Abstract
Sargassum horneri (Turner) C. Agardh (S. horneri) is edible brown seaweed that grows along the coast of East Asia and has been traditionally used as a folk medicine and a local food. In this study, we evaluated the effects of S. horneri on the development of obesity and related metabolic disorders in C57BL/6J mice fed a high-fat diet. S. horneri was freeze-dried, fine-powdered, and mixed with a high-fat diet at a weight ratio of 2% or 6%. Feeding a high-fat diet to mice for 13 weeks induced obesity, diabetes, hepatic steatosis, and hypercholesterolemia. Supplementation of mice with S. horneri suppressed high-fat diet-induced body weight gain and the accumulation of fat in adipose tissue and liver, and the elevation of the serum glucose level. In addition, S. horneri improved insulin resistance. An analysis of the feces showed that S. horneri stimulated the fecal excretion of triglyceride, as well as increased the fecal polysaccharide content. Furthermore, extracts of S. horneri inhibited the activity of pancreatic lipase in vitro. These results showed that S. horneri can ameliorate diet-induced metabolic diseases, and the effect may be partly associated with the suppression of intestinal fat absorption.
Keywords: obesity, diabetes, hepatic steatosis, high-fat diet, lipase inhibition, seaweed, Sargassum horneri (Turner) C. Agardh
1. Introduction
Seaweed has few calories and contains a wide variety of nutritional components including protein, polysaccharides, unsaturated fatty acids, minerals, vitamins, and amino acids [1,2,3]. Some of these nutrients are found at higher levels in seaweed than in terrestrial plant-derived foods. In addition, seaweed is rich in bioactive compounds, such as polyphenols and carotenoids [3]. Recent epidemiological evidence indicates that a seaweed intake is associated with a reduced incidence of cardiovascular disease mortality and increased life expectancy [4,5]. Seaweed has been regularly consumed in the daily diet since ancient times in Japan, Korea, and China, which may contribute to the longevity noted in these countries [6].
Obesity is a chronic metabolic disorder characterized by abnormal or excessive fat accumulation in the body. The prevalence of obesity has increased worldwide, reaching pandemic levels [7]. Obesity has been linked to a number of serious diseases, such as type 2 diabetes, cardiovascular disease, fatty liver disease, sleep apnea, and some cancers [8,9]. Various kinds of compounds, including polyphenols, polyunsaturated fatty acids, and dietary fiber, from fruits, vegetables, grains, seaweed, and medical plants have been reported to exhibit an anti-obesity effect in experimental animal models [10,11,12,13]. Although these compounds are expected to help attenuate the development of obesity, human data are scarce, in contrast to the large number of animal experiments that have been performed.
Sargassum horneri (Turner) C.Agardh (S. horneri), also known as Akamoku in Japan, is a brown seaweed that grows on the coast of East Asia [14]. S. horneri has been used as a food source and traditional medicine to treat several disorders for centuries in Japan, Korea, and China [15]. In Japan, S. horneri has been used as a local foodstuff in the Tohoku region since ancient times. Recently, S. horneri has been attracting increasing attention, as several studies using animal models have shown that higher levels of some active constituents exert beneficial effects on health promotion and disease prevention [16,17]. S. horneri contains high concentrations of polysaccharides, such as fucoidan and alginate. Fucoidan, a sulfated fucose-containing polysaccharide, has been shown to have a wide variety of biological activities, including anticancer, anticoagulant, immune-regulatory, anti-inflammatory, antiviral, anti-obesity, and antidiabetic effects in animal and in vitro studies [18,19]. The algal polysaccharide alginate has been isolated from the cell walls of brown seaweed and widely used in the food industry as a stabilizer or emulsifying agent [20]. Alginate acts as a dietary fiber and prevents the progression of cardiovascular and gastrointestinal diseases [21,22]. In human studies, alginate has been reported to suppress hunger and reduce the percentage of body fat [23,24]. Fucoxanthin, a marine carotenoid mainly found in brown seaweed, being especially rich in S. horneri, has been shown to exert multiple biological effects, including antioxidant, anticancer, anti-inflammatory, anti-angiogenic, anti-obesity and antidiabetic activities [25]. Thus, S. horneri has high levels of active components, and the health benefits of each component have been demonstrated in experimental animals and humans. However, few data on the preventive effects of whole S. horneri on metabolic diseases are available. It is important to understand the health-promoting effect of whole S. horneri as a traditional foodstuff. In the present study, we evaluated whether or not S. horneri could ameliorate the development of obesity and its related diseases in mice fed a high-fat diet.
2. Materials and Methods
2.1. Preparation of Powder and Extracts of S. horneri
The S. horneri sample was harvested on the coast of Fukui Prefecture in April 2019 and washed with water. It was freeze-dried, reduced to a fine powder using a food mixer, and mixed into a high-fat diet (HF). For enzyme inhibition experiments, powdered S. horneri was extracted with ethanol or water. Ethanol extract was prepared by homogenizing the sample powder in 70% ethanol and left at room temperature for two days. The sample was centrifuged at 3000× g for 15 min. The supernatant was then evaporated to dryness. Water extract was prepared by homogenizing the sample powder with water and left in boiling water for 30 min. The sample was centrifuged at 3000× g for 15 min and lyophilized.
2.2. Animal Experiments and Dietary Treatment
Six-week-old male C57BL/6J mice were purchased from CLEA Japan Inc. (Tokyo, Japan) and housed in a controlled atmosphere (22 ± 1 °C at 50% relative humidity) with a 12 h light/dark cycle. After 1 week of acclimation, the animals were randomly divided into four groups, as follows: (1) normal diet (Normal) group (n = 13), (2) high-fat diet (HF) group (n = 12), (3) group with a HF diet supplemented with 2% S. horneri (HF + S. horneri low-dose (ShL) group) (n = 12), and (4) a group with a HF diet supplemented with 6% S. horneri (HF + S. horneri high-dose (ShH) group) (n = 12). The compositions of the experimental diets were adjusted by considering the nutritional components of S. horneri (Table 1). The normal diet provided 354 kcal/100 g of energy (14.4% calories from protein, 11.1% calories from fat, and 74.4% calories from carbohydrate), while the HF provided 493 kcal/100 g of energy (17.9% calories from protein, 60.7% calories from fat, and 21.4% calories from carbohydrate). All experimental diets were based on the AIN-76 diet (Oriental Yeast Co. Ltd., Tokyo, Japan). Animals were allowed free access to diets and drinking water. The body weight and food intake were monitored every other day. After 13 weeks of feeding, the mice were deprived food overnight, and blood samples were withdrawn from the ophthalmic vein under a mixed anesthetic agent (0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol; Fujifilm Wako Pure Chemical Co., Osaka, Japan). Liver tissue and epididymal, peritoneal, and mesenteric white adipose tissues were removed, weighed, and stored at −80 °C. Some of the mice (n = 3−4) were used for histological examinations. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Fukui Prefectural University (approval No. 19–14).
