Abstract
Background & aims
Although primarily a disease with liver-specific complications, nonalcoholic fatty liver disease (NAFLD) is a systemic disease with extrahepatic complications. We aim to evaluate the association between NAFLD and cardiovascular disease (CVD), stroke and cerebrovascular disease, and extrahepatic cancers.
Methods
We searched MEDLINE, EMBASE, and Cochrane Systematic Review Database from January 1, 2000 to July 1, 2019 to identify peer-reviewed English language literature using predefined keywords for NAFLD, CVD, stroke and cerebrovascular disease, and extrahepatic cancers among adults. Two reviewers independently selected studies for inclusion. Measures of association between NAFLD and CVD, stroke and cerebrovascular disease, and extrahepatic cancers were extracted. Quality assessed using Newcastle-Ottawa scale and Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Results
Thirty studies were included evaluating CVD, 16 studies evaluating stroke or cerebrovascular disease, and 13 studies evaluating extrahepatic cancers. On pooled meta-analysis assessment, NAFLD was associated with increased risk of CVD (risk ratio [RR]: 1.78; 95% confidence interval [CI]: 1.52–2.08) and stroke or cerebrovascular disease (RR: 2.08, 95% CI: 1.72–2.51). Significant heterogeneity in assessing extrahepatic cancers prevented applying meta-analysis methods, but NAFLD seemed to be associated with increased risk of breast and colorectal cancers. Overall level of quality of studies were very low by GRADE.
Conclusions
NAFLD is associated with increased risks of CVD and stroke or cerebrovascular disease among adults. There appears to be increased risk of breast and colorectal cancers. Given low quality of evidence, it is premature to make any strong conclusions to modify CVD, stroke, or cancer screening policies in patients with NAFLD.
Keywords: nonalcoholic fatty liver disease, cardiovascular disease, extrahepatic cancer, meta-analysis
Abbreviations: CV, Cardiovascular disease; NAFLD, Nonalcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey
Nonalcoholic fatty liver disease (NAFLD) is estimated to affect up to 25% of the general adult population worldwide.1, 2, 3, 4 The overall prevalence of NAFLD is projected to rise dramatically over the next decade along with the aging population, as well as the increasing prevalence of metabolic disorders (e.g. obesity, diabetes mellitus, dyslipidemia) that are associated with significantly higher risks of developing NAFLD.3,5
Although NAFLD primary affects the liver, with potential to progress to severe liver disease, it is generally accepted that NAFLD is a syndrome in a larger spectrum of metabolic disorders and that NAFLD should be considered more of a systemic disease.6 Strong evidence supports that the clinical and economic burden of NAFLD is not only restricted to severe liver-related complications but also includes major extrahepatic diseases such as cardiovascular diseases (CVDs) and extrahepatic cancers.7 Most of the morbidity and mortality observed in patients with NAFLD are caused by the increased risk of these diseases. Indeed, it is known that CVD is the leading cause of mortality among patients with NAFLD, followed by extrahepatic cancers and liver-related complications.7, 8, 9, 10 However existing studies evaluating the association between NAFLD and CVDs as well as extrahepatic cancers are primarily observational cohort studies, with many based on single-center cohort samples. Previous meta-analyses evaluating the association between NAFLD and CVD have been conducted through January 2016.11 Our present study provides an update to include more recently published literature, as well as to incorporate not only CVDs but also cerebrovascular disease or stroke and extrahepatic cancers as an outcome measure. Thus our study aimed to perform an updated comprehensive systematic review and meta-analysis of the existing literature among adults with NAFLD.
Materials and methods
Data sources
We performed a systematic literature search to identify English language published studies that evaluated the association of NAFLD with CVD, stroke or cerebrovascular disease, and extrahepatic solid cancers. We searched MEDLINE, EMBASE, and the Cochrane Systematic Review Database from January 1, 2000 to July 1, 2019. Search terms for NAFLD included “NAFLD”, “nonalcoholic fatty liver disease”, “NASH”, “nonalcoholic steatohepatitis”, or “hepatic steatosis”; for CVD included “heart attack”, “cardiovascular outcomes”, “CVD”, “myocardial infarct”, “myocardial ischemia”, “coronary calcification”, “left ventricular hypertrophy”, “cardiac hypertrophy”, “cardiac dysfunction”, or “ventricular dysfunction”; for stroke included “stroke”, “CVA”, “cerebrovascular accident”, or “transient ischemic attack”; for extrahepatic solid cancers included “cancer”, “breast cancer”, or“colorectal cancer”. References of relevant articles were additionally reviewed to identify any additional studies meeting inclusion criteria that were not identified on the initial query.
Study selection
Our inclusion criteria included full length studies in the English language literature that included adults (age ≥ 18 years). Studies were excluded if the primary study population did not have NAFLD or NASH or the primary outcome was not the ones aforementioned as focuses of our study. In particular, the present study was specifically focused on evaluation of NAFLD and its association with extrahepatic cancers, and thus studies that focused solely on evaluating risk of hepatocellular carcinoma only, were excluded as well.
Data extraction and quality assessment
Study identification and data abstraction from identified articles were performed independently by two investigators (N.V. and R.W.). The Newcastle-Ottawa scale was used to assess the quality of observational studies.12 The Newcastle-Ottawa scale incorporates a standardized method for assessing the quality of studies via an assessment of three main study characteristics: selection of study groups, comparability of groups, and ascertainment of exposure/outcome. On a scale of 0–9, those studies achieving 8 or more points were categorized as high quality, those with 5–7 points were fair quality, and those with 4 or fewer points were poor quality. After this initial assessment, we applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method to determine the overall certainty of the evidence as it relates to the association of NAFLD with CVD and stroke separately.13 GRADE was not applied for the evaluation of NAFLD and extrahepatic cancers (as detailed in the following context), given significant heterogeneity of the outcomes of the included studies that prevented the application of meta-analysis methods. The GRADE method is used to evaluate the overall evidence of all studies for each association assessed and is categorized as high, moderate, low, or very low. The domains that are incorporated into the GRADE assessment include study design, consistency, precision, directness, and potential for other biases (e.g. publication bias).13
Data synthesis and analysis
Aggregate data on the association between NAFLD and CVD and stroke from included studies were evaluated with meta-analysis. Pooled risk ratios were generated using random effects models. Heterogeneity was assessed using χ2 and I2 tests with I2 > 50% indicating significant heterogeneity between studies. Potential publication bias was assessed using funnel plots. Analyses were performed using Review Manager (RevMan) [Computer program], version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014. Meta-analysis was not performed for evaluating the association between NAFLD and extrahepatic cancers given variation in the outcome between different studies. For example, one study focused on pancreatic cancers, three focused on all cancers, two studies focused on breast cancers, and seven studies focused on colorectal cancers. Thus we did not proceed with a meta-analysis to determine pooled effects after completing our systematic review.
Results
Cardiovascular disease
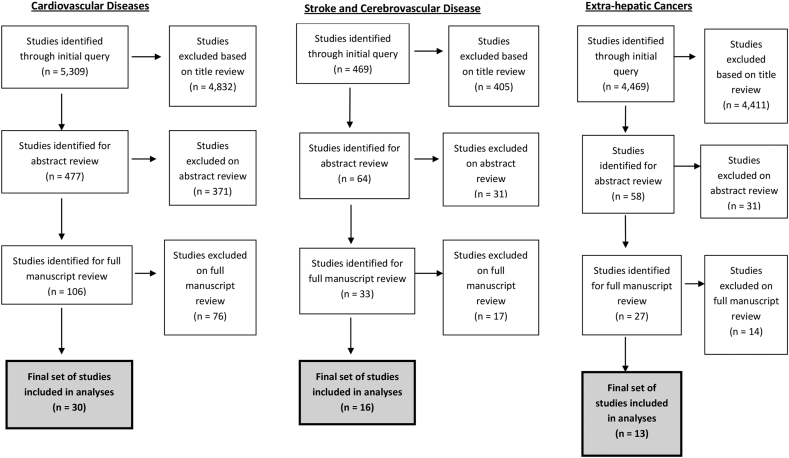
Our initial query identified 5309 studies, among which 4832 were excluded based on title review, 371 additionally excluded based on abstract review, and 76 studies were excluded based on full-length manuscript review. The final study cohort included 30 studies that met inclusion and exclusion criteria for further analyses (Figure 1).14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43
Figure 1.
PRISMA flow diagram for selection of included studies.
Study characteristics and outcomes
The study characteristics of the included studies are shown in Table 1. All studies were observational studies including 9 retrospective cohort studies, 12 cross-sectional studies, 7 prospective observational studies, one nested case-control study, and one case-control study. The majority of studies were conducted in the USA (n=7), Italy (n=6), and China (n=5). Table 1 also includes details regarding the determination of NAFLD diagnosis in the various studies, which included methods such as the Fatty Liver Index, the FibroMax algorithm, liver biopsy, ICD-9 and ICD-10 codes, as well as imaging-based modalities (Table 1). Most studies excluded individuals with excessive alcohol consumption, hepatitis and other chronic liver diseases, those on hepatotoxic medications, or history of CVD. Table 1 also provides specific details on how each study defined the CVD outcome assessed.
Table 1.
Main Characteristics of the Included Studies Evaluating Association Between NAFLD and (A) Cardiovascular Diseases, (B) Stroke and Cerebrovascular Diseases, and (C) Extrahepatic Cancers.
| Author (Year) | Country/Region | Study Design | Study Period | Study Population | NAFLD Diagnosis Criteria | Exclusion Criteria | Outcomes Assessed |
|---|---|---|---|---|---|---|---|
| A. Cardiovascular Diseases | |||||||
| Golabi (2019)22 | USA | Cross-sectional study | Individuals from 2011 to 2016 | 3197 individuals (816 Asian American adults and 2381 Non-Hispanic Whites) | US-Fatty Liver Index ≥ 30 | Excessive alcohol consumption (≥20g/day in men and ≥10g/day in women), positive hepatitis C virus RNA, positive hepatitis B surface antigen, iron overload (defined as serum transferrin saturation ≥ 50%) | Prevalence of atherosclerotic cardiovascular disease using ASCVD: 10-year ASCVD risk score ≥7.5% |
| Viglino (2018)39 | France | Single-center prospective cohort study | Individuals from 2007 to 2012 | 111 individuals with chronic obstructive pulmonary disease | FibroMax algorithm, which incorporates 3 non-invasive tests: FibroTest, SteatoTest, and NashTest | Active pulmonary infection, chronic heart failure, left ventricular ejection fraction <45%, active smoking >10 cigarettes per day, neoplasia, prior antioxidant treatment, pregnancy, alcohol ≥20g for women and ≥30 g for men daily, viral hepatitis | Incidence of new cardiovascular events during follow-up, which include acute myocardial infarction, stroke, peripheral arterial disease or acute limb ischemia, venous thromboembolic disease and/or pulmonary embolism, and new-onset arrhythmias |
| Chinnadurai (2019)17 | England | Retrospective Cohort Study | Individuals from 1/2000–12/2014 with follow-up through 12/2015 | 149 individuals with diabetic kidney disease | Hepatic ultrasonography | Concurrent renal replacement therapy, excessive alcohol intake, hepatitis and other chronic liver diseases | Incidence of nonfatal cardiovascular events: myocardial infarctions, acute coronary syndromes, nonfatal cardiac arrest, congestive cardiac failure, peripheral vascular disease and cerebrovascular disease |
| Vanjiappan (2018)38 | India | Single-center prospective cohort study | Individuals from 4/2014–5/2016 | 300 individuals with type 2 diabetes mellitus | Hepatic ultrasonography | hepatitis B & C infections, chronic liver disease, those on hepatotoxic drugs | Incidence of cardiovascular disease |
| Allen (2018)14 | USA | Retrospective cohort study | Individuals from 1997 to 2014 with follow-up through 10/1/2016 | 19,078 individuals | Hospital International Classification of Diseases Adapted (HICDA) codes and ICD codes | Other liver diseases such as viral hepatitis, alcoholic liver disease, alcohol use, cholestatic liver disease, short follow-up time of less than 1 year | Incidence of clinical cardiovascular events (myocardial infarction, angina/ischemic heart disease, atrial fibrillation, cardiac arrest, congestive heart failure and stroke) |
| Mantovani (2016)28 | Italy | Single-center retrospective cohort study | Individuals enrolled from 1999 to 2001 | 286 individuals with type 1 diabetes | Findings on liver ultrasound which include diffuse hyperechogenicity of the liver relative to kidneys, beam attenuation, poor visualization of the intrahepatic vessel border and diaphragm | Missing liver ultrasound data, concurrent end-stage renal disease or malignancy, cirrhosis, and liver diseases due to secondary causes, such as excessive alcohol consumption, viral hepatitis, iron overload or use of steatogenic medications | Incidence of cardiovascular events: combined endpoint of nonfatal ischemic heart disease, nonfatal ischemic stroke, carotid endarterectomy, coronary or lower extremity artery revascularization |
| Zeb (2016)43 | USA | Multicenter retrospective cohort study | Not clearly stated, but MESA study started enrolling July 2000 | 4119 individuals | Computed tomography | Heavy alcohol intake (defined as >14 drinks per week for men and >7 drinks per week for women), use of oral steroids, cirrhosis | Incidence of nonfatal coronary heart disease: myocardial infarction, resuscitated cardiac arrest, angina with or without coronary revascularization |
| Stolic (2016)32 | Serbia | Single-center observational study | Not stated | 72 individuals over the age of 65 years on chronic hemodialysis | Findings on liver ultrasound demonstrating increased hepatic parenchyma echogenicity compared to right kidney cortex | Hemodialysis, patients hospitalized in the past 6 months, infection with hepatotropic virus, diabetes mellitus, hepatobiliary surgery, body mass index higher than 30 kg/m2, concurrent use of statins or glucocorticosteroids | Prevalence of cardiovascular disease |
| Fracanzani (2016)21 | Italy | Prospective cohort study | Individuals from 6/2002–12/2004 | 273 individuals | Liver ultrasound assessment of hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring | Chronic viral hepatitis, autoimmune hepatitis, hereditary hemochromatosis, Wilson's disease, and drug-induced liver disease were excluded in NAFLD patients; Controls were negative for hepatitis B and C and had normal liver function tests | Incidence of major cardiovascular events |
| Wong (2016)41 | Hong Kong | Prospective cohort study | Individuals from 10/2007–11/2008 | 612 consecutive individuals who underwent coronary angiogram | Liver ultrasound findings including diffusely increased liver echogenicity compared to kidney or spleen, vascular blurring, and deep attenuation of the ultrasound signal | Excessive alcohol intake (>20 g/day in men and >10 g/day in women), secondary causes of fatty liver (e.g., systemic steroids or methotrexate), HBV or HCV, or antinuclear antibody titer >1/160 | Incidence of cardiovascular events: cardiovascular deaths, nonfatal myocardial infarction, heart failure, coronary interventions, and congestive heart failure |
