Abstract
Aims/hypothesis
We aimed to evaluate the relationship between childhood growth measures and risk of developing islet autoimmunity (IA) and type 1 diabetes in children with an affected first-degree relative and increased HLA-conferred risk. We hypothesised that being overweight or obese during childhood is associated with a greater risk of IA and type 1 diabetes.
Methods
Participants in a randomised infant feeding trial (N=2149) were measured at 12 month intervals for weight and length/height and followed for IA (at least one positive out of insulin autoantibodies, islet antigen-2 autoantibody, GAD autoantibody and zinc transporter 8 autoantibody) and development of type 1 diabetes from birth to 10–14 years. In this secondary analysis, Cox proportional hazard regression models were adjusted for birthweight and length z score, sex, HLA risk, maternal type 1 diabetes, mode of delivery and breastfeeding duration, and stratified by residence region (Australia, Canada, Northern Europe, Southern Europe, Central Europe and the USA). Longitudinal exposures were studied both by time-varying Cox proportional hazard regression and by joint modelling. Multiple testing was considered using family-wise error rate at 0.05.
Results
In the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) population, 305 (14.2%) developed IA and 172 (8%) developed type 1 diabetes. The proportions of children overweight (including obese) and obese only were 28% and 9% at 10 years, respectively. Annual growth measures were not associated with IA, but being overweight at 2–10 years of life was associated with a twofold increase in the development of type 1 diabetes (HR 2.39; 95% CI 1.46, 3.92; p<0.001 in time-varying Cox regression), and similarly with joint modelling.
Conclusions/interpretation
In children at genetic risk of type 1 diabetes, being overweight at 2–10 years of age is associated with increased risk of progression from multiple IA to type 1 diabetes and with development of type 1 diabetes, but not with development of IA. Future studies should assess the impact of weight management strategies on these outcomes.
Trial registration:
Keywords: Beta cell autoimmunity, Childhood growth, Genetic risk, Length, Type 1 diabetes, Weight
Graphical Abstract
Introduction
The incidence of type 1 diabetes in children and young adults continues to increase in most of the developed countries (1), although levelling off is seen in some (2). The rise in the disease incidence has been reported to be due to increased rates of disease in younger age groups. A recent report from the US population observed racial differences in incidence rates, with increases occurring at 10–14 years and 0–4 years of age in white and black children, respectively (3). The autoimmune process that results in beta cell destruction and leads to type 1 diabetes is believed to be the result of a combination of genetic predisposition and environmental or lifestyle risk factors. Environmental triggers considered to influence the expression of the disease include viruses, the intestinal microbiome, rapid growth and weight gain, and nutritional factors (4–5).
The causes of childhood obesity are multifactorial, with predisposing genetic factors and environmental components that include excessive energy intake in relation to physical activity (6). Observations of greater birthweight as well as increasing trends for childhood obesity worldwide have led researchers to hypothesise that accelerated childhood growth may contribute to the increasing incidence of type 1 diabetes (7–10). Increased growth could either initiate or accelerate islet autoimmunity (IA). One possible mechanism is that excess weight gain and sedentary lifestyle could cause insulin resistance and hyperinsulinaemia and further increased beta cell metabolism (11). These metabolically active beta cells have been shown to be more vulnerable to autoimmune attack by cytokines than cells at rest (12). A second possibility is that obesity-related insulin resistance with associated increased insulin demand may accelerate an ongoing beta cell destructive process and/or result in earlier or accelerated presentation of type 1 diabetes (13). Maternal diabetes during pregnancy, including type 1 diabetes, has been associated with an increased prevalence of childhood obesity largely as a result of fetal exposure to hyperinsulinaemia. This may be due partly to increased concentrations of glucose stimulating the fetal beta cells and partly as a consequence of transplacental transfer of insulin-insulin antibody complexes from the maternal circulation, increased fat deposition and macrosomia (14). Factors associated with maternal type 1 diabetes, including shorter duration of breastfeeding and higher birth size, are believed to contribute to this observed increase in being overweight and obese in offspring during childhood (15). Despite these risk factors for obesity, maternal type 1 diabetes increases the risk of type 1 diabetes in the offspring less than type 1 diabetes in other first-degree relatives (16).
There is some inconsistency in the literature on the effect of growth measures during the first years of life on the development of IA or type 1 diabetes (17–22). A meta-analysis of 29 primarily European studies reported that children who have a higher birthweight have a small increase in the risk of type 1 diabetes (23). Increased rate of weight gain during childhood has also been associated with development of type 1 diabetes (9, 17, 24) and progression from IA to type 1 diabetes (25–26). However, another study reported that weight, BMI, and weight and BMI velocities were not associated with development of IA or progression from IA to type 1 diabetes (27). A recent prospective study reported that a lower rate of linear growth during infancy and higher rate of linear growth in early childhood (up to 4 years) were associated with increased risk of progression from IA to type 1 diabetes (21). Two other studies reported that an increased rate of linear growth during the first two years of life was associated with developing multiple IA (28) and during childhood (2–11 years) was associated with earlier development of IA (27).
