Abstract
For over 35 years since Mosmann and Coffman proposed the seminal “type 1 T helper (Th1)/type 2 T helper (Th2)” hypothesis in 1986, the immunological community has appreciated that naïve CD4 T cells need to make important decisions upon their activation, namely to differentiate towards a Th1, Th2, Th17 (interleukin-17-producing T helper), follicular T helper (Tfh), or regulatory T cell (Treg) fate to orchestrate a variety of adaptive immune responses. The major molecular underpinnings of the Th1/Th2 effector fate choice had been initially characterized using excellent reductionist in vitro culture systems, through which the transcription factors T-bet and GATA3 were identified as the master regulators for the differentiation of Th1 and Th2 cells, respectively. However, Th1/Th2 cell differentiation and their cellular heterogeneity are usually determined by a combinatorial expression of multiple transcription factors, particularly in vivo, where dendritic cell (DC) and innate lymphoid cell (ILC) subsets can also influence T helper lineage choices. In addition, inflammatory cytokines that are capable of inducing Th17 cell differentiation are also found to be induced during typical Th1- or Th2-related immune responses, resulting in an alternative differentiation pathway, transiting from a Th17 cell phenotype towards Th1 or Th2 cells. In this review, we will discuss the recent advances in the field, focusing on some new players in the transcriptional network, contributions of DCs and ILCs, and alternative differentiation pathways towards understanding the Th1/Th2 effector choice in vivo.
Keywords: Th1/Th2 effector choice, dendritic cells, innate lymphoid cells
Introduction
The ability of naïve CD4 T cells to differentiate into distinct cytokine-producing effector T helper (Th) cell subsets has been well appreciated over the last 35 years. The initial hypothesis set forth by Mosmann and Coffman in 1986 that at least two subsets of CD4 Th cell clonotypes could be distinguished based on the production of interferon (IFN)γ or IL-41 has since been expanded to encompass new Th subsets, including type 1 IFNγ-producing Th (Th1) cells, type 2 IL-4/IL-5/IL-13-secreting Th (Th2) cells, IL-17A/IL-17F/IL-22-secreting Th (Th17) cells, T follicular helper (Tfh) cells, and regulatory T cell (Treg) populations2. Indeed, the initial hypothesis set forth sparked a period of discovery in which the major molecular and cellular events leading up to the differentiation of naïve CD4 T cells towards Th1 and Th2 effector cells were characterized using excellent reductionist in vitro models.
Th1 cells are key players in helping to mount a host defense against intracellular pathogens, including protozoa, bacteria, and viruses, but are also involved in the development of certain types of autoimmune diseases3–5. Lineage-specific master transcription factors often play decisive roles in determining cell fate. Following Mosmann and Coffman’s hypothesis, T-bet was identified6–8 as the Th1-lineage master transcription factor, as T-bet directly regulates the production of IFNγ. Soon after, several distinct upstream regulatory pathways were described to promote Th1 cell differentiation. As T-bet can positively regulate IFNγ production, autocrine IFNγ–IFNγR–Stat1 signaling can reinforce T-bet expression to solidify the Th1 phenotype9,10. IL-12 can also potently induce T-bet expression and Th1 polarization independent of IFNγ signaling11,12. Additionally, at the onset of an infection, IL-27 can induce IL-12R on naïve CD4 T cells, making them more susceptible to IL-12-mediated T-bet expression and Th1 polarization13. Lastly, T-bet was reported to induce its own expression14. However, T-bet autoregulation may not be required in the presence of either IL-12 or IFNγ. Nevertheless, T-bet and IL-12-induced pStat4 may synergize to remodel the Ifng locus and optimally induce IFNγ production12.
In contrast to Th1 cells, Th2 cells are primarily important in helping to mount a defense against helminth infections and exposure to venoms, but they also participate in different types of allergic diseases including asthma, atopic dermatitis, allergic rhinitis, and food allergy15–19. Ten years after the Th1/Th2 hypothesis, GATA3 was identified as the master transcription factor responsible for Th2 cell differentiation20–23. However, unlike T-bet, which is induced during Th1 cell differentiation, GATA3 is already expressed by naïve CD4 T cells at low levels and is required for CD4 T cell development in the thymus24,25. Upon encountering antigen presentation and IL-4, activation of Stat6 is sufficient to induce GATA3 upregulation and Th2 polarization. However, GATA3 is also sensitive to the strength of T cell receptor (TCR) stimulation, as low-dose/weaker TCR stimulation is sufficient to upregulate GATA3 expression in the absence of IL-4/Stat6 signaling26, consistent with the notion that TCR signaling strength could affect the fate of T cell differentiation27–29. Thus, there are IL-4-dependent and IL-4-independent mechanisms of GATA3 induction and Th2 cell differentiation, particularly in vivo, and GATA3 is critical for Th2 cell differentiation both in vitro and in vivo22. GATA3 directly binds to the Il4/Il13 gene locus. While GATA3 can induce Il5 and Il13 transcription through binding to their promoters30,31, GATA3 mainly affects Il4 expression through regulating epigenetic modifications at the Th2 cytokine gene locus25.
Following the identification of T-bet and GATA3 as Th1- and Th2-polarizing transcription factors, respectively, it became readily apparent that lineage cross-regulation occurs in order to solidify one T effector fate over the other. For example, T-bet was shown to suppress GATA3 transcription12,32 and inhibit GATA3 function through direct protein–protein interaction33. In addition, T-bet and GATA3 both colocalize at key Th1- and Th2-related genes, and endogenous T-bet is sufficient to inhibit GATA3 function during Th1 polarization, thereby enforcing a Th1 program12,34,35. In contrast, during Th2 polarization, GATA3 may suppress Stat4 expression, suppress Runx3-mediated IFNγ production, and epigenetically silence the Tbx21 locus to ensure Th2 polarization25,36,37.
In this review, we will discuss some recent interesting advances towards understanding the Th1/Th2 effector cell “choice”, particularly during in vivo immune responses, which include the role of new players in the transcriptional network, the contributions of dendritic cells (DCs) and innate lymphoid cells (ILCs) in the initiation of T cell differentiation, and the alternative differentiation pathways transiting from Th17 cells to Th1 or Th2 cells. While some of the topics that will be discussed are also relevant to Th17-, Treg-, and Tfh-mediated cellular responses as well as their plasticity, these subsets will not be discussed in detail, and we refer the reader to several excellent reviews2,38–45.
New roles for known transcription factors in regulating the differentiation and functions of Th1 and Th2 cells
Despite all that we have learned about the Th1/Th2 dichotomy in the past 35 years, there is still much to learn about the Th1/Th2 choice in the context of transcriptional networks. Specifically, non-lineage-specifying transcription factor networks can influence the quality of a Th1 or Th2 response by influencing their cytokine repertoire. Interestingly, several recent studies have highlighted non-lineage-restricted transcription factors, Bhlhe40 and B cell lymphoma 11B (Bcl11b), in affecting the cytokine repertoires of Th1 and Th2 cells.
