Abstract
Common gamma receptor–dependent cytokines and their JAK/STAT pathways play pivotal roles in T cell immunity. Abnormal activation of this system was pervasive in diverse T cell malignancies assessed by pSTAT3/pSTAT5 phosphorylation. Activating mutations were described in some but not all cases. JAK1 and STAT3 were required for proliferation and survival of these T cell lines whether or not JAKs or STATs were mutated. Activating JAK and STAT mutations were not sufficient to initiate leukemic cell proliferation but rather only augmented signals from upstream in the cytokine pathway. Activation required the full pathway, including cytokine receptors acting as scaffolds and docking sites for required downstream JAK/STAT proteins. JAK kinase inhibitors have depressed leukemic T cell line proliferation. The insight that JAK/STAT system activation is pervasive in T cell malignancies suggests novel therapeutic approaches that include antibodies to common gamma cytokines, inhibitors of cytokine-receptor interactions, and JAK kinase inhibitors that may revolutionize therapy for T cell malignancies.
Keywords: γc cytokines, JAK/STAT, T cell lymphoma
INTRODUCTION
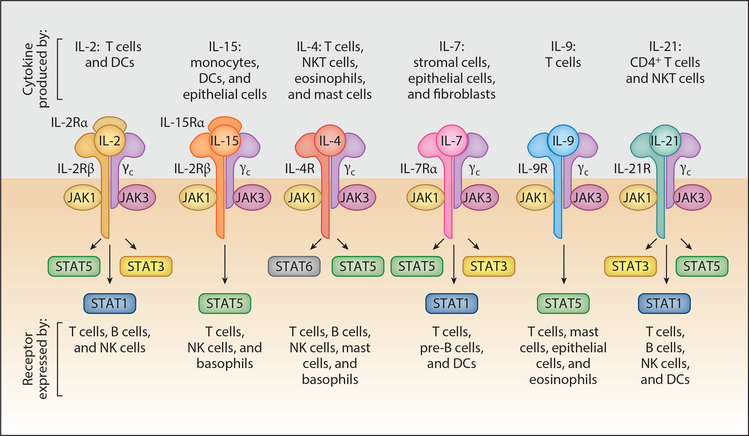
The common gamma (γc) receptor–dependent cytokines IL-2, -4, -7, -9, -15, -21 and their receptors and signaling pathways play critical roles in T cell immune responses (1–5) (Figure 1). The receptors for these cytokines contain γc as well as cytokine-specific a chains. IL-2 and IL-15 receptors share an additional subunit, IL-2/IL-15Rβ. These receptors have no intrinsic kinase activity; hence, they are tightly associated with Janus kinase (JAK) cytoplasmic tyrosine kinases. There are four JAK cytoplasmic kinases: JAK1, JAK2, JAK3, and TYK2. Cytokine binding to cytokine receptors induces conformational changes that yield receptor dimerization, cross-JAK tyrosine phosphorylation, and phosphorylation of the intracellular tail of the cytokine receptors. This action creates docking sites for signal transducer and activator of transcription (STAT), which leads to STAT phosphorylation homodimerization and nuclear translocation where phosphorylated dimers act as transcription factors. There are seven mammalian STAT proteins: STAT1, STAT2, STAT3, STAT4, STAT5A and STAT5B (referred to together as STAT5), and STAT6 (1–5). In addition to canonical cytokine signaling, there are noncanonical effects of JAK signaling that are important in normal and lymphoma biology (6–10). In particular, JAK2 cooperating with the histone demethylase JMJD2C can remodel the epigenome, augmenting lymphomagenesis (6). Furthermore, in natural killer/T cell lymphomas (NKTCLs) JAK3 may phosphorylate EZH2, the functional enzymatic component of the Polycomb Repression Complex (PRC2), converting it from a transcriptional repressor, its canonical role, into a transcriptional activator via methylation of lysine 27 in histone 3 (H3K27), thereby augmenting expression of a series of target genes that are involved in malignant T cell proliferation (9). In addition, the phosphorylation of STAT3 may induce its translocation to the mitochondria, where it induces cell death (10).
Figure 1.
Receptors for γc family cytokines. Shown are the receptors for IL-2, -15, -4, -7, -9, and -21. The receptors share the γc chain and express a cytokine-specific a chain. Furthermore, IL-2 and IL-15 use a third receptor chain, the IL-2/IL-15R β chain. The receptors for each γc family cytokine activate JAK1 and JAK3. The STAT activated by the cytokine receptors is shown, with STAT5 referring to both STAT5A and STAT5B. Abbreviations: DC, dendritic cell; NK cell, natural killer cell; NKT cell, natural killer T cell. Modified from Figure 1 in Reference 1, with permission.
Recently, disorders involving activation of the γc/JAK/STAT system were identified in virtually all forms of T cell leukemia/lymphomas (11–34). Migone and coworkers (12) demonstrated activation of the JAK/STAT pathway in T cells transformed with human T cell lymphotropic virus 1 (HTLV-1). Subsequently, activation of the γc/JAK/STAT system was identified in large granular lymphocytic (LGL) leukemia (13–15), ALK-negative and ALK-positive anaplastic large cell lymphoma (ALCL) (16–18), nasal-type natural killer cell lymphoma (NKCL) (19–22), γδ T cell lymphoma (23, 24), prolymphocytic leukemia (PLL) (25–27), Sézary syndrome (28–30), early T cell precursor (ETP) acute lymphoblastic leukemia (ALL) (31), angioimmunoblastic T cell lymphoma (AITL) (32), and HTLV-1-associated adult T cell leukemia/lymphoma (ATLL) (33–34).
Most studies of T cell lymphomas have focused on JAK/STAT mutations. Historically, acute leukemias were the first T cell malignancies associated with a JAK mutation. The TEL-JAK2 fusion protein was observed as a gene product of (9;12) (p24; p13) in a patient with T cell ALL (T-ALL) (35–37). The chimeric protein contained the oligomerization domain of ETS protein TEL and the JH1 tyrosine domain of JAK2. STAT5 was essential for lymphoproliferative disease in mice with TEL-JAK2. Subsequently, several similar JAK2 fusion proteins were described in ALL and ALCL (38). As noted, JAK2 may enter the nucleus and act as an epigenetic regulator (6). Furthermore, JAK2 mutations, in particular substitutions of valine for phenylalanine at codon 617 of JAK2 (V617F), were identified in patients with chronic myeloproliferative disorders (39–46).
STAT and JAK mutations were shown to be prevalent in variable proportions of diverse T cell malignancies, indicating that the mutations alone do not account for the pathogenesis of all lymphomas. Koskela et al. (13) identified somatic STAT3 mutations in 40% of 77 patients with LGL leukemia with hotspots in the Src homology-2 (SH2) domain, which mediates dimerization and activation of STAT. Activating mutations of STAT3 and/or STAT5B have subsequently been demonstrated in patients with ALK-negative ALCL (16–18), peripheral T cell lymphoma not otherwise specified(PTCL-NOS)(47),or PLL(25–27). Uponanalysis, the mutational hotspot of STAT 5BN642H was not sufficient to initiate leukemic cell proliferation; rather, this mutation only augmented signals from above in the cytokine-cytokine/receptor JAK pathway. Activating mutations of JAK1 and/or JAK3 were identified in a major proportion both among ALK-negative ALCL patients and among PLL patients and were found in a minority of patients with ATLL as well as among those with ETP-ALL and in those with Sezarý syndrome (16, 25, 30, 34). In general, however, even in the presence of STAT or JAK mutations the whole functional cytokine receptor, JAK, and STAT were required to maintain activation and malignant cell proliferation, and there are other mechanisms by which activation of the JAK/STAT pathway can be found in nearly all lymphomas.
