Abstract
STUDY QUESTION
Does the oxygen concentration in the culture medium [either physiologic (5%) or atmospheric (20%)] affect mitochondrial ultrastructure and function in preimplantation mouse embryos generated by IVF?
SUMMARY ANSWER
Embryos cultured in 20% oxygen show increased mitochondrial abnormalities compared to embryos cultured in 5% oxygen.
WHAT IS KNOWN ALREADY
ART are widely used and have resulted in the birth of more than 8 million children. A variety of media and oxygen concentrations are used to culture embryos. Embryos cultured under physiological O2 tension (5%) reach the blastocyst stage faster and have fewer alterations in gene expression when compared with embryos cultured under atmospheric oxygen conditions (20%). The mechanisms by which oxygen tension affects preimplantation development remain unclear, but mitochondria are believed to play an important role. The aim of this study was to evaluate how mitochondrial ultrastructure and function in IVF embryos were affected by culture under physiologic (5%) or atmospheric (20%) oxygen concentrations.
STUDY DESIGN, SIZE, DURATION
Zygotes, 2-cell, 4-cell, morula and blastocyst were flushed out of the uterus after natural fertilization and used as controls. IVF was performed in CF1 x B6D2F1 mice and embryos were cultured in Potassium simplex optimized medium (KSOM) with amino acids (KAA) under 5% and 20% O2 until the blastocyst stage. Embryo development with the addition of antioxidants was also tested.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Mitochondrial function was assessed by measuring mitochondrial membrane potential, reactive oxygen species (ROS) production, ATP levels, and the expression of selected genes involved in mitochondrial function. Mitochondria ultrastructure was evaluated by transmission electron microscopy (TEM).
MAIN RESULTS AND THE ROLE OF CHANCE
Embryos cultured under 20% O2 had fewer mitochondria and more vacuoles and hooded (abnormal) mitochondria compared to the other groups (P < 0.05). At the blastocyst stage the mitochondria of IVF embryos cultured in 20% O2 had lower mtDNA copy number, a denser matrix and more lamellar cristae than controls. Overall IVF-generated blastocysts had lower mitochondrial membrane potential, higher ROS levels, together with changes in the expression of selected mitochondrial genes (P < 0.05). ATP levels were significantly lower than controls only under 5% O2, with the 20% O2 IVF group having intermediate levels. Unexpectedly, adding antioxidant to the culture medium did not improve development.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
Findings in mice embryos might be different from human embryos.
WIDER IMPLICATIONS OF THE FINDINGS
This study suggests that changes in the mitochondria may be part of the mechanism by which lower oxygen concentration leads to better embryo development and further emphasize the importance of mitochondria as a locus of reprogramming.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by R01 HD 082039 to PFR, the Department of Life, Health and Environmental Sciences, University of L’Aquila, Italy (RIA 2016–2018) and the Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, La Sapienza University of Rome, Italy (University grants 2016–2017). The authors declare no competing interests.
Keywords: IVF, oxygen levels, mitochondria, ROS, embryo culture
Introduction
The protocols used for human IVF involve the utilization of different culture media and different oxygen concentrations (Chronopoulou and Harper, 2015). The O2 concentration used for human in vitro fertilization and culture has been either 5% (physiologic) or 20% (atmospheric). The use of atmospheric oxygen to culture human embryos has decreased over time, although a sizable percentage of clinics still use 20% O2 (Christianson et al., 2014). In fact, numerous studies in different species show that embryo development is improved by culturing embryos under low O2 tension (Adam et al., 2004), (Preis et al., 2007; Morin, 2017). Overall, embryos cultured under a lower O2 tension (5%) have higher cleavage, implantation, pregnancy, and birth rates in humans (Ciray et al., 2009; Waldenström et al., 2009). Interestingly, in several mammalian species, there is a decreasing oxygen gradient from the fallopian tubes (~5–8.7%), to the uterus (~1.5–8%) (Yedwab et al., 1976; Fischer and Bavister, 1993).
The mechanism underlying the improved development of embryos cultured under low oxygen is not known, but mitochondria are likely mediators of this effect. Mitochondria are the most abundant organelle in the mammalian oocyte and preimplantation embryo. They play a fundamental role in O2 utilization and the generation of ATP (Ramalho-Santos et al., 2009) importantly, several studies have correlated mitochondrial function with developmental competence (Van Blerkom, 2011).
During oogenesis and the early preimplantation period, mitochondria undergo spatial rearrangements (Van Blerkom, 2009). Mitochondria undergo an active selection process throughout oogenesis and early embryogenesis that involves multiple stage-specific bottlenecks and different patterns of mitochondrial segregation that shift from being homogeneous (i.e. uniformly distributed in the cytoplasm) to heterogeneous (localized in selected areas, e.g. perinuclear) (Howell et al., 2000). The mitochondria of oocytes and developing embryos appear to be in an immature state with a spherical shape, dense matrix and few cristae (Van Blerkom and Motta, 1979; Makabe and Blerkom, 2004). This phenotype persists through the late morula stages, when the mitochondria show a gradual transition to an elongated form and the matrix becomes less dense. Mitochondria at the blastocyst stage show an increased number of lamellar cristae, which completely cross the inner mitochondrial matrix. These morphological changes reflect the fact that by the late morula stage, embryonic metabolism changes (Van Blerkom, 2011). While during the cleavage stage there is a prevalent utilization of pyruvate via the Krebs cycle, at the morula blastocyst stage there is an increase in glucose utilization and an increase in oxidative phosphorylation (Schatten et al., 2014).
Indeed, mitochondrial function and efficiency are linked to the organelle’s morphology, which changes with the organism, the tissue and environmental conditions (Ma et al., 2017). However, up to now, a detailed morphological analysis of how different oxygen levels affect the structure and function of mitochondria in mouse embryos was not available.
In this paper, we aimed to describe the morphological alterations occurring in mitochondria following culture in vitro under different oxygen concentrations and using in vivo embryos as control at the zygote, 2-cell, 4 cell, morula and blastocyst stage. Further, we assessed mitochondrial function at the blastocyst stage by measuring ATP production, mitochondrial membrane potential, reactive oxygen species (ROS) production and gene expression of a selected groups of genes known to affect preimplantation embryo development.
Materials and Methods
Chemicals
All materials were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless stated otherwise.
