Abstract
The adaptability of the central nervous system has been revealed in several model systems. Of particular interest to central nervous system-injured individuals is the ability for neural components to be modified for regain of function. In both types of neurotrauma, traumatic brain injury and spinal cord injury, the primary parasympathetic control to the gastrointestinal tract, the vagus nerve, remains anatomically intact. However, individuals with traumatic brain injury or spinal cord injury are highly susceptible to gastrointestinal dysfunctions. Such gastrointestinal dysfunctions attribute to higher morbidity and mortality following traumatic brain injury and spinal cord injury. While the vagal efferent output remains capable of eliciting motor responses following injury, evidence suggests impairment of the vagal afferents. Since sensory input drives motor output, this review will discuss the normal and altered anatomy and physiology of the gastrointestinal vagal afferents to better understand the contributions of vagal afferent plasticity following neurotrauma.
Keywords: gastrointestinal functions, microbiome, neurotrauma, nodose ganglia, sensory neuropathy, spinal cord injury, traumatic brain injury, vagal afferents, visceral reflexes
Introduction
Central nervous system (CNS) trauma, or neurotrauma, is a result of sudden insult or mechanical force to the head or spine (Chang and Badjatia, 2014; Wang et al., 2018; Yates et al., 2019). The types of neurotrauma vary but include concussion, skull fractures, traumatic brain injury (TBI), spinal column fractures, and spinal cord injuries (SCI; Icahn, 2020). The World Health Organization (WHO) has recognized neurotrauma as a “critical public health problem” which leads to significant losses to the injured individual, the individual’s family, and the entire community as a result of lifelong disabilities or death (WHO, 2020). One comorbidity that is often overlooked following neurotrauma is dysregulation of the gastrointestinal (GI) organs associated with nutrient homeostasis. The function of the GI tract in health and disease is well-studied in the neurally-intact organism. Due to increased survivability after neurotrauma, it is imperative to understand the long-term consequences of neural injury and plasticity towards repair and regain of function of this vital organ system.
In the USA, there are approximately 1.7–2.0 million incidents of TBI annually (Huang, 2013; Wang et al., 2018), and there are about 5.3 million Americans currently living with permanent TBI-related comorbidities (Wang et al., 2018). These comorbidities include derangements in physical, emotional, and behavioral functions, neurocognitive disabilities (Langlois et al., 2006; McCrory et al., 2009; Daneshvar et al., 2011) and a vegetative state (McKee and Daneshvar, 2015). While there are both intracranial and extracranial complications post-TBI, the extracranial deficits are systemic and may involve any organ system. The most commonly affected organ systems include the vagally-innervated respiratory, cardiovascular, and GI systems. GI dysfunctions following TBI (Pilitsis and Rengachary, 2001; Hang et al., 2003; Zygun et al., 2005; Kemp et al., 2008; Masel and DeWitt, 2010; Olsen et al., 2013; Katzenberger et al., 2015; Vance et al., 2015) affect greater than 50% (Qi et al., 2011) of TBI individuals and include impaired swallowing (Mackay et al., 1999; Morgan and Mackay, 1999), decreased pharyngeal sensation, prolonged transit, (Morgan and Mackay, 1999), GI bleeding (Kamada et al., 1977; Brown et al., 1989; Lu et al., 1997), gastric reflux (Saxe et al., 1994; Kao et al., 1998), delayed gastric emptying (Qi et al., 2011), decreased tone in the lower esophageal sphincter (Kamada et al., 1977; Brown et al., 1989; Philip, 1992; Saxe et al., 1994; Pedoto et al., 1995; Lu et al., 1997; Kao et al., 1998; Pilitsis and Rengachary, 2001), gastroparesis (Brown et al., 1989; Jackson and Davidoff, 1989; Pilitsis and Rengachary, 2001), decreased intestinal peristalsis (Philip, 1992; Pedoto et al., 1995), mucosal damage with increased gut permeability (Bansal et al., 2009; Katzenberger et al., 2015), and altered GI motility (Jackson and Davidoff, 1989). Of the post-TBI GI complications, the most common comorbidity is gastritis with a reported incidence of 74–100% (Kamada et al., 1977; Brown et al., 1989). Lastly, 68% of TBI individuals suffer from chronic malnourishment (Horneman et al., 2005) which further exacerbates system-wide deficits from lack of nutrients. GI dysfunctions contribute to post-TBI morbidity and mortality (Qi et al., 2011); yet, the exact mechanisms for GI dysfunctions following TBI remain unclear.
The devastating losses in motor, GI, bladder, and sexual function are continually rated as the top regain of function priorities for SCI individuals (Anderson, 2004; Simpson et al., 2012; van Middendorp et al., 2016). Bladder and GI dysfunctions have been described as the “most debilitating” of these burdens because they not only impact the health of SCI individuals, but greatly limit their relationships and social interactions, further diminishing their quality of life (Simpson et al., 2012; Braaf et al., 2017). Specifically, reports indicate that high thoracic or cervical spinal trauma most often lead to delayed gastric emptying, early satiety, dysphagia, constipation, incontinence, nausea, bloating, and abdominal pain (Wolf and Meiners, 2003; Park and Camilleri, 2006; Fynne et al., 2012).
Gastric functions and other upper GI functions are dominated by and dependent on extrinsic parasympathetic nervous system circuits for regulation (Browning and Travagli, 2014; Travagli and Anselmi, 2016). The vagus nerve (VN) contains the visceral afferent and efferent fibers essential for parasympathetic nervous system-mediated reflexes. In both TBI and SCI, the VN remains anatomically intact following injury; however, evidence suggests that the parasympathetic nervous system and the enteric nervous system (ENS) within the gut no longer cooperatively coordinate GI functions suggesting a disruption in the vago-vagal reflexes. The normal anatomy and physiology of the VN has been extensively discussed by others (Lal et al., 2001; Browning and Mendelowitz, 2003; Undem et al., 2004; Blackshaw et al., 2007; Wang and Powley, 2007; Browning and Travagli, 2011; Potenzieri et al., 2012; Holmes et al., 2013; Bonaz et al., 2016; Grabauskas and Owyang, 2017; Breit et al., 2018; Driessen, 2019; Fülling et al., 2019). For our purposes, the following brief summary of VN anatomy and physiology is provided to give a frame of reference for our discussion of vagal neuroplasticity following CNS trauma. This review highlights the role of the VN in GI sensory signaling and the neuroplasticity that occurs following TBI and SCI.
The PubMed database was used between November 2019 and January 2020, using the search terms: traumatic brain injury/TBI, spinal cord injury/SCI, stroke, nodose ganglia, vagal afferents, vagus nerve, microbiome, and various combinations of these search terms.
Normal Anatomy and Physiology of the Vagus Nerve
Gastrointestinal vago-vagal reflex
The autonomic regulation of the GI tract in the context of SCI has recently been reviewed (Holmes and Blanke, 2019). Briefly, the VN is a central link in the brain-gut axis and contributes to the neural modulation of digestive (i.e., vago-vagal) reflexes. The vagal afferents transmit glutamatergic signals to the nucleus tractus solitarius (NTS) in response to GI mechanical and chemical stimuli (Figure 1). Within the caudal medulla, NTS interneurons may inhibit or excite cholinergic vagal efferents of the dorsal motor nucleus of the vagus (DMV). These DMV efferent neurons complete the vago-vagal circuit (Browning and Travagli, 2010) by synapsing onto parasympathetic postganglionic cholinergic or non-adrenergic non-cholinergic neurons in the submucosal and myenteric plexuses of the ENS. The functional response of the postganglionic enteric neurons may be excitatory causing an increase in GI tone, motility, and secretion or inhibitory preventing GI digestive functions (Travagli et al., 2006; Browning and Travagli, 2011). Proper GI functioning is dependent upon vagal reflexes, and any disruption in the circuit leads to GI pathologies in the neurally intact including but not limited to gastroparesis, dyspepsia, esophageal reflux, colitis, anorexia and bulimia nervosa (Yamano et al., 1997; Hornby and Abrahams, 2000; Andrews and Sanger, 2002; Thumshirn, 2002; Saito et al., 2006; Ghia et al., 2007; Faris et al., 2008).
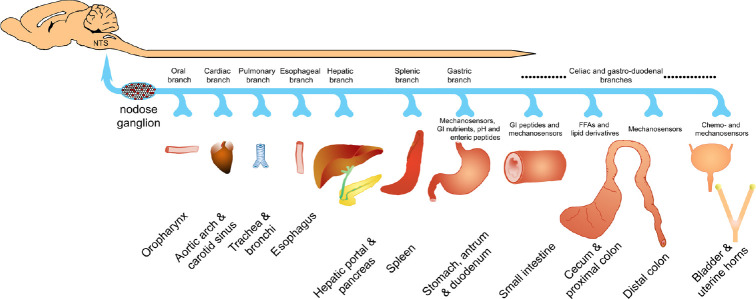
Figure 1.
Major vagal afferent innervation.
