Abstract
Redox signalling in the gastrointestinal mucosa is held in an intricate balance. Potent microbicidal mechanisms can be used by infiltrating immune cells, such as neutrophils, to protect compromised mucosae from microbial infection through the generation of reactive oxygen species. Unchecked, collateral damage to the surrounding tissue from neutrophil-derived reactive oxygen species can be detrimental; thus, maintenance and restitution of a breached intestinal mucosal barrier are paramount to host survival. Redox reactions and redox signalling have been studied for decades with a primary focus on contributions to disease processes. Within the past decade, an upsurge of exciting findings have implicated subtoxic levels of oxidative stress in processes such as maintenance of mucosal homeostasis, the control of protective inflammation and even regulation of tissue wound healing. Resident gut microbial communities have been shown to trigger redox signalling within the mucosa, which expresses similar but distinct enzymes to phagocytes. At the fulcrum of this delicate balance is the colonic mucosal epithelium, and emerging evidence suggests that precise control of redox signalling by these barrier-forming cells may dictate the outcome of an inflammatory event. This Review will address both the spectrum and intensity of redox activity pertaining to host–immune and host–microbiota crosstalk during homeostasis and disease processes in the gastrointestinal tract.
Mucosae are selectively permeable host surfaces, necessary for interaction with the environment and for facilitating crucial functions including gaseous exchange and nutrient absorption1. Protecting these surfaces from both pathogenic and commensal microorganisms, while maintaining immune homeostasis, requires the ability to rapidly and potently induce danger signals when appropriate and to promptly neutralize these signals to limit collateral damage to the mucosa. The colonic mucosa consists of a single layer of epithelia derived from the crypt stem cell niche. As crypt stem cells proliferate, daughter cells migrate along the crypt axis, differentiating into specialized epithelia of either secretory or absorptive lineages2. Absorptive enterocytes are responsible for water reabsorption, whereas secretory epithelia are tasked with mucus and antimicrobial peptide secretion into the lumen of the gut2,3. These secretions provide an essential carbon source for the microbial niche, in the form of glycosylated mucins, but they also maintain a sterile margin directly adjacent to the epithelial cells to prevent inappropriate responses to resident gut microbiota4,5.
Immune and inflammatory responses within the gastrointestinal mucosae are characterized by profound shifts in tissue metabolism. These changes include the utilization of large amounts of energy and diminished availability of oxygen (hypoxia)6. Such shifts in tissue metabolism result, at least in part, from recruitment of inflammatory cells, particularly neutrophils and other polymorphonuclear leukocytes (PMNs) and monocytes7. A particularly prominent phenotype of acute inflammatory lesions within the intestine is localized accumulation of PMNs, termed crypt abscesses. Given the large amounts of reactive oxygen species (ROS) that can be generated by activated PMNs, the crypt abscess represents a major signalling node for reduction–oxidation (redox) signalling (defined as an elicited signalling response to a particular ROS)8. Resident immune cells in the intestine, which include intraepithelial lymphocytes and professional antigen presenting cells (dendritic cells and macrophages), are poised as sentinels to respond to host threats such as bacterial and viral infections, but also contribute to homeostasis by immune surveillance and by promoting a regulatory immune response9-11. Most of these cell types — immune, epithelial and microorganism — are capable of eliciting and/or circumventing redox signalling with profound implications for mucosal homeostasis.
An important result of active inflammation in the intestinal mucosae is the localized conversion of molecular oxygen to ROS by immune cells with subsequent tissue hypoxia. At the tissue and cellular level, hypoxia induces an array of genes pivotal to adaptation to low-oxygen states (for example, those involved with glycolysis, angiogenesis or erythropoiesis). As a global regulator of oxygen homeostasis, the aβ-heterodimeric transcription factor hypoxia-inducible factor (HIF) facilitates both tissue oxygen delivery and adaptation to hypoxia12,13. HIF1 and HIF2 (also known as EPAS1) are members of the Per-ARNT-Sim family of basic helix–loop–helix transcription factors. HIF activation is dependent upon stabilization of an oxygen-dependent degradation domain of the HIF1 α-subunit (HIF1α) and the subsequent nuclear translocation of this subunit to form a functional complex with HIF1β and cofactors, such as CREB-binding protein (CBP) and its orthologue histone acetyltransferase p300 (REFS12,13). When oxygen supply exceeds demand, iron-dependent and oxygen-dependent hydroxylation of two prolines (Pro564 and Pro402) within the oxygen-dependent degradation domain of HIF1α or HIF2α initiates the association with the von Hippel–Lindau disease tumour suppressor protein and degradation of the α-subunit via ubiquitin E3 ligase proteasomal targeting14,15. A second hypoxic switch operates in the carboxy-terminal transactivation domain of HIF1α or HIF2α. Here, hypoxia blocks the hydroxylation of Asn803, thereby facilitating the recruitment of CBP–p300 (REF.16).
A unique feature of the intestinal mucosa, particularly the colon, is the juxtaposition to large numbers of microorganisms, termed the gut microbiota. Indeed, the mammalian gastrointestinal tract is home to >1013 microorganisms, which approximates the number of eukaryotic cells constituting the human body17. The epithelium, a single layer of specialized absorptive and secretory cells, is all that separates this biomass from the host immune system18. A finely regulated relationship exists within the intestinal mucosa, whereby microorganisms, essential for host health, can also initiate and perpetuate mucosal disease19. Nutrient provision by microorganisms is one benefit to the host, However, in addition to aiding in digestion, microorganisms benefit the host through the local synthesis of short-chain fatty acids (SCFAs), including butyrate, propionate and acetate20. SCFAs can reach luminal concentrations of 130 mM in the proximal colon and function as the primary metabolic fuel for intestinal epithelial cells20. Reduced abundance of SCFA-producing microbial species has been associated with colonic disease, including IBD21-23. The low-oxygen (anaerobic) conditions that enable SCFA production place unusual metabolic demands on the colonic epithelium24 and are enhanced during inflammation8. It is particularly notable that the gut microbiota are a key regulator of redox potential in the mucosa25.
In addition to homeostatic and regulatory functions, ROS are well characterized to be produced by, and contribute to, disease processes — acutely during ischaemic damage, tissue injury and repair, and chronically in inflammatory conditions, such as IBD, and in colorectal cancer. In this Review, we provide an overview of redox reactions in the gastrointestinal tract and describe how various sources of redox-sensitive pathways contribute to the function of the healthy and diseased mammalian intestine. We will also discuss exciting new findings that highlight the contributions that different intensities of redox signalling in microbial–host crosstalk have towards maintaining homeostasis or facilitating disease processes within the gastrointestinal tract.
Redox signalling in the gut
ROS generation.
ROS constitute a major group of potent antimicrobial mediators and redox signalling factors. Both the gastrointestinal mucosa and associated immune cells are sources of free radicals, which are defined as chemical species with one or more unpaired electrons in the outermost orbital shell, making them chemically reactive26. The reduction and oxidation (redox) state of the gastrointestinal tract is contingent on the balance of antioxidants (for example, haem oxygenase or glutathione, a tripeptide consisting of glutamate, cysteine and glycine) and oxidants (for example, free radicals, reactive oxygen and nitrogen species). When an imbalance in redox state occurs, owing either to increased oxidants or insufficient neutralizing antioxidants, the tissue experiences oxidative stress or nitrosative stress27. In the gastrointestinal tract, a variety of reactive oxygen radicals, including superoxide (O2·−) and hydroxyl (OH·), and non-radicals, including hypochlorous acid (HOCl) and hydrogen peroxide (H2O2), are generated by epithelial cells, endothelial cells and innate immune cells to implement mucosal defence28 (FIG. 1). Tissue homeostasis is influenced in a variety of ways by the redox state of the tissue, including modulation of signal-transduction pathways (for example, HIF, nuclear factor-κB (NF-κB) and nuclear factor erythroid 2-related factor 2 (NRF2))29 that elicit adaptive gene expression to minimize bystander tissue damage. Through reduction of disulfide bonds found in many gut peptides, redox state can also modulate the activity of antimicrobial peptides involved in mucosal defence and cytokine secretion30. Of particular significance is the redox state of the ubiquitously expressed human β–defensin 1 (BD1). In the oxidized state, BD1 exhibits limited antimicrobial activity; however, following reduction of the disulfide bridges, BD1 alters conformation and displays an enhanced antimicrobial efficacy31. Indeed findings from the Wehkamp group demonstrate that the reduced form of BD1 is capable of forming net-like structures around bacteria to limit bacteria invasion32.
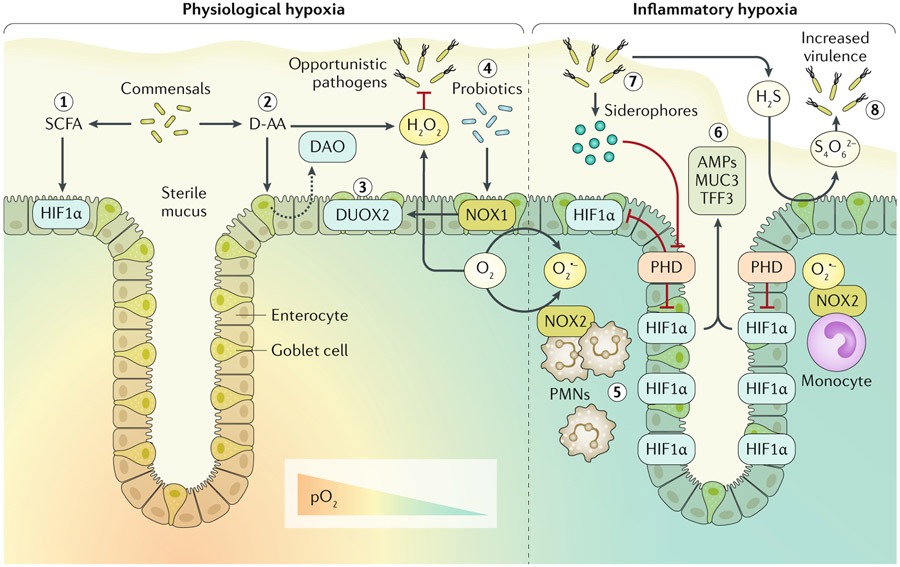
Fig. 1 ∣. Host-microbial redox signalling during hypoxia.
