Abstract
Background and Purpose:
Life’s Simple 7 (LS7) is a metric for cardiovascular health based on the seven domains of smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and fasting glucose. Because they may be targeted for secondary prevention purposes, we hypothesized that stroke survivors would experience improvement in LS7 score over time compared to people who did not experience a stroke. We addressed this hypothesis in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort of African American and white adults enrolled between 2003-2007.
Methods:
Participants who had LS7 data at baseline, were stroke-free at baseline, had a 10-year follow-up visit, and either did not have a stroke or had an ischemic stroke more than one year before follow-up were included (N=7569). Among these participants, 149 (2.0%) had an adjudicated ischemic stroke between the LS7 assessments. LS7 scores were classified as 0-2 points for each domain for a maximum score of 14, with higher scores representing better health. Multivariable linear regression was used to test the association of ischemic stroke with change in LS7 score. Covariates included baseline LS7 score, age, race, sex, education, and geographic region.
Results:
The 149 stroke survivors had an average of 4.9 years (SD=2.5) of follow up from the stroke event to the second LS7 assessment. After adjusting for covariates, participants who experienced an ischemic stroke showed 0.28 points more decline in total LS7 score (p=0.03) than those who did not experience a stroke.
Conclusions:
Stroke survivors did not experience improvements in cardiovascular health due to secondary prevention after ischemic stroke. On the contrary, they experienced significantly greater decline, indicating the need for greater efforts in secondary prevention after a stroke.
Keywords: stroke, lifestyle, risk factors, secondary prevention, epidemiology
Introduction
Life’s Simple 7 (LS7) was developed by the American Heart Association in 2010 as a measure of cardiovascular health based on seven domains of smoking, diet, physical activity, body mass index (BMI), blood pressure, total cholesterol, and fasting glucose.1 Higher LS7 scores indicate better cardiovascular health. A study with a representative sample of U.S. adults who are stroke survivors found that none of the participants met all seven ideal metrics and that stroke survivors with lower scores on the LS7 had higher rates of all-cause mortality2. In a cross-sectional study, less than 15% of stroke survivors had an ideal score on four or more out of the seven domains, and the prevalence of ideal cardiovascular health was lower for African American stroke survivors compared to white stroke survivors, and among those with lower compared to higher levels of education.3 Since few studies have LS7 scores before and after stroke, it is not known whether or how LS7 scores change after a stroke and how that change compares with those who have not had stroke over a similar time interval.
Recurrent strokes are a critical public health issue. On an annual basis, they comprise approximately 23% of all strokes in the United States4 and are more likely to be fatal or disabling than first stroke.5–8 Due to secondary prevention efforts to improve modifiable risk factors such as hypertension and dyslipidemia9, stroke survivors may experience improvements in LS7 over time compared to those who did not experience a stroke, though we are not aware of any studies that have evaluated this possibility.
In this study, we sought to determine whether persons who developed ischemic stroke had improvements in LS7 score over the course of up to 10 years compared to persons who did not experience a stroke over the same time period. Additionally, we aimed to determine whether associations differed by age, sex, and race, as well as whether time since stroke influences this change among the subgroup of stroke survivors.
Materials and Methods
In cooperation with the Institutional Review Board of the University of Alabama at Birmingham, the REasons for Geographic and Racial Differences in Stroke project facilitates data sharing through formal data use agreements. Investigators who wish to access the data should send their requests to regardsadmin@uab.edu.
Study participants.
REGARDS is a population-based epidemiological study that enrolled African American and white adults over 45 years of age in the continental United States, oversampling African Americans and residents of the stroke belt states (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee). A total of 30,239 participants were enrolled between 2003-2007, with socio-demographic, lifestyle and risk factor data collected at baseline and approximately 10 years later (2013-2016) through a computer-assisted telephone interview. There were 16,150 participants who completed at least one part of the 2nd follow-up assessment. Of those who did not complete the 2nd in-home exam, 59% had died and 41% withdrew from follow-up. Additional clinical information was collected at an in-home examination and self-administered questionnaires at the two time points. Further details on recruitment, enrollment, and assessment procedures for REGARDS have been described elsewhere.10,11
Among REGARDS participants who completed at least one part of the follow-up assessment, we excluded 1609 participants who were missing all non-self-report items on LS7 other than diet (blood cholesterol, blood glucose, blood pressure, BMI) as an indicator of not completing the 2nd in-home visit. We imputed missing follow-up diet scores as 0 (poor) due to significantly higher missingness compared to other LS7 variables, and because most participants with available data had poor diet and very few were ideal. Of the remaining 14,541 participants who completed the 2nd in-home assessment, we excluded 46 participants who had a hemorrhagic stroke or had an ischemic stroke less than one year before the follow-up LS7 assessment and 682 participants with a self-reported pre-baseline history of stroke. Lastly, with the exception of missing diet data, we excluded 6244 participants who did not have complete LS7 data at baseline or 10-year follow-up (6068 stroke-free participants, 176 stroke survivors). Overall LS7 missingness was not systematically different by stroke status. The reasons for exclusion are summarized in Figure 1.
