Abstract
Up to 85% of adult cancer survivors and 99% of adult survivors of childhood cancer live with an accumulation of chronic conditions, frailty, and/or cognitive impairments resulting from cancer and its treatment. Thus, survivors often show an accelerated development of multiple geriatric syndromes and need therapeutic interventions. To advance progress in this area, the National Cancer Institute convened the second of 2 think tanks under the auspices of the Cancer and Accelerated Aging: Advancing Research for Healthy Survivors initiative. Experts assembled to share evidence of promising strategies to prevent, slow, or reverse the aging consequences of cancer and its treatment. The meeting identified research and resource needs, including geroscience-guided clinical trials; comprehensive assessments of functional, cognitive, and psychosocial vulnerabilities to assess and predict age-related outcomes; preclinical and clinical research to determine the optimal dosing for behavioral (eg, diet, exercise) and pharmacologic (eg, senolytic) therapies; health-care delivery research to evaluate the efficacy of integrated cancer care delivery models; optimization of intervention implementation, delivery, and uptake; and patient and provider education on cancer and treatment-related late and long-term adverse effects. Addressing these needs will expand knowledge of aging-related consequences of cancer and cancer treatment and inform strategies to promote healthy aging of cancer survivors.
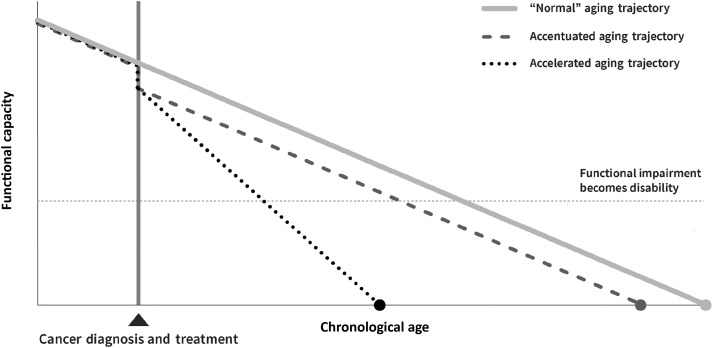
The rapidly aging US population coupled with improved cancer survival rates has led to predictions of unprecedented growth in the number of cancer survivors over the next decade (1–3). Unfortunately, many modalities used to cure or control cancer damage healthy tissue, leading to unintended consequences that appear to accelerate (eg, altered aging trajectory with a faster rate of functional decline) or accentuate the aging process (eg, paralleled “normal” aging trajectory with weakened reserve) (Figure 1) (4). Clinical observations supported by phenotypic, genomic, and molecular data (4–13) suggest that cancer survivors treated with adjuvant therapies are at risk for early onset of multimorbidity commonly seen in older patients. Estimates indicate that up to 85% of adult cancer survivors (14) and 99% of adult survivors of childhood cancer (15) live with cancer- and treatment-related comorbidities, including frailty, sarcopenia, cognitive impairment, and/or subsequent neoplasms (8,15–22). Adult cancer survivors report engaging in healthy behaviors at levels similar to adults with no history of cancer (23) and are more likely to adhere to physical activity recommendations (24). However, there are limited data on how physical activity and other strategies mitigate age-related conditions for cancer survivors.
Figure 1.
Hypothesized trajectories of the aging consequences of cancer and cancer treatment (used with permission) (4).
Strategies to Prevent or Mitigate Cancer- and Treatment-Associated Aging
Aging involves multifaceted, interdependent biological processes that can be altered by cancer and its treatments (25,26). The Geroscience Hypothesis postulates that many age-related conditions can be slowed or delayed by targeting drivers, or hallmarks, of aging (eg, genomic instability, stem cell exhaustion, cellular senescence, inflammation, mitochondrial dysfunction, and epigenetic alterations) (26–28). Given the complementarity of hallmarks that undergird aging, cancer, and cancer treatments (26,27,29), geroscience-guided interventions might delay or avert the age-related conditions observed in cancer survivors (30).
To consider emerging strategies that might prevent, mitigate, or reverse cancer- and treatment-related aging consequences, the National Cancer Institute (NCI) convened the second of two think tanks (4) under the Cancer and Accelerated Aging: Advancing Research for Healthy Survivors initiative. The think tank considered strategies that have demonstrated efficacy in clinical trials or showed preclinical promise to avert or alleviate age-related outcomes. Emphasis was placed on therapies linked to age-related conditions or underlying aging processes (hallmarks of aging) that could be potential targets for interventions. This report summarizes strategies identified during the think tank that have the potential to address the long-term effects of cancer and cancer treatment, and highlights novel opportunities to establish efficacy and expand the evidence base.
Exercise Therapy Strategies
Exercise is a relatively safe, cost-effective treatment strategy with demonstrated efficacy to reduce morbidity and mortality and preserve functional capacity (31–36). Robust evidence now indicates that routine physical activity attenuates many hallmarks of aging (37). In mice, modest exercise can suppress certain cellular senescent phenotypes, even in animals fed a high-fat diet, which accelerates many age-related pathologies (38). However, exercise is not currently considered a standard of care therapy for cancer survivors. In 2018, the Physical Activity Guidelines Advisory Committee and American College of Sports Medicine published separate reports endorsing exercise to improve cancer-related health outcomes (39–42). Given the pleiotropic nature of exercise, there is ample opportunity to ameliorate aging phenotypes (25) by initiating exercise therapy across the cancer diagnosis, treatment, and survivorship continuum.
Exercise Prehabilitation
“Window of opportunity” trials are used to test new drugs during the “window” between a cancer diagnosis and initiation of standard treatment (43,44). This trial design can be used to evaluate “prehabilitation,” or short-term exercise or nutrition therapy before cancer surgery or receipt of adjuvant treatment, to evaluate the impact on aging outcomes (45,46). Initial evidence indicates that prehabilitation may modulate tumor biology and can improve physiological function (eg, cardiorespiratory fitness), lower postoperative complications, and support recovery following therapy (47–50). During preoperative chemotherapy (51), patients with pancreatic cancer participating in a pilot exercise intervention exhibited statistically significant tumor vascular remodeling compared with controls (52). Randomization of 40 patients with lung cancer undergoing lobectomy to aerobic exercise or usual care for 3 weeks showed that exercise improved cardiorespiratory fitness by 17% compared with the usual care group (48). A meta-analysis investigating the effects of preoperative exercise therapy in patients with lung cancer showed that compared with usual care, exercise decreased hospital stay and reduced the risk of postoperative complications (49). Additional evidence is needed to establish efficacy for different exercise types, timing relative to treatment initiation, and dosing in other cancers and pretreatment contexts (40–42).
