Abstract
Atypical behavioral responses to environmental sounds are common in autistic children and adults, with 50–70% of this population exhibiting decreased sound tolerance (DST) at some point in their lives. This symptom is a source of significant distress and impairment across the lifespan, contributing to anxiety, challenging behaviors, reduced community participation, and school/workplace difficulties. However, relatively little is known about its phenomenology or neurocognitive underpinnings. The present article synthesizes a large body of literature on the phenomenology and pathophysiology of DST-related conditions to generate a comprehensive theoretical account of DST in autism. Notably, we argue against conceptualizing DST as a unified construct, suggesting that it be separated into three phenomenologically distinct conditions: hyperacusis (the perception of everyday sounds as excessively loud or painful), misophonia (an acquired aversive reaction to specific sounds), and phonophobia (a specific phobia of sound), each responsible for a portion of observed DST behaviors. We further elaborate our framework by proposing preliminary neurocognitive models of hyperacusis, misophonia, and phonophobia that incorporate neurophysiologic findings from studies of autism.
Keywords: autism spectrum disorder, auditory, sensory, decreased sound tolerance, sensitivity, hyperacusis, misophonia, phonophobia, central gain, salience, anxiety, specific phobia, review, theory
1. Introduction
Autism spectrum disorder (hereafter referred to as “autism”) is a heterogeneous, lifelong neurodevelopmental condition characterized by difficulties with social communication and the presence of restricted, repetitive patterns of behavior, interests, and activities (American Psychiatric Association, 2013). In addition to these cardinal features, autistic1 people commonly find a number of everyday sensory stimuli to be quite aversive (Ben-Sasson et al., 2009, 2019; Cascio et al., 2016; Schauder & Bennetto, 2016), now considered a core feature of the condition (American Psychiatric Association, 2013). Although this so-called “sensory hyperreactivity” can be present in any modality (Ausderau et al., 2014; Crane et al., 2009; Hazen et al., 2014; Leekam et al., 2007; Tavassoli et al., 2014), decreased sound tolerance (DST; i.e., an inability to tolerate everyday sounds) is among the most prevalent, persistent, and disabling sensory features of autism (Gomes et al., 2008; O’Connor, 2012; Stefanelli et al., 2020; Stiegler & Davis, 2010). A recent meta-analysis estimated that the current prevalence of DST in the autistic population is 38–45%, with 50 to 70% of individuals on the autism spectrum having experienced DST at some point in their lives (Williams, Suzman, et al., 2020b). In the largest single study investigating this phenomenon, Law and colleagues (2016) reported current and lifetime DST prevalence rates of 77.6% and 86.6%, respectively, in an online sample of 814 autistic children. The majority of children exhibited DST-related challenging behaviors daily or weekly, and over one third had physically injured themselves or others as a result of these behaviors (Law et al., 2016). Even when not a safety concern, DST contributes significantly to autism-related functional impairment, as many caregivers report that their children’s reactions to sounds prevent them from participating in a wide range of family, school, and community activities (Hussein et al., 2019; E. K. Jones et al., 2020; Law et al., 2016). Furthermore, aversions to sensory stimuli, such as DST, are often cited as reasons that autistic individuals find it difficult to seek medical care (Carter et al., 2017; Giarelli et al., 2014; Muskat et al., 2015; Nicholas et al., 2016). DST symptoms in autism are known to persist into adulthood (Elwin et al., 2013; Kuiper et al., 2019; Landon et al., 2016; A. E. Robertson & Simmons, 2015; Tavassoli et al., 2014), contributing to workplace difficulties (Hayward et al., 2019; Hedley et al., 2018; Lorenz et al., 2016; A. E. Robertson & Simmons, 2015), anxiety (Landon et al., 2016; Simonoff, 2020), avoidance behavior (Landon et al., 2016), and general distress (Griffith et al., 2011; R. S. P. Jones et al., 2003; Smith & Sharp, 2013). Notably, although autism is also associated with hyporeactivity to everyday auditory stimuli (Ben-Sasson et al., 2009, 2019; Law et al., 2016; Watts et al., 2016), this response pattern is far less prevalent than DST in the autistic population, and its effects on activity participation and functional impairment appear to be less pronounced (Law et al., 2016). Thus, we choose to focus this review solely on DST in autistic individuals, which we believe to be the sensory feature of autism with the largest overall impact on quality of life in this population.
Despite the prevalence and impact of DST in autism, there is currently insufficient evidence to support recommendations of any behavioral or pharmacologic treatment to reduce the severity or functional impact of this symptom (Fung et al., 2012; Sandbank et al., 2020; Schoen et al., 2019; Sinha et al., 2006; Weitlauf et al., 2017). One potential reason for the lack of evidence-based intervention in this area is the fact that the underlying mechanisms of autism-associated DST are still largely unknown, hindering the development of targeted interventions. Though a number of physiological and psychological processes have been suggested as potential sources of DST in autism (Marriage & Barnes, 1995; McCullagh et al., 2020; Pillion et al., 2018; Stiegler & Davis, 2010), there is still debate in the literature over whether DST behaviors reflect a disturbance in low-level auditory processing, abnormally strong emotional reactions to specific auditory stimuli, or a combination of the two. The purpose of the present article is to review the literature on DST in autism, incorporating published experimental and clinical observations into a unified theoretical framework. Synthesizing findings from the clinical and neuroscientific literature, we propose several potential neurocognitive models to explain the phenomenon of DST in autism. By doing so, we aim to generate testable hypotheses about the relationships between physiology, perception, and behavior, setting the stage for future research on DST mechanisms and bringing the field one step closer to the development of targeted interventions.
2. Definitions
The terminology used to describe DST in the medical literature has been extremely varied, with the words hyperacusis (Fagelson & Baguley, 2018; Tyler et al., 2014), misophonia (P. J. Jastreboff & Jastreboff, 2015; Schröder et al., 2013), phonophobia (Møller, 2011; Phillips & Carr, 1998), auditory over-responsivity (Tavassoli et al., 2019; Van Hulle et al., 2012, 2018), noise sensitivity (Stansfeld, 1992), auditory defensiveness (Goldsmith et al., 2006; Kern et al., 2006), noxacusis (Auerbach, 2019), and auditory hypersensitivity (Gomes et al., 2008) all used to describe specific patterns of sound intolerance across populations. The terminology used to describe DST as a symptom of autism specifically has also been highly inconsistent (Gomes et al., 2008; Landon et al., 2016; Stefanelli et al., 2020; Stiegler & Davis, 2010; Tavassoli et al., 2019), and thus we seek to provide definitions of several key terms before reviewing the literature on these topics. Although there is no unified standard of nomenclature for disorders of sound tolerance, we align our terminology with the first major academic text on this topic (Fagelson & Baguley, 2018), separating disorders of DST into the specific conditions of hyperacusis, misophonia, and phonophobia (Table 1; see also Tyler et al., 2014 for a similar framework).
Table 1.
Comparison of the Hyperacusis, Misophonia, and Phonophobia
| Hyperacusis | Misophonia | Phonophobia | |
|---|---|---|---|
| Definition | A hearing disorder in which sound of moderate intensity is perceived as excessively loud, painful, and/or overwhelming. | A neuropsychiatric condition in which individuals have excessive and inappropriate emotional responses to specific “trigger” sounds (e.g., chewing, tapping, sniffling), even when presented at a low level. | A specific phobia of particular sounds or classes of sounds, resulting in anticipatory responses and avoidance of potential sound sources. |
| Limited to specific sounds | No | Yes | Yes |
| Primary response to sound | Excessive loudness sensation and/or pain in ears/head | Anger, disgust, and/or extreme irritation | Fear and/or panic, anticipatory anxiety |
| Psychoacoustic loudness perception | Steeper loudness growth, reduced LDL | Normal | Unknown (possibly reduced LDL) |
| Influence of contextual factors | Low | High | High |
| Results in avoidance behavior | Yes | Yes | Yes |
| Associated with psychopathology | Yes | Yes | Yes |
| Occurs secondarily to | Cochlear hearing loss, noise trauma, brain injury | Unknown | Hyperacusis, reactive tinnitus, misophonia, posttraumatic stress disorder, other anxiety disorders |
| Proposed cognitive mechanism | Amplification of low-level sensory information resulting in a steeper growth of subjective loudness with increasing sound level. | Attribution of excess salience to particular sounds or associated perceptual phenomena (e.g., sights), resulting in conditioned aversive emotional response and heightened sympathetic arousal. | Pathological fear learning, combining excess attribution of threat to specific sounds with poor experience-dependent extinction. Maintained by avoidance of feared stimulus. |
| Implicated brain regions | Primary/secondary auditory cortex, inferior colliculus, auditory brainstem (superior olivary complex) | Salience network (anterior insular cortex, dorsal anterior cingulate cortex), ventromedial prefrontal cortex, posteromedial cortex, hippocampus, amygdala | Amygdala, hippocampus, anterior cingulate cortex, ventromedial prefrontal cortex, anterior insular cortex, bed nucleus of the stria terminalis/extended amygdala |
| Increased prevalence in autism | Yes | Unknown | Yes |
| Unique pathophysiology in autism | Yes | No | No |
Note. LDL = loudness discomfort level (the decibel level at which a sound starts being perceived as uncomfortably loud).
2.1. Hyperacusis
Hyperacusis is defined here as a hearing disorder involving an increased sensitivity or decreased tolerance to sound at levels that would not trouble most individuals. For the person experiencing hyperacusis, everyday sounds can be unpleasant, intense, painful, and/or overwhelming, and these sounds are often perceived as much louder than they actually are (Fackrell et al., 2019). This definition is more expansive than many that have previously been proposed, which have tended to focus exclusively on the perception of moderate-intensity sounds as overly loud (Fackrell et al., 2017; Phillips & Carr, 1998; Tyler et al., 2014). In their pivotal review on the topic, Tyler and colleagues (2014) proposed that hyperacusis could be separated into four subtypes characterized by loudness, annoyance, fear, and pain, occurring either singly or in combination. A more recent consensus definition of hyperacusis (Fackrell et al., 2019) differentiates hyperacusis from both misophonia (an acquired aversive reaction to specific “trigger” sounds characterized by anger, extreme annoyance, and disgust) and phonophobia (a persistent, abnormal and unwarranted fear of certain sounds). Thus, within our framework, hyperacusis refers specifically to loudness hyperacusis and pain hyperacusis, whereas the terms misophonia and phonophobia are proposed to refer to the constructs of annoyance hyperacusis and fear hyperacusis proposed by Tyler et al., respectively. Furthermore, despite the consensus definition of hyperacusis including the words “increased sensitivity,” it is well established that patients with hyperacusis do not have increased sensitivity to sounds in the psychoacoustic sense (i.e., lower auditory detection thresholds), and that many non-autistic individuals with hyperacusis exhibit some degree of hearing loss (Anari et al., 1999; Sheldrake et al., 2015; Tyler et al., 2014). Within the framework proposed by Ward (2019), a distinction is made between subjective sensory sensitivity (i.e., higher self-reported intolerance of sensory stimuli), neural sensory sensitivity (i.e., increased neural responses evoked by a given sensory stimulus), and behavioral sensory sensitivity (i.e., lower thresholds for stimulus detection and/or discrimination). However, as these three types of “sensory sensitivity” are generally unrelated to each other within the autistic population (Donkers et al., 2015; Dunlop et al., 2016; Gravel et al., 2006; Hudac et al., 2018; C. R. G. Jones et al., 2009; Kuiper et al., 2019; Millin et al., 2018; Tharpe et al., 2006), we believe the use of this single term to describe constructs at the behavioral, perceptual, and neural levels will lead researchers to commit the “jingle fallacy” (Dang et al., 2020), in which measures of “sensory sensitivity” at different levels are thought to tap the same construct. Thus, in accordance with Tyler et al. (2014) we refrain from using the term “hypersensitivity” to describe hyperacusis or any other form of DST, and we encourage autism researchers to use the term “sensory sensitivity” only when referring to behavioral stimulus detection or discrimination thresholds.
Loudness hyperacusis is a condition in which the threshold for loudness discomfort is reduced and sounds of moderate intensity are judged to be very loud (Phillips & Carr, 1998). Psychoacoustically, loudness hyperacusis can be thought of as an increased slope of the loudness growth function (i.e., steeper growth of perceived loudness as a function of sound intensity; Brandy & Lynn, 1995; Hébert et al., 2013; Noreña & Chéry-Croze, 2007). This condition is distinguishable from loudness recruitment, a steepening of the loudness growth function that occurs in the setting of cochlear hearing loss due to outer hair cell damage (Fowler, 1965; B. C. J. Moore et al., 1985; Preyer & Gummer, 1996). Notably, in loudness recruitment, the loudness of a sound “catches up” to that of a normal hearing listener, and at high levels the loudness growth functions of recruiting and normal-hearing ears will again overlap (B. C. J. Moore et al., 1985). This is in contrast to loudness hyperacusis, where the loudness growth curve diverges from that of a normal hearing listener without again merging with the normal loudness function (Brandy & Lynn, 1995; Noreña & Chéry-Croze, 2007).
Pain hyperacusis (Auerbach, 2019; Pollard, 2019; Tyler et al., 2014), also referred to as noxacusis, is a less well understood form of the condition in which an individual perceives physical pain in the ear when exposed to certain sounds at levels far below those needed to cause pain in a typical listener (i.e., approximately 120 dB sound pressure level [SPL], as loud as the front row of a rock concert; Plutchik, 1963). Although the character of the pain varies greatly across individuals (e.g., dull ache, burning, sharp, stabbing, or throbbing), 62% of hyperacusis patients responding to an online survey reportedly experienced ear pain at least daily (Pollard, 2019). It is currently unclear whether the pain experienced in this condition is the same pain experienced by typical listeners at extremely high sound levels or an entirely separate phenomenon with distinct neurophysiologic substrates. Recent discoveries have implicated the population of type II cochlear afferent neurons in mediating the perception of noxious or painful auditory stimuli (Flores et al., 2015; Liu et al., 2015; Zhang & Coate, 2017), but the role of these cells in pain hyperacusis remains to be determined.
Throughout this review, the term “hyperacusis” will be used to refer generally to both loudness and pain hyperacusis, regardless of neurological, psychiatric, otologic, or idiopathic etiology (Tyler et al., 2014). The specific terms “loudness hyperacusis” and “pain hyperacusis” will be utilized when referring to only one of the subtypes.
2.2. Misophonia
Misophonia, also called selective sound sensitivity syndrome (Neal & Cavanna, 2013) or annoyance hyperacusis (Danesh & Aazh, 2020; Tyler et al., 2014), is a newly described neuropsychiatric condition in which individuals have excessive and inappropriate emotional responses to specific “trigger” sounds (e.g., chewing, tapping, sniffling), even when presented at a low level (Brout et al., 2018; Claiborn et al., 2020; Jager et al., 2020; Palumbo et al., 2018; Potgieter et al., 2019; Schröder et al., 2013). The specific triggers and their elicited responses vary greatly across individuals and may change over time, although the predominant emotional response tends to involve some form of anger, disgust, or irritation (Jager et al., 2020) and increased autonomic arousal (Edelstein et al., 2013; Kumar et al., 2017). This emotional response is often accompanied by an idiosyncratic set of physical symptoms (e.g., muscle tension, paresthesias, abdominal discomfort, or other physical sensations; Claiborn et al., 2020; Dozier & Morrison, 2017). Moreover, misophonic reactions are in some cases evoked by specific visual stimuli as well (e.g., the sight of a person chewing can trigger anger and physical sensations, even in the absence of a sound), a phenomenon that has been termed “misokinesia” (Potgieter et al., 2019). Interestingly, the context in which these triggers are encountered modulates the response substantially, with more intense responses being reported when triggers are experienced in a familiar context (Potgieter et al., 2019) and fewer or less intense responses occurring when the triggering sound is made by a toddler or an adult with cognitive impairment (Jager et al., 2020). Triggering sounds also do not seem to typically evoke a response when produced by the individuals with misophonia themselves (Potgieter et al., 2019).
Compared with hyperacusis, misophonia is a relatively new concept, first described in the academic literature in 2002 (M. M. Jastreboff & Jastreboff, 2002). However, it was not until 2013, when diagnostic criteria for this disorder were proposed (Schröder et al., 2013), that misophonia began to attract the attention of researchers in psychology, psychiatry, and audiology. Although misophonia research remains in its infancy, there is sufficient evidence to indicate that misophonia is a distinct behavioral syndrome that can result in substantial distress and functional impairment (Brout et al., 2018; Jager et al., 2020; Potgieter et al., 2019; Schröder et al., 2013).
2.3. Phonophobia
The term phonophobia, literally meaning “fear of sound,” is commonly used in neurology to describe the sound intolerance that often accompanies migraine headaches (Ashkenazi et al., 2009; Irimia et al., 2008; Vingen et al., 1998; Woodhouse & Drummond, 1993). However, in the current framework, we define phonophobia as a specific phobia of particular sounds or classes of sounds, resulting in anticipatory responses and avoidance of potential sound sources (Baguley & McFerran, 2011; Landgrebe & Langguth, 2011; Møller, 2011; Stiegler & Davis, 2010). Our definition of phonophobia is nearly synonymous with that of Tyler’s (2014) “fear hyperacusis,” whereas the “phonophobia” described in migraine could be better classified within this framework as episodic loudness hyperacusis (Suhnan et al., 2017). Although a fear of loud noises is common in childhood (Meltzer et al., 2009; Ollendick et al., 2002), a diagnosis of phonophobia would require an amount of fear and impairment out of proportion to what one would expect for that individual’s developmental level (American Psychiatric Association, 2013). Furthermore, while both misophonia and phonophobia can involve inappropriate emotional reactions to specific sounds, the misophonic reaction primarily involves anger and/or disgust, whereas phonophobia is characterized by fear and/or anxious preoccupation. Notably, phonophobia as defined here often develops secondary to other forms of DST, as some patients with hyperacusis or misophonia may develop excessive anxiety with respect to situations in which they may encounter painful or otherwise distressing sounds (Blaesing & Kroener-Herwig, 2012; Goebel & Floezinger, 2008; Jager et al., 2020; Jüris et al., 2014; Landgrebe & Langguth, 2011).
