Abstract
Pulmonary melioidosis is a bacterial disease with high morbidity and a mortality rate that can be as high as 40% in resource-poor regions of South Asia. This disease burden is linked to the pathogen’s intrinsic antibiotic-resistance and protected intracellular localization in alveolar macrophages. Current treatment regimens require several antibiotics with multi-month oral and intravenous administrations that are difficult to implement in the developing world. Herein, we report that a macrophage-targeted polyciprofloxacin prodrug acts as a surprisingly effective pre-exposure prophylactic in highly lethal murine models of aerosolized human pulmonary melioidosis. A single dose of the polymeric prodrug maintained high lung drug levels and targeted an intracellular depot of ciprofloxacin to the alveolar macrophage compartment that was sustained over a period of 7 days above minimal inhibitory concentrations. This intracellular pharmacokinetic profile provided complete pre-exposure protection in a BSL-3 model with a drug-resistant, aerosolized clinical isolate of Burkholderia pseudomallei from Thailand. This total protection was achieved despite the bacteria’s intrinsic resistance to ciprofloxacin and where an equivalent dose of pulmonary-administered ciprofloxacin was ineffective. For the first time, we demonstrate that targeting the intracellular macrophage compartment with extended antibiotic dosing can achieve pre-exposure prophylaxis in a model of pulmonary melioidosis. This fully synthetic and modular therapeutic platform could be an important therapeutic approach with new or re-purposed antibiotics for melioidosis prevention and treatment, especially as portable inhalation devices in high-risk, resource-poor settings.
Keywords: Global health, melioidosis, ciprofloxacin, drug conjugate, prodrug, controlled release, alveolar macrophage, pulmonary infection, polymer drug conjugate
Introduction
Pulmonary infections remain one of the most challenging anti-infective settings, with diseases such as tuberculosis, non-tuberculous mycobacterial infections, influenza, legionellosis, Q fever, melioidosis, and antimicrobial resistant (AMR) infections exacting high and rising mortality and morbidity costs across the globe [1–5]. Furthermore, lung pathogens such as the causative agents of tularemia, melioidosis, and the SARS-coronavirus are also classified as CDC select agents [6]. The sudden emergence of pandemic SARS-CoV-2 further illustrates the special threat of pulmonary infections. While vaccine approaches would provide the preferred prophylactic approach to pulmonary intracellular infections, they are currently not available for the viral threats or the bacterial lung diseases tularemia and melioidosis. Even where available, e.g. for influenza, the vaccines provide limited efficacy [7, 8]. Pulmonary therapeutic approaches thus remain important in concert with vaccines for current and future threats.
The current standard oral and IV administered therapies for pulmonary drug therapies are generally limited by off-target exposure and poor pharmacokinetics (PK) in the lung due to poor biodistribution [9]. These dosing regimens also result in drug resistance acquisition by pathogens [10]. These problems are magnified in polytherapy approaches that are often required for intracellular viruses and bacterial infections, where drug-drug interactions and drug metabolism effects magnify toxicity [11]. The dosing limitations enforced by these issues can lead to poor drug efficacy in the pulmonary compartment when they might achieve activity and acceptable toxicity if optimal PK in the lungs could be achieved by inhalation administration routes and optimized therapeutic systems.
Inhaled antibiotics deliver higher drug doses directly to the lung [12], but suffer from rapid clearance kinetics in the lung, leading to the need for more frequent administration [13]. In recent years, drug carriers have been developed to improve drug PK. Liposomal ciprofloxacin has been shown to enhance PK and efficacy in pulmonary infection animal models and progressed to human clinical trials [14–16]. Most recently, the liposomal amikacin product ARIKAYCE® has been approved by the FDA for the treatment of mycobacterium avium complex (MAC) [17], and represents the first carrier-based, inhalable antibiotic product to reach clinical impact.
Here, we demonstrate the efficacy of a new inhalable “drugamer” therapeutic designed to achieve high and greatly extended dosing in the lung. This drugamer platform is shown to repotentiate ciprofloxacin and to achieve full prophylaxis where the parent drug is ineffective against the antibiotic-resistant, Tier 1 select agent Burkholderia pseudomallei. This pulmonary drug platform builds delivery properties such as targeting and PK extension into the drug itself, rather than utilizing liposomal [14–16] or other nanoparticle [18, 19] formulations. This new therapeutic platform is a fully synthetic and modular version of past polymer therapeutics [20–22].
This platform serves as a strikingly efficacious prophylactic in an aerosolized model of antibiotic-resistant B. pseudomallei. B. pseudomallei causes an estimated 165,000 cases of melioidosis per year, 89,000 annual deaths, and has a mortality rate of up to 50% in rural Thailand and Vietnam [23, 24]. Pneumonia is a common presentation of melioidosis and is associated with higher mortality rates [25]. A major reason for the high mortality rate is that B. pseudomallei achieves protection from antibiotic exposure and adaptive immune responses through sequestration within the alveolar macrophage [26]. Moreover, B. pseudomallei is classified as the highest Tier 1 select agent due to its bioterrorism threat [23]. These threats for military and civilian healthcare workers responding to a release event provide the strong rationale for effective and fully prophylactic antibiotic products. Poor rural farmers in Southeast Asia and other similar tropical locations are also one of the at-risk groups for melioidosis in select rainy seasons [27].
Current standard of care treatments rely on exhaustive antibiotic dosing regimens unchanged in decades [28]. Recommended treatments include 10–14 days or longer of intravenous therapy, followed by further 3–6 months of multi-daily oral antibiotic eradication therapy [23, 28]. This lengthy and intensive treatment course is unavailable to many poor rural farmers, is associated with patient nonadherence, leads to well-documented patient side-effects, and promotes the emergence of antibiotic-resistant bacteria [29]. While prophylactic use of antibiotics can be problematic in the context of drug resistance, the select and targeted use of prophylactic antibiotics has been advocated for in settings where health impacts are severe [30, 31]. This may be especially true for this current work that demonstrates repotentiation of the antibiotic ciprofloxacin to which B. pseudomallei is resistant clinically [32].
This drugamer platform targets the lung macrophage compartment as a reservoir to increase dosing against intracellular lung pathogens, as well as to extend drug PK out to seven days from a single dose (Fig. 1).The inhaled ciprofloxacin drugamer achieved 100% survival for the two-week study period, where the corresponding inhaled ciprofloxacin groups showed no efficacy and uniformly reached euthanasia endpoints after just 3 days. The modular drugamer platform is fully synthetic and thus manufacturing-ready with combination drug potential that can be exploited for response to other existing or emergent pulmonary infections [33–36].
Fig. 1. Macrophage-targeted drugamer enables a sustained delivery of ciprofloxacin to alveolar macrophages.