This review focuses on the abnormal activation of the JAK/STAT system that is pervasive in diverse T cell malignancies. Furthermore, it characterizes activating mutations of STAT identified in many but not all T cell malignancies. Taken as a whole, mutations of the JAK and STAT system in T cell malignancies have involved predominantly the SH2 domain of STAT3 and STAT5B and the pseudokinase domain of JAKs 1 and 3. Furthermore, the activating mutations were not sufficient on their own to drive cell proliferation but only augmented signals from above, that is, from cytokine interaction with cytokine receptors. The review also explores the activation of the JAK/STAT pathway in T cell malignancies in the absence of JAK/STAT mutations.
The demonstration of activation of the JAK/STAT pathway in a major proportion of T cell malignancies suggests JAKs as potential therapeutic targets that may help revolutionize multiagent therapy in select patients. The JAK1/2 inhibitor, ruxolitinib, and pan-JAK inhibitor, tofacitinib, were effective in inhibiting proliferation of a variety of T cell malignant cell lines and ex vivo malignant cells. To translate these insights, these inhibitors and many alternative, more specific JAK1 and JAK3 inhibitors are being evaluated in treatment of select patients with T cell malignancies (48–51).
JAK/STAT ACTIVATION IN T CELL LYMPHOMA
JAK/STAT activation can be assessed by phospho-STAT3 and STAT5 expression and nuclear localization, as well as by monitoring the negative effects of JAK inhibitors. Such JAK/STAT activation was shown to be present in a proportion (ranging from rare to 86%) of patients with virtually any form of T cell malignancy. In particular, activation of the γc cytokine, JAK1/3, STAT3, STAT5 pathway in malignant T cells has been a prominent feature in NK/TCL (19–22), ALK-positive and ALK-negative ALCL (16–18), LGL leukemia (13–15), PLL (25–27), Sézary syndrome, and mycosis fungoides (28–30), as well as in smoldering and chronic ATLL (33, 34). Phosphorylation, nuclear translocation, and a pattern of gene dysregulation of STAT3 were demonstrated in patients with LGL leukemia (13). However, many of these features were found not only in patients whose leukemic cells manifested STAT3 mutations but also in leukemias with wild-type STAT3. Treatment with a STAT3 inhibitor induced apoptosis in an increased proportion of leukemic LGLs but had little effect on normal lymphocytes. Again, effects of inhibitors were not specific for STAT3 mutant cells in that leukemic cells of patients without STAT3 mutations showed a similar response. JAK3 phosphorylation was identified on tyrosine 980 in 3 of 4 nasal-type NKCL cell lines and 20 of 23 NKCL tumor samples (20). However only in 1 cell line and 4 of 19 NKCL primary tumor samples was JAK3 activation associated with JAK/STAT mutations. In ALK-positive ALCL the ALK element interacted directly with STAT3 to activate it without involving cytokine receptors or JAKs (52, 53). Therefore, JAK inhibitors were not effective. Examination of ALCL tumors demonstrated an overall pSTAT3Tyr705 positivity with nuclear localization in 49 (82%) of 60 ALCL tumors, 26 (84%) of 31 ALK-positive ALCL tumors, and 23 (47%) of 49 ALK-negative ALCL tumors (16–18). Nonetheless, the majority—20 (74%) of 27—of pSTAT3-positive ALK-negative ALCLs displayed no recurrent JAK/STAT3 mutations. The spontaneous ex vivo proliferation of ATLL cells that was due to HTLV-1-Tax transactivation of γc cytokines IL-2, IL-9, and IL-15 was associated with activation of JAK/STAT proteins, and the proliferation was inhibited by the addition of tofacitinib (12, 33, 54, 55). STAT3 activation was demonstrated in Sézary syndrome leukemic cells, and tyrphostin AG490, a JAK inhibitor, diminished growth of such leukemic Sézary cells (28). These observations support the view that the activation of the γc cytokine receptor, JAK/STAT system is common in virtually all forms of T cell malignancy with and without mutations.
STAT3/STAT5B MUTATIONS IN T CELL LYMPHOMAS
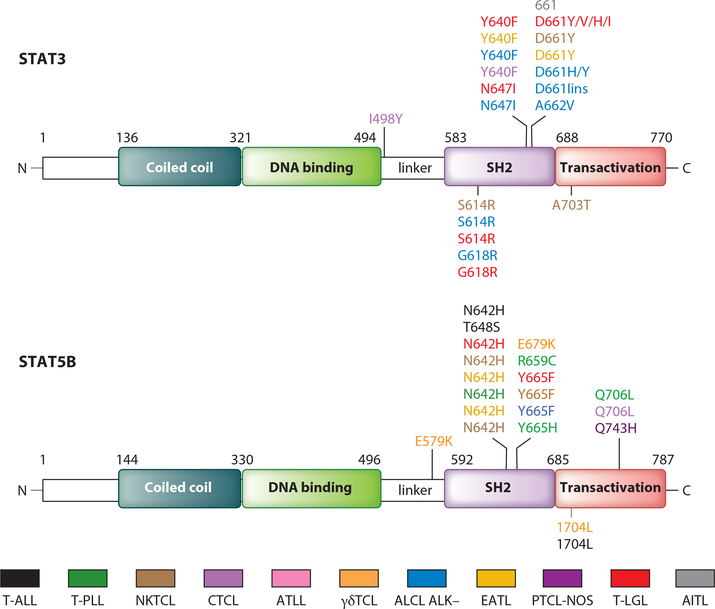
In light of frequent activation of the JAK/STAT system in T cell malignancies, such leukemias were examined for activating mutations of STAT proteins. Many T cell malignancies manifested STAT3/STAT5B mutations that involved their SH2 domain, which has multiple functions, including docking to phosphorylated tyrosine residues, transient binding to cytokine receptors, and binding to another STAT protein, which mediates their dimerization and nuclear localization (56, 57). Koskela et al. (13) first identified somatic STAT3 mutations in 31 (40%) of 77 patients with LGL leukemia (Figure 2, Table 1). Mutational hotspots were identified including Y640F in 11–17%, D661Y in 9–11%, and rare mutations in N647l, D661H, D661l, S614R, and G618R in exon 21 of the SH2 domain (13, 14). STAT3 mutations were also common in various T cell malignancies, including ALK-ALCL (N647I, D661H), D661ins and D661Y (16–18), GD-HSTCL (Y640F, D661, YG618R) (23–25), NKTCL (S614R, D661Y) (19–22), PTCL-NOS (Q743H) (47), and CTCL (I498V, Y640F) (30). STAT5B mutations in the SH2 domain at N642H and Y665F were reported in a small proportion of patients with mycosis fungoides (58) and aggressive LGL leukemia (59–60). The mutations were activating and led to increased phosphorylation and transcriptional activity of STAT5B. Comparable SH2 STAT5B mutations were demonstrated in hepatosplenic γδ lymphoma (N642H, E679K, I704L), NKTCL (N642H, Y665F) enteropathy associated T cell lymphoma (EATL) (N642H), CTCL (N642H, Q706L), T cell PLL (T-PLL) (N642H, R659C, Y665H, Q706L), and T-ALL (T648S, N642H, and Q706L) (16–31). Frequent STAT5B mutations were also defined in γδ hepatosplenic T cell lymphomas (23, 24) with STAT3 mutation hotspots Y640F and D661Y, whereas with STAT5B, N642H was a mutational hotspot (Figure 2). Küçük et al. (23) demonstrated that STAT5B mutations were associated with increased expression of phosphorylated STAT5B protein and a growth advantage of transduced cell lines or normal NK cells. Nevertheless, such activating STAT mutations were not sufficient to initiate leukemic cell proliferation but only enhanced upstream signals from the cytokine, cytokine receptor, JAK/STAT pathway. This group demonstrated with molecular modeling and surface plasmon resonance measurements of N642H mutants that there was an increased binding affinity of phosphotyrosine-Y699 with mutant histidine. To determine if this STAT5B-N642H mutation was associated with prolonged STAT5B activation in the presence of upstream JAK/STAT pathway activation, Küçük performed Western blot analyses on IL-2-activated YT cells in a time-course experiment where they evaluated pSTAT5 expression (23). They incubated STAT5B wild type– or N642H mutant–transduced YT cells with IL-2 for 30 min and evaluated pSTAT5 expression for up to 6 h following IL-2 withdrawal. pSTAT5 expression disappeared 1 h after transient IL-2 stimulation with STAT5B wild type–transduced cells, whereas it persisted for more than 6 h in N642H-transduced cells (23). These studies suggested that N642H mutations increased affinity of the phospho-STAT5B dimer element interaction and resulted in a more persistent activation, but only when provided with a signal from upstream in the pathway. In accordance with this view, the growth-promoting activity of the mutant was partially inhibited by addition of the JAK1/2 inhibitor, AZD1480.
