Abstract
The lack of physical exercise during the COVID-19 pandemic-related quarantine measures is challenging, especially for patients with Parkinson’s disease (PD). Without regular exercise not only patients, but also nursing staff and physicians soon noticed a deterioration of motor and non-motor symptoms. Reduced functional mobility, increased falls, increased frailty, and decreased quality of life were identified as consequences of increased sedentary behavior. This work overviews the current literature on problems of supplying conventional physiotherapy and the potential of telerehabilitation, allied health services, and patient-initiated exercise for PD patients during the COVID-19 period. We discuss recent studies on approaches that can improve remote provision of exercise to patients, including telerehabilitation, motivational tools, apps, exergaming, and virtual reality (VR) exercise. Additionally, we provide a case report about a 69-year-old PD patient who took part in a 12-week guided climbing course for PD patients prior to the pandemic and found a solution to continue her climbing training independently with an outdoor rope ladder. This case can serve as a best practice example for non-instructed, creative, and patient-initiated exercise in the domestic environment in difficult times, as are the current. Overall, many recent studies on telemedicine, telerehabilitation, and patient-initiated exercises have been published, giving rise to optimism that facilitating remote exercise can help PD patients maintain physical mobility and emotional well-being, even in phases such as the COVID-19 pandemic. The pandemic itself may even boost the need to establish comprehensive and easy-to-do telerehabilitation programs.
Subject terms: Parkinson's disease, Rehabilitation, Quality of life
Introduction
The global COVID-19 pandemic, caused by the coronavirus SARS-CoV-2, poses a high risk on old and chronically ill patients, including patients with Parkinson’s disease (PD)1–3. Next to devastating direct consequences caused by the acute illness, COVID-19 also poses risks of long-term effects on PD patients4,5. The worldwide pandemic forced countries all over the globe to implement severe quarantine measures. Not only social and public life is reduced to a minimum, but healthcare services have also been cut down considerably. Physiotherapy was among the canceled services, with grave consequences for chronically ill patients6–10. Since physical therapy is the basic pillar of their overall therapy, its interruption severely affected PD patients. When the COVID-19 pandemic forced public and private fitness providers alike to close their services abruptly, unable to provide information on reopening dates etc, patients and caregivers were left with the frustrating reality of losing a highly relevant therapeutic strategy11–13. This narrative review aims at presenting and discussing the effects of the forced absence of physical therapy in PD and their specific countermeasures. We summarize what is known about exercise-based treatment in PD, and how discontinuity brought about by the COVID-19-lockdown affects PD patients. We then describe in more detail the status of international recommendations on how to continue physiotherapy during COVID-19 and the current state of telerehabilitation, virtual reality (VR), tools for keeping motivation for physical activity, and exergaming, for patients with PD. At last, we present one patient and her unconventional individual solution to the lack of public climbing classes to add empirical evidence for the relevance of patient-driven engagement to keep exercising.
The importance of rehabilitation in PD
Exercise and physical therapy are known to improve motor and non-motor symptoms of PD, which is highlighted by several meta-analyses underline the importance and positive effects of physiotherapy on motor symptoms. Significant improvements of gait, functional mobility, balance, motor symptoms, increased muscle strength, and reduced falls in PD patients who received physical therapy in short- and long-term follow-ups ranging from 1 to 12 months have been shown14–17. Another meta-analysis, covering 20 randomized controlled trials, showed significant improvement of non-motor symptoms in PD including neurocognitive manifestations, mood disorders, sleep disorders, and fatigue by physical therapy18. Trials investigating alternative sports, such as Tai Chi and dancing, revealed similar benefits on motor- and non-motor symptoms of PD19–22.
Next to physiological, structural, and clinical changes through exercise, physical therapy might even hold the potential to change the course of disease by activating neuroprotective mechanisms23–25. Based on animal studies, both neuroprotective and disease-modifying effects seem to be induced by exercise. For example, brain neurotrophic factors can induce neuronal protection and repair mechanisms in the dopaminergic system, which, in turn, increases angiogenesis and functional compensation mechanisms via glutamatergic and serotonergic circuits26–28. In PD patients, it has been shown that an exercise-induced increase of neurotrophic factors is associated with increased gray matter volume and symptomatic changes16,27,29.
Different types of physical exercise show different effects on motor and non-motor symptoms. This is, for example, reflected by treadmill studies leading to improvements mainly of gait, compared to exergaming studies, which have been associated with changes of balance and quality of life30. In view of the different PD types and degrees of severity, subjectively varying degrees of disturbing symptoms, as well as personal preferences, it becomes clear that one single type of training can hardly meet all requirements of all PD patients28,31. Therefore, large-scale comparative studies to recommend a single, “best” sport for motor and non-motor symptoms of PD and different PD subgroups are still needed31–36. In the meantime, an individual approach with choice of the sport in consideration of the mentioned aspects is necessary31,35. In COVID-19 pandemic times there is an additional argument for physical rehabilitation and exercising in PD. A worsening of PD symptoms and possible higher susceptibility to viral infections including COVID-19-infection seems almost inevitable once physiotherapy is stopped for a longer period. This is also gaining in relevance during these times as physical exercise reduces the risk of upper respiratory tract viral infections and the associated mortality in a dose-dependent manner. Here, moderate intensity exercise (in contrast to high intensity) has the best cell- and cytokine-based effect on the immune system and should be kept in mind when it comes to trainings plans37. Summarizing, an overwhelming body of evidence proves that multi-faceted physical therapy might offer preventive and moderating effects on the course of PD, is a vital mainstay of treatment covering all aspects of PD symptoms and might reduce susceptibility to viral infections and associated mortality.
Impact of the COVID-19 lockdown on PD patients
COVID-19 and PD appear to interact with each other: there is evidence of an increased risk of COVID-19-associated mortality in PD as high as 20–40%1 compared to a 7–19% mortality risk of the general population. On the other hand, as with other infections, PD symptoms aggravate during COVID-19 infection. As PD patients are an elderly, often chronically ill patient group they are already within the high-risk groups for COVID-associated morbidity and mortality1. Furthermore, there is a potential link between COVID-19 and PD, which has shown high titers of anti-coronavirus antibodies in the cerebrospinal fluid of PD patients compared to healthy controls and other types of neurological illnesses5. This seems highly relevant, as the aggregation-susceptible alpha-synuclein might be also implicated in the innate anti-viral immune response5. At present, only self-quarantining offers sufficient protection against the virus. While effective against infection, the concept of quarantine opposes the usual recommendations for PD patients of keeping physically active. Due to their reduced cognitive flexibility and their dependence on regular physical exercise, they are vulnerable to serious collateral damage from the COVID-19 pandemic on their physical and mental health11. The combination of psychological stress and reduced physical activity can lead to a deterioration in motor symptoms that may start a vicious circle of depressed mood, lack of motivation, and further decline of mobility38,39. Although not infected, 10–28% of PD patients reported a worsening of their symptoms due to restricted mobility during the lockdown40–43. Italian PD patients spontaneously lamented the lack of rehabilitation programs after the suspension of standard clinical visits and trials when the country was ravaged by the pandemic. An observational study on 100 PD patients identified a marked decrease in exercise during the COVID-19 lockdown and later reports of deterioration of PD symptoms in half the study population. The patients who continued with their exercise regimen suffered from significantly less subjective aggravation of their PD symptoms, although the difference in the motor part of the MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III) score between the groups did not reach significance42. Another aspect to consider is the direct and indirect effects of the pandemic on the psychological state of PD patients. Up to 30% of Parkinson’s patients suffer from concomitant depressive symptoms and self-quarantining can increase symptoms of depression and anxiety4,44,45. It is particularly important to consider this area of non-motor symptoms of PD patients and to offer treatment and support.