| Mellinger (2015)29 | USA | Cross-sectional study from a large prospective longitudinal cohort study | Individuals from 2002 to 2005 | 3014 individuals | CT scan liver-phantom ratio with a liver-phantom ratio of 0.33 or lower representing the presence of thirty percent or more of hepatic steatosis | Pregnancy, weight >160 kg, CT scan results uninterpretable for hepatic steatosis, missing a complete profile | Prevalent cardiovascular disease: composite of nonfatal myocardial infarction, heart failure, stroke, transient ischemic attack, or peripheral arterial disease |
| Pisto (2014)30 | Finland | Population-based prospective cohort study | Individuals from 1991 to 2009 | 988 individuals | Liver ultrasound assessment of hepatic steatosis. | Previous hospital-diagnosed myocardial infarction or stroke | Incidence of cardiovascular disease: major coronary heart disease event and stroke based on ICD-10 coding. |
| Lai (2013)25 | Taiwan | Single-center retrospective cohort study | Individuals from 7/1998 to 10/2012 | 278 individuals undergoing hemodialysis | Liver ultrasound assessment that includes increased hepatic echogenicity compared to kidneys, blurring of the gallbladder wall, hepatic veins, or portal vein | Chronic drug or alcohol abuse, cirrhosis, history of CVD, malignancies, medications known to cause hepatic steatosis, insufficient medical records | Development of nonfatal cardiovascular events: angina pectoris, nonfatal myocardial infarction, acute pulmonary edema, congestive heart failure, and peripheral vascular disease |
| Choi (2013)18 | Korea | Single-center cross-sectional study | Individuals from 1/2009–6/27/2011 | 134 individuals undergoing elective coronary angiography | Liver ultrasound with characteristic echo patterns such as a diffuse increase in hepatic echogenicity compared to the kidney | Viral hepatitis, history of heavy alcohol ingestion, medications reported to affect hepatic steatosis within 3 months of enrollment, or other history of chronic liver disease. | Prevalence of coronary artery disease: at least 50% stenosis in at least one major coronary artery |
| Dunn (2013)19 | USA | Single-center retrospective cohort study | Individuals from 1/1/2002 to 12/31/2003 with follow-up through the end of 2008 | 2343 individuals with type 2 diabetes | CT scan assessment of liver and spleen attenuation difference of −10 or less (indicating ≥30% steatosis) | Patients with ICD-9 diagnostic codes for alcohol abuse, alcoholic liver disease, chronic hepatitis B and C, autoimmune hepatitis, biliary cirrhosis, Wilson disease, hemochromatosis, alpha-1 antitrypsin disease, or a prior liver transplant. | Development of cardiovascular outcomes: cardiovascular deaths, myocardial infarctions, strokes, angina, arrhythmias and congestive heart failure based on ICD-9 coding |
| Feitosa (2013)20 | USA | Multicentered cross-sectional study | Individuals recruited from 1992 to 1996 | 2756 individuals in 510 extended random and high coronary heart disease risk families recruited from the Framingham Heart Study, the Utah Family Tree Study, and the North Carolina and Minnesota sites of the Atherosclerosis Risk in Communities Study. | CT scan liver attenuation of 40 Hounsfield units predicted hepatic steatosis, | Alcohol consumption over 21 drinks/week for men and >14 drinks/week) for women; Amiodarone use; HCV antibody positive. | Prevalence of coronary heart disease: coronary bypass, myocardial infarction, coronary angioplasty, balloon angioplasty, atherectomy, stent, percutaneous transluminal coronary angioplasty, or percutaneous coronary intervention |
| Wong (2011)42 | Hong Kong | Single-center prospective cohort study | Individuals from 10/2007 to 11/2008 | 612 individuals who underwent coronary angiogram | Liver ultrasound showing diffusely increased hepatic echogenicity compared to kidney or spleen, vascular blurring and deep attenuation of the ultrasound signal | Contraindications to coronary angiogram, excessive alcohol intake, and secondary causes of fatty liver (e.g., chronic use of systemic corticosteroids or methotrexate), positive hepatitis B surface antigen, antibody against hepatitis C virus and antinuclear antibody titer >1/160 | Incidence of coronary artery disease: presence of at least 50% stenosis at one or more coronary arteries |
| Hamaguchi (2007)24 | Japan | Prospective cohort study | Individuals from 1/1998 to 12/1998 with follow-up through 12/2004 | 1647 individuals who completed health checkups on an annual or biennial basis | Abdominal ultrasonography evidence of hepatorenal contrast and liver brightness | Previous myocardial infarction, angina pectoris, ischemic stroke, cerebral hemorrhage or cancer, alcohol intake of more than 20 g/d, concurrent HBV or HCV, other liver diseases | Incidence of cardiovascular disease via self-administered questionnaire: coronary heart disease, ischemic stroke, cerebral hemorrhage, unstable angina, acute MI, silent MI |
| Targher (2006)34 | Italy | Single-center retrospective cohort study | Not stated | 800 individuals with type 2 diabetes | Liver ultrasonography evidence of characteristic echo patterns such as increased echogenicity of the liver compared to kidneys | Heavy alcohol use, other known causes of chronic liver disease (e.g. viral hepatitis, autoimmune hepatitis, use of hepatotoxic medications such as glucocorticoids, antibiotics, amiodarone, methotrexate, tamoxifen or other anti-neoplastic drugs) | Prevalence of cardiovascular disease: coronary artery disease (myocardial infarction, angina pectoris, heart failure or revascularization procedures), cerebrovascular disease, or peripheral vascular disease |
| Targher (2005)36 | Italy | Prospective nested case-control study | Individuals from 1/1/2000 to 12/31/2000 with follow-up through 5/31/2005 | 248 cases (with nonfatal coronary heart disease) and 496 controls (without coronary heart disease) | Liver ultrasound findings of increased diffuse hyperechogenicity compared to kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures | Alcohol abuse, other known causes of chronic liver disease (viral hepatitis, autoimmune hepatitis, use of hepatotoxic medications) | Prevalence of NAFLD in patients with and without any nonfatal coronary heart disease, ischemic stroke, or cardiovascular death. |
| Liu (2019)26 | China | Matched case-control study | Individuals from 3/2011 to 7/2016 who underwent coronary angiography for evaluation of angina-like chest pain and/or positive treadmill exercise test and/or significant stenosis by coronary computer tomography | 324 individuals with stable, new-onset coronary artery disease | Liver ultrasound evidence of diffusely increased liver echogenicity compared to kidney or spleen, vascular blurring, and deep attenuation of the ultrasound signal | Patients without abdominal ultrasound examination, HBV or HCV; autoimmune hepatitis; hereditary liver disease; excessive alcohol consumption; secondary causes of fatty liver (e.g., chronic use of systemic corticosteroids or methotrexate) or drug-induced liver disease | Incidence of cardiovascular events: all-cause death (death mainly cause by cardiovascular disease), nonfatal myocardial infarction and stroke |
| Hagstrom (2019)23 | Sweden | Retrospective cohort study | Individuals from 1971 to 2009 with biopsy proven NAFLD | 6872 individuals | Liver biopsy assessment of NAFLD activity score | Other causes of steatosis, low liver biopsy quality, CVD at baseline, liver outcome within 6 months | Incidence of cardiovascular outcomes: first event of either acute ischemic heart disease or stroke (ischemic or hemorrhagic) |
| Wild (2018)40 | Scotland | Retrospective cohort study | Individuals from 1/1/2004 to 12/31/2013 | 132,661 hospitalized individuals with type 2 diabetes | NAFLD ICD-9 and ICD-10 codes: 571.8, K76, K75.8 | Viral hepatitis, autoimmune hepatitis, hemochromatosis, and any cirrhosis, fibrosis, sclerosis, or portal hypertension with no mention of ALD or NAFLD | Incident/recurrent cardiovascular disease: ICD-9/10 codes for acute coronary syndrome, myocardial infarction, stroke, heart failure, coronary revascularization procedure and carotid revascularization procedure |
| Chan (2014)16 | Malaysia | Cross-sectional study | Individuals from 11/2011 to 4/2012 | 399 individuals with type 2 diabetes | Liver ultrasound showing evidence of increased echogenicity, posterior attenuation and loss of intrahepatic and architectural details | Heavy alcohol intake, other causes of chronic liver disease and use of drugs that could cause fatty liver. | Prevalence of ischemic heart disease: previous admission for acute coronary syndrome, previous coronary intervention, previous coronary angiography showing coronary artery disease, or under follow-up and treatment for ischemic heart disease |
| Stepanova (2012)31 | USA | Population-based cross-sectional study | Individuals from 1988 to 1994 | 11,613 individuals from the National Health and Nutrition Examination Survey III (NHANES III) | Abdominal ultrasonography evidence of moderate to severe hepatic steatosis | Excessive alcohol, iron overload, or positive for HBV or HCV | Prevalence of cardiovascular disease: self-reported history of congestive heart failure, stroke, angina, or myocardial infarction. |
| Targher (2012)37 | Italy | Single-center cross-sectional study | Individuals from 2008 to 2010 | 343 individuals with type 1 diabetes | Liver ultrasonography evidence of diffuse hyperechogenicity of the liver relative to the kidneys, ultrasound beam attenuation and poor visualization of the intrahepatic vessel borders and diaphragm | Unavailable liver ultrasound exam, history of end-stage renal disease, cirrhosis, malignancy, known causes of chronic liver disease (alcohol-induced or drug-induced liver disease, hemochromatosis, autoimmune or viral hepatitis) | Prevalence of cardiovascular disease composite endpoint including coronary heart disease, cerebrovascular disease, or peripheral vascular disease |
| Sun (2011)33 | China | Cross-sectional study | Individuals from 9/2008 to 9/2009 | 542 consecutive individuals with suspected coronary artery disease | Abdominal CT scan evidence of liver attenuation less than the spleen, blurred intrahepatic vessels, or markedly reduced attenuation of the liver with evident contrast between the liver and the intrahepatic vessels | Heavy alcohol consumption; other chronic liver diseases; medications known to induce fatty liver disease such as steroids, estrogens, amiodarone, tamoxifen, or other chemotherapeutic agents within the previous 6 months; creatinine >2 mg/dl; history of acute coronary syndrome or heart failure | Significant coronary artery disease: >70% stenosis of lumen diameter |
| Targher (2010)35 | Italy | Cross-sectional study | Individuals from 1/2009 to 12/2009 | 301 individuals with type 1 diabetes | Liver ultrasonography evidence of diffuse hyperechogenicity of liver relative to kidneys, ultrasound beam attenuation and poor visualization of intrahepatic vessel borders and diaphragm | Excessive alcohol consumption, other known causes of chronic liver disease, missing liver ultrasound data | Prevalence of cardiovascular disease composite endpoint including coronary heart disease, cerebrovascular disease, or peripheral vascular disease |
| Lu (2009)27 | China | Cross-sectional study | Individuals from 1/2002 to 1/2009 | 560 individuals with type 2 diabetes mellitus | Liver ultrasonography | Alcohol intake more than 20 g/day, no known etiologies of liver disease and negative tests for the presence of viral hepatitis | Prevalence of coronary heart disease |
| Arslan (2007)15 | Turkey | Cross-sectional study | Not stated | 92 consecutive individuals undergoing first coronary angiography | Liver ultrasound findings of diffuse increase in the echogenicity of the liver compared to kidney | coronary artery disease, or with a history of percutaneous surgical revascularization, prior acute coronary syndrome, chronic alcohol consumption (more than 20 g/day), HBV or HCV, systemic diseases that might cause fatty liver, use of drugs like statins and insulin-sensitizing agents (metformin and glitazones) | Prevalence of coronary artery disease: presence of at least 50% stenosis in at least one major coronary artery |
| B. Stroke and cerebrovascular diseases | |||||||
| Hagstrom (2019)23 | Sweden | Retrospective cohort study | Individuals from 1971 to 2009 with biopsy proven NAFLD | 6872 individuals | Liver biopsy assessment of NAFLD activity score | Other causes of steatosis, low liver biopsy quality, CVD at baseline, liver outcome within 6 months | Incidence of cardiovascular outcomes: first event of either acute ischemic heart disease or stroke (ischemic or hemorrhagic) |
| Alexander (2018)44 | USA | Retrospective case cohort study | Individuals from 2003 to 2007 with follow-up through 9/1/2011 | 1676 individuals (572 with incident ischemic stroke and a stratified stroke-free cohort random sample of 1017) | Fatty Liver Index > 60 | Medical conditions that precluded involvement, prior stroke, excessive alcohol consumption (14 drinks/week for men, 7 drinks/week for women) | Incidence of ischemic stroke |
| Mantovani (2016)28 | Italy | Single-center retrospective cohort study | Individuals enrolled from 1999 to 2001 | 286 individuals with type 1 diabetes | Liver ultrasound showing diffuse hyperechogenicity of the liver compared to kidneys, beam attenuation, poor visualization of the intrahepatic vessel border and diaphragm | Missing ultrasound data end-stage renal disease or malignancy, cirrhosis or other chronic liver diseases, excessive alcohol consumption, viral hepatitis, iron overload hepatitis or use of steatogenic medications | Incidence of cardiovascular events: combined endpoint of nonfatal ischemic heart disease, nonfatal ischemic stroke, carotid endarterectomy, coronary or lower extremity artery revascularization |
| Moshayedi (2014)45 | Iran | Cross-sectional study | Individuals from 5/2012 to11/2013 | 220 individuals (110 brain magnetic resonance imaging confirmed ischemic stroke patients and 110 age- and sex-matched controls) | Liver ultrasonography assessment of increased hepatic echogenicity and visualization of the diaphragm and intrahepatic vessel borders | Chronic HBV or HCV, heavy alcohol consumption more than 20 g/day and chronic hepatotoxic drug use | Prevalence of NAFLD in patients with and without imaging confirmed ischemic stroke |
| Pisto (2014)30 | Finland | Population-based prospective cohort study | Individuals from 1991 to 2009 | 988 individuals | Liver ultrasound assessment of steatosis | Previous hospital-diagnosed myocardial infarction or stroke | Incidence of cardiovascular disease: major coronary heart disease event and stroke based on ICD-10 coding. |
| Dunn (2013)19 | USA | Single-center retrospective cohort study | Individuals from 1/1/2002 to 12/31/2003 with follow-up through the end of 2008 | 2343 individuals with type 2 diabetes | Abdominal CT Scan assessment of liver and spleen attenuation difference of −10 or less (indicating ≥30% steatosis) | Patients with ICD-9 diagnostic codes for alcohol abuse, alcoholic liver disease, chronic hepatitis B and C, autoimmune hepatitis, biliary cirrhosis, Wilson disease, hemochromatosis, alpha-1 antitrypsin disease, or a prior liver transplant. | Development of stroke based on ICD-9 coding |
