Abstract
Background and objective
Data regarding the effects of omega-3 polyunsaturated fatty acids (PUFA) supplementation on metabolic status of pregnant women are limited. This systematic review and meta-analysis were done based on randomized controlled trials (RCTs) dealing with the effects of omega-3 PUFA supplementation on glycemic control, lipoproteins, inflammation and oxidative stress in pregnant women.
Methods
Following databases were searched for eligible studies published from inception to until 2019: MEDLINE, EMBASE, Web of Science, PubMed, Scopus, Cochrane Library, and Google scholar. Studies that evaluated the effect of omega-3 PUFA supplementation on parameters of glycemic control, lipoproteins, inflammation and oxidative stress in pregnant women were found by using the key MeSH. A study quality assessment was performed using the Cochrane Collaboration risk of bias tool and heterogeneity between studies was statistically computed using Cochrane’s Q test and I-square (I2). Data were pooled using a random-effects model and weighted mean difference (WMD) was considered as the overall effect size.
Results
No significant effects of omega-3 PUFA supplementation on FPG, insulin, insulin resistance, total cholesterol, triglycerides, LDL-cholesterol, total cholesterol/HDL-cholesterol, interleukin 6 (IL-6), IL-8, and malondialdehyde were found. However, omega-3 PUFA significantly increased serum concentrations of HDL-cholesterol (WMD: 3.10; 95% CI: 0.18, 6.03) and reduced C-reactive protein (WMD: -1.85; 95% CI: -2.61, -1.09).
Conclusion
Based on the results of this meta-analysis omega-3 PUFA supplementation during pregnancy has a significant beneficial effect on HDL-cholesterol, and C-reactive protein.
Keywords: Omega 3 fatty acids, Insulin resistance, Lipoproteins, Inflammation, Oxidative stress, Pregnant woman
Introduction
Pregnancy is associated with a variety of physiological changes in maternal metabolism including maternal insulin resistance, dyslipidemia [1], a moderate inflammation [2], and increased oxidative stress [3]. In pregnancies complicated by obesity, gestational diabetes mellitus (GDM) and pre-eclampsia these changes are even more expressed [4]. Impaired metabolic function in pregnant women probably also affects fetal growth and development [5]. In recent years, attention has been focused on maternal supplementation with different nutrients in order to support additional nutritional demands during pregnancy, improving mother's health and fetal development, and preventing metabolic disorders and adverse pregnancy outcomes [6, 7].
Omega-3 fatty acids are long chain polyunsaturated fatty acids (PUFA). The most important are alpha linoenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).The sources of ALA are vegetable oils like flaxseed and canola, while EPA and DHA are found in fish oils [8]. EPA and DHA are important for brain and retinal development of fetus [9]. Lower levels of omega-3 PUFA have been reported in a number of pregnancy complications such as intrauterine growth restriction (IUGR), pre-eclampsia and GDM [10]. It has been shown that taking omega-3 PUFA during pregnancy increased mean gestational length and decreased the risk of preterm birth and low birthweight [11]. Zhong et al.[12] in a meta-analysis concluded that omega-3 PUFA supplementation in women with GDM was associated with decreased fasting plasma glucose (FPG) levels, insulin resistance and C-reactive protein (CRP) concentrations, but it did not change the pregnancy outcomes. Since omega-3 PUFA have anti-inflammatory effects and participate in the regulation of metabolic pathways, a number of studies have investigated the efficacy of these PUFA on metabolic status in different conditions. A meta-analysis by AbuMweis et al. [13] indicated that taking EPA and DHA supplements decreased plasma levels of CRP and improved some serum lipoproteins. Zhang et al. [14], have found in their meta-analysis that omega-3 PUFA supplementation in overweight and obese adult's decreased serum triglycerides (TG) but did not change total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C) levels and FPG.
The efficacy of omega-3 PUFAs supplementation during pregnancy has not been well stablished and the results of studies have been inconclusive. Several randomized controlled trials (RCTs) indicated that omega-3 supplementation in pregnant women might improve some metabolic parameters [15, 16], while several others have not shown beneficial effects [17, 18]. Discrepancies between studies may be due to difference in study design, length of treatment, bioavailability and doses of omega-3 supplements, whether they were performed in healthy or sick pregnant women, previous PUFAs status of mother and the existence of pregnancy complications. We have performed a meta-analysis of RCTs to determine the effects of omega-3 PUFA supplementation on glycemic status, serum lipoproteins concentrations, as well as biomarkers of inflammation and oxidative stress in healthy and sick pregnant women.
Methods
Search strategy
Eligible RCTs were identified using Cochrane Library, Embase, Medline, Scopus, Web of Science, PubMed and Google scholar databases for relevant articles published from inception until 2019, and by manually searching the reference list of the located articles. Studies that evaluated the effects of omega-3 PUFA supplementation on parameters of glycemic control, lipoproteins, inflammation and oxidative stress were found by using MeSH and the following text words: intervention ["omega 3" OR "omega-3" OR "n-3 fatty acid*" OR "polyunsaturated fatty acid" OR PUFA OR "n-3 oil" OR "eicosapentaenoic acid" OR "alpha-linolenic acid" OR "alpha linolenic acid" OR "docosahexaenoic acid" OR "fish oil" OR "cod liver oil"], outcomes ["glycemic control" OR "glucose" OR "fasting plasma glucose" OR "fasting blood glucose" OR "FPG" OR "FBG" OR "FBS" OR "HbA1c" OR "insulin" OR "HOMA" OR "homeostatic model of insulin resistance" OR "lipid profile*" OR "lipoprotein" OR "triglyceride*" OR "cholesterol " OR "LDL" OR "HDL" OR "inflammation" OR "inflammatory markers" OR "C-Reactive Protein" OR "CRP" OR "Interleukin*" "IL" OR "oxidative stress" OR "malondialdehyde" OR "MDA"] and population ["gestation" OR "pregnancy" OR "pregnant" OR "gestational"]. Additional manual searches including reference lists of related studies as well as n reviews were reviewed to increase sensitivity of the search strategy. Studies included in this meta-analysis had to meet the following criteria: 1) original trials, 2) trials on humans, 3) intervention and control groups receiving omega-3 supplementation, and placebo or control, respectively and 4) the trials that reported mean changes or mean difference of metabolic parameters with standard deviation (SD) for the intervention and control groups. The search was restricted to clinical RCTs on humans and those published in English. In this study we did not include trials investigating the effects of flax seed oil supplementation or combined therapy of fish oil with other nutrients.
Data extraction and quality assessment
Two authors (EA and OA) independently extracted the data and assessed its quality using standard forms and the Cochrane Collaboration risk of bias tool [19, 20], respectively. This tool is based on information on the following domains: randomization generation, allocation concealment, blinding of subjects and outcome assessment, incomplete outcome data, and selective outcome reporting, and other sources of bias. When there was a disagreement between them, it was resolved by third author (JH). From eligible studies the following data were obtained: 1) first authors’ name 2) publication year 3) age, sex, and anthropologic parameters and/or metabolic parameters of study participants 4) study location 5) number of subjects in the intervention and control groups 6) study design 7) duration of the intervention.