Three reports have recently shown Bhlhe40 to be a key non-lineage-related cytokine modulator, demonstrating a role for Bhlhe40 in Th1 immunity in Toxoplasma gondii and Mycobacterium tuberculosis infection models and in Th2 immunity in a model of Heligmosomoides polygyrus infection46–48. Two groups independently demonstrated that Bhlhe40 plays a key role in suppressing IL-10 production by Th1 cells, functioning as a key inflammation/anti-inflammation switch. Yu and colleagues46 demonstrated that a CD4 T cell-restricted knockout of Bhlhe40 resulted in increased IL-10 production and decreased IFNγ production by T cells in a T. gondii infection model. Bhlhe40 may suppress IL-10 production via suppression of c-Maf and/or Aiolos but promotes IFNγ production in a T-bet-independent manner. Similarly, Huynh and colleagues47 demonstrated that Bhlhe40 is an essential repressor of IL-10 during M. tuberculosis infection. In the context of H. polygyrus helminth infection, Jarjour and colleagues48 found that Th2 cells require Bhlhe40 in order to mount an effective anti-helminth immune response. Interestingly, in their model, Bhlhe40 controlled the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-5 cytokines within gut Th2 cells, and both cytokines are required for efficient eosinophil recruitment and helminth control, suggesting that Bhlhe40 plays a key role in controlling the production of GM-CSF, IL-10, and other cytokines in multiple T cell subsets, including Th1, Th17, and Th2 cells. All together, these data suggest that Bhlhe40 plays an important lineage-independent cytokine-modifying role in Th1, Th2, and Th17 cells by promoting inflammation via inducing GM-CSF and suppressing IL-10.
There have also been several recent reports that have highlighted a non-lineage modulatory role for Bcl11b on Th1 and Th2 responses. Bcl11b is a critical transcription factor for early T cell development and is expressed by all T cells starting from the CD4/CD8 double negative (DN) stage 249. Bcl11b is critically required for Vβ-DJβ recombination and Tcrb expression at the DN3 to DN4 transition, as well as positive selection at the CD4+CD8+ DP stage50,51. In addition, Bcl11b plays an important role in regulating the development and functions of mature T cell subsets52. Furthermore, Bcl11b suppresses the cell fate of natural killer cells and is important for the development of ILC2s53–57. Recently, Fang and colleagues58 have demonstrated a novel role for Bcl11b in suppressing Th1 cell differentiation while simultaneously limiting the expression of Th2 cell-associated genes. In this study, it has been shown that Bcl11b physically interacts with GATA3 through protein–protein interaction and binds to common cis-regulatory elements of lineage-related genes that GATA3 binds in Th2 cells and thus limits IL-4, IL-5, and IL-13 production both in vitro and in vivo. Interestingly, GATA3 and Bcl11b also simultaneously suppressed Th1-associated genes by modulating H3K27ac and DNase I hypersensitivity sites within these gene loci. Strikingly, while Bcl11b limits Th2 cell responses at a later stage, it plays an important role in the initiation of Th2 responses59. Deletion of Bcl11b in naïve CD4 T cells results in a reduced Th2 response during helminth infection and in allergic asthma models, presumably because of a dysregulated balance between GATA3 and Runx3 expression in the absence of Bcl11b. Interestingly, Bcl11b may also play a role in restricting the expression of Th2 lineage genes within Th17 cells, as Bcl11b-deficient Th17 cells were shown to express GΑΤΑ3, IL-4, α4β7, and CCR9 in an experimental autoimmune encephalomyelitis (EAE) model60. Therefore, Bcl11b is not only critical for T cell development in the thymus but also important for T cell differentiation in the periphery, and its functions are highly cell type (or developmental stage) specific.
Interestingly, the aforementioned effects of Bhlhe40 and Bcl11b on the activation and differentiation of Th2 cells were recently confirmed by Henriksson and colleagues61. Expanding on the previous work focused on the network of transcription factors involved with Th17 cell activation versus differentiation62, Henriksson and colleagues utilized a combination of genome-wide CRISPR knockout libraries combined with RNAseq, ATAC-Seq, and ChIP-seq to dissect out the regulatory circuitry controlling the activation versus differentiation of Th2 cells in vitro. As a result of their efforts, they not only confirmed GATA3, Stat6, Batf, PPARγ, and IRF4 as key transcription factors that are involved in Th2 cell differentiation and activation but also revealed Bhlhe40, Bcl11b, and Xbp1 as transcription factors that are involved in Th2 cell activation. While more factors that are involved in regulating Th1/Th2 differentiation and functions are still being discovered, including the p53 family protein p73, which affects Th1 cell differentiation63, and Blimp-1, which regulates GATA3 expression and thus Th2 cell differentiation64, the combination of novel genome-level technologies such as CRISPR knockout with high-throughput sequencing will hopefully reveal more non-lineage-related transcription factors in modulating CD4 T cell differentiation.
DC subsets in making the Th1/Th2 decision
In order for naïve T cells to differentiate towards Th1 or Th2 effector cell fates in vivo, TCR stimulation via antigen presentation, co-stimulation, and polarizing cytokine cues, such as IL-12, IL-27, etc., are required. DCs are the premier antigen-presentation cell population in vivo, and they are ultimately required to activate and expand antigen-specific CD4 T cells via peptide–MHCII–TCR interactions (Figure 1). As such, DCs and DC subsets have garnered attention in the literature based on their differences in antigen presentation and T cell polarization capabilities. While the topic of DC subsets has been expertly reviewed elsewhere43,65,66, briefly, there are several subsets of DCs: conventional type 1 DCs (cDC1s), conventional type 2 DCs (cDC2s), plasmacytoid DCs (pDCs), monocyte-derived DCs (moDCs), and Langerhans cells (LCs), which differ based on their anatomical locations, ontogeny, and antigen-presentation capacities. Interestingly, out of the aforementioned subsets, conventional DCs are the most abundant and have been demonstrated to differ in terms of their abilities to induce Th1 or Th2 responses.
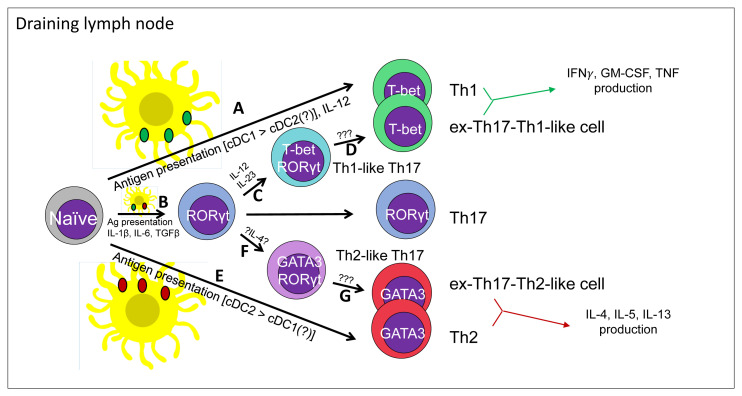
Figure 1. Contributions of dendritic cell subsets in the initiation of classical and alternative differentiation pathways for the generation of Th1 and Th2 cells in vivo.
Presented here is our updated view of the Th1/Th2 T effector decision in vivo. In response to an in situ immunological insult, local antigen-presenting dendritic cells acquire Ag and home to the nearest draining lymph node to present Ags. In the case of a pro-Th1 insult, such as a bacterial infection, bacterial Ag-laden dendritic cells can present bacterial Ags to naïve T cells and produce IL-12 in order to help generate bacterial Ag-specific Th1 cells (A). In parallel, some bacterial PAMPs can trigger Ag-laden dendritic cells to produce pro-Th17 cytokines, including IL-1β, IL-6, and TGFβ (B). Bacterial Ag-specific Th17 cells can subsequently respond to IL-12 and/or IL-23 in order to generate T-bet+ Th1-like Th17 cells (C) and ex-Th17-Th1-like cells (D) via unclear mechanisms. In contrast to a pro-Th1 insult, allergen exposure or a helminth infection is able to elicit a Th2 Ag-specific T cell response. In this scenario, helminth- or allergen-Ag-bearing dendritic cells home to the nearest draining lymph node, where they may select for Ag-specific naïve T cells to generate an Ag-specific Th2 cell response (E). In parallel, some helminth- or allergen-Ag-laden dendritic cells may instead help to generate Th17 cells, which may subsequently give rise to GATA3+ Th2-like Th17 cells (F) and ex-Th17-Th2-like cells (G). Ag, antigen; cDC, conventional dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; PAMP, pathogen-associated molecular pattern; TGFβ, transforming growth factor beta; Th1, type 1 T helper; Th2, type 2 T helper; Th17, interleukin-17-producing T helper; TNF, tumor necrosis factor.
