Abstract
Background
Gastric cancer (GC) is one of the most common carcinomas of the digestive tract, and the prognosis for these patients may be poor. There is evidence that some long non-coding RNAs(lncRNAs) can predict the prognosis of patients with GC. However, few lncRNA signatures have been used to predict prognosis. Herein, we aimed to construct a risk score model based on the expression of five lncRNAs to predict the prognosis of patients with GC and provide new potential therapeutic targets.
Methods
We performed differentially expressed and survival analyses to identify differentially expressed survival-ralated lncRNAs by using GC patient expression profile data from The Cancer Genome Atlas (TCGA) database. We then established a formula including five lncRNAs to predict the prognosis of patients with GC. In addition, to verify the prognostic value of this risk score model, two independent Gene Expression Omnibus (GEO) datasets, GSE62254 (N = 300) and GSE15459 (N = 200), were employed as validation groups.
Results
Based on the characteristics of five lncRNAs, patients with GC were divided into high or low risk subgroups. The prognostic value of the risk score model with five lncRNAs was confirmed in both TCGA and the two independent GEO datasets. Furthermore, stratification analysis results showed that this model had an independent prognostic value in patients with stage II–IV GC. We constructed a nomogram model combining clinical factors and the five lncRNAs to increase the accuracy of prognostic prediction. Enrichment analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) suggested that the five lncRNAs are associated with multiple cancer occurrence and progression-related pathways.
Conclusion
The risk score model including five lncRNAs can predict the prognosis of patients with GC, especially those with stage II-IV, and may provide potential therapeutic targets in future.
Keywords: Long non-coding RNA, Gastric cancer, Prognosis, LINC00205, TRHDE-AS1, OVAAL, LINC00106, MIR100HG
Introduction
Gastric cancer (GC) is one of the most common carcinomas of the gastrointestinal (GI) tract and is particularly prevalent in Asian countries. It is estimated that approximately 679,100 individuals were diagnosed with GC in 2015 in China and approximately 498,000 of them died that same year (Saka et al., 2011; Chen et al., 2016). The standard therapies for GC are surgery and chemotherapy. However, most patients with advanced GC show recurrence of the malignancy and metastasis after treatment, resulting in poor prognosis. Despite considerable research in therapies for GC, the prospects of survival of patients with GC remain bleak (Saka et al., 2011). The identification of patients with GC with poor prognosis and the administration of effective treatment as early as possible are key to improving survival. The investigation of potential therapeutic and prognostic biomarkers for GC is of considerable importance.
Long non-coding RNAs (lncRNAs) are RNAs with lengths of ≥200 nucleotides with no or limited protein-coding potential. There is considerable evidence that lncRNAs play crucial roles in the initiation and developments of cancers. For example, lncRNA-ATB disorders contribute to cancer cell proliferation, migration, invasion, and drug-resistance as well as induce epithelial-mesenchymal transition by competitively binding to microRNAs (Li et al., 2017; Balas & Johnson, 2018). Some researchers have suggested that lncRNAs serve as new prognostic biomarkers in various cancers, including CCAT2 (Yu et al., 2017), HOXB-AS3 (Huang et al., 2017), and ASLNC07322 (Li et al., 2019) in colon cancer. Many lncRNAs closely related to the prognosis of patients with GC have been identified, including MEG3 (Wei & Wang, 2017), SNHG7 (Wang et al., 2017), and DANCR (Mao et al., 2017). Risk score models have also been constructed to predict the prognosis of human cancers. The differences in prognosis in non–small-cell lung cancer can be identified by its eight-lncRNA signature (Miao et al., 2019). However, the identification of lncRNAs related to the prognosis of patients with GC is still in its early stages and additional research is warranted.
In this study, we analyzed the data of 450 patients with GC from The Cancer Genome Atlas (TCGA) database to identify differentially expressed lncRNAs for the prognostic prediction. We used two independent Gene Expression Omnibus (GEO) (Barrett et al., 2013) datasets to validate the selected-lncRNAs. In addition, we analyzed the accuracy of the prediction of five lncRNAs in different clinical subgroups using lncRNA data in combination with the clinical characteristics of the patients. Furthermore, we constructed a nomogram model by combining clinical factors and five lncRNAs to increase the accuracy of prognostic prediction. Finally, we performed pathway enrichment analysis to determine the potential functions of these lncRNAs in GC.
Materials & Methods
Preparation of GC datasets
We acquired a training dataset of GC samples from TCGA at UCSC Xena (https://xenabrowser.net/) before February 1st, 2019 comprising 450 samples and 14147 lncRNAs (case: normal = 414:36). We used these 450 samples to perform differential expression analysis. After excluding six cases with missing overall survival (OS) prognostic information, 408 cases were included for further univariate Cox proportional hazard regression analysis and subsequent analysis in the training group. The microarray data for the validation group and survival data of the patients are publicly available at GEO with accession numbers GSE62254 (N = 300; 1397 lncRNAs) and GSE15459 (N = 200; 1397 lncRNAs).
Normalization of GEO data
Because the two GEO datasets (GSE62254 and GSE15459) had different expression profiles, we performed quantile normalization on the original data and downloaded it as a probe-level CEL file. Affymetrix U133 Plus 2.0 was used as the probe matching platform. Data were downloaded from the Affymetrix website (http://www.affymetrix.com), and a total of 2986 lncRNA-specific probes were included.
Construction of an lncRNA-based risk score model from the training group
The lncRNAs that were differentially expressed between GC and normal gastric tissue in TCGA dataset were identified using the “limma” R package of the R statistical computing environment (log2—fold change— >1 and adjusted P < 0.05), and the adjusted P value was used to reduce false positives (Deng, Xu & Wang, 2019; Zeng et al., 2019). The candidate lncRNAs were analyzed using univariate Cox proportional hazard regression analysis (P < 0.05). The cutoff values of lncRNA expression were determined as the median of all expression values in Cox survival analysis. In total, we identified 278 lncRNAs with statistically significant differences. After identifying the lncRNAs common to both TCGA and GEO (GSE62254) datasets, we performed multivariate Cox proportional hazards analysis to identify independent prognostic lncRNAs. Finally, we constructed an lncRNA-based risk score model from a linear combination of the expression levels of these lncRNAs, multiplied by the regression coefficients obtained from the multivariate Cox proportional hazards regression analysis.
Validation of the lncRNA-based model for prognostic prediction
We calculated the risk scores of each patient and used the corresponding median score as the cutoff value to classify them into two groups: high risk and low risk subgroups. We used Kaplan–Meier analysis to compare the survival of the two groups and time-dependent receiver operating characteristic (ROC) curves to assess our lncRNA-based risk model. We used two GEO datasets to validate the model for prognostic prediction. Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) of the model with 95% confidence intervals to further evaluate the predictive value of the model for each clinical subgroup. Clinical subgroups were determined based on sex, tumor–node–metastases (TNM) stage, histologic grade, race, and age. Finally, we constructed a nomogram combining the model with clinical factors using the “rms” package. We also calculated the concordance index (C-index) and plotted a calibration curve to determine its predictive accuracy and discriminatory capacity.
Potential functions of the five lncRNAs
To determine the potential functions of the five lncRNAs, which appeared to be discriminatory, we performed linear regression analysis of the relationship between the lncRNAs and the protein-coding genes in TCGA dataset. The screening criteria for the protein-coding genes were a positive association with at least one lncRNA (Pearson coefficient > 0.4). After identifying the candidate genes, we screened out aberrantly activated signaling pathways using the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis via web-based Gene Set Analysis Toolkit (http://www.webgestalt.org/), a popular software tool for functional enrichment analysis related to KEGG pathways (Yang et al., 2019; Wang et al., 2013).
Statistical analysis
We used the R software (version 3.6.1) for statistical analyses. Differentially expressed analysis was performed using the “limma” R package. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify prognosis-related lncRNAs. The “survival” and “survminer” packages were used for Cox proportional hazards regression analyses, Kaplan–Meier survival analysis and calculation of C-index. A time-dependent ROC curve to assess the specificity and sensitivity of the risk score model was constructed using the “survivalROC” package. A nomogram combining the risk score model with the clinical factors was constructed using the “rms” package. The Review Manager software (version 5.3) was used to construct a forest plot. Chi-square tests were used to compare the recurrence and mortality rates between the high and low risk subgroups. A P-value of <0.05 was considered statistically significant, and all tests were two sided. Pearson’s linear regression analysis was used to determine the relationship between lncRNAs and protein-coding genes.
