Abstract
The worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demands unprecedented attention. We report four X-ray crystal structures of three synthetic nanobodies (sybodies) (Sb16, Sb45 and Sb68) bind to the receptor-binding domain (RBD) of SARS-CoV-2: binary complexes of Sb16–RBD and Sb45–RBD; a ternary complex of Sb45–RBD–Sb68; and Sb16 unliganded. Sb16 and Sb45 bind the RBD at the ACE2 interface, positioning their CDR2 and CDR3 loops diametrically. Sb16 reveals a large CDR2 shift when binding the RBD. Sb68 interacts peripherally at the ACE2 interface; steric clashes with glycans explain its mechanism of viral neutralization. Superposing these structures onto trimeric spike (S) protein models indicates these sybodies bind conformations of the mature S protein differently, which may aid therapeutic design.
One Sentence Summary:
X-ray structures of synthetic nanobodies complexed with the receptor-binding domain of the spike protein of SARS-CoV-2 reveal details of CDR loop interactions in recognition of distinct epitopic sites.
SARS-CoV-2, a β-coronavirus, is remarkable for its high infectivity, rapid worldwide dissemination, and evolution of highly infectious new variants (1–4). The virus exploits its trimeric S glycoprotein to adsorb to the host cell-surface receptor, angiotensin converting enzyme (ACE) ACE2 (5) resulting in proteolytic processing and conformational changes required for membrane fusion and cell entry (6). Understanding the fundamental molecular and cell biology and chemistry of the viral life cycle and the nature of the host immune response, offers rational avenues for developing diagnostics, therapeutics, and vaccines (7, 8). Exploring the detailed structures of anti-viral antibodies can provide critical understanding of the means to attenuate viral adsorption and entry, preventing or retarding ongoing infection and communal spread. An evolving database of X-ray and cryo-EM structures of the SARS-CoV-2 S and its interactions with ACE2 or various antibodies contributes to the design of effective antibodies or immunogens (9). Recent studies indicate the value of single domain antibodies derived from camelids (nanobodies) (10) or camelid-inspired synthetic libraries (sybodies) (11), and the value of generating multivalent constructs (12) for effective treatment (11). Many properties of nanobodies make them well suited for structural studies and drug development (13).
Here, we take advantage of available sequences of three SARS-CoV-2 RBD-directed sybodies - Sb16, Sb45, and Sb68 (previously designated Sb#16, Sb#45, and Sb#68 (14)). We describe binding studies and X-ray structures of complexes of these with the RBD, and also the structure of Sb16 unliganded. The sybodies had been shown to be effective inhibitors of the ACE2–RBD interaction (14), and neutralizers of viral infectivity (14). These sybodies (see Supplementary Materials and Methods) behaved as monomers by size exclusion chromatography (SEC) (15) (Figure S1), and we confirmed their activity in binding to the re-engineered RBD and S using surface plasmon resonance (SPR) (Figure S2). All three sybodies bind to surface immobilized RBD with KD values of 0.038 to 0.77 μM (Figure S2A to S2D) - measurements that are similar to those determined using RBD-YFP or RBD-Fc molecules by related techniques (14). Binding of Sb16, Sb45, and Sb68 to S consistently revealed lower affinities, in the range of 0.07 to 2.6 μM (Figure S2E to S2H). Experiments using SEC of premixed solutions of sybodies and RBD confirmed that all three sybodies bound the RBD (Figure S3). RBD consistently eluted at 13 min. Mixtures of RBD with Sb16 or Sb45 eluted at ~11.3 min and with Sb68 at ~11.7 min consistent with complex formation. For unliganded Sb16, the large change in elution time suggests that its RBD binding site is that which interacts with the chromatographic column matrix (Figure S3).