To efficiently polarize a naïve T cell to a Th1 phenotype, pro-Th1 polarizing cytokines are required, such as IL-12 and IL-27. Interestingly, cDC1s are a major source of IL-12 in vivo67–69 and have been reported to be superior in terms of their ability to generate Th1 cells in ex vivo coculture systems70 when they are compared to cDC2s. cDC1s constitutively express Il12b transcript and produce IL-12p40 protein in vivo71,72. Additionally, in experimental models of Th1 inflammation in the absence of cDC1s, the Th1 response is significantly compromised69,73–77, suggesting that cDC1s play a major role in generating Th1 responses in vivo. However, the following question arises: why are cDC1s able to produce basal levels of IL-12? The constitutive production of IL-12p40 doesn’t seem to depend on commensals or the specific acquisition of antigen, as cDC1 IL-12p40 production is maintained in germ-free, naïve mice, suggesting that IL-12 production is either an intrinsic property of the cDC1 lineage or maintained by the microenvironment78. Homeostatic cDC1-derived IL-12 might function to support the generation of innate-like T-bethigh CD4 memory phenotype cells. Interestingly cDC1s aren’t the only DC subset that is able to initiate a Th1 response. There is some evidence to suggest that moDCs are also capable of driving Th1 responses during T. gondii and Salmonella infections as well as in immunizations with CpG or CFA-based adjuvants79–82, possibly in coordination with cDC1s. Furthermore, during inflammation, cDC2s may acquire a hybrid inflammatory cDC2 phenotype in a manner that is reminiscent of cDC1s and moDCs, and type 1 IFN drives the generation of inflammatory cDC2s, which are capable of priming naive CD4 T cells to become IFNγ-producing Th1 cells83. Another recent study has also demonstrated that TNFR2+ cDC2 cells are able to drive Th1 responses following an intranasal immunization with cyclic dinucleotide as an adjuvant84, suggesting that both the cDC subsets and the adjuvant/PAMPs involved are important in determining the T cell differentiation outcome. Lastly, as one might expect, TLR3 and TLR9 agonists enhance DC IL-12p40 production and thus Th1 cell differentiation.
In contrast to the role of cDC1s in generating and recruiting Th1 cells, cDC2s, including IRF4+ cDC2s (some of which also express the transcription factor Klf4) in the skin, lungs, and intestinal lamina propria, are necessary for triggering Th2 responses in models of helminth infection or allergic diseases76,85–88. The exposure of cDC2s to helminth products, like Schistosoma mansoni egg antigen (SEA)-derived protein Omega1, can endow cDC2s with the capacity to induce Th2 cell differentiation by inhibiting IL-12 production and limiting contact time with CD4 T cells, resulting in Th2-favorable antigen presentation conditions88–94. Interestingly, transcriptomic analyses of helminth or allergen-conditioned DCs have identified TSLP as a key upstream pathway involved in the upregulation of pro-Th2 OX40L expression. CD301b+ dermal DCs can also support Th2 cell differentiation, as an immunization with OVA mixed with papain or alum is sufficient to drive Th2 cell polarization95. Interestingly, allergen-activated TRPV1 neurons may trigger the migration of CD301b+ DCs to the draining lymph node via substance P to induce Th2 cell differentiation96. Thus, a combination of select DC subsets, pro-Th1 or pro-Th2 adjuvants, PAMPs, and the site(s) of antigen acquisition seem to ultimately determine the resulting Th1 or Th2 response in vivo rather than a T cell “choice” within the draining lymph node.
The involvement of local ILC populations on determining T cell differentiation
ILCs are innate lymphocytes that lack specific antigen receptors but are able to respond to alarmin cytokines in order to closely mirror T cell subsets in terms of their subsets and cytokine repertoires. As a result, ILCs have drawn interest in how they might regulate the initiation or quality of a Th1/Th2 response in situ. As ILCs have been expertly reviewed elsewhere97–99, we will briefly re-introduce them here. ILCs can be divided up into five major subsets: NK cells, which mirror CD8 T cells, express T-bet and Eomes, and produce IFNγ, Perforin, and Granzyme B; group 1 ILCs (ILC1s), which closely mirror Th1 cells, express T-bet, and produce IFNγ and TNF; group 2 ILCs (ILC2s), which closely mirror Th2 cells, express high levels of GATA3, and produce IL-5, IL-13, and IL-9; group 3 ILCs (ILC3s), which closely mirror Th17 cells, express RORγt (with some of them also expressing T-bet), and produce IL-22 and GM-CSF (also IFNγ for T-bet+ ILC3s); and lymphoid tissue inducer (LTi) cells, which express RORγt, produce RANK, lymphotoxins, TNF, and IL-17A, and are required for the formation of lymphoid tissues during development. Thus, as one might expect, ILC subsets are active participants during immune responses and have garnered interest in how they might influence the development of Th1 or Th2 immune responses.
The effects of ILC-derived cytokines on Th responses
So how might ILCs influence the generation of a T cell response? One obvious way would be via the secretion of pro-Th1 or pro-Th2 cytokines in response to tissue alarmins (Figure 2). As one might expect, type 2 immunity-inducing agents (e.g. helminth products, papain, Der p1, etc.) can disrupt the integrity of the epithelium, resulting in the release of alarmins, including IL-33, IL-25, and TSLP. ILC2s can respond to IL-33 via T1/ST2 (IL-33R) and IL-25 via IL-17RB to locally produce the type 2 cytokines IL-4, IL-5, and IL-13, thereby setting up a pro-Th2 milieu in situ and helping to initiate and maintain a Th2 response17,100. With respect to IL-4, while ILC2s are relatively poor IL-4 producers compared to Th2 cells, there is some evidence to suggest that ILC2-derived IL-4 may support Th2 cell differentiation. During H. polygyrus infection, it seems that ILC2-derived IL-4 plays an important role in inducing a Th2 response101. In addition, it has been shown that other inflammatory mediators, such as the leukotriene LTD4, may induce IL-4 production by ILC2s101–103. Furthermore, IL-13 produced by ILC2s may promote the migration of lung DCs into the draining lymph node to initiate Th2 responses104. Similarly, in situ production of IL-4 and IL-13 by ILC2s or Th2 cells may further induce the expression of IL-25 to amplify type 2 immune responses through recruiting more activated ILC2s105. Lastly, early production of IL-5 by in situ ILC2s can potentially support a Th2 response via the recruitment of IL-4-producing eosinophils106–110. Therefore, the crosstalk between ILC2s and Th2 cells may play an important role in mounting a robust type 2 response, and such crosstalk may also serve as a target for treating chronic type 2 inflammation.
