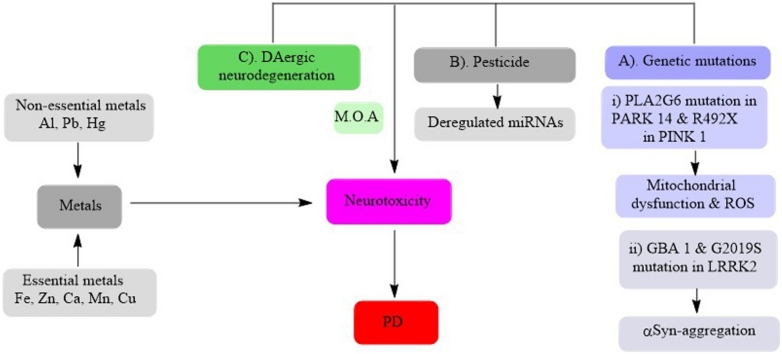
Graphical abstract
Keywords: Parkinson’s disease, Metals, Oxidative stress, Dopamine, Pesticides, miRNAs
Highlights
-
•
Common miRNA association between Parkinson's Disease (PD) and pesticides exist.
-
•
Pesticide-deregulated miRNAs affect PD-related molecules, e.g. α-synuclein.
-
•
There exist an association between essential, non-essential metals and PD.
-
•
UPS and mitochondrial impairment, oxidative stress, gene mutation and α-Syn aggregation are prime mechanisms involved in essential, non-essential metals neurotoxicity in PD.
Abstract
Essential metals including iron (Fe) and manganese (Mn) with known physiological functions in human body play an important role in cell homeostasis. Excessive exposure to these essential as well as non-essential metals including mercury (Hg) and Aluminum (Al) may contribute to pathological conditions, including PD. Each metal could be toxic through specific pathways. Epidemiological evidences from occupational and ecological studies besides various in vivo and in vitro studies have revealed the possible pathogenic role and neurotoxicity of different metals. Pesticides are substances that aim to mitigate the harm done by pests to plants and crops, and are extensively used to boost agricultural production. This review provides an outline of our current knowledge on the possible association between metals and PD. We have discussed the potential association between these two, furthermore the chemical properties, biological and toxicological aspects as well as possible mechanisms of Fe, Mn, Cu, Zn, Al, Ca, Pb, Hg and Zn in PD pathogenesis. In addition, we review recent evidence on deregulated microRNAs upon pesticide exposure and possible role of deregulated miRNA and pesticides to PD pathogenesis.
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD) [1,2]. In urbanized countries, the approximated incidence of PD is 0.3 % in the general population, 1.0 % in people older than 60 years and 3.0 % in people older than 80 years. PD incidence rates are approximated to range between 8–18 per 100,000 person per year [3,4]. Sex also plays an important role in PD pathogenesis. Several studies found that male exhibits greater prevalence and earlier onset of PD as compared to female [5,6]. During PD patients experience progressive extrapyramidal symptoms, including tremor, bradykinesia, rigidity and postural imbalance, additionally a variety of non-motor symptoms such as sleep and mood disorders. Augmentation of these symptoms occurs with the disease progression [[7], [8], [9]]. The pathological hallmarks of PD are the reduction of dopamine levels due to loss of dopaminergic neurons in the substantia nigra (SN) pars compacta (SNpc), and accumulation of misfolded alpha-synuclein (αSyn), in abnormal intra-cytoplasmic inclusions called Lewy bodies (LBs) [[10], [11], [12]]. Genetic mutations such as Glucocerebrosidase 1 (GBA1), Leucine-rich repeat kinase 2 (LRRK2) and PTEN-induced putative kinase 1 (PINK1) also play a prime role in PD pathogenesis as it exaggerates synucleopathies and ROS generation [[13], [14], [15], [16], [17], [18]] Other contributory factors comprises environmental toxicants such as pesticides, mitochondrial dysfunctions, oxidative stress, chronic neuroinflammation and calcium imbalance [[19], [20], [21]]. Medications, cerebrospinal meningitis and poisoning account for about 25 % cases of parkinsonism. The PD's 75 % cases are idiopathic [22,23]. Out of the 75 % idiopathic cases, more than 90 % are sporadic, while only 5–10 % have a genetic background. Circadian rhythm disorder is also one of the risk factors for PD development [[24], [25], [26]]. In PD development, circadian rhythm disorder also plays an important role, and it is estimated that 60–90 % of PD patients present sleep disturbance during early stages of disease progression [27]. In addition to the aforementioned contributory factors, metals alteration in the brain also play a pivotal role in PD pathogenesis. Owing to the significance of metals in the maintenance of cell homeostasis they play a pivotal role in the body functions maintenance. Among 23 elements with known physiological functions, 12 are metals including iron (Fe), manganese (Mn), zinc (Zn) and copper (Cu) [28]. Xenobiotic metals, including aluminum (Al), lead (Pb) and mercury (Hg) with unknown physiological functions are also present in a significant concentration in the human body [29]. Mounting shreds of evidence from the previous research has revealed that dyshomeostasis and exposition to these aforementioned metals are connected to increased PD risk [[30], [31], [32]]. A huge amount of in-vivo and in-vitro studies have been illustrated metals neurotoxicity through the generation of oxidative stress and other specific pathways [33]. Numerous metals are essential for physiological functions, and their dyshomeostasis have been associated with neurodegenerative disorders [28]. Metals produce reactive oxygen species (ROS), and due to this ROS, DNA damage occurs and leads to apoptosis [29,34]. Metals may also increase the expression of genes associated with neurodegenerative diseases [35]. In medical chemistry and neurotoxicology, the involvement of metals in PD pathogenesis is still a matter of great concern [36]. Its contribution is either through metallic toxicants or by depletion in essential metals for human health [37]. Numerous epidemiological studies have shown significant association between PD and long term exposure to metals [38,39]. The primary resources of metals exposure are medications, contaminated seafood, occupational exposure, environmental pollution and amalgam fillings in dental metals restorations [[40], [41], [42]]. A positive correlation was found between industrialization and PD prevalence in developed countries where environmental exposure to metals occur [43,44]. Metals exposure have an adverse effect on the health and cognition in children [45,46]. Occupational exposure to Fe, Al and Mn double the risk of PD. In contrast, those workers who are exposed for more than 20 years to Pb and Cu have shown 2–10 fold increase in PD prevalence [30,47]. The Elevated level of Hg is associated with a high prevalence of PD [48]. Vanadium pentaoxide (V2O5) inhalation in PD's rodent model is associated with the nigrostriatal dopaminergic system damage [49,50]. Metals ions usually exaggerate the aggregation properties of αSyn in PD [51].
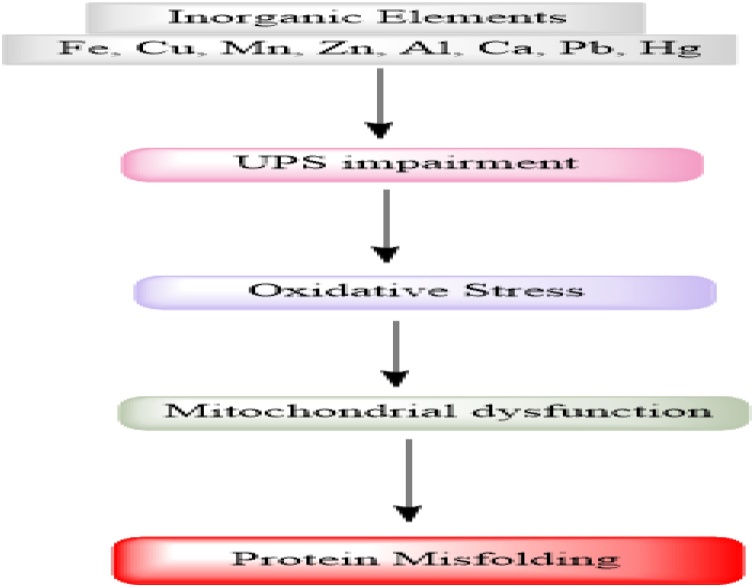
Acknowledging the potential role of metals on PD, in this review we have discussed the potential association between metals and PD, furthermore the chemical properties, biological and toxicological aspects as well as possible mechanisms of Fe, Mn, Cu, Zn, Al, Ca, Pb, Hg and Zn in PD pathogenesis (Fig. 1).
