Abstract
The aim of this systematic review and meta-analysis is to assess the effectiveness of probiotics in inducing body weight loss in patients with overweight or obesity with related metabolic diseases. The research was carried out on PubMed and Scopus, focusing on studies reporting the effect on anthropometric measures (weight, body mass Index (BMI), waist circumference (WC), and hip circumference (HC) after administration of various probiotic strains compared to placebo. Twenty randomized controlled trials, that included 1411 patients, were considered. The meta-analyzed mean differences (MD) for random effects showed no significant decrease in body weight after probiotic supplementation (−0.26 kg [−075, 0.23], p = 0.30), while a significant BMI decrease was found (−0.73 kg/m2 [−1.31, −0.16], p = 0.01). For WC and HC, the meta-analyzed MD for random effects showed a significant decrease (WC: −0.71 cm [−1.24; −0.19], p = 0.008 and HC: −0.73 cm [−1.16; −0.30], p = 0.0008). The risk of bias was also evaluated considering a high risk and a low risk according to PRISMA criteria. In conclusion, the results of this meta-analysis highlight a positive trend of probiotics supplementation on the amelioration of anthropometric measures of overweight and obese patients with related metabolic diseases. However, further research is needed before recommending the use of probiotics as a therapeutic strategy for these patients. The focus of the future research should be to evaluate the efficacy of different probiotic strains, the quantities to be administered, and the duration of the intervention.
Keywords: probiotics, weight loss, obesity, body weight
1. Introduction
The role of gut microbiota in metabolic disorders is increasingly considered. Although microbiota is influenced by different factors, diet seems to be the major contributor of its diversity [1]. Both the type of diet and its caloric content are able to modify the relative proportion of gut microbes (increase of Firmicutes with parallel decrease of Bacteroides) and consequently their capability of harvesting energy from food [2,3].
The “energy harvest” hypothesis refers to the body’s ability to extract energy from resistant starch or dietary fiber that remains indigestible in the small intestine, which is well observed in subjects affected by obesity [4,5]. The fermentation of these residues produces short-chain fatty acids (SCFAs), which are used for lipid or glucose synthesis [6]. In some studies, subjects with obesity showed higher SCFAs (first propionate, followed by butyrate, valerate, and acetate) in the feces than their leaner controls, without differences in the characterization of the main bacterial phyla [7,8].
A relationship between human gut microbiota and metabolic disease exists, but what has to be clarified is whether the change in intestinal microbiota occurs before the development of inflammation or vice versa [9]. In humans, some studies showed that obesity is associated with a reduced bacterial diversity and an altered representation of bacterial species [10,11]; while Kasai et al. showed that bacterial diversity was significantly greater in subjects with obesity compared with subjects without obesity [12]. A metanalysis published in 2014 failed to show changes in microbial diversity between obese and non-obese populations [13].
Firmicutes and Bacteroidetes represent the two predominant phyla in murine and human microbiota and an alteration in this ratio is implicated in many diseases. It was first reported by Ley et al. that an increase in Firmicutes and a decrease in Bacteroidetes is associated with obesity [14]. This was subsequently confirmed by Kasai et al., who analyzed the gut microbiota of obese and non-obese Japanese subjects [12]. Their results showed a significant reduction of the number of Bacteroidetes and a higher Firmicutes to Bacteroidetes ratio in subjects with obesity compared to subjects with normal body weight. On the contrary, Schwiertz et al. reported a lower ratio of Firmicutes to Bacteroidetes in adults affected by overweight or obesity compared with individuals without weight problems, while Duncan et al. found no differences between Firmicutes and Bacteroidetes in subjects with different BMI [8,15]. Other studies showed a different pattern, characterized by a reduction of Bacteroidetes in individuals with obesity without differences in Firmicutes [10,16].
Angelakis et al. analyzed the duodenal microbial population in obese and non-obese subjects. They found that the phylum taxonomic profile was similar when subjects with obesity and the control group were compared, with small differences for Firmicutes (62% in the control group vs. 67% in the group with obesity) and Proteobacteria (9.5% in the control group vs. 4% in the obesity group) [17]. Unlike what is observed in the distal gut microbiota, Bacteroidetes were almost completely absent in the duodenum: This is probably due to a limited availability of mucin as a carbon source for Bacteroidetes [17].
In a study investigating the correlation between bacterial concentration and BMI, it was observed that the fecal concentration of Lactobacillus reuteri was positively correlated with BMI, while Bifidobacterium animalis and Methanobrevibacter smithii were negatively associated with BMI [18]. The gut microbiota associated with human obesity is depleted in M. smithii [19].
Since the discovery of the link between gut microbiota and metabolic health, attention was focused on the possible use of ingredients like probiotics as a therapeutically active or preventive strategy in the management of metabolic disease. According to the definition of the Food and Agriculture Organization (FAO) and World Health Organization (WHO), probiotics are live microorganism which, when administered in adequate amounts, confer a health benefit on the host [20]. Gram-positive bacteria, Lactobacillus and Bifidobacterium, are the two most common genera. Probiotics seem to have beneficial effects on obesity and related metabolic disorders [21]. A meta-analysis reported that Lactobacillus gasseri and Lactobacillus plantarum have positive effects on weight loss, while other species (L. acidophilus, L. ingluviei and L. fermentum) are associated with weight gain [22].
The present review focuses on the effectiveness of the probiotics in the reduction of body weight in overweight and obese subjects with metabolic diseases—previous reviews done by Aoun, Darwish and Hamod 2020 [23], and by Ballini et al., 2020 [24] indicated that probiotics modify the secretion of hormones, neurotransmitters, and inflammatory factors, thus preventing food intake triggers that lead to weight gain. The novelty of this review as compared to others is that the outcomes are related to the changes of anthropometric measures (weight, body mass Index (BMI), waist circumference (WC), and hip circumference (HC) after administration of various probiotics in overweight or obese subjects with metabolic diseases.
Given this background, the aim of this systematic review and meta-analysis is to evaluate the efficacy of probiotics as a potential treatment option to reduce body weight and ameliorate other anthropometric measures in overweight and obese patients with metabolic related diseases.
2. Materials and Methods
The present systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement. The process of reporting was carried out as follows: (1) Formulation of working of research question stating that “is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases?”, (2) definition of participants: Adult women and men affected by overweight and obesity, (3) search strategy for identification of relevant intervention studies that include the effect of probiotic supplements on metabolic disease, and (4) analysis of data through the systematic review and meta-analysis.
2.1. Search Strategy
Articles that were written in the English language and published over the course of the last 10 years (2009–2019) were identified by searching PubMed and Scopus [25,26]. The search strategy was based on the following search terms: “probiotics” AND “obesity” AND “weight loss” AND “microbiota” OR “gut microbiota” AND “weight” AND “BMI” OR “WC” AND “HC”.
2.2. Study Eligibility
Eligible studies were required to report baseline and follow-up values, the mean change (∆-change) and relative standard deviation from baseline, and/or the mean difference among intervention groups vs. control group, concerning body weight or BMI and in addition other anthropometric measures, such as waist circumference (WC) and hip circumference (HC).
