Abstract
The gastrointestinal mucosa, structurally formed by the epithelium and lamina propria, serves as a selective barrier that separates luminal contents from the underlying tissues. Gastrointestinal mucosal wound repair is orchestrated by a series of spatial and temporal events that involve the epithelium, recruited immune cells, resident stromal cells, and the microbiota present in the wound bed. Upon injury, repair of the gastrointestinal barrier is mediated by collective migration, proliferation, and subsequent differentiation of epithelial cells. Epithelial repair is intimately regulated by a number of wound-associated cells that include immune cells and stromal cells in addition to mediators released by luminal microbiota. The highly regulated interaction of these cell types is perturbed in chronic inflammatory diseases that are associated with impaired wound healing. An improved understanding of prorepair mechanisms in the gastrointestinal mucosa will aid in the development of novel therapeutics that promote mucosal healing and reestablish the critical epithelial barrier function.
Keywords: gastrointestinal tract, inflammation, repair, mucosa, epithelia, lamina propria
INTRODUCTION
Epithelial barriers line body surfaces that reside in borders between the internal organs and the external environment. Epithelial cells associate with connective tissue in the lamina propria, line a cavity, or cover the surface of an internal organ, thereby serving as mucosal barriers. In luminal organs, mucosal membranes are further lined by mucus, which provides additional protection of tissues from microorganisms, toxins, and trauma (1).
The gastrointestinal (GI) tract, consisting of the stomach, small intestine, and large intestine, exhibits mucosal architecture that varies along the gut axis depending on the specific regional function. In addition to serving as a critical barrier, epithelial cells control secretion and absorption of select substances, while the subepithelial lamina propria provides support and has immune cells that control mucosal homeostasis and host defense (2).
GI mucosal damage resulting in epithelial injury or wounds is observed in several pathologic states that include inflammatory diseases, ischemic events, or mechanical injury. Adequate and efficient repair of the epithelial barrier is critical to regain mucosal homeostasis. Upon mucosal injury, wound repair is established through a spatial and temporal cross talk between epithelial cells, recruited and resident immune cells, microbiota, and other lamina propria cells such as mesenchymal stem cells (MSCs) and fibroblasts. These complex cellular networks prevent bacterial translocation across the mucosa while ensuring repair of the epithelium and removal of damaged cells (3). This review focuses on repair mechanisms in the GI tract.
Mucosal wound repair consists of sequential yet overlapping stages: hemostasis, inflammation, epithelial migration, proliferation, and ultimately, resolution of the inflammatory response. Hemostasis after mucosal injury is accompanied by vascular constriction that prevents excessive blood loss and platelet recruitment that contributes to a fibrin-rich clot, which further helps to seal the injured area. Concomitantly with hemostasis, inflammation triggered by mucosal injury contributes to the host defense and initiates the reparative response (4). The inflammatory phase is characterized by secretion of cytokines and chemokines that trigger neutrophil recruitment for host defense. Additionally, neutrophils secrete antimicrobial peptides, chemokines, and cytokines, which control the subsequent recruitment of monocytes that differentiate into macrophages in the wound bed. Monocytes/macrophages engulf apoptotic neutrophils and trigger epithelial proliferation by releasing prorepair molecules such as specialized proresolving mediators (SPMs) (5). In concert with the inflammatory response, mucosal repair requires the precise balance of epithelial migration, proliferation, and differentiation. Epithelial cells adjoining wounds flatten out and remodel their cytoskeleton to migrate and achieve mucosal repair, a process also referred to as epithelial restitution. Proliferation of the GI epithelial cells adjoining the wound increases the number of cells available to achieve repair and regain homeostasis. Associated with such epithelial events are deposition and remodeling of the extracellular matrix and endothelial cells in vascular structures. The last phase of mucosal wound healing requires differentiation of wound-associated epithelial (WAE) cells with strengthening of the barrier (6).
Another important player in intestinal mucosal wound repair is the microbiota, which includes more than 1,000 different species of bacteria residing near the gut mucus. These bacteria and their products have been proposed to interact with WAE cells to influence repair events. Evidence supporting the importance of intestinal microbiota in mucosal wound healing includes (a) delayed wound repair in germ-free mice with enhanced epithelial repair after colonization, (b) intestinal commensal flora that induce mucin expression in the colon, and (c) the bacterial metabolite butyrate associated with increased intestinal epithelial cell (IEC) proliferation (7).
This review focuses on the interplay of mechanisms by which epithelial cells, leukocytes, stromal cells, and microbiota coordinate cellular events required for mucosal healing and restoration of tissue homeostasis.
OVERVIEW OF THE GASTROINTESTINAL MUCOSA
The GI tract epithelium serves as a regulated barrier that also controls nutrient and fluid absorption. The overall structural organization of the GI tract is somewhat consistent, as all the individual regions comprise the following components: (a) mucosa, consisting of a monolayer of epithelial cells supported by the lamina propria; (b) muscularis mucosa that separates the lamina propria from the underlying submucosa and provides flexibility to the mucosa; (c) submucosa, consisting of connective tissue containing blood vessels, lymphatics, and peripheral neuronal components; and (d) muscularis externa, formed by two muscle layers (the inner circular and the outer longitudinal) that are oriented to coordinate directional movement of the gut or peristalsis.
Along the GI tract, the mucosal architecture demonstrates important spatial structural variations that are dependent of the function of the specific region. Epithelial cells that line the gastric mucosa consist of surface foveolar mucus cells. Additionally, specialized epithelial cells in the body and fundus oxyntic glands differentiate into parietal cells that secrete hydrochloric acid and intrinsic factor and chief cells that release digestive enzymes. Additionally, endocrine D cells in oxyntic glands produce somatostatin. In addition to surface mucus foveolar cells, pyloric glands also contain endocrine (G and D) cells that secret gastrin and somatostatin. Foveolar epithelial cells secrete neutral mucin that serves to protect tissues from the luminal acid environment (8).
The intestinal tract is lined by a monolayer of columnar epithelial cells that are organized in densely packed invaginations referred to as crypts. IECs differentiate into many cell types, including (a) enterocytes that regulate nutrients and water absorption; (b) goblet cells, which secrete mucus that has barrier function; (c) enteroendocrine cells that generate hormones; and (d) tuft cells, which produce interleukin (IL)-25. In the small intestine, Paneth cells secrete antimicrobial peptides, and microfold cells transport antigens to underlying lymphoid aggregates, thereby playing an important role in the adaptive immune response. Several innate and adaptive immune cells ranging from lymphocytes, mast cells, neutrophils, dendritic cells, and macrophages reside in the lamina propria. Spatiotemporal interactions of these cell types orchestrate homeostasis as well as repair after injury (9).
In the following sections, we focus on the cell populations described above and address their role in coordinating mucosal repair in the GI tract.
