Abstract
Background:
Intravenous immunoglobulin (IVIG) has been commonly used to treat myasthenia gravis exacerbation, but is still ineffective in nearly 30% of patients. A variable number of tandem repeat (VNTR) polymorphism in the FCGRT gene has been found to reduce the efficiency of IgG biologics. However, whether the polymorphism influences the efficacy of IVIG in generalized myasthenia gravis (MG) patients with exacerbations remains unknown.
Methods:
The distribution of VNTR genotypes was analyzed in 334 patients with MG. Varied VNTR alleles were determined by capillary electrophoresis and confirmed by Sanger sequencing. Information of endogenous IgG levels were collected in patients without previous immunotherapy (n = 26). Medical records of patients who received IVIG therapy were retrospectively analyzed for therapeutic outcomes of IVIG treatment (n = 61). Patients whose Activities of Daily Living scores decreased by 2 or more points on day 14 were considered responders to the treatment.
Results:
The VNTR3/3 and VNTR2/3 genotypes were detected in 96.7% (323/334) and 3.4% (11/334) patients, respectively. Patients with VNTR2/3 heterozygosity had lower endogenous IgG levels than those with VNTR3/3 homozygosity (9.81 ± 2.61 g/L versus 12.41 ± 2.45g/L, p = 0.016). The response rate of IVIG therapy was 78.7% (48/61). All responders and nine non-responders were VNTR3/3 homozygotes, whereas all the patients with VNTR2/3 genotypes were non-responders (n = 4). In patients who took IVIG treatments, endogenous IgG levels were significantly lower in non-responders compared with responders (12.93 ± 2.24 g/L versus 8.85 ± 2.69 g/L, p = 0.006), especially in VNTR2/3 heterozygotes (7.86 ± 1.78 g/L, p = 0.001).
Conclusion:
The VNTR2/3 genotype could influence endogenous IgG levels and serve as a predictive marker for poor responses to IVIG in MG patients.
Keywords: FCGRT gene, intravenous immunoglobulin treatment, myasthenia gravis, variable number of tandem repeat polymorphism
Introduction
Myasthenia gravis (MG) is an acquired autoimmune disease characterized by fatigability and fluctuant weakness in extraocular muscles, limbs, bulbar muscles and respiratory muscles.1 Some MG patients progress quickly to impending myasthenic crisis status, which is exemplified by a rapid worsening of clinical symptoms within a very short time period (days to weeks).1,2 In rare instances, the symptoms worsen into manifest myasthenic crisis, leading to increased respiratory muscle and/or bulbar muscle weakness which is severe enough to necessitate intubation.2 The incidence of MG patients experiencing myasthenic crisis ranges from 10% to 20%, with an approximate mortality rate of 5%.3–5 Thus, preventing impending myasthenic crisis from progressing into manifest myasthenic crisis is crucial to improving a patient’s quality of life. Currently, intravenous immunoglobulin (IVIG) and plasma exchange (PE) are the most effective therapies for treating the patient who experiences a rapid MG exacerbation.2,6
Although similar outcomes of IVIG and PE for MG exacerbations have been demonstrated,7 the choice of PE may be constrained by availability, age, comorbidities, venous access or specialized hospital settings, whereas IVIG is much easier to manage.3,8 PE is only available in a subset of advanced medical centers in our country, with IVIG being more preferable. However, nearly 30% of patients with MG respond poorly to IVIG treatment.7,9,10 It is important to screen for a patient’s response potential to IVIG treatment for the sake of both the patients and the clinicians. Early identification of IVIG-resistant patients would allow for a swift transition to alternative therapies, which reduces the risk and time course of the treatment and lessens the financial burdens associated with IVIG therapy.
While the exact underpinnings of IVIG’s therapeutic action in MG are not fully understood, some possible mechanisms include neutralization of autoimmune antibodies, depression of the deposition of complements, immunomodulation through inhibitory FcγIIb receptors, and acceleration of pathologic antibody clearance through neonatal Fc receptor (FcRn) saturation.11–16 FcRn, which is encoded by the FCGRT gene, extends the half-life of IgG by preventing intracellular degradation.17 A variable number tandem repeat (VNTR) polymorphism within the promoter region of the FCGRT gene was identified to be involved in modulating the expression of FcRn.18 VNTR2/3 individuals were associated with a decreased expression level of FcRn compared with VNTR3/3 individuals.18 Similarly, the VNTR2/3 genotype was associated with decreased effectiveness of IVIG treatment in patients with common variable immunodeficiency (CVID), possibly by interfering with infused IgG recycling through varied FcRn expression. This suggested that the VNTR polymorphism influenced the IgG catabolism dynamics.19 Based on these findings, we hypothesized that the VNTR polymorphism could influence endogenous IgG levels and was related to the efficacy of IVIG treatment in MG patients.
We investigated the possible factors that influenced the effectiveness of IVIG treatment in MG patients, including the presence of the VNTR2 allele. We found for the first time that the VNTR2/3 genotype may be considered as a predictor of non-response to IVIG treatment in MG patients.