Figure 1.
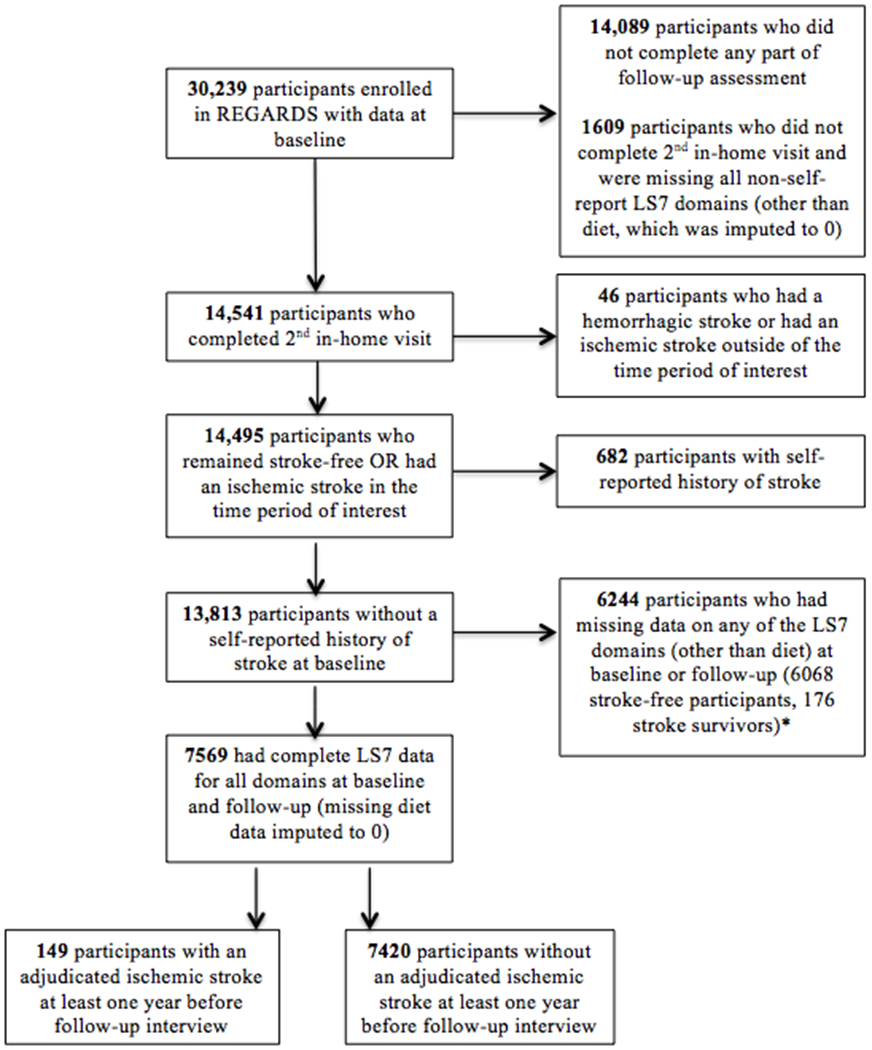
Flowchart of inclusion criteria for participants. Missing only at baseline, only at follow-up or both: 556 for physical activity, 1028 for cholesterol, 3981 for blood glucose, 63 for blood pressure, 154 for BMI, 2905 for diet (missing only at baseline; all missing imputed to 0 at follow-up); none missing at baseline or follow-up for smoking.
In the final study sample of 7569 participants, 149 (2.0%) were participants who experienced an adjudicated ischemic stroke between the first LS7 assessment and at least 1 year prior to the second LS7 assessment. The REGARDS study protocol was approved by the Institutional Review Boards at the collaborating centers. All participants provided written informed consent at both in-home examinations.
Variables.
The independent variable was whether or not a participant had at least one adjudicated ischemic stroke between the baseline visit and more than one year before the 10-year follow-up visit. Medical records from possible stroke events were examined by at least two trained adjudicators and classified as stroke or not. More information about the stroke adjudication process in REGARDS has been described elsewhere.12 Variables collected for participants with confirmed stroke were the date of stroke, type of stroke (ischemic or hemorrhagic), length of the acute hospital stay (if any), and discharge status.
Covariates included baseline LS7 score, self-reported age, race (white, African American), sex, education level (less than high school, high school graduate, some college, college graduate and above), and geographic location (non-belt and non-buckle, stroke belt, stroke buckle).
LS7 score was calculated at baseline and 10-year follow-up. As previously reported, scores on each of the seven domains of LS7 range were assigned from 0-2 (0=poor; 1=intermediate, 2=ideal) and summed, for a total score that ranged from 0-14 with higher scores indicating better cardiovascular health.13 The dependent variable was the change in LS7 score from baseline to follow-up and was modeled as a continuous variable. A positive value indicated improvement in LS7, a negative value indicated decline in LS7, and 0 indicated no change from baseline to follow-up.