2.3. Data analysis and Presentation of Results
Randomized clinical trials investigating the effectiveness of the administration of different probiotic strains on body composition outcomes (especially weight loss) were included. For each study, the following data were specified: First author and the year of publication, the study design, the setting, the inclusion criteria, the number, and age of participants enrolled in the study, the intervention of the control and experimental group/s, the duration of the intervention and change of body measures observed in each group.
3. Results
A synthesis of the 20 published studies with 1411 patients is presented in Table 1.
Table 1.
Intervention studies on the effect of different probiotics on anthropometric parameters.
| First Author, Year | Study Design | Participants (Age) |
Intervention Group(s) | Placebo Group(s) | Duration | Changes in Intervention Group(s) a | Changes in Control Group(s) a |
|---|---|---|---|---|---|---|---|
| Gomes, 2017 | Randomized controlled trial (RCT) | 43 (20–59 years) |
n = 21 Diet and 4 sachets/day: 1 × 109 CFU of Lactobacillus acidophilus LA-14, L. casei LC-11, L. lactis LL-23, Bifidobacterium bifidum BB-06, and B. lactis BL-4 |
n = 22 diet |
8 weeks | BW (kg): −0.98 BMI (kg/m2): −0.45 WC (cm): −5.14 |
BW (kg): −0.95 BMI (kg/m2): −0.72 WC: (cm) −3.32 |
| Lee, 2014 | RCT | 50 (19–65 years) |
n = 25 Twice/day Bofutsushosan, containing 18 components, 3 g per admnistration and priobiotic capsules (Duolac 7 included 5 billion viable of Streptococcus thermophiles, L. Plantarum, L. acidophilus, L. rhamnosus, B. Lactis, B. longum, and B. breve |
n = 25 Twice/dayBTS (3 g per admnistration) and placebo capsules |
8 weeks | BW (kg): 1.02 ± 1.69 BMI (kg/m2): 0.38 ± 0.67 WC (cm): 1.56 ± 1.53 |
BW (kg): 1.87 ± 1.28 BMI: 0.75 ± 0.52 WC (cm): 1.21 ± 2.00 |
| Sanchez, 2014 | RCT | 125 (18–55 years) |
n = 62 two capsules daily (6 × 108cfu of L. rhamnosus CGMCC1.3724 (LPR)) |
n = 63 Two capsules daily |
24 weeks | BW (kg): −5.3 ± 4.3 | BW (kg): −3.9 ± 4.2 |
| Zarrati, 2013 | RCT | 75 (20–50 years) |
Group 1, -, n = 25: diet and 200 g/day of probiotic yogurt (PLCD), containing S. thermophiles and L. bulgaricus - enriched with the L. acidophilus LA5, L. casei DN001 and, B. lactis Bb12 (1 × 108 cfu/g each strain) |
Group 2, n = 25: diet and 200 g/die of regular yogurt (RLCD)- Group 3, n = 25: 200 g/day of probiotic yogurt without any diet (PWLCD) |
8 weeks | PLCD: BW (kg): −4.23 BMI (kg/m2): −1.3 WC (cm): −2.78 HC (cm): −2 |
RLCD: BW (kg): −4.87 BMI (kg/m2): −1.9 WC (cm): −2.3 HC (cm): −3.18 PWLCD: BW (kg): −0.04 HC (cm): −0.03 |
| Jung, 2013 | RCT | 62 (19–60 years) |
n = 31 6 capsules/day composed of 1010 cfu of L. gasseri BNR17 |
n = 31 6 placebo capsules/day |
12 weeks | BW (kg): (−1.1 ± 2.2) BMI (kg/m2): (−0.5 ± 0.9) WC (cm): (−2 ± 4.4) HC (cm): (−2.8 ± 3.5) |
BW (cm): (0.2 ± 2.4) BMI (kg/m2): (0.3 ± 1.0) WC (cm): (1.1 ± 4.2) HC (cm): (−1.1 ± 2.3) |
| Sharafedtinov, 2013 | RCT | 40 (30–69 years) |
n = 25 50 g/day of probiotic product (semi-hard cheese) containing L. plantarum TENSIA, added in amounts of 1.5 × 1011 |
n = 15 50 g/day of cheese without probiotics |
3 weeks | BW (kg): −5.7 BMI (kg/m2): −2 |
BW (kg): −4.4 BMI (kg/m2): −2.3 |
| Kadooka, 2013 |
RCT |
210 (35–60 years) |
n = 69 Fermented milk (FM) containing 107 cfu LG2055/g n = 71 FM containing 106 cfu LG2055/g |
n = 70 Control FM containing 0 cfu LG2055/g |
12 weeks | 107 dose BMI (kg/m2): (−0.3) WC (cm): (−1.3) HC (cm): (−1.2) 106 dose BMI (kg/m2): (−0.4) WC (cm): (−1.1) HC (cm): (−0.9) |
BMI (kg/m2): (0.1) WC (cm): (−0.1) HC (cm): (−0.2) |
| Kadooka, 2010 | RCT |
87 (33–63 years) |
n = 43 200 g daily of FM with L. gasseri SBT2055 (LG2055), 5 × 1010 cfu/100 g of FM |
n = 44 200 g (2 portions of 100 g each) daily of FM without LG2055 |
12 weeks | BW (kg): −1.1 BMI (kg/m2): −0.4 WC (cm): −1.7 HC (cm): −1.5 |
BW (kg): 0.3 BMI (kg/m2): 0.1 HC (kg): −0.3 |
| Woodard, 2009 | RCT | 44 (median age of treated group was 48.6 years, of placebo group was 41.2) |
n = 22 1 pill/day of Puritan’s Pride®, probiotic supplement containing 2.4 billion live cells of Lactobacillus species. |
n = 22 placebo |
24 weeks | Weight loss % (6 weeks postoperative): 29.90 |
Weight loss % (6 weeks postoperative):25.50 |
| Asemi, 2014 | RCT | 70 (35–70 years) |
n = 35 3 times/day of synbiotic food with L sporogenes (1 × 107 cfu) and 0.04 inulin as prebiotic. Then they received 27 × 107 cfu L. sporogenes and 1.08 g of inulin each day |
n = 35 Control food: the same substance without probiotic bacteria and prebiotic inulin |
6 weeks | BW (kg): (−0.12 ± 1.57) BMI (kg/m2): (−0.05 ± 0.62) |
BW (kg): (−0.03 ± 2.44) BMI (kg/m2): (−0.02 ± 1) |
| Asemi, 2013 |
RCT | 54 (35–70 years) |
n = 27 The probiotic supplement has L. acidophilus (2 × 109 cfu), L. casei (7 × 109 cfu), L.rhamnosus (1.5 × 109 cfu), L.bulgaricus (2 × 108 cfu), B. breve (2 × 1010 cfu), B.longum (7 × 109 cfu), S. thermophilus (1.5 × 109 cfu) and 100 mg fructo-oligosaccharides |
n = 27 Placebo: the same substance without bacteria |
8 weeks | BMI (kg/m2): −0.65 | BW (kg): −0.61 BMI (kg/m2): −0.26 |
| Shakeri, 2014 |
RCT | 78 (35–70 years) |
n = 26 The synbiotic bread contained probiotic L. sporogenes (1 × 108 cfu) and 0.07 g inulin as prebiotic per 1 g. n = 26 The probiotic bread contained L. sporogenes (1 × 108 cfu) per 1 g. |
n = 26 Control bread: the same substance without probiotic bacteria and prebiotic inulin |
8 weeks | Synbiotic bread: BW (kg): (0.03 ± 1.9) BMI (kg/m2): (0.02 ± 0.8) Probiotic bread: BW (kg): (-0.2 ± 1.4) BMI (kg/m2): (−0.04 ± 0.6) |
Control bread: BW (kg): (−0.05 ± 1.6) BMI (kg/m2): (−0.02 ± 0.6) |