Materials and methods
Participants
MG patients (N = 334) were recruited from the Xuanwu Hospital Myasthenia Gravis database (Beijing, China). Patients in this database had provided their consent to be included in the registry and to have their medical records and serum and DNA samples to be used for research purposes. Genetic analysis of the VNTR polymorphism in the FCGRT gene was performed in all the patients. Among the recruited patients, endogenous IgG levels (normal range: 7.51–15.60 g/L) were analyzed in patients without previous immunotherapies including corticosteroids, non-steroidal immunosuppressants, plasma exchange and IVIG. (n = 26) (Supplemental material Figure 1 online). Medical records of generalized MG patients who received an IVIG dose of 0.4 g/kg per day for five consecutive days (n = 61) were retrospectively reviewed and analyzed for therapeutic outcomes of IVIG. Judged by treating physicians, IVIG treatments were prescribed to the patients on conditions of impending myasthenic crisis or manifest myasthenic crisis.2 Definition of impending myasthenic crisis is rapid clinical worsening of MG that, in the opinion of the treating physician, could lead to crisis in the short term (days to weeks). And the definition of manifest myasthenic crisis is worsening of myasthenic weakness requiring intubation or non-invasive ventilation to avoid intubation, except when these measures are employed during routine postoperative management.2 Patients with muscle specific tyrosine kinase antibody positive or with infections during the period of IVIG administration to the time for evaluation of the curative effects were excluded. Sixty-one patients were analyzed for therapeutic outcomes of IVIG. Patients were scored for MG-Activities of Daily Living (ADL) before and 14 days after the treatment. The ADL scores of the patients before the IVIG therapy increased by at least two points attributable to the following non-ocular items within 1 month: difficulty in swallowing, dysarthria, acute respiratory failure, or worsening limb weakness.20,21 Other MG medications prior to MG exacerbations remained unchanged during the evaluation of IVIG therapy outcomes. This study was approved by the Ethics Committee of Xuanwu Hospital (No. 2017084) and written informed consents were acquired from all participants.
Clinical data collection
To investigate the influential factors of treatment response, clinical data was collected including age, gender, course of disease, Osserman classification, thymoma, causes for exacerbations, presence of anti- acetylcholine receptor (AChR) antibody, and treatment schedule of MG medication. Causes of exacerbations were categorized as first manifestation of MG, idiopathic or unknown, post-thymectomy, infection, childbirth and reduction of drug dose. Patients who had taken IVIG treatment were scored for MG-ADL scores before and 14 days after the first infusion of IVIG. A good response to treatment was defined as a decrease of at least two points in the ADL score on day 14 after the first IVIG infusion.22 For patients with data of IgG concentrations acquirable (n = 26), clinical characteristics were analyzed to investigate the influential factors on endogenous IgG levels, including the VNTR genotype. Albumin levels of the 26 patients with data of IgG concentrations were reviewed and compared between different VNTR genotypes.
Genetic analysis of VNTR polymorphism within the FCGRT promotor
Genomic DNA was obtained from 334 patients. Capillary electrophoresis coupled with polymerase chain reaction (PCR) assays were used to analyze the VNTR region of the FCGRT gene. PCR amplification was conducted using a thermal cycler (Life pro, Hangzhou Bioer Technology Co. Ltd) in a volume of 20 μL containing 10 ng of DNA. Master mix solutions were composed of 10 mM of sense (5’- TGGATTCCTGGGTCTGAGAG-3’) and antisense primers (5’- GACACTTGATAGGCTGAGAGTCC-3’), 10 mM of dNTP and 5 U of Taq polymerase diluted in double distilled H2O. The forward primer was labeled with 5’- FAM. The PCR cycle was as follows: 2 min at 94°C, then 35 cycles, each consisting of denaturation (94°C for 30 s), annealing (60°C for 30 s) and extension (68°C for 30 s). Finally, a complete extension was set for 7 min at 72°C. PCR products were separated by capillary electrophoresis on a DNA analyzer (Applied Biosystems, ABI 3730 × l). Results were analyzed with GeneMarker 2.0. The PCR amplification fragments of VNTR3 alleles were identified at 327 bp in length while VNTR2 alleles were identified at 37 bp shorter than VNTR3 alleles. The sizes of VNTR alleles were detected by referring each fragment to the relative migration of molecular weight markers. Presence of two peaks corresponded to heterozygotes, which was further confirmed by Sanger sequencing. Primers for Sanger sequencing were as follows: sense primer, 5’-GGAGCGAGGCTGAAGGGAAC-3’; anti-sense primer 5’-CTCGGTCCAGACTGACAACA-3’; and the primer for sequencing was 5’-CCCCTGAACTGGATCTCAGTTG-3’.
Statistical analysis
Summary statistics (i.e. mean ± standard deviation, median and range, number and percentage) were computed for quantitative and qualitative variables. Correlations between clinical factors and endogenous IgG concentrations were evaluated using Spearman rank correlations. To determine differences in clinical characteristics between IVIG treatment responders and non-responders, continuous data were analyzed by t-test or Wilcoxon rank-sum test, depending on the data distribution. Categorical data was compared with Fisher’s exact test. A p value of 0.05 or less was considered statistically significant. SPSS (Version 22, IBM) was used for all statistical analyses.
Results
A total of 334 adult patients were included in the study. The allelic frequencies of VNTR polymorphisms in the promoter region of the FCGRT gene observed in our cohort, VNTR3 (98.4%) and VNTR2 (1.6%), were similar to those previously observed in Caucasians,18,19 while other less frequent alleles were not detected. Most patients (n = 323, 96.7%) were VNTR3/3 homozygotes, and 11 patients (3.3%) were VNTR2/3 heterozygotes. Clinical features of the 11 VNTR2/3 patients are presented in Table 1.
