Abstract
Uncontrolled diabetes results in several metabolic alterations including hyperglycemia. Indeed, several preclinical and clinical studies have suggested that this condition may induce susceptibility and the development of more aggressive infectious diseases, especially those caused by some bacteria (including Chlamydophila pneumoniae, Haemophilus influenzae, and Streptococcus pneumoniae, among others) and viruses [such as coronavirus 2 (CoV2), Influenza A virus, Hepatitis B, etc.]. Although the precise mechanisms that link glycemia to the exacerbated infections remain elusive, hyperglycemia is known to induce a wide array of changes in the immune system activity, including alterations in: (i) the microenvironment of immune cells (e.g., pH, blood viscosity and other biochemical parameters); (ii) the supply of energy to infectious bacteria; (iii) the inflammatory response; and (iv) oxidative stress as a result of bacterial proliferative metabolism. Consistent with this evidence, some bacterial infections are typical (and/or have a worse prognosis) in patients with hypercaloric diets and a stressful lifestyle (conditions that promote hyperglycemic episodes). On this basis, the present review is particularly focused on: (i) the role of diabetes in the development of some bacterial and viral infections by analyzing preclinical and clinical findings; (ii) discussing the possible mechanisms by which hyperglycemia may increase the susceptibility for developing infections; and (iii) further understanding the impact of hyperglycemia on the immune system.
Keywords: infections, diabetes, immune system, hyperglycemia, COVID-19
Introduction
Diabetes mellitus is a chronic and complex illness characterized by several metabolic alterations including dyslipidemia and hyperglycemia, among others (1). According to the American Diabetes Association (A.D.A.), diabetes mellitus (DM) can be classified into the following categories: (i) type 1 diabetes mellitus (T1DM), characterized by the loss of pancreatic β-cells induced by an autoimmune response; (ii) type 2 diabetes mellitus (T2DM), identified by the gradual loss of insulin secretion and/or the development of insulin resistance; (iii) gestational DM, developed in some pregnant women; and (iv) other types of DM that are due to miscellaneous causes (2, 3). Interestingly, patients with uncontrolled DM (regardless of type) have alterations in healing latency and susceptibility for developing some emerging infectious (mainly bacterial) diseases. In addition, compared to non-diabetic normoglycemic patients, DM patients are at higher risk for developing the current severe acute respiratory syndrome coronavirus 2 (SARS-COV2) caused by the coronavirus 2 (CoV2) that has shocked the world economy and created a global health pandemic emergency named COVID-19 (4–7). Moreover, the restoration of normoglycemia seems to be related to a better prognosis for bacterial infections (5); whereas in COVID-19 diabetic patients, no obvious conclusions have been reached about the impact of normoglycemic treatments on the development and outcome of this particular disease (8).
People with metabolic impairments (i.e., fasting hyperglycemia, postprandial hyperglycemia and DM) show greater ranges of glucose levels (2). Indeed, fasting hyperglycemia (when food has not been taken for at least 8 h) is a metabolic disorder characterized by levels of plasma glucose above 110 mg/dL, a condition commonly observed in diabetic patients (9). Fasting hyperglycemia (from now on simply referred to as hyperglycemia) has been involved in deleterious effects such as tissue damage associated with oxidative stress and immunological impairments (10), which increase the susceptibility to acquire bacterial infections and COVID-19 (8, 11–17) (see below).
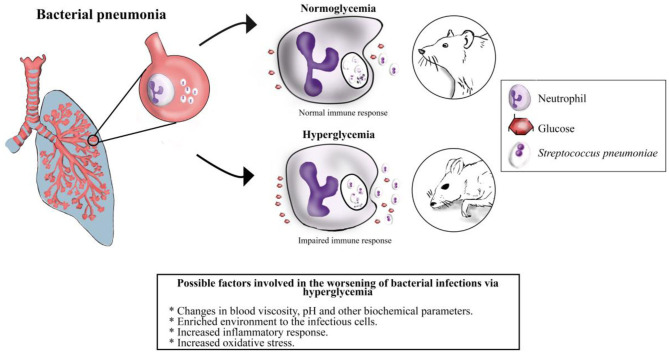
Remarkably, the effects of hypoglycemia induced by anorexia on the clinical outcome of infected patients have been discussed with no consensus (18). Moreover, Wang et al. (19) have suggested that: (i) the pathogenic nature (i.e., bacterial, viral, etc.) and infection profile may be key factors for the prognosis and clinical outcome in a preclinical model of bacterial infection; (ii) glucose plays a key role in the outcome of infected animals; and (iii) survival of animals under bacterial sepsis (with Listeria monocytogenes) was dramatically decreased when they were gavage-fed. In contrast, if these animals received glucose (i.p.), all animals died (19). As anorexia is an important stereotypic behavior of the sickness response, it could be an adaptive strategy for combating some infectious illnesses. In this sense, it has also been reported that bacteria from other groups (e.g., Salmonella) may induce inhibition of anorexia via Salmonella leucine rich repeat protein (SlrP) which inhibits interleukin-1β (IL-1β). This effect seems to maintain the conditions for increasing the opportunity for Salmonella to infect other hosts (20). Although these experiments were carried out in preclinical models, the results suggest that glycemia is so important that hosts and infectious agents have developed adaptive strategies to control glucose levels during the progression of an infectious process (11). Moreover, the control of glucose levels may determine the infection course and/or the recovery times (Figure 1); therefore, the understanding of the mechanisms involved on the hijacking of glucose control during infections may have an enormous medical utility.
Figure 1.
Illustration of a hypothetical outcome in an experimental model of bacterial pneumonia in normal conditions vs. during hyperglycemia. Under normoglycemia conditions the immune response handles successfully bacterial infections. Nevertheless, hyperglycemia impairs the immune response by inducing several glucose-related factors, including those mentioned above. These scenarios could determine the outcome during bacterial infections.
The relationship between hyperglycemia and susceptibility to infections has been described extensively in diabetic patients. Nevertheless, very few reports have analyzed the appropriate management of glycemia according to the infection type, immunological responsiveness, and clinical variables (i.e., patient age, time or period elapsed with diabetes, etc.). To focus on these aspects, the present review has considered information on: (i) the effect of glycemia on infection outcome and immune cells physiology; (ii) the biochemical alterations in cell physiology during diabetes and/or hyperglycemia; and (iii) the impact of pharmaceutical care interventions for glycemia control on some of the most frequent emerging infectious diseases.
Methods and Inclusion Criteria
To consider the relevant literature in this theoretical review, we searched for studies published in various databases such as Science direct, Pubmed central and Google Scholar. These databases included any combination of the main key terms “bacterial infections,” “COVID-19,” “diabetes,” “influenza A virus,” “hepatitis B and C viruses,” “human immunodeficiency virus,” and “hyperglycemia” among themselves and with important topics such as: “rheological properties of blood,” “biochemical alterations of diabetic immune cells,” “immune response on hyperglycemic environment,” “hypoglycemic drugs,” “bacterial infection outcome,” and “comorbidities with COVID-19.” Around 600 articles published from 1966 up to 2020 were perused, and only 260 of those articles with experimental and/or theoretical information which related hyperglycemia and/or diabetes to bacterial infections and/or COVID-19 and some other viruses were included in this review.
The Association Between Hyperglycemia and Common Infectious (Mainly Bacterial) Diseases in Diabetes
Effect of Hyperglycemia on the Immune Response
In general, alterations in the immune system during hyperglycemia seem to be associated with mechanisms that include lower secretion of inflammatory cytokines, depression in neutrophils and T cells function, as well as decreases in humoral immunity (21, 22). Moreover, it is documented that hyperglycemia may delay the recuperation of tissues (e.g., via changes in the secretion of growth factors and collagenase levels) (12, 23); this, in turn, may lead to increased susceptibility of these tissues to develop secondary emerging infections (mainly bacterial). Other alterations in the immune response induced by hyperglycemia can be explained by biochemical and/or cellular events, such as: (i) creation of advanced glycation end-products (AGEs), which reduce the expression of myeloid cells surface proteins known as class I major histocompatibility complex (24); (ii) decreased migration of polymorphonuclear leukocytes, chemotaxis, and/or phagocytic activity (25); (iii) inhibition on G6PD (see below) (26); and (iv) increased apoptosis of polymorphonuclear leukocytes and reduced transmigration through the endothelium (27). Clearly, the control of glycemia may be mandatory for dealing with emerging infections (mainly bacterial), in view that: (i) some bacteria grow better in a high glucose environment (28); and (ii) a hyperglycemic state seems to negatively affect the body's ability to respond to antimicrobial therapy (29).
Common infections related to T1DM and T2DM are those of the respiratory and urinary tracts. Indeed, it has been reported that patients with T2DM have alterations in chemotaxis, phagocytosis, antigen presentation and proliferation/function of T cells in response to Mycobacterium tuberculosis, which facilitates infection and its symptomatic progression (30). Certainly, the impaired chemotaxis of leukocytes does not depend on the type of diabetes mellitus (31). Other tissues/organs that are also commonly compromised in diabetic patients include the skin, bone marrow, gastrointestinal tract and liver, among others (21, 22, 32). This susceptibility for developing infections may lead to complications in the management of diabetic patients, such as post-operative infections, sepsis, chronic periodontitis, emphysematous cholecystitis, emphysematous pyelonephritis, malignant external otitis, rhinocerebral mucormycosis, gangrenous cholecystitis, and others (21, 22, 32, 33).
It is noteworthy that foot infections are highly common in patients with diabetes, which usually start after a foot wound that eventually leads to ulceration. In this respect, neuropathy seems to be an important component of foot ulceration which, in turn, increases the risk of amputation (34). The wound is predisposed to a loss of sensitivity because of the damage in neuron fibers by pathophysiological mechanisms not fully understood (34–36). It has been suggested that a vascular endothelium damage produced by inflammation and oxidative stress (36) may produce alterations in the microcirculation and, finally, nerve damage (37).
In many cases, these infections cause ischemia at the wound site, ultimately leading to amputations (38). Moreover: (i) immunological disturbances in neutrophil functions such as chemotaxis, phagocytosis and intracellular killing may contribute to exacerbate infections (12, 31, 38–41); and (ii) AGEs may influence the appearance of a chronic immune imbalance by activating pro-inflammatory cells which, in turn, would lead to a chronic subclinical inflammation that hinders the correct function of the immune system to fight infections and to deal with wound healing (42).
Thus, the typical hyperglycemia present in patients with diabetes could be related to an increased risk of different types of infections. Interestingly, cancer patients treated with glucocorticoids showed increased infection rates (43). Indeed, glucocorticoids are direct immunosuppressors that may increase hyperglycemia by hepatic gluconeogenesis and inhibition of glucose intake (43, 44). All these lines of evidence, strongly suggest that hyperglycemia may induce an adequate environment for several infectious pathogens; and hence, a suitable glycemic control would decrease the rate of infection risk (45–49).
Finally, it is logical to assume that the changes in the immune system produced by hyperglycemia as an occasional (transient) event (e.g., stress) should be quite different from the changes induced by a chronic hyperglycemia. Nevertheless, in any hyperglycemic condition (i.e., transient or chronic), patients may be susceptible to some of the same clinical complications, including poor wound healing and an increased rate of infection (50). In fact, acute glucose elevation in critically injured trauma patients may be predictive of infections (51); whereas hyperglycemia at admission (with no indication about the cause) is a predictor of infections in critically ill trauma patients (52). Clearly, chronic hyperglycemia involves compensatory mechanisms (not discussed here) that are absent when it is due to an occasional event; in both cases, normalization of the glucose levels seems to be a useful practice to improve the nosocomial outcome (50, 53, 54).
Stress-Induced Hyperglycemia and Infections
Besides diabetes, another condition that commonly predisposes to hyperglycemia is stress. The stress-induced hyperglycemia (SIH) generally refers to a metabolic condition with a transient hyperglycemia associated with clinical illness (55). The SIH is a common problem in patients admitted to intensive care units (ICU) (50), even in the absence of pre-existing diabetes (55), and it is defined as an increase above 200 mg/dL of blood glucose (52, 56, 57).
The SIH is especially dangerous in chronic critical illnesses (58), as the organs' functions become aberrant increasing the risk of death. In less severe cases, SIH seems to affect the normal immune response, since hyperglycemia (as discussed above) is associated with an increased risk to infections (50). Hyperglycemia is certainly related to a higher risk of infections and sepsis in patients of ICU (59), an increased risk of complications in patients who underwent orthopedic trauma surgery (60), and surgical site infections in non-diabetic orthopedic trauma patients (61) (see Table 1).
Table 1.
