Abstract
Background:
The menopausal transition is a hormonally sensitive period associated with changes in body weight. Phthalates are ubiquitous endocrine disrupting chemicals that could disrupt weight homeostasis, but it is unknown whether this occurs during the menopausal transition.
Objectives:
Our objectives were to (1) determine if phthalate exposure in pre- and perimenopausal women was associated with one-year change in body mass index (BMI), and (2) determine if these associations differed across the menopausal transition.
Methods:
We addressed our objectives using data from 524 participants enrolled in the Midlife Women’s Health Study. We calculated change in BMI from baseline to first follow-up visit approximately one year later. Phthalate exposures were approximated by measuring urinary metabolites in pools of two-to-four spot urine samples collected across a four-week period at baseline. We molar-converted and summed mono-(2-ethylhexyl) phthalate (mEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (mEHHP), mono(2-ethyl-5-oxohexyl) phthalate (mEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (mECPP) to approximate exposure to di-(2-ethylhexyl) phthalate (∑DEHP); ∑DEHP, mono(3-carboxypropyl) phthalate (mCPP), and monobenzyl phthalate (mBzP) to approximate exposure to plasticizer phthalates (∑Plastics); and monoethyl phthalate (mEP), monobutyl phthalate (mBP), and monoisobutyl phthalate (miBP) to approximate exposure to phthalates from personal care products (∑PCP). We used multivariable linear regression models to evaluate associations of specific gravity-adjusted ln-transformed phthalate metabolites or sums with one-year BMI change, and also considered whether associations differed depending on each woman’s menopausal status change from baseline to first follow-up.
Results:
At baseline, most women were premenopausal (67.8%), non-Hispanic white (67.9%), and college educated (65.8%). Overall, urinary phthalate metabolites or sums were not associated with one-year BMI change. Stratified analysis identified positive associations between ∑DEHP (and three of its metabolites: MEHP, MEHHP, and MEOHP) and one-year BMI change among women who transitioned from peri- to post-menopause from baseline to first follow-up. For example, in these women, with each doubling of ∑DEHP, BMI increased by 0.65 kg/m2 (95%CI: 0.17, 1.13) from baseline to first follow-up. Personal care product-associated phthalate metabolites (mBP and mEP) were negatively associated with one-year BMI change among women who remained perimenopausal from baseline to first follow-up, while miBP and mEP were positively associated with one-year BMI change among women who transitioned from peri- to post-menopause.
Conclusion:
We found the strongest associations between some phthalates and one-year BMI change in women who transitioned from peri- to post-menopause from baseline to first follow-up. This supports previous evidence that the menopausal transition is a hormonally sensitive period in women’s lives. To establish whether phthalate exposure contributes to body weight changes associated with the menopausal transition, substantially more research is needed to corroborate our findings.
Keywords: phthalates, endocrine disrupting chemicals, body mass index, weight, menopause, women
1. Introduction
The menopausal transition is marked by the cessation of menstruation with a concomitant decline in female sex hormones produced by the ovary (Hale et al., 2014). Because of these changes in hormone production and availability, menopause is considered a hormonally sensitive period (Hoyt and Falconi, 2015). Multiple deleterious health conditions have been specifically linked to the hormonal shifts that occur during the menopausal transition, including the onset of cardiovascular disease, cancer, and excessive weight gain (Hoyt and Falconi, 2015). Weight gain is associated with a number of health challenges in mid-life women, including cardiovascular diseases such as myocardial infarction and stroke (Eckel et al., 2018), depression (Mulugeta et al., 2018), dementia (Singh-Manoux et al., 2018), and many types of cancers, including postmenopausal breast and ovarian cancers (Lauby-Secretan et al., 2016).
Because the menopausal transition is a hormonally sensitive period marked by a drop in available estrogen, exposure to exogenous factors that further impact estrogen levels may contribute to dysregulated weight homeostasis and thus contribute to the development of chronic health conditions. This is because estrogen is an important component of appetite control, partly by increasing serum leptin levels (Fungfuang et al., 2013). Furthermore, adipose tissue contains both estrogen receptor (ER) α and ER β, which mediate the effects of estrogen on weight maintenance in women (Pallottini et al., 2008). Phthalates are ubiquitous, nonpersistent endocrine disrupting chemicals to which humans are exposed through ingestion, inhalation, and dermal absorption (National Research Council (US) Committee on the Health Risks of Phthalates)(2008; Fromme et al., 2007). Two important vehicles for human phthalate exposure are plastics used in food packaging/processing materials and personal care products (Giovanoulis et al., 2018; Husøy et al., 2019; Rudel et al., 2011). Animal models suggest that phthalate exposures impact weight regulation through multiple mechanisms (Blair et al., 2000; Feige et al., 2007; Schmidt et al., 2012), and phthalates are known to target estrogenic pathways (Gore et al., 2015). While the impact of phthalates on estrogen-mediated weight maintenance in women undergoing the menopausal transition is unknown, phthalates have been associated with weight gain in cross-sectional studies, though the associations are not consistent. A study in the National Health and Nutrition Examination Survey (NHANES) 1999–2002 found that mono-(2-ethylhexyl) phthalate (mEHP), a metabolite of di-(2-ethylhexyl) phthalate (DEHP), was inversely associated with weight among women age 20–59 (Hatch et al., 2008). However, a study in NHANES 2007–2010 noted a positive association between the sum of DEHP metabolites (∑DEHP) and obesity in adults, which appeared to be driven by the association in women (Buser et al., 2014). Similarly, a study of postmenopausal women found cross-sectional associations between ∑DEHP and obesity; specifically, postmenopausal women with ∑DEHP levels in the fourth quartile had 3.3 fold higher odds of obesity compared to postmenopausal women in the first quartile of ∑DEHP exposure (95% CI: 1.8, 6.0) (Díaz Santana et al., 2019). A different study that stratified by menopause status found no statistically significant association of urinary phthalate metabolites and general obesity (defined as a body mass index (BMI) >=25 kg/m2) or abdominal obesity in premenopausal and postmenopausal women, but the sample size of postmenopausal women was small (n=55) (Lim et al., 2020).
Longitudinal studies are a more robust approach for exploring exposure/outcome relationships and have also explored the association between phthalates exposure and weight change. One recent longitudinal study found an association with mono(2-ethyl-5-oxohexyl) phthalate (mEOHP), a metabolite of DEHP, and continuous change in weight, such that weight increased by 1.4 kg over three years in women who were in the highest compared to the lowest mEOHP quartile (95% CI: 0.1, 2.8) (Díaz Santana et al., 2019). The study also noted associations of monoethyl phthalate (mEP) and mono hydroxyisobutyl phthalate (mHiBP) with increasing weight (2.3 kg [95%CI: 0.9, 3.7] and 2.0 kg [95%CI: 0.6, 3.3] over three years for the highest compared to the lowest quartiles of mEP and mHiBP, respectively). Similarly, in women (but not men) over 70 years of age, monoisobutyl phthalate (miBP) was associated with higher waist circumference, total fat mass, and trunk fat mass two years later (Lind et al., 2012).
Although studies connecting phthalates to weight regulation across the female lifecycle are not entirely consistent, phthalates may be especially disruptive during periods of drastic hormonal change, though few have explored these association across the menopausal transition. (Banack et al., 2018; Sun et al., 2010) Therefore, our objectives were to 1) evaluate associations of phthalate exposures in pre- and peri-menopausal women with one-year change in BMI, and 2) determine if these associations differed by menopause status change from baseline to first follow-up visit. We chose to focus on change in BMI as our outcome because it yields correlations with cardiometabolic outcomes comparable to those of dual-energy x-ray absorptiometry, making it an easily obtainable measure of weight with utility in epidemiologic studies (Sun et al., 2010).