Recommendations for rehabilitation measures during the COVID-19 pandemic
The Centers for Disease Control and Prevention46 and the Centers for Medicare and Medicaid Services47 recommend to curb the spreading of COVID-19 and reschedule elective procedures and treatments. Healthcare professionals were asked to resort to telemedicine for all other, either time-sensitive or functionally disabling, indications. This led to a quick implementation of telemedicine to replace routine or emergency clinical visits with remote check-ups via telephone or internet to extend care into the patients’ homes41. While the chronic movement restrictions caused by PD do not meet the criteria for time-sensitive indications (except recharging of empty Deep Brain Stimulation-batteries), they qualify as functionally disabling9.
Continuing with adapted physical activity and rehabilitation is therefore strongly recommended with either on-site measures, including reduction of patient count, prohibition of escorts, symptoms screening, physical distancing, and personal protective equipment, or via the increasing opportunity of telemedicine and telerehabilitation4,11,12,48–50. Ideally, a regime of moderate-intensity exercise at home 5–7 days per week is recommended, wherever possible outside51–53.
Telerehabilitation
Telerehabilitation comprises home-based, professionally guided training sessions accessed via telecommunication devices such as video calls either conducted in a life, one-on-one trainer-based fashion, or via pre-recorded classes, often combined with apps. It allows for professionally instructed training sessions without the risk of infection for neither the participant nor the trainer or therapist54. In addition to the advantages described above, telerehabilitation in PD patients is at least non-inferior to traditional rehabilitation in improving gait and balance as well as speech55–61. Telerehabilitation is recommended by the American Physical Therapy Association62, the Chartered Society of Physiotherapy54, the World Confederation for Physical Therapy, and the International Network of Physiotherapy Regulatory Authorities63. The successful implementation in other conditions such as stroke64–67, cerebellar ataxia8, muscular dystrophies68, hereditary spastic paraplegia6, and multiple sclerosis69 makes it a promising therapeutic possibility in PD. A recent study showed the readiness of PD patients to take part in telerehabilitation70. They offered a Zoom-based exercise program for newly diagnosed PD patients, which led to an unexpectedly high recruitment rate within weeks. In their ongoing study, they use up to four web-based individual training lessons to promote long-term changes to a higher level of physical activity70. In a randomized controlled trial, PD patients significantly improved their upper limb-mobility and functional mobility after a 3 month-telerehabilitation course while the control group’s balance and functional mobility deteriorated71. For an individual approach, an increasing number of physical therapists has started to offer remote physical therapy with the outbreak of the pandemic. Internet-based video calls connect the trainer and the patient at home. They then carry out the training session in the usual manner apart from adaptations made necessary by spatial requirements and equipment in the patient’s home54. To complement the one-on-one trainer-based fashion of telerehabilitation, video tutorials, and app support, delivered by experts are among emerging web-based exercise options (see Table 1 and Fig. 1)11,72–75.
Table 1.
Overview of telerehabilitation methods.
| gait | rigidity | dexterity | balance/ posture | flexibility | speech | non-motor symptoms | medication adherence | symptom tracking | Advantage | Disadvantage | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Virtual reality | |||||||||||
| NintendoWii®, XboxKinect®, customized VR tools | ✓ | ✓ | ✓ | SE74,75 | EN | ||||||
| Exergaming | |||||||||||
| NintendoWii® | ✓ | ✓ | ✓ | ✓ | SE113–123 | EN | |||||
| Apps | |||||||||||
| 9zest Parkinson’s Therapy & Exercises[®107 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | SE98 | charges apply | ||
| ABF-gait app®, FOG-cue app[®93 | ✓ | SE93 | EN, ST | ||||||||
| APDA Symptom Tracker[®104 | ✓ | ✓ | SR | LE; mainly non-instructional | |||||||
| Beats Medical Parkinsons Treat[®106 | ✓ | ✓ | ✓ | ✓ | wide variety of target symptoms | LE | |||||
| Charity Miles[®102 | ✓ | RF | LE; non-instructional | ||||||||
| Parkinson Exercises Mobile[®109 | ✓ | ✓ | ✓ | ✓ | wide variety of target symptoms | LE, charges apply | |||||
| Parkinson mPower 2[®105 | ✓ | SE94,95, SR | mainly non-instructional | ||||||||
| Parkinson’s Moving Day[®103 | ✓ | RF | LE; non-instructional | ||||||||
| PD Warrior[®131 | ✓ | ✓ | ✓ | ✓ | wide variety of target symptoms | LE | |||||
| uMotif[®92 | ✓ | ✓ | SE132 | ST | |||||||
| Voice analyst[®113 | ✓ | real-time analysis | LE, charges apply | ||||||||
| Yoga against Parkinson’s[®111 | ✓ | ✓ | ✓ | mind–body approach | LE | ||||||
The main target areas of the respective method are indicated by a checkmark.
EN equipment needed, LE lack of scientific evidence, RF research funding, generates funding for future research, SE scientific evidence of feasibility and/ or effectiveness; see text/ citations for details, SR symptom reports, improves doctor–patient-communication by generating reports of symptoms to monitor treatment response and optimize care, ST only in the context of the study.