Table 1.
Clinical factors of 11 patients with VNTR2/3 genotype from 334 myasthenia gravis patients.
| Number | Gender/age | Course of disease (months) | Osserman classification | Presence of thymoma | AChR antibody titer (nmol/L)* | ADL score | Concurrent immunosuppressant | |
|---|---|---|---|---|---|---|---|---|
| Patients without clinical exacerbations | 1 | F/60 | 5 | I | N | 9.49 | 5 | Null |
| 2 | F/54 | 23 | IIB | P | N | 0 | Prednisone | |
| 3 | M/61 | 5 | IIB | N | 81.74 | 3 | Null | |
| 4 | F/66 | 37 | I | N | 6.26 | 3 | Null | |
| 5 | F/59 | 18 | IIB | P | 3.75 | 0 | Prednisone | |
| 6 | M/67 | 2 | I | N | N | 6 | Null | |
| 7 | M/42 | 6 | IIB | N | 0.75 | 6 | Null | |
| Patients with clinical exacerbations and taking IVIG | 8 | M/64 | 10 | IV | N | 18.84 | 6 | Null |
| 9 | F/75 | 1 | III | N | 2.58 | 9 | Null | |
| 10 | F/49 | 6 | IV | N | N | 8 | Null | |
| 11 | M/49 | 2 | IIB | P | 5.80 | 3 | Prednisone |
AChR-antibodies were tested by immunoradioassay.
AChR, acetylcholine receptor; ADL, Activities of Daily Living; F, female; IVIG, intravenous immunoglobulin; M, male; N, negative; P, positive; VNTR, variable number tandem repeat.
Among the recruited patients, data of endogenous IgG concentrations were available only for 26 patients who had not previously received immunotherapies. There was a significant difference in endogenous IgG levels between VNTR3/3 patients (n = 15, 12.41 ± 2.45 g/L) and VNTR2/3 patients (n = 11, 9.81 ± 2.61 g/L, p = 0.016, 95% confidence interval (CI) 0.541–4.664; Figure 1). Besides VNTR genotype, no correlation was found between other clinical factors and endogenous IgG concentrations (Table 2). The albumin levels did not differ between the VNTR3/3 and VNTR2/3 patients (39.88 ± 5.23 g/L versus 41.81 ± 3.34 g/L, p = 0.342, 95% CI –2.203 to 6.063).
Figure 1.
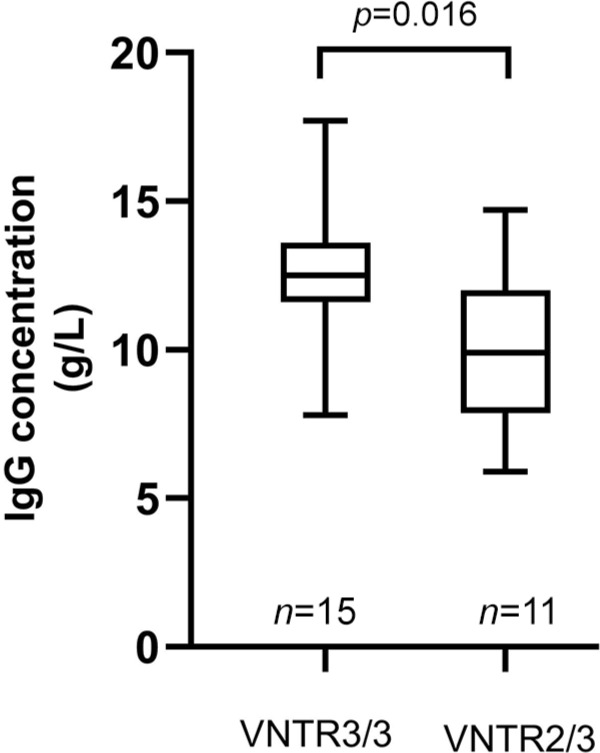
Comparison of endogenous IgG levels according to patients’ VNTR genotypes. Data are shown in box-plots with the center line, lower and upper borders of the box representing medians, 25th and 75th percentiles of the sample. The whiskers indicate minimal and maximal extreme values of the sample.
VNTR, variable number tandem repeat.
Table 2.
Correlation between clinical factors and endogenous IgG concentrations (n = 26).
| Clinical factor | Correlation | |
|---|---|---|
| Gender, male | 11 (42.31%) | r = 0.244 (p = 0.229) |
| Age, years | 55 ± 14.72 | r = –0.183 (p = 0.372) |
| Course of the disease, months | 3 (1–37) | r = –0.017 (p = 0.936) |
| ADL score | 5.73 ± 4.12 | r = 0.193 (p = 0.345) |
| Osserman classification III–IV | 8 (30.77%) | r = –0.195 (p = 0.341) |
| Thymoma | 11 (42.31%) | r = –0.068 (p = 0.743) |
| AChR antibody positive | 23 (88.46%) | r = 0.281 (p = 0.164) |
| VNTR3/3 genotype | 15 (57.69%) | r = 0.478 (p = 0.016) |
| Serum albumin levels, g/L | 40.67 ± 4.56 | r = –0.025 (p = 0.911) |
Data are presented as number (%), mean ± standard deviation or median (range).
AChR, acetylcholine receptor; ADL, Activities of Daily Living; VNTR, variable number tandem repeat.
