SUMMARY
Stem cells (SCs) maintain tissue homeostasis and repair wounds. Despite marked variation in tissue architecture and regenerative demands, SCs often follow similar paradigms in communicating with their microenvironmental “niche” to transition between quiescent and regenerative states. Here we use skin epithelium and skeletal muscle—among the most highly-stressed tissues in our body—to highlight similarities and differences in niche constituents and how SCs mediate natural tissue rejuvenation and perform regenerative acts prompted by injuries. We discuss how these communication networks break down during aging and how understanding tissue SCs has led to major advances in regenerative medicine.
INTRODUCTION
Billions of cells are lost daily from our body’s tissues, which are in a perpetual state of flux throughout our lifetime. The molecular engines that drive this turnover are self-renewing tissue stem cells. The work horses are stem cell-generated, short-lived progenitors that balance proliferation and differentiation, thereby maintaining tissue homeostasis. The homeostatic requirements for cellular replacements are tissue and context specific. They are continual in blood, epidermis, and intestine, episodic in the hair follicle and lactating mammary gland, and limited in brain and muscle. However, even largely quiescent stem cells, such as those of the muscle, can be mobilized into action when their tissue is injured. To guard against infection and heal wounds, the normal homeostatic cues—termed the “milieu interieur” in 1865 by Claude Bernard—must be overridden in ways that are still being determined.
Understanding stem cell behaviors necessitates knowledge of their local environment or “niche.” Increasing evidence shows that whether quiescent or active, stem cells are not simple passive responders to their niches; rather, they play an integral role in creating and communicating with their niches that envelope them. Regenerative signals, emanating either from a build up in crosstalk with niche factors or from marked environmental changes upon injury, alter stem cell behavior and disrupt the homeostatic equilibrium of the tissue. In part, the stem cells’ own progenies can become important niche components: while early short-lived progenitors can send transient activating cues back to their stem cell parents to fuel their self-renewal and boost tissue growth (Blau et al., 2015; Hsu et al., 2014; Mondal et al., 2014; Porpiglia et al., 2017), differentiated progeny can home back to their niche to halt further proliferation and tissue regeneration and restore homeostasis (Montarras et al., 2005; Sato et al., 2011; Yu and Scadden, 2016). In this way, tissue regeneration is orchestrated by a delicate balance of temporally coordinated cellular interactions and molecular feedback circuits in which stem cells play a central role.
Heterologous stem cell niche components include the basement membrane, rich in extracellular matrix and stem cell growth factors, as well as blood vessels, lymphatic capillaries, nerves, stromal, adipose, and a variety of tissue-resident immune cells that function with stem cells to guard against tissue damage and pathogens. The beauty of having immune cells as integral constituents of stem cell niches is that many are mobile, able to migrate to local lymph nodes and stimulate non-resident immune cells, which can then travel through the circulation to the site of tissue damage and contribute to the inflammatory response that clears pathogens and damaged cells from the tissue (Fan and Rudensky, 2016). Reinstating homeostasis, however, relies upon tissue repair, which is incompatible with inflammation. Since the stem cells are responsible for the reparative phase of the response, there must be intricate immune-stem cell communication to ensure not only that the pathogen invasion is under control, but also that the inflammation is subsequently dampened in order to facilitate repair (Arnold et al., 2007; Burzyn et al., 2013; Fan and Rudensky, 2016). How this happens is still largely a mystery, but a few clues are beginning to emerge.
Here we review how stem cells serve as architects of their own niche. This microenvironment envelops the stem cell and dictates its function during homeostasis while allowing it to rapidly mobilize its tissue regenerating energies when an injury occurs. We focus on two markedly different tissues—the skin epithelium and the skeletal muscle—both of which are subjected to stress and damage throughout life. We highlight features of the stem cells and their niches and discuss how they combine context-specific and universal mechanisms to maintain tissue fitness. We discuss increasing evidence that tissue stem cells sense and communicate with an amazingly diverse array of niche components. As we are beginning to learn, this complexity enables stem cells to not only deflect minor insults and maintain homeostasis, but also remain poised to sense and respond to natural regenerative stimuli and to the diverse array of tissue damage and other stresses they encounter throughout their lifetime. Given the complexity of stem cell niches, it is also perhaps not surprising that across many different tissues, including muscle and skin, aging often involves a breakdown of extrinsic niche components, rather than the intrinsic self-renewal capacity of its stem cell residents (Blau et al., 2015; Ge et al., 2020; Pentinmikko et al., 2019; Raaijmakers, 2019; Segel et al., 2019; Tierney and Sacco, 2016). An additional consequence of aging-associated changes is an increase in tissue stiffness that can disrupt homeostatic stem cell mechanosensing (Cosgrove et al., 2014; Gilbert et al., 2010; Madl et al., 2018). Finally, our review concludes with a discussion of the clinical implications and the therapeutic potential of tissue stem cells and the signals they receive from their microenvironment, in human diseases and regenerative medicine.
SKIN STEM CELLS AND THEIR NICHES
The skin is the largest organ of our body and its epithelial surface serves as our primary interface with the external environment. As such it is subjected to a barrage of assaults, ranging from mechanical stress to ultraviolet radiation to wounds and various pathogens, microbes, and allergens. To protect themselves, guard against pathogens, and retain the body’s fluids, the epithelial stem cells of the skin must generate a tough, resilient cellular barrier. For humans, the epidermis is the major barrier; for most mammals, the epidermis is thinner while the hair coat is more elaborate and acts as the major protector of the body surface. Both are derived from the same embryonic precursor cells.
Shortly after gastrulation, mammalian skin epithelium begins as a layer of unspecified progenitors. Through temporal and spatial cues, some progenitors become epidermal stem cells (EpdSCs) which will fuel the constant outward flux of cells through the stratified, differentiated layers (Ellis et al., 2019; Fan et al., 2018; Jones et al., 1995; Quiroz et al., 2020; Rompolas et al., 2016). By contrast, hair follicle stem cells (HFSCs) are born when WNTs from neighboring epidermal progenitors and BMP inhibitors from underlying mesenchyme specify hair buds which progress into mature hair follicles by postnatal day P6 (Matos et al., 2020; Mok et al., 2019; Noramly et al., 1999; Ouspenskaia et al., 2016; Reddy et al., 2001). In mature skin, the stem cells of the epidermis and its appendages form a contiguous monolayer at the epithelial-dermal interface, demarcated by a basement membrane rich in extracellular matrix (ECM) and growth factors (Dekoninck et al., 2020; Has and Nyström, 2015).
The skin epithelial stem cells produce the major components of their underlying basement membrane, which plays a central role in controlling the stem cells’ polarity, proliferation, and maintenance (Breitkreutz et al., 1998; Fiore et al., 2020; Nikolopoulos et al., 2005). Interestingly, however, many features of the basement membrane as well as the other cells surrounding the stem cells are markedly different, imparting to the epithelial skin stem cells distinct identities, gene expression programs, and tissue-regenerating responsibilities.
Epidermal Stem Cells: Orchestrators of Their Niche
Adult skin EpdSCs exist as a single (basal) layer adjacent to the basement membrane. There, EpdSCs balance self-renewal with a defined ascendant program of terminal differentiation that produces a tough, resilient stratified tissue barrier (Figure 1A). Basal epidermal progenitors express keratins 5 and 14 (K5/14), but they switch to K1/K10 as they activate NOTCH and other signals and enter the first suprabasal phase of terminal differentiation (Gonzales and Fuchs, 2017).
Figure 1. Natural Regeneration during Tissue Homeostasis in the Skin.
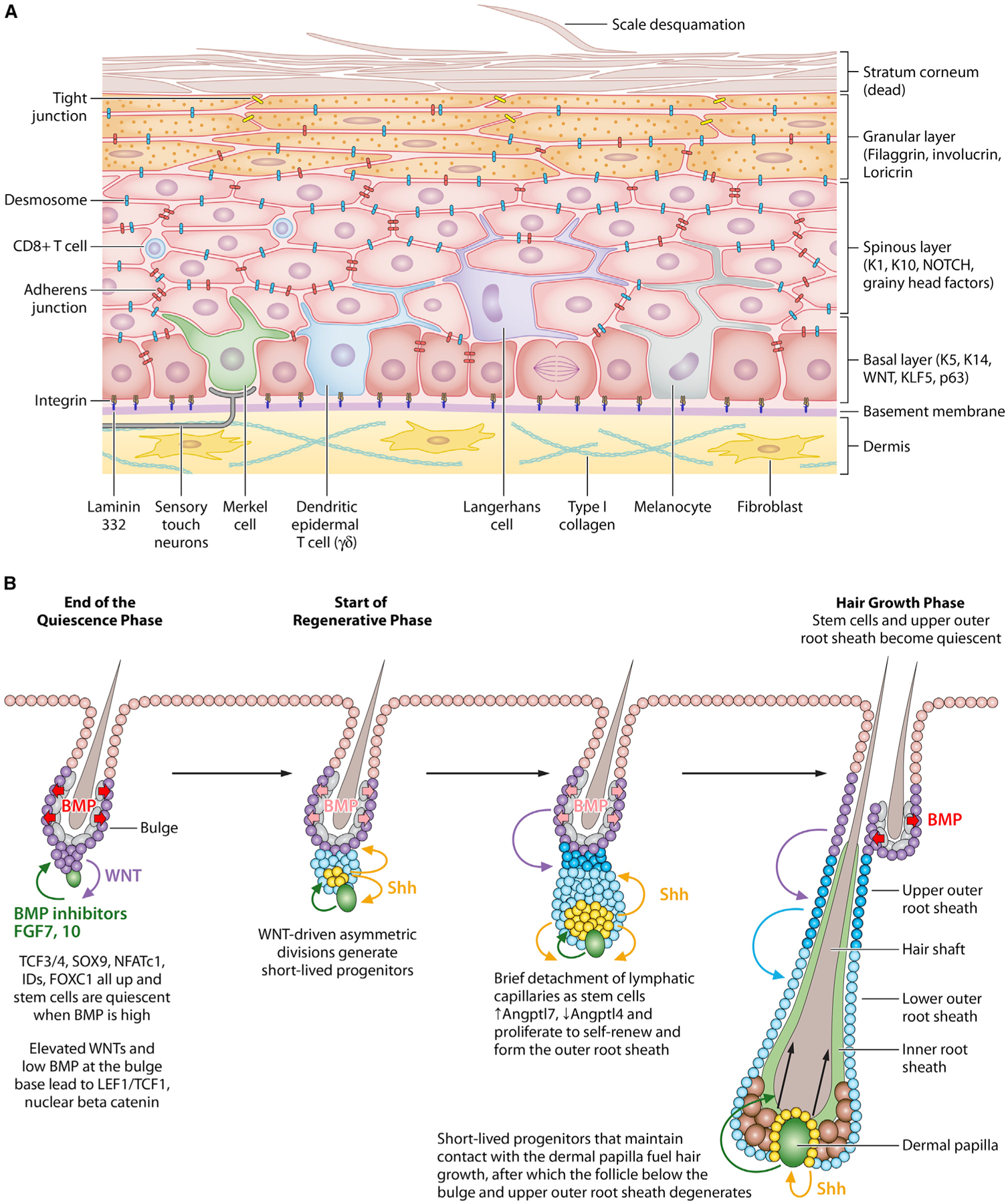
(A) Epidermal stem cells (EpdSCs) reside within the innermost (basal) layer of the skin epidermis. A basement membrane, rich in extracellular matrix (ECM) proteins and growth factors, resides at the epidermal-dermal border and is produced and secreted mostly by the EpdSCs. In adult skin, EpdSCs divide parallel to the basement membrane. As their proliferative progeny commit to terminally differentiate, they exit the basal layer and move outward, undergoing three morphologically and biochemically distinct stages: spinous cells, which are so-named because of their abundant desmosomes; granular cells, which are characterized by the presence of keratohyalin granules; and finally, stratum corneum cells, which are flattened dead cells devoid of organelles but packed with bundles of keratin filaments and sandwiched by lipid bilayers. Stratum corneum cells provide the body’s barrier to the external environment. These cells are continually sloughed from the skin surface and replaced by inner layer cells moving outward. Orchestrated by EpdSCs, the balance between proliferation and differentiation must be finely controlled to maintain equilibrium and keep the skin barrier rejuvenated. Within the epidermis are sentinels such as dendritic epidermal T cells (DETCs), macrophage-like Langerhans cells, and CD8+ T cells to warn of a barrier breach and invasion of harmful microbes. In human epidermis, melanocytes also exist within the basal epidermal layer, where they transfer sun-protective melanin to the EpdSCs. In both mouse and human epidermis, Merkel cells connect with sensory touch neurons that can relay a touch response to the brain and specialized nerve fibers to sense thermal fluctuations and pain terminate within the epidermis.
(B) Hair follicles (HFs) undergo cyclical bouts of rest (telogen), active hair growth (anagen), and destruction (catagen). During the resting phase, hair follicle stem cells (HFSCs) reside in quiescence at the base of the non-cycling portion of the HF in an anatomical niche known as the bulge. HFSC quiescence is maintained by BMPs and FGF18, which are expressed at high levels by an inner layer of terminally differentiated bulge cells. Just beneath the telogen bulge is a specialized dermal cluster known as the dermal papilla (DP), which undergoes stimulatory molecular crosstalk with neighboring HFSCs and establishes a blueprint for what will happen in anagen. When a threshold of activating WNT and BMP inhibitory factors override the quiescence factors, HFSCs at the bulge base (sometimes referred to as the hair germ) begin to divide asymmetrically. Daughter cells that retain their close proximity to the DP produce sonic hedgehog (SHH), a stimulatory factor for both the DP and the HFSCs. These cells maintain their contact with DP and give rise to the asymmetrically dividing unilineage progenitors that produce the inner root sheath and the hair shaft. By contrast, daughters more distant from the DP continue to proliferate as long as the SHH signal is sufficiently near. They form the outer root sheath, a layer of cells extending from the bulge to the hair bulb in the mature anagen HF. For the bulge, HFSCs return to quiescence shortly after the hair cycle has been launched; for the ORS cells in the lower part of the HF, they continue to proliferate throughout anagen and may help to fuel hair growth, which lasts ~3 weeks in mice. Not shown in this figure, catagen results in the terminal differentiation and apoptosis of the HF, sparing the DP and the upper ORS, which forms a new bulge for the next hair cycle.
Both pairs of keratins assemble into 10 nm intermediate filaments imparting mechanical strength to the epidermal cells. However, K1 and K10 both have long, unstructured amino head and carboxy tail domains that protrude along the surface of the filaments. Another key differentiation protein is filaggrin, composed of large >300 amino acid residue repeats that are rich in histidine and also unstructured. As differentiation proceeds and the levels of these proteins rises, their features drive liquid-liquid phase transitions, resulting in filaggrin-rich “keratohyalin” granules that grow, gain viscosity, and become caged by K1/K10 keratin bundles (Quiroz et al., 2020). The outcome is a crowding of the cytoplasm and mechanical distortion that leads to loss of nuclei and other organelles. The resultant dead, flattened squames of the stratum corneum layer are sealed by lipids, constituting an impenetrable barrier at the skin surface. By designing a terminal differentiation program based upon conformational changes in intrinsically disordered proteins, the epidermal stem cells beautifully exploit the harsh extremes in pH, temperature, and humidity at the body surface, which drive these protein dynamics (Quiroz et al., 2020). As squames are shed, they are replaced by upwardly differentiating cells fueled by the stem cells beneath.
Mutations that abrogate filaggrin’s ability to undergo liquid-phase transitions are at the root of atopic dermatitis, a human genetic disorder typified by a paucity of granules, retention of nuclei, and skin barrier breaches (Quiroz et al., 2020). Mutations in keratins result in skin-blistering disorders, with K5/K14 at the roots of stem cell mechanical fragility and K1/K10 at the roots of spinous cell mechanical defects (Coulombe et al., 2009). As important to mechanical strength and prevention of skin blistering is the ability of the epidermal stem cells to adhere to their underlying basement membrane. Mutations in epidermal laminin (332) chains, α6β4 integrins, or collagen VII all lead to devastating blistering disorders in humans (Pulkkinen and Uitto, 1999).
A fascinating facet is that by generating the basement membrane and the stratified layers of the skin barrier, epidermal stem cells essentially design their own niche and participate heavily in controlling their own destiny. The factors that govern their behavior during homeostasis are all localized in close proximity to EpdSCs. ECM-integrin interactions, growth factors, and other secreted ligands within the vicinity have all been shown to influence EpdSC self-renewal and epidermal progenitor proliferation (Giancotti and Ruoslahti, 1999). Conversely, E-cadherin-mediated intercellular (adherens) junctions place the brakes on proliferation, establishing a natural means of crowd control within the basal layer (Schlegelmilch et al., 2011; Vasioukhin et al., 2001). A key component of these junctions is α-catenin, which not only coordinates association with the actin cytoskeleton but also sequesters transcriptional cofactor YAP, which can translocate to the nucleus and trigger a proliferative response upon mechanical stress (Schlegelmilch et al., 2011).
The findings of Camargo and coworkers (Schlegelmilch et al., 2011) heightened the awareness that the mechanical properties of the epidermis are intertwined with the conventional signaling cues that together orchestrate stem cell behavior. Moreover, in assessing the mechanical forces exerted on the stem cells within stratified epithelial tissues such as the epidermis, both the overlying stiffness and tension of the differentiating layers and the underlying stiffness and the assembly rates of the basement membrane can profoundly influence stem cell behavior and tissue production (Fiore et al., 2020). Stretching also induces skin expansion by creating a transient bias in the renewal activity of epidermal stem cells (Aragona et al., 2020).
