Key Points
Question
Is obesity associated with better prognosis in patients with cancer?
Findings
This meta-analysis of 203 studies with more than 6.3 million participants found that obesity was associated with increased overall and cancer-specific mortality, especially among patients with breast, colon, and uterine cancer. In contrast, patients with obesity and renal cell carcinoma, lung cancer, or melanoma had better survival than patients without obesity.
Meaning
These findings suggest that survival outcomes are poor among patients with obesity and cancer, except in lung cancer and melanoma.
This systematic review and meta-analysis assesses the association between obesity and survival outcomes following a diagnosis of cancer.
Abstract
Importance
Obesity, defined as a body mass index (BMI) greater than 30, is associated with a significant increase in the risk of many cancers and in overall mortality. However, various studies have suggested that patients with cancer and no obesity (ie, BMI 20-25) have worse outcomes than patients with obesity.
Objective
To assess the association between obesity and outcomes after a diagnosis of cancer.
Data Sources
PubMed, the Cochrane Library, and EMBASE were searched from inception to January 2020.
Study Selection
Studies reporting prognosis of patients with obesity using standard BMI categories and cancer were included. Studies that used nonstandard BMI categories, that were limited to children, or that were limited to patients with hematological malignant neoplasms were excluded. Screening was performed independently by multiple reviewers. Among 1892 retrieved studies, 203 (17%) met inclusion criteria for initial evaluation.
Data Extraction and Synthesis
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines were reporting guideline was followed. Data were extracted by multiple independent reviewers. Risk of death, cancer-specific mortality, and recurrence were pooled to provide an adjusted hazard ratio (HR) with a 95% CI . A random-effects model was used for the retrospective nature of studies.
Main Outcomes and Measures
The primary outcome of the study was overall survival (OS) in patients with cancer, with and without obesity. Secondary end points were cancer-specific survival (CSS) and progression-free survival (PFS) or disease-free survival (DFS). The risk of events was reported as HRs with 95% CIs, with an HR greater than 1 associated with a worse outcome among patients with obesity vs those without.
Results
A total of 203 studies with 6 320 365 participants evaluated the association of OS, CSS, and/or PFS or DFS with obesity in patients with cancer. Overall, obesity was associated with a reduced OS (HR, 1.14; 95% CI, 1.09-1.19; P < .001) and CSS (HR, 1.17; 95% CI, 1.12-1.23; P < .001). Patients were also at increased risk of recurrence (HR, 1.13; 95% CI, 1.07-1.19; P < .001). Conversely, patients with obesity and lung cancer, renal cell carcinoma, or melanoma had better survival outcomes compared with patients without obesity and the same cancer (lung: HR, 0.86; 95% CI, 0.76-0.98; P = .02; renal cell: HR, 0.74; 95% CI, 0.53-0.89; P = .02; melanoma: HR, 0.74; 95% CI, 0.57-0.96; P < .001).
Conclusions and Relevance
In this study, obesity was associated with greater mortality overall in patients with cancer. However, patients with obesity and lung cancer, renal cell carcinoma, and melanoma had a lower risk of death than patients with the same cancers without obesity. Weight-reducing strategies may represent effective measures for reducing mortality in these patients.
Introduction
Obesity, defined as a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) greater than 30, is a chronic disease with increasing prevalence around the world, largely contributing to important health issues in most countries.1 Alongside body fat, which is a general risk factor for serious illness (eg, metabolic syndrome), greater cardiometabolic risk has also been associated with the localization of excess fat in the visceral adipose tissue and ectopic deposits.2 Several large epidemiologic studies have evaluated the association between obesity and mortality. In particular, a meta-analysis of 230 cohort studies including more than 30 million individuals3 found that both obesity and overweight were associated with an increased risk of all-cause mortality. Despite the evidence that excess mortality increases with increasing BMI, some studies have reached the conclusion that elevated BMI may improve survival in patients with cardiovascular disease, a phenomenon called the obesity paradox.4
Increased BMI is also associated with an increased risk of multiple cancer types.5 In addition, obesity and overweight may increase cancer mortality.6 During last decades, we have observed a more rapid increase in obesity among adult cancer survivors compared with the general population.7 The mechanisms contributing to higher cancer incidence and mortality may include alterations in sex hormone metabolism, insulin and insulin-like growth factor levels, and adipokine pathways.8,9
Various studies have suggested that patients with cancer and a normal BMI (ie, 20-25) have worse outcomes than patients with obesity. This phenomenon (ie, the obesity paradox) in cancer is not well understood and presents controversial explanations.10,11,12 Three different meta-analyses have led to different results, in particular in lung and renal cell carcinomas.13,14,15 In lung cancer, obesity is favorably associated with long-term survival of surgical patients. Moreover, in renal cell carcinoma, an inconsistent association of BMI with cancer-specific survival (CSS) was found. Conversely, breast, ovarian, and colorectal cancer are invariably associated with increased mortality in patients with obesity.16,17,18 The main explanations for these observations include the general poor health status of patients with very low BMI. Additionally, weight loss may be associated with frailty and other risk factors (eg, smoking).11 In cancer, obesity is also associated with increased efficacy of programmed cell death 1 and programmed cell death ligand 1 (PD-1/PD-L1) blockade in both tumor-bearing mice and patients.12 This updated systematic review and meta-analysis was conducted to evaluate the prognosis of patients with cancer who have obesity vs those without obesity.
Methods
Search Strategy and Inclusion Criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline19,20 A systematic search was conducted of EMBASE, PubMed, and the Cochrane Library for articles published from database inception until September 30, 2020. The following search terms were used: ((carcinoma or cancer or sarcoma or melanoma or (“Neoplasms”[MESH])) AND (obese OR obesity OR 30 kg/m2 OR “body mass index”) AND (hazard ratio) AND survival AND (multivariate OR cox or multivariable). The reference lists of identified articles were then manually searched to identify potentially relevant omitted citations. Articles that were not published in English were not included.
Retrospective and observational studies (ie, cohort, case-control) or prospective trials were selected when they reported the association of obesity, defined as a BMI of at least 30, with the risk of death (ie, overall survival [OS]), CSS, disease-free survival (DFS), or progression-free survival (PFS) in patients with cancer compared with counterparts without obesity (ie, BMI <30). We placed no restrictions on study setting, size, race, or country. Included studies were limited to those reporting hazard ratios (HRs) and their corresponding 95% CIs. Studies were restricted to adult patients with solid tumors. Hematologic malignant neoplasms were excluded. Short-term survival studies (eg, postsurgical mortality) were also excluded. Baseline-only BMI evaluation was considered (eg, BMI captured at cancer diagnosis in early-stage cancers or at metastatic disease in advanced-stage cancers).
The most up-to-date versions of full-text publications were included. Study selection was performed in 2 stages. First, titles and abstracts were screened; then, selected full-text articles were included according to the eligibility criteria. If pooled analyses of more than 1 study were evaluated for inclusion, the included articles were manually evaluated for duplicate inclusion compared with the other eligible articles. Screening was performed independently by 10 authors (M.G., G.T., A.G., A. Indini, A.C., O.N., V.R., A. Iaculli, L.D., M.S.), and conflicts were handled by consensus with a senior author (F.P.).
Data Collection and Quality Assessment
Data were collected independently by using a predesigned spreadsheet (Excel version 2007 [Microsoft Corp]). Collected data items included authors, year of publication, study setting and design, median follow-up, treatments received, outcomes, and type of analysis. The primary outcome was OS; secondary end points were CSS and PFS or DFS. Along with data extraction, 1 author (F.P.) assessed study quality according to a modified Newcastle Ottawa Scale (NOS; range 1-9, with 1-3 indicating low quality, 4-6 indicating moderate quality, and 7-9 indicating high quality).21
Statistical Analysis
First, pooled HRs with 95% CIs were estimated using random-effects meta-analysis with the generic inverse-variance method for studies that provided fully adjusted HRs. Inconsistency across studies was measured with the I2 method. Cutoff values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively. When I2 was larger than 50%, a random-effects model was primarily used because of the retrospective nature of included studies. To examine heterogeneity, we performed exploratory analyses of predefined subgroups based on type of disease, type of study, duration of follow-up, and race. Additionally, to address potential bias and verify our results, we performed sensitivity analyses using a leave-one-out method and the trim-and-fill method.22 These methods explore whether there are potential dominant studies that may have driven the results. Finally, to investigate the risk of publication bias, we applied the Egger test and visually inspected the funnel plots (ie, the Begg test).23 If the distribution of studies is symmetrical, the meta-analysis most likely does not have problems with publication bias. All statistical tests were 2-sided using a significance level of P < .05. All analyses were carried out using Comprehensive Meta-Analysis software version 3.3.070.
Results
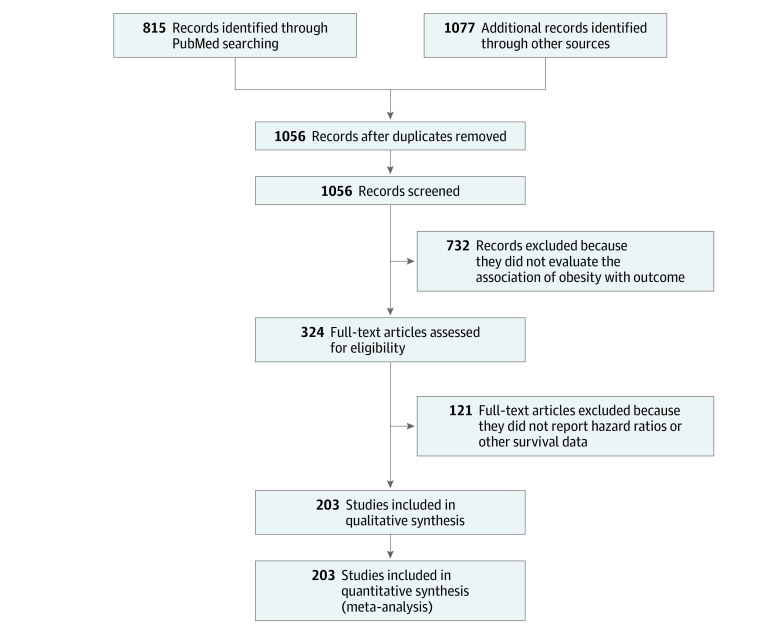
Our literature search yielded 1892 articles, of which 203 (17%) met the inclusion criteria for our overall systematic review of the association of obesity with cancer outcomes (Figure). Most excluded studies did not use the prespecified cutoff value for obesity (ie, BMI values different from 30 in 437 studies) or used a continuous cutoff for risk of death (eg, 1 unit-increase in BMI in 235 studies). Of the 203 articles, 170 (84%) were eligible for inclusion in the systematic review of the association of obesity with OS, 109 (54%) for association with CSS, and 79 (39%) for association with DFS or PFS. Descriptive data for studies included in our meta-analysis are listed in Table 1.12,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225 Overall, the included studies included a total of 6 320 365 patients. Sample sizes ranged from 41 to 1 096 492 patients, with a median of 1543. Most studies were retrospective in nature (132 studies [63%]); the minority were prospective cohort or observational studies (63 studies [31%]) or pooled analyses or randomized trials (8 studies [4%]). Multivariable analysis was performed in 197 studies. Overall, 136 studies (63%) reported a significant association of obesity with the outcome in at least 1 end point. The mean NOS score was 7 (median, 7.5; range, 5-9), indicating that the overall quality of articles was good.
Figure. Flow Diagram of Included Studies.
Table 1. Characteristics of Included Studies.