To gain insight into the precise topology of the interaction of each of the three sybodies with the RBD, we determined crystal structures of these complexes. We obtained crystals of several complexes: Sb16–RBD, Sb45–RBD, and the ternary Sb45–RBD–Sb68; and of Sb16 alone. These crystals diffracted X-rays to resolutions from 2.1 to 2.6 Å (Table S1). After molecular replacement, model building, and crystallographic refinement (see Materials and Methods), we obtained structural models with Rwork/Rfree (%) of 25.4/28.4, 18.6/21.6, 20.6/25.5 and 22.5/25.6, respectively, that satisfied standard criteria for fitting and geometry (Table S1). Illustrations of the quality of the final models as compared with the electron density maps are shown in Figure S4.
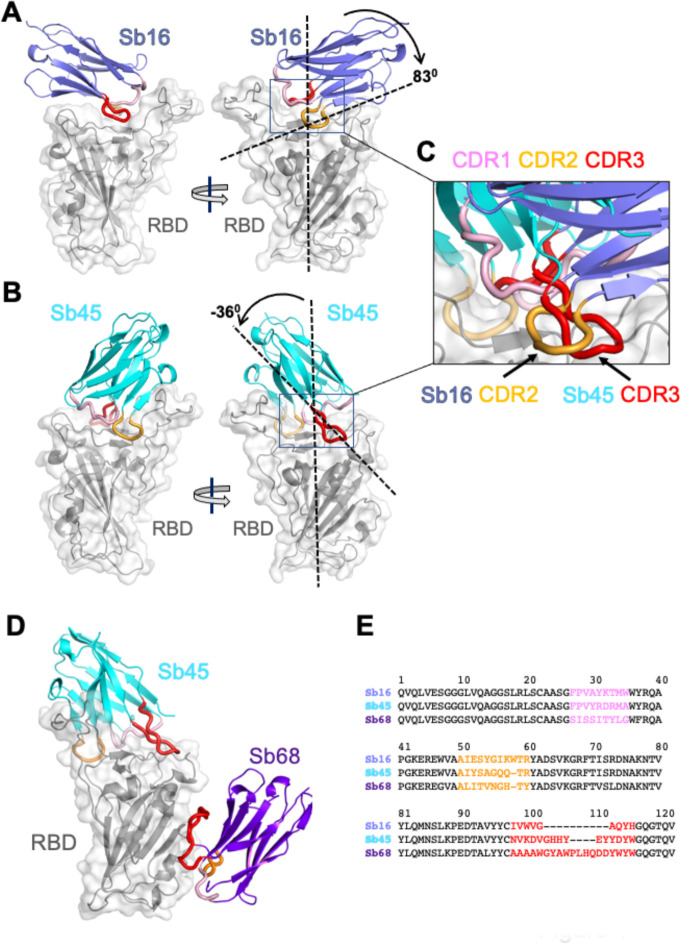
The structure of the RBD domain of these complexes (Figure 1A and 1B) revealed little difference between insect-expressed (16) and bacteria-expressed and refolded RBD. Each of the sybodies has a barrel of two β-sheets stabilized by a single disulfide-linked loop of 75 or 76 amino acids characteristic of an IgV fold (17, 18). The Sb16–RBD complex (Figure 1A and 2A) illustrates that CDR2 (residues 50–60) and CDR3 (residues 98–106) bestride the saddle-like region of the ACE2-binding surface of the RBD (see sequence alignment in Figure 1E). Sb16 angulates over the RBD by 83°. However, Sb45 (Figure 1B and 2B) straddles the RBD saddle in the opposite orientation, at an angle of −36°, and frames the interface with CDR2 (residues 50–59) and CDR3 (residues 97–111). CDR1s of both sybodies (residues 27–35) lie between the CDR2 and CDR3 loops. Superposition of the two structures, based on the RBD, emphasizes the diametrically opposite orientation of the two (Figure 1C), revealing that the CDR2 of Sb16 and CDR3 of Sb45 recognize the same epitopic regions.
Fig. 1.