Although epidermal stem cells are remarkably adept at orchestrating their niche, they still receive guidance from other cell types, including the papillary fibroblasts just beneath the basement membrane (Driskell et al., 2013).The ultimate goal is for EpdSCs to fine-tune their own proliferation in the basal layer, and at the same time, generate a constant flux of terminally differentiating protective cells so that homeostasis is maintained while creating a brand new skin barrier every few weeks. Additional components of the EpdSC niche are thermal, pain, and touch sensing neurons (Jenkins and Lumpkin, 2017; Neubarth et al., 2020; Paricio-Montesinos et al., 2020; Sharif et al., 2020; Zimmerman et al., 2019; Zylka et al., 2005) and resident immune cells (Jameson et al., 2002; Otsuka et al., 2018). These features endow the stem cells with an ability to sense and respond to diverse changes in their environment.
The Hair Follicle Stem Cell Niche: Coordinating Episodic Bouts of Natural Tissue Generation in the Absence of Wounding
In contrast to epidermal stem cells, which continually regenerate tissue, hair follicle stem cells (HFSCs) alternate between extended bouts of quiescence and active tissue regeneration (Figure 1B). In mice, much of the regeneration energy in the absence of injury is devoted to synchronous cycles of hair coat production, which is fueled by stem cells residing in an anatomical niche, known as the bulge. During periods of stem cell inactivity (quiescence), HFs are synchronized across the hair coat by connecting to an expansive vasculature (Li et al., 2019) now known to be a lymphatic capillary network (Gur-Cohen et al., 2019; Peña-Jimenez et al., 2019).
During the resting phase (telogen), the quiescent bulge niche exists at the base of the non-cycling portion of the HF, where it anchors the old hair and a surrounding layer of BMP6hiFGF18hi terminally differentiated cells (“inner bulge” cells) from the previous hair cycle (Figure 2A; Hsu et al., 2011). Above this niche is the arrector pili muscle and then the sebaceous gland, which has its own stem cells (Horsley et al., 2006). Between the gland and the bulge are also four sensory neurons that sense various inputs, such as mechanical hair pulling or stroking (Brownell et al., 2011; Cheng et al., 2018; Li et al., 2011) as well as anguish and other types of emotional stress (Zhang et al., 2020). Melanocyte stem cells co-reside with bulge epithelial stem cells (Nishimura et al., 2002) and are particularly sensitive to norepinephrine, a neurotransmitter released by stress-activated sympathetic nerves. This triggers quiescent melanocyte stem cells to proliferate and differentiate, likely resulting in the premature hair greying seen in some individuals with high-powered jobs (Zhang et al., 2020).
Figure 2. The Quiescent Niches of the Hair Follicle and Muscle Stem Cells.
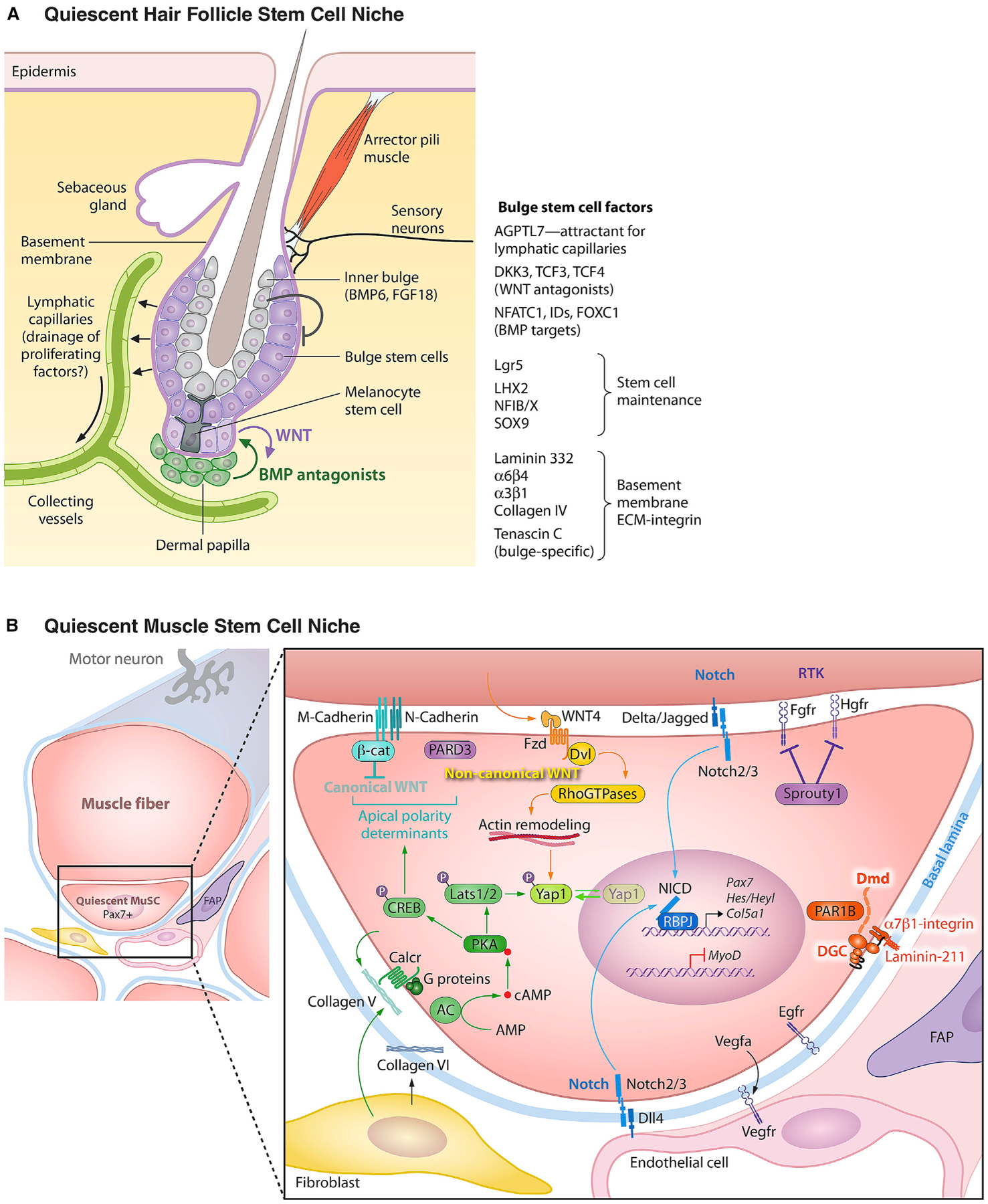
(A) The hair follicle bulge stem cell niche. In young adult mice, the resting phase of the hair cycle lasts only a few days, but this increases for up to several months as mice age. Since hair cycling is synchronized and happens in the absence of wounding, the HF has become an excellent model to study how its stem cells transition from quiescence to active tissue growth. Shown is a schematic of the quiescent bulge niche. Like EpdSCs, bulge HFSCs orchestrate their niche. They exist as a monolayer adjacent to the basement membrane which they produce and secrete. Some constituents such as laminin 332 and collagen IV are shared between EpdSC and HFSC basement membranes, but others such as tenascin C are unique to the HFSC basement membrane. Melanocyte stem cells also reside in this quiescent niche, where they respond to some but not all of the same signals that hold HFSCs in quiescence. Additionally, the two stem cell populations undergo crosstalk with each other, the nature of which is still unfolding. Both the club hair and the inner bulge layer are derived from the bulge HFSCs, but indirectly so, as they come from their terminally differentiating progeny at the end of each hair cycle. The inner bulge layer is important not only for providing a barrier against microbe entry from the hair orifice, but also for producing BMP6 and FGF18 which are important for maintaining HFSCs in quiescence. The lymphatic capillaries interconnect HFSC niches across the tissue and are also required to maintain HFSC quiescence. They are attracted to the quiescent niche by Angptl7, but how they participate in HFSC quiescence is still a mystery. On the activating side, the dermal papilla (DP) stimulates SCs at the bulge base, a crosstalk that results in the production of WNT activating and BMP inhibitory cues necessary to overcome quiescence cues. Although not shown in this diagram, adipocyte progenitors and certain immune cells, such as Tregs and macrophages, have also been implicated in regulating HFSC activation. Additional niche components include an arrector pili muscle and an array of sensory neurons, each tuned for a special purpose, including mechanical stroking of the hair, sensing thermal changes (e.g., goosebumps), and feeling stress. Neuronal signaling upon severe stress to the niche is particularly deleterious for melanocyte stem cells, which terminally differentiate. While many of the intricate details of the bulge niche still await to be elucidated, it has become increasingly clear that the niche complexity enables a multitude of long distant “macroenvironmental” signals, emanating from the brain, the external environment, the circulation, and the immune system to impact stem cell behavior and vice versa.
(B) Quiescent muscle stem cell niche. In homeostasis, muscle stem cells (MuSCs) reside as single cells in a non-dividing quiescent state in a niche juxtaposed to a muscle fiber under a shared basal lamina in close proximity to capillaries. MuSCs orchestrate their niche. The cellular and extracellular matrix components of the niche provide non-canonical WNT and NOTCH signals while inhibiting canonical WNT, receptor tyrosine kinase (RTK), and YAP signaling to facilitate and maintain the quiescent state. The basal lamina (basement membrane) contains laminin-211 that binds α7β1-integrins on the MuSC cell surface and prevents cell cycle entry. Muscle fiber M-cadherin and N-cadherin form homotypic adherens junctions with MuSCs that sequester beta-catenin (β-cat) at the apical surface. The polarity determinant PARD3 is also localized to the apical surface and opposes the basal surface localization of PAR1B which is sequestered by the dystrophin (Dmd)-containing, laminin-binding dystroglycan complex (DGC). Muscle fibers are the source of Wnt4 and Notch ligands Delta and Jagged. MuSCs express several receptor tyrosine kinases (RTKs) including HGFR, FGFR, and EGFR that prime their activation, cell cycle entry, and asymmetric cell division, respectively. Signaling through these RTKs is held in check by Sprouty1 in quiescent MuSCs. MuSCs secrete Vegfa to attract endothelial cells that in turn provide the Notch ligand Dll-4 in a feedback loop to maintain quiescence via the action of notch intracellular domain (NICD) to maintain transcription of the MuSC hallmark transcription factor, Pax7. Fibroblasts and MuSCs themselves secrete Collagen V, which signals via the Calcitonin receptor to activate adenyl cyclase (AC) and phosphorylation of protein kinase A (PKA). PKA-Lats1/2 signaling maintains YAP phosphorylation and prevent its translocation to the nucleus. Concurrently, PKA-CREB signaling is required for the maintenance of the localization of the apical polarity determinants (β-catenin, PARD3) to prevent precocious activation. Collagen VI provides essential structural support, as in its absence MuSCs escape the niche.
During telogen, which can last for months, bulge stem cells are quiescent, experiencing a BMP-rich WNT-restricted microenvironment that keeps them in an undifferentiated, non-proliferative state (Figure 2A; Hsu et al., 2011; Lien et al., 2014; Merrill et al., 2001; Wang et al., 2016). During this time, however, transcriptional crosstalk begins to intensify between the HFSCs at the bulge base (sometimes referred to as the hair germ) and the underlying specialized dermal papilla (DP) cells (analogous to the dermal condensate in embryonic HF morphogenesis). When a threshold of activating WNT and BMP inhibitory cues is reached, the quiescence cues from the inner bulge and other niche components are overpowered, launching the hair growth phase of the cycle (anagen) (Figure 1B; Greco et al., 2009; Hagner et al., 2020).
Single-cell analyses of the telogen hair germ cells unveil a molecular blueprint (Yang et al., 2017), explaining why stem cells in closest proximity to the dermal papilla are always the first to be activated at the telogen:anagen transition (Rompolas et al., 2013). These WNT-signaling hair germ stem cells divide asymmetrically relative to the basement membrane, generating a short-lived daughter cell that expresses sonic hedgehog (SHH) (Yang et al., 2017). For a brief period, SHH+ progeny are part of the niche, stimulating both the dermal papilla and the bulge stem cells more distant from the DP to self-renew and drive the formation of a new hair follicle (Hsu et al., 2014; Woo et al., 2012). In contrast to the stem cells that maintain contact with the dermal papilla, these more distant bulge stem cells divide symmetrically, giving rise to the outer root sheath, the upper portion of which will become the new bulge for the next hair cycle (Figure 1B; Hsu et al., 2011; Lay et al., 2018).
As the outer root sheath grows downward, the dermal papilla signaling center becomes distanced from the bulge, whose stem cells return to quiescence throughout the remainder of the hair cycle. By contrast, the SHH-expressing stem cell progeny, which begin as multipotent, maintain their proximity to the dermal papilla and proceed to form the uni-lineage progenitors of the six concentric cell layers of the hair shaft and its channel (the inner root sheath) (Yang et al., 2017). In this way, the hair follicle stem cell lineages are as complex as those of the hematopoietic system. The growth period is naturally short lived, and after about 3 weeks of hair growth, the follicle degenerates, drawing the dermal papilla upward to rest at the base of the quiescent bulge (Heitman et al., 2020).
Interestingly, the hair follicle stem cells also play an integral role in making and maintaining their niche. At the beginning of each hair cycle, their SHH progeny fuel bulge stem cell self-renewal (Hsu et al., 2014). At the end of the hair cycle, stem cell progeny late in the differentiation program home back to the niche as BMP6/FGF18+ inner bulge cells, raising the threshold for the next regenerative phase (Hsu et al., 2011). And whether in telogen, anagen, or catagen, the quiescent bulge stem cells produce angiopoietin like 7 (Angptl7), which promotes contact with lymphatic capillaries (Gur-Cohen et al., 2019). Only during the brief period of bulge stem cell proliferation do they downregulate Angptl7 and instead express Angptl4, which repels the lymphatic network. A fascinating question still to be addressed is whether the lymphatic network, known for draining fluids and immune cells from tissues, might be draining some key factors or metabolites required for stem cell activation (Gur-Cohen et al., 2019). Whatever the mechanism, these collective findings suggest that the bulge stem cells participate heavily in shaping their niche to regulate their activity.
While the multiplicity of cellular inputs to the bulge niche continues to unfold, additional environmental inputs worthy of mention here are the dermal sheath (Heitman et al., 2020), adipocyte progenitors (Shook et al., 2016), arrector pili muscle (Fujiwara et al., 2011), sympathetic neurons (Shwartz et al., 2020), and immune cells (Kobayashi et al., 2020). In particular, two tissue-resident immune cell types, macrophages and regulatory T cells (Tregs), have emerged as stem cell regulators under normal physiological conditions (Burzyn et al., 2013; Fujisaki et al., 2011; Pinho and Frenette, 2019; Wang et al., 2019a). Exactly how and in what context is still a matter of debate. When resident macrophages near the bulge niche die, they release WNTs, which could contribute to the activation of HFSCs to help launch a new hair cycle (Castellana et al., 2014). That said, an apparently distinct type of bulge-associated macrophage was recently discovered to express factors that synergize with regulators downstream of BMP signaling to maintain HFSC quiescence (Wang et al., 2019a). Like the perifollicular macrophages described by Castellana et al. (2014), these macrophages also die and promote hair cycle entry, but in this case by purportedly lowering the BMP signaling threshold in the niche.
Defined by their expression of transcription factor FOXP3, regulatory T cells (Tregs) are a subset of CD4+ helper T lymphocytes (Sakaguchi et al., 2013). Tregs potently suppress inflammatory immune cells and hence are beneficial in maintaining SC niches as immune privileged sites (Josefowicz et al., 2012). Notably, many stem cell niches have a paucity of inflammatory cells in their vicinity, suggesting a universal need for Tregs in protecting stem cells from catastrophic attack in autoimmune and other chronic inflammatory conditions (Fujisaki et al., 2011; Hirata et al., 2018). Underscoring the importance of Tregs as guardians of the stem cell niche, when Tregs are depleted, stem cell numbers decline (Biton et al., 2018; Hirata et al., 2018). A role for Tregs in maintaining HF stemness and quiescence would parallel the role of Tregs in hematopoietic stem cells and intestinal stem cells (Ali et al., 2017; Biton et al., 2018; Fujisaki et al., 2011). If so, Tregs would be added to the list of cellular sources of HF quiescence signals, which thus far have been traced to terminally differentiated stem cell progeny that line the “inner bulge” (Hsu et al., 2011), attachment to lymphatic capillaries (Gur-Cohen et al., 2019), and longer-range signals from the dermis (Plikus et al., 2008).