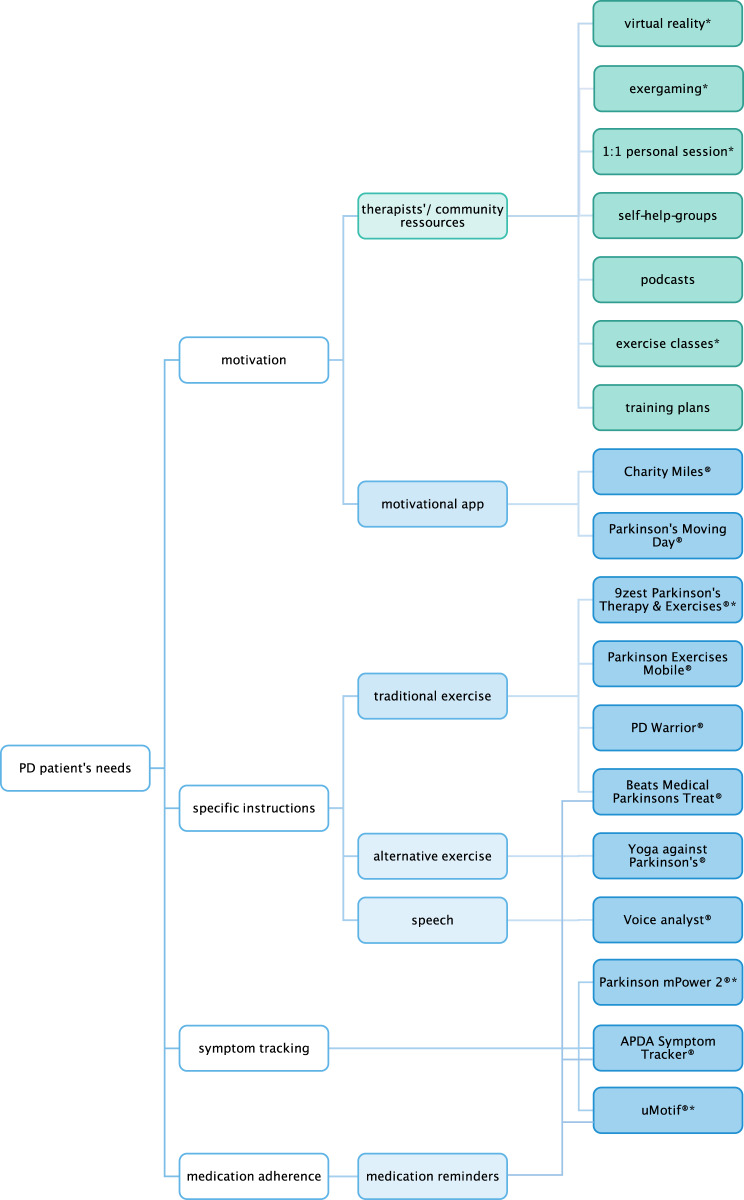
Fig. 1. Decision tree for remote exercise-based treatment options.
The figure shows the various needs of PD patients and their respective telemedical solution. White boxes: patient’s needs. Green boxes: online resources. Blue boxes: mobile applications. Virtual reality: patient-controlled avatar performs playful exercises to train balance, gait, or fine motor skills using motion sensors (either hand-held, body-mounted, or via a pressure-sensitive platform) and a headset or screen76,77. Exergaming: videogames demanding physical participation designed to improve motor skills58,59. 1:1 personal session: live therapeutic session with trainer or therapist via internet-based video calls55–61. Self-help groups and podcasts: motivational community resources on social media85–89. Exercise classes: web-based exercise options delivered by experts72–75. Training plans: downloadable plans for individual use90–92. Motivational apps: encouragement to stay active by donating to PD research (Charity Miles104, Parkinson’s Moving Day105). Traditional exercise: combination of motor, speech, and dexterity exercises (9zest Parkinson’s Therapy & Exercises110, Parkinson Exercises Mobile111, PD Warrior112, Beats Medical Parkinsons Treat109). Alternative exercise: yoga postures potentially beneficial for postural control and against rigidity (Yoga against Parkinson’s113). Speech: speech exercises for correction of hypophonia (Voice analyst114). Symptom-tracking: symptom tracking apps to monitor treatment response and optimize care by generating reports for discussion with physician and/ or physical therapist (Parkinson mPower 2107, APDA Symptom tracker106, uMotif94,108). Options with scientific evidence of feasibility and/ or effectiveness are indicated by an asterisk*.
Virtual reality
A subspecialty of telerehabilitation based on VR exercises has shown promising results on motor and non-motor symptoms in PD and is increasingly recognized as a valuable alternative to traditional physiotherapy. Using motion sensors (either hand-held, body-mounted, or via a pressure-sensitive platform) and a VR headset or screen, VR exercises enable interaction with a computer-generated scenario in which a patient-controlled avatar performs playful exercises to train balance, gait, or fine motor skills. A systematic review covering eight trials with 263 patients comparing VR training with standard physical therapy found improved gait characteristics, balance, and quality of life, as well as satisfactory regime adherence, though the level of evidence was judged to be low due to methodological concerns76. Later, another randomized trial of 76 PD patients revealed significant improvement in balance in the intervention group after a 7-week VR-based balance training compared to traditional sensory integration balance training of the control group (see Table 1 and Fig. 1)77.
Motivational tools
A huge challenge associated with unsupervised activity is the lack of motivation to start and keep a regular exercise regime. Apathy in PD patients can further aggravate demotivation, though exercise itself reduces apathy and fatigue18,21,78–81. The World Health Organization (WHO) recommends measures to promote motivation not only at the professional level by health professionals but also at the community level (formation of training groups) and the personal level by promoting the patient’s rights of self-determination82. The patient’s autonomy has a major influence on motivation. Being able to choose the type, time, and place of training independently achieves better long-term adherence than fixed plans83,84. Telecommunications-based resources are available for both community-based and individual motivation. An online community of fellow patients and professionals supplies encouragement and support through self-help-groups on social media, networking podcasts, instructional videos, and follow-along exercise classes85–89. Exercise programs and instructions in the form of downloadable training plans are also available for individual use (see Table 1 and Fig. 1)90–92.
Apps
A variety of apps covering relaxation and meditation, motivation, symptom tracking, and exercises for balance, speech, gait, cognitive functions, and coordination are available4,93. Despite the increasing use, scientific evidence on apps for PD is still sparse. In the light of the increasing field, we present hereafter a selection of current smartphone applications, useful for PD during the COVID-19 pandemic (see Table 1 and Fig. 1). In a randomized controlled study, a 16-week implication of the “Parkinson’s Tracker App uMotif” was able to significantly improve medication adherence, satisfaction with the medical consultation, and non-motor symptoms94. One randomized controlled trial has shown some first positive effects of app use on gait in 40 PD patients: although both groups showed significant improvement of gait speed, only the intervention group who performed a 6-week, thrice-weekly gait training course guided by the “ABF-gait app” and the “FOG-cue app” improved postural control and quality of life compared to the active control group who performed the same gait exercises without app-support95. The app relies on two external motion-sensor units, which might limit the app’s implementation in real-world telerehabilitation. Several observational studies have already shown that mobile apps are feasible both for recording and registering symptoms in a highly reliably manner for scientific purposes and for training to improve motor functions in PD96–99. A recent study on 28 PD patients evaluated the App “9zest Parkinson’s Therapy & Exercises” and reported it to be both feasible and effective. After 3 months of training, functional lower extremity strength, mobility, and quality of life improved100. Other feasibility studies on software programs supplemented by wearable motion-sensing technology are currently on their way101,102. A randomized controlled study of 51 PD patients showed superior effectiveness of a 12-month multimodal telerehabilitation course, using individualized training plans, video tutorials, and app support with significant improvement of mobility compared to the physically active control group without telerehabilitation, in which the subjective mobility-related quality of life as measured by the Parkinson’s Disease Questionnaires-39 (PDQ 39) mobility domain scores even deteriorated during the study period103.