| Mohamadshahi, 2014 | RCT | 44 (18–70 years) |
n = 22 300 g/day of probiotic yogurt (L. delbrueckii subsp. bulgaricus and S. thermophilus + 3.7×106 cfu/g of both B. animalis subsp. lactis Bb12 and L. acidophilus strain La5 |
n = 22 300 g/day of conventional yogurt |
8 weeks | BW (kg): −0.33 BMI (kg/m2): −0.12 WC (cm): 0.5 HC (cm): −0.15 |
BW (kg): −0.72 BMI (kg/m2): −0.04 WC (cm): 0.34 HC (cm): 0.19 |
| Nabavi, 2014 | RCT | 72 (23–63 years) |
n = 36 300 g/day of probiotic yogurt containing L. acidophilus La5 (4.42 × 106 cfu/g) and B. lactis Bb12 (3.85 × 106 cfu/g) |
n = 36 300 g/day of conventional yogurt |
8 weeks | BW (kg): −1.74 BMI (kg/m2): −0.68 |
BW (kg): −0.25 BMI (kg/m2): −0.11 |
| Alisi, 2014 | RCT | 48 (median age of treated group was 11 years, of placebo group was 10 years) |
n = 24 Probiotic VLS#3, 1 sachet/day <10 years old or 2 sachet/day >10 years old |
n = 24 Placebo, 1 sachet/day <10 years old or 2 sachet/day >10 years old |
16 weeks | BMI (kg/m2): −2.2 | BMI (kg/m2): 0.1 |
| Shavakhi, 2013 | RCT | 70 (18–75 years) |
n = 34 Two tablets/day of metformin 500 mg + two tablets/day of Protexin (L. acidophilus 1 × 108 CFU, L. casei 5 × 108 CFU, L. rhamnosus 7.5 × 107 CFU, L. bulgaricus 1.5 × 108 CFU, B. breve 5 × 107 CFU, B. longum 2.5 × 107 CFU, S. thermophilus 5 × 107 CFU, fructooligosaccharides 350 mg) |
n = 36 Two tablets/die of metformin 500 mg + two placebo tablets (120 mg of starch)/day |
24 weeks | BMI (kg/m2): −5.2 | BMI (kg/m2): −0.44 |
| Leber, 2012 | RT | 28 (24–66 years) |
n = 13 3 bottles/day (65 ml) containing L. casei Shirota at a concentration of 108/ml (3 × 6.5 × 109 cfu L. casei Shirota) |
n = 15 not received the product and served as a control group (standard). |
12 weeks |
BW (kg): (−0.58 ± 2.54) BMI (kg/m2): (−0.18 ± 0.78) |
BW (kg): (−0.13 ± 1.68) BMI (kg/m2): (−0.05 ± 0.60) |
| Chang, 2011 | RCT | 101 (20–65 years) |
n = 53 Functional yogurt containing S. thermophilus ≥3 × 109c.f.u./g, L. acidophilus ≥3 × 109c.f.u./g, B. infantis ≥1 × 1010c.f.u./g and functional ingredients |
n = 48 The control yogurts contained the same ingredients of S. thermophilus, L. acidophilus, B. infantis except functional ingredients |
8 weeks | BW (kg): (−0.24 ± 1.50) BMI (kg/m2): (−0.10 ± 0.58) WC (cm): (−0.45 ± 2.78) |
BW (kg): ( + 0.64 ± 1.39) BMI (kg/m2): ( + 0.24 ± 0.50) WC (cm): ( + 0.42 ± 2.78) |
| Ogawa, 2014 | Single-blind, CT | 30 (27–69 years) |
n = 15 200 g (2 portions of 100 g each) daily of FM with L. gasseri SBT2055 (LG2055) The viable cell count of LG2055 waproximately 5 × 1010 cfu/100g of FM on the initial day |
n = 15 200 g (2 portions of 100 g each) daily of control FM without LG2055 L. gasseri SBT2055 (LG2055) |
Control FM for 4 weeks; 4 weeks of washout period, active FM for 4 weeks | BW (kg): (−0.04 ± 0.12) BMI (kg/m2): (−0.01 ± 0.04) WC (cm): (−0.75 ± 0.35) |
BW (kg): (−0.23 ± 0.26) BMI (kg/m2): (−0.09 ± 0.09) WC (cm): (−1.78 ± 0.53) |
| Sadrzadeh-Yaganeh, 2010 | RCT | 90 (19–49 years) |
Group 1: n = 30 consumed daily 300 g probiotic yogurt containing L acidophilus La5 and B. lactis Bb12 (3.9 × 107 of both Bb12 and La5) Group 2: n = 30 consumed daily 300 g conventional yogurt |
Group 3: n = 30 did not consume any fermented and probiotic products |
6 weeks | Group 1 BW (kg): 0.2 Group 2 BW (kg): 0.4 BMI (kg/m2): 0.2 |
Group 3 No changes |
as Changes expressed as: (∆ change) ± SD where data are available. Abbreviations: CFU, colony forming unit; BW, body weight; BMI, body mass index; WC, waist circumference; HC, hip circumference; PLCD, probiotic yogurt with low calorie diet; RLCD, regular yogurt with low calorie diet; PWLCD, probiotic yogurt without low calorie diet; FM, fermented milk.
The table summarizes the studies that have evaluated as outcomes the changes of one or more anthropometric parameters (body weight, BMI, WC, and HC), after the administration of probiotics as supplements or food with a comparison between intervention and placebo treatment. In our analysis, we considered different study populations, including individuals with diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) metabolic syndrome, or altered lipid profile.
3.1. Overweight and Obesity
Kadooka et al. were the first who evaluated the effect of the probiotic L. gasseri (LG2055) in overweight adults. They reported that subjects who had consumed fermented milk containing L. gasseri 2055 at a total dose of 1011 cfu/day showed a 4.6% reduction in visceral fat area after 12 weeks of treatment, which was significantly different from the placebo group [27]. Furthermore, the intervention group showed significant decreases in body weight, BMI, WC, HC, and waist-to-hip ratio at both weeks 8 and 12 of treatment, as compared with the control group. Three years later, the same study group confirmed these results, using a fermented milk containing a lower dose of LG2055. BMI, WC, HC, body fat mass, and visceral fat areas, in both 107 and 106 dose groups decreased significantly at weeks 8 and 12 from baseline [28].
On the other hand, another study showed no statistically significant changes of anthropometric parameters between subjects with hypertriaciglycerolemia, after taking fermented milk with or without LG2055. However, the active fermented milk appeared able to reduce postprandial and fasting serum non-esterified fatty acid levels, two important components of the risk for obesity and type 2 diabetes mellitus [29]. Jung et al. examined the efficacy of a treatment with L. gasseri BNR17 in adults affected by obesity and overweight [30]. The 12-week intervention revealed a slight reduction in body weight, WC, and HC.
