Abstract
Background:
Atrial fibrillation (AF) and obstructive sleep apnea (OSA) are common conditions, but little is known about OSA and cardiovascular risk among AF patients.
Methods:
Using the Truven Health MarketScan databases we constructed a prospective cohort of AF patients from 2007–2014. AF, OSA, stroke, myocardial infarction (MI), and confounders were defined using International Classification of Disease (ICD)-9-CM codes. We matched individuals with an OSA diagnosis with up to five individuals without a diagnosis by age, sex, and enrollment date. Cox proportional hazards models adjusted for confounders and high-dimensional propensity scores. We included migraines as a control outcome. Bias analysis used published sensitivities and specificities to generate rate ratios adjusted for OSA misclassification.
Results:
We matched 56,969 individuals with an OSA diagnosis to 323,246 without. During a mean follow-up of 16 months, 3,234 incident strokes and 4,639 incident MIs occurred. After adjustment, OSA diagnosis was strongly associated with reduced risk of incident stroke (hazard ratio =0.48, 95% confidence interval [0.43, 0.53]) and MI (0.40, [0.37, 0.44]) and a smaller reduced risk of migraines (0.82 [0.68, 0.99]). Bias analysis produced wide ranging or inestimable rate ratios adjusted for misclassification of OSA.
Conclusions:
OSA diagnosis in AF patients was strongly associated with reduced risk of incident cardiovascular disease. We discuss misclassification, selection bias, and residual confounding as potential explanations.
Background
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. For men and women over 40, the lifetime risk of AF is 1 in 41 and the prevalence of AF is expected to reach 12.1 million cases in the United States by the year 2030.2 Those with AF are at increased risk of cardiovascular disease (CVD), such as stroke and myocardial infarction (MI),3–5 among other complications. Because of the high burden of AF in the population, it is necessary to better understand modifiable factors associated with adverse outcomes.
Obstructive sleep apnea (OSA) is also increasing in prevalence,6 possibly due to the obesity epidemic. In the general population, OSA has also been associated with increased risk of AF7–12 as well as stroke and coronary heart disease.13–15 Because AF patients are at especially elevated risk of CVD,3–5 and OSA has also been independently associated with CVD risk, it is possible that OSA may further increase CVD risk in the context of AF. Although OSA has been associated with AF,7–9 and stroke and MI are common outcomes among AF patients,3–5 the few studies examining the relationship between OSA and atherosclerotic CVD in AF patients have reported mixed results, with one finding a positive association and two finding no association.16–18 Additional research is needed, as these studies either used diagnostic polysomnography in small samples or did not employ optimal methodology to control for confounding.
Large administrative claims databases provide an opportunity to examine large populations of patients, such as those with AF, but these datasets are also subject to systematic error. Methods like high dimensional propensity scores can be used to help account for confounding, but misclassification is also a concern, especially for underdiagnosed exposures such as OSA, where 85% of individuals who meet the diagnostic criteria are unaware of their status.19 Quantitative bias analysis can help estimate the magnitude and direction of such errors by quantifying the uncertainty.20,21 Therefore, our goal for this study was to determine the association of diagnosed OSA with risk of MI and ischemic stroke in a large cohort of patients with non-valvular AF, and to use bias analysis to examine the influence of exposure misclassification on the observed associations.
Methods
The present analysis utilized commercially available de-identified data from two databases licensed by Truven Health MarketScan. The Commercial Claims and Encounters Database is a de-identified data source for privately insured healthcare plan enrollees under age 65, while the Medicare Supplemental database consists of adults 65 and older with employer-paid Medicare supplemental insurance. The databases contain individual-level information on enrollment and health insurance claims for inpatient and outpatient services, as well as outpatient pharmacy claims. All claims were paid and adjudicated. We used these healthcare claims to conduct a prospective cohort study of patients with AF from 1 January 2007 to 31 December 2014 among those aged 22–99. Because this secondary data is de-identified and commercially available, the University of Minnesota Institutional Review Board deemed it exempt from review.
Sample Selection and Matching
All participants in the analytic sample had non-valvular AF, as defined by at least one inpatient or two outpatient claims 7 to 365 days apart using the ICD-9-CM codes 427.3, 427.31, or 427.32. We excluded from the sample those with ICD-9-CM codes for valvular AF or procedural codes for valvular repair or replacement. The use of administrative claims data to identify AF was summarized in a systematic review, which found the algorithms had a median positive predictive value of 87% and a median sensitivity of 79%.22 In order to be included in the present analysis, we also required at least 90 days of continuous enrollment before first AF diagnosis, in order to obtain adequate information on confounding variables, as well as to ensure we were capturing incident AF events. For those participants who discontinued enrollment and then re-enrolled, only the first enrollment period was included in the analysis. Additionally, those with prevalent stroke, heart failure, MI, or TIA prior to the start of follow-up (index date) were excluded from the analysis.