Overall structures of Sb16, Sb45 and Sb68 complexes with SARS-CoV-2 RBD. Ribbons (sybodies) and ribbons plus surface (RBD) representations of the complex of (A) Sb16 (slate) with RBD (grey) (7KGK); (B) Sb45 (cyan) with RBD (7KGJ), and (C) Sb45 and Sb68 (purple) with RBD (7KLW). Sb16-RBD and Sb45-RBD, superimposed based on the RBD are shown in (D) to highlight CDR loops, which are color coded as indicated. The CDR2 of Sb16 and CDR3 of Sb45 interact similarly with the RBD surface.
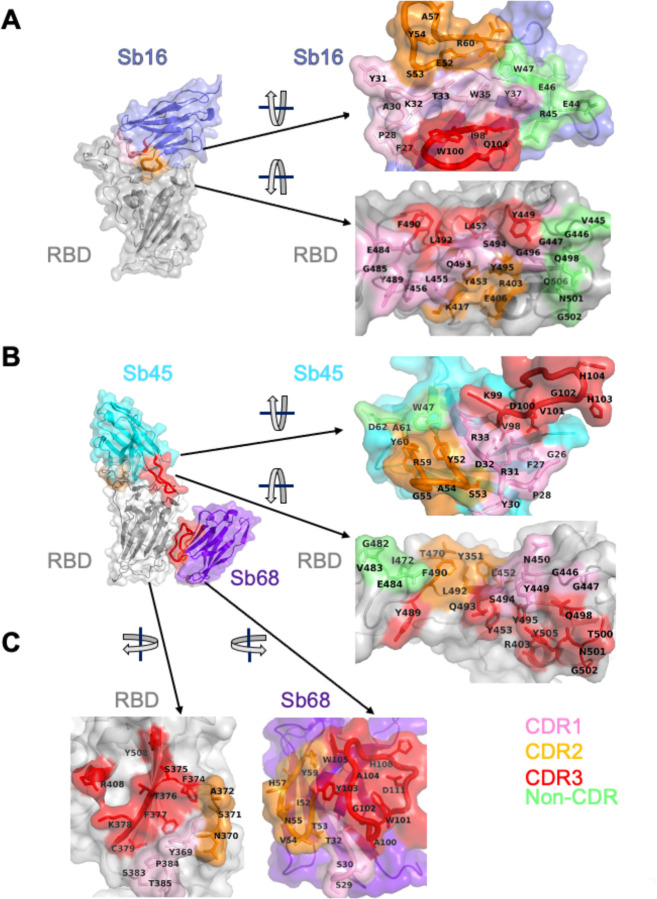
Fig. 2.
Interface and interaction of (A) Sb16-RBD, (B) Sb45-RBD and (C) Sb68-RBD. (Individual contacting residues are listed in TableS2 in Supplemental Materials). CDR1, CDR2, CDR3 regions are painted pink, orange and red respectively. Additional non-CDR region contacting residues are colored lime. On the RBD surface, the epitopic residues that contact the sybodies are colored according to the sybody CDR.
Exploring conditions using mixtures of two or three sybodies and the RBD, we obtained crystals and solved the structure of a ternary complex consisting of Sb45–RBD–Sb68 at 2.6 Å (Table S1 and Figure 1D). The refined model revealed Sb45 and Sb68 interacting at two different faces of the RBD (Figure 1D and 2B). Here, Sb45 binds in an identical orientation to that observed in the binary Sb45–RBD structure (RMSD of superposition, 0.491 Å for 1981 atoms), but Sb68 addresses a completely different face of the RBD - similar to that bound by Fab of CR3022 on RBD of SARS-CoV-2 (19) and by VHH72 on RBD of SARS-CoV-1 (20). Of particular interest, whereas Sb45 CDR2 and CDR3 span the RBD saddle as noted above, the distinct contacts of Sb68 to the RBD are through the longer CDR3, with only minor contributions from CDR1 and CDR2. Walter et al visualized similar distinct interactions in cryo-EM maps of two sybodies (Sb15 and Sb68) bound to S protein with local resolution of 6–7 Å (14).
