Abstract
Malnutrition and sarcopenia are common in patients with chronic liver disease and are associated with increased risk of decompensation, infections, wait-list mortality and poorer outcomes after liver transplantation. Assessment of nutritional status and management of malnutrition are therefore essential to improve outcomes in patients with chronic liver disease. This consensus statement of the Indian National Association for Study of the Liver provides a comprehensive review of nutrition in chronic liver disease and gives recommendations for nutritional screening and treatment in specific clinical scenarios of malnutrition in cirrhosis in adults as well as children with chronic liver disease and metabolic disorders.
Keywords: Sarcopenia, malnutrition, nutrition, cirrhosis, chronic liver disease
Abbreviations: ACLF, acute on chronic liver failure; ASM, appendicular skeletal muscle mass; BCAA, branched chain amino acids; BIA, bioimpedance analysis; BMI, body mass index; BMD, bone mineral densitometry; CLD, chronic liver disease; CS, corn-starch; CT, computed tomography; CTP, Child–Turcotte–Pugh; DEXA, dual-energy X-ray absorptiometry; EASL, European Association for the Study of the Liver; ESPEN, European society for Clinical Nutrition and Metabolism; GSD, glycogen storage disease; HGS, hand-grip strength; IBW, ideal body weight; IEM, inborn error of metabolism; INASL, Indian National Association for Study of the Liver; L3, third lumbar; LFI, Liver Frailty Index; MELD, model for end-stage liver disease; MCT, medium-chain triglyceride; MLD, metabolic liver disease; MRI, magnetic resonance imaging; REE, NASH; non-alcoholic liver disease, resting energy expenditure; RDA, recommended daily allowance; RFH-NPT, Royal Free Hospital-Nutritional Prioritizing Tool; SMI, skeletal muscle index; TEE, total energy expenditure
The liver plays a major role in the digestion, absorption, storage, synthesis and metabolism of macro- and micronutrients. Malnutrition is commonly seen in patients with liver disease and is associated with increased complications such as hepatic encephalopathy (HE), ascites and increased susceptibility to infections. Malnutrition and muscle mass loss (sarcopenia), which is a surrogate marker for severe malnutrition, are well recognized as a predictor of morbidity and mortality in patients with advanced liver disease.1
The importance of nutrition in patients with liver disease and transplant candidates has been well recognized, and there are up-to-date guidelines issued by leading authorities including (European Association for the Study of the Liver (EASL) and European Society for Clinical Nutrition and Metabolism (ESPEN).2, 3, 4 However, these guidelines cannot be universally applied to the Indian population, and there are specific issues that merit consideration in the assessment of nutritional status and management of malnutrition in the Indian context.
While there has been significant progress in health and nutrition interventions in India5 and under-nutrition rates are steadily decreasing, there is still a high prevalence of malnutrition, especially in rural India.6,7 Tribal populations are particularly vulnerable to undernutrition because of their geographical isolation, uncertainty of food supply, lack of adequate healthcare facilities and irrational belief systems and taboos.
Indians have lower muscle mass and higher prevalence of sarcopenia. In a health survey conducted in China, Ghana, India, Mexico, Russia and South Africa, between 2007 and 2012, the skeletal muscle mass was calculated with specific indirect population formulas based on age, sex, weight, height and race. The prevalence of sarcopenia in adults >65 years of age was highest in India (17.5%) and lowest in Poland (12.6%).8 There are ethnic differences in lean mass, and South Asian men and women have significantly less lean mass than Aboriginal, Chinese and European men and women of the same body size.9 The mean muscle mass of Asians is approximately 15% lower than that of western population even after height adjustments.10,11 Considering the lower muscle mass in Indians, there is a need to have normative values of sarcopenia in the Indian population. While some work has been done in evaluating normal values of computed tomography (CT) skeletal muscle index (SMI), hand-grip strength (HGS), gait velocity and chair stand in non-cirrhosis Indian population,12 further prospective studies are needed for the assessment of sarcopenia to establish criteria and standardize muscularity assessment according to ethnicity, gender and age.
Social and cultural issues like diet and physical activity may also explain the higher sarcopenia rates in Indians. Indian diets derive almost 60% of their protein from cereals with relatively low digestibility and quality.13 Besides diet, physical activity also plays a role in development of sarcopenia. Although moderate–vigorous physical activity is an important factor in counteracting sarcopenia,14 Indians have lower exercise levels as occupations have become less labour intensive and leisure time physical activity is not very popular.15
These considerations prompted the Indian National Association for Study of the Liver (INASL) to set up a Task Force to formulate consensus guidelines for management of nutrition in liver disease, relevant to the Indian scenario.
The available evidence and recommendations were adapted from the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system for evaluating evidence (Table 1).16
Table 1.
Level of Evidence and Grade of Recommendations (Adapted from GRADESystem).
| Level of evidencea | Confidence in the evidence | |
|---|---|---|
| High | Data derived from meta-analyses or systematic reviews or from (multiple) randomized trials with high quality. | Further research is unlikely to change our confidence in the estimate of benefit and risk. |
| Moderate | Data derived from a single randomised controlled trail or multiple non-randomized studies. | Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate. |
| Low | Small studies, retrospective observational studies, registries. | Any estimate of effect is uncertain. |
| Recommendations – Gradeb | Wording associated with the grade of recommendation | |
| Strong | ‘must’, ‘‘should’ or ‘‘INASL recommends’ | |
| Weak | ‘can’, ‘may’ or ‘‘INASL suggests’ | |
Level was graded down if there is a poor quality, strong bias or inconsistency between studies; level was graded up if there is a large effect size.
Recommendations were reached by consensus of the panel and included the quality of evidence, presumed patient important outcomes and costs.
Mechanism and etiology of malnutrition in cirrhosis
Malnutrition in cirrhosis is multifactorial and may be due to inadequate dietary intake, poor absorption or metabolic disturbances. Dietary intake can be decreased due to nausea, vomiting or early satiety secondary to ascites, gastroparesis, active alcoholism, reduced palatability due to salt restriction, aphthous ulcers secondary to vitamin B complex deficiency, dysgeusia secondary to zinc deficiency and poor socio-economic status especially in the developing countries. Restriction of oral intake due to HE along with iatrogenic fasting for diagnostic or therapeutic procedures further adds to the problem.
Maldigestion and malabsorption of nutrients can occur as a result of intraluminal bile acid deficiency due to decreased production from the cirrhotic liver, bacterial overgrowth, intestinal dysmotility and portal hypertensive enteropathy.17, 18, 19 Maldigestion may also be due to concomitant chronic pancreatitis among patients with alcohol-related cirrhosis.
Cirrhosis is a state of altered metabolism. Müller et al.20 found raised resting energy expenditure (REE) by indirect calorimetry in 34% of cirrhotic patients. This may be due to increased pro-inflammatory cytokines and abnormalities in carbohydrate, protein and lipid metabolism. Metabolism also increases due to infection, ascites and portal hypertension.21 Altered carbohydrate metabolism results in reduced synthesis of glycogen in the liver leading to increased gluconeogenesis from protein and fat breakdown.22 A study in patients with cirrhosis has shown that during an overnight fast, 58% of energy came from fat oxidation, whereas healthy controls derived 55% of their energy from carbohydrates.23
Pathogenesis of sarcopenia in cirrhosis
The liver has a very important role in maintaining muscle homeostasis by maintaining a balance between muscle growth and degradation. The primary pathway for muscle formation involves the mTOR signalling pathway leading to increased protein synthesis this pathway is activated by phosphokinase B. This pathway is increased by physical exercise, testosterone, insulin and insulin-like growth factor 1(IGF-1).24,25 The second pathway leading to muscle growth is by the proliferation and activation of the satellite cells. This is increased by branched chain amino acids (BCAAs) pool in the body, exercise and testosterone and negatively affected by myostatin, which deactivates the stellate cells.26,27 Ubiquitin-dependent proteasomal degradation is the major pathway involved in muscle degradation, and the second is autophagy. Increased level of inflammation leads to increased muscle degradation and overproduction of pro-inflammatory cytokines like adiponectin, interleukin (IL)-6 and tumour necrosis factor (TNF)-alpha, which have fibrogenic and oxidative effects.28,29 Autophagy levels are higher in cirrhotics, and alcohol can stimulate autophagy pathway.30 Reduced physical activity also leads to reduction in the release of myokines, which normally help to maintain muscle mass.31
The molecular basis of sarcopenia in patients with cirrhosis is centred on the myotoxic effects of hyperammonaemia.32 The proposed mechanism is that ammonia leads to an increase in myostatin via the NF Kappa B pathway leading to reduction in muscle protein synthesis and strength.33 Moreover, hyperammonaemia interferes with the tricyclic acid cycle and reduces ATP generation.33 The prevalence of sarcopenia in cirrhosis is also influenced by alcohol use disorder, a cause of myopathy and non-alcoholic fatty liver disease (NAFLD) in which there are other mechanisms leading to muscle weakness and sarcopenia.34,35
The overall turnover of muscle is higher in patients with cirrhosis.36,37 More BCAAs are used for energy due to increased protein degradation and turnover and an increased muscle uptake of BCAA to detoxify ammonia.38, 39, 40
Hormonal alterations contribute to alterations in muscle mass. Testosterone inhibits myostatin, thereby increasing the activity of the satellite cells in muscles. It also maintains a higher level of IGF-1, which promotes muscle protein synthesis. Data suggest that 90% of individuals with cirrhosis have decreased testosterone levels and increased levels of sex hormone–binding globulin further reducing the level of free testosterone.41,42 Reduced testosterone levels have been associated with increased mortality in individuals with cirrhosis.43 The levels of IGF inversely correlated with the disease severity.44,45
Pathogenesis of hepatic osteodystrophy in chronic liver disease
Hepatic osteodystrophy refers to the changes in bone metabolism in chronic liver disease (CLD), which are clinically represented by osteoporosis, osteopenia and less commonly osteomalacia. The clinical implication of these abnormalities is bone pains, skeletal deformities and frequent fractures. While these complications are commonly seen in patients with cholestatic diseases (primary biliary cholangitis and primary sclerosing cholangitis), these are also seen in other CLD as well.
The bone remodeling and osteoclastogenesis are regulated by the system of the receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG) system, in which RANKL is a promoter of osteoclast differentiation and activation and OPG is an inhibitor. Serum RANKL levels are significantly lower, and OPG levels are higher in osteopenic/osteoporotic patients with CLD. There are multiple risk factors for hepatic osteodystrophy in liver disease including genetic factors, vitamin D deficiency and calcium disorders, vitamin K deficiency, IGF-1 deficiency, hyperbilirubinemia, hypogonadism, medication, fibronectin, hyperhomocysteinemia, leptin and lifestyle.46 Osteoprogenitor cells are reduced in patients with cirrhosis.47
Magnitude of malnutrition in cirrhosis: global and Indian perspectives
Malnutrition and sarcopenia are common complications of cirrhosis, which are neglected and often not assessed or are under reported. Body weight and body mass index (BMI) are affected by fluid retention from ascites and oedema, which can result in underassessment of malnutrition. Moreover, the prevalence of malnutrition may be affected by the various definitions, methods and different cut-offs used to assess the nutritional status in patients with cirrhosis.
The global prevalence rate of sarcopenia in cirrhosis was mean 48.1% and appeared more among men (61.6%) than in women (36%).48 A high prevalence of malnutrition and sarcopenia in cirrhosis has been reported in India varying between 47% and 84%.49, 50, 51, 52, 53, 54 The prevalence of malnutrition increases with severity of liver disease.49
The high sarcopenia in cirrhotics in Indians may be related to high prevalence of malnutrition in Indian population, dietary differences and lower mean muscle mass in Indians. However, further studies are needed to establish criteria and standardize muscularity assessment according to the gender and age for Indian population.
Consensus statements
-
1.
Prevalence of malnutrition in cirrhosis is higher among Indians compared with global population. (Level of evidence – moderate)
-
2.
Malnutrition increases with severity of liver disease, and sarcopenia is more frequent in males. (Level of evidence – moderate)
Impact of malnutrition and sarcopenia on severity of liver disease and mortality
The severity and prevalence of sarcopenia in cirrhosis correlates with the Child–Pugh score.55 When added to the model for end-stage liver disease (MELD) score, sarcopenia improves the utility for predicting survival. It is particularly useful in patients with MELD scores <15 and Child–Pugh class A/B.55, 56, 57, 58 Therefore, sarcopenia can help in risk-stratifying patients with compensated and early decompensated cirrhosis.
Sarcopenia has a negative impact on morbidity and mortality in patients with liver cirrhosis.59, 60, 61, 62 Sarcopenia in patients with NAFLD is associated with a higher likelihood of having steatohepatitis or advanced liver fibrosis.63 Patients with cirrhosis and sarcopenia are more likely to develop HE, refractory ascites and sepsis-related complications.55,58,64 Sarcopenic patients are also more likely to develop acute on chronic liver failure (ACLF) after transjugular intrahepatic portosystemic shunt (TIPS).65 The treatment of refractory ascites by TIPS has improved sarcopenia; failure of reversal of sarcopenia after TIPS was accompanied by a higher mortality.66 Sarcopenia is associated with decreased survival, increased treatment-related mortality and tumour recurrence in patients with hepatocellular carcinoma (HCC).67
As per a recent meta-analysis, sarcopenia in the Asian populations (including Japan and Korea) was associated with higher mortality compared with Western populations (HR of mortality 2.45 (95% CI = 1.44–4.16) compared with the Western patients (HR of mortality 1.45(95% CI = 1.002–2.09.48 This higher mortality in Asian patients with cirrhosis and sarcopenia has been attributed to differences in racial characteristics, body size, dietary regimes, and life quality between Asian and Western individuals in different countries.
Consensus statements
-
3.
Malnutrition and sarcopenia are associated with increased risk of decompensation, infections and increased wait-list mortality in patients with cirrhosis. (Level of evidence – moderate)
Implications of malnutrition and sarcopenia in liver transplantation
Pre-transplant malnutrition and sarcopenia are associated with increased risk of decompensation, infections and increased waitlist mortality.68 Post-transplant these complications predict poorer outcomes and are associated with longer time to extubation after transplantation, increased post-operative infections, prolonged ICU stay and hospitalization, and decreased survival.69, 70, 71, 72
Sarcopenia does not always improve after transplantation.73, 74, 75 While some patients demonstrate improvement of sarcopenia after liver transplant,76 up to one-fourth may develop de novo sarcopenia after transplant.77 Plank et al.78 have reported 1 kg loss of total body protein immediately after surgery which was not replenished after 12 months. Post-transplant progression of sarcopenia may be related to the persistent catabolic state, immunosuppression, corticosteroids, prolonged hospital stay and at times due to recurrence of liver disease.74
Consensus statements
-
4.
Post-transplant malnutrition and sarcopenia predict poorer outcomes and are associated with longer hospitalization, longer ICU stay and increased risk of mortality. (Level of evidence- Low)
-
5.
Some patients may develop sarcopenia after liver transplantation. (Level of evidence- Low)
Obesity in patients with cirrhosis
While undernutrition is common, obesity can also be a cause of concern in patients with CLD. Obesity leads to overproduction of pro-inflammatory cytokines, which have fibrogenic and oxidative effects.79 Genetic factors like PNPLA3 polymorphisms add to the pro-inflammatory state.80
Obesity is linked with insulin resistance and the metabolic syndrome. A Swedish study has reported that adolescent obesity is associated with a significantly higher risk of developing severe liver disease later in life.81 Patients with non-alcoholic steatohepatitis (NASH) and alcohol-related cirrhosis have a higher prevalence of obesity.82,83 Despite having increased adipose tissue, these patients have reduced muscle mass which is referred to as sarcopenic obesity.84,85
Obesity is an independent risk factor for progression of underlying liver disease irrespective of the aetiology of liver disease.86, 87, 88 Alcohol-induced liver disease is more severe in obese compared with lean individuals. In an Italian study, 46% of patients with heavy drinking had steatosis compared with 95% in heavy drinkers with obesity.89 In another study, fibrosis progression was found to be more in obese patients with NAFLD with moderate drinking (<140 gm/week) compared with obese non-drinkers.90 A large epidemiologic study from the United Kingdom that analysed 1.3 million women found the risk of cirrhosis was increased by six times in the women who were obese and heavy drinkers compared with non-drinkers and two times higher compared with heavy drinkers alone.91 Similar effect of obesity has been shown in HCV-related liver disease. The HALT-C trial showed a higher rate of death or decompensation in patients who had a higher BMI. Each quartile increase in BMI was associated with a 14% increase in clinical events in the follow-up.92 Obesity has also been independently associated with infections in hospitalised patients with end-stage liver disease.93 Weight loss in obese patients with cirrhosis and portal hypertension was significantly associated with decrease in HVPG and reduced the progression of fibrosis and cirrhosis.94
Morbid obesity has also been associated with an increased risk of death of patients on liver transplant waiting list, a decreased probability of liver transplantation and decreased post-transplant survival.95,96 The impact of obesity in patients with end-stage liver disease has been investigated in a large UNOS database retrospective study. The waitlist mortality was higher in morbidly obese patients.97
Developing countries like India are facing a high risk of obesity and its adverse effects. As per a 2015 study, the prevalence of obesity in India is approximately 11.8%, and it was estimated that there were more than 135 million individuals affected by obesity in India.98 Hence, many patients with cirrhosis may be obese but still have muscle wasting and patients with advanced disease warrant additional screening for malnutrition and muscle wasting irrespective of BMI.99
Consensus statements
-
6.
Obesity is a risk factor for the presence of severe fibrosis in alcohol and viral-related CLD, fibrosis progression, and cirrhosis. (Level of evidence: moderate)
-
7.
Obesity is associated with increased morbidity and mortality, irrespective of the underlying aetiology of the liver disease. (Level of evidence – moderate)
Nutritional requirements in cirrhosis
Patients with cirrhosis are in a catabolic state characterized by reduced protein synthesis and enhanced proteolysis to provide fuel for gluconeogenesis. The process of gluconeogenesis requires energy and consequently increases the REE of patients with cirrhosis.100 Hence, a relatively higher intake of total energy and proteins are needed in such patients. The REE of a healthy adult is 1 kcal/kg body weight/hour, that is, 24 kcal/kg/day. The total energy expenditure (TEE), of a hemodynamically stable patient with cirrhosis with a sedentary life style is 1.3 times the estimated REE (1.3 × 24 kcal/kg/day, i.e. 32 kcal/kg/day [range 30–35 kcal/kg/day]).2,3 In patients with obesity, recent guidelines support that a target of 5–10% weight loss could be achieved by reducing the estimated TEE by 500–800 Kcal/day.2
If possible, REE should actually be measured instead of formula-based estimation because measured REE could be higher than estimated REE in up to 35% of cirrhosis patients.101 While the gold standard to measure REE is indirect calorimetery, it is not available in most places.102
A hand-held calorimeter is an easily available alternative bedside instrument but requires further validation in cirrhosis.103,104 The calculation of TEE should be based on dry weight. In a patient with ascites or oedema, we could estimate the dry weight by one of the following methods: (i) use of pre-ascites weight, if available (ii) calculate ideal body weight (IBW) based on height; the ideal BMI for Indian population ranges from 18 to 22.9 kg/m2 (iii) post-paracentesis weight or (iv) empirically corrected body weight68 by subtracting a percentage of weight based on severity of ascites (mild, 5%; moderate, 10%; severe, 15%) without or with bilateral pedal oedema (additional 5% subtracted if oedema is present).
While a healthy adult person needs 0.66 gm/kg of proteins every day,105 the daily protein requirement in patients with cirrhosis is increased to 1.2 gm/kg106 in the absence of malnutrition or 1.5 gm/kg in the presence of malnutrition.2,3 The diet should provide around 50–60% of total calories from carbohydrates, preferably complex carbohydrates, and 20–30% from fat. Among fats, saturated fatty acid should not contribute more than 10% of total calories, whereas monounsaturated fatty acids and polyunsaturated fatty acids should contribute equally to the remaining portion of calories provided by fats.107
Consensus statements
-
8.
Patients with cirrhosis who is in hemodynamically stable state and have a sedentary life style, require 30–35 kcal/kg dry body weight/day. The protein intake should be 1.2 gm of proteins/kg/day in the absence of malnutrition or 1.5 gm/kg/day in the presence of malnutrition. Carbohydrates should account for 50–60% of the calories and fats should be 20–30% of the calories. (Level of evidence – moderate; grade of recommendation – strong)
Assessment of nutritional status in cirrhosis
BMI is an inaccurate tool for nutritional assessment in patients with cirrhosis in the presence of ascites and oedema.
Nutritional screening tools
The waitlisted patient with low BMI [< 18.5 kg/m2] or the one with high BMI [>40 kg/m2] and those who are Child–Turcotte–Pugh (CTP) C do not require nutritional screening for risk stratification as it is clear they are already at risk and that they require more detailed nutritional assessment.2,102 Nutritional screening is recommended in all other patients with cirrhosis.
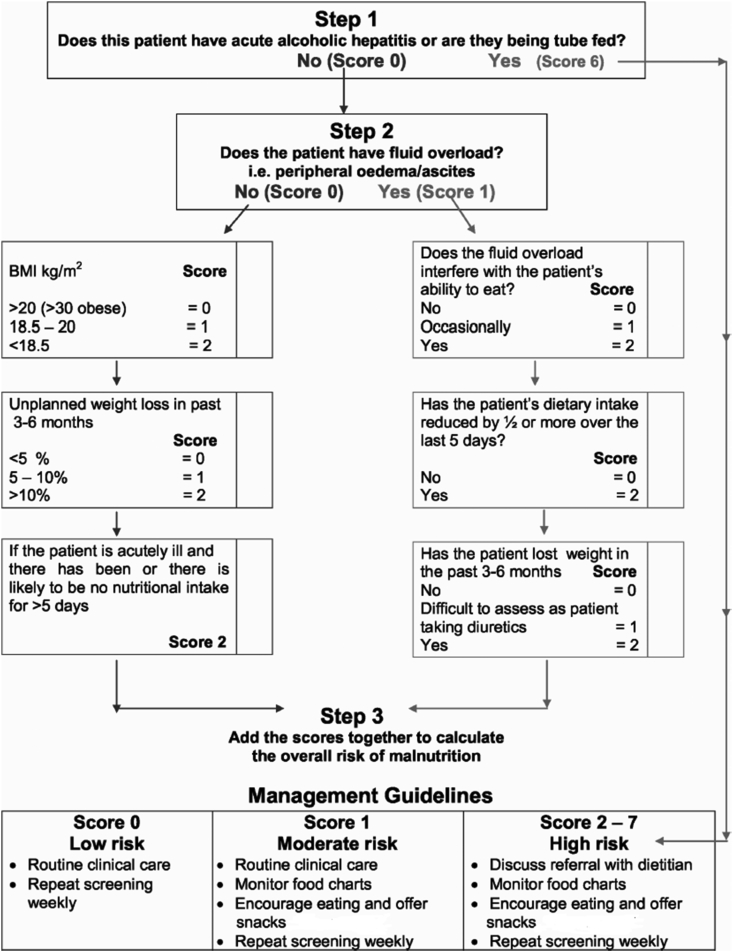
To date, there are limited data on cirrhosis-specific nutrition screening tools. Within cirrhosis, there have been three tools, which have undergone preliminary evaluation,2,102 the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT), the Nutrition Risk Screening-2002(NRS 2002) and the Liver Disease Undernutrition Screening tool (LDUST).108,109 Although the Malnutrition Universal Screening Tool (MUST) has been recommended as the screening tool of choice by societies3 this incorporates BMI and weight loss, which are inaccurate in the presence of ascites/fluid retention. With the available evidence to date, the RFH-NPT is the most promising nutritional screening tool in patients with cirrhosis (Box 1). It discriminates patients into low-, medium- and high-risk categories. In the original study, 3% of patients scored 0 (low risk), 50% scored 1 (moderate risk) and 47% scored 2–7 (high risk) with dietitian referral recommended for the high-risk group. The RFH-NPT has been reported to correlate with liver-related complications including ascites, hepatorenal syndrome and HE.110 The various methods of nutritional screening are shown in Box 1. RFH-NPT for determining nutritional risk in cirrhosis is depicted in Figure 1.
Box 1. Nutritional screening.
-
•Overt malnutrition/high risk of malnutrition:
-
oBMI of <18.5 kg/m2
-
oBMI >40 kg/m2
-
oChild–Pugh C disease
-
o
-
•Nutrition screening tests for other cirrhosis patients:
-
oRoyal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT)
-
oNutrition Risk Screening-2002 (NRS 2002)
-
oLiver Disease Undernutrition Screening Tool (LDUST).
-
o
Alt-text: Box 1
Figure 1.
Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) for determining nutritional risk in cirrhosis (Reprinted with permission from Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325-36.).
Consensus statements
-
9.
Patients who are CTP C or have a BMI of <18.5 kg/m2 or >40 kg/m2 have overt malnutrition or are at high risk of malnutrition. All other cirrhosis patients should undergo a rapid nutritional screen to risk stratify them for a more detailed nutritional assessment and intervention (Level of evidence – moderate; grade of recommendation – strong).
-
10.
Until further studies are performed, the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) is a rapid evidence-based nutritional screen that can be used in patients with cirrhosis (Level of evidence – moderate; grade of recommendation – weak).
Assessment of sarcopenia
The definition of sarcopenia has evolved over a period of time from generalized loss of skeletal muscle mass to loss of muscle strength and low physical performance. In non-cirrhosis populations, there are data to suggest that muscle strength may be more sensitive than muscle mass in predicting adverse outcomes.59
Cross-sectional imaging with CT scan or MRI is the accepted imaging tool to quantify the skeletal muscle mass. Abdominal CT scan is routinely done in patients with cirrhosis as second line imaging for screening of HCC and for evaluation for liver transplantation. CT scan provides an accurate, objective and reproducible measure of skeletal muscle mass, which is not affected by fluid retention.
