Abstract
Hepatocellular carcinoma highly occurs in chronic hepatitis livers, where hepatocyte apoptosis is frequently detected. Apoptosis is a mechanism that eliminates mutated cells. Hepatocyte apoptosis induces compensatory liver regeneration, which is believed to contribute to tumor formation. Hepatocyte-specific Mcl-1 knockout mice (Mcl-1Δhep mice) developed persistent hepatocyte apoptosis and compensatory liver regeneration with increased oxidative stress in adulthood but had not yet developed hepatocyte apoptosis at the age of 2 weeks. When diethylnitrosamine (DEN) was administered to 2-week-old Mcl-1Δhep mice, multiple liver tumors were formed at 4 months, while wild-type mice did not develop any tumors. These tumors contained the B-Raf V637E mutation, indicating that DEN-initiated tumorigenesis was promoted by persistent hepatocyte apoptosis. When N-acetyl-L-cysteine was given from 6 weeks of age, DEN-administered Mcl-1Δhep mice had reduced oxidative stress and suppressed tumorigenesis in the liver but showed no changes in hepatocyte apoptosis or proliferation. In conclusion, enhanced tumor formation from DEN-transformed hepatocytes by persistent hepatocyte apoptosis is mediated by increased oxidative stress, independent of compensatory liver regeneration. For patients with livers harboring transformed cells, the control of oxidative stress may suppress hepatocarcinogenesis based on chronic liver injury.
Subject terms: Hepatology, Liver diseases
Introduction
Hepatocellular carcinoma (HCC) is the major form of primary liver cancer and is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths owing to its poor 5-year survival rate1. Approximately 30–40% of HCC patients are suitable to undergo potential curative therapies (e.g., surgical resection, radiofrequency ablation (RFA), transarterial chemoembolization (TACE) and liver transplantation); however, the remaining 60–70% of patients are eligible for only palliative treatment2. Although some molecular targeted drugs have been developed3,4, there is still a strong need for novel treatments or preventive strategies for HCC.
The strongest risk factor for HCC is chronic liver disease, a condition that is related to liver inflammation caused by exposure to one or several risk factors, including hepatitis B virus (HBV), hepatitis C virus (HCV), excessive consumption of alcohol, and metabolic syndrome5. Other cofactors, such as tobacco smoke inhalation and intake of aflatoxin B1, are well-characterized contributors to HCC6. Independent of the underlying etiology, all chronic liver diseases exhibit persistent hepatocyte damage with serum alanine aminotransferase (ALT) elevation. Hepatocyte apoptosis is a type of cell death frequently observed in human chronic liver diseases7. In livers with chronic liver diseases, various factors, such as oxidative stress, ER stress, HCV core protein, HBx protein or genome integration by HBV, induce the malignant transformation of hepatocytes8–11. These transformed cells should be eliminated by apoptosis to result in the suppression of tumor formation. On the other hand, hepatocyte apoptosis leads to compensatory liver regeneration, which is believed to contribute to tumor formation12. It is unclear whether hepatocyte apoptosis eventually suppresses or enhances tumor formation in livers harboring transformed cells.
Apoptosis was originally thought to have a tumor suppressor action to remove tumor cells, but since the enhancement of apoptosis in the liver causes compensatory liver regeneration, it was also related to the growth progression of tumor cells. Mcl-1 is an anti-apoptotic bcl-2 family proteins. In hepatocytes, Mcl-1 protects against mitochondrial pathway-induced apoptosis by inhibiting Bak/Bax activation through cooperation with Bcl-xL, another anti-apoptotic bcl-2 family proteins13. Hepatocyte-specific deficiency of Mcl-1 as well as Bcl-xL in mice results in persistent hepatocyte apoptosis by Bak/Bax activation in adulthood13–15. In the present study, we used hepatocyte-specific Mcl-1 knockout mice to evaluate the effect of hepatocyte apoptosis on tumor formation. Here, we show that the enhancement of oxidative stress associated with apoptosis may promote tumor progression independent of compensatory liver regeneration.
