Abstract
Background
Little is known about the implications of hyperinsulinemia on energy metabolism, and such knowledge might help understand the pathophysiology of insulin dysregulation.
Objectives
Describe differences in the metabolic response to an oral glucose test, depending on the magnitude of the insulin response.
Animals
Twelve Icelandic horses in various metabolic states.
Methods
Horses were subjected to 3 oral glucose tests (OGT; 0.5 g/kg body weight glucose). Basal, 120 and 180 minutes samples were analyzed using a combined liquid chromatography tandem mass spectrometry and flow injection analysis tandem mass spectrometry metabolomic assay. Insulin concentrations were measured using an ELISA. Analysis was performed using linear models and partial least‐squares regression.
Results
The kynurenine : tryptophan ratio increased over time during the OGT (adjusted P‐value = .001). A high insulin response was associated with lower arginine (adjusted P‐value = .02) and carnitine (adjusted P‐value = .03) concentrations. A predictive model using only baseline samples performed well with as few as 7 distinct metabolites (sensitivity, 86%; 95% confidence interval [CI], 81%‐90%; specificity, 88%; 95% CI, 84%‐92%).
Conclusions and Clinical Importance
Our results suggest induction of low‐grade inflammation during the OGT. Plasma arginine and carnitine concentrations were lower in horses with high insulin response and could constitute potential therapeutic targets. Development of screening tools to identify insulin‐dysregulated horses using only baseline blood sample appears promising.
Keywords: biomarker, EMS, insulin dysregulation, metabolomics, oral glucose test
Abbreviations
- AUCins
area under the insulin curve over time
- EMS
equine metabolic syndrome
- HI
hyperinsulinemia
- ID
insulin dysregulation
- LysoPC
lysophosphatidylcholine
- NPV
negative predictive value
- OGT
oral glucose test
- PC
phosphatidylcholine
- PLS‐DA
partial least‐squares discriminant analysis
- PPV
positive predictive value
- SM
sphingomyelin
1. INTRODUCTION
Equine metabolic syndrome (EMS) encompasses a range of disorders of energy metabolism, bearing some similarities with metabolic syndrome as defined in humans. 1 Insulin dysregulation (ID), including insulin resistance and transient or long lasting hyperinsulinemia (HI), 2 and regional or generalized adiposity are seen as major risk factors for laminitis, 3 which is central to the definition of EMS. This disorder of the dermoepidermal attachment within the hoof in fact can be directly induced by HI, either experimentally 4 , 5 or as a result of an exaggerated pancreatic insulin secretion in response to PO carbohydrate intake, 6 but also might be promoted by proinflammatory factors observed in ID or EMS patients. 7 , 8 , 9
The oral glucose test (OGT) consists of administration of a fixed amount of glucose via nasogastric tube. By subsequently measuring insulin concentrations in blood, the insulin response can be quantified, providing a diagnostic tool for identification of HI 10 and prediction of laminitis risk. 6 Furthermore, the insulin response to the OGT appears to be correlated with the insulin response to grazing. 11
Many studies have been undertaken to identify markers of the inflammatory processes associated with HI, laminitis, or obesity in horses. 12 , 13 , 14 , 15 By using a metabolomics approach, cellular processes of this kind can be identified. The mechanisms triggered by carbohydrate intake during the OGT are of interest, because they might reflect what happens when hyperinsulinemic horses are grazing. Analysis of baseline samples could identify long sought biomarkers of HI useful for diagnostic screening and limit the requirement for OGT and other complex tests.
As a result, our aim was to investigate the impact of the OGT on the metabolome in healthy and hyperinsulinemic horses. Metabolites involved in inflammatory processes or linked to metabolic diseases were targeted. In contrast to previous studies of the metabolomic response of horses to the OGT, 16 , 17 the area under the curve of insulin over time (AUCins) was used as a continuous predictor in a linear model, allowing for a more detailed description of the relationship between the insulin response and the metabolome. Additionally, the performance of predictive models was explored to investigate the discriminatory potential of the candidate biomarkers.
2. MATERIALS AND METHODS
2.1. Horses
Twelve Icelandic horses (5 geldings and 7 mares) aged 9 to 29 years (median, 19 years) were enrolled in the study. They were fed hay ad libitum and kept in barns and paddocks. Access to pasture was allowed every day for up to 6 hours. A full clinical examination and thyrotropin releasing hormone stimulation test were performed after the standard protocol 18 and before the beginning of the experiments to rule out clinical disorders other than ID. The State Office for Consumer Protection and Food Safety (LAVES) approved the study in accordance with the German Animal Welfare Law (file number: 33.19‐42 502‐05‐17A099).