We matched individuals identified as having diagnosed OSA by age, sex, and enrollment date with up to five patients from the same sample but with no OSA diagnosis. The index date was defined as the date of the sleep diagnosis for those exposed, while the index date for the unexposed was the same date as the exposed person to whom they were matched.
Sleep variables
For the primary exposure definition, we identified claims for diagnosed OSA from the inpatient and outpatient databases using the following ICD-9-CM codes: 327.20 (organic sleep apnea, unspecified), 327.23 (obstructive sleep apnea adult pediatric), 327.29 (other organic sleep apnea), 780.51 (insomnia with sleep apnea, unspecified), 780.53 (hypersomnia with sleep apnea), and 780.57 (unspecified sleep apnea). We required one OSA claim after the AF diagnosis. We also performed two sensitivity analyses with different exposures definitions. One, a more specific definition, required one of the codes listed above ICD-9 codes as well as Current Procedural Terminology (CPT) codes for polysomnography and positive airway pressure (PAP) devices (G8759, G8839, G8846, G8848, 95808, 95810, 95811, G0398, G0399, G0400). The other sensitivity analysis used a broader definition of ICD-9 defined OSA (327.2x and 780.5x) similar to other publications.23,24 Validation studies comparing outcomes based on ICD-9 codes versus polysomnography found that definitions requiring codes for both polysomnography and a PAP device had sensitivities ranging from 0.02–0.70 and specificities ranging from 0.36–0.99, depending on definition and data source.23,24
Outcome ascertainment
Ischemic stroke was defined using the ICD-9-CM codes 434.xx (occlusion of cerebral arteries) and 436.xx (acute but ill-defined cerebrovascular disease) in the primary position. These algorithms had a positive predictive value of 80% or greater in a systematic review.25 MI was defined using the ICD-9-CM codes 410.xx in the first or second position. Positive predictive values for this algorithm range from 88%−94%.26,27 Migraines, defined using ICD-9-CM codes 346.xx, were selected as a control outcome, because no association with OSA was expected and if rates of migraines were different between those with and without diagnosed OSA it would suggest residual confounding.
Covariate ascertainment
Information on pre-determined covariates was identified using claims. In order to have adequate covariate information, a minimum of 90 days of enrollment was required prior to the participant index date. Claims information came from inpatient, outpatient, and pharmacy databases, and included information on demographics, comorbidities, and medications. Validated algorithms28 were used to define pre-specified comorbidities such as depression and alcohol abuse. Prescription medications included hypnotics, anti-depressants, thyroid medications, smoking cessation medications, and weight-loss medications. CHA2DS2-VASc score was also calculated.29 Because diagnoses of our exposure, outcomes, and covariates may depend on a person’s propensity toward healthcare utilization, we included the number of hospitalizations and outpatient visits as a covariate.
Analysis
We estimated high-dimensional propensity scores (HDPS) using previously published SAS macros available at www.drupepi.org to help control for potential confounding.30 To calculate these scores, database information was categorized into five domains: inpatient diagnostic codes, inpatient procedure codes, outpatient diagnostic codes, outpatient procedure codes, and medications. The algorithm identified the top 200 most prevalent claims from each of the five domains, which resulted in 1,000 covariates. We ranked these covariates based on the ratio of the prevalence of the covariate in those with OSA versus those without. In addition to the variables identified by HDPS, we forced the pre-determined covariates described above into the HDPS calculation. Based on this prioritization, the HDPS algorithm selected the top 500 variables and included them in a logistic regression model to estimate the probability of OSA exposure. A list of the top 20 covariates in the main model for each of the three exposures can be found in eTable 1. We then used the HDPS in two ways: as a continuous covariate in model adjustment in the main analysis and 5:1 matching using a 0.25 standard deviation caliper in a sensitivity analysis.
We used Cox proportional hazards models to model time to incident ischemic stroke, MI, and migraine events according to diagnosed OSA status. We calculated person–time by using the time from the index date until an outcome event, health plan disenrollment, or the end of the follow-up period. For each outcome, models were adjusted for age, sex, pre-specified covariates, and HDPS (continuous). We used SAS version 9.3 (SAS Institute, Cary, NC) to analyze the data.
