Abstract
Objective:
The Menopause Strategies: Finding Lasting Answers for Symptoms and Health clinical trials network was funded by the National Institutes of Health to find new ways to alleviate the most common, bothersome menopausal symptoms by designing and conducting multiple concurrent clinical intervention studies, accommodating a wide scope of populations and intervention strategies.
Methods:
Trials were conducted in Boston, Indianapolis, Minneapolis, Oakland, Philadelphia, and Seattle, with the Data Coordinating Center in Seattle, and were designed with standardized eligibility criteria and endpoints. Primary outcomes focused on vasomotor symptoms, sleep quality and insomnia symptoms, and vaginal symptoms. Secondary outcomes included quality of life, sexual function, and mood.
Results:
We completed five randomized clinical trials and three ancillary studies, testing nine interventions in over 1,300 women and collecting nearly 16,000 bio-specimens. Escitalopram, venlafaxine XR, and low dose estradiol diminished hot flashes by approximately 50% as compared with a 30% decrease by placebo. No benefits on vasomotor symptoms were observed with yoga or exercise compared to usual activity, nor with omega-3 supplementation compared to placebo. Cognitive behavioral therapy for insomnia reduced self-reported insomnia symptoms and improved overall sleep quality compared to menopause education control. We did not find significant benefit from a vaginal estradiol tablet or a vaginal moisturizer compared to placebo tablet and gel in diminishing the severity of vaginal symptoms.
Conclusions:
The MsFLASH trials contributed substantially to our understanding of bothersome menopausal symptom treatment. It is important that clinicians counseling women about available treatment options consider all therapies – both non-hormonal and hormonal.
Keywords: MsFLASH, menopause, RCT, review, hot flashes
INTRODUCTION
The Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) clinical trials network was funded by the National Institutes of Health (NIH) in 2008 with renewal in 2015. The original Request for Application (RFA) to establish a clinical trials network was spearheaded by Sherry Sherman, PhD,1 project officer at the National Institutes on Aging (NIA), following an NIH State of the Science Conference on management of menopause symptoms. The conference was held in the wake of the Women’s Health Initiative trial results which discouraged ubiquitous hormone therapy (HT) use for managing menopause and promoting healthy aging, and after Study of Women’s health Across the Nation (SWAN) results provided new data on the natural history of menopause in various race/ethnicity groups. Menopause experts were tasked with providing recommendations to NIH to expedite prospective studies on menopausal symptom treatments and thus provide women with better evidence upon which to make choices. The RFA issued by the National Institutes of Aging (NIA), National Institute of Child Health and Human Development (NICHD), National Center for Complementary and Alternative Medicine (NCCAM), and Office of Research on Women’s Health (ORWH) invited applications “to establish a Menopausal Symptoms Clinical Research Network to facilitate clinical intervention studies targeting bothersome vasomotor symptoms (VMS) and related sleep disturbance, mood disorders and vaginal dryness in a collaborative, multidisciplinary, multicenter setting”. The stated goal of the MsFLASH Network, in keeping with guidelines from the RFA, was to find new ways to alleviate the most common, bothersome symptoms of the menopausal transition by designing and conducting multiple concurrent clinical intervention studies, accommodating a wide scope of populations and intervention strategies.
To that end, MsFLASH investigators have completed five randomized clinical trials (RCTs) and three ancillary studies testing nine interventions in over 1,300 women. MsFLASH studies collected nearly 16,000 bio-specimens including blood samples, salivary, vaginal, and rectal swabs, vaginal biopsies, and vaginal specimens for vaginal maturation indices, and Nugent’s scores. The trials were designed with standard eligibility criteria so that pooled analyses were possible for major outcomes, such as VMS,2 sleep disturbances,3 sexual function, and quality of life. All results presented in this manuscript have been published previously. MsFLASH has provided evidence-based information, free of commercial bias (federally funded by NIH), for patients and clinicians about the efficacy and safety of treatments for menopausal symptoms. Novel aspects of MsFLASH trials included a focus on non-hormonal interventions, in addition to parallel placebo-controlled evaluations of non-hormonal pharmacologic therapies with gold-standard hormonal therapies.4, 5 Current MsFLASH activities aim to translate trial findings into multi-media translational resources (web and mobile apps) for patients and providers with the goal to educate women about menopause and to assist individual women in choosing optimal menopause therapy based on their symptoms, their treatment goals and their health concerns. A summary of findings and lessons learned-to-date follows.
METHODS
Study designs, interventions, control groups, primary outcomes, and study durations are shown in Table 1; methods for all trials have been reported extensively elsewhere.4–12 VMS interventions, over 8–12 weeks, included escitalopram 10–20 mg daily, omega-3 supplement 1.8 mg daily, exercise (individual, facility-based, aerobic training three times per week), yoga (weekly 90-minute classes and 20-minute daily home practices four times per week), venlafaxine XR 75 mg daily, and oral 17β-estradiol 0.5 mg daily. In addition, we evaluated telephone-based cognitive behavioral therapy for insomnia (CBT-I: six individual weekly sessions focused on sleep restriction, stimulus control, sleep hygiene, cognitive restructuring, and behavioral homework) and estradiol 10 mcg vaginal tablet (nightly for two weeks, then two times per week) and a vaginal moisturizer (three times per week) to treat vulvovaginal symptoms. Outcomes for each intervention were compared to those in a control arm, either an identical appearing placebo (oral pill, vaginal tablet, or vaginal gel), usual activity for the exercise and yoga interventions in the VMS trials (asked to not to start a new yoga or exercise practice, then offered a yoga workshop or a one-month gym membership after the 12-week intervention period), or telephone-based menopause education as an attention control for the cognitive behavioral therapy for insomnia.
Table 1.
Summary of MsFLASH RCT Study Designs
| Trial | Sample Size | Design | Primary Outcome | Intervention Length | Primary Results References |
|---|---|---|---|---|---|
| 01 | 205 | 2-arm: Escitalopram vs. placebo tablet | Frequency and severity of hot flashes | 8 weeks | Freeman EW, Guthrie KA, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305(3):267–274. |
| 02 | 355 | 3×2 factorial: Aerobic exercise and yoga vs. usual activity, plus omega-3 supplementation vs. placebo capsule | Frequency and bother of hot flashes | 12 weeks | Sternfeld BS, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014;21(4):330–338. Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of Omega-3 treatment for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21(4):347–354. Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21(4):339–346. |
| 03 | 339 | 3-arm: Low dose oral estradiol and venlafaxine vs. placebo, all in identical capsules | Frequency of hot flashes | 8 weeks | Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Int Med 2014;174(7):1058–1066. |
| 04 | 106 | 2-arm: Telephone cognitive behavior therapy for insomnia vs. menopause education control | Insomnia Severity Index | 8 weeks | McCurry S, Guthrie KA, Morin DM, et al. Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial. JAMA Intern Med 2016;176(7):913–920. |
| 05 | 302 | 3-arm: Vaginal estradiol+placebo gel and vaginal moisturizer+placebo tablet, vs. placebo gel+placebo tablet | Severity of most bothersome vulvovaginal symptom | 8 weeks | Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med 2018;178(5):681–690. |
Unique aspects of the trials increased their scientific value. The first trial recruited equal numbers of White and Black women to evaluate whether escitalopram’s efficacy varied by race.8 In addition, all women were given placebo pills for three weeks after completing eight weeks of treatment to allow assessment of intervention durability. The second trial efficiently tested yoga, exercise, and omega-3 supplements in a 3×2 factorial design that simultaneously randomized women to one of three behavioral arms and one of two oral tablet arms.9, 11 The third trial evaluated comparative efficacy of a serotonin and norepinephrine reuptake inhibitor (SNRI) and oral low dose estradiol, each compared to placebo4; no prior RCTs existed with side-by-side comparisons. The fourth trial evaluated a unique telephone-based CBT-I for menopausal sleep disturbances.12 The fifth trial evaluated an over-the-counter (OTC) product side by side with FDA-approved vaginal estrogen, with each compared to placebo, and collected an extensive biobank of specimens to evaluate possible pathophysiologic mechanisms underlying postmenopausal vaginal symptoms.5
Common MsFLASH eligibility criteria and common data collection instruments are shown in Supplementary Digital Content A, with trial-specific exclusions shown in Supplementary Digital Content B. All eligibility criteria, measures, and exclusions are published in detail elsewhere.4–12 Details for implementation of telephone-based CBT-I are shown in Supplementary Digital Content C. Women were recruited primarily via mass mailings to age-appropriate women, with targeted zip codes for women of color, but we also piloted Facebook recruitment in the fifth trial.13 MsFLASH investigators conducted focus groups prior to design of recruitment materials for the Vaginal Health Trial (05).
Primary outcomes focused on vasomotor symptoms (VMS) (hot flashes and night sweats), sleep quality, insomnia symptoms, and vaginal symptoms. Secondary outcomes included quality of life, sexual function, and mood (symptoms of depression and anxiety). Pilot studies provided clues to mechanistic processes that may underlie menopausal symptoms and their treatment including changes in cortisol, heart rate variability, and Kisspeptin/Neurokinin B/Dynorphin (KNDy) neuron activation, and perturbations in the postmenopausal vaginal ecosystem.
The designs and implementation of all five trials adhered to key RCT principles for rigorous randomized controlled trial principles to attain accurate, comprehensive, and meaningful assessments of each intervention. These included randomization to treatment arms and double-blinding in all cases except the behavioral intervention arm in the second trial. Trial design, including eligibility screening and follow-up timing, and investigator and staff training reinforced the goals of high adherence and retention to support robust estimates of efficacy. Sample size assumptions, such as statistical power, type I error levels, and effect sizes, were chosen to provide definitive evidence for clinically meaningful differences. Given preliminary or established data regarding the efficacy of the interventions tested, we assumed trial parameters appropriate for confirmatory trials: 90% statistical power and 2-sided 5% type I error. In trials with two primary comparisons (either co-primary outcomes or two active groups compared to a placebo group), we applied a 2-sided 2.5% type I error. An effect size of approximately 0.5 standard deviation was chosen for most trials; for the primary outcome of VMS frequency, this assumption translated to a difference between a 30% mean decrease with placebo and a 50% mean decrease with active treatment. A 5% significance level threshold was applied for analyses of secondary outcomes.