2.2. Oral glucose tests
Three OGTs were performed over a period of 7 weeks with 3‐ and 4‐week intervals between the first and second, and second and third OGT, respectively. The horses were fasted overnight before testing. The next morning an indwelling catheter (Intraflon 2 12 G, Vygon, Ecouen, France) was placed in a jugular vein for blood sample collection. After collection of a basal blood sample, 0.5 g/kg body weight glucose (Glucose, WDT, Garbsen, Germany) dissolved in 2 L of water was administered via a nasogastric tube. Additional blood samples were taken at 30, 60, 120, 180, and 240 minutes. All samples were collected into potassium EDTA and Z serum clot activator vacuum tubes (Vacuette, greiner bio‐one, Kremsmünster, Austria). The EDTA tubes were chilled at 4°C and the serum tubes were allowed to clot at room temperature. They were centrifuged at 4000g for 10 minutes within 6 hours of collection, and the plasma and serum supernatants collected, aliquoted, and stored at −80°C.
2.3. Insulin measurement
Serum insulin concentrations from all samples were measured in duplicate using a previously validated 19 equine insulin ELISA (Mercodia Equine Insulin ELISA, Mercodia AB, Uppsala, Sweden; interassay coefficient of variation, 7.7%) following manufacturer's instructions. When insulin concentration exceeded the range of quantification, serum samples were diluted 1:4 using diabetes sample buffer (Mercodia Diabetes Sample Buffer, Mercodia AB).
2.4. Metabolomic assay
Metabolic profiling of basal, 120 and 180 minutes EDTA plasma samples was performed using the Biocrates AbsoluteIDQ p180 Kit (Biocrates Life Sciences AG, Innsbruck, Austria). This assay includes up to 188 metabolites related to glycolysis, oxidative processes, lipid degradation, and inflammatory signaling. For example, acylcarnitines are related to fatty acid oxidation and fatty acid profiles 20 whereas the phospholipids (phosphatidylcholines [PCs], lysophosphatidylcholines [LysoPCs], and sphingomyelins [SMs]), which are major components of lipid membranes, also are involved in cell signaling. 21 Many such molecules previously have been linked to insulin action 22 or metabolic conditions in several species. 9 , 20 , 23 , 24 The total length of the fatty acid chains, number of double bonds, and bond types are indicated in the molecule annotation. For example, PC aa C34:3 represents PC, the 2 fatty acids of which are bound to glycerol via ester bonds (aa, acyl‐acyl; ae, acyl‐alkyl). Its 2 fatty acids have a combined length of 34 C atoms and 3 double bonds. Because acylcarnitines, hexoses, PC, LysoPC, and SMs were quantified using flow injection analysis‐tandem mass spectrometry, the lipid species can correspond to several isomers. In contrast, amino acids, and biogenic amines were measured by liquid chromatography‐tandem mass spectrometry. These measurements were performed at the Fraunhofer Institute of Toxicology and Experimental Medicine ITEM, Hanover, Germany.
2.5. Statistical analysis
The methods used for statistical analysis are described in detail in supplementary file 1. Briefly, metabolites that did not pass quality control were removed. Data were adjusted for batch effects, log2‐tranformed, scaled, and quantile normalized. 25
Linear models, as implemented in the “limma” R‐package,50 were used to identify metabolites significantly associated with time in the OGT and AUCins. P‐values were adjusted for multiple comparisons using the procedure of Benjamini and Hochberg. 26
Partial least‐squares discriminant analyses (PLS‐DA) were conducted using the “DiscriMiner” R‐package 27 to identify the most important metabolites for classification of horses depending on their total insulin response (2 arbitrarily defined, equally sized groups with either high or low AUCins). This analysis was performed separately for the basal and 120 minutes time point.
Metabolite importance was quantified using the variable importance in projection (VIP) score. This score can be interpreted as an indicator of the diagnostic value of the individual metabolites. Metabolites strongly correlated with HI and displaying a good separation between both groups generally are associated with higher VIP scores. To compare theses scores across models, they were scaled to a percentage value of the max VIP score within each model. As a result, the most important variable in each model was attributed a scaled VIP score of 100%.
Lastly, PLS‐DA was repeated on the baseline dataset while varying the number of metabolites included in the model as a hyperparameter during a bootstrap cross‐validation. Metabolites were removed by order of increasing importance as determined in the full model. Model performance (accuracy, sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) was estimated using holdout data. These estimates were adjusted for the mean reported prevalence of HI. 28 , 29 , 30 The aim of this second approach was to determine the accuracy of smaller metabolite sets as predictors of HI in basal samples.