Figure 2.
STAT mutations discovered in T cell malignancies. Color-coded representation of the location of each mutant residue within the domain structure of each STAT protein. The majority of mutations in STAT proteins were found within the SH2 domain with mutational hotspots at STAT3 Y640F and D661Y, and for STAT5B at N642H and Y665F. Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T cell leukemia/lymphoma; CTCL, cutaneous T cell lymphoma; EATL, enteropathy-associated T cell lymphoma; γδTCL, hepatocellular γδ peripheral T cell lymphoma; NKTCL, extranodal natural killer T cell lymphoma; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; T-ALL, early T cell precursor acute lymphoblastic leukemia; T-LGL, T cell large granular lymphocytic leukemia; T-PLL, T cell prolymphocytic leukemia.
Table 1.
JAK and STAT mutations in T cell lymphoma
| T cell disease | JAK mutation | STAT mutation | Other mutations/fusions | Reference(s) |
|---|---|---|---|---|
| Extranodal NKTCL | JAK1: Y652D JAK3: C565DEL, A572V, A573V, V722I | STAT3: S614R, D66IY, A703T, STAT5B: N642H, Y665F | 20, 22, 23 | |
| Hepatosplenic γδTCL | STAT3: Y640F, D661Y STAT5B: N642H, Y665F, E679K | 23 | ||
| CTCL, including Sézary syndrome | JAK1: Y654F, L710VJAK3: Y123H, A573V, S989I | STAT3: I498Y, Y640F STAT5B: N642H, Q706L | 26, 30, 58 | |
| HTLV-1 ATLL | JAK3: L156P, R172Q, E183G | 34,64 | ||
| PTCL-NOS | JAK3: L1073F | STAT5B: Q743H | IL-7RQ445* | 95 |
| ETP-ALL | JAK1: S512L, A634D, V658F, R724H/Q, V782M, L783F, R879S/C/H JAK2: R683S/G/Q, I682F, R867Q, D873N, P933R JAK3: V715ITYK2: V15A, G36D, S47N, R274H, R425H, V731I, E957D, R1027H | IL-7R, TEL-JAK2 | 31,35,37, 65, 69, 70 | |
| AITL | JAK2: V617F, G511S | 614,661 | 32 | |
| NK, LGL leukemia | JAK1: Y652DJAK3: C365del, A572V, A573V, V722I, L1073F | STAT3: S614R, G618R, Y640F, N647I, D661Y/VHI, STAT5B: N642H, Y665F | 13–15, 59 | |
| ALK-negative ALCL | JAK1: G1097D/S/V, Y640F, L910P | STAT3: S614R, G618R, Y640F, N647I, D661H/Y, lins, A662V | NFB2-ROS1, NFB2-TYK2, PABPC4-TYK2, lead to constitutive pSTAT3 activation | 16–18, Chen etal. 2016a |
| T-PLL | JAK1: L653F, Y658FJAK3: Q503H, Q507P/H, M511I, A572V, A573V, R629D, 630del, L653F, R657Q/W, V658F, V674F/A, V678L, V722I, Y824D, L857P | STAT5B: N642H, R659C, Y665H, Q706L | IL-2RG: M270 del, K315E | 25–27 |
| EATL | STAT5B: N642H | 23 |
J. Chen, Y. Zhang, M. Petrus, W. Xiao, A. Nicolae, et al., submitted manuscript.
Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ALL, acute lymphoblastic leukemia; ATLL, adult T cell leukemia/lymphoma; CTCL, cutaneous T cell lymphoma; EATL, enteropathy-associated T cell lymphoma; ETP, acute lymphoblastic leukemia; γδTCL, γδ T cell lymphoma; HTLV-1, human T cell lymphotropic virus 1; LGL, large granulocytic leukemia; NKTCL, natural killer cell lymphoma; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; T-PLL, T cell prolymphocytic leukemia.
In studies with ALK-negative T cell lines using shRNA loss-of-function analyses, our group has demonstrated that cell lines that manifested pSTAT3 were addicted to STAT3 whether or not STAT elements were mutated, suggesting importance of STAT3 activation in leukemic cell survival and that there are multiple mechanisms for STAT3 activation (J. Chen & T.A. Waldmann, unpublished observations). Nevertheless, when mutations in the SH2 domain of STAT proteins are present in T cell malignancy, they appear to augment dimerization and nuclear localization of the STAT proteins when there were upstream signals.
JAK MUTATIONS
Since STAT-activating mutations were not sufficient but rather only enhanced upstream signaling, requirement for JAK activation was explored. We investigated whether upstream signaling through JAK1 was involved in activation of STAT3 in phospho-STAT3-positive, ALK-negative cell lines (J. Chen & T.A. Waldmann, unpublished observations). Using shRNA loss-of-function of JAK1, we found cell death was induced in phospho-STAT3-positive, ALK-negative cell lines, whereas it had no effect on phospho-STAT3-negative cell lines. JAK1 shRNA treatment not only decreased expression of JAK1 protein but also decreased STAT3 phosphorylation.
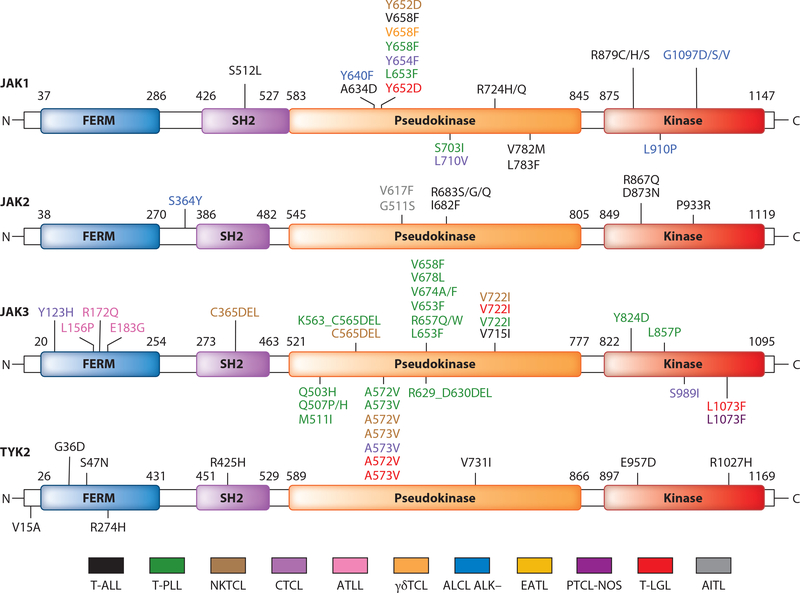
Given the requirement for activated JAKs, especially JAK1 and JAK3, for activation of the γc cytokine, JAK/STAT pathway, leukemic T cells were examined for JAK mutations (Figure 3, Table 1). Again, activation of the system was observed both in the presence and in the absence of activating JAK mutations. In contrast to JAK2-V617F mutations found in myelodysplastic syndromes, the majority of mutations in T cell malignancies were in JAK1 or JAK3. The majority of these mutations were in the pseudokinase domain that was reported to function as a protein kinase inhibiter that phosphorylates two residues that negatively regulate JAK kinases to suppress activity of tyrosine kinase (61, 62). This regulation is required to keep JAKs inactive in the absence of ligand stimulation. In the presence of a cytokine the pseudokinase domain has a second function: connection of signaling events to receptor activation (61, 62). Convergent activating mutations of JAK1 and/or STAT3 genes were present in 20% of 88 ALK-negative ALCLs, and 7% of systemic ALK-negative ALCLs displayed double lesions (16). The mutation hotspot of JAK1 involved Y658F, and with JAK3, A572V and A573V were hotspots of mutation (16). Ruxolitinib, an inhibitor of JAK1/JAK2, was associated with significant tumor growth inhibition in ALK-negative ALCL–bearing mice carrying the JAK1 and STAT3 mutations, supporting an essential role for the JAK/STAT pathway in tumor growth.