We defined diet, physical activity, BMI and smoking as behavioral domains of LS7. Diet in LS7 was measured by administering the Block 98 Food Frequency Questionnaire (FFQ), with the healthy diet score defined using the following criteria: 1) ≥4.5 servings per day of fruits and vegetables, 2) ≥200 g per week of fish, 3) >1 g of fiber per 10 g of carbohydrate, 4) <1500 mg per day of sodium, and 5) ≤450 kcal per week of sugar-sweetened foods and beverages.13 Physical activity and smoking were based on self-report. BMI was calculated from measured weight and height. We defined blood pressure, total cholesterol and fasting blood glucose as medication-controlled domains of LS7. Blood pressure was taken from the average of two measurements. Total cholesterol and fasting glucose were measured by colorimetric reflectance spectrophotometry. Treatment with antihypertensive medication, lipid-lowering medication, insulin or oral hypoglycemic medication was determined by self-report. Additional information about definitions of LS7 domains have been described elsewhere.1, 13, 14
Statistical analysis.
We tested differences in baseline characteristics between stroke survivors and those without stroke using t-tests for continuous variables and chi-squared tests for categorical and binary variables. We used general linear regression modeling to test the association of having an ischemic stroke on the change in LS7 score, adjusted for covariates (baseline LS7 score, age, race, sex, education level and geographic region). We used two multiple linear regression models to examine the change in LS7 score: 1) all covariates (baseline LS7, age, sex, race, education and region), and 2) all covariates and an interaction term between stroke and baseline LS7. Next, we conducted separate regressions with scores of individual domains of LS7 (blood pressure, smoking, BMI, diet, physical activity, blood glucose, cholesterol) as the respective outcomes instead of LS7 as a composite score. We further tested differential associations between having a stroke and change in LS7 by age, sex and race by adding the appropriate interaction term separately to each model. To evaluate the association of time since stroke with change in LS7 after stroke, we conducted a separate analysis among only those who had an incident ischemic stroke, including all covariates and adding a continuous variable for the number of years from stroke to the 2nd LS7 assessment.
All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). All analyses were conducted at the p<0.05 significance level.
Results
Participant statistics.
Table 1 compares demographic and clinical characteristics between stroke survivors (n=149) and those who did not have a stroke (n=7420). On a scale of 0-14, stroke survivors had an average baseline LS7 score of 7.1 (standard deviation [SD] = 2.1) and participants without stroke had an average score of 7.9 (SD=2.0). The mean follow up to the 2nd visit for stroke survivors was 9.4 years (SD=0.8) and for those without a stroke was 9.2 years (SD=0.9). The mean baseline age of the stroke survivors was higher than that of the stroke-free participants. A lower proportion of stroke survivors were women or college graduates, compared to stroke-free participants.
Table 1.
Baseline demographic characteristics and Life’s Simple 7 scores of participants
| N(%) or mean(SD) | Stroke (N=149) |
No stroke (N=7420) |
P-value |
|---|---|---|---|
| Age | 67.5 (8.1) | 62.7 (8.3) | <0.001 |
| Race (African American) | 43 (28.9%) | 2088 (28.1%) | 0.37 |
| Sex (Female) | 69 (46.3%) | 4201 (56.6%) | 0.023 |
| Education level Less than high school High school graduate Some college College graduate and above |
13 (8.7%) 37 (24.8%) 47 (31.5%) 52 (34.9%) |
388 (5.2%) 1683 (22.7%) 1928 (26.0%) 3421 (46.1%) |
<0.001 |
| Geographic region Non-belt, non-buckle Stroke belt Stroke buckle |
66 (44.3%) 58 (38.9%) 25 (16.8%) |
3245 (43.7%) 2388 (32.2%) 1787 (24.1%) |
0.005 |
| Life’s Simple 7 score at baseline Total Blood pressure Total cholesterol Fasting glucose Physical activity Diet BMI Smoking |
7.1 (2.1) 0.87 (0.59) 1.1 (0.68) 1.5 (0.67) 0.96 (0.74) 0.18 (0.39) 0.83 (0.74) 1.7 (0.72) |
7.9 (2.0) 1.1 (0.63) 1.2 (0.64) 1.6 (0.61) 1.0 (0.77) 0.22 (0.42) 0.92 (0.78) 1.8 (0.61) |
0.004 <0.001 0.010 0.006 0.22 0.24 0.19 0.037 |
| Life’s Simple 7 score change from baseline to follow-up* Total Blood pressure Total cholesterol Fasting glucose Physical activity Diet BMI Smoking |
−0.14 (1.9) 0.027 (0.66) 0.15 (0.75) −0.13 (0.67) −0.35 (0.79) −0.054 (0.46) 0.13 (0.58) 0.081 (0.51) |
−0.18 (1.7) 0.0005 (0.68) 0.018 (0.63) −0.096 (0.59) −0.15 (0.90) −0.025 (0.48) 0.010 (0.56) 0.067 (0.45) |
0.80 0.64 0.013 0.52 0.009 0.47 0.008 0.71 |
Negative values indicate worsening of Life’s Simple 7 score (change was calculated as the follow-up score minus the baseline score)
Change in LS7.
