Abstract
Neurodegenerative disorders, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), are the most concerning disorders due to the lack of effective therapy and dramatic rise in affected cases. Although these disorders have diverse clinical manifestations, they all share a common cellular stress response. These cellular stress responses including neuroinflammation, oxidative stress, proteotoxicity, and endoplasmic reticulum (ER)-stress, which combats with stress conditions. Environmental stress/toxicity weakened the cellular stress response which results in cell damage. Small molecules, such as flavonoids, could reduce cellular stress and have gained much attention in recent years. Evidence has shown the potential use of flavonoids in several ways, such as antioxidants, anti-inflammatory, and anti-apoptotic, yet their mechanism is still elusive. This review provides an insight into the potential role of flavonoids against cellular stress response that prevent the pathogenesis of neurodegenerative disorders.
Keywords: flavonoids, cellular stress response, neurodegenerative disorders, ER stress proteotoxicity, oxidative stress, neuroinflammation
1. Introduction
Neurodegenerative disorders are marked by different clinical features including memory and cognitive impairment, motor dysfunction, speaking disability, and breathing problems [1,2,3,4,5]. These symptoms are the consecutive results of stress conditions. Exposure to any stress, such as oxidative stress, environmental stress (metals and pesticides), and pharma chemicals, lead to disruption of cellular homeostasis by changing the normal cellular function. Cellular homeostasis includes neuroinflammation, protein quality control (PQC), and endoplasmic reticulum (ER) stress that are consistent in combating stress conditions [6,7,8,9,10]. A compromised cellular stress response condition leads to an imbalance in cellular homeostasis that results in cell death. Recent studies have found that flavonoids can prevent cell death by attenuating the cellular stress response [11,12]. Natural flavonoids are present in food and these are the most ingested polyphenolic compounds. These flavonoids have many therapeutic properties, such as anti-microbial, anti-oxidant, anti-inflammatory, and immune-modulatory [13,14,15,16,17,18,19]. Recent studies show the effectiveness of flavonoids in neurodegenerative disorders [20]. Diet rich in flavonoids have shown benefits against oxidative stress, inflammation [21], cardiovascular disease [22,23,24], apoptosis, and cancer [25,26]. The potential roles of flavonoids in neurodegenerative disorders were also confirmed by many studies. Citrus flavonoids, such as naringenin and hesperidin, both can cross the blood-brain barrier (BBB) and prevent neuronal deterioration [15,27,28,29]. Nobiletin (citrus flavonoid) shows the anti-neuroinflammatory effect by alleviating the inflammatory response. These pieces of evidence suggest that the therapeutic property of flavonoids against cellular stress and that could be used as a targeted drug for neurodegenerative disorders. This review provides insight on those flavonoids that prevent cellular death by alleviating the toxic impact of the cellular stress response.
2. Search Strategy
A comprehensive literature search was conducted to identify relevant research articles showing the beneficial effects of flavonoids in different models of neurodegenerative diseases. We searched Web of Science, PubMed, Google Scholar, Embase, and Cochrane Library databases to identify all relevant studies. We used different keywords for the search, such as “neurodegeneration, neuroprotective, neuroprotection, and neurodegenerative diseases, combined with “bioflavonoids, flavonols, flavan-3-ols, anthocyanin, flavone, flavones, isoflavones isoflavonoids or flavonones, and flavonoid”. Studies were included by studying the abstracts of the collected articles.
Selection Criteria
All studies showing the effects of flavonoids on in vitro and in vivo models of neurodegenerative disease were selected. Administration of drugs, mode of administration, and treatment schedule were not considered. Studies conducted on any species, age, and sex were included. Studies where a comparison between different groups was given (e.g., control group, diseased group, and treated with flavonoids group) were included. We did not include incomplete data, unpublished data, abstracts, conference proceedings, commentary, editorials/letters, and duplicate references.
3. Environmental Stress and Cellular Stress Response
3.1. Environmental Stress
A gradual rise in hazardous chemicals, such as heavy metals, pesticides, and pharma chemicals, causes an imbalance in the environment that adversely affects human health [30]. Epidemiological studies have suggested that environmental chemical exposure to humans was associated with several disorders. The toxic effect of these chemicals is due to the imbalance in cellular stress response. Cells have a well-evolved cellular homeostasis system; however, stress exposure leads to disruption of cellular homeostasis by causing an imbalance between the reactive oxygen species (ROS) and the antioxidant system. Under oxidative stress, the generation of superoxide radical (•O2−) in mitochondria is the former step in the formation and proliferation of other ROS. These free radicals react with hydrogen peroxide (H2O2) via the iron-catalyzed Haber–Weiss reaction that generates the hydroxyl radical (•OH) [31,32]. Another ROS ‘peroxynitrite’ (ONOO−) formation is accompanied by the reaction of free radical nitric oxide and O2. The presence of peroxynitrite causes severe toxic effects due to its interactions with amino acids that alter the structure/function of the protein [33,34]. Exposure to pesticides and heavy metals leads to a rise in ROS production. These ROS cause irreversible damage to the cellular macro-molecules that are associated with the alteration of mitochondrial membrane functions, thus causing mitochondrial dysfunction and apoptosis [35,36,37]. These environmental stress factors also induce proteotoxicity by altering the structure of proteins, or affecting the nascent polypeptide chain folding, e.g., arsenic induces protein aggregation [38]. Furthermore, studies show that arsenic exposure causes protein misfolding that might affect protein-protein interactions, thereby causing proteotoxicity and thus affecting cell viability [39,40]. Cadmium exposure to yeast cells leads to the unfolded protein response induction through impairment of protein folding in the endoplasmic reticulum [41,42]. Exposure to chromium results in protein damage by oxidation. Chromium also induces protein aggregation by enhancing mRNA mistranslation. Mistranslation appears to be a primary cause of cellular chromium toxicity [43]. Copper toxicity induces oxidative stress, inflammation, apoptosis, astrocytosis, and excitotoxicity in the corpus striatum, hippocampus, and frontal cortex region of the brain [44,45]. Pesticides are also known to show similar effects. Rotenone and dieldrin induce the aggregation of alpha-synuclein and mutant huntingtin (mthtt) protein [46,47]. Paraquet treatment of SHSY-5Y cells induces the decrease in levels of proteasome 19S subunits and causes proteasome dysfunction [48]. Thus, exposure to environmental or intracellular stress could initiate the cellular stress response to protect the cellular homeostasis, while exaggerated stress conditions could lead to cell death.
3.2. Cellular Stress Response
Cells eliminate toxic substances in many ways. Several types of stress, such as heat stress, provoke various protective responses including oxidative stress response, heat shock response, and unfolded protein response (UPR). All these stress responses work to balance the cellular homeostasis either by monitoring and protecting the protein quality control or by neutralizing the toxic effect of reactive nitrogen and oxygen species (RNS; ROS). These heat shock responses and UPR are generally enhanced either by intercellular (oxidative stress) or, extra-cellular (pesticide/metals) stresses. Both stress conditions lead to disruption of the PQC by damaging the protein and making large aggregates. Under stress conditions, cells enhance the expression of various heat shock proteins (HSPs) that maintain the protein structure and refolds a misfolded protein. These HSPs are grouped into different subfamily according to their molecular weight. These include: HSP110, HSP90, HSP70, HSP60, HSP40, and small HSPs (sHSPs). All these HSPs are ATP-dependent except the sHSPs [49,50,51,52]. Hsp27, Hsp70, and Hsp32 (Heme Oxygenase, HO-1) are generally responding to neuronal injuries including ischemia and hemorrhage. Hsp27 is a sHSP and works by making a multimer post phosphorylation [53]. HSP90 is associated with the maturation of substrates, especially those that have a role in various cellular pathways, such as E3 ubiquitin ligases, kinases, and transcription factors. HSP90 attains certain specific conformational states that are stabilized by co-chaperones [54,55].
Exposure to stress that interferes with the glucose level, protein glycosylation, and Ca2+ disturbance causes the accumulation of unfolded proteins in the endoplasmic reticulum. This results in the activation of the UPR [56]. This UPR activates a set of different proteins including inositol-requiring protein-1 (IRE1), protein kinase RNA (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6). UPR signaling protects a cell from an imbalanced unfolded protein load by increasing the folding capacity of the ER [57,58]. However, excessive protein overload in the ER or defects in the UPR may induce cell death, known as ER stress-induced cell death.
Generally, cells maintain a healthy balance by monitoring the ratio of pro-oxidant: antioxidant levels, but oxidative stress arises when the cells’ antioxidant systems, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, and other antioxidant proteins, fail to work [59]. ROS and RNS may interfere with the electron transport system. Furthermore, ROS and RNS also induce peroxidation of lipids in the plasma membrane and impair the functional activities of DNA and proteins [30,60,61]. All these cellular stress responses try to protect the cell from stress, but under extreme conditions, the cellular defense system fails to recover, thus promoting cell death.
4. Flavonoids
Flavonoids are polyphenolic compounds present in plants and are synthesized by the phenylpropanoid pathway [62,63,64]. They have antioxidative and anti-inflammatory properties [63,64,65]. Several case studies suggest that the intake of flavonoids reduce the risk of dementia [66]. Flavonoids have a neuroprotective property and they reduce the oxidative stress in epilepsy. In the central nervous system (CNS) several flavonoids bind to the benzodiazepine site on the γ-Aminobutyric acid type A (GABAA)-receptor resulting in anticonvulsive effects [67]. Intake of berry flavonoids improves memory in elderly people. Dietary cocoa flavanols improve cognition in older adults by enhancing dentate gyrus function [68]. Intake of cocoa flavanols improves human cognition and counteracts different types of cognitive decline [69]. Gratton et al. found that intake of cocoa flavanols enhances cerebral cortical oxygenation and cognition in healthy adults [70].