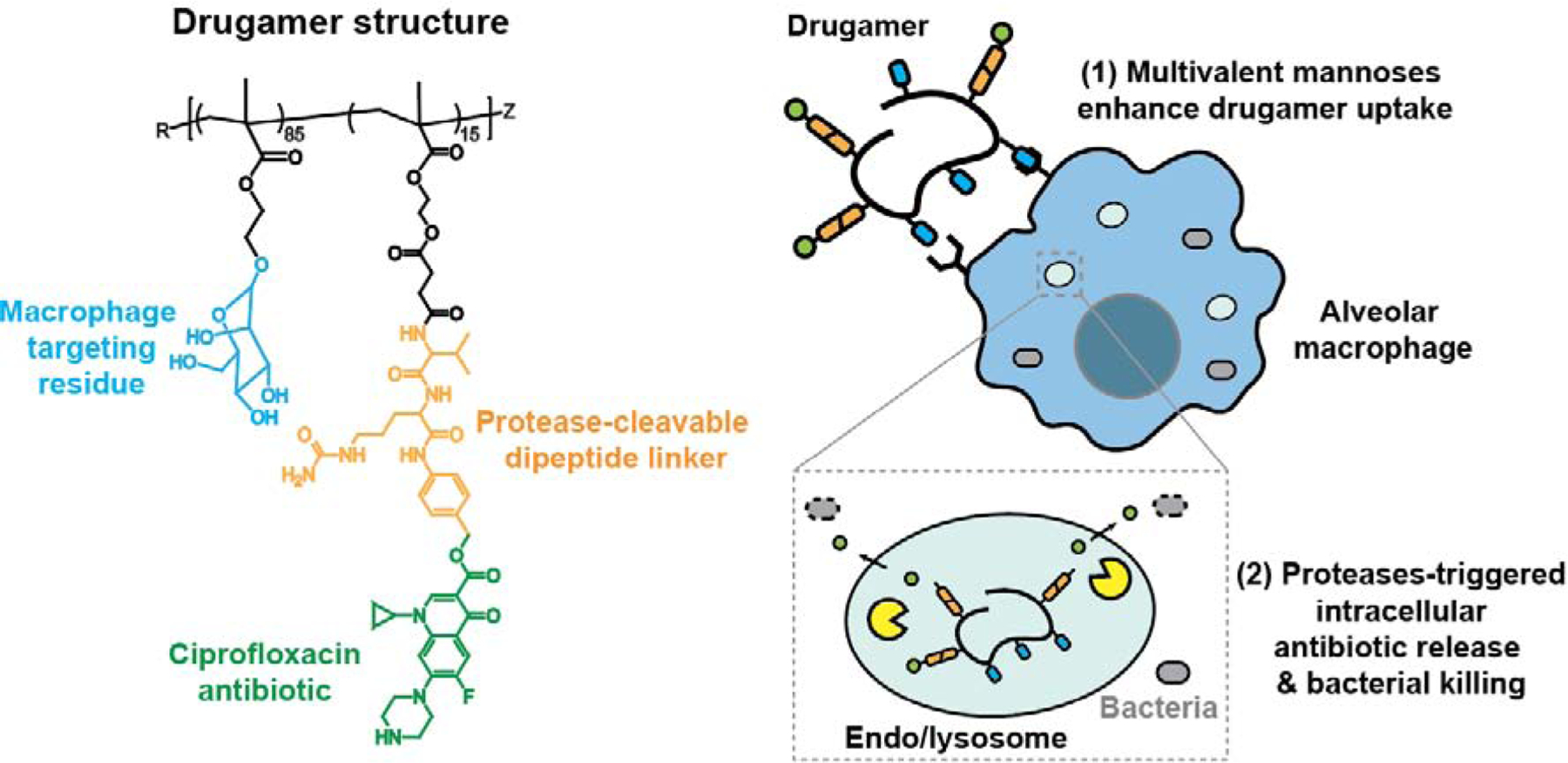
Schematic showing the composition polymeric ciprofloxacin prodrug and its mechanism for enhancing alveolar macrophage uptake and intracellular antibiotic release. (1) The drugamer is composed of multivalent mannose ligands that act both as solubilizing agents and targeting residues, thus enhancing drugamer internalization by alveolar macrophages. (2) Drugamer binding to mannose receptors induces receptor-mediated endocytosis. The protease-cleavable dipeptide motif (Valine-Citrulline) linking ciprofloxacin to the polymer backbone is subsequently cleaved by intracellular proteases, such as cathepsins, allowing release of the antibiotic in the alveolar macrophage and killing of intracellular bacteria.
Results
Drugamer properties.
The information pertaining to the drugamer’s synthesis was reported previously [36]. Its composition, molecular weight (Mn), and molar mass dispersity (Đ) are summarized in Supplementary Table S1 and Supplementary Figure S1.
Causal prophylactic treatment in the surrogate Burkholderia thailandensis infection model.
The activity of the mannose-targeted, polymeric prodrug was initially evaluated in a murine model of pulmonary melioidosis brought about by exposure to B. thailandensis (Table 1, Fig. 2). This model is highly lethal in mice but not humans. Two hours prior to infection, the mice were dosed with the aerosolizing microsprayer with the drugamer at a ciprofloxacin dose of 20 mg/kg, at which point they were challenged with a lethal dose of B. thailandensis (>105 colony forming units, CFU per lung) (Fig. 2A). Two further doses of the drugamer were administered at 24h and 48h post-infection (0, +1, +2 day dosing). To control for this challenge study, one group was treated with PBS (vehicle control), and another with an equivalent dose of free ciprofloxacin. Vehicle control mice in this model rapidly reach euthanasia criteria by 72h after infection, as seen in the morbidity plots of body temperature and weight (n = 8/8) (Fig. 2B–D).
Table 1.
Overall comparison of the three challenge studies reported. (MIC: Minimum inhibitory concentration)
| Figure 2 | Figure 4 | Figure 5 | |
|---|---|---|---|
| Bacterial strain | |||
| (Laboratory surrogate) | (Clinical isolate) | ||
| MIC50/MIC90 (μg/mL) | 2/4 | 2/2 | |
| Deposition dose* (CFU/Lung) | 4.53 × 105 | 6.25 × 104 | 3.80 ×103 |
| Dosing regimen† | Day 0, 1, 2 | Day −2, −1, 0 | Day −2, −1, 0 |
| Suvival rate of drugamer treated mice | 100% (8/8) | 100% (8/8) | 100% (10/10) |
| Suvival rate of free ciprofloxacin treated mice | 25% (2/8) | 12.5% (1/8) | 0% (0/10) |
Bacterial deposition in the lungs was determined from quantitative culture of lung tissue from mice sacrificed immediately after infection (n=4).
Bacterial infection was conducted at 2 h after the dosing on day 0.
Fig. 2. Efficacy of the ciprofloxacin drugamer against aerosolized Burkholderia thailandensis E264.
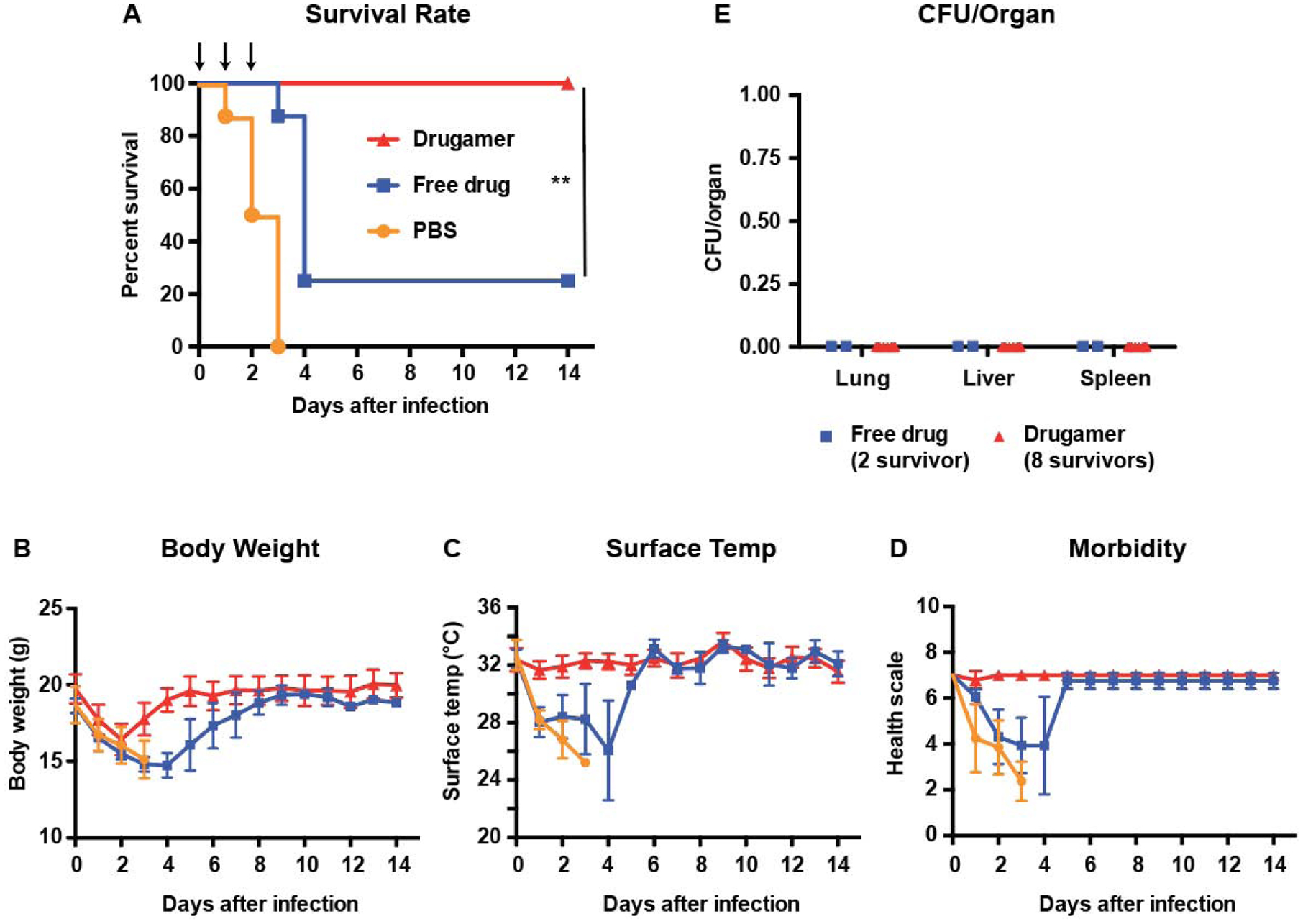
Free ciprofloxacin (free drug) or ciprofloxacin drugamer were intratracheally administered (50 μL aerosolization, 20mg ciprofloxacin/kg) at day 0 (2h prior to the infection), 1, and 2 using a MicroSprayer® (n = 8 for each treatment group). PBS was used as a vehicle control. All mice were challenged with aerosolized B. thailandensis 2h after dosing on day 0. Survival rate and health condition of mice were monitored for 14 days. (A) Survival rate of infected mice. Arrows indicate dosing day. (B & C) Body weight and surface temperature of mice over the course of the experiment. (D) Clinical scores of mice. Mice were scored on seven categories: activity, coat, eyes, breathing, posture, isolation, and resistance to handling, with scores of 0, 0.5, and 1 specified for each category. E Bacterial burden in organs retrieved from mice that survived to the experimental endpoint (14 days). CFU: Colony-forming unit. Data are presented as mean ± SD. ** denotes p < 0.01.