Figure 2 shows the LS7 score at baseline and follow-up in stroke and stroke-free participants. When assessing the unadjusted change from baseline to follow-up, stroke survivors had non-significantly smaller decline in LS7 than participants who were stroke-free. After adjusting for LS7 baseline score and all other covariates, stroke survivors experienced 0.28 points more decline (i.e. effect estimate of −0.28; 95% confidence interval [CI]: −0.53, −0.03; p=0.03) in LS7 score compared to those without stroke (Table 2). When an interaction term between baseline LS7 and stroke status was added to the model, we found that baseline LS7 did not modify the relationship between stroke status and the change in LS7 (estimate: −0.064; 95% CI: −0.19, 0.06; p=0.30).
Figure 2.
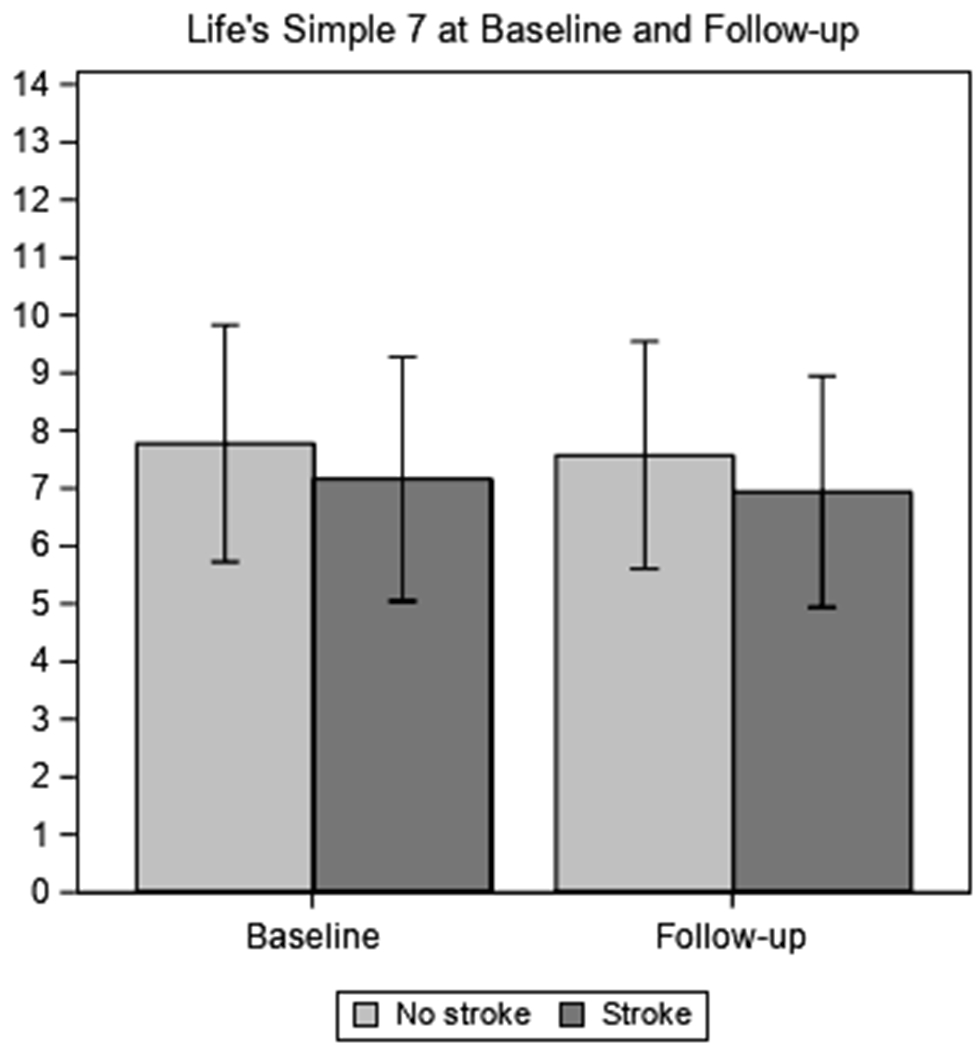
Total score for Life’s Simple 7 at baseline and follow-up (range=0-14)
Table 2.
Linear regression estimate, standard error (SE) and 95% confidence intervals (CI) for the change in Life’s Simple 7 scores
| Predictors | Estimate | SE | 95%CI |
|---|---|---|---|
| Age | 0.005* | 0.002 | 0.0008, 0.009 |
| Race (African American) | −0.21** | 0.04 | −0.29, −0.12 |
| Sex (Female) | −0.02 | 0.04 | −0.09, 0.05 |
| Education level Less than high school High school graduate Some college College graduate and above |
REF 0.14 0.23** 0.46** |
REF 0.09 0.09 0.08 |
REF −0.02, 0.31 0.06, 0.40 0.29, 0.62 |
| Geographic region Non-belt, non-buckle Stroke belt Stroke buckle |
REF −0.04 −0.07 |
REF 0.04 0.05 |
REF −0.13, 0.04 −0.16, 0.02 |
| Life’s Simple 7 score at baseline | −0.42** | 0.009 | −0.43, −0.40 |
| Stroke | −0.28* | 0.13 | −0.53, −0.03 |
SE: standard error; 95%CI: 95% confidence interval. Negative values indicate worsening of Life’s Simple 7 score (change was calculated as the follow-up score minus the baseline score). Estimates adjusted for baseline LS7, age, sex, race, education and region.
p<0.05
p<0.01
Figure 3a shows the change in the LS7 score for the two groups among the medication-controlled LS7 domains (blood pressure, blood glucose, total cholesterol). Neither group experienced significant changes in blood pressure or blood glucose from baseline to follow-up and both groups experienced some improvement in total cholesterol. This was supported by data on mean values at baseline and follow-up for total cholesterol, blood pressure and glucose shown in Supplemental Table I.