3. RESULTS
One horse was diagnosed with pituitary pars intermedia dysfunction (PPID). No treatment was initiated before the end of the trials.
3.1. Data preparation
The Biocrates AbsoluteIDQ p180 Kit measures plasma concentrations of up to 188 metabolites belonging to 6 substance classes. By summarizing these classes and adding the kynurenine : tryptophan ratio, 194 features are obtained. Data preprocessing decreased this number to 145, as detailed in Table 1. Twelve horses were subjected to 3 OGTs for each of which the time points 0, 120 and 180 minutes were considered in the metabolome, resulting in 108 samples. These time points were selected because of cost constraints to include baseline, insulin peak, and standard diagnostic time points. No outliers were found using the “bagplot” method.
TABLE 1.
Metabolites available before and after data preprocessing. Summarized values are the sums of plasma concentrations of metabolites by groups (eg, sum of acylcarnitines) or ratios such as the kynurenine : tryptohphan ratio, which is of interest in the scope of inflammatory processes
| Metabolite class | Before preprocessing | After preprocessing |
|---|---|---|
| Acylcarnitines | 40 | 7 |
| Amino acids | 21 | 21 |
| Biogenic amines | 21 | 20 |
| Glycerophospholipids | 90 | 75 |
| Sphingolipids | 15 | 15 |
| Sugars | 1 | 1 |
| Summarized values | 6 | 6 |
| Sum | 194 | 145 |
3.2. Linear model
Figure 1A graphically displays the progression of the significant features sorted by class. The sum of hexoses (H1) and dihydroxyphenylalanine (DOPA) increased upon glucose administration. Of all amino acids, only glycine (Gly) and tryptophan (Trp) increased over time whereas the others decreased. Similarly, among the glycerophospholipids, LysoPCs decreased whereas PCs increased, and except for the increasing carnitine (C0) and propionylcarnitine (C3), all acylcarnitines decreased.
FIGURE 1.

Heatmap of the relative metabolite concentrations for the metabolites significantly associated with (A) time during the oral glucose test (OGT) and (B) area under the insulin curve over time (AUCins). Each column of the heatmap represents a sample and each row a metabolite. In A, the samples are grouped by time point, whereas in B they are ordered by AUCins in ascending order. Metabolite names are displayed on the right side with associated fold change and adjusted P‐values. In the case of numeric predictors like “Time” or “AUCins,” the log2 fold change (logFC) given by the “limma” package represents the slope of the regression line. For each unit of the predictor (eg, time in minutes), the log2‐transformed normalized metabolite concentrations thus increase by log2 FC. Note that all lysophosphatidylcholines decreased over time—as on average the colored tiles are darker at 0 than 180 minutes—whereas phosphatidylcholines increased. The associations between metabolites and AUCins were less apparent, because there was more individual variability
The patterns associated with AUCins were less clear . All differentially concentrated acylcarnitines but also arginine (Arg) and spermidine were negatively associated with AUCins, in contrast to the only represented glycerophospholipid (PC ae C38:6), which was found in higher concentrations in horses with high insulin response (Figure 1B).
3.3. Variable importance in PLS‐DA
Indicators of model performance for both the baseline and 120 minutes model are summarized in Table 2. Overall, similar values were observed, but the baseline model appeared to be slightly more specific.
TABLE 2.
Indicators of model performance for the baseline and 120 minutes partial least‐squares discriminant analysis (PLS‐DA) as obtained by leave‐one‐out‐cross‐validation on all samples. Positive and negative predictive values were calculated using a prevalence of 22.5%
| Parameter | Baseline | 120 minutes |
|---|---|---|
| Accuracy | 83% (67%‐94%) | 83% (67%‐94%) |
| Sensitivity | 78% (52%‐94%) | 83% (59%‐96%) |
| Specificity | 89% (65%‐99%) | 83% (59%‐96%) |
| Positive predictive value | 68% (32%‐93%) | 60% (28%‐86%) |
| Negative predictive value | 93% (76%‐99%) | 94% (77%‐100%) |
Figure 2 displays the scaled VIP scores for both models. Acetylcarnitine (C2) and the sum of acylcarnitines appear to be among the most important predictors for a high insulin response both at baseline and 120 minutes after glucose intake. In contrast, although still among the most important metabolites, some molecules such as symmetric (SDMA) and asymmetric (ADMA) dimethylarginine or alanine (Ala) had more variation in their associated VIP scores, indicating that their discriminatory potential differs more clearly between the baseline and 120 minutes models.