The majority of the vagus nerve is comprised of sensory fibers and the cell bodies of vagal afferents are contained within the co-joined jugular and nodose ganglia. The afferent vagus innervates from the oral cavity to the transverse colon in humans, but tracing studies in rodent models have demonstrated afferent projections as caudal as the rectum, bladder, and reproductive tract. Vagal afferents carry mechanical and/or chemical sensory information to the caudal medulla, specifically the NTS and nucleus ambiguus. The cell bodies of the gastrointestinal viscera are distributed throughout the nodose ganglia (depicted in red) and vagally-mediated reflexes dominate esophageal and gastric reflexes. Functional alterations within all visceral organs following pathophysiological remodeling may become more pronounced in some disease and injury states. GI: Gastrointes tinal; NTS: nucleus tractus solitarius.
Our research focuses on how CNS trauma modifies the GI vago-vagal reflex and leads to GI dysfunction. Previously, we have shown that gastric vagal efferents are intact following experimental SCI, and DMV neurons projecting to the stomach respond similarly to uninjured controls (Swartz and Holmes, 2014). Vagal afferents demonstrate a significant amount of plasticity during both normal physiology and pathophysiology such as high fat diet, diabetes, inflammation, and direct vagal injury (Browning, 2003; Browning and Mendelowitz, 2003; Li and Owyang, 2003; Raybould, 2010; Dockray and Burdyga, 2011; Kentish and Page, 2014; de Lartigue, 2016; Travagli and Anselmi, 2016; Browning et al., 2017; Grabauskas and Owyang, 2017; Cawthon and de La Serre, 2018; de Lartigue and Xu, 2018). Therefore, we propose that vagal afferent alterations may be, in part, responsible for GI dysfunction following neurotrauma.
Gastrointestinal vagal afferent physiology
The GI vagal afferent cell bodies are contained within the nodose ganglia (NG) and have both peripheral and central projections (Figure 1). The vagal afferent terminal nerve endings are diffusely spread throughout layers of the walls of the proximal GI tract (reaching from mouth to transverse colon in humans (Rea, 2014) and distal colon in rats (Altschuler et al., 1993; Herrity et al., 2014). They consist of mechanoreceptor morphological specializations, known as intraganglionic laminar endings, for detecting tension and stretch in the muscle wall and intramuscular arrays which transduce stretch of the organ wall (Powley and Phillips, 2002). Additionally, chemical stimuli such as gut peptides like cholecystokinin (CCK) and ghrelin are detected by chemoreceptors (Powley and Phillips, 2002) on vagal afferent peripheral endings within GI mucosa or on the vagal afferent somas within the NG. CCK and ghrelin elicit opposite responses on the vagal afferents. CCK is released from enterochromaffin I-cells when proteins and lipids are taken into the GI tract. CCK then binds to CCK 1/A receptors on the vagal afferents or NG, thus causing the vagal afferent nerve firing to increase (Moran et al., 1990; Berthoud and Patterson, 1996; Moriarty et al., 1997; Broberger et al., 2001; Patterson et al., 2002). Ghrelin on the other hand, is released from enterochromaffin X/A-cells during the fasting state. Ghrelin acts on the growth-hormone secretgogue receptor A on both the vagal afferents and NG to inhibit vagal afferent firing (Kojima et al., 1999; Date et al., 2000, 2005; Page et al., 2007). Other gut peptides act on and alter vagal afferent firing such as: leptin, bombesin, PYY, secretin, GLP-1, serotonin, and VIP. The actions of CCK, ghrelin, and these other gut peptides have been thoroughly reviewed (Grabauskas and Owyang, 2017). The expression of the receptors of these gut peptides have been shown to change based on the nutrient state of the organism (Grabauskas and Owyang, 2017); demonstrating plasticity under normal physiological conditions.
The fibers which carry the detected mechanical and chemical stimuli can be classified into two primary groups: lightly myelinated A-type and unmyelinated C-type afferent fibers (Schild et al., 1994). A- and C-fibers have different biophysical properties which have been thoroughly reviewed (Browning, 2003). The types of fibers can be distinguished by their myelination, firing rate, conduction velocity, and action potential kinetics. A-fibers are lightly myelinated, tonic firing neurons with a conduction velocity of 3–30 m/s and unremarkable action potentials. While C-fibers are unmyelinated, phasic firing neurons with a conduction velocity of 0.8–1.2 m/s and distinguishing action potentials. C-fibers make up 80–90% of the vagal afferents and have a noticeable hump during the repolarization phase of their action potential. A- and C- fibers are also distinguished by their different ion channels which contribute to their action potential. Of particular importance, IKCaS, ID, IA, IH, TTX-S and TTX-R INa, and T- and L- type ICa currents give vagal afferent fibers unique action potential features depending upon the ion channel expression (Schild et al., 1994).
Beyond A- and C-fibers, vagal afferents can also be classified by their neurochemistry. The neurochemistry of the vagal afferent neurons of the NG includes the neurotransmitters glutamate, catecholamines, serotonin, and acetylcholine, as well as a considerable number of neuropeptides including substance P, neurokinin A, vasoactive intestinal peptide, calcitonin gene-related peptide, galanin, encephalin, somatostatin, CCK, neuropeptide Y, calcium-binding proteins, and other neuroactive molecules (i.e., nitric oxide; Zhuo et al., 1997). Additionally, beyond the mechanical and chemical stimuli, there is emerging evidence that vagal afferents transmit nociceptive information (Surdenikova et al., 2012). Transient receptor potential (TRP) channels have been shown to be expressed in GI-projecting vagal afferents (Yu et al., 2016). These fibers respond to acid, heat, cold, stretch, capsaicin, menthol, inflammatory mediators, and other stimuli which are associated with pain. Together under normal conditions, these vagal afferents contribute to the precise control and modulation of GI functions.
Altered Physiology of Gastrointestinal Vagal Afferents Following Neurotrauma
Since the mid-nineteenth century, GI comorbidities have been noted with CNS traumas (Cushing, 1932). These profound changes to the GI system after CNS trauma suggest there is VN involvement. In other pathophysiological states such as high fat diet, diabetes, inflammation, and direct vagal injury (Browning, 2003; Browning and Mendelowitz, 2003; Li and Owyang, 2003; Raybould, 2010; Dockray and Burdyga, 2011; Kentish and Page, 2014; de Lartigue, 2016; Travagli and Anselmi, 2016; Browning et al., 2017; Grabauskas and Owyang, 2017; Cawthon and de La Serre, 2018; de Lartigue and Xu, 2018), vagal afferents have been shown to be extremely plastic. The combination of GI dysfunction and vagal afferent plasticity in other pathophysiological models suggests vagal afferent plasticity after CNS trauma, as well. Therefore, this section focuses on the altered physiology of vagal afferents following neurotrauma and identifies major knowledge gaps within the field.
Traumatic brain injury
Vagal afferent plasticity following TBI is significantly understudied, yet, it is known that brain-gut axis (or neuroenteric axis; Bansal et al., 2010) disturbances are a major cause of GI dysfunction post-TBI (Browning and Travagli, 2011; Kharrazian, 2015). Vago-vagal circuit disturbances contribute to several GI dysfunctions (Browning and Travagli, 2011). Yet, the exact mechanisms of GI dysfunction following TBI remain unclear. Several functional bowel disorders, which lead to chronic GI disorders, are associated with long-term dysregulation of the autonomic nervous system (Tougas, 2000). TBI commonly presents with neural deficits and autonomic dysfunctions and is capable of causing chronic GI disorders. However, the neural and autonomic dysregulation is variable across studies but often results in decreased GI motility among other GI complaints (Tougas, 2000). Autonomic imbalances, or dysautonomia, are hypothesized to occur when the sympathetic and parasympathetic divisions of the ANS fail to properly integrate signals between the brain and viscera (Baguley et al., 2008). Dysautonomia has been reported in 8–33% of TBI individuals and is characterized by chronic sympathetic over-activity (Kirk et al., 2012) which may suggest parasympathetic dysfunction. ANS dysfunction is evident following TBI (Esterov and Greenwald, 2017) and persistent disruption of brain-visceral axes may be one culprit of multi-organ dysfunction following TBI. However, we are interested in the vagal afferent signaling following the axis disturbances.
The vago-vagal synaptic transmission is modulated by a variety of signals including afferent inputs and neurohormones (such as CCK; Browning and Travagli, 2011). Vagal afferent sensitivity can decrease depending on specific receptor activation. Particularly, CCK-A receptors have been found on vagal afferents (Li, 2007) and electrophysiological activation of these receptors alters the sensitivity of the fibers which then modulates efferent output to the GI tract. Decreased sensitivity, from activation of CCK-A receptors on vagal afferents, can directly affect second messenger systems in NTS neurons and activate GI-inhibitory efferent pathways in the circuit. This modulation and subsequent GI-inhibition has been shown experimentally with CCK (Browning and Travagli, 2011). In an animal model of TBI, several GI pathologies were discovered including gastric distension, intestinal dilation, and intestinal effusion suggesting delayed gastric emptying and paralytic ileus. Additionally, plasma and jejunum CCK levels were elevated in the post-TBI rats potentially altering the GI vagal afferent responses (Forster et al., 1990; Higham et al., 1997; Shoji et al., 1997; Hang et al., 2004). Increased CCK levels following TBI may be one source of GI dysfunction through activation of the vago-vagal NTS-inhibitory pathway from altered vagal afferent input.