Figure 3.
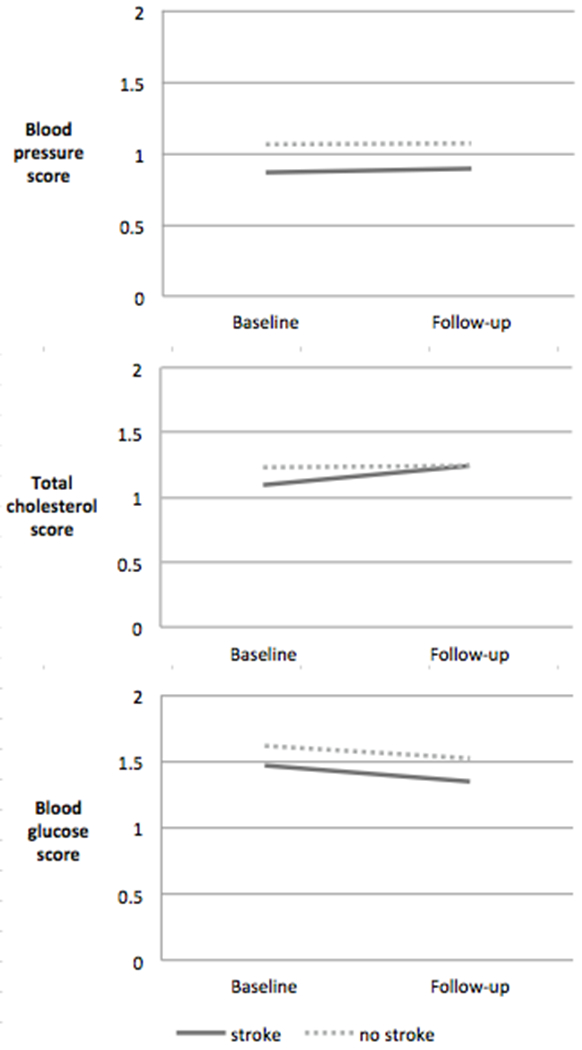
a. Scores for medication-controlled domains in Life’s Simple 7 (0=poor, 1=intermediate, 2=ideal). Ideal health for medication-controlled domains was defined as: untreated systolic blood pressure (SBP) < 120 mmHg and diastolic blood pressure (DBP) < 80 mm Hg, untreated total cholesterol < 200 mg/dL, and untreated fasting glucose < 100 mg/dL.
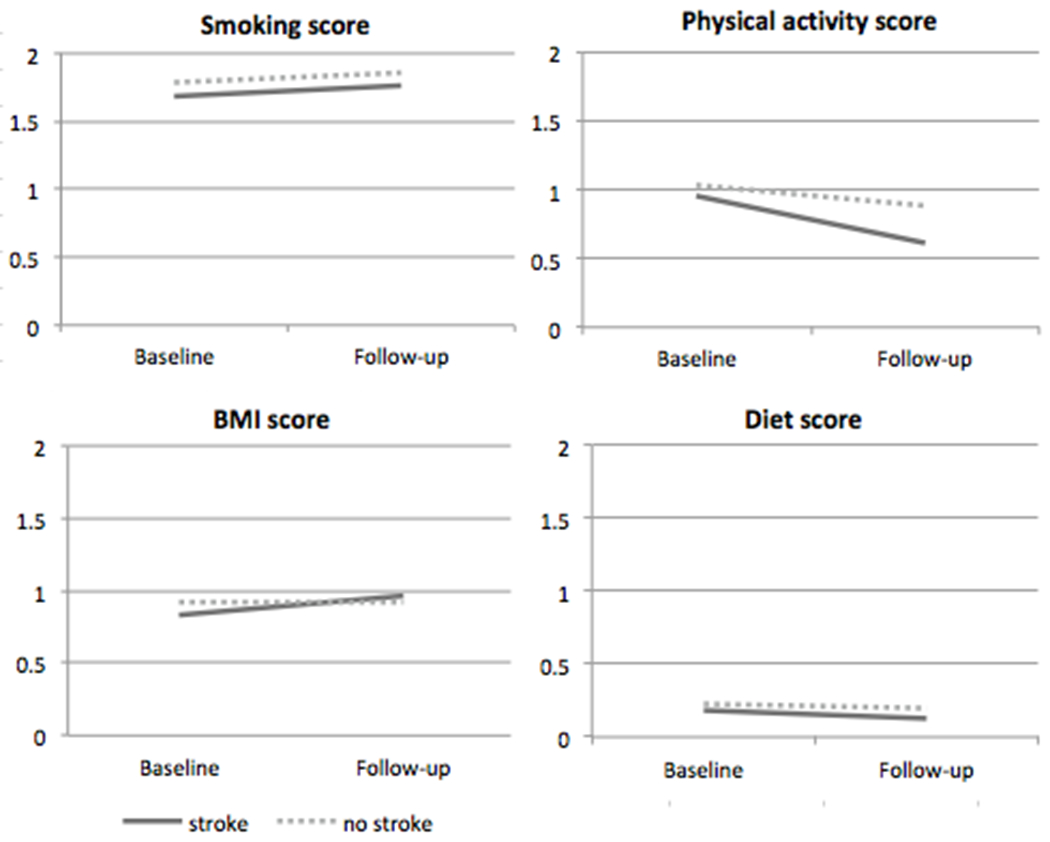
b. Scores for behavioral domains in Life’s Simple 7 (0=poor, 1=intermediate, 2=ideal). Ideal health for behavioral domains was defined as: never smoking or quitting more than 1 year ago, meeting four or five out of the five healthy diet components in the FFQ, having ≥ 4 bouts per week of intense physical activity sufficient to work up a sweat, and BMI < 25 kg/m2.