| Source | Patients, No. | Patients with obesity, No. (%) | Study type | Country | Disease | Follow-up, median, mo | Disease stage | Treatment | Type of analysis | OS | CSS | DFS/PFS | Qualitya |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromecki et al,24 2013 | 4118 | 1739 (42) | Retrospective | Various | Bladder | 44 | Early | S with or without adjuvant CT | MVA | ✓ | ✓ | ✓ | 7 |
| Ferro et al,25 2019 | 1155 | 224 (21) | Retrospective | Italy | Bladder | 48 | Early | TURBT with BCG vaccine | MVA | ✓ | ✓ | 7 | |
| Siegel et al,26 2013 | 853 | 216 (25) | Prospective | United States | Brain | 19 | Early | NA | MVA | ✓ | 8 | ||
| Abrahamson et al,27 2006 | 1254 | NA | Retrospective | United States | Breast | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Abukabar et al,28 2018 | 3012 | 433 (13) | Retrospective | Malaysia | Breast | 24 | Early and advanced | S with or withouth RT and/or CT | MVA | ✓ | ✓ | 6 | |
| Alarfi et al,29 2017 | 82 | 27 (33) | Prospective | Syria | Breast | 40 | Advanced | CT | MVA | ✓ | ✓ | 6 | |
| Alsaker et al,30 2011 | 2640 | 432 (16) | Retrospective | Norway | Breast | 69 | Early and advanced | NA | MVA | ✓ | 7 | ||
| Arce-Salinas et al,31 2014 | 819 | 596 (74) | Retrospective | Mexico | Breast | 28 | Advanced | CT | UVA | ✓ | ✓ | 6 | |
| Beasley et al,32 2012 | 13 302 | 2330 (18) | Pooled analysis, meta-analysis | United States, China | Breast | NA | Early | All | MVA | ✓ | ✓ | ✓ | 6 |
| Blair et al,33 2019 | 859 | 195 (23) | Cohort study | United States | Breast | 94 | Early and advanced | CT, RT, HT | MVA | ✓ | 8 | ||
| Braithwaite et al,34 2010 | 2202 | 500 (23) | Retrospective | United States | Breast | 88 | Early | NA | MVA | ✓ | ✓ | 8 | |
| Buono et al,35 2017 | 841 | 231 (27) | Retrospective | Italy | Breast | 58.9 | Early | CT, HT, S | MVA | ✓ | ✓ | 7 | |
| Caan and Kwan,36 2008 | 1692 | 409 (24) | Retrospective | United States | Breast | NA | Early | S and/or adjuvant systematic therapy | MVA | ✓ | ✓ | ✓ | 5 |
| Cecchini et al,37 2016 | 5265 | 1794 (34) | Phase 3, NSABP-B30 | United States | Breast | 108 | Early | CT | MVA | ✓ | NA | ||
| Cecchini et al,37 2016 | 2102 | 664 (32) | Phase 3, NSABP-B31 | United States | Breast | 99.6 | Early | CT and TTZ | MVA | ✓ | 8 | ||
| Cecchini et al,37 2016 | 3311 | 1186 (36) | Phase 3, NSABP-B34 | United States | Breast | 100.8 | Early | BPS vs Placebob | MVA | ✓ | 8 | ||
| Cecchini et al,37 2016 | 4860 | 1917 (39) | Phase 3, NSABP-B38 | United States | Breast | 70.8 | Early | CT | MVA | ✓ | 7 | ||
| Chang et al,38 2000 | 177 | 64 (36) | Retrospective | United States | Breast | 100 | Advanced | Induction CT and S | MVA | ✓ | 8 | ||
| Chen et al,39 2010 | 5042 | 283 (6) | Retrospective | China | Breast | 6.5 | Early and advanced | S, CT, RT, HT | MVA | ✓ | ✓ | 6 | |
| Chung et al,40 2017 | 8742 | 75 (9) | Retrospective | South Korea | Breast | 92 | Early | All | MVA | ✓ | ✓ | 9 | |
| Cleveland et al,41 2012 | 1447 | 319 (22) | Prospective, case-control | United States | Breast | NA | NA | NA | MVA | ✓ | ✓ | 8 | |
| Connor et al,42 2016 | 2478 | 142 (6) | Prospective, registry | United States | Breast | 129.6 | Early and advanced | NA | MVA | ✓ | ✓ | 9 | |
| Conroy et al,43 2011 | 3842 | 901 (23) | Prospective, cohort | United States | Breast | 74.4 | Early and advanced | S with or without CT/HT and/or RT | MVA | ✓ | ✓ | 8 | |
| Copson et al,44 2015 | 2843 | 533 (19) | Prospective | United Kingdom | Breast | 70.4 | Early and advanced | S, RT, CT, HT | MVA | ✓ | ✓ | 8 | |
| Crozier et al,45 2013 | 3017 | 1298 (43) | Retrospective | United States | Breast | 63.6 | Early | NA | MVA | ✓ | ✓ | 8 | |
| Dal Maso et al,46 2008 | 1453 | 172 (12) | Retrospective | Italy | Breast | NA | Early | S and/or adjuvant systematic therapy | UVA | ✓ | ✓ | 5 | |
| Dignam et al,47 2003 | 3385 | 395 (12) | Retrospective | United States | Breast | NA | Early | S and HT | MVA | ✓ | ✓ | ✓ | 5 |
| Dignam et al,48 2006 | 4077 | 1056 (26) | Retrospective | United States | Breast | NA | NA | NA | MVA | ✓ | ✓ | ✓ | 5 |
| Elwood et al,49 2018 | 1049 | 225 (21) | Retrospective | New Zealand | Breast | 49.2 | Early and advanced | CT, HT | MVA | ✓ | ✓ | 6 | |
| Emaus et al,50 2010 | 1364 | 147 (11) | Retrospective | Norway | Breast | 98.4 | Early and advanced | NA | MVA | ✓ | ✓ | 8 | |
| Feliciano et al,51 2017 | 1559 | 471 (30) | Retrospective | United States | Breast | 108 | Early | All | MVA | ✓ | ✓ | 8 | |
| Goodwin et al,52 2012 | 535 | NA | Retrospective | Canada | Breast | 145.2 | Early | S with or without adjuvant CT and/or HT | MVA | ✓ | ✓ | 9 | |
| He et al,53 2012 | 1983 | 546 (28) | Retrospective | United States | Breast | 47.6 | Early and advanced | All | MVA | ✓ | 7 | ||
| Hellmann et al,54 2010 | 528 | 76 (14) | Prospective | Denmark | Breast | 93.6 | Early and advanced | NA | MVA | ✓ | ✓ | 7 | |
| His et al,55 2016 | 3160 | 194 (6) | Prospective | France | Breast | 109.2 | Early | NA | MVA | ✓ | ✓ | ✓ | 8 |
| Jeon et al,56 2015 | 41 021 | 1632 (4) | Prospective, registry | Korea | Breast | 92 | Early | CT, HT | MVA | ✓ | ✓ | 8 | |
| Jiralerspong et al,57 2013 | 6342 | 1779 (30) | Retrospective | United States | Breast | 64.8 | Early | NA | MVA | ✓ | ✓ | ✓ | 7 |
| Kawai et al,58 2016 | 20 090 | 897 (5) | Prospective, registry | Japan | Breast | 80.4 | Early | CT, HT | MVA | ✓ | ✓ | ✓ | 7 |
| Keegan et al,59 2010 | 4153 | 127 (3) | Retrospective | United States | Breast | NA | NA | NA | MVA | ✓ | 5 | ||
| Kwan et al,60 2012 | 14 948 | 2440 (16) | Prospective, cohort | United States | Breast | 93.6 | Early | S with or without adjuvant CT and/or HT and/or RT | MVA | ✓ | ✓ | 8 | |
| Kwan et al,61 2014 | 11 351 | 3405 (30) | 3 pooled case-control, 3 prospective cohort | United States | Breast | 132 | Early and advanced | All | MVA | ✓ | ✓ | 8 | |
| Ladoire et al,62 2014 | 5009 | 666 (13) | Pooled analysis, 2 phase 3 | France | Breast | 70.8 | Early | CT | MVA | ✓ | ✓ | 7 | |
| Larsen et al,63 2015 | 1229 | 167 (14) | Prospective, cohort | Denmark | Breast | 115.2 | Early | NA | MVA | ✓ | 9 | ||
| Loi et al,64 2005 | 1101 | 131 (12) | Retrospective | Australia | Breast | 60 | Early | S and/or adjuvant systematic therapy | MVA | ✓ | ✓ | 6 | |
| Maskarinec et al,65 2011 | 382 | 71 (19) | Prospective, cohort | Hawaii | Breast | 158.4 | Early and advanced | S with or without adjuvant CT and/or HT | MVA | ✓ | ✓ | 9 | |
| McCullough et al,66 2005 | 430 236 | 5433 (1) | Prospective | United States | Breast | 240 | Early and advanced | All | MVA | ✓ | 9 | ||
| McCullough et al,67 2016 | 1308 | 301 (23) | Population-based | United States | Breast | 180 | Early | NA | MVA | ✓ | ✓ | 9 | |
| Nichols et al,68 2010 | 3993 | 639 (16) | Retrospective | United States | Breast | 76.8 | Early | S | MVA | ✓ | ✓ | 7 | |
| Nur et al,69 2019 | 49 259 | 202 (4) | Retrospective | Sweden | Breast | 91.6 | Early | S | MVA | ✓ | ✓ | 8 | |
| Oh et al,70 2011 | 747 | 251 (34) | Cohort study | Korea | Breast | 62.2 | Early | S with or without CT | MVA | ✓ | 7 | ||
| Oudanonh et al,71 2020 | 3747 | 1790 (48) | Retrospective, registry | Canada | Breast | NA | Early | HT, CT, RT, anti-ERBB2 | MVA | ✓ | 5 | ||
| Pajares et al,72 2013 | 5683 | 1376 (24) | Retrospective | Spain | Breast | NA | Early | CT, HT, S | MVA | ✓ | ✓ | ✓ | 5 |
| Pfeiler et al,73 2013 | 1509 | 315 (21) | Retrospective | Austria | Breast | 60 | Early | NA | MVA | ✓ | ✓ | ✓ | 7 |
| Pierce et al,74 2007 | 1490 | 380 (26) | Prospective | United States | Breast | 80.4 | Early | S and/or adjuvant systematic therapy | MVA | ✓ | 8 | ||
| Probst-Hensch et al,75 2010 | 855 | 72 (20) | Retrospective | Switzerland | Breast | 43.8 | Early | S with or without HT | MVA | ✓ | 6 | ||
| Senie et al,76 1992 | 923 | 207 (22) | Prospective | United States | Breast | 120 | Early | NA | MVA | ✓ | 9 | ||
| Sparano et al,77 2012 | 4817 | 1745 (46) | Retrospective, phase 3 | United States | Breast | 95 | Early | S with CT and HT | MVA | ✓ | ✓ | ✓ | 8 |
| Sparano et al,78 2012 | 6885 | 2547 (37) | Retrospective, 3 phase 3 | United States | Breast | 95 | Early | S with or without CT and/or HT | MVA | ✓ | ✓ | ✓ | 9 |
| Su et al,79 2013 | 1030 | 312 (30) | Randomized study | United States | Breast | NA | Early | S with or without CT and/or HT | MVA | ✓ | 5 | ||
| Sun et al,80 2015 | 1109 | 410 (37) | Population-based | United States | Breast | 162 | Early | NA | MVA | ✓ | ✓ | 8 | |
| Sun et al,81 2018 | 1017 | 192 (19) | Retrospective | China | Breast | 80 | Early | CT, HT, RT, S | MVA | ✓ | ✓ | 8 | |
| Tait et al,82 2014 | 501 | 202 (40) | Retrospective | United States | Breast | 40.1 | Early and advanced | S, CT | MVA | ✓ | ✓ | 6 | |
| Warren et al,83 2016 | 878 | NA | Retrospective | United States | Breast | 129.6 | Early | S | UVA | ✓ | 9 | ||
| Widschwendter et al,84 2015 | 3754 | 788 (21) | Phase 3, SUCCESS A | Germany | Breast | NA | Early | CT with HT | MVA | ✓ | ✓ | 5 | |
| Xiao et al,85 2014 | 5785 | 1680 (29) | Retrospective | China | Breast | 70 | Early | CT, HT | MVA | ✓ | 7 | ||
| Mazzarella et al,86 2013 | 1250 | 101 (8) | Retrospective | Europe | Breast, ERBB2 | NA | Early | S, RT, CT | MVA | ✓ | ✓ | 5 | |
| Rosenberg et al,87 2009 | 2640 | 376 (14) | Retrospective | Sweden | Breast, HR positive | NA | Early and advanced | S, CT, HT, RT | MVA | ✓ | 5 | ||
| Ademuyiawa et al,88 2011 | 418 | 164 (39) | Retrospective | United States | Breast, TN | 37.2 | Early | S | MVA | ✓ | ✓ | 6 | |
| Dawood et al,89 2012 | 2311 | 825 (36) | Retrospective | United States | Breast, TN | 39 | Early | S | MVA | ✓ | NA | ✓ | 6 |
| Melhem-Bertrandt et al,90 2011 | 1413 | 460 (33) | Retrospective | United States | Breast, TN | 59 | Early | S with or without CT | MVA | ✓ | NA | ✓ | 7 |
| Frumovitz et al,91 2014 | 3086 | 1026 (33) | Retrospective | United States | Cervical | 133 | Early and advanced | S, RT | MVA | ✓ | ✓ | 9 | |
| Fedirko et al,92 2014 | 3924 | 689 (18) | Prospective | Western Europe | CRC | 49 | Early and advanced | NA | MVA | ✓ | ✓ | 7 | |
| Boyle et al,93 2013 | 879 | 258 (29) | Retrospective | Australia | CRC | 67.2 | Early | NA | MVA | ✓ | ✓ | ✓ | 7 |
| Campbell et al,94 2015 | 5615 | 1483 (26) | Prospective | Various | CRC | NA | Early and advanced | S, CT | MVA | ✓ | ✓ | 5 | |
| Cespedes Feliciano et al,95 2017 | 2470 | NA | Retrospective | United States | CRC | 72 | Early | NA | MVA | ✓ | ✓ | 7 | |
| Clark et al,96 2013 | 99 | 40 (41) | Retrospective | United States | CRC | 39.4 | Early | CT and RT | MVA | ✓ | ✓ | 6 | |
| Dahdaleh et al,97 2018 | 1543 | 529 (34) | Retrospective, cohort | United States | CRC | 30.9 | Early | S, adjuvant CT | MVA | ✓ | ✓ | 6 | |
| Dignam et al,98 2006 | 4288 | 812 (10) | Retrospective | North America | CRC | NA | Early | CT | MVA | ✓ | ✓ | ✓ | 5 |
| Jayasekara et al,99 2018 | 724 | 164 (23) | Cohort study | Australia | CRC | 108 | Early | NA | MVA | ✓ | ✓ | 8 | |
| Kaidar-Person et al,100 2015 | 184 | 46 (25) | Retrospective | Israel | CRC | 27.6 | Advanced | CT, bevacizumab | MVA | ✓ | ✓ | 6 | |
| Kalb et al,101 2019 | 612 | 127 (21) | Retrospective | Germany | CRC | 58 | Early | CT, RT, S | MVA | ✓ | ✓ | 7 | |
| Meyerhardt et al,102 2003 | 3561 | 500 (17) | Cohort study | United States | CRC | 112 | Early | S and CT | MVA | ✓ | ✓ | 9 | |
| Meyerhardt et al,103 2004 | 1688 | 306 (18) | Cohort study | United States | CRC | 118 | Early | S and CT | MVA | ✓ | ✓ | 9 | |