Treatment with free ciprofloxacin provided a modest improvement in survival rates, with 25% (n = 2/8) of the mice remaining at the endpoint of 14 days. Administration of the polymeric ciprofloxacin prodrug led to full protection, with all mice surviving to 14 days (n = 8/8) (Fig. 2A). Though both free drug and drugamer-treated mice exhibited weight loss as soon as 24h post-infection, the latter had regained their baseline weight 5 days after the infection, compared with 9 days for the survivors of the former group. Of note, mice treated with free ciprofloxacin became visibly ill (e.g. decreased body temperature and increased morbidity) approximately 24h after infection, whereas these metrics remained stable for the drugamer-treated mice throughout the experiment (Fig. 2B–D). 14 days after infection, drugamer-treated mice (and the 2 survivors from the free ciprofloxacin-treated group) showed no detectable levels of bacteria in the lungs, spleen and liver (Fig. 2E), three organs that are commonly affected by melioidosis [37]. This is a strong indication that these mice had resolved the infection.
Single-dose PK study of ciprofloxacin release in alveolar macrophages.
A comparative PK study of the drugamer and free ciprofloxacin was performed in mice (Fig. 3). The animals received a single intratracheal dose of 20 mg/kg ciprofloxacin – either in drugamer or free drug form – and were subsequently euthanized at pre-determined timepoints (4 hours – 7 days). The lung macrophages of the euthanized mice were collected through bronchoalveolar lavage, and liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to measure the levels of ciprofloxacin in those cells. Inhaled ciprofloxacin was rapidly cleared as previously characterized [36, 38, 39] and after 4h, 33% of mice had quantifiable levels of the drug in the alveolar macrophages. This is in stark contrast with what was observed for the drugamer. The concentration of ciprofloxacin observed in the alveolar macrophage compartment was one to three orders of magnitude larger than that observed in mice dosed with the free drug, across all timepoints. All animals dosed with the drugamer also displayed detectable levels of ciprofloxacin in the alveolar macrophage compartment up to 5 days after administration, and two out of three mice exhibited detectable levels of ciprofloxacin in the alveolar macrophage compartment after 7 days.
Fig. 3. In vivo pharmacokinetics of drugamer-released ciprofloxacin in alveolar macrophages.
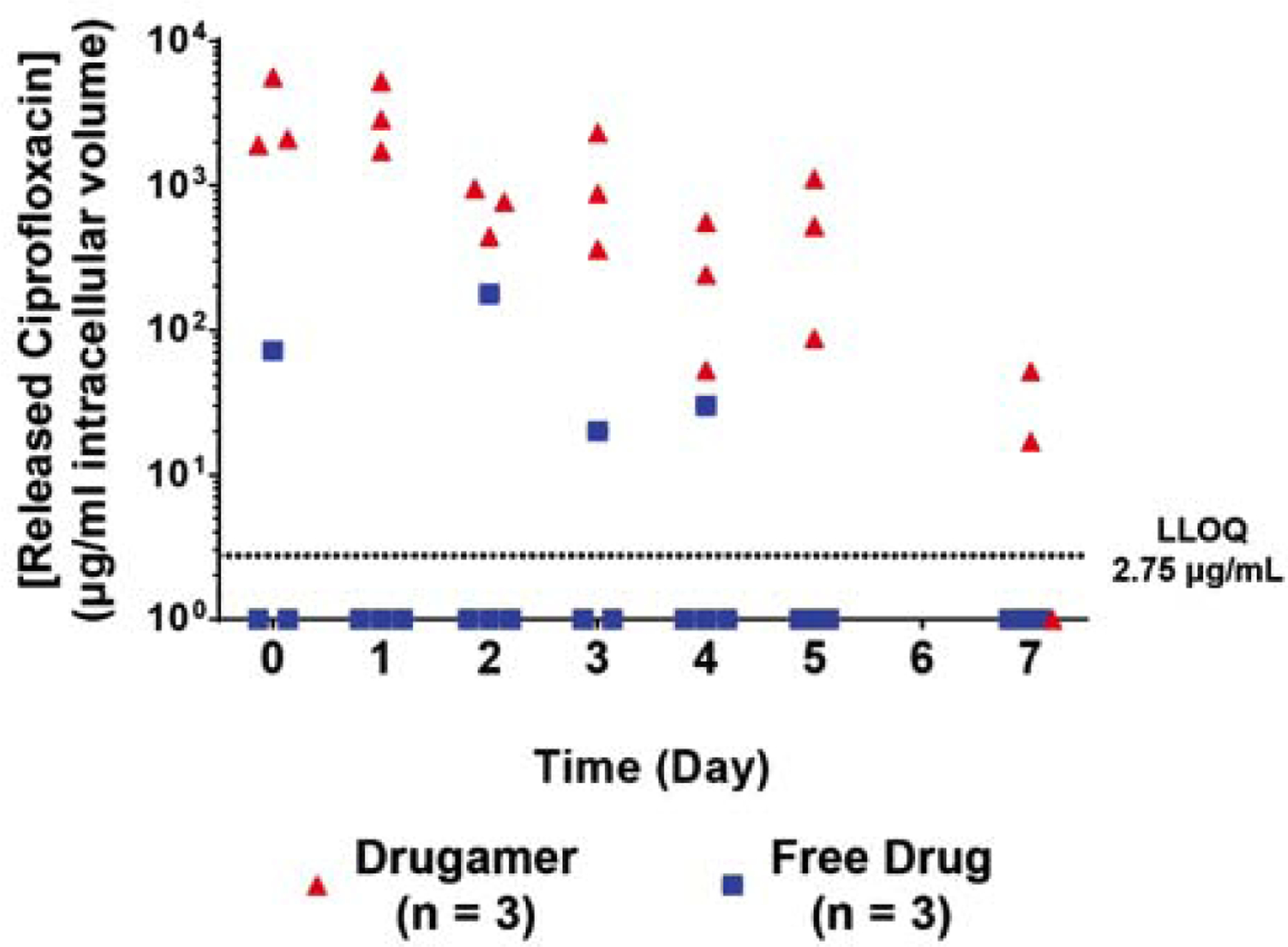
Released ciprofloxacin pharmacokinetics ranging from 4 hours (day 0) to 7 days after dosing. Day 0 was defined as 4h after the drugamer administration. The lines indicate the average of the 3–4 data points of each time point. (n = 3–4) LLOQ: Lower limit of quantification of the LC-MS/MS assay in this pharmacokinetics study.
The MIC of ciprofloxacin against B. thailandensis and B. pseudomallei are 2 and 4 μg/mL, respectively (Table 1). All the mice dosed with the drugamer had a ciprofloxacin concentration in the lungs that was above the MIC through the 7-day experiment, save for one mouse at the 7-day timepoint. Together, these observations demonstrate that the drugamer enables a delivery of ciprofloxacin to the alveolar macrophage compartment that was sustained over 5 to 7 days, with its concentration remaining orders of magnitude higher than when ciprofloxacin is administered as the free drug. Combined with the results from our successful challenge study with B. thailandensis, these observations prompted us to evaluate the efficacy of the drugamer in a fully prophylactic dosing regime
Activity of the mannose-targeted ciprofloxacin drugamer as a pre-exposure prophylactic against B. thailandensis infections.