EPITHELIAL REPAIR
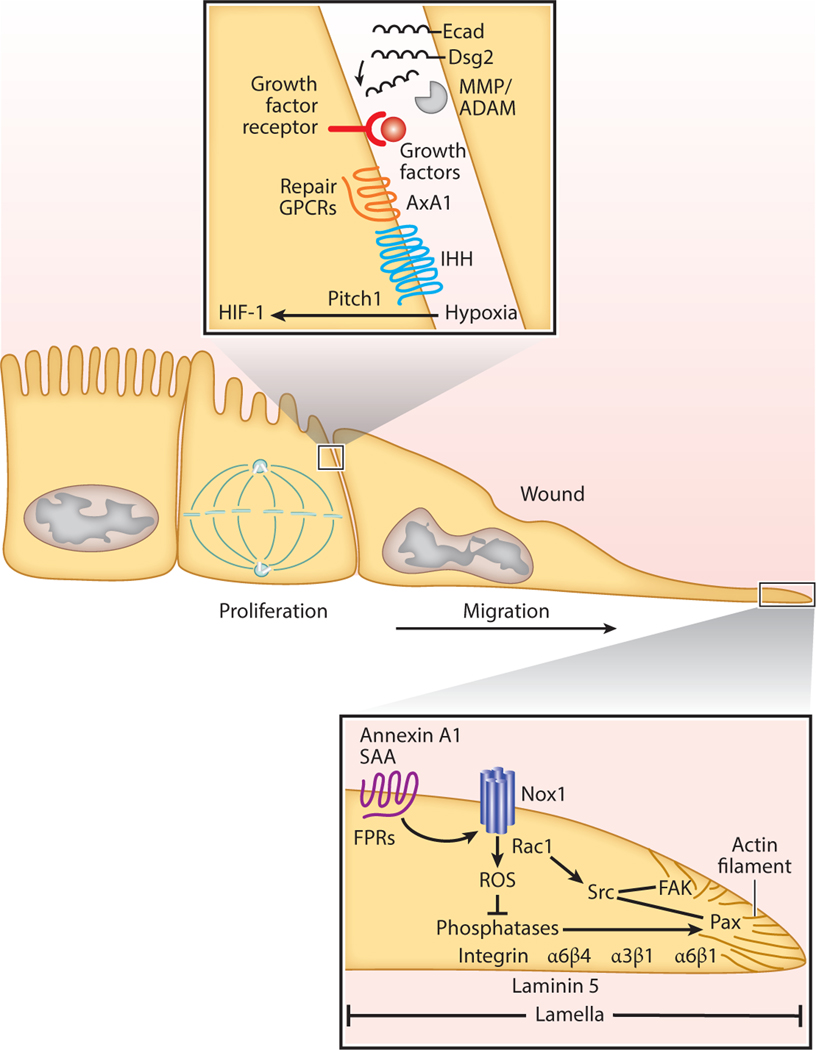
Under homeostatic conditions, the GI tract epithelium is actively turned over as progenitor and stem cells proliferate, differentiate, and are shed in a regulated manner into the lumen. Stem cell location and cell turnover rates vary in the different tissues along the length of the GI tract. In the intestine, where the epithelium is replaced every few days, stem cells are located in the base of the crypts. In response to injury, epithelial cells in crypts adjacent to the damaged mucosa migrate as a collective sheet to cover the denuded mucosa. This process, also referred to as restitution, is initiated within minutes of epithelial cell loss (10). Epithelial cells flatten out, change polarity, and undergo remodeling of the cytoskeleton to achieve forward movement to cover the wound. Furthermore, integrin-containing cell matrix associations are remodeled in the front versus rear of migrating cells. Specialized basal structures derived from integrin-containing focal complexes transform into focal and fibrillar adhesions that facilitate movement of the epithelium (11). Key integrins that control matrix adhesion and movement of IECs include β1 integrin that pairs with α6. Additionally, α6β4 integrin has been shown to function in synergy with α3β1 during cellular extrusion and matrix adhesion. These integrins mediate bidirectional signaling between the cells and the remodeling matrix during wound repair. Matrix components that have been shown to play an important role in controlling epithelial wound repair include laminin 5, 6, and 7, as well as fibronectin. Laminin 5 and α6β4 integrin are targeted into the basal matrix of the leading edge of migrating cells (12, 13) (Figure 1).
Figure 1.
Signaling pathways involved in gastrointestinal epithelial restitution. In order to regain homeostasis after injury, gastrointestinal epithelial cells need to migrate and proliferate. Cells in the edges of wounds migrate to cover denuded surfaces while cells behind the migration front proliferate to accelerate this process. Abbreviations: Dsg2, desmoglein-2 Ecad, E-cadherin; FAK, focal adhesion kinase; FPR, formyl peptide receptor; GPCR, G protein–coupled receptor; HIF-1, hypoxia-induced factor 1; IHH, Indian Hedgehog; MMP, metalloproteinase; Nox1, NADPH oxidase 1; ROS, reactive oxygen species; SAA, serum amyloid factor A.
In addition to the aforementioned structural elements, a number of signaling molecules coordinate repair events. These include small GTPases in the Rho and Rap family that control remodeling of the cytoskeleton and cell matrix adhesions (14–16). GTPase activity of the proteins is controlled by a number of regulatory molecules that include exchange factors and GTPases in addition to the RNA-binding protein, human antigen R (HuR) that increases Cdc42 expression (17) and cellular inhibitor of apoptosis 2 (BIRC3), which promotes activation of Rac1 (18). Similarly, integrin localization and remodeling in focal cell matrix contacts of IECs is influenced by a number of proteins that include annexin A2, annexin A1, and serum amyloid A1. Of interest, ligation of the formylpeptide receptor (FPR) family of G protein–coupled receptors (GCPRs) by annexin A1 and serum amyloid A has been shown to orchestrate intestinal epithelial repair by promoting reactive oxygen species (ROS) signaling with consequent modification of regulatory phosphatases, activation of focal adhesion kinase, and cell movement (15, 19, 20) (Figure 1; see sidebar titled In Vitro Models to Study Intestinal Mucosal Wound Repair).
Metalloproteinases
Movement of cells requires matrix remodeling. The transmembrane and cleaved matrix metalloproteinase (MMP) family of proteins contributes to this process. MMPs cleave and degrade structural proteins in the matrix and basal lamina, thus promoting cell migration. MMP-9 and ADAM 10 and 17 have been reported to influence GI epithelial cell wound repair (21–23). In addition to cell matrix adhesion, collective migration requires orchestrated modification of intercellular junctions. MMPs and ADAM proteases cleave intercellular junctional proteins to decrease intercellular adhesion while promoting forward movement of the epithelial sheet. These proteases target cadherins in the adherens junction (E-cadherin) and desmosomes [desmoglein-2 (Dsg2)] of IECs, resulting in the generation of extracellular cleaved products that are biologically active. We have observed that proinflammatory cytokines released into the epithelial milieu activate proteases in a spatiotemporal manner (24). In damaged cells, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) activate caspase 8, which promotes intracellular cleavage of Dsg2, and the resulting cleaved product [Dsg2 intracellular fragment (ICF)] sensitizes cells to apoptosis. Of interest, MMP-9 and ADAM 10 also promote extracellular cleavage of Dsg2 and E-cadherin (25). We believe that the significance of these events is that Dsg2 ICF signaling mediates death of damaged cells, while the subsequent generation of Dsg2 extracellular cleaved products serves to promote proliferation of surrounding epithelial cells to promote repair (Figure 1).
Cytokines
Cytokines, chemokines, growth factors, and specialized protein and lipid mediators are released into the milieu of the repairing epithelium to activate autocrine and paracrine signaling and orchestrate mucosal repair. Cytokines that display prorepair properties include oncostatin M, TNF-α, IL-2, IL-6, IL-10, IL-22, IL-28, and IL-36 (26–35). IL-10 and IL-22 activate STAT3, whereasIL-28 and on cost at in Mtrigger STAT1 signaling in epithelial cells (26, 30, 31, 35). STAT1 signaling not only activates proliferative pathways but also upregulates the expression of proteins involved in extracellular matrix remodeling and cell migration (36). The prorepair properties of IL-10 are also mediated by epithelial Creb signaling, leading to synthesis and secretion of Wnt1-induced secreted protein (WISP-1), which promotes epithelial migration and proliferation and ultimately mucosal repair (30). TNF-α is a canonical proinflammatory cytokine that contributes to the pathogenesis of inflammatory bowel disease (IBD), a condition where wound repair is compromised. Monoclonal antibodies targeting TNF-α have been successfully used to dampen the inflammatory response in subgroups of IBD patients (37). However, etanercept, a decoy receptor that binds to soluble TNF-α, has not been effective in remission of IBD disease symptoms (38). Of interest is a recent study that highlighted prohealing properties of TNF-α, which are mediated by epithelial nuclear factor-kappa B (NF-κB) and Wnt/β-catenin signaling to promote colonic epithelial stem cell proliferation and mucosal healing (27). It is therefore important to consider the stage of the disease for designing therapy to achieve maximal beneficial effects in promoting disease remission (27) (Figure 2).
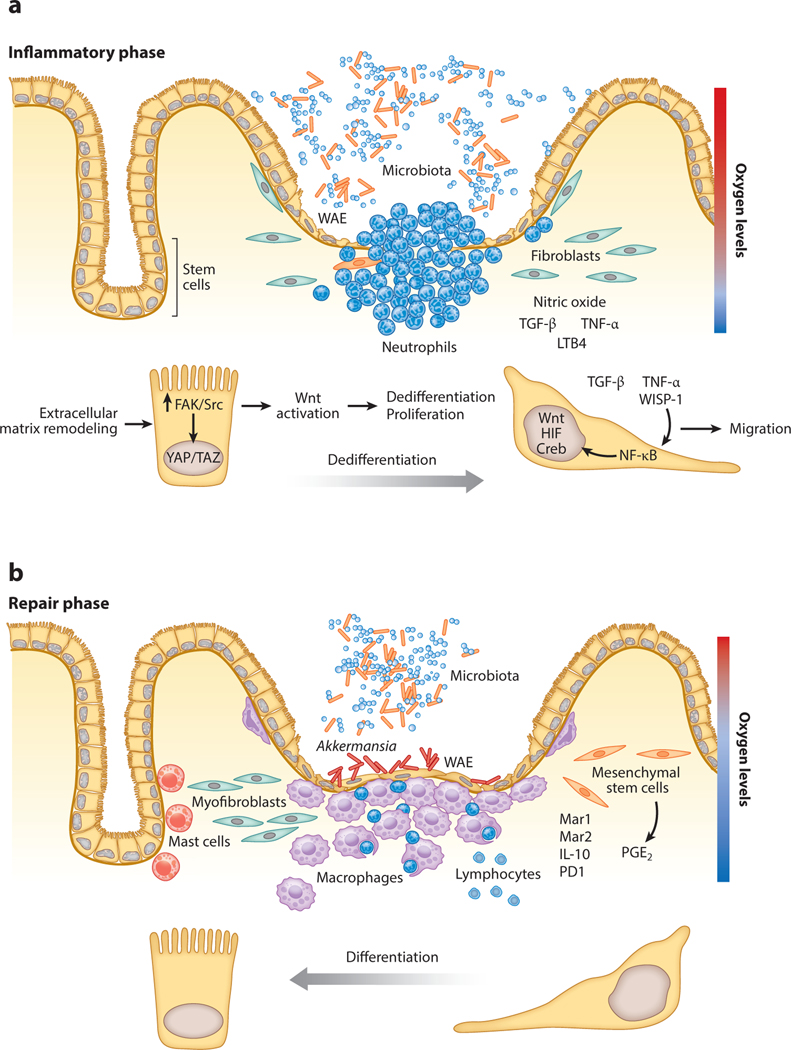
Figure 2.