Main complications during bacterial infections in diabetic patients.
| Pathogen | Emerging disease | Main complications in diabetics | References |
|---|---|---|---|
| RESPIRATORY INFECTIONS | |||
| Streptococcus pneumoniae | Pneumonia | Respiratory failure, pleural effusion, bacteremia, septic shock | (62–64) |
| Mycobacterium tuberculosis | Tuberculosis (TB) | Impaired cell-mediated immunity, renal failure, micronutrient deficiency and pulmonary microangiopathy | (65, 66) |
| URINARY TRACT INFECTIONS | |||
| Escherichia coli and Proteus sp. | Pyelonephritis | Pherinephric and/or renal abscesses, emphysematous pyelonephritis, renal papillary necrosis, urosepsis, and hemolytic-uremic syndrome | (5, 29, 67–70) |
| GASTROINTESTINAL INFECTIONS | |||
| Helicobacter pylori | Gastritis | Macroangiopathy, neuropathy, proteinuria and microalbuminuria | (71–74) |
| SKIN AND SOFT TISSUE INFECTIONS | |||
| Staphylococcus aureus and Staphylococcus epidermidis | Foot infection | Amputation, osteomyelitis, and death | (13, 75–78) |
| Combination of one anaerobic and many aerobic microorganisms | Necrotizing fasciitis | Fulminant local tissue destruction, microvascular thrombosis, and systemic signs of toxicity | (79–81) |
| Escherichia coli, Klebsiella sp., Proteus sp., and Peptostreptococcus | Fournier gangrene | Sepsis, multiple organ failure, and death | (82, 83) |
| HEAD AND NECK INFECTIONS | |||
| Pseudomonas aeruginosa | Invasive external otitis | Periostitis, osteitis, chondritis, osteomyelitis, multiple cranial nerve palsies, and facial paralysis | (84, 85) |
| Listeria monocytogenes | Listeriosis | Sepsis, meningitis, hydrocephalus | (86–88) |
A stress response is associated with an increased pro-inflammatory response characterized by the release of several cytokines, including tumor necrosis factor-alpha (TNF-α), IL-1 and IL-6, which are related to insulin resistance (59). Indeed: (i) TNF-α inhibits tyrosine kinases and decreases tyrosine phosphorylation of the insulin receptor (92); (ii) IL-1 suppresses glucose transporter-4 (GLUT-4) translocation by a decreased activation of the phosphoinositide-3-kinase (PI3K) mechanism (93); and (iii) IL-6 increases the release of adrenocorticotrophic hormone (94), with all the scenarios resulting in insulin resistance.
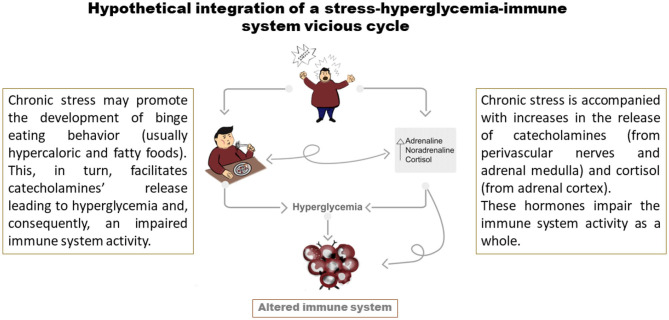
Additionally, there may be an integration of a vicious cycle when taking a hypercaloric fatty diet, which can also induce an increase in catecholamines' release (95), in combination with lifestyle factors leading to chronic stress (resulting in a plasma increase in catecholamines and cortisol). In this scenario, catecholamines via β-adrenoceptors expressed in adipocytes, liver, skeletal and smooth muscle cells may increase the metabolism of glycogen and triglycerides for increasing blood glucose, fatty acids, glycerol, and other local vascular actions (96–98). Accordingly, this SIH may lead to immunosuppression (see Figure 2).
Figure 2.
Development of a vicious cycle induced by a stressful lifestyle. Stressors promote the behavior of hypercaloric food binge which, in turn, increases the release of catecholamines and cortisol (immunosuppressants). Subsequently, catecholamines induce glycolysis, reinforce the maladaptive eating patterns, and negatively modulate immune cell activity. On the other hand, cortisol induces chronic immunosuppression.
The contribution of SIH in the diabetic patient is a complex issue that seems to worsen the glycemic status of patients with type 2 diabetes; whereas the autonomic damage induced by neuropathy in type 1 diabetes leads to contradictory and non-conclusive data (99). Patients with necrotizing fasciitis (and diabetes) developed adverse outcomes when SIH generated glycemic gaps with increases in glucose >146 mg/dL (86). Thus, synergy of diabetes and SIH is a phenomenon of high medical impact (both in infectious and non-infectious clinical admission) that remains to be completely characterized (53, 100–104).
Infectious Diseases in Patients With Diabetes: Exacerbation and Susceptibility to SARS-CoV-2 and Other Viral Diseases
Diabetes is a condition that may potentiate infectious diseases and predispose patients to acquiring more severe diseases. To support this notion, a recent matched cohort study analyzed the incidence infection rate from 306,011 patients (102,493 patients with type 1 and 2 diabetes), and reported that patients with diabetes (especially type 1) are more susceptible to developing severe infectious diseases (105). In addition, patients with diabetes are more vulnerable to fungal, viral, and bacterial infections than the non-diabetic population, exhibiting a worse prognosis once the infection is installed (106).
According to epidemiological studies, the most common infectious diseases of hospitalization in children, adolescents and adults with diabetes are lower tract respiratory infections (pneumonia, among others), diabetic foot infection, skin and soft tissue abscesses, and urinary tract infections (107, 108). The respiratory tract infections are the primary comorbidity associated with severe or lethal infections that increases hospitalizations in individuals with diabetes (109, 110).
Pneumonia is the hospitalization leading cause of severe lower respiratory tract infections, and it is an important risk factor for infectious illnesses in diabetic patients (111). The main fungal and bacterial pathogens associated with pneumonia infections are Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, methicillin-resistant Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae (109). This diversity of pneumonia development pathogens may denote a complex biological interaction between wild microorganisms residing in the human body, the host immunophysiology, and the pathogenic pneumonia specificity.
On the other hand, hormones like cortisol, glucagon and catecholamines released during certain conditions, such as trauma, infection and surgery (57) increase gluconeogenesis and decrease peripheral glucose uptake (52). Interestingly, the association between sympathetic hyperactivity (e.g., induced by chronic stress), hyperglycemia, hypothermia and immunosuppression of the acquired immunity seems to be mainly mediated by activation of α-adrenoceptors (112, 113).
Emerging global health studies have reported that other respiratory tract infections with high mortality rate in patients with diabetes, besides pneumonia (114), are those promoted by viral agents. These include the influenza viruses, the Middle East respiratory syndrome coronavirus (MERS-CoV), the severe acute respiratory syndrome coronavirus (SARS-CoV) and, most recently, the SARS-CoV-2 (see below), the last viral infection outbreak across the globe (7, 17, 115, 116).
SARS-CoV-2 and Other Viral Diseases: Impact of Hyperglycemia
Patients with hyperglycemia have been reported to be susceptible to develop a severe form of COVID-19, which is a risk factor for fatality (6, 17). Newsworthy, diabetes provides a ~3-fold higher risk of fatality as compared to the non-diabetic population among the COVID-19 sufferers (7). Moreover, diabetes increased the length of hospital stays for COVID-19 patients from 9.8 days in non-diabetic patients to 14.4 days in diabetic patients in a retrospective cross-sectional study that was conducted in England (14). In this regard, it has been described that a proper control of glycemia by antidiabetic drugs can be beneficial in reducing the risk of death in diabetic patients with COVID-19 (16). Indeed, it was inferred that DPP-4 inhibitors might be beneficial to prevent or treat COVID-19 disease (117). Although this certainly opens a new field of interest in the treatment of SARS-CoV-2 pneumonia, further studies and research are required on this topic.
Bacterial infections are frequently identified after typical viral respiratory infections and they are important causes of morbidity and mortality. In patients with COVID-19, bacterial comorbidity has been reported to be low (i.e., an overall proportion of 6.9 %) in a recent metanalysis reported by Langford et al. (118). Notoriously, the comorbidity was slightly higher in critical patients (i.e., 8.1%) (118).
DM, hypertension, cardiovascular diseases, and obesity are the top four comorbidities worldwide associated to critically ill patients with COVID-19 and mortality (15, 119–121). Indeed, 5 to 10% of patients with SARS-CoV-2 pneumonia require intensive care unit (ICU) admission and mechanical ventilation. Patients requiring invasive mechanical ventilation are strongly related to poor outcome with high mortality rate in Chinese and American populations (122, 123).
Unfortunately, studies on the unfavorable outcomes and mortality rate related to pathogenic co-infections that worsen respiratory tract function in people with diabetes and COVID-19 infection are limited (124). However, bacterial, and viral pathogenic co-infections have been studied in patients with SARS-CoV-2 pneumonia requiring ICU admission. These studies showed that methicillin-sensitive Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, Enterobacteriaceae, Pseudomonas aeruginosa, Moraxella catarrhalis, and Acinetobacter baumannii were the 28% of bacterial strains isolated by experimental laboratory procedures (cultures or PCRs assays) (125). Importantly, no viral co-infection has been detected in the critically ill COVID-19 patients, supporting the idea that respiratory infections are often depending on combinatorial factors associated to geography, season, human physiology, and behavior, as well as pathogenic interactions. Therefore, it is mandatory to determine the different biological mechanisms used for each viral infection and/or co-infection of pathogens that aggravate states of health or disease to pursue appropriate treatments.
Admittedly, pathogenesis of COVID-19 viremia remains unclear. However, some lines of evidence suggest that high levels of systemic glucose increase glucose concentration in the epithelial secretion of the respiratory tract, disrupting the orchestration of the innate and humoral immunological response. This includes, in particular, hyperglycemia-induced changes in coagulation, worsening of endothelial function, and reproduction of inflammatory cytokines (126).
Data about hospitalization for infectious diseases in diabetic and non-diabetic subjects have been associated with various hyperglycemic conditions on admission, increasing poor outcomes and mortality rates. Moreover, hyperglycemia on admission was clearly associated with undiagnosed DM, strongly suggesting that an optimal glycemic control that reduces glycemic fluctuations during hospitalization should be a beneficial clinical practice for viremia control (127).
The American Diabetes Association (A.D.A.) recommends a blood glucose level of 140 to 180 mg /dL (7.8 to 10.0 mmol/L) for most critically ill patients and patients who are not in good health. Glycemic control during clinical procedures could be accompanied by insulin therapy if the hyperglycemia persists starting at a threshold ≥ 180 mg/dL (128). Thus, patients with COVID-19 and with/without DM should have a well-controlled blood glucose (129).
Influenza A Virus
The influenza A virus (IAV) induces a self-limited infection in most patients, which is characterized by several symptoms such as myalgia, fever, and dry cough (130). Nevertheless, patients with diabetes experience a more severe type of this disease (131) that is represented by a triple risk of hospitalization and double risk of fatality compared with non-diabetic sufferers (132). Despite the fact that IAV infects upon 15% of the world's population every year (133), the full mechanisms underlying its pathogenesis, especially on patients with diabetes, remain thus far inconclusive. In this sense, Hulme et al. (132) reported that the IAV infection in hyperglycemic conditions increases the endothelial damage leading to a pronounced inflammatory response; this explains, at least in part, the severity of the symptoms in patients with diabetes (132). In support of this notion, Kohio and Adamson (134) reported an enhanced IAV replication rate in pulmonary epithelial cells under elevated glucose concentrations in vitro.
Admittedly, the specific mechanisms that underline susceptibility factors for viral infections development in patients with diabetes (Table 2) are poorly understood. However, an experimental study (132) that used an in vitro and in vivo model of pulmonary epithelial-endothelial cells exposed to a high glucose concentration (12 mM) demonstrated an increased barrier damage after co-cultured cells were infected with IAV; this, in turn, augmented pulmonary edema associated with a pro-inflammatory response (132). Thus, controlling hyperglycemia seems to be important for hospitalized patients with severe viral infections and diabetes (144).
Table 2.