Fig. 1.
Inorganic elements i-e Fe, Cu, Mn, Zn, Al, Ca, Pb and Hg causing UPS impairment and as a result oxidative stress, mitochondrial dysfunction and protein misfolding which are possible mechanisms of PD pathogenesis.
2. Iron
2.1. Iron and its role in the PD pathogenesis
Mounting evidences indicate increased levels of Fe in specific brain regions in particular neurodegenerative diseases, especially in PD. In the brain of PD patient's SNpc and lateral Globus pallidus, an approximately two fold increased in Fe levels occurred. In neurodegenerative disorders including PD, inflammation associated with dysfunction of metals ion homeostasis (Fe) accompanied by concomitant oxidative stress is the main factor in disease progression [52,53]. The role of Fe as risk factor for PD was suggested when the levels of total Fe was found 176 % increased, and that of ferric ion was increased by 225 % in SNpc of PD relative to age-matched control. Three lines of evidence indicated Fe as a risk factor in PD, including Fe levels, are increased in SN but not in other regions of the brain. Postmortem studies have shown elevated iron levels in DAergic neurons in PD. PD animal models showed neuroprotection by genetic or pharmacological chelation of Fe. Spectrophotometric method and Perl’s staining, inductively coupled plasma spectroscopy, MRI, laser microprobe mass analysis, susceptibility weighted imaging (SWI) and enhanced T2 star weighted angiography (ESWAN) studies have shown elevated Fe levels in SNpc of the brain [54,55].
2.2. Possible mechanism of iron in PD pathogenesis
2.2.1. Iron and oxidative stress
The Brain requires high concentration of Fe due to its role in energy metabolism, myelin formation and synthesis of neurotransmitters. Due to Fe strong redox ability, it participates in Fenton chemistry leading to ROS generation, which in turn induces oxidative stress causing cell dysfunction and ultimately cell death [56].
2.2.2. Iron and ubiquitin-proteasome system (UPS)
In addition to Fe accumulation, UPS impairment is also implemented in PD pathogenesis. UPS impairment, which is also responsible for IRPs degradation, may also cause accumulation of IRPs and Fe [[57], [58], [59]].
2.2.3. Iron and αSyn aggregation
αSyn aggregation is a key event in PD pathogenesis. The misfolding and aggregation of αSyn, which may be resistant to ubiquitination is suggested to contribute to PD. αSyn has been shown to directly bind Fe in both the Fe3+ and Fe+2 states, and ferric iron is able to foster its aggregation. ROS present in the cells could catalyze the oxidation of Fe toward its ferric form resulting in an exacerbation of α-Sync aggregation. Therefore, increased levels of Fe could result in an increased translation of αSyn protein [60,61].
2.2.4. Iron and gene mutation
Parkin, αSyn, LRRK2, PINK1, and DJ-1 are among several genes known to be linked to PD pathogenesis. Fe has a relationship among genes involved in monogenic PD. The Hyper echogenicity of the SN has also been found to be a typical sign in idiopathic PD and increased Fe level is responsible for this SN hyper echogenicity. By using transcranial sonography in PD patients having mutations in either of αSyn, LRRK2, Parkin, PINK1, or DJ-1 genes, showed significantly larger echogenicity in the SN relative to healthy controls [[62], [63], [64]].
3. Copper (Cu)
3.1. Copper content distribution in the brain
Humans body contains Cu as the third most abundant essential transition metal. The main sources of copper exposure to the body, in form of dietary intake are cereals, fresh fruits and vegetables [65,66]. Following liver, the brain is the organ contains the highest Cu because this organ has a high metabolic rate and is prone to oxidative stress. Total brain Cu content in human has been approximated to be 3.1 μg/g wet weight. However, due to physiological and anatomical heterogeneity of brain, different regions contain different Cu contents. In human, locus coeruleus and SN contains the highest Cu contents. Cerebrospinal fluid (CSF) 0.2 μM of Cu content. While synaptic cleft contains 250 μM of Cu [67,68]. During development, with age and in neurodegenerative diseases, brain Cu content and distribution changes. In PD, approximately 50 % reduction in Cu content reported in substantia nigra and locus coeruleus [69,70].
Different transport systems including copper transporter receptor (Ctr)1, copper transporter receptor (Ctr)2, DMT1, metallothioneins (MTs), glutathione (GSH), Copper-transporting P-type ATPases ATP7A and ATP7B play a pivotal role in the maintenance of mammalian cellular and systemic Cu homeostasis [71,72]. While the blood brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCB) are the main regulatory systems of the brain Cu homeostasis requiring combined action of Ctr 1 and ATP7 [73,74]
3.2. Role of copper in PD pathogenesis
The main pathological hallmarks in PD are protein aggregation and oxidative stress, although triggers for these events are not known, but changes in bio metals have long been suspected of playing a role in these events. Cu is an important bio metal and play a role in degenerating SNpc in PD. A Substantial decrease in tissue Cu has been reported over the decades. According to recent research, peripheral Cu dyshomeostasis occurs in PD [75]. Literature studies has proved that free Cu is more deleterious for neurodegenerative diseases due to its redox capacity and in turn, generation of free radicals as in the Fenton reaction [76].
3.3. Possible mechanisms of Cu toxicity in PD pathogenesis
Following are the possible mechanisms of Cu toxicity in PD
3.3.1. Cu and αSyn interactions and their toxicity in PD
PD can arise from αSyn accumulation, overexpression and aggregation as well as autosomal dominant mutations in αSyn gene resulting in A53 T, H50Q, E46 K, A53E, G51D, and A30 P variants. αSyn aggregation and oligomerization within the neuronal cytoplasm has a deleterious effect and linked to neurotoxicity [77,78]. Cu accumulates in the brain with aging and has the ability to bind with the αSyn and initiate its aggregation. Asp and Glu residues, abundantly present at the C terminus of αSyn, were identified as Cu (II) binding donors. At normal pH, αSyn exhibits two binding sites for Cu (II) at M1-D2 and H50. At the same time, at pH 5.0, H50 binding site at αSyn is abated replacing with D119-E123. While Cu (I) binds at M1-M5 and M116-M127. In cells both species of Cu co-exists, and transition between these species facilitates amyloid aggregates leading to ROS generation and cell damage [79]. Point mutation to major Cu (II) site H50 leads to the familial form of PD [80,81]. The Copper-αSyn complex is represented by post translational modifications induced by oxidative stress, and this is one of the main perspectives because synucleinopathies and neurodegenerative diseases, in general, are linked with high levels of oxidative stress in the brain [82,83]. Some studies have linked neuroinflammation with synucleopathies [84].