Wound-associated cells and soluble mediators in a microenvironment during different phases of gastrointestinal mucosal wound repair. After injury, the gastrointestinal mucosa goes through a dynamic process that involves immune cell recruitment and proliferation of epithelial and stromal cells. The inflammatory phase (a) of wound healing requires the recruitment of neutrophils and proinflammatory mediators, whereas the repair phase (b) involves the removal of dead cells and the presence of factors that stimulate proliferating and prosurvival signaling pathways. Abbreviations: FAK, focal adhesion kinase; HIF, hypoxia-inducible factor; IL-10, interleukin-10; LTB4, leukotriene B4; Mar, maresin; PD1, protectin D1; PGE2, prostaglandin E2; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; WAE, wound-associated epithelia; WISP-1, Wnt-induced secreted protein 1.
Growth Factors
Several growth factors, such as transforming growth factor-alpha and -beta (TGF-α and -β), epidermal growth factor (EGF), fibroblast growth factor 2 (FGF-2), keratinocyte growth factor (KGF), insulin-like growth factor 1 and 2 (IGF-1 and −2), and hepatocyte growth factor (HGF), enhance GI epithelial repair (39–45). TGF-β1 induces FGFβ expression, which activates extracellular signal-regulated protein kinase 1/2 (ERK1/2) to promote epithelial proliferation (46). FGFβ acts in synergy with the proinflammatory cytokine IL-17. The adaptor protein Act-1 suppresses FGFβ signaling, while also serving as an essential signaling component of the IL-17 receptor. In response to IL-17 binding, Act-1 forms a complex with the IL-17 receptor, resulting in release of the FGFβ suppressive effect that facilitates epithelial repair (46). Other growth factors such as IGF-1 and TGF-β activate proliferative ERK1/2 signaling via β-arrestin 2, which is also induced by TNF-α signaling during recovery from inflammation (39, 44).
Bioactive Lipids and Specialized Proresolving Mediators
Lipids such as prostaglandins, lysophosphatidic acid (LPA), and short-chain fatty acids (SCFAs), as well as SPMs such as resolvins and maresins, have been shown to influence epithelial repair (47–50). PGE2 has been shown to influence differentiation of intestinal stem cells to WAE cells that migrate over denuded wound surfaces to reestablish the epithelial barrier (47). WAE cells are derived from crypts adjacent to the injured mucosa and are replaced by columnar epithelial cells in a subsequent repair phase (51) (Figure 2). Additionally, LPA stimulates migration and proliferation of epithelial cells in the stomach and colon, and these effects are mediated by Rac1 and cyclin D1/Cdk4 signaling (48). Several SCFAs that have been identified in the intestinal mucosa include formate, acetate, propionate, and butyrate (49). Butyrate is the preferred fuel utilized by colon epithelial cells and has been shown to promote proliferation of healthy epithelial cells and enhance intestinal barrier function through increased expression of tight junction proteins, such as claudin-1 and zonula occludens-1 (52).
SPMs derived from polyunsaturated fatty acids, such as docosahexaenoic and eicosapentaenoic acids, limit the inflammatory response and ultimately promote repair through several mechanisms that include modulation of immune cell recruitment, augmented leukocyte phagocytosis, and regulation of apoptosis and efferocytosis. Recent studies have highlighted the contribution of the SPM lipids resolvin D1, D2, and D5, as well as resolvin E1, maresin 1, protectin D1, and lipoxin A4 in promoting resolution of inflammation (53–57). However, detailed mechanisms and their influence on epithelial repair remain incompletely understood.
SPMs serve as ligands for GPCRs, many of which are upregulated during an inflammatory response and set the stage for mucosal repair. GPCRs that have been shown to influence GI epithelial repair include corticotropin-releasing hormone receptor 2 (CRHR2), prostaglandin E receptor 4 (PTGER4), formyl peptide receptor 1 (FPR1) and 2 (FPR2), G protein–coupled receptor 35 (GPR35), adenosine A2b (A2B), and chemokine-like receptor 1 (CMKLR1) (20, 47, 54, 58–61). Activation of such GPCRs promotes intracellular calcium release, with subsequent activation of proliferative and migratory signaling pathways. Recently, we reported a role of epithelial FPR1 activation by annexin A1 cleavage peptide Ac2–26 (20). Ac2–26 promotes ROS generation by epithelial oxidase, Nox1, and small GTPase Rac1. Localized ROS signaling serves to inactivate the regulatory phosphatases PTEN and PTP-PEST, leading to tyrosine phosphorylation and activation of focal adhesion kinase (FAK) and paxillin, which regulate cell matrix adhesion and forward cell movement (20) (Figure 1). Additionally, intestinal microbiota such as lactobacilli activate this FPR1 signaling pathway to facilitate mucosal repair (62). FPR1-null mice exhibit delayed intestinal mucosal wound healing and recovery from acute colitis (20). These observations suggest that FPRs function as pattern recognition receptors to exogenous as well as endogenous mediators to promote the mucosal reparative response.
Signaling Proteins and Transcription Factors
A combination of vascular damage and high oxygen demand creates a hypoxic environment in healing mucosal wounds. The hypoxia-inducible factor (HIF) family of transcription factors regulates oxygen homeostasis and activates restorative signaling cascades that include proteins such as vascular-endothelial growth factor (VEGF), heme oxygenase-1, C-X-C chemokine receptor type 4 (CXCR4), and the CXCR4-ligand stromal cell-derived factor 1 (SDF-1) (63). The role of HIF-1α during intestinal epithelial wound repair remains incompletely understood, as reports have attributed its contribution to both decreasing and increasing the inflammatory response in different colitis models. In one study, lack of epithelial HIF-1α expression was associated with severe colitis, and expression of a constitutively active protein had protective effects (64). Additionally, increased HIF-1 expression has been correlated with more severe clinical symptoms and histological damage that was associated with an increase in proinflammatory mediators (65). These reports likely indicate that HIF levels are “finely tuned” during mucosal repair.
Hedgehog signaling has been explored more extensively in gastric than in intestinal epithelial cells. In a homeostatic state, gastric parietal cells secrete Sonic hedgehog (SHH), which regulates acid secretion, cell differentiation and proliferation, and tissue regeneration (66). Following damage to the gastric epithelium, chief and parietal cells decrease in number and either dedifferentiate or are replaced by proliferating regenerative cells. This phenotype switch is accompanied by loss of SHH secretion with a concomitant increase in Indian hedgehog that is highly expressed in undifferentiated epithelial cells with a proliferative phenotype (67).
β-catenin is a key mediator of canonical Wnt signaling that regulates epithelial proliferation in the GI tract under homeostatic conditions (68, 69). Of interest is the noncanonical Wnt5a, which has been shown to influence intestinal mucosal repair by influencing migration of WAE cells. Epithelial proliferation in crypts adjoining the wound contribute to subsequent mucosal repair (70). This proliferative stage has been recently linked to Hippo signaling mediated by YAP/TAZ proteins, which are two highly related transcriptional activators that serve as primary sensors of the cellular microenvironment, integrating cell polarity and growth factor/cytokine signaling (71). Although YAP/TAZ have been reported to be dispensable under homeostatic conditions, recent studies have highlighted their contribution in promoting intestinal repair (72–75). Wnt ligands can activate YAP/TAZ signaling and induce conversion of committed cells back to progenitor/stem cells expressing some fetal proteins that influence extracellular matrix remodeling and increased FAK/Src signaling. Upon tissue damage, cells that are distinct from adult intestinal stem cells contribute to wound repair by replenishing lost stem cells and restoring tissue architecture (75) (Figure 2). These observations are consistent with the notion that, upon mucosal injury, regeneration involves transcriptional reprogramming of epithelial cells to a more primitive state. In a recent study, we observed that during the establishment of the repair phase after intestinal mucosal injury, WISP-1 derived from epithelial cells stimulates IEC wound repair by increasing the expression of POU5F1 and NANOG, transcription factors that contribute to stem cell pluripotency and embryonic development (30).