In contrast, no significant change in body composition was observed by Lee et al. in obese patients when the probiotics and placebo groups were compared [31]. In addition, Sanchez et al., showed that the administration of L. rhamnosus during the energy-restriction period (from week 1 to 12) did not significantly decrease the body weight or fat mass in a population of male and female obese patients [32]. The probiotic-treated group did, however, lose more fat mass than the placebo group at the end of the maintenance phase (from week 12 to 24). The analysis of the sex-specific results revealed significantly higher body weight and fat mass losses in women but not in men.
Another study investigated the changes in anthropometric parameters, in subjects affected by obesity or overweight, after the administration of probiotic yogurt (containing L. acidophilus La5, Bifidobacterium BB12, and L. casei DN001) combined or not with a low-calorie diet. The results showed a reduction in WC when probiotics were associated with a dietary restriction [33]. A recent study reported that the supplementation of a probiotic mix reduced abdominal adiposity and increased antioxidant enzyme activity in a more effective way when compared with an isolated dietary intervention. Participants taking the probiotic mix had a greater decrease in WC (−5.47% vs. −3.40%, p = 0.03), waist–height ratio (−5.00% vs. −3.27%, p = 0.02), and conicity index (−4.09% vs. −2.43%, p = 0.03) than the group receiving only the dietary intervention [34].
In addition, probiotics may improve weight loss after bariatric surgery. The results from the study of Woodard et al. suggested the use of a daily probiotic for all patients undergoing roux-en-Y gastric bypass, in order to reduce postoperative morbidity and maximize the weight loss [35].
3.2. Type 2 Diabetes Mellitus
Various studies have evaluated the effects of probiotics in overweight and obese patients affected by diabetes. They considered different kinds of probiotic supplementation, from multispecies probiotic supplements (with various strains of Lactobacillus, Bifidobacterium, and Streptococcus), to symbiotic food with Lactobacillus sporogenes and inulin. Comparing the anthropometric measures at baseline and after intervention, these studies failed to find a significant difference in weight and BMI between the two groups [36,37,38,39]. At the moment, other ongoing studies are evaluating the effects of probiotic supplementation in overweight and obese patients with prediabetes and diabetes [40,41].
3.3. NASH
A significant reduction (p < 0.001) of BMI was observed by Shavakhi et al. in overweight and obese patients affected by NASH with excess body weight, when treated with a combination of metformin and probiotics (different strains of Lactobacillus and Bifidobacterium) instead of metformin alone [42]. Also in children with NASH the administration of VLS#3 (a mixture of eight probiotic strains: S. thermophilus, bifidobacteria [B. breve, B. infantis, B. longum], L. acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. bulgaricus) significantly decreased (p < 0.001) the BMI during a four-month supplementation period, with respect to the placebo group [43]. Moreover, Nabavi et al. showed that body weight and BMI decreased in a significant manner in patients affected by obesity or overweight and NAFLD receiving a probiotic yogurt (4.42 × 106 of L. acidophilus La5 and 3.85 × 106 cfu/g of B. lactis Bb12), when compared with conventional yogurt, after an eight-week intervention [44].
The same bacterial strains, La5 and Bb12, were administered in a female population to assess their effects on the lipid profile. The participants were divided into three groups and were instructed to consume a daily dose of 300 g of probiotic yogurt, containing 3.9 × 107 of both Bb12 and La5 or 300 g of conventional yogurt or consume any fermented and probiotic products. The authors reported mainly neutral effects of yogurt consumption on the lipid profile [45].
3.4. Metabolic Syndrome
Chang et al. reported benefits after the daily consumption of 300 mL of functional yogurt NY-YP901 consisting of several probiotics for eight weeks, on metabolic syndrome traits [46]. In particular, this kind of yogurt was associated with decreased low-density lipoprotein (LDL) cholesterol, body weight, and BMI following a daily consumption for eight weeks. Although there was no significant effect on the parameters of metabolic syndrome such as blood pressure, fasting blood glucose, triglycerides, and high-density lipoprotein (HDL), the decreases in LDL-cholesterol and body weight were expected to favor the decrease of cardiovascular risk [46]. In contrast, a study that aimed to investigate the effect of L. casei Shirota on gut permeability in patients with metabolic syndrome did not find any effect of this probiotic administration on BMI and WC [47].
The administration of 50 g/day of cheese containing L. plantarum TENSIA®, in subjects with hypertension, showed a reduction of BMI and blood pressure, that is, symptoms involved in metabolic syndrome. In these patients, a significant decrease in body weight was also observed when the intervention group was compared with controls (−5.7 vs. −4.4 kg, p = 0.083) [48].
3.5. Meta-Analyzed Data
The meta-analyzed mean differences for random effects (MD) showed no significant decrease in body weight after probiotic supplementation in patients with type 2 diabetes mellitus (Figure 1).
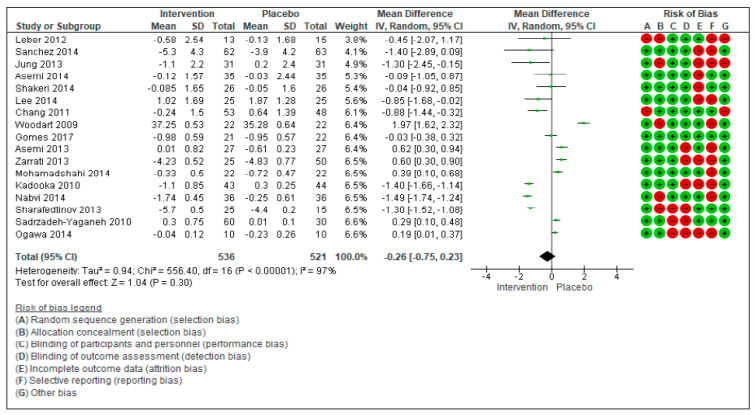
Figure 1.
Forest plot for randomized controlled trials of probiotic supplementation included in body weight (kg) subgroup meta-analysis (n = 1057). The studies are listed by first author and year. IV = equation that can be estimated by inverse variance (linear, exponential). The square represents the measures of effect (i.e., an odd ratio) for each study; the area of each square is proportional to the study’s weight in the meta-analysis. Horizontal line represents the confidence interval (CI) at the 95% level. The diamond represents the meta-analyzed measure of effect; the lateral points of diamond indicate CIs for this estimate. The vertical line represents no effects; if the CI for an individual study overlaps with this line, the given level of confidence for the effect size does not differ from no effect for that study. Risk of bias indicates the level of high and low risk associated with the article. With green signal for low risk and red for high risk of bias.
In the 17 studies [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48], with a total of 1057 subjects (536 in the intervention group and 521 in the control group), the overall effects showed that the treatment with probiotics did not significantly change the body weight (−0.26[−0.75, 0.23], p = 0.30) in the considered studies. τ2 (estimate of the between-studies variance in random-effect meta-analysis) = 0.94, χ2 = 556.40, df = 16 (p < 0.00001). I2 (statistically describing the percentage of variation across studies that is due to heterogeneity) = 100%.