Results
Identification of five prognostic lncRNAs
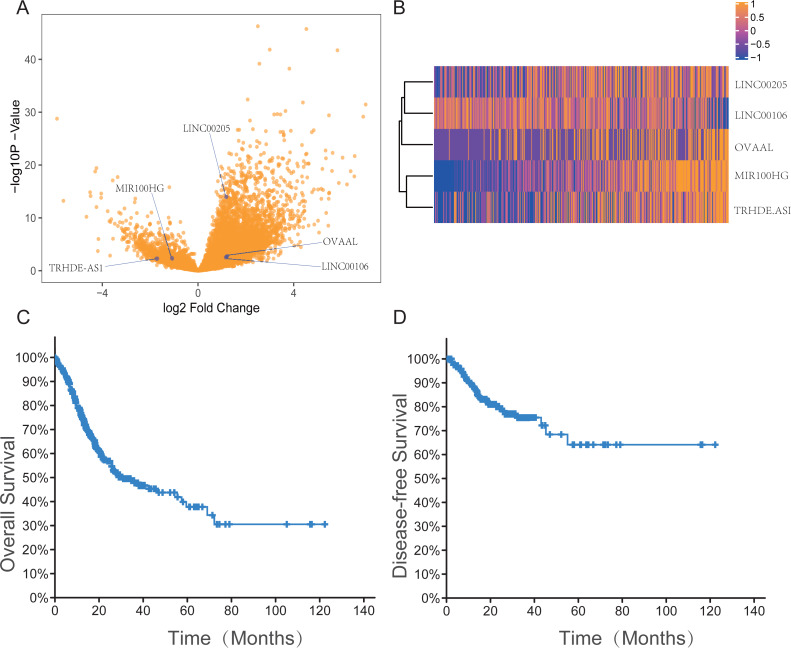
The datasets are publicly available and recruitment has already happened. We performed differentially expressed analysis (log2—fold change— >1 and adjusted P < 0.05) and univariate Cox proportional hazard regression analysis (P < 0.05) to identify survival-related lncRNAs. A total of 278 lncRNAs were analyzed further. To validate the predictive accuracy, we compared the lncRNAs selected from TCGA database with the GEO validation group. We found that 37 lncRNAs were common between the 278 lncRNAs and the validation dataset (GSE62254) (Table S1). Multivariate Cox proportional hazards regression analyses identified five lncRNAs as independent prognostic factors of GC: LINC00205, TRHDE-AS1, OVAAL, LINC00106, and MIR100HG (Table 1). Figs. 1A–1B shows the expression profiles of the five lncRNAs in patients with GC as volcano and heat maps, and Figs. 1C–1D shows the survival curves based on the OS and disease-free survival (DFS) of the 408 patients. Owing to the lack of clinical data in GSE15459, Table 2 shows the clinical features of patients with GC in the training group and GSE62254.
Table 1. Five lncRNAs significantly associated with prognosis of GC patients in the training group.
Derived from the multivariable Cox proportional hazards regression analysis in the training group.
| LncRNA name | Ensemble ID | Chr. | Coordinate | Coefficient | Hazard ratio | P value |
|---|---|---|---|---|---|---|
| LINC00205 | ENSG00000223768.1 | 21 | 45288052–45297354 | 0.249092 | 1.373451497 | 0.047216345 |
| TRHDE-AS1 | ENSG00000236333.3 | 12 | 72253507–72273509 | 0.182045 | 1.846654514 | 0.000109193 |
| OVAAL | ENSG00000236719.2 | 1 | 180558974–180566518 | 0.271169 | 1.880897277 | 0.0000744 |
| LINC00106 | ENSG00000236871.6 | X&Y | 1397025–1399412 | −0.207942 | 0.624972486 | 0.003469142 |
| MIR100HG | ENSG00000255248.6 | 11 | 122028329-122422871 | 0.502539 | 1.396343319 | 0.036829012 |
Figure 1. The expression information of five lncRNAs, overall survival and disease free survival in gastric cancer patients in the TCGA dataset.
(A) Volcano plot with blue dots indicating five lncRNAs expression levels which is significantly different between tumor and normal tissue based on the criteria of an absolute log2 fold change (FC) >1 and adjusted P < 0.05. (B) Heatmap of the five-lncRNA expression profile of the 414 patients in the TCGA dataset. Among five lncRNAs, MIR100HG and TRHDE-AS1 have a similar expression in 414 patients in the TCGA dataset, otherwise the other three lncRNAs do as well. (C–D) The survival curves based on the OS and DFS of the 408 patients in TCGA dataset.
Table 2. The clinical features of GC patients in training group and GSE62254.
| Training group | Validation group-1 (GSE62254) | |||
|---|---|---|---|---|
| Variables | n = 408 | % | n = 300 | % |
| Gender | ||||
| Male | 263 | 64.46 | 199 | 66.33 |
| Female | 145 | 35.54 | 101 | 33.67 |
| Age | ||||
| Old (≥50 years old) | 377 | 92.40 | 262 | 87.33 |
| Young (<50 years old) | 31 | 7.60 | 38 | 12.67 |
| TNM stage | ||||
| Stage I | 55 | 13.48 | 30 | 10.00 |
| Stage II | 120 | 29.41 | 96 | 32.00 |
| Stage III | 167 | 40.93 | 95 | 31.67 |
| Stage IV | 41 | 10.05 | 79 | 26.33 |
| Not Available | 25 | 6.13 | 0 | |
| T stage | ||||
| T1 | 20 | 4.90 | 2 | 0.67 |
| T2 | 87 | 21.32 | 186 | 62.00 |
| T3 | 178 | 43.63 | 91 | 30.33 |
| T4 | 114 | 27.94 | 21 | 7.00 |
| TX | 9 | 2.21 | 0 | |
| N stage | ||||
| N0 | 120 | 29.41 | 38 | 12.67 |
| N1 | 110 | 26.96 | 131 | 43.67 |
| N2 | 77 | 18.87 | 80 | 26.67 |
| N3 | 82 | 20.10 | 51 | 17.00 |
| NX | 17 | 4.17 | 0 | |
| Not Available | 2 | 0.49 | 0 | |
| M stage | ||||
| M0 | 362 | 88.73 | 273 | 91.00 |
| M1 | 27 | 6.62 | 27 | 9.00 |
| MX | 19 | 4.66 | 0 | |
| Survival status | ||||
| Alive | 251 | 61.52 | 148 | 49.33 |
| Dead | 157 | 38.48 | 152 | 50.67 |
Construction of an lncRNA-based risk model from the training group
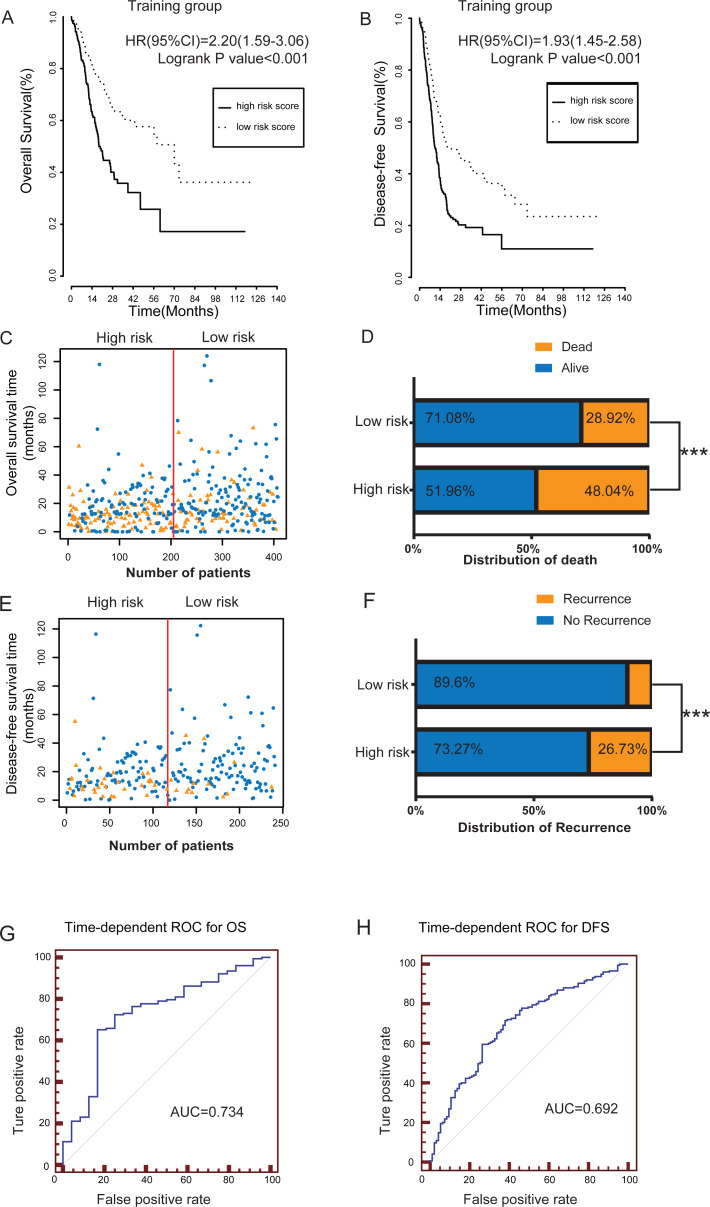
According to the schematic workflow of this study (Table 3), using the coefficients of the five lncRNAs identified by multivariate Cox hazard analysis, we created a risk-score formula as follows: risk score = (0.249092 × expression level of LINC00205) + (0.182045 × expression level of TRHDE-AS1) + (0.271169 × expression level of OVAAL) + (−0.20794 × expression level of LINC00106) + (0.502539 × expression level of MIR100HG). Among the five lncRNAs, LINC00106, which had a negative coefficient, was considered as a protective factor. The remaining four lncRNAs with positive coefficients, namely LINC00205, TRHDE-AS1, OVAAL, and MIR100HG, were risk factors. The risk scores of each patient in the training group were calculated (Table S2), ranging from −2.086959745 to 2.270305234. The patients in the training group were divided into two subgroups: high risk (n = 204) and low risk (n = 204) subgroups, with the median score (−0.001085) as the cutoff value. We performed Kaplan–Meier survival analysis to assess the effect of the lncRNA-based risk model on the OS and DFS of patients with GC in the training group (Figs. 2A–2B). Our results indicated that the high-risk group had a significantly poorer prognosis than the low risk group for both OS and DFS (P = 1 × 10−6 and 6 ×10−6, respectively). Figsures 2C–2F shows the scatter plots of the recurrence and mortality rates of patients with GC. The recurrence and mortality rates were significantly higher in the high risk group than in the low risk group (P < 0.001). To accurately evaluate the prognostic value of the five-lncRNA signature, we performed time-dependent ROC analysis using the 1–4 years cutoff value of OS and the 1–2 years cutoff of DFS as the ROC ending points (Figs. 2G–2H and Figs. S1A–S1D). The area under the ROC curve (AUC) was 0.734 for the 4-year cutoff value of OS and 0.692 for the 2-year cutoff value of DFS, respectively, and had the highest predictive value among those years, indicating that our model can be used for survival prediction in patients with GC (Figs. 2G–2H).
