Abstract
Tissue engineering (TE) is a rapidly growing interdisciplinary field, which aims to restore or improve lost tissue function. Despite that TE was introduced more than 20 years ago, innovative and more sophisticated trends and technologies point to new challenges and development. Current challenges involve the demand for multifunctional bioscaffolds which can stimulate tissue regrowth by biochemical curves, biomimetic patterns, active agents and proper cell types. For those purposes especially promising are carefully chosen primary cells or stem cells due to its high proliferative and differentiation potential. This review summarized a variety of recently reported advanced bioscaffolds which present new functions by combining polymers, nanomaterials, bioactive agents and cells depending on its desired application. In particular necessity of study biomaterial-cell interactions with in vitro cell culture models, and studies using animals with in vivo systems were discuss to permit the analysis of full material biocompatibility. Although these bioscaffolds have shown a significant therapeutic effect in nervous, cardiovascular and muscle, tissue engineering, there are still many remaining unsolved challenges for scaffolds improvement.
Keywords: Innovative materials, Biomimetric materials, Scaffolds, Regenerative medicine
Graphical abstract
Highlights
-
•
Challenges, limitations and future prospects in tissue engineering are presented.
-
•
Strategies for enhanced neural, cardiovascular and muscle regeneration are discussed.
-
•
Topographical, mechanical and chemical guiding enhance nerves regeneration.
-
•
Multifunctional scaffolds provide cardiac tissue-like environment.
-
•
Hybrid biomaterials mimic and improve regeneration of muscle tissue.
1. Introduction
Currently, one of the most intensively studied field of medicine is regenerative medicine (RM), it is a broad field about the potential and ability to regenerate and replace damaged tissues and organs. Recently regenerative medicine has shown a number of promising results for the regeneration variety of tissues and organs including joints, bones, skin, cardiovascular and nervous system [[1], [2], [3], [4], [5], [6], [7], [8]]. The main strategies of RM are i. cell therapies, that aim to injection of stem cells to induce direct regeneration and rebuild tissues and organs; ii. Immunomodulation therapies which involve biologically active molecules which stimulate tissues to regenerate; and iii. Tissue engineering. The tissue engineering (TE) field is mainly based on applying scaffold for cell attachment and growth by designing and fabricating three-dimensional cell-containing matrices that can be implanted into the body to disease treatment or defect repair [9]. Analogous to the natural extracellular matrix topography of scaffold regulate cell behavior. Scaffolds morphology and composition influence on cell adhesion, proliferation, differentiation and migration. There are multiple requirements for scaffolds usage in TE. Such scaffolds should be biocompatible, immunologically inert and support the normal functioning of cells and tissues. The most important requirement of biomaterials for scaffold applications is biocompatibility which refers to a wide range of effects that access possible clinical usage. The most intensively studied is material cytotoxicity which is determined by cell lysis leading to apoptosis or the inhibition of cell proliferation. Scaffolds should exhibit a lack of cytotoxic effect toward cells, which should be deeply investigated over a long period of time. Another aspect of biocompatibility is also the absence of genotoxicity in particular DNA destruction, chromosomal aberrations and gene mutations [10]. Carcinogenicity is another aspect of material biocompatibility that should be carefully investigated, especially according to tissue organization field theory (TOFT). TOFT claiming that cancer arises from the deregulation of extracellular matrix (ECM) architecture. It is well-known that changes in native ECM micro/nano environment and composition lead to local stiffening, tissue fibrosis which enhanced cancer development [11]. Therefore materials structure, architecture and composition should imitate the architecture of native ECM as more precise as it’s possible to fully mimic target tissue environment. Furthermore, scaffolds should be immunologically inert or influence minimal immunological reaction. When biomaterial induce inflammatory response by inducing foreign body reaction, that can lead to rejection of the implant [12]. The degradation products also cannot cause toxicity toward cells. It should be also considered while designing natural biomaterials due to their possible bioactive degradation products which can stimulate immunological response [12]. Therefore scaffolds for possible biomedical applications should be carefully examinated in terms of their long-term toxicity which can be crucial for their clinical trials. In vitro cell culture studies are valuable in investigating the effects of biomaterial-cell interactions, while in vivo studies using animals permit the analysis of full material biocompatibility.
There are numerous materials used to fabricate scaffolds, but polymers are the most popular basal materials for scaffolds production [13,14]. Those polymers can be categorized on two groups natural and synthetic which can be divided into biodegradable and non-degradable. Usage of certain polymer type and its composition depends on the target application. Table 1 compares the most popular polymers, their advantages, limitations and promising usage in different tissue engineering fields. Natural biomaterials are often processed from either whole ECMs or purified certain ECM components. Alternatively, pure ECM architecture and composition can be obtained by removing the cellular components from tissues by a process called decellularization of ECM. Many reports show the possibility of decellularization of tissues and even organs. Decellular scaffolds have no cells in structure and require recellularization by proper cell type. Their clinical use has been documented for TE applications such as blood, cardiac valves and renal bladders. Nevertheless, these acellular constructs differ depending on the source and isolation method which is one of the main disadvantages. The natural origin of that biomaterials is the potential danger of infection which potentially can lead to donor-derived infection. However, the main limitation is necessity for chemical usage during isolation and complicated preparation process. This can potentially trigger high immune response and inflammation [15,16].
Table 1.
The most popular polymers for scaffolds fabrication, their main advantages and limitations and current potential application in different tissue engineering fields.
| POLYMER | TYPE | EXAMPLE | ADVANTAGES | LIMITATIONS | PROMISING IN | REF. |
|---|---|---|---|---|---|---|
| Natural | polysaccharides | chitosan | biocompatibility, hemostatic activity, biodegradability, antibacterial activity, easily metabolized | stiff, brittle, low mechanical resistance | skin, nervous, bone, cartilage, cardiac, liver, and muscle tissue engineering | [[17], [18], [19], [20], [21], [22], [23]] |
| cellulose | biocompatibility, bioactivity, good mechanical properties depending on the source | non-biodegradable | skin, neural, bone, cardiovascular, muscle, tendons, cartilage regeneration | [[24], [25], [26], [27], [28]] | ||
| alginate | biocompatibility, non-immunogenicity, biodegradability, non-antigenicity, bioactivity | limited strength, toughness, difficulty in controlled gelation | skin, cartilage, bone, neural regeneration | [20,[29], [30], [31], [32], [33], [34]] | ||
| hyaluronic acid | biocompatibility, biodegradability, easy chemical modification, bioactivity | poor mechanical properties, rapid degradation | neural, skin, regeneration | [[35], [36], [37], [38], [39], [40], [41], [42]] | ||
| proteins | collagen | biocompatible, biodegradable, ECM mimicking, poorly immunogenic, bioactive | poor mechanical properties, | skin, cornea, dental, vascular, cartilage, bone regeneration | [41,[43], [44], [45], [46], [47], [48]] | |
| gelatin | biocompatible, biodegradable, ECM mimicking, low immunogenic, inexpensive, water-soluble, bioactive | poor mechanical properties, fast enzymatic degradation, low solubility in concentrated aqueous media | skin, bone, cartilage, adipose neural, regeneration | [[49], [50], [51], [52], [53], [54]] | ||
| fibrin | biocompatible, biodegradable, ECM mimicking, low immunogenic | rapid degradation rate, poor mechanical properties, expensive, risk of contamination | liver, retina, cartilage, vascular, neural regeneration | [[55], [56], [57], [58], [59]] | ||
| silk fibroin | biocompatibility, biodegradability, bioactivity, low immunogenic, high tensile strength, excellent mechanical properties, water-based processing, low cost | Weak, brittle as scaffolds. | skin, vascular, bone, cartilage, tendon, cornea, hepatic, Neural regenration | [[60], [61], [62], [63], [64], [65]] | ||
| elastin | biocompatibility, bioactivity, good biophysical and biomechanical properties | Water-insoluble, difficult to manipulate in vitro, risk of contamination, risk of inflammation, difficulties in sourcing | skin, cartilage, cardiovascular, tendon, skin, liver regeneration | [[66], [67], [68], [69], [70], [71], [72]] | ||
| Synthetic | Biodegradable | PCL | biocompatible, easy to modificate and fabricate, good organic solvent solubility, controllable degradation rate, inexpensive, good mechanical properties, thermoplastic | poor cellular adhesion due to hydrophobicity, relatively slow degradation rate (2–4 years), | skin, bone, vascular | [[73], [74], [75], [76], [77], [78]] |
| PLA | biocompatibility, easy to modificate and fabricate, obtained from renewable sources, | lack of bioactivity, low cell adhesion, biological inertness, acid degradation by-products, risk of inflammation, low porosity, low degradation rate (but faster than PCL) | skin, bone, cardiovascular, cartilage, ligament, neural regeneration | [[79], [80], [81], [82], [83], [84], [85]] | ||
| PGA | biocompatible, bioresorbability, high tensile strength, | fast degradation rate, acidic degradation products, low solubility | bone, cartilage, ligament regeneration | [[86], [87], [88], [89], [90], [91]] | ||
| Non-biodegradable | PDMS | biocompatibility, easy to fabricate, flexible, thermo-tolerant, tunable hardness, good biostability, the high solubility of oxygen in PDMS, | non-bioactivity due to hydrophobicity, non-biodegradable | skin, bone, neural regeneration | [[92], [93], [94], [95], [96]] | |
| PPy | electrical conductivity, easy to synthesized, environmental stability, low inflammatory response, | non-biodegradable, not easy to modify, non-thermoplastic, water insoluble, mechanically rigid, brittle, possible long-term toxicity, non-biodegradable | neural cardiovascular, liver regeneration | [[97], [98], [99], [100], [101], [102], [103], [104]] | ||
| PVDF | piezoelectric properties, high flexibility, non-toxicity, chemical and physical resistance | hydrophobicity, insufficient biocompatibility, non-bioactive, non-biodegradable | bone, neural, bladder, skeletal muscle regeneration | [[105], [106], [107], [108], [109], [110]] |
Natural polymers can be classified as polysaccharides (chitosan, cellulose, alginate, chitin, hyaluronic acid, and dextran) and proteins (collagen, gelatin, fibrin, elastin, silk, keratin, actin, and myosin). The greatest advantage of scaffolds made of naturally derived sources is their great biocompatibility and more closely mimicking natural ECM. Bioscaffolds refer to naturally-derived scaffolds made by natural polymers or with the addition of active bioagents. Because of their natural origin natural polymers tend to be highly bioactive what support cell attachment and growth. Scaffolds are environmental friendly what is another advantage of their usage in tissue engineering. However, materials derived from humans and animals hold a serious risk of potential diseases. Moreover, most of the natural polymers exhibit poor mechanical properties and a fast degradation rate. Proper chemical modification as well as crosslinking can overcome these disadvantages, contributing to enhanced mechanical properties. On the other hand, synthetic materials are also often used as scaffolds. The main advantage of synthetic polymers is their excellent mechanical properties, such as viscosity, strength, solubility and controllable degradation. There are many examples of synthetic polymers with conductive and piezoelectric properties which makes them attractive in electrically sensitive tissues such as nerve and heart muscle. Another benefit of some synthetic polymers is thermoplastic properties which make them easy-to-fabricate leading to versatility in fabrication. However polymeric degradation products could induce long term toxicity causing inflammation. Another drawback is the lack of cell-binding sites due to their hydrophobicity which makes them unattractive for the biomedical field. Fully synthetic scaffolds are generally composed of manufactured polymers, metals, or other synthetically derived substrates. Synthetic polymers can be precisely manufactured and therefore their properties such as mechanical strength and degradation rate can be readily tuned. Consequently, multiple polymers can be easily integrated within one material to obtain composite. An especially promising approach is to combine synthetic polymers characterized by good mechanical properties with natural biomaterials as they provide natural micro/nano environmental niche for functional tissue regeneration. To improve biological properties, scaffolds can also be enriched with bioactive signaling molecules. Commonly it could be adhesive peptides, extracellular matrix proteins, growth factors, cytokines, or hormones. These bioactive agents can have profound biologic activity leading to direct cell adhesion, proliferation, modulate cell survival, vascularization and targeting differentiation fate of stem cells. Such bioscaffolds achieve both the 3D matrix structure of the native ECM and the natural ligand landscape [111]. Designing and fabricating an ECM scaffold that fully mimics the biochemistry and architecture of native tissue ECM can be achieved by careful selection of the materials, bioactive additives and fabrication technique. The proper method for obtaining the 3D bioscaffolds enables their desire application and functional character. Typical scaffold architecture is made by 3D printing, electrospinning, lithography methods that enable to obtain fibers, hydrogels, meshes, sponges or foams.
Proper choice of a cell type model is another crucial aspect of basic research and possible transplantation success. Cell lines are broadly available, easy to maintain and cultivate. Mostly are immortalized through genetic manipulations by e.g. integration of relevant genes by viral transfections. Companies provide a wide range of immortal cell lines under constant growing conditions derived from healthy and unhealthy donors. However, during numerous passages, cells exhibit alterations in morphology, growth rates and response to stimuli compared to lower passage cells. Mentioned alterations often occur in parallel with cellular mutations, therefore continual cell lines subculture intensify genomic instabilities. Additionally, because of high immunogenicity, cell lines are not proper for clinical use. Despite those disadvantages, cell lines are useful as a proof of concept and basic research study. Primary cells are mature cells derived directly from tissue or organ of interest without viral transfections and any modifications, representing a better physiological model than cell lines [15]. These cells can be isolated from certain patients, cultivated in vitro on the scaffold and then transplanted in the target place of the host body. Primary cell transplantation gives less immunogenic response than cell lines which gives great clinical potential. The main limitation of the use of primary cells in tissue engineering tends to dedifferentiation followed by a low proliferation rate. On the other hand, primary cells have low capacity to differentiate, and many cell types should be isolated to rebuilding multicellular construct which could be challenging due to their limited quantity and accessibility [112]. Another useful cell model is stem cells. A wide range of stem cells are used in tissue engineering, including mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), cardiac stem cells (CSCs), neural stem cells (NSCs), muscle stem cells, dental pulp stem cells (DPSCs), and induced pluripotent stem cells (iPSCs). In general, their availability in hosts is limited and the origin of certain stem cells raises ethical doubts. Commercially available stem cells should be considered only as in vitro model, because of their immortalization and potentially triggering immune responses. Moreover, immortal stem cells, similar to cell lines often differ in function from their in vivo counterparts. iPSCs reprogramed from host somatic cells have gained increasing attention. That stem cells can differentiate into cell types of all three germ layers giving huge opportunities in tissue engineering. In the beginning, scientists assumed no risk of rejection after iPSCs transplantation, but the immune rejection was observed after transplantation of autologous iPSC-derived cells. That suggests the impact of in vitro operations on the immunogenicity of the iPSC [113]. An interesting approach is that iPSCs offer the opportunity to correct pathogenic genetic variants in advance of transplantation in the mutation-carrying patient. The limitation is time-consuming protocols that require multiple complicated intermediate steps [15]. Despite that, iPSCs exhibit a low risk for teratoma formation and immune response but reveal the risk of tumorigenesis. Over that unknown is the impact of reprogramming somatic cells on the epigenetic modifications and their overall safety.
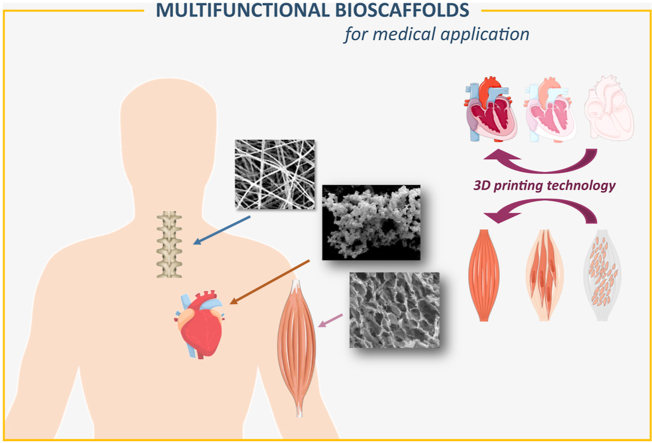
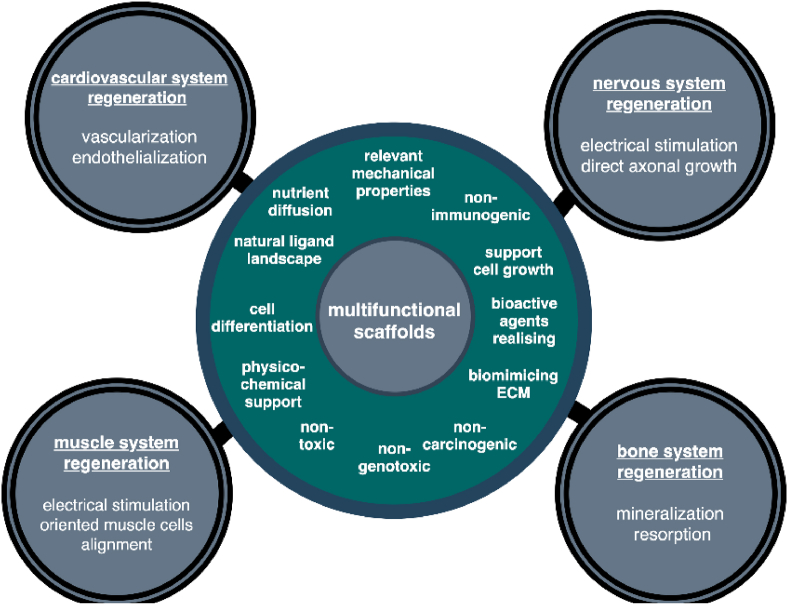
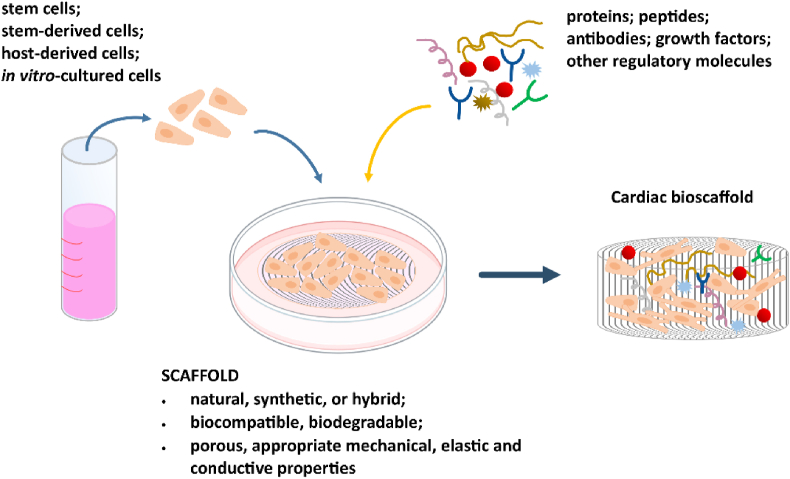
Bioscaffolds beyond mimicking of native ECM and interaction with cells, can influence more than one cell type and provide additional advanced functions. This includes releasing bioactive agents such as antibacterial molecules to prevent infection; growth factors to induce direct cell differentiation and anti-inflammatory agents to prevent excessive inflammation. Specific cell types incorporation within scaffold structure also provide new function, by their active spreading, releasing their growth factors leading to active tissue regeneration. In this regard, highly desirable are multifunctional scaffolds that provide physicochemical support to many cell types and deliver bioagents/drugs/antibacterial molecules. Such multifunctional bioscaffolds gained attention as the new generation of biomaterials for applied cardiovascular, nervous, muscle and bone tissue engineering as shown in Fig. 1. This review highlight recent insights of multifunctional biomaterials fabricating in order to be applied in clinical practice. The review provides crucial information about the biological effect of biomaterials in cardiovascular, muscle and nervous tissues regeneration as electrical sensitive systems. Due to the many works in this area in recent years, the aim of the review was to identify the latest trends in this field, with particular emphasis on the role of primary materials, which not only provide scaffolds but also support that enhance cell adhesion, proliferation, and differentiation. This approach allows for a broader view of bioactive materials, both in the research context but also in the application context, and an analysis of the polymers used, taking into account their nature and structure. It should be noted that the authors deliberately omitted the aspect related to the regeneration of the bone tissue due to many interesting and very detailed reviews in this area [[114], [115], [116], [117], [118]]. These works include scaffolds based on hydroxyapatite, as well as a number of polymers, including bio-polymers (e.g. cellulose, chitosan, gelatin, alginate and fibroin as well as and synthetic polymers (e.g. poly(lacticacid) (PLA), poly(glycolic acid) (PGA), and their copolymers PLGA] [[119], [120], [121], [122], [123], [124]]. The hybrid solutions such as hydroxyapatite/collagen [[125], [126], [127]], poly l-lactic acid [128,129] or κ-carrageenan [[130], [131], [132]] scaffolds or lanthanide-doped hydroxyapatite [8,[133], [134], [135]] for bone and osteochondral regeneration were also proposed and described in literature. Therefore this review highlights the new achievements, emerging trends and strategies in the field of neural, cardiovascular and muscle tissue engineering. Challenges, limitations and future prospects in tissue engineering are discussed.
Fig. 1.
Multifunctional bioscaffold's requirements and their possible usage in different areas of tissue engineering.
2. Bioscaffolds for nervous system regeneration
The nervous system is the most significant and complex tissue in the human body. The nervous system is a highly specialized network which can be divided into two main parts: the central nervous system (CNS includes the brain and the spinal cord) and the peripheral nervous system (PNS include the spinal and automatic nerves). Hundreds of millions of people worldwide are affected by numerous neurological disorders. The symptoms of nervous system abnormalities depend on their localization and the generating factors. Neurological disorders such as traumatic injuries (spinal cord injuries), strokes and neurodegenerative disorders belong to incurable diseases. Neurological disorders can be caused by loss of neurons and glia cells functionality in the central nervous system (CNS) and peripheral nervous system. The most important stem cells for the nervous system is neural stem cells (NSCs), which are multipotent stem cells, precursors of both neurons and neuroglia (oligodendrocytes and astrocytes) during not only embryonic development but also in the adult mammalians Mentioned process called neurogenesis appears in specific brain regions. Lately developed strategies in PNS and CNS by using multifunctional bioscaffolds were presented in Fig. 2.
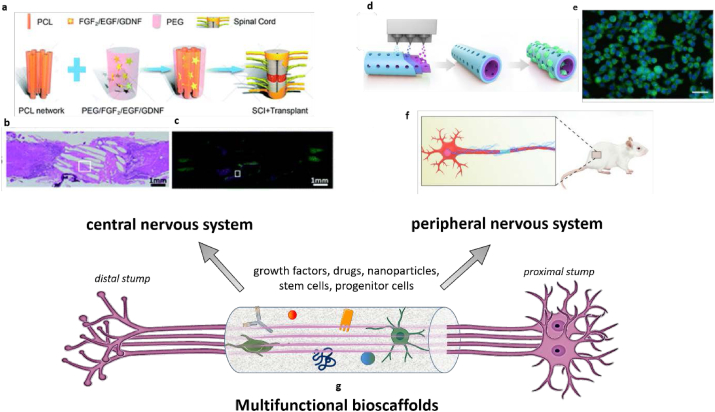
Fig. 2.
Recent strategies for regeneration of CNS (left) and PNS (right) by multifunctional bioscaffolds. CNS approach a) Scheme of cytokine-containing hydrogel embedded in a electrospun PCL scaffold composite b) Tissue bridging and neuronal axon regeneration observed by hematoxylin and eosin (H&E) staining and c) immunofluorescence staining of anti-microtubule-associated protein-2 (MAP2) neuron marker. PNS approach d,f) Scheme of fabrication of scaffolds composed of (−)-epigallocatechin gallate-loaded polycaprolactone using integrated molding and nerve conduit implantation in rat models e) anti‐oxidant marker NF-E2-related factor (Nrf2) immunofluorescent staining for RSCs on EGCG/PCL scaffolds. Reproduced with permission from Ref. [136]. Copyright © 2019 Cell Proliferation published by John Wiley & Sons Ltd. Reproduced with permission from Ref. [137]. Copyright 2020 RSC.
2.1. Peripheral nervous system
While the nervous system belongs to the most significant system with contemporaneously highly histological and anatomical structure and compound, the main issues with regeneration is a small number of NSCs and their progenitors in the specific niches. A low number of stem cells essential in CNS provide to the limited ability of the central nervous system regeneration. On the other hand, peripheral nerve axons have an intrinsic capacity to regenerate after injuries by making functional connections between two ends of a severed nerve. However, it is challenging to achieve full functional recovery after injury of the proximal nerve causing nerve gaps. Several approaches are typically used to induce increased regeneration in the gap between injured axons, including nerve autografts, nerve allografts and biologically-derived and synthetic scaffolds as an alternative. Autografts are the gold standard in PNI treatment however, it has several critical limitations, including donor site morbidity. Alternatively to autografts, nerve allografts are human decellularized nerve available commercially (e.g. Avance™). While traditional, artificial PNS scaffolds can occur in form of nerve guidance multi-channels and nerve guide conduits (NGCs) [43]. Guidant scaffolds for PNS regeneration have often tubular shape designed to bridge axonal gaps, prevent scarring and non-physiological accumulation of neurotropic and neurotrophic factors locally, protect the injured nerve from mechanical disruption and finally mechanically guide regenerating axons from proximal and distal nerve segment.