MUSCLE STEM CELLS: A BEAUTIFUL EXAMPLE OF STEM CELL DORMANCY DURING HOMEOSTASIS
Muscle comprises 40% of the body mass and is integral to mobility and strength, and loss of muscle mass and function with aging has a major impact on quality of life. The integrity of muscle tissues relies on a population of dedicated stem cells that is poised to repair and replace cells that are damaged by injury. Muscle has remarkable potential to regenerate which entails an orchestrated process that begins with an inflammatory response, followed by necrosis, then expansion and fusion of stem cells to restore the myofibers. In contrast to the skin, intestine, and blood, skeletal muscle and brain undergo relatively little tissue turnover, and consequently, their stem cells spend most of their time in a dormant state. Skeletal muscle stem cells (MuSCs), initially referred to as “satellite cells” by Alexander Mauro (Mauro, 1961), are particularly interesting in that under most conditions, they are unipotent: dedicated to the generation of a single specialized cell type. An exception is brown adipose tissue (BAT). MicroRNA-133 expression suppresses PRDM16, a master regulator of BAT, in satellite cells in vivo and prevents their adipogenic commitment during muscle regeneration and differentiation, as revealed by perturbations using antisense oligonucleotides. Notably, exposure to cold temperatures suffices to cause downregulation of miR-133 and generation of satellite cell-derived brown adipocytes in certain muscles, a finding with interesting implications for obesity (Seale et al., 2008; Yin et al., 2013). Nonetheless, under normal circumstances MuSCs are destined to contribute to the development, maintenance, and repair of muscle. MuSCs are loners, residing as individual cells in circumscribed niches that are scattered throughout the tissue. Like the stem cells of the skin epithelium, MuSCs are sandwiched between a basement membrane that protects them from the external environment and the mature contractile multinucleated muscle myofiber (Figure 2B; Mauro, 1961).
Quiescence and the Muscle Stem Cell Niche
Muscle stem cells, like hair follicle stem cells (Nowak et al., 2008), arise from progenitors that are “set aside” during embryonic development (Lepper and Fan, 2010; Relaix et al., 2005). Initially identified solely based on their anatomical location and appearance in electron micrographs, decades later MuSCs were prospectively isolated using antibodies to cell surface markers and shown via single-cell transplantation to be capable of both self-renewal and differentiation, meeting the quintessential definition of a tissue-specific stem cell (Cerletti et al., 2008; Montarras et al., 2005; Sacco et al., 2008).
MuSCs express a hallmark transcription factor Pax7 and are largely present in a mitotically and metabolically quiescent state (Cheung and Rando, 2013; Seale et al., 2004; Wang and Rudnicki, 2011; Yucel et al., 2019). Conditional genetic ablation of these cells revealed definitively that satellite cells are bona fide stem cells and play a crucial role in muscle repair (Lepper et al., 2011; McCarthy et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). Because Pax7 is not expressed by any other cell type in muscle tissue, it has served as an invaluable marker for lineage tracing to detect the contribution of MuSCs to myofibers in adulthood. Additionally, Pax7 has enabled a definitive determination of MuSC contributions to the creation of the niche through conditional genetic deletion of expressed genes at specific times using tamoxifen-induced Pax7-Cre drivers.
Like the highly quiescent long-term hematopoietic stem cells (Wilson et al., 2008), but in contrast to the episodic behavior of hair follicle stem cells (Hsu et al., 2014), MuSCs have long been thought to rarely undergo self-renewal except following wounding, exercise, or transplantation. Remarkably, lineage tracing studies revealed that in the normal resting state, MuSCs can fuse to as many as 20%, 50%, and 80% of multinucleated myofibers of hindlimb, extraocular, and diaphragm muscles, respectively (Keefe et al., 2015; Pawlikowski et al., 2015). Given this unexpectedly high frequency of contribution to myofibers in the absence of injury, it was surprising that genetic ablation of MuSCs throughout adulthood did not accelerate the onset of sarcopenia, the age-related loss of muscle mass and function (Fry et al., 2015; Keefe et al., 2015). A recent study indicates that this was because the mice studied were sedentary; when mice lacking MuSCs exercised daily by running on a wheel, they precociously manifested features of age-associated dysfunction, such as loss of balance, coordination, and capacity to run (Englund et al., 2020), underscoring the important role of MuSCs in muscle aging. Additionally, loss of MuSCs triggers early aging by a different mechanism; in the absence of MuSCs, neuromuscular junctions degenerate precociously (Liu et al, 2015).
Basement Membrane Interactions between MuSCs and Their Niche
The MuSC niche serves as an instructive environment that maintains quiescence in homeostasis (Figure 2B). Upon injury, MuSC states change due to the loss of these structural and biochemical signals. This physical disruption in the niche architecture also enables other cell types to access the niche and provide extrinsic stimuli essential to muscle repair. Quiescence, like the differentiated state, requires continuous active regulation to be maintained (Blau and Baltimore, 1991). Changes in basement membrane (basal lamina) composition provide chemical and biophysical cues to MuSCs and influence their maintenance or exit from quiescence, inducing activation, self-renewal, and differentiation (Gilbert et al., 2010; Madl et al., 2018). Similar to the role of the basement membrane in skin stem cells, this specialized extracellular matrix not only anchors MuSCs to their niche but also regulates MuSC function by sequestering growth factors, which are released if the tissue integrity is disrupted.
Repair of the basement membrane and the return of the ECM to homeostatic structural protein and growth factor signaling enables MuSCs to find their niche once integrity has been restored. This has been definitively shown by transplantation experiments. MuSCs injected into muscles harboring niches devoid of stem cells, such as in genetically Pax7-null mice, homed to and occupied the empty niches. Similarly, following depletion of niches by gamma irradiation, transplanted MuSCs homed to and repopulated the specialized anatomically distinct local microenvironment of the satellite cell niche as single cells in a quiescent state (Kuang et al., 2007; Sacco et al., 2008)
Like skin epithelial stem cells, MuSCs are the architects of their polarized niche. They synthesize key structural components of the ECM that entrap quiescence signals and encase the MuSCs juxtaposed to the multinucleated contractile myofiber to which they ultimately contribute. Additionally, the presence of the basement membrane establishes the polarized adhesive contacts that tether satellite cells to their niche.
Despite these similarities between skin epithelial and muscle stem cell niches, the basement membrane of the muscle niche is tailored to the unique requirements of its stem cell residents (Figure 2B). Laminin 2-1-1 is the major laminin in the basement membrane of skeletal muscle (Holmberg and Durbeej, 2013; Xu et al., 1994). MuSCs adhere to this laminin through integrin α7β1. This interaction is critical: the absence of laminin is associated with a severe human genetic muscle wasting disease and reduced lifespan (Helbling-Leclerc et al., 1995; Mohassel et al., 2018) and a lack of β1-integrin expression by murine MuSCs leads to a decrease in stem cell numbers and precocious MuSC differentiation and fusion into myofibers (Rozo et al., 2016). Collagen VI, on the other hand, which is synthesized by interstitial fibroblasts as well as MuSCs, serves a structural function, as mice that genetically lack this protein have muscle tissue of diminished elasticity (7 kPa compared to 12 kPa of controls) which results in an accumulation of MuSCs outside of the niche (Urciuolo et al., 2013).
Recent reports provide unexpected evidence that collagen V, which is secreted by MuSCs in quiescence, acts in an autocrine signaling loop via the calcitonin receptor, a G-protein coupled receptor (Baghdadi et al., 2018). This discovery was made by analysis of ChIP-seq data that revealed that intracellular NOTCH (NICD) and RBPJ bind to the enhancers of several collagen genes expressed by MuSCs. The expression of these target genes was tightly correlated with NOTCH activity in MuSCs. Moreover, in the absence of MuSC-derived collagen V, MuSCs escaped quiescence and precociously activated and differentiated, a phenotype similar to that seen in mice lacking Notch (Bjornson et al., 2012; Mourikis et al., 2012). Surprisingly, addition to the medium, not as an underlying adhesion substrate, impeded cell cycle entry of cultured MuSCs. This finding suggests that this structural protein does not act as a biomechanical modulator by altering substrate rigidity and geometry, but instead as a signaling molecule. Calcitonin Receptor has been known to mediate the maintenance of quiescence in MuSCs (Yamaguchi et al., 2015), but its regulation was thought to be systemic via calcitonin peptide hormones, not via a localized ligand in the niche. These intriguing findings show that both physically and functionally, collagen V acts to maintain MuSC quiescence.
Cell-Cell Interactions
The polarity established by basement membrane-integrin connections on the basal side of MuSCs also sets the stage for intercellular connections between MuSCs and the myofiber on the apical side of the niche (Goel et al., 2017). Here, MuSCs make elaborate adherens junctions composed of core transmembrane muscle and neural cadherins (M-Cad and N-Cad, respectively). Genetic loss-of-function experiments reveal that cadherin-mediated intercellular adhesion and signaling between MuSCs and their myofiber niche are essential for maintaining quiescence, likely through their binding and sequestering of β-catenin, thereby diminishing canonical WNT signaling. Indeed, loss of both M-Cad and N-Cad leads to nuclear accumulation of β-catenin and loss of MuSC quiescence, triggering aberrant cell cycle re-entry and fusion into myofibers.
Further insights into the regulation of quiescence by Calcitonin receptor have implicated the mechanosensitive transcriptional coactivator YAP as a major downstream target (Zhang et al., 2020). The Calcitonin receptor is sharply downregulated upon MuSC activation (Yamaguchi et al., 2015) and although this GPCR often signals via cAMP to induce genes that are targets of the cAMP response element (CREB) transcription factor, in the context of quiescent MuSCs, it does not (Li and Fan, 2017). Instead, Calcitonin receptor signaling leads to quiescience in MuSCs via cAMP activation of protein kinase A (PKA) which phosphorylates kinases in the Hippo pathway (LATS1/2), which in turn phosphorylate YAP (Zhang et al., 2020). This leads to preservation of quiescence, as activated YAP is unable to translocate to the nucleus, which would lead to MuSC activation.
Although the precise players and mechanisms are tailored to the particular needs of each tissue, a general paradigm has emerged whereby stem cell quiescence is maintained by raising the threshold for WNT and/or Hippo signaling, which can be achieved through the sequestration in the cytoplasm of β-catenin and YAP, respectively. Both canonical and non-canonical WNT signaling regulate YAP, as evidenced by the WNT4a-mediated activation of RhoGTPases and cytoskeletal remodeling that repress YAP and maintain MuSC quiescence (Eliazer et al., 2019). Additionally, increasing evidence suggests that for both epithelial and muscle stem cells or their progeny, there is an interplay between WNT and YAP signaling, which dictates whether cells remain quiescent or divide (Azzolin et al., 2014; Eliazer et al., 2019).
In contrast to WNT and YAP signaling, which share common functions in skin and muscle stem cells, NOTCH signaling is more context specific in its functions. In muscle, NOTCH signaling in the stem cell requires its membrane-associated NOTCH receptor to engage with a membrane-associated NOTCH ligand present on the myofiber “niche” cell. Upon engagement, NOTCH is cleaved and translocated to the stem cell nucleus to activate its DNA binding cofactor RBP-j, where it maintains a gene expression program required for the quiescent MuSC state (Bjornson et al., 2012; Mourikis et al., 2012). Abrogation of NOTCH signaling leads to a decline in MuSCs in resting muscle, concomitant with expression of differentiation-associated transcription factor MYOD and fusion into myofibers, often without MuSC divisions. Additionally, in the absence of NOTCH signaling, the quiescent MuSC pool is not replenished post-injury. By contrast, in the skin, loss of NOTCH signaling in HFSCs does not overtly alter their quiescent state, but rather alters their crosstalk with adjacent melanocyte stem cells, causing their precocious differentiation and exhaustion (Lu et al., 2020). Thus even within similarly quiescent niches, different tissue stem cells respond to NOTCH signaling in distinct ways.
Finally, some factors that profoundly impact stem cell quiescence appear to be entirely niche context specific. For example, oncostatin M (OSM) is a muscle-specific member of the interleukin-6 family of cytokines which induces quiescence in MuSCs (Sampath et al., 2018), while quiescence in HFSCs is maintained by high levels of BMP6 and FGF18 signaling (Hsu et al., 2011). In MuSCs, Sprouty1 keeps tyrosine kinase growth factors in check and blunts their effects on MuSC activation and proliferation (Chakkalakal et al., 2012; Shea et al., 2010).
Another intriguing feature of resting MuSCs is that they can exist in two different functional states—deep quiescence G0 and primed GAlert (Rodgers et al., 2014). GAlert is a state acquired by MuSCs exposed to systemic factors, such as HGF, resulting from a distal muscle wound, for example in the contralateral leg (Rodgers et al., 2017). MuSCs in GAlert exhibit an increase in cell size and are “primed” to activate, which shortens their time of response to enter the cell cycle and propensity to divide and heal the muscle in which they reside. mTORC1 signaling is crucial to the alert state and conditional deletion in MuSCs of Raptor, an essential component of mTORC1 signaling, causes MuSCs to be unresponsive to injury. The effects of receptor tyrosine kinases, HGF, and FGF, on MuSC activation are blunted by Sprouty1, which declines with age (Bigot et al., 2015; Chakkalakal et al., 2012; Shea et al., 2010). The alert MuSC state represents a form of “cellular memory,” similar to the adaptive response seen in immune cells or to the memory harbored by epidermal stem cells following a wound to the skin, in that prior experience influences future responses (Naik et al., 2017).
The Role of the Vasculature in the Muscle Stem Cell Niche
Since quiescent MuSCs are located underneath the basal lamina on the muscle fibers, direct cell-cell contact between MuSCs and other cell types such as endothelial cells (ECs) seems impossible. However, such interactions clearly do occur. A remarkable bi-directional paracrine mechanism has recently been revealed in which VEGFA secreted by MuSCs induces expression of the NOTCH ligand DLL4 in ECs that are in direct contact with MuSCs (Verma et al., 2018). Tissue clearing techniques resolved the proximity of MuSCs to the capillary vasculature network. In vitro transwell studies showed that MuSC-derived VEGFA recruits ECs. In agreement, abrogation of MuSC-derived VEGFA resulted in endothelial cells being located at a greater distance from MuSCs than untreated controls. Moreover, ECs in close proximity to VEGFA-secreting quiescent MuSCs secrete DLL4 which in turn interacts with NOTCH receptors on MuSCs to maintain their quiescent state. This feedback loop ensures that MuSCs remain inactive in the homeostatic resting state and underscores the important role of a juxtacrine vascular niche in maintaining MuSC quiescence, a feedback loop that is lost with aging.
MOBILIZING STEM CELLS AFTER INJURY
In response to injury, most if not all tissue stem cells must be able to respond. This is exemplified by muscle injuries. Regeneration fails following ablation of Pax7, which encodes the central transcription factor for MuSCs, underscoring their essential role in forming new muscle fibers in response to damage (Lepper et al., 2011; McCarthy et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). Mobilization of tissue stem cells is instigated by changes in the local environment. For muscle, myofiber degeneration destroys the associated MuSC niches, liberating the stem cells from quiescence. The concomitant release of wound-induced stimulatory factors from the basement membrane surrounding the myofiber and from cellular niche components, such as FGF2 and HGF, contributes to the mobilization process (Chakkalakal et al., 2012; Rodgers et al., 2017; Yablonka-Reuveni et al., 1999). An influx of diverse cell types in a temporally orchestrated succession mediates an acute inflammatory response, then necrosis, and finally myofiber and niche repair.
Of paramount importance to efficacious regeneration is a transient inflammatory response, the first step in muscle and skin healing after injury. Prostaglandin E2 (PGE2), a metabolite derived from phospholipid membranes, is a component of that response and a natural mediator of wound healing. The requirement for PGE2 signaling in regeneration was demonstrated in loss-of-function experiments (Ho et al., 2017). If the EP4 receptor, the GPCR through which PGE2 signals on MuSCs, is conditionally ablated, they fail to divide and regeneration is so impaired post-injury that 1 month later young mice exhibit a significant loss of strength. Similarly, inhibition of innate PGE2 synthesis post-injury through nonsteroidal anti-inflammatory drug (NSAID) administration hinders repair and compromises muscle strength. Mechanistically, PGE2 confers increased cell survival and augments proliferation by triggering a cAMP/phosphoCREB pathway that activates the transcription factor Nurr1. Remarkably, acute treatment with PGE2 post-injury suffices to robustly augment muscle regeneration by endogenous or transplanted MuSCs.
Once MuSCs are activated, they enter the cell cycle. High-dimensional single-cell mass cytometry revealed that activated Pax7+ MuSCs upregulate CD44 and CD98 and differentiate into two distinct Myogenin+ progenitor states distinguished by CD104 expression (Porpiglia et al., 2017). These cell fate transitions are marked by changes in glucose metabolism and mitochondrial respiration states. Activated MuSCs are less dependent on oxidative phosphorylation for metabolic demands and utilize glucose-derived acetyl-CoA for histone acetylation leading to changes in the chromatin landscape and an epigenetic signature that maintains the expression of stemness and cell cycle genes (Yucel et al., 2019). Concurrently, reduced cellular NAD+ levels decrease the deacetylase activity of SIRT1 (Ryall et al., 2015). Cell cycle exit and myogenic commitment are contingent upon mitochondrial remodeling and a transient loss of histone acetylation that abrogates the expression of genes characteristic of the stem cell state (Bracha et al., 2010; Sin et al., 2016; Yucel et al., 2019). Upon commitment, histone acetylation is redirected to muscle-specific genes by MyoD and drives muscle fiber hypertrophy (Berkes and Tapscott, 2005; Das et al., 2017). Thus, metabolic and epigenetic regulation work hand in hand to determine MuSC fate choices during regeneration and changes in nutrient availability can trigger changes in MuSC function in regeneration, aging, and muscular dystrophies (Cerletti et al., 2008; Zhang et al., 2016).