Exercise During Cancer Therapy
Growing evidence suggests that exercise during chemotherapy may prevent functional decline by protecting cardiovascular health and maintaining the integrity and function of lean muscle mass (53–55). A meta-analysis of randomized controlled trials (RCTs) revealed that exercise during and after treatment improved cardiorespiratory fitness compared with usual care in patients with adult-onset cancers (53). Exercise during treatment may also indirectly affect the aging consequences of cancer and cancer treatment by remodeling the tumor vasculature and improving chemotherapy delivery and efficacy (53). In animal models, exercise modifies the tumor vasculature in prostate (56,57), breast (58,59), pancreatic (60), melanoma (60), and Ewing sarcoma tumors (61). For example, in murine Ewing sarcoma, moderate aerobic exercise 5 d/wk led to statistically significantly more delivery of doxorubicin to the tumor, but not other organs, suggesting that exercise can increase chemotherapy efficacy without increasing toxicity to healthy tissue (61).
Exercise may improve aging outcomes in combination with other strategies. In the Exercise for Cancer Patients trial, nonmetastatic cancer patients receiving chemotherapy plus a 6-week exercise intervention demonstrated improved perceived cognitive function and less inflammation compared with patients receiving chemotherapy alone (62,63). A different Exercise for Cancer Patients intervention trial is currently evaluating aerobic and resistance exercise with or without low-dose ibuprofen on cancer-related cognitive impairment (CRCI) during chemotherapy in breast, gastrointestinal, and colorectal cancer survivors (NCT01238120). Further research is needed to understand the mechanisms that underlie the beneficial effects of exercise on health during treatment.
Studies have demonstrated that exercise is feasible and provides positive benefits in older patients receiving chemotherapy and those with existing age-related conditions (64,65). A clinical framework exists to provide tailored exercise prescriptions for breast cancer patients with complex health profiles undergoing chemotherapy (eg, comorbidities, adverse treatment effects, exercise restrictions) (66). Although refinement is needed to address personal and environmental factors (eg, pain, fatigue, patient preference, attainability, cost), the framework provides a useful foundation to address the inherent complexity of personalized cancer care.
Exercise Postcancer Therapy
Several observational studies indicate that exercise after first-line therapy lowers the long-term risk of morbidity, including cardiovascular disease (CVD) and CRCI (67) and mortality (53,68–70). Given that cancer survivors have a higher risk of death due to CVD than the general population (71), such findings further highlight the importance of exercise. For instance, compared with self-reported nonadherence to national exercise guidelines (∼9 metabolic equivalent of task-h/wk) (72), adherence was associated with a 23% (73) and 51% (69) lower risk of cardiovascular events among survivors of breast cancer and Hodgkin lymphoma, respectively. Moreover, in 15 450 adult survivors of childhood cancer (median follow-up of 10 years), exercising at least 3 metabolic equivalent of task-h/wk was associated with a 19% (P = .026) and 11% (P = .17) reduction in all-cause and health-related mortality, respectively, and a 39% (P = .026) reduction in recurrence or progression (70). Together, findings from observational studies indicate that exercise may mitigate treatment-related morbidity and mortality. Prospective trials are needed to determine the direct exercise-induced effects. Evidence from RCTs indicate that exercise improves cardiorespiratory fitness posttherapy (53); however, there is insufficient evidence regarding other markers of cardiovascular health (eg, blood pressure, insulin sensitivity) (47).
Diet and Nutrition Strategies
Diet and nutrition have been shown to influence cancer risk, progression, and treatment response through shared aging pathways (74). Suboptimal nutrition, as well as overnutrition, detrimentally affects metabolic function and changes aging processes by altering adipokine regulation, interfering with normal immune function, and promoting systemic inflammation, insulin resistance, and dysbiosis (75–78). Decades of nonprimate animal studies suggest that caloric restriction delays tumor progression and prolongs overall lifespan (79). Rodent models show that different regimens of caloric restriction (eg, intermittent fasting) can slow cancer, CVD, diabetes, and neurodegenerative disorders (80–82). Long-term human studies suggest that caloric restriction can slow biological aging (83). Thus, diet and nutrition may alter both aging and cancer outcomes. However, because sarcopenia and sarcopenic obesity can cooccur with cancer and cancer treatment, there is concern about how diet and nutritional approaches are recommended for cancer patients and survivors (84–86). Moreover, less is known about the relationship between diet and nutrition, aging outcomes, and the long-term effects of cancer and cancer treatment. In the following section, we discuss diet and nutrition strategies linked to age-related outcomes in a cancer context.
Diet and Nutrition Prehabilitation
Modulating diet before cancer therapy may reduce treatment toxicity and improve survival (87–91). Preclinical studies indicate that fasting decreases chemotherapy toxicity (92) and sensitizes a wide variety of cancer cell types to chemotherapy, radiotherapy, kinase inhibitors, and metabolic drugs without affecting healthy cells (93–101). For instance, mice fasted for 48 hours before treatment with cisplatin and doxorubicin survived longer than mice given a standard diet (102). To date, a few small human trials have shown that fasting diets are safe, reduce toxicity, and increase the efficacy of anticancer therapies (89,91). In a randomized crossover trial of 34 patients with gynecological cancer, patients who fasted for 36 hours before and 24 hours after three chemotherapy treatments (350 kcal maximum daily calorie intake) demonstrated better chemotherapy tolerance, higher quality of life (QOL), and less fatigue than patients on a eucaloric Mediterranean diet (103). A prehabilitation exercise and nutrition optimization (dietary assessment, whey protein supplementation, nutrition therapy) RCT in patients with gastric cancer showed improved function before and after surgery compared with controls (104). In ancillary analyses of the Women's Health Initiative trial (n = 48 835 postmenopausal women without breast cancer from 1993 to 1998), participants assigned to the dietary modification arm (ie, reduced-fat diet with increased fruit, vegetable, and whole-grain consumption) experienced lower mortality after breast cancer (88) and increased overall survival compared with the usual diet arm (87). In two window-of-opportunity trials, one conducted in 40 men with prostate cancer and another in 32 women with breast cancer, presurgical caloric restriction was found to have no impact on Ki67 tumor proliferation rates in breast tumors (105), whereas in prostate cancer, a weight loss of roughly 0.65 kg/wk increased (rather than decreased) tumor proliferation compared with a weight loss of approximately 0.34 kg/wk (106). Larger trials are needed to determine the impact of pretreatment diet and nutrition on reducing chemotherapy toxicity and long-term outcomes (90,107,108).
