Abstract
Purpose of review
In this review, we present recent insights into the role of the gut microbiota on gastrointestinal (GI) peptide secretion and signalling, with a focus on the orexigenic hormone, ghrelin.
Recent findings
Evidence is accumulating suggesting that secretion of GI peptides is modulated by commensal bacteria present in our GI tract. Recent data shows that the gut microbiome impacts on ghrelinergic signalling through its metabolites, at the level of the ghrelin receptor (growth hormone secretagogue receptor) and highlights concomitant changes in circulating ghrelin levels with specific gut microbiota changes. However, the mechanisms by which the gut microbiota interacts with gut peptide secretion and signalling, including ghrelin, are still largely unknown.
Summary
The gut microbiota may directly or indirectly influence secretion of the orexigenic hormone, ghrelin, similar to the modulation of satiety inducing GI hormones. Although data demonstrating a role of the microbiota on ghrelinergic signalling is starting to emerge, future mechanistic studies are needed to understand the full impact of the microbiota-ghrelin axis on metabolism and central-regulated homeostatic and non-homeostatic controls of food intake.
Keywords: appetite, ghrelin, gut peptides, metabolism, microbiota
INTRODUCTION
Scattered throughout the epithelial cells of the gastrointestinal (GI) tract, enteroendocrine cells (EECs) are responsible for regulation of appetite, digestion, intestinal absorption and motility [1]. Despite the fact that these cells only constitute 1% of the total gut epithelial population, they comprise the largest endocrine system of humans and express receptors capable of sensing and responding to luminal contents [1]. EECs are capable of secreting up to ∼20 specific GI peptides depending on cell type and location (Table 1). GI peptides are subsequently distributed in the GI mucosa to control digestion, appetite and metabolism [2,3]. EECs can be either open type, located in the intestinal epithelium at the GI lumen surface with extended microvilli structures, or closed type, near the basal membrane that lack microvilli [4]. Locations of EECs differ, with X/A, G, D and EC cells populating the stomach, G, D, I, K, L and EC cells being present in the small intestines, and L and EC cells being found in the large intestines [4]. The release of GI peptides by EECs throughout the digestive tract is mediated in response to nutrient availability and ultimately serves to communicate metabolic and nutrient status to the central nervous system (CNS). The brain subsequently determines and directs appetite and decisions on food seeking, food intake and food choice to balance the body's energy needs. This dynamic two-way communication of the gut–brain axis determines ongoing eating behaviour and impacts on overall energy homeostasis.
Table 1.
Common gastrointestinal (GI) peptides and the microbiome
| GI Peptide | EEC Type | Location of secretion | Evidence of microbial metabolite influence | Evidence of microbiome influence | Reference |
| Cholecystokinin (CCK) | I | Proximal Small Intestine | Lys restriction – decreased intestinal CCK expressionAcetate and Butyrate – increased plasma CCK | N/A | [11,12] |
| Gastrin | G | Stomach (Pyloric Antrum) | Increases Acetate – increased plasma Gastrin | N/A | [13] |
| Ghrelin | X/A | Stomach | Acetate, Propionate, Butyrate – decreased ghrelin secretion and attenuated ghrelin-mediated GHR1a stimulationLPS – decreased plasma ghrelinGln, Glu, Lys, Thr, Val – increased ghrelin releaseCys – reduced plasma acyl ghrelinH2S – inhibited ghrelin secretionFormyl Peptides – decreased ghrelin secretion | Total Bacteria, Clostridium, Ruminococcus – positively associated with ghrelinIncreased Bacteroidetes/Firmicutes, Faecalibacterium, Prevotellaceae – negatively associated with ghrelinBacteroides, Bifidobacterium, Lactobacillus – both positively and negatively associated with ghrelin | [14–38] |
| Glucagon-like peptide-1 (GLP-1) | L | Distal Small Intestine and Colon | Acetate, Butyrate, Propionate – trigger GLP-1 secretionIndole – short exposures and long exposures increased and decreased GLP-1 secretion, respectivelyLPS – metabolic changes mediated by LPS attenuated in GLP-1R knock-out mice | Decreased Firmicutes, Bacteroidetes – increased serum GLP-1Increased Proteobacteria – increased GLP-1 | [39–42] |
| Glucagon-like peptide-2 (GLP-2) | L | Distal Small Intestine and Colon | N/A | Increased total bacteria count, Lactobacillus, Bifidobacterium, C. coccoides-E. rectale due to prebiotics – increased GLP-2 production | [43] |
| Glucose-dependent insulinotrophic polypeptide (GIP) | K | Proximal Small Intestines | Butyrate, Propionate – increased plasma GIP | N/A | [44] |
| Leptin | P | Stomach | Lys restriction – reduced mesenteric vein leptin concentration | N/A | [12] |
| Motilin | M | Proximal Small Intestines | N/A | N/A | N/A |
| Nesfatin | X/A | Stomach | N/A | N/A | N/A |
| Neurotensin | N | Distal Small Intestines and Large Intestines | N/A | N/A | N/A |
| Obestatin | X/A | Stomach | N/A | N/A | N/A |
| Oxyntomodulin | L | Distal Small Intestines and Colon | N/A | N/A | N/A |
| Peptide YY (PYY) | L | Distal Small Intestines and Colon | Butyrate, Propionate – increased basal levels, expression and secretion of PYY | Antibiotic-induced fluctuations in enterococci, coliforms, bifidobacteria, aerobic/facultative anaerobic bacteria– increased PYY secretion | [45,46] |
| Secretin | S | Proximal Small Intestines | N/A | Presence/absence of faecal microbiome – secretin degraded within 5 min/no degradation | [47] |
| Somatostatin | D | Stomach, Small Intestines | N/A | Presence/absence of faecal microbiome – somatostatin degraded within 5 min/no degradation | [47] |
An increasing number of studies highlight a key role for the GI microbiota in host metabolism and energy balance (for reviews, see [5–8]). In addition, the gut microbiota is capable of modulating gut peptide (including Protein YY and Glucagon-like peptide-1) secretion and signalling (for reviews, see [9,10]). Here, we examine the current literature for interactions between the GI microbiota and gut hormone secretion, with particular emphasis on the orexigenic ghrelinergic system. Moreover, we will discuss the underlying mechanisms by which the microbiome may modulate ghrelinergic signalling in the gut–brain axis.
