Abstract
Ozone is known to cause lung injury, and resident cells of the respiratory tract (i.e., epithelial cells and macrophages) respond to inhaled ozone in a variety of ways that affect their survival, morphology, and functioning. However, a complete understanding of the sex-associated and the cell type-specific gene expression changes in response to ozone exposure is still limited. Through transcriptome profiling, we aimed to analyze gene expression alterations and associated enrichment of biological pathways in three distinct cell type-enriched compartments of ozone-exposed murine lungs. We subchronically exposed adult male and female mice to 0.8 ppm ozone or filtered air. RNA-Seq was performed on airway epithelium-enriched airways, parenchyma, and purified airspace macrophages. Differential gene expression and biological pathway analyses were performed and supported by cellular and immunohistochemical analyses. While a majority of differentially expressed genes (DEGs) in ozone-exposed versus air-exposed groups were common between both sexes, sex-specific DEGs were also identified in all of the three tissue compartments. As compared with ozone-exposed males, ozone-exposed females had significant alterations in gene expression in three compartments. Pathways relevant to cell division and DNA repair were enriched in the ozone-exposed airways, indicating ozone-induced airway injury and repair, which was further supported by immunohistochemical analyses. In addition to cell division and DNA repair pathways, inflammatory pathways were also enriched within the parenchyma, supporting contribution by both epithelial and immune cells. Further, immune response and cytokine-cytokine receptor interactions were enriched in macrophages, indicating ozone-induced macrophage activation. Finally, our analyses also revealed the overall upregulation of mucoinflammation- and mucous cell metaplasia-associated pathways following ozone exposure.
Keywords: airways, gene expression, macrophages, ozone, parenchyma
INTRODUCTION
Ozone, a constituent of photochemical smog, causes lung injury and inflammation (5), and elevated levels of ambient ozone compromises lung function (27, 29, 56, 63) and exacerbates pulmonary symptoms due to mucoinflammatory lung diseases, such as asthma (1, 42, 65) and chronic obstructive pulmonary disease (51, 59). Ambient ozone concentration, and in turn, the prevalence of ozone-related adverse health outcomes, is strongly correlated with hot climate (13, 39) and increasingly higher levels of vehicle emissions, both of which are on a continuous rise (53). Despite these concerns, our understanding of the molecular disturbances caused by ozone inhalation and their relevance in the pathogenesis of ozone-induced lung diseases is limited.
After inhalation, ozone reacts with the contents of the epithelial lining fluid in airways, as well as alveolar spaces to generate ozonized biomolecules that act on resident cells (50). Resident cells, including airway epithelial cells, alveolar epithelial cells, and airspace macrophages constitute the first-responder cellular defense against environmental pollutants, including ozone. Airway epithelial cells exhibit responses, including epithelial disruption, epithelial proliferation, and mucous cell metaplasia (33, 48). Similarly, ozone exposure compromises the permeability of the blood-gas barrier (28), induces alveolar epithelial cell injury and proliferation (26), and disrupts surfactant metabolism (44, 67). Ozone also affects airspace macrophage plasticity and overall population heterogeneity (50). Therefore, molecular adjustments within these resident cells either dictate their protective functional adaptations or reflect their contribution toward pathological outcomes.
Transcriptomic studies reveal critical information on the molecular perturbations caused by prevailing stresses. Previously, a number of acute and subacute transcriptomic studies on whole lung tissues from ozone-exposed rodents have revealed interesting findings, including suppression of cell cycle pathways in ozone-exposed neonatal mice (19), activation of repair pathways in ozone-exposed adult mice (46), the protective role for notch receptors against ozone-induced lung injury (71), modulation of immune responses by α-tocopherol in ozone-exposed adult mice (70), the contribution of IL-17 signaling in responsiveness to ozone in adult mice (41), protective roles of IL-10 against ozone-induced lung injury (4), dispensability of transcription-coupled repair process in ozone-induced injury (31), protective role of mannose-binding lectin in ozone-exposed adult mice (11), and the transcriptional responses of rat lungs to acute ozone exposure (45). In addition to significantly enhancing our current understanding of the pathogenesis of ozone-induced lung diseases, these studies have generated interesting questions regarding cell- and compartment-specific transcriptomic changes.
Three of the immediate questions arose from previous studies, i.e., to what extent does ozone influence the transcriptome of various resident cells within the respiratory airspaces; whether the compartment-specific changes in gene expression and biological pathways align with the pathological manifestations associated with those compartments; and whether these responses are sex-dependent? To address these questions, we hypothesized that molecular signatures and biological pathways associated with ozone-induced lung disease could be identified by evaluating transcriptomic signatures in resident macrophages, parenchyma, and airway epithelial cell-enriched compartments harvested from ozone exposed males and females. To test our hypothesis, C57BL/6J males and females were subchronically exposed to filtered air or 0.8 ppm ozone. Specifically, we performed gene expression profiling and biological pathway enrichment analyses on 1) airway epithelium enriched extra-pulmonary airways, 2) parenchyma, and 3) airspace macrophages. The results from this study identified transcriptomic signatures and biological pathways associated with sex-dependent tissue- and compartment-specific responses to ozone.
METHODS
Animal husbandry.
Seven-week-old male and female C57BL/6J mice were procured from Jackson Laboratory (Bar Harbor, ME). Mice were allowed to acclimatize for a period of 3 wk after arrival at Louisiana State University Division of Laboratory Animal Medicine. Mice were maintained in individually ventilated, hot-washed cages on a 12:12-h dark-light cycle. Male and female mice were housed separately during transport, acclimatization, and exposures, and they were fed a regular diet and water ad libitum. All animal use procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University.
Experimental design and ozone exposure.
Sex has been reported to influence ozone-induced lung injury (6, 7, 10, 62); therefore, to identify sex-dependent effects, we included males and females in our analyses. Mice were housed in polycarbonate cages with perforated lids and were deprived of feed and water during exposure. Ozone was generated by ozone generator (TSE Systems, Chesterfield, MO), and the concentration of ozone gas in a real-time sampled air from sampling cage from within the chamber was monitored by UV photometric ozone analyzer (Envia Altech Environment, Geneva, IL). Data acquisition was done through DACO monitoring and control software (TSE Systems, Chesterfield, MO). Control mice were kept in a chamber supplied with filtered air (Air). Because mice are nocturnal (52), i.e., more active during night, we performed ozone and filtered air exposures strictly at night. Loading of animals into the light-protected chambers was coordinated with the start of the night cycle at the vivarium. Animals were unloaded using night-vision red light goggles. All the exposures took place between 6:00 PM and 10:00 PM. Mice were exposed to 0.8 ppm ozone for 4 h/day for a period of 14 days (five consecutive days of exposures, followed by a two-day rest, five consecutive days of exposures, followed by a two-day rest, four consecutive days of exposures). Hatch et al. (22–24) reported that ∼4–5 times higher ozone concentration is required in rodents to reproduce lung inflammation comparable to exercising humans. The ozone concentration of 0.8 ppm used in this study is ∼11.5 fold higher than the 8-h National Ambient Air Quality Standards for ozone i.e., 0.07 ppm. We selected this regimen of subchronic nightly exposure to ozone based on various considerations: 1) to factor previously reported rodent studies (23, 24, 33, 34, 48), 2) to simulate real-life relevance to humans following repeated exposures when the ozone levels rise in summers (ozone levels may rise in summers for a few days to few weeks), 3) to simulate the effects in humans during their peak time of activity (day in humans, night in rodents), and 4) to consider the responsiveness of rodents to ozone with end-points reflected by epithelial injury and inflammation (0.8 ppm).
Necropsy and tissue harvesting.
Mice were anesthetized with an intraperitoneal injection of 2,2,2-tribromoethanol (250 mg/kg; Sigma-Aldrich, St. Louis, MO). Since the exposures were conducted at night, all the necropsies were conducted on the following day, i.e., within 12–16 h of the conclusion of exposures. After midline laparotomy, inferior vena cava was severed to assure exsanguination. Thereafter, the thoracic cavity was exposed, and a 20G cannula was secured into the trachea through an opening between the cricoid cartilage and the first cartilaginous ring, and a suture was tightened between the first and the second cartilaginous ring of trachea. Lungs were lavaged with a calculated volume (body weight in grams × 0.035 × 1000 = volume in μL) of ice-cold Dulbecco’s phosphate-buffered saline (DPBS) without calcium and magnesium. The first two lavages were pooled and stored on ice. To recover more airspace immune cells, further lavages were performed to collect an additional 9 mL of bronchoalveolar lavage fluid (BALF). Three-hundred microliters of BALF from the first two lavages was centrifuged at 500 g for 5 min at 4°C. Cell-free BALF was saved in −80°C for further analyses. After resuspension in 200 μL of DPBS, the cell pellet was used for the determination of total cell counts using a cell counter (Bio-Rad, Horsham, PA). Cytospins were prepared with 150 μL cell suspension and differentially stained (modified Giemsa kit; Newcomer Supply, Middleton, WI). The remaining portion of the first two lavages and additional 9-mL lavages were processed for macrophage purification.
Microdissection of the extrapulmonary airways and the parenchyma.
After lavaging, the lungs were inflated with RNAlater (Thermo Fisher Scientific, Waltham, MA), the cannula was removed, and a suture was tightened to prevent leakage of the RNAlater out of the lungs. Lungs were then dissected out of the abdominal cavity and stored in 5 mL RNAlater solution at 4°C. After 24 h, the extra-pulmonary airways (trachea (minus the first three cartilaginous rings) and the first and second generation of extralobular airways) (Supplemental Fig. S1, A and B; all supplemental data are available at https://doi.org/10.6084/m9.figshare.12950234) were dissected using dissection microscope, snap-frozen, and stored at −80°C. The connective tissues and the lymphoid nodules surrounding airways were removed. For parenchyma, 1-mm margins of the left lung lobes were trimmed (Fig. 1, A and B, and Supplemental Fig. S1, A and B), snap-frozen, and stored at −80°C.
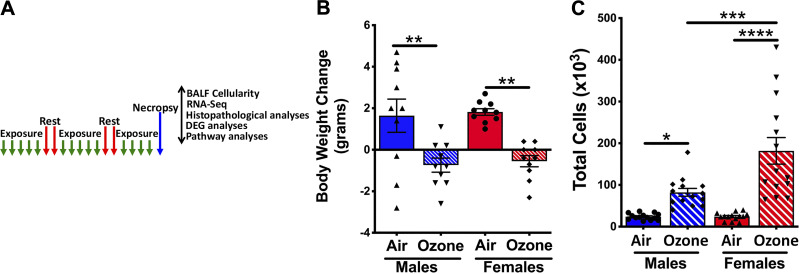
Figure 1.
A: ozone exposure disrupts body weight gain and alters bronchoalveolar lavage fluid (BALF) immune cell counts. A: experimental design depicting exposure regimen and designated outcomes examined. B: increase (positive values) or decrease (negative values) in body weight over the 3 wk of exposure to filtered air or ozone. C: total cells recovered in 300 μL of the first two lavages from air- and ozone-exposed males and females. Error bars represent means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, using one-way ANOVA followed by the Tukey multiple-comparison post hoc test. (n = 13 or 14 per group). BALF, bronchoalveolar lavage fluid.
Purification of airspace macrophages.
Since ozone-exposed mice had significant infiltration of neutrophils and eosinophils in the airspaces, magnetic-activated cell sorting (MACS) was used to purify airspace macrophages. Both neutrophils and eosinophils express CD11b marker, and airspace macrophages do not express this marker (12); therefore, we used a CD11b-microbead kit (Miltenyi Biotech, MA). BALF cell pellet from the remaining portion of the first two lavages and additional 9-mL lavages were resuspended in 180 μL of ice-cold degassed MACS buffer. Twenty microliters of CD11b-microbeads suspension was added followed by incubation at 4°C for 20 min. Following incubation, 2 mL of MACS buffer was added to the incubation mixture, and cells were pelleted at 500 g for 5 min at 4°C. The cell pellet was resuspended in 500 μL of MACS buffer and was added to the cold columns placed in a magnetic field. CD11b negative population (macrophages, essentially no neutrophils and eosinophils) was recovered from the flow through. CD11b-negative population was analyzed for purity, pelleted, snap-frozen, and stored at −80°C. CD11b-positive population (mostly neutrophils, eosinophils, and occasionally macrophage or DC-like cells) was recovered in flow-through after the column was removed from the magnetic field. Macrophage populations used for RNA isolation had ∼97.5% purity. To determine the purity of CD11b-negative population, we analyzed differentially stained cytospins (modified Giemsa kit; Newcomer Supply, Middleton, WI) (Supplemental Fig. S1, C–E). To minimize post-BALF recovery changes in gene signatures, all the BALF processing was performed on ice.
Histopathological analyses.
Trachea from a separate group of mice was cannulated, and lungs were inflated with a calculated volume (body weight in grams × 0.035 × 1000 = volume in μL) of 10% neutral buffered formalin. Formalin-fixed trachea and transverse sections of lung lobes were paraffin embedded, and 5-μm-thick sections were mounted onto glass slides. Sections were stained with hematoxylin and eosin.
Immunohistochemistry for Ki-67 and FOXJ1.
Formalin-fixed paraffin-embedded 5-μm lung sections were used for immunohistochemical localization of Ki-67 and FOXJ1. Sections were deparaffinized with Citrisolv and rehydrated with graded ethanol (100, 95, 70, 30%, with deionized water). Antigen retrieval was performed using a citrate buffer (pH 6.0)-based heat-induced antigen-retrieval method. To quench endogenous peroxidases, sections were incubated in 3% hydrogen peroxide for 10 min at room temperature, followed by PBS washes. After blocking with 3% goat serum for 30 min at room temperature, sections were incubated for 2 h at room temperature with rabbit monoclonal Ki-67 primary antibody (ab16667; ABCAM Cambridge, MA) and rabbit monoclonal FOXJ1 primary antibody (ab235445; ABCAM Cambridge, MA). The sections were then rinsed in PBS and further processed using VECTASTAIN Elite ABC HRP kit (Vector Laboratories, Burlingame, CA), followed by chromogenic substrate conversion to insoluble colored precipitate using ImmPACT NovaRED HRP substrate Kit (Vector Laboratories). Sections were counterstained with Gill’s hematoxylin-I, dehydrated, and coverslipped with mounting media (H-5000, Vector Laboratories).
In situ localization of Muc5b mRNA.
Formalin-fixed, paraffin-embedded 5-μm lung sections were used for in situ localization of Muc5b mRNA using RNAscope technologies, as reported previously (38).
RNA isolation and quality assessment.
Total RNA was isolated from three tissues, i.e., purified macrophages, extrapulmonary airways, and parenchyma, using Illustra RNAspin Mini-RNA isolation kit, according to the manufacturer’s recommendations (GE Healthcare and Biosciences, Pittsburgh, PA). RNA quantity and integrity were analyzed spectrophotometrically (NanoDrop 8000, Thermo Fisher Scientific) followed by RNA quality number (RQN) assessment using a fragment analyzer (ABI 3130 Genetic Analyzer, Thermo Fisher Scientific).
Construction of sequencing library.
One microgram of total RNA was used for the construction of cDNA sequencing libraries using NEBNext Ultra RNA Library prep kit for Illumina (NEB, Ipswich, MA), following the manufacturer’s protocol. Briefly, mRNA was enriched using oligo(dT) beads followed by two rounds of purification and fragmented randomly by adding fragmentation buffer. The first-strand cDNA was synthesized using random hexamer primers, after which a custom second-strand synthesis buffer (Illumina), dNTPs, RNase H, and DNA polymerase I were added to generate the second strand [double-strand (ds) cDNA)]. After a series of terminal repair, poly-adenylation, and sequencing adaptor ligation, the double-stranded cDNA library was completed following size selection and PCR enrichment. The resulting 250–350 bp insert libraries were quantified using a Qubit 2.0 fluorometer (Thermo Fisher Scientific) and quantitative PCR. Size distribution was analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
RNA sequencing.
RNA sequencing on qualified libraries was performed using the Illumina NovaSeq 6000 Platform (Illumina, San Diego, CA) to generate paired-end, 150-base pair reads. Averages of 52.4, 57.9, and 54.8 million raw reads were obtained from airways, parenchyma, and macrophage samples, respectively (Supplemental Table S1).
Data availability.
All of the raw data have been submitted to the Gene Expression Omnibus (GEO) database (GSE156799).
Gene expression analyses.
Paired-end clean reads were aligned to the mouse reference genome, using STAR (v2.5). Differential expression analysis between two groups was performed using the DESeq2 R package (1.14.1). DESeq2 provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P values were adjusted for multiple test correction using the false discovery rate (adjusted P value) by Benjamini-Hochberg’s method. Genes with an adjusted P value < 0.05 and fold change (FC) >2 (Log2 FC >1) were considered differentially expressed. The Venn diagrams were prepared using the function Venn Diagram in R based on the gene lists from different groups.
Pathway analyses.
To determine the biological and functional relevance of differentially expressed genes, we employed three different approaches for pathway analyses, i.e., ingenuity pathway analysis (IPA; https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis) (32), gene ontology (GO) enrichment analysis (2, 66), and STRING protein-protein interaction network analysis (https://string-db.org; version 11.0) (61). IPA identifies molecular and functional pathways in which differentially expressed genes are significantly involved and enriched. Differentially expressed genes (adjusted P value < 0.05 and fold change > 2) were uploaded into IPA software, and their contribution toward the enrichment of canonical pathways was analyzed. IPA upstream regulator analyses were performed on DEGs from purified macrophages to identify the cascades of upstream transcriptional regulators in the context of observed changes in the transcriptome. GO analysis identifies enriched pathways in the context of molecular functions (MF), cellular components (CC, location of cell structure in which DEGs perform a function), and biological processes (BP). The STRING maintains a database of known and predicted protein-protein interaction (PPI) networks based on the input information, e.g., a list of upregulated genes. STRING analysis was performed for identifying PPI network enrichment in upregulated genes in various comparisons.
Statistical analyses.
One-way analysis of variance (ANOVA), followed by the Tukey post hoc test for multiple comparisons, was used to determine significant differences among groups. All data were expressed as means ± SE. P < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA).
RESULTS
Ozone exposure differentially compromises body weight gain and induces airspace immune cell recruitment.
Ten-week-old male and female mice were exposed to ozone (806.1 ± 2.68 ppb) or filtered air (air) for a period of 14 days (Fig. 1A). Designated end points were examined 12–16 h after the conclusion of the last exposure. Air-exposed mice gained body weight (mean body weight gain, males ∼1.64 ± 0.8 g; females ∼1.8 ± 0.16 g) during the 3-wk air exposure time period (Fig. 1B). In contrast, the mean body weights for both sexes were reduced (males: approximately−0.74 ± 0.34 g; females: approximately −0.55 ± 0.27) during the 3 wk of ozone exposure (Fig. 1B). The differences in body weight change between air and ozone exposure were significant in both sexes (Fig. 1B).
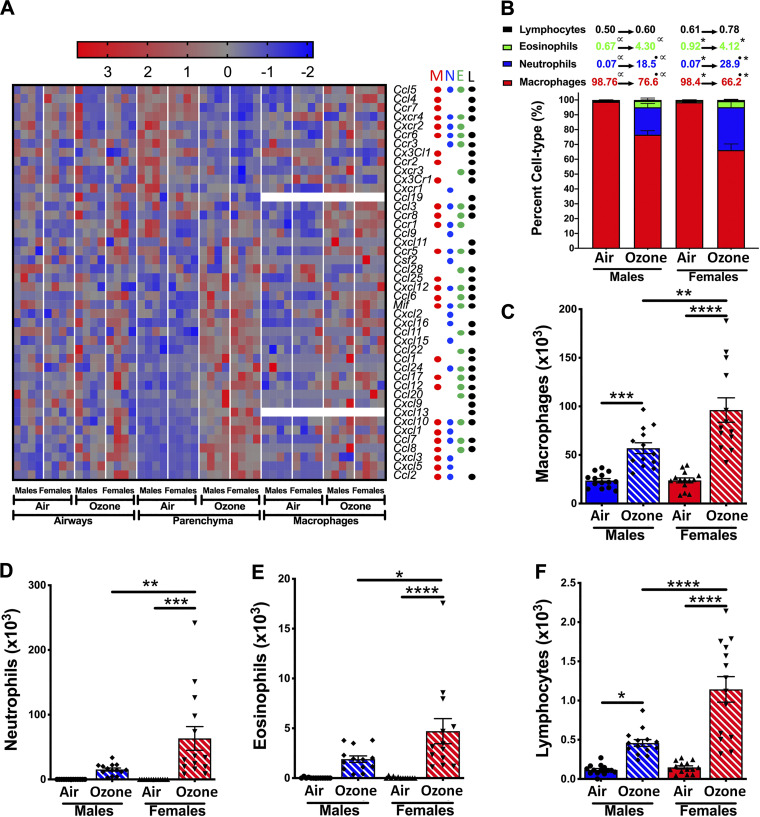
Next, to assess the effect of ozone exposure on airspace cellular recruitment, we analyzed BALF for immune cell counts and proportions. Ozone exposure resulted in a significant increase in total counts in both sexes, as compared with air-exposed mice. The total counts were comparable between air-exposed males and females (Fig. 1C; males: 23.9 × 103 ± 2.02 × 103; females: 24.2 × 103 ± 2.53 × 103). As compared with ozone-exposed males, the ozone-exposed females had significantly higher total cell counts (Fig. 1C; males: 82.2 × 103 ± 9.4 × 103; females: 181.7 × 103 ± 31.9 × 103).