Figure 3.
JAK mutations discovered in T cell malignancies. Color-coded representation of the location in each mutant residue within the domain structure of each JAK protein. The majority of mutations in the JAK proteins were found within the pseudokinase with mutational hotspots at JAK1 Y658F and at JAK3 A572V, A573V. Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T cell leukemia/lymphoma; CTCL, cutaneous T cell lymphoma; EATL, enteropathy-associated T cell lymphoma; γδTCL, hepatocellular γδ peripheral T cell lymphoma; NKTCL, extranodal natural killer T cell lymphoma; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; T-ALL, early T cell precursor acute lymphoblastic leukemia; T-LGL, T cell large granular lymphocytic leukemia; T-PLL, T cell prolymphocytic leukemia.
JAK3 mutations in the FERM domain were detected in 3 of 36 cases of HTLV-1-associated ATLL (34, 64), CTCL, and extranodal nasal-type NKCL. Activating mutations, predominantly A572V and A573V, were demonstrated by Koo et al. (22) in 23 (35.4%) of 65 NKCL cases. STAT activation with JAK3 phosphorylation on tyrosine 980 was demonstrated in 20 (87%) of 23 NKCL tumor samples (20). In 1 (25%) of 4 cell lines and 4 (21%) of 19 NKCL primary tumor samples, JAK3 activation was related to a mutation (A573V or V722I) in the JAK3 pseudokinase domain. In a human NKCL xenograft mouse model, tumor growth was significantly delayed by administration of tofacitinib.
Fourteen mutations were identified in the JAK3 gene in 11 (34%) of 32 patients with T-PLL; the most frequent mutation was M511I (25). Missense JAK3 mutations were also described in 19 (42%) of 45 T-PLL cases (26) and were observed affecting IL-2Rα, JAK1, JAK3, or STAT5B in 38 (76%) of 50 patients with T-PLL (27). These mutations were known to induce proliferation in in vitro models (64).
Many distinct JAK1 mutations were associated with ETP-ALL (31). Somatically acquired JAK1 mutations were identified in 18% of patients with T-ALL that conferred IL-3-independent growth in Ba/F3 cells (65). JAK1 mutations were reported in 1 (0.5%) and JAK2 mutations in 16 of 187 cases of high-risk ALL (66). Somatic gain-of-function mutations affecting JAK1 or JAK3 were revealed in a small proportion [2/66 (3%) each] of patients with Sézary syndrome and a small proportion [3/36 (8%)] of those with ATLL. It is clear from our JAK1 knockdown studies, discussed above, that JAK1 is required in cells that manifest activation of the cytokine receptor JAK/STAT system whether mutations are present or absent. As with the STAT mutations, JAK mutations appear to be incapable of initiating proliferation of observed cell responses but rather only augment responses initiated by upstream signals. In summary, upstream signaling through either JAK1 or JAK3 by leukemic T cells appears to be necessary but not sufficient for the survival of pSTAT5-activated malignant T cells.
FUNCTIONAL CYTOKINE RECEPTORS ARE REQUIRED IN THE pSTAT EXPRESSION OF T CELL MALIGNANCIES
Studies by Lu and coworkers (67) and Hornakova and coworkers (68) indicate that even with activating JAK mutations there is a requirement for expression of a functional cytokine receptor that plays two roles, first as a scaffold for cross-activation of JAK kinases, and second as a docking site for recruitment of STAT transcription factors (67, 68). Lu and coworkers (67) demonstrated that expression of a functional homodimeric type 1 cytokine receptor such as the erythropoietin receptor (EpoR) was required for transformation of hematopoietic cells to growth factor independence (67). Furthermore, EpoR mutations that impaired erythropoietin-mediated JAK2 or STAT5 activation also impaired transformation mediated by the JAK2V617F kinase, indicating that this mutant JAK kinase required a cytokine receptor scaffold for its transforming and signaling activities (67). Supporting this view, Hornakova and coworkers (68) examining JAK1 mutations demonstrated that either the IL-9Rα or IL-2/15Rβ receptor was required for STAT5 activation, whereas STAT3 activation was detected only in the presence of IL-9Rα. Thus, a cytokine receptor was required to obtain pathway activation, even when tumors expressed activating JAK mutations.
MOLECULAR DISORDERS OF GAMMA CYTOKINE/RECEPTORS ASSOCIATED WITH JAK/STAT ACTIVATION
As noted above, although mutations of STAT3/STAT5B and JAK1/JAK3 were frequent, activation of the receptor-signaling pathway was much more pervasive. A number of other disorders were responsible for the activation of the pathway, including mutations of the cytokine receptors, increased expression of select gamma cytokines, and disorders of protein phosphatases normally involved in negative regulation of the JAK/STAT system.
In addition to the requirement for a cytokine receptor as a scaffold and docking site, Shochat et al. (69) and Zenatti et al. (70) demonstrated gain-of-function mutations of the IL-7 receptor, IL-7Rα, exon 6, in patients with childhood ALL. These mutations involved either a serine-to-cysteine substitution at amino acid 185 in the extracellular domain or in-frame insertions and deletions in the transmembrane domain. Impaired cysteine receptors promoted formation of intermolecular disulfide bonds between IL-7Rα subunits (69). These mutations were associated with aberrant expression of cytokine receptor like–factor 2 (CRLF2). Mutant IL-7R proteins formed a functional receptor with CRLF2 for thymic stromal lymphopoietin (TSLP) (69). Thus, molecular abnormalities of the IL-7 receptor are responsible for some of the activation of the receptor-signaling pathway.
DISORDERS IN ADDITION TO γc RECEPTOR JAK/STAT MUTATIONS THAT YIELD ACTIVATION OF THE γc/JAK/STAT SYSTEM
In addition to activating mutations of the receptor, JAK/STAT system, certain T cell malignancies were shown to have an increased expression of select γc cytokines that led to augmented JAK/STAT signaling. Such increases in γc cytokine production were observed in HTLV-1-associated ATLL.
In particular, although JAK3 FERM domain mutations were only rarely observed in adult HTLV-1-associated ATLL, the γc cytokine, JAK/STAT pathway was usually activated in patients with smoldering and chronic forms of ATLL (12, 33, 54, 55). We demonstrated that the HTLV-1-encoded Tax protein transactivated two autocrine loops (IL-2/IL-2Rα, IL-15/IL-15Rα)and one paracrine loop (IL-9/IL-9Rα) in such patients (55, 71, 72). These cytokine/cytokine receptor loops led to activation of the JAK1/3 and STAT5 signaling pathway and were associated with ex vivo spontaneous proliferation of ATLL cells that was inhibited by tofacitinib (49).