Outlook of ongoing App projects
The mobile apps “Charity Miles” and “Parkinson’s Moving Day” encourage PD patients to stay active by donating to PD research according to their walking distances. This effectively serves two purposes in one: immediate benefit for the individual through exercise and long-term influence by funding research104,105. The “APDA Symptom Tracker” app by the American Parkinson Disease Association eases the increasingly vital remote care by letting PD patients record their symptoms to discuss their report with their neurologist at their next telehealth appointment106. “Parkinson mPower 2“, which has already been validated by an observational study, not only lets PD patients record their symptoms, but also gives them the opportunity to make their data available for scientific study purposes96,97,107. “Parkinson Exercises Mobile,” “PD Warrior108,” “9zest Parkinson’s Therapy & Exercises,” and “Beats Medical Parkinsons Treat” offer a combination of motor, speech, and dexterity exercises109–112. In the app “Yoga against Parkinson’s,” PD patients are guided through selected yoga postures that are considered beneficial for postural control and greater flexibility, as well as relaxation113. “Voice analyst” helps to correct hypophonia by analysing the speech of PD patients in real-time114.
Exergaming
For a more entertaining and therefore motivational approach, exergames, i.e., videogames demanding physical participation designed to improve motor skills, are increasingly recognized as a valuable alternative to traditional exercise. Two reviews of 7 and 22 studies on Nintendo Wii™ based exergaming interventions in patients with PD or the elderly, respectively, showed significant improvements in upper limb function and strength, balance and functional mobility, a reduction of falls, and an improvement of both neurocognitive abilities and psychosocial aspects115,116. In PD patients, two randomized controlled trials revealed a significant improvement of balance after an 8- or 12-week exergaming training regime117,118. Exergaming proved to be at least as effective in improving functional mobility and gait as traditional balance exercising, physiotherapy, or bicycle training in a randomized trial of 32 PD patients119. It furthermore significantly improved upper limb function in two series of PD patients120,121. Although a randomized trial failed to reproduce the same significant results of dexterity improvement after a 12-week exergaming course, it still showed increased speed in the nine-hole peg test but at the cost of accuracy. This was explained as a direct effect of those exergames which prioritize speed training while neglecting precision122. Even cognitive-motor dual-tasking functions improved significantly after exergaming sessions compared to controls123. A systematic review on exergaming in PD summarized found exergaming to be equally effective, as well as practical and safe, as traditional rehabilitation58. An elaborate recent study showed the feasibility of guided home exercising in PD patients, using app support and motivating exergaming experience. Within this large randomized study, 65 PD patients who underwent aerobic training for 6 months showed significant motoric improvement in the motor part of the MDS-UPDRS III compared to the control group who only performed regular stretching59.
Creative self-initiative on the part of the patients: A case presentation
An extraordinary example of self-engagement as presented here can be highly inspirational to other patients who cannot follow their conventional training schedule and routines during the COVID-19 pandemic. In February 2019, a 69-year-old woman presented in our outpatient clinic with a tremor-dominant PD, with symptoms starting in 2004. Neurological examination revealed a left dominant rest and postural tremor, left dominant mild bradykinesia, moderate axial and limb rigidity, a typical hypokinetic gait, mild dyskinesia, and a slightly stooped posture. She complained about neck pain and sleeping problems. Her daily PD medication included levodopa 200 mg, rotigotine 8 mg, and rasagiline 1 mg.
Without earlier climbing experience, she took part in a 12-week guided climbing course for patients with PD once a week for 90 min, where she learned top-rope climbing and belaying skills. She strongly benefitted from the course: her motor and non-motor symptoms improved, for example, and the neck pain disappeared.
When the climbing hall closed during the COVID-19 pandemic, her motor and non-motor symptoms deteriorated, and her neck pain reoccurred. In the week after the lockdown, she started climbing again, using an extra-long rope ladder that she attached to a branch of a tree in her garden for top-rope climbing. Secured by her husband, she continues climbing up the rope ladder at least 1–2 times per week.
When she started with her garden climbing experience in April 2020, she could climb the first 3–4 rungs and made progress within 2 weeks. Since then, she has been regularly climbing the whole ladder with 14 rungs 3–4 times without interruption for 7 months now (see Video, Supplemental Digital Content 1, which shows the patient climbing her garden rope ladder).
She can still perfectly control her neck pain with this exercise. It disappears during or after the training and does not return for 4–6 days. She also reported that during the regular climbing exercises in the garden, her sleep quality, her sense of body balance, and her left-sided weakness improved. Even if we perceive this type of exercise to be a great innovation and to have high potential in PD treatment, it is essential to readers to not mistake the exercise (and extreme training method) described above as a general recommendation for PD patients. This patient’s individual training method only worked well because she and her husband had previously gone through special climbing training sessions and had carefully examined the climbing mount and the tree for stability. We would like to emphasize the importance of performing physical exercise for PD patients only in a safe environment as osteoporosis is pandemic in PD and fractures due to falls must be avoided at any time. Patients and caregivers must consider this aspect when selecting and planning sports giving preference to low-risk sports with adequate (remote or personal) supervision.
Conclusion
Discontinuity in exercise-based therapy due to the COVID-19 pandemic has already had a detrimental effect on motor and non-motor symptoms, as well as on the wellbeing of PD patients. Most of the consequences are not yet visible and will only show later in long-term after-effects. Counterstrategies are based primarily on implementing comprehensive telerehabilitation programs, as they have shown great potential in the long-term remote care and support for PD patients. All the articles discussed earlier proposed the implementation of telehealth or telerehabilitation during the ongoing COVID-19 pandemic as an alternative to conventional physical therapy and allied health since internet-based technology cuts the risks of infection through personal contact. This enables carers and patients to continue with the most important non-pharmaceutical therapy principle in the treatment of PD even in pandemic and lockdown times. A growing body of evidence suggests that telemedicine and telerehabilitation could be as useful as treatments concerning functional outcomes124. Other benefits of remotely supplied treatment are the reduced costs and improved convenience by cutting travel expenses and burden51–53.