Vagal afferent inputs to NTS neurons are further influenced by tumor necrosis factor alpha (TNF-α; Travagli et al., 2006). In a preclinical model, TNF-α has been shown to favor the GI-inhibitory state by acting on the vago-vagal circuit (Emch et al., 2000). Specifically, it has been shown to cause gastric stasis (Hermann et al., 2005) through modulation of NTS-DMV synaptic transmission. Experimental models have revealed increased circulating TNF-α following TBI (Taupin et al., 1993; Tchelingerian et al., 1993; Shohami et al., 1994; Fan et al., 1996; Gourin and Shackford, 1997; Knoblach et al., 1999; Schmidt et al., 2005), alters synaptic transmission by selectively activating inhibitory or inhibiting excitatory NTS neurons which then synapse with DMV neurons for efferent outflow. The modulation occurs through changes in the glial environment, specifically through astrocyte gene expression changes which may cause long-term changes in the sensitivity of the vago-vagal circuit. The glial-neuronal communication may be responsible for leading to the activation of the GI-inhibitory efferent output and potentially be a therapeutic target. However, while TNF is elevated post-TBI, TNF has displayed both neuroprotective and neurotoxic effects in various models of CNS injury (Longhi et al., 2013).
Additional preclinical studies investigating the glial-neuronal communication between astrocytes and NTS neurons (Hermann et al., 2009; Vance et al., 2015) have also been found to disrupt vago-vagal circuitry. In a rat model, Hermann et al. (2009) showed a significant reduction in gastric transit by using a protease-activated receptor agonist to mimic the autonomic disruption first described by Cushing (1932) which occurs following a bleeding head injury. In this study, the interaction of thrombin with protease-activated receptors in the dorsal vagal complex activated astrocytes which in turn excited inhibitory-NTS neurons evoking inhibition of gastric motility. It is important to note that the autonomic astrocytes may play a role similar to that of neurons in the vago-vagal circuitry. The glial-neural interactions are multi-directional and visceral afferent inputs regulate astrocyte activity (McDougal et al., 2011; Accorsi-Mendonça et al., 2013). Therefore, alterations in vagal afferent input following TBI, may affect more than the neuronal synapses in the vago-vagal circuit. Modification of the vago-vagal reflex circuit following TBI, remains a contributing factor of brain-gut axis disruption (Kharrazian, 2015). These vago-vagal alterations are largely involved in GI dysfunction and elicit greater research on the vagal afferent changes post-TBI.
Lastly, in a mouse model of TBI, Bansal et al. (2009) showed that following TBI, intestinal permeability increased while protein expression of cell junction proteins decreased. Furthermore, there was an increase in intestinal TNF-α and other investigators have also reported increased expression of inflammatory cytokines post-TBI (Hang et al., 2005a, b; Katzenberger et al., 2015). While it is not clear whether the inflammation leads to the increased intestinal permeability, a preclinical study used a knockout mouse model equipped against inflammation to support the hypothesis that specific pro-inflammatory cytokines correlate with higher intestinal permeability following TBI (Jin et al., 2008). Yet, the source of intestinal inflammation post-TBI remains unclear. Alternatively, the source of increased intestinal permeability has been proposed as a heightened adrenergic state post-TBI, further highlighting the significance of a healthy brain-gut axis in proper gut functioning (Bansal et al., 2009).
The significance of diminished barrier integrity and heightened inflammatory state is revealed in an animal study by Ammori et al. (2008) that reported cellular degeneration in DMV vagal efferent neurons following experimental colitis. The potential for inflammatory cytokines to traverse the gut-brain axis (or enteric-neuro axis) and negatively affect neuronal survival provides another source of vago-vagal alteration (Bansal et al., 2010). Furthermore, preclinical studies reveal significant ENS changes caused by intestinal inflammation such as neuronal loss (Sanovic et al., 1999), hyperexcitability (Linden et al., 2003), and altered neurotransmitter release (Linden et al., 2005) causing changes in sensory interoceptive input and DMV structure (Ammori et al., 2008). Consistent with the negative impact of intestinal inflammation on neuronal populations of DMV and ENS neurons, DMV apoptosis is reversed by the anti-inflammatory role of ghrelin in an animal model (Qi et al., 2011). In addition to the cell loss, the time course of neuronal damage and plasticity caused by intestinal inflammation post-TBI is unknown.
Spinal cord injury
There is limited research investigating GI vagal function and plasticity in human SCI individuals. Of the few clinical studies examining SCI vagal function in general, one study utilized functional magnetic resonance imaging to measure brain activation from bladder fullness in complete SCI individuals (Krhut et al., 2017). Krhut et al. (2017) found a subset of subjects that had activation in nuclei (NTS and parabrachial nucleus) associated with vagal afferent activity. Under normal conditions these nuclei are not activated by bladder fullness, and suggest following SCI, there is vagal afferent plasticity or collateral sprouting occurring to re-innervate the bladder. Studies investigating female genital sensation after complete SCI also demonstrated new NTS activation, similarly suggesting new vagal afferent plasticity after SCI (Whipple and Komisaruk, 2002; Komisaruk et al., 2004). These studies demonstrate vagal plasticity after SCI, and GI vagal afferent dysfunction could be a promising avenue for further clinical research.
Preclinical rodent models of SCI also exhibit GI dysfunction. Our lab along with Gondim et al. have shown delayed gastric emptying and decreased GI transit time in SCI rats (Gondim et al., 1998; Tong and Holmes, 2009; Qualls-Creekmore et al., 2010) indicating a disruption in the vago-vagal reflexes. Gondim and colleagues’ studies were the first to observe an upper thoracic (T1 or T5) spinal cord transection in rats leads to delayed gastric emptying and delayed GI transit of liquid (Gondim et al., 1998, 2001; Rodrigues et al., 2001). We expanded these findings by demonstrating that upper thoracic T3-SCI animals show a diminished food intake (Primeaux et al., 2007), a significant reduction in gastric motility (Tong and Holmes, 2009) that lasts as long as 6 weeks after injury, and a delay in gastric emptying of a13 C-tagged solid meal (Qualls-Creekmore et al., 2010). It has been hypothesized that GI dysfunction is due to a vagally-mediated mechanism (Gondim et al., 2001; Tong and Holmes, 2009; Holmes, 2012).
Our lab has further found that vagal efferents remain anatomically and functionally intact after T3-SCI (Swartz and Holmes, 2014) but are silent due to lack of presynaptic stimuli (Tong et al., 2011). For example, NTS neurons are not activated after central or peripheral administration of CCK-8s in a T3-SCI model but respond to other depolarizing drugs, such as 4-aminopyridine, suggesting that afferents within the NTS are capable of releasing neurotransmitters but are not stimulated by GI peptides (Tong et al., 2011). However, the NTS receives afferent input from many brain regions, vagal afferents, and spinal afferents; therefore, this study could not conclude that the vagal afferents were altered specifically.
Our most recent study demonstrates directly that vagal afferents are hyposensitive to stimuli, both chemical and mechanical, after SCI (Besecker et al., 2020). We confirmed hyposensitivity of vagal chemoreceptors to CCK-8s and demonstrated novel mechanical hyposensitivity to von Frey Hair stimuli, and interestingly, TRPV1 receptor agonist, capsaicin, showed hypersensitivity in vagal afferents after SCI. These data suggest SCI provokes exceptional functional vagal plasticity and impairs vagal afferent signaling necessary for proper GI reflexes. Furthermore, another recent study of ours has reported this hyposensitivity is not specific to CCK and mechanical stimuli, but includes ghrelin (Besecker et al., 2018) and may involve other GI peptides not yet tested (Besecker et al., 2017).
A recent investigation in a chronic SCI rodent model, studied vagal afferents as a whole (i.e., not organ specific; Herrity et al., 2015). They demonstrated that SCI impacts the neurochemical characteristics of vagal afferents, by increasing P2X3 receptor expression and decreasing isolectin 4 binding in whole NG (Herrity et al., 2014). This study demonstrated SCI has the ability to change the phenotype of vagal afferents, but specific changes within GI vagal afferents remain to be determined.