Table 3. The schematic workflow of the present study.
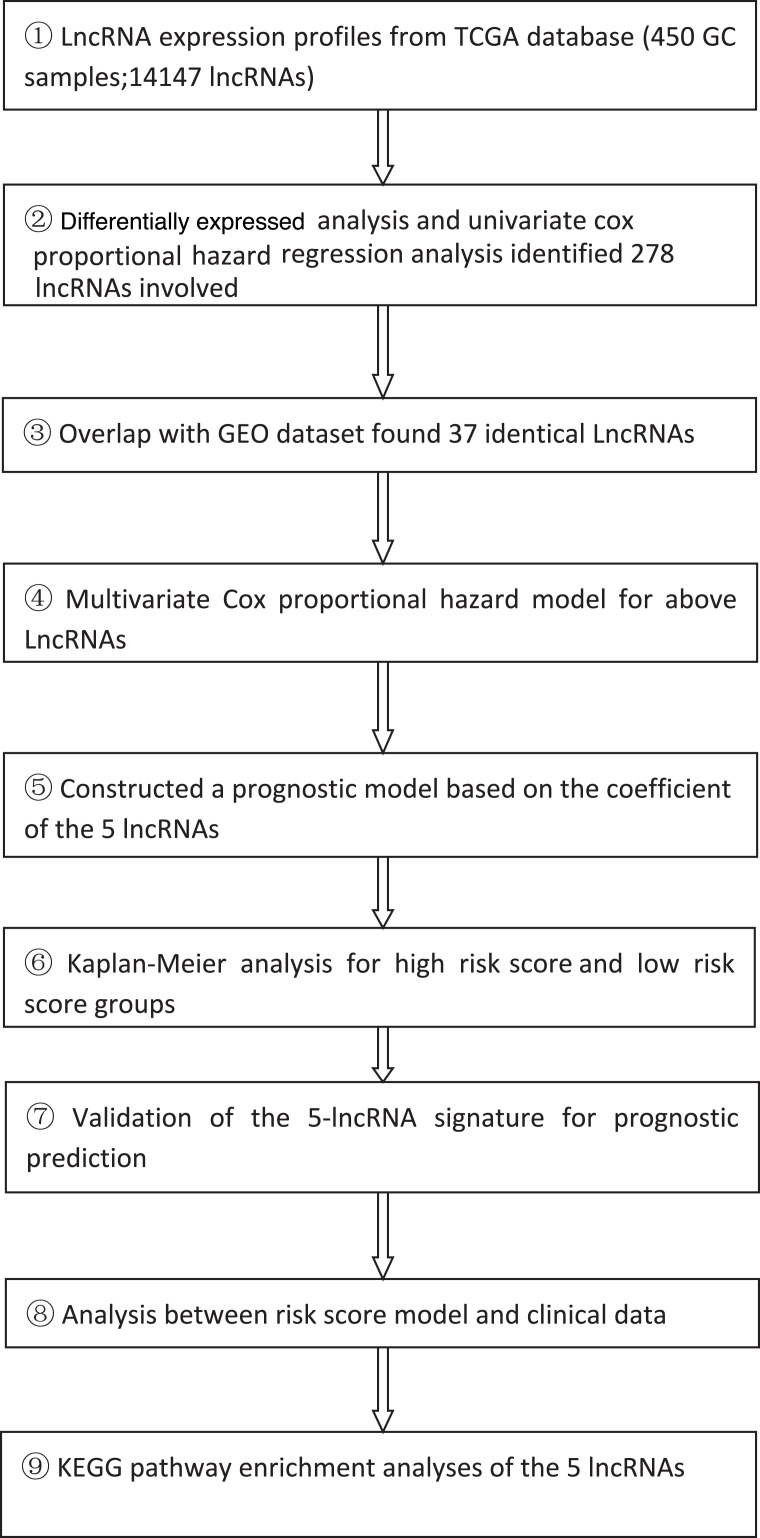
|
Figure 2. The prognostic value of lncRNA-based risk model in training group.
(A–B) Kaplan–Meier analysis of patients’ OS and DFS in the high risk (n = 204) and low risk (n = 204) subgroups of the training group. (C) The scatter plot of lncRNA-based risk model distribution for patient survival status. (D) The percentage of patient survival status in the high risk and low risk subgroups of the training group. (E) The lncRNA-based risk model distribution for patient recurrence. (F) The percentage of patient recurrence in the high risk and low risk subgroups of the training group. (G–H) The time-dependent ROC analysis of the risk score for prediction the 4-year cutoff OS and 2-year cutoff DFS of the training group. The area under the curve was calculated for ROC curve. *** P < 0.001.