Some aspects should however be specifically addressed in future studies. While patients receiving telemedicine are satisfied with the provided service124, those who do not have access to the technology or the necessary knowledge and confidence to use the resources must have a chance to receive treatment of comparable quality.
Future outlook
The COVID-19 pandemic, as terrible as it undoubtedly is, holds the potential for an unplanned but an unavoidable test phase for telemedicine. This raises the question, if telemedicine can and should support, and partly substitute, traditional personal medicine in view of the advantages described above even after the pandemic.
Despite the general patient satisfaction with telemedicine, prior research and recent experience of COVID-19 lockdown phases show that patients sorely miss the personal contact with doctors and therapists. Both sides are not willing to completely abandon personal care in future11,41,43,125. Despite comparable quality of remote appointments, they cannot provide a perfect substitute for traditional in-person visits with a direct physical examination126–128.
On the other hand, telerehabilitation offers the best possible treatment to patients in areas without easily accessible neurologists or therapists and also patients who live in the vicinity of therapy facilities should get the opportunity to supplement their standard treatment with telerehabilitation. It seems to be an inexpensive alternative to conventional physiotherapy and should be promoted as one way of high-quality care for patients without routine access to healthcare institutions129,130. However, long-term cost-effectiveness calculations are not available yet and should be investigated in future research51–53. From today’s perspective, it is very likely that an individual “hybrid model“ of traditional in-person medicine and some form of telerehabilitation will prevail.
Methods
Narrative Review
We performed a literature search in the PubMed database using the search criteria “COVID-19”, “Parkinson Disease”, “telerehabilitation”, “physiotherapy”, “exercise”, “virtual reality”, “exergaming”, “application”, screened the references of relevant articles for additional relevant publications, and searched official regulatory website of health authorities for recommendation on telemedicine. Relevant cell phone applications were identified in the app stores “App Store (iOS)”131 and “Google Play Store”132,133 using the search term “Parkinson Disease”.
Case report
After having completed a guided climbing course as a part of a clinical study (approved by the ethical committee of the Medical University of Vienna No: 1369/2017), the patient decided to continue her climbing training at home. The patient volunteered the information about her individual climbing training during a routine visit and spontaneously provided the Supplementary Video 1. The patient provided written informed consent to participate in this case report. The authors affirm that the human participants provided written informed consent for publication of the video in Supplementary Video 1.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank the patient for supplying the video and sharing her innovative idea.
Author contributions
A.L., H.Z., and W.M. designed and conceptualized the study. A.L., L.G., A.F., S.H., J.G., L.W., R.P., and H.Z. handled basic research, preparation, and interpretation of data. W.M., H.Z., and R.P. giving substantial input to the last final version of the manuscript.
Data availability
Data are available on request from the authors.
Code availability
No software or custom code was used for data collection or analysis.
Competing interests
The authors declare no competing interests (financial/non-financial).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00160-3.
References
- 1.Antonini A., Leta V., Teo J. & Chaudhuri K. R. Outcome of Parkinson’s disease patients affected by COVID-19. Mov Disord. 35, 905–908 (2020). [DOI] [PMC free article] [PubMed]
- 2.Ferini-Strambi L., Salsone M. COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? J Neurol. 1–11 10.1007/s00415-020-10070-8 (2020) Advance online publication. [DOI] [PMC free article] [PubMed]
- 3.Bhidayasiri R, Virameteekul S, Kim JM, Pal PK, Chung SJ. COVID-19: an early review of its global impact and considerations for Parkinson’s disease patient care. J. Mov. Disord. 2020;13:105–114. doi: 10.14802/jmd.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri KR. COVID_19 and Parkinson’s disease. Kinetic. 2020;2:4–5. [Google Scholar]
- 5.Sulzer D, et al. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Parkinsons Dis. 2020;6:18. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Venis L. et al. COVID-19 reveals influence of physical activity on symptom severity in hereditary spastic paraplegia. J. Neurol. 267, 1–3 (2020). [DOI] [PMC free article] [PubMed]
- 7.Boldrini P, et al. Living with a disability during the pandemic. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020;56:331–334. doi: 10.23736/S1973-9087.20.06373-X. [DOI] [PubMed] [Google Scholar]
- 8.Manto M, et al. Management of patients with cerebellar ataxia during the COVID-19 pandemic: current concerns and future implications. Cerebellum. 2020;19:562–568. doi: 10.1007/s12311-020-01139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karri, J., Seymour, M. L., Verduzco-Gutierrez, M. & Jayaram, P. Point-of-care procedures in physiatry: practice considerations during the Covid-19 pandemic. Am. J. Phys. Med. Rehabil. 99, 567–570 (2020). [DOI] [PMC free article] [PubMed]
- 10.Carda S. et al. The role of physical and rehabilitation medicine in the COVID-19 pandemic: the clinician’s view. Ann. Phys. Rehabil. Med. 63, 554–556 (2020). [DOI] [PMC free article] [PubMed]
- 11.Helmich RC, Bloem BR. The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J. Parkinsons Dis. 2020;10:351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoessl AJ, Bhatia KP, Merello M. Movement disorders in the World of COVID-19. Mov. Disord. 2020;35:709–710. doi: 10.1002/mds.28069. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S. et al. Parkinson’s disease and COVID-19: perceptions and implications in patients and caregivers. Mov Disord. 35, 912–914 (2020). [DOI] [PMC free article] [PubMed]
- 14.Uhrbrand A, Stenager E, Pedersen MS, Dalgas U. Parkinson’s disease and intensive exercise therapy–a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 2015;353:9–19. doi: 10.1016/j.jns.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Shen X, Wong-Yu IS, Mak MK. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: a meta-analysis. Neurorehabil. Neural. Repair. 2016;30:512–527. doi: 10.1177/1545968315613447. [DOI] [PubMed] [Google Scholar]
- 16.Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017;13:689–703. doi: 10.1038/nrneurol.2017.128. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson CL, et al. Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Syst. Rev. 2014;2014:Cd002815. doi: 10.1002/14651858.CD002815.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cusso ME, Donald KJ, Khoo TK. The impact of physical activity on non-motor symptoms in Parkinson’s disease: a systematic review. Front. Med. 2016;3:35. doi: 10.3389/fmed.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein PJ. Tai Chi Chuan in the management of Parkinson’s disease and Alzheimer’s disease. Med. Sport Sci. 2008;52:173–181. doi: 10.1159/000134298. [DOI] [PubMed] [Google Scholar]