Many synthetic and naturally-derived NGCs have been approved for clinical use. Natural, biodegradable conduits based on collagen type I (Neuromatrix™, Neuroflex™) are fully biodegradable and widely used. Synthetic tubes made by synthetic biodegradable polymers such as: poly(glycolic acid) (PGA) (Neurotube™) and poly(d,l-lactide-co-e-caprolactone) (PLCL): Neurolac™, NeuroMend™) is resorbable and semipermeable. Non-biodegradable polyvinyl alcohol (PVA) polymer has been used as nerve grafts (SaluTunnel™, SaluBridge™), however clinical utilization of non-resorbable conduits has declined with the advent of absorbable natural and synthetic grafts. The main limitation of using those systems is their ability to bridge longer axonal gaps was highly questionable and non-optimal [138]. Nevertheless, the studies on the above conduits suggest that those scaffolds are effective in the case of only small gaps up to 3 cm which gives similar outcomes to nerve autograft. Moreover, traditional NGC remains insufficient for their effectiveness in nerve regeneration, and failures were reported due to persistent loss of nerve function and neuroma formation. Therefore the huge need for advanced multifunctional scaffolds for full PNI regeneration remains one of the principal goals of neural tissue engineering [139]. Advanced conduit should be biocompatible, biodegradable, flexible and additional have electrical conductivity. One of the promising electrical conductive materials are carbon-based nanomaterials, such as carbon nanotubes (CNT) and graphene (G) which have been widely used as neuronal electrodes. CNT and G have excellent electrical properties, which may have great potential in the development of scaffolds. Carbon-based materials are capable to increase the neural activity and these results were confirmed by experimental models [140].
Lately, Junggeon Park et al. fabricated conductive hydrogel-based NGCs by combining widely-used gelatin methacryloyl (GelMA) and conductive reduced graphene oxide (GO). Conductive r(GO/GelMA) hydrogel had excellent mechanical (flexibility and durability) and electrical properties. Biological in vitro studies performed on PC12 cell line after 5 DIV show relevant cell attachment via integrin binding and cell spreading on the construct. Cultured PC12 cells with differentiation medium result in significant neuritis outgrowth compared to GO-free GelMA. In vivo studies on adult male SD rats with a 10 mm peripheral injury successfully demonstrated facilitate neural myelination and regrowth after 4 and 8 weeks. Importantly, r(GO/GelMA) conduits supported functional regeneration of both nerve tissues and muscle tissues without long-term toxicity to other organs. A developed multifunctional scaffold was as effective as traditional autografts in peripheral nerve regeneration positively influenced nerve regeneration in a relatively short period of time. The report strongly suggests the potential for the treatment of PNI using electrically conductive hybrid conduits [141]. It is well-known that scaffold morphology influences cell adhesion, proliferation, differentiation and migration. Analogous to the natural extracellular matrix topography of the scaffold can regulate cell behavior and even stem cell fate. This phenomenon was used by fabricating defined micropatterns of nerve tissue on the inner surface of the construct coupled with interconnected permeable pores. Conduit made by PLGA was coated by 3,4-dihydroxy-l-phenylalanine (DOPA) for the hydrophilicity of the inner surface (PP-NGC DOPA). Construct enhanced the neuritis elongation and migration of PC12 cells as well as neural differentiation of fetal mouse NSCs comparing to patterns without patterns. In vivo studies on rats with a 12 mm peripheral injury show significant acceleration of host neuronal tissue migration, improved neurofilament elongation, Schwann cell deposition at the distal region, contributing to enhanced neural regeneration. However sciatic function index and velocity of electrophysiological analysis were not significantly different comparing other groups. Nevertheless presented multifunctional conduit not only promotes cell migration and alignment of nerve cells in vitro but also guiding Schwann cell deposition and accelerates nerve regeneration in vivo [142]. Multifunctional effect on axon and muscle tissue regeneration by using environmentally safe natural agents is especially desirable. An interesting approach was suggested by Yun Qian et al. about nerve repair after peripheral neuropathy caused by radiation treatment. They used a porous PCL scaffold loaded with active natural bioagents. An example of a polyphenolic compound is (−)-epigallocatechin gallate (EGCG) which is abundant in green tea. EGCG is considered as one of the most natural effective free radical oxygen scavenger. Effect of EGCG loaded PCL with aligned pores (20 μm in diameter) was investigated in vitro on rat Schwann cells (ESCs) and rat skeletal muscle cells (RSMCs). Results indicated that hybrid scaffold reduced ROS levels and stimulated RSCs and RSMCs proliferation more discernably than the PCL scaffold without active bioagent. In a rat peripheral radiation injury model with 15 mm of 40-Gy radiation, studies on hybrid PCL-EGCG scaffold showed improvement of not only nerve but also muscle recovery with significantly increased nerve myelination as well as muscle fibre proliferation. Results proved reduced lipid peroxidation, macrophage infiltration, oxidative stress indicators, and inflammation. That combined strategy gives new insights into research on polyphenols for peripheral nerve regeneration [136].
2.2. Central nervous system
Spinal cord injury (SCI) causes permanent sensory and motor dysfunction. Traumatic insults of the central nervous system (CNS) such as traumatic brain injury and spinal cord injury (SCI) often affect sensory and motor function disorders [143]. This neuronal disturbance causes interruption of signaling pathways. Central nervous system regeneration is more challenging than PNS, due to more complex anatomical and histological structure. In contrast to PNS, CNS axons do not spontaneously regenerate after injury in adult mammals. Moreover, the CNS environment acts inhibitory for axon outgrowth [144]. In place of CNS injury glia cells express inhibiting factors, that inhibitors of regeneration. That factors include specific CNS myelin proteins and molecules associated with the astroglial scar formation [144]. Axon growth-supportive effect can be achieved by a variety of molecules such as growth factors (e.g. glial-derived growth factor (GDNF)) and extracellular matrix molecules (e.g. laminin) [145]. Lastly, Wang et al. fabricated a hybrid PCL-PEG based composite system, embedded with axonal growth factors. PCL provided physical curves for axonal outgrowth while growth factors (FGF2 and EGF) stimulated increase axon growth-supportive substrates (such as laminin). Additionally, for further chemoattract propriospinal axons GDNF was incorporated within the hydrogel. In vitro studies on PC12 cell line cultured with scaffolds exhibited no significant cytotoxicity after 3 DIV. However long-time toxicity studies were not performed. Neurite's elongation/directional growth was not clearly presented enough. In vivo studies on rats with a 2 mm spinal cord injury show promoted the axon's directional regeneration after 8 weeks of scaffolds implantation. Promotion of the motor function recovery after SCI was observed and preceded by the production of laminin which played an important role in the axon growth-supportive substrates. This data indicates the utility of incorporating growth factors in bioscaffolds for increase regeneration of the spinal cord after SCI [137]. The composition of scaffold primary material is essential for mimicking nervous tissue followed by a proper regeneration process. Hyaluronic acid, known also as hyaluronan (HA) is one of the main, highly abundant natural compounds of the normal central nervous system. The presence of HA with bioactive agents (neurotrophic factors, growth factors) provides a pivotal role in axonal guidance formation of synapses. HA usage as bioscaffolds gives many advantages including biocompatibility, bioactivity, but also limitation due to its poor mechanical properties (Table 1). Nonetheless, HA is known for neuroprotective effect after SCI and reduction of the formation of the glial scar by inhibition the chemotaxis, migration and lymphocytes proliferation [146]. Interdisciplinary research publicated on ACS Nano presented new combined approach in biomaterial engineering for spinal cord regeneration. HA hydrogel with dotted MnO2 NPs as antioxidant bioactive agents was used as primary scaffold. Hydrogel was additionally modificated by the laminin-derived peptide called PPFLMLLKGSTR, that was chosen or possible promototion of stem cells adhesion and bridging of damaged nerve tissue. In vitro studies on MSCs derived from human placenta cultured on hydrogels after 3 DIV exhibit no obvious toxicity. Hybrid hydrogel with MnO2 NP significantly reduced the H2O2 content after MSC incubation for 1 and 2 h, indicating an efficient antioxidant function of hybrid scaffolds. In vivo investigation on a 4 mm rat transection SCI model with implanted multifunctional hydrogel-containing multipotent MSC cells exhibit scaffold integration and increased neural differentiation, followed by efficient spinal cord regeneration. Composition studies showed partial elimination of Mn from the site of the lesion during 4 weeks. Finally, a multifunctional construct containing MSC enhanced motor function restoration after on a long-span rat spinal cord transection, which remains one of the principal goals of neural regenerative medicine [147]. Stem cells are widely used in regenerative medicine due to their differentiation capability and releasing their own growth factors [148]. But it should be carefully policed to enable ethical and safe usage. The main issue of introducing commercial multipotent stem cells to clinical use is possible immunogenicity, risk of teratoma, and tumorigenesis. Therefore for clinical application should be considered the only host-derived stem cells (iPSC, adult stem cells) which can significantly reduce the immune response. In general, undifferentiated stem cells (ESCs, iPS) by stemness potential have a relatively high capacity to form teratomas and tumors [149]. Therefore promising perspective for the treatment of neural disorders brings more specialized stem cell therapies. An example is neural progenitor cells (NPCs) which hold lower potential for tumorigenesis than e.g. ESCs. A combined approach of NPCs incorporation in personalized scaffold was recently investigated by J. Koffler et al. Complex CNS structure for spinal cord regeneration was printed using microscale continuous projection printing method (μCPP). Poly(ethylene glycol) diacrylate (PEGDA)–GelMa scaffold architecture was tailored precisely to the dimensions of 1.8 mm SCI rat lesion. NPCs suspended in the collection of fibrin matrix and growth factors (BDNF to support NPCs survival, bFGF to promote angiogenesis and calpain inhibitor for neuroprotection) were incorporated in scaffold channels. In vivo studies on rat SCI model at 1 month, post-implantation showed scaffold-NPCs the ability to support stem cell survival. Scaffolds loaded with NPCs induced host serotonergic axons regeneration, which modulates spinal motor systems. Injured host axons regenerated into multifunctional 3D biomimetic scaffolds providing synapse onto implanted NPCs, which lead to restoring not only synaptic transmission but also improve functional outcomes [150]. After SCI in the damaged spinal cord occurs complex physiological and pathological changes. Conventional treatment of SCI focuses on preventing further injury by using potent anti-inflammatory drugs, such as corticosteroids. One of them is methylprednisolone (MP) which was used to improved neurological functions recovery after acute spinal cord injuries. However, since 2013 use of MP has decreased dramatically due to comparative recent studies that have shown the potential side effects, such as blood clots, respiratory, urinary tract, wound infections, and steroid-induced myopathy [151]. Despite that, MP was recently used to fabricate multifunctional scaffold. The hybrid scaffold was fabricated via electrospinning from both natural materials (Polysialic acid (PSA)) and synthetic polymer (PCL) with incorporated MP. The nanofiber scaffold was biodegradable, and actively release MP over a short period of time. In vitro cytotoxicity studies on human neuroblastoma cell line (SH-SY5Y) and primary astrocytes indicated no significant differences between different scaffolds composition for cell proliferation for 7 DIV. In vivo studies on rats with 2 mm SCI effectively showed that the transplantation of hybrid PCL/PSA/MP scaffold effectively suppressed apoptosis and acute inflammation. Moreover, it attenuated glia scar formation. Construct supported axonal regeneration, leading to improvement of the functional recovery after SCI. Actively releasing MP from a multifunctional scaffold could be incorporated in could be beneficial through lesion site-specific drug administration [152].
Multifunctional bioscaffolds have great potential in providing cell support, inhibiting the glial scar formation and damaged neurons guidance by tubular conduits, actively releasing bioagents and drugs and combining stem/progenitor cells therapy which stimulates the release of axon regeneration-promoting neurotrophic factors. It has been confirmed that multifunctional scaffolds are an effective strategy to improve therapeutic benefits in animal models, resulting in the functional recovery of SCI rats in many cases. However, it is still a challenge to build an ideal scaffold for the full regeneration of damaged nervous tissue.
3. Bioscaffolds for cardiovascular system regeneration
Cardiac regeneration has been a subject of scientific reports for over 100 years [153,154]. Heart regeneration can be defined as the restoration of damaged heart tissues and their impaired function. Restoration of the injured human heart is limited in comparison with other vital organs, such as muscles, skin, lung, or liver, and deteriorates with age [155]. There are many types of cardiovascular diseases (CVD) responsible for heart tissue disorders, i.e. heart failure, myocardial infarction, dilated cardiomyopathy, or coronary artery disease [156]. According to the WHO data, CVD are the main cause of death worldwide and results in more than 50% of all deaths in Europe [157]. The WHO mortality statistics show also that most of these premature deaths could be avoided by changing a human lifestyle. Unfortunately, the change in health-related behavior is difficult, thus searching for new treatment methods is extremely important.
Numerous approaches for regeneration of injured heart tissues are currently investigated, ranging from surgical implantation of cardiac grafts over the biomolecules or cell injection, and advanced cell-modified scaffolds implementation. Heart surgeries entail various risks, such as infections, bleeding, stroke, or even death. Therefore, scientists are constantly looking for ways to boost current procedures and find new minimally invasive treatment methods based on the self-renewal of tissues [154]. Regeneration of heart tissues requires cardiomyocytes proliferation, but the cardiomyogenesis is very slow (less than 1% of cardiomyocytes can renew per year) and decreases with age. Thus, cardiomyocyte's loss exceeds its renewal, causing cardiac pathologies [158].
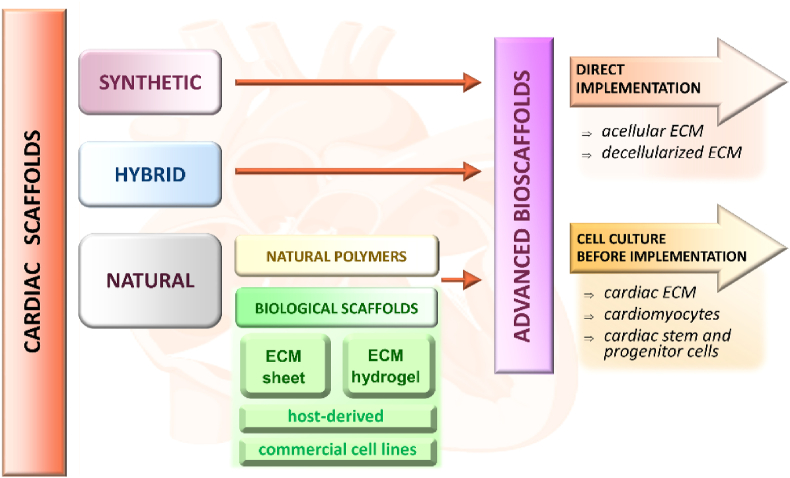
Currently, one of the most extensively investigated strategies to stimulate cardiomyocytes generation is a therapy based on advanced bioscaffolds. There are two main strategies to employ bioscaffolds for cardiovascular system regeneration (Fig. 3). The first one is based on the direct implementation of bioscaffolds into impaired heart tissue. In the second strategy, bioscaffolds serve as cardiac cells (and/or biomolecules) delivery system for myocardial repair.
Fig. 3.
Cardiac scaffolds classification based on materials and implementation techniques.
In the last decade, the extracellular matrix (ECM) from myocardium tissues has been intensively examined to design new optimal ECM bioscaffold for cardiac tissue regeneration [[159], [160], [161]]. ECM plays a crucial role in the regulation of cell functions (such as survival, proliferation, differentiation, migration, and adhesion), both, in homeostasis, and a response to injury [162,163]. The composition of ECM is different among particular tissues. Generally, ECM consists of four types of proteins, i.e. collagens, elastin, glycoproteins, and proteoglycans, as well as carbohydrates [161]. For instance, collagens (I and II) and elastin provide the strength and elasticity of tissues and organs. In turn, proteoglycans and glycoproteins (mainly fibronectin and laminin) are responsible for various growth factors binding, and regulation of their activity [164]. The ECM-bioscaffold in tissue engineering is a promising one due to its basic functions: i) it provides tissue maintenance, ii) ensures the formation of boundaries between different tissues, iii) regulates the activity of growth factors, and iv) regulates of signal transduction via cell interactions [165].
ECM bioscaffolds can be acellular or decellularized. Acellular ECMs bioscaffolds are usually surgically implemented into impaired heart region to facilitate the vasculogenesis and angiogenesis (endogenous cardiac regeneration) [165]. Additionally, these bioscaffolds can prevent the infract-derived scar thickening via inhibition of cardiac fibroblast activation [166]. Svystonyuk and co-workers demonstrated the fibroblast-mediated post-injury remodeling of cardiac tissues, stimulated by acellular ECM bioscaffold (neutralized SiS-ECM; porcine small intestinal submucosal extracellular matrix) [167]. The authors indicated that cardiac fibroblast combined with SiS-ECM-based bioscaffold may promote blood vessel formation and avoid scar expansion, due to upregulated gene expression and release of robust paracrine factors. There are some reasons to prefer decellularized ECM (dECM) bioscaffolds over the acellular ones for heart tissue engineering. The dECM bioscaffolds reveal the naturally bioactive composition and ability to partial recellularization in vivo. However, decellularization procedure is complicated and usually requires several physical, chemical, and enzymatic methods to remove all cellular components, while preserving the native ECM composition [168]. Several tissues or even whole organs can be decellularized to produce dECM bioscaffolds for regeneration of injured tissues, such as hearts, heart valves, lungs, kidneys, small intestine or urinary bladder [[169], [170], [171]]. Decellularization of tissues results in planar ECM sheets formation, which can be applied as patch graft materials [172] or processed into hydrogels [173]. Whole organ decellularization is used for 3D ECM bioscaffolds preparation. These 3D biostructures after further repopulation with host-derived cells may help to design the human organs for transplantation. Decellularization can be also employed to harvest ECM components in vitro. Cell-derived ECM scaffolds are useful for regeneration of damaged tissues, but also to examine the stem cells differentiation and proliferation [174].
After successful decellularization, ECM scaffolds must be recellularized by specific cell types to mimic the natural functions of tissue, such as drug response or electrical conduction. Moreover, cardiac dECM scaffolds should be modified with various pro-angiogenic factors and additional proteins to improve cell attachment/seeding and vasculogenesis (Fig. 4). For instance, pluripotent stem cells represent a source of cell that can differentiate into various cellular building blocks. Therefore, they hold a promise for regenerative medicine. Wang and co-workers designed and prepared human cardiac patches based on dECM from rat heart, pluripotent stem cells-derived cardiac cells, and fibroblasts [175]. The authors showed that this cardiac scaffold can reduce the infarct area of the heart of rats with induced-myocardial infarction, as well as enhance its function, such as normal beating, electrophysiological activity, and pharmaceutical response. In turn, Chamberland et al. demonstrated that embryonic decellularized cardiac scaffold reseeded with specific progenitor cells can serve as efficient support for cardiac cell growth. These progenitor cells were able to graft into the scaffold structure and form beating cardiac tissue [176].
Fig. 4.
Preparation of bioscaffolds for cardiac tissue engineering.
Godier-Furnémont designed a biological composite scaffold produced by seeding mesenchymal progenitor cells (MPCs) dispersed in fibrin hydrogel on decellularized ventricular human myocardium. The implanted scaffold improved the formation of the vascular network in the infarct area of the heart, leading to its functional recovery (rat ischemic myocardium model). The revascularization was related to MPCs migration and their ability to secrete SDF-1 (stromal cell-derived factors), which induced migration of further cells, and preservation of myocardial functions [177]. Some promising results were presented by a scientists team from Spain [178]. Perea-Gil et al. designed a cell-enriched myocardial graft based on a decellularized myocardial matrix modified with adipose tissue-derived progenitor cells (EMG-ATDPC) to regenerate the infarcted area of a swine heart. The in vivo studies showed that EMG-ATDPC- based bioscaffolds significantly enhanced cardiac function, promoted a new blood vessel formation, and inhibited progression of fibrosis in the impaired myocardium [178]. It should be pointed out that various types of cells can graft and differentiate into functional cardiomyocytes in vitro and in vivo, including bone marrow-derived cells, skeletal myoblasts, or mesenchymal stem cells [[179], [180], [181], [182]].
Most of the experimental studies suggest that the transfer of stem cells and progenitors may facilitate the regeneration of myocardium. The ECM offers an excellent source of various pluripotent cells, however, the decellularization and recellularization procedures still face many challenges.
Generally, cardiac scaffolds can be classified into three main groups on the basis of the biomaterial type: i) natural materials, including ECM-based scaffolds, and biocompatible polymers, ii) synthetic materials and iii) hybrid materials (Fig. 3).
Several natural polymers, such as collagen, chitosan, fibrin, hyaluronic acid, alginate, several self-assembling peptides, and polymer composites, can be applied as a structural template for heart tissue formation (Table 2). They are excellent candidates for tissue engineering due to their biocompatibility, biodegradability, renewability, and structure that can be easily modified with various stimuli and growth factors, or biomolecules to promote specific cell growth and proliferation.
Table 2.
Examples of natural polymers widely applied for cardiac regeneration (preclinical stage).
| BIOSCAFFOLD | COMPOSITION | FUNCTION | REF. |
|---|---|---|---|
| NATURAL POLYMER MATERIALS | |||
| Stem cell-capturing collagen scaffold | Collagen scaffolds covalently conjugated with stem cell specific antibody Sca-1 | Collagen scaffold facilitated the regeneration of cardiomyocytes and improved the tissue regeneration | [183] |
| Chitosan-collagen (C/C) scaffold | Stem cell-derived human cardiomyocyte seeded on the mico-structured chitosan-collagen scaffold | C/C scaffolds allowed the attachment, spreading, and orientation of human cardiomyocytes | [184] |
| 3-D collagen scaffold | Porous collagen sponge (type I) | Collagen scaffold promoted angiogenesis and arteriogenesis in the cryoinjured heart | [185] |
| Stem cells‐CREKA‐fibrin | Bone marrow stem cells modified with CREKA peptides | Stem cells-CREAKA-fibrin-targeting system revealed the ability to localize the stem cells to the fibrin-rich injured heart | [186] |
| Hyaluronic acid-based bioscaffold | HA-based hydrogel and mesenchymal stem cells; mixed esters of HA with butyric acid and retinoic acid; HA/silk fibroin-based scaffold |
HA-based sponges, meshes and hydrogels improved the myocardial structure formation, promote cell survival, reduce the inflammatory reaction, and increase neovascularization | [187] |
| Peptide‐functionalized alginate scaffold | Embryonic stem cell-derived cardiomyocyte co-seeded with dermal fibroblast in macroporous alginate scaffolds, modified with RGD and HBP peptide | RGD/HBP-modified alginate scaffolds promoted the formation of functional cardiac tissue from embryonic stem cell-derived cardiomyocytes co-cultured with dermal fibroblasts. | [188] |
| Self-assembling peptide scaffold | VEGF combined with RADA16-heparin domain | Combined RADA16-scaffold induced angiogenesis, recruitment, and differentiation of cardiac stem cells into cardiomyocytes | [189] |
| Chitosan-hyaluronan-silk fibroin cardiac scaffold | Silk fibroin modified with chitosan, and hyaluronan (in situ formulated) | Composite scaffold improved left ventricle functions and angiogenesis in myocardial infarction regions | [190] |
| SYNTHETIC POLYMER MATERIALS | |||
| PEEUU-PCL scaffold | Poly(ester-ether urethane urea) - poly-caprolactone blend | PEEUU-PCL scaffold enhanced functional activities of the cardiomyoblast cells | [191] |
| PLGA-IGF-1 scaffold | Poly(D,L-lactide-co-glycolide) nanoparticles modified with insulin-like growth factor | PLGA-IGF-1 NPs inhibited the cardiomyocyte cells apoptosis and reduced the infarct sizes | [192] |
| Cardiomyoctes-modified PU scaffold | Polyurethane film modified with lamin and gelatin | PU film supported the formation of cardiomyocyte multilayered construct of heart tissues | [193] |
| Stem cell-derived cardiomyocytes-modified PU scaffold | Polyurethane film modified with lamin, gelatin and collagen (type IV) | PU films supported the formation of fully contractile cardiomyocyte cells layers | [194] |
| Protein-functionalized PLA:PGS scaffold | Poly(lactic acid)- poly(glycerol sebacate) fibres modified by lamin or Matrigel | PLA:PGS scaffold induced neovascularization after implantation into mouse heart | [195] |
| HYBRID MATERIALS | |||
| Chitosan/Carbon scaffold | Carbon nanofibres dispersed into chitosan matrix | Chitosan/carbon scaffold improved the mechanical properties of cardiac tissue constructs and enhanced transmission of electrical signals between cells | [196] |
| PLL-GO scaffold | Graphene oxide sheet coated with poly-l-lysine | PLL-GO sheets improved electrophysiological function and mechanical integrity of tissue | [197] |
| rGO-GelMA scaffold | Reduced graphene Oxide‐ gelatin methacryloyl hybrid hydrogels | Cardiac cells cultured on rGO‐GelMA scaffolds exhibited excellent biological activities, i.e. cell viability, proliferation, and maturation | [198] |
| Au NPs- PCL scaffolds | Fibres modified embedded with gold nanoparticles | Scaffold induced the formation of tissue with structure resembled cardiac cell bundles | [199] |
| AdSCs-statin-PLGA scaffold | Adipose‐derived stem cell and statin-modified poly(lactic‐co‐glycolic) acid nanoparticles | Facilitated endogenous functional cardiac regeneration | [200] |
For instance, stem cells-collagen scaffolds modified with monoclonal specific antibody Sca-1, was applied as a patch to promote regeneration of surgical heart defects (C57/BL6 mouse, in vivo model). The authors highlighted the double efficiency of the collagen-based scaffold, i.e. it serves as a scaffold for stem cell proliferation and differentiation, and increases the enriching capacity for autologous stem cells [183]. In turn, Huang and co-workers reported the use of clot-binding pentapeptide (CRECA: cysteine-arginine-glutamic acid-lysine-alanine) to target the exogenous stem cells to the injured heart. Based on the fibrin-targeting theory, fibrin exhibits potential as a target in stem cell therapy for the myocardial infarction, due to its spatial‐specific distribution in myocardial injury. The CRECA-functionalized stem cells injected to the left ventricle of the fibrin‐rich rat heart (in vivo model of myocardial ischemia-reperfusion injury) revealed the ability to localize the damaged region and promoted the cardiomyocyte proliferation [201]. An interesting in vivo studies were published by Chi et al. [190]. Natural silk fibroin modified with chitosan and hyaluronan was examined as a cardiac patch to repair myocardial infraction hearts of rats. These three polymers were selected due to their biological activity and low inflammatory response. Silk fibrous proteins are known as a material for tendon regeneration. Chitosan is commonly applied for the regeneration of nerves and bones, and hyaluronic compounds can promote angiogenesis and cartilage repair. The performed studies indicated that chitosan-hyaluronan-silk fibroin cardiac scaffold markedly increased the thickness of the left ventricle of heart walls and enhanced their fractional shortening.