Data analysis
Heterogeneity and publication biases
The statistical heterogeneity of the results of the included studies was tested using chi-square test [21], and quantified by the I2 statistic [22]. Publication bias was assessed by the funnel plot and tested for statistical significance using the Egger's test [23].
Results
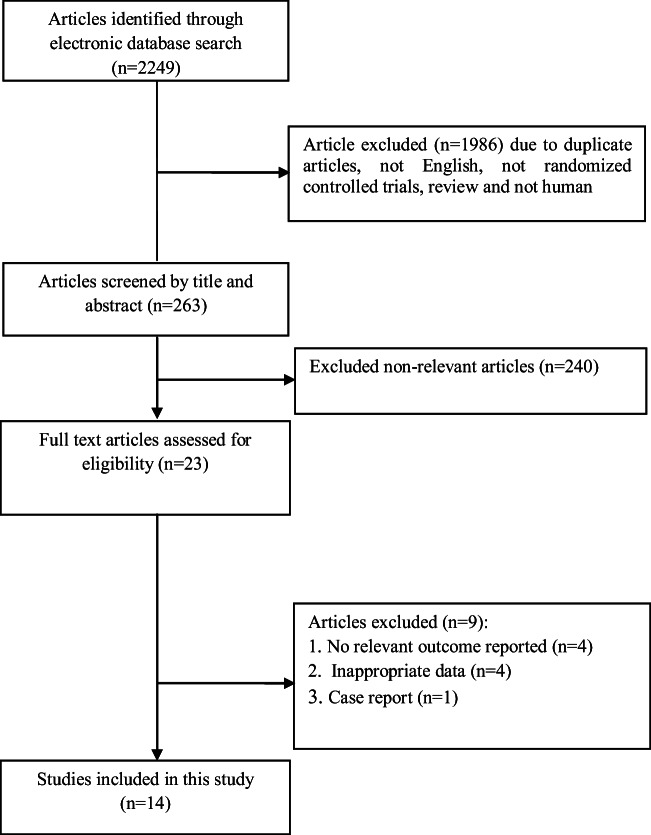
14 Studies with 15 effect sizes were included in this systematic review and meta-analysis. Flow-diagram of studies selection is presented in Fig. 1. Included studies were published from 2006 to 2019. 1468 subjects, including 718 controls, were enrolled in these studies. Participants were healthy, overweight or obese, and allergic pregnant women or those with gestational diabetes mellitus or at risk of depression. Studies were done in Australia, Norway, Germany, USA, Iran, Spain, New Mexico, and Finland. The trials used different doses of omega 3 fatty acids ranging from 1 g/day to 10 ml/day. The