Forty-eight out of 61 (78.7%) patients improved after IVIG treatment. When divided into responders and non-responders to IVIG treatment, no significant differences were found between the two groups in age, gender, course of disease, Osserman classification, thymoma, causes of exacerbations or presence of anti-AChR antibody (Table 3). All 48 responders and nine non-responders were VNTR3/3 homozygotes while four non-responders were VNTR2/3 heterozygotes. Responders had a higher frequency of the VNTR3/3 genotype (100.0% versus 69.2%, p = 0.001, odds ratio = 21.333, 95% CI 2.130–213.648) than non-responders. The brands of IVIG used by responders included RonsenIVIG 5%® (Chengdu Rongsheng Pharmaceuticals Co. Ltd, Sichuan, China) and human immunoglobulin (Hualan Biological Engineering Inc., Henan, China). All the non-responders were taking RonsenIVIG 5%®.
Table 3.
Clinical factors of responders and non-responders to IVIG therapy in MG patients.
| Clinical factor | Patients received IVIG n = 61 |
Responders n = 48 |
Non-responders n = 13 |
p value* (two-tailed) |
|---|---|---|---|---|
| Gender, male | 28 (25.9%) | 21 (43.8%) | 7 (53.8%) | 0.547 |
| Age, years | 51.36 ± 17.38 | 50.56 ± 18.03 | 54.31 ± 15.03 | 0.495 |
| Course of the disease, months | 4 (1–156) | 5.5 (1–156) | 2 (1–108) | 0.452 |
| Osserman classification | 0.291 | |||
| IIB | 35 (57.4%) | 30 (62.5%) | 5 (38.5%) | |
| III | 9 (14.8%) | 6 (12.5%) | 3 (23.1%) | |
| IV | 17 (27.9%) | 12 (25%) | 5 (38.5%) | |
| ADL score | 10 (3–22) | 10 (3–21) | 13 (6–22) | 0.191 |
| Thymoma | 22 (36.1%) | 18 (37.5%) | 4 (30.8%) | 0.753 |
| Causes of exacerbations | 0.770 | |||
| First manifestation of MG | 18 (28.5%) | 13 (27.1%) | 5 (38.5%) | |
| Idiopathic/unknown | 25 (41.0%) | 20 (41.7%) | 5 (38.5%) | |
| Post-thymectomy | 13 (21.3%) | 11 (22.9%) | 2 (15.4%) | |
| Infection | 2 (3.3%) | 1 (4.2%) | 1 (7.7%) | |
| Childbirth | 1 (1.6%) | 1 (4.2%) | 0 (0.0%) | |
| Reduction of drug dose | 2 (3.3%) | 2 (4.2%) | 0 (0.0%) | |
| AChR antibody positive | 55 (90.2%) | 45 (93.8%) | 10 (76.9%) | 0.105 |
| VNTR3/3 genotype | 57 (93.4%) | 48 (100.0%) | 9 (69.2%) | 0.001 |
Data are presented as number (%), mean ± standard deviation or median (range).
p value compared between responders and non-responders.
AChR, acetylcholine receptor; ADL, Activities of Daily Living; IVIG, intravenous immunoglobulin; MG, myasthenia gravis; VNTR, variable number tandem repeat.
In the 61 patients who underwent IVIG treatment, endogenous IgG concentrations before IVIG infusion were obtained from 12 responders and five non-responders without any previous immunotherapy (Supplemental Figure 1). Responders had higher endogenous IgG concentrations than non-responders (12.93 ± 2.24 g/L versus 8.85 ± 2.69 g/L, p = 0.006, 95% CI 1.396–6.769; Figure 2). Further analysis revealed that endogenous IgG levels were significantly lower in VNTR2/3 non-responders (n = 4, 7.86 ± 1.78 g/L) than in VNTR3/3 responders (n = 12, 12.93 ± 2.24 g/L, p = 0.001, 95% CI 2.411–7.730; Figure 2), suggesting that the statistically significant difference was driven by the VNTR2/3 heterozygotes. Of the nine VNTR3/3 non-responders in the study, the endogenous IgG level was acquired from only one patient (12.8g/L), which was relatively higher than the IgG levels of VNTR2/3 non-responder (median 7.68, range 5.89–10.20; Figure 2). The IgG levels of the other eight patients were excluded from analysis because five patients took IVIG therapy before visiting our hospital and three patients were taking other immunosuppressants before using IVIG.
Figure 2.
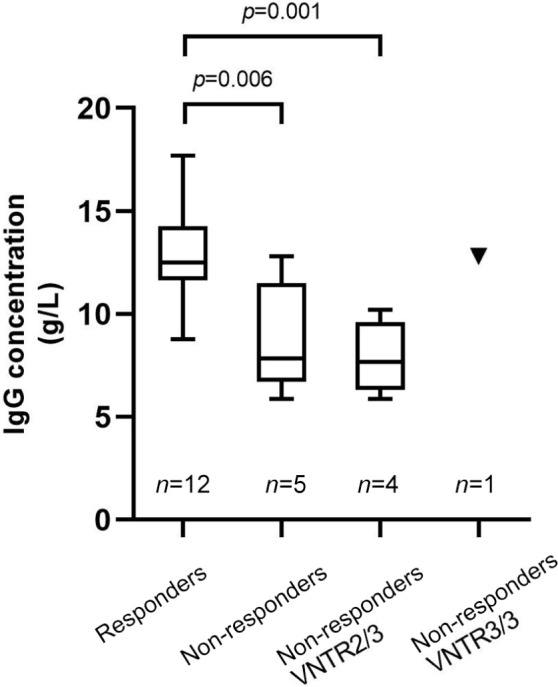
Comparison of endogenous IgG levels before intravenous immunoglobulin therapy in myasthenia gravis patients. Grouping was based on therapeutic effects and VNTR genotypes. The comparison of IgG levels was performed between 12 responders (who were all VNTR3/3 genotypes) and five non-responders. Among the non-responders, four patients were VNTR2/3 genotypes (presented in the third column) and one patient was VNTR3/3 (presented as the triangle on the rightmost column). Data are shown in box-plots with the center line, lower and upper borders of the box representing medians, 25th and 75th percentiles of the sample. The whiskers indicate minimal and maximal extreme values of the sample.