The Contribution of Calcium Signaling and pH in Gastric Repair
Calcium signaling, hydrogen ion concentration (pH), and proteins such as trefoil factor (TFF) and cyclooxygenase-1 (COX-1) have been implicated in modulating wound repair in the stomach. Ca2+ is a ubiquitous second messenger that influences numerous gastric cellular processes, including acid/bicarbonate secretion, mucus secretion, and cell migration (76). Both intracellular and extracellular Ca2+ influence gastric mucosal wound repair in vivo, and an interplay between intraand extracellular Ca2+ levels is required for appropriate gastric epithelial restitution (77). The mucus layer covering the gastric epithelium contains high levels of bicarbonate (HCO3), creating a neutral pH microenvironment that protects epithelial cells from the highly acidic gastric luminal contents. The pH microdomain adjacent to the surface epithelium is elevated (through HCO3 secretion) following epithelial damage and facilitates establishment of the healing process. COX-1 influences such reparative processes (78, 79). It is not well understood whether the pH increase is due to a decrease in acid secretion, an increase in bicarbonate secretion/leak, or a combination of both. Pharmacological inhibition of acid secretion has beneficial wound healing effects that are likely mediated by mechanisms beyond simple change in luminal pH (80). TFFs are peptides that have been reported to play an important role in epithelial defense and repair, and the underlying mechanisms are not well understood (81, 82) (see sidebar titled In Vivo Models to Study Intestinal Mucosal Wound Repair).
Contribution of Immune Cells to Mucosal Repair
During the epithelial restitution phase, immune cells are recruited to sites of mucosal injury to mediate host defense while also participating in reparative responses in the mucosa. The next section highlights their contribution to repair.
Neutrophils.
Neutrophils are one of the first responders and are recruited to injured sites within hours of damage (83) (Figure 2). In addition to mediating antimicrobial host defenses through phagocytosis of pathogens and release of cytotoxic mediators such as elastase and myeloperoxidase, neutrophils secrete several mediators that directly participate in reparative responses (84). Furthermore, neutrophils are a major source of ROS at sites of mucosal injury. The oxidative burst associated with ROS generation consumes a large amount of oxygen, thereby creating a hypoxic environment. Importantly, such localized hypoxia is associated with stabilization of epithelial hypoxia–sensing proteins such as HIF-1α, which has been shown to facilitate epithelial repair (85).
Neutrophils are recruited from the circulation, and their trafficking across endothelial cells has been extensively investigated. However, their subsequent journey in the lamina propria and across epithelial cells is less well understood. It is known that, when migrating through intercellular epithelial spaces, neutrophils release enzymes and activate epithelial proteases that result in the rearrangement of intercellular junction proteins and barrier compromise. However, more recently it has become apparent that neutrophils can act as a double-edged sword in that they play an important role in promoting mucosal repair (83, 84).
Neutrophil-derived proteases can directly or indirectly cleave intestinal epithelial junctional proteins such as E-cadherin and desmoglein-2, and these cleaved products can activate paracrine signaling to promote proliferation of surrounding epithelial cells (86, 87). After migrating across the intestinal epithelium, neutrophils bind and activate an apically expressed epithelial protein, intercellular adhesion molecule 1 (ICAM-1), resulting in downstream β-catenin activation and epithelial proliferation and repair (88). These observations suggest that modulation of ICAM-1 by neutrophils can potentially be used to promote epithelial repair.
In addition to having direct binding effects on epithelial cells, neutrophils produce or are involved in the biosynthesis of growth factors, such as VEGF, and of proresolution mediators such as lipoxins, resolvins, and protectins (89–92). Importantly, resolvin E1, lipoxin A4, and protectin D1 dampen neutrophil recruitment while increasing phagocytosis of apoptotic neutrophils by inflammatory macrophages (56, 93, 94). Nitric oxide and TGF-β derived from neutrophils could also promote mucosal wound repair. Furthermore, antimicrobial activity of neutrophils mediated by formation of neutrophil extracellular traps promotes resolution of inflammation by degrading cytokines/chemokines and reducing further neutrophil recruitment and activation (95). Neutrophils also play an important role in clearance of accumulated cell debris from wounded mucosal areas. In support of the importance of neutrophils for effective mucosal repair, impaired neutrophil function (or recruitment) results in impaired pathogen clearance and perturbed wound repair (96, 97). Therefore, although excessive neutrophilic infiltration is associated with the pathogenesis of many inflammatory diseases, neutrophils also release key mediators that are necessary for the resolution of inflammation.
Macrophages.
In response to damage and after the initial influx of neutrophils, inflammatory monocytes are recruited into the wound bed. In the healthy GI tract, a small population of subepithelial resident macrophages have been proposed to patrol the barrier and release local paracrine soluble mediators that control mucosal homeostasis (98). In addition to resident macrophages, recruited monocytes differentiate into wound-associated macrophages and represent a major inflammatory cell component in healing wounds (99). Thus, after the initial wave of neutrophil influx into wounds, macrophages contribute to host defense and the reparative response. Both the resident and recruited macrophages proliferate and undergo important functional changes in response to the changing tissue microenvironment (100). Broadly, macrophage subpopulations encompass pro- and anti-inflammatory phenotypes, although a spectrum of monocyte–macrophage phenotypes has recently been described (101, 102). Wound-associated macrophages have been reported to have a number of attributes that include their contribution to resolution of inflammation and repair and inhibition of fibrosis (103). It is still not known whether macrophages can transition into different phenotypes or whether these cells are terminally differentiated into subpopulations in response to the microenvironment. However, recent studies have found plasticity among macrophages, suggesting that a macrophage can evolve into different phenotypes depending on external cues (104).
Infiltrating monocytes that are recruited into the injured mucosa secrete soluble mediators that mediate protection from pathogens, promote clearance of inflammatory cells, and facilitate resolution of inflammation (105). Macrophages phagocytose neutrophils to prevent further release of their proteins, thereby further inhibiting tissue injury. Inflammatory monocytes/macrophages have been reported to secrete HGF, TNF-α, IL-1, IL-6, and IL-12 to help in host defense and contribute to wound repair (106). In addition to the host defense functions, macrophage-derived mediators directly influence not only epithelial repair but also matrix restructuring and angiogenesis (107, 108). Interestingly, inflammatory and nonresident macrophages are the main sources of IL-10 in healing biopsy-induced colonic wounds. IL-10 secretion by Ly6Chigh macrophages appears to suggest a transition toward a proresolution phenotype that facilitates mucosal repair (30) (Figure 2). Thus, the plasticity of macrophage phenotypes is likely context and microenvironment dependent.
Previous studies have identified resident macrophages that localize near the epithelium and function to control mucosal homeostasis as well as repair (99). Prorepair macrophages secrete SPMs such as maresins, resolvins, and protectins (109). In the skin, macrophage depletion inhibits repair that is dependent on the stage at which these cells are ablated, resulting in outcomes that range from impaired reepithelialization if ablated in the early stages of inflammation to no effects if they are depleted at a late stage of repair (110, 111). Macrophages, but not neutrophils or lymphocytes, may be necessary for the appropriate amplification of colonic epithelial progenitors that contribute to wound repair (112). During repair, macrophages surrounding the stem cell niche physically contact epithelial progenitors and release factors involved in epithelial proliferation and matrix remodeling (113). Macrophages can integrate cues from the surrounding environment, including MSCs, luminal microbiome, and the injured epithelia, to influence the colonic epithelial progenitor cell niche and promote a regenerative response (113). On the opposite end of the spectrum, uncontrolled inflammatory mediator generation by macrophages retards wound repair in chronic disorders such as diabetes mellitus or IBD as well as aging (114).
In summary, while the spectrum of macrophage phenotypes that contribute to homeostasis and mucosal repair is not completely understood, it is evident that a balanced monocyte–macrophage recruitment is pivotal in orchestrating mucosal repair.
Other immune cells.
Several other immune cell types encompassing mast cells, eosinophils, and lymphoid cells play an important role in orchestrating mucosal repair; details pertaining to their contribution to repair are extensively discussed in other reviews (115, 116). Although excess mast cells in chronic inflammation can negatively impact repair, their presence is important in maintaining mucosal homeostasis and reparative processes. Whereas mast cells release several mediators, histamine and prostaglandin D2 have been shown to influence mucosal repair (117, 118). The contribution of lymphocytes in mucosal repair is a rapidly evolving field. Studies examining tissues from patients with IBD have suggested a role of regulatory T cells (Tregs) in wound repair. These cell populations, including γδT cells, secrete growth factors and cytokines that promote repair (119, 120). In addition to Tregs, B lymphocytes have also been implicated in promoting repair of chronic dermal wounds (121). In summary, mucosal repair is influenced by many immune cell populations, some of which are resident, whereas others are recruited to sites of injury.
