Abstract
Background
Many trials supporting the benefits of pulmonary rehabilitation (PR) have used specialist exercise equipment, such as treadmills and cycle ergometers. However, access to specialist equipment may not be feasible in some settings. There is growing interest in delivering PR programmes with minimal, low-cost equipment, but uncertainty remains regarding their efficacy compared with programmes using specialist equipment.
Methods
Using propensity score matching, 318 consecutive patients with COPD undergoing supervised PR using minimal equipment (PR-min) were compared 1:1 with a control group of 318 patients with COPD who underwent supervised PR using specialist equipment (PR-gym). A non-inferiority analysis was performed for the primary outcome (incremental shuttle walk (ISW)) and secondary outcomes (Chronic Respiratory Disease Questionnaire (CRQ)—domain and total scores).
Results
Similar improvements in ISW and CRQ-domains were observed in PR-min and PR-gym groups (mean difference ISW: 3 m (95% CI −16 to 9); CRQ-total: 0.9 (95% CI −2.7 to 4.5)). The 95% CI between group differences for ISW and CRQ-total did not cross the predefined non-inferiority margins. However, completion rates were lower in PR-min compared with PR-gym (64% vs 73%; p=0.014).
Conclusions
In patients with COPD, PR delivered using minimal equipment produces clinically significant benefits in exercise capacity and health-related quality of life that are non-inferior to rehabilitation delivered using specialist equipment. This study provides support for the provision of PR using minimal exercise equipment, particularly in areas where access to specialist exercise equipment is limited.
Keywords: pulmonary rehabilitation, equipment evaluations
Key messages.
What is the key question?
Does supervised pulmonary rehabilitation that uses minimal equipment produce similar results to supervised pulmonary rehabilitation that uses specialist exercise equipment in patients with COPD?
What is the bottom line?
Supervised pulmonary rehabilitation undertaken with minimal equipment achieved clinically significant improvements in exercise capacity and health-related quality of life, which were non-inferior to pulmonary rehabilitation delivered using specialist equipment in patients with COPD.
Why read on?
The results of this study support the ongoing expansion of pulmonary rehabilitation programmes that use minimal equipment. This should allow greater access to this crucial form of treatment, especially in areas where access to specialist exercise equipment is limited.
Introduction
Pulmonary rehabilitation (PR) improves exercise capacity, dyspnoea and health-related quality of life in patients with COPD,1 and is established within international guidelines for COPD and other chronic respiratory diseases. A core component of PR is individually tailored and progressive aerobic and resistance exercise training,2 with a consensus that higher intensity training produces greater training benefits.3
Although many landmark trials were conducted in centres that used specialist exercise equipment, such as treadmills, cycle ergometers and fixed weight machines,1 there is growing interest in delivering PR with minimal, low-cost and portable equipment. There is considerable unmet need for PR4 and a minimal equipment approach has the potential to increase the geographical coverage and accessibility of PR by expanding the number and type of settings where PR can be delivered, including the home,5 or via telerehabilitation.6 For example, in the 2018 audit of PR services in England and Wales, a significant proportion of PR sites were located in community and health centres, church halls, community hospitals and general practitioner surgeries where access to specialist exercise equipment was limited.7
However, there remains uncertainty regarding the efficacy of PR programmes delivered using minimal equipment.8 A previous systematic review identified eight randomised controlled trials that compared exercise training using minimal equipment to usual care without exercise training.8 Although improvements in functional exercise capacity and health-related quality of life were observed, the studies were not able to show a significant difference in maximal exercise capacity (as measured by the incremental shuttle walk (ISW) test) between exercise training with minimal equipment and no exercise training.8 Furthermore, supervised PR programmes that use minimal equipment have not been directly compared with traditional ‘gold standard’ supervised PR delivered using specialist exercise equipment.
The aim of this study was to compare the outcomes of patients with COPD undergoing face-to-face supervised outpatient PR delivered using either minimal or specialist exercise equipment. We hypothesised that PR delivered using minimal equipment would produce similar improvements in exercise capacity and health-related quality of life as PR delivered using specialist equipment in patients with COPD.
Methods
Participants
Participants were prospectively recruited from community and hospital-based PR clinics in Northwest London between September 2011 and January 2016. Inclusion criteria were: a diagnosis of COPD according to the Global Initiative for Chronic Obstructive Pulmonary Disease guidelines,9 and consent to attend an 8-week supervised PR programme. Patients with a contraindication to exercise training (eg, unstable cardiac disease) or declining to take up PR were excluded. All patients were provided with written and verbal information on six PR locations across Northwest London, including location, travel directions, class times and available equipment. All assessments were performed in the Pulmonary Rehabilitation Department at Harefield Hospital, with data prospectively collected. As per routine clinical care, patients referred to the programme were allowed a free choice of which location they wished to attend.
The ‘experimental treatment’ group comprised those who chose to attend a twice-weekly, 8-week supervised PR programme at one of four community sites (three church halls and one community centre hall) with access to minimal exercise equipment only (PR-min).
The ‘control’ group comprised matched patients referred over the same time period who chose to attend a twice-weekly, 8-week supervised programme at one of two sites (acute hospital gymnasium and community leisure centre gymnasium) with access to specialist exercise equipment (PR-gym).