Enzymatic utilization of molecular oxygen (O2) within the intestinal mucosa facilitates redox signalling and results in both spatial and dynamic patterns of O2 availability. In the healthy intestinal mucosa, a steep O2 gradient exists between the highly vascularized mucosa and the anoxic lumen. Thus, cells within the crypt stem cell niche normally experience higher partial pressures of oxygen (pO2; ~100 mmHg) than the luminal-facing epithelia (<10 mmHg), which are known to normally experience hypoxia at homeostasis. This physiological hypoxia is experienced by epithelia adjacent to the lumen and results in stabilization of hypoxia-inducible factor (HIF). Gut microbiota secreting short chain fatty acids (SCFAs), particularly butyrate, contribute to this physiological hypoxia and associated stabilization of HIF1α through increased oxidative phosphorylation (step 1). Luminal redox signalling initiated by resident microorganisms releasing d-amino acids (D-AA) stimulates the epithelium to secrete D-AA oxidase (DAO) into the lumen, which subsequently yields hydrogen peroxide (H2O2) (step 2). Apical expression of epithelial dual oxidase 2 (DUOX2) probably results in luminal secretion of H2O2, which contributes to limiting opportunistic pathogen niche expansion (step 3). Probiotic lactobacilli upregulate epithelial NADPH oxidase 1 (NOX1) expression, which in turn induces DUOX2 (step4). Epithelial-expressed NOX1 and DUOX2, utilizing microenvironmental O2, generate oxygen radicals to further contribute to luminal release of H2O2. During inflammatory hypoxia, infiltrating polymorphonuclear leukocytes (PMNs) and monocytes expressing NOX2 generate superoxide (O2·−), resulting in inhibition of prolyl hydroxylase enzymes (PHD) and stabilization of HIF deep into the crypt (step 5). HIF transcriptional activity induces expression of barrier protective factors such as antimicrobial peptides (AMPs), mucin 3 (MUC3) and trefoil factor 3 (TFF3) (step 6). Certain opportunistic pathogens release siderophores, sequestering iron and inhibiting PHD (step 7). Sulfur metabolism of the mucosa can be hijacked by opportunistic pathogens. Hydrogen sulfide (H2S) is routinely detoxified to thiosulfate; however, high levels of reactive oxygen species within the mucosa can result in tetrathionate (S4O62−) generation, which can be utilized by Salmonella serotypes to provide a competitive advantage (step 8).
Reactive nitrogen species.
Nitric oxide (NO) is a short-lived, lipophilic and freely diffusible signalling molecule synthesized by mammalian cells with a broad spectrum of activities including regulation of blood flow, immune reactions and smooth muscle contraction33. NO is generated by the nitric oxide synthase (NOS) enzymes, which convert l-arginine to l-citrulline, liberating NO as a by-product34. In the gastrointestinal tract, NO functions as an inhibitory nonadrenergic, noncholinerigic neurotransmitter and smooth muscle cell relaxant via activation of guanylate cyclase35,36. To date, three isoforms of NOS have been cloned: neuronal NOS (nNOS; NOS1), endothelial NOS (eNOS; NOS3) and inducible NOS (iNOS; NOS2)37-39. Interaction of O2·− with NO leads to the formation of peroxynitrite (ONOO−)40. Further reactivity of peroxynitrite leads to the generation of various other NO-derived mediators termed reactive nitrogen species (RNS), including the reactive radical compounds nitrogen dioxide (NO2·) and hydroxyl radical (HO·), and nonradical dinitrogen trioxide (N2O3)40. ONOO−, together with RNS, are in turn responsible for nitrosylation of protein tyrosine residues, mitochondrial energy depletion, lipid peroxidation and induction of DNA strand breaks41. Nitrosative and oxidative stress have been implicated in a plethora of disease states, including conditions that affect the gastrointestinal tract (namely ischaemia–reperfusion injury (IRI) and inflammatory bowel diseases)29.
Sources of ROS.
Both exogenous and endogenous sources of ROS contribute to the overall redox state of the gastrointestinal tract. Endogenous sources contributing to ROS generation include the mitochondrial respiratory chain42, enzymes within the mucosal epithelia and submucosal lamina propria fibroblasts and myofibroblasts, such as NADPH oxidases (NOXs), xanthine oxidase and immune-expressed cyclooxygenases, lipoxygenases and myeloperoxidase27. Exogenous or environmental sources of ROS can also trigger oxidative stress, such as ionizing and nonionizing radiation, chemotherapeutics, xenobiotics, heavy metals and drugs43,44. Generation of ROS by cancer chemotherapeutic agents is a major contributor to the toxic adverse effects associated with these compounds44. Cigarette smoke comprises >7,000 chemical compounds and oxidative agents, containing >1014 free radicals per inhalation45. Tobacco use is known to modulate gastrointestinal diseases, and active smokers have an increased risk for colorectal cancer46 and increased severity of Crohn’s disease47. For reasons that are not completely clear, tobacco smoke seems to confer a somewhat protective influence to patients with ulcerative colitis48.
Mitochondrial metabolism and ROS
Although mitochondrial ROS (mtROS) are renowned for causing cellular damage (for example, during IRI)49, mtROS are now thought to contribute to healthy cellular function in terms of oxygen sensing, as well as disease50. Physiological production of mtROS occurs during oxidative phosphorylation and generation of high-energy ATP. The tricarboxylic acid cycle is tightly regulated; however, <2% of O2 consumption results in conversion to O2·−, whereby electrons leak out from the mitochondrial electron transport chain (ETC) and are aberrantly transferred to molecular oxygen51. Mitochondrial ETC complexes are capable of generating ROS at various sites. Complex I and II release O2·− into the mitochondrial matrix52, whereas manganese superoxide dismutase (SOD) converts it to H2O2. Complex III can produce O2·− within the inner membrane, but it is ejected into the intermembrane space owing to a large transmembrane electrical gradient53. If O2·− generated by the mitochondrial ETC is not efficiently converted to H2O2, NO radicals produce ONOO−, leading to subsequent irreversible nitration of proteins and enzyme inactivation41.
Cellular stressors such as ROS and hypoxia are hallmarks of pathogen invasion but also reflect the local environmental fluctuations experienced by intestinal epithelial cells during active inflammation or infection6. Interest exists in autophagy as a substantial contributor to intestinal disease mechanisms, especially IBD54. Autophagy represents a primordial cellular degradation pathway that facilitates cell survival under conditions of metabolic stress, in which cytoplasmic targets are engulfed by a double-membrane vacuole <1 μm in diameter termed the autophagosome that is subsequently fused with lysosomes for hydrolase-mediated digestion55. Considerable overlap exists between cellular stimuli for selective autophagy of damaged organelles (self) and invading microorganisms (non-self)56. Mitophagy is a particular type of autophagy, in which mitochondria are specifically targeted for autophagic lysosomal degradation57. Mitophagy is a highly regulated event and some studies indicate that the mitochondrial 18 kDa translocator protein (TSPO) is central to both regulation of mtROS generation and the induction of mitophagy58. Interestingly, the overexpression of TSPO in animal models of IBD has revealed that TSPO localizes with epithelial mitochondria59. Considering the endosymbiotic theory, which postulates the ancient common origin between mitochondria and proteobacteria60, it is curious to speculate how a pathway such as autophagy evolved to ignore functionally competent mitochondria and their proteobacterial ancestors but be triggered by invasive pathogenic organisms or damaged mitochondria.
H2O2 as a signalling molecule.
Oxygen radicals have a limited range of effect owing to their short-lived and highly reactive nature61. Specialized enzymes, such as SODs, convert oxygen radicals to the more stable and readily diffusible H2O2. Owing to its reduced reactivity, increased half-life and ability to induce reversible protein modification, H2O2 can act as a signalling molecule in its own right61. H2O2 has been demonstrated to oxidize cysteinyl thiol, induce disulfide bond formation and mediate glutathionylation of cysteine or sulfoxidation of methionine residues in numerous proteins. Such modifications can alter protein activity (increased or decreased) but also represent an important antioxidant defence mechanism62. In this Review, we focus primarily on the role of H2O2 in mucosal–microbiota crosstalk, but it is noteworthy that other redox signalling mechanisms (for example, nitrosylation) provide important signalling cues during host–bacterial interactions63.
Antioxidant pathways.
Regulators of the antioxidant response include enzymes that catalyse and neutralize ROS, ensuring their potent activity is short-lived to minimize collateral damage to the host tissue. Within the gastrointestinal mucosa antioxidant defence systems, SODs and glutathione peroxidase enzymes act as detoxification pathways for ROS. SODs are metal ion cofactor-requiring enzymes that catalyse the dismutation (that is, partitioning) of O2·− to H2O2 and O2 (REF.64). In humans, there are three SOD isoforms: mitochondrial SOD (manganese-requiring) and cytosolic SOD and extracellular SOD (both requiring copper and zinc). Mucosal injury mediated by H2O2 can be mitigated by SOD activity in the gastrointestinal tract65. Indeed, increased SOD activity is associated with mucosal healing of human gastric ulcers, whereas reduced SOD correlates with increased ulcer severity66.
Conversion of glutathione into oxidized glutathione is performed by the glutathione peroxidase (GPX) enzyme system. In this process, H2O2 is enzymatically reduced to H2O (REF.67). Within the human gastrointestinal tract, expression of GPX1 is ubiquitous, but GPX2 is expressed specifically in epithelial cells68 and is postulated to protect the mucosa from transporting luminal-derived lipid hydroperoxides69. Deletion of either Gpx1 or Gpx2 in mice had no phenotypic effect, but double-knockout mice develop spontaneous colitis70. Dismutation of H2O2 can also be achieved by the enzyme catalase, which converts 2H2O2 to 2H2O and O2 (REF.71). Peroxiredoxins (PRDXs) are another important family of thiol-specific antioxidant enzymes, designated PRDX1–6 and encoded by six different genes (reviewed extensively elsewhere72). It is notable that little redundancy exists within this family of proteins, where the loss of individual PRDXs lead to numerous pathologies, including haematological disorders, tumours and increased susceptibility to diseases associated with oxidative stress73. Somewhat surprisingly, mice deficient in PRDX2 and PRDX6 are protected from acute colitis74,75. Although not completely clear, the mechanism of PRDX2-mediated protection might involve ROS-dependent stability of fork-head box protein O1 (FOXO1) and FOXP3 regulatory T cell development.
NRF2 is a crucial regulator of the antioxidant response. NRF2 forms heterodimers with small MAF proteins and binds to antioxidant response elements in the regulatory region of promoters of cytoprotective and antioxidant enzymes, regulating de novo transcription. Kelch-like ECH-associated protein 1, an adaptor subunit of cullin 3 ubiquitin ligase, regulates the function of NRF2 by acting as a redox sensor (reviewed elsewhere76). Thus, antioxidant pathways provide an equally important and substantial balance to redox signalling responses in the gastrointestinal tract.
Redox signalling in the immune system
Active mucosal inflammation can rapidly deplete both nutrients and oxygen in the immediate environment. For example, when activated, PMNs can increase their O2 demand by as much as 50-fold in the generation of ROS (the so-called respiratory burst mediated by NOX) necessary to kill microorganisms following phagocytosis77. By contrast, proliferating T cells only moderately increase oxygen consumption during immune responses78. Mucosal tissues possess both the ability to generate and attenuate redox signals; however, it is widely accepted that in the context of inflammation, the majority of radicals and reactive species are derived predominantly from the activity of resident and infiltrating immune cells, in particular, professional phagocytes of the innate immune system, such as neutrophils, monocytes, macrophages, dendritic cells and mast cells.
NADPH oxidases and ROS.