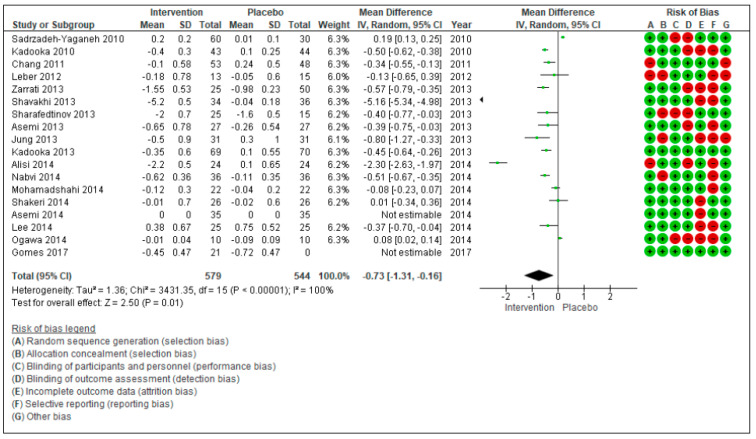
Figure 2 describes the meta-analyzed mean difference for random effects showing a significant decrease in BMI for the consumption of probiotic supplements. In a total of 18 studies, with a total of 1123 subjects (579 in the intervention group and 544 in the control group), the test of overall effects indicates that the treatment effect was significantly different (−0.73[−1.31, −0.16], p = 0.01) between the considered studies. τ2 (estimate of the between-studies variance in random-effect meta-analysis) = 1.36, χ2 = 3431.35, df = 15 (p < 0.00001).
Figure 2.
Forest plot for randomized controlled trials of probiotic supplementation included in body mass index (kg/m2) subgroup meta-analysis (n = 1123). The studies are listed by first author and year. IV = equation that can be estimated by inverse variance (linear, exponential). The square represents the measures of effect (i.e., an odd ratios) for each study; the area of each square is proportional to the study’s weight in the meta-analysis. Horizontal line represents the confidence interval (CI) at the 95% level. The diamond represents the meta-analyzed measure of effect; the lateral points of diamond indicate CIs for this estimate. The vertical line represents no effects; if the CI for an individual study overlaps with this line, the given level of confidence for the effect size does not differ from no effect for that study. Risk of bias indicates the level of high and low risk associated with the article, with green signal for low risk and red for high risk of bias.
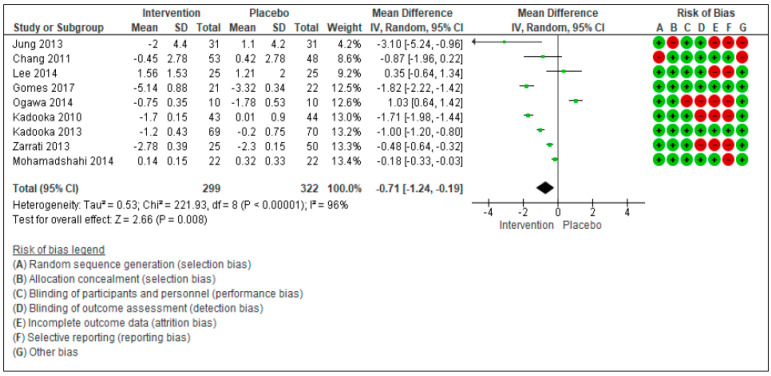
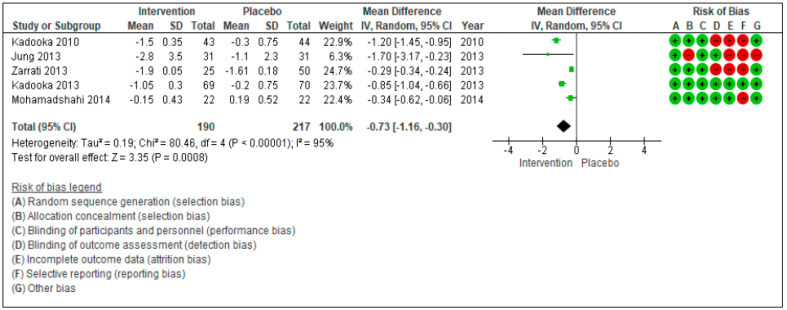
For WC (Figure 3) and HC (Figure 4), the meta-analyzed difference for random effects (MD) showed a significant decrease. For WC, 9 studies were included [27,28,29,30,31,32,33,34,35,36], with a total of 641 subjects (299 n the intervention group and 322 in the control group). Only 5 studies on HC were included [27,28,29,30,33], with a total of 407 subjects (190 in the intervention group and 217 in the control group). The test of overall effects for WC indicates that the treatment effect was significantly different (−0.71[−1.24, −0.19], p = 0.008) between the considered studies. τ2 (estimate of the between-studies variance in random-effect meta-analysis) = 0.53, χ2 = 221.93, df = 8 (p < 0.00001). I2 (statistically describing the percentage of variation across studies that is due to heterogeneity) = 100%. Similarly, the test of overall effects for HC indicates that the treatment effect was significantly different (−0.73[−1.16, −0.30], p = 0.00008) between the considered studies. τ2 (estimate of the between-studies variance in random-effect meta-analysis) = 0.19, χ2 = 80.46, df = 4 (p < 0.00001).
Figure 3.
Forest plot for randomized controlled trials of probiotic supplementation included in waist circumference (cm) subgroup meta-analysis (n = 621). The studies are listed by first author and year. IV = equation that can be estimated by inverse variance (linear, exponential). The square represents the measures of effect (i.e., an odd ratios) for each study; the area of each square is proportional to the study’s weight in the meta-analysis. The horizontal line represents the confidence interval CI) at the 95% level. The diamond represents the meta-analyzed measure of effect; the lateral points of diamond indicate CIs for this estimate. The vertical line represents no effects; if the CI for an individual study overlaps with this line, the given level of confidence for the effect size does not differ from no effect for that study. Risk of bias indicate the level of high and low risk associated with the article, with green signal for low risk and red for high risk of bias.
Figure 4.
Forest plot for randomized controlled trials of probiotic supplementation included in hip circumference (cm) subgroup meta-analysis (n = 621). The studies are listed by first author and year. IV = equation that can be estimated by inverse variance (linear, exponential). The square represents the measures of effect (i.e., an odd ratios) for each study; the area of each square is proportional to the study’s weight in the meta-analysis. The horizontal line represents the confidence interval CI) at the 95% level. The diamond represents the meta-analyzed measure of effect; the lateral points of diamond indicate CIs for this estimate. The vertical line represents no effects; if the CI for an individual study overlaps with this line, the given level of confidence for the effect size does not differ from no effect for that study. Risk of bias indicate the level of high and low risk associated with the article, with green signal for low risk and red for high risk of bias.
The risk of bias was also evaluated for the 20 studies: It was considered as high and low risk according to seven different criteria. Green indicates the risk of bias to be low, while red indicates the risk of bias to be high.
4. Discussion
The results of this meta-analysis show that probiotic supplementation significantly decreases the BMI (−0.73 kg/m2 [−1.31, −0.16], p = 0.01), WC (−0.71 cm [−1.24, −0.19], p = 0.008), and HC (−0.73 cm [−1.16, −0.30], p = 0.0008), but not the body weight (−0.26 kg [−0.75, 0.23], p = 0.30) of adults of both sexes affected by overweight and obesity with metabolic related diseases. Probiotics seem to be mostly effective in NASH and metabolic syndrome patients.