Box 1.
no caption available
GASTROINTESTINAL PEPTIDES AND THE GUT–BRAIN AXIS
GI peptides play diverse roles and are secreted from EECs in response to different nutrients, meal anticipation, microbial metabolites, and other circulating factors [11], typically following the modulation of EEC-expressed G protein-coupled receptors (GPCRs) [12]. The GI peptides secreted by EECs depend on the local rates of nutrient absorption but is primarily determined by the EEC type and location in the GI tract (Table 1). EECs may secrete 1 type of hormone, such as K cells that produce glucose-dependent insulinotropic polypeptide (GIP), whereas others secrete several, such as the L cell that secretes glucagon-like peptide 1 (GLP-1), Peptide YY (PYY), and GLP-2 [12]. Secretion of the anorexigenic peptide CCK, is stimulated by amino acids (the most effective of which are L-phenylalanine and L-tryptophan) and fatty acids [13]. Ghrelin is primarily produced in the stomach from X/A-like oxyntic gland cells (P/D1 cells in humans), but also by EECs of the small intestines, and circulating ghrelin levels are modified following secretion, via acylation and degradation [14]. CCK, GLP-1 and PYY interact with their respective GPCRs to promote satiety and inhibit food intake, whereas ghrelin secretion has the opposite effect and stimulates appetite [15]. GPCRs for CCK (CCKR) are located in the gallbladder, pancreas and stomach, GLP-1 GPCRs (GLP1R) are primarily located in the pancreas and stomach, and PYY receptors are scattered throughout the body [16–18]. As well as their effects locally, most gut peptides are essential for proper signalling crosstalk between the GI tract and the CNS [2]. This interaction is facilitated via the gut–brain axis, a complex communication network that incorporates both neural and hormonal signalling pathways for regulation of metabolic function and homeostasis [10].
Many of these gut peptides, including CCK, GLP-1 and PYY, have been shown to participate in the gut–brain axis communication via indirect mechanisms through modulation of GPCR signalling on afferent fibres of the vagus nerve (VN) (the primary element of the parasympathetic nervous system) or directly through traversing the blood-brain barrier and stimulating endogenous receptors in different parts of the brain [19–21]. The arcuate nucleus (ARC) of the hypothalamus is primarily targeted, specifically at the median eminence, a circumventricular organ with more permeable capillaries and is the brain region primarily involved in controlling homeostatic feeding behaviour and balance hunger and satiety [15,22]. Hormones CCK, GLP-1 and PYY have been shown to directly stimulate the VN via GPCRs on nerve endings [23]. Additionally, a recent discovery in mice revealed that EECs can interact physically with enteric glial cells via neuropod structures [24–26]. Neuropods are basal processes of EECs that contain large, dense vesicles of gut peptides and small, clear vesicles containing neurotransmitters [27,28]. These features extend into the lumen and interact with vagal neurons via release of vesicle contents for rapid signal transduction to the brain [29]. Thus, the interaction of gut peptides with the gut–brain axis orchestrates metabolic function, communicates nutrient status to the brain and modulates central-regulated appetite and homeostatic processes, which together drive food seeking and eating behaviour, to maintain energy balance.
MICROBIOTA AND GUT PEPTIDES
The GI tract is colonized by an enormous collection of microorganisms comprising niche populations that increase in density from the stomach (low microbial density of ≈101–103 colony forming units [CFU]/mL) to the colon (very densely populated, ≈1011CFU/mL) [30,31]. Noteworthy, the microbiota of the stomach, the site of ghrelin secretion, is less diverse and fewer in numbers than populations found throughout other parts of the GI tract; culture-independent methods have identified the dominant phyla as being Bacteroidetes, Firmicutes, Actinobacteria, Fusobacteria and Proteobacteria [32]. It is estimated that between 101 and 103 CFU /mL of microbes are found in the stomach, which have the potential to influence host gastric cells and functions [33,34]. The host and the gut microbiome form a symbiotic relationship whereby the host serves as a habitat to the microorganisms and provides it with necessary nutrition, whereas the microbiome metabolises macronutrients into smaller subunits for absorption, that would otherwise remain indigestible [30]. Although human gut microbiomes show variation between individuals, ‘healthy’ adults will display certain ‘typical’ community aspects, such as the dominant phyla being Bacteroidetes and Firmicutes [35].
The interaction between the microbiome, the GI tract and the CNS is facilitated by the microbiota-gut-brain axis [36]. The microbiome influences the brain through production of bioactive molecules and metabolites, including short-chain fatty acids (SCFAs) and neuroactive signalling molecules, such as γ aminobutyric acid (GABA), serotonin and dopamine (for reviews, see [37,38]. In addition, the gut microbiota significantly impacts the production and secretion of gut peptides by EECs [39]. Changes in microbial composition and diversity have been correlated with changes in gut peptide secretion, and specific gut bacteria have been shown to be capable of modulating EECs (Table 1). For example, EECs of germ-free (GF) mice exhibit altered hormone secretion and functionality in comparison to mice with conventional diets. One study demonstrated that GF mice had a higher concentration of K-, L- and enterochromaffin cells, with higher corresponding GLP-1 serum concentrations, than the microbial-colonised counterpart mice [40▪]. A separate study showed decreased expression of CCK, PYY, GLP-1, ghrelin and leptin, lower ileal EEC concentrations and higher colonic EEC counts in GF over control mice fed the same fat emulsions [41]. This highlights that targeting the EEC-microbiota interaction may have potential as novel therapeutic strategies in metabolic disorders such as type 2 diabetes, where hormone secretion levels between sufferers and healthy patients differs [42]. Nevertheless, the mechanisms by which the microbiota regulates gut hormone levels are still poorly understood.
GHRELIN AND THE GHRELIN RECEPTOR
Ghrelin is a 28 amino acid GI hormone, discovered 2 decades ago by Kojima et al., and is primarily released from the empty stomach by X/A-like oxyntic gland cells (P/D1 cells in humans) [43,44]. Ghrelin is also expressed in the small intestines, kidneys, pancreas, heart, lungs, and placenta [45,46]. Although previous reports hypothesise the production of ghrelin in the brain, this opinion remains still somewhat controversial [47,48]. The ghrelin hormone is cleaved from preproghrelin (117 amino acids) to give proghrelin [44]. Next, the ghrelin O-acyltransferase (GOAT) enzyme acylates or, more specifically, octanoylates proghrelin at the serine-3 residue, and the mature, 28-amino acid ghrelin is secreted [43]. Acyl ghrelin (AG) and desacyl ghrelin (DAG) can both be found in the blood stream, but only AG is capable of high-affinity binding to the growth hormone secretagogue receptor (GHSR) [49,50].
