Abstract
Purpose
Re-examine the current metabolic models.
Methods
Review of literature and gene networks.
Results
Insulin activates Pi uptake, glutamine metabolism to stabilise lipid membranes. Tissue turnover maintains the metabolic health. Current model of intermediary metabolism (IM) suggests glucose is the source of energy, and anaplerotic entry of fatty acids and amino acids into mitochondria increases the oxidative capacity of the TCA cycle to produce the energy (ATP). The reduced cofactors, NADH and FADH2, have different roles in regulating the oxidation of nutrients, membrane potentials and biosynthesis. Trans-hydrogenation of NADH to NADPH activates the biosynthesis. FADH2 sustains the membrane potential during the cell transformations. Glycolytic enzymes assume the non-canonical moonlighting functions, enter the nucleus to remodel the genetic programmes to affect the tissue turnover for efficient use of nutrients. Glycosylation of the CD98 (4F2HC) stabilises the nutrient transporters and regulates the entry of cysteine, glutamine and BCAA into the cells. A reciprocal relationship between the leucine and glutamine entry into cells regulates the cholesterol and fatty acid synthesis and homeostasis in cells. Insulin promotes the Pi transport from the blood to tissues, activates the mitochondrial respiratory activity, and glutamine metabolism, which activates the synthesis of cholesterol and the de novo fatty acids for reorganising and stabilising the lipid membranes for nutrient transport and signal transduction in response to fluctuations in the microenvironmental cues. Fatty acids provide the lipid metabolites, activate the second messengers and protein kinases. Insulin resistance suppresses the lipid raft formation and the mitotic slippage activates the fibrosis and slow death pathways.
Keywords: Tissue turnover, mTORC1, Fatty acids, Glutamine, Leucine, CD98, Mitochondrial pyruvate carrier proteins (MPC1&2)
Introduction
Diabetes and cancer are known as ancient diseases or diseases of civilization [1]. They are the neuro-metabolic disorders caused by the inappropriate uptake and utilization of protein-rich diets. Neurological disorders manifesting in excessive circulating blood sugar and its’ excretion in urine were recognized as symptoms of diabetes quite early in ancient history [2]. The two diseases differ in the fundamental issue of cell survival. While the cancer cell has perfected the survival pathways at the cost of its ageing host, insulin resistance (IR) and type-2 diabetes promote the slow-acting cell death pathways during ageing [3, 4].
The role of oxygen (O2), lactic acid in cell metabolism
Antoine Lavoisier in the eighteenth century defined the respiration as an exchange of O2 with the CO2 in the lungs. Lavoisier, however, suggested that the CO2 is not the direct product of the O2, but produced during metabolism. Besides, Lavoisier recognized that O2 is toxic, but animals survive the O2 toxicity by managing the CO2 produced in the body [5]. Parallel to the Lavoisier’s works on O2, the German physician, Karl Wilhelm Scheele (1780), demonstrated that sour milk produces lactic acid. Lactic acid remained at the centre stage of research in pathology in the nineteenth century. Berzelius in 1807/1808 observed that hunted stags accumulated the lactate in their muscles. Liebig, in a subsequent study, suggested that lactic acid accumulated in the dead muscles but not in living body. Scherer reported that lactic acid circulates in the blood of patients [6, 7]. Pasteur’s studies on Yeast and alcohol production in the middle of nineteenth century suggested that contamination of lactic acid-producing bacteria suspended fermentation and increased the growth of Yeast, causing losses to alcohol-producing units[8–10]. In two decades of extensive studies on frog’s muscle at the turn of the nineteenth century, Fletcher suggested that lactic acid is produced in contracting muscle during fatigue under anaerobic conditions but disappeared when exposed to pure oxygen. Lactic acid accumulated in muscles when the blood vessels are damaged or upon the development of rigour mortis (muscle death), during which the CO2 production decreased steeply, and lactic acid production increased exponentially. Fletcher did not speculate on the carbon source of lactic acid production, nor on CO2. Commenting on the Boehm ‘s suggestion, that glycogen could be the carbon source of lactic acid, Fletcher was categorical in suggesting that any suggestion on the role of glycogen in lactic acid production need to wait till the outcome of the effect of pancreatic juice (insulin) on the carbohydrate metabolism [11]. In a subsequent paper on the lactate vs CO2 production in the red and white muscles, Fletcher indicated that lactic acid could be the source of CO2 production in surviving excised muscle [12]. Contrary to this, Meyerhof and Warburg suggested that cells utilize the glycolytic energy and adapt to the anaerobic capacity for survival during the limitation of O2 availability or due to respiratory impairment. Meyerhof first proposed the glucose-lactate energetic theory to suggest that muscles utilize the energy of the anaerobic glycolysis for contraction and produce lactic acid during exercise and oxidise part of the lactic acid under aerobic conditions to reproduce glycogen. Warburg adopted Meyerhof’s lactate theory and proposed two hypotheses, the ‘Pasteur effect’ and ‘aerobic glycolysis’, to suggest that actively proliferating cells are glycolytic [13, 14]. Since then, glucose became the source of energy production in the cells, despite the demonstration that there is an inverse relationship between the O2 consumption and glucose uptake by the cells [15]. Pasteur was an advisor to alcohol industry. When some units stopped producing alcohol and exhibited growth of Yeast, Pasteur found that microbial contamination which produced lactic acid suspended fermentation and promoted the growth of Yeast. Besides he demonstrated that O2 accelerated growth, which required nitrogen as the nutrient and phosphate (Pi) as the mineral. Yeast converted the nitrogen in the nutrient medium during growth into the protein (Pasteur used the term “albuminoid like material”) [8, 9]. There is no difference in the amount ATP utilized per g of Yeast for the quantity of glucose consumed for fermentation or growth [10].In an extensive study on the lactate shuttles in muscles and mitochondrial metabolism, Brooks reported that lactate is a fuel for mitochondrial metabolism and lactate entry and exit into and out of cells is regulated by the monocarboxylate transporter isoforms in various tissues(MCT1, MCT2 and MCT4) [16, 17]. Recent studies in cancer cells by Sonveaux et al. demonstrated that lactate fuels the cancer cell respiration and targeting the monocarboxylic acid transporter1 (MCT1), prevents lactate entry into the cells and reduces the cancer cell proliferation [18].
Insulin and insulin resistance
The role of islets of Pancreas and insulin in diabetes came into focus during the nineteenth century; Paul Langerhans (1867) demonstrated the presence of non-pigmentary dendritic cells or stellate corpuscles in Pancreas, Oskar Minkowski and Joseph von Mering (1989) demonstrated that removal of pancreas from dogs resulted in development of symptoms of diabetes and dogs died soon [19]. Laguesse (1893) named the non-pigmentary dendritic cells as the Islets of Langerhans and suggested that they produce internal secretions [20]. Albert Sharpey-Schafer (1910) hypothesized that a single chemical, he named it insulin, produced by the islet cells could cause diabetes. A decade later in Macleod’s laboratory, Banting and Best (1921) purified insulin from the islets and demonstrated that insulin injection rescued the pancreatectomized dogs from death [21]. The diabetic patient Leonard Thompson, who was declared close to death was first to receive the purified insulin injections on January 11, 1922. Thomson lived for another 13 years. Enlisting the observations on the serum biochemical profiles of insulin treated subjects in clinical trials in his Nobel lecture, Macleod (1925) reported that insulin injection resulted in the disappearance of the inorganic phosphate (Pi) from the blood of patients along with the glucose but reappeared in the blood during the recovery period and was excreted in the urine. The glucose in the cells was neither catabolized to CO2 nor incorporated into glycogen but recycled as some unknown hexose phosphates. Macleod suggested that insulin injection causes hypoglycaemia and the patient could fall into coma unless supplemented with the dietary sugar, and suggested that the mechanism of insulin action in lowering the blood sugar needs to be understood to understand the carbohydrate metabolism in cells [22]. A decade after the success of insulin in treating diabetes, Himsworth (1930) distinguished diabetes patients into two categories, the insulin-sensitive and insulin insensitive (subsequently called the insulin resistant (IR) [23]. Both Macleod and Himsworth recognized that higher levels of circulating blood sugar (hyperglycaemia) is a compensatory mechanism for survival. Himsworth suggested that hyperglycaemia facilitates the utilization of glucose by the tissues in times of need. Recent studies demonstrate that there is a tissue-specific variation in glucose uptake and insulin sensitivity in diabetic patients [24, 25]. IR is recognized as the primary cause of metabolic syndromes (syndrome X) [26] caused by hypoxia and lipodystrophy but gives survival advantage to the patient [27], although manifesting in several neuroendocrine and vascular disorders. Some of the markers of lipodystrophy caused by IR include the enhanced circulation of branched chain amino acids (BCAA) [28–30].
The mechanism of insulin action in lowering the blood sugar is an unresolved issue
In teleological terms, insulin reduces the blood sugar by activating the uptake by the tissues, but we are yet to understand the mechanism of insulin action in lowering the blood sugar. In a detailed review on the complexity of the mechanism of insulin action and insulin resistance, Petersen and Shulman recently concluded “Regardless of its physiological provenance, insulin resistance is maladaptive in the setting of chronic over nutrition. Understanding insulin action and resistance more completely will facilitate the intelligent use of existing antidiabetic therapies, enable the development of new therapeutics, and, perhaps most importantly, inform prevention strategies to stem the tide of type 2 diabetes-[sic]” [31]. It reminds the concluding statement of Macleod’s Nobel lecture in 1925 “ At present, we are entirely at a loss to account for the disappearing glucose. When this problem is solved, it may be anticipated that a great advance will become possible in our knowledge of the intermediary metabolism of the carbohydrates [sic]” [22], which has lost the attention of global scientific community, perhaps by the dust created by the controversy on the Nobel prize [32]. Petersen and Shulman attributed insulin resistance to the lipodystrophy caused by the excess nutrients and the branched-chain amino acids and suggested it to be an adaptation mechanism to starvation. The authors took the example of the cave-dwelling Mexican fish, Astyanax mexicanus, which has mutations in the insulin receptor and develops fat deposits in their tissues as an adaptation for survival [33]. Semenkovich, in the 2016 Edwin Bierman Award Lecture suggested that the deregulated de novo lipogenesis, which is sensitive to insulin signalling, is the principal cause of dyslipidaemia and the microvascular disorders in diabetes [34]. In subsequent studies, Semenkovich reported that defects in myelination of Schwan cells in peripheral neurons and the oligodendrocytes in the central nervous system cause neuronal disorders in diabetes [35, 36]. The microvascular disorders have opposite effects on cancer and diabetes. Angiogenesis aggravates the proliferation and promotes the metastasis of tumours [37–39]. Hyperglycaemia and hyperlipidaemia cause the endothelial death, insulin resistance, and the microvascular disorders in retina, kidney and heart in diabetes [40, 42]. Microvascular pathology has multicellular origin, which include, the endothelial cells, smooth muscles, macrophages and immune cells. A miscommunication between the inter-connecting metabolic networks of different cells and tissues cause pathology [43–45]. The in vitro co-culturing of cells of the tumour micro-environment provide the evidence for such cross talk [46]. The cell transformation induced by the epithelial-mesenchymal transition (EMT) produces the anchorage independent cells. An interaction between the cytokines and nutrients between the anchorage independent cells in the micro-environment activate the re-programming of the cells’ metabolism and tissue remodelling [47–50].
Insulin resistance increases the lipolysis and the circulating fatty acids binding to the cell surface receptors produces the second messengers and protein kinases
Fatty acids and their metabolites act as ligands for the G protein coupled receptors (GPCRs) [51–53]. About 800 GPCRs are reported in cell membranes, out of which four classes of GPCRs (FFA1–4) are reported to bind the free fatty acids and their breakdown products including those of fibrates and activate the downstream cell signalling pathways of [53]. The GPCRs comprise seven helical transmembrane proteins, with an N-terminal region exposed to the exoplasmic side, while the C-terminal region in the cytoplasmic side binds to three heterotrimeric G-proteins, the Gα, Gβ and Gγ. Each of these proteins is polymorphic; in humans, the Gα subunit comprises four subunits, the Gαs, Gαi/o, Gαq/11 and Gα12/13, Gβ and Gγ subunits comprises 5 and 12 subunits respectively [54, 55]. In general, the long-chain fatty acids, the docosahexaenoic acid (DHA), α-linolenic acid, oleic acid and myristic acid activate the Gαq/11 family of G-proteins. The short-chain carboxylic acids and the keto acids, which are the digestive/oxidized products of fats and the BCAA activate the FFA 2 and FFA3 receptors. The FFA3 receptors are more widely distributed, in the gut, immune cells, adipose tissues and the spleen. These receptors have distinct downstream effects, while the FFA2 couples the signalling functions with Gαq/11, the FFA3 receptors couple their functions with the Gαi/o alone [55, 56]. The ligand binding to the GPCRs results in the production of secondary messengers, which include the cyclic AMP (cAMP), inositol trisphosphate (IP3), diacylglycerol, and calcium (Ca2+), each of which modulates distinct functions in the cells [56]. The sympathetic nervous system is a crucial regulator of the metabolic and endocrine functions of adipose tissue. Catecholamines and the neuro endocrine secretions activate the Gαs subunit, which promotes the cAMP dependent protein kinase A (PKA) signalling. PKA signalling increases the release of insulin in pancreatic β-cells, which results in hypoglycaemia and increased lipolysis in adipocytes. Gi-coupled α and β-adrenergic receptor signalling, which is involved in regulation of stress induced defence withdrawal [57], and in turn regulates the leptin expression and glucose and lipid metabolism in adipocytes [56]. The cAMP activates the PKA in mesenchymal stem cells and inhibits the insulin-stimulated mTORC1 activity by phosphorylation of Raptor, but does not reduce the amino acid-induced mTORC1 activity [58, 59]. The cAMP/PKA pathway inhibits the PI3K dependent AKT phosphorylation of GSK3β in human embryonic kidney cell lines, epidermal melanocytes and dopaminergic neurons, which releases the GSK3β mediated inhibition of Snail family transcriptional repressor 1 (SNAI1) [60, 61]. PKA also stabilizes the β-catenin pathway and activates the β- catenin signalling and promotes progesterone synthesis in corpus luteum [62, 63]. Adrenergic receptors (ARs) and the catecholamines induce the cyclic AMP (cAMP) activation and the transport of the amino acids and inhibit the autophagy. AR activate the lipolysis and pancreatic β cell insulin secretion and the glycogenolysis, and inhibits gluconeogenesis, which is controlled by the phosphodiesterases (PDE). PDE breaks the phosphodiester bonds of the cyclic nucleotides and regulates the overall lipid hydrolysis [64–66]. Prolonged activation cAMP/PKA pathway and the enhanced nitric oxide (NO) production increase the tumorigenesis. Blockade of adrenergic receptors induces inflammation, causes vascular dysfunction and liver cirrhosis and sepsis in cardiac and muscle tissues [67–70]. Phosphodiesterases rescue the endothelial cells from death in several tissues [71–76].
A reappraisal of the current model intermediary metabolism
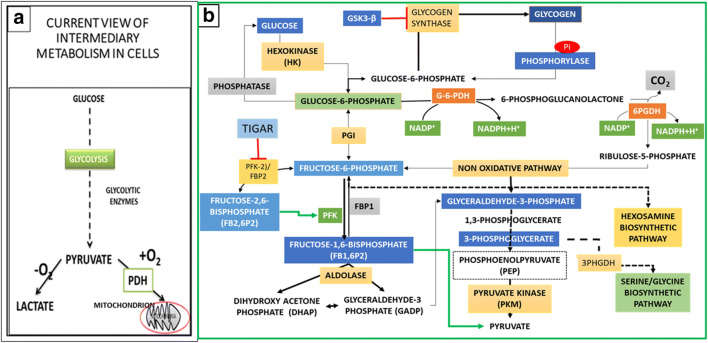
Parallel to the developments on the role of insulin in diabetes, Meyerhof and Warburg examined the metabolic alterations in muscle physiology and cancer cells, respectively. Meyerhof suggested that contracting muscles adapt to the anaerobic capacity during exercise due to the limitation O2 availability and Warburg et al. suggested that respiratory impairment, even in the presence of oxygen causes the carcinogenesis [13], which formed the basis for the current models of intermediary metabolism (Fig.1a). The discovery of ATP by Fiske and Subbarao [76], and subsequently by Lohmann [77], led to the identification of pyruvate kinase (PK) as one of the key regulatory enzymes in the production of ATP in glycolysis [78] Warburg proposed the hypothesis ‘aerobic glycolysis’ to suggest that proliferative cancer cells consume excess glucose and survive on reduced energy production. Lipmann suggested that ATP transfers the high energy phosphate group to the reacting components and the energy of ATP hydrolysis drives the metabolic reactions [79]. The emergence of the theories of bioenergetics in the middle of 1950s suggested that autotrophs utilize sun energy to produce carbohydrates and heterotrophs utilize the carbohydrates to produce the energy in the body for biological functions. These concepts stabilized the thinking that glucose is the source of energy and lactate is the product of anaerobic glycolysis and that the energy (ATP) is the principal driver of cell function [80, 81]. A critical review of the carbohydrate metabolism in the cytoplasm indicates glycogen hydrolysis activates the fluxes of two hexose monophosphates, the glucose-6 phosphate (G6P), Fructose-6 phosphate (Fr6P), and a bisphosphate fructose-1,6 bisphosphate (Fr1,6BP), which split into diverse triose phosphates in the cytoplasm in four distinct pathways; 1. the canonical glycolysis pathway, 2. the oxidative (oxPPP) and the non-oxidative pentose phosphate pathway (PPP), 3. The hexosamine biosynthetic pathway, and 4. the phosphoglycerate dehydrogenase (PHGDH) dependent serine biosynthesis (one-carbon metabolic) pathway (Fig. 1b). Two of these four pathways, the hexosamine and serine biosynthetic pathways integrate the metabolism of amino acids with that of mitochondria for production of metabolites for biosynthesis of synthesis of nucleotides, phospholipids for membrane synthesis and glycosylation of nutrient transporters and transcription factors involved in reprogramming of the gene expression during the cell cycle progression and tissue remodelling. Macleod highlighted two significant observations of the effect of insulin from the clinical trials in 1920s; 1) Following the insulin injection, Pi disappeared from the blood at the same rate as that of sugar, with a significant fall in excretion in the urine for several hours. During the recovery period, Pi entered the blood and was excreted in urine. 2) Sugar in the cells circulated as some unknown hexose phosphates. The lactacidogen, which was reported by Embden group as the precursor of lactic acid and Pi, was not detected in the patients treated with insulin. Bastedo and Irving (1928) subsequently reported [82] that Pi occurs as the labile organic phosphate mainly in the form of creatine phosphate (PCr).
Fig. 1.
Reappraisal of Glucose Metabolism in Cytoplasm; Effect of Insulin Action. a The Current Model of Intermediary Metabolism: Glucose is first oxidised in the cytoplasm to pyruvate. In the presence of O2 pyruvate enters the mitochondria to be oxidised to CO2. In the absence of O2 pyruvate is reduced to lactate to regenerate NAD+ required for the oxidation of glyceraldehyde-3phosphate (GA3P) to increase the flux of glucose oxidation to produce energy in times of intense cellular activity. b Insulin Activates the Inorganic Phosphate (Pi) Dependent Fluxes of Hexose Phosphates During Biosynthesis: During proliferation, cells utilise the glycogen hydrolysis for increasing the flux to glucose 6-phosphae (G6P) to produce pentose sugars and NADPH to maintain the redox homeostasis. Insulin induced Pi uptake activates the glycogen phosphorylase (GP) and produces the glucose 1-phosphae (G1P), which is the branch point between glycogen synthase (GS) and GP. Insulin inhibits GSK3β by activating the PI3K- Akt pathway and which increases flux of G1P to G6P, which is metabolised either in the pentose phosphate pathway (PPP) or converted to Fructose 1 phosphate (Fr-1P) in the canonical glycolysis. A bifunctional enzyme, the Fructose2,6 bisphosphate kinase2 (PFK2)/ Fructose2,6 bisphosphate phosphatase (PFK2/FBP2) phosphorylates the accumulating Fr6P and produces the Fr2,6 bis phosphate (Fr2,6BP). Fr2,6BP activates Fructose1,6 bisphosphate kinase1 (PFK1), which phosphorylates the Fr6P to produce Fr 1,6 bisphosphate (Fr1,6 BP). Fr1,6 BP has a feedforward effect on the pyruvate kinase (PK) and increases the activity of PK. In proliferative cells tyrosine kinases and ROS convert the PK to an enzymatically inactive form PKM2. The PPP has two branches, the oxidative (oxPPP) and the non-oxidative PPP. G6P is oxidised in oxPPP to produce the 5carbon ribulose -5 phosphate (RuP), NADPH and Co2, RuP is converted to ribose phosphate, Fr6P and the GA3P by a complex network of enzymes in non-oxidative PPP. The transaldolase and transketolase family of enzymes produce two Fr6P and three GA3P. Fr1,6 BP is hydrolysed to GA3P and the dihydroxy acetone phosphate (DHAP); the triose isomerase interconverts the two triosephosphates to increase the flux of GA3P. The tumour suppressor p53 produces the TP53 induced glycolysis and apoptosis regulator (TIGAR), which inhibits the kinase activity of PFK2/FBP2 and reduces the flux of carbons in glycolysis and increase the reverse flux of triose phosphates into the non-oxidative PPP. Glutamine/ glucosamine activates a parallel hexosamine biosynthetic pathway (HBP). The inhibition of the PFK2/FBP2 reduces the flux of Fr6P to Fr1,6BP (see Fig. 2a), which activates the glycosylation of several membrane proteins and the transcription factors to activate the nutrient transporters and the transcription factors (see Fig. 2a)
Insulin/Mitogenic signals Alter the fluxes of hexose and triose phosphates
Insulin is a mitogenic anabolic hormone, and the enzymes modulating the glycolysis assume non-canonical functions during cell cycle activation [83]. Huangyang and Simon (2018) recently reported that the glycolytic enzymes assume the non-canonical function and induce changes in gene transcription in diverse physiological and pathological states to maintain cell homeostasis [84], Park et al. (2020) reported that the dynamic alterations of the cellular cytoskeleton and the mechanical status of the matrix alter the functions of glycolytic enzymes, and disassembly of stress fibres activates the ubiquitination of the phosphofructokinase in bronchial epithelial cells [85]. The three hexose phosphates, the G6P, the Fr6P, and the Fr1,6BP, activate the canonical glycolysis or the PPP in the cytoplasm in response to the resting or insulin/mitogenic signals. The enzyme fructose-bisphosphate hydrolase (aldolase) splits the Fr1,6BP into two triose phosphates, the glyceradehyde-3 phosphate (GA3P) and the dihydroxyacetone phosphate (DHAP). The enzyme triosephosphate isomerase (TPI) interconverts the glyceraldehyde 3-phosphate (GA3P) and dihydroxyacetone phosphate (DHAP), which drives the reaction of aldolase in the forward direction to split the Fr1,6BP, and by oxidising the GA3P by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [86–88]. Fr1,6BP has allosteric functions and activate the enzyme pyruvate kinase to bind to its’ substrate the phosphoenolpyruvate (PEP) and activate the reaction to produce pyruvate and ATP. In respiring cells, the pyruvate kinase activity is drastically reduced and the PEP, which accumulates in the cytoplasm acts as feedback inhibitor of TPI and prevents the oxidative stress [87]. Besides, the accumulating PEP activates the reversible reactions of triose phosphates and increase the levels of the 3-phosphoglycerate, which has significant functional importance in metabolic remodelling (discussed below).
Inorganic phosphate (Pi) as the principal driver of fluxes of hexose phosphates and ATP production in glycolysis
Inorganic phosphate (Pi), which enters the cells following the insulin injection has two targets in the cytoplasm; 1. Pi activates the glycogen phosphorylase (GP), which hydrolyses the glycogen to an intermediate metabolite, glucose-1-phosphate (G1P; also called the Cori ester) and the enzyme phosphoglucomutase converts the G1P to G6P. 2. Pi is the substrate for GAPDH, which oxidises the GA3P to 1,3 bisphosphoglyceric acid (1,3BPG) and produce the reduced co-factor NADH. In the next step, Phosphoglycerate kinase (PGK) produces 3- Phosphoglycerate and the ATP [88–90]. Cori and Cori in 1920s demonstrated that muscle and tumour tissues possess abundant glycogen reserves and the G1P is an intermediate between the glycogen synthesis activated by glycogen synthase (GS) and breakdown by GP. Insulin regulates this balance through two antagonistic pathways; insulin dependent PI3K-Akt pathway inhibits glycogen synthase kinase 3β (GSK3β) which inhibits GS; the pathway is inactive in the proliferative metastatic cells and activated only during the lineage specific differentiation [91, 92]. The insulin induced Pi entry into cytoplasm activates the GP, which hydrolyses the glycogen and increase the flux to G1P (Fig. 1b) [93]. Recent studies by Witney et al. (2014) and Zois and Harris (2016) demonstrated that glycogen reserves are high in cancer tissue, and that glycogen metabolism remodels the cancer cell metabolism under stress conditions [94, 95]. Insulin activates the breakdown of glycogen by inhibiting the glycogen synthase-3 kinase-β (GSK3α/β) and through the Pi dependent phosphorylase [96–99]. Besides, GSK3β is a multifunctional protein which is also a target of the cyclic AMP (cAMP) and immune suppressants. The cAMP and GSK3β have reciprocal relationship; cAMP inhibits GSK3β, by phosphorylating the kinase on serine 9 and promotes neuronal survival independent of the Akt and PKA, but in melanoma cAMP inhibits phosphatidylinositol 3-kinase (PI3K)-Akt pathway and activates the GSK3β [60] and phosphodiesterases play a critical role in regulating the cAMP function in proliferative cells by forming cAMP, PKA, and GSK3 regulatory loop in regulating the proliferation and differentiation [100], GSK3β inhibition releases the transcriptional repressor SNAI1 and activates the Wnt- β-catenin pathway [101]. SNAI1 inhibits the transcription of E-cadherin and promotes EMT and the anchor independent cell proliferation [102]. Berzal et al. (2012) reported that the immunosuppressant cyclosporine A inhibited GSK3β and upregulated transcriptional repressors (Snail, Slug, and Twist) and other adherent and tight junction proteins, which resulted in fibrosis of renal tubular cells [103]. Post-translational modifications and cellular trafficking of GSK3β are implicated in several disorders, including diabetes, cancer, Alzheimer’s and bipolar disorders [104]. The isoform GSK3α is upregulated in diabetes implicating its’ role in glucose release from liver in diabetic patients [105, 106]. The allosteric regulation of glycogen phosphorylase rather than the GSK3 isoforms are suggested to regulate the fluxes between the glycogen synthesis or its’ break down. Proliferative cells depend on the increased production of G6P, which is a substrate for the oxidative pentose phosphate pathway (oxPPP) required to produce the pentose sugars and the reduced co-factor NADPH [31]. Glucose phosphate isomerase (GPI) converts the G6P to Fr6P; besides a complex network of enzymes in a non-oxidative PPP produce Fr6P. The ox PPP is the principal source of NADPH, redox homeostasis and the ribulose 5-phosphate. NADPH activates the reduction reactions in the biosynthetic pathways of fatty acids and reductive carboxylation. The ribulose phosphate isomerase (RPI) produces the ribose-5phosphate, which is a substrate for nucleotide biosynthesis [107, 108].