Main complications during viral infections in patients with diabetes.
| Pathogen | Emerging disease | Main complications in patients with diabetes | References |
|---|---|---|---|
| RESPIRATORY INFECTIONS | |||
| Influenza virus | Pneumonia | Risk of admission to the intensive care unit, fatal outcome after infection, increasing influenza severity, and secondary bacterial infections | (130, 135, 136) |
| Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV2) virus | COVID-19 | Inflammatory storm in atherosclerotic plaques, increased viral secondary infection to lung, acute respiratory distress syndrome, acute renal failure, acute cardiac injury and heat failure, and increased risk for patient mortality | (137, 138) |
| LIVER INFECTIONS | |||
| Hepatitis B virusa | Hepatitis B | Elevated serum triglyceride level, blood glucose abnormalities steatosis and cirrhosis | (22, 139) |
| Hepatitis C virus | Hepatitis C | Reduced rate of sustained virological response, progression to fibrosis and cirrhosis, and higher risk for development of hepatocellular carcinoma | (22, 140, 141) |
| OTHER INFECTIONS | |||
| Human immunodeficiency virus |
HIV/AIDS | Hypertension, dyslipidemia, and acute myocardial infarction | (142, 143) |
Hepatitis B and C Viruses
The Hepatitis C virus (HCV) and Hepatitis B virus (HBV) are known causes of hepatic decompensation, liver cirrhosis, and hepatocellular carcinoma (HCC), being two major public health problems worldwide (145–147). The evidence for a link between HCV and DM has been proposed several decades ago (148). In this sense, based on the meta-analysis of Gou and colleagues (145), patients with T2DM are more prone to HCV infection (~3.5-fold increase) compared with the risk in the non-diabetic group. In the case of HBV, the diabetic condition predisposes to acquiring the infection (147, 149). Moreover, there is a high association between diabetes and the higher risk for a worse outcome of HCV and HBV infection (139, 141, 150, 151). HCV patients with diabetes have a higher incidence of HCC compared to non-diabetic HCV patients (radio 1.73) (152). Interestingly, the use of several hypoglycemic drugs improves the prognosis for this type of cancer (153, 154).
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) increases the risk for developing T2DM (155, 156). Likewise, patients with HIV are prone to diabetes in younger people and in the absence of obesity (157, 158). In this sense, several hypotheses have been proposed to understand the mechanisms for this link, including the effects of antiretroviral drugs (ARVD), lipodystrophy, co-infections, and autoimmunity (156). The use of ARVD in patients with HIV, which include atazanavir, darunavir, and saquinavir, interfere with the GLUT-4 dynamics by increasing insulin resistance and reducing insulin secretion (159). On the other hand, it has been recognized that the HIV infection and/or its treatment can induce lipodystrophy (i.e., an abnormal distribution of fat in the body); this raises the levels of TNF-α which, in turn, contributes to increasing insulin resistance and finally triggering diabetes (160). The third hypothesis to understand the relation HIV-diabetes includes the co-participation of HCV; in this sense, the increased intrahepatic TNF-α may be a trigger to develop diabetes (161). Finally, the autoimmune hypothesis explains that some HIV-patients may undergo beta cell destruction, developing the autoimmune diabetes observed in some HIV-infected patients (162).
Physicochemical Changes During Hyperglycemia: Effects on the Immune System
Rheological Properties and Blood Viscosity
Rheological properties of blood may impact function, metabolism, motility and even the latency for clearing toxins of blood cells (163, 164). Changes in rheological conditions have been reported during diabetes and hyperglycemia, which may alter red blood cells physiology and the local microcirculation (163, 165). Indeed, some of the blood rheological properties that have been reported to be disturbed during hyperglycemia and/or diabetes include: (i) an increment in serum osmolarity (166); (ii) erythrocyte deformation that is produced by glycosylation of membrane proteins (167, 168); (iii) changes in pH (169); and (iv) an increase in blood viscosity (164, 165). All these alterations may impair the immune system activity and could explain the impact that glycemia has on the clinical outcome (Figure 1).
Furthermore, increased blood viscosity may lead to hemoconcentration and vasodilatation that increases edema (164). In close connection with this response, coagulation directly affects blood viscosity, increasing the risk for developing microangiopathy (168). In fact, anomalous erythrocyte deformability and platelet aggregation impair microcirculation, which leads to hypoxia in hyperglycemia and diabetes (170, 171). In this sense, a decrease in oxygen supply could impair the immune response because in those cells oxygen is essential for destroying infectious microorganisms (172). As a result, oxygen supplementation: (i) avoids surgical infections during the perioperative period (173, 174); and (ii) can be used to prevent infections and promote wound healing (175).
To round off and complete the above rheological scenario, it is to be noted that the concentration of fibrinogen and globulins are also important factors involved in blood viscosity (168). In fact, an increase in plasma fibrinogen in diabetic patients is a determining factor for blood viscosity (176). This, in turn, will alter oxygen supply resulting in an impaired immune response.
pH
Any change in pH may be detrimental for the proper functioning of the whole body (169), including the diabetic sufferers. In this sense, diabetic ketoacidosis (DKA) is a common hyperglycemic condition that affects both T1DM and T2DM patients, resulting in a decreased venous blood pH (below 7.3) (177). DKA results from an altered metabolism of glucose mainly produced by a decreased or abolished production of insulin (178). This, in turn, promotes the metabolism of triglycerides into glycerol and fatty acids, with the latter being further oxidized to ketone bodies, mainly acetoacetate and β-hydroxybutyrate (178, 179). Ketone bodies are weak acids that weigh down blood buffering capacity (carried out by bicarbonate anion), altering pH and resulting in a metabolic acidosis (177, 180).
As the most severe complication of DM, patients with DKA have more difficulty to handle infections (179, 181). Admittedly, it is not clear whether DM may increase the susceptibility for all infections; however, many of them (mainly the bacterial ones) are more severe, frequent and/or typical of diabetic patients (21). For instance, some of the most common infections in these patients are pneumonia and urinary tract infections (179, 182), as well as other infections difficult to manage, such as mucormycosis (183, 184), aspergillosis (185), tuberculous meningitis (186), and pulmonary coccidioidomycosis (187).
Several reports have shown the role of pH in the immune response. For example, with a pH below 6.5: (i) the mobility of polymorphonuclear leukocytes was impaired (188), which could result in delayed migration of leukocytes; (ii) chemotaxis was inhibited (188, 189); and (iii) the production of superoxide anion was decreased in neutrophils (190), resulting in an impaired “respiratory burst” (191). However, phagocytosis in bovine neutrophils was hardly affected when they were challenged with Staphylococcus aureus at acidic pH (192). Moreover, Loeffler et al. (193) reported an inhibition in lymphocytes proliferation induced by interleukin-2 (IL-2) at acidic pH. Nevertheless, only some functions seem to be affected in lymphocytes at an acidic pH, namely, at pH 6.7 (as compared with pH 7.1) an increase in lymphocytes mobilization was reported (194, 195). A possible explanation for this finding is that every cell type and specific functions are differentially altered by pH gradients. Obviously, further studies are required to understand the molecular mechanisms underlying each cellular type and the corresponding physiological phenomena.
Other important alterations induced by hyperglycemia in the circulatory system are related to a miss-functionality of the enzymatic machinery of blood cells, including Na+/K+-ATPase activity and glucose-6-phosphate dehydrogenase (G6PD) (see below).
Alterations in Na+/K+-ATPase Activity
Na+/K+-ATPase is a transmembrane protein responsible for maintaining intracellular Na+/K+ balance by generating the gradients of Na+ and K+ (196). This enzyme is expressed ubiquitously in almost all cell types, regulating a plethora of functions such as the reabsorption of glucose and amino acids (which depends on a Na+ gradient) in distal convoluted tubule, motility in sperm cells, action potentials in synaptic neurons, etc. (197). In erythrocytes, this enzyme is involved in maintaining their volume and water homeostasis (198); while in lymphocytes, their proliferation induced by a variety of stimulus is dependent on Na+/K+-ATPase activity (199). Interestingly, Na+/K+-ATPase activity is decreased in the erythrocytes from T2DM sufferers (198, 200), but its expression remains unaltered (201). These findings suggest that the activity of Na+/K+-ATPase may be used as a potential biomarker for detecting early phases of T2DM (202). Within this context, one theory that explains the effects of hyperglycemia on Na+/K+-ATPase is by glycosylation, which induces the impairment of the ATPase activity in erythrocytes (202). In fact, this enzyme has several glycosylation sites located at β-subunits, some of them related to protein maturation (203) and other functional processes (197). These lines of evidence show the importance of glycosylation in Na+/K+-ATPase activity.
On the other hand, Na+/K+-ATPase partake in the functionality of immune cells (199, 204, 205). Indeed, proliferation of lymphocytes is dependent on Na+/K+-ATPase activity (199) and the expression of nuclear factor of activated T cells transcription complex (NFAT) of thymocytes (206); this factor is essential for the production of Interleukin-2 (207), a cytokine produced by lymphocytes during a microbial infection (208). Hence, immunologic and hematologic deficiencies in diabetic patients are related to multiple alterations, which may include aberrant activity of the Na+/K+-ATPase.
In agreement with the above findings, a reasonable possibility to explain the alterations in immune system activity during diabetes is that the Na+/K+-ATPase activity could be equally decreased in both lymphocytes and erythrocytes (since these cell types are in the same environment) (201). Besides this, protein glycosylation can occur by enzymatic, but also by non-enzymatic ways; in this respect, glucose is chemically attached to proteins by Schiff base and Amadori product adducts, resulting in a variety of biological effects, including inactivation of enzymes (209), such as Na+/K+-ATPase. It has even been reported that a deficiency in glucose-6-phosphate dehydrogenase, an enzyme altered in diabetes, increases protein glycosylation (210), supporting the idea previously proposed (see below).
Glucose-6-Phosphate Dehydrogenase
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme expressed ubiquitously in all mammalian tissues. It plays an important role in the pentose pathway catalyzing the first reaction in this metabolic route, which is necessary to convert glucose into pentose sugars (211). This pathway produces nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), an antioxidant molecule that catalyzes the reaction to regenerate reduced glutathione (212).
Many studies have reported the importance of G6PD in antioxidant defense against toxicity of reactive oxygen species (ROS) (211, 213). Interestingly, a relationship is established between diabetes and a decrease in G6PD activity in a variety of cells from rats (212) and humans (214). Additionally, this enzyme plays an important role against infections (213, 215) and in T cell proliferation (216). In keeping with this view, a deficiency of this enzyme in leukocytes is related to serious infectious diseases, such as chronic granulomatous disease (172, 217).
Admittedly, the specific molecular mechanisms that explain the effects of chronic hyperglycemia on G6PD activity in immune cells remain uncertain. For example, Xu et al. (26) showed evidence of inhibition of this enzyme via phosphorylation by protein kinase A in kidney cortex of diabetic rats pretreated with streptozotocin. Similar results were observed in aortic endothelial cells cultured under hyperglycemic conditions (218). Another possibility to explain the effect of glucose on G6PD activity is via protein glycosylation produced by a high glucose concentration (219).
In summary, the above physicochemical alterations resulting from hyperglycemia impair the immune response, predisposing diabetic subjects to acquire infections as well as exacerbates them.
Potential Benefits of Hypoglycemic Drugs on the Outcome of Clinical Infections
Hypoglycemic Drugs and Their Clinical Effects on Bacterial Infections
An uncontrolled blood glucose level is associated with an increase in microvascular and macrovascular complications in diabetic patients (220). Likewise, a hyperglycemic state results in multiple consequences, including osmotic diuresis, fluid/electrolyte imbalance, poor wound healing, impaired immune response, and increased susceptibility to infections, among others (22, 221). Accordingly, these pathophysiological conditions have led to the implementation of therapeutic strategies for a tight glycemic control in patients with T2DM, resulting in the development of the so-called glucose-lowering drugs (i.e., Oral Antidiabetic Drugs; OADs).
Several lines of evidence have shown that the use of OADs to maintain tight blood glucose concentrations between 80 and 110 mg/dl decreases infection-related complications and mortality (see Table 3). For example, metformin, which is the first-line pharmacological agent for T2DM treatment (233), reduced airway glucose permeability and prevented the higher load of Staphylococcus aureus (S. aureus) induced by hyperglycemia (224). Similarly, metformin pre-treatment inhibited the glucose-induced growth of Pseudomonas aeruginosa, increased transepithelial electrical resistance (TEER) and decreased glucose flux in an epithelial cell culture model (234). In this sense, mutants of genes affecting glucose uptake of P. aeruginosa decreased the bacterial loads on streptozotocin-induced hyperglycemic mice compared to control.
Table 3.