The MsFLASH Network follows a data-sharing plan as required by the NIH. Datasets and specimens may be obtained under a Data and Materials Use Agreement by contacting Dr. Katherine Guthrie at the DCC (kguthrie@fredhutch.org).
FINDINGS AND DISCUSSION
Participant characteristics by trial are described in Table 2. All trials maintained excellent participant study retention (98% in trial 01; 97% in trials 02, 03, and 05; and 83% in trial 04, our only telephone-only trial).4, 5, 8–12 We summarize our findings below, first by efficacy of the interventions to improve symptoms, followed by details on recruitment success, placebo effects, studies to validate measures used, and finally by pilot and mechanistic studies.
Table 2.
MsFLASH Participant Characteristics by Trial
| MsFLASH 01 (n=205) | MsFLASH 02 (n=355) | MsFLASH 03 (n=339) | MsFLASH 04 (n=110) | MsFLASH 05 (n=302) | P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristic | n | % | n | % | n | % | n | % | n | % | |
| Age at screening, mean (SD) | 53.9 | (4.1) | 54.7 | (3.7) | 54.6 | (3.8) | 54.7 | (4.2) | 60.9 | (4.1) | <0.001 |
| < 50 | 24 | 11.7 | 19 | 5.4 | 30 | 8.8 | 9 | 8.2 | 0 | 0.0 | |
| 50–54 | 95 | 46.3 | 162 | 45.6 | 147 | 43.4 | 46 | 41.8 | 4 | 1.3 | |
| 55–59 | 66 | 32.2 | 130 | 36.6 | 123 | 36.3 | 41 | 37.3 | 124 | 41.1 | |
| ≥ 60 | 20 | 9.8 | 44 | 12.4 | 39 | 11.5 | 14 | 12.7 | 174 | 57.6 | |
| Race | <0.001 | ||||||||||
| Black | 95 | 46.3 | 93 | 26.2 | 116 | 34.2 | 1 | 0.9 | 12 | 4.0 | |
| White | 102 | 49.8 | 228 | 64.2 | 203 | 59.9 | 101 | 91.8 | 267 | 88.4 | |
| Other | 8 | 3.9 | 34 | 9.6 | 20 | 5.9 | 8 | 7.3 | 23 | 7.6 | |
| Hispanic | 0 | 0.0 | 5 | 1.4 | 1 | 0.3 | 3 | 2.7 | 1 | 0.3 | |
| American Indian | 1 | 0.5 | 8 | 2.3 | 2 | 0.6 | 1 | 0.9 | 6 | 2.0 | |
| Asian / Pacific Islander | 3 | 1.5 | 12 | 3.4 | 5 | 1.5 | 0 | 0.0 | 12 | 4.0 | |
| Undisclosed | 4 | 2.0 | 9 | 2.5 | 12 | 3.5 | 4 | 3.6 | 4 | 1.3 | |
| Education | <0.001 | ||||||||||
| ≤ High school diploma/GED | 38 | 18.5 | 21 | 5.9 | 55 | 16.2 | 5 | 4.5 | 11 | 3.6 | |
| Post-high school | 87 | 42.4 | 112 | 31.5 | 111 | 32.7 | 20 | 18.2 | 89 | 29.5 | |
| College graduate | 80 | 39.0 | 221 | 62.3 | 172 | 50.7 | 85 | 77.3 | 200 | 66.2 | |
| Smoking | <0.001 | ||||||||||
| Never | 99 | 48.3 | 232 | 65.4 | 174 | 51.3 | 85 | 77.3 | 199 | 65.9 | |
| Past | 59 | 28.8 | 89 | 25.1 | 107 | 31.6 | 24 | 21.8 | 96 | 31.8 | |
| Current | 47 | 22.9 | 32 | 9.0 | 55 | 16.2 | 1 | 0.9 | 6 | 2.0 | |
| BMI (m/kg2), mean (SD) | 29.1 | (6.5) | 27.0 | (4.4) | 28.3 | (6.8) | 24.9 | (5.0) | 26.4 | (5.3) | |
| < 25 | 54 | 26.3 | 123 | 34.6 | 118 | 34.8 | 71 | 64.5 | 134 | 44.4 | |
| 25 ≤ 30 | 72 | 35.1 | 144 | 40.6 | 107 | 31.6 | 21 | 19.1 | 106 | 35.1 | |
| ≥ 30 | 78 | 38.0 | 88 | 24.8 | 107 | 31.6 | 18 | 16.4 | 57 | 18.9 | |
|
Menopause status |
<0.001 | ||||||||||
| Postmenopausal | 142 | 69.3 | 266 | 74.9 | 256 | 75.5 | 70 | 63.6 | 302 | 100.0 | |
| Perimenopausal | 41 | 20.0 | 65 | 18.3 | 52 | 15.3 | 32 | 29.1 | 0 | 0.0 | |
| Indeterminate | 22 | 10.7 | 24 | 6.8 | 31 | 9.1 | 8 | 7.3 | 0 | 0.0 | |
| Site | <0.001 | ||||||||||
| Boston | 43 | 21.0 | 0 | 0.0 | 100 | 29.5 | 0 | 0.0 | 0 | 0.0 | |
| Indianapolis | 35 | 17.1 | 118 | 33.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Minneapolis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 145 | 48.0 | |
| Oakland | 57 | 27.8 | 110 | 31.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Philadelphia | 70 | 34.1 | 0 | 0.0 | 121 | 35.7 | 0 | 0.0 | 0 | 0.0 | |
| Seattle | 0 | 0.0 | 127 | 35.8 | 118 | 34.8 | 110 | 100.0 | 157 | 52.0 | |
| Mood | |||||||||||
| PHQ depressionb ≥ 10 | 12 | 5.9 | 29 | 8.2 | 29 | 8.6 | 32 | 29.1 | 18 | 6.0 | <0.001 |
| GAD-7 Anxiety ≥ 10 | 9 | 4.4 | 28 | 7.9 | 23 | 6.8 | 13 | 11.8 | 28 | 9.3 | 0.22 |
| MENQOL Total, mean (SD) | 3.8 | (1.3) | 3.8 | (1.2) | 3.6 | (1.1) | 3.9 | (1.2) | 3.3 | (1.1) | <0.001 |
| Sleep | |||||||||||
| ISI, mean (SD) | 11.4 | (6.3) | 11.9 | (5.4) | 11.0 | (6.0) | 16.2 | (3.5) | 7.5 | (5.2) | <0.001 |
| PSQI, mean (SD) | 8.0 | (3.7) | 8.0 | (3.3) | 7.5 | (3.4) | 9.1 | (2.8) | - | - | <0.001 |
| VMS | |||||||||||
| Frequency, mean (SD) | 9.8 | (5.6) | 7.6 | (3.8) | 8.1 | (5.3) | 7.5 | (4.2) | - | - | <0.001 |
| Severity (1–3), mean (SD) | 2.2 | (0.5) | 2.0 | (0.4) | 2.0 | (0.5) | 1.8 | (0.4) | - | - | <0.001 |
SD = standard deviation; BMI = body mass index; PHQ = Patient Health Questionnaire; GAD = Generalized Anxiety Disorder; MENQOL = Menopause-Specific Quality of Life; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index; VMS = vasomotor symptoms.
Homogeneity across trials assessed via chi-squared or F-test, as appropriate. For race, assessment based on collapsed race categories (Black, White, and Other).
PHQ depression scores based on PHQ-9 for MsFLASH 01 and 03 and PHQ-8 in MsFLASH 02 and 04, but only the first 8 items were included in these scores.
Efficacy of Interventions by Menopausal Symptom
Estimated effect sizes are provided for primary outcomes (VMS, sleep, vaginal symptoms), but only qualitatively described for secondary outcomes (sexual function, quality of life, mood, and pain). Details on secondary outcomes are published elsewhere.7
Vasomotor symptoms
The MsFLASH Network evaluated escitalopram, yoga, exercise, omega-3 supplements, oral low-dose estradiol, and venlafaxine for changes in the frequency, severity, and bother of hot flashes and night sweats (Figure 1). Hot flash interference was evaluated for these interventions as well as for CBT-I. These studies provided new information regarding effectiveness of escitalopram, yoga, exercise, and omega-3 supplements, and an opportunity for side-by-side comparisons of the SNRI, venlafaxine XR, and estrogen, each relative to placebo.
Figure 1.