Table 1.
Composition of the experimental diets.
| Ingredients (g/100 g) | Normal Diet | High-Fat (HF) | HF + ShL (2%) | HF + ShH (6%) |
|---|---|---|---|---|
| Cornstarch | 46.57 | |||
| α-Cornstarch | 15.5 | 16.0 | 15.0 | 13.0 |
| Maltodextrin | 6.0 | 6.0 | 6.0 | |
| Sucrose | 10.0 | 5.5 | 5.5 | 5.5 |
| Casein | 14.0 | 25.6 | 25.6 | 25.6 |
| Cellulose | 5.0 | 6.6 | 5.6 | 3.6 |
| Soybean oil | 4.0 | 2.0 | 2.0 | 2.0 |
| Lard | 33.0 | 33.0 | 33.0 | |
| Mineral mix | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 | 1.0 | 1.0 |
| L-Cysteine | 0.18 | 0.36 | 0.36 | 0.36 |
| Cholin bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
| Calcium carbonate | 0.18 | 0.18 | 0.18 | |
| S. horneri powder | 2.0 | 6.0 |
2.3. Serum Biochemical Analyses
Serum was obtained by centrifugation at 1500× g for 15 min at 4 °C. The serum levels of total cholesterol, high-density lipoprotein (HDL)-cholesterol, triglyceride, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and leucine aminopeptidase (LAP) were analyzed using a Hitachi 7060 Automatic Analyzer (Hitachi, Tokyo, Japan) with commercial kits (Fujifilm Wako Pure Chemical Co., Osaka, Japan). The non-HDL cholesterol levels were calculated by subtracting the HDL-cholesterol from the total cholesterol. Serum insulin (Morinaga Institute of Biological Science, Yokohama, Japan), adiponectin (Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan), and tumor necrosis factor-α (TNF-α; Fujifilm Wako Pure Chemical Co., Osaka, Japan) levels were also determined using a commercial ELISA kit.
2.4. Liver Lipid Analyses
Lipids were extracted from the liver according to the method described previously [26]. In brief, the frozen liver tissues (50 mg) were homogenized (20%, w/v) in isopropanol. The homogenate was kept at room temperature for 2 days and then centrifuged at 1000× g for 10 min. Aliquots of the supernatant were analyzed for triglyceride content using a commercial kit (Fujifilm Wako Pure Chemical Co., Osaka, Japan).
2.5. Histological Analyses
The mice were anesthetized with an intraperitoneal injection of mixed anesthetic agent (0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol; Fujifilm Wako Pure Chemical Co., Osaka, Japan), and perfused transcardially with a fixative containing 4% paraformaldehyde and 1.5% glutaraldehyde in phosphate-buffered saline (PBS). After the perfusion, the liver and white adipose tissue were removed and allowed to stand in the same fixative for one day. The tissues were rinsed several times with PBS and embedded in paraffin. Sections of tissues were cut into 5-μm-thick sections, mounted on slides, and stained with hematoxylin eosin (HE).
2.6. Glucose Tolerance Test
A glucose tolerance test was performed one week before the end of experiment. The mice fasted overnight and were intraperitoneally injected with glucose (2 g/kg body weight). The blood samples were collected from the tail veins of the mice, and glucose levels were measured at 0, 30, 60, 90, and 120 min after injection using a blood glucometer Nipro Stat Strip (Nipro, Osaka, Japan).
2.7. Fecal Analyses
During the fecal collection, mice were separated, and fecal samples for a 24-h period were collected from each mouse and weighed. These samples were ground into a powder in a mortar, and 50 mg of feces was extracted with 300 μL of distilled water. After centrifugation (16,000× g, 30 min, 4 °C), ethanol was added to the supernatant (final concentration of 85%), and polysaccharides were obtained as the precipitate. The resulting residue was washed with 85% ethanol and dried. The residue was then resuspended in distilled water and centrifuged (16,000× g, 10 min, 4 °C), and polysaccharide content in the supernatant was measured using a phenol-sulfuric acid method described elsewhere with galactose as the standard [27]. For the measurement of triglycerides, lipids were extracted by adding isopropanol (10 times the weight) to the fecal powder. The sample was then dried and dissolved in isopropanol. The concentration of triglyceride was measured using a commercial kit (Fujifilm Wako Pure Chemical Co., Osaka, Japan).
2.8. Lipase Assay
The inhibitory activity of S. horneri extracts on lipase was determined as previously described [28] with some modification. In brief, lipase (type II, from porcine pancreas, 400 units/mg protein; Sigma-Aldrich Corp., Saint Louis, MO, USA) was dissolved in distilled water at 5 mg/mL and then centrifuged (1000× g, 5 min), and the supernatant was used as the enzyme source. Next, 4-Nitrophenyl butyrate (4-NPB; Sigma-Aldrich Corp., Saint Louis, MO, USA) was dissolved in dimethylsulfoxide. The reaction mixture contained 100 μL of enzyme solution and 100 μL of S. horneri extract in 4 mL of 20 mM Tris-HCl buffer pH 8.5. The mixture was pre-incubated at 37 °C for 10 min. The reaction was started by the addition of 100 μL of 5 mM 4-NPB solution and then incubated for 30 min at 37 °C. The absorbance was measured at 400 nm.
2.9. Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple range test. All of the results were considered statistically significant at p<0.05.
3. Results
3.1. Effects of S. horneri on Food Intake and Body Weight
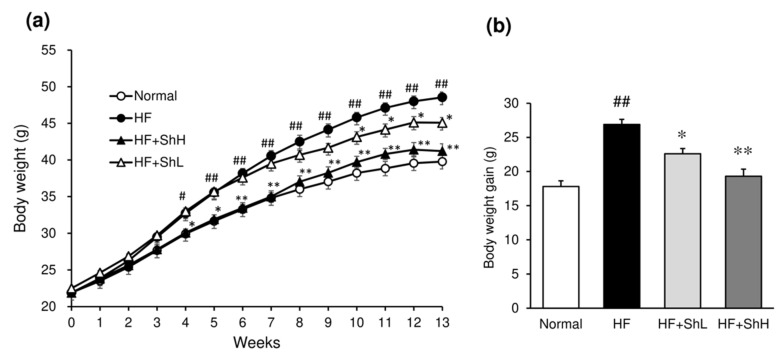
The addition of S. horneri to an HF diet did not affect the amount of food consumption. The average daily food intake of each group throughout the experimental period was 3.1 g (Normal), 2.4 g (HF), 2.6 g (HF + ShL), and 2.4 g (HF + ShH). The energy intake of mice on the normal diet was not significantly different from those with the HF (11.9 vs. 12.6 kcal/mouse/day). Feeding HF to mice for 13 weeks induced marked weight gain. The HF mice showed a significantly higher body weight gain than the Normal mice (Figure 1b). The body weights began to differ significantly between the HF and HF + ShH mice after 4 weeks of treatment (Figure 1a). The body weights between the HF and HF + ShL mice differed significantly after 10 weeks of treatment. In the last week of the experiment, the body weight for both doses of S. horneri was significantly lower than that in the HF mice (Figure 1a).