The plasma-membrane NOX family of enzymes are a group of paralogous enzymes, sharing common subunits. The complexes are made up of both membrane and cytosolic protein subunits that, upon activation, organize in the membrane to catalyse the conversion of O2 to O2·− (REF.79). The spectrum of NOX-mediated activity ranges from potent bactericidal capacity of professional phagocytes to critical intracellular signalling in numerous cell types.
In terms of enzymatic capacity, the redox factors produced by phagocyte oxidases and peroxidases exemplify the extreme end of the redox spectrum. In addition to phagocytes expressing NOX2, fibroblasts, endothelial and epithelial cells all express enzymes that enable generation of ROS, including NOX1, NOX3, NOX4, NOX5, as well as dual oxidases DUOX1 and DUOX2 (REF.80). DUOX2 and NOX4 are expressed throughout the human gastrointestinal tract, whereas NOX1 expression is highest in the distal colon, where it is restricted to the cytosol, presumably to transduce intracellular signalling28. By comparison, DUOX2 is expressed on the apical surface of epithelia, ostensibly enabling luminal secretion of ROS81. Others have examined the influence of NOX1-derived or DUOX2-derived ROS on Campylobacter jejuni infection and discovered that ROS impaired bacterial capsule formation and virulence by altering C. jejuni gene expression82.
ROS and innate immunity.
Innate immune cells, including neutrophils, macrophages and dendritic cells, represent the front line of immune surveillance and defence, and generation of ROS is a crucial microbicidal mechanism used by these cells. Activation of the NOX complex in innate immune cells elicits a rapid and potent respiratory burst83. Defects in phagocyte NOX function, such as in patients with chronic granulomatous disease (CGD), lead to leukocytes capable of phagocytosing but with impaired bacterial clearance84. The hallmark of CGD is recurrent bacterial and fungal infections. Typically, ~40% of patients with CGD develop IBD-like symptoms85.
Following their recruitment to sites of inflammation, monocytes can polarize into either classically activated (M1) or alternatively activated (M2) macrophages, depending on the redox state and cytokine milieu of the mucosa86. Typically, TNF and IFNγ are accepted to elicit an M1 phenotype and T helper type 2 cytokines result in M2 polarization; however, it is also apparent that macrophage phenotypes can be mixed87. These differentially polarized macrophages exhibit a spectrum of functionalities. The M1 phenotype is regarded as pro-inflammatory and characterized by expression of iNOS and, consequently, these cells are an important source of RNS88. M2 macrophages are thought to demonstrate various activities ranging from wound healing (release of transforming growth factor-β) to suppressing T cell function11. Expression of the enzyme arginase 1 by M2 macrophages depletes l-arginine, resulting in a downregulation of the T cell receptor (TCR) ζ chain89, impairing T lymphocyte function and resulting in immunosuppression. Aside from suppressing T cell function, ROS also contribute to regulatory T cell polarization and function90,91, but the exact molecular mechanisms have yet to be elucidated. Taken together, the net influence of ROS in macrophage polarization might promote a state of immune tolerance as it relates to regulation of T cell function.
Host–microbial interactions and ROS
The mammalian large intestine is host to trillions of bacteria, viruses and fungi, collectively termed the microbiota. A finely balanced mutualism exists within the intestinal mucosa, in which microorganisms, essential for host health, might also initiate and perpetuate mucosal disease92. The epithelium that lies juxtaposed to the mucosal immune system serves as a selective conduit between the host and microbial world. Recognizing that both the host and the gut microbiota (both commensals and pathogens) can generate a variety of ROS, the contribution of redox signalling to such interactions has emerged as a critical interface to host–microorganism interactions in the gut (FIG. 2).
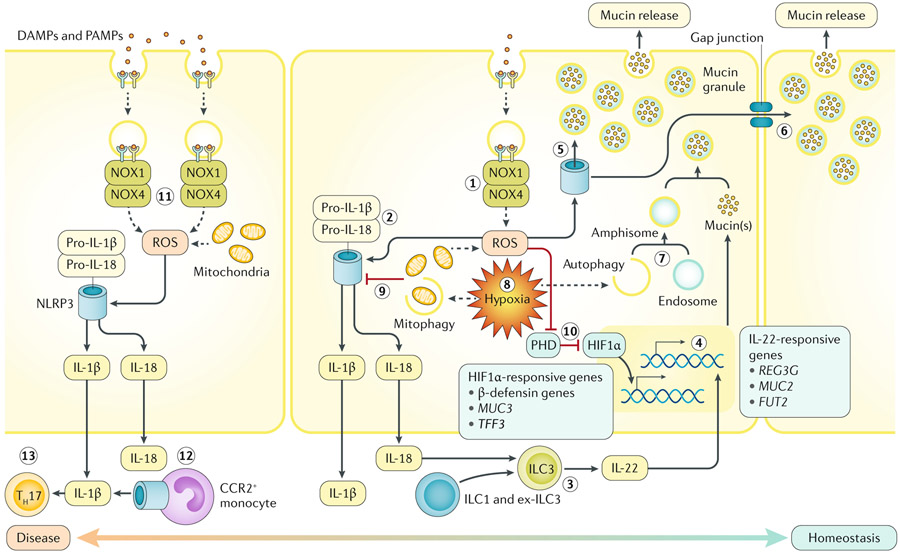
Fig. 2 ∣. Host redox–hypoxia crosstalk in the gastrointestinal mucosa.
The two major sources of endogenous reactive oxygen species (ROS) within the intestinal epithelium originate from mitochondria and NADPH oxidase 1 (NOX1) or NOX4 (step 1). In response to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), epithelia recruit and activate the NOX1–NOX4 complex, stimulating superoxide and hydrogen peroxide generation (sources of ROS). Both enzymatic and mitochondria-derived ROS can trigger the activity of epithelial inflammasomes. In colonic epithelia, ROS-stimulated NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome assembly leads to IL-18 (and IL-1β) production (step 2). Although excessive mature secreted IL-18 is detrimental to epithelial integrity, the presence of IL-18 is necessary for IL-22 release by type 3 innate lymphoid cells (ILC3s) (step 3). ILC3-derived IL-22 promotes mucosal barrier protection by inducing mucin synthesis and goblet cell function (step 4). In goblet cells, ROS triggers the NLRP6 inflammasome to elicit mucin granule secretion (step 5). Sentinel goblet cells responding to microbial triggers can signal to adjacent goblet cells to degranulate via gap junctions (step 6). A combination of autophagic proteins, endosomes and NOX-derived ROS are necessary for mucin granule formation in goblet cells (step 7). Both autophagy and mitophagy are induced by hypoxia (step 8). Mitophagy might decrease NLRP3 inflammasome activity, reducing processing of IL-1β and IL-18 (step 9). Inhibition of prolyl hydroxylase enzymes (PHD) by ROS or hypoxia stabilizes hypoxia-inducible factor-1α (HIF1α), regulating barrier protective genes (step 10). Unimpeded or excessive ROS generation during active inflammation can lead to abundant maturation of IL-1β or IL-18 or even inflammasome-mediated cell death (necroptosis and pyroptosis) (step 11). Inflammasome-activation of infiltrating CC-chemokine receptor 2 (CCR2)+ monocytes contributes to active IL-1β (step 12). Mucosal IL-1β may lead to accumulation of IL-17A-secreting immune cells, such as T helper 17 cells (TH17) (step 13).
Resident gut microbiota and ROS.
A number of studies from the Neish group have highlighted a beneficial influence of probiotic and resident microorganisms in eliciting ROS generation from epithelial sources93 (FIG. 1). In both Drosophila melanogaster and mouse models, Lactobacillus spp. were shown to induce epithelial-derived ROS via NOX1 activity, which stimulated epithelial proliferation94. Subsequent studies by this group demonstrated ROS signalling dependence on the redox-sensitive transcription factor NRF2 through mechanisms that involve cytoprotection and decreased epithelial apoptosis95. Further studies from this group and others have elegantly implicated a role for epithelial-expressed formyl peptide receptor (FPR), responding to microbial N-formyl-methionine-leucine-phenylalanine, in intestinal epithelial wound healing96,97. This wound healing response was found to occur through oxidative inactivation of the regulatory phosphatases PTEN and PTP-PEST, with associated activation of focal adhesion kinase and paxillin98. Central to such mucosal wound healing responses seems to be the regulation of epithelial cell migration. For example, redox sensitive tyrosine phosphatases (for example, SE1P2 and LMW-PTP) that are expressed at the edge of wounded epithelial monolayers are crucial to the organization of focal adhesions that organize epithelial migration and wound closure99. Likewise, the FPR ligand annexin A1 (ANXA1) activates the generation of ROS through NOX1 and activates focal adhesion kinases necessary for wound healing and tissue resolution98. Loss and gain of function studies have shown that both ANXA1-null and NOX1-null mice show substantial deficits in mucosal wound healing responses and that ANXA1 delivery promotes wound healing98. These investigators have also demonstrated that FPR-mediated or NOX2-mediated ROS generation in mice at local intestinal tissue sites selects for mucus-resident microorganisms, including Akkermansia muciniphila, that accelerate epithelial wound healing in an intestinal epithelial NOX1-dependent fashion100. Although not completely clear, this wound healing response seems to require ERK phosphorylation within the epithelium.
ROS and pathogen niche expansion.
Similar to resident gut microorganisms, opportunistic pathogens also use redox reactions to subvert host defences and establish a niche. One of the most studied in this regard is the invasive enteric pathogen Salmonella enterica subsp. enterica serovar Typhimurium. This pathogen is associated with acute gastrointestinal inflammation and diarrhoea, and elicits neutrophil chemotaxis into the mucosa101. Invasion is achieved through two type III secretion systems that facilitate S. Typhimurium to enter and persist inside intestinal epithelial cells and mucosal macrophages101. Before invasion, S. Typhimurium must outcompete the resident gut microbiota. Some studies indicate that inflammation amplifies proliferation of luminal S. Typhimurium, enabling it to overgrow other microorganisms102. In one report, the Bäumler group demonstrated that inflammation-induced intestinal ROS reacted with luminal thiosulfate to form a new respiratory electron acceptor, tetrathionate103. Moreover, S. Typhimurium express genes to enable utilization of tetrathionate as an electron acceptor that enables the pathogen to use respiration to outcompete fermenting microorganisms and establish a niche103. The authors subsequently demonstrated that this tetrathionate-enabled respiration provided another growth advantage to S. Typhimurium, enabling the utilization of epithelial-derived ethanolamine under anaerobic conditions104.
ROS and pathogen niche restriction.