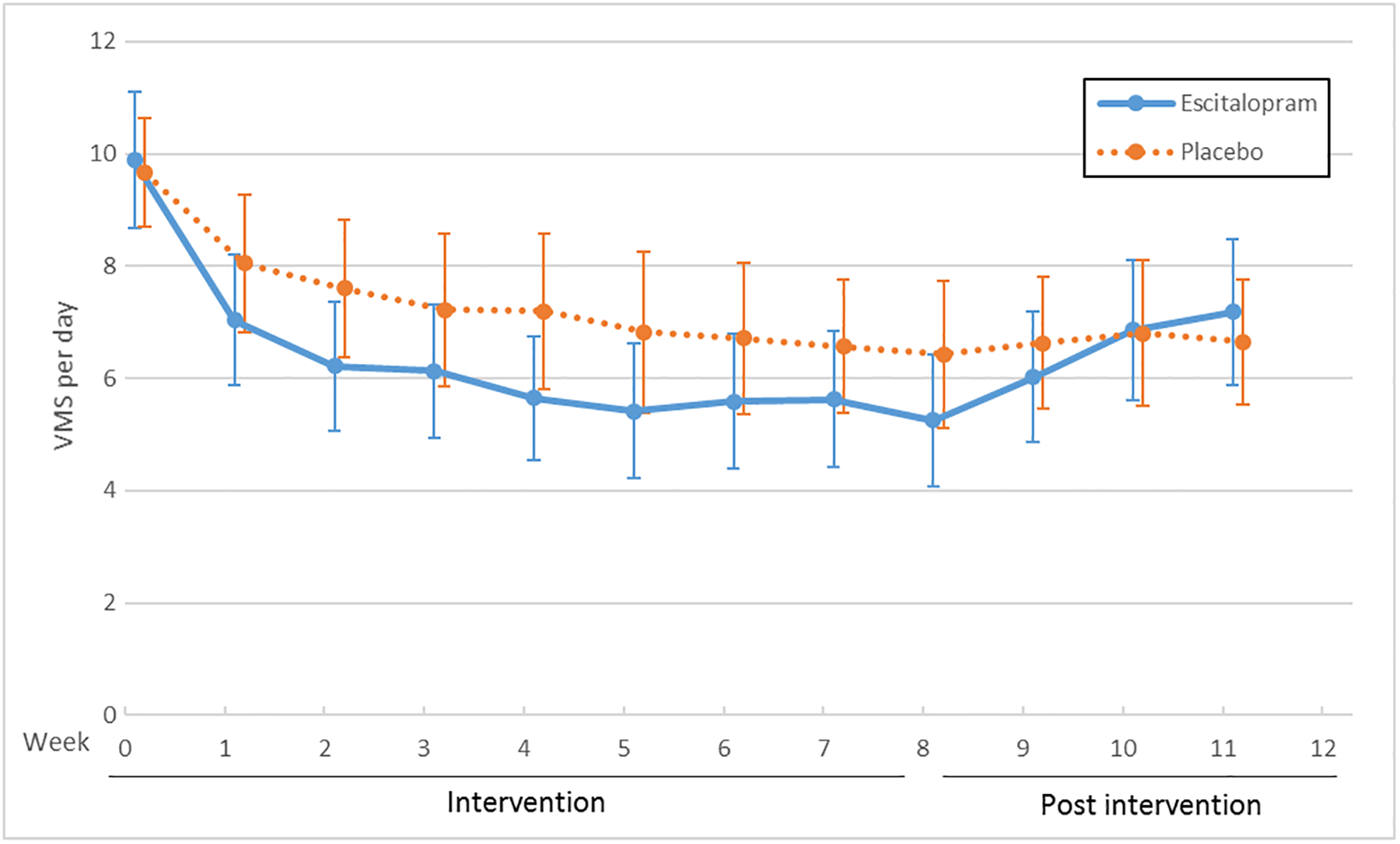
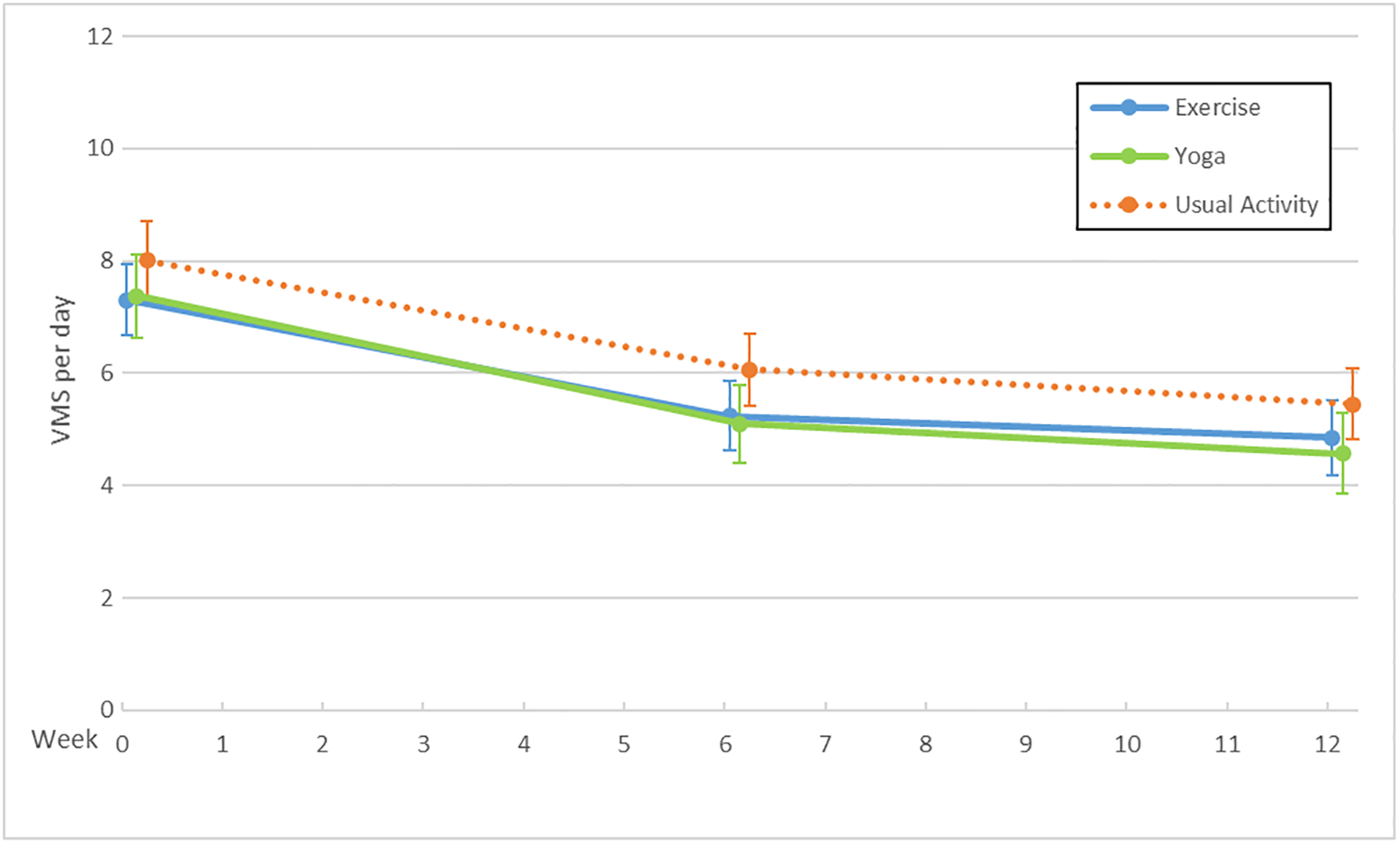
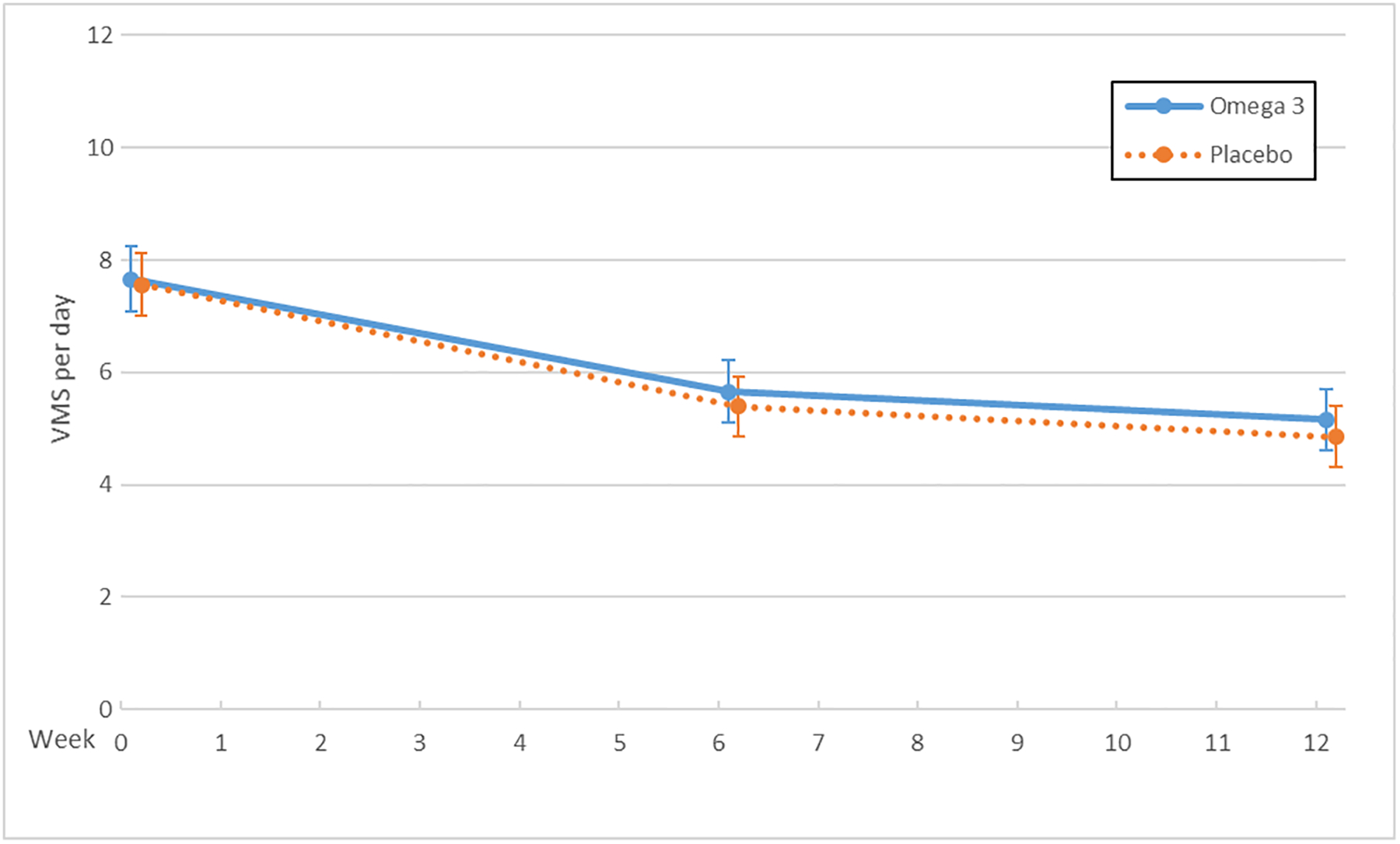
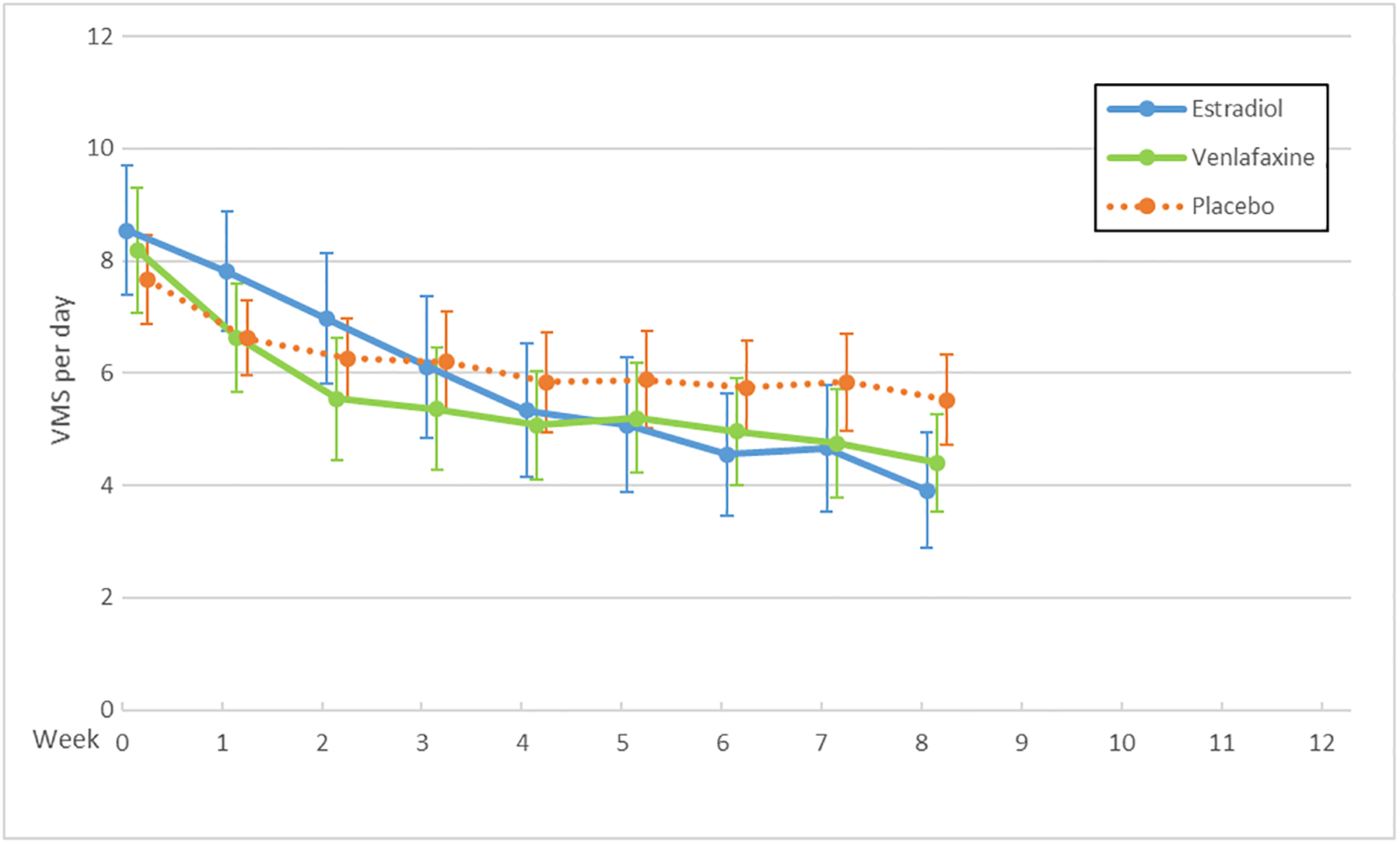
Vasomotor Symptoms (VMS) by Trial and Treatment Arm: Escitalopram (A), Exercise and Yoga (B), Omega-3 Supplements (C), Venlafaxine and Estradiol (D)
X axis: Time in weeks
Y axis: Vasomotor symptom number per day
The serotonergic formulations and low-dose oral estradiol showed benefit over placebo, each providing an approximate 50–60% decrease in mean daily hot flash frequency over eight weeks and placebo providing a decrease of approximately 30%. For escitalopram vs. placebo, the mean difference in hot flash frequency reduction was 1.41 (95% CI: 0.13, 2.69; P<0.001) fewer hot flashes per day.8 For venlafaxine vs. placebo, the mean difference in hot flash frequency reduction was 1.8 (95% CI: 0.8, 2.7; P=0.005) fewer hot flashes per day. For estradiol vs. placebo, the mean difference in hot flash frequency reduction was 2.3 (95% CI: 1.3, 3.4; P<0.001) fewer hot flashes per day.4 Thus, these pharmacologic therapies provided a benefit of reduced hot flash frequency by 1.4–2.3 hot flashes per day.
No benefits on vasomotor symptoms were observed over twelve weeks with the yoga or exercise interventions compared to usual activity, nor with omega-3 supplementation compared to placebo. Compared to the usual activity arm, the mean difference in hot flash frequency reduction was 0.3 (95% CI: −0.6, 1.2; P=0.12) fewer hot flashes in the yoga group and 0.2 (95% CI: −1.1, 0.6; P=0.43) in the exercise group.9, 11 Mean hot flash frequency decreased slightly more in the placebo arm than in the omega-3 supplement arm, giving a mean difference of −0.3 (95% CI: −1.0, 0.5; P=0.28).10
In a pooled analysis of individual-level data from MsFLASH participants enrolled in the first three trials, the 8-week reduction in hot flash frequency from baseline relative to placebo was similar for escitalopram, venlafaxine XR, and estradiol.2 All three of these interventions reached the threshold for minimal clinically important reduction in hot flashes reported by women at approximately 50%.14 In addition, women reported significantly less hot flash interference with escitalopram,15 venlafaxine,4 estradiol,4 and CBT-I.12
Sleep disturbances