Diet and Nutrition Strategies During Therapy
Studies of whey protein supplementation have recently gained attention as a strategy to improve health (109), including physical performance in frail older adults (110–112) and cancer patients (112–116). Trials of colorectal, lung, and advanced cancer patients reported that whey protein supplementation improves lean body mass, sarcopenia, muscle strength, and functional capacity and prevents chemotherapy toxicity (113–115). Currently, an RCT is examining a multimodal program that includes whey protein supplementation, exercise, and psychological treatment during neoadjuvant chemotherapy on several outcomes, including postoperative morbidity, disease-free survival, overall survival, and functional reserve in patients with esophageal and gastric cancers (117). However, high-protein diets increase insulin-like growth factor 1 (IGF-1) levels in both mice and humans, and mouse studies indicate that high-protein intake and elevated IGF-1 sensitize normal cells to chemotherapy toxicity and enhance the progression of different tumor types (118). Thus, the effects of protein supplementation on lean body mass and sarcopenia must be weighed against the well-established role of proteins in increasing IGF-1 and other progrowth signaling pathways, which could increase tumor growth and inhibit apoptosis in cancer cells.
Diet and Nutrition Strategies Postcancer Therapy
Observations regarding the relationship between posttreatment diet and nutrition and survival suggest that higher dietary intake of isoflavone, the major phytoestrogen in soy, correlates with a reduced risk of all-cause mortality in breast cancer survivors (119). Adherence to Mediterranean and Nordic diets posttreatment correlates with better overall survival among long-term colorectal cancer survivors (120). Next-generation diet and nutrition studies will likely be multi-component, exploring different dietary patterns among patients and survivors. The Reach-Out to Enhance Wellness trial was a two-arm, wait-list controlled, single-blinded, cross-over study conducted in 641 older, overweight or obese, long-term survivors of breast, prostate, or colorectal cancer. The intervention showed statistically significantly improved diet quality, physical activity, weight loss, and the trajectory of functional decline (121,122). At present, the breast cancer weight loss trial, a phase III trial of women recently diagnosed with stage II-III, HER2-negative breast cancer with a body mass index of at least 27 kg/m2, is evaluating the impact of weight loss after cancer diagnosis through caloric restriction and exercise on the risk of cancer recurrence and mortality. If effective, this intervention has the potential to make weight loss programs a standard part of breast cancer treatment (36,123). Future studies should include aging outcomes and consider multi-behavior approaches to address the complex disease profiles and needs of cancer survivors, who often have differing levels of baseline health, health behaviors, and function (124).
Opportunities to Expand the Evidence Base
The think tank aimed to discuss modalities that could be implemented in clinical settings in the near term. Further, we highlight promising areas that, in the longer term, will provide a better understanding of aging outcomes in cancer survivors and the mechanisms that contribute to cancer- and treatment-associated aging to guide the development of novel interventions. Several opportunities to expand the evidence base were noted (Boxes 1-3).
Considerations for Preclinical Research
Accelerated-aging mouse models can elucidate processes that drive aging phenotypes and provide opportunities for rapidly testing novel interventions (125,126). Studying cancer in aged rodents to model the aging consequences of cancer and treatment was discussed (Box 1). Additionally, animals other than mice may more closely mimic human aging or provide insights into mammalian aging processes that are more relevant to humans. For example, the bat has evolved transcriptomic signatures known to promote longevity, and its lifespan is longer than other mammals (127).
Box 1.
Opportunities for preclinical research to expand the evidence base for intervention studies targeting the aging consequences of cancer and cancer treatment
Explore alternative animal models that may more accurately model cancer and therapy-induced aging in humans (eg, bat, rat, pig, and dog models)
Explore new experimental systems that allow modeling and compound testing in human tissues, including human tissue explants and multi-cell organoids, microfluidic devices, and bioengineered platforms
Determine the appropriate time point(s) during the postcancer treatment aging trajectory to administer senolytic drugs
Develop models to predict patient-specific toxicity to senolytic agents (ie, potential for cytokine storm if senescent cell burden is inordinately high)
Explore the molecular mechanisms governing different exercise outcomes desired (ie, maintenance of muscle mass, vascular remodeling, or cardiovascular protection)
Identify biomarkers to assess when sufficient exercise has been performed to achieve the desired outcome(s)
Explore the impact of calorie restriction and diets high in fruits and vegetables on cancer biomarkers and cancer treatment outcomes
Evaluate caloric restriction, fasting-mimicking diets, and diet quality as strategies to reduce treatment toxicity, reverse anticancer therapy resistance, and increase cancer-free survival with conventional and newer targeted cancer therapies
Conduct basic and mechanistic research to discern how diet and physical activity affect both cancer and aging to determine optimal dose, intensity (rate), mode, and duration
Conduct preclinical and clinical studies to substantiate biomarkers that identify those at risk for treatment intolerance and accelerated aging as a consequence of cancer treatment (eg, senescent cell burden as measured by CD3+ T cell p16INK4a expression)
Considerations for Clinical Research
Therapies Targeting Senescence
Although several age-related processes provide potential targets for interventions, meeting discussions focused on cellular senescence. Cellular senescence is a cell fate that includes an irreversible proliferative arrest (128–130). Senescent cells accumulate in multiple tissues, and, interestingly, transplanting small numbers of senescent cells into young animals induces frailty and age-related disease (131,132). Senescent cells also develop a proinflammatory senescence-associated secretory phenotype that can disrupt tissue and immune function and create a permissive microenvironment for cancer growth (133).
Senescent cells are a promising target for aging interventions because these cells do not divide and can be eliminated by intermittent dosing using drugs with short half-lives (128,131,134–138). The senescence-associated secretory phenotype is also modifiable: it can be up- or down-regulated by hormones, pathogens, and drugs (136,139–141). Rapamycin, a mammalian target of rapamycin inhibitor, is a promising agent that has been implicated in both aging and senescence. Rapamycin fed to older mice was shown to delay aging and extend lifespan (142). Preclinical studies have also shown that rapamycin prevents cognitive decline, protects from skeletal muscle decline (143), and counteracts age-related functional decline in multiple tissues (143,144). In healthy older adults, a pilot RCT showed that short-term rapamycin treatment was feasible and safe (145).
Senolytics have also achieved success in recent preclinical studies (131,146–148); notably, several senolytics are repurposed cancer drugs (138,149). The first trial in humans, a pilot, open-label study of dasatinib plus quercetin for idiopathic pulmonary fibrosis, a progressive, fatal, senescence-driven disease, was recently published (147). After 9 doses over 3 weeks, participants showed improved physical function 1 week later. If shown to be safe and effective in larger trials, the hope is that mammalian target of rapamycin inhibitors and senolytics can be tested as preventatives of age-related conditions in cancer populations. Research is needed to determine the safety and efficacy of dosing intervals and systemic, as opposed to local, administration (131).