2.4. Noise Sensitivity
Unlike the previous terms, noise sensitivity is not a diagnosable medical condition but is instead a stable personality trait that moderates one’s reactions to noise. It is not, however, a true “sensory sensitivity,” as this trait is unrelated to auditory detection thresholds (Stansfeld et al., 1985). Defined by Job (1999, p. 58), noise sensitivity refers to “the internal states (be they physiological, psychological [including attitudinal], or related to life style or activities conducted) of any individual which increase their degree of reactivity to noise in general.” Noise sensitivity is thought to moderate the degree to which a person finds any given noise annoying, irrespective of overall noise exposure (Kliuchko et al., 2016; van Kamp et al., 2004). Compared to the general population, noise-sensitive individuals attend more to noises, find noises more threatening and out of their control, and adapt to noises more slowly (Stansfeld, 1992; Stansfeld & Clark, 2019). Unlike hyperacusis, noise sensitivity is not associated with changes in loudness functions (Ellermeier et al., 2001; Stansfeld et al., 1985), although self-reports of hyperacusis symptoms and noise sensitivity may correlate highly in some populations (Viziano et al., 2017). Notably, although most noise-sensitive individuals do not have hyperacusis, patients with hyperacusis report high levels of noise sensitivity (Paulin et al., 2016), likely as a result of the discomfort they experience in response to many everyday sounds.
2.5. Decreased Sound Tolerance
We define decreased sound tolerance (DST) as an umbrella term encompassing hyperacusis, misophonia, and phonophobia (P. J. Jastreboff & Jastreboff, 2014, 2015). This term is preferred over equally general terms such as “auditory defensiveness” and “auditory over-responsivity,” as it refers to the subjective percept of the sound as intolerable without describing any particular behavioral reaction. The personality trait of noise sensitivity is not included within this definition, though high levels of noise sensitivity may be seen in those with DST (Paulin et al., 2016). Similarly, individuals with chronic subjective tinnitus are not necessarily classified as having DST, although comorbid hyperacusis (Tyler et al., 2014) and phonophobia (Blaesing & Kroener-Herwig, 2012; Landgrebe & Langguth, 2011) are commonly observed in this population and can be diagnosed separately. While the labels of hyperacusis, misophonia, and phonophobia are better able to describe the phenomenology of an individual’s sound intolerance, the label of DST is useful when there is a lack of certainty about the underlying reasons for an individual’s sound-induced distress or discomfort (e.g., in nonverbal individuals who cannot describe why certain sounds are aversive). Thus, we use DST to refer to the behavioral hyperreactivity to auditory stimuli seen in individuals on the autism spectrum, as it is currently unclear whether these behaviors are due to hyperacusis, misophonia, phonophobia, or some combination thereof (Stiegler & Davis, 2010). As we argue in the following section, all three conditions likely contribute to the high rates of DST behavior seen in autistic individuals, and further research is needed to determine their relative contributions to the autistic DST phenotype.
3. Phenomenology of Decreased Sound Tolerance in Autism
Although a sizable body of literature has been published on DST in autism (Gomes et al., 2008; Moossavi & Moallemi, 2019; O’Connor, 2012; Stefanelli et al., 2020; Stiegler & Davis, 2010), there has been relatively little research to date systematically examining the specific sounds that autistic individuals find distressing or which aspects of the stimuli these individuals find difficult to tolerate. Although some first-person accounts of autistic people contain descriptions of subjective DST (Bemporad, 1979; Cesaroni & Garber, 1991; DePape & Lindsay, 2016; Elwin et al., 2012, 2013; Howe & Stagg, 2016; R. S. P. Jones et al., 2003; Kirby et al., 2015; Landon et al., 2016; Preece & Jordan, 2010; A. E. Robertson & Simmons, 2015; Smith & Sharp, 2013; Stiegler & Davis, 2010; Volkmar & Cohen, 1985), these accounts often do not provide enough detail of the individual’s perception of sound to confidently classify the reported DST as hyperacusis, misophonia, or phonophobia. An exception is the qualitative study by Landon and colleagues, which focused exclusively on the phenomenology of DST in autistic adults (Landon et al., 2016). In this study, researchers interviewed 10 adults with diagnosed autism and self-reported DST, asking questions about the participants’ experiences of sounds, the qualities of sounds that they felt caused their distress, specific events that produced aversive reactions, and strategies employed to cope with sounds in everyday life. Participants consistently regarded loud, sharp, or otherwise high-pitched sounds to be the most distressing, although several also indicated that specific sounds elicited strong emotional reactions regardless of level. The authors concluded that the participants reported experiences were consistent with both hyperacusis and “an intolerance to specific sounds” (i.e., misophonia). Anxiety and avoidance also played a prominent role in the participants’ responses to noise, indicating that a number of participants likely would be considered to have phonophobia as well.
Another frequently reported symptom in the Landon study was a tendency to be easily distracted by noises, even in relatively innocuous or quiet situations (Landon et al., 2016). Although auditory distractibility is pertinent to the overall sensory phenotype of autism, this process is distinct from DST as it is typically defined (Fagelson & Baguley, 2018; P. J. Jastreboff & Jastreboff, 2015). Moreover, auditory distractibility is likely mediated by higher-order cognitive factors such as working memory capacity and selective attention (Sörqvist, 2010; Sörqvist & Marsh, 2015; Sörqvist & Rönnberg, 2014), which are known to differ in the autistic population (Habib et al., 2019). Individuals with attention-deficit/hyperactivity disorder (ADHD) are also particularly susceptible to the effects of auditory distractions (Micoulaud-Franchi et al., 2015; Pelletier et al., 2016; Zentall & Shaw, 1980), and thus the high prevalence of co-occurring ADHD in autistic children and adults (Lai et al., 2019; Lugo-Marín et al., 2019) may contribute to the high rates of auditory distractibility seen in this population. A failure to habituate to low-level sounds may also play a role, as preliminary work has demonstrated reductions in simple loudness adaptation in autistic adults relative to neurotypical controls (Lawson et al., 2015). Alternatively, this symptom may be related to the ability of individuals on the autism spectrum to simultaneously process a larger amount of auditory information than neurotypical controls, a difference supported by data from laboratory-based auditory detection tasks (Brinkert & Remington, 2020; Remington & Fairnie, 2017). Auditory distractibility is also a known correlate of noise sensitivity (Belojević et al., 1992; Kjellberg et al., 1996; Sandrock et al., 2008), and the enhanced distractibility seen in autism could potentially contribute to more autistic individuals developing noise sensitivity. However, as the correlates of auditory distractibility in autism have yet to be empirically tested, these relationships remain purely speculative at this time.
In sum, the most detailed phenomenological account of DST in autism indicates that the auditory challenges in this population encompass hyperacusis, misophonia, phonophobia, and potentially noise sensitivity as well (Landon et al., 2016). Accordingly, questions of whether the DST seen in autism is physiological or psychological in origin (Stiegler & Davis, 2010) present a false dichotomy, as abnormalities in lower-level perceptual processes (i.e., hyperacusis) and higher-level cognitive processes (i.e., misophonia and phonophobia) each likely contribute to DST in a portion of autistic individuals (Danesh et al., 2015; Demopoulos & Lewine, 2016; Khalfa et al., 2004; Landon et al., 2016; B. Y. Lau et al., 2020; A. E. Robertson & Simmons, 2015). Still, it remains possible that a “dominant” pattern of sound intolerance (e.g., loudness hyperacusis) exists in this population. Thus, in sections 3.1–3.3, we review the published literature suggesting that DST in autism is characterized by (a) hyperacusis, (b) misophonia, and (c) phonophobia.
3.1. Hyperacusis in Autism
An aversion to loud stimuli is one of the most prominent forms of DST reported in both personal accounts and clinical descriptions of autism (O’Connor, 2012; Phillips & Carr, 1998; Stiegler & Davis, 2010; Tyler et al., 2014). Furthermore, when autistic people complete standardized self-report questionnaires assessing hyperacusis symptoms, they routinely endorse higher symptom levels than neurotypical controls (Danesh et al., 2015; Dunlop et al., 2016; Keith et al., 2019). Though such reactions are highly consistent with hyperacusis, it remains unclear whether individuals on the autism spectrum are more likely to manifest the loudness or pain subtype. Personal accounts of autism often describe certain sounds as physically painful (Elwin et al., 2012; Howe & Stagg, 2016; R. S. P. Jones et al., 2003; Kirby et al., 2015; Landon et al., 2016; A. E. Robertson & Simmons, 2015; Smith & Sharp, 2013); however, there are no empirical studies quantifying the number of autistic individuals who experience physical pain in response to everyday sounds. Alternatively, several studies indicate that loudness growth functions assessed via categorical loudness scaling are steeper in persons on the autism spectrum (Dunlop et al., 2016; Khalfa et al., 2004; Ohmura et al., 2019), confirming the presence of loudness hyperacusis in those participants. Notably, an unpublished study by our group of 243 patients with self-reported hyperacusis contained four individuals on the autism spectrum (Williams, Suzman, et al., 2020a), two of whom reported pain hyperacusis (one with comorbid misophonia and the other with comorbid phonophobia) and two of whom reported loudness hyperacusis (one with comorbid phonophobia and the other reporting no other DST conditions). Three of these individuals (two loudness, one pain) had also received diagnoses of hyperacusis from physicians or audiologists. These data, although preliminary, support the contention that both loudness and pain hyperacusis are present in some individuals on the autism spectrum, although the portion of individuals experiencing each subtype has yet to be precisely determined.
As further evidence of hyperacusis in autism, individuals on the autism spectrum also tend to exhibit lower loudness discomfort levels (LDLs) than neurotypical controls (Demopoulos & Lewine, 2016; Khalfa et al., 2004; Rosenhall et al., 1999; Steigner & Ruhlin, 2014). During LDL testing, a discrete sound stimulus (typically about one second in duration) is repeatedly presented with increasing intensity until the participant verbally or behaviorally indicates that the sound has become uncomfortably loud (Punch et al., 2004). In one of the first studies objectively assessing sound tolerance in autism, Rosenhall et al. (1999) reported that 18% of autistic children were unable to tolerate an 80 dB nHL broadband click stimulus as part of an auditory brainstem testing protocol (although all could tolerate be tested at 70 dB nHL). Notably, only one autistic child with sound tolerance problems had a sensorineural hearing loss, indicating that loudness recruitment could not account for this phenomenon in the majority of the sample. A smaller but more comprehensive study by Khalfa et al. (2004) confirmed the presence of enhanced loudness perception in autism, reporting that 63% of their autism group had LDLs for pure tones below 80 dB HL. In a higher-IQ group of autistic children, Demopoulos and Lewine (2016) tested LDLs for speech stimuli, finding that 37% of their autism group reported LDLs three or more standard deviations below the neurotypical group’s mean in at least one ear. Steigner and Ruhlin (2014) also reported the case of an autistic man with profoundly reduced tolerance of low-frequency sounds, confirmed by abnormally low LDLs to tones in the low frequencies and normal LDLs in the high frequencies (≥2000 Hz). Two additional studies have used behavioral observations to test sound tolerance in autistic children, finding that only a small minority of individuals exhibited behavioral signs of distress when exposed to high-level sounds (Gomes et al., 2004; Lucker, 2013). However, both of these studies were limited by their reliance solely on observable behavioral reactions; they were unable to rule out the possibility that additional participants experienced the sounds as uncomfortable but failed to communicate that discomfort in a “typical” way (Keating & Cook, 2020; D. J. Moore, 2015). Despite mixed results concerning behavioral loudness tolerance, multiple studies demonstrating reduced LDLs in autism suggest that loudness hyperacusis is prevalent in this population, although other forms of DST (i.e., pain hyperacusis or phonophobia) may also contribute to these abnormalities.
To further assess the role of loudness in autism-associated DST, we also consider the literature on intensity discrimination in this population. In persons with cochlear hearing loss and loudness recruitment (increased loudness growth due to outer hair cell damage), it has been hypothesized that steepened loudness functions translate into superior intensity discrimination ability (Buus et al., 1982; although see Huettel & Collins, 2004; Schroder et al., 1994 for an alternative explanation based on spread of excitation). Thus, based on the steeper loudness functions observed in autism, it would stand to reason that autistic people may also possess superior intensity discrimination ability compared to neurotypical listeners. However, the literature on psychophysical intensity discrimination in autism has not confirmed this hypothesis, with the majority of studies finding intensity discrimination abilities in persons on the autism spectrum to be normal (Alcántara et al., 2012; Bonnel et al., 2010; C. R. G. Jones et al., 2009) or even impaired (Kargas et al., 2015) relative to neurotypical controls. While these data are inconsistent with the theory that abnormal loudness growth in autism leads to enhanced intensity discrimination, it is notable that none of these studies concomitantly measured loudness functions and intensity discrimination thresholds. It is therefore still possible that the subset of individuals on the autism spectrum with steep loudness growth curves would exhibit superior intensity discrimination when compared to typical controls. Further work on loudness and intensity processing in autism is necessary to extend these findings, specifically addressing whether subjective reports of hyperacusis can be linked to psychoacoustic disturbances of loudness perception.
3.2. Misophonia in Autism
As misophonia has only recently been recognized as a distinct DST condition, far fewer studies have considered it as a potential explanation for observed behavioral reactions to auditory stimuli in autism (Stiegler & Davis, 2010). Furthermore, no study has specifically been conducted to assess the prevalence of misophonia in autistic individuals. Nevertheless, in the clinical sample of misophonia patients described by Jager et al. (2020), 14 of 575 (3%) were diagnosed with comorbid autism spectrum disorder. Notably, individuals with “primary autism spectrum conditions” referred for misophonia evaluation were excluded from the sample (i.e., misophonia in the aforementioned 14 individuals was judged to be “separate” from autism), indicating that the overlap between these conditions could potentially be substantially larger. Additionally, 38 of 1061 individuals (3.6%) in a large online sample of adults with self-reported misophonia reported having been diagnosed with autism (Claiborn et al., 2020), a prevalence nearly double that of the general population (Dietz et al., 2020). In another large clinical sample, over one fourth of children referred to a specialist pediatric hyperacusis center (60% of whom were diagnosed with autism) identified classic misophonia triggers (i.e., “people chewing, breathing, and snoring”) as the specific sounds causing distress (Amir et al., 2018). Lastly, in the survey of DST in autism performed by Law et al. (2016), many parents reported that environmental sounds made their children irritable (61.3%), frustrated (43.9%) or annoyed (40.9%), raising the possibility that some of these episodes may arise from misophonic reactions (Potgieter et al., 2019).
A number of personal accounts also support the contention that autistic individuals with DST may be experiencing misophonia-like symptoms (Cesaroni & Garber, 1991; Grinker, 2007; Landon et al., 2016; A. E. Robertson & Simmons, 2015; Smith & Sharp, 2013). A particularly classic account of an autistic person displaying misophonic reactions comes from anthropologist Roy Grinker, who described his autistic teenage daughter Isabel’s reaction to very specific sounds such as throat-clearing and the sounds of certain words:
…she still hates certain sounds, like a baby crying, the car alarm that tells you your seatbelt isn’t fastened, or the sound of a bathtub draining. When she hears them, she gets agitated, holds her hands over her ears, and vocalizes to block out the sound. She has the same reaction when she hears me clear my throat, or when someone says words associated with bathing, such as “bath,” “shower” or “shampoo.” (Grinker, 2007, p. 299)
Other examples have been published of autistic adults reacting strongly to sounds considered to be common misophonic triggers, “Small noises annoy me, like breathing, crunching food or … someone whistling … it makes me ratty” (A. E. Robertson & Simmons, 2015, p. 575) or describing experiences of being emotionally “triggered” by sounds in a way they retrospectively view as excessive:
… there is all this energy racing around inside me out of irritation and thinking about these things… its building up inside and you can’t let it out, you can’t go “ahhhhh be quiet” or whatever and can’t be jumping up and down or doing anything… (Landon et al., 2016, p. 47).
Remarkably, many of these individuals reporting misophonia-like experiences also seemed to concurrently experience problems tolerating loud noises (Landon et al., 2016), suggesting that a number of autistic individuals with DST may meet criteria for both misophonia and hyperacusis. Overall, these results together provide substantial evidence that at least some cases of DST in autism are likely due to misophonia, although additional research is needed to estimate the prevalence of misophonia, its co-occurrence with other types of DST, and its functional consequences in this population.
3.3. Phonophobia in Autism
Comorbid anxiety disorders are exceedingly common in autistic individuals (Hollocks et al., 2019; Lai et al., 2019), presenting as a mixture of “typical” anxiety symptoms and “atypical” fears that are unique to autism (Kerns et al., 2014, 2016; Kerns & Kendall, 2012; B. Y. Lau et al., 2020; Rodgers et al., 2016; Scahill et al., 2019; Wood & Gadow, 2010). Among these autism-specific fears, a number of studies have listed loud noises (either loud noises in general or specific noises such as toilets flushing) as the object of a child’s specific phobia (Gjevik et al., 2011; Kerns et al., 2014, 2016; B. Y. Lau et al., 2020; Leyfer et al., 2006; Mayes et al., 2013; Mukaddes & Fateh, 2010; Ozsivadjian et al., 2012), and multiple autism-specific anxiety questionnaires include items directly assessing a fear of loud noise (Rodgers et al., 2016; Scahill et al., 2019). This particular fear is present in a sizable minority of autistic children, with one large study finding that 23% had a phobia of loud sounds (e.g., babies crying, hairdryer sounds), and an additional 6% had fears of other specific sounds (B. Y. Lau et al., 2020). In addition, over half of autistic children with DST in the study by Law et al. (2016) were reported to become “scared” (55%) or “nervous” (54%) in response to bothersome sounds. No study has investigated the prevalence of phonophobia in adults on the autism spectrum, but qualitative reports indicate that many autistic adults continue to experience anxiety in response to loud noises (Simonoff, 2020), resulting in patterns of avoidance consistent with a specific phobia of these sounds (Bemporad, 1979; Cesaroni & Garber, 1991; Landon et al., 2016; Smith & Sharp, 2013).