- 20.Song R, et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 2017;41:3–13. doi: 10.1016/j.parkreldis.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Šumec R, Filip P, Sheardová K, Bareš M. Psychological benefits of nonpharmacological methods aimed for improving balance in Parkinson’s disease: a systematic review. Behav. Neurol. 2015;2015:620674. doi: 10.1155/2015/620674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, et al. Effect of exercise on quality of life in Parkinson’s disease: a systematic review and meta-analysis. Parkinsons Dis. 2020;2020:3257623. doi: 10.1155/2020/3257623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotty GF, Schwarzschild MA. Chasing protection in Parkinson’s disease: does exercise reduce risk and progression? Front. Aging Neurosci. 2020;12:186. doi: 10.3389/fnagi.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petzinger GM, et al. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenkman M, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75:219–226. doi: 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francardo V, Schmitz Y, Sulzer D, Cenci MA. Neuroprotection and neurorestoration as experimental therapeutics for Parkinson’s disease. Exp. Neurol. 2017;298:137–147. doi: 10.1016/j.expneurol.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 27.O’Callaghan A, et al. Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with Parkinson’s disease: a pilot study. Aging Clin. Exp. Res. 2020;32:1731–1738. doi: 10.1007/s40520-019-01353-w. [DOI] [PubMed] [Google Scholar]
- 28.Ellis T, Rochester L. Mobilizing Parkinson’s disease: the future of exercise. J. Parkinsons Dis. 2018;8:S95–S100. doi: 10.3233/JPD-181489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch MA, Iyer SS, Sanjak M. Exercise-induced neuroplasticity in human Parkinson’s disease: what is the evidence telling us? Parkinsonism Relat. Disord. 2016;22(Suppl 1):S78–81. doi: 10.1016/j.parkreldis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Radder DLM, et al. Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities. Neurorehabil Neural Repair. 2020;34:871–880. doi: 10.1177/1545968320952799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis TD, Dibble LE, Peterson DS. Moving Beyond Effectiveness. J. Neurol. Phys. Ther. 2019;43:1–2. doi: 10.1097/NPT.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 32.Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat. Disord. 2016;22(Suppl. 1):S60–64. doi: 10.1016/j.parkreldis.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho A, et al. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson’s disease: pilot study. Clin. Inter. Aging. 2015;10:183–191. doi: 10.2147/CIA.S68779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawson KS, et al. Exercise and Parkinson disease: comparing tango, treadmill, and stretching. J. Neurol. Phys. Ther. 2019;43:26–32. doi: 10.1097/NPT.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeely ME, Duncan RP, Earhart GM. A comparison of dance interventions in people with Parkinson disease and older adults. Maturitas. 2015;81:10–16. doi: 10.1016/j.maturitas.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HY, et al. Exercise therapies for Parkinson’s disease: a systematic review and meta-analysis. Parkinsons Dis. 2020;2020:2565320. doi: 10.1155/2020/2565320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall, M. E. & Church, F. C. Exercise for older adults improves the quality of life in Parkinson’s disease and potentially enhances the immune response to COVID-19. Brain Sci. 10, 612 (2020). [DOI] [PMC free article] [PubMed]
- 38.Zach, H., Dirkx, M.F., Pasman, J.W., Bloem, B.R. & Helmich, R.C. Cognitive stress reduces the effect of levodopa on Parkinson’s resting tremor. CNS Neurosci. Ther. 23, 209–215 (2017). [DOI] [PMC free article] [PubMed]
- 39.Subramanian I, Farahnik J, Mischley LK. Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. npj Parkinsons Dis. 2020;6:28. doi: 10.1038/s41531-020-00128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad S, et al. Parkinson’s disease and COVID-19: perceptions and implications in patients and caregivers. Mov. Disord. 2020;35:912–914. doi: 10.1002/mds.28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schirinzi T, et al. Self-reported needs of patients with Parkinson’s disease during COVID-19 emergency in Italy. Neurol. Sci. 2020;41:1373–1375. doi: 10.1007/s10072-020-04442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J, et al. The changes of exercise pattern and clinical symptoms in patients with Parkinson’s disease in the era of COVID-19 pandemic. Parkinsonism Relat. Disord. 2020;80:148–151. doi: 10.1016/j.parkreldis.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown EG, et al. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J. Parkinsons Dis. 2020;10:1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 45.Pontone GM, et al. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. npj Parkinsons Dis. 2019;5:30. doi: 10.1038/s41531-019-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (2020).
- 47.CMS. Non-emergent, elective medical services, and treatment recommendations. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf (2020).
- 48.Hcp H. P. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. National Center for Chronic Disease Prevention and Health Promotion. Division of Diabetes, Translation (Atlanta, GA, 2020) [Available from: https://stacks.cdc.gov/view/cdc/86043].
- 49.Ceravolo MG, de Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid “living” review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur. J. Phys. Rehabil. Med. 2020;56:347–353. doi: 10.23736/S1973-9087.20.06329-7. [DOI] [PubMed] [Google Scholar]
- 50.Leocani L., Diserens K., Moccia M. & Caltagirone C. Disability through COVID-19 pandemic: neurorehabilitation cannot wait. Eur. J. Neurol. 27, e50–e51 (2020). [DOI] [PMC free article] [PubMed]
- 51.Chen P, et al. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020;9:103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amekran, Y. & El Hangouche, A. J. Coronavirus disease (COVID-19) and the need to maintain regular physical activity. J. Sports Med. Phys. Fitness. 61, 159–160 (2021). [DOI] [PubMed]
- 53.Jiménez-Pavón D, Carbonell-Baeza A, Lavie CJ. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Prog. Cardiovasc. Dis. 2020;63:386–388. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Physiotherapy CSo. https://www.csp.org.uk/publications/covid-19-guide-rapid-implementation-remote-consultations.
- 55.Lei C, et al. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: a systematic review. PLoS ONE. 2019;14:e0224819. doi: 10.1371/journal.pone.0224819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Constantinescu G, et al. Treating disordered speech and voice in Parkinson’s disease online: a randomized controlled non-inferiority trial. Int J. Lang. Commun. Disord. 2011;46:1–16. doi: 10.3109/13682822.2010.484848. [DOI] [PubMed] [Google Scholar]
- 57.Theodoros DG, Hill AJ, Russell TG. Clinical and quality of life outcomes of speech treatment for Parkinson’s disease delivered to the home via telerehabilitation: a noninferiority randomized controlled trial. Am. J. Speech Lang. Pathol. 2016;25:214–232. doi: 10.1044/2015_AJSLP-15-0005. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Agundez A, et al. Recent advances in rehabilitation for Parkinson’s disease with exergames: a systematic review. J. Neuroeng. Rehabil. 2019;16:17. doi: 10.1186/s12984-019-0492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Kolk NM, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18:998–1008. doi: 10.1016/S1474-4422(19)30285-6. [DOI] [PubMed] [Google Scholar]
- 60.Chen YY, et al. Application of telehealth intervention in Parkinson’s disease: a systematic review and meta-analysis. J. Telemed. Telecare. 2020;26:3–13. doi: 10.1177/1357633X18792805. [DOI] [PubMed] [Google Scholar]
- 61.Schneider RB, Biglan KM. The promise of telemedicine for chronic neurological disorders: the example of Parkinson’s disease. Lancet Neurol. 2017;16:541–551. doi: 10.1016/S1474-4422(17)30167-9. [DOI] [PubMed] [Google Scholar]
- 62.Association APT. https://www.apta.org/your-practice/practice-models-and-settings/telehealth-practice.