There is evidence supporting a role for the γc uptake for IL-15 in CTCL, including IL-15 expression in CTCL tumor tissues (73–76). Furthermore, IL-15 was stimulatory for CTCL cells in vitro and was a growth factor for Sézary cell lines (29). Analysis of ex vivo patient Sézary cells and three CTCL lines by RT-PCR indicated that these cells expressed IL-15 mRNA. In CTCL cells there was a downregulation of ZEB1, a candidate tumor suppressor, that normally inhibits IL-2 and IL-15 expression (75, 76). On the basis of these studies, Döbbeling et al.(74)concluded that IL-15 is a growth viability factor for CTCL-derived cell lines and plays an important role in CTCL biology. The T cell malignancy AITL has been associated with an increase in IL-21 expression (77). Both ATLL and CTCL are characterized by increased γc cytokine expression: IL-2, -9, and -15 in ATLL and probably IL-15 in CTCL.
These observations taken as a whole support the view that persistent activation of the JAK/STAT pathway in T cell malignancy requires that all elements of the pathway be present, and disorders of each of these elements have been reported to play a role in select patients with a T cell malignancy.
DEFECTS IN THE NEGATIVE REGULATION OF THE JAK/STAT SYSTEM
Enhanced signal trafficking through the JAK/STAT system can arise from either upregulated cytokine, tyrosine kinase, STAT function, as discussed above, or loss of negative regulation. Protein tyrosine phosphatases are involved in negative regulation of the JAK/STAT system. Loss-of-function mutations in protein tyrosine phosphatase nonreceptor type 2 (PTPN2) have been identified in 6% of T-ALL cases (78, 79). Knockdown of PTPN2 increased the duration of JAK1 and STAT1 phosphorylation, cellular proliferation, and sensitivity of T-ALL cell lines or primary mouse T-ALL cells when stimulated with IL-2 or IL-7. Loss-of-function mutations of receptor tyrosine phosphatase PTPRC (CD45) were identified in T-ALL. Furthermore, downregulation of phosphatase CD45 was associated with increased signaling downstream of phosphatase (80).
Suppressors of cytokine signaling (SOCS) are a family of intracellular proteins that negatively regulate JAK/STAT signal transduction by combining direct inhibitory interactions with cytokine receptors and signaling proteins with a mechanism of targeting proteins for degradation (81). SOCS proteins are silenced in many tumors by hypermethylation of CpG islands in their promoters, including that of SOCS3 in NKCL (81). SOCS3 binds and directly inhibits the catalytic domains of JAK1 and JAK2 proteins. Furthermore, aberrant hypermethylation of SOCS3 was identified in the promoter in 3 of 16 T cell lymphoblastic lymphoma samples (82). This observation suggests that epigenetic alterations in activation of SOCS3 might be a modulatory event in T cell lymphoblastic lymphoma development. Thus, activation of the JAK/STAT system may arise from either upregulation of the function of any of the elements of the gamma cytokine, JAK/STAT pathway or loss of normal negative regulation of this pathway.
THE γc CYTOKINE, JAK/STAT SYSTEM AS A TARGET IN THE TREATMENT OF T CELL MALIGNANCIES
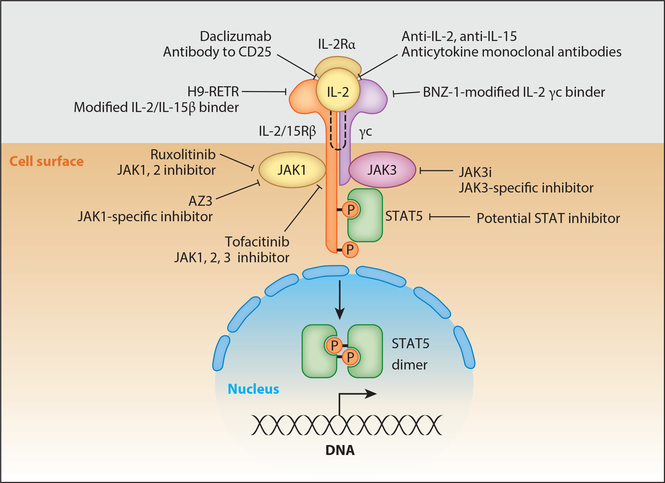
Disorders of the gamma cytokine and JAK/STAT signaling pathways are pervasive in T cell malignancies, suggesting new molecular targets and novel therapeutic opportunities that may revolutionize the treatment of these tumors, which are usually associated with a very poor prognosis. The insight that disorders of the γc cytokine, JAK/STAT system are pervasive in T cell malignancies provides the rationale for new therapeutic approaches, including those that target the γc cytokine directly, those that block cytokine-receptor interactions, and especially JAK kinase inhibitors that may revolutionize the therapy of T cell malignancies. Such diverse approaches include the use of neutralizing antibodies to γc receptor–dependent cytokines (IL-2, IL-15, IL-21), blocking antibodies to their receptors (anti-IL-2Rα, daclizumab, basiliximab, anti-IL-2/IL-15Rβ,Hu-Mik-β-1), small-molecule inhibitors interdicting cytokine-cytokine receptor interactions (BNZ-1, H9-RETR), JAK kinase inhibitors (ruxolitinib, tofacitinib) as well as JAK1-and JAK3 (JAK3i)-specific agents, and finally inhibitors of STAT action (Figure 4). Anti-cytokine antibodies to IL-15 have been evaluated in rheumatoid arthritis and may be of value in the treatment of CTCL where disorders of this cytokine have been identified (83).
Figure 4.
Examples of therapeutic agents that are of value in the treatment of T cell malignancies with associated disorders of the γc cytokine, JAK/STAT signaling pathway.
The studies discussed above suggest that, in select T cell malignancies where abnormal cytokine/cytokine-receptor interactions are involved (e.g., ATLL, CTCL), agents that inhibit the interaction of cytokines with their receptors might be of value. Monoclonal antibodies directed toward γc cytokine receptors including the IL-2 receptor subunit, IL-2Rα, have been used in immunotherapy (84, 85). The rationale for this target was that IL-2Rα is not expressed by most normal resting cells with the exception of regulatory T cells but is expressed by various T cell leukemias. Daclizumab (anti-Tac, Zenapax) was shown to be of value in the treatment of patients with smoldering and chronic ATLL (84, 85).
Recently two modifications of IL-2 have been generated that block binding of normal IL-2 and IL-15 to IL-2/IL-15Rβ and γc receptors, thereby simultaneously inhibiting the actions of both IL-2 and IL-15. BNZ-1 binds only to the γc chain, not IL-2/ IL-15Rβ, whereas H9-RETR binds tightly to IL-2/IL-15β but not to γc. Both agents prevent the heterodimerization of IL-2/IL-15Rβ with γc chains that is required for signaling (86, 87). These agents are being evaluated in murine xenograft models of ATLL.