The role of H2O2 secretion into the lumen of the gut is poorly understood, but several roles have been proposed. Some studies suggest a pro-inflammatory function for DUOX-derived H2O2, acting as a chemotactic signal for neutrophils in a zebrafish wound healing model105 and a mouse allergic airway model106. Other findings suggest that apical secretion of H2O2 into the lumen of the gut is implicated in restricting Helicobacter felis colonization in mice through increased bacterial oxidative stress107. Another study examined the influence of NOX1-derived or DUOX2-derived ROS on C. jejuni infection and discovered that ROS impaired bacterial capsule formation and virulence by altering C. jejuni gene expression82. During Citrobacter rodentium infection in wild-type mice, the Knaus group discovered that NOX1 regulates DUOX2 expression in the mucosal epithelium, with a resultant decrease in both O2·− and H2O2 production108. An unexpected but intriguing finding from this study was that ablation of epithelium-derived ROS, using an epithelium-restricted CYBA-deficient mouse (absence of the obligatory NOX dimerization partner), led to protection from colitis induced by C. rodentium. The authors attribute their findings to an altered gut microbiota with an expansion in H2O2-producing lactobacilli, which exert antimicrobial activity through release of urease, lactic acid and H2O2 (REF.109). Pircalabioru et al.108 demonstrated through the use of catalase to degrade H2O2 that H2O2-producing lactobacilli were responsible for attenuating C. rodentium virulence factors. Other findings to support H2O2 exerting an antimicrobial function include disruption of microbial intracellular signalling, which affects antioxidant defence and polysaccharide biosynthesis110. In the human body, l-amino acids are essential for protein synthesis; however, d-amino acids function in necessary non-ribosome-based roles111. Bacteria synthesize and secrete distinct d-amino acids into the lumen of the colon112, and the Waldor group demonstrated that microbiota-derived d-amino acids upregulate expression of the host epithelial-expressed enzyme, d-amino acid oxidase, which is secreted into the lumen. Oxidative deamination of d-amino acids by d-amino acid oxidase yields H2O2 as a by-product and protects from Vibrio cholerae pathogenicity113.
Consequences of redox signalling
Redox-sensitive signalling pathways are often limited by the availability of extracellular and intracellular oxygen114. Despite this understanding, ROS generation can occur at surprisingly low oxygen tensions. The neutrophil NOX, for example, is fully functional at ambient oxygen concentrations as low as 1%77. Such observations highlight the importance of differentiating oxygen and ROS diffusion within the tissue microenvironment, as well as the variability of oxygen availability within individual tissues. These differences often determine end-point tissue function and define the adaptability of tissues to hypoxic stress115.
Colonic tissue oxygen metabolism.
The partial pressure of oxygen (pO2) at sea level is ~145 mmHg, and the alveoli of healthy lungs experience a pO2 of ~110 mmHg (REF.116). The lumen of the colon is virtually anoxic, mainly due to the microbial biomass117, and colonic epithelia adjacent to the lumen experience and withstand a pO2 < 10 mmHg (REF.118). Thus, it might be surprising to discover that epithelial stem cells at the crypt base are highly oxygenated (experiencing a pO2 of ~100 mmHg)92. Such differences are compounded by epithelial metabolism and the arrangement of the microvasculature network with countercurrent blood flow dynamics in each villous structure92. Epithelia adjacent to the lumen effectively exist in a state of physiological hypoxia119 and are uniquely susceptible to changes in the redox state. Experimental use of oxygen-sensitive nitroimidazole compounds, such as pimonidazole, has enabled visualization of hypoxia in these tissues both basally and during inflammation120. It is notable that these dyes are neither dependent on redox enzymes nor changed by NADH and NADPH levels121. This technology, coupled with immunostaining, has been used to visualize oxygenated (or lack thereof) regions of large solid tumours in rats122. It is notable that pimonidazole adducts might serve as a more reliable marker than staining for HIF1, as it is retained in chronically hypoxic cells123. Such physiological hypoxia (FIG. 1) is reversible by oxygenation of the colonic lumen (for example, using oxygenated perfluorodecalin)124.
Adaptive responses to hypoxia involve the stabilization of HIF, a master regulator of oxygen homeostasis12. Prolyl hydroxylase (PHD) enzymes continually utilize cellular O2, in conjunction with 2-oxoglutarate and iron, to target HIF for hydroxylation and subsequent ubiquitylation and degradation125. Various factors in addition to limited O2 availability influence the activity of the PHD enzymes and HIF stabilization. Among them, H2O2 has been demonstrated to stabilize HIF via inhibition of the PHD2 enzyme126. Transcriptional activity of the HIF transcription factors regulates the genes involved in adaptive responses to hypoxia, the most widely acknowledged include angiogenesis-related and glycolysis-related genes (for example, VEGFA, NOS2, SLC2A1 and PGK1). Less well characterized, but rapidly increasing in number, are genes with associated mucosa-protective functions that enable colonic epithelial cells to restore impaired barrier function (for example, TFF3, MUC3A, CLDN1 and ABCB1)118. Original studies using genetic loss and gain of intestinal epithelial Hif1a expression in mice revealed a protective role for HIF in chemically-induced colitis models that corresponded to mucosal barrier protection120. Studies with cultured intestinal epithelial cells exposed to conditions that activate HIF have identified the regulation of a number of barrier-related protective genes127 that have now been validated in animal models of colitis120,128-132 and in human-derived colonic tissue8,133-135. The intestinal epithelial barrier proteins encoded by HIF target genes include those that localize to the apical surface of polarized epithelia, including mucins and mucin modifiers (for example, intestinal trefoil factor), tight junction proteins (claudin 1), antimicrobial peptides and proteins important for xenobiotic clearance118. Each of these components is a direct transcriptional target of HIF and contributes fundamentally to the 3D intestinal tissue architecture that forms an intact barrier during homeostasis.
Induction of epithelial HIF.
Following recruitment of immune cells to the mucosa, for instance following induction of experimental colitis, hypoxia extends deeper into tissue120, a phenomenon termed inflammatory hypoxia136 (FIG. 1). A study by Campbell et al.8, demonstrated that during experimental murine colitis, neutrophil influx was primarily responsible for inflammatory hypoxia. By use of a combination of neutrophil antibody depletion, hypoxia-reporter mice and NOX2-deficient (Gp91phox−/−) mice, the authors demonstrated that functional NOX2 not only disseminated mucosal hypoxia but also stabilized HIF within the intestinal epithelium. This HIF signature within the epithelium resulted in an adaptive transcriptional response, that the authors coined transcriptional imprinting8. Biopsy samples from patients with ulcerative colitis with evidence of crypt abscess formation — a pathological hallmark of transmigrated neutrophils within the lumen of the crypt — revealed induction of HIF (monitored by increases in the HIF-target gene SLC2A1). However, it is unclear from these studies if HIF stabilization is due to depletion of oxygen or generation of O2·− or H2O2, as all are capable of inhibiting the PHD enzymes8,79.
Another means to stabilize HIF by inhibition of PHD enzyme function is via depletion of another crucial cofactor: iron. Some findings indicate that certain microorganisms, such as Klebsiella pneumoniae and Pseudomonas aeruginosa, can stabilize HIF in lung epithelia via secretion of low-molecular-weight, high-affinity iron chelators, termed siderophores137,138. Presumably, these factors function to chelate iron bound within the active site of the PHD enzymes, although this has not been shown conclusively. Fermenting microbiota have also been shown to stabilize HIF in the colon, via SCFA release, particularly via butyrate production139. Butyrate is used as the preferred enterocyte fuel source, oxidizing butyrate to CO2 (REF.140). The net effect is epithelial hypoxia owing to increased oxygen consumption, likely resulting in PHD enzyme inhibition to facilitate HIF stabilization139. Indeed, a study from the Bäumler group in 2016 demonstrated that streptomycin-treated mice exhibited a decline in butyrate-producing clostridia, which led to increased oxygenation of the mucosal epithelium, enabling enhanced Salmonella expansion141.
Inflammasome activation.
The NLRP3 inflammasome is an intracellular multiprotein complex involved in perceived cellular danger response142. Pathogen-associated molecular patterns and host-derived danger-associated molecular patterns can trigger inflammasome activation143. Stimulation of NLRP3 leads to assembly of this inflammasome complex and, ultimately, to caspase-1 activation and downstream cleavage of pro-inflammatory cytokines IL-1β and IL-18 (REF.144). The role of IL-1β is widely studied in autoimmune diseases; however, in gastrointestinal inflammation, its involvement is not fully understood. Studies using mouse models of chronic colitis have demonstrated a role of IL-1β in accumulation of IL-17A-secreting CD4+ T helper 17 cells145. One study in 2017 by Neudecker et al.146 implicated CC-chemokine receptor 2 (CCR2)+ monocytes and NLRP3 activity leading to IL-1β production in the pathogenesis of acute colitis in mice. Surprisingly, mice lacking NLRP3 are hypersensitive to experimental colitis, displaying exacerbated immune infiltration and epithelial damage, primarily due to a loss of IL-18 (REF.147). Despite the intense interest in the field, relatively little is known about how the NLRP3 inflammasome is regulated at a molecular level. Some studies suggest that autophagy can negatively regulate the NLRP3 inflammasome148. Conversely, activation of ROS, ostensibly by NOXs149, has been shown to stimulate the NLRP3 inflammasome. However, patients with CGD with NADPH-deficient macrophages display normal inflammasome activation in several studies150,151, implicating other stimuli aside from NOX-derived ROS. Another abundant source of cellular ROS are mitochondria, which release ROS in response to elevated metabolism, hypoxia or membrane damage152. The Tschopp group demonstrated that inhibition of mitophagy (encapsulation and degradation of old or damaged mitochondria via cellular autophagic machinery) results in the accumulation of damaged ROS-generating mitochondria, which leads to NLRP3 inflammasome activation153. The authors subsequently demonstrated that both ROS generation and NLRP3 activation were suppressed when mitochondrial activity was disrupted by blockade of the voltage-dependent anion channel. These findings suggest that the NLRP3 inflammasome can perceive mitochondrial dysfunction153.
Goblet cell mucus secretion.
Goblet cells are specialized epithelial cells that protect the barrier from microbial invasion by secretion of a mucus hydrogel154. The principal components of mucus are large mucin peptides arranged in polymeric structures. Following translation, mucins undergo extensive N-linked and O-linked glycosylation modifications and are packaged into vesicles4. Goblet-cell-derived glycosylated mucins provide a major carbon source for the gut microbiota (reviewed elsewhere5). Interest has centred on understanding the regulation of mucin packaging and secretion at baseline and in response to microorganism detection155, which has led to the suggestion that goblet cells are actually an unappreciated and distinct innate immune cell4,156. Another secretory epithelial lineage, Paneth cells, which are tasked with antimicrobial peptide secretion and defence of the intestinal stem cell niche, rely on autophagy to organize secretory granules157. By contrast, autophagy-compromised goblet cells displayed normal mucin packaging into granules158. However, a combination of autophagy and NOX-derived ROS were found to be essential for mucin release by goblet cells (FIG. 2). Patel et al.158 demonstrated that amphisome-like vesicles form in goblet cells following autophagosome and endosome fusion and these specialized organelles regulate mucin secretion. It was subsequently demonstrated that the NLRP6 inflammasome is crucial for promoting goblet cell mucin release in response to proximity with microorganisms159. In 2016, the Hansson group proposed the existence of a ‘sentinel’ goblet cell160 based on proximity to the crypt entrance. This sentinel goblet cell nonspecifically endocytoses and responds to Toll-like receptor ligands, stimulating NOX1-dependent or DUOX2-dependent ROS production, through downstream activation of the NLRP6 inflammasome. Moreover, via intercellular gap junctions, signals are transduced down the crypt axis to elicit mucin secretion from neighbouring goblet cells160.