2. Methods
2.1. Recruitment of women into the Midlife Women’s Health Study
This study is a secondary analysis of data from the Midlife Women’s Health Study (MWHS), a longitudinal, population-based cohort study designed to evaluate the natural menopausal transition, which has previously been described elsewhere (Ziv-Gal et al., 2017). The original purpose of the MWHS was to identify factors that increase the risk of hot flash and the underlying physiological mechanisms of hot flashes (Ziv-Gal et al., 2017). From 2006–2015, women who were 45–54 years old and had not reached natural menopause were recruited from the Baltimore, Maryland metropolitan area to participate in the study. The majority of participants were recruited between 2008 and 2010. To be eligible, participants had to have intact uteri and ovaries and at least three menstrual periods in the previous 12 months (not be postmenopausal). Women who had a history of ovarian or uterine cancer, a hysterectomy, who were currently pregnant, or who were using hormonal or hormone-like supplements were excluded. Women were recruited by mail, and those who were interested in the study called the clinic for determination of eligibility. If eligible, women visited the clinic four times at one-week intervals in order to capture data across the menstrual cycle. Of the 2,507 women who responded to the letter, 780 were eligible and were enrolled in the study (31.1%) (Ziv-Gal et al., 2017). Eligible participants completed a baseline questionnaire and trained study staff measured each woman’s weight and height. Additionally, at each clinic visit, women gave samples of blood and urine. Annually, enrolled women completed the four consecutive clinic visits (including collection of blood and urine specimens) until they reached menopause or the study period ended. In total, the MWHS enrolled 780 women who gave informed consent using procedures approved by the Institutional Review Boards of the University of Illinois and Johns Hopkins University.
2.2. Assessment of one-year change in BMI
Our outcome of interest was one-year change in BMI from baseline to first follow-up visit of the study (referred to as change in BMI). Weight (pounds) and height (inches) were measured without shoes by trained clinic staff and were rounded to the nearest 0.5 pound or 0.5 inch, respectively. We multiplied height in inches by 0.0254 to convert to meters (m) and weight in pounds by 0.453592 to convert to kilograms (kg). We calculated BMI using weight and height measured by clinic staff at the first clinic visits at baseline and first follow-up approximately one year later using the following formula: kg/m2. Change in BMI was calculated by subtracting BMI at baseline from BMI at first follow-up.
2.3. Assessment of phthalates
Our exposures of interest were urinary phthalate concentrations measured at the baseline visit. Spot urine samples were collected up to four times at baseline and the subsequent annual visits for each participant, which were used for urinary phthalate metabolite assessment. Because phthalates have a short half-life in the body and there is substantial fluctuation in individual daily and weekly phthalate metabolite concentrations (Fromme et al., 2007; Johns et al., 2015a; Preau et al., 2010), a pool of several samples may better predict exposure to non-persistent chemicals like phthalates (Shin et al., 2019). Therefore, two- to- four urine specimens (per participant) from baseline were physically pooled for baseline phthalate assessment, and two- to- four urine specimens from the first follow-up visit cycle were pooled to assess first follow-up phthalate metabolites. Baseline urine pools were analyzed in 2018, and urine pools from first follow-up were analyzed in 2019. To account for urine dilution, specific gravity (SG) was measured for each pooled urine sample at the end of the study in samples that were thawed to room temperature and then immediately frozen before phthalate analyses as described below. Urinary phthalate metabolites have been shown to be stable across multiple freeze-thaw cycles given proper storage (Samandar et al., 2009).
The following phthalate metabolites were measured in the urine of MWHS participants: mono(2-ethyl-5- hydroxyhexyl) phthalate (mEHHP), mEOHP, mEHP, mono(2-ethyl-5-carboxypentyl) phthalate (mECPP), mCPP, mBzP, monobutyl phthalate (mBP), miBP, and mEP. Concentrations were reported in ng/mL. Phthalate metabolites were analyzed by the University of Illinois Roy J. Carver Metabolomics Center using methods adapted from those previously published by the CDC (Silva et al., 2007). Briefly, 200 µL of urine was mixed with 100 µL 10 mM ammonia formate, 10 µL 0.5 ug/mL internal standard mixture, 5 µL β-glucuronidase, and 10 µL 0.5 µg/mL 4-methylumbelliferone glucuronide. The mixture was incubated on a heat block at 37 °C for 90 minutes, followed by centrifugation for 5 minutes at 8, 000 rpm. The resulting supernatant was processed using solid-phase extraction (SPE) with Phenomenex Strata-X-A 33 µm cartridges (30 mg/3 mL) and acidic methanol to elute. The collected elution was dried completely with a SpeedVac and reconstituted into 20% acetonitrile solution (with 0.1 % formic acid) before instrument injection. Samples (10 μL) were analyzed using isotope dilution high-performance liquid chromatography negative-ion electrospray ionization-tandem mass spectrometry (HPLC-MS/MS) using the 5500 QTRAP LC/MS/MS system (Sciex, Framingham, MA) and the 1200 series HPLC system (Agilent Technologies, Santa Clara, CA). Data acquisition and analysis of the samples used Software Analyst 1.7.1. The 1200 series HPLC system (Agilent Technologies, Santa Clara, CA) includes a degasser, an autosampler, and a binary pump. The LC separation was performed on a Phenomenex (Torrance, CA) Gemini C6-phenyl column (2 × 100 mm, 3 μm) with mobile phase A (0.1 % formic acid in water) and mobile phase B (0.1 % formic acid in acetontrile). The flow rate was 0.25 mL/min. The linear gradient was as follows: 0–1 min, 70 % A; 10–15 min, 50 % A; 20 min, 10 % A; 21–27 min, 70 % A. The autosampler was set at 10°C. Mass spectra were acquired under negative electrospray ionization (ESI) with the ion spray voltage of −4500 V and source temperature of 500 °C. The curtain gas, ion source gas 1, and ion source gas 2 were 32, 50, and 65 psi, respectively. Multiple reaction monitoring (MRM) was used for quantitation using transitions specified in Supplement Table S1. To evaluate the enzyme β-glucuronidase activity, 4-methylumbelliferone was monitored at m/z 175.0 --> m/z 133.0 with 13C4-MEP as the internal standard (m/z 197.0 --> m/z 79.0).
Concentrations of phthalate metabolites that were lower than the limit of detection (LOD) or were undetectable were assigned a value of LOD/√2 in analyses. We adjusted urinary phthalate metabolite concentrations for SG using the following formula: Pc = P[(1.018 − 1)/( SGi − 1)], where Pc is the specific gravity adjusted phthalate metabolite concentration, P is the measured phthalate metabolite concentration (ng/mL), 1.018 is the median SG of MWHS population, and SGi is the specific gravity of each pooled urine sample (Meeker et al., 2009). We used the molar sum (nmol/mL) of the following SG-adjusted metabolites to represent exposure to the parent compound DEHP by dividing urine concentrations of each metabolite (ng/mL) by their molar weights: (∑DEHP = (mEHP/278) + (mEHHP/294) + (mEOHP/292) + (mECPP/308). We also created molar sums of SG-adjusted metabolites to represent exposure to all plastic-associated phthalates (∑Plastic = ∑DEHP + (mCPP/252) + (mBzP/256)) and personal care product-associated phthalates (∑PCP = (mBP/222) + (miBP/222) + (mEP/194)). SG-adjusted concentrations (ng/mL) of mCPP, mBzP, mBP, miBP, and mEP were used to approximate exposures to DOP, BBzP, DBP, DiBP, and DEP, respectively. We considered P-values less than or equal to 0.05 as statistically significant (Rothman, 1990). This study includes analyses for baseline to first follow-up only because chemical analyses had not yet been completed for visits beyond the first follow-up.