Figure 1.
Effects of S. horneri on the body weight in C57BL/6J mice fed a high-fat diet for 13 weeks. (a) Weekly changes in the body weight; (b) body weight gain. Normal, normal diet; high-fat (HF), high-fat diet; HF + ShL, high-fat diet mixed with 2% S horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. Each value represents the mean ± SEM (n = 12−13). Significant difference: * p < 0.05, ** p < 0.01 vs. HF group, # p < 0.05, ## p < 0.01 vs. Normal group.
3.2. Effects of S. horneri on the Mass and Morphology of White Adipose Tissue
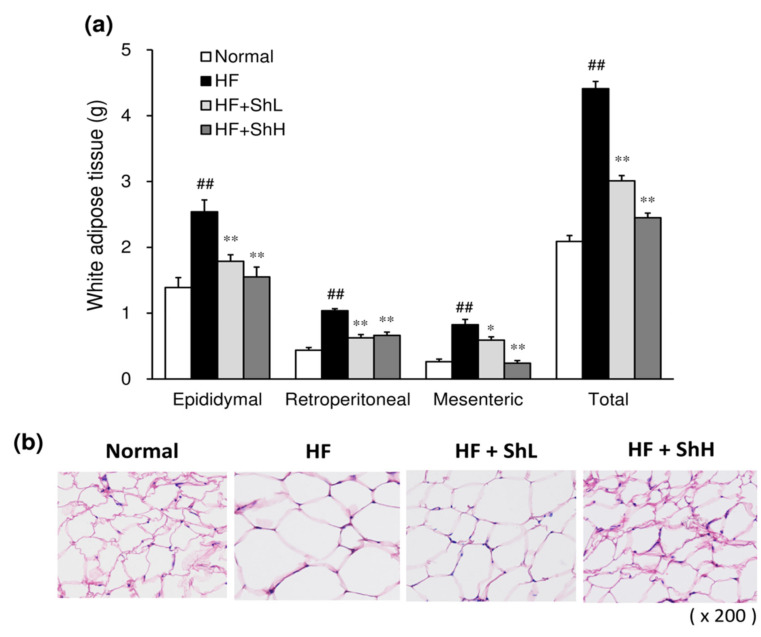
Consistent with the increase in body weight, feeding an HF to mice significantly increased the weight of white adipose tissue, including epididymal, retroperitoneal, and mesenteric adipose tissues, compared to those in the Normal group (Figure 2a). The total fat weight of the HF group was also significantly higher than that of the Normal group. Thirteen-week-treatment of mice with S. horneri suppressed the HF-induced increase in fat weight in all white adipose tissues examined, including epididymal, retroperitoneal, and mesenteric adipose tissues. A morphological analysis by hematoxylin and eosin (HE) staining showed that adipocytes were enlarged in the HF group compared to those in the Normal group (Figure 2b). In contrast, the adipocyte size was smaller in the S. horneri-treated mice than in the HF group.
Figure 2.
Effects of S. horneri on the mass and morphology of white adipose tissue in C57BL/6J mice fed a high-fat diet for 13 weeks. After the mice were sacrificed, masses of epididymal, retroperitoneal, and mesenteric white adipose tissue were determined (a). Adipose tissue was fixed, and the section of epididymal adipose tissue was stained with hematoxylin and eosin (HE) (b). Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S. horneri; and HF + ShH, high-fat diet mixed with 6% S. horneri. Each value represents the mean ± SEM (n = 8). Significant difference: * p < 0.05, ** p < 0.01 vs. HF group, ## p < 0.01 vs. Normal group.
3.3. Effects of S. horneri on Serum Levels of Glucose, Insulin and Adipokines, and Insulin Resistance
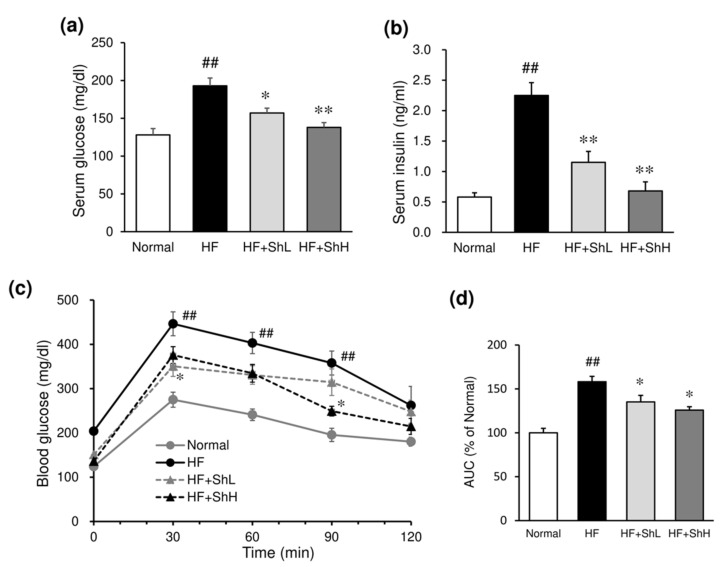
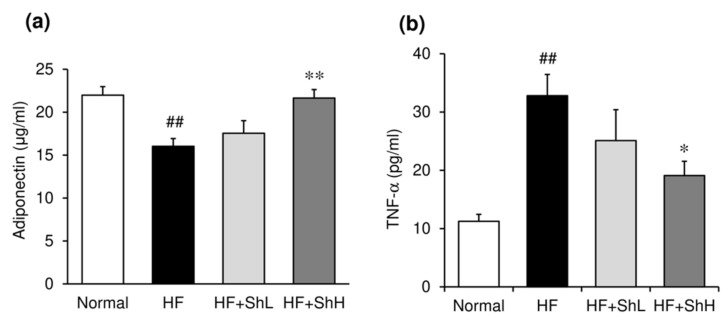
Serum glucose and insulin levels were significantly increased by HF diet (Figure 3a,b). Supplementation with S. horneri suppressed the increased levels of glucose and insulin in a dose-dependent manner (Figure 3a,b). The effect of S. horneri on insulin resistance was assessed using an intraperitoneal glucose tolerance test. The peak of blood glucose was lower, and the glycemic response was improved in S. horneri-treated mice, compared to mice in the HF group (Figure 3c). The area under the curve (AUC) for glucose was also significantly lower in S. horneri-treated mice compared to mice in the HF group (Figure 3d). Serum level of anti-inflammatory adiponectin was significantly reduced by feeding of HF diet (Figure 4a), while inflammatory cytokine TNF-α level was markedly elevated by HF diet (Figure 4b). Supplementation with S. horneri normalized these changes in a dose-dependent manner.