Validation of the lncRNA-based risk score model for prognostic prediction in independent groups
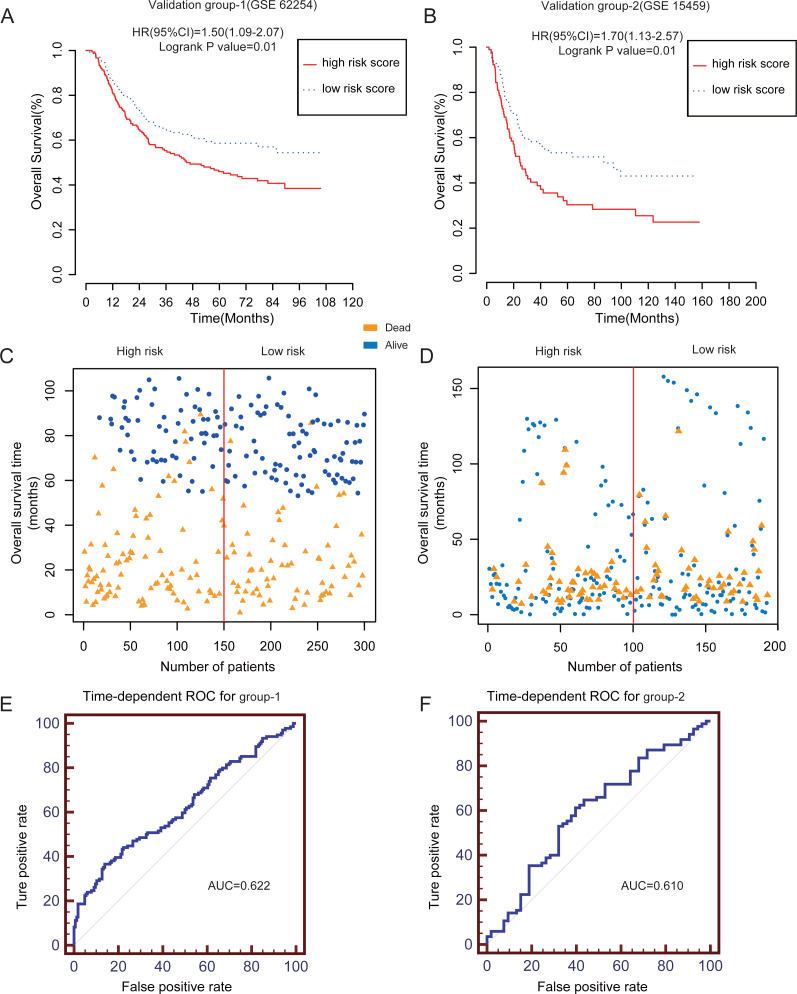
To assess the prognostic significance of this novel lncRNA-based risk model including the five-lncRNA signature in patients with GC, we used the other two independent validation datasets from the GEO database. We calculated the risk score using the formula mentioned above (Table S2). The patients with GC in GSE62254 (validation group-1; N = 300) and GSE15459 (validation group-2; N = 200) datasets were divided into high risk and low risk groups according to the median risk score. Owing to the lack of DFS data in GSE15459, we only calculated the OS of the patients. The high risk group had a poorer OS than the low risk group (log-rank P = 0.01) (Figs. 3A–3B). Figures 3C–3D shows the scatter plots for the death events. The mortality rates were significantly higher in the high risk group than in the low risk group (P < 0.001). The AUC for the two validation groups in the 4-year cutoff OS was 0.622 and 0.610 for validation group 1 and 2, respectively (Figs. 3E–3F). Figures S2A–S2F shows the ROC curve for the 1–3 year cutoff OS for the validation groups 1 and 2. Furthermore, we verified the performance of our risk score model for DFS of the GSE62254 dataset (Figs. S3A–Fig. S3D). Our results further confirmed the value and robustness of this risk score model for prognostic prediction in patients with GC.
Figure 3. The prognostic value of lncRNA-based risk model in two independent GEO validation groups.
(A–B) Kaplan–Meier analysis of predicting OS of GC patients based on the high risk and low risk subgroups in two independent validation groups (GSE62254 and GSE15459). (C–D) The scatter plot of five-lncRNA-based risk score distribution for patient survival status in two independent validation groups.(E–F) The time-dependent ROC analysis of the risk score for prediction the 4-year cutoff OS of the two independent validation groups. The area under the curve was calculated for ROC curve.
The lncRNA-based risk model has a favorable prognostic prediction in patients with stage II, III, and IV
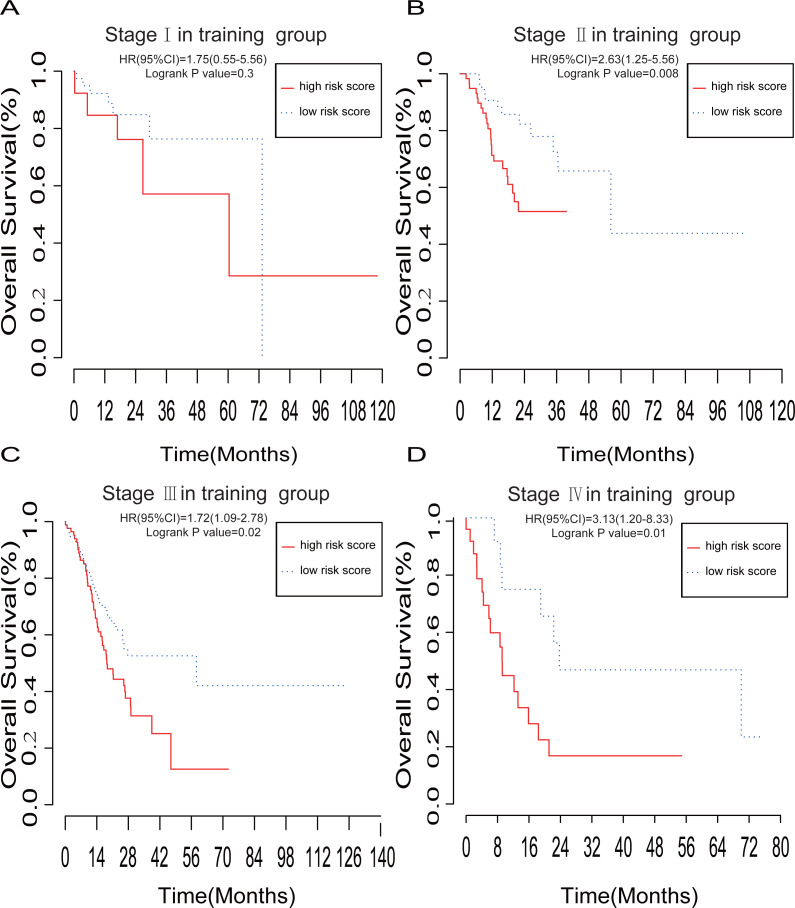
To further investigate the performance of our lncRNA-based risk model, we performed stratified Kaplan–Meier survival analysis of OS in the training group based on the AJCC TNM stages I, II, III, or IV (Figs. 4A–4D). The five-lncRNA signature showed good predictive value for OS of stages II (P = 0.008), III (P = 0.02), and IV (P = 0.01), but not I (P = 0.3).
Figure 4. The prognostic value of lncRNA-based risk model in subgroups according to the TNM stage.
(A–D) Kaplan–Meier analysis of the OS of GC patients with stage I, II, III and IV, respectively.
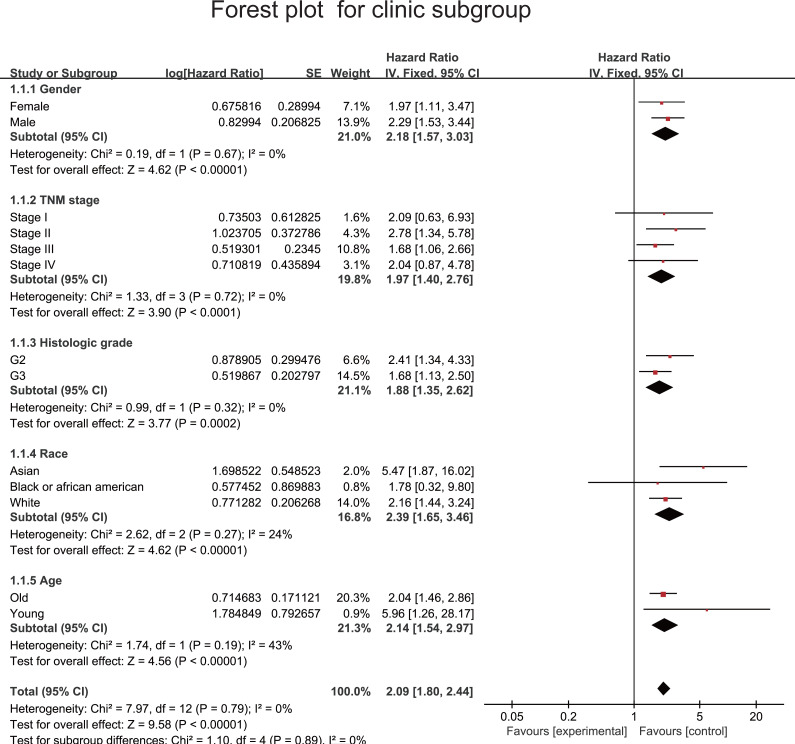
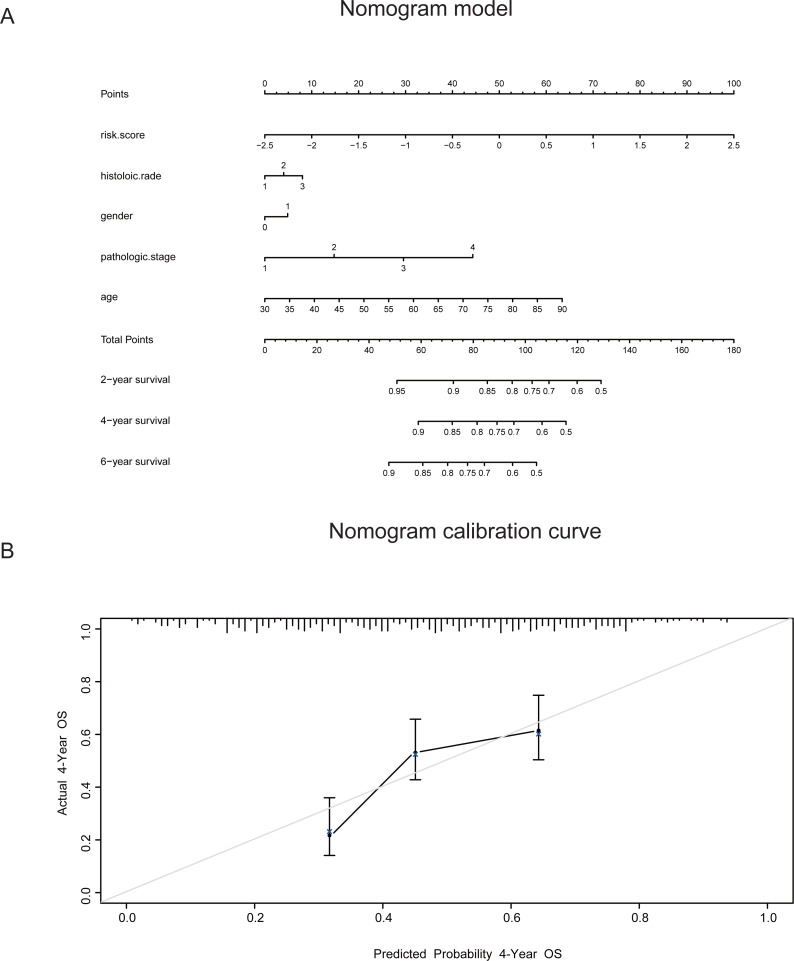
To estimate the HR of each subgroup of patients as defined by sex, TNM stage, histologic grade, race and age(≥ or < 50 years) (Table 4), we used our model to divide the patients into two risk groups on the basis of the median cutoff value. Forest plots are shown in Fig. 5. Table S3 shows the HR of each subgroup of patients in GSE62252. The risk score model had a relatively good prognostic value in the clinical subgroups of sex, histologic grade and age. To improve the prognostic value of this model, we combined the clinical factors with the risk score model to construct a nomogram model for prognostic prediction. The nomogram model and calibration curve are shown in Figs. 6A–6B. To evaluate the effect of the nomogram model, we calculated its C-index. The C-index for predicting the 4-year OS of patients with GC was 0.69668, indicating that this model is a valuable indicator for prognostic prediction.