We were concerned about exposure misclassification of OSA based on ICD codes and, as a result, we conducted a bias analysis using the crude data, which produced similar estimates to those adjusted for confounding. We adapted publicly available Excel for Microsoft Office 365 spreadsheets for rate ratios20,31 and estimates of sensitivity and specificity from validation studies23,24 were used to adjust for misclassification, allowing for estimation of expected observed frequencies of ischemic stroke events and person-years by exposure (OSA), as well as OSA misclassification adjusted rate ratios. Prior published validation studies reported sensitivities between 0.02–0.70 and specificities from between 0.36–0.99.23,24 We conducted bias analyses using these ranges.
Results
A total of 56,969 individuals met our primary OSA exposure definition. These individuals were matched to 323,246 controls. In this sample, the mean age at baseline was 63.2 years and 29.8% were female. There were 3,234 new cases of ischemic stroke and 4,639 new cases of MI over an average of 16 months of follow-up. The incidence rate was 6.2 (95% CI 6.0–6.4) stroke cases and 8.9 MI cases (95% CI 8.6–9.1) per 1000 person-years. For our more specific OSA definition, which required both a claim for OSA and a PAP device, 18,241 individuals met the definition who were matched to 95,418 controls. For the least specific definition, based on broad OSA claims only, 79,337 met the definition and were matched to 406,595 controls. Using these alternate exposure definitions, baseline population characteristics and incidence rates were similar to those observed with the primary definition.
Table 1 shows characteristics of AF patients by diagnosed OSA status. Due to matching, age and sex were similar in both groups. Compared to those without an OSA diagnosis, a greater proportion of those with an OSA diagnosis had diabetes and a claim for anti-depressant. Additionally, those with diagnosed OSA had more outpatient visits compared to those without an OSA diagnosis.
Table 1:
Characteristics of atrial fibrillation patients by sleep apnea status, MarketScan 2007–2014
| Sleep apnea (ICD)a | No sleep apnea | |
|---|---|---|
| N | 59,969 | 323,246 |
| Age, mean (SD) | 62.8 ± 11.7 | 63.3 ± 11.8 |
| CHA2DS2-VASc score, mean (SD) | 1.5 ± 1.2 | 1.5 ± 1.3 |
| Mean inpatient visits, mean (SD) | 0.3 ± 0.8 | 0.2 ± 0.7 |
| Mean outpatient visits, mean (SD) | 49.6 ± 89.5 | 41.9 ± 83.4 |
| Age, y | ||
| >65, N (%) | 13,100 (22) | 73,109 (23) |
| >75, N (%) | 10,427 (17) | 60,766 (19) |
| Female, N (%) | 17,335 (29) | 97,023 (30) |
| Diabetes, N (%) | 9,872 (17) | 45,006 (14) |
| Hypertension, N (%) | 24,135 (40) | 127,120 (39) |
| Comorbidities | ||
| Depression, N (%) | 3,208 (5) | 15,656 (5) |
| Alcohol abuse, N (%) | 76 (0) | 1,022 (0) |
| Medications | ||
| Hypnotics, N (%) | 3,759 (6) | 17,692 (6) |
| Anti-depressants, N (%) | 8,350 (14) | 37,429 (12) |
| Varenicline, N (%) | 211 (0) | 1,307 (0) |
| Weight loss medication, N (%) | 373 (1) | 1,103 (0) |
| Thyroid medication, N (%) | 4,799 (8) | 26,013 (8) |
Primary exposure definition
Table 2 shows, among AF patients, adjusted hazard ratios and 95% confidence intervals for ischemic stroke, MI and migraines, according to diagnosed OSA status. After adjustment for age, sex, depression, alcohol, diabetes, hypertension, inpatient and outpatient visits, medications, and propensity score, an OSA diagnosis was strongly associated with a reduced hazard of ischemic stroke (HR = 0.48, 95% CI 0.43, 0.53) and MI (HR = 0.40, 95% CI 0.37, 0.44). Results were similar when OSA was defined by both ICD-9 and CPT codes (HR for stroke = 0.46, 95% CI 0.38, 0.56; HR for MI = 0.38, 95% CI 0.32, 0.45), as well as when the broad definition of OSA was used (HR for stroke = 0.52, 95% CI 0.48, 0.57; HR for MI = 0.43, 95% CI 0.39, 0.46). Similar results were found in a sensitivity analysis where we matched on the propensity score (eTable 2).