Pharmacodynamics of some hypoglycemic drugs and their reported effects on infectious processes.
| Drug | Mechanism of action | Reported effects on infectious processes |
|---|---|---|
| Metformin | It decreases hepatic production and intestinal absorption of glucose with an improvement in insulin sensitivity (222) | Decreased risk of chronic lower respiratory diseases (223) Reduced infection with S. aureus and P. aeruginosa in mice (224) Inhibited growth of P. aeruginosa in airway epithelial cell line (Calu-3) in vitro (225) Reduced risk of Mycobacterium tuberculosis infection compared to those which received sulfonylureas as initial treatment for diabetes (226) Reduction of ~20% in the risk of sepsis (227) |
| Sulfonylureas | It increases insulin secretion via ATP-sensitive potassium channel- pathway (228) | Inefficient to reduce the risk of sepsis (227) |
| Acarbose | It inhibits the alpha-glucosidase enzymes in the small intestine, delaying the breaking down complex carbohydrates and sucrose (229) | N/D |
| Thiazolidinediones | Interaction with the nuclear peroxisome proliferator-activated receptor-gamma (PPAR- γ), regulating the transcription of several insulin responsive genes (230) | Moderate reduction in the risk of sepsis (227) |
| Dipeptidyl peptidase 4(DPP-4) inhibitors | It increases insulin secretion and inhibits the release of glucagon (231) | No association between DPP-4 inhibitors and risk of sepsis (227) |
| Sodium-glucose co-transporter 2 (SGLT2) inhibitors | Inhibit renal reabsorption of glucose (232) | N/D |
Interestingly: (i) metformin pre-treatment of hyperglycemic animals reduced both airway glucose and bacterial load (234); (ii) the incidence of tuberculosis has been related to abnormal glucose levels, whereas metformin is a protective agent in the treatment of tuberculosis in diabetic patients (235); (iii) metformin treatment was also associated with an increased risk of bacterial pneumonia in patients with chronic obstructive pulmonary disease from a nationwide cohort study (Taiwan) (236); and (iv) pneumonia is a swelling disease usually caused by a bacterial infection commonly associated with diabetic patients (237).
Consistent with the above findings, diabetic patients with community-acquired pneumonia (CAP) developed worse results and longer hospital stays in comparison to patients with CAP without diabetes (238); accordingly, it is important to discuss the relationship between the use of OADs and pneumonia. Indeed, these data support airway glucose as a critical determinant of increased bacterial load during diabetes (225).
Moreover, Mendy et al. (223) analyzed data from the National Health and Nutrition Examination Survey during 1988–1994 and 1999–2010 for participants aged 40 years or older who had diabetes and were followed up for mortality through 2011. Their results showed that metformin was associated with a decreased risk for chronic lower respiratory diseases (CLRD) mortality in the overall population (HR: 0.39, 95% CI: 0.15–0.99) and among participants with baseline CLRD (HR: 0.30, 95% CI: 0.10–0.93) (223).
Likewise, Pan et al. (226) investigated the effect of metformin vs. sulfonylureas on tuberculosis risk in patients with T2DM. The study demonstrated that patients with T2DM treated with metformin in the initial 2 years, had a significant reduced risk of tuberculosis as compared to those receiving sulfonylureas as initial treatment (226).
Furthermore, Shih et al. (227) reported the relationship between the use of AODs and the risk of hospitalization for sepsis. The authors found that the use of metformin was associated with ~20% reduced risk of sepsis as compared with non-use. In contrast, meglitinides and sulfonylureas were associated with increased risk of future sepsis events, but this association was not evident among recent and current sulfonylurea users. Moreover, the DPP-4 inhibitors and thiazolidinediones on sepsis were neutral, nevertheless, the occurrence of sepsis in current thiazolidinediones users was reduced (227).
On the other hand, some studies have shown that pretreatment with dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, reduced blood and bronchoalveolar lavage glucose concentrations and P. aeruginosa CFU in leptin receptor-deficient (db/db) mice, as compared to those seen in wild type (WT) mice (239).
In summary, the available evidence thus far has established the increased susceptibility to certain types of infections related to hyperglycemia in T2DM. Clearly, further studies on the mechanisms regulating OADs and bacterial action on specific tissues/organs are required. Such studies could yield potential alternatives to prevent/suppress hyperglycemia and bacterial infections.
Moreover, the risk for developing infections is increased in hyperglycemic environments, where there is a lower production of interleukins, a reduced chemotaxis and phagocytic activity, and a gastrointestinal dysmotility (22). The use of specific OADs such as metformin is associated with reduced hospital-treated infections, septicemia prognosis, and some kinds of respiratory illnesses (224, 240). Indeed, another study in diabetic patients has shown a reduction in autoimmune diseases by an acute intervention with OADs, such as DPP-4 in combination with other hypoglycemic drugs (241). One mechanism that may improve those immune response effects is through GLP-1 action that induces insulin secretion and inhibits glucagon secretion, ameliorating the glycemic variability (242).
To conclude this section, it is to be noted that experimental anorexia seems to play an important protective role in supporting the recuperation of bacterial infections (19). Moreover, mortality in critical illnesses (e.g., sepsis, severe burning, etc.) may increase via alterations in immune cell activity that, in turn, may be mediated by the release of stress hormones (cortisol, catecholamines, etc.) and the hyperglycemia that these hormones induce (57). As hyperglycemia impairs periphery glucose usage, administration of insulin improves cellular uptake and attenuates the inflammatory response (57).
A higher risk of CAP was found with other OADs, except with dipeptidyl-peptidase 4 (DPP-4) inhibitors (237). Indeed, in a retrospective cohort and a meta-analysis study, DPP-4 inhibitors failed to increase the risk of pneumonia during diabetes (243). These controversial data about the use OADs and the outcome of bacterial infections in diabetic patients point out the necessity for more detailed analyses and clinical observations.
In view that hyperglycemia may be a determinant factor in the outcome for bacterial infections, any effort for controlling the increases glucose levels is valuable. Another interesting approach may be the supplementation with calcium and vitamin D because it decreases insulin resistance and hyperglycemia (244); nevertheless, some strategies must be considered to ponder the risks and benefits.
Further Considerations
Hypoglycemia occurs when there exists a lack of adequate food intake, excessive exercise, a stressful experience, excessive alcohol consumption, concurrent infections, severe digestive and urologic diseases, and/or after taking antidiabetic medications (245). This suggests that hypoglycemia is an endocrine alarming signal that is triggered to level the required concentrations of blood glucose in the body.
Considering that fasting plasma glucose is normally maintained between 70 and 99 mg/dL (2), a biomarker associated with high blood glucose levels is HbA1c, whose normal range is between 4 and 5.7% in healthy people. Less than 7% of HbA1c is found in controlled people with diabetes, and above 8% is found in people with uncontrolled diabetes (246). Low or high levels of HbA1c have been related to severe hypoglycemic episodes with a glucose-lowering regimen in patients with diabetes (247).
Glycemic control and reduction of hyperglycemia or hypoglycemia events are the main challenges in the clinical experience to achieve decreases of blood glucose variability (248). Indeed, levels of glucose and its constant fluctuations are good indicators of organ dysfunctions such as those associated with infections (249). Patients with diabetes often suffer from chronic low-grade infections such as periodontitis and foot ulceration. Surgery-site infections and susceptibility to septic shock increase with pre and post-operative glucose levels and their variability (250, 251), suggesting that glucose monitoring is one of the key elements in hyperglycemia and hypoglycemia management diseases where the immune system is compromised.
Glucose variability is currently considered more deleterious than chronic hyperglycemia in the development of diabetes-related complications (252). However, some studies suggest that an intensive glucose control does not improve some of the diabetes-associated complications such as cardiovascular failures, raising the mortality rate (253). Furthermore, a tight glucose control induces hypoglycemic episodes and the increased response of the immune system, impacting on coagulant factors, pro-inflammatory cytokines, proatherogenic cell adhesion molecules, and nitric oxide-mediated vasodilatation. Innate immunity response is activated nearly after acute or chronic infections are experienced by diabetes sufferers. For this, the study of the suppression of innate immune system is a key factor, since it exacerbates the inflammatory response after an acute hypoglycemia episode, inducing prothrombotic changes and increasing platelet reactivity (254).
Another consideration is that under normal and pathophysiological metabolic functions, individuals course with glucose swings during the day (255), correlating them with the gastric emptying rate and postprandial glucose levels. Glycemic fluctuations are limited by low glucose levels that slow the gastric emptying or by high glucose that accelerate it (256). However, a hypoglycemic state promotes reverse effects; hence, the gastric emptying is accelerated and the absorption speed of nutrients is increased to reach the physiological glycemic levels, suggesting that gastrointestinal motility and gastric motor function are important factors to consider for a therapy of glycemic control (257).
During physiological gastric emptying, carbohydrates and proteins are evacuated faster than lipids for their caloric content. The evacuation of these macronutrients begins at 20 to 40 min after food intake and when they reach the intestine, incretin hormones are secreted to blood. Glucagon-like peptide-1 (GLP-1) is an incretin hormone that stimulates insulin secretion, reduces glucagon secretion, and delays gastric emptying in a glucose-dependent manner (256, 258).
Significantly, glucose-lowering therapies through the use of diverse drug classes have been reported as an important source of heart failure risk, particularly with differential effects on insulin (259). Consequently, older patients are the most affected population, especially if a diminished food intake, excessive alcohol use, combination of non-prescribed medications, concomitant infections, and diabetic complications are also taken into account (260). Because of this, an intervention with a forced hypoglycemia should be considered with caution according to disease timing, age, nutritional behaviors, type of medications and concomitant infections.
General Conclusion
Hyperglycemia clearly induces physiological and immunological disorders in body tissues/organs that may predispose and exacerbate some infectious diseases. Therefore, the control of glucose levels could be an alternative tool to contribute to the fight against infections not only in diabetic patients, but also in other conditions that induce hyperglycemia, such as SIH. In addition, several studies have shown the potential benefits of controlling it (e.g., pharmacological approaches), opening a new option to improve the outcome of some infections (bacterial and viral). It is worth noting that the authors of this review agree that glycemic control is necessary as part of good intervention strategies to treat current and emerging infectious diseases. Admittedly, the clinical evidence for reducing glycemic exposure requires more supportive data, specifically for hypoglycemia as a tool to fight infections in humans. Notwithstanding, this review summarizes enough preclinical evidence to increase our chances of beating infections by focusing on the key role of glycemic control.
Author Contributions
JC-R, CE-G, and BM-C developed the central idea of this article and wrote the manuscript. BM-C proposed the central idea, made the graphs, and obtained funding. PV-L, EC-S, AL-B, and AQ-S provided original ideas, developed some sections, and reviewed the manuscript. CMV and JM-R discussed the central ideas, reviewed, edited, and corrected the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor and reviewer AV declared a shared affiliation with one of the authors EC-S at time of review.
Acknowledgments
We thank Verónica Rodríguez Gallegos for her support in the elaboration of graphics.
Footnotes
Funding. BM-C was supported by Dirección General de Investigación y Posgrado from Autonomous University of Aguascalientes by the research grant: PIBB19-1. CMV was financially supported by the SEP-Cinvestav Research Support Fund (grant No. 50). JM-R was supported financially by PAICYT 2019–2020 and 2020–2021, Science Grants from the Universidad Autónoma de Nuevo León and by the Fronteras de la Ciencia CONACyT grant 1502. AL-B was a recipient of a Beca de Posdoctorado Nacional 2018–2020.