Contribution of Lamina Propria Stromal Cells to Mucosal Repair
MSCs secrete growth factors and cytokines, which recruit progenitor cells or endogenous stem cells to the injured site to mediate wound repair (122). MSCs reside in the stroma of mouse GI mucosa. MSCs secrete prostaglandin E2 that activates Wnt signaling in progenitor epithelial cells to maintain colonic epithelial proliferation. Following injury, MSCs undergo division and serve to either give structural support or secrete prorepair and immunomodulatory molecules. MSC-derived prostaglandin E2 downregulates macrophage secretion of proinflammatory cytokines and activates the epithelial Wnt pathway (123). MSCs are uniquely positioned to serve as a communication network between epithelial cells and wound-associated macrophages. MSCs can skew the wound microenvironment cytokine composition toward an anti-inflammatory profile, suggesting that these cells can function as immune regulators during repair (123) (Figure 2).
In addition to MSCs, fibroblasts in the injured sites secrete soluble mediators, such as TGF-β, IL-1α, IL-1β, IL-4, and VEGF, that promote fibroblast migration into wounds, thereby modulating the matrix composition that influences repair (124, 125). Wound bed–associated fibroblasts can differentiate into myofibroblasts by upregulating the expression of smooth muscle actin, thereby creating contractile forces that facilitate healing of wounds (126). In summary, spatiotemporal recruitment and activation of mesenchymal cells play important roles in coordinating reparative events in healing mucosal wounds.
Contribution of Microbiota to Mucosal Repair
A diverse and complex population of microorganisms resides in the GI tract lumen. Evolving studies have highlighted the importance of the microbiome not only in modulating mucosal homeostasis but also in facilitating repair of injured tissues (127). Colonization of germ-free mice with Bacteroides thetaiotaomicron, a member of mouse and human intestinal microflora, positively regulated expression of genes involved in nutrient absorption, mucosal barrier fortification, xenobiotic metabolism, angiogenesis, and postnatal intestinal maturation (128). However, the molecular basis of these responses is less well understood. Regeneration of colonic epithelial progenitor cells is markedly diminished in germ-free mice (112). In Drosophila, microbiota members of the genus Lactobacillus can stimulate intestinal epithelial ROS signaling that serves to promote proliferation of intestinal epithelial stem cells (62). In a murine model, an anaerobic mucinophilic gut symbiont Akkermansia muciniphila was shown to be enriched in hypoxic intestinal mucosal wounds and activate formyl peptide receptor signaling in epithelial cells to promote repair (129) (Figure 2). The prorepair properties of microbiota are not limited to the gut. Enhanced dermal wound healing was observed in mice that were given oral supplementation of Lactobacillus reuteri in their drinking water (130). Taken together, these studies highlight a crucial role of microbiota–host cross talk in controlling homeostatic and reparative responses in the gut.
HEALING OF WOUNDS IN CHRONIC INFLAMMATORY DISORDERS
Chronic inflammatory disorders of the GI tract, such as chronic gastritis and IBD, are associated with wounds that exhibit delayed or incomplete wound-healing abilities (131). Perturbed mucosal wound healing is also observed in aging individuals (132). In these pathologies, imbalanced immune responses and disordered epithelial repair result in failure of wounds to heal in an orderly and timely manner (133). Thus, although inflammation is a pivotal and necessary component of the healing process, uncontrolled inflammation is detrimental to tissue regeneration. Numerous studies have shed light on mechanisms that orchestrate wound repair. However, the basis of aberrant mucosal healing in chronic pathologies is not well understood and is likely related to an imbalance of inflammation, translocation of commensal bacteria, and impaired epithelial repair responses (114). During acute regulated mucosal repair, neutrophils are actively removed from the wounds within 24–76 h (134). However, in chronic wounds, neutrophils continue to reside at sites of injury, with resulting release of proteases that contribute to the tissue damage. Failure to heal in chronic wounds has also been attributed to deregulation of proteases and their inhibitors. Increased activity of MMPs such as collagenase and gelatinase A and B has been observed in chronic wounds compared to acute wounds. MMP activity influences epithelial adhesion and degradation of growth factors and other peptides involved in repair (135–138). Moreover, persistence of soluble inflammatory mediators in the wound bed of chronic injuries has been reported to negatively influence migration of fibroblasts and epithelial cells and ultimately repair (139, 140). This area of research clearly needs further investigation to identify the imbalance of mediators that are involved in the resolution of inflammation and epithelial repair.
Novel Approaches to Promote Mucosal Healing
For many GI chronic inflammatory pathologies, induction of clinical response and maintenance of remission are the primary goals that ultimately determine clinical success (141). These goals include treatment of chronic ulcers that are associated with patient symptoms. Given the pivotal role of the epithelium in functioning as a barrier to luminal contents, therapies aimed at suppressing the mucosal inflammatory response and promoting recovery of the epithelial barrier are important in achieving homeostasis. Current therapies for chronic IBD include immunosuppression and biological agents like TNF-α and α4 integrin antibodies. These treatment modalities have been extensively reviewed elsewhere (142). Here, we briefly address a few strategies in regenerative medicine that can be used to promote mucosal repair.
Regenerative medicine has focused on regeneration of functional tissue that can be used to promote repair (143). Strategies have included harnessing endogenous prorepair molecules to facilitate repair of damaged tissue and congenital defects. Local delivery of proresolving peptides encapsulated within nanoparticles may represent a potential therapeutic strategy to facilitate repair of chronic mucosal injuries. We have used polyethylene glycol (PEG) nanoparticles containing the proresolution molecule Ac2–26, a peptide derived from annexin A1 that promotes intestinal epithelial wound repair (144). The nanoparticles contain collagen IV peptide, which helps to target nanoparticles to sites of damage. Collagen IV is highly expressed during angiogenesis and participates in repair processes (144). Intramucosal delivery of these nanoparticles accelerates healing of murine colonic wounds after biopsy-induced injury. These nanoparticles can also be delivered systemically to accelerate recovery following experimentally induced colitis (145).
The system of local delivery into the wound bed has been used to deliver proresolving agents as well as stem cells that can accelerate mucosal wound healing. Strategies have included the use of organoids that are generated from in vitro stem cell cultures and can potentially be derived from a small number of donor cells and expanded and differentiated ex vivo. Following this, they could be transplanted back into injured sites to promote repair in the same individual. A few studies have shown that murine colonic organoids can localize, engraft, and differentiate into injured areas of dextran sodium sulfate–induced colitis mice without subsequent tumor formation (146). Furthermore, using a PEG-based hydrogel, we have delivered human intestinal organoids into injured intestinal mucosa of immunocompromised mice, resulting in engraftment and improved colonic wound repair (147). This strategy is an initial step for the development of human intestinal organoid-based therapies to treat chronic wounds in both the GI tract and other mucosal surfaces. Additionally, colonic MSCs have been delivered in a similar fashion to facilitate mucosal repair. Mucosal delivery of MSCs prevented the progress of gastric ulcers and stimulated angiogenesis in a VEGF-dependent manner, indicating that local injection of MSCs could potentially be used to treat pathologic conditions with impaired angiogenesis and repair (123).
Other strategies to promote epithelial restitution include use of an artificial gastric wall with a bioabsorbable polymer and the oral administration of a recombinant cholera toxin B (148). The bioabsorbable polymer sheets help to restore extensive gastric defects and could potentially be developed as a new therapeutic strategy after extensive gastrectomy and following intestinal anastomosis. The administration of cholera toxin B subunit was shown to promote mucosal healing in the colon, highlighting the potential of using modification of this toxin to promote repair (148, 149).
CONCLUDING REMARKS
The GI epithelium serves as a vital barrier that interfaces the external environment containing bacteria and antigens and internal tissue compartments. Injury resulting in a breach of this barrier has detrimental local and systemic effects. The epithelium has a remarkable capacity to repair wounds. It is orchestrated by a spatial and temporal interplay between pro- and anti-inflammatory molecules that mediate cross talk of wound-associated epithelia, neutrophils, macrophages, stromal cells, and microbiota. An imbalance of these interactions can result in delayed wound repair, which is observed in chronic inflammatory diseases. An improved understanding of mediators that coordinate mucosal repair is important not only in understanding the biology of repair but also in designing of novel therapies to promote healing of wounds in chronic inflammatory diseases.