Figure 3.
Effects of S. horneri on serum glucose and insulin levels and on the insulin resistance in C57BL/6J mice fed a high-fat diet. Serum levels of glucose (a) and insulin (b) were determined 13 weeks after high-fat diet ingestion. Insulin resistance (c) was evaluated in glucose tolerance test on the 12th week after high-fat diet ingestion, and the area under the curve (AUC) was calculated (d). Normal, normal diet; HF, high-fat diet; HF + ShL: high-fat diet mixed with 2% S horneri; HF + ShH: high-fat diet mixed with 6% S horneri. Each value represents the mean ± SEM (n = 6−8). Significant difference: * p < 0.05, ** p < 0.01 vs. HF group, ## p < 0.01 vs. Normal group.
Figure 4.
Effects of S. horneri on serum adipokine levels in C57BL/6J mice fed a high-fat diet for 13 weeks. Serum levels of adiponectin (a) and TNF-α (b) were determined using a commercial ELISA kit. Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. Each value represents the mean ± SEM (n = 6−8). Significant difference: * p < 0.05, ** p < 0.01 vs. HF group, ## p < 0.01 vs. Normal group.
3.4. Effects of S. horneri on Serum Lipid Levels
The serum levels of total cholesterol and non-HDL cholesterol were significantly increased in the HF group compared to those in the Normal group (Table 2). By contrast, the levels of HDL cholesterol and triglyceride were unchanged by feeding an HF. Although low-dose S. horneri had little effect on the total cholesterol and non-HDL levels in the serum, high-dose S. horneri significantly decreased these parameters.
Table 2.
Effects of S. horneri on serum lipid levels.
| Normal | HF | HF + ShL | HF + ShH | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 150 ± 9 | 217 ± 6 ## | 215 ± 7 | 189 ± 7 * |
| HDL cholesterol (mg/dL) | 86 ± 2 | 88 ± 2 | 85 ± 2 | 87 ± 2 |
| Non-HDL cholesterol (mg/dL) | 64 ± 7 | 128 ± 7 ## | 124 ± 6 | 99 ± 5 ** |
| Triglyceride (mg/dL) | 69 ± 8 | 48 ± 6 | 33 ± 3 | 45 ± 4 |
Each value represents the mean ± SEM for 7–8 mice. Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. Significantly different from the HF group, * p < 0.05; ** p < 0.01. Significantly different from the Normal group, ## p < 0.01.
3.5. Effects of S. horneri on Hepatic Steatosis
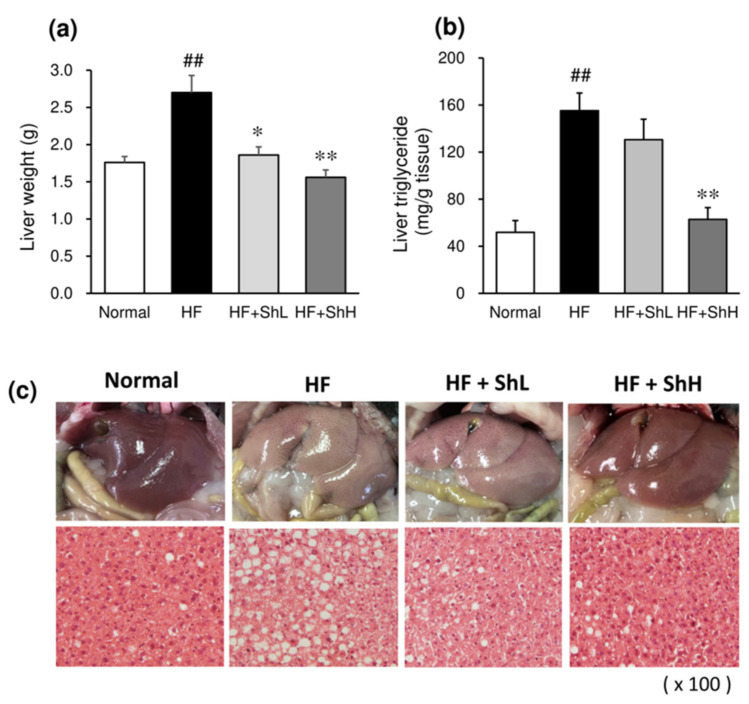
Feeding an HF to mice increased the liver weight, accompanied by the marked elevation of the liver triglyceride content (Figure 5a,b). Supplementation with S. horneri significantly suppressed the increase in the liver weight. It also suppressed the liver triglyceride accumulation in a dose-dependent manner. Gross morphology showed that the liver of the HF group was larger and exhibited a paler color than the Normal group (Figure 5c). A histological examination revealed that feeding an HF for 13 weeks caused hepatic steatosis, as evidenced by vacuoles, lipid droplets, and hepatocyte swelling (Figure 5c). Treatment of HF mice with S. horneri attenuated these pathological changes. In particular, HF + ShH normalized HF-induced hepatic steatosis. Consistent with histological observations, the ingestion of an HF increased the serum parameters of the liver function, including ALT, AST, ALP, and LAP (Table 3). HF + ShH decreased these elevated parameters to the normal level.
Figure 5.
Effects of S. horneri on the hepatic lipid accumulation in C57BL/6J mice fed a high-fat diet for 13 weeks. (a) Liver weight; (b) liver triglyceride content; (c) representative gross morphology, and histological sections of the liver. Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. Each value represents the mean ± SEM (n = 8). Significant difference: * p < 0.05, ** p < 0.01 vs. HF group, ## p < 0.01 vs. Normal group.
Table 3.
Effects of S. horneri on the serum parameters of the liver function.
| Normal | HF | HF + ShL | HF + ShH | |
|---|---|---|---|---|
| ALT (IU/L) | 32.7 ± 6.7 | 126.7 ± 23.5 ## | 102.4 ± 17.2 | 40.1 ± 4.9 ** |
| AST (IU/L) | 107.4 ± 10.6 | 155.0 ± 17.4 ## | 120.3 ± 10.6 | 103.0 ± 7.8 * |
| ALP (IU/L) | 223.7 ± 12.3 | 287.3 ± 27.1 ## | 255.8 ± 18.6 | 195.6 ± 8.8 ** |
| LAP (IU/L) | 33.8 ± 1.4 | 43.6 ± 3.7 # | 40.6 ± 2.7 | 32.1 ± 0.93 * |
Each value represents the mean ± SEM for 7–8 mice. Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LAP, leucine aminopeptidase. Significantly different from the HF group, * p < 0.05; ** p < 0.01. Significantly different from the Normal group, # p < 0.05; ## p < 0.01.