The application of natural polymers in regenerative medicine is limited to some extent, due to poor mechanical properties, low electrical conductivity, and rapid degradation in physiological conditions. The main challenge in myocardium tissue regeneration is to design advanced cardiac scaffolds, which is elastic and at the same time mechanically strong to endure the dynamic contractions of heart. Currently, synthetic polymers or hybrid materials consisting of synthetic and natural polymers, polymers modified with micro- or nanoparticles, or surface-functionalized organic and inorganic nanostructures, may provide the enhancement of mechanical, electrical, and surface properties of bioscaffolds. However, their surface should be also functionalized with biomolecules or growth/differentiation factors to improve biocompatibility and provide a tissue-like environment for cell attachment, growth, proliferation, and differentiation (Table 2).
High metabolic activity of cardiomyoblast cells was observed after the implementation of a porous scaffold made of poly(ester-ether urethane urea) and poly-caprolactone blend (PEEUU-PCL scaffold) The PCL-additive provided excellent mechanical properties, similar to those of heart tissues. Based on in vitro and in vivo studies, it was proven that the designed scaffold was surrounded by connective tissue and new-formed blood vessels [191]. Chang and co-authors demonstrated the application of poly(D,L-lactide-co-glycolide) nanoparticles (PLGA) modified with insulin-like growth factor (IGF)-1 as a new scaffold for cardioprotection. The IGF-1 plays a crucial role in the regulation of myocardial functions, including cardiomyocyte survival, growth, and protection from ischemia. Additionally, IGF-1 can improve myocardial function after heart infarction. The authors indicated that PLGA-IGF-1 NPs prolonged IGF-1 retention in heart tissue, and significantly inhibited the cardiomyocyte cells apoptosis (in vitro and in vivo studies) [192]. Another interesting biodegradable synthetic polymer for cardiac repair is polyurethane (PU). McDevitt et al. reported PU films as a scaffold for cardiomyocytes' growth (in vitro studies). To improve the adhesion of cells to the PU layer, its surface was coated with proteins, i.e. laminin and gelatin. Cardiomyocytes cultured on the PU dishes formed a multilayered construct of tissues with mechanical properties similar to native heart matrix [193]. The mechanical and conductive properties of scaffolds can be also improved by functionalization with various nanoparticles [202]. For instance, the conduction of electrical signals through cardiac tissue was enhanced by the incorporation of electrically conductive carbon nanofibres into the chitosan matrix [196]. Chitosan/carbon scaffolds supported the cultivation of the cardiac cells and improved their cardiogenic properties. In another study, a nano-patterned PEG scaffold was modified with graphene [203]. The authors indicated that the graphene-PEG scaffold improved the myofibrils and sarcomere structures and increased the electrical coupling of cardiac cells. Fleischeret and co-workers fabricated the conductive nanocomposite scaffold consists of gold nanoparticles and PCL fibers [199]. The addition of gold nanoparticles induced the formation of tissue with structure resembled cardiac cell bundles in vivo.
The structure of scaffolds allows delivering of nutrients, metabolites, nucleic acids, regulatory molecules, and cardioprotective drugs within the cells [204]. Delivery of active substances via nanocarriers is a promising tool to restore the injured heart function [205]. Somasuntharam et al. demonstrated DNAzyme gold NPs conjugates as a drug delivery system for the regulation of TNF-α expression in the rat model of myocardial infarction [206]. The authors showed that injection of DNAzyme gold scaffold in the myocardium resulted in the improvement of acute cardiac function due to significant TNF- α gene silencing. Yokoyama et al. examined adipose‐derived stem cells (AdCs) and statin-loaded PLGA nanoparticles as multifunctional bioscaffolds to stimulate the infarcted myocardium regeneration. The AdSCs were seeded to the scaffold structure to reduce the risk of inflammation, and statin was attached to recruit the circulating progenitor cells for angiogenesis [200]. The authors showed that AdCs-statin-PLGA scaffolds can facilitate cardiac regeneration, and may serve also as an efficient statin (or other active substance) delivery carrier. Diaz-Herraez et al. formulated PLGA microparticles loaded with neuregulin-1 (NRG) and further modified with ADCs. The presence of NRG (growth factor) promoted cardiomyoctyes proliferation and reduced infarct size (rat and pig models). The authors reported that ADCs-PLGA-NRG delivery system allowed to control the release of NRG in the infarcted region, accompanied by stimulation of vessel, arterioles and capillaries formation [207].
Synthetic materials in comparision with natural biomaterials exhibit improved mechanical, elastic, and conductive properties, better durability, stability, and controlled degradation rate [208]. However, there are many concerns related to their toxicity and potential hazardous health effects. Regardless of the type of material used to heart tissue regeneration, bioscaffold must be biocompatible, biodegradable, and possess a naturally cardiac tissue-like environment to facilitate cell attachment, growth, proliferation, and differentiation into mature. The degradation rate must be sufficient to support cell integration with native tissues. Additionally, bioscaffold should act as a reservoir of nutrients, and regulatory molecules and provide their slow release.
The outcomes of these in vitro, in vivo, and ex-vivo studies mark the future direction for the application of both, natural and synthetic materials for cardiac tissue regeneration. However, despite the positve premises, the use of bioscaffolds for cardiomyocytes regeneration is still lagging at the preclinical stage.
4. Regeneration of muscle system
There are three main types of muscle tissue: skeletal (or striated) muscle, smooth (or non-striated) muscle, and cardiac muscle. This chapter is focused on the first two types of muscles, while the last one is discussed in the chapter Bioscaffolds for cardiovascular system regeneration. The main difference between skeletal and smooth muscles is the presence or absence, respectively, of organized, regularly repeated arrangements of myofibrillar contractile proteins – myofilaments. The skeletal muscles are used in locomotion and to maintain posture, while smooth ones are part of the walls of organs and structures such as the uterus, esophagus, stomach, blood vessels. Because, depending on the type of muscle tissues, their structure as well as their functions differ, the way of regeneration is different. It should be noted that repairing an injured muscle is a multi-stage process that uses immune, muscle, perivascular and nerve cells. Without this repair, it leads to structural and functional deficits in the body, which in turn leads to a reduction in the quality of life not only due to a deficiency in the functioning of the muscles, but often also for aesthetic reasons.
4.1. Hydrogels
Hydrogels are water-swollen high-dimensional polymer chain networks which specific properties which depending on the origin source exhibit high biocompatibility, that makes them an ideal class of materials in tissue engineering. Hydrogels may display reversible structural or just volume deformations, induced by various stimuli, such as temperature, pH, wave length of light, ionic strength, and specific molecules [[209], [210], [211], [212]]. Moreover, they can withstand significant stress, which proves their flexibility, and combined with sensitivity to stimuli makes them ideal materials to compose artificial muscles [209,[213], [214], [215], [216], [217]]. The above features are important, however, not sufficient to fully replace natural muscle tissue. Actuation characteristics are required in special tissues reconstruction such as skeletal or to provide mechanical support to injured cardiac tissues [[218], [219], [220], [221], [222], [223], [224], [225]]. Therefore over last years, structure modulation has become a crucial step for developing hydrogel‐based artificial muscles. Various graft materials have been tested to promote skeletal muscle regeneration. Natural hydrogels are a popular choice for tissue engineering due to their low immunogenicity, porosity, good permeability, biodegradability and structural biocompatibility towards tissues, which minimizes the inflammatory response just at the outset. This type of hydrogel can not only act as a gentle scaffold for cell alignment in connective tissue, but also plays a dynamic and flexible role that determines cell behaviour and tissue function as scaffold for the growth of many types of tissue. Collagen is a fibrous protein found most commonly in the extracellular matrix and can be formulated as a scaffold for the growth of many types of tissues [226] (Table 1). It supports proliferation, differentiation and myotube formation of immortalized and primary murine myoblasts [[227], [228], [229]]. Cheema et al. have indicated that contraction forces depend on mioblast morphology. At low contraction forces myoblasts maintained a rounded morphology, and when contraction forces increases, myoblasts started to align and form myotubes under uniaxial tension [230]. Disadvantage of the collagen scaffolds is lack mechanical strength and structural stability upon hydration, which limit their applications in particular tissues. Problem can be solved throughout physical or chemical methods leading to intermolecular cross-linking of collagen scaffolds, but blending with other materials, such as synthetic polymers is also used. The effectiveness of myoblasts and mesenchymal stem cells in combination with fibrin gel in repairing volumetric muscle loss was also assessed by Matthias et al. [231] The obtained results have confirmed muscle mass restoration as well as fibrosis reduction with active contribution of transplanted cells in the muscle and vascular regeneration. In further studies Neal et al. have proposed method according which using fibrin hydrogel skeletal muscle tissue with a high volumetric density and perfect cell alignment along the axis can be created [232]. In these studies artificial muscle was accomplished by integration of gel fiber based fibrin containing mouse C2C12 immortalized myoblast cell line [232]. Fibrin scaffold with a populated satellite cell niche, enable to vascular integration and functional in vivo maturation was also used to construct a highly functional biometric muscle tissue [233], and functional neuromuscular junctions [234]. It was also confirmed the applicability of fibrin hydrogel in seeding of human umbilical cord mesenchymal stem cells (HUCMSCs) [235], and in production an engineered skeletal muscle with structural resemblance to in vivo tissue [236]. The microfabrication of new skeletal muscle tissue using smooth muscle cells incorporated in fibrin hydrogel was also tested to fabricate ureteral replacements [237]. Although high potential fibrin gel has been demonstrated, the most promising seems to be fibrin scaffolds with microthread architecture, in which scaffolds favour the ingrowth of nascent myofibers into the wound site, and the functional regeneration of skeletal muscle [238]. Alginate hydrogels have also been tested as a material supporting the regeneration of muscle tissues [239]. This type of material is mostly chemically modified to provide tighter control over properties such as stiffness and degradability. Its structure also allows for various types of use, e.g. in the form of a hydrated gel, microspheres or as highly porous, freeze-dried cryogenic gels [240,241]. Borselli et al. reported that an injected alginate gel can provide long-term delivery of incorporated myogenic and angiogenic growth factors, and when injected into the hind limbs of ischemic mice, it promote functional muscle regeneration by stimulating myogenesis, angiogenesis, and re-innervation [242]. As cryogels, alginate scaffolds promoted muscle regeneration by secreting bioactive factors that have a profound effect on the functioning of C2C12 mouse-derived myogenic progenitor cell line [243]. The RGD-alginate porous hydrogel provided a sustained release of incorporated IGF-1 and VEGF165 and adherence MSCs to the biomaterial walls (Fig. 5). Indeed the outward migration of muscle cells has been shown to be of vital importance on subsequent muscle regeneration. For example, Hill et al. have indicated that transplanting the cells with the highly porous alginate scaffold that simultaneously delivers of growth factors (hepatocyte growth factor (HGF) and fibroblast growth factor 2(FGF2)) led to increase in muscle mass and the overall extent of regeneration [244]. Passipieri and Chris have also shown that the alginate three-dimensional scaffolds can be used to deliver growth factors into a variety of volumetric muscle loss injuries [245]. But in newest work Quigley et al. have tested alginate fibers with enclosed muscle precursor cells for delivery of dystrophin-expressing cells to dystrophic muscle, and the constructed material reported more robust regenerative results than did myoblasts attached to synthetic fibers [246,247].
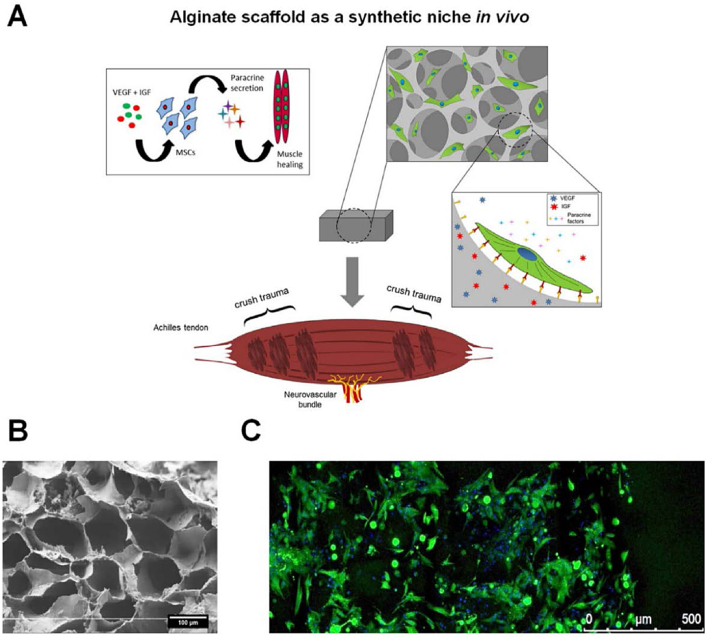
Fig. 5.
An engineered synthetic niche provides MSCs with a structural and chemical environment that is optimal for paracrine secretion. (A) Strategy of using porous alginate scaffold in muscle regeneration. (B) Representative SEM image showing the macroporous structure of the alginate scaffold. (C) Representative fluorescent image of rat bone marrow derived MSCs 24 h after seeding on the scaffold. Reproduced with permission from Ref. [243]. Copyright 2016 Elsevier.
Hyaluronic acid is a popular scaffold material for the regeneration of different tissues because it is biocompatible, promotes skeletal myoblast proliferation, and differentiation, regulates tissue hydration and facilitates the diffusion of nutrients [248] (Table 1). However, fabrication of hyaluronic acid-based scaffolds has been achieved through different chemical modifications such as a Michael addition reaction with thiol as nucleophile [249,250], photopolymerization of methacrylated or thioglycated hyaluronic acid [251,252]. The first one is dedicated to fibres scaffolds formation, the second one to preparation of hydrogel beads. For example the hyaluronic acid based photopolymerizable hydrogel was used for transplantation satellite cells and muscle progenitor cells, which enable generation of new myofibers, and recovery of muscle contraction strength [253]. It was also shown that modifying hyaluronic acid with both methacrylate and 3,4-dihydroxyphenylalanine groups obtained materials, which can be use in minimally invasive procedures to foster maxillofacial tissue repair [254]. Tanaka et al. have found combination scaffolds of salmon fibrin and hyaluronic acid form compliant hydrogels matching the physical properties of most tissues [255]. Other natural polymers such as chitosan and gelatin have also a good capacity of supporting cell attachment, however, their main drawback is immunogenicity [256]. The potential was found for gelatin-based hydrogels stabilized through reaction with lysine diisocyanate ethyl ester [257] or using gelatin as a component of other natural hydrogels e.g. cross-linked oxidized alginate-gelatin hydrogel [258].
The natural hydrogels due to their resemblance to native tissue are the preferred materials in tissue engineering especially for controlling cell growth, proliferation and differentiation, however as biological materials they have mostly and nonreplicable structural composition, limiting their in vivo application. Synthetic hydrogels mainly composed of poly(ethylene oxide) (polyethylene glycol), poly(vinyl alcohol), poly(lactic acid) or polypeptides, unlike their natural counterparts, can be closely adapted to certain requirements of a cell therapy application, in particular the mechanics that most closely resemble the native cellular microenvironment. Using synthetic polymers enables important material properties such as viscoelasticity, modulus, permeability and degradability. Another advantage over natural materials is that synthetic hydrogels have a relatively low risk of transmitting pathogens. Polyethylene glycol is one of the most widely used macromers in tissue engineering because its hydrophilicity, cytocompatibility, low non-specific protein adsorption, and is nondegradable under mammalian enzymes. Mechanical properties of PEG-based hydrogels can also be easy controlled, but active hydroxyl groups can be easily chemically functionalized through photopolymerization or Michael addition. Example of using functionalized PEG as hydrogel Han et al. presented [259,260]. In the studies a synthetic bioactive hydrogel based on a branched poly(ethylene glycol) with ends maleimide functionalized groups was used for incorporation muscle satellite cells to dystrophic skeletal muscles also with comorbid trauma. This material may also be suitable for treating craniofacial and limb muscle trauma. In newest papers the co-delivery of muscle satellite cells and Wingless-type MMTV Integrated 7a protein using the maleimide functionalized PEG hydrogel was studied. This work has confirmed that the hydrogel-encapsulated Wnt7a significantly increases hypertrophy of the muscle fiber, endogenous muscle satellite cells expansion, and exogenous cells migration during the implantation process [166]. The effect of the different Wnt7a-loaded PEG-4MA hydrogel on C2C12 myotubes hypertrophy is illustrated in Fig. 6. A major drawback of the polyethylene glycol-maleimide hydrogel is that the fast gelation speed can result in crosslinking heterogeneities. The decreasing of reaction kinetic and hence uniformity of particle dispersion can be achieved by the coupling a glutamate near the cysteine of the peptide crosslinker, as well as appropriate pH and ionic strength [261,262]. A wide range of protein-based hydrogels have also been developed as scaffold. They are very attractive due to their inherent cell adhesively as conferred by the presence of integrin-recoginizing peptide sequences [263]. The polysaccharides hydrogels are not bioactive and lack integrin binding domains, since such modification of polysaccharide molecules requires the attachment of chemical molecules that can facilitate cell adhesion [264,265].
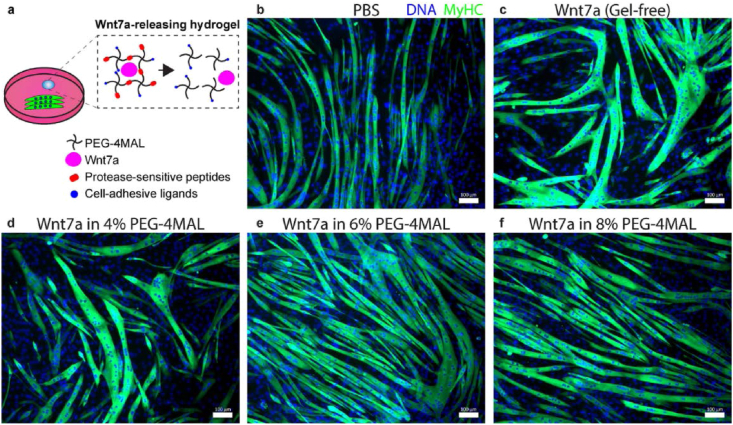
Fig. 6.
Hydrogel-released Wnt7a retains its bioactivity in vitro. (a) Schematic diagram of the experiment. Differentiating C2C12 myotubes treated with (b) PBS, (c) Wnt7a (gel-free), (d) Wnt7a in 4% PEG-4MAL hydrogel, (e) Wnt7a in 6% PEG-4MAL hydrogel, and (f) Wnt7a in 8% PEG-4MAL hydrogel. Day 5. Scale bar 100 lm. Reproduced with permission from Ref. [259]. Copyright 2019 Elsevier.
An alternative approach to pre-vascularization of engineered muscle involves plating endothelial cells, fibroblasts, and myoblasts onto the poly(lactic-co-glycolic acid) scaffold [266]. This studies have confirmed that flap consisting both endothelial cells, fibroblasts and myoblasts underwent the most effective integration and caused the most advanced regeneration of host tissue. The used material enabled successful muscle flap engineering. Furthermore, the increased mechanical strength of the transplanted tissue, which was caused by the myocytes became vascularized and innervated and finally, mature as myofibers. Worth attention are also hybrids natural and synthetic polymers. An example is photopolymerizable hydrogel based upon polyethyleneglycol and fibrinogen, which is enable to generate a complete and functional artificial muscle [267]. This type of hydrogel supports myogenic differentiation, cell survival after transplantation and angiogenic infiltration in vitro and in vivo [268]. The amine-reactive polyethylene glycol modified fibrinogen hydrogel with a decellularized extracellular matrix scaffold showed a high expression of ITGA5, ITGB1, and FN and a synergistic up-regulation of ang1 and tie-2 transcripts [269]. Scaffolds composed of collagen and polylactic acid is also a promising choice as it combines the good mechanical and processing properties of a synthetic component with the bioactivity of a natural polymer [270]. Conductive polymers such as polypyrrole, polyaniline, and polythiophene have formed hydrogels not only showing good biocompatibility, but also possessed suitable electroconductivity [271]. Sasaki et al. have developed a series of molecular permeable electronic devices to help to regenerate the muscle tissues. The hybrid of poly(3,4-ethylenedioxythiophene) and polyurethane have been biocompatible with muscle, as well as neural cells. Moreover, this displays excellent stability and high conductivity over physiological strain levels, making them highly suitable for low-invasive electrical stimulation [272]. Poly(acrylic acid) were modified with polyaniline, which provided not only a microfluidic pattern, but also a three-dimensional environment of nanofiber tissue formation [273]. In another work as the main body polyaniline grafted quaternary chitosan and cross-linked with oxidized dextran was fabricated to obtain a conductive hydrogel [274]. C2C12 cells have also exhibited a higher proliferation on conductive hydrogel than, for example on the chitosan hydrogel, indicating their potential application in skeletal muscle tissue engineering [275]. The micro-patterned electrically conductive reduced graphene oxide-incorporated/polyacrylamide hydrogel was found as an ideal multifunctional and high performance biomaterial platform to construct muscle tissue scaffolds [276].
4.2. Electrospun
The primary purpose of tissue engineering is to mimic the native tissue. This has been the reason for production of electrospun nanofibers via electrospinning. This method is, on the one hand, relatively simple and versatile, allowing the processing of solutions, suspensions or melts into nano-/micro-scale diameters’ continuous fibers, on the other hand, it is the only available method for the mass fabrication of long continuous nanofibers [[277], [278], [279], [280], [281]]. Such solution allows to encouragement the regeneration of skeletal muscles by creating, similar to natural, orientation scaffold, which is a pattern to alignment to encourage this organization in myoblasts by fuse and differentiate them to form multinucleated myofibers. In addition, this method can also be used to regenerate smooth muscles. The main advantage of the electrospinning technique is the possibility to control the properties of materials obtained by this method [277,[282], [283], [284], [285]]. For example, by changing the polymer concentration or operating conditions such as flow rate or distance from the needle to collector plate, it is possible to adjust the size of the fibres from nanometric to micrometric range. Moreover, by changing the collector plate, it is possible to control the alignment of nanofibers from those randomly oriented when using a stationary or very slow-rotating collector plate, or in the case of a fast-rotating, aligned fibers. The flexibility of this method means that new improved methods of nanofibers creation are still being sought in both literature and commercial solutions. Recent proposals indicate that this technique still needs to be studied in order to reproduce natural solutions in the most realistic way. These researches are aimed at finding new materials with improved performance and biocompatibility with the body as well as improving muscle recovery methods based on novel techniques to engineer 3D muscle grafts for therapeutic treatments for volumetric muscle loss (VML). The investigations related to this technology are carried out in two ways: by looking for new materials or additives to the scaffolds production in order to improve its performance, including biocompatibility, or by enriching the scaffolds with cells and active compounds to enhance the regeneration effect. In addition, depending on the type of scaffolds produced, various modifications of the electrospinning technique are proposed in the literature, including cell electrospinning (CE) or divergence electrospinning. The research directions, with particular reference to new trends, will be discussed below and summed up in Table 3. The first works related to the formation of scaffolds for muscle regeneration were based on poly(ε-caprolactone (PCL) [286,287], mainly due to its biocompatibility and low immunogenicity in body (Table 1). Current trends indicate the possibility of using synthetic copolymers, natural polymers or hybrid systems. In work [288] electrospun scaffolds from poly(butylene 1,4-cyclohexandicarboxylate-co-triethylene cyclohexanedicarboxylate) (P(BCE-co-TECE)) was proposed. The obtained results, based on in vitro and in vivo studies, showed that the presence of ether linkages had impact on mechanical properties, degradation rate, surface wettability, as well as density of cell anchoring points. Moreover this scaffolds enhance cell adhesion, proliferation, and differentiation by promoting cell orientation along fiber direction, as well as by enhance cell infiltration and oxygen and nutrient diffusion. Narayanan et al. [289] showed the possibility to use poly(lactide-co-glycolide) (PLGA) for scaffolds production. Authors concluded that control of mechanical properties and degradation kinetics could be obtain by changing the ratio of lactide to glycolide. In vivo study using an mdx mouse model, thus popular model for studying Duchenne muscular dystrophy, showed the potential of applying optimized fiber scaffolds to enhance myogenic potential of transplanted cells. Whereas among the examples of natural polymers, the work of Manchineella and co-workers can be indicated [290]. Combination of silk fibroin and melanin allowed to obtain antioxidant and electroactive biomaterial scaffolds which improved the myogenic differentiation of myoblasts into myotubes in vitro. An interesting solution was presented by Laurencin and co-workers [291]. For the reason that fibrin, on the one hand, is an optimal scaffold for tissue engineering applications, because it mimics extracellular matrices, and on the other hand has poor mechanical properties, authors proposed to obtain a bilayer fibrin-polyurethane scaffold by combining the electrospinning method (in order to obtain a nanofiber structure of fibrinogen) and the spray, phase-inversion technique to prepare the synthetic layer. The final polymerization of fibrin by spraying thrombin solution on the electrospun nanofibers allowed to obtain nanostructured layer of fibrin fixed on microporous poly(ether-urethane) support layer. According to the authors' suggestion the obtained material can be used i.e. in soft tissue regeneration processes including muscle, skin. Moreover polyesterurethane could be applied as potential scaffolds for skeletal muscle tissue engineering [292]. On the other hand, based on polyurethane it is possible to formation a hierarchical electrospun muscle inspired structure [293]. According to the results of citied work, it could be concluded that by applying the electrospinning method, materials development that mimic the alignment and geometry of nano- and micrometric systems, such as myofibers, myofibers/fascicles and surrounding membranes, as well as the entire muscle was possible. The obtained materials indicated slightly higher yields to the passive muscles with a similar biomimetic non-linear behaviour which could closely resemble the complex morphology of skeletal muscle tissue. It can be assumed that the introduction of active components into the structure will allow to realize a highly biomimetic artificial muscle. Thus, in addition to the base of polymer scaffolds the incorporation of bioactive factors and cells is promising topic of investigation for muscle regeneration engineering. Guo et al. demonstrated the possibility of using the electrospinning process, based on aqueous solution-electrospinning method to encapsulate C2C12s and electrospin them into fibrin/polyethylene oxide (PEO) microfiber bundles, to evenly distribute immortalized mouse myoblast cell C2C12s inside the fibrin scaffolds, as well as the lack of inhibition of cell growth after the process [294]. Despite low density of myotube, this method allowed for the elongation and differentiation of cells inside the fibres as well as the expression of mature muscle markers e.g. myosin heavy chain (MHC). Moreover, in order to improve the cells growth on the scaffold, the use of gold coatings was proposed [295,296]. In this case, the properties of gold nanoparticles, such as their biocompatibility, good conductivity and possibility to functionalization with various organic and biological compounds are implemented. Zhang et al. [296] proposed application of 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for regeneration of skeletal muscle based on aligned electrospun nanofibers and gold nanolayer coating. This solution allow to enhance myoblast alignment and the formation of myotubes thanks to gold nanolayer coating as a consequence of improving electrical signal transfer between cells. As suggested by the authors, on the one hand, hierarchically organized scaffolds and, on the other hand, their conductive properties allowed to create a platform that not only supports the desired growth but also myoblast differentiation, which translates into further assembly of the implantable fascia to repair skeletal muscle tissue. Enhancement of the muscle regeneration effect with the addition of active compounds was also confirmed by Liu et al. [287] for polycaprolactone (PCL) fibrous membranes coated by mussel-inspired poly norepinephrine (pNE), which originally functions in the brain and body as a hormone and neurotransmitter. Investigations showed a better effect of cell adhesion and proliferation both in vitro and in vivo. The tests on the rat skeletal muscle cell line L6 and in vivo experiments using six week Sprague-Dawley female rats showed the possibility of correct integration of the regenerated muscle layer with fiber membranes and surrounding tissues at the site of the impairment.