Integrative Strategies
Cancer survivors experience greater psychosocial distress, depression, and anxiety and worse QOL compared with the general population (14,15,21). A range of integrative strategies has been evaluated in recent years to improve QOL and reduce treatment effects, including imagery (150,151), yoga, meditation, mindfulness and similar approaches (151–155), music therapy (156), early palliative care (157), cognitive behavioral therapy (158), cognitive rehabilitation (159–161), transcranial direct current stimulation (162,163), and psychotherapy (159,164). Most of these interventions have not been studied with aging endpoints in mind. Yoga and meditation are associated with slowed cellular senescence, as measured by DNA damage markers, reactive oxygen species, interleukin-6, and telomere length (165,166), and are associated with improved sleep and cognitive function in patients with CRCI (167–170). Early palliative care correlates with reduced mortality in lung cancer survivors (157). Other strategies should be explored in relation to aging endpoints and biological aging drivers.
Models of Clinical Research
Clinical trials guided by geroscience principles hold promise to prevent, slow, or reverse the aging consequences of cancer and its treatments by targeting multiple, interrelated aging processes. The Targeting Aging with Metformin trial is a blueprint for such trials, because this drug impacts multiple hallmarks of aging and age-related disease outcomes. Additionally, this trial will assess a consensus-based set of biomarkers associated with aging (28). Intervention studies of aging outcomes (eg, frailty, cognitive decline, comorbidities, death) need to use biomarkers as intermediate endpoints to demonstrate modification of underlying aging processes before sufficient accumulation of clinical events, which may take years (171,172). Further, some biomarkers, such as DNA methylation, may help identify or evaluate promising anti-accelerated or anti-accentuated aging interventions because epigenetic changes are plastic (173,174). Although no standard set of aging biomarkers yet exists, those measured in blood or other easily accessed biofluids (eg, urine, saliva) are of particular interest because they can be measured in large observational studies and clinical trials (76). The first meeting of the Cancer and Accelerated Aging: Advancing Research for Healthy Survivors initiative highlighted promising biomeasures for studies on cancer and aging, including DNA methylation- and physiology-based measures (4). Lifestyle factors, including smoking, alcohol use, and sedentary behavior, should be explored as effect modifiers in clinical studies, because they have been shown to contribute to an aging phenotype in cancer survivors (5,11,175–177) and can accelerate epigenetic aging (178,179).
Considerations for Intervention Study Design
Measurement
Understanding the molecular mechanisms governing different desired outcomes (eg, maintenance of muscle mass, cardiovascular reserve, gait speed, cognitive function) and the therapies needed to achieve each outcome may help identify biomarkers that can be used to benchmark progress. Experts also discussed the need to characterize the specific cognitive processes affected by cancer and its treatments to improve assessment of subtle domain-specific cognitive changes because traditional neuropsychological measures were not designed to identify subtle deficits in cognitive function (18,19). Measures based on improved cognitive neuroscience and neuroimaging techniques (180) hold promise to detect specific cognitive processes affected, including those that occur outside conscious awareness. With an increased understanding of the mechanisms underlying cognitive deficits in cancer patients, several ongoing clinical trials are now testing pharmacotherapeutic strategies that target mechanisms linked to aging processes, including increased oxidative stress and depleted stem cell reserves (181,182). Further research defining both the biological mechanisms and cognitive processes affected is critical to develop targeted cognitive interventions.
Intervention Design and Delivery
Experts discussed the need for optimal dosing of exercise and nutritional modification to maximize patient benefit and minimize toxicities. Exercise interventions should be designed to clarify which mode of exercise is most effective and sustainable for improving or recovering functional reserve in subgroups of patients, at what timepoint, and at what frequency, intensity, and duration (183). Diet and nutrition trials should consider nutritional status as well as cancer- and treatment-induced changes in nutrients and body composition (eg, lean mass) before determining the appropriate intervention. Given that multiple modalities may act synergistically, multicomponent interventions should be further explored to determine which combinations are most effective at preventing and reversing cancer- and treatment-associated effects for different types and stages of cancer. Studies of safety and adherence are also needed because insufficient reporting diminishes study rigor and can lead to erroneous conclusions about harm-to-benefit ratios (49,53). The geriatric assessment (GA) should be used to identify individuals with age-related conditions typically excluded from clinical trial participation (184).
Several challenges related to intervention delivery were discussed, including caregiver inclusion and burden, functional and sensory deficits, pain, fatigue, cumulative disease burden, and social determinants of health (eg, transportation issues, low health literacy, insurance status, education). Potential solutions include designing interventions that include caregivers and/or integrate social engagement into study protocols, improving usability of technology-driven interventions and activity monitoring, providing study participants with materials at the appropriate reading level, and using telemedicine.
Considerations for Health-Care Delivery
The projected growth in the number of cancer survivors coupled with clinician and caregiver shortages and a transition to value-based care present imminent challenges and opportunities (185,186). The aging consequences of cancer and cancer treatments must be addressed within a health-care delivery framework because the biobehavioral and psychosocial mechanisms that influence aging are inherently multi-system and multi-outcome in nature and, if left unabated, translate to more healthcare resources and higher costs.
Health-care delivery could be improved by employing innovative, integrated care models that address the complex needs of cancer survivors through screening tools, early interventions, coordinated care, and addressing the wider social determinants of health (180). Given the increasing prevalence of multimorbidity and risk of poor psychosocial well-being for cancer survivors, a screening tool that identifies health vulnerabilities associated with compromised aging trajectories could be implemented at diagnosis and collected longitudinally. Although there is considerable variability in the data collected, the GA provides a holistic evaluation of the physical, cognitive, affective, social, financial, environmental, and spiritual components that influence aging trajectories (187). The GA utilized by Hurria et al. demonstrated feasibility in clinical settings and is predictive of cancer treatment toxicity and survival (188,189). Such a tool could be implemented into routine clinical practice to identify baseline and emerging vulnerabilities and address them with appropriate prescriptions or referrals (187,190).
Referring survivors into evidence-based interventions based on current health needs and risk of cancer-related aging consequences should be explored to offer the “right care at the right time” (personalized medicine) (185,186). Prehabilitation interventions that include exercise, diet and nutrition, and mental health services could be used to prevent or reduce the risk of adverse events and treatment toxicity, facilitate recovery, and improve treatment tolerance (45,191). Prehabilitation interventions were shown to improve physical capacity (192–194), and reduce morbidity (195), complications (193), health-care costs (196), hospital length of stay (196,197), and readmissions (196). Some evidence suggests that combined prehabilitation and rehabilitation interventions improve gait speed and physical function better than prehabilitation alone (45). Larger prehabilitation or rehabilitation trials are needed to determine who benefits most (eg, which cancer types, treatments, and patient populations), whether intervention inequities occur, and if it is efficacious to promote behavior and self-management strategies during diagnosis and treatment when survivors may not be in an ideal psychological state (198). Self-management strategies, clinician training, and appropriate resources are needed to educate survivors about cancer- and treatment-associated aging throughout the care continuum (17,199).