3.3.2. Oxidative mechanism of Cu and its role in PD pathogenesis
The ability of Cu to cycle between its oxidized state (Cu2+) and reduced state (Cu+) enables it to act as a redox catalyst and to coordinate a large variety of ligands [85]. This feature suggests its involvement in Fenton chemistry, as well as Haber-Weiss cycle, both are involved in the initiation of the generation of highly reactive hydroxyl radicals from hydrogen peroxide and results in the generation of unstable active radicals which attacks macromolecules. These radicals are the powerful oxidizing agents capable of induction of DNA strands breaks and lipid peroxidation [76]. Metals overload causes extensive genome damage, especially base modification and strand breaks demonstrated experimentally in vitro with isolated DNA [86]. Cu exerts its genotoxic effect via generation of singlet oxygen and/or hydroxyl radicals bound to or in close connection in copper-binding sites on double-stranded DNA rather than via the generation of free hydroxyl radicals [87]. The main targets of Cu induced oxidative stress is mitochondria which are accompanied by the functional impairment of mitochondrial respiratory enzymes [88,89]. Cu induced oxidative stress, triggers apoptosis via activation of tumor suppressor proteins p53, which in turn trigger cascade of anti or pro-apoptotic proteins or direct action at mitochondria [90]. An in-vitro study revealed that Cu assists the dopamine and related catechols such as L-Dopa and 6−OH-Dopamine oxidation. The complexes arise from these dopamine oxidations causes DNA damage [91,92].
3.4. Miscellaneous mechanisms associated with Cu toxicity
Although Cu toxicity is attributed to its oxidative stress, there are certain proteins to which Cu directly attached and induces its toxicity. Cu binds to X-linked inhibitor of apoptosis, induces a conformational change and decreases its half-life, thereby making the cell more susceptible to apoptotic stimuli and exerts its toxic effect [93]. Cu may bind to Cu metabolism independent protein's thiol and amino groups, thereby modifying their structure and biological functions [94]. Cu inhibits enzymatic activities of cytochrome P450, GSH transferases, and lactate dehydrogenase [95]. Cu hampers the activities of DNA glycosylases NEIL1 and NEIL2 and inhibit both phosphatase and kinase activities of polynucleotide kinase 3′-phosphatase (PNKP) that is responsible for preparation of nicked DNA for ligation. Also, Cu inhabits DNA-binding affinity of the DNA nick sensor poly(ADP-ribose) polymerase-1 (PARP-1) and H2O2-induced poly(ADPribosyl)ation in HeLa S3 cells. Alterations in neuromodulatory functions and their effect on voltage-gated ion channels and synaptic receptors are also contributed to some extent in Cu toxicity [96,97].
4. Manganese (Mn)
4.1. Mn adequacy
Even though its necessity, excessive and prolonged inhalation of Mn particulates in mining, welding and industries results in its accumulation in selected brain regions that causes CNS dysfunctions and neurological consequences. One of the prominent hazards is in the form of manganism an extra pyramidal syndrome related to PD. Mn can block dopamine synthesis at the level of tyrosine hydroxylation and can also induce the release of dopamine from intracellular stores. Positron emission tomography imaging of nonhuman primate brains has revealed impaired DA transmission, a feature of PD, in the striatum of Mn-exposed animals. The link in the clinical features and pathophysiology has been established between Mn-induced parkinsonism and PD [[98], [99], [100], [101]].
4.2. Mn distribution in the central nervous system
Human's brain Mn concentration in ordinary physiological conditions is approximately 5.32–14.03 ng Mn/mg protein (20.0–52.8 Mm Mn), whereas in pathological conditions it reaches to 15.96–42.09 ng Mn/mg protein (60.1–158.4 Mm Mn). Mn accumulation and distribution is affected by various groups of factors which results in its dyshomeostasis and toxicity [102]. Highest Mn concentrations is found in human's brain putamen, caudate nucleus and Globus pallidus, while lowest in pons and medulla. These highest levels of Mn are correlated with age. In PD highest level were found, especially in putamen while lowest in superior and middle temporal gyrus and Globus pallidus [[103], [104], [105]]. Mn has acquired an importance in the brain physiology and biology due to its role as a cofactor in several enzymatic processes. Following are some of the Mn-dependent and Mn-activated enzymes that play a crucial role in brain physiology and maintenance and function such as arginase with its two isoforms ARG1 and ARG2. Glutamine synthetase (GS), superoxide dismutase (Mn-SOD1/SOD2), pyruvate carboxylase and protein serine/threonine phosphatase-1 (PP1) [[106], [107], [108]].
Mn is toxic at high levels. In order to maintain its homeostasis in the CNS, the outflow mechanism play a pivotal role. Recently four main proteins facilitate this outflow including Ferroportin (Fpn), Solute carrier family 30 member 10 (SLC30A10), Secretory pathway Ca2+-ATPase 1 (SPCA1) and ATPase 13A2 (ATP13A2 or PARK9). Fpn and SLC30A10 directly export cytosolic Mn out of neurons, while SPCA1 indirectly control Mn efflux through the Golgi apparatus and ATP13A2 via lysosome. In the presence of Mn, mice, and human embryonic kidney cells display more Fpn levels. People with mutations in the SLC30A10 suffer from hypermanganesemia. ATP13A2 has been associated with early-onset parkinsonism and Kufor-Rakeb syndrome [109,110].
4.3. Mn possible pathogenic mechanisms in PD
Mn pathogenic mechanism is still not been completely understood, but evidence from different studies imply that Mn shows its toxicity through several mechanisms.
Reduction in dopamine levels in SNpc by 80 % is the hallmark of idiopathic PD. It is hypothesized that the locus coeruleus degeneration potentially exceeds dopaminergic degeneration in SNpc. Mn exposure affects brain tissue and extracellular NE concentrations as well as its intake. Mn effect on NE biology results in clocus coeruleus disturbance and in turn, the nigrostriatal dopaminergic pathway. Mn causes a two-fold reduction in both protein and mRNA levels of α2-adrenergic receptors in clocus coeruleus and SNpc [111,112].
Mn exposure elevates the extracellular concentration of γ-aminobutyric Acid (GABA) its nigrostriatal pathway intake, and alteration in its receptors and transport, which results in an alteration in locomotor effects such as hyperkinesia and ataxia. As GABA neurons receives dopaminergic terminals from the SNpc, so its perturbance results in dysregulation of nigrostriatal pathways [113,114].
Mitochondria are one of the most important targets for Mn-induced cellular dysfunction. It contributes to mitochondrial dysfunction by inhibiting mitochondrial complexes I and II of the electron transport chain. In addition, it decreases mitochondrial membrane potential and increases intracellular ROS levels [115,116].
Mn can trigger glial activation and neuro inflammation involving microglia and astrocytes and indirectly damage neurons [117].
Association between environmental exposure and genetic factors play a pivotal role in PD pathogenesis. Mn and genetic mutations associated with PD alters biochemical pathways and as a result, augment PD pathology. In a genome-wide study on cultured human astrocytes, Mn-induced expression changes were observed in genes associated with inflammation and DNA repair [118]. Non-human primates study revealed that Mn-induced changes in the brain gene, involved in apoptosis and inflammation [119].
Mn and αSyn combination have a deleterious effect on cells survival. Mn can accelerate the in-vitro fibrillation of αSyn. Moreover, a mutation in genes such as G2019S LRRK2, ATP13A2 and Parkin (PARK2) play a crucial role in Mn-induced neuronal toxicity [[120], [121], [122]].
MicroRNAs (miRNAs) are single stranded RNAs that regulate gene expression post-transcriptionally via binding to the 3′-untranslated region (UTR) of mRNAs. Additionally, mir-7 and mir-433 play a pivotal role in the regulation of the SNCA gene in normal and PD brain. Mn exposure leads to dysregulation of several miRNAs’ which regulate apoptosis and synaptic transmission. Dysregulation of miRNA expression was recently identified as contributing to a higher risk of neurodegeneration [[123], [124], [125]].