Although published studies confirm the presence of phonophobia in many individuals on the autism spectrum, one major unresolved question in this area is whether this phonophobia is secondary to hyperacusis or unrelated to abnormalities in loudness perception. As noted by Jastreboff and Jastreboff (2015), phonophobia is practically inevitable in cases of severe hyperacusis, as anxious apprehension and hypervigilance directed toward everyday sounds can easily develop from repeated exposures to extremely unpleasant/painful stimuli. Kerns and colleagues (2016) provide a relevant case example, in which an autistic child develops an anticipatory fear of fire alarms as a result of pre-existing hyperacusis. However, these authors argue that the majority of DST-related impairment experienced by this patient is due to hyperacusis, and therefore a diagnosis of specific phobia is not warranted unless a more impairing pattern of avoidance behavior develops. Interestingly, there is some limited evidence that behavioral tolerance of the aversive sound stimulus can be achieved by treating the phobia with graded exposure (Johnston et al., 2020; Koegel et al., 2004). This may indicate a bidirectional relationship between sound-induced discomfort and anxiety, whereby interventions targeting either hyperacusis or phonophobia symptoms have the potential to reduce symptoms in the other domain. As acute stress can decrease LDLs in some individuals (Hasson et al., 2013), reducing phobic anxiety could feasibly improve both the emotional reaction to the feared sound and its perceived loudness. However, as these studies did not include judgments of subjective loudness or pain, any effects of exposure therapy on the perceptual qualities of aversive sounds remain speculative. An alternative possibility is that the participants in these studies did not suffer from hyperacusis at all, and thus treatment of phonophobia was sufficient to eliminate all DST in those individuals. Additional research is necessary to determine whether autistic people with psychoacoustically-confirmed hyperacusis can experience a reduction in subjective DST from interventions targeting phonophobia or anxiety in general.
4. Potential Mechanisms of Decreased Sound Tolerance in Autism
While DST in autism is frequently discussed from a clinical perspective, the neurobiological correlates of this symptom cluster remain poorly understood. A wide range of abnormalities in the structure and function of the auditory system have been demonstrated in both autistic individuals and preclinical animal models of autism (Beers et al., 2014; Chin et al., 2013; McCullagh et al., 2020; O’Connor, 2012; Pillion et al., 2018; Williams, Abdelmessih, et al., 2020), but few theoretical models have been constructed to link these findings to behavioral sound intolerance. Instead, current theoretical frameworks for sensory hyperreactivity in autism tend to focus on multi-modality sensory processing (Mottron et al., 2006; Pellicano & Burr, 2012; C. E. Robertson & Baron-Cohen, 2017; Van de Cruys et al., 2014; Ward, 2019) in an attempt to explain the wide range of sensory differences seen in autistic individuals. Alternatively, a number of theoretical mechanisms of DST have been proposed based on findings in non-autistic individuals (Auerbach, 2019; Auerbach et al., 2014; Brout et al., 2018; Diehl & Schaette, 2015; P. J. Jastreboff & Jastreboff, 2014, 2015; Kumar et al., 2017; Palumbo et al., 2018; Pienkowski, 2019; Pienkowski et al., 2014; Sheppard et al., 2020; Suhnan et al., 2017), few of which have been specifically considered in the context of autism-associated DST. Thus, the purpose of this section is to synthesize the literature on these related topics, generating explanatory neurocognitive models to account for the presence of hyperacusis, misophonia, and phonophobia in individuals on the autism spectrum. Although evidence to support our proposed mechanisms of DST in autism is limited at this time, these theoretical models will provide a framework for future research in this area, generating hypotheses about the underlying neurobiology of DST and providing context for findings related to auditory physiology in autism and related conditions.
4.1. Model of Hyperacusis: Enhanced Central Gain
Although the underlying biology of hyperacusis is still poorly understood, a growing body of evidence suggests that the symptoms of loudness hyperacusis are generated by a pathological increase in central auditory gain (Auerbach, 2019; Auerbach et al., 2014; Diehl & Schaette, 2015; Pienkowski, 2019; Salvi et al., 2020; Sheppard et al., 2020) in which “neural activity from more central auditory structures is paradoxically increased at suprathreshold intensities” (Auerbach et al., 2014, p. 1). In particular, animal models of hyperacusis display steeper neural input-output functions in multiple cortical and subcortical areas, with the largest increases seen in the inferior colliculus (IC) and primary auditory cortex (Auerbach et al., 2019; Y.-C. Chen et al., 2015; Ma et al., 2020; Salvi et al., 2020; Stolzberg et al., 2012; Wong et al., 2020). Although there have been few studies examining sound-evoked neural activity in humans with hyperacusis (J.-H. Hwang et al., 2009), those that have examined hyperacusis in tinnitus patients have typically found increased activity comparable to that seen in these animal models (Gu et al., 2010; Lanting et al., 2008; Melcher et al., 2009). As activity in the primary and secondary auditory cortex is thought to encode loudness rather than intensity (Behler & Uppenkamp, 2016, 2020; Langers et al., 2007; Röhl & Uppenkamp, 2012; Schmidt et al., 2020; Schreiner & Malone, 2015; Uppenkamp & Röhl, 2014), hyperactive responses to sounds in these areas are hypothesized to be the substrate of loudness hyperacusis. While some studies have indicated that this enhanced neural activity may be insufficient to explain hyperacusis-like behavior in animal models (Möhrle et al., 2019; Radziwon et al., 2019), the central gain model remains the most empirically-supported model of hyperacusis pathophysiology proposed to date (Auerbach, 2019; Auerbach et al., 2014; Diehl & Schaette, 2015; Pienkowski, 2019; Sheppard et al., 2020).
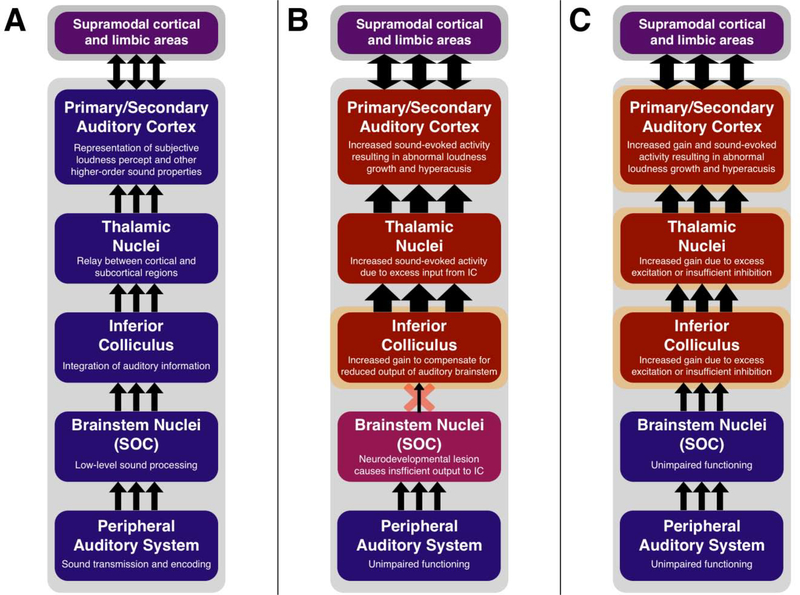
Given the strong associations between hyperacusis, tinnitus, and noise exposure (Pienkowski et al., 2014; Tyler et al., 2014), the central gain model of hyperacusis typically posits that an increase in central gain is a homeostatic consequence of reduced neural output from the cochlea (i.e., sensorineural hearing loss or potentially “cochlear synaptopathy”; Auerbach et al., 2014; Hickox & Liberman, 2014). However, rates of peripheral hearing loss are not greatly elevated in persons on the autism spectrum (Beers et al., 2014), and the majority of autistic individuals with reduced LDLs have hearing acuity within normal limits (Demopoulos & Lewine, 2016; Dunlop et al., 2016; Khalfa et al., 2004; Rosenhall et al., 1999), indicating that the central gain mechanism that putatively drives hyperacusis in autism may arise via a different mechanism. Though we are only able to speculate about the ways in which aberrant neurodevelopment in autism could contribute to enhanced central auditory gain, multiple plausible mechanisms exist based on current understandings of autism neuropathology (Figure 1).
Figure 1.
(A) Schematic of ascending information pathway in the central auditory system. Descending and recurrent connections have been omitted for simplicity. (B) Hypothesized model of enhanced central gain and hyperacusis in autism resulting from impaired brainstem transmission. Homeostatic upregulation of gain in the inferior colliculus (IC) results in excessive sound-evoked activity in the higher structures of the auditory system, including primary/secondary auditory cortex, the putative source of enhanced loudness perception. (C) Alternate model of enhanced central gain and hyperacusis in autism as a result of excitation/inhibition imbalance in the IC, thalamus, and/or auditory cortex. Notably, enhanced auditory cortical activity is likely sufficient to cause hyperacusis, and while other central auditory structures may contribute, pathology could feasibly be limited to cortical areas. SOC = Superior Olivary Complex.
One likely source of enhanced central gain in autism is auditory brainstem dysfunction, which has been observed in both idiopathic autism and several neurodevelopmental syndromes that frequently cause autism, such as fragile X syndrome (McCullagh et al., 2020; Pillion et al., 2018). Postmortem human and rodent studies have documented a number of abnormalities in auditory brainstem nuclei, with multiple studies converging on pathology in the superior olivary complex (Eto et al., 2017; Kulesza et al., 2011; Kulesza & Mangunay, 2008; Li et al., 2003; Lukose et al., 2011, 2015; Mansour et al., 2019; Mansour & Kulesza, 2020; McCullagh et al., 2020; Rodier et al., 1997; Rotschafer et al., 2015; Rotschafer & Cramer, 2017; Toyoshima et al., 2009; Zimmerman et al., 2018, 2020), a group of nuclei that mediates aspects of binaural processing and the efferent auditory pathways (Guinan, 2006, 2018; Laumen et al., 2016). Though data are mixed regarding the presence and nature of functional auditory brainstem abnormalities in autism (Bennetto et al., 2017; Dabbous, 2012; Danesh & Kaf, 2012; ElMoazen et al., 2020; Gravel et al., 2006; Kaf & Danesh, 2013; B. K. Lau et al., 2018; Lukose et al., 2013; McCullagh et al., 2020; Mercati et al., 2017; Miron et al., 2018, 2020; Ohmura et al., 2019; Pillion et al., 2018; Santos et al., 2017; Talge et al., 2018; Tharpe et al., 2006; Wilson et al., 2017), preliminary studies have indicated that certain auditory brainstem assays may differentiate autistic individuals with and without concurrent hyperacusis (Mercati et al., 2017; Ohmura et al., 2019; Wilson et al., 2017). In cases where such brainstem dysmorphology does exist, data from animal models suggests that fewer inhibitory axons project from various areas of the auditory brainstem to the IC (Li et al., 2003; Zimmerman et al., 2020). As the central gain model typically posits increased spontaneous and sound-evoked activity in the IC and higher auditory regions (Auerbach et al., 2014), a plausible model of central gain in autism would likely include similar enhancements of activity in these areas, brought on by homeostatic adaptations to aberrant brainstem information transfer. We therefore propose a model wherein structural abnormalities in the auditory brainstem during early neurodevelopment lead to compensatory changes in the IC and auditory cortex in autism, which result in an overall increase in central auditory gain and hyperacusis symptoms (Figure 1B). As the percept of subjective loudness (rather than sound intensity) is not seemingly represented until auditory cortex (Behler & Uppenkamp, 2016; Langers et al., 2007; Röhl & Uppenkamp, 2012; Schreiner & Malone, 2015; Uppenkamp & Röhl, 2014), increased central gain occurring only in cortical structures may also reasonably explain the phenomenon of hyperacusis in autism. Further research using both animal models and human neuroimaging is thus necessary to validate this model, investigating the presence of increased central auditory gain in autism at either the cortical or subcortical level.
An alternative explanation for increased central auditory gain in autism is the presence of an imbalance in the ratio of excitatory and inhibitory neurotransmission (E/I ratio) in key subcortical and cortical auditory structures (McCullagh et al., 2020), leading to an overall increase in activity of the IC, thalamus, and/or auditory cortex (Figure 1C). Such an increase in E/I ratio is not inconsistent with the previous hypothesis of brainstem dysfunction, as an imbalanced E/I ratio could feasibly serve as the circuit-level substrate of decreased brainstem information transfer or the homeostatic activity upregulation seen in the IC and/or auditory cortex (McCullagh et al., 2020; Zimmerman et al., 2020). Furthermore, it is quite likely that an increased E/I ratio is responsible for increases in central gain seen in models of hyperacusis elicited by salicylates (G.-D. Chen et al., 2013; Gong et al., 2008; Lu et al., 2011; W. Sun et al., 2009) or acoustic trauma (Heeringa & van Dijk, 2016; Ma et al., 2020; Salvi et al., 2000; Scholl & Wehr, 2008; Wei Sun et al., 2012).
A model of increased E/I ratio has long been posited as a common final pathway to explain the core features of autism (Culotta & Penzes, 2020; Foss-Feig et al., 2017; Lee et al., 2017; Nelson & Valakh, 2015; Rubenstein & Merzenich, 2003; Sohal & Rubenstein, 2019) as well as some associated conditions such as epilepsy (Bozzi et al., 2018). Although some recent data have suggested that this imbalance is an epiphenomenon of autism rather than its underlying cause (Antoine et al., 2019), this distinction is irrelevant when determining whether E/I imbalance is responsible for hyperacusis and enhanced central gain in some autistic individuals. The E/I imbalance model was further elaborated by Ward (2019) as a potential explanation for the wide range of sensory features seen in autism. In this framework, an increased E/I ratio results in both an increased signal level but also increased noise within a neural circuit’s computations. Thus, a given sensory stimulus will elicit a stronger neural response, activating larger areas of cortex and corresponding to a more intense (and in the case of hyperacusis, louder) percept. Although evidence for this model of sensory hyperreactivity is mixed (Dickinson et al., 2016; Ward, 2019), there are no studies specifically investigating the relationship of loudness hyperacusis and E/I imbalance in humans. A single study (Naples et al., 2020) has examined the relationship between self-reported “auditory sensitivity” (as measured by the Glasgow Sensory Questionnaire; A. E. Robertson & Simmons, 2013) and E/I imbalance (as measured by the Hurst exponent of the resting EEG power spectrum) in a sample of adults with and without diagnoses of autism or schizophrenia, finding a moderate correlation between the two metrics. In addition, comorbid epilepsy is associated with DST in autistic children (Law et al., 2016), implicating E/I imbalance as a potential cause of both co-occurring conditions. Furthermore, both rodent and human studies suggest that the phenotype of DST and increased auditory cortical activity seen in fragile X syndrome (Ethridge et al., 2016; McCullagh et al., 2020; Rotschafer & Razak, 2013) can be attributed to an E/I imbalance in auditory cortex (Ethridge et al., 2017; Lovelace et al., 2020; Wen et al., 2018). Given the plausibility of E/I imbalance as a mechanism for central auditory gain and hyperacusis in autism, future studies on this topic should incorporate human neuroimaging metrics of E/I balance (Bruining et al., 2020; Dickinson et al., 2016; Trakoshis et al., 2020), testing whether psychoacoustically-defined loudness growth is associated with an excess of excitatory neurotransmission.
4.2. Model of Misophonia: Conditioned Allocation of Excess Salience
In contrast to hyperacusis, there has been relatively little research seeking to understand the pathological basis of misophonia in non-autistic individuals. Based on neurophysiologic models of tinnitus and hyperacusis, Jastreboff and Jastreboff (2014, 2015) proposed a model of misophonia/phonophobia (considered to be the same construct in their framework) in which the processing of low-level auditory information is intact, but “the functional connections between the auditory and the limbic and autonomic nervous systems are enhanced for specific patterns of sound,” (P. J. Jastreboff & Jastreboff, 2014, p. 111). This model suggests that the aversive responses to sounds seen in misophonia are developed and maintained, at least in part, by associative learning mechanisms, and thus that the brain areas most likely implicated in misophonia are those involved in emotion, memory, and learning (Brout et al., 2018). So far, a small number of studies have begun to validate parts of this model. For instance, it is now fairly well established that individuals with misophonia have normal hearing acuity (Jager et al., 2020) and that exposure to misophonic triggers induces a degree of autonomic arousal greater than that seen in healthy controls (Edelstein et al., 2013; Kumar et al., 2017; Schröder et al., 2019). Furthermore, preliminary neuroimaging studies of misophonia indicate that misophonic triggers elicit intense emotional responses that are coupled with hyperactivation of anterior insular cortex (Kumar et al., 2017; Schröder et al., 2019), a key node of the brain’s salience network (Menon & Uddin, 2010; Uddin, 2015). One of these studies also investigated functional connectivity of the anterior insula during the presentation of misophonic trigger sounds (Kumar et al., 2017), finding an increase in connectivity between the insula and ventromedial prefrontal cortex, posteromedial cortex, hippocampus and amygdala, limbic regions involved in emotional processing and regulation. Thus, while misophonic reactions are likely mediated in part by the limbic system, the primary process at work may instead be the attribution of excessive salience to specific sounds.
The attribution of salience to specific auditory stimuli is an important neurocognitive process that allows individuals to efficiently focus attention on important environmental signals (e.g., a fire alarm or police siren) and to enact a timely behavioral response (Huang & Elhilali, 2017; Kaya & Elhilali, 2017). Of particular note, the human auditory system has been evolutionarily primed to detect and respond to the cries of our young (Arnal et al., 2015; Soltis, 2004), and thus, the acoustic properties of an infant’s cry cause this sound to be exceptionally salient. Using acoustic analysis, psychophysical experiments, and neuroimaging, Arnal et al. (2015) demonstrated that a wide range of natural and artificial alarm signals (including human screams, air horns, and sports arena buzzers) all contain amplitude modulations in the 30–150 Hz range, which correlate with the psychoacoustic attribute of roughness (see also Trevor et al., 2020). The roughness of a sound is highly predictive of its salience and subjective aversiveness (Arnal et al., 2015, 2019; Zhao et al., 2019), and individuals can localize rough stimuli both more quickly and more accurately than other sounds (Arnal et al., 2015). Notably, the perception of rough sounds is associated with unique patterns of brain activity, including the synchronization of large-scale salience-related networks that include superior temporal regions, subcortical and cortical limbic areas, frontal areas (e.g., inferior frontal gyrus, orbitofrontal cortex), and insular cortex (Arnal et al., 2019). As preliminary neuroimaging studies implicate many of these areas in the pathophysiology of misophonia (Kumar et al., 2017; Schröder et al., 2019), we hypothesize that misophonia is characterized by dysfunctional hyperactivation of this specialized “auditory salience detection” network, causing individuals with misophonia to categorize a number of everyday sounds as disproportionately salient and aversive.