Improved communication among specialties is needed to ensure seamless integration of care (199). Patient navigation programs will be essential to help survivors traverse fragmented care systems (16). Infrastructure is needed to improve communication between specialist teams and cancer survivors. Secure communication through online patient portals may be useful to share care plans, survey patient-reported outcomes, and provide links to eligible programs and services based on patient symptoms and needs. Improving these aspects of care will create a more patient-centric health-care system that may assuage late and long-term effects by identifying early symptoms and preventing progression to age-related outcomes.
With a higher number of cancer survivors living longer and aging into older adulthood, evidence-based strategies must be developed and implemented to prevent and mitigate the aging consequences of cancer and cancer treatment. This report summarized expert-informed deliberations of promising strategies to consider for implementation into clinical settings and highlights gaps in our understanding of approaches that avert or ameliorate age-related outcomes. Addressing these research gaps will facilitate the development of novel evidence-based strategies to enhance healthy aging for all cancer survivors.
Funding
This work was supported by the National Institutes of Health (R01CA172119, R01CA129769, U54CA137788, R01CA218496, R01CA129769, and P30CA008748 to TAA; R21/R33AG059206 to WD, SGM, and AH; DP2CA195765 and R01CA231014 to MCJ; R37AG013925 and R33AG061456 to JLK; P01AG062413 to JLK and LJN; P30AG021334, U01AG020478, U01AG020487, U01AG020480 to SNM; K24AG056589 to SGM; AG043376 and AG056278 LJN; R21CA218732 to KS; R21AG053198 and U01AG057545 to JAS; P30CA008748 to JMS; and R01AG052964 to RT), USDA (Agreement # 58–1950-0–014 and 58–805-9–004 to SNM), the American Cancer Society (CRP 14–111-01-CPPB to WDW), and AKTIV Against Cancer (JMS).
Notes
Role of the funder: Funds from the NCI were used to convene experts for the think tank that partially informed the manuscript’s scientific scope. NCI and National Institute on Aging staff had roles in the design of the think tank; contributed to the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. Moreover, the manuscript was reviewed and approved for submission through the NCI Division of Cancer Control and Population Sciences manuscript clearance process.
Disclosures: JLK has a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic, Buck Institute for Research on Aging, and Unity Biotechnology. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. VL has a financial interest and owns a company related to his research. JC is the scientific founder and shareholder in Unity Biotechnology. There are no other conflicts of interest to disclose.
Acknowledgments The authors dedicate this report in remembrance of Dr Arti Hurria (1970–2018), the founding chair of the NCI Cancer and Accelerated Aging Initiative and an international leader in the field of geriatric oncology. She was a cherished friend, mentor, and highly respected member of our community and we strive to bring her scientific vision to fruition and honor her legacy. We thank Kevin Howcroft and Felipe Sierra for their leadership and scientific contributions to the think tank. We thank Kirsten Ness for her leadership as Think Tank Chair.
References
- 1. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. Bethesda, MD: National Cancer Institute; 2019. https://seer.cancer.gov/csr/1975_2016/. Accessed November 2019. [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4. Guida JL, Ahles TA, Belsky D, et al. Measuring aging and identifying aging phenotypes in cancer survivors. J Natl Cancer Inst. 2019;111(12):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smitherman AB, Anderson C, Lund JL, et al. Frailty and comorbidities among survivors of adolescent and young adult cancer: a cross-sectional examination of a hospital-based survivorship cohort. J Adolesc Young Adult Oncol. 2018;7(3):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ness KK, Kirkland JL, Gramatges MM, et al. Premature physiologic aging as a paradigm for understanding increased risk of adverse health across the lifespan of survivors of childhood cancer. J Clin Oncol. 2018;36(21):2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67(2):212–215. [DOI] [PubMed] [Google Scholar]
- 8. Henderson TO, Ness KK, Cohen HJ.. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014;(34):e423–e430. [DOI] [PubMed] [Google Scholar]
- 9. Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2(5):e000250–e000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown JC, Harhay MO, Harhay MN.. The prognostic importance of frailty in cancer survivors. J Am Geriatr Soc. 2015;63(12):2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennett JA, Winters-Stone KM, Dobek J, et al. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40(3):E126–E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the bone marrow transplant survivor study. JAMA Oncol. 2016;2(10):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurria A, Jones L, Muss HB.. Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book. 2016;36:e516–e522. [DOI] [PubMed] [Google Scholar]
- 14. Dowling EC, Chawla N, Forsythe LP, et al. Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer. 2013;119(18):3393–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude lifetime cohort study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams J, Chen Y, Rubin P, et al. The biological basis of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):182–188. [DOI] [PubMed] [Google Scholar]
- 17. Ahles TA, Root JC.. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14(1):425–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Root JC, Andreotti C, Tsu L, et al. Learning and memory performance in breast cancer survivors 2 to 6 years post-treatment: the role of encoding versus forgetting. J Cancer Surviv. 2016;10(3):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Root JC, Ryan E, Barnett G, et al. Learning and memory performance in a cohort of clinically referred breast cancer survivors: the role of attention versus forgetting in patient-reported memory complaints. Psychooncology. 2015;24(5):548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ness KK, Armstrong GT, Kundu M, et al. Frailty in childhood cancer survivors. Cancer. 2015;121(10):1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan NF, Mant D, Carpenter L, et al. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105(S1):S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarleton HP, Ryan-Ibarra S, Induni M.. Chronic disease burden among cancer survivors in the California behavioral risk factor surveillance system, 2009-2010. J Cancer Surviv. 2014;8(3):448–459. [DOI] [PubMed] [Google Scholar]
- 23. Mayer DK, Terrin NC, Menon U, et al. Health behaviors in cancer survivors. Oncol Nurs Forum. 2007;34(3):643–651. [DOI] [PubMed] [Google Scholar]
- 24. Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–8893. [DOI] [PubMed] [Google Scholar]
- 25. Hill A, Sadda J, LaBarge MA, et al. How cancer therapeutics cause accelerated aging: insights from the hallmarks of aging. J Geriatr Oncol. 2020;11(2):169–179. [DOI] [PubMed] [Google Scholar]
- 26. Austad SN, The geroscience hypothesis: is it possible to change the rate of aging? In: Sierra F, Kohanski R, eds. Advances in Geroscience. Cham, Switzerland: Springer International Publishing; 2016:1–36. [Google Scholar]
- 27. Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Justice JN, Niedernhofer L, Robbins PD, et al. Development of clinical trials to extend healthy lifespan. Cardiovasc Endocrinol Metab. 2018;7(4):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fouad YA, Aanei C.. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 30. Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann NY Acad Sci. 2016;1386(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iyengar NM, Jones LW.. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 2019;5(11):1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daum CW, Cochrane SK, Fitzgerald JD, et al. Exercise interventions for preserving physical function among cancer survivors in middle to late life. J Frailty Aging. 2016;5(4):214–224. [DOI] [PubMed] [Google Scholar]
- 33. Naughton J, Lategola MT, Shanbour K.. A physical rehabilitation program for cardiac patients: a progress report. Am J Med Sci. 1966;252(5):545–553. [PubMed] [Google Scholar]
- 34. Bethell HJ. Cardiac rehabilitation: from Hellerstein to the millennium. Int J Clin Pract. 2000;54(2):92–97. [PubMed] [Google Scholar]
- 35. Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown JC, Ligibel JA.. Putting exercise into oncology practice: state-of-the-science, innovation, and future directions. Cancer J. 2019;25(5):316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18(1):57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schafer MJ, White TA, Evans G, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report Washington, DC: U.S. Department of Health and Human Services; 2018.