Embryo collection, in vitro fertilization and embryo culture
All experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. In vitro fertilization was performed as previously described (Feuer et al., 2014). Briefly, for each experiment, 5 CF1 female mice (8–9 weeks) were super ovulated by injecting 5 IU pregnant mare serum gonadotrophin (PMSG) and 48 h later 5 IU HCG. Oocytes were collected and incubated with sperm obtained from the cauda epididymis of male B6D2F1/J mice (8-9 weeks). The fertilized oocytes were washed and cultured to the blastocyst stage at 37°C under Ovoil™ (Vitrolife, #10029) using potassium simplex optimization medium with amino acids (KAA; Millipore, MR-106-D) in 5% CO2 in humidified air (20% oxygen: IVF 20% group) or 5% CO2 and 5% oxygen at 37°C, (IVF 5% group).
Control (called FB for flushed blastocysts) embryos at different stages of development were flushed out of the uterus. For each experiment, 5 CF1 female mice (8–9 weeks) were superovulated by injecting 5 IU PMSG and 42–46 h later 5 IU HCG. The CF1 females were then mated to B6D2F1/J males. The following morning the vaginal plug was checked (the presence and was considered Day 0.5).
To assess the effect of antioxidants on development, a group of zygotes (4 replicates, ~50 embryos per replicate) were cultured under 5% oxygen in the presence of a combination of antioxidants: α-lipoic acid (ALA; 10 μM) and N-acetyl cysteine (NAC; 1 mM), as described (Silva et al., 2015) for the entire duration of the culture period. The collections of various developmental stages from the IVF and control groups were based on the morphology of the embryos and was approximately as follows, following HCG injection: Zygote: FB- 12–15 hours (h), IVF 18 h; 2-cell: FB- 24–28 h, IVF 38 h; 4-cell: FB- 36–40 h, IVF 48–50 h; 8-cell, FB-45–50 hours (h), IVF 65–70 h; Morula, FB- 60–70 h, IVF 75–85 h; Blastocyst, FB-88–92 h, IVF 108–110 h. Five embryos per stage in three biological replicates were collected. Of note, 4-cell and 8-cell embryos showed a varied developmental timing (both stages of embryos were found at different collection times). Hence, the two groups were merged in a single group, hereafter named 4-8 cell.
Quantitation of ATP
Individual blastocyst (n = 63 in FB group, n = 63 in 5% IVF and n = 35 in 20% IVF) were rapidly frozen to − 80°C in about 2 μl KSOM and ATP was measured using the bioluminescent somatic cell assay kit (FLASC, Sigma Chemical Co., St Louis, MO, USA). Further detail is given in Supplementary methods.
Mitochondrial membrane potential
Mitochondrial membrane potential was measured in living blastocysts (n = 45 in FB group, n = 61 in 5% IVF and n = 32 in 20% IVF) using the JC-1 assay kit (Abcam, Cambridge MA) according to the manufacturer’s instructions. Further detail is given in Supplementary methods.
ROS levels
Embryo ROS was assessed with CellRox green reagent (Thermo Fischer Scientific, Waltham MA).
For a positive control, morulae were treated with 5 μM menadione (which generates ROS) for an hour (de Assis et al., 2015). Images were captured with a confocal microscope (Leica SP5, Germany) and quantified using Image J software. Further detail is given in Supplementary methods.
Light microscopy (LM) and transmission electron microscopy (TEM) and assessment of mitochondria numerical density
Five embryos for each developmental stage in triplicate were analyzed as previously described (Palmerini et al., 2014). Briefly, embryos were recovered, washed in PBS and immediately fixed in 2.5% glutaraldehyde (Agar Scientific, Cambridge Road Stansted Essex, UK) in phosphate buffered saline (PBS). Fixed specimens were stored at 4°C for 2-5 days, and then processed as previously described (Nottola et al., 2011; Palmerini et al., 2014). Briefly, samples were rinsed and postfixed with 1% osmium tetroxide (Agar Scientific, Stansted, UK) in PBS and rinsed again in PBS. Each embryo was then embedded in small blocks of 1% agar of about 5 × 5 × 1 mm in size, dehydrated in an ascending series of ethanol, immersed in propylene oxide for solvent substitution, embedded in epoxy resin EMbed-812 (Electron Microscopy Sciences, 1560 Industry Road, Hatfield, PA, USA) and sectioned by means of a Reichert-Jung Ultracut E ultramicrotome. Semi-thin sections (1 mm thick) were stained with Toluidine Blue, examined using LM (Zeiss Axioskop) and photographed using a digital camera (Leica DFC230). Ultrathin sections (60–80 nm) were cut with a diamond knife, mounted on copper grids and contrasted with saturated uranyl acetate and lead citrate (SIC, Rome, Italy). They were examined and photographed using Zeiss EM10 and Philips TEM CM100 Electron Microscopes operating at 80 kV.
Mitochondrial numerical density was evaluated as previously reported (Leoni et al., 2015). Detailed description available in Supplemental method section.
MtDNA copy number
MtDNA copy number was assessed in single blastocyst as described (Gonzalez-Hunt et al., 2016). Further detail is given in Supplementary methods.
RT qPCR of selected genes
Blastocysts developed in vivo or in vitro (KAA with 5% oxygen or KAA with 20% oxygen) were collected as described above for detection of mtDNA, and quantitative real-time PCR was conducted on four independent biological replicates containing ten pooled blastocysts. Total RNA was extracted using a PicoPure RNA isolation kit (Arcturus, Sunnyvale, CA, USA), cDNA was synthesized (iScript cDNA synthesis kit, Bio-Rad) and was normalized to the expression of the histone variant H2A. Data was analyzed within the log linear phase of the amplification curve obtained for each primer using the comparative threshold cycle method (Bio-Rad Laboratories, Hercules, CA, USA). Primer sequence is provided in Supplementary Table S1. Further detail is given in Supplementary methods.
Statistical analysis
All data were expressed as means ± standard deviation (SD). Statistical comparisons were performed using one-way or two-way ANOVA with Tukey’s HSD tests for post-hoc analysis (GraphPad InStat or Prism 5, La Jolla CA). Differences in values were considered significant if P < 0.05. Variance between the groups was calculated using the Levene test for equal variance.