- 63.Lee A. Report of the WCPT/INPTRA Digital Physical Therapy Practice Task Force. 2019.
- 64.Chang MC, Boudier-Revéret M. Usefulness of telerehabilitation for stroke patients during the COVID-19 pandemic. Am. J. Phys. Med. Rehabil. 2020;99:582. doi: 10.1097/PHM.0000000000001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tenforde AS, et al. Evidence-based physiatry: efficacy of home-based telerehabilitation versus in-clinic therapy for adults after stroke. Am. J. Phys. Med. Rehabil. 2020;99:764–765. doi: 10.1097/PHM.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 66.Laver KE, et al. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020;1:CD010255. doi: 10.1002/14651858.CD010255.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dial HR, et al. Investigating the utility of teletherapy in individuals with primary progressive aphasia. Clin. Inter. Aging. 2019;14:453–471. doi: 10.2147/CIA.S178878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veerapandiyan A, et al. The care of patients with Duchenne, Becker, and other muscular dystrophies in the COVID-19 pandemic. Muscle Nerve. 2020;62:41–45. doi: 10.1002/mus.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai B, et al. COVID-19 modifications for remote teleassessment and teletraining of a complementary alternative medicine intervention for people with multiple sclerosis: protocol for a randomized controlled trial. JMIR Res. Protoc. 2020;9:e18415. doi: 10.2196/18415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quinn, L., Macpherson, C., Long, K. & Shah, H. Promoting physical activity via telehealth in people with Parkinson disease: the path forward after the COVID-19 pandemic? Phys Ther. 100, 1730–1736 (2020). [DOI] [PMC free article] [PubMed]
- 71.Isernia S, et al. Effects of an innovative telerehabilitation intervention for people with Parkinson’s disease on quality of life, motor, and non-motor abilities. Front. Neurol. 2020;11:846. doi: 10.3389/fneur.2020.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parkinson’s on the Move. https://pdonthemove.com.
- 73.Davis Phinney Foundation. https://davisphinneyfoundation.org/parkinsons-exercise-essentials-video/#ExerciseGetStarted.
- 74.American Parkinson Disease Association. https://www.apdaparkinson.org/community/st-louis/resources-support-stl/exercise-classes/joy-of-movement/.
- 75.The Brian Grant Foundation. https://briangrant.org/exercise-videos/.
- 76.Dockx K, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016;12:CD010760. doi: 10.1002/14651858.CD010760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gandolfi M, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized, controlled trial. Biomed. Res. Int. 2017;2017:7962826. doi: 10.1155/2017/7962826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson’s disease. Mov. Disord. 2013;28:1587–1596. doi: 10.1002/mds.25658. [DOI] [PubMed] [Google Scholar]
- 79.Speelman AD, et al. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 80.Mele B, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10:e037632. doi: 10.1136/bmjopen-2020-037632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedman JH, et al. Fatigue in Parkinson’s disease. J Parkinsons Dis. 2016;2:15025. doi: 10.1038/npjparkd.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Organization W. H. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World. (World Health Organization, 2018).
- 83.Carl, J., Sudeck, G. & Pfeifer K. Competencies for a healthy physically active lifestyle-reflections on the model of physical activity-related health competence. J. Phys. Act. Health. 1–10 https://doi-org.ez.srv.meduniwien.ac.at/10.1123/jpah.2019-0442 (2020). [DOI] [PubMed]
- 84.Buetow SA, Martínez-Martín P, Hirsch MA, Okun MS. Beyond patient-centered care: person-centered care for Parkinson’s disease. NPJ Parkinson’s Dis. 2016;2:16019. doi: 10.1038/npjparkd.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dancing Through Parkinson’s ® . https://www.invertigodance.org/dtp.
- 86.European Parkinson’s Disease Association (EPDA). https://www.epda.eu.com/exercisecast.
- 87.The Parkinson’s Fitness Project: The Daily Dose PD. https://www.dailydosepd.com/free-workouts-2?_ga=2.251054467.426691946.1588676011-35375576.1588676011.
- 88.The Michael J. Fox Foundation. twitter.com/michaeljfoxorg.
- 89.Parkinson’s Social Network MyHealthTeams, Inc V. 12.0.4(Mobile application software, 2019). https://apps.apple.com/us/app/parkinsons-social-network/id1339366057.
- 90.Exercises for People with Parkinson’s. https://www.parkinsons.va.gov.
- 91.Foundation Ps. Fitness Counts. A Body Guide to Parkinson’s Disease: Parkinson’s Foundation https://www.parkinson.org/sites/default/files/Fitness_Counts.pdf.
- 92.How to stay active and exercise at home Parkinson’s Disease Society of the United Kingdom https://www.parkinsons.org.uk/information-and-support/exercise-progressing-symptoms.
- 93.Srivastav AK, Samuel AJ. E-rehabilitation: one solution for patients with Parkinson’s disease in COVID-19 era. Parkinsonism Relat. Disord. 2020;75:128–129. doi: 10.1016/j.parkreldis.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lakshminarayana R, et al. Using a smartphone-based self-management platform to support medication adherence and clinical consultation in Parkinson’s disease. NPJ Parkinson’s Dis. 2017;3:2. doi: 10.1038/s41531-016-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ginis P, et al. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: a pilot randomized controlled trial. Parkinsonism Relat. Disord. 2016;22:28–34. doi: 10.1016/j.parkreldis.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Bot BM, et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data. 2016;3:160011. doi: 10.1038/sdata.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trister AD, Dorsey ER, Friend SH. Smartphones as new tools in the management and understanding of Parkinson’s disease. NPJ Parkinson’s Dis. 2016;2:16006. doi: 10.1038/npjparkd.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ivkovic V, Fisher S, Paloski WH. Smartphone-based tactile cueing improves motor performance in Parkinson’s disease. Parkinsonism Relat. Disord. 2016;22:42–47. doi: 10.1016/j.parkreldis.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Samà A, et al. A double closed loop to enhance the quality of life of Parkinson’s disease patients: REMPARK system. Stud. Health Technol. Inform. 2014;207:115–124. [PubMed] [Google Scholar]
- 100.Landers MR, Ellis TD. A mobile app specifically designed to facilitate exercise in Parkinson disease: single-cohort pilot study on feasibility, safety, and signal of efficacy. JMIR Mhealth Uhealth. 2020;8:e18985. doi: 10.2196/18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegert C, Hauptmann B, Jochems N, Schrader A, Deck R. ParkProTrain: an individualized, tablet-based physiotherapy training programme aimed at improving quality of life and participation restrictions in PD patients - a study protocol for a quasi-randomized, longitudinal and sequential multi-method study. BMC Neurol. 2019;19:143. doi: 10.1186/s12883-019-1355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hulbert S, Fullam J, Hunt C, Goodwin VA. ‘Digital dancing’ - “can you see, what I feel” - an exploration of the physical ‘experience’ of dance for Parkinson’s through 3-dimensional motion analysis. Complement Ther. Med. 2020;52:102508. doi: 10.1016/j.ctim.2020.102508. [DOI] [PubMed] [Google Scholar]
- 103.Ellis TD, et al. Comparative effectiveness of mHealth-supported exercise compared with exercise alone for people with Parkinson disease: randomized controlled pilot study. Phys Ther. 2019;99:203–216. doi: 10.1093/ptj/pzy131. [DOI] [PubMed] [Google Scholar]
- 104.Charity Miles Charity Miles LLC V. 7.2.1 (Mobile application software, 2020). https://apps.apple.com/us/app/charity-miles/id505253234.