Our fourth trial concentrated on the primary outcome of menopausal sleep disturbance and tested a telephone-based CBT-I (Supplementary Digital Content C) that targeted insomnia symptoms (ISI) and subjective sleep quality (PSQI) with highly positive results that were sustained at 24 weeks. The ISI measures self-reported nocturnal and diurnal symptoms of insomnia (e.g., difficulty initiating and staying asleep, impairments attributed to poor sleep) whereas the PSQI measures self-reported sleep quality and sleep disturbances (e.g., duration, use of sleep medication, and daytime dysfunction) Cognitive behavioral therapy has been shown to improve sleep disorders in the general population but had not been studied specifically for menopausal sleep disturbances. From baseline to eight weeks, insomnia severity on the ISI (scale 0–28) decreased 9.9 points in women receiving CBT-I and 4.7 points in women in the menopause education control group, for a mean between-group difference of −5.2 (95% CI: −6.1, −3.3; P<0.001).12 Sleep quality measured with the PSQI (scale 0–21) improved from baseline to eight weeks by 4.0 points in women receiving CBT-I and 1.4 points in women in the control group, for a mean between-group difference of −2.7 (95% CI: −3.9, −1.5; P<0.001).
Insomnia symptoms and subjective sleep quality were assessed as secondary outcomes in the first three MsFLASH trials, providing unique information about the effects on sleep of escitalopram, yoga, exercise, omega-3 supplements, low-dose oral estradiol, and venlafaxine in women with hot flashes. Moderate but significant reductions in insomnia symptoms were seen with escitalopram compared to placebo, exercise and yoga compared to usual activity, and venlafaxine compared to placebo.9, 11, 16, 17 Improvements in sleep quality were significantly better than control with escitalopram compared to placebo, exercise compared to usual activity, and estradiol compared to placebo.11, 16, 17
In a pooled analysis of individual-level data from all MsFLASH participants with baseline ISI ≥ 12 from the first four trials (Figure 2), CBT-I was the most effective of all MsFLASH interventions at reducing insomnia symptoms (ISI) and improving sleep quality (PSQI).3 A clinically significant improvement in sleep disturbances at menopause has not been established. In other populations with sleep disorders, others have suggested a 6-point score reduction in the ISI18 and a 3-point score reduction in PSQI19 as clinically meaningful. In MsFLASH 04, CBT-I ISI scores dropped nearly ten points, but the between-group difference was 5.2 points, which although statistically significant, doesn’t reach the 6-point threshold.18 Similarly, PSQI scores dropped four points in CBT-I, but the between-group difference was 2.7, just below the 3-point threshold.19
Figure 2.