Table 4. The association between five-lncRNA signature and OS of GC patients in training group.
| Number (High Risk score/Low Risk score) | HR (95% CI) | P value | |
|---|---|---|---|
| Total | 204/204 | 2.09 (1.80, 2.44) | 0.000001 |
| Gender | |||
| Male | 129/134 | 2.29 (1.53, 3.44) | 0.00002 |
| Female | 75/70 | 1.97 (1.11, 3.47) | 0.01 |
| Histologic grade | |||
| G2 | 47/97 | 2.41 (1.34, 4.33) | 0.0006 |
| G3 | 146/97 | 1.68 (1.13, 2.50) | 0.02 |
| Race | |||
| Asian | 44/41 | 5.47 (1.87, 16.02) | 0.001 |
| Black or african american | 4/8 | 1.78 (0.32, 9.80) | 0.6 |
| White | 138/120 | 2.16 (1.44, 3.24) | 0.0003 |
| Age | |||
| Old (≥50 years old) | 186/191 | 2.04 (1.46, 2.86) | 0.00001 |
| Young(<50 years old) | 18/13 | 5.96 (1.26, 28.17) | 0.008 |
| TNM stage | |||
| Stage I | 14/41 | 2.09 (0.63, 6.93) | 0.3 |
| Stage II | 62/58 | 2.78 (1.34, 5.78) | 0.008 |
| Stage III | 88/79 | 1.68 (1.06, 2.66) | 0.02 |
| Stage IV | 25/16 | 2.04 (0.87, 4.78) | 0.01 |
Notes.
Abbreviations
- HR
- Hazard ratio
- 95%CI
- 95% confidence interval
Figure 5. Forest plot to evaluate prognostic value of lncRNA-based risk model in subgroups divided by clinical factors.
Figure 6. The prognostic value of a nomogram model combining five-lncRNA signature with the clinical factors.
(A) A nomogram model combining five-lncRNA signature with the clinical factors for predicting the 4-year OS of GC patients. (B) The nomogram calibration curve to evaluate the prediction of 4-year OS of GC patients. The C index of this model was also calculated.
Potential functions of the five lncRNAs
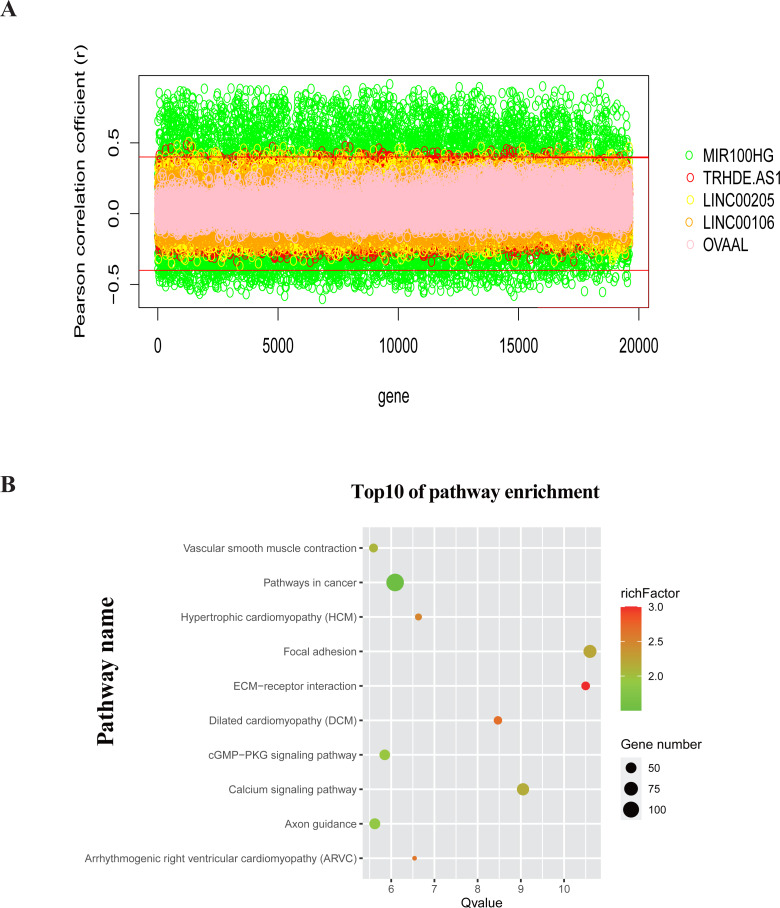
To investigate the functions of the five lncRNAs in patients with GC, we calculated Pearson correlations between the five-lncRNA signature and 19,605 protein-coding genes in TCGA dataset. A total of 3069 genes (Table S4) were positively correlated with at least one lncRNA (Pearson’s coefficient > 0.4) (Fig. 7A). We further selected these genes for KEGG pathway enrichment analysis. By ranking based on –logP value (Q value), we selected the top 10 pathways for construction of a bubble plot (Fig. 7B) (Zeng et al., 2019; Deng, Xu & Wang, 2019). For biological processes, the co-expressed genes were mainly enriched in pathways involved in cancer, such as the focal adhesion pathway, cGMP −PKG signaling pathway and calcium signaling pathway. This finding indicates that the five lncRNAs may be involved in the regulation of tumor initiation and progression.
Figure 7. Potential functions of the five lncRNAs.
(A) The Pearson correlation coefficient between 19,605 protein-coding genes and five lncRNAs in TCGA dataset. (B) The functional enrichment bubble map of pathways by KEGG pathway analysis. Bubble size represents the number of gene enriched in the pathway.
Discussion
In this study, we identified a potential signature involving five lncRNAs that are differentially expressed in tumor and normal tissues, and might be valuable for prognostic prediction in GC. The prognostic performance of this lncRNA-based risk score model was verified using both TCGA and GEO datasets. Stratified analysis suggested that the risk score model is valuable for prognostic prediction in patients with stage II-IV GC. To enhance the predictive accuracy of the model, we combined clinical parameters with the five-lncRNA signature to construct a nomogram model and confirmed its performance using a calibration curve and C index.