References
- 1.Schofield JD, Liu Y, Balakrishna PR, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Ther. (2016) 7:17. 10.1007/s13300-016-0167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A.D.A . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl. 1):S13–27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 3.A.D.A . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42(Suppl. 1):S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg CS. Wound healing in the patient with diabetes mellitus. Nurs Clin North Am. (1990) 25:247–61. [PubMed] [Google Scholar]
- 5.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. (2007) 23:3–13. 10.1002/dmrr.682 [DOI] [PubMed] [Google Scholar]
- 6.Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. (2020) 14:513–7. 10.1016/j.dsx.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, et al. Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2. (COVID-19) infection. Diabetes Obes Metab. (2020) 22:1907–14. 10.1111/dom.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. (2020) 162:108142. 10.1016/j.diabres.2020.108142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed Pharmacother. (2018) 107:306–28. 10.1016/j.biopha.2018.07.157 [DOI] [PubMed] [Google Scholar]
- 10.Vichaibun V, Khananurak K, Sophonnithiprasert T. Comparative analysis of plasma total antioxidant capacity in patients with hyperglycemia and hyperglycemia plus dyslipidemia. Diabetes Metab Syndr. (2019) 13:90–4. 10.1016/j.dsx.2018.08.029 [DOI] [PubMed] [Google Scholar]
- 11.Bistrian BR. Hyperglycemia and Infection: which is the chicken and which is the egg? (2001) 25:180–1. 10.1177/0148607101025004180 [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. (2007) 21:617–38. 10.1016/j.idc.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Ahmadishooli A, Davoodian P, Shoja S, Ahmadishooli B, Dadvand H, Hamadiyan H, et al. frequency and antimicrobial susceptibility patterns of diabetic foot infection of patients from Bandar Abbas District, Southern Iran. J Pathog. (2020) 2020:1–10. 10.1155/2020/1057167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Res Clin Pract. (2020) 165:108263. 10.1016/j.diabres.2020.108263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung SM, Lee YY, Ha E, Yoon JS, Won KC, Lee HW, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. (2020) 44:405–13. 10.4093/dmj.2020.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Deng Q, Feng J, Li F, Xiong N, He Q. Clinical characteristics of diabetic patients with COVID-19. J Diabetes Res. (2020) 2020:1652403. 10.1155/2020/1652403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. (2020) 146:110–8. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudsk KA, Laulederkind A, Hanna MK. Most infectious complications in parenterally fed trauma patients are not due to elevated blood glucose levels. J Parenter Enteral Nutr. (2001) 25:174–9. 10.1177/0148607101025004174 [DOI] [PubMed] [Google Scholar]
- 19.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. (2016) 166:1512–25.e1512. 10.1016/j.cell.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao S, Schieber AMP, O'Connor CP, Leblanc M, Michel D, Ayres JS. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell. (2017) 168:503–16.e512. 10.1016/j.cell.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. (1999) 341:1906–12. 10.1056/nejm199912163412507 [DOI] [PubMed] [Google Scholar]
- 22.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. (2012) 16(Suppl. 1):S27–36. 10.4103/2230-8210.94253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. (2007) 117:1219–22. 10.1172/jci32169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price CL, Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. (2010) 14:1806–15. 10.1111/j.1582-4934.2009.00803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. (2007) 40:1037–44. 10.1590/s0100-879x2006005000143 [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol. (2005) 289:F1040–7. 10.1152/ajprenal.00076.2005 [DOI] [PubMed] [Google Scholar]
- 27.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. (2010) 2010:792393. 10.1155/2010/792393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolowczuk I, Verwaerde C, Viltart O, Delanoye A, Delacre M, Pot B, et al. Feeding our immune system: impact on metabolism. Clin Dev Immunol. (2008) 2008:639803. 10.1155/2008/639803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristea OM, Avramescu CS, Balasoiu M, Popescu FD, Popescu F, Amzoiu MO. Urinary tract infection with Klebsiella pneumoniae in Patients with Chronic Kidney Disease. Curr Health Sci J. (2017) 43:137–48. 10.12865/chsj.43.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Ma A, Han X, Chan L, Liang H, Litifu A, et al. T cell profile was altered in pulmonary tuberculosis patients with type 2 diabetes. Med Sci Monit. (2018) 24:636–42. 10.12659/msm.905651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. (1997) 14:29–34. [DOI] [PubMed] [Google Scholar]
- 32.Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. (2015) 21 Suppl 2:S27–32. 10.1016/j.cmi.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 33.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. (2005) 41:281–8. 10.1086/431587 [DOI] [PubMed] [Google Scholar]
- 34.Sanz-Corbalán I, Lázaro-Martínez JL, García-Morales E, Molines-Barroso R, Álvaro-Afonso F, García-Álvarez Y. Advantages of early diagnosis of diabetic neuropathy in the prevention of diabetic foot ulcers. Diabetes Res Clin Pract. (2018) 146:148–54. 10.1016/j.diabres.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 35.Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J Clin Endocrinol Metab. (2016) 101:3401–8. 10.1210/jc.2016-2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleftheriadou I, Dimitrakopoulou N, Kafasi N, Tentolouris A, Dimitrakopoulou A, Anastasiou IA, et al. Endothelial progenitor cells and peripheral neuropathy in subjects with type 2 diabetes mellitus. J Diabetes Complicat. (2020) 34:107517. 10.1016/j.jdiacomp.2019.107517 [DOI] [PubMed] [Google Scholar]
- 37.Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. (2016) 17:917. 10.3390/ijms17060917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. (2006) 29:1288–93. 10.2337/dc05-2425 [DOI] [PubMed] [Google Scholar]
- 39.Chang BB, Darling RC, III, Paty PS, Lloyd WE, Shah DM, Leather RP. Expeditious management of ischemic invasive foot infections. Cardiovasc Surg. (1996) 4:792–5. [DOI] [PubMed] [Google Scholar]
- 40.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. (1999) 26:259–65. 10.1111/j.1574-695X.1999.tb01397.x [DOI] [PubMed] [Google Scholar]
- 41.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections? A meta-analysis. Diabetes Care. (2005) 28:454–60. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Jiang H, Ren H, Hu X, Wang X, Han C. AGEs and chronic subclinical inflammation in diabetes: disorders of immune system. Diabetes Metab Res Rev. (2015) 31:127–37. 10.1002/dmrr.2560 [DOI] [PubMed] [Google Scholar]
- 43.Zylla D, Gilmore G, Eklund J, Richter S, Carlson A. Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. J Diabetes Complications. (2019) 33:335–9. 10.1016/j.jdiacomp.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 44.Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest. (2012) 122:3854–7. 10.1172/jci66180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. (1995) 12:916–20. [DOI] [PubMed] [Google Scholar]
- 46.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. 10.1056/NEJMoa011300 [DOI] [PubMed] [Google Scholar]
- 47.Furnary AP, Wu Y. Eliminating the diabetic disadvantage: the Portland Diabetic Project. Semin Thorac Cardiovasc Surg. (2006) 18:302–8. 10.1053/j.semtcvs.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 48.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. (2016) 4:148–58. 10.1016/s2213-8587(15)00379-4 [DOI] [PubMed] [Google Scholar]
- 49.Hine JL, de Lusignan S, Burleigh D, Pathirannehelage S, McGovern A, Gatenby P, et al. Association between glycaemic control and common infections in people with Type 2 diabetes: a cohort study. Diabet Med. (2017) 34:551–7. 10.1111/dme.13205 [DOI] [PubMed] [Google Scholar]
- 50.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. (2001) 17:107–24. 10.1016/s0749-0704(05)70154-8 [DOI] [PubMed] [Google Scholar]
- 51.Bochicchio GV, Bochicchio KM, Joshi M, Ilahi O, Scalea TM. Acute glucose elevation is highly predictive of infection and outcome in critically injured trauma patients. Ann Surg. (2010) 252:597–602. 10.1097/SLA.0b013e3181f4e499 [DOI] [PubMed] [Google Scholar]
- 52.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. (2005) 59:80–3. 10.1097/01.ta.0000171452.96585.84 [DOI] [PubMed] [Google Scholar]
- 53.Chang MW, Huang CY, Liu HT, Chen YC, Hsieh CH. Stress-induced and diabetic hyperglycemia associated with higher mortality among intensive care unit trauma patients: cross-sectional analysis of the propensity score-matched population. Int J Environ Res Public Health. (2018) 15:992. 10.3390/ijerph15050992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CH, Wang JL, Wu LC, Chuang LM, Lin HH. Diabetes, glycemic control, and risk of infection morbidity and mortality: a cohort study. Open Forum Infect Dis. (2019) 6:ofz358. 10.1093/ofid/ofz358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. 10.1016/s0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. (2004) 30:748–56. 10.1007/s00134-004-2167-y [DOI] [PubMed] [Google Scholar]
- 57.Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. (2014) 2014:486403. 10.1155/2014/486403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mechanick JI. Metabolic mechanisms of stress hyperglycemia. JPEN J Parenter Enteral Nutr. (2006) 30:157–63. 10.1177/0148607106030002157 [DOI] [PubMed] [Google Scholar]
- 59.Bar-Or D Rael LT Madayag RM Banton KL Tanner A Acuna DL . Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front Med. (2019) 6:54. 10.3389/fmed.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karunakar MA, Staples KS. Does stress-induced hyperglycemia increase the risk of perioperative infectious complications in orthopaedic trauma patients? J Orthop Trauma. (2010) 24:752–6. 10.1097/BOT.0b013e3181d7aba5 [DOI] [PubMed] [Google Scholar]
- 61.Richards JE, Kauffmann RM, Obremskey WT, May AK. Stress-induced hyperglycemia as a risk factor for surgical-site infection in nondiabetic orthopedic trauma patients admitted to the intensive care unit. J Orthop Trauma. (2013) 27:16–21. 10.1097/BOT.0b013e31825d60e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bader MS, Yi Y, Abouchehade K, Haroon B, Bishop LD, Hawboldt J. Community-acquired pneumonia in patients with diabetes mellitus: predictors of complications and length of hospital stay. Am J Med Sci. (2016) 352:30–5. 10.1016/j.amjms.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 63.Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. (2007) 30:2251–7. 10.2337/dc06-2417 [DOI] [PubMed] [Google Scholar]
- 64.Principi N, Iughetti L, Cappa M, Maffeis C, Chiarelli F, Bona G, et al. Streptococcus pneumoniae oropharyngeal colonization in school-age children and adolescents with type 1 diabetes mellitus: impact of the heptavalent pneumococcal conjugate vaccine. Hum Vaccin Immunother. (2016) 12:293–300. 10.1080/21645515.2015.1072666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baghaei P, Marjani M, Javanmard P, Tabarsi P, Masjedi MR. Diabetes mellitus and tuberculosis facts and controversies. J Diabetes Metab Disord. (2013) 12:58. 10.1186/2251-6581-12-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Lopez N, Martinez AGR, Garcia-Hernandez MH, Hernandez-Pando R, Castañeda-Delgado JE, Lugo-Villarino G, et al. Type-2 diabetes alters the basal phenotype of human macrophages and diminishes their capacity to respond, internalise, and control Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. (2018) 113:e170326. 10.1590/0074-02760170326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raya S, Belbase A, Dhakal L, Govinda Prajapati K, Baidya R, Kishor Bimali N. In-vitro biofilm formation and antimicrobial resistance of Escherichia coli in diabetic and nondiabetic patients. Biomed Res Int. (2019) 2019:1474578. 10.1155/2019/1474578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suri RS, Mahon JL, Clark WF, Moist LM, Salvadori M, Garg AX. Relationship between Escherichia coli O157:H7 and diabetes mellitus. Kidney Int Suppl. (2009) 112:S44–6. 10.1038/ki.2008.619 [DOI] [PubMed] [Google Scholar]
- 69.Wang MC, Tseng CC, Wu AB, Lin WH, Teng CH, Yan JJ, et al. Bacterial characteristics and glycemic control in diabetic patients with Escherichia coli urinary tract infection. J Microbiol Immunol Infect. (2013) 46:24–9. 10.1016/j.jmii.2011.12.024 [DOI] [PubMed] [Google Scholar]