The GHSR (also known as the ghrelin receptor) is a 41 kDa GPCR consisting of seven transmembrane domains that exists in 2 forms, GHSR-1a and GHSR-1b [49]. AG is the endogenous ligand of only the GHSR-1a variant, with GHSR-1b being constitutively expressed in certain tissues and appearing to serve as a regulator of GHSR-1a [49]. The ghrelin receptor has been found to regulate a wide array of functions from energy homeostasis, muscle atrophy, cardiac functions, bone metabolism, neurogenesis, and immune function in the periphery, to central appetite mechanisms that regulate both homeostatic and hedonic feeding [51–54]. The recently described role of ligand-dependent and ligand-independent actions of the GHSR in the mesocorticolimbic pathway highlights the impact of this receptor on reward-related behaviour [55]. Ghrelin mainly acts as an orexigenic hormone involved in energy homeostasis, with serum levels being elevated prior to feeding and falling postmeal [56]. Ghrelin modulates food intake, appetite, and weight regulation, and abnormal ghrelin plasma levels are associated with metabolic disorders including obesity and Prader–Willi Syndrome [57]. Moreover, ghrelin plays a role in regulation of glucose homeostasis and metabolic stress [51]. Ghrelin has recently also been explored for its therapeutic potential in stress-related psychiatric disorders [58].
GHSR-1a is expressed ubiquitously in both the central and peripheral nervous system, in line with its breath of signalling functions on expression [59,60]. Communication between ghrelin and the brain is essential for the correct regulation of metabolic function and energy storage [51]. Ghrelin is able to interact with homeostatic appetite centres directly and impact on non-homeostatic reward centres of the brain [57,61]. Additionally, ghrelin can interact with the VN for regulation of feeding behaviour and metabolism [62]. Rats with ghrelin receptor knockdown on the vagal afferent nerve displayed metabolic disturbances, meal pattern disruption, and hippocampal-dependant memory impairment [63].
Thus, ghrelin is a key hormone that relays important nutritional information along the gut–brain axis [64]. An imbalance or dysregulation of the ghrelin-gut–brain axis will result in damaging outcomes for the host, that therefore likely go beyond metabolic and homeostatic dysfunction. Interestingly, a significant overlap exists between the ghrelinergic system and the gut microbiome in the regulation of metabolic and central homeostatic processes across the gut–brain axis, which suggest a potential interaction between microbiota and ghrelin. The following paragraph will highlight the recent data on microbiota and ghrelinergic system and discuss proven and potential mechanisms of this interaction.
MICROBIOTA-DRIVEN MECHANISMS OF GHRELINERGIC SIGNALLING
The interactions between the ghrelinergic system and the GI microbiota are only just beginning to emerge [65]. Positive associations between ghrelin and total bacteria, Clostridium, and Ruminococcus have been identified [66–68]. Additionally, a negative association has also been observed between an increased Bacteroidetes/Firmicutes ratio, Faecalibacterium, Prevotellaceae and ghrelin levels [69–71]. However, some associations are unclear and differ among studies, with Bacteroides, Bifidobacterium and Lactobacillus being both positively and negatively associated with ghrelin [66,67,72–74]. GF models have also been used to examine ghrelin signalling, although not all studies provide specific data on microbial group differences. One study observed a 10-fold higher ghrelin concentration in ex-GF mice fed a high fat diet in comparison to the GF control mice, attributed to the increased acetate production of the microbiome in the former group [75]. A separate group confirmed lower levels of ghrelin in GF mice fed an intralipid emulsion in comparison to the normal controls, although (similar to the Perry et al. (2016) study) no microbiome data was provided for this study [41]. Conversely, GF mice and GF mice infected with Helicobacter pylori exhibited significantly higher ghrelin concentrations than the pathogen-free and infected microbiome control groups at the final experimental timepoint [76]. One study implicated lactate-producing bacteria, specifically lactobacilli, as the primary cause of elevated plasma ghrelin levels in GF rats that received faecal transplants from human patients with short-bowel syndrome [77].
Additionally, the use of antibiotics for perturbation of the mouse microbiome and downstream effects on ghrelin have also been examined. One such study showed that serum ghrelin concentrations were significantly lower in sub-therapeutic antibiotic treatment mice on a high fat diet than control mice on the same diet [78]. Conversely, higher plasma AG levels were observed in mice that had received a fat-free diet and a cocktail of antibiotics in comparison to control mice on the same diet, although neither plasma DAG nor the AG/total ghrelin ratio differed significantly between groups [79]. The differences seen here may be explained by the different antibiotics and differing diets administered in each study, however, both show that microbial perturbation by antibiotics can have an effect on ghrelinergic signalling. Although this highlights a potential microbiota-mediated regulation of ghrelin, very little is understood with regards to the putative mechanism by which the gut microbiota may affect circulating ghrelin levels and ghrelinergic signalling.
Microbiota and ghrelin receptor signalling
A recent study has shown that specific bacteria strains common to the human GI tract (primarily strains of Bifidobacteria and Lactobacillus) are able to attenuate ghrelinergic signalling [80▪▪]. Specifically, addition of Bifidobacterium supernatants prior to ghrelin exposure increased the potency of ghrelin on downstream signalling kinases, and B. longum APC1472, Lb. rhamnosus DPC6118 and Lb. gasseri DPC6106 significantly reduced ghrelin-mediated GHSR internalisation [80▪▪]. This highlights for the first time the ability of specific bacterial strains to affect the complex signalling cascades of the ghrelin receptor, via the direct modification of ghrelin-mediated activation of the GHSR-1a (Fig. 1). Noteworthy, it was subsequently shown that B. longum APC1472 positively impacted on markers of obesity and stress in a preclinical mouse model of obesity as well as in healthy overweight and obese individuals (Schellekens et al., ‘Bifidobacterium longum Counters the Effects of Obesity: Partial Successful Translation from Rodent to Human’, EBIOMEDICINE, unpublished).
FIGURE 1.