Regardless of site, all patients were offered a programme comprising two supervised sessions per week for 8 weeks (16 supervised sessions in total), and were encouraged to participate in at least one additional weekly home unsupervised exercise session in accordance with the British Thoracic Society Guidelines for PR.2 Supervised sessions comprised 1 hour of exercise training and 45 min of education. There were a maximum of 12 patients participating in each supervised session, supervised by a minimum of two members of staff from the Harefield Pulmonary Rehabilitation Unit including one senior physiotherapist (minimum ratio: 1 staff:6 patients). Senior physiotherapists had a minimum of 3 years of postqualification clinical experience including at least 6 months of dedicated experience in delivering PR and had passed local competency assessments.
Patients in the PR-gym group had access to aerobic and resistance training equipment such as treadmills, cycle ergometers and fixed weight machines for lower limb resistance, for example, leg press, knee extension. In contrast, those attending PR-min only had access to a walking course and simple portable resistance training equipment such as steps, free weights and elastic resistance bands (TheraBand®). Aerobic and resistance exercise prescription and progression was tailored to each patient and conducted according to local standard operating procedures and international guidance.10 Further information on exercise prescription and progression for both groups is available in table 1.
Table 1.
Exercise equipment, prescription and progression used for each group
| PR-gym | PR-min | ||
| Aerobic exercise | Exercise equipment | Treadmill or cycle ergometer. | Walking course. |
| Prescription | Treadmill: 60%–80% of peak-predicted oxygen consumption based on baseline ISWT performance, with the aim of achieving 3–4 on Borg CR10-Dyspnoea scale. Cycling: based on achieving 3–4 on Borg CR10-Dyspnoea scale. |
Initial target walking distance and time based on 60%–80% of peak-predicted oxygen consumption based on baseline ISWT performance, with the aim of achieving 3–4 on Borg CR10-Dyspnoea scale. | |
| Progression | Increased as tolerated with the aim of performing 30 min of aerobic exercise by the end of the programme. | Increased as tolerated with the aim of walking for 30 min by the end of the programme. | |
| Resistance training | Exercise equipment | Leg press or knee extension. | Elastic resistance bands (TheraBand®), portable steps and free weights. |
| Prescription | Strength: 2–4 sets of 8–12 repetitions at 60% of one-repetition maximum (1RM), with the aim of achieving a rate of perceived exertion (RPE) of 13–15. Endurance: 1–2 sets of 15–20 repetitions at <50% 1RM, with the aim of achieving an RPE of 11–13. |
Strength: 2–4 sets of 8–12 repetitions with the aim of achieving an RPE of 13–15. Endurance: 1–2 sets of 15–20 repetitions, with the aim of achieving an RPE of 11–13. |
|
| Progression | Both strength and endurance training were progressed by increasing the number of sets and repetitions, as well as increasing the weight or resistance. | Both strength and endurance training were progressed by increasing the number of sets and repetitions, as well as increasing the weight or resistance. | |
ISWT, incremental shuttle walk test; PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Both groups received the same education programme comprising 16 separate talks delivered by a multidisciplinary team, with the aim of promoting self-management. The list of topics covered is included in table 2.
Table 2.
Education topics provided by the Harefield Pulmonary Rehabilitation programme
| Education topics | Profession |
| Lung anatomy and disease | Physiotherapist or nurse |
| Medication | Pharmacist |
| Smoking cessation | Respiratory nurse specialist in smoking cessation and health promotion |
| Breathing techniques | Physiotherapist |
| Chest clearance techniques | Physiotherapist |
| Energy conservation/pacing | Occupational therapist |
| SALT (Speech and Language Therapist)—swallowing advice | Speech and language specialist |
| Diet and nutrition | Dietician |
| Lifestyle (parts 1 and 2) | Physiotherapist or nurse |
| Inhaler techniques | Physiotherapist or nurse |
| Coping with your lung condition | Clinical psychologist |
| Self-management | Physiotherapist |
| Chest infections | Physiotherapist |
| Benefits of exercise | Physiotherapist |
| Onward exercise | Physiotherapy assistant practitioner |
| Peer support | Patient representative |
All patients provided informed consent to participate in PR and for their anonymised data to be used for service evaluation purposes.
Assessments
Assessments were performed 1 week before and after the 8-week PR programme. Exercise capacity was measured using the ISW11 as described by the American Thoracic Society field walking guidelines.12 Health status was measured using the self-report Chronic Respiratory Disease Questionnaire (CRQ).13 Further outcomes measured included anthropometric measurements, Medical Research Council (MRC) dyspnoea scale, and spirometry. Comorbidities were evaluated using the Charlson Comorbidity Index.14 Completion rates were defined a priori as attendance at a post-PR assessment, as per the National Asthma and COPD Audit Programme (UK), and the completion of a minimum of eight supervised sessions. Attendance at a minimum of eight supervised sessions was included as a criterion as this has been shown to produce clinically significant short-term and medium-term responses.15 Adherence rate (number of supervised sessions attended) was also recorded. Completers were asked to rate their satisfaction with the programme on a 5-point Likert scale (1: Very satisfied; 2: Satisfied; 3: Indifferent; 4: Dissatisfied; 5: Very dissatisfied).
Matching
The control group was determined using propensity score matching,16 using a logistic regression model. The covariates entered into the model were age, sex, FEV1 % predicted, body mass index (BMI), MRC dyspnoea score, smoking status and baseline exercise capacity (measured by ISW), as these factors may influence response to PR. The matching was performed by a researcher blinded to whether participants had completed PR. Patients who undertook PR-gym were matched 1:1 to the closest propensity score in those who undertook PR-min. Assessment of baseline matching of the groups was performed using independent t-tests or Mann-Whitney U tests. All participants used in the propensity score matching had all the variables above recorded.