Results
DEN-induced tumor formation is promoted in Mcl-1Δhep mice
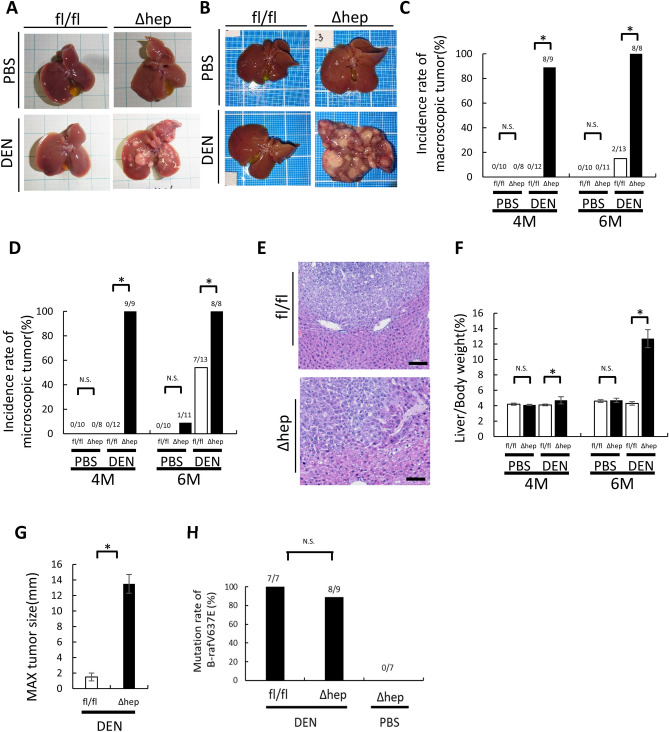
To clarify the effect of hepatocyte apoptosis on tumor formation, we injected diethylnitrosamine (DEN) into two-week-old hepatocyte-specific Mcl-1 knockout mice (Mcl-1Δhep mice), which showed persistent hepatocyte apoptosis in adulthood13. Six months after DEN injection, 54% (7/13) of the DEN-injected wild-type mice developed microscopic liver tumors, and 15% (2/13) of them developed macroscopic liver tumors (Fig. 1B,C,D,E). All developed liver tumors had the B-Raf V637E mutation (Fig. 1H), which is characteristic of DEN-induced liver tumors16. In contrast, all (8/8) DEN-injected Mcl-1Δhep mice developed microscopic and macroscopic liver tumors (Fig. 1B,C,D,E). Most liver tumors also had the B-Raf V637E mutation (Fig. 1H). The liver/body weight ratio and maximum tumor size were significantly higher in the DEN-injected Mcl-1Δhep mice than in the DEN-injected wild-type mice (Fig. 1F,G). Four months after DEN injection, the incidence rates of microscopic and macroscopic liver tumors were also higher in the DEN-injected Mcl-1Δhep mice than in the DEN-injected wild-type mice (Fig. 1A,C,D). We previously reported that Mcl-1Δhep mice developed liver tumors at the age of 1 year17,18. Indeed, one of eleven phosphate buffered saline (PBS)-injected Mcl-1Δhep mice developed microscopic HCC 6 months after PBS injection (Fig. 1D). However, none of the liver tumors that developed in Mcl-1Δhep mice at the age of 1 year had the B-Raf V637E mutation (Fig. 1H).
Figure 1.
Mcl-1 deficiency enhanced DEN-induced liver tumor development. At the age of 2 weeks, male Mcl-1fl/fl and Mcl-1Δhep mice were intraperitonially injected with PBS or DEN (20 mg/kg BW), and their livers were harvested after 4 or 6 months. (A,B) Representative images of the livers of PBS- or DEN-treated male Mcl-1 fl/fl and Mcl-1Δhep mice at the ages of 4 months (A) and 6 months (B). (C,D) Incidence rate of macroscopic (C) or microscopic (D) tumors at the age of 4 and 6 months (n ≥ 8 per group, chi-squared test). (E) Representative H&E staining images of liver sections from the DEN-treated male 6-month-old Mcl-1fl/fl and 6-month-old Mcl-1Δhep mice. The scale bar is 100 µm. (F) Ratio of liver weight to body weight (mean ± SEM, Mann–Whitney test). (G) Maximum tumor size (diameters by caliper) at the age of 6 months (mean ± SEM, Mann–Whitney test). (H) Mutation rate of B-Raf V637E in liver tumors (chi-squared test, *p < 0.05).