2.4. Collection of data to evaluate effect modification by menopause status change
Our second objective was to evaluate whether change in menopause status from baseline to first follow-up modifies associations of phthalates with change in BMI. Women were categorized as pre- or perimenopausal at baseline and at first follow-up according to the timing and number of menstrual periods they had experienced in the preceding year. Women were classified as premenopausal if they had at least one menstrual period in the last three months and reported 11 or more menstrual periods in the previous year. Women were classified as perimenopausal if they had their last menstrual period in the last year but not the previous three months or if they had experienced a menstrual period in the last three months but had ten or fewer menstrual periods in the last year. Women were classified as postmenopausal if they had not had a menstrual period in the past 12 months using guidance from the Stages of Reproductive Aging Workshop +10 (Harlow et al., 2012). To categorize change in menopause status, we classified women from baseline to first follow-up as those who remained in premenopause, those who remained in perimenopause, those who transitioned from pre- to perimenopause, or those who transitioned from peri- to postmenopause.
2.5. Collection and reporting of covariate information
We selected covariates a priori based on literature review (age, race and ethnicity, education, alcohol use, smoking status, family income) (Díaz Santana et al., 2019; Hatch et al., 2008; James-Todd et al., 2016) and based on important predictors of urinary phthalate metabolite concentrations in the MWHS cohort (marital status and diagnosis of depression). We specified our assumptions about relationships using a directed acyclic graph (DAGitty version 3.0) (Greenland et al., 1999; Textor et al., 2017) (Supplemental Figure S1). Covariate data were collected at baseline using a self-administered questionnaire that included questions about socio-demographic information, lifestyle habits, and health conditions. Questionnaires were checked for completeness by clinic staff.
2.6. Phthalate metabolites in the NHANES 2005–2016
We downloaded NHANES data files for waves 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016, which roughly align with the MWHS study recruitment period (available at https://wwwn.cdc.gov/nchs/nhanes/) to compare our study population’s phthalate metabolite concentrations to those of a nationally representative sample of women age 45–54. We examined numerical differences in phthalate metabolite concentrations between the MWHS and the NHANES but did not test for statistical differences in concentrations between the two studies.
2.7. Statistical analyses
To be included in this study, women 1) had to be pre- or perimenopausal at baseline, had to have 2) phthalate metabolites measured at baseline, 3) height and weight data for the baseline visit and for first follow-up visit, and 4) complete data for covariates included in models. Of the 780 participants, 197 did not have first follow-up data available. Of the remaining 583 women, two were excluded for missing first follow-up menopause status, and six were excluded for missing SG measures. After careful review of the data, we observed 21 women’s menopause status changed to an earlier stage (for example they were classified as perimenopausal at baseline and premenopausal at first follow-up). These 21 participants were excluded for likely misclassification of menopause stage. Additionally, 27 women did not have complete covariates for analysis, and three were excluded for implausible changes in BMI from baseline to first follow-up (10 – 17 kg/m2), likely due to height or weight mis-recording. Therefore, the final analysis included 524 women who met all inclusion criteria.
We used SAS 9.4 (SAS Institute, Cary, NC) for statistical analyses, and R Studio 1.2.5033 (RStudio Team, Boston, MA) for figure generation. We described the sample using median (range) and frequencies (percentages). To describe phthalate concentrations in our population, we calculated the geometric means of unadjusted phthalate metabolites at baseline for the MWHS and for the NHANES using SAS PROC SURVEYMEANS and did not SG-adjust urinary phthalate metabolites in order to make our data comparable to NHANES. Selected covariates ascertained at baseline included menopause status (pre- to premenopause (reference), pre- to perimenopause, peri- to perimenopause, peri- to postmenopause), age (45–47 (reference), 48–51, 52–54 years), race and ethnicity (non-Hispanic white (reference), non-Hispanic black, and Hispanic and other or mixed race groups), family income (less than $50,000 per year, $50,000 to less than $80,000 per year, $80,000 to less than $100,000 per year, and $100,000 per year or higher (reference)), education (some college or less, college graduate or higher (reference)), current use of alcohol (none (reference), occasional, regular), current smoking status (current, former, never (reference)), marital status (married/living with partner (reference), single/divorced/widowed), and depression (ever diagnosed, never diagnosed (reference)).
We used multivariable linear regression models to characterize the association between individual urinary phthalate metabolite concentrations or sums, and the change in BMI from baseline to first follow-up. Urinary phthalate metabolite concentrations and sums were used as continuous variables and were SG-adjusted and natural log transformed (ln-transformed). We reported associations as the change in BMI for each doubling of urinary phthalate metabolite concentrations or sums. We back-calculated the change in BMI for each doubling of urinary phthalate metabolite using the following equation: transformed estimate = β-estimate*(log(2.00).
To evaluate associations of phthalate exposures in pre- and peri-menopausal women with one-year change in BMI, we generated models that tested for a linear association between phthalate metabolite concentrations or sums and change in BMI for all eligible participants. We also generated models in which the urinary phthalate metabolites or sums were categorized in quartiles to check for non-linear (non-monotonic) relationships of phthalates with change in BMI. We controlled for the covariates listed previously. To determine if these associations differed by change in menopause status, we a priori stratified the analysis by change in menopause status from baseline to first follow-up. We also generated linear regression models with an interaction term for each metabolite and menopause status to test if the differences in slope were statistically significant. All models included the covariates used in the models for objective one, except menopause status, which was accounted for by stratification on menopause status.
In sensitivity analyses, we evaluated the above relationships after concurrently accounting for phthalate exposure at first follow-up. We first tested correlations between urinary phthalate metabolite concentrations and sums measured at baseline and at first follow-up for the 516 women with complete phthalate data in both years. We generated a second set of linear models for objective one (evaluating associations of baseline urinary phthalate metabolites and sums with change in BMI) that additionally controlled for first follow-up urinary phthalate metabolite concentrations. We also generated models that used percent change in BMI as an outcome, and models that excluded participants who had urinary phthalate metabolite concentrations below the LOD. Finally, we tested models that used the covariate age as a continuous variable and models that excluded depression as a covariate.
3. Results
3.1. Characteristics of MWHS participants
The majority of women (76.5%, n= 401) were 50 years of age or younger at baseline (Table 1). Almost 70% (n= 356) reported their race/ethnicity as non-Hispanic white, while 28.3% (n= 148) reported being non-Hispanic black. A small percentage of women reported their race and ethnicity in a category other than non-Hispanic white and non-Hispanic black (n = 20). About 66% (n = 345) of women reported an educational attainment of a college degree or higher. Alcohol use at baseline was evenly distributed between never, occasional, and regular drinkers. Most women never smoked cigarettes in their lifetime (55%, n = 291). Almost 70% (n= 353) of women were married or living with a partner at baseline, and almost 70% (n= 371) of women had never been diagnosed with depression. Household income was high, with the majority of women living in a household with an income of $100,000 per year or greater (n= 239). Full characteristics of the 516 women included in the sensitivity analyses that additionally controlled for phthalate levels at first follow-up can be found in Supplement Table S2.
Table 1.