5. Zinc (Zn)
5.1. The brain function and Zn importance
The Brain contains the average Zn concentrations between 10−15 μg/g of wet tissue. Different regions of the brain have different Zn levels with highest in hippocampus, amygdala and cerebellum. Zn is transported through BBB in the form of complexes with amino acids L-histidine and cysteine. Zn is present in the brain in three forms, first pool: in metals bound form which constitutes 90 %, second pool: in ions form and constitutes about 10 % and stores in presynaptic vesicles of glutamatergic neurons. Third pool: consists of free ions and present in non-precise form and constitutes 1%. Zn acts as a second messenger. It inhibits NMDA receptors and GABA receptors-mediated response which leads to the reduction of neuronal excitability. Zn enhances AMPA receptors in postsynaptic cells and regulates cell excitation. Therefore sustainable Zn homeostasis is required for proper brain functioning [[126], [127], [128]].
5.2. Zn neurotoxicity and possible mechanisms concerned with PD pathogenesis
Zn is important for proper brain functioning. Zn dyshomeostasis in the brain leads to the pathogenesis of numerous neurodegenerative disorders, including PD. Evidence from recent research revealed that Zn ions are capable of directly binding to PARK9. High Zn concentrations are a possible cause of PD as elevated Zn levels were found in SNpc of post mortal brain of PD patients [[129], [130], [131]]. Taking into consideration the aforementioned studies, it is revealed that Zn may play an important role in PD pathogenesis by following possible mechanisms
Until now, three main hypotheses are devised by which Zn may possibly cause cells damage and death, including excessive Zn causes excitotoxicity, induces oxidative stress and impairs the production of cellular energy. These all may act synergistically and cause cell death [132,133]. In addition, oxidative stress due to ROS production contributes significantly to PD pathogenesis. Zn is involved in this process because its dyshomeostasis causes free ROS generation, decrease superoxide dismutase (SOD) activity as well as expression levels of metallothioneins, which in turn induces oxidative stress. ROS generation in the mitochondria disrupts metabolic enzymatic activities and in turn, activates apoptotic processes [134]. In PD pathogenesis proteins misfolding and aggregation plays an important role. Protein aggregation results in Lewy bodies (LB) formation in dopaminergic neurons. αSyn is the main constituents of LB. Under normal conditions, UPS is responsible for its degradation. The malfunctioning of UPS is responsible for aberrant protein aggregation and protein death. Ubiquitin-conjugated proteins serve as a marker for impaired UPS. Numerous studies revealed that Zn dyshomeostasis causes induction of UPS impairment which in turn causes αSyn aggregation and increased expression of ubiquitin-conjugated proteins in the dopaminergic neurons and augment PD pathogenesis [[135], [136], [137], [138], [139]].
6. Aluminum (Al)
6.1. Al neurotoxicity
Al neurotoxicity is well-established fact now both in human and rodents. Al distributes in various brain regions and in human, its content increases with aging. Epidemiological and molecular studies have been linked the long-term exposure to Al along with its cellular concentrations, with multiple neurological disorders including PD. Elevated levels of Al have been found in dopamine-related brain regions in PD patients. The mechanism involving Al neurotoxicity is poorly understood. But the following mechanisms are considering as possible key contributors in Al-induced neurodegenerative diseases.
Oxidative stress is one of the major mechanisms behind metal induced neurotoxicity. It is a biochemical process resulting from the generation of ROS in the electron transport chain. Several studies have shown the presence of oxidative products of lipids, proteins and DNA in PD postmortem tissues. Even though Al is not a transition metal, but it reacts with other metals to generate ROS. Numerous researchers have shown the involvement of Al induced ROS and oxidative stress in Al neurotoxicity [140,141]. Numerous studies have revealed that in PD, Al affect tyrosine hydroxylase (TH) activity and inhibits DNA synthesis. Presence of Al is associated with increased aggregation of α-synuclein proteins. Mitochondria are important organelles serve as the power house of cells. It helps in the maintenance of cell functions. Several studies have revealed the involvement of Al induced oxidative stress in mitochondrial dysfunction [142,143]. Al induced oxidative stress results in proteins misfolding, reduced microtubule transport of neuronal vesicles, lipid peroxidation and eventually results in apoptosis. In addition to the aforementioned mechanisms, Al may create pro-inflammatory signals, innate immune disruption, altering NF-kβ, p53 and JNK pathways and play a pivotal role in PD pathogenesis [[144], [145], [146]].
7. Calcium (Ca)
7.1. Calcium dyshomeostasis and its role in PD pathogenesis
Brain Ca2+ dyshomeostasis has been associated with PD and other neurodegenerative disorders. The peculiar dependence of SNpc DA neurons on voltage-dependent L-type Ca2+ channels make it more vulnerable for damage. The demands of sustained Ca2+ entry enhance the aging process and make SNpc DA neurons more vulnerable to genetic and environmental challenges [[147], [148], [149]]. Ca2+ dyshomeostasis is associated with PD pathology through various mechanisms. Ca2+ promotes αSyn toxicity through Ca2+-dependent protein phosphatase calcineurin. In addition, some studies have proven that αSyn interacts with membrane to cause Ca2+ dyshomeostasis. Aggregates of αSyn activate calcium pumps SERCA, which in turn causes Ca2+ dyshomeostasis [[150], [151], [152]]. Genetic mutations such as mutations in genes sequence of αSyn, Parkin, and DJ-1 play an important role in Ca2+ dyshomeostasis and PD [153]. Cysteine proteases such as calpains and caspases, are activated by Ca2+ that degrade various substrates, including cytoskeleton proteins, metabolic enzymes and membrane receptors. It triggers apoptosis, through activation of pro-apoptotic proteins such as Bax, Par-4 and p53 [154]. Mitochondria are responsible for the “fine tuning” of Ca2+. Impairment of mitochondrial influx/efflux leads to Ca2+ dyshomeostasis and Ca2+ overload. This overload causes opening of permeability transition membrane pore (PTP). This mitochondrial Ca2+ dyshomeostasis is a key factor in PD pathogenesis. Ca2+ induces oxidative stress through various mechanisms, including activation of oxygenase such as arachidonic acid metabolism, mitochondrial Ca2+ perturbation, energy metabolism [[155], [156], [157]].
7.2. Lead (Pb)
Pb leads to alterations and decreases in dopamine, serotonin and other metabolites in the cerebral cortex, the basal and medial hypothalamus and hippocampus. In addition, it may affect the functional capacity of noradrenergic, dopaminergic, cholinergic, and GABAergic systems. In PD, Pb damages morphology of DA neurons that alters DAT. The DAT alteration causes extracellular DA levels and as a consequence causes neurotoxicity in CNS. Evidence from previous work have shown that Pb causes an alteration in Ca2+- mediated cellular processes and mimics Ca2+ binding to regulatory proteins. Also, it affects the release and reuptake of several neurotransmitters controlled by voltage gated Ca2+ channels. In the hippocampus, Pb causes αSyn accumulation, resulting in apoptosis and autophagy. It also activates protein kinase C (PKC) leading to ROS generation. Pb also induces ER stress. Pb is involved in epigenetic changes. Glial cells reactivity in response to brain injury is one of the pathological hallmarks of neurotoxicity because reactive microglia and astrocytes release numerous neurotoxic products including ROS, nitric oxide, proinflamatory cytokines and excitatory amino acids. Pb is involved in the induction of glial reactivity. Pb toxicity can be ameliorated by Pb chelating therapy [[158], [159], [160], [161], [162]].
