Abstract
Candida albicans is a commensal organism and opportunistic pathogen that can form biofilms that colonize surfaces such as implants, catheters, and dentures. Compared to planktonic C. albicans cells, cells in biofilms exhibit increased resistance to treatment. Histatin 5 (Hst-5) is an antimicrobial peptide that is natively secreted by human salivary glands and has strong antifungal activity against C. albicans. However, C. albicans produce secreted aspartic proteases (Saps) that can cleave and inactivate Hst-5, limiting its antifungal properties. We previously showed that residue substitutions K11R and K17R within Hst-5 improve its antifungal activity and prevent proteolytic degradation by Saps when treating planktonic C. albicans. We investigated the use of the K11R-K17R peptide as an alternative therapeutic against C. albicans biofilms by assessing its ability to reduce viability of pre-formed biofilms and to inhibit the formation of biofilms and showed that K11R-K17R had improved activity compared to Hst-5. Based on these results, we incorporated K11R-K17R and Hst-5 into polyelectrolyte multilayer (PEM) surface coatings and demonstrated that films functionalized with K11R-K17R reduced the formation of C. albicans biofilms. Our results demonstrate the therapeutic potential of the K11R-K17R Hst-5 variant in preventing and treating biofilms.
Keywords: histatin 5, Candida albicans, biofilm, antimicrobial peptides, polyelectrolyte multilayer film
Graphical Abstract
1. Introduction
One of the most important human fungal pathogens is Candida albicans, which is a commensal organism and opportunistic pathogen. C. albicans can cause a range of infections, including mild rashes, oral thrush, genital yeast infections, and potentially life-threatening systemic infections (Pfaller and Diekema, 2007). Resistance of C. albicans infections to currently available antifungal agents is a rising problem, with nearly 7% of bloodstream isolates exhibiting resistance (Centers for Disease Control and Prevention, 2019). Complicating infections further, C. albicans cells can colonize the surfaces of medical devices, such as implants, catheters, and dentures, and form biofilms that exhibit increased resistance to antifungal agents (Cavalheiro and Teixeira, 2018; Chandra and Mukherjee, 2015; Lohse et al., 2018; Nett and Andes, 2020; Nobile and Johnson, 2015). C. albicans cells from biofilms exhibit minimum inhibitory concentrations (MICs) up to 20,000-fold those of planktonic cells (Hawser and Douglas, 1995).
The challenges associated with resistance to current antifungal agents and biofilms have motivated the search for new approaches to treating and preventing infections caused by C. albicans. One class of molecules that has been explored as a source of new antifungal agents is antimicrobial peptides, which are a diverse class of molecules that are often cationic. One of these peptides, histatin 5 (Hst-5), is particularly interesting as a potential agent to combat C. albicans. Hst-5 is member of the histatin family of histidine-rich peptides that are secreted from human salivary glands (Xu et al., 1991). Of the histatin peptides, the 24-amino-acid Hst-5 has the strongest antifungal activity, and the peptide has been implicated in the maintenance of C. albicans at non-virulent levels in the oral cavity (Khan et al., 2013; Meiller et al., 2009; Peters et al., 2010). Hst-5 must enter C. albicans cells to exert its antimicrobial activity, and it enters the cells via the fungal polyamine transporters Dur3 and Dur31 in a nonlytic, energy-dependent manner (Mochon and Liu, 2008; Puri and Edgerton, 2014). Entry into the cell leads to an imbalance of ATP, potassium, and magnesium that causes cell volume loss and cell death (Mochon and Liu, 2008; Puri and Edgerton, 2014; Pusateri et al., 2009). Although Hst-5 has strong antifungal activity, the peptide is susceptible to degradation by secreted aspartic proteases (Saps) produced by C. albicans (Ikonomova et al., 2018; Ikonomova et al., 2020; Meiller et al., 2009). Degradation of Hst-5 by Saps leads to reduced antifungal activity against planktonic cells, suggesting a possible mechanism for C. albicans to evade its activity (Meiller et al., 2009) and presenting a potential limitation to its development as an antifungal agent.
To improve the potential of Hst-5 as an antifungal agent, our lab designed variants of the antimicrobial peptide histatin 5 with reduced susceptibility to Saps 2 and 9. Since the Sap enzymes cut Hst-5 at lysine residues (Ikonomova et al., 2018; Meiller et al., 2009), we explored variants with substitutions at these lysine residues. We found that modifying the K17 residue to either arginine or leucine led to a substantial reduction in proteolytic degradation by the Saps (Ikonomova et al., 2018). Substitution of the K11 residue with an arginine residue led to a modest improvement in antifungal activity against planktonic cells, though the overall degradation of the peptide was not reduced (Ikonomova et al., 2018). We combined the modifications at these two sites to create a new peptide, K11R-K17R, that exhibits both reduced degradation by Saps and an increase in antifungal activity without leading to toxicity towards mammalian cells (Ikonomova et al., 2018).
Because biofilms contain a large number of C. albicans cells potentially leading to a large concentration of Saps, we hypothesized that our protease resistant Hst-5 variant K11R-K17R may provide an advantage over native Hst-5 in inhibiting the growth of cells in C. albicans biofilms. To evaluate this hypothesis, we studied the ability of the Hst-5 and K11R-K17R peptides to inhibit viability in C. albicans biofilms and found the variant had improved antifungal activity. Based on this result, we developed a surface coating containing peptide and showed its potential in preventing growth of C. albicans biofilms.
2. Materials and Methods
2.1. Peptides
The parent Hst-5 peptide and the K11R-K17R peptides (Table 1) were commercially synthesized (Genscript, Piscataway, NJ). The peptides were synthesized with an amidated C-terminus and purified to ≥95%. The trifluoroacetic acid salt was removed by exchange with hydrochloride. Peptides were solubilized in water, prior to diluting in appropriate diluent for use in assays.
Table 1.
Peptide sequences
| Peptide | Sequence |
|---|---|
| Hst-5 | D-S-H-A-K-R-H-H-G-Y-K-R-K-F-H-E-K-H-H-S-H-R-G-Y |
| K11R-K17R | D-S-H-A-K-R-H-H-G-Y-R-R-K-F-H-E-R-H-H-S-H-R-G-Y |
2.2. Strain and routine culture conditions
C. albicans strain ATCC 90028 (American Type Culture Collection, Manassas, VA) was used for all experiments. The strain was maintained on a yeast-peptone-dextrose (YPD; 10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose) agar plate. Prior to use in experiments, a single colony from the agar plate was used to inoculate YPD liquid medium, and the culture was grown overnight at 30 °C while shaking.