According to this meta-analysis, the Lactobacillus (e.g., L. Casei strain Shirota (LAB13), L. Gasseri, L. Rhamnosus, L. Plantarum) and Bifidobacterium (e.g., B. Infantis, B. Longum, and B. Breve B3) show the most promising effects against obesity. A recent review shows promising in vivo and vitro effects of the same strains [49].
The definition of obesity was based on BMI and the intake of probiotics was followed by a significant decrease of this outcome. The decrease of WC and HC may also be linked to the decrease of BMI. The difference between the results obtained for BMI and body weight may be due to the lack of homogeneity among the studies included in this meta-analysis because some studies considered only BMI while other studies evaluated only body weight or both of them. Very important is the observed significant reduction of WC and HC because these parameters, particularly WC, are strictly related to cardiovascular risk [50]. One of the mechanisms involved in the reduction of BMI after probiotic intake is the regulation of gut microbiota. Obesity favors a change of the gut microbiota composition, which can affect the energy harvest from food, the secretory functions and the composition of adipose tissue, the metabolism of carbohydrates and lipids in the liver and could also influence the activity of specific centers in the brain [51]. The regulation of gut microbiota by means of probiotics is attained by enhancing the epithelial barrier integrity, increasing adhesion to intestinal mucosa (e.g., by increasing the amount of Akkermansia muciniphila), producing health-promoting and antimicrobial substances, excluding pathogenic microbes, and regulating the host immune system [51,52]. An increase in the amount of Firmicutes to Bacteroidetes leads to methylation of the obesity- and CVD-related genes and influences the activity of hormones affecting the metabolic function by increasing the ability to harvest energy [53].
Probiotics can play a significant role against obesity through species- and strain-specific mechanisms [49] Lactobacillus reuteri has shown that it can prevent the intestinal colonization of pathogenic microbes, by remodeling the commensal microbiota composition, by decreasing the production of pro-inflammatory cytokines, and by increasing the strength of the intestinal barrier [52]. Lactobacullus paracasei has shown in an animal model that it can decrease the fat storage by increasing the levels of angiopoietin-like 4 protein (ANGPTL4) [54]. Lactobacillus gasseri SBT2055 (LG2055) has shown that it can reduce lipid absorption and promote fecal fat excretion in humans [55].
Probiotics can influence effective on obese and diabetic patients, through positively influencing the lipid profile and insulin sensitivity—both mechanisms can have an ultimate positive effect on the body weight, BMI, WC, and HC [53]. Probiotics have shown that they decrease the total cholesterol, total triglycerides, and LDL levels, while they increase the level of HDL [53]. An increasing number of studies suggests that the oral and the intestinal microbiota may indirectly or directly influence cardiovascular risk. Besides diet, the other therapeutic and preventive route that could be traveled is that of microbiota modification, via the use of appropriate pro- and prebiotics [56].
In addition, probiotics also increase the production of short-chain fatty acids that eventually influence the appetite and energy homeostasis [51]. The enhanced production of short-chain fatty acids can affect inflammation resolution pathways in the mucosa [57]. A study done by Peng, Luying et al. concluded that butyrate enhances the intestinal barrier by regulating the assembly of tight junctions, mediated by the activation of AMPK [58]. An indirect mechanism of the anti-obesity activity of probiotics is through reversing the source of pro-inflammatory stimuli linked with low-grade endotoxemia and thus affecting the inflammatory response [57].
The administration of probiotics for the management of obesity may represent an attractive therapeutic strategy but, even though encouraging results emerged from experiments on rodents, the efficacy of probiotics in obese humans remains highly debatable [59].
The major limitations of this meta-analysis are due to the heterogeneity of the included studies. In particular, the age of patients showed a wide difference in the different studies and the same was for the duration on the intervention. The age of the participants ranged from 18 up to 75 years. The treatment duration also widely varied among the included studies, starting from 3 weeks up to 24 weeks. Both of these aspects negatively influence the data analysis and limit the understanding of the anti-obesity potential of probiotics.
Thus, in future research, it is essential to define several smaller age ranges while conducting clinical trials so that the effect of age becomes clearer. The treatment duration also widely varied between the studies, starting from 3 weeks up to 24 weeks. This wide range of duration contributes to limit the understanding of the anti-obesity potential of probiotics with respect to treatment duration.
This meta-analysis has various limitations based on the available scientific research, which is characterized by contradictory evidence. Part of the controversy is due to a lack of precise cost-effectiveness data and the lack of data on the correct dosage and type of probiotic that has to be supplemented. In addition, data on the correlation between specific claims and specific probiotics in obesity management are missing.
A second point of weakness of this meta-analysis is due to the absence of RCT in which the population sample is normalized for colonic content of bacteria.
Conceivably, the obese patients have an increased Firmicutes/Bacteroidetes (F/B) ratio and might require different probiotic doses. In overweight/obese humans, in addition, the low fecal bacterial diversity is associated with more marked overall adiposity and dyslipidemia, impaired glucose homeostasis and higher low-grade inflammation [11].
Moreover, what constitutes a healthy microbiota is far from being established. For example, determining what constitutes a healthy microbiota and the variability found across populations is another important question mark. Recent studies raise questions about the widespread use of probiotics to impart general wellness [60].
There is huge variability of fermentable substrates that have bulk effects on bowel functions. day-by-day and within a day, since many studies have revealed only minor effects on overall microbiome composition and usually show only few species changing in population size [61].
Last, but not least, there is also no validated method to evaluate microbiota, most of which escapes current techniques, and in order to advance microbiome research to a more standardized and routine medical diagnostic procedure, it is essential to establish uniform standard operating procedures throughout laboratories and to initiate regular proficiency testing [62].
The “energy extraction” hypothesis should be interpreted with extreme caution. Daily energy output in feces is about 500 KJ and the microbiota produces SCFA, hence contributing to energy production rather than extraction. Note that rodents, especially the gnotobiotic models, are poor models of human microbiota behavior [61].
5. Conclusions
The results of this meta-analysis highlight a positive trend of probiotics supplementation on anthropometric measures of overweight and obese patients with related metabolic diseases. However, further research is needed before recommending the use of probiotics as a therapeutic strategy for these patients. The focus of the future research should be to evaluate the efficacy of different probiotic strains, the quantities to be administered, and the duration of the intervention.
Acknowledgments
None.