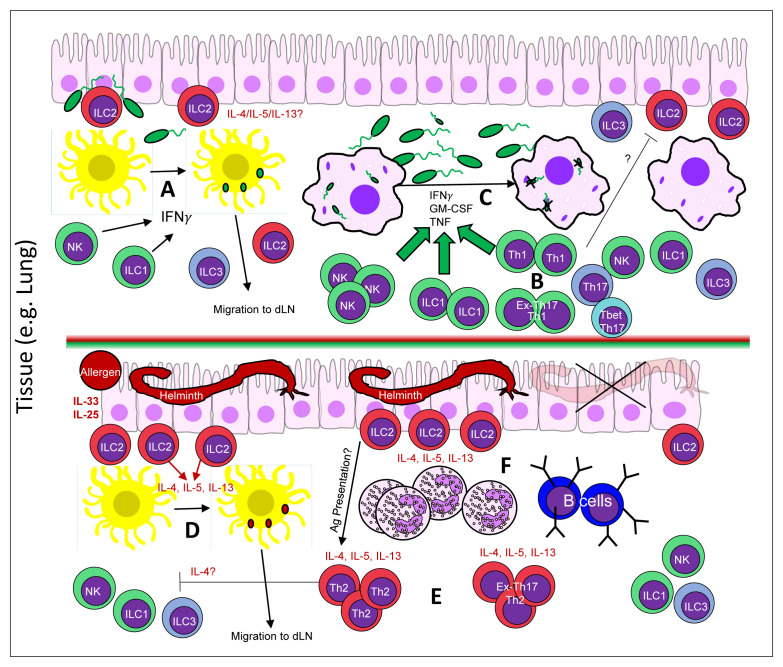
Figure 2. An updated view of the players in situ that help to shape a Th1/Th2 response.
Presented here is our updated view of the Th1/Th2 T effector decision in situ/in vivo. In response to an immunological insult in a complex tissue, such as the lung, local innate immune cells respond appropriately to guide the downstream selection of Th1 or Th2 cells. In response to a pro-Th1 bacterial infection (A–C), in situ NK cells and ILC1s respond to alarmins to produce IFNγ and local dendritic cells acquire bacterial Ags (A). Ag-laden dendritic cells may then travel to the nearest dLN to present bacterial Ags and generate bacterial Ag-specific Th1 cell or a mixed Th17 and Th1 cell response. Bacterial Ag-specific Th1, Th17, T-bet+ Th1-like Th17, and ex-Th17-Th1-like cells can then home back to the site of infection or Th17 cells can generate T-bet+ Th1-like Th17/ex-Th17-Th1-like cells in situ (B) in order to coordinate with local NK cells, ILC1 cells, and macrophages to control the bacterial infection (C). The potential antagonistic effects between Th1 cells and lung-resident ILC2s in this context are unclear. In contrast to a pro-Th1 insult, allergen exposure or a helminth infection is able to elicit a Th2 Ag-specific cell response (D–F). In this scenario, a helminth infection is sufficient to drive the production of alarmins (IL-33/IL-25) from the lung epithelium and activate local tissue ILC2s (D). In situ ILC2s can produce IL-4/IL-13 in response and promote helminth Ag-bearing dendritic cells homing to the nearest dLN. Helminth Ag-specific naïve T cells are selected by the Ag-laden dendritic cell and helminth Ag-specific Th2 or a mixture of Th17 and Th2 cells are generated (E). Helminth Ag-specific Th2, Th17, Th2-like Th17 cells, and ex-Th17-Th2-like cells may then home back to the infected tissue in order to work in conjunction with locally recruited and activated eosinophils, B cells, and ILC2s in order to expel or kill the invading helminth (F). Ag, antigen; dLN, draining lymph node; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; ILC, innate lymphoid cell; NK, natural killer; Th2, type 2 T helper; Th17, interleukin-17-producing T helper; TNF, tumor necrosis factor.
In response to an acute pro-Th1 infectious insult, such as T. gondii or MCMV infection, ILC1s are stimulated by cDC-sourced IL-12 in order to produce IFNγ and can help mount a Th1 response. However, one open-ended question considering this paradigm concerns how ILCs and DCs might interact or crosstalk in order to influence the generation of a Th1 or Th2 response. As noted in the preceding section, while cDC1s are great at generating a Th1 response, given the right adjuvants TNFR2+ cDC2s can also elicit Th1 cells. Depending on the adjuvants and infection route/immunization route involved, the involvement of ILC1s versus ILC2s might differ, as the tissue distributions of both are quite different. Another open-ended question that has not been addressed in the literature concerns how opposed ILCs might respond during a Th1 or Th2 response. For example, the lung is host to a large population of ILC2s and a smaller population of ILC3s and ILC1s, and, as one might expect, ILC2s actively participate in Th2-mediated lung pathologies including allergy. However, what might happen to those ILC2s in the context of a strong Th1 infectious insult such as a viral infection or bacterial pneumonia? Is it possible that the activation status of ILC2s in the lung may determine the severity of SARS-CoV2-infected patients? Thus, while ILCs are active participants in setting up a local pro-Th1 or pro-Th2 environment, there is still much to learn.
The effects of ILC-mediated antigen presentation on Th responses
Another possible mechanism through which ILCs might affect the Th1/Th2 response is through potential antigen presentation. Unlike MHC-I, which is expressed by almost every cell, the expression of MHC-II is restricted to antigen-presenting cells, and, remarkably enough, some ILC2s and ILC3s are also endowed with the machinery to process and present peptides on MHC-II molecules and are thus potentially able to interact with T cells via TCR–peptide–MHCII complexes99,111,112. Interestingly, ILC2s can stimulate T cells via peptide-loaded MHC-II, and one report demonstrated that the secretion of IL-2 by ILC2s in T cell/ILC2 co-cultures resulted in an expansion of ILC2s112. Thus, ILC2s might also be able to influence in situ Th2 responses via antigen presentation. At present, it isn’t clear how important this mechanism is in comparison to antigen presentation via professional antigen-presenting DCs.
Alternative differentiation pathways to Th1 & Th2 cells via Th17 intermediates
In addition to Th1 and Th2 cells, a third type of CD4 effector T cell termed Th17 cells are interesting in the context of the Th1/Th2 fate decision owing to their less-committed, plastic properties. Briefly, Th17 cells are a bona fide IL-17A/IL-17F/IL-22-secreting CD4 T effector cell subset that plays a key role in the defense against opportunistic fungal or bacterial pathogens but may also participate in autoimmune and allergic diseases38. Following the discovery of IL-17A-producing CD4 T cells in 2005113,114, the mechanisms of Th17 polarization were quickly described115–118. Conceptually, extracellular bacterial or fungal PAMPs result in antigen-presenting cell-mediated antigen presentation and the production of the pro-inflammatory cytokines IL-1β, IL-6, and IL-23, which drive the generation of Th17 cells in a RORγt (TCR/NFAT/NFkB/AP-1) and pSTAT3 (IL-6 and IL-23) dependent manner38,119. In addition, the master Th17 lineage transcription factor RORγt can collaborate with other transcription factors, including IRF4, BATF, and Runx1/Runx3, to optimally induce the expression of Th17 lineage genes2,62,120. However, following these seminal discoveries, it became readily apparent that Th17 cell-mediated responses can exert both protective and pathogenic effects during immunological challenges, suggesting that Th17 cells can be divided into “pathogenic” and “non-pathogenic” subsets. Functionally distinct homeostatic and inflammatory Th17 cells can be found in the intestine121. Several putative regulators of Th17 “pathogenicity” have been described, including IL-23, CD5L, REV-ERBα, and various environmental factors such as commensal organisms and tissue salinity; however, the precise mechanisms governing the protective/pathogenic switch are still unclear and may involve supplemental non-lineage-related transcription factors122–128. To further complicate things, it has become readily apparent that Th17 cells can co-opt T-bet or GATA3 expression to assume aspects of the Th1 or Th2 lineage (termed Th17/Th1 or Th17/Th2 cells) or to fully assume a functional Th1 or Th2 phenotype (ex-Th17-Th1-like cell or ex-Th17-Th2-like cell).