2.3. Antifungal activity against planktonic cells
The antifungal activity of the peptides against planktonic C. albicans cells was confirmed using a microplate assay as we have done previously (Ikonomova et al., 2018; Ikonomova et al., 2020). C. albicans cells grown overnight were subcultured into YPD and grown until the OD600 = 1.0 to 1.2. Cells from the subculture were harvested and washed with sodium phosphate buffer (NaPB) before resuspending at 5 × 107 cells/mL in 2 mM NaPB. Twofold serial dilutions of peptide were prepared in water at 1.56–800 μM. The peptide dilutions (20 μL) and cells (20 μL) were added to the wells of 96-well round-bottom plates and mixed to give final peptide concentrations of 0.78–400 μM and final buffer concentration of 1 mM NaPB. Controls containing no peptide were also included. Plates were incubated at 30 °C for 30 minutes, and the interaction of the peptides and cells was slowed by adding 320 μL of 1 mM NaPB per well (Li et al., 2003). To measure the viability of the cells following treatment with the peptides, approximately ~250 cells from each well were transferred to a new plate containing 200 μL of an equal mixture of YPD and NaPB. Wells containing no cells served as a sterility control and provided the background signal for the assay. After incubating the plate overnight at 30 °C on a microplate shaker, the OD600 if each well was measured. The decrease in viability was calculated as follows:
| (1) |
The assay was performed on three different days with two replicates on each day (N=6).
2.4. Antifungal activity against biofilms
To evaluate the ability of the Hst-5 peptides to reduce the viability of C. albicans biofilms, the antifungal effect of peptides on biofilms formed in 96-well plates was quantified using a protocol adapted from previous work (Karlsson et al., 2009; Ramage and Lopez-Ribot, 2005). Cells from an overnight culture were washed twice with phosphate-buffered saline (PBS) and resuspended at a density of 1 × 106 cells/mL in RPMI 1640 medium prepared at half its normal strength (0.5× RPMI 1640). A volume of 100 μL of cell suspension was transferred to each well of a 96-well ELISA plate (Corning, Tewksbury, MA), leaving the last column empty. Plates were covered, sealed with parafilm, and incubated at 37 °C for 24 hours to allow biofilms to form. After biofilms were formed in the wells, they were washed three times with PBS. Twofold serial dilutions of peptides (0.012–6 mM) were prepared in 0.5× RPMI 1640 medium, and 100 μL was added to the first ten columns containing biofilms. The final column containing biofilm received PBS with no peptide. The biofilms were incubated with the peptides for 24 hours at 37 °C.
After incubation with the peptides, the biofilms were washed three times with PBS, and viability of the biofilms was assayed using a colorimetric metabolic assay based on the reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT; Invitrogen, Carlsbad, CA). A solution containing 0.5 g/L XTT and 1 μM menadione (MP Biomedicals, Irvine, CA) was prepared, and 100 μL of the solution was added to each well of the plate, including the peptide-free biofilms wells and the biofilm-free (XTT only) wells. Plates were incubated in the dark at 37 °C for 1.5 hours and centrifuged at 2,000 × g for 5 minutes. A volume of 75 μL of supernatant from each well was transferred to the corresponding well of a new 96-well flat-bottom plate, and the absorbance of each well at 490 nm was measured. The decrease in metabolic activity due to treatment with the peptides was calculated as follows:
| (2) |
The assay was performed on three different days with two replicates on each day (N=6).
2.5. Inhibition of biofilm formation by peptide in solution
The ability of the peptides to inhibit the formation of C. albicans biofilms was evaluated using a protocol adapted from previous work (Karlsson et al., 2009). Cells from an overnight culture were washed twice with phosphate-buffered saline (PBS) and resuspended at a density of 1 × 106 cells/mL in full-strength RPMI 1640 medium. Twofold serial dilutions of peptides were prepared in water at twice the desired assay concentrations. The peptide dilutions and the cells were added to wells of a 96-well ELISA plate in equal volumes (50 μL each) to yield final peptide concentrations of 0.012–6 mM in 0.5× RPMI 1640. One column of the plate contained a control containing cells but no peptide, and one column remained empty for use as the XTT background control. Plates were covered, sealed with parafilm, and incubated at 37 °C for 24 hours to allow biofilms to form. Following incubation, wells were washed three times with PBS, and XTT was used to quantify the metabolic activity in each well as described in Section 2.4. Differential interference contrast (DIC) microscopy images of selected wells were obtained using an Olympus IX83 inverted microscope (Waltham, MA). The assay was performed on three different days with two replicates on each day (N=6).
2.6. Statistical analysis of antifungal activity data
Statistical analysis of the data for the antifungal activity assays in 96-well plates was performed using Prism (GraphPad Software, San Diego, CA). A regression was used to compare the curves for the two peptides in each set of experiments. A log transformation was performed on the concentration values, and a non-linear regression was completed on the transformed data, using the “Dose-response – Stimulation” model (four-parameter dose-response curve) of the following form:
| (3) |
where Y is the decrease in viability or metabolic activity, Ybottom and Ytop are the bottom and top plateaus of the S-shaped curve, X is the log-transformed peptide concentration, EC50 is the concentration of peptide at the half-maximal level of Y, and nH is the Hill Slope that describes curve steepness. A least-squares fitting method was used, and the likelihood of the same set of parameters fitting the data for both peptides was determined using the Akaike information criteria (AIC). Fitted parameter values are provided in Supplementary Table S1, and the fitted models are plotted in Supplementary Figure S1.
In addition to fitting the dose-response curve to the data sets, results were further analyzed using two-tailed t-tests to compare the decreases in viability or metabolic activity at each concentration. The false discovery rate (Q) was set to 0.05. P-values for each concentration are provided in Supplementary Tables S2–S4.
2.7. Polyelectrolyte multilayer film fabrication and characterization
Polyelectrolyte multilayer (PEM) films with and without peptide were fabricated on quartz or silicon using methods adapted from previous work (Breitbach et al., 2011; Etienne et al., 2005; Etienne et al., 2004; Jewell et al., 2007; Karlsson et al., 2010; Tostanoski et al., 2019). Quartz slides (VWR, Radnor, PA) and silicon wafers (Silicon Inc., Boise, ID) were cut into 18 mm × 7 mm substrates for film deposition. The substrates were serially cleaned with acetone, ethanol, methanol, and water before drying under a filtered air stream. After cleaning, substrates were treated with oxygen plasma (Branson 3000 Barrel Resist Stripper) to impart a positive charge on the substrate surface. Polymer base layers were fabricated from polyethyleneimine (PEI; linear MW = 25,000; Polysciences, Inc., Warrington, PA) and poly(sodium 4-styrenesulfonate) (SPS; MW = 70,000; Sigma-Aldrich St. Louis, MO). To deposit the base layers, the following steps were performed using a DR3 dipping robot (Riegler & Kirstein GmbH, Potsdam, Germany): (1) substrates were dipped in a 20 mM SPS solution containing 50 mM NaCl and 5 mM HCl in water for 5 minutes, (2) substrates were washed twice by dipping in water for 1 min per wash, (3) substrates were dipped in a 20 mM PEI solution containing 50 mM NaCl in water for 5 min, and (4) substrates were washed twice in water for 1 min per wash. Steps (1) through (4) were repeated until 10 SPS/PEI bilayers were formed.