Ozone exposure perturbs gene expression patterns in the extrapulmonary airways.
Ozone inhalation causes epithelium-specific pathological outcomes in airways, including epithelial sloughing, proliferation, and epithelial remodeling (34). To identify transcriptional changes associated with these pathological outcomes, we performed transcriptomic profiling of the extrapulmonary airways [tracheal segment (minus the first three cartilaginous rings) and the first and second generation of extralobular airways]. (Supplemental Fig. S1, A and B). RNA-Seq was performed on 16 extrapulmonary airway RNA samples (four samples/treatment/sex). All the RNA samples selected for RNA-Seq analyses had RQN values in the range of 8.1–9.6. On average, ∼52.4 and 50.8 million raw and mapped reads, respectively, were obtained per sample with ∼97.1% mapping rate (Supplemental Table S1).
The principal component (PC) analysis of airways was performed to identify distinct separation between the four experimental groups. Our analyses using the top three PCs that contribute to ∼68% variance revealed that both treatment and sex were the primary drivers of variation in global gene expression. PC2, which accounts for 21.33% of the variance, separated air-exposed animals from ozone-exposed animals, and PC3, which accounts for 14.52% of the variance, separated air-exposed males and air-exposed females (Fig. 2, A and B). None of the top three principal components separated ozone-exposed males and ozone-exposed females (Fig. 2, A and B).
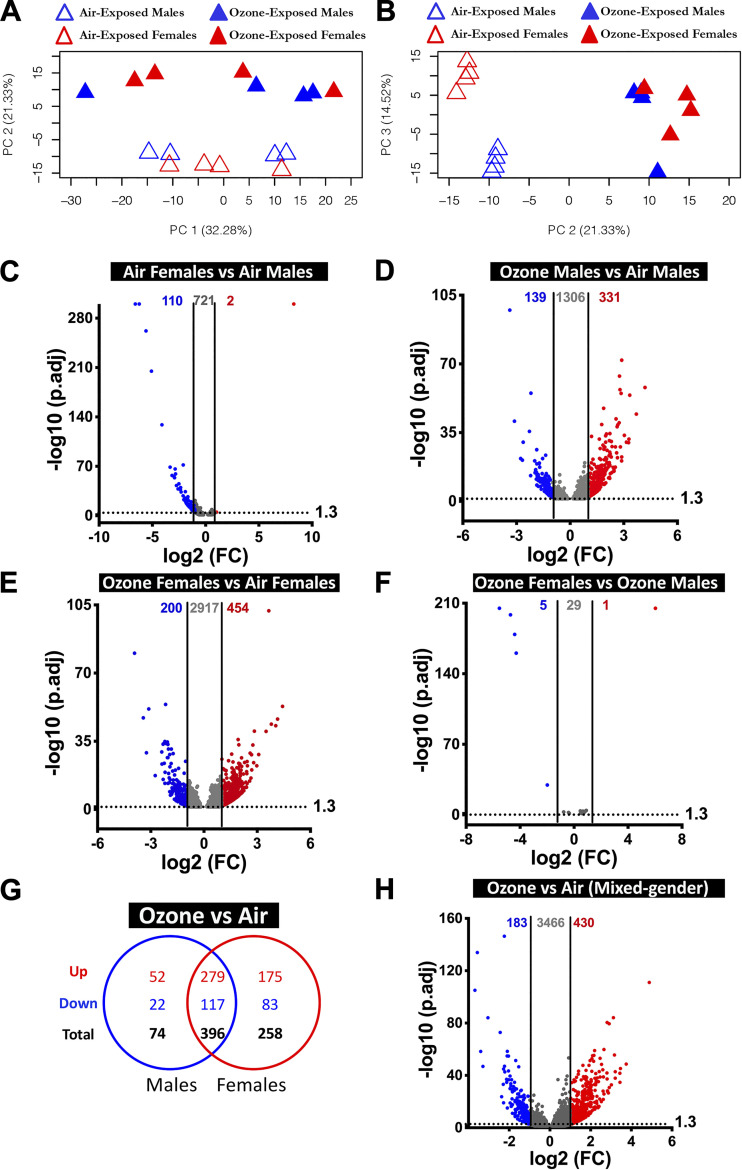
Figure 2.
Transcriptional responses in airway epithelial cell-enriched compartment. A: two-dimensional principal component (PC) analysis plot using PC1 and PC2 on all the detected genes (after normalization) in air- and ozone-exposed airways. B: two-dimensional PC analysis plot using PC2 and PC3 on all the detected genes (after normalization) in air- and ozone-exposed airways. C–F: volcano plots depicting differentially expressed genes (DEGs; upregulated and downregulated) in four different comparisons that were identified using cutoff criteria (Log2 Fold change>1, adjusted P < 0.05). C: air-exposed females vs. air-exposed males (DEGs = 112). D: ozone-exposed males vs. air-exposed males (DEGs = 470). E: ozone-exposed females vs. air-exposed females (DEGs = 654). F: ozone-exposed females vs. ozone-exposed males (DEGs = 6). (n = 4 per sex per treatment). G: Venn diagram depicting common and unique DEGs (upregulated and downregulated) in ozone-exposed males vs. air-exposed males and ozone-exposed females vs. air-exposed females. H: volcano plots depicting DEGs (upregulated and downregulated) in airways from ozone-exposed mice vs. air-exposed mice. DEG, differentially expressed genes.
To assess sex-driven gene expression differences in airway transcriptome in homeostasis, we compared gene expression data from air-exposed females with air-exposed males. Using stringent cutoff criteria [Supplemental Table S2, (fold change > 2; adjusted P value < 0.05)], only 112 genes (upregulated, 2; downregulated, 110) were differentially expressed (DEGs) (Fig. 2C). However, on relaxing the cutoff criteria (fold change>1; adjusted P value < 0.05), 833 DEGs were identified (upregulated, 168; downregulated, 665). A list of DEGs in air-exposed females versus air-exposed males is included in Table 1 (top 20 differentially upregulated and top 20 differentially downregulated.
Table 1.
Top 20 upregulated genes and top 20 downregulated genes in airways in designated comparisons
| Air |
Ozone |
Males |
Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females vs. Males |
Females vs. Males |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P-Value |
|
Top 20 upregulated genes | |||||||||||
| Xist | 311.48 | 0 | Xist | 65.38 | 0 | Psca | 18.22 | 1.09E−58 | Psca | 21.55 | 1.36E−53 |
| Oxtr | 2.04 | 7.66E−06 | Rpl23a | 1.88 | 4.85E−05 | Foxn4 | 13.04 | 3.56E−45 | Anxa10 | 17.75 | 3.83E−47 |
| Dusp5 | 1.84 | 9.85E−05 | Rtp4 | 1.78 | 0.00028167 | Krt13 | 10.09 | 7.58E−55 | Krt13 | 16.52 | 1.06E−43 |
| Nts | 1.81 | 7.69E−05 | Phf11d | 1.67 | 0.005181 | Cdc20b | 9.79 | 1.85E−32 | Serpinb2 | 13.87 | 1.44E−44 |
| Foxn4 | 1.80 | 0.00042413 | Ifit1 | 1.67 | 0.0074655 | Mcidas | 9.65 | 1.18E−30 | Upk3bl | 12.57 | 7.39E−103 |
| Bpifb5 | 1.77 | 0.00012054 | Usp18 | 1.65 | 0.005181 | Ccno | 8.86 | 7.13E−31 | Cwh43 | 11.32 | 8.29E−41 |
| Pnpla3 | 1.68 | 3.96E−05 | I830012O16Rik | 1.62 | 0.0091607 | Ccna1 | 8.19 | 2.45E−34 | Calcb | 8.50 | 7.03E−29 |
| Lctl | 1.60 | 0.0040662 | Oas2 | 1.61 | 0.020394 | Krt4 | 7.40 | 1.38E−72 | Vsig1 | 7.88 | 4.22E−23 |
| Adamts9 | 1.60 | 0.0043958 | Isg15 | 1.61 | 0.019942 | Anxa10 | 7.39 | 2.75E−23 | Krt4 | 7.17 | 6.86E−41 |
| Clca3 | 1.60 | 0.013739 | Ifi44 | 1.60 | 0.023295 | Sprr2a3 | 7.37 | 2.55E−21 | Ly6g6c | 6.96 | 1.08E−24 |
| Col6a5 | 1.59 | 4.72E−05 | Igkv8–30 | 1.59 | 0.017466 | Ly6g6c | 7.23 | 9.61E−56 | Serpinb10 | 6.75 | 8.55E−34 |
| Eci3 | 1.59 | 0.01583 | Oas1g | 1.59 | 0.023295 | Stil | 7.01 | 2.23E−28 | Sptssb | 6.47 | 4.63E−21 |
| Kdm6a | 1.56 | 1.72E−08 | Rsad2 | 1.59 | 0.031367 | Pbk | 6.91 | 1.33E−57 | Syt8 | 6.42 | 8.34E−29 |
| Mc2r | 1.56 | 0.024325 | Apol9a | 1.58 | 0.023295 | Upk3bl | 6.78 | 1.65E−64 | Cdc20b | 6.27 | 8.17E−17 |
| Aldh1a2 | 1.55 | 1.23E−07 | Ifit3 | 1.58 | 0.019704 | Cwh43 | 6.69 | 1.01E−40 | Pbk | 6.06 | 1.23E−29 |
| Kdm5c | 1.54 | 4.43E−08 | Kdm6a | 1.57 | 0.00023265 | Ccdc18 | 6.34 | 1.34E−39 | Muc13 | 5.95 | 2.53E−15 |
| Tpsab1 | 1.54 | 0.023899 | Atpif1 | 1.57 | 0.0024485 | Ccdc67 | 6.27 | 6.49E−23 | Atg9b | 5.45 | 3.92E−23 |
| C1qtnf3 | 1.53 | 0.0087593 | Dnm3os | 1.55 | 0.0024485 | Cdk1 | 6.20 | 1.12E−38 | Dsg3 | 5.40 | 5.31E−14 |
| Wt1 | 1.53 | 0.014613 | Rps29 | 1.54 | 0.0080438 | Serpinb2 | 6.07 | 1.98E−25 | Cdk1 | 5.39 | 2.49E−25 |
| Thbs1 | 1.51 | 0.045931 | Mx2 | 1.53 | 0.045486 | Spag5 | 5.92 | 1.05E−42 | Esco2 | 5.33 | 3.99E−19 |
|
Top 20 downregulated genes | |||||||||||
| Ddx3y | −96.90 | 0 | Ddx3y | −46.14 | 0 | Cdh26 | −10.37 | 4.22E−98 | Aoc1 | −15.11 | 6.74E−81 |
| Eif2s3y | −75.29 | 0 | Eif2s3y | −26.18 | 3.07E−199 | Aoc1 | −8.66 | 1.75E−41 | Cdh26 | −10.72 | 1.02E−47 |
| Kdm5d | −48.31 | 1.26E−262 | Uty | −21.15 | 8.88E−180 | Erich5 | −6.81 | 1.52E−22 | Slc35g3 | −9.50 | 8.15E−30 |
| Uty | −34.03 | 1.28E−205 | Kdm5d | −19.49 | 3.72E−161 | Slc35g3 | −6.36 | 9.07E−22 | Unc79 | −8.65 | 2.46E−52 |
| Slc26a7 | −17.13 | 1.61E−129 | RP24–540G19.3 | −3.96 | 2.69E−30 | Unc79 | −6.16 | 8.44E−31 | Erich5 | −6.72 | 3.75E−18 |
| Scg2 | −10.05 | 1.69E−69 | Sult1e1 | −1.70 | 0.0015006 | Gm281 | −4.84 | 2.70E−36 | Gm281 | −5.21 | 3.27E−30 |
| Calca | −8.88 | 8.80E−58 | Fbxo21 | −1.32 | 0.005181 | 4833428L15Rik | −4.63 | 7.20E−14 | Tbata | −5.16 | 5.42E−24 |
| Iyd | −7.85 | 2.81E−56 | Snd1 | −1.27 | 0.041251 | 4930562C15Rik | −4.58 | 7.35E−56 | Gm12695 | −4.95 | 3.17E−34 |
| Ano5 | −7.56 | 1.71E−54 | Ldlrad1 | −3.96 | 2.63E−21 | Gtsf1l | −4.93 | 4.11E−16 | |||
| Prlr | −7.23 | 6.26E−67 | Gm12695 | −3.90 | 3.24E−15 | BC007180 | −4.83 | 1.59E−34 | |||
| Slc26a10 | −7.06 | 4.99E−60 | BC007180 | −3.82 | 4.87E−16 | Wdr95 | −4.72 | 1.92E−24 | |||
| Folr1 | −6.64 | 1.74E−43 | Fam166b | −3.79 | 8.80E−17 | 2010001K21Rik | −4.61 | 3.96E−14 | |||
| RP24–540G19.3 | −5.98 | 1.01E−38 | 2010109I03Rik | −3.71 | 5.29E−12 | Ankrd65 | −4.60 | 1.14E−35 | |||
| Kcnh3 | −5.70 | 6.52E−46 | Erich3 | −3.70 | 1.56E−15 | Erich3 | −4.57 | 7.87E−20 | |||
| Prom2 | −5.15 | 8.38E−38 | Cdhr3 | −3.65 | 4.48E−27 | 4930562C15Rik | −4.47 | 1.38E−54 | |||
| Gjb1 | −5.09 | 1.07E−40 | Stmnd1 | −3.64 | 8.07E−20 | Stmnd1 | −4.36 | 1.47E−34 | |||
| Scg5 | −4.91 | 2.52E−34 | Gm20661 | −3.64 | 8.99E−15 | Ccdc33 | −4.18 | 2.51E−35 | |||
| Fxyd4 | −4.30 | 6.27E−29 | Pklr | −3.63 | 5.56E−09 | Cdhr3 | −4.17 | 2.35E−27 | |||
| Wisp1 | −4.28 | 9.53E−73 | Gtsf1l | −3.60 | 1.00E−13 | Ldlrad1 | −4.10 | 3.55E−34 | |||
| Gm12446 | −4.13 | 1.07E−23 | Wdr95 | −3.46 | 2.33E−14 | Ky | −4.10 | 2.75E−27 | |||
Next, to assess the effect of ozone exposure on gene expression in male airways, we compared gene expression data from ozone-exposed males with air-exposed males. We found that 331 and 139 DEGs were upregulated and downregulated, respectively, at stringent cutoff criteria (Fig. 2D). Upon relaxing the cutoff criteria, 1,776 (upregulated, 1,100; downregulated, 676) DEGs were identified (Supplemental Table S2). A list of DEGs in ozone-exposed males versus air-exposed males is included in Table 1 (top 20 differentially upregulated and top 20 differentially downregulated).
Next, to assess the effect of ozone exposure on gene expression in female airways, we compared gene expression data from ozone-exposed females with air-exposed females. On the basis of stringent cutoff criteria, 454 and 200 DEGs were upregulated and downregulated, respectively (Fig. 2E). On the basis of relaxed cutoff criteria, a total of 3,571 (upregulated, 1,953; downregulated, 1,618) DEGs were identified (Supplemental Table S2). A list of DEGs in ozone-exposed females versus air-exposed females is included in Table 1 (top 20 differentially upregulated and top 20 differentially downregulated).
Lastly, to assess differences in the ozone-induced gene expression that are contributed by sex status, we compared gene expression data from ozone-exposed females with ozone-exposed males. Only 1 and 5 DEGs were upregulated (Xist, located on the X-chromosome) and downregulated (all five genes located on Y-chromosome), respectively, at stringent cutoff criteria (Fig. 2F). Using relaxed cutoff criteria, 35 (upregulated, 27; downregulated, 8) DEGs were identified (Supplemental Table S2).
Comparison of significantly upregulated genes (fold change >2; adjusted P < 0.05) in airways from ozone-exposed males and ozone-exposed females identified 279 genes that were found common to both sexes (Fig. 2G). An additional 52 and 175 genes were upregulated exclusively in males and females, respectively (Fig. 2G). Similarly, a comparison of significantly downregulated genes (fold change > 2; adjusted P value < 0.05 in airways from ozone-exposed males and ozone-exposed females showed 117 genes that were found common to both sexes. Additional 22 and 83 genes were downregulated only in males and females, respectively (Fig. 2G). A list of sex-specific and common DEGs identified in ozone-exposed mice is presented in Table 2 and Supplemental Table S3. Airway transcriptome from ozone-exposed mice was enriched in gene signatures associated with responses, including epithelial remodeling, antibacterial defense, DNA replication, DNA repair, cell cycle and division, detoxification, cell-cell junction, protease inhibitory activity, and extracellular matrix rearrangement. (Supplemental Table S4A).
Table 2.