VNTR, variable number tandem repeat.
Discussion
Our study demonstrated that most MG patients had VNTR3/3 homozygotic alleles in the promoter region of the FCGRT gene. Patients with the VNTR2/3 genotype had significantly lower endogenous IgG levels than those of the patients with VNTR3/3 homozygosity. The overall effectiveness rate of IVIG was 78.7% on day 14, and poor responses to IVIG treatment were mostly associated with carrying the VNTR2/3 genotype. To our knowledge, this is the first study investigating the association between the VNTR polymorphism and the efficacy of IVIG therapy in MG patients.
The FCGRT gene encodes the heavy chain of FcRn. FcRn is mainly expressed in syncytiotrophoblasts and endothelial cells. In several organs, FcRn mediates transcytosis or functions as a recycling receptor for IgG.23–25 FcRn binds internalized IgG under acidic conditions and protects it from lysosomal degradation. Thus, FcRn extends serum IgG life span and plays a central role in IgG homeostasis.17
Previous studies have identified a VNTR polymorphism in the promoter region of the FCGRT gene, with VNTR3/3 homozygosity being the predominant genotype in Caucasians.18,19 The distribution of VNTR genotypes in our study was in accordance with previous reports. Importantly, the VNTR2 allele was related to decreased promoter activity and lower expression level of FcRn compared with the more common VNTR3 allele.18 Sachs et al. found that monocytes from VNTR3/3 homozygous individuals expressed 1.66-fold more FcRn transcripts than monocytes from VNTR2/3 heterozygous individuals and possessed a much higher binding capacity.18 Our results showed that VNTR2/3 heterozygosity was associated with decreased endogenous IgG levels. Lower endogenous IgG concentrations could be evidence of reduced FcRn quantity and increased IgG elimination. Similar associations have been identified in animal studies.26,27 For example, Shubin et al. observed that the minority of VNTR genotypes were related to lower levels of serum IgG in rhesus macaques.27 However, no correlation was found between the VNTR polymorphism and endogenous IgG concentration in Guillain–Barre syndrome patients, perinatal women or patients with ovarian cancer.28–30 The difference between these findings might be due to the distinct pathophysiology involved in MG among the above-mentioned conditions. Meanwhile, it is important to mention that MG patients who experience exacerbations requiring IVIG treatment, but have no previous immunotherapies, are rare. Combined with the fairly low proportion of the VNTR2/3 genotype in MG patients, the amount of available IgG data used in this study was limited. Therefore, future studies utilizing data from multiple health care centers are necessary to fully elucidate these interactions.
FcRn also binds albumin and protects it from degradation with distinct binding site from FcRn–IgG interaction.31 However, the albumin levels of VNTR2/3 patients are not distinguished from VNTR3/3 patients in our study. Previous studies reported that higher serum albumin levels were related to reduced eliminations of therapeutic monoclonal antibodies and were thought to be a reflection of FcRn activity,30,32 whereas no significant difference was found in the albumin levels regarding VNTR genotypes.30 The albumin synthesis is influenced by many factors,33 and is especially affected by a decrease in food intake.34 In our study, most patients with IgG and albumin levels analyzed were having difficulties in swallowing. Thus, the albumin levels may not reveal the real effect of regulating capacities of FcRn. The influence of the VNTR polymorphism on the albumin levels should be assessed in patients without factors disturbing the synthesis of albumin to confirm the role of FcRn activity in the future. Due to the functional consequences of varied FcRn expression, VNTR allelic variation could serve as a pharmacogenetic marker, thereby affecting the catabolism of IgG biologics. For example, in one study VNTR2/3 patients had a higher cetuximab distribution clearance than VNTR3/3 patients, indicating that in VNTR3/3 patients the drug was more efficiently retained in peripheral tissues.35 Similarly, in a different study, VNTR2/3 patients were associated with decreased infliximab concentrations during the induction therapy, which was linked with worse disease outcomes.36 IVIG treatment efficacy was also reduced in CVID patients with non-VNTR3/3 genotypes.19 Saturation of FcRn receptors caused by high IgG peak concentrations, after an intravenous infusion, leads to unspecific IgG clearance.37 Importantly, VNTR3/3 patients were reported to have superior FcRn binding capacities,18 resulting in the extended life span of infused IgG. Our research revealed that none of the VNTR2/3 patients responded to IVIG therapy, suggesting that the VNTR polymorphism influenced IVIG treatment efficacy in MG patients. The VNTR2 allele could be an indicator of reduced FcRn binding capacity and consequently diminished efficacy of IVIG. Therefore, MG patients with the VNTR2/3 genotype should be aware of the risk of poor response to IVIG treatment.