Flavonoids are categorized into different subgroups, summarized in Table 1. The application of flavonoids could mitigate the harsh effect of stress-induced cellular events. Hence, the use of these flavonoids could attenuate the toxic effect of environmental stress and cellular stress response.
Table 1.
A subgroup of flavonoids, their natural resources, and example.
| Subgroup | Chemical Structure | Plant Source | Example | Ref. |
|---|---|---|---|---|
| Isoflavones |
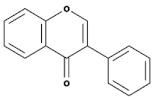
|
Soybeans, leguminous plants, microbes, | Genistein, Daidzein, Glycerin, Formanantine | [71,72,73,74] |
| Flavones |
|
Leaves, flowers, and fruits | Luteolin, Apigenin | [75,76] |
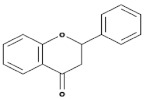
| Flavanones |
|
All citrus fruits | Hesperidin, Naringenin | [77] |
| Flavonols |
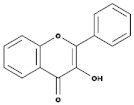
|
Onions, berries, lettuce, tomatoes, grapes, and apples | Kaempferol, Quercetin | [78] |
| Neoflavonoids |
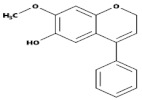
|
Sri Lankan endemic plant Mesuathwaitesii | Calophyllolide | [79,80] |
| Flavanols(Flavan-3-ols) |
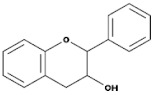
|
Peaches, pears, blueberries, bananas, and apples | Catechins, Epicatechins, Epigallocatechin | [81,82] |
| Anthocyanins |
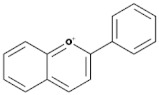
|
Bilberries, cranberries, merlot grapes, blackberries, black currants, red grapes, strawberries, blueberries, and raspberries | Cyanidin, Delphinidin, Malvidine | [83] |
| Flavones |
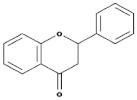
|
Leaves, flowers, and fruits | Luteolin, Apigenin | [84,85] |
| Flavanones |
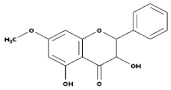
|
All citrus fruits | Hesperidin, Naringenin | [77] |
5. Flavonoids and Cellular Stress Response
5.1. Role of Flavonoids in Neuroinflammation
Neuroinflammation is an immune response of the CNS. During neuroinflammation, glial cells (microglia) get activated and release inflammatory mediators, such as cytokines, chemokines, and ROS/RNS [86]. The flavonoids can interact with neuronal receptors and modulate kinase signaling pathways, transcription factors, and gene and/or protein expression, which control memory and learning processes in the hippocampus [87]. The level of prostaglandins (PGs) increases in the inflamed neuronal region, a feature of acute inflammation [88]. In an aging brain, neuroinflammation is marked by an increase in prostaglandin E2 (PGE2) levels. Once the neuroinflammation achieves the threshold and becomes over-activated, it leads to cellular damage and loss of neuronal function. Microglia activation/proliferation and reactive astrogliosis are commonly observed during neuroinflammation. Activated microglia are involved in the onset and maintenance of astrocyte proliferation. Lipopolysaccharide treatment in primary enriched astrocyte cultures results in increased proliferation of astrocytes. PGE2 released from activated microglia enhances astrocyte proliferation [89].
Flavonoids have a neuroprotective role in both in-vitro and in-vivo models against neuroinflammation [15,65,90]. Flavonoids can suppress the microglial activation and reduce the neurotoxicity induced by neurotoxic species released by microglia. The plant flavonoid wogonin inhibits activation-induced death of C6 glial cells by suppressing nitric oxide (NO) production. These inhibitory effects of wogonin on NO production are exerted through inhibition of NF-kappaB-mediated inducible nitric oxide synthase (iNOS) induction [91].
Flavonoids, luteolin, and apigenin protect the dopaminergic neurons by reducing oxidative stress, neuroinflammation and microglial activation along with enhanced neurotrophic potential in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced parkinsonism mice model. Luteolin and apigenin-treated mice model shows increased brain-derived neurotrophic factor (BDNF) levels in the substantia nigra region of the brain compared to MPTP treatment mice [92]. Li et al. found that treatment with apigenin (20 mg/kg, intragastrically) for three weeks remarkably ameliorated chronic unpredictable mild stress (CUMS)-induced behavioral abnormalities, such as: decreased locomotor activity and reduced sucrose consumption. Apigenin inhibits IL-1β and caspase-1 via disrupting the NLRP3 assembly. Apigenin inhibits the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome activation through the upregulation of peroxisome proliferator-activated receptor-gamma (PPARγ) [93]. Apigenin ameliorated dopaminergic neuronal loss and improved behavioral, biochemical, and mitochondrial enzyme activities by suppression of oxidative stress and neuroinflammation [94].
Another flavonoid, rutin, when given to male albino Wistar rats, decreases mRNA expression of cytokines, caspase-1, apoptosis-associated speck-like CARD-containing protein (ASC), and ASC-NLRP3 [95]. Daidzein (flavonoid) ameliorates the inflammatory process and alleviates the risk of Alzheimer’s disease (AD) progression. Daidzein treatment down-regulates the expression of TNF-α, IL-1, and IL-6 in the primary astrocytes which are stimulated with amyloid-beta or lipopolysaccharide [96]. Catechin (flavonoid) protected murine microglia N9 cells from tert-butylhydroperoxide induced cell death by the inhibition of NF-kB, p53 activity, and activation of extracellular signal-regulated protein kinase (ERK) [97]. Blueberry extract (rich in flavonoids) inhibits the production of inflammatory mediators iNOS and COX-2 and reduces the level of NO, TNF-α, IL-1β, and ROS in lipopolysaccharide-activated BV2 microglial cells [98].
Naringenin treatment prevents neuronal cell death in LPS/IFNγ stimulated glial cells by the reduction in iNOS, NO, and TNF-α level and inhibition of p38 signaling cascades and STAT-1 transcription factor [99]. Biochanin A protects dopaminergic neurons against LPS-induced damage through inhibition of microglia activation and reduction in superoxide, TNFα, and NO [100]. Nobiletin prevents neuroinflammation in LPS-stimulated BV-2 microglial cells by inhibiting the release of TNF-α, IL-1β, ERK, c-Jun NH(2)-terminal kinase (JNK), and p38 mitogen-activated protein kinases (MAPKs) [101]. Adjunctive treatment with genistein and daidzein preserve neuronal functioning and sustain neurocognitive abilities of HIV-1 infected persons via a selective ER-mediated mechanism in neurons [102].
Transgenic Parkinson’s disease (PD) mice (C57BL/6 mice) received grape polyphenol concentrate (1.5 mL/kg/day) from the age of 6–8 weeks for four months have improved their behavioral and cognitive function. Grape polyphenol exhibits neuroprotective activity by reducing the α-synuclein accumulation in the frontal cortex and neuroinflammatory response in the frontal cortex and hippocampus [103]. Luteolin protects dopaminergic neurons against inflammation-induced neurotoxicity by inhibiting microglial activation [104]. Naringin (present in grape and orange) protects dopaminergic neurons by induction of the activation of the mammalian target of rapamycin complex-1 and inhibited microglial activation in the 6-OHDA treated mouse model [105]. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the nuclear factor E2-related factor 2 (Nrf2) and antioxidant response element (ARE) signaling pathway [106]. Baicalein inhibits the upregulation of tumor necrosis factor-α and interleukin-1β in the substantia nigra and striatum in MPTP-induced PD mice models [107]. Baicalein inhibits α-synuclein aggregation, inflammasome activation, and cathepsin B production in Sprague-Dawley rats treated with 1-methyl-4-phenylpyridinium [108].
5.2. Role of Flavonoids in Oxidative Stress
ROS are the major cause of oxidative stress and are linked with the pathogenesis of several neurological disorders [109]. Accumulation of ROS, such as hydroxyl radicals (•OH), superoxide radicals (•O2−), and hydrogen peroxide (H2O2), are associate with neuronal cell death [110,111]. The elevation in ROS induces protein oxidation, DNA damage, and lipid peroxidation (LPO), collectively leading to apoptosis in neuronal cells [112]. Uses of antioxidants, such as flavonoids, might be beneficial in reducing the toxicity of the oxygen-free radicals. These flavonoids have the potential to counter the toxicity of oxidative stress and decrease the pathogenesis of several neurological disorders [113]. Treatment with flavonoids, namely quercitrin, isoquercitrin, and afzelin, in human neuronal SH-SY5Y neuronal cells has shown beneficial effects through regulating inflammation, apoptosis, and ROS-scavenging. These flavonoids attenuated inflammation by inhibiting the expression of nitric oxide synthase, cyclooxygenase-2, and caspase activation [114]. Treatment with quercetin and luteolin and their metabolites 3,4-dihydroxytoluene (DHT) and 3,4- 3,4-dihydroxyphenylacetic acid (DHPAA), respectively, in neuronal PC12 cells, prevents oxidative stress. These metabolites are less efficient than parent flavonoids [115].
Two novel prenylated flavonoids, morachalcone D and morachalcone E, isolated from mulberry leaf, have antioxidant properties since their exposure to HT22 cells. Morachalcone D has higher efficiency than morachalcone E as it inhibits glutamate and erastin-induced cellular damage. Morachalcone D inhibits ROS production, glutathione (GSH) depletion, and iron accumulation. It is also involved in the upregulation of the expression of several genes of the antioxidant systems including Nrf2, GPx4, SOD2, SLC7A11, HMOX1, and CAT [116].