In addition to neuroimaging studies, several other factors suggest that an inappropriate attribution of salience to auditory stimuli could lie at the heart of misophonia. Subjectively, patients with misophonia routinely describe an intense hyper-focus on their various trigger stimuli (da Silva & Sanchez, 2019; Edelstein et al., 2013; Osuagwu et al., 2020; Schröder et al., 2017), which may serve to further amplify the already intense emotional reactions elicited by those stimuli. Moreover, in the first published study to systematically evaluate cognitive behavioral therapy in misophonia, Schröder and colleagues (2017) targeted hyper-focus on trigger sounds as a core symptom of misophonia in addition to the patients’ negative emotional reactions (see also Aazh et al., 2019). The potential interactions between hyper-focus on triggers, intense emotional experiences, and autonomic arousal can be further explained by the large degree of functional overlap between neural circuits responsible for sympathetic arousal, emotion, and salience detection (Beissner et al., 2013). As the salience of an auditory stimulus is highly associated with its perceived emotional intensity (Anikin, 2020), it is quite possible that the intense negative emotional reactions seen in misophonia could be coupled with an over-activation of salience neurocircuitry, along with associated projections to limbic areas. Moreover, as auditory salience attribution is remarkably context dependent (Huang & Elhilali, 2017), a salience mechanism helps account for the variable reactions that individuals with misophonia can have to the same stimulus across different situations. Furthermore, the inability of self-produced sounds to trigger a misophonic reaction can potentially be explained by the reduced salience attributed to self-generated stimuli, a phenomenon known as self-suppression (Hughes et al., 2013; Whitford, 2019). Like other authors, we contend that the idiosyncratic nature of misophonic triggers is the result of each individual’s unique learning history; however, as some sounds have been reported to trigger the vast majority of individuals with misophonia (e.g., chewing, nasal/breathing sounds; Jager et al., 2020), we also believe that some acoustic property of these sounds preferentially activates the aforementioned salience attribution neurocircuitry in persons with misophonia, increasing the likelihood that a given individual will find them aversive.
With regard to the neural underpinnings of misophonia, we have no a priori reason to expect that the pathophysiology of this condition would differ in autistic and non-autistic individuals. Moreover, the insula and salience network have been heavily implicated in the pathology of autism (Nomi et al., 2019; Uddin, 2015), and changes in insular structure/function may contribute to a higher rate of misophonia in individuals on the autism spectrum. This hypothesis is further supported by neuroimaging studies in autistic children that indicate that nonspecific (auditory-tactile) sensory hyperreactivity is related to increased resting-state connectivity between the salience network and amygdala (Green et al., 2016; Green & Wood, 2019). This finding also raises the question of whether the intolerance of stimuli in other modalities seen in autism could similarly be attributed to the mechanism of excessive salience attribution. Additional research is necessary to understand the manner in which the salience network contributes to sensory processing abnormalities in autism, particularly in the context of comorbid misophonia.
4.3. Model of Phonophobia: Pathological Fear Learning and Extinction
As a form of specific phobia, the neurobiological underpinnings of phonophobia are far more established than those of either hyperacusis or misophonia. A large body of research has been published on the neural correlates of fear conditioning and anxiety disorders (Chavanne & Robinson, 2020; Garcia, 2017; Herry & Johansen, 2014; Ipser et al., 2013; LeDoux et al., 2017; Tovote et al., 2015), as well as the ways in which auditory stimuli acquire affective valence (Frühholz et al., 2016). While a comprehensive overview of both topics is beyond the scope of this review, we seek to integrate these two lines of research into a neurocognitive model of phonophobia, which can be applied to autistic as well as non-autistic individuals.
The predominant neurocircuitry involved in fear and fear conditioning centers on connections within and between nuclei of the amygdala, hippocampus, and multiple areas of medial prefrontal cortex, including anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC; Chavanne & Robinson, 2020; Fullana et al., 2020; Tovote et al., 2015). The amygdala is essential for the acquisition, storage, and expression of learned fear associations, serving as a major hub in a number of fear- and anxiety-related neural circuits (LeDoux, 2000). Additionally, the amygdala is highly connected to auditory areas of the thalamus and cortex (Frühholz et al., 2016; Frühholz & Staib, 2017; LeDoux et al., 1991), and during sound processing, the amygdala is implicated in both the modulation of auditory cortical activity (Aizenberg et al., 2019) and encoding multiple features of the sound stimulus, including its perceived aversiveness (Kumar et al., 2012; Viinikainen et al., 2012). This hippocampus is primarily involved in the learning, storage, and retrieval of fear memories, as well as inhibiting the retrieval of these memories in specific contexts. The ACC, which plays a role in assessing the salience of a stimulus and regulating emotional responsiveness, has been associated with the expression of conditioned fear responses (Ipser et al., 2013). Via connections with the anterior insula, the ACC also makes up part of a “central autonomic–interoceptive network,” which is responsible for integrating representations of one’s cognitive, affective, and physical state to facilitate homeostatic autonomic and behavioral responses (Fullana et al., 2016). The vmPFC plays a role in the inhibition of threat expression by downregulating amygdala activity (Fullana et al., 2020), and this region is also implicated in the generation, storage, and retrieval of “safety” signals (i.e., extinction memories; Fullana et al., 2016; Milad & Quirk, 2012). Working in concert, this fear conditioning network serves to evaluate the content of a stimulus within the current context, triggering downstream signals of “danger” or “safety” and priming the individual to enact an appropriate behavioral response.
Although appropriate fear learning is key to survival, dysfunctional fear neurocircuitry can lead to phobias, in which the fear experienced by an individual is disproportionate to any potential danger. Neuroimaging studies have indicated that specific phobia is associated with hyperactivation of the amygdala and insular cortex when exposed to a phobic stimulus (Ipser et al., 2013), seemingly indicating that the salience and subjective aversiveness of the stimulus are both exaggerated in this condition. As a fear of loud noises is common during development (Meltzer et al., 2009; Ollendick et al., 2002), phonophobia is considered a nonexperiential or nonassociative phobia (i.e., a phobia not developed through direct experience with the phobic object; Garcia, 2017; Poulton & Menzies, 2002). While for the majority of children, the fear of loud noises is transient, there exists a small group of individuals in whom these fears intensify over time rather than resolving, potentially due to a genetic vulnerability (Muris et al., 2002). According to a recent model proposed by Garcia (2017), nonexperiential phobias are generated and maintained by both a sensitization of the fear response and a deficit in fear extinction learning, resulting in overly exaggerated initial fear responses and a persistence of the irrational fear despite nontraumatic exposure to the feared stimulus. This sensitized fear response is likely due to hyperactive amygdala responses to loud noises, which could result from baseline differences in the extent of sound-evoked auditory cortical activity, amygdala E/I balance, or auditory-limbic connectivity. Individuals with hyperacusis in particular may find specific sounds extremely painful or aversive, leading to a similar avoidant phenotype seen in chronic pain patients with high levels of pain anxiety (Greenberg & Burns, 2003). In addition to this sensitized fear response, individuals with specific phobias display persistent amygdala activity to repeated stimulation that does not habituate effectively (Garcia, 2017). An inability to habituate to repeated exposures likely indicates an underlying deficit in the ability to encode the “safety” signal necessary to extinguish a learned fear response. Thus, abnormalities in neurocircuitry involved in fear extinction, including the vmPFC and hippocampus (Fullana et al., 2016), likely play a role in the pathological persistence of phobic responses. Additionally, the extinction process may be hindered in patients with hyperacusis, whose adverse reactions to loud and/or painful sounds may serve to reinforce their maladaptive responses to phobic stimuli.
Although this model of phonophobia is equally applicable to autistic and non-autistic individuals, neuroimaging studies of autism provide further evidence of its utility in this population. Autistic children and adolescents display elevated amygdala activity in response to mildly aversive auditory stimuli (Green et al., 2013, 2015), with stronger amygdala activation predicting higher levels of parent-reported sensory hyperreactivity (Green et al., 2013, 2015). In addition, autistic individuals with high levels of sensory hyperreactivity demonstrate reduced amygdala habituation over repeated presentations of auditory and tactile stimuli (Green et al., 2015, 2019), which is thought to result from altered functional connectivity of the amygdala with prefrontal regions (Green et al., 2015, 2019). These findings provide preliminary evidence that the pattern of neural activity elicited by sensory stimuli in autistic individuals may be altered in such a way as to predispose these individuals to developing specific phobias, including phonophobia. This hypothesis is further supported by clinical studies demonstrating correlations between self/parent reports of sensory hyperreactivity and anxiety symptoms in autistic children and adults (Glod et al., 2015; Y. I. J. Hwang et al., 2020; Neil et al., 2016, 2017; Pickard et al., 2020; Syu & Lin, 2018; Wigham et al., 2015), with particularly strong relationships between sensory hyperreactivity and symptoms of specific phobia (Bitsika et al., 2016; Black et al., 2017; MacLennan et al., 2020). Longitudinal studies further indicate that increased sensory hyperreactivity early in development is predictive of later anxiety symptoms, both in autistic children and the general population (Carpenter et al., 2019; Green et al., 2012). This body of research generally supports the theory that hyperreactivity to sensory stimuli in autism may contribute to the high rates of anxiety disorders seen in this population (Green & Ben-Sasson, 2010; Kerns et al., 2016; McVey, 2019; Muskett et al., 2019; South & Rodgers, 2017). However, as sensory reactivity to date has primarily been measured using parent reports of a child’s observable behaviors, it remains possible that many aversive responses to sensory stimuli are actually the manifestations of specific phobia (Koegel et al., 2004). Additional research relating observed sound intolerance behaviors, psychoacoustic loudness growth, and phonophobia symptoms is thus necessary to clarify the relative contributions of hyperacusis and phonophobia to the observable phenotype of DST in autism.
5. Conclusion and Future Directions
Based on our review of the literature on DST in autism, it is likely that autistic individuals with DST present with a wide range of subjective complaints, including hyperacusis, misophonia, and phonophobia. Evidence from personal accounts, behavioral studies, and psychophysical tests all tend to support the notion that autistic people frequently perceive everyday sounds as unbearably loud or painful, suggesting that a substantial portion of DST behaviors can be explained by the presence of hyperacusis. In addition, many autistic children and adults exhibit clinically significant anxiety directed towards specific sounds (i.e., phonophobia), which may arise on its own or secondary to hyperacusis. However, the interplay between hyperacusis and phonophobia has not been studied in autism, and it remains unclear whether these conditions are comorbid in the majority of cases. Lastly, while much less research has examined the overlap of misophonia and autism, there is moderate evidence to suggest that a minority of autistic individuals with DST complaints likely meet diagnostic criteria for misophonia, regardless of whether hyperacusis is present.
In addition to describing the phenomenology of DST in autism, we propose neurocognitive models to explain each subtype of DST and its occurrence in people on the autism spectrum. Although hyperacusis in non-autistic individuals is typically associated with cochlear damage, we instead hypothesize that an alteration in the subcortical processing of auditory information leads to enhanced central auditory gain, resulting in the same sound-evoked auditory cortex hyperactivity seen in patients with tinnitus and hyperacusis. As opposed to our autism-specific model of hyperacusis, we believe that the neurocognitive processes leading to misophonia and phonophobia are the same regardless of the presence of autism, although certain neurobiological alterations seen in autism may predispose autistic individuals to these conditions. We propose that misophonia is a disorder of excessive salience attribution, in which neural machinery to detect evolutionarily important stimuli is abnormally sensitive to the acoustic properties of certain repetitive sounds, causing widespread neuronal synchronization that results in a disproportionate emotional and autonomic response. This misophonic reaction becomes associated with the “trigger” stimulus through associative learning mechanisms, potentially generalizing to other stimuli over time. Similarly, we hypothesize that phonophobia results from pathological fear learning, including an abnormally strong initial fear response to aversive sound stimuli and a failure to extinguish this response in the presence of non-threatening stimuli. Our model further suggests that hyperacusis strongly predisposes an individual to phonophobia, as aversive reactions to loud or painful sounds sensitize the amygdala to these stimuli, reinforcing and maintaining the fear response in the presence of repeated sound exposures. Though we find preliminary support for these explanatory models in the autism literature, additional behavioral, psychophysical, and neuroimaging research is necessary to validate model predictions and further refine the proposed DST constructs. By developing this theoretical framework, we hope to set the stage for future research on DST in autism, encouraging the field to move beyond descriptive science and into a new era of hypothesis-driven investigations and mechanism-based therapeutic development.
Although this work represents a major step forward in conceptualizing DST in autism, substantially more research in this area is necessary to improve our theoretical understanding of this construct and its mechanistic underpinnings. The body of literature describing DST in autism is limited by variable and inconsistent terminology and a tendency to group hyperacusis, misophonia, and phonophobia symptoms together into the construct of “auditory over-responsiveness.” Moreover, no study simultaneously assesses multiple types of DST in individuals on the autism spectrum, making it difficult to determine the relative contributions of hyperacusis, misophonia, and phonophobia to DST in this population. Perhaps most importantly, the most widely used measures of sensory responsiveness in autistic children and adults (DuBois et al., 2017; Jorquera-Cabrera et al., 2017; Williams et al., 2018) do not effectively differentiate between DST subtypes, substantially limiting the inferences that can be made about DST using these tools. Important future directions in this area of research include (a) the abandonment of “auditory over-responsiveness” and generalized sensory hyperreactivity as unitary constructs, (b) the establishment of a common nomenclature for DST conditions across disciplines, (c) the development and validation of novel DST questionnaires with adequate content coverage of hyperacusis, misophonia, and phonophobia, (d) the simultaneous assessment of all DST subtypes and their developmental trajectories in autistic individuals, (e) the investigation of DST in relation to other sensory features of autism, such as hyporeactivity and sensory seeking, (f) the comparison of psychoacoustic and neurophysiologic indices of auditory processing between groups of individuals with each form of DST, and (g) the evaluation of candidate interventions specifically targeting individual DST subtypes in controlled clinical trials. By partitioning DST into the distinct conditions of hyperacusis, misophonia, and phonophobia, we can reduce the heterogeneity inherent in autism and potentially derive subgroups of autistic individuals who differ in neuropathology, clinical course, or response to treatment (Feczko et al., 2019; Harris, 2019; Uljarević et al., 2017).
Highlights.
Decreased sound tolerance (DST) is a common and disabling feature of autism
DST in autism is a combination of hyperacusis, misophonia, and phonophobia
Hyperacusis is thought to result from excessive gain in the central auditory system
Misophonia may be caused by the attribution of excess salience to certain sounds
Phonophobia is a specific phobia of sound maintained by impaired fear extinction
Acknowledgements
This work was supported by National Institute of General Medical Sciences grant T32- GM007347 (ZJW) and the Nancy Lurie Marks family foundation (ZJW/TGW). The authors would like to thank Patrick Dwyer, Jane Burton, and Ira Kraemer for their helpful comments on earlier versions of this manuscript.
Footnotes
Declaration of Interests
ZJW has received consulting fees from Roche. He is also a member of the Family Advisory Committee of the Autism Speaks Autism Treatment Network site at Vanderbilt University and the Autistic Researcher Review Board of the Autism Intervention Research Network on Physical Health (AIR-P). The remaining authors declare no competing interests, financial or otherwise.