Hepatocyte apoptosis and compensatory liver regeneration were observed in DEN-induced Mcl-1Δhep mice after 4 weeks of age
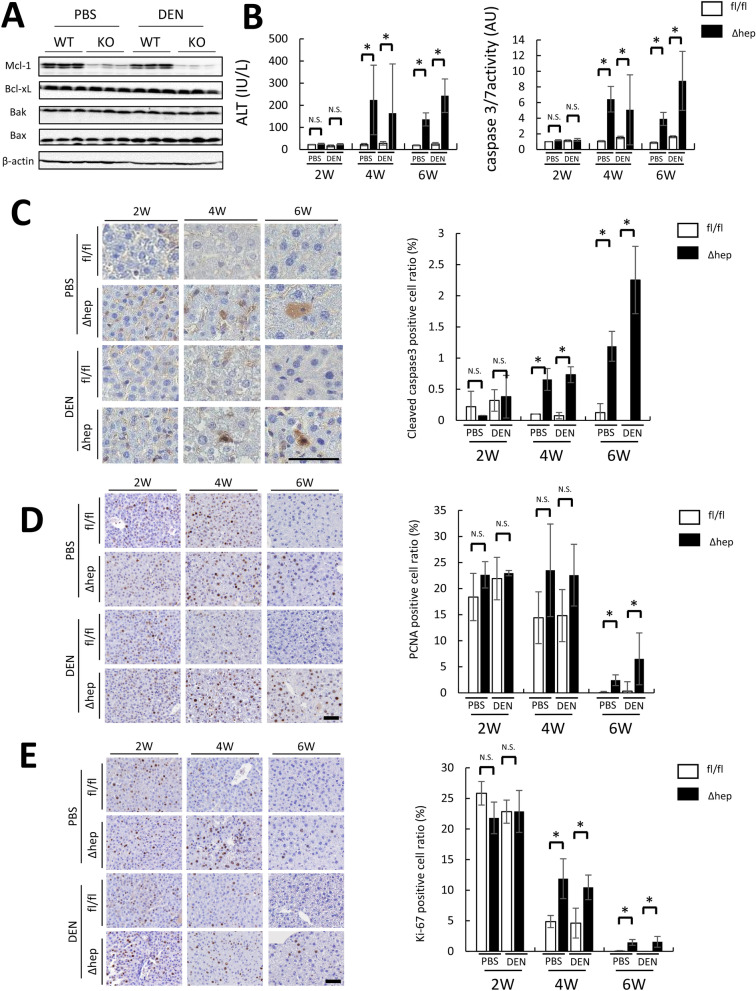
Mcl-1 is an anti-apoptotic protein, and we previously reported that hepatocyte-specific Mcl-1 deficiency induced continuous hepatocyte apoptosis after 6 weeks of age, leading to the promotion of liver regeneration13,17. To elucidate the underlying mechanism of promoted tumor formation in DEN-treated Mcl-1Δhep mice, we first examined hepatocyte apoptosis, liver regeneration and DNA damage after 2 weeks of age when DEN was administered. We confirmed that the expression levels of Mcl-1 decreased in Mcl-1Δhep mice at the age of 2 weeks and that DEN treatment did not affect Mcl-1 expression (Fig. 2A). At the age of 2 weeks, there were no differences in the serum ALT levels, serum caspase-3/7 activity, or cleaved caspase-3-positive hepatocyte ratios between wild-type and Mcl-1Δhep mice regardless of DEN administration (Fig. 2B,C), indicating that hepatocyte apoptosis was not enhanced in Mcl-1Δhep mice. At the ages of 4 and 6 weeks, the cleaved caspase-3-positive hepatocyte ratios, serum ALT levels and caspase-3/7 activity were higher in Mcl-1Δhep mice than in wild-type mice with or without DEN injection, indicating that Mcl-1 deficiency in hepatocytes induced persistent hepatocyte apoptosis after 4 weeks (Fig. 2B,C). There were no differences in the PCNA-positive hepatocyte ratios or Ki-67-positive hepatocyte rates between wild-type and Mcl-1Δhep mice with or without DEN injection at the age of 2 weeks, while they were higher at the age of 6 weeks (Fig. 2D,E).
Figure 2.
Mcl-1 deficiency resulted in increased hepatocyte apoptosis and subsequent compensatory liver regeneration after 4 weeks of age. Two-week-old male Mcl-1fl/fl and Mcl-1Δhep mice injected with PBS or DEN (20 mg/kg BW) were sacrificed at 2, 4 and 6 weeks of age. (A) Representative immunoblots of the indicated proteins in the liver lysates of two-week-old mice. Full-length blots/gels are presented in Supplementary Fig. 1. (B) Serum ALT levels and caspase 3/7 activity (n ≥ 8 per group, mean ± SEM, Mann–Whitney test). (C–E) Representative images of liver sections stained with anti-cleaved caspase-3 antibody (C), PCNA antibody, and Ki-67 antibody (E) (left) (n ≥ 4 per group). The scale bar is 50 µm. Each positive hepatocyte ratio was quantified and is shown as a percentage (right) (mean ± SEM, Mann–Whitney test, *p < 0.05).