The third lumbar (L3) SMI is used for quantifying sarcopenia. The skeletal muscles area is quantified using tissue-specific Hounsfield unit thresholds of −29 to +150.111 There are different image analysis software packages available for calculating the total cross-sectional area of abdominal skeletal muscles at the L3 level. Cross-sectional area of muscles (psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques and rectus abdominis) are measured at this level and are then normalised for stature (cm2/m2).112 Cut-off values for the diagnosis of sarcopenia, derived from cirrhotic patients on the liver transplant list have been suggested to be less than 50 cm2/m2 for men and less than 39 cm2/m2 for women but need to be further validated, particularly outside of North America.113
Previous studies reported that the mean muscle mass of Asians is approximately 15% lower than that of Westerners even after height adjustments.114,115 Hence, it is important to establish criteria for evaluating and measuring sarcopenia in diverse ethnicities. Sidhu et al.12 evaluated 3087 non-cirrhotic Indian patients without cirrhosis and found that the mean CT SMI was 41.25 ±4.42 in females and 44.33 ±6.56 in males, which is much less than that reported from West. Similarly, lower mean muscle mass has been reported in 149 Japanese subjects in whom the optimal cut-off values for CT L3 SMI to identify sarcopenia were calculated as 42 cm2/m2 for men (AUC, 0.83; sensitivity, 89%; specificity, 57%) and 38 cm2/m2 for women.116
Data with regard to MRI are even more scarce, and normal values are not available. Psoas muscle area and psoas muscle thickness/height (PMTH) have also been used to quantify sarcopenia although this method of assessment has been criticized.117 Cut-off value of <16.8 mm/m has been reported for sex-nonspecific PMTH sarcopenia.118 Sex-specific cut-offs of PMTH have been reported to be 17.3 mm/m in men and 10.4 mm/m is women.119 Ultrasound has also been used to identify sarcopenia. Tandon et al.120 used thigh ultrasound to measure the thigh muscle index using both compression and no compression at two predetermined points on the thigh. They took two sets of readings a compression reading taken by pressing the probe downward until no further compression of the muscles was possible; and a featherweight reading where the probe was held without pressure on the thigh. Measurements were averaged and corrected for stature (height squared) to calculate an average compression index and an average feather index. They found that the average feather index was most strongly associated with sarcopenia in cirrhotic patients. Although promising, further validation is required.
Muscle attenuation on CT indirectly measures the infiltration of fat in the muscles or myosteatosis. The cut-off values associated with higher mortality risk in cirrhotics are <41 HU in patients with BMI up to 24.9 kg/m2 and <33 HU in patients with BMI ≥25 kg/m2.121
Assessment of body composition
The methods available for this are total body electrical conductivity, bioelectrical impedance, air displacement plethysmography, dual-energy X-ray absorptiometry (DEXA) and magnetic resonance spectroscopy, the last two being imaging techniques. These methods can quantify body composition based on specific components like water, proteins and minerals. They can calculate the total body weight, the fat mass and the lean body mass.122 However, some of these methods are limited by routine availability, reproducibility and/or accuracy. Although DEXA is reproducible and involves only low-dose X-rays, it has the disadvantage of radiation exposure. Water retention due to ascites can also lead to overhydration and overestimation of the fat-free mass while using DEXA.123 Both DEXA and CT can also be used to measure the bone mineral density (BMD), which can get depleted in patients with cirrhosis.
DEXA enables the estimation of the absolute level of skeletal muscle mass or appendicular skeletal muscle mass, which can be adjusted for body size in different ways, namely using height squared (ASM/height2), weight (ASM/weight) or body mass index (ASM/BMI).124 However, the preferred adjustment and whether the same method can be used for all populations is still debatable. Despite the minimal radiation exposure from DEXA, using DEXA in community screening of sarcopenia is still difficult. The Asian Working Group for Sarcopenia (AWGS) recommends using height-adjusted skeletal muscle mass instead of weight-adjusted skeletal muscle mass, and the suggested cut-off values were 7.0 kg/m2 in men and 5.4 kg/m2 in women by using DEXA.125
Bio-impedance analysis (BIA) has also been used for the assessment of sarcopenia. BIA has particularly become an attractive index because it is portable, non-invasiveness, no radiation exposure and is not costly. Sarcopenia is defined as patients with SMI using BIA <7.0 cm2/m2 for males and <5.7 cm2/m2 for females.126 However, the major drawback of DEXA and BIA is distortion by hydration status and presence of oedema in cirrhosis.
Assessment of sarcopenic obesity
Patients with sarcopenic obesity develop simultaneous loss of skeletal muscle and gain of adipose tissue.53,121,127, Not only is there muscle depletion, there may also be myosteatosis. Myosteatosis is defined by fat deposition in skeletal muscle, which can occur in both sarcopenic and non-sarcopenic patients, with or without obesity. Myosteatosis is associated with decreased strength and increases with age and adiposity.128
There is no consensus on the definition of sarcopenic obesity due to the wide heterogeneity of diagnostic criteria, different modalities of body composition analysis and absence of well-defined population-based cut-offs.129 BMI of ≥23 or ≥25 kg/m2 corrected for ascites should be used to define obesity, and sarcopenia should be defined based on CT-based height corrected L3-SMI.130
Assessment of muscle strength and physical performance
Measurements of muscle strength HGS) and physical performance (gait speed) reflect overall functional reserve and frailty.131 The assessment of muscle strength can be easily done by measuring the HGS by a hand dynamometer. HGS is a simple, inexpensive and effective method to detect sarcopenia in cirrhotic patients. Because of the lack of outcome-based cut-off values, AWGS recommends using the lower 20th percentile of HGS of the study population as the cut-off value for low muscle strength before outcome-based data are available.125 In Indians, mean HGS reported in non-cirrhotic population was 25.19 ± 7.57 kg in females versus 35.14 ± 8.56 kg in males (p < 0.0001).12
Gait speed is considered a quick, safe and highly reliable test for physical performance in sarcopenia. It can be widely used in clinical practice. The European consensus for gait speed recommends a cut-off speed ≤0.8 m/s for the 4-m usual walking speed test to be an indicator of severe sarcopenia.59 However, the AWGS has revised the cut-off for gait speed. They recommend a cut-off of <1.0 m/s for a 6-m walk test to define reduced physical performance.125
Assessment of frailty
Physical frailty overlaps with many other concepts in which muscle health is an objective feature – including concepts such as sarcopenia, disability, decreased energy expenditure and malnutrition. Sarcopenia in particular is a central component of frailty, but as discussed in a recent American Society of Transplantation consensus statement, it is important to note that frailty extends beyond sarcopenia to include muscle function and the patient's experience of their frailty state.132 Across a range of studies, physical frailty has been shown to be a robust independent predictor of transplant waitlist and post-transplant mortality, mortality after hospitalization, hospital length of stay and discharge location.132, 133, 134, 135, 136, 137, 138, 139, 140, 141
A wide range of frailty tools have been evaluated and found to have prognostic utility. These have included objective performance-based tools such as the Liver Frailty Index (LFI), the 6-min walk test and cardiopulmonary exercise testing as well as more subjective provider or patient-assessed frailty metrics such as the Karnofsky Performance Scale, Clinical Frailty Scale and Activities of Daily Living. It is suggested that at least one frailty tool be incorporated during the initial evaluation and longitudinal follow-up.132 In liver transplant listed patients, an objective performance-based tool is suggested compared with more subjective provider or patient-assessed frailty metrics. The more subjective frailty metrics have are time-efficient for a busy clinical practice setting and have also demonstrated a robust association with relevant clinical outcomes.136, 137, 138, 139,142
A recent study on the LFI, which includes grip strength, timed chair stands and balance testing, demonstrated that frailty was a stronger predictor of waitlist mortality than traditional factors such as ascites and HE.143
Bedside tools for assessment of nutritional status
Physicians often assess muscle wasting and physical ability by the patients overall look and activity levels. However, these are subjective and may vary between clinicians. While it is not possible to objectively measure muscle mass at the bedside, muscle strength can be assessed easily by HGS and physical ability by walking speed. The cut-off of HGS is < 26 kg for men and <18 kg for women, and the cut-off walking speed is < 0.8 m/s. The addition of an objective measure like the LFI gives a more accurate prediction of frailty. The LFI includes grip strength, which is a marker of nutritional status; balance testing, which assesses neuromuscular function and chair stands, which are a marker of lower extremity weakness. It is a simple test that can be carried out on the bedside or in the outpatient setting. LFI score of 4.5 indicates that patients are frail instructions of LFI are available at http://liverfrailtyindex.ucsf.edu. The details of LFI calculation are shown in Box 2. The various methods to assess sarcopenia are given in Table 2.
Box 2. Liver Frailty Index.
-
•Components
-
oDominant hand-grip strength: the average of three attempts using a hand dynamometer
-
oTime to do five chair stands: the time in seconds to stand up and down in a chair five times with the subject's arms folded across the chest
-
oBalance testing: measured as the number of seconds that the subject can balance in three positions (feet placed side to side, semi-tandem and tandem) for a maximum of 10 s each.
-
o
-
•Formula
-
o(-0:330 x gender-adjusted grip strength) + (- 2:529 × number of chair stands per second) + (−0.040 × balance time) + 6
-
o
Alt-text: Box 2
• Online calculator with instructions are available at: http://liverfrailtyindex.ucsf.edu.
LFI scores of 4.5 indicated that patients are frail.
Table 2.
Assessment of Sarcopenia.
| Diagnostic parameter | Method of assessment | Cut-off of defined sarcopenia |
|---|---|---|
| Muscle mass | DEXA∗ | Men: 7.0 kg/m2 Women: 5.4 kg/m2 |
| BIA∗ | Men: <7.0 cm2/m2 Women: <5.7 cm2/m2 |
|
| CT SMI index: measure total muscle mass at the level of L3 divided by height squared | Men: < 42 cm2/m2 Women: 38 cm2/m2 |
|
| Muscle strength | Hand-grip strength | Men: <27 kg Women: <16 kg |
| Physical ability | Gait speed (4 m usual walking speed) | ≤0.8 m/s |
CT, computed tomography; BIA, bioelectrical impedance analysis; DEXA, dual-energy X-ray absorptiometry; SMI, skeletal muscle index.
Consensus statements
-
11.
Cross-sectional imaging (CT or MRI) scans done for other indications can also be used to evaluate for sarcopenia using the L3-level SMI. (Level of evidence – moderate; grade of recommendation – strong)
-
12.
Dual-energy X-ray absorptiometry (DEXA) and Bioimpedance analysis (BIA) can also be used for assessment of skeletal muscle mass. However, these modalities may be affected by hydration status and presence of oedema in cirrhosis. (Level of evidence – low)
-
13.
HGS can be used to detect muscle strength in cirrhotic patients (Level of evidence – moderate; grade of recommendation – strong)
-
14.
Gait speed can be used for assessment of physical performance in sarcopenia (Level of evidence – moderate, grade of recommendation – strong)
-
15.
Physical frailty is a robust predictor of adverse clinical outcomes in cirrhosis. Every patient with cirrhosis, particularly those on the liver transplant waiting list, should be assessed both at baseline and in longitudinal follow-up using a standardized frailty tool. Although rapid frailty screens may be useful for an initial screen, to be sensitive enough to detect a change over time, objective performance-based frailty tools are suggested. The most suitable tool may vary depending on site-specific experience and resources and preference (level of evidence – moderate; grade of recommendation – strong).
Assessment of hepatic osteodystrophy
Osteoporosis is common in patients with cirrhosis making them at an increased risk of fractures. Bone loss in liver cirrhosis is more severe among trabecular bones, such as vertebrae, with a lesser impact on the cortical ones. This pattern of increased vertebral damage is similar to some findings observed in the elderly, leading to compression fractures, disability and spinal deformities. Assessment of BMD by using DEXA at lumbar vertebrae and femoral neck is considered as gold standard in the diagnosis of hepatic osteodystrophy. According to the World Health Organization (WHO), osteoporosis is defined as BMD less than 2.5 standard deviations compared with normal average value (T-score < −2.5); osteopenia is defined as T-score between −1 and −2.5.144 In individuals less than 50 years of age, the Z-score is used, which represents BMD of patient compared with mean BMD of age-, race- and sex-matched controls.145
A two-dimensional X-ray examination can be used to check the vertebrae before ordering a more expensive examination. Lumbar spine measurements may be unreliable in the elderly due to the presence of osteophytes, extraskeletal calcification and vertebral and/or spinal deformity.146 Therefore, a lateral vertebral X-ray can be important as a complimentary examination to search for dorsal or lumbar spine fractures.147
Presence of ascites in patients with cirrhosis may affect the accuracy of bone density measurement in the spine. Ascites can cause fluid artefact in the soft tissue and bone interface that can underestimate the real BMD value particularly in the lumbar spine. After paracentesis vertebral BMD values of 4.2–7% higher were observed. Paracentesis modified the diagnosis of osteoporosis or osteopenia in 12% of patients. Therefore, in patients with ascites, BMD should be preferentially measured soon after paracentesis, to avoid over-diagnosis of osteoporosis and osteopenia, particularly in the lumbar spine.148,149
The prevalence of osteoporosis is higher in patients with cholestatic liver diseases and those who have received long-term steroids. The prevalence of osteoporosis in patients eligible for liver transplant is 30%.150 Hence, these patients should be screened for osteoporosis. In patients with normal DEXA scan, it should be repeated every 2–3 years. DEXA scan should be repeated at 1–2 years in patients with osteopenia and in those receiving prolonged corticosteroids.
Consensus statements
-
16.
A lateral vertebral X-ray may be done to search for dorsal or lumbar spine fractures, deformities. (Level of evidence – moderate; grade of recommendation – strong)
-
17.
BMD should be preferentially measured soon after paracentesis in patients with cirrhosis with ascites to avoid over-diagnosis of osteoporosis and osteopenia, particularly in the lumbar spine. (Level of evidence – high; grade of recommendation – strong)
-
18.
Evaluation of BMD measurement by DEXA should be done in patients with cirrhosis and in patients with chronic cholestatic diseases, those receiving long-term corticosteroid treatment and before liver transplantation. (Level of evidence – moderate; grade of recommendation – strong)
-
19.
DEXA should be repeated after 2 to 3 years in patients within normal BMD, and within 2 years in patients with osteopenic BMD and within 1 year in patients receiving prolonged glucocorticoids. (Level of evidence – moderate; grade of recommendation – strong)
Treatment of malnutrition in liver disease
Common misconceptions in dietary advice in cirrhosis
There are several myths regarding pathogenesis and treatment of malnutrition in cirrhosis. For more than half a century, protein restriction has been one of the main treatments for HE.151, 152, 153 Older clinical observations had been reported that high protein intake may worsen encephalopathy in patients with cirrhosis154, and it had become a universal practice to recommend low-protein diet to patients with cirrhosis. More recent studies have, however, shown that protein restriction has no major contribution in the treatment or prevention of HE.155,156 On the other hand, protein restriction/starvation has deleterious effect on muscles, which have an important function of buffering ammonia and providing amino acids for gluconeogenesis.157
Another myth is that patients with cirrhosis should not consume fat. Patients with cirrhosis have poor glycogen reserves; hence, the energy extraction often shifts to fatty acid oxidation.158,159 The ready availability of fatty acids may spare muscle breakdown to some extent. Indian patients also have a belief that herbal products are safe, and there is no harm in trying them. There are now several reports of hepatotoxicity from these indigenous medicines and should be avoided in patients with liver disease.160
Nutritional management principles in patients with cirrhosis
A good nutritional status plays an important role in the outcome of patients with cirrhosis and may even influence their survival.161, 162, 163, 164, 165 Nutritional management needs to start from a complete nutritional assessment, be followed by development of a nutritional care program and be maintained by monitoring the nutritional modifications over time. Ideally a registered dietitian needs to be involved in the development of the nutritional program.166 When carried out within the principles of strong patient engagement, inquiry into their beliefs around the benefit of a healthy diet and personalized patient education, nutritional counselling has the potential to adjust a patients' behavior.167 In one study, counselling involving a multidisciplinary team (physicians, nurses, pharmacists and dieticians) was associated with better survival than counselling by just one professional.168
Spontaneous dietary intake is usually inadequate in patients with cirrhosis, and unnecessary dietary restrictions are frequently adopted due to misunderstandings.169,170 Low dietary intake may be harmful in patients with cirrhosis where the hypermetabolism may be associated with a poor prognosis.171 As stated above, to accurately determine a nutrition prescription for these patients, a direct measurement of energy expenditure is recommended.172
Patients with cirrhosis need to be supported by an adequate amount of energy and protein to avoid the activation of endogenous catabolic processes to derive energy. An adequate diet should reach the target of 30–35 kcal/kg/dry body weight/day (50–60% of calories as carbohydrates; 20–30% as fat) with 1.2–1.5 g protein/kg body weight/day.
Furthermore, the meal pattern during the day should prevent prolonged periods of fasting: early morning breakfast and late evening snacks have both been found to be beneficial in this regard. The composition and quality of the small snacks still need to be defined, but the presence of proteins has been shown to improve nitrogen balance and increase muscle mass in some studies. In a study by Plank and colleagues,173 103 patients with cirrhosis were randomized to either daytime or night-time supplementary nutrition of 710 Kcal per day. There was a significant improvement in total body protein and fat-free mass in the patients who received nocturnal supplementation. Similarly, a systematic analysis and review, showed that late-evening snack reverses the aberrant substrate utilization pattern, improved substrate utilization and nitrogen retention than daytime calorie supplementation alone, may improve health-related quality of life and survival and also may reduce the frequency and severity of HE.174 Therefore, it is recommended that patients with cirrhosis should have their caloric and protein intake split into multiple, small, frequent meals (4–6 hourly). Higher protein content of breakfast and an energy-dense late evening snack comprising of complex polysaccharide (50 g) is also recommended to avoid an early onset, gluconeogenic starvation like state that further worsens the nutritional state of the patient.173
Overweight or obese patients may benefit from a progressive weight normalization.175 Energy intake in these patients should not be increased, and a moderate hypocaloric diet (−500 Kcal daily reduction) may be planned, following the patient periodically for adjustments. Protein intake needs to be maintained or even increased (1.5 g/kg weight/day) to achieve weight loss without inducing muscle catabolism.176
In patients with HE, the protein intake should not be restricted but preferably be enriched by vegetable and dairy proteins.177 BCAA can be used in case of protein intolerance to achieve the desired target of protein assumption.178,179
Due to salt and water retention a moderate dietary sodium intake (2 g of sodium corresponding to 5 g of salt) is usually recommended in patients with ascites. However, evidence in this respect is controversial.180 A reduction in sodium intake may, however, interfere with the patients approach to the diet compromising energy and protein intake.181
Consensus statements
-
20.
Multidisciplinary nutrition support teams should do nutritional counselling and regular individualized follow-up. (Level of evidence – moderate; grade of recommendation – strong)
-
21.
The optimal recommended daily calorie and protein requirements in patients with cirrhosis patients are calories 30–35 kcal/kg/day and proteins: 1.2–1.5 g/kg/d). (Level of evidence – moderate; grade of recommendation – strong)
-
22.
Multiple, small, frequent meals (4–6 hourly) with complex carbohydrate-dense (50 g) bed-time snack and protein-rich breakfast are recommended. (Level of evidence – moderate; grade of recommendation – strong)
Micronutrient and vitamin requirements
Patients with cirrhosis may develop deficiencies in water-soluble vitamins, particularly thiamine, and lipid-soluble vitamins such as vitamin D.2 Decreased serum vitamin D levels182 and high prevalence of osteodystrophy183 are seen in patients with cirrhosis. Supplementation of vitamin D may improve survival in vitamin D–deficient patients with cirrhosis.184
At autopsy, histological features of Wernicke's encephalopathy were found in a quarter of patients who died of alcoholic cirrhosis and HE.185 It is advisable to give thiamine to malnourished patients with chronic alcohol intake prior to glucose administration as it can precipitate Wernicke's encephalopathy.186,187
Reduced magnesium content in muscle (with normal serum magnesium levels) have been correlated with presence of HE.188 Zinc deficiency is common in patients with cirrhosis; however, the data are not convincing that zinc supplementation is beneficial in these patients.189
Consensus statements
-
23.
In patients with cirrhosis, micronutrient and vitamin deficiency, if identified clinically or by laboratory tests, should be corrected. (Level of evidence – low; grade of recommendation – strong)
-
24.
Fat-soluble vitamin supplementation is advisable to prevent deficiency in chronic cholestatic conditions. (Level of evidence – low; grade of recommendation – weak)
-
25.
Water-soluble vitamin supplementation is advisable to prevent deficiency in alcohol-related liver disease. Level of evidence – low; grade of recommendation – weak)
Feeding methods: oral/enteral/parenteral
Patients with CLD may be candidates for supplementation of diet either by the enteral or parenteral route. The oral route is preferred for its many benefits – easy, cheap, less complications, more physiological. However, if adequate calories are not met via the oral route then the enteral route should be tried after due consideration given to the presence of varices, ileus and coagulopathy. The use of the parenteral route should be considered if enteral feeds are not an option.
While a meta-analysis of enteral nutritional supplementation has not shown any reduction in mortality, some studies either had very sick patients or a very short intervention duration, which may have impacted the potential for benefit.190 Meta-analyses of nutritional supplementation in patients with alcoholic liver disease and alcoholic hepatitis (AH) showed a trend towards a better nitrogen balance with parenteral nutrition.191,192
While both parenteral nutrition and early enteral nutrition (EN) after liver transplantation may be effective with regard to the maintenance of nutritional state, EN has been shown to reduce complication rates and costs.193 Early EN (12 h after liver transplantation) may be associated with fewer viral infections and better nitrogen retention.194
A contentious situation, is nasogastric tube feeding in patients with varices, especially after a recent variceal bleed and variceal ligation. While a study has shown no adverse effect of EN, it has been recommended to withhold EN for 48–72 h after acute bleeding.195,196
Approach to the management of sarcopenia in patients with cirrhosis
Sarcopenia in cirrhosis should be treated by a combined approach based on adequate energy and protein dietary intake, oral nutrient supplementation when needed and regular physical exercise. Additional pharmacological therapy has also been proposed in some circumstances.197
Patients with cirrhosis experience protein depletion and require an increased amount of protein to achieve positive nitrogen balance.198, 199, 200 Increased protein intake is generally well tolerated and safe in cirrhotic patients and has been shown to improve protein anabolism.201
Vitamin D deficiency has been associated with sarcopenia in older adults.202 Vitamin D has also been shown to preserve muscle mass203 or ameliorate the low-grade inflammatory syndrome in sarcopenic older individuals when associated with leucine-enriched whey protein204 and has been recommended in all patients with cirrhosis with low vitamin D levels.2
Chronic hyperammonemia (HA) has been shown to be involved in the pathophysiology of sarcopenia in patients with liver cirrhosis.205,206 Ammonia-lowering treatments may theoretically be of advantage for improving sarcopenia.207,208 In one study, l-carnitine supplementation suppressed the progression of sarcopenia and was associated with the improvement of HA in patients with liver cirrhosis.209
Testosterone levels have been found to be decreased in men with advanced CLD and are associated with decreased muscle mass, increased risk of mortality, need for liver transplantation and increased risk of major infections.210 In a randomized controlled trial (RCT), testosterone therapy in men with cirrhosis and low serum testosterone safely increased muscle mass, bone mass and haemoglobin in the treated group.211
Immunonutrition is another aspect of nutritive modulation and involves modification of either activation of immune system or the consequences of activation by nutrients or specific foods.212 Major nutrients that fall under this category include amino acids (glutamine and arginine), fatty acids (mega-3 fatty acid supplements) and nucleotides. The use of immunonutrition for patients with cirrhosis has been a matter of debate. Most of the studies have looked at use of arginine-enriched, glutamine-enriched, or w-3 fatty acid–enriched supplements in these patients. The studies have shown variable results, and consistent with existing guidelines,2 there is insufficient evidence for prescribing their use in cirrhosis.213
Advice regarding exercise in cirrhotics and post liver-transplant patient
Patients with cirrhosis have very high rates of frailty, sarcopenia and deconditioning. In a systematic review of 1107 patients awaiting liver transplantation cardiopulmonary exercise test results were pooled. Despite a mean age of 55 (SD 3.2) years, the weighted mean peak VO2 of participants was 17.4 ml/kg/minute a value corresponding to expected VO2 levels of a sedentary female in the eighth decade of life and below the threshold required for full and independent living.214 Notably, patients with cirrhosis have amongst the highest levels of physical inactivity, spending ∼76% of their waking hours in the sedentary state.215 In comparison with other organ failure populations (e.g. lung, heart), the evidence to support exercise training in patients with cirrhosis is still in its beginning stages.216
Physical exercise was previously discouraged in patients with cirrhosis due to the fear of increasing portal hypertension or ammonia levels.217, 218, 219 There is accumulating evidence about the potential beneficial effect of regular physical activity in these patients.220, 221, 222, 223, 224 A program of physical activity has been able to increase aerobic capacity and improve sarcopenia. Resistance exercise may also be essential to the preservation of lean body mass and bone density in obese cirrhosis patients who are undergoing weight loss. To perform a program of physical activity in patients with cirrhosis requires some key considerations: an accurate examination of feasibility considering cardiovascular or pulmonary contraindication, presence of HE, tense ascites, frailty, history of falls, risk of GI bleeding; personalized program starting with a progressive approach from very light exercise to mild–moderate intensity physical activity including counselling to increase patient's motivation to the tasks to be reached and adequate energy dietary assumption before starting to exercise.216,220 It has been proposed the final target to be a total of 150 min exercise per week to be reached gradually.216 Both aerobic and resistance training may be combined in different proportion to favour either aerobic or sarcopenia improvement.216
What have the exercise studies shown in cirrhosis?
The last 5 years have seen a growing number of studies evaluating the effects of exercise in cirrhosis.225, 226, 227, 228, 229, 230, 231 To date, studies have had modest sample sizes with programs of up to 14 weeks of activity and a predominance of patients with compensated cirrhosis. Both supervised and home-based exercise studies have been carried out. The exercise studies have not reported significant adverse events. Notably, across individual studies, the short training durations have been associated with significant improvements in peak VO2 levels, aerobic endurance as measured by the 6-min walk test, muscle mass as measured by anthropometrics and thigh ultrasound, quality of life, reductions in fatigue and increases in muscle strength. Several studies have associated the exercise interventions with a reduction in the hepatic venous pressure gradient from the beginning to the end of the study.228,229 A recent Cochrane review of six RCTs did not find a clear pooled benefit of exercise on morbidity, mortality or health-related quality of life.232
What steps can clinicians follow to prescribe exercise in cirrhosis?
If clinicians do not have ready access to an exercise professional to assist them to prescribe exercise, they can follow three steps outlined in a recent review to initiate a basic exercise prescription in their patients:216
-
i)
Screen for safety to exercise – If activities are kept to the demand of a brisk walk, routine pre-participation cardiac clearance is not required. Patients at high risk of falls require supported activities and ideally should be supervised by a caregiver.
-
ii)
Baseline assessment of sarcopenia/frailty – As described in sections above, an objective baseline assessment allows for an accurate assessment of response to the intervention.