Figure 3b shows the change in the LS7 score for the two groups among the behavioral LS7 domains (smoking, physical activity, BMI, diet). Differences in unadjusted mean changes between stroke survivors and non-stroke participants (Figure 3b) and adjusted mean changes (Table 3) indicate that the greater adjusted decline in overall LS7 score occurred primarily in the physical activity domain (p<0.01), while both groups experienced little change in the smoking, BMI or diet domains.
Table 3.
Adjusted mean change in LS7 domains from baseline to follow-up by stroke status
| Stroke | No stroke | Difference between groups | |||
|---|---|---|---|---|---|
| Estimate | SE | 95%CI | |||
| Blood pressure | −0.12 | −0.04 | −0.08 | 0.04 | −0.17, 0.005 |
| Cholesterol | 0.06 | 0.04 | 0.02 | 0.04 | −0.07, 0.10 |
| Glucose | −0.22 | −0.14 | −0.08 | 0.05 | −0.17, 0.01 |
| Physical activity | −0.38 | −0.18 | −0.20** | 0.06 | −0.32, −0.08 |
| Diet | −0.09 | −0.06 | −0.03 | 0.03 | −0.09, 0.03 |
| BMI | 0.05 | −0.01 | 0.06 | 0.04 | −0.03, 0.15 |
| Smoking | 0.01 | 0.05 | −0.03 | 0.03 | −0.09, 0.03 |
SE: standard error; 95%CI: 95% confidence interval. Estimates adjusted for baseline LS7 component score, age, sex, race, education and region.
p<0.05
p<0.01
Other analyses.
Among all participants, we found no interactions between stroke status and age, sex or race on the change in LS7 score. Among stroke survivors, years since stroke (mean=4.9; SD=2.5) was not associated with the change in LS7 score (Supplemental Figure I).
Discussion
In this national study of stroke survivors and participants who did not have a stroke during follow-up, stroke survivors worsened by 0.28 more points on a scale of 0-14 for LS7 score compared to people who did not have a stroke after adjusting for covariates including baseline LS7 scores. Stroke survivors had significantly worse cardiovascular health at baseline prior to their stroke and a non-significantly smaller amount of decline from baseline to follow-up compared to non-stroke participants. After adjusting for the differences in LS7 score at baseline, the decline among stroke participants was relatively worse compared to the decline for non-stroke participants.
Several other analyses from the REGARDS study have shown the importance of cardiovascular health and found that baseline LS7 is associated with incident stroke, atrial fibrillation, cognitive impairment and depressive symptoms. One study found that each point increase in LS7 score was associated with 8% lower risk of incident stroke, and the association was not modified by race.13 Those with poor cardiovascular health, as measured by LS7, had 47% higher odds of developing atrial fibrillation, a condition that is associated with a 5-fold higher risk of stroke.15 Another analysis found that less favorable LS7 was associated with substantially higher incidence of cognitive impairment.14 Our results build on this evidence, finding that LS7 declines more over 10 years among people who had an ischemic stroke than those who remained stroke-free.
In our study, physical activity scores on LS7 declined more among stroke survivors compared to those who did not have a stroke. This may be due to some stroke survivors being more sedentary and inactive as a result of impairment from the stroke.16 The change in score for BMI was non-significantly better for stroke survivors compared to those who did not have a stroke, though an improved score in this domain indicates weight loss and may have deleterious effects among older adults and stroke survivors.17 Stroke survivors may have lost more weight due to stroke-induced muscle loss, a downstream effect of decreased physical activity post-stroke.18 Weight loss may also be related to diet and nutrition, which we observed to be worse than other domains at baseline and worsened among stroke survivors over follow-up. We expected smoking scores to improve more among stroke survivors, but improvement in smoking score did not differ between stroke survivors and stroke-free participants. Since smoking significantly increases the risk of stroke recurrence19, this suggests the need to emphasize smoking cessation over the course of stroke patient education in the clinic as well as in community-based health promotion efforts.