| Hamaguchi (2007)24 | Japan | Prospective cohort study | Individuals from 1/1998 to 12/1998 with follow-up through 12/2004 | 1647 individuals who completed health checkups on an annual or biennial basis | Abdominal ultrasonography evidence of hepatorenal contrast and liver brightness | Previous myocardial infarction, angina pectoris, ischemic stroke, cerebral hemorrhage or cancer, alcohol intake of more than 20 g/d, HBV or HCV positive, or other chronic liver diseases | Incidence of cardiovascular disease via self-administered questionnaire: coronary heart disease, ischemic stroke, cerebral hemorrhage, unstable angina, acute MI, silent MI |
| Targher (2005)36 | Italy | Prospective nested case-control study | Individuals from 1/1/2000 to12/31/2000 with follow-up through 5/31/2005 | 248 cases with cardiovascular outcomes and 496 controls (without cardiovascular outcomes) | Liver ultrasound findings of increased diffuse hyperechogenicity compared to kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures | Alcohol abuse, other known causes of chronic liver disease (viral hepatitis, autoimmune hepatitis, use of hepatotoxic medications) | Prevalence of NAFLD in patients with and without cardiovascular outcomes (any nonfatal coronary heart disease, ischemic stroke, or cardiovascular death) |
| Viglino (2018)39 | France | Single-center prospective cohort study | Individuals from 2007 to 2012 | 111 individuals with chronic obstructive pulmonary disease | FibroMax algorithm, which incorporates 3 non-invasive tests: FibroTest, SteatoTest, and NashTest | Active pulmonary infection, chronic heart failure, left ventricular ejection fraction <45%, active smoking >10 cigarettes per day, neoplasia, antioxidant treatment, pregnant women, a daily consumption of alcohol ≥20g for women and ≥30 g for men, viral hepatitis | Incidence of new cardiovascular events during follow-up: acute myocardial infarction; stroke; new diagnosis of peripheral arterial disease or acute limb ischemia; venous thromboembolic disease and/or pulmonary embolism and new-onset arrhythmias |
| Mellinger (2015)29 | USA | Cross-sectional study from a large prospective longitudinal cohort study | Individuals from 2002 to 2005 | 3014 individuals | CT scan liver-phantom ratio with a liver-phantom ratio of 0.33 or lower representing the presence of thirty percent or more of hepatic steatosis | Pregnancy, weight >160 kg, CT scan results uninterpretable for hepatic steatosis, missing a complete profile | Prevalent cardiovascular disease: composite of non-fatal myocardial infarction, heart failure, stroke, transient ischemic attack, or peripheral arterial disease |
| Targher (2006)34 | Italy | Single-center retrospective cohort study | Not stated | 800 individuals with type 2 diabetes | Liver ultrasonography evidence of characteristic echo patterns such as increased echogenicity of the liver compared to kidneys | Heavy alcohol use, other known causes of chronic liver disease (e.g. viral hepatitis, autoimmune hepatitis, use of hepatotoxic medications such as glucocorticoids, antibiotics, amiodarone, methotrexate, tamoxifen or other anti-neoplastic drugs) | Prevalence of cardiovascular disease: coronary artery disease, cerebrovascular disease,or peripheral vascular disease |
| Liu (2019)26 | China | Matched case-control study | Individuals from 3/2011 to7/2016 who underwent coronary angiography for evaluation of angina-like chest pain and/or positive treadmill exercise test and/or significant stenosis by coronary computer tomography | 324 individuals with stable, new-onset coronary artery disease | Liver ultrasound evidence of diffusely increased liver echogenicity compared to kidney or spleen, vascular blurring, and deep attenuation of the ultrasound signal | Patients without abdominal ultrasound examination, HBV or HCV; autoimmune hepatitis; hereditary liver disease; excessive alcohol consumption; secondary causes of fatty liver (e.g., chronic use of systemic corticosteroids or methotrexate) or drug-induced liver disease | Incidence of cardiovascular events: all-cause death (death mainly cause by cardiovascular disease), non-fatal myocardial infarction and stroke |
| Wild (2018)40 | Scotland | Retrospective cohort study | Individuals from 1/1/2004 to12/31/2013 | 132,661 hospitalized individuals with type 2 diabetes | NAFLD ICD-9 and ICD-10 codes: 571.8, K76, K75.8 | Viral hepatitis, autoimmune hepatitis, hemochromatosis, and any cirrhosis, fibrosis, sclerosis, or portal hypertension with no mention of ALD or NAFLD | Incident/recurrent cardiovascular disease: ICD-9/10 codes for acute coronary syndrome, myocardial infarction, stroke, heart failure, coronary revascularization procedure and carotid revascularization procedure |
| Stepanova (2012)31 | USA | Population-based cross-sectional study | Individuals from 1988 to 1994 | 11,613 individuals from the National Health and Nutrition Examination Survey III (NHANES III) | Abdominal ultrasonography evidence of moderate to severe hepatic steatosis | Excessive alcohol, iron overload, or positive for HBV or HCV | Prevalence of cardiovascular disease: self-reported history of congestive heart failure, stroke, angina, or myocardial infarction. |
| Targher (2012)37 | Italy | Single-center cross-sectional study | Individuals from 2008 to 2010 | 343 individuals with type 1 diabetes | Liver ultrasonography evidence of diffuse hyperechogenicity of the liver relative to the kidneys, ultrasound beam attenuation and poor visualization of the intrahepatic vessel borders and diaphragm | Unavailable liver ultrasound exam, known history of end-stage renal disease, cirrhosis, malignancy, known causes of chronic liver disease (alcohol-induced or drug-induced liver disease, hemochromatosis, autoimmune or viral hepatitis) | Prevalence of cardiovascular disease composite endpoint including coronary heart disease, cerebrovascular disease, or peripheral vascular disease |
| Targher (2010)35 | Italy | Cross-sectional study | Individuals from 1/2009 to12/2009 | 301 individuals with type 1 diabetes | Liver ultrasonography evidence of diffuse hyperechogenicity of liver relative to kidneys, ultrasound beam attenuation and poor visualization of intrahepatic vessel borders and diaphragm | Excessive alcohol consumption, other known causes of chronic liver disease, missing liver ultrasound data | Prevalence of cardiovascular disease composite endpoint including coronary heart disease, cerebrovascular disease, or peripheral vascular disease |
| C. Extrahepatic Cancers | |||||||
| Kim (2017)49 | Korea | Single-center retrospective cohort study | Individuals from 9/1/2004 to 12/31/2005 with follow-up through 12/31/2015 | 25,947 individuals who underwent a comprehensive health checkup | Liver ultrasound evidence of hepatic steatosis | Excessive alcohol consumption; HBV or HCV; HIV; cancer or diagnosis of cancer at baseline; prior organ transplantation; cirrhosis; chronic kidney disease; subjects who had not visited hospital for > 2 years but later returned after cancer diagnosis; | Incidence of all cancers |
| Chang (2018)47 | Taiwan | Single-center cross-sectional study | Individuals diagnosed with pancreatic cancer between 1/2009 and 12/2013 | 143 individuals with pancreatic cancer and 414 randomly selected control patients without pancreatic cancer | Unenhanced CT scanning evidence of hepatic steatosis | HBV, HCV, or cirrhosis; history of alcohol drinking; prior major operation involving abdominal organ; history of medications that may have contribute to hepatotoxicity | Prevalence of NAFLD in cases and controls |
| Wild (2018)40 | Scotland | Retrospective cohort study of national population-based registry of patients with type 2 diabetes mellitus | Individuals diagnosed with type 2 diabetes between 1/1/2004 and 12/31/2013 | 132,661 adults age 40–89 with type 2 diabetes mellitus | Presence of the ICD-9 and ICD-10 codes for NAFLD: 571.8, K76, K75.8 | ICD-9 and ICD-10 codes for viral hepatitis, autoimmune hepatitis, hemochromatosis, and any cirrhosis, fibrosis, sclerosis, or portal hypertension with no mention of ALD or NAFLD | Incident or recurrent cancer, excluding HCC |
| Nseir (2017)53 | Israel | Single-center cross-sectional study | Individuals referred for a mammography screening exam from 1/2008 to 12/2011 | 73 patients with malignant breast cancer with abdominal CT imaging data within one month of diagnosis and 73 controls with normal screening mammography and breast ultrasonography who had abdominal CT imaging within 3 months of screening examinations | Presence of hepatic steatosis on abdominal CT exam | For NAFLD diagnosis: alcohol consumption > 20 g per day, positive serology for hepatitis B, hepatitis C, positive autoimmune hepatitis antibodies, or any history of another known liver disease | Prevalence of NAFLD in cases and controls |
| Seko (2015)55 | Japan | Single-center retrospective cohort study | Individuals from 1/1999 to 4/2013 | 312 individuals with liver biopsy confirmed NAFLD | Liver biopsy findings of steatosis in 5% or more of hepatocytes | Presence of viral hepatitis, autoimmune hepatitis, drug-induced liver disease, primary biliary cirrhosis, biliary obstruction, hemochromatosis, Wilson's disease and α-1-antitrypsin-deficiency-associated liver disease, heavy alcohol use; decompensated cirrhosis or HCC | Incidence of all cancers |
| Kwak (2019)50 | Korea | Single-center case-control study | Individuals from 1/2008 to 5/2017 | 270 patients with breast cancer diagnosed by screening and 270 controls with normal breast mammography and/or ultrasonography | Hepatic steatosis by ultrasonography | Other chronic liver disease, including HBV or HCV, significant alcohol consumption (defined as >20 g/d for women) | Prevalence of NAFLD in cases and controls |
| Cho (2019)48 | Korea | Single-center cross-sectional study | Individuals from 1/2013 to 11/2018 | 476 individuals with biopsy proven NAFLD | Liver biopsy in which at least 5% of hepatocytes displayed macrovesicular steatosis | Hepatitis B or C virus infection; autoimmune hepatitis or primary biliary cholangitis; drug-induced liver injury or steatosis; Wilson disease or hemochromatosis; excessive alcohol consumption; diagnosis of malignancy within the prior year; family history of CRC in first-degree relatives; having an inherited syndrome (e.g. Lynch syndrome, Peutz-Jeghers syndrome, MYH-associated polyposis or familial adenomatous polyposis); past medical history of colorectal neoplasm; inflammatory bowel disease; bowel symptoms (e.g. hematochezia, melena, or bowel habit change); patients who underwent polypectomy within the last 5 years; patients who declined to undergo colonoscopy | Prevalence of advanced colorectal neoplasm (defined as adenomatous polyp 10 mm or larger and/or with a feature of villous adenoma, and/or high grade dysplasia or adenocarcinoma |
| Yang (2017)56 | Korea | Single-center retrospective cohort study | Individuals from 1/2009 to 12/2013 who underwent surveillance colonoscopy after index colonoscopy | 441 patients with NAFLD and 441 patients without NAFLD – propensity score matched cohorts | Liver ultrasound or CT scan showing diffuse increased hepatic echogenicity compared to kidney, blurring of vascular structure, lower hepatic parenchymal attenuation than that of the spleen. | Prior history of colorectal surgery or colorectal disease; inflammatory bowel disease; an incomplete index colonoscopy; any colonoscopy within the previous 3 years of the index colonoscopy; chronic liver disease, including HBV, HCV or cirrhosis; significant alcohol consumption; no diagnostic examination for NAFLD within 3 months of the index colonoscopy; incomplete clinical information (missing variables ≥2) | Incidence of advanced colorectal neoplasm (defined as cancer or adenomatous polyp 10 mm or larger, any adenoma with tubulovillous or villous histology, high grade dysplasia) |
| Pan (2017)54 | China | Single-center cross-sectional study | Individuals from 1/2011–to 11/2015 | 1793 individuals undergoing colonoscopy as part of routine health checkup (27 with colorectal cancer and 1767 without colorectal cancer) | Hepatic ultrasonography with features including hepatomegaly, diffusely increased echogenicity, and blurring of vasculature | viral hepatitis; cirrhosis; liver cancer or other liver disease; excess alcohol consumption | Prevalence of NAFLD in cases and controls |
| Ahn (2017)46 | Korea | Single-center cross-sectional study | Individuals from 1/2003 to 12/2012 | 26,540 adults undergoing routine comprehensive health check up | Liver ultrasound showing increased parenchymal brightness, liver-to-kidney contrast, deep beam attenuation and bright vessel walls during examination | Cirrhosis, HBV or HCV, excess alcohol consumption >30g/day in men or 20g/day in women | Incidence of advanced colorectal neoplasia, defined as an invasive cancer or adenoma that was at least 10 mm in diameter, had high grade dysplasia, villous or tubulovillous histology or any combination thereof |
| Lin (2014)52 | China | Single-center cross-sectional study | Individuals enrolled from 10/2007–12/2011 | 2315 individuals undergoing routine screening colonoscopy | Ultrasonography features including hepatomegaly, diffusely increased echogenicity of liver parenchyma, and blurring of vasculature | History of colorectal cancer, adenoma and polyp; history of other extraintestinal malignancies; contraindications to colonoscopy; viral hepatitis, cirrhosis, liver cancer or other liver diseases; heavy alcohol consumption | Prevalence of colorectal malignant neoplasm (adenocarcinoma) |
| Lee (2012)51 | Korea | Single-center retrospective cohort study | Individuals enrolled from 7/1/2002–6/30/2006 with follow-up through 12/31/2008 | 5517 women aged 35–80 years undergoing life insurance health examinations | Abdominal ultrasonography assessment of hepatorenal contrast, liver brightness, deep attenuation, and blurring of the vasculature | HBV or HCV positive, alcohol consumption of more than 40 g/week; history of chronic liver disease, including viral, toxic, and autoimmune liver diseases; history of receiving previous medical insurance benefits | Incidence of colorectal cancer |
| Wong (2011)42 | Hong Kong | Multicenter cross-sectional study | Individuals enrolled from 1/2008–7/2010 (two cohorts: community subjects and hospital patients) | 380 individuals | Liver biopsy (hospital cohort) and proton-magnetic resonance spectroscopy (community cohort) | HBV or HCV positive; excess alcohol consumption (men >30g/day and women >20g/day; history of colorectal cancers or polyps; inflammatory bowel disease; bowel symptoms including per rectal bleeding and altered bowel habit; prior colorectal cancer screening; contraindications to colonoscopy | Prevalence of advanced colorectal neoplasms (cancer or adenoma with high grade dysplasia or villous architecture) |
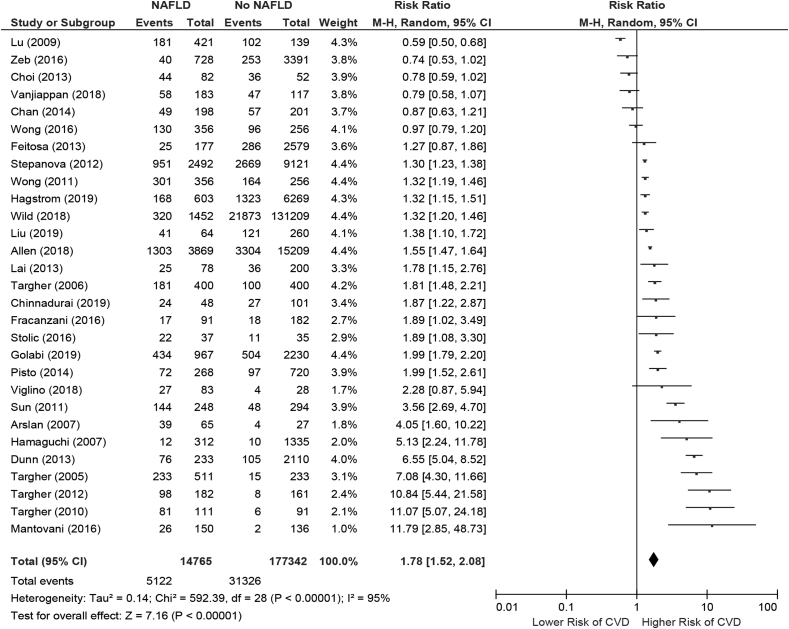
Table 2 shows the main outcomes of the studies. Although not all studies included adjusted regression analyses in evaluating the association between NAFLD and CVD, there was a consistent positive signal demonstrating increased risk of CVD in patients with NAFLD. The majority of patients among the included studies were middle-aged individuals and predominantly of male sex. Among studies that did include multivariate analyses, the majority included important potential confounders that are commonly considered in affecting the risk of both NAFLD and CVD (e.g. metabolic disease risk factors). On meta-analysis, the pooled risk ratio demonstrated a significantly increased risk of CVD associated with NAFLD diagnosis (overall pooled risk ratio: 1.78; 95% confidence interval [CI]: 1.52–2.08) (Figure 2). However, significant heterogeneity was present (I2 of 95%).