Results
IVF embryos had more vacuoles and fewer morphologically normal mitochondria
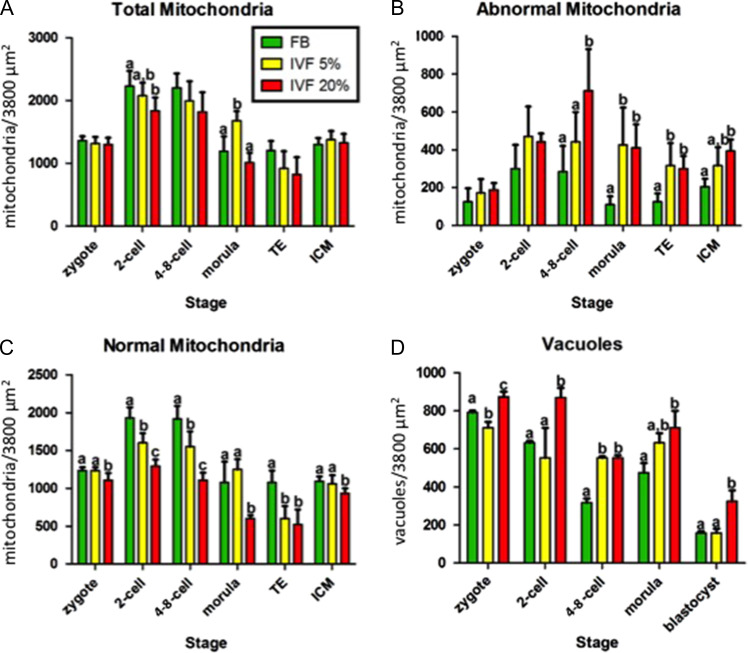
Morphological analysis of embryos generated after spontaneous mating (FB) or after IVF (IVF-5%, IVF-20%) revealed some important findings. First, total mitochondrial numerical density changed over the course of preimplantation development. Compared to zygotes, embryos of all three groups showed an increase in the density of mitochondria at the 2- and 4-8 cell stages and then a decrease at the morula and blastocyst stages (both in ICM and TE cells) (Fig. 1A).
Figure 1.
Mitochondrial density by developmental stage. The numerical density (mitochondria per 3800 μm2) of total (A), abnormal (B) and normal (C) mitochondria, as well as vacuoles (D) varies with developmental stage. IVF embryos have more abnormal mitochondria and vacuoles and fewer normal mitochondria. Bars with a different letter superscript (within a stage) are statistically different. The absence of superscript letter indicates that there were no statistically significant differences between any of the treatment groups. TE, trophectoderm; ICM, inner cell mass; FB, in vivo fertilized embryos flushed from the uterus; IVF 5%/20%, IVF embryos cultured under 5 / 20% oxygen.
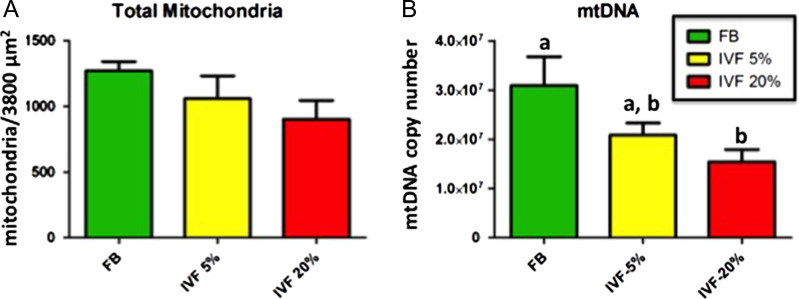
Secondly, IVF embryos had fewer total as well as normal mitochondria (Figs 1A and C) compared to control embryos. This trend was particularly evident in IVF-20% embryos. The decrease in total mitochondrial density within blastocysts in IVF group was confirmed by an independent parameter, mtDNA copy number (Fig. 2). Two-way analysis of variance showed significant effects of both the stage of development and conception/culture conditions on the number of mitochondria.
Figure 2.
Numerical density of mitochondria and mtDNA content. The numerical density (mitochondria per 3800 μm2) of total mitochondria in blastocysts tended to decline from in vivo fertilized embryos flushed from the uterus (FB) to IVF embryos cultured under-5% group and again to those cultured under 20% oxygen (IVF5%/20% groups) (A). The mtDNA copy number (B) reflects the pattern seen morphologically. Bars with a different letter superscript are statistically different. The absence of superscript letter indicates that there were no statistically significant differences between any of the treatment groups.
Thirdly there was an increase in abnormal mitochondria (Fig. 1B).
Finally, while vacuoles were noted at every stage of development, their number tended to decrease as development proceeded (Fig. 1D) in both the FB and IVF groups. The IVF-20% embryos always had more vacuoles than control and IVF 5% embryos. This was statistically significant at all stages except 2-cells.
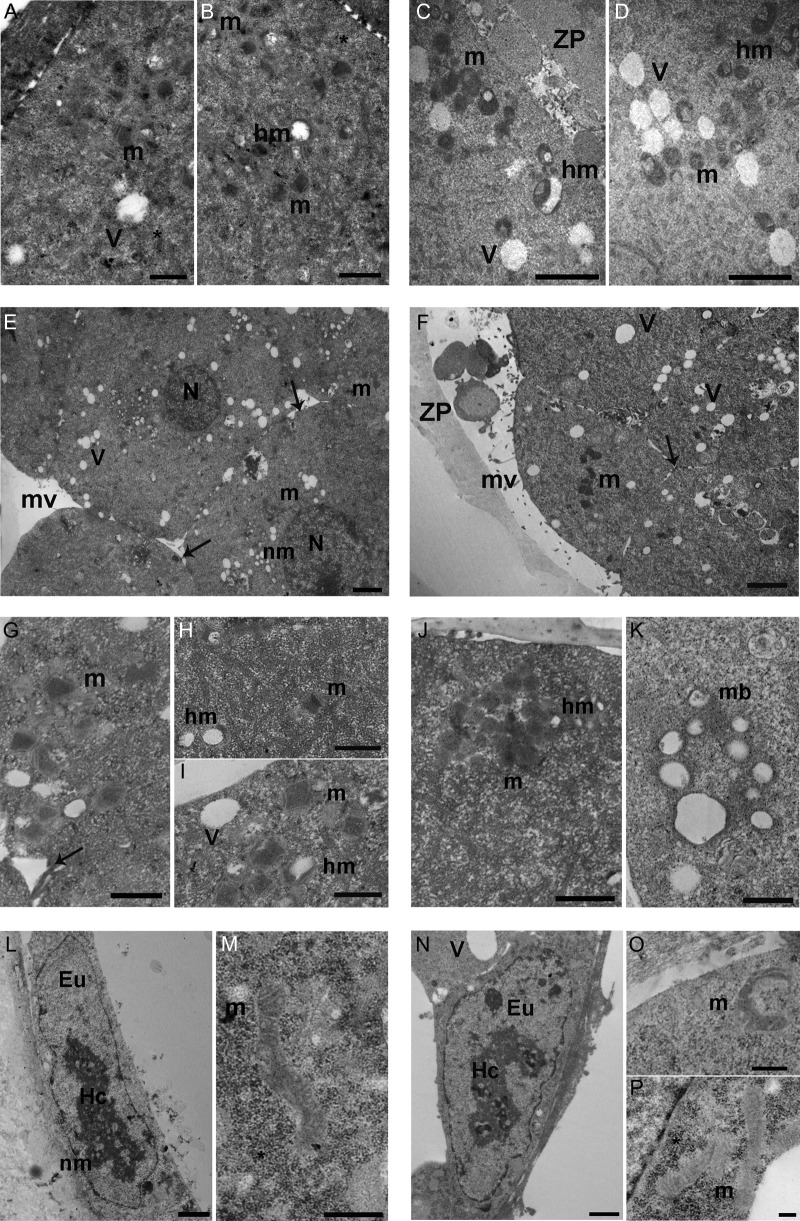
Specific morphological findings for each stage of development are described in Table I and illustrated in Fig. 3. Briefly, mitochondria in all groups had a round to ovoid shape up to the morula stage, at which point they started to acquire a tubular shape. The tubular shape was predominant at the blastocyst stage. The IVF20% group showed a higher incidence of branching mitochondria compared to other groups (Fig. 3P). The electron-density of the mitochondrial matrix increased from the zygote to the blastocyst stage. IVF groups had higher mitochondrial electron-density at the two cell stage (Fig. 3D). While mitochondrial distribution was uniform throughout the cytoplasm, starting at the two cell stage mitochondria were often found associated with vacuoles (Fig. 3D). Hooded mitochondria were more common in the IVF 20% group than in other groups starting at the 4-8 cell stage (Fig. 3E).