Table 2:
Adjusted hazard ratios (95% confidence intervals) for atherosclerotic cardiovascular disease by sleep apnea among patients with atrial fibrillation: MarketScan 2007–2014
| OSA ICD Only (Primary definition) | OSA ICD + CPT (More specific definition) | OSA Broad ICD (Less specific definition) | ||||
|---|---|---|---|---|---|---|
| No OSA | OSA | No OSA | OSA | No OSA | OSA | |
| N | 323,246 | 59,969 | 95,418 | 18,241 | 406,595 | 79,337 |
| Ischemic Stroke | ||||||
| N Events | 2,809 | 425 | 816 | 132 | 3,560 | 667 |
| Model 1a | Ref. | 0.43 (0.39, 0.48) | Ref. | 0.45 (0.38, 0.55) | Ref. | 0.45 (0.41, 0.49) |
| Model 2b | Ref. | 0.42 (0.38, 0.47) | Ref. | 0.42 (0.34, 0.50) | Ref. | 0.45 (0.41, 0.49) |
| Model 3c | Ref. | 0.48 (0.43, 0.53) | Ref. | 0.46 (0.38, 0.56) | Ref. | 0.52 (0.48, 0.57) |
| Myocardial Infarction | ||||||
| N Events | 4,116 | 523 | 1,193 | 160 | 4,748 | 707 |
| Model 1a | Ref. | 0.36 (0.33, 0.39) | Ref. | 0.38 (0.32, 0.45) | Ref. | 0.36 (0.33, 0.39) |
| Model 2b | Ref. | 0.35 (0.32, 0.38) | Ref. | 0.34 (0.29, 0.40) | Ref. | 0.35 (0.32, 0.38) |
| Model 3c | Ref. | 0.40 (0.37, 0.44) | Ref. | 0.38 (0.32, 0.45) | Ref. | 0.43 (0.39, 0.46) |
| Migraine | ||||||
| N Events | 673 | 153 | 219 | 55 | 659 | 223 |
| Model 1a | Ref. | 0.73 (0.61, 0.87) | Ref. | 0.86 (0.64, 1.2) | Ref. | 0.89 (0.76, 1.0) |
| Model 2b | Ref. | 0.73 (0.61, 0.88) | Ref. | 0.89 (0.65, 1.2) | Ref. | 0.83 (0.71, 0.97) |
| Model 3c | Ref. | 0.82 (0.68, 0.99) | Ref. | 0.90 (0.66, 1.2) | Ref. | 0.93 (0.79, 1.1) |
Abbreviations – OSA: obstructive sleep apnea, ICD: International Classification of Diseases, CPT: Current Procedural Terminology
Model 1 adjusted for age and sex
Model 2 added depression, alcoholism, diabetes, hypertension, sleep medications, anti-depressants, thyroid medication, weight-loss medication, anti-smoking medication, and inpatient and outpatient visits
Model 3 added continuous high dimensional propensity score
Table 2 also shows adjusted hazard ratios and 95% confidence intervals for migraines, the control outcome by diagnosed OSA status. After adjustment for age, sex, confounders, and propensity score, there was evidence of an association between OSA diagnosis and migraines (HR= 0.82, 95% CI 0.68, 0.99). However, most other OSA definitions did not find an association.
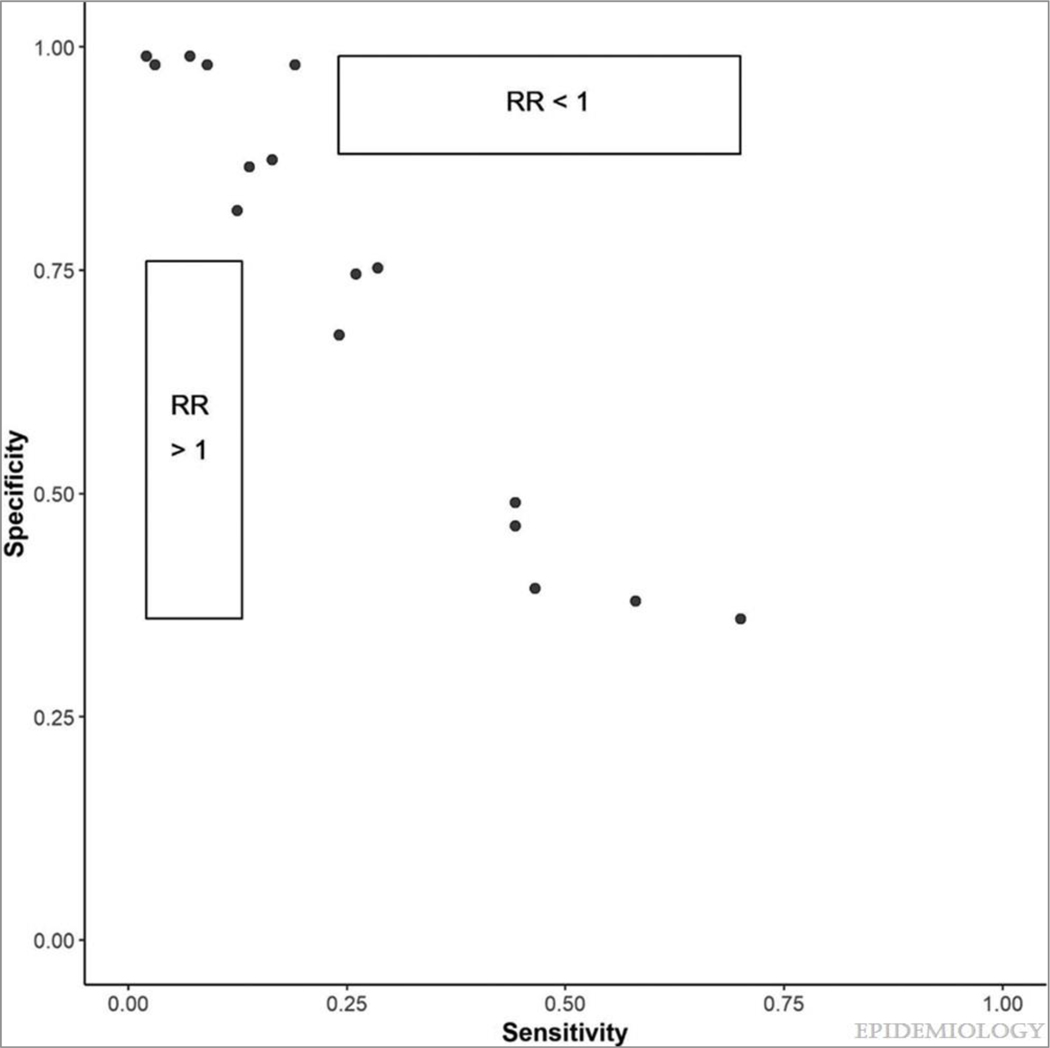
Bias analysis was used to evaluate the influence that misclassification of OSA diagnosis based on ICD codes may have had on our results. The sensitivity and specificity values from previous literature resulted in numerous adjusted expected frequencies having negative values, suggesting that the sensitivity and specificity values were not compatible with the observed data. As a result, we plotted values from the range of published sensitivities (0.02–0.70) and specificities (0.36–0.99), and reported whether the OSA misclassification adjusted relative rate was >1 or <1 (Figure). We found that the combination of sensitivities between 0.02–0.14 and specificities between 0.36–0.76 could result in rate ratios adjusted for OSA misclassification that were greater than 1, while the combination of sensitivities between 0.24–0.70 and specificities between 0.86–0.99 could result in rate ratios less than 1 in this dataset. (eTable 3).