| Meyerhardt et al,104 2008 | 1043 | 236 (23) | Prospective | United States and Canada | CRC | 63.6 | Early | S and/or adjuvant systematic therapy | MVA | ✓ | ✓ | 8 | |
| Morikawa et al,105 2012 | 1060 | 200 (19) | Prospective, cohort | United States | CRC | 162 | Early and advanced | All | MVA | ✓ | ✓ | 9 | |
| Ogino et al,106 2009 | 546 | 84 (16) | Retrospective | United States | CRC | NA | Early and advanced | NA | MVA | ✓ | ✓ | 5 | |
| Patel et al,107 2015 | 1174 | 462 (39) | Retrospective | Australia | CRC | NA | Advanced | CT | MVA | ✓ | 5 | ||
| Pelser et al,108 2014 | 5727 | NA | Retrospective | United States | CRC | NA | Early and advanced | S, RT, CT | MVA | ✓ | ✓ | 5 | |
| Prizment et al,109 2010 | 1096 | 295 (27) | Retrospective, registry | United States | CRC | 240 | Early and advanced | CT, RT, S | MVA | ✓ | ✓ | 9 | |
| Schlesinger et al,110 2014 | 2143 | 397 (19) | Prospective | Germany | CRC | 42 | Early and advanced | NA | MVA | ✓ | 6 | ||
| Shah et al,111 2015 | 242 | 59 (25) | Prospective | United States | CRC | NA | Advanced | S, CT | MVA | ✓ | 7 | ||
| Sinicrope et al,112 2012 | 2693 | 630 (23) | Pooled analysis, randomized trial | United States | CRC | NA | Early | S with or without CT | MVA | ✓ | ✓ | 5 | |
| Sinicrope et al,113 2013 | 25 291 | 4463 (18) | Retrospective | United States | CRC | 93.6 | Early | NA | MVA | ✓ | ✓ | 8 | |
| Sorbye et al,114 2012 | 342 | 67 (20) | Retrospective | Europe | CRC | NA | Advanced | S with or without CT | MVA | ✓ | 5 | ||
| Wang et al,115 2017 | 1452 | NA | Retrospective | China | CRC | 40.8 | Early and advanced | All | MVA | ✓ | 6 | ||
| Zheng et al,116 2016 | 226 | 52 (23) | Cohort study | China | CRC | NA | Early and advanced | NA | MVA | ✓ | ✓ | 5 | |
| Doria-Rose et al,117 2006 | 633 | 96 (15) | Retrospective | United States | CRC among women | 112.8 | Early | S and/or other, unspecified treatments | MVA | ✓ | ✓ | 8 | |
| Kristensen et al,118 2017 | 4330 | NA | Retrospective | Denmark | Endometrial | NA | Early and advanced | S | MVA | ✓ | 5 | ||
| Nagle et al,119 2018 | 1359 | 568 (42) | Retrospective | Australia | Endometrial | 85.2 | Early and advanced | S with or without CT | MVA | ✓ | ✓ | 8 | |
| Nicholas et al,120 2014 | 490 | 203 (41) | Retrospective | United States | Endometrial | 54 | Early and advanced | S | MVA | ✓ | 6 | ||
| Todo et al,121 2014 | 716 | 99 (14) | Retrospective | Japan | Endometrial | 74 | Early and advanced | S, CT | MVA | ✓ | ✓ | 7 | |
| Yoon et al,122 2015 | 2987 | 417 (14) | Retrospective, cohort | United States | Endometrial | NA | Early and advanced | S, CT, RT | MVA | ✓ | 5 | ||
| Hynes et al,123 2017 | 390 | 64 (17) | Prospective, cohort | Sweden | Esophagus | NA | Early | S | MVA | ✓ | ✓ | 5 | |
| Spreafico et al,124 2017 | 564 | 76 (13) | Retrospective | Canada | Esophagus | 32.5 | Early and advanced | All | MVA | ✓ | ✓ | 6 | |
| Sundelöf et al,125 2008 | 580 | 55 (10) | Retrospective | Sweden | Esophagus | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Yoon et al,126 2011 | 778 | 46 (19) | Retrospective | United States | Esophagus, adenocarcinoma | 154.8 | Early | S with or without adjuvant CT, RT and/or CTRT | MVA | ✓ | ✓ | ✓ | 9 |
| Thrift et al,127 2012 | 783 | 263 (33) | Retrospective | Australia | Esophagus or gastric | 76.8 | Early and advanced | S with or without CTRT and/or BSC | MVA | ✓ | 8 | ||
| Trivers et al,128 2005 | 1142 | 156 (4) | Retrospective | United States | Esophagus or gastric | NA | Early and advanced | S and/or other, unspecified treatments | MVA | ✓ | ✓ | 5 | |
| Potharaju et al,129 2018 | 392 | 40 (10) | Retrospective | India | GBM | 48.6 | NA | S, RT, and TMZ | MVA | ✓ | 6 | ||
| Gama et al,130 2017 | 1279 | 243 (21) | Retrospective | Canada | HN | 30 | Early and advanced | RT, S, CT | MVA | ✓ | ✓ | 6 | |
| Grossberg et al,131 2016 | 190 | 65 (34) | Retrospective | United States | HN | 68.6 | Early | CT with RT | MVA | ✓ | ✓ | ✓ | 7 |
| Hu et al,132 2019 | 576 | 33 (6) | Retrospective | China | HN, oral SCC | 64 | Early | S | MVA | ✓ | ✓ | 7 | |
| Ata et al,133 2019 | 8352 | 2841 (34) | Retrospective | United States | HCC | 60 | NA | Liver transplantation | MVA | ✓ | ✓ | 7 | |
| Carr et al,134 2018 | 521 | NA | Retrospective | Italy | HCC | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Yang et al,135 2019 | 2442 | 86 (4) | Retrospective | United States | HCC | 50.5 | Early | S | MVA | ✓ | ✓ | 8 | |
| Roque et al,136 2016 | 128 | 72 (56) | Retrospective | United States | Leiomyosarcoma | 49 | Early and advanced | All | MVA | ✓ | ✓ | 6 | |
| McMahon et al,137 2017 | 1080 | NA | Retrospective | United States | Liver | 123.6 | Early and advanced | All | MVA | ✓ | 9 | ||
| Abdel-Rahman,138 2019 | 145 544 | 18 131 (24) | Population-based, randomized | United States | Lung | 135 | Early and advanced | NA | MVA | ✓ | 9 | ||
| Leung et al,139 2011 | 58 931 | 3520 (6) | Prospective | Japan | Lung | NA | NA | NA | MVA | ✓ | 7 | ||
| Nonemaker et al,140 2009 | 2054 | Black participants: 50 (13); White participants: 46 (8) | Retrospective | United States | Lung | NA | NA | NA | MVA | ✓ | ✓ | 5 | |
| Qi et al,141 2009 | 420 | 79 (23) | Retrospective | United States | Lung | NA | Advanced | All | MVA | ✓ | 5 | ||
| Shepshelovich et al,142 2019 | 29 217 | 418 (1) | Pooled analysis | Canada | Lung | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Turner et al,143 2011 | 188 699 | 22 054 (12) | Prospective | United States | Lung | 312 | NA | NA | MVA | ✓ | 9 | ||
| Xie et al,144 2017 | 624 | NA | Retrospective | China | Lung | 63.2 | Early | S | MVA | ✓ | 6 | ||
| McQuade et al,145 2019 | 1918 | 513 (27) | Pooled analysis | United States | Melanoma | NA | Advanced | CT, IT, TT | MVA | ✓ | ✓ | 5 | |
| Aldrich et al,146 2013 | 501 | 126 (25) | Prospective (cohort) | United States | NSCLC | 16 | Early and advanced | NA | MVA | ✓ | 7 | ||
| Kichenadasse et al,147 2020 | 1434 | 239 (7) | Prospective | Various | NSCLC | NA | Advanced | Atezolizumab vs docetaxel | UVA | ✓ | ✓ | 7 | |
| Nakagawa et al,148 2016 | 1311 | 25 (2) | Retrospective | Japan | NSCLC | 59 | Early | S | MVA | ✓ | ✓ | 7 | |
| Bandera et al,149 2015 | 1846 | 547 (30) | Cohort study | United States | Ovarian | NA | Early and advanced | CT | MVA | ✓ | ✓ | 5 | |
| Kotsopoulos et al,150 2012 | 1423 | 230 (18) | Retrospective | Canada | Ovarian | 120 | Early and advanced | All | MVA | ✓ | 9 | ||
| Minlikeeva et al,151 2019 | 7022 | 1557 (22) | Retrospective, pooled data | United States and Australia | Ovarian | NA | Early and advanced | NA | MVA | ✓ | ✓ | 5 | |
| Previs et al,152 2014 | 81 | 28 (34) | Retrospective | United States | Ovarian | NA | Early and advanced | S, RT | MVA | ✓ | ✓ | ✓ | 5 |
| Tyler et al,153 2012 | 425 | 28 (7) | Prospective, case-control | United States | Ovarian | 116.4 | Early and advanced | All | MVA | ✓ | ✓ | 9 | |
| Yang et al,154 2008 | 635 | 81 (13) | Prospective | Europe | Ovarian | 96 | Early and advanced | NA | MVA | ✓ | 7 | ||
| Dalal et al,155 2012 | 41 | 8 (20) | Prospective | United States | Pancreas | NA | Advanced | CTRT | MVA | ✓ | 7 | ||
| Genkinger et al,156 2015 | 1 096 492 | NA | Cohort study | United States | Pancreas | 152.4 | NA | NA | MVA | ✓ | 8 | ||
| Gong et al,157 2012 | 510 | 51 (10) | Retrospective | United States | Pancreas | 121.2 | Early and advanced | All | MVA | ✓ | 9 | ||
| Li et al,158 2009 | 841 | 163 (19) | Retrospective | United States | Pancreas | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Lin et al,159 2013 | 799 542 | 19 988 (3) | Retrospective | Various | Pancreas | 37.2 | NA | NA | MVA | ✓ | 6 | ||
| Olson et al,160 2010 | 475 | 108 (23) | Retrospective | United States | Pancreas | NA | Early and advanced | S | MVA | ✓ | ✓ | 5 | |
| Yuan et al,161 2013 | 902 | 136 (15) | Prospective, cohort | United States | Pancreas | 480 | Early and advanced | NA | MVA | ✓ | 9 | ||
| Tsai et al,162 2010 | 795 | 103 (13) | Retrospective | United States | Pancreas | NA | Early and advanced | S | MVA | ✓ | 5 | ||
| Bassett et al,163 2012 | 16 525 | 247 (18) | Prospective, cohort | Australia | Prostate | 180 | NA | NA | MVA | ✓ | 9 | ||
| Bonn et al,164 2014 | 4376 | 483 (11) | Retrospective | Sweden | Prostate | 48 | Early | S, RT | MVA | ✓ | ✓ | ✓ | 6 |
| Dickerman et al,165 2017 | 5158 | 564 (11) | Retrospective | United States | Prostate | NA | Early | All | MVA | ✓ | 5 | ||
| Efstathiou et al,166 2007 | 945 | 145 (15) | Prospective | United States | Prostate | 97.2 | Advanced | RT with or without goserelin | MVA | ✓ | ✓ | 8 | |
| Farris et al,167 2018 | 987 | 192 (19) | Prospective, cohort | Canada | Prostate | 228 | Early and advanced | S, RT, HT | MVA | ✓ | ✓ | 9 | |
| Froehner et al,168 2014 | 2131 | 356 (17) | Retrospective | Germany | Prostate | 110 | Early | All | UVA, MVA | ✓ | ✓ | 9 | |
| Gong et al,169 2007 | 752 | 128 (17) | Retrospective | United States | Prostate | 116.4 | Early and advanced | S, ADT, RT, and other, unspecified treatments | MVA | ✓ | 9 | ||
| Han et al,170 2010 | 2511 | 211 (8) | Retrospective | United States | Prostate | 156 | Early | All | UVA | ✓ | 9 | ||
| Ho et al,171 2012 | 1038 | 337 (32) | Retrospective | United States | Prostate | 41 | Early and advanced | S | MVA | ✓ | 6 | ||
| Kelly et al,172 2016 | 7822 | 1612 (21) | Retrospective | United States | Prostate | 156 | Early and advanced | All | MVA | ✓ | 9 | ||
| Kenfield et al,173 2015 | 112 185 | 9984 (9) | Prospective | United States | Prostate | 170 | NA | NA | MVA | ✓ | 9 | ||
| Khan et al,174 2017 | 822 | NA | Retrospective | United States | Prostate | 60 | Early and advanced | All | MVA | ✓ | 7 | ||
| Ma et al,175 2008 | 2546 | 87 (3) | Retrospective | United States | Prostate | 84 | Early and advanced | NA | MVA | ✓ | ✓ | 7 | |
| Maj-Hes et al,176 2017 | 6519 | 2462 (38) | Retrospective | Austria | Prostate | 28 | Early | S | MVA | ✓ | 6 | ||
| Møller et al,177 2015 | 26 877 | 4140 (15) | Cohort study | Denmark | Prostate | 43.2 | Early and advanced | NA | MVA | ✓ | 8 | ||
| Rudman et al,178 2016 | 273 | 59 (22) | Retrospective | United Kingdom | Prostate | 139.2 | Early | HT | MVA | ✓ | ✓ | 9 | |
| Schiffmann et al,179 2017 | 16 014 | 2403 (15) | Retrospective | Germany | Prostate | 36.4 | Early | S | MVA | ✓ | 6 | ||
| Spangler et al,180 2007 | 924 | 286 (31) | Prospective | Various | Prostate | 36 | Early | S | MVA | ✓ | 7 | ||
| Vidal et al,181 2017 | 4268 | 1372 (32) | Retrospective | United States | Prostate | 81.6 | Early | S | MVA | ✓ | ✓ | 8 | |
| Wu et al,182 2015 | 333 | 118 (35) | Retrospective | United States | Prostate | NA | Advanced | CT | MVA | ✓ | 5 | ||
| Montgomery et al,183 2007 | 1006 | 160 (16) | Retrospective | United States | Prostate, AD | NA | Advanced | Bilateral orchiectomy with or without flutamide | MVA | ✓ | ✓ | 5 | |
| Halabi et al,184 2007 | 1296 | 405 (31) | Retrospective | United States | Prostate, AI | 33.8 | Early and advanced | NA | MVA | ✓ | ✓ | 6 | |
| Montgomery et al,183 2007 | 671 | 253 (38) | Retrospective | United States | Prostate, AI | NA | Advanced | Mitoxantrone and prednisone vs docetaxel and estramustine | MVA | ✓ | ✓ | 5 | |
| Keizman et al,185 2014 | 278 | 67 (24) | Retrospective | Israel | RCC | 55 | Advanced | TKI | MVA | ✓ | ✓ | 7 | |
| Lee et al,186 2010 | 2750 | 120 (4) | Retrospective | South Korea | RCC | 34.8 | Early | S | MVA | ✓ | ✓ | 6 | |
| Parker et al,187 2006 | 970 | 336 (35) | Retrospective | United States | RCC | 56.4 | Early | S | MVA | ✓ | ✓ | 7 | |