Table I.
Mitochondrial morphology
| Stage | Shape | Cristae density | Distribution | Other | Conclusion |
|---|---|---|---|---|---|
| Zygote | Round ovoid | Intensely stained | Throughout cytoplasm | No differences among the groups | |
| 2-cell | Round ovoid | IVF groups denser than in vivo group | Often close to vacuoles and similar among the groups | Vacuoles are present; inclusions of glycogen; hooded mitochondria are first identified | IVF mitochondria are denser than vivo |
| 4/8-cell | Round ovoid | Denser than prior developmental stages | Association to tubular elements of smooth endoplasmic reticulum | Hooded mitochondria more common in IVF 20% | |
| Morula | Some round ovoid but also tubular shape | Denser than in prior developmental stages | Close to vacuoles | IVF 20% showed compacted blastomere more than other groups. Multivesicular bodies present only in IVF groups | |
| ICM | Prevalent elongated and tubular shape; occasionally branching mitochondria | Highest density | More mitochondria in ICM than TE | Abundant glycogen more so in IVF 20% | IVF 20% show higher incidence of branching mitochondria |
| TE |
Figure 3.
Mitochondrial ultrastructure. (A and B): Ultrastructure of mitochondria in in vivo fertilized embryos flushed from the uterus (FB) 2-cell and 4-8 cell embryos. A—2-cell embryo mitochondria with cristae (m) and vacuoles (V). *: granules of mono-particulate form of glycogen (Transmission electron microscopy [TEM] Bar: 0.6 μm). B—ultrastructure of 4-8 cell embryo hooded mitochondria (hm) and normal mitochondria (m) with cristae. *: granules of glycogen (TEM. Bar: 0.8 μm). (C and D): ultrastructure of round/ovoid mitochondria in a 2-cell and a 4-8 cell IVF embryo cultured under 20% oxygen (IVF20%). C—IVF 20% 2-cell embryo mitochondria (m) and hooded mitochondria (hm). V: vacuoles; ZP: zona pellucida. (TEM. Bar: 1 μm). D—IVF 20% 4-8 cell embryo mitochondria (m), hooded mitochondria (hm) and vacuoles (V) (TEM. Bar: 1 μm). E—FB morula with large round nucleus (N). Numerous blastomeres are visible. Arrows show the intercellular contacts. m: mitochondria; V: vacuole; nm: nuclear membrane; mv: microvilli (TEM. Bar: 2 μm). F—ultrastructure of IVF-20% morula. TEM micrograph shows blastomeres in a compaction stage. Arrows indicate the intercellular contacts. m: mitochondria; V: vacuoles; ZP: zona pellucida; mv: microvilli (TEM. Bar: 2 μm). (G–I): FB morula mitochondria. G—FB morula mitochondria (m) with visible cristae. Arrow shows intercellular contacts (TEM. Bar: 0.8 μm). H—high magnification of FB morula isolated mitochondria (m) and hooded mitochondria (hm) (TEM. Bar: 0.8 μm). I—high magnification of group of mitochondria (m), hooded mitochondria (hm) and vacuoles (V) (TEM. Bar: 0.6 μm). (J and K): IVF 20% morula. J—ultrastructure of high electron-density mitochondria (m) with round shape. hm: hooded mitochondria (TEM. Bar: 0.8 μm). K—high magnification of a multivesicular body (mb) (TEM. Bar: 0.6 μm).(L and M): ultrastructure of FB blastocyst. L—trophoblast cell with large nucleus. The blastomeres shows nuclear heterochromatin (He) and euchromatin (Eu). nm: nuclear membrane (TEM. Bar: 1 μm). M—elongated mitochondria with visible cristae. *: monoparticulate form of glycogen (TEM. Bar: 0.6 μm). (N–P:) ultrastructure of IVF blastocyst cultured under 20% oxygen (IVF-20%). N—trophoblast cell with large nucleus with pacth of heterochromatin (He) and euchromatin (Eu). Large vacuole (V) is visible. O—IVF 20% Trophectoderm (TE) branching mitochondria (m). (TEM. Bar: 0.4 μm). P—representative TEM micrograph of IVF TE mitochondria (m) and monoparticulate form of glycogen (*) (TEM. Bar: 0.2 μm).
Three additional observations are relevant: IVF20% embryos appeared to undergo compaction earlier than FB embryos (Fig. 3G). IVF embryos had more glycogen at the blastocyst stage (Fig. 3Q). Further, multivesicular bodies were present only in the two IVF groups, and absent in the in vivo group (Figure 3L)
Mitochondria in IVF blastocysts showed functional alterations
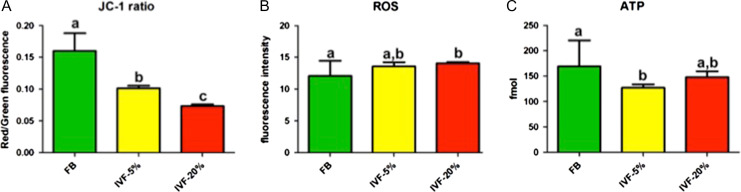
Mitochondrial membrane potential
Mitochondrial membrane potential (ΔΨm) was examined by staining blastocysts with the fluorescent probe, JC-1. The ΔΨm (i.e. ratio of red to green fluorescence) of FB blastocysts (0.16 ± 0.028, n = 45) was higher than those of IVF-5% (0.10 ± 0.025, n = 61; P < 0.01) and IVF-20% (0.07 ± 0.016, n = 32; P < 0.01) (Fig. 4A). The variance in the FB group was greater than in the IVF-groups (P < 0.05).