- 40. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalinsky K, Hershman DL.. Cracking open window of opportunity trials. J Clin Oncol. 2012;30(21):2573–2575. [DOI] [PubMed] [Google Scholar]
- 44. Schmitz S, Caballero C, Locati LD.. Perspectives on window of opportunity trials in head and neck cancer: lessons from the EORTC 90111-24111-NOCI-HNCG study. Eur J Cancer. 2018;104:219–223. [DOI] [PubMed] [Google Scholar]
- 45. Silver JK, Baima J.. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–727. [DOI] [PubMed] [Google Scholar]
- 46. Koelwyn GJ, Jones LW.. Exercise as a candidate antitumor strategy: a window into the future. Clin Cancer Res. 2019;25(17):5179–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scott JM, Nilsen TS, Gupta D, et al. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137(11):1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. 2013;44(4):e260–e265. [DOI] [PubMed] [Google Scholar]
- 49. Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–497. [DOI] [PubMed] [Google Scholar]
- 50. Åsgård R, Rytter E, Basu S, et al. High intake of fruit and vegetables is related to low oxidative stress and inflammation in a group of patients with type 2 diabetes. Scand J Food Nutr. 2007;51(4):149–158. [Google Scholar]
- 51. Ngo-Huang A, Parker NH, Wang X, et al. Home-based exercise during preoperative therapy for pancreatic cancer. Langenbecks Arch Surg. 2017;402(8):1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Florez Bedoya CA, Cardoso ACF, Parker N, et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep. 2019;9(1):13966–13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Focht BC, Clinton SK, Devor ST, et al. Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol. 2013;11(2):45–60. [PubMed] [Google Scholar]
- 55. Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCullough DJ, Stabley JN, Siemann DW, et al. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCullough DJ, Nguyen L-D, Siemann DW, et al. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol. 2013;115(12):1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Betof AS, Lascola CD, Weitzel DH, et al. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107(5):djv040–djv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Faustino-Rocha AI, Silva A, Gabriel J, et al. Long-term exercise training as a modulator of mammary cancer vascularization. Biomed Pharmacother. 2016;81:273–280. [DOI] [PubMed] [Google Scholar]
- 60. Schadler KL, Thomas NJ, Galie PA, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–65440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morrell M, Alvarez Florez C, Zhang A, et al. Vascular modulation through exercise improves chemotherapy efficacy in Ewing sarcoma. Pediatr Blood Cancer. 2019;66(9):e27835–e27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mustian KM, Janelsins MC, Peppone LJ, et al. EXCAP exercise effects on cognitive impairment and inflammation: a URCC NCORP RCT in 479 cancer patients. J Clin Oncol. 2015;33(15_suppl):9504–9504. [Google Scholar]
- 63. Kamen CS, Janelsins MC, Heffner K, et al. Standardized, progressive exercise program (EXCAP) to reduce psychological distress and improve inflammatory cytokines of distress among prostate cancer survivors. J Clin Oncol. 2014;32(15_suppl):9510–9510. [Google Scholar]
- 64. Loh KP, Kleckner IR, Lin PJ, et al. Effects of a home-based exercise program on anxiety and mood disturbances in older adults with cancer receiving chemotherapy. J Am Geriatr Soc. 2019;67(5):1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loh KP, Lin PJ, Uth J, et al. Exercise for managing cancer- and treatment-related side effects in older adults. J Geriatr Oncol. 2018;9(4):405–410. [DOI] [PubMed] [Google Scholar]
- 66. van der Leeden M, Huijsmans RJ, Geleijn E, et al. Tailoring exercise interventions to comorbidities and treatment-induced adverse effects in patients with early stage breast cancer undergoing chemotherapy: a framework to support clinical decisions. Disabil Rehabil. 2018;40(4):486–496. [DOI] [PubMed] [Google Scholar]
- 67. Campbell KL, Zadravec K, Bland KA, et al. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: a systematic review of randomized clinical trials. Phys Ther. 2020;100(3):523–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fuller JT, Hartland MC, Maloney LT, et al. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311–1322. [DOI] [PubMed] [Google Scholar]
- 69. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scott JM, Li N, Liu Q, et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4(10):1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. [DOI] [PubMed] [Google Scholar]
- 73. Jones LW, Habel LA, Weltzien E, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Riscuta G. Nutrigenomics at the interface of aging, lifespan, and cancer prevention. J Nutr. 2016;146(10):1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lopez-Otin C, Galluzzi L, Freije JMP, et al. Metabolic control of longevity. Cell. 2016;166(4):802–821. [DOI] [PubMed] [Google Scholar]
- 76. Lu Y, Tao F, Zhou MT, et al. The signaling pathways that mediate the anti-cancer effects of caloric restriction. Pharmacol Res. 2019;141:512–520. [DOI] [PubMed] [Google Scholar]
- 77. Yu B, Xu L, Cai M, et al. Effect of tumor necrosis factor-alpha-induced protein 8 on the immune response of cd4+ t lymphocytes in mice following acute insult. Mol Med Rep. 2018;17(5):6655–6660. [DOI] [PubMed] [Google Scholar]
- 78. White MJ, Beaver CM, Goodier MR, et al. Calorie restriction attenuates terminal differentiation of immune cells. Front Immunol. 2016;7:667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hursting SD, Lavigne JA, Berrigan D, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54(1):131–152. [DOI] [PubMed] [Google Scholar]
- 80. Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111(47):16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Longo VD, Mattson MP.. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Eissenberg JC. Hungering for immortality. Mol Med. 2018;115(1):12–17. [PMC free article] [PubMed] [Google Scholar]
- 83. Belsky DW, Huffman KM, Pieper CF, et al. Change in the rate of biological aging in response to caloric restriction: CALERIE Biobank analysis. J Gerontol A Biol Sci Med Sci. 2018;73(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Villasenor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. [DOI] [PubMed] [Google Scholar]
- 86. Carneiro IP, Mazurak VC, Prado CM.. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep. 2016;18(10):62–75. [DOI] [PubMed] [Google Scholar]
- 87. Chlebowski RT, Aragaki AK, Anderson GL, et al. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the Women's Health Initiative Randomized Clinical Trial. JAMA Oncol. 2018;4(10):e181212–e181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-fat dietary pattern and breast cancer mortality in the Women's Health Initiative Randomized Controlled Trial. J Clin Oncol. 2017;35(25):2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38(1):209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Groot S, Vreeswijk MP, Welters MJ, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15(1):652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vernieri C, Signorelli D, Galli G, et al. Exploiting fasting-mimicking diet and metformin to improve the efficacy of platinum-pemetrexed chemotherapy in advanced LKB1-inactivated lung adenocarcinoma: the FAME trial. Clin Lung Cancer. 2019;20(3):e413–e417. [DOI] [PubMed] [Google Scholar]
- 92. Rautiainen S, Wang L, Lee IM, et al. Higher intake of fruit, but not vegetables or fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of normal BMI at baseline. J Nutr. 2015;145(5):960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Elgendy M, Ciro M, Hosseini A, et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3beta-MCL-1 Axis. Cancer Cell. 2019;35(5):798–815. [DOI] [PubMed] [Google Scholar]