Top 20 upregulated and top 20 downregulated genes in airways of ozone-exposed mice (common and unique)
| Common in Both Sexes |
Unique to Males |
Unique to Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ozone vs. Air |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC (Males) | Adjusted P Value (Males) | FC (Females) | Adjusted P Value (Females) | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value |
|
Top 20 upregulated genes* | ||||||||||
| Psca | 18.22 | 1.1E−58 | 21.55 | 1.4E−53 | AI848285 | 5.32 | 5.5E−18 | Gsta1 | 5.23 | 3.42E−12 |
| Anxa10 | 7.39 | 2.8E−23 | 17.75 | 3.8E−47 | Cnfn | 4.65 | 2.0E−12 | Grhl3 | 4.65 | 6.97E−19 |
| Krt13 | 10.09 | 7.6E−55 | 16.52 | 1.1E−43 | Necab2 | 3.26 | 5.8E−08 | Prss32 | 3.92 | 6.28E−08 |
| Serpinb2 | 6.07 | 2.0E−25 | 13.87 | 1.4E−44 | Dpy19l2 | 2.93 | 1.0E−06 | Ano9 | 3.88 | 2.14E−24 |
| Upk3bl | 6.78 | 1.7E−64 | 12.57 | 7.4E−103 | Slc17a8 | 2.75 | 9.2E−06 | Tmprss11e | 3.75 | 1.87E−07 |
| Cwh43 | 6.69 | 1.0E−40 | 11.32 | 8.3E−41 | Rptoros | 2.65 | 8.8E−08 | Cryba2 | 3.71 | 2.54E−07 |
| Calcb | 2.82 | 7.9E−07 | 8.50 | 7.0E−29 | RP23–29D20.3 | 2.49 | 1.6E−04 | Gp2 | 3.67 | 3.79E−09 |
| Vsig1 | 3.86 | 7.4E−10 | 7.88 | 4.2E−23 | Ccne1 | 2.48 | 2.8E−07 | Gcnt3 | 3.67 | 3.38E−07 |
| Krt4 | 7.40 | 1.4E−72 | 7.17 | 6.9E−41 | Smim24 | 2.43 | 2.1E−07 | Sprr1a | 3.46 | 0.0000012 |
| Ly6g6c | 7.23 | 9.6E−56 | 6.96 | 1.1E−24 | Dnah8 | 2.42 | 4.1E−05 | Ifi202b | 3.44 | 0.00000013 |
| Serpinb10 | 3.14 | 6.5E−12 | 6.75 | 8.6E−34 | Ncan | 2.41 | 2.5E−05 | Gjb4 | 3.38 | 1.39E−07 |
| Sptssb | 4.58 | 1.9E−20 | 6.47 | 4.6E−21 | Ldhc | 2.36 | 3.1E−04 | Il1rn | 3.22 | 2.84E−12 |
| Syt8 | 3.74 | 4.2E−16 | 6.42 | 8.3E−29 | Sfn | 2.35 | 2.7E−12 | RP23–362B7.1 | 3.12 | 0.0000125 |
| Cdc20b | 9.79 | 1.9E−32 | 6.27 | 8.2E−17 | Efcab11 | 2.30 | 1.6E−06 | 1100001G20Rik | 3.05 | 4.88E−12 |
| Pbk | 6.91 | 1.3E−57 | 6.06 | 1.2E−29 | Mdm1 | 2.30 | 7.7E−07 | Diap3 | 3.02 | 8.5E−20 |
| Muc13 | 5.49 | 4.6E−16 | 5.95 | 2.5E−15 | Incenp | 2.30 | 2.6E−10 | B3galt5 | 3.01 | 0.0000089 |
| Atg9b | 3.59 | 1.8E−14 | 5.45 | 3.9E−23 | Stmn1 | 2.29 | 8.3E−34 | Aifm3 | 3.00 | 5.88E−10 |
| Dsg3 | 2.27 | 2.3E−04 | 5.40 | 5.3E−14 | Pla2g10 | 2.29 | 4.9E−04 | Foxe1 | 2.89 | 0.0000595 |
| Cdk1 | 6.20 | 1.1E−38 | 5.39 | 2.5E−25 | Adam5 | 2.29 | 1.7E−04 | Slc9a4 | 2.88 | 2.59E−08 |
| Esco2 | 4.43 | 1.3E−19 | 5.33 | 4.0E−19 | Gsdmc3 | 2.26 | 5.0E−04 | Sprr2a2 | 2.87 | 0.0000452 |
|
Top 20 downregulated genes* | ||||||||||
| Cdh26 | −10.37 | 4.2E−98 | −10.72 | 1.0E−47 | Ccl28 | −2.64 | 2.2E−09 | A930001A20Rik | –3.179 | 0.00000582 |
| Aoc1 | –8.66 | 1.8E−41 | –15.11 | 6.7E−81 | Wisp1 | –2.64 | 1.2E−13 | Gm13187 | –2.978 | 0.0000314 |
| Erich5 | –6.81 | 1.5E−22 | –6.72 | 3.8E−18 | Slc5a8 | –2.55 | 1.7E−07 | D130043K22Rik | –2.749 | 4.87E−12 |
| Slc35g3 | –6.36 | 9.1E−22 | –9.50 | 8.2E−30 | Gm14964 | –2.47 | 2.9E−05 | Pcp4l1 | –2.686 | 2.22E−12 |
| Unc79 | –6.16 | 8.4E−31 | –8.65 | 2.5E−52 | S100a9 | –2.40 | 9.6E−05 | Prr18 | –2.665 | 2.02E−10 |
| Gm281 | –4.84 | 2.7E−36 | –5.21 | 3.3E−30 | 8430408G22Rik | –2.29 | 8.2E−08 | Ccdc81 | –2.559 | 0.00000483 |
| 4833428L15Rik | −4.63 | 7.2E−14 | –3.81 | 7.3E−10 | Slc14a2 | –2.23 | 1.3E−03 | Dpp6 | –2.529 | 0.0000439 |
| 4930562C15Rik | −4.58 | 7.4E−56 | –4.47 | 1.4E−54 | P2rx6 | –2.23 | 1.7E−12 | Col6a5 | –2.509 | 6.24E−12 |
| Ldlrad1 | –3.96 | 2.6E−21 | –4.10 | 3.6E−34 | 1700007G11Rik | –2.23 | 5.9E−08 | Ttll8 | –2.431 | 0.000044 |
| Gm12695 | –3.90 | 3.2E−15 | –4.95 | 3.2E−34 | S100a8 | –2.19 | 7.6E−04 | Gm867 | –2.425 | 1.47E−10 |
| BC007180 | –3.82 | 4.9E−16 | –4.83 | 1.6E−34 | Rec8 | –2.16 | 3.9E−04 | Ccdc135 | –2.417 | 0.0000911 |
| Fam166b | –3.79 | 8.8E−17 | –3.81 | 7.0E−29 | A330033J07Rik | –2.15 | 7.5E−04 | Kcna1 | –2.411 | 0.00000047 |
| 2010109I03Rik | –3.71 | 5.3E−12 | –2.76 | 2.5E−05 | Snx31 | –2.15 | 9.4E−05 | Proc | –2.393 | 0.00000422 |
| Erich3 | –3.70 | 1.6E−15 | –4.57 | 7.9E−20 | Slfn4 | –2.13 | 1.8E−03 | Pmp2 | –2.391 | 0.0014621 |
| Cdhr3 | –3.65 | 4.5E−27 | –4.17 | 2.4E−27 | Aard | –2.11 | 7.6E−06 | Gm1965 | –2.367 | 0.0012316 |
| Stmnd1 | –3.64 | 8.1E−20 | –4.36 | 1.5E−34 | Krt9 | –2.10 | 3.0E−03 | Dnah11 | –2.363 | 1.69E−08 |
| Gm20661 | –3.64 | 9.0E−15 | –3.58 | 1.0E−31 | Nr1d1 | –2.08 | 3.4E−06 | 1810020O05Rik | –2.361 | 0.00035387 |
| Pklr | –3.63 | 5.6E−09 | –3.54 | 1.7E−08 | Mup3 | –2.07 | 4.6E−03 | Odf3b | –2.344 | 0.0000089 |
| Gtsf1l | –3.60 | 1.0E−13 | –4.93 | 4.1E−16 | Lhb | –2.06 | 2.7E−06 | Unc13c | –2.335 | 0.00024664 |
| Wdr95 | –3.46 | 2.3E−14 | –4.72 | 1.9E−24 | Cyp2a5 | –2.02 | 1.3E−05 | Adgb | –2.297 | 9.95E−08 |
A complete list of differentially expressed genes is included in Supplemental Table S3.
Ozone exposure results in the enrichment of biological pathways involved in cell division and DNA repair in extrapulmonary airways.
To determine whether the observed ozone-induced changes in the airway transcriptome contribute to significant changes in biological pathways, we performed pathway analyses using three different approaches for biological pathway analyses, including ingenuity pathway (IP) analysis (32), gene ontology (GO) analysis (2, 66), and STRING protein-protein interaction network analysis (61).
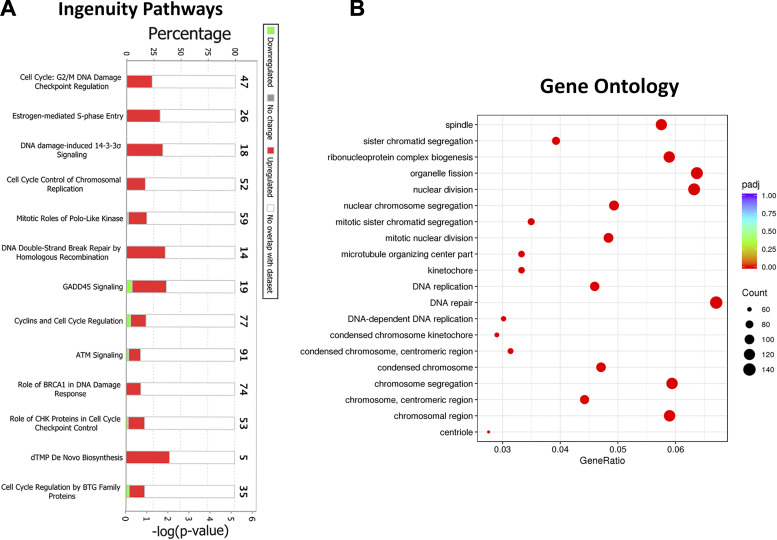
IP analysis identifies molecular and functional pathways in which DEGs are significantly involved and enriched. To determine associations between DEGs and canonical pathways, we analyzed DEGs [613 genes (430 (upregulated); 183 (downregulated)); fold change (FC) >2; adjusted P value < 0.05] in airway transcriptome from ozone-exposed versus air-exposed mice (Fig. 2H). Our analyses identified upregulation of pathways involved in the cell cycle, including G2/M DNA damage checkpoint, DNA damage-induced signaling, mitotic roles of polo-like kinase, and GADD45 signaling, (Fig. 3A).
Figure 3.
Biological pathway analyses on differentially expressed genes in airway transcriptome from ozone-exposed mice. A: stacked bar graph depicting most enriched biological pathways identified using ingenuity pathway (IP) analysis approach. B: dot plot showing enrichment of gene ontology biological processes, including biological processes, cell component, and molecular function. ATM, ataxia-telangiectasiamutated; BRCA1, breast cancer type 1 susceptibility protein; BTG, B cell translocation gene; CHK, checkpoint kinase; dTMP, deoxythymidine monophosphate.
Next, we performed gene ontology (GO) analysis, which identifies enriched pathways in the context of molecular functions (MF), cellular components (CC), and biological processes (BP). Our analyses revealed enrichment of CC associated with cell division, such as spindle, kinetochore, chromosomal region, centriole, and BP, such as chromosomal segregation, ribonucleoprotein biogenesis, organelle fission, nuclear division, DNA replication, and DNA repair (Fig. 3B). The enrichment scores for the MF category were not high enough for the top 20 ranks.
Finally, we performed protein-protein interaction network enrichment analyses using the STRING database (61). The STRING (https://string-db.org; version 11.0) maintains a database of known and predicted protein-protein interaction (PPI) networks. We interrogated the list of differentially upregulated genes (430 genes) in airways from ozone-exposed mice versus air-exposed mice for their contribution toward the enrichment of PPI networks. The enriched PPI networks in the airways from ozone-exposed mice versus air-exposed mice included cell cycle, cell division, and DNA repair (Supplemental Fig. S2A). In addition, a PPI network associated with keratinization was also enriched.
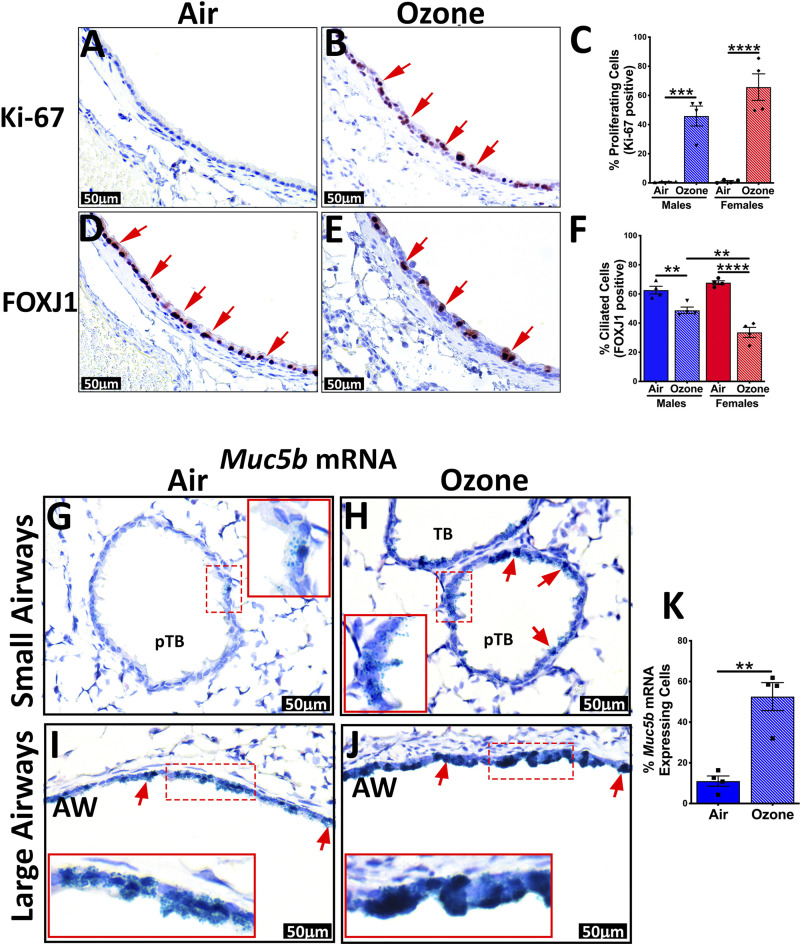
These data are consistent with the widespread staining of airway epithelial cells for Ki-67, a cellular proliferation marker, in ozone-exposed mice (Fig. 4, A–C). The airway epithelium had a significant loss of ciliated cells, as indicated by FOXJ1 staining, a cell-specific marker for ciliated cells (Fig. 4, D–F).
Figure 4.
Immunohistochemical and in situ RNAscope staining of airways for epithelial remodeling-associated changes. Representative photomicrographs of Ki-67 stained lung sections from air-exposed (A) and ozone-exposed mice (B). C: percentage of Ki-67 stained cells in the first-generation airways from air- and ozone-exposed mice. Representative photomicrographs of FOXJ1-stained lung sections from air-exposed (D) and ozone-exposed mice (E). F: percentage of FOXJ1-stained cells in the first-generation airways from air- and ozone-exposed mice. Error bars represent means ± SE **P < 0.01, ***P < 0.001, ****P < 0.0001 using one-way ANOVA followed by the Tukey multiple-comparison post hoc test. (n = 4 per group). G–J: mRNA expression of Muc5b in airways of air-exposed and ozone-exposed mice was detected by RNAscope assay. Representative photomicrographs depicting Muc5b mRNA signal (green dots, as well as green-stained cells, red arrows) air-exposed (G; small airways, I; large airways) and ozone-exposed mice (H; small airways, J; large airways). Inset outlined with red solid box is the higher magnification of what is depicted in the red dashed box. K: percentage of Muc5b mRNA-expressing cells in the smaller airways from air- and ozone-exposed mice. AW, 1st generation airway; pTB, preterminal bronchiole; TB, terminal bronchiole.
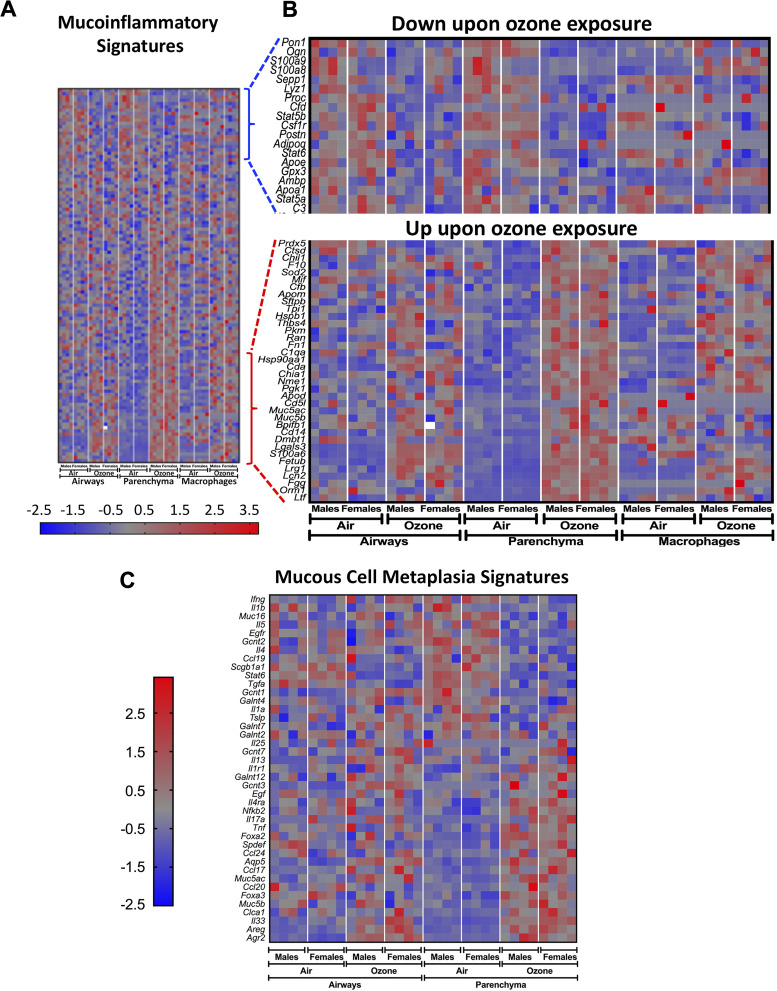
The reduction in the proportion of ciliated cells was more pronounced in ozone-exposed female mice versus ozone-exposed male mice (Fig. 4, D–F). In situ localization for Muc5b mRNA revealed that while the smaller airways from air-exposed mice had occasional presence of Muc5b mRNA expressing mucous secretory cells (Fig. 4G), the smaller airways from ozone-exposed mice showed widespread presence of Muc5b mRNA-expressing mucous secretory cells (Fig. 4H). Further, although the Muc5b mRNA-expressing mucous secretory cells were frequently present in the large airways from air-exposed mice (Fig. 4I), the signal intensity of Muc5b mRNA staining was remarkably higher in the large airways from ozone-exposed mice (Fig. 4J). Analyses for Muc5b mRNA expressing cells in the smaller airways revealed significantly more frequent cells in the ozone-exposed mice (Fig. 4K). These data suggest that subchronic ozone causes cellular injury in the airway epithelial cells, and adaptive responses such as cell division, proliferation, and DNA repair scramble to restore the normal structure and function of airways. Further, in this process, while the goblet cells within the larger airways start producing more mucins; the smaller airways undergo changes consistent with mucous cell metaplasia.
Ozone exposure perturbs gene expression patterns in the parenchyma.
Parenchyma is a heterogeneous mixture of a variety of cell types, including alveolar epithelial cells, immune cells, endothelial cells, smooth muscle cells, and fibroblasts, with the majority of cells encountering ozone or ozonized products being the alveolar epithelial cells. Accordingly, we hypothesized that gene signatures relevant to the functioning of the constituent cells in alveoli will be differentially regulated in ozone-exposed mice. To identify these signatures, 1-mm wide parenchymal sections closer to the edges of the lung lobes were harvested and processed for RNA extraction (Supplemental Fig. S1, A and B). The RNA quality number (RQN) values for all the parenchyma samples were in the range of 7.9–9.3. RNA-Seq was performed on the 16 parenchyma samples (four samples/treatment/sex). On average, ∼57.9 and 56.3 million raw and mapped reads, respectively, were obtained per sample with ∼97.21% mapping rate (Supplemental Table S1).
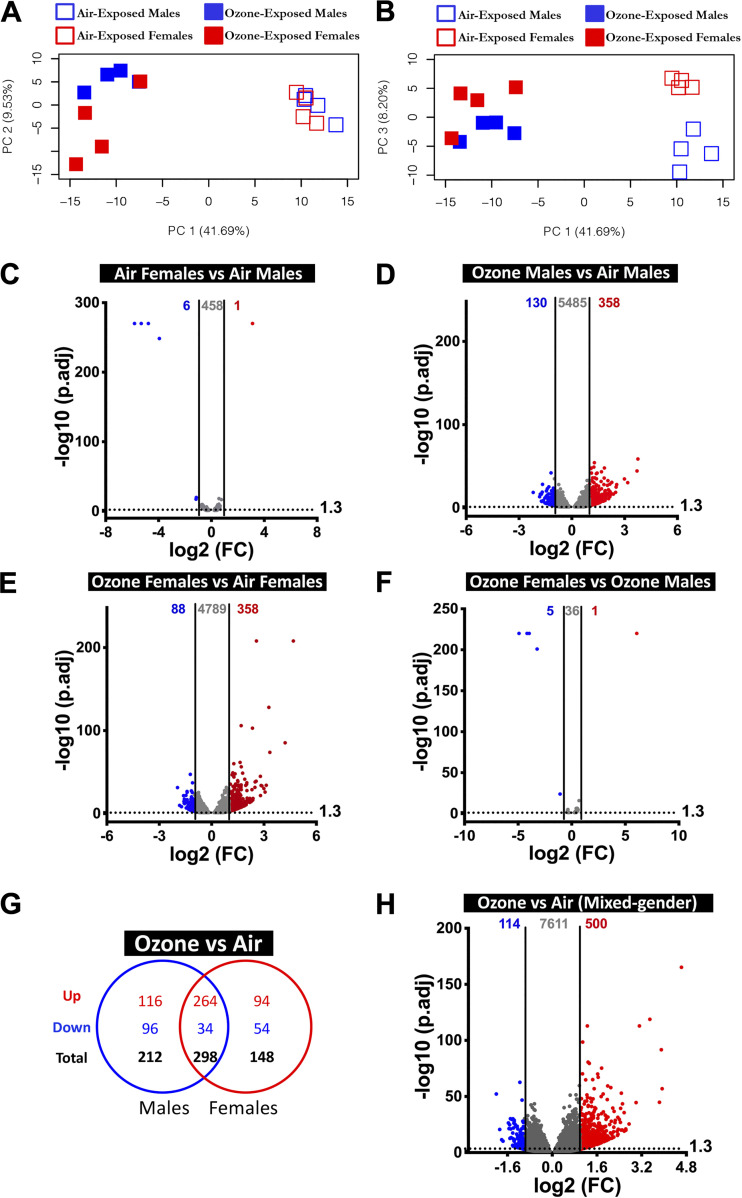
The PC analyses of parenchyma were performed to identify distinct separation between four experimental groups. Our analyses using the top three PCs, equivalent to ∼60% variance, revealed that treatment and sex were the primary drivers of global gene expression variation. PC1 (41.69% of variance) separated air-exposed animals from ozone-exposed animals, PC3 (8.2% of variance) separated air-exposed males and air-exposed females (Fig. 5, A and B). None of the top three principal components completely separated ozone-exposed males and ozone-exposed females (Fig. 5, A and B).