Mcl-1Δhep mice showed persistent DEN-induced DNA damage with increased hepatocyte oxidative stress
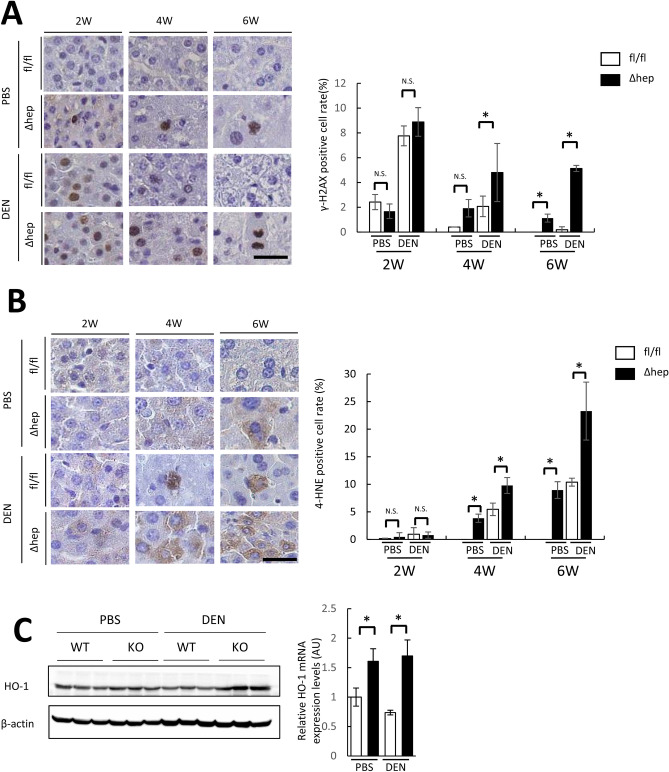
DEN is a potent alkylating agent that induces DNA damage upon conversion into alkyldiazohydroxide by cytochrome P450. DEN injection (2 weeks after birth) increased the positive hepatocyte ratio of γ-H2AX, which is one of central component in DNA repair and exerts its actions at nascent DNA double strand break sites19, regardless of the Mcl-1 genotype (Fig. 3A). DNA double strand break are repaired by DNA repair system19. The DEN-induced high γ-H2AX positive cell rate decreased in the time course (Fig. 3A), consistent with a previous report20. At 6 weeks of age, the positive hepatocyte ratio of γ-H2AX in the Mcl-1Δhep mice was significantly higher than that in the wild-type mice (Fig. 3A).
Figure 3.
Mcl-1 deficiency impaired DNA damage repair with elevation of oxidative stress. Two-week-old male Mcl-1fl/fl and Mcl-1Δhep mice injected with PBS or DEN (20 mg/kg BW) were sacrificed at 2, 4 and 6 weeks of age. (A,B) Representative images of liver sections stained with anti-γ-H2AX (A) or anti-4-HNE (B) antibody (left). The scale bar is 50 µm. γ-H2AX-positive and 4-HNE-positive hepatocyte ratios were quantified and are shown as percentages (right) (n ≥ 4 per group, mean ± SEM, Mann–Whitney test, *p < 0.05). (C) Representative immunoblots of HO-1 in the liver of six-week-old mice (left), and expression levels of HO-1 by real- time PCR in the liver of six-week-old mice (right) (n = 8 per group, mean ± SEM, Mann–Whitney test, *p < 0.05). Full-length blots/gels are presented in Supplementary Fig. 2.
Next, we evaluated oxidative stress in the liver, which was previously reported to increase in Mcl-1Δhep mouse livers in adulthood17. The 4-hydroxy-2-nonenal (4-HNE)-positive hepatocyte ratio, a marker of lipid peroxidation, was significantly higher in the Mcl-1Δhep mice than in the wild-type mice (Fig. 3B). The expression levels of heme oxygenase-1 (HO-1), which is induced by oxidative stress though Nrf2 activation21, were significantly higher in DEN-treated the Mcl-1Δhep mice than in the DEN-treated wild-type mice (Fig. 3C).
