbing defect [267] |
| D42-Gal4 (motoneurons) |
38377 (VDRC) | Normal larval locomotion [265] Increased larval turning, age progressive climbing deficit [289] |
| 38379 (VDRC) | Increased larval turning, age progressive climbing deficit [289] | |
| OK371-Gal4 (glutamatergic neurons) |
38377 (VDRC) | Normal larval locomotion [290] |
| OK371-Gal4/MARCM (individual leg motoneuron) |
38379 (VDRC) | Normal larval locomotion [290] |
| Unknown | No effect on leg motoneuron/axon [291] | |
| Repo-Gal4 (pan-glial) |
38379 (VDRC) | Larval locomotion and climbing defects, reduced lifespan, wrapping defect at larval NMJ [271] |
| Homemade RNAi targeting exon 5 | Normal lifespan and climbing, impaired overall motor activity in 30 day old flies [270] | |
| BG57-Gal4 (muscles) |
Homemade RNAi targeting exon 5 | Impaired synaptic transmission at larval NMJ (normal quantal content) [266] |
| Mef2-Gal4 (muscles) |
38379 (VDRC) | Impaired larval locomotion and climbing, reduced lifespan [282] |
| Homemade RNAi targeting exon 5 | Normal lifespan and climbing, impaired overall motor activity in 30 day old flies [270] | |
| Mhc-Gal4 (muscles) |
38377 (VDRC) | Normal larval locomotion [265] Impaired larval locomotion and climbing [282] |
| GMR-Gal4 (eye) |
38377 (VDRC) | No effect [290] Mild rough eye [269,292] |
| 38379 (VDRC) | No effect [290] | |
| 104401 (VDRC) | Mild rough eye [269] | |
| 29517 (BDSC) | Mild rough eye [269] | |
| 39014 (BDSC) | Mild rough eye [269] | |
| Unknown | No effect [272], weak eye degeneration [293] | |
| EB1-Gal4 (ellipsoid body neurons) |
Homemade RNAi targeting exon 5 | Impaired adult motor behavior, age dependent axonal/synaptic degeneration and loss of upper motoneuron [266] |
| Gal4221-Gal4 (sensory neurons) |
38377 (VDRC) | Reduced dendritic branching [274] |
| 38379 (VDRC) | Reduced dendritic branching [274] | |
| OK107-Gal4 (mushroom bodies) |
38377 (VDRC) | Normal mushroom bodies, weak learning inability [265] Axonal loss and neuronal death [290] |
| 38379 (VDRC) | Normal mushroom bodies and learning ability [265] Axonal loss and neuronal death [290] |
This table describes the Gal4 lines used to express TBPH RNAi, the type/origin of RNAi construct, and the associated phenotypes/references. It is important to note that all experiments were not done at the same temperature. In addition, some experiments have combined RNAi and Dicer-2 expression to enhance the effect of the RNAi (not mentioned). Altogether, these different experimental conditions may explain some discrepancies. Data obtained at larval NMJs are not described.
TBPH mutant adult escapers also have ectopic bristles and sensory neurons on their notum [287], indicating that the specification of sensory organ precursor (SOP) cells is altered in absence of TBPH. The precise generation of SOP cell number is regulated by microRNA (miR)-9a [294], which has reduced expression level in TBPH mutant [287]. Thus, it is likely that TBPH modulates miR-9a production to ensure proper neuronal specification. Another report has described that the wing phenotype induced by miR-1 overexpression was enhanced in heterozygous TBPH flies, suggesting that TBPH may dampen miR-1 activity [295].
Numerous studies have used RNA interference to examine the function of TBPH in different tissues (Table 8). While some divergences exist between the different experiments, certain conclusions may be drawn. First, the ubiquitous knockdown of TPBH was lethal with few adult escapers showing climbing defects [266,269,274], mimicking what was observed in TBPH mutant flies. Interestingly, when TBPH knockdown was induced at the adult stage, the flies had reduced lifespan and impaired climbing capacities, showing that TBPH is required throughout adult life [288]. Second, flies with pan-neuronal TBPH knockdown showed age-dependent impaired climbing and have reduced lifespan [266,267,269]. Similar phenotypes were described when TBPH expression was suppressed specifically in motoneurons [289]. Larval locomotion seems also affected by pan-neuronal or motoneuronal TBPH knockdown, depending on the RNAi and the Gal4 lines used [265,289]. Third, pan-muscle, pan-glial, or mushroom body TBPH downregulation gave opposite results, making it difficult to determine whether TBPH plays effectively an important role or not in such tissues [265,266,270,271,282,290]. Similarly, some studies reported that eye-specific TBPH knockdown has no effect or may induce a weak degeneration [269,272,290,292,293]. However, TBPH mutant adult escapers have a normal eye but survive only a few days, making it impossible to determine whether TBPH is clearly required for eye integrity [159,267].
5.1.2. TBPH and TDP-43 Share the Same Functions
Several studies have shown that TBPH and TDP-43 share redundant functions and can substitute for each other. For example, rescue experiments of TBPH mutant phenotypes have shown that TDP-43 is as effective as TBPH for recovery of lifespan or locomotion defects [159,267,296]. To exclude any side effect due to overexpression, Chang and Morton used the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 genome editing system to replace the TBPH gene by its human ortholog, allowing TDP-43 to be expressed at endogenous level. These flies had normal development and life span and they did not show any behavioral defects [297]. This clearly indicates that TDP-43 has the same function than TBPH, which is reinforced by overexpression studies (see below). Furthermore, one report showed that TBPH and TDP-43 share the ability to associate in vitro with Hrp38/Hrb98DE/CG9983 and Squid/Hrp40/CG16901 [298]. These two genes are the orthologs of human hnRNP A1 and A2/B1, which are known to interact with TDP-43 [299]. Thus, functional interaction of TBPH/TDP-43 with some hnRNPs is conserved during evolution. Moreover, TDP-43 and TBPH were shown to be interchangeable regarding their nucleic acid binding specificity and their role in splicing [300].
5.2. TBPH and TDP-43 Gain of Function Mutations Are Toxic
5.2.1. Toxicity
TBPH and TDP-43 GOF experiments have demonstrated that a high level of TBPH or TDP-43 is very toxic (Table 9 and Table 10).
Table 9.
Gain-of-function phenotypes induced by TBPH.
| Gal4 Line | Phenotype/Reference |
|---|---|
| Elav-Gal4 (pan-neuronal) | 100% lethality (90% at first instar larvae) [275] Semi-lethal [274] Lethal throughout development, larval locomotion defect, adult escapers with reduced lifespan and impaired climbing [266] |
| Elav-Gal4, Tubulin-Gal80TS (pan-neuronal, induced at adult stage) |
Climbing defect [288] |
| D42-Gal4 (motoneurons) | Impaired larval locomotion [265] Increased larval turning, weak motoneuronal death in LIII ventral ganglia [289] Lethal pupal stage [265,289] Lethal pupal/reduced lifespan depending on the dose [265] Age-dependent climbing defect [265,275] |
| D42-Gal4, Tubulin-Gal80TS (motoneurons, induced at adult stage) |
Weak reduction of neuron number in thoracic ganglia, reduced lifespan [301] |
| OK371-Gal4 (glutamatergic neurons) | Lifespan severely reduced [302] |
| OK371-Gal4/MARCM (individual leg motoneuron) |
Leg motor axon and NMJ degeneration [291] |
| Repo-Gal4 (pan-glial) | Premature lethality (prepupal stage) [270] |
| Mef2-Gal4 (muscles) | Irregular sarcomere (2nd instar larval stage), impaired larval locomotion, prepupal lethality [270] |
| GMR-Gal4 (eye) | Eye degeneration [270,274,289,292,302,303,304] 100% lethal at 29 °C [289] |
| EB1-Gal4 (ellipsoid body neurons) |
Impaired adult motor behavior, age-dependent axonal/synaptic degeneration, and loss of upper motoneuron [266] |
| Gal4221-Gal4 (sensory neurons) | Increased dendritic branching [274] |
| OK107-Gal4 (mushroom bodies) | Smaller axonal lobe, impaired learning ability [265] |
This table describes the Gal4 lines used to overexpress TBPH and the associated phenotypes/references.
Table 10.
Gain-of-function phenotypes induced by wildtype TDP-43.
| Gal4 Line | Phenotype/Reference |
|---|---|
| Act5C-Gal4 (ubiquitous) | Premature lethality [305] |
| Daughterless-Gal4 (ubiquitous) | Lethal pupal [306] |
| Elav-Gal4 (pan-neuronal) |
Embryonic lethal [306] L1 lethal [307] Reduced lifespan [305,308,309,310] Age progressive climbing deficit [309,311] |
| Elav-Gal4, Tubulin-Gal80TS (pan-neuronal, induced at adult stage) |
Premature lethality, reduced lifespan [305,307] Age progressive climbing deficit [312] |
| ElavGS (pan-neuronal, induced at adult stage) |
Reduced lifespan [307,313] Age progressive climbing deficit [314] |
| D42-Gal4 (motoneurons) |
Reduced survival, lethal at 2nd instar larval stage with high dose [315] Semi-lethal at 25 °C but viable at 18 °C, impaired climbing, no motoneuronal death in LIII ventral ganglia, motoneuronal death in adult thoracic ganglia [289] Larval locomotion defect [316] Increased larval turning [289,291,317,318,319] Age progressive climbing deficit [305,308,316,320,321] Reduced lifespan [293,313,318,320,322,323] |
| D42-Gal4, Tubulin- Gal80TS (motoneurons, induced at adult stage) |
Age progressive climbing deficit, reduced lifespan [324] |
| OK371-Gal4 (glutamatergic neurons) |
Lethal [306] Lethal pupal, motoneuron death in LIII [290] |
| OK371-Gal4/MARCM (individual leg motoneuron) |
Leg motor axon and NMJ degeneration [291] |
| RN2-Gal4 (sub-population of adult motoneurons) |
Age progressive climbing deficit [290] |
| Repo-Gal4 (pan-glial) |
Pupal lethal [307] Age progressive climbing deficit, reduced lifespan [309,325] Terminal deoxynucleotidyl tranferase (TdT) dUTP Nick-end labeling (TUNEL)-positive neural cells [309] Little loss of glial cells and neurons in adult brains [326] |
| Repo-Gal4, Tubulin-Gal80TS (pan-glial, induced at adult stage) |
Dramatic loss of glial cells associated to neuronal loss in adult brains [326] |
| 24B-Gal4 (muscles) | Lethal [306,307] |
| Mef2-Gal4 (muscles) | Lethal [306] |
| GMR-Gal4 (eye) |
Neurodegeneration [310,312,316,317,319,320,324,327,328,329] Age dependent progressive neurodegeneration [289,290,293,307,308,314,315,318,330,331,332,333,334] |
| GMR-Gal4, Tubulin-Gal80TS (eye, induced at adult stage) |
Age-dependent progressive neurodegeneration [293,312] |
| Gal4221-Gal4 (sensory neurons) |
Promoted dendritic branching [274] |
| OK107-Gal4 (mushroom bodies) |
No TUNEL-positive cells [309] Axonal loss and neuronal death (TUNEL-positive cells) [290] |
This table describes the Gal4 lines used to overexpress TDP43 and the associated phenotypes/references.
Pan-neuronal overexpression of TDP-43 or TBPH induced premature lethality [266,274,275,306,307], reduced lifespan [266,305,309,310], larval locomotion defect [266], and age-dependent climbing deficit [309,311]. Similar phenotypes were observed when TBPH or TDP-43 was expressed in motoneurons [265,273,275,289,290,293,302,305,306,313,315,316,317,318,319,320,322,323]. By using the temporal and regional gene expression targeting (TARGET) system, it was shown that adult-restricted overexpression of TBPH or TDP-43 in all neurons or motoneurons induced age progressive climbing defects and reduced lifespan [288,301,305,307,312,324]. This indicates that the level of TDP-43 must be tightly regulated throughout life. GOF experiments also revealed that excess of TBPH or TDP-43 is highly toxic in muscles [270,306,307], glial cells [270,307,309,325,326], and in the eye [266,274,289,290,292,293,302,303,304,306,307,310,312,314,315,316,317,318,319,320,324,327,328,329,330,331,332,333,334]. Moreover, it was reported that TBPH or TDP-43 overexpression induces cell death, as was shown in motoneurons [266,289,290,301], glial cells [309,326], mushroom bodies [265,290], and in the eye. At larval NMJs, TDP-43 or TBPH GOF gave some conflicting results (Table 11).
Table 11.
Gain-of-function phenotypes induced by TBPH or TDP-43 at larval neuromuscular junctions.
| Gal4 | TBPH or TDP-43 | Active Zone Number | Synaptic Boutons Number | Axonal Branch Number | Axonal Branch Length | Larval Locomotion |
|---|---|---|---|---|---|---|
| Elav-Gal4 (pan-neuronal) |
TDP-43 | nd | Decreased [316] | nd | nd | nd |
| D42-Gal4 (motoneurons) |
TBPH | nd | Increased [289] Decreased, dose dependent [265] |
Normal [289] Decreased, dose dependent [265] |
nd | Altered larval turning [289] Impaired, dose dependent [265] Normal [275] |
| TDP-43 | Increased [289] | Decreased [11,289,335,336] Normal [293] |
Decreased [11,289,335] | nd | Altered larval turning [289] Normal [293] |
|
| OK6-Gal4 (motoneurons) |
TBPH | nd | Increased [159] | nd | Increased [159] | nd |
| TDP-43 | nd | Increased [159] | nd | Increased [159] | nd | |
| OK371-Gal4 (glutamatergic neurons) |
TBPH | nd | Normal [302] | nd | nd | nd |
| TDP-43 | Normal [337] | Decreased [281,290] | Decreased [143] | Decreased [281] | Altered [281,290] | |
| Repo-Gal4 (pan-glial) |
TDP-43 | Normal [336] | Decreased [336] | nd | nd | Altered larval turning [336] |
This table describes the Gal4 lines used to overexpress TBPH or TDP-43 and the associated phenotypes/references at larval neuromuscular junctions (nd: not determined).
5.2.2. TBPH and TDP-43 Gain-of-Function Phenotypes Are Dose- and Age-Dependent
It is important to keep in mind that all the phenotypes observed following TBPH or TDP-43 overexpression are dose-dependent, i.e., the severity of the phenotypes increases with higher levels of TBPH/TDP-43. For example, upon pan-neuronal expression, the reduction in lifespan is correlated with TDP-43 expression level, with a very high dose inducing lethality [305]. Similarly, eye degeneration is correlated with the expression levels of TBPH/TDP-43 that can lead to death at very high levels [289,293,315,333,336]. This may explain some discrepancies between different GOF studies (Table 9 and Table 10). Several phenotypes induced by TBPH/TDP-43 GOF, such as eye degeneration and climbing defects, show age-dependent progression [265,275,289,290,293,305,307,308,309,311,312,314,315,316,318,320,321,324,325,330,331,332,333,334]. As TBPH expression decreased with aging [338], one may speculate that reduced endogenous TBPH levels exacerbate GOF effects without excluding that phenotype aggravation may be due to continuous abnormal accumulation of TBPH/TDP-43.
5.2.3. TDP-43/TBPH Toxicity Requires RNA Binding
An important point is that the toxicity of TBPH/TDP-43 is clearly associated with its RNA-binding property. For example, TDP-43 motoneuronal GOF causes reduced lifespan [293], age-dependent climbing deficit [305], and NMJ/axon degeneration of leg motoneurons [291]. These defects were not observed with TDP-43RRM1mut, a mutant form carrying mutations into the RRM1 domain, which abolish its RNA-binding capacity [291,293,305]. Similarly, TDP-43RRM1mut GOF does not induce eye degeneration contrary to the wildtype form [293]. Importantly, the RNA-binding property of TPBH is also required to induce climbing defects upon pan-neuronal expression [298]. Altogether, these data show that RNA binding is crucial for TBPH/TDP-43 toxicity. One hypothesis is that TBPH/TDP-43 toxicity is linked to the nuclear functions of TDP-43 in RNA processing. However, RRM1 mutation abolished the strong toxicity of TDP-43 mutated in its NLS domain, which is mainly cytoplasmic [293]. Thus, it is very likely that TDP-43-associated toxicity requires RNA binding both in the nucleus and in the cytoplasm.
5.2.4. TDP-43/TBPH Toxicity and Nucleocytoplasmic Localization
Overexpressed TDP-43WT is predominantly present in the nucleus, although it was also detected in the cytoplasm of developing photoreceptors [289,307,336], motoneurons [296], and glial cells [336]. Interestingly, TDP-43 cytoplasmic localization extends until the larval NMJs when expressed in motoneurons [296] or glial cells [336], strongly suggesting that TDP-43 plays a local role at NMJs. Endogenous TBPH, which is mainly nuclear, was also found in the cytoplasm of neurons and glial cells [266,270]. Currently, no study has described the presence of TBPH at larval NMJs, maybe because its endogenous expression level is too low. Several groups have asked whether the subcellular localization of wildtype TDP-43 may influence its toxicity. To answer this issue, mutant forms of TDP-43 lacking the NLS or the NES were created (Table 12).
Table 12.
Comparison of phenotypes induced by gain of function of TDP-43 wildtype, mutated in the NLS or in the NES.
| GAL4 Line | TDP-43Ω | TDP-43NLSmut | TDP-43NESmut |
|---|---|---|---|
| Act5C-Gal4 (ubiquitous) | Lethal [305] | Lethal [305] | nd |
| Elav-Gal4 (pan-neuronal) |
L1 larval lethality [307] Viable, strong reduced life span [305] |
Pupal lethal [307] Viable, modest reduced life span [305] |
Pupal lethal, rare escapers [307] |
| ElavGS (pan-neuronal, induced at adult stage) |
Strong reduced lifespan [307] | Modest reduced lifespan [307] | Weak reduced lifespan [307] |
| D42-Gal4 (motoneurons) |
Age progressive climbing defect [321] | Strong age progressive climbing defect [321] | No climbing defect [321] |
| Repo-Gal4 (pan-glial) |
Pupal lethal [307] | Pupal lethal [307] | No effect [307] |
| 24B-Gal4 (muscles) | Larval lethal [307] | Embryonic lethal [307] | No effect [307] |
| GMR-Gal4 (eye) |
Modest degeneration [292,293,307,321,327] |
Strong degeneration [292,293,307,321,327] | No degeneration [292,307,321] |
This table describes the Gal4 lines used to overexpress the wildtype or NLS/NES mutant form of TDP-43 and the associated phenotypes/references.
Accordingly, TDP-43NLSmut and TDP-43NESmut were predominantly cytoplasmic and nuclear, respectively [292,305,307,321]. Pan-neuronal overexpression experiments have shown that the wildtype TDP-43 form is more toxic than the cytoplasmic form, which is more toxic than the strict-nuclear form [305,307]. However, eye degeneration or age-dependent climbing defects, induced by eye or motoneuronal overexpression, were stronger with TDP-43NLSmut compared to TDP-43WT, while TDP-43NESmut had no effect [292,293,307,321,327]. In addition, one study found that TDP-43WT or TDP-43NLSmut GOF in muscles or glial cells was lethal but TDP-43NESmut had no effect [307]. Altogether, these data indicate that cytoplasmic excess of TDP-43 is very toxic, and that nuclear accumulation of TDP-43 may be toxic depending on the cell type.