FIGURE 2.
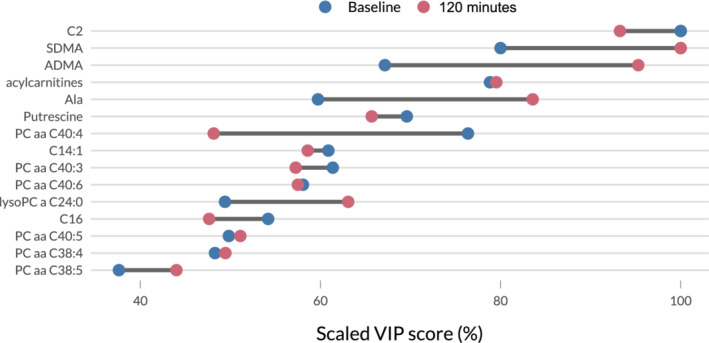
Dumbbell plot of the scaled Variable Importance in Projection (VIP) scores of the top 10 metabolites from the baseline and 120 minutes partial least‐squares discriminant analysis (PLS‐DA) models. The scaling of the scores allows for a better comparability between models. As there is some overlap between the 10 metabolites in each model, the combination of both rankings results in the 15 metabolites displayed here. The dark segments between pairs of points represent the difference in relative importance of the metabolites. Large differences indicate that although the metabolite is very helpful in distinguishing horses with a high area under the insulin curve over time (AUCins) from horses with a low 1in‐ model, the difference between both groups regarding this metabolite is less striking at the other time point
3.4. Performance of reduced PLS‐DA models on baseline samples
To investigate if identification of horses with high AUCins also was possible with fewer metabolites, the baseline PLS‐DA model was rerun repeatedly with fewer and fewer metabolites in a bootstrap approach. Model performance for each of these repetitions is presented in Figure 3. The metabolites included in each run can be derived from the VIP scores in the full baseline PLS‐DA model provided as supplemental Table S1. Because a bootstrap approach with more validation samples was used in comparison to the leave‐one‐out cross‐validation used beforehand (see variable importance in PLS‐DA), the overfitting often present in PLS‐DA models with more features than samples resulted in a loss of performance when more metabolites were used, because fewer samples were available to train the model. Overall, model performance increased when decreasing the number of predictors. Specificity and PPV were maximized at 7, accuracy at 30, and sensitivity and NPV at 59 metabolites. With as few as 2 metabolites, accuracy, sensitivity, and NPV were within their respective 5 highest values.
FIGURE 3.
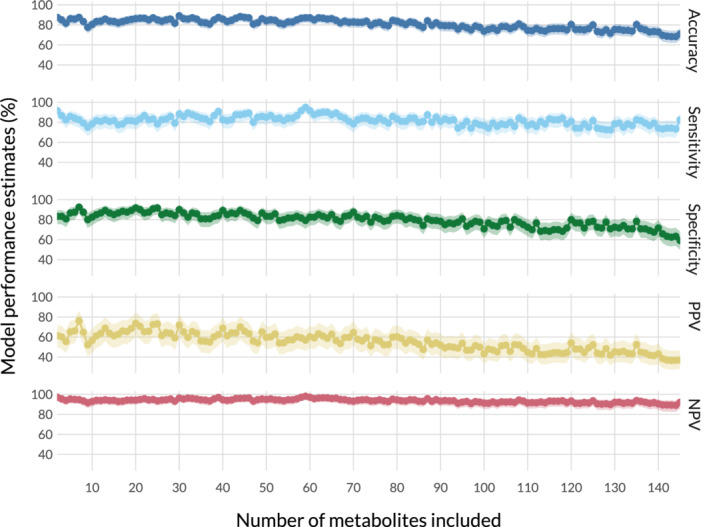
Model performance estimates on the baseline samples obtained by bootstrap cross‐validation depending on the number of metabolites included. Positive Predictive Value (PPV) and Negative Predictive Value (NPV) were obtained using abovementioned formulas and the mean of previously reported prevalence of hyperinsulinemia. 28 , 29 , 30 The 95% confidence interval is shown as a shaded area behind each estimate. Overall, best model performance is reached with the top 7 and top 20 metabolites as determined by the baseline partial least‐squares discriminant analysis (PLS‐DA) model including all metabolites
4. DISCUSSION
Our objective was to investigate the metabolic response of horses during the OGT with a targeted metabolomics approach. The time course of metabolite concentrations and their relationship to the total insulin response, approximated as AUCins, were analyzed and the predictive power of the metabolite set was explored.