Figure.
An exploratory bias analysis of published sensitivities and specificities for ICD-9 codes for OSA compared to polysomnography.
Points represent published sensitivities and specificities from validation articles, all of which resulted in adjusted expected frequencies that had negative values in the bias analysis. Rate ratio (RR): OSA misclassification adjusted rate ratios for the association between OSA and ischemic stroke. RR >1 represents the combination of sensitivities and specificities that could result in OSA misclassification adjusted rate ratios greater than 1 in this dataset. RR <1 represents the combination of sensitivities and specificities that could result in OSA misclassification adjusted rate ratios less than 1 in this dataset.
Discussion
In this analysis of a large administrative database of AF patients, counter to our hypothesis, an OSA diagnosis was associated with a strikingly lower rate of incident stroke and MI, which we believe is implausible. Our bias analysis to examine misclassification of the OSA exposure produced many impossible values, and those that were possible yielded a wide range of OSA misclassification adjusted rate ratios. Estimates of sensitivity and specificity from previous studies all yielded negative corrected cell counts. As such, our findings raise questions about whether administrative data can be used to test hypotheses about exposures that are believed to be substantially underdiagnosed, such as OSA,32 where it is estimated that approximately 85% of individuals who meet the diagnostic criteria for OSA are unaware of their status.19
Comparison to prior literature
Previous research exploring the association between OSA and atherosclerotic CVD risk among AF patients are summarized in Table 3. In one study of 17,000 AF patients, a Taiwanese national health insurance database found no association between ICD-9 defined OSA and stroke although, similar to our findings, hazard ratios were in a protective direction (HR~0.80).18 In a study using the ORBIT-AF registry, OSA defined via clinician diagnosis or history was not associated with a composite cardiovascular outcome, which included cardiovascular death, MI, and stroke/transient ischemic attack.17 However, in a small study of patients referred for polysomnography, OSA was strongly associated with first-time stroke among AF patients (OR~3.84).16 Thus, there is a pattern whereby large studies using administrative or self-reported measures of OSA found protective or no associations, while a study with a small number of cases that used polysomnography found a greatly increased risk of stroke among those with OSA.