Table 3.
Recent trends in electrospinning technique in muscle tissue engineering strategies.
| SCAFFOLD/METHODS | TEST OBJECT | MAJOR OUTCOME | REF. |
|---|---|---|---|
| Polycaprolactone (PCL) with outer polylactic acid (PLA) frame/Divergence Electrospinning | Nanofiber properties (spatial distribution of fiber density) | Easy to control the fiber density by changing the experimental conditions (collector heights, inclination angle); Lack of in vitro and in vivo experiments. | [297] |
| PCL and nanoclays (phyllosilicate) to enhance the homogeneity of fiber distribution/Divergence Electrospinning | Nanofiber properties (fiber diameter, density, alignment) | Addition of nanoclays improves the overall homogeneity of the 3D nanofiber scaffolds microstructure; Lack of in vitro and in vivo experiments. | [298] |
| PCL and decellularized bovine muscle ECM/Electrospinning | Male C57/BL6 adult (14–16 weeks old) mice | Increased activity of anti-inflammatory M2 macrophages (arginase+); Increased myofiber (MHC+) regeneration; No effects in muscle weights and force production. | [299] |
| PCL and gelatin functionalized with the addition of heparin/Electrospinning with heparinization of PCL/gelatin scaffolds | Male (weight 280–300 g) Sprague–Dawley rats | Addition of gelatin improves the hydrophilicity, cytocompatibility, and biodegradation, while heparin improves hemocompatibility; Heparin is covalently attached to the free amines of gelatin using the 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide hydrochloride and N-hydroxysulfosuccinimide; Obtained small-diameter vascular grafts are beneficial to the development of small-diameter artificial blood vessels. | [300] |
| PCL modified with graphene oxide (GO) with skeletal muscle cells (C2C12)/Cell Electrospinning and oxygen plasma modification of scaffold | The cellular interaction, morphology, and orientation changes | Oxygen plasma allows to change hydrophilic surface of electrospun fibers in order to improve the interaction with GO; GO-modified PCL nanofibers scaffolds impact cell elongation. | [301] |
| PCL and collagen struts with endothelial cells (HUVECs) and C2C12 cells/Cell Electrospinning and 3D printing | The cellular activities (myoblast proliferation, alignment, and differentiation/maturation) | Higher degree of the myosin heavy chain (MHC) with striated patterns and enhanced myogenic-specific gene expressions (MyoD, troponin T, MHC and myogenin) is obtained for scaffold with myoblasts and HUVECs in comparison to scaffold without HUVECs; HUVEC-electrospinning with modification in fiber direction is simple and effective method to provide biophysical/biochemical cues for facilitating myoblast alignment and differentiation. | [302] |
| Poly(L-lactideco-caprolactone) and poly(l-lactic acid) (PLCL/PLLA)/Coaxial Electrospinning | Vascular smooth muscle cells (VSMCs) | Flow rates of the PLLA-core and PLCL-shell solutions determines modulus/stiffness of the aligned fibers, without negative effects to the fiber topography and surface chemistry; Stiffness effect of electrospun fibers on phenotypic modulation in vascular smooth muscle cells (SMCs) is observed. | [303] |
| Poly(lactide-co-glycolide) (PLGA) with induced pluripotent stem cells (iPSCs)/Electrospinning | SMC differentiation by evaluation of the five SMC related genes and two SMC related proteins | Enhanced smooth muscle cell (SMC) differentiation potential of the human iPSCs; iPSCs-seeded PLGA shows potential potential for use in bladder tissue engineering. | [304] |
| Decellularized extracellular matrix (dECM) scaffolds without the polymer carrier/Electrospinning | New Zealand White rabbits | dECM contains many biochemical cues that help in cell adhesion, proliferation, and differentiation; There is possibility to produce dECM scaffolds with tunable physicochemical properties while retaining proregenerative matrix components. | [305] |
| Decellularized ECM-methacrylate with poly(lactide-co-glycolide) (PLGA) carrier/Electrospinning and 3D printing | Human muscle progenitor cells (hMPCs) | Promotion of the cellular orientation and myotube formation of human muscle progenitor cells by dECM-MA/PLGA composite scaffold. | [306] |
An important issue in tissue engineering is the application of an appropriate scaffolds production method. Here, modifications of the electrospinning technique come to the aid. Although 3D scaffolds is already widely used for tissue reconstruction [307] and electrospinning procedures are often used to generate scaffolds with alignment cues that lead to uniaxial alignment of seeded cells, there is still a problem due to the specificity of this solution. In this procedure, the cells mainly adhere to the outside of the scaffold, which results in uneven distribution on it. Therefore, it was proposed to include cells in the biomaterial during electrospinning. The answer to this is cell electrospinning (CE) that based on the basic process of electrospinning encapsulated viable cells in the micro/nanofibers [282,294,308]. This issue is difficult because traditional electrospinning subjects biomaterial to high voltages and currents that are harmful to cell survival [309]. The advantages of using electrospinning in formation of scaffolds for tissue engineering applications have contributed to the development of many methods based on this technique. In addition to mentioned above the cell electrospinning method, divergence electrospinning is proposed in the literature [[297], [298], [310]]. This technique allows to produce a scaffolding from nanofiber with a thickness of centimeter in a short period of time, showing the advantages of scalability in comparison with traditional electrospinning and high resolution in comparison with 3D printing techniques. By changing the height and angle of inclination of the two cone collector, the density of the produced fibers as well as microstructure gradient of a 3D nanofiber matrix can be changed and controlled. This technique promote the development of biomimetic artificial tissues with patterned nanofiber structures, thus not only muscle but also ligament, cartilage, tendon.
The development of such electrospun nanofiber materials has led to some of them being already in the commercialisation phase. For example, P&P Biotech Company offers patches as class III medical devices based on the development of the He Group at Fudan University Affiliated Zhongshan Hospital, China [283]. Research has shown the possibility of using electrospun nanofibers made of a mixture of poly(l-lactide-co-caprolactone) and porcine fibrinogen as a patch for abdominal wall regeneration. In vivo experiments were carried out on dog showing that after 36 weeks the skeletal muscle regeneration of the abdominal cavity was effectively induced, while being restored within two weeks after implantation.
It should be noted that electrospun nanofibers are proposed also as effective drug delivery system [284,291,[311], [312], [313]]. For example Bagheri et al. [314] proposed PVA/chitosan-aniline oligomer, which indicated suitable biocompatibility, cellular activity and cell adhesion. Addition of dexamethasone to the electrospinning solution allow to obtain new material which exhibit anti-inflammatory and immunosuppressive properties. Electrospun poly-l-lactide (PLA) scaffold with the cell death-inducing drug Diclofenac (DCF) encapsulated has been successfully tested on human dermal fibroblasts (HDFs) [315]. Controlled drug delivery allow to changes in cell morphology and glycolytic activity. The possibility to control the release of sirolimus, also known as rapamycin, drug which prevent organ transplant rejection has been proved using electrospun polyurethanes [316]. In the review focused on electrospun cellulose acetate [317], the possibility of its use as a drug carrier was indicated, including: anti-cancer, anti-inflammatory, antioxidant, antibacterial agents, as well as vitamins and amino acids. Such solutions can be applied in transdermal or local delivery systems, wound dressings and in biomedical applications. At the same time, the authors pointed out in this case that CA nanofibers cannot be completely biodegradable in the human body due to the lack of cellulase enzyme and are degraded by microorganisms, which in case of potential application should be improved. Khodadadi and co-workers [318] in their work summarized the possibility of using electrospun nanofiber in drug delivery system (with chemotherapeutic agents such as 5‐fluorouracil, cisplatin, curcumin, dichloroacetate (DCA), docetaxel, doxorubicin (DOX), paclitaxel (PTX), and platinum complexes) for localized cancer chemotherapy. It was confirmed for DOX in the case of pH-sensitive polyvinyl alcohol/polycaprolactone (PVA/PCL) core-shell nanofibers obtained by coaxial electrospinning technique [319]. In works [[320], [321], [322]] authors have shown the possibility of applying these materials for ocular drug delivery, while for oral drug delivery system in works [[323], [324], [325], [326]]. Moreover, electrospun nanofibers characteristics such as a large surface area with controlled conformation and relatively simple modification possibilities, as well as complex pore structure and high biocompatibility make these materials a promising example for the construction of biosensors at the nanoscale [277,[327], [328], [329], [330], [331]] as well as wound healing patches [[332], [333], [334], [335]], i.e. multilayer alginate–polycaprolactone electrospun membranes [333]. The discussed works indicate the wide use of electrospun nanofibers in medicine, which at the same time indicates their high potential for muscle regeneration combined with specialized treatment methods. Going forward, based on the already existing knowledge in muscle tissue engineering and the cited work related to other medical applications of the electrospinning technique, significant work needs to be done to assess the potential use of these materials and its possible improvements and limitations in muscle regeneration.
5. Perspectives in scaffold fabrication
5.1. Additive manufacturing in scaffold fabrication
An ideal scaffold for tissue engineering not only needs to be made from a biocompatible material but also supports cell adhesion, growth and migration by specific, designed micro/nanoarchitecture. Advanced, functional scaffolds should simultaneously provide structural support for cells and mimic the native tissue structure. The wide range of commonly used in laboratories scaffold fabrication techniques such as phase separation, solvent casting, soft lithography, molding, fiber bonding, gas foaming, emulsification, freeze-drying, membrane lamination and particulate leaching enable to form 3D scaffolds, but has a major limitations [336], which includes difficulties in controlling complex micro/nanoarchitecture, pore size, porosity and its network. 3D printing technologies overcome these issues, and enable the production of repetitive, customized scaffolds with controlled parameters and also provide highly complex shapes. There are more than 40 different types of 3D-printing techniques currently. The most promising techniques of 3D-printing scaffolds for tissue engineering are presented below.
5.1.1. Near-field electrospinning (NFES)
NFES is an alternative approach to the traditional electrospinning method, where the electrode-to-collector distance is decreased to control the electrospun fibers deposition [337]. The shorter spinning distance causes that the fibers can be deposited in straight-line stage. Moreover, the short distance results in reduction of the applied electrostatic voltage from hundreds to tens of volts, making this process cheaper and more safe. Several materials can be applied to formulate nanofibers by using NFES, for instance PEO, PVP, PCL, PVDF, PS, PMMA or bioactive glass [338,339]. Depending on the physical and chemical properties, they can be used as materials for fabrication of 3D biomimetic scaffolds in the field of tissue engineering. Kolan et al. designed PCL/bioactive glass scaffold with microstructure similar to the cancellous bone [339]. The authors improved that NFES scaffolds improved high human adipose-derived mesenchymal stem cell proliferation and distribution, compared to 3D printed scaffolds. Ren and co-authors fabricated PCL/collagen fibers in order to control the growth and differentiation of human peridontal ligament stem cells (hPDLSCs) [340]. By using NFES technique, they produced ordered scaffold with unique topography (controlled intervals and directions of fibres). Ren et al. improved that differentiation of hPDLSCs into cementum-forming cells, collagen-forming cells, or bone-forming cells can be controlled by topographic guidance of prepared scaffolds [340]. The NFES provides a powerful, simple and low-cost technique for the ultrafine fibres deposition. However, it still has some limitations: i) the small droplet size restricts the large-scale preparation of fibers, ii) the shortened distance between electrode and collector limits the thinning and stretching of fibres, iii) ambient (environmental) factors, such as humidity and temperature, as well as viscosity, conductivity of polymer solution/mixture may also affect the morphology of nanofibres.
5.1.2. 3D printing technologies
Additive manufacturing, commonly known as 3D printing, established several approaches, but each of them enables to form of highly complex 3D scaffolds. Conventional 3D printing involves producing of objects by a layer-by-layer approach. Most of additive manufactured scaffolds require two-step fabrication of acellular scaffolds which are further seeded with cells and cell-laden constructs developed to mimic their native analogs.
5.1.3. Stereolithography (SLA)
SLA is the first rapid prototyping process developed in the late 1980s. In SLA, the ultraviolet (UV) light is use to induce curing of a liquid layer of polymer resin via photopolymerization. UV light is irradiated on the photosensitive resin surface in precise patterns. Excitation of photoinitiator molecules by UV light induces releasing reactive species such as free radicals upon causing polymerization of the resin which leads to the formation of a solid material. The first fabrication step involves the adhesion of the first layer of a photopolymerized polymer directly to a build platform. This important step provides support for 3D structures as they are fabricated. When the first layer is completely polymerized, the build platform is moved to defined step height for polymerization of the subsequent layer. The moving process then repeats, with each new layer cured onto the previous layer until the three-dimensional structure is completed. Once the 3D structure is polymerized, the scaffold should be rinsed in the solvent to remove the uncured resin [341]. The main advantage of using stereolithography is the control over the internal and external geometry of the scaffold structure, which involves pore size, porosity, patterns [342] as well as the ability to the remove of unpolymerized resin, and extremely high feature resolution (~1.2 μm). The disadvantage of SLA is the poor range of biocompatible resins that simultaneously have proper processing properties. Another drawback is the necessity of usage of photoinitiators and radicals which can be cytotoxic toward cells, possible entrapment of unreacted monomer and other residual photoinitiators, poor mechanical properties of photopolymerized resin and relatively long processing time. Finally in SLA challenging is the completely removal of support structures and the inability to fabricate compositional gradients along horizontal planes [343]. Besides that, scientists all the time publish improvements in the field of scaffolds 3D printing and new materials combination. Recently H. Kumar et al. presented digital light processing (DLP)-based SLA (DLP-SLA) bioprinting of biocompatible scaffolds made by gelatin methacryloyl (GelMA). GelMA synthesized in reverse osmosis (RO) filtered water (RO-GelMAs) results in rapid fabrication of high resolution and mechanically stable 3D constructs. Obtained bioinks exhibited excellent biocompatibility and cell-organization over three weeks in culture with 3T3 fibroblasts and U118 astrocytes [344].
5.1.4. Selective laser sintering (SLS)
SLS is another 3D printing method, in which scanning laser fuses particles with a diameter around 50 μm, in order to build a designed part layer by layer from a fine powder. The sintering (recrystallization) of fine powders takes place once illuminated by a high-power beam of a laser. The process is generally performed under inert atmosphere to limit contamination or undesired oxidation of powders [345]. It should be pointed out that SLS method, due to significant material restriction, is mainly applied to fabricate 3D scaffolds for bone tissue engineering [346]. For instance, the incorporation of biomolecules is limited due to the use of a high-power beam laser to sinter powdered material. Another major limitations of SLS are: i) poor surface finishing of designed parts, ii) presence of defects in the fabricated parts as a results of large shrinkage rates, and iii) need to apply post-processing treatments to improve the quality of the surface. Despite all these disadvantages, SLS is commonly applied to fabricate bioactive bone scaffolds [[347], [348], [349], [350]]. Tan et al. demonstrated successful incorporation of hydroxyapatite into polyetheretherketone (PEEK) polymer matrix to enhance the bioactivity of designed scaffold. The authors highlighted that SLS provided excellent control over the microstructures of scaffold by adjusting SLS process paramterers, such as temperature, and laser power [349]. Sun et al. reported fabrication of PLLA porous scaffold containing encapsulated dexamethasone (Dex) as a scaffold for bone regeneration. Based on the ex vivo studies, the authors showed that implantation of prepared scaffold in rat cranium defects enhanced the formation of new bone and blood vessel, due to the controllable release of Dex molecule [351].
5.1.5. Bioprinting
Generally, two strategies are in use: fabrication of acellular functional scaffolds which are further seeded with cells and cell-laden constructs developed to mimic their native analogs. Different technologies that utilizes living cells to form 3D cell-laden scaffolds are known as bioprinting. The principle of this process consists the deposition of cells loaded in bioink by nozzle-based techniques or laser-assisted techniques:
5.1.6. Nozzle-based 3D printing
Nozzle-based techniques include material extrusion or Inkjet printing, as described below. Inkjet bioprinters are frequently use for tissue engineering applications. Thermal inkjet bioprinting uses a prepolymer solution containing cells (the bioinks), loaded in an ink cartridge. Then printer head with cartridge eject droplets of ink through air bubbles created by the heat in the printing head. The advantages of those techniques are fast fabrication, their widespread usage caused by the affordability of the device. Extrusion Bioprinting is a type of inkjet bioprinting, which aims to dispense of bioink dispense by pneumatic (air pressure) or mechanical (piston, screw) systems. The most popular is the pneumatic system, where bioink is extruded from the nozzle or needle by continuously applying air pressure instead of single droplets. This approach provides structural integrity to the 3D structure [352]. The disadvantages of nozzle-based 3D printing is clogging of the nozzle because of high viscosity of the ink, cell aggregation and drying of the injected biomaterial in the nozzle. Moreover, the high mechanical stresses during extrusion may be harmful to cells and could lead to a decrease in cell survival [353]. Prototype on an innovative injecting/extruding 3D cellular printer based on remote magnetic control for dual effect of 3D bioprinted scaffolds with controlled cells seeding via magnetic guiding was recently reported. The new approach of designed magnetic scaffolds with magnetic gradients, were able to orient and trap the magnetized cells on the chosen side of the scaffold fibres. In vitro separation of two cell populations MSCs and human umbilical vein endothelial cells (HUVECs), on the opposite sides of the magnetic scaffold fibres were described for the first time which potentially can be used at in vivo environment [354].
5.1.7. Laser-assisted bioprinting (LAB)
LAB is another possibility of advanced 3D printing of living cells. This approach involves the usage of the pulsed laser source, a donor layer, and a receiving substrate. The cells suspended in bioink are transferred to the donor layer, by focusing a laser on a membrane that is coated with cell-containing bioink. The pulsed laser source is focused on the laser absorbing-layer that generates a vapor bubble. This bubble forms pressure to deform the bioink and forms droplets. By this method, cells are transferred directly from the side of the membrane facing the printing surface to the donor layer (receiver) following by their crosslinking. The main advantage of LAB is an absence of an orifice, which lead to the decreased shear stress on cells, also the resolution of printing is better than in other bioprinting methods [355].
5.2. Current advantages of multifunctional scaffolds
The literature review shows a recent trend in scaffolds development especially using ECM-based or naturally derived biomaterials with incorporated active agents (e.g. growth factors) and delivering therapeutic agents (sections 2, 3, 4). Despite scaffolds clear biological potential, it is challenging to compare those biomaterials due to lack of detail physico-chemical characterization (such as mechanical strength, viscosity, degradation rate, swelling rate, Young's modulus etc.). Table 4 describes selected publications which connect advanced scaffold processing, accurate physico-chemical characterization and excellent biological properties. The obtained materials were found to be non-cytotoxic to skeletal, vascular or neural cells. Most of the developed biosystems mimic living tissues by improvement of architectural organization of artificial tissues [[356], [357], [358], [359], [360]]. The electrospinning and additive manufacturing were frequently used to develop tissue substitutes [356,357,359,361,362]. All the developed scaffolds for soft tissue engineering, was successfully examinated in vitro, ex vivo or in vivo systems [357,363]. Tissue-specific stem cells and progenitor cells, were frequently used as they are able to regenerate the tissue from which they are isolated. Presented scaffolds induced accelerate cells growth and differentiation [359,360,363].
Table 4.
Comparison of recently fabricated scaffolds properties in nervous, cardiovascular and muscle tissue engineering.
| SCAFFOLDS FOR TISSUE ENGINEERING | |||
|---|---|---|---|
| NERVOUS SYSTEM REGENERATION | |||
| SCAFFOLD | Photocured gelatin fibres packed with NGF, laminin and fibronectin [364] | 3D multichannel silk electrospun bifunctionalized with NGF and CNTF [362] | Two-component collagen nerve guides (Neuromaix) [358] |
| FABRICATION TECHNIQUE | photopolymerization | electrospinning | commercial scaffold |
| DEGRADATION TIME | after 12 months without inflammantory reactions | from 72 h to 168 h | after 12 months without non-toxic degradation products |
| MECHANICAL STRENGHT | – | 8.47 ± 1.33 MPa (elastic modulus) | – |
| BIOLOGICAL MODEL | in vivo (Lewis rats) | in vitro (neural cells) | in vivo (Lewis rats) |
| REGENERATION | Functional recovery of nerve tissue after 6 months | it supports the growth, development and migration of cultured neural cells | Functional recovery of nerve tissue after 12 weeks |
| FUNCTIONAL RECOVERY | 10000 of myelinated axons/mm2 (after 24 weeks)* | – | 200 of regenerated axons/mm2 (after 12 weeks)- |
| ADDITIONAL COMMENTS | diameters of the regenerated tissue prostheses (0.84 ± 0.2 mm) were close to the normal sciatic nerve (1.0 ± 0.2 mm) | elastic modulus of scafflod was close to rat sciatic nerves (13.79 ± 5.48 MPa) | it exhibits reduced myelin sheath thickness, it allows to axonal regeneration across large nerve gaps, the regenerating axons were able to functionally reinnervate the muscles |
| CARDIOVASCULAR SYSTEM REGENERATION | |||
| SCAFFOLD | PU-based scaffold [363] | ECM-based cardiac patch [359] | PLGA/gelatin scaffolds [365] |
| FABRICATION TECHNIQUE | melt-extrusion additive manufacturing technique | decellularization, solubilization, and electrospinning | soft lithography |
| DEGRADATION | melt-extrusion AM technique helps to avoid PU thermal degradation | degradation process starts below 100 °C | after 15 days weight loss of about 50% |
| BIOCOMPATIBILITY | cardiac progenitor cell viability > 95% | 7-fold increase in human bone marrow mesenchymal stem cell number after 4 weeks | long-term viability of hMSCs up to 15 days |
| BIOLOGICAL MODEL | ex vivo (CD117-positive CPCs isolated from left ventricle from pathological hearts with ischemic cardiomyopathy) | ex vivo (left ventricular tissues, isolated from healthy commercial slaughter-weight pigs) | in vitro (Human mesenchymal stem cells) |
| PHYSICAL PROPERTIES | Tg = 45.4 °C Tm1 = 76.0 °C, Tm2 = 155.0 °C |
Tpeak = 300.12 °C Tendset = 448.02 °C |
– |
| MECHANICAL PROPERTIES | 10.2 ± 2.2 MPa (Young's modulus) | 203 ± 13.4 kPa (Young's modulus) | 0.78–1.20 MPa (Young's modulus) |
| ADDITIONAL COMMENTS | it supports the adhesion and spreads of human cardiac progenitor cells (CPCs), whereas does not stimulate CPC proliferation | it support proliferation and growth of human bone marrow mesenchymal stem cells (hMSCs) | It promotes adhesion, ordered disposition and early myocardial commitment of hMSCs |
| SKELETAL MUSCLE REGENERATION | |||
| SCAFFOLD | PCL/collagen nanofiber meshes [357] | chitosan/PVA scaffold [361] | cells into 3D constructs composed of PEG-Fibrinogen hydrogel fibers [356] |
| FABRICATION TECHNIQE | electrospinning | electrospinning | 3D bioprinting |
| MECHANICAL STRENGHT | 3.06–4.88 MPa (tensile strenght) | 6.63 MPa (tensile strenght) | 48 kPa (tensile stiffness) |
| BIOLOGICAL MODEL | ex vivo (human skeletal muscle tissues taken from male patients, age 50–65) | in vivo (New Zealand white rabbit) | in vivo (Immunodeficient mouse) |
| BIOCOMPATIBILITY | the muscle cells readily adhered and proliferated to myotubes after 7 days | there was not any significant immunological symptoms, i.e. fever, pain, or fainting until 2 weeks | After 21 days myotubes underwented sarcomerogenesis, guarantees their proper contractile function |
| VISCOSITY | – | 14563.85 cP (RT) CS/PVA solution (5% w/v) |
– |
| DEGRADATION TIME |
– | after 16 h | after 5 days |
| SWELLING PROPERTIES | high fluid uptake ability (325 ± 7%) | swelling ration more than 200% after 16 h | – |
| OTHER FEATURES | it facilitates cell adhesion, proliferation and differentiation | it promotes cell attachment, acts as mechanical support for muscle, helps to store nutrients for cell attachment and growth | 3D scaffold leds to a substantial improvement of architectural organization of artificial muscle tissue |
| ADDITIONAL COMMENTS | PCL/collagen scaffold is able to guide and orient skeletal muscle cells into organized structures | it exhibits higher stress strength than native required strength for skeletal muscle tissue (0.2 MPa) | Young's modulus of scaffold is well above the optimal range of substrate modulus for myotube differentiation (8–11 kPa) |
In the future advanced biomaterials studies about specific physico-chemical characterization should be done prior to better understanding of scaffolds performance. It seems that obvious physico-chemical parameters are overlooked by authors, which makes it difficult to learn about all the scaffolds properties and compare these systems. Lack of biophysical characterization hinder the full scaffolds potential.