The niche provides polarity and localized signals that determine the fate of MuSC progeny through either symmetric or asymmetric cell divisions (Kuang et al., 2007; Le Grand et al., 2009; Rocheteau et al., 2012; Yennek et al., 2014). Symmetric stem cell divisions lead to an expansion of the stem cell pool available for the regeneration of damaged muscles. If MuSCs undergo only symmetric cell divisions, the stem cell reservoir required to surmount future wounds is depleted. That is what occurs in lethal Duchenne muscular dystrophy (DMD) (Blau et al., 1983). Nonetheless, symmetric MuSC divisions mediated by non-canonical WNT7a signaling are required to accelerate and augment the stem cell pool (Le Grand et al., 2009). In conjunction with fibronectin, WNT7a binds to FZD7 and syndecan4 co-receptors to activate RAC1 and downstream RhoGTPases and execute symmetric cell divisions via a planar cell polarity pathway (Bentzinger et al., 2013). MuSCs play a key role in this process by dynamically regulating fibronectin synthesis and secretion in response to injury.
Of equal importance is a means of maintaining the stem cell reservoir to overcome additional insults. This requires asymmetric cell divisions, wherein one daughter resumes a quiescent state and the other reproduces to yield more committed daughters that heal the damaged myofiber (Kuang et al., 2007; Rocheteau et al., 2012). Unexpectedly, dystrophin, the gene product whose absence underlies DMD, was identified as playing a seminal role, not only in myofiber contractile function, but also as a cell-intrinsic regulator of asymmetric MuSC self-renewal. In activated MuSCs, dystrophin is expressed and localized by the cell polarity regulator PARD3, which regulates mitotic spindle orientation and asymmetric self-renewing divisions (Dumont et al., 2015). These findings have upended our current understanding of the etiology of this devastating disease as not only due to increased myofiber fragility but also to a dystrophin-dependent loss of stem cell function in regeneration. A small molecule screen identified epidermal growth factor receptor (EGFR) and Aurora kinase A (Aurka) as regulators of MuSC mitotic polarity leading to asymmetric divisions, which if conditionally ablated causes a reduction in progenitors and exhaustion of the MuSC pool. Strikingly, EGF treatment in vivo rescues the reduction in asymmetric cell divisions in dystrophin-deficient MuSCs in the DMD mouse model, mdx, leading to increased numbers of progenitors, augmented regeneration, and enhanced strength (Wang et al., 2019b).
Of increasing importance are fibroadipogenic progenitors (FAPs), which mirror the kinetics of satellite cell behavior: they rapidly expand within 3 days following injury and then recede to normal levels upon repair (Joe et al., 2010; Lemos et al., 2015; Uezumi et al., 2010). FAPs secrete various MuSC stimulants, including IGF1 and WNTs. These cells also generate interleukin IL-6, which, like OSM, triggers the JAK/STAT3 kinase pathway, albeit with opposite effects; OSM signaling represses myogenin expression and induces quiescence, whereas IL6 promotes myogenin expression and MuSC activation and commitment (Sampath et al., 2018). Notably, OSM is an anomaly of the IL6 family and the JAK/STAT3 pathway, which generally leads to stem cell activation in diverse tissues exposed to damage, including stem cells of muscle and skin. Nuclear pSTAT3 is a well-established and potent stimulus of the repair process (Figure 3; Keyes et al., 2016; Sala et al., 2019; Tierney et al., 2014).
Figure 3. The Two Phases of Wound Repair in the Skin.
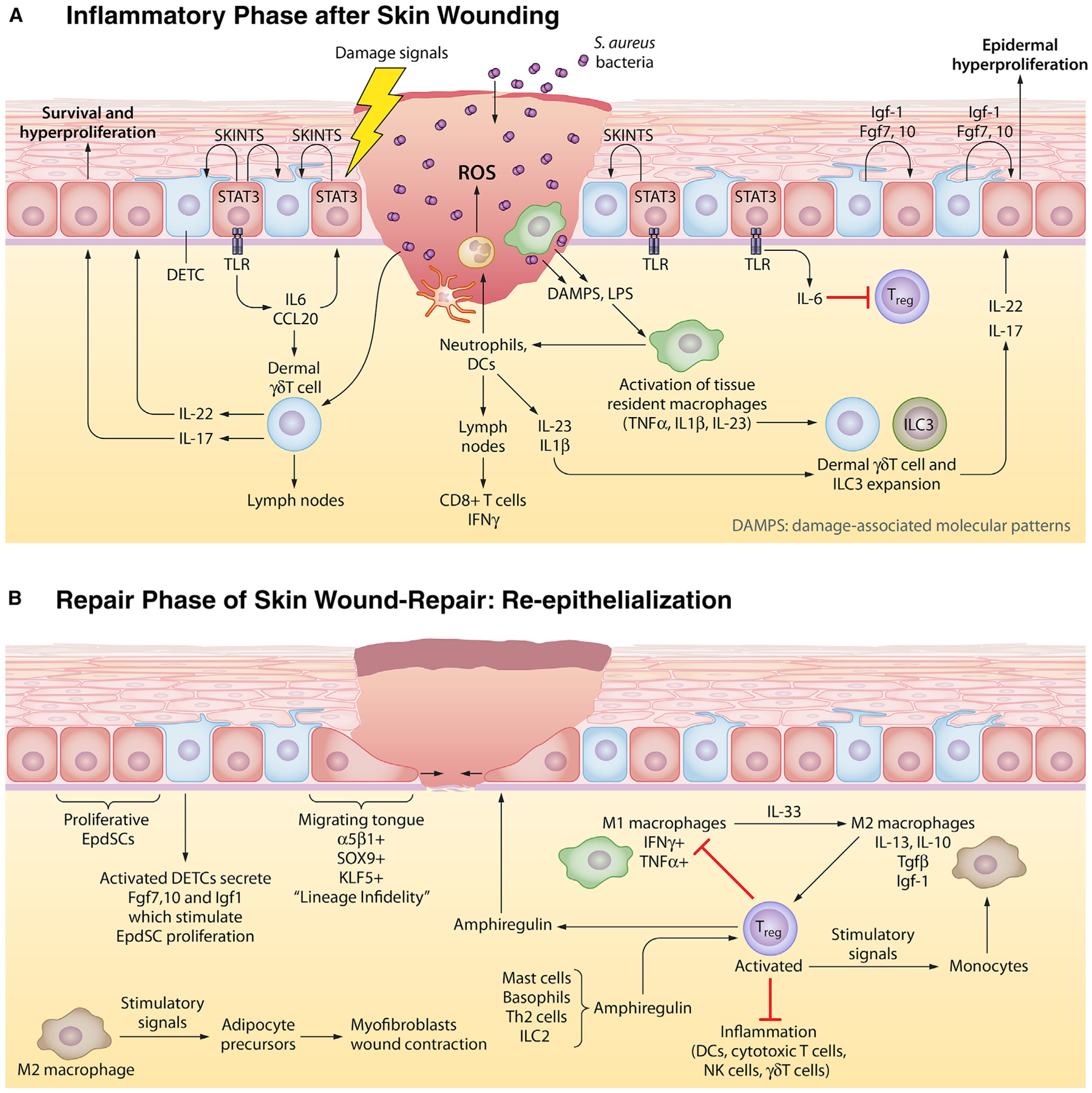
In the skin, the stem cell niche closest to the site of the wound is mobilized to respond. Here, we schematize the response to full-thickness skin wounds, where EpdSCs adjacent to the wound site become mobilized. Wound responses involve two steps. In the first phase, a blood clot forms to seal off the wound and an inflammatory response is triggered to guard against microbial invasion and to clear out damaged cells. In the second phase, inflammation must be dampened to permit re-epithelialization of the damaged skin barrier.
(A) Epithelial inflammatory phase. Damage-associated molecular patterns (DAMPs) and reactive oxygen species (ROS) emanating from wound-damaged tissue and from pathogens, such as Staphylococcus aureus, signal to circulating neutrophils and to resident macrophages to become activated to form so-called M1 macrophages and begin the inflammatory response. Activated antigen-presenting dendritic cells (DCs) migrate to the lymph nodes to activate and recruit cytotoxic T cells to the skin. EpdSCs are not silent during this time. Those adjacent to the wound edge produce SKINTs, factors that mobilize resident DETCs to produce factors that in turn fuel epidermal hyperproliferation next to the wound site. Nearby epidermal cells phosphorylate and activate STAT3, which in turn stimulate production of interleukins and chemokines that stimulate dermal δγ T cells which have been purported to be able to transit to lymph nodes like DCs do. The outcome from this heightened immune response is a plethora of cytokines that further stimulate other immune cells, including innate lymphoid cells (ILCs) to mobilize into action as shown here.
(B) Epithelial repair phase. While inflammation is essential to fight infections, it is incompatible with the re-epithelialization process. For this to occur, inflammation must be dampened. Exactly how is still not clear, but it is known that the expansion of resident regulatory T cells (Tregs) is key. Tregs respond to a number of factors, including amphiregulin, generated by a number of inflammatory cells. Tregs both dampen/exhaust cytotoxic T cell activity and also stimulate the conversion of inflammatory M1 macrophages to repair M2 macrophages. M2 macrophages have multiple functions, one of which is to produce factors such as TGFβ that further stimulate Treg expansion and another is to stimulate adipocyte precursors to differentiate and stimulate wound contraction. As the inflammatory response is dampened and dead cells have been cleared, EpdSCs at the wound edge begin to migrate into the wound bed to re-epithelialize the epidermis. They are fueled by proliferating EpdSCs behind them to generate a one-two punch in repair.
In the skin, the multiple types of stem cell niches add complexity to the wound response. A major question is which niches become mobilized after injury and how this affects the repair process. Lineage tracing has shown that when the skin is wounded, the nearest stem cells to the wound are stimulated to respond. If the wound is deep and wide, the epidermal stem cells at the wound edge are mobilized to re-epithelialize the missing skin epidermis and initiate de novo hair follicle morphogenesis (Ito et al., 2007). If the wound is shallow, the bulge stem cells in the hair follicles beneath the wound are mobilized to move upward and re-epithelialize the upper HF and the epidermis (Ge et al., 2017; Page et al., 2013).
Underscoring the importance of the microenvironment in stem cell behavior, skin stem cells that have exited their niche adopt a new gene expression program reflective of their wound microenvironment. This program is typified by dual signs of both epidermal and hair follicle identities, a state referred to as “lineage infidelity” (Ge et al., 2017) that is observed irrespective of whether epidermal stem cells or hair follicle stem cells are called into action. Many of the features of lineage infidelity remain to be interrogated, but the state is essential for the stem cells to survive in a wound microenvironment (Ge et al., 2017). A key driver appears to be the RAS/MAPK pathway, which is transiently activated in wound repair and causes phosphorylation of the ETS2 transcription factor (Ge et al., 2017). In squamous cell carcinomas of the skin, where RAS/MAPK signaling is constitutively active, chromatin regions rich in ETS2 binding sites are opened and genes associated with these peaks are activated (Ge et al., 2017). A phosphomimetic (T72:D) ETS2 is sufficient to shift the chromatin of an epidermal stem cell to a squamous cell carcinoma landscape (Yang et al., 2015). The data at hand have started to place a molecular understanding of the long-held adage that “cancer is a wound that never heals.” This is also reflected by the finding that many of the signals that are crucial in stimulating stem cell function in regeneration can be tumorigenic, for instance WNTs, Hedgehog (Hh), Prostaglandin E2, IGF1, and EGF.
SENSING TISSUE DAMAGE AND ORCHESTRATING IMMUNE CELL RESPONSES AFTER INJURY
Major questions in the stem cell and regenerative medicine field are how stem cells sense tissue damage and the extent to which they participate in orchestrating the inflammatory response that prevents pathogen invasion but then must be placed in check during tissue repair.
Fighting Infection
Upon injury, damage-associated molecular patterns (DAMPS) are released from dying cells (Janeway and Medzhitov, 2002). While precise details are still unfolding, stem cells near a skin wound edge respond to DAMPs. Similar to muscle FAPs, DAMPs appear to induce nuclear pSTAT3, which in addition to impacting stem cell behavior, as described above, can also induce expression of a number of cytokines and chemokines to affect resident immune cells and mount an inflammatory response (Figures 3 and 4; Nelson et al., 2015). This is particularly important not only for the epidermis, which resides at the interface between the body and the external environment, but also for muscle injuries, where immune cells infiltrate soon after tissue damage.
Figure 4. The Muscle Stem Cell Niche in Regeneration and Wound Repair.
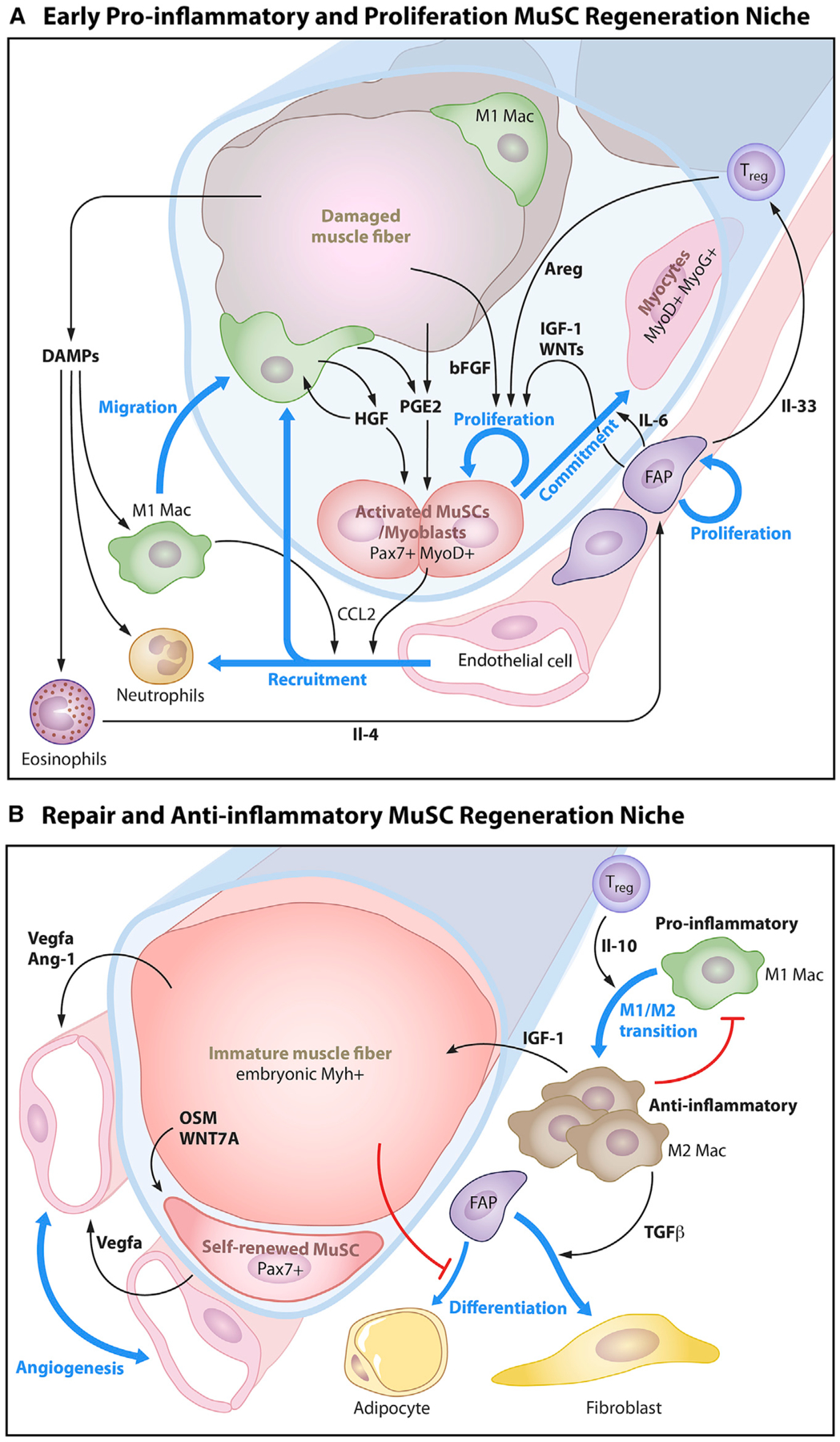
(A) Early pro-inflammatory and proliferation phase. Post injury, myofiber-derived factors, immune cell invasion, and inflammatory cytokines mobilize MuSCs to self-renew and expand to repair the damaged myofiber. Damage-associated molecular patterns (DAMPs) are released from damaged muscle fibers that cause tissue-resident macrophages to migrate to the site of injury and secrete cytokines that promote the recruitment of circulating immune cells (neutrophils and macrophages). This early pro-inflammatory environment also promotes the activation of resident muscle stem cells which secrete CCL2 to recruit endothelial cells and induce angiogenesis. Damaged muscle fibers secrete prostaglandin E2 (PGE2) and basic fibroblast growth factor (bFGF or Fgf2). Activated macrophages release paracrine signals such as hepatocyte growth factor (HGF) and prostaglandin E2 (PGE2) to activate MuSCs. In parallel, eosinophils release interleukin 4 (Il-4) to activate fibroadipogenic precursors (FAPs). FAPs secrete insulin-like growth factor (IGF-1) and interleukin 6 (Il-6) to promote the proliferation and commitment of MuSCs and interleukin 33 (IL-33) to promote the expansion of Foxp3+ regulatory T cells (Tregs). Tregs in this early phase of regeneration secrete amphiregulin (Areg) to stimulate MuSC expansion of Pax7-expressing activated progenitors (P1 and P2), which give rise to committed progenitors that fuse to and heal injured myofibers.