Sample size estimation
Previous audits of the Harefield Pulmonary Rehabilitation Unit have shown that participants undergoing PR-gym achieve a mean (SD) change in ISW of 58 (67) m. The non-inferiority margin was defined as half the known minimum clinically important difference (MCID) using the fixed margin method with a preserved effect of 50% as recommended by previous guidance, including from the US Food and Drug Administration.17 18 The MCID of the ISW is 48 m19 and therefore 24 m was considered the non-inferiority margin. The null hypothesis was that PR-min is inferior to the standard treatment (PR-gym). The alternative hypothesis was that PR-min is not inferior to PR-gym. If there is truly no difference between the standard and experimental treatment, then 406 patients (203 in each arm) were required to be 95% sure that the lower limit of a one-sided 97.5% CI (or equivalently a 95% two-sided CI) would be above the non-inferiority limit of −24. Assuming a 65% completer rate, we aimed to recruit a minimum of 313 participants in each intervention group.
Analysis
Data are presented as proportions and mean (SD or median (25th, 75th centiles) depending on the normality of the data). Baseline characteristics, adherence and completion rates were compared between groups using independent t-tests (or Mann-Whitney U test for data that were not normally distributed) and χ2 test. We performed a completer analysis and compared change from pre-PR to post-PR between groups using independent t-tests. The primary outcome was change in ISW, and change in overall health-related quality of life (as measured by the CRQ-total) was the main secondary outcome. We also compared individual CRQ-domains as well as PR programme completion and adherence rates, and programme satisfaction scores.
We performed non-inferiority analysis with the null hypothesis that experimental treatment (PR-min) was inferior to the standard treatment (PR-gym) by the non-inferiority margin. The non-inferiority margin was defined a priori as half the known MCID.18 As described above, the non-inferiority margin was set at 24 m. Similarly, given that the MCID for the CRQ is a mean change of 0.5 per question,20 the non-inferiority margins for the CRQ-dyspnoea (5 questions), CRQ-fatigue (4 questions), CRQ-emotion (7 questions), CRQ-mastery (4 questions) domains and CRQ-total score (20 questions) were set at 1.25, 1.0, 1.75, 1.0 and 5, respectively. Post hoc analysis to investigate any differences in socioeconomic deprivation using Index of Multiple Deprivation was also performed.21
Analyses were performed using SPSS (V.22, IBM) and graphs were produced using Prism V.7 (GraphPad Software, San Diego, California, USA). A one-sided p value <0.025 was considered statistically significant.
Results
During the study period, 318 patients with COPD opted to attend PR-min and 955 patients with COPD opted to attend PR-gym. From the 955 patients attending PR-gym, data from 318 patients were used for the propensity-matched control group. Baseline characteristics according to PR-min and PR-gym are detailed in table 3. The PR-min and PR-gym groups were successfully matched at baseline for age, sex, FEV1 % predicted, BMI, smoking status, home oxygen status, ISW and CRQ-total. A post hoc comparison of socioeconomic deprivation between PR-min and PR-gym demonstrated a lower Index of Multiple Deprivation in the PR-min group (PR-min 18 259 (7374) vs PR-gym 19 229 (8242); p=0.028). All those that completed PR had complete data for the primary and secondary analyses.
Table 3.
Baseline clinical characteristics of PR-min and PR-gym groups
| Whole group | Completers | |||||||
| PR-min (n=318) |
PR-gym (n=318) |
Test statistic | P value | PR-min (n=204) |
PR-gym (n=232) |
Test statistic | P value | |
| Male: n (%) | 145 (45.6) | 157 (49.4) | 0.91 | 0.34 | 115 (56.9) | 113 (48.9) | 2.78 | 0.10 |
| Age (years) | 71 (10) | 71 (9) | −0.20 | 0.84 | 72 (9) | 71 (9) | 0.24 | 0.81 |
| FEV1 (L) | 1.12 (0.57) | 1.08 (0.95) | 0.99 | 0.32 | 1.02 (0.74, 1.43) | 0.99 (0.75, 1.44) | −0.18 | 0.85 |
| FEV1 (% predicted) | 46.8 (19.7) | 45.8 (18.7) | −0.60 | 0.55 | 47.9 (19.4) | 47.7 (18.8) | 0.08 | 0.94 |
| GOLD staging (A/B/C/D) (%) | 3/12/8/77 | 3/18/4/74 | 7.01 | 0.07 | 5:13:9:72 | 4:21:5:70 | 6.43 | 0.09 |
| BMI (kg/m2) | 27.5 (6.7) | 27.4 (6.7) | −0.18 | 0.86 | 27.6 (6.4) | 27.2 (6.1) | 0.82 | 0.41 |
| Home oxygen user: n (%) | 28 (8.8) | 35 (11.0) | 0.86 | 0.35 | 16 (7.8) | 27 (11.7) | 1.71 | 0.19 |
| Smoking status: current:former:never (%) |
19:75:6 | 20:75:5 | 0.57 | 0.75 | 16:78:6 | 15:80:5 | 0.25 | 0.88 |
| MRC | 4 (3, 4) | 4 (3, 4) | 0.27 | 0.79 | 3 (1) | 3 (1) | −1.24 | 0.22 |
| ISW (m) | 192 (134) | 195 (144) | 0.31 | 0.76 | 207 (136) | 190 (90, 270) | −0.60 | 0.55 |
| CRQ-total | 75.2 (21.9) | 73.3 (20.5) | −1.10 | 0.27 | 78.3 (21.2) | 74.6 (21.6) | 1.71 | 0.09 |
| Charlson Comorbidity Index | 1 (1, 3) | 1 (1, 3) | −0.64 | 0.52 | 1.5 (1, 3) | 1 (1, 3) | −1.11 | 0.27 |
| Self-reported exacerbations in previous year (n) | 2 (1, 4) | 2 (1, 3) | −0.91 | 0.37 | 2 (1, 4) | 2 (1, 3) | −0.37 | 0.71 |
| Self-reported hospital days in previous year | 0 (0, 3) | 0 (0, 4) | −0.03 | 0.97 | 0 (0, 3) | 0 (0, 3) | 0.50 | 0.62 |
Data expressed as n, mean (SD) or median (25th and 75th percentiles).