GC is a common malignancy of the GI tract (Siegel, Miller & Jemal, 2019). Despite continuous improvements in treatment, the 5-year survival rate of patients with advanced GC is only approximately 20% (Min et al., 2019; Misawa et al., 2019). Therefore, early diagnosis, early identification of high-risk patients and implementation of effective treatment measures as early as possible are necessary to improve survival. It is also important to develop novel prognostic indicators of GC. Over the past few decades, research has shown that protein-coding genes(Ghoorun et al., 2019; Luo et al., 2019) and microRNAs (Li et al., 2020; Zhou, Wu & Bi, 2019), play vital roles in the occurrence and development of various cancers, and can also be used to predict patient prognosis. Several nomogram models involving clinical factors have been constructed to predict the prognosis of patients with GC. For example, Yu (Yu & Zhang, 2019) used tumor size and tumor site, as independent prognostic factors, to construct OS nomograms for predicting outcomes in patients with GC, and the C-index of this model indicated that it could predict prognosis. Recently, more lncRNAs related to GC prognosis have been discovered; however, prognostic prediction models involving lncRNAs still lack consensus. We present a nomogram including clinical factors and the five-lncRNA signature that might be of value for prognostic prediction in GC.
It is necessary to explore novel biomarkers to improve the diagnostic accuracy and prognosis of GC because of limitations of TNM staging and some related scoring systems. Many lncRNAs have been identified, of which only few have been functionally annotated. However, evidence indicates that lncRNAs, acting either as oncogenes or tumor suppressors, participate in the tumorigenesis and development of various cancers by regulating chromatin remodeling, transcription and post-transcriptional modification (Bartonicek, Maag & Dinger, 2016; Iyer et al., 2015), and therefore might be valuable for cancer diagnosis and prognosis. Some studies have found that GC-related lncRNAs are involved in biological behaviors including the proliferation, migration, invasion, and autophagy of GC cells, thereby affecting the initiation and prognosis of GC (Mao et al., 2017; Wei & Wang, 2017). For example, the lncRNA MEG3 inhibits the proliferation, metastasis, and prognosis of GC cells by upregulating the expression p53—a key tumor suppressor (Wei & Wang, 2017). We identified five lncRNAs—LINC00205, TRHDE-AS1, OVAAL, LINC00106, and MIR100HG—as predictors of GC prognosis, and developed a risk-score model. Kaplan–Meier analysis suggested that our lncRNA-based risk model is valuable for predicting GC prognosis. We used two independent GEO datasets as validation datasets. Our results confirmed that our risk score model is stable and performs well in the prognostic prediction of GC.
Of the five lncRNAs, LINC00205, TRHDE-AS1, OVAAL, and MIR100HG, act as risk factors of GC, whereas LINC00106 is a protective factor. Apart from LINC00205 and MIR100HG, the other three lncRNAs have not been reported much in the literature. Our study identified LINC00205, TRHDE-AS1, OVAAL, and MIR100HG as potential prognostic biomarkers of GC for the first time. Consistent with our result, it has previously been reported that high expression of LINC00106 indicates prolonged OS of patients with GC (Qi et al., 2020). Nevertheless, the role of this lncRNA in GC as well as its specific mechanism need to be further investigated. Interestingly, in hepatocellular carcinoma (HCC), comprehensive genome-wide analysis revealed that the expression of LINC00205, a tumor suppressor, is positively associated with OS and recurrence-free survival (Cui et al., 2017). A study showed that, as a competing endogenous RNA with lower expression levels in tumor tissues, LINC00205 may negatively regulate HCC progression via the miR-184/EPHX1 axis (Long et al., 2019), While another study indicated that LINC00205, can serve as an oncogene, and can promote the proliferation, migration and invasion of HCC cells by targeting miR-122-5p (Zhang et al., 2019a). In addition, LINC00205 can act as a protective factor in pancreatic cancer [HR = 0.58, P (log rank) = 0.0091] (Giulietti et al., 2018). The reported role and therefore prognostic prediction value of LINC00205 in various cancers shows significant discrepancies. These discrepancies might be associated with the specificities of different cancers. The upregulation of TRHDE-AS1 inhibits the growth of lung carcinoma through competitive combination with the miRNA-103-KLF4 axis (Zhuan et al., 2019). A study has found that OVVAL is highly expressed in colon cancer and melanoma, and further experimental results showed that OVAAL promotes the proliferation of cancer cells via dual mechanisms controlling RAF/MEK/ERK signaling and p27-mediated cell senescence (Sang et al., 2018). The lncRNA MIR100HG has been studied as an oncogene in acute megakaryoblastic leukemia (Emmrich et al., 2014), and laryngeal squamous cell carcinoma (Huang, Zhang & Zhou, 2019), as well as for its role in mediating cetuximab resistance via Wnt/ β-catenin signaling (Lu et al., 2017) in colorectal cancer. Although the roles of these lncRNAs in cancer need to be further investigated, our results may provide a novel approach to study GC.
To further investigate the functions of the five lncRNAs in GC, we performed pathway enrichment analysis. These genes are enriched in cancer regulation, including the cGMP−PKG signaling pathway, calcium signaling pathway, and focal adhesion pathway etc. This finding suggests that the five lncRNAs may play an important role in the occurrence and development of GC. There is evidence that lncRNAs can promote tumorigenesis through the cGMP−PKG signaling pathway. For example, the overexpression of SRRM2-AS accelerates angiogenesis in nasopharyngeal carcinoma via the cGMP−PKG signaling pathway (Chen et al., 2019). The calcium signaling pathway has been reported to be mainly involved in metabolic diseases and heart diseases over the past years (Berridge, 2016; Dewenter et al., 2017). A recent research showed that the calcium signaling pathway was associated with cancer cell survival, but more details on its effects remain to be studied (Reczek & Chandel, 2018). Focal adhesion sites are special sites where integrin receptors aggregated in cells interact with extracellular matrix and intracellular actin skeleton (Burridge, 2017), and they play a critical role in tumor invasion and migration (Shen et al., 2018). There is evidence that knockdown of Linc01060 could promote the progression of pancreatic cancer via the vinculin-mediated focal adhesion pathway turnover (Shi et al., 2018). However, whether the lncRNA can mediate the progression of GC through the focal adhesion pathway is less reported. In short, lncRNAs may participate in the genesis and development of various tumors via the above pathways.
Risk score model is a common and widely used method to predict the prognosis of patients with multiple diseases (Lemke et al., 2017; Li et al., 2018; Yang et al., 2017; Sobotka et al., 2018). Our risk score was determined by the expression of independent survival-lncRNAs obtained after Cox hazard analyses and its corresponding coefficients. It was calculated using binary lncRNA expression values according to the medians of original lncRNA expression values. This adjustment helps to improve the clinical application of the prognostic model in other study population (Zhang et al., 2018). In general, the higher is the risk score, the poorer is the prognosis, which is consistent with our analysis. Our Kaplan–Meier survival analysis showed that the patients in the high risk group had a significantly poorer prognosis than those in the low risk group. Our risk score model based on lncRNAs has several advantages. This model based on the expression of five lncRNAs provides a novel noninvasive method for predicting the prognosis of patients with GC before surgery. Compared with conventional invasive pathological examinations, it reduces unnecessary pain for patients. Second, this five-lncRNA risk model can provide preoperative risk predictive probability of individual mortality and recurrence in different clinical endpoints. It is simple and convenient for clinicians and patients to understand. Third, our model used the median of five-lncRNA-based risk score as the cutoff value to divide patients into high risk and low risk groups. It can identify patients at high risk of mortality or recurrence in a timely manner and prompt clinical interventions as early as possible to improve their prognosis.
There have been several reports on lncRNA signatures for GC. A previous study reported a 24-lncRNA signature that can predict outcomes in patients with GC by applying the random survival forest-variable hunting algorithm using GEO datasets (Zhu et al., 2016). However, because of the limited amount of data in the GEO datasets, the lncRNAs identified in this study might not represent the complete population of lncRNAs involved in GC. In this study, we integrated 950 samples from TCGA and GEO databases to comprehensively investigate the potentially prognostic lncRNAs. This greatly improved the accuracy, reliability and robustness of our model. A six-lncRNA prognostic signature was established by robust likelihood-based survival and LASSO model (Ma, Li & Ren, 2019). Whether the six-lncRNA signature combined with other clinical features can enhance the predictive power remains to be determined. To improve the accuracy of the five-lncRNA prognosis model, we combined it with clinical factors to develop a nomogram model that could predict the OS of patients with GC. Zhu et al. (2018) et al. constructed an 11-lncRNA signature by univariate and multivariate Cox regression analyses. Although an internal validation was validated using the bootstrap resampling method, external validation studies are needed to further evaluate the value of this model. We not only included two external verification datasets, but also performed survival analysis, ROC curve analysis, and constructed a forest plot for predictive verification, indicating a favorable effectiveness of our model.