- 70.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. (2015) 8:129–36. 10.2147/dmso.S51792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. (2009) 14:144–50. 10.1111/j.1523-5378.2009.00705.x [DOI] [PubMed] [Google Scholar]
- 72.Hosseininasab Nodoushan SA, Nabavi A. The interaction of Helicobacter pylori infection and type 2 diabetes mellitus. Adv Biomed Res. (2019) 8:15. 10.4103/abr.abr_37_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schimke K, Chubb SA, Davis WA, Phillips P, Davis TM. Antiplatelet therapy, Helicobacter pylori infection and complicated peptic ulcer disease in diabetes: the Fremantle Diabetes Study. Diabet Med. (2009) 26:70–5. 10.1111/j.1464-5491.2008.02637.x [DOI] [PubMed] [Google Scholar]
- 74.Shi Y, Duan JY, Liu DW, Qiao YJ, Han QX, Pan SK, et al. Helicobacter pylori infection is associated with occurrence of proteinuria in type 2 diabetes patients: a systemic review and meta-analysis. Chin Med J. (2018) 131:2734–40. 10.4103/0366-6999.245269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cervantes-García E, García-González R, Reséndiz-Albor A, Salazar-Schettino PM. Infections of diabetic foot ulcers with methicillin-resistant Staphylococcus aureus. Int J Low Extrem Wounds. (2015) 14:44–9. 10.1177/1534734614564053 [DOI] [PubMed] [Google Scholar]
- 76.Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia. (2010) 53:914–23. 10.1007/s00125-010-1672-5 [DOI] [PubMed] [Google Scholar]
- 77.Nicolau DP, Stein GE. Therapeutic options for diabetic foot infections: a review with an emphasis on tissue penetration characteristics. J Am Podiatr Med Assoc. (2010) 100:52–63. 10.7547/1000052 [DOI] [PubMed] [Google Scholar]
- 78.Smit J, Thomsen RW, Schønheyder HC, Nielsen H, Frøslev T, Søgaard M. Outcome of community-acquired staphylococcus aureus bacteraemia in patients with diabetes: a historical population-based cohort study. PLoS ONE. (2016) 11:e0153766. 10.1371/journal.pone.0153766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chattar-Cora D, Tulsyan N, Cudjoe EA, Onime GD, Pyo DJ, Weinstein L. Necrotizing fasciitis of the head and neck: a report of two patients and review. Head Neck. (2002) 24:497–501. 10.1002/hed.10060 [DOI] [PubMed] [Google Scholar]
- 80.Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. (2010) 49:1051–7. 10.2169/internalmedicine.49.2964 [DOI] [PubMed] [Google Scholar]
- 81.Zhang WJ, Cai XY, Yang C, Zhou LN, Cai M, Lu XF, et al. Cervical necrotizing fasciitis due to methicillin-resistant Staphylococcus aureus: a case report. Int J Oral Maxillofac Surg. (2010) 39:830–4. 10.1016/j.ijom.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 82.Thwaini A, Khan A, Malik A, Cherian J, Barua J, Shergill I, et al. Fournier's gangrene and its emergency management. Postgrad Med J. (2006) 82:516–9. 10.1136/pgmj.2005.042069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran HA, Hart AM. Fournier's gangrene. Intern Med J. (2006) 36:200–1. 10.1111/j.1445-5994.2006.01031.x [DOI] [PubMed] [Google Scholar]
- 84.Carfrae MJ, Kesser BW. Malignant otitis externa. Otolaryngol Clin North Am. (2008) 41:537–49. 10.1016/j.otc.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 85.Prasanna Kumar S, Ravikumar A, Somu L, Ismail NM. Malignant otitis externa: an emerging scourge. J Clin Gerontol Geriatr. (2013) 4:128–31. 10.1016/j.jcgg.2013.02.003 [DOI] [Google Scholar]
- 86.Chen PC, Tsai SH, Wang JC, Tzeng YS, Wang YC, Chu CM, et al. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE. (2019) 14:e0223126. 10.1371/journal.pone.0223126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yildiz O, Aygen B, Esel D, Kayabas U, Alp E, Sumerkan B, et al. Sepsis and meningitis due to Listeria monocytogenes. Yonsei Med J. (2007) 48:433–9. 10.3349/ymj.2007.48.3.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang JJ, He XY, Ye H. Rhombencephalitis caused by Listeria monocytogenes with hydrocephalus and intracranial hemorrhage: A case report and review of the literature. World J Clin Cases. (2019) 7:538–47. 10.12998/wjcc.v7.i4.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bali A, Chadha I, Sharma A. Necrotizing fasciitis of the chest wall caused by infected dentigerous cyst: a case report. J Maxillofacial Oral Surg. (2012) 11:347–50. 10.1007/s12663-011-0214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solá E, Rivera C, Mangual M, Martinez J, Rivera K, Fernandez R. Diabetes mellitus: an important risk factor for reactivation of tuberculosis. Endocrinol Diabetes Metab Case Rep. (2016) 2016:16-0035. 10.1530/edm-16-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen SY, Lee JJ, Chien CC, Tsai WC, Lu CH, Chang WN, et al. High incidence of severe neurological manifestations and high mortality rate for adult Listeria monocytogenes meningitis in Taiwan. J Clin Neurosci. (2020) 71:177–85. 10.1016/j.jocn.2019.08.072 [DOI] [PubMed] [Google Scholar]
- 92.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1. (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. (1995) 270:23780–4. 10.1074/jbc.270.40.23780 [DOI] [PubMed] [Google Scholar]
- 93.Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z. The alpha and beta subunits of IkappaB kinase. (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor. (TNF) receptor 1 in response to TNF. Mol Cell Biol. (2001) 21:3986–94. 10.1128/mcb.21.12.3986-3994.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. (1993) 77:1690–4. 10.1210/jcem.77.6.8263159 [DOI] [PubMed] [Google Scholar]
- 95.Bo S, Broglio F, Settanni F, Parasiliti Caprino M, Ianniello A, Mengozzi G, et al. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: a pilot study. Nutr Diabetes. (2017) 7:303. 10.1038/s41387-017-0010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, et al. Glucose metabolism and catecholamines. Crit Care Med. (2007) 35:S508–18. 10.1097/01.Ccm.0000278047.06965.20 [DOI] [PubMed] [Google Scholar]
- 97.Escarcega Gonzalez CE, Gonzalez Hernandez A, Villalon CM, Rodriguez MG, Marichal Cancino BA. beta-adrenoceptor blockade for infantile hemangioma therapy: do beta3-adrenoceptors play a role? J Vasc Res. (2018) 55:159–68. 10.1159/000489956 [DOI] [PubMed] [Google Scholar]
- 98.Marichal-Cancino BA, González-Hernández A, Muñoz-Islas E, Villalón CM. Monoaminergic receptors as modulators of the perivascular sympathetic and sensory CGRPergic outflows. Curr Neuropharmacol. (2020) 18:790–808. 10.2174/1570159x18666200503223240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. (1992) 15:1413–22. 10.2337/diacare.15.10.1413 [DOI] [PubMed] [Google Scholar]
- 100.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. (2000) 355:773–8. 10.1016/s0140-6736(99)08415-9 [DOI] [PubMed] [Google Scholar]
- 101.Marcovecchio ML, Chiarelli F. The effects of acute and chronic stress on diabetes control. Sci Signal. (2012) 5:pt10. 10.1126/scisignal.2003508 [DOI] [PubMed] [Google Scholar]
- 102.Chao H-Y, Liu P-H, Lin S-C, Chen C-K, Chen J-C, Chan Y-L, et al. Association of in-hospital mortality and dysglycemia in septic patients. PloS ONE. (2017) 12:e0170408. 10.1371/journal.pone.0170408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rau CS, Wu SC, Chen YC, Chien PC, Hsieh HY, Kuo PJ, et al. Higher mortality in trauma patients is associated with stress-induced hyperglycemia, but not diabetic hyperglycemia: a cross-sectional analysis based on a propensity-score matching approach. Int J Environ Res Public Health. (2017) 14:1161. 10.3390/ijerph14101161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rau CS, Wu SC, Chen YC, Chien PC, Hsieh HY, Kuo PJ, et al. Stress-induced hyperglycemia in diabetes: a cross-sectional analysis to explore the definition based on the trauma registry data. Int J Environ Res Public Health. (2017) 14:1527. 10.3390/ijerph14121527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. (2018) 41:513–21. 10.2337/dc17-2131 [DOI] [PubMed] [Google Scholar]
- 106.Erben N, Ozgunes I, Aksit F, Doyuk Kartal E, Colak E, Usluer G. Healthcare-associated infections and the distribution of causative pathogens in patients with diabetes mellitus. Eur J Clin Microbiol Infect Dis. (2013) 32:821–5. 10.1007/s10096-013-1816-x [DOI] [PubMed] [Google Scholar]
- 107.Korbel L, Easterling RS, Punja N, Spencer JD. The burden of common infections in children and adolescents with diabetes mellitus: a Pediatric Health Information System study. Pediatr Diabetes. (2018) 19:512–9. 10.1111/pedi.12594 [DOI] [PubMed] [Google Scholar]
- 108.Ahmadi F, Moogahi S, Bahrami H. Determining frequency and pattern of infections associated with diabetes based educational hospitals in Ahvaz city; Iran. Diabetes Metab Syndr. (2019) 13:2441–4. 10.1016/j.dsx.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 109.Klekotka RB, Mizgała E, Król W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. (2015) 83:401–8. 10.5603/PiAP.2015.0065 [DOI] [PubMed] [Google Scholar]
- 110.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. (2019) 4:e131774. 10.1172/jci.insight.131774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jensen AV, Faurholt-Jepsen D, Egelund GB, Andersen SB, Petersen PT, Benfield T, et al. Undiagnosed diabetes mellitus in community-acquired pneumonia: a prospective cohort study. Clin Infect Dis. (2017) 65:2091–8. 10.1093/cid/cix703 [DOI] [PubMed] [Google Scholar]
- 112.Shimizu T, Kawamura T, Miyaji C, Oya H, Bannai M, Yamamoto S, et al. Resistance of extrathymic T cells to stress and the role of endogenous glucocorticoids in stress associated immunosuppression. Scand J Immunol. (2000) 51:285–92. 10.1046/j.1365-3083.2000.00695.x [DOI] [PubMed] [Google Scholar]
- 113.Watanabe M, Tomiyama-Miyaji C, Kainuma E, Inoue M, Kuwano Y, Ren H, et al. Role of alpha-adrenergic stimulus in stress-induced modulation of body temperature, blood glucose and innate immunity. Immunol Lett. (2008) 115:43–9. 10.1016/j.imlet.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 114.Li S, Wang J, Zhang B, Li X, Liu Y. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J. (2019) 43:319–41. 10.4093/dmj.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci. (2018) 22:4956–61. 10.26355/eurrev_201808_15635 [DOI] [PubMed] [Google Scholar]
- 116.Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. (2020) 24:2012–9. 10.26355/eurrev_202002_20379 [DOI] [PubMed] [Google Scholar]
- 117.Strollo R, Pozzilli P. DPP4 inhibition: preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. (2020) 36:e3330. 10.1002/dmrr.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. (2020) 26:1622–9. 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hernández-Garduño E. Obesity is the comorbidity more strongly associated for Covid-19 in Mexico. A case-control study. Obes Res Clin Pract. (2020) 14:375–9. 10.1016/j.orcp.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. (2020) 8:823–33. 10.1016/s2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. (2020) 105:dgaa346. 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. 10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mirzaei R, Goodarzi P, Asadi M, Soltani A, Aljanabi HAA, Jeda AS, et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life. (2020) 72:2097–111. 10.1002/iub.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cox MJ, Loman N, Bogaert D, O'Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. (2020) 1:e11. 10.1016/s2666-5247(20)30009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. (2020) 75:1564–81. 10.1111/all.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. (2020) 31:1068–77.e1063. 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.American Diabetes Association . 14. Diabetes care in the hospital: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl. 1):S144–51. 10.2337/dc18-S014 [DOI] [PubMed] [Google Scholar]
- 129.Longo M, Caruso P, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Treating type 2 diabetes in COVID-19 patients: the potential benefits of injective therapies. Cardiovasc Diabetol. (2020) 19:115. 10.1186/s12933-020-01090-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hulme KD, Gallo LA, Short KR. Influenza virus and glycemic variability in diabetes: a killer combination? Front Microbiol. (2017) 8:861. 10.3389/fmicb.2017.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Short KR, Kroeze E, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. (2014) 14:57–69. 10.1016/s1473-3099(13)70286-x [DOI] [PubMed] [Google Scholar]
- 132.Hulme KD, Yan L, Marshall RJ, Bloxham CJ, Upton KR, Hasnain SZ, et al. High glucose levels increase influenza-associated damage to the pulmonary epithelial-endothelial barrier. eLife. (2020) 9:e56907. 10.7554/eLife.56907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goeijenbier M, van Sloten TT, Slobbe L, Mathieu C, van Genderen P, Beyer WEP, et al. Benefits of flu vaccination for persons with diabetes mellitus: a review. Vaccine. (2017) 35:5095–101. 10.1016/j.vaccine.2017.07.095 [DOI] [PubMed] [Google Scholar]