5.3. Future perspectives of the scaffolds
Depending on tissue type there are requirements for different architecture. The architecture including pores and topography of biomaterial regulates cellular behavior and determines stem cell fate. Biophysical properties of the natural nano/microenvironment where cells exist, such as topography and stiffness provide extracellular support for stem cells. This microenvironment denoted as “niche” modulate cell adhesion, growth, self-renewal, migration and differentiation of stem cells. In recent decades much more attention to developing biocompatible materials has been paid for extracellular matrix (ECM) mimicking. ECM mimicking not only rely on mimicking its composition (primary material, growth factors) but also stiffness and geometry. In vitro ECM-mimicking can be performed by a selection of pores and topographical cues (patterns) for controlling cell shape [366]. Such materials should have well-defined compositions, structures and properties. It was confirmed that both macro and nanotopography influence cell behavior by similarities to native ECM. The interaction of nanotopographical features with cells integrin receptors alters cells adhesion, alignment and even differentiation [367]. The most promising approaches for scaffold fabrication connect controlled manufacturing of complex nano/microarchitecture and mechanical tuned scaffolds made up of bioactive material. This allows for scaffold integration with cells followed by transformation into the intended artificial organ or tissue.
5.3.1. Neural engineering
The main challenge in neural tissue engineering is the fabrication of scaffolds with controlled topography, biochemical cues capable of directing damaged nerves and restoring the function of neuronal cells toward the recovery from neurological disorders and injuries [368]. Numbers of studies showed that the most effective topographical cues for neural cell adhesion, growth, migration, differentiation and regeneration are grooves, aligned fibers, or channels [369]. One of the most intensively studied are scaffolds for increased peripheral nerve regeneration after injuries. A nerve conduit is a tubular structure made of synthetic or biological materials designed to bridge the gap of a sectioned nerve. The purpose of the conduit is to protect the nerve from scar formation, to prevent fluid from leaking from the nerve stump and to guide the axon nerve cone into the distal nerve stump [138]. Patterned topographies influence attachment, alignment and orientation of stem cells by changes in the shape of the nucleus, in cytoskeleton rearrangement as well as by the expression level of genes. It has been reported that micro or nanopatterns can effectively induce neuronal differentiation of various stem cell types. Recently platform modified with homogeneous nanohole patterns of three different sizes (500 nm, 700 nm, and 900 nm) by laser interference lithography (LIL), exhibit effective guiding neurogenesis of mouse neural stem cells (mNSCs). Such nanoplatforms could be useful for controlling various differentiation lineages of stem cells [370]. Additionally highly desired in neural TE are conductive scaffolds which have beneficial properties due to connecting the bioelectric flow in the body. External electrical stimulation of such constructs was confirmed to modulate cell migration, differentiation, maturation, synaptogenesis and finally enhance damage nerves regeneration [371]. Electrical stimulation was directly applied to electrospun nanofibrous scaffolds made by conductive block copolymer of PPy and PCL (PPy-b-PCL) to enhance the nerve regeneration process. Biodegradable and conductive 3D porous scaffold with superior was constructed by means of a novel electrohydrodynamic jet 3D printing technique. Authors obtained superior control over the pore size, porosity, precisely controlled fiber diameter and fiber alignment. PCL/PPy scaffolds supported the differentiation and maturation of hESC-NCSCs to peripheral neurons, exhibiting potential clinical value as cell-laden or cell-free NGCs for peripheral neuronal regeneration [372]. Novel 3D nanofibrous hydrogels have been recently demonstrated. Scaffolds were made by fibrin/polyurethane/multiwall carbon nanotube (fibrin/PU/MWCNT), for improve advanced scaffold electrical conductivity and mechanical properties. Results conformed an appropriate microenvironment for enhancing cell adhesion, proliferation and high viability [373]. Nanotopographical cues in combination with chemical cues are highly desired in 3D scaffold fabrication. Researchers have proposed various strategies to enhance or accelerate nerve outgrowth, however multidimensional regeneration of both neurons and glial cells is the real challenge. Regeneration of oligodendrocytes can reestablish myelin sheaths and restore their functions. Simultaneously preventing the formation of glial scars, and promoted axonal, myelin regeneration is highly desirable. Many scientific reports show the wide diversity of active biomaterials with topographical cues, but despite many studies in this field, the successful combination of material with high mechanical and biological properties is yet to be achieved.
5.3.2. Cardiovascular engineering
The challenge in cardiovascular engineering remains to create functional tissue constructs that can reestablish the structure and function of injured tissue by mimicking and regulating the microenvironments, and physiochemical stimuli, to control the maturation of cells toward cardiovascular cell phenotypes [220]. Additionally, the critical aspect of cardiovascular tissue engineering is the lack of vascularization in constructs. Cardiac scaffolds should have a highly porous structure with efficiently interconnected pores to allow the vascularization, the flow of nutrients and the elimination of waste products. It was observed that pore parameters inside scaffold can enhance vascularization [374]. The last results show that poly(vinyl) alcohol (PVA) scaffold with a designed interconnected pore size ranging from 10 μm to 370 μm enables spreading through scaffold and proliferation of human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) [375]. Another promising scaffold architecture refers to force direct cell orientation. By controlling the scaffold shape linearly, the cells are directionally influenced by patterning and tensile force which influence growth and maturation. Y. Tsukamoto et al. reported on a method for the fabrication of 3D cardiac tissue with heart-specific structure, exhibiting cell orientation and vascular network. Hydroxybutyl chitosan (HBC) scaffold were fabricated by combining orientation-controlled 3D tissue by using an LbL technique, cell accumulation method and 3D printing technology. Obtained by co-cultured hiPSC-CM, NHCF and human cardiac microvascular endothelial cells (HMVEC) native-like 3D cardiac tissue exhibit orientation and vascular network within the constructs [376]. Recently bio-inspired scaffolds made of the crosslinked gelatin and cellulose nanofibrils (CNF) were raported. Combining gelatin with biomimicry properties with structural reinforcement by the CNF and suitable pore size and interconnection allowed fibroblasts effective colonization and proliferation. The designed 3D nanocomposite polymers, exhibited chemical stability, good mechanical properties and biocompatibility [377]. An ideal cardiovascular scaffold should have proper architecture of interconnected pores, also should enable for effective cell migration and vascularization. Another promising strategy is the usage of electrostimulation, which was showed to enhance efficiency of cardiac differentiation and promote cardiomyocyte maturation [378]. Recently M. Valls-Margarit from E. Martínez and A. Raya's groups reported on implementation of a platform for the production of engineered cardiac macrotissues from human pluripotent stem cells (PSCs) named ‘‘CardioSlice.’’ 3D porous scaffolds made by collagen and elastin-based sponges were used for culturing PSC-derived cardiomyocytes and human fibroblasts. Cell-laden scaffold was used under parallelized perfusion bioreactor together with electrical stimulation. Continuous electrical stimulation for 2 weeks promotes cardiomyocytes alignment, synchronization, and the development of cardiac tissue-like properties. Continuous electrical stimulation of cardiac macrotissues resulted in minor (but measurable) improvements in cardiomyocytes maturation, however significantly enhanced maturation at the tissue level. Developed in vitro system is highly promising in many applications including disease modeling, drug screening and toxicology, and regenerating damaged heart tissue [379].
Encapsulating, medical applications of 3D printing include the fabrication of anatomical models for pre-surgical studies, fabrication of acellular scaffolds, medical devices and finally direct 3D printing of cell-laden scaffolds and organs. Due to interactions between scaffolds and cells are a key to cell adhesion, viability, proliferation and differentiation, detail characteristics of biomaterials such as viscosity, mechanical strength, charge, degradation, roughness, swelling, reactivity, hydrophilicity/hydrophobicity need to be considered.
5.3.3. Muscle engineering
Skeletal muscle has ability to regenerate after injuries but endogenous self-regeneration is impaired due to a complex and highly regulated process included inflammatory or destruction phase, phase of the repair and remodeling phase. Crucial role in regeneration of inured muscle have basal lamina which acts as regenerative template, and secrete chemotactic factors which recruit stem cells to differentiate. When at the site of the injury, the basal lamina is damaged, occurs the harmful impact on the myogenesis process [380]. Additionally the natural regeneration process could be hindered due to volumetric muscle loss (VML) injuries. VML are caused by critical loss of skeletal muscle tissues which lead to severe functional impairment. Therefore scaffolds with incorporated biochemical cues (chemotactic factors and growth factors) which stimulate stem cells to differentiate and mature are highly promising for TE of muscle tissue. In addition, parallel alignment of regenerating muscle cells is essential for optimal tissue integration. Bioscaffolds which mimick the architecture and physicochemical cues was recently developed by N. Narayanan et al. Implantable glycosaminoglycan-based hydrogel made of thiolated hyaluronic acid (HA) and thiolated chondroitin sulfate (CS) scaffold cross-linked by poly(ethylene glycol) diacrylate offer appropriate biophysical cues for muscle engineering. Developed biomimetic scaffold support 3D encapsulation of murine myoblasts as well as progressive cell proliferation and facilitated myoblast to differentiate into myotube in vitro. Finally HA-CS scaffolds enhanced angiogenesis, innervation at the defect and promote skeletal muscle regeneration of VML injuries in mice [381]. Another important role in muscle regeneration process fulfill the satellite cells which are a skeletal muscle-specific stem cells. Satellite cells in normal conditions are quiescent between the basal lamina of the mature muscle fiber and sarcolemma. After muscle tissue damage, satellite cells play a major role in formation of new muscle cells and therefore reassembling of the contractile apparatus [382]. Cell-laden functional scaffolds were recently presented by Y. Zhang et al. Hierarchically organized, anisotropic and conductive scaffold with microscale melt electrowriting (MEW) grooves were manually rolled with myoblast cells to mimic the fascicle assembly. Parallel aligned oriented nanofibrous mesh was constructed to guide myoblast cell alignment, elongation and differentiation into myotubes. Results demonstrated that aligned nanofibers were crucial for myoblast alignment, while microgrooves were more effective in increasing both the elongation and maturation of myotubes, which brings new insight to development of novel scaffolds for muscle biomimicing [296]. Consequently, bioactive or cell-laden advance scaffolds are promising tools for improving skeletal muscle cells proliferation.
6. Conclusions
The type of material used for the production of scaffolds, as well as the sources of cells and bioactive molecules, supports the regeneration process. Despite the encouraging premises, this area still requires further studies. Effective cell-based therapy is possible by using bio-synthetic-and hybrid-material scaffolds. The most promising bioscaffolds fulfill many biological functions, i.e. provide migration of a large number of cells towards the injured tissue, their successful engraftment, and differentiation into mature, as well as serve as a delivery system to target growth factors, cytokines, genes, and other regulatory biomolecules. However, there is still a need to develop artificial scaffolds to successfully imposing in the clinical stage.
One of the main limitations of the peripheral neural tissue engineering is the incomplete alignment of axons from proximal to distal nerve segment due to insufficient regeneration properties of the scaffolds. This issue can be solved by topographical, mechanical and chemical guiding regenerating axons. Another obstacles with in vivo application of neural scaffolds are poor multidimensional regeneration of both neurons and glia. Lack of regeneration of Schwann cells for reestablish myelin sheaths and restore their functions is limitation in current peripheral neural tissue engineering. Simultaneously preventing the formation of glial scars and promotion of axonal, myelin regeneration is highly desirable in advance central and peripheral TE. Dual regeneration effect can be achieved by using electrically conductive hybrid conduits with incorporated biochemical cues and topographical features which enhance multimodal tissue regeneration. Combined strategies gives new perspectives into not only axonal outgrowth but also nerve myelination and muscle regeneration.
The current challenge in cardiovascular engineering remains to mimicking and regulating the microenvironments and physiochemical stimuli of native cardiovascular tissue. The critical aspect of cardiovascular TE is the poor vascularization of constructs which can be improved by using of biomimicking interconnected porous scaffolds that allow the vascularization, the flow of nutrients and the elimination of waste products. Acceleration of cardiovascular tissue maturation was received by continuous electrical stimulation of the scaffold. Therefore biomimicking, conductive, porous bioactive scaffolds are highly desirable in cardiovascular TE.
Effective incorporation of hierarchically organized scaffolds with biochemical cues (chemotactic factors and growth factors) is currently a great challenge for providing parallel alignment of regenerating muscle cells. Therefore such bioactive scaffolds which stimulate muscle cells to differentiate and maturation are highly promising for TE of muscle tissue. The potential of the use of electrospinning for muscle regeneration, including the possibility of targeting cell development and supporting it by strengthening cell infiltration and diffusion of oxygen and nutrients, is by far one of the most important trends to assume that such a solution is an opportunity to significantly improve the quality of life of patients with atrophy or damage to muscle tissue.
CRediT authorship contribution statement
Jagoda Litowczenko: Conceptualization, Writing - original draft, Writing - review & editing, Funding acquisition. Marta J. Woźniak-Budych: Writing - original draft, Writing - review & editing, Visualization. Katarzyna Staszak: Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Karolina Wieszczycka: Writing - original draft, Writing - review & editing, Funding acquisition. Stefan Jurga: Writing - original draft. Bartosz Tylkowski: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financed by the Ministry of Science and Higher Education, Poland, Grants no. 0912/SBAD/2010 and 0912/SBAD/2000 and the National Science Centre (NSC) grant no. 2016/23/N/ST5/00955.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jagoda Litowczenko, Email: jagoda.litowczenko@amu.edu.pl.
Bartosz Tylkowski, Email: bartosz.tylkowski@eurecat.org.
References
- 1.Yu L., Dawson L.A., Yan M., Zimmel K., Lin Y.L., Dolan C.P., Han M., Muneoka K. BMP9 stimulates joint regeneration at digit amputation wounds in mice. Nat. Commun. 2019 doi: 10.1038/s41467-018-08278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalirfardouei R., Jamialahmadi K., Jafarian A.H., Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019 doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 3.Tong Z., Jin L., Oliveira J.M., Reis R.L., Zhong Q., Mao Z., Gao C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021;6:1375–1387. doi: 10.1016/j.bioactmat.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöqvist S., Ishikawa T., Shimura D., Kasai Y., Imafuku A., Bou-Ghannam S., Iwata T., Kanai N. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles. 2019 doi: 10.1080/20013078.2019.1565264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathi E., Farahzadi R., Sheikhzadeh N. Immunophenotypic characterization, multi-lineage differentiation and aging of zebrafish heart and liver tissue-derived mesenchymal stem cells as a novel approach in stem cell-based therapy. Tissue Cell. 2019 doi: 10.1016/j.tice.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Edamura K., Takahashi Y., Fujii A., Masuhiro Y., Narita T., Seki M., Asano K. Recombinant canine basic fibroblast growth factor-induced differentiation of canine bone marrow mesenchymal stem cells into voltage- and glutamate-responsive neuron-like cells. Regen. Ther. 2020;15:121–128. doi: 10.1016/j.reth.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdolmaleki A., Zahri S., Bayrami A. Rosuvastatin enhanced functional recovery after sciatic nerve injury in the rat. Eur. J. Pharmacol. 2020;882:173260. doi: 10.1016/j.ejphar.2020.173260. [DOI] [PubMed] [Google Scholar]
- 8.Wieszczycka K., Staszak K., Woźniak-Budych M.J., Jurga S. Lanthanides and tissue engineering strategies for bone regeneration. Coord. Chem. Rev. 2019;388:248–267. doi: 10.1016/j.ccr.2019.03.003. [DOI] [Google Scholar]
- 9.Wang P., Sun Y., Shi X., Shen H., Ning H., Liu H. Bioscaffolds embedded with regulatory modules for cell growth and tissue formation: A review. Bioact. Mater. 2021;6:1283–1307. doi: 10.1016/j.bioactmat.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cvetković V.J., Miladinov D.T., Stojanović S. Biomater. Clin. Pract. Adv. Clin. Res. Med. Devices. 2017. Genotoxicity and mutagenicity testing of biomaterials. [DOI] [Google Scholar]
- 11.Bizzarri M., Cucina A. Tumor and the microenvironment: A chance to reframe the paradigm of carcinogenesis? Biomed Res. Int. 2014 doi: 10.1155/2014/934038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehler R.M., Graham J.G., Shea L.D. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011 doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratton S., Shelke N.B., Hoshino K., Rudraiah S., Kumbar S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016;1:93–108. doi: 10.1016/j.bioactmat.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R., Chen F., Guo J., Zhou D., Luan S. Recent advances in polymeric biomaterials-based gene delivery for cartilage repair. Bioact. Mater. 2020;5:990–1003. doi: 10.1016/j.bioactmat.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilodeau C., Goltsis O., Rogers I.M., Post M. Limitations of recellularized biological scaffolds for human transplantation. J. Tissue Eng. Regen. Med. 2020 doi: 10.1002/term.3004. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y., Alkhawaji A., Ding Y., Mei J. Decellularized scaffolds in regenerative medicine. Oncotarget. 2016 doi: 10.18632/oncotarget.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y., Zhang T., Song Y., Sun W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020 doi: 10.1088/1748-605X/ab452d. [DOI] [PubMed] [Google Scholar]
- 18.Maged A., Abdelkhalek A.A., Mahmoud A.A., Salah S., Ammar M.M., Ghorab M.M. Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur. J. Pharm. Sci. 2019 doi: 10.1016/j.ejps.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Niu X., Wei Y., Liu Q., Yang B., Ma N., Li Z., Zhao L., Chen W., Huang D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020 doi: 10.1016/j.eurpolymj.2020.109861. [DOI] [Google Scholar]
- 20.Sadeghi A., Moztarzadeh F., Aghazadeh Mohandesi J. Investigating the effect of chitosan on hydrophilicity and bioactivity of conductive electrospun composite scaffold for neural tissue engineering. Int. J. Biol. Macromol. 2019 doi: 10.1016/j.ijbiomac.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Azuma K., Izumi R., Osaki T., Ifuku S., Morimoto M., Saimoto H., Minami S., Okamoto Y. Chitin, Chitosan, and Its Derivatives for Wound Healing: Old and New Materials. J. Funct. Biomater. 2015 doi: 10.3390/jfb6010104. [DOI] [Google Scholar]
- 22.Rodríguez-Vázquez M., Vega-Ruiz B., Ramos-Zúñiga R., Saldaña-Koppel D.A., Quiñones-Olvera L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res. Int. 2015 doi: 10.1155/2015/821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam M.M., Shahruzzaman M., Biswas S., Nurus Sakib M., Rashid T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020;5:164–183. doi: 10.1016/j.bioactmat.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S., Ul-Islam M., Ikram M., Islam S.U., Ullah M.W., Israr M., Jang J.H., Yoon S., Park J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Luo H., Cha R., Li J., Hao W., Zhang Y., Zhou F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019 doi: 10.1016/j.carbpol.2019.115144. [DOI] [PubMed] [Google Scholar]
- 26.Kuzmenko V., Karabulut E., Pernevik E., Enoksson P., Gatenholm P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018 doi: 10.1016/j.carbpol.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 27.Hickey R.J., Pelling A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019 doi: 10.3389/fbioe.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X., Wang C., Liao M., Dai L., Tang Y., Zhang H., Coates P., Sefat F., Zheng L., Song J., Zheng Z., Zhao D., Yang M., Zhang W., Ji P. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019 doi: 10.1016/j.carbpol.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Naghieh S., Sarker M.D., Abelseth E., Chen X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019 doi: 10.1016/j.jmbbm.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Sun J., Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 2013 doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández-González A.C., Téllez-Jurado L., Rodríguez-Lorenzo L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020 doi: 10.1016/j.carbpol.2019.115514. [DOI] [PubMed] [Google Scholar]
- 32.Farokhi M., Jonidi Shariatzadeh F., Solouk A., Mirzadeh H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020 doi: 10.1080/00914037.2018.1562924. [DOI] [Google Scholar]
- 33.Prasadh S., Wong R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018 doi: 10.1016/S1348-8643(18)30005-3. [DOI] [Google Scholar]
- 34.Deepthi S., Jayakumar R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018;3:194–200. doi: 10.1016/j.bioactmat.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson R.E., Pardieck J., Smith L., Kenny P., Crawford L., Shoichet M., Sakiyama-Elbert S. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials. 2018;162:208–223. doi: 10.1016/j.biomaterials.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spearman B.S., Agrawal N.K., Rubiano A., Simmons C.S., Mobini S., Schmidt C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. - Part A. 2020 doi: 10.1002/jbm.a.36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Movahedi M., Asefnejad A., Rafienia M., Khorasani M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020;146:627–637. doi: 10.1016/j.ijbiomac.2019.11.233. [DOI] [PubMed] [Google Scholar]
- 38.Bejoy J., Wang Z., Bijonowski B., Yang M., Ma T., Sang Q.X., Li Y. Differential Effects of Heparin and Hyaluronic Acid on Neural Patterning of Human Induced Pluripotent Stem Cells. ACS Biomater. Sci. Eng. 2018 doi: 10.1021/acsbiomaterials.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chircov C., Mihai Grumezescu A., Everard Bejenaru L. R RE EV VI IE EW W Hyaluronic acid-based scaffolds for tissue engineering. Rom J Morphol Embryol. 2018;59:71–76. [PubMed] [Google Scholar]
- 40.Monteiro I.P., Shukla A., Marques A.P., Reis R.L., Hammond P.T. Spray-assisted layer-by-layer assembly on hyaluronic acid scaffolds for skin tissue engineering. J. Biomed. Mater. Res. - Part A. 2015 doi: 10.1002/jbm.a.35178. [DOI] [PubMed] [Google Scholar]
- 41.Bacakova L., Pajorova J., Zikmundova M., Filova E., Mikes P., Jencova V., Kuzelova Kostakova E., Sinica A. Curr. Futur. Asp. Nanomedicine, IntechOpen. 2020. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Nature-Derived Polymers. [DOI] [Google Scholar]
- 42.Huang S., Wang C., Xu J., Ma L., Gao C. In situ assembly of fibrinogen/hyaluronic acid hydrogel via knob-hole interaction for 3D cellular engineering. Bioact. Mater. 2017;2:253–259. doi: 10.1016/j.bioactmat.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Zhao Y., Li X., Xiao Z., Yao Y., Chu Y., Farkas B., Romano I., Brandi F., Dai J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018 doi: 10.1002/adhm.201800315. [DOI] [PubMed] [Google Scholar]
- 44.Nabavi M.H., Salehi M., Ehterami A., Bastami F., Semyari H., Tehranchi M., Nabavi M.A., Semyari H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-019-00666-7. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Jang J., Park J., Lee K.P., Lee S., Lee D.M., Kim K.H., Kim H.K., Cho D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication. 2019 doi: 10.1088/1758-5090/ab1a8b. [DOI] [PubMed] [Google Scholar]