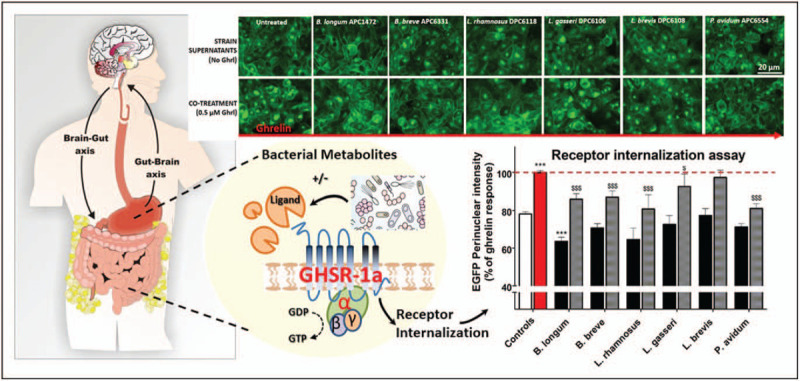
Bacterial strains supernatants attenuate ghrelin-mediated GHSR-1a signalling. Representative images of GHSR-1a-EGFP internalization in HEK293 cells and quantified fluorescence intensity in bar graph following treatment with bacterial supernatants (upper panel, black bars) and following cotreatment with 0.5 μM ghrelin (lower panel, light grey bars). Ghrelin-mediated GHSR-1a-EGFP internalization is depicted by the red bar. Bacterial strains: B. longum APC1472, B. breve APC6331, L. rhamnosus DPC6118, L. gasseri DPC6106, L. brevis DPC6108, and P. avidum APC6544. ∗∗∗P # 0.001 vs. untreated control; $$$ P # 0.01 vs. ghrelin control, demonstrates attenuation of ghrelin-mediated GHSR-1a internalization. (adapted from [80▪▪]).
Microbiota-derived short chain fatty acids and ghrelin signalling
SCFAs are produced by gut microbes through fermentation of ingested, otherwise undigestible complex carbohydrates, with the most common including acetate, butyrate and propionate [81]. SCFAs play a role in gut health maintenance and present an energy source for colonic cells and are also implicated in CNS homeostasis [37,81,82]. SCFAs play a role in maintaining blood brain barrier integrity, the maturation of microglia, and regulating levels of neurotransmitters and neurotrophic factors, and administration of these compounds in experimental models of various CNS disorders have shown therapeutic potential [83]. Although in vivo studies exist that have monitored concentration changes of SCFAs in conjunction with ghrelin levels in the body, the molecular mechanisms are not yet fully understood.
SCFAs are negatively associated with ghrelin concentrations [84] and microbial SCFAs can target free fatty acid receptor 2 (FFAR2), a GPCR which has a downstream inhibitory effect on ghrelin secretion [85–87]. Interestingly, recent evidence has shown that SCFAs regulate ghrelin in a more direct manner, through antagonism of its primary GHR1a receptor [80▪▪]. In vitro experiments showed that cells expressing the GHR1a exhibited a significant decrease in ghrelin-mediated calcium influx when cotreated with ghrelin and propionate or butyrate. Additionally, ghrelin-mediated GHR1a internalization was decreased in the presence of sodium acetate, sodium butyrate and sodium propionate [80▪▪]. Overall, gut microbes that produce SFCAs may influence ghrelinergic signalling, either indirectly via FFAR2-mediated ghrelin regulation or via direct antagonism or allosteric modulation of the ghrelin-specific receptor, GHR1a (Fig. 2). Together, this highlights a novel putative mechanism by which the microbiota may interact on the ghrelinergic axis, and future studies are warranted.
FIGURE 2.
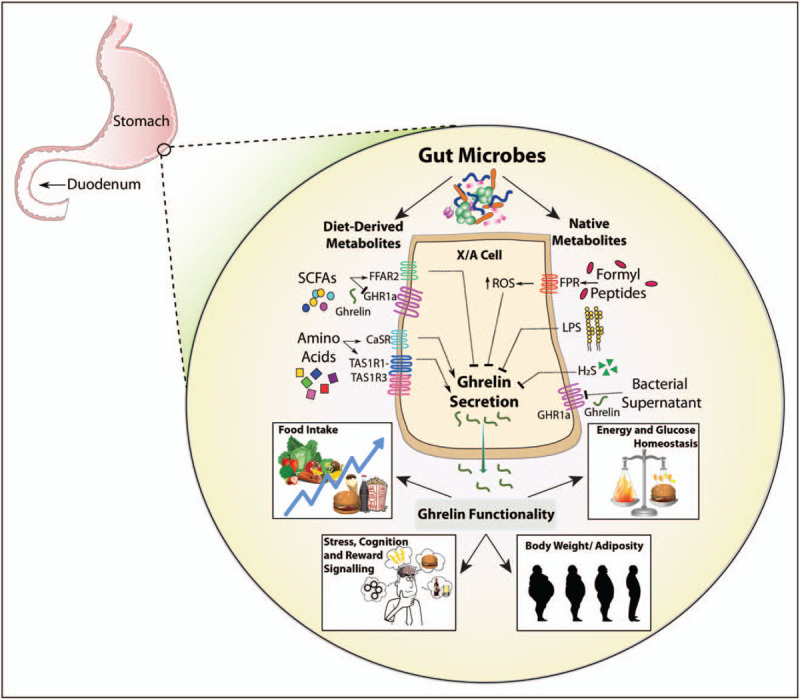
Putative mechanisms by which the microbiota influences ghrelinergic signalling.