Although there are diverse mechanisms for activation of the JAK/STAT pathway in T cell malignancies, essentially all require the activation of JAK for malignant cell proliferation, thus providing the scientific basis for the use of JAK inhibitors for the treatment of patients with T cell malignancy (46, 88, 89). JAK inhibitors depress proliferation of T cell lymphoma lines or that of ex vivo cells transfected with activating JAK or STAT mutations that were observed with T cell leukemias, also supporting use of such inhibitors as an element in the therapy of T cell malignancies (20–22, 49). The best biomarker suggesting that JAKs would be a rational target was the presence of activation (e.g., pSTAT3 or pSTAT5 and their nuclear translocation) rather than less frequent JAK/STAT mutations affecting this system. HTLV-1 Tax transactivates IL-2, IL-15, and IL-9 that signal through JAK1/JAK3 STAT5, suggesting use of a JAK1/3 inhibitor. The JAK1/2 inhibitor, ruxolitinib, and pan-JAK1/2/3 inhibitor, tofacitinib, inhibited ex vivo proliferation and phospho-STAT expression of ATLL cells (49). To translate these observations, a clinical trial of ruxolitinib is underway in patients with smoldering and chronic ATLL (51). In addition, tofacitinib, an inhibitor approved by the FDA for treatment of patients with rheumatoid arthritis, was proposed as a novel salvage therapeutic agent for refractory LGL leukemia (90). Among 9 patients with LGL leukemia and rheumatoid arthritis treated with tofacitinib, there was a hematologic response in 6, with 8 manifesting an improvement of arthritis symptoms and improvement in neutropenia in 5 of 7 evaluable patients.
With rare exceptions, T cell malignancies are associated with activation of γc cytokine, JAK1/JAK3, STAT3/STAT5 signaling but not activation of JAK2. However, both ruxolitinib and tofacitinib inhibit the off-target JAK2 kinase in addition to the desired JAK1 and JAK3. JAK2 inhibition interferes with signaling mediated by thrombopoietin, erythropoietin, IL-3, IL-5, and GM-CSF, leading to thrombocytopenia, anemia, and neutropenia. This cytopenia was associated with an increased incidence of infections including herpes zoster and tuberculosis (46). In light of toxicities, due to JAK2 inhibition, agents with greater specificity for JAK1 or JAK3 are being developed and are in the clinic. An example is filgotinib, which preferentially inhibits JAK1. Nevertheless, as a JAK1 inhibitor, it has activities that inhibit cytokines other than the γc cytokines.
JAK3 is an alternative, attractive target for specific inhibition. JAK3 expression is restricted to cells of hematopoietic lineage and is exclusively associated with the γc chain (2). Mutations of JAK3 in humans or mice result in abnormalities restricted to severe combined immunodeficiency (2). However, Haan and coworkers (91) have reported that JAK1 has a dominant role over JAK3 in signal transduction through γc-containing cytokine receptors. These authors concluded that JAK3 predominantly plays a role as a scaffold, and they disagree with the proposal that selective ATP-competitive JAK3 kinase inhibitors would be effective therapeutic agents. In contrast, Smith et al. (92) demonstrated that a selective covalent inhibitor of JAK3 (JAK3i) blocked IL-2-stimulated T cell proliferation at low-nanomolar concentrations with great selectivity. This observation reflected temporal dissection of IL-2 signaling wherein they revealed a biphasic role for JAK3 catalytic activity in CD4+ T cells. JAK3i blocked a second temporal wave of IL-2-mediated signaling. Smith et al.’s (92) results contradict the prior conclusions of Haan et al. (91) that discounted the catalytic role of JAK3 and revealed a preferential requirement for JAK3 kinase activity in a second wave of IL-2-mediated signaling.
As an alternative focus, STATs would appear to be logical targets for therapeutic agents (43, 46, 89, 93). However, despite considerable efforts, development of effective STAT inhibitors has been challenging. Nevertheless, STAT3 decoys to block STAT binding are being evaluated, and efforts are being made to block phosphorylation of STATs to disrupt SH2 domains to interfere with dimerization or with STAT DNA binding. In addition, a number of drugs have been repurposed as nonspecific STAT inhibitors; these include lisofylline, fludarabine, pimozide, sulforaphane, pyrimethamine, and curcumin (43, 46).
If they are to be of major value in the treatment of T cell malignancies, JAK/STAT inhibitors will probably have to be used in combination therapy. To develop such a combination, we evaluated ruxolitinib in a high-throughput matrix screen combined with other potential therapeutic agents (94). The Bcl-2/Bcl-xL inhibitor, navitoclax, was identified as a strong candidate for multicomponent therapy with ruxolitinib. Ruxolitinib and navitoclax independently demonstrated modest antitumor efficacy, whereas the combination dramatically lowered tumor burden and prolonged survival in an ATLL tumor xenograft murine model (94).
CONCLUSIONS
The discovery of the JAK/STAT signaling pathway and the demonstration of pervasive activation of this pathway in most forms of T cell malignancies represent one of the most exciting developments in T cell biology and in defining the pathogenesis of T cell malignancy. Activating mutations of STATs and JAKs were identified in many but not all cases. These mutations were not sufficient to support leukemic cell proliferation but only augmented the action of upstream signals in the cytokine receptor, JAK/STAT pathway. Each participant in the γc cytokine, cytokine receptor JAK/STAT pathway was required to maintain pathway activation. This insight that disorders of the γc/JAK/STAT system are pervasive in T cell malignancies suggests that novel pathway therapeutic directed approaches, including those that involve antibodies to γc cytokines, agents that block cytokine-receptor interaction, and especially JAK kinase inhibitors, may revolutionize therapy for T cell malignancies.
SUMMARY POINTS.
Activation of the γc cytokine, JAK/STAT system is pervasive in T cell malignancies, supporting JAK kinase inhibitor therapy.
Activating mutations in STAT3/STAT5B, JAK1, JAK3 were frequent but not sufficient to initiate proliferation and only augmented signals from upstream in the cytokine-cytokine receptor, JAK/STAT pathway.
pSTAT malignant T cell lines were addicted to JAK1 and STAT3 whether or not they were mutated.
Even with activating JAK mutants there was a requirement for expression of a functional cytokine receptor that played two roles, first as a scaffold for cross-activation of JAK kinases and second as a docking site for recruitment of STAT transcription factors.
The fundamental insight that disorders of the γc/JAK/STAT system are pervasive in T cell malignancies suggests novel therapeutic approaches, especially the use of JAK kinase inhibitors, that may revolutionize therapy for T cell malignancies.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. We thank Dr. Richard Bamford and Dr. Arthur Shaffer for their helpful suggestions.