When the distinctions between Th17 and Th1 cells blur: Th17/Th1 and ex-Th17-Th1-like cells
To date, there are several lines of evidence to support the concept that Th17 cells may co-opt aspects of the Th1 lineage or even assume an ex-Th17-Th1-like cell fate in various in vivo contexts. In a study utilizing IL-17A+ Th17 cell fate mapping reporter mice (Il17aCreRosa26eYFP mice) and mouse models of Th17- and Th1-related inflammation (EAE and subcutaneous immunization with Candida albicans), a population of fate mapped ex-Th17 cells (IL-17A–eYFP+ cells) was identified129. Interestingly, subpopulations of T-bet+RORγt+IL-17A+ cells and IFNγ+IL-17A– arose amongst the IL-17A fate mapped cells, suggesting that a Th17 cell may generate a mixture of IFNγ+IL-17A+ Th17 cells and IFNγ+IL-17A– ex-Th17-Th1-like cell subpopulations. Furthermore, the generation of IL-17A fate mapped eYFP+ cells depends on IL-23-induced expression of T-bet, as an Il17aCreRosa26eYFPIl23r–/– variant failed to generate IFNγ+IL-17A+eYFP+ or IFNγ+IL-17A–eYFP+ fate mapped cells. Runx1 together with T-bet plays an important role in the generation of IFNγ-producing Th17 cells130. A similar phenotypic change from Th17 to Th1 cells has also been observed in a model of Helicobacter hepaticus-induced colitis, an in vitro polarized Th17 T cell transfer Rag2–/– colitis model, and an IL-22 Th17 fate mapping model in the gut, suggesting that Th17 cells are intrinsically plastic and can generate a population of T-bet+RORγt+ Th1-like Th17 cells and a population of ex-Th17 cells that assumed a Th1 phenotype (ex-Th17-Th1-like cells)131–133. In addition, IL-23 was shown by Jain and colleagues to induce Blimp-1 expression within Th17 cells, and Blimp-1 was shown to be necessary for the induction of T-bet-, GM-CSF-, and IFNγ-expressing Th17 cells within the gut, suggesting that IL-23 along with other transcription factors may synergize to induce a Th1-like phenotype within Th17 cells134. However, from a Th1/Th2-centric viewpoint, the later ex-Th17-Th1-like cell population poses an interesting philosophical dilemma: if Th17 cells can generate a subpopulation of cells that are essentially Th1 cells and naïve T cells can directly differentiate into Th1 cells as well, what, if anything, would distinguish the two in vivo? In addition, are there any meaningful differences between former Th17-derived Th1 cells and de novo Th1 cells? Do they have distinct functionalities in host defense versus in inflammation? While these open questions remain to be addressed in the literature, Th1-like subpopulations of Th17 cells have been observed in various human patient populations, including multiple sclerosis, rheumatoid arthritis, psoriasis, inflammatory bowel disease, and M. tuberculosis patients and others, so Th1-like Th17 cells and ex-Th17-Th1-like cells may have some clinical relevance.
Th17 plasticity towards the other fate: Th17/Th2 and ex-Th17-Th2-like cells
Similarly, there is some limited evidence to suggest that Th17 cells may also assume Th2-like properties in the context of allergic disease. In a study profiling human PBMC CD4 memory cells from atopic asthma patients, Wang and colleagues observed a population of CD4+CRTH2+CCR6+ T cells that were elevated in allergic asthmatic patients versus healthy controls and co-expressed IL-4, IL-17A, IL-22, IL-5, IL-13, RORγt, and GATA3, suggesting that a population of Th2-like Th17 cells are generated during the development of atopic asthma and may be associated with the pathology. Interestingly, a similar population of IL-4GFP+IL-17A+ Th17 cells were isolated from the bronchoalveolar lavage (BAL) fluid and lungs of Aspergillus Orazae + OVA challenged IL-4-GFP knock-in (4Get) mice, suggesting that both mouse and human Th17 cells may assume a partial Th2 phenotype in the context of allergy135. Similar results were observed by Irvin and colleagues in a study examining BAL T cell phenotypes from a different cohort of atopic asthmatic patients. In that study, IL-4+IL-17A+GATA3+RORγt+ T cells were observed and IL-4+IL-17A+ T cells correlated with eosinophil counts and occurred in the most severe subgroup of asthmatic patients136. Together, these observations of Th2-like Th17 cells suggest that Th17 cells may also assume characteristics of Th2 cells and suggest that the formation of ex-Th17-Th2-like cells (the Th2 equivalent of ex-Th17-Th1-like cells) may be theoretically feasible in the context of allergy or helminth infections. Indeed, a recent study showed that ~10% of the IL-17A–IL-4/5/13+ cells in the lung are IL-17A fate mapping positive137. Taken together, the available data indicate that close encounters between bona fide Th1/Th2 cells and Th17 plastic approximations of Th1/Th2 cells, which develop through the Th17 intermediate stage, may occur in vivo. While it is likely that the pro-inflammatory cytokines such as IL-1β and IL-6, both of which can be induced even during Th1 and Th2 responses, may determine these alternative differentiation pathways to Th1 and Th2 cells, the precise regulation and contributions of these unconventional Th1/Th2 subsets relative to classical Th1/Th2 cells during immune responses remain an open question (Figure 1).
Conclusion
In summary, despite the tremendous amount of work that has been accomplished since Mossman and Coffman’s seminal Th1/Th2 hypothesis in 1986, there is clearly more to discover about how Th1/Th2 effector fate decisions are made in vivo. While the present work was in no way designed to be all encompassing, we have highlighted some recent advances in the field that have contributed to our understanding of how Th1/Th2 immune responses are initiated and amplified in vivo. Ultimately, the decision to launch or modify the quality of a Th1 or Th2 immune response can be distilled down to several variables: 1) the site(s) of antigen encounter and which DC subset(s) acquire and present the antigen(s), 2) which ILC subsets are activated in situ to support the de novo adaptive immune response, 3) which differentiating cytokines are produced by the antigen-presenting DCs within the draining lymph node, 4) whether or not there is Th17 cell involvement to contribute towards the overall Th1/Th2 cell response, and 5) the expression of Th1/Th2 response-modifying transcription factors, such as Bhlhe40 and Bcl11b, etc. New technologies including single cell RNA-Seq138 and single cell ATAC-Seq analysis of antigen-specific CD4 T cells as well as advanced imaging to visualize cell–cell interactions in vivo at different stages during immune responses will greatly help further our understanding of the differentiation process of Th1 and Th2 cells and ultimately contribute to the design of better and precise strategies in treating immunological diseases involving these two important lymphocyte subsets.
The peer reviewers who approve this article are:
Kenneth J Oestreich, Department of Microbial Infection and Immunity, The Ohio State University College of Medicine and Wexner Medical Center, Columbus, OH, USA
Dorina Avram, Department of Immunology, Moffitt Cancer Center, Tampa, FL, USA
Namrata Gautam, Department of Immunology, Moffitt Cancer Center, Tampa, FL, USA
Funding Statement
This work is supported by the Division of Intramural Research of NIAID (US National Institutes of Health).
Contributor Information
Matthew J Butcher, Email: matthew.butcher@niaid.nih.gov.
Jinfang Zhu, Email: jfzhu@niaid.nih.gov.