The association of the occurrence of being overweight and obese with the development of IA and type 1 diabetes is unknown. We examined growth measures (weight, length/height and occurrence of being overweight and obese) annually and over time (up to 10 years of age) and the risk of development of IA and type 1 diabetes in the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) population who have at least one first-degree relative with type 1 diabetes and carry HLA-conferred susceptibility. We hypothesised that a higher weight and length/height z score and occurrence of being overweight and obese (annually and over time) during childhood are associated with islet autoantibody positivity and a greater risk of type 1 diabetes. If confirmed in this large international population, weight loss treatment programmes to slow excess weight gain should be considered in the prevention of type 1 diabetes.
Methods
Design and participants
The TRIGR study is an international type 1 diabetes prevention trial designed to determine whether weaning to a hydrolysed infant formula reduces the incidence of type 1 diabetes in children with a first-degree relative with type 1 diabetes and increased HLA-defined genetic risk. The trial was approved by the Ethics Institutional Review Boards and Committees of Human Experimentation in all participating TRIGR centre institutions. Written informed consent was obtained from the family prior to enrolment and assent was obtained for underage participants during the study, if required. A full description of the TRIGR study design has been reported previously (29). In total, 2159 children at increased risk for developing type 1 diabetes from the USA, Canada, Australia and 12 countries in Europe were recruited for the trial between May 2002 and January 2007. Children were monitored until the last child reached 10 years of age for the frequency of type 1 diabetes-associated autoantibodies and/or the development of clinical diabetes. HLA genotyping for selected DQB1 and DQA1 alleles was performed using sequence-specific oligonucleotide hybridisation. Children with the following risk genotypes were included: (1) HLA DQB1*02/DQB1*03:02 (high risk); (2) HLA DQB1*03:02/x (moderate risk; x not DQB1*02, DQB1*03:01 or DQB1*06:02); (3) HLA DQA1*05-DQB1*02/y (y not DQA102:01-DQB1*02, DQB1*03:01, DQB1*06:02 or DQB1*06:03) or HLA DQA1*03-DQB1*02/y (mild risk; y not DQA102:01-DQB1*02, DQB1*03:01, DQB1*06:02 or DQB1*06:03).
Variable measures
As part of the TRIGR study protocol, anthropometric indices (weight in kilograms and length before 2 years of age or height beginning at 2 years of age in centimetres) were obtained at birth and at each study visit (every 3 months until 1 year of age and annually thereafter to age 10–14 years). Participants with anthropometric data are included in this secondary analysis (N=2149). BMI was calculated beginning at 2 years of age. The International Obesity Task Force (IOTF) cut-off points for being overweight (corresponding BMI=25 kg/m2 in adults) and obese (corresponding BMI=30 kg/m2 in adults) by age and sex were used (available from 2 years of age onwards) (30). Because there were no differences in any of these measures between the two intervention groups, their data were combined. Any IA was defined as positivity for one or more of the following biochemical autoantibodies: insulin autoantibodies (IAA), islet antigen-2 autoantibody (IA-2A), GAD autoantibody (GADA) and zinc transporter 8 autoantibody (ZnT8A), on two or more sequential study visits. IAA first antibody is IAA being the first positive antibody out of GADA, IAA, IA-2A and ZnT8A. GADA first antibody is GADA being the first positive antibody out of GADA, IAA, IA-2A and ZnT8A. Multiple IA was defined as positivity for two or more autoantibodies on two or more sequential study visits. Progression from multiple IA to type 1 diabetes was defined as the development of type 1 diabetes after being positive for multiple IA. Growth data up to the date of seroconversion to IA or within 3 months of the date of diagnosis of type 1 diabetes were used in the analyses. IAA, IA-2A, GADA and ZnT8A were quantified with the use of specific radiobinding assays in the Scientific Laboratory, Children’s Hospital, University of Helsinki, Helsinki, Finland (31). Type 1 diabetes was diagnosed according to the World Health Organization criteria (32).
Statistical analysis
An imputation algorithm was used to correct errant entered weight and length/height values. Missing data were ignored in the time-dependent Cox models. Twenty-nine per cent of the participants had one or more missing calculated BMI values from the time of birth to their last visit. However, joint modelling by design inputs missing data based on the longitudinal mixed modelling that was performed. Ten participants had no weight or length/height measures and were not included in the analyses.
Cox proportional hazard models were used to assess the association of fixed measures for weight z score, length/height z score and being overweight and/or obese by age with the development of any IA, IAA first antibody, GADA first antibody, multiple IA, progression from multiple IA to type 1 diabetes and type 1 diabetes. Similarly, Cox proportional hazard models with time-dependent covariates (last value carried forward) for weight z score, length/height z score and being overweight and/or obese were constructed for the development of any IA, IAA first antibody, GADA first antibody, multiple IA, from multiple IA to type 1 diabetes and type 1 diabetes. These models were adjusted for birthweight z score, birth length z score, sex, HLA risk and maternal type 1 diabetes (yes/no), and, to account for possible non-proportional hazards, were stratified by region of residence (Australia, Canada, Northern Europe [Finland, Sweden], Southern Europe [Italy, Spain], Central Europe I [Czech Republic, Estonia, Hungary, Poland], Central Europe II [Germany, Luxembourg, the Netherlands, Switzerland] and the USA) (Table 1).
Table 1.