(B) Repair and anti-inflammatory phase. For muscle to heal, the inflammatory response must be quenched and blood vessels restored. During this phase, Tregs secrete interleukin 10 (IL-10) that promotes a shift in macrophages from the M1 phenotype toward the anti-inflammatory M2 phenotype. M2 macrophages secrete IGF-1 which facilitates the growth and maturation of regenerated immature muscle fibers, which in turn secrete transforming growth factor beta (TGFβ) that induces fibrogenic differentiation of FAPs. The successful formation of the immature muscle fiber from fusion of MuSC progeny signals the residual MuSCs to self-renew via WNT7a and re-quiesce via oncostatin M (OSM). The immature muscle fiber also stimulates the re-establishment of the capillary network through the release of angiogenic factors, vascular endothelial growth factor A (VEGFa), and angiotensin 1 (Ang-1). The self-renewed MuSCs also secrete VEGFa to recruit capillaries to remodel and rebuild their local niche.
Inflammatory (M1) macrophages are among the first to appear at the damage scene of both muscle and skin injuries (Figures 3 and 4). Intriguingly, these professional phagocytes not only act as scavengers and clear damaged cellular corpses, but they also secrete cytokines. In muscle, M1 macrophages produce factors including IL-1β and IFNγ which stimulate recruitment of neutrophils, dendritic cells (DCs), and other immune cells. Intriguingly, by generating TNFα, M1 macrophages may also function by inducing apoptosis and by restricting expansion of FAPs, which might otherwise fuel fibrosis (Lemos et al., 2015).
Interestingly, in both wounded epidermis and in hair plucking, a mechanical means of inducing local injury to the HFSC niche (Hsu et al., 2011), macrophage recruitment also occurs (Figure 3; Chen et al., 2015). Mechanically stimulated HFSCs and MuSCs have been shown to express chemokine CCL2, a stimulus for macrophages that surround the locally damaged follicle niche. Canonical WNT signaling is known to be involved in MuSC expansion and overrides the quiescence-inducing non-canonical WNT4a signaling pathway (Figure 2B). WNTs may also be involved in skin repair, as they are elevated in many different injured tissues; they are produced by M1 macrophages, and they are known to be general stimulants of stem cells (Clevers et al., 2014; Ito et al., 2007; Wu et al., 2011). Moreover, as evidenced in wounded zebrafish tailfin, when WNTs are diminished, wound repair is not only impeded but there are also fewer macrophages recruited to the wound site (Petrie et al., 2014). Taken together, the studies on muscle and skin injuries illustrate that M1 macrophages do not function solely as scavengers in wound repair, but also key mediators of signaling.
While the involvement of macrophages is similar across most wounded tissues, specialized immune cell:stem cell communication lines also come into play. A case in point is the epidermis, which harbors an elaborate array of resident immune cells. In response to DAMPs, wound-activated, nuclear pSTAT3+ EpdSCs produce chemokines, which promote the survival and expansion of neighboring dermal γδ T cells, as well as SKINTs, surface antigens that stimulate specialized dendritic epithelial γδ T cells (DETCs), the sentinels of the first line of a barrier breach (Jameson et al., 2002; Keyes et al., 2016). Once stimulated, active γδ T cells and dendritic cells (DCs) migrate to nearby lymph nodes, where they activate and recruit other immune cells to the skin, including basophils, eosinophils, T helper cells, and cytotoxic T cells, to fuel an inflammatory response (Cruz et al., 2018; Jameson and Havran, 2007; Jameson et al., 2002; Saito et al., 2017). Concordantly, in the epidermis, the stem cell:DETC circuitry is bi-directional: DETCs secrete growth factors and chemokines, including IGF-1 and FGF-7/10, that fuel epidermal hyperproliferation (Figure 3; Jameson et al., 2002). At these early stages of inflammation, the hyperproliferation of the surrounding skin epithelium is likely to be a transient effort by EpdSCs to halt further invasion of pathogens.
The beauty of such teamwork between stem cells and immune cells is that many immune cells are mobile, enabling some to be recruited from the circulation to the wound site, while others within the tissue can transit to nearby lymph nodes where they can activate and recruit other inflammatory immune cells. An interesting twist comes from considering deep skin wounds, where a number of indirect circuits surface that are otherwise not seen in shallower wounds. Here, the subcutaneous fascia, composed of a gelatinous extracellular matrix embedded with specialized fibroblasts, lymphatics, immune cells, and nerves, is exposed. As the fibroblasts become mobilized, they drag fascia, including macrophages and other immune cells with it, into the wound bed, eliminating the need for direct stem cell signaling to recruit immune cells (Correa-Gallegos et al., 2019). While the gelatinous matrix acts as a protective sealant over the wound, it contributes to scarring long term, as the matrix is abnormal.
Tissue Repair after Injury
While inflammation is necessary to fight infection immediately following a barrier breach, it is incompatible with wound-repair (Figures 3 and 4; Aurora and Olson, 2014; Karin and Clevers, 2016). The relative simplicity of muscle repair and its choreographed sequence of events following injury has made it an ideal model for dissecting the temporal signals that govern this process and the cellular inputs involved. Two key immune cell types come into play in dampening the inflammatory response. First is the transition of inflammation-associated M1 macrophages to regenerative M2 macrophages, a step that in muscle necessitates suppression of NOTCH function, thereby releasing satellite cells from quiescence and promoting new tissue growth (Arnold et al., 2007; Du et al., 2017). M2 macrophages also produce factors such as IGF-1, stimulatory for both MuSCs and EpdSCs, as well as repressors of differentiation (Figure 3; Tonkin et al., 2015). Second are specialized T lymphocytes, known as regulatory T cells (Tregs), that also function integrally in the reparative phase of both muscle and skin injuries (Burzyn et al., 2013; Kuswanto et al., 2016; Lay et al., 2018; Mathur et al., 2019). When Tregs are ablated using a conditional diptheria toxin (DTR) approach, the regenerative response is negatively impacted both in muscle and in skin (Ali et al., 2017; Burzyn et al., 2013; Lay et al., 2018). While the precise mechanisms underlying the role of Tregs in stimulating stem cells remains unclear and indirect avenues have been described (Heredia et al., 2013), the stimulatory effect of wound-activated Tregs on HFSCs can be direct, perhaps mediated through amphiregulin, related to epidermal growth factor. This would be similar to the effects on hematopoietic stem cells (Hirata et al., 2018). In muscle, Tregs express amphiregulin which acts via EGFR on MuSCs and myoblasts to stimulate their proliferation and subsequently their differentiation into myofibers (Burzyn et al., 2013; Kuswanto et al., 2016).
The truly special feature of Tregs in wound repair is that they function robustly in dampening the inflammatory response initiated after a wound. In the skin, Tregs target cytotoxic T cells, Th17 immune cells, and neutrophils, all critical in mounting the inflammatory response (Mathur et al., 2019). Studies from muscle suggest the possibility that like macrophages, Tregs may exist in two states, an early state compatible with inflammation and a subsequent “regenerative” state that exhausts the inflammatory response to stimulate the repair process (Burzyn et al., 2013; Gerriets et al., 2016; Nosbaum et al., 2016). Since skin stem cells must also be mobilized to migrate into the wound bed, but then wait to repair the wound until the inflammation is dampened, it is tempting to speculate that Tregs act as orchestrators of the temporal steps in immune-stem cell crosstalk that must occur for efficient wound repair.
Neither immune cells nor stem cells can heal a wound alone. Future studies are needed to dissect the dynamics of this communication and unravel how much of their participation emanates from macrophage and/or Treg autonomous signaling, as opposed to taking their cues from the stem cells themselves or from other cells within the niche microenvironment, such as FAPs. Finally, whether direct or indirect, stem cell-immune cell interactions include not only macrophages and Tregs, but also many other tissue-resident immune cells (Lindemans et al., 2015; von Moltke et al., 2016). Probing stem cell:immune cell interactions in tissue repair still poses a plethora of avenues to explore in the future.
Memories of Inflammatory Encounters
Stem cells encounter many inflammatory stresses throughout an organism’s lifetime. For the muscle, this includes exercise, which although physically does not lead to broken blood vessels and pathogen invasion, nonetheless involves mechanical injury that triggers an inflammatory response. Epithelial tissues encounter a diverse array of inflammatory encounters that extend beyond wounding. For the skin, these include chemicals and other noxious agents, allergens such as poison ivy, pathogens, and ultraviolet radiation. For the gastrointestinal tract, this includes toxic bacteria, helminths, and foodstuffs. For the nasal and lung epithelia, it can include airborne viruses and toxins. Remarkably, stem cells register and recordtheir inflammatory encounterswithin their chromatin landscape, a feature that subsequently impacts the stem cells’ behavior upon the next inflammatory confrontation.
Inflammatory imprinting was first described for macrophages, which can retain epigenetic memory of an experience throughout their brief 6 day lifetime (Netea et al., 2020; Netea and Joosten, 2016). More recent studies revealed that the macrophage’s parents, long-lived hematopoietic SCs (HSCs), can also retain memory of their experiences, including sepsis, Mycobacterium tuberculosis vaccine, and fatty “Western” diets. Intriguingly, this skews HSC activity toward a myeloid lineage, enabling their macrophage progeny to “inherit” the epigenetic information (Christ et al., 2018; Kaufmann et al., 2018; Mitroulis et al., 2018). The discovery of this longer-term epigenetic memory harbored by HSCs came on the heels of studies from skin showing that following exposure to the inflammation-inducing drug imiquimod, EpdSCs undergo marked changes in their chromatin, some of which can be detected even 6 months after the pathology has returned to normal (Naik et al., 2017).
Notably, IL1-β is required for both EpdSCs and HSCs to remember these encounters (Naik et al., 2018). Given that IL1-β is often induced following injury, this suggests the fascinating possibility that certain features of inflammatory memory may be conserved across stem cells. Mitogen activated protein kinase (p38 MAPK) is also central to a number of inflammatory responses induced by wounding, and intriguingly, transient exposure of aged MuSCs to p38MAPK inhibitors leads to a heritable rejuvenated potential to regenerate damaged myofibers (Cosgrove et al., 2014); whether this feature is propagated at the epigenetic level as seen in response to a shift in MuSC metabolic state awaits further investigation (Yucel et al., 2019). As the precise mechanisms underlying inflammatory memory are un-raveled, it might in the future be possible to develop drugs to erase it. While this may slow wound healing and repair, it might in the short term dampen inflammation in chronic wounds and in a variety of inflammatory disorders, ranging from psoriasis to atopic dermatitis, allergic lung inflammation, and inflammatory bowel disease. Similarly, chronic inflammatory myopathies such as polymyositis, dermatomyositis, and necrotizing autoimmune myopathy may also benefit from such a therapeutic strategy. For all of these disease states, it would be beneficial if stem cells could rapidly revert to their homeostatic functions once inflammation resolves, which would relieve what is otherwise likely to be a major cumulative burden (Netea et al., 2020).
AGING
Age-Associated Inflammation and Stem Cells
Age-associated decline in tissue function is usually marked by increased levels of pro-inflammatory mediators, low-grade systemic inflammation, and impaired wound healing (Blau et al., 2015; Goldberg and Dixit, 2015). Recent studies suggest that some of these age-related defects result from miscommunication between immune cells and tissue stem cells and chronic accumulation of pro-inflammatory mediators in the tissue (Blau et al., 2015; Keyes et al., 2016).
Skin is ideal for interrogating this failed conversation between resident immune cells and aged stem cells. Already discussed is the DETC:EpdSC communication circuitry that follows skin injury, and in aging, the ability of the EpdSCs to sense wounds and transmit signals to the nearby immune cells breaks down (Keyes et al., 2016). Although human skin lacks DETCs, similar impairments in the dialog between other tissue-resident T cells and EpdSCs could underlie the chronic wounds often seen in aged individuals.
In the skin, the proliferative capacity of stem cells also diminishes, as witnessed by thinning of the epidermis and reduced capacity for hair regeneration during homeostasis (Matsumura et al., 2016). While some of this diminished capacity appears to arise from diminished stem cell numbers and perturbed gene expression, recent studies show that age-related skewing of resident immune cell populations can impact these activities. One of these changes is a decline in resident Tregs (Ge et al., 2020), which could explain the exacerbated inflammatory response often seen in aging. Notably, blocking the over-active inflammatory response is sufficient to reverse the proliferative defects in skin stem cells. Similarly, in the intestine, aged macrophages increase expression of TNFα, resulting in diminished intestinal stem cell function and perturbations in the epithelial barrier, creating a feed-forward circuit of inflammation that perpetuates the aged phenotype (Thevaranjan et al., 2017). Thus, a key to reversing the aging process in stem cells and rejuvenating tissues will be therapies that not only stimulate tissue stem cell self-renewal, but also target inflammatory mediators that accumulate with age.
Niche Dysfunction with Aging
In addition to breakdown between immune cells and stem cells, other perturbations in the stem cell’s milieu are critical to the aging process (Figure 5). In muscle, upon injury or exercise, cytokines and growth factors are released in the tissue milieu, prompting MuSCs to undergo multiple rounds of self-renewing symmetric and asymmetric divisions to replenish the pool and yield committed progenitors that fuse into and restore myofiber function (Evano et al., 2020; Kuang et al., 2007; Le Grand et al., 2009; Shinin et al., 2009; Wang et al., 2019b; Yennek et al., 2014). The balance of self-renewal and commitment is crucial to maintaining a quiescent MuSC reservoir throughout life in order to meet the needs of successive regenerative demands. With age, MuSC numbers and function decline. This depletion aggravates muscle weakness and leads to premature degeneration of neuromuscular junctions (NMJ) (Liu et al., 2015, 2017). Loss of NMJ function and loss of proximity to the vasculature foretell the atrophy of muscle fibers seen in sarcopenia.
Figure 5. Dysregulated Aged Muscle Stem Cell Niche.
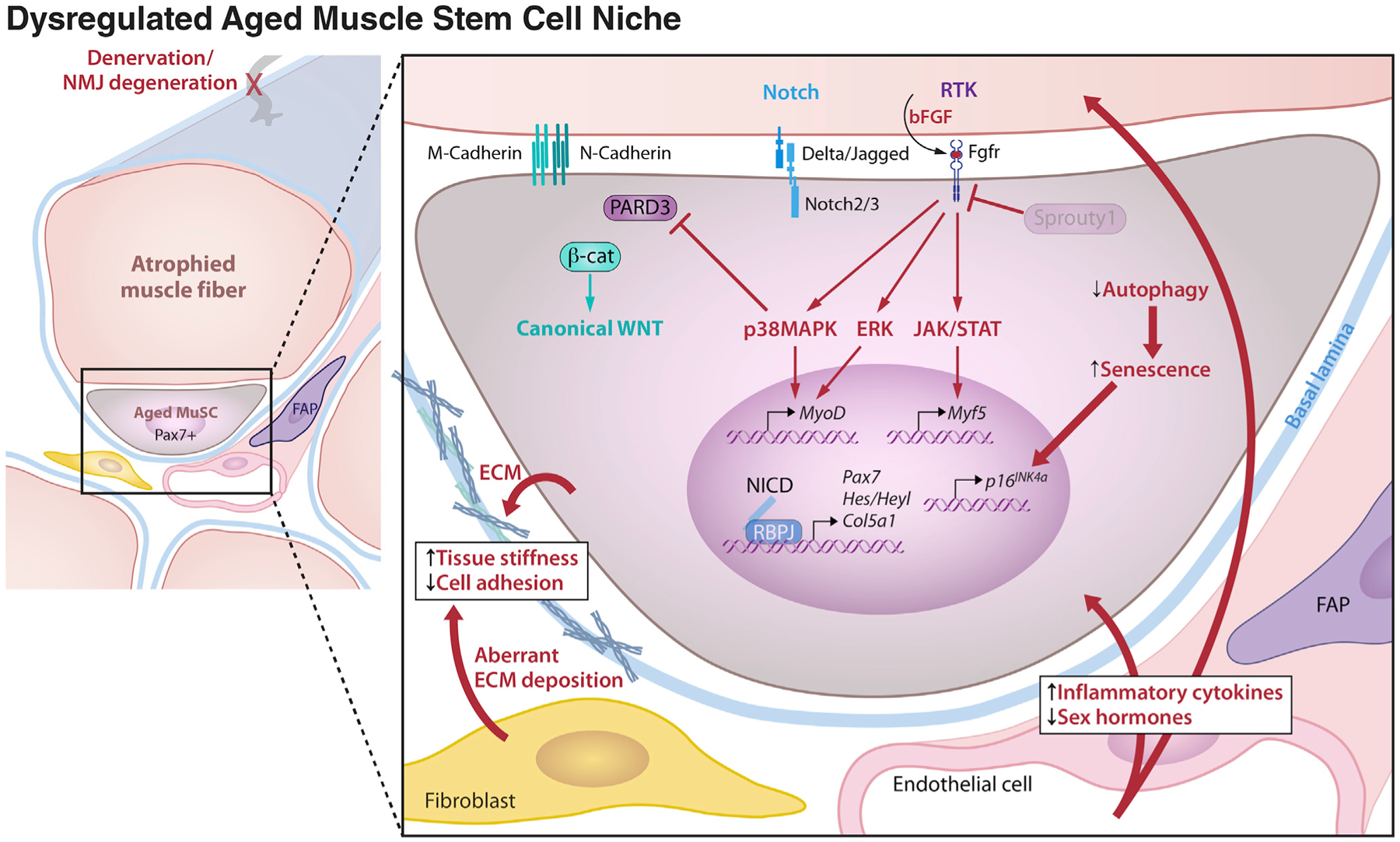
Changes to the cellular and systemic environment that occur with aging are deleterious to muscle stem cell quiescence and function. Denervation of the host muscle fiber can reduce quiescence cues and promote the release of damage signals such as bFGF that trigger persistent downstream extracellular signal-regulated kinase (ERK), p38 mitogen activated protein kinase (p38MAPK), and the Janus kinases (JAK)-signal transducer and activator of transcription proteins (STAT) signaling pathways. Diminished Sprouty1 levels are no longer able to hold these pathways in check. A reduction in Notch signals from the muscle fiber and disruption of apical polarity signaling in aged MuSCs cause a switch from Notch to canonical WNT signaling that impedes MuSC proliferation and drives precocious commitment in response to injury. Decreased autophagy in aged MuSCs leads to senescence. Aberrant deposition of extracellular matrix increases tissue stiffness and leads to reduced MuSC adhesion, altering mechanosensitive signaling pathways.