- 94. Lu Z, Xie J, Wu G, et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat Med. 2017;23(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Di Biase S, Shim HS, Kim KH, et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017;15(3):e2001951–E2001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Caffa I, D’Agostino V, Damonte P, et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget. 2015;6(14):11820–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bianchi G, Martella R, Ravera S, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6(14):11806–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brandhorst S, Wei M, Hwang S, et al. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48(10):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):ra27–ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Safdie F, Brandhorst S, Wei M, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7(9):e44603–e44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lee C, Longo VD.. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30(30):3305–3316. [DOI] [PubMed] [Google Scholar]
- 102. Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105(24):8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bauersfeld SP, Kessler CS, Wischnewsky M, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Minnella EM, Awasthi R, Loiselle SE, et al. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153(12):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Demark-Wahnefried W, Rogers LQ, Gibson JT, et al. Randomized trial of weight loss in primary breast cancer: impact on body composition, circulating biomarkers and tumor characteristics. Int J Cancer. 2020;146(10):2784–2796. doi: 10.1002/ijc.32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Demark-Wahnefried W, Rais-Bahrami S, Desmond RA, et al. Presurgical weight loss affects tumour traits and circulating biomarkers in men with prostate cancer. Br J Cancer. 2017;117(9):1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dorff TB, Groshen S, Garcia A, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16(1):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: a case series report. Aging. 2009;1(12):988–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McPherson RA, Hardy G.. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care. 2011;14(6):562–568. [DOI] [PubMed] [Google Scholar]
- 110. Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):720–726. [DOI] [PubMed] [Google Scholar]
- 111. Lorenzo-Lopez L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rozich NS, Jones CE, Morris KT.. Malnutrition, frailty, and sarcopenia in pancreatic cancer patients: assessments and interventions for the pancreatic surgeon. Ann Pancreat Cancer. 2019;2:31–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mazzuca F, Roberto M, Arrivi G, et al. Clinical impact of highly purified, whey proteins in patients affected with colorectal cancer undergoing chemotherapy: preliminary results of a placebo-controlled study. Integr Cancer Ther. 2019;18:153473541986611–153473541986692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cereda E, Turri A, Klersy C, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019;8(16):6923–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu Z, Qiu T, Pei L, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg. 2019;1:1–9. [DOI] [PubMed] [Google Scholar]
- 116. Bumrungpert A, Pavadhgul P, Nunthanawanich P, et al. Whey protein supplementation improves nutritional status, glutathione levels, and immune function in cancer patients: a randomized, double-blind controlled trial. J Med Food. 2018;21(6):612–616. [DOI] [PubMed] [Google Scholar]
- 117. Le Roy B, Pereira B, Bouteloup C, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB Study. BMJ Open. 2016;6(12):e012876–e012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nencioni A, Caffa I, Cortellino S, et al. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11):707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhang FF, Haslam DE, Terry MB, et al. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: the breast cancer family registry. Cancer. 2017;123(11):2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ratjen I, Schafmayer C, di Giuseppe R, et al. Postdiagnostic Mediterranean and healthy Nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J Nutr. 2017;147(4):636–644. [DOI] [PubMed] [Google Scholar]
- 121. Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Presley CJ, Dotan E, Soto-Perez-de-Celis E, et al. Gaps in nutritional research among older adults with cancer. J Geriatr Oncol. 2016;7(4):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Banga S, Heinze-Milne SD, Howlett SE.. Rodent models of frailty and their application in preclinical research. Mech Ageing Dev. 2019;179:1–10. [DOI] [PubMed] [Google Scholar]
- 126. Gurkar AU, Niedernhofer LJ.. Comparison of mice with accelerated aging caused by distinct mechanisms. Exp Gerontol. 2015;68:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Huang Z, Whelan CV, Foley NM, et al. Longitudinal comparative transcriptomics reveals unique mechanisms underlying extended healthspan in bats. Nat Ecol Evol. 2019;3(7):1110–1120. [DOI] [PubMed] [Google Scholar]
- 128. Kirkland JL, Tchkonia T.. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. [DOI] [PubMed] [Google Scholar]
- 130. Tchkonia T, Zhu Y, van Deursen J, et al. Cellular Senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu M, Bradley EW, Weivoda MM, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Bio Sci Med Sci. 2017;72(6):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Coppé JP, Patil C, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the P53 tumor suppressor. PLoS Biol. 2008;6(12):e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tchkonia T, Kirkland JL.. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. 2018;320(13):1319–1320. [DOI] [PubMed] [Google Scholar]
- 135. Khosla S, Farr JN, Kirkland JL.. Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J Clin Endocrinol Metab. 2018;103(4):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kirkland JL, Tchkonia T, Zhu Y, et al. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65(10):2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, et al. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging. 2017;9(11):2411–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4(4):e12997–e13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tang H, Shrager JB, Goldman D.. Rapamycin protects aging muscle. Aging (Albany NY). 2019;11(16):5868–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Walters HE, Cox LS.. mTORC Inhibitors as broad-spectrum therapeutics for age-related diseases. Int J Mol Sci. 2018;19(8):E2325–E2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kraig E, Linehan LA, Liang H, et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: immunological, physical performance, and cognitive effects. Exp Gerontol. 2018;105:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Freeman LW, White R, Ratcliff CG, et al. A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: reducing symptoms and barriers to care. Psychooncology. 2015;24(8):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. JNCI Monogr. 2014;2014(50):346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Galantino ML, Greene L, Daniels L, et al. Longitudinal impact of yoga on chemotherapy-related cognitive impairment and quality of life in women with early stage breast cancer: a case series. Explore (NY )2012;8(2):127–135. [DOI] [PubMed] [Google Scholar]