Author Contributions
Conceptualization, S.P. and M.R.; methodology, S.P.; validation, A.R. and G.P. (Giovanna Petrangolini); formal analysis, Z.I.; data curation, S.P.; writing—original draft preparation, S.P., M.R., and A.A.R.; writing—review and editing, G.P. (Gabriella Peroni), A.A.R., and A.G.; visualization, C.G., M.A.F., C.R., M.N., and Z.I.; project administration, S.P. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baothman O.A., Zamzami M.A., Taher I., Abubaker J., Abu-Farha M. The Role of Gut Microbiota in the Development of Obesity and Diabetes. Lipids Health Dis. 2016;15:1–8. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A., et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I., Krakoff J. Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E.F., Cotter P.D., Healy S., Marques T.M., O’Sullivan O., Fouhy F., Clarke S.F., O’Toole P.W., Quigley E.M., Stanton C., et al. Composition and Energy Harvesting Capacity of the Gut Microbiota: Relationship to Diet, Obesity and Time in Mouse Models. Gut. 2010;59:1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 5.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Tagliabue A., Elli M. The Role of Gut Microbiota in Human Obesity: Recent Findings and Future Perspectives. Nutr. Metab. Cardiovasc. Dis. 2013;23:160–168. doi: 10.1016/j.numecd.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes J., Su W., Rahat-Rozenbloom S., Wolever T.M.S., Comelli E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwiertz A., Taras D., Schäfer K., Beijer S., Bos N.A., Donus C., Hardt P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 9.Sanmiguel C., Gupta A., Mayer E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015;4:250–261. doi: 10.1007/s13679-015-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A Core Gut Microbiome in Obese and Lean Twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 12.Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015;15:1–10. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters W.A., Xu Z., Knight R. Meta-Analyses of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Duncan S.H., Lobley G.E., Holtrop G., Ince J., Johnstone A.M., Louis P., Flint H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 16.Furet J.-P., Kong L.-C., Tap J., Poitou C., Basdevant A., Bouillot J.-L., Mariat D., Corthier G., Doré J., Henegar C., et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery-Induced Weight Loss: Links with Metabolic and Low-Grade Inflammation Markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelakis E., Armougom F., Carrière F., Bachar D., Laugier R., Lagier J.C., Robert C., Michelle C., Henrissat B., Raoult D. A Metagenomic Investigation of the Duodenal Microbiota Reveals Links with Obesity. PLoS ONE. 2015;10:1–15. doi: 10.1371/journal.pone.0137784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Million M., Angelakis E., Maraninchi M., Henry M., Giorgi R., Valero R., Vialettes B., Raoult D. Correlation between Body Mass Index and Gut Concentrations of Lactobacillus Reuteri, Bifidobacterium Animalis, Methanobrevibacter Smithii and Escherichia Coli. Int. J. Obes. 2013;37:1460–1466. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Million M., Maraninchi M., Henry M., Armougom F., Richet H., Carrieri P., Valero R., Raccah D., Vialettes B., Raoult D. Obesity-Associated Gut Microbiota Is Enriched in Lactobacillus Reuteri and Depleted in Bifidobacterium Animalis and Methanobrevibacter Smithii. Int. J. Obes. 2012;36:817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.World Health Organization (WHO) Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Fao Who. 2001:1–34. doi: 10.1201/9781420009613.ch16. [DOI] [Google Scholar]
- 21.Gérard P. Gut Microbiota and Obesity. Cell. Mol. Life Sci. 2016;73:147–162. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Million M., Angelakis E., Paul M., Armougom F., Leibovici L., Raoult D. Comparative Meta-Analysis of the Effect of Lactobacillus Species on Weight Gain in Humans and Animals. Microb. Pathog. 2012;53:100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Aoun A., Darwish F., Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Preventive Nutrition Food Sci. 2020;25:113–123. doi: 10.3746/pnf.2020.25.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballini A., Scacco S., Boccellino M., Santacroce L., Arrigoni R. Microbiota and Obesity: Where Are We Now? Biology. 2020;9:415. doi: 10.3390/biology9120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Home-PubMed-NCBI. [(accessed on 27 September 2018)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed.
- 26.Scopus Preview-Scopus-Welcome to Scopus. [(accessed on 27 September 2018)]; Available online: https://www.scopus.com/home.uri.
- 27.Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., Okano M., Kagoshima M., Tsuchida T. Regulation of Abdominal Adiposity by Probiotics (Lactobacillus Gasseri SBT2055) in Adults with Obese Tendencies in a Randomized Controlled Trial. Eur. J. Clin. Nutr. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 28.Kadooka Y., Sato M., Ogawa A., Miyoshi M., Uenishi H., Ogawa H., Ikuyama K., Kagoshima M., Tsuchida T. Effect of Lactobacillus Gasseri SBT2055 in Fermented Milk on Abdominal Adiposity in Adults in a Randomised Controlled Trial. Br. J. Nutr. 2013;110:1696–1703. doi: 10.1017/S0007114513001037. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa A., Kadooka Y., Kato K., Shirouchi B., Sato M. Lactobacillus Gasseri SBT2055 Reduces Postprandial and Fasting Serum Non-Esterified Fatty Acid Levels in Japanese Hypertriacylglycerolemic Subjects. Lipids Health Dis. 2014;13:1–8. doi: 10.1186/1476-511X-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung S.-P., Lee K.-M., Kang J.-H., Yun S.-I., Park H.-O., Moon Y., Kim J.-Y. Effect of Lactobacillus Gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial. Korean J. Fam. Med. 2013;34:80–89. doi: 10.4082/kjfm.2013.34.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.J., Bose S., Seo J.G., Chung W.S., Lim C.Y., Kim H. The Effects of Co-Administration of Probiotics with Herbal Medicine on Obesity, Metabolic Endotoxemia and Dysbiosis: A Randomized Double-Blind Controlled Clinical Trial. Clin. Nutr. 2014;33:973–981. doi: 10.1016/j.clnu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez M., Darimont C., Drapeau V., Emady-Azar S., Lepage M., Rezzonico E., Ngom-Bru C., Berger B., Philippe L., Ammon-Zuffrey C., et al. Effect of Lactobacillus Rhamnosus CGMCC1.3724 Supplementation on Weight Loss and Maintenance in Obese Men and Women. Br. J. Nutr. 2014;111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- 33.Zarrati M., Shidfar F., Nourijelyani K., Mofid V., Hossein zadeh-Attar M.J., Bidad K., Najafi F., Gheflati Z., Chamari M., Salehi E. Lactobacillus Acidophilus La5, Bifidobacterium BB12, and Lactobacillus Casei DN001 Modulate Gene Expression of Subset Specific Transcription Factors and Cytokines in Peripheral Blood Mononuclear Cells of Obese and Overweight People. BioFactors. 2013;39:633–643. doi: 10.1002/biof.1128. [DOI] [PubMed] [Google Scholar]
- 34.Gomes A.C., de Sousa R.G.M., Botelho P.B., Gomes T.L.N., Prada P.O., Mota J.F. The Additional Effects of a Probiotic Mix on Abdominal Adiposity and Antioxidant Status: A Double-Blind, Randomized Trial. Obesity. 2017;25:30–38. doi: 10.1002/oby.21671. [DOI] [PubMed] [Google Scholar]