The presence of TDP-43-positive cytoplasmic inclusions in almost all ALS patients led to the hypothesis that they were directly linked to neurodegeneration. In Drosophila, cytoplasmic inclusions were not observed in neurons overexpressing TDP-43NLSmut, strongly suggesting that cytoplasmic inclusions are not absolutely required for toxicity [307]. From the studies cited above, it appears that accumulation of the nuclear restricted form of TDP-43 is less toxic than the WT or cytoplasmic forms. Fly neuronal expression of TDP-43NESmut, but not WT, was associated with TDP-43-positive nuclear inclusions, which very likely correspond to NBs [321]. NBs contain specific nuclear proteins and RNAs to modulate nuclear functions and homeostasis [339]. Interestingly, motoneuronal GOF-induced climbing defects associated with TDP-43 expression were worse with TDP-43NLSmut compared to wildtype, and TDP-43NESmut induced no defect [321]. Similar degrees of severity phenotypes were observed in the eye [321]. This suggests that TDP-43NESmut-associated NBs may be protective and may explain why nuclear accumulation of TDP-43 is less toxic.
5.2.5. TDP-43 Toxicity of ALS-Linked TDP-43 Mutations
In order to better understand how TDP-43 mutations may induce motoneuronal death, several ALS-linked TDP-43 mutations have been studied in Drosophila. One study replaced the endogenous TBPH gene by TARDBP gene carrying either the G294A or M337V ALS-associated mutations [277]. Homozygous flies for each mutation had normal development and lifespan. In addition, the larval locomotion was unaltered, and their climbing capacity was only weakly reduced with aging compared to control flies and those expressing wildtype TDP-43 [277]. Interestingly, the two mutant proteins showed a higher level of cytoplasmic puncta in the adult brain compared to wildtype. Altogether, these data indicate that G294A and M337V mutations are not highly toxic, at least when expressed at endogenous levels [297]. In contrast, the overexpression of ALS-linked TDP-43 mutants (TDP-43mutALS) induces dramatic defects (Table 13).
Table 13.
Gain-of-function phenotypes induced by ALS-linked TDP-43 mutants.
| TDP-43 Mutant |
Gal4 Line | Phenotype/Reference |
|---|---|---|
| D169G | D42-Gal4 (motoneurons) |
Decreased bouton number [336] Increased active zone number [336] Increased larval turning time [331,336] Abnormal nuclear shape [336] |
| Repo-Gal4 (pan-glial) |
Normal bouton number (reduced with WT) [336] Normal active zone number [336] Increased GluRIIC [336] Increased larval turning time [336] |
|
| GMR-Gal4 (eye) |
Age and dose dependent degeneration [331,336] | |
| G287S | Act5C-Gal4 (ubiquitous) | Lethal [305] |
| Elav-Gal4 (pan-neuronal) |
Viable, reduced lifespan (as WT but better) [305] | |
| G298S | Elav-Gal4 (pan-neuronal) |
Impaired climbing [311] |
| D42-Gal4 (motoneurons) |
Decreased bouton number [336], (normal with WT) [293] Increased active zone number [336] Abnormal nuclear shape [336] Increased larval turning time [317,318,319,331,336,340], (normal with WT) [293] Decreased larval locomotion (Normal with WT) [293] Reduced lifespan [318], (as WT but worse) [293] |
|
| Repo-Gal4 (pan-glial) |
Decreased bouton number [336] Normal number of active zones [336] Increased GluRIIC [336] Increased larval turning time [336] |
|
| GMR-Gal4 (eye) |
Degeneration [317,318,319] Age- and dose-dependent degeneration [336] Age-dependent degeneration (as WT but worse) [293] |
|
| A315T | Act5C-Gal4 (ubiquitous) | Lethal [305] |
| Elav-Gal4 (pan-neuronal) |
Viable, reduced lifespan (as WT but better) [305] | |
| Elav-Gal4, Tubulin-Gal80TS (pan-neuronal, induced at adult stage) |
Age-dependent climbing deficit (as WT but worse) [312] | |
| D42-Gal4 (motoneurons) |
No change in bouton number (WT decreased) [289], decreased at high dose [289,336] No change in branch number, even at high dose (WT decreased) [289] Increased larval turning time (as WT but worse) [289] Increased active zone number [336] Abnormal nuclear shape [336] Viable but 100% pupal lethal at high dose (WT semi-lethal, escapers) [289] Reduced lifespan (as WT but better) [289] Impaired climbing (as WT but better) [289] No motoneuron apoptosis in LIII ganglia [289] Rare motoneuron apoptosis in adult thoracic ganglia (as WT but better) [289] Age progressive climbing deficit [305] Upregulation of HDAC6 mRNA (as WT but more) [340] |
|
| OK371-Gal4 (glutamatergic neurons) |
Cell soma and proximal axon (WT reaches distal axon and NMJ) [296] Impaired anterograde movement (compared to WT) [296] |
|
| Repo-Gal4 (pan-glial) |
Normal number of active zones [336] Increased GluRIIC [336] |
|
| GMR-Gal4 (eye) |
Age- and dose-dependent degeneration [289,331], (as WT but worse) [293] Degeneration with mitochondrial damage [312] |
|
| GMR-Gal4, Tubulin-Gal80TS (eye, induced at adult stage) |
Age-dependent degeneration with mitochondrial damage [312] | |
| Q331K | Daughterless-Gal4 (ubiquitous) | Lethal pupal [306] |
| Elav-Gal4 (pan-neuronal) |
Embryonic lethal [306] | |
| D42-Gal4 (motoneurons) |
Age progressive climbing deficit (as WT but worse) [308] | |
| OK371-Gal4 (glutamatergic neurons) |
Lethal [306] | |
| OK371-Gal4/MARCM (individual leg motoneuron) |
Degeneration of leg motor axon and NMJ (as WT but worse) [291] | |
| 24B-Gal4 or Mef2-Gal4 (muscles) |
Lethal [306] | |
| GMR-Gal4 (eye) |
Cytoplasmic puncta (not observed with WT) [306] | |
| Gal4221-Gal4 (sensory neurons) |
Increased dendritic branching (as WT but less) [274] | |
| OK107-Gal4 (mushroom bodies) |
Lethal (WT is viable) [306] | |
| TH-Gal4 (dopaminergic neurons) | Viable [306] | |
| Vestigial-Gal4 (wing imaginal discs) | Ectopic production of scutellar bristle (as WT but less) [287] | |
| M337V | Armadillo-Gal4 (ubiquitous) |
No rescue of viability of TBPH mutant (rescue with WT) [296] |
| Daughterless-Gal4 (ubiquitous) |
Lethal pupal [306] | |
| Elav-Gal4(pan-neuronal) | Embryonic lethal [306] | |
| D42-Gal4 (motoneurons) |
Normal bouton number [293] Increased larval turning time (normal with WT) [293] Decreased larval locomotion (normal with WT) [293] Reduced lifespan (as WT but worse) [293] |
|
| OK371-Gal4 (glutamatergic neurons) |
Cell soma and proximal axon (WT reaches distal axon and NMJ) [296] Impaired anterograde movement (compared to WT) [296] Lethal [306] |
|
| OK6-Gal4 (motoneurons) | Normal bouton number (increased with WT) [159] | |
| 24B-Gal4 or Mef2-Gal4 (muscles) |
Lethal [306] | |
| GMR-Gal4 (eye) |
Age dependent degeneration (as WT but worse) [293,334] | |
| CCAP-Gal4 (bursicon neurons) |
Impaired vesicle transport (no effect with WT) [341] | |
| Gal4221-Gal4 (sensory neurons) |
Increased dendritic branching (as WT but less) [274] | |
| OK107-Gal4 (mushroom bodies) |
Viable [306] | |
| TH-Gal4 (dopaminergic neurons) | Viable [306] | |
| Vestigial-Gal4 (wing imaginal disc) | Ectopic production of scutellar bristle (as WT but less) [287] | |
| Q343R | GMR-Gal4 (eye) |
Age-dependent degeneration (as WT but worse) [293] |
| N345K | D42-Gal4 (motoneurons) |
Decreased bouton number [336] Increased active zone number [336] Increased larval turning time [336] Abnormal nuclear shape [336] |
| Repo-Gal4 (pan-glial) |
Decreased bouton number [336] Normal number of active zones [336] Increased GluRIIC [336] Increased larval turning time [336] |
|
| GMR-Gal4 (eye) |
Age- and dose-dependent degeneration [336] | |
| G348C | Act5C-Gal4 (ubiquitous) | Lethal [305] |
| Elav-Gal4 (pan-neuronal) |
Viable, reduced lifespan (as WT but better) [305] | |
| A382T | Act5C-Gal4 (ubiquitous) | Lethal [305] |
| Elav-Gal4 (pan-neuronal) |
Viable, reduced lifespan (as WT but better) [305] | |
| D42-Gal4 (motoneurons) |
Upregulation of HDAC6 mRNA (as WT but more) [340] | |
| N390D | Act5C-Gal4 (ubiquitous) | Lethal [305] |
| Elav-Gal4 (pan-neuronal) |
Viable, reduced lifespan (as WT but better) [305] |
Most of the phenotypes induced by ALS-linked TDP-43 mutants are similar to those induced by wildtype except when mentioned (text in bold). Note that studies that used ALS-linked mutants without comparing them to the wildtype are not mentioned.
Indeed, GOF of TDP-43mutALS causes lethality, reduced lifespan, altered locomotion at larval and adult stages, and eye degeneration, depending on the tissue in which the mutant form was overexpressed. However, these defects were not so different from those observed with overexpression of wildtype TDP-43. In some cases, the defects were worse, and it was suggested that the ALS-linked mutations enhance the toxic function of TDP-43. In other cases, the mutant forms were a little less toxic and it was concluded that these ALS-linked mutations induce partial loss of TDP-43 function. Thus, it is still unclear how mutations of TDP-43 may induce motor deficit in the Drosophila ALS model.
However, a few reports found some differences between the wildtype and the mutated forms of TDP-43. One report described that the nuclei of motoneurons overexpressing mutants but not wildtype TDP-43 were misshapen, suggesting that nuclear stress may be associated with TDP-43 mutations [336]. When expressed in motoneurons, TDP-43WT was detected in dynamic cytoplasmic granules present in axons and extending up to NMJs [296,336]. On the contrary, TDP-43mutALS accumulated in the cell body and proximal axons due to impaired anterograde transport compared to wildtype [296]. In addition, the mobility of TDP-43mutALS within cytoplasmic granules was highly reduced compared to wildtype [336]. Altogether, these data strongly suggest that TDP-43mutALS are not transported correctly along axons and that the delivery of TDP-43-interacting mRNAs to distal neurites may be compromised by ALS-linked mutations into TDP-43.
5.3. Molecular Mechanisms Underlying TDP-43 Toxicity
5.3.1. Splicing Repression
RNA-sequencing of TBPH mutant heads showed cryptic exon incorporation, suggesting impaired splicing repression [280]. To go further, the authors used a chimeric construct consisting of the N-terminal region of TDP-43, including the two RNA-binding domains, fused to the splicing repressor ribonucleoprotein, PTB-binding 1 (RAVER1) (TDP-43Nter-RAVER1). When TDP-43Nter-RAVER1 was expressed in motoneurons of TBPH mutants, it highly extended lifespan [280]. On the contrary, the TDP-43Nter-RAVER1 construct carrying two mutations in the RRM1 that abolish RNA binding had no effect [280]. This confirms that the protective effect of TDP-43Nter-RAVER1 was due to TDP-43 binding onto its target genes. Altogether, these data strongly suggest that a major function of TBPH is splicing repression, which is very likely involved in the drastic phenotypes induced by loss of TBPH.
5.3.2. Mitochondrial Dysfunction
Abnormal fragmentation of mitochondria was described in the adult brain upon pan-neuronal expression of TDP-43 [311]. Highly fragmented mitochondria were also observed in thoracic muscles and axons of leg motoneurons expressing TDP-43 [185]. Fusion of mitochondria is promoted by Mitofusin/Marf, which has reduced expression level in fly heads overexpressing TDP-43 [185,311]. This suggests that TDP-43 GOF alters mitochondrial dynamic by inducing excessive fission of mitochondria due to Mitofusin/Marf downregulation. When overexpressed in the eye, TDP-43WT or TDP-43A315T induced mitochondrial damages including reduced size and abnormal cristae [312]. In addition, abnormally elevated ROS levels were detected in larval motoneurons overexpressing TDP-43WT or TDP-43A315T [312]. Pan-neuronal TDP-43WT or TDP-43A315T GOF induced an activation of the mitochondrial unfolded protein response (mitoUPR), characterized by the increased expression of known genes involved in mitoUPR, including Lon, the Drosophila ortholog of LonP1 [312]. LonP1 is a mitochondrial protease that plays an important role in mitochondrial protein quality control. Lon silencing exacerbated TDP-43-induced retinal degeneration and mitochondrial damages, as well as increased the level of mitochondrial TDP-43 without affecting the cytoplasmic level [312]. This suggests that Lon plays a protective role against TDP-43 proteinopathy. Altogether, these data strongly suggest that mitochondrial dysfunction may be associated with TDP-43 excess and that reversing mitochondrial damages may provide a potential therapeutic approach.
5.3.3. Transposon Upregulation
RNA-sequencing experiments have shown that pan-neuronal or pan-glial expression of TDP-43 induced upregulation of some retrotransposable element (RTE) in adult brain [309]. Then, the authors focused on gypsy RTE, for which level was highly increased in response to TDP-43 expression in glial cells. This was due to a reduction in the siRNA pathway, which is the major post-transcriptional silencing component of RTE in somatic tissue. Interestingly, reducing the glial expression of gypsy by RNA interference ameliorated the lifespan alteration induced by TDP-43 glial expression, strongly suggesting that loss of gypsy silencing was at least partially involved in TDP-43-induced toxicity [309]. This was further confirmed by the finding that glial cells undergo programmed cell death due to DNA damage caused by TDP-43-induced RTE replication [309]. In addition, DNA damage and apoptosis of nearby neurons were also observed upon TDP-43 glial GOF, suggesting that glial toxicity may spread to adjacent neurons [326]. Altogether, these studies suggest that TDP-43 overexpression alters the silencing of RTE, leading to DNA damage and subsequently cell death, which may contribute to neurodegeneration processes in ALS. Interestingly, upregulation of RTE expression was also reported in TBPH mutant [286], showing that TDP-43 GOF and TBPH LOF share similar phenotypes.
5.3.4. Excitotoxicity
Two-dimensional gel electrophoresis followed by mass spectrometry analyses on TBPH mutant heads showed that the levels of glutamic acid decarboxylase (Gad1) protein were reduced [342]. This downregulation occurred also at the mRNA level. Pan-neuronal expression of Gad1 in TBPH mutant partially restored the defects in larval locomotion and the abnormal distribution of the post-synaptic GluRIIA glutamate receptors and Discs-large (Dlg) scaffolding protein. On the contrary, pan-neuronal silencing of Gad1 triggered larval locomotion defects and abnormal organization of GluRIIA and Dlg, as was observed in TBPH mutants. Interestingly, pan-glial expression of Gad1 completely rescued larval locomotion defects and abnormal GluRIIA distribution of TBPH mutants but not the Dlg one. Conversely, Gad1 silencing in glial cells induced locomotion defects and abnormal GluRIIA distribution but did not affect Dlg. Increased glutamate levels were found in the hemolymph of TBPH mutant or Gad1 knockdown larvae [342], suggesting that an abnormal concentration of the neurotransmitter glutamate may be responsible for these defects. Thus, it would be conceivable to pharmacologically modulate glutamate signaling to interfere with neurodegenerative progression in ALS patients.
5.4. Genetic Modifiers of TDP-43 Toxicity
5.4.1. Stress Granules
Kim et al. found that Drosophila pan-neuronal TDP-43 expression resulted in increased phosphorylation of eIF2α [314]. In response to cellular stress, several kinases phosphorylate eIF2α, leading to translation repression and SG formation [343,344]. It was also shown that TDP-43 is recruited to SG upon stress [153], suggesting that TDP-43 may confer toxicity by increasing SG accumulation. RNAi-induced knockdown or pharmacological inhibition of poly(ADP-ribose)ylation glycohydrolase (PEK), a kinase that phosphorylates eIF2α, suppressed the climbing deficit induced by TDP-43. Conversely, downregulation of Gadd34, a phosphatase of eIF2α, enhanced the locomotor defects [314]. Altogether, these data showed that modulating the phosphorylation level of eIF2α might be therapeutically interesting to mitigate TDP-43 toxicity.
Using a candidate approach to search for RBPs that modulate TDP-43 toxicity, Coyne et al. identified dFMRP [318], the Drosophila ortholog of FMRP, which encodes a translational regulator and component of neuronal RNA granules [345]. LOF and GOF of dFMRP enhanced and reduced TDP-43-induced eye degeneration and larval locomotor dysfunction, respectively [318]. In primary larval motoneuron cultures, TDP-43 was detected in some cytoplasmic puncta, which were positive for dFMRP and other markers of SGs. In addition, immunoprecipitation experiments from adult heads indicated that TDP-43 associates to endogenous dFMRP in vivo. Fractionation experiments showed that dFMRP GOF decreased the insoluble fraction of TDP-43, suggesting that excess of dFMRP may modify TDP-43 aggregation properties to restore translation [318]. Thus, this study indicates that dFMRP may mitigate TDP-43 toxicity by remodeling RNA granules and restoring translation.
Ataxin-2 is known to participate to SG assembly [346,347]. In Drosophila, dAtx2 GOF aggravated the lifespan defects and eye degeneration induced by TDP-43. On the contrary, reducing dAtx2 dose rescued these phenotypes [308]. From these observations, one may conclude that excess of Atx-2 may exacerbate TDP-43 toxicity. Accordingly, reduction of the Atx-2 level has beneficial effects in a mouse model of TDP-43 proteinopathy [348].
The poly(A)-binding protein nuclear 1 (PABPN1) was identified as a partner of TDP-43 [331]. PABPN1 (also known as PABP2) is a ubiquitous protein that controls the poly(A) tail length of RNA transcripts [349]. Furthermore, PABPN1 was shown to form a complex with TDP-43 and FUS in mammalian cells [350]. LOF of PABP2, the Drosophila ortholog of PABPN1, enhanced the eye degeneration and the larval locomotor defect induced by TDP-43 GOF [331]. In primary motoneuron cultures, TDP-43 disturbed SG dynamics, and this defect was suppressed by PABPN1 co-expression [331]. Thus, PABP2 might modify TDP-43 toxicity by modulating SG dynamics. Another study found that loss of PABP2 slightly increased the median lifespan of flies expressing TDP-43 in motoneurons [313], suggesting a different role during aging.