- 153. Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12(1):559–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wirth MD, Franco R, Wagner Robb S, et al. Randomized controlled trial of a 4-week mindfulness intervention among cancer survivors compared to a breathing control. Cancer Invest. 2019;37(4-5):227–232. [DOI] [PubMed] [Google Scholar]
- 155. Huang HP, He M, Wang HY, et al. A meta-analysis of the benefits of mindfulness-based stress reduction (MBSR) on psychological function among breast cancer (BC) survivors. Breast Cancer. 2016;23(4):568–576. [DOI] [PubMed] [Google Scholar]
- 156. Gramaglia C, Gambaro E, Vecchi C, et al. Outcomes of music therapy interventions in cancer patients-a review of the literature. Crit Rev Oncol Hematol. 2019;138:241–254. [DOI] [PubMed] [Google Scholar]
- 157. Ambroggi M, Biasini C, Toscani I, et al. Can early palliative care with anticancer treatment improve overall survival and patient-related outcomes in advanced lung cancer patients? A review of the literature. Support Care Cancer. 2018;26(9):2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Clark K, Greene P, DuHamel K, et al. A unique interactive cognitive behavioral training program for front-line cancer care professionals. J Canc Educ. 2012;27(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ye M, Du K, Zhou J, et al. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology. 2018;27(7):1695–1703. [DOI] [PubMed] [Google Scholar]
- 160. Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. 2015;121(11):1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Abrahams HJG, Gielissen MFM, Donders RRT, et al. The efficacy of internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: a randomized controlled trial. Cancer. 2017;123(19):3825–3834. [DOI] [PubMed] [Google Scholar]
- 162. Knotkova H, Malamud SC, Cruciani RA.. Transcranial direct current stimulation (TDCS) improved cognitive outcomes in a cancer survivor with chemotherapy-induced cognitive difficulties. Brain Stimul. 2014;7(5):767–768. [DOI] [PubMed] [Google Scholar]
- 163. Park SH, Seo JH, Kim YH, et al. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25(2):122–126. [DOI] [PubMed] [Google Scholar]
- 164. Nelson CJ, Saracino RM, Roth AJ, et al. Cancer and aging: reflections for elders (CARE): a pilot randomized controlled trial of a psychotherapy intervention for older adults with cancer. Psychooncology. 2019;28(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tolahunase M, Sagar R, Dada R.. Impact of yoga and meditation on cellular aging in apparently healthy individuals: a prospective, open-label single-arm exploratory study. Oxid Med Cell Longev. 2017;2017:1–7928990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow the rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172(1):34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS(c)(R) yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther. 2016;15(3):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Cifu G, Power MC, Shomstein S, et al. Mindfulness-based interventions and cognitive function among breast cancer survivors: a systematic review. BMC Cancer. 2018;18(1):1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Johns SA, Von Ah D, Brown LF, et al. Randomized controlled pilot trial of mindfulness-based stress reduction for breast and colorectal cancer survivors: effects on cancer-related cognitive impairment. J Cancer Surviv. 2016;10(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the tame biomarkers workgroup. Geroscience. 2018;40(5-6):419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Niedernhofer LJ, Kirkland JL, Ladiges W.. Molecular pathology endpoints useful for aging studies. Ageing Res Rev. 2017;35:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Horvath S, Raj K.. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. [DOI] [PubMed] [Google Scholar]
- 174. Konar A, Singh P, Thakur MK.. Age-associated cognitive decline: insights into molecular switches and recovery avenues. Aging Dis. 2016;7(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hayek S, Gibson TM, Leisenring WM, et al. Prevalence and predictors of frailty in childhood cancer survivors and siblings: a report from the childhood cancer survivor study. J Clin Oncol. 2019;38(3):232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wilson CL, Chemaitilly W, Jones KE, et al. Modifiable factors associated with aging phenotypes among adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(21):2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9(2):419–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zhao W, Ammous F, Ratliff S, et al. Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. Int J Environ Res Public Health. 2019;16(17):E3141–E3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chen BT, Jin T, Patel SK, et al. Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: a pilot longitudinal study. Breast Cancer Res Treat. 2019;176(1):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Karschnia P, Parsons MW, Dietrich J.. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 2019;20(2):e92–e102. [DOI] [PubMed] [Google Scholar]
- 182. Dietrich J, Baryawno N, Nayyar N, et al. Bone marrow drives central nervous system regeneration after radiation injury. J Clin Invest. 2018;128(6):2651–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Scott JM, Dolan LB, Norton L, et al. Multisystem toxicity in cancer: lessons from NASA's countermeasures program. Cell. 2019;179(5):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. DuMontier C, Sedrak MS, Soo WK, et al. Arti Hurria and the progress in integrating the geriatric assessment into oncology: Young International Society of Geriatric Oncology review paper. J Geriatr Oncol. 2020;11(2):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mayer DK, Alfano CM.. Personalized risk-stratified cancer follow-up care: its potential for healthier survivors, happier clinicians, and lower costs. J Natl Cancer Inst. 2019;111(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Alfano CM, Leach CR, Smith TG, et al. Equitably improving outcomes for cancer survivors and supporting caregivers: a blueprint for care delivery, research, education, and policy. CA Cancer J Clin. 2019;69(1):35–49. [DOI] [PubMed] [Google Scholar]
- 187. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Faithfull S, Turner L, Poole K, et al. Prehabilitation for adults diagnosed with cancer: a systematic review of long-term physical function, nutrition and patient-reported outcomes. Eur J Cancer Care (Care ) 2019;28(4):e13023–e13045. [DOI] [PubMed] [Google Scholar]
- 192. Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–514. [DOI] [PubMed] [Google Scholar]
- 193. Minnella EM, Bousquet-Dion G, Awasthi R, et al. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol. 2017;56(2):295–300. [DOI] [PubMed] [Google Scholar]
- 194. Gillis C, Loiselle SE, Fiore JF Jr, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. 2016;116(5):802–812. [DOI] [PubMed] [Google Scholar]
- 195. Jack S, West M, Grocott MP.. Perioperative exercise training in elderly subjects. Best Pract Res Clin Anaesthesiol. 2011;25(3):461–472. [DOI] [PubMed] [Google Scholar]
- 196. Philipson TJ, Snider JT, Lakdawalla DN, et al. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121–128. [PubMed] [Google Scholar]
- 197. Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg. 2005;53(5):237–243. [DOI] [PubMed] [Google Scholar]
- 198. Giles C, Cummins S.. Prehabilitation before cancer treatment. BMJ. 2019;366:l5120–l5121. [DOI] [PubMed] [Google Scholar]
- 199. Loonen JJ, Blijlevens NM, Prins J, et al. Cancer survivorship care: person centered care in a multidisciplinary shared care model. Int J Integr Care. 2018;18(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]