- 35.Woodard G.A., Encarnacion B., Downey J.R., Peraza J., Chong K., Hernandez-Boussard T., Morton J.M. Probiotics Improve Outcomes after Roux-En-Y Gastric Bypass Surgery: A Prospective Randomized Trial. J. Gastrointest. Surg. 2009;13:1198–1204. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 36.Asemi Z., Khorrami-Rad A., Alizadeh S.A., Shakeri H., Esmaillzadeh A. Effects of Synbiotic Food Consumption on Metabolic Status of Diabetic Patients: A Double-Blind Randomized Cross-over Controlled Clinical Trial. Clin. Nutr. 2014;33:198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Shakeri H., Hadaegh H., Abedi F., Tajabadi-Ebrahimi M., Mazroii N., Ghandi Y., Asemi Z. Consumption of Synbiotic Bread Decreases Triacylglycerol and VLDL Levels While Increasing HDL Levels in Serum from Patients with Type-2 Diabetes. Lipids. 2014;49:695–701. doi: 10.1007/s11745-014-3901-z. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi-Sartang M., Bellissimo N., Totosy de Zepetnek J.O., Brett N.R., Mazloomi S.M., Fararouie M., Bedeltavana A., Famouri M., Mazloom Z. The Effect of Daily Fortified Yogurt Consumption on Weight Loss in Adults with Metabolic Syndrome: A 10-Week Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. NMCD. 2018;28:565–574. doi: 10.1016/j.numecd.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Asemi Z., Zohreh Z., Shakeri H., Sabihi S., Esmaillzadeh A. Effect of Multispecies Probiotic Supplements on Metabolic Profile, Hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013;63:1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 40.Kassaian N., Aminorroaya A., Feizi A., Jafari P., Amini M. The Effects of Probiotic and Synbiotic Supplementation on Metabolic Syndrome Indices in Adults at Risk of Type 2 Diabetes: Study Protocol for a Randomized Controlled Trial. Trials. 2017;18:148. doi: 10.1186/s13063-017-1885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palacios T., Vitetta L., Coulson S., Madigan C.D., Denyer G.S., Caterson I.D. The Effect of a Novel Probiotic on Metabolic Biomarkers in Adults with Prediabetes and Recently Diagnosed Type 2 Diabetes Mellitus: Study Protocol for a Randomized Controlled Trial. Trials. 2017;18:7. doi: 10.1186/s13063-016-1762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shavakhi A., Minakari M., Firouzian H., Assali R., Hekmatdoost A., Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-Alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int. J. Prev. Med. 2013;4:531–537. [PMC free article] [PubMed] [Google Scholar]
- 43.Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., Giammaria P., Reali L., Anania F., Nobili V. Randomised Clinical Trial: The Beneficial Effects of VSL#3 in Obese Children with Non-Alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabavi S., Rafraf M., Somi M.H., Homayouni-Rad A., Asghari-Jafarabadi M. Effects of Probiotic Yogurt Consumption on Metabolic Factors in Individuals with Nonalcoholic Fatty Liver Disease. J. Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 45.Sadrzadeh-Yeganeh H., Elmadfa I., Djazayery A., Jalali M., Heshmat R., Chamary M. The Effects of Probiotic and Conventional Yoghurt on Lipid Profile in Women. Br. J. Nutr. 2010;103:1778–1783. doi: 10.1017/S0007114509993801. [DOI] [PubMed] [Google Scholar]
- 46.Chang B.J., Park S.U., Jang Y.S., Ko S.H., Joo N.M., Kim S.I., Kim C.H., Chang D.K. Effect of Functional Yogurt NY-YP901 in Improving the Trait of Metabolic Syndrome. Eur. J. Clin. Nutr. 2011;65:1250–1255. doi: 10.1038/ejcn.2011.115. [DOI] [PubMed] [Google Scholar]
- 47.Leber B., Tripolt N.J., Blattl D., Eder M., Wascher T.C., Pieber T.R., Stauber R., Sourij H., Oettl K., Stadlbauer V. The Influence of Probiotic Supplementation on Gut Permeability in Patients with Metabolic Syndrome: An Open Label, Randomized Pilot Study. Eur. J. Clin. Nutr. 2012;66:1110–1115. doi: 10.1038/ejcn.2012.103. [DOI] [PubMed] [Google Scholar]
- 48.Sharafedtinov K.K., Plotnikova O.A., Alexeeva R.I., Sentsova T.B., Songisepp E., Stsepetova J., Smidt I., Mikelsaar M. Hypocaloric Diet Supplemented with Probiotic Cheese Improves Body Mass Index and Blood Pressure Indices of Obese Hypertensive Patients—A Randomized Double-Blind Placebo-Controlled Pilot Study. Nutr. J. 2013;12:1. doi: 10.1186/1475-2891-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abenavoli L., Scarpellini E., Colica C., Boccuto L., Salehi B., Sharifi-Rad J., Aiello V., Romano B., De Lorenzo A., Izzo A.A., et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients. 2019;11:2690. doi: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papadaki A., Nolen-Doerr E., Mantzoros C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients. 2020;12:3342. doi: 10.3390/nu12113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiciński M., Gębalski J., Gołębiewski J., Malinowski B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms. 2020;8:1148. doi: 10.3390/microorganisms8081148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazloom K., Siddiqi I., Covasa M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients. 2019;11:258. doi: 10.3390/nu11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniali M., Nikfar S., Abdollahi M. A Brief Overview on the Use of Probiotics to Treat Overweight and Obese Patients. Expert Rev. Endocrinol. Metab. 2020;15:1–4. doi: 10.1080/17446651.2020.1719068. [DOI] [PubMed] [Google Scholar]
- 54.Aronsson L., Huang Y., Parini P., Korach-André M., Håkansson J., Gustafsson J.-Å., Pettersson S., Arulampalam V., Rafter J. Decreased Fat Storage by Lactobacillus Paracasei Is Associated with Increased Levels of Angiopoietin-Like 4 Protein (ANGPTL4) PLoS ONE. 2010;5:e13087. doi: 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa A., Kobayashi T., Sakai F., Kadooka Y., Kawasaki Y. Lactobacillus Gasseri SBT2055 Suppresses Fatty Acid Release through Enlargement of Fat Emulsion Size in Vitro and Promotes Fecal Fat Excretion in Healthy Japanese Subjects. Lipids Health Dis. 2015;14:20. doi: 10.1186/s12944-015-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarmozzino F., Poli A., Visioli F. Microbiota and cardiovascular disease risk: A scoping review. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104952. [DOI] [PubMed] [Google Scholar]
- 57.Lescheid D.W. Probiotics as Regulators of Inflammation: A Review. Funct. Foods Health Dis. 2014;4:299. doi: 10.31989/ffhd.v4i7.2. [DOI] [Google Scholar]
- 58.Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q., Wu Y., Fei X. Effect of Probiotics on Body Weight and Body-Mass Index: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Int. J. Food Sci. Nutr. 2016;67:571–580. doi: 10.1080/09637486.2016.1181156. [DOI] [PubMed] [Google Scholar]
- 60.Abbasi J. Are Probiotics Money Down the Toilet? or Worse? JAMA J. Am. Med. Assoc. 2019;321:633–635. doi: 10.1001/jama.2018.20798. [DOI] [PubMed] [Google Scholar]
- 61.Daniel H. Diet and the gut microbiome: From hype to hypothesis. Br. J. Nutr. 2020;124:521–530. doi: 10.1017/S0007114520001142. [DOI] [PubMed] [Google Scholar]
- 62.Hiergeist A., Reischl U., Gessner A., Garzetti D., Stecher B., Gálvez E.J.C., Strowig T., Yang I., Suerbaum S., Fischer N., et al. Multicenter quality assessment of 16S ribosomal DNA-sequencing for microbiome analyses reveals high inter-center variability. Int. J. Med. Microbiol. 2016;306:334–342. doi: 10.1016/j.ijmm.2016.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.