NAC treatment suppresses tumor formation in DEN-treated Mcl-1Δhep mice with a reduction in DNA damage
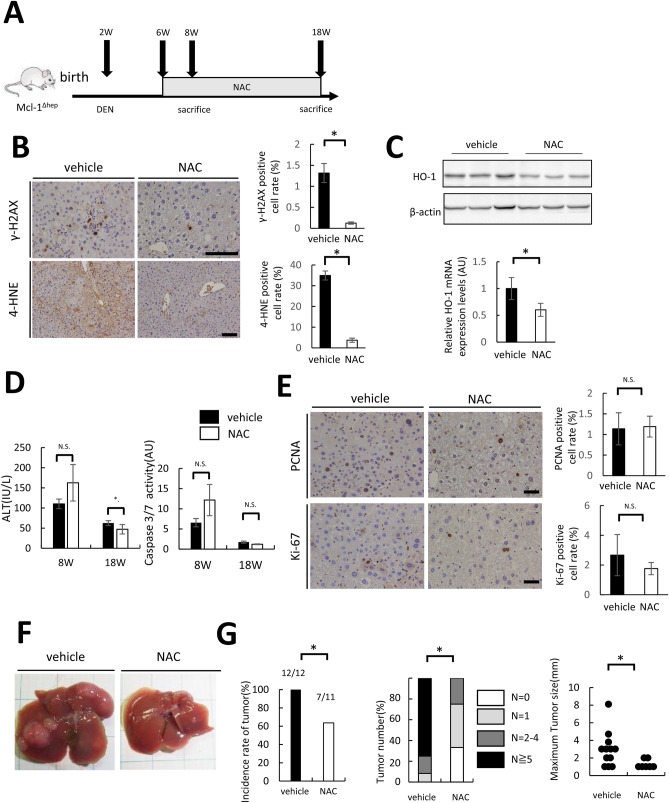
To examine the significance of oxidative stress on the tumor formation of DEN-injected mice, we administered N-acetyl-L-cysteine (NAC) in drinking water for 12 weeks to DEN-treated Mcl-1 KO mice from the age of 6 weeks, which was 4 weeks after DEN injection. Then, we explored the phenotype of these mice at the age of 18 weeks (Fig. 4A). While there was a significant decrease in the 4-HNE-positive cell ratio and the HO-1 expression levels in the NAC group (Fig. 4B, 4C), there were no significant differences in the serum caspase-3/7 activity between the vehicle group and the NAC group (Fig. 4D). The PCNA- and Ki-67-positive hepatocyte ratios also did not differ between the vehicle group and the NAC group (Fig. 4E). In contrast, NAC treatment significantly decreased the phospho-H2AX-positive hepatocyte ratio (Fig. 4B). While all 12 mice treated with vehicle developed macroscopic liver tumors, only 7 of 11 mice treated with NAC did (Fig. 4F,G). The frequency of multiple liver tumors and the maximum size of liver tumors were significantly decreased in the NAC group compared with the vehicle group (Fig. 4G).
Figure 4.
NAC administration significantly decreased liver tumor formation via the improvement of DNA damage in DEN-treated Mcl-1 KO mice. Two-week-old male Mcl-1Δhep mice injected with DEN (20 mg/kg BW) were administered NAC in drinking water from the age of 6 weeks. (A) Schematic of the experimental design. (B) Representative images of liver sections stained with anti- γ-H2AX or anti-4-HNE antibody (left). The scale bar is 100 µm. Each positive hepatocyte ratio was quantified and is shown as a percentage (right) (N = 7 or more per group, mean ± SEM, Mann–Whitney test). (C) Serum ALT levels and caspase-3/7 activity at the indicated ages (n = 8 or more per group, mean ± SEM, Mann–Whitney test, *p < 0.05). (D) Representative immunoblots of HO-1 in the liver (upper), and expression levels of HO-1 by real-time PCR in the liver (lower) (n = 8 per group, mean ± SEM, Mann–Whitney test, *p < 0.05). Full-length blots/gels are presented in Supplementary Fig. 3. (E) Representative images of liver sections stained with anti-PCNA or Ki-67 antibody (left). The scale bar is 50 µm. Each positive hepatocyte ratio was quantified and is shown as a percentage (right) (n ≥ 7 per group, mean ± SEM, Mann–Whitney test). (F) Representative images of mice with the indicated genotype at the age of 18 weeks. (G) Incidence rate of tumors (chi-squared test), incidence rate of tumors by tumor number (chi-squared test), maximum size of liver tumors (Mann–Whitney test, *p < 0.05).