Secretion of additional mucins in response to detection of microbial proximity is obviously one approach to repel a microbial onslaught, but goblet cell hyperplasia is an alternative mechanism. As mucus erosion and goblet cell depletion are pathological hallmarks of ulcerative colitis161, repletion of both goblet cells and their mucin granule contents is necessary for epithelial barrier restitution. As mentioned previously, IL-18 secretion is stimulated by ROS-mediated inflammasome activation144, where some findings reveal distinct and opposing roles for IL-18 and IL-22 signalling in regulating goblet cell homeostasis. For example, the Flavell group demonstrated, using various intestinal-epithelial-specific knockout mice to target IL-18 signalling, that excess IL-18 promotes goblet cell depletion and colitis162. Moreover, IL-18 seems to suppress goblet cell differentiation markers. Contrastingly, immune-cell-derived IL-22 has well-recognized protective mucosal effects via promoting stem cell differentiation, mucin synthesis (mucin 2), antimicrobial peptide (REG3γ) and goblet cell function (Fut2 expression)163-165. The recently characterized type 3 innate lymphoid cells (ILC3s) are the major source of IL-22 within the intestinal mucosa166. In fact, during Toxoplasma gondii infection, ILCs and T cells required epithelial-derived inflammasome-processed IL-18 in order to release IL-22 (REF.167). Thus, a combination of redox signalling, inflammasome activity and immune crosstalk might hold the key to homeostasis between IL-18 and IL-22 signalling and indeed mucosal-microbiota homeostasis. Moreover, IL-1β can both induce activation of ILC3s and contribute to plasticity (in concert with other factors, including retinoic acid) and reprogramming of ILC1s and ex-ILC3s to ILC3s168.
Redox signalling in intestinal disorders
Ischaemia–reperfusion injury.
Ischaemia is defined as insufficient blood flow to tissues, resulting in disruption of cell function and ultimately necrosis. Various tissue insults can lead to intestinal ischaemia, including trauma, stroke and atherosclerosis, and reperfusion (restitution of blood supply) following ischaemia can result in aggravated tissue damage. The intestine is particularly sensitive to IRI169. Ischaemia rapidly induces expression of cyclooxygenase (COX) and accumulation of cells expressing lipoxygenase enzymes, which are responsible for generating pro-inflammatory eicosanoids from membrane-liberated arachidonic acid, such as prostaglandins and leukotrienes170. Constitutively expressed COX1 and the inducible isoform COX2 are responsible for catalysing the conversion of arachidonic acid to prostaglandins171. The primary prostaglandin studied in this context is prostaglandin E2 (REF.172), which elicits a bifunctional influence on the intestinal mucosa, promoting injury via vasodilatory influences on the endothelium but simultaneously conferring cytoprotection to the intestinal epithelium173. Infiltrating leukocytes, recruited by endothelial-derived leukotriene B4, facilitate neutrophil adhesion, activation and degranulation174. Following reperfusion, a necessary substrate (O2) becomes available to enable the de novo synthesis of an ‘eicosanoid storm’, in which bioactive lipids of the eicosanoid family become substantially amplified in their production175. This combination of lipid mediator generation, complement activation176 and neutrophil accumulation in the tissue milieu results in another consequence of IRI: lipid peroxidation177 (the oxidative degradation of lipids that result in plasma membrane and organelle damage). ROS generated by re-oxygenated neutrophils have long been recognized as instigators of lipid peroxidation in intestinal IRI, resulting in barrier disruption49,135,169,177. Indeed, treatment with SOD in a murine intestinal IRI model limits the contribution of ROS to both lipid peroxidation and mucosal permeability177. Experimental strategies to circumvent the deleterious exaggerated inflammation and resultant tissue damage occurring in IRI mostly hinge on reducing neutrophil recruitment signals and leukocyte activation178. The anti-inflammatory influences of carbon monoxide (CO), derived from endogenous haem oxygenase, might be a promising therapeutic strategy to attenuate damage from IRI179. Multiple lines of evidence have revealed that the activation of haem oxygenase effectively promotes cytoprotection and inhibits the pro-inflammatory signatures elicited by multiple cell types during intestinal IRI179. Strategies to induce haem oxygenase and CO release are in development and include haem oxygenase 1 fusion proteins, small molecule haem oxygenase inducers, bilirubin, glutamine and inhaled CO179.
Ischaemic preconditioning.
Aside from leukocyte-derived sources of ROS, the mucosa itself can substantially contribute to redox-mediated damage during intestinal ischaemia. High concentrations of ATP are released extracellularly during ischaemia, which are ultimately catabolized to hypoxanthine180. Concomitantly, ischaemic stress results in the conversion of xanthine dehydrogenase to xanthine oxide181. Following tissue reperfusion, the combination of hypoxanthine, xanthine oxide and newly available O2 yields additional sources of tissue O2·− (REF.182). Although some limited therapeutic success has arisen from scavenging ROS or targeting inflammatory mediators, one of the more promising strategies to reduce IRI is ischaemic preconditioning (or hypoxic preconditioning), whereby cells or tissues are exposed to brief and intermittent periods of non-lethal ischaemia. Such treatments have been shown to protect organs that experience a major ischaemic event, which is best studied in the heart183. The mechanisms involved in ischaemic preconditioning are complex but ultimately result in reduced pro-inflammatory factors, decreased lipid peroxidation and elevated levels of natural antioxidants including glutathione, SOD and haem oxygenase 1 (REF.184). Khoury et al.185 identified extracellular adenosine released by hypoxic preconditioned intestinal epithelia as the major anti-inflammatory factor responsible for hypoxic preconditioning. This protective role of adenosine in ischaemic preconditioning corresponded with the inhibition of NF-κB via deneddylation (where NEDD8 is removed from a conjugated protein) of cullin 1, a component of the proteasomal degradation pathway important in the activation of NF-κB186. It was shown that adenosine might regulate HIF through similar mechanisms; for example, a small molecule deneddylator of the cullin family proteins has become commercially available. This compound, MLN4924, is an AMP analogue that functionally inhibits NEDD8-activating enzyme and results in the deneddylation of cullin 1 and cullin 2 (REFS187,188) and has proven to be a potent HIF stabilizer in cultured cells189. In this regard, HIF might function to promote tissue ischaemic preconditioning, which has been shown in some studies13, and small molecule stabilizers of HIF (especially PHD inhibitors) show promise in protection from damage associated with IRI190.
Extracellular adenosine is derived from the enzymatic degradation of ATP via the action of surface apyrases (for example, CD39 (also known as NTPDase 1)) and ecto-5′-nucleotidase (CD73)191. CD73 expression is increased on intestinal epithelia during hypoxia in a HIF-dependent manner, resulting in increased extracellular adenosine accumulation127. Moreover, HIF stabilization in hypoxia was also demonstrated to decrease expression of the equilibrative nucleoside transporter 1 and 2, resulting in reduced uptake of extracellular adenosine providing more available for extracellular signalling192. Extracellular adenosine signals through activation of any of four surface G-protein-coupled receptors. Activation of the adenosine receptors A1 or A3 results in decreased intracellular cAMP levels (Gαi-coupled), whereas adenosine binding to the high-affinity adenosine receptor A2a (A2AAR) or the low affinity adenosine receptor A2b (A2BAR) is associated with elevation of cAMP (Gαs-coupled)193. The predominant receptor-mediated signalling associated with intestinal epithelial cells is A2BAR, and the crystal structure of agonist-bound and antagonist-bound A2AAR has been resolved194. The majority of evidence suggests that the induction of A2BAR by HIF translates to a strong anti-inflammatory phenotype in the intestinal mucosa, at least in part associated with barrier protection195,196. These studies have shown important protective roles for A2BAR in experimental colitis195 and intestinal IRI197,198 that results in diminished inflammation199.
A number of sources of nucleotides exist in inflamed and ischaemic tissue. Many cell types actively release nucleotides, particularly in the form of ATP or ADP193. Programmed cell death (apoptosis) is associated with the generation of large amounts of ATP during ischaemia and inflammation. The ATP released by apoptotic cells has been demonstrated to function as a ‘find-me’ signal to promote phagocytic clearance during inflammatory resolution200. Other studies have shown that inflammatory cells, such as neutrophils, can release ATP in an active manner though connexin 43 (also known as GJA1) hemichannels201,202. Platelets release nucleotides at high concentration upon activation and are also an important source of extracellular nucleotides during ischaemia. In the intestinal mucosa, for example, platelets and neutrophils have been shown to co-migrate across the epithelium and into the lumen in the formation of crypt abscesses203. As originally described by Madara et al.204, this local generation of luminal nucleotides results in adenosine-mediated activation of electrogenic chloride secretion and associated water movement into the intestinal lumen. This fluid transport process provides an important flushing of the mucosal surface during ongoing inflammation.
Role of ROS in IBD.
IBD includes ulcerative colitis and Crohn’s disease and is characterized as a chronic inflammatory condition of the gastrointestinal mucosae in susceptible individuals54. Ulcerative colitis and Crohn’s disease exhibit distinct pathophysiology in terms of effector immune functions. Common features of IBD include abdominal pain and diarrhoea, and susceptibility to IBD is dictated by a combination of genetic, environmental and microbial risk factors54. The microenvironment of active IBD lesions is considered to be strongly redox active, in which ROS are considered to play an important part in both inflammatory signalling and in bystander damage to surrounding tissue29.
Does excessive ROS or insufficient ROS contribute to IBD? Evidence exists to support both excessive ROS and insufficient ROS as contributing to IBD. Considering the number and functional diversity of susceptibility genes in IBD identified by genome-wide association studies, it is likely that the answer to this question depends on the individual combination of aetiological factors and not merely the diagnosis of ulcerative colitis versus Crohn’s disease. For instance, a study of 157 patients with CGD profiled IBD risk alleles among this cohort and concluded that defective O2·− generation in CGD is a major risk factor for IBD205.