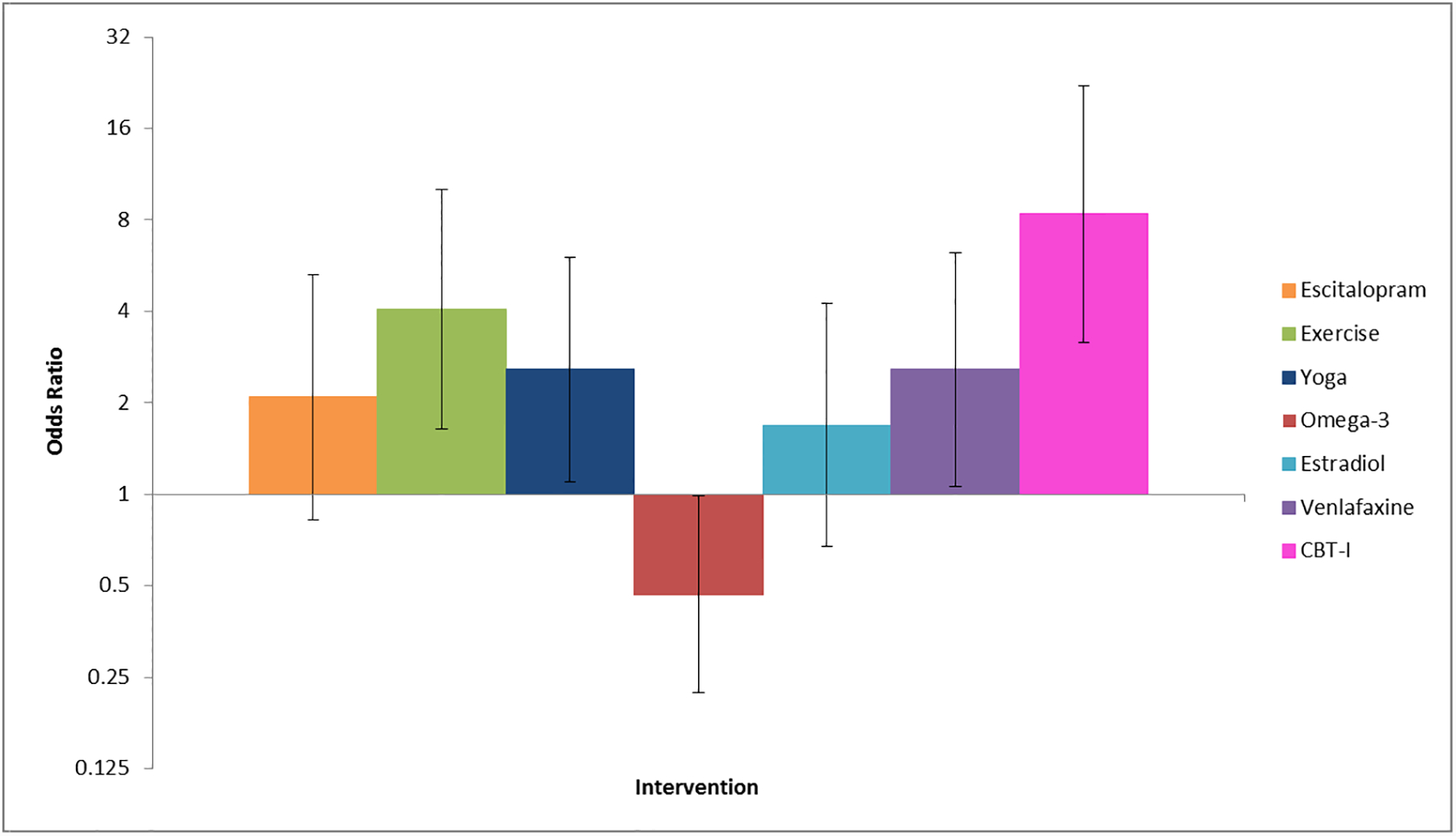
Odds of Insomnia Symptom Remission (ISI < 8) by Intervention Relative to Control in a Pooled Trial Analysis3
X axis: Interventions left to right: Escitalopram 10–20 mg, Exercise, Yoga, Omega 3, Oral estradiol 0.5 mg Venlafaxine 75 mg, Cognitive Behavioral Therapy for Insomnia
Y axis: Odds of intervention improving or worsening sleep as compared with control
Vaginal symptoms
Bothersome vaginal symptoms are observed in 40–70% of midlife women and are particularly bothersome in late menopause.5 The primary outcome of our fifth RCT was bothersome vaginal symptoms; we evaluated an FDA-approved 10 mcg vaginal estradiol tablet and an OTC vaginal moisturizer, compared to placebo tablet and gel.
The MsFLASH Vaginal Health Trial did not find significant benefit from use of a 10 mcg vaginal estradiol tablet or a vaginal moisturizer compared to placebo tablet and gel in diminishing the severity of vaginal symptoms (Figure 3).5 In women who reported moderate to severe symptoms of vaginal itching, dryness, irritation or pain with sexual activity at baseline, all treatment groups had similar mean reductions in the severity of their most bothersome symptom (scale 0–3) over twelve weeks: estradiol −1.4 (95% CI: −1.6, −1.2), moisturizer −1.2 (95% CI: −1.4, −1.0), and placebo −1.3 (95% CI: −1.5, −1.1; P=0.25 estradiol vs. placebo, P=0.31 moisturizer vs. placebo). The trial was designed with power to detect an effect size of 0.5 SD, which translates to a difference in decrease from baseline between active and placebo of 0.5 point. The clinical significance of smaller effect sizes observed in larger trials20, 21 has not been established.
Figure 3.
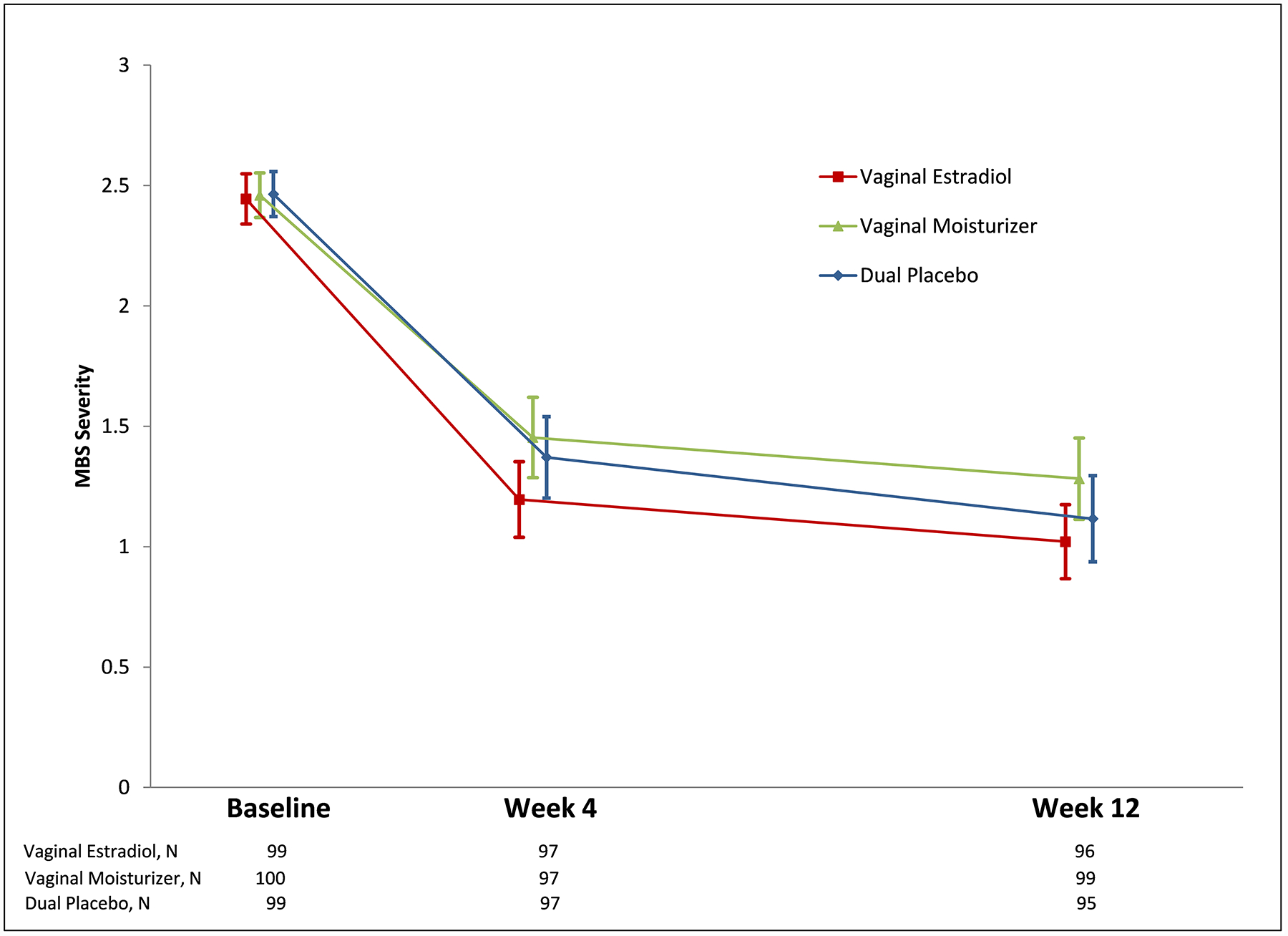
Change in Severity of the Most Bothersome Vaginal Symptom (MBS) at 4 and 12 Weeks
X axis: Time in weeks and number of women at each time point in each randomization group
Y axis: Most bothersome vaginal symptom severity score (scale 0–3)
Sexual function
All MsFLASH trials collected data on sexual function as secondary outcomes (Supplementary Digital Content A). There was no overall benefit or harm of treatment with escitalopram, venlafaxine, oral or vaginal estradiol on sexual function (total Female Sexual Functioning Index, FSFI), but minor effects on FSFI domains should be mentioned. Treatment with escitalopram was associated with small reduced lubrication relative to placebo among women who were sexually active women at baseline.22 Women taking venlafaxine XR showed slight diminished orgasm but also decreased pain relative to placebo. Women taking oral low-dose estradiol had slight improved desire relative to placebo.23 Differences from placebo in these domains were small and clinical significance has not been established.
Quality of life
The MsFLASH Network included quality of life as measured by the Menopause-Specific Quality of Life Questionnaire (MENQOL) as a secondary outcome in all trials. Overall, we saw improvements in the MENQOL that were significantly greater than placebo, but small in magnitude, for escitalopram, yoga, venlafaxine, low-dose oral estradiol, and vaginal estradiol.
Treatment with escitalopram resulted in significantly greater improvement in total MENQOL scores relative to placebo, as well as in the vasomotor, psychosocial, and physical domain scores, with the largest difference seen in the vasomotor domain.24 Yoga improved the total MENQOL score relative to usual activity control, driven by improvements in the vasomotor and sexual domain scores. Exercise did show benefit in the MENQOL physical domain score at 12-weeks compared to control, but not in the total score.25 Both low-dose estradiol and venlafaxine were efficacious pharmacologic agents for improving menopause-related quality of life in healthy women with vasomotor symptoms, based on total MENQOL scores.26 Treatment with vaginal estradiol 10 mcg tablet resulted in significantly greater improvement from baseline to week 12 in total MENQOL scores compared to dual placebo, with improvement in the sexual domain.27 Treatment with vaginal moisturizer did not provide improvement compared to placebo in total MENQOL scores. The clinical significance of the small but statistically significant changes in total MENQOL scores in our studies compared with placebo (0.2 for venlafaxine, 0.3 for yoga, and 0.4 for oral low dose estradiol, escitalopram, and vaginal estradiol)24–27 remains uncertain. A change of 1 point has been proposed as a significant difference to use in sample size calculations.28, 29
Mood
Anxiety (Generalized Anxiety Disorder 7-item scale, GAD-7) and depressive symptoms (Patient Health Questionnaire depression scale, PHQ-8 or PHQ-9) were measured in all trials. At baseline, approximately 13% of women had moderate anxiety or depressive symptoms (Table 2). A moderate but significant reduction in depressive symptoms was seen with exercise compared to usual activity.11 Otherwise, we found no effects of escitalopram, yoga, omega-3, estradiol, and venlafaxine relative to control on symptoms of anxiety or depression.8–10, 26 Given the low baseline prevalence and the numbers of women in our trials, one would not expect significant changes.