References
- 1. Mosmann TR, Cherwinski H, Bond MW, et al. : Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986; 136(7): 2348–57. [PubMed] [Google Scholar]
- 2. Zhu J: T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb Perspect Biol. 2018; 10(10): a030338. 10.1101/cshperspect.a030338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szabo SJ, Sullivan BM, Peng SL, et al. : Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003; 21: 713–58. 10.1146/annurev.immunol.21.120601.140942 [DOI] [PubMed] [Google Scholar]
- 4. Zhu J, Paul WE: CD4 T cells: Fates, functions, and faults. Blood. 2008; 112(5): 1557–69. 10.1182/blood-2008-05-078154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu J, Yamane H, Paul WE: Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010; 28: 445–89. 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szabo SJ, Kim ST, Costa GL, et al. : A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment. Cell. 2000; 100(6): 655–69. 10.1016/S0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- 7. Szabo SJ, Sullivan BM, Stemmann C, et al. : Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002; 295(5553): 338–42. 10.1126/science.1065543 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 8. Lazarevic V, Glimcher LH, Lord GM: T-bet: A bridge between innate and adaptive immunity. Nat Rev Immunol. 2013; 13(11): 777–89. 10.1038/nri3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lighvani AA, Frucht DM, Jankovic D, et al. : T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001; 98(26): 15137–42. 10.1073/pnas.261570598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Afkarian M, Sedy JR, Yang J, et al. : T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002; 3(6): 549–57. 10.1038/ni794 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 11. Yang Y, Ochando JC, Bromberg JS, et al. : Identification of a distant T-bet enhancer responsive to IL-12/Stat4 and IFNgamma/Stat1 signals. Blood. 2007; 110(7): 2494–500. 10.1182/blood-2006-11-058271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu J, Jankovic D, Oler AJ, et al. : The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012; 37(4): 660–73. 10.1016/j.immuni.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hölscher C: The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med Microbiol Immunol. 2004; 193(1): 1–17. 10.1007/s00430-003-0186-x [DOI] [PubMed] [Google Scholar]
- 14. Mullen AC, High FA, Hutchins AS, et al. : Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001; 292(5523): 1907–10. 10.1126/science.1059835 [DOI] [PubMed] [Google Scholar]
- 15. Lambrecht BN, Hammad H: Biology of lung dendritic cells at the origin of asthma. Immunity. 2009; 31(3): 412–24. 10.1016/j.immuni.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 16. Licona-Limón P, Kim LK, Palm NW, et al. : TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14(6): 536–42. 10.1038/ni.2617 [DOI] [PubMed] [Google Scholar]
- 17. Zhu J: T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015; 75(1): 14–24. 10.1016/j.cyto.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker JA, McKenzie ANJ: T H 2 cell development and function. Nat Rev Immunol. 2018; 18(2): 121–133. 10.1038/nri.2017.118 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 19. Paul WE, Zhu J: How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010; 10(4): 225–35. 10.1038/nri2735 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 20. Zheng Wp, Flavell RA: The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell. 1997; 89(4): 587–96. 10.1016/S0092-8674(00)80240-8 [DOI] [PubMed] [Google Scholar]
- 21. Zhang DH, Cohn L, Ray P, et al. : Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997; 272(34): 21597–603. 10.1074/jbc.272.34.21597 [DOI] [PubMed] [Google Scholar]
- 22. Zhu J, Min B, Hu-Li J, et al. : Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004; 5(11): 1157–65. 10.1038/ni1128 [DOI] [PubMed] [Google Scholar]
- 23. Pai SY, Truitt ML, Ho IC: GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A. 2004; 101(7): 1993–8. 10.1073/pnas.0308697100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ho IC, Tai TS, Pai SY: GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009; 9(2): 125–35. 10.1038/nri2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei G, Abraham BJ, Yagi R, et al. : Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011; 35(2): 299–311. 10.1016/j.immuni.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 26. Yamane H, Zhu J, Paul WE: Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005; 202(6): 793–804. 10.1084/jem.20051304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosken NA, Shibuya K, Heath AW, et al. : The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995; 182(5): 1579–84. 10.1084/jem.182.5.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brogdon JL, Leitenberg D, Bottomly K: The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J Immunol. 2002; 168(8): 3825–32. 10.4049/jimmunol.168.8.3825 [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharyya ND, Feng CG: Regulation of T Helper Cell Fate by TCR Signal Strength. Front Immunol. 2020; 11: 624. 10.3389/fimmu.2020.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 30. Yamashita M, Ukai-Tadenuma M, Kimura M, et al. : Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. J Biol Chem. 2002; 277(44): 42399–408. 10.1074/jbc.M205876200 [DOI] [PubMed] [Google Scholar]
- 31. Tanaka S, Motomura Y, Suzuki Y, et al. : The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat Immunol. 2011; 12(1): 77–85. 10.1038/ni.1966 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 32. Usui T, Preiss JC, Kanno Y, et al. : T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006; 203(3): 755–66. 10.1084/jem.20052165 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 33. Hwang ES, Szabo SJ, Schwartzberg PL, et al. : T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005; 307(5708): 430–3. 10.1126/science.1103336 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Jenner RG, Townsend MJ, Jackson I, et al. : The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009; 106(42): 17876–81. 10.1073/pnas.0909357106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanhere A, Hertweck A, Bhatia U, et al. : T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012; 3: 1268. 10.1038/ncomms2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Usui T, Nishikomori R, Kitani A, et al. : GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003; 18(3): 415–28. 10.1016/S1074-7613(03)00057-8 [DOI] [PubMed] [Google Scholar]
- 37. Yagi R, Junttila IS, Wei G, et al. : The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010; 32(4): 507–17. 10.1016/j.immuni.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 38. Korn T, Bettelli E, Oukka M, et al. : IL-17 and Th17 Cells. Annu Rev Immunol. 2009; 27: 485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- 39. Cannons JL, Lu KT, Schwartzberg PL: T follicular helper cell diversity and plasticity. Trends Immunol. 2013; 34(4): 200–7. 10.1016/j.it.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakaguchi S, Vignali DAA, Rudensky AY, et al. : The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013; 13(6): 461–7. 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- 41. Crotty S: T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014; 41(4): 529–42. 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shevach EM: Foxp3 + T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front Immunol. 2018; 9: 1048. 10.3389/fimmu.2018.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 43. Hilligan KL, Ronchese F: Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 2020; 17(6): 587–99. 10.1038/s41423-020-0465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 44. Song W, Craft J: T follicular helper cell heterogeneity: Time, space, and function. Immunol Rev. 2019; 288(1): 85–96. 10.1111/imr.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 45. Ruterbusch M, Pruner KB, Shehata L, et al. : In Vivo CD4 + T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol. 2020; 38: 705–25. 10.1146/annurev-immunol-103019-085803 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 46. Yu F, Sharma S, Jankovic D, et al. : The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J Exp Med. 2018; 215(7): 1813–21. 10.1084/jem.20170155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huynh JP, Lin CC, Kimmey JM, et al. : Bhlhe40 is an essential repressor of IL-10 during Mycobacterium tuberculosis infection. J Exp Med. 2018; 215(7): 1823–38. 10.1084/jem.20171704 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48. Jarjour NN, Bradstreet TR, Schwarzkopf EA, et al. : BHLHE40 Promotes T H 2 Cell-Mediated Antihelminth Immunity and Reveals Cooperative CSF2RB Family Cytokines. J Immunol. 2020; 204(4): 923–32. 10.4049/jimmunol.1900978 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Li L, Leid M, Rothenberg EV: An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010; 329(5978): 89–93. 10.1126/science.1188989 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 50. Inoue J, Kanefuji T, Okazuka K, et al. : Expression of TCR alpha beta partly rescues developmental arrest and apoptosis of alpha beta T cells in Bcl11b-/- mice. J Immunol. 2006; 176(10): 5871–9. 10.4049/jimmunol.176.10.5871 [DOI] [PubMed] [Google Scholar]