Phloretin and phlorizin (dihydrochalcone, a type of natural phenol, a dihydrochalcone, a family of bicyclic flavonoids) have neuroprotective effects against rotenone-induced toxicity in human SH-SY5Y neuroblastoma cells. They reduce rotenone-induced cell death by actively scavenging ROS, normalizing mitochondrial transmembrane potential, inhibiting caspase 3 activity, and DNA damage [117]. Administration of 6′′′-p-coumaroylspinosin (P-CS) (flavonoid isolated from Ziziphi Spinosae Semen) on PC12 neuronal cells significantly prevents acrylamide-induced cell death, decreases GSH content, and ROS overproduction. P-CS was also suppressing the expression of Bax (apoptosis regulator) and Bim (pro-apoptotic protein) induced by acrylamide and inhibits the JNKs pathway [16].
Baicalein exerts protective effects in vivo and in vitro against 6-hydroxydopamine (6-OHDA) [118]. Baicalein prevented abnormal behavior by increasing dopaminergic neurons and dopamine and serotonin levels in the striatum and also inhibited oxidative stress and astroglia response [119]. Similarly, baicalein protects cells against the toxicity of a point mutation in α-synuclein [120], and inhibited the formation of α-synuclein oligomers, and consequently prevents its oligomerization [121]. Mitochondrial dysfunction in SH-SY5Y cells and upregulation of DJ-1 protein expression induced by 6-OHDA are prevented by baicalein [122]. Baicalein downregulates the activation of NF-κB, ERK, and JNK and attenuates astrocyte activation in MPTP mice [123].
Rutin protects dopaminergic neurons against 6-OHDA-induced neurotoxicity by and activating SOD, catalase, GPx, and total GSH activity and inhibition of LPO [124,125]. Kaempferol improves motor coordination, raises striatal dopamine and its metabolite levels, increases SOD and GSH activity, and reduces the content of LPO, also preventing the loss of TH-positive neurons induced by MPTP [126]. Kaempferol exhibit neuroprotection in models of rotenone-mediated acute toxicity by protecting SH-SY5Y cells and primary neurons from rotenone toxicity [127].
Quercetin protects against oxidative stress and increases activities of ATPase, SOD, GPx, Acetylcholinesterase, and dopamine depletion in MPTP-treated mice [128]. Furthermore, in a rotenone model, quercetin has been shown to upregulate mitochondrial complex-I activity and increase catalase and SOD activity [129]. In the 6-OHDA rat model, treatment of quercetin increased levels of antioxidants and striatal dopamine and reduced dopaminergic neuronal loss [130]. Luteolin also reduces cytotoxicity induced by 6-OHDA and ROS production in neuronal PC12 cells by modulating changes in the stress response pathway [131]. In MPTP-treated mice, luteolin and apigenin protect dopaminergic neurons by reducing oxidative damage, neuroinflammation, and microglial activation and also improve muscular and locomotor activity [92].
Baicalein prevented the progression of α-synuclein accumulation and protected dopaminergic neurons, and also inhibited the formation of α-synuclein oligomers in a rotenone mouse model [132]. Hesperidin (found in oranges and lemons) protects against iron-induced oxidative damage in the Drosophila melanogaster model of PD. Hesperidin restores dopamine levels, cholinergic activity, and improves motor function [133]. Antunes et al. found that hesperidin protects against neurotoxicity by reducing oxidative damage, increasing dopamine levels, and also improving the behavioral parameters in 6-OHDA-treated mice [134].
5.3. Role of Flavonoids in Proteotoxicity
Neurological disorders are marked by the presence of protein aggregates termed as amyloid, malfunctioned ubiquitin-proteasome system (UPS), and disrupted PQC network. These aggregates are present as insoluble prefibrillar amyloid-β oligomers (AβO) or insoluble amyloid-β oligomers [135,136,137,138]. In AD, the aggregated protein species, known as amyloid-β, are considered as the most neurotoxic species, while, in PD, the presence of α-synuclein aggregates and Lewy bodies are prominent hallmarks of PD pathology. These aggregates or disrupted UPS are the consecutive resultants of various stress conditions. Under stress conditions, the PQC system fails, thus being unable to combat proteotoxicity. Under such conditions, it has been found that flavonoids can effectively exclude proteotoxicity by preventing the formation of protein aggregates. Different cell model studies suggest that flavan-3-ols (especially their metabolites) could serve as great therapeutic targets for AD prevention. ‘Phenyl-γ-valerolactones (PVL)’ a flavan-3-ols’s metabolite efficiently reduces the Aβ-mediated toxicity. In yeast and mammalian cells, these PVLs especially monohydroxylated PVL, exclude the β-oligomer-induced toxicity and prevent cell death. Another PVL ‘(4′-OH)-PVL’ has been found to disrupt the Aβ assembly. Atomic force microscopy (AFM) images have shown the remodeling of toxic AβO aggregates into non-toxic amorphous aggregates [139]. Cellular protein aggregates hamper the PQC, thus causing disrupted protein homeostasis [140]. Myricetin (a type of flavone) inhibits aggregation of different aberrant proteins and modulates the HSP70 chaperone and quality control (QC)-E3 ubiquitin ligase E6-AP levels. Myricetin alleviates cytotoxicity by stabilizing the E6-AP, thus reducing the misfolded protein inclusions [141].
Modified flavonoids could be a promising candidate against various diseases. Dihydroquercetin, a modified form of quercetin, enhances the quercetin quality. Under physical stress conditions (thermal and chemical), quercetin fails to prevent stress-induced cell death. In contrast, dihydroquercetin has successfully prevented cellar injuries. Moreover, under hyperthermic stress, as well as sodium arsenite exposure to cells, quercetin led to a reduction in HSP70 synthesis and accumulation [142].
Pesticides cause various diseases as evidenced by many epidemiological studies. Mechanistic studies have shown their association with proteotoxicity as they induce the formation of Aβ amyloids. Silymarin, a flavonolignan extracted from the seeds of the milk thistle Silybummarianum, promotes the reduction of paraquat-induced Aβ aggregates formation in C.elegans [143]. Epimedium treatment on two C. elegans models of human proteotoxic disease namely CL4176 (expressing amyloid-β (1–42) peptide) and AM140 (expressing apolyglutamine protein), have shown the anti-proteotoxic property. Moreover, it also involves the reduction of Aβ1–42 and polyglutamine-induced paralysis in both models [144].
Treatment of 6′′′-feruloylspinosin (6-FS), one of the main active flavonoid components in Sour Jujube seeds, on the β-amyloid protein of transgenic C. elegans (GMC101) and PC12 cells resulted in delaying the aging process, reduced the rate of paralysis, enhanced the resistance to heat stress, increased the chemotaxis ability and promoted autophagy activity through the autophagy/lysosome pathway. Furthermore, 6-FS reduced the β-amyloid-induced toxicity by suppressing the deposition of β-amyloid and aggregation of the protein. It also increased the level of mitophagy in PC12 cells by promoting the expression of Pink1/Parkin in the mitophagy pathway [145].
5.4. Role of Flavonoids in Endoplasmic Reticulum (ER) Stress
ER stress is a condition caused by the accumulation of misfolded proteins and alterations in the calcium homeostasis which leads to the disruption of the structure and function of the ER. The ER stress response uplifts the expression of specific proteins including ER chaperones and proteins associated with the degradation of misfolded proteins. In ER stress, the accumulation of unfolded proteins disrupts the cellular proteostasis balance. This condition triggers the downstream signaling cascade in the ER, termed unfolded protein response (UPR). Prolonged ER stress induces several pathological conditions and aggravated ER stress may even lead to cell death. In several human neuronal pathologies, such as PD, AD, and Huntington’s disease (HD), ER stress has been reported. In recent years, the discovery of small molecules that could inhibit the UPR and ER stress have gained much attention to produce potential therapeutics [12,146,147,148,149,150].
Case studies on intake of diet rich in flavonoids have shown potential against many diseases. Kaempferol a natural flavonol attenuates the ER stress-induced cell death in human neuroblastoma cell line IMR32 via inhibiting the UPR signaling. Kaempferol significantly reduces the Brefeldin-A (BFA) induced mRNA expression of UPR markers like glucose-regulated protein (GRP78) and C/EBP homologous protein (CHOP) in IMR32 [146]. Luteolin, flavanol, is present in various plant products, such as celery and broccoli. Treatment with luteolin in PC12 cells has shown the attenuation of GRP78 and CHOP upregulation [131]. Apigenin treatment on murine HT22 hippocampal neuronal cells has shown a reduced level of ER stress-associated proteins including CHOP, GRP 78, and GRP94. Additionally, it has a role in the cleavage of activating transcription factor 6a, phosphorylation of eukaryotic initiation factor 2a, and inositol-requiring enzyme 1a, and the activation of mitogen-activated protein kinases, such as p38, c-Jun NH2-terminal kinase, and extracellular-regulated kinase [151]. Epicatechin (EC), a type of flavan-3-ol, has antibacterial, antitumor, antimutagenic, antiviral, and antioxidant properties. EC treatment on HT22 hippocampal neuronal cells successfully prevents the methamphetamine (METH) induced neurotoxicity. EC inhibits the activation of ERK, p38, CHOP, and DR4 expression [12]. Thus, ER-stress may be prevented using EC flavonoids. Effect of different flavonoids on the cellular stress response is summarized in Table 2.
Table 2.