7.3. Mercury (Hg)
In developing countries, Hg toxicity accounts for 8-fold increase in PD incidence [163,164]. Hg is associated with nervous tissues damage in mammalian species as well as young and adult humans as high levels of Hg is found in deceased brain tissues of patients suffering from neurodegenerative diseases. Epidemiological, in-vivo and in-vitro studies have revealed that Hg toxicity depends upon its chemical forms, times and doses of exposure. MeHg toxicity and its involvement in neurological dysfunction have been proven by two epidemics. First epidemics; The Minamata bay disaster in Japan (1953) by ingestion of MeHg fish and shell fish and second epidemic; in Iraq (1971–1972) by ingestion of bread contaminated by organomercury fungicide. Both took 1043 and 452 lives, respectively [165]. Hg can disrupt neurobiological processes such as synaptic transmission through over activation of NMDA receptors [166]. As Hg toxicity depends upon its form, different forms of Hg exert different toxicity levels through different mechanisms. Hg2+ form is very toxic for the brain because of its long half-life. It can cause brain damage due to its ability to bind irreversibly to thiol sulfhydryl (–SH) group altering protein structure and inhibiting enzymatic functions, binding with cysteine residue and glutathione causes inactivation of hormones and Sulphur cofactor. It can cause tubule disruption by binding to the thiol group of α- and β-tubulin. It can impair Ca-ATP pumps alter Ca homeostasis. It has the ability to inhibits glutamate uptake, promoting its release in extra cellular space. MeHg can cross the BBB as well as its take up by neuronal cells through MeHg-L-cysteine complex where it targets CNS. Epidemiological studies have shown that MeHg can transfer from pregnant mother to fetus, and causes neuronal deficits in their offspring. MeHg interacts with, and oxidized nucleophilic groups of biomolecules, sulfhydryl groups (thiol/thiolate; – SH/–S) are the main targets in biological system. MeHg interaction with sulfhydryl-containing proteins such as neurotransmitter receptors, transporters, antioxidant enzymes and non-proteins thiols such as glutathione and cysteine are main neurotoxicity mediators. MeHg direct exposure causes depletion in GSH levels. Its interaction with selenohydryl (selenol/selenolate; –SeH/–Se) is important target of neurotoxicity [[167], [168], [169], [170]].
Due to the induction of neuronal toxicity, Hg represents itself as one of the etiological factors for PD. Hg ingestion causes loss of DA neurons and glutathione depletion in SNpc, glutamate increase and mitochondrial dysfunction. Occupational exposure to Hg causes parkinsonism. Many scientists have investigated and proved the correlation of PD with Hg levels [171]. Blood Hg levels in PD patients have six folds higher as compared with control group. Another study conducted in Taiwan has proved the correlation of PD with the presence of dental amalgam. Among several professionals’ dentist were most common in PD patients [172]. Another study investigated the correlation between PD and airborne metals in female nurses and found that among other metals, only Hg is associated with highest PD risk [173]. Numerous in vitro and in vivo experimental studies have proved the Hg toxicity and its possible mechanisms in the etiology of PD, Such as oxidative stress and ROS generation, Ca2+ dyshomeostasis, mitochondrial dysfunction, apoptosis and neuroinflammation [[174], [175], [176], [177], [178], [179], [180]].
7.4. Possible role of pesticides and deregulated miRNA to PD
In spite of their beneficial role in agriculture, they have been associated with severe side effects on human health, such as acute poisoning and chronic consequences, even neurodegenerative diseases. PD is also strongly associated with environmental exposures to numerous substances and pollutants, amongst them several pesticides [[181], [182], [183]]. Glutathione-S-transferase (GST) and total antioxidant capacity are greatly influenced by pesticides. Oxidative stress (OS) has been proven to play a major role in the manifestation of neurodegenerative diseases such as PD. Pesticides may lead to these neurotoxic processes via OS [184,185]. A meta-analysis concluded that prolonged pesticide exposure was associated with a higher risk for PD of up to 11 % [186]. Another meta-analysis of case-control studies concluded that pesticides are associated with a higher risk for PD and alterations in genes related to PD. Moreover, in France, PD has even considered a professional disease of farmers applying pesticides [187,188]. In another case-control study Paraquat and rotenone were closely associated with higher risk of developing PD [189,190].
MicroRNAs (miRNAs) are small non-coding RNA molecules, that control the expression of genes in several cellular processes and the translation of mRNAs following the transcription [191,192]. miRNAs are considered to modify more than half of protein-coding genes [193]. Several studies have validated the involvement of numerous miRNAs in the disease's manifestation. In view of the wide interest of the scientific community on PD and miRNAs, many studies have been published [194,195].
7.5. Some key deregulated miRNAs involvement in pesticide-related neuronal death and PD pathogenesis are as following
Paraquat (PQ) is one of the most widely distributed herbicides. Since 1882, before the discovery of its herbicidal activities, it was known as a redox agent. Its redox potential, that produces reactive oxygen species (ROS) and consumes NADPH is toxic to plants and mammals [196]. PQ has been linked with a significantly higher risk of developing PD. Possible mechanisms underlying its links to PD have been proposed, employing a multi-faceted theory [197]. It has been shown that it can enter dopaminergic neurons via dopamine transporters (DAT), and mutations in the DAT gene also confer susceptibility to PD upon exposure to PQ [198]. A study on PQ-exposed SHSY5Y dopaminergic neurons revealed an upregulation of the highly brain-specific miRNA−153, which was linked to the researchers' previous finding, that PQ leads to cellular death via oxidative stress [199,200]. Concerning PD, miR-153 has increased in CSF samples from late onset of PD (LOPD) patients [201]. The in-vitro studies of pesticide exposure and the PD CSF results both show an upregulation, a common finding that could hint towards PQ being involved in PD pathogenesis [202]. A recent study on neuro-2a cells exposed to PQ indicated a significant downregulation of miR − 17−5p, a molecule which promotes cell proliferation and suppresses apoptosis, alongside −210−3p, −503−5p and − 374−5p. The researchers proposed that these molecules are involved in PD-pathogenesis via cellular death through endocytosis, ubiquitin-mediated proteolysis, cell cycle changes and the MAPK signaling pathway, especially the −17−5p molecule [203]. Numerous human sample investigations and in-vitro studies evident the fact that PD seems to be a derivative of environmental and genetic interactions in the individuals. We cannot deny the possibility that the miRNA deregulation noted in the cell lines could be due a global response against the neurotoxicant applied. The PQ study found the miRNA let-7 family downregulated, which is in line with the studies with CSF and plasma samples from PD patients, hinting towards another common feature between PD and PQ. miR-29, which has been implicated in epithelial–mesenchymal transition (EMT), skeletal muscle cell and osteoblast differentiation, as well as in cardiac fibrosis and systemic sclerosis which was found downregulated in human neural progenitor cells exposed to PQ [204]. It was also reported to decrease in CSF samples from PD patients [205]. It also possibly represents a common link between PQ and PD. miR-181, with -181b downregulated in the PQ study. Two studies have shown decreased levels of miR-181a in serum and brain tissue of late onset PD patients [206].
As a whole, PQ seems to be the main culprit in the oxidative procedures that may link pesticides with PD. Regarding microRNAs, several of those that have been reported as deregulated in a PD context have emerged in PQ-related research as well.