-
iii)
Formulate an exercise prescription – This can be based on the FITT (Frequency, Intensity, Type, Time) format dividing activity components into aerobic, resistance and flexibility/balance with overall time increasing as the patient becomes more comfortable. Although patients will often start at a more basic level, eventual targets include 150 min per week of aerobic activity and 2 or more days per week of resistance, flexibility and balance work at an intensity of 4–5 on a 10-point Borg scale. Resistance, flexibility and balance exercises can range from supported to more advanced with examples provided online at the www.wellnesstoolbox.ca.216
After liver transplantation, overweight and obesity are common and sarcopenia and frailty persist in most patients. More evidence is required to evaluate the optimal regimen and effects of exercise in the post-transplant setting.233
Consensus statements
-
26.
Individual exercise studies in cirrhosis have demonstrated beneficial effects on exercise capacity (VO2), muscle mass, muscle strength, the hepatic venous pressure gradient and quality of life. Larger high-quality studies with global representation, longer follow-up and inclusion of CTP B and C patients are required to clarify the extent of the pre- and post-transplantation effects (level of evidence – moderate).
-
27.
Wherever possible, all patients with cirrhosis should be given an exercise prescription consisting of recommendations for frequency, intensity, type and time of activity including aerobic, resistance and flexibility/balance components. The tenant of ‘start low and go slow’ can be used to advance exercise in this population. Those patients at fall risk require consideration for supervised, supported activities (level of evidence – moderate; grade of recommendation – strong).
-
28.
After liver transplantation, overweight and obesity are common and sarcopenia and frailty often persist. Exercise therapy in conjunction with nutritional counselling is advised (level of evidence – moderate; grade of recommendation – strong).
Ideal nutritional supplements
Over the last 20–25 years, Indian markets and pharmacies have been flooded with a plethora of EN supplements or formulas. There are very few RCTs in support of their use for most of the formulas.234, 235, 236 The nutritional formulas can be polymeric (generic or disease specific), pre-digested formulas (elemental or semi-elemental) or modular formulas. While commercially available supplements give a more accurate delivery of nutrients than food, the cost of the supplements may be a deterrent in resource constraints.
Standard polymeric formulas
The nutrient composition of these formulas matches a typical diet consumed by healthy individuals. These formulas are nutritionally complete, by and large contain ‘intact’ or ‘non-hydrolysed’ nutrients and are best suited for patients with an intact and functional digestive tract. Most of these formulas are lactose-free and gluten-free with an osmolarity close to the physiological range ∼300 mOsmol/litre. They contain ∼40–60% of carbohydrates as their main macronutrient. Most commonly used sources of carbohydrates are maltodextrin, corn syrup solids, hydrolysed starch, fructose, sugar alcohols and sometimes sucrose. Some formulas may contain starch; however, this may decrease the solubility of the formula. The addition of sucrose usually increases the palatability of a formula. Standard polymeric formulas contain intact or whole proteins in the order of 15–25% of the total energy; hence, they require normal levels of pancreatic and digestive enzymes for digestion and absorption. The total content of protein may range from 30 to 80 g/L with a non-protein calorie to nitrogen ratio (NPC:N) between 75:1 and 200:1. Most common sources of protein include milk proteins (casein, delactosed lactalbumin, whey protein concentrates), soy protein isolates, egg white albumin and peanut protein hydrolysate. Addition of fat to the enteral formulas provides not only a concentrated source of energy but also a source of essential fatty acids and even helps in regulating the osmolarity of the formula. Lipids are present in the concentration ranging from 25 to 40% of the total calories mainly as triglycerides of long-chain or medium-chain fatty acids. The sources of lipids could be corn oil, soybean oil, sunflower oil, canola oil, palm oil or even some amounts of medium-chain triglycerides (MCTs). If a patient is fed on exclusive tube feeding with these formulas then typically a 1–1.5 L of the standard formula would meet 100% of the recommended dietary allowances (RDAs) of most vitamins and minerals. However, certain formulas may contain increased amounts of some micronutrients like copper, sodium, potassium etc., which may require close monitoring and clinical judgement.
Monomeric and oligomeric formulas
Monomeric formulas
These formulas are also called the elemental formulas (a misnomer, as their chemical composition does not have simple elements C, N, O). These formulas contain individual amino acids, glucose, oligosaccharides and low amounts of lipids (2–3% of calories) in the form of MCTs or even essential fatty acids along with essential micronutrients like minerals, vitamins and trace elements. Nonetheless these products have high osmolarity (∼500–900 mOsmol/L); hence, they may cause increased losses of fluids in some cases of short bowel syndrome etc.
Oligomeric formulas
These formulas are also called semi-elemental formulas. The macronutrients of these formulas unlike the polymeric formulas have been enzymatically hydrolyzed or pre-digested to promote easy digestion and absorption. These are usually lactose-free and gluten-free but have a high osmolarity. These formulas typically have peptides of varying chain length (mostly as dipeptide or tripeptide), simple sugars, glucose polymers (disaccharides and maltodextrin) or fat primarily as MCTs) or even omega 3 and omega 6 essential fatty acids. They contain the recommended doses of all micronutrients. The osmolarity of semi-elemental diets is lower than elemental formulas. These formulas are best suited for patients with inflammatory bowel disease, short bowel syndrome, fistulas, radiation enteritis in cancer patients, pancreatitis or critically ill patients. However, these semi-elemental formulas often have poor taste (caused by amino acids), and higher costs (∼400% more than polymeric formulas),237 and may cause complications like osmotic diarrhoea due to high osmolarity. These products may even hamper glycemic control due to a rapid gastric emptying rate.238 Routine use of elemental or semi-elemental formulas is not recommended except in cases of malabsorption, pancreatic dysfunction, chyle leak or other evidence of GI diseases. Hence, these formulas are preserved for patients who have failed a trial of standard polymeric formulas.236
Hepatic formulas
Characteristically, these formulas have lower percentage of total protein and electrolytes, a higher percentage of carbohydrates, high calorie to nitrogen ratio (>180:1) and high MCT in the lipid fraction (MCT:LCT ratio 70:30 compared with standard formula with a ratio of 20:80). These products may also contain S-adenosyl methionine (SAMe), taurine and carnitine. These are calorie-dense formulas, which have higher proportions of BCAA and low levels of aromatic amino acids (AAAs) and methionine. It is well known that protein should not be restricted in patients with cirrhosis due to the rampant problem of reduced muscle mass. Addition of BCAA to the formulas increases the overall cost. Addition of SAMe, taurine and carnitine act as a precursor for the synthesis of glutathione, protect against xenobiotic injury and act as essential components for the beta oxidation of fat, respectively.
Clinical scenarios requiring special considerations in a patient with cirrhosis
Nutritional treatment options for hepatic encephalopathy
Malnutrition and sarcopenia directly play a significant role in development of recurrent and overt HE apart from predisposing patients to infections.55,84,239, 240, 241 Hence, the nutritional management options in HE should focus on prevention and/or delaying progression of sarcopenia, long-term ammonia-lowering drugs/dietary interventions and supplementation of micronutrient deficiencies. The pertinent questions for nutritional treatment options for HE are given below.
Is there any benefit of tailored nutritional therapy in cirrhosis with HE?
Maharshi et al.242 in a RCT, in 120 patients with minimal HE (MHE), showed that nutritional intervention (30–35 kcal/kg IBW/d, 1.0–1.5 g vegetable protein/kg/d for 6 months) improved neuropsychiatric performance in these patients with MHE and decreased their risk of developing overt HE compared with no nutritional intervention.
Hence, all patients with cirrhosis and HE should undergo detailed nutritional assessment via anthropometric and imaging tools available and should be recommended and followed up with adequate nutritional therapy and advised as opposed to normal diet, by a multidisciplinary nutrition support team.
Is caloric requirement different in patients with cirrhosis with HE versus cirrhosis alone?
Cirrhosis is a hypercatabolic state and energy requirements in patients with cirrhosis per se and cirrhosis with HE are considered to be the similar.177 Higher protein content should be given for breakfast as it improves cognitive function in cirrhotic patients with cognitive impairment.243
Is there any role of protein restriction?
In 1950s, based on largely uncontrolled observations, restriction of protein intake in patients with cirrhosis became an accepted standard of care. In 2004, Cordoba et al.244 assessed hospitalized patients with cirrhosis and HE who received different amounts of dietary proteins. Authors concluded that a normal-protein diet was safe and did not exacerbate HE and suggested that low-protein diets should be abandoned. In light of this evidence, nutrition guidelines then proposed that protein restriction should be avoided in patients with HE as protein requirements are increased in cirrhosis. Studies also showed that cirrhotic patients are able to use up to 1.8 g/kg IBW/d of protein.245 In a study of plasma amino acid response to meals in patients with liver cirrhosis, while there was accumulation of some amino acids in response to a high protein meal in patients with decompensated cirrhosis, it did not precipitate HE.246
Does source of protein matter: vegetable/dairy proteins versus animal proteins?
Vegetable/dairy proteins are presumably better tolerated than animal proteins in patients with advanced liver disease and cirrhosis as they have low levels of ammonia-genic amino acids like methionine and AAAs like tryptophan, phenylalanine and tyrosine.247 Bianchi et al.248 in their randomized, crossover comparison study, associated vegetable protein with improved nitrogen balance, increased average daytime integrated blood glucose (BG) and improved clinical grading of HE. Vegetable protein with an abundance of dietary fibre can increase nitrogen incorporation and elimination by the gut by decreasing the intestinal transit time and increasing the intraluminal pH and faecal ammonia excretion.249,250 Moreover, dairy (casein) proteins may be better tolerated than are proteins from mixed sources in patients with HE. Gheorghe et al.249 reported improvement of HE using a modified high-calorie, high-protein diet. Vegetable- and milk-derived protein was initiated to ensure that an adequate energy requirement of 30 kcal/kg/d and protein requirement of 1.2 protein g/kg/d were met. This high-calorie, high-protein diet improved mental status in about 80% of the study population. However, evidence emerging from clinical studies is not yet conclusive, primarily due to the heterogeneity of the diets used, the small number of patients treated, their different clinical conditions and the poor assessment of encephalopathy. Moreover, long-term vegetarian diets are often associated with insufficient calcium, iron, energy and protein intake.251
Therefore, patients may be recommended to increase their intake of vegetable proteins. However, to meet protein requirements, these may need to be supplemented with the consumption of BCAA or other high biological value proteins such as eggs, lean animal meats such as fish, chicken, turkey and dairy, while avoiding excessive red meat consumption.252
Role of supplementation with oral BCAA
Plasma levels of BCAAs (leucine, isoleucine, valine) are decreased as part of a deranged amino acid homeostasis in cirrhosis. There is increasing evidence for BCAAs being beneficial in HE by their effect on ammonia detoxification outside the liver via effects on skeletal muscle protein synthesis.253 A recently published Cochrane review,254 assessing the effects of BCAAs on HE in cirrhosis included 16 RCTs comprised of 827 participants with HE classified as OHE (12 trials) or MHE (four trials). Seven trials assessed intravenous BCAAs, and eight trials assessed oral BCAA supplements. The control groups received placebo/no intervention (2 trials), diets (10 trials), lactulose (2 trials) or neomycin (2 trials). The meta-analyses showed that BCAAs have a beneficial effect on HE manifestations with an NNT of five patients and a relative risk reduction to 0.73. BCAAs had no effect on mortality. The evidence associated was oral but not intravenous BCAAs with beneficial effects. In sarcopenic patients with cirrhosis, in addition to the beneficial effects on HE, the muscle build-up resulting from BCAAs may carry important improvements in daily living and quality of life.
Consensus statements
-
29.
Restriction of protein intake is detrimental to already malnourished cirrhosis patients and is not recommended. (Level of evidence – moderate; grade of recommendation – strong)
-
30.
There is weak evidence that vegetable proteins are better than animal proteins. To meet protein requirement, vegetable proteins may need to be supplemented by BCAA or animal proteins. (Level of evidence – weak; grade of recommendation – strong)
-
31.
BCAA supplementation (leucine rich) is recommended to reach adequate dietary nitrogen intake. (Level of evidence – moderate; grade of recommendation – weak)
-
32.
Oral dietary intake is preferred in patients with early HE and in those who can tolerate recommended intake. In patients with advanced HE or with protected airways and in those who are not able to take recommended intake, nasogastric tube feeding or parenteral nutrition should be considered. (Level of evidence – moderate; grade of recommendation – strong)
Nutritional treatment options for critically ill patient with cirrhosis
Patients with cirrhosis who have ACLF, are septic or in HE or have spontaneous bacterial peritonitis (SBP) or hepatorenal syndrome and are being managed in ICU, have higher nutritional requirements due to a net catabolic state.255,256 Further, their nutrient intake can be compromised because of additional factors such as vomiting, GI bleeding, SBP and ileus, hours of fasting for procedures, protein restriction in advanced encephalopathy and inadequate tube feeding protocols. For these reasons, the nutrient requirements for critically ill patients with cirrhosis are higher, similar to any sick ICU patient,257 and should be replenished as a priority.
TEE and REE are increased in cirrhotics.100 Although there are no direct studies comparing the TEE or REE in critically ill patients with cirrhosis, it is anticipated that patients admitted to ICU with complications will have a net catabolic state and therefore the caloric requirement would be higher. One study has documented that higher REE in patients with cirrhosis (a proportion of which had severe liver disease) was associated with lower survival.258 The recommended calorie intake in critically ill patients with cirrhosis should be at least 30–35 kcal/kg/day. The recommended protein intake in critically ill patients with cirrhosis should be at least 1.2–1.5 gm/kg/day.
Nutritional treatment options for alcoholic hepatitis
Among all aetiologies of CLD, malnutrition is most common among patients with alcoholic liver disease and almost all patients admitted with AH have malnutrition.259 Overall, AH has a high mortality.260 Combination of malnutrition,261 infections262 and organ failure263 determines the outcome in AH.
The first and most important step in the management of AH is abstinence from alcohol. Data from the STOPAH trial264 suggest that abstinence is associated with better survival among AH patients compared with those who reduce drinking or continue drinking.
A study from the United States, which included Veterans, reported higher mortality (>80%) among AH patients with total calorie intake less than 1000 kcal/d, compared with those consuming >3000 kcal/d. The risk of mortality varied inversely with the daily calorie consumption.259 In an RCT, 6 months of survival was shown to be similar among patients who received intensive EN plus methylprednisolone compared with those who received conventional nutrition plus methylprednisolone. Importantly, a greater proportion of patients who consumed daily calories <21.5 kcal/kg/d died, compared with those who consumed more calories (65.8% vs. 33.1%, P < 0.001).265
In an RCT, patients received either enteral (n = 35) nutrition (2000 kcal/d) or prednisolone (n = 36) 40 mg/d for a total of 28 days.266 The patients were followed up for a 1-year duration. Overall, on the intention to treat analysis, the mortality was similar in both groups (31% vs. 36%). Importantly, patients randomized to enteral feeding died early (median 7 days) compared with those receiving steroids (median 23 days). The mortality during follow-up was higher in patients randomized to steroids compared with those who received EN (37.0% vs. 8.3%, P = 0.04). The higher mortality among the steroid group was predominantly due to infections. A metanalysis of seven RCTs did not show survival benefit among AH patients with nutritional supplementation compared with a normal balanced diet.267 However, there was a significant improvement in HE with nutritional supplementation.
Vitamin A, folic acid, thiamine, pyridoxine, vitamin B12, vitamin D and vitamin E are commonly deficient in alcoholics, and these need to be supplemented. Apart from vitamins, supplementation of elements such as iron, calcium, magnesium, phosphorous, selenium and zinc should be done when appropriate.
Consensus statements
-
33.
Patients should be counselled for complete abstinence of alcohol. (Level of evidence – moderate; grade of recommendation – strong)
-
34.
Assessment for nutritional deficiencies – calories, proteins, vitamins and minerals should be done in all patients. In the presence of deficiencies, supplementation should be provided. (Level of evidence – moderate; grade of recommendation – strong)
Nutritional support in gastrointestinal bleeding
Traditionally, early feeding after gastrointestinal (GI) bleeding had been avoided due to risk of rebleeding and the risk of precipitating encephalopathy by increased protein load in GI bleeding.
It was believed that early feeding could cause a shift in blood flow to the splanchnic circulation, which could increase portal pressure and increase risk of variceal rebleeding.268 However, in an RCT, there was no difference in rebleeding, nutritional status, liver function, duration of hospital stay and mortality among those with and without nasogastric tube feed for 4 days immediately after bleeding when observed over a follow-up of 35 days.269 A recent meta-analysis of five trials involving 313 patients concluded that compared with delayed EN, early EN group had similar rebleeding rate and mortality but reduced hospitalized days.270 In patients with high risk of rebleeding, patient may be kept nil orally for 24–48 h if a repeat endoscopic procedure is anticipated.
The second concern about feeding after a variceal bleed is that the increased protein load in the intestines may lead to increased ammonia production and precipitates HE. Brief protein restriction may be advisable in patients where persistent bleeding leads to prolonged HE.2 Parenteral nutritional supplementation is rarely needed but is likely to have a beneficial role during prolonged periods of poor oral intake in refractory GI bleeding or associated ileus.
Consensus statements
-
35.
Early EN within 24 h after GI bleeding may reduce hospital stay without higher risk of rebleeding and mortality compared with delayed EN. After treatment for bleeding, patients at low risk for rebleeding can be fed early. (Level of evidence – moderate; strength of recommendation – weak)
-
36.
Protein restriction is usually not advisable but may be considered in those with refractory bleed–related persistent HE. (Level of evidence – low; strength of recommendation – weak)
Salt intake in ascites/hyponatremia
Salt restriction is the main stay of ascites management because cirrhosis, portal hypertension resultant splanchnic vasodilatation and neuro-humoral responses, induce positive sodium balance due to impaired renal sodium excretion.271 But the major issue with salt restriction is higher risk for malnutrition as there is poor intake due to impaired palatability.272 Also, strict salt restriction (<5gm/day) is associated with higher incidence of hyponatraemia and diuretic-induced renal impairment.273 With moderate salt restriction (5–6 gm/day) the development of above is hyponatremia, and renal impairment is less frequent, but the dose of diuretics required is higher.274
Hyponatremia in cirrhosis can be hypervolemic or hypovolemic. Intravenous normal saline along with diuretic withdrawal is the main treatment in hypovolemic hyponatremia. Fluid restriction is needed in hypervolemic type. Hypertonic saline is indicated only in cases presenting with life-threatening complications (seizures, cardiopulmonary distress or coma). The use of vaptans is restricted to clinical trials only.2
Nutritional management of patients with cirrhosis around liver transplantation and non-transplant surgery
Nutritional management of a waitlisted patients is as important a component of patient management as prescription medicines. Nutritional rehabilitation of waitlisted patients is similar to that of the general recommendations. For patients who have a long wait list period [1–3 months or longer, which is generally the case in most deceased donor programs in the country], the effect of dietary intervention should be assessed using objective parameters such as gait speed, 6 min walk test, improvement in frailty scores, HGS and anthropometric measurements.
Post liver transplant, patients should be initiated on early enteral feeds in the post-operative period [less than 12 h after surgery]. In the first 48 h after liver transplant, the caloric intake should be pegged at 20 kcal/kg IBW/day, although the protein intake should be maintained at 1.2–1.5 gm/kg/day. ParEN should be reserved for those in whom the bowel cannot be engaged. The negative nitrogen balance of a cirrhosis patient persists after the liver transplant, and this may last for 12 months post-liver transplant. After liver transplant, the general well-being and increased oral intake allows components of metabolic syndrome to get well and truly established, while the poor physical activity scale and ongoing negative nitrogen balance does not permit a commensurate increase in muscle mass. This syndrome of sarcopenic obesity with one or more components of metabolic syndrome should be looked for and be a part of active intervention, apart from regular immunosuppression.
Elective non-liver transplant surgery is rarely performed in patients with decompensated CLD. In situations such as elective umbilical hernia repair, hepatectomy for HCC, surgery for diverticular disease of the colon, the patient may benefit from nutritional intervention or rehabilitation. In addition, importance should be given to physical exercise for improving muscle strength. For early recovery after surgery, there should ideally be shortening of period of fasting pre-operatively, specific avoidance of opiates for pain management and early mobility after the procedure.275
Consensus statements
-
37.
Nutritional status of patients with cirrhosis waitlisted for liver transplantation influences the waitlist mortality as well as post-transplant complications and survival. (Level of evidence – high; strength of recommendation – strong)
-
38.
Diagnosis of sarcopenia and frailty should be an integral part of pre-transplant assessment, as they affect post-transplant outcome and are amenable to correction while the patient awaits transplantation. (Level of evidence – high; strength of recommendation – strong)
-
39.
Patients with cirrhosis who are awaiting non-transplant surgery should be nutritionally rehabilitated if the surgery is elective. (Level of evidence – moderate; grade of recommendation – strong)
Approach and management of obesity in patients with cirrhosis
Management of obesity in patients with cirrhosis may differ depending on the disease severity (compensated or decompensated). In compensated cirrhosis, weight reduction is largely based on lifestyle modifications with no data on the use of anti-obesity drugs and a small amount of data on bariatric surgery. A weight reduction of 5–10% is usually an adequate goal.2 Due to the risk of worsening sarcopenia, only moderate calorie restriction (500–800 Kcal/day) is recommended while maintaining adequate protein intake (>1.5 g/kg ideal BW/day).2 There is lack of data regarding the type and intensity of exercise; because of the risk worsening of portal pressure with increasing abdominal pressure, provided varices are surveyed for and managed, moderate intensity exercise (a combination of aerobic and resistance), tailored as per the patients’ ability can be recommended in patients with compensated cirrhosis with obesity.276,277 There are no data on the use of anti-obesity drugs like orlistat, lorcaserin, phentermine, bupropion-naltrexone, liraglutide or newer drugs in patients with compensated cirrhosis; hence, these cannot be recommended in this population.
Of the various bariatric surgeries, there are data regarding the use of laparoscopic sleeve gastrectomy (LSG), adjustable gastric banding and Roux-en-Y gastric bypass in patients.278 LSG is usually the preferred bariatric surgery in patients with cirrhosis because of some evidence of less operative time and reduced morbidity, availability of gastric tube for future endoscopic interventions to tackle varices and access to biliary tract post liver transplant and better absorption of drugs.279,280 Bariatric surgery with standard indications has been done in patients with either known compensated cirrhosis or cirrhosis detected incidentally at the time of bariatric surgery. Sharpton et al.281 have reported good results of sleeve gastrectomy in 32 obese patients with decompensated cirrhosis who were liver transplant candidates.
Obese patients with decompensated cirrhosis subjected to liver transplantation have high peri-operative morbidity and mortality, hence, ideally would need weight reduction prior to liver transplantation. Even though practically difficult, lifestyle modifications can be individualized in obese patients with decompensated cirrhosis. Only one case report has shown the efficacy of the anorectic anti-obesity drug lorcaserin (selective 5-HT2c [serotonergic] receptor agonist) in a patient with decompensated cirrhosis prior to liver transplantation.282 Hence, the only option in obese patients with decompensated cirrhosis would be the endoscopic bariatric therapy and bariatric surgery done before, during or after the liver transplantation. Of the various endoscopic bariatric therapies, there is only one report on the use of intra-gastric balloon in cirrhotic patients with obesity prior to liver transplantation. Eight patients with either decompensated cirrhosis (n = 7) or HCC (n = 1) listed for liver transplantation with BMI >40 kg/m2 or BMI between 35 and 40 (with a low graft to recipient weight ratio) underwent intragastric balloon placement and dietary counselling. All patients except one had weight loss, and five of them had successful liver transplantation. None of patients had any serious complications, and three of five patients maintained weight loss post-transplant as well.283 Intra-gastric balloons, however, cannot be placed in all patients with cirrhosis and are contraindicated in those with large or high-risk oesophageal or gastric varices, severe coagulopathy, ulceration, in addition to the usual contraindications like prior gastro-oesophageal surgery, large hiatal hernia, oesophageal stenosis or motility disorders and unwilling for modified diet and behaviour modification. There are no data on the use of endoscopic gastroplasty techniques like endoscopic sleeve gastroplasty and primary obesity surgery endoluminal in for the treatment of obesity in compensated and decompensated cirrhosis. Even though small intestinal endoscopic devices like endobarrier, gastrodudeonojejunal bypass sleeve, duodenal mucosal resurfacing and self-assembling magnets look promising in non-cirrhotic obese patients, there are no data of their use in patients with cirrhosis. Morbidity and mortality in patients with cirrhosis undergoing bariatric surgery is higher than patients without cirrhosis and further increases in patients with decompensated cirrhosis.284,285 Thus, the decision to perform bariatric surgery before, during and after liver transplantation in patients with decompensated cirrhosis has to be individualized.286, 287, 288, 289
Dietary modification and exercise are the cornerstone of management of patients with NASH. A reduction in the consumption of saturated fatty acids, total fat, trans-fatty acids and fructose is recommended. Mediterranean diet appears to beneficial in patients with NASH.290 Mediterranean diet has a high intake of olive oil, which is rich in monounsaturated fatty acids, nuts, fruits, legumes, vegetables and fish, and a low intake of red meat and high sugar food. However, Mediterranean diet also includes red wine in moderation, which cannot be recommended in patients with cirrhosis. The Mediterranean diet was also associated with a lower risk of liver cancer.291
Weight losses of at least 5% can improve hepatic steatosis with a weight loss of 7–10% necessary to have significant improvements in the liver histology of obese and overweight patients with NASH.292 However, there is less evidence in NASH with cirrhosis, and these patients are at increased risk of debilitation and frailty.
Consensus statements
-
40.
A weight reduction of 5–10% is usually adequate in obese patients with cirrhosis and can be achieved with moderate calorie restriction with adequate protein intake and exercise as per patient's ability (Level of evidence – Low; grade of recommendation – weak)
-
41.
Because of the lack of data, anti-obesity drugs cannot currently be recommended for use in patients with cirrhosis (Level of evidence – low; grade of recommendation – strong)
-
42.
In the absence of contraindications, endoscopic bariatric therapy with intra-gastric balloon can be attempted in obese patients with decompensated cirrhosis prior to liver transplantation (Level of evidence – low; grade of recommendation – weak)
-
43.
Bariatric surgery is associated with high morbidity and mortality in obese patients with decompensated cirrhosis and can be individualized before, during and after liver transplantation (Level of evidence – moderate; grade of recommendation – weak)
-
44.