As alluded to earlier, the majority of patients with CGD develop IBD-like symptoms85. A potentially confounding issue for research in this field is the mouse models used to address the roles of phagocyte-derived ROS versus ROS from mucosal sources. Campbell et al.8, using a 2,4,6-trinitrobenzenesulfonic acid (TNBS) model of colitis, demonstrated that Nox2−/− mice develop substantially more severe colitis, reflected by increased weight loss, increased intestinal permeability and the failure to resolve ongoing inflammation compared with wild-type control mice. Conversely, Bao et al.206 used the same mice in a dextran sulfate sodium (DSS) model of colitis and found no difference in weight loss or disease severity compared with wild-type controls. They concluded that less tissue damage was associated with decreased oxidative burst, though no evidence was provided for increased ROS-mediated damage. A possible explanation for the discrepancy between these studies is in the nature of the models used to ascertain the relative importance of phagocyte NOX. DSS models of colitis rely on denudation of epithelial cells, beginning with erosion of apical mucus, apoptosis of epithelia and resulting innate immune infiltrate207. It could be argued that, under such circumstances, phagocyte-derived ROS might not be contributing to denudation of colonic epithelia; thus, only genes or therapeutic intervention that influence epithelial viability or turnover will have an appreciable effect. By contrast, TNBS models involve pre-sensitizing the host immune system to haptenized microbial antigens, with subsequent colonic exposure to the haptenizing agent207. DSS results in progressive tissue damage, extending proximally from the rectum, and incremental loss of body weight over the course of the experiment. TNBS-treated animals lose and regain weight rapidly over time and tend to exhibit skip lesions with relatively intact epithelia207. Moreover, immune infiltrates and inflammatory mediators differ substantially between the models207. As such, the DSS model represents a wound model, whereas the TNBS model represents an acute-to-chronic inflammation and resolution model. Thus, it is conceivable that Nox2−/− mice do not exhibit enhanced mucosal wounding but rather fail to resolve inflammatory insults.
Despite the dependence of IBD on genetic susceptibility and observed chronic adaptive immune responses, numerous aspects of disease progression are comparable between IBD and IRI. For instance, pro-inflammatory mediators such as TNF and prostaglandin E2 are implicated in both IRI and Crohn’s disease49,208. Involvement of neutrophils, monocytes and leukocyte-derived ROS have been implicated in both ulcerative colitis and IRI in the colon and intestine49,209. Similarly, epithelial barrier disruption and enhanced microbial translocation are features of both IBD and IRI49,210. Studies have also suggested that shifts in the gut microbiome (dysbiosis) might contribute to both IRI and IBD49,211. One important caveat to this understanding is the observation that antibody-mediated neutrophil depletion strategies in intestinal IRI models do not seem to influence injury end points212, whereas neutrophil depletion substantially enhances tissue damage in multiple colitis models213. Another common feature between IBD and IRI models is that accumulation of extracellular ATP in colitis models has been demonstrated to contribute to the inflammatory process, in part via stimulation of the P2X7 receptor214. Also similar to ischaemic preconditioning is that extracellular adenosine seems to confer mucosal protection during colitis, principally via A2BAR signalling. Indeed, murine models of whole-body and conditional epithelial deletion of A2BAR results in more severe DSS-induced colitis associated with decreased barrier function and diminished mucosal wound healing195,196.
ROS-induced collateral tissue damage.
Substantial evidence exists that collateral tissue damage, the bystander effect, might result from increased oxidative stress associated with active intestinal inflammation215. Implications of excessive ROS-mediated tissue damage in the gastrointestinal tract include alterations of absorptive function, barrier dysfunction and dysmotility216. Numerous studies have, for example, indicated malabsorption of nutrients in the intestine following IRI and in IBD217,218. By contrast, colonic epithelia are tasked predominantly with the re-uptake of water from the faecal stream, so disruption to colonic absorption in IBD manifests as diarrhoea219. Extensive tissue damage from excessive ROS (for example, via lipid peroxidation or protein chlorination of mucosal barrier proteins) and from immune mediators, such as TNF and IFNγ, increases mucosal permeability by modulating tight junction function220 (FIG. 3). One well-documented mechanism of barrier disruption is via induction of so-called ‘leaky’ claudin tight junction proteins, such as claudin 2 and claudin 5 (REF.221). Under such conditions, so-called claudin switching occurs in which physiologically distinct tight junctions, defined by their constellation of claudin components, determine the structural integrity of the epithelial barrier221. It is also notable that increases in vascular permeability might precede increases in epithelial permeability during active mucosal inflammation. Tolstanova et al.222 used four murine models of colitis to demonstrate that early endothelial damage resulted in perivascular oedema and epithelial hypoxia, which contributed to the stabilization of HIF within the mucosa. Evidence for early ROS-mediated endothelial damage were demonstrated at the level of electron microscopy and were consistent in genetic models of colitis, including Il10−/− and Gnai2−/− animals (REF.222). Finally, it is likely that gut motility is affected by redox-sensitive mechanisms. For example, Brown et al.223 showed that enteric neuron death during active colitis was mediated by NO derived from glial cells. This neurotoxic activity was driven by NO influences on connexin 43 activity. Moreover, exposure of submucosal smooth muscle cells to microbial lipopolysaccharide probably contributes to dysmotility through the generation of large amounts of ROS and RNS224. Taken together, these studies implicate collateral tissue damage associated with oxidative stress in active intestinal inflammation.
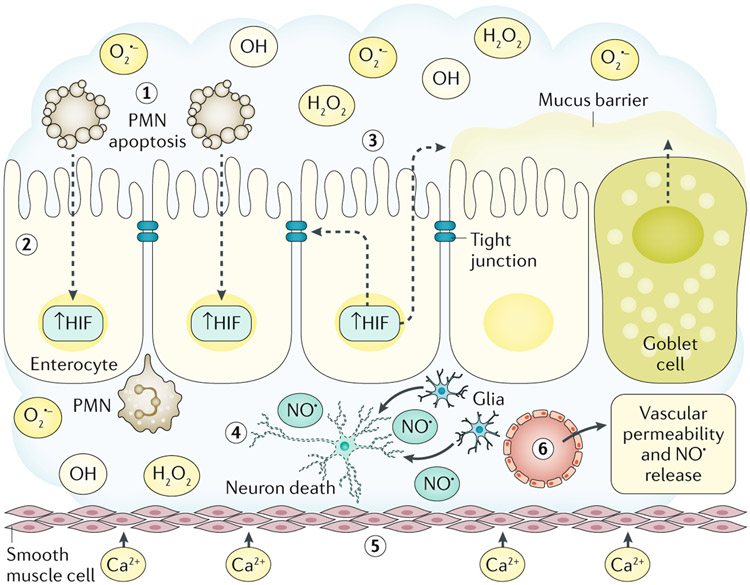
Fig. 3 ∣. ROS collateral damage and gastrointestinal disease.
During active inflammation, reactive oxygen species (ROS; O2·−, OH· and H2O2) and reactive nitrogen species generated in the local microenvironment cause collateral tissue damage. Activated, transmigrating polymorphonuclear leukocytes (PMNs) consume large amounts of O2 in the generation of ROS in the local milieu (step 1). Under these conditions, PMN ROS generation is limited by rapid induction of PMN apoptosis. Such O2 consumption results in localized hypoxia and the stabilization of epithelial hypoxia-inducible factor (HIF) (step 2). Epithelial HIF stabilization activates a cascade of gene transcription that promotes expression of barrier protective function genes (for example, TFF3, ABCB1 and CLDN1) and mucins in goblet cells (step 3). Within the lamina propria, activation of glial cell inducible nitric oxide synthase and the generation of nitric oxide (NO·) leads to enteric nerve cell death, resulting in intestinal dysmotility (step 4). Smooth muscle responses to oxidative stress include increased Ca2+ permeability that perpetuates intestinal dysmotility (step 5). An early event in acute mucosal inflammation within the gastrointestinal tract is increased vascular permeability through the generation of NO· by multiple cell types, such as smooth muscle cells, endothelial cells and enteric glia (step 6).
Conclusions
The gastrointestinal tract represents a particularly austere environment for redox-sensitive signalling. The combination of multiple sources of ROS or RNS in the setting of trillions of microorganisms requires the presence of important gatekeeper mechanisms to avoid the potential chaos that could occur during active inflammation. Just as important is the need to maintain a well-poised antimicrobial environment, in large part driven by epithelial and leukocyte-derived oxygen radicals. It is notable that the profound differences in local O2 tension within mucosal tissues and substantial increases in energy demand during inflammation provide a unique setting to understand tissue metabolism under stress. Of particular interest is the metabolic shift toward hypoxia and the associated stabilization of HIF target pathways that associates with tissue barrier function, wound healing, autophagy and inflammation resolution. Redox signals derived from immune cells, parenchymal cells (epithelia, endothelia and fibroblasts), as well as the gut microbiota, are coupled with the differing potencies, toxicities and half-lives of the redox products produced locally that require tight control for tissue homeostasis. Studies in vitro and in vivo have provided new insights toward a better understanding of productive inflammatory responses and mechanisms that promote inflammatory resolution. Also relevant is the shift in tissue redox potential that mediates collateral tissue damage and end-point organ function. A better understanding of the basic molecular signals, transcriptional programmes and the environmental cues that regulate mucosal redox state (BOX 1) are likely to provide new insight into the development of novel therapies for diseases such as IBD.
Box 1 ∣. Knowledge gaps and future research directions.
Which host metabolic factors control the redox state threshold and under what conditions are they generated?
Could microbiota-derived factors that influence redox state be enriched to benefit the host in health or during disease?
Does the low oxygen partial pressure environment of the gut provide an opportunity for drug targeting and/or drug delivery?
How does overall tissue redox state influence acute inflammatory resolution versus progression toward chronic inflammation?
Is innate immunity more amenable to therapeutic targeting than adaptive immunity, or vice versa?
Is pharmacological stabilization of hypoxia-inducible factor (for example, via prolyl hydroxylase inhibition) a viable therapeutic option for mucosal disease?
Which pharmacological approaches best mimic ischaemic preconditioning and under which circumstances might these approaches benefit the host?
For therapeutic targeting of redox pathways, how might we maximize the beneficial influence of redox signalling and minimize bystander tissue damage?
Key points.
Immune cells, microorganisms and the epithelium all generate and respond to redox signals in the colonic mucosa during homeostasis and in disease.
Redox signals, particularly H2O2, are generated by the host and the gut microbiota to impede overgrowth of opportunistic pathogens; similarly, certain pathogens utilize these systems to subvert host defences.
Host responses to reactive oxygen species (ROS) produced in situ and hypoxia act in concert and opposition to regulate homeostasis in the gut.
Host–immune and host–microbiota crosstalk can both contribute to excessive ROS production, participating in collateral damage at the tissue level.
Acknowledgements
E.L.C. is supported by NIH grant DK103639, and S.P.C. is supported by NIH grants DK50189, DK104713, DK095491, DK103712 and a Merit Award from the Veterans Administration.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
PubMed was searched from 1999 to 2017 for articles using the terms: “reactive oxygen species”, “hydrogen peroxide”, “hypoxia”, “microbiota”, “mucosa” and “epithelium” alone or in combination. Articles in English were considered on the basis of their relevance to this article’s topic. The reference lists of articles were crosschecked for additional references.