Scrutiny of the different interfaces provides insights into the distinct ways each sybody exploits its unique CDR residues for interaction with epitopic residues of the RBD (Figure 2). Both Sb16 and Sb45 use longer CDR2 and CDR3 to straddle the RBD, positioning CDR1 residues over the central crest of the saddle (Figure 2A and 2B). Also, several non-CDR residues (Y37, E44, R45, E46, and W47 for Sb16; and W47 for Sb45), derived from framework 2 (21), provide additional contacts to the RBD. The interface of Sb68 with RBD (Figure 2C) is quite different, predominantly exploiting eight CDR3, six CDR2, and four CDR1 residues, along with non-CDR residues at the interface. (Table S2 lists all individual contacts between each sybody and the RBD).
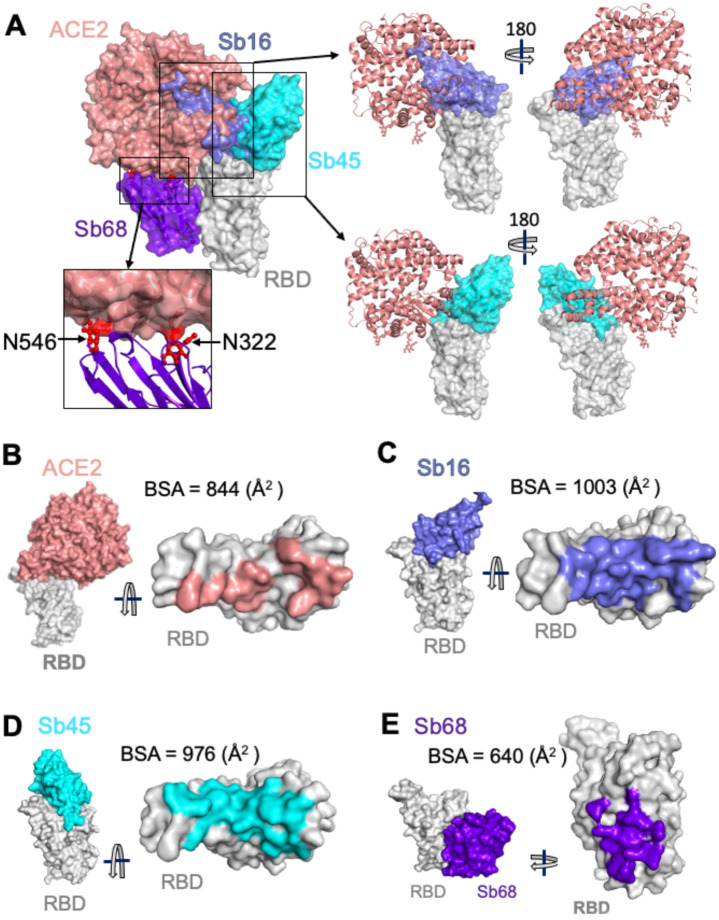
To evaluate the structural basis for the ability of these three sybodies to block the interaction of RBD with ACE2, we superposed each of the three sybody–RBD structures onto the ACE2–RBD structure and examined the steric clashes (Figure 3A). Sb16 and Sb45 directly impinge on the ACE2 binding site, offering a structural rationale for their viral neutralization capacity (14). Sb68, which also blocks viral infectivity, binds to RBD at a site which appears to be noncompetitive for ACE2 binding. The carbohydrate at ACE2 residues N322 and N546 provides an explanation (Figure 3A, and below).
Fig. 3.