Data were analysed using independent t-tests (or Mann-Whitney U test for data that were not normally distributed) and χ2 test. All participants had data for each of the listed variables.
BMI, body mass index; CRQ, Chronic Respiratory Disease Questionnaire; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease guidelines; ISW, incremental shuttle walk; MRC, Medical Research Council dyspnoea score; PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Response to PR
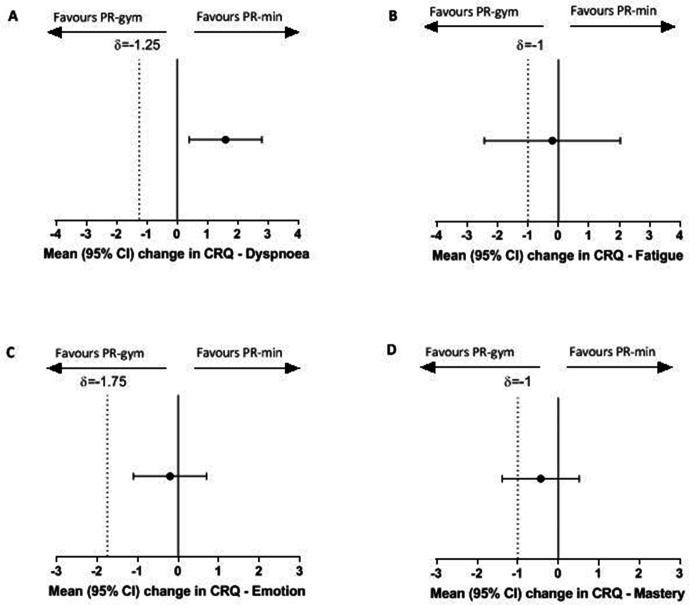
Table 4 shows the completer response in both PR-min (n=204) and PR-gym (n=232) groups. Clinically and statistically significant improvements in ISW, CRQ-total and all CRQ-domains were observed in both groups following PR. The magnitude of changes in ISW, CRQ-total and CRQ-domains was greater than the commonly accepted MCID for these outcomes.19 20 No significant between-group differences were seen in changes in ISW, CRQ-total, CRQ-fatigue, CRQ-emotion and CRQ-mastery. However, there was a statistically greater change in CRQ-dyspnoea domain in PR-min compared with PR-gym, although there was uncertainty about the clinical significance given that the between-group difference was smaller than the commonly accepted MCID (table 4).
Table 4.
Comparison of clinical outcomes following pulmonary rehabilitation between PR-min and PR-gym groups
| Change with PR | Between-group differences | Test statistic | P value | ||
| PR- min (n=204) |
PR-gym (n=232) |
||||
| ISW (m) | 56.6 (47.8 to 65.4) | 59.7 (50.9 to 68.6) | −3.1 (−15.6 to 9.4) | −0.49 | 0.63 |
| CRQ-dyspnoea | 5.8 (4.9 to 6.7) | 4.2 (3.4 to 5.0) | 1.6 (0.4 to 2.8) | 2.63 | 0.009 |
| CRQ-fatigue | 2.8 (2.1 to 3.4) | 3.0 (2.4 to 3.6) | −0.2 (−1.1 to 0.7) | −0.51 | 0.61 |
| CRQ-emotion | 4.3 (3.2 to 5.3) | 4.3 (3.3 to 5.3) | −0.02 (−1.5 to 1.4) | −0.04 | 0.97 |
| CRQ-mastery | 2.6 (1.9 to 3.2) | 3.0 (2.4 to 3.7) | −0.4 (−1.4 to 0.5) | −0.90 | 0.37 |
| CRQ-total | 15.5 (12.9 to 18.2) | 14.6 (12.2 to 17.2) | 0.9 (−2.7 to 4.5) | 0.49 | 0.62 |
Data expressed as mean (95% CI) or median (25th and 75th percentiles) pre-to-post PR.
Data were analysed using independent t-tests (or Mann-Whitney U test for data that were not normally distributed). All participants had data for each of the listed variables.