Table 2.
Main Outcomes of the Included Studies Evaluating the Association Between NAFLD and Cardiovascular Disease, Stroke and Cerebrovascular Diseases, and Extrahepatic Cancers.
| Author/Year | Median Follow-up | Age and Sex Distribution | NAFLD | No NAFLD | Univariate Outcomes | Adjusted Outcomes | Variables Included in Multivariate Analyses |
|---|---|---|---|---|---|---|---|
| Cardiovascular diseases | |||||||
| Golabi (2019)22 | N/A | Asian Americans: Age (mean, SE): 48.90, 1.72 (NAFLD); 42.68, 0.93 (No NAFLD); Male: 58.99% (NAFLD), 44.66% (No NAFLD); Non-Hispanic Whites: Age (mean, SE): 53.42, 0.59 (NAFLD); 47.08, 0.66 (no NAFLD); Male: 56.28% (NAFLD), 44.56% (no NAFLD) | Total N = 967 (Asian Americans N = 159 and Non-Hispanic Whites N = 808) | Total N = 2230 (Asian Americans N = 657 and Non-Hispanic Whites N = 1573) | ASCVD risk score >7.5%: Asian Americans: 35.39% (NAFLD) vs. 17.85% (no NAFLD), p < 0.05; non-Hispanic whites: 46.75% (NAFLD) vs. 24.58% (no NAFLD), p < 0.05 | N/A | N/A |
| Viglino (2018)39 | Not stated | Age (median, range): 65.3, 61.1–70.9 (NAFLD), 55.4, 51–64.8 (no liver disease); Male: 85.5% (NAFLD), 53.6% (no liver disease) | N = 83 | N = 28 | Total cardiovascular events: 32.5% (NAFLD) vs. 14.3% (no liver disease), p = 0.09; Univariate 5-year composite outcome: HR 3.06, 95% CI 1.08–8.63, p = 0.035 | Cardiovascular events and death at 5-years: Steatosis vs. no steatosis: HR 1.66, 95% CI 0.72–3.84, p = 0.236; NASH vs. no NASH: HR 0.80, 95% CI 0.34–1.85, p = 0.596; Fibrosis vs. no fibrosis: HR 2.94, 95% CI 1.18–7.33, p = 0.02 | Age, BMI, gender, inhaled corticosteroids, dyslipidemia and diabetes |
| Chinnadurai (2019)17 | 69 months | Age (median, range): 65, 56–71 (NAFLD); 65, 56–72 (no NAFLD); Male: 72.9% (NAFLD), 65.8% (no NAFLD) | N = 48 | N = 101 | NAFLD vs. no NAFLD: HR 3.48, 95% CI 1.59–7.6, p = 0.002 | NAFLD vs. no NAFLD: HR 2.95, 95% CI 1.31–6.60, p = 0.01 | ischemic heart disease, cerebrovascular accident, age |
| Vanjiappan (2018)38 | Not stated | Age (mean, SD): 53.8 ± 10.5 (NAFLD), 53.9 ± 10.4 (no NAFLD); Male: 58.6% (NAFLD), 41.3% (no NAFLD) | N = 183 | N = 117 | NAFLD vs. no NAFLD: 58/183 (31.6%) vs. 47/117 (40.1%) | N/A | N/A |
| Allen (2018)14 | 7 years | Age (median, IQR): 53, 42–63 (NAFLD); 53, 43–64 (no NAFLD); Female: 52% (NAFLD), 52% (no NAFLD) | N = 3869 | N = 15,209 | Cardiovascular events at baseline: 28% (NAFLD) vs. 18% (no NAFLD), p < 0.0001; Cardiovascular events after 10 years: 34% (NAFLD) vs. 22% (no NAFLD), p < 0.0001; Cardiovascular events in patients with no comorbidities (NAFLD vs. no NAFLD): RR: 1.96, 95% CI = 1.35–2.86, p < 0.001; Cardiovascular events in patients with 1 comorbidity (NAFLD vs. no NAFLD): RR: 1.21, 95% CI: 0.96–1.53, p = 0.10; Cardiovascular events in patients with 2 comorbidities (NAFLD vs. no NAFLD): RR 1.24, 95% CI 1.05–1.47, p = 0.01; Cardiovascular events in patients with 3 comorbidities (NAFLD vs. no NAFLD): RR 1.02, 95% CI 0.86–1.21, p = 0.76 | N/A | Outcomes compared with age and sex-matched controls |
| Mantovani (2016)28 | mean ± SD: 5.3 ± 2.1 years | Age (mean, SD): 47.5 ± 14.2 (NAFLD), 38.8 ± 13.1 (no NAFLD); Male: 48% (NAFLD), 36% (no NAFLD) | N = 150 | N = 136 | NAFLD vs. no NAFLD: HR 8.16, 95% CI 1.9–35.1, p < 0.005 | NAFLD vs. no NAFLD: HR 5.86, 95% CI 1.1–30.5, p = 0.035 | Age, sex, diabetes duration, smoking history and temporal changes in BMI, hemoglobin A1c, eGFR, hypertension and dyslipidemia |
| Zeb (2016)43 | 7.6 years | Age (mean, SD): 61 ± 9 (NAFLD), 63 ± 10 (no NAFLD) | N = 728 | N = 3391 | No data provided | NAFLD vs. no NAFLD: Adjusted HR 1.74, 95% CI 1.25–2.41, p = 0.01 | Age, sex, ethnicity, and MESA study sites |
| Stolic (2016)32 | Not stated | Age: No data; Male: 51% (NAFLD), 63% (no NAFLD) | N = 37 | N = 35 | Cardiovascular disease: 22/37 (56%) in NAFLD vs. 11/35 (31%) in no NAFLD, p = 0.017 | NAFLD vs. no NAFLD: OR 3.01, 95% CI 1.08–8.4, p = 0.035 | aspartate aminotransferase, alanine aminotransferase, c-reactive protein |
| Fracanzani (2016)21 | Not stated | Age at baseline (mean, SD): 51 ± 11 (NAFLD), 52 ± 12 (no NAFLD) | N = 125 | N = 250 | NAFLD vs. no NAFLD: HR 2.43, 95% CI 1.25–4.73, p = 0.009 | NAFLD vs. no NAFLD: HR 1.99, 95% CI 1.01–3.94, p = 0.04 | sex, hypertension, smoking habits, diabetes, presence of carotid plaque |
| Wong (2016)41 | 3679 patient-years | Age (mean, SD): 63 ± 10 (NAFLD), 63 ± 12 (no NAFLD); Male: 74.2% (NAFLD), 66% (no NAFLD) | N = 356 | N = 256 | All cardiovascular events: 36.5% (NAFLD) vs. 37.1% (no NAFLD); Cardiovascular deaths: 2.5% (NAFLD) vs. 7.0% (no NAFLD) | NAFLD vs. no NAFLD: All cardiovascular events (HR 0.90, 95% CI 0.69–1.18, p = 0.46); Cardiovascular deaths (HR 0.33, 95% CI 0.15–0.73, p = 0.007) | age and sex |
| Mellinger (2015)29 | N/A | Age (mean, SD): 62.3 ± 10.2 (CVD), 50.4 ± 10.1 (no CVD); Male: 62.7% (CVD), 48.7% (no CVD) | N = 512 | N = 2502 | No data provided | NAFLD vs. no NAFLD: OR: 1.06, 95% CI: 0.90–1.25, p = 0.494 | Clinical covariate profile: Composite of the following: age, sex, alcohol use, smoking, menopause, HRT use, diabetes, BMI, HDL, total cholesterol, HTN, and presence of lipid-lowering medications |
| Pisto (2014)30 | 212 months | Age (mean, SD): 50.9 ± 6.0 (no fatty liver), 51.9 ± 6.1 (moderate fatty liver), 51.5 ± 5.5 (severe fatty liver); Male: 44.3% (no fatty liver), 65.3% (moderate fatty liver), 59.9% (severe fatty liver) | N = 268 (124 with moderate fatty liver, 144 with severe fatty liver) | N = 720 | Total cardiovascular events: 13.5% (no fatty liver), 24.2% (moderate fatty liver), 29.2% (severe fatty liver) | Compared to no fatty liver: moderate fatty liver (OR: 1.49, 95% CI: 0.99 to 2.26), severe fatty liver (OR 1.76, 95% CI, 1.21 to 2.56). | study group, age, gender, smoking, alcohol consumption, systolic blood pressure, LDL cholesterol level, BMI, waist circumference, alcohol consumption, triglycerides, systolic blood pressure, fasting insulin, fasting glucose, alanine aminotransferase, type 2 diabetes, c-reactive protein, lipid lower treatment, antihypertensive treatment |
| Lai (2013)25 | 2245 patient-years | Age (mean, SD): 60.12 ± 12.46 (NAFLD), 59.86 ± 12.71 (no NAFLD); Male: 41% (NAFLD), 49% (no NAFLD) | N = 78 | N = 200 | NAFLD vs. no NAFLD: HR: 1.84, 95% CI: 1.10–3.07, p = 0.021 | HR: 2.82, 95% CI: 1.51–5.86, p = 0.001 | BMI, ALT, HDL, triglyceride levels, age, sex, diabetes, hypertension, obesity, smoking, dyslipidemia, Kt/V, Ca × P, albumin level, and hs-CRP level |
| Choi (2013)18 | N/A | Age (mean, SD): 62.5 ± 10.8 (no significant CAD), 65.2 ± 9.2 (significant CAD); Male: 22.7% (no significant CAD), 37% (significant CAD) | N = 82 | N = 52 | Prevalence of NAFLD: 51.2% (no significant CAD) vs. 78.3% (significant CAD), p = 0.002 | OR 1.685, 95% CI 1.051–2.702, p = 0.030 | age, total cholesterol, triglycerides, low-density lipoprotein levels, presence of NAFLD, glucose, HbA1c, BMI |
| Dunn (2013)19 | Not stated | Age (mean, SD): 66.6 ± 15.1 (no NAFLD), 58.1 ± 13.7 (NAFLD); Female: 54% (no NAFLD), 58% (NAFLD) | N = 233 | N = 2110 | Cardiovascular-related death: 1% (NAFLD) vs. 5% (no NAFLD), p = 0.35; MI: 23% (NAFLD) vs. 28% (no NAFLD), p = 0.11; Stroke: 2% (NAFLD) vs. 3% (no NAFLD), p = 0.66; Angina: 6% (NAFLD) vs 8% (no NAFLD), p = 0.53; Arrhythmia: 22% (NAFLD) vs. 32% (no NAFLD), p = 0.001; CHF: 23% (NAFLD) vs. 34% (no NAFLD), p = 0.001. | NAFLD vs. no NAFLD: Cardiovascular deaths (HR 0.30, 95% CI 0.07–1.23, p = 0.09); MI (HR 0.77, 95% CI 0.58–1.02, p = 0.07); CHF (HR 0.87, 95% CI 0.65–1.16, p = 0.33); Angina (HR: 0.72, 95% CI: 0.42–1.22, p = 0.22); Arrhythmia (HR 0.80, 95% CI 0.60–1.07, p = 0.14) | Age, sex, BMI, LDL, triglyceride, AST, hemoglobin A1C levels, cirrhosis |
| Feitosa (2013)20 | N/A | Age (mean, SD): 68.1 ± 9.1 (with CHD), 55.6 ± 13.1 (without CHD); Male: 71.3% (with CHD), 41.3% (without CHD) | 8.0% (with CHD); 6.5% (without CHD) | 92.0% (with CHD); 93.5% (without CHD) | NAFLD vs. no NAFLD: HR 1.116, 95% CI 1.043–1.329, p = 0.0084 | NAFLD vs. no NAFLD: HR: 0.996, 95% CI: 0.980–1.012, p = 0.598 | Age, sex, alanine aminotransferase, BMI, diabetes, insulin resistance, smoking, alcohol intake |
| Wong (2011)42 | 87 ± 22 weeks | Age (mean, SD): 63 ± 10 (fatty liver), 63 ± 12 (no fatty liver); Male: 74.2% (fatty liver), 66.0% (no fatty liver) | N = 356 | N = 256 | NAFLD vs. no NAFLD: Coronary artery disease (OR 3.07, 95% CI 2.09–4.51, p < 0.001) | NAFLD vs. no NAFLD: Coronary artery disease (OR: 2.31, 95% CI: 1.46–3.64, p < 0.001) | Age, sex, smoking, alcohol, diabetes, waist circumference, fasting glucose, HDL cholesterol, alanine aminotransferase |
| Hamaguchi (2007)24 | 7115 person-years | Age (mean, SD): 49.1 ± 8.7 (NAFLD), 47.8 ± 8.6 (no NAFLD); Male: 80.1% (NAFLD), 54.7% (no NAFLD) | N = 312 | N = 1335 | NAFLD vs. no NAFLD: OR 5.37, 95% CI 2.29–12.58, p < 0.001 | OR: 4.12, 95% CI: 1.58–10.75, p = 0.004 | age, smoking, LDL cholesterol, metabolic syndrome |
| Targher (2006)34 | Not stated | Age (mean, SD): 58 ± 4 (NAFLD), 59 ± 4 (without NAFLD); Male: 54% (NAFLD), 54% (no NAFLD) | N = 400 | N = 400 | NAFLD vs. no NAFLD: OR 1.82, 95% CI 1.5–2.0, p = 0.001 | NAFLD vs. no NAFLD: OR 1.1, 95% CI 0.9–1.4, p = 0.21 | smoking history, diabetes duration, duration, HbA1c, LDL cholesterol, GGT levels and use of medications (i.e. oral hypoglycemic, antihypertensive, lipid-lowering or antiplatelet drugs), metabolic syndrome |
| Targher (2005)36 | 5 years | Age (mean, SD): 66 ± 4 (coronary heart disease), 65 ± 3 (no coronary heart disease); Male: 62% (coronary heart disease), 62% (no coronary heart disease) | Coronary heart disease: n = 233; no coronary heart disease: 278 | Coronary heart disease: n = 15; no coronary heart disease: n = 218 | NAFLD vs. no NAFLD: OR 1.91, 95% CI 1.4–2.2, p = 0.001 | NAFLD vs. no NAFLD: OR 1.53, 95% CI 1.1–1.7, p = 0.02 | Age and sex, smoking history, diabetes duration, A1C, LDL cholesterol, GGT levels, and use of medications, metabolic syndrome |