Changes in scores on medication-controlled domains of LS7 were not significantly different among stroke survivors compared to stroke-free participants. Most stroke survivors are prescribed antihypertensive and lipid-lowering agents after a stroke. Blood pressure, total cholesterol and fasting glucose could not have been scored as “ideal” when participants are taking antihypertensive medication, lipid-lowering medication, insulin or oral hypoglycemic medication.1 As such, the scores on these three domains could have improved by a maximum of one unit and may have lacked sensitivity to detect any changes in the medication-controlled domains of cardiovascular health.
Overall, we observed a small but significantly greater adjusted decline in LS7 scores among stroke survivors, compared to stroke-free survivors. The worsening among stroke survivors was counter to our hypothesis, given that we expected stroke survivors to decline less due to secondary prevention measures. This may indicate a lack of adherence or a lack of implementation of these measures, particularly those related to lifestyle domains of LS7 (smoking, physical activity, BMI, diet). With the exception of physical activity, assessing only the change in any of the other individual domains without analyzing the change in the composite score would not have shown the difference in cardiovascular health between stroke survivors and stroke-free participants. This suggests that assessing cardiovascular health as a composite outcome is necessary and informative beyond measuring the contributions of individual domains.
We did not find any interactions between stroke status and age, sex or race, though African Americans with or without stroke experienced greater declines than white participants with or without stroke. Greater decline among African American participants with incident stroke is consistent with previous findings showing that they have over two times higher odds of having low LS7 scores, defined as having an ideal score in one or less LS7 domains, compared to white participants with a self-reported history of stroke.3 Interestingly, we found that time since stroke did not influence the amount of change in LS7 among the stroke survivors. This may be because those who had more time to recover from the stroke may have had less severe strokes in order to survive for a long period of time, but may also have stopped engaging in secondary prevention measures. Surprisingly, age did not modify the association between stroke status and change in LS7. We had expected to find greater decline among younger participants with stroke than no stroke due to survival bias, since older participants would have already survived to an older age to remain in the study and may have better cardiovascular health due to health behaviors or genetic predisposition. The lack of association between age and LS7 change may be due to those with no stroke history comprising a majority of the study population; they were younger and declined non-significantly less in LS7 scores than stroke survivors in the unadjusted analysis, which could have obscured any associations between younger age and greater decline.
Raising LS7 scores should be a priority for stroke survivors. Our study found that stroke survivors had worse baseline LS7 compared to participants who did not have a stroke, with the greatest decline occurring primarily in the physical activity domain. A study of U.S. stroke survivors using the National Health and Nutrition Examination Surveys from 1988 to 2014 found that the proportion of those with poor LS7 scores has increased over time, a rise that was associated with a concurrent increase in poor blood pressure.3 Additional attention should be paid to several domains in particular: the American Heart Association and American Stroke Association recommends that physical activity be incorporated into risk factor management with stroke survivors,20 while the domains of blood pressure, smoking, total cholesterol, and blood glucose have been shown to be important domains of secondary prevention.9
Our study has several strengths and limitations. A strength was the use of adjudicated strokes, which increased the validity of our categorization of stroke status and allowed us to draw inferences with minimal bias. REGARDS is a population-based study, which improved our ability to generalize these findings to the U.S. population. On the other hand, all stroke survivors in REGARDS are community-dwelling and may be healthier than the overall population of stroke survivors in the United States. Some participants in the stroke-free group may have had stroke events that were not identified through medical record retrieval.12 There were around 10 years between baseline and follow-up, which makes our outcome of change in LS7 a crude measure of the change in cardiovascular health with limited information about why and how the changes took place in the intervening period. Furthermore, since there is no a priori definition for a clinically significant change in LS7, the finding of 0.28 points greater decline for stroke survivors compared to non-stroke participants should be interpreted with caution.
Another limitation was the large number of participants who were excluded from analysis because they did not complete the second assessment (N=15,698) or due to missing LS7 data (N=6244). We believe that LS7 missingness did not significantly impact our findings on the main association of interest. First, those who did not return for the second assessment likely had more severe strokes, and it follows that the decline in LS7 among stroke survivors may have been even greater in the complete sample. As such, the effect size estimate of the difference in decline between stroke survivors and stroke-free participants may have been underestimated in our study and our results were most likely biased in the conservative direction, if at all. Among the 6244 participants who were missing data on any of the LS7 domains, 4493 (72%) were missing LS7 data at baseline, and missingness at baseline was non-differentially associated with later occurrence of stroke. About half of those excluded due to missing LS7 data were missing diet data at baseline. We imputed missing diet scores to 0 (poor), as was consistent with the diet score of a majority of the participants with available data, which partially mitigated missingness.