IN VITRO MODELS TO STUDY INTESTINAL MUCOSAL WOUND REPAIR
Epithelial cells migrate as a collective sheet to efficiently cover denuded surfaces. The scratch-wound healing assay is a simple and reproducible method that can be used to evaluate different parameters of repair. Either primary cultures or cell lines are plated and grown to confluence and scratch wounded using a fine pipette tip. The ability to perform this assay in primary cultured cells combined with time-lapse microscopy and fluorescent tracking of probes can yield valuable information on cell morphology, protein localization, and rate of wound closure. Proliferating cells can be detected by analysis of the thymidine analog EdU incorporation or Ki67 immunostaining. Polarity of cells migrating into the wound can also be evaluated with this model (150).
IN VIVO MODELS TO STUDY INTESTINAL MUCOSAL WOUND REPAIR
Chemical ulcers: This model recapitulates chemical peptic injury in the stomach and duodenum. The most commonly used chemical agent is acetic acid. This model is simple and reproducible, and its pathological features and healing mechanisms resemble human disease (151).
Laser-induced injury: ROS and free radicals can be generated by photoexcitation causing irreversible damage and cell death. The precision and potency of two-photon confocal microscopes allow for the creation of laser-induced damage that is dependent on the laser power and pulse duration. In this model, wound repair can be followed in real time (76).
DSS and recovery: Dextran sodium sulfate (DSS)-induced injury is a commonly used experimental colitis model. DSS is administered in the drinking water of mice, resulting in epithelial injury. To analyze mucosal healing, DSS is replaced with drinking water, and the healing response is then evaluated over the subsequent 3–5 days (152).
Biopsy-induced mucosal injury: A veterinary colonoscope equipped with biopsy forceps is used to injure the colonic mucosa and visualize wound repair. This technique is useful in identifying important players during intestinal mucosal repair and is amenable to analysis of wound repair in transgenic mice (153).
ACKNOWLEDGMENTS
This work was supported by US National Institutes of Health grants (RO1DK055679, RO1DK089763, DK059888) to A. Nusrat and the Crohn’s and Colitis Foundation of America Career Development Award (544599) to M. Quirós. We would like to acknowledge Jay Mierzwiak for the help provided during the preparation of this manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Moens E, Veldhoen M. 2012. Epithelial barrier biology: good fences make good neighbours. Immunology 135:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng LK, O’Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. 2010. Gastrointestinal system. Wiley Interdisc. Rev. Syst. Biol. Med. 2:65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizuka M, Konno S. 2011. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 17:2161–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature 453:314–21 [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. 2016. Resolution of inflammation: Whatcontrols its onset? Front. Immunol. 7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnemann KJ, Bement WM. 2011. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 27:237–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L, Zhang J. 2017. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saenz JB, Mills JC. 2018. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat. Rev. Gastroenterol. Hepatol. 15:257–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14:141–53 [DOI] [PubMed] [Google Scholar]
- 10.Sturm A, Dignass AU. 2008. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 14:348–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt S, Friedl P. 2010. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 339:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotz MM, Nusrat A, Madara JL, Ezzell R, Wewer UM, Mercurio AM. 1997. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am. J. Pathol. 150:747–60 [PMC free article] [PubMed] [Google Scholar]
- 13.Koivisto L, Heino J, Hakkinen L, Larjava H. 2014. Integrins in wound healing. Adv. Wound Care 3:762–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer RA, Wendt MK, Johan esen PA, Turner JR, Dwinell MB. 2007. RhoactivationregulatesCXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab. Investig. 87:807–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, et al. 2007. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am. J. Pathol. 170:951–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro AC, Luissint AC, Sumagin R, Lai C, Vielmuth F, et al. 2014. Trans-dimerization of JAM-A regulates Rap2 and is mediated by a domain that is distinct from the cis-dimerization interface. Mol. Biol. Cell 25:1574–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Zhuang R, Xiao L, Chung HK, Luo J, et al. 2017. HuR enhances early restitution of the intestinal epithelium by increasing Cdc42 translation. Mol. Cell. Biol. 37:e00574–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidelin JB, Larsen S, Linnemann D, Vainer B, Coskun M, et al. 2015. Cellular inhibitor of apoptosisprotein 2 controls human colonic epithelial restitution, migration, and Rac1 activation. Am. J. Physiol. Gastrointest. Liver Physiol. 308:G92–99 [DOI] [PubMed] [Google Scholar]
- 19.Hinrichs BH, Matthews JD, Siuda D, O’Leary MN, Wolfarth AA, et al. 2018. Serum amyloid A1 is anepithelial prorestitutive factor. Am. J. Pathol. 188:937–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni G,Alam A,Neumann PA,Lambeth JD,Cheng G,et al. 2013AnnexinA1,formylpeptidereceptor,and NOX1 orchestrate epithelial repair. J. Clin. Investig. 123:443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, et al. 2005. ADAM10 mediates E-cadherinshedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. PNAS 102:9182–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Escudero J, Moreno V, Martin-Alonso M, Hernandez-Riquer MV, Feinberg T, et al. 2017´ E-cadherin cleavage by MT2-MMP regulates apical junctional signaling and epithelial homeostasis in the intestine. J. Cell Sci. 130:4013–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamekura R, Nava P, Feng M, Quiros M, Nishio H, et al. 2015. Inflammation-induced desmoglein-2 ectodomain shedding compromises the mucosal barrier. Mol. Biol. Cell 26:3165–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luissint AC, Parkos CA, Nusrat A. 2016. Inflammation and the intestinal barrier: leukocyte-epithelialcell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151:616–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yulis M, Quiros M, Hilgarth R, Parkos CA, Nusrat A. 2018. Intracellular Desmoglein-2 cleavage sensitizes epithelial cells to apoptosis in response to pro-inflammatory cytokines. Cell Death Dis. 9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beigel F,Friedrich M,Probst C,Sotlar K,Goke B,et al. 2014OncostatinMmediatesSTAT3-dependentintestinal epithelial restitution via increased cell proliferation, decreased apoptosis and upregulation of SERPIN family members. PLOS ONE 9:e93498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford EM, Ryu SH, Singh AP, Lee G, Goretsky T, et al. 2017. Epithelial TNF receptor signalingpromotes mucosal repair in inflammatory bowel disease. J. Immunol. 199:1886–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N, Mishra J, Narang VS, Waters CM. 2007. Janus kinase 3 regulates interleukin 2-inducedmucosal wound repair through tyrosine phosphorylation of villin. J. Biol. Chem. 282:30341–45 [DOI] [PubMed] [Google Scholar]
- 29.Kuhn KA,Manieri NA,Liu TC,Stappenbeck TS.2014IL-6stimulatesintestinalepithelialproliferationand repair after injury. PLOS ONE 9:e114195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiros M, Nishio H, Neumann PA, Siuda D, Brazil JC, et al. 2017. Macrophage-derived IL-10 mediatesmucosal repair by epithelial WISP-1 signaling. J. Clin. Investig. 127:3510–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, et al. 2009. STAT3 links IL-22 signaling inintestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206:1465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, et al. 2017. IL-36R signalling activates intestinalepithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 66:823–38 [DOI] [PubMed] [Google Scholar]
- 33.Medina-Contreras O, Harusato A, Nishio H, Flannigan KL, Ngo V, et al. 2016. Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J. Immunol. 196:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mooseker MS, Pollard TD, Wharton KA. 1982. Nucleated polymerization of actin from the membraneassociated ends of microvillar filaments in the intestinal brush border. J. Cell Biol. 95:223–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiriac MT, Buchen B, Wandersee A, Hundorfean G, Gunther C, et al. 2017. Activation of epithelial¨ signal transducer and activator of transcription 1 by interleukin 28 controls mucosal healing in mice with colitis and is increased in mucosa of patients with inflammatory bowel disease. Gastroenterology 153:123–38.e8 [DOI] [PubMed] [Google Scholar]
- 36.Hou SX, Zheng Z, Chen X, Perrimon N. 2002. The Jak/STAT pathway in model organisms: emergingroles in cell movement. Dev. Cell 3:765–78 [DOI] [PubMed] [Google Scholar]
- 37.Shah B,Mayer L.2010Currentstatusofmonoclonalantibodytherapyforthetreatmentofinflammatorybowel disease. Expert Rev. Clin. Immunol. 6:607–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, et al. 2001. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121:1088–94 [DOI] [PubMed] [Google Scholar]
- 39.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. 2003. Transforming growth factorβmediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am. J. Pathol. 162:597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhodes JA, Tam JP, Finke U, Saunders M, Bernanke J, et al. 1986. Transforming growth factor alphainhibits secretion of gastric acid. PNAS 83:3844–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dignass AU, Tsunekawa S, Podolsky DK. 1994. Fibroblast growth factors modulate intestinal epithelialcell growth and migration. Gastroenterology 106:1254–62 [DOI] [PubMed] [Google Scholar]