The terms ‘autistic person’ and ‘person on the autism spectrum’ are the preferred language of the majority of people diagnosed with autism (Bury et al., 2020; Kenny et al., 2016). Out of respect for these preferences, we use these terms to refer to individuals on the spectrum rather than exclusively using person-first language.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aazh H, Landgrebe M, Danesh AA, & Moore BCJ (2019). Cognitive behavioral therapy for alleviating the distress caused by tinnitus, hyperacusis and misophonia: Current perspectives. Psychology Research and Behavior Management, 12, 991–1002. 10.2147/PRBM.S179138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenberg M, Rolón-Martínez S, Pham T, Rao W, Haas JS, & Geffen MN (2019). Projection from the amygdala to the thalamic reticular nucleus amplifies cortical sound responses. Cell Reports, 28(3), 605–-615.e4.. 10.1016/j.celrep.2019.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántara JI, Cope TE, Cope W, & Weisblatt EJ (2012). Auditory temporal-envelope processing in high-functioning children with autism spectrum disorder. Neuropsychologia, 50(7), 1235–1251. 10.1016/j.neuropsychologia.2012.01.034 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®) (5th ed.). American Psychiatric Association Publishing. [Google Scholar]
- Amir I, Lamerton D, & Montague M-L (2018). Hyperacusis in children: The Edinburgh experience. International Journal of Pediatric Otorhinolaryngology, 112, 39–44. 10.1016/j.ijporl.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Anari M, Axelsson A, Eliasson A, & Magnusson L (1999). Hypersensitivity to sound: Questionnaire data, audiometry and classification. Scandinavian Audiology, 28(4), 219–230. 10.1080/010503999424653 [DOI] [PubMed] [Google Scholar]
- Anikin A (2020). The link between auditory salience and emotion intensity. Cognition and Emotion, 1–14. 10.1080/02699931.2020.1736992 [DOI] [PubMed] [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, & Feldman DE (2019). Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron, 101(4), 648–661.e4. 10.1016/j.neuron.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Flinker A, Kleinschmidt A, Giraud A-L, & Poeppel D (2015). Human screams occupy a privileged niche in the communication soundscape. Current Biology, 25(15), 2051–2056. 10.1016/j.cub.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Kleinschmidt A, Spinelli L, Giraud A-L, & Mégevand P (2019). The rough sound of salience enhances aversion through neural synchronisation. Nature Communications, 10(1), 3671 10.1038/s41467-019-11626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Mushtaq A, Yang I, & Oshinsky ML (2009). Ictal and interictal phonophobia in migraine—A quantitative controlled study. Cephalalgia, 29(10), 1042–1048. 10.1111/j.1468-2982.2008.01834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD (2019, January 1). Physiological mechanisms of hyperacusis: An update. ENT & Audiology News, 27(6). https://www.entandaudiologynews.com/features/audiology-features/post/physiological-mechanisms-of-hyperacusis-an-update [Google Scholar]
- Auerbach BD, Radziwon K, & Salvi R (2019). Testing the central gain model: Loudness growth correlates with central auditory gain enhancement in a rodent model of hyperacusis. Neuroscience, 407, 93–107. 10.1016/j.neuroscience.2018.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, & Salvi RJ (2014). Central gain control in tinnitus and hyperacusis. Frontiers in Neurology, 5(6), 206 10.3389/fneur.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, & Baranek GT (2014). National survey of sensory features in children with ASD: Factor structure of the sensory experience questionnaire (3.0). Journal of Autism and Developmental Disorders, 44(4), 915–925. 10.1007/s10803-013-1945-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM, & McFerran DJ (2011). Hyperacusis and disorders of loudness perception In Møller AR, Langguth B, De Ridder D, & Kleinjung T (Eds.), Textbook of Tinnitus (pp. 13–23). Springer; 10.1007/978-1-60761-145-5_3 [DOI] [Google Scholar]
- Beers AN, McBoyle M, Kakande E, Dar Santos RC, & Kozak FK (2014). Autism and peripheral hearing loss: A systematic review. International Journal of Pediatric Otorhinolaryngology, 78(1), 96–101. 10.1016/j.ijporl.2013.10.063 [DOI] [PubMed] [Google Scholar]
- Behler O, & Uppenkamp S (2016). The representation of level and loudness in the central auditory system for unilateral stimulation. NeuroImage, 139, 176–188. 10.1016/j.neuroimage.2016.06.025 [DOI] [PubMed] [Google Scholar]
- Behler O, & Uppenkamp S (2020). Activation in human auditory cortex in relation to the loudness and unpleasantness of low-frequency and infrasound stimuli. PLoS One, 15(2), e0229088 10.1371/journal.pone.0229088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär K-J, & Napadow V (2013). The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience, 33(25), 10503–10511. 10.1523/JNEUROSCI.1103-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belojević G, Öhrström E, & Rylander R (1992). Effects of noise on mental performance with regard to subjective noise sensitivity. International Archives of Occupational and Environmental Health, 64(4), 293–301. 10.1007/BF00378288 [DOI] [PubMed] [Google Scholar]
- Bemporad JR (1979). Adult recollections of a formerly autistic child. Journal of Autism and Developmental Disorders, 9(2), 179–197. 10.1007/BF01531533 [DOI] [PubMed] [Google Scholar]
- Bennetto L, Keith JM, Allen PD, & Luebke AE (2017). Children with autism spectrum disorder have reduced otoacoustic emissions at the 1 kHz mid-frequency region. Autism Research, 10(2), 337–345. 10.1002/aur.1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Gal E, Fluss R, Katz-Zetler N, & Cermak SA (2019). Update of a meta-analysis of sensory symptoms in ASD: A new decade of research. Journal of Autism and Developmental Disorders, 49(12), 4974–4996. 10.1007/s10803-019-04180-0 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. 10.1007/s10803-008-0593-3 [DOI] [PubMed] [Google Scholar]
- Bitsika V, Sharpley CF, & Mills R (2016). How are sensory features associated with seven anxiety disorders in boys with autism spectrum disorder? International Journal of Developmental Neuroscience, 50(1), 47–54. 10.1016/j.ijdevneu.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Black KR, Stevenson RA, Segers M, Ncube BL, Sun SZ, Philipp-Muller A, Bebko JM, Barense MD, & Ferber S (2017). Linking anxiety and insistence on sameness in autistic children: The role of sensory hypersensitivity. Journal of Autism and Developmental Disorders, 47(8), 2459–2470. 10.1007/s10803-017-3161-x [DOI] [PubMed] [Google Scholar]
- Blaesing L, & Kroener-Herwig B (2012). Self-reported and behavioral sound avoidance in tinnitus and hyperacusis subjects, and association with anxiety ratings. International Journal of Audiology, 51(8), 611–617. 10.3109/14992027.2012.664290 [DOI] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, Burack JA, & Mottron L (2010). Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia, 48(9), 2465–2475. 10.1016/j.neuropsychologia.2010.04.020 [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Provenzano G, & Casarosa S (2018). Neurobiological bases of autism–epilepsy comorbidity: A focus on excitation/inhibition imbalance. European Journal of Neuroscience, 47(6), 534–548. 10.1111/ejn.13595 [DOI] [PubMed] [Google Scholar]
- Brandy WT, & Lynn JM (1995). Audiologic findings in hyperacusic and nonhyperacusic subjects. American Journal of Audiology, 4(1), 46–51. 10.1044/1059-0889.0401.46 [DOI] [Google Scholar]
- Brinkert J, & Remington A (2020). Making sense of the perceptual capacities in autistic and non-autistic adults. Autism, 1362361320922640 10.1177/1362361320922640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brout JJ, Edelstein M, Erfanian M, Mannino M, Miller LJ, Rouw R, Kumar S, & Rosenthal MZ (2018). Investigating misophonia: A review of the empirical literature, clinical implications, and a research agenda. Frontiers in Neuroscience, 12, 373 10.3389/fnins.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H, Hardstone R, Juarez-Martinez EL, Sprengers J, Avramiea A-E, Simpraga S, Houtman SJ, Poil S-S, Dallares E, Palva S, Oranje B, Matias Palva J, Mansvelder HD, & Linkenkaer-Hansen K (2020). Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Scientific Reports, 10(1), 9195 10.1038/s41598-020-65500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SM, Jellett R, Spoor JR, & Hedley D (2020). “It defines who I am” or “It’s something I have”: What language do [autistic] Australian adults [on the autism spectrum] prefer? Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04425-3 [DOI] [PubMed] [Google Scholar]
- Buus S, Florentine M, & Redden RB (1982). The SISI test: A review: Part I. Audiology, 21(4), 273–293. 10.3109/00206098209072744 [DOI] [PubMed] [Google Scholar]
- Carpenter KLH, Baranek GT, Copeland WE, Compton S, Zucker N, Dawson G, & Egger HL (2019). Sensory over-responsivity: An early risk factor for anxiety and behavioral challenges in young children. Journal of Abnormal Child Psychology, 47(6), 1075–1088. 10.1007/s10802-018-0502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Broder-Fingert S, Neumeyer A, Giauque A, Kao A, & Iyasere C (2017). Brief Report: Meeting the needs of medically hospitalized adults with autism: A provider and patient toolkit. Journal of Autism and Developmental Disorders, 47(5), 1510–1529. 10.1007/s10803-017-3040-5 [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Woynaroski T, Baranek GT, & Wallace MT (2016). Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Research, 9(9), 920–925. 10.1002/aur.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni L, & Garber M (1991). Exploring the experience of autism through firsthand accounts. Journal of Autism and Developmental Disorders, 21(3), 303–313. 10.1007/BF02207327 [DOI] [PubMed] [Google Scholar]
- Chavanne AV, & Robinson OJ (2020). The overlapping neurobiology of induced and pathological anxiety: A meta-analysis of functional neural activation. American Journal of Psychiatry, appi.ajp.2020.19111153 10.1176/appi.ajp.2020.19111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-D, Stolzberg D, Lobarinas E, Sun W, Ding D, & Salvi R (2013). Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hearing Research, 295, 100–113. 10.1016/j.heares.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Li X, Liu L, Wang J, Lu C-Q, Yang M, Jiao Y, Zang F-C, Radziwon K, Chen G-D, Sun W, Muthaiah VPK, Salvi R, & Teng G-J (2015). Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. ELife, 4, e06576 10.7554/elife.06576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RY, Moran T, & Fenton JE (2013). The otological manifestations associated with autistic spectrum disorders. International Journal of Pediatric Otorhinolaryngology, 77(5), 629–634. 10.1016/j.ijporl.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Claiborn JM, Dozier TH, Hart SL, & Lee J (2020). Self-Identified misophonia phenomenology, impact, and clinical correlates. Psychological Thought, 13(2), 349–375. 10.37708/psyct.v13i2.454 [DOI] [Google Scholar]
- Crane L, Goddard L, & Pring L (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13(3), 215–228. 10.1177/1362361309103794 [DOI] [PubMed] [Google Scholar]
- Culotta L, & Penzes P (2020). Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD. Molecular Autism, 11(1), 32 10.1186/s13229-020-00339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva FE, & Sanchez TG (2019). Evaluation of selective attention in patients with misophonia. Brazilian Journal of Otorhinolaryngology, 85(3), 303–309. 10.1016/j.bjorl.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous AO (2012). Characteristics of auditory brainstem response latencies in children with autism spectrum disorders. Audiological Medicine, 10(3), 122–131. 10.3109/1651386X.2012.708986 [DOI] [Google Scholar]
- Danesh AA, & Aazh H (2020). Misophonia: A neurologic, psychologic, and audiologic complex. The Hearing Journal, 73(3), 20 10.1097/01.HJ.0000657984.74790.d5 [DOI] [Google Scholar]
- Danesh AA, & Kaf WA (2012). DPOAEs and contralateral acoustic stimulation and their link to sound hypersensitivity in children with autism. International Journal of Audiology, 51(4), 345–352. 10.3109/14992027.2011.626202 [DOI] [PubMed] [Google Scholar]
- Danesh AA, Lang D, Kaf W, Andreassen WD, Scott J, & Eshraghi AA (2015). Tinnitus and hyperacusis in autism spectrum disorders with emphasis on high functioning individuals diagnosed with Asperger’s syndrome. International Journal of Pediatric Otorhinolaryngology, 79(10), 1683–1688. 10.1016/j.ijporl.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Dang J, King KM, & Inzlicht M (2020). Why are self-report and behavioral measures weakly correlated? Trends in Cognitive Sciences, 24(4), 267–269. 10.1016/j.tics.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C, & Lewine JD (2016). Audiometric profiles in autism spectrum disorders: Does subclinical hearing loss impact communication? Autism Research, 9(1), 107–120. 10.1002/aur.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePape A-M, & Lindsay S (2016). Lived experiences from the perspective of individuals with autism spectrum disorder: A qualitative meta-synthesis. Focus on Autism and Other Developmental Disabilities, 31(1), 60–71. 10.1177/1088357615587504 [DOI] [Google Scholar]
- Dickinson A, Jones M, & Milne E (2016). Measuring neural excitation and inhibition in autism: Different approaches, different findings and different interpretations. Brain Research, 1648, 277–289. 10.1016/j.brainres.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Diehl PU, & Schaette R (2015). Abnormal auditory gain in hyperacusis: Investigation with a computational model. Frontiers in Neurology, 6, 157 10.3389/fneur.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Rose CE, McArthur D, & Maenner M (2020). National and state estimates of adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, Schipul SE, Baranek GT, Cleary KM, Willoughby MT, Evans AM, Bulluck JC, Lovmo JE, & Belger A (2015). Attenuated auditory event-related potentials and associations with atypical sensory response patterns in children with autism. Journal of Autism and Developmental Disorders, 45(2), 506–523. 10.1007/s10803-013-1948-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier TH, & Morrison KL (2017). Phenomenology of misophonia: Initial physical and emotional responses. The American Journal of Psychology, 130(4), 431–438. JSTOR. 10.5406/amerjpsyc.130.4.0431 [DOI] [Google Scholar]
- DuBois D, Lymer E, Gibson B, Desarkar P, & Nalder E (2017). Assessing sensory processing dysfunction in adults and adolescents with autism spectrum disorder: A scoping review. Brain Sciences, 7(8), 108 10.3390/brainsci7080108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop WA, Enticott PG, & Rajan R (2016). Speech discrimination difficulties in high-functioning autism spectrum disorder are likely independent of auditory hypersensitivity. Frontiers in Human Neuroscience, 10(77), 2382 10.3389/fnhum.2016.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein M, Brang D, Rouw R, & Ramachandran VS (2013). Misophonia: Physiological investigations and case descriptions. Frontiers in Human Neuroscience, 7, 296 10.3389/fnhum.2013.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier W, Eigenstetter M, & Zimmer K (2001). Psychoacoustic correlates of individual noise sensitivity. The Journal of the Acoustical Society of America, 109(4), 1464–1473. 10.1121/1.1350402 [DOI] [PubMed] [Google Scholar]
- ElMoazen D, Sobhy O, Abdou R, & AbdelMotaleb H (2020). Binaural interaction component of the auditory brainstem response in children with autism spectrum disorder. International Journal of Pediatric Otorhinolaryngology, 131, 109850 10.1016/j.ijporl.2019.109850 [DOI] [PubMed] [Google Scholar]
- Elwin M, Ek L, Kjellin L, & Schröder A (2013). Too much or too little: Hyper- and hypo-reactivity in high-functioning autism spectrum conditions. Journal of Intellectual & Developmental Disability, 38(3), 232–241. 10.3109/13668250.2013.815694 [DOI] [PubMed] [Google Scholar]
- Elwin M, Ek L, Schröder A, & Kjellin L (2012). Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Archives of Psychiatric Nursing, 26(5), 420–429. 10.1016/j.apnu.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Ethridge LE, White SP, Mosconi MW, Wang J, Byerly MJ, & Sweeney JA (2016). Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Translational Psychiatry, 6, e787 10.1038/tp.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, White SP, Mosconi MW, Wang J, Pedapati EV, Erickson CA, Byerly MJ, & Sweeney JA (2017). Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Molecular Autism, 8(1), 22 10.1186/s13229-017-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto MI, Hara N, Ohkawara T, & Narita M (2017). Mechanism of auditory hypersensitivity in human autism using autism model rats. Pediatrics International, 59(4), 404–407. 10.1111/ped.13186 [DOI] [PubMed] [Google Scholar]
- Fackrell K, Potgieter I, Shekhawat GS, Baguley DM, Sereda M, & Hoare DJ (2017). Clinical interventions for hyperacusis in adults: A scoping review to assess the current position and determine priorities for research. BioMed Research International, 2017, 1–22. 10.1155/2017/2723715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackrell K, Stratmann L, Kennedy V, MacDonald C, Hodgson H, Wray N, Farrell C, Meadows M, Sheldrake J, Byrom P, Baguley DM, Kentish R, Chapman S, Marriage J, Phillips J, Pollard T, Henshaw H, Gronlund TA, & Hoare DJ (2019). Identifying and prioritising unanswered research questions for people with hyperacusis: James Lind Alliance Hyperacusis Priority Setting Partnership. BMJ Open, 9(11), e032178 10.1136/bmjopen-2019-032178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagelson MA, & Baguley DM (Eds.). (2018). Hyperacusis and disorders of sound intolerance: Clinical and research perspectives. Plural Publishing. [Google Scholar]
- Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, & Fair DA (2019). The heterogeneity problem: Approaches to identify psychiatric subtypes. Trends in Cognitive Sciences, 23(7), 584–601. 10.1016/j.tics.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, & García-Añoveros J (2015). A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Current Biology, 25(5), 606–612. 10.1016/j.cub.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, Krystal JH, Murray JD, & Anticevic A (2017). Searching for cross-diagnostic convergence: Neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biological Psychiatry, 81(10), 848–861. 10.1016/j.biopsych.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler EP (1965). Some attributes of “loudness recruitment” and “loudness decruitment”. Annals of Otology, Rhinology & Laryngology, 74(2), 500–506. 10.1177/000348946507400218 [DOI] [PubMed] [Google Scholar]
- Frühholz S, & Staib M (2017). Neurocircuitry of impaired affective sound processing: A clinical disorders perspective. Neuroscience & Biobehavioral Reviews, 83, 516–524. 10.1016/j.neubiorev.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Frühholz S, Trost W, & Kotz SA (2016). The sound of emotions—Towards a unifying neural network perspective of affective sound processing. Neuroscience & Biobehavioral Reviews, 68, 96–110. 10.1016/j.neubiorev.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Fullana MA, Dunsmoor JE, Schruers KRJ, Savage HS, Bach DR, & Harrison BJ (2020). Human fear conditioning: From neuroscience to the clinic. Behaviour Research and Therapy, 124, 103528 10.1016/j.brat.2019.103528 [DOI] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, & Radua J (2016). Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21(4), 500–508. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Fung LK, Chahal L, Libove RA, Bivas R, & Hardan AY (2012). A retrospective review of the effectiveness of aripiprazole in the treatment of sensory abnormalities in autism. Journal of Child and Adolescent Psychopharmacology, 22(3), 245–248. 10.1089/cap.2010.0103 [DOI] [PubMed] [Google Scholar]