Baseline demographic and lifestyle characteristics of women enrolled in the Midlife Women’s Health Study.
| Demographic or lifestyle characteristics | n (%)1 |
|---|---|
| Age at baseline | |
| 45–47 | 217 (41.4) |
| 48–50 | 184 (35.1) |
| 51–54 | 123 (23.5) |
| Race and Ethnicity | |
| White | 356 (67.9) |
| Black | 148 (28.2) |
| Other2 | 20 (3.8) |
| Education level at baseline | |
| Some college/technical school or less | 179 (34.2) |
| College graduate or higher | 345 (65.8) |
| Use of alcohol at baseline | |
| Never | 165 (31.5) |
| Occasional | 156 (29.8) |
| Regular | 203 (38.7) |
| Smoking status at baseline | |
| Current | 44 (8.4) |
| Former | 189 (36.1) |
| Never | 291 (55.5) |
| Marital Status at baseline | |
| Married/ living with partner | 353 (67.4) |
| Single/Widowed/Divorced | 171 (32.6) |
| Diagnosis of depression at baseline | |
| Yes | 153 (29.2) |
| No | 371 (70.8) |
| Household income at baseline | |
| <$50,000 | 106 (20.2) |
| $50,000 to <$100,000 | 179 (34.2) |
| $100,000 or higher | 239 (45.6) |
| BMI at baseline3 | |
| Underweight/Normal | 213 (40.7) |
| Overweight | 139 (26.5) |
| Obese | 172 (32.8) |
| BMI at first follow-up3 | |
| Underweight/Normal | 211 (40.3) |
| Overweight | 147 (28.1) |
| Obese | 166 (31.7) |
| Change in Menopause Status | |
| Pre-to-Premenopause | 290 (55.3) |
| Pre-to-Perimenopause | 65 (12.4) |
| Peri-to-Perimenopause | 109 (20.8) |
| Peri-to-Postmenopause | 60 (11.5) |
| Change in BMI from baseline to first follow-up visit (median, range) | 0.09 (−6.1, 6.5) |
May not add to 100% due to rounding
Other includes Hispanic/Latina ethnicity, Asian, and other races/ethnicities specified by participants including multiple race groups
BMI categories: Underweight: <18.5 kg/m2, Normal weight: 18.5 - <25 kg/m2, Overweight: 25 - <30 kg/m2, Obese: 30 kg/m2 or higher
Abbreviations: BMI, body mass index
The majority of women included in the current study were premenopausal at baseline and remained in premenopause at first follow-up (55.3%, n= 290), and 20.8% (n= 109) started the study in perimenopause and remained in perimenopause at first follow-up (Table 1). About 12% (n= 65) of the sample transitioned from premenopause at baseline to perimenopause at first follow-up, and 11.5% (n= 60) transitioned from perimenopause at baseline to postmenopause by first follow-up (Table 1).
3.2. Change in BMI from baseline to first follow-up
The distribution of BMI stayed stable from baseline to follow-up, with about 40% of women in the under/normal weight category, 26–27% of women in the overweight category, and 32–33% of women in the obese category. The median change in BMI from baseline to follow-up was 0.09 kg/m2, indicating weight was generally stable (Table 1).
3.3. Geometric means of urinary phthalate metabolites in the MWHS and NHANES
Although we did not test for statistical differences, urinary phthalate metabolite concentrations in MWHS were numerically higher than those in NHANES 2005–2016 (Table 2, Supplemental Figure S2). Geometric means of urinary phthalate metabolite concentrations in MWHS changed little from baseline to first follow-up (first follow-up concentrations can be found in Supplemental Table S3, Supplemental Figure S3). Correlations between urinary phthalate metabolite concentrations from baseline to first follow-up were moderate (range of r values: 0.32–0.68), except for mCPP, which showed weak correlation from baseline to first follow-up (Supplemental Table S4).
Table 2.
Geometric Means (GM) of phthalate concentrations (ng/mL) in Midlife Women’s Health Study participants.
| Parent Compound | Metabolite | MWHS LOD (ng/mL) | % Above LOD | Baseline GM (95% CI) (ng/mL) | NHANES 2006–15 GM (95% CI) (ng/mL) |
|---|---|---|---|---|---|
| DEHP | mEHP | 0.2 | 100% | 5.2 (4.8, 5.7) | 1.6 (1.4, 1.8) |
| mEHHP | 0.05 | 100% | 34.0 (31.6, 36.5) | 9.7 (8.4, 11.2) | |
| mEOHP | 0.05 | 100% | 13.3 (12.3, 14.3) | 5.9 (5.1, 6.8) | |
| mECPP | 0.1 | 100% | 28.5 (26.4, 30.7) | 14.6 (12.7, 16.8) | |
| DOP | mCPP | 0.02 | 100% | 2.9 (2.7, 3.2) | 1.5 (1.3, 1.7) |
| BBzP | mBzP | 0.01 | 100% | 9.8 (9.1, 10.5) | 4.2 (3.7, 4.9) |
| DBP | mBP | 0.02 | 100% | 21.2 (19.9, 22.7) | 11.3 (9.8, 12.9) |
| DiBP | miBP | 0.05 | 100% | 16.6 (15.6, 17.7) | 5.5 (4.9, 6.2) |
| DEP | mEP | 0.01 | 99.8% | 99.0 (89.4, 109.5) | 63.0 (54.3, 73.0) |
Abbreviations: CI, confidence interval; ∑DEHP, sum of DEHP metabolites; LOD, limit of detection; mBP, monobutyl phthalate; mBzP, monobenzyl phthalate; mono(3-carboxypropyl) phthalate (mCPP), mEP, monoethyl phthalate; mECPP, mono(2-ethyl-5-carboxypentyl), mEHP, mono-(2-ethylhexyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; phthalate; miBP, monoisobutyl phthalate; NHANES, National Health and Nutrition Examination Survey.
Phthalate concentrations have not been specific gravity adjusted.
MWHS n = 524, NHANES n = 919
3.4. Overall associations of urinary phthalate concentrations with change in BMI
Table 3 presents results from overall adjusted linear regression models for associations of individual urinary phthalate metabolites or sums with change in BMI from baseline to first follow-up. Model 1 included the baseline urinary phthalate metabolites described above, while model 2 was additionally adjusted for first follow-up phthalate metabolite concentrations. Generally, the overall associations of baseline urinary phthalate metabolites or sums with one-year change in BMI were null and controlling for phthalate concentrations at first follow-up changed the results minimally (Table 3). Analyses where phthalates were assessed in quartiles of exposure also yielded null results (Supplemental Table S5). The results were unchanged when we used percent change in BMI as the outcome, when we excluded women whose urinary phthalate metabolites were less than the LOD, when we used age as a continuous variable, and when we excluded depression as a covariate (data not shown).
Table 3.
Associations of urinary phthalate metabolites and molar sums with change in BMI from baseline to first follow-up visit in Midlife Women’s Health Study participants.
| Phthalate Metabolite/Molar Sum | Model 1: β (95% CI) | P | Model 2: β (95% CI) | P |
|---|---|---|---|---|
| mEHP | −0.01 (−0.10, 0.08) | 0.84 | 0.02 (−0.08, 0.12) | 0.71 |
| mEHHP | 0.002 (−0.12, 0.12) | 0.98 | 0.05 (−0.08, 0.17) | 0.47 |
| mEOHP | 0.01 (−0.09, 0.12) | 0.80 | 0.06 (−0.05, 0.17) | 0.31 |
| mECPP | 0.001 (−0.10, 0.11) | 0.99 | 0.06 (−0.06, 0.17) | 0.33 |
| ∑DEHP | −0.001 (−0.12, 0.11) | 0.98 | 0.05 (−0.07, 0.18) | 0.41 |
| mCPP | 0.03 (−0.05, 0.12) | 0.43 | 0.04 (−0.05, 0.12) | 0.36 |
| mBzP | −0.01 (−0.12, 0.09) | 0.78 | 0.03 (−0.11, 0.17) | 0.70 |
| ∑Plastic | 0.01 (−0.12, 0.13) | 0.90 | 0.06 (−0.07, 0.19) | 0.37 |
| mBP | 0.02 (−0.11, 0.15) | 0.78 | 0.02 (−0.13, 0.17) | 0.79 |
| miBP | −0.004 (−0.13, 0.12) | 0.95 | −0.03 (−0.18, 0.13) | 0.71 |
| mEP | −0.03 (−0.11, 0.05) | 0.52 | −0.01 (−0.10, 0.07) | 0.95 |
| ∑PCP | −0.03 (−0.14, 0.07) | 0.52 | −0.03 (−0.14, 0.09) | 0.65 |
Model 1: adjusted for menopause status, age, race/ethnicity, family income, education, current use of alcohol, current smoking status, marital status, and depression diagnosis. n = 524
Model 2: adjusted for the variables in Model 1 and firs follow-up visit phthalate metabolite or molar sum concentrations. n = 516.