| Psutka et al,188 2016 | 387 | 166 (43) | Retrospective | United States | RCC | 86.4 | Early | S | MVA | ✓ | ✓ | ✓ | 8 |
| Spiess et al,189 2012 | 99 | 43 (43) | Retrospective | United States | RCC | 44.4 | Early and advanced | S | MVA | ✓ | 6 | ||
| Yu et al,190 1991 | 360 | 44 (12) | Retrospective | United States | RCC | 53 | Early | S | MVA | ✓ | 7 | ||
| Hung et al,191 2018 | 33 551 | 2362 (7) | Retrospective, cohort | Taiwan | Solid cancers | 43.8 | Early and advanced | S | MVA | ✓ | ✓ | 6 | |
| Houdek et al,192 2019 | 261 | 71 (9) | Retrospective | Canada | STS | 48 | NA | RT vs none | MVA | ✓ | ✓ | 6 | |
| Iyengar et al,193 2014 | 155 | 30 (19) | Retrospective | United States | Tongue | NA | Early | S | MVA | ✓ | ✓ | ✓ | 5 |
| Xu et al,194 2019 | 644 | 92 (14) | Retrospective | China | Upper tract urothelial | 39 | Early | S | MVA | ✓ | ✓ | ✓ | 6 |
| Arem et al,195 2013 | 1400 | 610 (43) | Retrospective | United States | Uterine | 61.2 | Early and advanced | NA | MVA | ✓ | ✓ | 7 | |
| Matsuo et al,196 2016 | 665 | 459 (69) | Retrospective | United States | Uterine | 36.4 | Early and advanced | S with CTRT | MVA | ✓ | ✓ | 6 | |
| Ruterbusch et al,197 2014 | 627 | 184 (29) | Retrospective | United States | Uterine | NA | Early and advanced | S with or without CT | MVA | ✓ | ✓ | 5 | |
| Seidelin et al,198 2016 | 3638 | 984 (27) | Population-based | Denmark | Uterine | NA | Early and advanced | NA | MVA | ✓ | 7 | ||
| Abdullah et al,199 2011 | 5036 | 567 (11) | Retrospective, cohort | Various | Various | NA | NA | NA | MVA | ✓ | 5 | ||
| Akinyemiju et al,200 2018 | 22 514 | 8786 (39) | Prospective | United States | Various | 78 | NA | NA | MVA | ✓ | 8 | ||
| Barroso et al,201 2018 | 54 446 | 15 158 (28) | Retrospective | Spain | Various | NA | NA | NA | MVA | ✓ | ✓ | 5 | |
| Boggs et al,202 2011 | 51 695 | 23 656 (46) | Prospective | United States | Various | NA | NA | NA | MVA | ✓ | 8 | ||
| Cortellini et al,203 2019 | 976 | 377 (39) | Retrospective | Italy | Various | 17.2 | Advanced | Anti–PD-1/PD-L1 | MVA | ✓ | ✓ | 6 | |
| Drake et al,204 2017 | 7061 | 3220 (46) | Prospective, cohort | Sweden | Various | 202 | Early and advanced | All | MVA | ✓ | ✓ | 9 | |
| Han et al,205 2014 | 13 901 | 708 (5) | Retrospective | United States | Various | NA | NA | NA | MVA | ✓ | 5 | ||
| Izumida et al,206 2019 | 10 824 | 235 (2) | Cohort study | China | Various | 220.8 | NA | NA | MVA | ✓ | 9 | ||
| Janssen et al,207 2015 | 927 | NA | Retrospective | United States | Various | NA | Early and advanced | NA | MVA | ✓ | 5 | ||
| Jenkins et al,208 2018 | 502 631 | 12 539 (25) | Cohort study | United Kingdom | Various | 93.6 | NA | NA | MVA | ✓ | 7 | ||
| Katzmarzyk et al,209 2012 | 10 522 | 1972 (19) | Retrospective | Canada | Various | 168 | Early and advanced | All | MVA | ✓ | 8 | ||
| Kitahara et al,210 2014 | 313 575 | 9564 (3) | Retrospective | Various | Various | NA | NA | NA | MVA | ✓ | 5 | ||
| Martini et al,211 2020 | 90 | 23 (26) | Retrospective | United States | Various | NA | Advanced | IT | MVA | ✓ | ✓ | 5 | |
| Mathur et al,212 2010 | 279 | 97 (35) | Retrospective | United States | Various | 31 | Advanced | Hepatectomy | MVA | ✓ | ✓ | 6 | |
| Meyer et al,213 2015 | 35 703 | 2820 (8) | Population-based | Switzerland | Various | NA | Early and advanced | NA | MVA | ✓ | 7 | ||
| Nechuta et al,214 2010 | 71 243 | 8264 (12) | Cohort study | China | Various | 109.2 | NA | NA | MVA | ✓ | 9 | ||
| Parr et al,215 2010 | 401 215 | 16 978 (4) | Retrospective | All | Various | NA | NA | NA | MVA | ✓ | 5 | ||
| Sasazuki et al,216 2011 | 353 422 | 7327 (2) | Prospective, cohort | Japan | Various | 150 | NA | NA | MVA | ✓ | 9 | ||
| Silventoinen et al,217 2014 | 734 438 | 9187 (1) | Retrospective | Finland, Sweden | Various | 403.2 | NA | NA | MVA | ✓ | ✓ | 9 | |
| Song et al,218 2012 | 135 745 | NA | Prospective | Europe | Various | 201.6 | NA | NA | MVA | ✓ | 9 | ||
| Taghizadeh et al,219 2015 | 8645 | 683 (8) | Cohort study | Netherlands | Various | 480 | NA | NA | MVA | ✓ | 9 | ||
| Tseng,220 2013 | 89 056 | NA | Prospective | Taiwan | Various | 144 | Early and advanced | All | MVA | ✓ | 9 | ||
| Tseng,221 2016 | 92 546 | NA | Retrospective | Taiwan | Various | 204 | Early and advanced | All | MVA | ✓ | 9 | ||
| Valentijn et al,222 2013 | 10 247 | 1851 (18) | Retrospective | The Netherlands | Various | 64.8 | NA | NA | MVA | ✓ | 7 | ||
| Wang et al,12 2019 | 250 | 81 (12) | Retrospective | United States | Various | NA | Advanced | IT | MVA | ✓ | ✓ | 5 | |
| Xu et al,223 2018 | 6197 | 1885 (30) | Cohort study | United States | Various | 204 | NA | NA | MVA | ✓ | 9 | ||
| Yano et al,224 2013 | 3641 | 792 (22) | Prospective | Japan | Various | 122 | NA | NA | MVA | ✓ | 9 | ||
| You et al,225 2015 | 1314 | NA | Prospective, cohort | China | Various | 52.7 | Early and advanced | NA | MVA | ✓ | ✓ | 7 |
Abbreviations: AD, androgen dependent; ADT, androgen deprivation therapy; AI, androgen independent; BCG, Bacillus Calmette-Guérin; BPS, bisphosphonate; BSC, best supportive care; CRC, colorectal cancer; CSS, cancer specific survival; CT, chemotherapy; CTRT, chemotherapy with radiotherapy; DFS, disease-free survival; GBM, glioblastoma multiforme; HCC, hepatocellular carcinoma; HN, head and neck tumors; HR, hormone receptor; HT, hormone therapy; IT, immunotherapy; NSCLC, non–small cell lung cancer; MVA, multivariate analysis; NA, not applicable; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; RCC, renal cell carcinoma; RT, radiotherapy; STS, soft tissue sarcoma; SCC, squamous cell carcinoma; S, surgery; TKI, tyrosine kinase inhibitor; TMZ, temozolomide; TN, triple negative; TT, targeted therapy; TTZ, trastuzumab; TURBT, transurethral resection of bladder tumor; UVA, univariate analysis.
Quality assessed according to a modified Newcastle Ottawa Scale (range 1-9, with 1-3 indicating low quality, 4-6 indicating moderate quality, and 7-9 indicating high quality).
Clinical trial randomizing breast cancer patients to receive BPS vs placebo; patients received concomitant systemic anticancer treatment according to physicians’ decision (following institutional guidelines).
OS and Obesity in Patients With Cancer
A total of 170 studies reported data on OS. Because the heterogeneity test showed a high level of heterogeneity (I2 = 79.7%; P < .001) among studies, a random-effects model was used for the analysis. OS among patients with obesity was significantly worse than that among patients without obesity (HR, 1.14; 95% CI, 1.09-1.19; P < .001) (eFigure 1 in the Supplement). The association of obesity with outcomes was independent by other main cancer prognostic factors, including stage (100%), sex (85%), age (100%), race (80%), smoking status (83%), and other comorbidities according to multivariable analysis.
CSS and Obesity in Patients With Cancer
Similarly, obesity was associated with reduced CSS in 109 studies (HR, 1.17; 95% CI, 1.12-1.23; P < .001) (eFigure 2 in the Supplement). Heterogeneity was high (I2 = 73.9%; P < .001), so a random-effects model was used.
DFS or PFS and Obesity in Patients With Cancer
In 79 studies, obesity was associated with worse DFS or PFS compared with not having obesity (HR, 1.13; 95% CI, 1.07-1.19; P < .001) (eFigure 3 in the Supplement). Heterogeneity was high (I2 = 73.7%; P < .001), so a random-effects model was used.
Subgroup Analysis
A subgroup analysis for OS was performed according to type of disease (Table 2, Table 3, and Table 4). Patients with breast, colorectal, or uterine cancers and obesity had higher overall mortality than those without obesity (breast: HR, 1.26; 95% CI, 1.2-1.33; P < .001; colorectal: HR, 1.22; 95% CI, 1.14-1.31; P < .001; HR, 1.20; 95% CI, 1.04-1.38; P = .01). Patients with obesity and lung cancer, renal cell carcinoma, or melanoma had better survival outcomes compared with patients without obesity and the same cancer (lung: HR, 0.86; 95% CI, 0.76-0.98; P = .02; renal cell: HR, 0.74; 95% CI, 0.53-0.89; P = .02; melanoma: HR, 0.74; 95% CI, 0.57-0.96; P < .001). CSS was decreased in patients with obesity and breast, colorectal, prostate, and pancreatic cancers (breast: 1.23; 95% CI, 1.15-1.32; P < .001; colorectal: HR, 1.24; 95% CI, 1.16-1.32; P < .001; prostate: HR, 1.26; 95% CI, 1.08-1.47; P = .01; pancreatic: HR, 1.28; 95% CI, 1.05-1.57; P = .01). DFS was decreased in patients with obesity and breast, colorectal, prostate, and gastroesophageal cancers (breast: HR, 1.14; 95% CI, 1.1-1.19; P < .001; colorectal: HR, 1.15; 95% CI, 1.01-1.3; P = .01; prostate: HR, 1.29; 95% CI, 1.07-1.56; P < .001; gastroesophageal: HR, 1.62; 95% CI, 1.13-2.32; P < .001). Additional subgroup analyses included type of study (retrospective: HR, 1.07; 95% CI, 1.07-1.18; P < .001; prospective: HR, 1.14; 95% CI, 1.05-1.23; P < .001), duration of follow up (>10 years: HR, 1.16; 95% CI, 0.86-1.58; P = .08; <10 years: HR, 1.23; 95% CI, 0.84-1.63; P = .09), race (non-Asian race: HR, 1.22; 95% CI, 0.86-1.66, P = .06; Asian race: HR, 1.22; 95% CI, 0.74-1.72; P = .09), and stage of disease (early: HR, 1.20; 95% CI, 0.99-1.25; P = .07; advanced: HR, 1.2; 95% CI, 1.12-1.28; P = .01). Regression analysis according to NOS score was not significant.
Table 2. Association of Obesity With Overall Mortality, by Cancer.
| Disease | Studies, No. | HR (95% CI) | P value | I2 % | Type of analysis |
|---|---|---|---|---|---|
| Bladder or UTUC | 3 | 1.08 (0.98-1.20) | .11 | 0 | Random |
| Brain | 2 | 0.96 (0.50-1.84) | .90 | 88.5 | Random |
| Breast | 59 | 1.26 (1.20-1.33) | .004 | 51.3 | Random |
| CRC | 30 | 1.22 (1.14-1.31) | .001 | 54.5 | Random |
| Gastroesophageal | 7 | 1.08 (0.77-1.52) | .62 | 80.2 | Random |
| Head and neck | 7 | 0.59 (0.33-1.05) | .07 | 65.4 | Random |
| Hepatobiliary | 5 | 1.06 (0.89-1.25) | .48 | 73.6 | Random |
| Lung | 11 | 0.86 (0.76-0.98) | .02 | 60.4 | Random |
| Melanoma | 1 | 0.74 (0.63-0.89) | .004 | 0 | Random |
| Ovarian | 4 | 1.03 (0.75-1.41) | .84 | 64.7 | Random |
| Pancreas | 6 | 1.36 (0.95-1.93) | .08 | 80.5 | Random |
| Prostate | 12 | 1.07 (0.91-1.25) | .38 | 69.7 | Random |
| RCC | 5 | 0.78 (0.57-0.96) | .02 | 89.5 | Random |
| Uterine | 12 | 1.20 (1.04-1.38) | .01 | 60.8 | Random |
| Various | 9 | 1.10 (1.05-1.16) | .008 | 96.1 | Random |
Abbreviations: CRC, colorectal cancer; HR, hazard ratio; RCC, renal cell carcinoma; UTUC, upper tract urothelial carcinoma.
Table 3. Association of Obesity With Cancer-Specific Mortality by Cancer Type.
| Disease | Studies, No. | HR (95% CI) | P value | I2, % | Type of analysis |
|---|---|---|---|---|---|
| Bladder or UTUC | 3 | 1.36 (0.96-1.93) | .08 | 59.4 | Random |
| Breast | 36 | 1.23 (1.15-1.32) | .004 | 58.8 | Random |
| CRC | 13 | 1.24 (1.16-1.33) | .002 | 0 | Random |
| Gastroesophageal | 2 | 0.83 (0.58-1.16) | .28 | 0 | Random |
| Head and neck | 3 | 1.35 (0.27-6.74) | .70 | 90.5 | Random |
| Hepatobiliary | 1 | 0.79 (0.50-1.24) | .31 | 0 | Random |
| Lung | 3 | 0.53 (0.30-0.92) | .02 | 0 | Random |
| Ovarian | 4 | 1.06 (0.82-1.37) | .61 | 33.3 | Random |
| Pancreas | 3 | 1.28 (1.05-1.57) | .01 | 61.1 | Random |
| Prostate | 15 | 1.26 (1.08-1.47) | .001 | 57.9 | Random |
| RCC | 4 | 1.08 (0.58-2.00) | .80 | 89.5 | Random |
| Uterine | 6 | 1.02 (0.75-1.39) | .86 | 69.1 | Random |
| Various | 16 | 1.08 (0.97-1.19) | .14 | 83.3 | Random |
Abbreviations: CRC, colorectal cancer; HR, hazard ratio; RCC, renal cell carcinoma; UTUC, upper tract urothelial carcinoma.