PEMs containing peptide or control films without peptide were formed on the base layers using poly-L-glutamic acid (PGA; MW = 50,000 – 100,000; Sigma-Aldrich) and poly-L-lysine (PLL; MW = 15,000 – 30,000; Sigma-Aldrich). The PGA and PLL dipping solutions were prepared at 1 mg/mL polymer in water containing 20 mM NaCl. The peptide solution was prepared at either 1 mg/mL or 3 mg/mL in water containing 20 mM NaCl. The layers were deposited using the following steps: (1) substrates with base layers were dipped in the PGA solution for 5 minutes, (2) substrates were washed twice by dipping in water for 1 min per wash, (3) substrates were dipped in the peptide solution (films containing peptide) or water (control films) for 5 minutes, (4) substrates were washed twice by dipping in water for 1 min per wash, (5) substrates were dipped in the PLL solution for 5 minutes, and (6) substrates were washed twice by dipping in water for 1 min per wash. Steps (1) through (6) were repeated until 60 bilayers (PGA/PLL; control films) or trilayers (PGA/peptide/PLL; peptide films) were deposited.
For experiments to measure the film thickness, PEMs were formed on silicon substrates. The final film thickness was measured at 5 points along the central axis of the film (N=5) using an ellipsometer (Gaertner Scientific, Skokie, Illinois) with an incident angle of 70°. Data were analyzed in Prism using a one-way ANOVA with a post-hoc Tukey’s multiple comparison test (α=0.05).
2.8. Inhibition of biofilm formation by peptides in PEMs
To study the effect of peptide-functionalized PEMs on the formation of C. albicans, PEMs formed on quartz substrates were used. Cells from an overnight culture were subcultured into fresh YPD and grown until the OD600 ≈ 1. Cells were harvested, washed three times with PBS, and resuspended in 0.5× RPMI 1640 at a final concentration of 5 × 105 cells/mL. Substrates with PEMs fabricated using 0 (control), 1, or 3 mg/mL of peptide were placed in individual wells of a Nunc Lab-Tek II coverglass-bottomed, 4-well chamber slide (Thermo Scientific, Waltham, MA). The cell suspension (500 μL) was added to each well containing a substrate and fresh 0.5× RPMI 1640 was added to empty wells as a sterility control. The chamber slides were incubated at 37 °C for 2 h to allow adherence of the cells on the PEM surface. The substrates were then transferred to new chamber slides after gently washing with PBS to removed non-adherent cells. Fresh 0.5× RPMI 1640 was added to each substrate well (0.5 mL/well). The sterility controls were also transferred to new unoccupied wells. The chamber slides were sealed and incubated at 37 °C for 24 h to allow biofilm growth. Inhibition of biofilm formation was assessed via microscopy at 6, 12, and 24 h at several positions on each substrate at each time point. The assay was performed on three different days (N=3).
3. Results and Discussion
In a previous study, we showed that a variant of Hst-5 with two lysine residues substituted with arginine residues (K11R-K17R; Table 1) exhibited marked resistance to degradation by the Sap2 and Sap9 proteases produced by the fungal pathogen C. albicans (Ikonomova et al., 2020). Additionally, the K11R-K17R peptide showed improved antifungal activity compared to the parent Hst-5 peptide (Ikonomova et al., 2020). Biofilms formed by C. albicans present an important biomedical challenge, and Sap enzymes, including Sap2 and Sap9, are reported to have high levels of expression in biofilms and to be upregulated in biofilm growth (Dutton et al., 2016; Joo et al., 2013; Nailis et al., 2010; Ramage et al., 2012; Tobouti et al., 2016). In this work, we evaluated the ability of the protease-resistant K11R-K17R variant of Hst-5 to reduce the viability of biofilms and prevent their formation.
3.1. K11R-K17R peptide has enhanced antifungal activity against biofilms
To evaluate the antifungal activity of the K11R-K17R peptide and the parent Hst-5 peptide, we assayed their ability to reduce the viability of planktonic and biofilm C. albicans. The antifungal activity against planktonic cells was evaluated by incubating a suspension of cells with dilutions of the peptides and measuring the growth of the surviving cells. As we saw in our previous work, the antifungal activity of K11R-K17R was greater than the antifungal activity of Hst-5 (Figure 1A). We compared the activity by fitting a dose-response model (Equation 3) to the data for each peptide and found a >99.99% probability that the curves that fit the two data sets are different. (Values for the fitted parameters are provided in Supplementary Table S1.) Statistically significant differences between the measured decreases in viability for discrete peptide concentrations were identified near the middle and top of the viability curves.
Figure 1.
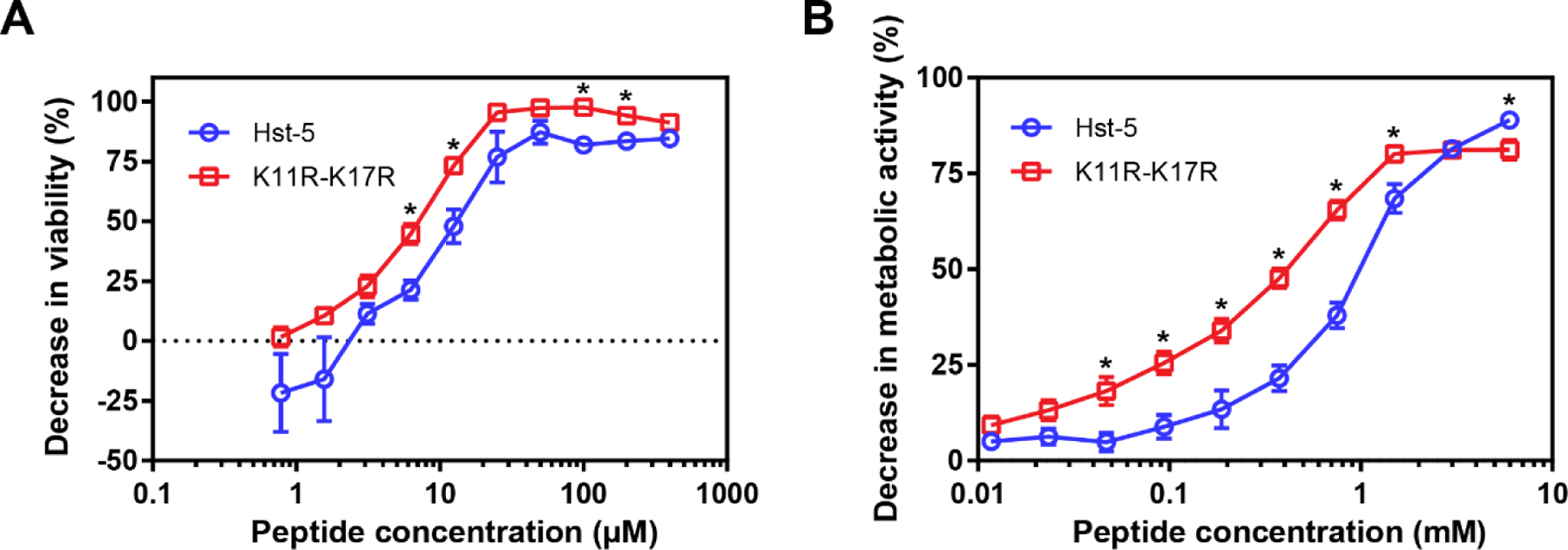
Antifungal activity of Hst-5 and K11R-K17R against C. albicans. (A) Activity against planktonic cells was evaluated by serially diluting peptides and incubating them with 2.5 × 107 cells/mL for 30 min at 30 °C before quantifying the viability of the cells compared to untreated cells. (B) Activity against biofilms was evaluated by forming biofilms for 24 h, adding serially diluted peptides, incubating the peptides with biofilms for 24 h, and then quantifying the metabolic activity of the treated biofilms compared to untreated biofilms. Error bars indicate the standard error of the mean (N = 6). Asterisks indicate concentrations with substantial differences between the peptides (Q = 0.05).