- 51. Albu DI, Feng D, Bhattacharya D, et al. : BCL11B is required for positive selection and survival of double-positive thymocytes. J Exp Med. 2007; 204(12): 3003–15. 10.1084/jem.20070863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Avram D, Califano D: The multifaceted roles of Bcl11b in thymic and peripheral T cells: Impact on immune diseases. J Immunol. 2014; 193(5): 2059–65. 10.4049/jimmunol.1400930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li P, Burke S, Wang J, et al. : Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010; 329(5987): 85–9. 10.1126/science.1188063 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. Califano D, Cho JJ, Uddin MN, et al. : Transcription Factor Bcl11b Controls Identity and Function of Mature Type 2 Innate Lymphoid Cells. Immunity. 2015; 43(2): 354–68. 10.1016/j.immuni.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walker JA, Oliphant CJ, Englezakis A, et al. : Bcl11b is essential for group 2 innate lymphoid cell development. J Exp Med. 2015; 212(6): 875–82. 10.1084/jem.20142224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu Y, Wang C, Clare S, et al. : The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J Exp Med. 2015; 212(6): 865–74. 10.1084/jem.20142318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhong C, Zhu J: Bcl11b drives the birth of ILC2 innate lymphocytes. J Exp Med. 2015; 212(6): 828. 10.1084/jem.2126insight1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fang D, Cui K, Hu G, et al. : Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation. J Exp Med. 2018; 215(5): 1449–62. 10.1084/jem.20171127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lorentsen KJ, Cho JJ, Luo X, et al. : Bcl11b is essential for licensing Th2 differentiation during helminth infection and allergic asthma. Nat Commun. 2018; 9(1): 1679. 10.1038/s41467-018-04111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Califano D, Sweeney KJ, Le H, et al. : Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J Clin Invest. 2014; 124(1): 174–87. 10.1172/JCI70103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Henriksson J, Chen X, Gomes T, et al. : Genome-wide CRISPR Screens in T Helper Cells Reveal Pervasive Crosstalk between Activation and Differentiation. Cell. 2019; 176(4): 882–896.e18. 10.1016/j.cell.2018.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 62. Ciofani M, Madar A, Galan C, et al. : A validated regulatory network for Th17 cell specification. Cell. 2012; 151(2): 289–303. 10.1016/j.cell.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ren M, Kazemian M, Zheng M, et al. : Transcription factor p73 regulates Th1 differentiation. Nat Commun. 2020; 11(1): 1475. 10.1038/s41467-020-15172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 64. He K, Hettinga A, Kale SL, et al. : Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J Exp Med. 2020; 217(7): e20190742. 10.1084/jem.20190742 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 65. Granot T, Senda T, Carpenter DJ, et al. : Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017; 46(3): 504–15. 10.1016/j.immuni.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson DA, 3rd, Dutertre CA, Ginhoux F, et al. : Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol. 2021; 21(2): 101–115. 10.1038/s41577-020-00413-x [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. Mashayekhi M, Sandau MM, Dunay IR, et al. : CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011; 35(2): 249–59. 10.1016/j.immuni.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 68. Ruffell B, Chang-Strachan D, Chan V, et al. : Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014; 26(5): 623–37. 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martínez-López M, Iborra S, Conde-Garrosa R, et al. : Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol. 2015; 45(1): 119–29. 10.1002/eji.201444651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maldonado-López R, de Smedt T, Michel P, et al. : CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999; 189(3): 587–92. 10.1084/jem.189.3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Everts B, Tussiwand R, Dreesen L, et al. : Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12. J Exp Med. 2016; 213(1): 35–51. 10.1084/jem.20150235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Conejero L, Khouili SC, Martínez-Cano S, et al. : Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. JCI Insight. 2017; 2(10): e90420. 10.1172/jci.insight.90420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Igyártó BZ, Haley K, Ortner D, et al. : Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011; 35(2): 260–72. 10.1016/j.immuni.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 74. Luda KM, Joeris T, Persson EK, et al. : IRF8 Transcription-Factor-Dependent Classical Dendritic Cells Are Essential for Intestinal T Cell Homeostasis. Immunity. 2016; 44(4): 860–74. 10.1016/j.immuni.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 75. Muzaki ARBM, Tetlak P, Sheng J, et al. : Intestinal CD103(+)CD11b(-) dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016; 9(2): 336–51. 10.1038/mi.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Demiri M, Müller-Luda K, Agace WW, et al. : Distinct DC subsets regulate adaptive Th1 and 2 responses during Trichuris muris infection. Parasite Immunol. 2017; 39(10). 10.1111/pim.12458 [DOI] [PubMed] [Google Scholar]
- 77. Arnold IC, Zhang X, Artola-Boran M, et al. : BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 2019; 15(6): e1007866. 10.1371/journal.ppat.1007866 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 78. Kawabe T, Jankovic D, Kawabe S, et al. : Memory-phenotype CD4 + T cells spontaneously generated under steady-state conditions exert innate T H 1-like effector function. Sci Immunol. 2017; 2(12): eaam9304. 10.1126/sciimmunol.aam9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nakano H, Lin KL, Yanagita M, et al. : Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009; 10(4): 394–402. 10.1038/ni.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Flores-Langarica A, Marshall JL, Bobat S, et al. : T-zone localized monocyte-derived dendritic cells promote Th1 priming to Salmonella. Eur J Immunol. 2011; 41(9): 2654–65. 10.1002/eji.201141440 [DOI] [PubMed] [Google Scholar]
- 81. Goldszmid RS, Caspar P, Rivollier A, et al. : NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012; 36(6): 1047–59. 10.1016/j.immuni.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Koker S, van Hoecke L, de Beuckelaer A, et al. : Inflammatory monocytes regulate Th1 oriented immunity to CpG adjuvanted protein vaccines through production of IL-12. Sci Rep. 2017; 7(1): 5986. 10.1038/s41598-017-06236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bosteels C, Neyt K, Vanheerswynghels M, et al. : Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity. 2020; 52(6): 1039–1056.e9. 10.1016/j.immuni.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 84. Mansouri S, Patel S, Katikaneni DS, et al. : Immature lung TNFR2- conventional DC 2 subpopulation activates moDCs to promote cyclic di-GMP mucosal adjuvant responses in vivo. Mucosal Immunol. 2019; 12(1): 277–89. 10.1038/s41385-018-0098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 85. Gao Y, Nish SA, Jiang R, et al. : Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013; 39(4): 722–32. 10.1016/j.immuni.2013.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 86. Williams JW, Tjota MY, Clay BSI, et al. : Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013; 4: 2990. 10.1038/ncomms3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tussiwand R, Everts B, Grajales-Reyes GE, et al. : Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015; 42(5): 916–28. 10.1016/j.immuni.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mayer JU, Demiri M, Agace WW, et al. : Different populations of CD11b + dendritic cells drive Th2 responses in the small intestine and colon. Nat Commun. 2017; 8: 15820. 10.1038/ncomms15820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. MacDonald AS, Straw AD, Bauman B, et al. : CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001; 167(4): 1982–8. 10.4049/jimmunol.167.4.1982 [DOI] [PubMed] [Google Scholar]
- 90. de Jong EC, Vieira PL, Kalinski P, et al. : Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002; 168(4): 1704–9. 10.4049/jimmunol.168.4.1704 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 91. Everts B, Perona-Wright G, Smits HH, et al. : Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009; 206(8): 1673–80. 10.1084/jem.20082460 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 92. Steinfelder S, Andersen JF, Cannons JL, et al. : The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J Exp Med. 2009; 206(8): 1681–90. 10.1084/jem.20082462 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 93. Connor LM, Tang SC, Camberis M, et al. : Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo. J Immunol. 2014; 193(6): 2709–17. 10.4049/jimmunol.1400374 [DOI] [PubMed] [Google Scholar]