Phthalate metabolites and molar sums were SG adjusted and ln-transformed for analysis. β estimates were transformed to represent a one-unit change in BMI for each doubling of phthalates.
Abbreviations: BMI, body mass index; CI, confidence interval; ΣDEHP, sum of DEHP metabolites; mBP, monobutyl phthalate; mBzP, monobenzyl phthalate; mono(3-carboxypropyl) phthalate (mCPP), mEP, monoethyl phthalate; mECPP, mono(2-ethyl-5-carboxypentyl), mEHP, mono-(2-ethylhexyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; phthalate; miBP, monoisobutyl phthalate
ΣPCP, sum of phthalate metabolites of personal care products; SG, specific gravity.
ΣDEHP = molar sum of mEHP, mEHHP, mEOHP, and mECPP
ΣPlastic = molar sum of mEHP, mEHHP, mEOHP, mECPP, mCPP, and mBzP
ΣPCP = molar sum of mBP, miBP, and mEP
3.5. Associations of urinary phthalate concentrations with change in BMI stratified by menopause status
In stratified models, several phthalate metabolites or their sums were differentially associated with change in BMI depending on menopause status transition from baseline to first follow-up (Figure 1 and Supplemental Table S6, which includes Model 1 Pinteraction). For example, a doubling of ∑DEHP was associated with a 0.65 kg/m2 (95%CI: 0.17, 1.13) increase in BMI among women who had transitioned from peri-to postmenopause from baseline to first follow-up (n = 60); similar results were observed for ∑Plastic. DEHP metabolites mEHHP and mEOHP were associated with change in BMI only in women who transitioned from peri- to postmenopause, where each doubling of mEHHP and mEOHP was associated with 0.75 kg/m2 (95%CI: 0.29, 1.22) and 0.48 kg/m2 (95%CI: 0.05, 0.90) increase in BMI, respectively. The other two DEHP metabolites, mEHP and mECPP, demonstrated smaller associations with change in BMI only among women who had transitioned from peri- to post-menopause, and the confidence intervals crossed the null.
Figure 1. Associations of urinary phthalate molar sums and metabolites with change in body mass index (BMI) from baseline to first follow-up visit, stratified by menopause status.
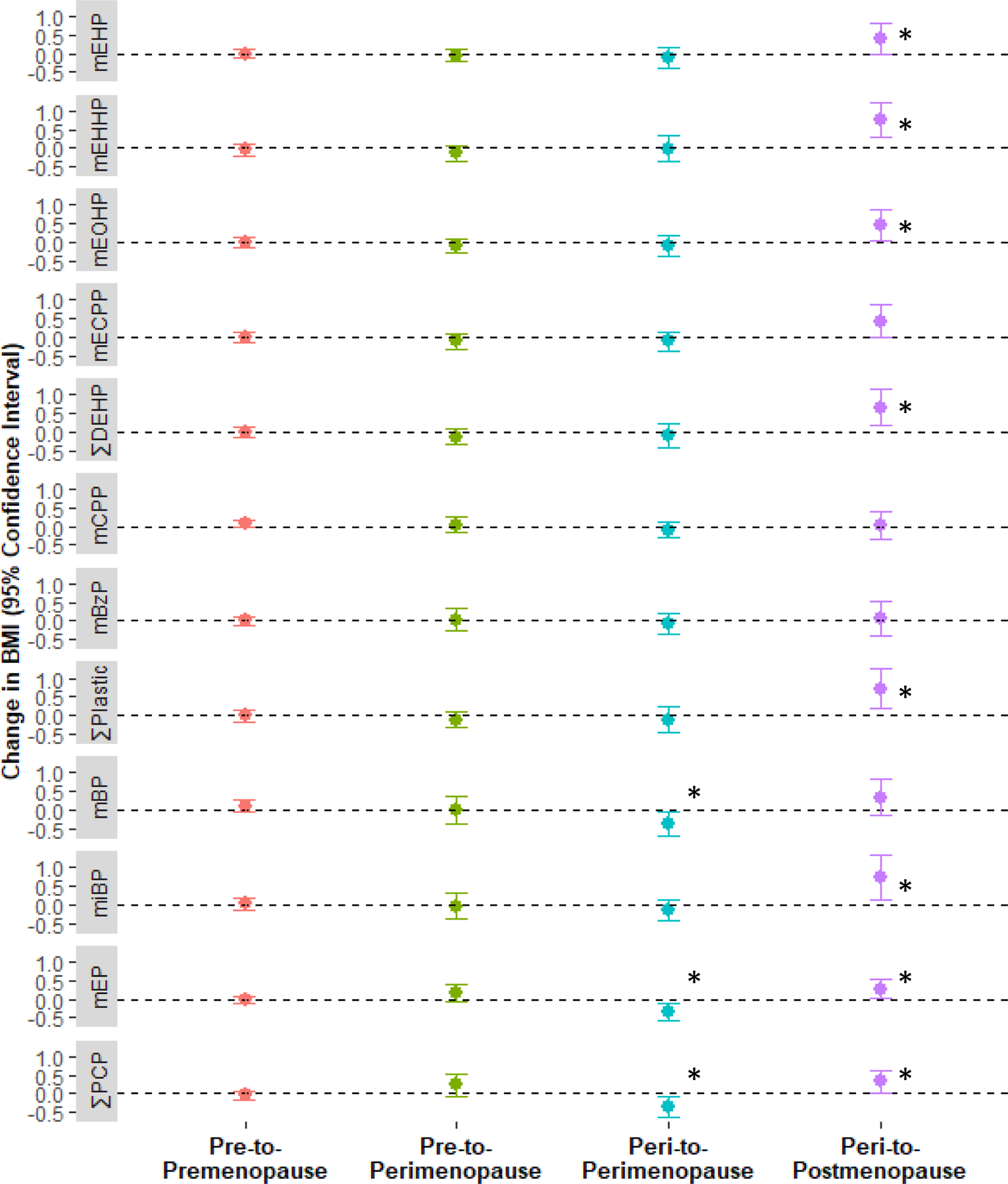
Linear regression models evaluated the associations between log-transformed and specific gravity adjusted urinary phthalate metabolites or molar sums and one-year change in BMI (kg/m2). Beta estimates were transformed to express an estimate of the change in BMI for each doubling of urinary phthalate concentration or molar sums. Results are presented as a point estimate of the change in BMI (filled circle) and the 95% confidence intervals (solid lines) for the adjusted models. Analyses were stratified by menopause status and control for age, race/ethnicity, family income, education, current use of alcohol, current smoking status, marital status, and depression diagnosis. Significant associations (P ≤ 0.05) are indicated by an asterisk (*). Abbreviations: BMI, body mass index; CI, confidence interval; ∑DEHP, sum of DEHP metabolites; mBP, monobutyl phthalate; mBzP, monobenzyl phthalate; mono(3-carboxypropyl) phthalate (mCPP), mEP, monoethyl phthalate; mECPP, mono(2-ethyl-5-carboxypentyl), mEHP, mono-(2-ethylhexyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; phthalate; miBP, monoisobutyl phthalate; ∑PCP, sum of phthalate metabolites of personal care products; SG, specific gravity.