Footnotes
This is a work of the U.S. Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Rochman Y, Spolski R, Leonard WJ. 2009. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol 9(7):480–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard WJ. 2001. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol 1(3):200–8 [DOI] [PubMed] [Google Scholar]
- 3.Kovanen PE, Leonard WJ. 2004. Cytokines and immunodeficiency diseases: critical roles of the γc-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev 202:67–83 [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol 6(8):595–601 [DOI] [PubMed] [Google Scholar]
- 5.Waldmann TA. 2015. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol. Res 3(3):219–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rui LX, Emre NCT, Kruhlak MJ, Chung HJ, Steidl C, et al. 2010. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell 18(6):590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen E, Staudt LM, Green AR. 2012. Janus kinase deregulation in leukemia and lymphoma. Immunity 36(4):529–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, et al. 2009. JAK2 phosphorylates histone H3Y41 and excludes HP1a from chromatin. Nature 461(7265):819–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J, Li B, Lin B, Lee PT, Chung TH, et al. 2016. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood 128(7):948–58 [DOI] [PubMed] [Google Scholar]
- 10.Wegrzyn J, Potla R, Chwae Y-J, Sepuri NBV, Zhang Q, et al. 2009. Function of mitochondrial Stat3 in cellular respiration. Science 323(5915):793–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vainchenker W, Constantinescu SN. 2013. JAK/STAT signaling in hematological malignancies. Oncogene 32(21):2601–13 [DOI] [PubMed] [Google Scholar]
- 12.Migone TS, Lin JX, Cereseto A, Mulloy JC, O’Shea JJ, et al. 1995. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 269(5220):79–81 [DOI] [PubMed] [Google Scholar]
- 13.Koskela HLM, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, et al. 2012. Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med 366(20):1905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerez A, Clemente MJ, Makishima H, Koskela H, LeBlanc F, et al. 2012. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood 120(15):3048–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohgami RS, Ma L, Merker JD, Martinez B, Zehnder JL, Arber DA. 2013. STAT3 mutations are frequent in CD30+ T-cell lymphomas and T-cell large granular lymphocytic leukemia. Leukemia 27(11):2244–47 [DOI] [PubMed] [Google Scholar]
- 16.Crescenzo R, Abate F, Lasorsa E, Tabbo F, Gaudiano M, et al. 2015. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 27(4):516–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiarle R, Simmons WJ, Cai HY, Dhall G, Zamo A, et al. 2005. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med 11(6):623–29 [DOI] [PubMed] [Google Scholar]
- 18.Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, et al. 2003. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin. Cancer Res 9(10):3692–699 [PubMed] [Google Scholar]
- 19.Kucuk C, Jiang B, Hu XZ, Gaulard P, Zhang Y, et al. 2013. Frequent activating mutations of JAK-STAT pathway genes in natural killer cell lymphomas. Blood 122(21):812 (Abstr.) [Google Scholar]
- 20.Bouchekioua A, Scourzic L, de Wever O, Zyang Y, Cervera P, et al. 2014. JAK3 deregulation by activating mutations confers invasive growth advantage in extranodal nasal-type natural killer cell lymphoma. Leukemia 28(2):338–48 [DOI] [PubMed] [Google Scholar]
- 21.Coppo P, Gouilleux-Gruart V, Huang Y, Bouhlal H, Bouamar H, et al. 2009. STAT3 transcription factor is constitutively activated and is oncogenic in nasal-type NK/T-cell lymphoma. Leukemia 23(9):1667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo GC, Tan SY, Tang T, Poon SL, Allen GE, et al. 2012. Janus kinase 3-activating mutations identified in natural/killer/T-cell lymphoma. Cancer Discov 2(7):591–97 [DOI] [PubMed] [Google Scholar]
- 23.Küçük C, Jiang B, Hu XZ, Zhang WY, Chan JKC, et al. 2015. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat. Commun 6:6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolae A, Xi L, Pittaluga S, Abdullaev Z, Pack SD, et al. 2014. Frequent STAT5B mutations in γδ hepatosplenic T-cell lymphomas. Leukemia 28(11):2244–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmann AK, Schneppenheim S, Seifert M, Betts MJ, Haake A, et al. 2014. Recurrent mutation of JAK3 in T-cell prolymphocytic leukemia. Genes Chromosomes Cancer 53(4):309–16 [DOI] [PubMed] [Google Scholar]
- 26.Kiel MJ, Velusamy T, Rolland D, Sahasrabuddhe AA, Chung F, et al. 2014. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood 124(9):1460–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellanger D, Jacquemin V, Chopin M, Pierron G, Bernard OA, et al. 2014. Recurrent JAK1 and JAK3 somatic mutations in T-cell prolymphocytic leukemia. Leukemia 28(2):417–19 [DOI] [PubMed] [Google Scholar]
- 28.Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, et al. 2001. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia 15(5):787–93 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Nowak I, Vonderheid EC, Rook AH, Kadin ME, et al. 1996. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. PNAS 93(17):9148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J, Goh G, Walradt T, Hong BS, Bunick CG, et al. 2015. Genomic landscape of cutaneous T cell lymphoma. Nat. Genet 47(9):1011–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JH, Ding L, Holmfeldt L, Wu G, Heatley SL, et al. 2012. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481(7380):157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odejide O, Weigert O, Lane AA. 2014. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 123(9):1293–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BKR, et al. 1997. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. PNAS 94(25):13897–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, et al. 2015. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet 47(11):1304–15 [DOI] [PubMed] [Google Scholar]
- 35.Lacronique V, Boureux A, DellaValle V, Poirel H, Quang CT, et al. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278(5341):1309–12 [DOI] [PubMed] [Google Scholar]
- 36.Schwaller J, Parganas E, Wang DM, Cain D, Aster JC, et al. 2000. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol. Cell 6(3):693–704 [DOI] [PubMed] [Google Scholar]
- 37.Carron C, Cormier F, Janin A, Lacronique V, Giovannini M, et al. 2000. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood 95(12):3891–99 [PubMed] [Google Scholar]
- 38.Feldman AL, Vasmatzis G, Asmann YW, Davila J, Middha S, et al. 2013. Novel TRAF1-ALK fusion identified by deep RNA sequencing of anaplastic large cell lymphoma. Genes Chromosomes Cancer 52(11):1097–102 [DOI] [PubMed] [Google Scholar]
- 39.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, et al. 2005. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365(9464):1054–61 [DOI] [PubMed] [Google Scholar]
- 40.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, et al. 2005. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7(4):387–97 [DOI] [PubMed] [Google Scholar]
- 41.Kralovics R, Passamonti F, Buser AS, Teo S, Tiedt R, et al. 2005. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med 352(17):1779–90 [DOI] [PubMed] [Google Scholar]
- 42.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, et al. 2005. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434(7037):1144–48 [DOI] [PubMed] [Google Scholar]
- 43.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. 2015. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med 66:311–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, et al. 2012. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med 366(9):787–98 [DOI] [PubMed] [Google Scholar]
- 45.Firwana B, Sonbol MB, Diab M, Raza S, Hasan R, et al. 2016. Tyrosine kinase inhibitors as a first-line treatment in patients with newly diagnosed chronic myeloid leukemia in chronic phase: a mixed-treatment comparison. Int. J. Cancer 138(6):1545–53 [DOI] [PubMed] [Google Scholar]
- 46.O’Shea JJ, Holland SM, Staudt LM. 2013. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med 368(2):161–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schatz JH, Horwitz SM, Lunning MA, Dolgalev I, Huberman K, et al. 2013. Next-generation sequencing suggests complex, heterogeneous pathogenesis in peripheral T-cell lymphoma unspecified. Blood 122(21):843 (Abstr.) [Google Scholar]
- 48.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ. 2008. Therapeutic targeting of Janus kinases. Immunol. Rev 223:132–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju W, Zhang ML, Jiang JK, Thomas CJ, Oh U, et al. 2011. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood 117(6):1938–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei M, Koshy N, van Besien K, Inghirami G, Horwitz SM. 2015. Refractory T-cell prolymphocytic leukemia with JAK3 mutation: in vitro and clinical synergy of tofacitinib an druxolitinib. Blood 126(23):5486 (Abstr.) [Google Scholar]