The non-oxidative PPP is the principal source of Fructose-6 phosphate (Fr6P) and the glyceraldehyde 3-phosphate (GA3P) in proliferative cells
The non-oxidative PPP is the major platform of the carbon fluxes between the Fr6P, pentose sugars and trioses, which are catalysed mainly by the transaldolase (TALDO) and the transketolase (TKT) [109, 110]. The oxPPP oxidises the G6P, and produces 2 NADPH, one CO2 and one ribulose-5-phosphate (Ru5P). The nonoxidative PPP activates the reversible enzymes belonging to the transketolase (TKT) family (transketolase-like 1(TKTL1) and TKTL2) and converts the Ru5P to xylulose-5-phosphate (X5P) and Ru5P by the Ru5P epimerase and isomerase, respectively. Besides, the TKT transfers two-carbon groups from X5P to ribose-5phosphate (R5P) and generates the sedoheptulose-7-phosphate (S7P) and glyceraldehyde-3-phosphate (GA3P). TALDO transfers three-carbon groups from S7P to GA3P to generate erythrose-4-phosphate (E4P) and Fr6P and two-carbon groups from X5P to E4P to generate GA3P and Fr6P, which enter the glycolysis [107]. When cells experience high oxidative stress, metabolites from the non-oxidative pathway activate the PHGDH dependent one-carbon metabolic pathways (Fig. 2b) to refill the NADP+ to sustain the oxidative arm with NADP+ for production of NADPH and the pentose sugars. In proliferative cells, the antioxidant Nuclear Factor, Erythroid 2-Like 2 (NRF2)/Kelch-Like ECHAssociated Protein 1 (KEAP1) antagonises the BTB and CNC Homolog 1 (BACH1). BACH1 is a haem-binding transcription factor, which negatively regulates transcription of the genes of mitochondrial electron transport chain (ETC) during the differentiation and protect the cancer and vascular cells from apoptosis [111]. NRF2)/ KEAP1 pathway activates the expression TKT family of proteins and the TP53-induced glycolysis and apoptosis regulator (TIGAR) to protect the survival of glioblastomas by enhancing the activity of TKTL1 and NADP+ production and sustain the carbon flux to increase the OxPPP and redox homeostasis [112, 113]. Deficiencies in the enzymes of the non-oxidative PPP are reported to result in pre-natal growth retardation, haemolytic anaemia, neuromotor dysfunctions, neuro-muscular disorders, the hepatic and kidney diseases [114, 115].
Fig. 2.
Hexose Shuttles Interact with Amino Acids and Regulate Membrane Transporters and The Hexosamine Biosynthetic Pathway: Glutamine activates the rate limiting enzyme of the hexosamine biosynthetic pathway (GFAT) in response to the depleting levels of 2-oxoglutarate (2-OG), which in a series of reactions involving the fatty acid β-oxidation and the nucleotide uridine trisphosphate (UTP) produces the UDP-N acetyl glucosamine (UDPGlcNAc). UDPGlcNAc is the substrate for O-linked glycosylation (OGlcNAc) and N-linked GlcNAc (NGlcNAc) of several membrane proteins and transcription factors. Glycosylation of the multifunctional surface antigen and the Solute Carrier Family 3 Member 2 (SLC3A2/ 4F2HC/CD98), which complexes with the glutamine, cysteine and the essential amino acids transporters across the plasma membrane involved in the de novo fatty acid and the cholesterol biosynthesis (see the text) b Serine-Glycine (One Carbon Metabolic) Biosynthetic Pathway: TIGAR mediated inhibition of kinase activity of PFK2/FBP2 inhibits the PFK1 and tyrosine phosphorylation downregulates the PKM2, which results in the accumulation of the phosphoenolpyruvate (PEP). PEP accumulation activates the reversible reactions and increase the levels of 3-phosphoglycerate. The enzyme phosphoglycerate dehydrogenase (PHGDH) diverts the 3-phosphoglycerate to activate the serine-glycine (one-carbon metabolic) biosynthetic pathway. The one-carbon metabolic pathway activates multiple pathways for the synthesis of the nucleotides, phospholipids, inositol phosphates, S adenosine methionine, and the creatine phosphate (PCr), which are involved in the whole carbon metabolic homeostasis (see the text)
Hexosamine biosynthetic pathway
Fr6P is a substrate for the hexosamine biosynthetic pathway (Fig. 2a), which is activated in response to the metabolic stress induced by reduced mitochondrial glutamine metabolism and requires the glutamine uptake to produce end product uridine diphosphate N-acetyl glucosamine (UDP-GlcNAc), [47, 116]. Under the normal/ healthy conditions, the Fr6P levels are controlled by the fructose 2,6 bisphosphate (Fr2,6P2). The bi-functional enzyme PFK-2/ FBP-2 (6-phosphofructo-2-kinase)/(fructose-2,6-bisphosphatase) converts the Fr6P to Fr2,6P2, which is an allosteric activator of phosphofructokinase-1 (PFK-1), which phosphorylates Fr6P to fructose 1,6 bisphosphate (Fr1,6P2). The enzyme fructose-1,6-bisphosphate-1-phosphohydrolase (FBP1) hydrolyses the 1-phosphate group of Fr1,6P2 to increase the levels of Fr6P. These inter-conversions are regulated by the Phosphatase and Tensin Homolog (PTEN), which regulates the production of Fru2,6P2 [117], AMP is an allosteric inhibitor of the FBPase1 [118]. FBPase1senses the depleting levels of Fr1,6P2 and together with the aldolase, activates the AMP-activated protein kinase complex (AMPK) at the lysosomal surface and suppresses the mTORC1 [119–121]. Canonical model of glycolysis proposes that pyruvate kinase produces two net ATP molecules and protects cells against hypoxic stress. The stoichiometric analysis of the number of molecules of glyceraldehyde 3-phosphates produced in the non-oxidative PPP (3molecules) plus 1GA3P + 1DHAP = 2 molecules produced by the aldolase reaction and the ATP produced by the GAPDH and PGK by incorporating the Pi into ADP is at least three times higher per molecule of glucose oxidized through PPP compared to the pyruvate kinase in canonical glycolysis [107]. It is interesting to note that Warburg identified 1,3 bisphosphoglyceric acid and Meyerhof suggested that an ATPase is a mandatory requirement for driving the GA3P oxidation, which couples the ATP production in glycolysis with the mitochondrial inner membrane (IMM) potential (Δψm) described by Mitchel and Paul Boyer [122]. Martinus et al. (1996) and Buchet and Godinot (1998) reported the presence of the functional Δψm in rho degrees cells (r° cells) depleted of mtDNA during electron transfer or during ATP hydrolysis catalysed by the ATPase-ATP synthase [123, 124]. Appleby et al. (1999) reported that the maximum value of this potential was approximately 110 mV in permeabilized cells and approximately 67 mV in intact cells, which is enough to import nuclear-encoded proteins and consumed about 13% of the ATP produced by glycolysis [125]. It may not be out of place to mention that the glycerol-3 phosphate dehydrogenase, which oxidizes the glycerol-3phosphate to the DHAP transports the hydrogens to the complex-II of ETC during the phospholipid and triglyceride biosynthesis during nucleotide synthesis, the cytosolic enzyme dihydro-orotate dehydrogenase (DHODH) physically associates with complex-II of respiratory chain [126] Several laboratories in Europe [127] [reviewed by Ibsen (1961)], and the works of Wu and Racker in the middle of twentieth century demonstrated that Pi is a limiting factor in the ATP production in glycolysis [128] and that the ATP production in mitochondrial oxidative phosphorylation lasts only 30s to 2 min. and glucose suppresses the ATP production in ETC and activates the biosynthesis. Loomis and Lipmann (1948) demonstrated that di-nitrophenol inhibits the Pi uptake and oxidative phosphorylation but activates the glucose uptake and glycolysis [129]. Recent studies report that Sirtuin 3 (Sirt3) inhibits the MDM2 and activates p53, which arrests the cell cycle and activates the senescence [130, 131]. The non-oxidative flux of PPP also produces the Fr6P and inhibition of the PFK-2 /FBPase-2 by TIGAR results in the accumulation of Fr6P, which activates the hexosamine biosynthetic pathway (HBP), which requires the glutamine as the substrate to activate the rate limiting enzyme of the pathway, the glutamine-fr6P amino transferase (GFAT1) to synthesize the glucosamine-6Phosphate. In subsequent steps catalysed by the glucosamine-6-phosphate N-acetyltransferase followed by phosphoglucomutase and UDP-N-acetyl-hexosamine pyro-phosphorylase integrates the fatty acid oxidation and nucleotide (uridine trisphosphate; UTP) metabolism to produce the uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) [306]. UDP-GlcNAc is a substrate for the O-linked glycosylation (OGlcNAc) and N-linked GlcNAc (NGlcNAc) in a reversible pathway catalysed by the O-GlcNAc-transferase (OGT) and O-GlcNAcase respectively the OGlcNAc regulates the activities of several proteins through the dynamic addition/ removal of glycosylated moieties. The pathway plays an important role in regulation of the proteins involved in nutrient sensing, transcription factors, signal transduction, and maintains the communication between the microenvironment, intracellular organelles and chromatin material in the nucleus in response to the nutrient, hormonal and environmental clues [132, 133]. The glutamine transporter SLC1A5 (also known as the ASCT2) requires the glycosylation of two asparagine residues, the N163 and N212, for localization into the plasma membrane. ASCT2 expression increases in inflammation and cancer stem cells become auxotrophic for glutamine for survival [134]. These changes are temporally regulated.
PHGDH activates the de novo serine synthesis, and coordinates the central carbon metabolism
The TIGAR inhibits the kinase active isoform PFKFB3 of the bifunctional enzyme PFK-2 /FBPase-2 and reduces the activity of PFK-1and the Fr1,6P2, resulting in the reduction of Pyruvate kinase activity and accumulation of PEP. Accumulating PEP has dual roles; (1) it inhibits the TPI leading to the activation of the FBPase1 and accumulation of Fr6P and activates AMPK [121, 135] and the hexosamine biosynthetic pathway. (2) PEP accumulation activates the reversible reaction catalysed by the enolase and the phosphoglycerate mutase, which results in the accumulation of 3-phosphoglycerate. The Phosphoglycerate dehydrogenase (PHGDH) activates the one-carbon metabolic pathway leading to the de novo serine and glycine biosynthesis, nucleotide production, which simultaneously alters the PPP and mitochondrial metabolism (Fig. 2b). The pathway has a vital role in Heme biosynthesis in vascular cells and maintains redox homeostasis in cancer stem cells [136, 137] and central carbon metabolism and utilises the glucose, glutamine, serine, and glycine through folate cycle and S-adenosylmethionine (SAM) to produce the membrane phospholipids, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol [138–141]. SAM plays an important role in the histone methylation and CrP, cholesterol synthesis, and promotes senescence [142–144] by terminating the cell cycle and activation of growth of differentiated cells [145–147].
Role of mitochondria in cell cycle regulation and metabolism
Proliferative cells increase the respiration (O2 consumption) was demonstrated by Pasteur and Crabtree. The present model of IM depicts mitochondria as catabolic hubs and the mitochondria respiration promotes ATP (energy) production. Recent studies by Patel and others suggest that ATP acts as a biological hydrotrope [148, 149] and acts as the solubilising agent of intrinsically disordered proteins and the complex chromatin material by phosphorylation of histones. Phosphorylation of histone proteins opens the chromatin material for genetic remodelling by demethylating and acetylating agents [150, 151]. Lipmann (1941) hypothesised that ATP hydrolysis provides the energy for phosphate transfers to activate the metabolic reactions [79]. A careful study of the signalling pathways indicates that the kinases and phosphatases regulate the pathways by phosphorylation and dephosphorylation of proteins alter the protein structure to facilitate the substrate binding/ inhibition to perform their functional role, which is no different from acetylation/deacetylation or methylation/ demethylation dynamics in epigenetic control of protein function. Depleting 2-OG levels inhibit the dioxygenases and demethylases and upregulate the HIF1, which activates methylation of histones and the differentiation of proliferative cells [152–154]. One of the Key steps, in which the fatty acids are activated by fatty acyl-CoA synthesis requires the hydrolysis of ATP to produces the AMP and pyrophosphate (PP). Both AMP and phosphate have epigenetic functions in controlling the fatty acid catabolism. The carnitine palmitoyl-CoA transferase (CPT1) transfers the activated fatty acids into mitochondria for β-oxidation in mitochondria [155]. Malonyl-CoA regulates fatty acid oxidation by inhibiting the CPT1. AMP activates the AMPK, which phosphorylates and inhibits the acetyl-CoA carboxylase and malonyl-CoA synthesis, to regulate the substrate flux during the fatty acid synthesis [156].
The paradox of the TCA cycle in Lipogenesis, a historical review
The current model of the mitochondrial metabolism (Fig. 3a) depicts the pyruvate decarboxylation to acetyl-CoA activates the TCA cycle to produce the CO2, the dicarboxylates and energy. The review of the historical development of TCA cycle (BOX) suggests that Krebs and Johnson (1937) proposed the cyclic nature of the TCA cycle when they observed that addition of small quantities of pyruvate to the pigeon breast muscle extract increased succinate [157] and based on the earlier reports of Szent Gyorgyi that the oxidation of the dicarboxylates increases respiration and those of Martius group, who reported that citrate is oxidised to isocitrate by aconitase and the isocitrate dehydrogenase (IDH) catalyses the oxidative decarboxylation of the isocitrate to the 2-OG (2-oxoglutarate, also called the α- ketoglutarate), but the equilibrium of the reaction favoured the citrate production, which was later confirmed by Johnson (1939) [158]. In a later study, Krebs and Holzach (1952) demonstrated that aconitase produces an intermediate product, the cis-aconitase, between citrate and isocitrate [159]. In a series of experiments between 1945 and 55, on the citrate production, Ochoa Stern, Kornberg, Lynen and other collaborators proposed two models for citrate production, the reductive carboxylation and the condensing reaction (also known as the citrate synthase; CS). Ochoa demonstrated that the reductive carboxylation of 2-OG produces 90% of the citrate when the reaction is carried out in the presence of oxidative pentose phosphate pathway (oxPPP) and aconitase, when the Mg2+ / Mn2+ and NADPH are the cofactors. The condensing reaction takes place only when the OXA concentration is less than 2 µM (micro molar). The concentration of OXA exceeding 2 µM (micro molar) inhibits the reaction (BOX) [160–173].
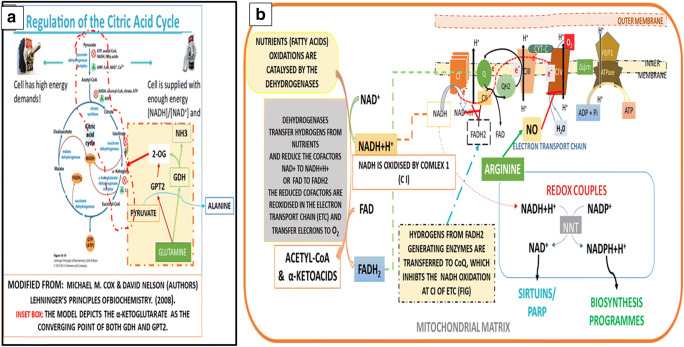
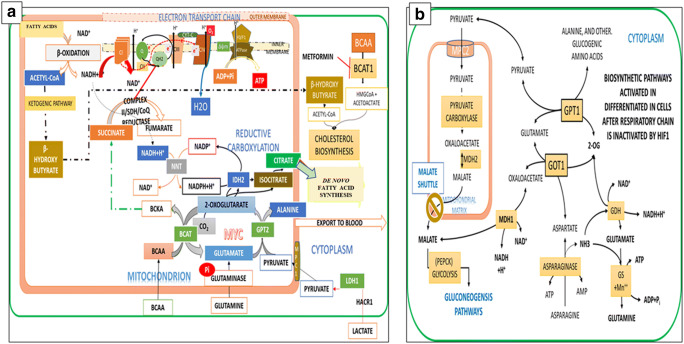
Fig. 3.
Reappraisal of Mitochondrial Metabolism. a Current Model of The TCA cycle: The current model of TCA cycle supports the hypotheses that oxidation reactions take place only in the mitochondria, and that the glutamine metabolism (highlighted in the orange colour) by the glutamate dehydrogenase (GLUD) and the glutamate pyruvate amino transferase converge on 2-OG to replenish the oxidative potential of the TCA cycle to increase the production of ATP. The discovery of two mitochondrial pyruvate carrier proteins (MPC1& MPC2), and the subsequent knock out experiments suggest that pyruvate entry through MPC1 into mitochondria promotes reductive carboxylation and succinate oxidation for de novo fatty acid synthesis for mitotic citrate synthesis (see the Fig. 8). b Nutrient Oxidations Produce Two Types of Cofactors the NADH and FADH2, which Partition the Hydrogens Between the Production of ATP and NADPH: Nutrient oxidations by dehydrogenases produce two types of reduced cofactors, the NADH and FADH2, which perform dual functions, production of ATP, and preserving the inner mitochondrial membrane (IMM) potential. Inhibition NADH production by complex I by the Complex II activates biosynthesis of NADPH to regenerate NAD+ and maintain the redox homeostasis (the inset box) and conserve the nutrient hydrogens for reduction reactions of the biosynthesis pathway. The nicotinamide nucleotide transhydrogenase (NNT) activates the transhydrogenation of NADH to NADPH. NAD+ activates the deacetylases, and the transcription factors, which regulate the gene transcription and metabolism. FADH2 as the component of respiratory super complex supports ATP synthesis, but maintains the inner mitochondrial membrane (IMM) potential (Δψm) in an uncoupled state, which supports the recycling of Pi between the ADP and ATP. Several FADH2 dependent dehydrogenases, in the cytoplasm, in addition to the mitochondria SDH, inhibit NADH production by complex I by activating the Q-Cycle (see the Table)
Krebs adopted the CS reaction in his Nobel lecture (1953) for cyclic production of citrate in the TCA cycle. Paradoxically, both Krebs and Johnson and the OCHOA group worked on the tissue extracts of pigeon breast muscles, and rat heart muscle but not on isolated mitochondria. Krebs neither mentioned mitochondria in his 1937 and 1952 articles nor in his Nobel lecture. Recent studies indicate that there are three isoforms of the isocitrate de-hydrogenases (IDH1–3) and the IDH1 produces the 2-OG from the isocitrate in the cytoplasm. The inhibition of respiratory complexes by NO and the suppression of aconitase in the cytoplasm leads to the production of ROS, and the manganese superoxide dismutase (Mn SOD) protects survival of proliferative cells [174]. The mutant forms IDH1 divert the 2-OG to produce the oncometabolite 2-hydroxyglutarate (2-HG), with simultaneous lactic acid production (Fig. 4a). The IDH isoforms play a significant role in carcinogenesis and fatty acid synthesis [175–177]. Studies by Jiang et al., Metallo et al. and others, demonstrated that citrate is produced through the reductive carboxylation in the anchorage independent proliferative cells when the hypoxia inducible factor1 (HIF1) is active, and the pyruvate dehydrogenase (PDH) is inhibited [178]. Although initially discovered as the sensor of O2 levels, the HIF1 activity is regulated by 2-OG and suppression of 2-OG activates the HIF1 [179, 180]. Sun and Denko (2014) recently reported that HIF1 supports the reductive carboxylation of 2-OG to citrate and by suppressing the oxidation of 2-OG by the oxoglutarate dehydrogenase2 (OGDH2; also known as the α-ketoglutarate dehydrogenase) and that the OGDH2-expressing cells require exogenous lipids or citrate for growth [179]. Krebs proposed that oxidation of carbohydrates produces the keto acids. The present model of IM depicts the citrate production in mitochondria connects the carbohydrate as the carbon source for lipogenesis, a term used both for the cholesterol and fatty acid biosynthesis. The model fails to explain one of the fundamental questions on the relation between citrate metabolism and the cholesterol and fatty acid synthetic pathways. If mitochondrial dysfunction is the cause of diabetes, how can mitochondrial citrate increase the cholesterol biosynthesis, while decreasing the de novo fatty acid synthesis?
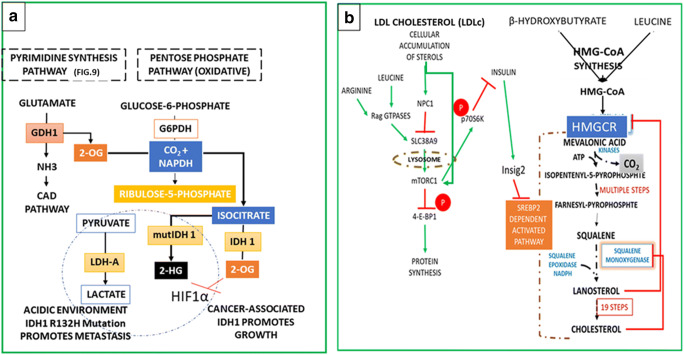
Fig. 4.
Metabolism of Proliferative Progenitor cells: Regulation by IDH1, HIF1 and The Cholesterol: a Pyrimidine Synthesis and the IDH1 Promote the Reductive Carboxylation and Partition the Cell Transformation Between Metastasis and Growth: In proliferating progenitor cells proline produces the glutamate for the synthesis of pyrimidines (see the details in FIG 9). Glutamate oxidation by GLUD isoforms in cytoplasm produces 2-OG and ammonia for pyrimidine synthesis. IDH1 carboxylates the 2-OG to isocitrate by reductive carboxylation by the NADPH and the CO2 produced in the oxPPP. The IDH1 also converts the isocitrate to 2-OG in the cytoplasm, which suppresses HIF1 by ubiquitination of HIF1α subunit. The mutant forms of the IDH1R132H suppress the 2-OG production and decarboxylate the isocitrate to produce the oncometabolite 2-hydroxyglutarate (2-HG). 2-HG production consumes NADPH and activates the LDHA to produce lactate to export protons into the microenvironment, which acidifies the microenvironment and the proliferative cells tend to migrate to O2 rich environments for respiration (see the text). b Dietary Sterols circulating as Low-Density Lipoprotein cholesterols (LDLc) activate the cholesterol dependent mTORC1 by Suppressing the Amino Acids Sensing SLC38A9. The Nieman-Pick proteins, NPC1 and NPC2, regulate the transport of LDLc in and out of the cells. Within the cells NPC1 protein carries LDLc to the lysosomes, and the lysozymes hydrolyse the cholesterol esters to liberate the cholesterol. Liberated cholesterol enters the endoplasmic reticulum (ER) and the NPC1 inhibits the SLC38A9 mediated amino acids sensing, but low cholesterol activates the mTORC1 in cells independent of amino acid sensing, which promotes the cholesterol biosynthesis. Progenitor cells have low uptake of leucine, and the inhibition of mTORC1 upregulates the insulin signalling, which activates the Insig2 genes and inhibits the cholesterol synthesis. Low levels of free cholesterol in the cytoplasm binds to the CD44-Ezrin complex and inhibit the formation of lipid rafts, which activates the metastasis of progenitor cells
Dietary sterols differentially regulate the cholesterol and fatty acid biosynthesis pathways in progenitor and differentiated cells
Cholesterol is an important component of the lipid rafts, which is required for activation of nutrient uptake, cell signalling and cell growth [180]. Circulating free cholesterols or supplementation of cholesterol to the culture medium inhibits the MAP kinase and PI3K-Akt pathways and cause cell death by activating the TNFα induced apoptotic pathways in endothelia as well as in macrophages [181–183]. Free cholesterol binds to the CD44-Ezrin complex in the cytoplasm and inhibits the formation of lipid rafts in the plasma membranes, the CD44 association with the lipid rafts requires palmitoylation, while the acetylation of CD44 activates the endocytosis of hyaluronan, a component of the extracellular matrix, which is involved in inflammation, angiogenesis, cell proliferation and metastasis [184–187]. Cholesterol biosynthetic pathway has two regulatory steps, the HMGC-CoA reductase (HMGCR) which controls squalene biosynthesis and the squalene monooxygenase (SM), which catalyzes the first oxygenation step in cholesterol biosynthesis. Three transcription factors, the sterol regulatory element-binding proteins, the SREBP1a, SREBP1c, and the SREBP2, activate the fatty acid synthesis and cholesterol synthesis [188–190]. The nascent proteins of these transcription factors remain in the endoplasmic reticulum in inactive form. The SREBP cleavage-activating protein (SCAP) escorts them to the Golgi complex to processes them into the truncated forms [189, 190]. The truncated forms enter the nucleus and transcribe the genes responsible for the fatty acid and cholesterol synthesis. Dietary sterols circulating as low-density lipoprotein cholesterols (LDLc) enter the cells and activate the biosynthesis of cholesterol in cells. The elevated synthesis of cholesterol or the intermediate sterols of the cholesterol biosynthetic pathway inhibit the cholesterol biosynthesis in a feedback mechanism (Fig. 4b). The sterols bind to the insulin induced Insig proteins (Insig1 and 2) and SCAP and degrade the HMGCR and partition the cholesterol and fatty acid synthesis, by differentially activating the SREBP2 and the SRBP1 [191–193]. A historical study reveals that leucine plays the principal role in regulation of the cholesterol and apolipoproteins.
Leucine metabolites and the β-hydroxybutyrate (BHB) promote Mevalonate biosynthesis
A historical review of the studies on cholesterol biosynthesis indicates that leucine metabolites and the β-hydroxybutyrate (BHB) are the carbon sources of the HMG-CoA and mevalonate synthesis. Bloch was first to suggest that small molecule metabolites of leucine are the carbon source for cholesterol synthesis in 1940s [194] and dysregulated cholesterol metabolism is the cause of the arterial diseases [195]. In a subsequent paper, Zabin and Bloch (1951) reported that the two-carbon carbonyl atom of the butyrate produced in the oxidation of the saturated fatty acids could contribute to cholesterol synthesis [196]. Elwood et al., (1960), demonstrated that the acetoacetate derived from fatty acids contributes to mevalonate and cholesterol synthesis, but not the fatty acid synthesis in diabetic rats [197]. The description of the TCA cycle was only in the formative stages when Bloch proposed leucine metabolites are the carbon source of the cholesterol. Ruzicka (1925) proposed a unifying hypothesis that terpenes and steroids have a common origin [198] and Robinson (1947) suggested that cyclisation of the hydrocarbon squalene results in cholesterol production [199]. Bonner and Arreguin (1949) suggested that acetate is the carbon source for the isoprene polymer and speculated that three acetate molecules combine to form the isoprenoid subunits by way of acetoacetate and 9 -methyl crotonic acid [200]. Rosenthal et al. (1974) reported that insulin increased the conversion of leucine into the saponifiable lipids and incorporated significant amounts of leucine derived acetoacetate into cholesterol in the liver compared to that of adipose tissue and the muscle [201]. The role of leucine in cholesterol research took a backseat due to the migration of several scientists (including Bloch) from European labs caused by the political disturbances in Europe and the Second World War. Following the model proposed by Bonner and Arreguin for the isoprenoid synthesis (1949), Bloch proposed the AcCoA is the substrate for cholesterol synthesis and remained silent on his earlier papers on the role of leucine metabolites in cholesterol synthesis [202, 203]. AcCoA became the model substrate for mevalonate synthesis in all subsequent publications [204–206]. Later reports suggested that lanosterol and its demethylated intermediate, the 3-β-hydroxylanost-8-en-32-al, could regulate the HMGCR and cholesterol biosynthesis [207]. Dietary sterols circulating as apolipoproteins induce the production of cholesterol and activate the fatty acid synthesis pathways to sustain cell survival; recent reports suggest that LDLc activates the mTORC1 and the cholesterol synthesis by modulating the lysosomal arginine/ leucine sensor SLC38A9 independent of the amino acid sensing [208–210], Chen et al. in a parallel study, reported that the endogenous lanosterol degrades HMGCR, and that the C4-dimethylated sterol intermediates may regulate both HMGCR degradation and SREBP-2 cleavage [211]. Recent studies indicate a significant role of leucine in the production of cholesterol and the apolipoproteins. The short-chain fatty acids (SCFA) produced by the metabolism of fatty acids and amino acids activate the insulin signalling and the short branched-chain fatty acids (BCFA) especially the isovaleric acid produced by the metabolism of BCAA inhibits lipolysis, reduces the insulin signalling, activates the secretion of defensins by Paneth cells and improves immunity and the intestinal smooth muscle relaxation [212–214]. The enzyme, the HMGCL (HMG-CoA lyase) in leucine metabolic pathway controls the production of ketone bodies from the HMG-CoA is produced both by the fatty acid and leucine oxidation in mitochondria and cytoplasm [215, 216].