To test whether the polymeric prodrug was efficacious in a fully prophylactic dosing regimen, mice were dosed with the drugamer at 48h, 24h and 2h before being infected with a deposited ~105 CFU/lung of B. thailandensis (Fig. 4A). The control group consisted of mice treated with an equivalent dose of free ciprofloxacin at the same time points. Apart from a modest and temporary decrease in body weight that was concurrent with the infection, the drugamer-treated group remained largely unaffected by the challenge with 100% survival (n = 8/8) at 14 days (Fig. 4B–D). This is in clear contrast with the 12.5% survival rate that resulted from treatment with free ciprofloxacin (1/8 survivors). A very significant drop in body weight, surface temperature and health score was observed for all mice, from which 7/8 did not recover. The bacterial burden in the survivors was significantly reduced from the lethal inoculum (~ 105 CFU/lung) to sub-lethal levels (Fig. 4E).
Fig. 4. Efficacy of the drugamer against a pulmonary Burkholderia thailandensis E264 infection in a pre-exposure prophylactic setting.
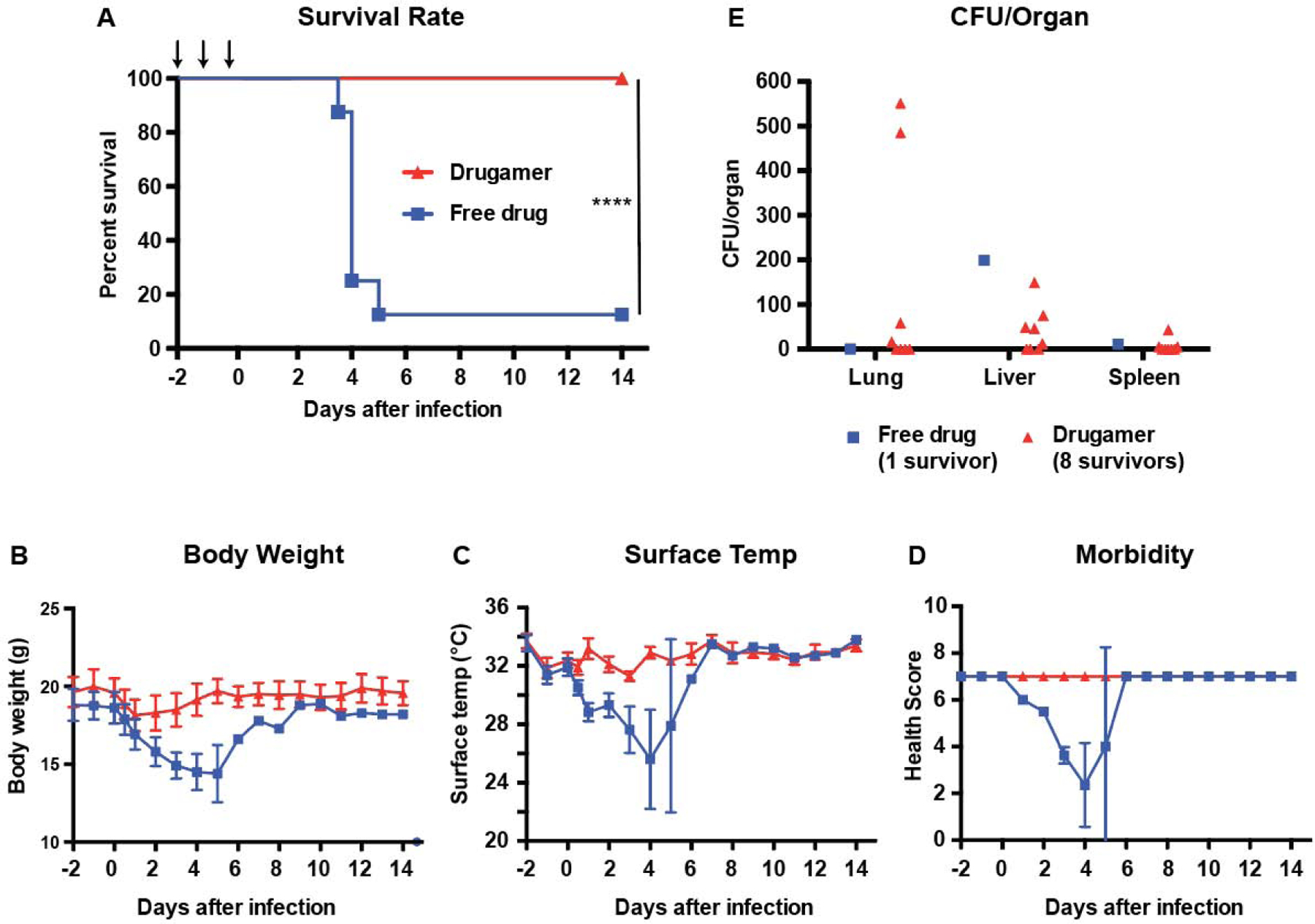
Free ciprofloxacin (free drug) or drugamer were intratracheally administered (50 μL aerosolization, 20mg ciprofloxacin/kg) at day −2, −1, and 0 using a MicroSprayer® (n = 8 for each treatment group). All mice were challenged with aerosolized B. thailandensis 2h after dosing on day 0. Survival rate and health condition of mice were monitored for 14 days. (A) Survival rate of infected mice. Arrows indicate dosing day. (B & C) Body weight and surface temperature of mice over the course of the experiment. (D) Clinical scores of mice. (E) Bacterial burden in organs retrieved from mice that survived to the experimental endpoint (14 days). Mice were scored on seven categories: activity, coat, eyes, breathing, posture, isolation, and resistance to handling, with scores of 0, 0.5, and 1 specified for each category. CFU: Colony-forming unit. Data are presented as mean ± SD.
Pre-exposure prophylaxis against an antibiotic-resistant, human B. pseudomallei clinical isolate in a BSL-3 pulmonary infection mouse model.
Having optimized our prophylactic treatment regimen against the surrogate strain, we characterized its efficacy in a virulent human-specific pathogen, B. pseudomallei 1026b [40]. Under conditions similar to our prophylactic challenge study against B. thailandensis, mice were dosed with the drugamer at 48h, 24h and 2h before being infected with a deposited ~ 4×103 CFUs of B. pseudomallei (Fig. 5A). Control groups received an equivalent dose of the free drug (ciprofloxacin), or vehicle-treatment (PBS). The lethality of B. pseudomallei 1026b was apparent from the morbidity parameters: the surface temperature of vehicle-treated mice dropped below 25°C within two days of the infection, with a concomitant decrease in health score. As a result, all untreated mice had reached euthanasia criteria by day 4 (n = 10/10) as shown in Fig. 5A. With respect to body weight, temperature, morbidity scale, and survival rates, mice treated with free ciprofloxacin did not fare any better than vehicle-treated mice (Fig. 5B–D).
Fig. 5. Pre-exposure prophylaxis efficacy of ciprofloxacin drugamer against a clinical isolate of Burkholderia pseudomallei.