3.6. Effects of S. horneri on the Fecal Content of Triglycerides and Polysaccharides
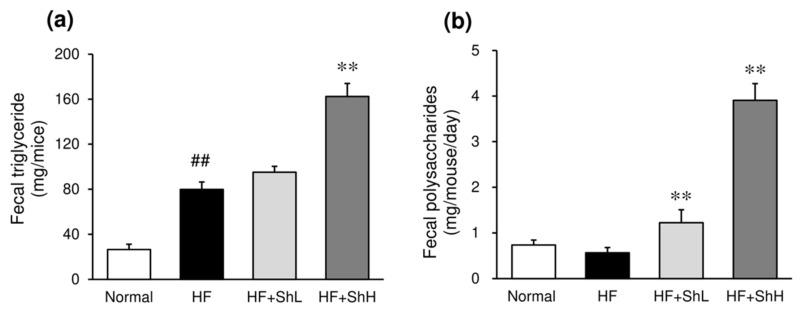
An HF increased the triglyceride content in the feces more than three-fold compared to the normal mice (Figure 6a). High-dose S. horneri increased the fecal triglyceride levels significantly further. In addition, treatment with S. horneri significantly increased the fecal content of polysaccharides (Figure 6b).
Figure 6.
Effects of S. horneri on the feces components in C57BL/6J mice fed a high-fat diet. Feces were collected from each mouse 10 weeks after high-fat diet ingestion, and the contents of triglyceride (a), and polysaccharide (b) were measured after extraction with isopropanol and distilled water, respectively. Normal, normal diet; HF, high-fat diet; HF + ShL, high-fat diet mixed with 2% S. horneri; HF + ShH, high-fat diet mixed with 6% S. horneri. Each value represents the mean ± SEM (n = 6−8). Significant difference: ** p < 0.01 vs. HF group, ## p < 0.01 vs. Normal group.
3.7. Effects of S. horneri on the Pancreatic Lipase Activity
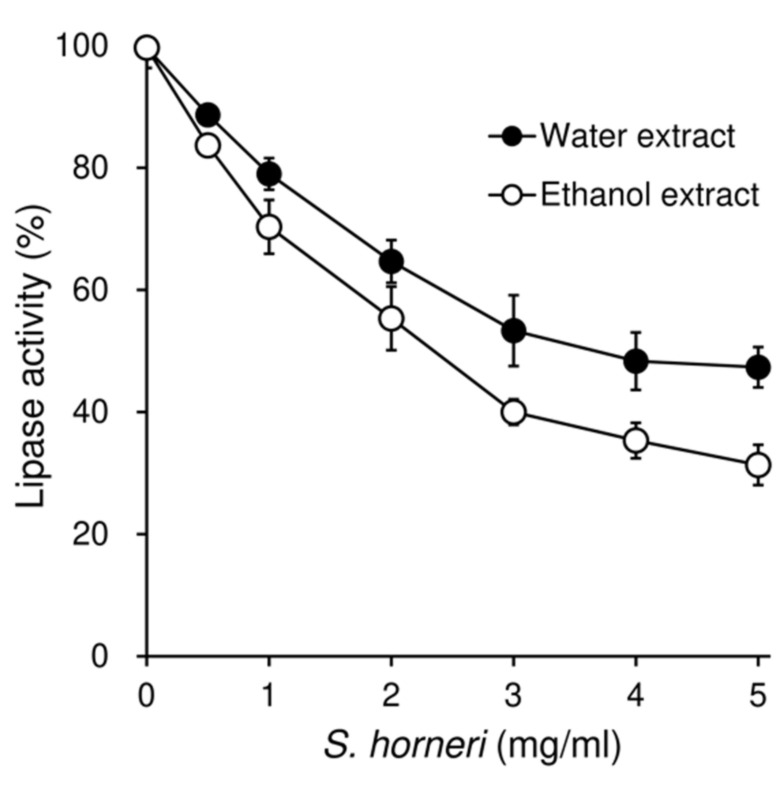
Two different extracts were prepared from S. horneri, and their inhibitory activity on pancreatic lipase was tested in vitro. Both water extract and ethanol extract dose-dependently inhibited the activity of lipase, with IC50 values of 3.7 mg/mL and 2.3 mg/mL, respectively (Figure 7).
Figure 7.
Effects of S. horneri extracts on the activity of pancreatic lipase in vitro. S. horneri was extracted with water (water extract) or 70% ethanol (ethanol extract), and its inhibitory activity was measured in vitro. Each point represents the mean ± SEM of triplicate experiments.
4. Discussion
The present study showed that dietary supplementation with S. horneri was able to ameliorate the development of obesity and related metabolic disorders, including diabetes, hepatic steatosis, and hypercholesterolemia, in mice fed a high-fat diet (HF). S. horneri is characterized by a higher content of bioactive polysaccharides and fucoxanthin than other popular edible brown seaweeds, including Wakame seaweed (Undaria pinnatifida) and Japanese tangle (Saccarina japonica). We confirmed that the S. horneri used in the present study contained approximately 35−52% (wt) alginate and 5−11% (wt) fucoidan as polysaccharides. The anti-obesity effects of these components have been previously reported. The effect of the sulfated polysaccharide fucoidan on obesity was examined in mice fed an HF [29]. This animal experiment revealed that fucoidan reduces body weight gain, epididymal fat mass, plasma triglyceride, and liver steatosis, which are accompanied by the down-regulation of the mRNA expression of PPARγ, adipose-specific fatty acid-binding protein, and acetyl CoA carboxylase in adipose tissue. In 3T3-L1 adipocytes, fucoidan ameliorates the lipid accumulation by increasing the expression of hormone-sensitive lipase, a key enzyme involved in lipolysis, together with suppression of inflammation and reactive oxygen species [30]. In vitro and animal studies have suggested that the anti-obesity effects of fucoidan are associated with antioxidative and anti-inflammatory action and enhanced lipolysis. A randomized, double-blind, placebo-controlled study showed the beneficial effects of fucoidan on insulin secretion and serum cholesterol levels in overweight or obese adults [31]. Another major polysaccharide in S. horneri, alginate, has also been shown to have anti-obesity effects. Alginate inhibits the activity of pancreatic lipase, leading to decreased breakdown and retarded absorption of triacylglycerol [32]. Furthermore, alginate is a gelling polysaccharide that increases satiety and reduces energy intake [33]. Several studies, including human experiments have suggested that these effects of alginate may be related to delayed gastric clearance and the stimulation of stretch receptors leading to attenuated fat absorption.