Of the various bariatric surgeries, LSG is the preferred surgery in obese patients with cirrhosis (Level of evidence – moderate; grade of recommendation – strong)
Nutrition in children with chronic liver disease
Poor nutrition is observed in up to 80% of children with CLD.293,294 Infants and those with severe cholestatic liver disease are at particularly high risk of malnutrition.
Growth failure is an important predictor of survival in children with CLD and therefore has been incorporated in the paediatric end-stage liver disease (PELD) scores. Nutritional assessment in children with CLD should include physical assessment for signs of nutrient deficiency, anthropometry and biochemical tests including serum micronutrient levels.
Anthropometry in children
Malnutrition in children with CLD may be under recognized, as routine parameters as weight-for-age, height-for-age, and weight-for-height percentiles frequently overestimate nutritional status. In contrast to adults, malnutrition is often manifest as growth failure and negative impact on neurodevelopment. Weight measurement alone may be misleading and is affected by fluid retention, ascites and organomegaly. Weight in infants needs to be measured without diapers to the nearest 10 g. Older children should be measured to the nearest 100gm while wearing little or no outer clothing and no shoes.295 Height/length and weight for age can be checked on the Indian Association of Paediatrics (IAP) growth charts.296 Growth failure is manifested as < 3rd percentile (z scores < -2) for height/length, weight and weight for height (50th percentile is z score of 0). The interpretation of linear growth is improved by an adjustment based on the mid-parental height. Height/length of younger children (<2 years) responds better to nutritional intervention compared with older children.297 Pubertal status needs to be assessed for determining growth velocity in older children and adolescents. This is based on growth of pubic hair in both sexes, breast development in girls and genital development in boys.298
Nutritional status is better assessed with triceps skin fold thickness (TSFT) and mid-upper arm circumference (MUAC). These are compared with age- and height-matched normal values and are used to estimate body fat and muscle bulk, respectively. In children between 6 months and 5 years, an MUAC value less than 12.5–30 cm, respectively (z score < −2) suggests moderate to severe malnutrition299 and estimates the risk of death.300 Upper limb measurements are less affected by oedema compared with lower limb measurements. TSFT is a better indicator of acute malnutrition and wasting compared with height and weight.301
Body composition analysis has also been studied in children.302 Sarcopenia, which has emerged as an important determinant in adults, has not been well studied in children. A recent Brazilian study of 85 children with CLD assessed weight, height, muscle strength (assessed by manual grip strength) and muscle mass (estimated through dual-energy X-ray absorptiometry). Sarcopenia was diagnosed based on the simultaneous presence of muscle mass and muscle strength deficits, defined as the values below the mean for muscle mass and strength of the studied population, according to gender. Forty percent of children were found to be sarcopenic.303 There are several nutrition screening tools for assessing malnutrition in adult patients with cirrhosis, which have been studied and compared, but these have not been validated in children. Physical examination and serum levels of vitamins and trace elements further help diagnose specific nutritional deficiencies.304
Nutritional requirements in children with chronic liver disease
Many children with CLD have cholestatic liver disease especially infants, with biliary atresia being the commonest. Children awaiting transplant need specific nutritional intervention as a part of preoperative preparation as they have a significantly higher incidence of infections and surgical complications.305 The energy, macronutrient and micronutrient requirements in children with CLD are depicted in Table 3.293,304 High calorie intake 120–150 kcal/kg/day (up to 150 times the RDA for age) comprising carbohydrate 15–20 g/kg/day, protein 2–4 g/kg/day and fat 8 g/kg/day, which includes 30–50% from MCTs should ideally be provided.
Table 3.
Dailyrequirement ofnutrients in Childrenwith Chronicliverdisease.
| Constituent | Daily requirement in CLD | Deficiency manifestation |
|---|---|---|
| Calories | 130–150% of RDA∗ | Growth failure |
| Protein | 2–4 g/kg | Lower MUAC∗∗, oedema, ascites |
| Vitamin A | 1000 IU/kg/d up to 25,000 IU orally of water- soluble preparation <10-kg start with 5000 IU/d >10-kg start with 10,000 IU/d |
Bitot's spots, night blindness, dryness of eyes, corneal ulcers |
| Vitamin D | <40 kg: 120–200 IU/kg >40 kg: depending on S. 25OH vit D
|
Enamel hypoplasia; hypotonia; rachitic rosary; delayed closure of fontanelles; parietal and frontal bossing; widening of wrist and bowing of distal radius and ulna; lateral bowing of femur and tibia |
| Vitamin E | TPGS∗∗∗ 15–25 IU per kg/d α-Tocopherol (acetate): 10–200 IU/kg/d |
Delayed deep tendon reflexes; progressive ataxia; peripheral neuropathy; visual field defects; dementia |
| Vitamin K | 2.5–5 mg/day; 2–7 times weekly orally or 1–10 mg IV | Bleeding diathesis |
| Iron | 6 mg/kg/day | Anaemia |
| Calcium | 25–100 mg elemental calcium/kg/day in divided doses | Bone pains, tooth decay |
| Zinc | 1 mg/kg/day of elemental zinc | Acral dermatitis, altered taste, diarrhoea, fatigue, hair loss |
| Selenium | 1–2 mcg/kg/day | Fatigue, hair loss |
Modified from Young S et al297.
RDA∗: Recommended daily allowance, MUAC:∗∗ Mid-upper arm circumference; TPGS∗∗∗ D-alpha tocopheryl PEG-1000 succinate.
Vitamin E deficiency needs to be corrected prior to iron supplementation as it improves response; calcium citrate preferred if patient is on proton pump inhibitors.
The highest risk for hypoglycaemia is in infants because of smaller glycogen stores. Older children are able to use fat and protein as fuel sources. Therefore, infants who are kept starving, need to have frequent monitoring of BG and will require intravenous dextrose infusion if required to fast or nil per oral for durations longer than 4 h.306 Protein restriction is not warranted in most cases of CLD, except in cases of refractory encephalopathy, and even in these cases, protein restriction to <2 g/kg/d should be avoided as this will lead to endogenous muscle protein consumption. In children, there are limited data showing that BCAA supplementation may exert favourable effects on weight, fat mass, fat-free mass and serum albumin level.307 In a small RCT of 19 children comparing BCAA supplemented versus standard feeds, weight gain was seen with the BCAA feeds and not on the standard formula feed.308 In the setting of cholestasis, deficiency of fat-soluble vitamins (FSVs) (A, D, E and K) and essential fatty acids and is common since micelle formation (which needs bile) is important for absorption of these nutrients.309 In a small but significant study of 23 patients, FSV deficiency was prevalent in majority of patients with serum bilirubin >3 mg/dl and more than half of those with serum bilirubin <3 mg/dl.309,310 The deficiency and toxic limits of FSV are mentioned in Table 3.309 It has been recommended that FSV levels need to be regularly monitored as excess supplementation may lead to toxicity, especially vitamins A and D.304,309 This is challenging in countries like India due to lack of easy availability of these assays and cost. Ideally, FSVs need to be given in a water-soluble oral form to be absorbable in the setting of cholestasis. Availability of a water-soluble oral formulation of FSV mixture as in the West would make compliance better. Vitamin D deficiency in children manifests differently compared with adults. X rays reveal widening of the epiphyseal plate, cupping, splaying, formation of cortical spurs, and stippling of growth plate.293 In India, vitamin E is mostly available as alpha tocopherol, which is not water soluble. Tocotrienol may have better water solubility than tocoferol. The preferred form of vitamin E in the presence of cholestasis is D-alpha tocopheryl PEG-1000 succinate (TPGS; tocophersolan).311 TPGS forms its own micelles at low concentrations and hence might not require the presence of bile acids. This form of vitamin E also improves vitamin D absorption. Vitamin K deficiency usually manifests as a bleeding diathesis typically epistaxis, bleeding from gums and easy bruisability. Breast-fed infants with cholestatic liver disease are particularly deficient in vitamin D and vitamin K and can present with catastrophic bleeds at any site including life-threatening intracranial bleeds, and this may be the first manifestation of their liver disease.312 Oral absorption is poor and therefore the parenteral route (IM/IV) is preferred. Prothrombin time is used to assess vitamin K deficiency but identifies <50% patients with vitamin K deficiency, which is best identified by PIVKA II (protein induced in vitamin K absence).313 PFIC and Alagille patients with partial biliary diversion may be at an increased risk of developing FSV deficiency despite improvement in their cholestasis due to loss of a large amount of bile through the stoma. All fat-soluble vitamin supplements are best administered in the morning (when bile flow may be maximal), with a meal and in the absence of bile acid binding drugs (cholestyramine) or pro-oxidants (such as iron sulfate). FSV deficiency and toxic levels are shown in Table 4.
Table 4.
Fat-soluble Vitamin deficiency and toxic levelsa.
| Measured levels | Deficiency limit | Toxicity limit | |
|---|---|---|---|
| Vitamin A | Plasma retinol (mmol/L) | <1 | >3 |
| Vitamin D | 25OH vitamin D (ng/ml) | <14 | >80 |
| Vitamin E | Alpha tocoferol (mmol/L) | <23 | >80 |
| Vitamin K | INR | >1.3 | – |
From Yu-Mei Shen et al302
MCTs are more water soluble and are readily absorbed by enterocytes in the absence of micelles and are therefore the preferred source of fat. However, long-chain triglycerides (LCTs)/long-chain poly unsaturated fatty acids are needed to provide essential fatty acids (i.e. linoleic, linolenic acids), which assist in absorption of fat-soluble vitamins. These include soyabean oil, fish oil egg yolk, Canola and sunflower oil and are important for neurodevelopment in children. LCTs also improve palatability compared with MCTs. Considering this, about 30–50% of fat requirement should come from MCT.314
Water-soluble vitamins–B complex and vitamin C have limited storage in the body and need to be supplemented in children with CLD.293 Calcium and magnesium deficiency usually arise secondary to reduced vitamin D stimulated intestinal absorption of these trace elements. Iron deficiency is seen in a third of children with CLD.315 Zinc deficiency can also arise from malabsorption. Copper and manganese increase in patients with CLD as primarily excreted in bile excretion is reduced in cholestatic children.316 Water-soluble vitamins are given in the RDA doses317, and trace element nutritional requirements in children with CLD are mentioned in Table 3.
Feeding in children with chronic liver disease
Breast mild is dilute with a caloric density of 0.67 kcal/ml. A vegetarian mother would increase the risk of vitamin B12 deficiency in the child. In exclusively breast-fed children, breast milk can be expressed, fortified and refed. In older kids, MCT oil may be added to the oral diet. Nasogastric feeds through a soft fine bore tube should be considered when oral intake is inadequate. Bolus feeds to top up oral intake with continuous feed through a feeding pump at night may be considered. Nocturnal feeds have been shown to improve anthropometric indices in cholestatic children.318 If day time bolus feeds are not tolerated, continuous feeds during the day can be started. Increasing the concentration of the formula increases the osmolality and may cause diarrhoea.
In children with ascites, salt needs to be restricted. In infants and smaller children not more than 1 gm of salt (NaCl) and in older children and adolescents 2–3gm of salt or 1–2 mEq/kg/d is permissible.319 Fluid restriction is advised only in the presence of dilutional hyponatremia.
While malnutrition is the burning issue in children with CLD in most countries, the West also experiences obesity as a nutritional problem and may soon become an issue in India. Less than 15% of children who are transplanted are obese (BMI z-scores >3).320 These children had increased late (>12 years) mortality and were more likely to experience post-transplant obesity.
Consensus statements
-
45.
Nutritional assessment in a child with CLD should include a growth chart with height and weight for age; TSFT and mid-arm circumference measurement (Level of evidence – moderate; grade of recommendation – strong)
-
46.
Children with CLD need up to 150% of RDA of calories and 2–4 g/kg/day of proteins/day (Level of evidence – moderate; grade of recommendation – strong)
-
47.
In children with cholestatic CLD, fat-soluble vitamin deficiency is almost universal and supplementation is needed. MCT-containing formulas should be included to provide up to 50% of fat requirements. (Level of evidence – high; grade of recommendation – strong)
-
48.
Naso-gastric tube feeding including continuous nocturnal feeds are warranted in malnourished children with reduced oral intake and awaiting transplantation. (Level of evidence – moderate; grade of recommendation – strong)
Dietary advise for metabolic disorders causing liver disease in paediatric patients
Inborn errors of metabolism (IEMs) are disorders with an enzyme deficiency in a metabolic pathway. When the IEM is associated with hepatomegaly or abnormal liver function, it is labelled as metabolic liver diseases (MLD). The presentation of MLD is variable and encompasses the entire spectrum of clinical liver disease.321,322
The MLD in which dietary therapy has an important role in the treatment including galactosemia, hereditary fructose intolerance (HFI), glycogen storage disorders (GSDs), tyrosinemia, Wilson's disease (WD), fatty acid oxidation and carnitine metabolism defects, urea cycle defects (UCDs) and citrin deficiency.
General measures
In MLD, the enzyme deficiency results in accumulation of certain substrates that may be toxic and deficiency of some products which are essential. The dietary manipulations needed for management increase the risk of micronutrient/vitamin deficiencies requiring monitoring and supplementation. With age and growth, the dietary requirements change necessitating continuous involvement of a trained metabolic dietician. Careful monitoring of diet, clinical status (including growth and development) and biochemical evidence of both disease control (levels of both toxic and deficient substances) and specific nutrient deficiencies is essential.323 The age-based dietary requirements and nutrient composition of various food items are essential for planning the diet.324
Consensus statement
-
49.
Dietary therapy is an important aspect of management of various MLD. Involvement of a trained dietician is essential. Regular monitoring of diet, clinical status and metabolites for adequacy of disease control, along with identification of changing dietary needs with growth, infection and illness is needed (Level of evidence – moderate; grade of recommendation – strong)
Galactosemia
Galactose is a monosaccharide derived from the hydrolysis of lactose, by the lactase enzyme, in the brush border membranes of the enterocytes. Galactose thereafter is transported across enterocyte through the sodium-dependent glucose–galactose transporter.325 Three enzymes involved in the metabolic pathway by which galactose is converted to glucose include galactokinase (GALK), galactose-1-phosphate uridyl transferase (GALT) and uridine diphosphate (UDP) galactose-4-epimerase (GALE). GALT deficiency results in classical galactosemia and presents with vomiting, hypoglycaemia, cataract, progressive liver disease with ascites, haemolysis and infections. In some cases with GALE deficiency the clinical features are similar to the classical disease.326
Classical galactosemia
Presence of non-glucose-reducing sugar in urine in a patient with suggestive clinical feature supports the diagnosis but confirmation requires estimation of RBC GALT activity.
Lifelong dietary restriction of galactose is the treatment for classic galactosemia. Soy formula and elemental formula are used for infants as a replacement of the lactose containing milk formulas or breast milk. But soy formulas are not recommended for premature infants and in them, elemental formulas are preferred.327 There has been a debate on whether mature cheese should be allowed in subjects with galactosemia and studies have found most of them to be safe.328 The galactose content of pulses, fruits, vegetables, offal meats and cereals is lower than the dairy-based food.329 Also the galactose present is these food items is in a complex form which is not digestible.327 Berry et al.330 challenged galactosemia patients with additional dietary galactose in the form of fruits and vegetables for three weeks and did not find any significant change in erythrocyte galactose-1-phosphate concentrations and/or urinary galactitol excretion. In addition, it has been recognised that a substantial amount of endogenous production of galactose occurs in the body.331 Keeping the above facts in mind, the current recommendations allow intake of all fruits, vegetables, legumes, unfermented soy products, mature cheese and foods containing caseinates.327 There are no data on the exact amount of galactose that these patients may take normally at different ages. Complementary feeding is started at appropriate age with non-dairy products.
Parents must be taught the importance of reading the nutrition labels and ingredient list as many non-dairy packaged foods do contain lactose and galactose. After starting the dietary galactose restriction, the high RBC Gal-1-P (GALT) concentrations start showing a reduction which is useful for monitoring. Treatment with a galactose-free diet results in improvement of symptoms and recovery of liver function with normal growth.
As there are no controlled trials in this condition, the management varies from centre to centre. Keeping this in mind a group of experts, that is, members of The Galactosemia Network (GalNet) have developed an evidence-based and internationally applicable guideline for galactosemia.332 Patients with generalised epimerase deficiency require dietary galactose restriction similar to classic galactosemia.333
Consensus statements
-
50.
Galactose-restricted diet (milk and milk product–free diet) should be started with a suspicion of classical galactosemia. Breast feeding and regular milk-based infant formulas need to be stopped (Level of evidence – low; grade of recommendation – strong)
-
51.
Soy formulas and elemental formulas are allowed (Level of evidence – low; grade of recommendation – strong)
-
52.
Galactosemia patients need a life-long galactose-restricted diet, that is, no dairy products (Level of evidence – low; grade of recommendation – weak)
-
53.
Galactose from non-milk sources, that is, fruits, vegetables, legumes, unfermented soy-based products, mature cheeses (with galactose content <25 mg/100 g), are allowed in the diet without any restrictions (Level of evidence – low; grade of recommendation – weak)
-
54.
There are no data for age-specific recommendation of the amount of galactose allowed in the diet of a patient (Level of evidence – low; grade of recommendation – weak)
-
55.
Calcium and vitamin D should be supplemented in diet as per the age-based RDA. Annual assessment for intake along with measurement of blood levels of calcium and vitamin D should be done to assess adequacy of therapy (Level of evidence – low; grade of recommendation – weak)
-
56.
During infancy, complimentary feeding can be started at the recommended age (Level of evidence – low; grade of recommendation – weak)
-
57.
Regular follow-up with clinician and dietician is required to assess growth, development and dietary compliance. RBC Gal-1-P levels can be measured at diagnosis, and in follow-up at 3 months, 9 months and then yearly to assess dietary compliance (Level of evidence – low; grade of recommendation – strong).
Hereditary fructose intolerance
HFI is an autosomal-recessive disorder, characterized by deficiency of aldolase B enzyme. The fructose is first phosphorylated into fructose 1- phosphate by the enzyme fructokinase. This fructose 1-phosphate is then broken down by the aldolase B (fructose 1,6-bisphosphate aldolase) enzyme into triose sugars (dihydroxy acetone phosphate and glyceraldehyde).334
In patients with HFI, ingestion of large amounts of fructose leads to accumulation of fructose 1-phosphate, which causes reduction in intracellular ATP concentration and hypophosphatemia due to depletion of inorganic phosphate. Hypoglycemia occurs due to inhibition of both gluconeogenesis and glycogenolysis by the accumulated fructose 1 phosphate.335
Infants become symptomatic on first exposure to fructose in honey or sucrose (simple sugar). Continued exposure to fructose leads to vomiting, hepatomegaly, jaundice, hypoglycaemia, renal Fanconi syndrome, poor feeding, growth failure and eventually liver failure.336 Dietary removal of fructose leads to resolution of symptoms, improvement in liver functions and restoration of normal growth and development.337 Adults with HFI develop aversion to sweet foods.
Removal of all fructose, sucrose and sorbitol from the diet is the therapy for HFI. All fruits, fruit juices, honey, sorghum, palm or coconut sugar, maple syrup, etc. are to be avoided. A detailed list of foods to be avoided and those which can be consumed is available.335 Special precaution should be taken before consuming medications in syrup form as many of them contain sucrose and sorbitol.338 Similarly, many nutritional drinks also have fructose, and it is essential to check the labels for ingredients. Fructose-containing intravenous fluids need to be avoided. If required, glucose powder can be used as a sweetener. Although some people believe that the dietary restrictions may be relaxed after 2 years of age339, but there are no recommendations about age-based fructose intake in HFI patients.
Patients with HFI are at risk of micronutrient and water-soluble vitamin deficiencies due to the dietary restrictions and need supplementation (sugar-free multivitamins).339
Consensus statement
-
58.
Dietary therapy is the main stay of treatment of HFI. Fructose, sucrose and sorbitol must be completely removed from the diet of a patient with suspected HFI (Level of evidence – low; grade of recommendation – strong)
-
59.
Dietary elimination leads to recovery of symptoms, normalization of liver functions and normal growth (Level of evidence – low; grade of recommendation – strong)
Glycogen storage disease
In the human body, glucose is stored in the form of glycogen with liver and muscle being the main storage organs. The hepatic glycogen is used to release glucose during fasting and to prevent hypoglycaemia. Various IEM characterized by abnormal storage or utilization of glycogen are grouped together as GSDs and classified based on the specific enzyme deficiency and tissues affected.340 GSD type I, III, VI and IX are described as the hepatic GSD. Enlarged liver and hypoglycaemia are the hallmark features of hepatic GSD. Muscle involvement presents as muscle weakness, exercise intolerance and cardiomyopathy. Various long-term complications can occur, which highlight the need of proper treatment with good metabolic control and regular follow-up.341
GSD1: There are two types of GSD1 -
-
(i)
Ia -deficiency of glucose-6-phosphatase (G6Pase-α), more common ∼80% cases
-
(ii)
Ib-deficiency of glucose-6-phosphate transporter (G6PT), less common ∼20% cases and they have neutropenia, impaired neutrophil function and Crohn's like colitis in addition.342
In GSD1, glucose can neither be released from the liver glycogen nor produced by gluconeogenesis or glycogenolysis; hence, hypoglycaemia is most marked in GSD1 in comparison with other hepatic GSD.
Nutritional therapy is the backbone of treatment of GSD1. The nutritional requirements change with age, growth and periods of illness. Regular BG monitoring to detect asymptomatic hypoglycaemia is essential and target is to keep the BG at >70 mg/dl and avoid rapid glucose fluxes. Monitoring of BG helps in improving the metabolic control, growth and development.343 Small frequent feeds that are high in complex carbohydrates and evenly distributed both during day and night are the best.
Most experts recommend that 60–70% of calories should come from carbohydrates, 10–15% from proteins and the remaining from fat (<30% for children older than 2 years).
Due to deficiency of the G6Pase enzyme, both fructose and galactose cannot be used in GSD1 and so lactose, which has galactose, sucrose and fructose, needs to be restricted. Given the dietary restrictions, a soy infant formula may be a good choice in infants. This restrictive diet can lead to micronutrient and vitamin deficiencies including calcium and vitamin D.
Infants: – As hypoglycaemia may be associated with seizures, brain damage and even death, all efforts are targeted at avoiding this complication. The options for feeding every 3–4 h during both day and night include waking up the child and oral feeds or using nasogastric tube/gastrostomy for overnight drip feed. In our experience, parent education and understanding is vital for achieving this. Complementary feed is started similar to other infants except that fruits, juices, sucrose and dairy products need restriction.
Young child: Uncooked corn-starch (CS) is used for prevention of hypoglycemia as it is digested slowly and releases glucose over a longer period of time. As amylase is required for its digestion and infants (up to 2 years of age) have deficiency of amylase, they may not be able to tolerate CS in full doses. CS may be introduced from 6 months to 1 year of age but in small amounts in the beginning and then increased as per tolerance. Excess gas, bloating and even loose stools may occur after intake of CS, but these symptoms usually subside with continued use.344
Dose of CS is kept around 1.6 g/kg body weight every 3–4 h in young children and 1.7–2.5 g/kg every 4–6 h for older children and adolescents. Some adolescents/adults may need only a single bed-time dose (1.7–2.5 g/kg) of CS to have a BG of >70 mg/dl and lactate of <2 mmol/l throughout night.345 Detailed age-wise guidelines for feeding and CS and metabolic control are given in the 2002 European guidelines.346 Parents are instructed about recognition and treatment of hypoglycaemia. Presence of lethargy, nausea, irritability, sweating or light headedness may suggest hypoglycaemia. If these are present, sugar should be checked immediately. Both symptomatic/asymptomatic BG of <60 mg/dl needs to be treated. First glucose powder in water is given and then the regular food or CS should be given quickly.
Consensus statements
-
60.
Regular BG monitoring before feeds is recommended. BG levels should be kept at 70 mg/dl to obtain good metabolic control (Level of evidence – low; grade of recommendation – strong)
-
61.
The amount of sucrose, fructose and galactose in diet should be restricted (Level of evidence – low; grade of recommendation – strong)
-
62.
All dietary recommendations target two things: (1) prevention of hypoglycaemia and other metabolic complications and (2) adequate growth. Even overtreatment is harmful as it can cause insulin resistance. Multivitamins, calcium and vitamin D supplementation is required (Level of evidence – low; grade of recommendation – strong)
-
63.
In infants and children, feeding should be done at 3–4 h intervals, even during night. Either oral, nasogastric or Gastrostomy tube can be used. Raw, uncooked CS may be introduced between 6 and 12 months of age (Level of evidence – low; grade of recommendation – strong)
-
64.
In adolescents and adults: Avoid fasting for more than 5–6 h. Use uncooked CS and/or frequent feeds. Small, frequent meals (calories – 60–70% carbohydrates, 10–15% protein, <30% fat) should be prescribed (Level of evidence – low; grade of recommendation – strong)
GSD III
The diet in GSD III is planned according to the age at diagnosis, severity of manifestations, especially hypoglycaemia and type of GSD III (IIIa or IIIb). Type IIIa has myopathy/cardiomyopathy while IIIb does not. In infants and young children with GSD IIIa and IIIb, the aim is to give frequent feeds, avoid fasting and prevent hypoglycaemia similar to GSDI. The exact distribution of calories from carbohydrate, protein and fat in infants is still controversial. A high protein diet helps GSD type III cases by three ways:347
-
1)
As gluconeogenesis is not affected, the alanine from protein can be used as an alternate source for glucose in periods of fasting.
-
2)
High dietary protein enhances muscle protein synthesis and thus improves muscle function.
-
3)
Avoiding excess carbohydrate intake and replacing some of it with protein may help in reducing unnecessary glycogen storage.
Thus the child with GSD IIIa should be given a high protein diet.348,349 Few small studies have shown that myopathy and cardiomyopathy improves after increasing dietary protein intake in GSD IIIa patients.350,351 Although in type IIIb, the benefit of high protein is less compared with type IIIa, but it is still beneficial by mechanism 1 and 3 above. As gluconeogenesis is normal in GSD III, no dietary restriction of sucrose, fructose and lactose are needed. As dietary restrictions in GSD III are less severe than in type I, vitamin and micronutrient deficiencies are less common but still they need periodic assessment. Recommendations suggest that around 20%–30% calories come from protein, 35%–55% from carbohydrates, and 20%–35% from fat in children/adolescents.
The amount of CS needs to be adjusted as per the response. Mostly 1 g/kg body weight CS every 4 h is sufficient for maintenance of BG. However, some patients have severe hypoglycaemia similar to GSD I and they need CS in doses similar to that in type I. CS may be mixed in water or cold milk or yogurt.