Our work showed that IVIG non-responders had lower levels of endogenous IgG before treatment. Combined with the correlation between lower IgG level and VNTR2/3 genotype, and reduced expression of FcRn in VNTR2/3 heterozygosity,18 it could be inferred that lower endogenous IgG levels reflected the decreased binding capacities of FcRn and indicated fast metabolism of infused IgG. In our study, VNTR2/3 patients with estimated lower FcRn expression and fast IgG degradation also experienced exacerbation. We speculate that the production rates of pathogenic antibodies were elevated in those patients. After VNTR2/3 patients took IVIG treatment, the duration of IVIG action was shortened due to the fast elimination of IgG, which weakened the elimination effect on the pathogenic antibodies, although it was not supported experimentally in the current study. The production rate of pathogenic antibodies could be greater than the removal of the pathogenic antibodies by the remaining IVIG, thus resulting in IVIG ineffectiveness. Also, the decreased duration of IVIG retention also reduced the functions, including neutralization of cytokines, inhibition of anti-idiotype autoantibodies and binding to inhibitory FcγRIIb.31 Our work investigated only the differences in endogenous IgG levels between the groups with differing response to IVIG therapy, and fell short of investigating the IgG change of each patient. Further research on the metabolic rate of infused IgG is needed, specifically between patients with varied therapeutic outcomes to IVIG treatments. Efgartigimod was a promising FcRn antagonist, which possessed one of the therapeutic properties of IVIG as inhibiting the IgG recycling activity of FcRn and inducing the clearance of pathogenic IgG autoantibodies.38,39 Patients benefited from the drug as evidenced by the decreased endogenous IgG and AChR antibody levels compared with their respective baselines.40 Similarities of the therapeutic mechanism in reducing AChR antibody between efgartigimod and IVIG raised the assumption that IVIG non-responders may not respond to efgartigimod either. Taken together, this suggests that incremental doses or repetitive administration of IVIG, or even alternative therapeutic approaches altogether, may be necessary in patients with lower endogenous IgG concentrations or VNTR2/3 genotypes.
The 61 patients were analyzed for IVIG efficacy because they took IVIG treatment when experiencing exacerbation or myasthenic crisis. Patients included for IVIG effectiveness analysis were not from a typical MG population. Thymoma was present in 36.1% (22/61) of the patients in our study. A similar incidence was previously reported as 32% of patients with myasthenic crisis having thymoma.41 Except for the VNTR2/3 genotype, we found no relationship between gender, age, illness duration, causes of exacerbations, thymoma or the presence of AChR antibody with IVIG-resistance (Table 3). Further analysis in patients with VNTR3/3 revealed no correlation between clinical characteristics as mentioned above and response to IVIG therapy (data not shown). Therefore, the reason for the VNTR3/3 genotype patients being unresponsive to the initial IVIG treatment was unclear. The percentages of Osserman class III–IV and ADL scores were relatively higher in the non-responder group, which indicated that there were more non-responders experienced myasthenic crisis and the disease tended to be more severe; however, our study lacked power to show statistically significant differences. The severity and complex pathogenesis of MG might contribute to instances of IVIG resistance. Therapeutic responsiveness to IVIG involves a variety of immunologic mechanisms.42 Most studies on predicting IVIG resistance were performed in patients with Kawasaki disease.43 It has been proposed that functional polymorphisms or epigenetic changes in the coding genes of Fc gamma receptors could be used to predict IVIG response in patients with Kawasaki disease.44 However, in a study with a small number of MG patients, a negative correlation was found between the Fc gamma receptors polymorphisms and the treatment response,45 suggesting it may not be a good predictive marker. Alternatively, numerous coding variants of interleukins and relevant receptors have been associated with IVIG resistance.46,47 Therefore, genome-wide association studies may provide insight into risk factors associated with IVIG treatment resistance in MG patients.48 Similarly, serum cytokine profiles may prove helpful in predicting poor response to IVIG, as post-IVIG levels of IL-6 and IL-10 were found to be significantly higher in non-responders than in responders in patients with Kawasaki disease.49 Nevertheless, further investigation is required to establish a risk scoring system or a new algorithm model based on genetic and non-genetic biomarkers that can predict IVIG treatment resistance in MG patients.
Our study was a retrospective study and has the following limitations. (1) The endogenous IgG level was acquirable from only one non-responder with VNTR3/3 genotype, and a critical comparison of IgG levels between non-responders with different VNTR genotypes was not performed due to the small sample size and restricted data. (2) We did not test the binding capacities of FcRn in our patients and the varied expression quantities of FcRn among different genotypes were unknown. (3) The ADL scores were assessed according to medical records or telephone follow-up interviews in some patients. Quantitative MG scores and MG-composite scores were not available from all patients. There was a lack of relatively objective evaluation for therapeutic outcomes for analysis. (4) Most patients did not measure IgG levels after IVIG infusion, and the data on IgG level changes were not collected at the time. (5) Moreover, the pathological autoantibody titers needed to be traced before and after the IVIG treatment to verify the associations between the decline rates of autoantibodies with VNTR genotypes and IVIG efficacy. Our work provides an observation that VNTR2/3 patients are associated with lower IgG levels and possibly fast IgG catabolism, which consequently results in shortened retention of IVIG and reduced efficacy thereafter. In the future, a well-designed prospective study is needed to further investigate the relationship between VNTR genotypes and IVIG efficacy by studying the real effect of the genotypes on IVIG catabolism rates and the elimination of pathological autoantibodies.