- 42.Frey MR, Golovin A, Polk DB. 2004. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J. Biol. Chem. 279:44513–21 [DOI] [PubMed] [Google Scholar]
- 43.Han DS, Li F, Holt L, Connolly K, Hubert M, et al. 2000. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G1011–22 [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Nezu R, Wasa M, Sando K, Kamata S, et al. 1999. Insulin-like growth factor-1 modulation ofintestinal epithelial cell restitution. J. Parenter. Enter. Nutr. 23:S89–92 [DOI] [PubMed] [Google Scholar]
- 45.Nusrat A, Parkos CA, Bacarra AE, Godowski PJ, Delp-Archer C, et al. 1994. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J. Clin. Investig. 93:2056–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song X, Dai D, He X, Zhu S, Yao Y, et al. 2015. Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity 43:488–501 [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, et al. 2017. Prostaglandin E2 promotesintestinal repair through an adaptive cellular response of the epithelium. EMBO J. 36:5–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU. 1999. Modulation of intestinal epithelialwound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 117:368–77 [DOI] [PubMed] [Google Scholar]
- 49.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, et al. 2015. Crosstalk between microbiotaderived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell EL, Serhan CN, Colgan SP. 2011. Antimicrobial aspects of inflammatory resolution in themucosa: a role for proresolving mediators. J. Immunol. 187:3475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyoshi J, Takai Y. 2008. Structural and functional associations of apical junctions with cytoskeleton.Biochim. Biophys. Acta 1778:670–91 [DOI] [PubMed] [Google Scholar]
- 52.Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. 2012. Butyrate promotes the recovering of intestinalwound healing through its positive effect on the tight junctions. J. Anim. Sci. 90(Suppl. 4):266–68 [DOI] [PubMed] [Google Scholar]
- 53.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. 2011. Omega-3 fatty acid-derived mediators17(R)-hydroxydocosahexaenoicacid,aspirin-triggeredresolvinD1andresolvinD2preventexperimental colitis in mice. J. Immunol. 187:1957–69 [DOI] [PubMed] [Google Scholar]
- 54.Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, et al. 2010. Resolvin E1-inducedintestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. PNAS 107:14298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. 2013. Maresin 1, a proresolving lipidmediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 191:4288–98 [DOI] [PubMed] [Google Scholar]
- 56.Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, et al. 2017. Protectin D1n-3 DPA andresolvin D5n-3 DPA are effectors of intestinal protection. PNAS 114:3963–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vong L, Ferraz JG, Dufton N, Panaccione R, Beck PL, et al. 2012. Up-regulation of Annexin-A1 andlipoxin A4 in individuals with ulcerative colitis may promote mucosal homeostasis. PLOS ONE 7:e39244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. 2016. Corticotropin-releasing hormone receptor 2 signaling promotes mucosal repair responses after colitis. Am. J. Pathol. 186:134–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen K, Liu M, Liu Y, Yoshimura T, Shen W, et al. 2013. Formylpeptide receptor-2 contributes tocolonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Investig. 123:1694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukahara T, Hamouda N, Utsumi D, Matsumoto K, Amagase K, Kato S. 2017. G protein-coupledreceptor 35 contributes to mucosal repair in mice via migration of colonic epithelial cells. Pharmacol. Res. 123:27–39 [DOI] [PubMed] [Google Scholar]
- 61.Eltzschig HK, Rivera-Nieves J, Colgan SP. 2009. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets 13:1267–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, et al. 2013. Symbiotic lactobacilli stimulate gutepithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32:3017–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colgan SP, Eltzschig HK. 2012. Adenosine and hypoxia-inducible factor signaling in intestinal injuryand recovery. Annu. Rev. Physiol. 74:153–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karhausen J,Furuta GT,Tomaszewski JE,Johnson RS,Colgan SP,Haase VH.2004Epithelialhypoxiainducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 114:1098–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, et al. 2013. Endothelial PAS domainprotein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145:831–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engevik AC, Feng R, Yang L, Zavros Y. 2013. The acid-secreting parietal cell as an endocrine sourceof Sonic Hedgehog during gastric repair. Endocrinology 154:4627–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng R, Aihara E, Kenny S, Yang L, Li J, et al. 2014. Indian Hedgehog mediates gastrin-inducedproliferation in stomach of adult mice. Gastroenterology 147:655–66.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fevr T, Robine S, Louvard D, Huelsken J. 2007. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 27:7551–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koch S. 2017. Extrinsic control of Wnt signaling in the intestine. Differentiation 97:1–8 [DOI] [PubMed] [Google Scholar]
- 70.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. 2012. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338:108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. 2017. Mechanobiology of YAP and TAZ in physiologyand disease. Nat. Rev. Mol. Cell Biol. 18:758–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, et al. 2013. Restriction of intestinal stemcell expansion and the regenerative response by YAP. Nature 493:106–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. 2010. The Hippo signaling pathway restrictsthe oncogenic potential of an intestinal regeneration program. Genes Dev. 24:2383–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng F, Peng L, Li Z, Tan G, Liang E, et al. 2018. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yui S,Azzolin L,Maimets M,Pedersen MT,Fordham RP,et al. 2018YAP/TAZ-dependentreprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22:35–49.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aihara E, Montrose MH. 2014. Importance of Ca2+ in gastric epithelial restitution—new views revealed by real-time in vivo measurements. Curr. Opin. Pharmacol. 19:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aihara E,Hentz CL,Korman AM,Perry NP,Prasad V,Shull GE,Montrose MH.2013Invivoepithelialwound repair requires mobilization of endogenous intracellular and extracellular calcium. J. Biol. Chem. 288:33585–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH. 2008. Disruption of the Cox-1 geneslowsrepair ofmicroscopiclesionsin the mousegastric epithelium.Am. J. Physiol. Cell Physiol. 294:C223–32 [DOI] [PubMed] [Google Scholar]
- 79.Demitrack ES, Soleimani M, Montrose MH. 2010. Damage to the gastric epithelium activates cellularbicarbonate secretion via SLC26A9 Cl−/HCO3−. Am. J. Physiol. Gastrointest. Liver Physiol. 299:G255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demitrack ES, Aihara E, Kenny S, Varro A, Montrose MH. 2012. Inhibitors of acid secretion can benefitgastric wound repair independent of luminal pH effects on the site of damage. Gut. 61:804–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue L, Aihara E, Podolsky DK, Wang TC, Montrose MH. 2010. In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase. Gut. 59:1184–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue L, Aihara E, Wang TC, Montrose MH. 2011. Trefoil factor 2 requires Na/H exchanger 2 activityto enhance mouse gastric epithelial repair. J. Biol. Chem. 286:38375–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brazil JC, Parkos CA. 2016. Pathobiology of neutrophil-epithelial interactions. Immunol. Rev. 273:94–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J. 2018. Neutrophils in tissue injury and repair. Cell Tissue Res. 371:531–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, et al. 2014. Transmigrating neutrophilsshapethemucosalmicroenvironmentthroughlocalizedoxygendepletiontoinfluenceresolution of inflammation. Immunity 40:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu H, Ma Y, Yang M, Zhang C, Huang H, et al. 2016. Persisting and increasing neutrophil infiltrationassociates with gastric carcinogenesis and E-cadherin downregulation. Sci. Rep. 6:29762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boxio R, Wartelle J, Nawrocki-Raby B, Lagrange B, Malleret L, et al. 2016. Neutrophil elastase cleavesepithelial cadherin in acutely injured lung epithelium. Respir. Res. 17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sumagin R, Brazil JC, Nava P, Nishio H, Alam A, et al. 2016. Neutrophil interactions with epithelialexpressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 9:1151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crawford J. 2002. Neutrophil growth factors. Curr. Hematol. Rep. 1:95–102 [PubMed] [Google Scholar]
- 90.Chandrasekharan JA,Sharma-Walia N.2015Lipoxins:nature’swaytoresolveinflammation.J.Inflamm. Res 8:181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kohli P, Levy BD. 2009. Resolvins and protectins: mediating solutions to inflammation. Br. J. Pharmacol. 158:960–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Kebir D, Gjorstrup P, Filep JG. 2012. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. PNAS 109:14983–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, et al. 2003. Lipoxin A4 and aspirin triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with micro vessel endothelial cells and epithelial cells. J. Immunol. 170:2688–94 [DOI] [PubMed] [Google Scholar]
- 95.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, et al. 2014. Aggregated neutrophil extracellular¨ traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20:511–17 [DOI] [PubMed] [Google Scholar]