In most aging tissues, a decline in regenerative capacity is rooted in both stem cell-intrinsic changes and extrinsic changes in the stem cell niche microenvironment. Recent studies show that in skin, although the positional relationship of fibroblasts to the EpdSC niche remains constant with age (Marsh et al., 2018), the fibroblasts themselves progressively lose their identity, showing increasing characteristics of adipogenesis (Salzer et al., 2018). In muscle, the age-related extrinsic signals, such as components of the ECM that anchor the stem cells, are diminished. Fibronectin is a case in point; its loss impedes integrin signaling via FAK and MAP kinase pathways. Whereas in young organisms a sharp peak in fibronectin levels occurs in response to injury, in aged organisms this surge occurs but is blunted and never reaches the same high level. In vivo ubiquitous knockout of fibronectin in young mice led to a 90% reduction in this ECM component post injury, which phenocopied levels in aged mice and led to a dramatic reduction in Pax7+ MuSCs. This loss of adhesion capacity causes MuSC anoikis, anchorage-dependent programmed cell death. That fibronectin is the culprit is clear as injection of fibronectin into aged mouse muscles rescued the niche-dependent loss of MuSC function (Lukjanenko et al., 2016).
Stem cells are exquisitely sensitive not only to biochemical cues, but also to biophysical cues within the niche. In addition to adhesive properties, substrate stiffness has a profound impact on the ability of MuSCs to self-renew (Cosgrove et al., 2014; Gilbert et al., 2010; Madl et al., 2018). If cultured on hydrogels with an equivalent stiffness to native muscle tissue, MuSCs maintain their regenerative potential during expansion in culture and after transplantation in vivo. However, if cultured on hydrogels with an elasticity characteristic of fibrotic aged muscle tissue, their self-renewal capacity is markedly reduced.
MuSCs in aged niches are also exposed to chronically dysregulated signals that lead to a loss of MuSC quiescence and numbers and impair MuSC function in regeneration (Blau et al., 2015). For example, in young mice the NOTCH pathway holds MuSCs in check in a quiescent state from which they escape after injury and induction of canonical WNT signaling. With aging, this regulatory sequence is lost, as Notch ligand Delta 1 expression on the muscle fiber membrane is markedly reduced and WNT3A stimulation of β-catenin signaling is increased, antagonizing NOTCH signaling and triggering MuSC exit from quiescence, decreasing regenerative function, and increasing fibrosis (Brack et al., 2007, 2008). Another signaling node that is dysregulated with aging is mediated by Hedgehog, whose GPCR receptor is localized on cilia, sensory organelles that protrude from MuSCs and are truncated with aging, leading to loss of signaling and reduced regenerative capacity (Jaafar Marican et al., 2016; Palla et al., 2020). The endocrine milieu also changes and as levels of sex hormones and estrogen receptor levels on MuSCs decline after menopause, muscle regeneration is further impeded (Collins et al., 2019).
Additionally, in contrast to the acute sequential changes that mediate tissue repair in young, several signaling pathways become constitutively activated in aged MuSCs that lead to their dysfunction (Blau et al., 2015). These include the p38 mitogen-activated protein kinase (p38MAPK) pathway the fibroblast growth factor (FGF)-ERK MAP kinase cascades and the Janus kinase (JAK-STAT) transcription factor pathway (Bernet et al., 2014; Cosgrove et al., 2014; Price et al., 2014; Tierney et al., 2014). This is due in part to constitutively increased levels of FGF2 in aged muscles which causes MuSCs to overcome the inhibitor, Sprouty1, and escape quiescence leading to failure of regeneration. FGF signaling is known to activate the JAK-STAT, PI3K-Akt, and MAPK pathways (Lanner and Rossant, 2010). In aging, increased FGF signaling is exacerbated by a reduction in Sprouty1, which buffers quiescent MuSCs from entering the cell cycle (Chakkalakal et al., 2012), and the endogenous peptide apelin, which is induced by muscle contraction (Vinel et al., 2018). Notably, reducing signaling via these pathways using inhibitors restores MuSC self renewal and improves muscle healing in aged mice (Bernet et al., 2014; Cosgrove et al., 2014; Price et al., 2014; Tierney et al., 2014).
“Rejuvenation” of aged MuSC function in regeneration requires not only disruption of intrinsic signaling pathways that go awry in aging but also an alteration in the extrinsic biomechanical properties of the niche. Substrate stiffness plays a crucial role in the ability to rejuvenate MuSCs derived from aged mice and augment regenerative function (Cosgrove et al., 2014). Accordingly, a combination of culturing aged MuSCs on compliant substrates with young muscle-like stiffness and pharmacological inhibition of p38 MAP kinase resulted in expansion of a stem cell pool with improved engraftment and regenerative capacity that was heritable across multiple stem cell generations and serial mouse transplantations. This synergy of biochemical and biophysical cues had the remarkable capacity to rejuvenate aged MuSC function, regain engraftment potential, and restore strength of aged muscles post-injury to be on par with young (Cosgrove et al., 2014).
Finally, it is interesting to compare the skin and muscle with regards to the strong link between aging and the increased incidence of most types of cancers. Although squamous cell carcinomas of the skin are the second most common cancer world-wide and their incidence increases markedly with age, muscle cancers are rare. Indeed, the remarkably rare rhabdomyosar-comas that do occur are most prevalent in young individuals. While muscle and skin differ in their level of exposure to ultraviolet radiation and other damaging agents from the environment, the natural turnover of skin stem cells during homeostasis and their robust proliferative response in wound repair distinguish them from muscle stem cells. Thus, in contrast to skin, where a single EpdSC is sufficient to regenerate the body surface (Green, 1991; Hirsch et al., 2017), muscle stem cells largely remain quiescent until injury, but then exhibit transient robust regenerative capacity. MuSCs not withstanding, it is intriguing that other cell types in muscle tissue that give rise to tumors in other tissues do not become neoplastic in muscle, even with aging, suggesting that the microenvironment of skeletal muscle may be tumor suppressive, for reasons worthy of further investigation.
TRANSLATING THE KNOWLEDGE OF STEM CELLS: FROM NICHE CROSS-TALK TO THE CLINIC
Skin
Pioneered by Howard Green (Gallico et al., 1984), epidermal cell culture for clinical use in skin replacement to treat badly burned patients has been the gold standard for several decades. In third degree burns, only a few stem cells were needed to regenerate the epidermis even from children whose body surface was nearly entirely burned. Therapeutic strategies for enhancing repair of wounds in the skin will speed recovery not only from acute damage due to burns or trauma, but also from chronic debilitating problems such as ulcers or heritable skin disorders.
A recent therapeutic breakthrough for the use of skin stem cells in a clinical setting was reported by De Luca and colleagues in Modena, Italy (Figure 6A). These investigators cultured the EpdSCs from a 7-year-old boy with a devastating autosomal-recessive blistering skin disease, junctional epidermolysis bullosa (JEB). JEB is caused by loss-of-function mutations in either an epidermal laminin chain or a hemidesmosomal integrin (α6 or β4), an ECM-receptor complex that is essential for maintaining strong adhesion of the epidermis to its underlying basement membrane (Has et al., 2018). The researchers used gene therapy to deliver the wild-type gene to one of the mutant EpdSCs, and following expansion and engraftment, the child’s body surface was largely restored (Hirsch et al., 2017). Without gene replacement, afflicted children are largely confined to the hospital, as even a minor abrasion has serious consequences.
Figure 6. Therapeutic Applications of Skin and Muscle Stem Cells.
(A) Cell-based therapies for skin: epidermolysis bullosa. Recent advances in the treatment of the severe heritable blistering disorder, epidermolysis bullosa (EBD), highlight the potential for cell-based therapies for the skin. Epidermolysis bullosa is caused by mutations in components of the extracellular matrix, such as laminin 332 (LAMB3), that are essential for maintaining the adhesion of the epidermis to the dermis via type VII collagens. The loss of this adhesion results in severe blistering and skin compromise. By transplanting skin grafts produced from autologous epidermal stem cells obtained from patient biopsies and genetically corrected by transduction with retrovirus carrying functional LAMB3, 80% of the skin of an EB patient was replaced.
(B) Therapeutic targeting of MuSCs within muscle: sarcopenia and DMD. Due to the integral role MuSCs play in maintaining muscle function, therapies targeting MuSCs hold significant regenerative potential. (Top) Muscle strength commonly decreases with aging, a condition known as sarcopenia. To counter sarcopenia, potential therapeutic approaches entail targeting signaling pathways that are dysregulated in aged MuSCs to restore the regenerative function lost with aging. Alternative or combinatorial approaches seek to counter atrophy pathways in myofibers. (Bottom) Muscular dystrophies are heritable muscle wasting disorders that culminate in premature patient death due to the absence of crucial structural or contractile proteins. For Duchenne muscular dystrophy, a number of strategies entail restoring dystrophin by read through drugs that overcome premature stop codons, gene correction of the myofiber by adeno-associated virus (AAV)-mediated micro-dystrophin delivery, or CRISPR-Cas9-mediated exon skipping. These approaches could be used in combination with approaches to enhance MuSC function, e.g., stimulating asymmetric divisions or stem cell expansion, to enhance the rate of repair and ameliorate the disease. Anti-fibrotic agents that target FAPs could promote MuSC function by creating a favorable, pro-regenerative microenvironment.
The success of this clinical trial stemmed in part from the use of fibrin as a culture substrate, a platelet-derived protein that comprises the natural scaffold of skin wounds (Kubo et al., 2001). Siprashvili and colleagues successfully engineered smaller skin grafts to express collagen Col7a, another recessive EB subtype, using FDA-approved culture materials (Clinicaltrials.gov: NCT01263379) (Siprashvili et al., 2016).
Even for successful therapies, however, epithelial restorations through engraftments have been largely limited to regeneration of the epidermis. For third-degree burns, for example, although the patients’ epidermis lasted for years, the patients were unable to sweat or grow hair (Green, 1991). We now know that this is due not only to the different stem cell populations that exist in the skin for the sweat gland and HFs, but also the complexity of each stem cell niche, necessitating replacement of both stem cells and scaffolds to mimic niche components.
For dominant-negative disorders of epidermal stem cells, such as epidermolysis bullosa simplex, a disorder of K5 and K14 keratins, homologous recombination would be needed to replace the mutant gene. The long-term culture of EBS keratinocytes to perform these studies is problematic, due to the mechanical fragility of the cells. Although skin fibroblast derivation of induced pluripotent stem cells (iPSCs) circumvents this issue, deriving healthy keratinocytes from iPSCs has to date proven problematic, as the cells exhibited mutations over time and did not maintain long-term engraftment (Sebastiano et al., 2014). Although umbilical cord blood, bone marrow, and mesenchymal stem cell transplants have been reported (Sebastiano et al., 2014; Wagner et al., 2010), the autologous epidermal tissue-specific stem-cell transplant is most efficacious and currently serves as a benchmark (Hirsch et al., 2017).
Muscle
MuSCs are crucial for skeletal muscle regeneration throughout life. Sarcopenia, the age-dependent loss of skeletal muscle mass and strength, is a major public-health problem that affects an estimated 5% of individuals over 65 years of age. Aging is accompanied by a decrease in functional MuSCs that reduces the capacity of muscle to respond to exercise and injury and to efficiently repair damage to muscle fibers. This is compounded in aging by a loss of MuSC numbers which markedly impedes healing of muscle after injury or surgery. In addition to aging, diabetes, obesity, and cancer cachexia are all accompanied by a loss of MuSC function and a dramatic decline in regenerative capacity of skeletal muscle. Prolonged recovery leads to prolonged immobility which in turn leads to loss of muscle mass in a vicious cycle. As a result, inefficient muscle healing constitutes a major clinical problem and there is a great need for therapeutic approaches to restore MuSC function and augment regeneration.
In addition to muscle atrophy due to sarcopenia, there are a number of devastating genetic muscle wasting disorders that compromise quality of life and lead to premature death. Cell therapy has been envisioned as a treatment. Duchenne muscular dystrophy (DMD), the most common and severe genetic disorder, is caused by mutations in the dystrophin gene that encodes a structural protein crucial to membrane integrity upon contraction (Anderson and Kunkel, 1992). Spanning the last three decades, a number of clinical trials have employed highly proliferative human myogenic progenitors, myoblasts. Restoration of the missing dystrophin gene and protein was achieved (Gussoni et al., 1992), but at very low efficiencies owing to the limited potential of progenitors to proliferate and restore myofiber function. Nonetheless, such trials are still ongoing (Skuk et al., 2006) (Clinicaltrials.gov: NCT02196467 [Laval University]). MuSCs are clearly needed to establish a renewable long-term reservoir for sustained restoration of muscle. Although markers for FACS isolation of human MuSCs have been identified (Alexander et al., 2016; Charville et al., 2015; Garcia et al., 2018), the drastic loss in stem cell potential limits their therapeutic application. This loss is ameliorated by culturing MuSCs on biomaterials with elasticity matching healthy muscle tissue, but expansion to yield a clinically useful supply remains a challenge. Human patient iPSC-derived MuSCs are under development and could constitute an unlimited cell therapeutic that allows for CRISPR-mediated gene replacement or correction (Chal et al., 2015; Darabi et al., 2011; Hicks et al., 2018). One advantage of skeletal muscle over other tissues like skin for iPSC-cell replacement may be muscle tissue’s anti-tumorigenic microenvironment.
Gene therapy, CRISPR editing, and small molecule therapeutic approaches for DMD are advancing rapidly toward the clinic to replace or restore dystrophin (Figure 6B). Minimal truncated, yet functional versions of dystrophin can be delivered by AAV variants (AAV9 and rAAVrh74) that have demonstrated efficient muscle transduction and varying degrees of efficacy in preclinical studies (Chamberlain and Chamberlain, 2017) (ClinicalTrials. gov: NCT03368742 [Solid Bioscience], Clinicaltrials.gov: NCT03375164 [Nationwide Children’s Hospital], Clinicaltrials.gov: NCT03362502 [Pfizer]). In parallel, exon-skipping approaches are being used to by-pass premature stop codons or out-of-frame indels using AAV-delivered CRISPR-Cas9 to target amenable mRNA splice sites and generate truncated but inframe dystrophin protein (Zhang et al., 2020). One limitation is that due to immune response, AAV vectors will likely only be well tolerated once. Second, although these approaches show great promise in restoring dystrophin expression in muscle fibers, the stem cells in the tissues are not efficiently targeted by these approaches (Goldstein et al., 2019; Nance et al., 2019). As a result, muscle wasting may be arrested, but de novo muscle formation may fail. Since muscle stem cells are required for muscle maintenance and growth throughout life, novel vectors that target MuSCs in situ need to be developed to complement the current myofiber targeting approaches. An alternative strategy entails exploiting the dedicated stem cells that are present within the muscle tissue and harbor remarkable regenerative capacity.
DISCUSSION
In this review we have highlighted the autoregulatory role of tissue stem cells as architects that design and create their own niches. This entails elaboration of structural components of the ECM that tether the stem cells to their niches, synthesis of growth factors and cytokines that engage other cell types, and presentation of receptors essential to receiving signals from cellular neighbors. We find remarkable commonalities among stem cells that function quite differently in the regeneration of their respective tissues: continuously (epidermal stem cells), occasionally (muscle stem cells), and episodically (hair follicle stem cells).
Quiescence is not a passive state, but instead, like the differentiated state (Blau and Baltimore, 1991), requires continuous active regulation to be maintained. In skin and muscle stem cells, canonical WNT signaling mediates exit from quiescence and signals stem cell expansion. Thus WNTs, or their downstream target β-catenin, must be held in check in quiescence. This can be achieved by sequestration of β-catenin by membrane components such as neural and muscle cadherins in MuSCs, or by expressing transcription factors that raise the threshold for WNT-signaling, as in HFSCs (Adam et al., 2018; Goel et al., 2017). To restrict proliferation, stem cells also deploy diverse signaling mechanisms to regulate the mechanosensitive transcription factor YAP and maintain its phosphorylation, thereby preventing its translocation to the nucleus, where it induces an activated stem cell state (Eliazer et al., 2019; Schlegelmilch et al., 2011; Zhang et al., 2020). These mechanisms may well be universal properties shared by diverse stem cell types and their niches.