PolyADP ribosylation (PARylation) is a reversible PTM in which polymers of ADP-ribose are attached to proteins by PAR polymerase enzymes (PARPs) and removed by PAR glycohydrolases (PARG). PARylation was shown to regulate SG dynamics with a decreased level suppressing SG formation [351]. In Drosophila, both Parp knockdown and Parg overexpression suppressed the eye degeneration caused by TDP-43 GOF [351]. Parp silencing also rescued age progressive climbing defects and reduced lifespan of pan-neuronal TDP-43 expressing flies [351]. A genetic screen based on the modification of the rough eye phenotype induced by TDP-43 GOF showed that downregulation of tankyrase (Tnks), which encodes a PARP, restored eye structure [310]. Moreover, Tnks knockdown rescued the lifespan defect induced upon pan-neuronal TDP-43 expression. Interestingly, the authors found PAR-binding motifs in the NLS region of TDP-43 that are required to localize TDP-43 to SGs [310]. They also found that Tnks downregulation induced a redistribution of TDP-43 protein, with an increase in the nucleus and a decrease in the cytoplasm. This was likely due to an impairment of TDP-43 incorporation into SGs. Altogether, these studies showed that decreased PARylation is protective against TDP-43 toxicity.
The discovery that modulation of SG dynamics may modify the TDP-43-induced phenotypes opens the way to future studies targeting SGs for potential therapeutic strategy.
5.4.2. Cytoplasmic Aggregation
Choksi et al. found that TDP-43Q331K mutant, but not wildtype or M337V mutant, was detected in cytoplasmic puncta when expressed in the developing eye [306]. Co-expression of TDP-43Q331K with doubletime, the fly ortholog of casein kinase Iε, strongly enhanced the degenerative eye phenotype induced by TDP-43Q331K but not the one induced by wildtype or M337V TDP-43 [306]. Interestingly, when co-expressed with doubletime, the Q331K mutant showed a higher level of S409/410 phosphorylation, which is detected in cytoplasmic inclusions of ALS patients [352]. In addition, high-molecular-weight oligomeric species were detected upon Q331K expression, and they were enhanced when doubletime was co-expressed [306]. These data suggest that the toxicity of TDP-43Q331K was linked to abnormal phosphorylation and cytoplasmic aggregation. However, the reason why Q331K was more toxic compared to wildtype or M337V is still unclear. Nevertheless, this study suggests that targeting a specific kinase to modulate TDP-43 phosphorylation may be therapeutically interesting.
Budini et al. developed a cellular model of TDP-43 aggregation by using a GFP-tagged construct composed of repetitions of the Q/N-rich C-terminal region of TDP-43 (GFP-12xQ/N) [353]. In cell lines, the co-expression of TDP-43 and GFP-12xQ/N triggered the formation of TDP-43 aggregates, which were cytoplasmic, ubiquitinated, and phosphorylated, as was observed in ALS patients [31,253]. TBPH overexpression induced strong neurodegeneration in the eye that was completely abolished when GFP-12xQ/N was co-expressed [304]. In addition, TBPH and GFP-12xQ/N were found co-localized in cytoplasmic aggregates in retinal cells. Biochemical fractionation experiments indicated that TBPH was mainly present in the soluble fraction upon eye expression, while it was predominantly present in the insoluble fraction when co-expressed with GFP-12xQ/N [304]. Altogether, these data strongly suggest that the excess of soluble TBPH is toxic and that aggregates have a protective effect, likely by sequestrating excess of TBPH. The same group showed that pan-neuronal expression of GFP-12xQ/N induced locomotion defects in mid-adult life [338]. They also found that endogenous TBPH expression dropped during aging, which likely explains why TBPH sequestration by GFP-12xQ/N was deleterious during aging [338].
By using a proximity-dependent biotin identification approach, Chou et al. found that components of the nuclear pore complex and nucleocytoplasmic transport machinery were enriched in detergent-insoluble TDP-43 aggregates [317]. They also showed that TDP-43 aggregates sequestered and/or mislocalized nucleoporins (Nup). To test for functional interaction, the authors asked whether Nup function might modify TDP-43 toxicity in flies. They found that LOF of Nup50, Nup93, Nup98-96, Nup107, or Nup214 suppressed the TDP-43-mediated rough eye phenotype and rescued the larval locomotor phenotype induced by motoneuronal TDP-43 GOF [317]. This study suggests that some nucleocytoplasmic transport alteration may be part of TDP-43 toxicity. Another group found that mutation in Nup50 rescued lifespan defects of motoneuronal TDP-43-expressing flies [313]. Interestingly, TDP-43 was still predominantly nuclear in larval neurons lacking Nup50 [313], suggesting that the protective effect of Nup50 mutation was not due to a major nucleocytoplasmic redistribution of TDP-43 but rather to nucleocytoplasmic transport modulations. Thus, targeting some Nup proteins may have a beneficial effect on ALS disease.
5.4.3. Unfolded Protein Response
The co-expression of TDP-43 with a GFP reporter of the unfolded protein response revealed that ER stress was elevated in Drosophila retinal cells compared to control [324]. Then, the authors asked whether Clusterin, a normally secreted chaperone that is redirected to the cytosol under ER stress condition [354], might modulate TDP-43-induced toxicity. Note that Clusterin has no Drosophila ortholog. When expressed in adult fly motoneurons, TDP-43 was found in cytoplasmic foci distributed in soma and neuronal processes. In contrast, co-expression of Clusterin restored the predominant nuclear localization of TDP-43 [324]. In addition, co-expression of Clusterin with TDP-43 partially restored the climbing defect, the reduced lifespan, and the eye degeneration induced by TDP-43 GOF [324]. Thus, this study suggests that Clusterin may be protective upon TDP-43-induced ER stress.
The deubiquitinating enzyme UBPY (ubiquitin isopeptidase Y) was identified as an interactor of TDP-43 [332]. The knockdown of its Drosophila ortholog, dUBPY (also known as Ubiquitin specific protease 8) resulted in higher amounts of insoluble and likely ubiquitinated TDP-43 in fly heads [332]. Additionally, dUBPY silencing enhanced the age-dependent eye degeneration induced by TDP-43 expression [332]. Another study found that the TDP-43M337V-induced eye degeneration was suppressed or enhanced by GOF and LOF of CG5445 gene, respectively [334]. CG5445 encodes a protein that interacts with ubiquitinated proteins and promotes their degradation [334]. Accordingly, the authors found that CG5445 GOF and LOF increased or decreased, respectively, the soluble form of TDP-43M337V, likely facilitating its degradation.
Altogether, these findings showed that modulating the unfolded protein response and ubiquitination might influence TDP-43 aggregation and toxicity, which may open the way for the discovery of potential therapeutic strategy.
5.4.4. Inflammation
A genetic screening, aiming to find modifiers of reduced lifespan induced by motoneuronal expression of TDP-43, identified the mitogen-activated protein kinase kinase kinase (MAP3K) Wallenda (Wnd), the Drosophila ortholog of dual leucine kinase (DLK) [323]. Wnd LOF and GOF extended and reduced lifespan, respectively. Effector kinases downstream of Wnd are p38 and JNK, two stress-activated kinases. The JNK pathway had a protective effect as the GOF of Basket, the JNK Drosophila ortholog, extended lifespan of flies expressing TDP-43 in motoneurons. Conversely, a reduced dose of Basket reduced lifespan. Contrary results were obtained with p38b, one the three p38 Drosophila orthologs, indicating that the p38 pathway was rather deleterious. This report also showed that oxidative stress and neuroinflammation were differentially modulated by Basket and p38b [323], suggesting that these two processes may be part of TDP-43 toxicity.
Drosophila pan-glial TDP-43 increased the expression of the inflammatory genes Dorsal (the ortholog of human NF-kappa B,NF-κB and inducible Nitric Oxyde Synthase, iNOS) [325]. This was rescued by RNAi-induced glial knockdown of Ptp61F, the Drosophila ortholog of human Ptp1b encoding tyrosine phosphatase 1B, which is a ubiquitous enzyme anchored in the ER membrane, upregulated in neuroinflammatory conditions [355]. In addition, Ptp61F silencing mitigated the age-dependent climbing defects and the reduced lifespan induced by glial GOF of TDP-43 [325]. This study suggests that the Ptp1b-dependent inflammatory response may be associated with TDP-43 toxicity.
Thus, inflammation processes may represent a target to counteract disease progression in ALS.
5.4.5. Mammalian Target of Rapamycin (mTOR) Pathway
In TBPH mutant larvae, the phosphorylation levels of ribosomal protein S6 kinase (S6K) and eukaryotic translation initiation factor 4E (4E-BP1), two downstream effectors of mTORC1 [356], were decreased [174]. In addition, the expression level of raptor mRNA was decreased in TPBH mutants. Raptor is an essential component of mTORC1 that regulates mTORC1 lysosomal localization. TBPH mutants also displayed an upregulation of some lysosomal and autophagic genes, as well as alteration in autophagosome–lysosome fusion [174]. Administration of the mTOR inhibitor rapamycin to TBPH mutant aggravated larval locomotion. On the contrary, the mTOR agonist phosphatic acid ameliorated this phenotype. In both cases, the effects of rapamycin or phosphatic acid were dose-dependent [174]. Another report found that rapamycin administration ameliorated the reduced lifespan and locomotion defects induced by adult motoneuronal TBPH GOF [301]. Altogether, these studies suggest that impaired mTORC1 signaling influences TDP-43 toxicity and that restoring mTOR signaling may be beneficial.
5.4.6. Autophagosomes
A genome-wide RNAi screening identified the inositol-1,4,5-trisphosphate receptor type 1 (ITPR1) as a strong modulator of TDP-43 subcellular localization [322]. The silencing of ITPR1, which is an inositol 1,4,5-trisphosphate (IP3)-gated Ca2+ channel localized in the endoplasmic reticulum, promoted cytoplasmic accumulation of TDP-43 and potentiated its recruitment to phagosomes [322]. This suggests that ITPR1 silencing promotes TDP-43 clearance by autophagosomes. Mutations in Itp-r83A, the only fly ortholog of ITPR1, increased the cytosolic fraction of TDP-43 in larval neurons and rescued the climbing defects and the reduced lifespan induced by motoneuronal expression of TDP-43 [322]. Thus, it is likely that phagosome metabolism can influence TDP-43 toxicity, and future studies will be needed to determine whether modulating phagosome function may have a therapeutic effect.
5.4.7. Chaperone
Co-expression of HSP67Bc, the Drosophila ortholog of human HSPB8, rescued the strong eye degenerative phenotype induced by TDP-43 mutated in its NLS domain (TDP-43NLSmut) but had no effect on the rough eye phenotype induced by wildtype TDP-43 [327]. HSP67Bc facilitates the degradation of misfolded proteins, which likely explains its protective effect as the protein level of TDP-43NLSmut but not wildtype was reduced when they were co-expressed. Conversely, RNAi-induced knockdown of HSP67Bc aggravated the TDP-43NLSmut-associated eye phenotype, which was correlated with a high accumulation of ubiquitinated proteins [327]. Thus, HSP67Bc is protective against the abnormal protein homeostasis induced by TDP-43NLSmut.
5.4.8. Chromatin
Berson et al. selected some genes related to various aspects of chromatin regulation and asked whether their downregulation may modify the TDP-43-induced rough eye phenotype [330]. They found that TDP-43-induced toxicity was strongly enhanced by knockdown of Chd1, which encodes a nucleosome-remodeling enzyme required for heat-shock gene expression following stress [357]. Upon heat stress, the upregulation of several heat-shock genes was lower in flies expressing TDP-43, and this defect was abolished by Chd1 co-expression [330]. Co-immunoprecipitation experiments on whole-fly lysates indicated that TDP-43 interacts with Chd1. In addition, chromatin immunoprecipitation (Chip)-qPCR analyses showed that TDP-43 impaired the recruitment of Chd1 to chromatin [330]. These data suggest that TDP-43 may prevent the proper recruitment of Chd1 to chromatin, leading to dampened stress-related gene expression. Thus, modulating chromatin dynamics may be an interesting way to fight against cellular stress induced by TDP-43.
5.4.9. hnRNPs
Appocher et al. investigated whether RNAi-induced knockdown of Drosophila hnRNPs may modify the degenerative phenotype induced by TBPH expression in the eye. In this way, Hrb27c, CG42458, Glo, Syncrip/Syp, and Hrp38 were identified as strong suppressors, while Hrb87F, Sm, Heph, and Rump were mild suppressors of eye degeneration [303]. Thus, TBPH toxicity may be modulated by hnRNPs.
5.4.10. Metabolic Deregulation
Clinical observations indicate that ALS patients have metabolic disturbances including body weight loss [358], hypermetabolism [359,360], glucose intolerance [361], and defects in lipid metabolism [362]. Metabolomic analyses performed on whole larvae revealed that glucose metabolism was increased when TDP-43 (wildtype or G298S) was expressed in motoneurons [363]. In addition, mRNA levels of phosphofructokinase, an enzyme that control the rate of glycolysis, was increased, strongly suggesting that glycolysis was upregulated by TDP-43 motoneuronal expression. By using a glucose sensor, it was found that the entry of glucose in motoneurons was enhanced by the expression of TDP-43 G298S but not wildtype [363]. Interestingly, a high glucose diet was sufficient to mitigate the larval locomotor defect, as well as the reduced survival induced by TDP-43 (wildtype or G298S) expression, in motoneurons. In addition, motoneuronal co-expression of the human glucose transporter GLUT-3 suppressed the larval locomotor defects, as well as the alterations at larval NMJs induced by TDP-43 [363]. These data strongly suggest that increasing glucose availability is protective against the deleterious effect of TDP-43.
Some alterations of lipid metabolism, such as a decrease in the carnitine shuttle and reduced lipid beta oxidation, were also found in larvae expressing TDP-43 (wildtype or G298S) in motoneurons [364]. In addition, the mRNA levels of some carnitine shuttle components were misregulated by TDP-43. To bypass the carnitine shuttle, medium-chain fatty acids or beta-hydroxybutyrate were administrated in the food, and this was sufficient to mitigate the larval locomotor defects induced by TDP-43 [364].
Altogether, these studies showed that dietary intervention might be an interesting way to alleviate TDP-43 induced defects.
5.5. Target RNAs
One challenge when studying RBPs is to identify their target RNAs. Several RNA targets of TBPH have been identified in Drosophila (see also [276,365,366]).
5.5.1. Futsch
One report found that the level of Futsch protein, a neuronal microtubule-binding protein homolog to human Microtubule associated protein 1B (MAP1B), was decreased in TBPH mutant heads and at larval NMJs [279]. Immunoprecipitation from heads expressing pan-neuronally Flag-tagged TPBH showed that futsch mRNA was highly enriched [279]. In addition, an RNA-binding defective TBPH construct was not able to restore the endogenous Futsch level as was observed with wildtype, indicating that the RNA-binding activity of TBPH was required to maintain futsch expression level [279]. Altogether, these data strongly suggest that TBPH binds the futsch transcript to positively regulate its expression. The futsch mRNA was also enriched in immunoprecipitation experiments on flies expressing YFP-tagged TDP-43 in the eye [335], indicating that both TBPH and TDP-43 may interact with futsch mRNA. When TDP-43 was expressed in motoneurons, the levels of futsch mRNA and protein were increased in the cell bodies, while they were decreased at the NMJs compared to control [335], suggesting a failure in futsch mRNA transport. In addition, polysome fractionation experiments indicated that TDP-43 induces the futsch mRNA to shift toward the untranslated fractions, strongly suggesting that TDP-43 represses the translation of fustch mRNA [335]. This is in agreement with RNA-sequencing data that showed a downregulation of futsch in TBPH GOF and no change in LOF condition [275]. It was shown that both TBPH and TDP-43 directly bind the 5′-UTR of futsch mRNA [367], which contains UG-rich sequences known to be target of TDP-43. A luciferase assay performed with the 5′-UTR of the futsch mRNA region suggested that TBPH positively modulates the translational efficiency of futsch [367]. It is noteworthy that no evidence for a repressor role of TDP-43 was provided in this study.
5.5.2. Histone Deacetylase 6 (HDAC6)
HDAC6 is a cytoplasmic deacetylase that plays an important role in the detection and degradation of ubiquitinated cellular aggregates [368]. In TBPH mutants, HDAC6 mRNA level was strongly decreased [268,340], while TDP-43 motoneuronal GOF increased HDAC6 mRNA level [340]. In addition, TDP-43 and TBPH bound HDAC6 mRNA [268,279]. Interestingly, HDAC6 is necessary and sufficient for deacetylation of Bruchpilot (Brp) [340], an important player at presynaptic density that tethers vesicles [369]. Thus, it is tempting to speculate that HDAC6 dysregulation observed upon TDP-43 GOF or in TBPH LOF might alter Brp acetylation and synaptic transmission.
5.5.3. Cacophony
Transcriptome analysis revealed that the splicing of cacophony transcripts was altered in TBPH mutant CNS [275]. The cacophony gene codes for a voltage-gated calcium channel localized at the active zone of NMJs that is required for neurotransmitter release. The level of cacophony protein was reduced in TBPH mutant larval NMJs, which have abnormal crawling behavior [277]. Interestingly, pan-neuronal or motoneuronal expression of cacophony fully rescues the locomotion behavior phenotype but not the pupal lethality associated with TBPH mutation, suggesting that the locomotion phenotype is mainly due to the lack of cacophony protein in motoneurons [277]. However, another study reported that cacophony may also be required in a few central neurons [278]. RT-PCR experiments on immunoprecipitates from adult fly using an anti-TBPH antibody revealed the presence of cacophony transcripts, strongly suggesting that TPBH binds cacophony mRNA [277]. In addition, Lembke et al. showed that the subtle changes in the splicing pattern of cacophony observed in TBPH mutant decreased the cacophony protein expression level [370]. Altogether, these studies strongly suggested that TBPH is required for proper splicing of cacophony transcripts. Thus, these findings revealed that the splicing alteration of genes involved in synaptic transmission is a consequence of TBPH dysfunction, which might open new ways to better understand ALS pathogenesis.
6. Conclusions
Drosophila is a powerful genetic tool that has made major contributions to further our understanding of several neurodegenerative disorders, including ALS (Figure 2). Furthermore, large-scale screenings in Drosophila have allowed the identification of numerous ALS disease modifiers, paving the way for future studies in mammals. Genome-wide association studies and whole-exome/genome sequencing have accelerated the pace of identification of new ALS-associated genes, as illustrated for instance by the recent discovery of ALS-linked mutations in the kinesin family member 5A gene [371,372]. Moreover, large-scale analysis using next-generation sequencing has significantly increased the rate of new mutation detection in already known ALS-causing genes. The subsequent challenging question relates to the pathogenicity of these new variants. The wide variety of genetic tools available in Drosophila combined with their short lifespan will undoubtedly help to gain insights into the pathogenic mechanisms associated with these novel genes or variants.
Despite the well-recognized motoneuronal death in ALS, motoneuron neighboring cells including astrocytes, oligodendrocytes, and interneurons degenerate before motoneuron loss [10], strongly suggesting that they contribute to ALS pathogenesis. Recently, one report showed that TDP-43 overexpression in glial cells induced neuronal death in adult brains [326], showing that non-cell-autonomous mechanisms also take place in flies. However, this non-cell-autonomous degeneration of motoneurons is still a neglected subject of research in flies, despite all the genetic tools that are available in Drosophila.