Inhibition of planktonic cells is an important achievement, but biofilms present a more challenging test of antifungal activity. Biofilms contain large numbers of cells surrounded by a matrix of extracellular polymeric substances (EPS). Cells in biofilms exhibit resistance to therapeutics and the EPS can bind to and inhibit activity and transport of antifungal agents (Cavalheiro and Teixeira, 2018; Chandra and Mukherjee, 2015; Lohse et al., 2018; Nett and Andes, 2020; Nobile and Johnson, 2015). To evaluate the performance of the peptides against this more challenging target, we first formed biofilms in the wells of 96-well plates. After forming the biofilms for 24 h, we added serial dilutions of Hst-5 and K11R-K17 and incubated the biofilms with the peptides for 24 h. We quantified the effect of the peptides on the biofilms using XTT, a tetrazolium salt which is converted to a water-soluble formazan dye by metabolically active cells. The model fit to these data revealed >99.99% probability that the biofilm inhibition curves for the peptides are different (see Supplementary Table S1 for parameter values). Both Hst-5 and K11R-K17 were able to substantially reduce the metabolic activity compared to the untreated control, with K11R-K17R showing a statistically significant enhancement antifungal activity against C. albicans compared to Hst-5, at most peptide concentrations. The benefit of the modified peptide was most apparent at concentrations between 0.05–1 mM. For example, at 0.75 mM, the decrease in metabolic activity due treatment with K11R-K17R was 1.7 times that due to Hst-5; the relative benefit was even higher for concentrations below 0.75 mM, with the largest benefit occurring at 0.05 mM, where K11R-K17R resulted in 3.7 times the decrease in metabolic activity compared to Hst-5. Interestingly, the activity of Hst-5 was greater than that of K11R-K17R at the highest concentration tested in this assay. The reason for this is not clear, though differences in aggregation of the peptides at high concentrations could play a role. Although differences between the biofilm and planktonic assays do not allow a direct comparison of the inhibition data for these assays, we note that the concentrations required for inhibition of biofilms were substantially higher than the concentrations leading to inhibition for planktonic cells. Many more cells are present in the biofilms requiring more peptide to be effective, and previous work to evaluate the effect of antimicrobial peptides against biofilms has also found that a higher concentration is required to reduce viability of biofilm cells (Karlsson et al., 2009; Munusamy et al., 2018; Paulone et al., 2017; Roscetto et al., 2018). Although the concentrations required are high, our results show that Hst-5-derived peptides could be used to reduce viability of biofilms that have formed on surfaces and that our modified peptide provides an advantage over the parent peptide.
3.2. K11R-K17R peptide improves inhibition of biofilm formation
The ability to reduce viability of biofilms that have formed on the surface of medical devices is an important therapeutic goal; however, preventing biofilm formation could prevent infections due to device-associated biofilms. As a starting point to explore the use of Hst-5 peptides to reduce biofilm formation, we evaluated the formation of biofilms on the bottom of 96-well plates in the presence of Hst-5 and K11R-K17R. Both Hst-5 and K11R-K17R substantially reduced growth of cells on the surface, as the reduced metabolic activities compared to biofilms formed without peptide illustrate (Figure 2A). As with the planktonic and preformed biofilm assays, K11R-K17R showed a statistically significant improvement in activity compared to the parent Hst-5 peptide, with a >99.99% probability that the curves require different parameters to properly describe the data. At the highest concentration tested (6 mM), K11R-K17R resulted in a 95.8% decrease in metabolic activity compared to the control, while Hst-5 led to an 81.5% decrease. Microscopy images of the bottom surface of the wells highlight the effect of the peptides (Figure 2B). With no peptide present, a biofilm forms on the bottom of the plate, and the cells grow in predominantly hyphal form. The hyphae are long and intertwined. In contrast, wells containing Hst-5 and K11R-K17 had some cells attached to the surface, but without the dense growth indicative of biofilm formation. Additionally, the cells that were grown in the presence of the peptides exhibit substantially reduced hyphal growth compared to the biofilm grown without peptide. With the K11R-K17R peptide, the overall density of cells on the surface is lower than with Hst-5 and minimal filamentous growth is present, with no long hyphae. The microscopy images suggest the peptides, especially K11R-K17R, may inhibit hyphal formation and/or elongation in addition to reducing the viability of the cells. Inhibiting hyphal formation or elongation could play a role in reducing the virulence of cells in an infection, as the ability to form hyphae is a well-known virulence factor of C. albicans and inhibitors of filamentation reduce virulence and biofilm formation (Lu et al., 2014; Romo et al., 2017; Vila et al., 2017). As with treating the preformed biofilms, inhibition of biofilm formation requires concentrations that are quite high compared to concentrations required to inhibit the planktonic cells; however, the results for inhibition of biofilm formation suggest that the presence of a locally high concentration of peptide could prevent the formation of biofilms on surfaces.
Figure 2.