Table 4. Association of Obesity With Recurrence by Cancer Type.
| Disease | Studies, No. | HR (95% CI) | P value | I2, % | Type of analysis |
|---|---|---|---|---|---|
| Bladder or UTUC | 3 | 1.42 (0.92-2.20) | .11 | 88.3 | Random |
| Breast | 34 | 1.14 (1.10-1.19) | .002 | 0 | Random |
| CRC | 12 | 1.15 (1.01-1.30) | .02 | 67.6 | Random |
| Gastroesophageal | 1 | 1.62 (1.13-2.32) | .005 | 0 | Random |
| Head and neck | 3 | 1.03 (0.48-2.20) | .92 | 75.7 | Random |
| Hepatobiliary | 2 | 1.06 (0.73-1.53) | .73 | 88.9 | Random |
| Lung | 2 | 0.55 (0.18-1.62) | .28 | 77.5 | Rando |
| Melanoma | 1 | 0.79 (0.69-0.90) | .006 | 0 | Random |
| Ovarian | 2 | 1.04 (0.92-1.17) | .52 | 0 | Random |
| Prostate | 11 | 1.29 (1.07-1.56) | .003 | 85.1 | Random |
| RCC | 4 | 0.69 (0.41-1.14) | .15 | 62.4 | Random |
| Sarcoma | 1 | 0.89 (0.47-1.68) | .72 | 0 | Random |
| Uterine | 2 | 0.98 (0.45-2.11) | .97 | 74.3 | Random |
| Various | 1 | 0.72 (0.49-1.05) | .09 | 0 | Random |
Abbreviations: CRC, colorectal cancer; HR, hazard ratio; RCC, renal cell carcinoma; UTUC, upper tract urothelial carcinoma.
Publication Bias
A funnel plot was used to assess publication bias in the studies evaluating OS in patients with and without obesity. No publication bias was detected by funnel plot inspection (Begg test). Egger test was instead significant (eFigure 4 in the Supplement). According to the trim-and-fill method, 18 studies were placed to the left of the mean, and according the random-effect model, the final result for OS was similar (HR, 1.08; 95% CI, 1.03-1.13). After the leave-one-out procedure, HRs for OS ranged from 1.14 to 1.15.
Discussion
This meta-analysis found that overall mortality was increased in patients with obesity and breast, colorectal, or uterine cancers. Cancer mortality was increased in breast, colorectal, prostate, and pancreatic cancers. Finally, the relapse rate was increased in breast, colorectal, prostate and gastroesophageal cancers. The obesity paradox, which describes improved cancer and all-cause mortality rates among patients with obesity, was observed in lung cancer and in melanoma; however, these data derive from only 12 studies. We used a categorical BMI definition of obesity (ie, BMI ≥30), because a more standardized definition would permit the comparison and synthesis of studies better than other categories (eg, continuous measures or unit of BMI increase).
The magnitude of effect size was similar for both OS and CSS in breast, colorectal, and lung cancer. This means that obesity may affect both the natural history of cancer and noncancer-related deaths.
Various factors are potentially associated with increased cancer mortality in some malignant neoplasms. Hormonal factors, reduced physical activity, more lethal or aggressive disease behavior, metabolic syndromes, and potential undertreatment in patients with obesity are possible reasons. It is well known that postmenopausal women with higher BMI have an increased risk of breast cancer because of higher estrogen levels resulting from the peripheral conversion of estrogen precursors (from adipose tissue) to estrogen.226 In these patients, weight loss and exercise may reduce cancer risk by lowering exposure to breast cancer biomarkers.227 In colorectal cancer, prediagnosis BMI was associated with increased all-cause, cardiovascular, and colorectal cancer–specific mortality.228 The reason for this association is not presently understood, although insulin, insulin-like growth factors, their binding proteins, chronic inflammation, oxidative stress, and impaired immune surveillance have been supposed to be causative factors.229 In pancreatic cancer, higher prediagnostic BMI was associated with more advanced stage at diagnosis, with 72.5% of patients with obesity presenting with metastatic disease vs 59.4% of patients with reference-range BMI (P = .02) in 2 large prospective cohort studies.161 Lastly, in prostate cancer, obesity may be a consequence of androgen deprivation therapy but seems also associated with more aggressive disease (ie, Gleason score ≥7)230 or more advanced disease at diagnosis.231
Our results showed that patients with obesity and lung cancer had significantly prolonged CSS and OS compared with patients without obesity. When considering these findings, we must take into account that 9 of 11 evaluated studies included patients with advanced and/or metastatic disease. Cancer cachexia mechanisms are not completely defined, but research has shown that the systemic inflammatory status induced either by the tumor or host response is a key moment in the development of cachexia.232 Lung cancers are indeed known to be aggressive, and patients with advanced disease usually have poorer performance statuses and experience significant weight loss at the time of diagnosis, which underlies a systemic inflammatory response.233 In our studies, obesity was positively associated with OS, independent of smoking status, in patients with lung cancer. Interestingly, a post hoc pooled analysis of randomized prospective trials comparing a PD-L1 checkpoint inhibitor (atezolizumab) with docetaxel in patients with advanced non–small cell lung cancer (NSCLC), revealed that the OS benefit for patients with obesity vs those with reference-range BMI was restricted to patients who received immunotherapy; no association was found in the group receiving docetaxel.147 Another study also explored the role of baseline BMI and BMI variation during treatment in a cohort of patients with advanced NSCLC and PD-L1 expression of at least 50% who received first-line pembrolizumab (a PD-1 checkpoint inhibitor) and in a control cohort of patients with NSCLC receiving first-line standard chemotherapy, confirming that the survival benefit for patients with obesity was restricted to those receiving immunotherapy.234
Similar findings have been described in patients with melanoma receiving immunotherapy, and a survival benefit for patients with obesity was reported in the single study205 included in our meta-analysis. However, despite some evidence showing that patients with obesity and melanoma who were receiving immune-checkpoint inhibitors achieved better outcomes,235,236 the association is currently questioned, given that opposite results have been reported in a multicenter study.237
Interestingly, patients with obesity and renal cell carcinoma also had a significantly longer OS compared with the patients without obesity. It has been hypothesized that the perinephric white adipose tissue acts as a reservoir of activated immune cells, with increased characteristics of hypoxia, infiltration of T helper type 1 cells, regulatory T cells, dendritic cells, and type 1 macrophages. However, only 1 of 6 studies included patients who were receiving immunotherapy.238,239
Intriguingly, we found that the association between obesity and better clinical outcomes was confirmed for those malignant neoplasms in which immune checkpoint inhibitors have first (and strongly) proved to be effective; however, studies involving patients receiving immune checkpoint inhibitors are poorly represented in this meta-analysis. Such results might be an epiphenomenon; however, we speculate that white adipose tissue could be considered an immune organ, which somehow plays a role in the antitumor immune response. It has been observed that the adipocyte-derived hormone leptin could alter T cell function, resulting in improved response to anti–PD-1 therapy.12 Moreover, another preclinical study reported that white adipose tissue acts as a reservoir for a peculiar population of memory T cells, which elicit some effective responses in the case of antigenic re-exposure during infections (and why not in case of exposure to cancer-specific antigens?).240 Finally, considering that immune checkpoint inhibitors exert their action within the tumor microenvironment, modulating the interactions between the tumor and the host, it has been proposed that systemic metabolic conditions, including high blood cholesterol, obesity, hyperglycemia and diabetes, atherosclerosis, and hypertension, may represent the epiphenomena of an inflamed patient. Such a patient might be characterized by an enrichment of cytokines and pro-inflammatory mediators (both in the innate and adaptive compartments) and by a condition of T cell exhaustion, with defective cellular-mediated mechanisms. Nevertheless, in these patients, immune checkpoint blockade might be more effective in reversing this immunological anergy both at the tumor and at the systemic levels.241
Patients with obesity are also at increased risk of reduced physical activity. Various studies highlighted this concept. Physical activity decreases over time in patients with obesity.242,243 In particular, physical activity is strictly associated with breast cancer and colorectal cancer mortality.244,245 Therefore physical activity (or inactivity) should be a major target of obesity prevention and treatment in particular for patients with cancer. Type 2 diabetes is strongly associated with obesity in the metabolic syndrome. More than 80% of cases of type 2 diabetes can be attributed to obesity, which may also account for many diabetes-related deaths. The association between BMI and cause-specific mortality was also illustrated in the Prospective Studies Collaboration analysis.246 In the upper BMI range (ie, 25 to 50), each 5-unit increase in BMI was associated with a significant increase in mortality from coronary heart disease, stroke, diabetes, chronic kidney disease, and many cancers. In the same analysis, individuals with BMI less than 22.5 had higher mortality compared with individuals with a BMI of 22.5 to 25. The excess mortality was predominantly associated with smoking-related diseases (ie, respiratory disease and cancer). However, there are no clear recommendations about dosing of chemotherapy in patients with obesity, so caution is recommended for high-risk regimens.247 The hypothesis that a reduced dose according to ideal body weight may lead to a worse outcome cannot be confirmed by prospective studies but may be considered a potential reason for the observed results in some settings (eg, breast cancer). In a pooled analysis of toxic effects in patients with and without obesity, rates of toxic effects were similar or lower in patients with obesity.248
Limitations
This study has several limitations. First, we combined data for patients with obesity and compared their prognosis with patients with different weights (ie, normal weight or normal weight and overweight). Second, accurate measures of potentially self-reported weight and height are always a challenge in observational studies. The evaluation often takes place before diagnosis, but in some studies the timing of the obesity diagnosis was not described. Patients with obesity have a generally poor prognosis in terms of overall mortality and noncancer mortality, so it seems obvious that their prognosis would be worse than patients without obesity. However, almost all studies provided a multivariate analysis according to main prognostic factor for oncological outcome so that obesity remains generally an independent prognostic factor in patients with cancer. The outcome was almost never adjusted for private medical insurance, but obesity can increase costs for cancer treatment and complications. Therefore, patients with a lower socioeconomic status may have had reduced access to medical facilities (ie, access to anticancer treatments), rehabilitation, or follow-up intensity and therefore had inferior outcomes. Duration of follow-up, treatments received, and countries were heterogeneous even if subgroup analyses did not explain results with these different variables. Furthermore, this meta-analysis compared mortality between patients belonging to a fixed category of obesity (ie, BMI >30), and thus, we are not able to provide an effect size per unit increment.
Conclusions
In this study, the results supported the notion that obesity is a competing risk factor for overall and cancer specific mortality as well as recurrence in various cancers treated with curative intent or for metastatic disease, except for lung cancer and melanoma, in which obesity was associated with reduced mortality (obesity paradox). These results suggest that oncologists should increase their efforts to manage patients in multidisciplinary teams for care and cure of both cancer and obesity. Improving lifestyle factors (eg, physical activity, caloric intake, care and prevention of cardiovascular complications), more intensive follow-ups of cancer in patients with obesity, and adequate dose of medical therapies are all proven measures that may improve prognosis for patients with cancer and obesity.