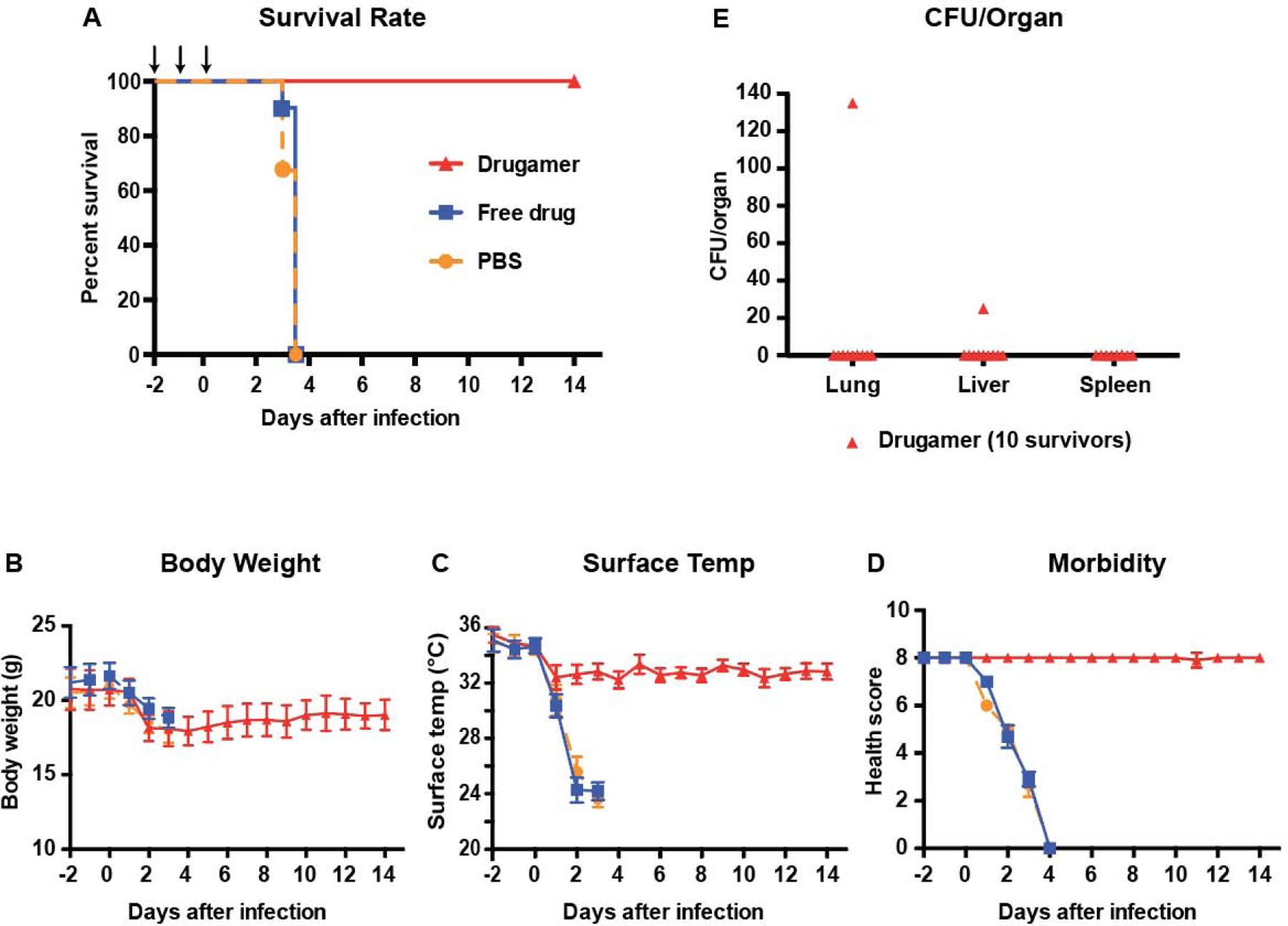
PBS, free ciprofloxacin (free drug) or drugamer were intratracheally administered (50 μL aerosolization, 20 mg ciprofloxacin/kg) at day −2, −1, and 0 using a MicroSprayer® (n = 10 for each treatment group). PBS was used as a carrier control. All mice were challenged with aerosolized B. pseudomallei 2h after dosing on day 0. Survival rate and health condition of mice were monitored for 14 days. (A) Survival rate of infected mice. Arrows indicate dosing day. (B & C) Body weight and surface temperature of mice over the course of the experiment. (D) Clinical scores of mice. Mice were scored on seven categories: activity, coat, eyes, breathing, posture, isolation, and resistance to handling, with scores of 0, 0.5, and 1 specified for each category. (E) Bacterial burden in organs retrieved from mice that survived to the experimental endpoint (14 days). CFU: Colony-forming unit. Data are presented as mean ± SD.
This result reinforces the high lethality of B. pseudomallei 1026b apart from its surrogate strain B. thailandensis, where a modest increase in survival rates was seen with ciprofloxacin treatment (Fig. 4A). All mice treated with the drugamer survived to the experimental two-week endpoint (n = 10/10). The mice suffered from a modest and temporary decrease in body weight and temperature following the infection, promptly followed by recovery and their health scores remained high throughout the study. Postmortem analysis of lung and disseminated organ bacterial burdens showed that 80% of the mice treated with the drugamer were sterile at the experimental endpoint (n = 8/10, Fig. 5E). One mouse exhibited residual bacteria (~ 102 CFUs) in the lungs and one separate mouse exhibited residual bacteria in the liver (~ 101 CFUs).
Discussion
Pulmonary infections are leading contributors to the global disease burden. Non-tuberculous pulmonary infections killed 2.74 million people in 2015 and constitute the leading infectious cause of death and the fifth leading cause of death overall [41]. The intracellular pulmonary bacteria Mycobacterium tuberculosis latently infected 1.7 billion of the world’s population in 2014 [42] and killed 1.3 million [43]. B. pseudomallei is a less well-known pulmonary pathogen that causes pulmonary melioidosis when inhaled. Some 165,000 cases of melioidosis (including infection caused both by cutaneous inoculation and inhalation) are estimated to occur annually [24]. Despite appropriate therapy – even in modern intensive care units – 40 to 50 percent of patients died from pulmonary melioidosis [44, 45]. Accordingly, the pathogen is classified as a US CDC and USDA Tier 1 select agent [6]. Successful eradication of the pathogen is difficult: treatment requires at least two weeks of intravenous therapy with ceftazidime or a carbapenem followed by 3–6 months oral trimethoprim/sulfamethoxazole [23, 28]. Worryingly, acquired resistance to ceftazidime is now well described [46, 47] and there are recent reports of a decreased susceptibility of B. pseudomallei to carbapenems [48, 49]. As both a public health and bioweapon threat, there is a well-established need for better therapies against B. pseudomallei, and such approaches may also provide new routes to treating other pulmonary infections.
Given the small pipeline of new antibiotics, repurposing and re-potentiating existing antibiotics is therefore an important goal for pulmonary infections and B. pseudomallei in particular. Effective repurposing strategies must mechanistically alter the drug’s PK and target it more effectively to key bacterial reservoirs and sites of pathogenesis. We present a successful re-potentiation approach for ciprofloxacin via the drugamer platform, which effectively targets the drug to the alveolar macrophage compartment, where a dramatic lengthening in intracellular dosing is demonstrated. Ciprofloxacin is a bactericidal antibiotic that inhibits DNA replication by inhibiting bacterial DNA topoisomerase and DNA-gyrase [50]. Despite its broad-spectrum activity, ciprofloxacin is not considered a lead antibiotic for treating melioidosis due to its poor intrinsic treatment efficacy when freely administered [32, 51, 52]. Where free ciprofloxacin completely failed to protect the mice from B. pseudomallei (0% survival rates), our drugamer therapeutic offered full protection until the experimental endpoint of 14 days, by which 80% of the drugamer-treated group had completely cleared the pathogen (n = 8/10).
This is a particularly significant result, given the relatively low intensity and duration of the regimen – a total of three doses of 20 mg/kg ciprofloxacin equivalent, spread over three days – when compared with the current standard of care in humans (2–3 doses/day for more than 3 months) [23, 28]. As a reference point, reports characterizing the ciprofloxacin treatment of melioidosis in murine models have typically used more intensive regimens of ciprofloxacin: Barnes et al. dosed mice infected with B. pseudomallei K96243 with two daily doses of 30 mg/kg ciprofloxacin for 14 days [53], whilst Steward et al. treated mice infected with B. pseudomallei 576 with two daily doses of 100 mg/kg ciprofloxacin for 14 days [54]. Both studies reported high but not 100% survival in their treatment groups at 14 days after infection (90% and 95%, respectively), despite these lengthy and high intensity dosing regimens. The high activity of the drugamer therapeutics may enable the development of dose-sparing regimens by reducing duration and/or dose. Such properties would potentially avoid the emergence of antibiotic resistance facilitated by the current standard of care for melioidosis, which involves intensive antibiotic treatment (multiple daily doses for 3–6 months) [23, 28]. Moreover, the dose-sparing regimens that may be enabled by drugamers would be important to increase patient compliance, particularly in low-resource settings (e.g., rural Southeast Asia).
The improved efficacy of our drugamer can be attributed to its ability to modulate the biodistribution and PK properties of the antibiotic in the lungs and alveolar macrophages [36]. As observed in our PK studies, a single dose of the drugamer enables a delivery of ciprofloxacin to the alveolar macrophage compartment that is sustained over 5 days for all mice, and up to 7 days for two of the three mice sampled at that timepoint. Ciprofloxacin use for the treatment of melioidosis is ill-advised, both in humans [32, 51, 52] and in murine models [53, 54]. This suggests that delivery of this antibiotic to the right target compartment and its short half-life are key factors limiting its efficacy. Repotentiating antibiotics through the use of this modular platform could provide a rationale for selected prophylactic use for global health since B. pseudomallei is resistant to fluoroquinolones. The number of doses and the dosing regimen for the future clinical development will need to be carefully decided based on the pharmacokinetic properties of antibiotics released from drugamers to avoid toxicities and minimize antibiotic usage.