Figure 5.
Transcriptional responses in the parenchyma. A: two-dimensional principal component (PC) analysis plot using PC1 and PC2 on all the detected genes (after normalization) in the parenchyma from air- and ozone-exposed mice. B: two-dimensional principal component (PC) analysis plot using PC1 and PC3 on all of the detected genes (after normalization) in the parenchyma from air- and ozone-exposed mice. C–F: volcano plots depicting differentially expressed genes (DEGs, upregulated and downregulated) in four different comparisons that were identified using cutoff criteria (Log2 Fold change > 2, adjusted P values < 0.05). C: air-exposed females vs. air-exposed males (DEGs = 7). D: ozone-exposed males vs. air-exposed males (DEGs = 488). E: ozone-exposed females vs. air-exposed females (DEGs = 446). F: ozone-exposed females vs. ozone-exposed males (DEGs = 6). (n = 4 per sex per treatment). G: Venn diagram depicting common and unique DEGs (upregulated and downregulated) in ozone-exposed males vs. air-exposed males and ozone-exposed females vs. air-exposed females. H: volcano plots depicting differentially expressed genes (DEGs; upregulated and downregulated) in the parenchyma from ozone-exposed mice vs. air-exposed mice.
To assess differences by sex in the gene expression in healthy mice, we first compared gene expression data from air-exposed females with air-exposed males. On the basis of the stringent cutoff criteria, only 7 DEGs (upregulated, 1; downregulated, 6) were identified, (Fig. 5C). Upon relaxing cutoff criteria, a total of 465 (upregulated, 165; downregulated, 300) DEGs were identified (Supplemental Table S2). A list of DEGs in air-exposed females versus air-exposed males is included in Table 3 (top 20 upregulated and top 20 downregulated).
Table 3.
Top 20 upregulated genes and top 20 downregulated genes in the parenchyma in designated comparisons
| Air |
Ozone |
Males |
Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females vs. Males |
Females vs. Males |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value |
|
Top 20 upregulated genes | |||||||||||
| Xist | 8.63 | 2.5E−138 | Xist | 67.38 | 0.00 | Ltf | 13.51 | 2E−59 | Retnla | 25.74 | 9E−209 |
| Mfap4 | 1.69 | 1.4E−17 | Kdm6a | 1.61 | 0.00 | Retnla | 13.03 | 4E−45 | Saa3 | 18.54 | 5E−86 |
| AI838599 | 1.58 | 1.4E−08 | Lrat | 1.51 | 0.00 | Orm1 | 9.06 | 5E−31 | S100a14 | 10.10 | 1E−74 |
| Gsg1l | 1.56 | 2.6E−05 | Wt1 | 1.46 | 0.00 | S100a14 | 7.94 | 2E−35 | Timp1 | 9.71 | 1E−128 |
| Kdm6a | 1.51 | 7.2E−09 | Gm14420 | 1.45 | 0.00 | Syt12 | 5.86 | 1E−28 | Ltf | 8.76 | 6E−35 |
| Cpa3 | 1.51 | 1.3E−04 | Eif2s3x | 1.42 | 0.00 | Timp1 | 5.72 | 8E−26 | Sprr1a | 8.34 | 1E−26 |
| Iqgap3 | 1.50 | 1.9E−04 | Pbdc1 | 1.42 | 0.00 | Phgr1 | 5.62 | 2E−19 | Chl1 | 7.98 | 6E−31 |
| Entpd8 | 1.50 | 3.5E−04 | RP23–32A8.1 | 1.41 | 0.00 | Chl1 | 5.15 | 3E−17 | Mcidas | 7.37 | 3E−34 |
| Eif2s3x | 1.49 | 6.2E−19 | 5530601H04Rik | 1.40 | 0.00 | Kng2 | 5.09 | 2E−17 | Phgr1 | 7.14 | 1E−34 |
| Cnr1 | 1.49 | 2.3E−04 | Gabra3 | 1.39 | 0.01 | Mfsd2a | 4.83 | 2E−31 | Gjb4 | 6.98 | 2E−22 |
| Rps29 | 1.48 | 5.3E−11 | Ifit1 | 1.37 | 0.00 | AU018091 | 4.81 | 2E−17 | Cldn4 | 6.95 | 1E−45 |
| Slc7a10 | 1.48 | 2.1E−08 | Ppp1r3a | 1.36 | 0.02 | Pbk | 4.55 | 1E−19 | Lrg1 | 5.94 | 9E−209 |
| Kdm5c | 1.47 | 1.4E−10 | Ddx3x | 1.34 | 0.00 | Atp10b | 4.48 | 3E−14 | Tgm1 | 5.86 | 8E−40 |
| Adamts17 | 1.47 | 2.7E−05 | Peg3 | 1.34 | 0.03 | AU040972 | 4.48 | 3E−29 | Mfsd2a | 5.62 | 7E−39 |
| Phex | 1.46 | 1.5E−05 | Ccl2 | 1.34 | 0.04 | Rasl10b | 4.46 | 1E−20 | Rasl10b | 5.60 | 2E−32 |
| Kcne4 | 1.45 | 3.0E−05 | Kdm5c | 1.34 | 0.00 | Rgs16 | 4.35 | 1E−16 | Fgg | 5.18 | 4E−19 |
| Gbp4 | 1.45 | 1.7E−04 | Entpd8 | 1.32 | 0.05 | Krtap17–1 | 4.35 | 1E−19 | Orm1 | 5.13 | 5E−17 |
| Ano5 | 1.43 | 2.4E−03 | Omd | 1.30 | 0.04 | Gjb3 | 4.31 | 2E−15 | Lcn2 | 5.06 | 7E−104 |
| Adam12 | 1.43 | 2.1E−03 | Pydc4 | 1.29 | 0.03 | Prss22 | 4.26 | 1E−11 | Slc26a4 | 4.96 | 1E−29 |
| Zmat4 | 1.42 | 2.8E−03 | Cftr | 1.28 | 0.05 | Dtl | 4.24 | 2E−22 | Pbp2 | 4.83 | 3E−15 |
|
Top 20 downregulated genes | |||||||||||
| Ddx3y | –56.61 | 0.0E+00 | Ddx3y | –46.14 | 0.00 | Ces1f | –4.57 | 2E−19 | Igfbp3 | –3.87 | 4E−32 |
| Eif2s3y | –39.79 | 0.0E+00 | Eif2s3y | –17.92 | 0.00 | Pon1 | –3.55 | 4E−14 | Ighv1–64 | –3.57 | 9E−11 |
| Kdm5d | –27.42 | 0.0E+00 | Kdm5d | –15.60 | 0.00 | Igfbp3 | –3.46 | 3E−17 | Ighv7–3 | –3.30 | 4E−09 |
| Uty | –15.35 | 2.9E−249 | Uty | –9.40 | 0.00 | Ifitm6 | –3.31 | 3E−17 | Gzmb | –3.05 | 2E−22 |
| Serpina3m | –2.24 | 4.7E−18 | RP24–540G19.3 | –2.12 | 0.00 | Tbata | –3.31 | 8E−09 | Colq | –2.99 | 1E−15 |
| RP24–540G19.3 | –2.21 | 1.7E−20 | Camk2b | –1.37 | 0.01 | Asgr1 | –3.26 | 2E−08 | Klre1 | –2.98 | 2E−13 |
| Mefv | –1.81 | 2.5E−09 | Arhgdig | –1.34 | 0.00 | Gm14964 | –3.21 | 1E−19 | Ncr1 | –2.95 | 3E−18 |
| Pla2g7 | –1.79 | 1.4E−09 | Col23a1 | –1.29 | 0.00 | Fmo3 | –3.17 | 5E−29 | Gm14085 | –2.92 | 7E−13 |
| F5 | –1.70 | 6.3E−09 | Myh14 | –1.29 | 0.04 | RP23–458B6.6 | –3.07 | 3E−09 | Prf1 | –2.89 | 5E−14 |
| Ms4a4a | –1.70 | 2.6E−07 | Pitpnc1 | –1.28 | 0.00 | Sult1d1 | –3.06 | 1E−09 | Gzma | –2.77 | 8E−23 |
| RP23–458B6.6 | –1.69 | 2.8E−07 | Hnrnpa0 | –1.26 | 0.01 | Mefv | –2.89 | 1E−12 | Pon1 | –2.74 | 1E−07 |
| Atp1a3 | –1.68 | 2.6E−07 | Ighv1–64 | –1.26 | 0.05 | Slfn4 | –2.89 | 1E−06 | S1pr5 | –2.67 | 1E−16 |
| Ccr2 | –1.67 | 6.3E−10 | Ehd4 | –1.24 | 0.04 | Gfy | –2.86 | 1E−06 | Npr3 | –2.66 | 2E−27 |
| Wfdc17 | –1.67 | 5.0E−07 | Tnrc18 | –1.24 | 0.04 | Ngp | –2.79 | 3E−06 | Nkg7 | –2.64 | 2E−17 |
| Itgam | –1.67 | 8.6E−08 | H2-Eb1 | –1.24 | 0.02 | Slfn1 | –2.72 | 7E−15 | Ccl5 | –2.64 | 2E−26 |
| Gad1-ps | –1.65 | 2.0E−06 | Crispld2 | –1.20 | 0.03 | Sell | –2.72 | 4E−21 | Eomes | –2.58 | 2E−09 |
| Ttn | –1.64 | 2.6E−07 | Zc3h7b | –1.18 | 0.04 | Irg1 | –2.71 | 5E−06 | Gm14964 | –2.57 | 2E−13 |
| Asprv1 | –1.64 | 1.0E−06 | Emr4 | –2.67 | 5E−13 | Klra9 | –2.53 | 9E−11 | |||
| Fgr | –1.62 | 1.8E−10 | Trpm2 | –2.63 | 2E−12 | Klrc2 | –2.53 | 2E−09 | |||
| Cd300lf | –1.60 | 2.0E−06 | Galnt15 | –2.62 | 1E−23 | Gprasp2 | –2.53 | 1E−10 | |||
Next, to assess the effect of ozone exposure on parenchymal gene expression in males, we compared gene expression data from ozone-exposed males with air-exposed males. Although 358 and 130 DEGs were upregulated and downregulated, respectively, at stringent criteria (Fig. 5D), 5,995 (upregulated, 3,060; downregulated, 2,935) genes were differentially expressed in ozone-exposed males versus air-exposed males using relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in ozone-exposed males versus air-exposed males is included in Table 3 (top 20 upregulated and top 20 downregulated).
Next, to assess the effect of ozone exposure on gene expression in females, we compared gene expression data from ozone-exposed females with air-exposed females. 358 and 88 DEGs were upregulated and downregulated, respectively, at stringent cutoff criteria (Fig. 5E). A total of 5,235 (upregulated, 2,679; downregulated, 2,556) DEGs were identified on the basis of relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in ozone-exposed females versus air-exposed females is included in Table 3 (top 20 upregulated and top 20 downregulated).
To assess differences by sex in the gene expression in ozone-exposed mice, we compared gene expression data from ozone-exposed females with ozone-exposed males. Only 1 and 5 DEGs were upregulated (Xist, located on X-chromosome) and downregulated (all located on Y-chromosome), respectively, at stringent cutoff criteria (Fig. 5F). However, 42 (upregulated, 25; downregulated, 17) DEGs were identified on the basis of relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in ozone-exposed females versus ozone-exposed males is included in Table 3 (top 20 upregulated and top 20 downregulated).
Comparison of significantly upregulated genes (fold change >2; adjusted P value < 0.05) in the parenchyma from ozone-exposed males and females identified 264 genes that were found common to both sexes (Fig. 5G). An additional 116 and 94 genes were upregulated exclusively in males and females, respectively (Fig. 5G) Similarly, comparison of significantly downregulated genes (fold change > 2; adjusted P value < 0.05) in ozone-exposed males and females identified 34 genes that were found common to both the sexes. An additional 96 and 54 genes were downregulated only in males and females, respectively (Fig. 5G). A list of common and sex-specific DEGs identified in ozone-exposed mice is presented in Table 4 and Supplemental Table S5. Parenchyma transcriptome from ozone-exposed mice was enriched in gene signatures associated with epithelial remodeling, antibacterial defense, DNA replication, DNA repair, cell cycle and division, detoxification, cell-cell junction, protease inhibitory activity, lectin-binding activity, acute phase proteins, extracellular matrix rearrangement, and inflammatory responses (Supplemental Table S4B).
Table 4.
Top 20 upregulated and top 20 downregulated genes in the parenchyma of ozone-exposed mice (common and unique)
| Common in Both Sexes |
Unique to Males |
Unique to Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ozone vs. Air |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC (Males) | Adjusted P Value (Males) | FC (Females) | Adjusted P Value (Females) | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value |
|
Top 20 upregulated genes* | ||||||||||
| Retnla | 13.03 | 4.0E−45 | 25.74 | 9.4E−209 | Kng2 | 5.09 | 1.83E−17 | Saa3 | 18.54 | 4.88E−86 |
| S100a14 | 7.94 | 1.7E−35 | 10.10 | 1.2E−74 | Ccna1 | 3.68 | 4.42E−10 | Mcidas | 7.37 | 3.16E−34 |
| Timp1 | 5.72 | 7.9E−26 | 9.71 | 9.7E−129 | Klk10 | 3.68 | 5.83E−10 | Syt8 | 4.56 | 2.39E−14 |
| Ltf | 13.51 | 1.7E−59 | 8.76 | 6.5E−35 | Nrcam | 3.22 | 4.36E−44 | Cdc20b | 3.33 | 1.77E−10 |
| Sprr1a | 3.34 | 9.6E−09 | 8.34 | 1.1E−26 | Cdca5 | 3.12 | 1.13E−11 | Fga | 3.20 | 2.49E−08 |
| Chl1 | 5.15 | 2.6E−17 | 7.98 | 6.0E−31 | Exo1 | 3.04 | 1.85E−09 | Serpina3m | 3.10 | 4.75E−08 |
| Phgr1 | 5.62 | 1.9E−19 | 7.14 | 1.2E−34 | Pgc | 3.03 | 1.11E−08 | Wfdc17 | 3.05 | 8.83E−20 |
| Gjb4 | 2.61 | 6.4E−06 | 6.98 | 1.7E−22 | Kifc1 | 2.89 | 3.47E−11 | Vcan | 2.81 | 1.6E−40 |
| Cldn4 | 3.44 | 2.3E−09 | 6.95 | 1.1E−45 | Krt4 | 2.89 | 0.00000113 | Cxcl1 | 2.79 | 5.07E−08 |
| Lrg1 | 2.44 | 2.7E−06 | 5.94 | 9.4E−209 | Ccnf | 2.88 | 1.32E−16 | Msr1 | 2.75 | 8.75E−12 |
| Tgm1 | 3.29 | 1.0E−09 | 5.86 | 7.8E−40 | Eme1 | 2.82 | 1.99E−07 | Psca | 2.68 | 1.05E−06 |
| Mfsd2a | 4.83 | 2.0E−31 | 5.62 | 6.9E−39 | Tubb3 | 2.69 | 1.98E−07 | Shcbp1 | 2.65 | 1.48E−10 |
| Rasl10b | 4.46 | 1.2E−20 | 5.60 | 2.0E−32 | Adam12 | 2.68 | 7.03E−11 | Cd14 | 2.64 | 1.06E−18 |
| Fgg | 3.05 | 2.0E−07 | 5.18 | 3.9E−19 | Mxd3 | 2.65 | 2.98E−07 | Syt2 | 2.63 | 6.08E−09 |
| Orm1 | 9.06 | 4.8E−31 | 5.13 | 5.2E−17 | RP24–503F14.1 | 2.63 | 0.00000526 | 1100001G20Rik | 2.63 | 7.45E−14 |
| cn2 | 3.17 | 1.8E−32 | 5.06 | 7.5E−104 | Fam25c | 2.62 | 0.0000103 | AA467197 | 2.61 | 4.6E−10 |
| Slc26a4 | 3.84 | 4.3E−12 | 4.96 | 1.2E−29 | Ces1g | 2.62 | 3.7E−11 | Hdc | 2.54 | 9.02E−49 |
| Pbp2 | 2.85 | 1.3E−06 | 4.83 | 2.8E−15 | Slfn9 | 2.60 | 8.62E−13 | Vsig4 | 2.53 | 1.39E−05 |
| Gjb3 | 4.31 | 1.7E−15 | 4.57 | 4.2E−19 | E2f7 | 2.55 | 3.12E−08 | Dlk2 | 2.51 | 1.38E−05 |
| Ccl2 | 3.30 | 1.1E−10 | 4.35 | 2.6E−16 | Pask | 2.55 | 1.24E−10 | Arg1 | 2.49 | 4.66E−10 |
|
Top 20 downregulated genes* | ||||||||||
| Igfbp3 | –3.46 | 3.0E−17 | –3.87 | 4.4E−32 | Ces1f | –4.57 | 2.31E−19 | Ighv1–64 | –3.57 | 9.03E−11 |
| Gzmb | –2.24 | 2.3E−10 | –3.05 | 1.9E−22 | Ifitm6 | –3.31 | 2.87E−17 | Ighv7–3 | –3.30 | 3.68E−09 |
| Klre1 | –2.53 | 6.7E−11 | –2.98 | 2.0E−13 | Tbata | –3.31 | 8.17E−09 | Colq | –2.99 | 1.43E−15 |
| Ncr1 | –2.42 | 1.2E−12 | –2.95 | 3.2E−18 | RP23–458B6.6 | –3.07 | 2.82E−09 | Eomes | –2.58 | 2.16E−09 |
| Gm14085 | –2.45 | 2.3E−07 | –2.92 | 7.3E−13 | Sult1d1 | –3.06 | 1.06E−09 | Gprasp2 | –2.53 | 1.42E−10 |
| Prf1 | –2.61 | 2.8E−16 | –2.89 | 4.9E−14 | Mefv | –2.89 | 1E−12 | Cma1 | –2.49 | 7.49E−06 |
| Gzma | –2.19 | 5.2E−12 | –2.77 | 7.6E−23 | Slfn4 | –2.89 | 0.00000112 | Wnt10b | –2.48 | 1.03E−13 |
| Pon1 | –3.55 | 3.6E−14 | –2.74 | 1.4E−07 | Gfy | –2.86 | 0.00000134 | Gm16485 | –2.35 | 3.49E−06 |
| S1pr5 | –2.41 | 4.8E−13 | –2.67 | 1.0E−16 | Ngp | –2.79 | 0.00000283 | Igkv4–57–1 | –2.34 | 7.13E−05 |
| Npr3 | –2.35 | 4.4E−05 | –2.66 | 1.9E−27 | Slfn1 | –2.72 | 7.16E−15 | Klra7 | –2.33 | 1.39E−07 |
| Nkg7 | –2.30 | 1.6E−11 | –2.64 | 1.5E−17 | Sell | –2.72 | 3.64E−21 | Gbp4 | –2.32 | 3.77E−48 |
| Ccl5 | –2.20 | 1.9E−09 | –2.64 | 1.7E−26 | Irg1 | –2.71 | 0.0000046 | Klrb1a | –2.31 | 0.000064 |
| Gm14964 | –3.21 | 1.1E−19 | –2.57 | 1.5E−13 | Emr4 | –2.67 | 4.59E−13 | Ighv1–76 | –2.25 | 0.000178 |
| Klra9 | –2.28 | 1.6E−07 | –2.53 | 8.7E−11 | Trpm2 | –2.63 | 2.28E−12 | Chst8 | –2.25 | 3.74E−07 |
| Klrc2 | –2.51 | 1.8E−12 | –2.53 | 2.3E−09 | Galnt15 | –2.62 | 1.07E−23 | Ecm2 | –2.23 | 4.24E−28 |
| Siglech | –2.59 | 8.6E−08 | –2.52 | 4.6E−07 | Fam71f2 | –2.53 | 7.5E−10 | Slc4a1 | –2.23 | 0.000112 |
| Klrb1c | –2.48 | 1.5E−17 | –2.42 | 3.1E−11 | Cd300e | –2.52 | 1.18E−09 | Pcolce2 | –2.22 | 5.14E−21 |
| Acaa1b | –2.53 | 1.4E−12 | –2.41 | 1.7E−14 | Ms4a4a | –2.48 | 2.07E−09 | Tbx21 | –2.21 | 1.96E−08 |
| Itgad | –2.02 | 7.5E−04 | –2.39 | 2.5E−06 | Ifi204 | –2.48 | 9.07E−11 | Hbb-bt | –2.21 | 3.19E−10 |
| Klri2 | –2.10 | 4.0E−07 | –2.35 | 2.4E−08 | Pyhin1 | –2.47 | 5.01E−26 | Ces2e | –2.19 | 9.41E−05 |
A complete list of differentially expressed genes is included in Supplemental Table S5.