| Liu (2019)26 | 11,484 patient-years | Age (mean, SD): 61.2 ± 9.1 (CV events), 61.1 ± 8.8 (no CV events); Male: 64.8% (CV events), 64.8% (no CV events) | CV events: n = 41; no CV events: n = 23 | CV events: n = 121; no CV events: n = 139 | NAFLD vs. no NAFLD: HR 1.66, 95% CI 1.15–2.42, p = 0.007 | NAFLD vs. no NAFLD: HR 1.62, 95% CI 1.09–2.39, p = 0.017 | age, sex, MS, Gensini score, left ventricular ejection fraction, creatinine, and high-sensitivity C-reactive protein |
| Hagstrom (2019)23 | 18.6 years | Age (mean, SD): 47.4 ± 13.4 (NAFLD); Male: 63% (NAFLD); Non-NAFLD cases were age and sex-matched to NAFLD cases | N = 603 | N = 6269 | 27.9% in NAFLD vs. 21.1% in non-NAFLD, p < 0.001 | NAFLD vs. no NAFLD: HR: 1.54, 95% CI: 1.30–1.83, p < 0.001 | Age, sex, BMI, hypertension, hyperlipidemia, type 2 diabetes, smoking |
| Wild (2018)40 | 4.3 years | Age (mean, SD): 58.7 ± 11 (NAFLD), 62.7 ± 12 (no liver disease); Male: 47.2% (NAFLD), 54.9% (no liver disease) | N = 1452 | N = 131,209 | Incident/recurrent CVD: 51.5 events per 1000 person-years (NAFLD) vs. 38.6 events per 1000 person-years (no liver disease) | NAFLD vs. no NAFLD: HR: 1.70, 95% CI: 1.52–1.90 | Age, sex, socioeconomic status, smoking status, hypertension/antihypertensive treatment, high cholesterol/lipid-lowering treatment, glycated hemoglobin (HbA1c), and record of CVD history before T2DM diagnosis |
| Chan (2014)16 | N/A | Age (mean, SD): 66.4 ± 8.9 (ischemic heart disease), 61.5 ± 10.7 (no ischemic heart disease); Male: 50% (ischemic heart disease), 40.6% (no ischemic heart disease) | N = 198 | N = 201 | NAFLD vs. no NAFLD: OR 1.19, 95% CI 0.76–1.86, p = 0.450 | No data | N/A |
| Stepanova (2012)31 | N/A | 35–44 years: 22.04% (NAFLD), 23.91% (no NAFLD); 45–54 years 20.33% (NAFLD), 14.53% (no NAFLD); 55–64 years: 18.78% (NAFLD), 11.81% (no NAFLD); 65–74 years: 15.53% (NAFLD), 10.26% (no NAFLD) | N = 2492 | N = 9121 | Prevalence of cardiovascular disease: 38.18% ± 1.68 (NAFLD) vs. 29.26% ± 0.88 (no NAFLD) | NAFLD vs. no NAFLD: OR 1.23; 95% CI, 1.04–1.44 | age, sex, race/ethnicity, obesity, diabetes mellitus, smoking, family history of cardiovascular disease |
| Targher (2012)37 | N/A | Age (mean, SD): 49 ± 15 (NAFLD), 39 ± 13 (no NAFLD); Male: 51.1% (NAFLD), 39.1% (no NAFLD) | N = 182 | N = 161 | NAFLD vs. no NAFLD: OR 10.3, 95% CI 5.7–20.3, p < 0.001 | NAFLD vs. no NAFLD: OR: 8.2, 95% CI: 4.3–22.7, p < 0.001 | Age, sex, duration of diabetes, glycated hemoglobin, smoking status, alcohol consumption, physical activity level, family history of cardiovascular disease, LDL cholesterol, BMI, systolic blood pressure, HDL cholesterol, triglycerides and current use of antihypertensive, lipid-lowering or antiplatelet medications |
| Sun (2011)33 | N/A | Age (mean, SD): 62 ± 10 (NAFLD), 58 ± 10 (no NAFLD); Male: 62.1% (NAFLD), 68.0% (no NAFLD) | N = 248 | N = 294 | Significant CAD: 58.1% NAFLD; Non-significant CAD: 16.3% | NAFLD vs. no NAFLD: OR 7.585, 95% CI 4.617–12.461, p < 0.001 | Age, sex, previous history of myocardial infarction, total cholesterol, aspartate aminotransferase |
| Targher (2010)35 | N/A | Age (mean, SD): 47 ± 12 (NAFLD), 37 ± 12 (no NAFLD); Male: 63% (NAFLD), 40% (no NAFLD) | N = 111 | N = 91 | NAFLD vs. no NAFLD: OR 11.7, 95% CI 4.4–31.2, p < 0.0001 | NAFLD vs. no NAFLD: OR 7.17, 95% CI 1.6–31.5, p < 0.01 | Age, sex, diabetes duration, HbA1c, smoking status, LDL cholesterol, BMI, systolic blood pressure, HDL cholesterol, triglycerides and medication use (i.e., antihypertensive, lipid-lowering or antiplatelet drugs |
| Lu (2009)27 | N/A | Age (mean, SD): 56.42 ± 6.57 (NAFLD), 57.19 ± 6.61 (no NAFLD); Male: 62.7% (NAFLD), 46.0% (no NAFLD) | N = 421 | N = 139 | Prevalence of coronary heart disease: 43.0% (NAFLD) vs. 73.4% (without NAFLD) | No data | N/A |
| Arslan (2007)15 | N/A | Age (mean, SD): 56.6 ± 10.3; Male: 65.0% | N = 65 | N = 27 | No data provided | NAFLD vs. no NAFLD: OR:7.92, 95% CI: 1.57–40.04, p = 0.012 | Age, sex, BMI, LDL cholesterol, smoking history, metabolic syndrome |
| Stroke and cerebrovascular disease | |||||||
| Hagstrom (2019)23 | 18.6 years | Age (mean, SD): 47.4 ± 13.4 (NAFLD); Male: 63% (NAFLD); Non-NAFLD cases were age and sex-matched to NAFLD cases | N = 603 | N = 6269 | 27.9% in NAFLD vs. 21.1% in non-NAFLD, p < 0.001 | NAFLD vs. no NAFLD: HR 1.54, 95% CI 1.30–1.83, p < 0.001 | Age, sex, BMI, hypertension, hyperlipidemia, type 2 diabetes, smoking |
| Alexander (2018)44 | 5.8 years | Age (mean): 64.7; Male: 45% | N = 447 of non-stroke cohort, no data provided for stroke cohort | N = 193 of non-stroke cohort, no data provided for stroke cohort | No data provided | NAFLD vs. no NAFLD: HR 0.65, 95% CI 0.43–1.00, p < 0.05 | age, race, and age∗race, Framingham stroke risk factors (systolic blood pressure (SBP), left ventricular hypertrophy (LVH), smoking, prevalent CVD, atrial fibrillation, diabetes, and hypertension medication use) |
| Mantovani (2016)28 | mean ± SD: 5.3 ± 2.1 years | Age (mean, SD): 47.5 ± 14.2 (NAFLD), 38.8 ± 13.1 (no NAFLD); Male: 48% (NAFLD), 36% (no NAFLD) | N = 150 | N = 136 | NAFLD vs. no NAFLD: HR 8.16, 95% CI 1.9–35.1, p < 0.005 | NAFLD vs. no NAFLD: HR 5.86, 95% CI 1.1–30.5, p = 0.035 | Age, sex, diabetes duration, smoking history and temporal changes in BMI, hemoglobin A1c, eGFR, hypertension and dyslipidemia |
| Moshayedi (2014)45 | N/A | Age (mean, SD): 66.42 ± 11.31 (stroke), 66.51 ± 11.27 (no stroke); Male: 62.7% (stroke), 62.7% (no stroke) | n = 47 in stroke group; n = 25 in no stroke group | n = 63 in stroke group; n = 85 in no stroke group | Prevalence of NAFLD: 42.7% (stroke) vs. 22.7% (no stroke), p = 0.001 | OR 1.68, 95% CI 0.42–6.76, p = 0.460 | Age, sex, waist circumference, hypertension, diabetes mellitus, low-density lipoprotein, triglyceride, alanine aminotransferase, aspartate aminotransferase, creatine, body mass index, cigarette smoking, and ischemic heart disease |
| Pisto (2014)30 | 212 months | Age (mean, SD): 50.9 ± 6.0 (no fatty liver), 51.9 ± 6.1 (moderate fatty liver), 51.5 ± 5.5 (severe fatty liver); Male: 44.3% (no fatty liver), 65.3% (moderate fatty liver), 59.9% (severe fatty liver) | N = 268 (124 with moderate fatty liver, 144 with severe fatty liver) | N = 720 | Total cardiovascular events: 13.5% (no fatty liver), 24.2% (moderate fatty liver), 29.2% (severe fatty liver) | Compared with no fatty liver: moderate fatty liver (OR 1.49, 95% CI, 0.99 to 2.26), severe fatty liver (OR 1.76, 95% CI, 1.21 to 2.56). | Study group, age, gender, smoking, alcohol consumption, systolic blood pressure, LDL cholesterol level, BMI, waist circumference, alcohol consumption, triglycerides, systolic blood pressure, fasting insulin, fasting glucose, alanine aminotransferase, type 2 diabetes, c-reactive protein, lipid lower treatment, antihypertensive treatment |
| Dunn (2013)19 | Not stated | Age (mean, SD): 66.6 ± 15.1 (no NAFLD), 58.1 ± 13.7 (NAFLD); Female: 54% (no NAFLD), 58% (NAFLD) | N = 233 | N = 2110 | Stroke: 2% (NAFLD) vs. 3% (no NAFLD), p = 0.66 | NAFLD vs. no NAFLD: HR 0.69, 95% CI 0.28–1.75, p = 0.44 | Age, sex, race, prior MI, prior stroke |
| Hamaguchi (2007)24 | 7115 person-years | Age (mean, SD): 49.1 ± 8.7 (NAFLD), 47.8 ± 8.6 (no NAFLD); Male: 80.1% (NAFLD), 54.7% (no NAFLD) | N = 312 | N = 1335 | NAFLD vs. no NAFLD: OR 5.37, 95% CI 2.29–12.58, p < 0.001 | OR 4.12, 95% CI 1.58–10.75, p = 0.004 | age, smoking, LDL cholesterol, metabolic syndrome |
| Targher (2005)36 | 5 years | Age (mean, SD): 66 ± 4 (cardiovascular disease), 65 ± 3 (no cardiovascular disease); Male: 62% (cardiovascular disease), 62% (no cardiovascular disease) | Cardiovascular disease: n = 233; no cardiovascular disease: 278 | Cardiovascular disease: n = 15; no cardiovascular disease: n = 218 | NAFLD vs. no NAFLD: OR 1.91, 95% CI 1.4–2.2, p = 0.001 | NAFLD vs. no NAFLD: OR 1.53, 95% CI 1.1–1.7, p = 0.02 | Age and sex, smoking history, diabetes duration, A1C, LDL cholesterol, GGT levels, and use of medications, metabolic syndrome |
| Viglino (2018)39 | Not stated | Age (median, range): 65.3, 61.1–70.9 (NAFLD), 55.4, 51–64.8 (no liver disease); Male: 85.5% (NAFLD), 53.6% (no liver disease) | N = 83 | N = 28 | Total cardiovascular events: 32.5% (NAFLD) vs. 14.3% (no liver disease), p = 0.09; Univariate 5-year composite outcome: HR 3.06, 95% CI 1.08–8.63, p = 0.035 | Cardiovascular events and death at 5-years: Steatosis vs. no steatosis: HR 1.66, 95% CI 0.72–3.84, p = 0.236; NASH vs. no NASH: HR 0.80, 95% CI 0.34–1.85, p = 0.596; Fibrosis vs. no fibrosis: HR 2.94, 95% CI 1.18–7.33, p = 0.02 | Age, BMI, gender, inhaled corticosteroids, dyslipidemia and diabetes |
| Mellinger (2015)29 | N/A | Age (mean, SD): 62.3 ± 10.2 (CVD), 50.4 ± 10.1 (no CVD); Male: 62.7% (CVD), 48.7% (no CVD) | N = 512 | N = 2502 | No data provided | NAFLD vs. no NAFLD: OR 1.06, 95% CI 0.90–1.25, p = 0.494 | Clinical covariate profile: Composite of the following: age, age2, sex, alcohol use, smoking, menopause, HRT use, diabetes, BMI, HDL, total cholesterol, HTN, and presence of lipid-lowering medications |
| Targher (2006)34 | Not stated | Age (mean, SD): 58 ± 4 (NAFLD), 59 ± 4 (without NAFLD); Male: 54% (NAFLD), 54% (no NAFLD) | N = 400 | N = 400 | NAFLD vs. no NAFLD: OR 1.82, 95% CI 1.5–2.0, p = 0.001 | NAFLD vs. no NAFLD: OR 1.1, 95% CI 0.9–1.4, p = 0.21 | Smoking history, diabetes duration, duration, HbA1c, LDL cholesterol, GGT levels and use of medications (i.e. oral hypoglycemic, antihypertensive, lipid-lowering or antiplatelet drugs), metabolic syndrome |