Metabolic stress alters the role of enzymes of glycolysis in somatic cells and activates EMT and mitochondrial metabolism
Despite the central role of the mTOR signalling in nutrient uptake and the biosynthetic pathways, the hyper activation of the mTORC1 pathway induces metabolic stress, resulting in diabetes, lipodystrophy, neurodegenerative disorders and cancers, [217–219]. Amino acid composition of dietary proteins determines the muscle health. Dietary leucine is one of the important amino acids, which regulate the overall metabolic homeostasis of the body [219, 220]. Depletion of leucine levels inside the cells is sensed by the leucyl-tRNA Synthetase [221], which suppresses mTORC1, protein synthesis and activates the autophagy [222]. Developments over the past few decades recognised that the growth factor targeted therapies cause drug resistance, and cancer cells transform to the mesenchymal/ stem cell state and increase the proliferative potential [223, 224]. Following the aggressive radiation/ chemotherapies, the relapsing cancer cells transform to the mesenchymal stage (EMT) and acquire respiratory genome and increase the proliferative potential (Figs. 5 and 6). Mandal et al. (2011) demonstrated that undifferentiated self-renewing embryonic stem cells (ESC) are glycolytic and have functionally defective mitochondria, with prominent stem cell markers, NANOG, OCT4, and SOX2, and during the differentiation, but during the transformation, ESC reduce the stem cell markers and acquire normal mitochondrial function for proliferation and differentiated cells with tumorigenic potential lose their respiratory function [225]. In a parallel study, Tan et al. (2015) reported that a horizontal transfer of mtDNA from host cells genome activates the assembly of respiratory complexes in the tumour micro-environment and tumour cells increases the proliferative capacity. The metastatic cells exhibit higher rate and migrate to O2 rich environments [226]. The summary of the chronological events in metabolic reprogramming and cell cycle progression, and corresponding alterations in the gene network analysis are presented in (Figs. 5, 6, and 7).
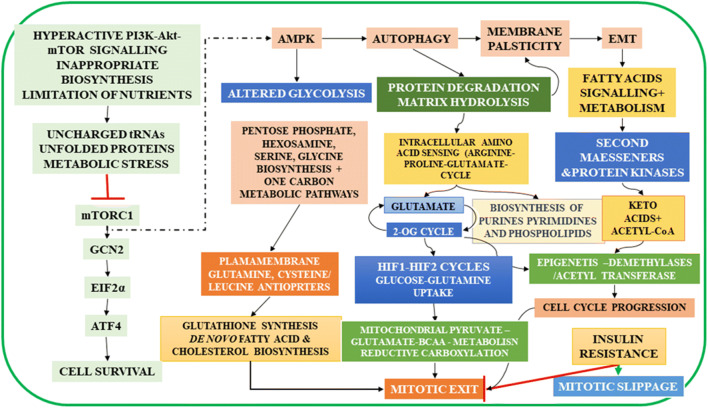
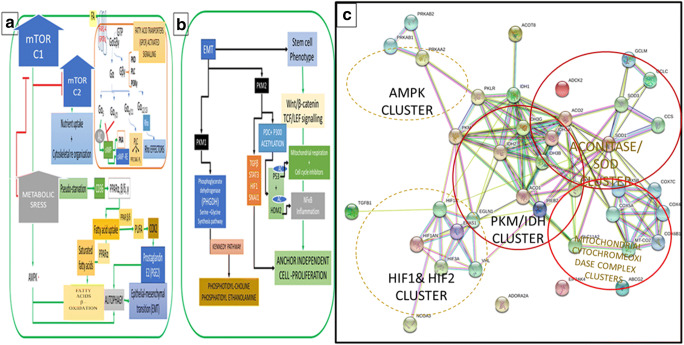
Fig. 5.
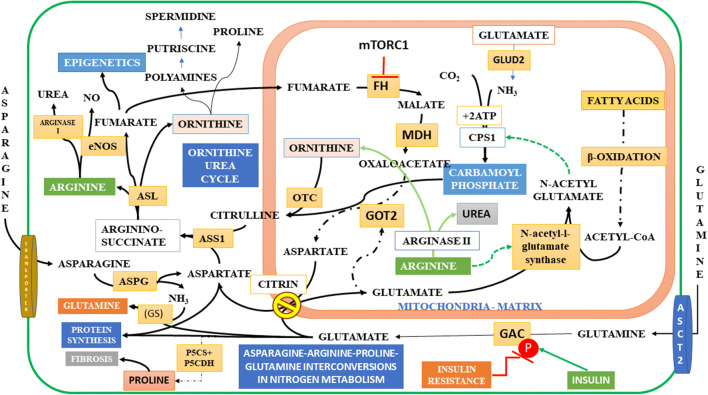
Schematic Representation of Metabolic Reprogramming During Cell Transformations: The nutrient, mechanical, or proliferative stress induces the EMT in cells, and the cells transform to the mesenchymal state. Suppression of mTORC1 arrests the translation of proteins, which results in the accumulation of unfolded proteins and enhances the ER stress and activates two parallel pathways, the general control nonderepressible 2 (GCN2) and the AMPK. GCN2 suppresses the translation process and rescues cells from apoptosis by phosphorylating the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2 α). But under limited supply of amino acids GCN2 activates translation of the transcription factor, ATF4, which controls the transcription of several genes involved in cellular homeostasis. AMPK pathway activates the autophagy in cells, which hydrolyses several intracellular membrane proteins and the matrix proteins and increase the intracellular non-essential amino acids, induces the membrane plasticity, and activates EMT. AMPK suppresses PFK1 and activates the PPP, one-carbon metabolic pathways and the hexosamine biosynthesis pathways, which modulate temporal changes in nutrient transporters (see Fig. 1b). Protein degradation increases the availability of amino acids and the arginine -proline-glutamate cycle activates the NO production and the nucleotide synthesis. The GLUD1 produces ammonia for pyrimidine synthesis, and 2-OG activates the isocitrate synthesis, when coupled to the oxPPP (FIG.9). 2-OG is an epigenetic modifier and activates the demethylases and suppresses the HIF1 transcription factor. It has lesser effect on the HIF2 transcription and the glutamine and BCAA transporters. Stress induces the activation of adrenergic receptors, and the catecholamines activate the cAMP pathway, which activates the lipolysis and the anchorage independent proliferation. Fatty acids and their metabolites activate the GPCRs and produce the second messengers and protein kinases as well as the acetyl-CoA and ketoacids, which remodel the cell metabolism by epigenetic modulation, cholesterol synthesis. Insulin activates the Pi dependent mitochondrial glutamine metabolism and activates the cell cycle exit by coordinating the synthesis of membrane lipids and tubulins for mitotic spindle formation. Insulin resistance inhibits the mitotic exit, but ASNS activates the asparagine synthesis and cell survival. Surviving cells evade the G2 check point by mitotic slippage (See also Fig. 10)
Fig. 6.
Stress Induced Reprogrammes of Metabolism, Cellular Respiration and Fatty acid oxidation. a Hyperactive mTORC1 inhibits mTORC2, resulting in the depletion of nutrients, which has a starvation effect and inhibits the mTOC1. Stress activates the sympathetic nervous system, and cAMP pathway and the anchorage independent cell proliferation. Starvation effect activates the fatty acids uptake and metabolism. The binding of fatty acids to the cell surface receptors activate the downstream GPCR signalling (Inset box), which activates the second messengers, the diacyl glycerol, Inositol phosphates, Ca++ ions, as well as the protein kinases, PKA and PKC signalling (see of the text for details). Fatty acids entry into the cells activates the peroxisome proliferator activated receptors (PPARα,β and γ), which activate the fatty acids uptake and β-oxidation in mitochondria, inhibition of mTORC1 activates AMPK, which activates the autophagy and increases the membrane plasticity for enhanced communication between the cytoplasm, nucleus and organelles. b EMT induces the PKM2 translocation into mitochondria, and together with PDH and the p300 activates the epigenetic trans acetylation of histones, p53 and MDM2. The tumour protein p53 is inactive in stem cells, acetylation activates the protein. P53 has multiple functions in the survival of pluripotent stem cells, in response to amino acids deprivation, it transcribes the cell cycle inhibitors and arrests the cell cycle, it activates the transcription of components of the cytochrome oxidase (COX2) (complex IV) of the respiratory chain in mitochondria and activates respiration. The mitochondrial respiratory chain assembly and increases the respiration and metabolic reprogramming. GCN2 dependent activation of ATF4 activates the survival pathways of proliferative cells (see Fig. 5). c String gene network produced by feeding the putative genes (TGFB1 , EPAS1 , ACO1 , IDH1 , SOD1 , PKM2, HIF1, GCN2 and AMPK) involved in the EMT and metabolic reprogramming demonstrating the interacting networks between the AMPK, HIF1&2, mitochondrial respiratory complex (COX2) assembly, the IDH and aconitase and SOD pathways, which link the mitochondrial respiration to activation of biosynthesis
Fig. 7.
Temporal Changes in Pluripotent cell Gene Networks: a Metabolism of Arginine and Essential Amino Acids During Cell Cycle Progression: Stem cell phenotype depicting the Notch signalling (an embryonic phenotype marker), Ras-Map kinase pathway, Myc activation, and the inflammatory pathways, which activate cell cycle progression and differentiation. Acetylation programmes integrate the activation of MYC transcription factors, which activate the glutamate/ glutamine metabolism and mTORC1 function for biosynthesis. The transcriptional activation of genes involved in lysosomal function, RNA polymerases, and mRNA synthesis. b Arginine metabolism indices the mitochondria NO oxide production, which limits the mitochondria oxidative phosphorylation and activates the epigenetic modifying trans acetylases for biosynthesis of ribosomes and translation activating mediator complex. c Arginine sensor SLC38A9, activates the transceptors involved in non-essential amino acids for uptake of non-essential amino acids and lysosomal genes for mTORC1 activation. d Leucine dependent activation requires the ROS induced sestrins, and the activation of genes related to lysosome function and surface transporters LAT1and LAT2. e Ribosome biogenesis prepares cells for active translation processes required for growth of cells. Poly polyphosphorylation of lysine residues of multiple non-enzymatic proteins are reported to activate the ribosome biogenesis in humans and in Yeast
P53 reprograms glycolysis through TIGAR, and glycolytic enzymes assume non-canonical functions in response to stress and promote EMT
Altered glycolysis and the mitochondrial metabolism promote the tissue remodelling. Inhibition of glycolysis to curb the aerobic glycolysis/ Warburg effect remained the principal target in cancer therapies [79]. The tumour suppressor p53 has multiple roles to maintain the cellular homeostasis, in response to the DNA damage, mechanical and metabolic stress. P53 transcribes TIGAR and the cell cycle inhibitors to arrest cell division and regulates apoptosis to rescue cells from apoptosis; the transcription of TIGAR alters the hexose and triose phosphate fluxes in glycolysis and PPP (Fig. 1b) [227–229]. TIGAR inhibits the kinase active isoform of bifunctional enzyme PFK-2 /FBPase-2 and reduces the PFK1 activity, which results in the activation of Fructose 1,6 bisphosphatase (FBP1) and increased levels of Fr6P. Besides, TIGAR targets the TKT family of enzymes to increase the NADPH production and protects the cancer cells from apoptosis promoting drugs [112, 228]. The function of p53 is tightly regulated; in the stem cells, p53 is kept in an inactive deacetylated state by OCT4 and Sirtuin1, but activated p53 suppresses the OCT4, KLF4, LIN28A, and SOX2 and promotes differentiation and lineage commitment. The proto-oncogene MDM2 (HDM2 in humans) ubiquitinates the p53 in somatic cells to keep the apoptosis of cancer cells in check [230]. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirtuin 1 mediated deacetylation [231]. Both differentiated and ESC have higher number of mitochondria, the protein Parkin, a ring protein of E3 ubiquitin ligase is involved in mitochondrial biogenesis, Parkin is located within the mitochondrial organelle of proliferating cells and protects the mitochondrial genome by repairing the DNA damage [232]. Parkin is released from the mitochondria, when permeability transition pore is opened, but localises to the extra-mitochondrial membrane in differentiated cells. The PTEN-induced kinase 1 (PINK1) protein kinase phosphorylates the Parkin and maintains the quality control by inducing the dynamin-related protein 1 (Drp1) and culling the defective mitochondria [233]. Cytoplasmic localisation of p53 suppresses the mitochondrial fission by destabilising the PINK1 and Parkin association and suppresses the mitophagy [234]. Upregulation and activation of cell cycle inhibitor p21 and the p53 in proliferating pluripotent stem cells results in the mitofusin1/ 2 activation, to increase the mitochondrial respiration, which is also associated with ROS production [235]. Since pluripotent stem cells have different metabolic demands, the activation of the two hypoxia-inducible factors, HIF1α and HIF2α in a stage-specific manner and initiate the metabolic switch between the glycolysis and mitochondria. HIF2 is more tolerant to O2 levels; inactivation of HIF1 facilitates the biosynthetic reprogramming. The stabilisation of HIF2α during the differentiation upregulates the TNF-related apoptosis-inducing ligand (TRAIL), which represses the apoptotic caspase 3 activity specifically in cells undergoing metabolic reprogramming [236]. HIF1 and p53 have antagonistic relations in activation of glycolysis and the oxidative phosphorylation, while HIF1 suppresses the mitochondrial respiration, p53 supresses glycolysis by inducing TIGAR and activates the mitochondrial respiration by transcribing the synthesis of cytochrome C oxidase 2 (SCO2), a protein coding gene, which is involved in the biogenesis of cytochrome c oxidase (COX) subunit II, required for the assembly of COX2, the complex IV of the ETC, which transfers electrons to O2 [237]. HIF1 regulates the SCO2 and TIGAR by occupying the promoter regions of both SCO2 and TIGAR. Acetylation induced by the p300/CBP-associated factor (PCAF) inhibits HIF1 and activates the mitochondrial respiration through activation of the transcription factor SCO2 in the nucleus [238]. During the EMT, glycolytic enzymes translocate into the nucleus to re-programme the genetic programmes to rewire the metabolism in tissue remodelling [84].
Catalytically inactive pyruvate kinase 2 (PKM2) translocates into the nucleus and activates two parallel pathways HIF1 and p53
Nuclear translocation of the catalytically inactive pyruvate kinase 2 (PKM2) activates two parallel pathways HIF1 and p53 (Fig. 6). The transcriptional function of the PKM2 and STAT3 in the nucleus activates the HIF1 [239–241], which transcribes genes responsible for angiogenesis [242, 243]. PKM2 complexes with the pyruvate dehydrogenase (PDH) complex (PDC) to form a complex in the nucleus, which produces the AcCoA from phosphoenolpyruvate (PEP). PCAF acetylates the tumour protein p53 and its negative regulator MDM2. While the p53 dependent activation of the SCO2 activates the mitochondrial respiration [244–247], MDM2 activates the NFκB dependent inflammation. P53 is inactive in stem cells and ageing cells but activated in progenitor cells by acetylation [248, 249]. HIF1activates the transcription of pyruvate dehydrogenase kinase (PDK) and inactivates the pyruvate dehydrogenase, delinks it from the acetylation and activation of p53 leading to the inhibition of respiration [250, 251]. The transforming growth factor-β (TGFβ) activates the TGF-beta-activated kinase-1 (TAK1), an upstream activator of the LKB1/AMPK and induces AMPK dependent metabolic reprogramming [252, 253]. Autophagy is associated with EMT; epigenetic modifications, especially the lysine-specific methylation/ demethylation and acetylation/deacetylation of specific histones initiates the chromatin remodelling [254–256] and promotes the anchorage independence and Stemness [257–262]. Kim et al. (2011) reported that ULK1 is the target of both AMPK and mTORC1 in activation or inactivation of autophagy [262]. Initiation of autophagy is modulated by a complex of proteins, which comprise ATG13, FIP200, ATG101, and ULK1 (Jung et al., 2009). mTORC1 phosphorylates ULK1 at S758 and ATG13 at S259 to repress autophagy amino acid depletion rapidly dephosphorylates these proteins and activates autophagy. Sancak et al., (2010) reported that the localisation of mTORC1 to the lysosomal surface is required for the amino acids (RAG GTPase) dependent and independent pathways, and prevention of its localisation inhibits the mTORC1 activation. Three small proteins, MP1, p14 and p18, encoded by MAPKSP1, ROBLD3, and c11orf59 respectively localise with RAG GTPases to activate the mTORC1 to lysosomal surface [263]. In an earlier study, Nada et al. (2009) reported that p18 is a lipid raft protein and requires palmitoylation for its incorporation into the lipid rafts of late endosomes, which specifically binds to the MP1 and the p14; loss of p18 (p18−/−) disrupts the scaffold complex, which results in embryonic lethality [264]. The three proteins form a scaffold for MEK1 and activate the MEK-Erk pathway, which is essential for activation of cyclin B--cdc2 complex for entry into mitosis [265]. Mitosis entry is associated with the disruption of nuclear membranes for spindle formation and segregation of chromosomes; and the inhibition of autophagy protects the cells from the autophagic death, even under low levels of nutrients; which inhibit the mTORC1 [266–268]. Odle et al. (2020) in a recent study demonstrated that mitosis inhibits the cyclin dependent kinase1 (CDK1), which phosphorylates raptor and inhibits the mTORC1 and autophagy through hyper phosphorylation of the lysosomal proteins, ULK1, ATG13, ATG14, and the transcription factor TFEB, which is involved in the lysosomal biogenesis and distribution in the cytoplasm [269–271]. Mitotic spindle formation requires the association of several microtubules made of α/β-tubulin heterodimers and associated proteins, which regulate the microtubule polymerization dynamics, transport, and nucleation to generate bipolar spindles, which attach to the chromosomes for segregation during metaphase of cell division [272–274]. Several post translational modifications of tubulins which include phosphorylation, palmitoylation, S-nitrosylation, ubiquitylation, sumoylation, glycosylation and methylation contribute to the diversity and dynamics of microtubules, which have diverse functions in the spindle assembly and organisation of cytoskeleton in proliferative and differentiated cells [275–278].
Mitochondrial respiration initiates the cell proliferation, fatty acids and amino acids provide the carbon and nitrogen for biosynthesis of nucleotides, proteins and the membrane lipids and the cytoskeletal architecture
Pasteur’s work in 1860s on Yeast demonstrated that lactic acid suspends fermentation and O2 accelerates the proliferation and nitrogen and phosphate providing the nutrient and support for growth. Commenting on the Warburg’s aerobic glycolysis, Crabtree (1929) reported that the aerobic glycolysis is a common feature of all malignant growths, and demonstrated that there is an inverse relation between O2 consumption and glucose uptake, but proliferative cells increase the respiration over those of normal cells and that the rate of respiration of tumour cells grafted in subcutaneous regions is 50% higher than those grafted in the abdominal region, indicating the rate of respiration of proliferative cells increases in hypoxic environments. Researches in Chance laboratory demonstrated that succinate oxidation in mitochondria takes place only under ATP rich environments and that succinate oxidation by the SDH-CoQ reductase (complex-II of the respiratory chain) inhibits the NADH oxidation by complex-I by reverse electron transport leading to the accumulation of NADH in mitochondria [279, 280]. Besides, Klingenberg’s works on uncoupling proteins demonstrated that uncoupling proteins delink ATP production from respiration [281, 282], which results in the export of C4 metabolites out of mitochondria into cytoplasm for biosynthesis [283]. Recent reports by Scialò et al. and Hüttemann et al. report that ETC respiratory complex formation is essential to activate apoptosis or survival [284].
Respiratory super complexes and reactive oxygen species (ROS)
The respiratory chain [also called the electron transport chain (ETC)] has dual roles in mitochondria. Four respiratory complexes, Complex-I to IV (CI-CIV) with two linkers, the coenzyme Q (Q) and cytochrome C (Cyt-C) constitute the ETC. Recent reports suggest that the ETC comprises a supramolecular structure named the respiratory super complex comprising the CI-Q-CIII-Cyt-c-CIV of the ETC oxidises the reduced cofactors NADH and FADH2, and maintains the optimal flux of electrons to O2 and the Δψm of the IMM CI oxidizes the NADH and reduces Q to QH2. Hydrogens from the FADH2 directly reduce Q to QH2. QH2 transfers only one electron at a time to CIII; the flow from CIII through Cyt-C to CIV to reduce the O2 to H2O. The protons liberated at CI and CIII are transported across the internal mitochondrial membrane (IMM) to generate the membrane potential (Δψm). The energy of Δψm is harvested for ATP production by the complex V. A second route of electron flux from FAD dependent dehydrogenases converges on CoQ and transports electrons from CII-to CIV, which sustains the inner mitochondrial membrane (IMM) Δψm at low levels and uncouples the ATP production from electron transport [285, 286] (Fig. 3b). Inhibition of NADH oxidation in ETC results in the production of ROS [287, 288]. The maintenance of the Δψm is essential for the IMM permeability, which regulates the influx of ions and metabolites into mitochondria. The succinate dehydrogenase and its mutant forms, which form the complex of the CII protect cancer cells from apoptosis [289–291] and targeting the complex results in apoptosis. This improves the efficacy of cancer therapeutic drugs [292, 293]. Mitochondrial glycerol-3-phosphate dehydrogenase (mGPDH) generated from high fat diets sequesters the SDH from the CII activates respiration and ATP production as part of energy regenerating cell survival mechanisms which utilise the PPAR-γ induced SCD2 activation for desaturation and elongation of fatty acids in proliferative progenitor cells and preadipocytes [258–263]. There are several hypotheses on the role of Q in the ROS production [294, 295]; a recent study suggests that the hydrogens from FADH2 produced from the oxidation of long-chain fatty acids are physically associated with ETC and directly transfer the electrons to the CIII of super complex [296]. The fatty acid oxidations produce equal amounts FADH2 to NADH. A competition of oxidation of C1 and FADH2 at Q site imposes an electron cloud at Q site, which causes the ROS generation at complex I/III [295]. Excessive utilisation of fatty acids and the production of reactive oxygen species (ROS) is one of the attributed features of lipodystrophy in metabolic pathologies, which disturbs the NRF2 (NF-E2-related factor 2) signalling pathways [247, 297, 298]. The FADH2 generated from non-fatty acid sources reduce Q through complex II. The FAD-dependent dehydrogenases of different pathways protect ETC in uncoupled state; the dihydro-orotate dehydrogenase of CAD pathway, physically binds the C II of ETC and protects cells from oxidative stress and apoptosis [126] and inhibition of pyrimidine synthesis activates the p53 dependent oxidative stress and apoptosis [299, 300].
Mitochondrial pyruvate carrier proteins (MPC1&2)
The mechanism by which Pyruvate enters the mitochondria remained unknown until 2012. Two independent groups discovered two mitochondrial pyruvate carrier proteins (MPC1 and MPC2) [169, 170]. In mammals, the proteins are reported to function autonomously. MPC1 has a critical role in cell survival and growth; the pyruvate transport into mitochondria through MPC1 increases the ROS production [307, 308]. Vanderperre et al. reported that the global knock out of MPC1 in mice is embryologically lethal, which was rescued by the ketogenic diets. Zou et al.(2018) reported that the mitochondria fatty acid metabolism rescues cells from the MPC deficiency [309, 310]. Activation of MPC1 and 2 suppresses the Tumorigenesis and promotes growth [311, 307, 312]. Genetic deletion of the mitochondrial pyruvate carrier (MPC1) accelerated the proliferation and transformed the cells into stem cell phenotype but activated the arginine metabolism in progenitor cells [172, 173, 307, 313]. MPC2 knockout suppresses gluconeogenesis and activated the glutamate-alanine transamination, and liver-specific deletion of MPC1 suppresses the gluconeogenesis and activates the arginine-urea metabolism in hepatocytes [171, 313]. The contradiction relates to glutamine metabolism, which is partitioned between cytosol and mitochondria. The placental homologue of MPC1, the MPC1 like (MPC1L), activates MPC2 and rescues the respiration-deficient cells, which are deficient in the MPC1 [169, 170].
Glutamate-pyruvate aminotransferase partitions the cell metabolism between cell proliferation, fatty acid synthesis and cell growth
Glutamine metabolism in cells is complex and the entry of glutamine into cells is tightly regulated. Its’ metabolism in the mitochondria depends on the phosphate availability [314]. Glutamine has an essential role in the glutathione biosynthesis to maintain the redox homeostasis and the cell fate between survival or death [315, 316]. The primary effect of glutamine deprivation is the reduced cell division and oxidative stress. Glutamine deprivation results in cell death, which can be rescued by the supplementation of nucleotides or by the activation of cell cycle inhibitors [317, 318]. The microvascular dysfunction resulting from the endothelial cell death is the cause of diabetic retinal, renal and lower limb disorders [34, 319]. Krebs in 1930s discovered that mammalian cells have two types of glutaminases and suggested that glutamine is a fuel for respiration; subsequent studies recognised that Myc mediated glutamine metabolism is active in cancers, and it remains the target for cancer therapies [316, 320]. Krebs recognised the presence of two glutaminases, which are recently classified as the kidney type GLS (often mentioned as GLS1) and the liver type GLS2. The two enzymes are encoded by two different genes; while the GLS is the target of the oncoprotein MYC and expresses at higher levels in aggressive tumours, the GLS2 is the target of the tumour suppressor p53 and reduces the tumour burden [321, 322]. The two enzymes have splice variants; GLS has two splice variants, the GAC and the KGA, while GLS2 has LGA and the GAB [323, 324]. Although initial studies reported that GAC is active in tumours and stabilised by the Pi in mitochondria [325], recent reports indicate a differential role for the cytosolic and mitochondrial glutaminases. While the KGA is involved in tumour formation and migration in the hypoxic environment, the GAC suppresses the proliferation and promotes the growth [326]. Glutamine availability or its transport by the ASCT2 downstream of MYC differentially regulates the two enzymes. The metastatic cancers depend on the glutamine metabolism for nucleotide production, ribosome biogenesis and the angiogenesis; polyphosphates attached to lysine residues of non-enzymatic protein are reported to activate ribosome biogenesis in both microbial, yeast and mammalian systems [327, 328]. Zhang et al. recently reported that ASCT2 dependent glutamine transport, in addition to LAT1 and GLS is over-expressed in HNSCC both in vivo and in vitro models, and suppression of GLS resulted in suppressed glutathione production, attenuated growth and proliferation, and increased apoptosis [329]. Li et al. reported that targeting the GLS suppresses the β-catenin pathway, increases the stemness and oxidative stress in hepatic cancers [330]. MPC1 and the phosphate-dependent stabilisation of GAC activate the differentiation by suppressing the proliferation [325, 331] and the knockdown of MPC1 increases the stemness of inhibits the proliferation of cells [311, 332]. In a recent study on neuroendocrine tumours of pancreas and the gut, Szeliga et al. demonstrated that the KGA isoform is expressed highly in the proliferative cells, while the GAC isoform is expressed both in proliferative and non-proliferative cells, but the ratio of difference between the proliferative and non-proliferative cells was not significant [326]. The reduced citrate synthesis is a marker for proliferation [334]; GAC dependent mitochondrial glutamine metabolism activates the citrate synthesis and shifts the cell metabolism to lipogenesis programmes during growth, which requires the fatty acid oxidation and maintenance of ETC for FADH2 oxidation and the NADPH production for reductive carboxylation (Fig. 8a). A recent report suggests that the glutamine deprivation suppresses tumour formation, glutaminase inhibitors remodel the metabolism of the amino acids and increase the biosynthesis of proline, glutamate, glycine, and the glutamine in the triple-negative breast cancers [335–338]. Defects in the glutamine metabolism suppress the mitochondrial respiratory functions in the neurodegenerative disorders, which result from the demyelination of neurons [339, 340]. Suspension of mitochondrial respiration protects the cells and shifts the mitochondrial glutamine aspartate metabolism. Pyruvate enters the mitochondria through MPC2 and activates the pyruvate carboxylation to OXA and aspartate metabolism, which shifts the cell metabolism from lipogenesis to gluconeogenesis, glutamine synthesis ureagenesis and senescence (Fig. 8b) [341–346].