Addition of extra protein to the CS, like whey protein supplement, may be helpful in maintaining the BG.352,353 The best time to check BG is before meals, before CS and first thing in the morning. Guidance regarding healthy eating habits and importance of exercise especially in patients with GSD IIIa cannot be over emphasized.
There is no specific recommendation for adults with GSD IIIa and IIIb. The risk of hypoglycaemia is less but exists, and they should consume a regular well-balanced diet with adequate proteins and avoid fasting. The practice guidelines from the American College of Medical Genetics offers detailed information about management of GSD III.347 While adjusting the dietary recommendations in follow-up, age-based RDA, severity of hypoglycemic episodes and laboratory findings (ALT, AST, creatine kinase, triglyceride, lactate, ketones) need to be considered.354
Consensus statement
-
65.
Small, frequent feeds containing complex carbohydrates and protein both in day and night with less of simple sugars. Fasting should not be done (Level of evidence – low; grade of recommendation – strong)
-
66.
If child has hypoglycaemia then corn starch to be started at 6–12 months of age (Level of evidence – low; grade of recommendation – strong)
-
67.
In adolescents and adults, a diet with high protein (25% of total calories) is important for GSD IIIa, that is, with muscle involvement (Level of evidence – low; grade of recommendation – strong)
-
68.
Fructose, sucrose and lactose need not be restricted in GSD III (Level of evidence – low; grade of recommendation – strong)
GSD VI and IX
GSD types VI and IX primarily affect the liver and are usually milder than GSD I. The enzyme glycogen phosphorylase is deficient in GSD VI while GSD XI is due to deficiency of phosphorylase kinase. There is variability in the clinical manifestations of GSD VI and IX and differentiation between them or other hepatic GSD often requires genetic/enzymatic analysis. Patients with minimal metabolic abnormality need a normal diet while those with hypoglycaemia, raised transaminases and poor growth need frequent feeds with avoidance of fasting.355, 356, 357 Diet containing around 2–3 g protein/kg body weight is advised. Similar to GSD III, lactose, sucrose and fructose are not prohibited, but the amount of simple sugars should not be excessive. About 30% of calories should come from fat including poly and monounsaturated fats.
Vitamin and mineral supplements are similar to that in type III GSD. Children can maintain BG with a dose of 1 g/kg/body weight of CS for longer periods of 4–8 h and dose of CS is adjusted as per response. The overnight dose of CS is decided based on the midnight and early morning sugar levels. Adults usually have lesser requirement of CS than children. The goal is to maintain BG between 70 and 100 mg/dl, and blood ketones between 0.0 and 0.2 mmol/L.358
Consensus statement
-
69.
Diet should be adjusted to provide, ∼45–50% of total calories from carbohydrates, ∼20–25% from proteins and ∼30% from fats (Level of evidence – low; grade of recommendation – strong)
-
70.
All meals and snacks should have complex carbohydrates and proteins including the bedtime meal (Level of evidence – low; grade of recommendation – strong)
-
71.
CS ∼1 g/kg body weight may be required at bedtime to prevent hypoglycemia in the night time. Some patients may need more frequent CS to maintain euglycemia (Level of evidence – low; grade of recommendation – strong)
-
72.
No restriction of dairy products or fruits is required but these should be consumed in moderation just like simple sugars to avoid problems of excessive glycogen accumulation and rapid changes in BG (Level of evidence – low; grade of recommendation – strong)
Tyrosinemia
Tyrosinemia type 1 (hepatorenal tyrosinemia HT-1) is an autosomal recessive condition, which occurs due to the deficiency of the enzyme fumaryl acetoacetate hydrolase that breaks down the fumaryl acetoacetate (FAA) into fumarate and acetoacetate. Blockage at this step leads to increase in FAA and succinylacetone (SA), which are responsible for the hepatic, renal and neurological manifestations of HT1.359 Diagnosis is established by demonstrating high blood/urine levels of succinyl acetone.360 The management of HT1 rests on two main pillars-use of nitisinone(2-[2-nitro-4-trifluoromethylbenzoyl]-1,3-cyclohexanedione, NTBC) to reduce the formation of toxic metabolites and a special diet to maintain the tyrosine/phenylalanine (PA) levels in the recommended range while sustaining normal growth and development. Treatment with NTBC leads to increase in plasma tyrosine level and its associated complications.361 Dietary restriction of PA and tyrosine, even if started in infancy, does not stop the renal and hepatic complications of HT1, that is, dietary therapy alone does not work for HT1. Weight gain in childhood, infections and dietary lapses often result in big fluctuations in the tyrosine levels and so regular and lifelong adherence to the diet is needed to prevent complications (keratitis, impaired cognitive development) of high tyrosine levels.362
Nutritional intake should meet the age-appropriate calorie and nutrient requirements as per RDA. Nearly three-fourth of dietary PA is converted to tyrosine and so it is important to restrict both PA and tyrosine in the diet.363 This is possible by restricting the normal dietary protein and replacing it with special protein supplements free of tyrosine and PA. The total required protein intake is calculated based on the RDA with ‘some’ extra amount as the proteins from medicinal foods have a slightly lower absorption.364 The two types of food, normal diet and special food together should supply the required energy, carbohydrate, fat, proteins and also vitamins and other micronutrients. It is suggested that young infants should be given a diet with ∼120 kcal/kg/day and 3.5 g/kg/day of protein from special formulas. Normal infant formula or breast milk is adjusted to provide 185–550 mg/day of PA and 95–275 mg/day of tyrosine. Excellent review of age-based protein from normal diet and special food, tyrosine and PA content of food items are available for planning the diet.365 In general, carbohydrate rich and low-protein vegetarian food are allowed in the diet while monitoring and targeting a tyrosine concentration of 200–600 μmol/L (normal: 35–90 μmol/L) and PA concentration of 20–80 μmol/L.366,367 Experts feel that additional protein from milk or other items should be added if the blood PA levels fall to <20 μmol/L.366 Some clinicians suggest using PA supplements to correct these low levels.367 Dietary management of HT1 is very complicated and requires a team of trained dieticians and clinicians with experience.
Consensus statement
-
73.
Dietary therapy and medication (NTBC) should be started as soon as diagnosis is made (Level of evidence – moderate; grade of recommendation – strong)
-
74.
Dietary protein intake should be restricted, and protein should be replaced by tyrosine and PA-free special formula/supplements with a target to maintain plasma tyrosine concentration of 200–600 μmol/L (Level of evidence – low; grade of recommendation – strong)
-
75.
The intake of PA should also be adjusted to maintain plasma PA concentrations within the normal range (Level of evidence – low; grade of recommendation – weak)
-
76.
Age appropriate supplement of vitamins and minerals should be given to maintain normal growth in patients with HT1 (Level of evidence – low; grade of recommendation – weak)
Wilson's disease
WD is an autosomal recessive disorder, which affects the ATP7B gene on chromosome 13. There is a defect in biliary excretion of copper, which leads to abnormal copper accumulation and liver damage.368,369 Chelators are efficacious in reducing the copper overload, and there are no data to show that addition of dietary copper restriction improves the outcome in comparison with chelator therapy alone2,368 and recent paediatric guidelines recommend that patients should avoid intake of food and water with high concentration of copper especially in the first year of therapy while on treatment with chelators.368,370,371 This is based on the assumption that the body should not be overloaded with extra dietary copper in the beginning of the chelation therapy.
Intestinal copper absorption is normal in patients with WD. Intestinal copper absorption is dependent on the dietary copper content, proportion of absorbed dietary copper varies from 12% at high intake to 56% at very low intake.372 The normal daily adequate intake of copper as per the European dietary reference value recommendation is 1.3 mg/day in women and 1.6 mg/day in men.373 The food items with very high copper content (>1 mg copper/per 100 gm of food, range 1.3–4.4 mg) include liver, shell fish (oyster/lobster), nuts and cocoa powder (chocolates).374 It has been suggested that occasional intake (once/week) of small amounts of liver and shellfish may be permitted once the patient has been adequately cheated, that is, after the first year of therapy.375 Excessive restriction of dietary copper is disadvantageous as it reduces the protein intake to less than the RDA in CLD patients especially vegetarians.369 Drinking water should contain <0.1 mg copper per litre of water. Copper or brass pipes used for water supply may be a source of increased copper intake as copper content of water may range from 0.005 to 30 mg/L.375 It is recommended to discard the first 1–2 L of water from the copper pipes as stagnant water in copper pipes is likely to have higher copper than flowing water. Studies in another copper overload disease (Indian childhood cirrhosis) have shown that boiling and storing (>6 h) milk or water in copper/brass vessels increases the copper content of the milk and water.376 Patients with WD should preferably be asked to use aluminium/steel or glass utensils. Patient and their family should be counselled that the main focus of therapy is chelators and not diet.
Consensus statement
-
77.
Copper-rich food should be restricted in the diet of patients with WD, especially in the first year until clinical remission while on therapy with chelating agents (Level of evidence – low; grade of recommendation – weak).
-
78.
Intake of water with high copper content should be avoided especially in the first year of treatment (Level of evidence – low; grade of recommendation – weak).
Fatty acid oxidation disorders
FAODs are a group of disorders affecting beta-oxidation of fatty acids. During fasting, beta-oxidation of fatty acids in the mitochondria provides ketone bodies and high-energy phosphates that act as major fuels for various tissues. Fatty acids enter the mitochondria through the carnitine shuttle (except medium-chain fatty acids) and thereafter undergo beta-oxidation through several steps catalyzed by different enzymes. FAODs can have defects in the carnitine shuttle or in beta-oxidation proper.377 Defects in beta-oxidation enzymes are classified based on the chain length of the fatty acid substrate; thus, it can affect very-long-chain-, long-chain or medium-chain fatty acid metabolism. Manifestations of FAODs are because of energy depletion and accumulation of toxic intermediaries.
The cornerstone of dietary management of FAODs is feeding at regular intervals and avoid prolonged fasting so that there is no hypoglycemia that can trigger oxidation of fatty acids. Feeding with glucose polymers or CS (oral or by nasogastric route) or intravenous glucose are required only during periods of stress or severe illness.377,378 The details of management of different subtypes are covered elsewhere.379, 380, 381
Consensus statement
-
79.
The maximum periods of fasting during wellness are as follows: neonates – 3 h, < 6 months – 4 h, > 6 months – 4 h in day time and 6 h at night, > 1 year–4 h in day time and 10 h at night (Level of evidence – low; grade of recommendation – weak)
-
80.
Supplementation with glucose polymers or cornstarch is needed during periods of stress. Intravenous 10% glucose should be given during severe illnesses. Nasogastric feeding with glucose polymers (maltodextrin) can be given when oral intake is poor (Level of evidence – moderate; grade of recommendation – strong)
-
81.
Subjects with very-long-chain acyl-CoA dehydrogenase (VLCAD) and long-chain 3-hydroxy-acyl-CoA Dehydrogenase (LCHAD) deficiency need supplementation with MCT and restriction of LCTs. Essential fatty acids should be supplemented in these cases (Level of evidence – moderate; grade of recommendation – strong)
-
82.
MCT supplements should not be given in medium-chain acyl CoA dehydrogenase deficiency (Level of evidence – low; grade of recommendation – weak)
-
83.
Subjects on restriction of LCT, may develop deficiencies of fat-soluble vitamins, which needs supplementation (Level of evidence – low; grade of recommendation – weak)
-
84.
The role of carnitine supplementation (100 mg/kg/day) is unequivocal only in carnitine transport defect (OCTN2 defect). In all other disorders, carnitine supplementation is controversial (Level of evidence – moderate; grade of recommendation – strong)
Urea cycle defects
Urea cycle is an important metabolic cycle, which enables excretion of nitrogen waste products, generated due to protein catabolism. The most common presentation with complete enzyme defect is HA that may lead to coma and death and occurs in neonatal period. Partial defects in enzymes result in HA, developmental disabilities, coma and even death and may manifest an any age.382,383
Nutritional management of urea cycle defects
The nutritional management can be classified into that of acute episode (acute HA) and long-term management.
Acute hyperammonemic Acrisis
All protein intake should be stopped, and energy should be provided with glucose (10%) and if needed intravenous lipids (after exclusion of FAODs). This should be done even on clinical suspicion, while the diagnostic work-up is being done. If the patient can take orally, glucose polymer–based high energy, protein-free diet is initiated.384,385 Enteral feeding/breast feeding should be reintroduced as soon as possible. Protein intake should not be restricted beyond 24–48 h to avoid catabolic state.383 It is started in small amounts and increased to required daily needs with ammonia monitoring. If the patient cannot take orally, formulations containing essential amino acids (EAAs) should be given through nasogastric tube or intravenously. Energy intake is kept at 120% of daily requirement. If the ammonia increases (>100 μmol/L), special EAA mixtures (for UCD) can be used alone or in combination with dietary proteins. Details of protein and energy needs during various ages have been defined by FAO/WHO/UNU report 2007.383,386
Long-term nutritional management of urea cycle defects
This is based on a diet of low protein, with adequate EAA and supplementation of vitamins and minerals.
The diet in UCD should be low in protein (∼1.7 g/kg infants to 0.8 g/kg in older child and adults per day) but still adequate to sustain normal growth while having BA in the range of <80 μmol/L.383, 384, 385, 386 Some patients, who can tolerate only minimal protein intake, need EAA supplementation with regular meals (3–4 times/day) to provide for 20–30% of protein intake as EAA.387 EAA formulations should be rich in BCAA but not in tryptophan, phenylalanine and tyrosine. Carbohydrate and fats should be given at libitum to provide adequate energy and avoid catabolism. Feeds should be evenly spaced throughout the day, avoiding prolonged periods of fasting. The protein and energy intake should be titrated as per needs of the patient, that is, age, any catabolic state, special situations like pregnancy and lactation etc. Female patients with mild Ornithine transcarbamylase deficiency do not need protein restriction, except during decompensation, pregnancy and lactation. In patients with arginase deficiency, severe protein restriction is needed and EAA should form up to 50% of protein requirement. Vitamin and mineral should be supplemented in diet as patients are at risk of deficiency of Fe, Zn, Cu, Ca and cobalamin.384
Practical aspects of low-protein diet: Infants should be managed with either breast feeding or standard infant formula.388,389 If needed, protein intake may be restricted by use of special protein-free formula along with breast feeding. Weaning may be achieved with initial introduction of protein-free fruits and vegetables and then a gradual introduction of dietary proteins replacing gram by gram from breast/formula feed to protein containing foods. Regular adjustments in diet are needed during childhood and adolescence to adjust for increased needs and appetite during puberty. The last meal of the day should provide approximately 25% of daily energy and natural protein to reduce risk of catabolism during overnight fasting.
Consensus statement
-
85.
During an episode of HA, immediate treatment without waiting for diagnostic workup should be initiated in form of intravenous glucose and stopping of protein intake. Enteral feeding is the preferred route and should be initiated as soon as possible. Proteins or EAA should be reintroduced when the ammonia levels fall below 100 μmol/L, usually within 24–48 h (Level of evidence – low; grade of recommendation – strong)
-
86.
Long-term management of UCD is centred on nutritional management in form of low-protein diet with EAA and mineral supplementation. A dietician, expert in nutritional management of metabolic disorders is required for regular monitoring and fine-tuning of nutritional management, especially for various individual disorders (Level of evidence – low; grade of recommendation – strong)
-
87.
During different stages of development from infancy to adulthood and special situations like pregnancy and lactation, individualized dietary planning should be done (Level of evidence – low; grade of recommendation – strong)
Citrin deficiency
Citrin is a liver-specific mitochondrial aspartate/glutamate (ASG) carrier, and its deficiency results in an IEM with varied presentation.390 In neonates it presents as intrahepatic cholestasis (NICCD), in older children it causes failure to thrive and dyslipidemia (FTTDCD) and in adults it presents with episodic hyperammonaemia with neuropsychiatric symptoms (citrullinemia type 2, CTLN2).391 These patients often have a strong preference for protein- and/or lipid-rich food with aversion for carbohydrates.
Citrin transports aspartate from mitochondria to cytosol and also plays a role in the transport of NADH reducing equivalent from cytosol to mitochondria as a component of malate–aspartate shuttle. The pathophysiology is explained by limitation of transport of ASG from cytosol to mitochondria and reduction of aspartate formation from citric acid cycle in mitochondria. This results in decreased oxaloacetate and aspartate and further blockage of ureagenesis.
Nutritional management: depends on the type of presentation.
NICCD: lactose-free and MCT-rich formula is given along with supplementation of fat-soluble vitamins.
FTTDCD and CTLN 2: protein-rich, carbohydrate-limited natural diet is advised.
Arginine administration helps in reducing the blood ammonia.392 Beans and peanuts are rich in aspartate/asparagine along with arginine. Sodium pyruvate and MCT oil supplementation is beneficial.391 High-carbohydrate diets and alcohol should not be given as they result in high NADH/NAD + ratio due to non-utilization of NADH in citric acid cycle in mitochondria.
Consensus statement
-
88.
In patients with citrin deficiency (NICCD, FTTDCD and CTLN 2), normal-high-protein diet with adequate fat should be prescribed. Carbohydrates should be restricted and alcohol should be avoided (CTLN 2). Supplementation with fat-soluble vitamins and MCT may be beneficial especially for NICCD and FTTDCD (Level of evidence – low; grade of recommendation – weak)
Conclusions
Assessing and treating malnutrition is an essential component of treating patients with CLD. Nutritional assessment and management can be improved by close cooperation between attending interns, medical residents, physicians, hepatologists, house staff and dieticians. Reuter et al.393 assessed the nutritional status in inpatients with cirrhosis using RFH-NPT and showed there was improvement in nutritional consultations after educational training regarding nutritional guidelines of the stakeholders and was associated with lower readmissions.
Additional studies are required to determine the nutrition screen of choice in cirrhosis. Ideally, these studies should compare screens with an accepted nutritional assessment tool (i.e. dietitian assessment), evaluate the validity of screens both in inpatients and outpatients and compare several screens in a single study. Future studies can evaluate whether adding simple measures of muscle mass and/or function (currently done as part of a nutritional assessment) may increase the effectiveness of existing nutrition screens without sacrificing efficiency. Additionally, screens may themselves have independent prognostic utility for the prediction of complications such as hospitalization and mortality and this can be evaluated in further studies.
The appendices with this document include nutritional values of common Indian foods and sample dietary advice for cirrhotics. However, there is enormous complexity of socio-cultural practices throughout India and South Asia, and while the tables can only serve as rough guides, protein, fat and carbohydrate intake need to be individualized for patients from different countries or parts of the country.
CRediT authorship contribution statement
Pankaj Puri: Conceptualization, Methodology, Writing – original draft, Visualization. Radha K. Dhiman: Conceptualization, Methodology, Writing – original draft, Supervision. Sunil Taneja: Writing . Puneeta Tandon: Writing . Manuela Merli: Writing . Anil C. Anand: Writing . Anil Arora: Writing . Subrat K. Acharya: Writing . Jaya Benjamin: Writing . Yogesh K. Chawla: Writing . Sunil Dadhich: Writing . Ajay Duseja: Writing . C.E. Eapan: Writing . Amit Goel: Writing . Naveen Kalra: Writing . Dharmesh Kapoor: Writing . Ashish Kumar: Writing . Kaushal Madan: Writing . Aabha Nagral: Writing . Gaurav Pandey: Writing . P.N. Rao: Writing. Sanjiv Saigal: Writing , Writing . Neeraj Saraf: Writing . Vivek A. Saraswat: Writing . Anoop Saraya: Writing . Shiv K. Sarin: Writing . Praveen Sharma: Writing . Shalimar: Writing . Akash Shukla: Writing . Sandeep S. Sidhu: Writing . Namrata Singh: Writing . Anshu Srivastava: Writing . Manav Wadhawan: Writing .
Conflicts of interest
The authors have none to declare.
Funding
None.
Appendix 1. Appendix 1: Nutritional values of common Indian foods
Food composition tables are data repository for the content of nutritionally relevant chemical constituents and energy values of foods. The first Indian Food Composition Tables was brought out in the year 1937, since then National Institute of Nutrition (NIN) has been constantly updating the compositional database of Indian foods. The Indian Food Composition Tables (IFCT) 20171 is the major source of food composition data in India, generated, developed, managed and maintained by the National Institute of Nutrition (ICMR), Hyderabad.
Foods with common characteristics have been placed together and arranged in groups (Table1). All foods have been categorized into 20 food groups, and the number in Table1 indicates the total number of foods present in each group. A total of 528 foods have been analysed for more than 150 parameters and presented under different nutrient component parameters. None of the food sampled for analysis is fortified, and it represents only inherent values.
Table 2 is a compilation of commonly eaten foods with the portion size and approximate nutrient content. Table 3 gives the sodium content of foods/100 gm of edible portion.
References to Appendix 1:
-
1.
Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian Food Composition Tables. National Institute of Nutrition.ICMR.2017
-
2.
Gopalan, C., Sastri, B. V. R., & Balasubramanian, S. C. Nutritive Values of Indian Foods (Rev.ed). India, Hyderabad: National Institute of Nutrition. 1989
-
3.
Bajaj M. Diet metrics. Hand book of food exchanges.Notionpress.2018
-
4.
‘Diet cal’ Version 9.0 (Profound Tech Solutions; http://dietcal.in/), which is based on values from Indian Food Composition Tables 2017
Appendix 2. Appendix 2: Sample dietary advice for patients with cirrhosis
(2000 Kcal, 75 grams protein, fat – 30%, 2 g sodium).
The various food groups and servings are given in Table 1. Incorporation of protein-rich items in dietary plan are depicted in Table 2.
Points to be kept in mind:
-
1.
Diet for liver disease patients should be simple and balanced one.
-
2.
Meals cooked for the whole family can be eaten, but with less salt.
-
3.
Food is your therapy and treatment. Eat it regularly like medicines. Cook foods with spices and oil. Spices (turmeric, coriander, garam masala and other spices) make the food tasty; include them in your food.
-
4.
Use vinegar, tamarind, lemon, tomato to make the food tasty.
-
5.
The above-mentioned quantity of food should be divided between 7 and 8 meals in a day. Eat something before sleeping (necessarily)
-
6.
Do not drink along with food.
-
7.
Eat fresh fruits and avoid fruit juices.
-
8.
Do not put extra salt on salad, curd, fruits.
-
9.
Eat all pulses and vegetables, with less salt.
-
10.
Eat homemade chutneys and pickles without salt.
-
11.
Eat the food regularly according to the mentioned ideal timings.
-
12.
Keep time gap of 2–3 h between meals.
-
13.
Do not drinks lot of water along with food.
-
14.
Tamarind and lemon can be used in the food.
-
15.
Boiling, steaming, roasting, etc. are better forms of cooking food. Avoid use excessive fat or frying of the food.
Foods to be avoided:
-
1.
Salted food items: salted chips, kurkure, pickle, chutney, papad, sauce, churan etc.
-
2.
Bread, biscuits, Chinese food, sea food (fish, prawn etc), baking soda etc.
-
3.
Beverages: Carbonated soft drinks, packed juice etc.
-
4.
Excessive fried foods.
Lifestyle modifications:
-
1.
Do not consume alcohol, tobacco, cigarette etc.
-
2.
Continue with your job/business/study etc.
-
3.
Weakness of muscles is normal in this disease. So, exercise should be necessarily done – ½ h in the morning and 1/2 h in the evening. It will strengthen your muscles.
Table 1.
Food groups in the Indian Food Composition Table.
| Code | Food groups | No. of food entries |
|---|---|---|
| A | Cereals and millets | 24 |
| B | Grain legumes | 25 |
| C | Green leafy vegetables | 34 |
| D | Other vegetables | 78 |
| E | Fruits | 68 |
| F | Roots and tubers | 19 |
| G | Condiments and spices | 33 |
| H | Nuts and oil seeds | 21 |
| I | Sugars | 2 |
| J | Mushrooms | 4 |
| K | Miscellaneous foods | 2 |
| L | Milk and milk products | 4 |
| M | Egg and egg products | 15 |
| N | Poultry | 19 |
| O | Animal meat | 63 |
| P | Marine fish | 92 |
| Q | Marine shellfish | 8 |
| R | Marine mollusks | 7 |
| S | Fresh water fish and shellfish | 10 |
| T | Edible oils and fats | 9 |
Table 2.
Portion size of commonly eaten food (edible portion) and approximate nutrient content.
| Food items | Raw weight (g) | Portion size (cooked) | Energy (kcal) | Proteins (g) | Carbohydrates (g) | Fats (g) | Foods included |
|---|---|---|---|---|---|---|---|
| Cereals and millets | 25 | 1 chapati (6″ diameter) | 85 | 2.5 | 17 | 0.5 | Barley, cornflakes, maize, oats, rice, rice flakes, vermicelli, semolina, wheat |
| Bread, white | 20 | 1 slice | 50 | 1.5 | 10 | 0 | Bread-standard size |
| Pulses and legumes | 25 | 1 bowl (volume 210 ml, height 4.5 cm, diameter 8 cm | 80 | 7 | 13 | 0.5 | All dehusked pulses |
| Whole gram | 25 | 1 bowl (same as pulses | 75 | 5 | 12 | 0 | All whole pulses with husk |
| Milk (whole, cow) | 100 ml | ½ cup (200 ml, height 7 cm, diameter 7 cm) | 75 | 3 | 5 | 4 | Milk |
| Curd (cow's milk) | 100 | ½ cup (200 ml, height 7 cm, diameter 7 cm) | 60 | 3 | 3 | 4 | Curd |
| Fresh paneer | 25 | 1″x1″x1/2″ | 65 | 4.75 | 3 | 3.75 | Fresh paneer |
| Roots and tubers | 100 | 1 bowl (volume 155 ml, height 3.8 cm, diameter 7.5 cm) | 50 | 1 | 10 | 0 | Potato, onion, sweet potato, colocasia, carrot, radish |
| Green leafy vegetables | 100 | 1bowl( volume 155 ml, height 3.8 cm, diameter 7.5 cm) | 30 | 2 | 3 | 1.0 | Spinach, Fenugreek leaves, Bathua, mustard leaves, mint, radish leaves, cabbage |
| Other vegetables | 100 | 1 bowl (volume 155 ml, height 3.8 cm, diameter 7.5 cm) | 30 | 2 | 5 | 0 | Beans, brinjal, capsicum, tomato cauliflower, cucumber, gourd, |
| Fruits | 100 | 1 medium size | 50 | 0.7 | 13 | 0 | Apple, grapes, guava, litchi, papaya, watermelon |
| Banana | 100 | 1 large | 105 | 1.23 | 23.63 | 0.33 | Banana |
| Fats and Oils | 5 | 1 tea spoon | 45 | – | – | 5.0 | All refined oils, ghee |
| Nuts | 15 | Almonds – 12; cashew – 6; walnut – 5; | 85 | 3 | 2 | 7 | Nuts |
| Sugars | 5 | 1 tea spoon | 20 | – | 5 | – | Sugar, jaggery, jams |
| Egg, poultry | 50 | 1 no. | 75 | 7 | – | 5.27 | Egg |
| Meat, chicken, fish | 50 | Palm size | 75 | 10 | – | 4 | Non-vegetarian foods |
Nutrient values have been rounded to the nearest number.