7.6. Organophosphates
Organophosphate (OP) pesticides are mostly insecticides, which are frequently applied in agricultural production and occasionally for industrial or residential use. OPs mechanism of action is the inhibition of acetylcholinesterase (Ache) [207]. Following acute OP poisoning, let-7 g were found downregulated, which are also downregulated in the CSF of PD patients [208]. Moreover, miR-141 was also found downregulated (miR-141–5p) upon acute OP poisoning, was reported downregulated (miR-141−3p) in neuron samples from PD patients. MiR-126 was found upregulated in the serum of patients exposed to OP and in two studies on dopaminergic neuron samples from PD patients [209].
7.7. Triazines
Triazines are herbicides. Atrazine, a widely-used triazine has been shown to be directly toxic for the dopaminergic neurons. It has been confirmed that miR-126 contributes to parkinsonism via the downregulation of factors in IGF-1/PI3K signaling and the rendering of cells susceptible to neurotoxins [210,211].
7.8. Organochlorines
Organochlorine (OC) pesticides represent another well-known insecticide class. OC substances exert their action due to their similarity to γ-aminobutyric acid (GABA) receptor antagonist picrotoxin, consequently blocking the inhibitory action of GABA [212]. The upregulation of the miRNA−190 family in animal models exposed to DDT, in several tissue samples, while their target gene product, Tp53inp1, was unsurprisingly decreased [213]. Tp53inp1 has involved in oxidative stress response, and its product acts as an antioxidant. Thus, miRNA's deregulation could also be involved in the OS procedure that leads to dopaminergic neuron death [214]. OCs have been shown to induce CYP polymorphisms, and thus they have been implicated in the etiology of neurodegenerative processes, such as in Alzheimer's disease and PD [207,215].
7.9. Rotenone
Rotenone is the prototypical member of the rotenoid family of naturally-occurring substances with insecticidal abilities. It inhibits mitochondrial complex I, resulting in reduced ATP generation, ROS generation and oxidative stress [216]. An odds ratio of 2.5 for humans exposed to rotenone to develop PD and attributed the rotenone-induced mitochondrial dysfunction [217]. The researchers studied the concentrations of several PD-related miRNAs in the striatum of the rotenone exposed rats and found a significant increase in miR-26a and miR-34a, and a significant decrease in miR − 7and let-7a [218]. MiR-34 was found increased in PQ-exposed neural progenitor cells. Taken together, this persistent upregulation in experiments with three pesticide categories (rotenone, OCs, PQ) have been associated with PD literature, could indicate that pesticides may be indeed associated with PD. MiR-7, in the rotenone experiment shown decreased, was also found decreased in an in-vitro study of manganese exposure, which also causes a Parkinsonian syndrome, and in brain samples of LOPD patients. miR-26a, here significantly increased, was also reported upregulated in both neuronal, and CSF samples from PD patients [219].
The aforementioned studies suggest a common pattern indicating that specific miRNAs are deregulated in a PD context, and several of those are also deregulated as a result of pesticide exposure, raising the possibility of a likely link between the two.
8. Conclusion
Metals ions which are essential for the operation of many physiological processes in the human body have a prominent role in the sustenance of healthy life. Furthermore, the homeostasis and forms of these metals in the human body is also of great concern. Even though the fundamental molecular mechanisms of PD etiology and pathogenesis is still obscure, metals toxicity have been documented in the implication of PD pathogenesis by several epidemiological studies with several potential mechanisms. In many cases, the metals equilibrium is disturbed, which leads to deleterious effect on the entire body including the brain. Such changes in the brain lead to impairment of neurons by different mechanisms including oxidative stress, mitochondrial dysfunctions, neuroinflammation and apoptosis. Consequently, the development of neurodegenerative diseases including PD occurs. Several metals such as Fe, Cu, Pb have a synergistic effect among themselves and with Hg, which adds to their neuronal toxicity. Among metals, Hg is the most toxic because it is neurotoxic in every chemical form. Due to high tubulin content, nigral dopaminergic neurons are most sensitive to Hg. Taken together, metals have a fundamental role in PD etiology and pathogenesis. In addition to metals, numerous studies have revealed the deregulation of specific miRNAs due to pesticides exposure, and their involvement in PD pathogenesis. The results finding from various studies suggest that in addition to standard treatment, using of chelating agents and antioxidants might contribute to the treatment of PD. Metals toxicity are still unclear; understanding the underlying mechanisms is essential for designation of novel therapeutic approaches. A Better understanding of these mechanisms will assist in the development of multifactorial approaches to delay or cure PD progression.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 81403145, 81560715, 51501080]; the Sub-Project of National Science and Technology Major Projects for “Major New Drugs Innovation and Development” [grant number 2015ZX09501-004-003-008]; The Fundamental Research Funds for the Central Universities of China [lzujbky-2018-136, lzujbky-2017-206, lzujbky-2018-40].
Edited by Dr. A.M Tsatsaka
Contributor Information
Xin Wang, Email: wx@lzu.edu.cn.
Hongyu Li, Email: lihy@lzu.edu.cn.
References
- 1.Shulman J.M., De Jager P.L., Feany M.B. Parkinson’s disease: genetics and pathogenesis. Annu. Rev. Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 2.Cuenca L. Parkinson’s disease: a short story of 200 years. Histol. Histopathol. 2018:18073. doi: 10.14670/HH-18-073. [DOI] [PubMed] [Google Scholar]
- 3.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A., Gilbert R.M. Epidemiology of Parkinson disease. Neurol. Clin. 2016;34(4):955–965. doi: 10.1016/j.ncl.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Pringsheim T. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 6.Gillies G.E. Sex differences in Parkinson’s disease. Front. Neuroendocrinol. 2014;35(3):370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schilder J.C. The terminology of akinesia, bradykinesia and hypokinesia: past, present and future. Parkinsonism Relat. Disord. 2017;37:27–35. doi: 10.1016/j.parkreldis.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Danoudis M., Iansek R., Simpson P. Freezing of gait in Parkinson’s disease: further insights into pathophysiological mechanisms. Parkinsonism Relat. Disord. 2012;18(5):543–547. doi: 10.1016/j.parkreldis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Titova N., Chaudhuri K.R. Nonmotor Parkinson’s and future directions. Int. Rev. Neurobiol. 2017;134:1493–1505. doi: 10.1016/bs.irn.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Bridi J.C., Hirth F. Mechanisms of alpha-synuclein induced synaptopathy in parkinson’s disease. Front. Neurosci. 2018;12:80. doi: 10.3389/fnins.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rekatsina M. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv. Ther. 2020;37(1):113–139. doi: 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Xu S.-Y.K., Lee Frank J.S., Song Weihong, Jin Lee-Way, Yankner Bruce A. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002;(June) doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 13.Casani Sandra, Emilia Matallana R.G.-P., Paricio Nuria. Antioxidant compound supplementation prevents oxidative damage in a Drosophila model of Parkinson disease. Free Radic. Biol. Med. 2013;(March 23) doi: 10.1016/j.freeradbiomed.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Johnson Parker, N.J.W James Cloyd, Tuite Paul J., Kartha R.V. GBA1 mutations: prospects for exosomal biomarkers in αsynuclein pathologies. Mol. Genet. Metab. 2019;12(October) doi: 10.1016/j.ymgme.