- 134.Kohio HP, Adamson AL. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. (2013) 444:301–9. 10.1016/j.virol.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 135.Bechini A, Ninci A, Del Riccio M, Biondi I, Bianchi J, Bonanni P, et al. Impact of influenza vaccination on all-cause mortality and hospitalization for pneumonia in adults and the elderly with diabetes: a meta-analysis of observational studies. Vaccines. (2020) 8:263. 10.3390/vaccines8020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wiwanitkit V. Influenza and diabetes mellitus. Diabetes Metab Syndrome Clin Res Rev. (2010) 4:99–100. 10.1016/j.dsx.2009.07.001 [DOI] [Google Scholar]
- 137.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. (2020) 8:782–92. 10.1016/s2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ganesan SK, Venkatratnam P, Mahendra J, Devarajan N. Increased mortality of COVID-19 infected diabetes patients: role of furin proteases. Int J Obes. (2020) 44:2486–8. 10.1038/s41366-020-00670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen Y, Zhang J, Cai H, Shao JG, Zhang YY, Liu YM, et al. Identifying patients with chronic hepatitis B at high risk of type 2 diabetes mellitus: a cross-sectional study with pair-matched controls. BMC Gastroenterol. (2015) 15:32. 10.1186/s12876-015-0263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ambachew S, Eshetie S, Geremew D, Endalamaw A, Melku M. Prevalence of type 2 diabetes mellitus among hepatitis C virus-infected patients: a protocol for systematic review and meta-analysis. Syst Rev. (2019) 8:60. 10.1186/s13643-019-0976-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol. (2015) 6:134. 10.3389/fendo.2015.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. (2011) 3:2. 10.1186/1758-5996-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalra S, Agrawal N. Diabetes and HIV: current understanding and future perspectives. Curr Diab Rep. (2013) 13:419–27. 10.1007/s11892-013-0369-9 [DOI] [PubMed] [Google Scholar]
- 144.Corsino L, Dhatariya K, Umpierrez G. Management of Diabetes and Hyperglycemia in Hospitalized Patients. South Dartmouth, MA: MDText.com, Inc. (2000). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK279093/ [Google Scholar]
- 145.Guo X, Jin M, Yang M, Liu K, Li JW. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: a systematic review. Sci Rep. (2013) 3:2981. 10.1038/srep02981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li X, Gao Y, Xu H, Hou J, Gao P. Diabetes mellitus is a significant risk factor for the development of liver cirrhosis in chronic hepatitis C patients. Sci Rep. (2017) 7:9087. 10.1038/s41598-017-09825-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang X, Zhu X, Ji Y, Li H, Hou F, Xiao C, et al. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus. Biosci Rep. (2019) 39:BSR20181715. 10.1042/bsr20181715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. (1994) 21:1135–9. 10.1016/s0168-8278(05)80631-2 [DOI] [PubMed] [Google Scholar]
- 149.Reilly ML, Schillie SF, Smith E, Poissant T, Vonderwahl CW, Gerard K, et al. Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol. (2012) 6:858–66. 10.1177/193229681200600417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. (2016) 61:636–45. 10.1007/s10620-015-3983-3 [DOI] [PubMed] [Google Scholar]
- 151.Villar LM, Geloneze B, Vasques ACJ, Pires MLE, Miguel JC, da Silva EF, et al. Prevalence of hepatitis B and hepatitis C among diabetes mellitus type 2 individuals. PLoS ONE. (2019) 14:e0211193. 10.1371/journal.pone.0211193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. (2012) 107:46–52. 10.1038/ajg.2011.384 [DOI] [PubMed] [Google Scholar]
- 153.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. (2010) 33:1674–85. 10.2337/dc10-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zelenko Z, Gallagher EJ. Diabetes and cancer. Endocrinol Metab Clin North Am. (2014) 43:167–85. 10.1016/j.ecl.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 155.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: a cross-sectional study. PLoS ONE. (2018) 13:e0194199. 10.1371/journal.pone.0194199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. (2018) 18:125. 10.1007/s11892-018-1076-3 [DOI] [PubMed] [Google Scholar]
- 157.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care. (2017) 5:e000304. 10.1136/bmjdrc-2016-000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Samad F, Harris M, Puskas CM, Ye M, Chia J, Chacko S, et al. Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care. (2017) 5:e000457. 10.1136/bmjdrc-2017-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV AIDS Rep. (2012) 9:206–17. 10.1007/s11904-012-0124-x [DOI] [PubMed] [Google Scholar]
- 160.Vigouroux C, Maachi M, Nguyên TH, Coussieu C, Gharakhanian S, Funahashi T, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. (2003) 17:1503–11. 10.1097/00002030-200307040-00011 [DOI] [PubMed] [Google Scholar]
- 161.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. (2000) 133:592–9. 10.7326/0003-4819-133-8-200010170-00009 [DOI] [PubMed] [Google Scholar]
- 162.Takarabe D, Rokukawa Y, Takahashi Y, Goto A, Takaichi M, Okamoto M, et al. Autoimmune diabetes in HIV-infected patients on highly active antiretroviral therapy. J Clin Endocrinol Metab. (2010) 95:4056–60. 10.1210/jc.2010-0055 [DOI] [PubMed] [Google Scholar]
- 163.Barnes AJ, Locke P, Scudder PR, Dormandy TL, Dormandy JA, Slack J. Is hyperviscosity a treatable component of diabetic microcirculatory disease? Lancet. (1977) 2:789–91. 10.1016/s0140-6736(77)90724-3 [DOI] [PubMed] [Google Scholar]
- 164.Melkumyants AM, Balashov SA. Effect of blood viscocity on arterial flow induced dilator response. Cardiovasc Res. (1990) 24:165–8. [DOI] [PubMed] [Google Scholar]
- 165.Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during chronic and acute hyperglycemia. Stroke. (1987) 18:52–8. 10.1161/01.STR.18.1.52 [DOI] [PubMed] [Google Scholar]
- 166.Franca EL, Ribeiro EB, Scherer EF, Cantarini DG, Pessoa RS, Franca FL, et al. Effects of Momordica charantia L. on the blood rheological properties in diabetic patients. Biomed Res Int. (2014) 2014:840379. 10.1155/2014/840379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Watala C, Zawodniak M, Bryszewska M, Nowak S. Nonenzymatic protein glycosylation. I. Lowered erythrocyte membrane fluidity in juvenile diabetes. Ann Clin Res. (1985) 17:327–30. [PubMed] [Google Scholar]
- 168.Cho YI, Mooney MP, Cho DJ. Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol. (2008) 2:1130–8. 10.1177/193229680800200622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, Al-Rawi PG, Chatfield D, et al. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery. (2004) 55:877–81; discussion 882. 10.1227/01.neu.0000137658.14906.e4 [DOI] [PubMed] [Google Scholar]
- 170.Le Devehat C, Khodabandehlou T, Vimeux M. Relationship between hemorheological and microcirculatory abnormalities in diabetes mellitus. Diabetes Metab. (1994) 20:401–4. [PubMed] [Google Scholar]
- 171.Kim S, Popel AS, Intaglietta M, Johnson PC. Effect of erythrocyte aggregation at normal human levels on functional capillary density in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. (2006) 290:H941–7. 10.1152/ajpheart.00645.2005 [DOI] [PubMed] [Google Scholar]
- 172.Agudelo-Flórez P, Costa-Carvalho BT, López JA, Redher J, Newburger PE, Olalla-Saad ST, et al. Association of glucose-6-phosphate dehydrogenase deficiency and X-linked chronic granulomatous disease in a child with anemia and recurrent infections. Am J Hematol. (2004) 75:151–6. 10.1002/ajh.10477 [DOI] [PubMed] [Google Scholar]
- 173.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. (2000) 342:161–7. 10.1056/nejm200001203420303 [DOI] [PubMed] [Google Scholar]
- 174.Al-Niaimi A, Safdar N. Supplemental perioperative oxygen for reducing surgical site infection: a meta-analysis. J Eval Clin Pract. (2009) 15:360–5. 10.1111/j.1365-2753.2008.01016.x [DOI] [PubMed] [Google Scholar]
- 175.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. (2008) 34:1159–69. 10.1111/j.1524-4725.2008.34254.x [DOI] [PubMed] [Google Scholar]
- 176.Lowe GD, Lowe JM, Drummond MM, Reith S, Belch JJ, Kesson CM, et al. Blood viscosity in young male diabetics with and without retinopathy. Diabetologia. (1980) 18:359–63. [DOI] [PubMed] [Google Scholar]
- 177.Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. (2003) 2:95–108. 10.2165/00024677-200302020-00003 [DOI] [PubMed] [Google Scholar]
- 178.Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. (2006) 29:1150–9. 10.2337/diacare.2951150 [DOI] [PubMed] [Google Scholar]
- 179.Charfen MA, Fernández-Frackelton M. Diabetic ketoacidosis. Emerg Med Clin North Am. (2005) 23:609–28. 10.1016/j.emc.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 180.Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. (2010) 6:274–85. 10.1038/nrneph.2010.33 [DOI] [PubMed] [Google Scholar]
- 181.Azoulay E, Chevret S, Didier J, Barboteu M, Bornstain C, Darmon M, et al. Infection as a trigger of diabetic ketoacidosis in intensive care-unit patients. Clin Infect Dis. (2001) 32:30–5. 10.1086/317554 [DOI] [PubMed] [Google Scholar]
- 182.Slovis CM, Mork VG, Slovis RJ, Bain RP. Diabetic ketoacidosis and infection: leukocyte count and differential as early predictors of serious infection. Am J Emerg Med. (1987) 5:1–5. 10.1016/0735-6757(87)90280-4 [DOI] [PubMed] [Google Scholar]
- 183.Dökmetaş HS, Canbay E, Yilmaz S, Elaldi N, Topalkara A, Oztoprak I, et al. Diabetic ketoacidosis and rhino-orbital mucormycosis. Diabetes Res Clin Pract. (2002) 57:139–42. 10.1016/s0168-8227(02)00021-9 [DOI] [PubMed] [Google Scholar]
- 184.Gen R, Horasan E, Vaysoglu Y, Arpaci RB, Ersöz G, Özcan C. Rhino-orbito-cerebral mucormycosis in patients with diabetic ketoacidosis. J Craniofac Surg. (2013) 24:e144–7. 10.1097/SCS.0b013e31827c7eb8 [DOI] [PubMed] [Google Scholar]
- 185.Grizzanti JN, Knapp A. Diabetic ketoacidosis and invasive aspergillosis. Lung. (1981) 159:43–9. 10.1007/bf02713896 [DOI] [PubMed] [Google Scholar]
- 186.Elmas ÖN, Akinci A, Bilir P. Tuberculous meningitis associated with diabetic ketoacidosis. J Clin Res Pediatr Endocrinol. (2011) 3:222–4. 10.4274/jcrpe.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Westphal SA, Sarosi GA. Diabetic ketoacidosis associated with pulmonary coccidioidomycosis. Clin Infect Dis. (1994) 18:974–8. 10.1093/clinids/18.6.974 [DOI] [PubMed] [Google Scholar]
- 188.Nahas GG, Tannieres ML, Lennon JF. Direct measurement of leukocyte motility: effects of pH and temperature. Proc Soc Exp Biol Med. (1971) 138:350–2. 10.3181/00379727-138-35894 [DOI] [PubMed] [Google Scholar]
- 189.Rotstein OD, Fiegel VD, Simmons RL, Knighton DR. The deleterious effect of reduced pH and hypoxia on neutrophil migration in vitro. J Surg Res. (1988) 45:298–303. 10.1016/0022-4804(88)90079-0 [DOI] [PubMed] [Google Scholar]
- 190.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. (1979) 53:1133–9. [PubMed] [Google Scholar]
- 191.Rotstein OD, Nasmith PE, Grinstein S. The Bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun. (1987) 55:864–70. 10.1128/iai.55.4.864-870.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Craven N, Williams MR, Field TR, Bunch KJ, Mayer SJ, Bourne FJ. The influence of extracellular and phagolysosomal pH changes on the bactericidal activity of bovine neutrophils against Staphylococcus aureus. Vet Immunol Immunopathol. (1986) 13:97–110. 10.1016/0165-2427(86)90052-8 [DOI] [PubMed] [Google Scholar]
- 193.Loeffler DA, Juneau PL, Masserant S. Influence of tumour physico-chemical conditions on interleukin-2-stimulated lymphocyte proliferation. Br J Cancer. (1992) 66:619–22. 10.1038/bjc.1992.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ratner S. Lymphocytes stimulated with recombinant human interleukin-2: relationship between motility into protein matrix and in vivo localization in normal and neoplastic tissues of mice. J Natl Cancer Inst. (1990) 82:612–6. 10.1093/jnci/82.7.612 [DOI] [PubMed] [Google Scholar]
- 195.Ratner S. Motility of IL-2-stimulated lymphocytes in neutral and acidified extracellular matrix. Cell Immunol. (1992) 139:399–410. 10.1016/0008-8749(92)90081-y [DOI] [PubMed] [Google Scholar]
- 196.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. (2002) 269:2434–9. 10.1046/j.1432-1033.2002.02910.x [DOI] [PubMed] [Google Scholar]
- 197.Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol. (2017) 8:371. 10.3389/fphys.2017.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Radosinska J, Vrbjar N. The role of red blood cell deformability and Na,K-ATPase function in selected risk factors of cardiovascular diseases in humans: focus on hypertension, diabetes mellitus and hypercholesterolemia. Physiol Res. (2016) 65(Suppl. 1):S43–54. 10.33549/physiolres.933402 [DOI] [PubMed] [Google Scholar]