The anti-obesity effect of S. horneri was demonstrated by a decrease in the body-weight gain and visceral-fat weight in the present HF-induced obese mice. It is well known that white adipose tissue is an active endocrine organ that expresses and secretes various adipokines such as adiponectin, leptin, tumor-necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) [34,35]. These factors play significant roles in the regulation of energy, glucose, and lipid metabolism. The hypertrophy of adipocytes during excessive visceral-fat accumulation causes the alteration of the adipokine-secretion pattern and induces an inflammatory condition that contributes to the onset of obesity-related comorbidities. Adipose-tissue macrophages are also involved in obesity-related inflammation and systemic insulin resistance [31,32]. Thus, it is suggested that an increase in visceral adipose tissue induced insulin resistance, hepatic steatosis, and dyslipidemia in mice fed an HF.
Adiponectin is the most abundant adipokine that exerts insulin-sensitizing actions in obesity-related metabolic disorders. The circulating adiponectin level is inversely related to metabolic dysregulation, the inflammatory process, and oxidative stress and is lower in obese subjects and animals with insulin resistance than healthy subjects and animals [36]. Although ingestion of an HF for 13 weeks reduced the serum adiponectin level, treatment with S. horneri suppressed this decrease in a dose-dependent manner. Consistent with previous findings, the present study indicates a negative correlation between the serum adiponectin level and obesity-related metabolic disorders, including diabetes, hepatic steatosis, and dyslipidemia in mice. TNF-α is an important pro-inflammatory cytokine that is critically involved in the development of insulin resistance and pathogenesis of type 2 diabetes [34,35]. Our previous study using the same HF-induced obese mice indicated that elevation of the serum TNF-α level was correlated with the increased mRNA expression of TNF-α in white adipose tissue [37]. Although the serum level of TNF-α was markedly increased by the ingestion of an HF, treatment with S. horneri suppressed this increase in serum TNF-α, suggesting that S. horneri may improve the inflammatory condition in adipocytes. In fact, ethanol extract from S. horneri has been shown to exert an anti-inflammatory effect. S. horneri extract exerts anti-inflammatory actions in RAW 264.7 macrophage cells through the inhibition of ERK, p-p38, NF-κB, and pro-inflammatory gene expression [38]. In addition, the anti-inflammatory effects of major components of S. horneri (e.g., fucoidan [39], alginate [40], and fucoxanthin [41]), have also been reported. Thus, supplementation with S. horneri may alleviate inflammation in white adipose tissue, which is partly associated with its anti-obesity effect. Furthermore, it is also well documented that adipose-tissue-derived adipokines play a central role in the development of hepatic steatosis by causing liver inflammation [42].
The present in vitro study showed that both water extract and ethanol extract of S. horneri inhibited the activity of pancreatic lipase. Although we did not perform a component analysis of S. horneri, water extract is presumed to mainly contain polysaccharides, such as alginate and fucoidan, while 70% ethanol extract is expected to be rich in polyphenols. Hundreds of extracts isolated from a wide variety of plants, seaweed, and bacteria have been reported to inhibit the activity of pancreatic lipase [43]. The active compounds include polyphenols, triterpenes, saponins, and polysaccharides. Some brown seaweed extracts, including Ascophyllum nodosum, Fucus vesuculosus, and Pelvetia canaliculata, have been shown to inhibit lipase activity [44]. Extracts of these seaweeds contain polysaccharides, such as alginate, fucoidan, and laminarin, as well as low-molecular-weight active compounds, such as polyphenols. These polysaccharides and polyphenols may be mainly involved in the inhibition of lipase activity. Alginate has been shown to inhibit the activity of pancreatic lipase and increase fat excretion in rats [45] and human [46]. Several possible mechanisms concerning the inhibitory effect of alginate on lipase have been reported. Alginate may interact with both the substrate and the enzyme through electrostatic interactions, as negatively charged alginate can associate with positively charged proteins [47]. Furthermore, alginate has been shown to decrease the diffusion of lipid in the porcine intestinal mucus layer, leading to reduced lipid absorption [48]. Thus, certain active components of S. horneri, including polysaccharides and polyphenols, are suggested to inhibit pancreatic lipase.
Pancreatic lipase is responsible for the absorption of dietary fat, hydrolyzing triacylglycerols to monoacylglycerols, and fatty acids [49]. Since excess dietary fat is the major source of undesirable calories, the inhibition of this enzyme is a possible mechanism by which S. horneri reduces fat absorption and ameliorates obesity. Orlistat, the only authorized anti-obesity drug, acts by inhibiting digestive lipase, reducing hydrolysis of ingested fat, and thereby increasing fecal-fat excretion [50]. Our results indicate that S. horneri inhibits pancreatic lipase and thereby suppresses the hydrolysis of fat, leading to reduced fat absorption. A reduction in fat absorption results in not only the amelioration of obesity but the improvement of insulin resistance, hepatic steatosis, and dyslipidemia. Analyses of feces revealed that the fecal triglyceride content was markedly elevated by high-dose S. horneri, accompanied by an increase in the fecal polysaccharide content. Elevation of the fecal polysaccharide levels suggests that alginate and fucoidan play an important role in the inhibition of intestinal fat absorption and obesity development by S. horneri.
Fucoxanthin is a characteristic marine carotenoid present in brown seaweed. It should be noted that the content of fucoxanthin in S. horneri is higher than in other brown seaweed [51]. Fucoxanthin exhibits various benefits including anti-obesity and antidiabetic activities [52]. Sixteen-week, double-blind, randomized, placebo-controlled studies have demonstrated the effectiveness of fucoxanthin in the treatment of obese humans [53]. Fucoxanthin and its metabolite fucoxanthinol have also been reported to inhibit the activity of lipase [54]. The half-maximal inhibitory concentration for enzymatic activity (IC50) was around 700 nmol/L, which is 100-fold stronger than that of orlistat. The inhibition of triglyceride absorption in vivo was demonstrated in conscious rats. These previous findings support the notion that active components characteristic of S. horneri, including alginate, fucoidan, and fucoxanthin, may be involved in the inhibition of pancreatic lipase, which results in the stimulation of fecal-fat excretion and suppression of fat absorption.