Sybodies compete with ACE2 for RBD binding. (A) Sb16 (slate), Sb45 (cyan) and Sb68 (purple) - RBD complexes were superposed on the ACE2—RBD structure (salmon) (6M0J) based on the RBD. Sb16 is buried inside ACE2; Sb45 is partially buried in ACE2; and Sb68 has major clashes with two N-glycan sites (N322 and N546) of ACE2. (B) Epitopic areas (on RBD) captured by ACE2 (salmon), BSA = 844 (Å2); (C) by Sb16 (slate), BSA = 1003 (Å2); (D) by Sb45 (cyan), BSA = 976 (Å2); and (E) by Sb68 (purple), BSA = 640 (Å2).
To compare the epitopic areas captured by these sybodies, we evaluated the buried surface area (BSA) interfaces between RBD and ACE2 or the sybodies. The BSA at the ACE2–RBD, Sb16–RBD, Sb45–RBD, and Sb68–RBD interfaces are 844 Å2, 1,003 Å2, 976 Å2, and 640 Å2, respectively (Figure 3B to 3E). Sb16 and Sb45 capture more surface area than ACE2 or other published nanobody or sybody–RBD complexes (see Table S3). The interface with Sb68 is the smallest (640 Å2) (Figure 3E). The total BSA captured by Sb45 and Sb68 in the ternary complex is 1,650 (1,010 plus 640) Å2 (Table S4) and is consistent with the view that a linked bispecific sybody, as described by Walter et al (14), would exert strong avidity effects. Table S3 summarizes these BSA values and those of other nanobody–RBD interactions.
A reasonable explanation for the ability of Sb68 to block the ACE2–RBD interaction arises on inspection of the sites where Sb68, bound to the RBD, might clash with ACE2. Scrutiny of a superposition of Sb68–RBD with ACE2–RBD reveals several areas of steric interference. Sb68 loop 40–44 clashes with amino acid side chains of ACE2 (residues 318–320 and 548–552), loop 61–64 with ACE2 N322 carbohydrate, and loop 87–89 (a 3,10 helix) with ACE2 N546 carbohydrate as well as residues 313 and 316–218 (Figure 3A). The ACE2 used in the crystallographic visualization of ACE2–RBD (22) was expressed in Trichoplusia ni insect cells, which produce biantennary N-glycans terminating with N-acetylglucosamine residues (23, 24). Electron density was observed only for the proximal N-glycans at residues N322 and N546, but larger, complex, non-sialylated, biantennary carbohydrates have been detected in glycoproteomic analysis of ACE2 in mammalian cells (25). These are highly flexible carbohydrates adding greater than 1500 Da at each position, so are larger than the single carbohydrate residues visualized in the crystal structure. Additionally, molecular dynamics simulations of RBD–ACE2 implicated the direct interaction of carbohydrate with the RBD (26). Thus, the ability of Sb68 to impinge on ACE2 interaction with RBD likely involves the steric clash of the N322- and N546-linked glycans.
We also obtained a 2.1 Å structure of free Sb16 (Figure S5). Remarkably, the CDR2 of Sb16 shows Y54 in starkly different positions in the unliganded structure as compared to the complex: the Cα carbon is displaced by 6.0 Å, while the Oη oxygen of Y54 is 15.2 Å distant, indicative of dynamic flexibility.