While NOTCH is a ubiquitous regulator of stem cells, the outcome is context dependent. In MuSCs, engaging Delta and Jagged ligands presented by other cells within the niche ensures that stem cells remain inactive and non-proliferative (Baghdadi et al., 2018; Bi et al., 2016; Bjornson et al., 2012; Lu et al., 2020; Mourikis et al., 2012). In EpdSCs, NOTCH ligands presented by the stem cells ensures differentiation by their NOTCH-expressing progeny (Gonzales and Fuchs, 2017). In this regard, it is important to note that for many of the key pathways that regulate stem cell activity, signaling levels matter. Even for universal regulators such as WNTs, low levels of WNT signaling may favor stem cell quiescence, moderate levels may favor stem cell proliferation, and high WNT signaling can favor differentiation (Brack et al., 2008; Eliazer et al., 2019; Ito et al., 2007; Lien et al., 2014; Rudolf et al., 2016; Seale et al., 2003).
Since 1978, when the concept of a stem cell niche was first raised, stem cell:niche communications have been increasingly shown to be at the heart of maintaining stemness, repairing wounds, and ensuring efficacious healing. As in quiescence, the stem cells are instrumental in orchestrating this cellular crosstalk, as following tissue injury and disruption of their niche they secrete cytokines and growth factors, sounding an alarm that serves to attract diverse cell types. Neutrophils and eosinophils are early invaders of the niche that produce cytokines (Tidball, 2017). M1 macrophages are crucial secondary responders that act as scavengers to eliminate cellular debris resulting from tissue damage (Brigitte et al., 2010). In addition, M1 macrophages contribute growth factors and cytokines that can elicit FAP proliferation or FAP apoptosis at different stages of repair (Lemos et al., 2015). The profound impact of FAPS has been generally underappreciated. They are not only the source of fibroblasts and adipocytes, an abundance of which can impede regeneration, but they are also serve as a potent positive influence, a reservoir of cytokines that attract diverse immune cells and stimulate proliferation and commitment of stem cells (Arnold et al., 2007; Castellana et al., 2014; Chazaud et al., 2003; Du et al., 2017). M2 macrophages play a restorative role supplying growth factors and cytokines that stimulate the stem cells to differentiate and contribute to the reconstruction of the damaged tissue. In the absence of macrophages, wound repair is severely impeded (Arnold et al., 2007; Chazaud, 2020; Naik et al., 2017; Tonkin et al., 2015). Similarly, the proximity to the stem cell niche of either blood vessels, conduits for systemic growth factors that nurture the niche, or lymphatic capillaries, which could be draining activating metabolites, growth factors, or immune cells to maintain stem cell behavior, cannot be underestimated. Stem cells can also secrete VEGF, angiopoeitins, and other factors in order to attract the vasculature (Verma et al., 2018).
While still a field in its infancy, cellular memory of experiences that influence future responses has been reported for several diverse stem cells. This type of memory is a well known feature of the adaptive response seen in immune cells. It is also evident in epidermal stem cells that have experienced an inflammatory insult and upon a second inflammatory encounter, such as a wound, respond with far greater alacrity (Naik et al., 2017). The GAlert quiescent state of MuSCs represents a form of cellular memory that accelerates the response to a second wound that is manifested even at a distance from the original damage, for example the MuSCs of the contralateral leg (Rodgers et al., 2014). Aging and disease are known to be associated with an increase in fibrosis that leads to increased rigidity in many tissues, including muscle and skin. The heritable rejuvenated function of aged MuSCs in repairing wounds in secondary transplants after a transient in vitro exposure to an inhibitor of p38MAPK, a mediator of inflammation, in conjunction with a substrate of youthful muscle stiffness, is suggestive of a mechanical memory (Cosgrove et al., 2014). It will be of interest to determine the molecular nature of these regeneration-enhancing cellular memories and to assess the extent to which common molecular paradigms can be drawn across tissue stem cell types. On the other hand, cellular memories are not always good memories, for example those resulting from persistent exposure to inflammatory cells and signals can engender an epigenetic memory for negative encounters that can delay or impede regeneration, as reported for long-term HSC and EpdSCs (reviewed by Naik et al., 2018). In wounds or other inflammatory conditions, the ability of stem cells to quickly revert back to their homeostatic functions once inflammation resolves is crucial to efficacious healing (Netea et al., 2020). The existence of cellular memory raises the prospect of devising therapeutic agents that might mimic the molecular underpinnnings of positive regenerative memories or erase the bad memories that occur in chronic conditions and work against tissue fitness.
Despite more than four decades of success with skin stem cells as a therapeutic application for burn patients, stem cell therapeutics have only recently begun to be revitalized. This is due to the ability to culture most tissue stem cells coupled with strategies to replace or correct the gene mutations involved in heritable diseases. Hurdles persist, especially in the use of MuSCs in cell therapy, as despite advances (e.g., elastic hydrogels mimicking young tissue stiffness that delay loss of stemness in culture) (Gilbert et al., 2010), sufficient numbers of tissue-derived regenerative MuSCs are not readily generated. Although significant strides have been made in converting iPSC to MuSCs, their maturation still leaves something to be desired (Chal et al., 2015; Darabi et al., 2011; Hicks et al., 2018). Thus, stem cell strategies have not kept pace with strategies to correct dystrophin mutations in mature myofibers of DMD patients using CRISPR-Cas9, which have advanced rapidly in mouse and dog with clinical trials on the horizon (Amoasii et al., 2018; Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016). A challenge that remains to be overcome is a means to efficiently achieve CRISPR-Cas9 correction of the MuSCs that fuel the continuous need for regeneration of the chronically degenerating myofibers in DMD patients.
During aging or heritable muscle wasting disorders, muscle stem cell regenerative potential is progressively lost. In conjunction with approaches to counter muscle atrophy or gene replacement, innovative therapeutic strategies currently under pre-clinical investigation entail restoring the potency of endogenous aged MuSCs in situ in their niches. Breakthroughs will come from a better understanding of the complex crosstalk that exists between stem cells and their niches. Sifting through the myriad of communication networks involved offers a rich future for stem cell biologists and as knowledge continues to unfold, a new generation of therapeutic agents will evolve that capitalizes on the remarkable potential of tissue stem cells.
ACKNOWLEDGMENTS
We thank Patrick Lane for illustrating our figures. We are also grateful to the many friends and colleagues in the stem cell and niche fields whose work inspired us to write this review. We centered this review largely on skin stem cells and muscle stem cells, drawing parallels and distinctions between the two types. While text constraints limited our focus, we tried to illustrate paradigms gained from other tissue stem cells and their niches. Our limited discussion of other stem cells is by no means an inference of their lessened importance. Similarly, due to reference constraints, we regret that we could not cite all of the worthy papers on this topic. From our treatise on skin and muscle stem cells, we hope that the exciting questions in stem cell biology and future avenues for exploring how tissue stem cells cope with stress will shine through and inspire new work. E.F. is an Investigator of the Howard Hughes Medical Institute and the Rebecca C. Lancefield Professor. H.M.B. is the Donald E. and Delia B. Baxter Foundation Professor. This work was supported by grants from the NIH (R01-AR050452, R01-AR31737, and R01-AR27883 to E.F. and R01-AG020961 and R01-HG00967401 to H.M.B.), the Starr Foundation (E.F.), the Melanoma Research Alliance (E.F.), NYSTEM C32585GG (E.F.), the Baxter Foundation (H.M.B.), the Li Ka Shing Foundation (H.M.B.), California Institute for Regenerative Medicine (CIRM) grants DISC1-10036 and DISC2-10604 (H.M.B.), and American Heart Association grant AHA-17CSA33590101 (H.M.B.).
Footnotes
DECLARATION OF INTERESTS
E.F. is a member of the Scientific Advisory Boards of L’Oreal and Aresenal Bio-sciences. H.M.B. is a founder and consultant to Myoforte Therapeutics and a cofounder of Rejuvenation Technologies, and has two relevant issued patents. (1) Blau HM, Ho ATV, Palla A. Compositions and methods for muscle regeneration using prostaglandin E2. US9918994B1 3/20/18. (2) Ramunas, J., Yakubov, E, Blau, H.M., and Cooke, J. Compounds, compositions, methods, and kits relating to telomere extension US10525075B2 1/7/20.
REFERENCES
- Adam RC, Yang H, Ge Y, Lien WH, Wang P, Zhao Y, Polak L, Levorse J, Baksh SC, Zheng D, and Fuchs E (2018). Temporal Layering of Signaling Effectors Drives Chromatin Remodeling during Hair Follicle Stem Cell Lineage Progression. Cell Stem Cell 22, 398–413.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MS, Rozkalne A, Colletta A, Spinazzola JM, Johnson S, Rahimov F, Meng H, Lawlor MW, Estrella E, Kunkel LM, and Gussoni E (2016). CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell 19, 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. (2017). Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169, 1119–1129.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoasii L, Hildyard JCW, Li H, Sanchez-Ortiz E, Mireault A, Caballero D, Harron R, Stathopoulou TR, Massey C, Shelton JM, et al. (2018). Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, and Kunkel LM (1992). The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biochem. Sci 17, 289–292. [DOI] [PubMed] [Google Scholar]
- Aragona M, Sifrim A, Malfait M, Song Y, Van Herck J, Dekoninck S, Gargouri S, Lapouge G, Swedlund B, Dubois C, et al. (2020). Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, and Chazaud B (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med 204, 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, and Olson EN (2014). Immune modulation of stem cells and regeneration. Cell Stem Cell 15, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. (2014). YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170. [DOI] [PubMed] [Google Scholar]
- Baghdadi MB, Castel D, Machado L, Fukada SI, Birk DE, Relaix F, Tajbakhsh S, and Mourikis P (2018). Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature 557, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, and Rudnicki MA (2013). Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes CA, and Tapscott SJ (2005). MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol 16, 585–595. [DOI] [PubMed] [Google Scholar]
- Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, and Olwin BB (2014). p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med 20, 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi P, Yue F, Sato Y, Wirbisky S, Liu W, Shan T, Wen Y, Zhou D, Freeman J, and Kuang S (2016). Stage-specific effects of Notch activation during skeletal myogenesis. eLife 5, e17355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot A, Duddy WJ, Ouandaogo ZG, Negroni E, Mariot V, Ghimbovschi S, Harmon B, Wielgosik A, Loiseau C, Devaney J, et al. (2015). Age-Associated Methylation Suppresses SPRY1, Leading to a Failure of Requiescence and Loss of the Reserve Stem Cell Pool in Elderly Muscle. Cell Rep. 13, 1172–1182. [DOI] [PubMed] [Google Scholar]
- Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, et al. (2018). T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 175, 1307–1320.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, and Rando TA (2012). Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, and Baltimore D (1991). Differentiation requires continuous regulation. J. Cell Biol 112, 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Webster C, and Pavlath GK (1983). Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 80, 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Cosgrove BD, and Ho AT (2015). The central role of muscle stem cells in regenerative failure with aging. Nat. Med 21, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha AL, Ramanathan A, Huang S, Ingber DE, and Schreiber SL (2010). Carbon metabolism-mediated myogenic differentiation. Nat. Chem. Biol 6, 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, and Rando TA (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, and Rando TA (2008). A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, and Fusenig NE (1998). Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur. J. Cell Biol 75, 273–286. [DOI] [PubMed] [Google Scholar]
- Brigitte M, Schilte C, Plonquet A, Baba-Amer Y, Henri A, Charlier C, Tajbakhsh S, Albert M, Gherardi RK, and Chrétien F (2010). Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 62, 268–279. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, and Joyner AL (2011). Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, and Mathis D (2013). A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, and Perez-Moreno M (2014). Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 12, e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Good-year LJ, and Wagers AJ (2008). Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, and Brack AS (2012). The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, et al. (2015). Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol 33, 962–969. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, and Chamberlain JS (2017). Progress toward Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther 25, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, and Rando TA (2015). Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells. Stem Cell Reports 5, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B (2020). Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages!. Trends Immunol. 41, 481–492. [DOI] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, and Gherardi RK (2003). Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol 163, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. (2015). Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CC, Tsutsui K, Taguchi T, Sanzen N, Nakagawa A, Kakiguchi K, Yonemura S, Tanegashima C, Keeley SD, Kiyonari H, et al. (2018). Hair follicle epidermal stem cells define a niche for tactile sensation. eLife 7, e38883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, and Rando TA (2013). Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol 14, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, et al. (2018). Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 172, 162–175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, and Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012. [DOI] [PubMed] [Google Scholar]
- Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi HK, Laakkonen EK, Sipilä S, et al. (2019). Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 28, 368–381.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallegos D, Jiang D, Christ S, Ramesh P, Ye H, Wannemacher J, Kalgudde Gopal S, Yu Q, Aichler M, Walch A, et al. (2019). Patch repair of deep wounds by mobilized fascia. Nature 576, 287–292. [DOI] [PubMed] [Google Scholar]
- Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, and Blau HM (2014). Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med 20, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Kerns ML, and Fuchs E (2009). Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J. Clin. Invest 119, 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MS, Diamond A, Russell A, and Jameson JM (2018). Human αβ and γδ T Cells in Skin Immunity and Disease. Front. Immunol 9, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Santos FN, Filareto A, Pan W, Koene R, Rudnicki MA, Kyba M, and Perlingeiro RC (2011). Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors. Stem Cells 29, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Morvan F, Morozzi G, Jourde B, Minetti GC, Kahle P, Rivet H, Brebbia P, Toussaint G, Glass DJ, and Fornaro M (2017). ATP Citrate Lyase Regulates Myofiber Differentiation and Increases Regeneration by Altering Histone Acetylation. Cell Rep. 21, 3003–3011. [DOI] [PubMed] [Google Scholar]
- Dekoninck S, Hannezo E, Sifrim A, Miroshnikova YA, Aragona M, Malfait M, Gargouri S, de Neunheuser C, Dubois C, Voet T, et al. (2020). Defining the Design Principles of Skin Epidermis Postnatal Growth. Cell 181, 604–620.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, and Watt FM (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Shih CH, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, Rando TA, and Feldman BJ (2017). Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat. Commun 8, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, and Rudnicki MA (2015). Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med 21, 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S, Muncie JM, Christensen J, Sun X, D’Urso RS, Weaver VM, and Brack AS (2019). Wnt4 from the Niche Controls the Mechano-Properties and Quiescent State of Muscle Stem Cells. Cell Stem Cell 25, 654–665.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Gomez NC, Levorse J, Mertz AF, Ge Y, and Fuchs E (2019). Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 569, 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ Jr., Dupont-Versteegden EE, McCarthy JJ, and Peterson CA (2020). Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity. Am. J. Physiol. Cell Physiol 318, C1178–C1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evano B, Khalilian S, Le Carrou G, Almouzni G, and Tajbakhsh S (2020). Dynamics of Asymmetric and Symmetric Divisions of Muscle Stem Cells In Vivo and on Artificial Niches. Cell Rep. 30, 3195–3206.e7. [DOI] [PubMed] [Google Scholar]
- Fan X, and Rudensky AY (2016). Hallmarks of Tissue-Resident Lymphocytes. Cell 164, 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang D, Burgmaier JE, Teng Y, Romano RA, Sinha S, and Yi R (2018). Single Cell and Open Chromatin Analysis Reveals Molecular Origin of Epidermal Cells of the Skin. Dev. Cell 47, 133. [DOI] [PubMed] [Google Scholar]
- Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, and Fuchs E (2020). Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature. 10.1038/s41586-41020-42695-41589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, and Peterson CA (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. (2011). In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, and Watt FM (2011). The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallico GG 3rd, O’Connor NE, Compton CC, Kehinde O, and Green H (1984). Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med 311, 448–451. [DOI] [PubMed] [Google Scholar]
- Garcia SM, Tamaki S, Lee S, Wong A, Jose A, Dreux J, Kouklis G, Sbitany H, Seth R, Knott PD, et al. (2018). High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Reports 10, 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP, Polak L, Yuan S, Elemento O, and Fuchs E (2017). Stem Cell Lineage Infidelity Drives Wound Repair and Cancer. Cell 169, 636–650.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Miao Y, Gur-Cohen S, Gomez N, Yang H, Nikolova M, Polak L, Hu Y, Verma A, Elemento O, et al. (2020). The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. USA 117, 5339–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, et al. (2016). Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol 17, 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, and Ruoslahti E (1999). Integrin signaling. Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, and Blau HM (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel AJ, Rieder MK, Arnold HH, Radice GL, and Krauss RS (2017). Niche Cadherins Control the Quiescence-to-Activation Transition in Muscle Stem Cells. Cell Rep. 21, 2236–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EL, and Dixit VD (2015). Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev 265, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Tabebordbar M, Zhu K, Wang LD, Messemer KA, Peacker B, Ashrafi Kakhki S, Gonzalez-Celeiro M, Shwartz Y, Cheng JKW, et al. (2019). In Situ Modification of Tissue Stem and Progenitor Cell Genomes. Cell Rep. 27, 1254–1264.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KAU, and Fuchs E (2017). Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 43, 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, and Fuchs E (2009). A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H (1991). Cultured cells for the treatment of disease. Sci. Am 265, 96–102. [DOI] [PubMed] [Google Scholar]
- Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, and Fuchs E (2019). Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, and Blau HM (1992). Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 356, 435–438. [DOI] [PubMed] [Google Scholar]
- Hagner A, Shin W, Sinha S, Alpaugh W, Workentine M, Abbasi S, Rahmani W, Agabalyan N, Sharma N, Sparks H, et al. (2020). Transcriptional Profiling of the Adult Hair Follicle Mesenchyme Reveals R-spondin as a Novel Regulator of Dermal Progenitor Function. iScience 23, 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, and Nyström A (2015). Epidermal Basement Membrane in Health and Disease. Curr. Top. Membr 76, 117–170. [DOI] [PubMed] [Google Scholar]
- Has C, Nyström A, Saeidian AH, Bruckner-Tuderman L, and Uitto J (2018). Epidermolysis bullosa: Molecular pathology of connective tissue components in the cutaneous basement membrane zone. Matrix Biol 71–72, 313–329. [DOI] [PubMed] [Google Scholar]