∑DEHP = molar sum of mEHP, mEHHP, mEOHP, and mECPP
∑Plastic = molar sum of mEHP, mEHHP, mEOHP, mECPP, mCPP, and mBzP
∑PCP = molar sum of mBP, miBP, and mEP
n = 524
Patterns of associations for PCP-associated phthalate metabolites and sum appeared to depend on menopause transition status from baseline to first follow-up (Figure 1 and Supplemental Table S6). Among women who remained perimenopausal from baseline to first follow-up, each doubling of mBP and mEP was associated with a 0.36 kg/m2 (95% CI: −0.67, −0.05) and 0.33 kg/m2 (95%CI: −0.56, −0.10) decrease in BMI, respectively. However, among women who transitioned from peri- to postmenopause, a doubling in mEP and miBP was associated with a 0.27 kg/m2 (95% CI: 0.01, 0.53) and 0.73 kg/m2 (95% CI: 0.15, 1.32) increase in BMI, respectively. Additionally, a doubling in ∑PCP was associated with a 0.35 kg/m2 (95% CI:−0.61, −0.08) decrease in BMI among women who remained perimenopausal from baseline to first follow-up, while it was associated with a 0.32 kg/m2 (95% CI: 0.02, 0.63) increase in BMI among women who transitioned from peri- to postmenopause. The addition of first follow-up visit urinary phthalate metabolites to these models yielded similar results, with the exception of ∑PCP, which had an attenuated effect estimate (Supplemental Table S6).
4. Discussion
Overall, we found no association of baseline urinary phthalate metabolites with change in BMI one year later in a cohort of women who were pre- or perimenopausal at the start of the study. However, in women who transitioned from peri- to postmenopause from baseline to first follow-up, we found that ∑Plastics, ∑DEHP (and its metabolites mEHHP and mEOHP), ∑PCP, miBP, and mEP were significantly associated with increasing BMI. However, among women who remained perimenopausal from baseline to first follow-up, ∑PCP, mBP, and mEP were significantly associated with decreasing BMI. These data contribute to the body of evidence associating phthalates with weight change in older women, and particularly point to the menopausal transition as a period sensitive to potential endocrine disruption by phthalates.
4.1. Differences in urinary phthalate metabolite concentrations in the MWHS and the NHANES
The geometric means of the urinary phthalate metabolites measured in the MWHS women were higher than the geometric means of women age 45–54 in the NHANES (2005–2016). However, the range of the metabolite concentrations in the two studies overlapped, which suggests that the distributions may be more similar than the geometric means alone indicate. There are several possibilities for the differences between the geometric means of the MWHS and the NHANES. One possibility is the heaviest recruiting for MWHS took place early (2008–2010), and the detection of many phthalate metabolites in NHANES is declining over time (National Research Council (US) Committee on the Health Risks of Phthalates, 2019). When we calculated geometric means by year of recruitment in the MWHS, we also saw a decline in many of the concentrations (data not shown). Additionally, our cohort used multiple pooled urine samples to quantify phthalate metabolites, while the NHANES only used a single spot urine.
4.2. Null overall associations of urinary phthalate concentrations with BMI change
In the current study, we did not observe overall associations of urinary phthalate metabolites or sums with BMI change, which is inconsistent with previous cross-sectional studies in mid-life women. A study of the NHANES 1999–2002 waves found that among women 20–59 years of age, as mEHP quartile increased, the adjusted mean BMI decreased (p-trend: 0.02) (Hatch et al., 2008), whereas, an analysis of the NHANES 2007–2010 waves noted that the odds of obesity were 1.7 times higher among females with ∑DEHP metabolite concentrations in the fourth compared to the first quartile (Buser et al., 2014). However, cross-sectional associations between exposures to phthalates like DEHP and measures of adiposity are difficult to interpret because people with high body weight also likely have higher energy intake compared to people with lower body weight. Higher energy intake may increase exposure to plasticizers like DEHP because such plasticizers are often found in food processing and packaging materials (Campbell et al., 2018). It is important to note that both of the previous studies did not focus on the menopause transition and included adults age 20 years and older, which is a substantially different target population compared to our study, which recruited women approaching the menopause transition whose ages fell into a narrow range of 45–54 years.
In contrast to our findings, other longitudinal studies have found phthalate exposures are associated with weight change. One longitudinal study of postmenopausal women found that three years after baseline, mEOHP was associated with a 1.4 kg (95%CI: 0.1, 2.8) increase in weight among women in the highest compared to the lowest quartile (Díaz Santana et al., 2019). The study also noted that mEP and mHiBP were associated with 2.3 kg (95%CI: 0.9, 3.7) and 2.0 kg (95%CI: 0.6, 3.3) weight increases over three years for the highest compared to lowest quartiles of mEP and mHiBP, respectively. The study found no associations between phthalate exposures at baseline and weight change six years after baseline (Díaz Santana et al., 2019). Key differences that may have contributed to incongruencies between this study and ours include the timeframes of weight ascertainment (3 years versus 1 year in our study) and the stage of menopause at recruitment (postmenopause versus to pre-and peri-menopause in our study). In a study of adults 70 years of age or older, miBP at baseline was positively associated with waist circumference, total fat mass, and trunk fat mass 2 years later (Lind et al., 2012). Important differences again include the time frames of weight ascertainment and the age at enrollment (70 years versus to 45–54 years in the present study). Additional differences include the types of body composition measures utilized in their study versus ours. The study by Lind et al. used dual X-ray absorptiometry and abdominal MRI to assess changes in fat mass, whereas we were only able to evaluate BMI. Our null overall results also contrast with a study nested in the Nurses’ Health Study (NHS) and NHSII, which found positive, longitudinal associations of phthalic acid, mBzP, the sum of butyl phthalates, total phthalate metabolite burden with bi-annually measured weight gain among the women enrolled in the study (Song et al., 2014). The estimates of annual weight change (kg/year) suggested bigger changes compared to our study that used BMI as a measure of weight change. For example, Song et al. found a monotonic increase in weight change across quartiles of mBzP, ranging from an additional 0.29 kg/year in the second quartile to 0.43 kg/year in the fourth quartile compared to the first (Song et al., 2014), whereas in our study, mBzP was not associated with one-year change in BMI. The study by Song et al. also found monotonic increases in weight according to quartile of the sum of butyl phthalate metabolites mBP and miBP, whereas we noted no associations of mBP or miBP with one-year BMI change. Important differences between the MWHS and the NHS and NHSII include the anthropometric measure used (weight in kg versus BMI kg/m2), the participants age ranges (NHS: 30–55 years and NHSII: 25–42 years versus our study: 45–54 years), follow-up period (2 years versus 1 year in our study), urine collection (one first morning void sample versus to 2–4 spot samples over a single menstrual cycle). It is also likely that the number of women in our study who transitioned to postmenopause was too small to observe a shift in weight across the entire sample, and that the span of one year was too small to see substantial changes in BMI.