Inhalable antibiotic therapeutics offer unique advantages in delivering concentrated antibiotics to the site of bacterial persistence [55]. Aerosolized ceftazidime, however, only showed a comparable efficacy to intraperitoneal injection in a murine melioidosis model [56], which is likely due to a short retention of the drug in mouse lungs (6 hours after exposure) [57]. This short retention in the lungs was also observed in aerosolized ciprofloxacin, which is rapidly cleared within 1–2 h post-administration [39]. To overcome the rapid clearance kinetics of free antibiotics, inhalable liposomal antibiotics have been extensively studied [14–17]. Liposomal ciprofloxacin achieved sustained levels of ciprofloxacin in the lungs for 24h [39], leading to an improved therapeutic efficacy against lung infections caused by Francisella tularensis, Coxiella burnetii, and Yersinia pestis [14–16]. Despite its promise against relevant pulmonary bacterial infections, inhalable liposomal ciprofloxacin has not been tested against B. pseudomallei. Although not suitable for inhalational administration, a bile salts-enriched liposome (bilosome) was recently used to develop oral formulations of levofloxacin and doxycycline to treat inhalational melioidosis [58]. Despite the significant survival advantage that they confer over the corresponding free antibiotics, bilosomes still require intense treatment courses (50 mg/kg, once a day for 7 days). Contrasting with those liposome-based formulations that rely on the physical encapsulation of antibiotics and passive targeting to control PK, our drugamer exploits a cleavable peptide linker coupled with active macrophage-targeting ligands to achieve significantly better PK properties (sustained over 5–7 days) and striking therapeutic efficacy (full survival with merely 3 doses).
Conclusion
Looking forward, we envision that the drugamers can be used clinically for on-demand protection both in biodefense settings and potentially in tropical global health settings. The inhalable drugamer product is envisioned for military and civilian personnel entering into the battlefield or civilian locations where the Tier 1 threats have been suspected of release. Prophylactic use of antibiotics is a concern in the context of antibiotic-resistance generation, but are necessary in bioterrorism settings that are non-recurring events. Prophylactic use may also be acceptable in global health settings where the antibiotic has been repotentiated as in this report, since the bacteria are already resistant to the parent drug. These prophylactic applications of drugamers are important as the current treatment regimen for melioidosis is often not available for people such as poor rural farmers in resource-limited settings. In terms of the administration route, this inhalable platform could be administered to humans in using either nebulizers or portable aerosolization devices. The modular nature of the platform may also enable repurposing of additional antibiotics and drug combinations, thus helping to close the gap between increasingly drug-resistant pulmonary pathogens and the shrinking antibiotic pipeline. The modularity of the drug repertoire has been shown with other prodrug monomers [33–35], and could thus be expanded to other pulmonary infection space, including other antibiotics, antivirals and host-directed drugs. The fully synthetic platform has intrinsic scalability, streamlined CMC, and an on-demand, rapid response potential using existing GMP manufacturing infrastructure. These features make the platform a promising candidate for pulmonary infection therapy against intransient existing pathogens and current and future emergent pandemic threats.
Materials and Methods
Study design.
The primary objective of this study was to develop a macrophage-targeted polymeric prodrug that provides fully prophylactic protection in a BSL3 aerosolized model of human pulmonary melioidosis model. We first tested this polymeric prodrug of ciprofloxacin in B. thailandensis, a laboratory surrogate model of B. pseudomallei that is highly lethal in mice. The first dosing regimen design used a pre- and post-exposure dosing schedule. We next tested this polymeric prodrug using a fully pre-exposure prophylactic dosing regimen in B. thailandensis. Finally, the prophylactic and repurposing activity was tested in the BSL3 model with an aerosolized clinical isolate of B. pseudomallei from Thailand. To mechanistically elucidate the therapeutic effectiveness, we evaluated the intracellular ciprofloxacin PK in alveolar macrophages recovered by bronchoalveolar lavage from mice across a week period. Sample sizes were chosen based on our prior data to achieve statistical significance. Statistical analyses are described in the Methods section.
Bacterial strains and growth conditions.
B. pseudomallei 1026b was originally obtained from a bacteremic patient from Thailand (36). B. thailandensis E264 was provided by Dr. Donald Woods at the University of Calgary, Alberta, Canada. All bacterial strains were subcultured from stocks kept at −80°C in 20% glycerol. For use in the experiments, they were cultured in Luria broth (LB) overnight at 37°C with shaking at 200 rpm. The overnight cultures were washed twice in cold phosphate-buffered saline (PBS) and then enumerated using a spectrophotometer at OD600 to the desired concentration (107 CFU/mL for B. pseudomallei and 109 CFU/mL for B. thailandensis). Bacterial suspensions were serially diluted and plated onto LB agar for quantitative culture. Colonies were counted after incubation at 37°C for 24 h. All procedures involving B. pseudomallei 1026b were performed in a Select Agent approved Biosafety Level 3 (BSL-3) facility at the University of Washington using compliant procedures and protocols. The minimal inhibitory concentration (MIC) of ciprofloxacin against B. pseudomallei 1026b and B. thailandensis E264 was determined by broth microdilution following CLSI protocols [59].
Animals.
Wild type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All animals were housed in cages under specific pathogen free conditions and permitted unlimited access to sterile food and water. Studies using B. thailandensis E264 were conducted in ABSL2 facilities, whereas studies using B. pseudomallei 1026b were conducted in ABSL3 facilities. Euthanasia was accomplished with intraperitoneal injection of pentobarbital or carbon dioxide followed by exsanguination from cardiac puncture. All animal procedures and handling were conducted under protocols (4047-02 and 2671-10) approved by the Institutional Animal Care and Use Committee at the University of Washington.
Drugamer synthesis and characterization.
The synthesis and characterization of our macrophage-targeted, protease-cleavable drugamer was reported previously (29). Representative NMR and GPC profiles of polymer prodrugs used in this study are presented in supplementary materials. The drugamer is a unimer and fully water soluble at the concentrations used, though its upper solubility limit has not been established.
Antibacterial efficacy against pulmonary infection of B. thailandensis.
The antibiotic activity of drugamers was tested in C57Bl/6 mice (female, 8 weeks old, n = 8–10) challenged with aerosolized B. thailandensis E264 or B. pseudomallei 1026b. The mice were first anesthetized with 5% isoflurane in O2 at 1 L/min, then treated with either PBS, free ciprofloxacin (20 mg/kg in 50 μL, 5% dextrose in water) or the drugamer (20 mg/kg ciprofloxacin equivalent in 50 μL PBS) via intratracheal aerosolization using an endobronchial MicroSprayer® Aerosolizer (model IA-1C, Penn-Century, Wyndmoor Inc., PA, USA) and a mouse laryngoscope (model LS-2-M, Penn-Century, PA, USA). The MicroSprayer® Aerosolizer gives aerosol droplets of 16 – 22 μm in size with water. Each mouse received three treatments with a 24h interval between each dose. For the first pre-exposure prophylaxis study with B. thailandensis (Fig. 2A), the first dose started at 2h before infection. For the second prophylaxis B. thailandensis (Fig. 4A) and the prophylaxis study with B. pseudomallei (Fig. 5A), the first dose started at 48 h before infection.
Mice were simultaneously exposed to aerosolized bacteria for a duration of 15 min, with total airflow through the chamber maintained at 19.5 L/min. Bacteria aerosol was generated from 9 mL of bacteria suspension using a MiniHEART hi-flo nebulizer (Westmed, Arizona) connected to a Biaera whole-body exposure chamber through which pressures and flows were controlled via a computer interface (Biaera Technologies, Maryland). Bacterial deposition in each experiment was determined from quantitative culture of lung tissue from sentinel mice euthanized immediately post-exposure. Animals were monitored daily for health, body weight, and temperature. Animals with temperatures of less than 25°C, body weight loss of greater than 20%, or a combination of ruffled fur, eye crusting, hunched posture, lack of resistance to handling, and isolation from cage mates were deemed terminal for study purposes and euthanized.