CRQ, Chronic Respiratory Disease Questionnaire; ISW, incremental shuttle walk; PR, pulmonary rehabilitation; PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Completion, adherence and satisfaction rates
The overall completion rate for the whole cohort from attending pre-PR assessment was 68.6%. Completion rates were lower in the PR-min group (64.2%) than the PR-gym group (73.0%) (z-score=2.46, p=0.014). This was corroborated by overall group adherence data; those in the PR-min group attended fewer supervised sessions (mean=9.1, SD=5.9) than those in PR-gym ((mean=10.4, SD=5.2) (t-statistic=2.88; p=0.004)). However, for completers, there was no difference in the median (25th, 75th centiles) number of supervised sessions attended (PR-min (n=204): 14 (12, 15) vs PR-gym (n=232): 14 (12, 15); p=0.598). There was no between-group difference in the proportion of patients reporting they were either very satisfied or satisfied following intervention (PR-min: 99.5% vs PR-gym: 98.3%; p=0.348). In patients for whom a reason for non-completion was provided, dropout was largely attributed to exacerbation of their COPD or worsening of a comorbidity (figure 1).
Figure 1.
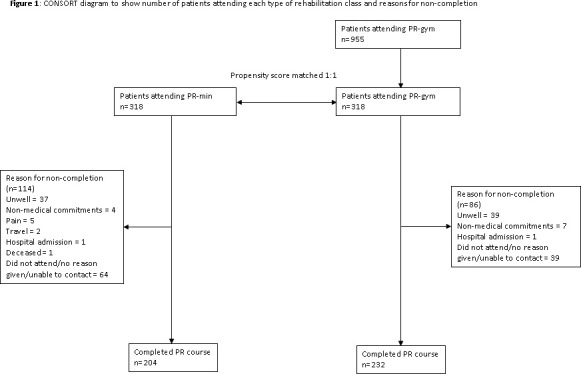
Consolidated Standards of Reporting Trials (CONSORT) diagram to show the number of patients attending each type of rehabilitation class and reasons for non-completion. PR, pulmonary rehabilitation; PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Non-inferiority analysis
For the primary outcome, change in ISW, figure 2A shows that the 95% CI between-group difference did not cross the predefined non-inferiority margin of −24 m, indicating that PR-min was non-inferior to PR-gym. Similar results were seen for CRQ-total (figure 2B), CRQ-dyspnoea and CRQ-emotion (figure 3). However, for CRQ-fatigue and CRQ-mastery, the 95% CI of the between-group difference in change did cross the predefined non-inferiority margin. For these outcomes, it was not possible to conclude non-inferiority of PR-min.
Figure 2.
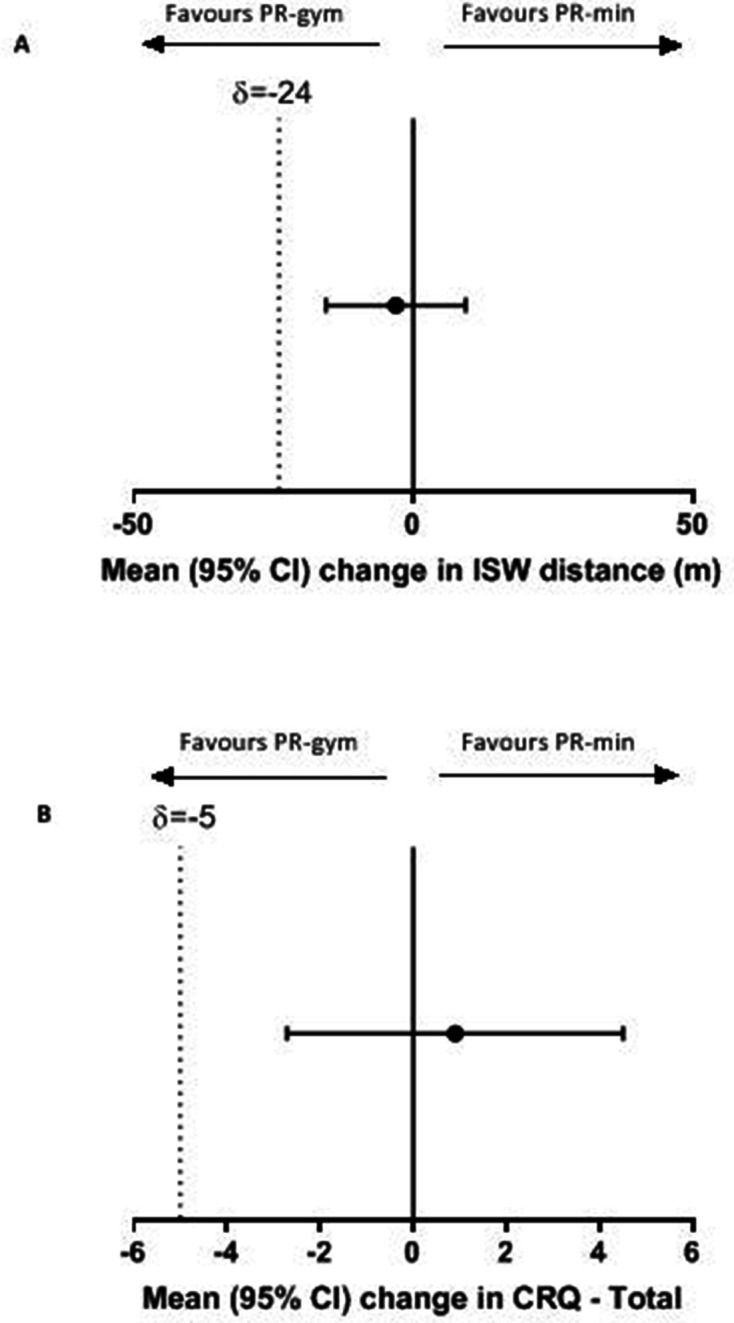
Between-group changes in (A) incremental shuttle walk (ISW). (B) Chronic Respiratory Disease Questionnaire (CRQ)—total and non-inferiority limits. Data expressed as mean (95% CIs). The dotted line represents the non-inferiority limit. PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Figure 3.