- 199.Rodrigues-Mascarenhas S Da Silva de Oliveira A Amoedo ND Affonso-Mitidieri OR Rumjanek FD Rumjanek VM . Modulation of the immune system by ouabain. Ann N Y Acad Sci. (2009) 1153:153–63. 10.1111/j.1749-6632.2008.03969.x [DOI] [PubMed] [Google Scholar]
- 200.De La Tour DD, Raccah D, Jannot MF, Coste T, Rougerie C, Vague P. Erythrocyte Na/K ATPase activity and diabetes: relationship with C-peptide level. Diabetologia. (1998) 41:1080–4. 10.1007/s001250051033 [DOI] [PubMed] [Google Scholar]
- 201.Zadhoush F, Sadeghi M, Pourfarzam M. Biochemical changes in blood of type 2 diabetes with and without metabolic syndrome and their association with metabolic syndrome components. J Res Med Sci. (2015) 20:763–70. 10.4103/1735-1995.168383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Abosheasha M, Fm Z, Bessa S, Mohamed T. Na+/K+-ATPase activity as a potential biomarker for type 2 diabetes mellitus. Res J Pharm Biol Chem Sci. (2018) 9:1227–31. Available online at: https://www.researchgate.net/publication/324825574_NaK-ATPase_activity_as_a_potential_biomarker_for_Type_2_Diabetes_mellitus/stats [Google Scholar]
- 203.Tokhtaeva E, Munson K, Sachs G, Vagin O. N-glycan-dependent quality control of the Na,K-ATPase beta(2) subunit. Biochemistry. (2010) 49:3116–28. 10.1021/bi100115a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Redondo JM, López Rivas A, Fresno M. Activation of the Na+/K+-ATPase by interleukin-2. FEBS Lett. (1986) 206:199–202. 10.1016/0014-5793(86)80980-2 [DOI] [PubMed] [Google Scholar]
- 205.Brodie C, Tordai A, Saloga J, Domenico J, Gelfand EW. Ouabain induces inhibition of the progression phase in human T-cell proliferation. J Cell Physiol. (1995) 165:246–53. 10.1002/jcp.1041650205 [DOI] [PubMed] [Google Scholar]
- 206.Rodrigues-Mascarenhas S, Bloise FF, Moscat J, Rumjanek VM. Ouabain inhibits p38 activation in thymocytes. Cell Biol Int. (2008) 32:1323–8. 10.1016/j.cellbi.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 207.Chow CW, Rincón M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol. (1999) 19:2300–7. 10.1128/mcb.19.3.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol. (2011) 2:88. 10.3389/fimmu.2011.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. (1984) 101:527–37. 10.7326/0003-4819-101-4-527 [DOI] [PubMed] [Google Scholar]
- 210.Jain SK. Glutathione and glucose-6-phosphate dehydrogenase deficiency can increase protein glycosylation. Free Radic Biol Med. (1998) 24:197–201. 10.1016/s0891-5849(97)00223-2 [DOI] [PubMed] [Google Scholar]
- 211.Mehta A, Mason PJ, Vulliamy TJ. Glucose-6-phosphate dehydrogenase deficiency. Baillieres Best Pract Res Clin Haematol. (2000) 13:21–38. 10.1053/beha.1999.0055 [DOI] [PubMed] [Google Scholar]
- 212.Díaz-Flores M, Ibáñez-Hernández MA, Galván RE, Gutiérrez M, Durán-Reyes G, Medina-Navarro R, et al. Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci. (2006) 78:2601–7. 10.1016/j.lfs.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 213.Hsieh YT, Lin MH, Ho HY, Chen LC, Chen CC, Shu JC. Glucose-6-phosphate dehydrogenase. (G6PD)-deficient epithelial cells are less tolerant to infection by Staphylococcus aureus. PLoS ONE. (2013) 8:e79566. 10.1371/journal.pone.0079566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.West IC. Glucose-6-phosphate dehydrogenase: a candidate gene for diabetes. Diabet Med. (2002) 19:172–4. 10.1046/j.1464-5491.2002.690_1.x [DOI] [PubMed] [Google Scholar]
- 215.Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. (2003) 29:642–5. 10.1007/s00134-002-1628-4 [DOI] [PubMed] [Google Scholar]
- 216.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. (2011) 35:871–82. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bellanti JA, Cantz BE, Schlegel RJ. Accelerated decay of glucose 6-phosphate dehydrogenase activity in chronic granulomatous disease. Pediatr Res. (1970) 4:405–11. 10.1203/00006450-197009000-00003 [DOI] [PubMed] [Google Scholar]
- 218.Zhang Z, Apse K, Pang J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem. (2000) 275:40042–7. 10.1074/jbc.M007505200 [DOI] [PubMed] [Google Scholar]
- 219.Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. (1989) 38:1031–5. 10.2337/diab.38.8.1031 [DOI] [PubMed] [Google Scholar]
- 220.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. (2008) 88:1322–35. 10.2522/ptj.20080008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. (2014) 2:488–96. 10.12998/wjcc.v2.i10.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brunetti L, Kalabalik J. Management of type-2 diabetes mellitus in adults: focus on individualizing non-insulin therapies. P T. (2012) 37:687–96. [PMC free article] [PubMed] [Google Scholar]
- 223.Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology. (2019) 24:646–51. 10.1111/resp.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, Gill S, et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax. (2013) 68:835–45. 10.1136/thoraxjnl-2012-203178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gill SK, Hui K, Farne H, Garnett JP, Baines DL, Moore LS, et al. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep. (2016) 6:27636. 10.1038/srep27636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pan SW, Yen YF, Kou YR, Chuang PH, Su VY, Feng JY, et al. The risk of TB in patients with type 2 diabetes initiating metformin vs sulfonylurea treatment. Chest. (2018) 153:1347–57. 10.1016/j.chest.2017.11.040 [DOI] [PubMed] [Google Scholar]
- 227.Shih CJ, Wu YL, Chao PW, Kuo SC, Yang CY, Li SY, et al. Association between Use of oral anti-diabetic drugs and the risk of sepsis: a nested case-control study. Sci Rep. (2015) 5:15260. 10.1038/srep15260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marin-Penalver JJ, Martin-Timon I, Sevillano-Collantes C, Del Canizo-Gomez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. (2016) 7:354–95. 10.4239/wjd.v7.i17.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Martin AE, Montgomery PA. Acarbose: an alpha-glucosidase inhibitor. Am J Health Syst Pharm. (1996) 53:2277–90. [DOI] [PubMed] [Google Scholar]
- 230.Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs. (2003) 63:1373–405. 10.2165/00003495-200363130-00004 [DOI] [PubMed] [Google Scholar]
- 231.Pathak R, Bridgeman MB. Dipeptidyl Peptidase-4. (DPP-4) inhibitors in the management of diabetes. P T. (2010) 35:509–13. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2957740/ [PMC free article] [PubMed] [Google Scholar]
- 232.Baruah MP, Makkar BM, Ghatnatti VB, Mandal K. Sodium glucose co-transporter-2 inhibitor: benefits beyond glycemic control. Indian J Endocrinol Metab. (2019) 23:140–9. 10.4103/ijem.IJEM_160_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.A.D.A . 9. pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42(Suppl. 1):S90–102. 10.2337/dc19-S009 [DOI] [PubMed] [Google Scholar]
- 234.Patkee WR, Carr G, Baker EH, Baines DL, Garnett JP. Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. J Cell Mol Med. (2016) 20:758–64. 10.1111/jcmm.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marupuru S, Senapati P, Pathadka S, Miraj SS, Unnikrishnan MK, Manu MK. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz J Infect Dis. (2017) 21:312–6. 10.1016/j.bjid.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yen FS, Wei JC, Yang YC, Hsu CC, Hwu CM. Respiratory outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Sci Rep. (2020) 10:10298. 10.1038/s41598-020-67338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gorricho J, Garjón J, Alonso A, Celaya MC, Saiz LC, Erviti J, et al. Use of oral antidiabetic agents and risk of community-acquired pneumonia: a nested case-control study. Br J Clin Pharmacol. (2017) 83:2034–44. 10.1111/bcp.13288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kofteridis DP, Giourgouli G, Plataki MN, Andrianaki AM, Maraki S, Papadakis JA, et al. Community-acquired pneumonia in elderly adults with type 2 diabetes mellitus. J Am Geriatr Soc. (2016) 64:649–51. 10.1111/jgs.14011 [DOI] [PubMed] [Google Scholar]
- 239.Astrand A, Wingren C, Benjamin A, Tregoning JS, Garnett JP, Groves H, et al. Dapagliflozin-lowered blood glucose reduces respiratory Pseudomonas aeruginosa infection in diabetic mice. Br J Pharmacol. (2017) 174:836–47. 10.1111/bph.13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mor A, Petersen I, Sørensen HT, Thomsen RW. Metformin and other glucose-lowering drug initiation and rates of community-based antibiotic use and hospital-treated infections in patients with type 2 diabetes: a Danish nationwide population-based cohort study. BMJ Open. (2016) 6:e011523. 10.1136/bmjopen-2016-011523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis. (2015) 74:1968–75. 10.1136/annrheumdis-2014-205216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. (2006) 368:1696–705. 10.1016/s0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 243.Morieri ML, Bonora BM, Longato E, Di Camilo B, Sparacino G, Tramontan L, et al. Exposure to dipeptidyl-peptidase 4 inhibitors and the risk of pneumonia among people with type 2 diabetes: retrospective cohort study and meta-analysis. Diabetes Obes Metab. (2020) 22:1925–34. 10.1111/dom.14142 [DOI] [PubMed] [Google Scholar]
- 244.Tournis S, Mitrakou A. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults: response to Pittas et al. Diabetes Care. (2007) 30:e81. 10.2337/dc07-0665 [DOI] [PubMed] [Google Scholar]
- 245.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. (2009) 94:709–28. 10.1210/jc.2008-1410 [DOI] [PubMed] [Google Scholar]
- 246.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. (2016) 11:95–104. 10.4137/bmi.S38440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lipska KJ, Warton EM, Huang ES, Moffet HH, Inzucchi SE, Krumholz HM, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. (2013) 36:3535–42. 10.2337/dc13-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chico A, Aguilera E, Ampudia-Blasco FJ, Bellido V, Cardona-Hernández R, Escalada FJ, et al. Clinical approach to flash glucose monitoring: an expert recommendation. J Diabetes Sci Technol. (2020) 14:155–64. 10.1177/1932296819841911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chiu PF, Wu CL, Huang CH, Liou HH, Chang CB, Chang HR, et al. Lower blood glucose and variability are associated with earlier recovery from renal injury caused by episodic urinary tract infection in advanced type 2 diabetic chronic kidney disease. PLoS ONE. (2014) 9:e108531. 10.1371/journal.pone.0108531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sehgal R, Berg A, Figueroa R, Poritz LS, McKenna KJ, Stewart DB, et al. Risk factors for surgical site infections after colorectal resection in diabetic patients. J Am Coll Surg. (2011) 212:29–34. 10.1016/j.jamcollsurg.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 251.Jeon CY, Furuya EY, Berman MF, Larson EL. The role of pre-operative and post-operative glucose control in surgical-site infections and mortality. PLoS ONE. (2012) 7:e45616. 10.1371/journal.pone.0045616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. (2006) 295:1681–7. 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- 253.Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. (2011) 34:855–7. 10.2337/dc10-1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Iqbal A, Prince LR, Novodvorsky P, Bernjak A, Thomas MR, Birch L, et al. Effect of hypoglycemia on inflammatory responses and the response to low-dose endotoxemia in humans. J Clin Endocrinol Metab. (2019) 104:1187–99. 10.1210/jc.2018-01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Daly ME, Vale C, Walker M, Littlefield A, Alberti KG, Mathers JC. Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet. Am J Clin Nutr. (1998) 67:1186–96. 10.1093/ajcn/67.6.1186 [DOI] [PubMed] [Google Scholar]
- 256.Mihai BM, Mihai C, Cijevschi-Prelipcean C, Grigorescu ED, Dranga M, Drug V, et al. Bidirectional relationship between gastric emptying and plasma glucose control in normoglycemic individuals and diabetic patients. J Diabetes Res. (2018) 2018:1736959. 10.1155/2018/1736959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. (2013) 36:1396–405. 10.2337/dc12-1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. (2015) 11:112–28. 10.1038/nrendo.2014.202 [DOI] [PubMed] [Google Scholar]
- 259.Bailey CJ. Glucose-lowering therapies in type 2 diabetes: opportunities and challenges for peptides. Peptides. (2018) 100:9–17. 10.1016/j.peptides.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 260.Pilemann-Lyberg S, Thorsteinsson B, Snorgaard O, Zander M, Vestergaard H, Røder ME. Severe hypoglycaemia during treatment with sulphonylureas in patients with type 2 diabetes in the Capital Region of Denmark. Diabetes Res Clin Pract. (2015) 110:202–7. 10.1016/j.diabres.2015.09.006 [DOI] [PubMed] [Google Scholar]