Characteristics of participating children according to IA and type 1 diabetes development
| Characteristic | IAa (n=305) | No IA (n=1844) | HR (95% CI) | Type 1 diabetes (n=172) | No type 1 diabetes (n=1977) | HR (95% CI) |
|---|---|---|---|---|---|---|
| Age at first autoantibody-positive visit/diagnosis of type 1 diabetes or most recent visit (months)b | 36.4 (17.8–64.3) | 120.0 (119.4–120.0) | 71.5 (35.1–108.6) | 142.3 (127.0–155.2) | ||
| Country regionsc | ||||||
| Northern Europe | 77 (25.3) | 478 (25.9) | 49 (28.5) | 506 (25.6) | ||
| Central Europe I | 42 (13.8) | 237 (12.9) | 20 (11.6) | 259 (13.1) | ||
| Central Europe II | 32 (10.5) | 153 (8.3) | 12 (7.0) | 173 (8.8) | ||
| Southern Europe | 11 (3.6) | 101 (5.5) | 6(3.5) | 106 (5.4) | ||
| USA | 50 (16.4) | 343 (18.6) | 32 (18.6) | 361 (18.3) | ||
| Canada | 83 (27.2) | 441 (23.9) | 45 (26.2) | 479 (24.2) | ||
| Australia | 10 (3.3) | 91 (4.9) | 8 (4.7) | 93 (4.7) | ||
| Maternal type 1 diabetesc | 131 (43.0) | 976 (52.9) | 0.71 (0.55, 0.91) | 69 (40.1) | 1038 (52.5) | 0.67 (0.48, 0.94) |
| HLAc | ||||||
| HLA-DQB1*0302/DQB1*02 | 117 (38.4) | 398 (21.6) | 1 | 73 (42.4) | 442 (22.4) | 1 |
| HLA-DQB1*0302/x | 123 (40.3) | 824 (44.7) | 0.54 (0.42, 0.69) | 63 (36.6) | 884 (44.7) | 0.45 (0.32, 0.63) |
| HLA-DQA1*05-DQB1 *02/y or HLA- DQA1 *03-DQB1*02/y | 65 (21.3) | 622 (33.7) | 0.38 (0.28, 0.52) | 36 (20.9) | 651 (32.9) | 0.36 (0.24, 0.53) |
| Sex (female)c | 136 (44.6) | 879 (47.7) | 0.87 (0.70, 1.10) | 81 (47.1) | 934 (47.2) | 0.93 (0.69, 1.26) |
| Birthweight z scorec | 1.02 (0.30) | 1.07 (0.29) | 0.95 (0.82, 1.09) | 0.98 (0.26) | 1.07 (0.29) | 0.95 (0.78, 1.14) |
| Birth length z scorec | 1.01 (0.58) | 0.98 (0.48) | 1.12 (0.97, 1.30) | 0.93 (0.51) | 0.99 (0.49) | 1.05 (0.86, 1.28) |
| Mode of deliveryc | ||||||
| Vaginal delivery | 174 (57.1) | 1037 (56.2) | 1 | 104 (60.5) | 1107 (56.0) | 1 |
| Caesarean section | 131 (43.0) | 807 (43.8) | 1.20 (0.94, 1.54) | 68 (39.5) | 870 (44.0) | 1.07 (0.77, 1.50) |
| Breastfeeding duration ≥6 monthsc | 190 (62.3) | 1025 (55.6) | 1.10 (0.87, 1.39) | 109 (63.4) | 1106 (55.9) | 1.18 (0.86, 1.63) |
HRs based on models adjusted for birthweight z score, birth length/height z score, sex, HLA risk, maternal type 1 diabetes status, mode of delivery and breastfeeding duration, and stratified by region of residence (Northern Europe: Finland, Sweden; Central Europe I: Czech Republic, Estonia, Hungary, Poland; Central Europe II: Germany, Luxembourg, the Netherlands, Switzerland; Southern Europe: Italy, Spain)
Antibodies include IAA, IA-2A, GADA and ZnT8A
Median (IQR)
n (%)
Joint models (models that fit jointly generalised mixed models for longitudinal data and proportional hazard models for time-to-event response) for each measure (weight z score, length/height z score and being overweight and/or obese based on IOTF cut-off points) were constructed to predict the time to IA and type 1 diabetes. These models, likewise, were stratified by region and adjusted for birthweight z score, birth length z score, sex, HLA risk and maternal type 1 diabetes (yes/no). The joint model analysis could not be completed for obesity (IA, type 1 diabetes) and for being overweight (IA) as the joint model failed to converge.
For participants who were lost to follow-up, all information available up until the date of their last study visit was used in the time-dependent Cox models as well as in the joint modelling. To account for multiple testing, the Bonferroni method was used to control the family-wise error rate at 0.05. As such,p values less than 0.005 were considered statistically significant. All analyses were performed using Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Of the 2149 TRIGR participants, 305 (14%) developed IA and 172 (8%) type 1 diabetes (Table 1). The median (IQR) age of seroconversion to IA was 36.4 (17.8 – 64.3) months and the onset of type 1 diabetes 71.5 (35.1 – 108.6) months. The demographic characteristics of the TRIGR study population are shown in Table 1. Sex and anthropometric measures at birth did not predict the development of IA or type 1 diabetes. Compared with the highest-risk genotype, fewer children with the moderate- and lower-risk genotypes developed IA and type 1 diabetes. The proportions of participants who were overweight and obese by sex at annual visits from 2 to 10 years of age are shown in electronic supplemental material (ESM) Fig. 1. In both female and male children, the proportions of children overweight (including obese) and obese rose steadily from ∼9% and ∼1.5%, respectively, at 2 years to ∼28% and ∼9%, respectively, by age 10 years.