Discussion
DEN is a well-known chemical carcinogen that induces liver tumors in mice. In the present study, we injected DEN into mice, which was followed by persistent hepatocyte apoptosis by the ablation of Mcl-1, an anti-apoptotic protein. We demonstrated that DEN-induced, B-Raf-driven, liver tumor formation is promoted by persistent hepatocyte apoptosis. The promotion was suppressed by oxidative stress reduction without any change in hepatocyte apoptosis or compensatory liver regeneration.
We previously reported that persistent hepatocyte apoptosis causes gene mutations via oxidative stress, which is related to liver tumor development18. In the present study, we demonstrated that persistent hepatocyte apoptosis-induced oxidative stress accelerates tumor formation in livers harboring transformed hepatocytes. Collectively, oxidative stress in livers with persistent hepatocyte apoptosis works at both points of hepatocyte transformation and accelerates tumor formation after acquiring transformation. Gentric et al22 reported that antioxidant treatment restored hepatocyte pathological polyploidization, so they concluded that oxidative stress promoted pathological polyploidization, which was suggested to be an early event in the hepatocarcinogenesis of nonalcoholic fatty liver disease (NAFLD). Maeda et al23 reported that hepatocyte-specific IkKβ deletion increased oxidative stress and enhanced DEN-induced liver tumor development. In Ma’s report24, ROS decreased CD4 + T lymphocytes, which promoted hepatocarcinogenesis in NAFLD livers. Oxidative stress promotes liver tumor formation through various pathways.
It has been reported that DEN-induced liver tumor formation in mice is suppressed by the deficiency of BH3-only protein, Puma25, Bid26, or Bok27. In these reports, based on the results that Puma, Bid or Bok deficiency suppressed both hepatocyte apoptosis and ompensatory liver regeneration after DEN injection, it is speculated that hepatocyte apoptosis or compensatory liver regeneration contributes to the acceleration of DEN-induced liver tumor formation. Consistent with these reports, in the present study, hepatocyte apoptosis efficiently promoted DEN-induced liver tumor formation. However, we clearly demonstrated that the promoting effect was attenuated by antioxidants without any change in hepatocyte apoptosis or liver regeneration. Based on our findings, since hepatocyte apoptosis generates oxidative stress18, we speculate that Puma, Bid, and Bok deficiency reduces not only hepatocyte apoptosis but also oxidative stress in the livers, which may be involved in the suppressive mechanism of DEN-induced tumors in Puma-, Bid-, and Bok-deficient mice.
In clinical settings, it has been previously reported that serum ALT levels are a risk factor for HCC incidence in patients with various chronic liver diseases, including viral hepatitis28 and nonviral hepatitis29. Given that the presence of hepatocyte apoptosis is reflected as high serum ALT, one of the reasons high ALT is a risk for the development of HCC may be that persistent hepatocyte apoptosis accelerates the process from the appearance of transformed cells to tumor formation. From this viewpoint, it is important to aim to normalize ALT in patients with chronic liver disease. Recently, HCV has been eliminated with direct-acting antiviral (DAA) treatment in most cases30. However, some genetic or epigenetic changes have been detected in HCV-eliminated cirrhotic livers31,32. HBV DNA levels can be maintained at undetectable levels by nucleos(t)ide analog (NA) treatment33; however, inserted HBV DNA cannot be eliminated by NA treatment. In these patients already treated or undergoing treatment for chronic viral hepatitis, from a carcinogenic point of view, although ALT elevation due to viral infection is controlled, attention should also be paid to ALT elevation due to other factors, such as metabolic dysfunction-associated fatty liver disease and alcoholic liver disease. For patients whose ALT elevation cannot be controlled, even if the patients’ hepatocytes already have a genetic mutation, treatments with antioxidants may be an option for delaying liver cancer development.
In conclusion, persistent hepatocyte apoptosis accelerates tumor formation in livers harboring DNA-damaged hepatocytes via an increase in oxidative stress, which is independent of compensatory liver regeneration. For these livers, apoptosis no longer plays a positive role in preventing liver tumors but works negatively.