- 46.Rahmani Del Bakhshayesh A., Mostafavi E., Alizadeh E., Asadi N., Akbarzadeh A., Davaran S. Fabrication of Three-Dimensional Scaffolds Based on Nano-biomimetic Collagen Hybrid Constructs for Skin Tissue Engineering. ACS Omega. 2018 doi: 10.1021/acsomega.8b01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim Y.S., Ok Y.J., Hwang S.Y., Kwak J.Y., Yoon S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs. 2019 doi: 10.3390/md17080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D., Wu X., Chen J., Lin K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018;3:129–138. doi: 10.1016/j.bioactmat.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezaeeyazdi M., Colombani T., Memic A., Bencherif S.A. Injectable hyaluronic acid-co-gelatin cryogels for tissue-engineering applications. Materials (Basel) 2018 doi: 10.3390/ma11081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celikkin N., Mastrogiacomo S., Jaroszewicz J., Walboomers X.F., Swieszkowski W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. - Part A. 2018 doi: 10.1002/jbm.a.36226. [DOI] [PubMed] [Google Scholar]
- 51.Conrad B., Han L.H., Yang F. Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions. Tissue Eng. - Part A. 2018 doi: 10.1089/ten.tea.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye W., Li H., Yu K., Xie C., Wang P., Zheng Y., Zhang P., Xiu J., Yang Y., Zhang F., He Y., Gao Q. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020 doi: 10.1016/j.matdes.2020.108757. [DOI] [Google Scholar]
- 53.Tytgat L., Van Damme L., Van Hoorick J., Declercq H., Thienpont H., Ottevaere H., Blondeel P., Dubruel P., Van Vlierberghe S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 54.Afewerki S., Sheikhi A., Kannan S., Ahadian S., Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019 doi: 10.1002/btm2.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Liu C. Fibrin hydrogels for endothelialized liver tissue engineering with a predesigned vascular network. Polymers (Basel) 2018 doi: 10.3390/polym10101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soleimannejad M., Ebrahimi-Barough S., Soleimani M., Nadri S., Tavangar S.M., Roohipoor R., Yazdankhah M., Bayat N., Riazi-Esfahani M., Ai J. Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif. Cells, Nanomedicine Biotechnol. 2018 doi: 10.1080/21691401.2017.1345922. [DOI] [PubMed] [Google Scholar]
- 57.Bachmann B., Spitz S., Rothbauer M., Jordan C., Purtscher M., Zirath H., Schuller P., Eilenberger C., Ali S.F., Mühleder S., Priglinger E., Harasek M., Redl H., Holnthoner W., Ertl P. Engineering of three-dimensional pre-vascular networks within fibrin hydrogel constructs by microfluidic control over reciprocal cell signaling. Biomicrofluidics. 2018 doi: 10.1063/1.5027054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abelseth E., Abelseth L., De La Vega L., Beyer S.T., Wadsworth S.J., Willerth S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019 doi: 10.1021/acsbiomaterials.8b01235. [DOI] [PubMed] [Google Scholar]
- 59.Song H.H.G., Rumma R.T., Ozaki C.K., Edelman E.R., Chen C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell. 2018 doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atrian M., Kharaziha M., Emadi R., Alihosseini F. Silk-Laponite® fibrous membranes for bone tissue engineering. Appl. Clay Sci. 2019 doi: 10.1016/j.clay.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 61.Gupta P., Lorentz K.L., Haskett D.G., Cunnane E.M., Ramaswamy A.K., Weinbaum J.S., Vorp D.A., Mandal B.B. Bioresorbable silk grafts for small diameter vascular tissue engineering applications: In vitro and in vivo functional analysis. Acta Biomater. 2020 doi: 10.1016/j.actbio.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keirouz A., Zakharova M., Kwon J., Robert C., Koutsos V., Callanan A., Chen X., Fortunato G., Radacsi N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C. 2020 doi: 10.1016/j.msec.2020.110939. [DOI] [PubMed] [Google Scholar]
- 63.Kundu B., Rajkhowa R., Kundu S.C., Wang X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013 doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 64.Gholipourmalekabadi M., Sapru S., Samadikuchaksaraei A., Reis R.L., Kaplan D.L., Kundu S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z., Zhang Q., Li H., Wei Q., Zhao X., Chen F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact. Mater. 2021;6:589–601. doi: 10.1016/j.bioactmat.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian L., Prabhakaran M.P., Ramakrishna S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen. Biomater. 2015 doi: 10.1093/rb/rbu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalili S., Khorasani S.N., Razavi S.M., Hashemibeni B., Tamayol A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2019;187:1193–1203. doi: 10.1007/s12010-018-2871-7. [DOI] [PubMed] [Google Scholar]
- 68.Silva R., Singh R., Sarker B., Papageorgiou D.G., Juhasz-Bortuzzo J.A., Roether J.A., Cicha I., Kaschta J., Schubert D.W., Chrissafis K., Detsch R., Boccaccini A.R. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Ali M.S., Lacerda C.M.R. A three-dimensional collagen-elastin scaffold for heart valve tissue engineering, Bioengineering. 2018 doi: 10.3390/bioengineering5030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen T.U., Shojaee M., Bashur C.A., Kishore V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication. 2019 doi: 10.1088/1758-5090/aaeab0. [DOI] [PubMed] [Google Scholar]
- 71.Miranda-Nieves D., Chaikof E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017 doi: 10.1021/acsbiomaterials.6b00250. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez de Torre I., Alonso M., Rodriguez-Cabello J.C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020 doi: 10.3389/fbioe.2020.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao Q., Liu Y., Pan Y., Miszuk J.M., Sun H. One-pot porogen free method fabricated porous microsphere-aggregated 3D PCL scaffolds for bone tissue engineering. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34601. [DOI] [PubMed] [Google Scholar]
- 74.Kudryavtseva V., Stankevich K., Kibler E., Golovkin A., Mishanin A., Bolbasov E., Choynzonov E., Tverdokhlebov S. The deposition of thin titanium-nitrogen coatings on the surface of PCL-based scaffolds for vascular tissue engineering. Appl. Phys. Lett. 2018 doi: 10.1063/1.5017580. [DOI] [Google Scholar]
- 75.Sharif S., Ai J., Azami M., Verdi J., Atlasi M.A., Shirian S., Samadikuchaksaraei A. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2018 doi: 10.1002/jbm.b.33966. [DOI] [PubMed] [Google Scholar]
- 76.Felice B., Sánchez M.A., Socci M.C., Sappia L.D., Gómez M.I., Cruz M.K., Felice C.J., Martí M., Pividori M.I., Simonelli G., Rodríguez A.P. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C. 2018 doi: 10.1016/j.msec.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Longmire M.N., Swain K., Vig K. Designing Scaffolds For Skin Tissue Engineering. FASEB J. 2019;33:603.4. doi: 10.1096/FASEBJ.2019.33.1_SUPPLEMENT.603.4. [DOI] [Google Scholar]
- 78.Dwivedi R., Kumar S., Pandey R., Mahajan A., Nandana D., Katti D.S., Mehrotra D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020 doi: 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kung F.C., Kuo Y.L., Gunduz O., Lin C.C. Dual RGD-immobilized poly(L-lactic acid) by atmospheric pressure plasma jet for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2019 doi: 10.1016/j.colsurfb.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 80.Muniyandi P., Palaninathan V., Veeranarayanan S., Ukai T., Maekawa T., Hanajiri T., Mohamed M.S. ECM mimetic electrospun porous poly (l-lactic acid) (PLLA) scaffolds as potential substrates for cardiac tissue engineering. Polymers (Basel) 2020 doi: 10.3390/polym12020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin C., Liu C., Zhang L., Huang Z., Zhao P., Chen R., Pang M., Chen Z., He L., Luo C., Rong L., Liu B. Interaction of iPSC-derived neural stem cells on poly(L-lactic acid) nanofibrous scaffolds for possible use in neural tissue engineering. Int. J. Mol. Med. 2018 doi: 10.3892/ijmm.2017.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao D., Li Q., Li D., Chen Y., Chen X., Xu X. Fabrication of 3D porous poly(lactic acid)-based composite scaffolds with tunable biodegradation for bone tissue engineering. Mater. Des. 2018;142:1–10. doi: 10.1016/j.matdes.2018.01.016. [DOI] [Google Scholar]
- 83.Narayanan G., Vernekar V.N., Kuyinu E.L., Laurencin C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016;107:247–276. doi: 10.1016/j.addr.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S., Qin S., He M., Zhou D., Qin Q., Wang H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020;199:108238. doi: 10.1016/j.compositesb.2020.108238. [DOI] [Google Scholar]
- 85.Barbeck M., Serra T., Booms P., Stojanovic S., Najman S., Engel E., Sader R., Kirkpatrick C.J., Navarro M., Ghanaati S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components – Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017;2:208–223. doi: 10.1016/j.bioactmat.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J., Yang S., Yang X., Xi Z., Zhao L., Cen L., Lu E., Yang Y. Novel Fabricating Process for Porous Polyglycolic Acid Scaffolds by Melt-Foaming Using Supercritical Carbon Dioxide. ACS Biomater. Sci. Eng. 2018 doi: 10.1021/acsbiomaterials.7b00692. [DOI] [PubMed] [Google Scholar]
- 87.Otsuki S., Nakagawa K., Murakami T., Sezaki S., Sato H., Suzuki M., Okuno N., Wakama H., Kaihatsu K., Neo M. Evaluation of Meniscal Regeneration in a Mini Pig Model Treated With a Novel Polyglycolic Acid Meniscal Scaffold. Am. J. Sports Med. 2019 doi: 10.1177/0363546519850578. [DOI] [PubMed] [Google Scholar]
- 88.Song R., Murphy M., Li C., Ting K., Soo C., Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018 doi: 10.2147/DDDT.S165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Y., Xia H., Zhang B., Zhao Y., Wang Y. Assessment of polyglycolic acid scaffolds for periodontal ligament regeneration. Biotechnol. Biotechnol. Equip. 2018 doi: 10.1080/13102818.2018.1437358. [DOI] [Google Scholar]
- 90.Cervelló I., Medrano J.V., Simón C. Transl. Regen. Med. to Clin. Elsevier Inc.; 2016. Regenerative Medicine and Tissue Engineering in Reproductive Medicine: Future Clinical Applications in Human Infertility; pp. 139–151. [DOI] [Google Scholar]
- 91.Shuai C., Yang W., Feng P., Peng S., Pan H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021;6:490–502. doi: 10.1016/j.bioactmat.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S., Severino F.P.U., Ban J., Wang L., Pinato G., Torre V., Chen Y. Improved neuron culture using scaffolds made of three-dimensional PDMS micro-lattices. Biomed. Mater. 2018 doi: 10.1088/1748-605X/aaa777. [DOI] [PubMed] [Google Scholar]
- 93.Varshney N., Sahi A.K., Vajanthri K.Y., Poddar S., Balavigneswaran C.K., Prabhakar A., Rao V., Mahto S.K. Culturing melanocytes and fibroblasts within three-dimensional macroporous PDMS scaffolds: towards skin dressing material. Cytotechnology. 2019 doi: 10.1007/s10616-018-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montazerian H., Mohamed M.G.A., Montazeri M.M., Kheiri S., Milani A.S., Kim K., Hoorfar M. Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 95.Jothi L., Nageswaran G. Non-Thermal Plasma Technol. Polym. Mater. Elsevier; 2019. Plasma Modified Polymeric Materials for Biosensors/Biodevice Applications; pp. 409–437. [DOI] [Google Scholar]
- 96.Li J., Liu X., Crook J.M., Wallace G.G. Development of a porous 3D graphene-PDMS scaffold for improved osseointegration. Colloids Surfaces B Biointerfaces. 2017 doi: 10.1016/j.colsurfb.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 97.Ma C., Jiang L., Wang Y., Gang F., Xu N., Li T., Liu Z., Chi Y., Wang X., Zhao L., Feng Q., Sun X. 3D printing of conductive tissue engineering scaffolds containing polypyrrole nanoparticles with different morphologies and concentrations. Materials (Basel) 2019 doi: 10.3390/ma12152491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hatamzadeh M., Sarvari R., Massoumi B., Agbolaghi S., Samadian F. Liver tissue engineering via hyperbranched polypyrrole scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2019 doi: 10.1080/00914037.2019.1667800. [DOI] [Google Scholar]
- 99.Sun X., Lin H., Zhang C., Huang X., Jin J., Di S. Electrosynthesized nanostructured polypyrrole on selective laser melted titanium scaffold. Surf. Coatings Technol. 2019 doi: 10.1016/j.surfcoat.2019.04.078. [DOI] [Google Scholar]
- 100.Ning C., Zhou Z., Tan G., Zhu Y., Mao C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018 doi: 10.1016/j.progpolymsci.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ashtari K., Nazari H., Ko H., Tebon P., Akhshik M., Akbari M., Alhosseini S.N., Mozafari M., Mehravi B., Soleimani M., Ardehali R., Ebrahimi Warkiani M., Ahadian S., Khademhosseini A. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alegret N., Dominguez-Alfaro A., González-Domínguez J.M., Arnaiz B., Cossío U., Bosi S., Vázquez E., Ramos-Cabrer P., Mecerreyes D., Prato M. Three-Dimensional Conductive Scaffolds as Neural Prostheses Based on Carbon Nanotubes and Polypyrrole. ACS Appl. Mater. Interfaces. 2018;10:43904–43914. doi: 10.1021/acsami.8b16462. [DOI] [PubMed] [Google Scholar]
- 103.Granato A.E.C., Ribeiro A.C., Marciano F.R., Rodrigues B.V.M., Lobo A.O., Porcionatto M. Polypyrrole increases branching and neurite extension by Neuro2A cells on PBAT ultrathin fibers. Nanomedicine Nanotechnology, Biol. Med. 2018 doi: 10.1016/j.nano.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Ferrigno B., Bordett R., Duraisamy N., Moskow J., Arul M.R., Rudraiah S., Nukavarapu S.P., Vella A.T., Kumbar S.G. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 2020;5:468–485. doi: 10.1016/j.bioactmat.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacob J., More N., Kalia K., Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018 doi: 10.1186/s41232-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitsara M., Blanquer A., Murillo G., Humblot V., De Bragança Vieira S., Nogués C., Ibáñez E., Esteve J., Barrios L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale. 2019 doi: 10.1039/c8nr10384d. [DOI] [PubMed] [Google Scholar]
- 107.Sengupta P., Ghosh A., Bose N., Mukherjee S., Roy Chowdhury A., Datta P. A comparative assessment of poly(vinylidene fluoride)/conducting polymer electrospun nanofiber membranes for biomedical applications. J. Appl. Polym. Sci. 2020;137:49115. doi: 10.1002/app.49115. [DOI] [Google Scholar]
- 108.Wu S., Chen M.S., Maurel P., Lee Y.S., Bunge M.B., Arinzeh T.L. Aligned fibrous PVDF-TrFE scaffolds with Schwann cells support neurite extension and myelination in vitro. J. Neural Eng. 2018 doi: 10.1088/1741-2552/aac77f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ardeshirylajimi A., Ghaderian S.M.H., Omrani M.D., Moradi S.L. Biomimetic scaffold containing PVDF nanofibers with sustained TGF-β release in combination with AT-MSCs for bladder tissue engineering. Gene. 2018 doi: 10.1016/j.gene.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 110.Szewczyk P.K., Metwally S., Karbowniczek J.E., Marzec M.M., Stodolak-Zych E., Gruszczyński A., Bernasik A., Stachewicz U. Surface-Potential-Controlled Cell Proliferation and Collagen Mineralization on Electrospun Polyvinylidene Fluoride (PVDF) Fiber Scaffolds for Bone Regeneration. ACS Biomater. Sci. Eng. 2019 doi: 10.1021/acsbiomaterials.8b01108. [DOI] [PubMed] [Google Scholar]
- 111.Costa A., Naranjo J.D., Londono R., Badylak S.F. Biologic scaffolds. Cold Spring Harb. Perspect. Med. 2017 doi: 10.1101/cshperspect.a025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buttery L.D.K., Bishop A.E. Biomater. Artif. Organs Tissue Eng. Elsevier Inc.; 2005. Introduction to tissue engineering; pp. 193–200. [DOI] [Google Scholar]
- 113.Vanneaux V. Updat. Mesenchymal Induc. Pluripotent Stem Cells. IntechOpen; 2020. Induced Pluripotent Stem Cells for Clinical Use. [DOI] [Google Scholar]
- 114.Elgali I., Omar O., Dahlin C., Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017;125:315–337. doi: 10.1111/eos.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bai X., Gao M., Syed S., Zhuang J., Xu X., Zhang X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018;3:401–417. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ho-Shui-Ling A., Bolander J., Rustom L.E., Johnson A.W., Luyten F.P., Picart C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bandyopadhyay A., Mitra I., Bose S. 3D Printing for Bone Regeneration. Curr. Osteoporos. Rep. 2020;18:505–514. doi: 10.1007/s11914-020-00606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu L., Luo D., Liu Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020;12:6. doi: 10.1038/s41368-020-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sprio S., Sandri M., Iafisco M., Panseri S., Adamiano A., Montesi M., Campodoni E., Tampieri A. Bio-inspired assembling/mineralization process as a flexible approach to develop new smart scaffolds for the regeneration of complex anatomical regions. J. Eur. Ceram. Soc. 2016;36:2857–2867. doi: 10.1016/j.jeurceramsoc.2016.01.005. [DOI] [Google Scholar]
- 120.Polo-Corrales L., Latorre-Esteves M., Ramirez-Vick J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014;14:15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Filippi M., Born G., Chaaban M., Scherberich A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020;8:474. doi: 10.3389/fbioe.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Donnaloja F., Jacchetti E., Soncini M., Raimondi M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers (Basel) 2020;12 doi: 10.3390/POLYM12040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guerrieri A.N., Montesi M., Sprio S., Laranga R., Mercatali L., Tampieri A., Donati D.M., Lucarelli E. Innovative Options for Bone Metastasis Treatment: An Extensive Analysis on Biomaterials-Based Strategies for Orthopedic Surgeons. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.589964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zou L., Zhang Y., Liu X., Chen J., Zhang Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2019;178:222–229. doi: 10.1016/j.colsurfb.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 125.Taraballi F., Cui W., Jiao S., University T., Serra C.T., Sandri M., Dellaquila A., Campodoni E., Tampieri A. 2020. Overcoming the Design Challenge in 3D Biomimetic Hybrid Scaffolds for Bone and Osteochondral Regeneration by Factorial Design. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Z., Du T., Ruan C., Niu X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021;6:1491–1511. doi: 10.1016/j.bioactmat.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sridharan R., Genoud K.J., Kelly D.J., O’brien F.J. Hydroxyapatite Particle Shape and Size Influence MSC Osteogenesis by Directing the Macrophage Phenotype in Collagen-Hydroxyapatite Scaffolds. ACS Appl. Bio Mater. 2020 doi: 10.1021/acsabm.0c00801. [DOI] [PubMed] [Google Scholar]
- 128.De Luca A., Vitrano I., Costa V., Raimondi L., Carina V., Bellavia D., Conoscenti G., Di Falco R., Pavia F.C., La Carrubba V., Brucato V., Giavaresi G. Improvement of osteogenic differentiation of human mesenchymal stem cells on composite poly L-lactic acid/nano-hydroxyapatite scaffolds for bone defect repair. J. Biosci. Bioeng. 2020;129:250–257. doi: 10.1016/j.jbiosc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 129.Paredes C., Martínez-Vázquez F.J., Pajares A., Miranda P. Development by robocasting and mechanical characterization of hybrid HA/PCL coaxial scaffolds for biomedical applications. J. Eur. Ceram. Soc. 2019;39:4375–4383. doi: 10.1016/j.jeurceramsoc.2019.05.053. [DOI] [Google Scholar]
- 130.Aslam Khan M.U., Raza M.A., Mehboob H., Abdul Kadir M.R., Abd Razak S.I., Shah S.A., Iqbal M.Z., Amin R. Development and: In vitro evaluation of κ-carrageenan based polymeric hybrid nanocomposite scaffolds for bone tissue engineering. RSC Adv. 2020;10:40529–40542. doi: 10.1039/d0ra07446b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mirza S., Jolly R., Zia I., Saad Umar M., Owais M., Shakir M. Bioactive Gum Arabic/κ-Carrageenan-Incorporated Nano-Hydroxyapatite Nanocomposites and Their Relative Biological Functionalities in Bone Tissue Engineering. ACS Omega. 2020;5:11279–11290. doi: 10.1021/acsomega.9b03761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pacheco-Quito E.-M., Ruiz-Caro R., Veiga M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs. 2020;18:583. doi: 10.3390/md18110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agid R.S., Kaygili O., Bulut N., Dorozhkin S.V., Ates T., Koytepe S., Ates B., Ercan I., İnce T., Mahmood B.K. Investigation of the effects of Pr doping on the structural properties of hydroxyapatite: an experimental and theoretical study. J. Aust. Ceram. Soc. 2020;56:1501–1513. doi: 10.1007/s41779-020-00495-9. [DOI] [Google Scholar]
- 134.Liu M., Shu M., Yan J., Liu X., Wang R., Hou Z., Lin J. Luminescent Net-like Inorganic Scaffolds with Europium Doped Hydroxyapatite for Enhanced Bone Reconstruction. Nanoscale. 2020 doi: 10.1039/D0NR05608A. [DOI] [PubMed] [Google Scholar]
- 135.Tang Y.Q., Wang Q.Y., Ke Q.F., Zhang C.Q., Guan J.J., Guo Y.P. Mineralization of ytterbium-doped hydroxyapatite nanorod arrays in magnetic chitosan scaffolds improves osteogenic and angiogenic abilities for bone defect healing. Chem. Eng. J. 2020;387:124166. doi: 10.1016/j.cej.2020.124166. [DOI] [Google Scholar]
- 136.Qian Y., Yao Z., Wang X., Cheng Y., Fang Z., Yuan W.E., Fan C., Ouyang Y. (-)-Epigallocatechin gallate-loaded polycaprolactone scaffolds fabricated using a 3D integrated moulding method alleviate immune stress and induce neurogenesis. Cell Prolif. 2020;53 doi: 10.1111/cpr.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang P., Wang H., Ma K., Wang S., Yang C., Mu N., Yang F., Feng H., Chen T. Novel cytokine-loaded PCL-PEG scaffold composites for spinal cord injury repair. RSC Adv. 2020 doi: 10.1039/c9ra10385f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du J., Chen H., Qing L., Yang X., Jia X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018 doi: 10.1039/c8bm00260f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moore A.M., Kasukurthi R., Magill C.K., Farhadi F.H., Borschel G.H., Mackinnon S.E. Limitations of conduits in peripheral nerve repairs. Hand. 2009 doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tadyszak K., Wychowaniec J.K., Litowczenko J. Biomedical applications of graphene-based structures. Nanomaterials. 2018;8 doi: 10.3390/nano8110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park J., Jeon J., Kim B., Lee M.S., Park S., Lim J., Yi J., Lee H., Yang H.S., Lee J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020:1–14. doi: 10.1002/adfm.202003759. 2003759. [DOI] [Google Scholar]
- 142.Kim S.M., Lee M.S., Jeon J., Lee D.H., Yang K., Cho S.W., Han I., Yang H.S. Biodegradable Nerve Guidance Conduit with Microporous and Micropatterned Poly(lactic-co-glycolic acid)-Accelerated Sciatic Nerve Regeneration. Macromol. Biosci. 2018 doi: 10.1002/mabi.201800290. [DOI] [PubMed] [Google Scholar]
- 143.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019 doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huebner E.A., Strittmatter S.M. Axon regeneration in the peripheral and central nervous systems. Results Probl. Cell Differ. 2009 doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cortés D., Carballo-Molina O.A., Castellanos-Montiel M.J., Velasco I. The non-survival effects of Glial cell line-derived neurotrophic factor on neural cells. Front. Mol. Neurosci. 2017;10:1–13. doi: 10.3389/fnmol.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu S., Xie Y.Y., Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019 doi: 10.4103/1673-5374.253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li L., Xiao B., Mu J., Zhang Y., Zhang C., Cao H., Chen R., Patra H.K., Yang B., Feng S., Tabata Y., Slater N.K.H., Tang J., Shen Y., Gao J. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano. 2019 doi: 10.1021/acsnano.9b07598. [DOI] [PubMed] [Google Scholar]
- 148.Rhee Y.H., Yi S.H., Kim J.Y., Chang M.Y., Jo A.Y., Kim J., Park C.H., Cho J.Y., Choi Y.J., Sun W., Lee S.H. Neural stem cells secrete factors facilitating brain regeneration upon constitutive Raf-Erk activation. Sci. Rep. 2016 doi: 10.1038/srep32025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Poulos J. The limited application of stem cells in medicine: A review. Stem Cell Res. Ther. 2018 doi: 10.1186/s13287-017-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., Chen S., Tuszynski M.H. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Evaniew N., Noonan V.K., Fallah N., Kwon B.K., Rivers C.S., Ahn H., Bailey C.S., Christie S.D., Fourney D.R., Hurlbert R.J., Linassi A.G., Fehlings M.G., Dvorak M.F. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Propensity Score-Matched Cohort Study from a Canadian Multi-Center Spinal Cord Injury Registry. J. Neurotrauma. 2015 doi: 10.1089/neu.2015.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang S., Wang X.J., Li W.S., Xu X.L., Hu J.B., Kang X.Q., Qi J., Ying X.Y., You J., Du Y.Z. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018 doi: 10.1016/j.actbio.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 153.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011 doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Steinhauser M.L., Lee R.T. Regeneration of the heart. EMBO Mol. Med. 2011 doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Breckwoldt K., Weinberger F., Eschenhagen T. Heart regeneration. Biochim. Biophys. Acta - Mol. Cell Res. 2016;1863:1749–1759. doi: 10.1016/J.BBAMCR.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 156.Papageorgiou N. Environmental Factors and their Interaction; 2016. Cardiovascular Diseases: Genetic Susceptibility. [Google Scholar]
- 157.WHO . World Heal. Organ; 2013. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 978 92 4 1506236. [Google Scholar]
- 158.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabé-Heide F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H., Jovinge S., Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;80 doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011 doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 160.Chan B.P., Leong K.W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 2008;17(Suppl 4):467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim T.G., Shin H., Lim D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012;22:2446–2468. doi: 10.1002/adfm.201103083. [DOI] [Google Scholar]