duration of intervention varied from 6 to 25 weeks. Studies reported no significant difference concerning side effects between intervention and control groups. TC, LDL-C, HDL-C, TG, TC/HDL-C ratio, FPG, insulin, HOMA-IR, MDA, CRP, IL-6 and IL-8 were measured as outcome in these studies. General characteristics of included studies are summarized in Table 1.
Fig. 2.
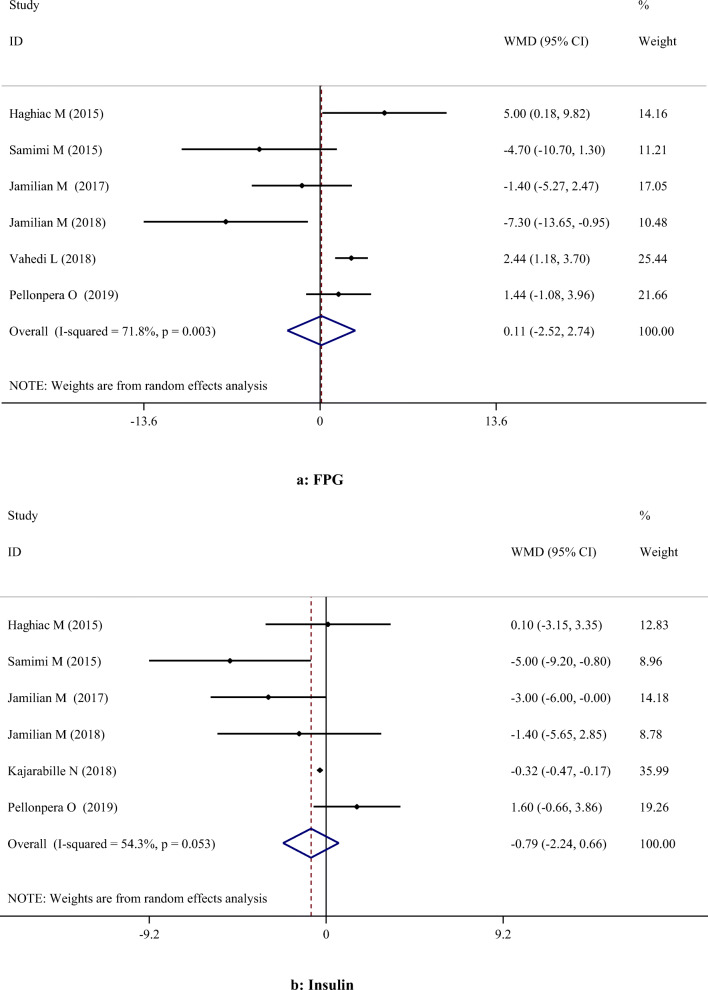
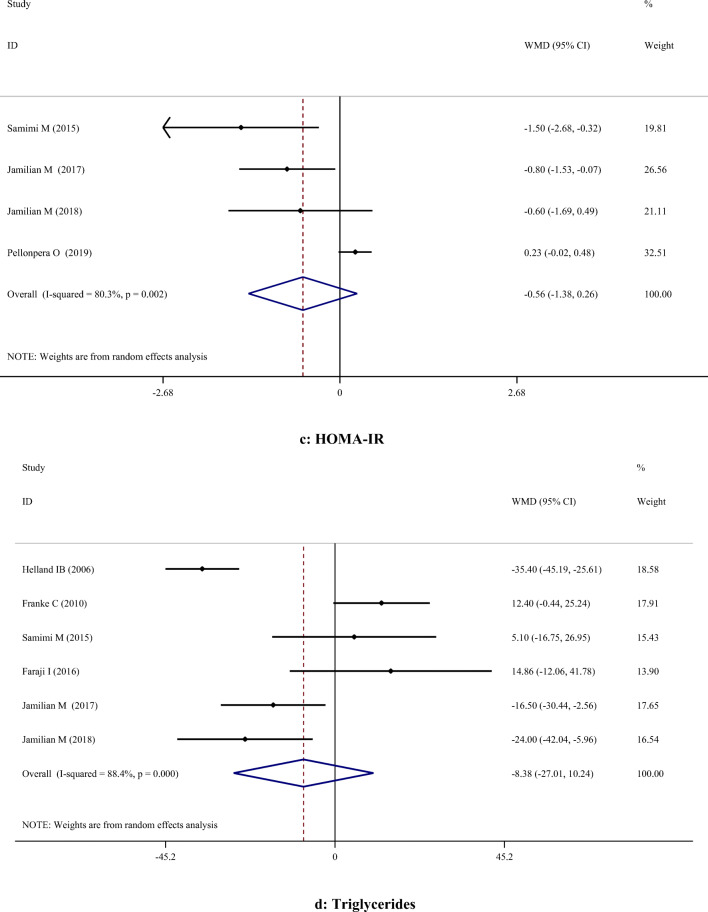
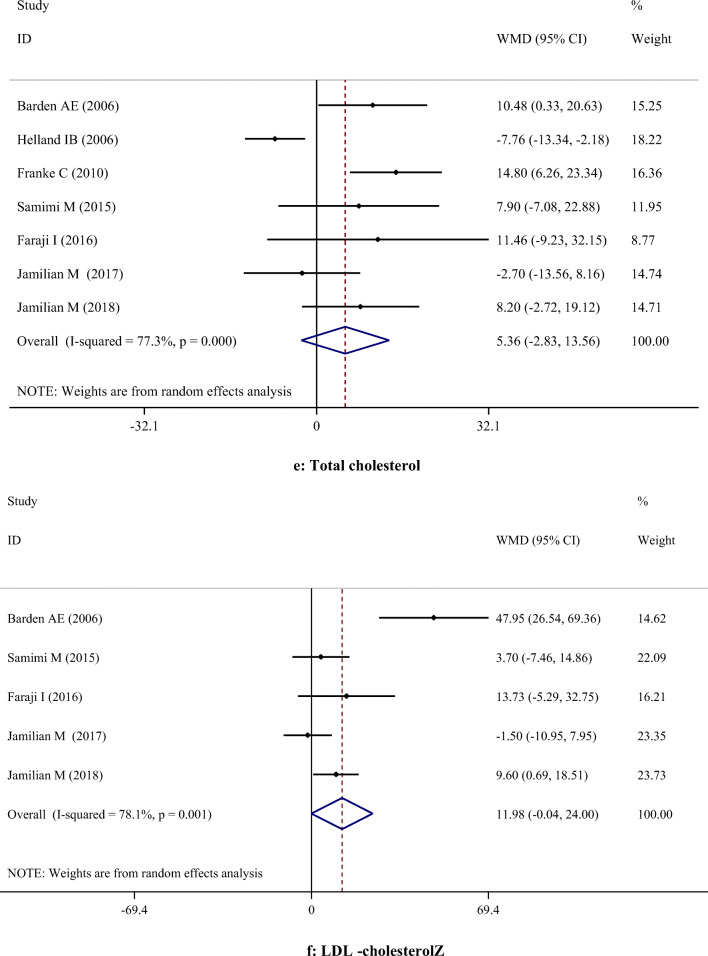
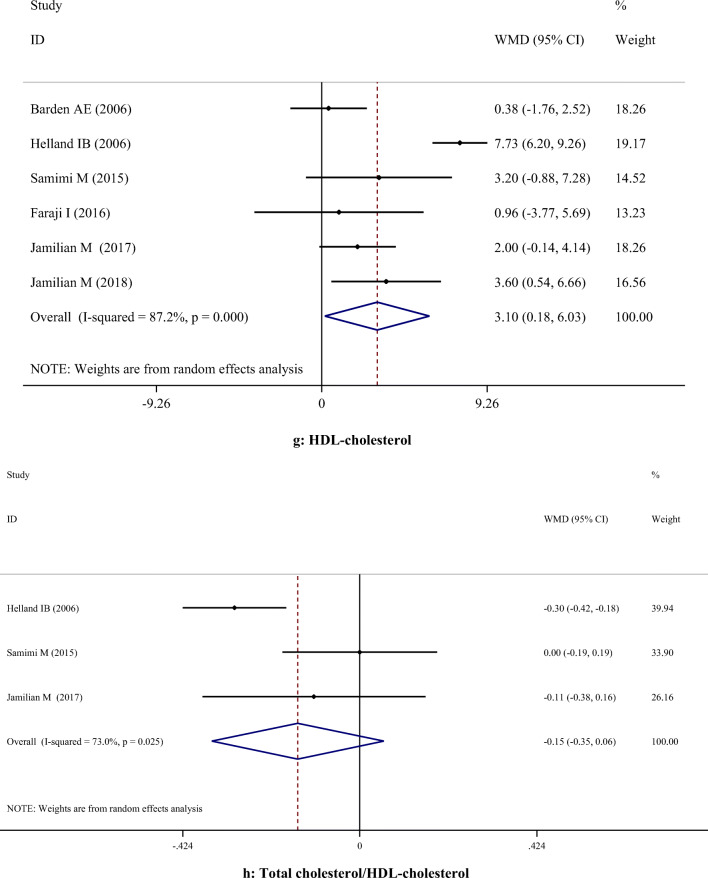
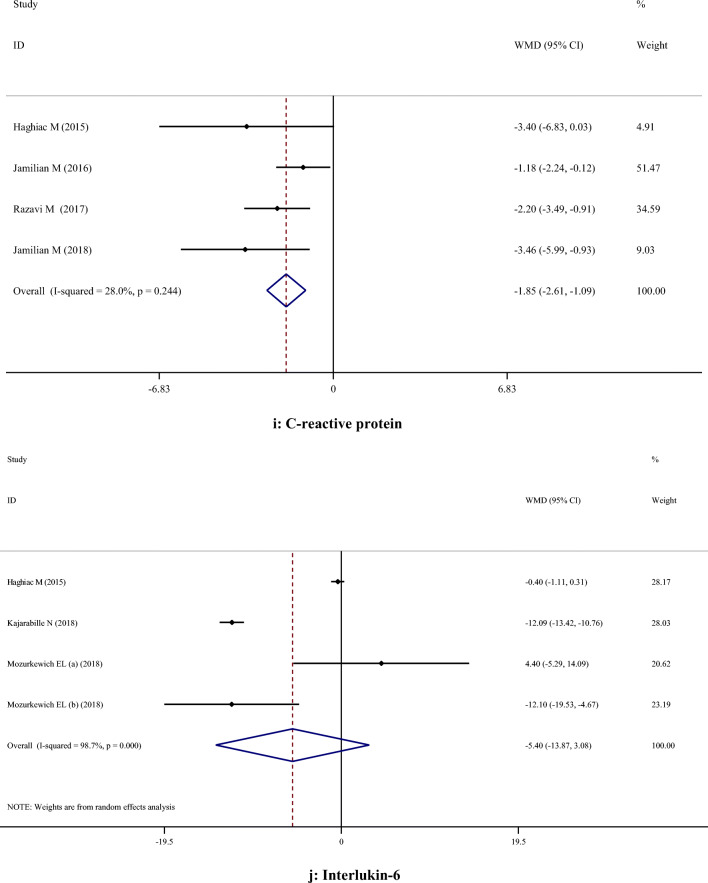
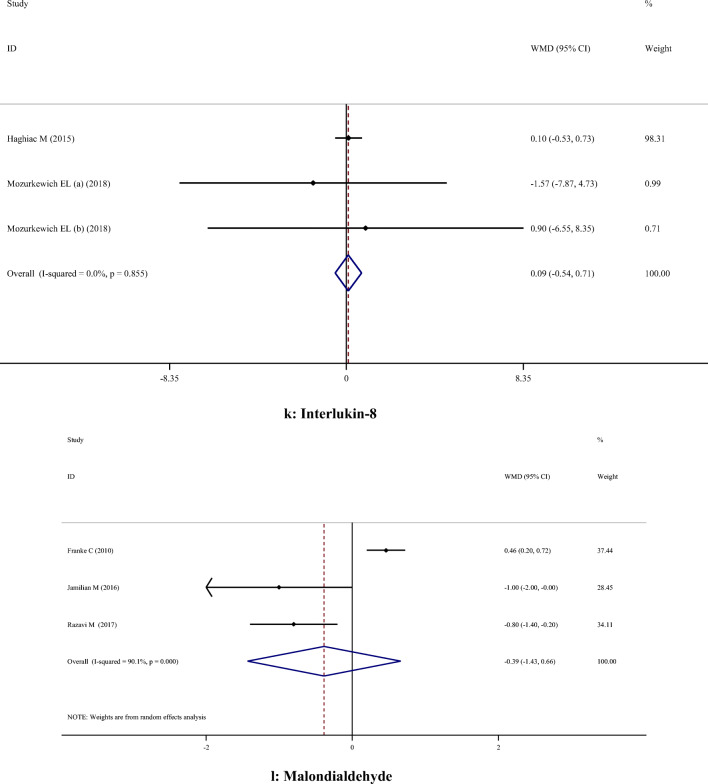
A-H. Meta-analysis metabolic profiles weighted mean difference estimates for A) FPG, B) Insulin, C) HOMA-IR, D) Triglycerides, E) Total cholesterol, F) LDL-cholesterol, G) HDL-cholesterol, H) Total cholesterol/HDL-cholesterol, I) C-reactive protein, J) Interleukin-6, K) Interlukin-8, I) Malondialdehyde in the omega-3 and placebo groups (CI = 95%)
Fig. 1.