- 51.Conlon KC, Waldmann TA. 2016. Ruxolitinib for adult T-cell leukemia. Clin. Study Rec NCT01712659, updated Sep. 28. Natl. Inst. Health. https://clinicaltrials.gov/ct2/show/NCT01712659
- 52.Zamo A, Chiarle R, Piva R, Howes J, Fan Y, et al. 2002. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 21(7):1038–47 [DOI] [PubMed] [Google Scholar]
- 53.Roskoski R Jr. 2013. Anaplastic lymphoma kinase (ALK): structure, oncogenic activation, and pharmacological inhibition. Pharmacol. Res 68(1):68–94 [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Petrus M, Bryant BR, Nguyen VP, Stamer M, et al. 2008. Induction of the IL-9 gene by HTLV-1 Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood 111(10):5163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Petrus M, Bryant BR, Nguyen VP, Goldman CK et al. 2010. Autocrine/paracrine cytokine stimultion of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood 116(26):5948–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filippakopoulos P, Muller S, Knapp S. 2009. SH2 domains: modulators of nonreceptor tyrosine kinase activity. Curr. Opin. Struct. Biol 19(6):643–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heim MH, Kerr IM, Stark GR, Darnell JE. 1995. Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Sci 267(5202):1347–49 [DOI] [PubMed] [Google Scholar]
- 58.McGirt LY, Jia P, Baerenwald DA, Duszynski RJ, Dahlman KB, et al. 2015. Whole-genome sequening reveals oncogenic mutations in mycosis fungoides. Blood 126(4):508–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajala HLM, Eldfors S, Kuusanmaki H, van Adrichem AJ, Olson T, et al. 2013. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood 121(22):4541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajala HLM, Porkka K, Maciejewski JP, Loughran TP, Mustjoki S. 2014. Uncovering the pathogenesis of large granular lymphocytic leukemia-novel STAT3 and STAT5b mutations. Ann. Med 46(3):114–22 [DOI] [PubMed] [Google Scholar]
- 61.Saharinen P, Silvennoinen O. 2002. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem 277(49):47954–63 [DOI] [PubMed] [Google Scholar]
- 62.Saharinen P, Takaluoma K, Silvennoinen O. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell Biol 20(10):3387–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott NE, Cleveland SM, Grann V, Janik J, Waldmann TA, Dave UP. 2011. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood 118(14):3911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamashita Y, Shimada A, Yamada T, Yamaji K, Hon T, et al. 2013. IKZF1 and CRLF2 gene alterations correlate with poor prognosis in Japanese BCR-ABL1-negative high-risk B-cell precursor acute lymphoblastic leukemia. Pediatr. Blood Cancer 60(10):1587–92 [DOI] [PubMed] [Google Scholar]
- 65.Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, et al. 2008. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J. Exp. Med 205(4):751–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullighan CG, Zhang JH, Harvey RC, Collins-Underwood JR, Schulman BA, et al. 2009. JAK mutations in high-risk childhood acute lymphoblastic leukemia. PNAS 106(23):9414–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu XH, Levine R, Tong W, Wernig G, Pikman Y, et al. 2005. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. PNAS 102(52):18962–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hornakova T, Staerk J, Royer Y, Flex E, Tartaglia M, et al. 2009. Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor ahomodimers. J. Biol. Chem 284(11):6773–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, et al. 2011. Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias. J. Exp. Med 208(5):901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zenatti PP, Ribeiro D, Li WQ, Zuurbier L, Silva MC, et al. 2011. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat. Genet 43(10):932–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tendler CL, Greenberg SJ, Blattner WA, Manns A, Murphy E, et al. 1990. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. PNAS 87(13):5218–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azimi N, Brown K, Bamford RN, Tagaya Y, Siebenlist U, Waldmann TA. 1998. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-κB site. PNAS 95(5):2452–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leroy S, Dubois S, Tenaud I, Chebassier N, Godard A, et al. 2001. Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Br.J.Dermatol 144(5):1016–23 [DOI] [PubMed] [Google Scholar]
- 74.Döbbeling U, Dummer R, Laine E, Potoczna N, Gin JZ, Burg G. 1998. Interleukin-15 is an autocrine/ paracrine viability factor for cutaneous T-cell lymphoma cells. Blood 92(1):252–58 [PubMed] [Google Scholar]
- 75.Mishra A, Kwiatkowski S, Sullivan L, Grinshpun L, Russo G, et al. 2015. Epigenetic disruption of ZEB1 binding causes constitutive activation of IL-15 in cutaneous T-cell lymphoma. Blood 126(23):226138535 [Google Scholar]
- 76.Nakahata S, Yamazaki S, Nakauchi H, Morishita K. 2010. Downregulation of ZEB1 and overexpression of Smad7 contribute to resistance to TGF-β1-mediated growth suppression in adult T-cell leukemia/lymphoma. Oncogene 29(29):4157–69 [DOI] [PubMed] [Google Scholar]
- 77.Jain S, Chen J, Nicolae A, Wang HS, Shin DM, et al. 2015. IL-21-driven neoplasms in SJL mice mimic some key features of human angioimmunoblastic T-cell lymphoma. Am. J. Pathol 185(11):3102–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kleppe M, Lahortiga I, El Chaar T, De Keersmaecker K, Mentens N, et al. 2010. Deletion of the protein tyrosine phosphatase gene PTPN2 in T-cell acute lymphoblastic leukemia. Nat. Genet 42(6):530–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleppe M, Soulier J, Asnafi V, Mentens N, Hornakova T, et al. 2011. PTPN2 negatively regulates oncogenic JAK1 in T-cell acute lymphoblastic leukemia. Blood 117(26):7090–98 [DOI] [PubMed] [Google Scholar]
- 80.Porcu M, Kleppe M, Gianfelici V, Geerdens E, De Keersmaecker K, et al. 2012. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood 119(19):4476–79 [DOI] [PubMed] [Google Scholar]
- 81.Alexander WS. 2002. Suppressors of cytokine signalling(SOCS) in the immune system. Nat. Rev. Immunol 2(6):410–16 [DOI] [PubMed] [Google Scholar]
- 82.Küçük C, Hu X, Jiang B, Klinkebiel D, Geng HM, et al. 2015. Global promoter methylation analysis reveals novel candidate tumor suppressor genes in natural killer cell lymphoma. Clin.Can.Res 21(7):1699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, et al. 2005. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum 52(9):2686–692 [DOI] [PubMed] [Google Scholar]
- 84.Waldmann TA. 2007. Anti-Tac(daclizumab,Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J. Clin. Immunol 27(1):1–18 [DOI] [PubMed] [Google Scholar]
- 85.Berkowitz JL, Janik JE, Stewart DM, Jaffe ES, Stetler-Stevenson M, et al. 2014. Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin. Immunol 155(2):176–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nata T, Basheer A, Cocchi F, van Besien R, Massoud R, et al. 2015. Targeting the binding interface on a shared receptor subunit of a cytokine family enables the inhibition of multiple member cytokines with selectable target spectrum. J. Biol. Chem 290(37):22338–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitra S, Ring AM, Amarnath S, Spangler JB, Li P, et al. 2015. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42(5):826–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Shea JJ, Notarangelo LD, Johnston JA, Candotti F. 1997. Advances in the understanding of cytokine signal transduction: the role of Jaks and STATs in immunoregulation and the pathogenesis of immunodeficiency. J. Clin. Immunol 17(6):431–47 [DOI] [PubMed] [Google Scholar]
- 89.Leonard WJ, O’Shea JJ. 1998. JAKS AND STATS: Biological implications. Annu. Rev. Immunol 16:293–322 [DOI] [PubMed] [Google Scholar]
- 90.Bilori B, Thota S, Clemente MJ, Patel B, Jerez A, et al. 2015. Tofacitinib as a novel salvage therapy for refractory T-cell large granular lymphocytic leukemia. Leukemia 29(12):2427–29 [DOI] [PubMed] [Google Scholar]
- 91.Haan C, Rolvering C, Raulf F, Kapp M, Druckes P, et al. 2011. Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors. Chem. Biol 18(3):314–23 [DOI] [PubMed] [Google Scholar]
- 92.Smith GA, Uchida K, Weiss A, Taunton J. 2016. Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nat. Chem. Biol 12(5):373–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buettner R, Mora LB, Jove R. 2002. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res 8(4):945–54 [PubMed] [Google Scholar]
- 94.Zhang ML, Griner LAM, Ju W, Duveau DY, Guha R, et al. 2015. Selective targeting ofJAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. PNAS 112(40):12480–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schatz JH, Horwitz SM, Teruya-Feldstein J, Lunning MA, Viale A, et al. 2015. Targeting mutational profiling of peripheral T-cell lymphoma not otherwise specified highlights new mechanisms in a heterogeneous pathogenesis. Leukemia 29(1):237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]