Based upon these studies, we propose that SCI-induced pathophysiological plasticity occurs within vagal afferents and NG though the degree remains unknown. Multiple mechanisms can be proposed to account for the loss-of-function in vagal afferents, such as altered trafficking of receptors, receptor plasticity and/or adaptation, disruption of intracellular signaling pathways, ionic imbalances, degeneration of nerve endings, or loss of function of gated ion channels. In the case of SCI, it is unknown what electrophysiological phenotype is exhibited in vagal afferents after injury. However, following SCI in rodent models, other sensory afferents, such as the dorsal root ganglia, have shown to have both decreased (Yoshimura and de Groat, 1997; Black et al., 2003) and increased (Black et al., 2003; Yang et al., 2014) expression and function of TTX-R NaV channels: thus, resulting in hyperexcitability or hypoexcitability of sensory afferents depending on the target organ they innervate similar to what is seen in vagal afferents in our SCI model (Besecker et al., 2020). Other voltage-gated channels, such as KV3.4 (Ritter et al., 2015), in dorsal root ganglia have been shown to be altered by SCI, as well. The SCI-induced changes in electrophysiological properties of sensory afferents have not been expanded to the vagal afferents, thus opening more promising avenues for further research.
Gastrointestinal Microbiome
The GI microbiome plays a critical role in health and disease (Kigerl et al., 2016; Sherwin et al., 2016; O'Connor et al., 2018). The bidirectional pathway or the microbiota-gut-brain axis (Rhee et al., 2009; Bercik, 2011; Cryan and O'Mahony, 2011; Cryan and Dinan, 2012; Burokas et al., 2015; Mayer et al., 2015) is emerging as a critical communication line between the GI microbiome and brain (Sherwin et al., 2018) via the VN, immune mediators, microbial metabolites, and modulation of circulating tryptophan (O'Mahony et al., 2015; Scott et al., 2015; Foster et al., 2016; Sarkar et al., 2016; Sherwin et al., 2016). GI microbes indirectly influence neural communication, such as the vagal afferents, via the production of metabolites (e.g., short-chain fatty acids), synthesis of neurotransmitters (e.g., gamma-aminobutyric acid, noradrenaline, and dopamine), and immune system modulation (Sherwin et al., 2016). Disruptions in the microbiota-gut-brain axis of the VN contribute to both GI (Mulak and Bonaz, 2004; Bonaz and Bernstein, 2013; Oświęcimska et al., 2017) and neurodegenerative disorders (Cenit et al., 2017; Kobayashi et al., 2017; Quigley, 2017). Vagal afferents use gut endocrine cell interactions (Raybould, 2010), direct and receptor activation (Goehler et al., 1999; Lal et al., 2001), and CCK-mediated mechanisms (Lal et al., 2001) to detect GI microbial compounds or metabolites and carry the information to the brain. The NG contributes to the brain-relay by responding to bacterial products such as lipopolysaccharide (Hosoi et al., 2005). Information along the vagal pathway reaches the brain to elicit adaptations or inappropriate responses, the latter contributes to GI and neurodegenerative pathophysiologies (Eisenstein, 2016; Tse, 2017).
While several preclinical studies have shown vagal nerve activity in response to GI microbes (Gaykema et al., 2004; Tanida et al., 2005; Raybould, 2010; Bravo et al., 2011; Perez-Burgos et al., 2013), clinical vagal afferent activation via GI microbiota remains hypothetical (Bonaz et al., 2018). For further review of GI microbiota and the microbiome-gut-brain axis, please see (Burnet and Cowen, 2013; Wang and Kasper, 2014; Kelly et al., 2015; Moos et al., 2016). Additionally, a preclinical model has shown evidence of gut microbiota modulating satiety signals, specifically increasing GLP-1 and PYY plasma levels, favoring anorexigenic signaling to the brain (Breton et al., 2016); such GI microbiota influence on feeding behaviors may be responsible for the GI dysfunction following neurotrauma. Gaining a better understanding of the influence altered gut microbes have on the brain following trauma, may lead to interventions for reduced comorbidities and improved functional outcomes. However, there are limited clinical studies on the microbiome-gut-brain axis following TBI and SCI (O'Connor et al., 2018).
Dysbiosis following traumatic brain injury
Dysbiosis is a result of pathogenic bacteria outnumbering beneficial nonpathogenic gut bacteria resulting in undesired health outcomes. GI dysbiosis occurs within 2 to 24 hours after TBI (Treangen et al., 2018; Nicholson et al., 2019) and remains altered in chronic TBI (Ma et al., 2017) in rodent models. In the study by Treangen et al. (2018) the microbiota changes post-TBI include decreased Ruminococcus flavieciens, increased Eubacterium ventriosum, and increased Marvinbryantia formatexigens. Thus, this study demonstrated a change in Firmicutes/Bacteroidetes ratio and succinate-producing microbes. Sherwin et al. (2018) noted that the surge in SNS activation may be responsible for altering post-TBI microbiota as observed in a study in mice (Houlden et al., 2016). This work demonstrated a decrease in the intestinal microbiota family Prevotellaceae post-TBI, while levels of norepinephrine release and noradrenergic innervation in the cecum increased in mice (Houlden et al., 2016). Additionally, norepinephrine levels have been shown to increase, in both animal and human studies, following trauma (Millen et al., 1985; Woolf et al., 1992; Lyte and Bailey, 1997) exuding a sympathetic dominance which may contribute to the GI dysfunction. However, norepinephrine has recently been shown to reduce the relative abundance of certain species of the genus Prevotella (from the family Prevotellaceae) in vitro (Jentsch et al., 2013). Less is understood about the family Peptococcaceae, but increases (as demonstrated in mice (Houlden et al., 2016)) have been correlated with increased expression of the proinflammatory cytokine (CCL5) which has been linked to colitis development (Elinav et al., 2011) in mice.
The work by Durham et al. (2016) as well as several other studies (Rogers et al., 2016; Duvallet et al., 2017; Sun and Shen, 2018; Winter et al., 2018) indicate the involvement of the brain-gut axis in TBI-related neuropathologies which has recently expanded to include TBI-GI pathologies suggesting microbiome-gut-brain-axis (Mayer et al., 2015; Martin and Mayer, 2017; Sundman et al., 2017) disruption. The paradox remains in that GI microbial shifts alter VN activity (microbiota-gut-brain axis) and GI dysfunction alters GI microbial populations (brain-gut-microbiota axis). However, evidence is beginning to indicate that probiotic treatments may be capable of healing the injured brain by repairing post-TBI gut dysbiosis (Treangen et al., 2018).
Dysbiosis following spinal cord injury
Reviews of the current SCI microbiome literature can be found in (Holmes and Blanke, 2019; Wallace et al., 2019) but briefly, investigators have shown that gut dysbiosis occurs following SCI. Similar to the TBI-microbiome preclinical study, Kigerl et al. (2016) showed that the Firmicutes/Bacteroidetes ratio also increased following SCI in mice. Additionally, in another SCI rodent model, the following GI microbiota changes were detected: increased Lactobacillus intestinalis, Clostridium disporicum, and Bifidobacterium choerinum while relative abundance of Clostridium saccharogumia decreased (O'Connor et al., 2018). High prevalence of Lactobacillaceae family has been correlated with carbohydrate fermentation to lactic acid, and Kigerl et al. (2016) revealed post-SCI functional recovery correlations to lactic acid supplementation. Clostridium spp. have been found to be both helpful and harmful but the functional implications of C. disporicum remain to be further understood. In humans, the family of Bifidobacteriaceae is probiotic and found in healthy guts conferring positive health benefits (O'Callaghan et al., 2015). Lastly, while literature reveals deleterious effects on the host when C. saccharogumia is depleted, the significance of this is low with the increased relative abundance of the other commensal microbes (O'Connor et al., 2018).
These microbiota changes were inversely correlated with intestinal inflammatory changes. The intestinal proinflammatory cytokines likely suppressed microbial taxa responsible for butyrate production in the GI tract. Butyrate plays a critical role in gut health through its anti-inflammatory properties by supporting intestinal cell cycles, its pathogen-protection activities, modulation of the immune system, and reduction of cancer progression (Gungor et al., 2016; O'Connor et al., 2018). Previous preclinical studies analyzed the effects of Faecalibacterium prausnitzii, a known butyrate-producing microbe, and found it to have protective effects on the gut (Miquel et al., 2013). O'Connor et al. (2018) noted F. prausnitzii to be depleted in SCI mice with significantly increased levels of proinflammatory cytokines (Miquel et al., 2013; Kigerl et al., 2016). Similarly, a decrease in the abundance of butyrate-producing bacteria correlates with gut dysbiosis in clinical SCI (Ferrante et al., 2003; Sun et al., 2015; Butchbach et al., 2016; Gungor et al., 2016; Li et al., 2016). As observed with the TBI-microbiota changes, the microbial shift post-SCI is towards a recovery profile to the host; yet many health-related microbes are depleted due to the chronic inflammatory state of the GI tract post-neurotrauma.
While much remains to be understood with microbiota effects on system physiology, characterizing GI microbiota is critical following neurotrauma. The characterization, identification of physiological roles, and determination of critical time-points of the changing microbiota will help in designing therapeutic interventions for TBI- and SCI-related GI comorbidities.