eFigure 1. Forest Plot for Overall Survival
eFigure 2. Forest Plot for Cancer-Specific Survival
eFigure 3. Forest Plot for Disease-Free Survival
eFigure 4. Funnel Plot for Overall Survival Analysis
References
- 1.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. World Health Organization; 2000. Accessed February 24, 2021. https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed] [Google Scholar]
- 2.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 3.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie CJ, Sharma A, Alpert MA, et al. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58(4):393-400. doi: 10.1016/j.pcad.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 7.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in obesity prevalence in adults with a history of cancer: results from the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133-3140. doi: 10.1200/JCO.2016.66.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727-748. doi: 10.1152/physrev.00030.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886-895. doi: 10.1038/nrc3174 [DOI] [PubMed] [Google Scholar]
- 10.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. doi: 10.1007/s11912-016-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shachar SS, Williams GR. The obesity paradox in cancer-moving beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26(6):981. doi: 10.1158/1055-9965.EPI-17-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141-151. doi: 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the “obesity paradox” really exist? Eur J Cardio-thoracic Surg. 2017;51(5):817-828. doi: 10.1093/ejcts/ezw386 [DOI] [PubMed] [Google Scholar]
- 14.Bagheri M, Speakman JR, Shemirani F, Djafarian K. Renal cell carcinoma survival and body mass index: a dose-response meta-analysis reveals another potential paradox within a paradox. Int J Obes (Lond). 2016;40(12):1817-1822. doi: 10.1038/ijo.2016.171 [DOI] [PubMed] [Google Scholar]
- 15.Shen N, Fu P, Cui B, Bu C-Y, Bi J-W. Associations between body mass index and the risk of mortality from lung cancer: a dose-response PRISMA-compliant meta-analysis of prospective cohort studies. Medicine (Baltimore). 2017;96(34):e7721. doi: 10.1097/MD.0000000000007721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901-1914. doi: 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Liu J, Wang X, Li M, Gan Y, Tang Y. Association of obesity and overweight with overall survival in colorectal cancer patients: a meta-analysis of 29 studies. Cancer Causes Control. 2014;25(11):1489-1502. doi: 10.1007/s10552-014-0450-y [DOI] [PubMed] [Google Scholar]
- 18.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5(7):901-910. doi: 10.1158/1940-6207.CAPR-12-0048 [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed August 13, 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 22.Shi L, Lin L, Omboni S. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). 2019;98(23):e1598. doi: 10.1097/MD.0000000000015987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. [PubMed] [Google Scholar]
- 24.Chromecki TF, Cha EK, Fajkovic H, et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. 2013;111(2):249-255. doi: 10.1111/j.1464-410X.2012.11322.x [DOI] [PubMed] [Google Scholar]
- 25.Ferro M, Vartolomei MD, Russo GI, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2019;37(3):507-514. doi: 10.1007/s00345-018-2397-1 [DOI] [PubMed] [Google Scholar]
- 26.Siegel EM, Nabors LB, Thompson RC, et al. Prediagnostic body weight and survival in high grade glioma. J Neurooncol. 2013;114(1):79-84. doi: 10.1007/s11060-013-1150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1871-1877. doi: 10.1158/1055-9965.EPI-06-0356 [DOI] [PubMed] [Google Scholar]
- 28.Abubakar M, Sung H, Bcr D, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20(1):114. doi: 10.1186/s13058-018-1033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alarfi H, Salamoon M, Kadri M, et al. The impact of baseline body mass index on clinical outcomes in metastatic breast cancer: a prospective study. BMC Res Notes. 2017;10(1):550. doi: 10.1186/s13104-017-2876-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaker MDK, Opdahl S, Åsvold BO, Romundstad PR, Vatten LJ. The association of reproductive factors and breastfeeding with long term survival from breast cancer. Breast Cancer Res Treat. 2011;130(1):175-182. doi: 10.1007/s10549-011-1566-3 [DOI] [PubMed] [Google Scholar]
- 31.Arce-Salinas C, Aguilar-Ponce JL, Villarreal-Garza C, et al. Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. Breast Cancer Res Treat. 2014;146(1):183-188. doi: 10.1007/s10549-014-2977-8 [DOI] [PubMed] [Google Scholar]
- 32.Beasley JM, Kwan ML, Chen WY, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131(2):637-643. doi: 10.1007/s10549-011-1770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair CK, Wiggins CL, Nibbe AM, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5(1):33. doi: 10.1038/s41523-019-0128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braithwaite D, Satariano WA, Sternfeld B, et al. Long-term prognostic role of functional limitations among women with breast cancer. J Natl Cancer Inst. 2010;102(19):1468-1477. doi: 10.1093/jnci/djq344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buono G, Crispo A, Giuliano M, et al. Combined effect of obesity and diabetes on early breast cancer outcome: a prospective observational study. Oncotarget. 2017;8(70):115709-115717. doi: 10.18632/oncotarget.22977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caan BJ, Kwan ML. Pre-diagnosis body mass index, post-diagnosis weight change and prognosis among women with early stage breast cancer. Cancers Causes Control. 2008;19(10):1319-1328. doi: 10.1007/s10552-008-9203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cecchini RS, Swain SM, Costantino JP, et al. Body mass index at diagnosis and breast cancer survival prognosis in clinical trial populations from NRG oncology/NSABP B-30, B-31, B-34, and B-38. Cancer Epidemiol Biomarkers Prev. 2016;25(1):51-59. doi: 10.1158/1055-9965.EPI-15-0334-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S, Alderfer JR, Asmar L, Buzdar AU. Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64(2):157-163. doi: 10.1023/A:1006489100283 [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122(3):823-833. doi: 10.1007/s10549-009-0708-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung IY, Lee JW, Lee JS, et al. Interaction between body mass index and hormone-receptor status as a prognostic factor in lymph-node-positive breast cancer. PLoS One. 2017;12(3):e0170311. doi: 10.1371/journal.pone.0170311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, Gammon MD. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23(7):1193-1203. doi: 10.1007/s10552-012-9989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor AE, Visvanathan K, Baumgartner KB, et al. Ethnic differences in the relationships between diabetes, early age adiposity and mortality among breast cancer survivors: the Breast Cancer Health Disparities Study. Breast Cancer Res Treat. 2016;157(1):167-178. doi: 10.1007/s10549-016-3810-3 [DOI] [PubMed] [Google Scholar]
- 43.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129(2):565-574. doi: 10.1007/s10549-011-1468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copson ER, Cutress RI, Maishman T, et al. ; POSH Study Steering Group . Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26(1):101-112. doi: 10.1093/annonc/mdu509 [DOI] [PubMed] [Google Scholar]
- 45.Crozier JA, Moreno-Aspitia A, Ballman KV, Dueck AC, Pockaj BA, Perez EA. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer. 2013;119(13):2447-2454. doi: 10.1002/cncr.28051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dal Maso L, Zucchetto A, Talamini R, et al. ; Prospective Analysis of Case-control studies on Environmental factors and health (PACE) study group . Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123(9):2188-2194. doi: 10.1002/ijc.23747 [DOI] [PubMed] [Google Scholar]
- 47.Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95(19):1467-1476. doi: 10.1093/jnci/djg060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245-254. doi: 10.1007/s10549-005-9118-3 [DOI] [PubMed] [Google Scholar]
- 49.Elwood JM, Tin Tin S, Kuper-Hommel M, Lawrenson R, Campbell I. Obesity and breast cancer outcomes in chemotherapy patients in New Zealand—a population-based cohort study. BMC Cancer. 2018;18(1):76. doi: 10.1186/s12885-017-3971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emaus A, Veierød MB, Tretli S, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat. 2010;121(3):651-660. doi: 10.1007/s10549-009-0603-y [DOI] [PubMed] [Google Scholar]
- 51.Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS Study. JAMA Oncol. 2017;3(12):e172319. doi: 10.1001/jamaoncol.2017.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30(2):164-171. doi: 10.1200/JCO.2011.36.2723 [DOI] [PubMed] [Google Scholar]
- 53.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23(7):1771-1780. doi: 10.1093/annonc/mdr534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellmann SS, Thygesen LC, Tolstrup JS, Grønbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19(5):366-373. doi: 10.1097/CEJ.0b013e32833b4828 [DOI] [PubMed] [Google Scholar]
- 55.His M, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F, Dossus L. Prediagnostic body size and breast cancer survival in the E3N cohort study. Int J Cancer. 2016;139(5):1053-1064. doi: 10.1002/ijc.30158 [DOI] [PubMed] [Google Scholar]
- 56.Jeon YW, Kang SH, Park MH, Lim W, Cho SH, Suh YJ. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer. 2015;15(1):865. doi: 10.1186/s12885-015-1879-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506-2514. doi: 10.1093/annonc/mdt224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawai M, Tomotaki A, Miyata H, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 2016;5(6):1328-1340. doi: 10.1002/cam4.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keegan THM, Milne RL, Andrulis IL, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat. 2010;123(2):531-542. doi: 10.1007/s10549-010-0774-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132(2):729-739. doi: 10.1007/s10549-011-1914-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwan ML, John EM, Caan BJ, et al. Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol. 2014;179(1):95-111. doi: 10.1093/aje/kwt233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladoire S, Dalban C, Roché H, et al. Effect of obesity on disease-free and overall survival in node-positive breast cancer patients in a large French population: a pooled analysis of two randomised trials. Eur J Cancer. 2014;50(3):506-516. doi: 10.1016/j.ejca.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 63.Larsen SB, Kroman N, Ibfelt EH, Christensen J, Tjønneland A, Dalton SO. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol. 2015;54(5):780-788. doi: 10.3109/0284186X.2014.998774 [DOI] [PubMed] [Google Scholar]
- 64.Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1686-1691. doi: 10.1158/1055-9965.EPI-05-0042 [DOI] [PubMed] [Google Scholar]
- 65.Maskarinec G, Pagano I, Lurie G, Bantum E, Gotay CC, Issell BF. Factors affecting survival among women with breast cancer in Hawaii. J Womens Health (Larchmt). 2011;20(2):231-237. doi: 10.1089/jwh.2010.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCullough ML, Feigelson HS, Diver WR, Patel AV, Thun MJ, Calle EE. Risk factors for fatal breast cancer in African-American women and White women in a large US prospective cohort. Am J Epidemiol. 2005;162(8):734-742. doi: 10.1093/aje/kwi278 [DOI] [PubMed] [Google Scholar]
- 67.McCullough LE, Chen J, Cho YH, et al. DNA methylation modifies the association between obesity and survival after breast cancer diagnosis. Breast Cancer Res Treat. 2016;156(1):183-194. doi: 10.1007/s10549-016-3724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2010;18(5):1403-1409. doi: 10.1158/1055-9965.EPI-08-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nur U, El Reda D, Hashim D, Weiderpass E. A prospective investigation of oral contraceptive use and breast cancer mortality: findings from the Swedish women’s lifestyle and health cohort. BMC Cancer. 2019;19(1):807. doi: 10.1186/s12885-019-5985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res. 2011;13(2):R34. doi: 10.1186/bcr2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oudanonh T, Nabi H, Ennour-Idrissi K, et al. Progesterone receptor status modifies the association between body mass index and prognosis in women diagnosed with estrogen receptor positive breast cancer. Int J Cancer. 2020;146(10):2736-2745. doi: 10.1002/ijc.32621 [DOI] [PubMed] [Google Scholar]
- 72.Pajares B, Pollán M, Martín M, et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res. 2013;15(6):R105. doi: 10.1186/bcr3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeiler G, Stöger H, Dubsky P, et al. ; ABCSG . Efficacy of tamoxifen ± aminoglutethimide in normal weight and overweight postmenopausal patients with hormone receptor-positive breast cancer: an analysis of 1509 patients of the ABCSG-06 trial. Br J Cancer. 2013;108(7):1408-1414. doi: 10.1038/bjc.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345-2351. doi: 10.1200/JCO.2006.08.6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Probst-Hensch NM, Steiner JHB, Schraml P, et al. IGFBP2 and IGFBP3 protein expressions in human breast cancer: association with hormonal factors and obesity. Clin Cancer Res. 2010;16(3):1025-1032. doi: 10.1158/1078-0432.CCR-09-0957 [DOI] [PubMed] [Google Scholar]
- 76.Senie RT, Rosen PP, Rhodes P, Lesser ML, Kinne DW. Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med. 1992;116(1):26-32. doi: 10.7326/0003-4819-116-1-26 [DOI] [PubMed] [Google Scholar]
- 77.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104(5):406-414. doi: 10.1093/jnci/djr543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937-5946. doi: 10.1002/cncr.27527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su HI, Sue LY, Flatt SW, Natarajan L, Patterson RE, Pierce JP. Endogenous estradiol is not associated with poor physical health in postmenopausal breast cancer survivors. J Womens Health (Larchmt). 2013;22(12):1043-1048. doi: 10.1089/jwh.2013.4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26(12):1803-1811. doi: 10.1007/s10552-015-0673-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: an analysis by menstruation status when breast cancer diagnosis. Medicine (Baltimore). 2018;97(26):e11220. doi: 10.1097/MD.0000000000011220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat. 2014;146(1):189-197. doi: 10.1007/s10549-014-3002-y [DOI] [PubMed] [Google Scholar]
- 83.Warren LEG, Ligibel JA, Chen YH, Truong L, Catalano PJ, Bellon JR. Body mass index and locoregional recurrence in women with early-stage breast cancer. Ann Surg Oncol. 2016;23(12):3870-3879. doi: 10.1245/s10434-016-5437-3 [DOI] [PubMed] [Google Scholar]
- 84.Widschwendter P, Friedl TWP, Schwentner L, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17(1):129. doi: 10.1186/s13058-015-0639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. 2014;35(3):2035-2045. doi: 10.1007/s13277-013-1270-5 [DOI] [PubMed] [Google Scholar]
- 86.Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49(17):3588-3597. doi: 10.1016/j.ejca.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg L, Czene K, Hall P. Obesity and poor breast cancer prognosis: an illusion because of hormone replacement therapy? Br J Cancer. 2009;100(9):1486-1491. doi: 10.1038/sj.bjc.6605025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117(18):4132-4140. doi: 10.1002/cncr.26019 [DOI] [PubMed] [Google Scholar]
- 89.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer. 2012;12(5):364-372. doi: 10.1016/j.clbc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 90.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29(19):2645-2652. doi: 10.1200/JCO.2010.33.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frumovitz M, Jhingran A, Soliman PT, Klopp AH, Schmeler KM, Eifel PJ. Morbid obesity as an independent risk factor for disease-specific mortality in women with cervical cancer. Obstet Gynecol. 2014;124(6):1098-1104. doi: 10.1097/AOG.0000000000000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fedirko V, Romieu I, Aleksandrova K, et al. Pre-diagnostic anthropometry and survival after colorectal cancer diagnosis in Western European populations. Int J Cancer. 2014;135(8):1949-1960. doi: 10.1002/ijc.28841 [DOI] [PubMed] [Google Scholar]