Ozone exposure results in the enrichment of pathways involved in cell division, DNA repair, and immune responses in the parenchyma.
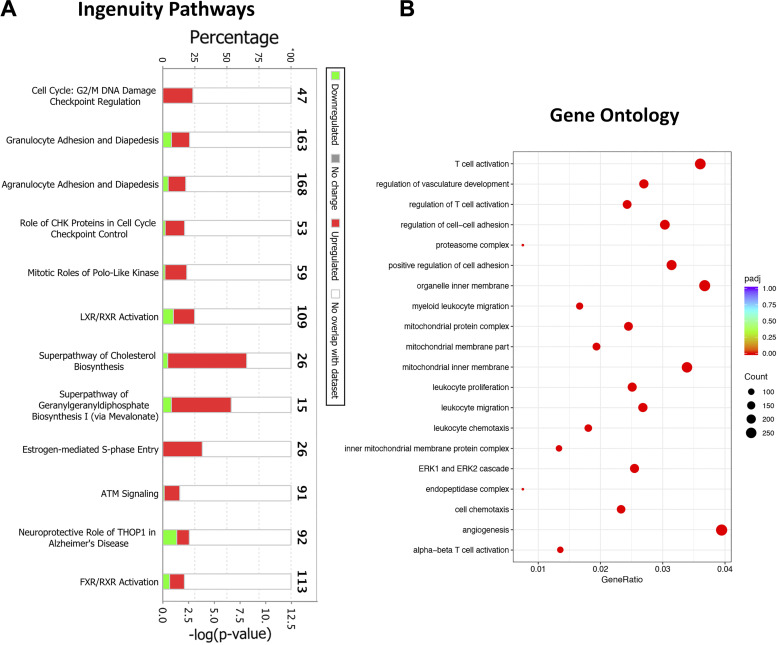
We hypothesized that differentially expressed genes in parenchyma will reflect enrichment of pathways relevant to the constituent cells in the alveoli. Accordingly, we analyzed DEGs in the parenchyma from ozone-exposed mice versus air-exposed mice by performing IP analyses on combined (both sexes) DEGs [614 genes (500, upregulated; 114, downregulated); FC > 2; adjusted P value < 0.05] (Fig. 5H).
As seen in the airways, the IPA on parenchyma also revealed enrichment of canonical pathways related to cell cycle and DNA repair, i.e., G2/M DNA damage checkpoint regulation and mitotic roles of Polo-like kinase (Fig. 6A). Interestingly, GO analyses did not identify the highest ranked enrichment of cell cycle and DNA repair pathways (Fig. 6B). Consistent with IPA, the STRING analyses identified networks relevant to PPI in the cell cycle, cell division, and DNA repair (Supplemental Fig. S2B).
Figure 6.
Biological pathway analyses on differentially expressed genes in the parenchyma from ozone-exposed mice. A: stacked bar graph depicting enrichment of biological pathways identified using the ingenuity pathway (IP) analysis approach. B: dot plot showing enrichment of gene ontology biological processes, including biological processes, cell component, and molecular function. ATM, ataxia-telangiectasiamutated; CHK, checkpoint kinase; FXR/RXR, farnesoid X receptor/retinoid X receptor; LXR/RXR, liver X receptor/retinoid X receptor; THOP1, thimet oligopeptidase 1.
In addition to the pathways seen in the airways, the IP, GO, and STRING analyses revealed enrichment of pathways relevant to immune responses. The IP analyses identified enrichment of immune pathways, such as granulocyte adhesion and diapedesis, LXR/RXR activation, and FXR/RXR activation in the parenchyma (Fig. 6A). The IP analyses also revealed enrichment of canonical pathways related to cholesterol and geranylgeranyl diphosphate biosynthesis. The GO analyses revealed enrichment of immune pathways such as T-cell activation, myeloid migration, leukocyte proliferation, and leukocyte chemotaxis (Fig. 6B).
Ozone exposure perturbs gene expression patterns in the purified macrophages.
While BALF from air-exposed mice contains macrophages predominantly, the BALF from ozone-exposed mice also contains additional immune cell populations, including neutrophils and eosinophils. To deplete neutrophil and eosinophil populations, we used a magnetic activated cell sorting (MACS) approach (55) to perform CDl1b microbead-mediated depletion of granulocytes (Supplemental Fig. S1C). CD11b+ population was enriched in granulocytes (neutrophils and eosinophils), and CD11bneg population was enriched in macrophage/DC-like cells (Supplemental Fig. S1, D and E). CD11bneg population used for RNA isolation had ∼97.5% purity (Supplemental Fig. 1, D and E). The RNA quality number (RQN) for purified macrophages were in the range of 7.5–9.4. RNA-Seq was performed on 16 macrophage samples (four samples/treatment/sex). On average, ∼54.8 and 53.3 million raw and mapped reads, respectively, were obtained per sample with ∼97.28% mapping rate (Supplemental Table S1).
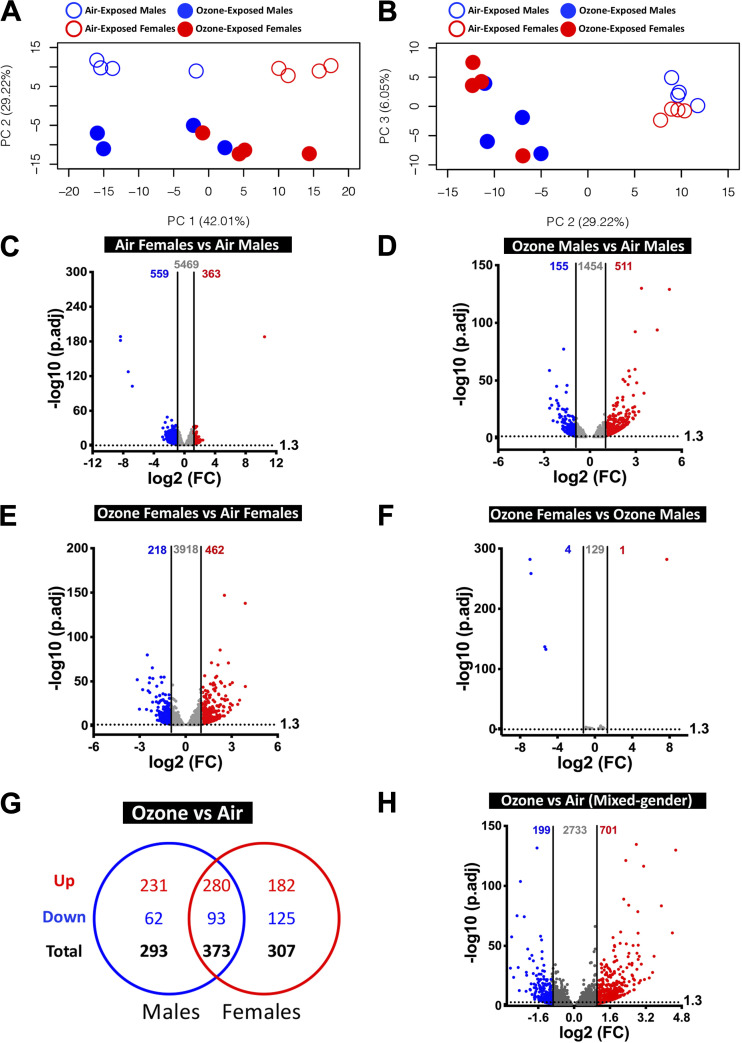
The PC analysis of macrophage transcriptome was performed to identify distinct separation between the four experimental groups. Similar to the airways and parenchyma, sex and treatment contributed to most of the ∼77% variance (from the top three PCs) in macrophage gene expression. While PC1, which accounts for 42.01% of the variance, separated air-exposed males from air-exposed females, PC2, which accounts for 29.22% of the variance, separated air-exposed animals and ozone-exposed animals. None of the top three principal components separated ozone-exposed males and ozone-exposed females (Fig. 7, A and B).
Figure 7.
Transcriptional responses in purified macrophages. A: two-dimensional principal component (PC) analysis plot using PC1 and PC2 on all the detected genes (after normalization) in macrophages from air- and ozone-exposed mice. B: two-dimensional PC analysis plot using PC2 and PC3 on all the detected genes (after normalization) in macrophages from air- and ozone-exposed mice. C–F: volcano plots depicting differentially expressed genes (DEGs, upregulated and downregulated) in four different comparisons that were identified using cutoff criteria (Log2 fold change > 2, adjusted P values < 0.05). C: air-exposed females vs. air-exposed males (DEGs = 922). D: ozone-exposed males vs. air-exposed males (DEGs = 666). E: ozone-exposed females vs. air-exposed females (DEGs = 680). F: ozone-exposed females vs. ozone-exposed males (DEGs = 5). (n = 4 per sex per treatment). G: Venn diagram depicting common and unique DEGs (upregulated and downregulated) in ozone-exposed males vs. air-exposed males and ozone-exposed females vs. air-exposed females. H: volcano plots depicting DEGs (upregulated and downregulated) in macrophages from ozone-exposed mice vs. air-exposed mice.
To assess sex-associated differences in gene expression under homeostasis, we compared gene expression data from air-exposed females with air-exposed males. 363 and 559 DEGs were upregulated and downregulated, respectively, at stringent cutoff (Fig. 7C). A total of 6,391 (upregulated, 3,062; downregulated, 3,329) DEGs were identified on the basis of relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in air-exposed females versus air-exposed males is included in Table 5 (top 20 upregulated and top 20 downregulated).
Table 5.
Top 20 upregulated genes and top 20 downregulated genes in macrophages in designated comparisons
| Air |
Ozone |
Males |
Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females vs. Males |
Females vs. Males |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value |
|
Top 20 upregulated genes | |||||||||||
| Xist | 1418.35 | 0.0E+00 | Xist | 207.53 | 0.00 | C4b | 36.59 | 1E−129 |
Lrg1 |
14.85 | 5E−45 |
| RP24–210C4.1 | 5.31 | 4.7E−10 | 2700099C18Rik | 1.96 | 0.02 | Lrg1 | 21.10 | 2E−94 |
Mafb |
14.82 | 8E−139 |
| 9430025C20Rik | 4.19 | 5.4E−11 | 1190002F15Rik | 1.93 | 0.02 | Mafb | 11.60 | 9E−40 |
Arg1 |
11.47 | 1E−29 |
| Gimap3 | 4.16 | 3.2E−08 | Erdr1 | 1.93 | 0.01 | Msr1 | 10.32 | 1E−130 |
C4b |
10.53 | 7E−25 |
| Gm26772 | 4.08 | 2.8E−07 | Pif1 | 1.92 | 0.02 | C1qb | 9.15 | 8E−24 |
Wfdc17 |
8.26 | 3E−49 |
| Tagln3 | 3.98 | 6.0E−08 | Troap | 1.90 | 0.02 | Clu | 8.33 | 1E−48 |
Ocstamp |
8.21 | 6E−24 |
| Thy1 | 3.86 | 3.6E−07 | Arhgef39 | 1.89 | 0.02 | Spp1 | 7.78 | 5E−93 |
Awat1 |
7.57 | 1E−25 |
| Gm3235 | 3.82 | 4.3E−06 | Cenpw | 1.87 | 0.02 | C1qa | 7.77 | 1E−20 |
C1qb |
7.56 | 1E−18 |
| Slc7a3 | 3.70 | 1.8E−08 | Ndc80 | 1.87 | 0.02 | Anpep | 7.73 | 2E−60 |
Tnfsf14 |
7.42 | 3E−47 |
| Muc6 | 3.70 | 7.5E−07 | Cdkn3 | 1.86 | 0.03 | Fads2 | 7.59 | 5E−28 |
Ear6 |
7.29 | 2E−21 |
| S100a6 | 3.67 | 6.5E−16 | Cd209b | 1.85 | 0.03 | Cldn4 | 7.44 | 3E−23 |
Msr1 |
6.92 | 2E−71 |
| Gm26668 | 3.55 | 1.8E−05 | Cdkn2d | 1.83 | 0.00 | Sash1 | 7.15 | 6E−38 |
Atf7ip2 |
6.77 | 8E−16 |
| Ltb | 3.51 | 1.4E−08 | Ckap2 | 1.82 | 0.02 | F13a1 | 7.15 | 1E−17 |
Mmp14 |
6.61 | 3E−30 |
| Gm26762 | 3.34 | 1.3E−05 | Cenph | 1.82 | 0.03 | Ablim1 | 7.05 | 1E−23 |
Socs3 |
6.13 | 2E−34 |
| Gm11973 | 3.32 | 3.1E−07 | Stmn1 | 1.82 | 0.03 | C1qc | 6.98 | 3E−18 |
Sash1 |
5.94 | 3E−29 |
| S100a4 | 3.24 | 2.0E−06 | Tacc3 | 1.81 | 0.03 | Syngr1 | 6.93 | 4E−27 |
Slc6a8 |
5.91 | 1E−29 |
| Gm12689 | 3.24 | 7.8E−12 | Knstrn | 1.81 | 0.03 | Wfdc17 | 6.04 | 3E−54 |
Anpep |
5.85 | 4E−45 |
| Gm8738 | 3.23 | 6.3E−07 | Cdk1 | 1.80 | 0.03 | Cfh | 5.98 | 5E−22 |
Spp1 |
5.76 | 1E−147 |
| Rps19-ps3 | 3.21 | 7.1E−12 | Cdca5 | 1.79 | 0.02 | Gimap4 | 5.82 | 1E−14 |
Fads2 |
5.74 | 8E−12 |
| Prkag2os1 | 3.19 | 9.0E−05 | Sapcd2 | 1.79 | 0.04 | Socs3 | 5.80 | 4E−59 |
Timp1 |
5.74 | 1E−30 |
|
Top 20 downregulated genes | |||||||||||
| Eif2s3y | –327.74 | 4.1E−189 | Ddx3y | –122.71 | 0.00 | Htra3 | –6.26 | 2E−59 |
Dnah11 |
–8.91 | 1E−52 |
| Ddx3y | –326.76 | 1.9E−182 | Eif2s3y | –114.76 | 0.00 | Dnah11 | –6.22 | 1E−26 |
Hmcn1 |
–8.06 | 2E−19 |
| Kdm5d | –162.74 | 2.7E−128 | Kdm5d | –40.98 | 0.00 | D630039A03Rik | –6.01 | 4E−35 |
Itgad |
–6.96 | 2E−41 |
| Uty | –111.81 | 2.8E−103 | Uty | –37.99 | 0.00 | Itgad | –5.66 | 2E−29 |
Adra2a |
–5.87 | 4E−19 |
| Pdpr | –7.21 | 1.4E−15 | Adap2 | –1.92 | 0.00 | Adra2a | –5.31 | 7E−16 |
Gm8113 |
–5.65 | 2E−80 |
| Gm14548 | –7.01 | 4.5E−31 | Kcnh4 | –1.92 | 0.00 | Gm8113 | –4.54 | 1E−45 |
Cd300lg |
–5.43 | 4E−40 |
| RP24–540G19.3 | –6.46 | 7.5E−11 | Gdap10 | –1.88 | 0.03 | Acaa1b | –4.54 | 2E−33 |
Htra3 |
–5.16 | 4E−49 |
| Krt80 | –5.67 | 1.3E−40 | Slfn10-ps | –1.85 | 0.03 | Sdk1 | –4.37 | 1E−14 |
Gabbr1 |
–5.07 | 9E−55 |
| Cog5 | –5.50 | 7.4E−20 | Gm15931 | –1.85 | 0.02 | Spag11b | –4.32 | 4E−26 |
D630039A03Rik |
–5.07 | 3E−38 |
| Hmgxb3 | –5.45 | 2.0E−24 | Hpse | –1.84 | 0.03 | Fabp1 | –4.28 | 5E−31 |
1810011O10Rik |
–4.72 | 2E−17 |
| Snx29 | –5.32 | 8.2E−18 | Hmgxb3 | –1.82 | 0.03 | Rbpms | –4.09 | 8E−29 |
Spag11b |
–4.50 | 5E−66 |
| Vcl | –5.16 | 1.1E−12 | Gm15635 | –1.82 | 0.04 | Gm12349 | –3.98 | 3E−21 |
Tgfb2 |
–4.45 | 2E−27 |
| Zfp526 | –5.15 | 5.5E−18 | Gm16185 | –1.80 | 0.03 | Kcnh4 | –3.94 | 1E−18 |
Hr |
–4.39 | 5E−54 |
| Rnf24 | –5.02 | 2.7E−17 | Sik2 | –1.80 | 0.03 | Slc1a3 | –3.67 | 1E−19 |
Klk8 |
–3.79 | 3E−33 |
| Mob3a | –4.92 | 1.3E−22 | Setd1b | –1.79 | 0.02 | Gabbr1 | –3.54 | 8E−15 |
Hepacam2 |
–3.78 | 1E−12 |
| Xylt1 | –4.90 | 4.3E−12 | Uprt | –1.79 | 0.03 | Mamdc2 | –3.49 | 6E−26 |
Fabp1 |
–3.76 | 1E−27 |
| Pira2 | –4.75 | 6.8E−50 | Hgf | –1.79 | 0.05 | Cd209b | –3.45 | 8E−09 |
Clcf1 |
–3.69 | 3E−45 |
| 4931406P16Rik | –4.59 | 1.4E−09 | Echdc1 | –1.78 | 0.03 | Atp2b4 | –3.43 | 3E−11 |
Cox6a2 |
–3.60 | 5E−07 |
| Mical3 | –4.58 | 2.1E−15 | Zfp955a | –1.77 | 0.03 | Tppp | –3.41 | 4E−11 |
Fam212a |
–3.58 | 5E−18 |
| Arhgef11 | –4.57 | 4.7E−24 | 9930111J21Rik1 | –1.77 | 0.05 | Gm13546 | –3.39 | 4E−17 | Trf | –3.47 | 2E−21 |
Next, to assess the effect of ozone exposure on gene expression in males, we compared gene expression data from ozone-exposed males with air-exposed males. 511 and 155 DEGs were upregulated and downregulated, respectively, at stringent criteria (Fig. 7D). A total of 2,120 (upregulated, 1,227; downregulated, 893) DEGs were identified on the basis of relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in ozone-exposed males versus air-exposed males is included in Table 5 (top 20 upregulated and top 20 downregulated). Further, to assess the effect of ozone exposure on gene expression in females, we compared gene expression data from ozone-exposed females with air-exposed females. 462 and 218 DEGs were upregulated and downregulated, respectively, at stringent criteria (Fig. 7E). A total of 4,598 (upregulated, 2,447; downregulated, 2,151) DEGs were identified on the basis of relaxed cutoff criteria (Supplemental Table S2). A list of DEGs in ozone-exposed females versus air-exposed females is included in Table 5 (top 20 upregulated and top 20 downregulated).
To assess sex-associated differences in gene expression in ozone-exposed mice, we compared gene expression data from ozone-exposed females with ozone-exposed males. Only 1 and 4 DEGs were upregulated (Xist, located on X-chromosome) and downregulated (all located on Y-chromosome), respectively, at stringent cutoff criteria (Fig. 7F). However, 134 (upregulated, 64; downregulated, 70) DEGs were identified on the basis of relaxed cutoff criteria. A list of DEGs in ozone-exposed females versus ozone-exposed males is included in Table 5 (top 20 upregulated and top 20 downregulated).