Materials and methods
Mice
Hepatocyte-specific Mcl-1 knockout mice (Mcl-1Δhep mice) were generated by crossing Mcl-1fl/fl with albumin-Cre transgenic mice, as described previously13. We injected a single intraperitoneal dose of DEN (Sigma-Aldrich) (20 mg/kg) into 2-week-old male mice to induce a gene alteration in hepatocytes. As a control for DEN injection, we injected PBS. In the NAC treatment group, hepatocyte-specific Mcl-1 KO mice were given NAC (Sigma-Aldrich) water at a dose of 1 g/L from 6 to 8 weeks or 18 weeks and then sacrificed. The mice were maintained in a specific pathogen-free facility and treated with humane care. All mouse experiments were approved by the Animal Care and Use Committee of Osaka University Medical School (30-015), and we performed all mouse experiments in accordance with the approved protocol. All experiments were carried out in compliance with the ARRIVE guideline, the relevant guidelines and regulations.
HE staining and immunohistochemistry
Freshly dissected liver samples were fixed in 10% formalin for 24 h and embedded in paraffin. For hematoxylin and eosin (HE) staining, 4-µm tissue sections were stained with HE. For immunohistochemistry, cleaved caspase-3, γ-H2AX, 4-HNE, PCNA and Ki-67 were labeled in paraffin-embedded liver sections using an anti-cleaved caspase-3 antibody, anti-PCNA antibody, anti-Ki-67 antibody (Cell Signaling Technology), and anti-4-HNE antibody (Abcam), respectively. The detection of immunolabeled proteins was performed using an avidin–biotin complex with the Vectastain ABC Kit (Vector Laboratories). Four visual fields of a magnified image (× 20 or × 40) were randomly selected for each immune-stained section, and the positive cell ratio was calculated.
Serum ALT levels and caspase-3/7 activity measurement
For measurement of serum ALT levels and caspase-3/7 activity, blood was collected from the inferior vena cava of the mice. After centrifugation, serum was stored at − 20 °C until use. Serum ALT levels were measured at the Oriental Kobo Life Science Laboratory (Nagahama, Japan). Serum caspase-3/7 activity was measured using a luminescent substrate assay for caspase-3 and caspase-7 (Caspase-Glo assay, Promega, Tokyo, Japan).
Detection of B-Raf mutation
To detect B-Raf mutations in liver tumors, we performed direct sequencing as previously published. In brief, DNA was extracted from frozen materials with the DNeasy Blood and Tissue Kit (Qiagen). The region that included the B-Raf codon 637 was amplified by PCR using appropriate primers (forward: 5′-gacctcacggtaaaaataggtgac-3′; reverse: 5′-gcaattatgcctggcttacaa-3′) and Platinum PCR SuperMix High Fidelity (Invitrogen). The PCR products were purified with the Genomic DNA Purification Kit (Promega) and sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) on an Applied Biosystems 3730 DNA Analyzer (Thermo Fisher Scientific).
Western blots
Murine liver lysates were prepared for Western blots as previously described34. We used following primary antibodies for detection of the specific proteins: Mcl-1 (#5453, Cell Signaling Technology), Bcl-xL (#2764, Cell Signaling Technology), Bak (#3814, Cell Signaling Technology), Bax (#2772, Cell Signaling Technology), HO-1(#43966, Cell Signaling Technology) and beta actin (A5316, Sigma-Aldrich). We detected the signals using Fusion Solo S (Vilber Lourmat, Collegien, France).
Real-time PCR
Complementary DNA form murine livers was prepared as previously described34.
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) were performed using Thunderbird qPCR master mix (Toyobo, Osaka, Japan) and TaqMan probes (Thermo Fisher Scientific). We used following probes; HO-1 (Mm00516005_m1) and Actb (Mm02619580_g1). HO-1 expression levels were normalized to beta-actin expression levels.
Statistical analysis
Statistical analysis was performed using JMP software. The data are shown as the mean ± standard error of the mean unless otherwise indicated. Comparisons of continuous variables between wild-type mice and Mcl-1Δhep mice were performed using the Mann–Whitney test. Comparisons of noncontinuous variables between wild-type mice and Mcl-1Δhep mice were performed using the chi-squared test. P < 0.05 was considered significant.
Supplementary Information
Acknowledgements
This study was partly supported by a Grant-in-Aid for Research on Hepatitis from the Japanese Agency for Medical Research and Development (JP 20fk0210064; to T.Tak.) and JSPS KAKENHI (Grant Number JP18H02795 to T.Tak. and JP 20K08307 to H.H.).