Fig. 8.
Two Mitochondrial Pyruvate Carrier proteins (MPC1 and 2) Differentially regulate the Mitochondrial Pyruvate and Glutamine Metabolism during Cell Differentiation: a Pyruvate entry through MPC1 activates Reductive Carboxylation and de novo fatty acid synthesis: Pyruvate and glutamine entry into mitochondria through MPC1 activates the glutamate pyruvate amino transferase (GPT2) and the reductive carboxylation (RC). GPT2 promotes the production of 2-OG as a substrate for reductive carboxylation (RC) to citrate in mitochondria. RC requires the functional respiratory chain, NADPH, and mitochondrial aconitase 2 (ACO2). RC also activates the simultaneous oxidation of the 2-OG to succinate (not shown in the figure. See the text). Succinate oxidised in mitochondria by SDH-CoQ (complex II of the respiratory complexes) inhibits the NADH oxidation by Complex-I, and the NNT transhydrogenates the accumulating NADH to NADPH, which is utilised as the co-factor by mutant form of IDH2 to activate the citrate production. Citrate exported into cytoplasm is the target of ATP citrate lyase (ACLY), which produces the acetyl-CoA (AcCoA) and the OXA. AcCoA carboxylase (ACC), a complex multifunctional enzyme, which carboxylate the AcCoA to malonyl-CO-A. ACC is a rate limiting step in the de novo fatty acid synthesis. The β-hydroxy butyrate produced by fatty acid oxidation is exported to cytoplasm and activates the cholesterol biosynthesis in coordination with the leucine metabolites produced by the BACT1. b Pyruvate entry through MPC2 Suppresses mitochondrial the glutamine metabolism and activates the pyruvate carboxylase. The glutamate-oxaloacetate amino transferase (GOT) increases the production of aspartate, ASNS supports the enhanced aspartate synthesis from glutamine. Glucogenic amino acids and GPT1 increase the pyruvate production in cytoplasm. Pyruvate entry into mitochondria through MPC2 enhances the OXA production, which activates the malate synthesis. Malate export into the cytoplasm activates the gluconeogenesis, while proline and aspartate metabolism produces de novo arginine and activates urea cycle enzymes, which activates poly amine biosynthesis, eNOS in differentiated cells. (see Figure 10 for further details)
Transhydrogenase shuttles Hydrogens derived from the nutrient oxidations between ATP production and the biosynthesis
Oxidation of nutrients involves the dehydrogenase reactions and the biosynthesis reactions require hydrogens for the reductive reactions. The dehydrogenases transfer hydrogens from the nutrients to two types of cofactors, the NAD+ or the FAD and reduce them to NADH and FADH2. The ETC oxidises the reduced cofactors for increasing the oxidation of nutrients to metabolites required for the biosynthesis of macromolecules. The ratio of FADH2 to NADH produced varies with the nutrients oxidised; the fatty acid oxidation in mitochondria generates the bulk of the acetyl-CoA, ketone bodies, and an equal proportion of the reduced cofactors NADH and FADH2. Activation of the ETC also induces the production of the reactive oxygen species (ROS), which suppresses the ATP production; trans-hydrogenation of NADH to NADPH reduces the oxidative stress, by uncoupling respiration from ATP production by reducing the Δψm. NADPH activates the biosynthesis. There are two mechanisms of production of NADPH from the NADH in the mitochondria; the nicotinamide nucleotide transhydrogenase (NNT) promotes the bulk of NADPH production (Fig. 8a) to maintain the redox homeostasis during the uncoupled state of the respiration and reductive biosynthesis of citrate [347–349]. The glutamate dehydrogenase (GLUD2) [350–352], and the isocitrate dehydrogenase 2 (IDH2) in mitochondria [353, 354] catalyse the production of NADPH during the active respiration and ATP production.
Amino acids activate mTORC1 and biosynthesis Programmes
Pasteur was first to recognize that nitrogen source is required for growth and Yeast convert nutrient nitrogen into protein. Twentieth century bioenergetic theories put glucose at the centre stage of both catabolism and anabolism. Citrate produced in the mitochondria was linked to the carbohydrate as the source of lipid synthesis, anaplerotic and cataplerotic reactions of amino acids were projected to contribute to gluconeogenesis [355]. Recent studies, however, recognise the role of proteins and the amino acids as the major contributors of the biomass [356–361]. Hyper activation of mTORC1 and protein synthesis depletes amino acid levels in cells and induces a starvation effect, which suppresses the mTORC1 [262, 360]. Starvation induces the autophagy and increases the utilisation of fatty acids and the amino acids in mitochondria to activate mTORC1 [361]. Dysregulation of autophagy is one of the attributed factors in insulin resistance [362–364]. The transition from the epithelial to mesenchymal cells (EMT) reprograms the tissue remodelling of stressed out tissues, to bring them back to normalcy. Insulin suppresses the lipolysis and promotes the anabolic programmes, especially the protein synthesis and de novo fatty acid synthesis in muscles, adipose tissue and the liver. The mechanism by which insulin activates these anabolic programmes remained elusive until the discovery of the phosphoinositide 3-kinase (PI3K) by Cantley and colleagues in 1980s, and the two serine/threonine kinases, the Akt and the mechanistic target of rapamycin (mTOR) as the cell survival and the nutrient sensor pathways at the beginning of the 1990s. Growth factor induced PI3K-Akt-mTOR signalling remained at the centre stage of cell proliferation, metabolism, hormone function and tissue homeostasis [365–367]. The aggressive relapse of cancers to apoptosis promoting chemotherapeutics revived the interest in metabolism. Apoptotic cell death also increases nutrients and toxic chemicals in the microenvironment, which can be causative factors of aggressive relapse of cancers [368, 369]. The mTOR exists in two multiprotein complexes, mTORC1 and the mTORC2; the insulin and the insulin-like growth factor signalling activates the mTORC1 by activating the PI3K and Akt at the cell membrane. The PI3K-Akt-mTORC1 pathway remains the target for therapies in cancer [370–373]. Oxygen and the hypoxia-induced transcription factors play a significantl role in modulating the PI3K signalling [374–377]. Both insulin and the insulin-like growth factor signalling (IIS) mediate their effects through activation of PI3Kinase and Akt, which activate the mTORC1. In a feedback mechanism, mTORC1 regulates the insulin signalling by the phosphorylation of the serine residues of IRS proteins through its’ downstream target S6Kinase [378–380].
Differentiating cells are auxotrophic for amino acids during growth
Cachexia/ sarcopenia (muscle loss) is one of the common problems of diabetes, cancer and ageing populations. Cramer’s group (1907–1913) reported that glycogen depletes very fast in growing tumours and the tumour grows at the expense of host tissue proteins; Cramer suggested that lipids provide energy, while carbohydrates contribute to build the protoplasm [381, 382]. Summarising two decade of works on cancers of the rat models and clinical trials in the National Cancer Institute (NIH, USA) and European laboratories, Guido Mider (1953) suggested that tumour has three different stages of growth. In the first phase, the transplanted mass of tumour cells in the subcutaneous tissue becomes palpable as tumour at the site of implantation after 7 to 10 days and grows slowly but progressively and the host gains weight during this period. The second phase is the rapid neoplastic growth, during which the host tissues lose weight as the diet’s contribution to the nitrogen developing tumour may be inadequate. The onset of anorexia (loss of appetite) coincides with the beginning of the second phase and the host may die during this stage and anorexia activates the translocation of nitrogen from body tissues to neoplasm, which is accompanied by a progressive loss of total body lipid, which provides the major fuel for growing cancer. When fat is depleted, host tissue proteins and amino acids are degraded and excrete the nitrogen as urea and ammonia. Mider concluded “Excessive loss of lipid suggests increased energy expenditure by cancerous subjects and a better knowledge of nutritional requirements, and the metabolic processes of the cancer patients might help the palliative relief to the patients” [383].
Arginine and Leucine as Signalling molecules in initiating the biosynthesis
One of the breakthrough researches, which brought into focus that nutrient molecules act as signal molecules and that intracellular membranes and lysosomes can sense the availability of the nutrients independent of hormonal clues was recognized by two parallel studies by Hara et al. and Wang et al. in 1998. The two groups demonstrated that the amino acids activate mTORC1 at the basal level, independent of PI3K-Akt signalling [384, 385]. Further, Hara et al. demonstrated that depletion of amino acids suppresses the mTORC1 activity even when stimulated by the serum and insulin, which varied with specific amino acids. Depletion of arginine and leucine suppress more than 70–90% of mTORC1 activity respectively. More importantly, the addition of these amino acids to the feed did not restore the mTOC1 activity immediately; addition of arginine or leucine alone restored <10% activity, while the two amino acids together could restore <29% of activity. Proud’s group, in a subsequent study, demonstrated the presence of intracellular amino acid sensors in the amphibian oocytes, which increased the translation capacity of the oocytes for the pyrimidine synthesis in response to the small increases in the concentrations of total amino acids or leucine or tryptophan alone [386]. In a series of papers on the nutrient sensing in Yeast and plant species, Tavleen’ group demonstrated the central role of the Snf1 protein kinase (AMPK), the GPCR system, the role of Ras in cAMP signalling, and the role of the general control nonderepressible 2 (GCN2) in sensing intracellular amino acids. In the absence of essential amino acids, GCN2 activates translation of the transcription factor, ATF4, which controls the transcription of several genes involved in cellular homeostasis [387, 388]. During initiation of protein synthesis, the intracellular transporters and the intracellular metabolic intermediates sense the nutrients and control cellular growth (reviewed by Conrad et al. (2014) [389] and Steyfkens et al. (2018) [390]. Besides, eIF-2 α phosphorylation activates the HIF1 dependent vascular endothelial growth factor (VEGF) and promotes angiogenesis [391]. Deprivation of individual amino acids have differential effects; arginine starvation, for example, stimulates the p53 dependent transcription of the cell cycle inhibitors [392]. ATF4 activates the asparagine synthetase (ASNS), which inhibits GCN2 through feedback inhibition; overexpression of ASNS arrests proliferation and promotes growth [388, 393]. In a series of papers on the role of lysosomes in sensing the amino acids, Sabatini group demonstrated that amino acids activate the mTORC1 at the lysosomal surface through the Ras-related guanosine triphosphatases (Rag GTPases) [263, 394, 395]. The cytosolic arginine sensor for mTORC1 (CASTOR1) and stress-induced sestrins sense the depletion of arginine and leucine respectively and inhibit the GTPase activating protein (GAP) of TOR, GATOR2 and suppress the activity of the Rag GTPases. The reactivation of the mTORC1 by supplementation of these amino acids is a temporal process. Early stages of activation of mTORC1is promoted by the intracellular amino acids, which accumulate several essential and non-essential amino acids in cells due to the degradation of the cytoplasmic proteins during autophagy, which include the matrix and collagen molecules [396, 397]. In parallel studies Goberdhan et al. demonstrated the role of the intracellular amino acid transceptors and the role of the solute carriers, the Proton-assisted Amino acid Transporter (PAT or SLC36) and Sodium-coupled Neutral Amino acid Transporter (SNAT or SLC38) in activating the mTORC1in mammalian cells [398, 399]. Three amino acids, glutamine, arginine and leucine play crucial role in activating the mTORC1, nucleotides, lipid and protein synthesis. Both starvation and high-fat diets upregulate the solute carrier family 38member 9 (SLC38A9) [400]. SLC38A9 senses the arginine depletion and promotes the transport of the amino acids from the lysosomes into the cytoplasm [401–404].Arginine metabolism produces the nitric oxide, polyamines, nucleotides and activates the ornithine-urea pathways in different cell types [405–407].
Glutamine transport into cells through ASCT2 is highly regulated
Bhutia and Ganapathy (2016) reported 14 transporters in the mammalian cell membrane that accept glutamine as a substrate, which belong to four distinct gene families, SLC1, SLC6, SLC7, and SLC38, and suggested that the sodium/proton coupled SLC38 is the principal glutamine transporter in the CNS, which maintains the glutamate/glutamine cycles between neurons and astrocytes or promotes channelling of ammonia to pyrimidine synthesis in cancer cells [408]. Metastatic cells depend on proline or the intracellular transceptors SNAT1 (SLC38A1) and SNAT2 (SLC38A2) for glutamate in cancer cells [409–411]. Besides, SLC6A14 is reported to be associated with the transport of cationic amino acids and activates cystic fibrosis and pathogenic infections [412, 413]. In a detailed study on the amino acid transporters, Palacín et al. (1998) reported that the amino acid transporters belonging to the group system A and ASC act as the symporters, which transport the sodium coupled neutral amino acids with small side chains, like alanine, serine and cystine, while system L amino acid transporters are the uniport transport system and transport the bulk chain aromatic and BCAA transport and that these transporters are present in in plasma membranes of every cell. System L is subdivided to L1 and L2, which recognise the amino acids at nanomolar and millimolar range, respectively [414]. Several recent reports suggest that LAT1 (SLC7A5) and LAT2 (SLC7A8) differentially activate the leucine uptake in a sodium dependent and independent manner [415]. LAT1 forms a heteromeric amino acid transporter complex with the surface antigen, a type II membrane glycoprotein, called 4F2 heavy chain (4F2hc, also known as CD98 and SLC3A2), and catalyses the cross-membrane flux of large neutral amino acids in a sodium- and pH-independent and acts as antiporter of the amino acid-polyamine-organocation superfamily and tumour cells overexpress LAT1 [416–418]. Besides, Dickens et al. (2008), reported that soluble cholesterol is required to stabilise the complex at the plasma membrane [419]. LAT2 on the other hand, is reported to promote glycolysis and chemoresistance by activating the glutamine mediated mTORC1 [420] and transports the thyroid hormones [421], which are involved in the pathogenesis of glomerulonephritis [422], hearing loss and cataract formation during ageing [422, 423], Glutamine uptake into the cells from the microenvironment is facilitated through the neutral amino acid transporter, the SLC7A5 (also called the ASCT2) expression, which increases in inflammatory and stem cells to meet the glutamine demand of cancer cells for survival [134]. Depleting glutamine catabolites, especially the 2-OG depletion activate the mTORC2, activate the HBP to produce the UDP-GlcNAc, for activation of O-linked and N-linked glycosylation of proteins involved in the nutrient transport [424–426]. Tumour cells auxotrophic to glutamine exhibit elevated expression of LAT1 (SLC7A5), 4F2hc (SLC3A2), xCT (SLC7A11) and ASCT2 (SLC1A5), and are reported to be poor prognostic factors in cancer therapy. Interestingly, these amino acids have differential effects on the amino acid fluxes across the plasma membrane. 4F2HC is co-expressed with all the three transporters. While ASCT2 promotes the glutamine uptake into the cells and promotes growth and the de novo fatty acid, palmitoyl–CoA, synthesis to completes the cell Cycle and exit the mitosis [427, 326]. Association of the 4F2HC and LAT1, promotes the efflux of the glutamine in exchange for the leucine and essential amino acids into the cells [428]. Indiveri group demonstrated that a reconstituted glutamine/amino acid transporter, which functionally corresponded to the ASCT2 protein into liposomes transported efficiently the amino acids glutamine, alanine, serine, asparagine, threonine translocated from outside to inside and from inside to outside the proteo-liposomes. Cysteine and valine were translocated preferentially from outside to inside. A bisubstrate kinetic analysis suggested that the glutamine export was strongly stimulated by internal nucleoside triphosphates and to a lower extent, by pyrophosphate [429]. Similarly, cysteine/ cystine uptake into the cells in exchange is promoted by the glutamate export out of the cells, which has a role in glutathione biosynthesis and redox homeostasis and the levels of the glutathione (GSH) synthesis or glutamate, cystine exchanges depend upon the rate limiting enzyme, the Glutamate-Cysteine Ligase Catalytic subunit (GCLC) which is the gene of limiting synthesizing enzyme for GSH. Deficiency of cysteine/ cystine transport through xCT is implicated in neurological disorders [430], viral and bacterial infections [431]. The antagonistic relationship between the leucine uptake and glutamine export has a role in cell cycle exit or slippage.
Role of amino acids in cell cycle progression, cell differentiation and growth
In parallel studies to those of Pasteur on Yeast, Gobley, Hoppe-Seyler, Parke, and Tubingen’s laboratories isolated phospholipids from bovine brains, egg yolk and red blood cell (RBC) membranes. They classified the mixture of these constituents as the Proteids, Cholesterin, and Protagon. Hoppe-Seyler hypothesized that they perform important biological functions. Of the three substances, the composition of Protagon, a white crystalline powder consisting of a mixture of lipids obtained from the brain remained controversial. In a detailed study on the composition of protagon, Posner and Gies (1905) reported that protagon is not a well-defined substance, but a mixture of substances comprising phosphorus free and one or more compounds of the phosphorus containing substances [432]. Contradicting these reports, and Crammer (1907) reported that water content of the isolating solvents could play mischief in the results and suggested that repeated crystallization of protagon by water free chloroform yielded, pure phosphorus rich protagon, which in brain tissues exists in association with cerebrin or pseudo-cerebrin [433]. In a subsequent study, Rosenheim and Tebb (1909), demonstrate that protagon is not an independent chemical, but a mixture comprising the phosphorus rich sphingomyelin, found associated with cerebrosides and glycosides, which exhibit identical properties to protagon, leading to confusions [434].
Arginine metabolism limits the mitochondrial respiration in favour of cell proliferation/survival
Arginine metabolism limits ATP production and promotes the production of nucleotides, polyamines, ribosomes and amino acids [406]. Nitric oxide (NO) production requires the arginine oxidation in mitochondria. NO competes with the binding of O2 to CIV and inhibits the reduction of O2 and suppresses the NADH oxidation by CI of ETC, which results in the ROS production in the ETC. Oxidative stress causes death, which is rescued by the supplementation of the glutamine or by the nucleotides [435]. Glutaminases produce glutamate; the redox homeostasis in cells requires the amino acid cysteine and glutamate acts as an antiporter for cysteine at the plasma membrane [436, 437]. The living cells can synthesise arginine, glutamine and cysteine, but proliferative cells are auxotrophic for these amino acids as the demand for these amino acids during metabolic reprogramming far exceeds the biosynthetic capacity of cells [438, 439]. Arginine is a substrate for three mitochondrial enzymes, the mitochondrial arginine decarboxylase (ADC), the arginase2(ARG2) and the mitochondrial nitric oxide synthase (mtNOS). Arginine decarboxylase produces the polyamine agmatine (AGM), which limits NOS and stimulates the whole-body fatty acid metabolism and activates the tissue level cyclic AMP (cAMP) pathway. AGM downregulates the high fat-induced obesity and stimulates the β-oxidation, the PPAR-α, its coactivator PGC1α, and increases the activity of the PPAR-γ, and the genes regulating the thermogenesis, gluconeogenesis, and prevents atherosclerosis [440–442]. Although nitric oxide synthases, eNOS, nNOS, and iNOS remained at the central stage of vascular health for long, the mitochondrial nitric oxide synthetase discovered by Ghafourifar and Richter in 1997 [443–445], as the enzyme producing the mitochondrial NO, and as the regulator of matrix pH, which limits the mitochondrial respiration and transmembrane potential produces citrulline, opened a new role for the NO in promoting the pyrimidine synthesis and as regulator of the urea cycle enzymes. There are however controversies on the existence of the mtNOS [446]. The mtNOS protein cross reacts with the three well studied nitric oxide synthases, the endothelial NOS (eNOS), neuronal NOS (nNOS), and the inducible NOS (iNOS) (reviewed by Xu et al. [447] and Tegan and Moraes [448], the mitochondrial NO synthesis inhibits the mitochondrial respiration and the electron transport at complex I and IV and produces the reaction oxygen/nitrogen species [448–450]. But this inhibition is a temporary phenomenon, as the depletion of NO restores the respiration by activating the complex -II/ SDH-CoQ reductase [451, 452]. Besides mitochondrial ARG2 expression limits the peroxide stress by limiting mtNOS production [453]. ARG2 modulates NO synthesis through small amounts of the endogenous L-arginine pools [454], which quickly depletes the arginine availability for the reaction. The de novo synthesis of arginine requires glutamine metabolism to sustain the biosynthesis of nucleic acids [455]. In the absence of glutamine, the cells apoptose as the de novo synthesis of arginine requires the carbamoyl phosphate (CP) synthesis in mitochondria [335, 456] (Fig. 9a). The mitochondrial GLUD2 activates the synthesis the carbamoyl phosphate (CP) in mitochondria and activates the ornithine transcarboxylase to produce citrulline and its downstream metabolic product argininosuccinate [457, 458]. ARG2 mediated ornithine production activates the polyamine spermines, putrescine and spermine. The polyamine spermine increases mitochondria glutamine metabolism [459]. ARG2 deficiency extends the life span of female mice [460]. Arginase activity is self-regulatory; the urea produced in the reaction can inhibit the arginase activity [461], but it is not clear whether the urea produced by ARG2 in mitochondria inhibits ARG2. Recent studies suggested that the activation of the epidermal growth factor receptor (EGFR), the two hypoxia transcription factors, the HIF1 and HIF2 and the vascular endothelial growth factor (VEGF) increases the ARG2 levels and the proliferation of the pulmonary microvascular artery endothelial cells and metastatic tumours [46, 462, 463]. Hypoxia is the principal regulator of the endothelial cell function and angiogenesis [464–466]. The microvascular dysfunction resulting out of the endothelial cell death is the cause of diabetic retinal, renal and lower limb disorders [34, 319]. The mtNOS produces NO in the mitochondria, which competes with the O2 at complex IV of ETC and inhibits the ATP and ROS production and protects cells under hyperoxia [467, 446, 445]. While the low levels of NO are protective, increasing levels of NO (1micromolar/L and above) are damaging to the mitochondrial Δψm, which may lead to apoptosis [468–471]. NO inhibits O2 consumption in the ETC in a reversible manner and suspends the ATP production [472, 473] (Fig. 9a). Different isoforms of nitric oxide synthases (NOS), arginases, and glutaminases target the arginine and glutamine in mitochondria and cytoplasm at temporally different phases of cell growth.
Fig. 9.
Intracellular Amino Acids Activates Proliferation Membrane Uptake of Intercellular Glutamine and leucine Activates Cell cycle Exit: a Arginine Metabolism in Mitochondria, proline, and the GCN2 dependent glutamate and aspartate synthesis activate the CAD pathway for pyrimidine synthesis in proliferative cells: Mitochondrial arginine is the target of three enzymes: the mitochondrial nitric oxide synthase (mtNOS), mitochondrial arginase (ARG2), and the arginine decarboxylase (ADC). The mtNOS produces the NO and citrulline. Citrulline is exported into the cytoplasm and activates the CAD pathway. NO inhibits the O2 consumption by complex IV and the NADH oxidation by complex-I of the respiratory chain. ARG2 produces the ornithine and urea. Urea has feedback effect on the ARG2, but ornithine activates the polyamine biosynthesis (not shown in the figure). ADC produces the CO2 and the agmatine. Agmatine has activating effect on the fatty acid β-oxidation. The GCN2 dependent asparagine synthesis supplies the aspartate for CAD pathway, while proline contribute to glutamate synthesis (FIG. b) which produces the carbamoyl phosphate for pyrimidine synthesis in CAD pathway. b Proline Contributes to Glutamate Production for CAD Pathway in Proliferative cells EMT induces the matrix degradation and the collagen hydrolysis liberates the proline. Pyrroline-5-carboxylate synthase (P5CS) is a bifunctional enzyme which produces an intermediate delta(1)-pyrroline-5-carboxylate and inter converts the proline to glutamate and vice versa. The enzyme ALDH1A1 and the ALDH18A1, members of the aldehyde dehydrogenase 1 family member A1 and aldehyde dehydrogenase gene family 18 member A1/2, respectively catalyse the reversible conversion of the delta(1)-pyrroline-5-carboxylate to glutamate in cytoplasm and mitochondria, respectively. The delta(1)-pyrroline-5-carboxylate reductase (P5CR) produces the intermediate the delta(1)-pyrroline-5-carboxylate from the proline. (ALDH1A1, is one of the markers of cancer stem cell (CSC), and it has been related to tumorigenesis and drug resistance in several cancers)
Proline metabolism and cytoplasmic aspartate availability supports the cell proliferation under glutamine deprivation
During the cell transformation, leucine uptake is limited in cancer epithelial cells as TGFβ1 reduces the expression of SLC3A2 and proline metabolism plays a vital role in cell proliferation [474, ]. Proline metabolism produces glutamate for biosynthesis of carbamoyl phosphate in the cytoplasm for CAD pathway in pluripotent stem cells (Fig. 9a, b) and cisplatin resistant cancers [475–479]. The mtNOS supports the nucleotide synthesis in proliferative cells, which requires the glutamine, cytoplasmic glutaminase and aspartate [480]. Citrulline produced by the mtNOS and de novo aspartate synthesis promotes the argininosuucinate synthesis, which upon hydrolysis promotes de novo arginine, fumarate and polyamine biosynthesis (reference 457). The delta(1)-pyrroline-5-carboxylate reductase (P5CR) produces the intermediate the delta(1)-pyrroline-5-carboxylate from the proline. ALDH1A1 which is one of the markers of cancer stem cell (CSC), and it has been related to tumorigenesis and drug resistance in several cancers [481–483]. Aspartate has multiple functions ranging from pyrimidine synthesis to production of glutamate to increase the amino acid fluxes for protein synthesis. to activate the carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) pathway for nucleotide synthesis and ribosome biogenesis [306]. The SNAT7 (SLC38A7), which is a selective lysosomal glutamine and asparagine transporter, supports the biosynthesis of nucleotides in the anchor-independent cells in an uncoupled state [484]. Inhibition of respiration suppresses the glutamine metabolism, which results in apoptosis [485, 486]. The cell metabolism shifts from glutamine to aspartate metabolism when ETC is dismantled, which supports the survival of cells which are resistant to glutamine deprivation [341, 487]. Aspartate becomes a limiting factor in tumours insensitive to respiration; the enzyme asparaginase 1 (ASNase1), which produces aspartate from asparagine, aspartate/ glutamate transporter SLC1A3 provide the required aspartate for tumours insensitive to inhibition of respiration [488, 489].