At day 14 after infection, the number of mice that survived the otherwise lethal infection was recorded. The surviving mice were euthanized, and the left lung, median hepatic lobe, and spleen were harvested for quantitative culture to determine viable bacteria surviving after treatment. Organs were homogenized in 1 mL sterile Dulbecco’s PBS and serial dilutions plated on LB agar. Colonies were counted after 2–4 day of incubation at 37°C in humid air with 5% CO2. Verification of B. thailandensis/B. pseudomallei growth was confirmed by plating on Ashdown’s agar, a selective medium that contains crystal violet and gentamicin to inhibit growth of other bacteria. Plates were counted after 4 day of incubation at 37°C in humid air with 5% CO2. Typical Burkholderia colonies on Ashdown’s appear purple and wrinkled.
Intracellular ciprofloxacin PK profiles in alveolar macrophages.
The PK properties of free ciprofloxacin and drugamer-released ciprofloxacin were studied using C57BL/6 mice (female, 6–9 weeks old, n = 3–4). The mice were first anesthetized with 5% isoflurane in O2 at 1 L/min, then treated with either free ciprofloxacin (20 mg/kg in 50 μL, 5% dextrose in water) or the drugamer (20 mg/kg ciprofloxacin equivalent in 50 μL PBS) via intratracheal aerosolization using a MicroSprayer® Aerosolizer (model IA-1C, Penn-Century, Wyndmoor Inc., PA, USA) and a mouse laryngoscope (model LS-2-M, Penn-Century, PA, USA). At pre-determined timepoints after administration (4h, 24h, 48h, 72h, 96h, 110h, 158h), the animals were euthanized by intraperitoneal pentobarbital overdose followed by cardiac puncture. Bronchoalveolar lavage was immediately conducted with a 0.85% NaCl solution containing 0.6 mM EDTA (3 flushes of 1.0 mL). The bronchoalveolar lavage fluid was centrifuged to collect alveolar macrophages (4 °C, 400 g, 15 min). The collected cells were washed with PBS and centrifuged again to remove the supernatant (4°C, 400 g, 15 min). The cell pellet was resuspended in PBS (50 μL) and the obtained cell number was counted using crystal violet staining (0.1% in 0.1 M citric acid) to determine the total cell volume. To the cell suspension was added a solution of ciprofloxacin-d8 (5 μL of a 500 ng/mL solution in water), followed by 150 μL of acetonitrile. The suspension was incubated on ice for 1 hour to lyse the cells. The cell lysates were then dried to completion and reconstituted in 150 μL of a 2/1 solution of acetonitrile/water, and centrifuged at 18000 g for 20 min at 4 °C. The supernatants were stored on ice until analyzed via LC-MS/MS. Details of the LC-MS/MS method for ciprofloxacin quantification have been described in our previous publication (29).
Statistical Analysis.
Student’s two-tailed t-tests were performed to compare two groups and analysis of variance (ANOVA) was performed for comparisons of multiple groups, through GraphPad Prism (version 5.0, GraphPad Software Inc.). Data reported denote the means ± SD. Survival analyses were performed using a log-rank test in GraphPad Prism. p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the Defense Threat Reduction Agency (Grant #HDTRA1-13-1-0047), and the NIH (R01AI134729). F.Y.S. was supported by an international student research fellowship from the Howard Hughes Medical Institute. We dedicate this paper to Professor Kopecek and his remarkable contributions to the field of drug delivery, to polymer therapeutics which underlie this work, and finally for his even more remarkable friendship and inspiring spirit that has enlivened our group over the past three decades.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kersh GJ, Antimicrobial therapies for Q fever, Expert review of anti-infective therapy 11(11) (2013) 1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hussain M, Galvin HD, Haw TY, Nutsford AN, Husain M, Drug resistance in influenza A virus: the epidemiology and management, Infection and drug resistance 10 (2017) 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koch A, Cox H, Mizrahi V, Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment, Current opinion in pharmacology 42 (2018) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson ES, Legionellosis, infections of the central nervous system, John Wiley & Sons Ltd; 2020, pp. 383–391. [Google Scholar]
- [5].Chin KL, Sarmiento ME, Alvarez-Cabrera N, Norazmi MN, Acosta A, Pulmonary non-tuberculous mycobacterial infections: current state and future management, European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 39(5) (2020) 799–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].CDC, CDC Federal Select Agent Program, October 28th, 2019. https://www.selectagents.gov/SelectAgentsandToxinsList.html.
- [7].Titball RW, Burtnick MN, Bancroft GJ, Brett P, Burkholderia pseudomallei and Burkholderia mallei vaccines: Are we close to clinical trials?, Vaccine 35(44) (2017) 5981–5989. [DOI] [PubMed] [Google Scholar]
- [8].Amanat F, Krammer F, SARS-CoV-2 Vaccines: Status Report, Immunity 52(4) (2020) 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wenzler E, Fraidenburg DR, Scardina T, Danziger LH, Inhaled antibiotics for gram-negative respiratory infections, Clinical Microbiology Reviews 29(3) (2016) 581–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leekha S, Terrell CL, Edson RS, General principles of antimicrobial therapy, Mayo Clin Proc 86(2) (2011) 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mehta KC, Dargad RR, Borade DM, Swami OC, Burden of antibiotic resistance in common infectious diseases: role of antibiotic combination therapy, J Clin Diagn Res 8(6) (2014) ME05–ME8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Quon BS, Goss CH, Ramsey BW, Inhaled antibiotics for lower airway infections, Annals of the American Thoracic Society 11(3) (2014) 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Strong P, Ito K, Murray J, Rapeport G, Current approaches to the discovery of novel inhaled medicines, Drug discovery today 23(10) (2018) 1705–1717. [DOI] [PubMed] [Google Scholar]
- [14].Hamblin KA, Armstrong SJ, Barnes KB, Davies C, Laws T, Blanchard JD, Harding SV, Atkins HS, Inhaled liposomal ciprofloxacin protects against a lethal infection in a murine model of pneumonic plague, frontiers in microbiology 8 (2017) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Norville IH, Hatch GJ, Bewley KR, Atkinson DJ, Hamblin KA, Blanchard JD, Armstrong SJ, Pitman JK, Rayner E, Hall G, Vipond J, Atkins TP, Efficacy of liposome-encapsulated ciprofloxacin in a murine model of Q fever, Antimicrob Agents Chemother 58(9) (2014) 5510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamblin KA, Armstrong SJ, Barnes KB, Davies C, Wong JP, Blanchard JD, Harding SV, Simpson AJH, Atkins HS, Liposome encapsulation of ciprofloxacin improves protection against highly virulent Francisella tularensis strain Schu S4, Antimicrob Agents Chemother 58(6) (2014) 3053–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, Nashed M, Joseph J, Perkins WR, DiPetrillo K, Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages, Frontiers in microbiology 9 (2018) 915–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moghimi SM, Hunter AC, Andresen TL, Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective, Annual review of pharmacology and toxicology 52 (2012) 481–503. [DOI] [PubMed] [Google Scholar]
- [19].Yamashita F, Hashida M, Pharmacokinetic considerations for targeted drug delivery, Advanced drug delivery reviews 65(1) (2013) 139–47. [DOI] [PubMed] [Google Scholar]
- [20].Duncan R, Seymour LCW, Scarlett L, Lloyd JB, Rejmanová P, Kopeček J.i., Fate of N-(2-hydroxypropyl)methacrylamide copolymers with pendent galactosamine residues after intravenous administration to rats, Biochimica et Biophysica Acta (BBA) - General Subjects 880(1) (1986) 62–71. [DOI] [PubMed] [Google Scholar]
- [21].Ringsdorf H, Structure and properties of pharmacologically active polymers, Journal of Polymer Science: Polymer Symposia 51(1) (1975) 135–153. [Google Scholar]
- [22].Kopeček J, Duncan R, Targetable polymeric prodrugs, Journal of Controlled Release 6(1) (1987) 315–327. [Google Scholar]
- [23].Currie BJ, Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment, Seminars in respiratory and critical care medicine 36(1) (2015) 111–25. [DOI] [PubMed] [Google Scholar]
- [24].Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI, Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis, Nature microbiology 1(1) (2016). [DOI] [PubMed] [Google Scholar]