Summary/Conclusions
Current literature on secondary stroke prevention focuses primarily on pharmacological interventions. We provide a holistic view of secondary prevention efforts using LS7 as an overall measure of cardiovascular health, which may serve to inform behavioral interventions to improve secondary prevention after a stroke event. We found that stroke survivors had greater decline in cardiovascular health after adjusting for covariates and baseline LS7 score compared to stroke-free participants. Although the effect size was small, it nonetheless points to the need for more secondary prevention efforts, whether through health education in the clinic during visits with patients and their family members or in a community setting. Additionally, further research is needed to determine whether or not the decline is due to insufficient secondary prevention efforts or other factors leading to changes to lifestyle, and whether further counseling efforts could lead to positive lifestyle modifications.
Supplementary Material
Supplemental Figure I
Supplemental Table I
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Sources of Funding: This research project is supported by a cooperative agreement U01 NS041588 co-funded from the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Aging (NIA), National Institutes of Health, Department of Health and Human Service. Additional funding was provided by an investigator-initiated grant (R01 NS075047) from NINDS. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Dr. Rebecca F. Gottesman is supported by a grant from the National Institute on Aging (K24AG052573).
Disclosures: Chelsea Liu received the American Heart Association Student Scholarship in Cerebrovascular Disease and Stroke (2019). Rebecca F. Gottesman is the former Associate Editor for the journal Neurology (from the American Academy of Neurology).
Abbreviations:
- LS7
Life’s Simple 7
- REGARDS
REasons for Geographic and Racial Differences in Stroke
- BMI
body mass index
- SD
standard deviation
- CI
confidence interval
Contributor Information
Chelsea Liu, Johns Hopkins School of Public Health.
David L. Roth, Johns Hopkins University School of Medicine.
Rebecca F. Gottesman, Johns Hopkins University School of Medicine.
Orla C. Sheehan, Johns Hopkins University School of Medicine.
Marcela D. Blinka, Johns Hopkins University School of Medicine.
Virginia J. Howard, University of Alabama at Birmingham School of Public Health.
Suzanne E. Judd, University of Alabama at Birmingham School of Public Health.
Mary Cushman, University of Vermont Medical Center.
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2.Lin MP, Ovbiagele B, Markovic D, Towfighi A. “Life’s simple 7” and long-term mortality after stroke. J Am Heart Assoc. 2015;4:e001470. doi: 10.1161/JAHA.114.001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin AM, Lin MP, Markovic D, Ovbiagele B, Sanossian N, Towfighi A. Less Than Ideal. Stroke. 2018:STROKEAHA118022644. doi: 10.1161/STROKEAHA.118.022644 [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 5.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–337. doi: 10.1161/01.str.25.2.333 [DOI] [PubMed] [Google Scholar]
- 6.Hardie K Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. 2004;35:731–735. doi: 10.1161/01.STR.0000116183.50167.D9 [DOI] [PubMed] [Google Scholar]
- 7.Hier DB, Foulkes MA, Swiontoniowski M, Sacco RL, Gorelick PB, Mohr JP, Price TR, Wolf PA. Stroke recurrence within 2 years after ischemic infarction. Stroke. 1991;22:155–161. doi: 10.1161/01.str.22.2.155 [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology. 1997;49:S39–S44. doi: 10.1212/wnl.49.5_suppl_4.s39 [DOI] [PubMed] [Google Scholar]
- 9.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 11.Howard G, Safford MM, Moy CS, Howard VJ, Keindorfer DO, Unverzagt FW, Soliman EZ, Flaherty ML, McClure LA, Lackland DT, et al. Racial differences in the incidence of cardiovascular risk factors in older black and white adults. J Am Geriatr Soc. 2017;65:83–90. doi: 10.1111/jgs.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neuro. 2011;69:619–627. doi: 10.1002/ana.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple Seven and risk of incident stroke: REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life’s Simple 7 and incident cognitive impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635. doi: 10.1161/JAHA.113.000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg PK, O’Neal WT, Ogunsua A, Thacker EL, Howard G, Soliman EZ, Cushman M. Usefulness of the American Heart Association’s Life Simple 7 to Predict the Risk of Atrial Fibrillation (from the REasons for Geographic And Racial Differences in Stroke [REGARDS] Study). Am J Cardiol. 2018;121:199–204. doi: 10.1016/j.amjcard.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wondergem R, Veenhof C, Wouters EMJ, de Bie RA, Visser-Meily JMA, Pisters MF. Movement Behavior Patterns in People With First-Ever Stroke. Stroke. 2019:STROKEAHA119027013. doi: 10.1161/STROKEAHA.119.027013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aparicio HJ, Himali JJ, Beiser AS, Davis-Plourde KL, Vasan RS, Kase CS, Wolf PA, Seshadri S. Overweight, Obesity, and Survival After Stroke in the Framingham Heart Study. J Am Heart Assoc. 2017;6:e004721. doi: 10.1161/JAHA.116.004721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced Sarcopenia: muscle wasting and disability after stroke. Int J Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, Dai Z, Gu M, Xia Y, Zhao M, et al. Impact of Smoking Status on Stroke Recurrence. J Am Heart Assoc. 2019;8:e011696. doi: 10.1161/JAHA.118.011696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I
Supplemental Table I