Growth measures and development of IA
The growth measures at any age point (weight z score, length/height z score and occurrence of being overweight and obese) adjusted for birthweight z score, birth length/height z score, sex, HLA risk, maternal type 1 diabetes status, mode of delivery and breastfeeding duration, and stratified by region of residence, were not associated with the development of any IA, IAA first, GADA first or multiple IA in Cox proportional hazard models when multiple testing was considered (ESM Tables 1 and 2). In the time-varying model of growth from 1 year up to 10 years of age, weight z scores, length/height z scores and being overweight and obese were not associated with the development of any IA, first antibody or multiple IA. Similarly, joint modelling analysis for weight z score, length/height z score and occurrence of being overweight indicated that the measures of growth over time were not associated with the development of any IA or multiple IA.
Growth measures and development of type 1 diabetes
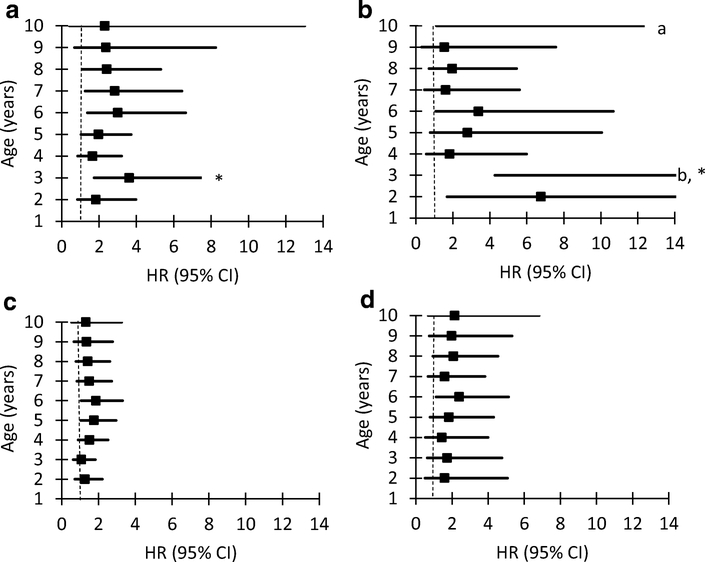
The association of growth measures with the progression from multiple IA to type 1 diabetes and with the development of type 1 diabetes was assessed using fixed measures and longitudinal exposures. In the time-varying model of growth from 1 year up to 10 years of age, weight z score was associated with the progression from multiple IA to type 1 diabetes (HR 1.53; 95% CI 1.16, 2.03; p=0.003) and with the development of type 1 diabetes (HR 1.43; 95% CI 1.20, 1.72; p<0.001; ESM Table 3). In addition, the time-varying Cox regression model showed that the risk of developing type 1 diabetes was two times as high over time in children who were overweight from 2 years up to 10 years of age (HR 2.39; 95% CI 1.46, 3.92; p<0.001) (Table 2). A similar result was observed according to joint modelling (HR 2.16; 95% CI 1.40, 3.21; p=0.004). Cox proportional hazard regression analysis of fixed measures revealed an increase in risk of progressing from multiple IA to type 1 diabetes for weight z score at 3 years of age (HR 1.48; 95% CI 1.13, 1.92; p=0.004) after adjustment for covariates and when multiple testing was considered (ESM Table 3). An increase in the risk of progression from multiple IA to type 1 diabetes was also observed at 3 years of age in children who were overweight (HR 3.61; 95% CI 1.73, 7.54; p<0.001) and in children who were obese (HR 18.88; 95% CI 4.27, 83.45; p<0.001) (Table 2 and Fig. 1a–d).
Table 2.