Glutamine uptake by cells suppresses metastasis, promotes De novo Palmitoyl-CoA synthesis and mitotic exit
The production of lipid droplets, microtubule formation is essential for mitotic exit, which requires the de novo fatty acid synthesis for the formation of microtubules and the lipid droplets to complete cell division [427]. HIF1 activates the GLUT1 mediated glucose uptake and oxPPP, which supplies NADPH for de novo fatty acid synthesis [490]. Citrate produced in mitochondria by reductive carboxylation is transported into the cytoplasm and ATP Citrate lyase (ACLY) hydrolases citrate to Acetyl CoA and OXA, which reduced to malate. NADP dependent malic enzyme (ME) hydrolyses malate to pyruvate and CO2 and produces NADPH. This NADPH so produced supplies reducing hydrogen for initial activation of fatty acid synthesis [490, 491]. High fat diets bypass the de novo fatty acid synthesis by activating the SREBP1c and stearoyl-CoA desaturase (SCD), which results in lipid accumulation and mitotic slippage [492–495]. Huang et al. (2017), Pavlova et al. (2018) reported that defects in glutamine metabolism by inhibiting the glutaminase (GLS) or by depletion of glutamine in endothelial cells (EC) resulted in vessel sprouting defects due to defective proliferation and migration [496, 497]. Asparagine supplementation and TCA cycle intermediates in glutamine-deprived ECs, activated the glutamine and amino acid synthesis to support the protein synthesis, suppressed ER stress, and reactivated mTOR signalling. De novo asparagine synthesis rather than the supplementation of asparagine restored the metabolic aberrations and proliferation defects when GLS1 is inhibited. Glutamine provides nitrogen for asparagine synthesis and silencing asparagine synthetase (ASNS), which converts aspartate to asparagine or depletion of ASNS impaired EC sprouting even in the presence of glutamine and asparagine. Intracellular asparagine levels regulate the uptake of amino acids, especially serine, arginine and histidine; serine uptake influences one-carbon metabolism and activates mTORC1 dependent protein and nucleotide synthesis for cancer cell growth [497–500]. Inhibition of mitochondrial glutamine metabolism also inhibits the BCAT2 and valine deprivation in mitochondria suppresses the succinate production [501–503] and its oxidation by succinate dehydrogenase (SDH) (Fig. 8a, b). SDH deficiency activates the pyruvate carboxylase, which produces the aspartate in mitochondria through GOT2 [504]. Glycosylation also activates the heterodimeric amino acid transporter interacting with the glycoprotein CD98 (SLC3A2) and the large neutral amino acid transporter 1 (LAT1, or SLC7A5), which acts as an antiporter for glutamine, in exchange for the uptake of the essential branched chain amino acids and phenylalanine [416, 417, 505]. Enhanced BCAA uptake and their catabolism especially valine by the mitochondrial branched-chain aminotransferase (BCAT2) and the branched-chain keto acid dehydrogenase (BCKDH) promotes the succinate synthesis in mitochondria and suppresses the mitochondrial respiration and promotes immunity, differentiation by activating gluconeogenesis and amino acid synthesis [506–508, 503]. Valine deprivation was reported to protect the bone marrow stem cell grafts against apoptosis by suppressing the immune reaction [509–511].
The mechanism of Palmitoylation Caveolins and lipid rafts
Palmitoylation of proteins is catalysed by the palmitoyl acyl transferases which share a common amino acids sequence referred to as a DHHC (aspartate-histidine-histidine-cysteine) domains and produce several acylated proteins modulating cell function [512–514]. The process of acylation involves two steps, the binding of the palmitoyl-CoA followed by the hydrolysis of the CoA, which results in the formation of an intermediate between enzyme and palmitate; palmitate is then transferred to the target proteins, dietary fats and high cholesterol disrupt the palmitoylation process and vascularisation [515, 516]. High fat diets directly activate the Δ9 de-saturation, inhibit de novo palmitoyl-CoA synthesis, by activating the stearoyl-Coenzyme A desaturase 1 (SCD1), and insulin resistance [517, 518]. Insulin regulates the fatty acid and cholesterol synthesis through transcription of two insulin induced genes (INSIG1 & 2). Insulin increases the de novo fatty acid synthesis by down regulating the transcription of the Insig2 protein [191, 351], which inhibits the sterol regulatory element binding transcription factor (SREBF) chaperone protein (SCAP) and prevents the proteolytic processing of the transcription factor SREBF2in Golgi complex.
Perspective summary
For over a century, the intermediary metabolism (IM) in the cells projected glucose, ATP, and lactate production as the focus of attention in health and metabolic pathology. Mitochondria remained as the compartmentalised catabolic organelles for oxidation of nutrients to produce energy in the TCA cycle, and the failure to produce ATP is projected as the mitochondrial dysfunction. Three enzymes, one glycolytic enzyme, pyruvate kinase (PK) and the two mitochondrial enzymes, the pyruvate dehydrogenase (PDH) complex (PDC) and the condensing enzyme (the citrate synthase) in the TCA cycle drive the functioning of the present model of IM. The role of PDC in mitochondria remained controversial from the beginning. The enzyme was shown to be inhibited by acetyl-CoA, fatty acyl-CoA, succinyl-CoA, and the high ratio of NADH/NAD+. Two alternative hypotheses were proposed questioning the role of Pyruvate oxidation in mitochondria. The Randle cycle suggested that fatty acids inhibit the glucose oxidation [519], and the Cahill cycle, also known as the glucose-alanine cycle, proposed that the amino acids released from muscles produce pyruvate, which produces glucose in the liver [520]. Latic acid remained the marker of anaerobic glycolysis. In extensive studies for over a half century, Brooks proposed that lactate shuttles modulating the mitochondrial function in muscles; lactate produced in one cell/ tissue becomes fuel for its neighbours to promote the mitochondrial oxidative metabolism [521, 522]. However, the story of lactate is much more complex than just being a fuel; lactate enters the cells through the GPCR, HCA1, and activates the G protein, Gi/o [523, 524], which inhibits the cAMP/PKA pathway and suppresses the cAMP-dependent acetylation pathways. More importantly, lactate stabilises the HIF2/ARNT transcriptional activity, which upregulates glutamine uptake, biosynthesis of the erythropoietin, FGF23, phosphate, vitamin-D, iron metabolism and red cell production [525–527]. HIF2 transactivates the MYC, and together they promote the glutamine uptake through ASC2, BCAA uptake through LAT1 and activates the mitochondrial glutamine and BCAA metabolism [528, 529] Interestingly, MYC suppresses the PDH and increases the respiration; and PDH KO cells have increased respiration in response to mitochondrial glutamate levels [251]. Deregulated Pi dependent mitochondrial glutamine metabolism is at the centre stage of two critical problems of diabetes/insulin resistance in ageing populations, the dysregulated cholesterol biosynthesis and the cachexia/ sarcopenia (the debilitating muscle loss), which causes death in terminal stages of cancer and diabetes.
Tissue turnover reprograms metabolism to maintain systemic homeostasis
The ‘chicken or the egg’ is causality dilemma, a classical metaphoric adjective used to describe the confusions in determining the cause and the effect [530]. This is more relevant to the metabolism of living bodies. The aggressive relapse of cancers following the growth factor, surgical and radiation therapies provided the answer; the embryonic cells produce healthy tissues and ageing cells regenerate the immortal cells by accumulating stress factors caused by the nutrient imbalance and hyperactive mTORC1 (see the Hay flick concept of finite replicative capacity and telomerase contributing to immortality of pathology in cultured cells [531, 532] and the role mTORC1’s in promoting the quasi programmed senescence [533]. Claude Bernard was the first to suggest that turnover of the tissues in the body is essential for systemic homeostasis [534]. Schoenheimer, Ratner and others in the early twentieth century used the isotopic techniques to demonstrate that the body fats and proteins are regularly turned over to maintain homeostasis [535, 536]. Glutamine deprivation causes cell death, but fatty acids and nucleotides rescue cells from apoptotic cell death and allow the cell cycle progression [537, 435]. Tissue renewal activates autophagy and the degradation of cytoplasmic organelles and unfolded proteins support the cell transition to mesenchymal state and reprogram the cell metabolism for proliferation and activate the cell survival or apoptosis, depending upon nutrients availability. The plasticity of extracellular matrix, especially reduction in collagen density activate the EMT and shifts the cell metabolism from glycolytic to oxidative phenotype and directs the metabolic reprogramming, the stem cells fate and lineage specification.
To make the long story short, hyperactive mTORC1 inhibits mTORC2, and nutrients uptake (glucose and amino acids) and induces the starvation effects. The depleting nutrients, mechanical and replicative stress has the feedback effect and inhibits the mTORC1 and protein synthesis. Inhibition of mTORC1 activates the AMPK and the autophagy and hydrolyses the cytoplasmic proteins. Starvation effect increases the fatty acids uptake and oxidation of fatty acids and the amino acids in mitochondria and induce temporal changes to activate mTORC1. Stress induced secretion of catecholamines/ neuro-transmitters [538], the fatty acids and their metabolites bind to the cell surface GPCRs and activate the second messengers like cAMP, DAG, calcium and inositol phosphates, which activate the Ras-MAP kinase pathway and induce the anchor independent cell proliferation. The hyperactive mTORC1 also induces disturbances in cell polarity and tissue remodelling. Pluripotent proliferative cells are anchorage independent and depend on the metabolism of fatty acids, arginine, proline and cytoplasmic aspartate for reprogramming cellular biosynthesis and repair of damaged cells. The intracellular transceptors and the non-essential amino acids support nucleotide synthesis for proliferation and cell cycle progression. The cell cycle exit requires the de novo cholesterol and fatty acid biosynthesis for lipid droplet and lipid rafts. In the absence of lipid rafts the pluripotent cells resort to metastasis and migrate to O2 enriched environments to sustain the respiration and survival. TGFβ limits the leucine uptake in progenitor cells. The dietary sterols support the cholesterol biosynthesis in the absence of leucine availability in progenitor cells. Cancer cells are auxotrophic for cholesterol and the glutamine. Glutamine uptake into the cells from the microenvironment is facilitated through the neutral amino acid transporter, the SLC7A5 (ASCT2). Depleting glutamine catabolites, the glutamate and the 2-OG, activate the mTORC2, and the HBP to produce the UDP-GlcNAc, for activation of O-linked and N-linked glycosylation of nutrient transporters and intra-organelle communication. 4F2 HC (CD98) protein mobilises the cell surface transporters for glutamine and essential amino acids uptake. Elevated expression of LAT1 (SLC7A5), 4F2hc (SLC3A2), xCT (SLC7A11) and ASCT2 (SLC1A5) in tumour cells promotes the glutamine uptake into the cells and promotes growth and the de novo fatty acid, palmitoyl–CoA synthesis to completes the cell cycle, and exit the mitosis. The 4F2HC with SLC7A11 activates the cysteine/ cystine uptake in exchange of the glutamate and activates the glutathione (GSH) synthesis and redox homeostasis. The 4F2HC and LAT1 complex promotes the efflux of the glutamine in exchange for the leucine and essential amino acids into the cells. The antagonistic relationship between the transport of cysteine and glutamate, the leucine and glutamine uptake has a role in cell cycle exit or slippage. Glutamine catabolism in mitochondria requires Pi [539, 325]; insulin resistance suppresses the pi uptake and the mitochondrial glutamine metabolism suppresses the reductive carboxylation and the de novo lipogenesis. Inhibition of glutamine uptake activates the asparagine uptake, which increases the leucine uptake. The basal levels of glutamine in cytoplasm promotes the asparagine synthesis, which results in mitotic slippage and the incompletely divided cells become tetraploid/ aneuploid and the surviving cells either become fibrotic or face a delayed death of vascular and smooth muscle cells. Insulin-like Growth Factor 2 (IGF2), over expressed in cancer cells, sustains the angiogenesis and progenitor cell proliferation; in diabetes, the protein inhibits the β-cell function by activating the dedifferentiation of β-cells and vascular atherosclerosis [540, 541]. The 2-OG shuttles the ammonia and carbon from the essential and non- essential amino acids to activate the biosynthesis of nucleotide, isocitrate and amino acid synthesis. The role of GDH is to supply NH3 pyrimidine and nucleotide synthesis, while the pyruvate-glutamate aminotransferase (GPT) activates the de novo fatty acid synthesis, which requires the functional ETC in uncoupled state to provide the reduced NADH for transhydrogenation to NADPH, the glutamate-oxaloacetate aminotransferase (GOT) promotes the senescence and mTORC1 dependent biosynthesis programmes, when the ETC is disrupted. Insulin resistance activates the lipolysis and increased uptake of lipids into the cells suppresses the glutamine metabolism, promotes cell survival by supressing the mitotic exit and activating the mitotic slippage (Fig. 10). Asparagine becomes essential amino acid in the absence of glutamine. under insulin resistance, leucine uptake, activates the metabolic syndromes by promoting the excess cholesterol biosynthesis, atherosclerosis and fibrosis.
Fig. 10.
Metabolic Alterations Induced by Insulin Resistance During Mitotic Slippage, Fibrosis and Pathology: In the absence of de novo fatty acid synthesis, the cell cycle progression will be arrested, and the tetraploid dividing cells resort to mitotic slippage and will delay apoptosis. Insulin resistance, however, supresses the glutamine hydrolysis and activates the asparagine synthesis and uptake into cells induced by mitotic slippage. Although such cells also take up extracellular asparagine, the exact transporter of asparagine transport is not fully understood; the SLC1A3, an aspartate and glutamate transporter in solid tumours and the neutral amino acid transporter solute transporters 2 (SNAT2)/ SLC38A3 and the electrogenic SLC6A14 are reported to support the asparagine and glutamine uptake across the membranes. ASNS and asparagine-glutamine proline biosynthesis activates the protein biosynthesis and under anorexia, the peripheral cells, especially muscles increase the hydrolysis of proteins to supply the amino acids to the developing tumours
Conclusions
Type-2 diabetes and cancer are the pathologies of ageing population. During the postnatal life, the child growing to adult stage has demand for the excess nutrients, for tissue growth. As he/ she reaches the adult stage, only few pockets of tissues, like epithelial cells in skin, the gut, air way passages, the vascular cells, the gonads and the haematopoietic cells need nutrients for rapid turnover. Physical exercise, fasting activate the tissue turnover, which balances the nutrient uptake, utilization with biosynthesis. Insulin as a mild mitogenic anabolic hormone responds to nutrients uptake, activates the biosynthesis programmes to suit to the remodelling of the proliferative tissues within the adult body by activating the PPP and mitochondrial metabolic pathways to maintain systemic homeostasis and health. Although every cell has the insulin receptors, the muscle, liver and adipose tissue are the principal modulators of insulin action. Skeletal muscles, which constitute more than 40% of the body mass are the principal storage organs of the protein; physical activity, and starvation induces the turnover of much of the protein in muscles and releases the bulk of amino acids into circulation. The circulating amino acids are used to reprogramme the cell metabolism. The smooth muscles line the walls of the gut, pulmonary bronchioles, uterus, urethra, bladder, blood vessels, the skin, and involuntarily respond to the material movements through the lumen of theses organs. Cardiac muscle (myocardium), which is similar in structure to the skeletal muscle is actively involved in the contraction relaxation cycles, which help the blood circulation and supply of the nutrients, O2 and the hormones to every cell of the body. The extensive network of vasculature delivers the nutrients and collect the metabolites from the cells. Organisms regenerate the capillary network (angiogenesis) to meet the demands of O2 and metabolites for tissue repair and growth. Inability to regenerate the capillary networks results in the vascular pathology in diabetes, and hyperactive angiogenesis promotes tumorigenesis. Glutamine attracted the early attention as an alternative fuel to glucose in carcinogenesis. Krebs (1935) discovered two types of glutaminases GLS and GLS2 in kidney and liver. The oncogene MYC promotes the glutamine metabolism in tumours. Myc activates expression of GLS genes, while the tumour suppressor p53 promotes the expression of GLS2. GLS gene in turn codes for two types of isoforms, the cytosolic kidney type (KGA) and the mitochondrial GAC and the GLS2 codes two liver type isoforms, the GAB and LGA (reviewed by Altman et al. (2016) [320]; Szeliga and Obara-Michlewska [542]. Within the cell, the metabolism of glutamine produces two metabolites, the glutamate and the ammonia (NH3). Both the glutamate and glutamine are antiporters for cysteine and leucine uptake at the plasma membrane, which activate the glutathione and cholesterol biosynthesis respectively. Glutamate in the cells is metabolized by two different types of enzymes, the glutamate dehydrogenase (GLUD) and the amino transferases, the glutamate-pyruvate aminotransferase (GPT) and the glutamate -oxaloacetate aminotransferase (GOT), which are also called the alanine amino transferase (ALT) and the aspartate amino transferase (AST) respectively. Each of these enzymes have the cytosolic (GLUD1, GPT1, GOT1) and the mitochondrial (GLUD2, GPT2, GOT2) isoforms. The mitochondrial glutamine metabolism, and the enzymology attracted early attention as the current model of TCA cycle depicted the 2-OG produced both by GLUD2 and GPT2 as the converging point to replenish the oxidative capacity of the TCA cycle for ATP production. The 2-OG is a metabolite for both synthesis and decarboxylation of isocitrate. The isocitrate dehydrogenase (IDH) isoforms both in cytoplasm and the mitochondria modulate the cell metabolism and systemic health by differentially regulating the production of 2-OG, the oncometabolite 2-HG in the cytoplasm, and reductive carboxylation to produce citrate in the cytoplasm (FIG.4A& 8A). The discovery of two mitochondrial pyruvate carrier proteins (MPC1 &2) in 2012, and the following knockout studies demonstrated that the entry of pyruvate and glutamine into mitochondria through MPC1 suspends proliferation and promotes de novo fatty acid synthesis and mitotic exit. The disruption of respiratory chain activates the pyruvate entry into mitochondria through MPC2 and activates the pyruvate carboxylase to produce the OXA. OXA is reduced to malate or transaminated to aspartate and activate the gluconeogenesis and amino acid synthesis and urea production in fully differentiated cells.
Glutamine transport into the cells is temporally regulated during cell cycle progression, proliferative progenitor cells depend on proline or the intracellular transceptors SNAT1 (SLC38A1) and SNAT2 (SLC38A2) for glutamate in cancer cells. Proline and glutamine can be interconverted through a reciprocal regulation of Δ1pyrroline-5-carboxylate (P5C). During proliferation Aldehyde dehydrogenase 1 family, member A1 (ALDH1A1) produces glutamate in the cytoplasm to activate the pyrimidine synthesis. Reduced 2-OG during proliferation and production of the oncometabolites activates the hexosamine biosynthetic pathway activates the 4F2HC/CD 98, suppresses proliferation and activates the cell surface transporters. Glutamine uptake thorough ASCT2 and the uptake of BCAA through LAT1/LAT2 complex is essential for uptake of cysteine and leucine for production of glutathione and cholesterol. A variant of SLC1A5 (ASCT2) promotes the glutamine uptake into mitochondria [543]. Insulin activates the Pi dependent glutamine metabolism in the mitochondria, which in turn activates the reductive carboxylation and citrate synthesis and the de novo fatty acid synthesis. Succinate production in mitochondria is activate by two different pathways, the reductive carboxylation is also associated with simultaneous oxidation of 2-OG to succinate [544], which also produces the GTP required for the cytoskeletal reorganisation. Alternatively, the transamination of BCAA especially the valine produces the succinate (Fig. 8a). One of the perplexing question that remained a puzzle in insulin resistance, IR activates the hypercholesterolemia, but disrupts de novo fatty acid synthesis. Present model proposed that acetyl-CoA produced from citrate is the carbon source for lipogenesis (a term used for fatty acid and cholesterol synthesis). A historical review of cholesterol biosynthesis and the model presented in this article suggests that dietary sterols promote the cholesterol synthesis in proliferative cells and leucine uptake through LAT1 and LAT2 complex activates the mevalonate synthesis as well as the apolipoprotein synthesis. Glutamine metabolism in mitochondria is essential for de novo palmitoylation and several proteins, especially the lipid rafts, the lipid droplets and the mitotic spindle proteins (tubulins) require palmitoylation for membrane stability and mitotic exit. Insulin resistance inhibits mitochondrial glutamine metabolism, and the de novo palmitoyl-CoA synthesis, which results asparagine synthesis and mitotic slippage, which activates the proline metabolism and fibrosis in pathology of neurovascular disorders. During mitotic slippage, when the respiratory chain, and mitochondrial glutamine metabolism is inhibited glutamine is converted to asparagine, which contribute the biosynthesis of amino acids and fibrosis in pathology [305, 477, 545].
Figures (1to 10) and the legends of the figures give the details of temporal changes associated during cell cycle progression and metabolic remodelling.
Acknowledgments
LV acknowledges umpteen number of authors and former students (both cited and not cited in the article), who shared their research articles on request, which helped in shaping the hypothesis and development of the article to its final shape. MI would like to acknowledge all staff of the Institute of Biotechnology, University of Gondar, Gondar, Ethiopia, for their constant encouragement and support extended. The University of Hyderabad partly supported ABMR laboratory through institutional funding from DST-FIST (DBT-RNAi, DST-SERB), Government of India. GBM acknowledges UGC-CSIR for student fellowship.
Authors’ contribution
The corresponding author (LV) formulated the hypothesis and developed the scheme and the write-up. MI participated in the discussion and development of gene networks by using the geneMANIA and the String databases.SK contributed to literature collection and compilation of literature. GBM, NN, and ABMR participated in the day to day discussions and supported in literature collection, during the write-up, which helped in shaping the article in its’ final form.
Compliance with ethical standards
Conflict of interest
The article is not supported by any funding agency; the authors do not have any conflict of interest.
Footnotes
Corresponding author is a retired professor from Kakatiya University
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lakshmipathi Vadlakonda, Email: lvadlakonda@gmail.com.
Meera Indracanti, Email: drmeerabio@yahoo.com.
Suresh K. Kalangi, Email: skkalangi@ggn.amity.edu
B. Meher Gayatri, Email: meher.gaya3@gmail.com.
Navya G Naidu, Email: navyanaidu570@gmail.com.
Aramati B. M. Reddy, Email: abmsl@uohyd.ernet.in
References
- 1.Clatici VG, Voicu C, Voaides C, Roseanu A, Icriverzi M, Jurcoane S. Diseases of civilization - cancer, diabetes, obesity and acne - the Implication of Milk, IGF-1 and mTORC1. Maedica (Buchar) 2018;13(4):273–281. doi: 10.26574/maedica.2018.13.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ertel A, Tsirigos A, Whitaker-Menezes D, Birbe RC, Pavlides S, Martinez-Outschoorn UE, et al. Is cancer a metabolic rebellion against host aging? In the quest for immortality, tumor cells try to save themselves by boosting mitochondrial metabolism. Cell Cycle. 2012;11(2):253–263. doi: 10.4161/cc.11.2.19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabadkai G, Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14(12):1405–1423. doi: 10.1007/s10495-009-0363-5. [DOI] [PubMed] [Google Scholar]
- 4.Song Z, Wang W, Li N, Yan S, Rong K, Lan T, et al. Sphingosine kinase 2 promotes lipotoxicity in pancreatic beta-cells and the progression of diabetes. FASEB J. 2019;33(3):3636–3646. doi: 10.1096/fj.201801496R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokes MA. Antoine Lavoisier and the study of respiration: 200 years old. Aust N Z J Surg. 1991;61(3):229–232. doi: 10.1111/j.1445-2197.1991.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 6.Gladden LB. 200th anniversary of lactate research in muscle. Exerc Sport Sci Rev. 2008;36(3):109–115. doi: 10.1097/JES.0b013e31817c0038. [DOI] [PubMed] [Google Scholar]
- 7.Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med. 2007;33(11):1967–1971. doi: 10.1007/s00134-007-0788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett JA. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850-1880. Yeast. 2000;16(8):755–771. doi: 10.1002/1097-0061(20000615)16:8<755::AID-YEA587>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Latour B. Pasteur on lactic acid yeast: a partial semiotic analysis. Configurations. 1993;1(1):129–145. doi: 10.1353/con.1993.0004. [DOI] [PubMed] [Google Scholar]
- 10.Vadlakonda L, Reddy VD, Pasupuleti M, Reddanna P. The Pasteur’s Dictum: Nitrogen Promotes Growth and Oxygen Reduces the Need for Sugar. Front Oncol. 2014;4:51. doi: 10.3389/fonc.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher WM. Lactic acid in amphibian muscle. J Physiol. 1907;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher WM. Lactic acid formation, survival respiration and rigor mortis in mammalian muscle. J Physiol. 1913;47(4-5):361–380. doi: 10.1113/jphysiol.1913.sp001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 15.de Bari L, Atlante A. Including the mitochondrial metabolism of L-lactate in cancer metabolic reprogramming. Cell Mol Life Sci. 2018;75(15):2763–2776. doi: 10.1007/s00018-018-2831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587(Pt 23):5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;101454 10.1016/j.redox.2020.101454. [DOI] [PMC free article] [PubMed]
- 18.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft R. Oskar Minkowski: discovery of the pancreatic origin of diabetes, 1889. Diabetologia. 1989;32(7):399–401. doi: 10.1007/bf00271257. [DOI] [PubMed] [Google Scholar]
- 20.Sakula A. Paul Langerhans (1847-1888): a centenary tribute. J R Soc Med. 1988;81(7):414–415. doi: 10.1177/014107688808100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol (Lausanne). 2018;(9):613. 10.3389/fendo.2018.00613. [DOI] [PMC free article] [PubMed]
- 22.Macleod J. The physiology of insulin and its source in the animal body. Nobel Lecture 1925. 1925.