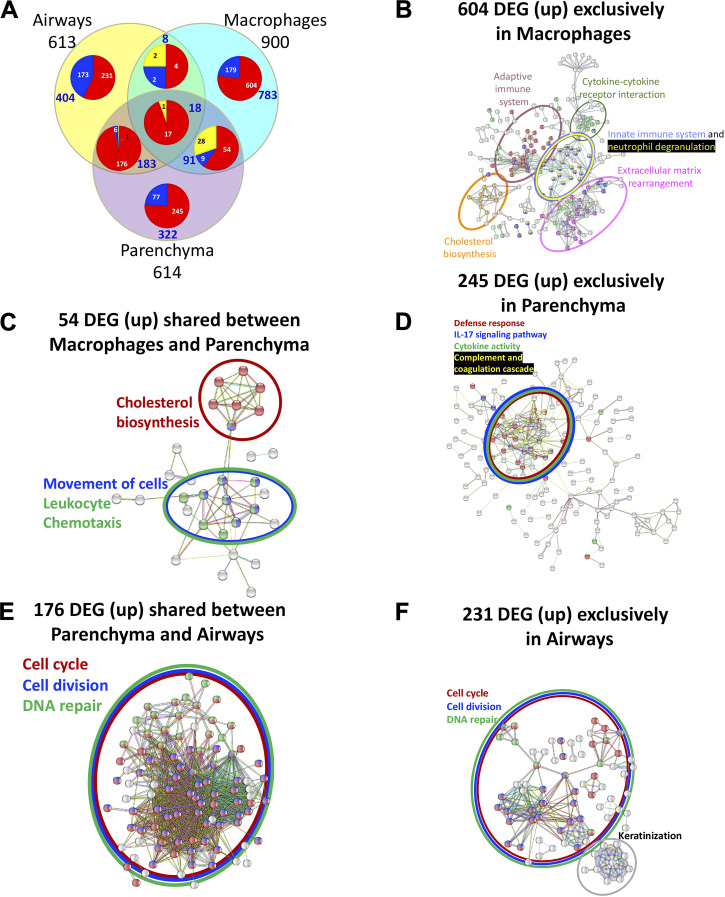
Comparison of ozone-induced upregulated genes (fold change > 2; adjusted P value < 0.05) in males and females identified 280 genes that were found common in both sexes (Fig. 7G). Additional 231 and 182 genes were upregulated only in males and females, respectively (Fig. 7G). Similarly, comparison of ozone-induced downregulated genes (fold change > 2; adjusted P value < 0.05) in males and females identified 93 genes that were found common to both sexes. An additional 62 and 125 genes were downregulated only in males and females, respectively (Fig. 7G). A list of common and sex-specific DEGs identified in ozone-exposed mice is presented in Table 6 and Supplemental Table S6. Macrophage transcriptome from ozone-exposed mice was enriched in gene signatures associated with categories, including regulation of complement cascade, cholesterol biosynthesis, extracellular matrix organization, cytokine-cytokine receptor interaction, and innate immune system (Supplemental Table S4C).
Table 6.
Top 20 upregulated and top 20 downregulated genes in macrophages from ozone-exposed mice (common and unique)
| Common in Both Sexes |
Unique to Males |
Unique to Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ozone vs. Air |
Ozone vs. Air |
Ozone vs. Air |
||||||||
| Gene | FC (Males) | Adjusted P Value (Males) | FC (Females) | Adjusted P Value (Females) | Gene | FC | Adjusted P Value | Gene | FC | Adjusted P Value |
|
Top 20 upregulated genes* | ||||||||||
| Lrg1 | 14.85 | 5.0E−45 | 21.10 | 1.7E−94 | Gimap4 | 5.82 | 1.3E−14 | Arg1 | 11.47 | 1.3E−29 |
| Mafb | 14.82 | 8.0E−139 | 11.60 | 9.1E−40 | Cd8b1 | 5.48 | 7.3E−13 | Ocstamp | 8.21 | 5.5E−24 |
| C4b | 10.53 | 7.4E−25 | 36.59 | 1.2E−129 | Clic5 | 5.30 | 1.7E−16 | AA467197 | 4.13 | 2.5E−23 |
| Wfdc17 | 8.26 | 2.6E−49 | 6.04 | 2.9E−54 | Sh2d2a | 5.17 | 1.5E−12 | Uaca | 4.03 | 8.4E−11 |
| Awat1 | 7.57 | 1.2E−25 | 4.03 | 1.1E−10 | Thy1 | 5.08 | 6.1E−13 | Tarm1 | 3.93 | 1.5E−07 |
| C1qb | 7.56 | 1.2E−18 | 9.15 | 8.1E−24 | Lax1 | 4.82 | 3.0E−11 | Mmp12 | 3.31 | 1.8E−07 |
| Tnfsf14 | 7.42 | 2.9E−47 | 4.93 | 2.2E−16 | Cd3e | 4.74 | 3.4E−11 | Fasn | 3.27 | 3.9E−19 |
| Ear6 | 7.29 | 1.8E−21 | 4.52 | 1.3E−14 | Lck | 4.71 | 5.2E−12 | Cyp2b10 | 3.08 | 2.9E−06 |
| Msr1 | 6.92 | 1.6E−71 | 10.32 | 1.4E−130 | Cd3g | 4.36 | 1.0E−09 | Slfn9 | 3.08 | 1.0E−14 |
| Atf7ip2 | 6.77 | 8.1E−16 | 3.88 | 1.9E−08 | Ikzf3 | 4.32 | 7.9E−10 | Cds1 | 3.02 | 4.6E−07 |
| Mmp14 | 6.61 | 3.3E−30 | 5.70 | 4.2E−19 | Cd163l1 | 4.25 | 9.0E−11 | Itgb6 | 3.02 | 4.5E−05 |
| Socs3 | 6.13 | 2.2E−34 | 5.80 | 3.6E−59 | Bcl2 | 4.19 | 1.7E−10 | Nt5dc2 | 2.87 | 1.5E−07 |
| Sash1 | 5.94 | 3.5E−29 | 7.15 | 6.1E−38 | Gimap8 | 4.15 | 3.2E−09 | Il1bos | 2.81 | 6.5E−05 |
| Slc6a8 | 5.91 | 1.5E−29 | 5.13 | 1.3E−21 | Hspb1 | 4.13 | 3.0E−10 | Acss2 | 2.80 | 3.7E−12 |
| Anpep | 5.85 | 3.9E−45 | 7.73 | 2.1E−60 | Tcf7 | 4.05 | 4.0E−09 | Lrrc8b | 2.75 | 3.4E−08 |
| Spp1 | 5.76 | 1.4E−147 | 7.78 | 4.7E−93 | Acsbg1 | 4.03 | 6.2E−09 | Gm13571 | 2.74 | 4.0E−07 |
| Fads2 | 5.74 | 8.5E−12 | 7.59 | 5.3E−28 | Gimap6 | 3.87 | 2.4E−08 | Pgam1 | 2.73 | 8.6E−15 |
| Timp1 | 5.74 | 1.5E−30 | 5.06 | 1.3E−14 | Il18r1 | 3.85 | 2.1E−10 | Slc11a1 | 2.69 | 2.0E−04 |
| Cldn4 | 5.66 | 5.9E−22 | 7.44 | 2.8E−23 | Gimap1 | 3.79 | 5.1E−08 | Lmbrd2 | 2.67 | 1.4E−06 |
| Gpr84 | 5.52 | 1.3E−22 | 2.64 | 1.9E−05 | Cd28 | 3.55 | 2.5E−07 | Ambp | 2.64 | 3.9E−04 |
|
Top 20 downregulated genes* | ||||||||||
| Dnah11 | –8.91 | 1.4E−52 | –6.22 | 9.8E−27 | Sdk1 | –4.37 | 1.1E−14 | Trf | –3.47 | 1.6E−21 |
| Hmcn1 | –8.06 | 2.0E−19 | –2.72 | 1.1E−04 | Cd209b | –3.45 | 8.0E−09 | Slpi | –3.40 | 2.7E−26 |
| Itgad | –6.96 | 1.9E−41 | –5.66 | 2.4E−29 | Cd300e | –3.07 | 6.8E−17 | Gbp8 | –3.35 | 1.7E−26 |
| Adra2a | –5.87 | 3.7E−19 | –5.31 | 7.0E−16 | Bpifa1 | –2.80 | 1.6E−07 | Gm13889 | –3.33 | 1.1E−06 |
| Gm8113 | –5.65 | 1.7E−80 | –4.54 | 1.1E−45 | Tlr5 | –2.77 | 9.4E−08 | Gm7061 | –3.32 | 2.9E−13 |
| Cd300lg | –5.43 | 3.6E−40 | –3.10 | 3.5E−10 | Bcam | –2.76 | 8.3E−09 | Dusp1 | –3.31 | 5.5E−17 |
| Htra3 | –5.16 | 4.3E−49 | –6.26 | 1.9E−59 | Shc3 | –2.73 | 4.2E−06 | Rpl10-ps3 | –3.00 | 5.1E−09 |
| Gabbr1 | –5.07 | 9.3E−55 | –3.54 | 7.6E−15 | Prss30 | –2.72 | 1.1E−12 | S100a4 | –2.94 | 1.3E−13 |
| D630039A03Rik | –5.07 | 3.1E−38 | –6.01 | 3.8E−35 | Gpr33 | –2.69 | 1.8E−05 | Gm16168 | –2.78 | 1.0E−06 |
| 1810011O10Rik | –4.72 | 1.5E−17 | –2.45 | 1.6E−04 | Gm16576 | –2.61 | 7.2E−06 | H2-DMb2 | –2.78 | 1.1E−36 |
| Spag11b | –4.50 | 5.1E−66 | –4.32 | 4.2E−26 | Scgb3a1 | –2.57 | 3.2E−05 | Fam3b | –2.76 | 1.8E−06 |
| Tgfb2 | –4.45 | 2.2E−27 | –2.08 | 3.8E−04 | Kctd17 | –2.50 | 8.5E−08 | Bcl2a1b | –2.74 | 6.1E−11 |
| Hr | –4.39 | 4.7E−54 | –2.97 | 1.9E−17 | Slc35g3 | –2.49 | 4.7E−04 | Amigo2 | –2.74 | 1.8E−19 |
| Klk8 | –3.79 | 2.8E−33 | –3.05 | 1.2E−13 | Krt80 | –2.44 | 1.3E−07 | Gm11973 | –2.74 | 1.3E−05 |
| Hepacam2 | –3.78 | 1.4E−12 | –2.61 | 3.0E−07 | Slc16a14 | –2.42 | 1.6E−04 | Gm5601 | –2.71 | 1.3E−06 |
| Fabp1 | –3.76 | 1.1E−27 | –4.28 | 4.5E−31 | Ric3 | –2.40 | 2.4E−08 | 4930516B21Rik | –2.71 | 4.1E−15 |
| Clcf1 | –3.69 | 2.5E−45 | –2.17 | 5.5E−09 | Gm5936 | –2.39 | 9.0E−04 | Clca3 | –2.71 | 2.8E−04 |
| Cox6a2 | –3.60 | 4.6E−07 | –2.81 | 2.9E−05 | Sec16b | –2.39 | 9.8E−05 | Ccpg1 | –2.65 | 3.5E−04 |
| Fam212a | –3.58 | 5.3E−18 | –2.85 | 3.9E−08 | Clec3a | –2.38 | 9.8E−04 | 1700023L04Rik | –2.59 | 7.8E−08 |
| Tnfsf13b | –3.29 | 1.9E−16 | –2.84 | 2.5E−08 | Pzp | –2.34 | 3.8E−07 | Gm12689 | –2.55 | 9.6E−11 |
A complete list of differentially expressed genes is included in Supplemental Table S6.
Ozone exposure results in the enrichment of pathways involved in immune responses and cholesterol biosynthesis in airspace macrophages.
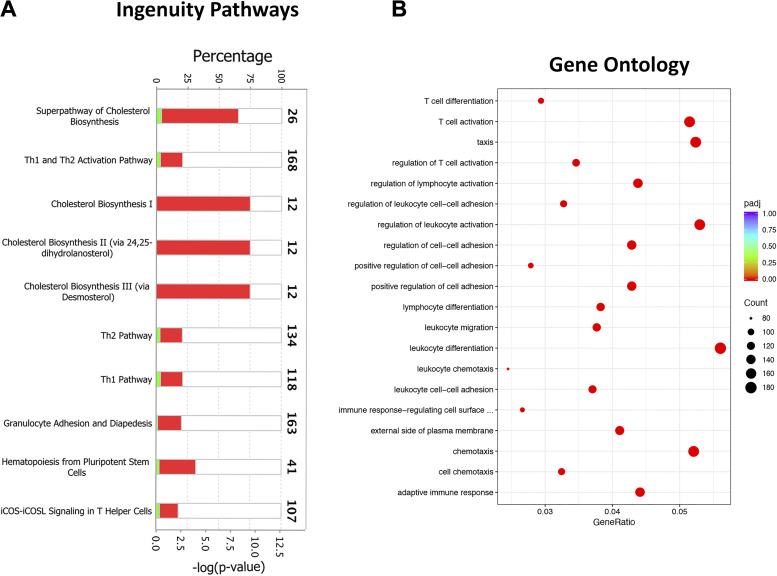
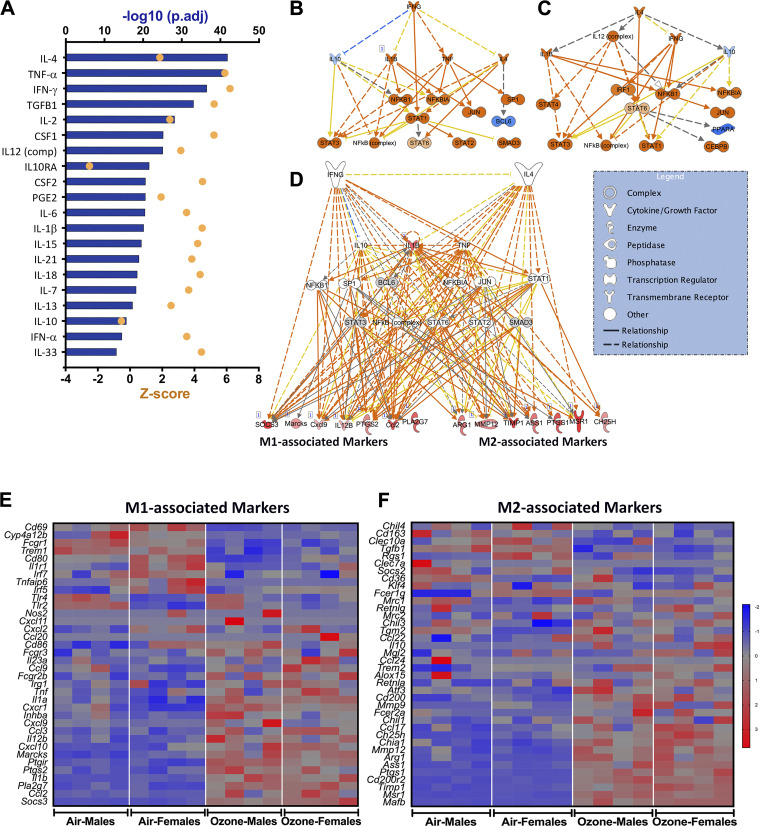
Combined (both sexes) DEGs [900 genes (upregulated, 701; downregulated, 199); FC > 2; adjusted P value < 0.05] (Fig. 7H) were examined for their functional relevance using IP, GO, and STRING analyses. The IP analyses revealed significant enrichment of canonical pathways associated with immune response (Th1 and Th2 pathway activation, granulocyte adhesion and diapedesis, hematopoiesis, and iCOS-iCOSL signaling in Th cells) and metabolic alterations (cholesterol biosynthesis) (Fig. 8A).
Figure 8.
Biological pathway analyses on differentially expressed genes in purified macrophages from ozone-exposed mice. A: stacked bar graph depicting enrichment of biological pathways identified using ingenuity pathway analysis approach. B: dot plot showing enrichment of gene ontology biological processes, including biological processes, cell component, and molecular function. iCOS-iCOSL, inducible T-cell costimulator-inducible T-cell costimulator-ligand.
The GO analyses revealed enrichment of immune response-related pathways [T-cell activation, Taxis, regulation of leukocyte activation, cell-cell adhesion, leucocyte differentiation, chemotaxis, and adaptive immune response (ranked within top 20)] (Fig. 8B). Consistent with IP and GO enrichment data, STRING analyses identified enriched networks relevant to cytokine-cytokine receptor interaction, innate immune system, neutrophil degranulation, adaptive immune system, extracellular matrix rearrangement, and cholesterol biosynthesis (Supplemental Fig. S2C).
Upstream regulator analysis indicates the presence of an airspace microenvironment responsible for mixed-macrophage activation responses.
To identify the cascade of upstream transcriptional regulators in the context of observed changes in the macrophage transcriptome, we performed upstream regulator analyses using the IPA tool. This application exploits the Ingenuity knowledge base that has been developed in literature-based scientific knowledge on the effects of transcriptional regulators and their downstream target genes. We reasoned that macrophage-derived cytokines establish airspace microenvironment that dictates the function of various airspace cells, including macrophages, other immune cells, and epithelial cells. Accordingly, combined 900 DEGs (Fig. 7H) were subjected to upstream regulator analyses by specifically limiting the search criteria for cytokines as upstream regulators. We identified cytokines that were predicted to be responsible for the observed changes in the macrophage transcriptome. IL-4, TNF-α, IFN-γ, CSF2, IL-1β, and IL-13 ranked higher based on the Z-scores and adjusted P value criteria (Fig. 9A), suggesting that these regulators are likely present in the airspaces and influence the expression profiles of 900 DEGs.
Figure 9.
Upstream regulator and differentially expressed genes (DEG) analyses reveal mixed macrophage activation patterns. A: ingenuity pathway analysis (IPA) for upstream regulators shown as a bar graph depicting upstream cytokines predicted to drive differential expression of genes in macrophages. Orange dots represent Z-score, and blue bars represent -log10 (P-adjusted) values. Mechanistic transcriptional network regulated by IFN-γ (B) and IL-4 (C). D: integrated mechanistic network depicting crosstalk between IL-4- and IFN-γ-regulated pathways and effector genes associated with classical (M1) and alternatively (M2) activated macrophages. The functional networks were generated using the Qiagen IPA analyses tool. Heat maps for normalized gene expression values (Z-scores) of classically (M1) (E) and alternative (M2) activation-associated genes (F) in purified macrophages. Higher and lower expressions of each gene are represented by red and blue colors, respectively.
Among the identified regulators, IFN-γ and IL-4/IL-13 are well-established inducers of classical (M1) and alternative (M2) macrophage activation responses, respectively. To identify downstream transcriptional regulators, we analyzed IFN-γ regulated (Fig. 9B) and IL-4 regulated (Fig. 9C) activation of mechanistic networks, which revealed that a number of transcription factors, including STAT1, STAT3, STAT6, and NF-κB regulate the expression of signatures associated with M1 and M2 activation (Fig. 9D). A majority of DEGs that were associated with either of the two regulators, IL-4 and INF-γ, were upregulated in ozone-exposed mice (Supplemental Fig. S3, A and B). Activation of macrophages in ozone-exposed mice has been shown previously in the acute exposure models of ozone-induced lung injury in mice (21, 40, 60). Consistent with these reports, the gene signatures associated with both M1 classical (Fig. 9E) and M2 alternative activation (Fig. 9F) were upregulated in ozone-exposed mice.
Integrated analyses of DEGs from the three tissues identified tissue-specific enriched pathways.
To determine common and unique DEGs between three tissue/cell transcriptomes and their functional relevance in the context of ozone-induced lung injury, we compared lists of DEGs that were significantly affected (FC > 2; adjusted P value < 0.05) in tissues/cells from ozone-exposed mice versus air-exposed mice. First, we compared 613 DEGs (Fig. 2H) from airways with 614 DEGs (Fig. 5H) from parenchyma, which identified tissue-specific and shared gene signatures between these two compartments but was not present in macrophages (Supplemental Table S7). 404 and 322 unique genes were present in airways and parenchyma, respectively (Fig. 10A and Supplemental Table S7). Out of 183 shared signatures between airways and parenchyma, 176 and 6 signatures were upregulated and downregulated, respectively (Fig. 10A and Supplemental Table S7). One uncharacterized gene (AU040972) was significantly upregulated in parenchyma but significantly downregulated in airways (Fig. 10A and Supplemental Table S7).
Figure 10.
Comparison of differentially expressed genes (DEGs) between three tissue compartments and their STRING analyses reveals enrichment of tissue-specific biological networks. A: integrated Venn diagram showing unique and shared DEGs across three tissue compartments in ozone-exposed mice vs. air-exposed mice. 613 (yellow-filled circle), 614 (pink-filled circle), and 900 (aqua-filled circle) DEGs from airways, parenchyma, and macrophages, were analyzed to identify the number of upregulated (depicted by red sector of the pie chart), downregulated (depicted by blue sector of the pie chart), and differentially regulated genes in the opposite direction in different tissues (depicted by yellow sector of the pie chart), respectively. The list of unique and shared DEGs between the three compartments is included in Supplemental Table S7. B: STRING database protein-protein interaction network analyses on 604 genes that were exclusively upregulated in macrophages from ozone-exposed mice vs. air-exposed mice. C: STRING database protein-protein interaction network analyses on 54 genes that were upregulated in both tissues, i.e., macrophages and parenchyma, from ozone-exposed mice vs. air-exposed mice. D: STRING database protein-protein interaction network analyses on 245 genes that were exclusively upregulated in the parenchyma from ozone-exposed mice vs. air-exposed mice. E: STRING database protein-protein interaction network analyses on 176 genes that were upregulated in both tissues, i.e., airways and parenchyma, from ozone-exposed mice vs. air-exposed mice. F: STRING database protein-protein interaction network analyses on 231 genes that were exclusively upregulated in airways from ozone-exposed mice vs. air-exposed mice. Interactions were determined on the basis of evidence, using the highest confidence level (0.9) setting.