Pain and perceived stress
Pain (Pain, Enjoyment of life, General Activity scale, PEG) and perceived stress (Perceived Stress Scale, PSS) were evaluated in most of the trials (Supplementary Digital Content A). The PEG showed improvement with escitalopram treatment compared to placebo, and the PSS showed improvement with venlafaxine compared to placebo.24, 26
Additional Secondary Findings
Recruitment success
Mass mailings to age-appropriate women allowed us to reach out to the general population, making our findings relevant to most healthy, midlife women rather than specialized populations. Conventional recruitment for menopausal trials has been via specialty clinics. Targeted mailings to specific zip codes provided successful recruitment for women of color.4, 8 Facebook recruitment for our Vaginal Health Trial was equally as successful as mass mailing, but we found that response rates to Facebook advertising varied by region with higher response rates in the Pacific Northwest as compared to the Midwest.13
Placebo effect
As expected in RCTs with subjective endpoints, symptom improvement was observed in all control arms. The placebo effects for vasomotor symptom frequency included a 33% decrease at eight weeks in the escitalopram trial, a 36% decrease (supplement placebo arm) and a 33% decrease (usual activity arm) at twelve weeks in the omega-3, exercise, and yoga trial, and a 29% decrease at eight weeks in the estradiol and venlafaxine trial.4, 8, 9–11, 30 In all trials, clinically relevant improvements with placebo accrued throughout the treatment period with a time course similar to the observed improvements in the active arm.
Placebo effects on insomnia symptoms and sleep quality were equally pronounced for behavioral and medical interventions. In the CBT-I trial telephone-delivered menopause education attention control arm, mean improvements were 40% in the ISI and 29% in the PSQI.12 The ISI placebo responses were decreases of 24% and 29% in the SSRI/SNRI trials and decreases of 31% in the placebo supplement arm and 25% in the usual activity control group in the omega-3, exercise, and yoga trial.9–11, 16, 17
Vaginal symptom improvement in our fifth trial’s placebo arm was larger than observed in other trials for vaginal symptom therapies (Figure 4). Our design was unique in providing a double-placebo, including an inert tablet matching the active estradiol tablet and an inert gel matching the active vaginal moisturizer. The placebo hydroxyethylcellulose gel was designed to maintain vaginal ecosystem stasis, but also had excellent lubricity, an acidic pH, and had neutral osmolality (neither hypo- or hyperosmolar), all optimal qualities for a vaginal lubricant.31
Figure 4.
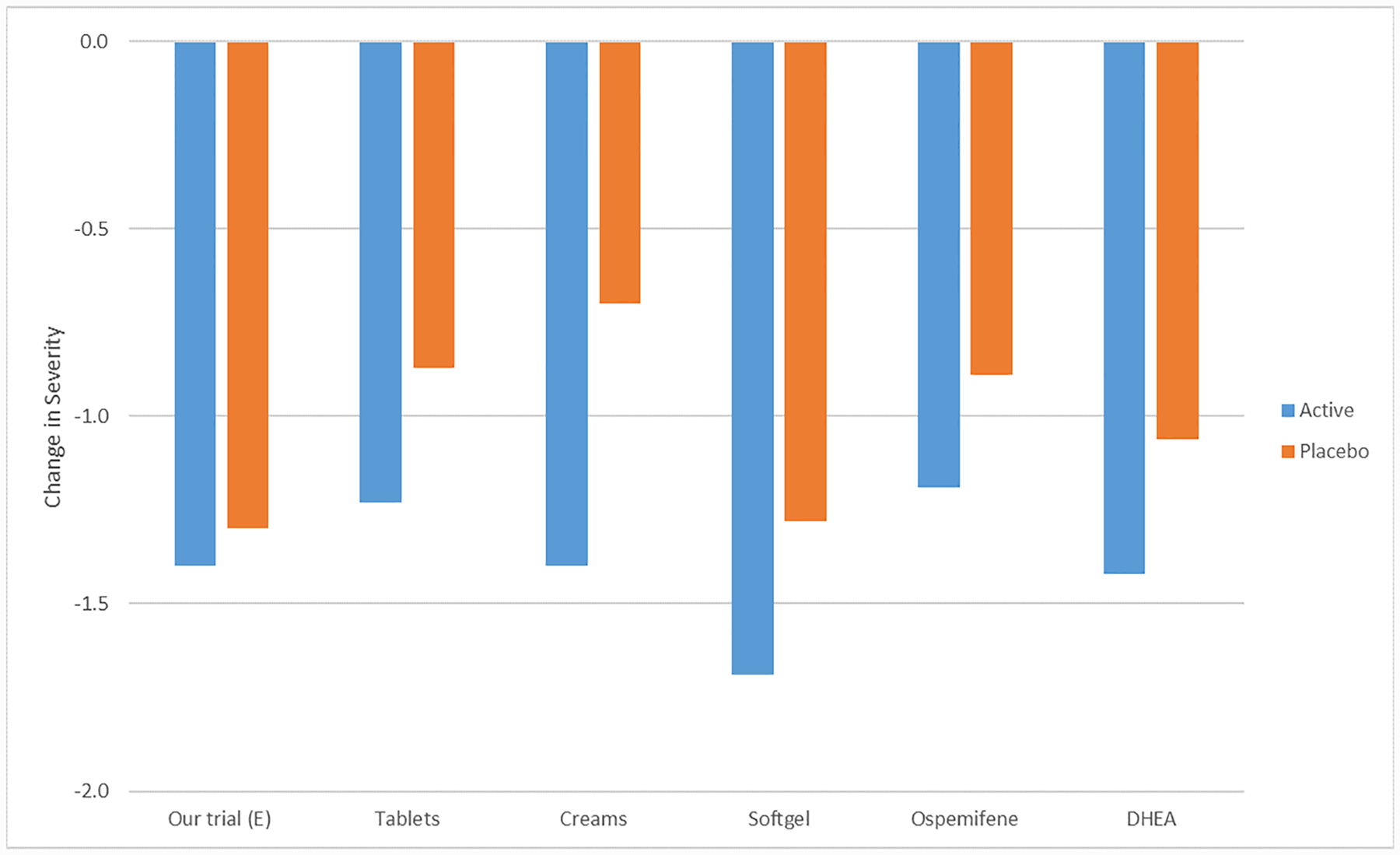
Mean Reduction in Severity of Vaginal Symptoms Across Interventions, Active vs. Placebo
X axis: Intervention vs. Placebo from left to right: vaginal estradiol tablet plus placebo gel vs. vaginal placebo tablet and vaginal placebo gel5; vaginal estradiol tablet vs. placebo tablet21; vaginal conjugated estrogen cream vs. placebo cream59; vaginal soft gel vs. placebo gel20; oral ospemifene vs. oral placebo tablet60; vaginal dehydroepiandosterone suppository vs. vaginal placebo suppository61
Y axis: Reduction in vaginal symptom severity score (scale 0–3)
Menopausal symptom measure evaluations
MsFLASH trials utilized validated measures for all outcomes that were standardized across trials (Supplementary Digital Content A). In addition, hot flash monitors, as objective measures of hot flashes, were pilot tested; our studies showed that at the time of our trials, the ambulatory monitors were not sufficiently accurate to objectively measure hot flashes in the outpatient setting.32 In our published account we concluded: The Bahr Monitor™ (Simplex Scientific, Middleton, WI) and Biolog™ (UFI, Morro Bay, CA) appeared suitable for use in controlled, laboratory conditions over short periods of time. However, the current versions of these monitors may not be suitable for ambulatory clinical trials at this time.” This study was published in 2012 and the work was performed almost 10 years ago, thus these findings may no longer apply as further technological development has occurred. In addition, wrist accelerometers were provided to a subset of participants to relate objectively measured sleep disturbance symptoms to intervention effects of yoga and exercise.33
Our first trials utilized the full Female Sexual Function Scale (FSDS) with 13 questions. A shorter version, FSDS-R, was also analyzed and our results suggested that a single question (item #1) provided a relatively robust screen for the presence or absence of female sexual function distress.34 Subsequent trials adopted the FSDS-R. Psychometric properties of several scales were examined in MsFLASH trial cohorts, including the Pittsburgh Sleep Quality Index,35 Female Sexual Function Index,36 the Hot Flash Related Daily Interference Scale,37 and the Insomnia Severity Index.38
Using a “card sort” of menopausal symptoms, MsFLASH 02 participants were asked to prioritize symptoms for treatment. The most common symptom priorities were: VMS (n = 322), sleep (n = 191), concentration (n = 140), and fatigue (n = 116). Evaluation suggested an accurate mechanism that could be used in the clinic or research setting to identify individual priorities for targeting symptom-based treatments.39 In addition, symptom clusters (i.e., statistically derived groups of co-occurring symptoms) including interference and severity of VMS, sleep, depression, anxiety, and pain symptoms, were identified in women enrolled in our first three trials.40 These symptom clusters may represent useful phenotypes for differentiating treatment effects or evaluating associations with biomarkers or genes.
Mechanistic study findings
In addition to evaluating treatments for menopausal symptoms, the MsFLASH Network’s studies provide insight into pathophysiologic mechanisms that may underlie menopausal symptoms and treatment benefits/risks.
Bone turnover and SSRIs
Observational studies have suggested that use of Selective Serotonin Reuptake Inhibitors (SSRIs) is associated with an increased fracture risk and an accelerated bone loss, although conflicting results have been reported. We measured bone turnover markers in the third MsFLASH study and did not find significant differences between escitalopram and placebo over eight weeks,41 suggesting minimal or no short-term impact of SSRIs on bone metabolism.
Cortisol and hot flashes
Compared with reported normal ranges for salivary free cortisol in the general population,42, 43 women enrolled in the second MsFLASH trial had abnormally low, or low range of normal, cortisol values at baseline.42 In our cross-sectional ancillary study, the median cortisol rise at 30 minutes after awakening was only 18% higher than the wake value, as compared to a normal 50–100% rise in the general population.42, 43 Black women, irrespective of hot flash frequency, had significantly higher evening free cortisol concentrations, and significantly lower awakening responses (difference between awakening cortisol value and the value 30 minutes later), than those of White women (both P=0.05). These pattern differences have also been observed in other general populations and are hypothesized to be related to chronic stress.42, 43
Heart Rate Variability
We hypothesized that women with severe hot flashes would have diminished heart rate variability (HRV), but there was no significant association between hot flash severity and heart rate variability in our studies.44 Neither yoga nor exercise increased HRV in middle-aged women with vasomotor symptoms.45 We did not measure HRV as a response to a hot flash, rather we evaluated the cross-sectional correlation of HRV and hot flash frequency and found no significant association.