Effect of flavonoids on the cellular stress response.
| Flavonoids | Cellular Stress Response | Host Model | Ref |
|---|---|---|---|
| Kaempferol | Inhibits the expression of GRP78 (a chaperone) and CHOP (ER stress associated pro-apoptotic transcription factor) | Human IMR32 | [146] |
| Quercetin | Reduction in the expression of glucose-regulated protein 78 (GRP78) and C/EBP-homologous protein (CHOP) | Human umbilical vein endothelial cells | [152] |
| Morin | Inhibition of the expression of GRP78, Decreased ROS and apoptosis |
renal proximal tubular HK-2 cells | [153] |
| Methoxyflavones | Activation of the UPR pathway via activating eIF2α and Nrf2 and induces the expression of downstream genes, such as GRP78, HO-1, and CHOP, without causing ER stress | Mouse insulinoma MIN6 cells | [147] |
| Agathisflavone | Increases the remyelination and alters microglial activation state. Neuroprotective effect via increase the expression of neurotrophic factors ciliary neurotrophic factor (Cntf), epidermal growth factor receptor (Egfr), and neuronal GABA b1 receptor subunit (Gabrb1) | Mice belonging to the C57BL/6 background | [154] |
| Apigenin | Neuroprotection, astrocytes integrity and have an anti-neuro-inflammatory response. These responses are generated via the modulation of inflammatory cytokines mRNA expression and reduce the expression of OX42, IL-6, and gp130. Induces the expression of brain-derived neurotrophic factor (BDNF). | Wistar rats’ hemispheres brain’s Glial cells and neurons | [154] |
| Hesperetin | Reduction of the expression of inflammatory Cytokines by ameliorating Toll-like receptor-4 (TLR4)-mediated ionized calcium-binding adapter molecule 1/glial fibrillary acidic protein (Iba-1/GFAP) expression. Attenuation in the LPS-induced generation of reactive oxygen species/lipid peroxidation (ROS/LPO) and improved the antioxidant protein level, such as nuclear factor erythroid 2-related factor 2 (Nrf2) and Haem-oxygenase (HO-1), in the mouse brain |
C57BL/6 N mice | [155] |
| Epimedium | Have anti-proteotoxic potency as it reduces the Aβ1–42- and polyQ-induced paralysis in CL4176 and AM140 | C. elegans human proteotoxic disease models (CL4176, AM140) | [144] |
| Rutin | Rutin treatment reduces polyglutamine (polyQ) protein aggregation in muscle, reduced polyQ-mediated neuronal death in ASH sensory neurons, and extended lifespan. | C. elegans model of Huntington’s disease | [156] |
| phenyl-γ-valerolactones (metabolites of flavan-3-ols) | (4′-OH)-PVL interferes with AβO (but not fibril) assembly and actively remodels performed AβOs into nontoxic amorphous aggregate. | Yeast strains expressing different variants of the human Aβ42 and β23 peptides | [139] |
6. Pre-Clinical/Clinical Studies of Flavonoids
After knowing the beneficial effects of flavonoids, several pre-clinical studies have been conducted to know the way of administration and the doses in animal models specific to AD, PD, Amyotrophic lateral sclerosis (ALS), and HD. A systematic review of the preclinical study on AD and PD suggested that flavonoids could be a potential drug to treat neurodegenerative diseases [157]. The possible mode of action, dose, and route of administration are summarized in Table 3.
Table 3.
Studies related to the effect of flavonoids on the animal model.
| Disease | Clinical Onsets | Behavioral Onsets | Disease Model | Flavonoids | Dose | Effect of Flavonoids Treatment on the Animal Model | Ref. |
|---|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Presence of extracellular neuritic plaques containing (Aβ) peptide and intracellular neurofibrillary tangles containing tau | AD results in a progressive loss of cognitive ability and eventually daily function activities | 5 × FAD model | 7,8-dihydroxyflavone (7,8-DHF) | IP injection (5 mg/kg) | Improved memory | [160] |
| Oral administration (5 mg/kg/day) | Improvement in memory and reduction in synapse loss | [161] | |||||
| 2 × FAD model | Apigenin | Oral administration (40 mg/kg/day) | Improvement in learning and memory, reduction in deposition of insoluble Aβ | [162] | |||
| 1 × FAD model, 3 × FAD model, SAMP8 mice | Nobiletin | IP injection (10 mg/kg) | Improvement in memory and reduction in levels of both soluble and insoluble Aβ | [163] | |||
| IP injections (10 and 30 mg/kg) | Improvement in memory; reduction in soluble Aβ levels | [164] | |||||
| 1 × FAD model | Baicalein | IP injections 10 and 50 mg/kg | Improves the memory, reduces some markers of oxidative stress | [162] | |||
| (SAMP8) | Quercetin | IP injections (10 mg/kg) | Improves working memory and reduces the production of Aβ | [165] | |||
| Oral administration (25 mg/kg/day) | Reduces the markers of oxidative stress, LPO and activates the ERK pathway | ||||||
| Huntington’s disease (HD) | Presence of a trinucleotide repeat (CAG) that encodes an abnormally long polyglutamine tract in the huntingtin protein | Movement and psychiatric disturbances, as well as cognitive impairment | 3-NP model of HD in rats | Chrysin | Oral administration (50 mg/kg/day) | Improvement in behavior and reduction in markers of oxidative stress and cell death, and enhancement in the survival of striatal neurons | [166] |
| R6/1 N-terminal transgenic mouse model | 7,8-DHF | Oral administration (5 mg/kg/day) | Delay the development of motor and cognitive deficits, prevention of the loss of striatal volume, enhances the marker of neurotrophic factor signaling, and reduction in some markers of inflammation | [167] | |||
| 3-NP model | Quercetin | oral administration (25 mg/kg/day) | Reduce motor deficits, improve mitochondrial function, and attenuate some markers of oxidative stress | [168] | |||
| R6/1 N-terminal transgenic mouse model | Anthocyanins | 100 mg/kg/day | Delay the loss of motor function | [169] | |||
| 3-NP model in rats | Hesperidin | Oral administration (100 mg/kg/day) | Reduce motor deficits, as well as markers of inflammation and oxidative stress | [170] | |||
| Amyotrophic Lateral Sclerosis (ALS) | Heritable gene mutations | Loss of the motor neurons that control the voluntary movement of muscles, resulting in paralysis and death | SOD1-G93A model | 7,8-DHF | IP injection (5 mg/kg) | Reduction in the age-dependent decrease in motor performance and preserving the total motor neuron count and dendritic spine density on motor neurons | [171] |
| Fisetin | Oral administration (9 mg/kg) | Delay the development of motor deficits, reduction in their rate of progression, and increases lifespan | [172] | ||||
| (−)-epigallocatechin gallate (EGCG) | oral administration (5.8–10 mg/kg) | Delay symptom onset and extend the lifespan | [173] |
An epidemiological study on 808 adults Italian cohort found that higher dietary intake of anthocyanins, flavan-3-ols, catechins, and flavonols are associated with better cognitive health [158]. Intake of dietary flavonoids can mitigate the pathogenesis of neurological disorders by reducing oxidative stress. A cohort study performed on 1367 elderly (more than 65 years) depicted that flavonoid intake is inversely related to the risk of incident dementia [159].
7. Flavonoid Metabolism
To use flavonoids as a therapeutic agent, it is important to know their pharmacokinetics. As these dietary flavonoids are used as traditional medicines from past decades, many studies have been conducted to know their absorption and metabolism to rule out their possible way of action. Dietary flavonoids are mostly found in the glycoside form. After ingestion of these dietary flavonoids, the deglycosylation process occurs in the small and large intestine. Lactase-phlorizin hydrolase (LPH) is the first enzyme reported for the hydrolysis of quercetin 3-O-glucoside (Q3G) and quercetin 4′-O-glucoside (Q4′G) that are monoglucosides of genistein and daidzein to produce aglycons in-vitro [174]. Before hydrolysis, the glucosides are taken up into the cells via sodium-glucose co-transporter type 1 (SGLT1) membrane transporter and this is reported for Q4ʹG, which was found using human Caco-2 cells and SGLT1 transfected rodent G6D3 cells [175]. After the hydrolysis, the produced aglycons are inserted in the epithelial cells and metabolized via phase II enzymes to produce corresponding conjugated metabolites. These phase II enzymes are uridine-5ʹ-diphosphate-glucuronosyltransferases (UGT), sulfotransferases (SULT), and catechol-O-methyltransferases (COMT) [176]. After intestinal conjugation, further conjugation including sulfation and methylation occurs in the liver. Post metabolism, several chemical forms of flavonoids are found in the urine, and systemic circulation [177,178,179]. After excretion, metabolites are further deconjugated by the microbiota and reabsorbed. The transportation of these absorbed flavonoids is conducted via the lymph in the body [180]. Flavonoids are found in the form of conjugated metabolites in the blood and tissues which are reported to have lower activity than the aglycon form. The functions of flavonoid metabolites are controlled by the balance of the conjugation-deconjugation process.
8. Neuronal Access of Flavonoids
There exists a lacuna of experimental evidence of whether flavonoids can cross the BBB [181]. This lacuna hinders the development of flavonoids-based therapeutics. Daily intake of dietary flavonoids is beneficial for many neurodegenerative disorders as supported by epidemiological studies. Therefore, numerous studies are being conducted to enhance access and promote the neuronal accessibility of flavonoids.
A study on the human brain endothelial cell line (HBMEC) model has revealed that amongst three flavonoids: quercetin, epigallocatechin gallate (EGCG), and cyanidin-3-glucoside (C3G), EGCG crosses the BBB more rapidly than C3G while quercetin was unable to cross BBB. Another study conducted on eighteen, three-month-old male Sprague-Dawley rats showed that quercetin can cross the BBB if administered with α-tocopherol (Vitamin E) [182]. These studies have proven that flavonoids can be used in combating neuronal disorders since they can reach the site of damage (by crossing the BBB) and exert their therapeutic effect.
9. Flavonoid Extraction: A Key to Improved Flavonoid Property
The traditional method for flavonoid extraction reduces its quality. Thus, recent studies have gained attention for improving the flavonoid property by modulating the extraction method. Many approaches, such as solvent extraction (SE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), and ultra-high pressure extraction (UHPE), are gradually being used for improving the content and quality of flavonoid [183].