Conclusion
Neurotrauma causes profound changes to ANS control of the GI system. GI dysfunction contributes to increased morbidity and mortality. However, neural plasticity offers the ability to target specific mechanisms and treat GI dysfunctions following CNS injury, but the exact mechanisms of disruption remain largely under-researched. Following TBI, the GI vagal afferents exhibit modified receptor-, peptide-, and glial-interactions which favor the inhibitory-NTS vagal efferent pathway. Vagal efferent and ENS neuronal populations decrease following intestinal inflammatory insult, and may be an on-going cycle across synapses in the vago-vagal reflex circuit. These vagal afferent and efferent interactions and reactions may serve as suitable therapeutic targets in repairing GI dysfunction post-TBI, but the exact mechanisms which contribute to these changes and the system-wide interactions from such plasticity need to be better understood.
The effects of SCI on GI dysfunction still remains a significant knowledge gap. From the above studies, we know human vagal afferents show significant re-wiring post-SCI to activate once-quiet NTS neurons. This modified afferent input following SCI remains unclear but shows potential for re-activation of vago-vagal reflexes. In animal models of SCI, it has been shown that GI vago-vagal reflexes are disrupted. While the vagal efferents remain intact, they fail to respond spontaneously due to lack of presynaptic stimuli. This further suggests vagal afferent alterations. Furthermore, we have reported that GI vagal afferents are hyposensitive to chemical and mechanical stimuli but selectively hypersensitive to noxious chemical stimuli. Lastly, SCI changes phenotypic profiles of NG by increasing P2X3 receptor expression and decreasing isolectin 4 binding. While little is known about the plasticity of GI-vagal afferents following SCI, the research suggests that vago-vagal reflexes are affected and responsive to changing conditions. Upon further understanding of the mechanisms of plasticity in the vago-vagal circuit, we will strengthen the efforts of finding treatments for GI pathologies following neurotrauma.
There is an emerging discussion that neurotrauma is a systemic pathology (Figure 2). The vago-vagal reflex circuit is anatomically intact after TBI and SCI, suggesting the derangements to the GI vago-vagal reflexes must be due to a secondary cascade of events. The components of the vago-vagal reflex are optimally poised to sense secondary events such as systemic inflammation. Immediately following trauma to the CNS, regardless of location, a systemic inflammatory response (SIR) ensues (Anthony and Couch, 2014; Schwab et al., 2014; Sun et al., 2016). SIR following SCI has been thoroughly reviewed (Sun et al., 2016; Bloom et al., 2020). For SCI, the SIR is characterized by increased levels of circulating inflammatory mediators and cells, activation of resident glial and immune cells, and infiltration of neutrophils, macrophages and lymphocytes, all of which culminate in multiple organ failure (Sun et al., 2016; Bloom et al., 2020). The SCI-induced SIR is triggered within hours and persists for weeks following injury, and currently there is no clinical evidence to support the use of any neuroprotective drugs to improve functional outcomes after SCI (Popovich et al., 2001; Campbell et al., 2005; Gris et al., 2008; Wing, 2008; Sauerbeck et al., 2015; Bloom et al., 2020). Studies of other inflammatory disease models have examined the effects of systemic inflammation on vagal afferents and have been reviewed (Browning et al., 2017). Briefly, inflammation has shown to decrease vagal afferent response to leptin and CCK, while increasing mechanosensitivity, TRP and P2X receptors and NaV channel expression (Browning et al., 2017). Our preclinical SCI reports of decreased GI vagal afferent response to CCK (Tong et al., 2011; Besecker et al., 2020), increased TRPV1 expression and hypersensitivity (Besecker et al., 2019), along with the preclinical report by Herrity et al. (2015) of increased P2X3 receptor expression in NG after SCI, insinuate a similar mechanism. This suggests systemic inflammation may cause pathophysiological plasticity within vagal afferents after SCI (Figure 2). Lastly, inflammation has been shown to alter the GI microbiota preclinically (O'Connor et al., 2018). The inflammatory profile causes dysbiosis causing disruption to the microbiota-gut-brain axis. This disruption may be the culprit of the chronic GI and neurological disorders seen in neurotrauma individuals. By understanding the GI microbiome, and developing a healthy gut profile, we may be equipped with probiotics necessary to reverse the long-term GI and neural deficits in TBI- and SCI individuals.
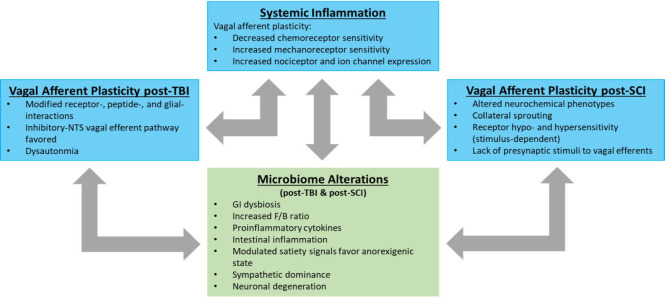
Figure 2.
Vagal afferent plasticity following neurotrauma.
Neurotrauma is emerging as systemic phenomenon whereby multiple organ systems demonstrate post-injury pathophysiology. Vagal afferents remain anatomically intact after TBI and SCI, yet they demonstrate significant remodeling following injury. Systemic inflammation contributes to multi-organ compromise, including the gastrointestinal tract. Whether this systemic inflammation causes vagal afferent plasticity or the plasticity of the vagal afferents contributes to the systemic inflammation has yet to be determined. This dysregulation may exist in concert with, or independent of, alterations in the gut microbiome. F/B: Firmicutes/Bacteroidetes ratio; GI: gastrointestinal.NTS: Nucleus tractus solitarius; SCI: spinal cord injury; TBI: traumatic brain injury.
Additional file: Open peer review reports 1 (86.2KB, pdf) and 2 (87.6KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by grants from the National Institutes of Health (NINDS 49177; NINDS 105987) and Craig H. Neilsen Foundation Senior Research award (295319) to GMH and a grant from the National Institutes of Health (NINDS F31 NS 087834) to EMB.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: David Benn Lovejoy, Macquarie University, Australia; Shaojun Liu, Institute of Military Cognition and Brain Sciences, Beijing, China.
P-Reviewers: Lovejoy DB, Liu S; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Funding: This work was supported by grants from the National Institutes of Health (NINDS 49177; NINDS 105987) and Craig H. Neilsen Foundation Senior Research award (295319) to GMH and a grant from the National Institutes of Health (NINDS F31 NS 087834) to EMB.