Next, we compared 613 DEGs (Fig. 2H) from airways with 900 DEGs (Fig. 7H) from macrophages that identified mostly as tissue-specific, with few shared gene signatures between these two compartments, but not present in the parenchyma (Fig. 10Aand Supplemental Table S7). 404 and 783 genes were present exclusively in airways and macrophages, respectively (Fig. 10A and Supplemental Table S7). Additionally, eight DEGs were shared between airways and macrophages that included four and two genes that were upregulated and downregulated in both tissues. In addition, two (yellow) additional signatures were also changed but in the opposite direction, i.e., Cd177 was upregulated in macrophages but downregulated in airways, and Slpi was upregulated in airways but downregulated in macrophages (Fig. 10A and Supplemental Table S7).
For the third comparison, i.e., parenchyma and macrophages, 614 DEGs from parenchyma were compared with 900 DEGS from macrophages (Fig. 10A and Supplemental Table S7). 322 and 783 genes were present exclusively in parenchyma and macrophages, respectively (Fig. 10A and Supplemental Table S7). Out of 91 shared signatures between parenchyma and macrophages, 54 (red) and 9 (blue) signatures were upregulated and downregulated, respectively. Twenty-eight (yellow) additional signatures were also changed in the opposite direction; 7 signatures were upregulated in parenchyma but downregulated in macrophages, and 21 signatures were upregulated in macrophages but downregulated in the parenchyma (Fig. 10A and Supplemental Table S7).
Next, we compared DEGs from the three compartments and identified 18 shared signatures among the three compartments. Seventeen gene signatures were found upregulated in all the compartments (Fig. 10A and Supplemental Table S7). One signature, i.e., Fam3b, was upregulated in airways and parenchyma but downregulated in macrophages (Fig. 10A and Supplemental Table S7).
These comparisons also categorized signatures that were upregulated only in one compartment (e.g., 231 signatures in airways, 245 signatures in the parenchyma, and 604 signatures in macrophages) and those that were shared exclusively in two of the compartments (e.g., 176 upregulated signatures shared between airways and parenchyma exclusively, 54 upregulated signatures shared between macrophages and parenchyma exclusively, 4 upregulated signatures shared between airways and macrophages exclusively) (Fig. 10A and Supplemental Table S7).
First, using the STRING approach, we analyzed the 604 genes that were upregulated exclusively in the macrophages (Fig. 10B). The output of the analyses was comparable to the one that we obtained from the entire set of genes (701) that were upregulated in macrophages (Supplemental Fig. S2C). Next, we analyzed 54 shared upregulated signatures between the macrophages and the parenchyma. Three networks, i.e., cholesterol biosynthesis, movement of cells, and leukocyte chemotaxis, were found enriched in this analysis (Fig. 10C). Of note, these pathways were also enriched in the gene signatures that were upregulated exclusively in the macrophages, which suggests that these shared signatures are of macrophage origin and likely contributed by residual airspace or interstitial macrophages in the parenchyma. It is noteworthy that a significant number of macrophages remain within the parenchyma despite a large number of lavages that we performed to obtain a good harvest of the macrophages. Further, the parenchyma also contains interstitial macrophages that are not removed during repeated lavages. Accordingly, we hypothesized that shared signatures between the macrophages and the parenchyma are most likely contributed by the residual alveolar macrophages in the parenchyma or the macrophage populations within the interstitium.
Next, we compared STRING analyses using shared and unique gene signatures between the parenchyma and airway compartments. 176 upregulated signatures between these two compartments caused the enrichment of networks related to cell cycle, cell division, and DNA repair (Fig. 10E). Because of the extraction of these 176 signatures, the revised network output from 245 uniquely upregulated genes in the parenchyma (Fig. 10D) was completely devoid of these pathways (cell cycle, cell division, and DNA repair) that were present when the entire set of upregulated genes (501 genes) within the parenchyma was analyzed (Supplemental Fig. S2B). The intensity of genes related to these pathways was also diminished in airways when 176 shared signatures were extracted from the original set of 430 genes that were upregulated in the airways (Fig. 10F). Of note, while the lavaged extrapulmonary airways do not contain airspace immune cells and alveolar epithelial cells, the parenchyma is mostly enriched with alveolar epithelial cells, with little or no airway epithelial cells. Accordingly, we believe that ozone encountered by airways, as well as alveolar epithelial cells, results in cellular injury/cell death, which triggers pathways of cellular proliferation. In summary, these analyses suggest that both airway and alveolar epithelial cells are injured upon exposure to ozone, which results in the activation of cellular proliferation, whereas the encounter of ozone or ozonation products by macrophages results in the activation of immune cell pathways.
Immune cell recruitment response to ozone is more robust in females.
Ozone exposure is known to trigger the recruitment of inflammatory macrophages (16, 50), neutrophils (9, 73), lymphocytes, and eosinophils (34). To assess alterations in the expression patterns of genes for chemoattractants and their receptors known to mediate recruitment of these cell types (35, 47, 58, 64), we again analyzed the transcriptome from the three compartments (Fig. 11A). Monocyte-specific chemoattractant genes i.e., Ccl1 and Ccl2, were upregulated in the parenchyma of ozone-exposed mice versus air-exposed mice. In contrast, Cx3cl1, another monocyte-specific chemoattractant gene, was downregulated in the parenchyma of ozone-exposed mice versus air-exposed mice. Genes encoding neutrophil-specific, eosinophil-specific, and lymphocyte-specific chemokines were all upregulated in the parenchyma of ozone exposed mice versus air-exposed mice.
Figure 11.
A: ozone exposure alters gene expression of chemokines and bronchoalveolar lavage fluid immune cell composition. A: heat maps for normalized gene expression values (Z-scores) of chemokine genes in the three compartments. Higher and lower expressions of each gene are represented by red and blue colors, respectively. The relevance of each chemokine in the recruitment of macrophages (M), neutrophils (N), eosinophils (E), and lymphocytes (L) is indicated with red, blue, green, or black dots, respectively. B: percent distributions of immune cells are shown as a stacked bar graph [macrophages (red), neutrophils (blue), eosinophils (green), and lymphocytes (black)]. Values with identical superscript symbols represent a significant difference (P < 0.05). Differential cell counts for macrophages (C), neutrophils (D), eosinophils (E), and lymphocytes (F). Error bars represent means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using ANOVA followed by the Tukey multiple-comparison post hoc test. (n = 13–14 per group).
While the majority of immune cells in the BALF from air-exposed males were macrophages (∼98.8%), immune cells from ozone-exposed males were an admixture of macrophages (∼76.6%), neutrophils (∼18.5%), eosinophils (∼4.30%), and lymphocytes (0.60%) (Fig. 11B). The alteration in the composition of the immune cell population was contributed by increases in the absolute number of macrophages (Fig. 11C), neutrophils (Fig. 11D), eosinophils (Fig. 11E), and lymphocytes (Fig. 11F). The absolute numbers of macrophages (Fig. 11C) and lymphocytes (Fig. 11F) in the analyzed fraction were significantly higher in ozone-exposed males versus air-exposed males. Although trending higher, the numbers of neutrophils (Fig. 11D) and eosinophils (Fig. 11E) in ozone-exposed males versus air-exposed males did not reach significance.
Similar to the air-exposed males, the majority of immune cells in the BALF from air-exposed females were macrophages (∼98.4%), and ozone exposure resulted in altered BALF cell composition, i.e., macrophages (∼66.2%), neutrophils (∼28.9%), eosinophils (∼4.12%), and lymphocytes (0.78%) (Fig. 11B). As compared with air-exposed females, ozone-exposed females had a significant increase in the absolute number of all the four BALF cell types, i.e., macrophages (Fig. 11C), neutrophils (Fig. 11D), eosinophils (Fig. 11E), and lymphocytes (Fig. 11F). The absolute number of macrophages, neutrophils, eosinophils, and lymphocytes were significantly higher in ozone-exposed females versus ozone-exposed males (Fig. 11, C–F). These data suggest that inflammatory cell recruitment response to ozone exposure is relatively more robust in females versus males.
Ozone exposure results in an enrichment of gene signatures relevant to mucoinflammatory airway disease.
Although it is known that ozone exposure results in airway disease, it is not fully understood how closely the murine airway responses resemble human mucoinflammatory disease pathology. Accordingly, we queried the transcriptome from the three compartments for the expression of customized gene sets relevant to human mucoinflammatory lung diseases (Fig. 12A). The majority of the genes in the customized gene set were found differentially regulated in at least one of the tissues (Fig. 12A). Genes including Muc5ac, Muc5b, Lgals3, Lcn2, Chia1, Fn1, Sfpta, Sfptb, F10, Prdx5, Mrc1, and Chil1 were found to be upregulated in the parenchyma of ozone-exposed mice (Fig. 12B).
Figure 12.
Gene expression analyses for genes relevant to mucoinflammatory lung diseases and mucous cell metaplasia responses. A: heat maps for normalized gene expression values (Z-scores) of genes associated with mucoinflammatory lung diseases in mice and humans. Low-resolution heat map (left) depicting expression patterns for the entire mucoinflammatory gene set (high-resolution heat map with gene names is presented in Supplemental Fig. S4). Heat map corresponding to gene signatures that were downregulated (B, top) or upregulated (B, bottom) in one or more tissues from ozone-exposed mice was amplified for better resolution. C: heat map for normalized gene expression values (Z-scores) of genes associated with mucous cell metaplasia in mice and humans.
Mucous cell metaplasia (MCM) is a hallmark feature of the epithelium in the mucoinflammatory lung diseases (14, 15, 36, 37). To determine whether the MCM-relevant gene signatures are differentially regulated in the epithelial cell-enriched tissues, we analyzed the expression patterns of a customized gene set in airways and parenchyma (Fig. 12C). Genes including Agr2, Areg, Il33, Il13, Gcnt1, Gcnt3, Gcnt7, and Galnt7 were upregulated in the airways of ozone-exposed mice. Apart from these gene signatures, parenchyma transcriptome was enriched in additional MCM-relevant gene signatures, including Clca1, Muc5b, Muc5ac, Foxa3, Ccl17, Ccl24, Spdef, Foxa2, Il4ra, and Nfkb2.
Comparative analyses on transcriptome between the current subchronic study and a previously published acute study reveal upregulation of common and unique gene signatures.
A recently published acute study explored transcriptomic changes in alveolar macrophages and conducting airways from ozone-exposed female mice (69). To determine common, as well as unique, gene signatures that upregulate in each compartment between the acute and subchronic exposure paradigms, we compared upregulated DEGs obtained from both studies. We hypothesized that a significant number of genes that upregulate during acute exposure sustain throughout the subchronic exposure paradigm. Further, we hypothesized that some genes will exhibit exposure-specific gene expression changes. To minimize sex-associated variation, we specifically compared DEGs identified in macrophage and airway samples from females.
First, 477 upregulated DEGs in alveolar macrophages from acutely exposed females were compared with 464 upregulated DEGs in alveolar macrophages from subchronically exposed females. Our analyses revealed 84 gene signatures (Arg1, C4b, Cd200r2, Ccl17, Cd38, Il1rl1, Saa3, Marcks, Lcn2, Timp1, and Mmp14) that were found upregulated in alveolar macrophages from both studies (Log2 FC > 1.0; adjusted P value < 0.05) (Supplemental Table S8). In addition, 393 and 380 DEGs were upregulated exclusively in the macrophages from acute and chronic exposure studies, respectively (Supplemental Table S8). These exclusive DEGs from each study were correlated with the other study to identify genes that might have missed the cutoff criteria by a narrow margin, and the genes that showed remarkable differences in the expression values. For this, on the basis of their expression values in the other study, we divided these genes in to three categories, i.e., relaxed (Log2 FC = 0.0–1.0; adjusted P value < 0.05), nonsignificant (adjusted P value > 0.05), opposite (Log2 FC < 0 or not found) (Supplemental Table S8). Out of 393 DEGs that were identified to be exclusively upregulated in macrophages from the acute study, 107, 175, and 111 genes were subdivided into relaxed (relaxed fold-change criteria for upregulated), nonsignificant (no significant change), and opposite (changing in opposite direction) categories, respectively (Supplemental Table S8). Out of 380 DEGs that were identified to be exclusively upregulated in macrophages from our subchronic study, 48, 160, and 172 genes were subdivided into relaxed, nonsignificant, and opposite categories, respectively (Supplemental Table S8).
Next, we compared upregulated 527 DEGs identified in the extrapulmonary airways from acutely exposed females with upregulated 456 DEGs identified in the conducting airways from subchronically exposed females. Our analyses revealed 182 gene signatures (Psca, Anxa10, Gsta1, Vsig1, S100a14, Gcnt3, Gjb4, Lgals3, Lcn2, Mt2, Ereg, Slpi, Saa3, and Krt13) that were found upregulated in both studies. In addition, 345 and 274 DEGs were upregulated exclusively in the airways from acute and subchronic exposure studies, respectively (Supplemental Table S9). Out of 345 DEGs that were identified to be exclusively upregulated in the airways from the acute study, 94, 131, and 120 genes belonged to the relaxed, nonsignificant, and opposite categories, respectively (Supplemental Table S9). Out of 274 DEGs that were identified to be exclusively upregulated in airways from our subchronic study, 20, 155, and 99 genes were subdivided into relaxed, nonsignificant, and opposite categories, respectively (Supplemental Table S9).
DISCUSSION
Ozone inhalation results in altered cellular survival and function, including cell death, cellular proliferation, increased immune cell recruitment, and altered cellular functioning, which in turn, constitute various pathophysiological responses in the respiratory tract, including cellular injury, epithelial remodeling, and airspace inflammation (34, 48). Although whole lung transcriptomic changes in ozone-exposed lungs have been extensively investigated (4, 11, 19, 31, 41, 45, 46, 70, 71), their cell-specific relevance remains poorly characterized. In the current study, we analyzed perturbations in gene expression in three distinct resident cell-type enriched compartments of the respiratory tract of ozone-exposed adult mice. Because ozone and ozonation products interact with resident cells in the airway and alveolar airspaces, we harvested compartments enriched in three different resident cell types, i.e., airway epithelial cells, alveolar epithelial cells, and macrophages (primary immune cells in airspaces). Using RNA-Seq-based gene sequencing, we obtained expression values of transcripts to identify differentially expressed genes (DEGs), enriched functional pathways, and protein-protein interaction networks. To the best of our knowledge, this is the first study of its kind in which comprehensive transcriptome analyses on three different cell type-enriched compartments have been performed using mouse model of subchronic ozone exposure.
Previous studies have demonstrated that acute ozone exposure results in exaggerated lung inflammation in females, as compared with males (6, 7, 10, 62). Consistent with these reports, as compared with ozone-exposed males, ozone exposure to females caused increased recruitment of immune cells. Accordingly, we hypothesized that, as compared with males, females show a relatively greater degree of perturbation in gene expression in response to ozone exposure. To our surprise, for all the tissue compartments analyzed, only five or six genes were differentially regulated when DEGs in ozone-exposed females were compared with DEGs in ozone-exposed males. Of note, these 5 or 6 DEGs were located on sex chromosomes. Interestingly, however, the fold changes for DEGs between ozone-exposed females versus air-exposed females were relatively greater than those in ozone-exposed males versus air-exposed males (Tables 2, 4, and 6). These findings suggest that while the directionality of transcriptomic changes to ozone-induced lung injury remains identical, the magnitude of these changes is more robust in females. However, because of the extended exposure paradigm (∼20 days), the analyses of estrous cyclicity on immune responses, as reported previously in acute study (18), could not be performed. This is because the estrous cycle generally repeats every 5 days in mice.
Ozone exposure results in three major categories of responses in the airways, i.e., epithelial sloughing, epithelial proliferation, and mucous cell metaplasia (34, 48). Gene signatures that were enriched in extrapulmonary airways, i.e., trachea, first-generation (main stem) bronchi, and second-generation bronchi were representative of cell cycle regulation, cell division, and DNA repair (Supplemental Table 4). Consistent with these responses and previous reports (33, 69), the airway epithelial cells exhibited marked cellular proliferation, as indicated by Ki-67 staining (Fig. 4, A–C). Similarly, the upregulation of gene signatures relevant to mucous cell metaplasia (Fig. 12) was consistent with the elevation of airway epithelial contents of Muc5b mRNA (Fig. 4, G–K), a marker of mucous cell metaplasia.
This study identifies novel airway signatures that have not been previously shown to be associated with ozone-induced lung disease. For instance, MUC13, transmembrane mucin encoded by Muc13, is significantly enriched in ozone-exposed airways. Since MUC13 is known to counter neutrophilic inflammation via reducing the release of neutrophil chemokines (57), its upregulation suggests a role of airway epithelial cells in protection against neutrophilic recruitment in ozone-exposed airspaces. Foxn4, a gene encoding FOXN4, alongside FOXJ1, promotes the expression of genes required for ciliogenesis (8). The expression of Foxn4 was ∼13- and ∼5-fold elevated in airways from ozone-exposed males and females, respectively, suggesting sex-specific association in ciliogenesis response. Calcb, yet another gene previously unidentified to be associated with ozone exposure, was upregulated in airways from ozone-exposed mice. CALCB is a neuropeptide that is expressed in neuroendocrine cells and sensory C fibers of airways (54), and its receptor, i.e., calcitonin gene-related peptide receptor 1 is known to mediate ozone-induced airway epithelial injury (49). Calcb was ∼9-fold upregulated in airways from ozone-exposed females as opposed to ∼2.8-fold in ozone-exposed males, which, consistent with exaggerated immune cell filtration in ozone-exposed females, suggests a relatively stronger stimulus toward neuroinflammation in females.
A large number of previously unknown signatures including Mafb, Fads2, C1q, Mmp14, and Awat1 were also upregulated in macrophages from ozone-exposed mice. MAFB (musculo-aponeurotic fibrosarcoma oncogene homolog B, encoded by Mafb), a transcription factor, is critical for commitment of hematopoietic stem cells toward myeloid lineage (72) and the suppression of macrophage self-renewal (3). MAFB is specifically expressed in the interstitial macrophages but not the alveolar macrophages (20). The fact that Mafb was upregulated in macrophages obtained from both, ozone-exposed males (FC = ∼15) and ozone-exposed females (FC = ∼12), provides interesting clues on mechanisms involving maintenance of macrophage population in ozone-exposed lungs. Specifically, it appears that the recruitment of interstitial macrophages, but not self-renewal, regulates macrophage population size in mice subchronically exposed to ozone. Interestingly, MAFB is also known to promote alternative activation of macrophages and regulates the efflux of cholesterol from foamy macrophages (30). The upregulated Mafb expression, together with enrichment of cholesterol biosynthetic pathways, suggests a critical role for MAFB in the regulation of macrophage function in ozone-exposed airspaces. However, the biological relevance of MAFB in ozone-exposed airways remains unclear and warrants further investigation.
Although it is well established that the elevated levels of ambient ozone are poorly tolerated by patients with ongoing mucoinflammatory lung diseases, such as asthma (1, 42, 65), it is unclear whether chronic ozone exposure results in asthma. To begin to address this question, we hypothesized that subchronic ozone exposure will induce differential expression of genes relevant to mucoinflammatory responses. Upregulation of mucous cell metaplasia-relevant gene signatures (Agr2, Areg, Slc26a4, Clca2, Clca4, Muc13, Muc15, Gcnt3, and Ereg) in the airway epithelium enriched tissues, Muc5b mRNA localization in the small and large airways, and upregulation of inflammatory signatures (Ccl2, Il1b, Ccl17, Areg, and Il33) in the airspace macrophages suggest similarities between airway disease due to subchronic ozone exposure and human asthma. While signatures commonly upregulated in asthmatic lungs of rodents and humans are clearly enriched in ozone-exposed murine lungs, further omics-based experiments and lung function studies are warranted.
In homeostasis, airspace macrophages remain functionally suppressed (25, 50). In ozone-exposed airways, macrophage activation, i.e., enhanced functioning, is a typical response that has been thoroughly investigated (21, 40, 60). In in vitro settings, classical (M1) and alternative (M2) activation responses are unequivocally induced by IFN-γ/LPS and IL-4 respectively (43). Gene signatures associated with macrophages could indicate the nature of their immediate microenvironment. Interestingly, gene signatures associated with both M1 and M2 were enriched in the macrophages from ozone-exposed mice, suggesting influences of upstream regulator cytokines, including both IFN-γ and IL-4 (Fig. 9). At this moment, it is unclear whether mixed M1/M2 signatures from harvested macrophage population reflect a mixed response at the population level (population heterogeneity) or at the cellular level (cellular plasticity). Future studies focusing on this aspect are desired, as M1 versus M2 signatures are generally divided alongside the dichotomy of pathogenic (proinflammatory) and protective (anti-inflammatory or tissue healing) functions.