Table 3:
Summary of previous studies on OSA and atherosclerotic cardiovascular disease among atrial fibrillation patients
| Authors | Sample Size | AF Population | Measurement of OSA | Outcome | Length of follow-up | Results |
|---|---|---|---|---|---|---|
| Current study | 100,000 + | U.S. Administrative Claims data | ICD-9 | Stroke and myocardial infarction via ICD-9 | 1.4 years | HR~0.48 |
| Chang et al.18 | 17,375 | Taiwan health insurance claims | ICD-9 | Stroke via ICD-9 | 2.5 years | HR~0.80 |
| Holmqvist et al.17 | 10,132 | ORBIT-AF registry | Physician report and medical records | Composite of cardiovascular death, coronary heart disease, stroke/TIA | 2 years | HR~1.10 |
| Yaranov et al.16 | 332 | Patients referred for sleep study | Polysomnography | Stroke via electronic medical records | 4.4 years | OR~3.84 |
Abbreviations: OSA – obstructive sleep apnea, ICD – International Classification of Diseases, HR – hazard ratio, OR – odds ratio
Our finding of OSA being associated with decreased atherosclerotic CVD risk is counterintuitive, as pathophysiology suggests that OSA results in hypoxia, hypercapnia, changes in autonomic nervous system activity, and inflammation, all of which could act in AF patients to increase atherosclerotic CVD risk.33 Additionally, epidemiologic studies in ‘healthy’ population-based samples without AF also suggest that OSA may be associated with increased risk of stroke and CHD.13,15
Potential influence of misclassification
A plausible explanation for these unexpected results is misclassification of exposure. The gold standard for measuring OSA is in-lab polysomnography; however, this method is too expensive and burdensome to implement in a sample this large. Two papers have examined the validity of administrative data as compared to polysomnography for defining OSA. Applying several different ICD-code definitions of OSA, one sample of Canadian surgical patients found a wide range of specificities (e.g. 36%−99%) and sensitivities (e.g. 2%−70%) that varied due to the case definition,23 while a second study of patients referred for sleep studies found that sensitivity decreased and specificity increased when multiple claims were required (e.g. with one code the sensitivity was 44%−47% and the specificity 39%−49%; with multiple codes, sensitivities were 12%−16% and specificities 82%−87%).24 In a secondary analysis, we tried to increase the specificity of our OSA definition by requiring both ICD and CPT codes. However, results remained essentially unchanged.
We attempted to use the published sensitivities and specificities to generate rate ratios adjusted for exposure misclassification to estimate the expected association between OSA and atherosclerotic CVD among AF patients. Applying the published estimates of sensitivity and specificity to the misclassification bias analysis equations resulted in numerous impossible (negative) expected cell frequencies. These impossible cell frequencies represent an incompatibility between the sensitivity and specificity and the observed data, which may manifest from different sources. It is possible that the published sensitivities and specificities are not applicable to our data, perhaps because the source populations are so different. On the other hand, it is possible that other sources of error in our data are responsible for this incompatibility. For instance, the misclassification of disease or confounding could result in this incompatibility. To demonstrate where errors occurred and how estimates may change with different combinations of sensitivities and specificities, we plotted sensitivities and specificities together with whether the OSA misclassification adjusted rate ratios were greater than 1 or less than 1. Finally, it is possible that the misclassification is, in fact, differential and we have misspecified our bias model.
In most instances, non-differential misclassification biases estimates of association toward the null and, consequently, correction for nondifferential misclassification results in measures of association further away from the null.31 In this study, we only considered non-differential misclassification, which would appear unlikely to explain the unusual protective association since the misclassification adjusted result would be even more protective. However, if sensitivity and specificity sum to less than one, as observed for some combinations used in the present study, it indicates that the classification is worse than random. In this context, the direction of the association can be reversed, and thus correction for this kind of misclassification could change not only the magnitude but direction of effect.31 Several combinations of sensitivities and specificities from the validation papers add up to less than one, including exposure definitions most similar to the ones used in this study. In addition, our exploratory analysis demonstrates that choosing sensitivities between 0.02–0.13 and specificities between 0.36–0.76, which were in the range of the published OSA validation studies and sum to less than one produce rate ratios suggesting OSA could, in fact, be adversely associated with atherosclerotic CVD among AF patients, as anticipated. As such, it is conceivable that misclassification bias as a result of the low sensitivity and specificity of ICD-defined OSA may have led to our surprising result of OSA being associated with strikingly lower risk of stroke and MI. However, it is important to note that the exploratory bias analysis produced a wide range of rate ratios, making it impossible to determine the direction of the misclassification bias.