References
- 1.Accorsi-Mendonça D, Zoccal DB, Bonagamba LG, Machado BH. Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats. Physiol Rep. 2013;1:e00080. doi: 10.1002/phy2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 3.Ammori JB, Zhang WZ, Li JY, Chai BX, Mulholland MW. Effects of ghrelin on neuronal survival in cells derived from dorsal motor nucleus of the vagus. Surgery. 2008;144:159–167. doi: 10.1016/j.surg.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 6.Anthony DC, Couch Y. The systemic response to CNS injury. Exp Neurol. 2014;258:105–111. doi: 10.1016/j.expneurol.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Baguley IJ, Heriseanu RE, Cameron ID, Nott MT, Slewa-Younan S. A critical review of the pathophysiology of dysautonomia following traumatic brain injury. Neurocrit Care. 2008;8:293–300. doi: 10.1007/s12028-007-9021-3. [DOI] [PubMed] [Google Scholar]
- 8.Bansal V, Costantini T, Kroll L, Peterson C, Loomis W, Eliceiri B, Baird A, Wolf P, Coimbra R. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. 2009;26:1353–1359. doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, Elicieri B, Baird A, Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria. Gut. 2011;60:288–289. doi: 10.1136/gut.2010.226779. [DOI] [PubMed] [Google Scholar]
- 11.Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat Basel. 1996;156:123–131. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- 12.Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2017;312:R146–156. doi: 10.1152/ajpregu.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besecker EM, White AR, Holmes GM. Diminished gastric prokinetic response to ghrelin in a rat model of spinal cord injury. Neurogastroenterol Motil. 2018;30:e13258. doi: 10.1111/nmo.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besecker EM, Blanke EN, Deiter GM, Holmes GM. Gastric vagal afferent neuropathy following experimental spinal cord injury. Exp Neurol. 2020;323:113092. doi: 10.1016/j.expneurol.2019.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black JA, Cummins TR, Yoshimura N, de Groat WC, Waxman SG. Tetrodotoxin-resistant sodium channels Nav1. 8/SNS and Nav1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res. 2003;963:132–138. doi: 10.1016/s0006-8993(02)03957-4. [DOI] [PubMed] [Google Scholar]
- 16.Blackshaw LA, Brookes SJH, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 17.Bloom O, Herman PE, Spungen AM. Systemic inflammation in traumatic spinal cord injury. Exp Neurol. 2020;325:113143. doi: 10.1016/j.expneurol.2019.113143. [DOI] [PubMed] [Google Scholar]
- 18.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Bonaz B, Sinniger V, Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol Motil. 2016;28:455–462. doi: 10.1111/nmo.12817. [DOI] [PubMed] [Google Scholar]
- 20.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braaf S, Lennox A, Nunn A, Gabbe B. Social activity and relationship changes experienced by people with bowel and bladder dysfunction following spinal cord injury. Spinal Cord. 2017;55:679–686. doi: 10.1038/sc.2017.19. [DOI] [PubMed] [Google Scholar]
- 22.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J, Goichon A, Guérin C, Peltier J, Pestel-Caron M, Chan P, Vaudry D, do Rego JC, Liénard F, Pénicaud L, Fioramonti X, Ebenezer IS, Hökfelt T, Déchelotte P, Fetissov SO. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 2016;23:324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–140. doi: 10.1016/s0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 26.Brown TH, Davidson PF, Larson GM. Acute gastritis occurring within 24 hours of severe head injury. Gastrointest Endosc. 1989;35:37–40. doi: 10.1016/s0016-5107(89)72683-3. [DOI] [PubMed] [Google Scholar]
- 27.Browning KN. Excitability of nodose ganglion cells and their role in vago-vagal reflex control of gastrointestinal function. Curr Opin Pharmacol. 2003;3:613–617. doi: 10.1016/j.coph.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Browning KN, Mendelowitz D. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes: II Integration of afferent signaling from the viscera by the nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G8–14. doi: 10.1152/ajpgi.00322.2002. [DOI] [PubMed] [Google Scholar]
- 29.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil. 2010;22:1154–1163. doi: 10.1111/j.1365-2982.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13. doi: 10.1016/j.autneu.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152:730–744. doi: 10.1053/j.gastro.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnet PW, Cowen PJ. Psychobiotics highlight the pathways to happiness. Biol Psychiatry. 2013;74:708–709. doi: 10.1016/j.biopsych.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Burokas A, Moloney RD, Dinan TG, Cryan JF. Chapter One - microbiota regulation of the mammalian gut–brain axis. In: Sariaslani S, Gadd GM, editors. Advances in Applied Microbiology. Waltham, MA, USA: Academic Press; pp. 1–62. [DOI] [PubMed] [Google Scholar]
- 35.Butchbach MER, Lumpkin CJ, Harris AW, Saieva L, Edwards JD, Workman E, Simard LR, Pellizzoni L, Burghes AHM. Protective effects of butyrate-based compounds on a mouse model for spinal muscular atrophy. Exp Neurol. 2016;279:13–26. doi: 10.1016/j.expneurol.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon CR, de La Serre CB. Gut bacteria interaction with vagal afferents. Brain Res. 2018;1693:134–139. doi: 10.1016/j.brainres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23:5486–5498. doi: 10.3748/wjg.v23.i30.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang WT, Badjatia N. Neurotrauma. Emerg Med Clin North Am. 2014;32:889–905. doi: 10.1016/j.emc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 41.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 42.Cushing H. Peptic ulcer and the interbrain. Surg Gynecol Obstet. 1932;55:1–34. [Google Scholar]
- 43.Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. 2011;30:1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 45.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 46.de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594:5791–5815. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Lartigue G, Xu C. Mechanisms of vagal plasticity influencing feeding behavior. Brain Res. 2018;1693:146–150. doi: 10.1016/j.brainres.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol. 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 49.Driessen AK. Vagal afferent processing by the paratrigeminal nucleus. Front Physiol. 2019;10:1110. doi: 10.3389/fphys.2019.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durham WJ, Foreman JP, Randolph KM, Danesi CP, Spratt H, Masel BD, Summons JR, Singh CK, Morrison M, Robles C, Wolfram C, Kreber LA, Urban RJ, Sheffield-Moore M, Masel BE. Hypoaminoacidemia characterizes chronic traumatic brain injury. J Neurotrauma. 2016;34:385–390. doi: 10.1089/neu.2015.4350. [DOI] [PubMed] [Google Scholar]
- 51.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. 2017;8:1784. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenstein M. Microbiome: bacterial broadband. Nature. 2016;533:S104–106. doi: 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- 53.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome is a regulator of colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582–586. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- 55.Esterov D, Greenwald BD. Autonomic dysfunction after mild traumatic brain injury. Brain Sci. 2017 doi: 10.3390/brainsci7080100. doi: 103390/brainsci7080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 57.Faris PL, Hofbauer RD, Daughters R, Vandenlangenberg E, Iversen L, Goodale RL, Maxwell R, Eckert ED, Hartman BK. De-stabilization of the positive vago-vagal reflex in bulimia nervosa. Physiol Behav. 2008;94:136–153. doi: 10.1016/j.physbeh.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci Off J Soc Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol. 1990;258:G552–556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- 60.Foster JA, Lyte M, Meyer E, Cryan JF. Gut microbiota and brain function: an evolving field in neuroscience. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyv114. doi: 101093/ijnp/pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fülling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Fynne L, Worsøe J, Gregersen T, Schlageter V, Laurberg S, Krogh K. Gastric and small intestinal dysfunction in spinal cord injury patients. Acta Neurol Scand. 2012;125:123–128. doi: 10.1111/j.1600-0404.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 63.Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 65.Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems. J Neurosci. 1999;19:2799–806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gondim FA, da-Graça JR, de-Oliveira GR, Rêgo MC, Gondim RB, Rola FH. Decreased gastric emptying and gastrointestinal and intestinal transits of liquid after complete spinal cord transection in awake rats. Braz J Med Biol Res. 1998;31:1605–1610. doi: 10.1590/s0100-879x1998001200015. [DOI] [PubMed] [Google Scholar]
- 67.Gondim FA, Rodrigues CL, da Graça JR, Camurça FD, de Alencar HM, dos Santos AA, Rola FH. Neural mechanisms involved in the delay of gastric emptying and gastrointestinal transit of liquid after thoracic spinal cord transection in awake rats. Auton Neurosci. 2001;87:52–58. doi: 10.1016/s1566-0702(00)00261-7. [DOI] [PubMed] [Google Scholar]
- 68.Gourin CG, Shackford SR. Production of tumor necrosis factor-alpha and interleukin-1beta by human cerebral microvascular endothelium after percussive trauma. J Trauma. 1997;42:1101–1107. doi: 10.1097/00005373-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 69.Grabauskas G, Owyang C. Plasticity of vagal afferent signaling in the gut. Medicina (Kaunas) 2017;53:73–84. doi: 10.1016/j.medici.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211:259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Gungor B, Adiguzel E, Gursel I, Yilmaz B, Gursel M. Intestinal microbiota in patients with spinal cord injury. PLoS One. 2016;11:e0145878. doi: 10.1371/journal.pone.0145878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hang CH, Shi JX, Li JS, Li WQ, Wu W. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J Surg Res. 2005a;123:188–193. doi: 10.1016/j.jss.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Hang CH, Shi JX, Li JS, Li WQ, Yin HX. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J Gastroenterol. 2005b;11:1149–1154. doi: 10.3748/wjg.v11.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hang CH, Shi JX, Li JS, Wu W, Li WQ, Yin HX. Levels of vasoactive intestinal peptide, cholecystokinin and calcitonin gene-related peptide in plasma and jejunum of rats following traumatic brain injury and underlying significance in gastrointestinal dysfunction. World J Gastroenterol. 2004;10:875–880. doi: 10.3748/wjg.v10.i6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hang CH, Shi JX, Li JS, Wu W, Yin HX. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 2003;9:2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hermann GE, Holmes GM, Rogers RC. TNF(alpha) modulation of visceral and spinal sensory processing. Curr Pharm Des. 2005;11:1391–1409. doi: 10.2174/1381612053507828. [DOI] [PubMed] [Google Scholar]