The risk of type 1 diabetes associated with being overweight (including obese) and obese by age and time-varying model of being overweight (including obese) and obese and up to 10 years of age using IOTF BMI cut-off points (N=2149)
| Variable | From multiple IAa to type 1 diabetes (n=133) | Type 1 diabetes (n=172) | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Overweight (%) | |||||||
| 2 years | 1.83 | 0.84, 3.98 | 0.13 | 1.26 | 0.71, 2.21 | 0.43 | |
| 3 years | 3.61 | 1.73, 7.54 | <0.001 | 1.07 | 0.62, 1.83 | 0.82 | |
| 4 years | 1.65 | 0.85, 3.21 | 0.14 | 1.50 | 0.89, 2.52 | 0.13 | |
| 5 years | 1.96 | 1.03, 3.72 | 0.04 | 1.75 | 1.03, 2.96 | 0.04 | |
| 6 years | 3.01 | 1.37, 6.64 | 0.006 | 1.86 | 1.05, 3.31 | 0.03 | |
| 7 years | 2.84 | 1.25, 6.44 | 0.01 | 1.49 | 0.82, 2.72 | 0.19 | |
| 8 years | 2.41 | 1.09, 5.31 | 0.03 | 1.42 | 0.77, 2.62 | 0.26 | |
| 9 years | 2.36 | 0.68, 8.25 | 0.18 | 1.35 | 0.66, 2.77 | 0.41 | |
| 10 years | 2.30 | 0.41, 13.02 | 0.34 | 1.31 | 0.53, 3.26 | 0.56 | |
| Obesity (%) | |||||||
| 2 years | 6.76 | 1.68, 27.28 | 0.007 | 1.59 | 0.50, 5.08 | 0.44 | |
| 3 years | 18.88 | 4.27, 83.45 | <0.001 | 1.73 | 0.63, 4.77 | 0.29 | |
| 4 years | 1.83 | 0.56, 5.99 | 0.32 | 1.44 | 0.52, 4.00 | 0.48 | |
| 5 years | 2.79 | 0.77, 10.05 | 0.12 | 1.83 | 0.78, 4.31 | 0.17 | |
| 6 years | 3.38 | 1.07, 10.68 | 0.04 | 2.40 | 1.12, 5.14 | 0.02 | |
| 7 years | 1.61 | 0.46, 5.61 | 0.46 | 1.60 | 0.67, 3.83 | 0.29 | |
| 8 years | 1.96 | 0.71, 5.45 | 0.20 | 2.07 | 0.94, 4.55 | 0.07 | |
| 9 years | 1.54 | 0.31, 7.57 | 0.59 | 1.98 | 0.74, 5.33 | 0.18 | |
| 10 years | 70.85 | 1.11, 4529.89 | 0.04 | 2.15 | 0.68, 6.83 | 0.19 | |
| Overweight up to 10 years | 2.17 | 1.12, 4.19 | 0.02 | 2.39 | 1.46, 3.92 | <0.001 | |
| Obesity up to 10 years | 0.98 | 0.21, 4.61 | 0.98 | 2.08 | 0.93, 4.64 | 0.07 | |
| Overweight (joint modelling) | 2.16 | 1.40, 3.21 | 0.004 | ||||
| Obesity (joint modelling) | |||||||
HRs adjusted for birthweight z score and birth length z score, sex, HLA risk, maternal type 1 diabetes status, mode of delivery and breastfeeding duration, and stratified by region of residence. Presented p values are nominal (uncorrected) p values, and were considered significant if <0.005. Shaded analyses: model failed to converge
Antibodies include IAA, IA-2A, GADA and ZnT8A; the number of children with multiple IA=190
Fig. 1.
Risk of type 1 diabetes associated with being overweight (including children who are obese) and obese by age (2 to 10 years). Progression from multiple islet autoimmunity to type 1 diabetes associated with being overweight (a) and obese (b). Development of type 1 diabetes associated with being overweight (c) and obese (d). *p<0.001; aactual values (HR 18.88, 95% CI 4.27, 83.45); bactual values (HR 70.85, 95% CI 1.11, 4529.89)
Discussion
In this international, large birth cohort of 2149 children with increased genetic risk of type 1 diabetes, we observed a strong positive association between being overweight over 2 to 10 years of age and development of type 1 diabetes. This association remained significant after controlling for the confounders and multiple testing, and the finding was the same using time-varying Cox proportional hazard regression and joint model analysis. Weight z score from 1 year up to 10 years of age was associated with greater risk of progression from multiple IA to type 1 diabetes. A similar association was found with weight z score and being overweight and obese at the 3 year age point. A possible explanation for this observation is that children who are heavier and taller require more insulin. If insulin secretion does not parallel an increase in growth, then insulin insufficiency results in the development of type 1 diabetes. Weight or height z scores or being overweight or obese were not significantly associated with development of IA or multiple IA. Neither did anthropometric measures at birth predict the development of IA or type 1 diabetes.
The strengths of the study include a large cohort with a carefully constructed study protocol and the use of strictly standardised and validated prospective data collection and measurement methods. Participation in the study remained excellent during the long follow-up. Compared with other prospective studies that examined associations between childhood growth measures and development of IA and type 1 diabetes, this study has a long follow-up and a relatively high number of children who developed any IA, multiple IA or type 1 diabetes. Frequent measurements of growth both before and after seroconversion enabled us to explore the associations of the growth measures with both initiation and acceleration of the IA development.
In the TRIGR population, the proportions of children overweight (including obesity) and obese increased by age, reaching 28% and 9%, respectively, at 10 years in both sexes (from 2012 to 2017). By comparison, in the general population a systematic analysis of being overweight and obese in children from developed countries by the Global Burden of Disease 2013 Obesity Collaboration revealed a prevalence of being overweight or obese of 24% (95% CI 23%, 25%) for boys and 23% (95% CI 22%, 24%) for girls aged 2–19 years using the IOTF cut-off points, which is lower than the prevalence of being overweight (including obese) for the TRIGR population at 10 years of age (33). The proportion of 11-year-old European children who were overweight or obese in the Health Behavior in School-Aged Children Study: International Report from the 2013–2014 Survey was 17% in girls and 27% in boys using IOTF BMI cut-off points, which is also lower than the TRIGR study population from the same time period (34).
The Diabetes Autoimmunity Study in the Young (DAISY) includes a US population of children at increased genetic risk for type 1 diabetes (24). At 8 years of age, DAISY children were observed to have a mean±SD BMI of 16.83±2.38 kg/m2. This is approximately the 50% percentile on the Centers for Disease Control and Prevention (CDC) Clinical Growth Charts for boys and girls (35). We previously reported differences in early childhood growth between TRIGR regions (36). The higher early childhood weight and length measures were consistent with the higher incidence of type 1 diabetes in Northern European TRIGR countries and Canada compared with southern countries (37), which supported a relationship but did not determine causality.