References
- 1.Luissint AC, Parkos CA & Nusrat A Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151, 616–632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crosnier C, Stamataki D & Lewis J Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet 7, 349–359 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Clevers HC & Bevins CL Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol 75, 289–311 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Johansson ME & Hansson GC Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol 16, 639–649 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tailford LE, Crost EH, Kavanaugh D & Juge N Mucin glycan foraging in the human gut microbiome. Front. Genet 6, 81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CT & Colgan SP Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol 17, 774–785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colgan SP, Dzus AL & Parkos CA Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J. Exp. Med 184, 1003–1015 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell EL et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SY, Ko HJ & Kweon MN Mucosal dendritic cells shape mucosal immunity. Exp. Mol. Med 46, e84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, He C, Cong Y & Liu Z Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 8, 969–978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies LC, Jenkins SJ, Allen JE & Taylor PR Tissue-resident macrophages. Nat. Immunol 14, 986–995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL Oxygen sensing, homeostasis, and disease. N. Engl. J. Med 365, 537–547 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol 9, 47–71 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Maxwell PH et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Tanimoto K, Makino Y, Pereira T & Poellinger L Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19, 4298–4309 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lando D, Peet DJ, Whelan DA, Gorman JJ & Murray LW Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Sender R, Fuchs S & Milo R Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14, e1002533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCracken VJ & Lorenz RG The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol 3, 1–11 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK & Knight R Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamer HM et al. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther 27, 104–119 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Machiels K et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Eeckhaut V et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62, 1745–1752 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Sokol H et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis 15, 1183–1189 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Tremaroli V & Backhed F Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Jones RM & Neish AS Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med 105, 41–47 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B & Gutteridge JM Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186, 1–85 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni AC, Kuppusamy P & Parinandi N Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal 9, 1717–1730 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Bedard K & Krause KH The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev 87, 245–313 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Biasi F, Leonarduzzi G, Oteiza PI & Poli G Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal 19, 1711–1747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bals R Epithelial antimicrobial peptides in host defense against infection. Respir. Res 1, 141–150 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroeder BO et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Raschig J et al. Ubiquitously expressed human β defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLOS Pathog. 13, e1006261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer RM, Ferrige AG & Moncada S Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Palmer RM, Ashton DS & Moncada S Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333, 664–666 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Stark ME, Bauer AJ, Sarr MG & Szurszewski JH Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology 104, 398–409 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Stark ME & Szurszewski JH Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology 103, 1928–1949 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Sessa WC et al. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J. Biol. Chem 267, 15274–15276 (1992). [PubMed] [Google Scholar]
- 38.Xie QW et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256, 225–228 (1992). [DOI] [PubMed] [Google Scholar]
- 39.Bredt DS et al. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351, 714–718 (1991). [DOI] [PubMed] [Google Scholar]
- 40.Beckman JS, Beckman TW, Chen J, Marshall PA & Freeman BA Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl Acad. Sci. USA 87, 1620–1624 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckman JS & Koppenol WH Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol 271, C1424–1437 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Poyton RO, Castello PR, Ball KA, Woo DK & Pan N Mitochondria and hypoxic signaling: a new view. Ann. NY Acad. Sci 1177, 48–56 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Riley PA Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol 65, 27–33 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Conklin KA Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther 3, 294–300 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Church DF & Pryor WA Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect 64, 111–126 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JC et al. Smokers as a high-risk group: data from a screening population. J. Clin. Gastroenterol 43, 747–752 (2009). [DOI] [PubMed] [Google Scholar]
- 47.van der Heide F et al. Differences in genetic background between active smokers, passive smokers, and non-smokers with Crohn’s disease. Am. J. Gastroenterol 105, 1165–1172 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Loftus EV Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126, 1504–1517 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Kalogeris T, Baines CP, Krenz M & Korthuis RJ Ischemia/Reperfusion. Compr. Physiol 7, 113–170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balaban RS, Nemoto S & Finkel T Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005). [DOI] [PubMed] [Google Scholar]
- 51.West AP, Shadel GS & Ghosh S Mitochondria in innate immune responses. Nat. Rev. Immunol 11, 389–402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genova ML et al. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 505, 364–368 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Sabharwal SS & Schumacker PT Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGovern DP, Kugathasan S & Cho JH Genetics of inflammatory bowel diseases. Gastroenterology 149, 1163–1176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohsumi Y Historical landmarks of autophagy research. Cell Res. 24, 9–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levine B, Mizushima N & Virgin HW Autophagy in immunity and inflammation. Nature 469, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemasters JJ Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuven. Res 8, 3–5 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Gatliff J & Campanella M TSPO is a REDOX regulator of cell mitophagy. Biochem. Soc. Trans 43, 543–552 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Ostuni MA et al. Overexpression of translocator protein in inflammatory bowel disease: potential diagnostic and treatment value. Inflamm. Bowel Dis 16, 1476–1487 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timmis JN, Ayliffe MA, Huang CY & Martin W Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet 5, 123–135 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Stone JR & Yang S Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal 8, 243–270 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Davies MJ Protein oxidation and peroxidation. Biochem. J 473, 805–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazquez-Torres A & Baumler AJ Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Curr. Opin. Microbiol 29, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelko IN, Mariani TJ & Folz RJ Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med 33, 337–349 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Klinowski E, Broide E, Varsano R, Eshchar J & Scapa E Superoxide dismutase activity in duodenal ulcer patients. Eur. J. Gastroenterol. Hepatol 8, 1151–1155 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Naito Y et al. Changes in superoxide dismutase activity in the gastric mucosa of peptic ulcer patients. J. Clin. Gastroenterol 14 (Suppl. 1), S131–S134 (1992). [DOI] [PubMed] [Google Scholar]
- 67.Toppo S, Vanin S, Bosello V & Tosatto SC Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal 10, 1501–1514 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Chu FF, Doroshow JH & Esworthy RS Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase. GSHPx-GI. J. Biol. Chem 268, 2571–2576 (1993). [PubMed] [Google Scholar]
- 69.Wingler K, Muller C, Schmehl K, Florian S & Brigelius-Flohe R Gastrointestinal glutathione peroxidase prevents transport of lipid hydroperoxides in CaCo-2 cells. Gastroenterology 119, 420–430 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Esworthy RS et al. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am. J. Physiol. Gastrointest. Liver Physiol 281, G848–G855 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Schrader M & Fahimi HD Peroxisomes and oxidative stress. Biochim. Biophys. Acta 1763, 1755–1766 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Knoops B, Argyropoulou V, Becker S, Ferte L & Kuznetsova O Multiple roles of peroxiredoxins in inflammation. Mol. Cells 39, 60–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hampton MB & O’Connor KM Peroxiredoxins and the regulation of cell death. Mol. Cells 39, 72–76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Won HY et al. Ablation of peroxiredoxin II attenuates experimental colitis by increasing FoxO1-induced Foxp3+ regulatory T cells. J. Immunol 191, 4029–4037 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Melhem H et al. Prdx6 deficiency ameliorates DSS colitis: relevance of compensatory antioxidant mechanisms. J. Crohns Colitis 11, 871–884 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Suzuki T & Yamamoto M Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem 292, 16817–16824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gabig TG, Bearman SI & Babior BM Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53, 1133–1139 (1979). [PubMed] [Google Scholar]
- 78.Fox CJ, Hammerman PS & Thompson CB Fuel feeds function: energy metabolism and the T cell response. Nat. Rev. Immunol 5, 844–852 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Campbell EL & Colgan SP Neutrophils and inflammatory metabolism in antimicrobial functions of the mucosa. J. Leukoc. Biol 98, 517–522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geiszt M NADPH oxidases: new kids on the block. Cardiovascular Res. 71, 289–299 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Rada B & Leto TL Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol 15, 164–187 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corcionivoschi N et al. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe 12, 47–59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McPhail LC, Henson PM & Johnston RB Jr. Respiratory burst enzyme in human neutrophils. Evidence for multiple mechanisms of activation. J. Clin. Invest 67, 710–716 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dinauer MC, Orkin SH, Brown R, Jesaitis AJ & Parkos CA The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327, 717–720 (1987). [DOI] [PubMed] [Google Scholar]
- 85.Werlin SL, Chusid MJ, Caya J & Oechler HW Colitis in chronic granulomatous disease. Gastroenterology 82, 328–331 (1982). [PubMed] [Google Scholar]
- 86.Mantovani A, Sica A & Locati M Macrophage polarization comes of age. Immunity 23, 344–346 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Hume DA The many alternative faces of macrophage activation. Front. Immunol 6, 370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacMicking J, Xie QW & Nathan C Nitric oxide and macrophage function. Annu. Rev. Immunol 15, 323–350 (1997). [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez PC et al. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J. Biol. Chem 277, 21123–21129 (2002). [DOI] [PubMed] [Google Scholar]
- 90.Efimova O, Szankasi P & Kelley TW Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLOS ONE 6, e16013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kraaij MD et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 17686–17691 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng L, Kelly CJ & Colgan SP Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol 309, C350–C360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lambeth JD & Neish AS Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol 9, 119–145 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Jones RM et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32, 3017–3028 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones RM et al. Lactobacilli modulate epithelial cytoprotection through the Nrf2 Ppathway. Cell Rep. 12, 1217–1225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alam A et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7, 645–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Babbin BA et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol 179, 8112–8121 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Leoni G et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest 123, 443–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitra SK, Hanson DA & Schlaepfer DD Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol 6, 56–68 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Alam A et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol 1, 15021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crump JA, Sjolund-Karlsson M, Gordon MA & Parry CM Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev 28, 901–937 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barman M et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infection Immun. 76, 907–915 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winter SE et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thiennimitr P et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl Acad. Sci. USA 108, 17480–17485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niethammer P, Grabher C, Look AT & Mitchison TJ A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang S et al. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic. Biol. Med 65, 38–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grasberger H, El-Zaatari M, Dang DT & Merchant JL Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology 145, 1045–1054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pircalabioru G et al. Defensive mutualism rescues NADPH oxidase inactivation in gut infection. Cell Host Microbe 19, 651–663 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Walter J, Britton RA & Roos S Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl Acad. Sci. USA 108, 4645–4652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alvarez LA et al. NADPH oxidase-derived H2O2 subverts pathogen signaling by oxidative phosphotyrosine conversion to PB-DOPA. Proc. Natl Acad. Sci. USA 113, 10406–10411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fujii N & Saito T Homochirality and life. Chem. Rec 4, 267–278 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Cava F, de Pedro MA, Lam H, Davis BM & Waldor MK Distinct pathways for modification of the bacterial cell wall by non-canonical d-amino acids. EMBO J. 30, 3442–3453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasabe J et al. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol 16125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chandel NS & Schumacker PT Cellular oxygen sensing by mitochondria: old questions, new insight. J. Appl. Physiol 88, 1880–1889 (1985). [DOI] [PubMed] [Google Scholar]