- 23.Himsworth HP. Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types. Diabet Med. 2011;28(12):1440–1444. doi: 10.1111/j.1464-5491.2011.3508.x. [DOI] [PubMed] [Google Scholar]
- 24.Kido Y, Burks DJ, Withers D, et al. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105(2):199-205. 10.1172/JCI7917). [DOI] [PMC free article] [PubMed]
- 25.Jelenik T, Sequaris G, Kaul K, Ouwens DM, Phielix E, Kotzka J, et al. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes. 2014;63(11):3856–3867. doi: 10.2337/db13-1794. [DOI] [PubMed] [Google Scholar]
- 26.Reaven G. A toast to Sir Harold Himsworth. Diabet Med. 2011;28(12):1436–1437. doi: 10.1111/j.1464-5491.2011.03339.x. [DOI] [PubMed] [Google Scholar]
- 27.Haider S, McIntyre A, van Stiphout RG, Winchester LM, Wigfield S, Harris AL, et al. Genomic alterations underlie a pan-cancer metabolic shift associated with tumour hypoxia. Genome Biol. 2016;17(1):140. doi: 10.1186/s13059-016-0999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deedwania PC. Diabetes is a vascular disease: the role of endothelial dysfunction in pathophysiology of cardiovascular disease in diabetes. Cardiol Clin. 2004;22(4):505–509. doi: 10.1016/j.ccl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Ageno W, Di Minno MN, Ay C, Jang MJ, Hansen JB, Steffen LM, et al. Association between the metabolic syndrome, its individual components, and unprovoked venous thromboembolism: results of a patient-level meta-analysis. Arterioscler Thromb Vasc Biol. 2014;34(11):2478–2485. doi: 10.1161/ATVBAHA.114.304085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart LK, Kline JA. Metabolic syndrome increases risk of venous thromboembolism recurrence after acute deep vein thrombosis. Blood Adv. 2020;4(1):127–135. doi: 10.1182/bloodadvances.2019000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenfeld L. Insulin: discovery and controversy. Clin Chem. 2002;48(12):2270–2288. [PubMed] [Google Scholar]
- 33.Riddle MR, Aspiras AC, Gaudenz K, Peuss R, Sung JY, Martineau B, et al. Insulin resistance in cavefish as an adaptation to a nutrient-limited environment. Nature. 2018;555(7698):647–651. doi: 10.1038/nature26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenkovich CF. We know more than we van tell about diabetes and vascular disease: The 2016 Edwin Bierman Award Lecture. Diabetes. 2017;66(7):1735–1741. doi: 10.2337/db17-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montani L, Pereira JA, Norrmen C, Pohl HBF, Tinelli E, Trotzmuller M, et al. De novo fatty acid synthesis by Schwann cells is essential for peripheral nervous system myelination. J Cell Biol. 2018;217(4):1353–1368. doi: 10.1083/jcb.201706010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimas P, Montani L, Pereira JA, Moreno D, Trotzmuller M, Gerber J, et al. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife. 2019;8 10.7554/eLife.44702. [DOI] [PMC free article] [PubMed]
- 37.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 38.Gialeli C, Viola M, Barbouri D, Kletsas D, Passi A, Karamanos NK. Dynamic interplay between breast cancer cells and normal endothelium mediates the expression of matrix macromolecules, proteasome activity and functional properties of endothelial cells. Biochim Biophys Acta. 2014;1840(8):2549–2559. doi: 10.1016/j.bbagen.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato T, Mizuno S, Ito A. A decrease in glomerular endothelial cells and endothelial-mesenchymal transition during glomerulosclerosis in the Tensin2-deficient mice (ICGN strain) Acta Histochem Cytochem. 2014;47(6):265–271. doi: 10.1267/ahc.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igari K, Kudo T, Toyofuku T, Inoue Y. The relationship between endothelial dysfunction and endothelial cell markers in peripheral arterial disease. PLoS One. 2016;11(11):e0166840. doi: 10.1371/journal.pone.0166840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 43.Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation. 2016;23(2):95–121. doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perbellini F, Watson SA, Bardi I, Terracciano CM. Heterocellularity and cellular cross-talk in the cardiovascular system. Front Cardiovasc Med. 2018;5:143. doi: 10.3389/fcvm.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pool CM, Jin Y, Chen B, Liu Y, Nelin LD. Hypoxic-induction of arginase II requires EGF-mediated EGFR activation in human pulmonary microvascular endothelial cells. Physiol Rep. 2018;6(10):e13693. doi: 10.14814/phy2.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40(2):323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucena MC, Carvalho-Cruz P, Donadio JL, Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, et al. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J Biol Chem. 2016;291(25):12917–12929. doi: 10.1074/jbc.M116.729236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu H, Schlegel V. Impact of nutrient overload on metabolic homeostasis. Nutr Rev. 2018;76(9):693–707. doi: 10.1093/nutrit/nuy023. [DOI] [PubMed] [Google Scholar]
- 50.DeBerardinis RJ. Tumor microenvironment, metabolism, and immunotherapy. N Engl J Med. 2020;382(9):869–871. doi: 10.1056/NEJMcibr1914890. [DOI] [PubMed] [Google Scholar]
- 51.Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13(2):183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1) 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed]
- 53.Glatz JF, Luiken JJ. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26. doi: 10.1016/j.biochi.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol. 2018;8(3):1091–1115. doi: 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichimura A. Polymorphic variation in FFA receptors: functions and consequences. Handb Exp Pharmacol. 2017;236:133–158. doi: 10.1007/164_2016_57. [DOI] [PubMed] [Google Scholar]
- 56.Caron A, Reynolds RP, Castorena CM, Michael NJ, Lee CE, Lee S, et al. Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Mol Metab. 2019;27:11–21. doi: 10.1016/j.molmet.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaihara KA, Dickson LM, Jacobson DA, Tamarina N, Roe MW, Philipson LH, et al. beta-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes. 2013;62(5):1527–1536. doi: 10.2337/db12-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105(20):7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jewell JL, Fu V, Hong AW, Yu FX, Meng D, Melick CH, et al. GPCR signaling inhibits mTORC1 via PKA phosphorylation of raptor. Elife. 2019;8 10.7554/eLife.43038. [DOI] [PMC free article] [PubMed]
- 60.Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, et al. Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 2002;277(37):33690–33697. doi: 10.1074/jbc.M202939200. [DOI] [PubMed] [Google Scholar]
- 61.Nagarajan D, Melo T, Deng Z, Almeida C, Zhao W. ERK/GSK3beta/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radic Biol Med. 2012;52(6):983–992. doi: 10.1016/j.freeradbiomed.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, et al. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150(11):5036–5045. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A. Epac and lipolysis. Cell Signal. 2009;21(5):760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, et al. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol. 2011;11(6):676–682. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad F, Degerman E, Manganiello VC. Cyclic nucleotide phosphodiesterase 3 signaling complexes. Horm Metab Res. 2012;44(10):776–785. doi: 10.1055/s-0032-1,312,646. [DOI] [PubMed] [Google Scholar]
- 67.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol. 2005;15(4):277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117(Pt 14):2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki T, Suzuki Y, Okuda J, Kurazumi T, Suhara T, Ueda T, et al. Sepsis-induced cardiac dysfunction and beta-adrenergic blockade therapy for sepsis. J Intensive Care. 2017;5:22. doi: 10.1186/s40560-017-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgdorff AM, Bucher M, Schumann J. Vasoplegia in patients with sepsis and septic shock: pathways and mechanisms. J Int Med Res. 2018;46(4):1303–1310. doi: 10.1177/0300060517743836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson PD, Horster MF. Differential response to hormones of defined distal nephron epithelia in culture. Am J Physiol. 1983;244(3):C166–C174. doi: 10.1152/ajpcell.1983.244.3.C166. [DOI] [PubMed] [Google Scholar]
- 72.Pullamsetti SS, Savai R, Schaefer MB, Wilhelm J, Ghofrani HA, Weissmann N, et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation. 2011;123(11):1194–1204. doi: 10.1161/CIRCULATIONAHA.110.941484. [DOI] [PubMed] [Google Scholar]
- 73.Neviere R, Delguste F, Durand A, Inamo J, Boulanger E, Preau S. Abnormal mitochondrial cAMP/PKA signaling is involved in sepsis-induced mitochondrial and myocardial dysfunction. Int J Mol Sci. 2016;17(12) 10.3390/ijms17122075. [DOI] [PMC free article] [PubMed]
- 74.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 75.Kresge N, Simoni RD, Hill RL. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. J Biol Chem. 2005;280(4):e3. [PubMed] [Google Scholar]
- 76.Simoni RD, Hill RL, Vaughan M. The determination of phosphorus and the discovery of phosphocreatine and ATP: the work of Fiske and SubbaRow. J Biol Chem. 2002;277(32):21e. [PubMed] [Google Scholar]
- 77.Langen P, Hucho F. Karl Lohmann and the discovery of ATP. Angew Chem Int Ed Engl. 2008;47(10):1824–1827. doi: 10.1002/anie.200702929. [DOI] [PubMed] [Google Scholar]
- 78.Buicher T, Pfleiderer G. Pyruvate kinase. In: SP C, Kaplan N, editors. Method in enzymology. 1. New York: Academic Press Inc.; 1955. p. 435. [Google Scholar]
- 79.Lipmann F. Metabolic Generation and Utilization of Phosphate Bond Energy. (In: Advances in Enzymology and Related Areas of Molecular Biology. 1941 p. 99-162.)
- 80.Szent-Györgyi A. Bioenergetics. J Sci. 1956;124(3227):873–875. doi: 10.1126/science.124.3227.873%. [DOI] [PubMed] [Google Scholar]
- 81.Albert L. LEHNINGER (1965) Bioenergetics The Molecular Basis of Biological Energy Transformations Second Edition (Published by W. A. Benjamin, New York (1965).
- 82.Bastedo GM, Irving L. The inorganic phosphorus of frog muscle in relation to lactacidogen and phosphocreatine. Am J Physiol. 1928;86(3):505–519. doi: 10.1152/ajplegacy.1928.86.3.505. [DOI] [Google Scholar]
- 83.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huangyang P, Simon MC. Hidden features: exploring the non-canonical functions of metabolic enzymes. Dis Model Mech. 2018;11(8) 10.1242/dmm.033365. [DOI] [PMC free article] [PubMed]
- 85.Park JS, Burckhardt CJ, Lazcano R, Solis LM, Isogai T, Li L, et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 2020;578(7796):621–626. doi: 10.1038/s41586-020-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grüning, NM, Du D, Keller MA, Luisi BF, Ralser M. Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biol. 2014;(3):130232. Published 2014 Mar 5. 10.1098/rsob.130232. [DOI] [PMC free article] [PubMed]
- 87.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6(2):195–210. doi: 10.1016/s0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 88.Reis M, Alves CN, Lameira J, Tunon I, Marti S, Moliner V. The catalytic mechanism of glyceraldehyde 3-phosphate dehydrogenase from Trypanosoma cruzi elucidated via the QM/MM approach. Phys Chem Chem Phys. 2013;15(11):3772–3785. doi: 10.1039/c3cp43968b. [DOI] [PubMed] [Google Scholar]
- 89.Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. 2014;1(12):777–802. doi: 10.18632/oncoscience.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kvassman J, Pettersson G. Mechanism of 1,3-bisphosphoglycerate transfer from phosphoglycerate kinase to glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1989;186(1-2):265–272. doi: 10.1111/j.1432-1033.1989.tb15205.x. [DOI] [PubMed] [Google Scholar]
- 91.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2) 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed]
- 92.Ramazzotti G, Ratti S, Fiume R, Follo MY, Billi AM, Rusciano I, et al. Phosphoinositide 3 kinase signaling in human stem cells from reprogramming to differentiation: a tale in cytoplasmic and nuclear compartments. Int J Mol Sci. 2019;20(8) 10.3390/ijms20082026. [DOI] [PMC free article] [PubMed]
- 93.Cori CG. Carl and Gerty Cori and carbohydrate metabolism. American Chemical Society National Historic Chemical Landmarks. Am Chem Soc. 2004;
- 94.Witney TH, Carroll L, Alam IS, Chandrashekran A, Nguyen QD, Sala R, et al. A novel radiotracer to image glycogen metabolism in tumors by positron emission tomography. Cancer Res. 2014;74(5):1319–1328. doi: 10.1158/0008-5472.CAN-13-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zois CE, Harris AL. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J Mol Med (Berl). 2016;94(2):137–154. doi: 10.1007/s00109-015-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2(2):101–120. doi: 10.2174/1566524024605761. [DOI] [PubMed] [Google Scholar]
- 97.Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51(7):2190–2198. doi: 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- 98.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48(10):2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 99.Hojlund K, Birk JB, Klein DK, Levin K, Rose AJ, Hansen BF, et al. Dysregulation of glycogen synthase COOH- and NH2-terminal phosphorylation by insulin in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(11):4547–4556. doi: 10.1210/jc.2009-0897. [DOI] [PubMed] [Google Scholar]
- 100.Dey S, Goswami S, Eisa A, Bhattacharjee R, Brothag C, Kline D, et al. Cyclic AMP and glycogen synthase kinase 3 form a regulatory loop in spermatozoa. J Cell Physiol. 2018;233(9):7239–7252. doi: 10.1002/jcp.26557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24(8):1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10) 10.3390/cells8101118. [DOI] [PMC free article] [PubMed]
- 103.Berzal S, Alique M, Ruiz-Ortega M, Egido J, Ortiz A, Ramos AM. GSK3, snail, and adhesion molecule regulation by cyclosporine A in renal tubular cells. Toxicol Sci. 2012;127(2):425–437. doi: 10.1093/toxsci/kfs108. [DOI] [PubMed] [Google Scholar]
- 104.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S49–S57. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 106.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev. Camb Philos Soc. 2015;90(3):927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin L, Zhou Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol Lett. 2019;17(5):4213–4221. doi: 10.3892/ol.2019.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samland AK, Sprenger GA. Transaldolase: from biochemistry to human disease. Int J Biochem Cell Biol. 2009;41(7):1482–1494. doi: 10.1016/j.biocel.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 110.Kowalik MA, Columbano A, Perra A. Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front Oncol. 2017;7:87. doi: 10.3389/fonc.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee J, Yesilkanal AE, Wynne JP, et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019;568(7751):254‐258. 10.1038/s41586-019-1005-x. [DOI] [PMC free article] [PubMed]
- 112.Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem. 2012;287(40):33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rotblat B, Melino G, Knight RA. NRF2 and p53: Januses in cancer? Oncotarget. 2012;3(11):1272–1283. doi: 10.18632/oncotarget.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pontremoli S, Bonsignore A, Grazi E, Horecker BL. A coupled reaction catalyzed by the enzymes transketolase and transaldolase. J Biol Chem. 1960;235:1881–1887. [PubMed] [Google Scholar]
- 115.Kathagen-Buhmann A, Schulte A, Weller J, Holz M, Herold-Mende C, Glass R, et al. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro Oncol. 2016;18(9):1219–1229. doi: 10.1093/neuonc/now024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arriola Apelo SI, Lamming DW. mTORC2 puts its shoulder to krebs’ wheel. Mol Cell. 2016;63(5):723–725. doi: 10.1016/j.molcel.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 117.Cordero-Espinoza L, Hagen T. Increased concentrations of fructose 2,6-bisphosphate contribute to the Warburg effect in phosphatase and tensin homolog (PTEN)-deficient cells. J Biol Chem. 2013;288(50):36020–36028. doi: 10.1074/jbc.M113.510289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skalecki K, Mularczyk W, Dzugaj A. Kinetic properties of D-fructose-1,6-bisphosphate 1-phosphohydrolase isolated from human muscle. Biochem J. 1995;310(Pt 3):1029–1035. doi: 10.1042/bj3101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y, et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013;18(4):546–555. doi: 10.1016/j.cmet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 120.Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL, et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20(3):526–540. doi: 10.1016/j.cmet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 121.Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Racker E. From Pasteur to Mitchell: a hundred years of bioenergetics. Fed Proc. 1980;39(2):210–215. [PubMed] [Google Scholar]
- 123.Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240(1):98–103. doi: 10.1111/j.1432-1033.1996.0098h.x. [DOI] [PubMed] [Google Scholar]
- 124.Buchet K, Godinot C. Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho degrees cells. J Biol Chem. 1998;273(36):22983–22989. doi: 10.1074/jbc.273.36.22983. [DOI] [PubMed] [Google Scholar]
- 125.Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, Wei YH, et al. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur J Biochem. 1999;262(1):108–116. doi: 10.1046/j.1432-1327.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- 126.Fang J, Uchiumi T, Yagi M, Matsumoto S, Amamoto R, Takazaki S, et al. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci Rep. 2013;33(2):e00021. doi: 10.1042/BSR20120097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ibsen KH. The Crabtree effect: a review. Cancer Res. 1961;21:829–841. [PubMed] [Google Scholar]
- 128.Wu R, Racker E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959;234(5):1029–1035. [PubMed] [Google Scholar]
- 129.Loomis WF, Lipmann F. Reversible inhibition of the coupling between phosphorylation and oxidation. J Biol Chem. 1948;173(2):807. [PubMed] [Google Scholar]
- 130.Zhang YY, Zhou LM. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem Biophys Res Commun. 2012;423(1):26–31. doi: 10.1016/j.bbrc.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 131.Chen J, Wang A, Chen Q. SirT3 and p53 deacetylation in aging and cancer. J Cell Physiol. 2017;232(9):2308–2311. doi: 10.1002/jcp.25669. [DOI] [PubMed] [Google Scholar]
- 132.Fardini Y, Dehennaut V, Lefebvre T, Issad T. O-GlcNAcylation: a new cancer hallmark? Front Endocrinol (Lausanne) 2013;4:99. doi: 10.3389/fendo.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52. doi: 10.1186/s12915-019-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scalise M, Pochini L, Console L, Losso MA, Indiveri C. The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front Cell Dev Biol. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Snaebjornsson MT, Schulze A. Non-canonical functions of enzymes facilitate cross-talk between cell metabolic and regulatory pathways. Exp Mol Med. 2018;50(4):34. doi: 10.1038/s12276-018-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Samanta D, Park Y, Andrabi SA, Shelton LM, Gilkes DM, Semenza GL. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016;76(15):4430–4442. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- 137.Vandekeere S, Dubois C, Kalucka J, Sullivan MR, Garcia-Caballero M, Goveia J, et al. Serine synthesis via PHGDH is essential for heme production in endothelial cells. Cell Metab. 2018;28(4):573–587. doi: 10.1016/j.cmet.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 138.Aveldano MI, Bazan NG. Molecular species of phosphatidylcholine, −ethanolamine, −serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J Lipid Res. 1983;24(5):620–627. [PubMed] [Google Scholar]
- 139.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev. Cancer. 2013;13(8):572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reid MA, Allen AE, Liu S, Liberti MV, Liu P, Liu X, et al. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat Commun. 2018;9(1):5442. doi: 10.1038/s41467-018-07868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao E, Hou J, Cui H. Serine-glycine-one-carbon metabolism: vulnerabilities in MYCN-amplified neuroblastoma. Oncogenesis. 2020;9(2):14. doi: 10.1038/s41389-020-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stekol JA, Anderson EI, Weiss S. S-Adenosyl-L-methionine in the synthesis of choline, creatine, and cysteine in vivo and in vitro. J Biol Chem. 1958;233(2):425–429. [PubMed] [Google Scholar]
- 143.Najbauer J, Orpiszewski J, Aswad DW. Molecular aging of tubulin: accumulation of isoaspartyl sites in vitro and in vivo. Biochemistry. 1996;35(16):5183–5190. doi: 10.1021/bi953063g. [DOI] [PubMed] [Google Scholar]
- 144.da Silva RP, Nissim I, Brosnan ME, Brosnan JT. Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab. 2009;296(2):E256–E261. doi: 10.1152/ajpendo.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fraineau S, Palii CG, Allan DS, Brand M. Epigenetic regulation of endothelial-cell-mediated vascular repair. FEBS J. 2015;282(9):1605–1629. doi: 10.1111/febs.13183. [DOI] [PubMed] [Google Scholar]
- 146.Alam H, Gu B, Lee MG. Histone methylation modifiers in cellular signaling pathways. Cell Mol Life Sci. 2015;72(23):4577–4592. doi: 10.1007/s00018-015-2023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15(2):196–212. doi: 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, et al. ATP as a biological hydrotrope. Science. 2017;356(6339):753–756. doi: 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- 149.Greiner JV, Glonek T. Hydrotropic function of ATP in the crystalline lens. Exp Eye Res. 2020;190:107862. doi: 10.1016/j.exer.2019.107862. [DOI] [PubMed] [Google Scholar]
- 150.Gupta J, Kumar S, Li J, Krishna Murthy Karuturi R, Tikoo K. Histone H3 lysine 4 monomethylation (H3K4me1) and H3 lysine 9 monomethylation (H3K9me1): distribution and their association in regulating gene expression under hyperglycaemic/hyperinsulinemic conditions in 3 T3 cells. Biochimie. 2012;94(12):2656–2664. doi: 10.1016/j.biochi.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 151.Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chem Biol. 2015;10(1):95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rendina AR, Pietrak B, Smallwood A, Zhao H, Qi H, Quinn C, et al. Mutant IDH1 enhances the production of 2-hydroxyglutarate due to its kinetic mechanism. Biochemistry. 2013;52(26):4563–4577. doi: 10.1021/bi400514k. [DOI] [PubMed] [Google Scholar]
- 153.Dang L, Su SM. Isocitrate dehydrogenase mutation and (R)-2-hydroxyglutarate: from basic discovery to therapeutics development. Annu Rev. Biochem. 2017;86:305–331. doi: 10.1146/annurev-biochem-061516-044732. [DOI] [PubMed] [Google Scholar]
- 154.Ribeiro ML, Reyes-Garau D, Armengol M, Fernandez-Serrano M, Roue G. Recent advances in the targeting of epigenetic regulators in B cell non-Hodgkin lymphoma. Front Genet. 2019;10:986. doi: 10.3389/fgene.2019.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Drysdale GR, Lardy HA. Fatty acid oxidation by a soluble enzyme system from mitochondria. J Biol Chem. 1953;202(1):119–136. [PubMed] [Google Scholar]
- 156.Berg JMTJ, Stryer L. Biochemistry: the utilization of fatty acids as fuel requires three stages of processing. 5. New York: W H Freeman Publishers; 2002. [Google Scholar]
- 157.Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett. 1980;117(Suppl):K1–10. doi: 10.4159/harvard.9780674366701.c143. [DOI] [PubMed] [Google Scholar]
- 158.Johnson WA. Aconitase. Biochem J. 1939;33(6):1046–1053. doi: 10.1042/bj0331046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krebs HA, Holzach O. The conversion of citrate into cis-aconitate and isocitrate in the presence of aconitase. Biochem J. 1952;52(3):527–528. doi: 10.1042/bj0520527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ochoa S. Biosynthesis of tricarboxylic acids by carbon dioxide fixation; enzymatic mechanisms. J Biol Chem. 1948;174(1):133–157. [PubMed] [Google Scholar]
- 161.Ochoa S, Weisz-Tabori E. Oxalosuccinic carboxylase. J Biol Chem. 1945;159(1):245–246. [PubMed] [Google Scholar]
- 162.Ochoa S. Isocitric dehydrogenase and carbon dioxide fixation. J Biol Chem. 1945;159(1):243–244. [Google Scholar]
- 163.Ochoa S, Weisz-Tabori E. Biosynthesis of tricarboxylic acids by carbon dioxide fixation; oxalosuccinic carboxylase. J Biol Chem. 1948;174(1):123–132. [PubMed] [Google Scholar]
- 164.Stern JR, Ochoa S. Enzymatic synthesis of citric acid by condensation of acetate and oxalacetate. J Biol Chem. 1949;179(1):491. [PubMed] [Google Scholar]
- 165.STERN JR, SHAPIRO B, OCHOA S. Synthesis and breakdown of citric acid with crystalline condensing enzyme. Nature. 1950;166(4218):403‐404. 10.1038/166403b0. [DOI] [PubMed]
- 166.STERN JR, OCHOA S. Enzymatic synthesis of citric acid. I. Synthesis with soluble enzymes. J Biol Chem. 1951;191(1):161‐172. [PubMed]
- 167.Korkes S, Del Campillo A, Gunsalas IC, Ochoa S. Enzymatic synthesis of citric acid. IV. Pyruvate as acetyl donor. J Biol Chem. 1951;193(2):721–735. [PubMed] [Google Scholar]
- 168.Stern JR, Ochoa S, Lynen F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952;198(1):313–321. [PubMed] [Google Scholar]
- 169.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 170.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 2015;22(4):682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rauckhorst AJ, Gray LR, Sheldon RD, Fu X, Pewa AD, Feddersen CR, et al. The mitochondrial pyruvate carrier mediates high fat diet-induced increases in hepatic TCA cycle capacity. Mol Metab. 2017;6(11):1468–1479. doi: 10.1016/j.molmet.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19(9):1027–1036. doi: 10.1038/ncb3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270(22):13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 175.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Icard P, Poulain L, Lincet H. Understanding the central role of citrate in the metabolism of cancer cells. Biochim Biophys Acta. 2012;1825(1):111–116. doi: 10.1016/j.bbcan.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 177.Al-Khallaf H. Isocitrate dehydrogenases in physiology and cancer: biochemical and molecular insight. Cell Biosci. 2017;7:37. doi: 10.1186/s13578-017-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.IDH1-driven reductive carboxylation supports anchorage independence. Cancer Discov. 2016;6(6):570. 10.1158/2159-8290.CD-RW2016-076. [DOI] [PubMed]
- 179.Sun RC, Denko NC. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19(2):285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–2231. [PubMed] [Google Scholar]
- 181.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–968. doi: 10.1172/JCI19935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lim SC, Parajuli KR, Duong HQ, Choi JE, Han SI. Cholesterol induces autophagic and apoptotic death in gastric carcinoma cells. Int J Oncol. 2014;44(3):805–811. doi: 10.3892/ijo.2014.2246. [DOI] [PubMed] [Google Scholar]
- 183.Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer. 2013;4(9-10):401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, et al. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146(4):843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281(45):34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Babina IS, McSherry EA, Donatello S, Hill AD, Hopkins AM. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 2014;16(1):R19. doi: 10.1186/bcr3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Garantziotis S, Savani RC. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019;78-79:1–10. doi: 10.1016/j.matbio.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Espenshade PJ. SREBPs: sterol-regulated transcription factors. J Cell Sci. 2006;119(Pt 6):973–976. doi: 10.1242/jcs02866. [DOI] [PubMed] [Google Scholar]
- 189.Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, Horton JD, et al. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15(10):1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shao W, Espenshade PJ. Sterol regulatory element-binding protein (SREBP) cleavage regulates Golgi-to-endoplasmic reticulum recycling of SREBP cleavage-activating protein (SCAP) J Biol Chem. 2014;289(11):7547–7557. doi: 10.1074/jbc.M113.545699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100(6):3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McPherson R, Gauthier A. Molecular regulation of SREBP function: the Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem Cell Biol. 2004;82(1):201–211. doi: 10.1139/o03-090. [DOI] [PubMed] [Google Scholar]
- 193.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104(16):6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bloch K. Some aspects of the metabolism of leucine and valine. J Biol Chem. 1944;155(1):255–263. [Google Scholar]
- 195.Bloch K. Some aspects of the intermediary metabolism of cholesterol. Am Heart J. 1948;36(3):471. doi: 10.1016/0002-8703(48)90376-7. [DOI] [PubMed] [Google Scholar]
- 196.Zabin I, Bloch K. Studies on the utilization of isovaleric acid in cholesterol synthesis. J Biol Chem. 1951;192(1):267–273. [PubMed] [Google Scholar]
- 197.Elwood JC, Marco A, Van Bruggen JT. Lipid metabolism in the diabetic rat. IV. Metabolism of acetate, acetoacetate, butyrate, and mevalonate in vitro. J Biol Chem. 1960;235:573–577. [PubMed] [Google Scholar]
- 198.Ruzicka L. The isoprene rule and the biogenesis of terpenic compounds. Experientia. 1953;9(10):357–367. doi: 10.1007/bf02167631. [DOI] [PubMed] [Google Scholar]
- 199.Cornforth JW, Robinson R. Towards the synthesis of cholesterol. Nature. 1947;160(4074):737–739. doi: 10.1038/160737a0. [DOI] [PubMed] [Google Scholar]
- 200.Bonner J, Arreguin B. The biochemistry of rubber formation in the guayule; rubber formation in seedlings. Arch Biochem. 1949;21(1):109–124. [PubMed] [Google Scholar]
- 201.Rosenthal J, Angel A, Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. Am J Physiol. 1974;226(2):411–418. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]
- 202.Bloch K. Summing up. Annu Rev. Biochem. 1987;56:1–19. doi: 10.1146/annurev.bi.56.070187.000245. [DOI] [PubMed] [Google Scholar]
- 203.Bloch K. The biological synthesis of cholesterol. Science. 1965;150(3692):19–28. doi: 10.1126/science.150.3692.19. [DOI] [PubMed] [Google Scholar]
- 204.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52(1):6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505(2):131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Okamura E, Tomita T, Sawa R, Nishiyama M, Kuzuyama T. Unprecedented acetoacetyl-coenzyme A synthesizing enzyme of the thiolase superfamily involved in the mevalonate pathway. Proc Natl Acad Sci U S A. 2010;107(25):11265–11270. doi: 10.1073/pnas.1000532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen HW, Leonard DA, Fischer RT, Trzaskos JM. A mammalian mutant cell lacking detectable lanosterol 14 alpha-methyl demethylase activity. J Biol Chem. 1988;263(3):1248–1254. [PubMed] [Google Scholar]