Abbreviations
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- DEN
Diethylnitrosamine
- 4-HNE
4-Hydroxy-2-nonenal
- HO-1
Heme oxygenase-1
- NAC
N-acetyl-L-cysteine
Author contributions
Y.N., H.H., T.K., T.Tat. and T.Tak. designed this study. Y.N., H.H., and T.Tak. wrote the manuscript. Y.N., S.T., K.F., Y.M., S.S. and Y.S. performed the tumor formation analysis in mice. Y.N., M.U., K.S., A.D. K.M. and R.S. performed the histology analysis, immunostaining, protein expression analysis and genomic mutation analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83082-7.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol. Res. 2018;48:597–607. doi: 10.1111/hepr.13181. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda K. Recent advances in medical management of hepatocellular carcinoma. Hepatol. Res. 2019;49:14–32. doi: 10.1111/hepr.13259. [DOI] [PubMed] [Google Scholar]
- 5.Baumeister SE, et al. Association between physical activity and risk of hepatobiliary cancers: a multinational cohort study. J. Hepatol. 2019;70:885–892. doi: 10.1016/j.jhep.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Hepatocellular carcinoma. Nat Rev Dis Primers2, 16019 (2016). [DOI] [PubMed]
- 7.Boege Y, et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell. 2017;32:342–359.e310. doi: 10.1016/j.ccell.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz K, et al. Ccne1 overexpression causes chromosome instability in liver cells and liver tumor development in mice. Gastroenterology. 2019;157:210–226.e212. doi: 10.1053/j.gastro.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa H, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoudvand S, Shokri S, Taherkhani R, Farshadpour F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J. Gastroenterol. 2019;25:42–58. doi: 10.3748/wjg.v25.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Berasain C, Avila MA. The EGFR signalling system in the liver: from hepatoprotection to hepatocarcinogenesis. J. Gastroenterol. 2014;49:9–23. doi: 10.1007/s00535-013-0907-x. [DOI] [PubMed] [Google Scholar]
- 13.Hikita H, et al. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–1226. doi: 10.1002/hep.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takehara T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Hikita H, et al. BH3-only protein bid participates in the Bcl-2 network in healthy liver cells. Hepatology. 2009;50:1972–1980. doi: 10.1002/hep.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, et al. Role of the BrafV637E mutation in hepatocarcinogenesis induced by treatment with diethylnitrosamine in neonatal B6C3F1 mice. Mol. Carcinog. 2017;56:478–488. doi: 10.1002/mc.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hikita H, et al. Bak deficiency inhibits liver carcinogenesis: a causal link between apoptosis and carcinogenesis. J. Hepatol. 2012;57:92–100. doi: 10.1016/j.jhep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Hikita H, et al. Activation of the mitochondrial apoptotic pathway produces reactive oxygen species and oxidative damage in hepatocytes that contribute to liver tumorigenesis. Cancer Prev. Res. (Phila) 2015;8:693–701. doi: 10.1158/1940-6207.CAPR-15-0022-T. [DOI] [PubMed] [Google Scholar]
- 19.Bonner WM, et al. GammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guedj A, et al. Early age decline in DNA repair capacity in the liver: in depth profile of differential gene expression. Aging (Albany NY) 2016;8:3131–3146. doi: 10.18632/aging.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentric G, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Invest. 2015;125:981–992. doi: 10.1172/JCI73957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu W, et al. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology. 2011;54:1249–1258. doi: 10.1002/hep.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wree A, et al. Hepatocyte-specific Bid depletion reduces tumor development by suppressing inflammation-related compensatory proliferation. Cell Death Differ. 2015;22:1985–1994. doi: 10.1038/cdd.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabachini T, et al. BOK promotes chemical-induced hepatocarcinogenesis in mice. Cell Death Differ. 2018;25:708–720. doi: 10.1038/s41418-017-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CF, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141(1240–1248):1248.e1241–1242. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Bhala N, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioannou GN, Feld JJ. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology. 2019;156:446–460.e442. doi: 10.1053/j.gastro.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Kim SK, et al. Comprehensive analysis of genetic aberrations linked to tumorigenesis in regenerative nodules of liver cirrhosis. J. Gastroenterol. 2019;54:628–640. doi: 10.1007/s00535-019-01555-z. [DOI] [PubMed] [Google Scholar]
- 32.Hamdane N, et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology. 2019;156:2313–2329.e2317. doi: 10.1053/j.gastro.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J. Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Saito, Y. et al. DNase II activated by the mitochondrial apoptotic pathway regulates RIP1-dependent non-apoptotic hepatocyte death via the TLR9/IFN-β signaling pathway. Cell Death Differ.26, 470–486 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.