| Liu (2019)26 | 11,484 patient-years | Age (mean, SD): 61.2 ± 9.1 (CV events), 61.1 ± 8.8 (no CV events); Male: 64.8% (CV events), 64.8% (no CV events) | CV events: n = 41; no CV events: n = 23 | CV events: n = 121; no CV events: n = 139 | NAFLD vs. no NAFLD: HR 1.66, 95% CI 1.15–2.42, p = 0.007 | NAFLD vs. no NAFLD: HR 1.62, 95% CI 1.09–2.39, p = 0.017 | Age, sex, MS, Gensini score, left ventricular ejection fraction, creatinine, and high-sensitivity C-reactive protein |
| Wild (2018)40 | 4.3 years | Age (mean, SD): 58.7 ± 11 (NAFLD), 62.7 ± 12 (no liver disease); Male: 47.2% (NAFLD), 54.9% (no liver disease) | N = 1452 | N = 131,209 | Incident/recurrent CVD: 51.5 events per 1000 person-years (NAFLD) vs. 38.6 events per 1000 person-years (no liver disease) | NAFLD vs. no NAFLD: HR 1.70, 95% CI 1.52–1.90 | Age, sex, socioeconomic status, smoking status, hypertension/antihypertensive treatment, high cholesterol/lipid-lowering treatment, glycated hemoglobin (HbA1c), and record of CVD history before T2DM diagnosis |
| Stepanova (2012)31 | N/A | 35–44 years: 22.04% (NAFLD), 23.91% (no NAFLD); 45–54 years 20.33% (NAFLD), 14.53% (no NAFLD); 55–64 years: 18.78% (NAFLD), 11.81% (no NAFLD); 65–74 years: 15.53% (NAFLD), 10.26% (no NAFLD) | N = 2492 | N = 9121 | Prevalence of cardiovascular disease: 38.18% ± 1.68 (NAFLD) vs. 29.26% ± 0.88 (no NAFLD) | NAFLD vs. no NAFLD: OR 1.23; 95% CI, 1.04–1.44 | age, sex, race/ethnicity, obesity, diabetes mellitus, smoking, family history of cardiovascular disease |
| Targher (2012)37 | N/A | Age (mean, SD): 49 ± 15 (NAFLD), 39 ± 13 (no NAFLD); Male: 51.1% (NAFLD), 39.1% (no NAFLD) | N = 182 | N = 161 | NAFLD vs. no NAFLD: OR 10.3, 95% CI 5.7–20.3, p < 0.001 | NAFLD vs. no NAFLD: OR 8.2, 95% CI 4.3–22.7, p < 0.001 | Age, sex, duration of diabetes, glycated hemoglobin, smoking status, alcohol consumption, physical activity level, family history of cardiovascular disease, LDL cholesterol, BMI, systolic blood pressure, HDL cholesterol, triglycerides and current use of antihypertensive, lipid-lowering or antiplatelet medications |
| Targher (2010)35 | N/A | Age (mean, SD): 47 ± 12 (NAFLD), 37 ± 12 (no NAFLD); Male: 63% (NAFLD), 40% (no NAFLD) | N = 111 | N = 91 | NAFLD vs. no NAFLD: OR 11.7, 95% CI 4.4–31.2, p < 0.0001 | NAFLD vs. no NAFLD: OR 7.17, 95% CI 1.6–31.5, p < 0.01 | age, sex, diabetes duration, HbA1c, smoking status, LDL cholesterol, BMI, systolic blood pressure, HDL cholesterol, triglycerides and medication use (i.e., anti-hypertensive, lipid-lowering or antiplatelet drugs |
| Extrahepatic cancers | |||||||
| Kim (2017)49 | 7.5 years (IQR 3.2–9.3) | Age (mean, SD): 50.1 ± 9.7 (NAFLD), 46.9 ± 10.2 (No NAFLD); Male: 71.1% (NAFLD), 45.1% (No NAFLD) | N = 8721 | N = 17,226 | IRR (NAFLD vs. no NAFLD): All Cancers (1.32, 95% CI 1.17–1.49); Stomach (1.36, 95% CI 1.00–1.86); Colon and rectum (2.04, 95% CI 1.30–3.19); Breast (1.77, 95% CI 1.15–2.74) | NAFLD vs. no NAFLD: All cancers (HR 1.08, 95% CI 0.94–1.24, p = 0.27); Stomach (HR 0.98, 95% CI 0.69–1.38, p = 0.91); Colon and Rectum (HR 1.45, 95% CI 0.88–2.38, p = 0.15); Breast (HR 1.92, 1.15–3.20, p = 0.01) | Age, sex, smoking status, diabetes, hypertension, GGT, HDL, LDL, and triglycerides |
| Chang (2018)47 | N/A | Age (mean, SD): 64.1 ± 14.9 (pancreatic cancer), 64.8 ± 15.9 (non cancer); Male: 58.7% (pancreatic cancer), 51.4% (non cancer) | 17/143 in pancreatic cancer; 21/414 in patients without pancreatic cancer | 126/143 in pancreatic cancer; 393/414 in patients without pancreatic cancer | Prevalence of NAFLD: Pancreatic cancer, 17/143 (11.9%) vs. non-pancreatic cancer, 21/414 (5.1%), p = 0.0095 | NAFLD vs. no NAFLD: Pancreatic cancer, OR 2.63, 95% CI 1.24–5.58, p = 0.011 | Diabetes, smoking, statin use, aspirin use |
| Wild (2018)40 | 4.3–4.7 years | Age (mean, SD): 58.7 ± 11.0 (NAFLD), 62.7 ± 12.0 (no liver disease); Male: 47.2% (NAFLD), 54.9% (no liver disease) | N = 1452 | N = 131,209 | NAFLD: 23.4 cancers per 1000 person-years; No liver disease: 25.6 per 1000 person-years | NAFLD vs. no NAFLD: Incident/recurrent cancers, excluding HCC, HR 1.10, 95% CI 0.94–1.29 | Age, sex, socioeconomic status, smoking status, hypertension, high cholesterol, glycated hemoglobin (HbA1c), and cardiovascular disease |
| Nseir (2017)53 | N/A | Age (mean, SD): 54.8 ± 12 (breast cancer), 57.5 ± 9.6 (no breast cancer); Female: 100% | 33/73 in breast cancer group; 12/73 in non-breast cancer group | 40/73 in breast cancer group; 61/73 in non-breast cancer group | Prevalence of NAFLD: Breast cancer, 33/73 (45.2%) vs. no breast cancer, 12/73 (16.4%), p = 0.002 | NAFLD vs. no NAFLD: Breast cancer (OR 2.82, 95%CI 1.20–5.50, p = 0.016) | Age at first delivery, estrogen use |
| Seko (2015)55 | 4.8 years (range, 0.3–15.7) | Age (median, range): 59 (16–92); Female: 49%, Male: 51% | N = 312 (NAFL = 136, NASH = 176) | No non-NAFLD comparator group | 20/312 (6.4%) developed extrahepatic cancers (annual rate of 1.5%), including stomach cancer (n = 5), lung cancer (n = 4), pancreatic cancer (n = 3), colorectal cancer (n = 3), breast cancer (n = 1), bile duct cancer (n = 1), prostate cancer (n = 1), malignant lymphoma (n = 1), spinal cord cancer (n = 1) | N/A | N/A |
| Kwak (2019)50 | N/A | Age (mean, SD): 51.7 ± 9.3 (breast cancer), 51.6 ± 9.3 (no breast cancer); Female: 100% | 81/270 in breast cancer group; 54/270 in no breast cancer group | 189/270 in breast cancer group; 216/270 in no breast cancer group | Prevalence of NAFLD Breast cancer 81/270 (30.0%) vs. no breast cancer 54/270 (20.0%), p = 0.008 | NAFLD vs. no NAFLD: Breast cancer (OR 1.63, 95% CI 1.01–2.62, p = 0.046) | Family history of breast cancer, body mass index, waist circumference, metabolic syndrome, GGT, triglycerides, systolic blood pressure, age at menarche |
| Cho (2019)48 | N/A | Age (mean, SD): 61.9 ± 12.5 (advanced colorectal neoplasm), 53.9 ± 12.7 (no colorectal adenoma); Male: 39.6% (advanced colorectal neoplasm), 47.7% (no colorectal adenoma) | Advanced colorectal neoplasm (24/53); no colorectal adenoma (246/323) | Advanced colorectal neoplasm (5/53); no colorectal adenoma (77/323) | NAFL vs. no NAFLD (OR 2.60, 95% CI 0.96–7.04, p = 0.060); NASH vs. no NAFLD (2.74, 95% CI 1.01–7.43, p = 0.047) | NASH vs no NAFLD: OR 2.81, 95% CI 1.01–7.87, p = 0.049 | Age, sex, diabetes |
| Yang (2017)56 | NAFLD: 52.2 ± 15.1 months; Without NAFLD: 51.8 ± 15.2 months | Age (mean, SD): 53.8 ± 10.4 (NAFLD), 54.5 ± 10.6 (without NAFLD); Male: 59.6% (NAFLD), 51.7% (without NAFLD) | N = 441 | N = 441 | NAFLD: 16/441 (3.6%); Without NAFLD 14/441 (3.2%) | NAFLD vs. no NAFLD: HR 1.07, 95% CI 0.51–2.26, p = 0.85 | age, body mass index, hypertension, diabetes, aspirin or NSAID use, lipid-lowering agent, and risk categories based on index colonoscopy findings |
| Pan (2017)54 | N/A | Age (mean, SD): 55.57 ± 9.45 (colorectal cancer), 49.02 ± 10.76 (without colorectal cancer); Male: 74.1% (colorectal cancer), 64.3% (without colorectal cancer) | Colorectal cancer (14/27); no colorectal cancer (559/1767) | Colorectal cancer (13/27); no colorectal cancer (1208/1767) | Prevalence of NAFLD: Colorectal cancer 14/27 (51.9%) vs. no colorectal cancer 559/1767 (31.6%) | NAFLD vs. no NAFLD: OR 2.164, 95% CI 1.289–3.271, p = 0.005 | age, metabolic syndrome |
| Ahn (2017)46 | N/A | Age (mean, SD): 55.0 ± 8.6 (advanced colorectal neoplasia), 48.5 ± 9.0 (no colorectal neoplasia); Male: 77.9% (advanced colorectal neoplasia), 58.9% (no colorectal neoplasia) | Advanced colorectal neoplasia (263/569); no colorectal neoplasia (5893/18011) | Advanced colorectal neoplasia (306/569); no colorectal neoplasia (12118/18011) | NAFLD vs. no NAFLD: OR 1.66 (95% CI 1.41–1.96) | NAFLD vs. no NAFLD: Model 1 (OR 1.32, 95% CI 1.12–1.57, p = 0.001); Model 2 (OR 1.28, 95% CI 1.06–1.54, p = 0.009); Model 3 (OR 1.21, 95% CI 0.99–1.47, p = 0.053) | Model 1: age, sex; Model 2: age, sex, smoking, alcohol, body mass index, first-degree family history of colorectal cancer, aspirin use; Model 3: age, sex, smoking, alcohol, body mass index, first-degree family history of colorectal cancer, aspirin use, fasting blood glucose, use of anti-diabetic medication, total cholesterol, triglyceride, use of anti-dyslipidemic medication, systolic blood pressure, and use of anti-hypertensive medication |
| Lin (2014)52 | N/A | Age (mean, SD): 63.1 ± 12.8 (males with NAFLD), 65.4 ± 13.8 (males without NAFLD), 64.8 ± 11.5 (females with NAFLD), 63.4 ± 14.3 (females without NAFLD); Male: 59.2%, Female: 40.8% | N = 263 | N = 2052 | Malignant colon neoplasm: 29.3% (n = 77) in NAFLD vs. 18.0% (n = 369) in non-NAFLD group (OR 2.043; 95% CI 1.512–2.761, p = 0.001) | NAFLD vs non-NAFLD: (OR 1.868; 95% CI 1.360–2.567, p = 0.001) | BMI, history of hypertension, triglycerides, uric acid, alanine aminotransferase, hemoglobin, platelet, albumin |
| Lee (2012)51 | up to 7 years | Age (mean, SD): 50.0 ± 7.7 (NAFLD), 46.2 ± 6.4 (without NAFLD); Female: 100% | N = 831 | N = 4686 | Incidence rate: 233.6 per 100,000 person-years (NAFLD) vs. 27.0 per 100,000 person-years (without NAFLD); Crude RR NAFLD vs. non-NAFLD: RR 8.71 (95% CI 3.10–24.48) | Adjusted RR (NAFLD vs. no NAFLD): Colorectal cancer (RR 3.08, 95% CI 1.02–9.34) | Age, body mass index, blood pressure, fasting glucose, total cholesterol, triglycerides, HDL cholesterol, smoking habits |
| Wong (2011)42 | N/A | Age (mean, SD): 50.8 ± 8.5 (NAFLD – hospital cohort), 50.3 ± 5.8 (NAFLD – community cohort), 48.5 ± 5.8 (Non-NAFLD cohort); Male: 54.8% (NAFLD-hospital cohort), 57.8% (NAFLD-community cohort), 36.5% (Non-NAFLD cohort) | N = 199 (135 NAFLD-hospital cohort, 64 NAFLD-community cohort) | N = 181 | Advanced colorectal neoplasms: 18.6% (NAFLD) vs. 5.5% (Non-NAFLD); OR 3.91, 95% CI 1.88 to 8.11, p < 0.001 | NAFLD vs. non-NAFLD: OR 3.04, 95% CI 1.29 to 7.20, p = 0.011 | Age, sex, smoking, colorectal cancer in first degree relatives, body mass index, diabetes, hypertension |
CV, cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; HCC, hepatocellular carcinoma; RR, risk ratio.