- 208.Castellano BM, Thelen AM, Moldavski O, Feltes M, van der Welle RE, Mydock-McGrane L, et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science. 2017;355(6331):1306–1311. doi: 10.1126/science.aag1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lim CY, Davis OB, Shin HR, Zhang J, Berdan CA, Jiang X, et al. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat Cell Biol. 2019;21(10):1206–1218. doi: 10.1038/s41556-019-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci U S A. 2010;107(10):4764–4769. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen L, Ma MY, Sun M, Jiang LY, Zhao XT, Fang XX, et al. Endogenous sterol intermediates of the mevalonate pathway regulate HMGCR degradation and SREBP-2 processing. J Lipid Res. 2019;60(10):1765–1775. doi: 10.1194/jlr.RA119000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ananieva EA, Powell JD, Hutson SM. Leucine Metabolism in T Cell Activation: mTOR Signaling and Beyond. Adv Nutr. 2016;7(4):798S–805S. doi: 10.3945/an.115.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Heimann E, Nyman M, Palbrink AK, Lindkvist-Petersson K, Degerman E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte. 2016;5(4):359–368. doi: 10.1080/21623945.2016.1252011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Takakuwa A, Nakamura K, Kikuchi M, Sugimoto R, Ohira S, Yokoi Y, et al. Butyric acid and leucine induce alpha-defensin secretion from small intestinal paneth cells. Nutrients. 2019;11(11) 10.3390/nu11112817. [DOI] [PMC free article] [PubMed]
- 215.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338(Pt 3):569–582. [PMC free article] [PubMed] [Google Scholar]
- 216.Arnedo M, Menao S, Puisac B, Teresa-Rodrigo ME, Gil-Rodriguez MC, Lopez-Vinas E, et al. Characterization of a novel HMG-CoA lyase enzyme with a dual location in endoplasmic reticulum and cytosol. J Lipid Res. 2012;53(10):2046–2056. doi: 10.1194/jlr.M025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Luo W, Qin L, Li B, et al. Inactivation of HMGCL promotes proliferation and metastasis of nasopharyngeal carcinoma by suppressing oxidative stress. Sci Rep. 2017;7(1):11954. Published 2017 Sep 20. 10.1038/s41598-017-11025-2. [DOI] [PMC free article] [PubMed]
- 218.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease Nat Rev Mol Cell Biol. 2020;21(4):183‐203. [published correction appears in Nat Rev Mol Cell Biol. 2020. 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed]
- 219.Cho CS, Kowalsky AH, Namkoong S, Park SR, Wu S, Kim B, et al. Concurrent activation of growth factor and nutrient arms of mTORC1 induces oxidative liver injury. Cell Discov. 2019;5:60. doi: 10.1038/s41421-019-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients. 2016;8(5) 10.3390/nu8050295. [DOI] [PMC free article] [PubMed]
- 221.Lee M, Kim JH, Yoon I, Lee C, Fallahi Sichani M, Kang JS, et al. Coordination of the leucine-sensing Rag GTPase cycle by leucyl-tRNA synthetase in the mTORC1 signaling pathway. Proc Natl Acad Sci U S A. 2018;115(23):E5279–E5E88. doi: 10.1073/pnas.1801287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoon I, Nam M, Kim HK, Moon HS, Kim S, Jang J, et al. Glucose-dependent control of leucine metabolism by leucyl-tRNA synthetase 1. Science. 2020;367(6474):205–210. doi: 10.1126/science.aau2753. [DOI] [PubMed] [Google Scholar]
- 223.Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106(12):1901–1906. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:5416923. doi: 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29(3):486–495. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21(1):81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 227.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 228.Xie JM, Li B, Yu HP, Gao QG, Li W, Wu HR, et al. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74(18):5127–5138. doi: 10.1158/0008-5472.CAN-13-3517. [DOI] [PubMed] [Google Scholar]
- 229.Liu Z, Wu Y, Zhang Y, Yuan M, Li X, Gao J, et al. TIGAR promotes tumorigenesis and protects tumor cells from oxidative and metabolic stresses in gastric cancer. Front Oncol. 2019;9:1258. doi: 10.3389/fonc.2019.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Haines DS. The mdm2 proto-oncogene. Leuk Lymphoma. 1997;26(3-4):227–238. doi: 10.3109/10428199709051772. [DOI] [PubMed] [Google Scholar]
- 231.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev. Cancer. 2009;9(10):749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rothfuss O, Fischer H, Hasegawa T, Maisel M, Leitner P, Miesel F, et al. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet. 2009;18(20):3832–3850. doi: 10.1093/hmg/ddp327. [DOI] [PubMed] [Google Scholar]
- 233.Garza-Lombo C, Pappa A, Panayiotidis MI, Franco R. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion. 2020;51:105–117. doi: 10.1016/j.mito.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 235.Lee JY, Kapur M, Li M, Choi MC, Choi S, Kim HJ, et al. MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria. J Cell Sci. 2014;127(Pt 22):4954–4963. doi: 10.1242/jcs.157321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mathieu J, Zhou W, Xing Y, et al. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14(5):592-605. 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed]
- 237.Timon-Gomez A, Nyvltova E, Abriata LA, Vila AJ, Hosler J, Barrientos A. Mitochondrial cytochrome c oxidase biogenesis: recent developments. Semin Cell Dev Biol. 2018;76:163–178. doi: 10.1016/j.semcdb.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rajendran R, Garva R, Ashour H, Leung T, Stratford I, Krstic-Demonacos M, et al. Acetylation mediated by the p300/CBP-associated factor determines cellular energy metabolic pathways in cancer. Int J Oncol. 2013;42(6):1961–1972. doi: 10.3892/ijo.2013.1907. [DOI] [PubMed] [Google Scholar]
- 239.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Demaria M, Poli V. PKM2, STAT3 and HIF-1alpha: the Warburg’s vicious circle. JAKSTAT. 2012;1(3):194–196. doi: 10.4161/jkst.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rubini E, Altieri F, Chichiarelli S, Giamogante F, Carissimi S, Paglia G, et al. STAT3, a hub protein of cellular signaling pathways, is triggered by beta-hexaclorocyclohexane. Int J Mol Sci. 2018;19(7) 10.3390/ijms19072108. [DOI] [PMC free article] [PubMed]
- 242.Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 245.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13(10):1272–1279. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carrasco-Garcia E, Moreno M, Moreno-Cugnon L, Matheu A. Increased Arf/p53 activity in stem cells, aging and cancer. Aging Cell. 2017;16(2):219–225. doi: 10.1111/acel.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang P, Li CG, Qi Z, Cui D, Ding S. Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp Physiol. 2016;101(3):410–420. doi: 10.1113/EP085493. [DOI] [PubMed] [Google Scholar]
- 248.Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545(7653):229–233. doi: 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Levine AJ, Berger SL. The interplay between epigenetic changes and the p53 protein in stem cells. Genes Dev. 2017;31(12):1195–1201. doi: 10.1101/gad.298984.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28(4):721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 251.Wang H, Lu J, Kulkarni S, Zhang W, Gorka JE, Mandel JA, et al. Metabolic and oncogenic adaptations to pyruvate dehydrogenase inactivation in fibroblasts. J Biol Chem. 2019;294(14):5466–5486. doi: 10.1074/jbc.RA118.005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103(46):17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23(5):537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ramadoss S, Chen X, Wang CY. Histone demethylase KDM6B promotes epithelial-mesenchymal transition. J Biol Chem. 2012;287(53):44508–44517. doi: 10.1074/jbc.M112.424903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sciacovelli M, Frezza C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J. 2017;284(19):3132–3144. doi: 10.1111/febs.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Anan K, Hino S, Shimizu N, Sakamoto A, Nagaoka K, Takase R, et al. LSD1 mediates metabolic reprogramming by glucocorticoids during myogenic differentiation. Nucleic Acids Res. 2018;46(11):5441–5454. doi: 10.1093/nar/gky234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Swick AG, Lane MD. Identification of a transcriptional repressor down-regulated during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1992;89(17):7895–7899. doi: 10.1073/pnas.89.17.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor gamma expression and adipogenesis in cultured 3 T3-L1 cells. J Biol Chem. 2008;283(5):2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- 259.Kaestner KH, Ntambi JM, Kelly TJ, Jr, Lane MD. Differentiation-induced gene expression in 3 T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1989;264(25):14755–14761. [PubMed] [Google Scholar]
- 260.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Beyaz S, Yilmaz OH. Molecular Pathways: Dietary Regulation of Stemness and Tumor Initiation by the PPAR-delta Pathway. Clin Cancer Res. 2016;22(23):5636–5641. doi: 10.1158/1078-0432.CCR-16-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Broer S, Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474(12):1935–1963. doi: 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, et al. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 2009;28(5):477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Harding A, Giles N, Burgess A, Hancock JF, Gabrielli BG. Mechanism of mitosis-specific activation of MEK1. J Biol Chem. 2003;278(19):16747–16754. doi: 10.1074/jbc.M301015200. [DOI] [PubMed] [Google Scholar]
- 266.Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X, et al. Inhibition of autophagy in mitotic animal cells. Traffic. 2002;3(12):878–893. doi: 10.1034/j.1600-0854.2002.31204.x. [DOI] [PubMed] [Google Scholar]
- 267.Neufeld TP. Autophagy and cell growth--the yin and yang of nutrient responses. J Cell Sci. 2012;125(Pt 10):2359–2368. doi: 10.1242/jcs.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mathiassen SG, De Zio D, Cecconi F. Autophagy and the Cell Cycle: A Complex Landscape. Front Oncol. 2017;7:51. doi: 10.3389/fonc.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129(13):2475–2481. doi: 10.1242/jcs.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Willett R, Martina JA, Zewe JP, Wills R, Hammond GRV, Puertollano R. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat Commun. 2017;8(1):1580. doi: 10.1038/s41467-017-01871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Odle RI, Walker SA, Oxley D, Kidger AM, Balmanno K, Gilley R, et al. An mTORC1-to-CDK1 switch maintains autophagy suppression during mitosis. Mol Cell. 2020;77(2):228–240. doi: 10.1016/j.molcel.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Brugues J, Nuzzo V, Mazur E, Needleman DJ. Nucleation and transport organize microtubules in metaphase spindles. Cell. 2012;149(3):554–564. doi: 10.1016/j.cell.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 273.Barisic M, Maiato H. The tubulin code: a navigation system for chromosomes during mitosis. Trends Cell Biol. 2016;26(10):766–775. doi: 10.1016/j.tcb.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Petry S. Mechanisms of mitotic spindle assembly. Annu Rev. Biochem. 2016;85:659–683. doi: 10.1146/annurev-biochem-060815-014528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fukushima N, Furuta D, Hidaka Y, Moriyama R, Tsujiuchi T. Post-translational modifications of tubulin in the nervous system. J Neurochem. 2009;109(3):683–693. doi: 10.1111/j.1471-4159.2009.06013.x. [DOI] [PubMed] [Google Scholar]
- 276.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev. Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 277.Liu N, Xiong Y, Ren Y, Zhang L, He X, Wang X, et al. Proteomic profiling and functional characterization of multiple post-translational modifications of tubulin. J Proteome Res. 2015;14(8):3292–3304. doi: 10.1021/acs.jproteome.5b00308. [DOI] [PubMed] [Google Scholar]
- 278.Ferreira LT, Figueiredo AC, Orr B, Lopes D, Maiato H. Dissecting the role of the tubulin code in mitosis. Methods Cell Biol. 2018;144:33–74. doi: 10.1016/bs.mcb.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961;236:1534–1543. [PubMed] [Google Scholar]
- 280.Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. III. Substrate requirements for pyridine nucleotide reduction in mitochondria. J Biol Chem. 1961;236:1555–1561. [PubMed] [Google Scholar]
- 281.Klingenberg M, Echtay KS. Uncoupling proteins: the issues from a biochemist point of view. Biochim Biophys Acta. 2001;1504(1):128–143. doi: 10.1016/s0005-2728(00)00242-5. [DOI] [PubMed] [Google Scholar]
- 282.Huang SG, Klingenberg M. Two-stage nucleotide binding mechanism and its implications to H+ transport inhibition of the uncoupling protein from brown adipose tissue mitochondria. Biochemistry. 1996;35(24):7846–7854. doi: 10.1021/bi960244p. [DOI] [PubMed] [Google Scholar]
- 283.Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A. 2014;111(3):960–965. doi: 10.1073/pnas.1317400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Scialo F, Fernandez-Ayala DJ, Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lobo-Jarne T, Ugalde C. Respiratory chain supercomplexes: structures, function and biogenesis. Semin Cell Dev Biol. 2018;76:179–190. doi: 10.1016/j.semcdb.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Protasoni M, Perez-Perez R, Lobo-Jarne T, Harbour ME, Ding S, Penas A, et al. Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. EMBO J. 2020;39(3):e102817. doi: 10.15252/embj.2019102817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int J Mol Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10(5):289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- 290.Bezawork-Geleta A, Wen H, Dong L, Yan B, Vider J, Boukalova S, et al. Alternative assembly of respiratory complex II connects energy stress to metabolic checkpoints. Nat Commun. 2018;9(1):2221. doi: 10.1038/s41467-018-04603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Powers JF, Cochran B, Baleja JD, Sikes HD, Zhang X, Lomakin I, et al. A unique model for SDH-deficient GIST: an endocrine-related cancer. Endocr Relat Cancer. 2018;25(11):943–954. doi: 10.1530/ERC-18-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kluckova K, Sticha M, Cerny J, Mracek T, Dong L, Drahota Z, et al. Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis. 2015;6:e1749. doi: 10.1038/cddis.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kruspig B, Valter K, Skender B, Zhivotovsky B, Gogvadze V. Targeting succinate:ubiquinone reductase potentiates the efficacy of anticancer therapy. Biochim Biophys Acta. 2016;1863(8):2065–2071. doi: 10.1016/j.bbamcr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 294.Speijer D, Manjeri GR, Szklarczyk R. How to deal with oxygen radicals stemming from mitochondrial fatty acid oxidation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1646):20130446. doi: 10.1098/rstb.2013.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Speijer D. Being right on Q: shaping eukaryotic evolution. Biochem J. 2016;473(22):4103–4127. doi: 10.1042/BCJ20160647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang Y, Palmfeldt J, Gregersen N, Makhov AM, Conway JF, Wang M, et al. Mitochondrial fatty acid oxidation and the electron transport chain comprise a multifunctional mitochondrial protein complex. J Biol Chem. 2019;294(33):12380–12391. doi: 10.1074/jbc.RA119.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 298.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Khutornenko AA, Roudko VV, Chernyak BV, Vartapetian AB, Chumakov PM, Evstafieva AG. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proc Natl Acad Sci U S A. 2010;107(29):12828–12833. doi: 10.1073/pnas.0910885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Khutornenko AA, Dalina AA, Chernyak BV, Chumakov PM, Evstafieva AG. The role of dihydroorotate dehydrogenase in apoptosis induction in response to inhibition of the mitochondrial respiratory chain complex III. Acta Naturae. 2014;6(1):69–75. [PMC free article] [PubMed] [Google Scholar]
- 301.Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernandez-Fernandez C, Mourino-Bayolo D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion. 2019;46:73–90. doi: 10.1016/j.mito.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 302.Siebels I, Drose S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta. 2013;1827(10):1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 303.Grivennikova VG, Kozlovsky VS, Vinogradov AD. Respiratory complex II: ROS production and the kinetics of ubiquinone reduction. Biochim Biophys Acta Bioenerg. 2017;1858(2):109–117. doi: 10.1016/j.bbabio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 304.MacDonald MJ, Brown LJ. Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch Biochem Biophys. 1996;326(1):79–84. doi: 10.1006/abbi.1996.0049. [DOI] [PubMed] [Google Scholar]
- 305.Tanner JJ, Fendt SM, Becker DF. The proline cycle as a potential cancer therapy target. Biochemistry. 2018;57(25):3433–3444. doi: 10.1021/acs.biochem.8b00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem. 2004;279(32):33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 307.Flores A, Schell J, Krall AS, Jelinek D, Miranda M, Grigorian M, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19(9):1017–1026. doi: 10.1038/ncb3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tai Y, Cao F, Li M, Li P, Xu T, Wang X, et al. Enhanced mitochondrial pyruvate transport elicits a robust ROS production to sensitize the antitumor efficacy of interferon-gamma in colon cancer. Redox Biol. 2019;20:451–457. doi: 10.1016/j.redox.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vanderperre B, Herzig S, Krznar P, Horl M, Ammar Z, Montessuit S, et al. Embryonic lethality of mitochondrial pyruvate carrier 1 deficient mouse can be rescued by a ketogenic diet. PLoS Genet. 2016;12(5):e1006056. doi: 10.1371/journal.pgen.1006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zou S, Lang T, Zhang B, Huang K, Gong L, Luo H, et al. Fatty acid oxidation alleviates the energy deficiency caused by the loss of MPC1 in MPC1(± ) mice. Biochem Biophys Res Commun. 2018;495(1):1008–1013. doi: 10.1016/j.bbrc.2017.11.134. [DOI] [PubMed] [Google Scholar]
- 311.Zhou X, Xiong ZJ, Xiao SM, Zhou J, Ding Z, Tang LC, et al. Overexpression of MPC1 inhibits the proliferation, migration, invasion, and stem cell-like properties of gastric cancer cells. Onco Targets Ther. 2017;10:5151–5163. doi: 10.2147/OTT.S148681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ohashi T, Eguchi H, Kawamoto K, Konno M, Asai A, Colvin H, et al. Mitochondrial pyruvate carrier modulates the epithelial-mesenchymal transition in cholangiocarcinoma. Oncol Rep. 2018;39(3):1276–1282. doi: 10.3892/or.2017.6172. [DOI] [PubMed] [Google Scholar]
- 313.Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 2015;22(4):669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Watford M, Smith EM, Erbelding EJ. The regulation of phosphate-activated glutaminase activity and glutamine metabolism in the streptozotocin-diabetic rat. Biochem J. 1984;224(1):207–214. doi: 10.1042/bj2240207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mates JM, Segura JA, Martin-Rufian M, Campos-Sandoval JA, Alonso FJ, Marquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med. 2013;13(4):514–534. doi: 10.2174/1566524011313040005. [DOI] [PubMed] [Google Scholar]
- 316.Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu S, et al. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 2013;1833(12):2996–3005. doi: 10.1016/j.bbamcr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 317.Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS One. 2009;4(3):e4715. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tran TQ, Lowman XH, Reid MA, Mendez-Dorantes C, Pan M, Yang Y, et al. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene. 2017;36(14):1991–2001. doi: 10.1038/onc.2016.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 320.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev. Cancer. 2016;16(10):619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Szeliga M, Bogacinska-Karas M, Rozycka A, Hilgier W, Marquez J, Albrecht J. Silencing of GLS and overexpression of GLS2 genes cooperate in decreasing the proliferation and viability of glioblastoma cells. Tumour Biol. 2014;35(3):1855–1862. doi: 10.1007/s13277-013-1247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Szeliga M, Albrecht J. Opposing roles of glutaminase isoforms in determining glioblastoma cell phenotype. Neurochem Int. 2015;88:6–9. doi: 10.1016/j.neuint.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 323.Katt WP, Lukey MJ, Cerione RA. A tale of two glutaminases: homologous enzymes with distinct roles in tumorigenesis. Future Med Chem. 2017;9(2):223–243. doi: 10.4155/fmc-2016-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Saha SK, Islam SMR, Abdullah-Al-Wadud M, Islam S, Ali F, Park KS. Multiomics analysis reveals that GLS and GLS2 differentially modulate the clinical outcomes of cancer. J Clin Med. 2019;8(3) 10.3390/jcm8030355. [DOI] [PMC free article] [PubMed]
- 325.Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci U S A. 2012;109(4):1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Szeliga M, Cwikla J, Obara-Michlewska M, Cichocki A, Albrecht J. Glutaminases in slowly proliferating gastroenteropancreatic neuroendocrine neoplasms/tumors (GEP-NETs): Selective overexpression of mRNA coding for the KGA isoform. Exp Mol Pathol. 2016;100(1):74–78. doi: 10.1016/j.yexmp.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 327.Bentley-DeSousa A, Holinier C, Moteshareie H, Tseng YC, Kajjo S, Nwosu C, et al. A screen for candidate targets of lysine polyphosphorylation uncovers a conserved network implicated in ribosome biogenesis. Cell Rep. 2018;22(13):3427–3439. doi: 10.1016/j.celrep.2018.02.104. [DOI] [PubMed] [Google Scholar]
- 328.McCarthy L, Bentley-DeSousa A, Denoncourt A, Tseng YC, Gabriel M, Downey M. Proteins required for vacuolar function are targets of lysine polyphosphorylation in yeast. FEBS Lett. 2020;594(1):21–30. doi: 10.1002/1873-3468.13588. [DOI] [PubMed] [Google Scholar]
- 329.Zhang Z, Liu R, Shuai Y, et al. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br J Cancer. 2020;122(1):82-93 10.1038/s41416-019-0637-9. [DOI] [PMC free article] [PubMed]
- 330.Zou H, Chen Q, Zhang A, Wang S, Wu H, Yuan Y, et al. MPC1 deficiency accelerates lung adenocarcinoma progression through the STAT3 pathway. Cell Death Dis. 2019;10(3):148. doi: 10.1038/s41419-019-1324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li X, Han G, Li X, Kan Q, Fan Z, Li Y, et al. Mitochondrial pyruvate carrier function determines cell stemness and metabolic reprogramming in cancer cells. Oncotarget. 2017;8(28):46363-80. 10.18632/oncotarget.18199. [DOI] [PMC free article] [PubMed]
- 332.Sandoval IT, Delacruz RG, Miller BN, Hill S, Olson KA, Gabriel AE, et al. A metabolic switch controls intestinal differentiation downstream of Adenomatous polyposis coli (APC). Elife. 2017;6 10.7554/eLife.22706. [DOI] [PMC free article] [PubMed]
- 333.Rauckhorst AJ, Taylor EB. Mitochondrial pyruvate carrier function and cancer metabolism. Curr Opin Genet Dev. 2016;38:102-9. 10.1016/j.gde.2016.05.003. [DOI] [PMC free article] [PubMed]
- 334.Icard P, Lincet H. The reduced concentration of citrate in cancer cells: an indicator of cancer aggressiveness and a possible therapeutic target. Drug Resist Updat. 2016;29:47–53. doi: 10.1016/j.drup.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 335.Song M, Kim SH, Im CY, Hwang HJ. Recent development of small molecule glutaminase inhibitors. Curr Top Med Chem. 2018;18(6):432–443. doi: 10.2174/1568026618666180525100830. [DOI] [PubMed] [Google Scholar]
- 336.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 337.Hoerner CR, Chen VJ, Fan AC. The ‘Achilles Heel’ of metabolism in renal cell carcinoma: glutaminase inhibition as a rational treatment strategy. Kidney Cancer. 2019;3(1):15–29. doi: 10.3233/KCA-180043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Grinde MT, Hilmarsdottir B, Tunset HM, Henriksen IM, Kim J, Haugen MH, et al. Glutamine to proline conversion is associated with response to glutaminase inhibition in breast cancer. Breast Cancer Res. 2019;21(1):61. doi: 10.1186/s13058-019-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Haider L, Zrzavy T, Hametner S, Hoftberger R, Bagnato F, Grabner G, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139(Pt 3):807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Desai RA, Davies AL, Tachrount M, Kasti M, Laulund F, Golay X, et al. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann Neurol. 2016;79(4):591–604. doi: 10.1002/ana.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166(5):1324–1337. doi: 10.1016/j.cell.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Vincent EE, Sergushichev A, Griss T, Gingras MC, Samborska B, Ntimbane T, et al. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell. 2015;60(2):195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 343.Montal ED, Dewi R, Bhalla K, Ou L, Hwang BJ, Ropell AE, et al. PEPCK coordinates the regulation of central carbon metabolism to promote cancer cell growth. Mol Cell. 2015;60(4):571–583. doi: 10.1016/j.molcel.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tajan M, Hock AK, Blagih J, Robertson NA, Labuschagne CF, Kruiswijk F, et al. A role for p53 in the adaptation to glutamine starvation through the expression of SLC1A3. Cell Metab. 2018;28(5):721–736. doi: 10.1016/j.cmet.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Martins TL, Chitto AL, Rossetti CL, Brondani CK, Kucharski LC, Da Silva RS. Effects of hypo- or hyperosmotic stress on lipid synthesis and gluconeogenic activity in tissues of the crab Neohelice granulata. Comp Biochem Physiol A Mol Integr Physiol. 2011;158(4):400–405. doi: 10.1016/j.cbpa.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 346.Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5(1):30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 347.Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6 J mice results in mitochondrial redox abnormalities. Free Radic Biol Med. 2013;63:446–456. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 348.Meimaridou E, Goldsworthy M, Chortis V, Fragouli E, Foster PA, Arlt W, et al. NNT is a key regulator of adrenal redox homeostasis and steroidogenesis in male mice. J Endocrinol. 2018;236(1):13–28. doi: 10.1530/JOE-16-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Francisco A, Ronchi JA, Navarro CDC, Figueira TR, Castilho RF. Nicotinamide nucleotide transhydrogenase is required for brain mitochondrial redox balance under hampered energy substrate metabolism and high-fat diet. J Neurochem. 2018;147(5):663–677. doi: 10.1111/jnc.14602. [DOI] [PubMed] [Google Scholar]
- 350.Shashidharan P, Plaitakis A. The discovery of human of GLUD2 glutamate dehydrogenase and its implications for cell function in health and disease. Neurochem Res. 2014;39(3):460–470. doi: 10.1007/s11064-013-1227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.McFarlane MR, Liang G, Engelking LJ. Insig proteins mediate feedback inhibition of cholesterol synthesis in the intestine. J Biol Chem. 2014;289(4):2148–2156. doi: 10.1074/jbc.M113.524041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Prakash P, Punekar NS, Bhaumik P. Structural basis for the catalytic mechanism and alpha-ketoglutarate cooperativity of glutamate dehydrogenase. J Biol Chem. 2018;293(17):6241–6258. doi: 10.1074/jbc.RA117.000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ronchi JA, Francisco A, Passos LA, Figueira TR, Castilho RF. The contribution of nicotinamide nucleotide transhydrogenase to peroxide detoxification is dependent on the respiratory state and counterbalanced by other sources of NADPH in liver mitochondria. J Biol Chem. 2016;291(38):20173–20187. doi: 10.1074/jbc.M116.730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108(49):19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277(34):30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 356.Scott E, Peter F, Sanders J. Biomass in the manufacture of industrial products--the use of proteins and amino acids. Appl Microbiol Biotechnol. 2007;75(4):751–762. doi: 10.1007/s00253-007-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, et al. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev Cell. 2016;36(5):540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Leithner K, Triebl A, Trotzmuller M, Hinteregger B, Leko P, Wieser BI, et al. The glycerol backbone of phospholipids derives from noncarbohydrate precursors in starved lung cancer cells. Proc Natl Acad Sci U S A. 2018;115(24):6225–6230. doi: 10.1073/pnas.1719871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol (Lausanne) 2018;9:790. doi: 10.3389/fendo.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nofal M, Zhang K, Han S, Rabinowitz JD. mTOR inhibition restores amino acid balance in cells dependent on catabolism of extracellular protein. Mol Cell. 2017;67(6):936–946. doi: 10.1016/j.molcel.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Wyant GA, Abu-Remaileh M, Frenkel EM, Laqtom NN, Dharamdasani V, Lewis CA, et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science. 2018;360(6390):751–758. doi: 10.1126/science.aar2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kim KH, Lee MS. Autophagy - a key player in cellular and body metabolism. Nat Rev. Endocrinol. 2014;10(6):322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 363.Viry E, Paggetti J, Baginska J, Mgrditchian T, Berchem G, Moussay E, et al. Autophagy: an adaptive metabolic response to stress shaping the antitumor immunity. Biochem Pharmacol. 2014;92(1):31–42. doi: 10.1016/j.bcp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 364.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1-2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 366.Roof AK, Gutierrez-Hartmann A. Consider the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol Cell Endocrinol. 2018;463:87–96. doi: 10.1016/j.mce.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 367.Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol Med Rep. 2019;19(2):783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Casey SC, Vaccari M, Al-Mulla F, Al-Temaimi R, Amedei A, Barcellos-Hoff MH, et al. The effect of environmental chemicals on the tumor microenvironment. Carcinogenesis. 2015;36(Suppl 1):S160–S183. doi: 10.1093/carcin/bgv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev. Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 372.Vadlakonda L, Dash A, Pasupuleti M, Anil Kumar K, Reddanna P. The paradox of Akt-mTOR interactions. Front Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Basho RK, Gilcrease M, Murthy RK, Helgason T, Karp DD, Meric-Bernstam F, et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017;3(4):509–515. doi: 10.1001/jamaoncol.2016.5281. [DOI] [PubMed] [Google Scholar]