Microbiota-derived lipopolysaccharides and ghrelin signalling
Ghrelin signalling may also be affected by endotoxins produced by gut microbes. Gram negative bacteria-derived Lipopolysaccharide (LPS) stimulate TLR-4 and inflammation responses in the host [88,89]. In vivo studies have shown that LPS administration alters circulating levels of ghrelin, albeit with some inconsistency, namely a decrease in fasting plasma ghrelin in rats and a biphasic change in healthy humans [90–92]. Exogenous ghrelin administration was shown to interact with the VN, resulting in a reduction of LPS-induced colonic hyperpermeability [93▪]. The LPS produced during a H. pylori infection was shown to stimulate TLR-4 in the stomach, which led to a signal cascade ultimately resulting in unwanted production of urease and ammonia and loss of proper epithelial permeability [94]. Specifically, H. pylori LPS and subsequent colonisation in the stomach induced secretion of proinflammatory cytokines (including IL-1β), and ghrelin-mediated activation of the GHR1a, resulting in a convergence of the ghrelin and TLR-4 signal pathways at MAPK and PLC/PKC/PI3K pathways [94]. Thus, the inflammatory response following H. pylori LPS is modulated via direct TLR-4 stimulation of LPS and via ghrelin-mediated GHR1a activation [94].Interestingly, H. pylori-infected obese individuals were shown to have less ghrelin-producing cells [95▪]. A separate study demonstrated an upregulation in GHSR mRNA and protein levels signalling in human osteoblast-like cells in the presence of a periodontopathogen and the IL-1β cytokine, which was enhanced further in the presence of ghrelin [96▪]. Noteworthy, EEC Toll-like receptor (TLR) 9 were shown to bind to CpG hexamers of typical enteropathogenic bacteria, resulting in the secretion of CCK [97▪]. Thus, evidence is accumulating demonstrating that the gut microbiome and specifically enteropathogens modulate gut peptide secretion and ghrelin signalling via TLRs expressed on EECs (Fig. 2).
Other microbiota-derived metabolites and Ghrelin signalling
The microbiota may mediate ghrelin expression through the generation of reactive oxygen species (ROS) in host epithelial cells. Endogenous ROS are produced in the host cell by mitochondria, through conversion of O2 to energy or as a response to exposure of cells to microbial factors [98]. Commensal gut microbes can produce formylated peptides capable of stimulating ROS generation in host cells through activation of epithelial GPCR formyl peptide receptor 1 (FPR1) [99]. Several studies have confirmed that the concentration of plasma ghrelin levels is increased by systemic oxidative stress, and that ghrelin may exert anti-inflammatory properties [100]. Finally, activation of antioxidant pathways in ghrelin cells decreases intracellular ROS, which increases ghrelin secretion [101▪]. Thus, microbial generation of ROS in X/A cells has the potential to modulate the concentration of secreted ghrelin (Fig. 2).
A significant quantity of the gaseous signalling Hydrogen Sulphide (H2S) is produced by the gut microbiome, and it has been demonstrated that the bound sulfane-sulfur fraction concentration is decreased by 50–80% in GF mice in comparison to their conventional counterparts, highlighting the importance of the gut microbiome in H2S bioavailability and metabolism [102]. Interestingly, a role for H2S in ghrelin secretion has been reported, whereby H2S inhibited ghrelin secretion in rat stomach cells in vitro and the administration of the same H2S donor molecule compound in mice exhibited delays in postprandial ghrelin secretion, as well as a reduced appetite [103▪]. This may suggest that microbial-produced H2S regulated ghrelin levels via modulation of its secretion (Fig. 2).
The gut microbiota also digest large dietary proteins into specific amino acids (AA), following catabolism by bacterial extracellular proteases and peptidases [104]. The colonic microbiota phyla of firmicutes have been reported to produce glutamine, glutamic acid, lysine, tryptophan and leucine [105]. The digestibility of threonine and valine by the microbiota in the small intestines was shown to be >50%, whereas cysteine degradation is a primary pathway for production of hydrogen sulphide by gut microbes [106,107]. Interestingly, specific AA have been shown to affect ghrelin levels in vivo with L-glutamine, L-glutamic acid, L-lysine, L-threonine and L-valine increasing [108,109,110], and L-cysteine, L-tryptophan and L-leucine reducing ghrelin plasma levels [111,112]. Gastric ghrelinoma cells secrete ghrelin in response to specific classes of amino acids via taste receptors mediated mechanism [113], which are affected in obesity [114] (Fig. 2).
In addition, the gut microbiota may be able to produce metabolites, yet to be discovered, that can either activate receptors on EECs for ghrelin secretion or act as allosteric modulators or ghrelin mimetics for GHSR modulation [80▪▪]. Overall, recent literature is highlighting the immense potential in exploration of microbiota-derived metabolites for GPCR modulation in the gut–brain axis [115,116,117▪].
CONCLUDING REMARKS
Evidence shows that the gut microbiome produces metabolites that can interact with GI peptide-producing EECs and indirectly regulate metabolism, appetite and satiety systems. Ghrelin is an important gut peptide produced in the stomach and primarily involved in peripheral metabolism, glucose homeostasis, energy balance and central homeostatic as well as hedonic mechanisms that regulate appetite and food intake. Ghrelin is one of several important appetite regulating proteins produced by EECs but is unique in that it is the only known peripherally produced orexigenic modulator. Ghrelin is an important signalling peptide in the brain–gut axis, either indirectly interacting with the CNS via the VN or directly by traversing the blood-brain barrier and stimulating its target receptor, the GHSR-1a, expressed in the periphery and various parts of the brain. Correlation studies demonstrate changes in levels of the GI peptide ghrelin in the presence of defined microbial compositions (Table 1). However, evidence for the exact mechanisms of how ghrelin is modulated by the microbiome or microbial metabolites are still largely unexplored, with the majority of the studies examining plasma ghrelin levels overall.
This review has identified the potential role of the GI microbiota and its metabolites to directly or indirectly interact with the ghrelin signalling system. Specifically, we highlighted the ability of specific bacterial strains and SCFAs to directly modify ghrelin-mediated activation of the GHSR [80▪▪] (see (Fig. 1). In addition, we highlighted the potential of microbiota-derived metabolites to modulate the ghrelinergic system and the specific biochemical pathways of these interactions now need to be investigated further (see (Fig. 2).
The gut microbiota plays a key role that extends beyond digestion and metabolic function, to central-regulated processes, which govern homeostatic and non-homeostatic mechanism controlling eating behaviour. Understanding how the gut microbiota contributes to GI peptide secretion, including ghrelin, will significantly contribute to the development of microbiota-targeted strategies to modulate metabolism, appetite and eating behaviour.
Acknowledgements
The authors would like to thank Ken O’Riordan for assistance with graphic work.
Financial support and sponsorship
Their research is funded by Science Foundation Ireland Research Centre Grant SFI/12/RC/2273 to the APC Microbiome Institute Ireland. They have research partnerships with a number of food and pharma companies including Cremo, Pharmavite, Dupont and Nutricia.