The fruits of Ziziphus jujube Mill., known as jujube or Chinese date have neuroprotective properties. Jujube protects neuronal cells against neurotoxin stress, promoting memory and learning, stimulating neuronal differentiation, and increasing the expression of neurotrophic factors [183]. Flavonoids extracted from jujube seed by using the UAE method improves its medicinal quality [184,185]. Moreover, jujube seed flavonoid extracted by UAE method displayed a higher capacity of scavenging ABTS, DPPH, superoxide, and hydroxyl radicals and reducing the level of ROS accumulation in PC12 cells. Moreover, administration of these flavonoids in the transgenic C. elegans model (GMC101) reduces the Aβ toxicity [17]. The UHPE method has many advantages, such as shortening the time, reducing the temperature, and reducing the solvent. Flavonoid extracted from jujube seed through UHPE shows higher concentrations of total flavonoids extracted and stronger DPPH and ABTS radical-scavenging activities in a shorter period [186]. Thus, applying the improved flavonoid extraction method would be beneficial for improving the flavonoid property. The role of flavonoids in prevention against oxidative stress, neuroinflammation, and ER stress is summarized in Figure 1.
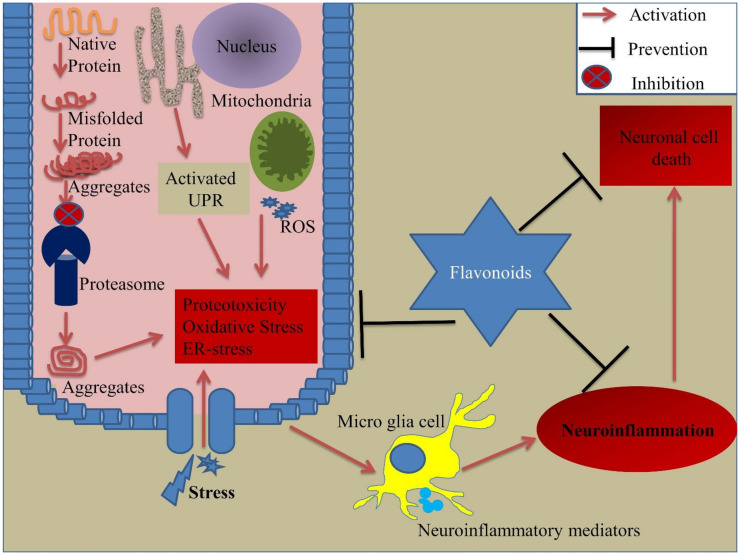
Figure 1.
Role of flavonoids in prevention against cellular stress response. Exposure to stress conditions leads to the activation of cellular stress responses, such as UPR, ER stress, oxidative stress, proteotoxicity, and neuroinflammation. When cells are exposed to any stress condition, it affects the cellular proteome, thus inducing the UPR in the ER and further activation of ER stress. Stress conditions also initiate proteotoxicity by affecting the proteins’ structure, as well as proteasome subunits of the proteasomal degradation machinery, causing the release of misfolded/aggregated proteins in the cytosol, thus inducing proteotoxicity. The release of ROS from mitochondria leads to the generation of oxidative stress. All these cellular stress responses try to eliminate the stress-induced toxicity, but extreme cellular stress responses may lead to cell death. During stress exposure, microglia start to release neuroinflammatory mediators thus causing neuroinflammation. This inflammation creates a hostile environment within the cell and under harsh conditions, leads to cell death. Flavonoids have the potential to combat and prevent these exaggerated cellular stress responses in-turn preventing cell death. ER: Endoplasmic reticulum, ROS: Reactive oxygen species, UPR: Unfolded protein response.
10. Conclusions
Current data on neurodegenerative disorders suggest the need for a potential therapeutic target. With a deep understanding of the neurological pathologies, it becomes easy to target the potential hallmarks that are responsible for these diseases. Flavonoids are phytochemicals, and many studies on these compounds depict their effective role against neurological disorders. Flavonoids have shown beneficial effects on the cellular stress response. As described by several studies, these flavonoids could be promising candidates for neurological disorders. Further studies are needed to focus on their clinical acceptance. Modified flavonoids also need to be studied in detail to assess their role as therapeutics in neurological disorders. Risk assessment and pharmacokinetics of flavonoids are essential parameters that need to be explored for their clinical use. Hence, a multi-fold increase in the number of in-vivo and clinical studies is the need of the hour.
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ER | Endoplasmic reticulum |
| PQC | Protein quality control |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| UPR | Unfolded protein response |
| HSP | Heat shock protein |
Author Contributions
Conceptualization, S.D., V.K.; methodology, S.D., V.K.; Visualization, Supervision, V.K.; writing—original draft preparation, S.D., V.K.; writing—review and editing J.-J.K., S.D., S.K.S., A.K.D., V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abeliovich A., Gitler A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 2.Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 3.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Focus on neurodegenerative disease. Nat. Neurosci. 2018;21:1293. doi: 10.1038/s41593-018-0250-x. [DOI] [PubMed] [Google Scholar]
- 6.Grippo A.J., Scotti M.A. Stress and neuroinflammation. Mod. Trends Pharm. 2013;28:20–32. doi: 10.1159/000343965. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima Y., Suzuki S. Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner. Int. J. Mol. Sci. 2013;14:7771–7783. doi: 10.3390/ijms14047771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohn A., Tramutola A., Cascella R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxidative Med. Cell. Longev. 2020;2020:5497046. doi: 10.1155/2020/5497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprenkle N.T., Sims S.G., Sanchez C.L., Meares G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017;12:42. doi: 10.1186/s13024-017-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang S.L., Shih P.H., Yen G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012;60:877–885. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 11.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001;30:433–446. doi: 10.1016/S0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y., Lee J.H., Seo Y.H., Jang J.H., Jeong C.H., Lee S., Jeong G.S., Park B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019;27:145–151. doi: 10.4062/biomolther.2018.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prochazkova D., Bousova I., Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Chaurasia J.K., Mishra A., Tripathi Y.B. Immunomodulation property of hexane fraction of leaves of Cinnamomum tamala Linn. in rats. Cell Biochem. Funct. 2010;28:454–460. doi: 10.1002/cbf.1677. [DOI] [PubMed] [Google Scholar]
- 15.Khan A., Ikram M., Hahm J.R., Kim M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants. 2020;9:609. doi: 10.3390/antiox9070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Zhou A., Cui X., Zhang Y., Xie J. 6’”-p-Coumaroylspinosin protects PC12 neuronal cells from acrylamide-induced oxidative stress and apoptosis. J. Food Biochem. 2020;44:e13321. doi: 10.1111/jfbc.13321. [DOI] [PubMed] [Google Scholar]
- 17.Yang T., Fang L., Lin T., Li J., Zhang Y., Zhou A., Xie J. Ultrasonicated sour Jujube seed flavonoids extract exerts ameliorative antioxidant capacity and reduces Abeta-induced toxicity in Caenorhabditis elegans. J. Ethnopharmacol. 2019;239:111886. doi: 10.1016/j.jep.2019.111886. [DOI] [PubMed] [Google Scholar]
- 18.Maher P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019;20:3056. doi: 10.3390/ijms20123056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao L.H., Jiang Y.M., Shi J., Tomas-Barberan F.A., Datta N., Singanusong R., Chen S.S. Flavonoids in food and their health benefits. Plant. Foods Hum. Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 20.Jones Q.R., Warford J., Rupasinghe H.P., Robertson G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012;33:602–610. doi: 10.1016/j.tips.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Maiti S., Nazmeen A., Medda N., Patra R., Ghosh T.K. Flavonoids green tea against oxidant stress and inflammation with related human diseases. Clin. Nutr. Exp. 2019;24:1–14. doi: 10.1016/j.yclnex.2018.12.004. [DOI] [Google Scholar]
- 22.Rees A., Dodd G.F., Spencer J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients. 2018;10:1852. doi: 10.3390/nu10121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suen J., Thomas J., Kranz A., Vun S., Miller M. Effect of Flavonoids on Oxidative Stress and Inflammation in Adults at Risk of Cardiovascular Disease: A Systematic Review. Healthcare. 2016;4:69. doi: 10.3390/healthcare4030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed S., Ahmed N., Rungatscher A., Linardi D., Kulsoom B., Innamorati G., Meo S.A., Gebrie M.A., Mani R., Merigo F., et al. Cocoa Flavonoids Reduce Inflammation and Oxidative Stress in a Myocardial Ischemia-Reperfusion Experimental Model. Antioxidants. 2020;9:167. doi: 10.3390/antiox9020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A., Busselberg D. Flavonoids in Cancer and Apoptosis. Cancers. 2018;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liskova A., Koklesova L., Samec M., Smejkal K., Samuel S.M., Varghese E., Abotaleb M., Biringer K., Kudela E., Danko J., et al. Flavonoids in Cancer Metastasis. Cancers. 2020;12:1498. doi: 10.3390/cancers12061498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajialyani M., Hosein Farzaei M., Echeverria J., Nabavi S.M., Uriarte E., Sobarzo-Sanchez E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules. 2019;24:648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nouri Z., Fakhri S., El-Senduny F.F., Sanadgol N., Abd-ElGhani G.E., Farzaei M.H., Chen J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules. 2019;9:690. doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youdim K.A., Dobbie M.S., Kuhnle G., Proteggente A.R., Abbott N.J., Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.J., Kim Y.S., Kumar V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019;54:226–231. doi: 10.1016/j.jtemb.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Kehrer J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 32.Koppenol W.H. The Haber-Weiss cycle--70 years later. Redox Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 33.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018;592:692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 37.Rego A.C., Oliveira C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003;28:1563–1574. doi: 10.1023/A:1025682611389. [DOI] [PubMed] [Google Scholar]