- 162.Hoshiba T., Yamaoka T. CHAPTER 1. Extracellular Matrix Scaffolds for Tissue Engineering and Biological Research. Royal Society of Chemistry. 2019:1–14. doi: 10.1039/9781788015998-00001. [DOI] [Google Scholar]
- 163.Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009 doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 164.Hynes R.O., Naba A. Overview of the matrisome-An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012 doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018 doi: 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- 166.Pattar S.S., Hassanabad A.F., Fedak P.W. Acellular extracellular matrix bioscaffolds for cardiac repair and regeneration. Front. Cell Dev. Biol. 2019 doi: 10.3389/fcell.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Svystonyuk D.A., Mewhort H.E.M., Hassanabad A.F., Heydari B., Mikami Y., Turnbull J.D., Teng G., Belke D.D., Wagner K.T., Tarraf S.A., DiMartino E.S., White J.A., Flewitt J.A., Cheung M., Guzzardi D.G., Kang S., Fedak P.W.M. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury. Sci. Rep. 2020 doi: 10.1038/s41598-020-66327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fernández-Pérez J., Ahearne M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019 doi: 10.1038/s41598-019-49575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rosario D.J., Reilly G.C., Salah E.A., Glover M., Bullock A.J., MacNeil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 2008 doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 170.Willemse J., Verstegen M.M.A., Vermeulen A., Schurink I.J., Roest H.P., van der Laan L.J.W., de Jonge J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater. Sci. Eng. C. 2020 doi: 10.1016/j.msec.2019.110200. [DOI] [PubMed] [Google Scholar]
- 171.Guyette J.P., Gilpin S.E., Charest J.M., Tapias L.F., Ren X., Ott H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014 doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- 172.Perea-Gil I., Gálvez-Montón C., Prat-Vidal C., Jorba I., Segú-Vergés C., Roura S., Soler-Botija C., Iborra-Egea O., Revuelta-López E., Fernández M.A., Farré R., Navajas D., Bayes-Genis A. Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci. Rep. 2018 doi: 10.1038/s41598-018-25115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duan Y., Liu Z., O’Neill J., Wan L.Q., Freytes D.O., Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res. 2011 doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoshiba T., Chen G., Endo C., Maruyama H., Wakui M., Nemoto E., Kawazoe N., Tanaka M. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016 doi: 10.1155/2016/6397820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Q., Yang H., Bai A., Jiang W., Li X., Wang X., Mao Y., Lu C., Qian R., Guo F., Ding T., Chen H., Chen S., Zhang J., Liu C., Sun N. Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials. 2016 doi: 10.1016/j.biomaterials.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 176.Chamberland C., Martinez-Fernandez A., Beraldi R., Nelson T.J. Embryonic Decellularized Cardiac Scaffold Supports Embryonic Stem Cell Differentiation to Produce Beating Cardiac Tissue. ISRN Stem Cells. 2014 doi: 10.1155/2014/625164. [DOI] [Google Scholar]
- 177.Godier-Furnémont A.F.G., Martens T.P., Koeckert M.S., Wan L., Parks J., Arai K., Zhang G., Hudson B., Homma S., Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc. Natl. Acad. Sci. U. S. A. 2011 doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Perea-Gil I., Prat-Vidal C., Gálvez-Montón C., Roura S., Llucià-Valldeperas A., Soler-Botija C., Iborra-Egea O., Díaz-Güemes I., Crisóstomo V., Sánchez-Margallo F.M., Bayes-Genis A. A Cell-Enriched Engineered Myocardial Graft Limits Infarct Size and Improves Cardiac Function: Pre-Clinical Study in the Porcine Myocardial Infarction Model. JACC Basic to Transl. Sci. 2016 doi: 10.1016/j.jacbts.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu Y., Guan J. Biomaterial property-controlled stem cell fates for cardiac regeneration. Bioact. Mater. 2016 doi: 10.1016/j.bioactmat.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Alrefai M.T., Murali D., Paul A., Ridwan K.M., Connell J.M., Shum-Tim D. Cardiac tissue engineering and regeneration using cell-based therapy, Stem Cells Cloning. Adv. Appl. 2015 doi: 10.2147/SCCAA.S54204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Durrani S., Konoplyannikov M., Ashraf M., Haider K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010 doi: 10.2217/rme.10.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo R., Morimatsu M., Feng T., Lan F., Chang D., Wan F., Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020;11:19. doi: 10.1186/s13287-019-1536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi C., Li Q., Zhao Y., Chen W., Chen B., Xiao Z., Lin H., Nie L., Wang D., Dai J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 184.Benzoni P., Ginestra P., Altomare L., Fiorentino A., De Nardo L., Ceretti E., Dell’Era P. Biomanufacturing of a Chitosan/Collagen Scaffold to Drive Adhesion and Alignment of Human Cardiomyocyte Derived from Stem Cells. Procedia CIRP. 2016;49:113–120. doi: 10.1016/J.PROCIR.2015.09.004. [DOI] [Google Scholar]
- 185.Callegari A., Bollini S., Iop L., Chiavegato A., Torregrossa G., Pozzobon M., Gerosa G., De Coppi P., Elvassore N., Sartore S. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts, Biomaterials. 2007 doi: 10.1016/j.biomaterials.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 186.Huang Z., Song Y., Pang Z., Li M., Guliya Y., Shen Y., Qian J., Ge J. Fibrin-targeting delivery: a novel platform for cardiac regenerative medicine. J. Cell. Mol. Med. 2016 doi: 10.1111/jcmm.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bonafè F., Govoni M., Giordano E., Caldarera C.M., Guarnieri C., Muscari C. Hyaluronan and cardiac regeneration. J. Biomed. Sci. 2014 doi: 10.1186/s12929-014-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hayoun-Neeman D., Korover N., Etzion S., Ofir R., Lichtenstein R.G., Cohen S. Exploring peptide-functionalized alginate scaffolds for engineering cardiac tissue from human embryonic stem cell-derived cardiomyocytes in serum-free medium. Polym. Adv. Technol. 2019 doi: 10.1002/pat.4602. [DOI] [Google Scholar]
- 189.Matson J.B., Stupp S.I. Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun. 2012 doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chi N.H., Yang M.C., Chung T.W., Chou N.K., Wang S.S. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr. Polym. 2013 doi: 10.1016/j.carbpol.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 191.Asadpour S., Yeganeh H., Ai J., Kargozar S., Rashtbar M., Seifalian A., Ghanbari H. Polyurethane-Polycaprolactone Blend Patches: Scaffold Characterization and Cardiomyoblast Adhesion, Proliferation, and Function. ACS Biomater. Sci. Eng. 2018 doi: 10.1021/acsbiomaterials.8b00848. [DOI] [PubMed] [Google Scholar]
- 192.Chang M.Y., Yang Y.J., Chang C.H., Tang A.C.L., Liao W.Y., Cheng F.Y., Yeh C.S., Lai J.J., Stayton P.S., Hsieh P.C.H. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J. Control. Release. 2013 doi: 10.1016/j.jconrel.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 193.McDevitt T.C., Woodhouse K.A., Hauschka S.D., Murry C.E., Stayton P.S. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J. Biomed. Mater. Res. - Part A. 2003 doi: 10.1002/jbm.a.10504. [DOI] [PubMed] [Google Scholar]
- 194.Alperin C., Zandstra P.W., Woodhouse K.A. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials. 2005 doi: 10.1016/j.biomaterials.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 195.Flaig F., Ragot H., Simon A., Revet G., Kitsara M., Kitasato L., Hébraud A., Agbulut O., Schlatter G. Design of Functional Electrospun Scaffolds Based on Poly(glycerol sebacate) Elastomer and Poly(lactic acid) for Cardiac Tissue Engineering. ACS Biomater. Sci. Eng. 2020 doi: 10.1021/acsbiomaterials.0c00243. [DOI] [PubMed] [Google Scholar]
- 196.Martins A.M., Eng G., Caridade S.G., Mano J.F., Reis R.L., Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules. 2014 doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shin S.R., Aghaei-Ghareh-Bolagh B., Gao X., Nikkhah M., Jung S.M., Dolatshahi-Pirouz A., Kim S.B., Kim S.M., Dokmeci M.R., Tang X., Khademhosseini A. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv. Funct. Mater. 2014 doi: 10.1002/adfm.201401300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shin S.R., Zihlmann C., Akbari M., Assawes P., Cheung L., Zhang K., Manoharan V., Zhang Y.S., Yüksekkaya M., Wan K.T., Nikkhah M., Dokmeci M.R., Tang X.S., Khademhosseini A. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small. 2016 doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fleischer S., Shevach M., Feiner R., Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale. 2014 doi: 10.1039/c4nr00300d. [DOI] [PubMed] [Google Scholar]
- 200.Yokoyama R., Ii M., Masuda M., Tabata Y., Hoshiga M., Ishizaka N., Asahi M. Cardiac Regeneration by Statin-Polymer Nanoparticle-Loaded Adipose-Derived Stem Cell Therapy in Myocardial Infarction. Stem Cells Transl. Med. 2019 doi: 10.1002/sctm.18-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang Z., Song Y., Pang Z., Li M., Guliya Y., Shen Y., Qian J., Ge J. Fibrin-targeting delivery: a novel platform for cardiac regenerative medicine. J. Cell. Mol. Med. 2016;20:2410–2413. doi: 10.1111/jcmm.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Saberi A., Jabbari F., Zarrintaj P., Saeb M.R., Mozafari M. Electrically conductive materials: Opportunities and challenges in tissue engineering. Biomolecules. 2019;9 doi: 10.3390/biom9090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Smith A.S.T., Yoo H., Yi H., Ahn E.H., Lee J.H., Shao G., Nagornyak E., Laflamme M.A., Murry C.E., Kim D.H. Micro-and nano-patterned conductive graphene-PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017 doi: 10.1039/c7cc01988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Santhakumar R., Vidyasekar P., Verma R.S. Cardiogel: A nano-matrix scaffold with potential application in cardiac regeneration using mesenchymal stem cells. PLoS One. 2014 doi: 10.1371/journal.pone.0114697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei M., Li S., Le W. Nanomaterials modulate stem cell differentiation: biological interaction and underlying mechanisms. J. Nanobiotechnology. 2017 doi: 10.1186/s12951-017-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Somasuntharam I., Yehl K., Carroll S.L., Maxwell J.T., Martinez M.D., Che P.L., Brown M.E., Salaita K., Davis M.E. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials. 2016 doi: 10.1016/j.biomaterials.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Díaz-Herráez P., Saludas L., Pascual-Gil S., Simón-Yarza T., Abizanda G., Prósper F., Garbayo E., Blanco-Prieto M.J. Transplantation of adipose-derived stem cells combined with neuregulin-microparticles promotes efficient cardiac repair in a rat myocardial infarction model. J. Control. Release. 2017 doi: 10.1016/j.jconrel.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 208.Cassani M., Fernandes S., Vrbsky J., Ergir E., Cavalieri F., Forte G. Combining Nanomaterials and Developmental Pathways to Design New Treatments for Cardiac Regeneration: The Pulsing Heart of Advanced Therapies. Front. Bioeng. Biotechnol. 2020 doi: 10.3389/fbioe.2020.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Han L., Lu X., Wang M., Gan D., Deng W., Wang K., Fang L., Liu K., Chan C.W., Tang Y., Weng L.T., Yuan H. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small. 2017;13:1601916. doi: 10.1002/smll.201601916. [DOI] [PubMed] [Google Scholar]
- 210.Ashammakhi N., Ahadian S., Darabi M.A., El Tahchi M., Lee J., Suthiwanich K., Sheikhi A., Dokmeci M.R., Oklu R., Khademhosseini A. Minimally Invasive and Regenerative Therapeutics. Adv. Mater. 2019;31:1804041. doi: 10.1002/adma.201804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mirvakili S.M., Hunter I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018;30 doi: 10.1002/adma.201704407. [DOI] [PubMed] [Google Scholar]
- 212.Wei W., Ma Y., Yao X., Zhou W., Wang X., Li C., Lin J., He Q., Leptihn S., Ouyang H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021;6:998–1011. doi: 10.1016/j.bioactmat.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li C.H., Wang C., Keplinger C., Zuo J.L., Jin L., Sun Y., Zheng P., Cao Y., Lissel F., Linder C., You X.Z., Bao Z. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016;8:618–624. doi: 10.1038/nchem.2492. [DOI] [PubMed] [Google Scholar]
- 214.Yesilyurt V., Webber M.J., Appel E.A., Godwin C., Langer R., Anderson D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016;28:86–91. doi: 10.1002/adma.201502902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strandman S., Zhu X.X. Self-Healing Supramolecular Hydrogels Based on Reversible Physical Interactions. Gels. 2016;2:16. doi: 10.3390/gels2020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Azevedo S., Costa A.M.S., Andersen A., Choi I.S., Birkedal H., Mano J.F. Bioinspired Ultratough Hydrogel with Fast Recovery, Self-Healing, Injectability and Cytocompatibility. Adv. Mater. 2017;29:1700759. doi: 10.1002/adma.201700759. [DOI] [PubMed] [Google Scholar]
- 217.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017:356. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaiser N.J., Coulombe K.L.K. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed. Mater. 2015;10 doi: 10.1088/1748-6041/10/3/034003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jang J. 3D bioprinting and in vitro cardiovascular tissue modeling. Bioengineering. 2017;4 doi: 10.3390/bioengineering4030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Duan B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017;45:195–209. doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- 221.Gaetani R., Doevendans P.A., Metz C.H.G., Alblas J., Messina E., Giacomello A., Sluijter J.P.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 222.Hu J.B., Tomov M.L., Buikema J.W., Chen C., Mahmoudi M., Wu S.M., Serpooshan V. Cardiovascular tissue bioprinting: Physical and chemical processes. Appl. Phys. Rev. 2018;5 doi: 10.1063/1.5048807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang Z., Lee S.J., Cheng H.J., Yoo J.J., Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le X., Lu W., Zhang J., Chen T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv. Sci. 2019;6 doi: 10.1002/advs.201801584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sano K., Ishida Y., Aida T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chemie - Int. Ed. 2018;57:2532–2543. doi: 10.1002/anie.201708196. [DOI] [PubMed] [Google Scholar]
- 226.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers (Basel) 2016;8 doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharples A.P., Player D.J., Martin N.R.W., Mudera V., Stewart C.E., Lewis M.P. Modelling in vivo skeletal muscle ageing in vitro using three-dimensional bioengineered constructs. Aging Cell. 2012;11:986–995. doi: 10.1111/j.1474-9726.2012.00869.x. [DOI] [PubMed] [Google Scholar]
- 228.Rhim C., Lowell D.A., Reedy M.C., Slentz D.H., Zhang S.J., Kraus W.E., Truskey G.A. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve. 2007;36:71–80. doi: 10.1002/mus.20788. [DOI] [PubMed] [Google Scholar]
- 229.Hinds S., Bian W., Dennis R.G., Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cheema U., Yang S.-Y., Mudera V., Goldspink G.G., Brown R.A. 3-D in vitro model of early skeletal muscle development. Cell Motil. Cytoskeleton. 2003;54:226–236. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 231.Matthias N., Hunt S.D., Wu J., Lo J., Smith Callahan L.A., Li Y., Huard J., Darabi R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs) Stem Cell Res. 2018;27:65–73. doi: 10.1016/j.scr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Neal D., Sakar M.S., Ong L.L.S., Harry Asada H. Formation of elongated fascicle-inspired 3D tissues consisting of high-density, aligned cells using sacrificial outer molding. Lab Chip. 2014;14:1907–1916. doi: 10.1039/c4lc00023d. [DOI] [PubMed] [Google Scholar]
- 233.Juhas M., Engelmayr G.C., Fontanella A.N., Palmer G.M., Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. n.d. [DOI] [PMC free article] [PubMed]
- 234.Martin N.R.W., Passey S.L., Player D.J., Mudera V., Baar K., Greensmith L., Lewis M.P. Neuromuscular junction formation in tissue-engineered skeletal muscle augments contractile function and improves cytoskeletal organization. Tissue Eng. - Part A. 2015;21:2595–2604. doi: 10.1089/ten.tea.2015.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu J., Xu H.H.K., Zhou H., Weir M.D., Chen Q., Trotman C.A. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9:4688–4697. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Martin N.R.W., Passey S.L., Player D.J., Khodabukus A., Ferguson R.A., Sharples A.P., Mudera V., Baar K., Lewis M.P. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials. 2013;34:5759–5765. doi: 10.1016/j.biomaterials.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 237.Seifarth V., Grosse J.O., Gossmann M., Janke H.P., Arndt P., Koch S., Epple M., Artmann G.M., Artmann A.T. Mechanical induction of bi-directional orientation of primary porcine bladder smooth muscle cells in tubular fibrin-poly(vinylidene fluoride) scaffolds for ureteral and urethral repair using cyclic and focal balloon catheter stimulation. J. Biomater. Appl. 2017;32:321–330. doi: 10.1177/0885328217723178. [DOI] [PubMed] [Google Scholar]
- 238.Grasman J.M., Do D.M., Page R.L., Pins G.D. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials. 2015;72:49–60. doi: 10.1016/j.biomaterials.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cezar C.A., Mooney D.J. Biomaterial-based delivery for skeletal muscle repair. Adv. Drug Deliv. Rev. 2015;84:188–197. doi: 10.1016/j.addr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen C.Y., Ke C.J., Yen K.C., Hsieh H.C., Sun J.S., Lin F.H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics. 2015;5:643–655. doi: 10.7150/thno.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu J., Zhou H., Weir M.D., Xu H.H.K., Chen Q., Trotman C.A. Fast-degradable microbeads encapsulating human umbilical cord stem cells in alginate for muscle tissue engineering. Tissue Eng. - Part A. 2012;18:2303–2314. doi: 10.1089/ten.tea.2011.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Borselli C., Storrie H., Benesch-Lee F., Shvartsman D., Cezar C., Lichtman J.W., Vandenburgh H.H., Mooney D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pumberger M., Qazi T.H., Ehrentraut M.C., Textor M., Kueper J., Stoltenburg-Didinger G., Winkler T., von Roth P., Reinke S., Borselli C., Perka C., Mooney D.J., Duda G.N., Geißler S. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials. 2016;99:95–108. doi: 10.1016/j.biomaterials.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 244.Hill E., Boontheekul T., Mooney D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Passipieri J.A., Christ G.J. The potential of combination therapeutics for more complete repair of volumetric muscle loss injuries: The role of exogenous growth factors and/or progenitor cells in implantable skeletal muscle tissue engineering technologies. Cells Tissues Organs. 2016;202:202–213. doi: 10.1159/000447323. [DOI] [PubMed] [Google Scholar]
- 246.Quigley A.F., Cornock R., Mysore T., Foroughi J., Kita M., Razal J.M., Crook J., Moulton S.E., Wallace G.G., Kapsa R.M.I. Wet-Spun Trojan Horse Cell Constructs for Engineering Muscle. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang L., Cao L., Shansky J., Wang Z., Mooney D., Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol. Ther. 2014;22:1441–1449. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Collins M.N., Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering - A review. Carbohydr. Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 249.Coogan K., Stone P., Sempertegui N., Rao S. Fabrication of micro-porous hyaluronic acid hydrogels through salt leaching. Eur. Polym. J. 2020;135 doi: 10.1016/j.eurpolymj.2020.109870. 109870. [DOI] [Google Scholar]
- 250.Nair D.P., Podgórski M., Chatani S., Gong T., Xi W., Fenoli C.R., Bowman C.N. The Thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014;26:724–744. doi: 10.1021/cm402180t. [DOI] [Google Scholar]
- 251.Ki H.B., Jun J.Y., Tae G.P. Biotechnol. Prog. Biotechnol Prog; 2006. Fabrication of hyaluronic acid hydrogel beads for cell encapsulation; pp. 297–302. [DOI] [PubMed] [Google Scholar]
- 252.Ding Y.L., Zhang H., Yin R.X., Zhang W. Mater. Sci. Forum. Trans Tech Publications Ltd; 2020. Photo-crosslinkable double-network hyaluronic acid based hydrogel dressing; pp. 59–66. [DOI] [Google Scholar]
- 253.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C., Vitiello L., Elvassore N., De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25:2296–2304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 254.Salzlechner C., Haghighi T., Huebscher I., Walther A.R., Schell S., Gardner A., Undt G., da Silva R.M.P., Dreiss C.A., Fan K., Gentleman E. Adhesive Hydrogels for Maxillofacial Tissue Regeneration Using Minimally Invasive Procedures. Adv. Healthc. Mater. 2020;9:1901134. doi: 10.1002/adhm.201901134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tanaka M., Nakahata M., Linke P., Kaufmann S. Stimuli-responsive hydrogels as a model of the dynamic cellular microenvironment. Polym. J. 2020;52:861–870. doi: 10.1038/s41428-020-0353-6. [DOI] [Google Scholar]
- 256.Akerele G., Ramadan N., Renu S., Renukaradhya G.J., Shanmugasundaram R., Selvaraj R.K. In vitro characterization and immunogenicity of chitosan nanoparticles loaded with native and inactivated extracellular proteins from a field strain of Clostridium perfringens associated with necrotic enteritis. Vet. Immunol. Immunopathol. 2020;224:110059. doi: 10.1016/j.vetimm.2020.110059. [DOI] [PubMed] [Google Scholar]
- 257.Tondera C., Hauser S., Krüger-Genge A., Jung F., Neffe A.T., Lendlein A., Klopfleisch R., Steinbach J., Neuber C., Pietzsch J. Gelatin-based hydrogel degradation and tissue interaction in vivo: Insights from multimodal preclinical imaging in immunocompetent nude mice. Theranostics. 2016;6:2114–2128. doi: 10.7150/thno.16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Baniasadi H., Mashayekhan S., Fadaoddini S., Haghirsharifzamini Y. Design, fabrication and characterization of oxidized alginate-gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016;31:152–161. doi: 10.1177/0885328216634057. [DOI] [PubMed] [Google Scholar]
- 259.Han W.M., Mohiuddin M., Anderson S.E., García A.J., Jang Y.C. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 2019;94:243–252. doi: 10.1016/j.actbio.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Han W.M., Anderson S.E., Mohiuddin M., Barros D., Nakhai S.A., Shin E., Amaral I.F., Pêgo A.P., García A.J., Jang Y.C. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aar4008. eaar4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jansen L.E., Negrón-Piñeiro L.J., Galarza S., Peyton S.R. Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 2018;70:120–128. doi: 10.1016/j.actbio.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cerletti M., Jurga S., Witczak C.A., Hirshman M.F., Shadrach J.L., Goodyear L.J., Wagers A.J. Highly Efficient, Functional Engraftment of Skeletal Muscle Stem Cells in Dystrophic Muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jabbari E. Challenges for Natural Hydrogels in Tissue Engineering. Gels. 2019;5:30. doi: 10.3390/gels5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Huettner N., Dargaville T.R., Forget A. Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018;36:372–383. doi: 10.1016/j.tibtech.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 265.Kirschning A., Dibbert N., Dräger G. Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications. Chem. - A Eur. J. 2018;24:1231–1240. doi: 10.1002/chem.201701906. [DOI] [PubMed] [Google Scholar]
- 266.Shandalov Y., Egozi D., Koffler J., Dado-Rosenfeld D., Ben-Shimol D., Freiman A., Shor E., Kabala A., Levenberg S. An engineered muscle flap for reconstruction of large soft tissue defects. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6010–6015. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fuoco C., Rizzi R., Biondo A., Longa E., Mascaro A., Shapira‐Schweitzer K., Kossovar O., Benedetti S., Salvatori M.L., Santoleri S., Testa S., Bernardini S., Bottinelli R., Bearzi C., Cannata S.M., Seliktar D., Cossu G., Gargioli C. In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol. Med. 2015;7:411–422. doi: 10.15252/emmm.201404062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ahadian S., Banan Sadeghian R., Yaginuma S., Ramón-Azcón J., Nashimoto Y., Liang X., Bae H., Nakajima K., Shiku H., Matsue T., Nakayama K.S., Khademhosseini A. Hydrogels containing metallic glass sub-micron wires for regulating skeletal muscle cell behaviour. Biomater. Sci. 2015;3:1449–1458. doi: 10.1039/c5bm00215j. [DOI] [PubMed] [Google Scholar]
- 269.Aurora A., Wrice N., Walters T.J., Christy R.J., Natesan S. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss. Acta Biomater. 2018;65:150–162. doi: 10.1016/j.actbio.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 270.Sensini A., Gualandi C., Zucchelli A., Boyle L.A., Kao A.P., Reilly G.C., Tozzi G., Cristofolini L., Focarete M.L. Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties. Sci. Rep. 2018;8:17167. doi: 10.1038/s41598-018-35536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dong R., Ma P.X., Guo B. Conductive biomaterials for muscle tissue engineering. Biomaterials. 2020;229:119584. doi: 10.1016/j.biomaterials.2019.119584. [DOI] [PubMed] [Google Scholar]
- 272.Sasaki M., Karikkineth B.C., Nagamine K., Kaji H., Torimitsu K., Nishizawa M. Highly Conductive Stretchable and Biocompatible Electrode-Hydrogel Hybrids for Advanced Tissue Engineering. Adv. Healthc. Mater. 2014;3:1919–1927. doi: 10.1002/adhm.201400209. [DOI] [PubMed] [Google Scholar]
- 273.Hosseinzadeh S., Rezayat S.M., Giaseddin A., Aliyan A., Soleimani M. Microfluidic system for synthesis of nanofibrous conductive hydrogel and muscle differentiation. J. Biomater. Appl. 2018;32:853–861. doi: 10.1177/0885328217744377. [DOI] [PubMed] [Google Scholar]