Conflicts of interest
John F. Cryan & Harriët Schellekens are inventors on a patent based on the activity of bacterial strains at the ghrelin receptor. The remaining author has no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Crooks B, Stamataki NS, Mclaughlin JT. Appetite, the enteroendocrine system, gastrointestinal disease and obesity. Proc Nutr Soc 2020; 1–9. [DOI] [PubMed] [Google Scholar]
- 2.Sam AH, Troke RC, Tan TM, Bewick GA. The role of the gut/brain axis in modulating food intake. Neuropharmacology 2012; 63:46–56. [DOI] [PubMed] [Google Scholar]
- 3.Murray S, Tulloch A, Gold MS, Avena NM. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 2014; 28:540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 2016; 28:620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira R, dos S, Mendonça LABM, et al. Relationship between intestinal microbiota, diet and biological systems: an integrated view. Crit Rev Food Sci Nutr 2020. [DOI] [PubMed] [Google Scholar]
- 6.Duca FA, Lam TKT. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab 2014; 16:68–76. [DOI] [PubMed] [Google Scholar]
- 7.van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr Am Soc Nutr 2017; 147:727–745. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol 2017; 2:747–756. [DOI] [PubMed] [Google Scholar]
- 9.Covasa M, Stephens RW, Toderean R, Cobuz C. Intestinal sensing by gut microbiota: targeting gut peptides. Front Endocrinol 2019; 10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss ES, Whiteside E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol 2018; 9:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin AM, Sun EW, Keating DJ. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J Endocrinol BioScientifica Ltd 2020; 244:R1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 2019; 15:226–237. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chandra R, Samsa LA, et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol - Gastrointest Liver Physiol 2011; 300:G528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdalla MMI. Ghrelin - physiological functions and regulation. Eur Endocrinol 2015; 11:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prinz P, Stengel A. Control of food intake by gastrointestinal peptides: mechanisms of action and possible modulation in the treatment of obesity. J Neurogastroenterol Motil 2017; 23:180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehfeld JF. Cholecystokinin-from local gut hormone to ubiquitous messenger. Front Endocrinol 2017; 8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker HE, Gribble FM, Reimann F. The role of gut endocrine cells in control of metabolism and appetite. Exp Physiol 2014; 99:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi M, Li H, Wu Z, et al. A promising therapeutic target for metabolic diseases: neuropeptide y receptors in humans. Cell Physiol Biochem 2018; 45:88–107. [DOI] [PubMed] [Google Scholar]
- 19.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018; 12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 2018; 15:36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon- like peptide-1 secretion. Endocrinology 1999; 140:1687–1694. [DOI] [PubMed] [Google Scholar]
- 22.Bentivoglio M, Kristensson K, Rottenberg ME. Circumventricular organs and parasite neurotropism: neglected gates to the brain? Front Immunol 2018; 9:2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye L, Liddle RA. Gastrointestinal hormones and the gut connectome. Curr Opin Endocrinol Diabetes Obes 2017; 24:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohórquez DV, Samsa LA, Roholt A, et al. An enteroendocrine cell - Enteric glia connection revealed by 3D electron microscopy. PLoS One 2014; 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohórquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Investig 2015; 125:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science (80-) 2018; 361:5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liddle RA. Neuropods. CMGH 2019; 7:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaelberer MM, Bohórquez DV. The now and then of gut-brain signaling. Brain Res 2018; 1693:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaelberer MM, Rupprecht LE, Liu WW, et al. Neuropod cells: the emerging biology of gut-brain sensory transduction. Ann Rev Neurosci 2020; 43:337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2015; 14:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016; 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardone G, Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur Gastroenterol J 2015; 3:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe 2019; 26:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens RW, Arhire L, Covasa M. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity 2018; 26:801–809. [DOI] [PubMed] [Google Scholar]
- 36.Cryan JF, O’riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev 2019; 99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 37.Silva YP, Bernardi A, Frozza RL. The Role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 2020; 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res 2018; 1693:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plovier H, Cani PD. Enteroendocrine cells: metabolic relays between microbes and their host. In:. Endocre Dev 2017; 139–164. [DOI] [PubMed] [Google Scholar]
- 40▪.Modasia A, Parker A, Jones E, et al. regulation of enteroendocrine cell networks by the major human gut symbiont Bacteroides thetaiotaomicron. Front Microbiol 2020; 11:2800. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that a single microbial strain has the potential to regulate enteroendorcine cells, even restore numbers of specific hormone-producing cells in mouse models.