Despite intensive research over the past decades, no efficient therapy is currently available for ALS. Drosophila will certainly help to identify and characterize novel molecular and cellular mechanisms involved in this disease, giving hope for the development of therapeutic strategies.
Acknowledgments
We are grateful to all members of the team for their helpful discussion.
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| arcRNA | Architectural RNA |
| Arg | Arginine |
| ATP | Adenosine triphosphate |
| Aub | Aubergine |
| BMAA | β-N-methylamino l-alanine |
| C9ORF72 | Chromosome 9 open reading frame 72 |
| Ca | Calcium |
| Cas9 | CRISPR-associated protein 9 |
| Caz | Cabeza |
| CCAP | Crustacean cardioactive peptide |
| Chip | Chromatin immunoprecipitation |
| CNS | Central nervous system |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Cu | Copper |
| DART5 | Drosophila arginine methyltransferase protein 5 |
| dDSIF | Drosophila DRB sensitivity-inducing factor |
| dEAAT1 | Drosophila excitatory amino acid transporter 1 |
| dFMR1 | Drosophila Fragile X mental retardation 1 |
| dFMRP | Drosophila Fragile X mental retardation protein |
| Dlg | Discs-large |
| DNA | Deoxyribonucleic acid |
| dPAF1 | Drosophila polymerase-associated factor 1 |
| DPR | Dipeptide proteins |
| eIF1A | Eukaryotic translation initiation factor 1A |
| eIF2α | Eukaryotic initiation factor 2α |
| EJP | Excitatory junction potential |
| Elav | Embryonic lethal abnormal visual protein |
| ER | Endoplasmic reticulum |
| EWS | Ewing sarcoma |
| fALS | Familial amyotrophic lateral sclerosis |
| FET | FUS EWS TAF15 family of proteins |
| FUS | Fused in sarcoma |
| FTD | Frontotemporal dementia |
| Gad1 | Glutamic acid decarboxylase 1 |
| GDP | Guanosine diphosphate |
| GFP | Green fluorescent protein |
| GluRIIA | Glutamate receptor IIA |
| Gly | Glycine |
| GMR | Glass multiple reporter |
| GOF | Gain of function |
| GRD | Glycine-rich domain |
| GS | GeneSwitch |
| GTP | Guanosine triphosphate |
| HDAC6 | Histone deacetylase 6 |
| HEK/HEK293 | Human embryonic kidney 293 |
| HeLa | Henrietta Lacks |
| Hpo | Hippo |
| hnRNP | Heterogenous nuclear ribonucleoprotein |
| HSP60 | Heat-shock protein 60 |
| HSP70 | Heat-shock protein 70 |
| HRE | Hexanucleotide repeat expansion |
| IP3 | Inositol 1,4,5-trisphosphate |
| iPSC | Induced pluripotent stem cell |
| ITPR1 | Inositol-1,4,5-trisphosphate receptor type 1 |
| JNK | c-Jun N-terminal kinase |
| Kapβ2 | Karyopherin-β2 |
| kDa | Kilodalton |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| LA | α-Lipoic acid |
| LAMP-1 | Lysosomal-associated membrane protein 1 |
| LCD | Low-complexity domain |
| lncRNA | Long non-coding RNA |
| LOF | Loss of function |
| MARCM | Mosaic analysis with a repressible cell marker |
| miRNA | MicroRNAs |
| mRNA | Messenger RNA |
| NB | Nuclear body |
| NES | Nuclear export signal |
| NIR | Nuclear import receptor |
| NMJ | Neuromuscular junction |
| NLS | Nuclear localization signal |
| Nup | Nucleoporin |
| Orz | γ-Oryzanol |
| PABPN1 | Poly(A)-binding protein nuclear 1 |
| PARG | Poly(ADP-ribose) glycohydrolase |
| PARP1 | Poly(ADP-ribose)ylation polymerase 1 |
| PEK | Poly(ADP-ribose)ylation glycohydrolase |
| PINK1 | PTEN-induced kinase 1 |
| piRNA | Piwi-interacting RNA |
| PrLD | Prion-like domain |
| poly-GA | Poly-glycine/alanine |
| poly-GP | Poly-glycine/proline |
| poly-GR | Poly-glycine/arginine |
| poly-PA | Poly-proline/alanine |
| poly-PR | Poly-proline/arginine |
| PTM | Post-translational modification |
| PTP1B | Tyrosine phosphatase 1B |
| qPCR | Quantitative polymerase chain reaction |
| QGSY | Glutamine, glycine, serine, and tyrosine residues |
| RAN | Repeat-associated non-AUG |
| RanGAP | Ras-related nuclear GTPase-activating protein |
| RAVER1 | Ribonucleoprotein, PTB-binding 1 |
| RBP | RNA-binding protein |
| Rbp1 | RNA-binding protein 1 |
| RGG | Arginine glycine rich domain |
| ROS | Reactive oxygen species |
| RRM | RNA recognition motif |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| RTE | Retrotransposable element |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| sALS | Sporadic amyotrophic lateral sclerosis |
| SF2 | Splicing factor 2 |
| Sf3b-1 | Splicing factor 3b subunit 1 |
| SG | Stress granule |
| siRNA | Small interfering RNA |
| SOD | Superoxide dismutase |
| SOP | Sensory organ precursor |
| TAF15 | TATA box-binding protein-associated factor 68 kDa |
| TARDBP | TAR DNA-binding protein |
| TARGET | Temporal and regional gene expression targeting |
| TBPH | TAR DNA-binding protein-43 homolog |
| TCERG1 | Transcription elongation regulator 1 |
| TDP-43 | TAR DNA-binding protein 43 |
| TDPBR | TDP-43 binding region |
| Ter94 | Transitional endoplasmic reticulum 94 |
| TLS | Translocated in liposarcoma |
| TMPyP4 | 5,10,15,20-Tetrakis-(N-methyl-4-pyridyl)porphine |
| UAS | Upstream activating sequence |
| UBPY | Ubiquitin isopeptidase Y |
| UPR | Unfolded protein response |
| UTR | Untranslated transcribed region |
| VCP | Valosin-containing protein |
| Wnd | Wallenda |
| WT | Wildtype |
| XPO1 | Exportin 1 |
| Zfp106 | Zing finger protein 106 |
| Zn | Zinc |
| ZnF | Zinc finger domain |
Author Contributions
Conceptualization, S.L. and L.S.; writing—original draft preparation, S.L., L.T., S.O., C.R., and L.S.; writing—review and editing, S.L., C.R., and L.S.; visualization, S.O.; supervision, S.L., C.R., and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the French National Institute for Health and Medical Research (Inserm), Association pour la Recherche sur la SLA (ARSLA, grant number R19101FF to S.L.), and Marie Sklodowska Curie Individual fellowship “ADELE” (C.R.). L.T. is a recipient of Montpellier University ATER Research fellowship. S.O. is a recipient of Ministère de l’enseignement Supérieur, de la Recherche et de L’innovation (MESRI) Ph.D. fellowship.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., van den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 3.Rowland L.P., Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 4.Van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., van den Berg L.H. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 5.Longinetti E., Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019;32:771–776. doi: 10.1097/WCO.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L., Liu T., Liu L., Yao X., Chen L., Fan D., Zhan S., Wang S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2020;267:944–953. doi: 10.1007/s00415-019-09652-y. [DOI] [PubMed] [Google Scholar]
- 7.Cappello V., Francolini M. Neuromuscular Junction Dismantling in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2017;18:2092. doi: 10.3390/ijms18102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland D.W., Rothstein J.D. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan L.M., Hollander D. Respiratory dysfunction in amyotrophic lateral sclerosis. Clin. Chest Med. 1994;15:675–681. [PubMed] [Google Scholar]
- 10.Crabé R., Aimond F., Gosset P., Scamps F., Raoul C. How Degeneration of Cells Surrounding Motoneurons Contributes to Amyotrophic Lateral Sclerosis. Cells. 2020;9:2550. doi: 10.3390/cells9122550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramzon Y.A., Fratta P., Traynor B.J., Chia R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2020;14:42. doi: 10.3389/fnins.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elamin M., Bede P., Byrne S., Jordan N., Gallagher L., Wynne B., O’Brien C., Phukan J., Lynch C., Pender N., et al. Cognitive changes predict functional decline in ALS: A population-based longitudinal study. Neurology. 2013;80:1590–1597. doi: 10.1212/WNL.0b013e31828f18ac. [DOI] [PubMed] [Google Scholar]
- 13.Phukan J., Elamin M., Bede P., Jordan N., Gallagher L., Byrne S., Lynch C., Pender N., Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. J. Neurol. Neurosurg. Psychiatry. 2012;83:102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- 14.Cappella M., Ciotti C., Cohen-Tannoudji M., Biferi M.G. Gene Therapy for ALS-A Perspective. Int. J. Mol. Sci. 2019;20:4388. doi: 10.3390/ijms20184388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boylan K. Familial Amyotrophic Lateral Sclerosis. Neurol. Clin. 2015;33:807–830. doi: 10.1016/j.ncl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mejzini R., Flynn L.L., Pitout I.L., Fletcher S., Wilton S.D., Akkari P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019;13:1310. doi: 10.3389/fnins.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shatunov A., Al-Chalabi A. The genetic architecture of ALS. Neurobiol. Dis. 2021;147:105156. doi: 10.1016/j.nbd.2020.105156. [DOI] [PubMed] [Google Scholar]
- 18.Zou Z.Y., Zhou Z.R., Che C.H., Liu C.Y., He R.L., Huang H.P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2017;88:540–549. doi: 10.1136/jnnp-2016-315018. [DOI] [PubMed] [Google Scholar]
- 19.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 20.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J. Biol. Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 21.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majounie E., Renton A.E., Mok K., Dopper E.G., Waite A., Rollinson S., Chiò A., Restagno G., Nicolaou N., Simon-Sanchez J., et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: A cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellier C., Campanari M.L., Julie Corbier C., Gaucherot A., Kolb-Cheynel I., Oulad-Abdelghani M., Ruffenach F., Page A., Ciura S., Kabashi E., et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016;35:1276–1297. doi: 10.15252/embj.201593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang W., Hu F. Cellular and physiological functions of C9orf72 and implications for ALS/FTD. J. Neurochem. 2020 doi: 10.1111/jnc.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratti A., Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016;138(Suppl. 1):95–111. doi: 10.1111/jnc.13625. [DOI] [PubMed] [Google Scholar]
- 27.Calvio C., Neubauer G., Mann M., Lamond A.I. Identification of hnRNP P2 as TLS/FUS using electrospray mass spectrometry. RNA. 1995;1:724–733. [PMC free article] [PubMed] [Google Scholar]
- 28.Morohoshi F., Ootsuka Y., Arai K., Ichikawa H., Mitani S., Munakata N., Ohki M. Genomic structure of the human RBP56/hTAFII68 and FUS/TLS genes. Gene. 1998;221:191–198. doi: 10.1016/S0378-1119(98)00463-6. [DOI] [PubMed] [Google Scholar]
- 29.Kwiatkowski T.J., Jr., Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 30.Vance C., Rogelj B., Hortobágyi T., de Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 32.Bolognesi B., Faure A.J., Seuma M., Schmiedel J.M., Tartaglia G.G., Lehner B. The mutational landscape of a prion-like domain. Nat. Commun. 2019;10:4162. doi: 10.1038/s41467-019-12101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams M.D., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 34.Myers E.W., Sutton G.G., Delcher A.L., Dew I.M., Fasulo D.P., Flanigan M.J., Kravitz S.A., Mobarry C.M., Reinert K.H., Remington K.A., et al. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- 35.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development (Cambridge, UK) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 36.McGuire S.E., Mao Z., Davis R.L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 37.Reiter L.T., Potocki L., Chien S., Gribskov M., Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd T.E., Verstreken P., Ostrin E.J., Phillippi A., Lichtarge O., Bellen H.J. A genome-wide search for synaptic vesicle cycle proteins in Drosophila. Neuron. 2000;26:45–50. doi: 10.1016/S0896-6273(00)81136-8. [DOI] [PubMed] [Google Scholar]
- 39.Marsh J.L., Thompson L.M. Drosophila in the study of neurodegenerative disease. Neuron. 2006;52:169–178. doi: 10.1016/j.neuron.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 40.McGurk L., Berson A., Bonini N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics. 2015;201:377–402. doi: 10.1534/genetics.115.179457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Şentürk M., Bellen H.J. Genetic strategies to tackle neurological diseases in fruit flies. Curr. Opin. Neurobiol. 2018;50:24–32. doi: 10.1016/j.conb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harman D. The aging process. Proc. Natl. Acad. Sci. USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 44.Harman D. Role of free radicals in mutation, cancer, aging, and the maintenance of life. Radiat. Res. 1962;16:753–763. doi: 10.2307/3571274. [DOI] [PubMed] [Google Scholar]
- 45.Harman D. Nutritional implications of the free-radical theory of aging. J. Am. Coll. Nutr. 1982;1:27–34. doi: 10.1080/07315724.1982.10718090. [DOI] [PubMed] [Google Scholar]
- 46.Harman D. The aging process. Basic Life Sci. 1988;49:1057–1065. doi: 10.1007/978-1-4684-5568-7_175. [DOI] [PubMed] [Google Scholar]
- 47.Harman D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 48.Seto N.O., Hayashi S., Tener G.M. Cloning, sequence analysis and chromosomal localization of the Cu-Zn superoxide dismutase gene of Drosophila melanogaster. Gene. 1989;75:85–92. doi: 10.1016/0378-1119(89)90385-5. [DOI] [PubMed] [Google Scholar]
- 49.Phillips J.P., Campbell S.D., Michaud D., Charbonneau M., Hilliker A.J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. USA. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips J.P., Tainer J.A., Getzoff E.D., Boulianne G.L., Kirby K., Hilliker A.J. Subunit-destabilizing mutations in Drosophila copper/zinc superoxide dismutase: Neuropathology and a model of dimer dysequilibrium. Proc. Natl. Acad. Sci. USA. 1995;92:8574–8578. doi: 10.1073/pnas.92.19.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staveley B.E., Phillips J.P., Hilliker A.J. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990;33:867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- 52.Orr W.C., Sohal R.S. Effects of Cu-Zn superoxide dismutase overexpression of life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- 53.Reveillaud I., Niedzwiecki A., Bensch K.G., Fleming J.E. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol. Cell. Biol. 1991;11:632–640. doi: 10.1128/MCB.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elia A.J., Parkes T.L., Kirby K., St George-Hyslop P., Boulianne G.L., Phillips J.P., Hilliker A.J. Expression of human FALS SOD in motorneurons of Drosophila. Free radic. Biol. Med. 1999;26:1332–1338. doi: 10.1016/s0891-5849(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 55.Parkes T.L., Elia A.J., Dickinson D., Hilliker A.J., Phillips J.P., Boulianne G.L. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 56.Mockett R.J., Radyuk S.N., Benes J.J., Orr W.C., Sohal R.S. Phenotypic effects of familial amyotrophic lateral sclerosis mutant Sod alleles in transgenic Drosophila. Proc. Natl. Acad. Sci. USA. 2003;100:301–306. doi: 10.1073/pnas.0136976100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Şahin A., Held A., Bredvik K., Major P., Achilli T.M., Kerson A.G., Wharton K., Stilwell G., Reenan R. Human SOD1 ALS Mutations in a Drosophila Knock-In Model Cause Severe Phenotypes and Reveal Dosage-Sensitive Gain- and Loss-of-Function Components. Genetics. 2017;205:707–723. doi: 10.1534/genetics.116.190850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Held A., Major P., Sahin A., Reenan R.A., Lipscombe D., Wharton K.A. Circuit Dysfunction in SOD1-ALS Model First Detected in Sensory Feedback Prior to Motor Neuron Degeneration Is Alleviated by BMP Signaling. J. Neurosci. 2019;39:2347–2364. doi: 10.1523/JNEUROSCI.1771-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernard E., Pegat A., Svahn J., Bouhour F., Leblanc P., Millecamps S., Thobois S., Guissart C., Lumbroso S., Mouzat K. Clinical and Molecular Landscape of ALS Patients with SOD1 Mutations: Novel Pathogenic Variants and Novel Phenotypes. A Single ALS Center Study. Int. J. Mol. Sci. 2020;21:6807. doi: 10.3390/ijms21186807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yim M.B., Kang J.H., Yim H.S., Kwak H.S., Chock P.B., Stadtman E.R. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu,Zn-superoxide dismutase mutant: An enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ezzi S.A., Urushitani M., Julien J.P. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J. Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 62.Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F., Wilcox H.M., Flood D.G., Beal M.F., Brown R.H., Jr., et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 63.Bruijn L.I., Houseweart M.K., Kato S., Anderson K.L., Anderson S.D., Ohama E., Reaume A.G., Scott R.W., Cleveland D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science (New York, NY) 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 64.Ciechanover A., Kwon Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciryam P., Lambert-Smith I.A., Bean D.M., Freer R., Cid F., Tartaglia G.G., Saunders D.N., Wilson M.R., Oliver S.G., Morimoto R.I., et al. Spinal motor neuron protein supersaturation patterns are associated with inclusion body formation in ALS. Proc. Natl. Acad. Sci. USA. 2017;114:E3935–E3943. doi: 10.1073/pnas.1613854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durham H.D., Roy J., Dong L., Figlewicz D.A. Aggregation of mutant Cu/Zn superoxide dismutase proteins in a culture model of ALS. J. Neuropathol. Exp. Neurol. 1997;56:523–530. doi: 10.1097/00005072-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Farrawell N.E., Lambert-Smith I.A., Warraich S.T., Blair I.P., Saunders D.N., Hatters D.M., Yerbury J.J. Distinct partitioning of ALS associated TDP-43, FUS and SOD1 mutants into cellular inclusions. Sci. Rep. 2015;5:13416. doi: 10.1038/srep13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnston J.A., Dalton M.J., Gurney M.E., Kopito R.R. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S., Kim H.J. Prion-like Mechanism in Amyotrophic Lateral Sclerosis: Are Protein Aggregates the Key? Exp. Neurobiol. 2015;24:1–7. doi: 10.5607/en.2015.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinder G.A., Lacourse M.C., Minotti S., Durham H.D. Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J. Biol. Chem. 2001;276:12791–12796. doi: 10.1074/jbc.M010759200. [DOI] [PubMed] [Google Scholar]
- 71.Silverman J.M., Fernando S.M., Grad L.I., Hill A.F., Turner B.J., Yerbury J.J., Cashman N.R. Disease Mechanisms in ALS: Misfolded SOD1 Transferred Through Exosome-Dependent and Exosome-Independent Pathways. Cell. Mol. Neurobiol. 2016;36:377–381. doi: 10.1007/s10571-015-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J., Xu G., Borchelt D.R. High molecular weight complexes of mutant superoxide dismutase 1: Age-dependent and tissue-specific accumulation. Neurobiol. Dis. 2002;9:139–148. doi: 10.1006/nbdi.2001.0471. [DOI] [PubMed] [Google Scholar]