- 77.Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci. 2009;29:9292–9300. doi: 10.1523/JNEUROSCI.6063-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrity AN, Rau KK, Petruska JC, Stirling DP, Hubscher CH. Identification of bladder and colon afferents in the nodose ganglia of male rats. J Comp Neurol. 2014;522:3667–3682. doi: 10.1002/cne.23629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrity AN, Petruska JC, Stirling DP, Rau KK, Hubscher CH. The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons. Am J Physiol RegulIntegr Comp Physiol. 2015;308:R1021–1033. doi: 10.1152/ajpregu.00445.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Higham A, Vaillant C, Yegen B, Thompson DG, Dockray GJ. Relation between cholecystokinin and antral innervation in the control of gastric emptying in the rat. Gut. 1997;41:24–32. doi: 10.1136/gut.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holmes GM. Upper gastrointestinal dysmotility after spinal cord injury: is diminished vagal sensory processing one culprit. Front Physiol. 2012;3:277. doi: 10.3389/fphys.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmes GM, Blanke EN. Gastrointestinal dysfunction after spinal cord injury. Exp Neurol. 2019;320:113009. doi: 10.1016/j.expneurol.2019.113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol. 2013;591:3081–3100. doi: 10.1113/jphysiol.2013.253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108:90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- 85.Horneman G, Folkesson P, Sintonen H, von Wendt L, Emanuelson I. Health-related quality of life of adolescents and young adults 10 years after serious traumatic brain injury. Int J Rehabil Res. 2005;28:245–249. doi: 10.1097/00004356-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Auton Neurosci Basic Clin. 2005;120:104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts IS, Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang JH. Traumatic brain injury. Neurol Res. 2013;35:221–222. doi: 10.1179/1743132813Y.0000000178. [DOI] [PubMed] [Google Scholar]
- 89.Icahn School of Medicine at Mount Sinai. Neurosurgery: Neurotrauma, 2020 from https://www.mountsinai. org/care/neurosurgery/services/neurotrauma. Neurosurgery: Neurotrauma, 2020 from https://wwwmountsinaiorg/care/neurosurgery/services/neurotrauma Accessed January 1, 2020. [Google Scholar]
- 90.Jackson MD, Davidoff G. Gastroparesis following traumatic brain injury and response to metoclopramide therapy. Arch Phys Med Rehabil. 1989;70:553–555. [PubMed] [Google Scholar]
- 91.Jentsch HF, März D, Krüger M. The effects of stress hormones on growth of selected periodontitis related bacteria. Anaerobe. 2013;24:49–54. doi: 10.1016/j.anaerobe.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Jin W, Wang H, Ji Y, Hu Q, Yan W, Chen G, Yin H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 2008;44:135–140. doi: 10.1016/j.cyto.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Kamada T, Fusamoto H, Kawano S, Noguchi M, Hiramatsu K. Gastrointestinal bleeding following head injury: a clinical study of 433 cases. J Trauma. 1977;17:44–47. [PubMed] [Google Scholar]
- 94.Kao CH, ChangLai SP, Chieng PU, Yen TC. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108–1112. doi: 10.1111/j.1572-0241.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 95.Katzenberger RJ, Ganetzky B, Wassarman DA. The gut reaction to traumatic brain injury. Fly (Austin) 2015;9:68–74. doi: 10.1080/19336934.2015.1085623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kemp CD, Johnson JC, Riordan WP, Cotton BA. How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am Surg. 2008;74:866–872. [PubMed] [Google Scholar]
- 98.Kentish SJ, Page AJ. Plasticity of gastrointestinal vagal afferent endings. Physiol Behav. 2014;136:170–178. doi: 10.1016/j.physbeh.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 99.Kharrazian D. Traumatic brain injury and the effect on the brain-gut axis. Altern Ther Health Med. 2015;21:28–32. [PubMed] [Google Scholar]
- 100.Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213:2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kirk KA, Shoykhet M, Jeong JH, Tyler-Kabara EC, Henderson MJ, Bell MJ, Fink EL. Dysautonomia after pediatric brain injury. Dev Med Child Neurol. 2012;54:759–764. doi: 10.1111/j.1469-8749.2012.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, Kondo T, Abe K, Xiao JZ. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 105.Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 2004;1024:77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 106.Krhut J, Tintera J, Bilkova K, Holy P, Zachoval R, Zvara P, Blok B. Brain activity on fMRI associated with urinary bladder filling in patients with a complete spinal cord injury. Neurourol Urodyn. 2017;36:155–159. doi: 10.1002/nau.22901. [DOI] [PubMed] [Google Scholar]
- 107.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 108.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Li H, Sun J, Wang F, Ding G, Chen W, Fang R, Yao Y, Pang M, Lu ZQ, Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–78. doi: 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 110.Li Y. Sensory signal transduction in the vagal primary afferent neurons. Curr Med Chem. 2007;14:2554–2563. doi: 10.2174/092986707782023334. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Owyang C. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes. V Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G461–469. doi: 10.1152/ajpgi.00119.2003. [DOI] [PubMed] [Google Scholar]
- 112.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 114.Longhi L, Perego C, Ortolano F, Aresi S, Fumagalli S, Zanier ER, Stocchetti N, De Simoni MG. Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor. J Cereb Blood Flow Metab. 2013;33:1182–1189. doi: 10.1038/jcbfm.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu WY, Rhoney DH, Boling WB, Johnson JD, Smith TC. A review of stress ulcer prophylaxis in the neurosurgical intensive care unit. Neurosurgery. 1997;41:416–426. doi: 10.1097/00006123-199708000-00017. [DOI] [PubMed] [Google Scholar]
- 116.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 117.Ma EL, Smith AD, Desai N, Cheung L, Hanscom M, Stoica BA, Loane DJ, Shea-Donohue T, Faden AI. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 2017;66:56–69. doi: 10.1016/j.bbi.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mackay LE, Morgan AS, Bernstein BA. Swallowing disorders in severe brain injury: Risk factors affecting return to oral intake. Arch Phys Med Rehabil. 1999;80:365–371. doi: 10.1016/s0003-9993(99)90271-x. [DOI] [PubMed] [Google Scholar]
- 119.Martin CR, Mayer EA. Gut-brain axis and behavior. Nestle Nutr Inst Workshop Ser. 2017;88:45–53. doi: 10.1159/000461732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 121.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J Athl Train. 2009;44:434–448. doi: 10.4085/1062-6050-44.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci. 2011;31:14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Millen JE, Glauser FL, Fairman RP. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J Neurosurg. 1985;62:587–591. doi: 10.3171/jns.1985.62.4.0587. [DOI] [PubMed] [Google Scholar]
- 126.Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 127.Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K. Microbiota and neurological disorders: a gut feeling. Biores Open Access. 2016;5:137–145. doi: 10.1089/biores.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res. 1990;526:95–102. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- 129.Morgan AS, Mackay LE. Causes and complications associated with swallowing disorders in traumatic brain injury. J Head Trauma Rehabil. 1999;14:454–461. doi: 10.1097/00001199-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 130.Moriarty P, Dimaline R, Thompson DG, Dockray GJ. Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience. 1997;79:905–913. doi: 10.1016/s0306-4522(96)00675-6. [DOI] [PubMed] [Google Scholar]
- 131.Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Med Sci Monit Int Med J Exp Clin Res. 2004;10:55–62. [PubMed] [Google Scholar]
- 132.Nicholson SE, Watts LT, Burmeister DM, Merrill D, Scroggins S, Zou Y, Lai Z, Grandhi R, Lewis AM, Newton LM, Eastridge BJ, Schwacha MG. Moderate traumatic brain injury alters the gastrointestinal microbiome in a time-dependent manner. Shock. 2019;52:240–248. doi: 10.1097/SHK.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 133.O'Callaghan A, Bottacini F, O'Connell Motherway M, van Sinderen D. Pangenome analysis of Bifidobacterium longum and site-directed mutagenesis through by-pass of restriction-modification systems. BMC Genomics. 2015;16:832. doi: 10.1186/s12864-015-1968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O'Connor G, Jeffrey E, Madorma D, Marcillo A, Abreu MT, Deo SK, Dietrich WD, Daunert S. Investigation of microbiota alterations and intestinal inflammation post-spinal cord injury in rat model. J Neurotrauma. 2018;35:2159–2166. doi: 10.1089/neu.2017.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsen AB, Hetz RA, Xue H, Aroom KR, Bhattarai D, Johnson E, Bedi S, Cox CS, Jr, Uray K. Effects of traumatic brain injury on intestinal contractility. Neurogastroenterol Motil. 2013;25:593–463. doi: 10.1111/nmo.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 137.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Page AJ, Slattery JA, Milte C, Laker R, O'Donnell T, Dorian C, Brierley SM, Blackshaw LA. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1376–1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- 139.Park MI, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101:1129–1139. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 140.Patterson LM, Zheng H, Berthoud HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec. 2002;266:10–20. doi: 10.1002/ar.10026. [DOI] [PubMed] [Google Scholar]
- 141.Pedoto MJ, O'Dell MW, Thrun M, Hollifield D. Superior mesenteric artery syndrome in traumatic brain injury: Two cases. Arch Phys Med Rehabil. 1995;76:871–875. doi: 10.1016/s0003-9993(95)80555-9. [DOI] [PubMed] [Google Scholar]
- 142.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G211–220. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- 143.Philip PA. Superior mesenteric artery syndrome: an unusual cause of intestinal obstruction in brain-injured children. Brain Inj. 1992;6:351–358. doi: 10.3109/02699059209034949. [DOI] [PubMed] [Google Scholar]