- 94. van Panhuys N, Klauschen F, Germain RN: T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014; 41(1): 63–74. 10.1016/j.immuni.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 95. Kumamoto Y, Linehan M, Weinstein JS, et al. : CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013; 39(4): 733–43. 10.1016/j.immuni.2013.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Perner C, Flayer CH, Zhu X, et al. : Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity. 2020; 53(5): 1063–1077.e7. 10.1016/j.immuni.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 97. Artis D, Spits H: The biology of innate lymphoid cells. Nature. 2015; 517(7534): 293–301. 10.1038/nature14189 [DOI] [PubMed] [Google Scholar]
- 98. Gurram RK, Zhu J: Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol. 2019; 16(3): 225–35. 10.1038/s41423-019-0210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klose CSN, Artis D: Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020; 30(6): 475–91. 10.1038/s41422-020-0323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 100. Kabata H, Moro K, Koyasu S, et al. : The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018; 286(1): 37–52. 10.1111/imr.12706 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 101. Pelly VS, Kannan Y, Coomes SM, et al. : IL-4-producing ILC2s are required for the differentiation of T H 2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016; 9(6): 1407–17. 10.1038/mi.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Doherty TA, Khorram N, Lund S, et al. : Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013; 132(1): 205–13. 10.1016/j.jaci.2013.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Salimi M, Stöger L, Liu W, et al. : Cysteinyl leukotriene E 4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D 2 and epithelial cytokines. J Allergy Clin Immunol. 2017; 140(4): 1090–1100.e11. 10.1016/j.jaci.2016.12.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Halim TYF, Steer CA, Mathä L, et al. : Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014; 40(3): 425–35. 10.1016/j.immuni.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 105. von Moltke J Ji M Liang HE,et al. : Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016; 529(7585): 221–5. 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 106. Moqbel R, Ying S, Barkans J, et al. : Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995; 155(10): 4939–47. [PubMed] [Google Scholar]
- 107. Nonaka M, Nonaka R, Woolley K, et al. : Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J Immunol. 1995; 155(6): 3234–44. [PubMed] [Google Scholar]
- 108. Möller GM, de Jong TA, van der Kwast TH, et al. : Immunolocalization of interleukin-4 in eosinophils in the bronchial mucosa of atopic asthmatics. Am J Respir Cell Mol Biol. 1996; 14(5): 439–43. 10.1165/ajrcmb.14.5.8624248 [DOI] [PubMed] [Google Scholar]
- 109. Nakajima H, Gleich GJ, Kita H: Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J Immunol. 1996; 156(12): 4859–66. [PubMed] [Google Scholar]
- 110. Davoine F, Lacy P: Eosinophil cytokines, chemokines, and growth factors: Emerging roles in immunity. Front Immunol. 2014; 5: 570. 10.3389/fimmu.2014.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hepworth MR, Monticelli LA, Fung TC, et al. : Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013; 498(7542): 113–7. 10.1038/nature12240 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Oliphant CJ, Hwang YY, Walker JA, et al. : MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014; 41(2): 283–95. 10.1016/j.immuni.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 113. Park H, Li Z, Yang XO, et al. : A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005; 6(11): 1133–41. 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 114. Harrington LE, Hatton RD, Mangan PR, et al. : Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005; 6(11): 1123–32. 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 115. Bettelli E, Carrier Y, Gao W, et al. : Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441(7090): 235–8. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 116. Langrish CL, Chen Y, Blumenschein WM, et al. : IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201(2): 233–40. 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 117. Mangan PR, Harrington LE, O'Quinn DB, et al. : Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006; 441(7090): 231–4. 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 118. Veldhoen M, Hocking RJ, Atkins CJ, et al. : TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006; 24(2): 179–89. 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 119. Ivanov II, McKenzie BS, Zhou L, et al. : The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006; 126(6): 1121–33. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 120. Lazarevic V, Chen X, Shim JH, et al. : T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011; 12(1): 96–104. 10.1038/ni.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Omenetti S, Bussi C, Metidji A, et al. : The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity. 2019; 51(1): 77–89.e6. 10.1016/j.immuni.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 122. Ivanov II, Atarashi K, Manel N, et al. : Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009; 139(3): 485–98. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 123. Kleinewietfeld M, Manzel A, Titze J, et al. : Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013; 496(7446): 518–22. 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 124. Wang C, Yosef N, Gaublomme J, et al. : CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell. 2015; 163(6): 1413–27. 10.1016/j.cell.2015.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 125. Wilck N, Matus MG, Kearney SM, et al. : Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017; 551(7682): 585–9. 10.1038/nature24628 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 126. Amir M, Chaudhari S, Wang R: REV-ERBα Regulates TH17 Cell Development and Autoimmunity. Cell Rep. 2018; 25(13): 3733–3749.e8. 10.1016/j.celrep.2018.11.101 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 127. Bacher P, Hohnstein T, Beerbaum E, et al. : Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell. 2019; 176(6): 1340–1355.e15. 10.1016/j.cell.2019.01.041 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 128. Britton GJ, Contijoch EJ, Mogno I, et al. : Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt+ Regulatory T Cells and Exacerbate Colitis in Mice. Immunity. 2019; 50(1): 212–224.e4. 10.1016/j.immuni.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 129. Hirota K, Duarte JH, Veldhoen M, et al. : Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011; 12(3): 255–63. 10.1038/ni.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 130. Wang Y, Godec J, Ben-Aissa K, et al. : The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity. 2014; 40(3): 355–66. 10.1016/j.immuni.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morrison PJ, Bending D, Fouser LA, et al. : Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal Immunol. 2013; 6(6): 1143–56. 10.1038/mi.2013.11 [DOI] [PubMed] [Google Scholar]
- 132. Ahlfors H, Morrison PJ, Duarte JH, et al. : IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol. 2014; 193(9): 4602–13. 10.4049/jimmunol.1401244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Harbour SN, Maynard CL, Zindl CL, et al. : Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A. 2015; 112(22): 7061–6. 10.1073/pnas.1415675112 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 134. Jain R, Chen Y, Kanno Y, et al. : Interleukin-23-Induced Transcription Factor Blimp-1 Promotes Pathogenicity of T Helper 17 Cells. Immunity. 2016; 44(1): 131–42. 10.1016/j.immuni.2015.11.009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 135. Wang YH, Voo KS, Liu B, et al. : A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010; 207(11): 2479–91. 10.1084/jem.20101376 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 136. Irvin C, Zafar I, Good J, et al. : Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014; 134(5): 1175–1186.e7. 10.1016/j.jaci.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tortola L, Jacobs A, Pohlmeier L, et al. : High-Dimensional T Helper Cell Profiling Reveals a Broad Diversity of Stably Committed Effector States and Uncovers Interlineage Relationships. Immunity. 2020; 53(3): 597–613.e6. 10.1016/j.immuni.2020.07.001 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 138. Tibbitt CA, Stark JM, Martens L, et al. : Single-Cell RNA Sequencing of the T Helper Cell Response to House Dust Mites Defines a Distinct Gene Expression Signature in Airway Th2 Cells. Immunity. 2019; 51(1): 169–184.e5. 10.1016/j.immuni.2019.05.014 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation