Abstract
Aim
Right‐half dissection of the superior mesenteric artery (SMA) nerve plexus in pancreatoduodenectomy for pancreatic cancer was initiated to accomplish R0 resection; however, subsequent refractory diarrhea was a major concern. This study aimed to evaluate the necessity of this technique.
Methods
From April 2014 to June 2018, 74 patients with pancreatic head cancer were randomly allocated to either Group A, in which right‐half dissection of the SMA nerve plexus was performed (n = 37), or Group B, in which total preservation of the nerve plexus was performed (n = 37). Short‐term, long‐term, and survival outcomes were prospectively compared between the groups.
Results
The patient demographics, including the R0 resection rate, were not significantly different between the groups. Postoperative diarrhea occurred in 26 (70.3%) patients in Group A and 18 (48.6%) patients in Group B. There was a tendency for the development of severe diarrhea in Group A within 1 year postoperatively, and the frequency of diarrhea gradually decreased within 2 years, although that did not affect tolerance to adjuvant chemotherapy. There was no difference in either locoregional recurrence (27.0% vs 32.4%) or systemic recurrence (46.0% vs 46.0%). The median overall survival time in Groups A and B was 37.9 and 34.6 months, respectively (P = 0.77).
Conclusion
We did not demonstrate a clinical impact of right‐half dissection of the SMA nerve plexus on locoregional recurrence or survival. Therefore, the prophylactic dissection of the SMA nerve plexus is unnecessary given that refractory diarrhea could be induced by this technique (UMIN000012241).
Keywords: pancreatic head cancer, pancreatoduodenectomy, phase II trial, superior mesenteric artery nerve plexus
The necessity of right‐half dissection of the SMA nerve plexus in pancreatoduodenectomy for pancreatic cancer was evaluated. We did not demonstrate the clinical impact of this technique on locoregional recurrence or survival. Therefore, the prophylactic dissection is unnecessary, given that refractory diarrhea could be induced by this technique.
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies worldwide. It currently ranks as the third leading cause of cancer‐related death and is estimated to become the second leading cause by 2030. 1 The survival outcome of PDAC is still dismal, with actual 5‐year survival rates ranging from 10% to 20%. 2 To conquer this disease, extended lymphadenectomy with dissection of the nerve plexus has been challenged in some randomized controlled trials; however, survival outcomes have not been improved. 3 , 4 , 5 , 6
Among some extended surgery techniques for PDAC, right‐half dissection of the superior mesenteric artery (SMA) nerve plexus has been considered a standard technique of pancreatoduodenectomy in Japan. 7 , 8 Although this concept was initiated to accomplish R0 resection and totally extirpate the tissue that had the potential for nerve plexus invasion, subsequent refractory diarrhea is a major concern. This refractory diarrhea often decreases the relative dose intensity of adjuvant chemotherapy, which has been demonstrated to be a significant prognostic factor in PDAC. 9 , 10 Therefore, unnecessary right‐half dissection of the SMA nerve plexus in pancreatoduodenectomy might induce refractory diarrhea, potentially worsening the prognosis.
This multicenter randomized phase II trial was performed to judge the necessity of right‐half dissection of the SMA nerve plexus in the context of the locoregional recurrence rate. We randomly assigned the enrolled patients into those who did and did not undergo right‐half dissection of the SMA nerve plexus and statistically analyzed the short‐term, long‐term, and survival outcomes between the groups.
2. METHODS
2.1. Patient recruitment
From April 2014 to June 2018, 80 patients from Japanese regional high‐volume centers (Nagoya University Hospital, Kansai Medical University Hospital, and Toyama University Hospital) were enrolled in this phase II study. The eligibility criteria were planned pancreatoduodenectomy for pancreatic head cancer, no invasion of the SMA nerve plexus on preoperative imaging studies and estimated R0 resection by preservation of the nerve plexus, and age of ≥20 to ≤75 years. The exclusion criteria were a malignant pancreatic tumor other than pancreatic cancer, invasion or suspected invasion of the SMA nerve plexus, liver cirrhosis or active hepatitis, interstitial pneumonia or emphysema, artificial dialysis for chronic renal failure, other active concomitant malignancies, and preoperative moderate diarrhea. Written informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of the affiliated hospital. The registration number of this clinical trial is UMIN000012241.
2.2. Study design
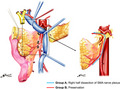
In this multicenter randomized phase II study, 80 enrolled patients were randomly allocated to Group A, in which right‐half dissection of the SMA nerve plexus was performed (n = 40), and Group B, in which total preservation of the nerve plexus was performed (n = 40). The modulators of allocation were the institution, portal system invasion, and preoperative radiation therapy. In Group A, the nerve plexus was dissected from the bifurcation of the middle colic artery to the root of the SMA, along with the pancreatic head. 8 The remnant SMA nerve plexus that was the nearest to the presenting part of the tumor was pathologically evaluated intraoperatively in at least two sites. 11 In Group B, the pancreatoduodenectomy was performed with exposure and preservation of the nerve plexus in its entire circumference (Figure 1). D2 lymph node dissection was conducted in both groups; thus, the only difference was the performance of right‐half nerve plexus dissection. A close‐up photograph of the SMA was taken to confirm the accuracy of the procedure (Figure S1). The CONSORT diagram of this study is shown in Figure 2. Other pathologies were diagnosed in three patients, and another three patients’ conditions were deemed unresectable; therefore, these six patients were excluded. Finally, 74 patients (37 in each group) were analyzed. The primary endpoint was the local recurrence rate at 2 years after surgery. The secondary endpoints were the frequency of diarrhea, accumulation of ascites, peritoneal recurrence rate, initiation ratio and start of adjuvant chemotherapy after surgery, recurrence‐free survival (RFS), and overall survival (OS). In the current study, zero mm rule was utilized to evaluate the residual tumor (R) status.
FIGURE 1.
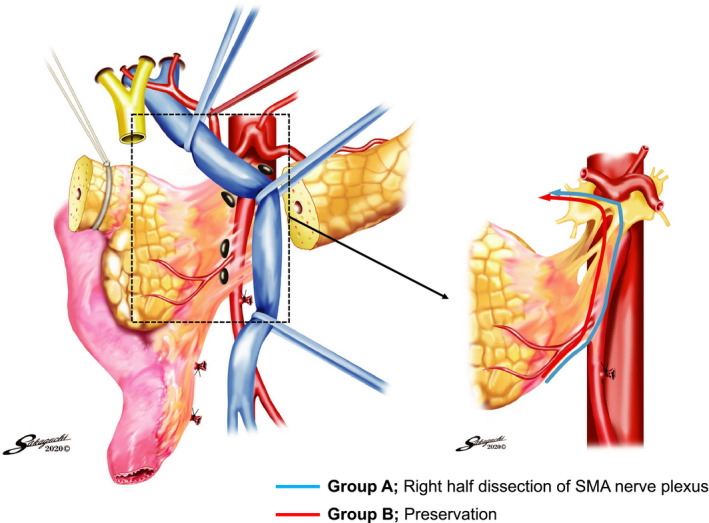
Study schema. The blue line indicates right‐half dissection, and the red line indicates preservation of the SMA nerve plexus. SMA, superior mesenteric artery
FIGURE 2.
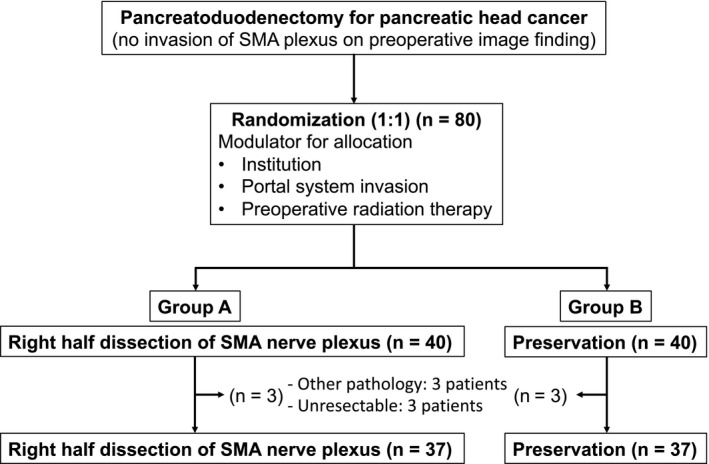
CONSORT diagram. SMA, superior mesenteric artery
2.3. Postoperative follow‐up
Oral S‐1 (oral 5‐fluorouracil prodrug tegafur with oteracil and gimeracil) was initiated as adjuvant chemotherapy from 3 to 10 weeks postoperatively. S‐1 was administered from days 1 to 28 followed by a 2‐week rest period. 10 In outpatient clinics, the patients were followed up once per month for 6 months and every 3 months thereafter. Blood examinations, including measurement of serum tumor markers, were performed at every outpatient care visit, and dynamic computed tomography or magnetic resonance imaging was performed every 3 months.
2.4. Statistical analysis
The sample size was calculated as follows. The local recurrence rate at 2 years after surgery in Group A was estimated to be 15% to 20%. Assuming a null hypothesis of 30% and an alternative hypothesis of 10% with a one‐sided type I error of 0.05 and power of 0.9, enrollment of 37 patients per arm was required. Continuous variables are expressed as mean ± standard deviation. RFS was defined as the time from surgery to confirmation of any recurrence. OS was defined as the time from surgery to death by any cause. Survival analysis based on the Kaplan‐Meier method and log‐rank tests were also performed. The last follow‐up date was April 2020. All statistical analyses were performed using JMP Pro version 14.2.0 (SAS Institute, Cary, NC, USA) and R version 3.5.3 (http//www.r‐project.org/). The level of statistical significance was set at P < 0.05.
3. RESULTS
3.1. Patient demographics
Patient demographics are compared between the two study groups in Table 1. There was no difference in age, sex distribution, performance status, or body mass index. Twenty‐nine (78.4%) resectable cancers based on the NCCN guidelines Version 1.2014 were observed in Group A and 30 (81.1%) were observed in Group B, with no difference in the resectability status between the groups. 12 Neoadjuvant therapy was performed in 16 (43.2%) and 12 (32.4%) patients, among whom preoperative radiation therapy was conducted in only two (5.4%) in Group A and four (10.8%) in Group B, respectively. Among the operative factors, there was no difference in the operation time or blood loss. Pathologically, invasion of the SMA nerve plexus was reported in two (5.4%) and three (8.1%) patients in Groups A and B, respectively, and the R0 resection rate was also comparable between the two groups. As for detailed site of R1, no patient with positive SMA margin was observed in Group A, whereas one was observed in Group B.
Table 1.
Comparison of demographics between patients with and without right‐half dissection of SMA nerve plexus
| Dissection (n = 37) | Preservation (n = 37) | P | |
|---|---|---|---|
| Clinical variables | |||
| Age, years | 64.8 ± 8.9 | 65.3 ± 9.1 | 0.8028 |
| Sex, male/female | 24 (64.9%) /13 (35.1%) | 23 (62.2%)/ 14 (37.8%) | 1.0000 |
| Performance status, 0/1 | 36 (97.3%)/ 1 (2.7%) | 36 (97.3%)/ 1 (2.7%) | 1.0000 |
| Body mass index, kg/m2 | 21.1 ± 2.8 | 21.4 ± 2.4 | 0.5954 |
| NCCN resectability status | |||
| R | 29 (78.4%) | 30 (81.1%) | 0.7105 |
| BR‐PV | 7 (18.9%) | 5 (13.5%) | |
| BR‐A | 1 (2.7%) | 2 (5.4%) | |
| Tumor size, mm | 23.0 ± 7.2 | 23.8 ± 8.1 | 0.6513 |
| Biliary drainage | 17 (46.0%) | 23 (62.2%) | 0.2433 |
| Neoadjuvant therapy | 16 (43.2%) | 12 (32.4%) | 0.4725 |
| Preoperative radiation therapy | 2 (5.4%) | 4 (10.8%) | 0.6741 |
| CEA, ng/ml | 27.6 ± 126.0 | 5.2 ± 5.8 | 0.2865 |
| CA19‐9, U/ml | 320.5 ± 827.5 | 765.2 ± 1713.9 | 0.1612 |
| DUPAN‐2, U/ml | 305.0 ± 415.8 | 282.8 ± 600.3 | 0.8756 |
| Operation time, min | 450.3 ± 111.7 | 491.4 ± 108.8 | 0.1134 |
| Blood loss, ml | 892.4 ± 565.8 | 1024.7 ± 649.1 | 0.3530 |
| Portal vein resection | 16 (43.2%) | 21 (56.8%) | 0.3525 |
| Blood transfusion | 5 (13.5%) | 5 (13.5%) | 1.0000 |
| Pathological invasion of SMA nerve plexus | 2 (5.4%) | 3 (8.1%) | 1.0000 |
| Residual tumor, R0/R1 | 35 (94.6%)/ 2 (5.4%)` | 31 (83.8%)/ 6 (16.2%) | 0.2611 |
| SMA margin | 0 (0.0%) | 1 (2.7%) | |
| Dissected pancreatic margin | 2 (5.4%) | 5 (13.5%) | |
| UICC stage (7th edition) | |||
| IA | 3 (8.1%) | 3 (8.1%) | 0.9188 |
| IB | 1 (2.7%) | 0 (0.0%) | |
| IIA | 9 (24.3%) | 7 (18.9%) | |
| IIB | 21 (56.8%) | 24 (64.9%) | |
| III | 1 (2.7%) | 1 (2.7%) | |
| IV | 2 (5.4%) | 2 (5.4%) | |
Data are expressed as mean ± standard deviation or n (%).
Abbreviations: BR‐A, borderline resectable with arterial invasion; BR‐PV, borderline resectable with portal vein invasion; CA19‐9, cancer antigen 19‐9; CEA, carcinoembryonic antigen; R, resectable; SMA, superior mesenteric artery; UICC, Union for International Cancer Control.
3.2. Short‐term outcomes
The short‐term outcomes after pancreatectomy are compared between the two study groups in Table 2. Postoperative diarrhea was evaluated based on CTCAE v4.0. A total of 26 (70.3%) patients in Group A and 18 (48.6%) patients in Group B developed postoperative diarrhea. Figure 3 shows the postoperative course of diarrhea between the two groups. There was a greater tendency for the development of severe diarrhea in Group A than B within 1 year postoperatively, and the frequency of diarrhea gradually decreased within 2 years in both groups. Also, the postoperative time‐course alteration of the incidence for clinically significant grade 2 or 3 diarrhea in each group was depicted in Figure S2, which showed the same pattern. Opioid use was found in four patients for Group A and two in Group B. With respect to other complications, we found no difference in the development of pancreatic fistula, ascites, intra‐abdominal infection, delayed gastric emptying, or intra‐abdominal bleeding. No reoperations or 30‐day mortality occurred in this trial. When the status of adjuvant chemotherapy was compared, we found no differences in the initiation ratio, start of treatment after surgery, or completion ratio between the groups.
Table 2.
Comparison of short‐term outcomes between patients with and without right‐half dissection of SMA nerve plexus
| Dissection (n = 37) | Preservation (n = 37) | P | |
|---|---|---|---|
| Complications | |||
| Diarrhea (CTCAE v4.0) | |||
| Absent | 11 (29.7%) | 19 (51.4%) | 0.2505 |
| Present | 26 (70.3%) | 18 (48.6%) | |
| Grade 1 | 13 (35.1%) | 11(29.7%) | |
| Grade 2 | 11 (29.7%) | 6(16.2%) | |
| Grade 3 | 2 (5.4%) | 1(2.7%) | |
| Opioid use as antidiarrheal agent | |||
| Yes | 4 (10.8%) | 2 (5.4%) | 0.6741 |
| Pancreatic fistula a | |||
| None | 30 (81.1%) | 29 (78.4%) | 0.6784 |
| Biochemical leakage | 3 (8.1%) | 5 (13.5%) | |
| Grade B | 3 (8.1%) | 3 (8.1%) | |
| Grade C | 1 (2.7%) | 0 (0.0%) | |
| Ascites | 13 (35.1%) | 12 (32.4%) | 1.0000 |
| Intra‐abdominal infection | 4 (10.8%) | 4 (10.8%) | 1.0000 |
| Delayed gastric emptying | 4 (10.8%) | 4 (10.8%) | 1.0000 |
| Intra‐abdominal bleeding | 1 (2.7%) | 0 (0.0%) | 1.0000 |
| Others | 9 (24.3%) | 7 (18.9%) | 0.7784 |
| Re‐operation | 0 (0.0%) | 0 (0.0%) | 1.0000 |
| Postoperative hospital stay, days | 18.7 ± 9.0 | 20.3 ± 13.8 | 0.5644 |
| 30‐day mortality | 0 (0.0%) | 0 (0.0%) | 1.0000 |
| 30‐day re‐admission | 5 (13.5%) | 2 (5.4%) | 0.4297 |
| Adjuvant chemotherapy | |||
| Initiation ratio | 35 (94.6%) | 33 (89.2%) | 0.6741 |
| Start of treatment after surgery, days | 40.6 ± 15.9 | 45.6 ± 23.2 | 0.3093 |
| Completion ratio | 29 (82.9%) | 21 (63.6%) | 0.1003 |
| Performance status, 0/1/2 | 27 (75.0%)/ 8 (22.2%)/ 1 (2.8%) | 28 (82.4%)/ 6 (17.7%)/ 0 (0.0%) | 0.5359 |
Data are expressed as mean ± standard deviation or n (%).
Abbreviations: CTCAE v4.0, Common Terminology Criteria for Adverse Events version 4.0; SMA, superior mesenteric artery.
Pancreatic fistula was defined based on the 2016 ISGPS definition.
FIGURE 3.
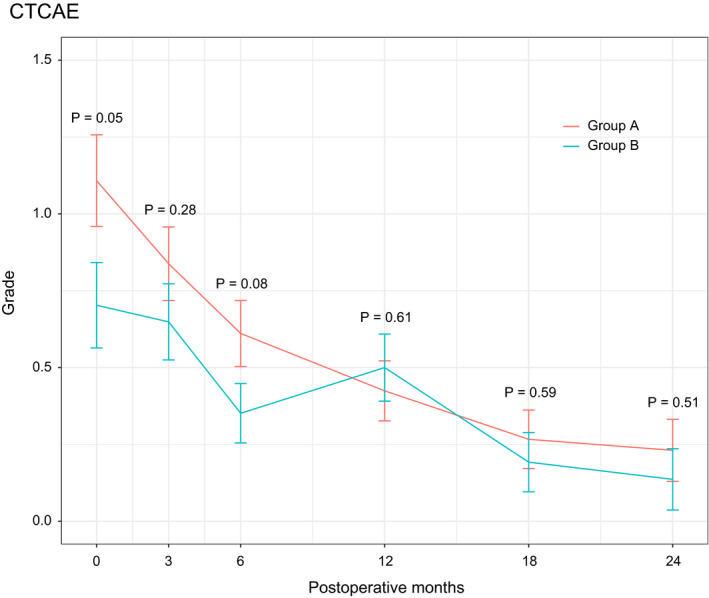
Postoperative course of diarrhea between the two groups. Group A, right‐half dissection of the superior mesenteric artery nerve plexus; Group B, preservation of the nerve plexus
3.3. Long‐term outcomes
The long‐term outcomes are compared between the two study groups in Table 3. Recurrence in all sites was observed in 21 (56.8%) patients in Group A and 22 (59.5%) patients in Group B, with no significant difference. When the recurrence rate was evaluated based on the initial recurrence patterns, there was no difference in either the locoregional or systemic (peritoneum, liver, lymph node, and lung) recurrence rates. The confirmed locoregional recurrence rate in Group A was 27.0% (90% confidence interval = 16.9%‐40.3%), whereas, that in Group B was 32.4% (90% confidence interval = 21.4%‐45.9%), so the null hypothesis for the primary endpoint (≤30%) was not rejected.
Table 3.
Comparison of long‐term outcomes between patients with and without right‐half dissection of SMA nerve plexus
| Dissection (n = 37) | Preservation (n = 37) | P | |
|---|---|---|---|
| All recurrence | 21 (56.8%, 40.9%‐71.3% a ) | 22 (59.5%, 46.1%‐75.9% a ) | 1.0000 |
| Locoregional | 10 (27.0%, 16.9%‐40.3% b ) | 12 (32.4%, 21.4%‐45.9% b ) | 0.7997 |
| Systemic | 17 (46.0%, 31.0%‐61.6% a ) | 17 (46.0%, 33.4%‐64.1% a ) | 1.0000 |
| Peritoneum | 7 (18.9%, 9.5%‐34.2% a ) | 9 (24.3%, 15.4%‐43.0% a ) | 0.7784 |
| Liver | 9 (24.3%, 13.4%‐40.1% a ) | 14 (37.8%, 24.1%‐53.9% a ) | 0.3151 |
| Lymph node | 5 (13.5%, 5.9%‐28.0% a ) | 3 (8.1%, 2.8%‐21.3% a ) | 0.7106 |
| Lung | 6 (16.2%, 7.7%‐31.1% a ) | 2 (5.4%, 1.5%‐17.7% a ) | 0.2611 |
Data are expressed as n (%).
Abbreviation: SMA, superior mesenteric artery.
95% confidence interval.
90% confidence interval.
3.4. Survival outcomes
Finally, RFS and OS are compared between the two study groups in Figure 4. The median RFS time in Group A was 19.0 months and that in Group B was 14.1 months, with no significant difference (2‐year OS, 42.4% vs 46.0%; P = 0.64). Similarly, the median OS time in Group A was 37.9 months and that in Group B was 34.6 months, which were also comparable (2‐year OS, 74.8% vs 59.5%; P = 0.77).
FIGURE 4.
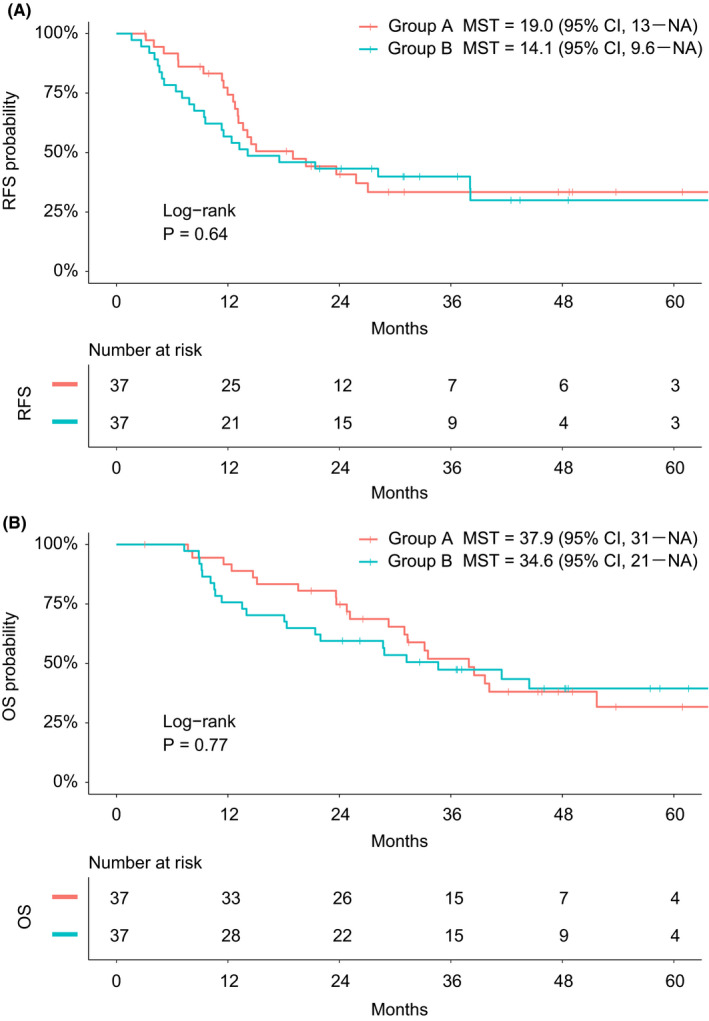
A, Recurrence‐free survival and B, overall survival were compared between the groups. Group A, right‐half dissection of the superior mesenteric artery nerve plexus; Group B, preservation of the nerve plexus. MST, median survival time; CI, confidence interval; NA, not available; RFS, recurrence‐free survival; OS, overall survival
4. DISCUSSION
In this trial, we randomized the enrolled patients into Group A, in which right‐half dissection of the SMA nerve plexus was performed, and Group B, in which the SMA nerve plexus was preserved. We then compared the clinical and survival outcomes between the groups. We found no significant difference in either the total recurrence rate or the locoregional recurrence rate between the groups. There was no clinical benefit with respect to long‐term survival in Group A. Rather, nerve plexus dissection induced postoperative diarrhea as expected, which might have a detrimental impact on postoperative adjuvant chemotherapy.
Fortner 13 first proposed the concept of extended pancreatectomy in the field of pancreatic cancer surgery. In Japan, Ishikawa et al 14 subsequently advocated that extensive surgery including complete dissection of the lymphatic and connective tissues around the common hepatic artery and SMA could provide a survival benefit compared with conventional surgery. However, later randomized controlled trials did not successfully demonstrate the clinical significance of this extended pancreatectomy. 3 , 4 , 5 , 6 With respect to dissection of the SMA nerve plexus, some previous reports revealed that perineural invasion around the SMA may be a prognostic factor in PDAC. 15 , 16 , 17 However, we found no randomized controlled trials that reached this conclusion. In 2014, Jang et al 18 reported that extended lymphadenectomy with dissection of the nerve plexus did not provide a significant survival benefit compared with standard resection in pancreatic head cancer; however, they did not genuinely evaluate the benefit of dissection of the SMA nerve plexus.
Generally, refractory postoperative diarrhea is a major concern after dissection of the SMA nerve plexus. Inoue et al 19 performed a unique tailored mesopancreas dissection technique and observed postoperative diarrhea in 65% of patients who underwent hemicircumferential nerve plexus dissection and 50% of patients who underwent nerve plexus preservation. In contrast, Jang et al 18 showed a comparable and relatively low rate of diarrhea between the standard and extended groups (12.0% vs 15.1%, respectively), indicating little effect on intestinal motility. The current study showed a high rate similar to that reported by Inoue et al, 19 but both studies revealed that most cases of diarrhea were well‐controlled by antidiarrheal opioids. Additionally, we found no difference in the impact on adjuvant chemotherapy between the two groups.
When considering the survival impact of nerve plexus dissection, we must refer to previous randomized controlled trials. However, these studies were conducted a generation ago, 3 , 4 , 5 when eminent procedures such as neoadjuvant and adjuvant chemotherapy were not as available as they are today. Even among studies published in the 2010s, the 2‐year overall survival rate was <50%. 6 , 18 In contrast, in the present study, the 2‐year OS rate in the dissection group was 74.8% and that in the preservation group was 59.5%. Thus, comparison with previous studies is difficult. The same holds true for the lack of clinical benefit of dissection of the SMA nerve plexus. The lack of a difference in either the locoregional or systemic recurrence rate in both groups could support this conclusion.
Besides lymphadenectomy, dissection of the nerve plexus has been considered an indispensable surgical technique to obtain R0 resection and subsequent reduction of locoregional recurrence. In particular, when we encounter a tumor with an extrapancreatic nerve plexus and perineural invasion such as borderline resectable cancer, or when conversion surgery for locally advanced cancer is performed, partial or half dissection of the SMA nerve plexus is definitely required to accomplish R0 resection. In this trial, the patients with invasion or suspected invasion of the SMA nerve plexus were excluded from the criteria, and we focused on the necessity of prophylactic dissection of the nerve plexus. Therefore, we believe that surgeons should strictly refrain from the prophylactic dissection of the nerve plexus because this technique can result in refractory diarrhea, malnutrition, and poor quality of life. 6 , 7 , 8 , 20
In recent years, preoperative chemotherapy or chemoradiotherapy plus adjuvant therapy in the treatment of resectable or borderline resectable pancreatic cancer have been widely spread as the multidisciplinary therapy. This trend basically indicates the difficulty to completely cure the pancreatic cancer by surgery alone. In this situation, one of the clinical benefits for this neoadjuvant therapy could be the prevention of microinvasion or micrometastasis around the primary tumor. Although our study could not demonstrate that the prophylactic dissection of the SMA right‐half nerve plexus could yield a prognostic benefit in the patients with resectable pancreatic cancer, the preoperative chemotherapy or chemoradiotherapy should be strongly recommended to reduce the incidence of local recurrence in future studies.
In conclusion, we conducted a multicenter randomized phase II trial to validate the necessity of right‐half dissection of the SMA nerve plexus in pancreatoduodenectomy for pancreatic head cancer. We did not demonstrate a clinical impact on the locoregional recurrence rate or survival outcomes. Therefore, the prophylactic dissection of the SMA nerve plexus is unnecessary given that refractory diarrhea could be induced by this technique.
DISCLOSURE
Conflict of Interest: The authors have no commercial affiliations that can pose any conflicts of interest in connection with this study.
Funding: No funding was received for this study.
Supporting information
Fig S1
Fig S2
ACKNOWLEDGMENT
We thank Angela Morben, DVM, ELS, from Edanz Group (https://en‐author‐services.edanzgroup.com/), for editing a draft of this manuscript.
Yamada S, Satoi S, Takami H, et al. Multicenter randomized phase II trial of prophylactic right‐half dissection of superior mesenteric artery nerve plexus in pancreatoduodenectomy for pancreatic head cancer. Ann Gastroenterol Surg.2021;5:112–119. 10.1002/ags3.12399
Sohei Satoi has equal contribution.
REFERENCES
- 1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 2. Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41(7):985–92. [DOI] [PubMed] [Google Scholar]
- 3. Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228(4):508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo CJ, Cameron JL, Sohn TA, Coleman JoAnn, Sauter PK, Hruban RH, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short‐term outcome. Ann Surg. 1999;229(5):613–22; discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138(4):618–28; discussion 28–30. [DOI] [PubMed] [Google Scholar]
- 6. Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long‐term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19(3):230–41. [DOI] [PubMed] [Google Scholar]
- 7. Takada T. Surgery for carcinoma of the pancreas in Japan. Past, present, and future aspects. Digestion. 1999;60(Suppl 1):114–9. [DOI] [PubMed] [Google Scholar]
- 8. Nakao A, Takeda S, Sakai M, Kaneko T, Inoue S, Sugimoto H, et al. Extended radical resection versus standard resection for pancreatic cancer: the rationale for extended radical resection. Pancreas. 2004;28(3):289–92. [DOI] [PubMed] [Google Scholar]
- 9. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative‐intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. [DOI] [PubMed] [Google Scholar]
- 10. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57. [DOI] [PubMed] [Google Scholar]
- 11. Fujii T, Satoi S, Yamada S, Murotani K, Yanagimoto H, Takami H, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52(1):81–93. [DOI] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN Guidelines). NCCN; 2014. [Google Scholar]
- 13. Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg. 1984;199(4):418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikawa O, Ohhigashi H, Sasaki YO, Kabuto T, Fukuda I, Furukawa H, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208(2):215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, Akiyama T, et al. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80(5):619–21. [DOI] [PubMed] [Google Scholar]
- 16. Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12(4):357–61. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi H, Ohigashi H, Ishikawa O, Gotoh K, Yamada T, Nagata S, et al. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine‐based chemoradiation therapy for resectable pancreatic cancer. Ann Surg. 2012;255(1):95–102. [DOI] [PubMed] [Google Scholar]
- 18. Jang J‐Y, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259(4):656–64. [DOI] [PubMed] [Google Scholar]
- 19. Inoue Y, Saiura A, Oba A, Kawakatsu S, Ono Y, Sato T, et al. Optimal extent of superior mesenteric artery dissection during pancreaticoduodenectomy for pancreatic cancer: balancing surgical and oncological safety. J Gastrointest Surg. 2019;23(7):1373–83. [DOI] [PubMed] [Google Scholar]
- 20. Satoi S, Yanagimoto H, Yamamoto T, Ohe C, Miyasaka C, Uemura Y, et al. Clinical outcomes of pancreatic ductal adenocarcinoma resection following neoadjuvant chemoradiation therapy vs chemotherapy. Surg Today. 2017;47(1):84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