Between-group change in Chronic Respiratory Disease Questionnaire (CRQ) domains (A) dyspnoea, (B) fatigue, (C) emotion, and (D) mastery and non-inferiority limits. Data expressed as mean (95% CIs). The dotted line represents the non-inferiority limit. PR-gym, pulmonary rehabilitation using specialist equipment; PR-min, pulmonary rehabilitation using minimal equipment.
Discussion
Our study shows that PR delivered using minimal equipment (PR-min) produces clinically significant improvements in exercise capacity and health-related quality of life in patients with COPD. These improvements were of similar magnitude to those observed in a well-matched group of patients with COPD undergoing PR delivered using specialist exercise equipment (PR-gym). With formal non-inferiority analysis, we found PR-min to be non-inferior to PR-gym in terms of improvements in exercise capacity and overall health-related quality of life. However, we also observed lower completion rates with PR-min.
Relation to previous studies
There remains uncertainty about the efficacy of PR programmes using minimal equipment. Alison and McKeough identified eight trials that used low-cost equipment for endurance exercise training and demonstrated statistically and clinically significant differences in functional exercise capacity and health-related quality of life compared with no training at all.8 However, these trials were small (six of the eight trials had a combined sample size of less than 50 patients) and of variable quality (selective patient population, varied length of intervention and intensity of training achieved). Furthermore, there were conflicting results. Whereas the pooled data from four trials that used the 6 min walk distance showed a positive effect on exercise capacity, no significant effect was seen in the four trials that used the ISW.8
To our knowledge, our study is the first to directly compare a supervised PR programme using minimal exercise equipment with a supervised programme using specialist exercise equipment. Previous studies have compared different settings where access to specialist equipment may not have been possible, such as in the home.5 22–24 However, in some trials, investigators were careful to ensure that exercise training was similar between groups and did not allow the gold standard control programme to have access to specialist equipment.23 25 In other trials that compared different settings and level of supervision and where with a difference in accessibility to equipment, the ‘gold-standard’ arm did not produce the anticipated improvements.5 22 26 A possible explanation is that the trial populations were selective with a preference for the alternative intervention to supervised rehabilitation. For example, in the trial from Holland and colleagues, a significant proportion of patients assessed for trial eligibility were not randomised because they wanted to attend supervised hospital-based PR with access to specialist exercise equipment.5
Methodological reflections
Our study has several strengths. First, our sample size was greater than the combined eight trials previously reported in a systematic review.8 As such, we had sufficient power to perform a non-inferiority analysis and conclude that PR-min was non-inferior to PR-gym in terms of effects on ISW and CRQ-total, our primary and main secondary outcomes. Second, the interventions were delivered by the same experienced team using standard operating procedures for exercise prescription, and both interventions were delivered according to national quality standards.2 27 The improvements in the outcome measures in both groups were clinically significant, with changes exceeding the MCID for the ISW and CRQ-domains, and exceeding the median changes observed in the national audit of PR services in England and Wales.7 Third, the groups were carefully matched at baseline using a validated statistical method, and the study design allowed patients to select the PR site of their choice. This might have reduced any potential recruitment bias that might be observed in randomised controlled trials, particularly when there may not be patient equipoise.
Although we accounted for potential confounders by using an established statistical approach (propensity score matching) and our groups were well balanced at baseline for clinical characteristics identified a priori, we cannot exclude the possibility that the results might be explained due to an imbalance between the groups. A randomised controlled trial of the two types of equipment would be apposite. However, the study population was typical of those attending PR programmes in England and Wales.7
Another limitation is that we only recorded data on short-term response to PR. An argument to support PR-min is that it uses relatively cheap, portable equipment that might be more feasible for regular, continued use at home. Hence, longer term follow-up data to identify the maintenance of benefits of PR would have been of interest.
Our outcome data were limited to exercise capacity, dyspnoea and health-related quality of life—the variables that are expected to improve with PR. However, a wider range of outcomes might have provided more information about the relative advantages and disadvantages of accessibility to specialist exercise equipment. For example, lower limb muscle strength, which is an independent prognostic factor in COPD,28 29 might have been of interest as the availability of specialist resistance equipment might particularly influence this outcome. Our study was also focused on patients with COPD, and therefore our findings cannot be extrapolated to patients with other chronic respiratory diseases undergoing PR.30 31
Unexpected findings
We observed a statistically greater improvement in dyspnoea in the PR-min group compared with PR-gym. However, this difference was small, and smaller than the accepted MCID for the CRQ-dyspnoea. The difference may have been due to the nature of the CRQ-dyspnoea domain which asks about the level of dyspnoea associated with five everyday functional activities chosen by the patient. We speculate that the exercises performed during PR-min might be more reflective of these functional activities than, for example, treadmill walking or cycle ergometry, which dominated the aerobic exercise training in PR-gym.