- 105.Parkinson’s Moving Day Charity Dynamics, Inc. V. 1.3 (Mobile application software, 2020). https://play.google.com/store/apps/details?id=com.charitydynamics.parkinson&hl=de_AT.
- 106.APDA Symptom Tracker American Parkinson Disease Association V. 1.0.1 (Mobile application software, 2020). https://play.google.com/store/apps/details?id=com.aoic.apdahealthcaretrackerapp&hl=de_AT.
- 107.Parkinson mPower 2 Sage Bionetworks, a Not-For-Profit Research Organization V. 2.1 (Mobile application software, 2018) https://apps.apple.com/us/app/parkinson-mpower-2/id1375781575.
- 108.uMotif: uMotif V.3.9.0 (Mobile application software, 2020). https://play.google.com/store/apps/details?id=com.umotif.umotif_wellbeing&hl=en_US.
- 109.Beats Medical Parkinsons Treat: Beats Medical V. 3.20 (Mobile application software, 2019). https://www.parkinsons.org.uk/information-and-support/exercise-progressing-symptoms.
- 110.9zest Parkinson’s Therapy & Exercises V.3.2.2 ed. 9zest (Mobile application software, 2018).
- 111.Parkinson Exercises Mobile European Foundation for Health and Exercise V. 1.0.1 (Mobile application software, 2013). https://play.google.com/store/apps/details?id=nl.efox.parkinsonss.en.phone&hl=en_US.
- 112.PD Warrior: NEURO PHYSIO V.1.0.6 (Mobile application software, 2019). https://play.google.com/store/apps/details?id=com.pd.warrior.
- 113.Yoga against Parkinson’s PhysUp eHealth V. 1.0 (Mobile application software, 2018). https://play.google.com/store/apps/details?id=com.mr_ingrisano.Yoga_against_Parkinsons&hl=de_AT.
- 114.Voice Analyst: pitch & volume Speechtools Ltd 2014-2020 V. 2.31 (Mobile application software, 2020. https://apps.apple.com/us/app/voice-analyst-pitch-volume.
- 115.Chao YY, Scherer YK, Montgomery CA. Effects of using Nintendo Wii™ exergames in older adults: a review of the literature. J. Aging Health. 2015;27:379–402. doi: 10.1177/0898264314551171. [DOI] [PubMed] [Google Scholar]
- 116.Barry G, Galna B, Rochester L. The role of exergaming in Parkinson’s disease rehabilitation: a systematic review of the evidence. J. Neuroeng. Rehabil. 2014;11:33. doi: 10.1186/1743-0003-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shih MC, Wang RY, Cheng SJ, Yang YR. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: a single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2016;13:78. doi: 10.1186/s12984-016-0185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ribas CG, Alves da Silva L, Corrêa MR, Teive HG, Valderramas S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: a pilot randomized controlled trial. Parkinsonism Relat. Disord. 2017;38:13–18. doi: 10.1016/j.parkreldis.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Ferraz DD, et al. The effects of functional training, bicycle exercise, and exergaming on walking capacity of elderly patients with Parkinson disease: a pilot randomized controlled single-blinded trial. Arch. Phys. Med. Rehabil. 2018;99:826–833. doi: 10.1016/j.apmr.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 120.van Beek JJW, van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons With Parkinson disease: a pilot feasibility study. J. Neurol. Phys. Ther. 2019;43:168–174. doi: 10.1097/NPT.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 121.Cikajlo I, et al. Can telerehabilitation games lead to functional improvement of upper extremities in individuals with Parkinson’s disease? Int. J. Rehabil. Res. 2018;41:230–238. doi: 10.1097/MRR.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allen NE, et al. An interactive videogame for arm and hand exercise in people with Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat. Disord. 2017;41:66–72. doi: 10.1016/j.parkreldis.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 123.Schaeffer E, et al. Effects of exergaming on attentional deficits and dual-tasking in Parkinson’s disease. Front. Neurol. 2019;10:646. doi: 10.3389/fneur.2019.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hatcher-Martin JM, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology. 2020;94:30–38. doi: 10.1212/WNL.0000000000008708. [DOI] [PubMed] [Google Scholar]
- 125.Spear KL, Auinger P, Simone R, Dorsey ER, Francis J. Patient views on telemedicine for Parkinson disease. J. Parkinsons Dis. 2019;9:401–404. doi: 10.3233/JPD-181557. [DOI] [PubMed] [Google Scholar]
- 126.Block VA, et al. Remote physical activity monitoring in neurological disease: a systematic review. PLoS ONE. 2016;11:e0154335. doi: 10.1371/journal.pone.0154335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mammen JR, et al. Patient and physician perceptions of virtual visits for Parkinson’s disease: a qualitative study. Telemed. J. E. Health. 2018;24:255–267. doi: 10.1089/tmj.2017.0119. [DOI] [PubMed] [Google Scholar]
- 128.Beck CA, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89:1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chirra M, et al. Telemedicine in neurological disorders: opportunities and challenges. Telemed. J. E. Health. 2019;25:541–550. doi: 10.1089/tmj.2018.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peacock D, et al. Perception of healthcare access and utility of telehealth among Parkinson’s disease patients. Can. J. Neurol. Sci. 2020;47:700–704. doi: 10.1017/cjn.2020.99. [DOI] [PubMed] [Google Scholar]
- 131.Apple Inc. https://www.apple.com/app-store.
- 132.Google L. L. C. https://play.google.com/store.
- 133.Chaudhuri KR, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov. Disord. 2006;21:916–923. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the authors.
No software or custom code was used for data collection or analysis.