- 144.Pilitsis JG, Rengachary SS. Complications of head injury. Neurol Res. 2001;23:227–236. doi: 10.1179/016164101101198389. [DOI] [PubMed] [Google Scholar]
- 145.Popovich PG, Stuckman S, Gienapp IE, Whitacre CC. Alterations in immune cell phenotype and function after experimental spinal cord injury. J Neurotrauma. 2001;18:957–966. doi: 10.1089/089771501750451866. [DOI] [PubMed] [Google Scholar]
- 146.Potenzieri C, Meeker S, Undem BJ. Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. J Physiol. 2012;590:5449–5459. doi: 10.1113/jphysiol.2012.237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- 148.Primeaux SD, Tong M, Holmes GM. Effects of chronic spinal cord injury on body weight and body composition in rats fed a standard chow diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1102–1109. doi: 10.1152/ajpregu.00224.2007. [DOI] [PubMed] [Google Scholar]
- 149.Qi L, Cui X, Dong W, Barrera R, Nicastro J, Coppa GF, Wang P, Wu R. Ghrelin attenuates brain injury after traumatic brain injury and uncontrolled hemorrhagic shock in rats. Mol Med. 2011;18:186–193. doi: 10.2119/molmed.2011.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qualls-Creekmore E, Tong M, Holmes GM. Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol Motil. 2010;22:62–69. doi: 10.1111/j.1365-2982.2009.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- 152.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci Basic Clin. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rea P, Rea P. Clinical Anatomy of the Cranial Nerves. San Diego, CA, USA: Academic Press; 2014. Vagus Nerve; pp. 105–116. [Google Scholar]
- 154.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ritter DM, Zemel BM, Hala TJ, O'Leary ME, Lepore AC, Covarrubias M. Dysregulation of Kv3. 4 channels in dorsal root ganglia following spinal cord injury. J Neurosci. 2015;35:1260–1273. doi: 10.1523/JNEUROSCI.1594-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodrigues CL, Gondim FA, Leal PR, Camurça FD, Freire CC, dos Santos AA, Rola FH. Gastric emptying and gastrointestinal transit of liquid throughout the first month after thoracic spinal cord transection in awake rats. Dig Dis Sci. 2001;46:1604–1609. doi: 10.1023/a:1010624730975. [DOI] [PubMed] [Google Scholar]
- 157.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saito Y, Kawashima Y, Kondo A, Chikumaru Y, Matsui A, Nagata I, Ohno K. Dysphagia-gastroesophageal reflux complex: complications due to dysfunction of solitary tract nucleus-mediated vago-vagal reflex. Neuropediatrics. 2006;37:115–120. doi: 10.1055/s-2006-924428. [DOI] [PubMed] [Google Scholar]
- 159.Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051–1057. doi: 10.1016/S0002-9440(10)65207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sauerbeck AD, Laws JL, Bandaru VV, Popovich PG, Haughey NJ, McTigue DM. Spinal cord injury causes chronic liver pathology in rats. J Neurotrauma. 2015;32:159–169. doi: 10.1089/neu.2014.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saxe JM, Ledgerwood AM, Lucas CE, Lucas WF. Lower esophageal sphincter dysfunction precludes safe gastric feeding after head injury. J Trauma. 1994;37:581–584. doi: 10.1097/00005373-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 163.Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type rat nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol. 1994;71:2338–2358. doi: 10.1152/jn.1994.71.6.2338. [DOI] [PubMed] [Google Scholar]
- 164.Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury—an inflammatory disease. Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 165.Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression and autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258:121–129. doi: 10.1016/j.expneurol.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scott KP, Antoine JM, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877. doi: 10.3402/mehd.v26.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 168.Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019–1041. doi: 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- 170.Shoji E, Okumura T, Onodera S, Takahashi N, Harada K, Kohgo Y. Gastric emptying in OLETF rats not expressing CCK-A receptor gene. Dig Dis Sci. 1997;42:915–919. doi: 10.1023/a:1018860313674. [DOI] [PubMed] [Google Scholar]
- 171.Simpson LA, Eng JJ, Hsieh JT, Wolfe DL. Spinal Cord Injury Rehabilitation Evidence Scire Research Team (2012) The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 29:1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun J, Wang F, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J, He Y, Liu J, Liu C. Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. Biomed Res Int. 2015;2015:395895. doi: 10.1155/2015/395895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res Rev. 2018;45:53–61. doi: 10.1016/j.arr.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 174.Sun X, Jones ZB, Chen XM, Zhou L, So KF, Ren Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship. J Neuroinflammation. 2016;13:260. doi: 10.1186/s12974-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44. doi: 10.1016/j.bbi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 176.Surdenikova L, Ru F, Nassenstein C, Tatar M, Kollarik M. The neural crest- and placodes-derived afferent innervation of the mouse esophagus. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2012;24:e517–525. doi: 10.1111/nmo.12002. [DOI] [PubMed] [Google Scholar]
- 177.Swartz EM, Holmes GM. Gastric vagal motoneuron function is maintained following experimental spinal cord injury. Neurogastroenterol Motil. 2014;26:1717–1729. doi: 10.1111/nmo.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389:109–114. doi: 10.1016/j.neulet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 179.Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- 180.Tchelingerian JL, Quinonero J, Booss J, Jacque C. Localization of TNF alpha and IL-1 alpha immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron. 1993;10:213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]
- 181.Thumshirn M. Pathophysiology of functional dyspepsia. Gut. 2002;51:63–66. doi: 10.1136/gut.51.suppl_1.i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tong M, Holmes GM. Gastric dysreflexia after acute experimental spinal cord injury in rats. Neurogastroenterol Motil. 2009;21:197–206. doi: 10.1111/j.1365-2982.2008.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterol Motil. 2011;23:e69–79. doi: 10.1111/j.1365-2982.2010.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tougas G. The autonomic nervous system in functional bowel disorders. Gut. 2000;47:78–80. doi: 10.1136/gut.47.suppl_4.iv78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389–401. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Travagli RA, Hermann GE, Browning KN, Rogers RC. Annu Rev Physiol 68:279-305. 2006 doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Treangen TJ, Wagner J, Burns MP, Villapol S. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757. doi: 10.3389/fimmu.2018.02757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tse JKY. Gut microbiota, nitric, oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem Neurosci. 2017;8:1438–1447. doi: 10.1021/acschemneuro.7b00176. [DOI] [PubMed] [Google Scholar]
- 189.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.van Middendorp JJ, Allison HC, Ahuja S, Bracher D, Dyson C, Fairbank J, Gall A, Glover A, Gray L, Masri WE, Uttridge A, Cowan K. Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnership. Spinal Cord. 2016;54:341–346. doi: 10.1038/sc.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vance KM, Rogers RC, Hermann GE. PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. J Neurosci. 2015;35:776–785. doi: 10.1523/JNEUROSCI.3105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wallace DJ, Sayre NL, Patterson TT, Nicholson SE, Hilton D, Grandhi R. Spinal cord injury and the human microbiome: beyond the brain–gut axis. Neurosurg Focus. 2019;46:E11. doi: 10.3171/2018.12.FOCUS18206. [DOI] [PubMed] [Google Scholar]
- 193.Wang FB, Powley TL. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007;329:221–230. doi: 10.1007/s00441-007-0413-7. [DOI] [PubMed] [Google Scholar]
- 194.Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein R, Tyndall JA, Manley GT. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18:165–180. doi: 10.1080/14737159.2018.1428089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Whipple B, Komisaruk BR. Brain (PET) responses to vaginal-cervical self-stimulation in women with complete spinal cord injury: preliminary findings. J Sex Marital Ther. 2002;28:79–86. doi: 10.1080/009262302317251043. [DOI] [PubMed] [Google Scholar]
- 197.WHO Neurotrauma 2020 from https://www.who. int/violence_injury_prevention/road_traffic/activities/neurotrauma/en/ Neurotrauma 2020 from https://wwwwhoint/violence_injury_prevention/road_traffic/activities/neurotrauma/en/ Accessed December 15, 2019. [Google Scholar]
- 198.Wing PC. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care providers. Who should read it. J Spinal Cord Med. 2008;31:360. doi: 10.1080/10790268.2008.11760737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Winter G, Hart RA, Charlesworth RPG, Sharpley CF. Gut microbiome and depression: what we know and what we need to know. Rev Neurosci. 2018;29:629–643. doi: 10.1515/revneuro-2017-0072. [DOI] [PubMed] [Google Scholar]
- 200.Wolf C, Meiners TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41:347–353. doi: 10.1038/sj.sc.3101440. [DOI] [PubMed] [Google Scholar]
- 201.Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 202.Yamano M, Kamato T, Nagakura Y, Miyata K. Effects of gastroprokinetic agents on gastroparesis in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:145–150. doi: 10.1007/pl00005022. [DOI] [PubMed] [Google Scholar]
- 203.Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET. Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci. 2014;34:10765–10769. doi: 10.1523/JNEUROSCI.5316-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yates AG, Anthony DC, Ruitenberg MJ, Couch Y. Systemic immune response to traumatic CNS injuries—are extracellular vesicles the missing link. Front Immunol. 2019;10:2723. doi: 10.3389/fimmu.2019.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurons innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu X, Yu M, Liu Y, Yu S. TRP channel functions in the gastrointestinal tract. Semin Immunopathol. 2016;38:385–396. doi: 10.1007/s00281-015-0528-y. [DOI] [PubMed] [Google Scholar]
- 207.Zhuo H, Ichikawa H, andHelke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 208.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654–660. doi: 10.1097/01.ccm.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.