- 38.Tamás M.J., Sharma S.K., Ibstedt S., Jacobson T., Christen P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules. 2014;4:252–267. doi: 10.3390/biom4010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibstedt S., Sideri T.C., Grant C.M., Tamas M.J. Global analysis of protein aggregation in yeast during physiological conditions and arsenite stress. Biol. Open. 2014;3:913–923. doi: 10.1242/bio.20148938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson T., Navarrete C., Sharma S.K., Sideri T.C., Ibstedt S., Priya S., Grant C.M., Christen P., Goloubinoff P., Tamas M.J. Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast. J. Cell Sci. 2012;125:5073–5083. doi: 10.1242/jcs.107029. [DOI] [PubMed] [Google Scholar]
- 41.Gardarin A., Chedin S., Lagniel G., Aude J.C., Godat E., Catty P., Labarre J. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbiol. 2010;76:1034–1048. doi: 10.1111/j.1365-2958.2010.07166.x. [DOI] [PubMed] [Google Scholar]
- 42.Le Q.G., Ishiwata-Kimata Y., Kohno K., Kimata Y. Cadmium impairs protein folding in the endoplasmic reticulum and induces the unfolded protein response. FEMS Yeast Res. 2016;16:fow049. doi: 10.1093/femsyr/fow049. [DOI] [PubMed] [Google Scholar]
- 43.Holland S.L., Ghosh E., Avery S.V. Chromate-induced sulfur starvation and mRNA mistranslation in yeast are linked in a common mechanism of Cr toxicity. Toxicol Vitr. 2010;24:1764–1767. doi: 10.1016/j.tiv.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalita J., Kumar V., Misra U.K., Bora H.K. Memory and Learning Dysfunction Following Copper Toxicity: Biochemical and Immunohistochemical Basis. Mol. Neurobiol. 2018;55:3800–3811. doi: 10.1007/s12035-017-0619-y. [DOI] [PubMed] [Google Scholar]
- 45.Kalita J., Kumar V., Misra U.K., Bora H.K. Movement Disorder in Copper Toxicity Rat Model: Role of Inflammation and Apoptosis in the Corpus Striatum. Neurotox. Res. 2020;37:904–912. doi: 10.1007/s12640-019-00140-9. [DOI] [PubMed] [Google Scholar]
- 46.Chaves R.S., Melo T.Q., Martins S.A., Ferrari M.F. Protein aggregation containing beta-amyloid, alpha-synuclein and hyperphosphorylated tau in cultured cells of hippocampus, substantia nigra and locus coeruleus after rotenone exposure. BMC Neurosci. 2010;11:144. doi: 10.1186/1471-2202-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deshmukh R.S., Chaudhary R.K., Roy I. Effect of pesticides on the aggregation of mutant huntingtin protein. Mol. Neurobiol. 2012;45:405–414. doi: 10.1007/s12035-012-8252-2. [DOI] [PubMed] [Google Scholar]
- 48.Yang W., Tiffany-Castiglioni E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. J. Toxicol. Environ. Health A. 2007;70:1849–1857. doi: 10.1080/15287390701459262. [DOI] [PubMed] [Google Scholar]
- 49.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 50.Garrido C., Paul C., Seigneuric R., Kampinga H.H. The small heat shock proteins family: The long forgotten chaperones. Int. J. Biochem. Cell Biol. 2012;44:1588–1592. doi: 10.1016/j.biocel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Kim J.Y., Yenari M. Heat Shock Proteins and the Stress Response. In: Caplan L.R., Biller J., Leary M.C., Lo E.H., Thomas A.J., Yenari M., Zhang J.H., editors. Primer on Cerebrovascular Diseases. Academic Press; San Diego, CA, USA: 2017. pp. 273–275. [Google Scholar]
- 52.Pockley A.G., Henderson B. Extracellular cell stress (heat shock) proteins-immune responses and disease: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20160522. doi: 10.1098/rstb.2016.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrido C. Size matters: Of the small HSP27 and its large oligomers. Cell Death Differ. 2002;9:483–485. doi: 10.1038/sj.cdd.4401005. [DOI] [PubMed] [Google Scholar]
- 54.Ehrlich E.S., Wang T., Luo K., Xiao Z., Niewiadomska A.M., Martinez T., Xu W., Neckers L., Yu X.F. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA. 2009;106:20330–20335. doi: 10.1073/pnas.0810571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theodoraki M.A., Caplan A.J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta. 2012;1823:683–688. doi: 10.1016/j.bbamcr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroder M., Kaufman R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 57.Tamatani M., Matsuyama T., Yamaguchi A., Mitsuda N., Tsukamoto Y., Taniguchi M., Che Y.H., Ozawa K., Hori O., Nishimura H., et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat. Med. 2001;7:317–323. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy D., Samali A., Jager R. Methods for studying ER stress and UPR markers in human cells. Methods Mol. Biol. 2015;1292:3–18. doi: 10.1007/978-1-4939-2522-3_1. [DOI] [PubMed] [Google Scholar]
- 59.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venditti P., Di Meo S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020;21:2173. doi: 10.3390/ijms21062173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemes R., Koltai E., Taylor A.W., Suzuki K., Gyori F., Radak Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants. 2018;7:85. doi: 10.3390/antiox7070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 63.Cotelle N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001;1:569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 64.Ferreyra M.L.F., Rius S.P., Casati P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 2012;3:222. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 66.Williams R.J., Spencer J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Diniz T.C., Almeida J.R.G.D.S., De Lima-Saraiva S.R.G., Ribeiro F.P.R.D.A., Pacheco A.G.M., De Freitas R.M., Quintans-Júnior L.J., Quintans J.D.S.S., Mendes D.F.R., De Almeida R.F.P.R. The role of flavonoids on oxidative stress in epilepsy. Oxidative Med. Cell. Longev. 2015;2015:171756. doi: 10.1155/2015/171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L.K., Suzuki W., Schroeter H., Wall M., Sloan R.P., Small S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014;17:1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Socci V., Tempesta D., Desideri G., De Gennaro L., Ferrara M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017;4:19. doi: 10.3389/fnut.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gratton G., Weaver S.R., Burley C.V., Low K.A., Maclin E.L., Johns P.W., Pham Q.S., Lucas S.J.E., Fabiani M., Rendeiro C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020;10:19409. doi: 10.1038/s41598-020-76160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y., Wang G.J., Song T.T., Murphy P.A., Hendrich S. Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity. J. Nutr. 1999;129:957–962. doi: 10.1093/jn/129.5.957. [DOI] [PubMed] [Google Scholar]
- 72.Krizova L., Dadakova K., Kasparovska J., Kasparovsky T. Isoflavones. Molecules. 2019;24:1076. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaufman P.B., Duke J.A., Brielmann H., Boik J., Hoyt J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997;3:7–12. doi: 10.1089/acm.1997.3.7. [DOI] [PubMed] [Google Scholar]
- 74.Yu O., Jung W., Shi J., Croes R.A., Fader G.M., McGonigle B., Odell J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant. Physiol. 2000;124:781–794. doi: 10.1104/pp.124.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Wang G., Gurley E.C., Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE. 2014;9:e107072. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hvattum E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002;16:655–662. doi: 10.1002/rcm.622. [DOI] [PubMed] [Google Scholar]
- 78.Cruickshank I.A.M., Biggs D.R., Perrin D.R., Whittle C.P. Phaseollin and phaseollidin relationships in infection-droplets on endocarp of Phaseolus vulgaris. Physiol. Plant. Pathol. 1974;4:261–276. doi: 10.1016/0048-4059(74)90014-9. [DOI] [Google Scholar]
- 79.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 80.Tripoli E., La Guardia M., Giammanco S., Di Majo D., Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 81.Arts I.C., van de Putte B., Hollman P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 82.Bernatoniene J., Kopustinskiene D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;23:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Truong V.D., Deighton N., Thompson R.T., McFeeters R.F., Dean L.O., Pecota K.V., Yencho G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010;58:404–410. doi: 10.1021/jf902799a. [DOI] [PubMed] [Google Scholar]
- 84.Andreeva O.A., Ivashev M.N., Ozimina I.I., Maslikova G.V. Diosmetin glycosides from caucasian vetch: Isolation and study of biological activity. Pharm. Chem. J. 1998;32:595–597. doi: 10.1007/BF02465832. [DOI] [Google Scholar]
- 85.Hertog M.G.L., Hollman P.C.H., van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993;41:1242–1246. doi: 10.1021/jf00032a015. [DOI] [Google Scholar]
- 86.Sochocka M., Diniz B.S., Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rendeiro C., Rhodes J.S., Spencer J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015;89:126–139. doi: 10.1016/j.neuint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 88.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang D., Hu X., Qian L., Wilson B., Lee C., Flood P., Langenbach R., Hong J.S. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol. Appl. Pharmacol. 2009;238:64–70. doi: 10.1016/j.taap.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim H., Kim Y.S., Kim S.Y., Suk K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001;309:67–71. doi: 10.1016/S0304-3940(01)02028-6. [DOI] [PubMed] [Google Scholar]
- 92.Patil S.P., Jain P.D., Sancheti J.S., Ghumatkar P.J., Tambe R., Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 93.Li R., Wang X., Qin T., Qu R., Ma S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1beta production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016;296:318–325. doi: 10.1016/j.bbr.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 94.Anusha C., Sumathi T., Joseph L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017;269:67–79. doi: 10.1016/j.cbi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 95.Aruna R., Geetha A., Suguna P. Rutin modulates ASC expression in NLRP3 inflammasome: A study in alcohol and cerulein-induced rat model of pancreatitis. Mol. Cell. Biochem. 2014;396:269–280. doi: 10.1007/s11010-014-2162-8. [DOI] [PubMed] [Google Scholar]