Another unexpected finding was that the completion and adherence rates were lower in PR-min compared with PR-gym. Unfortunately, only about one-third of those who dropped out from PR were contactable and therefore patient feedback was limited. The principal reason for dropout was either exacerbation of COPD or worsening of another comorbidity. Although we cannot exclude the possibility that the content of PR-min was directly responsible for this, we think it mechanistically unlikely. We speculate that the difference in completion rates was related to subtle baseline differences in demographics that were not identified in the matching process. For example, socioeconomic deprivation and frailty are two recently identified variables that influence completion rates.21 32 A post hoc comparison of socioeconomic deprivation using the Multiple Deprivation Index demonstrated a lower index (ie, lower socioeconomic status) in the PR-min group. We hypothesise that this baseline imbalance in socioeconomic deprivation may explain the difference in completion rates observed in our study.
Clinical implications
The major finding of our study was that PR-min produced clinically significant benefits in exercise capacity and health-related quality of life that were non-inferior to those observed in a matched group of patients undergoing PR-gym. Staff skill set is considered an important factor in determining the effects of rehabilitation, with 96% of multidisciplinary cardiopulmonary rehabilitation specialists agreeing that the way that rehabilitation is delivered by healthcare professionals has an important influence on success.33 This may be more influential than accessibility to specialist equipment.
Globally, demand for PR outstrips supply. In the UK, for example, the National Health Service Long Term Plan specifically highlights the importance of expanding PR services.34 PR programmes are increasingly provided in places that are convenient for patients to access, such as church halls and community centres, but might have limited access to specialist exercise equipment. Our study provides reassurance that such programmes, when supervised by skilled therapists, provide the same benefits as ‘gold-standard’ programmes that have access to specialist equipment. Limited access to specialist and more expensive exercise equipment need not be a barrier to developing clinically effective PR programmes and increasing accessibility to a wider group of patients. This is also relevant in low to middle-income countries where access to specialist exercise equipment may be limited.35
Future research
Although we used a validated statistical method to match our treatment groups, randomisation would have helped balance out any unknown confounding factors that might have influenced the results, for example, socioeconomic deprivation. We propose that future work should include a randomised controlled trial. The trial should incorporate a wider range of outcomes (eg, muscle strength measures which might be more influenced by the equipment used) as well as longer term follow-up to evaluate whether there are differences in maintenance of benefits. Such a trial should have an accompanying analysis to compare the relative cost-effectiveness of PR programmes that use minimal or specialist exercise equipment. We would also advocate both intention-to-treat and per-protocol analyses as it is important to understand how non-completion might influence overall group benefits.
In summary, we have demonstrated that PR delivered using minimal equipment produces clinically significant benefits in exercise capacity and health-related quality of life that are non-inferior to rehabilitation delivered using specialist equipment in patients with COPD. Our study provides reassurance that limited access to specialist exercise equipment is not a barrier to developing a clinically effective PR programme, thus potentially increasing accessibility to a wider group of patients.
Footnotes
Twitter: @suhani_patel1, @clairemnolan84, @COPDdoc, @toplungdoc
SP, MDP and CMN contributed equally.
Contributors: Concept and design of study: MDP, WDCM. Acquisition of data: MDP, CMN, SEJ, SP, REB, JAW, SCW. Interpretation and analysis of data: SP, SEJ, MDP, CMN, WG, MM, WDCM. Drafting of manuscript: SP, WDCM. Revision of manuscript critically for important intellectual content: all authors. Approval of final manuscript: all authors. WDCM acts as the guarantor of the data.
Funding: This study was funded by a National Institute for Health Research (NIHR) Research for Patient Benefit grant PB-PG-0816-20022. SP is supported by an NIHR Clinical Doctoral Research Fellowship (NIHR 300566). REB is supported by an NIHR Clinical Doctoral Research Fellowship (ICA-CDRF-2017-03-018). CMN is supported by an NIHR Clinical Trials Fellowship (CTF-2017-06-005) and a British Lung Foundation Project Grant (IPFPG17-15). SEJ is supported by an NIHR Doctoral Research Fellowship (DRF-2015-08-004). JAW and SCW are supported by an NIHR Research for Patient Benefit PB-PG-0816-20022. MM is supported by an NIHR Career Development Fellowship (CDF-2017-10-009). WG and MM are supported by NIHR Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. This paper presents independent research funded by the National Institute for Health Research (NIHR).