Values less than 0.5 have been considered as 0.
Adapted from References1,2,3,4.
Table 3.
Sodium content of foods/100 gm (edible portion).a
| <5 mg Na | 5–35 mg Na | 35–140 mg Na | High >140 mg Na | |
|---|---|---|---|---|
| Cereals | Chapathi, whole wheat flour | Barley | Dosa flour (dry) | |
| Quinoa | Idli flour (dry) | |||
| Rice flakes (dry) | ||||
| Rice, parboiled milled | ||||
| Rice, raw milled | ||||
| Wheat, flour fiend | ||||
| Wheat, semolina (dry) | ||||
| Wheat, vermicelli (dry) | ||||
| Millets | Bajra | Jowar | ||
| Jowar | ||||
| Ragi | ||||
| Samai | ||||
| Varagu | ||||
| Pulses | Soyabean, white | Bengal gram dhal | ||
| Bengal gram whole | ||||
| Black gram dal | ||||
| Black gram whole | ||||
| Cow pea white | ||||
| Green gram dhal | ||||
| Green gram whole | ||||
| Horse gram whole | ||||
| Peas dry | ||||
| Rajmah, red | ||||
| Red gram dhal | ||||
| Fruits | Apple, big | Musk melon orange flesh | ||
| Banana, ripe, poovam | Papaya ripe | |||
| Custard apple | Tamarind, pulp | |||
| Dates, processed | ||||
| Gosse berry | ||||
| Grapes, green round | ||||
| Guava, green round seeded | ||||
| Guava, white flesh | ||||
| Jackfruit, ripe | ||||
| Jambu fruit, ripe | ||||
| Mango, ripe, Banganapalli | ||||
| Orange, pulp | ||||
| Pear | ||||
| Pineapple | ||||
| Plum | ||||
| Sapota | ||||
| Strawberry | ||||
| Sweet lime, pulp | ||||
| Watermelon, dark green | ||||
| Green leafy vegetables | Agathi leaves | Fenugreek leaves | ||
| Amaranth leaves, green | Ponnaganni | |||
| Cabbage, green ` | Radish leaves | |||
| Cauliflower leaves | Spinach | |||
| Drumstick leaves | ||||
| Lettuce | ||||
| Mustard leaves | ||||
| Roots and tubers | Colocasia | Sweet potato, pink skin | Carrot, orange | |
| Potato, brown skin, big | Tapioca | Beetroot | ||
| Yam, elephant | ||||
| Other vegetables | Ash gourd | Bitter gourd, jagged, teeth, ridges, elongate | ||
| Beans scarlet, tender | Cauliflower | |||
| Bottle gourd, elongate, pale green | Cucumber, green elongate | |||
| Brinjal all varieties | Ladies finger | |||
| Ridge gourd | Plantain flower | |||
| Zucchini, gourd | Snake gourd, long, pale green | |||
| Tomato, green | ||||
| Tomato, red ripe | ||||
| Milk | Curd | |||
| Milk, whole, cow | ||||
| Milk, whole, buffalo | ||||
| Paneer | ||||
| Meat | Beef, chops | Chicken, poultry, breast skinless | ||
| Egg, poultry, whole raw | ||||
| Goat, chops | ||||
| Marine | Salmon fish | Paarai | Anchovy (Nethili) | |
| Vanjar (seer fish) | Pomfret, black | Prawns, big | ||
| Condiments and spices | Chilies, all varieties of green | Asafoetida | Coriander levels | Cloves |
| Onion, small | Cardamom, green | Cumin seed | ||
| Chilies, red | Fenugreek seed | |||
| Coriander seed | ||||
| Curry leaves | ||||
| Garlic, big, clove | ||||
| Ginger, fresh | ||||
| Mint leaves | ||||
| Omum | ||||
| Onion, big | ||||
| Pepper, black | ||||
| Poppy seeds | ||||
| Turmeric powder | ||||
| Nuts | Almond | Cashew | ||
| Mustard | Coconut, kernel, fresh | |||
| Walnut | Groundnut | |||
| Pistachio nut | ||||
| Sugars | Sugar | Jaggery | ||
| Processed products | Act II popcorn (golden sizzle) | Kissan jam | Kissan orange squash | 777 mango pickle |
| Bindu appalam | Mount dew | Ovaltin | Amul butter | |
| Kellogg's cornflakes | Pepsi | Real-pomegranate | Britannia cheese cube s | |
| Quaker oats | Tang Orange instant drink mix | Diary milk | ||
| Tropicana Orange | Haldiram bhujia | |||
| Kissan ketchup | ||||
| Maggie noodles | ||||
| Knorr mix vegetable soup |
Note: In labelled food products, care needs to be taken for conversion of common salt (sodium chloride) to sodium.
-
•Sodium chloride contains 40% (39.3%) sodium and 60% chloride.
-
•To covert sodium chloride to its sodium, multiply by 0.393.
-
•To convert mg of sodium to mEq, divide by the atomic weight of 23.
-
•To convert sodium to salt, multiply by 2.54.
- •Millimoles and milliequivalents of sodium are the same.
-
•
Sodium chloride contains 40% (39.3%) sodium and 60% chloride.
-
•
To covert sodium chloride to its sodium, multiply by 0.393.
-
•
To convert mg of sodium to mEq, divide by the atomic weight of 23.
-
•
To convert sodium to salt, multiply by 2.54.
-
•
Millimoles and milliequivalents of sodium are the same.
Table 1.
Food groups and servings.
| Food groups | Food items | Quantity |
|---|---|---|
| 1) Milk (light in protein) | Milk, curd, lassi, buttermilk, paneer (cottage cheese), milk leased sweet dishes, ice cream, mustard, kheer, tea, coffee etc. | 1000 ml (4 big glasses of milk) |
| 2) Cereals | Wheat, rice, jowar, bajra, maize, dalia (broken wheat), poha (rice flakes), pulses, semolina | 150 g (6–7 chapatis) |
| 3) Pulses (light in protein) | Pulses, roasted Bengal grain, rajma, germinated/sprouted pulses, sattu, soyabean, cheela, besan laddu, dhokla, peanuts, boiled channa, rajma etc. | 75 g (3 katori cooked) |
| 4) Vegetables | Potato | 100 g |
| Potato, seasonal and green vegetables | 300 g (2 katori) | |
| 5) Fruits | All seasonal fruits | 200 g (2 medium) |
| 6) Meat-fish (light in protein) | Fish and chicken | 50 g |
| 7) Oil and ghee | Any oil (mustard, refined, olive, soyabean, groundnut), white butter, ghee | 30 ml (5–6 tsp) |
| 8) Sugar | Jaggery, honey, chikki, gajak, sweets | 25 gm (5 tsp) |
| 9) Salt | Only in cooked pulses and vegetables | 1/2 tsp (2.5 g) |
| 10) Egg | 1 |
Table 2.
Incorporating protein rich foods in the dietary plana.
| 8 a.m | No. 1/3/6/10 + breakfast |
| 10 a.m | Egg/1 glass thick lassi/roasted Bengal gram (channa)/dry fruits/peanuts |
| 1 p.m | 1 katori dal/pulse + 1 katori curd + lunch |
| 5 p.m | Roasted Bengal gram (channa)/peanuts/dhokla/any besan dish + tea etc. |
| 8 p.m | 1 katori dal + dinner |
| 10 p.m | 1 glass milk/no. 1/3/6/10 |
serial numbers in table from food groups in Table 1.
References
- 1.Alveras-da-Silva M.R., Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plauth M., Bernal W., Dasarathy S. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazurak V.C., Tandon P., Montano-Loza A.J. Nutrition and the transplant candidate. Liver Transplant. 2017;23:1451–1464. doi: 10.1002/lt.24848. [DOI] [PubMed] [Google Scholar]
- 5.Menon P., Nguven P., Avula R., Mani S., Tran L., Victora C. A decade of progress on scaling up health and nutrition interventions in India: a countdown to 2030 case study (P04-115-19) Curr Dev Nutr. 2019;3 doi: 10.1093/cdn/nzz051.P04-115-19. pii: nzz051.P04-115-19. [DOI] [Google Scholar]
- 6.Ramachandran P. Vol. 37. 2016. India's nutritional challenges.https://www.nutritionfoundationofindia.res.in/nfi-bulletin/ (NFI Bulletin). Number 1. Available from: [Google Scholar]
- 7.Vijayaraghavan K., Rao D.H. Diet & nutrition situation in rural India. Indian J Med Res. 1998;108:243–253. [PubMed] [Google Scholar]
- 8.Tyrovolas S., Koyanagi A., Olaya B. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–321. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lear S.A., Kohli S., Bondy G.P., Tchernof A., Sniderman A.D. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. 2009;94:4696–4702. doi: 10.1210/jc.2009-1030. [DOI] [PubMed] [Google Scholar]
- 10.Lau E.M., Lynn H.S., Woo J.W., Kwok T.C., Melton L.J. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60:213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 11.Wu S.W., Wu S.F., Liang H.W., Wu Z.T., Huang S. Measuring factors affecting grip strength in a Taiwan Chinese population and a comparison with consolidated norms. Appl Ergon. 2009;40:811–815. doi: 10.1016/j.apergo.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Sidhu S., Saggar K., Goyal O., Kishore H., Sidhu S.S. Normative values of sarcopenia in the Indian population. Indian J Gastroenterol. 2018;37:A1–A137. doi: 10.1007/s12664-024-01565-7. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan S., Vaz M., Kurpad A.V. Protein intakes in India. Br J Nutr. 2012;108:S50–S58. doi: 10.1017/S0007114512002413. [DOI] [PubMed] [Google Scholar]
- 14.Mijnarends D.M., Koster A., Schols J.M. Physical activity and incidence of sarcopenia: the population-based AGES-Reykjavik Study. Age Ageing. 2016;45:614–620. doi: 10.1093/ageing/afw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anjana R.M., Ranjani H., Unnikrishnan R., Weber M.B., Mohan V., Narayan K.M. Exercise patterns and behaviour in Asian Indians: data from the baseline survey of the diabetes community lifestyle improvement program (D-CLIP) Diabetes Res Clin Pract. 2015;107:77–84. doi: 10.1016/j.diabres.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Vist G.E., GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romiti A., Merli M., Martorano M. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Ital J Gastroenterol. 1990;22:118–123. [PubMed] [Google Scholar]
- 18.Kalaitzakis E. Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol. 2014;20:14686–14695. doi: 10.3748/wjg.v20.i40.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigley E.M., Stanton C., Murphy E.F. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020–1027. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Müller M.J., Böttcher J., Selberg O. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- 21.Owen O.E., Trapp V.E., Reichard G.A. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72:1821–1832. doi: 10.1172/JCI111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merli M., Eriksson L.S., Hagenfeldt L., Wahren J. Splanchnic and leg exchange of free fatty acids in patients with liver cirrhosis. J Hepatol. 1986;3:348–355. doi: 10.1016/s0168-8278(86)80488-3. [DOI] [PubMed] [Google Scholar]
- 23.Henkel A.S., Buchman A.L. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:202–209. doi: 10.1038/ncpgasthep0443. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair M., Gow P.J., Grossmann M., Angus P.W. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765–777. doi: 10.1111/apt.13549. [DOI] [PubMed] [Google Scholar]
- 25.Drummond M.J., Dreyer H.C., Fry C.S., Glynn E.L., Rasmussen B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signalling. J Appl Physiol Bethesda Md. 2009;1985106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamman M.M., Shipp J.R., Jiang J. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–E390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura T., Morinaga Y., Fujitani S., Takehana K., Nishitani S., Sonaka I. Oral administration of branched-chain amino acids activates the mTOR signal in cirrhotic rat liver. Hepatol Res. 2005;33:27–32. doi: 10.1016/j.hepres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2015;15:12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 29.Tilg H., Wilmer A., Vogel W. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 30.Thapaliya S., Runkana A., McMullen M.R. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi F., Matsumoto Y., Momoki C. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res. 2013;43:1264–1275. doi: 10.1111/hepr.12085. [DOI] [PubMed] [Google Scholar]
- 32.Qiu J., Tsien C., Thapalaya S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983–E993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu J., Thapaliya S., Runkana A. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci USA. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullough A.J., Mullen K.D., Kalhan S.C. Measurements of total body and extracellular water in cirrhotic patients with and without ascites. Hepatol Baltim Md. 1991;14:1102–1111. [PubMed] [Google Scholar]
- 35.Carias S., Castellanos A.L., Vilchez V. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol. 2016;31:628–633. doi: 10.1111/jgh.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison W.L., Bouchier I.A., Gibson J.N., Rennie M.J. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin. Sci. Lond. Engl. 1990;197978:613–619. doi: 10.1042/cs0780613. [DOI] [PubMed] [Google Scholar]
- 37.Dunlop D.S., Kaufman H., Zanchin G., Lajtha A. Protein synthesis rates in rats with portacaval shunts. J Neurochem. 1984;43:1487–1489. doi: 10.1111/j.1471-4159.1984.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 38.Dam G., Sørensen M., Buhl M. Muscle metabolism and whole blood amino acid profile in patients with liver disease. Scand J Clin Lab Invest. 2015;75:674–680. [PubMed] [Google Scholar]
- 39.Dejong C.H.C., van de Poll M.C.G., Soeters P.B., Jalan R., Olde Damink S.W.M. Aromatic amino acid metabolism during liver failure. J Nutr. 2007;137:1579S–1585S. doi: 10.1093/jn/137.6.1579S. [DOI] [PubMed] [Google Scholar]
- 40.Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31:14–20. doi: 10.1016/j.nut.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Handelsman D.J., Strasser S., McDonald J.A., Conway A.J., McCaughan G.W. Hypothalamic-pituitary-testicular function in end-stage non-alcoholic liver disease before and after liver transplantation. Clin Endocrinol (Oxf) 1995;43:331–337. doi: 10.1111/j.1365-2265.1995.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 42.Grossmann M., Hoermann R., Gani L. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin Endocrinol. 2012;77:323–328. doi: 10.1111/j.1365-2265.2012.04347.x. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair M., Grossmann M., Angus P.W. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31:661–667. doi: 10.1111/jgh.13182. [DOI] [PubMed] [Google Scholar]
- 44.Khoshnood A., Nasiri Toosi M., Faravash M.J. A survey of correlation between insulin-like growth factor-I (igf-I) levels and severity of liver cirrhosis. Hepat Mon. 2013;13 doi: 10.5812/hepatmon.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assy N., Pruzansky Y., Gaitini D., Shen Orr Z., Hochberg Z., Baruch Y. Growth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunction. J Hepatol. 2008;49:34–42. doi: 10.1016/j.jhep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Barbu E.C., Chiu-Tiu C.E., Lazăr M. Hepatic osteodystrophy: a global (Re)view of the problem. Acta Clin Croat. 2017;56:512–525. doi: 10.20471/acc.2017.56.03.19. [DOI] [PubMed] [Google Scholar]
- 47.Bihari C., Lal D., Thakur M. Suboptimal level of bone-forming cells in advanced cirrhosis are associated with hepatic osteodystrophy. Hepatol Commun. 2018;2:1095–1110. doi: 10.1002/hep4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim G., Kang S.H., Kim M.Y., Baik S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maharshi S., Sharma B.C., Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. 2015;30:1507–1513. doi: 10.1111/jgh.12999. [DOI] [PubMed] [Google Scholar]
- 50.Kalal C.R., Benjamin J., Shashthry V., Joshi Y., Sarin S. No consensus among nutritional assessment tools for identification of malnutrition in patients with alcoholic liver disease. J Hepatol. 2017;66:S346. [Google Scholar]
- 51.Panackel C., Balan S., Rommel S. Prevalence, risk factors and prognostic significance of sarcopenia in liver cirrhosis in Indian population. Indian J Gastroenterol. 2018;37:A85. [Google Scholar]
- 52.Surakshit T.K., Kumar M., Ranjan P., Ghuman S., Arora A. Sarcopenia in cirrhosis: a risk factor for hospitalizations and short term mortality. Gut. 2019;68:A159–A160. [Google Scholar]
- 53.Choudhary N.S., Saigal S., Saraf N. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. 2015;29:211–215. doi: 10.1111/ctr.12505. [DOI] [PubMed] [Google Scholar]
- 54.Gajula U.N.M. Assessment of sarcopenia in patients with chronic liver disease. J Clin Expl Hepatol. 2018;8:S67. [Google Scholar]
- 55.Montano-Loza A.J., Duarte-Rojo A., Meza-Junco J. Inclusion of sarcopenia within MELD (MELD Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merli M., Riggio O., Dally L. Does malnutrition affect survival in cirrhosis? PINC (policentrica italiana nutrizione cirrosi) Hepatology. 1996;23:1041–1046. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 57.Kang S.H., Jeong W.K., Baik S.K., Cha S.H., Kim M.Y. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. 2018;9:860–870. doi: 10.1002/jcsm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang K.V., Chen J.D., Wu W.T., Huang K.C., Lin H.Y., Han D.S. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J Formos Med Assoc. 2019;118:833–842. doi: 10.1016/j.jfma.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Jentoft A.J., Bahat G., Bauer J. Writing group for the European working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Vugt J.L.A., Alferink L.J.M., Buettner S. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 61.Kim G., Kang S.H., Kim M.Y., Baik S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montano-Loza A.J., Meza-Junco J., Prado C.M. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Yu R., Shi Q., Liu L., Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol. 2018;18:51. doi: 10.1186/s12876-018-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merli M., Giusto M., Lucidi C. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 65.Praktiknjo M., Clees C., Pigliacelli A. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsien C., Shah S.N., McCullough A.J., Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 67.Chang K., Chen J., Wu W., Huang K., Hsu C., Han D. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2018;7:90–103. doi: 10.1159/000484950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tandon P., Ney M., Irwin I. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transplant. 2012;18 doi: 10.1002/lt.23495. 1209-16. [DOI] [PubMed] [Google Scholar]
- 69.Kalafateli M., Mantzoukis K., Choi Yau Y. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merli M., Giusto M., Gentili F. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208–214. doi: 10.1111/j.1478-3231.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 71.Englesbe M.J., Patel S.P., He K. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirai H., Kaido T., Hamaguchi Y. Preoperative low muscle mass has a strong negative effect on pulmonary function in patients undergoing living donor liver transplantation. Nutrition. 2018;45:1–10. doi: 10.1016/j.nut.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 73.Jeon J.Y., Wang H.J., Ock S.Y. Newly developed sarcopenia as a prognostic factor for survival in patients who underwent liver transplantation. PLoS One. 2015 Nov 30;10 doi: 10.1371/journal.pone.0143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013;58:3103–3111. doi: 10.1007/s10620-013-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsien C., Garber A., Narayanan A. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergerson J.T., Lee J.-G., Furian A. Liver transplantation arrests and reverses muscle wasting. Clin Transplant. 2015;29:216–221. doi: 10.1111/ctr.12506. [DOI] [PubMed] [Google Scholar]
- 77.Bhanji R.A., Takahashi N., Moynagh M.R. The evolution and impact of sarcopenia pre- and post- liver transplantation. Aliment Pharmacol Ther. 2019;49:807–813. doi: 10.1111/apt.15161. [DOI] [PubMed] [Google Scholar]
- 78.Plank L.D., Metzger D.J., McCall J.L. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001;234:245e55. doi: 10.1097/00000658-200108000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagström H., Stål P., Hultcrantz R., Hemmingsson T., Andreasson A. Overweight in late adolescence predicts development of severe liver disease later in life: a 39 years follow-up study. J Hepatol. 2016;65:363–368. doi: 10.1016/j.jhep.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 82.Duseja A., Sharma B., Kumar A. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatology. 2010;52:2248–2249. doi: 10.1002/hep.23838. [DOI] [PubMed] [Google Scholar]
- 83.Mehta M., Satsangi S., Duseja A., Taneja S., Dhiman R.K., Chawla Y. Can alcoholic liver disease and nonalcoholic fatty liver disease Co-exist? J Clin Exp Hepatol. 2017;7:121–126. doi: 10.1016/j.jceh.2017.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Periyalwar P., Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong H.C., Hwang S.Y., Choi H.Y. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 86.Berzigotti A., Garcia-Tsao G., Bosch J. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555–561. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raynard B., Balian A., Fallik D. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 88.Ortiz V., Berenguer M., Rayón J.M., Carrasco D., Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 89.Bellentani S., Saccoccio G., Masutti F. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 90.Ekstedt M., Franzén L.E., Holmqvist M. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 91.Liu B., Balkwill A., Reeves G., Beral V. Million Women Study Collaborators. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Everhart J.E., Lok A.S., Kim H.Y., HALT-C Trial Group Weight related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sundaram V., Kaung A., Rajaram A. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther. 2015;42:1271–1280. doi: 10.1111/apt.13426. [DOI] [PubMed] [Google Scholar]
- 94.Berzigotti A., Albillos A., Villanueva C., Ciberehd SportDiet Collaborative Group Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 95.Kardashian A.A., Dodge J.L., Roberts J., Brandman D. Weighing the risks: morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int. 2018;38:553–563. doi: 10.1111/liv.13523. [DOI] [PubMed] [Google Scholar]
- 96.Aguilar M., Liu B., Holt E.W., Bhuket T., Wong R.J. Impact of obesity and diabetes on waitlist survival, probability of liver transplantation and post-transplant survival among chronic hepatitis C virus patients. Liver Int. 2016;36:1167–1175. doi: 10.1111/liv.13091. [DOI] [PubMed] [Google Scholar]
- 97.Schlansky B., Naugler W.E., Orloff S.L., Enestvedt C.K. Higher mortality and survival benefit in obese patients awaiting liver transplantation. Transplantation. 2016;100:2648–2655. doi: 10.1097/TP.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 98.Ahirwar R., Mondal P.R. Prevalence of obesity in India: a systematic review. Diabetes Metab Syndr. 2019;13:318–321. doi: 10.1016/j.dsx.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 99.Vidot H., Kline K., Cheng R. The relationship of obesity, nutritional status and muscle wasting in patients assessed for liver transplantation. Nutrients. 2019 Sep 4;11 doi: 10.3390/nu11092097. pii: E2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greco A.V., Mingrone G., Benedetti G., Capristo E., Tataranni P.A., Gasbarrini G. Daily energy and substrate metabolism in patients with cirrhosis. Hepatology. 1998;27:346–350. doi: 10.1002/hep.510270205. [DOI] [PubMed] [Google Scholar]
- 101.Eslamparast T., Vandermeer B., Raman M. Are predictive energy expenditure equations accurate in cirrhosis? Nutrients. 2019;11 doi: 10.3390/nu11020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tandon P., Raman M., Mourtzakis M., Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- 103.Glass C., Hipskind P., Cole D., Lopez R., Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677–688. doi: 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hipskind P., Glass C., Charlton D., Nowak D., Dasarathy S. Do handheld calorimeters have a role in assessment of nutrition needs in hospitalized patients? A systematic review of literature. Nutr Clin Pract. 2011;26:426–433. doi: 10.1177/0884533611411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Indian Council of Medical Research . National Institute of Nutrition; Hyderabad: 2010. Nutrient Requirements and Recommended Dietary Allowances for Indians: A Report of the Expert Group of the Indian Council of Medical Research. [Google Scholar]
- 106.Swart G.R., van den Berg J.W., van Vuure J.K., Rietveld T., Wattimena D.L., Frenkel M. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin Nutr. 1989;8:329–336. doi: 10.1016/0261-5614(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 107.Dietary Guidelines for Indians: A Manual. National Institute of Nutrition; Hyderabad, India: 2011. [Google Scholar]
- 108.McFarlane M., Hammond C., Roper T. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin Nutr ESPEN. 2018;23:156–161. doi: 10.1016/j.clnesp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 109.Booi A.N., Menendez J., Norton H.J., Anderson W.E., Ellis A.C. Validation of a screening tool to identify undernutrition in Ambulatory patients with liver cirrhosis. Nutr Clin Pract. 2015;30:683–689. doi: 10.1177/0884533615587537. [DOI] [PubMed] [Google Scholar]
- 110.Borhofen S.M., Gerner C., Lehmann J. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61:1735–1743. doi: 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- 111.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaveric validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 112.Mourtzakia M., Prado C.M., Lieffers J.R., Reiman T., McCargar L.J., Baracos V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metabol. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 113.Carey E.J., Lai J.C., Wang C.W. Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 115.Lau E.M., Lynn H.S., Woo J.W., Kwok T.C., Melton L.J., 3rd Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60:213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 116.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 1st ed. Vol. 46. 2016. Japan society of hepatology guidelines for sarcopenia in liver disease; pp. 951–963. [DOI] [PubMed] [Google Scholar]
- 117.Ebadi M., Wang C.W., Lai J.C. From the Fitness, Life Enhancement, and Exercise in Liver Transplantation (FLEXIT) Consortium. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle. 2018;9:1053–1062. doi: 10.1002/jcsm.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Durand F., Buyse S., Francoz C. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 119.Gu D.H., Kim M.Y., Seo Y.S. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–330. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tandon P., Low G., Mourtzakis M. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 121.Montano-Loza A.J., Angulo P., Meza-Junco J. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montano-Loza A.J. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giusta M., Lattanzi B., Albanese C. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:328–334. doi: 10.1097/MEG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 124.Kim K.M., Jang H.C., Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med (Engl Ed) 2016;31:643–650. doi: 10.3904/kjim.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen L.K., Liu L.K., Woo J. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 126.Nishikawa H., Enomoto H., Iwata Y., Nishimura T., Iijima H., Nishiguchi S. Clinical utility of bioimpedance analysis in liver cirrhosis. J Hepatobiliary Pancreat Sci. 2017;24:409–416. doi: 10.1002/jhbp.455. [DOI] [PubMed] [Google Scholar]
- 127.Carias S., Castellanos A.L., Vilchez V. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol. 2016;31:628–633. doi: 10.1111/jgh.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gallagher D., Kuznia P., Heshka S. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polyzos S.A., Margioris A.N. Sarcopenic obesity. Hormones (Basel) 2018;17:321–331. doi: 10.1007/s42000-018-0049-x. [DOI] [PubMed] [Google Scholar]