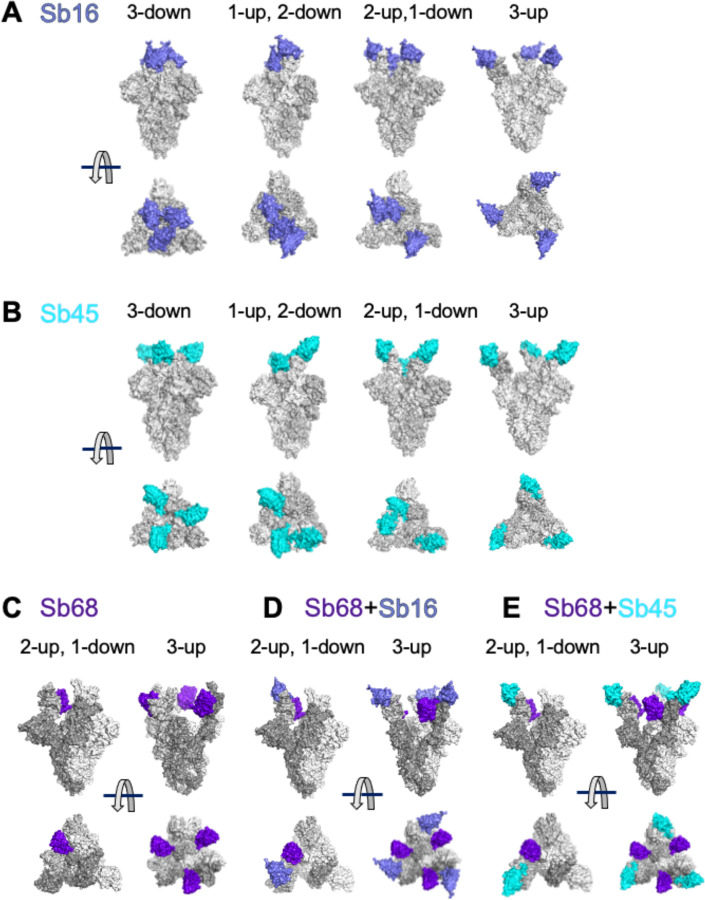
To gain additional insight into the structural consequences of the interactions of each of these sybodies with a trimeric S protein, we superposed each of the individual sybody–RBD complexes on each of several cryo-EM-determined models of S, including examples of different combinations of RBD orientation: three-down (6XEY (27)), one-up, two-down (6Z43 (28)), two-up, one-down (7A29 (29)), and three-up (7JVC (30)) (see Figure 4). Both Sb16 and Sb45 may dock on each of the three RBDs in the trimeric S in any of the four configurations, without any apparent clash (Figure 4A, 4B). However, Sb68 could not be superposed without clashes to any RBD of the three-down or to the one-up two down position. The only permissible superpositions were to two in the two-up, one-down (Figure 4C); and to all three in the three-up position (Figure 4C). For paired sybodies, either Sb16 and Sb68 or Sb45 and Sb68, superposition was possible without clashes, with two or more RBDs in the up conformation (Figure 4D and 4E). Walter et al (14) suggested that a covalent bispecific Sb15–Sb68 reagent could bind S in both the two-up and three-up configurations, based on cryo-EM maps of complexes of S with Sb15 and Sb68, with local resolution in the range of 6–7 Å. It appears that Sb16 binds to S in an orientation similar to, but in detail distinct from that of Sb15. This analysis demonstrates an advantage of the small size of sybodies or nanobodies in accessing epitopic regions of S.
Fig. 4.
Superposition of complexes on spike models reveals accessibility of sybodies. Superposition of (A) Sb16-RBD (slate) on spike (6XEY: 3-down; 6Z43: 1-up and 2-down; 7A29: 2-up and 1-down; 7JVC: 3-up); (B) Sb45-RBD (cyan); and (C) Sb68-RBD (purple), there is no accessible surface for Sb68 on 3-down of spike; (D) Sb68 and Sb16 on RBD; (E) SB68 and Sb45 on RBD.
Barnes et al (31) categorized a host of anti-S and anti-RBD Fabs into four classes (1–4) based on the location of the footprint, and whether the Fab has access to either the up only or up and down configuration of the RBD in the context of the full trimer (Figure S6A). Xiang et al (12) categorized anti-RBD nanobodies into five epitopic regions (I-V) (Figure S6A). By superposition, Sb16 would clash with the light chain of the B38 Fab (7BZ5), as in class 1, but it also clashes with the heavy chain of COVA2-39 (7JMP), as in class 2 (Figure S6B). Sb45 clashes effectively with the heavy chain of COVA2-39, and thus appears to be closer to a “true” class 2 sybody (Figure S6C). Both Sb16 and Sb45 are capable of binding the RBD of S in either the up or down position, a defining characteristic of class 2. By contrast, Sb68 competes mostly with the CR3022 heavy chain (6W41), VHH72 (6WAQ) (20) and VHH-U (7KN5) (32) placing it in class 4. Overall, our structural studies not only define the Sb16, Sb45, and Sb68 epitopes at high resolution, they suggest that a battery of sybodies or nanobodies have the potential to saturate the available RBD surface.