- 93.Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109(3):814-822. doi: 10.1038/bjc.2013.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell PT, Newton CC, Newcomb PA, et al. Association between body mass index and mortality for colorectal cancer survivors: overall and by tumor molecular phenotype. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1229-1238. doi: 10.1158/1055-9965.EPI-15-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cespedes Feliciano EM, Kwan ML, Kushi LH, et al. Body mass index, PAM50 subtype, recurrence, and survival among patients with nonmetastatic breast cancer. Cancer. 2017;123(13):2535-2542. doi: 10.1002/cncr.30637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216(6):1070-1081. doi: 10.1016/j.jamcollsurg.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dahdaleh FS, Sherman SK, Poli EC, et al. Obstruction predicts worse long-term outcomes in stage III colon cancer: a secondary analysis of the N0147 trial. Surgery. 2018;164(6):1223-1229. doi: 10.1016/j.surg.2018.06.044 [DOI] [PubMed] [Google Scholar]
- 98.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98(22):1647-1654. doi: 10.1093/jnci/djj442 [DOI] [PubMed] [Google Scholar]
- 99.Jayasekara H, English DR, Haydon A, et al. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer. 2018;142(2):238-250. doi: 10.1002/ijc.31049 [DOI] [PubMed] [Google Scholar]
- 100.Kaidar-Person O, Badarna H, Bar-Sela G. Bevacizumab for metastatic colon cancer: does patient BMI influence survival? Anticancer Drugs. 2015;26(3):363-366. doi: 10.1097/CAD.0000000000000201 [DOI] [PubMed] [Google Scholar]
- 101.Kalb M, Langheinrich MC, Merkel S, et al. Influence of body mass index on long-term outcome in patients with rectal cancer-a single centre experience. Cancers (Basel). 2019;11(5):E609. doi: 10.3390/cancers11050609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98(3):484-495. doi: 10.1002/cncr.11544 [DOI] [PubMed] [Google Scholar]
- 103.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22(4):648-657. doi: 10.1200/JCO.2004.07.121 [DOI] [PubMed] [Google Scholar]
- 104.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. ; Cancer and Leukemia Group B 89803 . Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26(25):4109-4115. doi: 10.1200/JCO.2007.15.6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morikawa T, Kuchiba A, Liao X, et al. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131(5):1169-1178. doi: 10.1002/ijc.26495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogino S, Nosho K, Baba Y, et al. A cohort study of STMN1 expression in colorectal cancer: body mass index and prognosis. Am J Gastroenterol. 2009;104(8):2047-2056. doi: 10.1038/ajg.2009.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel GS, Ullah S, Beeke C, et al. Association of BMI with overall survival in patients with mCRC who received chemotherapy versus EGFR and VEGF-targeted therapies. Cancer Med. 2015;4(10):1461-1471. doi: 10.1002/cam4.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pelser C, Arem H, Pfeiffer RM, et al. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014;120(10):1540-1547. doi: 10.1002/cncr.28573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2229-2237. doi: 10.1158/1055-9965.EPI-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlesinger S, Siegert S, Koch M, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014;25(10):1407-1418. doi: 10.1007/s10552-014-0435-x [DOI] [PubMed] [Google Scholar]
- 111.Shah MS, Fogelman DR, Raghav KPS, et al. Joint prognostic effect of obesity and chronic systemic inflammation in patients with metastatic colorectal cancer. Cancer. 2015;121(17):2968-2975. doi: 10.1002/cncr.29440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinicrope FA, Foster NR, Yoon HH, et al. Association of obesity with DNA mismatch repair status and clinical outcome in patients with stage II or III colon carcinoma participating in NCCTG and NSABP adjuvant chemotherapy trials. J Clin Oncol. 2012;30(4):406-412. doi: 10.1200/JCO.2011.39.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sinicrope FA, Foster NR, Yothers G, et al. ; Adjuvant Colon Cancer Endpoints (ACCENT) Group . Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528-1536. doi: 10.1002/cncr.27938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorbye H, Mauer M, Gruenberger T, et al. ; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK (CRUK); Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) . Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg. 2012;255(3):534-539. doi: 10.1097/SLA.0b013e3182456aa2 [DOI] [PubMed] [Google Scholar]
- 115.Wang N, Khankari NK, Cai H, et al. Prediagnosis body mass index and waist-hip circumference ratio in association with colorectal cancer survival. Int J Cancer. 2017;140(2):292-301. doi: 10.1002/ijc.30459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng J, Li Y, Zhu S, et al. NDRG4 stratifies the prognostic value of body mass index in colorectal cancer. Oncotarget. 2016;7(2):1311-1322. doi: 10.18632/oncotarget.6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States). Cancer Causes Control. 2006;17(1):63-70. doi: 10.1007/s10552-005-0360-0 [DOI] [PubMed] [Google Scholar]
- 118.Kristensen AB, Hare-Bruun H, Høgdall CK, Rudnicki M. Influence of body mass index on tumor pathology and survival in uterine cancer: a Danish register study. Int J Gynecol Cancer. 2017;27(2):281-288. doi: 10.1097/IGC.0000000000000874 [DOI] [PubMed] [Google Scholar]
- 119.Nagle CM, Crosbie EJ, Brand A, et al. ; Australian National Endometrial Cancer Study Group . The association between diabetes, comorbidities, body mass index and all-cause and cause-specific mortality among women with endometrial cancer. Gynecol Oncol. 2018;150(1):99-105. doi: 10.1016/j.ygyno.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 120.Nicholas Z, Hu N, Ying J, Soisson P, Dodson M, Gaffney DK. Impact of comorbid conditions on survival in endometrial cancer. Am J Clin Oncol. 2014;37(2):131-134. doi: 10.1097/COC.0b013e318277d5f4 [DOI] [PubMed] [Google Scholar]
- 121.Todo Y, Okamoto K, Minobe S, Kato H. Clinical significance of surgical staging for obese women with endometrial cancer: a retrospective analysis in a Japanese cohort. Jpn J Clin Oncol. 2014;44(10):903-909. doi: 10.1093/jjco/hyu106 [DOI] [PubMed] [Google Scholar]
- 122.Yoon LS, Goodman MT, Rimel BJ, Jeon CY. Statin use and survival in elderly patients with endometrial cancer. Gynecol Oncol. 2015;137(2):252-257. doi: 10.1016/j.ygyno.2015.01.549 [DOI] [PubMed] [Google Scholar]
- 123.Hynes O, Anandavadivelan P, Gossage J, Johar AM, Lagergren J, Lagergren P. The impact of pre- and post-operative weight loss and body mass index on prognosis in patients with oesophageal cancer. Eur J Surg Oncol. 2017;43(8):1559-1565. doi: 10.1016/j.ejso.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 124.Spreafico A, Coate L, Zhai R, et al. Early adulthood body mass index, cumulative smoking, and esophageal adenocarcinoma survival. Cancer Epidemiol. 2017;47:28-34. doi: 10.1016/j.canep.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 125.Sundelöf M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44(11):1566-1571. doi: 10.1016/j.ejca.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 126.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29(34):4561-4567. doi: 10.1200/JCO.2011.37.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thrift AP, Nagle CM, Fahey PP, Smithers BM, Watson DI, Whiteman DC. Predictors of survival among patients diagnosed with adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control. 2012;23(4):555-564. doi: 10.1007/s10552-012-9913-1 [DOI] [PubMed] [Google Scholar]
- 128.Trivers KF, De Roos AJ, Gammon MD, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol. 2005;3(3):225-230. doi: 10.1016/S1542-3565(04)00613-5 [DOI] [PubMed] [Google Scholar]
- 129.Potharaju M, Mangaleswaran B, Mathavan A, et al. Body mass index as a prognostic marker in glioblastoma multiforme: a clinical outcome. Int J Radiat Oncol Biol Phys. 2018;102(1):204-209. doi: 10.1016/j.ijrobp.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 130.Gama RR, Song Y, Zhang Q, et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck. 2017;39(6):1226-1233. doi: 10.1002/hed.24760 [DOI] [PubMed] [Google Scholar]
- 131.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782-789. doi: 10.1001/jamaoncol.2015.6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu Q, Peng J, Chen X, et al. Obesity and genes related to lipid metabolism predict poor survival in oral squamous cell carcinoma. Oral Oncol. 2019;89:14-22. doi: 10.1016/j.oraloncology.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 133.Ata N, Ayloo S, Tsung A, Molinari M. Recipient obesity does not affect survival after deceased donor liver transplantation for hepatocellular carcinoma: a national retrospective cohort study in the United States. HPB (Oxford). 2019;21(1):67-76. doi: 10.1016/j.hpb.2018.06.1797 [DOI] [PubMed] [Google Scholar]
- 134.Carr BI, Giannelli G, Guerra V, et al. Plasma cholesterol and lipoprotein levels in relation to tumor aggressiveness and survival in HCC patients. Int J Biol Markers. 2018;33(4):423-431. doi: 10.1177/1724600818776838 [DOI] [PubMed] [Google Scholar]
- 135.Yang T, Liu K, Liu CF, et al. Impact of postoperative infective complications on long-term survival after liver resection for hepatocellular carcinoma. Br J Surg. 2019;106(9):1228-1236. doi: 10.1002/bjs.11231 [DOI] [PubMed] [Google Scholar]
- 136.Roque DR, Taylor KN, Palisoul M, et al. Gemcitabine and docetaxel compared with observation, radiation, or other chemotherapy regimens as adjuvant treatment for stage I-to-IV uterine leiomyosarcoma. Int J Gynecol Cancer. 2016;26(3):505-511. doi: 10.1097/IGC.0000000000000634 [DOI] [PubMed] [Google Scholar]
- 137.McMahon BJ, Bruden D, Townshend-Bulson L, et al. Infection With hepatitis C virus genotype 3 is an independent risk factor for end-stage liver disease, hepatocellular carcinoma, and liver-related death. Clin Gastroenterol Hepatol. 2017;15(3):431-437.e2. doi: 10.1016/j.cgh.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abdel-Rahman O. Pre-diagnostic body mass index trajectory in relationship to lung cancer incidence and mortality; findings from the PLCO trial. Expert Rev Respir Med. 2019;13(10):1029-1035. doi: 10.1080/17476348.2019.1656532 [DOI] [PubMed] [Google Scholar]
- 139.Leung CC, Lam TH, Yew WW, Chan WM, Law WS, Tam CM. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40(1):174-182. doi: 10.1093/ije/dyq134 [DOI] [PubMed] [Google Scholar]
- 140.Nonemaker JM, Garrett-Mayer E, Carpenter MJ, et al. The risk of dying from lung cancer by race: a prospective cohort study in a biracial cohort in Charleston, South Carolina. Ann Epidemiol. 2009;19(5):304-310. doi: 10.1016/j.annepidem.2008.12.017 [DOI] [PubMed] [Google Scholar]
- 141.Qi Y, Schild SE, Mandrekar SJ, et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol. 2009;4(9):1075-1082. doi: 10.1097/JTO.0b013e3181ae27f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shepshelovich D, Xu W, Lu L, et al. Body mass index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the International Lung Cancer Consortium. J Thorac Oncol. 2019;14(9):1594-1607. doi: 10.1016/j.jtho.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Turner MC, Krewski D, Pope CA III, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184(12):1374-1381. doi: 10.1164/rccm.201106-1011OC [DOI] [PubMed] [Google Scholar]
- 144.Xie HJ, Zhang X, Wei ZQ, Long H, Rong TH, Su XD. Effect of body mass index on survival of patients with stage I non-small cell lung cancer. Chin J Cancer. 2017;36(1):7. doi: 10.1186/s40880-016-0170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McQuade JL, Daniel CR, Hess PKR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310-322. doi: 10.1016/S1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aldrich MC, Grogan EL, Munro HM, Signorello LB, Blot WJ. Stage-adjusted lung cancer survival does not differ between low-income Blacks and Whites. J Thorac Oncol. 2013;8(10):1248-1254. doi: 10.1097/JTO.0b013e3182a406f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6(4):512-518. doi: 10.1001/jamaoncol.2019.5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakagawa T, Toyazaki T, Chiba N, Ueda Y, Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. 2016;23(4):560-566. doi: 10.1093/icvts/ivw175 [DOI] [PubMed] [Google Scholar]
- 149.Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH. Impact of chemotherapy dosing on ovarian cancer survival according to body mass index. JAMA Oncol. 2015;1(6):737-745. doi: 10.1001/jamaoncol.2015.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kotsopoulos J, Moody JRK, Fan I, et al. Height, weight, BMI and ovarian cancer survival. Gynecol Oncol. 2012;127(1):83-87. doi: 10.1016/j.ygyno.2012.05.038 [DOI] [PubMed] [Google Scholar]
- 151.Minlikeeva AN, Cannioto R, Jensen A, et al. ; Australian Ovarian Cancer Study Group; Ovarian Cancer Association Consortium . Joint exposure to smoking, excessive weight, and physical inactivity and survival of ovarian cancer patients, evidence from the Ovarian Cancer Association Consortium. Cancer Causes Control. 2019;30(5):537-547. doi: 10.1007/s10552-019-01157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Previs RA, Kilgore J, Craven R, et al. Obesity is associated with worse overall survival in women with low-grade papillary serous epithelial ovarian cancer. Int J Gynecol Cancer. 2014;24(4):670-675. doi: 10.1097/IGC.0000000000000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tyler CP, Whiteman MK, Zapata LB, et al. The effect of body mass index and weight change on epithelial ovarian cancer survival in younger women: a long-term follow-up study. J Womens Health (Larchmt). 2012;21(8):865-871. doi: 10.1089/jwh.2012.3487 [DOI] [PubMed] [Google Scholar]
- 154.Yang L, Klint A, Lambe M, et al. Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer. 2008;123(3):672-679. doi: 10.1002/ijc.23429 [DOI] [PubMed] [Google Scholar]
- 155.Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage. 2012;44(2):181-191. doi: 10.1016/j.jpainsymman.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. 2015;26(11):2257-2266. doi: 10.1093/annonc/mdv355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gong Z, Holly EA, Bracci PM. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes Control. 2012;23(12):1929-1937. doi: 10.1007/s10552-012-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin Y, Fu R, Grant E, et al. Association of body mass index and risk of death from pancreas cancer in Asians: findings from the Asia Cohort Consortium. Eur J Cancer Prev. 2013;22(3):244-250. doi: 10.1097/CEJ.0b013e3283592cef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127(10):2412-2419. doi: 10.1002/ijc.25240 [DOI] [PubMed] [Google Scholar]
- 161.Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol. 2013;31(33):4229-4234. doi: 10.1200/JCO.2013.51.7532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg. 2010;14(7):1143-1150. doi: 10.1007/s11605-010-1201-3 [DOI] [PubMed] [Google Scholar]
- 163.Bassett JK, Severi G, Baglietto L, et al. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2012;131(7):1711-1719. doi: 10.1002/ijc.27414 [DOI] [PubMed] [Google Scholar]
- 164.Bonn SE, Wiklund F, Sjölander A, et al. Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control. 2014;25(8):933-943. doi: 10.1007/s10552-014-0393-3 [DOI] [PubMed] [Google Scholar]
- 165.Dickerman BA, Ahearn TU, Giovannucci E, et al. Weight change, obesity and risk of prostate cancer progression among men with clinically localized prostate cancer. Int J Cancer. 2017;141(5):933-944. doi: 10.1002/ijc.30803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer: analysis of RTOG 85-31. Cancer. 2007;110(12):2691-2699. doi: 10.1002/cncr.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. Anthropometric measurements and survival after a prostate cancer diagnosis. Br J Cancer. 2018;118(4):607-610. doi: 10.1038/bjc.2017.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Froehner M, Kellner AE, Koch R, Baretton GB, Hakenberg OW, Wirth MP. A combined index to classify prognostic comorbidity in candidates for radical prostatectomy. BMC Urol. 2014;14(1):28. doi: 10.1186/1471-2490-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109(6):1192-1202. doi: 10.1002/cncr.22534 [DOI] [PubMed] [Google Scholar]