- 96.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. 2013. Reduced neutrophil chemotax is and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol. 190:1746–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilgus TA, Roy S, McDaniel JC. 2013. Neutrophils and wound repair: positive actions and negative reactions. Adv. Wound Care 2:379–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, et al. 2018. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 215:1507–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, et al. 2013. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependentfates of thesameLy6Chi monocyte precursors. Mucosal Immunol. 6:498–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mowat AM, Bain CC. 2011. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 3:550–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mosser DM,Edwards JP.2008Exploringthefullspectrumofmacrophageactivation.Nat.Rev.Immunol. 8:958–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das A, Sinha M, Datta S, Abas M, Chaffee S, et al. 2015. Monocyte and macrophage plasticity in tissuerepair and regeneration. Am. J. Pathol. 185:2596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wynn TA, Vannella KM. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44:450–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tabas I, Bornfeldt KE. 2016. Macrophage phenotype and function in different stages of atherosclerosis.Circ. Res. 118:653–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bain CC, Mowat AM. 2014. The monocyte-macrophage axis in the intestine. Cell Immunol. 291:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectiousdiseases. Front. Immunol. 5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koh TJ, DiPietro LA. 2011. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13:e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Angelo F,Bernasconi E,Schafer M,Moyat M,Michetti P,et al. 2013Macrophagespromoteepithelialrepair through hepatocyte growth factor secretion. Clin. Exp. Immunol. 174:60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dalli J, Serhan C. 2016. Macrophage proresolving mediators-the when and where. Microbiol. Spectr. 4 10.1128/microbiolspec.MCHD-0001-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hesketh M, Sahin KB, West ZE, Murray RZ. 2017. Macrophage phenotypes regulate scar formationand chronic wound healing. Int. J. Mol. Sci. 18:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, et al. 2010. Differential roles of macrophages in diversephases of skin repair. J. Immunol. 184:3964–77 [DOI] [PubMed] [Google Scholar]
- 112.Pull SL,Doherty JM,Mills JC,Gordon JI,Stappenbeck TS.2005Activatedmacrophagesareanadaptiveelement of the colonic epithelial progenitor niche necessary for regenerative responses to injury. PNAS 102:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. 2009. Efficient colonic mucosalwound repair requires Trem2 signaling. PNAS 106:256–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neurath MF, Travis SP. 2012. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61:1619–35 [DOI] [PubMed] [Google Scholar]
- 115.Cader MZ, Kaser A. 2013. Recent advances in inflammatory bowel disease: mucosal immune cells inintestinal inflammation. Gut 62:1653–64 [DOI] [PubMed] [Google Scholar]
- 116.Kurashima Y, Kiyono H. 2017. Mucosal ecological network of epithelium and immune cells for guthomeostasis and tissue healing. Annu. Rev. Immunol. 35:119–47 [DOI] [PubMed] [Google Scholar]
- 117.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, et al. 2009. Mast cells regulate homeostaticintestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. PNAS 106:22381–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krystel-Whittemore M, Dileepan KN, Wood JG. 2015. Mast cell: a multi-functional master cell. Front. Immunol. 6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, Eberl M. 2017. Antigen-presenting humanγδ T cells promote intestinal CD4+ T cell expression of IL-22 and mucosal release of calprotectin. J.Immunol. 198:3417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. 2002. Protection of the intestinal mucosa byintraepithelial γδT cells. PNAS 99:14338–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sirbulescu RF, Boehm CK, Soon E, Wilks MQ, Ilies I, et al. 2017. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 25:774–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manieri NA, Stappenbeck TS. 2011. Mesenchymal stem cell therapy of intestinal disease: are their effectssystemic or localized? Curr. Opin. Gastroenterol. 27:119–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, et al. 2015. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J. Clin. Investig. 125:3606–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roulis M, Flavell RA. 2016. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiologyand disease. Differentiation 92:116–31 [DOI] [PubMed] [Google Scholar]
- 125.Goke M, Kanai M, Podolsky DK. 1998. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am. J. Physiol. 274:G809–18 [DOI] [PubMed] [Google Scholar]
- 126.Darby IA, Laverdet B, Bonte F, Desmouliere A. 2014. Fibroblasts and myofibroblasts in wound healing.Clin. Cosmet. Investig. Dermatol. 7:301–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McDermott AJ, Huffnagle GB. 2014. The microbiome and regulation of mucosal immunity. Immunology 142:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zocco MA, Ainora ME, Gasbarrini G, Gasbarrini A. 2007. Bacteroides thetaiotaomicron in the gut: molecular aspects of their interaction. Dig. Liver Dis. 39:707–12 [DOI] [PubMed] [Google Scholar]
- 129.Alam A, Leoni G, Quiros M, Wu H, Desai C, et al. 2016. The microenvironment of injured murine gutelicits a local pro-restitutive microbiota. Nat. Microbiol. 1:15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, et al. 2013. Microbial symbionts acceleratewound healing via the neuropeptide hormone oxytocin. PLOS ONE 8:e78898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao R, Liang H, Clarke E, Jackson C, Xue M. 2016. Inflammation in chronic wounds. Int. J. Mol. Sci. 17:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo S, Dipietro LA. 2010. Factors affecting wound healing. J. Dent. Res. 89:219–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rieder F, Karrasch T, Ben-Horin S, Schirbel A, Ehehalt R, et al. 2012. Results of the 2nd scientificworkshop of the ECCO (III): basic mechanisms of intestinal healing. J. Crohns Colitis 6:373–85 [DOI] [PubMed] [Google Scholar]
- 134.Diegelmann RF. 2003. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 11:490–95 [DOI] [PubMed] [Google Scholar]
- 135.Eming SA, Martin P, Tomic-Canic M. 2014. Wound repair and regeneration: mechanisms, signaling,and translation. Sci. Transl. Med. 6:265sr6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Sullivan S, Gilmer JF, Medina C. 2015. Matrix metalloproteinases in inflammatory bowel disease: anupdate. Mediat. Inflamm. 2015:964131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. 2000. Differential expression of matrix metal lo proteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, et al. 2005. Targeted deletionofmetalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129:1991–2008 [DOI] [PubMed] [Google Scholar]
- 139.Leeb SN, Vogl D, Gunckel M, Kiessling S, Falk W, et al. 2003. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology 125:1341–54 [DOI] [PubMed] [Google Scholar]
- 140.Tong Q, Vassilieva EV, Ivanov AI, Wang Z, Brown GT, et al. 2005. Interferon-γinhibits T84 epithelial cell migration by redirecting transcytosis of β1 integrin from the migrating leading edge. J. Immunol. 175:4030–38 [DOI] [PubMed] [Google Scholar]
- 141.Cintolo M, Costantino G, Pallio S, Fries W. 2016. Mucosal healing in inflammatory bowel disease: maintain or de-escalate therapy. World J. Gastrointest. Pathophysiol. 7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Danese S, Vuitton L, Peyrin-Biroulet L. 2015. Biologic agents for IBD: practical insights. Nat. Rev. Gastroenterol. Hepatol. 12:537–45 [DOI] [PubMed] [Google Scholar]
- 143.Bitar KN, Zakhem E. 2016. Bioengineering the gut: future prospects of regenerative medicine. Nat. Rev. Gastroenterol. Hepatol. 13:543–56 [DOI] [PubMed] [Google Scholar]
- 144.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, et al. 2013. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. PNAS 110:6506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, et al. 2015AnnexinA1-containingextracellularvesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Investig. 125:1215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, et al. 2012. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18:618–23 [DOI] [PubMed] [Google Scholar]
- 147.Cruz-Acuna R, Quir˜ os M, Farkas AE, Dedhia PH, Huang S, et al. 2017. Synthetic hydrogels for humań intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19:1326–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miyazawa M, Aikawa M, Watanabe Y, Takase K, Okamoto K, et al. 2015. Extensive regeneration of the stomach using bioabsorbable polymer sheets. Surgery 158:1283–90 [DOI] [PubMed] [Google Scholar]
- 149.Baldauf KJ, Royal JM, Kouokam JC, Haribabu B, Jala VR, et al. 2017. Oral administration of a recombinant cholera toxin B subunit promotes mucosal healing in the colon. Mucosal Immunol. 10:887–900 [DOI] [PubMed] [Google Scholar]
- 150.Cory G. 2011. Scratch-wound assay. Methods Mol. Biol. 769:25–30 [DOI] [PubMed] [Google Scholar]
- 151.Okabe S, Amagase K. 2005. An overview of acetic acid ulcer models—the history and state of the art of peptic ulcer research. Biol. Pharm. Bull. 28:1321–41 [DOI] [PubMed] [Google Scholar]
- 152.Whittem CG, Williams AD, Williams CS. 2010. Murine colitis modeling using dextran sulfate sodium(DSS). J. Vis. Exp. 35:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bruckner M, Lenz P, Nowacki TM, Pott F, Foell D, Betten worth D. 2014. Murine endoscopy for in vivo multimodal imaging of carcinogenesis and assessment of intestinal wound healing and inflammation. Vis. Exp. 90:51875. [DOI] [PMC free article] [PubMed] [Google Scholar]