Figure 4.
Mitochondrial functional tests. Mitochondrial membrane potential, as measured by JC-1 staining was significantly less in embryos cultured under 20% oxygen (IVF-20%) (A) and ROS levels were greater in IVF-20% embryos (B). ATP levels were lower in IVF embryos than FB, but the group of embryos cultured under 5% oxygen (IVF-5%) was the only group for which the difference reached statistical significance (C).
Reactive oxygen species
The level of ROS, measured with Cell ROx Green, in FB blastocysts (12.1 ± 2.37, n = 28) was significantly lower than those in the IVF-20% group (14.0 ± 1.13, n = 27; P < 0.01). Embryos in the IVF-5%O2 group had intermediate, and not significantly different, levels of ROS compared to the other two groups. (13.6 ± 3.74, n = 32 Fig. 4B). The variance in the FB group was greater than in the IVF groups (P < 0.05).
ATP levels
In order to assess net metabolic output, we measured the concentration of ATP in the lysates of individual blastocysts (Fig. 4C). We found that the concentration of ATP in FB blastocysts (169 ± 52 fmol, n = 63) was significantly higher than that in IVF-5% blastocysts, (127 ± 52 fmol, n = 63; P < 0.01). Blastocysts in the IVF-20% group had ATP levels that were intermediate (148 ± 69 fmol, n = 35), and not statistically different from the FB or IVF 5% blastocysts. The variance in the FB group was greater than in the IVF-groups (P < 0.05).
Selected mitochondrial genes were down-regulated
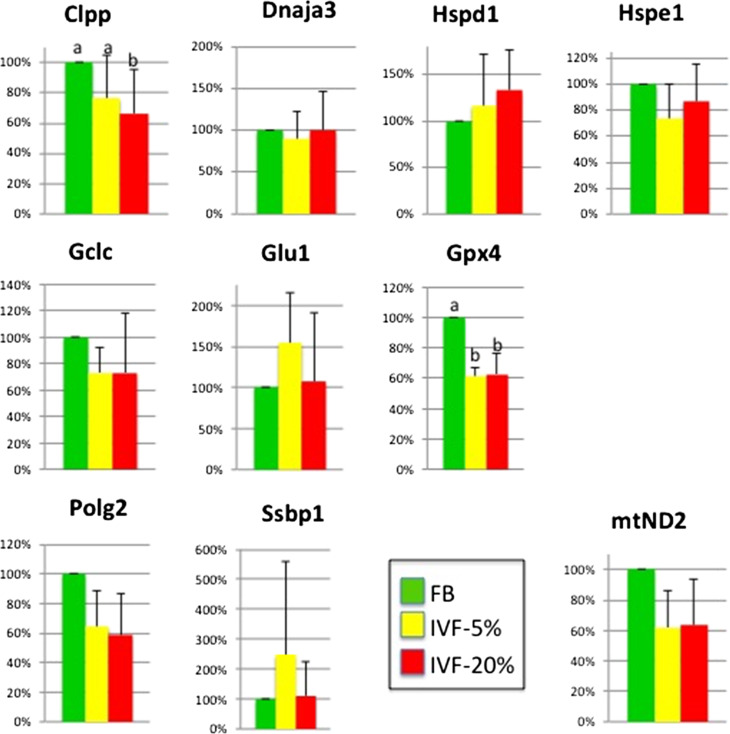
The levels of a selected a group of genes known to play important roles in mitochondrial physiology were measured. Among the genes tested (Gclc, Glul, Polg2, Gpx4, Clpp, DNAja3, Hspd1, Hspe1, mtND2), two genes (Gpx4, Clpp) were downregulated (P < 0.05) and one (mtND2) showed a trend for lower levels (P = 0.054) in IVF groups compared to control (Fig. 5).
Figure 5.
Expression of ten selected genes involved in mitochondrial function and cellular redox state. Top row genes involved in mitochondrial protein quality control, middle row genes involved in glutathione antioxidant pathway, bottom row genes involved in mitochondrial DNA synthesis and (mtND2) electron transport chain. Bars with no letter designation show no difference among the treatment groups. Bars with different letter designations are statistically different. FB, in vivo fertilized embryos flushed from the uterus; IVF 5%/20%, IVF embryos cultured under 5 / 20% oxygen.
Antioxidant treatment of embryos did not affect development
There were no statistically significant differences between the rate of development of embryos in the IVF 5% group cultured with or without antioxidants (Supplementary Table SII).
Discussion
While mitochondrial dysfunction following in vitro culture has been described before (Crosier et al., 2000), this is the first publication that describes mitochondrial morphology using electron microscopy during preimplantation development in mouse embryos generated in vivo or by IVF under physiologic or atmospheric oxygen concentration. Overall, we found that IVF and embryo culture result in a decrease in total number of mitochondria with an increase of abnormally shaped mitochondria. Further, analysis of mitochondrial function at the blastocyst stage revealed that IVF embryos had increased ROS levels, decreased mitochondrial membrane potential, changes in ATP levels and decreased expression of selected genes, suggesting an alteration in mitochondrial function. Importantly, the majority of these effects were more evident in the IVF-20% group, suggesting that the stresses induced by IVF and culture, combined with higher oxygen concentrations are more detrimental to embryo well-being.
First and foremost, we found that in vitro fertilization and culture affects, in a profound way, mitochondrial morphology and number. IVF embryos (in particular IVF-20% group) showed earlier compaction at the morula stage and an increase in visible cristae at the blastocyst stage compared to in vivo flushed (FB) embryos at the same stage. In addition, IVF embryos had a lower numerical density of normal, healthy mitochondria and higher numerical density of abnormal (i.e. hooded) mitochondria, with the IVF-20% group at the 4-8 cell stage showing a greater numerical density of hooded mitochondria compared to both the IVF-5% group and the FB group. At the same time, the IVF-20% embryos showed lower numerical density of normal mitochondria compared to the IVF-5% or FB group. This alteration in number was associated with a greater number of vacuoles in IVF embryos, a phenomenon particularly evident in the IVF-20% group.