Our results are inconsistent with some previous studies that showed direct association between linear growth parameters and the development of IA in children at increased genetic risk of type 1 diabetes. Greater linear growth velocity was associated with IA and type 1 diabetes in the at-risk Colorado DAISY study population (N=1714) (24) and with an increased risk of progression from IA to type 1 diabetes in The Environmental Determinants of Diabetes in the Young (TEDDY) study (N=7522) (21), while no association between length/height and IA or type 1 diabetes risk or progression from IA to type 1 diabetes was observed in TRIGR. The difference in results may be due to differences between the study populations or in measurement techniques in the varying studies. The DAISY population includes three groups of children at risk for developing type 1 diabetes (unaffected first-degree relatives of patients with type 1 diabetes, infants screened for HLA susceptibility at birth and siblings of the newborn-screened group). The TEDDY study population includes a cohort covering four countries of genetically high-risk children, the majority selected from the general population, whereas participants in the TRIGR study were recruited from a high-risk population with affected first-degree relatives in 15 countries and additionally were screened for HLA-conferred risk genes. In both TEDDY and TRIGR studies, hazard models were adjusted for similar variables (birth measures, sex, HLA risk, family history of type 1 diabetes [TEDDY] or maternal type 1 diabetes [TRIGR], duration of exclusive breastfeeding and country of residence).
Moreover, while we observed no association between birth measures and the development of type 1 diabetes, a primarily European study meta-analysis revealed an increase in type 1 diabetes risk of 3% per 500 g increase in birthweight after adjustment for gestational age, maternal age, birth order, Caesarean section, breastfeeding and maternal diabetes (23). In addition, children in the Diabetes Prediction in Skane study who developed type 1 diabetes were taller at birth compared with age- and sex-matched control participants born to mothers without type 1 diabetes (38). A systematic review and meta-analysis suggests that childhood obesity increases the risk of type 1 diabetes (17). Weight gain rate in early childhood was directly associated with progression from IA to type 1 diabetes in the TEDDY study but only in children with GADA appearing first (21). In the time-varying model of weight z score from 1 year up to 10 years of age, we observed an association with progression from multiple IA to type 1 diabetes but did not examine this based on appearance of first IA.
Our observation that being overweight is associated with progression from multiple IA to type 1 diabetes and with the development of type 1 diabetes is consistent with the observation of higher weight and body composition measures in a large population (N=9248) of young German and Austrian children (<5 years of age) diagnosed with type 1 diabetes (39). In a subgroup (n=1117) of participants of the TrialNet Pathway to Prevention (PTP) study, which includes first-, second- or third-degree relatives (2 to 18 years of age) of people with type 1 diabetes, consistent elevation of BMI >85th percentile was found to be associated with increased risk of progression from IA to type 1 diabetes (26). This increased risk occurred at a lower level of sustained elevated BMI in children <12 years of age, particularly in female children. In the TrialNet PTP subgroup of participants (n=706) who were single autoantibody positive, persistently elevated BMI was found to increase the risk of developing multiple IA in children >9 years of age who did not carry high-risk HLA haplotypes but not in children <9 years of age regardless of HLA type (27). The TrialNet PTP results are somewhat consistent with our observations. However, TrialNet examined the association between persistent excess weight in participants and risk of IA or progression to type 1 diabetes, whereas we examined being overweight and/or obese.
The lack of information on parental weight and height is a limitation of this study. Neither did we have information on puberty timing of the participating children. As the participants had a very high genetic risk of type 1 diabetes due to HLA-conferred risk genes and at least one of the first-degree relatives having type 1 diabetes, these findings are not necessarily generalisable to populations with lower genetic susceptibility. We did not assess insulin resistance by the homeostatic model of assessment. Therefore, we cannot confirm that the progression from multiple IA to type 1 diabetes was accelerated by insulin resistance. We used BMI as a measure of adiposity in our population. The assessment of excess adiposity may be strengthened in future studies by the use of objective measures of fat mass such as blood leptin level and adiponectin concentration (40).
Increased weight and consistent overweight status in young children increased the risk of developing type 1 diabetes in a large, international population of children at high genetic risk. This is the first study to examine the relationship between weight status, expressed as being overweight and obese, and the development of IA, multiple IA and type 1 diabetes in addition to the progression from multiple IA to type 1 diabetes. The World Health Organization has reported that childhood obesity is a serious global public health challenge that affects countries worldwide (41). In comparison with children at a healthy weight, children who are overweight or obese are at higher risk for pulmonary, endocrine (insulin resistance) and cardiovascular conditions during childhood that may have long-lasting effects (42). Treatment programmes to slow the progress of weight gain or encourage gradual weight loss in children may be implemented based on BMI status (overweight or obese) (43). In addition to setting targets for stopping the increase in obesity, expert committees have developed action steps and recommendations for healthcare professionals to aid with the prevention and treatment of childhood obesity (43, 44). The results of this study support prior research that has shown an association between growth and the development of type 1 diabetes in children and expand the literature by reporting associations using standardised definitions of being overweight and obese that guide clinical practice. Future studies should assess the impact of weight management strategies on these outcomes.
Supplementary Material
Research in context.
What is already known about this subject?
Increased rate of weight gain during childhood has been associated with development of type 1 diabetes and progression from islet autoimmunity to type 1 diabetes
A higher rate of linear growth in early childhood is reported to be related to an increased risk of progression from islet autoimmunity to type 1 diabetes
What is the key question?