Figure 2.
Forest plot diagram evaluating the association between NAFLD and cardiovascular diseases. NAFLD, Nonalcoholic fatty liver disease.
Quality assessment
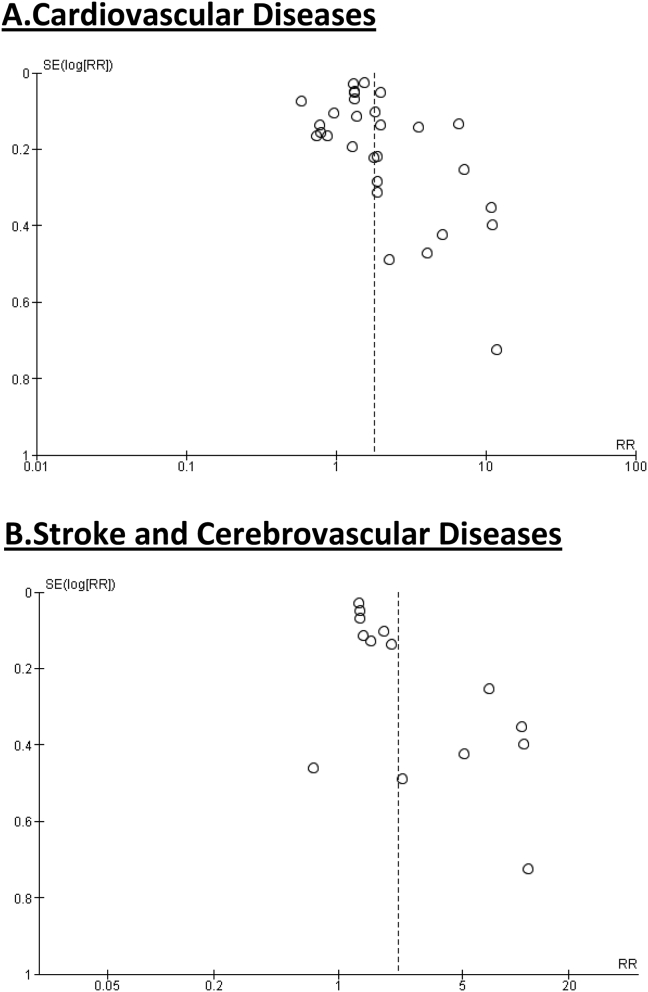
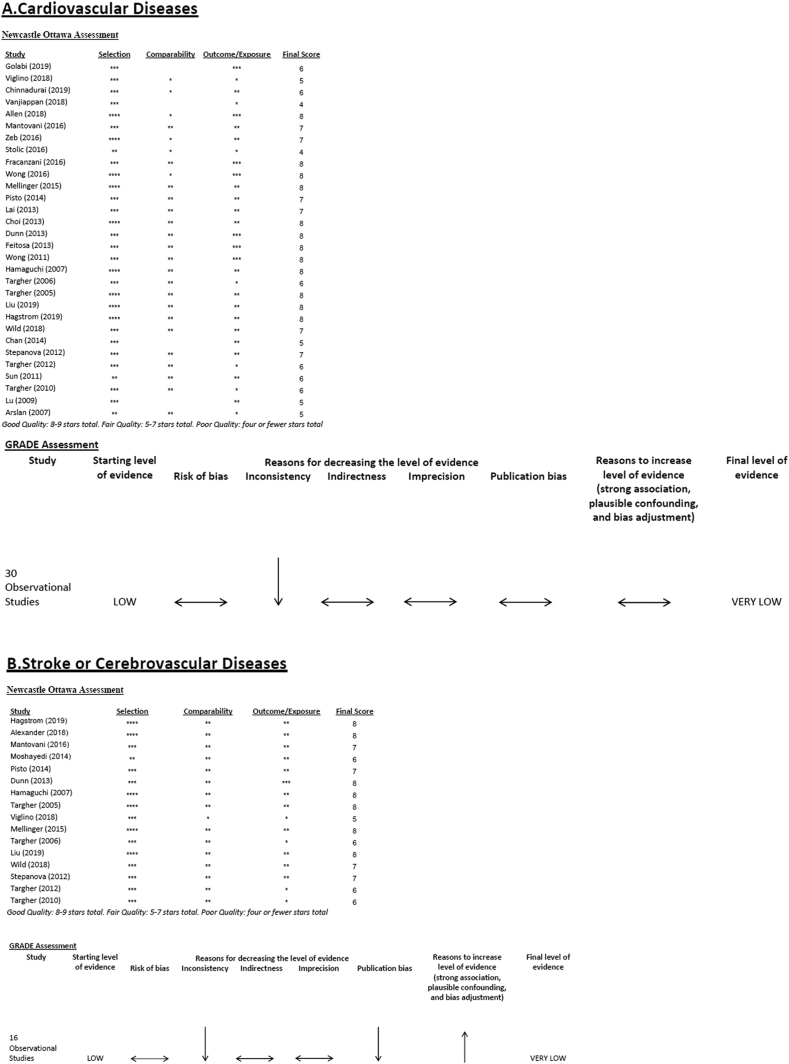
While accurate assessment of publication bias is limited in the setting of significant study heterogeneity, the funnel plots did not indicate severe publication bias present (Figure 4a). Our quality assessment of the individual studies included in our analysis using the Newcastle-Ottawa Scale demonstrated 12 studies meeting good quality, 16 studies meeting fair quality, and 2 studies meeting poor quality. Using the GRADE system for assessing the overall certainty of the evidence, given that all studies were observational in study design, we began with a “low” rating. Although there was some concern for risk of bias, when considered across all studies as a whole, the potential risk of bias was considered to be not serious and no downgrading for bias was given. However, concerns regarding heterogeneity and inconsistency led to downgrading the overall rating to “very low” (Figure 5a).
Figure 4.
Funnel plot diagram for association between NAFLD and (a) Cardiovascular diseases and (b) Stroke and cerebrovascular diseases. NAFLD, Nonalcoholic fatty liver disease.
Figure 5.
Quality assessment of included studies evaluating the association between NAFLD and (A) Cardiovascular diseases, (B) Stroke or cerebrovascular diseases, and (C) Extrahepatic cancers. NAFLD, Nonalcoholic fatty liver disease.
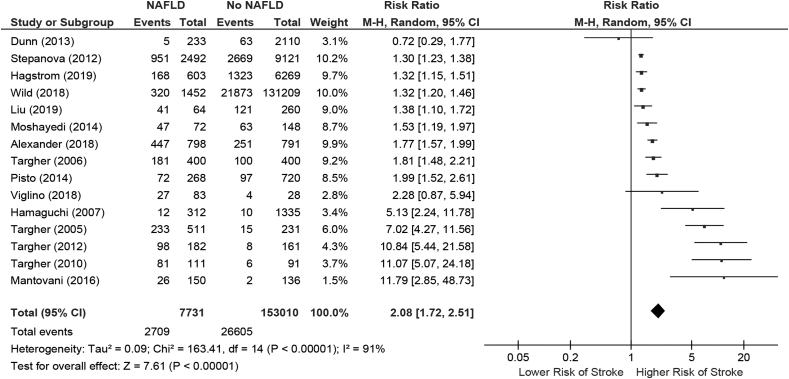
Stroke or cerebrovascular disease
Our initial query identified 469 studies, among which 405 were excluded based on title review, 31 additionally excluded based on abstract review, and 17 studies were excluded based on full-length manuscript review. The final study cohort included 16 studies that met inclusion and exclusion criteria for further analyses (Figure 1).19,23,24,26,28, 29, 30, 31,34, 35, 36, 37,39,40,44,45
Study characteristics and outcomes
The study characteristics of the included studies are shown in Table 1. All studies were observational in study design, including 6 retrospective cohort studies, 5 cross-sectional studies, 3 prospective observational studies, one case-control, and one prospective nested case-control study. 5 studies were carried out in Italy, 4 in the USA, and the remaining 7 from various countries. Determination of NAFLD diagnosis was mostly based on radiographic modalities, and most studies excluded individuals with excessive alcohol consumption, hepatitis and other chronic liver diseases, those on hepatotoxic medications. Table 1 also provides specific details on how each study defined stroke or cerebrovascular disease outcomes.
The main outcomes of the studies are shown in Table 2. For the assessment of stroke and cerebrovascular disease outcomes, all studies included in our analyses provided adjusted regression analyses with the majority including relevant variables that may confound the association between NAFLD and risk of stroke and cerebrovascular disease outcomes. Across all studies, there was a positive signal indicating increased risk of stroke and cerebrovascular disease in patients with NAFLD, and on meta-analysis the pooled outcome assessment demonstrated more than doubling the risk of stroke or cerebrovascular disease in patients with NAFLD (overall pooled risk ratio: 2.08, 95% CI: 1.72–2.51) (Figure 3). However, significant heterogeneity was observed between studies (I2 of 91%).
Figure 3.
Forest plot diagram evaluating the association between NAFLD and stroke and cerebrovascular diseases. NAFLD, Nonalcoholic fatty liver disease.
Quality assessment
Significant heterogeneity was also observed when evaluating stroke outcomes, which makes assessment of publication bias challenging. Review of the funnel plots did seem to demonstrate some mild publication bias (Figure 4b). Our quality assessment of the individual studies included in our analysis using the Newcastle-Ottawa Scale demonstrated 7 studies meeting good quality and 9 studies meeting fair quality. Using the GRADE system for assessing the overall certainty of the evidence, given that all studies were observational in study design, we began with a “low” rating. The overall certainty of evidence was further downgraded for inconsistency (given significant heterogeneity between studies), as well as for potential publication bias. Given the overall magnitude of the pooled effect measure from our meta-analysis, we allowed a one level upgrade, but the final level of evidence remained “very low” (Figure 5b).
Extrahepatic cancer
Our initial query identified 4469 studies, among which 4411 were excluded based on title review, 31 additionally excluded based on abstract review, and 14 studies were excluded based on full-length manuscript review. The final study cohort included 13 studies that met inclusion and exclusion criteria for further analyses (Figure 1).40,42,46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56
Study characteristics and outcomes
The study characteristics of the included studies are shown in Table 1. All studies were observational in study design, including 5 retrospective observation studies, 7 cross-sectional studies, and one case-control study. Six studies were conducted in Korea, three in China, one in Japan, one in Israel, one in Scotland, and one in Taiwan. Similar to the aforementioned studies, the determination of NAFLD used different modalities including liver biopsy, presence of ICD-9 and ICD-10 codes for NAFLD in hospital admission records, abdominal ultrasonography, and abdominal CT scan. Exclusion criteria varied, but individuals in all studies were excluded if they had a history of excessive alcohol consumption, hepatitis or other chronic liver diseases. Unlike CVD and stroke or cerebrovascular disease, there was significant heterogeneity in the outcomes assessed in this category. For example, one study focused on pancreatic cancers, three focused on all cancers, two studies focused on breast cancers, and seven studies focused on colorectal cancers.
In the one cross-sectional study with pancreatic cancer as an outcome, the investigators did observe a significant association between NAFLD and pancreatic cancer on adjusted regression analyses (OR: 2.63, 95% CI: 1.24–5.58, p = 0.011) (Table 2). Two single-center cross-sectional studies evaluated association between imaging modality determined NAFLD and risk of breast cancer. Both studies demonstrated a significantly increased association between NAFLD and breast cancer (OR: 2.82, 95%CI: 1.20–5.50, p = 0.016 and OR: 1.63, 95% CI: 1.01–2.62, p=0.046) (Table 2).50,53 Among the three studies that focused on all cancers overall, no significant association was observed. However in the study by Kim, et al49 although there was no increased risk for all cancers, the investigators did perform a stratified analysis and found a significant association between NAFLD and breast cancer (hazard ratio [HR]: 1.92, 1.15–3.20, p=0.01). Among the 7 studies that evaluated colorectal cancer outcomes, 6 studies reported a significant association and one did not observe a significant association (Table 2).
Quality assessment
As noted previously, given the heterogeneity of the outcomes in the articles assessing extrahepatic cancers, we did not perform meta-analysis or GRADE assessment. Our quality assessment of the individual studies included in our analysis using the Newcastle-Ottawa Scale demonstrated 8 studies meeting good quality and 5 studies meeting fair quality (Figure 5c).
Discussion
Although NAFLD contributes to significant liver disease burden worldwide, growing evidence supports that NAFLD is strongly correlated with and perhaps a manifestation of systemic metabolic diseases. Our current systematic review and meta-analysis aimed to comprehensively evaluate the association between NAFLD and three of the leading causes of morbidity and mortality – CVDs, stroke or cerebrovascular diseases, and extrahepatic cancers.
Meta-analysis of data from observational studies demonstrated a nearly doubling of CVD risk in patients with NAFLD compared with those without NAFLD. This observation supports clinical observations that underlying metabolic diseases are associated with increased risk of NAFLD and NAFLD progression,57 and it is these same metabolic disease risk factors that are also associated with increased risk of CVDs. Although all the studies included in our analyses were observational in nature, thereby only suggesting association without clear causation, it is important to note that significant heterogeneity was present and GRADE assessment of the quality of evidence overall was very low. Nevertheless, our observations along with existing studies demonstrating the leading cause of death in NAFLD patients is in fact CVD-related, emphasizing the importance of optimizing management of metabolic disease risk factors in patients with NAFLD.10 The clinical implications of these observations further highlight the need for refining CVD risk assessment in patients with NAFLD and earlier and consistent implementation of CVD risk assessment that allows for early preventative care to reduce long-term risk of CVD-related morbidity and mortality.
Our study also demonstrated a significantly increased risk of stroke or cerebrovascular disease associated with NAFLD. Although there was significant overlap in articles with the CVD section, there were additional studies that were identified in this query that specifically focused on stroke outcomes. The risk of stroke and cerebrovascular disease was more than double in patients with NAFLD compared with patients with non-NAFLD. As with CVD, this observation is clinically relevant given that the majority of strokes and cerebrovascular disease outcomes assessed were ischemic in nature, and thus subject to the same metabolic disease risk factors aforementioned.