- 115.Hagen T, Taylor CT, Lam F & Moncada S Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302, 1975–1978 (2003). [DOI] [PubMed] [Google Scholar]
- 116.Schaible B, Schaffer K & Taylor CT Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol 174, 235–243 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Kelly CJ et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 17, 662–671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glover LE, Lee JS & Colgan SP Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Invest 126, 3680–3688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Latz E NOX-free inflammasome activation. Blood 116, 1393–1394 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Karhausen J et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest 114, 1098–1106 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arteel GE, Thurman RG & Raleigh JA Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur. J. Biochem 253, 743–750 (1998). [DOI] [PubMed] [Google Scholar]
- 122.Arteel GE, Thurman RG, Yates JM & Raleigh JA Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br. J. Cancer 72, 889–895 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goethals L et al. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int. J. Radiat. Oncol. Biol. Phys 65, 246–254 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Hindryckx P et al. Intrarectal administration of oxygenated perfluorodecalin promotes healing of murine colitis by targeting inflammatory hypoxia. Lab Invest. 91, 1266–1276 (2011). [DOI] [PubMed] [Google Scholar]
- 125.Sang N, Fang J, Srinivas V, Leshchinsky I & Caro J Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1α is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol. Cell. Biol 22, 2984–2992 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niecknig H et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic. Res 46, 705–717 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Synnestvedt K et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest 110, 993–1002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cummins EP et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Han IO, Kim HS, Kim HC, Joe EH & Kim WK Synergistic expression of inducible nitric oxide synthase by phorbol ester and interferon-γ is mediated through NF-κB and ERK in microglial cells. J. Neurosci. Res 73, 659–669 (2003). [DOI] [PubMed] [Google Scholar]
- 130.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR & Eltzschig HK Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136, 607–618 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Robinson A et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shah YM et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134, 2036–2048 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giatromanolaki A et al. Hypoxia inducible factor 1α and 2α overexpression in inflammatory bowel disease. J. Clin. Pathol 56, 209–213 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mariani F et al. Cyclooxygenase-2 and hypoxia-inducible factor-1α protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett. 279, 221–229 (2009). [DOI] [PubMed] [Google Scholar]
- 135.Matthijsen RA et al. Enterocyte shedding and epithelial lining repair following ischemia of the human small intestine attenuate inflammation. PLOS ONE 4, e7045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colgan SP & Taylor CT Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol 7, 281–287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holden VI, Breen P, Houle S, Dozois CM & Bachman MA Klebsiella pneumoniae Siderophores induce inflammation, bacterial dissemination, and HIF-α stabilization during pneumonia. mBio 7, e01397–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirienko NV et al. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 13, 406–416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelly CJ et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donohoe DR, Wali A, Brylawski BP & Bultman SJ Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes. PLOS ONE 7, e46589 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rivera-Chavez F et al. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19, 443–454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zambetti LP & Mortellaro A NLRPs, microbiota, and gut homeostasis: unravelling the connection. J. Pathol 233, 321–330 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Wen H, Miao EA & Ting JP Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39, 432–441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schroder K & Tschopp J The inflammasomes. Cell 140, 821–832 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Coccia M et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med 209, 1595–1609 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neudecker V et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med 214, 1737–1752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zaki MH et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saitoh T et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Dostert C et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meissner F et al. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116, 1570–1573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van de Veerdonk FL et al. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl Acad. Sci. USA 107, 3030–3033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brookes PS, Yoon Y, Robotham JL, Anders MW & Sheu SS Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol 287, C817–C833 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Zhou R, Yazdi AS, Menu P & Tschopp J A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011). [DOI] [PubMed] [Google Scholar]
- 154.Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH & Hansson GC New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen GY & Stappenbeck TS Mucus, it is not just a static barrier. Sci. Signal 7, pe11 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Johansson ME & Hansson GC Is the intestinal goblet cell a major immune cell? Cell Host Microbe 15, 251–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cadwell K et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patel KK et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 32, 3130–3144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wlodarska M et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Birchenough GM, Nystrom EE, Johansson ME & Hansson GC A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352, 1535–1542 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sankey EA et al. Early mucosal changes in Crohn’s disease. Gut 34, 375–381 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nowarski R et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goto Y et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lindemans CA et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng Y et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med 14, 282–289 (2008). [DOI] [PubMed] [Google Scholar]
- 166.Eberl G, Colonna M, Di Santo JP & McKenzie AN Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munoz M et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42, 321–331 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Bernink JH et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Guan Y, Worrell RT, Pritts TA & Montrose MH Intestinal ischemia-reperfusion injury: reversible and irreversible damage imaged in vivo. Am. J. Physiol. Gastrointest. Liver Physiol 297, G187–196 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang D, Mann JR & DuBois RN The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology 128, 1445–1461 (2005). [DOI] [PubMed] [Google Scholar]
- 171.Vane JR, Bakhle YS & Botting RM Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol 38, 97–120 (1998). [DOI] [PubMed] [Google Scholar]
- 172.Moses T, Wagner L & Fleming SD TLR4-mediated Cox-2 expression increases intestinal ischemia/reperfusion-induced damage. J. Leukoc. Biol 86, 971–980 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blikslager AT, Roberts MC, Rhoads JM & Argenzio RA Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J. Clin. Invest 100, 1928–1933 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Samuelsson B & Hammarstrom S Leukotrienes: a novel group of biologically active compounds. Vitam. Horm 39, 1–30 (1982). [DOI] [PubMed] [Google Scholar]
- 175.Dennis EA & Norris PC. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol 15, 511–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Collard CD et al. Reoxygenation of hypoxic human umbilical vein endothelial cells (HUVEC’s) activates the classical complement pathway. Circulation 96, 326–333 (1997). [DOI] [PubMed] [Google Scholar]
- 177.Otamiri T Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery 105, 593–597 (1989). [PubMed] [Google Scholar]
- 178.Eltzschig HK, Bratton DL & Colgan SP Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov 13, 852–869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Katada K, Takagi T, Uchiyama K & Naito Y Therapeutic roles of carbon monoxide in intestinal ischemia-reperfusion injury. J. Gastroenterol. Hepatol 30, 46–52 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Younes M et al. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res. Exp. Med 184, 259–264 (1984). [DOI] [PubMed] [Google Scholar]
- 181.Parks DA, Williams TK & Beckman JS Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am. J. Physiol 254, G768–G774 (1988). [DOI] [PubMed] [Google Scholar]
- 182.Harrison R Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med 33, 774–797 (2002). [DOI] [PubMed] [Google Scholar]
- 183.Eisen A et al. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis 172, 201–210 (2004). [DOI] [PubMed] [Google Scholar]
- 184.Alchera E, Dal Ponte C, Imarisio C, Albano E & Carini R Molecular mechanisms of liver preconditioning. World J. Gastroenterol 16, 6058–6067 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khoury J, Ibla JC, Neish AS & Colgan SP Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J. Clin. Invest 117, 703–711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hatakeyama S et al. Ubiquitin-dependent degradation of IκBα is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc. Natl Acad. Sci. USA 96, 3859–3863 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boh BK, Smith PG & Hagen T Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J. Mol. Biol 409, 136–145 (2011). [DOI] [PubMed] [Google Scholar]
- 188.Soucy TA et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009). [DOI] [PubMed] [Google Scholar]
- 189.Ehrentraut SF et al. Central role for endothelial human deneddylase-1/SENP8 in fine-tuning the vascular inflammatory response. J. Immunol 190, 392–400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Colgan SP & Eltzschig HK Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol 74, 153–175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eltzschig HK, Sitkovsky MV & Robson SC Purinergic signaling during inflammation. N. Engl. J. Med 367, 2322–2333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eltzschig HK et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med 202, 1493–1505 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eltzschig HK Adenosine: an old drug newly discovered. Anesthesiology 111, 904–915 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu F et al. Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Frick JS et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol 182, 4957–4964 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aherne CM et al. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol. 8, 1324–1338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hart ML et al. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol 182, 4957–4964 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 198.Hart ML, Jacobi B, Schittenhelm J, Henn M & Eltzschig HK Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J. Immunol 186, 4367–4374 (2009). [DOI] [PubMed] [Google Scholar]
- 199.Eltzschig HK et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 104, 3986–3992 (2004). [DOI] [PubMed] [Google Scholar]
- 200.Elliott MR et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Eltzschig HK et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res 99, 1100–1108 (2006). [DOI] [PubMed] [Google Scholar]
- 202.Eltzschig HK et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med 198, 783–796 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weissmuller T et al. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J. Clin. Invest 118, 3682–3692 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Madara JL et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J. Clin. Invest 91, 2320–2325 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang C et al. Genetic risk for inflammatory bowel disease is a determinant of Crohn’s disease development in chronic granulomatous disease. Inflamm. Bowel Dis 22, 2794–2801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bao S, Carr ED, Xu YH & Hunt NH Gp91(phox) contributes to the development of experimental inflammatory bowel disease. Immunol. Cell Biol 89, 853–860 (2011). [DOI] [PubMed] [Google Scholar]
- 207.Strober W, Fuss IJ & Blumberg RS The immunology of mucosal models of inflammation. Annu. Rev. Immunol 20, 495–549 (2002). [DOI] [PubMed] [Google Scholar]
- 208.Abraham C & Cho JH Inflammatory bowel disease. N. Eng. J. Med 361, 2066–2076 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fournier BM & Parkos CA The role of neutrophils during intestinal inflammation. Mucosal Immunol. 5, 354–366 (2012). [DOI] [PubMed] [Google Scholar]
- 210.Schulzke JD et al. Epithelial tight junctions in intestinal inflammation. Ann. NY Acad. Sci 1165, 294–300 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Butto LF & Haller D Dysbiosis in intestinal inflammation: cause or consequence. Int. J. Med. Microbiol 306, 302–309 (2016). [DOI] [PubMed] [Google Scholar]
- 212.Simpson R et al. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann. Surg 218, 444–453 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kuhl AA et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology 133, 1882–1892 (2007). [DOI] [PubMed] [Google Scholar]
- 214.Wan P et al. Extracellular ATP mediates inflammatory responses in colitis via P2 × 7 receptor signaling. Sci. Rep 6, 19108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wright HL, Moots RJ, Bucknall RC & Edwards SW Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49, 1618–1631 (2010). [DOI] [PubMed] [Google Scholar]
- 216.Tian T, Wang Z & Zhang J Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev 2017, 1–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hebuterne X, Filippi J & Schneider SM Nutrition in adult patients with inflammatory bowel disease. Curr. Drug Targets 15, 1030–1038 (2014). [DOI] [PubMed] [Google Scholar]
- 218.Tso P, Lee T & Demichele SJ Lymphatic absorption of structured triglycerides versus physical mix in a rat model of fat malabsorption. Am. J. Physiol 277, G333–G340 (1999). [DOI] [PubMed] [Google Scholar]
- 219.Hering NA, Fromm M & Schulzke JD Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol 590, 1035–1044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Capaldo CT & Nusrat A Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788, 864–871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Capaldo CT & Nusrat A Claudin switching: Physiological plasticity of the Tight Junction. Semin. Cell Dev. Biol 42, 22–29 (2015). [DOI] [PubMed] [Google Scholar]
- 222.Tolstanova G et al. Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Lab Invest. 92, 9–21 (2012). [DOI] [PubMed] [Google Scholar]
- 223.Brown IA, McClain JL, Watson RE, Patel BA & Gulbransen BD Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol 2, 77–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Scirocco A et al. Exposure of Toll-like receptors 4 to bacterial lipopolysaccharide (LPS) impairs human colonic smooth muscle cell function. J. Cell. Physiol 223, 442–450 (2010). [DOI] [PubMed] [Google Scholar]