- Heitman N, Sennett R, Mok KW, Saxena N, Srivastava D, Martino P, Grisanti L, Wang Z, Ma’ayan A, Rompolas P, and Rendl M (2020). Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weis-senbach J, Tomé FM, Schwartz K, Fardeau M, Tryggvason K, et al. (1995). Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat. Genet 11, 216–218. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, and Chawla A (2013). Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MR, Hiserodt J, Paras K, Fujiwara W, Eskin A, Jan M, Xi H, Young CS, Evseenko D, Nelson SF, et al. (2018). ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol 20, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, Robson SC, Frenette PS, and Fujisaki J (2018). CD150high Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell 22, 445–453.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho ATV, Palla AR, Blake MR, Yucel ND, Wang YX, Magnusson KEG, Holbrook CA, Kraft PE, Delp SL, and Blau HM (2017). Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. USA 114, 6675–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, and Durbeej M (2013). Laminin-211 in skeletal muscle function. Cell Adhes. Migr 7, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, and Fuchs E (2006). Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, and Fuchs E (2011). Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, and Fuchs E (2014). Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, and Cotsarelis G (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320. [DOI] [PubMed] [Google Scholar]
- Jaafar Marican NH, Cruz-Migoni SB, and Borycki AG (2016). Asymmetric Distribution of Primary Cilia Allocates Satellite Cells for Self-Renewal. Stem Cell Reports 6, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J, and Havran WL (2007). Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol. Rev 215, 114–122. [DOI] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, and Havran WL (2002). A role for skin gammadelta T cells in wound repair. Science 296, 747–749. [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr., and Medzhitov R (2002). Innate immune recognition. Annu. Rev. Immunol 20, 197–216. [DOI] [PubMed] [Google Scholar]
- Jenkins BA, and Lumpkin EA (2017). Developing a sense of touch. Development 144, 4078–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, and Rossi FM (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol 12, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Harper S, and Watt FM (1995). Stem cell patterning and fate in human epidermis. Cell 80, 83–93. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, and Rudensky AY (2012). Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, and Clevers H (2016). Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, et al. (2018). BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172, 176–190.e19. [DOI] [PubMed] [Google Scholar]
- Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, and Kardon G (2015). Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun 6, 7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, and Fuchs E (2016). Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 167, 1323–1338.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ricardo-Gonzalez RR, and Moro K (2020). Skin-Resident Innate Lymphoid Cells - Cutaneous Innate Guardians and Regulators. Trends Immunol. 41, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, and Rudnicki MA (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Van de Water L, Plantefaber LC, Mosesson MW, Simon M, Tonnesen MG, Taichman L, and Clark RA (2001). Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J. Invest. Dermatol 117, 1369–1381. [DOI] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, and Mathis D (2016). Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 44, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner F, and Rossant J (2010). The role of FGF/Erk signaling in pluripotent cells. Development 137, 3351–3360. [DOI] [PubMed] [Google Scholar]
- Lay K, Yuan S, Gur-Cohen S, Miao Y, Han T, Naik S, Pasolli HA, Larsen SB, and Fuchs E (2018). Stem cells repurpose proliferation to contain a breach in their niche barrier. eLife 7, e41661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scimè A, and Rudnicki MA (2009). Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, and Rossi FM (2015). Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med 21, 786–794. [DOI] [PubMed] [Google Scholar]
- Lepper C, and Fan CM (2010). Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, and Fan CM (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, and Fan CM (2017). A CREB-MPP7-AMOT Regulatory Axis Controls Muscle Stem Cell Expansion and Self-Renewal Competence. Cell Rep. 21, 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. (2011). The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KN, Jain P, He CH, Eun FC, Kang S, and Tumbar T (2019). Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. eLife 8, e45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, and Fuchs E (2014). In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol 16, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. (2015). Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wei-LaPierre L, Klose A, Dirksen RT, and Chakkalakal JV (2015). Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. eLife 4, e09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, and Chakkalakal JV (2017). Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. eLife 6, e26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, and Olson EN (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xie Y, Huang H, Jiang K, Zhou B, Wang F, and Chen T (2020). Hair follicle stem cells regulate retinoid metabolism to maintain the self-renewal niche for melanocyte stem cells. eLife 9, e52712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, Karaz S, Jacot G, Schmidt M, Li L, et al. (2016). Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med 22, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl CM, Heilshorn SC, and Blau HM (2018). Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E, Gonzalez DG, Lathrop EA, Boucher J, and Greco V (2018). Positional Stability and Membrane Occupancy Define Skin Fibroblast Homeostasis In Vivo. Cell 175, 1620–1633.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, Mehta P, Lowe MM, Abbas AK, Ali N, and Rosenblum MD (2019). Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 50, 655–667.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos I, Asare A, Levorse J, Ouspenskaia T, de la Cruz-Racelis J, Schuhmacher LN, and Fuchs E (2020). Progenitors oppositely polarize WNT activators and inhibitors to orchestrate tissue development. eLife 9, e54304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, and Nishimura EK (2016). Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351, aad4395. [DOI] [PubMed] [Google Scholar]
- Mauro A (1961). Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. (2011). Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, and Fuchs E (2001). Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, 1688–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. (2018). Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172, 147–161.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohassel P, Foley AR, and Bönnemann CG (2018). Extracellular matrix-driven congenital muscular dystrophies. Matrix Biol. 71–72, 188–204. [DOI] [PubMed] [Google Scholar]
- Mok KW, Saxena N, Heitman N, Grisanti L, Srivastava D, Muraro MJ, Jacob T, Sennett R, Wang Z, Su Y, et al. (2019). Dermal Condensate Niche Fate Specification Occurs Prior to Formation and Is Placode Progenitor Dependent. Dev. Cell 48, 32–48.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Shim J, Evans CJ, and Banerjee U (2014). Pvr expression regulators in equilibrium signal control and maintenance of Drosophila blood progenitors. eLife 3, e03626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, and Buckingham M (2005). Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, and Tajbakhsh S (2012). A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30, 243–252. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, and Kardon G (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, and Fuchs E (2017). Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Cowley CJ, and Fuchs E (2018). Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 175, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance ME, Shi R, Hakim CH, Wasala NB, Yue Y, Pan X, Zhang T, Robinson CA, Duan SX, Yao G, et al. (2019). AAV9 Edits Muscle Stem Cells in Normal and Dystrophic Adult Mice. Mol. Ther 27, 1568–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, Chang E, Resnik SR, Page C, Kim D, et al. (2015). dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 17, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, and Joosten LA (2016). Master and commander: epigenetic regulation of macrophages. Cell Res. 26, 145–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. (2020). Defining trained immunity and its role in health and disease. Nat. Rev. Immunol 20, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang Q, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, et al. (2020). Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 368, eabb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, and Giancotti FG (2005). Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol. Cell. Biol 25, 6090–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, and Nishikawa S (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860. [DOI] [PubMed] [Google Scholar]
- Noramly S, Freeman A, and Morgan BA (1999). beta-catenin signaling can initiate feather bud development. Development 126, 3509–3521. [DOI] [PubMed] [Google Scholar]
- Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, and Rosenblum MD (2016). Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol 196, 2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, and Fuchs E (2008). Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Egawa G, and Kabashima K (2018). Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front. Immunol 9, 1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouspenskaia T, Matos I, Mertz AF, Fiore VF, and Fuchs E (2016). WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell 164, 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Göttgens B, and Jensen KB (2013). The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla AR, Hilgendorf KI, Yang AV, Kerr JP, Hinken AC, Demeter J, Kraft P, Mooney NA, Yucel N, Jackson PK, et al. (2020). Ciliation of muscle stem cells is critical to maintain regenerative capacity and is lost during aging. bioRxiv. 10.1101/2020.03.20.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio-Montesinos R, Schwaller F, Udhayachandran A, Rau F, Walcher J, Evangelista R, Vriens J, Voets T, Poulet JFA, and Lewin GR (2020). The Sensory Coding of Warm Perception. Neuron 106, 830–841.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski B, Pulliam C, Betta ND, Kardon G, and Olwin BB (2015). Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet. Muscle 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Jimenez D, Fontenete S, Megias D, Fustero-Torre C, Graña-Castro O, Castellana D, Loewe R, and Perez-Moreno M (2019). Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 38, e101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N, Iqbal S, Mana M, Andersson S, Cognetta AB 3rd, Suciu RM, Roper J, Luopajärvi K, Markelin E, Gopalakrishnan S, et al. (2019). Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie TA, Strand NS, Yang CT, Rabinowitz JS, and Moon RT (2014). Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, and Frenette PS (2019). Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol 20, 303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, and Chuong CM (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpiglia E, Samusik N, Ho ATV, Cosgrove BD, Mai T, Davis KL, Jager A, Nolan GP, Bendall SC, Fantl WJ, and Blau HM (2017). High-resolution myogenic lineage mapping by single-cell mass cytometry. Nat. Cell Biol 19, 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, and Rudnicki MA (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med 20, 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, and Uitto J (1999). Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 18, 29–42. [DOI] [PubMed] [Google Scholar]
- Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, and Fuchs E (2020). Liquid-liquid phase separation drives skin barrier formation. Science 367, eaax9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MHGP (2019). Aging of the Hematopoietic Stem Cell Niche: An Unnerving Matter. Cell Stem Cell 25, 301–303. [DOI] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, and Millar SE (2001). Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev 107, 69–82. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, and Buckingham M (2005). A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953. [DOI] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, and Tajbakhsh S (2012). A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, et al. (2014). mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 510, 393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Schroeder MD, Ma C, and Rando TA (2017). HGFA Is an Injury-Regulated Systemic Factor that Induces the Transition of Stem Cells into GAlert. Cell Rep. 19, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, and Greco V (2013). Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, Boucher J, Klein AM, and Greco V (2016). Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352, 1471–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozo M, Li L, and Fan CM (2016). Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat. Med 22, 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf A, Schirwis E, Giordani L, Parisi A, Lepper C, Taketo MM, and Le Grand F (2016). β-Catenin Activation in Muscle Progenitor Cells Regulates Tissue Repair. Cell Rep. 15, 1277–1290. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, and Sartorelli V (2015). The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, and Blau HM (2008). Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Yano M, Ohki Y, Tomura M, and Nakano N (2017). Occludin Expression in Epidermal γδ T Cells in Response to Epidermal Stress Causes Them To Migrate into Draining Lymph Nodes. J. Immunol 199, 62–71. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, and Waldmann H (2013). The plasticity and stability of regulatory T cells. Nat. Rev. Immunol 13, 461–467. [DOI] [PubMed] [Google Scholar]
- Sala D, Cunningham TJ, Stec MJ, Etxaniz U, Nicoletti C, Dall’Agnese A, Puri PL, Duester G, Latella L, and Sacco A (2019). The Stat3-Fam3a axis promotes muscle stem cell myogenic lineage progression by inducing mitochondrial respiration. Nat. Commun 10, 1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Stephan-Otto Attolini C, Prats N, Aguilera M, et al. (2018). Identity Noise and Adipogenic Traits Characterize Dermal Fibroblast Aging. Cell 175, 1575–1590.e22. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, and Galy A (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656. [DOI] [PubMed] [Google Scholar]
- Sampath SC, Sampath SC, Ho ATV, Corbel SY, Millstone JD, Lamb J, Walker J, Kinzel B, Schmedt C, and Blau HM (2018). Induction of muscle stem cell quiescence by the secreted niche factor Oncostatin M. Nat. Commun 9, 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, and Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, and Camargo FD (2011). Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Polesskaya A, and Rudnicki MA (2003). Adult stem cell specification by Wnt signaling in muscle regeneration. Cell Cycle 2, 418–419. [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Scimè A, and Rudnicki MA (2004). Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2, E130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P, Leung TL, Siprashvili Z, Tichy A, Li J, et al. (2014). Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci. Transl. Med 6, 264ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, et al. (2019). Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif B, Ase AR, Ribeiro-da-Silva A, and Séguéla P (2020). Differential Coding of Itch and Pain by a Subpopulation of Primary Afferent Neurons. Neuron 106, 940–951.e4. [DOI] [PubMed] [Google Scholar]
- Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, and Brack AS (2010). Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, and Tajbakhsh S (2009). Template DNA-strand co-segregation and asymmetric cell division in skeletal muscle stem cells. Methods Mol. Biol 482, 295–317. [DOI] [PubMed] [Google Scholar]
- Shook B, Rivera Gonzalez G, Ebmeier S, Grisotti G, Zwick R, and Horsley V (2016). The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches. Annu. Rev. Cell Dev. Biol 32, 609–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwartz Y, Gonzalez-Celeiro M, Chen CL, Pasolli HA, Sheu SH, Fan SM, Shamsi F, Assaad S, Lin ET, Zhang B, et al. (2020). Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 182, 578–593.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, and Gottlieb RA (2016). Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, Lorenz HP, Leung TH, Keene DR, Rieger KE, et al. (2016). Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA 316, 1808–1817. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugré FJ, Sylvain M, Lachance JG, Deschênes L, et al. (2006). Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol 65, 371–386. [DOI] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, et al. (2017). Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 21, 455–466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG (2017). Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol 17, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT, and Sacco A (2016). The role of muscle stem cell-niche interactions during aging. Nat. Med 22, 837–838. [DOI] [PubMed] [Google Scholar]
- Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, and Sacco A (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med 20, 1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, and Rosenthal N (2015). Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol. Ther 23, 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, and Tsuchida K (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, et al. (2013). Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun 4, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, and Fuchs E (2001). Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104, 605–617. [DOI] [PubMed] [Google Scholar]
- Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, and Asakura A (2018). Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 23, 530–543.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, Le Gonidec S, Dortignac A, Geoffre N, Pereira O, Karaz S, et al. (2018). The exerkine apelin reverses age-associated sarcopenia. Nat. Med 24, 1360–1371. [DOI] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, and Locksley RM (2016). Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, Chen M, Riddle MJ, Osborn MJ, Lund T, et al. (2010). Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N. Engl. J. Med 363, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, and Rudnicki MA (2011). Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol 13, 127–133. [DOI] [PubMed] [Google Scholar]
- Wang L, Siegenthaler JA, Dowell RD, and Yi R (2016). Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 351, 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ECE, Dai Z, Ferrante AW, Drake CG, and Christiano AM (2019a). A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 24, 654–669.e6. [DOI] [PubMed] [Google Scholar]
- Wang YX, Feige P, Brun CE, Hekmatnejad B, Dumont NA, Renaud JM, Faulkes S, Guindon DE, and Rudnicki MA (2019b). EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions. Cell Stem Cell 24, 419–432.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. (2008). Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129. [DOI] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, and Oro AE (2012). Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 26, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, and Fuchs E (2011). Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell 144, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu XR, Wewer UM, and Engvall E (1994). Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat. Genet 8, 297–302. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, and Rivera AJ (1999). Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem. Cytochem 47, 23–42. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Watanabe Y, Ohtani T, Uezumi A, Mikami N, Nakamura M, Sato T, Ikawa M, Hoshino M, Tsuchida K, et al. (2015). Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell Rep. 13, 302–314. [DOI] [PubMed] [Google Scholar]
- Yang H, Schramek D, Adam RC, Keyes BE, Wang P, Zheng D, and Fuchs E (2015). ETS family transcriptional regulators drive chromatin dynamics and malignancy in squamous cell carcinomas. eLife 4, e10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, and Fuchs E (2017). Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 169, 483–496.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yennek S, Burute M, Théry M, and Tajbakhsh S (2014). Cell adhesion geometry regulates non-random DNA segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Rep. 7, 961–970. [DOI] [PubMed] [Google Scholar]
- Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, et al. (2013). MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 17, 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VW, and Scadden DT (2016). Hematopoietic Stem Cell and Its Bone Marrow Niche. Curr. Top. Dev. Biol 118, 21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel N, Wang YX, Mai T, Porpiglia E, Lund PJ, Markov G, Garcia BA, Bendall SC, Angelo M, and Blau HM (2019). Glucose Metabolism Drives Histone Acetylation Landscape Transitions that Dictate Muscle Stem Cell Function. Cell Rep. 27, 3939–3955.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. (2016). NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, Gonçalves WA, Shwartz Y, Fast EM, Su Y, et al. (2020). Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AL, Kovatsis EM, Pozsgai RY, Tasnim A, Zhang Q, and Ginty DD (2019). Distinct Modes of Presynaptic Inhibition of Cutaneous Afferents and Their Functions in Behavior. Neuron 102, 420–434.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, and Anderson DJ (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45, 17–25. [DOI] [PubMed] [Google Scholar]