Literature search and review flowchart for selection of studies
Table 1.
Characteristics of included studies
| Authors (Ref) | Publication year | Sample size (control/ intervention) |
Country/population | Intervention (name and daily dose) | Duration | Age (y) (control vs. intervention) |
Presented data |
|---|---|---|---|---|---|---|---|
| Barden et al. [17] | 2006 | 43/40 | Australia/ Pregnant women with allergic disease |
4 g/d fish oil (56% DHA + 27.7% EPA) |
≈ 16 weeks (20 wk until delivery) |
32.4 ± 3.27, 31.0 ± 3.7 | TC, LDL-C, HDL-C |
| Helland et al. [24] | 2006 | 154/160 | Norway/ Pregnant women | 10 ml/d cod liver oil (1183 mg DHA + 803 mg EPA) |
17 weeks (18 wk until 35) |
19–35 | TG, TC, HDL-C, TC/HDL-C |
| Franke et al. [18] | 2010 | 59/57 | Germany/ Pregnant women | Modified fish oil in form of milk-based supplements (500 mg DHA + 150 mg EPA) |
≈ 20 weeks (20 wk until delivery) |
31.4 ± 4.7, 30.8 ± 4.8 | TG, TC, MDA |
| Haghiac et al. [15] | 2015 | 24/25 | USA/ Overweight or obese pregnant women | 2 g/d fish oil (800 mg DHA + 1200 mg EPA) | 25 weeks (before 16 wk until delivery) | 27 ± 5 | FPG, insulin, CRP, IL-6, IL-8 |
| Samimi et al. [25] | 2015 | 28/28 | Iran/ Pregnant women with GDM | 1 g/d fish oil (120 mg DHA + 180 mg EPA) |
6 weeks (24–28 wk until 6 wk later) |
30.3 ± 5.5, 29.8 ± 5 |
FPG, insulin, HOMA-IR, TG, TC, LDL-C, HDL-C, TC/HDL-C |
| Faraji et al. [26] | 2016 | 47/45 | Iran/ Pregnant women with GDM | 1 g/d fish oil (120 mg DHA + 180 mg EPA) |
≈ 20 weeks (21 wk until delivery) |
27.3 ± 4.2, 25.6 ± 4.7 | TG, TC, LDL-C, HDL-C |
| Jamilian et al. [16] | 2016 | 27/27 | Iran/ Pregnant women with GDM | 1 g/d fish oil (120 mg DHA + 180 mg EPA) |
6 weeks (24–28 wk until 6 wk later) |
30.0 ± 5.5, 30.1 ± 5.3 | CRP, MDA |
| Jamilian et al. [27] | 2017 | 35/35 | Iran/ Pregnant women with GDM | 2 g/d fish oil (240 mg DHA + 360 mg EPA) |
6 weeks (24–28 wk until 6 wk later) |
30.7 ± 4.1, 30.7 ± 3.5 | FPG, insulin, HOMA-IR, TG, TC, LDL-C, HDL-C, TC/HDL-C |
| Razavi et al. [28] | 2017 | 30/30 | Iran/ Pregnant women with GDM | 2 g/d fish oil (240 mg DHA + 360 mg EPA) |
6 weeks (24–28 wk until 6 wk later) |
29.2 ± 3.4, 29.7 ± 3.6 | CRP, MDA |
| Jamilian et al. [29] | 2018 | 20/20 | Iran/ Pregnant women with GDM | 2 g/d fish oil (240 mg DHA + 360 mg EPA) |
6 weeks (24–28 wk until 6 wk later) |
30.8 ± 2.4, 30.5 ± 3.8 |
FPG, insulin, HOMA-IR TG, TC, LDL-C, HDL-C CRP |
| Kajarabille et al. [30] | 2018 | 46/44 | Spain/ Pregnant women | Fish oil enriched dairy drink (320 mg DHA + 72 mg EPA) |
≈ 12 weeks (28 wk until delivery) |
29.9 ± 4.7, 30.5 ± 4.8 | Insulin, IL-6 |
| Mozurkewich et al. (a) [31] | 2018 | 20/37 | New Mexico/ Pregnant women at risk for depression |
EPA-rich fish oil (1060 mg EPA + 274 mg DHA) |
minimum: 14 weeks (between 12–20 wk and 34–36 wk) | 18–40 | IL-6, IL-8 |
| Mozurkewich et al. (b) [31] | 2018 | 20/36 | New Mexico/ Pregnant women at risk for depression |
DHA-rich fish oil (900 mg DHA + 180 mg EPA) |
minimum: 14 weeks (between 12–20 wk and 34–36 wk) | 18–40 | IL-6, IL-8 |
| Vahedi et al. [32] | 2018 | 75/75 | Iran/ Pregnant women |
1 g fish oil (120 mg DHA + 180 mg EPA) |
20 weeks (6–10 wk until 26–30) |
26.9 ± 4.5, 25.9 ± 4.8 | FPG |
| Pellonpera et al. [33] | 2019 | 91/90 | Finland/ Overweight and obese pregnant women | Fish oil containing 2.4 g W3 (1900 mg DHA + 220 mg EPA) |
≈ 21 weeks (14 wk until 35) |
30.4 ± 4.1, 30.4 ± 4.8 | FPG, insulin, HOMA-IR |
HOMA IR, homeostasis model assessment of insulin resistance; TG, triglyceride; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; CRP, C-Reactive Protein; NO, nitric oxide; TAC, total antioxidant capacity; GSH, glutathione; MDA, malondialdehyde; TNF-a, tumor necrosis factor α; IL, interleukin
The effects of omega-3 PUFA on glycemic control
Our meta-analysis showed no significant effects of omega-3 PUFA supplementation on serum concentrations of FPG [Weighted Mean Difference (WMD): 0.11; 95% Confidence Interval (CI): -2.52, 2.74)] and insulin (WMD: -0.79; 95% CI: -2.24, 0.66), and on HOMA-IR (WMD: -0.56; 95% CI: -1.38, 0.26) (Table 2). Different findings were obtained in subgroup analyses. A significant reduction was found in serum levels of FPG following omega-3 PUFA supplementation in studies which lasted ≤ 6 weeks and were performed on patients with GDM. However, omega-3 PUFA supplementation resulted in a significant increase in FPG in studies with a duration of > 6 weeks and those performed on healthy subject. On the other hand, omega-3 PUFA supplementation had a significant effect on reducing insulin level in studies which lasted ≤ 6 weeks, and those using intervention dosage < 1 g/day which were performed on both healthy and GDM subjects. However, duration of intervention for > 6 weeks decreased the insulin levels significantly (Table 3).