Since mitochondria play a key role in cellular metabolism, these morphological and numerical findings indicate that the IVF process and in particular high oxygen concentration alters significantly the metabolism of the developing embryos (Crosier et al., 2000). The most likely explanation of the findings is that the process of in vitro culture induces mitochondrial stress with an increase in abnormal mitochondria, and a decrease in normal mitochondrial number. Given that the vacuoles are located in proximity of mitochondria and that multivesicular bodies are observed only in the IVF group, we speculate the vacuoles represent mitochondrial degeneration and mitophagy (Ding and Yin, 2012). Of note, degenerating mitochondria have not been identified during the preimplantation stage (Makabe and Blerkom, 2004).
Although the exact significance of hooded mitochondria is unknown, some investigators suggest they represent immature mitochondria that limit the production of reactive oxygen species (Makabe and Blerkom, 2004; Crocco et al., 2011). Other studies suggest that the hooded morphology is associated with increased functional surface area by extending both outer and inner mitochondrial membranes (Dadarwal et al., 2015). These morphological findings together with the decrease in membrane potential (JC-1 ratio) found in IVF embryos would suggest that the IVF mitochondria are less efficient.
Of note, mitochondrial distribution did not vary among the groups and mitochondria appeared equally distributed in the cytoplasm. One study (Wang et al., 2009) demonstrated that the population of mitochondria in the fully grown mammalian oocyte is morphologically homogenous, undeveloped, and usually uniformly distributed within the cytoplasm. An increased level of mitochondrial aggregation around the nucleus indicates oocyte maturation. After fertilization, mitochondria accumulate to form a relatively dense peri-pronuclear sphere which normally disperses prior to the first cleavage division. Mitochondrial distribution changed from being homogeneous to heterogeneous. Specifically, in the heterogeneous state, the mitochondrial distribution changed from granulated aggregation to clustered aggregation. It is also observed that morphologically low quality embryos are characterized by the homogenous distribution of mitochondria (Wilding et al., 2001). By these criteria all of the embryos in the current study would be categorized as low quality.
This murine study shows that all embryos generated in vitro or in vivo showed mitochondrial number changes during preimplantation stage with number of total mitochondria being higher in 2-cell and 4-8-cell embryos than in zygotes, morula and blastocysts. This is an interesting observation, given that it is normally thought that preimplantation embryos do not undergo mitochondrial synthesis. It has been suggested that 120,000–350,000 mitochondria are present in MII human oocytes and early embryos (Jansen, 2000; Cummins, 2002). Further, others have found that mtDNA copy numbers in mouse embryos remained unchanged (Wai et al., 2010), while the mtDNA copy numbers in cattle, pigs and humans have been reported to decrease temporarily during embryo development (Hashimoto et al., 2017).
The reason of the different total count of mitochondria during different stages of development is intriguing, since it was observed independently in all three groups of embryos. It is possible that mitochondrial distribution might have changed during development, with more mitochondria being distributed in the equatorial plane, while being diminished in the periphery. However, observation under low magnification seems to exclude this possibility. Alternatively, there may indeed be an increase in mitochondrial numerical density during selected stages in the present study. Harvey et al. suggest that changes in mitochondrial number and morphology during the preimplantation stages could reflect fusion or fission processes and these changes could be associated with a different metabolic activity of the preimplantation embryo (Harvey et al., 2011).
Finally, it is possible that the stress of culture, especially in the presence of atmospheric oxygen, results in altered mitochondrial division (forming shorter filaments), generating an abnormal morphology with the net result that a lower number of total mitochondria is counted. It is also interesting to note that the increase in mitochondrial number starts at the 2-cell stage, the time of zygote genome activation in the mouse.
Importantly, we need to clarify that our counting method provides a numerical density and not a total number of mitochondria. This method is robust and reliable, since it allows to count inactive/abnormal mitochondrial population (Reader et al., 2015). Various methods have been developed to determine the mitochondrial quantity. For example, mitochondria quantity has been measured with MitoTracker dyes, which are strictly directed in active mitochondria. MitoTracker Green is relatively insensitive to mitochondrial membrane potential and therefore has been used as a means to quantify active mitochondrial mass or number. Inactive mitochondria are not stained by MitoTracker Green and, in the case of the oocyte/embryo which is thought to contain a high proportion of mitochondria that are relatively inactive/abnormal, MitoTracker Green fluorescence intensity may not provide an accurate estimation of overall mitochondrial mass (Reader et al., 2015). Given these findings, we consider numerical density as a reliable surrogate marker of total mitochondrial number.
Our data indicate that mtDNA copy number is lower in IVF embryos, and this parallels the findings of lower mitochondria numerical density in IVF embryos. Although others have described that the mtDNA number declines significantly during cleavage stage (Takeo et al., 2013), the literature on mtDNA copy number and embryo implantation is controversial, given the high level of variation in mtDNA levels in embryos (Hicks et al., 2012). In fact, some human studies suggest that high mtDNA numbers are associated with decreased implantation (Diez-Juan et al., 2015; Fragouli et al., 2015), while others studies do not (Treff et al., 2017; Victor et al., 2017).
The second important finding of this study is that IVF embryos show significant functional abnormalities that complement the morphological findings. We limited the functional analysis to blastocysts, because this stage of development represents the culmination of all the events of preimplantation development, when embryos have increased oxidative phosphorylation to generate ATP and because embryos are often transferred at the blastocyst stage. IVF blastocysts showed altered membrane potential, with decreased JC-1 ratio particularly evident in IVF 20% group. The reduced membrane potential indicates that not only do IVF blastocysts have fewer mitochondria, but these mitochondria are less functional. Interestingly IVF embryos had an increase in green fluorescence (an indication of viability) but decrease in red fluorescence (an indication of activation), suggesting that the mitochondria were indeed activated but unable to create a proper membrane potential. These findings would indicate that IVF embryos are farther from the optimal or goldilocks zone, in agreement with the hypothesis of Leese (Leese et al., 2016). It is further intriguing that the control embryos had higher variance in membrane potential and ROS levels compared to IVF embryos. While the significance of this finding is unclear, it suggests that the IVF process exerts a restrictive effect on functional mitochondrial parameters, limiting the number of outliers. We are not aware of similar findings in the literature; however, de Waal et al. found that mouse IVF concepti exhibited increased frequency of stochastic epigenetic errors in the placenta (de Waal et al., 2014).
The Casper group (Acton et al., 2004) analyzed embryo mitochondrial membrane potential in IVF and in vivo mouse embryos from zygotes to blastocysts. They found that the membrane potential showed a progressive increase with development. The membrane potential of FB blastocysts was higher (but non-statistically significant) than IVF blastocysts, similarly to our results. Moreover, analysis of mitochondrial gene expression by Ren et al. (Ren et al., 2015) also showed down-regulation of genes involved in transmembrane transport. Functional changes often result from structural ones. For example Crosier et al. found that excess of lipids in cytoplasm are associated with a reduction in the numerical density of mitochondria (Crosier et al., 2000).