- 374.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46(6):733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 375.Kazi AA, Molitoris KH, Koos RD. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol Reprod. 2009;81(2):378–387. doi: 10.1095/biolreprod.109.076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Rios C, D’Ippolito G, Curtis KM, Delcroix GJ, Gomez LA, El Hokayem J, et al. Low oxygen modulates multiple signaling pathways, increasing self-renewal, while decreasing differentiation, senescence, and apoptosis in stromal MIAMI cells. Stem Cells Dev. 2016;25(11):848–860. doi: 10.1089/scd.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF1 signaling pathway in hypoxiaischemia (Review) Mol Med Rep. 2018;18(4):3547–3554. doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167(3):399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30(1):35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 380.Sathe A, Chalaud G, Oppolzer I, Wong KY, von Busch M, Schmid SC, et al. Parallel PI3K, AKT and mTOR inhibition is required to control feedback loops that limit tumor therapy. PLoS One. 2018;13(1):e0190854. doi: 10.1371/journal.pone.0190854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cramer W, Pringle H, Sharpey-Schafer EA. Contributions to the biochemistry of growth.—The total nitrogen metabolism of rats bearing malignant new growths. Proc Royal Soc B. 1910;82(556):307–315. doi: 10.1098/rspb.1910.0021. [DOI] [Google Scholar]
- 382.Cramer W, Lochhead J, Schäfer EA. Contributions to the biochemistry of growth.—The glycogen-content of the liver of rats bearing malignant new growths. Proc Royal Soc B. 1913;86(588):302–307. doi: 10.1098/rspb.1913.0025. [DOI] [Google Scholar]
- 383.Mider GB. Neoplastic diseases; some metabolic aspects. Annu Rev. Med. 1953;4:187–198. doi: 10.1146/annurev.me.04.020153.001155. [DOI] [PubMed] [Google Scholar]
- 384.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273(23):14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 385.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334(Pt 1):261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277(12):9952–9957. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- 387.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20(9):436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29(12):2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, Thevelein JM. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2014;38(2):254–299. doi: 10.1111/1574-6976.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Steyfkens F, Zhang Z, Van Zeebroeck G, Thevelein JM. Multiple transceptors for macro- and micro-nutrients control diverse cellular properties through the PKA pathway in yeast: a paradigm for the rapidly expanding world of eukaryotic nutrient transceptors up to those in human cells. Front Pharmacol. 2018;9:191. doi: 10.3389/fphar.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wang Y, Ning Y, Alam GN, Jankowski BM, Dong Z, Nor JE, et al. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15(8):989–997. doi: 10.1593/neo.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Vynnytska-Myronovska BO, Kurlishchuk Y, Chen O, Bobak Y, Dittfeld C, Huther M, et al. Arginine starvation in colorectal carcinoma cells: Sensing, impact on translation control and cell cycle distribution. Exp Cell Res. 2016;341(1):67–74. doi: 10.1016/j.yexcr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 393.Nakamura A, Nambu T, Ebara S, Hasegawa Y, Toyoshima K, Tsuchiya Y, et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci U S A. 2018;115(33):E7776–E7E85. doi: 10.1073/pnas.1805523115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150(6):1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24(7):400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci U S A. 2008;105(8):2824–2829. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Gauza-Wlodarczyk M, Kubisz L, Wlodarczyk D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int J Biol Macromol. 2017;104(Pt A):987–991. doi: 10.1016/j.ijbiomac.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 398.Goberdhan DC, Wilson C, Harris AL. Amino acid sensing by mTORC1: intracellular transporters mark the spot. Cell Metab. 2016;23(4):580–589. doi: 10.1016/j.cmet.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Fan SJ, Goberdhan DCI. PATs and SNATs: amino acid sensors in disguise. Front Pharmacol. 2018;9:640. doi: 10.3389/fphar.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Hellsten SV, Eriksson MM, Lekholm E, Arapi V, Perland E, Fredriksson R. The gene expression of the neuronal protein, SLC38A9, changes in mouse brain after in vivo starvation and high-fat diet. PLoS One. 2017;12(2):e0172917. doi: 10.1371/journal.pone.0172917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519(7544):477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.You L, Wang Z, Li H, Shou J, Jing Z, Xie J, et al. The role of STAT3 in autophagy. Autophagy. 2015;11(5):729–739. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Jung J, Genau HM, Behrends C. Amino acid-dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol Cell Biol. 2015;35(14):2479–2494. doi: 10.1128/MCB.00125-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, et al. mTORC1 Activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell. 2017;171(3):642–654. doi: 10.1016/j.cell.2017.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Morris SM., Jr Arginine metabolism revisited. J Nutr. 2016;146(12):2579S–2586S. doi: 10.3945/jn.115.226621. [DOI] [PubMed] [Google Scholar]
- 407.Keshet R, Erez A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis Model Mech. 2018;11(8) 10.1242/dmm.033332. [DOI] [PMC free article] [PubMed]
- 408.Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863(10):2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Xie J, Li P, Gao HF, Qian JX, Yuan LY, Wang JJ. Overexpression of SLC38A1 is associated with poorer prognosis in Chinese patients with gastric cancer. BMC Gastroenterol. 2014;14:70. doi: 10.1186/1471-230X-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75(9):1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 411.Broer A, Rahimi F, Broer S. Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J Biol Chem. 2016;291(25):13194–13205. doi: 10.1074/jbc.M115.700534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Di Paola M, Park AJ, Ahmadi S, Roach EJ, Wu YS, Struder-Kypke M, et al. SLC6A14 is a genetic modifier of cystic fibrosis that regulates Pseudomonas aeruginosa attachment to human bronchial epithelial cells. mBio. 2017;8(6) 10.1128/mBio.02073-17. [DOI] [PMC free article] [PubMed]
- 413.Ruffin M, Mercier J, Calmel C, Mesinele J, Bigot J, Sutanto EN, et al. Update on SLC6A14 in lung and gastrointestinal physiology and physiopathology: focus on cystic fibrosis. Cell Mol Life Sci. 2020; 10.1007/s00018-020-03487-x. [DOI] [PMC free article] [PubMed]
- 414.Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78(4):969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 415.Yamamoto A, Akanuma S, Tachikawa M, Hosoya K. Involvement of LAT1 and LAT2 in the high- and low-affinity transport of L-leucine in human retinal pigment epithelial cells (ARPE-19 cells) J Pharm Sci. 2010;99(5):2475–2482. doi: 10.1002/jps.21991. [DOI] [PubMed] [Google Scholar]
- 416.Napolitano L, Scalise M, Galluccio M, Pochini L, Albanese LM, Indiveri C. LAT1 is the transport competent unit of the LAT1/CD98 heterodimeric amino acid transporter. Int J Biochem Cell Biol. 2015;67:25–33. doi: 10.1016/j.biocel.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 417.Singh N, Ecker GF. Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, LAT1. Int J Mol Sci. 2018;19(5) 10.3390/ijms19051278. [DOI] [PMC free article] [PubMed]
- 418.Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature. 2019;568(7750):127–130. doi: 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 419.Dickens D, Chiduza GN, Wright GS, Pirmohamed M, Antonyuk SV, Hasnain SS. Modulation of LAT1 (SLC7A5) transporter activity and stability by membrane cholesterol. Sci Rep. 2017;7:43580. doi: 10.1038/srep43580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J, et al. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37(1):274. doi: 10.1186/s13046-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Kinne A, Wittner M, Wirth EK, Hinz KM, Schulein R, Kohrle J, et al. Involvement of the L-type amino acid transporter Lat2 in the transport of 3,3′-Diiodothyronine across the plasma membrane. Eur Thyroid J. 2015;4(Suppl 1):42–50. doi: 10.1159/000381542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Kurayama R, Ito N, Nishibori Y, Fukuhara D, Akimoto Y, Higashihara E, et al. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest. 2011;91(7):992–1006. doi: 10.1038/labinvest.2011.43. [DOI] [PubMed] [Google Scholar]
- 423.Knopfel EB, Vilches C, Camargo SMR, Errasti-Murugarren E, Staubli A, Mayayo C, et al. Dysfunctional LAT2 amino acid transporter is associated with cataract in mouse and humans. Front Physiol. 2019;10:688. doi: 10.3389/fphys.2019.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24(24):2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Moloughney JG, Kim PK, Vega-Cotto NM, Wu CC, Zhang S, Adlam M, et al. mTORC2 responds to glutamine catabolite levels to modulate the hexosamine biosynthesis enzyme GFAT1. Mol Cell. 2016;63(5):811–826. doi: 10.1016/j.molcel.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Jacobs HG. Surgery in the dental practice. 4. Oroantral communication after tooth extraction. Niedersachs Zahnarztebl. 1977;12(7):326–329. [PubMed] [Google Scholar]
- 427.Scaglia N, Tyekucheva S, Zadra G, Photopoulos C, Loda M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle. 2014;13(5):859–868. doi: 10.4161/cc.27767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Oppedisano F, Pochini L, Galluccio M, Indiveri C. The glutamine/amino acid transporter (ASCT2) reconstituted in liposomes: transport mechanism, regulation by ATP and characterization of the glutamine/glutamate antiport. Biochim Biophys Acta. 2007;1768(2):291–298. doi: 10.1016/j.bbamem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 430.Fournier M, Monin A, Ferrari C, Baumann PS, Conus P, Do K. Implication of the glutamate-cystine antiporter xCT in schizophrenia cases linked to impaired GSH synthesis. NPJ Schizophr. 2017;3(1):31. doi: 10.1038/s41537-017-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Dai L, Noverr MC, Parsons C, Kaleeba JA, Qin Z. xCT, not just an amino-acid transporter: a multi-functional regulator of microbial infection and associated diseases. Front Microbiol. 2015;6:120. doi: 10.3389/fmicb.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Posner ER, Gies WJ. Is protagon a mechanical mixture of substances or a definite chemical compound? J Biol Chem. 1905;1(1):59–112. [Google Scholar]
- 433.Pringle H, Cramer W. On the assimilation of protein introduced enterally. J Physiol. 1908;37(2):158–164. doi: 10.1113/jphysiol.1908.sp001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Rosenheim O, Tebb MC. The non-existence of;protagon’ as a definite chemical compound. J Physiol. 1907;36(1):1–16. doi: 10.1113/jphysiol.1907.sp001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178(1):93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bridges RJ, Natale NR, Patel SA. System xc(−) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165(1):20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, et al. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(5):522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Grimm H, Kraus A. Immunonutrition--supplementary amino acids and fatty acids ameliorate immune deficiency in critically ill patients. Langenbecks Arch Surg. 2001;386(5):369–376. doi: 10.1007/s004230100241. [DOI] [PubMed] [Google Scholar]
- 439.Biolo G. Protein metabolism and requirements. World Rev. Nutr Diet. 2013;105:12–20. doi: 10.1159/000341545. [DOI] [PubMed] [Google Scholar]
- 440.Nissim I, Daikhin Y, Nissim I, Luhovyy B, Horyn O, Wehrli SL, et al. Agmatine stimulates hepatic fatty acid oxidation: a possible mechanism for up-regulation of ureagenesis. J Biol Chem. 2006;281(13):8486–8496. doi: 10.1074/jbc.M506984200. [DOI] [PubMed] [Google Scholar]
- 441.Wisniewska A, Olszanecki R, Toton-Zuranska J, Kus K, Stachowicz A, Suski M, et al. Anti-atherosclerotic action of agmatine in ApoE-knockout mice. Int J Mol Sci. 2017;18(8) 10.3390/ijms18081706. [DOI] [PMC free article] [PubMed]
- 442.Nissim I, Horyn O, Daikhin Y, Chen P, Li C, Wehrli SL, et al. The molecular and metabolic influence of long term agmatine consumption. J Biol Chem. 2014;289(14):9710–9729. doi: 10.1074/jbc.M113.544726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418(3):291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 444.Ghafourifar P, Richter C. Mitochondrial nitric oxide synthase regulates mitochondrial matrix pH. Biol Chem. 1999;380(7-8):1025–1028. doi: 10.1515/BC.1999.127. [DOI] [PubMed] [Google Scholar]
- 445.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26(4):190–195. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 446.Lacza Z, Pankotai E, Busija DW. Mitochondrial nitric oxide synthase: current concepts and controversies. Front Biosci (Landmark Ed) 2009;14:4436–4443. doi: 10.2741/3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Xu C, Yi C, Wang H, Bruce IC, Xia Q. Mitochondrial nitric oxide synthase participates in septic shock myocardial depression by nitric oxide overproduction and mitochondrial permeability transition pore opening. Shock. 2012;37(1):110–115. doi: 10.1097/SHK.0b013e3182391831. [DOI] [PubMed] [Google Scholar]
- 448.Tengan CH, Moraes CT. NO control of mitochondrial function in normal and transformed cells. Biochim Biophys Acta Bioenerg. 2017;1858(8):573–581. doi: 10.1016/j.bbabio.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33(11):1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- 450.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658(1-2):44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 451.Perales-Clemente E, Bayona-Bafaluy MP, Perez-Martos A, Barrientos A, Fernandez-Silva P, Enriquez JA. Restoration of electron transport without proton pumping in mammalian mitochondria. Proc Natl Acad Sci U S A. 2008;105(48):18735–18739. doi: 10.1073/pnas.0810518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes. 2011;60(11):3015–3022. doi: 10.2337/db11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther. 2006;318(3):1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- 455.Yelamanchi SD, Jayaram S, Thomas JK, Gundimeda S, Khan AA, Singhal A, et al. A pathway map of glutamate metabolism. J Cell Commun Signal. 2016;10(1):69–75. doi: 10.1007/s12079-015-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Lam SK, Yan S, Xu S, U KP, Cheng PN, Ho JC. Endogenous arginase 2 as a potential biomarker for PEGylated arginase 1 treatment in xenograft models of squamous cell lung carcinoma. Oncogenesis. 2019;8(3):18. doi: 10.1038/s41389-019-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol. 2011;2(1):8–23. [PMC free article] [PubMed] [Google Scholar]
- 458.Fultang L, Vardon A, De Santo C, Mussai F. Molecular basis and current strategies of therapeutic arginine depletion for cancer. Int J Cancer. 2016;139(3):501–509. doi: 10.1002/ijc.30051. [DOI] [PubMed] [Google Scholar]
- 459.Kovacevic Z, Day SH, Collett V, Brosnan JT, Brosnan ME. Activation of hepatic glutaminase by spermine. Biochem J. 1995;305(Pt 3):837–841. doi: 10.1042/bj3050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Xiong Y, Yepuri G, Montani JP, Ming XF, Yang Z. Arginase-II deficiency extends lifespan in mice. Front Physiol. 2017;8:682. doi: 10.3389/fphys.2017.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Aoyagi K. Inhibition of arginine synthesis by urea: a mechanism for arginine deficiency in renal failure which leads to increased hydroxyl radical generation. Mol Cell Biochem. 2003;244(1-2):11–15. [PubMed] [Google Scholar]
- 462.Setty BA, Pillay Smiley N, Pool CM, Jin Y, Liu Y, Nelin LD. Hypoxia-induced proliferation of HeLa cells depends on epidermal growth factor receptor-mediated arginase II induction. Physiol Rep. 2017;5(6) 10.14814/phy2.13175. [DOI] [PMC free article] [PubMed]
- 463.Chen D, Gu K, Wang H. Optimizing sequential treatment with anti-EGFR and VEGF mAb in metastatic colorectal cancer: current results and controversies. Cancer Manag Res. 2019;11:1705–1716. doi: 10.2147/CMAR.S196170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107(7):2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Lee JE, Shin SH, Shin HW, Chun YS, Park JW. Nuclear FGFR2 negatively regulates hypoxia-induced cell invasion in prostate cancer by interacting with HIF-1 and HIF-2. Sci Rep. 2019;9(1):3480. doi: 10.1038/s41598-019-39,843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Woolf DK, Li SP, Detre S, Liu A, Gogbashian A, Simcock IC, et al. Assessment of the spatial heterogeneity of breast cancers: associations between Computed Tomography and Immunohistochemistry. Biomark Cancer. 2019;11:1179299X19851513. doi: 10.1177/1179299X19851513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504(1):46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 468.Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17(25):3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- 469.Walford GA, Moussignac RL, Scribner AW, Loscalzo J, Leopold JA. Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways. J Biol Chem. 2004;279(6):4425–4432. doi: 10.1074/jbc.M310582200. [DOI] [PubMed] [Google Scholar]
- 470.Arab A, Wang J, Bausch K, von Schmadel K, Bode C, Hehrlein C. Transient hyperoxic reoxygenation reduces cytochrome C oxidase activity by increasing superoxide dismutase and nitric oxide. J Biol Chem. 2010;285(15):11172–11177. doi: 10.1074/jbc.M109.053181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Poderoso JJ, Helfenberger K, Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. 2019;88:61–72. doi: 10.1016/j.niox.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 472.Schweighofer H, Rummel C, Mayer K, Rosengarten B. Brain function in iNOS knock out or iNOS inhibited (l-NIL) mice under endotoxic shock. Intensive Care Med Exp. 2014;2(1):24. doi: 10.1186/s40635-014-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Singh S, Zhuo M, Gorgun FM, Englander EW. Overexpressed neuroglobin raises threshold for nitric oxide-induced impairment of mitochondrial respiratory activities and stress signaling in primary cortical neurons. Nitric Oxide. 2013;32:21–28. doi: 10.1016/j.niox.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Loayza-Puch F, Rooijers K, Buil LC, Zijlstra J, Oude Vrielink JF, Lopes R, et al. Tumour-specific proline vulnerability uncovered by differential ribosome codon reading. Nature. 2016;530(7591):490–494. doi: 10.1038/nature16982. [DOI] [PubMed] [Google Scholar]
- 475.Casalino L, Comes S, Lambazzi G, De Stefano B, Filosa S, De Falco S, et al. Control of embryonic stem cell metastability by L-proline catabolism. J Mol Cell Biol. 2011;3(2):108–122. doi: 10.1093/jmcb/mjr001. [DOI] [PubMed] [Google Scholar]
- 476.von Stechow L, Ruiz-Aracama A, van de Water B, Peijnenburg A, Danen E, Lommen A. Identification of cisplatin-regulated metabolic pathways in pluripotent stem cells. PLoS One. 2013;8(10):e76476. doi: 10.1371/journal.pone.0076476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18(1):71–77. doi: 10.1097/MCO.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Liu W, Hancock CN, Fischer JW, Harman M, Phang JM. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: involvement of pyridine nucleotides. Sci Rep. 2015;5:17206. doi: 10.1038/srep17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Phang JM. Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxid Redox Signal. 2019;30(4):635–649. doi: 10.1089/ars.2017.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN, et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 2018;28(5):706–720. doi: 10.1016/j.cmet.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kim D, Choi BH, Ryoo IG, Kwak MK. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis. 2018;9(9):896. doi: 10.1038/s41419-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Liesche F, Griessmair M, Barz M, Gempt J, Schlegel J. ALDH1 - A new immunohistochemical diagnostic marker for Schwann cell-derived tumors. Clin Neuropathol. 2019;38(4):168–173. doi: 10.5414/NP301190. [DOI] [PubMed] [Google Scholar]
- 483.Feng J, Zhou Z, Shi L, Yang X, Liu W. Cancer stem cell markers ALDH1 and Bmi1 expression in oral erythroplakia revisited: Implication for driving the process of field cancerization. J Oral Pathol Med. 2020;49(1):96–99. doi: 10.1111/jop.12955. [DOI] [PubMed] [Google Scholar]
- 484.Verdon Q, Boonen M, Ribes C, Jadot M, Gasnier B, Sagne C. SNAT7 is the primary lysosomal glutamine exporter required for extracellular protein-dependent growth of cancer cells. Proc Natl Acad Sci U S A. 2017;114(18):E3602–E3E11. doi: 10.1073/pnas.1617066114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Chen L, Cui H. Targeting glutamine induces apoptosis: a cancer therapy approach. Int J Mol Sci. 2015;16(9):22830–22855. doi: 10.3390/ijms160922830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Choi YK, Park KG. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul). 2018;26(1):19–28. doi: 10.4062/biomolther.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Valter K, Chen L, Kruspig B, Maximchik P, Cui H, Zhivotovsky B, et al. Contrasting effects of glutamine deprivation on apoptosis induced by conventionally used anticancer drugs. Biochim Biophys Acta Mol Cell Res. 2017;1864(3):498–506. doi: 10.1016/j.bbamcr.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 488.Sullivan LB, Luengo A, Danai LV, Bush LN, Diehl FF, Hosios AM, et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol. 2018;20(7):782–788. doi: 10.1038/s41556-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Garcia-Bermudez J, Baudrier L, La K, Zhu XG, Fidelin J, Sviderskiy VO, et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol. 2018;20(7):775–781. doi: 10.1038/s41556-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Liu L, Shah S, Fan J, Park JO, Wellen KE, Rabinowitz JD. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat Chem Biol. 2016;12(5):345–352. doi: 10.1038/nchembio.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282(4):2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 493.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791(2):85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Duarte JA, Carvalho F, Pearson M, Horton JD, Browning JD, Jones JG, et al. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J Lipid Res. 2014;55(12):2541–2553. doi: 10.1194/jlr.M052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wong A, Chen S, Yang LK, Kanagasundaram Y, Crasta K. Lipid accumulation facilitates mitotic slippage-induced adaptation to anti-mitotic drug treatment. Cell Death Discov. 2018;4:109. doi: 10.1038/s41420-018-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36(16):2334–2352. doi: 10.15252/embj.201695518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, et al. As extracellular glutamine levels decline, asparagine becomes an essential Amino acid. Cell Metab. 2018;27(2):428–438. doi: 10.1016/j.cmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Bott AJ, Maimouni S, Zong WX. The pleiotropic effects of glutamine metabolism in cancer. Cancers (Basel). 2019;11(6) 10.3390/cancers11060770. [DOI] [PMC free article] [PubMed]
- 500.Jiang J, Srivastava S, Zhang J. Starve Cancer cells of glutamine: break the spell or make a hungry monster? Cancers (Basel). 2019;11(6) 10.3390/cancers11060804. [DOI] [PMC free article] [PubMed]
- 501.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136(1 Suppl):207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 502.Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3(1):1. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Cardaci S, Zheng L, MacKay G, van den Broek NJ, MacKenzie ED, Nixon C, et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol. 2015;17(10):1317–1326. doi: 10.1038/ncb3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Scalise M, Galluccio M, Console L, Pochini L, Indiveri C. The human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018;6:243. doi: 10.3389/fchem.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Kakazu E, Kanno N, Ueno Y, Shimosegawa T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol. 2007;179(10):7137–7146. doi: 10.4049/jimmunol.179.10.7137. [DOI] [PubMed] [Google Scholar]
- 507.Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. 2016;1857(8):1086–1101. doi: 10.1016/j.bbabio.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 508.Anderson NM, Mucka P, Kern JG, Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018;9(2):216–237. doi: 10.1007/s13238-017-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21(3):579–596. doi: 10.1016/j.beha.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, Kasai M, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354(6316):1152–1155. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- 511.Rowe RG, Daley GQ. Stem cells: valine starvation leads to a hungry niche. Nature. 2017;541(7636):166–167. doi: 10.1038/nature21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47(6):1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 513.Tabaczar S, Czogalla A, Podkalicka J, Biernatowska A, Sikorski AF. Protein palmitoylation: palmitoyltransferases and their specificity. Exp Biol Med (Maywood). 2017;242(11):1150-1157. d10.1177/1535370217707732. [DOI] [PMC free article] [PubMed]
- 514.Manandhar SP, Calle EN, Gharakhanian E. Distinct palmitoylation events at the amino-terminal conserved cysteines of Env7 direct its stability, localization, and vacuolar fusion regulation in S. cerevisiae. J Biol Chem. 2014;289(16):11431–11442. doi: 10.1074/jbc.M113.524082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Jennings BC, Linder ME. DHHC protein S-acyltransferases use similar ping-pong kinetic mechanisms but display different acyl-CoA specificities. J Biol Chem. 2012;287(10):7236–7245. doi: 10.1074/jbc.M111.337246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Donatello S, Babina IS, Hazelwood LD, Hill AD, Nabi IR, Hopkins AM. Lipid raft association restricts CD44-ezrin interaction and promotion of breast cancer cell migration. Am J Pathol. 2012;181(6):2172–2187. doi: 10.1016/j.ajpath.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Yang N, Ying C, Xu M, Zuo X, Ye X, Liu L, et al. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007;76(1):167–174. doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 518.Garcia-Serrano S, Moreno-Santos I, Garrido-Sanchez L, Gutierrez-Repiso C, Garcia-Almeida JM, Garcia-Arnes J, et al. Stearoyl-CoA desaturase-1 is associated with insulin resistance in morbidly obese subjects. Mol Med. 2011;17(3-4):273–280. doi: 10.2119/molmed.2010.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 520.Felig P. The glucose-alanine cycle. Metabolism. 1973;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 521.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27(4):757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 522.Gladden LB. Lactate as a key metabolic intermediate in cancer. Ann Transl Med. 2019;7(10):210. doi: 10.21037/atm.2019.01.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Offermanns S, Colletti SL, Lovenberg TW, Semple G, Wise A, AP IJ. International Union of Basic and Clinical Pharmacology. LXXXII: nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B) Pharmacol Rev. 2011;63(2):269–290. doi: 10.1124/pr.110.003301. [DOI] [PubMed] [Google Scholar]
- 524.Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, et al. The lactate receptor, G protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res. 2015;93(7):1045–1055. doi: 10.1002/jnr.23593. [DOI] [PubMed] [Google Scholar]
- 525.Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299(1):F1–13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.van Vuren AJ, Gaillard C, Eisenga MF, van Wijk R, van Beers EJ. The EPO-FGF23 signaling pathway in erythroid progenitor cells: opening a new area of research. Front Physiol. 2019;10:304. doi: 10.3389/fphys.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Perez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hee VF, De Saedeleer CJ, et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle. 2016;15(1):72–83. doi: 10.1080/15384101.2015.1120930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Science Aao. Which came first: the chicken or the egg? 2018. https://www.science.org.au/curious/earth-environment/which-came-first-chicken-or-egg.
- 531.Hayflick L. Mortality and immortality at the cellular level. A review. Biochemistry (Mosc). 1997;62(11):1180–1190. [PubMed] [Google Scholar]
- 532.Hayflick L. The illusion of cell immortality. Br J Cancer. 2000;83(7):841–846. doi: 10.1054/bjoc.2000.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5(18):2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 534.Cooper SJ. From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite. 2008;51(3):419–427. doi: 10.1016/j.appet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 535.Ratner S. The dynamic state of body proteins. Ann N Y Acad Sci. 1979;325:188–209. doi: 10.1111/j.1749-6632.1979.tb14135.x. [DOI] [PubMed] [Google Scholar]
- 536.Guggenheim KY. Rudolf Schoenheimer and the concept of the dynamic state of body constituents. J Nutr. 1991;121(11):1701–1704. doi: 10.1093/jn/121.11.1701. [DOI] [PubMed] [Google Scholar]
- 537.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Tilan J, Kitlinska J. Sympathetic neurotransmitters and tumor angiogenesis-link between stress and cancer progression. J Oncol. 2010;2010:539706. doi: 10.1155/2010/539706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kovacevic Z, McGivan JD. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- 540.Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59(3):240–249. doi: 10.1159/000343995. [DOI] [PubMed] [Google Scholar]
- 541.Casellas A, Mallol C, Salavert A, Jimenez V, Garcia M, Agudo J, et al. Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem. 2015;290(27):16772–16785. doi: 10.1074/jbc.M115.642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Szeliga M, Obara-Michlewska M. Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem Int. 2009;55(1-3):71–75. doi: 10.1016/j.neuint.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 543.Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 2020;31(2):267–283. doi: 10.1016/j.cmet.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 544.Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7(5):1679–1690. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109(23):8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]