Table 2.
Effect of omerga-3 on glycemic control, lipid profile, inflammatory biomarkers and oxidative stress biomarkers
| Variables | Number of effect sizes | Weighted Mean Difference | Confidence Interval 95% | P-value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I-squared (%) | P-value | ||||||
| Glycemic control | FPG | 6 | 0.11 | -2.52, 2.74 | 0.935 | 71.8% | 0.003 |
| Insulin | 6 | -0.79 | -2.24, 0.66 | 0.285 | 54.3% | 0.053 | |
| HOMA-IR | 4 | -0.56 | -1.38, 0.26 | 0.181 | 80.3% | 0.002 | |
| Lipid profile | TC | 7 | 5.36 | -2.83, 13.56 | 0.200 | 77.3% | < 0.001 |
| TG | 6 | -8.38 | -27.01, 10.24 | 0.378 | 88.4% | < 0.001 | |
| LDL-C | 5 | 11.98 | -0.04, 24.00 | 0.051 | 78.1% | 0.001 | |
| HDL-C | 6 | 3.10 | 0.18, 6.03 | 0.038 | 87.2% | < 0.001 | |
| TC/HDL | 3 | -0.15 | -0.35, 0.06 | 0.157 | 73.0% | 0.025 | |
| oxidative stress and Inflammatory biomarkers | CRP | 4 | -1.85 | -2.61, -1.09 | < 0.001 | 28.0% | 0.244 |
| IL-6 | 4 | -5.40 | -13.87, 3.08 | 0.212 | 98.7% | < 0.001 | |
| IL-8 | 3 | 0.09 | -0.54, 0.71 | 0.780 | 0.0% | 0.855 | |
| MDA | 3 | -0.39 | -1.43, 0.66 | 0.470 | 90.1% | < 0.001 | |
FPG, fasting plasma glucose; HOMA IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin-sensitivity check index; TC, total cholesterol; TG, triglyceride; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; CRP, C-Reactive Protein; IL, interleukin; MDA, malondialdehyde
Table 3.
Subgroup analysis of omerga-3 on glycemic control, lipid profile
| Number of effect sizes | Weighted Mean Difference | Confidence Interval 95% | P-value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| I-squared (%) | P-value | Between-study I2 (%) | |||||
| Effect of omerga-3 on FPG | |||||||
| Study duration (week) | |||||||
| > 6 | 3 | 2.38 | 1.28, 3.48 | < 0.001 | 0.0% | 0.432 | < 0.001 |
| ≤ 6 | 3 | -3. 39 | -6.28, -0.49 | 0.022 | 24.8% | 0.265 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 2 | 2.20 | -0.02, 4.43 | 0.053 | 39.3% | 0.199 | 0.587 |
| < 1 g/d | 4 | 1.50 | 0.35, 2.66 | 0.011 | 81.0% | 0.001 | |
| Health status | |||||||
| Healthy | 3 | 2.38 | 1.28, 3.48 | < 0.001 | 0.0% | 0.432 | < 0.001 |
| GDM | 3 | -3.39 | -6.28, -0.49 | 0.022 | 24.8% | 0.265 | |
| Effect of omerga-3 on insulin | |||||||
| Study duration (week) | |||||||
| > 6 | 3 | -0.31 | -0.46, -0.15 | < 0.001 | 29.3% | 0.243 | 0.010 |
| ≤ 6 | 3 | -3.11 | -5.22, -0.99 | 0.004 | 0.0% | 0.496 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 2 | 1.11 | -0.74, 2.96 | 0.240 | 0.0% | 0.457 | 0.127 |
| < 1 g/d | 4 | -0.33 | -0.48, -0.18 | < 0.001 | 62.7% | 0.045 | |
| Health status | |||||||
| Healthy | 3 | -0.31 | -0.46, -0.15 | < 0.001 | 29.3% | 0.243 | 0.010 |
| GDM | 3 | -3.11 | -5.22, -0.99 | 0.004 | 0.0% | 0.496 | |
| Effect of omerga-3 on TG | |||||||
| Study duration (week) | |||||||
| > 6 | 3 | -15.31 | -22.79, -7.84 | < 0.001 | 94.9% | < 0.001 | 0.878 |
| ≤ 6 | 3 | -14.34 | -24.19, -4.49 | 0.004 | 52.8% | 0.120 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 1 | -35.40 | -45.18, -25.61 | < 0.001 | - | - | < 0.001 |
| < 1 g/d | 5 | -2.94 | -10.44, 4.56 | 0.442 | 75.8% | 0.002 | |
| Health status | |||||||
| Healthy | 3 | -15.31 | -22.79, -7.84 | < 0.001 | 94.9% | < 0.001 | 0.878 |
| GDM | 3 | -14.34 | -24.19, -4.49 | 0.004 | 52.8% | 0.120 | |
| Effect of omerga-3 on TC | |||||||
| Study duration (week) | |||||||
| > 6 | 4 | 1.42 | -2.73, 5.58 | 0.501 | 87.4% | < 0.001 | 0.561 |
| ≤ 6 | 3 | 3.80 | -3.04, 10.65 | 0.276 | 12.6% | 0.319 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 2 | -3.52 | -8.41, 1.37 | 0.158 | 89.5% | 0.002 | 0.001 |
| < 1 g/d | 5 | 8.31 | 3.14, 13.49 | 0.002 | 36.1% | 0.181 | |
| Health status | |||||||
| Healthy | 4 | 1.42 | -2.73, 5.58 | 0.501 | 87.4% | < 0.001 | 0.561 |
| GDM | 3 | 3.80 | -3.04, 10.65 | 0.276 | 12.6% | 0.319 | |
| Effect of omerga-3 on LDL-C | |||||||
| Study duration (week) | |||||||
| > 6 | 2 | 28.82 | 14.60, 43.04 | < 0.001 | 81.8% | 0.019 | 0.002 |
| ≤ 6 | 3 | 4.20 | -1.39, 9.81 | 0.141 | 29.0% | 0.245 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 1 | 47.95 | 26.53, 69.36 | < 0.001 | - | - | < 0.001 |
| < 1 g/d | 4 | 4.96 | -0.40, 10.34 | 0.070 | 18.9% | 0.296 | |
| Health status | |||||||
| Healthy | 2 | 28.82 | 14.60, 43.04 | < 0.001 | 81.8% | 0.019 | 0.002 |
| GDM | 3 | 4.20 | -1.39, 9.81 | 0.141 | 29.0% | 0.245 | |
| Effect of omerga-3 on HDL-C | |||||||
| Study duration (week) | |||||||
| > 6 | 3 | 4.96 | 3.75, 6.16 | < 0.001 | 93.9% | < 0.001 | 0.023 |
| ≤ 6 | 3 | 2.62 | 1.01, 4.24 | 0.001 | 0.0% | 0.672 | |
| Dosage (EPA + DHA) | |||||||
| ≥ 1 g/d | 2 | 5.23 | 3.99, 6.48 | < 0.001 | 96.7% | < 0.001 | 0.006 |
| < 1 g/d | 4 | 2.45 | 0.93, 3.98 | 0.002 | 0.0% | 0.748 | |
| Health status | |||||||
| Healthy | 3 | 4.96 | 3.75, 6.16 | < 0.001 | 93.9% | < 0.001 | 0.023 |
| GDM | 3 | 2.62 | 1.01, 4.24 | 0.001 | 0.0% | 0.672 | |
FPG, fasting plasma glucose; HOMA IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; GDM, gestational diabetes mellitus
The effects of omega-3 PUFA on serum lipoproteins
Supplementation with omega-3 PUFA had no significant effects on serum concentrations of TC (WMD: 5.36; 95% CI: -2.83, 13.56), TG (WMD: -8.38; 95% CI: -27.01, 10.24), LDL-C (WMD: 11.98; 95% CI: -0.04, 24.00), and TC/HDL-C (WMD: -0.15; 95% CI: -0.35, 0.06). However, omega-3 PUFA supplementation had a significant effect on increasing HDL-C levels (WMD: 3.10; 95% CI: 0.18, 6.03). Subgroup analysis demonstrated that omega-3 PUFA supplementation increased TC level in studies which used dosage < 1 g/day. All subgroups showed that TG level was significantly reduced after omega-3 PUFA supplementation, except when dosage was less than 1 g/day. LDL-C level was significantly increased in studies with the duration > 6 weeks, when the dosage was ≥ 1 g/day and in those which were performed on healthy women. However, findings of the subgroup analysis did not change the results concerning HDL-C (Table 3).