ROS levels and gene expression data provide some valuable information. ROS levels showed a step-wise increase with increase oxygen concentration in the culture condition (statistically increased in IVF cultured under high oxygen). These data confirm the findings of others that embryo culture induces an increase in ROS (Goto et al., 1993; Cebral et al., 2007; Martin-Romero et al., 2008) and it was expected. However, it was surprising to find that the addition of antioxidant to = embryos cultured under 5% O2 did not improve development. It is unclear why this was so as others have shown an improved development when antioxidant were added (Silva et al., 2015). It is possible that the different culture media and the different strain of mice used in our study can explain the findings. Given that ROS function as physiologic second messengers and have beneficial effects it is likely that unique antioxidant compositions and levels need to be found for every experimental condition. It is possible that a beneficial effect of antioxidant could be observed in embryos cultured under 20% oxygen. However, given the significant negative effects of 20% oxygen on embryo development and gene expression (Rinaudo et al., 2006) and that 5% oxygen is the preferred method of culture, we did not perform these additional experiments.
The gene expression data showed that selected genes were altered in IVF embryos. Gpx4 and Clpp and mtND2 were decreased in IVF embryos. Gpx4 encodes a selenium-dependent glutathione peroxidase whose main biological role is to protect the organism from oxidative damage. Interestingly, Gpx4 was also misregulated according to the study of Ren et al. (2015), although it was increased in IVF embryos. This discrepancy in findings might signify that the glutathione peroxidase (GPx) system is very sensitive to embryo manipulation and different cultured conditions used in the studies might alter a normal balance. The Clpp gene encodes ATP-dependent Clp protease proteolytic subunit (ClpP), which is a major contributor for mitochondrial protein quality control system whose function is to remove damaged or misfolded proteins in mitochondria. Clpp down-regulation could affect multiple aspects of normal mitochondria morphology and activity and it has been shown to be important for oocytes and embryo development (Wang, 2008). Finally, mtND2 encodes the core subunit of NADH dehydrogenase, which is the first enzyme of the mitochondrial electron transport chain. Lower expression of mtND2 on could lead to a reduction in NADH dehydrogenase activity, and subsequently a decrease of ATP generation.
Of note, we did not perform a comprehensive gene expression analysis of mitochondrial genes, since a comprehensive analysis of mitochondrial gene expression was performed by Ren et al. (Ren et al., 2015).
Finally, our ATP data offer a less univocal interpretation. We would have expected that lower quantities of mitochondria and abnormal membrane potential in IVF embryos would have resulted in reduced generation of ATP, more significant in the IVF20% group. Instead, we found that only the 5% IVF blastocysts (but not 20% IVF blastocysts) showed a decrease in ATP levels. However, since ATP levels are a static measure of metabolism in the cells it is difficult to comment if these results can be explained by relative changes in ATP production versus consumption or a combination of the two phenomena. One possible explanation is that the IVF20% embryos might be producing more ATP to compensate for their increased stress, as shown by higher ROS levels, lower membrane potential and decreased in mitochondria number. The ability of mitochondria to balance ATP supply and demand is considered the most critical factor with respect to fertilization competence for the oocyte and developmental competence for the embryo. Although we were careful to select blastocysts of similar morphology at time of analysis to decrease the variability existent in embryos at slightly different stages of development, it is however possible that some of the difference observed could be explained by different cell number existent in blastocysts cultured under different oxygen concentrations.
In summary, our findings indicate that embryos generated in vitro under physiologic or atmospheric oxygen show important changes in mitochondrial number, morphology and function compared to in vivo generated embryos; overall these changes were more severe in embryos cultured under 20% O2. This study confirms the need to culture embryos under physiologic oxygen and suggests that changes in the mitochondria may be part of the mechanism by which lower oxygen concentration leads to better embryo development and further suggest to focus future studies on mitochondria as a locus of reprogramming.
Supplementary Material
Authors’ roles
MB TEM analysis and counts and article writing; LZ functional experiments and article writing; XL performed the mt DNA and RT PCR experiments; AD assistance in experiment, article writing and data interpretation; ER embryo culture experiments; MGP analyzed the TEM and data interpreation; SAN, GM TEM data interpretation and analysis. SAN, GM and PR planned and designed the experiments and data analysis PR article writing
Funding
The study was funded by R01 HD092267-01 to PR, the Department of Life, Health and Environmental Sciences, University of L’Aquila, Italy (RIA 2016–2018) and the Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, La Sapienza University of Rome, Italy (University grants 2016–2017).
Conflict of interest
The authors declare no competing interests.
References
- Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod 2004;10:23–32. [DOI] [PubMed] [Google Scholar]
- Adam AAG, Takahashi Y, Katagiri S, Nagano M. In vitro culture of mouse preantral follicles using membrane inserts and developmental competence of in vitro ovulated oocytes. J Reprod Dev 2004;50:579–586. [DOI] [PubMed] [Google Scholar]
- Cebral E, Carrasco I, Vantman D, Smith R. Preimplantation embryotoxicity after mouse embryo exposition to reactive oxygen species. Biocell 2007;31:51–59. [PubMed] [Google Scholar]
- Christianson MS, Zhao Y, Shoham G, Granot I, Safran A, Khafagy A, Leong M, Shoham Z. Embryo catheter loading and embryo culture techniques: results of a worldwide Web-based survey. J Assist Reprod Genet 2014;31:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update 2015;21:39–55. [DOI] [PubMed] [Google Scholar]
- Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril 2009;91:1459–1461. [DOI] [PubMed] [Google Scholar]
- Crocco M, Alberio RH, Lauria L, Mariano MI. Effect of serum on the mitochondrial active area on developmental days 1 to 4 in in vitro-produced bovine embryos. Zygote 2011;19:297–306. [DOI] [PubMed] [Google Scholar]
- Crosier AE, Farin PW, Dykstra MJ, Alexander JE, Farin CE. Ultrastructural morphometry of bovine compact morulae produced in vivo or in vitro. Biol Reprod 2000;62:1459–1465. [DOI] [PubMed] [Google Scholar]
- Cummins JM. The role of maternal mitochondria during oogenesis, fertilization and embryogenesis. Reprod Biomed Online 2002;4:176–182. [DOI] [PubMed] [Google Scholar]
- Dadarwal D, Adams GP, Hyttel P, Brogliatti GM, Caldwell S, Singh J. Organelle reorganization in bovine oocytes during dominant follicle growth and regression. Reprod Biol Endocrinol 2015;13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis PM, Castro LS, Siqueira AF, Delgado Jde C, Hamilton TR, Goissis MD, Mendes CM, Nichi M, Visintin JA, Assumpcao ME. System for evaluation of oxidative stress on in-vitro-produced bovine embryos. Reprod Biomed Online 2015;31:577–580. [DOI] [PubMed] [Google Scholar]