- [25].Meumann EM, Cheng AC, Ward L, Currie BJ, Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 54(3) (2012) 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Allwood EM, Devenish RJ, Prescott M, Adler B, Boyce JD, Strategies for Intracellular Survival of Burkholderia pseudomallei, Frontiers in microbiology 2 (2011) 170–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peacock SJ, Schweizer HP, Dance DAB, Smith TL, Gee JE, Wuthiekanun V, DeShazer D, Steinmetz I, Tan P, Currie BJ, Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei, Emerg Infect Dis 14(7) (2008) e2–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dance D, Treatment and prophylaxis of melioidosis, International journal of antimicrobial agents 43(4) (2014) 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ventola CL, The antibiotic resistance crisis: part 1: causes and threats, P T 40(4) (2015) 277–283. [PMC free article] [PubMed] [Google Scholar]
- [30].Estes DM, Dow SW, Schweizer HP, Torres AG, Present and future therapeutic strategies for melioidosis and glanders, Expert review of anti-infective therapy 8(3) (2010) 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].MacLaren G, Lye DC, Lee VJ, Increasing experience with melioidosis and critical care: medical and military implications*, Critical Care Medicine 44(8) (2016). [DOI] [PubMed] [Google Scholar]
- [32].Chaowagul W, Suputtamongkul Y, Smith MD, White NJ, Oral fluoroquinolones for maintenance treatment of melioidosis, Transactions of the Royal Society of Tropical Medicine and Hygiene 91(5) (1997) 599–601. [DOI] [PubMed] [Google Scholar]
- [33].Son HN, Srinivasan S, Yhee JY, Das D, Daugherty BK, Berguig GY, Oehle VG, Kim SH, Kim K, Kwon IC, Stayton PS, Convertine AJ, Chemotherapeutic copolymers prepared via the RAFT polymerization of prodrug monomers, Polymer Chemistry 7(27) (2016) 4494–4505. [Google Scholar]
- [34].Kern HB, Srinivasan S, Convertine AJ, Hockenbery D, Press OW, Stayton PS, Enzyme-cleavable polymeric micelles for the intracellular delivery of proapoptotic peptides, Molecular pharmaceutics 14(5) (2017) 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Freeman H, Srinivasan S, Das D, Stayton PS, Convertine AJ, Fully synthetic macromolecular prodrug chemotherapeutics with EGFR targeting and controlled camptothecin release kinetics, Polymer Chemistry 9(42) (2018) 5224–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Su FY, Srinivasan S, Lee B, Chen J, Convertine AJ, West TE, Ratner DM, Skerrett SJ, Stayton PS, Macrophage-targeted drugamers with enzyme-cleavable linkers deliver high intracellular drug dosing and sustained drug pharmacokinetics against alveolar pulmonary infections, Journal of controlled release : official journal of the Controlled Release Society 287 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Warawa JM, Evaluation of surrogate animal models of melioidosis, Frontiers in microbiology 1 (2010) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen J, Su F-Y, Das D, Srinivasan S, Son H-N, Lee B, Radella F, Whittington D, Monroe-Jones T, West TE, Convertine AJ, Skerrett SJ, Stayton PS, Ratner DM, Glycan targeted polymeric antibiotic prodrugs for alveolar macrophage infections, Biomaterials 195 (2019) 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cipolla D, Blanchard J, Gonda I, Development of Liposomal Ciprofloxacin to Treat Lung Infections, Pharmaceutics 8(1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].West TE, Myers ND, Limmathurotsakul D, Liggitt HD, Chantratita N, Peacock SJ, Skerrett SJ, Pathogenicity of high-dose enteral inoculation of Burkholderia pseudomallei to mice, Am J Trop Med Hyg 83(5) (2010) 1066–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Collaborators GL, Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015, The Lancet. Infectious diseases 17(11) (2017) 1133–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cohen A, Mathiasen VD, Schön T, Wejse C, The global prevalence of latent tuberculosis: a systematic review and meta-analysis, The European respiratory journal 54(3) (2019). [DOI] [PubMed] [Google Scholar]
- [43].World Health Organization, Global tuberculosis report 2019, 2019. https://www.who.int/tb/publications/en/.
- [44].Currie BJ, Ward L, Cheng AC, The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study, PLoS neglected tropical diseases 4(11) (2010) e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Limmathurotsakul D, Wuthiekanun V, Chierakul W, Cheng AC, Maharjan B, Chaowagul W, White NJ, Day NP, Peacock SJ, Role and significance of quantitative urine cultures in diagnosis of melioidosis, Journal of clinical microbiology 43(5) (2005) 2274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sarovich DS, Price EP, Limmathurotsakul D, Cook JM, Von Schulze AT, Wolken SR, Keim P, Peacock SJ, Pearson T, Development of ceftazidime resistance in an acute Burkholderia pseudomallei infection, Infection and drug resistance 5 (2012) 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chantratita N, Rholl DA, Sim B, Wuthiekanun V, Limmathurotsakul D, Amornchai P, Thanwisai A, Chua HH, Ooi WF, Holden MTG, Day NP, Tan P, Schweizer HP, Peacock SJ, Antimicrobial resistance to ceftazidime involving loss of penicillin-binding protein 3 in Burkholderia pseudomallei, Proceedings of the National Academy of Sciences of the United States of America 108(41) (2011) 17165–17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Price EP, Smith ML, Paxinos EE, Tallon LJ, Sadzewicz L, Sengamalay N, Baird RW, Currie BJ, Sarovich DS, Whole-genome sequences of burkholderia pseudomallei isolates exhibiting decreased meropenem susceptibility, Genome Announc 5(14) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bugrysheva JV, Sue D, Hakovirta J, Loparev VN, Knipe K, Sammons SA, Ranganathan-Ganakammal S, Changayil S, Srinivasamoorthy G, Weil MR, Tatusov RL, Gee JE, Elrod MG, Hoffmaster AR, Weigel LM, Finished annotated genome sequence of burkholderia pseudomallei strain bp1651, a multidrug-resistant clinical isolate, Genome Announc 3(6) (2015) e01427–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Masadeh MM, Alzoubi KH, Khabour OF, Al-Azzam SI, Ciprofloxacin-Induced Antibacterial Activity Is Attenuated by Phosphodiesterase Inhibitors, Current therapeutic research, clinical and experimental 77 (2015) 14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ashdown LR, Currie BJ, Melioidosis: when in doubt leave the quinolone alone!, The Medical journal of Australia 157(6) (1992) 427–8. [DOI] [PubMed] [Google Scholar]
- [52].Chetchotisakd P, Chaowagul W, Mootsikapun P, Budhsarawong D, Thinkamrop B, Maintenance therapy of melioidosis with ciprofloxacin plus azithromycin compared with cotrimoxazole plus doxycycline, Am J Trop Med Hyg 64(1–2) (2001) 24–7. [DOI] [PubMed] [Google Scholar]
- [53].Barnes KB, Hamblin KA, Richards MI, Laws TR, Vente A, Atkins HS, Harding SV, Demonstrating the Protective Efficacy of the Novel Fluoroquinolone Finafloxacin against an Inhalational Exposure to Burkholderia pseudomallei, Antimicrob Agents Chemother 61(7) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Steward J, Piercy T, Lever MS, Nelson M, Simpson AJ, Brooks TJ, Comparison of gatifloxacin, moxifloxacin and ciprofloxacin for treatment of experimental Burkholderia pseudomallei infection, The Journal of antimicrobial chemotherapy 55(4) (2005) 523–7. [DOI] [PubMed] [Google Scholar]
- [55].Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK, Inhaled formulations and pulmonary drug delivery systems for respiratory infections, Advanced drug delivery reviews 85 (2015) 83–99. [DOI] [PubMed] [Google Scholar]
- [56].Ruiz SI, Bowen LE, Bailey MM, Berkland C, Pulmonary Delivery of Ceftazidime for the Treatment of Melioidosis in a Murine Model, Molecular pharmaceutics 15(3) (2018) 1371–1376. [DOI] [PubMed] [Google Scholar]
- [57].Ruiz SI, El-Gendy N, Bowen LE, Berkland C, Bailey MM, Formulation and Characterization of Nanocluster Ceftazidime for the Treatment of Acute Pulmonary Melioidosis, Journal of pharmaceutical sciences 105(11) (2016) 3399–3408. [DOI] [PubMed] [Google Scholar]
- [58].D’Elia RV, Woods S, Butcher W, McGahon J, Khadke S, Perrie Y, Williamson ED, Roberts CW, Exploitation of the bilosome platform technology to formulate antibiotics and enhance efficacy of melioidosis treatments, Journal of controlled release : official journal of the Controlled Release Society 298 (2019) 202–212. [DOI] [PubMed] [Google Scholar]
- [59].Melvin M Weinstein P, Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition, Clinical and Laboratory Standards Institute, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