The effects of omega-3 PUFA on MDA and inflammatory biomarkers
Supplementation with omega-3 PUFA reduced serum CRP concentrations (WMD: -1.85; 95% CI: -2.61, -1.09), but had no significant effect on IL-6 (WMD: -5.40; 95% CI: -13.87, 3.08), IL-8 (WMD: 0.09; 95% CI: -0.54, 0.71) and MDA (WMD: -0.39; 95% CI: -1.43, 0.66) concentrations (Table 2).
Publication bias
Findings from Egger’s regression test demonstrated that there was no considerable publication bias for FPG (P = 0.051), Insulin (P = 0.124), HOMA-IR (P = 0.068), TC (P = 0.233), TG (P = 0.137), LDL-C (P = 0.279), HDL-C (P = 0.085), TC/HDL (P = 0.350), CRP (P = 0.703), IL-6 (P = 0.841), IL-8 (P = 0.623), and MDA (P = 0.213).
Discussion
In this study, for the first time, the data of RCTs with omega-3 PUFA supplementation in pregnant women were analyzed. This meta-analysis showed that taking omega-3 PUFA supplements during pregnancy might increase HDL-C levels and decrease serum CRP concentrations.
Effects on parameters of glycemic control and serum lipoproteins
During the pregnancy different physiologic changes occur in metabolic status, including increased insulin resistance, dyslipidemia, increased inflammatory markers and decreased antioxidant defense system. Exacerbation of these changes is supposed to play an important role in pregnancy complications and negatively affects maternal and infant outcomes [4, 34]. The findings of this meta-analysis suggest that taking omega-3 PUFA supplements during pregnancy significantly increases HDL-C values while does not affect markers related to glycemic status including FPG, serum insulin levels, HOMA-IR, but neither TG, TC, LDL-C and total/HDL-C ratio. Several meta-analyses investigated the effects of omega-3 PUFAs intake in different populations. A meta-analysis by Gao et al.[35], indicated that fish oil supplementation could improve insulin sensitivity in individuals with metabolic disorders. Zhong et al.[12] reported that omega-3 PUFA supplementation decreased FPG and HOMA-IR in women with GDM. Another study demonstrated that taking EPA and DHA containing supplements resulted in a significant increase of both HDL-C and LDL-C levels [13]. A meta-analysis by Choi et al.[36] indicated that combination therapy with omega-3 PUFA and statins in patients with dyslipidemia improved lipid profiles except LDL-C when compared with statin monotherapy. Abdelhamid et al.[37] found that increased intake of fish and plant based omega-3 PUFAs in RCTs that lasted 12 months or more did not change lipid profiles and that increasing EPA and DHA has little or no effect on cardiovascular (CV) risk or mortality. Increased maternal insulin resistance plays a central role in the pathogenesis of GDM which is not a rare complication of pregnancy [38]. On the other hand, a recent study suggested that maternal lipid concentrations were associated with offspring DNA methylation metabolites and developmental epigenetic programming which might have an impact on lifelong disease risk [39]. Decreased insulin sensitivity can be associated with an altered lipid metabolism [40], and both are involved in the development of CV disease in mother and affect offspring health in later life [41, 42]. Omega-3 PUFA may promote insulin sensitivity and lipid profiles by regulation of transcription factors related to carbohydrate and lipid metabolism such as peroxisome proliferator-activated receptor (PPAR) alpha and gamma and sterol regulatory element binding protein-1c (SREBP-1c) [43], increased glucose transporter-4 (GLUT-4) and insulin receptor substrate-1 (IRS-1) expression [44], increased adiponectin secretion and anti-inflammatory functions [45]. Despite the established fact that omega-3 PUFA have TG-lowering effects in appropriate doses, conflicting results of different studies caused uncertainity whether they can prevent CV disease and events, particularly CV deaths [46]. Recently two studies were published analyzing the effects of omega-3 PUFA on serum lipoproteins and CV events on a large number of subjects. The first study was performed on 15,480 patients with diabetes who were treated with 1 g/day of omega-3 PUFA and no significant effect on reducing CV events could be seen [47]. However, in another study on 8,179 patients with elevated triglycerides and either diabetes or CV disease a much higher dose of 4 g/day resulted with a significant reduction of CV events [48, 49].