- 170.Han M, Trock BJ, Partin AW, et al. The impact of preoperative erectile dysfunction on survival after radical prostatectomy. BJU Int. 2010;106(11):1612-1617. doi: 10.1111/j.1464-410X.2010.09472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ho T, Gerber L, Aronson WJ, et al. Obesity, prostate-specific antigen nadir, and biochemical recurrence after radical prostatectomy: biology or technique? results from the SEARCH database. Eur Urol. 2012;62(5):910-916. doi: 10.1016/j.eururo.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kelly SP, Graubard BI, Andreotti G, Younes N, Cleary SD, Cook MB. Prediagnostic body mass index trajectories in relation to prostate cancer incidence and mortality in the PLCO cancer screening trial. J Natl Cancer Inst. 2016;109(3):1-9. doi: 10.1093/jnci/djw225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kenfield SA, Batista JL, Jahn JL, et al. Development and application of a lifestyle score for prevention of lethal prostate cancer. J Natl Cancer Inst. 2015;108(3):djv329. doi: 10.1093/jnci/djv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khan S, Cai J, Nielsen ME, et al. The association of diabetes and obesity with prostate cancer progression: HCaP-NC. Prostate. 2017;77(8):878-887. doi: 10.1002/pros.23342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039-1047. doi: 10.1016/S1470-2045(08)70235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maj-Hes AB, Mathieu R, Özsoy M, et al. Obesity is associated with biochemical recurrence after radical prostatectomy: a multi-institutional extended validation study. Urol Oncol. 2017;35(7):460.e1-460.e8. doi: 10.1016/j.urolonc.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 177.Møller H, Roswall N, Van Hemelrijck M, et al. Prostate cancer incidence, clinical stage and survival in relation to obesity: a prospective cohort study in Denmark. Int J Cancer. 2015;136(8):1940-1947. doi: 10.1002/ijc.29238 [DOI] [PubMed] [Google Scholar]
- 178.Rudman SM, Gray KP, Batista JL, et al. Risk of prostate cancer-specific death in men with baseline metabolic aberrations treated with androgen deprivation therapy for biochemical recurrence. BJU Int. 2016;118(6):919-926. doi: 10.1111/bju.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schiffmann J, Salomon G, Tilki D, et al. Radical prostatectomy neutralizes obesity-driven risk of prostate cancer progression. Urol Oncol. 2017;35(5):243-249. doi: 10.1016/j.urolonc.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 180.Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;178(5):1939-1944. doi: 10.1016/j.juro.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 181.Vidal AC, Howard LE, Sun SX, et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis. 2017;20(1):72-78. doi: 10.1038/pcan.2016.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu W, Liu X, Chaftari P, et al. Association of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective review. PLoS One. 2015;10(3):e0122047. doi: 10.1371/journal.pone.0122047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Montgomery RB, Goldman B, Tangen CM, et al. ; Southwest Oncology Group . Association of body mass index with response and survival in men with metastatic prostate cancer: Southwest Oncology Group trials 8894 and 9916. J Urol. 2007;178(5):1946-1951. doi: 10.1016/j.juro.2007.07.026 [DOI] [PubMed] [Google Scholar]
- 184.Halabi S, Ou SS, Vogelzang NJ, Small EJ. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110(7):1478-1484. doi: 10.1002/cncr.22932 [DOI] [PubMed] [Google Scholar]
- 185.Keizman D, Gottfried M, Ish-Shalom M, et al. Active smoking may negatively affect response rate, progression-free survival, and overall survival of patients with metastatic renal cell carcinoma treated with sunitinib. Oncologist. 2014;19(1):51-60. doi: 10.1634/theoncologist.2012-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee WK, Byun SS, Kim HH, et al. Characteristics and prognosis of chromophobe non-metastatic renal cell carcinoma: a multicenter study. Int J Urol. 2010;17(11):898-904. doi: 10.1111/j.1442-2042.2010.02630.x [DOI] [PubMed] [Google Scholar]
- 187.Parker AS, Lohse CM, Cheville JC, Thiel DD, Leibovich BC, Blute ML. Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma. Urology. 2006;68(4):741-746. doi: 10.1016/j.urology.2006.05.024 [DOI] [PubMed] [Google Scholar]
- 188.Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. 2016;195(2):270-276. doi: 10.1016/j.juro.2015.08.072 [DOI] [PubMed] [Google Scholar]
- 189.Spiess PE, Kurian T, Lin HY, et al. Preoperative metastatic status, level of thrombus and body mass index predict overall survival in patients undergoing nephrectomy and inferior vena cava thrombectomy. BJU Int. 2012;110(11 Pt B):E470-E474. doi: 10.1111/j.1464-410X.2012.11155.x [DOI] [PubMed] [Google Scholar]
- 190.Yu ML, Asal NR, Geyer JR. Later recurrence and longer survival among obese patients with renal cell carcinoma. Cancer. 1991;68(7):1648-1655. doi: [DOI] [PubMed] [Google Scholar]
- 191.Hung CY, Lai CC, Chen PT, et al. Impact of body mass index on long-term survival outcome in Asian populations with solid cancer who underwent curative-intent surgery: a six-year multicenter observational cohort study. J Cancer. 2018;9(18):3316-3325. doi: 10.7150/jca.25729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Houdek MT, Griffin AM, Ferguson PC, Wunder JS. Morbid obesity increases the risk of postoperative wound complications, infection, and repeat surgical procedures following upper extremity limb salvage surgery for soft tissue sarcoma. Hand (N Y). 2019;14(1):114-120. doi: 10.1177/1558944718797336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early‐stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120(7):983-991. doi: 10.1002/cncr.28532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu H, Tan P, Zheng X, et al. Metabolic syndrome and upper tract urothelial carcinoma: A retrospective analysis from a large Chinese cohort. Urol Oncol. 2019;37(4):291.e19-291.e28. doi: 10.1016/j.urolonc.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 195.Arem H, Park Y, Pelser C, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst. 2013;105(5):342-349. doi: 10.1093/jnci/djs530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Matsuo K, Moeini A, Cahoon SS, et al. Weight change pattern and survival outcome of women with endometrial cancer. Ann Surg Oncol. 2016;23(9):2988-2997. doi: 10.1245/s10434-016-5237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ruterbusch JJ, Ali-Fehmi R, Olson SH, et al. The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol. 2014;211(6):627.e1-627.e9. doi: 10.1016/j.ajog.2014.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seidelin UH, Ibfelt E, Andersen I, et al. Does stage of cancer, comorbidity or lifestyle factors explain educational differences in survival after endometrial cancer? a cohort study among Danish women diagnosed 2005-2009. Acta Oncol. 2016;55(6):680-685. doi: 10.3109/0284186X.2015.1136750 [DOI] [PubMed] [Google Scholar]
- 199.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985-996. doi: 10.1093/ije/dyr018 [DOI] [PubMed] [Google Scholar]
- 200.Akinyemiju T, Moore JX, Pisu M, et al. A prospective study of obesity, metabolic health, and cancer mortality. Obesity (Silver Spring). 2018;26(1):193-201. doi: 10.1002/oby.22067.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barroso M, Goday A, Ramos R, et al. ; FRESCO Investigators . Interaction between cardiovascular risk factors and body mass index and 10-year incidence of cardiovascular disease, cancer death, and overall mortality. Prev Med. 2018;107(107):81-89. doi: 10.1016/j.ypmed.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 202.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365(10):901-908. doi: 10.1056/NEJMoa1104119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Drake I, Gullberg B, Sonestedt E, et al. Type 2 diabetes, adiposity and cancer morbidity and mortality risk taking into account competing risk of noncancer deaths in a prospective cohort setting. Int J Cancer. 2017;141(6):1170-1180. doi: 10.1002/ijc.30824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Han X, Stevens J, Truesdale KP, et al. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014;135(12):2900-2909. doi: 10.1002/ijc.28930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Izumida T, Nakamura Y, Ishikawa S. Impact of body mass index and metabolically unhealthy status on mortality in the Japanese general population: the JMS cohort study. PLoS One. 2019;14(11):e0224802. doi: 10.1371/journal.pone.0224802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Janssen SJ, van der Heijden AS, van Dijke M, et al. 2015 Marshall Urist Young Investigator Award: prognostication in patients with long bone metastases: does a boosting algorithm improve survival estimates? Clin Orthop Relat Res. 2015;473(10):3112-3121. doi: 10.1007/s11999-015-4446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jenkins DA, Bowden J, Robinson HA, et al. Adiposity-mortality relationships in type 2 diabetes, coronary heart disease, and cancer subgroups in the UK Biobank, and their modification by smoking. Diabetes Care. 2018;41(9):1878-1886. doi: 10.2337/dc17-2508 [DOI] [PubMed] [Google Scholar]
- 209.Katzmarzyk PT, Reeder BA, Elliott S, et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health. 2012;103(2):147-151. doi: 10.1007/bf03404221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11(7):e1001673. doi: 10.1371/journal.pmed.1001673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Martini DJ, Kline MR, Liu Y, et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer. 2020;126(3):575-582. doi: 10.1002/cncr.32576 [DOI] [PubMed] [Google Scholar]
- 212.Mathur AK, Ghaferi AA, Sell K, Sonnenday CJ, Englesbe MJ, Welling TH. Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg. 2010;14(5):849-857. doi: 10.1007/s11605-010-1163-5 [DOI] [PubMed] [Google Scholar]
- 213.Meyer J, Rohrmann S, Bopp M, Faeh D; Swiss National Cohort Study Group . Impact of smoking and excess body weight on overall and site-specific cancer mortality risk. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1516-1522. doi: 10.1158/1055-9965.EPI-15-0415 [DOI] [PubMed] [Google Scholar]
- 214.Nechuta SJ, Shu XO, Li HL, et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med. 2010;7(9):e1000339. doi: 10.1371/journal.pmed.1000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Parr CL, Batty GD, Lam TH, et al. ; Asia-Pacific Cohort Studies Collaboration . Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741-752. doi: 10.1016/S1470-2045(10)70141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sasazuki S, Inoue M, Tsuji I, et al. ; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan . Body mass index and mortality from all causes and major causes in Japanese: results of a pooled analysis of 7 large-scale cohort studies. J Epidemiol. 2011;21(6):417-430. doi: 10.2188/jea.JE20100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Silventoinen K, Tynelius P, Rasmussen F. Weight status in young adulthood and survival after cardiovascular diseases and cancer. Int J Epidemiol. 2014;43(4):1197-1204. doi: 10.1093/ije/dyu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Song X, Pitkäniemi J, Gao W, et al. ; DECODE Study Group . Relationship between body mass index and mortality among Europeans. Eur J Clin Nutr. 2012;66(2):156-165. doi: 10.1038/ejcn.2011.145 [DOI] [PubMed] [Google Scholar]
- 219.Taghizadeh N, Boezen HM, Schouten JP, Schröder CP, Elisabeth de Vries EG, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10(4):e0125261. doi: 10.1371/journal.pone.0125261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226(1):186-192. doi: 10.1016/j.atherosclerosis.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 221.Tseng CH. Factors associated with cancer- and non-cancer-related deaths among Taiwanese patients with diabetes after 17 years of follow-up. PLoS One. 2016;11(12):e0147916. doi: 10.1371/journal.pone.0147916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg. 2013;37(11):2561-2568. doi: 10.1007/s00268-013-2162-y [DOI] [PubMed] [Google Scholar]
- 223.Xu H, Cupples LA, Stokes A, Liu CT. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Netw Open. 2018;1(7):e184587. doi: 10.1001/jamanetworkopen.2018.4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yano Y, Kario K, Ishikawa S, et al. ; JMS Cohort Study Group . Associations between diabetes, leanness, and the risk of death in the Japanese general population: the Jichi Medical School Cohort Study. Diabetes Care. 2013;36(5):1186-1192. doi: 10.2337/dc12-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.You J, Huang S, Huang GQ, et al. Nonalcoholic fatty liver disease: a negative risk factor for colorectal cancer prognosis. Medicine (Baltimore). 2015;94(5):e479. doi: 10.1097/MD.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Key TJ, Appleby PN, Reeves GK, et al. ; Endogenous Hormones Breast Cancer Collaborative Group . Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218-1226. doi: 10.1093/jnci/djg022 [DOI] [PubMed] [Google Scholar]
- 227.Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314-2326. doi: 10.1200/JCO.2011.37.9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30(1):42-52. doi: 10.1200/JCO.2011.38.0287 [DOI] [PubMed] [Google Scholar]
- 229.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71-83. doi: 10.1080/13813450801954303 [DOI] [PubMed] [Google Scholar]
- 230.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA. 2009;301(1):39-51. doi: 10.1001/jama.2008.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665-1671. doi: 10.1093/annonc/mdr603 [DOI] [PubMed] [Google Scholar]
- 232.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754-762. doi: 10.1038/nrc3829 [DOI] [PubMed] [Google Scholar]
- 233.Scott HR, McMillan DC, Forrest LM, Brown DJF, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87(3):264-267. doi: 10.1038/sj.bjc.6600466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cortellini A, Ricciuti B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403. doi: 10.1136/jitc-2020-001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Naik GS, Waikar SS, Johnson AEW, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. 2019;7(1):89. doi: 10.1186/s40425-019-0512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Richtig G, Hoeller C, Wolf M, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS One. 2018;13(10):e0204729. doi: 10.1371/journal.pone.0204729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rutkowski P, Indini A, De Luca M, et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: a multicenter international retrospective study. J Immunother Cancer. 2020;8(2):e001117. doi: 10.1136/jitc-2020-001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Santoni M, Cortellini A, Buti S. Unlocking the secret of the obesity paradox in renal tumours. Lancet Oncol. 2020;21(2):194-196. doi: 10.1016/S1470-2045(19)30783-1 [DOI] [PubMed] [Google Scholar]
- 239.Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21(2):283-293. doi: 10.1016/S1470-2045(19)30797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity. 2017;47(6):1154-1168.e6. doi: 10.1016/j.immuni.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bersanelli M, Cortellini A, Buti S. The interplay between cholesterol (and other metabolic conditions) and immune-checkpoint immunotherapy: shifting the concept from the “inflamed tumor” to the “inflamed patient.” Hum Vaccin Immunother. 2021;00(00):1-5. doi: 10.1080/21645515.2020.1852872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tucker JM, Tucker LA, Lecheminant J, Bailey B. Obesity increases risk of declining physical activity over time in women: a prospective cohort study. Obesity (Silver Spring). 2013;21(12):E715-E720. doi: 10.1002/oby.20415 [DOI] [PubMed] [Google Scholar]
- 243.Pietiläinen KH, Kaprio J, Borg P, et al. Physical inactivity and obesity: a vicious circle. Obesity (Silver Spring). 2008;16(2):409-414. doi: 10.1038/oby.2007.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Spei ME, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta-analysis on overall and breast cancer survival. Breast. 2019;44:144-152. doi: 10.1016/j.breast.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 245.Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905-1913. doi: 10.1002/ijc.28208 [DOI] [PubMed] [Google Scholar]
- 246.Whitlock G, Lewington S, Sherliker P, et al. ; Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083-1096. doi: 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Renehan AG, Harvie M, Cutress RI, et al. How to manage the obese patient with cancer. J Clin Oncol. 2016;34(35):4284-4294. doi: 10.1200/JCO.2016.69.1899 [DOI] [PubMed] [Google Scholar]
- 248.Hourdequin KC, Schpero WL, McKenna DR, Piazik BL, Larson RJ. Toxic effect of chemotherapy dosing using actual body weight in obese versus normal-weight patients: a systematic review and meta-analysis. Ann Oncol. 2013;24(12):2952-2962. doi: 10.1093/annonc/mdt294 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Forest Plot for Overall Survival
eFigure 2. Forest Plot for Cancer-Specific Survival
eFigure 3. Forest Plot for Disease-Free Survival
eFigure 4. Funnel Plot for Overall Survival Analysis