KNDy Neuron complex and thermoregulation
We hypothesized that hot flashes can be reduced by KNDy neuron manipulation. A double-blind placebo-controlled 3-arm crossover trial of healthy volunteer women admitted to an inpatient unit showed diminution in hot flash frequency among those given a kappa agonist as compared to placebo (P<0.05) and a nonsignificant reduced number of LH pulses in a small sample (P=0.12, n=12).46 LH pulsatility mirrored objective hot flashes in some, but not all, women confirming studies performed in the late 1970s, but not otherwise replicated.47 Our findings, along with work by others,48–51 suggest that menopausal thermoregulatory control is in part determined by the KNDy neuron complex.
Postmenopausal vaginal microbiome
Few studies have evaluated the postmenopausal vaginal microbiome. In a cross-sectional study of 88 postmenopausal women, the majority without bothersome vaginal symptoms, 38% displayed a Lactobacillus dominant flora and a full 34% had no Lactobacillus detected.52 Importantly, this pattern did not vary by those women who had or did not have vaginal symptoms. The lower prevalence of Lactobacillus among postmenopausal women, and particularly among women without symptoms, is in sharp contra-distinction to patterns observed among premenopausal women.53 Postmenopausal women appear to have a more diverse vaginal microbiome than premenopausal women without bacterial vaginosis. Lactobacillus appeared to increase over eight weeks of treatment with estradiol, but this change was not associated with symptom improvement.54
The MsFLASH Vaginal Health Trial collected longitudinal biologic samples from women in all three arms of the RCT, including vaginal swabs, vaginal biopsies, and cervicovaginal lavage fluid. Preliminary analyses demonstrated greater changes in pH and vaginal maturation index (VMI) in the estradiol arm compared to the moisturizer and placebo arms, although this did not correlate with greater improvement in symptom severity.5 Pilot analyses presented at the North American Menopause Society Annual meeting in 2018 demonstrated no statistically significant differences in vaginal fluid cytokines and chemokines between treatment arms, nor between women who had the largest improvement in symptoms (≥ 2-point decrease on a 0–3 scale) and those who did not improve. Additional planned analyses will examine the vaginal microbiome and metabolome between treatment arms and women whose symptoms did and did not improve with treatment. Using specimens from MsFLASH 03 and MsFLASH 05, differences by race will be evaluated.
Brief Summary of Findings by Intervention
Escitalopram (10–20 mg daily for eight weeks) significantly reduced the frequency and severity of hot flashes.8 Findings did not vary between Black and White women. Over half (55%) of women assigned to escitalopram reported a decrease in hot flash frequency of at least 50%, compared to 36% of women assigned to placebo. Improvement in symptoms was appreciated in the first week and symptoms returned abruptly with cessation of escitalopram. Hot flash interference,4 sleep quality, insomnia symptoms,16 and quality of life24 improved, and overall sexual function did not change.22 For escitalopram vs. placebo, the mean difference in hot flash frequency reduction was 1.41 (95% CI: 0.13, 2.69; P<0.001) fewer hot flashes per day.8
Exercise (individual, facility-based aerobic training three times/week for twelve weeks) did not significantly reduce the frequency, bother, or interference of hot flashes, but did modestly improve sleep quality compared to usual activity control.11 The intervention did not measurably affect quality of life.25
Yoga (weekly 90-minute classes and 20-minute daily home practices four times per week for twelve weeks) did not significantly reduce the frequency, bother, or interference of hot flashes, but did slightly improve quality of life and reduce insomnia symptoms9, 25 compared to usual activity control.
Omega-3 supplement (1.8 gm daily for twelve weeks) did not significantly reduce the frequency, bother, or interference of hot flashes, nor did it improve sleep quality, insomnia symptoms, quality of life, or mood, compared to placebo.10, 25
Venlafaxine XR (75 mg daily for eight weeks) had similar effects to Oral Estradiol (0.5 mg) on VMS frequency, an approximate 50% decrease from baseline compared to placebo.4 Venlafaxine XR improved insomnia symptoms and low-dose oral estradiol improved sleep quality, compared to placebo.17 Both venlafaxine and low-dose estradiol modestly improved hot flash interference4 and quality of life.26 There was no change in overall sexual function with either venlafaxine or low-dose oral estradiol, relative to placebo, although venlafaxine decreased sexual pain but resulted in diminished orgasm, and low-dose oral estradiol improved desire.23 For venlafaxine vs. placebo, the mean difference in hot flash frequency reduction was 1.8 (95% CI: 0.8, 2.7; P=0.005) fewer hot flashes per day. For estradiol vs. placebo, the mean difference in hot flash frequency reduction was 2.3 (95% CI: 1.3, 3.4; P<0.001) fewer hot flashes per day.4
Cognitive Behavioral Therapy for Insomnia (CBT-I; six 30-minute telephone-delivered individual sessions over eight weeks) compared to menopause education control was efficacious at 8- and 24-week follow-up in reducing self-reported insomnia symptoms, improving overall sleep quality, and increasing self-reported sleep efficiency. There was also a reduction in self-reported hot flash interference with CBT-I as compared to menopause education control. From baseline to eight weeks, the ISI (scale 0–28) decreased 9.9 points in women receiving CBT-I and 4.7 points in women in the menopause education control group, for a mean between-group difference of −5.2 (95% CI: −6.1, −3.3; P<0.001).12
Vaginal 10 mcg estradiol tablet (nightly for two weeks, then twice weekly for ten weeks), did not provide any added benefit over a dual placebo (vaginal gel + tablet) in reducing vulvovaginal discomfort, improving sexual function, or sexual activity in postmenopausal women.5 However, a modest improvement in menopausal quality of life was observed with vaginal estrogen, driven by the sexual domain.27 Changes in most bothersome symptom (MBS): Estradiol −1.4 (95% CI: −1.6, −1.2) and placebo −1.3 (95% CI: −1.5, −1.1; P=0.25).
Vaginal moisturizer (three times per week for twelve weeks) did not provide any added benefit over a dual placebo (vaginal gel + tablet) in reducing vulvovaginal discomfort, improving sexual function, or sexual activity in postmenopausal women.5 No improvement in menopausal quality of life was observed.27 Changes in MBS: −1.2 (95% CI: −1.4, −1.0) and placebo −1.3 (95% CI: −1.5, −1.1; P=0.31).
CONCLUSIONS
SSRI/SNRIs diminish hot flashes by approximately 50% as compared with a 30% decrease by placebo, as shown by the MsFLASH studies on escitalopram and venlafaxine.4, 8 The SNRI, low-dose venlafaxine XR, appears to provide similar benefit to low-dose oral estradiol (0.5 mg) for decreasing frequency and severity of hot flashes.4 Data from studies of SSRIs and SNRIs for mood and their negative impact on sexual function55–57 and sleep58 in the general population have been of concern. In this regard, data from the MsFLASH Network provides essential information for healthy women with menopausal symptoms without evidence of major symptoms of anxiety or depression (Table 2). We found that neither escitalopram nor venlafaxine XR showed a greater decrease than placebo in overall sexual function as measured by the FSFI. Also, escitalopram showed improved sleep quality, and both escitalopram and venlafaxine improved insomnia. Explanations for these findings in part are likely related to dose since the most effective doses for treatment of vasomotor symptoms are lower than doses prescribed for mood disorders which are associated with sexual and sleep dysfunction in depressed populations4, 8, 55–58.
Exercise, yoga, and omega-3 supplements were not beneficial in decreasing the frequency or severity of VMS, or VMS interference, but yoga did improve menopausal quality of life and reduce insomnia symptoms, and exercise did improve sleep quality and reduce insomnia symptoms9–11 No effect on sexual function was observed with these interventions. It is well understood that yoga and exercise may contribute to healthy aging and, therefore, should be encouraged.
A telephone-based cognitive behavioral therapy intervention for menopausal sleep disturbances was highly efficacious.12 Thus, CBT-I should be considered first-line therapy for menopausal related sleep disturbance. The program was relatively easy to implement and warrants further study.
A vaginal 10 mcg estradiol tablet given in conjunction with an inert placebo vaginal gel did not show benefit over a double-placebo arm (placebo tablet + inert placebo vaginal gel) in vaginal symptoms or sexual function, suggesting no added benefit of vaginal estradiol over the placebo gel.5 An OTC moisturizer similarly did not show benefit. Many postmenopausal women with moderate-to-severe vulvovaginal symptoms can be treated with a non-prescription vaginal lubricating gel. However, not all gel formulations may have the same effects, and some women may prefer non-gel formulations. Treatment choice should be based on individual patient preferences regarding cost and formulation.
In conclusion, the MsFLASH trials have contributed substantially to our understanding of treatment of bothersome menopausal symptoms. It is important that clinicians counseling women about available treatment options for hot flashes, sleep disturbances, quality of life, sexual function, and vaginal symptoms consider all non-hormonal and hormonal therapies. Though the benefits of many interventions are small in magnitude, particularly relative to the noteworthy placebo response, some women will find non-hormonal strategies helpful and perhaps preferable. Others might argue current treatments are inadequate, particularly current nonhormonal therapies for VMS. The methods used in the MsFLASH Network trials produced rigorous and reliable results, and we encourage their use for future trials evaluating new interventions for menopausal symptom relief. Our goal is to provide additional evidence-based options for women navigating bothersome menopausal symptoms experienced during this inevitable and, for the most part, natural aging process.