The significance of the ternary structure of Sb45–RBD–Sb68 (7KLW) is confirmed in a recent paper (32). Koenig et al determined a ternary nanobody structure of VHH-E–RBD–VHH-U (7KN5) which illustrates the binding to two distinct epitopic sites. Superposition of Sb45–RBD–Sb68 on VHH-E–RBD–VHH-U indicates that Sb45 and VHH-E represent class 2 in recognizing the epitope region but do so in different orientations (Figure S7A, middle panel). Sb45 uses both long CDR2 and CDR3 loops riding along both sides of RBD surface, while VHH-E uses a long CDR3 loop engaging one side of RBD surface.
Recently, several SARS-CoV-2 spike variants have been isolated and characterized with respect to their infectivity and severity of disease. The UK-SARS-CoV-2 variant has multiple substitutions including N501Y in the RBD (1). This is expected to impinge on the peripheral aspect of the footprint of Sb16 and Sb45 but would have no effect on the Sb68 site. Thus, precise mapping of anti-RBD antibody, nanobody, and sybody epitopes, especially for those that are developed for clinical trials, has implications not only for mechanistic understanding of the interactions of the RBD with ACE2, but also for evaluating the potential susceptibility of newly arising viral variants to currently administered vaccines and antibodies.
Supplementary Material
Acknowledgments:
We appreciate the help of Joy (Huaying) Zhao and Peter Schuck, NIBIB, NIH in analyzing SPR data, and thank Peter Sun, NIAID, NIH for access to his program, HINGE. We thank Barney Graham, NIAID, NIH for plasmids used in initial aspects of the work, and Apostolos Gittis, NIAID, NIH, for help in protein characterization. We appreciate the advice of Michael Mage and D. K. Taylor during this work.
Funding: This work was supported by the Intramural Research Program of the NIAID, NIH, including funds from the CARES Act. X-ray data were collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID (or 22-BM) beamline at the Advanced Photon Source, Argonne National Laboratory. SER-CAT is supported by its member institutions (www.ser-cat.org/members.html) and equipment grants (S10_RR25528 and S10_RR028976) from the National Institutes of Health. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38.
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability: All data are included in the paper or in the supplementary material. X-ray structure factors and coordinates are deposited at the protein data bank (www.pdb.org) under accession numbers 7KGK, 7KGJ, 7KLW and 7KGL for Sb16–RBD, Sb45–RBD, Sb45–RBD–Sb68, and Sb16 respectively.