Effects on inflammation and oxidative stress markers
Our meta-analysis showed that omega-3 PUFA supplementation in pregnant women had a significant effect on CRP plasma levels but did not improve IL-6, IL-8 and MDA. An earlier meta-analysis by AbuMweis et al.[13] indicated that EPA and DHA was effective in reducing CRP levels. Two other meta-analyses also demonstrated that omega-3 PUFA supplementation was associated with a significant decrease in inflammatory markers in patients with T2DM [50] and GDM [12]. According to some studies fish oil supplementation in patients on hemodialysis reduced CRP concentrations without changing IL-6 and TNF-α levels [51]. Sepidarkis et al. [52] in a meta-analysis suggested that co-administration of omega-3 PUFA and vitamin E decreased MDA but did not improve other markers of oxidative stress. Ren et al.[53] reported that taking flaxseed and its derivatives could not improve CRP levels except in obese populations. Another study reported that omega-3 PUFA supplementation did not affect CRP levels in patients with chronic kidney disease [54]. During pregnancy inflammatory state and oxidative stress are associated with an imbalance between angiogenic and antiangiogenic factors which leads to low-flow uteroplacental circulation and chronic fetal hypoxia. This consequently increases the risk of placental abruption, premature rupture of membranes, fetal growth restriction, preeclampsia and stillbirth [55, 56]. Modulation of maternal immune system function and anti-oxidant defense system may be a potential therapeutic target to reduce these adverse pregnancy outcomes [57, 58]. Probable mechanisms by which omega-3 PUFA might achieve anti-inflammatory and antioxidant effects most probably include suppression of generation of pro-inflammatory eicosanoids by competition for active sites of cyclooxygenase and lipoxygenase enzymes, inhibition of nuclear factor kappa B (NF-kB), decreased cytokines production, activation of anti-inflammatory transcription factor PPAR gamma, modulation of cell membrane phospholipids composition, participating in nitric oxide (NO) synthesis, increasing resolvins and protectins production and restored antioxidant capacity [59–61].
This meta-analysis has some limitations. One of the most important is that subjects in the included studies had different clinical characteristics, and the studies were performed on healthy, overweight or obese, and allergic pregnant women as well as those with gestational diabetes mellitus or at risk of depression which might have an influence on the results. Moreover, due to the heterogeneity between the studies concerning variations in duration of omega-3 PUFA intake, the dosage and frequency of omega-3 PUFA used, the results of this meta-analysis should be interpreted with caution. The number of studies and sample size of participant's that finally were included in this meta-analysis was relatively low.
Conclusions
Based on the results of this meta-analysis, it could be concluded that omega-3 PUFA supplementation during pregnancy has a significant beneficial effect on HDL-C and CRP levels. Therefore, omega-3 PUFA intake might play an indirect role in improved pregnancy outcomes due to its effect on HDL-C and CRP levels.
Abbreviations
- HOMA IR
homeostasis model assessment of insulin resistance
- TG
triglyceride
- TC
total cholesterol
- HDL-C
HDL cholesterol
- LDL-C
LDL cholesterol
- CRP
C-Reactive Protein
- MDA
malondialdehyde
- IL
interleukin
Author contributions
JH and SC contributed in conception, design, statistical analysis and drafting of the manuscript. EA, ZA, OA, AM, MAM, ZR and BM contributed in conception, data collection and manuscript drafting. The final version was confirmed by all authors for submission.
Availability of data and material
The primary data for this study is available from the authors on direct request.
Compliance with ethical standards
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elaheh Amirani, Email: elahe.amirani@rocketmail.com.
Zatollah Asemi, Email: asemi_r@yahoo.com.
Omid Asbaghi, Email: omid.asbaghi@gmail.com.
Alireza Milajerdi, Email: Alimila66@gmail.com.
Željko Reiner, Email: zeljko.reiner@kbc-zagreb.hr.
Mohammad Ali Mansournia, Email: mansournia_ma@yahoo.com.
Jamal Hallajzadeh, Email: jamal.hallaj@yahoo.com.
Bahram Moazzami, Email: bahrammoazzami@gmail.com.
Shahla Chaichian, Email: shchaichian@gmail.com.
References
- 1.Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investig. 2016;26:109–27. doi: 10.1515/hmbci-2015-0025. [DOI] [PubMed] [Google Scholar]
- 2.Kalagiri RR, Carder T, Choudhury S, Vora N, Ballard AR, Govande V, et al. Inflammation in Complicated Pregnancy and Its Outcome. Am J Perinatol. 2016;33:1337–56. doi: 10.1055/s-0036-1582397. [DOI] [PubMed] [Google Scholar]
- 3.Wu F, Tian FJ, Lin Y, Xu WM. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. 2016;76:258–71. doi: 10.1111/aji.12454. [DOI] [PubMed] [Google Scholar]
- 4.Hoch D, Gauster M, Hauguel-de Mouzon S, Desoye G. Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol Aspects Med. 2019;66:21–30. doi: 10.1016/j.mam.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. BioMed research international. 2014;2014:418975. doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milman N, Paszkowski T, Cetin I, Castelo-Branco C. Supplementation during pregnancy: beliefs and science. Gynecol Endocrinol. 2016;32:509–16. doi: 10.3109/09513590.2016.1149161. [DOI] [PubMed] [Google Scholar]
- 7.Hovdenak N, Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2012;164:127–32. doi: 10.1016/j.ejogrb.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Pothineni NV, Singhal M, Paydak H, Saldeen T, Mehta JL. Fish, fish oils and cardioprotection: promise or fish tale? Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 9.Lowensohn RI, Stadler DD, Naze C. Current concepts of maternal nutrition. Obstet Gynecol Surv. 2016;71:413–26. doi: 10.1097/OGX.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadhwani N, Patil V, Joshi S. Maternal long chain polyunsaturated fatty acid status and pregnancy complications. Prostaglandins, leukotrienes, and essential fatty acids. 2018;136:143–52. [DOI] [PubMed]
- 11.Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11:Cd003402. doi: 10.1002/14651858.CD003402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong N, Wang J. The efficacy of omega-3 fatty acid for gestational diabetes: a meta-analysis of randomized controlled trials. Gynecol Endocrinol. 2019;35:4–9. doi: 10.1080/09513590.2018.1480716. [DOI] [PubMed] [Google Scholar]
- 13.AbuMweis S, Jew S, Tayyem R, Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J Hum Nutr Diet. 2018;31:67–84. doi: 10.1111/jhn.12493. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YY, Liu W, Zhao TY, Tian HM. Efficacy of omega-3 polyunsaturated fatty acids supplementation in managing overweight and obesity: a meta-analysis of randomized clinical trials. J Nutr Health Aging. 2017;21:187–92. doi: 10.1007/s12603-016-0755-5. [DOI] [PubMed] [Google Scholar]
- 15.Haghiac M, Yang XH, Presley L, Smith S, Dettelback S, Minium J, et al. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PloS one. 2015;10:e0137309. [DOI] [PMC free article] [PubMed]
- 16.Jamilian M, Samimi M, Kolahdooz F, Khalaji F, Razavi M, Asemi Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. J Matern Fetal Neonat Med. 2016;29:669–75. doi: 10.3109/14767058.2015.1015980. [DOI] [PubMed] [Google Scholar]
- 17.Barden AE, Dunstan JA, Beilin LJ, Prescott SL, Mori TA. n -- 3 fatty acid supplementation during pregnancy in women with allergic disease: effects on blood pressure, and maternal and fetal lipids. Clin Sci (Lond) 2006;111:289–94. doi: 10.1042/CS20060096. [DOI] [PubMed] [Google Scholar]
- 18.Franke C, Demmelmair H, Decsi T, Campoy C, Cruz M, Molina-Font JA, et al. Influence of fish oil or folate supplementation on the time course of plasma redox markers during pregnancy. Br J Nutr. 2010;103:1648–56. doi: 10.1017/S0007114509993746. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansournia MA, Higgins JP, Sterne JA, Hernan MA. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology. 2017;28:54–9. doi: 10.1097/EDE.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53:764–70. doi: 10.1038/sj.ejcn.1600880. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helland IB, Saugstad OD, Saarem K, Van Houwelingen AC, Nylander G, Drevon CA. Supplementation of n-3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. J Matern Fetal Neonatal Med. 2006;19:397–406. doi: 10.1080/14767050600738396. [DOI] [PubMed] [Google Scholar]
- 25.Samimi M, Jamilian M, Asemi Z, Esmaillzadeh A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: randomized, double-blind, placebo-controlled trial. Clin Nutr. 2015;34:388–93. [DOI] [PubMed]
- 26.Faraji I, Ostadrahimi A, Farshbaf-Khalili A, Aslani H. The impact of supplementation with fish oil on lipid profile of pregnant mothers: a randomized controlled trial. Changes. 2016;2:5.