4.3. Associations of phthalates with change in BMI differed by menopause transition status
Although our overall findings were null, we did find evidence that change in menopause status across the follow-up period (baseline to first follow-up) appeared to modify associations between urinary phthalate metabolites or sums and change in BMI. Positive associations of several phthalate metabolites or sums with BMI change among women who transitioned from peri- to postmenopause suggest that this period of hormonal change may be especially sensitive to phthalate exposure. Phthalates interact with multiple pathways that regulate body weight, including steroid hormone, thyroid hormone, and peroxisome proliferator activated receptor pathways (Johns et al., 2016; Johns et al., 2015b; Kratochvil et al., 2019; Kusu et al., 2008; Maloney and Waxman, 1999; Romano et al., 2018). However, our findings that the impacts of phthalates on BMI change were limited to women who were approaching or experiencing the menopausal transition suggests hormonal changes associated with the menopause transition may be a key pathway to explore. Although the mechanisms underlying our findings are not well-understood, the decline in sex hormones, particularly estrogen, associated with menopause places women at an increased risk for weight gain during the transition from peri- to postmenopause (Guthrie et al., 1999; Hale et al., 2014; Melanson et al., 2015; Montazeri et al., 2019). As previously discussed, phthalates have been shown to modulate estrogen signaling through ERs (Blair et al., 2000; Chan Kang and Mu Lee, 2005; Engel et al., 2017; Takeuchi et al., 2005). Additional mechanistic studies are needed to establish what makes peri- and postmenopausal women especially susceptible to the actions of phthalates, though evidence in humans suggests that ER expression and activity may change after the menopausal transition. In a study of pre- and postmenopausal women, gene expression levels of ERα and the ratio of ERα to ERβ were lower in postmenopausal women compared to premenopausal women (Park et al., 2017). Given the critical role of ERs for weight regulation in women (Heine et al., 2000; Miao et al., 2016; Pallottini et al., 2008), it is possible the actions of phthalates on weight regulation actively change as ER expression changes throughout a woman’s life, which supports findings from the current study.
4.4. Limitations and strengths of the current study
This study has several limitations. The sample size was limited for the stratified analysis as not many women transitioned from their baseline menopause status in one year’s time. However, the stratified analysis still yielded robust results. We cannot rule out confounding by changes in personal habits that may be associated with both baseline phthalate levels and change in BMI (e.g. diet), though if there was a change in a confounding habit, we would expect this to be reflected in the geometric means of urinary phthalate metabolite concentrations from baseline to first follow-up. However, there is little evidence that phthalate exposure changed from baseline to first follow-up in our sample, with the exception of one metabolite of DEHP, mECPP. While BMI correlates with measures of adiposity-related conditions such as blood pressure and cholesterol (Sun et al., 2010), future studies evaluating more precise measures of adiposity (e.g. waist circumference, body composition assessment) may be needed to better characterize phthalates’ mode of action in human populations. The focus on phthalate exposures and the use of the single pollutant method of analysis may inflate the risk of a type I error and does not account for the milieu of chemicals to which people are exposed, including chemicals such as phenols (Kapraun et al., 2017). Given that this is an exploratory study, we chose not to adjust the type I error for multiple comparisons (Rothman, 1990), and note that future studies should consider using multi-pollutant models that can accommodate correlated metabolites and other pollutants associated with weight regulation. Diet quality and eating habits may influence both baseline phthalate exposure and change in BMI; unfortunately, we lack a measure of diet in this cohort. Additionally, women who were perimenopausal at baseline and women whose family income was less than $50,000 per year were more likely to have incomplete data at the first follow-up. The loss of women who were perimenopausal may lead to bias and results should be interpreted with caution.
This study also has several strengths. The MWHS is a large, prospective cohort study. Because of the large sample size at baseline, we were powered to follow women from baseline to first follow-up, which allowed us to leverage the baseline measurement of urinary phthalate metabolites and the longitudinal measure of change in BMI, which eliminates concern of reverse causation. Additionally, the longitudinal design of the MWHS allowed us to evaluate women as they transitioned through menopause, which is rare. Evaluating change in BMI as our outcome allowed us to specifically assess weight gain, rather than a single measure of weight at first follow-up. The women who had urinary phthalate metabolites analyzed at baseline only (n = 524) were also very similar to the women who urinary phthalate metabolites analyzed at both baseline and first follow-up (n =516). Finally, urinary phthalate metabolites were measured in pools of two- to- four spot urine samples for each participant, which provided a more reliable measured of typical phthalate exposure compared to a single spot urine (Fromme et al., 2007; Johns et al., 2015a; Preau et al., 2010).
5. Conclusion
Our findings suggest that exposure to some phthalates in midlife women may be associated with weight change, which also differs by change in menopausal status. Specifically, we observed that DEHP exposure may be associated with increasing BMI among women who transition from peri- to postmenopause. Exposure to personal care product phthalates was associated with a downward change in weight among women who remained perimenopausal but an upward change in weight among women who transitioned from peri- to postmenopause. Further research confirming our findings in a cohort with a larger number of women undergoing natural menopause is necessary, but this study is an important step for establishing the menopausal transition as a sensitive window for phthalate exposure.
Supplementary Material
Figure S1. Directed Acyclic Graph Directed acyclic graph of the assumptions underlying the multiple linear regression models. Abbreviations: BMI, body mass index. Figure was generated using DAGitty version 3.0 (www.dagitty.net). Green nodes indicate exposure. Blue nodes indicate outcome. White nodes indicate variables that were used for adjustment in models. Gray nodes indicate unmeasured variables. Green paths indicate the exposure-outcome path. Black paths indicate controlled paths in the expected relationships.
Figure S2. Boxplots of Urinary Phthalate Metabolites at Baseline for 45–54 Year Old Women in the Midlife Women’s Health Study (2006–2015) and in the National Health and Nutrition Examination Survey (2005–2016) Box plots display the median (dark horizontal line), 25th and 75th percentiles (box) and range (vertical line) for urinary phthalate metabolites in the Midlife Women’s Health Study (n = 524) and the NHANES waves 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016 (n = 919). NHANES metabolites are for women who were between the ages of 45 and 54. Urinary phthalate metabolite concentrations were not adjusted for specific gravity or creatinine. Abbreviations: mBP, monobutyl phthalate; mBzP, monobenzyl phthalate; mono(3-carboxypropyl) phthalate (mCPP), mEP, monoethyl phthalate; mECPP, mono(2-ethyl-5-carboxypentyl), mEHP, mono-(2-ethylhexyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; phthalate; miBP, monoisobutyl phthalate; NHANES, National Health and Nutrition Examination Survey.
Highlights.