- 130.Eslamparast T., Montano-Loza A.J., Raman M., Tandon P. Sarcopenic obesity in cirrhosis—the confluence of 2 prognostic titans. Liver Int. 2018;38:1706–1717. doi: 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 131.Lee D.C., Shook R.P., Drenowatz C., Blair S.N. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. 2016;2:FSO127. doi: 10.4155/fsoa-2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai J.C., Sonnenday C.J., Tapper E.B. Frailty in liver transplantation: an expert opinion statement from the American society of transplantation liver and intestinal community of practice. Am J Transplant. 2019;19:1896–1906. doi: 10.1111/ajt.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lai J.C., Feng S., Terrault N.A., Lizaola B., Hayssen H., Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai J.C., Covinsky K.E., Dodge J.L. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564–574. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ney M., Haykowsky M.J., Vandermeer B., Shah A., Ow M., Tandon P. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016;44:796–806. doi: 10.1111/apt.13771. [DOI] [PubMed] [Google Scholar]
- 136.Sundaram V., Lim J., Tholey D.M. The Braden Scale, A standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transplant. 2017;23:1153–1160. doi: 10.1002/lt.24789. [DOI] [PubMed] [Google Scholar]
- 137.Tandon P., Reddy K.R., O'Leary J.G. North American Consortium for the Study of End-Stage Liver Disease. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65:217–224. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 138.Orman E.S., Ghabril M., Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1189–1195.e1. doi: 10.1016/j.cgh.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tandon P., Tangri N., Thomas L. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111:1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 140.Dunn M.A., Josbeno D.A., Tevar A.D. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111:1768–1775. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 141.Carey E.J., Steidley D.E., Aqel B.A. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplant. 2010;16:1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 142.Tapper E.B., Finkelstein D., Mittleman M.A., Piatkowski G., Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584–590. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lai J.C., Rahimi R.S., Verna E.C. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology. 2019;56:1675–1682. doi: 10.1053/j.gastro.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 145.Fogelman I., Blake G.M. Different approaches to bone densitometry. J Nucl Med. 2000;41:2015–2025. [PubMed] [Google Scholar]
- 146.Collier J.D., Ninkovic M., Compston J.E. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50:i1–i9. doi: 10.1136/gut.50.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pares A., Guanabens N. Treatment of bone disorders in liver disease. J Hepatol. 2006;45:445–453. doi: 10.1016/j.jhep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 148.Labio E.D., Del Rosario D.B., Strasser S.I., McCaughan G.W., Crawford B.A. Effect of ascites on bone density measurement in cirrhosis. J Clin Densitom. 2007;10:391–394. doi: 10.1016/j.jocd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 149.Guañabens N., Monegal A., Muxi A. Patients with cirrhosis and ascites have false values of bone density: implications for the diagnosis of osteoporosis. Osteoporos Int. 2012;23:1481–1487. doi: 10.1007/s00198-011-1756-1. [DOI] [PubMed] [Google Scholar]
- 150.Monegal A., Navasa M., Peris P. Bone disease in patients awaiting liver transplantation. Has the situation improved in the last two decades? Calcif Tissue Int. 2013;93:571–576. doi: 10.1007/s00223-013-9797-4. [DOI] [PubMed] [Google Scholar]
- 151.Soulsby C.T., Morgan M.Y. Dietary management of hepatic encephalopathy in cirrhotic patients: survey of current practice in United Kingdom. BMJ. 1999;318:1391. doi: 10.1136/bmj.318.7195.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillips G.B., Schwartz R., Gabuzda G.J., Davidson C.S. The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med. 1952;247:239–246. doi: 10.1056/NEJM195208142470703. [DOI] [PubMed] [Google Scholar]
- 153.Riordan S.M., Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:413–419. doi: 10.1056/NEJM199708143370707. [DOI] [PubMed] [Google Scholar]
- 154.Seymour C.A., Whelan K. Dietary management of hepatic encephalopathy. BMJ. 1999;318:1364–1365. doi: 10.1136/bmj.318.7195.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Córdoba J., López-Hellín J., Planas M. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 156.Les I., Planas M., Cardenas G. Effects of the proteins of the diet in patientswith cirrhosis and a prior episode of hepatic encephalopathy. A long-term randomized study (#24) Hepatology. 2009;50:313A. [Google Scholar]
- 157.Anand A.C. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Merli M., Eriksson L.S., Hagenfeldt L., Wahren J. Splanchnic and leg exchange of free fatty acids in patients with liver cirrhosis. J Hepatol. 1986;3:348–355. doi: 10.1016/s0168-8278(86)80488-3. [DOI] [PubMed] [Google Scholar]
- 159.Chen X., Iqbal N., Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103:365–372. doi: 10.1172/JCI5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.The Lancet Gastroenterology Hepatology Herbal assault: liver toxicity of herbal and dietary supplements. Lancet Gastroenterol Hepatol. 2018;3:141. doi: 10.1016/S2468-1253(18)30011-6. [DOI] [PubMed] [Google Scholar]
- 161.Gunsar F., Raimondo M.L., Jones S. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563–572. doi: 10.1111/j.1365-2036.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 162.Ebadi M., Tandon P., Moctezuma-Velazquez C. Low subcutaneous adiposity Associates with higher mortality in female patients with cirrhosis. J Hepatol. 2018;69:608–616. doi: 10.1016/j.jhep.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 163.Huisman E.J., Trip E.J., Siersema P.D., van Hoek B., van Erpecum K.J. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 164.Sasidharan M., Nistala S., Narendhran R.T., Murugesh M., Bhatia S.J., Rathi P.M. Nutritional status and prognosis in cirrhotic patients. Trop Gastroenterol. 2012;33:257–264. [PubMed] [Google Scholar]
- 165.Lucidi C., Lattanzi B., Di Gregorio V. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38:851–857. doi: 10.1111/liv.13691. [DOI] [PubMed] [Google Scholar]
- 166.Alavinejad P., Hajiani E., Danyaee B., Morvaridi M. The effect of nutritional education and continuous monitoring on clinical symptoms, knowledge, and quality of life in patients with cirrhosis. Gastroenterol Hepatol Bed Bench. 2019 Winter;12:17–24. [PMC free article] [PubMed] [Google Scholar]
- 167.Chaney A.J., Heckman M.G. The benefit of supplemental nutrition education for severely malnourished patients awaiting liver transplant. Prog Transplant. 2018;28:390–393. doi: 10.1177/1526924818800052. [DOI] [PubMed] [Google Scholar]
- 168.Iwasa M., Iwata K., Hara N. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition. 2013;29:1418–1421. doi: 10.1016/j.nut.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 169.Gottschall C.B., Pereira T.G., Rabito E.I., Álvares-Da-Silva M.R. Nutritional Status and dietary intake in non-cirrhotic adult chronic hepatitis C patients. Arq Gastroenterol. 2015;52:204–209. doi: 10.1590/S0004-28032015000300010. [DOI] [PubMed] [Google Scholar]
- 170.Roongpisuthipong C., Sobhonlidsuk A., Nantiruj K., Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761–765. doi: 10.1016/s0899-9007(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 171.Mathur S., Peng S., Gane E.J., McCall J.L., Plank L.D. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition. 2007;23:398–403. doi: 10.1016/j.nut.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 172.Madden A.M., Morgan M.Y. Resting energy expenditure should be measured in patients with cirrhosis, not predicted. Hepatology. 1999;30:655–664. doi: 10.1002/hep.510300326. [DOI] [PubMed] [Google Scholar]
- 173.Plank L.D., Gane E.J., Peng S. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 174.Tsien C.D., McCullough A.J., Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 175.Berzigotti A., Albillos A., Villanueva C. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 176.Dasarathy S., Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Amodio P., Bemeur C., Butterworth R. The nutritional management of hepatic encephalopathy in patients with cirrhosis: international society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 178.Marchesini G., Bianchi G., Merli M. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 179.Matsuoka S., Tamura A., Nakagawara H., Moriyama M. Improvement in the nutritional status and clinical conditions of patients with liver failure using a liver diet combined with a branched chain amino acids-enriched elemental diet. Hepato-Gastroenterology. 2014;61:1308–1312. [PubMed] [Google Scholar]
- 180.Gu X.B., Yang X.J., Zhu H.Y., Xu B.Y. Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liver. 2012;6:355–361. doi: 10.5009/gnl.2012.6.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morando F., Rosi S., Gola E. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508–1515. doi: 10.1111/liv.12583. [DOI] [PubMed] [Google Scholar]
- 182.Kumar R., Kumar P., Saxena K.N. Vitamin D status in patients with cirrhosis of the liver and their relatives-A case control study from North India. Indian J Gastroenterol. 2017;36:50–55. doi: 10.1007/s12664-017-0727-7. [DOI] [PubMed] [Google Scholar]
- 183.Choudhary N.S., Tomar M., Chawla Y.K. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig Dis Sci. 2011;56:3323–3327. doi: 10.1007/s10620-011-1722-y. [DOI] [PubMed] [Google Scholar]
- 184.Jha A.K., Jha S.K., Kumar A., Dayal V.M., Jha S.K. Effect of replenishment of vitamin D on survival in patients with decompensated liver cirrhosis: a prospective study. World J Gastrointest Pathophysiol. 2017;8:133–141. doi: 10.4291/wjgp.v8.i3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kril J.J., Butterworth R.F. Diencephalic and cerebellar pathology in alcoholic and nonalcoholic patients with end-stage liver disease. Hepatology. 1997;26:837–841. doi: 10.1002/hep.510260405. [DOI] [PubMed] [Google Scholar]
- 186.Thomson A.D., Cook C.C., Touquet R., Henry J.A. Royal College of physicians, london. The royal College of physicians report on alcohol: guidelines for managing wernicke's encephalopathy in the accident and emergency department. Alcohol Alcohol. 2002;37:513–521. doi: 10.1093/alcalc/37.6.513. [DOI] [PubMed] [Google Scholar]
- 187.Ambrose M.L., Bowden S.C., Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25:112–116. [PubMed] [Google Scholar]
- 188.Chacko R.T., Chacko A. Serum & muscle magnesium in Indians with cirrhosis of liver. Indian J Med Res. 1997;106:469–474. [PubMed] [Google Scholar]
- 189.Marchesini G., Fabbri A., Bianchi G., Brizi M., Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23:1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 190.Ney M., Vandermeer B., van Zanten S.J., Ma M.M., Gramlich L., Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37:672–679. doi: 10.1111/apt.12252. [DOI] [PubMed] [Google Scholar]
- 191.Koretz R.L., Avenell A., Lipman T.O. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012;16 doi: 10.1002/14651858.CD008344.pub2. CD008344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Antar R., Wong P., Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463–467. doi: 10.1155/2012/945707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wicks C., Routley D., Williams R. Comparison of enteral feeding and total parenteral nutrition after liver transplantation. Lancet. 1994;344:837–840. doi: 10.1016/s0140-6736(94)92824-x. [DOI] [PubMed] [Google Scholar]
- 194.Hasse J.M., Blue L.S., Liepa G.U. Early enteral nutrition support in patients undergoing liver transplantation. J Parenter Enteral Nutr. 1995;19:437–443. doi: 10.1177/0148607195019006437. [DOI] [PubMed] [Google Scholar]
- 195.de Ledinghen V., Beau P., Mannant P.R. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci. 1997;42:536–541. doi: 10.1023/a:1018838808396. [DOI] [PubMed] [Google Scholar]
- 196.McClave S.A., Chang W.K. When to feed the patient with gastrointestinal bleeding. Nutr Clin Pract. 2005;20:544–550. doi: 10.1177/0115426505020005544. [DOI] [PubMed] [Google Scholar]
- 197.Carey E.J., Lai J.C., Sonnenday C. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kondrup J., Muller M.J. Energy and protein requirements of patients with chronic liver disease. J Hepatol. 1997;27:239–247. doi: 10.1016/s0168-8278(97)80308-x. [DOI] [PubMed] [Google Scholar]
- 199.Peng S., Plank L.D., McCall J.L., Gillanders L.K., McIlroy K., Gane E.J. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 200.Tsien C., Davuluri G., Singh D. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nielsen K., Kondrup J., Martinsen L. Longterm oral refeeding of patients with cirrhosis of the liver. Br J Nutr. 1995;74:557–567. doi: 10.1079/bjn19950158. [DOI] [PubMed] [Google Scholar]
- 202.Scott D., Blizzard L., Fell J., Ding C., Winzenberg T., Jones G. A prospective study of the associations between 25-hydroxyvitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. 2010;73:581–587. doi: 10.1111/j.1365-2265.2010.03858.x. [DOI] [PubMed] [Google Scholar]
- 203.Verreijen A.M., Verlaan S., Engberink M.F., Swinkels S., de Vogel-van den Bosch J., Weijs P.J. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101:279–286. doi: 10.3945/ajcn.114.090290. [DOI] [PubMed] [Google Scholar]
- 204.Liberman K., Njemini R., Luiking Y. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res. 2019;31:845–854. doi: 10.1007/s40520-019-01208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dasarathy A., Davuluri G., Silva R.N.E. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017;65:2045–2058. doi: 10.1002/hep.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kumar A., Davuluri G., Silva R.N.E. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017;65:2045–2058. doi: 10.1002/hep.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsien C., Davuluri G., Singh D. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Davuluri G., Krokowski D., Guan B.J. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65:929–937. doi: 10.1016/j.jhep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hiramatsu A., Aikata H., Uchikawa S. Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosis. Hepatol Commun. 2019;3:348–355. doi: 10.1002/hep4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sinclair M., Gow P.J., Grossmann M., Shannon A., Hoermann R., Angus P.W. Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the model for end-stage liver disease score. Liver Transplant. 2016;22:1482–1490. doi: 10.1002/lt.24607. [DOI] [PubMed] [Google Scholar]
- 211.Sinclair M., Grossmann M., Hoermann R., Angus P.W., Gow P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906–913. doi: 10.1016/j.jhep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 212.Calder P.C. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;98:S133–S139. doi: 10.1017/S0007114507832909. [DOI] [PubMed] [Google Scholar]
- 213.Annetta M.G., Pittiruti M., Vecchiarelli P., Silvestri D., Caricato A., Antonelli M. Immunonutrients in critically ill patients: an analysis of the most recent literature. Minerva Anestesiol. 2016;82:320–331. [PubMed] [Google Scholar]
- 214.Ney M., Haykowsky M.J., Vandermeer B., Shah A., Ow M., Tandon P. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016;44:796–806. doi: 10.1111/apt.13771. [DOI] [PubMed] [Google Scholar]
- 215.Dunn M.A., Josbeno D.A., Schmotzer A.R. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transplant. 2016;22:1324–1332. doi: 10.1002/lt.24506. [DOI] [PubMed] [Google Scholar]
- 216.Tandon P., Ismond K.P., Riess K. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69:1164–1177. doi: 10.1016/j.jhep.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 217.Bandi J.C., García-Pagán J.C., Escorsell A. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology. 1998;28:677–682. doi: 10.1002/hep.510280312. [DOI] [PubMed] [Google Scholar]
- 218.Garcia-Pagan J.C., Santos C., Barbera J.A. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300–1306. doi: 10.1053/gast.1996.v111.pm8898644. [DOI] [PubMed] [Google Scholar]
- 219.Dietrich R., Bachmann C., Lauterburg B.H. Exercise-induced hyperammonemia in patients with compensated chronic liver disease. Scand J Gastroenterol. 1990;25:329–334. doi: 10.3109/00365529009095494. [DOI] [PubMed] [Google Scholar]
- 220.Duarte-Rojo A., Ruiz-Margain A., Montano-Loza A.J., Macias-Rodriguez R.U., Ferrando A., Kim W.R. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transplant. 2018;24:122–139. doi: 10.1002/lt.24958. [DOI] [PubMed] [Google Scholar]
- 221.Keating S.E., Hackett D.A., George J., Johnson L.A. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 222.Katsagoni C.N., Georgoulis M., Papatheodoridis G.V., Panagiotakos D.B., Kontogianni M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 223.Orci L.A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 224.Hiraoka A., Michitaka K., Kiguchi D. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416–1423. doi: 10.1097/MEG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 225.Román E., García-Galcerán C., Torrades T. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zenith L., Meena N., Ramadi A. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–1926.e2. doi: 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 227.Kruger C., McNeely M.L., Bailey R.J. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep. 2018;8:99. doi: 10.1038/s41598-017-18320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Macías-Rodríguez R.U., Ilarraza-Lomelí H., Ruiz-Margáin A. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7 doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Berzigotti A., Albillos A., Villanueva C. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 230.Debette-Gratien M., Tabouret T., Antonini M.T. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145–150. doi: 10.1097/TP.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 231.Williams F.R., Vallance A., Faulkner T. Home-based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transplant. 2019;25:995–1006. doi: 10.1002/lt.25442. [DOI] [PubMed] [Google Scholar]
- 232.Aamann L., Dam G., Rinnov A.R., Vilstrup H., Gluud L.L. Physical exercise for people with cirrhosis. Cochrane Database Syst Rev. 2018 Dec 21;12:CD012678. doi: 10.1002/14651858.CD012678.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mathur S., Janaudis-Ferreira T., Wickerson L. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235–2245. doi: 10.1111/ajt.12874. [DOI] [PubMed] [Google Scholar]
- 234.Brown B., Roehl K., Betz M. Enteral nutrition formula selection: current evidence and implications for practice. Nutr Clin Pract. 2015;30:72–85. doi: 10.1177/0884533614561791. [DOI] [PubMed] [Google Scholar]
- 235.Zadak Z., Kent-Smith L. Basics in clinical nutrition: commercially prepared formulas. E-SPN. the European e-Journal of Clinical Nutrition and Metabolism. 2009;4:e212–e215. [Google Scholar]
- 236.Escuro A.A., Hummell A.C. Enteral formulas in nutrition support practice: is there a better choice for your patient? Nutr Clin Pract. 2016;31:709–722. doi: 10.1177/0884533616668492. [DOI] [PubMed] [Google Scholar]
- 237.Malone A. Enteral formula selection: a review of selected product categories. In: Nutrition Issues in Gastroenterology 2005, June Series #28. Ed: Parish CR, Shugar Publishing NY.
- 238.Davidson P., Kwiatkowski C.A., Wien M. Management of hyperglycemia and enteral nutrition in the hospitalized patient. Nutr Clin Pract. 2015;30:652–659. doi: 10.1177/0884533615591057. [DOI] [PubMed] [Google Scholar]
- 239.Huisman E.J., Trip E.J., Siersema P.D., van Hoek B., van Erpecum K.J. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 240.Merli M., Giusto M., Lucidi C. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 241.Montano-Loza A.J. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Maharshi S., Sharma B.C., Sachdeva S., Srivastava S., Sharma P. Efficacy of nutritional therapy for patients with cirrhosis and minimal hepatic encephalopathy in a randomized trial. Clin Gastroenterol Hepatol. 2016;14:454–460.e3. doi: 10.1016/j.cgh.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 243.Vaisman N., Katzman H., Carmiel-Haggai M., Lusthaus M., Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92:137–140. doi: 10.3945/ajcn.2010.29211. [DOI] [PubMed] [Google Scholar]
- 244.Córdoba J., López-Hellín J., Planas M. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 245.Nielsen K., Kondrup J., Martinsen L. Long-term oral refeeding of patients with cirrhosis of the liver. Br J Nutr. 1995;74:557–567. doi: 10.1079/bjn19950158. [DOI] [PubMed] [Google Scholar]
- 246.Campollo O., Sprengers D., Dam G., Vilstrup H., McIntyre N. Protein tolerance to standard and high protein meals in patients with liver cirrhosis. World J Hepatol. 2017;9:667–676. doi: 10.4254/wjh.v9.i14.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Keshavarzian A., Meek J., Sutton C., Emery V.M., Hughes E.A., Hodgson H.J. Dietary protein supplementation from vegetable sources in the management of chronic portal systemic encephalopathy. Am J Gastroenterol. 1984;79:945–949. [PubMed] [Google Scholar]
- 248.Bianchi G.P., Marchesini G., Fabbri A. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med. 1993;233:385–392. doi: 10.1111/j.1365-2796.1993.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 249.Gheorghe L., Iacob R., Vădan R., Iacob S., Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005;14:231–238. [PubMed] [Google Scholar]
- 250.Uribe M., Dibildox M., Malpica S. Beneficial effect of vegetable protein diet supplemented with psyllium plantago in patients with hepatic encephalopathy and diabetes mellitus. Gastroenterology. 1985;88:901–907. doi: 10.1016/s0016-5085(85)80006-8. [DOI] [PubMed] [Google Scholar]
- 251.Amodio P., Caregaro L., Pattenò E., Marcon M., Del Piccolo F., Gatta A. Vegetarian diets in hepatic encephalopathy: facts or fantasies? Dig Liver Dis. 2001;33:492–500. doi: 10.1016/s1590-8658(01)80028-1. [DOI] [PubMed] [Google Scholar]
- 252.Eghtesad S., Poustchi H., Malekzadeh R. Malnutrition in liver cirrhosis: the influence of protein and sodium. Middle East J Dig Dis. 2013;5:65–75. [PMC free article] [PubMed] [Google Scholar]
- 253.Tsien C., Davuluri G., Singh D. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gluud L.L., Dam G., Les I. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017 May 18;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kohno M., Fuji T., Hirayama C. [15N] glycine metabolism in normal and cirrhotic subjects. Biochem Med Metab Biol. 1990;43:201–213. doi: 10.1016/0885-4505(90)90026-w. [DOI] [PubMed] [Google Scholar]
- 256.Glass C., Hipskind P., Tsein C. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol. 1985;2013:559–565. doi: 10.1152/japplphysiol.01042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.McClave S.A., Taylor B.E., Martindale R.G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN - J Parenter Enter Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 258.Balarmino G., Singer P., Gonzales M.C. Prognostic value of energy expenditure and respiratory quotient measuring in patients with liver cirrhosis. Clin Nutr. 2019;38:1899–1904. doi: 10.1016/j.clnu.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 259.Mendenhall C.L., Anderson S., Weesner R.E., Goldberg S.J., Crolic K.A. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans administration cooperative study group on alcoholic hepatitis. Am J Med. 1984;76:211–222. doi: 10.1016/0002-9343(84)90776-9. [DOI] [PubMed] [Google Scholar]
- 260.Sonika U., Jadaun S., Ranjan G. Alcohol-related acute-on-chronic liver failure-Comparison of various prognostic scores in predicting outcome. Indian J Gastroenterol. 2018;37:50–57. doi: 10.1007/s12664-018-0827-z. [DOI] [PubMed] [Google Scholar]
- 261.Mendenhall C., Roselle G.A., Gartside P., Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19:635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 262.Shalimar, Rout G., Jadaun S.S., Ranjan G., Kedia S., Gunjan D. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018;50:1225–1231. doi: 10.1016/j.dld.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 263.Shalimar, Kumar D., Vadiraja P.K., Nayak B., Thakur B., Das P. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31:856–864. doi: 10.1111/jgh.13213. [DOI] [PubMed] [Google Scholar]
- 264.Thursz M.R., Richardson P., Allison M. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 265.Moreno C., Deltenre P., Senterre C. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology. 2016;150:903–910. doi: 10.1053/j.gastro.2015.12.038. e8. [DOI] [PubMed] [Google Scholar]
- 266.Cabré E., Rodríguez-Iglesias P., Caballería J. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 267.Antar R., Wong P., Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463–467. doi: 10.1155/2012/945707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.McClave S.A., Chang W.K. When to feed the patient with gastrointestinal bleeding. Nutr Clin Pract. 2005;20:544–550. doi: 10.1177/0115426505020005544. [DOI] [PubMed] [Google Scholar]
- 269.Hébuterne X., Vanbiervliet G. Feeding the patients with upper gastrointestinal bleeding. Curr Opin Clin Nutr Metab Care. 2011;14:197–201. doi: 10.1097/MCO.0b013e3283436dc5. [DOI] [PubMed] [Google Scholar]
- 270.De Ledinghen V., Beau P., Mannant P.R. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci. 1997;42:536–541. doi: 10.1023/a:1018838808396. [DOI] [PubMed] [Google Scholar]
- 271.Dudley F.J. Pathophysiology of ascites formation. Gastroenterol Clin N Am. 1992;21 215-35. [PubMed] [Google Scholar]
- 272.Cheung K., Lee S.S., Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10:117–125. doi: 10.1016/j.cgh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 273.Gauthier A., Levy V.G., Quinton A. Salt or no salt in the treatment of cirrhotic ascites: a randomised study. Gut. 1986;27:705–709. doi: 10.1136/gut.27.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gu X.B., Yang X.J., Zhu H.Y., Xu B.Y. Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liv. 2012;6:35. doi: 10.5009/gnl.2012.6.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Coolsen M.M., Wong-Lun-Hing E.M., Dam R.M. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB. 2013;15:245–251. doi: 10.1111/j.1477-2574.2012.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.García-Pagàn J.C., Santos C., Barberá J.A. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300–1306. doi: 10.1053/gast.1996.v111.pm8898644. [DOI] [PubMed] [Google Scholar]