- de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, Coutifaris C, Schultz RM, Bartolomei MS. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod 2014;90:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, Diaz-Gimeno P, Valbuena D, Simon C. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril 2015;104:534–541 e531. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012;393:547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer SK, Liu X, Donjacour A, Lin W, Simbulan RK, Giritharan G, Piane LD, Kolahi K, Ameri K, Maltepe Eet al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014;155:1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 1993;99:673–679. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, Kokocinski F, Cohen J, Munne S, Wells D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet 2015;11:e1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hunt CP, Rooney JP, Ryde IT, Anbalagan C, Joglekar R, Meyer JN. PCR-based analysis of mitochondrial DNA copy number, mitochondrial DNA damage, and nuclear DNA damage. Curr Protoc Toxicol 2016;67:20 11 21–20 11 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med 1993;15:69–75. [DOI] [PubMed] [Google Scholar]
- Harvey A, Gibson T, Lonergan T, Brenner C. Dynamic regulation of mitochondrial function in preimplantation embryos and embryonic stem cells. Mitochondrion 2011;11:829–838. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Morimoto N, Yamanaka M, Matsumoto H, Yamochi T, Goto H, Inoue M, Nakaoka Y, Shibahara H, Morimoto Y. Quantitative and qualitative changes of mitochondria in human preimplantation embryos. J Assist Reprod Genet 2017;34:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Howe DK, Leung A, Denver DR, Estes S. In vivo quantification reveals extensive natural variation in mitochondrial form and function in Caenorhabditis briggsae. PLoS One 2012;7:e43837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N, Chinnery PF, Ghosh SS, Fahy E, Turnbull DM. Transmission of the human mitochondrial genome. Hum Reprod 2000;15:235–245. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod 2000;15:112–128. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Guerif F, Allgar V, Brison DR, Lundin K, Sturmey RG. Biological optimization, the Goldilocks principle, and how much is lagom in the preimplantation embryo. Mol Reprod Dev 2016;83:748–754. [DOI] [PubMed] [Google Scholar]
- Leoni GG, Palmerini MG, Satta V, Succu S, Pasciu V, Zinellu A, Carru C, Macchiarelli G, Nottola SA, Naitana Set al. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS One 2015;10:e0124911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Chen HW, Tzeng CR. Low oxygen tension increases mitochondrial membrane potential and enhances expression of antioxidant genes and implantation protein of mouse blastocyst cultured in vitro. J Ovarian Res 2017;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabe S, Van Blerkom J. An Atlas of Human Female Reproduction: Ovarian Development to Embryogenesis In Vitro. London: Taylor and Francis Books Ltd, 2004. [Google Scholar]
- Martin-Romero FJ, Miguel-Lasobras EM, Dominguez-Arroyo JA, Gonzalez-Carrera E, Alvarez IS. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod Biomed Online 2008;17:652–661. [DOI] [PubMed] [Google Scholar]
- Morin SJ. Oxygen tension in embryo culture: does a shift to 2% O2 in extended culture represent the most physiologic system? J Assist Reprod Genet 2017;34:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PM, Nottola SA, Familiari G, Macchiarelli G, Vizza E, Correr S. Ultrastructure of human reproduction from folliculogenesis to early embryo development. A review. Ital J Anat Embryol 1995;100:9–72. [PubMed] [Google Scholar]
- Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Rep 2000;15:129–147. [DOI] [PubMed] [Google Scholar]
- Nottola SA, Heyn R, Camboni A, Correr S, Macchiarelli G. Ultrastructural characteristics of human granulosa cells in a coculture system for in vitro fertilization. Microsc Res Tech 2006;69:508–516. [DOI] [PubMed] [Google Scholar]
- Nottola SA, Cecconi S, Bianchi S, Motta C, Rossi G, Continenza MA, Macchiarelli G. Ultrastructure of isolated mouse ovarian follicles cultured in vitro. Reprod Biol Endocrinol 2011;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottola SA, Albani E, Coticchio G, Palmerini MG, Lorenzo C, Scaravelli G, Borini A, Levi-Setti PE, Macchiarelli G. Freeze/thaw stress induces organelle remodeling and membrane recycling in cryopreserved human mature oocytes. J Assist Reprod Genet 2016;33:1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, Macchiarelli G, Khalili MA, Antinori S. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J Reprod Dev 2014;60:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis KA, Seidel GE, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev 2007;74:893–903. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 2009;15:553–572. [DOI] [PubMed] [Google Scholar]
- Reader KL, Cox NR, Stanton JA, Juengel JL. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod Fertil Dev 2015;27:513–522. [DOI] [PubMed] [Google Scholar]
- Ren L, Wang Z, An L, Zhang Z, Tan K, Miao K, Tao L, Cheng L, Zhang Z, Yang Met al. Dynamic comparisons of high-resolution expression profiles highlighting mitochondria-related genes betweenin vivoandin vitrofertilized early mouse embryos. Hum Reprod 2015;30:2892–2911. dev228. [DOI] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril 2006;86:1252–1265. 1265 e1251-1236. [DOI] [PubMed] [Google Scholar]
- Schatten H, Sun Q-Y, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol 2014;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Strauss GA, Herrick K, Schoolcraft J, Krisher R W. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reprod Fertil Dev 2015;27:975–983. epub. [DOI] [PubMed] [Google Scholar]
- Takeo S, Goto H, Kuwayama T, Monji Y, Iwata H. Effect of maternal age on the ratio of cleavage and mitochondrial DNA copy number in early developmental stage bovine embryos. J Reprod Dev 2013;59:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, Morrison L, Morin SJ, Scott RT Jr. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod 2017;32:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol 2009;20:354–364. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011;11:797–813. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Motta PM. The Cellular Basis of Mammalian Reproduction. Baltimore and Munich: Urban and Schwartenberg, 1979. [Google Scholar]
- Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, Viotti M. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril 2017;107:34–42 e33. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 2010;83:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenström U, Engström A-B, Hellberg D, Nilsson S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril 2009;91:2461–2465. [DOI] [PubMed] [Google Scholar]
- Wang L-y, Wang D-h, Zou X-y, Xu C-m. Mitochondrial functions on oocytes and preimplantation embryos. J Zhejiang Univ Sci B 2009;10:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001;16:909–917. [DOI] [PubMed] [Google Scholar]
- Yedwab GA, Paz G, Homonnai TZ, David MP, Kraicer PF. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertil Steril 1976;27:304–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.