In conclusion, our results showed that in MG patients, the VNTR2/3 genotype was associated with lower endogenous IgG levels and a diminished response to IVIG treatment, which suggested that the VNTR2/3 genotype, as well as decreased endogenous IgG levels, may serve as a predictive indicator of IVIG non-responsiveness in MG patients.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286420986747 for VNTR2/VNTR3 genotype in the FCGRT gene is associated with reduced effectiveness of intravenous immunoglobulin in patients with myasthenia gravis by Shengyao Su, Qing Liu, Xueping Zhang, Xinmei Wen, Lin Lei, Faxiu Shen, Zhirong Fan, Jianying Duo, Yan Lu, Li Di, Min Wang, Hai Chen, Wenjia Zhu, Min Xu, Suobin Wang and Yuwei Da in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors would like to thank all the subjects for participation in our study.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Clinical Cohort Study of Myasthenia Gravis, National Key R&D Program of China, Precision Medicine Project (No. 2017YFC0907700) and Beijing Municipal Health Commission Fund (PXM2020_026283_000005).
ORCID iDs: Shengyao Su  https://orcid.org/0000-0002-5020-7056
https://orcid.org/0000-0002-5020-7056
Zhirong Fan  https://orcid.org/0000-0002-3118-9016
https://orcid.org/0000-0002-3118-9016
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shengyao Su, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Qing Liu, Department of Neurology, Xuanwu Hospital, Capital Medical University, Chang Chun Street, Beijing, China; Department of Neurology, Beijing Fengtai You’anmen Hospital, Beijing, China.
Xueping Zhang, Department of Neurology, Xuanwu Hospital, Capital Medical University, Chang Chun Street, Beijing, China; Department of Neurology, Beijing Fengtai You’anmen Hospital, Beijing, China.
Xinmei Wen, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Lin Lei, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Faxiu Shen, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Zhirong Fan, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Jianying Duo, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Yan Lu, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Li Di, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Min Wang, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Hai Chen, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Wenjia Zhu, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Min Xu, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Suobin Wang, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Yuwei Da, Department of Neurology, Xuanwu Hospital, Capital Medical University, Chang Chun Street, Beijing 100053, China.
References
- 1. Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers 2019; 5: 30. [DOI] [PubMed] [Google Scholar]
- 2. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 2016; 87: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos-Fransi A, Rojas-Garcia R, Segovia S, et al. Myasthenia gravis: descriptive analysis of life-threatening events in a recent nationwide registry. Eur J Neurol 2015; 22: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 4. Bedlack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4: 40–42. [DOI] [PubMed] [Google Scholar]
- 5. Alshekhlee A, Miles JD, Katirji B, et al. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009; 72: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 6. Lunemann JD, Quast I, Dalakas MC. Efficacy of intravenous immunoglobulin in neurological diseases. Neurotherapeutics 2016; 13: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barth D, Nabavi NM, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011; 76: 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalakas MC. Progress in the therapy of myasthenia gravis: getting closer to effective targeted immunotherapies. Curr Opin Neurol 2020; 33: 545–552. [DOI] [PubMed] [Google Scholar]
- 9. Hellmann MA, Mosberg-Galili R, Lotan I, et al. Maintenance IVIg therapy in myasthenia gravis does not affect disease activity. J Neurol Sci 2014; 338: 39–42. [DOI] [PubMed] [Google Scholar]
- 10. Edan G, Landgraf F. Experience with intravenous immunoglobulin in myasthenia gravis: a review. J Neurol Neurosurg Psychiatry 1994; 57(Suppl.): 55–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liblau R, Gajdos P, Bustarret FA, et al. Intravenous gamma-globulin in myasthenia gravis: interaction with anti-acetylcholine receptor autoantibodies. J Clin Immunol 1991; 11: 128–131. [DOI] [PubMed] [Google Scholar]
- 12. Fuchs S, Feferman T, Meidler R, et al. A disease-specific fraction isolated from IVIG is essential for the immunosuppressive effect of IVIG in experimental autoimmune myasthenia gravis. J Neuroimmunol 2008; 194: 89–96. [DOI] [PubMed] [Google Scholar]
- 13. Berger M, McCallus DE, Lin CS. Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies. J Peripher Nerv Syst 2013; 18: 275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alabdali M, Barnett C, Katzberg H, et al. Intravenous immunoglobulin as treatment for myasthenia gravis: current evidence and outcomes. Expert Rev Clin Immunol 2014; 10: 1659–1665. [DOI] [PubMed] [Google Scholar]
- 15. Tha-In T, Bayry J, Metselaar HJ, et al. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol 2008; 29: 608–615. [DOI] [PubMed] [Google Scholar]
- 16. Vincent A, Lang B, Kleopa KA. Autoimmune channelopathies and related neurological disorders. Neuron 2006; 52: 123–138. [DOI] [PubMed] [Google Scholar]
- 17. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 18. Sachs UJ, Socher I, Braeunlich CG, et al. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor alpha-chain promoter. Immunology 2006; 119: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gouilleux-Gruart V, Chapel H, Chevret S, et al. Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: correlations with clinical phenotype and polymorphism of the neonatal Fc receptor. Clin Exp Immunol 2013; 171: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gajdos P, Chevret S, Clair B, et al. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia gravis clinical study group. Ann Neurol 1997; 41: 789–796. [DOI] [PubMed] [Google Scholar]