In addition to the effect of fucoxanthin on lipase activity, several studies have shown that the anti-obesity effect of fucoxanthin is associated with fatty-acid oxidation and heat production by inducing uncoupling protein 1 (UCP-1) in white adipose tissue [55]. Nutrigenomic studies have shown that fucoxanthin induces UCP1 in the mitochondria of abdominal white adipose tissue, leading to the oxidation of fatty acids and heat production. Fucoxanthin improves insulin resistance and decreases blood glucose levels through the regulation of cytokine secretion from white adipose tissue [56].
In the present study, supplementation of mice with S. horneri decreased the HF-induced increase in serum non-HDL cholesterol levels. Possible mechanisms include a reduced intestinal reabsorption and an enhanced fecal excretion of bile acids. Dietary fiber is known to interact with bile acids and interfere with their reabsorption in the intestine. As a result, the fecal excretion of bile acids increases. Decreased intestinal circulation of bile acids up-regulates bile acid synthesis from cholesterol, which results in a decreased serum cholesterol level.
5. Conclusions
In conclusion, the present results show that whole S. horneri suppressed the development of HF-induced obesity and related metabolic disorders in mice. The anti-obesity effect of S. horneri is associated with the inhibition of pancreatic lipase, which leads to the suppression of intestinal lipid absorption and their subsequent accumulation in adipose tissue and liver. In addition, the anti-inflammatory action may be partly involved in the improvement of obesity and diabetes. The major components of S. horneri fucoidan, alginate, and fucoxanthin seem to be responsible for its beneficial effects. Thus, the traditionally eaten brown seaweed S. horneri may be useful as a foodstuff for reducing rates of obesity and diabetes. This study involved experiments using mice, so the results cannot be directly applied to humans. Further studies are needed to demonstrate the effectiveness of S. horneri in humans.
Author Contributions
Conceptualization, S.M.; formal analysis, S.M., C.H., T.O., R.Y., T.M., M.M., T.I., and C.M.; data curation, S.M., C.H., T.O., R.Y., and T.M.; writing—original draft preparation, S.M.; writing—review and editing, S.M. Investigation, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant for scientific research from Fukui Prefectural University.
Institutional Review Board Statement
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Fukui Prefectural University (approval No. 19–14)..
Informed Consent Statement
Not applicable
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown E.S., Allsopp P.J., Magee P.J., Gill C.I., Nitecki S., Strain C.R., McSorley E.M. Seaweed and human health. Nutr. Rev. 2014;72:205–216. doi: 10.1111/nure.12091. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso S.M., Pereira O.R., Seca A.M., Pinto D.C., Silva A.M. Seaweeds as preventive agents for cardiovascular diseases from nutrients to functional foods. Mar. Drugs. 2015;13:6838–6865. doi: 10.3390/md13116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry P., O’Hara C., Magee P.J., McSorley E.M., Allsopp P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019;77:307–329. doi: 10.1093/nutrit/nuy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murai U., Yamagishi K., Sata M., Kokubo Y., Saito I., Yatsuya H., Ishihara J., Inoue M., Sawada N., Iso H., et al. JPHC Study Group. Seaweed intake and risk of cardiovascular disease: The Japan Public Health Center-based Prospective (JPHC) Study. Am. J. Clin. Nutr. 2019;110:1449–1455. doi: 10.1093/ajcn/nqz231. [DOI] [PubMed] [Google Scholar]
- 5.Kishida R., Yamagishi K., Muraki I., Sata M., Tamakoshi A., Iso H. JACC Study Group. Frequency of seaweed intake and its association with cardiovascular disease mortality: The JACC Study. J. Atheroscler. Thromb. 2020;27:1340–1347. doi: 10.5551/jat.53447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sho H. History and characteristics of Okinawan longevity food. Asia Pac. J. Clin. Nutr. 2001;10:159–164. doi: 10.1046/j.1440-6047.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 7.Blüher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 8.Van Gaal L., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher E.J., LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol. Rev. 2015;95:727–948. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W.L., Zhu L., Jiang J.G. Active ingredients from natural botanicals in the treatment of obesity. Obes. Rev. 2014;15:957–967. doi: 10.1111/obr.12228. [DOI] [PubMed] [Google Scholar]
- 11.Sun N.N., Wu. T.Y., Chau C.F. Natural dietary and herbal products in anti-obesity treatment. Molecules. 2016;21:E1351. doi: 10.3390/molecules21101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero L., Del. Pilar Sánchez-Camargo A., Ibáñez E., Gilbert-López B. Phenolic compounds from edible algae Bioactivity and health benefits. Curr. Med. Chem. 2018;25:4808–4826. doi: 10.2174/0929867324666170523120101. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Pérez C., Segura-Carretero A., Del. Mar. Contreras M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food. Sci. Nutr. 2019;59:1212–1229. doi: 10.1080/10408398.2017.1399859. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu T., Fukuda M., Mikami A., Mizuno S., Kantachumpoo A., Tanoue H., Kawamiya M. Possible change in distribution of seaweed, Sargassum horneri, in northeast Asia under A2 scenario of global warming and consequent effect on some fish. Mar. Pollut. Bull. 2014;85:317–324. doi: 10.1016/j.marpolbul.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., Liu H.B. Research progress in chemical components and biological activity of Sargassum. Chin. J. Mar. Drugs. 2012;31:41–51. [Google Scholar]
- 16.Silchenko A.S., Rasin A.B., Kusaykin M.I., Kalinovsky A.I., Miansong Z., Changheng L., Malyarenko O., Zueva A.O., Zvyagintseva T.N., Ermakova S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017;175:654–660. doi: 10.1016/j.carbpol.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Herath K.H.I.N.M., Cho J., Kim A., Kim H.S., Han E.J., Kim H.J., Kim M.S., Ahn G., Jeon Y.J., Jee Y. Differential modulation of immune response and cytokine profiles of Sargassum horneri ethanol extract in murine spleen with or without Concanavalin A stimulation. Biomed. Pharmacother. 2019;110:930–942. doi: 10.1016/j.biopha.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Kwak J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs. 2014;12:851–870. doi: 10.3390/md12020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luthuli S., Wu S., Cheng Y., Zheng X., Wu M., Tong H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs. 2019;17:E487. doi: 10.3390/md17090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martău G.A., Mihai M., Vodnar D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers. 2019;11:E1837. doi: 10.3390/polym11111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson J.W., Baird P., Davis R.H., Ferreri S., Knudtson M., Koraym A., Waters V., Williams C.L. Health benefits of dietary fiber. Nutr. Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 22.Wan-Loy C., Siew-Moi P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs. 2016;14:E222. doi: 10.3390/md14120222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters H.P., Koppert R.J., Boers H.M., Ström A., Melnikov S.M., Haddeman E., Schuring E.A., Mela D.J., Wiseman S.A. Dose-dependent suppression of hunger by a specific alginate in a low-viscosity drink formulation. Obesity. 2011;19:1171–1176. doi: 10.1038/oby.2011.63. [DOI] [PubMed] [Google Scholar]