Phthalate metabolites were not associated with body mass index (BMI) change overall
However, associations varied by one-year menopause transition status
Strongest associations were in women transitioning from peri- to post-menopause
Longitudinal study allowed capture of one-year BMI and menopause status changes
6. Acknowledgements
This work was supported by the National Institute for Environmental Health Sciences (grant # R01 ES026956). Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the NIH. This project was also supported by the USDA National Institute of Food and Agriculture and Michigan AgBioResearch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of ethics: Authors have no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
7. References Cited
- Blair RM, et al. , 2000. The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands. Toxicological Sciences. 54, 138–153. [DOI] [PubMed] [Google Scholar]
- Buser MC, et al. , 2014. Age and sex differences in childhood and adulthood obesity association with phthalates: Analyses of NHANES 2007–2010. International Journal of Hygiene and Environmental Health. 217, 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL Jr., et al. , 2018. Excretion of Di-2-ethylhexyl phthalate (DEHP) metabolites in urine is related to body mass index because of higher energy intake in the overweight and obese. Environment international. 113, 91–99. [DOI] [PubMed] [Google Scholar]
- Chan Kang S, Mu Lee B, 2005. DNA Methylation of Estrogen Receptor α Gene by Phthalates. Journal of Toxicology and Environmental Health, Part A. 68, 1995–2003. [DOI] [PubMed] [Google Scholar]
- Díaz Santana MV, et al. , 2019. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environmental Health. 18, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel N, et al. , 2018. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 6, 714–724. [DOI] [PubMed] [Google Scholar]
- Engel A, et al. , 2017. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicology Letters. 277, 54–63. [DOI] [PubMed] [Google Scholar]
- Feige JN, et al. , 2007. The Endocrine Disruptor Monoethyl-hexyl-phthalate Is a Selective Peroxisome Proliferator-activated Receptor γ Modulator That Promotes Adipogenesis. Journal of Biological Chemistry. 282, 19152–19166. [DOI] [PubMed] [Google Scholar]
- Fromme H, et al. , 2007. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. International Journal of Hygiene and Environmental Health. 210, 21–33. [DOI] [PubMed] [Google Scholar]
- Fungfuang W, et al. , 2013. Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Laboratory animal research. 29, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoulis G, et al. , 2018. Multi-pathway human exposure assessment of phthalate esters and DINCH. Environment International. 112, 115–126. [DOI] [PubMed] [Google Scholar]
- Gore AC, et al. , 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 36, E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, et al. , 1999. Causal diagrams for epidemiologic research. Epidemiology. 10, 37–48. [PubMed] [Google Scholar]
- Guthrie JR, et al. , 1999. Weight gain and the menopause: a 5-year prospective study. Climacteric. 2, 205–211. [DOI] [PubMed] [Google Scholar]
- Hale GE, et al. , 2014. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 142, 121–31. [DOI] [PubMed] [Google Scholar]
- Harlow SD, et al. , 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause (New York, N.Y.). 19, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, et al. , 2008. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environmental Health. 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, et al. , 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proceedings of the National Academy of Sciences. 97, 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt LT, Falconi AM, 2015. Puberty and perimenopause: reproductive transitions and their implications for women’s health. Social science & medicine (1982). 132, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husøy T, et al. , 2019. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ Int. 132, 105103. [DOI] [PubMed] [Google Scholar]
- James-Todd TM, et al. , 2016. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment International. 96, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2015a. Exposure assessment issues in epidemiology studies of phthalates. Environment international. 85, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2016. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ Health Perspect. 124, 1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, et al. , 2015b. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapraun DF, et al. , 2017. A method for identifying prevalent chemical combinations in the U.S. population. Environmental Health Perspectives. 125, 087017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvil I, et al. , 2019. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor γ. Rapid Commun Mass Spectrom. 33 Suppl 1, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu R, et al. , 2008. Effects of phthalate ester derivatives including oxidized metabolites on coactivator recruiting by PPARα and PPARγ. Toxicology in Vitro. 22, 1534–1538. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, et al. , 2016. Body Fatness and Cancer — Viewpoint of the IARC Working Group. New England Journal of Medicine. 375, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JE, et al. , 2020. Urinary bisphenol A, phthalate metabolites, and obesity: do gender and menopausal status matter? Environ Sci Pollut Res Int. 27, 34300–34310. [DOI] [PubMed] [Google Scholar]
- Lind PM, et al. , 2012. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environmental Health. 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ, 1999. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 161, 209–18. [DOI] [PubMed] [Google Scholar]
- Meeker JD, et al. , 2009. Serum and follicular fluid organochlorine concentrations among women undergoing assisted reproduction technologies. Environmental Health. 8, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson EL, et al. , 2015. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol (1985). 119, 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y. f., et al. , 2016. An ERβ agonist induces browning of subcutaneous abdominal fat pad in obese female mice. Scientific Reports. 6, 38579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri SA, et al. , 2019. Effect of aging, menopause, and age at natural menopause on the trend in body mass index: a 15-year population-based cohort. Fertility and Sterility. 111, 780–786. [DOI] [PubMed] [Google Scholar]
- Mulugeta A, et al. , 2018. Obesity and depressive symptoms in mid-life: a population-based cohort study. BMC Psychiatry. 18, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee on the Health Risks of Phthalates, 2008. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Phthalate Exposure Assessment in Humans. National Academies Press (US), Washington DC. Last accessed on August 5, 2020 at https://www.ncbi.nlm.nih.gov/books/NBK215044/ [PubMed] [Google Scholar]
- Pallottini V, et al. , 2008. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infect Disord Drug Targets. 8, 52–60. [DOI] [PubMed] [Google Scholar]
- Park Y-M, et al. , 2017. Age- and menopause-related differences in subcutaneous adipose tissue estrogen receptor mRNA expression. Steroids. 121, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL Jr., et al. , 2010. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect. 118, 1748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, et al. , 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int J Hyg Environ Health. 221, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology. 1, 43–6. [PubMed] [Google Scholar]
- Rudel RA, et al. , 2011. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environmental Health Perspectives. 119, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samandar E, et al. , 2009. Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environ Res. 109, 641–6. [DOI] [PubMed] [Google Scholar]
- Schmidt J-S, et al. , 2012. Effects of Di(2-ethylhexyl) Phthalate (DEHP) on Female Fertility and Adipogenesis in C3H/N Mice. Environmental Health Perspectives. 120, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, et al. , 2019. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int. 122, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 860, 106–12. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, et al. , 2018. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 14, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, et al. , 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International Journal of Obesity. 38, 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, et al. , 2010. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. American journal of epidemiology. 172, 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, et al. , 2005. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology. 210, 223–233. [DOI] [PubMed] [Google Scholar]
- Textor J, et al. , 2017. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International Journal of Epidemiology. 45, 1887–1894. [DOI] [PubMed] [Google Scholar]
- U.S. Deprtment of Health and Human Services Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals, updated tables. Vol. One. Atlanta, Georgia, 2019. Last accessed on 25 October 2020 at https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf. [Google Scholar]
- Ziv-Gal A, et al. , 2017. The Midlife Women’s Health Study – a study protocol of a longitudinal prospective study on predictors of menopausal hot flashes. Women’s Midlife Health. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Directed Acyclic Graph Directed acyclic graph of the assumptions underlying the multiple linear regression models. Abbreviations: BMI, body mass index. Figure was generated using DAGitty version 3.0 (www.dagitty.net). Green nodes indicate exposure. Blue nodes indicate outcome. White nodes indicate variables that were used for adjustment in models. Gray nodes indicate unmeasured variables. Green paths indicate the exposure-outcome path. Black paths indicate controlled paths in the expected relationships.
Figure S2. Boxplots of Urinary Phthalate Metabolites at Baseline for 45–54 Year Old Women in the Midlife Women’s Health Study (2006–2015) and in the National Health and Nutrition Examination Survey (2005–2016) Box plots display the median (dark horizontal line), 25th and 75th percentiles (box) and range (vertical line) for urinary phthalate metabolites in the Midlife Women’s Health Study (n = 524) and the NHANES waves 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016 (n = 919). NHANES metabolites are for women who were between the ages of 45 and 54. Urinary phthalate metabolite concentrations were not adjusted for specific gravity or creatinine. Abbreviations: mBP, monobutyl phthalate; mBzP, monobenzyl phthalate; mono(3-carboxypropyl) phthalate (mCPP), mEP, monoethyl phthalate; mECPP, mono(2-ethyl-5-carboxypentyl), mEHP, mono-(2-ethylhexyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; phthalate; miBP, monoisobutyl phthalate; NHANES, National Health and Nutrition Examination Survey.