There are some limitations of the present study. We integrated data from TCGA and GEO databases to increase the number of the cases, thereby reducing bias from a small sample size. Integrated analysis has been proved to be an effective approach for multiple datasets with different platforms using R package (Zhang et al., 2019b; Nie et al., 2020; Zhao et al., 2018; Zhao et al., 2020), thereby promoting the reliability of our conclusion (Ma et al., 2017). However, TCGA dataset has a larger number of lncRNAs than the GEO dataset (14147:1397) because of different sequencing technologies: TCGA uses RNA sequencing technology, whereas GEO uses microarray chip technology. Intersection of three datasets has inevitably omitted potential prognostic lncRNAs. Moreover, the clinical characteristics of the patients in the three datasets are heterogeneous. This might have inevitably led to a bias. Besides, owing to the lack of DFS and clinical data in GSE15459, we used only one external validation group to verify the prognostic value of the five-lncRNA signature for the DFS of patients. In addition, many important variables affecting the prognosis of patients with GC are not provided in TCGA and GEO datasets, such as dietary habits, previous disease, history of chemotherapy or radiation therapy, and family history of cancer. Thus, on the one hand, it is necessary to perform a large-scale multi-center prospective clinical study based on the same sequencing technology to decrease the bias mentioned above. On the other hand, based on existing data, it is beneficial to develop innovative statistical algorithms to reduce the heterogeneity of different data sources. Last, because of the limited number of studies regarding these lncRNAs, experimental research on these lncRNAs is highly warranted to further understand their functions in GC.
Conclusions
We established a risk score model including five lncRNAs to predict the OS and DFS of patients with GC, particularly in those with stage II-IV GC. Our findings also provided evidence of developing effective prognostic biomarkers for patients with GC and potential therapeutic targets in the future.
Supplemental Information
(A) Kaplan–Meier analysis of predicting DFS of GC patients based on the high risk and low risk subgroups in validation group (GSE62254). (B) The lncRNA-based model distribution for patient recurrence in validation group (GSE62254). (C–D) The time-independent ROC analysis of the risk score for prediction the 1-2 year cutoff DFS in validation group (GSE62254). The area under the curve was calculated for ROC curve.
Abbreviations: HR, Hazard ratio; 95%CI, 95% confidence interval.
Acknowledgments
At the point of finishing this paper, we would thank to Jie Peng for technical assistance with the data analysis.
Funding Statement
This work was supported in part by grants from the Science and Technology project of Health Commission of Jiangxi Province, China (202130409) and the Science and Technology project of Jiangxi Provincial Administration of Traditional Chinese Medicine, China (2020A0051). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yujuan You, Email: 506737972@qq.com.
Jing Wu, Email: 446346807@qq.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yiguo Wu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Junping Deng and Shuhui Lai performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yujuan You conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jing Wu conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Data are available at UCSC Xena (https://xenabrowser.net/) and at NCBI GEO: GSE62254 and GSE15459.
References
- Balas & Johnson (2018).Balas MM, Johnson AM. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Research. 2018;3:108–117. doi: 10.1016/j.ncrna.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett et al. (2013).Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Research. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartonicek, Maag & Dinger (2016).Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Molecular Cancer. 2016;15:43. doi: 10.1186/s12943-016-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge (2016).Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiological Reviews. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- Burridge (2017).Burridge K. Focal adhesions: a personal perspective on a half century of progress. FEBS Journal. 2017;284:3355–3361. doi: 10.1111/febs.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen S, Lv L, Zhan Z, Wang X, You Z, Luo X, You H. Silencing of long noncoding RNA SRRM2-AS exerts suppressive effects on angiogenesis in nasopharyngeal carcinoma via activating MYLK-mediated cGMP-PKG signaling pathway. Journal of Cellular Physiology. 2019;235:7757–7768. doi: 10.1002/jcp.29382. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2017).Cui H, Zhang Y, Zhang Q, Chen W, Zhao H, Liang J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Medicine. 2017;6:2932–2941. doi: 10.1002/cam4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Xu & Wang (2019).Deng J, Xu Y, Wang G. Identification of potential crucial genes and key pathways in breast cancer using bioinformatic analysis. Frontiers in Genetics. 2019;10 doi: 10.3389/fgene.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewenter et al. (2017).Dewenter M, Von der Lieth A, Katus HA, Backs J. Calcium signaling and transcriptional regulation in cardiomyocytes. Circulation Research. 2017;121:1000–1020. doi: 10.1161/CIRCRESAHA.117.310355. [DOI] [PubMed] [Google Scholar]
- Emmrich et al. (2014).Emmrich S, Streltsov A, Schmidt F, Thangapandi VR, Reinhardt D, Klusmann JH. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Molecular Cancer. 2014;13:171. doi: 10.1186/1476-4598-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoorun et al. (2019).Ghoorun RA, Wu XH, Chen HL, Ren DL, Wu XB. Prognostic significance of fkbp14 in gastric cancer. OncoTargets and Therapy. 2019;12:11567–11577. doi: 10.2147/OTT.S221943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti et al. (2018).Giulietti M, Righetti A, Principato G, Piva F. LncRNA co-expression network analysis reveals novel biomarkers for pancreatic cancer. Carcinogenesis. 2018;39:1016–1025. doi: 10.1093/carcin/bgy069. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2017).Huang JZ, Chen M, Chen Gao, XC, Zhu S, Huang H, Hu M, Zhu H, Yan GR. A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth. Molecular Cell. 2017;68:171–184. doi: 10.1016/j.molcel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Huang, Zhang & Zhou (2019).Huang Y, Zhang C, Zhou Y. LncRNA MIR100HG promotes cancer cell proliferation, migration and invasion in laryngeal squamous cell carcinoma through the downregulation of miR-miR-204-5p. Onco Targets Ther. 2019;12:2967–2973. doi: 10.2147/OTT.S202528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer et al. (2015).Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM. The landscape of long noncoding RNAs in the human transcriptome. Nature Genetics. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke et al. (2017).Lemke M, Law CH, Li J, Dixon E, Tun AM, Hernandez AR, Bennett S, Martel G, Karanicolas PJ. Three-point transfusion risk score in hepatectomy. British Journal of Surgery. 2017;104:434–442. doi: 10.1002/bjs.10416. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018).Li CI, Li TC, Liu CS, Liao LN, Lin WY, Lin CH, Yang SY, Chiang JH, Lin CC. Risk score prediction model for dementia in patients with type 2 diabetes. European Journal of Neurology. 2018;25:976–983. doi: 10.1111/ene.13642. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, Jiang X. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Proliferation. 2017;50:e12381. doi: 10.1111/cpr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019).Li X, Lv X, Li Z, Li C, Li X, Xiao J, Liu B, Yang H, Zhang Y. Long noncoding RNA ASLNC07322 functions in VEGF-C expression regulated by Smad4 during colon cancer metastasis. Molecular Therapy - Nucleic Acids. 2019;18:851–862. doi: 10.1016/j.omtn.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020).Li JP, Zhang HM, Liu MJ, Xiang Y, Li H, Huang F, Li HH, Dai ZT, Gu CJ, Liao XH, Zhang TC. miR-133a-3p/FOXP3 axis regulates cell proliferation and autophagy in gastric cancer. Journal of Cellular Biochemistry. 2020;121:3392–3405. doi: 10.1002/jcb.29613. [DOI] [PubMed] [Google Scholar]
- Long et al. (2019).Long X, Li Q, Zhi LJ, Li JM, Wang ZY. LINC00205 modulates the expression of EPHX1 through the inhibition of miR - 184 in hepatocellular carcinoma as a ceRNA. Journal of Cellular Physiology. 2019;235:3013–3021. doi: 10.1002/jcp.29206. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2017).Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R, Cao Z, Singh B, Franklin JL, Wang J, Hu H, Wei T, Yang M, Yeatman TJ, Lee E, Saito-Diaz K, Hinger S, Patton JG, Chung CH, Emmrich S, Klusmann JH, Fan D, Coffey RJ. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/beta-catenin signaling. Nature Medicine. 2017;23:1331–1341. doi: 10.1038/nm.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2019).Luo X, He Y, Tang H, Cao Y, Gao M, Liu B, Hu Z. Effects of HER2 on the invasion and migration of gastric cancer. American Journal of Translational Research. 2019;11:7604–7613. [PMC free article] [PubMed] [Google Scholar]