- 96.Liu M.H., Lin Y.S., Sheu S.Y., Sun J.S. Anti-inflammatory effects of daidzein on primary astroglial cell culture. Nutr. Neurosci. 2009;12:123–134. doi: 10.1179/147683009X423274. [DOI] [PubMed] [Google Scholar]
- 97.Huang Q., Wu L.J., Tashiro S., Gao H.Y., Onodera S., Ikejima T. (+)-Catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced DNA damage and cell cycle arrest. J. Pharmacol. Sci. 2005;98:16–24. doi: 10.1254/jphs.FPJ04053X. [DOI] [PubMed] [Google Scholar]
- 98.Lau F.C., Bielinski D.F., Joseph J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007;85:1010–1017. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- 99.Vafeiadou K., Vauzour D., Lee H.Y., Rodriguez-Mateos A., Williams R.J., Spencer J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009;484:100–109. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 100.Chen H.Q., Jin Z.Y., Li G.H. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007;417:112–117. doi: 10.1016/j.neulet.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 101.Cui Y., Wu J., Jung S.C., Park D.B., Maeng Y.H., Hong J.Y., Kim S.J., Lee S.R., Kim S.J., Kim S.J., et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010;33:1814–1821. doi: 10.1248/bpb.33.1814. [DOI] [PubMed] [Google Scholar]
- 102.Adams S.M., Aksenova M.V., Aksenov M.Y., Mactutus C.F., Booze R.M. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 Tat(1-86) exposure. PLoS ONE. 2012;7:e37540. doi: 10.1371/journal.pone.0037540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tikhonova M.A., Tikhonova N.G., Tenditnik M.V., Ovsyukova M.V., Akopyan A.A., Dubrovina N.I., Amstislavskaya T.G., Khlestkina E.K. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules. 2020;25:5339. doi: 10.3390/molecules25225339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen H.Q., Jin Z.Y., Wang X.J., Xu X.M., Deng L., Zhao J.W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci. Lett. 2008;448:175–179. doi: 10.1016/j.neulet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 105.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016;28:140–146. doi: 10.1016/j.jnutbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 106.Lou H., Jing X., Wei X., Shi H., Ren D., Zhang X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 107.Xue X., Liu H., Qi L., Li X., Guo C., Gong D., Qu H. Baicalein ameliorated the upregulation of striatal glutamatergic transmission in the mice model of Parkinson’s disease. Brain Res. Bull. 2014;103:54–59. doi: 10.1016/j.brainresbull.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Hung K.C., Huang H.J., Wang Y.T., Lin A.M. Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016;194:522–529. doi: 10.1016/j.jep.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Facheris M., Beretta S., Ferrarese C. Peripheral markers of oxidative stress and excitotoxicity in neurodegenerative disorders: Tools for diagnosis and therapy? J. Alzheimers Dis. 2004;6:177–184. doi: 10.3233/JAD-2004-6210. [DOI] [PubMed] [Google Scholar]
- 113.Tan B.L., Norhaizan M.E., Liew W.P., Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018;9:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim J.H., Quilantang N.G., Kim H.Y., Lee S., Cho E.J. Attenuation of hydrogen peroxide-induced oxidative stress in SH-SY5Y cells by three flavonoids from Acer okamotoanum. Chem. Pap. 2019;73:1135–1144. doi: 10.1007/s11696-018-0664-7. [DOI] [Google Scholar]
- 115.Pavlica S., Gebhardt R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010;86:79–86. doi: 10.1016/j.lfs.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 116.Wen L., Shi D., Zhou T., Tu J., He M., Jiang Y., Yang B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020;315:126236. doi: 10.1016/j.foodchem.2020.126236. [DOI] [PubMed] [Google Scholar]
- 117.Barreca D., Curro M., Bellocco E., Ficarra S., Lagana G., Tellone E., Giunta M.L., Visalli G., Caccamo D., Galtieri A., et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. BioFactors. 2017;43:549–557. doi: 10.1002/biof.1358. [DOI] [PubMed] [Google Scholar]
- 118.Mu X., He G., Cheng Y., Li X., Xu B., Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009;92:642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Cheng Y., He G., Mu X., Zhang T., Li X., Hu J., Xu B., Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: Behavioral, biochemical and immunohistochemical profile. Neurosci. Lett. 2008;441:16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 120.Jiang M., Porat-Shliom Y., Pei Z., Cheng Y., Xiang L., Sommers K., Li Q., Gillardon F., Hengerer B., Berlinicke C., et al. Baicalein reduces E46K alpha-synuclein aggregation in vitro and protects cells against E46K alpha-synuclein toxicity in cell models of familiar Parkinsonism. J. Neurochem. 2010;114:419–429. doi: 10.1111/j.1471-4159.2010.06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu J.H., Ardah M.T., Durairajan S.S., Liu L.F., Xie L.X., Fong W.F., Hasan M.Y., Huang J.D., El-Agnaf O.M., Li M. Baicalein inhibits formation of alpha-synuclein oligomers within living cells and prevents Abeta peptide fibrillation and oligomerisation. ChemBioChem. 2011;12:615–624. doi: 10.1002/cbic.201000604. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y.H., Yu H.T., Pu X.P., Du G.H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules. 2013;18:14726–14738. doi: 10.3390/molecules181214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee E., Park H.R., Ji S.T., Lee Y., Lee J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J. Neurosci. Res. 2014;92:130–139. doi: 10.1002/jnr.23307. [DOI] [PubMed] [Google Scholar]
- 124.Khan M.M., Raza S.S., Javed H., Ahmad A., Khan A., Islam F., Safhi M.M., Islam F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox. Res. 2012;22:1–15. doi: 10.1007/s12640-011-9295-2. [DOI] [PubMed] [Google Scholar]
- 125.Magalingam K.B., Radhakrishnan A., Haleagrahara N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int. J. Mol. Med. 2013;32:235–240. doi: 10.3892/ijmm.2013.1375. [DOI] [PubMed] [Google Scholar]
- 126.Li S., Pu X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011;34:1291–1296. doi: 10.1248/bpb.34.1291. [DOI] [PubMed] [Google Scholar]
- 127.Filomeni G., Graziani I., De Zio D., Dini L., Centonze D., Rotilio G., Ciriolo M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging. 2012;33:767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 128.Lv C., Hong T., Yang Z., Zhang Y., Wang L., Dong M., Zhao J., Mu J., Meng Y. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2012;2012:928643. doi: 10.1155/2012/928643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karuppagounder S.S., Madathil S.K., Pandey M., Haobam R., Rajamma U., Mohanakumar K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 130.Haleagrahara N., Siew C.J., Mitra N.K., Kumari M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011;500:139–143. doi: 10.1016/j.neulet.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 131.Hu L.W., Yen J.H., Shen Y.T., Wu K.Y., Wu M.J. Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS ONE. 2014;9:e97880. doi: 10.1371/journal.pone.0097880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu Q., Uversky V.N., Huang M., Kang H., Xu F., Liu X., Lian L., Liang Q., Jiang H., Liu A., et al. Baicalein inhibits alpha-synuclein oligomer formation and prevents progression of alpha-synuclein accumulation in a rotenone mouse model of Parkinson’s disease. Biochim. Biophys. Acta. 2016;1862:1883–1890. doi: 10.1016/j.bbadis.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Poetini M.R., Araujo S.M., De Paula M.T., Bortolotto V.C., Meichtry L.B., De Almeida F.P., Jesse C.R., Kunz S.N., Prigol M. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson’s disease. Chem. Biol. Interact. 2018;279:177–186. doi: 10.1016/j.cbi.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 134.Antunes M.S., Goes A.T., Boeira S.P., Prigol M., Jesse C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30:1415–1422. doi: 10.1016/j.nut.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 135.Sharma S.K., Priya S. Expanding role of molecular chaperones in regulating alpha-synuclein misfolding; implications in Parkinson’s disease. Cell. Mol. Life Sci. 2017;74:617–629. doi: 10.1007/s00018-016-2340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roth D.M., Balch W.E. Modeling general proteostasis: Proteome balance in health and disease. Curr. Opin. Cell Biol. 2011;23:126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sontag E.M., Samant R.S., Frydman J. Mechanisms and Functions of Spatial Protein Quality Control. Annu. Rev. Biochem. 2017;86:97–122. doi: 10.1146/annurev-biochem-060815-014616. [DOI] [PubMed] [Google Scholar]
- 138.Wang X.F., Li S., Chou A.P., Bronstein J.M. Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson’s disease. Neurobiol. Dis. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 139.Ruotolo R., Minato I., La Vitola P., Artioli L., Curti C., Franceschi V., Brindani N., Amidani D., Colombo L., Salmona M., et al. Flavonoid-Derived Human Phenyl-gamma-Valerolactone Metabolites Selectively Detoxify Amyloid-beta Oligomers and Prevent Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2020;64:e1900890. doi: 10.1002/mnfr.201900890. [DOI] [PubMed] [Google Scholar]
- 140.Cook C., Stetler C., Petrucelli L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:a009423. doi: 10.1101/cshperspect.a009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Joshi V., Mishra R., Upadhyay A., Amanullah A., Poluri K.M., Singh S., Kumar A., Mishra A. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J. Cell. Physiol. 2019;234:20900–20914. doi: 10.1002/jcp.28695. [DOI] [PubMed] [Google Scholar]
- 142.Budagova K.R., Zhmaeva S.V., Grigor’ev A.N., Goncharova A.Y., Kabakov A.E. Flavonoid dihydroquercetin, unlike quercetin, fails to inhibit expression of heat shock proteins under conditions of cellular stress. Biochemistry. 2003;68:1055–1061. doi: 10.1023/a:1026081016923. [DOI] [PubMed] [Google Scholar]
- 143.Kumar J., Park K.C., Awasthi A., Prasad B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer’s model. CNS Neurol. Disord. Drug Targets. 2015;14:295–302. doi: 10.2174/1871527314666150116110212. [DOI] [PubMed] [Google Scholar]