- 41.Duca FA, Swartz TD, Sakar Y, Covasa M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS 2012; 7:e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorsal T, Rhee NA, Pedersen J, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 2018; 61:284–294. [DOI] [PubMed] [Google Scholar]
- 43.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402:656–660. [DOI] [PubMed] [Google Scholar]
- 44.Schellekens H, Dinan TG, Cryan JF. Lean mean fat reducing ‘ghrelin’ machinehypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology 2010; 58:2–16. [DOI] [PubMed] [Google Scholar]
- 45.Ghelardoni S, Carnicelli V, Frascarelli S, et al. Ghrelin tissue distribution: Comparison between gene and protein expression. J Endocrinol Invest 2006; 29:115–121. [DOI] [PubMed] [Google Scholar]
- 46.Sakata I, Sakai T. Ghrelin cells in the gastrointestinal tract. Int J Pept 2010; 2010:945056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabral A, López Soto EJ, Epelbaum J, Perelló M. Is ghrelin synthesized in the central nervous system? Int J Mol Sci 2017; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellman M, Abizaid A. Knockdown of central ghrelin O-acyltransferase by vivo-morpholino reduces body mass of rats fed a high-fat diet. Peptides 2015; 70:17–22. [DOI] [PubMed] [Google Scholar]
- 49.Yin Y, Li Y, Zhang W. The growth hormone secretagogue receptor: Its intracellular signaling and regulation. Int J Mol Sci 2014; 15:4837–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moose JE, Leets KA, Mate NA, et al. An overview of ghrelin O-acyltransferase inhibitors: a literature and patent review for 2010–2019. Expert Opinion on Therapeutic Patents 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metabol 2018; 27:786–804. [DOI] [PubMed] [Google Scholar]
- 52.Kim C, Kim S, Park S. Neurogenic effects of ghrelin on the hippocampus. Int J Mol Sci 2017; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pradhan G, Samson SL, Sun Y. Ghrelin: Much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013; 16:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereira JADS, Silva FC, Da, De Moraes-Vieira PMM. The impact of ghrelin in metabolic diseases: an immune perspective. J Diabetes Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornejo MP, Mustafá ER, Barrile F, et al. The intriguing ligand-dependent and ligand-independent actions of the growth hormone secretagogue receptor on reward-related behaviors. Neurosci Behav Rev 2020. [DOI] [PubMed] [Google Scholar]
- 56.Stengel A, Tache Y. Interaction between gastric and upper small intestinal hormones in the regulation of hunger and satiety: ghrelin and cholecystokinin take the central stage. Curr Protein Pept Sci 2011; 12:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howick K, Griffin BT, Cryan JF, Schellekens H. From belly to brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci 2017; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fritz EM, Singewald N, De Bundel D. The good, the bad and the unknown aspects of ghrelin in stress coping and stress-related psychiatric Disorders. Front Synaptic Neurosci 2020; 12:594484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gnanapavan S, Kola B, Bustin SA, et al. The Tissue Distribution of the mRNA of Ghrelin and Subtypes of Its Receptor, GHS-R, in Humans. J Clin Endocrinol Metab 2002; 87:2988–12988. [DOI] [PubMed] [Google Scholar]
- 60.McKee KK, Palyha OC, Feighner SD, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol 1997; 11:415–423. [DOI] [PubMed] [Google Scholar]
- 61.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 2015; 274:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Date Y. Ghrelin and the vagus nerve. Methods Enzymol 2012; 514:261–269. [DOI] [PubMed] [Google Scholar]
- 63.Davis EA, Wald HS, Suarez AN, et al. Ghrelin signaling affects feeding behavior, metabolism, and memory through the vagus nerve. Curr Biol 2020; 30:4510–4518. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schellekens H, Finger BC, Dinan TG, Cryan JF. Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol Ther 2012; 135:316–326. [DOI] [PubMed] [Google Scholar]
- 65.Schalla MA, Stengel A. Effects of microbiome changes on endocrine ghrelin signaling – a systematic. Peptides 2020; 133: [DOI] [PubMed] [Google Scholar]
- 66.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr 2012; 107:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang F, Li J, Pang G, et al. Effects of diethyl phosphate, a nonspecific metabolite of organophosphorus pesticides, on serum lipid, hormones, inflammation, and gut microbiota. Molecules 2019; 24:2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bo TB, Zhang XY, Wen J, et al. The microbiota–gut–brain interaction in regulating host metabolic adaptation to cold in male Brandt's voles (Lasiopodomys brandtii). ISME J 2019; 13:3037–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanagi H, Tsuda A, Matsushima M, et al. Changes in the gut microbiota composition and the plasma ghrelin level in patients with Helicobacter pylori-infected patients with eradication therapy. BMJ Open Gastroenterol 2017; 4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang C, Zhang Y, Zhu X, et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab 2016; 101:4681–4689. [DOI] [PubMed] [Google Scholar]
- 71.Gomez-Arango LF, Barrett HL, McIntyre HD, et al. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 2016; 65:2214–2223. [DOI] [PubMed] [Google Scholar]
- 72.Hooda S, Vester Boler BM, Kerr KR, et al. The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br J Nutr 2013; 109:1637–1646. [DOI] [PubMed] [Google Scholar]
- 73.Massot-Cladera M, Mayneris-Perxachs J, Costabile A, et al. Association between urinary metabolic profile and the intestinal effects of cocoa in rats. Br J Nutr 2017; 117:623–634. [DOI] [PubMed] [Google Scholar]
- 74.Liu R, Zhang C, Shi Y, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol 2017; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016; 534:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khosravi Y, Seow SW, Amoyo AA, et al. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci Rep 2015; 5:8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillard L, Mayeur C, Robert V, et al. Microbiota is involved in postresection adaptation in humans with short bowel syndrome. Front Physiol 2017; 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahana D, Trent CM, Kurtz ZD, et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med 2016; 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikenoya C, Takemi S, Kaminoda A, et al. (-Oxidation in ghrelin-producing cells is important for ghrelin acyl-modification. Sci Rep 2018; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪▪.Torres-Fuentes C, Golubeva AV, Zhdanov AV, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J 2019; 33:13546–13559. [DOI] [PubMed] [Google Scholar]; This paper describes direct interaction of both SCFA and bacterial supernatants on the Ghrelin receptor, which is novel, demonstrating the ability of microbes to attenuate ghrelin signalling.
- 81.Venegas DP, De La Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol 2018; 596:4923–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bogie JFJ, Haidar M, Kooij G, Hendriks JJA. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv Drug Deliv Rev 2020; 159:198–213. [DOI] [PubMed] [Google Scholar]
- 84.Rahat-Rozenbloom S, Fernandes J, Cheng J, Wolever TMS. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur J Clin Nutr 2017; 71:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mishra SP, Karunakar P, Taraphder S, Yadav H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view. Biomedicines 2020; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Engelstoft MS, Schwartz TW. Opposite regulation of ghrelin and glucagon-like peptide metabolite G-protein-coupled receptors. Trends Endocrinol Metab 2016; 27:665–675. [DOI] [PubMed] [Google Scholar]
- 87.Engelstoft MS, Park W, mee, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2013; 2:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salguero M, Al-Obaide M, Singh R, et al. Dysbiosis of gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med 2019; 18:3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.d’Hennezel E, Abubucker S, Murphy LO, Cullen TW. Total lipopolysaccharide from the human gut microbiome silences toll-like receptor signaling. mSystems 2017; 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Basa NR, Shaikh A, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol - Gastrointest Liver Physiol 2006; 291: [DOI] [PubMed] [Google Scholar]
- 91.Vila G, Maier C, Riedl M, et al. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab 2007; 92:3930–3934. [DOI] [PubMed] [Google Scholar]
- 92.Basa NR, Wang L, Arteaga JR, et al. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett 2003; 343:25–28. [DOI] [PubMed] [Google Scholar]
- 93▪.Ishioh M, Nozu T, Igarashi S, et al. Ghrelin acts in the brain to block colonic hyperpermeability in response to lipopolysaccharide through the vagus nerve. Neuropharmacology 2020; 173. [DOI] [PubMed] [Google Scholar]; This study shows that ghrelin has role in ameliorating LPS-induced hyperpermeability. LPS is a microbial metabolite that here is shown to affect ghrelinergic signalling in some way.