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu Ching-Chi, Yeh Tu-Hsueh, Lu Chin-Song, Huang Yin-Cheng, Cheng Yi-Chuan, Huang Ying-Zu, Weng Yi-Hsin, Liu Yu-Chuan, Lai Szu Chia, Chen Ying-Ling, Chen Yu-Jie, Chen Chao-Lang, Chen Hsin-Yi, Wang Y.-W.La.H.-L. PARK14 PLA2G6 mutants are defective in preventing rotenoneinduced mitochondrial dysfunction, ROS generation and activation of mitochondrial apoptotic pathway. Oncotarget. 2017;17(August) doi: 10.18632/oncotarget.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuana Xiang-li, J.-f.G, Shib Zhen-hua, Xiao Zhi-qian, Yana Xin-xiang, Bao-lu Zhaob B.-s.T. R492X mutation in PTEN-induced putative kinase 1 induced cellular mitochondrial dysfunction and oxidative stress. Brain Res. 2010;(June 1) doi: 10.1016/j.brainres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Khalaf Ossama, B.F Abid Oueslati, Dikiy Igor, Mahul-Mellier Anne-Laure, Ruggeri Francesco Simone, Mbefo Martial K., Vercruysse Filip, Dietler Giovanni, Lee Seung-Jae, Eliezer David, Lashue a.H.A. The H50Q mutation enhances the H50Q mutation enhances α-Synuclein aggregation, secretion, and toxicity. J. Biol. Chem. 2014;(June 16) doi: 10.1074/jbc.M114.553297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J.D. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review) Int. J. Mol. Med. 2018;41(4):1817–1825. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- 19.Biosa Alice, A.S.-M Roberta Filograna, Terriente-Felix Ana, Alam Sarah M., Beltramini Mariano, Bubacco Luigi, Bisaglia Marco, Whitworth Alexander J. Superoxide dismutating molecules rescue the toxic effects of PINK1 and parkin loss. Hum. Mol. Genet. 2018 doi: 10.1093/hmg/ddy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storz P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci. STKE. 2006;2006(332):re3. doi: 10.1126/stke.3322006re3. [DOI] [PubMed] [Google Scholar]
- 21.Nandipati S., Litvan I. Environmental exposures and parkinson’s disease. Int. J. Environ. Res. Public Health. 2016;13(9) doi: 10.3390/ijerph13090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada Hideyuki, T.O, Yamamoto Kenji, Umemura Atsushi, Tomita Satoshi, Hayashi Ryutaro, Kohsaka Masayuki, Kawamura Takashi. Trigger medications and patient-related risk factors for parkinson disease psychosis requiring anti-psychotic drugs: a retrospective cohort study. BMC Neurol. 2013 doi: 10.1186/1471-2377-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollerhage M. Secondary parkinsonism due to drugs, vascular lesions, tumors, trauma, and other insults. Int. Rev. Neurobiol. 2019;149:377–418. doi: 10.1016/bs.irn.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Nalls M.A. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J.W., Cannon J.R. LRRK2 mutations and neurotoxicant susceptibility. Exp. Biol. Med. (Maywood) 2015;240(6):752–759. doi: 10.1177/1535370215579162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Hao, P.W, Jankovic Joseph. The genetics of Parkinson disease. Ageing Res. Rev. 2018;42:72–85. doi: 10.1016/j.arr.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Lauretti E. Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol. Psychiatry. 2017;22(2):280–286. doi: 10.1038/mp.2016.47. [DOI] [PubMed] [Google Scholar]
- 28.Fraga C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005;26(4-5):235–244. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Farina M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem. Int. 2013;62(5):575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonilla-Ramirez L., Jimenez-Del-Rio M., Velez-Pardo C. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study Parkinsonism. Biometals. 2011;24(6):1045–1057. doi: 10.1007/s10534-011-9463-0. [DOI] [PubMed] [Google Scholar]
- 31.Bush A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000 doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 32.Perello G. Dietary intake of trace elements by the population of Catalonia (Spain): results from a total diet study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015;32(5):748–755. doi: 10.1080/19440049.2015.1018844. [DOI] [PubMed] [Google Scholar]
- 33.Ciceroa Calogero Edoardo, Mostilea Giovanni, Vastaa Rosario, Rapisardab Venerando, Signorellib Salvatore Santo, Ferrantea Margherita, Zappiaa Mario, Nicolettia Alessandra. ⁎ Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017;28(July) doi: 10.1016/j.envres.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Valko* M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005 doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 35.Kwok J.B. Role of epigenetics in Alzheimer’s and Parkinson’s disease. Epigenomics. 2010 doi: 10.2217/epi.10.43. [DOI] [PubMed] [Google Scholar]
- 36.McAllum E.J., Finkelstein D.I. Metals in alzheimer’s and parkinson’s disease: relevance to dementia with lewy bodies. J. Mol. Neurosci. 2016;60(3):279–288. doi: 10.1007/s12031-016-0809-5. [DOI] [PubMed] [Google Scholar]
- 37.Piao Y.S. Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci. Rep. 2017;7(1):10547. doi: 10.1038/s41598-017-10593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coon S. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ. Health Perspect. 2006;114(12):1872–1876. doi: 10.1289/ehp.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorell J.M., M.C.C.J, Rybicki B.A., Peterson E.L., Kortsha G.X., Brown G.G., Richardson R.J. ScD Occupational exposures to metals as risk factors for Parkinson’s disease. NEUROLOGY. 1997 doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- 40.Petersen M.S. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology. 2008;29(4):584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Dadar M., Peyghan R., Memari H.R. Evaluation of the bioaccumulation of heavy metals in white shrimp (Litopenaeus vannamei) along the Persian Gulf coast. Bull. Environ. Contam. Toxicol. 2014;93(3):339–343. doi: 10.1007/s00128-014-1334-2. [DOI] [PubMed] [Google Scholar]
- 42.Dadar M. A comparative study of trace metals in male and female Caspian kutum (Rutilus frisii kutum) from the southern basin of Caspian Sea. Environ. Sci. Pollut. Res. Int. 2016;23(24):24540–24546. doi: 10.1007/s11356-016-6871-2. [DOI] [PubMed] [Google Scholar]
- 43.Mezynska M., Brzoska M.M. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. Int. 2018;25(4):3211–3232. doi: 10.1007/s11356-017-0827-z. [DOI] [PubMed] [Google Scholar]
- 44.Anyanwu B.O. Heavy metal mixture exposure and effects in developing nations: an update. Toxics. 2018;6(4) doi: 10.3390/toxics6040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H. Multiple exposure pathways and health risk assessment of heavy metal(loid)s for children living in fourth-tier cities in Hubei Province. Environ. Int. 2019;129:517–524. doi: 10.1016/j.envint.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Lucchini R.G. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environ. Health. 2019;18(1):67. doi: 10.1186/s12940-019-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellou V. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat. Disord. 2016;23:1–9. doi: 10.1016/j.parkreldis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Sun H. Association of soil selenium, strontium, and magnesium concentrations with Parkinson’s disease mortality rates in the USA. Environ. Geochem. Health. 2018;40(1):349–357. doi: 10.1007/s10653-017-9915-8. [DOI] [PubMed] [Google Scholar]
- 49.Afeseh Ngwa H. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2009;240(2):273–285. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avila-Costa M.R. Ependymal epithelium disruption after vanadium pentoxide inhalation. A mice experimental model. Neurosci. Lett. 2005;381(1-2):21–25. doi: 10.1016/j.neulet.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 51.Bisaglia M. Interaction between alpha-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2009;11(4):239–251. doi: 10.1007/s12017-009-8082-1. [DOI] [PubMed] [Google Scholar]