- Ma, Li & Ren (2019).Ma B, Li Y, Ren Y. Identification of a 6 - lncRNA prognostic signature based on microarray re - annotation in gastric cancer. Cancer Medicine. 2019;9:335–349. doi: 10.1002/cam4.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2017).Ma T, Liang F, Oesterreich S, Tseng GC. A joint Bayesian model for integrating microarray and RNA sequencing transcriptomic data. Journal of Computational Biology. 2017;24:647–662. doi: 10.1089/cmb.2017.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao et al. (2017).Mao Z, Li H, Cui KDuB, Xing Y, Zhao X, Zai S. LncRNA DANCR promotes migration and invasion through suppression of lncRNA-LET in gastric cancer cells. Bioscience Reports. 2017;37:BSR20171070. doi: 10.1042/BSR20171070. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miao et al. (2019).Miao R, Ge C, Zhang X, He Y, Ma X, Xiang X, Gu J, Fu Y, Qu K, Liu C, Wu Q, Lin T. Combined eight-long noncoding RNA signature: a new risk score predicting prognosis in elderly non-small cell lung cancer patients. Aging. 2019;11:467–479. doi: 10.18632/aging.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min et al. (2019).Min SH, Won Y, Kim G, Lee Y, Park YS, Ahn SH, Park DJ, Kim HH. 15-year experience of laparoscopic gastrectomy in advanced gastric cancer: analysis on short-term and long-term oncologic outcome. Surgical Endoscopy and Other Interventional Techniques. 2019;34:4983–4990. doi: 10.1007/s00464-019-07292-x. [DOI] [PubMed] [Google Scholar]
- Misawa et al. (2019).Misawa K, Mochizuki Y, Sakai M, Teramoto H, Morimoto D, Nakayama H, Tanaka N, Matsui T, Ito Y, Ito S, Tanaka K, Uemura K, Morita S, Kodera Y. Randomized clinical trial of extensive intraoperative peritoneal lavage versus standard treatment for resectable advanced gastric cancer (CCOG 1102 trial) British Journal of Surgery. 2019;106:1602–1610. doi: 10.1002/bjs.11303. [DOI] [PubMed] [Google Scholar]
- Nie et al. (2020).Nie K, Deng Z, Zheng Z, Wen Y, Pan J, Jiang X, Yan Y, Liu P, Liu F, Li P. Identification of a 14-lncRNA signature and construction of a prognostic nomogram predicting overall survival of gastric cancer. DNA and Cell Biology. 2020;39:1532–1544. doi: 10.1089/dna.2020.5565. [DOI] [PubMed] [Google Scholar]
- Qi et al. (2020).Qi M, Yu B, Yu H, Li F. Integrated analysis of a ceRNA network reveals potential prognostic lncRNAs in gastric cancer. Cancer Medicine. 2020;9:1798–1817. doi: 10.1002/cam4.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek & Chandel (2018).Reczek CR, Chandel NS. ROS promotes cancer cell survival through calcium signaling. Cancer Cell. 2018;33:949–951. doi: 10.1016/j.ccell.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Saka et al. (2011).Saka M, Morita S, Fukagawa T, Katai H. Present and future status of gastric cancer surgery. Japanese Journal of Clinical Oncology. 2011;41:307–313. doi: 10.1093/jjco/hyq240. [DOI] [PubMed] [Google Scholar]
- Sang et al. (2018).Sang B, Zhang YY, Guo ST, Kong LF, Cheng Q, Liu GZ, Thorne RF, Zhang XD, Jin L, Wu M. Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:E11661–E11670. doi: 10.1073/pnas.1805950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen et al. (2018).Shen J, Cao B, Wang Y, Ma C, Zeng Z, Liu L, Li X, Tao D, Gong J, Xie D. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. Journal of Experimental & Clinical Cancer Research. 2018;37:175. doi: 10.1186/s13046-018-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2018).Shi X, Guo X, Li X, Wang M, Qin R. Loss of Linc01060 induces pancreatic cancer progression through vinculin-mediated focal adhesion turnover. Cancer Letters. 2018;433:76–85. doi: 10.1016/j.canlet.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Siegel, Miller & Jemal (2019).Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Sobotka et al. (2018).Sobotka LA, Husain SG, Krishna SG, Hinton A, Pavurula R, Conwell DL, Zhang C. A risk score model of 30-day readmission in ulcerative colitis after colectomy or proctectomy. Clinical and Translational Gastroenterology. 2018;9:e175. doi: 10.1038/s41424-018-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne set analysis toolkit (WebGestalt): update 2013. Nucleic Acids Research. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang MW, Liu J, Liu Q, Xu QH, Li TF, Jin S, Xia TS. LncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric cancer cells by repressing the P15 and P16 expression. European Review for Medical and Pharmacological Sciences. 2017;21:4613–4622. [PubMed] [Google Scholar]
- Wei & Wang (2017).Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:3850–3856. [PubMed] [Google Scholar]
- Yang et al. (2017).Yang HJ, Choi S, Park SK, Jung YS, Choi KY, Park T, Kim JY, Park DI. Derivation and validation of a risk scoring model to predict advanced colorectal neoplasm in adults of all ages. Journal of Gastroenterology and Hepatology. 2017;32:1328–1335. doi: 10.1111/jgh.13711. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2019).Yang M, Qin X, Qin G, Zheng X. The role of IRAK1 in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 2019;12:2171–2180. doi: 10.2147/OTT.S185662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu & Zhang (2019).Yu C, Zhang Y. Development and validation of prognostic nomogram for young patients with gastric cancer. Annals of Translational Medicine. 2019;7:641. doi: 10.21037/atm.2019.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu Y, Nangia-Makker P, Farhana L, Majumdar A. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Molecular Cancer. 2017;16:155. doi: 10.1186/s12943-017-0725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2019).Zeng J, Lu W, Liang L, Chen G, Lan H, Liang X, Zhu X. Prognosis of clear cell renal cell carcinoma (ccRCC) based on a six-lncRNA-based risk score: an investigation based on RNA-sequencing data. Journal of Translational Medicine. 2019;17:281. doi: 10.1186/s12967-019-2032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang Z, Liu Q, Wang P, Li J, He T, Ouyang Y, Huang Y, Wang W. Development and internal validation of a nine-lncRNA prognostic signature for prediction of overall survival in colorectal cancer patients. PeerJ. 2018;6:e6061. doi: 10.7717/peerj.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019a).Zhang L, Wang Y, Sun J, Ma H, Guo C. LINC00205 promotes proliferation, migration and invasion of HCC cells by targeting miR-122-5p. Pathology - Research and Practice. 2019a;215:152515. doi: 10.1016/j.prp.2019.152515. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019b).Zhang X, Zhang W, Jiang Y, Liu K, Ran L, Song F. Identification of functional lncRNAs in gastric cancer by integrative analysis of GEO and TCGA data. Journal of Cellular Biochemistry. 2019b;120:17898–17911. doi: 10.1002/jcb.29058. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2020).Zhao J, Guo C, Ma Z, Liu H, Yang C, Li S. Identification of a novel gene expression signature associated with overall survival in patients with lung adenocarcinoma: a comprehensive analysis based on TCGA and GEO databases. Lung Cancer. 2020;149:90–96. doi: 10.1016/j.lungcan.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao X, Sun S, Zeng X, Cui L. Expression profiles analysis identifies a novel three-mRNA signature to predict overall survival in oral squamous cell carcinoma. American Journal of Cancer Research. 2018;8:450–461. [PMC free article] [PubMed] [Google Scholar]
- Zhou, Wu & Bi (2019).Zhou HY, Wu CQ, Bi EX. MiR-96-5p inhibition induces cell apoptosis in gastric adenocarcinoma. World Journal of Gastroenterology. 2019;25:6823–6834. doi: 10.3748/wjg.v25.i47.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2018).Zhu M, Wang Q, Luo Z, Liu K, Zhang Z. Development and validation of a prognostic signature for preoperative prediction of overall survival in gastric cancer patients. OncoTargets and Therapy. 2018;11:8711–8722. doi: 10.2147/OTT.S181741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2016).Zhu X, Tian X, Yu C, Shen C, Yan T, Hong J, Wang Z, Fang J, Chen H. A long non-coding RNA signature to improve prognosis prediction of gastric cancer. Molecular Cancer. 2016;15:60. doi: 10.1186/s12943-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuan et al. (2019).Zhuan B, Lu Y, Chen Q, Zhao X, Li P, Yuan Q, Yang Z. Overexpression of the long noncoding RNA TRHDE - AS1 inhibits the progression of lung cancer via the miRNA - 103/KLF4 axis. Journal of Cellular Biochemistry. 2019;120:17616–17624. doi: 10.1002/jcb.29029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Kaplan–Meier analysis of predicting DFS of GC patients based on the high risk and low risk subgroups in validation group (GSE62254). (B) The lncRNA-based model distribution for patient recurrence in validation group (GSE62254). (C–D) The time-independent ROC analysis of the risk score for prediction the 1-2 year cutoff DFS in validation group (GSE62254). The area under the curve was calculated for ROC curve.
Abbreviations: HR, Hazard ratio; 95%CI, 95% confidence interval.
Data Availability Statement
The following information was supplied regarding data availability:
Data are available at UCSC Xena (https://xenabrowser.net/) and at NCBI GEO: GSE62254 and GSE15459.