- 24.Georg Jensen M., Kristensen M., Astrup A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012;96:5–13. doi: 10.3945/ajcn.111.025312. [DOI] [PubMed] [Google Scholar]
- 25.Peng J., Yuan J.P., Wu C.F., Wang J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs. 2011;9:1806–1828. doi: 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami S., Yamagishi I., Sato M., Tomisawa K., Nara Y., Yamori Y. ACAT inhibitor HL-004 accelerates the regression of hypercholesterolemia in stroke-prone spontaneously hypertensive rats (SHRSP): Stimulation of bile acid production by HL-004. Atherosclerosis. 1997;133:97–104. doi: 10.1016/S0021-9150(97)00121-4. [DOI] [PubMed] [Google Scholar]
- 27.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 28.Bendicho S., Trigueros M.C., Hernández T., Martin O. Validation and comparison of analytical methods based on the release of p-nitrophenol to determine lipase activity in milk. J. Dairy Sci. 2001;84:1590–1596. doi: 10.3168/jds.S0022-0302(01)74592-4. [DOI] [PubMed] [Google Scholar]
- 29.Kim M.J., Jeon J., Lee J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014;28:137–143. doi: 10.1002/ptr.4965. [DOI] [PubMed] [Google Scholar]
- 30.Park M.K., Jung U., Roh C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs. 2011;9:1359–1367. doi: 10.3390/md9081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández-Corona D.M., Martínez-Abundis E., González-Ortiz M. Effect of fucoidan administration on insulin secretion and insulin resistance in overweight or obese adults. J. Med. Food. 2014;17:830–831. doi: 10.1089/jmf.2013.0053. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox M.D., Brownlee I.A., Richardson J.C., Dettmar P.W., Pearson J.P. The modulation of pancreatic lipase activity by alginates. Food Chem. 2014;146:479–484. doi: 10.1016/j.foodchem.2013.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georg Jensen M., Pedersen C., Kristensen M., Frost G., Frost G., Astrup A. Review: Efficacy of alginate supplementation in relation to appetite regulation and metabolic risk factors: Evidence from animal and human studies. Obes. Rev. 2013;14:129–144. doi: 10.1111/j.1467-789X.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 34.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin S., Hirai S., Yamaguchi Y., Goto T., Takahashi N., Tani F., Mutoh C., Sakurai T., Murakami S., Yu R., et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol. Nutr. Food Res. 2013;57:2155–2165. doi: 10.1002/mnfr.201300150. [DOI] [PubMed] [Google Scholar]
- 38.Kim M.E., Jung Y.C., Jung I., Lee H.W., Youn H.Y., Lee J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-κB pathway regulation. Immunol. Invest. 2015;44:137–146. doi: 10.3109/08820139.2014.942459. [DOI] [PubMed] [Google Scholar]
- 39.Park J., Cha J.D., Choi K.M., Lee K.Y., Han K.M., Jang Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017;43:91–98. doi: 10.1016/j.intimp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Kawauchi S., Horibe S., Sasaki N., Tanahashi T., Mizuno S., Hamaguchi T., Rikitake Y. Inhibitory effects of sodium alginate on hepatic steatosis in mice induced by a methionine- and choline-deficient diet. Mar. Drugs. 2019;17:104. doi: 10.3390/md17020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan C.P., Hou Y.H. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation. 2014;37:443–450. doi: 10.1007/s10753-013-9757-1. [DOI] [PubMed] [Google Scholar]
- 42.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 43.de la Garza A.L., Milagro F.I., Boque N., Campión J., Martínez J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011;77:773–785. doi: 10.1055/s-0030-1270924. [DOI] [PubMed] [Google Scholar]
- 44.Chater P.I., Wilcox M., Cherry P., Herford A., Mustar S., Wheater H., Brownlee I., Seal C., Pearson J. Inhibitory activity of extracts of Hebridean brown seaweeds on lipase activity. J. Appl. Phycol. 2016;28:1303–1313. doi: 10.1007/s10811-015-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasahara F., Kato T., Idota Y., Takahashi H., Kakinuma C., Yano K., Arakawa H., Hara K., Miyajima C., Ogihara T. Reduction effect of calcium alginate on blood triglyceride levels causing the inhibition of hepatic and total body accumulation of fat in rats. Biol. Pharm. Bull. 2019;42:365–372. doi: 10.1248/bpb.b18-00530. [DOI] [PubMed] [Google Scholar]
- 46.Sandberg A.S., Andersson H., Bosaeus I., Carlsson N.G., Hasselblad K., Härröd M. Alginate, small bowel sterol excretion, and absorption of nutrients in ileostomy subjects. Am. J. Clin. Nutr. 1994;60:751–756. doi: 10.1093/ajcn/60.5.751. [DOI] [PubMed] [Google Scholar]
- 47.Chater P.I., Wilcox M.D., Houghton D., Pearson J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015;6:3420–3427. doi: 10.1039/C5FO00293A. [DOI] [PubMed] [Google Scholar]
- 48.Mackie A.R., Macierzanka A., Aarak K., Rigby N.M., Parker R., Channell G.A., Harding S.E., Bajka B.H. Sodium alginate decreases the permeability of intestinal mucus. Food Hydrocoll. 2016;52:749–755. doi: 10.1016/j.foodhyd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowe M.E. Pancreatic triglyceride lipase and colipase: Insights into dietary fat digestion. Gastroenterology. 1994;107:1524–1536. doi: 10.1016/0016-5085(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 50.Heck A.M., Yanovski J.A., Calis K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270–279. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terasaki M., Hirose A., Narayan B., Baba Y., Kawagoe C., Yasui H., Saga N., Hosokawa M., Miyashita K. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents (1) J. Phycol. 2009;45:974–980. doi: 10.1111/j.1529-8817.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 52.Gammone M.A., D’Orazio N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs. 2015;13:2196–2214. doi: 10.3390/md13042196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abidov M., Ramazanov Z., Seifulla R., Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010;12:72–81. doi: 10.1111/j.1463-1326.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto M., Hosokawa M., Matsukawa N., Hagio M., Shinoki A., Nishimukai M., Miyashita K., Yajima T., Hara H. Suppressive Effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur J. Nutr. 2010;49:243–249. doi: 10.1007/s00394-009-0078-y. [DOI] [PubMed] [Google Scholar]
- 55.Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005;332:392–397. doi: 10.1016/j.bbrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Woo M.N., Jeon S.M., Shin Y.C., Lee M.K., Kang M.A., Choi M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009;53:1603–1611. doi: 10.1002/mnfr.200900079. [DOI] [PubMed] [Google Scholar]