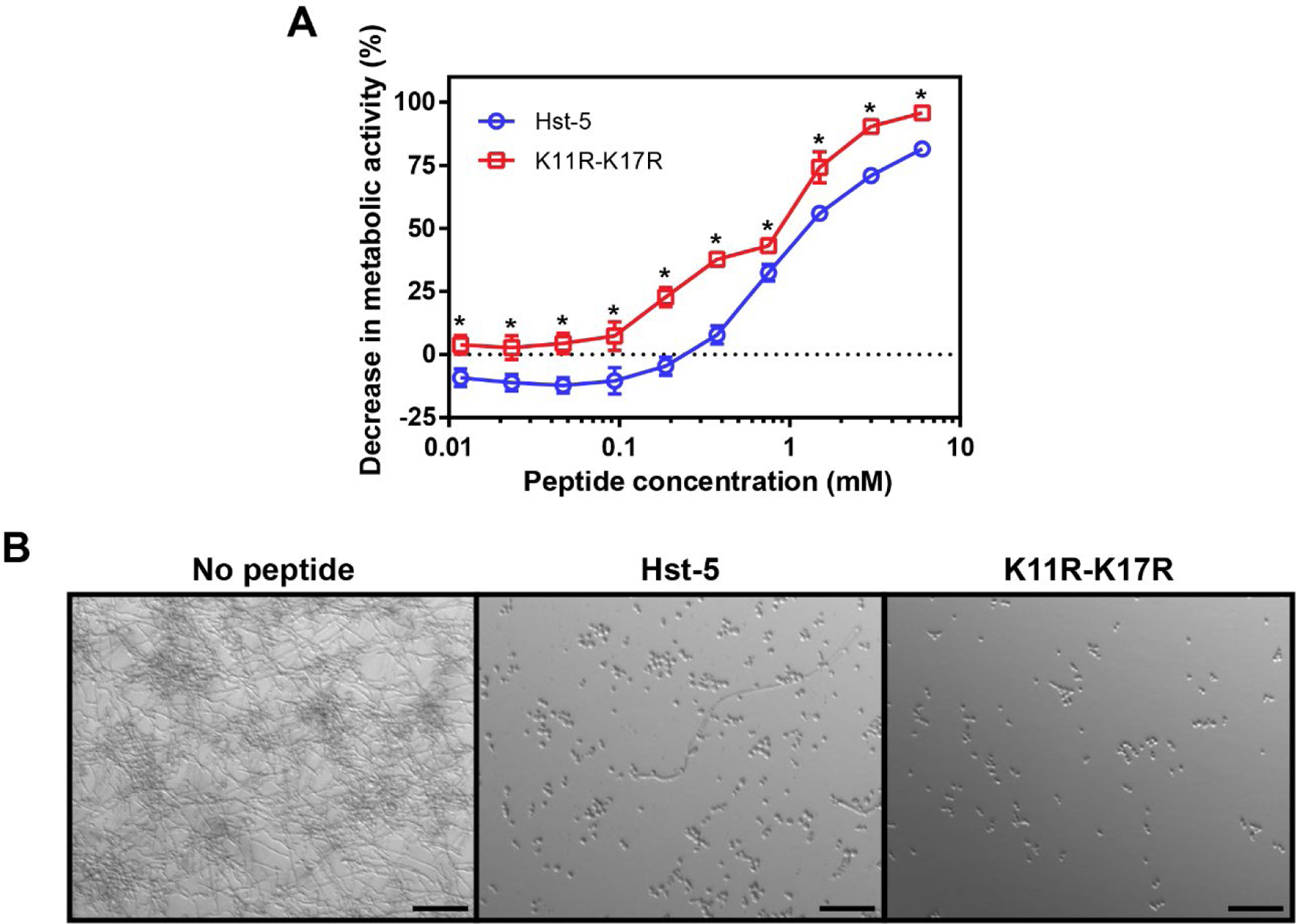
Reduction in biofilm formation by C. albicans due to the presence of Hst-5 and K11R-K17R evaluated by (A) metabolic activity and (B) microscopy. Cells were incubated with serially diluted peptides for 24 h to allow biofilm formation. Following incubation, the metabolic activity of the biofilms formed in the presence of peptide was compared with those formed in the absence of peptide, and DIC microscopy images were obtained. In (A), error bars indicate the standard error of the mean (N = 6), and asterisks indicate concentrations with substantial differences between the peptides (Q = 0.05). In (B), peptide concentration was 6 μM, and scale bars are 50 μm.
3.3. Peptides can be incorporated into polyelectrolyte multilayer coatings
Since C. albicans biofilms can grow on the surfaces of medical devices, one approach to achieving a locally high concentration to inhibit biofilm growth would be to use an antimicrobial coating on the surface. To evaluate the antifungal potential of Hst-5 and K11R-K17R incorporated into surface coating, we constructed PEMs containing the peptides. PEMs are fabricated by the alternating electrostatic deposition of polyanions and polycations in a layer-by-layer manner (Boudou et al., 2010; Seon et al., 2015; Tostanoski and Jewell, 2017) (Figure 3A). The opposite charges of the polycation and polyanion allow the polymers to deposit on one another and build a film that has a thickness dependent on the number of “bilayers” of polyanion and polycation deposited on the surface. Previous work has demonstrated the incorporation of antifungal peptides into these films and the maintenance of peptide antifungal activity against planktonic C. albicans and biofilms (Etienne et al., 2005; Karlsson et al., 2010; Raman et al., 2014; Raman et al., 2016a; Raman et al., 2016b; Seon et al., 2015).
Figure 3.
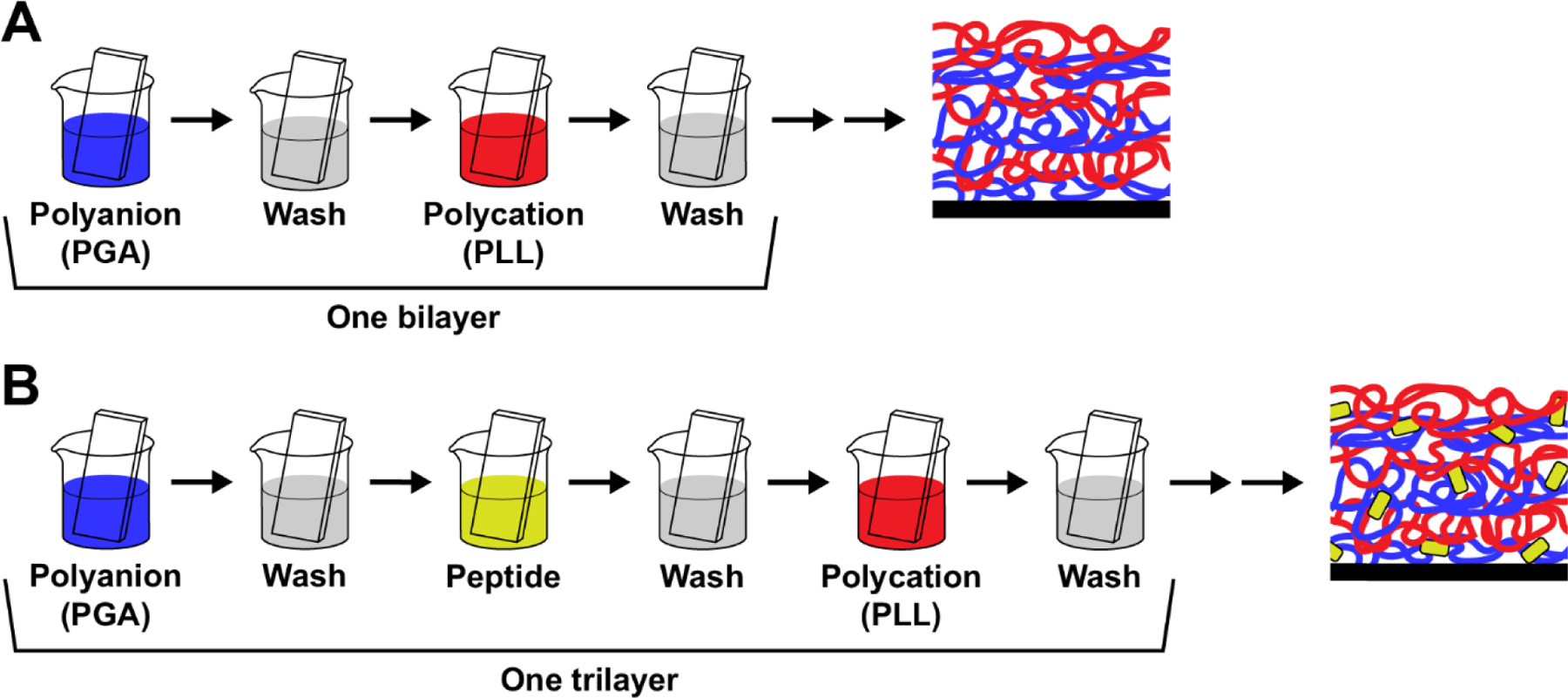
Construction of PEMs. (A) Following deposition of base layers on a surface, substrates are dipped in alternating solutions of a polyanion (poly-L-glutamic acid, PGA) and a polycation (poly-L-lysine, PLL), with washing steps after each polymer. The alternating charges allow a film to build on the surface. One bilayer is made up of the coating from dipping in the polyanion and the polycation. (B) A cationic peptide can be incorporated into the film by dipping the substrate into a peptide solution after the polyanion solution. The polyanion, peptide, and polycation deposition forms one trilayer on the surface.