- 27.Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol. 2017;11:459–68. doi: 10.1016/j.jacl.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab. 2017;14:80. doi: 10.1186/s12986-017-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamilian M, Samimi M, Mirhosseini N, Afshar Ebrahimi F, Aghadavod E, Taghizadeh M, et al. A Randomized double-blinded, placebo-controlled trial investigating the effect of fish oil supplementation on gene expression related to insulin action, blood lipids, and inflammation in gestational diabetes mellitus-fish oil supplementation and gestational diabetes. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed]
- 30.Kajarabille N, Peña M, Díaz-Castro J, Hurtado JA, Peña-Quintana L, Iznaola C, et al. Omega-3 LCPUFA supplementation improves neonatal and maternal bone turnover: a randomized controlled trial. J Funct Foods. 2018;46:167–74.
- 31.Mozurkewich EL, Berman DR, Vahratian A, Clinton CM, Romero VC, Chilimigras JL, et al. Effect of prenatal EPA and DHA on maternal and umbilical cord blood cytokines. BMC Pregnancy Childbirth. 2018;18:261. doi: 10.1186/s12884-018-1899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahedi L, Ostadrahimi A, Edalati-Fard F, Aslani H, Farshbaf-Khalili A. Is fish oil supplementation effective on maternal serum FBS, oral glucose tolerance test, hemoglobin and hematocrit in low risk pregnant women? A triple-blind randomized controlled trial. J Complement Integr Med. 2018;15. [DOI] [PubMed]
- 33.Pellonpera O, Mokkala K. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled. Double-Blind Clin Trial. 2019;42:1009–17. doi: 10.2337/dc18-2591. [DOI] [PubMed] [Google Scholar]
- 34.Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol. 2014;15:24–31. doi: 10.2174/1389201015666140330192345. [DOI] [PubMed] [Google Scholar]
- 35.Gao H, Geng T, Huang T, Zhao Q. Fish oil supplementation and insulin sensitivity: a systematic review and meta-analysis. Lipids Health Dis. 2017;16:131. doi: 10.1186/s12944-017-0528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HD, Chae SM. Comparison of efficacy and safety of combination therapy with statins and omega-3 fatty acids versus statin monotherapy in patients with dyslipidemia: a systematic review and meta-analysis. Medicine. 2018;97:e13593. [DOI] [PMC free article] [PubMed]
- 37.Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:Cd003177. doi: 10.1002/14651858.CD003177.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 39.Marchlewicz EH, Dolinoy DC, Tang L, Milewski S, Jones TR, Goodrich JM, et al. Lipid metabolism is associated with developmental epigenetic programming. Sci Rep. 2016;6:34857. doi: 10.1038/srep34857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wild R, Weedin EA, Wilson D. Dyslipidemia in pregnancy. Cardiol Clin. 2015;33:209–15. doi: 10.1016/j.ccl.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Palinski W, Nicolaides E, Liguori A, Napoli C. Influence of maternal dysmetabolic conditions during pregnancy on cardiovascular disease. J Cardiovasc Transl Res. 2009;2:277–85. doi: 10.1007/s12265-009-9108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendelson MM, Lyass A, O’Donnell CJ, D’Agostino RB, Sr, Levy D. Association of maternal prepregnancy dyslipidemia with adult offspring dyslipidemia in excess of anthropometric, lifestyle, and genetic factors in the Framingham heart study. JAMA Cardiol. 2016;1:26–35. doi: 10.1001/jamacardio.2015.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–92. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Lalia AZ, Lanza IR. Insulin-sensitizing effects of omega-3 fatty acids: lost in translation? Nutrients. 2016;8. [DOI] [PMC free article] [PubMed]
- 45.Pinel A, Morio-Liondore B, Capel F. n-3 Polyunsaturated fatty acids modulate metabolism of insulin-sensitive tissues: implication for the prevention of type 2 diabetes. J Physiol Biochem. 2014;70:647–58. doi: 10.1007/s13105-013-0303-2. [DOI] [PubMed] [Google Scholar]
- 46.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Nutr Rev. 2017;75:731–67. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 47.Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–50. [DOI] [PubMed]
- 48.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed]
- 49.Reiner Z, Laufs U, Cosentino F, Landmesser U. The year in cardiology 2018: prevention. Eur Heart J. 2019;40:336–44. doi: 10.1093/eurheartj/ehy894. [DOI] [PubMed] [Google Scholar]
- 50.O'Mahoney LL, Matu J, Price OJ, Birch KM, Ajjan RA, Farrar D, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovascular diabetology. 2018;17:98. doi: 10.1186/s12933-018-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He L, Li MS, Lin M, Zhao TY, Gao P. Effect of fish oil supplement in maintenance hemodialysis patients: a systematic review and meta-analysis of published randomized controlled trials. Eur J Clin Pharmacol. 2016;72:129–39. doi: 10.1007/s00228-015-1976-y. [DOI] [PubMed] [Google Scholar]
- 52.Sepidarkish M, Akbari-Fakhrabadi M, Daneshzad E, Yavari M, Rezaeinejad M, Morvaridzadeh M, et al. Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on oxidative stress parameters: a systematic review and meta-analysis. Clinical nutrition (Edinburgh, Scotland). 2019. [DOI] [PubMed]
- 53.Ren GY, Chen CY, Chen GC, Chen WG, Pan A, Pan CW, et al. Effect of flaxseed intervention on inflammatory marker c-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8:136. [DOI] [PMC free article] [PubMed]
- 54.Hu C, Yang M, Zhu X, Gao P, Yang S, Han Y, et al. Effects of omega-3 fatty acids on markers of inflammation in patients with chronic kidney disease: a controversial issue. Ther Apher Dial. 2018;22:124–32. [DOI] [PMC free article] [PubMed]
- 55.Polettini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: a systematic review of the literature. Placenta. 2015;36:969–73. [DOI] [PubMed]
- 56.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, et al. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22:143–57. doi: 10.1093/molehr/gav074. [DOI] [PubMed] [Google Scholar]
- 57.Girardi G. Can statins prevent pregnancy complications? J Reprod Immunol. 2014;101–102:161–7. doi: 10.1016/j.jri.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol. 2018;218:829-s40. doi: 10.1016/j.ajog.2017.11.565. [DOI] [PubMed] [Google Scholar]
- 59.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–15. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 60.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801:1260–73. [DOI] [PMC free article] [PubMed]
- 61.Jones ML, Mark PJ, Waddell BJ. Maternal dietary omega-3 fatty acids and placental function. Reproduction. 2014;147:R143-52. doi: 10.1530/REP-13-0376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.