Although the mechanisms behind the association between NAFLD and CVD are not well-understood, several explanations have been proposed. One explanation involves the role of NAFLD in mediating chronic low-grade systemic inflammation on the circulatory system. Chronic low-grade inflammation may lead to increased circulation of proinflammatory cytokines, which in turn can affect the electrophysiology and structural substrates of the myocardium. These series of effects may ultimately affect left ventricular structure and function. Patients with NAFLD may also have increased levels of inflammatory biomarkers such as C-reactive protein.58,59 Another hypothesis involves the mechanistic pathway of altered endothelial function and increased platelet activation, as well as dysregulation of fibrinolytic pathways, all of which have been observed in NAFLD. These mechanistic pathways may also contribute to increased risks of CVD.60,61 Insulin resistance is a common co-occurrence in patients with NAFLD, and insulin resistance has been associated with alterations in diastolic function. While the exact mechanism is not known, insulin signaling is a critical determinant of adult cardiac size and plays a role in regulating myosin gene expression and substrate use by the heart, and suppression of fatty acid oxidation and increased glucose use can be affected by development of insulin resistance.62
Existing literature has raised some concern with increased risk of extrahepatic malignancies associated with NAFLD. Our study demonstrated significant heterogeneity in the existing literature. Although there was a single study demonstrating strong association with pancreatic cancer, it is difficult to make definitive conclusions given the observational nature of this study and the overall fair quality of the study design. However, our study did demonstrate an association between NAFLD and breast cancer and NAFLD and colorectal cancer. Several possible mechanisms may explain the association between NAFLD and breast cancer. The first is that NAFLD is closely associated with increased levels of proinflammatory cytokines, as mentioned earlier.63 These cytokines may promote cancer through tumor cell proliferation, antiapoptotic effects, and angiogenesis.64 As previously mentioned, insulin resistance is often a key feature of NAFLD, and insulin may cross-bind to IGF-1 receptors expressed on breast cells, leading to proliferation.65 Elevated circulating insulin may also increase hepatic IGF-I synthesis and decrease expression of IGF-I binding proteins, leading to high levels of free IGF-I.53,65,66 These changes in NAFLD may contribute to the development of breast cancer, although the evidence does not support a clear strong causative pathway.67, 68, 69, 70 Other studies have shown that insulin resistance may also promote the occurrence and development of colorectal cancer. NAFLD patients have reduced expression of adiponectin, and in human studies, low levels of adiponectin is associated with increased risk of colorectal adenomas.71 Despite these data, the current paucity of data and overall poor-fair quality of existing observational studies in our analysis limit any strong conclusions to modify cancer screening policies in patients with NAFLD.
Our analysis has some important limitations that should be mentioned. All of the existing studies included in our analyses were observational in nature, and thus while aforementioned meta-analyses do in fact demonstrate significantly increased risk of CVD and stroke or cerebrovascular disease outcomes in patienst with NAFLD, the study designs only allows us to determine associations without definite causation. Furthermore, as noted in our quality assessment, given significant heterogeneity, the overall GRADE assessment of the quality of included studies was very low. In addition, while we were comprehensive to ensure a broad representation and capture of all relevant studies, it should be noted that definitions of NAFLD and outcomes of interest (CVD, stroke or cerebrovascular disease, extrahepatic cancers) were not uniform across all studies, and thereby may have contributed bias or inconsistency to our final assessments. Although almost all studies included in the meta-analysis have adjusted the results for age, sex, smoking, body mass index and pre-existing diabetes (or metabolic syndrome), the possibility of residual confounding by some unmeasured factors cannot be ruled out. The interpretation of some results of this meta-analysis requires some caution, given the high heterogeneity observed in the analysis of these studies. It is plausible to assume that the high heterogeneity of the observational studies likely reflects differences in the characteristics of study populations, in the study country, as well as in the methods used for diagnosing NAFLD and defining the outcomes. Notwithstanding these limitations, our meta-analysis has also important strengths. As previously discussed, the present meta-analysis provides the most comprehensive assessment to date on the association between NAFLD and CVD, stroke or cerebrovascular disease, and extrahepatic solid cancers. We used strict quality tools to assess the quality of individual studies, and for those in which we were able to perform meta-analyses, we used GRADE assessment on the overall quality of evidence.
In conclusion, the findings of this comprehensive systematic review and meta-analysis of observational studies suggest that NAFLD is associated with significantly increased risk of CVD, stroke and cerebrovascular disease. While the association between NAFLD and extrahepatic cancers is more heterogeneous, there appears to be an increased risk, particularly for breast cancer and colorectal cancer in patients with NAFLD, which is clinically relevant given that breast and colorectal cancers are the leading cancers among men and women in the USA and many regions worldwide. However, follow-up studies are needed to further explore this association and to determine whether existing breast and colorectal cancer screening protocols need to be adjusted for those with NAFLD. This study has important clinical implications given that nearly 90 million U.S. adults are affected with NAFLD and nearly 25% of the global population is estimated to have NAFLD. Our study highlights the need for early assessment of cardiovascular and cerebrovascular disease risk and the importance of optimizing metabolic disease risk factors that would contribute to these outcomes.
CRediT authorship contribution statement
Nicolette Veracruz: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Bilal Hameed: Formal analysis, Writing - review & editing. Sammy Saab: Formal analysis, Writing - review & editing. Robert J. Wong: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing - review & editing.
Conflicts of interest
All authors have none to declare.
Funding
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jceh.2020.04.018.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Wong R.J., Liu B., Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46:974–980. doi: 10.1111/apt.14327. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Stepanova M., Younossi Y. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 4.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. J Am Med Assoc. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar M., Bhuket T., Torres S. Prevalence of the metabolic syndrome in the United States, 2003-2012. J Am Med Assoc. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 6.Reccia I., Kumar J., Akladios C. Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism. 2017;72:94–108. doi: 10.1016/j.metabol.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z.M., Blissett D., Blissett R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 8.Adams L.A., Anstee Q.M., Tilg H. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 9.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Kim D., Kim W.R., Kim H.J. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Targher G., Byrne C.D., Lonardo A. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen A.M., Therneau T.M., Larson J.J. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslan U., Turkoglu S., Balcioglu S. Association between nonalcoholic fatty liver disease and coronary artery disease. Coron Artery Dis. 2007;18:433–436. doi: 10.1097/MCA.0b013e3282583c0d. [DOI] [PubMed] [Google Scholar]
- 16.Chan W.K., Tan A.T., Vethakkan S.R. Ultrasonography-diagnosed non-alcoholic fatty liver disease is not associated with prevalent ischemic heart disease among diabetics in a multiracial Asian hospital clinic population. Clin Res Hepatol Gastroenterol. 2014;38:284–291. doi: 10.1016/j.clinre.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Chinnadurai R., Chrysochou C., Kalra P.A. Increased risk for cardiovascular events in patients with diabetic kidney disease and non-alcoholic fatty liver disease. Nephron. 2019;141:24–30. doi: 10.1159/000493472. [DOI] [PubMed] [Google Scholar]
- 18.Choi D.H., Lee S.J., Kang C.D. Nonalcoholic fatty liver disease is associated with coronary artery disease in Koreans. World J Gastroenterol. 2013;19:6453–6457. doi: 10.3748/wjg.v19.i38.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn M.A., Behari J., Rogal S.S. Hepatic steatosis in diabetic patients does not predict adverse liver-related or cardiovascular outcomes. Liver Int. 2013;33:1575–1582. doi: 10.1111/liv.12285. [DOI] [PubMed] [Google Scholar]
- 20.Feitosa M.F., Reiner A.P., Wojczynski M.K. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis. 2013;227:420–424. doi: 10.1016/j.atherosclerosis.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fracanzani A.L., Tiraboschi S., Pisano G. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208–213. doi: 10.1016/j.atherosclerosis.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Golabi P., Paik J., Hwang J.P. Prevalence and outcomes of non-alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int. 2019;39:748–757. doi: 10.1111/liv.14038. [DOI] [PubMed] [Google Scholar]
- 23.Hagstrom H., Nasr P., Ekstedt M. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi M., Kojima T., Takeda N. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Y.C., Cheng B.C., Hwang J.C. Association of fatty liver disease with nonfatal cardiovascular events in patients undergoing maintenance hemodialysis. Nephron Clin Pract. 2013;124:218–223. doi: 10.1159/000357952. [DOI] [PubMed] [Google Scholar]
- 26.Liu H.H., Cao Y.X., Sun D. Impact of non-alcoholic fatty liver disease on cardiovascular outcomes in patients with stable coronary artery disease: a matched case-control study. Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H., Zeng L., Liang B. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res. 2009;40:571–575. doi: 10.1016/j.arcmed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A., Mingolla L., Rigolon R. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225:387–391. doi: 10.1016/j.ijcard.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Mellinger J.L., Pencina K.M., Massaro J.M. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63:470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisto P., Santaniemi M., Bloigu R. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanova M., Younossi Z.M. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Stolic R.V., Trajkovic G.Z., Kostic M.M. Correlation between nonalcoholic fatty liver and cardiovascular disease in elderly hemodialysis patients. Int Urol Nephrol. 2016;48:883–889. doi: 10.1007/s11255-016-1237-8. [DOI] [PubMed] [Google Scholar]
- 33.Sun L., Lu S.Z. Association between non-alcoholic fatty liver disease and coronary artery disease severity. Chin Med J. 2011;124:867–872. [PubMed] [Google Scholar]
- 34.Targher G., Bertolini L., Padovani R. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2006;23:403–409. doi: 10.1111/j.1464-5491.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 35.Targher G., Bertolini L., Padovani R. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Targher G., Bertolini L., Poli F. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 37.Targher G., Pichiri I., Zoppini G. Increased prevalence of cardiovascular disease in Type 1 diabetic patients with non-alcoholic fatty liver disease. J Endocrinol Invest. 2012;35:535–540. doi: 10.3275/7875. [DOI] [PubMed] [Google Scholar]
- 38.Vanjiappan S., Hamide A., Ananthakrishnan R. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diabetes Metab Syndr. 2018;12:479–482. doi: 10.1016/j.dsx.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Viglino D., Plazanet A., Bailly S. Impact of non-alcoholic fatty liver disease on long-term cardiovascular events and death in chronic obstructive pulmonary disease. Sci Rep. 2018;8:16559. doi: 10.1038/s41598-018-34988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wild S.H., Walker J.J., Morling J.R. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41:341–347. doi: 10.2337/dc17-1590. [DOI] [PubMed] [Google Scholar]
- 41.Wong V.W., Wong G.L., Yeung J.C. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology. 2016;63:754–763. doi: 10.1002/hep.28253. [DOI] [PubMed] [Google Scholar]
- 42.Wong V.W., Wong G.L., Yip G.W. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 43.Zeb I., Li D., Budoff M.J. Nonalcoholic fatty liver disease and incident cardiac events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2016;67:1965–1966. doi: 10.1016/j.jacc.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 44.Alexander K.S., Zakai N.A., Lidofsky S.D. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: the Reasons for Geographic and Racial Differences in Stroke cohort. PloS One. 2018;13 doi: 10.1371/journal.pone.0194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moshayedi H., Ahrabi R., Mardani A. Association between non-alcoholic fatty liver disease and ischemic stroke. Iran J Neurol. 2014;13:144–148. [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn J.S., Sinn D.H., Min Y.W. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther. 2017;45:345–353. doi: 10.1111/apt.13866. [DOI] [PubMed] [Google Scholar]
- 47.Chang C.F., Tseng Y.C., Huang H.H. Exploring the relationship between nonalcoholic fatty liver disease and pancreatic cancer by computed tomographic survey. Intern Emerg Med. 2018;13:191–197. doi: 10.1007/s11739-017-1774-x. [DOI] [PubMed] [Google Scholar]
- 48.Cho Y., Lim S.K., Joo S.K. Nonalcoholic steatohepatitis is associated with a higher risk of advanced colorectal neoplasm. Liver Int. 2019;39:1722–1731. doi: 10.1111/liv.14163. [DOI] [PubMed] [Google Scholar]
- 49.Kim G.A., Lee H.C., Choe J. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.012. S0168-8278(17)32294-8. [DOI] [PubMed] [Google Scholar]
- 50.Kwak M.S., Yim J.Y., Yi A. Nonalcoholic fatty liver disease is associated with breast cancer in nonobese women. Dig Liver Dis. 2019;51:1030–1035. doi: 10.1016/j.dld.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.I., Lim Y.S., Park H.S. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27:91–95. doi: 10.1111/j.1440-1746.2011.06816.x. [DOI] [PubMed] [Google Scholar]
- 52.Lin X.F., Shi K.Q., You J. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41:2989–2997. doi: 10.1007/s11033-014-3157-y. [DOI] [PubMed] [Google Scholar]
- 53.Nseir W., Abu-Rahmeh Z., Tsipis A. Relationship between non-alcoholic fatty liver disease and breast cancer. Isr Med Assoc J. 2017;19:242–245. [PubMed] [Google Scholar]
- 54.Pan S., Hong W., Wu W. The relationship of nonalcoholic fatty liver disease and metabolic syndrome for colonoscopy colorectal neoplasm. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seko Y., Sumida Y., Tanaka S. Predictors of malignancies and overall mortality in Japanese patients with biopsy-proven non-alcoholic fatty liver disease. Hepatol Res. 2015;45:728–738. doi: 10.1111/hepr.12407. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y.J., Bang C.S., Shin S.P. Clinical impact of non-alcoholic fatty liver disease on the occurrence of colorectal neoplasm: propensity score matching analysis. PloS One. 2017;12 doi: 10.1371/journal.pone.0182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong R.J., Tran T., Kaufman H. Increasing metabolic co-morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PloS One. 2019;14 doi: 10.1371/journal.pone.0220612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ndumele C.E., Nasir K., Conceicao R.D. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Targher G., Bertolini L., Rodella S. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16:1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 60.Haukeland J.W., Damas J.K., Konopski Z. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Hui J.M., Hodge A., Farrell G.C. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 62.Belke D.D., Betuing S., Tuttle M.J. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adolph T.E., Grander C., Grabherr F. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanna C., Rosso C., Marietti M. Non-Alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laudisio D., Muscogiuri G., Barrea L. Obesity and breast cancer in premenopausal women: current evidence and future perspectives. Eur J Obstet Gynecol Reprod Biol. 2018;230:217–221. doi: 10.1016/j.ejogrb.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 66.Khan S., Shukla S., Sinha S. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–513. doi: 10.1016/j.cytogfr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 68.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin N Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 69.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 70.Komninou D., Ayonote A., Richie J.P., Jr. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med. 2003;228:396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 71.Yamaji T., Iwasaki M., Sasazuki S. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Canc Res. 2010;70:5430–5437. doi: 10.1158/0008-5472.CAN-10-0178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.