- 21. Pasnoor M, He J, Herbelin L, et al. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 2016; 87: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muppidi S. The myasthenia gravis–specific activities of daily living profile. Ann N Y Acad Sci 2012; 1274: 114–119. [DOI] [PubMed] [Google Scholar]
- 23. Simister NE, Story CM, Chen HL, et al. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol 1996; 26: 1527–1531. [DOI] [PubMed] [Google Scholar]
- 24. Borvak J, Richardson J, Medesan C, et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 1998; 10: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 25. Shah U, Dickinson BL, Blumberg RS, et al. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res 2003; 53: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang R, Zhao Z, Zhao Y, et al. Association of FcRn heavy chain encoding gene (FCGRT) polymorphisms with IgG content in bovine colostrum. Anim Biotechnol 2009; 20: 242–246. [DOI] [PubMed] [Google Scholar]
- 27. Shubin Z, Tagaya Y, Poonia B. Functional polymorphisms in rhesus macaque FCGRT and beta2-m. Immunogenetics 2018; 70: 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fokkink WJ, Haarman AE, Tio-Gillen AP, et al. Neonatal Fc receptor promoter gene polymorphism does not predict pharmacokinetics of IVIg or the clinical course of GBS. Ann Clin Transl Neurol 2016; 3: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freiberger T, Ravcukova B, Grodecka L, et al. No association of FCRN promoter VNTR polymorphism with the rate of maternal-fetal IgG transfer. J Reprod Immunol 2010; 85: 193–197. [DOI] [PubMed] [Google Scholar]
- 30. O’Shannessy DJ, Bendas K, Schweizer C, et al. Correlation of FCGRT genomic structure with serum immunoglobulin, albumin and farletuzumab pharmacokinetics in patients with first relapsed ovarian cancer. Genomics 2017; 109: 251–257. [DOI] [PubMed] [Google Scholar]
- 31. Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: function and role in therapeutic intervention. J Allergy Clin Immunol 2020; 146: 467–478. [DOI] [PubMed] [Google Scholar]
- 32. Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009; 65: 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fanali G, di Masi A, Trezza V, et al. Human serum albumin: from bench to bedside. Mol Aspects Med 2012; 33: 209–290. [DOI] [PubMed] [Google Scholar]
- 34. Rothschild MA, Oratz M, Schreiber SS. Current concepts of albumin metabolism. A review. Gastroenterology 1970; 58: 402–408. [PubMed] [Google Scholar]
- 35. Passot C, Azzopardi N, Renault S, et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs 2013; 5: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Billiet T, Dreesen E, Cleynen I, et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol 2016; 111: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 37. Fuhrmann S, Kloft C, Huisinga W. Impact of altered endogenous IgG on unspecific mAb clearance. J Pharmacokinet Pharmacodyn 2017; 44: 351–374. [DOI] [PubMed] [Google Scholar]
- 38. Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 2018; 128: 4372–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peter HH, Ochs HD, Cunningham-Rundles C, et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol 2020; 146: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howard JJ, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019; 92: e2661–e2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist 2011; 1: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Negi VS, Elluru S, Siberil S, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol 2007; 27: 233–245. [DOI] [PubMed] [Google Scholar]
- 43. Galeotti C, Kaveri SV, Bayry J. Molecular and immunological biomarkers to predict IVIg response. Trends Mol Med 2015; 21: 145–147. [DOI] [PubMed] [Google Scholar]
- 44. Chang LS, Lo MH, Li SC, et al. The effect of FcgammaRIIA and FcgammaRIIB on coronary artery lesion formation and intravenous immunoglobulin treatment responses in children with Kawasaki disease. Oncotarget 2017; 8: 2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barnett C, Grinberg Y, Ghani M, et al. Fcgamma receptor polymorphisms do not predict response to intravenous immunoglobulin in myasthenia gravis. J Clin Neuromuscul Dis 2012; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- 46. Amano Y, Akazawa Y, Yasuda J, et al. A low-frequency IL4R locus variant in Japanese patients with intravenous immunoglobulin therapy-unresponsive Kawasaki disease. Pediatr Rheumatol Online J 2019; 17: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim HJ, Kim JJ, Yun SW, et al. Association of the IL16 Asn1147Lys polymorphism with intravenous immunoglobulin resistance in Kawasaki disease. J Hum Genet 2020; 65: 421–426. [DOI] [PubMed] [Google Scholar]
- 48. Kuo HC, Wong HS, Chang WP, et al. Prediction for intravenous immunoglobulin resistance by using weighted genetic risk score identified from genome-wide association study in Kawasaki disease. Circ Cardiovasc Genet 2017; 10: e001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Wang W, Gong F, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum 2013; 65: 805–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286420986747 for VNTR2/VNTR3 genotype in the FCGRT gene is associated with reduced effectiveness of intravenous immunoglobulin in patients with myasthenia gravis by Shengyao Su, Qing Liu, Xueping Zhang, Xinmei Wen, Lin Lei, Faxiu Shen, Zhirong Fan, Jianying Duo, Yan Lu, Li Di, Min Wang, Hai Chen, Wenjia Zhu, Min Xu, Suobin Wang and Yuwei Da in Therapeutic Advances in Neurological Disorders