- Garcia R (2017). Neurobiology of fear and specific phobias. Learning & Memory, 24(9), 462–471. 10.1101/lm.044115.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarelli E, Nocera R, Turchi R, Hardie TL, Pagano R, & Yuan C (2014). Sensory stimuli as obstacles to emergency care for children with autism spectrum disorder. Advanced Emergency Nursing Journal, 36(2), 145–163. 10.1097/TME.0000000000000013 [DOI] [PubMed] [Google Scholar]
- Gjevik E, Eldevik S, Fjæran-Granum T, & Sponheim E (2011). Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(6), 761–769. 10.1007/s10803-010-1095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod M, Riby DM, Honey E, & Rodgers J (2015). Psychological correlates of sensory processing patterns in individuals with autism spectrum disorder: A systematic review. Review Journal of Autism and Developmental Disorders, 2(2), 199–221. 10.1007/s40489-015-0047-8 [DOI] [Google Scholar]
- Goebel G, & Floezinger U (2008). Pilot study to evaluate psychiatric co-morbidity in tinnitus patients with and without hyperacusis. Audiological Medicine, 6(1), 78–84. 10.1080/16513860801959100 [DOI] [Google Scholar]
- Goldsmith HH, Van Hulle CA, Arneson CL, Schreiber JE, & Gernsbacher MA (2006). A population-based twin study of parentally reported tactile and auditory defensiveness in young children. Journal of Abnormal Child Psychology, 34(3), 378–392. 10.1007/s10802-006-9024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E, Pedroso FS, & Wagner MB (2008). Auditory hypersensitivity in the autistic spectrum disorder. Pró-Fono Revista de Atualização Científica, 20(4), 279–284. 10.1590/s0104-56872008000400013 [DOI] [PubMed] [Google Scholar]
- Gomes E, Rotta NT, Pedroso FS, Sleifer P, & Danesi MC (2004). Auditory hypersensitivity in children and teenagers with autistic spectrum disorder. Arquivos De Neuro-Psiquiatria, 62(3B), 797–801. [DOI] [PubMed] [Google Scholar]
- Gong N, Zhang M, Zhang X-B, Chen L, Sun G-C, & Xu T-L (2008). The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology, 54(2), 454–463. 10.1016/j.neuropharm.2007.10.017 [DOI] [PubMed] [Google Scholar]
- Gravel JS, Dunn M, Lee WW, & Ellis MA (2006). Peripheral audition of children on the autistic spectrum. Ear and Hearing, 27(3), 299–312. 10.1097/01.aud.0000215979.65645.22 [DOI] [PubMed] [Google Scholar]
- Green SA, & Ben-Sasson A (2010). Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? Journal of Autism and Developmental Disorders, 40(12), 1495–1504. 10.1007/s10803-010-1007-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Ben-Sasson A, Soto TW, & Carter AS (2012). Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. Journal of Autism and Developmental Disorders, 42(6), 1112–1119. 10.1007/s10803-011-1361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, & Dapretto M (2016). Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 618–626.e1. 10.1016/j.jaac.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Lawrence KE, Liu J, Tsang T, Yeargin J, Cummings K, Laugeson E, Dapretto M, & Bookheimer SY (2019). Distinct patterns of neural habituation and generalization in children and adolescents with autism with low and high sensory overresponsivity. American Journal of Psychiatry, 176(12), 1010–1020. 10.1176/appi.ajp.2019.18121333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, & Dapretto M (2015). Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry, 72(8), 778–786. 10.1001/jamapsychiatry.2015.0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Tottenham N, Dapretto M, & Bookheimer SY (2013). Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 52(11), 1158–1172. 10.1016/j.jaac.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, & Wood ET (2019). The role of regulation and attention in atypical sensory processing. Cognitive Neuroscience, 10(3), 160–162. 10.1080/17588928.2019.1592141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J, & Burns JW (2003). Pain anxiety among chronic pain patients: Specific phobia or manifestation of anxiety sensitivity? Behaviour Research and Therapy, 41(2), 223–240. 10.1016/S0005-7967(02)00009-8 [DOI] [PubMed] [Google Scholar]
- Griffith GM, Totsika V, Nash S, & Hastings RP (2011). ‘I just don’t fit anywhere’: Support experiences and future support needs of individuals with Asperger syndrome in middle adulthood. Autism, 16(5), 532–546. 10.1177/1362361311405223 [DOI] [PubMed] [Google Scholar]
- Grinker RR (2007). Unstrange minds: Remapping the world of autism. Basic Books. [Google Scholar]
- Gu JW, Halpin CF, Nam E-C, Levine RA, & Melcher JR (2010). Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. Journal of Neurophysiology, 104(6), 3361–3370. 10.1152/jn.00226.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ (2006). Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear and Hearing, 27(6), 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- Guinan JJ (2018). Olivocochlear efferents: Their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hearing Research, 362, 38–47. 10.1016/j.heares.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A, Harris L, Pollick F, & Melville C (2019). A meta-analysis of working memory in individuals with autism spectrum disorders. PLOS ONE, 14(4), e0216198 10.1371/journal.pone.0216198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JC (2019). The necessity to identify subtypes of autism spectrum disorder. JAMA Psychiatry, 76(11), 1116 10.1001/jamapsychiatry.2019.1928 [DOI] [PubMed] [Google Scholar]
- Hasson D, Theorell T, Bergquist J, & Canlon B (2013). Acute stress induces hyperacusis in women with high levels of emotional exhaustion. PloS One, 8(1), e52945 10.1371/journal.pone.0052945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SM, McVilly KR, & Stokes MA (2019). Autism and employment: What works. Research in Autism Spectrum Disorders, 60, 48–58. 10.1016/j.rasd.2019.01.006 [DOI] [Google Scholar]
- Hazen EP, Stornelli JL, O’Rourke JA, Koesterer K, & McDougle CJ (2014). Sensory symptoms in autism spectrum disorders. Harvard Review of Psychiatry, 22(2), 112–124. 10.1097/01.HRP.0000445143.08773.58 [DOI] [PubMed] [Google Scholar]
- Hébert S, Fournier P, & Noreña A (2013). The auditory sensitivity is increased in tinnitus ears. Journal of Neuroscience, 33(6), 2356–2364. 10.1523/JNEUROSCI.3461-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D, Cai R, Uljarević M, Wilmot M, Spoor JR, Richdale A, & Dissanayake C (2018). Transition to work: Perspectives from the autism spectrum. Autism, 22(5), 528–541. 10.1177/1362361316687697 [DOI] [PubMed] [Google Scholar]
- Heeringa AN, & van Dijk P (2016). The immediate effects of acoustic trauma on excitation and inhibition in the inferior colliculus: A Wiener-kernel analysis. Hearing Research, 331, 47–56. 10.1016/j.heares.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Herry C, & Johansen JP (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience, 17(12), 1644–1654. 10.1038/nn.3869 [DOI] [PubMed] [Google Scholar]
- Hickox AE, & Liberman MC (2014). Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? Journal of Neurophysiology, 111(3), 552–564. 10.1152/jn.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Lerh JW, Magiati I, Meiser-Stedman R, & Brugha TS (2019). Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychological Medicine, 49(4), 559–572. 10.1017/s0033291718002283 [DOI] [PubMed] [Google Scholar]
- Howe FEJ, & Stagg SD (2016). How sensory experiences affect adolescents with an autistic spectrum condition within the classroom. Journal of Autism and Developmental Disorders, 46(5), 1656–1668. 10.1007/s10803-015-2693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, & Elhilali M (2017). Auditory salience using natural soundscapes. The Journal of the Acoustical Society of America, 141(3), 2163–2176. 10.1121/1.4979055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudac CM, DesChamps TD, Arnett AB, Cairney BE, Ma R, Webb SJ, & Bernier RA (2018). Early enhanced processing and delayed habituation to deviance sounds in autism spectrum disorder. Brain and Cognition, 123, 110–119. 10.1016/j.bandc.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel LG, & Collins LM (2004). A theoretical analysis of normal- and impaired-hearing intensity discrimination. IEEE Transactions on Speech and Audio Processing, 12(3), 323–333. 10.1109/TSA.2004.825672 [DOI] [Google Scholar]
- Hughes G, Desantis A, & Waszak F (2013). Mechanisms of intentional binding and sensory attenuation: The role of temporal prediction, temporal control, identity prediction, and motor prediction. Psychological Bulletin, 139(1), 133–151. 10.1037/a0028566 [DOI] [PubMed] [Google Scholar]
- Hussein S, Bahmei B, Gustafson K, Fisher R, Iarocci G, Arzanpour S, & Birmingham E (2019, May). Family experiences of auditory hypersensitivity in ASD. International Society for Autism Research Annual Meeting, Montreal, QC https://insar.confex.com/insar/2019/webprogram/Paper30947.html [Google Scholar]
- Hwang J-H, Chou P-H, Wu C-W, Chen J-H, & Liu T-C (2009). Brain activation in patients with idiopathic hyperacusis. American Journal of Otolaryngology, 30(6), 432–434. 10.1016/j.amjoto.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Hwang YIJ, Arnold S, Srasuebkul P, & Trollor J (2020). Understanding anxiety in adults on the autism spectrum: An investigation of its relationship with intolerance of uncertainty, sensory sensitivities and repetitive behaviours. Autism, 24(2), 411–422. 10.1177/1362361319868907 [DOI] [PubMed] [Google Scholar]
- Ipser JC, Singh L, & Stein DJ (2013). Meta-analysis of functional brain imaging in specific phobia. Psychiatry and Clinical Neurosciences, 67(5), 311–322. 10.1111/pcn.12055 [DOI] [PubMed] [Google Scholar]
- Irimia P, Cittadini E, Paemeleire K, Cohen AS, & Goadsby PJ (2008). Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia, 28(6), 626–630. 10.1111/j.1468-2982.2008.01565.x [DOI] [PubMed] [Google Scholar]
- Jager I, de Koning P, Bost T, Denys D, & Vulink N (2020). Misophonia: Phenomenology, comorbidity and demographics in a large sample. PLOS One, 15(4), e0231390 10.1371/journal.pone.0231390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff MM, & Jastreboff PJ (2002). Decreased sound tolerance and tinnitus retraining therapy (TRT). The Australian and New Zealand Journal of Audiology, 24(2), 74–84. [Google Scholar]
- Jastreboff PJ, & Jastreboff M (2014). Treatments for decreased sound tolerance (hyperacusis and misophonia). Seminars in Hearing, 35(2), 105–120. 10.1055/s-0034-1372527 [DOI] [Google Scholar]
- Jastreboff PJ, & Jastreboff MM (2015). Decreased sound tolerance: Hyperacusis, misophonia, diplacousis, and polyacousis In Aminoff MJ, Boller F, & Swaab DF (Eds.), Handbook of clinical neurology (Vol. 129, pp. 375–387). Elsevier; 10.1016/B978-0-444-62630-1.00021-4 [DOI] [PubMed] [Google Scholar]
- Job RFS (1999). Noise sensitivity as a factor influencing human reaction to noise. Noise & Health, 1(3), 57–68. [PubMed] [Google Scholar]
- Johnston D, Egermann H, & Kearney G (2020). SoundFields: A virtual reality game designed to address auditory hypersensitivity in individuals with autism spectrum disorder. Applied Sciences, 10(9), 2996 10.3390/app10092996 [DOI] [Google Scholar]
- Jones CRG, Happé F, Baird G, Simonoff E, Marsden AJS, Tregay J, Phillips RJ, Goswami U, Thomson JM, & Charman T (2009). Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia, 47(13), 2850–2858. 10.1016/j.neuropsychologia.2009.06.015 [DOI] [PubMed] [Google Scholar]
- Jones EK, Hanley M, & Riby DM (2020). Distraction, distress and diversity: Exploring the impact of sensory processing differences on learning and school life for pupils with autism spectrum disorders. Research in Autism Spectrum Disorders, 72, 101515 10.1016/j.rasd.2020.101515 [DOI] [Google Scholar]
- Jones RSP, Quigney C, & Huws JC (2003). First-hand accounts of sensory perceptual experiences in autism: A qualitative analysis. Journal of Intellectual & Developmental Disability, 28(2), 112–121. 10.1080/1366825031000147058 [DOI] [Google Scholar]
- Jorquera-Cabrera S, Romero-Ayuso D, Rodriguez-Gil G, & Triviño-Juárez J-M (2017). Assessment of sensory processing characteristics in children between 3 and 11 years old: A systematic review. Frontiers in Pediatrics, 5, 57 10.3389/fped.2017.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüris L, Andersson G, Larsen HC, & Ekselius L (2014). Cognitive behaviour therapy for hyperacusis: A randomized controlled trial. Behaviour Research and Therapy, 54, 30–37. 10.1016/j.brat.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Kaf WA, & Danesh AA (2013). Distortion-product otoacoustic emissions and contralateral suppression findings in children with Asperger’s Syndrome. International Journal of Pediatric Otorhinolaryngology, 77(6), 947–954. 10.1016/j.ijporl.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Kargas N, López B, Reddy V, & Morris P (2015). The relationship between auditory processing and restricted, repetitive behaviors in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(3), 658–668. 10.1007/s10803-014-2219-2 [DOI] [PubMed] [Google Scholar]
- Kaya EM, & Elhilali M (2017). Modelling auditory attention. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1714), 20160101 10.1098/rstb.2016.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating CT, & Cook JL (2020). Facial expression production and recognition in autism spectrum disorders: A shifting landscape. Child and Adolescent Psychiatric Clinics of North America. 10.1016/j.chc.2020.02.006 [DOI] [PubMed] [Google Scholar]
- Keith JM, Jamieson JP, & Bennetto L (2019). The importance of adolescent self-report in autism spectrum disorder: Integration of questionnaire and autonomic measures. Journal of Abnormal Child Psychology, 47(4), 741–754. 10.1007/s10802-018-0455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E (2016). Which terms should be used to describe autism? Perspectives from the UK autism community: Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, Johnson DG, Mehta JA, & Schroeder JL (2006). The pattern of sensory processing abnormalities in autism. Autism, 10(5), 480–494. 10.1177/1362361306066564 [DOI] [PubMed] [Google Scholar]
- Kerns CM, & Kendall PC (2012). The presentation and classification of anxiety in autism spectrum disorder. Clinical Psychology: Science and Practice, 19(4), 323–347. 10.1111/cpsp.12009 [DOI] [Google Scholar]
- Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, Miller J, & Herrington J (2014). Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2851–2861. 10.1007/s10803-014-2141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Rump K, Worley J, Kratz H, McVey A, Herrington J, & Miller J (2016). The differential diagnosis of anxiety disorders in cognitively-able youth with autism. Cognitive and Behavioral Practice, 23(4), 530–547. 10.1016/j.cbpra.2015.11.004 [DOI] [Google Scholar]
- Khalfa S, Bruneau N, Rogé B, Georgieff N, Veuillet E, Adrien J-L, Barthélémy C, & Collet L (2004). Increased perception of loudness in autism. Hearing Research, 198(1–2), 87–92. 10.1016/j.heares.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Kirby AV, Dickie VA, & Baranek GT (2015). Sensory experiences of children with autism spectrum disorder: In their own words. Autism, 19(3), 316–326. 10.1177/1362361314520756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellberg A, Landström U, Tesarz M, Söderberg L, & Akerlund E (1996). The effects of nonphysical noise characteristics, ongoing task and noise sensitivity on annoyance and distraction due to noise at work. Journal of Environmental Psychology, 16(2), 123–136. 10.1006/jevp.1996.0010 [DOI] [Google Scholar]
- Kliuchko M, Heinonen-Guzejev M, Vuust P, Tervaniemi M, & Brattico E (2016). A window into the brain mechanisms associated with noise sensitivity. Scientific Reports, 6(1), 39236 10.1038/srep39236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Openden D, & Koegel LK (2004). A systematic desensitization paradigm to treat hypersensitivity to auditory stimuli in children with autism in family contexts. Research and Practice for Persons with Severe Disabilities, 29(2), 122–134. 10.2511/rpsd.29.2.122 [DOI] [Google Scholar]
- Kuiper MWM, Verhoeven EWM, & Geurts HM (2019). Stop making noise! Auditory sensitivity in adults with an autism spectrum disorder diagnosis: Physiological habituation and subjective detection thresholds. Journal of Autism and Developmental Disorders, 49(5), 2116–2128. 10.1007/s10803-019-03890-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Lukose R, & Stevens LV (2011). Malformation of the human superior olive in autistic spectrum disorders. Brain Research, 1367, 360–371. 10.1016/j.brainres.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, & Mangunay K (2008). Morphological features of the medial superior olive in autism. Brain Research, 1200, 132–137. 10.1016/j.brainres.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Kumar S, Tansley-Hancock O, Sedley W, Winston JS, Callaghan MF, Allen M, Cope TE, Gander PE, Bamiou D-E, & Griffiths TD (2017). The brain basis for misophonia. Current Biology, 27(4), 527–533. 10.1016/j.cub.2016.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, von Kriegstein K, Friston K, & Griffiths TD (2012). Features versus feelings: Dissociable representations of the acoustic features and valence of aversive sounds. Journal of Neuroscience, 32(41), 14184–14192. 10.1523/JNEUROSCI.1759-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Kassee C, Besney R, Bonato S, Hull L, Mandy W, Szatmari P, & Ameis SH (2019). Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. The Lancet Psychiatry, 6(10), 819–829. 10.1016/s2215-0366(19)30289-5 [DOI] [PubMed] [Google Scholar]
- Landgrebe M, & Langguth B (2011). Tinnitus and anxiety In Møller AR, Langguth B, De Ridder D, & Kleinjung T (Eds.), Textbook of tinnitus (pp. 499–503). Springer; 10.1007/978-1-60761-145-5_64 [DOI] [Google Scholar]
- Landon J, Shepherd D, & Lodhia V (2016). A qualitative study of noise sensitivity in adults with autism spectrum disorder. Research in Autism Spectrum Disorders, 32, 43–52. 10.1016/j.rasd.2016.08.005 [DOI] [Google Scholar]
- Langers DRM, van Dijk P, Schoenmaker ES, & Backes WH (2007). FMRI activation in relation to sound intensity and loudness. NeuroImage, 35(2), 709–718. 10.1016/j.neuroimage.2006.12.013 [DOI] [PubMed] [Google Scholar]
- Lanting CP, De Kleine E, Bartels H, & Van Dijk P (2008). Functional imaging of unilateral tinnitus using fMRI. Acta Oto-Laryngologica, 128(4), 415–421. 10.1080/00016480701793743 [DOI] [PubMed] [Google Scholar]
- Lau BK, Maddox RK, Estes A, Dager S, & Lee AK (2018). Combining clinical, behavioral, and neurophysiological measures to investigate auditory processing abnormalities in individuals with autism spectrum disorder. Proceedings of Meetings on Acoustics, 35(1), 050004 10.1121/2.0000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BY, Leong R, Uljarevic M, Lerh JW, Rodgers J, Hollocks MJ, South M, McConachie H, Ozsivadjian A, Van Hecke A, Libove R, Hardan A, Leekam S, Simonoff E, & Magiati I (2020). Anxiety in young people with autism spectrum disorder: Common and autism-related anxiety experiences and their associations with individual characteristics. Autism, 24(5), 1111–1126. 10.1177/1362361319886246 [DOI] [PubMed] [Google Scholar]