4.1. Effects attributable to insulin action
The time course of metabolite concentrations during the OGT (Figure 1A) was linked to the pharmacokinetics and pharmacodynamics of glucose intake and insulin secretion. Because of the high glucose influx, the sum of hexoses (H1) is roughly equivalent to the glucose concentration during the OGT. Unsurprisingly, an increase in glucose can be observed over time, with a slight decrease from 120 to 180 minutes. The fold change of H1 can be used as a scale to interpret the shifts in other metabolites, because it should have the highest magnitude.
Of the 14 amino acids that varied significantly over time, only Trp and Gly had a positive concentration gradient during the test, whereas all others were negative. The decrease in amino acids corroborates previous reports on the metabolome during the OGT in humans and horses and could be attributed to insulin‐induced decreased proteolysis and enhanced cellular amino acid uptake. 9 , 31 , 32
An increase of Trp during the OGT previously has been reported in ponies, 17 whereas kynurenine was shown to increase in horses. 9 In our study, both molecules and their ratio (kynurenine : tryptophan) exhibited a positive concentration gradient, which might be attributable to enhanced indoleamine 2,3‐dioxygenase (IDO) activity, considered to be induced by inflammatory processes and associated with metabolic syndrome in humans. 33 Thus, the OGT may elicit low‐grade inflammation. Assuming the OGT models processes that occur naturally during grazing or nonstructural carbohydrate intake, this finding would support an inflammatory component in the pathogenesis of endocrinopathic laminitis, which could be responsible for chronic lamellar structural damage or priming metabolic pathomechanisms.
To our knowledge, an increase of DOPA (a precursor of dopamine) during the OGT has not been reported previously in any species. Parkinson's disease is associated with a loss of dopaminergic innervation in several brain areas, similar to the loss of dopaminergic inhibition in the pars intermedia of the pituitary gland of horses with PPID, 34 but also with glucose intolerance and diabetes. 35 , 36 A possible lack of inhibition of insulin secretion in β‐cells of the pancreatic islets by DOPA and dopamine 37 , 38 could link the pathogenesis of PPID with ID.
4.2. Differential response of insulin‐dysregulated horses
Carnitine is necessary for the transportation of fatty acids into mitochondria for energy production via β‐oxidation. Therefore, it has been hypothesized that obese individuals with higher plasma fatty acid concentrations use more carnitine. 39 , 40 In our study, a negative association between carnitine (C0) and the insulin response (AUCins; Figure 1B) was observed, possibly indicating similar differences in energy metabolism between hyper‐ and normo‐insulinemic horses. Nevertheless, the benefits of carnitine supplementation were equivocal in this species. 41 , 42 Finally, if less carnitine is available for carnitine acetyltransferase, lower acetylcarnitine (C2) concentrations are to be expected (Figure 1B). The negative correlation between AUCins and acetylcarnitine observed in our study also emphasizes the importance of this metabolite in both PLS‐DA models (Figure 2).
Arginine is another molecule available as a dietary supplement, and it is said to improve metabolic conditions such as obesity and Type‐2 diabetes mellitus in rats, pigs, and humans. 43 Similar to its metabolites spermidine and putrescine, it was present in lower concentrations in horses with high insulin response (Figures 1B and 2). Arginine has been shown to increase oxidation of long‐chain fatty acids and glucose, 43 but also is known for its strong vasodilatory effect, mediated by nitric oxide. 43 , 44 Lower arginine concentrations in horses with high insulin response therefore could be associated with some form of endothelial dysfunction, potentially involved in the pathophysiology of endocrinopathic laminitis. 45
On the other hand, ADMA, the biologically active asymmetric stereoisomer of SDMA, which inhibits nitric oxide synthesis was slightly lower in horses with high insulin response (data not shown), and was given high importance in the 120 minutes PLS‐DA model (Figure 2). Because SDMA and ADMA are derived from the catabolism of proteins containing methylated arginine, and not from methylation of free arginine, 46 the potential implications of this finding for the pathophysiology of ID remain obscure.
In our study, ID was assessed in a cut‐off agnostic fashion using AUCins as an approximation of the total insulin response, 47 , 48 which encompasses basal HI and the response to glucose stimulation. The observed insulin responses at 120 minutes ranged from 20 to 240 μIU/mL. However, no horse was hyperinsulinemic at baseline 1 and insulin resistance was not assessed separately, whereas both factors could have impact on the metabolic phenotype. In addition, hypotheses have been made regarding the potential implications of some metabolites associated with a higher insulin response to glucose stimulation, but it remains unclear if these deviations are a cause or a consequence of ID.