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: WDCM reports grants from the National Institute for Health Research, during the conduct of the study; grants from Pfizer and the British Lung Foundation, non-financial support from GSK, personal fees from Jazz Pharmaceuticals, Mundipharma and Novartis, outside the submitted work. CMN reports personal fees from Novartis, outside the submitted work. SSCK reports personal fees from Novartis, outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: On completion of the HRA ethics approval decision tool (http://www.hra-decisiontools.org.uk), no ethical approval was required as this study was defined as service evaluation. Registration was completed with the Royal Brompton and Harefield Hospital NHS Foundation Trust clinical audit department as per local protocols (Registration No 1237).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;3 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68 Suppl 2:ii1–30. 10.1136/thoraxjnl-2013-203808 [DOI] [PubMed] [Google Scholar]
- 3. Ward TJC, Plumptre CD, Dolmage TE, et al. Change in V.O2peak in Response to Aerobic Exercise Training and the Relationship With Exercise Prescription in People With COPD: A Systematic Review and Meta-analysis. Chest 2020;158:131–44. 10.1016/j.chest.2020.01.053 [DOI] [PubMed] [Google Scholar]
- 4. Philip K, Gaduzo S, Rogers J, et al. Patient experience of COPD care: outcomes from the British lung Foundation patient Passport. BMJ Open Respir Res 2019;6:e000478. 10.1136/bmjresp-2019-000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holland AE, Mahal A, Hill CJ, et al. Home-Based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017;72:57–65. 10.1136/thoraxjnl-2016-208514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasilopoulou M, Papaioannou AI, Kaltsakas G, et al. Home-Based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J 2017;49:1602129. 10.1183/13993003.02129-2016 [DOI] [PubMed] [Google Scholar]
- 7. Steiner MMV, Lowe D, Holzhauer-Barrie J. Pulmonary rehabilitation: an exercise in improvement. National chronic obstructive pulmonary disease (COPD) audit programme: clinical and organisational audit of pulmonary rehabilitation services in England and Wales 2017. clinical audit data analysis and result. London: Royal College of Physicians, 2018. [Google Scholar]
- 8. Alison JA, McKeough ZJ. Pulmonary rehabilitation for COPD: are programs with minimal exercise equipment effective? J Thorac Dis 2014;6:1606–14. 10.3978/j.issn.2072-1439.2014.07.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 10. Medicine ACoS ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed Lippincott Williams & Wilkins, 2017. [Google Scholar]
- 11. Singh SJ, Morgan MD, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–24. 10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holland AE, Spruit MA, Troosters T, et al. An official European respiratory Society/American thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 13. Williams JE, Singh SJ, Sewell L, et al. Development of a self-reported chronic respiratory questionnaire (CRQ-SR). Thorax 2001;56:954–9. 10.1136/thorax.56.12.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15. Sewell L, Singh SJ, Williams JEA, et al. How long should outpatient pulmonary rehabilitation be? a randomised controlled trial of 4 weeks versus 7 weeks. Thorax 2006;61:767–71. 10.1136/thx.2005.048173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 17. Wangge G, Putzeist M, Knol MJ, et al. Regulatory scientific advice on non-inferiority drug trials. PLoS One 2013;8:e74818. 10.1371/journal.pone.0074818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 19. Singh SJ, Jones PW, Evans R, et al. Minimum clinically important improvement for the incremental shuttle walking test. Thorax 2008;63:775–7. 10.1136/thx.2007.081208 [DOI] [PubMed] [Google Scholar]
- 20. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–15. 10.1016/0197-2456(89)90005-6 [DOI] [PubMed] [Google Scholar]
- 21. Steiner MC, Lowe D, Beckford K, et al. Socioeconomic deprivation and the outcome of pulmonary rehabilitation in England and Wales. Thorax 2017;72:530–7. 10.1136/thoraxjnl-2016-209376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horton EJ, Mitchell KE, Johnson-Warrington V, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax 2018;73:29–36. 10.1136/thoraxjnl-2016-208506 [DOI] [PubMed] [Google Scholar]
- 23. Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open 2017;7:e014580. 10.1136/bmjopen-2016-014580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nolan CM, Kaliaraju D, Jones SE, et al. Home versus outpatient pulmonary rehabilitation in COPD: a propensity-matched cohort study. Thorax 2019;74:996–8. 10.1136/thoraxjnl-2018-212765 [DOI] [PubMed] [Google Scholar]
- 25. Waterhouse JC, Walters SJ, Oluboyede Y, et al. A randomised 2 X 2 trial of community versus Hospital pulmonary rehabilitation, followed by telephone or conventional follow-up. Health Technol Assess 2010;14:1–140. 10.3310/hta14060 [DOI] [PubMed] [Google Scholar]
- 26. Polkey MI, Qiu Z-H, Zhou L, et al. Tai chi and pulmonary rehabilitation compared for treatment-naive patients with COPD: a randomized controlled trial. Chest 2018;153:1116–24. 10.1016/j.chest.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 27. Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic Society/European respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 28. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007;62:115–20. 10.1136/thx.2006.062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Decramer M, Gosselink R, Troosters T, et al. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997;10:417–23. 10.1183/09031936.97.10020417 [DOI] [PubMed] [Google Scholar]
- 30. Patel S, Cole AD, Nolan CM, et al. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. Eur Respir J 2019;53:1801264. 10.1183/13993003.01264-2018 [DOI] [PubMed] [Google Scholar]
- 31. Nolan CM, Delogu V, Maddocks M, et al. Validity, responsiveness and minimum clinically important difference of the incremental shuttle walk in idiopathic pulmonary fibrosis: a prospective study. Thorax 2018;73:680–2. 10.1136/thoraxjnl-2017-210589 [DOI] [PubMed] [Google Scholar]
- 32. Maddocks M, Kon SSC, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016;71:988–95. 10.1136/thoraxjnl-2016-208460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Man WD-C, Chowdhury F, Taylor RS, et al. Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure. Chron Respir Dis 2016;13:229–39. 10.1177/1479972316642363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NHS The NHS long term plan, 2019. Available: https://wwwlongtermplannhsuk/
- 35. Jones R, Kirenga BJ, Katagira W, et al. A pre-post intervention study of pulmonary rehabilitation for adults with post-tuberculosis lung disease in Uganda. Int J Chron Obstruct Pulmon Dis 2017;12:3533–9. 10.2147/COPD.S146659 [DOI] [PMC free article] [PubMed] [Google Scholar]