- Laumen G, Ferber AT, Klump GM, & Tollin DJ (2016). The physiological basis and clinical use of the binaural interaction component of the auditory brainstem response. Ear and Hearing, 37(5), e276–e290. 10.1097/AUD.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JK, Rubenstein E, Marvin AR, Toroney J, & Lipkin PH (2016, May). Auditory sensitivity issues in children with autism spectrum disorders: Characteristics and burden. Pediatric Academic Societies Meeting, Baltimore, MD https://iancommunity.org/sites/default/files/galleries/conference-presentations/Law_PAS_2016.pdf [Google Scholar]
- Lawson RP, Aylward J, White S, & Rees G (2015). A striking reduction of simple loudness adaptation in autism. Scientific Reports, 5(1). 10.1038/srep16157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion Circuits in the Brain. Annual Review of Neuroscience, 23(1), 155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, & Romanski LM (1991). Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neuroscience Letters, 134(1), 139–144. 10.1016/0304-3940(91)90526-Y [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Moscarello J, Sears R, & Campese V (2017). The birth, death and resurrection of avoidance: A reconceptualization of a troubled paradigm. Molecular Psychiatry, 22(1), 24–36. 10.1038/mp.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lee J, & Kim E (2017). Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biological Psychiatry, 81(10), 838–847. 10.1016/j.biopsych.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, & Gould J (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37(5), 894–910. 10.1007/s10803-006-0218-7 [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, & Lainhart JE (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36(7), 849–861. 10.1007/s10803-006-0123-0 [DOI] [PubMed] [Google Scholar]
- Li H, Takeda Y, Niki H, Ogawa J, Kobayashi S, Kai N, Akasaka K, Asano M, Sudo K, Iwakura Y, & Watanabe K (2003). Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. European Journal of Neuroscience, 17(5), 929–936. 10.1046/j.1460-9568.2003.02514.x [DOI] [PubMed] [Google Scholar]
- Little LM, Ausderau K, Sideris J, & Baranek GT (2015). Activity participation and sensory features among children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(9), 2981–2990. 10.1007/s10803-015-2460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Glowatzki E, & Fuchs PA (2015). Unmyelinated type II afferent neurons report cochlear damage. Proceedings of the National Academy of Sciences, 112(47), 14723–14727. 10.1073/pnas.1515228112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Frischling C, Cuadros R, & Heinitz K (2016). Autism and overcoming job barriers: Comparing job-related barriers and possible solutions in and outside of autism-specific employment. PLoS One, 11(1), e0147040 10.1371/journal.pone.0147040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Rais M, Palacios AR, Shuai XS, Bishay S, Popa O, Pirbhoy PS, Binder DK, Nelson DL, Ethell IM, & Razak KA (2020). Deletion of Fmr1 from forebrain excitatory neurons triggers abnormal cellular, EEG, and behavioral phenotypes in the auditory cortex of a mouse model of fragile X syndrome. Cerebral Cortex, 30(3), 969–988. 10.1093/cercor/bhz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, & Sun W (2011). GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience, 189, 187–198. 10.1016/j.neuroscience.2011.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker JR (2013). Auditory hypersensitivity in children with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 28(3), 184–191. 10.1177/1088357613475810 [DOI] [Google Scholar]
- Lugo-Marín J, Magán-Maganto M, Rivero-Santana A, Cuellar-Pompa L, Alviani M, Jenaro-Rio C, Díez E, & Canal-Bedia R (2019). Prevalence of psychiatric disorders in adults with autism spectrum disorder: A systematic review and meta-analysis. Research in Autism Spectrum Disorders, 59, 22–33. 10.1016/j.rasd.2018.12.004 [DOI] [Google Scholar]
- Lukose R, Beebe K, & Kulesza RJ (2015). Organization of the human superior olivary complex in 15q duplication syndromes and autism spectrum disorders. Neuroscience, 286, 216–230. 10.1016/j.neuroscience.2014.11.033 [DOI] [PubMed] [Google Scholar]
- Lukose R, Brown K, Barber CM, & Kulesza RJ (2013). Quantification of the stapedial reflex reveals delayed responses in autism: Delayed brain stem reflexes in autism. Autism Research, 6(5), 344–353. 10.1002/aur.1297 [DOI] [PubMed] [Google Scholar]
- Lukose R, Schmidt E, Wolski TP, Murawski NJ, & Kulesza RJ (2011). Malformation of the superior olivary complex in an animal model of autism. Brain Research, 1398, 102–112. 10.1016/j.brainres.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Ma L, Ono M, Qin L, & Kato N (2020). Acoustic trauma induced the alteration of the activity balance of excitatory and inhibitory neurons in the inferior colliculus of mice. Hearing Research, 391, 107957 10.1016/j.heares.2020.107957 [DOI] [PubMed] [Google Scholar]
- MacLennan K, Roach L, & Tavassoli T (2020). The relationship between sensory reactivity differences and anxiety subtypes in autistic children. Autism Research, 13(5), 785–795. 10.1002/aur.2259 [DOI] [PubMed] [Google Scholar]
- Mansour Y, & Kulesza RJ (2020). Three dimensional reconstructions of the superior olivary complex from children with autism spectrum disorder. Hearing Research, 107974 10.1016/j.heares.2020.107974 [DOI] [PubMed] [Google Scholar]
- Mansour Y, Mangold S, Chosky D, & Kulesza RJ (2019). Auditory midbrain hypoplasia and dysmorphology after prenatal valproic acid exposure. Neuroscience, 396, 79–93. 10.1016/j.neuroscience.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Marriage J, & Barnes NM (1995). Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction? The Journal of Laryngology & Otology, 109(10), 915–921. 10.1017/s0022215100131676 [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Aggarwal R, Baker C, Mathapati S, Molitoris S, & Mayes RD (2013). Unusual fears in children with autism. Research in Autism Spectrum Disorders, 7(1), 151–158. 10.1016/j.rasd.2012.08.002 [DOI] [Google Scholar]
- McCullagh EA, Rotschafer SE, Auerbach BD, Klug A, Kaczmarek LK, Cramer KS, Kulesza RJ, Razak KA, Lovelace JW, Lu Y, Koch U, & Wang Y (2020). Mechanisms underlying auditory processing deficits in fragile X syndrome. The FASEB Journal, 34(3), 3501–3518. 10.1096/fj.201902435R [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey AJ (2019). The neurobiological presentation of anxiety in autism spectrum disorder: A systematic review. Autism Research, 12(3), 346–369. 10.1002/aur.2063 [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, & Norris B (2009). The auditory midbrain of people with tinnitus: Abnormal sound-evoked activity revisited. Hearing Research, 257(1–2), 63–74. 10.1016/j.heares.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H, Vostanis P, Dogra N, Doos L, Ford T, & Goodman R (2009). Children’s specific fears. Child: Care, Health and Development, 35(6), 781–789. 10.1111/j.1365-2214.2008.00908.x [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercati O, Huguet G, Danckaert A, André-Leroux G, Maruani A, Bellinzoni M, Rolland T, Gouder L, Mathieu A, Buratti J, Amsellem F, Benabou M, Van-Gils J, Beggiato A, Konyukh M, Bourgeois J-P, Gazzellone MJ, Yuen RKC, Walker S, … Bourgeron T (2017). CNTN6 mutations are risk factors for abnormal auditory sensory perception in autism spectrum disorders. Molecular Psychiatry, 22(4), 625–633. 10.1038/mp.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micoulaud-Franchi J-A, Lopez R, Vaillant F, Richieri R, El-Kaim A, Bioulac S, Philip P, Boyer L, & Lancon C (2015). Perceptual abnormalities related to sensory gating deficit are core symptoms in adults with ADHD. Psychiatry Research, 230(2), 357–363. 10.1016/j.psychres.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63(1), 129–151. 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millin R, Kolodny T, Flevaris AV, Kale AM, Schallmo M-P, Gerdts J, Bernier RA, & Murray S (2018). Reduced auditory cortical adaptation in autism spectrum disorder. ELife, 7, 20150355 10.7554/elife.36493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron O, Beam AL, & Kohane IS (2018). Auditory brainstem response in infants and children with autism spectrum disorder: A meta- analysis of wave V. Autism Research, 11(2), 355–363. 10.1002/aur.1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron O, Delgado RE, Delgado CF, Simpson EA, Yu K-H, Gutierrez A, Zeng G, Gerstenberger JN, & Kohane IS (2020). Prolonged auditory brainstem response in universal hearing screening of newborns with autism spectrum disorder. Autism Research. 10.1002/aur.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrle D, Hofmeier B, Amend M, Wolpert S, Ni K, Bing D, Klose U, Pichler B, Knipper M, & Rüttiger L (2019). Enhanced central neural gain compensates acoustic trauma-induced cochlear impairment, but unlikely correlates with tinnitus and hyperacusis. Neuroscience, 407, 146–169. 10.1016/j.neuroscience.2018.12.038 [DOI] [PubMed] [Google Scholar]
- Møller AR (2011). Misophonia, phonophobia, and “exploding head” syndrome In Møller AR, Langguth B, De Ridder D, & Kleinjung T (Eds.), Textbook of tinnitus (pp. 25–27). Springer; 10.1007/978-1-60761-145-5_4 [DOI] [Google Scholar]
- Moore BCJ, Glasberg BR, Hess RF, & Birchall JP (1985). Effects of flanking noise bands on the rate of growth of loudness of tones in normal and recruiting ears. The Journal of the Acoustical Society of America, 77(4), 1505–1513. 10.1121/1.392045 [DOI] [PubMed] [Google Scholar]
- Moore DJ (2015). Acute pain experience in individuals with autism spectrum disorders: A review. Autism, 19(4), 387–399. 10.1177/1362361314527839 [DOI] [PubMed] [Google Scholar]
- Moossavi A, & Moallemi M (2019). Auditory processing and auditory rehabilitation approaches in autism. Auditory and Vestibular Research. 10.18502/avr.v28i1.410 [DOI] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, & Burack J (2006). Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders, 36(1), 27–43. 10.1007/s10803-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Mukaddes NM, & Fateh R (2010). High rates of psychiatric co-morbidity in individuals with Asperger’s disorder. The World Journal of Biological Psychiatry, 11(2–2), 486–492. 10.3109/15622970902789130 [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, de Jong PJ, & Ollendick TH (2002). The etiology of specific fears and phobias in children: A critique of the non-associative account. Behaviour Research and Therapy, 40(2), 185–195. 10.1016/S0005-7967(01)00051-1 [DOI] [PubMed] [Google Scholar]
- Muskat B, Burnham Riosa P, Nicholas DB, Roberts W, Stoddart KP, & Zwaigenbaum L (2015). Autism comes to the hospital: The experiences of patients with autism spectrum disorder, their parents and health-care providers at two Canadian paediatric hospitals. Autism, 19(4), 482–490. 10.1177/1362361314531341 [DOI] [PubMed] [Google Scholar]
- Muskett A, Capriola-Hall NN, Radtke SR, Factor R, & Scarpa A (2019). Repetitive behaviors in autism spectrum disorder: Associations with depression and anxiety symptoms. Research in Autism Spectrum Disorders, 68, 101449 10.1016/j.rasd.2019.101449 [DOI] [Google Scholar]
- Naples A, Wolf J, Carlos CL, Foss-Feig JH, Anticevic A, Kala S, Bagdasarov A, Cummings EM, Srihari V, & McPartland J (2020, June 3). Dynamics of E/I activity predict social and sensory symptoms transdiagnostically. INSAR 2020 Virtual Meeting https://insar.confex.com/insar/2020/meetingapp.cgi/Paper/34960 [Google Scholar]
- Neal M, & Cavanna AE (2013). Selective sound sensitivity syndrome (misophonia) in a patient with Tourette wyndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 25(1), E01 10.1176/appi.neuropsych.11100235 [DOI] [PubMed] [Google Scholar]
- Neil L, Green D, & Pellicano E (2017). The psychometric properties of a new measure of sensory behaviors in autistic children. Journal of Autism and Developmental Disorders, 47(4), 1261–1268. 10.1007/s10803-016-3018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil L, Olsson NC, & Pellicano E (2016). The relationship between intolerance of uncertainty, sensory sensitivities, and anxiety in autistic and typically developing children. Journal of Autism and Developmental Disorders, 46(6), 1962–1973. 10.1007/s10803-016-2721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, & Valakh V (2015). Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron, 87(4), 684–698. 10.1016/j.neuron.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas DB, Zwaigenbaum L, Muskat B, Craig WR, Newton AS, Kilmer C, Greenblatt A, Roberts W, & Cohen-Silver J (2016). Experiences of emergency department care from the perspective of families in which a child has autism spectrum disorder. Social Work in Health Care, 55(6), 409–426. 10.1080/00981389.2016.1178679 [DOI] [PubMed] [Google Scholar]
- Nomi JS, Molnar-Szakacs I, & Uddin LQ (2019). Insular function in autism: Update and future directions in neuroimaging and interventions. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 412–426. 10.1016/j.pnpbp.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Noreña AJ, & Chéry-Croze S (2007). Enriched acoustic environment rescales auditory sensitivity. Neuroreport, 18(12), 1251–1255. 10.1097/wnr.0b013e3282202c35 [DOI] [PubMed] [Google Scholar]
- O’Connor K (2012). Auditory processing in autism spectrum disorder: A review. Neuroscience and Biobehavioral Reviews, 36(2), 836–854. 10.1016/j.neubiorev.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Ichikawa I, Kumagaya S, & Kuniyoshi Y (2019). Stapedial reflex threshold predicts individual loudness tolerance for people with autistic spectrum disorders. Experimental Brain Research, 237(1), 91–100. 10.1007/s00221-018-5400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH, King NJ, & Muris P (2002). Fears and phobias in children: Phenomenology, epidemiology, and aetiology. Child and Adolescent Mental Health, 7(3), 98–106. 10.1111/1475-3588.00019 [DOI] [Google Scholar]
- Osuagwu FC, Osuagwu VC, & Machoka AM (2020). Methylphenidate ameliorates worsening distractibility symptoms of misophonia in an adolescent male. The Primary Care Companion for CNS Disorders, 22(5), 19l02553 10.4088/PCC.19l02553 [DOI] [PubMed] [Google Scholar]
- Ozsivadjian A, Knott F, & Magiati I (2012). Parent and child perspectives on the nature of anxiety in children and young people with autism spectrum disorders: A focus group study. Autism, 16(2), 107–121. 10.1177/1362361311431703 [DOI] [PubMed] [Google Scholar]
- Palumbo DB, Alsalman O, De Ridder D, Song J-J, & Vanneste S (2018). Misophonia and potential underlying mechanisms: A perspective. Frontiers in Psychology, 9, 219 10.3389/fpsyg.2018.00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin J, Andersson L, & Nordin S (2016). Characteristics of hyperacusis in the general population. Noise & Health, 18(83), 178–184. 10.4103/1463-1741.189244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M-F, Hodgetts HM, Lafleur MF, Vincent A, & Tremblay S (2016). Vulnerability to the irrelevant sound effect in adult ADHD. Journal of Attention Disorders, 20(4), 306–316. 10.1177/1087054713492563 [DOI] [PubMed] [Google Scholar]
- Pellicano E, & Burr D (2012). When the world becomes “too real”: A Bayesian explanation of autistic perception. Trends in Cognitive Sciences, 16(10), 504–510. 10.1016/j.tics.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Phillips DP, & Carr MM (1998). Disturbances of loudness perception. Journal of the American Academy of Audiology, 9(5), 371–379. [PubMed] [Google Scholar]
- Pickard H, Hirsch C, Simonoff E, & Happé F (2020). Exploring the cognitive, emotional and sensory correlates of social anxiety in autistic and neurotypical adolescents. Journal of Child Psychology and Psychiatry, jcpp.13214 10.1111/jcpp.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkowski M (2019). Rationale and efficacy of sound therapies for tinnitus and hyperacusis. Neuroscience, 407, 120–134. 10.1016/j.neuroscience.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Tyler RS, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Coelho CB, Andersson G, Keiner AJ, Cacace AT, Martin N, & Moore BCJ (2014). A review of hyperacusis and future directions: Part II. Measurement, mechanisms, and treatment. American Journal of Audiology, 23(4), 420–436. 10.1044/2014_AJA-13-0037 [DOI] [PubMed] [Google Scholar]
- Pillion JP, Boatman-Reich D, & Gordon B (2018). Auditory brainstem pathology in autism spectrum disorder: A review. Cognitive And Behavioral Neurology, 31(2), 53–78. 10.1097/wnn.0000000000000154 [DOI] [PubMed] [Google Scholar]
- Plutchik R (1963). Auditory pain thresholds for intermittent, “beat” and steady signals. Perceptual and Motor Skills, 16(3), 863–870. 10.2466/pms.1963.16.3.863 [DOI] [PubMed] [Google Scholar]
- Pollard B (2019). Clinical advancements for managing hyperacusis with pain. The Hearing Journal, 72(10), 10 10.1097/01.HJ.0000602900.16223.0e [DOI] [Google Scholar]
- Potgieter I, MacDonald C, Partridge L, Cima R, Sheldrake J, & Hoare DJ (2019). Misophonia: A scoping review of research. Journal of Clinical Psychology, 75(7), 1203–1218. 10.1002/jclp.22771 [DOI] [PubMed] [Google Scholar]
- Poulton R, & Menzies RG (2002). Non-associative fear acquisition: A review of the evidence from retrospective and longitudinal research. Behaviour Research and Therapy, 40(2), 127–149. 10.1016/S0005-7967(01)00045-6 [DOI] [PubMed] [Google Scholar]
- Preece D, & Jordan R (2010). Obtaining the views of children and young people with autism spectrum disorders about their experience of daily life and social care support. British Journal of Learning Disabilities, 38(1), 10–20. 10.1111/j.1468-3156.2009.00548.x [DOI] [Google Scholar]
- Preyer S, & Gummer AW (1996). Nonlinearity of mechanoelectrical transduction of outer hair cells as the source of nonlinear basilar-membrane motion and loudness recruitment. Audiology and Neurotology, 1(1), 3–11. 10.1159/000259185 [DOI] [PubMed] [Google Scholar]
- Punch J, Joseph A, & Rakerd B (2004). Most comfortable and uncomfortable loudness levels: Six decades of research. American Journal of Audiology, 13(2), 144–157. 10.1044/1059-0889(2004/019) [DOI] [PubMed] [Google Scholar]
- Radziwon K, Auerbach BD, Ding D, Liu X, Chen G-D, & Salvi R (2019). Noise-Induced loudness recruitment and hyperacusis: Insufficient central gain in auditory cortex and amygdala. Neuroscience, 422, 212–227. 10.1016/j.neuroscience.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington A, & Fairnie J (2017). A sound advantage: Increased auditory capacity in autism. Cognition, 166, 459–465. 10.1016/j.cognition.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Robertson AE, & Simmons DR (2013). The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental Disorders, 43(4), 775–784. 10.1007/s10803-012-1608-7 [DOI] [PubMed] [Google Scholar]
- Robertson AE, & Simmons DR (2015). The sensory experiences of adults with autism spectrum disorder: A qualitative analysis. Perception, 44(5), 569–586. 10.1068/p7833 [DOI] [PubMed] [Google Scholar]
- Robertson CE, & Baron-Cohen S (2017). Sensory perception in autism. Nature Reviews Neuroscience, 18(11), 671–684. 10.1038/nrn.2017.112 [DOI] [PubMed] [Google Scholar]
- Rodgers J, Wigham S, McConachie H, Freeston M, Honey E, & Parr JR (2016). Development of the anxiety scale for children with autism spectrum disorder (ASC-ASD). Autism Research, 9(11), 1205–1215. 10.1002/aur.1603 [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, & Croog VJ (1997). Linking etiologies in humans and animal models: Studies of autism. Reproductive Toxicology, 11(2), 417–422. 10.1016/S0890-6238(97)80001-U [DOI] [PubMed] [Google Scholar]