Supplementary Material
SDC A: MsFLASH Common Eligibility Criteria and Data Collection Instruments
SDC B: MsFLASH Trial-specific Exclusion Criteria
SDC C: Cognitive Behavioral Therapy Intervention for Menopausal Sleep Disturbance
Acknowledgements:
We would like to acknowledge Ellen W. Freeman, PhD, a Principal Investigator and integral partner in the MsFLASH studies, Sherry Sherman, PhD (deceased), and Judy Hannah, PhD, from the National Institute for Aging for their wisdom and guidance, and Ms. Sheri Greaves for administrative support at the Data Coordinating Center, without whose effort our studies could not have been accomplished.
Sources of Funding:
RFA AG-08-004 for U01 AG032682 (2007), New Interventions for Menopausal Symptoms, awarded $22 million in 2008; Sponsored by: National Institute on Aging (NIA), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), The National Center for Complementary and Alternative Medicine (NCCAM), The Office of Research on Women’s Health (ORWH)
PAR 13-097 (2013) invites existing U01 agreements to submit revision and or renewal application; awarded $7.0 million in 2015 (1R01AG048209-01A1); Sponsored by: National Institute on Aging (NIA)
NIH Grant support:
U01 AG032699 (Anderson/LaCroix), U01AG032682 (Newton/Reed), U01AG032656 (Cohen/Joffe), U01AG032659 (Caan/Sternfeld), U01AG032659 (Carpenter), U01AG032699 (Freeman), 5R01AG048209 (Guthrie, LaCroix, Reed)
Administrative Supplements:
U01AG032682 from NIA (Newton/Reed) “Vasomotor Symptoms, Heart Rate Variability and Cortisol”, 3R01AG048209-03S1 from ORWH (Guthrie/LaCroix/Reed) “Racial Differences in the Postmenopausal Microbiome”, and 3R01AG048209-01A1S1 from NIA/OBSSR (Guthrie/LaCroix/Reed).
Additional Funding:
University of Washington KL2TR000421-06 Pilot from the National Center for Research Resources, Clinical and Translational Sciences Award, departmental funding from University of Washington Department of Obstetrics and Gynecology, Indiana Clinical and Translational Sciences Institute (UL1RR025761), from the National Center for Research Resources, Clinical and Translational Sciences Award.
Footnotes
Financial dislcosures/conflicts of interest: Janet Carpenter received funds for use of HFRDIS scale from Astellas Pharma and Clinical Ink/Sojournix. She received consultant funds from RoundGlass. Hadine Joffe receives grant funding for research from Pfizer. No other authors reported any conflicts of interest.
ClinicalTrials.gov Registration: MsFLASH 01 - Escitalopram for Menopausal Symptoms in Midlife Women, NCT00894543; MsFLASH 02 - Interventions for Relief of Menopausal Symptoms: A 3-by-2 Factorial Design Examining Yoga, Exercise, and Omega-3 Supplementation, NCT01178892; MsFLASH 03 - Comparative Efficacy of Low-Dose Estradiol and Venlafaxine XR for Treatment of Menopausal Symptoms, NCT01418209; MsFLASH 04 - Pilot Trial: Telephone Behavioral Therapy for Menopause-related Sleep Disturbance, NCT01936441; MsFLASH 05 – The Vaginal Health Trial, NCT02516202.
REFERENCES
- 1.Sherman S, Miller H, Nerurkar L, Schiff I. Research opportunities for reducing the burden of menopause-related symptoms. Am J Med 2005;118 Suppl 12B:166–171. [DOI] [PubMed] [Google Scholar]
- 2.Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol 2015;126:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie KA, Larson JC, Ensrud KE, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and subjective sleep quality in women with hot flashes: A pooled analysis of individual participant data from 4 MsFLASH trials. Sleep 2018:41;zsx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014;174:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell CM, Reed SD, Diem S, et al. Efficacy of Vaginal Estradiol or Vaginal Moisturizer vs Placebo for Treating Postmenopausal Vulvovaginal Symptoms: A Randomized Clinical Trial. JAMA Intern Med 2018;178:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternfeld B, LaCroix A, Caan BJ, et al. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: the MsFLASH experience. Contemp Clin Trials 2013;35:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause 2014;21:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of Omega-3 treatment for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternfeld BS, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014;21:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCurry S, Guthrie KA, Morin DM, et al. Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial. JAMA Intern Med 2016;176:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie KA, Caan B, Diem S, et al. Facebook advertising for recruitment of midlife women with bothersome vaginal symptoms: A pilot study. Clinical Trials 2019. May 6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butt DA, Deng LY, Lewis JE, Lock M. Minimal decrease in hot flashes desired by postmenopausal women in family practice. Menopause 2007;14:203–207. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril 2012;97:1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy menopausal women with hot flashes: a randomized controlled trial. Menopause 2012;19:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ensrud KE, Guthrie KA, Hohensee C, et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 2015;38:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Miao D, Sun Y. Cognitive behavioral therapy alone and with medication for persistent insomnia. JAMA 2009;302:1053. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med 2011;171:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantine GD, Simon JA, Pickar JH, et al. ; on behalf of the REJOICE Study Group. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2017;24:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol 2008;112:1053–1060. [DOI] [PubMed] [Google Scholar]
- 22.Reed SD, Guthrie KA, Joffe H, Shifren JL, Seguin RA, Freeman EW. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized trial. Obstet Gynecol 2012;119:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed SD, Mitchell CM, Joffe H, et al. Sexual function in women on estradiol or venlafaxine for hot flushes: a randomized controlled trial. Obstet Gynecol 2014;124(2 Pt 1):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCroix AZ, Freeman EW, Larson J, et al. Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial. Maturitas 2012;73:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed SD, Guthrie KA, Newton KM, et al. Menopausal quality of life: a RCT of yoga, exercise, and omega-3 supplements. Am J Obstet Gynecol 2014;210:244.e1–244.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related symptoms and quality of life in healthy postmenopausal women with hot flashes: a randomized controlled trial. Menopause 2015:22:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diem SJ, Guthrie KA, Mitchell CM, et al. Effects of vaginal estradiol tablets and moisturizer on menopause-specific quality of life and mood in healthy post-menopausal women with vaginal symptoms: a randomized clinical trial. Menopause 2018;25:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas 2005;50:209–221. [DOI] [PubMed] [Google Scholar]
- 29.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996;24:161–175. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EW, Ensrud K, Larson J, et al. Placebo improvement in pharmacologic treatment of menopausal hot flashes: time course, duration and predictors. Psychosom Med 2015;77:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards D and Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric 2016;19:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter JS, Newton KN, Sternfeld BS, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the Menopause Strategies Finding Lasting Answers for Symptoms and Health network. Menopause 2012;19:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchanan DT, Landis CA, Hohensee C, et al. Effects of yoga and aerobic exercise on actigraphic sleep parameters in menopausal women with hot flashes. J Clin Sleep Med 2017;13:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter JS, Reed SD, Guthrie KA, et al. Using an FSDS-R item to screen for sexually related distress: a MsFLASH analysis. Sex Med 2015;3:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otte JL, Rand KC, Landis C, et al. Confirmatory factor analysis of the Pittsburgh Sleep Quality Index in women with hot flashes. Menopause 2015;22:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter JS, Jones SMW, Studts CR, et al. Female Sexual Function Index Short Version: a MsFLASH response analysis. Arch Sex Behav 2016;45:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 2001;22:979–989. [DOI] [PubMed] [Google Scholar]
- 38.Otte JL, Bakoyannis G, Rand KL, et al. Confirmatory factor analysis of the Insomnia Severity Index (ISI) and invariance across race: A pooled analysis of MsFLASH data. Menopause 2019. April 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter JS, Woods NF, Otte JL, et al. MsFLASH participants’ priorities for alleviating menopausal symptoms. Climacteric 2015;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods NF, Hohensee C, Carpenter JS, et al. Symptom clusters among MsFLASH clinical trial participants. Menopause 2016;23:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diem SJ, Joffe H, Larson JC, et al. Effects of escitalopram on markers of bone turnover: a randomized clinical trial. J Clin Endocrinol Metab 2014;99:E1732–E1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed SD, Newton KM, Larson JC, et al. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol (Oxf) 2016;84:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlamangla AS, Friedman EM, Seeman T, et al. Daytime trajectories of cortisol: demographic and socioeconomic differences--findings from the National Study of Daily Experiences. Psychoneuroendocrinology 2018;38:2585–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones SW, Guthrie KA, LaCroix AZ, et al. Is heart rate variability associated with frequency and bother of vasomotor symptoms among healthy peri-menopausal and post-menopausal women? Clin Auton Res 2016;26:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones SM, Guthrie KA, Reed SD, et al. A yoga and exercise randomized controlled trial for vasomotor symptoms: Effects on heart rate variability. Complement Ther Med 2016;6:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakley AE, Steiner RA, Chavkin C, Clifton DK, Ferrara LK, Reed SD. K Agonists as a novel therapy for menopausal hot flashes. Menopause 2015;22:1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH and skin temperature during the menopausal hot flash. J Clin Endocrinol Metab 1979;49:152–154. [DOI] [PubMed] [Google Scholar]
- 48.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prague JK. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017;389: 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell CM, Srinivasan S, Zhan X, et al. Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause 2017;24:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014;21:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell CM, Srinivasan S, Plantinga A, et al. Associations between improvement in genitourinary symptoms of menopause and changes in the vaginal ecosystem. Menopause 2018;25:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry 2002;63:357–366. [DOI] [PubMed] [Google Scholar]
- 56.Gregorian RS, Golden KA, Bahce A, Goodman C, Kwong WJ, Khan ZM. Antidepressant-induced sexual dysfunction. Ann Pharmacother 2002;36:1577–1589. [DOI] [PubMed] [Google Scholar]
- 57.Cyranowski JM, Frank E, Cherry C, Houck P, Kupfer DJ. Prospective assessment of sexual function in women treated for recurrent major depression. J Psychiatr Res 2004;38:267–273. [DOI] [PubMed] [Google Scholar]
- 58.Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep 2017;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009;16:719–727. [DOI] [PubMed] [Google Scholar]
- 60.Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010;17:480–486. [DOI] [PubMed] [Google Scholar]
- 61.Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2016;23:243–256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC A: MsFLASH Common Eligibility Criteria and Data Collection Instruments
SDC B: MsFLASH Trial-specific Exclusion Criteria
SDC C: Cognitive Behavioral Therapy Intervention for Menopausal Sleep Disturbance