- 73.Wang J., Xu G., Gonzales V., Coonfield M., Fromholt D., Copeland N.G., Jenkins N.A., Borchelt D.R. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol. Dis. 2002;10:128–138. doi: 10.1006/nbdi.2002.0498. [DOI] [PubMed] [Google Scholar]
- 74.Watson M.R., Lagow R.D., Xu K., Zhang B., Bonini N.M. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J. Biol. Chem. 2008;283:24972–24981. doi: 10.1074/jbc.M804817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang C., Liang W., Wang H., Yang Y., Wang T., Wang S., Wang X., Wang Y., Feng H. γ-Oryzanol mitigates oxidative stress and prevents mutant SOD1-Related neurotoxicity in Drosophila and cell models of amyotrophic lateral sclerosis. Neuropharmacology. 2019;160:107777. doi: 10.1016/j.neuropharm.2019.107777. [DOI] [PubMed] [Google Scholar]
- 76.Islam R., Kumimoto E.L., Bao H., Zhang B. ALS-linked SOD1 in glial cells enhances ß-N-Methylamino L-Alanine (BMAA)-induced toxicity in Drosophila. F1000Research. 2012;1:47. doi: 10.12688/f1000research.1-47.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallart-Palau X., Ng C.H., Ribera J., Sze S.K., Lim K.L. Drosophila expressing human SOD1 successfully recapitulates mitochondrial phenotypic features of familial amyotrophic lateral sclerosis. Neurosci. Lett. 2016;624:47–52. doi: 10.1016/j.neulet.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Gong Y.H., Parsadanian A.S., Andreeva A., Snider W.D., Elliott J.L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lino M.M., Schneider C., Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J. Neurosci. 2002;22:4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pramatarova A., Laganière J., Roussel J., Brisebois K., Rouleau G.A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumoto G., Stojanovic A., Holmberg C.I., Kim S., Morimoto R.I. Structural properties and neuronal toxicity of amyotrophic lateral sclerosis-associated Cu/Zn superoxide dismutase 1 aggregates. J. Cell Biol. 2005;171:75–85. doi: 10.1083/jcb.200504050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stathopulos P.B., Rumfeldt J.A., Scholz G.A., Irani R.A., Frey H.E., Hallewell R.A., Lepock J.R., Meiering E.M. Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc. Natl. Acad. Sci. USA. 2003;100:7021–7026. doi: 10.1073/pnas.1237797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pickles S., Vande Velde C. Misfolded SOD1 and ALS: Zeroing in on mitochondria. Amyotrophic lateral sclerosis: Official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. Amyotroph. Lateral Scler. 2012;13:333–340. doi: 10.3109/17482968.2012.648645. [DOI] [PubMed] [Google Scholar]
- 84.Sábado J., Casanovas A., Hernández S., Piedrafita L., Hereu M., Esquerda J.E. Immunodetection of disease-associated conformers of mutant cu/zn superoxide dismutase 1 selectively expressed in degenerating neurons in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2013;72:646–661. doi: 10.1097/NEN.0b013e318297fd10. [DOI] [PubMed] [Google Scholar]
- 85.Hetz C., Martinon F., Rodriguez D., Glimcher L.H. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 86.Jaronen M., Vehviläinen P., Malm T., Keksa-Goldsteine V., Pollari E., Valonen P., Koistinaho J., Goldsteins G. Protein disulfide isomerase in ALS mouse glia links protein misfolding with NADPH oxidase-catalyzed superoxide production. Hum. Mol. Genet. 2013;22:646–655. doi: 10.1093/hmg/dds472. [DOI] [PubMed] [Google Scholar]
- 87.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science (New York, NY) 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 89.Kalmar B., Greensmith L. Cellular Chaperones as Therapeutic Targets in ALS to Restore Protein Homeostasis and Improve Cellular Function. Front. Mol. Neurosci. 2017;10:251. doi: 10.3389/fnmol.2017.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin W., Kunkler P.E., Harding H.P., Ron D., Kraig R.P., Popko B. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma. Am. J. Pathol. 2008;173:1508–1517. doi: 10.2353/ajpath.2008.080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin W., Lin Y., Li J., Fenstermaker A.G., Way S.W., Clayton B., Jamison S., Harding H.P., Ron D., Popko B. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J. Neurosci. 2013;33:5980–5991. doi: 10.1523/JNEUROSCI.1636-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valenzuela V., Collyer E., Armentano D., Parsons G.B., Court F.A., Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gifondorwa D.J., Robinson M.B., Hayes C.D., Taylor A.R., Prevette D.M., Oppenheim R.W., Caress J., Milligan C.E. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2007;27:13173–13180. doi: 10.1523/JNEUROSCI.4057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spencer P.S., Nunn P.B., Hugon J., Ludolph A.C., Ross S.M., Roy D.N., Robertson R.C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 95.Ilieva H., Polymenidou M., Cleveland D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trotti D., Danbolt N.C., Volterra A. Glutamate transporters are oxidant-vulnerable: A molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol. Sci. 1998;19:328–334. doi: 10.1016/S0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 97.Gorąca A., Huk-Kolega H., Piechota A., Kleniewska P., Ciejka E., Skibska B. Lipoic acid—biological activity and therapeutic potential. Pharmacol. Rep. 2011;63:849–858. doi: 10.1016/S1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 98.Wang T., Cheng J., Wang S., Wang X., Jiang H., Yang Y., Wang Y., Zhang C., Liang W., Feng H. α-Lipoic acid attenuates oxidative stress and neurotoxicity via the ERK/Akt-dependent pathway in the mutant hSOD1 related Drosophila model and the NSC34 cell line of amyotrophic lateral sclerosis. Brain Res. Bull. 2018;140:299–310. doi: 10.1016/j.brainresbull.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 99.Juliano C., Cossu M., Alamanni M.C., Piu L. Antioxidant activity of gamma-oryzanol: Mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int. J. Pharm. 2005;299:146–154. doi: 10.1016/j.ijpharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 100.Van Mossevelde S., van der Zee J., Cruts M., van Broeckhoven C. Relationship between C9orf72 repeat size and clinical phenotype. Curr. Opin. Genet. Dev. 2017;44:117–124. doi: 10.1016/j.gde.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Belzil V.V., Bauer P.O., Prudencio M., Gendron T.F., Stetler C.T., Yan I.K., Pregent L., Daughrity L., Baker M.C., Rademakers R., et al. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 2013;126:895–905. doi: 10.1007/s00401-013-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frick P., Sellier C., Mackenzie I.R.A., Cheng C.Y., Tahraoui-Bories J., Martinat C., Pasterkamp R.J., Prudlo J., Edbauer D., Oulad-Abdelghani M., et al. Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers. Acta Neuropathol. Commun. 2018;6:72. doi: 10.1186/s40478-018-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waite A.J., Bäumer D., East S., Neal J., Morris H.R., Ansorge O., Blake D.J. Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol. Aging. 2014;35:1779. doi: 10.1016/j.neurobiolaging.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ciura S., Lattante S., Le Ber I., Latouche M., Tostivint H., Brice A., Kabashi E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:180–187. doi: 10.1002/ana.23946. [DOI] [PubMed] [Google Scholar]
- 105.Therrien M., Rouleau G.A., Dion P.A., Parker J.A. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS ONE. 2013;8:e83450. doi: 10.1371/journal.pone.0083450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Rourke J.G., Bogdanik L., Yáñez A., Lall D., Wolf A.J., Muhammad A.K., Ho R., Carmona S., Vit J.P., Zarrow J., et al. C9orf72 is required for proper macrophage and microglial function in mice. Science (New York, NY) 2016;351:1324–1329. doi: 10.1126/science.aaf1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu Q., Jiang J., Gendron T.F., McAlonis-Downes M., Jiang L., Taylor A., Diaz Garcia S., Ghosh Dastidar S., Rodriguez M.J., King P., et al. Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72. Nat. Neurosci. 2020;23:615–624. doi: 10.1038/s41593-020-0619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iyer S., Acharya K.R., Subramanian V. A comparative bioinformatic analysis of C9orf72. PeerJ. 2018;6:e4391. doi: 10.7717/peerj.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gendron T.F., Bieniek K.F., Zhang Y.J., Jansen-West K., Ash P.E., Caulfield T., Daughrity L., Dunmore J.H., Castanedes-Casey M., Chew J., et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodman L.D., Prudencio M., Kramer N.J., Martinez-Ramirez L.F., Srinivasan A.R., Lan M., Parisi M.J., Zhu Y., Chew J., Cook C.N., et al. Toxic expanded GGGGCC repeat transcription is mediated by the PAF1 complex in C9orf72-associated FTD. Nat. Neurosci. 2019;22:863–874. doi: 10.1038/s41593-019-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kramer N.J., Carlomagno Y., Zhang Y.J., Almeida S., Cook C.N., Gendron T.F., Prudencio M., van Blitterswijk M., Belzil V., Couthouis J., et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science (New York, NY) 2016;353:708–712. doi: 10.1126/science.aaf7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu Z., Poidevin M., Li X., Li Y., Shu L., Nelson D.L., Li H., Hales C.M., Gearing M., Wingo T.S., et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc. Natl. Acad. Sci. USA. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanai Y., Dohmae N., Hirokawa N. Kinesin transports RNA: Isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 114.Celona B., Dollen J.V., Vatsavayai S.C., Kashima R., Johnson J.R., Tang A.A., Hata A., Miller B.L., Huang E.J., Krogan N.J., et al. Suppression of C9orf72 RNA repeat-induced neurotoxicity by the ALS-associated RNA-binding protein Zfp106. eLife. 2017;6:e19032. doi: 10.7554/eLife.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang K., Donnelly C.J., Haeusler A.R., Grima J.C., Machamer J.B., Steinwald P., Daley E.L., Miller S.J., Cunningham K.M., Vidensky S., et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burguete A.S., Almeida S., Gao F.B., Kalb R., Akins M.R., Bonini N.M. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. eLife. 2015;4:e08881. doi: 10.7554/eLife.08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tran H., Almeida S., Moore J., Gendron T.F., Chalasani U., Lu Y., Du X., Nickerson J.A., Petrucelli L., Weng Z., et al. Differential Toxicity of Nuclear RNA Foci versus Dipeptide Repeat Proteins in a Drosophila Model of C9ORF72 FTD/ALS. Neuron. 2015;87:1207–1214. doi: 10.1016/j.neuron.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moens T.G., Mizielinska S., Niccoli T., Mitchell J.S., Thoeng A., Ridler C.E., Grönke S., Esser J., Heslegrave A., Zetterberg H., et al. Sense and antisense RNA are not toxic in Drosophila models of C9orf72-associated ALS/FTD. Acta Neuropathol. 2018;135:445–457. doi: 10.1007/s00401-017-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mori K., Arzberger T., Grässer F.A., Gijselinck I., May S., Rentzsch K., Weng S.M., Schludi M.H., van der Zee J., Cruts M., et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 2013;126:881–893. doi: 10.1007/s00401-013-1189-3. [DOI] [PubMed] [Google Scholar]
- 120.Mori K., Weng S.M., Arzberger T., May S., Rentzsch K., Kremmer E., Schmid B., Kretzschmar H.A., Cruts M., van Broeckhoven C., et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 121.Mizielinska S., Grönke S., Niccoli T., Ridler C.E., Clayton E.L., Devoy A., Moens T., Norona F.E., Woollacott I.O.C., Pietrzyk J., et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science (New York, NY) 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wen X., Tan W., Westergard T., Krishnamurthy K., Markandaiah S.S., Shi Y., Lin S., Shneider N.A., Monaghan J., Pandey U.B., et al. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron. 2014;84:1213–1225. doi: 10.1016/j.neuron.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Freibaum B.D., Lu Y., Lopez-Gonzalez R., Kim N.C., Almeida S., Lee K.H., Badders N., Valentine M., Miller B.L., Wong P.C., et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee K.H., Zhang P., Kim H.J., Mitrea D.M., Sarkar M., Freibaum B.D., Cika J., Coughlin M., Messing J., Molliex A., et al. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell. 2016;167:774–788. doi: 10.1016/j.cell.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perry S., Han Y., Das A., Dickman D. Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Hum. Mol. Genet. 2017;26:4153–4167. doi: 10.1093/hmg/ddx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang D., Abdallah A., Li Z., Lu Y., Almeida S., Gao F.B. FTD/ALS-associated poly(GR) protein impairs the Notch pathway and is recruited by poly(GA) into cytoplasmic inclusions. Acta Neuropathol. 2015;130:525–535. doi: 10.1007/s00401-015-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morón-Oset J., Supèr T., Esser J., Isaacs A.M., Grönke S., Partridge L. Glycine-alanine dipeptide repeats spread rapidly in a repeat length- and age-dependent manner in the fly brain. Acta Neuropathol. Commun. 2019;7:209. doi: 10.1186/s40478-019-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boeynaems S., Bogaert E., Michiels E., Gijselinck I., Sieben A., Jovičić A., de Baets G., Scheveneels W., Steyaert J., Cuijt I., et al. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci. Rep. 2016;6:20877. doi: 10.1038/srep20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moens T.G., Niccoli T., Wilson K.M., Atilano M.L., Birsa N., Gittings L.M., Holbling B.V., Dyson M.C., Thoeng A., Neeves J., et al. C9orf72 arginine-rich dipeptide proteins interact with ribosomal proteins in vivo to induce a toxic translational arrest that is rescued by eIF1A. Acta Neuropathol. 2019;137:487–500. doi: 10.1007/s00401-018-1946-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li S., Wu Z., Li Y., Tantray I., de Stefani D., Mattarei A., Krishnan G., Gao F.B., Vogel H., Lu B. Altered MICOS Morphology and Mitochondrial Ion Homeostasis Contribute to Poly(GR) Toxicity Associated with C9-ALS/FTD. Cell Rep. 2020;32:107989. doi: 10.1016/j.celrep.2020.107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simone R., Balendra R., Moens T.G., Preza E., Wilson K.M., Heslegrave A., Woodling N.S., Niccoli T., Gilbert-Jaramillo J., Abdelkarim S., et al. G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol. Med. 2018;10:22–31. doi: 10.15252/emmm.201707850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andersson M.K., Ståhlberg A., Arvidsson Y., Olofsson A., Semb H., Stenman G., Nilsson O., Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guerrero E.N., Wang H., Mitra J., Hegde P.M., Stowell S.E., Liachko N.F., Kraemer B.C., Garruto R.M., Rao K.S., Hegde M.L. TDP-43/FUS in motor neuron disease: Complexity and challenges. Prog. Neurobiol. 2016;145–146:78–97. doi: 10.1016/j.pneurobio.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang C., Zhou H., Tong J., Chen H., Liu Y.J., Wang D., Wei X., Xia X.G. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 2011;7:e1002011. doi: 10.1371/journal.pgen.1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naumann M., Pal A., Goswami A., Lojewski X., Japtok J., Vehlow A., Naujock M., Günther R., Jin M., Stanslowsky N., et al. Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat. Commun. 2018;9:335. doi: 10.1038/s41467-017-02299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lagier-Tourenne C., Polymenidou M., Hutt K.R., Vu A.Q., Baughn M., Huelga S.C., Clutario K.M., Ling S.C., Liang T.Y., Mazur C., et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rogelj B., Easton L.E., Bogu G.K., Stanton L.W., Rot G., Curk T., Zupan B., Sugimoto Y., Modic M., Haberman N., et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci. Rep. 2012;2:603. doi: 10.1038/srep00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan A.Y., Manley J.L. TLS inhibits RNA polymerase III transcription. Mol. Cell. Biol. 2010;30:186–196. doi: 10.1128/MCB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uranishi H., Tetsuka T., Yamashita M., Asamitsu K., Shimizu M., Itoh M., Okamoto T. Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator. J. Biol. Chem. 2001;276:13395–13401. doi: 10.1074/jbc.M011176200. [DOI] [PubMed] [Google Scholar]
- 140.Zinszner H., Sok J., Immanuel D., Yin Y., Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. Pt 15J. Cell Sci. 1997;110:1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]
- 141.Lagier-Tourenne C., Cleveland D.W. Rethinking ALS: The FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Da Cruz S., Cleveland D.W. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr. Opin. Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Corrado L., Del Bo R., Castellotti B., Ratti A., Cereda C., Penco S., Sorarù G., Carlomagno Y., Ghezzi S., Pensato V., et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J. Med. Genet. 2010;47:190–194. doi: 10.1136/jmg.2009.071027. [DOI] [PubMed] [Google Scholar]
- 144.Dormann D., Rodde R., Edbauer D., Bentmann E., Fischer I., Hruscha A., Than M.E., Mackenzie I.R., Capell A., Schmid B., et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dini Modigliani S., Morlando M., Errichelli L., Sabatelli M., Bozzoni I. An ALS-associated mutation in the FUS 3’-UTR disrupts a microRNA-FUS regulatory circuitry. Nat. Commun. 2014;5:4335. doi: 10.1038/ncomms5335. [DOI] [PubMed] [Google Scholar]
- 146.Sabatelli M., Moncada A., Conte A., Lattante S., Marangi G., Luigetti M., Lucchini M., Mirabella M., Romano A., Del Grande A., et al. Mutations in the 3’ untranslated region of FUS causing FUS overexpression are associated with amyotrophic lateral sclerosis. Hum. Mol. Genet. 2013;22:4748–4755. doi: 10.1093/hmg/ddt328. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y., Yang M., Deng J., Chen X., Ye Y., Zhu L., Liu J., Ye H., Shen Y., Li Y., et al. Expression of human FUS protein in Drosophila leads to progressive neurodegeneration. Protein Cell. 2011;2:477–486. doi: 10.1007/s13238-011-1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kabashi E., Bercier V., Lissouba A., Liao M., Brustein E., Rouleau G.A., Drapeau P. FUS and TARDBP but not SOD1 interact in genetic models of amyotrophic lateral sclerosis. PLoS Genet. 2011;7:e1002214. doi: 10.1371/journal.pgen.1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murakami T., Qamar S., Lin J.Q., Schierle G.S., Rees E., Miyashita A., Costa A.R., Dodd R.B., Chan F.T., Michel C.H., et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sasayama H., Shimamura M., Tokuda T., Azuma Y., Yoshida T., Mizuno T., Nakagawa M., Fujikake N., Nagai Y., Yamaguchi M. Knockdown of the Drosophila fused in sarcoma (FUS) homologue causes deficient locomotive behavior and shortening of motoneuron terminal branches. PLoS ONE. 2012;7:e39483. doi: 10.1371/journal.pone.0039483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou Y., Liu S., Liu G., Oztürk A., Hicks G.G. ALS-associated FUS mutations result in compromised FUS alternative splicing and autoregulation. PLoS Genet. 2013;9:e1003895. doi: 10.1371/journal.pgen.1003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bosco D.A., Lemay N., Ko H.K., Zhou H., Burke C., Kwiatkowski T.J., Jr., Sapp P., McKenna-Yasek D., Brown R.H., Jr., Hayward L.J. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Y.R., King O.D., Shorter J., Gitler A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun Z., Diaz Z., Fang X., Hart M.P., Chesi A., Shorter J., Gitler A.D. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011;9:e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bourefis A.R., Campanari M.L., Buee-Scherrer V., Kabashi E. Functional characterization of a FUS mutant zebrafish line as a novel genetic model for ALS. Neurobiol. Dis. 2020;142:104935. doi: 10.1016/j.nbd.2020.104935. [DOI] [PubMed] [Google Scholar]
- 156.Jackson K.L., Dhaibar H.A., Dayton R.D., Cananzi S.G., Mayhan W.G., Glasscock E., Klein R.L. Severe respiratory changes at end stage in a FUS-induced disease state in adult rats. BMC Neurosci. 2016;17:69. doi: 10.1186/s12868-016-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma A., Lyashchenko A.K., Lu L., Nasrabady S.E., Elmaleh M., Mendelsohn M., Nemes A., Tapia J.C., Mentis G.Z., Shneider N.A. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat. Commun. 2016;7:10465. doi: 10.1038/ncomms10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lanson N.A., Jr., Maltare A., King H., Smith R., Kim J.H., Taylor J.P., Lloyd T.E., Pandey U.B. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum. Mol. Genet. 2011;20:2510–2523. doi: 10.1093/hmg/ddr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang J.W., Brent J.R., Tomlinson A., Shneider N.A., McCabe B.D. The ALS-associated proteins FUS and TDP-43 function together to affect Drosophila locomotion and life span. J. Clin. Investig. 2011;121:4118–4126. doi: 10.1172/JCI57883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xia R., Liu Y., Yang L., Gal J., Zhu H., Jia J. Motor neuron apoptosis and neuromuscular junction perturbation are prominent features in a Drosophila model of Fus-mediated ALS. Mol. Neurodegener. 2012;7:10. doi: 10.1186/1750-1326-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bogaert E., Boeynaems S., Kato M., Guo L., Caulfield T.R., Steyaert J., Scheveneels W., Wilmans N., Haeck W., Hersmus N., et al. Molecular Dissection of FUS Points at Synergistic Effect of Low-Complexity Domains in Toxicity. Cell Rep. 2018;24:529–537. doi: 10.1016/j.celrep.2018.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miguel L., Avequin T., Delarue M., Feuillette S., Frébourg T., Campion D., Lecourtois M. Accumulation of insoluble forms of FUS protein correlates with toxicity in Drosophila. Neurobiol. Aging. 2012;33:1008. doi: 10.1016/j.neurobiolaging.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 163.Deng J., Yang M., Chen Y., Chen X., Liu J., Sun S., Cheng H., Li Y., Bigio E.H., Mesulam M., et al. FUS Interacts with HSP60 to Promote Mitochondrial Damage. PLoS Genet. 2015;11:e1005357. doi: 10.1371/journal.pgen.1005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jäckel S., Summerer A.K., Thömmes C.M., Pan X., Voigt A., Schulz J.B., Rasse T.M., Dormann D., Haass C., Kahle P.J. Nuclear import factor transportin and arginine methyltransferase 1 modify FUS neurotoxicity in Drosophila. Neurobiol. Dis. 2015;74:76–88. doi: 10.1016/j.nbd.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Daigle J.G., Lanson N.A., Jr., Smith R.B., Casci I., Maltare A., Monaghan J., Nichols C.D., Kryndushkin D., Shewmaker F., Pandey U.B. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 2013;22:1193–1205. doi: 10.1093/hmg/dds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Steyaert J., Scheveneels W., Vanneste J., van Damme P., Robberecht W., Callaerts P., Bogaert E., van den Bosch L. FUS-induced neurotoxicity in Drosophila is prevented by downregulating nucleocytoplasmic transport proteins. Hum. Mol. Genet. 2018;27:4103–4116. doi: 10.1093/hmg/ddy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gogia N., Sarkar A., Mehta A.S., Ramesh N., Deshpande P., Kango-Singh M., Pandey U.B., Singh A. Inactivation of Hippo and cJun-N-terminal Kinase (JNK) signaling mitigate FUS mediated neurodegeneration in vivo. Neurobiol. Dis. 2020;140:104837. doi: 10.1016/j.nbd.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Machamer J.B., Collins S.E., Lloyd T.E. The ALS gene FUS regulates synaptic transmission at the Drosophila neuromuscular junction. Hum. Mol. Genet. 2014;23:3810–3822. doi: 10.1093/hmg/ddu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shahidullah M., Le Marchand S.J., Fei H., Zhang J., Pandey U.B., Dalva M.B., Pasinelli P., Levitan I.B. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. J. Neurosci. 2013;33:19590–19598. doi: 10.1523/JNEUROSCI.3396-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ito D., Seki M., Tsunoda Y., Uchiyama H., Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann. Neurol. 2011;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- 171.Kino Y., Washizu C., Aquilanti E., Okuno M., Kurosawa M., Yamada M., Doi H., Nukina N. Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res. 2011;39:2781–2798. doi: 10.1093/nar/gkq1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee B.J., Cansizoglu A.E., Süel K.E., Louis T.H., Zhang Z., Chook Y.M. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126:543–558. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stolow D.T., Haynes S.R. Cabeza, a Drosophila gene encoding a novel RNA binding protein, shares homology with EWS and TLS, two genes involved in human sarcoma formation. Nucleic Acids Res. 1995;23:835–843. doi: 10.1093/nar/23.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xia Q., Wang H., Hao Z., Fu C., Hu Q., Gao F., Ren H., Chen D., Han J., Ying Z., et al. TDP-43 loss of function increases TFEB activity and blocks autophagosome-lysosome fusion. EMBO J. 2016;35:121–142. doi: 10.15252/embj.201591998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frickenhaus M., Wagner M., Mallik M., Catinozzi M., Storkebaum E. Highly efficient cell-type-specific gene inactivation reveals a key function for the Drosophila FUS homolog cabeza in neurons. Sci. Rep. 2015;5:9107. doi: 10.1038/srep09107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shimamura M., Kyotani A., Azuma Y., Yoshida H., Binh Nguyen T., Mizuta I., Yoshida T., Mizuno T., Nakagawa M., Tokuda T., et al. Genetic link between Cabeza, a Drosophila homologue of Fused in Sarcoma (FUS), and the EGFR signaling pathway. Exp. Cell Res. 2014;326:36–45. doi: 10.1016/j.yexcr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 177.Azuma Y., Tokuda T., Kushimura Y., Yamamoto I., Mizuta I., Mizuno T., Nakagawa M., Ueyama M., Nagai Y., Iwasaki Y., et al. Hippo, Drosophila MST, is a novel modifier of motor neuron degeneration induced by knockdown of Caz, Drosophila FUS. Exp. Cell Res. 2018;371:311–321. doi: 10.1016/j.yexcr.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 178.Azuma Y., Tokuda T., Shimamura M., Kyotani A., Sasayama H., Yoshida T., Mizuta I., Mizuno T., Nakagawa M., Fujikake N., et al. Identification of ter94, Drosophila VCP, as a strong modulator of motor neuron degeneration induced by knockdown of Caz, Drosophila FUS. Hum. Mol. Genet. 2014;23:3467–3480. doi: 10.1093/hmg/ddu055. [DOI] [PubMed] [Google Scholar]
- 179.Lo Piccolo L., Jantrapirom S., Nagai Y., Yamaguchi M. FUS toxicity is rescued by the modulation of lncRNA hsrω expression in Drosophila melanogaster. Sci. Rep. 2017;7:15660. doi: 10.1038/s41598-017-15944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wakisaka K.T., Tanaka R., Hirashima T., Muraoka Y., Azuma Y., Yoshida H., Tokuda T., Asada S., Suda K., Ichiyanagi K., et al. Novel roles of Drosophila FUS and Aub responsible for piRNA biogenesis in neuronal disorders. Brain Res. 2019;1708:207–219. doi: 10.1016/j.brainres.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 181.Machamer J.B., Woolums B.M., Fuller G.G., Lloyd T.E. FUS causes synaptic hyperexcitability in Drosophila dendritic arborization neurons. Brain Res. 2018;1693:55–66. doi: 10.1016/j.brainres.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hirano A., Nakano I., Kurland L.T., Mulder D.W., Holley P.W., Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 1984;43:471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 183.Sasaki S., Horie Y., Iwata M. Mitochondrial alterations in dorsal root ganglion cells in sporadic amyotrophic lateral sclerosis. Acta Neuropathol. 2007;114:633–639. doi: 10.1007/s00401-007-0299-1. [DOI] [PubMed] [Google Scholar]
- 184.Smith E.F., Shaw P.J., de Vos K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019;710:132933. doi: 10.1016/j.neulet.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 185.Altanbyek V., Cha S.J., Kang G.U., Im D.S., Lee S., Kim H.J., Kim K. Imbalance of mitochondrial dynamics in Drosophila models of amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2016;481:259–264. doi: 10.1016/j.bbrc.2016.10.134. [DOI] [PubMed] [Google Scholar]
- 186.Chen Y., Deng J., Wang P., Yang M., Chen X., Zhu L., Liu J., Lu B., Shen Y., Fushimi K., et al. PINK1 and Parkin are genetic modifiers for FUS-induced neurodegeneration. Hum. Mol. Genet. 2016;25:5059–5068. doi: 10.1093/hmg/ddw310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mayer M.P. Gymnastics of molecular chaperones. Mol. Cell. 2010;39:321–331. doi: 10.1016/j.molcel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 188.Huang S. Review: Perinucleolar structures. J. Struct. Biol. 2000;129:233–240. doi: 10.1006/jsbi.2000.4247. [DOI] [PubMed] [Google Scholar]
- 189.Mao Y.S., Zhang B., Spector D.L. Biogenesis and function of nuclear bodies. Trends Genet. 2011;27:295–306. doi: 10.1016/j.tig.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meng F., Na I., Kurgan L., Uversky V.N. Compartmentalization and Functionality of Nuclear Disorder: Intrinsic Disorder and Protein-Protein Interactions in Intra-Nuclear Compartments. Int. J. Mol. Sci. 2015;17:24. doi: 10.3390/ijms17010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ninomiya K., Hirose T. Short Tandem Repeat-Enriched Architectural RNAs in Nuclear Bodies: Functions and Associated Diseases. Non-Coding RNA. 2020;6:6. doi: 10.3390/ncrna6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chujo T., Yamazaki T., Hirose T. Architectural RNAs (arcRNAs): A class of long noncoding RNAs that function as the scaffold of nuclear bodies. Biochim. Biophys. Acta. 2016;1859:139–146. doi: 10.1016/j.bbagrm.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 193.Sun Q., Hao Q., Prasanth K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shelkovnikova T.A., Robinson H.K., Troakes C., Ninkina N., Buchman V.L. Compromised paraspeckle formation as a pathogenic factor in FUSopathies. Hum. Mol. Genet. 2014;23:2298–2312. doi: 10.1093/hmg/ddt622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jolly C., Lakhotia S.C. Human sat III and Drosophila hsr omega transcripts: A common paradigm for regulation of nuclear RNA processing in stressed cells. Nucleic Acids Res. 2006;34:5508–5514. doi: 10.1093/nar/gkl711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Onorati M.C., Lazzaro S., Mallik M., Ingrassia A.M., Carreca A.P., Singh A.K., Chaturvedi D.P., Lakhotia S.C., Corona D.F. The ISWI chromatin remodeler organizes the hsrω ncRNA-containing omega speckle nuclear compartments. PLoS Genet. 2011;7:e1002096. doi: 10.1371/journal.pgen.1002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Piccolo L.L., Corona D., Onorati M.C. Emerging roles for hnRNPs in post-transcriptional regulation: What can we learn from flies? Chromosoma. 2014;123:515–527. doi: 10.1007/s00412-014-0470-0. [DOI] [PubMed] [Google Scholar]
- 198.Prasanth K.V., Rajendra T.K., Lal A.K., Lakhotia S.C. Omega speckles—A novel class of nuclear speckles containing hnRNPs associated with noncoding hsr-omega RNA in Drosophila. Pt 19J. Cell Sci. 2000;113:3485–3497. doi: 10.1242/jcs.113.19.3485. [DOI] [PubMed] [Google Scholar]
- 199.Singh A.K., Lakhotia S.C. Dynamics of hnRNPs and omega speckles in normal and heat shocked live cell nuclei of Drosophila melanogaster. Chromosoma. 2015;124:367–383. doi: 10.1007/s00412-015-0506-0. [DOI] [PubMed] [Google Scholar]
- 200.Terasawa K., Tomabechi Y., Ikeda M., Ehara H., Kukimoto-Niino M., Wakiyama M., Podyma-Inoue K.A., Rajapakshe A.R., Watabe T., Shirouzu M., et al. Lysosome-associated membrane proteins-1 and -2 (LAMP-1 and LAMP-2) assemble via distinct modes. Biochem. Biophys. Res. Commun. 2016;479:489–495. doi: 10.1016/j.bbrc.2016.09.093. [DOI] [PubMed] [Google Scholar]
- 201.Burke K.A., Janke A.M., Rhine C.L., Fawzi N.L. Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol. Cell. 2015;60:231–241. doi: 10.1016/j.molcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Han T.W., Kato M., Xie S., Wu L.C., Mirzaei H., Pei J., Chen M., Xie Y., Allen J., Xiao G., et al. Cell-free formation of RNA granules: Bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 203.Kato M., Han T.W., Xie S., Shi K., Du X., Wu L.C., Mirzaei H., Goldsmith E.J., Longgood J., Pei J., et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lin Y., Protter D.S., Rosen M.K., Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matsumoto T., Matsukawa K., Watanabe N., Kishino Y., Kunugi H., Ihara R., Wakabayashi T., Hashimoto T., Iwatsubo T. Self-assembly of FUS through its low-complexity domain contributes to neurodegeneration. Hum. Mol. Genet. 2018;27:1353–1365. doi: 10.1093/hmg/ddy046. [DOI] [PubMed] [Google Scholar]
- 206.Guo L., Kim H.J., Wang H., Monaghan J., Freyermuth F., Sung J.C., O’Donovan K., Fare C.M., Diaz Z., Singh N., et al. Nuclear-Import Receptors Reverse Aberrant Phase Transitions of RNA-Binding Proteins with Prion-like Domains. Cell. 2018;173:677–692. doi: 10.1016/j.cell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Couthouis J., Hart M.P., Shorter J., DeJesus-Hernandez M., Erion R., Oristano R., Liu A.X., Ramos D., Jethava N., Hosangadi D., et al. A yeast functional screen predicts new candidate ALS disease genes. Proc. Natl. Acad. Sci. USA. 2011;108:20881–20890. doi: 10.1073/pnas.1109434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hennig S., Kong G., Mannen T., Sadowska A., Kobelke S., Blythe A., Knott G.J., Iyer K.S., Ho D., Newcombe E.A., et al. Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles. J. Cell Biol. 2015;210:529–539. doi: 10.1083/jcb.201504117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schwartz J.C., Wang X., Podell E.R., Cech T.R. RNA seeds higher-order assembly of FUS protein. Cell Rep. 2013;5:918–925. doi: 10.1016/j.celrep.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boeynaems S., Alberti S., Fawzi N.L., Mittag T., Polymenidou M., Rousseau F., Schymkowitz J., Shorter J., Wolozin B., van den Bosch L., et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018;28:420–435. doi: 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., Mittag T., Taylor J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Patel A., Lee H.O., Jawerth L., Maharana S., Jahnel M., Hein M.Y., Stoynov S., Mahamid J., Saha S., Franzmann T.M., et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 213.March Z.M., King O.D., Shorter J. Prion-like domains as epigenetic regulators, scaffolds for subcellular organization, and drivers of neurodegenerative disease. Brain Res. 2016;1647:9–18. doi: 10.1016/j.brainres.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dudman J., Qi X. Stress Granule Dysregulation in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2020;14:598517. doi: 10.3389/fncel.2020.598517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hofmann S., Kedersha N., Anderson P., Ivanov P. Molecular mechanisms of stress granule assembly and disassembly. Biochim. Biophys. Acta. Mol. Cell Res. 2021;1868:118876. doi: 10.1016/j.bbamcr.2020.118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wolozin B., Ivanov P. Stress granules and neurodegeneration. Nature Rev. Neurosci. 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang X., Wang F., Hu Y., Chen R., Meng D., Guo L., Lv H., Guan J., Jia Y. In vivo stress granule misprocessing evidenced in a FUS knock-in ALS mouse model. Brain. 2020;143:1350–1367. doi: 10.1093/brain/awaa076. [DOI] [PubMed] [Google Scholar]
- 218.Ratovitski T., O’Meally R.N., Jiang M., Chaerkady R., Chighladze E., Stewart J.C., Wang X., Arbez N., Roby E., Alexandris A., et al. Post-Translational Modifications (PTMs), Identified on Endogenous Huntingtin, Cluster within Proteolytic Domains between HEAT Repeats. J. Proteome Res. 2017;16:2692–2708. doi: 10.1021/acs.jproteome.6b00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barrett P.J., Timothy G.J. Post-translational modification of α-synuclein in Parkinson’s disease. Brain Res. 2015;1628:247–253. doi: 10.1016/j.brainres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 220.Correia S.C., Carvalho C., Cardoso S., Moreira P.I. Post-translational modifications in brain health and disease. Biochim. Biophys. Acta. Mol. Basis dis. 2019;1865:1947–1948. doi: 10.1016/j.bbadis.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 221.Kurtishi A., Rosen B., Patil K.S., Alves G.W., Møller S.G. Cellular Proteostasis in Neurodegeneration. Mol. Neurobiol. 2019;56:3676–3689. doi: 10.1007/s12035-018-1334-z. [DOI] [PubMed] [Google Scholar]
- 222.Wang Y., Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 223.Rhoads S.N., Monahan Z.T., Yee D.S., Shewmaker F.P. The Role of Post-Translational Modifications on Prion-Like Aggregation and Liquid-Phase Separation of FUS. Int. J. Mol. Sci. 2018;19:886. doi: 10.3390/ijms19030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xue Y.C., Ng C.S., Xiang P., Liu H., Zhang K., Mohamud Y., Luo H. Dysregulation of RNA-Binding Proteins in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2020;13:78. doi: 10.3389/fnmol.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dormann D., Madl T., Valori C.F., Bentmann E., Tahirovic S., Abou-Ajram C., Kremmer E., Ansorge O., Mackenzie I.R., Neumann M., et al. Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS. EMBO J. 2012;31:4258–4275. doi: 10.1038/emboj.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hofweber M., Hutten S., Bourgeois B., Spreitzer E., Niedner-Boblenz A., Schifferer M., Ruepp M.D., Simons M., Niessing D., Madl T., et al. Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation. Cell. 2018;173:706–719. doi: 10.1016/j.cell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 227.Qamar S., Wang G., Randle S.J., Ruggeri F.S., Varela J.A., Lin J.Q., Phillips E.C., Miyashita A., Williams D., Ströhl F., et al. FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell. 2018;173:720–734. doi: 10.1016/j.cell.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Boulanger M.C., Miranda T.B., Clarke S., di Fruscio M., Suter B., Lasko P., Richard S. Characterization of the Drosophila protein arginine methyltransferases DART1 and DART4. Biochem. J. 2004;379:283–289. doi: 10.1042/bj20031176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scaramuzzino C., Casci I., Parodi S., Lievens P.M.J., Polanco M.J., Milioto C., Chivet M., Monaghan J., Mishra A., Badders N., et al. Protein arginine methyltransferase 6 enhances polyglutamine-expanded androgen receptor function and toxicity in spinal and bulbar muscular atrophy. Neuron. 2015;85:88–100. doi: 10.1016/j.neuron.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang Y.C., Li C. Evolutionarily conserved protein arginine methyltransferases in non-mammalian animal systems. FEBS J. 2012;279:932–945. doi: 10.1111/j.1742-4658.2012.08490.x. [DOI] [PubMed] [Google Scholar]
- 231.Lo Piccolo L., Mochizuki H., Nagai Y. The lncRNA hsrω regulates arginine dimethylation of human FUS to cause its proteasomal degradation in Drosophila. J. Cell Sci. 2019;132:jcs236836. doi: 10.1242/jcs.236836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Braun R.J., Zischka H. Mechanisms of Cdc48/VCP-mediated cell death: From yeast apoptosis to human disease. Biochim. Biophys. Acta. 2008;1783:1418–1435. doi: 10.1016/j.bbamcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 233.Kobayashi H., Tomari Y. Identification of an AGO (Argonaute) protein as a prey of TER94/VCP. Autophagy. 2020;16:190–192. doi: 10.1080/15548627.2019.1691351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Meyer H., Bug M., Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nature Cell Biol. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 235.Johnson J.O., Mandrioli J., Benatar M., Abramzon Y., van Deerlin V.M., Trojanowski J.Q., Gibbs J.R., Brunetti M., Gronka S., Wuu J., et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Harley J., Hagemann C., Serio A., Patani R. FUS is lost from nuclei and gained in neurites of motor neurons in a human stem cell model of VCP-related ALS. Brain. 2020;143:e103. doi: 10.1093/brain/awaa339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tyzack G.E., Luisier R., Taha D.M., Neeves J., Modic M., Mitchell J.S., Meyer I., Greensmith L., Newcombe J., Ule J., et al. Widespread FUS mislocalization is a molecular hallmark of amyotrophic lateral sclerosis. Brain. 2019;142:2572–2580. doi: 10.1093/brain/awz217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Baillon L., Germani F., Rockel C., Hilchenbach J., Basler K. Xrp1 is a transcription factor required for cell competition-driven elimination of loser cells. Sci. Rep. 2018;8:17712. doi: 10.1038/s41598-018-36277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Reeves R. Nuclear functions of the HMG proteins. Biochim. Biophys. Acta. 2010;1799:3–14. doi: 10.1016/j.bbagrm.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mallik M., Catinozzi M., Hug C.B., Zhang L., Wagner M., Bussmann J., Bittern J., Mersmann S., Klämbt C., Drexler H.C.A., et al. Xrp1 genetically interacts with the ALS-associated FUS orthologue caz and mediates its toxicity. J. Cell Biol. 2018;217:3947–3964. doi: 10.1083/jcb.201802151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Iwasaki Y.W., Murano K., Ishizu H., Shibuya A., Iyoda Y., Siomi M.C., Siomi H., Saito K. Piwi Modulates Chromatin Accessibility by Regulating Multiple Factors Including Histone H1 to Repress Transposons. Mol. Cell. 2016;63:408–419. doi: 10.1016/j.molcel.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 242.Le T.A., Rogers A.K., Webster A., Marinov G.K., Liao S.E., Perkins E.M., Hur J.K., Aravin A.A., Tóth K.F. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 2013;27:390–399. doi: 10.1101/gad.209841.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yamanaka S., Siomi M.C., Siomi H. piRNA clusters and open chromatin structure. Mob. DNA. 2014;5:22. doi: 10.1186/1759-8753-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ghildiyal M., Seitz H., Horwich M.D., Li C., Du T., Lee S., Xu J., Kittler E.L., Zapp M.L., Weng Z., et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science (New York, NY) 2008;320:1077–1081. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Perrat P.N., DasGupta S., Wang J., Theurkauf W., Weng Z., Rosbash M., Waddell S. Transposition-driven genomic heterogeneity in the Drosophila brain. Science (New York, NY) 2013;340:91–95. doi: 10.1126/science.1231965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ma X., Chen Y., Xu W., Wu N., Li M., Cao Y., Wu S., Li Q., Xue L. Impaired Hippo signaling promotes Rho1-JNK-dependent growth. Proc. Natl. Acad. Sci. USA. 2015;112:1065–1070. doi: 10.1073/pnas.1415020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ma X., Wang H., Ji J., Xu W., Sun Y., Li W., Zhang X., Chen J., Xue L. Hippo signaling promotes JNK-dependent cell migration. Proc. Natl. Acad. Sci. USA. 2017;114:1934–1939. doi: 10.1073/pnas.1621359114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kango-Singh M., Singh A. Regulation of organ size: Insights from the Drosophila Hippo signaling pathway. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009;238:1627–1637. doi: 10.1002/dvdy.21996. [DOI] [PubMed] [Google Scholar]
- 250.Manning S.A., Kroeger B., Harvey K.F. The regulation of Yorkie, YAP and TAZ: New insights into the Hippo pathway. Development (Cambridge, UK) 2020;147 doi: 10.1242/dev.179069. [DOI] [PubMed] [Google Scholar]
- 251.Sahu M.R., Mondal A.C. The emerging role of Hippo signaling in neurodegeneration. J. Neurosci. Res. 2020;98:796–814. doi: 10.1002/jnr.24551. [DOI] [PubMed] [Google Scholar]
- 252.Brunet M.A., Jacques J.F., Nassari S., Tyzack G.E., McGoldrick P., Zinman L., Jean S., Robertson J., Patani R., Roucou X. The FUS gene is dual-coding with both proteins contributing to FUS-mediated toxicity. EMBO Rep. 2020;22:e50640. doi: 10.15252/embr.202050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 254.Gitcho M.A., Baloh R.H., Chakraverty S., Mayo K., Norton J.B., Levitch D., Hatanpaa K.J., White C.L., 3rd, Bigio E.H., Caselli R., et al. TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C., Bouchard J.P., Lacomblez L., Pochigaeva K., Salachas F., et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 256.Rutherford N.J., Zhang Y.J., Baker M., Gass J.M., Finch N.A., Xu Y.F., Stewart H., Kelley B.J., Kuntz K., Crook R.J., et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science (New York, NY) 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Van Deerlin V.M., Leverenz J.B., Bekris L.M., Bird T.D., Yuan W., Elman L.B., Clay D., Wood E.M., Chen-Plotkin A.S., Martinez-Lage M., et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: A genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yokoseki A., Shiga A., Tan C.F., Tagawa A., Kaneko H., Koyama A., Eguchi H., Tsujino A., Ikeuchi T., Kakita A., et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann. Neurol. 2008;63:538–542. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- 260.Kapeli K., Martinez F.J., Yeo G.W. Genetic mutations in RNA-binding proteins and their roles in ALS. Human Genet. 2017;136:1193–1214. doi: 10.1007/s00439-017-1830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Picher-Martel V., Valdmanis P.N., Gould P.V., Julien J.P., Dupré N. From animal models to human disease: A genetic approach for personalized medicine in ALS. Acta Neuropathol. Commun. 2016;4:70. doi: 10.1186/s40478-016-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Buratti E., Baralle F.E. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010;7:420–429. doi: 10.4161/rna.7.4.12205. [DOI] [PubMed] [Google Scholar]
- 263.Suk T.R., Rousseaux M.W.C. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Molecular Neurodegener. 2020;15:45. doi: 10.1186/s13024-020-00397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang H.Y., Wang I.F., Bose J., Shen C.K. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/S0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 265.Lin M.J., Cheng C.W., Shen C.K. Neuronal function and dysfunction of Drosophila dTDP. PLoS ONE. 2011;6:e20371. doi: 10.1371/journal.pone.0020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Diaper D.C., Adachi Y., Sutcliffe B., Humphrey D.M., Elliott C.J., Stepto A., Ludlow Z.N., Vanden B.L., Callaerts P., Dermaut B., et al. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum. Mol. Genet. 2013;22:1539–1557. doi: 10.1093/hmg/ddt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Feiguin F., Godena V.K., Romano G., D’Ambrogio A., Klima R., Baralle F.E. Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotive behavior. FEBS Lett. 2009;583:1586–1592. doi: 10.1016/j.febslet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 268.Fiesel F.C., Voigt A., Weber S.S., van den Haute C., Waldenmaier A., Görner K., Walter M., Anderson M.L., Kern J.V., Rasse T.M., et al. Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. EMBO J. 2010;29:209–221. doi: 10.1038/emboj.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kushimura Y., Tokuda T., Azuma Y., Yamamoto I., Mizuta I., Mizuno T., Nakagawa M., Ueyama M., Nagai Y., Yoshida H., et al. Overexpression of ter94, Drosophila VCP, improves motor neuron degeneration induced by knockdown of TBPH, Drosophila TDP-43. Am. J. Neurodegener. Dis. 2018;7:11–31. [PMC free article] [PubMed] [Google Scholar]
- 270.Diaper D.C., Adachi Y., Lazarou L., Greenstein M., Simoes F.A., di Domenico A., Solomon D.A., Lowe S., Alsubaie R., Cheng D., et al. Drosophila TDP-43 dysfunction in glia and muscle cells cause cytological and behavioural phenotypes that characterize ALS and FTLD. Hum. Mol. Genet. 2013;22:3883–3893. doi: 10.1093/hmg/ddt243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Romano G., Appocher C., Scorzeto M., Klima R., Baralle F.E., Megighian A., Feiguin F. Glial TDP-43 regulates axon wrapping, GluRIIA clustering and fly motility by autonomous and non-autonomous mechanisms. Hum. Mol. Genet. 2015;24:6134–6145. doi: 10.1093/hmg/ddv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Llamusi B., Bargiela A., Fernandez-Costa J.M., Garcia-Lopez A., Klima R., Feiguin F., Artero R. Muscleblind, BSF and TBPH are mislocalized in the muscle sarcomere of a Drosophila myotonic dystrophy model. Dis. Models Mech. 2013;6:184–196. doi: 10.1242/dmm.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang S.J.H., Sinclair D.A.R., Kim H.Y., Kinsey S.D., Yoo B., Shih C.R.Y., Wong K.K.L., Krieger C., Harden N., Verheyen E.M. Homeodomain-interacting protein kinase (Hipk) plays roles in nervous system and muscle structure and function. PLoS ONE. 2020;15:e0221006. doi: 10.1371/journal.pone.0221006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lu Y., Ferris J., Gao F.B. Frontotemporal dementia and amyotrophic lateral sclerosis-associated disease protein TDP-43 promotes dendritic branching. Mol. Brain. 2009;2:30. doi: 10.1186/1756-6606-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hazelett D.J., Chang J.C., Lakeland D.L., Morton D.B. Comparison of parallel high-throughput RNA sequencing between knockout of TDP-43 and its overexpression reveals primarily nonreciprocal and nonoverlapping gene expression changes in the central nervous system of Drosophila. G3 Genes Genomes Genet. 2012;2:789–802. doi: 10.1534/g3.112.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vanden B.L., Naval-Sánchez M., Adachi Y., Diaper D., Dourlen P., Chapuis J., Kleinberger G., Gistelinck M., van Broeckhoven C., Lambert J.C., et al. TDP-43 loss-of-function causes neuronal loss due to defective steroid receptor-mediated gene program switching in Drosophila. Cell Rep. 2013;3:160–172. doi: 10.1016/j.celrep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 277.Chang J.C., Hazelett D.J., Stewart J.A., Morton D.B. Motor neuron expression of the voltage-gated calcium channel cacophony restores locomotion defects in a Drosophila, TDP-43 loss of function model of ALS. Brain Res. 2014;1584:39–51. doi: 10.1016/j.brainres.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lembke K.M., Scudder C., Morton D.B. Restoration of Motor Defects Caused by Loss of Drosophila TDP-43 by Expression of the Voltage-Gated Calcium Channel, Cacophony, in Central Neurons. J. Neurosci. 2017;37:9486–9497. doi: 10.1523/JNEUROSCI.0554-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Godena V.K., Romano G., Romano M., Appocher C., Klima R., Buratti E., Baralle F.E., Feiguin F. TDP-43 regulates Drosophila neuromuscular junctions growth by modulating Futsch/MAP1B levels and synaptic microtubules organization. PLoS ONE. 2011;6:e17808. doi: 10.1371/journal.pone.0017808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Donde A., Sun M., Ling J.P., Braunstein K.E., Pang B., Wen X., Cheng X., Chen L., Wong P.C. Splicing repression is a major function of TDP-43 in motor neurons. Acta Neuropathol. 2019;138:813–826. doi: 10.1007/s00401-019-02042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Deshpande M., Feiger Z., Shilton A.K., Luo C.C., Silverman E., Rodal A.A. Role of BMP receptor traffic in synaptic growth defects in an ALS model. Mol. Biol. Cell. 2016;27:2898–2910. doi: 10.1091/mbc.E16-07-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Strah N., Romano G., Introna C., Klima R., Marzullo M., Ciapponi L., Megighian A., Nizzardo M., Feiguin F. TDP-43 promotes the formation of neuromuscular synapses through the regulation of Disc-large expression in Drosophila skeletal muscles. BMC Biol. 2020;18:34. doi: 10.1186/s12915-020-00767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Neumann M., Kwong L.K., Truax A.C., Vanmassenhove B., Kretzschmar H.A., van Deerlin V.M., Clark C.M., Grossman M., Miller B.L., Trojanowski J.Q., et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J. Neuropathol. Exp. Neurol. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 284.Nishihira Y., Tan C.F., Onodera O., Toyoshima Y., Yamada M., Morita T., Nishizawa M., Kakita A., Takahashi H. Sporadic amyotrophic lateral sclerosis: Two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol. 2008;116:169–182. doi: 10.1007/s00401-008-0385-z. [DOI] [PubMed] [Google Scholar]
- 285.Zhang H., Tan C.F., Mori F., Tanji K., Kakita A., Takahashi H., Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;115:115–122. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 286.Romano G., Klima R., Feiguin F. TDP-43 prevents retrotransposon activation in the Drosophila motor system through regulation of Dicer-2 activity. BMC Biol. 2020;18:82. doi: 10.1186/s12915-020-00816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Li Z., Lu Y., Xu X.L., Gao F.B. The FTD/ALS-associated RNA-binding protein TDP-43 regulates the robustness of neuronal specification through microRNA-9a in Drosophila. Hum. Mol. Genet. 2013;22:218–225. doi: 10.1093/hmg/dds420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Romano G., Klima R., Buratti E., Verstreken P., Baralle F.E., Feiguin F. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiol. Dis. 2014;71:95–109. doi: 10.1016/j.nbd.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 289.Estes P.S., Boehringer A., Zwick R., Tang J.E., Grigsby B., Zarnescu D.C. Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Hum. Mol. Genet. 2011;20:2308–2321. doi: 10.1093/hmg/ddr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Li Y., Ray P., Rao E.J., Shi C., Guo W., Chen X., Woodruff E.A., 3rd, Fushimi K., Wu J.Y. A Drosophila model for TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sreedharan J., Neukomm L.J., Brown R.H., Jr., Freeman M.R. Age-Dependent TDP-43-Mediated Motor Neuron Degeneration Requires GSK3, hat-trick, and xmas-2. Curr. Biol. 2015;25:2130–2136. doi: 10.1016/j.cub.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ritson G.P., Custer S.K., Freibaum B.D., Guinto J.B., Geffel D., Moore J., Tang W., Winton M.J., Neumann M., Trojanowski J.Q., et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J. Neurosci. 2010;30:7729–7739. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ihara R., Matsukawa K., Nagata Y., Kunugi H., Tsuji S., Chihara T., Kuranaga E., Miura M., Wakabayashi T., Hashimoto T., et al. RNA binding mediates neurotoxicity in the transgenic Drosophila model of TDP-43 proteinopathy. Hum. Mol. Genet. 2013;22:4474–4484. doi: 10.1093/hmg/ddt296. [DOI] [PubMed] [Google Scholar]
- 294.Li Y., Wang F., Lee J.A., Gao F.B. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev. 2006;20:2793–2805. doi: 10.1101/gad.1466306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.King I.N., Yartseva V., Salas D., Kumar A., Heidersbach A., Ando D.M., Stallings N.R., Elliott J.L., Srivastava D., Ivey K.N. The RNA-binding protein TDP-43 selectively disrupts microRNA-1/206 incorporation into the RNA-induced silencing complex. J. Biol. Chem. 2014;289:14263–14271. doi: 10.1074/jbc.M114.561902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S.W., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A., et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chang J.C., Morton D.B. Drosophila lines with mutant and wild type human TDP-43 replacing the endogenous gene reveals phosphorylation and ubiquitination in mutant lines in the absence of viability or lifespan defects. PLoS ONE. 2017;12:e0180828. doi: 10.1371/journal.pone.0180828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Romano M., Buratti E., Romano G., Klima R., del Bel Belluz L., Stuani C., Baralle F., Feiguin F. Evolutionarily conserved heterogeneous nuclear ribonucleoprotein (hnRNP) A/B proteins functionally interact with human and Drosophila TAR DNA-binding protein 43 (TDP-43) J. Biol. Chem. 2014;289:7121–7130. doi: 10.1074/jbc.M114.548859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.D’Ambrogio A., Buratti E., Stuani C., Guarnaccia C., Romano M., Ayala Y.M., Baralle F.E. Functional mapping of the interaction between TDP-43 and hnRNP A2 in vivo. Nucleic Acids Res. 2009;37:4116–4126. doi: 10.1093/nar/gkp342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ayala Y.M., Pantano S., D’Ambrogio A., Buratti E., Brindisi A., Marchetti C., Romano M., Baralle F.E. Human, Drosophila, and C. elegans TDP43: Nucleic acid binding properties and splicing regulatory function. J. Mol. Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 301.Cheng C.W., Lin M.J., Shen C.K. Rapamycin alleviates pathogenesis of a new Drosophila model of ALS-TDP. J. Neurogener. 2015;29:59–68. doi: 10.3109/01677063.2015.1077832. [DOI] [PubMed] [Google Scholar]
- 302.Kankel M.W., Sen A., Lu L., Theodorou M., Dimlich D.N., McCampbell A., Henderson C.E., Shneider N.A., Artavanis-Tsakonas S. Amyotrophic Lateral Sclerosis Modifiers in Drosophila Reveal the Phospholipase D Pathway as a Potential Therapeutic Target. Genetics. 2020;215:747–766. doi: 10.1534/genetics.119.302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Appocher C., Mohagheghi F., Cappelli S., Stuani C., Romano M., Feiguin F., Buratti E. Major hnRNP proteins act as general TDP-43 functional modifiers both in Drosophila and human neuronal cells. Nucleic Acids Res. 2017;45:8026–8045. doi: 10.1093/nar/gkx477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cragnaz L., Klima R., Skoko N., Budini M., Feiguin F., Baralle F.E. Aggregate formation prevents dTDP-43 neurotoxicity in the Drosophila melanogaster eye. Neurobiol. Dis. 2014;71:74–80. doi: 10.1016/j.nbd.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 305.Voigt A., Herholz D., Fiesel F.C., Kaur K., Müller D., Karsten P., Weber S.S., Kahle P.J., Marquardt T., Schulz J.B. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS ONE. 2010;5:e12247. doi: 10.1371/journal.pone.0012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Choksi D.K., Roy B., Chatterjee S., Yusuff T., Bakhoum M.F., Sengupta U., Ambegaokar S., Kayed R., Jackson G.R. TDP-43 Phosphorylation by casein kinase Iε promotes oligomerization and enhances toxicity in vivo. Hum. Mol. Genet. 2014;23:1025–1035. doi: 10.1093/hmg/ddt498. [DOI] [PubMed] [Google Scholar]
- 307.Miguel L., Frébourg T., Campion D., Lecourtois M. Both cytoplasmic and nuclear accumulations of the protein are neurotoxic in Drosophila models of TDP-43 proteinopathies. Neurobiol. Dis. 2011;41:398–406. doi: 10.1016/j.nbd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 308.Elden A.C., Kim H.J., Hart M.P., Chen-Plotkin A.S., Johnson B.S., Fang X., Armakola M., Geser F., Greene R., Lu M.M., et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Krug L., Chatterjee N., Borges-Monroy R., Hearn S., Liao W.W., Morrill K., Prazak L., Rozhkov N., Theodorou D., Hammell M., et al. Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS Genet. 2017;13:e1006635. doi: 10.1371/journal.pgen.1006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.McGurk L., Gomes E., Guo L., Mojsilovic-Petrovic J., Tran V., Kalb R.G., Shorter J., Bonini N.M. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol. Cell. 2018;71:703–717. doi: 10.1016/j.molcel.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Khalil B., Cabirol-Pol M.J., Miguel L., Whitworth A.J., Lecourtois M., Liévens J.C. Enhancing Mitofusin/Marf ameliorates neuromuscular dysfunction in Drosophila models of TDP-43 proteinopathies. Neurobiol. Aging. 2017;54:71–83. doi: 10.1016/j.neurobiolaging.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 312.Wang P., Deng J., Dong J., Liu J., Bigio E.H., Mesulam M., Wang T., Sun L., Wang L., Lee A.Y., et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 2019;15:e1007947. doi: 10.1371/journal.pgen.1007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhan L., Hanson K.A., Kim S.H., Tare A., Tibbetts R.S. Identification of genetic modifiers of TDP-43 neurotoxicity in Drosophila. PLoS ONE. 2013;8:e57214. doi: 10.1371/journal.pone.0057214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kim H.J., Raphael A.R., LaDow E.S., McGurk L., Weber R.A., Trojanowski J.Q., Lee V.M., Finkbeiner S., Gitler A.D., Bonini N.M. Therapeutic modulation of eIF2α phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat. Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hanson K.A., Kim S.H., Wassarman D.A., Tibbetts R.S. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS) J. Biol. Chem. 2010;285:11068–11072. doi: 10.1074/jbc.C109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cha S.J., Han Y.J., Choi H.J., Kim H.J., Kim K. Glutathione S-Transferase Rescues Motor Neuronal Toxicity in Fly Model of Amyotrophic Lateral Sclerosis. Antioxidants (Basel, Switzerland) 2020;9:615. doi: 10.3390/antiox9070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chou C.C., Zhang Y., Umoh M.E., Vaughan S.W., Lorenzini I., Liu F., Sayegh M., Donlin-Asp P.G., Chen Y.H., Duong D.M., et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 2018;21:228–239. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Coyne A.N., Yamada S.B., Siddegowda B.B., Estes P.S., Zaepfel B.L., Johannesmeyer J.S., Lockwood D.B., Pham L.T., Hart M.P., Cassel J.A., et al. Fragile X protein mitigates TDP-43 toxicity by remodeling RNA granules and restoring translation. Hum. Mol. Genet. 2015;24:6886–6898. doi: 10.1093/hmg/ddv389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Liu G., Coyne A.N., Pei F., Vaughan S., Chaung M., Zarnescu D.C., Buchan J.R. Endocytosis regulates TDP-43 toxicity and turnover. Nat. Commun. 2017;8:2092. doi: 10.1038/s41467-017-02017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gregory J.M., Barros T.P., Meehan S., Dobson C.M., Luheshi L.M. The aggregation and neurotoxicity of TDP-43 and its ALS-associated 25 kDa fragment are differentially affected by molecular chaperones in Drosophila. PLoS ONE. 2012;7:e31899. doi: 10.1371/journal.pone.0031899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wang C., Duan Y., Duan G., Wang Q., Zhang K., Deng X., Qian B., Gu J., Ma Z., Zhang S., et al. Stress Induces Dynamic, Cytotoxicity-Antagonizing TDP-43 Nuclear Bodies via Paraspeckle LncRNA NEAT1-Mediated Liquid-Liquid Phase Separation. Mol. Cell. 2020;79:443–458. doi: 10.1016/j.molcel.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 322.Kim S.H., Zhan L., Hanson K.A., Tibbetts R.S. High-content RNAi screening identifies the Type 1 inositol triphosphate receptor as a modifier of TDP-43 localization and neurotoxicity. Hum. Mol. Genet. 2012;21:4845–4856. doi: 10.1093/hmg/dds321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhan L., Xie Q., Tibbetts R.S. Opposing roles of p38 and JNK in a Drosophila model of TDP-43 proteinopathy reveal oxidative stress and innate immunity as pathogenic components of neurodegeneration. Hum. Mol. Genet. 2015;24:757–772. doi: 10.1093/hmg/ddu493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Gregory J.M., Whiten D.R., Brown R.A., Barros T.P., Kumita J.R., Yerbury J.J., Satapathy S., McDade K., Smith C., Luheshi L.M., et al. Clusterin protects neurons against intracellular proteotoxicity. Acta Neuropathol. Commun. 2017;5:81. doi: 10.1186/s40478-017-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lee S., Kim S., Kang H.Y., Lim H.R., Kwon Y., Jo M., Jeon Y.M., Kim S.R., Kim K., Ha C.M., et al. The overexpression of TDP-43 in astrocytes causes neurodegeneration via a PTP1B-mediated inflammatory response. J. Neuroinflamm. 2020;17:299. doi: 10.1186/s12974-020-01963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chang Y.H., Dubnau J. The Gypsy Endogenous Retrovirus Drives Non-Cell-Autonomous Propagation in a Drosophila TDP-43 Model of Neurodegeneration. Curr. Biol. 2019;29:3135–3152. doi: 10.1016/j.cub.2019.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Crippa V., Cicardi M.E., Ramesh N., Seguin S.J., Ganassi M., Bigi I., Diacci C., Zelotti E., Baratashvili M., Gregory J.M., et al. The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity. Hum. Mol. Genet. 2016;25:3908–3924. doi: 10.1093/hmg/ddw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sanna S., Esposito S., Masala A., Sini P., Nieddu G., Galioto M., Fais M., Iaccarino C., Cestra G., Crosio C. HDAC1 inhibition ameliorates TDP-43-induced cell death in vitro and in vivo. Cell Death Dis. 2020;11:369. doi: 10.1038/s41419-020-2580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tazelaar G.H.P., Boeynaems S., de Decker M., van Vugt J., Kool L., Goedee H.S., McLaughlin R.L., Sproviero W., Iacoangeli A., Moisse M., et al. ATXN1 repeat expansions confer risk for amyotrophic lateral sclerosis and contribute to TDP-43 mislocalization. Brain Commun. 2020;2:fcaa064. doi: 10.1093/braincomms/fcaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Berson A., Sartoris A., Nativio R., van Deerlin V., Toledo J.B., Porta S., Liu S., Chung C.Y., Garcia B.A., Lee V.M., et al. TDP-43 Promotes Neurodegeneration by Impairing Chromatin Remodeling. Curr. Biol. 2017;27:3579–3590. doi: 10.1016/j.cub.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chou C.C., Alexeeva O.M., Yamada S., Pribadi A., Zhang Y., Mo B., Williams K.R., Zarnescu D.C., Rossoll W. PABPN1 suppresses TDP-43 toxicity in ALS disease models. Hum. Mol. Genet. 2015;24:5154–5173. doi: 10.1093/hmg/ddv238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hans F., Fiesel F.C., Strong J.C., Jäckel S., Rasse T.M., Geisler S., Springer W., Schulz J.B., Voigt A., Kahle P.J. UBE2E ubiquitin-conjugating enzymes and ubiquitin isopeptidase Y regulate TDP-43 protein ubiquitination. J. Biol. Chem. 2014;289:19164–19179. doi: 10.1074/jbc.M114.561704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pons M., Miguel L., Miel C., Avequin T., Juge F., Frebourg T., Campion D., Lecourtois M. Splicing factors act as genetic modulators of TDP-43 production in a new autoregulatory TDP-43 Drosophila model. Hum. Mol. Genet. 2017;26:3396–3408. doi: 10.1093/hmg/ddx229. [DOI] [PubMed] [Google Scholar]
- 334.Uechi H., Kuranaga E., Iriki T., Takano K., Hirayama S., Miura M., Hamazaki J., Murata S. Ubiquitin-Binding Protein CG5445 Suppresses Aggregation and Cytotoxicity of Amyotrophic Lateral Sclerosis-Linked TDP-43 in Drosophila. Mol. Cell. Biol. 2018;38 doi: 10.1128/MCB.00195-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Coyne A.N., Siddegowda B.B., Estes P.S., Johannesmeyer J., Kovalik T., Daniel S.G., Pearson A., Bowser R., Zarnescu D.C. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34:15962–15974. doi: 10.1523/JNEUROSCI.2526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Estes P.S., Daniel S.G., McCallum A.P., Boehringer A.V., Sukhina A.S., Zwick R.A., Zarnescu D.C. Motor neurons and glia exhibit specific individualized responses to TDP-43 expression in a Drosophila model of amyotrophic lateral sclerosis. Dis. Models Mech. 2013;6:721–733. doi: 10.1242/dmm.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Coyne A.N., Lorenzini I., Chou C.C., Torvund M., Rogers R.S., Starr A., Zaepfel B.L., Levy J., Johannesmeyer J., Schwartz J.C., et al. Post-transcriptional Inhibition of Hsc70-4/HSPA8 Expression Leads to Synaptic Vesicle Cycling Defects in Multiple Models of ALS. Cell Rep. 2017;21:110–125. doi: 10.1016/j.celrep.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Cragnaz L., Klima R., de Conti L., Romano G., Feiguin F., Buratti E., Baralle M., Baralle F.E. An age-related reduction of brain TBPH/TDP-43 levels precedes the onset of locomotion defects in a Drosophila ALS model. Neuroscience. 2015;311:415–421. doi: 10.1016/j.neuroscience.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 339.Staněk D., Fox A.H. Nuclear bodies: News insights into structure and function. Curr. Opin. Cell Biol. 2017;46:94–101. doi: 10.1016/j.ceb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 340.Miskiewicz K., Jose L.E., Yeshaw W.M., Valadas J.S., Swerts J., Munck S., Feiguin F., Dermaut B., Verstreken P. HDAC6 is a Bruchpilot deacetylase that facilitates neurotransmitter release. Cell Rep. 2014;8:94–102. doi: 10.1016/j.celrep.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 341.Baldwin K.R., Godena V.K., Hewitt V.L., Whitworth A.J. Axonal transport defects are a common phenotype in Drosophila models of ALS. Hum. Mol. Genet. 2016;25:2378–2392. doi: 10.1093/hmg/ddw105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Romano G., Holodkov N., Klima R., Grilli F., Guarnaccia C., Nizzardo M., Rizzo F., Garcia R., Feiguin F. Downregulation of glutamic acid decarboxylase in Drosophila TDP-43-null brains provokes paralysis by affecting the organization of the neuromuscular synapses. Sci. Rep. 2018;8:1809. doi: 10.1038/s41598-018-19802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Holcik M., Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 344.Kedersha N., Chen S., Gilks N., Li W., Miller I.J., Stahl J., Anderson P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lai A., Valdez-Sinon A.N., Bassell G.J. Regulation of RNA granules by FMRP and implications for neurological diseases. Traffic (Copenhagen, Denmark) 2020;21:454–462. doi: 10.1111/tra.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kaehler C., Isensee J., Nonhoff U., Terrey M., Hucho T., Lehrach H., Krobitsch S. Ataxin-2-like is a regulator of stress granules and processing bodies. PLoS ONE. 2012;7:e50134. doi: 10.1371/journal.pone.0050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Nonhoff U., Ralser M., Welzel F., Piccini I., Balzereit D., Yaspo M.L., Lehrach H., Krobitsch S. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol. Biol. Cell. 2007;18:1385–1396. doi: 10.1091/mbc.e06-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Becker L.A., Huang B., Bieri G., Ma R., Knowles D.A., Jafar-Nejad P., Messing J., Kim H.J., Soriano A., Auburger G., et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 2017;544:367–371. doi: 10.1038/nature22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Banerjee A., Apponi L.H., Pavlath G.K., Corbett A.H. PABPN1: Molecular function and muscle disease. FEBS J. 2013;280:4230–4250. doi: 10.1111/febs.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kim S.H., Shanware N.P., Bowler M.J., Tibbetts R.S. Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA. J. Biol. Chem. 2010;285:34097–34105. doi: 10.1074/jbc.M110.154831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Duan Y., Du A., Gu J., Duan G., Wang C., Gui X., Ma Z., Qian B., Deng X., Zhang K., et al. PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 2019;29:233–247. doi: 10.1038/s41422-019-0141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Inukai Y., Nonaka T., Arai T., Yoshida M., Hashizume Y., Beach T.G., Buratti E., Baralle F.E., Akiyama H., Hisanaga S., et al. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899–2904. doi: 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 353.Budini M., Buratti E., Stuani C., Guarnaccia C., Romano V., de Conti L., Baralle F.E. Cellular model of TAR DNA-binding protein 43 (TDP-43) aggregation based on its C-terminal Gln/Asn-rich region. J. Biol. Chem. 2012;287:7512–7525. doi: 10.1074/jbc.M111.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Nizard P., Tetley S., Le Dréan Y., Watrin T., Le Goff P., Wilson M.R., Michel D. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic (Copenhagen, Denmark) 2007;8:554–565. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 355.Song G.J., Jung M., Kim J.H., Park H., Rahman M.H., Zhang S., Zhang Z.Y., Park D.H., Kook H., Lee I.K., et al. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J. Neuroinflamm. 2016;13:86. doi: 10.1186/s12974-016-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 357.Morettini S., Tribus M., Zeilner A., Sebald J., Campo-Fernandez B., Scheran G., Wörle H., Podhraski V., Fyodorov D.V., Lusser A. The chromodomains of CHD1 are critical for enzymatic activity but less important for chromatin localization. Nucleic Acids Res. 2011;39:3103–3115. doi: 10.1093/nar/gkq1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Körner S., Hendricks M., Kollewe K., Zapf A., Dengler R., Silani V., Petri S. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): Impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bouteloup C., Desport J.C., Clavelou P., Guy N., Derumeaux-Burel H., Ferrier A., Couratier P. Hypermetabolism in ALS patients: An early and persistent phenomenon. J. Neurol. 2009;256:1236–1242. doi: 10.1007/s00415-009-5100-z. [DOI] [PubMed] [Google Scholar]
- 360.Desport J.C., Preux P.M., Magy L., Boirie Y., Vallat J.M., Beaufrère B., Couratier P. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am. J. Clin. Nutr. 2001;74:328–334. doi: 10.1093/ajcn/74.3.328. [DOI] [PubMed] [Google Scholar]
- 361.Pradat P.F., Bruneteau G., Gordon P.H., Dupuis L., Bonnefont-Rousselot D., Simon D., Salachas F., Corcia P., Frochot V., Lacorte J.M., et al. Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010;11:166–171. doi: 10.3109/17482960902822960. [DOI] [PubMed] [Google Scholar]
- 362.Dupuis L., Corcia P., Fergani A., Gonzalez de Aguilar J.L., Bonnefont-Rousselot D., Bittar R., Seilhean D., Hauw J.J., Lacomblez L., Loeffler J.P., et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 363.Manzo E., Lorenzini I., Barrameda D., O’Conner A.G., Barrows J.M., Starr A., Kovalik T., Rabichow B.E., Lehmkuhl E.M., Shreiner D.D., et al. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. eLife. 2019;8 doi: 10.7554/eLife.45114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Manzo E., O’Conner A.G., Barrows J.M., Shreiner D.D., Birchak G.J., Zarnescu D.C. Medium-Chain Fatty Acids, Beta-Hydroxybutyric Acid and Genetic Modulation of the Carnitine Shuttle Are Protective in a Drosophila Model of ALS Based on TDP-43. Front. Mol. Neurosci. 2018;11:182. doi: 10.3389/fnmol.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Brouns M.R., Matheson S.F., Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat. Cell Biol. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- 366.Langellotti S., Romano G., Feiguin F., Baralle F.E., Romano M. RhoGAPp190: A potential player in tbph-mediated neurodegeneration in Drosophila. PLoS ONE. 2018;13:e0195845. doi: 10.1371/journal.pone.0195845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Romano M., Feiguin F., Buratti E. TBPH/TDP-43 modulates translation of Drosophila futsch mRNA through an UG-rich sequence within its 5’UTR. Brain Res. 2016;1647:50–56. doi: 10.1016/j.brainres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 368.Boyault C., Zhang Y., Fritah S., Caron C., Gilquin B., Kwon S.H., Garrido C., Yao T.P., Vourc’h C., Matthias P., et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kittel R.J., Wichmann C., Rasse T.M., Fouquet W., Schmidt M., Schmid A., Wagh D.A., Pawlu C., Kellner R.R., Willig K.I., et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science (New York, NY) 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 370.Lembke K.M., Law A.D., Ahrar J., Morton D.B. Deletion of a specific exon in the voltage-gated calcium channel gene cacophony disrupts locomotion in Drosophila larvae. Pt 1J. Exp. Biol. 2019;222 doi: 10.1242/jeb.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Brenner D., Yilmaz R., Müller K., Grehl T., Petri S., Meyer T., Grosskreutz J., Weydt P., Ruf W., Neuwirth C., et al. Hot-spot KIF5A mutations cause familial ALS. Brain. 2018;141:688–697. doi: 10.1093/brain/awx370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Nicolas A., Kenna K.P., Renton A.E., Ticozzi N., Faghri F., Chia R., Dominov J.A., Kenna B.J., Nalls M.A., Keagle P., et al. Genome-wide Analyses Identify KIF5A as a Novel ALS Gene. Neuron. 2018;97:1268–1283. doi: 10.1016/j.neuron.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.