We used a trilayer assembly process (Figure 3B) to incorporate Hst-5 and K11R-K17R into films formed using these technologies. In particular, the structural components were oppositely charged amino acid-based polymers poly-L-glutamic acid (PGA) and poly-L-lysine (PLL). This combination of polymers has been used previously to incorporate other antimicrobial peptides into PEM coatings (Etienne et al., 2005; Etienne et al., 2004; Karlsson et al., 2010; Raman et al., 2014; Raman et al., 2016a; Raman et al., 2016b). To evaluate the incorporation of Hst-5-based peptides into PEMs, we first deposited films onto silicon substrates to allow measurement of film thickness by ellipsometry. Films fabricated with 0, 1, and 3 mg/mL Hst-5 in the dipping solution all formed films with measurable thickness on the silicon substrate (Figure 4). The films with no peptide in the dipping solution—formed only from PGA and PLL—exhibited a thickness of 142.6 nm, while the films containing peptide were significantly thicker (190.4 nm for the 1 mg/mL Hst-5 solution and 186.5 for the 3 mg/mL Hst-5 solution). This outcome is in agreement with our previous results that confirm incorporation of a peptide increases the thickness of similar PEMs (Karlsson et al., 2010; Raman et al., 2014). The 1 mg/mL and 3 mg/mL Hst-5 solutions had similar thicknesses. The lack of difference between the films may result from the loading being saturated at these concentrations or to a change in film density at the higher concentration that masks higher loading of the peptide. loading saturation at these doses or a change in film density that masks higher loading of the peptide.
Figure 4.
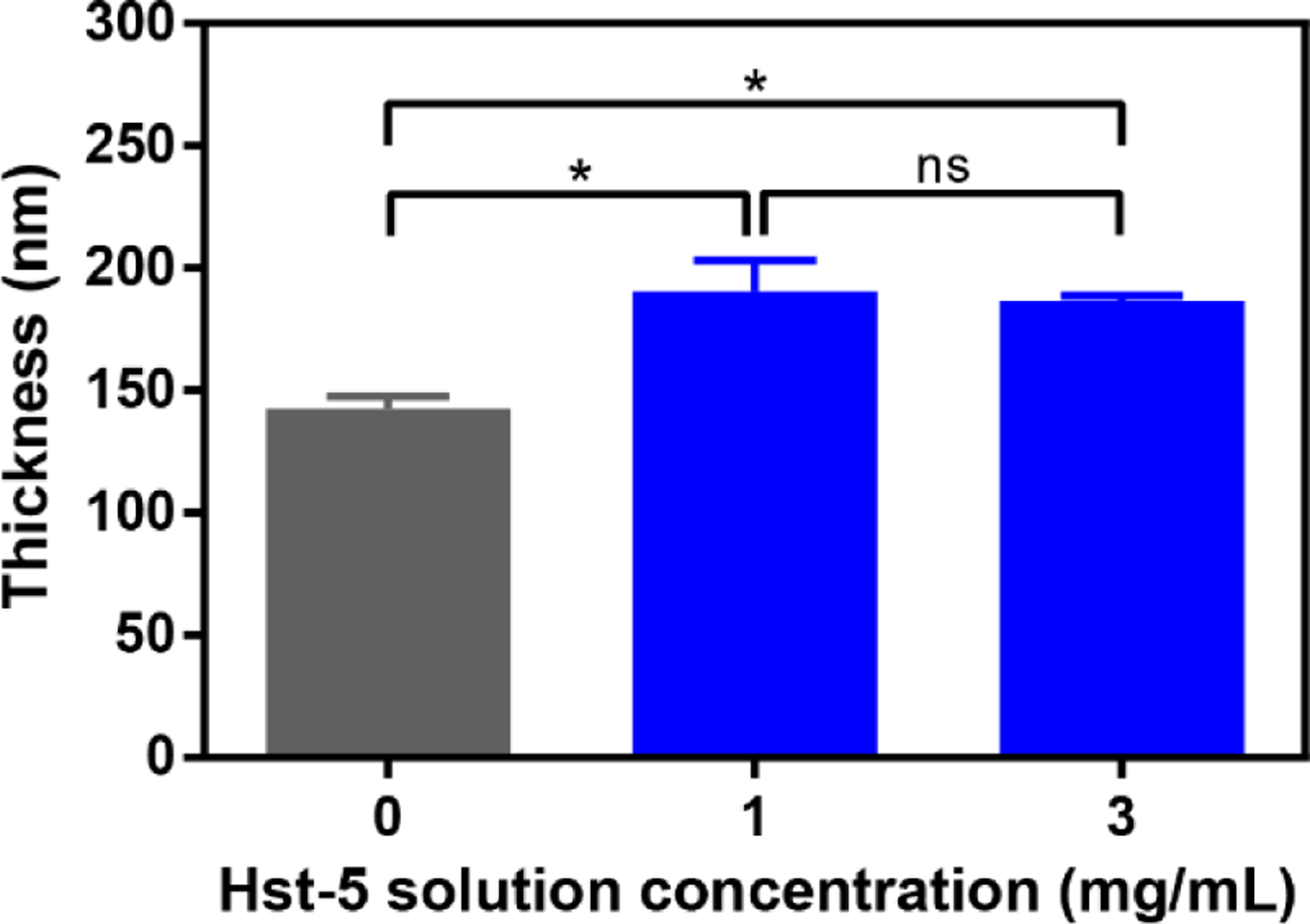
Thickness of PEMs formed on silicon substrate. After depositing base layers on the substrate, films were fabricated by dipping in alternating solutions of PGA, Hst 5, and PLL, as shown in Figure 3B. The concentration of Hst-5 is indicated, with the 0 mg/mL solution containing no peptide. The film thickness on each substrate was measured by ellipsometry at five points per substrate after depositing 60 trilayers (PGA/Hst-5/PLL), and the experiment was performed three times. Error bars indicate standard error of the mean (N=15). An asterisk indicates a statically significant difference between the indicated means (p≤0.05), while “ns” indicates no significant difference.