In children at especially high genetic risk of type 1 diabetes (a positive family history and increased HLA-conferred risk), are growth measures and occurrence of being overweight and obese during childhood associated with a greater risk of islet autoimmunity and type 1 diabetes?
What are the new findings?
In this large international cohort of children with genetic risk, we observed that being overweight at 2–10 years of age was associated with increased risk of developing type 1 diabetes
How might this impact on clinical practice in the foreseeable future?
Understanding of the association between body composition and type 1 diabetes may encourage preventive weight management strategies for children at risk of type 1 diabetes
Acknowledgments
Funding This work was supported by grant numbers HD040364, HD042444 and HD051997 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Special Statutory Funding Program for Type 1 Diabetes Research administered by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), the Canadian Institutes of Health Research, JDRF International, the Academy of Finland, the Commission of the European Communities (specific RTD programme ‘Quality of Life and Management of Living Resources’, contract number QLK1-2002-00372 ‘Diabetes Prevention’; this work does not reflect the Commission’s views and in no way anticipates the Commission’s future policy in this area) and the EFSD/JDRF/Novo Nordisk Focused Research Grant. The study formulas were provided free of charge by Mead Johnson Nutrition.
Abbreviations:
- DAISY
Diabetes Autoimmunity Study in the Young
- GADA
GAD autoantibody
- IA
Islet autoimmunity
- IAA
Insulin autoantibody
- IA-2A
Islet antigen-2 autoantibody
- IOTF
International Obesity Task Force
- PTP
Pathway to Prevention
- TEDDY
The Environmental Determinants of Diabetes in the Young
- TRIGR
Trial to Reduce IDDM in the Genetically at Risk
- ZnT8A
Zinc transporter 8 autoantibody
Footnotes
Authors’ relationships and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
The investigators and members of The TRIGR Investigators are listed in the electronic supplementary material.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability
The dataset used for these analyses can be requested by emailing TRIGR@epi.usf.edu.
References
- 1.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, EURODIAB Study Group. (2009) Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: A multicentre prospective registration study. Lancet 373:2027–2033. 10.1016/S0140-6736(09)60568-7 [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sund R, Knip M, Groop PH. (2013) Incidence of type 1 diabetes in Finland. JAMA 310:427–428. 10.1001/jama.2013.8399 [DOI] [PubMed] [Google Scholar]
- 3.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. (2013) Increasing incidence of type 1 diabetes in youth. Diabetes Care 36:1597–1603. 10.2337/dc12-0767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virtanen SM. (2016) Dietary factors in the development of type 1 diabetes. Pediatr Diabetes 17(Suppl 22):49–55. 10.1111/pedi.12341 [DOI] [PubMed] [Google Scholar]
- 5.Rewers M, Ludvigsson J. (2016) Environmental risk factors for type 1 diabetes. Lancet 387(10035):2340–2348. 10.1016%2FS0140-6736(16)30507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine (2012) Accelerating progress in obesity prevention: solving the weight of the nation. The National Academies Press, Washington, DC [Google Scholar]
- 7.Knip M, Reunanen A, Virtanen SM, Nuutinen M, Viikari J, Akerblom HK. (2008) Does the secular increase in body mass in children contribute to the increasing incidence of type 1 diabetes? Pediatr Diabetes 9:46–49. 10.1111/j.1399-5448.2007.00344.x [DOI] [PubMed] [Google Scholar]
- 8.Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK. (2000) The Childhood Diabetes in Finland Study Group. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 23:1755–1760. 10.2337/diacare.23.12.1755 [DOI] [PubMed] [Google Scholar]
- 9.Johansson C, Samuelsson U, Ludvigsson J. (1994) A high weight gain early in life is associated with an increased risk of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 37:91–94. 10.1007/BF00428783 [DOI] [PubMed] [Google Scholar]
- 10.Wilkin TJ. (2001) The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia 44:914–922. 10.1007/s001250100548 [DOI] [PubMed] [Google Scholar]
- 11.Dahlquist G (2006) Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 49:20–24. 10.1007/s00125-005-0076-4 [DOI] [PubMed] [Google Scholar]
- 12.Palmer JP, Helquist S, Spinas GA, et al. (1989) Interaction of beta-cell activity and IL-1 concentration and exposure time in isolated rat islets of Langerhans. Diabetes 38:1211–1216. 10.2337/diab.38.10.1211 [DOI] [PubMed] [Google Scholar]
- 13.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. (2003) Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care 26:2871–2875. 10.2337/diacare.26.10.2871 [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D (2007) The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 30(Suppl 2):S169–S174. 10.2337/dc07-s211 [DOI] [PubMed] [Google Scholar]
- 15.Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG. (2009) Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care 32:921–925. 10.2337/dc08-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale EA, Gillespie KM. Diabetes and gender. (2001) Diabetologia 44:3–15. 10.1007/s001250051573 [DOI] [PubMed] [Google Scholar]
- 17.Verbeeten KC, Elks CE, Daneman D, Ong KK. (2011) Association between childhood obesity and subsequent type 1 diabetes: a systematic review and meta-analysis. Diabet Med 28:10–18. 10.1111/j.1464-5491.2010.03160.x [DOI] [PubMed] [Google Scholar]