The bias analyses we conducted focused on nondifferential misclassification; however, as the validity of defining OSA using ICD codes has not been examined with regard to any outcome, it is possible that misclassification in the OSA exposure is differential (i.e. different for those with and without CVD in this sample). An example of how differential misclassification could occur is if those who were obese were more likely to be screened for OSA, and also more likely to experience an atherosclerotic CVD event during follow-up. In addition to uncertainty about whether misclassification was differential or non-differential, it is also unclear how the validity of using administrative data to define OSA compares to other measurement methods used in some prior publications in the absence of polysomnography (e.g. self-reported physician’s diagnosis of OSA or snoring as a surrogate).
In addition, the bias analyses we conducted focused on exposure misclassification because OSA is highly underdiagnosed; however, other forms of misclassification may also have led to our results. Although the positive predictive values for using ICD-9 codes to identify ischemic stroke and MI were high, there is still a possibility that outcome misclassification contributed to our results.25–27 Sensitivity and positive predictive value for AF were also high,22 but not perfect, potentially leading to another form of misclassification that may have influenced our results. Combined, these different forms of misclassification may have led the results found in this study.
It is also possible that those with an OSA diagnosis via ICD codes receive higher quality care than those without a diagnosis, regardless of their true sleep apnea status. Physicians trained in sleep medicine, or those in more affluent health-care settings, may be more likely to screen and diagnose their patients with sleep apnea. Because of the care they receive, these patients may be less likely to develop an incident atherosclerotic cardiovascular event compared to patients without access to specialty physicians. On the other hand, physicians may use an OSA ICD code to order a sleep study for their patient, but the results of the study may indicate that patient does not in fact have OSA. Thus, these are additional potential explanations for the inverse association found in this paper.
Potential influence of selection bias and residual confounding
Another possible explanation for our unanticipated results is selection bias. Because over twenty percent of our participants in this sample are older than 65, it is possible that those with OSA had already died of MI or stroke before the beginning of the study. As a result, those with OSA in this study may be healthier and thus less likely to develop atherosclerotic CVD than those in the general population, resulting in the counterintuitive associations. However, in our sample a greater proportion of those with diagnosed OSA compared to those without OSA had diabetes, making this explanation less likely.
Our unexpected finding that OSA is associated with lower risk of atherosclerotic CVD may be analogous to the established “obesity paradox”, where overweight or obese patients are at lower risk for some outcomes compared to those who are normal weight. This phenomenon has been documented for some CVD events in AF patients.34 Selection bias is one plausible explanation for this occurrence, due to conditioning on a collider (with the collider being a diagnosis of AF, which can be a consequence of OSA and other risk factors for stroke such as hypertension or obesity).35
Residual confounding may also be an explanation for our findings. We rigorously attempted to mitigate uncontrolled confounding by adjusting for healthcare utilization intensity, matching, and using high dimensional propensity scores. However, when we incorporated into the analysis a control outcome, migraines, the association with OSA was protective for some of the exposure definitions, albeit to a lesser degree than our primary outcomes. As a result, residual confounding may partially explain our results as well. Overall, it is likely that the unexpected findings of this paper result from a combination of the described biases, not just one.
Strengths and additional limitations
Other limitations of this study include the possibility that those diagnosed with OSA received treatment over the follow-up period, which may have reduced the incidence of stroke or MI. However, though OSA treatment, such as continuous positive airway pressure (CPAP), improves OSA and daytime sleepiness,36,37 adherence is low as patients do not always find the treatment acceptable.38,39 Additionally, randomized trials have found no effect of CPAP on the prevention of cardiovascular events.40,41 Thus, those prescribed treatment may not be at reduced risk. In the MarketScan dataset information on some potential confounders (e.g. BMI) was not present; however, high-dimensional propensity score methodology helped to control for residual confounding, though some may still exist.30 Additionally, follow-up time in this study was only 16 months, which may not be enough time for OSA to affect atherosclerotic cardiovascular disease if the relationship is causal. Besides the use of propensity scores, other strengths of this study include a large, real-world sample with extensive information on medications and comorbidities.