3.4. Films functionalized with K11R-K17R reduce C. albicans growth on surface
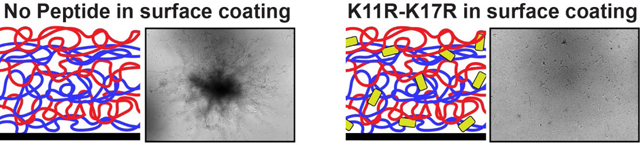
After confirming that Hst-5-based peptides can be incorporated into PEM films on surfaces, we fabricated PEMs containing Hst-5 and K11R-K17R on quartz substrates to facilitate microscopy studies. We fabricated the films in an analogous manner to the films on the (opaque) silicon substrates used for thickness measurements. Following fabrication of the films, we placed the substrates into chamber slides containing medium, added C. albicans cells, and incubated the slides for 2 h to allow adherence of cells to the surface. We transferred the substrates to fresh medium in new chamber slides and then incubated the slides for 24 h, taking images of the substrates after 6, 12, and 24 h of incubation (Figure 5). As the control films show, C. albicans cells readily grow on PEMs formed from PGA and PLL, with growth increasing over time. Interestingly, the presence of Hst-5 in the PEM did not appear to reduce growth of C. albicans on the surface and, in fact, may have enhanced growth. This was a consistent result in each of three replicate experiments. In contrast to Hst-5, PEM films fabricated with K11R-K17R substantially reduced growth of C. albicans on the film surface. The PEM films constructed with the higher concentration of K11R-K17R (3 mg/mL) had minimal cells on the surface at all time points; hyphal formation and/or elongation was also very limited compared to the films constructed without peptide or with Hst-5.
Figure 5.
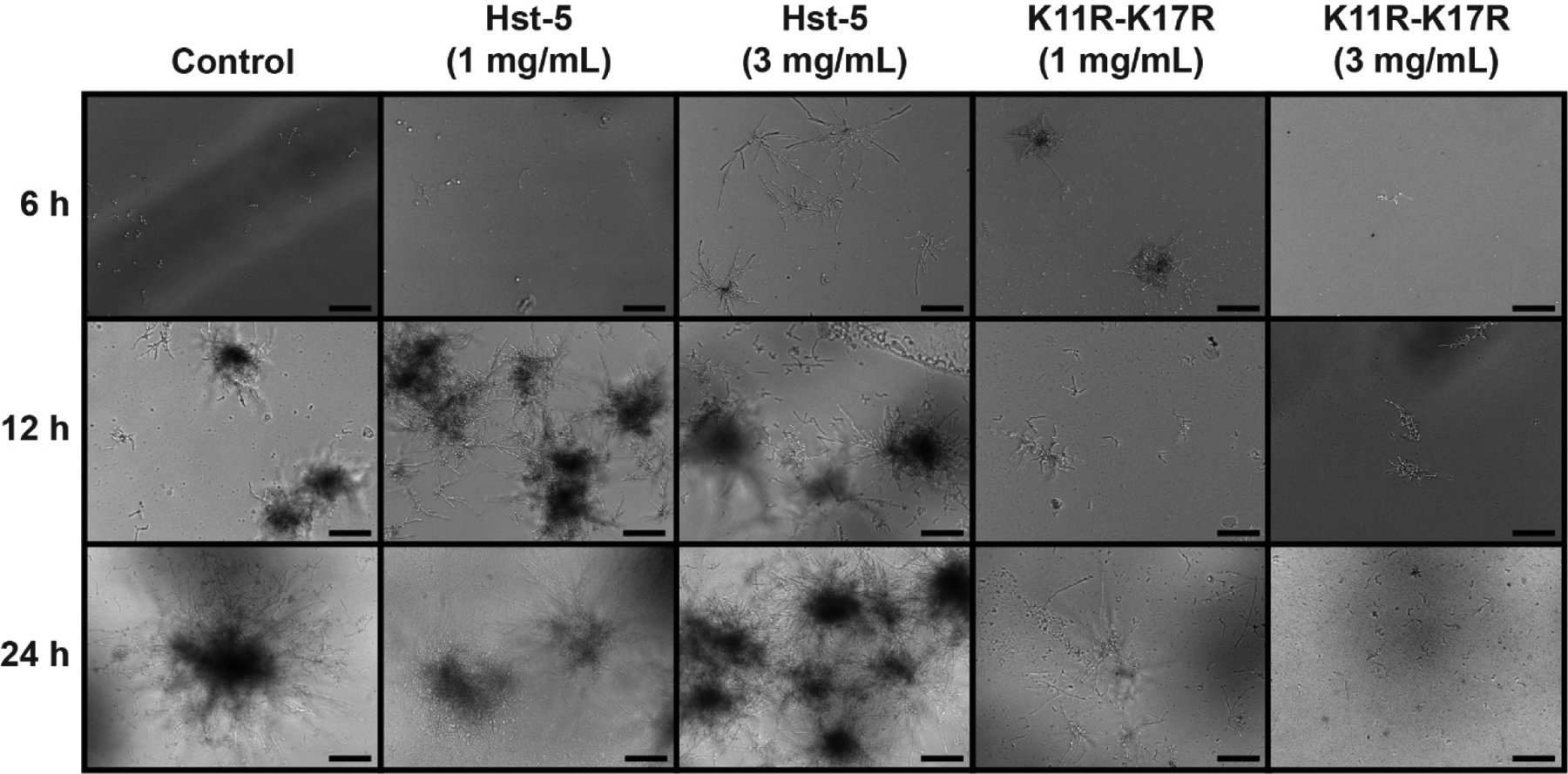
Growth of C. albicans on quartz surfaces coated with PEMs containing Hst-5 and K11R-K17R. Films were fabricated by dipping in alternating solutions of PGA, Hst-5, and PLL, as shown in Figure 3B. The concentration of peptide used in film fabrication is indicated, with the control film utilizing a solution of 0 mg/mL peptide. Films were imaged at 6, 12, and 24 h, and these images are representative of the results from three replicate experiments. The scale bar indicates 100 μm.
The difference in the inhibitory effect of Hst-5 and K11R-K17R was far more pronounced in the biofilm formation assay on PEMs than in our other assays. The lack of inhibitory activity of Hst-5 in the PEMs was surprising, given the peptide in solution has significant antifungal activity, and our data suggest Hst-5 was readily incorporated into the films. Although the reason for the different performance of the peptides in the PEMs is not obvious, one explanation could be related to the difference in susceptibility to proteolytic degradation of the two peptides. K11R-K17R was designed to be more stable in the presence of C. albicans proteases, which could increase the half-life of the peptide in the presence of the cells. This cannot fully explain the difference, though, since Hst-5 retained its antifungal activity in the assays with the peptide in solution. Another possible source of the difference in activity could be that Hst-5 interacts more strongly with the PEM polymers than K11R-K17R. A strong interaction or other solvent hydration factors could lead to distinct sequestration patterns of each peptide in the film, preventing diffusion to the film surface or release into the supernatant. Our observation that Hst-5 may actually enhance the growth of C. albicans on the surface is particularly interesting, though the reasons for the enhanced growth remain unclear.