- 18.Couper JJ, Beresford S, Hirte C, et al. (2009) Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care 32:94–99. 10.2337/dc08-0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyerlein A, Thiering E, Pfluegger M, et al. (2014) Early infant growth is associated with the risk of islet autoimmunity in genetically susceptible children. Pediatric Diabetes 15:534–542. 10.1111/pedi.12118 [DOI] [PubMed] [Google Scholar]
- 20.Larsson HE, Vehik K, Haller MJ, et al. (2016) Growth and risk for islet autoimmunity and progression to type 1 diabetes in early childhood: the environmental determinants of diabetes in the young study. Diabetes. 65:1988–1995. 10.2337/db15-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Vehik K, Huang Y, et al. (2020) Distinct growth phases in early life associated with the risk of type 1 diabetes: The TEDDY Study. Diabetes Care 43:556–562. 10.2337/dc19-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnus MC, Olsen SF, Granström C, et al. (2015) Infant growth and risk of childhood-onset type 1 diabetes in children from 2 Scandinavian birth cohorts. JAMA Pediatr 169: e153759. doi: 10.1001/jamapediatrics [DOI] [PubMed] [Google Scholar]
- 23.Caldwell CR, Stene LC, Joner G, et al. (2010) Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia 53:641–651. 10.1007/s00125-009-1648-5 [DOI] [PubMed] [Google Scholar]
- 24.Antvorskov JC, Aunsholt L, Buschard K, et al. (2018) Childhood body mass index in relation to subsequent risk of type 1 diabetes-A Danish cohort study. Pediatr Diabetes 19:265–270. 10.1111/pedi.12568 [DOI] [PubMed] [Google Scholar]
- 25.Ferrara CT, Geyer SM, Evans-Molina C, et al. (2017) Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development. Diabetes Care 40:698–701. 10.2337/dc16-2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara-Cook C, Geyer SM, Evans-Molina, et al. (2020) Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care 43:580–587. 10.2337/dc19-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb MM, Yin X, Zerbe GO, et al. (2009) Height growth velocity, islet autoimmunity and type 1 diabetes development: the Diabetes Autoimmunity Study in the Young. Diabetologia 52:2064–2071. 10.1007/s00125-009-1428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacaud D, Nucci AM, Cuthbertson D, et al. (2020) Association between family history, early growth and the risk of beta cell autoimmunity in children at risk for type 1 diabetes. Diabetologia 10.1007/s00125-020-05287-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.TRIGR Study Group. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). (2007) Pediatr Diabetes 8:117–137. 10.1007/s00125-010-1964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243. 10.1136/bmj.320.7244.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knip M, Virtanen SM, Seppa K, et al. (2010) Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med 363:1900–1908. 10.1056/NEJMoa1004809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberti KG, Zimmet PZ. (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 15:539–553. [DOI] [PubMed] [Google Scholar]
- 33.The Global Burden of Disease 2013 Obesity Collaboration. (2014) Global, regional and national prevalence of overweight and obesity in children and adults 1980–2013: a systematic analysis. Lancet 384:766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inchley J, Currie D, Young T, et al. (eds) (2016) Growing up unequal: gender and socioeconomic differences in young people’s health and well-being. Health Behavior in School-Aged Children (HBSC) Study: International Report from the 2013/2014 Survey. World Health Organization, Copenhagen [Google Scholar]
- 35.Centers for Disease Control and Prevention. Clinical Growth Charts. Available from https://www.cdc.gov/growthcharts/clinicalcharts.htm#Set1. Last reviewed 16 June 2017. Accessed 11 January 2020
- 36.Nucci AM, Becker DJ, Virtanen SM, et al. (2012) Growth differences between North American and European children at risk for type 1 diabetes. Pediatr Diabetes 13:425–431. 10.1111/j.1399-5448.2011.00840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DIAMOND Project Group. (2006) Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 23:857–866. 10.1111/j.1464-5491.2006.01925.x [DOI] [PubMed] [Google Scholar]
- 38.Larsson HE, Hansson G, Carlsson A, et al. (2008) Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia 51:1623–1630. 10.1007/s00125-008-1074-0 [DOI] [PubMed] [Google Scholar]
- 39.Knerr I, Wolf J, Reinehr T, et al. (2005) The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia 48:2501–2504. 10.1007%2Fs00125-005-0033-2 [DOI] [PubMed] [Google Scholar]
- 40.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. (2009) Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 123:682–689. 10.1542%2Fpeds.2008-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization (2018) Taking action on childhood obesity. Available from https://apps.who.int/iris/bitstream/handle/10665/274792/WHO-NMH-PND-ECHO-18.1-eng.pdf?ua=1. Accessed 8 October 2020
- 42.Lobstein T, Baur L, Uauy R for the IASO International Obesity Task Force. (2004) Obesity in children and young people: a crisis in public health. Obesity Reviews 5(Suppl 1):4–85 10.1111/j.1467-789X.2004.00133.x [DOI] [PubMed] [Google Scholar]
- 43.Barlow SE and the Expert Committee. (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4):S164–S192. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization (2017) Report of the Commission on Ending Childhood Obesity implementation plan: executive summary. Available from https://apps.who.int/iris/bitstream/handle/10665/259349/WHO-NMH-PND-ECHO-17.1-eng.pdf?sequence=1. Accessed 8 October 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used for these analyses can be requested by emailing TRIGR@epi.usf.edu.