4.3. Classification performance and future perspectives
During the first PLS‐DA approach, only 1 sample was excluded from each run of the model‐training step and kept for model validation (leave‐one‐out‐cross‐validation). Therefore, compared to the second approach, where model metrics were obtained by bootstrap cross‐validation, better model performance is achievable at the cost of a higher risk for overfitting.
Several metabolites not identified by the linear model approach had notable variable importance in projection (eg, SDMA, ADMA, Ala; Figure 2). The reason for this observation might be that AUCins was used as a continuous variable in the linear model, whereas it was dichotomized in the PLS‐DA approach. Therefore, linear correlations might be masked whereas nonlinear relationships could be uncovered. Additionally, during PLS‐DA, all metabolites were considered simultaneously, allowing detection of metabolites of predictive value in the scope of statistical interactions.
Because of the complexity and costs of metabolomics analysis, it does not appear feasible to use large metabolite panels for diagnostic purposes in animals. Therefore, we investigated the discriminatory potential of the panel while gradually decreasing the number of metabolites used, in order of descending variable importance from the initial baseline model. Our objective was to determine if similar accuracy was achievable using fewer metabolites, which could be brought to a different diagnostic platform, such as a multiplex point‐of‐care device. The results presented in Figure 3 are considered a proof‐of‐concept. The decrease in the number of metabolites appears beneficial to model performance. This finding possibly could be a result of the high dimensionality of the data (many more metabolites than samples), for which PLS‐DA is more sensitive than other classification algorithms. However, because sample size was small and the interpretation limited to a proof‐of‐concept, it was considered best not to introduce additional statistical methods. Best model performance was reached at 7 and 20 metabolites, which, depending on the detection technique, can be considered a feasible number of analytes to include into a point‐of‐care device. 49
In our study, the effect of artificial HI was investigated in a hypothesis‐driven metabolomics approach. It remains to be confirmed if naturally occurring HI has similar metabolic impact. Several metabolites involved in inflammatory processes and vascular dysfunction, potentially involved in the pathogenesis of ID or laminitis, were identified. However, because laminitis was not induced during the study, additional experiments on larger cohorts are warranted.
5. CONCLUSION
In our study, the response of horses to OGT was described on the metabolomic level. Results from previous experiments in horses were confirmed, but several new potential biomarkers for HI also were identified. Metabolites linked to β‐oxidation (eg, acetylcarnitine, carnitine) were strongly associated with total insulin response. In addition, signs of a low‐grade inflammatory response to the OGT (increased kynurenine : tryptophan) and potential vascular impairment associated with ID (decreased Arg and spermidine concentrations) were found. Oral supplementation of carnitine and Arg already have been used successfully against metabolic disorders in several species and could be investigated as potential therapeutic targets. Although confirmatory studies still are required, our results may aid in development of a point‐of‐care device to identify hyperinsulinemic horses using a single unstimulated blood sample.
6. CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The State Office for Consumer Protection and Food Safety (LAVES) approved the study in accordance with the German Animal Welfare Law (file number: 33.19‐42 502‐05‐17A099).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Table listing the VIP scores of each metabolite in the full baseline PLS‐DA model. In the reduced PLS‐DA models, the metabolites were removed by order of increasing VIP score (eg, the reduced model with two metabolites included only C2 and SDMA as predictors).
Appendix S1 Detailed description of the methods used for statistical analysis.
Appendix S2 Supporting Information.
ACKNOWLEDGMENTS
No funding was received for this study. The authors thank Professor Wolfgang Leibold for his support and providing the horses, and Dr Björn Steinbjörnsson for his help during the experiments and dedicated care to the horses.