- 274.Zhao X., Li P., Guo B., Ma P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015;26:236–248. doi: 10.1016/j.actbio.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 275.Berti F.V., Srisuk P., da Silva L.P., Marques A.P., Reis R.L., Correlo V.M. <sup/> Synthesis and Characterization of Electroactive Gellan Gum Spongy-Like Hydrogels for Skeletal Muscle Tissue Engineering Applications. Tissue Eng. Part A. 2017;23:968–979. doi: 10.1089/ten.tea.2016.0430. [DOI] [PubMed] [Google Scholar]
- 276.Park J., Choi J.H., Kim S., Jang I., Jeong S., Lee J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019;97:141–153. doi: 10.1016/j.actbio.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 277.Cleeton C., Keirouz A., Chen X., Radacsi N. Electrospun Nanofibers for Drug Delivery and Biosensing. ACS Biomater. Sci. Eng. 2019;5:4183–4205. doi: 10.1021/acsbiomaterials.9b00853. [DOI] [PubMed] [Google Scholar]
- 278.Ding J., Zhang J., Li J., Li D., Xiao C., Xiao H., Yang H., Zhuang X., Chen X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019;90:1–34. doi: 10.1016/j.progpolymsci.2019.01.002. [DOI] [Google Scholar]
- 279.Wang K., Tan H., Tian D., Xiong B., Zhang L., Zhu J. Generation of Aligned Electrospun Fibers by Using Insulating and Hydrophobic Collectors. ACS Appl. Polym. Mater. 2020;2:2151–2159. doi: 10.1021/acsapm.0c00121. [DOI] [Google Scholar]
- 280.Farr A.C., Hogan K.J., Mikos A.G. Nanomaterial Additives for Fabrication of Stimuli‐Responsive Skeletal Muscle Tissue Engineering Constructs. Adv. Healthc. Mater. 2020:2000730. doi: 10.1002/adhm.202000730. [DOI] [PubMed] [Google Scholar]
- 281.Tan G.Z., Zhou Y. Electrospinning of biomimetic fibrous scaffolds for tissue engineering: a review. Int. J. Polym. Mater. Polym. Biomater. 2019:1–14. doi: 10.1080/00914037.2019.1636248. [DOI] [Google Scholar]
- 282.Yeo M., Kim G.H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small. 2018;14:1803491. doi: 10.1002/smll.201803491. [DOI] [PubMed] [Google Scholar]
- 283.Wu T., Mo X., Xia Y. Moving Electrospun Nanofibers and Bioprinted Scaffolds toward Translational Applications. Adv. Healthc. Mater. 2020;9:1901761. doi: 10.1002/adhm.201901761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Doostmohammadi M., Forootanfar H., Ramakrishna S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater. Sci. Eng. C. 2020;109:110521. doi: 10.1016/j.msec.2019.110521. [DOI] [PubMed] [Google Scholar]
- 285.Chen Y., Shafiq M., Liu M., Morsi Y., Mo X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020;5:963–979. doi: 10.1016/j.bioactmat.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Smoak M.M., Mikos A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio. 2020;7:100069. doi: 10.1016/j.mtbio.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu Y., Zhou G., Liu Z., Guo M., Jiang X., Taskin M.B., Zhang Z., Liu J., Tang J., Bai R., Besenbacher F., Chen M., Chen C. Mussel Inspired Polynorepinephrine Functionalized Electrospun Polycaprolactone Microfibers for Muscle Regeneration. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-08572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bloise N., Berardi E., Gualandi C., Zaghi E., Gigli M., Duelen R., Ceccarelli G., Cortesi E.E., Costamagna D., Bruni G., Lotti N., Focarete M.L., Visai L., Sampaolesi M. Ether-oxygen containing electrospun microfibrous and sub-microfibrous scaffolds based on poly(Butylene 1,4-cyclohexanedicarboxylate) for skeletal muscle tissue engineering. Int. J. Mol. Sci. 2018;19:3212. doi: 10.3390/ijms19103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Narayanan N., Jiang C., Wang C., Uzunalli G., Whittern N., Chen D., Jones O.G., Kuang S., Deng M. Harnessing Fiber Diameter-Dependent Effects of Myoblasts Toward Biomimetic Scaffold-Based Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2020;8:203. doi: 10.3389/fbioe.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Manchineella S., Thrivikraman G., Khanum K.K., Ramamurthy P.C., Basu B., Govindaraju T. Pigmented Silk Nanofibrous Composite for Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2016;5:1222–1232. doi: 10.1002/adhm.201501066. [DOI] [PubMed] [Google Scholar]
- 291.Nagiah N., Murdock C.J., Bhattacharjee M., Nair L., Laurencin C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-57412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Riboldi S.A., Sampaolesi M., Neuenschwander P., Cossu G., Mantero S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 293.Gotti C., Sensini A., Fornaia G., Gualandi C., Zucchelli A., Focarete M.L. Biomimetic Hierarchically Arranged Nanofibrous Structures Resembling the Architecture and the Passive Mechanical Properties of Skeletal Muscles: A Step Forward Toward Artificial Muscle. Front. Bioeng. Biotechnol. 2020;8:767. doi: 10.3389/fbioe.2020.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Guo Y., Gilbert-Honick J., Somers S.M., Mao H.Q., Grayson W.L. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem. Biophys. Res. Commun. 2019;516:558–564. doi: 10.1016/j.bbrc.2019.06.082. [DOI] [PubMed] [Google Scholar]
- 295.Yang H.S., Lee B., Tsui J.H., Macadangdang J., Jang S.-Y., Im S.G., Kim D.-H. Electroconductive Nanopatterned Substrates for Enhanced Myogenic Differentiation and Maturation. Adv. Healthc. Mater. 2016;5:137–145. doi: 10.1002/adhm.201500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang Y., Zhang Z., Wang Y., Su Y., Chen M. 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater. Sci. Eng. C. 2020;116:111070. doi: 10.1016/j.msec.2020.111070. [DOI] [PubMed] [Google Scholar]
- 297.Zaman M.A.U., Sooriyaarachchi D., Zhou Y.G., Tan G.Z., Du D.P. Modeling the density gradient of 3D nanofiber scaffolds fabricated by divergence electrospinning. Adv. Manuf. 2020:1–16. doi: 10.1007/s40436-020-00307-0. [DOI] [Google Scholar]
- 298.Zhou Y., Mahurubin S., Sooriyaarachchi D., Tan G.Z. Procedia Manuf. Elsevier B.V.; 2019. The effect of nanoclays on nanofiber density gradient in 3D scaffolds fabricated by divergence electrospinning; pp. 110–117. [DOI] [Google Scholar]
- 299.Patel K.H., Talovic M., Dunn A.J., Patel A., Vendrell S., Schwartz M., Garg K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:2528–2537. doi: 10.1002/jbm.b.34584. [DOI] [PubMed] [Google Scholar]
- 300.Shi J., Chen S., Wang L., Zhang X., Gao J., Jiang L., Tang D., Zhang L., Midgley A., Kong D., Wang S. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin‐loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019;107:2040–2049. doi: 10.1002/jbm.b.34295. [DOI] [PubMed] [Google Scholar]
- 301.Uehara T.M., Paino I.M.M., Santos F.A., Scagion V.P., Correa D.S., Zucolotto V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold, Polym. Adv. Technol. 2020;31:1437–1443. doi: 10.1002/pat.4874. [DOI] [Google Scholar]
- 302.Yeo M., Kim G.H. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020;107:102–114. doi: 10.1016/j.actbio.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 303.Yi B., Shen Y., Tang H., Wang X., Li B., Zhang Y. Stiffness of Aligned Fibers Regulates the Phenotypic Expression of Vascular Smooth Muscle Cells. ACS Appl. Mater. Interfaces. 2019;11:6867–6880. doi: 10.1021/acsami.9b00293. [DOI] [PubMed] [Google Scholar]
- 304.Mirzaei A., Saburi E., Islami M., Ardeshirylajimi A., Omrani M.D., Taheri M., Moghadam A.S., Ghafouri-Fard S. Bladder smooth muscle cell differentiation of the human induced pluripotent stem cells on electrospun Poly(lactide-co-glycolide) nanofibrous structure. Gene. 2019;694:26–32. doi: 10.1016/j.gene.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 305.Smoak M.M., Han A., Watson E., Kishan A., Grande-Allen K.J., Cosgriff-Hernandez E., Mikos A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods. 2019;25:276–287. doi: 10.1089/ten.tec.2018.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lee H., Kim W.J., Lee J.U., Yoo J.J., Kim G.H., Lee S.J. Effect of Hierarchical Scaffold Consisting of Aligned dECM Nanofibers and Poly(lactide- co-glycolide) Struts on the Orientation and Maturation of Human Muscle Progenitor Cells. ACS Appl. Mater. Interfaces. 2019;11:39449–39458. doi: 10.1021/acsami.9b12639. [DOI] [PubMed] [Google Scholar]
- 307.Kim W.J., Jang C.H., Kim G.H. A Myoblast-Laden Collagen Bioink with Fully Aligned Au Nanowires for Muscle-Tissue Regeneration. Nano Lett. 2019;19:8612–8620. doi: 10.1021/acs.nanolett.9b03182. [DOI] [PubMed] [Google Scholar]
- 308.Hong J., Yeo M., Yang G.H., Kim G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20246208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Townsend-Nicholson A., Jayasinghe S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7:3364–3369. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 310.Tan G.Z., Zhou Y. Tunable 3D Nanofiber Architecture of Polycaprolactone by Divergence Electrospinning for Potential Tissue Engineering Applications. Nano-Micro Lett. 2018;10:73. doi: 10.1007/s40820-018-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Eskitoros‐Togay Ş.M., Bulbul Y.E., Dilsiz N. Controlled release of doxycycline within core/shell <scp>poly(ε‐caprolactone)</scp> /poly(ethylene oxide) fibers via coaxial electrospinning. J. Appl. Polym. Sci. 2020;137:49273. doi: 10.1002/app.49273. [DOI] [Google Scholar]
- 312.Kajdič S., Planinšek O., Gašperlin M., Kocbek P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019;51:672–681. doi: 10.1016/j.jddst.2019.03.038. [DOI] [Google Scholar]
- 313.Torres-Martinez E.J., Cornejo Bravo J.M., Serrano Medina A., Pérez González G.L., Villarreal Gómez L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018;15:1360–1374. doi: 10.2174/1567201815666180723114326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Bagheri B., Zarrintaj P., Samadi A., Zarrintaj R., Ganjali M.R., Saeb M.R., Mozafari M., Park O.O., Kim Y.C. Tissue engineering with electrospun electro-responsive chitosan-aniline oligomer/polyvinyl alcohol. Int. J. Biol. Macromol. 2020;147:160–169. doi: 10.1016/j.ijbiomac.2019.12.264. [DOI] [PubMed] [Google Scholar]
- 315.Piccirillo G., Carvajal Berrio D.A., Laurita A., Pepe A., Bochicchio B., Schenke-Layland K., Hinderer S. Controlled and tuneable drug release from electrospun fibers and a non-invasive approach for cytotoxicity testing. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-40079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bil M., Kijeńska-Gawrońska E., Głodkowska-Mrówka E., Manda-Handzlik A., Mrówka P. Design and in vitro evaluation of electrospun shape memory polyurethanes for self-fitting tissue engineering grafts and drug delivery systems. Mater. Sci. Eng. C. 2020;110:110675. doi: 10.1016/j.msec.2020.110675. [DOI] [PubMed] [Google Scholar]
- 317.Wsoo M.A., Shahir S., Mohd Bohari S.P., Nayan N.H.M., Razak S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020;491:107978. doi: 10.1016/j.carres.2020.107978. [DOI] [PubMed] [Google Scholar]
- 318.Khodadadi M., Alijani S., Montazeri M., Esmaeilizadeh N., Sadeghi‐Soureh S., Pilehvar‐Soltanahmadi Y. Recent advances in electrospun nanofiber‐ <scp>mediated drug</scp> delivery strategies for localized cancer chemotherapy. J. Biomed. Mater. Res. Part A. 2020;108:1444–1458. doi: 10.1002/jbm.a.36912. [DOI] [PubMed] [Google Scholar]
- 319.Yan E., Jiang J., Yang X., Fan L., Wang Y., An Q., Zhang Z., Lu B., Wang D., Zhang D. pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J. Drug Deliv. Sci. Technol. 2020;55:101455. doi: 10.1016/j.jddst.2019.101455. [DOI] [Google Scholar]
- 320.Göttel B., de Souza e Silva J.M., Santos de Oliveira C., Syrowatka F., Fiorentzis M., Viestenz A., Viestenz A., Mäder K. Electrospun nanofibers – A promising solid in-situ gelling alternative for ocular drug delivery. Eur. J. Pharm. Biopharm. 2020;146:125–132. doi: 10.1016/j.ejpb.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 321.Grimaudo M.A., Concheiro A., Alvarez-Lorenzo C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics. 2020;12:274. doi: 10.3390/pharmaceutics12030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yan D., Yao Q., Yu F., Chen L., Zhang S., Sun H., Lin J., Fu Y. Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Mater. Sci. Eng. C. 2020;111:110767. doi: 10.1016/j.msec.2020.110767. [DOI] [PubMed] [Google Scholar]
- 323.Celebioglu A., Uyar T. Hydrocortisone/cyclodextrin complex electrospun nanofibers for a fast-dissolving oral drug delivery system. RSC Med. Chem. 2020;11:245–258. doi: 10.1039/c9md00390h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Balusamy B., Celebioglu A., Senthamizhan A., Uyar T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems - A systematic review. J. Control. Release. 2020;326:482–509. doi: 10.1016/j.jconrel.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 325.Domokos A., Balogh A., Dénes D., Nyerges G., Ződi L., Farkas B., Marosi G., Nagy Z.K. Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning. Eur. J. Pharm. Sci. 2019;130:91–99. doi: 10.1016/j.ejps.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 326.Celebioglu A., Uyar T. Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast-dissolving drug delivery system. Int. J. Pharm. 2020;584:119395. doi: 10.1016/j.ijpharm.2020.119395. [DOI] [PubMed] [Google Scholar]
- 327.Verma R.S., Ramakrishna S. Biomaterials: Biosensors. Curr. Opin. Biomed. Eng. 2020;13:A3–A5. doi: 10.1016/j.cobme.2020.07.001. [DOI] [Google Scholar]
- 328.Liu Y., Hao M., Chen Z., Liu L., Liu Y., Yang W., Ramakrishna S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020;13:174–189. doi: 10.1016/j.cobme.2020.02.001. [DOI] [Google Scholar]
- 329.Murthe S.S., Mohamed Saheed M.S., Perumal V., Mohamed Saheed M.S., Mohamed N.M. Nanobiosensors Biomol. Target. Elsevier; 2018. Electrospun nanofibers for biosensing applications; pp. 253–267. [DOI] [Google Scholar]
- 330.Asghari S., Rezaei Z., Mahmoudifard M. Electrospun nanofibers: A promising horizon toward the detection and treatment of cancer. Analyst. 2020;145:2854–2872. doi: 10.1039/c9an01987a. [DOI] [PubMed] [Google Scholar]
- 331.Senthamizhan A., Balusamy B., Uyar T. Recent progress on designing electrospun nanofibers for colorimetric biosensing applications. Curr. Opin. Biomed. Eng. 2020;13:1–8. doi: 10.1016/j.cobme.2019.08.002. [DOI] [Google Scholar]
- 332.Sylvester M.A., Amini F., Tan C.K. Electrospun nanofibers in wound healing. Mater. Today Proc. 2020 doi: 10.1016/j.matpr.2020.05.686. [DOI] [Google Scholar]
- 333.Dodero A., Alloisio M., Castellano M., Vicini S. Multilayer Alginate-Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces. 2020;12:31162–31171. doi: 10.1021/acsami.0c07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Innocenti Malini R., Lesage J., Toncelli C., Fortunato G., Rossi R.M., Spano F. Crosslinking dextran electrospun nanofibers via borate chemistry: Proof of concept for wound patches. Eur. Polym. J. 2019;110:276–282. doi: 10.1016/j.eurpolymj.2018.11.017. [DOI] [Google Scholar]
- 335.Dodero A., Scarfi S., Pozzolini M., Vicini S., Alloisio M., Castellano M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Appl. Mater. Interfaces. 2020;12:3371–3381. doi: 10.1021/acsami.9b17597. [DOI] [PubMed] [Google Scholar]
- 336.An J., Teoh J.E.M., Suntornnond R., Chua C.K. Engineering; 2015. Design and 3D Printing of Scaffolds and Tissues. [DOI] [Google Scholar]
- 337.Sun D., Chang C., Li S., Lin L. Near-field electrospinning. Nano Lett. 2006 doi: 10.1021/nl0602701. [DOI] [PubMed] [Google Scholar]
- 338.He X.X., Zheng J., Yu G.F., You M.H., Yu M., Ning X., Long Y.Z. Near-Field Electrospinning: Progress and Applications. J. Phys. Chem. C. 2017 doi: 10.1021/acs.jpcc.6b12783. [DOI] [Google Scholar]
- 339.Kolan K.C.R., Li J., Roberts S., Semon J.A., Park J., Day D.E., Leu M.C. Near-field electrospinning of a polymer/bioactive glass composite to fabricate 3D biomimetic structures. Int. J. Bioprinting. 2019 doi: 10.18063/ijb.v5i1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ren S., Yao Y., Zhang H., Fan R., Yu Y., Yang J., Zhang R., Liu C., Sun W., Miao L. Aligned fibers fabricated by near-field electrospinning influence the orientation and differentiation of hPDLSCs for periodontal regeneration. J. Biomed. Nanotechnol. 2017 doi: 10.1166/jbn.2017.2451. [DOI] [PubMed] [Google Scholar]
- 341.Walker J.L., Santoro M. Bioresorbable Polym. Biomed. Appl. From Fundam. to Transl. Med. 2017. Processing and production of bioresorbable polymer scaffolds for tissue engineering. [DOI] [Google Scholar]
- 342.Skoog S.A., Goering P.L., Narayan R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014 doi: 10.1007/s10856-013-5107-y. [DOI] [PubMed] [Google Scholar]
- 343.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015 doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kumar H., Sakthivel K., Mohamed M.G.A., Boras E., Shin S.R., Kim K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2020 doi: 10.1002/mabi.202000317. [DOI] [PubMed] [Google Scholar]
- 345.Kazmer D. Appl. Plast. Eng. Handb. Process. Mater. Appl. Second Ed. 2017. Three-Dimensional Printing of Plastics. [DOI] [Google Scholar]
- 346.Hutmacher D.W., Woodfield T.B.F., Dalton P.D. Tissue Eng. Second Ed. Elsevier Inc.; 2014. Scaffold Design and Fabrication; pp. 311–346. [DOI] [Google Scholar]
- 347.Tan K.H., Chua C.K., Leong K.F., Naing M.W., Cheah C.M. Fabrication and characterization of three-dimensional poly(ether-ether-ketone)/-hydroxyapatite biocomposite scaffolds using laser sintering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2005 doi: 10.1243/095441105X9345. [DOI] [PubMed] [Google Scholar]
- 348.Hao L., Savalani M.M., Zhang Y., Tanner K.E., Harris R.A. Selective laser sintering of hydroxyapatite reinforced polyethylene composites for bioactive implants and tissue scaffold development. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006 doi: 10.1243/09544119JEIM67. [DOI] [PubMed] [Google Scholar]
- 349.Tan K.H., Chua C.K., Leong K.F., Cheah C.M., Cheang P., Abu Bakar M.S., Cha S.W. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003 doi: 10.1016/S0142-9612(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 350.Antonov E.N., Bagratashvili V.N., Howdle S.M., Konovalov A.N., Popov V.K., Panchenko V.Y. Fabrication of polymer scaffolds for tissue engineering using surface selective laser sintering. Laser Phys. 2006 doi: 10.1134/s1054660x06050070. [DOI] [Google Scholar]
- 351.Sun Z., Wu F., Gao H., Cui K., Xian M., Zhong J., Tian Y., Fan S., Wu G. A Dexamethasone-Eluting Porous Scaffold for Bone Regeneration Fabricated by Selective Laser Sintering. ACS Appl. Bio Mater. 2020;2020:8747. doi: 10.1021/acsabm.0c01126. [DOI] [PubMed] [Google Scholar]
- 352.Knowlton S., Anand S., Shah T., Tasoglu S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018 doi: 10.1016/j.tins.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 353.Tamay D.G., Usal T.D., Alagoz A.S., Yucel D., Hasirci N., Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019 doi: 10.3389/fbioe.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Goranov V., Shelyakova T., De Santis R., Haranava Y., Makhaniok A., Gloria A., Tampieri A., Russo A., Kon E., Marcacci M., Ambrosio L., Dediu V.A. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020 doi: 10.1038/s41598-020-58738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ahangar P., Cooke M.E., Weber M.H., Rosenzweig D.H. Current biomedical applications of 3D printing and additive manufacturing. Appl. Sci. 2019 doi: 10.3390/app9081713. [DOI] [Google Scholar]
- 356.Costantini M., Testa S., Mozetic P., Barbetta A., Fuoco C., Fornetti E., Tamiro F., Bernardini S., Jaroszewicz J., Święszkowski W., Trombetta M., Castagnoli L., Seliktar D., Garstecki P., Cesareni G., Cannata S., Rainer A., Gargioli C. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials. 2017;131:98–110. doi: 10.1016/J.BIOMATERIALS.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 357.Choi J.S., Lee S.J., Christ G.J., Atala A., Yoo J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 358.SGA van N., K H.-T., A B., T S., C D., K C., R D., GA B., J W., N P., A B. Two-component collagen nerve guides support axonal regeneration in the rat peripheral nerve injury model. J. Tissue Eng. Regen. Med. 2017;11 doi: 10.1002/TERM.2248. [DOI] [PubMed] [Google Scholar]
- 359.Efraim Y., Schoen B., Zahran S., Davidov T., Vasilyev G., Baruch L., Zussman E., Machluf M. 3D Structure and Processing Methods Direct the Biological Attributes of ECM-Based Cardiac Scaffolds. Sci. Rep. 2019 doi: 10.1038/s41598-019-41831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Cristallini C., Rocchietti E.C., Gagliardi M., Mortati L., Saviozzi S., Bellotti E., Turinetto V., Sassi M.P., Barbani N., Giachino C. Micro- and Macrostructured PLGA/Gelatin Scaffolds Promote Early Cardiogenic Commitment of Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2016 doi: 10.1155/2016/7176154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.M K., E V.-F., A G., F G. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. A. 2016;104 doi: 10.1002/JBM.A.35702. [DOI] [PubMed] [Google Scholar]
- 362.TM D., R E., G V., Q D., C D., DL K., C E., F M. 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration. J. Mech. Behav. Biomed. Mater. 2015;41 doi: 10.1016/J.JMBBM.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 363.Chiono V., Mozetic P., Boffito M., Sartori S., Gioffredi E., Silvestri A., Rainer A., Giannitelli S.M., Trombetta M., Nurzynska D., Meglio F. Di, Castaldo C., Miraglia R., Montagnani S., Ciardelli G. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus. 2014 doi: 10.1098/rsfs.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.E G., Y G., K N., T I., T S., T M. Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant. 2004;13 doi: 10.3727/000000004783983639. [DOI] [PubMed] [Google Scholar]
- 365.Cristallini C., Rocchietti E.C., Gagliardi M., Mortati L., Saviozzi S., Bellotti E., Turinetto V., Sassi M.P., Barbani N., Giachino C. Micro- and Macrostructured PLGA/Gelatin Scaffolds Promote Early Cardiogenic Commitment of Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2016;2016 doi: 10.1155/2016/7176154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Nicolas J., Magli S., Rabbachin L., Sampaolesi S., Nicotra F., Russo L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules. 2020 doi: 10.1021/acs.biomac.0c00045. [DOI] [PubMed] [Google Scholar]
- 367.Dalby M.J., Gadegaard N., Oreffo R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014 doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 368.Papadimitriou L., Manganas P., Ranella A., Stratakis E. Biofabrication for neural tissue engineering applications. Mater. Today Bio. 2020 doi: 10.1016/j.mtbio.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Meco E., Lampe K.J. Microscale architecture in biomaterial scaffolds for spatial control of neural cell behavior. Front. Mater. 2018 doi: 10.3389/fmats.2018.00002. [DOI] [Google Scholar]
- 370.Cho Y.W., Kim D.S., Suhito I.R., Han D.K., Lee T., Kim T.H. Enhancing neurogenesis of neural stem cells using homogeneous nanohole pattern-modified conductive platform. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhu W., Ye T., Lee S.J., Cui H., Miao S., Zhou X., Shuai D., Zhang L.G. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomedicine Nanotechnology, Biol. Med. 2018 doi: 10.1016/j.nano.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 372.Vijayavenkataraman S., Kannan S., Cao T., Fuh J.Y.H., Sriram G., Lu W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019 doi: 10.3389/fbioe.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hasanzadeh E., Ebrahimi-Barough S., Mirzaei E., Azami M., Tavangar S.M., Mahmoodi N., Basiri A., Ai J. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. - Part A. 2019 doi: 10.1002/jbm.a.36596. [DOI] [PubMed] [Google Scholar]
- 374.Rouwkema J., Rivron N.C., van Blitterswijk C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008 doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 375.Dattola E., Parrotta E.I., Scalise S., Perozziello G., Limongi T., Candeloro P., Coluccio M.L., Maletta C., Bruno L., De Angelis M.T., Santamaria G., Mollace V., Lamanna E., Di Fabrizio E., Cuda G. Development of 3D PVA scaffolds for cardiac tissue engineering and cell screening applications. RSC Adv. 2019 doi: 10.1039/C8RA08187E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tsukamoto Y., Akagi T., Akashi M. Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer. Sci. Rep. 2020 doi: 10.1038/s41598-020-59371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Campodoni E., Heggset E.B., Rashad A., Ramírez-Rodríguez G.B., Mustafa K., Syverud K., Tampieri A., Sandri M. Polymeric 3D scaffolds for tissue regeneration: Evaluation of biopolymer nanocomposite reinforced with cellulose nanofibrils. Mater. Sci. Eng. C. 2019 doi: 10.1016/j.msec.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 378.Ma R., Liang J., Huang W., Guo L., Cai W., Wang L., Paul C., Yang H.T., Kim H.W., Wang Y. Electrical Stimulation Enhances Cardiac Differentiation of Human Induced Pluripotent Stem Cells for Myocardial Infarction Therapy. Antioxidants Redox Signal. 2018 doi: 10.1089/ars.2016.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Valls-Margarit M., Iglesias-García O., Di Guglielmo C., Sarlabous L., Tadevosyan K., Paoli R., Comelles J., Blanco-Almazán D., Jiménez-Delgado S., Castillo-Fernández O., Samitier J., Jané R., Martínez E., Raya Á. Engineered Macroscale Cardiac Constructs Elicit Human Myocardial Tissue-like Functionality. Stem Cell Reports. 2019 doi: 10.1016/j.stemcr.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Mulbauer G.D., Matthew H.W.T. Biomimetic Scaffolds for Skeletal Muscle Regeneration. Discoveries. 2019 doi: 10.15190/d.2019.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Narayanan N., Jia Z., Kim K.H., Kuang L., Lengemann P., Shafer G., Bernal-Crespo V., Kuang S., Deng M. Biomimetic glycosaminoglycan-based scaffolds improve skeletal muscle regeneration in a Murine volumetric muscle loss model. Bioact. Mater. 2021 doi: 10.1016/j.bioactmat.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Mertens J.P., Sugg K.B., Lee J.D., Larkin L.M. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen. Med. 2014 doi: 10.2217/rme.13.81. [DOI] [PMC free article] [PubMed] [Google Scholar]