Supplementary Materials:
Materials and Methods
Figures S1–S8
Tables S1–S3
References (33–45)
References and Notes:
- 1.Conti P. et al. , The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents. 2021. ( 10.23812/21-3-E). [DOI] [PubMed] [Google Scholar]
- 2.Kirby T., New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021. ( 10.1016/S2213-2600(21)00005-9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J. W., Tambyah P. A., Hui D. S., Emergence of a new SARS-CoV-2 variant in the UK. J Infect. 2020. ( 10.1016/j.jinf.2020.12.024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wibmer C. K. et al. , SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv, 2021.2001.2018.427166 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Wang Q. et al. , Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020. ( 10.1016/j.cell.2020.03.045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang J. et al. , Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117, 11727–11734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L. R. et al. , Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020. ( 10.1056/NEJMoa2035389). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y. et al. , Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 182, 73–84 e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C. et al. , Conformational dynamics of SARS-CoV-2 trimeric spike glycoprotein in complex with receptor ACE2 revealed by cryo-EM. Sci Adv. 2020. ( 10.1126/sciadv.abe5575). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanke L. et al. , An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat Commun 11, 4420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoof M. et al. , An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science. 2020. ( 10.1126/science.abe3255). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang Y. et al. , Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science 370, 1479–1484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram J. R., Schmidt F. I., Ploegh H. L., Exploiting Nanobodies’ Singular Traits. Annu Rev Immunol 36, 695–715 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Walter J. D. et al. , Highly potent bispecific sybodies neutralize SARS-CoV-2. bioRxiv. 2020. ( 10.1101/2020.11.10.376822). [DOI] [Google Scholar]
- 15.Zimmermann I. et al. , Generation of synthetic nanobodies against delicate proteins. Nat Protoc 15, 1707–1741 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Wu Y. et al. , A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, 1274–1278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan K., Mage M. G., Margulies D. H., Immunoglobulin Superfamily. eLS. 2015. ( 10.1002/9780470015902.a0000926.pub2). [DOI] [Google Scholar]
- 18.Halaby D. M., Poupon A., Mornon J., The immunoglobulin fold family: sequence analysis and 3D structure comparisons. Protein Eng 12, 563–571 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Huo J. et al. , Neutralization of SARS-CoV-2 by Destruction of the Prefusion Spike. Cell Host Microbe 28, 445–454 e446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrapp D. et al. , Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell 181, 1004–1015 e1015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu T. T., Kabat E. A., An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med 132, 211–250 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan J. et al. , Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Rudd P. M. et al. , Hybrid and complex glycans are linked to the conserved N-glycosylation site of the third eight-cysteine domain of LTBP-1 in insect cells. Biochemistry 39, 1596–1603 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Hsu T. A. et al. , Differential N-glycan patterns of secreted and intracellular IgG produced in Trichoplusia ni cells. J Biol Chem 272, 9062–9070 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Shajahan A. et al. , Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2. Glycobiology. 2020. ( 10.1093/glycob/cwaa101). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao P. et al. , Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 28, 586–601 e586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L. et al. , Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584, 450–456 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Huo J. et al. , Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat Struct Mol Biol 27, 846–854 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Custodio T. F. et al. , Selection, biophysical and structural analysis of synthetic nanobodies that effectively neutralize SARS-CoV-2. Nat Commun 11, 5588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccoli L. et al. , Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 183, 1024–1042 e1021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes C. O. et al. , SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig P. A. et al. , Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021. ( 10.1126/science.abe6230). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan K. K. et al. , Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369, 1261–1265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Natarajan K., Malchiodi E., Margulies D., Mariuzza R., Three-dimensional structure of H-2D(d) complexed with an immunodominant peptide from human immunodeficiency virus envelope glycoprotein 120. Journal of Molecular Biology 283, 179–191 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Walter J. D. et al. , Highly potent bispecific sybodies neutralize SARS-CoV-2. bioRxiv, (2020). [Google Scholar]
- 36.Zimmermann I. et al. , Generation of synthetic nanobodies against delicate proteins. Nat Protoc 15, 1707–1741 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Zhao H., Boyd L. F., Schuck P., Measuring Protein Interactions by Optical Biosensors. Curr Protoc Protein Sci 88, 20 22 21–20 22 25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H., Gorshkova II, Fu G. L., Schuck P., A comparison of binding surfaces for SPR biosensing using an antibody-antigen system and affinity distribution analysis. Methods 59, 328–335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabsch W., Xds. Acta Crystallogr D Biol Crystallogr 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai B. K., Fiser A., Multiple mapping method: a novel approach to the sequence-to-structure alignment problem in comparative protein structure modeling. Proteins 63, 644–661 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Wu Y. et al. , A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, 1274–1278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCoy A. J. et al. , Phaser crystallographic software. J Appl Crystallogr 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams P. D. et al. , PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liebschner D. et al. , Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol 75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.