Delarocque J, Frers F, Feige K, Huber K, Jung K, Warnken T. Metabolic changes induced by oral glucose tests in horses and their diagnostic use. J Vet Intern Med. 2021;35:597–605. 10.1111/jvim.15992
REFERENCES
- 1. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ, American College of Veterinary Internal Medicine . Equine metabolic syndrome. J Vet Intern Med. 2010;24(3):467‐475. [DOI] [PubMed] [Google Scholar]
- 2. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 3. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33(2):335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174(3):530‐535. [DOI] [PubMed] [Google Scholar]
- 5. de Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42(2):129‐135. [DOI] [PubMed] [Google Scholar]
- 6. Meier AD, de Laat MA, Reiche DB, et al. The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates. Domest Anim Endocrinol. 2018;63(November):1‐9. [DOI] [PubMed] [Google Scholar]
- 7. Waller APP, Huettner L, Kohler K, Lacombe VAA. Novel link between inflammation and impaired glucose transport during equine insulin resistance. Vet Immunol Immunopathol. 2012;149(3–4):208‐215. [DOI] [PubMed] [Google Scholar]
- 8. Treiber K, Carter R, Gay L, Williams C, Geor R. Inflammatory and redox status of ponies with a history of pasture‐associated laminitis. Vet Immunol Immunopathol. 2009;129(3–4):216‐220. [DOI] [PubMed] [Google Scholar]
- 9. Kenéz À, Warnken T, Feige K, Huber K. Lower plasma trans‐4‐hydroxyproline and methionine sulfoxide levels are associated with insulin dysregulation in horses. BMC Vet Res. 2018;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49(5):570‐576. [DOI] [PubMed] [Google Scholar]
- 11. Fitzgerald DM, Walsh DM, Sillence MN, Pollitt CC, de Laat MA. Insulin and incretin responses to grazing in insulin‐dysregulated and healthy ponies. J Vet Intern Med. 2018;33(1):225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suagee JK, Corl BA, Crisman MV, Hulver MW, McCutcheon LJ, Geor RJ. Effects of acute hyperinsulinemia on inflammatory proteins in horses. Vet Immunol Immunopathol. 2011;142(3–4):141‐146. [DOI] [PubMed] [Google Scholar]
- 13. Banse HE, Frank N, Kwong GPS, McFarlane D. Relationship of oxidative stress in skeletal muscle with obesity and obesity‐associated hyperinsulinemia in horses. Can J Vet Res. 2015;79(4):329‐338. [PMC free article] [PubMed] [Google Scholar]
- 14. Vick MM, Adams AA, Murphy BA, et al. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007;85(5):1144‐1155. [DOI] [PubMed] [Google Scholar]
- 15. Holbrook TC, Tipton T, McFarlane D. Neutrophil and cytokine dysregulation in hyperinsulinemic obese horses. Vet Immunol Immunopathol. 2012;145(1–2):283‐289. [DOI] [PubMed] [Google Scholar]
- 16. Kenéz À, Dänicke S, Rolle‐Kampczyk U, von Bergen M, Huber K. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics. 2016;12(11):1‐11. [Google Scholar]
- 17. Jacob SI, Murray KJ, Rendahl AK, Geor RJ, Schultz NE, McCue ME. Metabolic perturbations in Welsh Ponies with insulin dysregulation, obesity, and laminitis. J Vet Intern Med. 2018;32(3):1215‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frank N, Andrews F, Durham A, et al. Recommendations for the diagnosis and treatment of pituitary pars intermedia dysfunction (PPID). 2015.
- 19. Öberg J, Bröjer J, Wattle O, Lilliehöök I. Evaluation of an equine‐optimized enzyme‐linked immunosorbent assay for serum insulin measurement and stability study of equine serum insulin. Comp Clin Path. 2011;21(6):1291‐1300. [Google Scholar]
- 20. Pallares‐Méndez R, Aguilar‐Salinas CA, Cruz‐Bautista I, Del Bosque‐Plata L. Metabolomics in diabetes, a review. Ann Med. 2016;48(1‐2):89‐102. [DOI] [PubMed] [Google Scholar]
- 21. Haucke V, Di Paolo G. Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol. 2007;19(4):426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klein MS, Buttchereit N, Miemczyk SP, et al. NMR metabolomic analysis of dairy cows reveals milk glycerophosphocholine to phosphocholine ratio as prognostic biomarker for risk of ketosis. J Proteome Res. 2012;11(2):1373‐1381. [DOI] [PubMed] [Google Scholar]
- 24. Ding M, Rexrode KM. A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Metabolites. 2020;10(4):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolstad BM, Irizarry R, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289‐300. [Google Scholar]
- 27. Sanchez G. DiscriMiner: Tools of the Trade for Discriminant Analysis. 2013.