- 277.Macías-Rodríguez R.U., Ilarraza-Lomelí H., Ruiz-Margáin A. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7 doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shimizu H., Phuong V., Maia M. Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis. 2013;9:1–6. doi: 10.1016/j.soard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 279.Clapp B., Wynn M., Martyn C., Foster C., O'Dell M., Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14:741–747. doi: 10.1016/j.soard.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 280.Rebibo L., Gerin O., Verhaeghe P., Dhahri A., Cosse C., Regimbeau J.M. Laparoscopic sleeve gastrectomy in patients with NASH-related cirrhosis: a case-matched study. Surg Obes Relat Dis. 2014;10:405–410. doi: 10.1016/j.soard.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 281.Sharpton S.R., Terrault N.A., Posselt A.M. Outcomes of sleeve gastrectomy in obese liver transplant candidates. Liver Transplant. 2019 Apr;25:538–544. doi: 10.1002/lt.25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gutierrez J.A., Landaverde C., Wells J.T., Poordad F. Lorcaserin use in the management of morbid obesity in a pre-liver transplant patient. Hepatology. 2016;64:301–302. doi: 10.1002/hep.28556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Choudhary N.S., Puri R., Saraf N. Intragastric balloon as a novel modality for weight loss in patients with cirrhosis and morbid obesity awaiting liver transplantation. Indian J Gastroenterol. 2016;35:113–116. doi: 10.1007/s12664-016-0643-2. [DOI] [PubMed] [Google Scholar]
- 284.Mosko J.D., Nguyen G.C. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 285.Cazzo E., Gestic M.A., Utrini M.P. Bariatric surgery in individuals with liver cirrhosis: a narrative review. Rev Assoc Med Bras. 2017;63:190–194. doi: 10.1590/1806-9282.63.02.190. [DOI] [PubMed] [Google Scholar]
- 286.Takata M.C., Campos G.M., Ciovica R. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4:159–164. doi: 10.1016/j.soard.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 287.Lin M.Y., Tavakol M.M., Sarin A. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9:653–658. doi: 10.1016/j.soard.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 288.Lin M.Y., Tavakol M.M., Sarin A. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013;27:81–85. doi: 10.1007/s00464-012-2410-5. [DOI] [PubMed] [Google Scholar]
- 289.Nesher E., Mor E., Shlomai A. Simultaneous liver transplantation and sleeve gastrectomy: prohibitive combination or a necessity? Obes Surg. 2017;27:1387–1390. doi: 10.1007/s11695-017-2634-5. [DOI] [PubMed] [Google Scholar]
- 290.Trovato F.M., Catalano D., Martines G.F., Pace P., Trovato G.M. Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin Nutr. 2015;34:86–88. doi: 10.1016/j.clnu.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 291.Turati F., Trichopoulos D., Polesel J. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606–611. doi: 10.1016/j.jhep.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 292.Promrat K., Kleiner D.E., Niemeier H.M. Randomized controlled trial testing the effects of weight loss on non-alcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sultan M.I., Leon C.D., Biank V.F. Role of nutrition in pediatric chronic liver disease. Nutr Clin Pract. 2011;26:401–408. doi: 10.1177/0884533611405535. [DOI] [PubMed] [Google Scholar]
- 294.Carter-Kent C., Radhakrishnan K., Feldstein A.E. Increasing calories, decreasing morbidity and mortality: is improved nutrition the answer to better outcomes in patients with biliary atresia? Hepatology. 2007;46:1329–1331. doi: 10.1002/hep.22043. [DOI] [PubMed] [Google Scholar]
- 295.Zemel B.S., Riley E.M., Stallings V.A. Evaluation of methodology for nutritional assessment in children: anthropometry, body composition, and energy expenditure. Annu Rev Nutr. 1997;17:211–235. doi: 10.1146/annurev.nutr.17.1.211. [DOI] [PubMed] [Google Scholar]
- 296.Khadilkar V., Yadav S., Agrawal K.K. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year- old Indian children. Indian Pediatr. 2015;52:47–55. doi: 10.1007/s13312-015-0566-5. [DOI] [PubMed] [Google Scholar]
- 297.Gorstein J., Sullivan K., Yip R. Issues in the assessment of nutritional status using anthropometry. Bull World Health Organ. 1994;72:273–283. [PMC free article] [PubMed] [Google Scholar]
- 298.Tanner J.M. Normal growth and techniques of growth assessment. Clin Endocrinol Metabol. 1986;15:411–451. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 299.Trowbridge F.L., Sommer A. Nutritional anthropometry and mortality risk. Am J Clin Nutr. 1981;34:2591–2592. doi: 10.1093/ajcn/34.11.2591. [DOI] [PubMed] [Google Scholar]
- 300.Briend A., Dykewicz C., Graven K., Mazumder R.N., Wojtyniak B., Bennish M. Usefulness of nutritional indices and classifications in predicting death of malnourished children. Br Med J. 1986;293:373–375. doi: 10.1136/bmj.293.6543.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sokol R.J., Stall C. Anthropometric evaluation of children with chronic liver disease. Am J Clin Nutr. 1990;52:203–208. doi: 10.1093/ajcn/52.2.203. [DOI] [PubMed] [Google Scholar]
- 302.Wasserman D., Zemel B.S., Mulberg A.E. Growth, nutritional status, body composition, and energy expenditure in prepubertal children with Alagille syndrome. J Pediatr. 1999;134:172–177. doi: 10.1016/s0022-3476(99)70411-7. [DOI] [PubMed] [Google Scholar]
- 303.Rezende I.F.B., Conceição-Machado M.E.P., Souza V.S., Santos E.M.D., Silva L.R. Sarcopenia in children and adolescents with chronic liver disease. J Pediatr. 2019;S0021–7557:31087–31088. doi: 10.1016/j.jped.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Young S., Kwarta E., Azzam R., Sentongo T. Nutrition assessment and support in children with end-stage liver disease. Nutr Clin Pract. 2013;28:317–329. doi: 10.1177/0884533612474043. [DOI] [PubMed] [Google Scholar]
- 305.Moukarzel A.A., Najm I., Vargas J., McDiarmid S.V., Busuttil R.W., Ament M.E. Effect of nutritional status on outcome of orthotopic liver transplantation in pediatric patients. Transplant Proc. 1990;22:1560–1563. [PubMed] [Google Scholar]
- 306.Hume R., Burchell A., Williams F.L., Koh D.K. Glucose homeostasis in the newborn. Early Hum Dev. 2005;81:95–101. doi: 10.1016/j.earlhumdev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 307.Ooi P.H., Gilmour S.M., Yap J., Mager D.R. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: a systematic review. Clin Nutr ESPEN. 2018;28:41–51. doi: 10.1016/j.clnesp.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 308.Chin S.E., Shepherd R.W., Thomas B.J. Nutritional support in children with end-stage liver disease: a randomized crossover trial of a branched- chain amino acid supplement. Am J Clin Nutr. 1992;56:158–163. doi: 10.1093/ajcn/56.1.158. [DOI] [PubMed] [Google Scholar]
- 309.Shen Y.M., Wu J.F., Hsu H.Y. Oral absorbable fat-soluble vitamin formulation in pediatric patients with cholestasis. J Pediatr Gastroenterol Nutr. 2012;55:587–591. doi: 10.1097/MPG.0b013e31825c9732. [DOI] [PubMed] [Google Scholar]
- 310.Shneider B.L., Magee J.C., Bezerra J.A. Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia. Pediatrics. 2012;130:e607–e614. doi: 10.1542/peds.2011-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thebaut A., Nemeth A., Le Mouhaer J. Oral tocofersolan corrects or prevents vitamin E deficiency in children with chronic cholestasis. J Pediatr Gastroenterol Nutr. 2016;63:610–615. doi: 10.1097/MPG.0000000000001331. [DOI] [PubMed] [Google Scholar]
- 312.Bancroft J., Cohen M.B. Intracranial hemorrhage due to vitamin K deficiency in breast-fed infants with cholestasis. J Pediatr Gastroenterol Nutr. 1993;16:78–80. doi: 10.1097/00005176-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 313.Strople J., Lovell G., Heubi J. Prevalence of subclinical vitamin K deficiency in cholestatic liver disease. J Pediatr Gastroenterol Nutr. 2009;49:78–84. doi: 10.1097/MPG.0b013e31819a61ff. [DOI] [PubMed] [Google Scholar]
- 314.Pettei M.J., Daftary S., Levine J.J. Essential fatty acid deficiency associated with the use of a medium-chain-triglyceride infant formula in pediatric hepatobiliary disease. Am J Clin Nutr. 1991;53:1217–1221. doi: 10.1093/ajcn/53.5.1217. [DOI] [PubMed] [Google Scholar]
- 315.Chin S.E., Shepherd R.W., Thomas B.J. The nature of malnutrition in children with end-stage liver disease awaiting orthotopic liver transplantation. Am J Clin Nutr. 1992;56:164–168. doi: 10.1093/ajcn/56.1.164. [DOI] [PubMed] [Google Scholar]
- 316.Goksu N., Ozsoylu S. Hepatic and serum levels of zinc, copper, and magnesium in childhood cirrhois. J Pediatr Gastroenterol Nutr. 1986;5:459–462. doi: 10.1097/00005176-198605000-00022. [DOI] [PubMed] [Google Scholar]
- 317.National Institute of Nutrition . National Institute of Nutrition; Hyderabad: 2010. Recommended Dietary Allowances. [Google Scholar]
- 318.Moreno L.A., Gottrand F., Hoden S., Turck D., Loeuille G.A., Farriaux J.P. Improvement of nutritional status in cholestatic children with supplemental nocturnal enteral nutrition. J Pediatr Gastroenterol Nutr. 1991;12:213–216. doi: 10.1097/00005176-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 319.Bes D.F., Fernandez M.C., Malla I. Pathophysiology, diagnostic evaluation, hospitalization criteria, treatment, nutritional management. Arch Argent Pediatr. 2017;115:385–390. doi: 10.5546/aap.2017.eng.385. [DOI] [PubMed] [Google Scholar]
- 320.R Glidden D., Roberts J.P., Rosenthal P. Overweight and obesity in pediatric liver transplant recipients: prevalence and predictors before and after transplant, United Network for Organ Sharing Data, 1987- 2010. Pediatr Transplant. 2012;16:41–49. doi: 10.1111/j.1399-3046.2011.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Alam S., Sood V. Metabolic liver disease: when to suspect and how to diagnose? Indian J Pediatr. 2016;83:1321–1333. doi: 10.1007/s12098-016-2097-z. [DOI] [PubMed] [Google Scholar]
- 322.Ferreira C.R., Cassiman D., Blau N. Clinical and biochemical footprints of inherited metabolic diseases. II. Metabolic liver diseases. Mol Genet Metabol. 2019;127:117–121. doi: 10.1016/j.ymgme.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Boyer S.W., Barclay L.J., Burrage L.C. Inherited metabolic disorders: aspects of chronic nutritional management. Nutr Clin Pract. 2015;30:502–510. doi: 10.1177/0884533615586201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian Food Composition Tables 2017. Publisher National Institute of Nutrition, ICMR, Department of Health Research Hyderabad, India.
- 325.Hopfer U. Membrane transport mechanisms for hexoses and amino acids in the small intestine. In: Johnson L.R., Christensen J., Jackson M.J., editors. Physiology of the Gastrointestinal Tract. 2nd ed. Raven Press; New York: 1987. pp. 1499–1526. [Google Scholar]
- 326.Holton J.B., Gillett M.G., MacFaul R., Young R. Galactosemia: a new severe variant due to uridine diphosphate galactose-4-epimerase deficiency. Arch Dis Child. 1981;56:885–887. doi: 10.1136/adc.56.11.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Van Calcar S.C., Bernstein L.E., Rohr F.J., Scaman C.H., Yannicelli S., Berry G.T. A re-evaluation of life-long severe galactose restriction for the nutrition management of classic galactosemia. Mol Genet Metabol. 2014;112:191–197. doi: 10.1016/j.ymgme.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 328.Portnoi P.A., Macdonald A. The lactose and galactose content of cheese suitable for galactosaemia: new analysis. JIMD Rep. 2016;29:85–87. doi: 10.1007/8904_2015_520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Van Calcar S.C., Bernstein L.E., Rohr F.J., Yannicelli S., Berry G.T., Scaman C.H. Galactose content of legumes, caseinates, and some hard cheeses: implications for diet treatment of classic galactosemia. J Agric Food Chem. 2014;62:1397–1402. doi: 10.1021/jf404995a. [DOI] [PubMed] [Google Scholar]
- 330.Berry G.T., Palmieri M., Gross K.C. The effect of dietary fruits and vegetables on urinary galactitol excretion in galactose-1-phosphate uridyl transferase deficiency. J Inherit Metab Dis. 1993;16:91–100. doi: 10.1007/BF00711320. [DOI] [PubMed] [Google Scholar]
- 331.Walter J.H., Collins J.E., Leonard J.V. Recommendations for the management of galactosaemia. UK galactosaemia steering group. Arch Dis Child. 1999;80:93–96. doi: 10.1136/adc.80.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Welling L., Bernstein L.E., Berry G.T. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. On behalf of the Galactosemia Network (GalNet) J Inherit Metab Dis. 2017;40:171–176. doi: 10.1007/s10545-016-9990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Fridovich-Keil J., Bean L., He M., Schroer R. Epimerase deficiency galactosemia. 2011 Jan 25. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews®. University of Washington, Seattle; Seattle (WA): 1993-2019. https://www.ncbi.nlm.nih.gov/books/NBK51671 updated 2016 Jun 16. internet. Available from: [Google Scholar]
- 334.Demirbas D., Brucker W.J., Berry G.T. Inborn errors of metabolism with hepatopathy. Metabolism defects of galactose, fructose, and tyrosine. Pediatr Clin. 2018;65:337–352. doi: 10.1016/j.pcl.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 335.Baker P., II, Ayres L., Gaughan S., Weisfeld-Adams J. Hereditary fructose intolerance. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews®. University of Washington, Seattle; Seattle (WA): 1993-2019. https://www.ncbi.nlm.nih.gov/books/NBK333439/ Internet. Available from: [Google Scholar]
- 336.Steinmann B., Gitzelmann R., Van den Berghe G. Disorders of fructose metabolism. In: Valle D., Beaudet A.L., Vogelstein B., editors. The Online Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2014. [Google Scholar]
- 337.Endres W., Sierck T., Shin Y.S. Clinical course of hereditary fructose intolerance in 56 patients. Acta Paediatr Jpn. 1988;30:452–456. doi: 10.1111/j.1442-200x.1988.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 338.Valayannopoulos V., Romano S., Mention K. What's new in metabolic and genetic hypoglycaemias: diagnosis and management. Eur J Pediatr. 2008;167:257–265. doi: 10.1007/s00431-007-0600-2. [DOI] [PubMed] [Google Scholar]
- 339.Tran C. Inborn errors of fructose metabolism. What can we learn from them? Nutrients. 2017;9:E356. doi: 10.3390/nu9040356. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen Y.-T., Kishnani P.S., Koeberl D. Glycogen storage diseases. In: Valle D., Beaudet A.L., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A., editors. The Online Metabolic and Molecular Bases of Inherited Disease. The McGraw-Hill Companies, Inc.; New York: 2013. [Google Scholar]
- 341.Burda P., Hochuli M. Hepatic glycogen storage disorders: what have we learned in recent years? Curr Opin Clin Nutr Metab Care. 2015;18:415–421. doi: 10.1097/MCO.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 342.Chou J.Y., Jun H.S., Mansfield B.C. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2015;38:511–519. doi: 10.1007/s10545-014-9772-x. [DOI] [PubMed] [Google Scholar]
- 343.Kishnani P.S., Austin S.L., Abdenur J.E. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. doi: 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 344.Correia C.E., Bhattacharya K., Lee P.J. Use of modified corn-starch therapy to extend fasting in glycogen storage disease types Ia and Ib. Am J Clin Nutr. 2008;88:1272–1276. doi: 10.3945/ajcn.2008.26352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chen Y.T., Cornblath M., Sidbury J.B. Cornstarch therapy in type I glycogen storage disease. N Engl J Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 346.Rake J.P., Visser G., Labrune J.V., Ullrich K., Smit G.P.A. Guidelines for management of glycogen storage disease type I-European study on glycogen storage disease type I (ESGSD I) Eur J Pediatr. 2002;161:S112–S119. doi: 10.1007/s00431-002-1016-7. [DOI] [PubMed] [Google Scholar]
- 347.Kishnani P.S., Austin S.L., Arn P. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446–463. doi: 10.1097/GIM.0b013e3181e655b6. [DOI] [PubMed] [Google Scholar]
- 348.Slonim A.E., Coleman R.A., Moses W.S. Myopathy and growth failure in debrancher enzyme deficiency: improvement with high-protein nocturnal enteral therapy. J Pediatr. 1984;105:906–911. doi: 10.1016/s0022-3476(84)80075-x. [DOI] [PubMed] [Google Scholar]
- 349.Slonim A.E., Weisberg C., Benke P., Evans O.B., Burr I.M. Reversal of debrancher deficiency myopathy by the use of high-protein nutrition. Ann Neurol. 1982;11:420–422. doi: 10.1002/ana.410110417. [DOI] [PubMed] [Google Scholar]
- 350.Mayorandan S., Meyer U., Hartmann H., Das A.M. Glycogen storage disease type III: modified Atkins diet improves myopathy. Orphanet J Rare Dis. 2014;9:196. doi: 10.1186/s13023-014-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sentner C.P., Caliskan K., Vletter W.B., Smit G.P. Heart failure due to severe hypertrophic cardiomyopathy reversed by low calorie, high protein dietary adjustments in a glycogen storage disease type IIIa patient. JIMD Rep. 2012;5:13–16. doi: 10.1007/8904_2011_111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Goldberg T., Slonim A.E. Nutrition therapy for hepatic glycogen storage diseases. J Am Diet Assoc. 1993;93:1423–1430. doi: 10.1016/0002-8223(93)92246-t. [DOI] [PubMed] [Google Scholar]
- 353.Borowitz S.M., Greene H.L. Cornstarch therapy in a patient with type III glycogen storage disease. J Pediatr Gastroenterol Nutr. 1987;6:631–634. doi: 10.1097/00005176-198707000-00024. [DOI] [PubMed] [Google Scholar]
- 354.Derks T.G., Smit G.P. Dietary management in glycogen storage disease type III: what is the evidence? J Inherit Metab Dis. 2015;38:545–550. doi: 10.1007/s10545-014-9756-x. [DOI] [PubMed] [Google Scholar]
- 355.Chen Y.T., Kishnani P.S., Koeberl D. Glycogen storage diseases. In: Valle D., Beaudet A., Vogelstein B., editors. Scriver's Online Metabolic & Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2016. [Google Scholar]
- 356.Dagli A., Weinstein D.A. Glycogen storage disease type VI. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington, Seattle; Seattle, WA: 1993. pp. 2009–2019.https://www.ncbi.nlm.nih.gov/books/NBK5941/ (updated 2011) [Google Scholar]
- 357.Herbert M., Goldstein J.L., Rehder C., Austin S., Kishnani P.S., Bali D.S. Phosphorylase kinase deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington–Seattle; Seattle, WA: 1993–2018. p. 2011.http://www.ncbi.nlm.nih.gov/books/NBK55061 (updated 2018) [Google Scholar]
- 358.Kishnani P.S., Goldstein J., Austin S.L. Diagnosis and management of glycogen storage diseases type VI and IX: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2019;21:772–789. doi: 10.1038/s41436-018-0364-2. [DOI] [PubMed] [Google Scholar]
- 359.De Laet C., Dionisi-Vici C., Leonard J.V. Recommendations for the management of tyrosinaemia type 1. Orphanet Rare Dis. 2013;8:8. doi: 10.1186/1750-1172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.De Jesus V.R., Adam B.W., Mandel D., Cuthbert C.D., Matern D. Succinylacetone as primary marker to detect tyrosinemia type I in new-borns and its measurement by new-born screening programs. Mol Genet Metabol. 2014;113:67–75. doi: 10.1016/j.ymgme.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lindstedt S., Holme E., Lock E.A., Hjalmarson O., Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 362.Rampini S., Vollmin J.A., Bosshard H.R., Muller M., Curtius H.C. Aromatic acids in urine of healthy infants, persistent hyperphenylalaninemia, and phenylketonuria, before and after phenylalanine load. Pediatr Res. 1974;8:704–709. doi: 10.1203/00006450-197407000-00003. [DOI] [PubMed] [Google Scholar]
- 363.Otten J., Hellwig J., Meyers L. National Academies Press; Washington, DC: 2006. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. [DOI] [Google Scholar]
- 364.Acosta P.B., Matalon K.M. Nutrition management of patients with inherited disorders of aromatic amino acid metabolism. In: Acosta P.B., editor. Nutrition Management of Patients with Inherited Metabolic Disorders. Jones and Bartlett; Sudbury, MA: 2010. pp. 119–174. [Google Scholar]
- 365.van Spronsen F.J., van Rijn M., Meyer U., Das A.M. Dietary considerations in tyrosinemia type I. In: Tanguar R., editor. Vol. 959. Springer; Cham: 2017. p. 197. (Hereditary Tyrosinemia. Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 366.Daly A., Gokmen-Ozel H., MacDonald A. Diurnal variation of phenylalanine concentrations in tyrosinaemia type 1: should we be concerned? J Hum Nutr Diet. 2012;25:111–116. doi: 10.1111/j.1365-277X.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 367.Wilson C.J., Van Wyk K.G., Leonard J.V., Clayton P.T. Phenylalanine supplementation improves the phenylalanine profile in tyrosinaemia. J Inherit Metab Dis. 2000;23:677–683. doi: 10.1023/a:1005666426079. [DOI] [PubMed] [Google Scholar]
- 368.Socha P., Janczyk W., Dhawan A. Wilson's disease in children: a position paper by the hepatology committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:334–344. doi: 10.1097/MPG.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 369.Nagral A., Sarma M.S., Matthai J. Wilson's disease: clinical practice guidelines of the Indian national association for study of the liver, the Indian society of pediatric gastroenterology, hepatology and nutrition, and the movement disorders society of India. J Clin Exp Hepatol. 2019;9:74–98. doi: 10.1016/j.jceh.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.European association for the study of the liver. EASL clinical practice guidelines: Wilson's disease. J Hepatol. 2012;56:671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 371.Roberts E.A., Schilsky M.L. American association for study of liver disease (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 372.Turnlund J.R., Keyes W.R., Anderson H.L., Acord L.L. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr. 1989;49:870–878. doi: 10.1093/ajcn/49.5.870. [DOI] [PubMed] [Google Scholar]
- 373.EFSA Panel on Dietetic Products, Nutrition and Allergies Scientific opinion on dietary reference values for copper. EFSA J. 2015;13:4253. doi: 10.2903/j.efsa.2015.4253. [DOI] [Google Scholar]
- 374.Lurie D.G., Holden J.M., Schubert A. The copper content of foods based on a critical evaluation of published analytical data. J Food Compos Anal. 1989;2:298–316. [Google Scholar]
- 375.Russel K., Gillanders L.K., Orr D.W., Plank L.D. Dietary restriction in Wilson's disease. Eur J Clin Nutr. 2018;72:326–331. doi: 10.1038/s41430-017-0002-0. [DOI] [PubMed] [Google Scholar]
- 376.Pandit A., Bhave S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am J Clin Nutr. 1996;63 doi: 10.1093/ajcn/63.5.830. 830S–5S. [DOI] [PubMed] [Google Scholar]
- 377.Spiekerkoetter U., Lindner M., Santer R. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- 378.Saudubray J.M., Martin D., De Lonlay P. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 379.Spiekerkoetter U., Lindner M., Santer R. Management and outcome in 75 individuals with long-chain fatty acid oxidation defects: results from a workshop. J Inherit Metab Dis. 2009;32:488–497. doi: 10.1007/s10545-009-1125-9. [DOI] [PubMed] [Google Scholar]
- 380.Spiekerkoetter U., Bastin J., Gillingham M., Morris A., Wijburg F., Wilcken B. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis. 2010;33:555–561. doi: 10.1007/s10545-010-9188-1. [DOI] [PubMed] [Google Scholar]
- 381.Gillingham M.B., Connor W.E., Matern D. Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Mol Genet Metabol. 2003;79:114–123. doi: 10.1016/s1096-7192(03)00073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Waisbren S., Gropman A., Members of U.D.C.C., Batshaw M. Improving long term outcomes in urea cycle disorders-Report from the urea cycle disorders consortium. J Inherit Metab Dis. 2016;39:573–584. doi: 10.1007/s10545-016-9942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Häberle J., Burlina A., Chakrapani A. Suggested guidelines for the diagnosis and management of urea cycle disorders: first revision. J Inherit Metab Dis. 2019;42:1192–1230. doi: 10.1002/jimd.12100. [DOI] [PubMed] [Google Scholar]
- 384.Dixon M. Clinical Pediatric Dietetics. Shaw; Lawson: 2007. Disorders of amino acid metabolism, organic acidemias and urea cycle defects. Organic acidemias and urea cycle disorders; pp. 357–389. [Google Scholar]
- 385.Singh R.H. Nutrition management of patients with inherited disorders of urea cycle enzymes. In: Acosta P.B., editor. Nutrition Management of Patients with Inherited Metabolic Disorders. Jones & Bartlett Learning; Sudbury: 2009. pp. 405–429. [Google Scholar]
- 386.WHO Technical Report Series . Report of a Joint WHO/FAO/UNU Expert Consultation Series. Vol. 935. 2007. Protein and amino acid requirement in human nutrition. [PubMed] [Google Scholar]
- 387.Adam S., Champion H., Daly A. British Inherited Metabolic Diseases Group (BIMDG) Dietitian's Group. Dietary management of urea cycle disorders: UK practice. J Hum Nutr Diet. 2012;25:398–404. doi: 10.1111/j.1365-277X.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 388.Dixon M, White F, Leonard J. Breast feeding in metabolic disease: how successful is this? Compilation of Papers Presented at the Fifth Dietitians Meeting at the VIII International Congress of Inborn Errors of Metabolism. Pp 4-8. Cambridge: ICIEM.
- 389.Huner G., Baykal T., Demir F., Demirkol M. Breastfeeding experience in inborn errors of metabolism other than phenylketonuria. J Inherit Metab Dis. 2005;28:457–465. doi: 10.1007/s10545-005-0457-3. [DOI] [PubMed] [Google Scholar]
- 390.Saheki T., Kobayashi K., Iijima M. Pathogenesis and pathophysiology of citrin (a mitochondrial aspartate glutamate carrier) deficiency. Metab Brain Dis. 2002;17:335–346. doi: 10.1023/a:1021961919148. [DOI] [PubMed] [Google Scholar]
- 391.Saheki T., Song Y.Z. Citrin deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews®. University of Washington, Seattle; Seattle (WA): 2005 Sep 16. pp. 1993–2019. Updated 2017 Aug 10. Internet. [Google Scholar]
- 392.Mutoh K., Kurokawa K., Kobayashi K., Saheki T. Treatment of a citrin-deficient patient at the early stage of adult-onset type II citrullinaemia with arginine and sodium pyruvate. J Inherit Metab Dis. 2008;31:S343–S347. doi: 10.1007/s10545-008-0914-x. [DOI] [PubMed] [Google Scholar]
- 393.Reuter B., Shaw J., Hanson J., Tatee V., Acharya C., Bajaj J.S. Nutritional assessment in inpatients with cirrhosis can be improved after training and is associated with lower readmissions. Liver Transplant. 2019;25:1790–1799. doi: 10.1002/lt.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]