- Röhl M, & Uppenkamp S (2012). Neural coding of sound intensity and loudness in the human auditory system. Journal of the Association for Research in Otolaryngology, 13(3), 369–379. 10.1007/s10162-012-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Sandström M, Ahlsén G, & Gillberg C (1999). Autism and hearing loss. Journal of Autism and Developmental Disorders, 29(5), 349–357. 10.1023/A:1023022709710 [DOI] [PubMed] [Google Scholar]
- Rotschafer SE, & Cramer KS (2017). Developmental emergence of phenotypes in the auditory brainstem nuclei of Fmr1 knockout mice. ENeuro, 4(6), ENEURO.0264–17.2017. 10.1523/ENEURO.0264-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Marshak S, & Cramer KS (2015). Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS One, 10(2), e0117266 10.1371/journal.pone.0117266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, & Razak K (2013). Altered auditory processing in a mouse model of fragile X syndrome. Brain Research, 1506, 12–24. 10.1016/j.brainres.2013.02.038 [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior, 2(5), 255–267. 10.1034/j.1601-183X.2003.00037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Auerbach BD, Lau C, Chen Y-C, Manohar S, Liu X, Ding D, & Chen G-D (2020). Functional neuroanatomy of salicylate- and noise-induced tinnitus and hyperacusis. 1–28. 10.1007/7854_2020_156 [DOI] [PubMed]
- Salvi RJ, Wang J, & Ding D (2000). Auditory plasticity and hyperactivity following cochlear damage. Hearing Research, 147(1), 261–274. 10.1016/S0378-5955(00)00136-2 [DOI] [PubMed] [Google Scholar]
- Sandbank M, Bottema-Beutel K, Crowley S, Cassidy M, Dunham K, Feldman JI, Crank J, Albarran SA, Raj S, Mahbub P, & Woynaroski TG (2020). Project AIM: Autism intervention meta-analysis for studies of young children. Psychological Bulletin, 146(1), 1–29. 10.1037/bul0000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock S, Schütte M, & Griefahn B (2008). Impairing effects of noise in high and low noise sensitive persons working on different mental tasks. International Archives of Occupational and Environmental Health, 82(6), 779 10.1007/s00420-008-0379-0 [DOI] [PubMed] [Google Scholar]
- Santos M, Marques C, Nóbrega Pinto A, Fernandes R, Coutinho MB, & Almeida e Sousa C (2017). Autism spectrum disorders and the amplitude of auditory brainstem response wave I: Autism spectrum disorders and the amplitude of ABR wave I. Autism Research, 10(7), 1300–1305. 10.1002/aur.1771 [DOI] [PubMed] [Google Scholar]
- Scahill L, Lecavalier L, Schultz RT, Evans AN, Maddox B, Pritchett J, Herrington J, Gillespie S, Miller J, Amoss RT, Aman MG, Bearss K, Gadow K, & Edwards MC (2019). Development of the parent-rated anxiety scale for youth with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 58(9), 887–896.e2. 10.1016/j.jaac.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Schauder KB, & Bennetto L (2016). Toward an Interdisciplinary Understanding of Sensory Dysfunction in Autism Spectrum Disorder: An Integration of the Neural and Symptom Literatures. Frontiers in Neuroscience, 10, 268 10.3389/fnins.2016.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FH, Mauermann M, & Kollmeier B (2020). Neural representation of loudness: Cortical evoked potentials in an induced loudness reduction experiment. Trends in Hearing, 24, 2331216519900595 10.1177/2331216519900595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen SA, Lane SJ, Mailloux Z, May- Benson T, Parham LD, Roley SS, & Schaaf RC. (2019). A systematic review of Ayres Sensory Integration intervention for children with autism. Autism Research, 12(1), 6–19. 10.1002/aur.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, & Wehr M (2008). Disruption of balanced cortical excitation and inhibition by acoustic trauma. Journal of Neurophysiology, 100(2), 646–656. 10.1152/jn.90406.2008 [DOI] [PubMed] [Google Scholar]
- Schreiner CE, & Malone BJ (2015). Representation of loudness in the auditory cortex In Aminoff MJ, Boller F, & Swaab DF (Eds.), Handbook of clinical neurology (Vol. 129, pp. 73–84). Elsevier; 10.1016/B978-0-444-62630-1.00004-4 [DOI] [PubMed] [Google Scholar]
- Schroder AC, Viemeister NF, & Nelson DA (1994). Intensity discrimination in normal- hearing and hearing- impaired listeners. The Journal of the Acoustical Society of America, 96(5), 2683–2693. 10.1121/1.411276 [DOI] [PubMed] [Google Scholar]
- Schröder AE, van Wingen G, Eijsker N, Giorgi RS, Vulink NC, Turbyne C, & Denys D (2019). Misophonia is associated with altered brain activity in the auditory cortex and salience network. Scientific Reports, 9(1), 7542 10.1038/s41598-019-44084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder AE, Vulink NC, van Loon AJ, & Denys DA (2017). Cognitive behavioral therapy is effective in misophonia: An open trial. Journal of Affective Disorders, 217, 289–294. 10.1016/j.jad.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Schröder AE, Vulink N, & Denys D (2013). Misophonia: Diagnostic criteria for a new psychiatric disorder. PloS One, 8(1), e54706 10.1371/journal.pone.0054706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake J, Diehl PU, & Schaette R (2015). Audiometric characteristics of hyperacusis patients. Frontiers in Neurology, 6(1), 105 10.3389/fneur.2015.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A, Stocking C, Ralli M, & Salvi R (2020). A review of auditory gain, low-level noise and sound therapy for tinnitus and hyperacusis. International Journal of Audiology, 59(1), 5–15. 10.1080/14992027.2019.1660812 [DOI] [PubMed] [Google Scholar]
- Simonoff E (2020, June 3). Mental health in autistic people: Setting a research agenda for the coming decade [INSAR 2020 Virtual - Keynote Webinar]. International Society for Autism Research Annual Meeting https://insar.confex.com/insar/2020/meetingapp.cgi/Paper/36311 [Google Scholar]
- Sinha Y, Silove N, Wheeler D, & Williams K (2006). Auditory integration training and other sound therapies for autism spectrum disorders: A systematic review. Archives of Disease in Childhood, 91(12), 1018–1022. 10.1136/adc.2006.094649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, & Sharp J (2013). Fascination and isolation: A grounded theory exploration of unusual sensory experiences in adults with Asperger syndrome. Journal of Autism and Developmental Disorders, 43(4), 891–910. 10.1007/s10803-012-1633-6 [DOI] [PubMed] [Google Scholar]
- Sohal VS, & Rubenstein JLR (2019). Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Molecular Psychiatry, 24(9), 1248–1257. 10.1038/s41380-019-0426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis J (2004). The signal functions of early infant crying. Behavioral and Brain Sciences, 27(4), 443–458. 10.1017/S0140525X0400010X [DOI] [PubMed] [Google Scholar]
- Sörqvist P (2010). The role of working memory capacity in auditory distraction: A review. Noise & Health, 12(49), 217–224. 10.4103/1463-1741.70500 [DOI] [PubMed] [Google Scholar]
- Sörqvist P, & Marsh JE (2015). How concentration shields against distraction. Current Directions in Psychological Science, 24(4), 267–272. 10.1177/0963721415577356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörqvist P, & Rönnberg J (2014). Individual differences in distractibility: An update and a model. PsyCh Journal, 3(1), 42–57. 10.1002/pchj.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, & Rodgers J (2017). Sensory, emotional and cognitive contributions to anxiety in autism spectrum disorders. Frontiers in Human Neuroscience, 11, 20 10.3389/fnhum.2017.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld SA (1992). Noise, noise sensitivity and psychiatric disorder: Epidemiological and psychophysiological studies. Psychological Medicine Monograph Supplement, 22, 1–44. 10.1017/S0264180100001119 [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, & Clark C (2019). Mental health effects of noise In Nriagu J (Ed.), Encyclopedia of Environmental Health (2nd ed., pp. 287–294). Elsevier; 10.1016/B978-0-12-409548-9.11814-7 [DOI] [Google Scholar]
- Stansfeld SA, Clark CR, Turpin G, Jenkins LM, & Tarnopolsky A (1985). Sensitivity to noise in a community sample: II. Measurement of psychophysiological indices. Psychological Medicine, 15(2), 255–263. 10.1017/S0033291700023539 [DOI] [PubMed] [Google Scholar]
- Stefanelli ACGF, Zanchetta S, Furtado EF, Stefanelli ACGF, & Furtado EF (2020). Auditory hyper-responsiveness in autism spectrum disorder, terminologies and physiological mechanisms involved: Systematic review. CoDAS, 32(3), e20180287 10.1590/2317-1782/20192018287 [DOI] [PubMed] [Google Scholar]
- Steigner J, & Ruhlin SU (2014). Systematic desensitization of hyperacusis and vocal pitch disorder treatment in a patient with autism. The Internet Journal of Allied Health Sciences and Practice, 12(1), 1–6. [Google Scholar]
- Stiegler LN, & Davis R (2010). Understanding sound sensitivity in individuals with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 25(2), 67–75. 10.1177/1088357610364530 [DOI] [Google Scholar]
- Stolzberg D, Chrostowski M, Salvi RJ, & Allman BL (2012). Intracortical circuits amplify sound-evoked activity in primary auditory cortex following systemic injection of salicylate in the rat. Journal of Neurophysiology, 108(1), 200–214. 10.1152/jn.00946.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhnan AP, Finch PM, & Drummond PD (2017). Hyperacusis in chronic pain: Neural interactions between the auditory and nociceptive systems. International Journal of Audiology, 56(11), 801–809. 10.1080/14992027.2017.1346303 [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, & Salvi RJ (2009). Salicylate increases the gain of the central auditory system. Neuroscience, 159(1), 325–334. 10.1016/j.neuroscience.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Wei, Deng A, Jayaram A, & Gibson B (2012). Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Research, 1485, 108–116. 10.1016/j.brainres.2012.02.008 [DOI] [PubMed] [Google Scholar]
- Syu Y-C, & Lin L-Y (2018). Sensory overresponsivity, loneliness, and anxiety in taiwanese adults with autism spectrum disorder. Occupational Therapy International, 2018, 1–7. 10.1155/2018/9165978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Tudor BM, & Kileny PR (2018). Click- evoked auditory brainstem responses and autism spectrum disorder: A meta- analytic review. Autism Research, 11(6), 916–927. 10.1002/aur.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Brandes-Aitken A, Chu R, Porter L, Schoen S, Miller LJ, Gerdes MR, Owen J, Mukherjee P, & Marco EJ (2019). Sensory over-responsivity: Parent report, direct assessment measures, and neural architecture. Molecular Autism, 10(1), 4 10.1186/s13229-019-0255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, & Baron-Cohen S (2014). Sensory overresponsivity in adults with autism spectrum conditions. Autism, 18(4), 428–432. 10.1177/1362361313477246 [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Bess FH, Sladen DP, Schissel H, Couch S, & Schery T (2006). Auditory characteristics of children with autism. Ear and Hearing, 27(4), 430–441. 10.1097/01.aud.0000224981.60575.d8 [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Lüthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16(6), 317–331. 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Sakurai K, Shimazaki K, Takeda Y, Shimoda Y, & Watanabe K (2009). Deficiency of neural recognition molecule NB-2 affects the development of glutamatergic auditory pathways from the ventral cochlear nucleus to the superior olivary complex in mouse. Developmental Biology, 336(2), 192–200. 10.1016/j.ydbio.2009.09.043 [DOI] [PubMed] [Google Scholar]
- Trakoshis S, Martínez-Cañada P, Rocchi F, Canella C, You W, Chakrabarti B, Ruigrok AN, Bullmore ET, Suckling J, Markicevic M, Zerbi V, MRC AIMS Consortium, Baron-Cohen S, Gozzi A, Lai M-C, Panzeri S, & Lombardo MV (2020). Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. ELife, 9, e55684 10.7554/eLife.55684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor C, Arnal LH, & Frühholz S (2020). Terrifying film music mimics alarming acoustic feature of human screams. The Journal of the Acoustical Society of America, 147(6), EL540–EL545. 10.1121/10.0001459 [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Coelho CB, Andersson G, Keiner AJ, Cacace AT, Martin N, & Moore BCJ (2014). A review of hyperacusis and future directions: Part I. Definitions and manifestations. American Journal of Audiology, 23(4), 402–419. 10.1044/2014_AJA-14-0010 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, & Lane A (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10(5), 703–710. 10.1002/aur.1747 [DOI] [PubMed] [Google Scholar]
- Uppenkamp S, & Röhl M (2014). Human auditory neuroimaging of intensity and loudness. Hearing Research, 307, 65–73. 10.1016/j.heares.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, & Wagemans J (2014). Precise minds in uncertain worlds: Predictive coding in autism. Psychological Review, 121(4), 649–675. 10.1037/a0037665 [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Lemery-Chalfant K, & Hill Goldsmith H (2018). Parent-offspring transmission of internalizing and sensory over-responsivity symptoms in adolescence. Journal of Abnormal Child Psychology, 46(3), 557–567. 10.1007/s10802-017-0300-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Schmidt NL, & Goldsmith HH (2012). Is sensory over-responsivity distinguishable from childhood behavior problems? A phenotypic and genetic analysis. Journal of Child Psychology and Psychiatry, 53(1), 64–72. 10.1111/j.1469-7610.2011.02432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kamp I, Job RFS, Hatfield J, Haines M, Stellato RK, & Stansfeld SA (2004). The role of noise sensitivity in the noise-response relation: A comparison of three international airport studies. The Journal of the Acoustical Society of America, 116(6), 3471–3479. 10.1121/1.1810291 [DOI] [PubMed] [Google Scholar]
- Viinikainen M, Kätsyri J, & Sams M (2012). Representation of perceived sound valence in the human brain. Human Brain Mapping, 33(10), 2295–2305. 10.1002/hbm.21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingen JV, Pareja JA, Støren O, White LR, & Stovner LJ (1998). Phonophobia in migraine. Cephalalgia : An International Journal of Headache, 18(5), 243–249. 10.1111/j.1468-2982.1998.1805243.x [DOI] [PubMed] [Google Scholar]
- Viziano A, Micarelli A, & Alessandrini M (2017). Noise sensitivity and hyperacusis in patients affected by multiple chemical sensitivity. International Archives of Occupational and Environmental Health, 90(2), 189–196. 10.1007/s00420-016-1185-8 [DOI] [PubMed] [Google Scholar]
- Volkmar FR, & Cohen DJ (1985). The experience of infantile autism: A first-person account by Tony W. Journal of Autism and Developmental Disorders, 15(1), 47–54. 10.1007/BF01837898 [DOI] [PubMed] [Google Scholar]
- Ward J (2019). Individual differences in sensory sensitivity: A synthesizing framework and evidence from normal variation and developmental conditions. Cognitive Neuroscience, 10(3), 139–157. 10.1080/17588928.2018.1557131 [DOI] [PubMed] [Google Scholar]
- Watts SJ, Rodgers J, & Riby D (2016). A systematic review of the evidence for hyporesponsivity in ASD. Review Journal of Autism and Developmental Disorders, 3(4), 286–301. 10.1007/s40489-016-0084-y [DOI] [Google Scholar]
- Weitlauf AS, Sathe N, McPheeters ML, & Warren ZE (2017). Interventions targeting sensory challenges in autism spectrum disorder: A systematic review. Pediatrics, 139(6), e20170347 10.1542/peds.2017-0347 [DOI] [PubMed] [Google Scholar]
- Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, Razak KA, & Ethell IM (2018). Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cerebral Cortex, 28(11), 3951–3964. 10.1093/cercor/bhx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ (2019). Speaking-induced suppression of the auditory cortex in humans and Its relevance to schizophrenia. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(9), 791–804. 10.1016/j.bpsc.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Wigham S, Rodgers J, South M, McConachie H, & Freeston M (2015). The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 943–952. 10.1007/s10803-014-2248-x [DOI] [PubMed] [Google Scholar]
- Williams ZJ, Abdelmessih PG, Key AP, & Woynaroski TG (2020). Cortical auditory processing of simple stimuli is altered in autism: A meta-analysis of auditory evoked responses. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ, Failla MD, Gotham KO, Woynaroski TG, & Cascio C (2018). Psychometric evaluation of the Short Sensory Profile in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(12), 4231–4249. 10.1007/s10803-018-3678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ, Suzman E, & Woynaroski TG (2020a). A Phenotypic Comparison of Loudness and Pain Hyperacusis: Symptoms, Comorbidity, and Associated Features in a Multinational Patient Registry. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed]
- Williams ZJ, Suzman E, & Woynaroski TG (2020b). Prevalence of decreased sound tolerance (hyperacusis) in individuals with autism spectrum disorder: A meta-analysis. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed]
- Wilson US, Sadler KM, Hancock KE, Guinan JJ, & Lichtenhan JT (2017). Efferent inhibition strength is a physiological correlate of hyperacusis in children with autism spectrum disorder. Journal of Neurophysiology, 118(2), 1164–1172. 10.1152/jn.00142.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Radziwon K, Chen G-D, Liu X, Manno FAM, Manno SHC, Auerbach B, Wu EX, Salvi R, & Lau C (2020). Functional magnetic resonance imaging of enhanced central auditory gain and electrophysiological correlates in a behavioral model of hyperacusis. Hearing Research, 389, 107908 10.1016/j.heares.2020.107908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, & Gadow KD (2010). Exploring the nature and function of anxiety in youth with autism spectrum disorders. Clinical Psychology: Science and Practice, 17(4), 281–292. 10.1111/j.1468-2850.2010.01220.x [DOI] [Google Scholar]
- Woodhouse A, & Drummond PD (1993). Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia, 13(6), 417–421. 10.1046/j.1468-2982.1993.1306417.x [DOI] [PubMed] [Google Scholar]
- Zentall SS, & Shaw JH (1980). Effects of classroom noise on performance and activity of second-grade hyperactive and control children. Journal of Educational Psychology, 72(6), 830–840. 10.1037/0022-0663.72.6.830 [DOI] [PubMed] [Google Scholar]
- Zhang KD, & Coate TM (2017). Recent advances in the development and function of type II spiral ganglion neurons in the mammalian inner ear. Seminars in Cell & Developmental Biology, 65, 80–87. 10.1016/j.semcdb.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Yum NW, Benjamin L, Benhamou E, Yoneya M, Furukawa S, Dick F, Slaney M, & Chait M (2019). Rapid ocular responses are modulated by bottom-up-driven auditory salience. Journal of Neuroscience, 39(39), 7703–7714. 10.1523/JNEUROSCI.0776-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R, Patel R, Smith A, Pasos J, & Kulesza RJ (2018). Repeated prenatal exposure to valproic acid results in auditory brainstem hypoplasia and reduced calcium binding protein immunolabeling. Neuroscience, 377, 53–68. 10.1016/j.neuroscience.2018.02.030 [DOI] [PubMed] [Google Scholar]
- Zimmerman R, Smith A, Fech T, Mansour Y, & Kulesza RJ (2020). In utero exposure to valproic acid disrupts ascending projections to the central nucleus of the inferior colliculus from the auditory brainstem. Experimental Brain Research, 238(3), 551–563. 10.1007/s00221-020-05729-7 [DOI] [PubMed] [Google Scholar]