- 144.Cai W., Wu J., Wang X., Huang J., Shen Z., Chen X. Epimedium flavonoids mitigate proteotoxicity and extend healthspan via DAF-16 in C. elegans. Tradit. Med. Mod. Med. 2019;2:19–25. doi: 10.1142/S2575900019500046. [DOI] [Google Scholar]
- 145.Yang T., Zhao X., Zhang Y., Xie J., Zhou A. 6‴-Feruloylspinosin alleviated beta-amyloid induced toxicity by promoting mitophagy in Caenorhabditis elegans (GMC101) and PC12 cells. Sci. Total. Environ. 2020;715:136953. doi: 10.1016/j.scitotenv.2020.136953. [DOI] [PubMed] [Google Scholar]
- 146.Abdullah A., Ravanan P. Kaempferol mitigates Endoplasmic Reticulum Stress Induced Cell Death by targeting caspase 3/7. Sci Rep. 2018;8:2189. doi: 10.1038/s41598-018-20499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takano K., Tabata Y., Kitao Y., Murakami R., Suzuki H., Yamada M., Iinuma M., Yoneda Y., Ogawa S., Hori O. Methoxyflavones protect cells against endoplasmic reticulum stress and neurotoxin. Am. J. Physiol. Cell Physiol. 2007;292:C353–C361. doi: 10.1152/ajpcell.00388.2006. [DOI] [PubMed] [Google Scholar]
- 148.Martucciello S., Masullo M., Cerulli A., Piacente S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020;21:1905. doi: 10.3390/ijms21061905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu H., Yang J., Li L., Shi W., Yuan X., Wu L. The Natural Occurring Compounds Targeting Endoplasmic Reticulum Stress. Evid. Based Complement. Alternat. Med. 2016;2016:7831282. doi: 10.1155/2016/7831282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Uddin M.S., Tewari D., Sharma G., Kabir M.T., Barreto G.E., Bin-Jumah M.N., Perveen A., Abdel-Daim M.M., Ashraf G.M. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2020;57:2902–2919. doi: 10.1007/s12035-020-01929-y. [DOI] [PubMed] [Google Scholar]
- 151.Chiang C.K., Hsu S.P., Wu C.T., Huang J.W., Cheng H.T., Chang Y.W., Hung K.Y., Wu K.D., Liu S.H. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol. Med. 2011;17:1295–1305. doi: 10.2119/molmed.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suganya N., Bhakkiyalakshmi E., Suriyanarayanan S., Paulmurugan R., Ramkumar K.M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014;47:231–240. doi: 10.1111/cpr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mo J.S., Choi D., Han Y.R., Kim N., Jeong H.S. Morin has protective potential against ER stress induced apoptosis in renal proximal tubular HK-2 cells. Biomed. Pharmacother. 2019;112:108659. doi: 10.1016/j.biopha.2019.108659. [DOI] [PubMed] [Google Scholar]
- 154.Dourado N.S., Souza C.D.S., De Almeida M.M.A., Da Silva A.B., Dos Santos B.L., Silva V.D.A., De Assis A.M., Da Silva J.S., Souza D.O., Costa M.D.F.D., et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020;12:119. doi: 10.3389/fnagi.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muhammad T., Ikram M., Ullah R., Rehman S.U., Kim M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-kappaB Signaling. Nutrients. 2019;11:648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cordeiro L.M., Machado M.L., Da Silva A.F., Baptista F.B.O., Da Silveira T.L., Soares F.A.A., Arantes L.P. Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in Caenorhabditis elegans model. Food Chem. Toxicol. 2020;141:111323. doi: 10.1016/j.fct.2020.111323. [DOI] [PubMed] [Google Scholar]
- 157.Teles R.B.D.A., Diniz T.C., Pinto T.C.C., Júnior R.G.D.O., Silva M.G.E., De Lavor É.M., Fernandes A.W.C., De Oliveira A.P., Ribeiro F.P.R.D.A., Da Silva A.A.M., et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxidative Med. Cell. Longev. 2018;2018:7043213. doi: 10.1155/2018/7043213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Godos J., Caraci F., Castellano S., Currenti W., Galvano F., Ferri R., Grosso G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules. 2020;10:1300. doi: 10.3390/biom10091300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Commenges D., Scotet V., Renaud S., Jacqmin-Gadda H., Barberger-Gateau P., Dartigues J.F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000;16:357–363. doi: 10.1023/A:1007614613771. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Z., Liu X., Schroeder J.P., Chan C.-B., Song M., Yu S.P., Weinshenker D., Ye K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology. 2014;39:638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Devi L., Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao L., Wang J.L., Liu R., Li X.X., Li J.F., Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–9965. doi: 10.3390/molecules18089949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Onozuka H., Nakajima A., Matsuzaki K., Shin R.W., Ogino K., Saigusa D., Tetsu N., Yokosuka A., Sashida Y., Mimaki Y., et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008;326:739–744. doi: 10.1124/jpet.108.140293. [DOI] [PubMed] [Google Scholar]
- 164.Nakajima A., Aoyama Y., Shin E.J., Nam Y., Kim H.C., Nagai T., Yokosuka A., Mimaki Y., Yokoi T., Ohizumi Y., et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD) Behav. Brain Res. 2015;289:69–77. doi: 10.1016/j.bbr.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 165.Moreno L., Puerta E., Suarez-Santiago J.E., Santos-Magalhaes N.S., Ramirez M.J., Irache J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017;517:50–57. doi: 10.1016/j.ijpharm.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 166.Thangarajan S., Ramachandran S., Krishnamurthy P. Chrysin exerts neuroprotective effects against 3-Nitropropionic acid induced behavioral despair-Mitochondrial dysfunction and striatal apoptosis via upregulating Bcl-2 gene and downregulating Bax-Bad genes in male wistar rats. Biomed. Pharmacother. 2016;84:514–525. doi: 10.1016/j.biopha.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 167.Barriga G.G.-D., Giralt A., Anglada-Huguet M., Gaja-Capdevila N., Orlandi J.G., Soriano J., Canals J.-M., Alberch J. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCgamma1 pathway. Hum. Mol. Genet. 2017;26:3144–3160. doi: 10.1093/hmg/ddx198. [DOI] [PubMed] [Google Scholar]
- 168.Sandhir R., Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta. 2013;1832:421–430. doi: 10.1016/j.bbadis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 169.Kreilaus F., Spiro A.S., Hannan A.J., Garner B., Jenner A.M. Therapeutic Effects of Anthocyanins and Environmental Enrichment in R6/1 Huntington’s Disease Mice. J. Huntingt. Dis. 2016;5:285–296. doi: 10.3233/JHD-160204. [DOI] [PubMed] [Google Scholar]
- 170.Menze E.T., Tadros M.G., Abdel-Tawab A.M., Khalifa A.E. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology. 2012;33:1265–1275. doi: 10.1016/j.neuro.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 171.Stephen M., Woo J., Hasan N.U., Whiting P.H., Thomson A.W. Immunosuppressive activity, lymphocyte subset analysis, and acute toxicity of FK-506 in the rat. A comparative and combination study with cyclosporine. Transplantation. 1989;47:60–65. doi: 10.1097/00007890-198901000-00014. [DOI] [PubMed] [Google Scholar]
- 172.Wang T.H., Wang S.Y., Wang X.D., Jiang H.Q., Yang Y.Q., Wang Y., Cheng J.L., Zhang C.T., Liang W.W., Feng H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience. 2018;379:152–166. doi: 10.1016/j.neuroscience.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Xu Z., Chen S., Li X., Luo G., Li L., Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006;31:1263–1269. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- 174.Day A.J., Canada F.J., Diaz J.C., Kroon P.A., McLauchlan R., Faulds C.B., Plumb G.W., Morgan M.R., Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 175.Walgren R.A., Lin J.T., Kinne R.K., Walle T. Cellular uptake of dietary flavonoid quercetin 4’-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000;294:837–843. [PubMed] [Google Scholar]
- 176.Van der Woude H., Boersma M.G., Vervoort J., Rietjens I.M. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem. Res. Toxicol. 2004;17:1520–1530. doi: 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]
- 177.Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006;96:107–116. doi: 10.1079/BJN20061809. [DOI] [PubMed] [Google Scholar]
- 178.Crozier A., Del Rio D., Clifford M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 179.Nakamura T., Murota K., Kumamoto S., Misumi K., Bando N., Ikushiro S., Takahashi N., Sekido K., Kato Y., Terao J. Plasma metabolites of dietary flavonoids after combination meal consumption with onion and tofu in humans. Mol. Nutr. Food Res. 2014;58:310–317. doi: 10.1002/mnfr.201300234. [DOI] [PubMed] [Google Scholar]
- 180.Murota K., Terao J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005;579:5343–5346. doi: 10.1016/j.febslet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 181.Schaffer S., Halliwell B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012;7:99–109. doi: 10.1007/s12263-011-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ferri P., Angelino D., Gennari L., Benedetti S., Ambrogini P., Del Grande P., Ninfali P. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with alpha-tocopherol. Food Funct. 2015;6:394–400. doi: 10.1039/C4FO00817K. [DOI] [PubMed] [Google Scholar]
- 183.Chen J., Liu X., Li Z., Qi A., Yao P., Zhou Z., Dong T.T.X., Tsim K.W.K. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Altern. Med. 2017;2017:3019568. doi: 10.1155/2017/3019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Song L., Zhang L., Xu L., Ma Y., Lian W., Liu Y., Wang Y. Optimized Extraction of Total Triterpenoids from Jujube (Ziziphus jujuba Mill.) and Comprehensive Analysis of Triterpenic Acids in Different Cultivars. Plants. 2020;9:412. doi: 10.3390/plants9040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Berkani F., Serralheiro M.L., Dahmoune F., Ressaissi A., Kadri N., Remini H. Ultrasound Assisted Extraction of Phenolic Compounds from a Jujube By-Product with Valuable Bioactivities. Processes. 2020;8:1441. doi: 10.3390/pr8111441. [DOI] [Google Scholar]
- 186.Zhang L., Liu P., Li L., Huang Y., Pu Y., Hou X., Song L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules. 2018;24:122. doi: 10.3390/molecules24010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.