4. Conclusions
We have demonstrated the ability of Hst-5 and its protease-resistant variant K11R-K17R to reduce the viability of C. albicans biofilms and to reduce biofilm formation. Furthermore, we have shown that the K11R-K17R variant can be incorporated into a PEM coated on a surface to reduce C. albicans growth on the surface. Although additional work is needed to better understand the incorporation of Hst-5 and K11R-K17R into PEM films and optimize the antifungal surfaces, our results suggest that K11R-K17R could be used to fabricate an effective surface coating to prevent biofilm formation. Our results also motivate continued work to improve the resistance of Hst-5 and other peptides to degradation by additional Saps prevalent in biofilms.
Supplementary Material
5. Acknowledgments
The authors thank the University of Maryland NanoCenter FabLab for the use of equipment. This work was supported by the National Science Foundation (CBET 1511718 to AJK), the National Institutes of Health (R03DE029270 to AJK, R01EB027143 to CMJ), and the Maryland VA Health Care System (I01BX003690 to CMJ). HBE is a trainee of the Cell and Molecular Biology Training Program (National Institutes of Health T32GM080201).
Footnotes
Declaration of competing interest
AJK is an inventor on a patent that includes the K11R-K17R peptide. CMJ is an employee of the VA Maryland Health Care System. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. CMJ has an equity position in Cellth Systems, LLC, and Avidea Technologies.
7. References
- Boudou T, et al. , 2010. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Adv Mater. 22, 441–67. [DOI] [PubMed] [Google Scholar]
- Breitbach AS, et al. , 2011. Surface-mediated release of a synthetic small-molecule modulator of bacterial quorum sensing: gradual release enhances activity. Chem Commun (Camb). 47, 370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro M, Teixeira MC, 2018. Candida biofilms: threats, challenges, and promising strategies. Front Med 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States, 2019. Vol. 2020, 2019.
- Chandra J, Mukherjee PK, 2015. Candida biofilms: development, architecture, and resistance. Microbiol Spectr. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton LC, et al. , 2016. Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog Dis. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne O, et al. , 2005. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials. 26, 6704–12. [DOI] [PubMed] [Google Scholar]
- Etienne O, et al. , 2004. Multilayer polyelectrolyte films functionalized by insertion of defensin: a new approach to protection of implants from bacterial colonization. Antimicrob Agents Chemother. 48, 3662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawser SP, Douglas LJ, 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 39, 2128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomova SP, et al. , 2018. Engineering improved variants of the antifungal peptide histatin 5 with reduced susceptibility to Candida albicans secreted aspartic proteases and enhanced antimicrobial potency. FEBS J. 285, 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomova SP, et al. , 2020. Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Sci. 29, 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell CM, et al. , 2007. Multilayered films fabricated from an oligoarginine-conjugated protein promote efficient surface-mediated protein transduction. Biomacromolecules. 8, 857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo MY, et al. , 2013. Expression of SAP5 and SAP9 in Candida albicans biofilms: comparison of bloodstream isolates with isolates from other sources. Med Mycol. 51, 892–6. [DOI] [PubMed] [Google Scholar]
- Karlsson AJ, et al. , 2010. Polyelectrolyte multilayers fabricated from antifungal b-peptides: design of surfaces that exhibit antifungal activity against Candida albicans. Biomacromolecules. 11, 2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson AJ, et al. , 2009. Effect of sequence and structural properties on 14-helical β-peptide activity against Candida albicans planktonic cells and biofilms. ACS Chem Biol. 4, 567–79. [DOI] [PubMed] [Google Scholar]
- Khan SA, et al. , 2013. Impaired histatin-5 levels and salivary antimicrobial activity against C. albicans in HIV infected individuals. J AIDS Clin Res. 4, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XS, et al. , 2003. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. Journal of Biological Chemistry. 278, 28553–28561. [DOI] [PubMed] [Google Scholar]
- Lohse MB, et al. , 2018. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 16, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, et al. , 2014. Candida albicans hyphal initiation and elongation. Trends Microbiol. 22, 707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiller TF, et al. , 2009. A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One. 4, e5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochon AB, Liu H, 2008. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathogens. 4, e1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munusamy K, et al. , 2018. A study on Candida biofilm growth characteristics and its susceptibility to aureobasidin A. Rev Iberoam Micol. 35, 68–72. [DOI] [PubMed] [Google Scholar]
- Nailis H, et al. , 2010. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression. BMC Microbiol. 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Andes DR, 2020. Contributions of the biofilm matrix to Candida pathogenesis. J Fungi (Basel). 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Johnson AD, 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol. 69, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulone S, et al. , 2017. The synthetic killer peptide KP impairs Candida albicans biofilm in vitro. PLoS One. 12, e0181278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, et al. , 2010. Protection of the oral mucosa by salivary histatin-5 against Candida albicans in an ex vivo murine model of oral infection. FEMS Yeast Res. 10, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 20, 133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Edgerton M, 2014. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell. 13, 958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusateri CR, et al. , 2009. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol. 54, 588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, et al. , 2012. In vitro Candida albicans biofilm induced proteinase activity and SAP8 expression correlates with in vivo denture stomatitis severity. Mycopathologia. 174, 11–19. [DOI] [PubMed] [Google Scholar]
- Ramage G, Lopez-Ribot JL, 2005. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 118, 71–9. [DOI] [PubMed] [Google Scholar]
- Raman N, et al. , 2014. Polymer multilayers loaded with antifungal β-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes. J Control Release. 191, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman N, et al. , 2016a. Antifungal activity of a β-peptide in synthetic urine media: Toward materials-based approaches to reducing catheter-associated urinary tract fungal infections. Acta Biomater. 43, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman N, et al. , 2016b. Intraluminal release of an antifungal β-peptide enhances the antifungal and anti-biofilm activities of multilayer-coated catheters in a rat model of venous catheter infection. ACS Biomater Sci Eng. 2, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo JA, et al. , 2017. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of Candida albicans filamentation. mBio. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscetto E, et al. , 2018. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci Rep. 8, 17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seon L, et al. , 2015. Polyelectrolyte multilayers: a versatile tool for preparing antimicrobial coatings. Langmuir. 31, 12856–72. [DOI] [PubMed] [Google Scholar]
- Tobouti PL, et al. , 2016. Expression of secreted aspartyl proteinases in an experimental model of Candida albicans-associated denture stomatitis. J Prosthodont. 25, 127–34. [DOI] [PubMed] [Google Scholar]
- Tostanoski LH, et al. , 2019. Engineering release kinetics with polyelectrolyte multilayers to modulate TLR signaling and promote immune tolerance. Biomater Sci. 7, 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostanoski LH, Jewell CM, 2017. Engineering self-assembled materials to study and direct immune function. Adv Drug Deliv Rev. 114, 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila T, et al. , 2017. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 8, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, et al. , 1991. Anticandidal activity of major human salivary histatins. Infect Immun. 59, 2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