Conclusions
In this study, diagnosed OSA was strongly associated with reduced risk of incident atherosclerotic CVD. This counterintuitive finding may be attributable to many sources of biases, with misclassification of OSA being particularly noteworthy. More longitudinal studies or trials with valid and reliable measurements of sleep apnea are thus needed to provide more definitive evidence on this association. The present findings also raise questions about the validity of research using administrative data to define OSA, and possibly other exposures that are known to be highly underdiagnosed.
Supplementary Material
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation.2004;110(9):1042–1046. [DOI] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol.2013;112(8):1142–1147. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke.1991;22(8):983–988. [DOI] [PubMed] [Google Scholar]
- 4.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med.2014;174(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neal WT, Sangal K, Zhang ZM, Soliman EZ. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol.2014;37(12):750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology.2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. Journal of the American College of Cardiology.2007;49(5):565–571. [DOI] [PubMed] [Google Scholar]
- 8.Kwon Y, Gharib SA, Biggs ML, et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax.2015;70(9):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin GM, Colangelo LA, Lloyd-Jones DM, et al. Association of Sleep Apnea and Snoring With Incident Atrial Fibrillation in the Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology.2015;182(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med.2006;173(8):910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra R, Stone KL, Varosy PD, et al. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med.2009;169(12):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qaddoura A, Kabali C, Drew D, et al. Obstructive sleep apnea as a predictor of atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. Can J Cardiol.2014;30(12):1516–1522. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. International journal of cardiology.2014;172(2):466–469. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med.2010;182(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis.2013;229(2):489–495. [DOI] [PubMed] [Google Scholar]
- 16.Yaranov DM, Smyrlis A, Usatii N, et al. Effect of obstructive sleep apnea on frequency of stroke in patients with atrial fibrillation. Am J Cardiol.2015;115(4):461–465. [DOI] [PubMed] [Google Scholar]
- 17.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). American heart journal.2015;169(5):647–654 e642. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, Chiu CC, Chiang CH, et al. Obstructive sleep apnea and the risk of ischemic stroke in patients with atrial fibrillation. International journal of cardiology.2015;181:144–146. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society.2008;5(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FMP Lash T.L., Fink AK Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Springer; 2010. [Google Scholar]
- 21.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol.2014;43(6):1969–1985. [DOI] [PubMed] [Google Scholar]
- 22.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf.2012;21 Suppl 1:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIsaac DI, Gershon A, Wijeysundera D, Bryson GL, Badner N, van Walraven C. Identifying Obstructive Sleep Apnea in Administrative Data: A Study of Diagnostic Accuracy. Anesthesiology.2015;123(2):253–263. [DOI] [PubMed] [Google Scholar]
- 24.Laratta CR, Tsai WH, Wick J, Pendharkar SR, Johannson KA, Ronksley PE. Validity of administrative data for identification of obstructive sleep apnea. J Sleep Res.2017;26(2):132–138. [DOI] [PubMed] [Google Scholar]
- 25.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf.2012;21 Suppl 1:100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal.2004;148(1):99–104. [DOI] [PubMed] [Google Scholar]
- 27.Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf.2010;19(6):596–603. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care.2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 29.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest.2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 30.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology.2009;20(4):512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman K, Greenland S, & Lash TL Modern Epidemiology, 3rd Edition.Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 32.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep & breathing = Schlaf & Atmung.2002;6(2):49–54. [DOI] [PubMed] [Google Scholar]
- 33.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Journal of the American College of Cardiology.2008;52(8):686–717. [DOI] [PubMed] [Google Scholar]
- 34.Proietti M, Guiducci E, Cheli P, Lip GY. Is There an Obesity Paradox for Outcomes in Atrial Fibrillation? A Systematic Review and Meta-Analysis of Non-Vitamin K Antagonist Oral Anticoagulant Trials. Stroke.2017;48(4):857–866. [DOI] [PubMed] [Google Scholar]
- 35.Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology.2013;24(3):461–462. [DOI] [PubMed] [Google Scholar]
- 36.Engleman HM, Cheshire KE, Deary IJ, Douglas NJ. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax.1993;48(9):911–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet.1994;343(8897):572–575. [DOI] [PubMed] [Google Scholar]
- 38.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J.2008;15(7):365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med.1999;159(4 Pt 1):1108–1114. [DOI] [PubMed] [Google Scholar]
- 40.McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med.2016;375(10):919–931. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Zhou Z, McEvoy RD, et al. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA.2017;318(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.