This study also presents a technological advancement aimed at collecting homogenous and/or cell type-enriched compartments. We have previously used a MACS approach to deplete Ly6G-expressing neutrophils (55). Ly6G-expressing microbeads effectively capture neutrophils but not eosinophils. Although murine alveolar macrophages do not express CD11b (12), neutrophils and eosinophils express CD11b and efficiently bind to CD11b-antibodies. Therefore, we used a CD11b-microbead-based MACS approach (Supplemental Fig. S1, D and E) to effectively deplete neutrophils as well as eosinophils. On a cautionary note, this approach alone would not be suitable to purify macrophages in conditions, where epithelial cells that are also CD11bneg, are present in the BALF.
Although airspace macrophages do not express CD11b in healthy mice, our unpublished observations and other studies (16, 68) report the recruitment of CD11b+ monocyte/macrophage populations in the airspaces of ozone-exposed mice. Therefore, as a caveat to this study, macrophage transcriptomes reported here correspond specifically to CD11bneg macrophages. Accordingly, we believe that the gene signatures and their associated pathways would have been different in relatively smaller CD11b+ myeloid (monocytic and dendritic cell) subpopulations. Further studies on comparison of CD11b+ versus CD11bneg myeloid cells might shed light on the comparative role of resident versus recruited macrophages.
Although our study is the first to explore compartment-specific transcriptomic perturbations in subchronically ozone-exposed mice, a recent study comprehensibly explored transcriptomic changes in alveolar macrophages and conducting airways from female mice that were acutely exposed to ozone (69). We compared DEGs that were upregulated in the two comparable compartments, i.e., alveolar macrophages and conducting airways, between our subchronic study and previously published acute exposure study (69). Despite differences in the exposure paradigm, i.e., ozone dose [1 ppm (acute) versus 0.8 ppm (sub-chronic)], airways sampling approach [conducting zone generation 0–5 (acute) versus conducting zone generation 0–2 (subchronic)], and macrophage populations [adherent alveolar macrophages (acute) versus CD11bneg alveolar macrophages (subchronic)], a large number of common upregulated transcripts were found between the two studies [Supplemental Tables S8 (macrophages) and S9 (conducting airways)]. These comparative analyses suggest that a significant proportion of the DEGs, which are induced by a one-time (acute) encounter to ozone, persist upon repeated encounters (subchronic). A significant proportion of the upregulated transcripts were also exposure paradigm-specific, indicating differential pathogenesis of the acute versus chronic ozone-induced lung injury. Because males were not investigated in the acute ozone exposure paradigm, comparative analyses between males could not be conducted.
In conclusion, gene expression and pathway analyses supported by cellular recruitment, immunostaining, and RNAscope analysis reported in this study enhance our scientific understanding of cell-specific molecular changes in murine ozone-induced lung diseases in multiple ways. First, sex influences the differential expression of genes more robustly in ozone-exposed females versus ozone-exposed males. Second, transcriptomic responses to ozone exposure in the airway epithelium are largely associated with enrichment of pathways of cell division and DNA repair. Genes associated with mucin synthesis, mucous cell metaplasia, and mucus production are also enriched in the airways following ozone exposure. Third, the transcriptomic profile of the parenchyma from ozone-exposed mice suggests mixed signatures from alveolar epithelial cells, and, most likely, immune and nonimmune cells of interstitial origin. Fourth, transcriptome of alveolar macrophages from ozone-exposed mice reflects dynamic responses encompassing a wide range of biological pathways associated with immune and inflammatory responses. Fifth, mixed M1/M2 macrophage activation responses suggest the presence of an admixture of upstream regulators. Collectively, our data highlight dynamic compartment/cell-specific and sex-specific gene expression responses in subchronically ozone-exposed mice. This study sets a stage for the future studies focused on identifying transcriptomic changes in more refined homogenous cell populations from different lung compartments.
GRANTS
The work was supported by National Institute of Environmental Health Sciences Grant R01 ES030125 (to Y.S.).
GeneLab is supported by Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences (Grants P20 GM103424 and P20 GM130555).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P. and Y.S. conceived and designed research; I.C., T.V., K.P., S.P. and Y.S. performed experiments; I.C., T.V., K.P., S.P. and Y.S. analyzed data; S.P. and Y.S. interpreted results of experiments; S.P. and Y.S. prepared figures; S.P. and Y.S. drafted manuscript; S.P. and Y.S. edited and revised manuscript; I.C., T.V., K.P., S.P. and Y.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sherry Ring for histological tissue processing. We thank Dr. Samir Kelada (University of North Carolina at Chapel Hill) for discussion and suggestions on the comparison of our study with their published acute exposure study. We acknowledge the GeneLab for providing core services and access to Bioinformatics software.
REFERENCES
- 1.Anenberg SC, Henze DK, Tinney V, Kinney PL, Raich W, Fann N, Malley CS, Roman H, Lamsal L, Duncan B, Martin RV, van Donkelaar A, Brauer M, Doherty R, Jonson JE, Davila Y, Sudo K, Kuylenstierna JCI. Estimates of the global burden of ambient PM2.5, ozone, and NO2 on asthma incidence and emergency room visits. Environ Health Perspect 126: 107004, 2018. doi: 10.1289/EHP3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science 326: 867–871, 2009. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 4.Backus GS, Howden R, Fostel J, Bauer AK, Cho H-Y, Marzec J, Peden DB, Kleeberger SR. Protective role of interleukin-10 in ozone-induced pulmonary inflammation. Environ Health Perspect 118: 1721–1727, 2010. doi: 10.1289/ehp.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA 292: 2372–2378, 2004. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova A, Cyphert-Daly J, Cumming RI, Yu Y-R, Gowdy KM, Que LG, Tighe RM. Sex modifies acute ozone-mediated airway physiologic responses. Toxicol Sci 169: 499–510, 2019. doi: 10.1093/toxsci/kfz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabello N, Mishra V, Sinha U, DiAngelo SL, Chroneos ZC, Ekpa NA, Cooper TK, Caruso CR, Silveyra P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol 309: L1150–L1163, 2015. doi: 10.1152/ajplung.00018.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell EP, Quigley IK, Kintner C. Foxn4 promotes gene expression required for the formation of multiple motile cilia. Development 143: 4654–4664, 2016. doi: 10.1242/dev.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- 10.Cho Y, Abu-Ali G, Tashiro H, Brown TA, Osgood RS, Kasahara DI, Huttenhower C, Shore SA. Sex differences in pulmonary responses to ozone in mice. role of the microbiome. Am J Respir Cell Mol Biol 60: 198–208, 2019. doi: 10.1165/rcmb.2018-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciencewicki JM, Verhein KC, Gerrish K, McCaw ZR, Li J, Bushel PR, Kleeberger SR. Effects of mannose-binding lectin on pulmonary gene expression and innate immune inflammatory response to ozone. Am J Physiol Lung Cell Mol Physiol 311: L280–L291, 2016. doi: 10.1152/ajplung.00205.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan M, Steinfort DP, Smallwood D, Hew M, Chen W, Ernst M, Irving LB, Anderson GP, Hibbs ML. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs. Mucosal Immunol 9: 550–563, 2016. doi: 10.1038/mi.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entwistle MR, Gharibi H, Tavallali P, Cisneros R, Schweizer D, Brown P, Ha SU. Ozone pollution and asthma emergency department visits in Fresno, CA, USA, during the warm season (June–September) of the years 2005 to 2015: a time-stratified case-crossover analysis. Air Qual Atmos Health 12: 661–672, 2019. doi: 10.1007/s11869-019-00685-w. [DOI] [Google Scholar]
- 14.Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med 6: 15–20, 2000. doi: 10.1097/00063198-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis M, Groves AM, Sun R, Cervelli JA, Choi H, Laskin JD, Laskin DL. Editor’s Highlight: ccr2 regulates inflammatory cell accumulation in the lung and tissue injury following ozone exposure. Toxicol Sci 155: 474–484, 2017. doi: 10.1093/toxsci/kfw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuentes N, Silveyra P. Lung microRNA profiling across the estrous cycle in ozone-exposed mice. J Vis Exp (143): 2019. doi: 10.3791/58664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabehart K, Correll KA, Yang J, Collins ML, Loader JE, Leach S, White CW, Dakhama A. Transcriptome profiling of the newborn mouse lung response to acute ozone exposure. Toxicol Sci 138: 175–190, 2014. doi: 10.1093/toxsci/kft276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, Leach SM, Bratton DL, Henson PM, Janssen WJ, Jakubzick CV. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol 57: 66–76, 2017. doi: 10.1165/rcmb.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves AM, Gow AJ, Massa CB, Hall L, Laskin JD, Laskin DL. Age-related increases in ozone-induced injury and altered pulmonary mechanics in mice with progressive lung inflammation. Am J Physiol Lung Cell Mol Physiol 305: L555–L568, 2013. doi: 10.1152/ajplung.00027.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch GE, Duncan KE, Diaz-Sanchez D, Schmitt MT, Ghio AJ, Carraway MS, McKee J, Dailey LA, Berntsen J, Devlin RB. Progress in assessing air pollutant risks from in vitro exposures: matching ozone dose and effect in human airway cells. Toxicol Sci 141: 198–205, 2014. doi: 10.1093/toxsci/kfu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatch GE, McKee J, Brown J, McDonnell W, Seal E, Soukup J, Slade R, Crissman K, Devlin R. Biomarkers of dose and effect of inhaled ozone in resting versus exercising human subjects: comparison with resting rats. Biomark Insights 8: 53–67, 2013. doi: 10.4137/BMI.S11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med 150: 676–683, 1994. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 25.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14: 81–93, 2014. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 26.Jang A-S, Choi I-S, Koh Y-I, Park C-S, Lee J-S. The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy 57: 737–740, 2002. doi: 10.1034/j.1398-9995.2002.23569.x. [DOI] [PubMed] [Google Scholar]
- 27.Jerrett M, Burnett RT, Pope CA III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 360: 1085–1095, 2009. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehrl HR, Vincent LM, Kowalsky RJ, Horstman DH, O’Neil JJ, McCartney WH, Bromberg PA. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis 135: 1124–1128, 1987. doi: 10.1164/arrd.1987.135.5.1124. [DOI] [PubMed] [Google Scholar]
- 29.Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, Schmitt MT, Case M, Devlin RB, Peden DB, Diaz-Sanchez D. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med 183: 1215–1221, 2011. doi: 10.1164/rccm.201011-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci Rep 7: 7591, 2017. doi: 10.1038/s41598-017-07381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooter IM, Pennings JL, Fokkens PH, Leseman DL, Boere AJ, Gerlofs-Nijland ME, Cassee FR, Schalk JA, Orzechowski TJ, Schaap MM, Breit TM, Dormans JA, van Oostrom CT, de Vries A, van Steeg H. Ozone induces clear cellular and molecular responses in the mouse lung independently of the transcription-coupled repair status. J Appl Physiol (1985) 102: 1185–1192, 2007. doi: 10.1152/japplphysiol.00796.2006. [DOI] [PubMed] [Google Scholar]
- 32.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumagai K, Lewandowski R, Jackson-Humbles DN, Li N, Van Dyken SJ, Wagner JG, Harkema JR. Ozone-induced Nasal Type 2 immunity in mice is dependent on innate lymphoid cells. Am J Respir Cell Mol Biol 54: 782–791, 2016. doi: 10.1165/rcmb.2015-0118OC. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai K, Lewandowski RP, Jackson-Humbles DN, Buglak N, Li N, White K, Van Dyken SJ, Wagner JG, Harkema JR. Innate lymphoid cells mediate pulmonary eosinophilic inflammation, airway mucous cell metaplasia, and type 2 immunity in mice exposed to ozone. Toxicol Pathol 45: 692–704, 2017. doi: 10.1177/0192623317728135. [DOI] [PubMed] [Google Scholar]
- 35.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol 130: 572–584, 2012. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis BW, Choudhary I, Paudel K, Mao Y, Sharma R, Wang Y, Deshane JS, Boucher RC, Patial S, Saini Y. The innate lymphoid system is a critical player in the manifestation of mucoinflammatory airway disease in mice. J Immunol 205: 1695–1708, 2020. doi: 10.4049/jimmunol.2000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis BW, Sultana R, Sharma R, Noël A, Langohr I, Patial S, Penn AL, Saini Y. Early postnatal secondhand smoke exposure disrupts bacterial clearance and abolishes immune responses in muco-obstructive lung disease. J Immunol 199: 1170–1183, 2017. doi: 10.4049/jimmunol.1700144. [DOI] [PubMed] [Google Scholar]
- 38.Lewis BW, Vo T, Choudhary I, Kidder A, Bathula C, Ehre C, Wakamatsu N, Patial S, Saini Y. Ablation of IL-33 suppresses Th2 responses but is accompanied by sustained mucus obstruction in the Scnn1b transgenic mouse model. J Immunol 204: 1650–1660, 2020. doi: 10.4049/jimmunol.1900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P, Zhou H, Xu Y, Shang B, Feng Z. The effects of elevated ozone on the accumulation and allocation of poplar biomass depend strongly on water and nitrogen availability. Sci Total Environ 665: 929–936, 2019. doi: 10.1016/j.scitotenv.2019.02.182. [DOI] [PubMed] [Google Scholar]
- 40.Mathews JA, Kasahara DI, Ribeiro L, Wurmbrand AP, Ninin FM, Shore SA. γδ T cells are required for M2 macrophage polarization and resolution of ozone-induced pulmonary inflammation in mice. PLoS One 10: e0131236, 2015. doi: 10.1371/journal.pone.0131236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, Williams AS, Wurmbrand AP, Ribeiro L, Cuttitta F, Sunday ME, Levy BD, Shore SA. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol 58: 341–351, 2018. doi: 10.1165/rcmb.2017-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res 80: 110–121, 1999. doi: 10.1006/enrs.1998.3894. [DOI] [PubMed] [Google Scholar]
- 43.Mosser DM, Zhang X. . Activation of murine macrophages. Curr Protoc Immunol 83: 14.2.1–14.2.8, 2008. doi: 10.1002/0471142735.im1402s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller B, Seifart C, Barth PJ. Effect of air pollutants on the pulmonary surfactant system. Eur J Clin Invest 28: 762–777, 1998. doi: 10.1046/j.1365-2362.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 45.Nadadur SS, Costa DL, Slade R, Silbjoris R, Hatch GE. Acute ozone-induced differential gene expression profiles in rat lung. Environ Health Perspect 113: 1717–1722, 2005. doi: 10.1289/ehp.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakes JL, O’Connor BP, Warg LA, Burton R, Hock A, Loader J, Laflamme D, Jing J, Hui L, Schwartz DA, Yang IV. Ozone enhances pulmonary innate immune response to a Toll-like receptor-2 agonist. Am J Respir Cell Mol Biol 48: 27–34, 2013. doi: 10.1165/rcmb.2012-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol 283: R7–R28, 2002. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 48.Ong CB, Kumagai K, Brooks PT, Brandenberger C, Lewandowski RP, Jackson-Humbles DN, Nault R, Zacharewski TR, Wagner JG, Harkema JR. Ozone-induced type 2 immunity in nasal airways. development and lymphoid cell dependence in mice. Am J Respir Cell Mol Biol 54: 331–340, 2016. doi: 10.1165/rcmb.2015-0165OC. [DOI] [PubMed] [Google Scholar]
- 49.Oslund KL, Hyde DM, Putney LF, Alfaro MF, Walby WF, Tyler NK, Schelegle ES. Activation of calcitonin gene-related peptide receptor during ozone inhalation contributes to airway epithelial injury and repair. Toxicol Pathol 37: 805–813, 2009. doi: 10.1177/0192623309345691. [DOI] [PubMed] [Google Scholar]
- 50.Patial S, Saini Y. Lung macrophages: current understanding of their roles in Ozone-induced lung diseases. Crit Rev Toxicol 50: 310–323, 2020. doi: 10.1080/10408444.2020.1762537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulin LM, Kaufman JD, Hansel NN. Concerns remain regarding long-term ozone exposure and respiratory outcomes-reply. JAMA Intern Med 180: 804, 2020. doi: 10.1001/jamainternmed.2020.0577. [DOI] [PubMed] [Google Scholar]
- 52.Peirson SN, Foster RG. Bad light stops play. EMBO Rep 12: 380, 2011. doi: 10.1038/embor.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfister GG, Walters S, Lamarque J-F, Fast J, Barth MC, Wong J, Done J, Holland G, Bruyère CL. Projections of future summertime ozone over the U.S. J Geophys Res: Atmospheres 119: 5559–5582, 2014. doi: 10.1002/2013JD020932. [DOI] [Google Scholar]
- 54.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142, 2014. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saini Y, Dang H, Livraghi-Butrico A, Kelly EJ, Jones LC, O’Neal WK, Boucher RC. Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics 15: 726, 2014. doi: 10.1186/1471-2164-15-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schelegle ES, Morales CA, Walby WF, Marion S, Allen RP. 6.6-hour inhalation of ozone concentrations from 60 to 87 parts per billion in healthy humans. Am J Respir Crit Care Med 180: 265–272, 2009. doi: 10.1164/rccm.200809-1484OC. [DOI] [PubMed] [Google Scholar]
- 57.Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, Sutton P, McGuckin MA. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol 6: 557–568, 2013. doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- 58.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strosnider HM, Chang HH, Darrow LA, Liu Y, Vaidyanathan A, Strickland MJ. Age-specific associations of ozone and fine particulate matter with respiratory emergency department visits in the United States. Am J Respir Crit Care Med 199: 882–890, 2019. doi: 10.1164/rccm.201806-1147OC. [DOI] [PubMed] [Google Scholar]
- 60.Sunil VR, Patel-Vayas K, Shen J, Laskin JD, Laskin DL. Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol Appl Pharmacol 263: 195–202, 2012. doi: 10.1016/j.taap.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47: D607–D613, 2019. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tashiro H, Kasahara DI, Osgood RS, Brown T, Cardoso A, Cho Y, Shore SA. Sex differences in the impact of dietary fiber on pulmonary responses to ozone. Am J Respir Cell Mol Biol 62: 503–512, 2020. doi: 10.1165/rcmb.2019-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatum AJ, Shapiro GG. The effects of outdoor air pollution and tobacco smoke on asthma. Immunol Allergy Clin North Am 25: 15–30, 2005. doi: 10.1016/j.iac.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5: 508, 2014. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tétreault LF, Doucet M, Gamache P, Fournier M, Brand A, Kosatsky T, Smargiassi A. Childhood exposure to ambient air pollutants and the onset of asthma: an administrative cohort study in Québec. Environ Health Perspect 124: 1276–1282, 2016. doi: 10.1289/ehp.1509838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Gene Ontology Consortium The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res 47: D330–D338, 2019. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson KC, Jones SH, Rennie AR, King MD, Ward AD, Hughes BR, Lucas CO, Campbell RA, Hughes AV. Degradation and rearrangement of a lung surfactant lipid at the air-water interface during exposure to the pollutant gas ozone. Langmuir 29: 4594–4602, 2013. doi: 10.1021/la304312y. [DOI] [PubMed] [Google Scholar]
- 68.Tighe RM, Li Z, Potts EN, Frush S, Liu N, Gunn MD, Foster WM, Noble PW, Hollingsworth JW. Correction: ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J Immunol 196: 2424, 2016. doi: 10.4049/jimmunol.1600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tovar A, Smith GJ, Thomas JM, Crouse WL, Harkema JR, Kelada SNP. Transcriptional profiling of the murine airway response to acute ozone exposure. Toxicol Sci 173: 114–130, 2020. doi: 10.1093/toxsci/kfz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasu VT, Oommen S, Lim Y, Valacchi G, Hobson B, Eiserich JP, Leonard SW, Traber MG, Cross CE, Gohil K. Modulation of ozone-sensitive genes in alpha-tocopherol transfer protein null mice. Inhal Toxicol 22: 1–16, 2010. doi: 10.3109/08958370902838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verhein KC, McCaw Z, Gladwell W, Trivedi S, Bushel PR, Kleeberger SR. Novel roles for Notch3 and Notch4 receptors in gene expression and susceptibility to ozone-induced lung inflammation in mice. Environ Health Perspect 123: 799–805, 2015. doi: 10.1289/ehp.1408852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X, Briseño CG, Durai V, Albring JC, Haldar M, Bagadia P, Kim KW, Randolph GJ, Murphy TL, Murphy KM. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J Exp Med 213: 2553–2565, 2016. doi: 10.1084/jem.20160600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol Lung Cell Mol Physiol 274: L39–L46, 1998. doi: 10.1152/ajplung.1998.274.1.L39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the raw data have been submitted to the Gene Expression Omnibus (GEO) database (GSE156799).