- 94.Slomiany BL, Slomiany A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin. Inflammopharmacology 2017; 25:415–429. [DOI] [PubMed] [Google Scholar]
- 95▪.Mihalache L, Arhire LI, Giuşcă SE, et al. Ghrelin-producing cells distribution in the stomach and the relation with Helicobacter pylori in obese patients. Rom J Morphol Embryol 2019; 60:219–225. [PubMed] [Google Scholar]; This study showed a decrease in ghrelin-producing cells in individuals with a Helicobacter pylori infection. As other papers investigated in this review examined the effect of H. pylori LPS on ghrelin signalling, the ability of the pathogen to modulate ghrelin cells is highly relevent.
- 96▪.Nokhbehsaim M, Nogueira AVB, Memmert S, et al. Regulation of ghrelin receptor by microbial and inflammatory signals in human osteoblasts. Braz Oral Res 2019. [DOI] [PubMed] [Google Scholar]; As this review examines the ability of ghrelin to be modulated by LPS, this particular study is important in substantiating the potential anti-inflammatory role of ghrelin and, thus, why ghrelineric signalling would be influenced by endotoxins such as LPS.
- 97▪.Daly K, Burdyga G, Al-Rammahi M, et al. Toll-like receptor 9 expressed in proximal intestinal enteroendocrine cells detects bacteria resulting in secretion of cholecystokinin. Biochem Biophys Res Commun 2020; 525:936–940. [DOI] [PubMed] [Google Scholar]; This suggests a role for Toll-like receptors in modulation of enterendocrine cells via interaction with gut microbes or microbial metabolites. This could be a potential mechanism for ghrelin modulation by the gut microbiome that has not yet been examined.
- 98.Marciano F, Vajro P. Oxidative stress and gut microbiota. In: Gastrointestinat tissue: oxidative stress and dietary antioxidants. Elsevier Inc.; 2017. p. 113–23. [Google Scholar]
- 99.Alam A, Leoni G, Wentworth CC, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor. Mucosal Immunol 2014; 7:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr 2011; 48:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101▪.Mani BK, Osborne-Lawrence S, Metzger N, Zigman JM. Lowering oxidative stress in ghrelin cells stimulates ghrelin secretion. Am J Physiol - Endocrinol Metab 2020; 319:E330–E337. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that removal of reactive oxygen species from ghrelin cells stimulates ghrelin secretion. Microbiobes can cause an increase in reactive oxygen species in host cells, potentially modulating ghrelin secretion.
- 102.Shen X, Carlström M, Borniquel S, et al. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 2013; 60:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103▪.Slade E, Williams L, Gagnon J. Hydrogen sulfide suppresses ghrelin secretion in vitro and delays postprandial ghrelin secretion while reducing appetite in mice. Physiol Rep 2018; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper looks at the direct result of Hygrogen sulfide in vitro on primary ghrelin cells and in vivo in mice, with both showing an effect on ghrelin secretion.
- 104.Portune KJ, Beaumont M, Davila AM, et al. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci Technol 2016; 57:213–232. [Google Scholar]
- 105.Matsumoto M, Kunisawa A, Hattori T, et al. Free D-amino acids produced by commensal bacteria in the colonic lumen. Sci Rep 2018; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma N, Ma X. Dietary amino acids and the gut-microbiome-immune axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Safy 2019; 18:221–242. [DOI] [PubMed] [Google Scholar]
- 107.Blachier F, Beaumont M, Kim E. Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr Opin Clin Nutr Metab Care 2019; 22:68–75. [DOI] [PubMed] [Google Scholar]
- 108.Yin J, Han H, Li Y, et al. Lysine restriction affects feed intake and amino acid metabolism via gut microbiome in piglets. Cell Physiol Biochem 2018; 44:1749–1761. [DOI] [PubMed] [Google Scholar]
- 109.Yin J, Li Y, Han H, et al. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Funct 2018; 9:4153–4163. [DOI] [PubMed] [Google Scholar]
- 110.Elsabagh M, Ishikake M, Sakamoto Y, et al. Postruminal supply of amino acids enhances ghrelin secretion and lipid metabolism in feed-deprived sheep. Anim Sci J 2018; 89:1663–1672. [DOI] [PubMed] [Google Scholar]
- 111.Mcgavigan AK, O’hara HC, Amin A, et al. L-cysteine suppresses ghrelin and reduces appetite in rodents and humans. Int J Obes 2015; 39:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinert RE, Ullrich SS, Geary N, et al. Comparative effects of intraduodenal amino acid infusions on food intake and gut hormone release in healthy males. Physiol Rep 2017; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vancleef L, Van Den Broeck T, Thijs T, et al. Chemosensory signalling pathways involved in sensing of amino acids by the ghrelin cell. Sci Rep 2015; 5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vancleef L, Thijs T, Baert F, et al. Obesity impairs oligopeptide/amino acid-induced ghrelin release and smooth muscle contractions in the human proximal stomach. Mol Nutr Food Res 2018; 62:1700804. [DOI] [PubMed] [Google Scholar]
- 115.Cully M. G protein-coupled receptors: gut feeling on bacterial GPCR agonists. Nat Rev Drug Discov 2017; 16:754. [DOI] [PubMed] [Google Scholar]
- 116.Cryan JF, Clarke G, Dinan TG, Schellekens H. A microbial drugstore for motility. Cell Host Microbe 2018; 23:691–692. [DOI] [PubMed] [Google Scholar]
- 117▪.Colosimo DA, Kohn JA, Luo PM, et al. Mapping interactions of microbial metabolites with human G-protein-coupled receptors. Cell Host Microbe 2019; 26:273–282. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies simple compounds produced from microbes that have the capacity to modulate various GPCR of host cells, implicating a potential role for these metablites in important signalling pathways (e.g. host immune system). A similar idea whereby microbes produce useful metabolites of unknown function has been put forward previously by Cryan et al., 2018 (see above). This current review has identified several GPCRs capable of affecting ghrelinergic signalling, that can be stimulated by microbial metabolites, affirming that microbial metabolites can utilize GPCRs to module ghrelin signalling and secretion.