- 28. Muno JD. Prevalence, Risk Factors and Seasonality of Plasma Insulin Concentrations in Normal Horses in Central Ohio. Columbus, Ohio: The Ohio State University; 2009. [Google Scholar]
- 29. Pleasant RS, Suagee JK, Thatcher CD, Elvinger F, Geor RJ. Adiposity, plasma insulin, leptin, lipids, and oxidative stress in mature light breed horses. J Vet Intern Med. 2013;27(3):576‐582. [DOI] [PubMed] [Google Scholar]
- 30. Morgan RA, McGowan TW, Mcgowan CM. Prevalence and risk factors for hyperinsulinaemia in ponies in Queensland, Australia. Aust Vet J. 2014;92(4):101‐106. [DOI] [PubMed] [Google Scholar]
- 31. Ho JE, Larson MG, Vasan RS, et al. Metabolite profiles during oral glucose challenge. Diabetes. 2013;62(8):2689‐2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4(214):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mangge H, Summers KL, Meinitzer A, et al. Obesity‐related dysregulation of the Tryptophan‐Kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity. 2014;22(1):195‐201. [DOI] [PubMed] [Google Scholar]
- 34. Millington WR, Dybdal NO, Dawson R, Manzini C, Mueller GP. Equine Cushing's disease: differential regulation of β‐endorphin processing in tumors of the intermediate pituitary. Endocrinology. 1988;123(3):1598‐1604. [DOI] [PubMed] [Google Scholar]
- 35. Lipman IJ, Boykin ME, Flora RE. Glucose intolerance in Parkinson's disease. J Chronic Dis. 1974;27:573‐579. [DOI] [PubMed] [Google Scholar]
- 36. Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol Med. 2013;19(3):176‐186. [DOI] [PubMed] [Google Scholar]
- 37. Lundquist I, Panagiotidis G, Stenstrom A. Effect of L‐DOPA administration on islet monoamine oxidase activity and glucose‐induced insulin release in the mouse. Pancreas. 1991;6(5):522‐527. [DOI] [PubMed] [Google Scholar]
- 38. Boyd AE, Lebovitz HE, Feldman JM. Endocrine function and glucose metabolism in patients with Parkinson's disease and their alteration by L‐dopa. J Clin Endocrinol Metab. 1971;33(5):829‐837. [DOI] [PubMed] [Google Scholar]
- 39. Xie B, Waters MJ, Schirra HJ. Investigating potential mechanisms of obesity by metabolomics. J Biomed Biotechnol. 2012;2012:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seiler SE, Martin OJ, Noland RC, et al. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J Lipid Res. 2014;55(4):635‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Weyenberg S, Buyse J, Janssens GPJ. Increased plasma leptin through l‐carnitine supplementation is associated with an enhanced glucose tolerance in healthy ponies. J Anim Physiol Anim Nutr. 2009;93(2):203‐208. [DOI] [PubMed] [Google Scholar]
- 42. Morgan R, Keen J, McGowan C. Equine metabolic syndrome. Vet Rec. 2015;177(7):173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKnight JR, Satterfield MC, Jobgen WS, et al. Beneficial effects of L‐arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39(2):349‐357. [DOI] [PubMed] [Google Scholar]
- 44. Bode‐Böger SM. Effect of L‐arginine supplementation on NO production in man. Eur J Clin Pharmacol. 2006;62(Suppl. 13):91‐99.16344921 [Google Scholar]
- 45. Morgan RA, Keen JA, Walker BR, Hadoke PWF. Vascular dysfunction in horses with endocrinopathic laminitis. PLoS One. 2016;11(9):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20(9):2032‐2037. [DOI] [PubMed] [Google Scholar]
- 47. Dühlmeier R, Deegen E, Fuhrmann H, et al. Glucose‐dependent insulinotropic polypeptide (GIP) and the enteroinsular axis in equines (Equus caballus). Comp Biochem Physiol A Mol Integr Physiol. 2001;129(2‐3):563‐575. [DOI] [PubMed] [Google Scholar]
- 48. Lerner RL, Porte D. Relationships between intravenous glucose loads, insulin responses and glucose disappearance rate. J Clin Endocrinol Metab. 1971;33(3):409‐417. [DOI] [PubMed] [Google Scholar]
- 49. Dincer C, Bruch R, Kling A, Dittrich PS, Urban GA. Multiplexed point‐of‐care testing – xPOCT. Trends Biotechnol. 2017;35(8):728‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ritchie Matthew E., Phipson Belinda, Wu Di, Hu Yifang, Law Charity W., Shi Wei, Smyth Gordon K.. limma powers differential expression analyses for RNA‐sequencing and microarray studies. Nucleic Acids Research. 2015;43 (7):e47–e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Table listing the VIP scores of each metabolite in the full baseline PLS‐DA model. In the reduced PLS‐DA models, the metabolites were removed by order of increasing VIP score (eg, the reduced model with two metabolites included only C2 and SDMA as predictors).
Appendix S1 Detailed description of the methods used for statistical analysis.
Appendix S2 Supporting Information.