- 52.Bandyopadhyay S., Rogers J.T. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: maintenance of brain iron homeostasis. Biochem. Pharmacol. 2014;88(4):486–494. doi: 10.1016/j.bcp.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z. Iron deposition in substantia nigra: abnormal iron metabolism, neuroinflammatory mechanism and clinical relevance. Sci. Rep. 2017;7(1):14973. doi: 10.1038/s41598-017-14721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zucca F.A. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richardsona Des R., Lanea Darius J.R. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. PNAS. 2010;(March 15) doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sian-Hulsmann J. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011;118(6):939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang J. Iron-dependent degradation of apo-IRP1 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 2007;27(7):2423–2430. doi: 10.1128/MCB.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang H. Brain Iron metabolism dysfunction in parkinson’s disease. Mol. Neurobiol. 2017;54(4):3078–3101. doi: 10.1007/s12035-016-9879-1. [DOI] [PubMed] [Google Scholar]
- 59.Le W. Role of iron in UPS impairment model of Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:S158–S161. doi: 10.1016/S1353-8020(13)70038-5. [DOI] [PubMed] [Google Scholar]
- 60.Anderson J.P. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006;281(40):29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 61.Lu Y. Phosphorylation of alpha-Synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-Synuclein in the pathogenesis of Parkinson’s Disease and related disorders. ACS Chem. Neurosci. 2011;2(11):667–675. doi: 10.1021/cn200074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leah HAarris Z., Y.s Takahashi, Miyajima Hiroaki, Serizawa Masahiro, Ross A.J.D.G., Macgillivray T.A. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc. Natl. Acad. Sci. U. S. A. 1995;(March) doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antharam V. High field magnetic resonance microscopy of the human hippocampus in Alzheimer’s disease: quantitative imaging and correlation with iron. Neuroimage. 2012;59(2):1249–1260. doi: 10.1016/j.neuroimage.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen G.F. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013;14(12):1127–1135. doi: 10.1038/embor.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filippini T. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018;50:508–517. doi: 10.1016/j.jtemb.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Noel L. Li, Cr, Mn, Co, Ni, Cu, Zn, Se and Mo levels in foodstuffs from the second french TDS. Food Chem. 2012;132(3):1502–1513. doi: 10.1016/j.foodchem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Strozyk D. Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol. Aging. 2009;30(7):1069–1077. doi: 10.1016/j.neurobiolaging.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies K.M. Localization of copper and copper transporters in the human brain. Metallomics. 2013;5(1):43–51. doi: 10.1039/c2mt20151h. [DOI] [PubMed] [Google Scholar]
- 69.Ayton S. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013;73(4):554–559. doi: 10.1002/ana.23817. [DOI] [PubMed] [Google Scholar]
- 70.Fu S., Jiang W., Zheng W. Age-dependent increase of brain copper levels and expressions of copper regulatory proteins in the subventricular zone and choroid plexus. Front. Mol. Neurosci. 2015;8:22. doi: 10.3389/fnmol.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizue Moriya Y.-H.H., Grana Anne, Nguyen Linh, Alvarez Arrissa, Jamil Rita, Leigh Ackland A.M.M., Hamer Pia, Ramos Danny, Kim Stephen, Mercer Julian F.B., Linder a.M.C. Copper is taken up efficiently from albumin and α2-macroglobulin by cultured human cells by more than one mechanism. Am. J. Physiol., Cell Physiol. 2008;(June 25) doi: 10.1152/ajpcell.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimnicka A.M., Maryon E.B., Kaplan J.H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes. J. Biol. Chem. 2007;282(36):26471–26480. doi: 10.1074/jbc.M702653200. [DOI] [PubMed] [Google Scholar]
- 73.Kuo Yien-Ming, A.A.G, y Joshua W.Pyatskowit, y Jane Gitschier, Prohaskay a.J.R. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J. Nutr. 2005;27(October) doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monnot A.D., Zheng G., Zheng W. Mechanism of copper transport at the blood–cerebrospinal fluid barrier: influence of iron deficiency in anin vitromodel. Exp. Biol. Med. 2012;237(3):327–333. doi: 10.1258/ebm.2011.011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larner F. High precision isotope measurements reveal poor control of copper metabolism in parkinsonism. Metallomics. 2013;5(2):125–132. doi: 10.1039/c3mt20238k. [DOI] [PubMed] [Google Scholar]
- 76.Wang H. The distribution profile and oxidation states of biometals in APP transgenic mouse brain: dyshomeostasis with age and as a function of the development of Alzheimer’s disease. Metallomics. 2012;4(3):289–296. doi: 10.1039/c2mt00104g. [DOI] [PubMed] [Google Scholar]
- 77.Shin J.H., Dawson V.L., Dawson T.M. SnapShot: pathogenesis of Parkinson’s disease. Cell. 2009;139(2):440 e1–2. doi: 10.1016/j.cell.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 78.Kostas Vekrellis H.J.R., Stefanis Leonidas. Neurobiology of -synuclein. Mol. Neurobiol. 2004 doi: 10.1385/MN:30:1:001. [DOI] [PubMed] [Google Scholar]
- 79.Karpenko M.N. Role of copper dyshomeostasis in the pathogenesis of parkinson’s disease. Bull. Exp. Biol. Med. 2018;164(5):596–600. doi: 10.1007/s10517-018-4039-4. [DOI] [PubMed] [Google Scholar]
- 80.Camponeschi F. Copper(I)-alpha-synuclein interaction: structural description of two independent and competing metal binding sites. Inorg. Chem. 2013;52(3):1358–1367. doi: 10.1021/ic302050m. [DOI] [PubMed] [Google Scholar]
- 81.Christos Proukakis P. A novel a-SYNUCLEIN missense mutation in Parkinson Disease. Neurology. 2013;12(March) doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liua Fang, J.H, Nguyena Jamie L., Rufa Katie J., Zhua Junyi, Jeremy L., Schielera C.C.B., Woodc Karl V., Jo Davissona V., Christophe Jean. Rocheta, Methionine sulfoxide reductase a protects dopaminergic cells from Parkinson’s disease-related insults. Free Radic. Biol. Med. 2008 doi: 10.1016/j.freeradbiomed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tiwari M.K. Early events in copper-ion catalyzed oxidation of alpha-synuclein. Free Radic. Biol. Med. 2018;121:38–50. doi: 10.1016/j.freeradbiomed.2018.04.559. [DOI] [PubMed] [Google Scholar]
- 84.Vieira B.D. Neuroinflammation in multiple system atrophy: response to and cause of alpha-synuclein aggregation. Front. Cell. Neurosci. 2015;9:437. doi: 10.3389/fncel.2015.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruces-Sande A. Copper increases brain oxidative stress and enhances the ability of 6-Hydroxydopamine to cause dopaminergic degeneration in a rat model of parkinson’s disease. Mol. Neurobiol. 2019;56(4):2845–2854. doi: 10.1007/s12035-018-1274-7. [DOI] [PubMed] [Google Scholar]
- 86.Mitra J. New perspectives on oxidized genome damage and repair inhibition by pro-oxidant metals in neurological diseases. Biomolecules. 2014;4(3):678–703. doi: 10.3390/biom4030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaetke L.M., Chow-Johnson H.S., Chow C.K. Copper: toxicological relevance and mechanisms. Arch. Toxicol. 2014;88(11):1929–1938. doi: 10.1007/s00204-014-1355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cobine P.A. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004;279(14):14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 89.Alimba C.G. Genotoxicity and cytotoxicity of chromium, copper, manganese and lead, and their mixture in WIL2-NS human B lymphoblastoid cells is enhanced by folate depletion. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016;798-799:35–47. doi: 10.1016/j.mrgentox.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Wang D.B. p53 and mitochondrial function in neurons. Biochim. Biophys. Acta. 2014;1842(8):1186–1197. doi: 10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spencer W.A. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: role of reactive oxygen species. Free Radic. Biol. Med. 2011;50(1):139–147. doi: 10.1016/j.freeradbiomed.2010.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gurung S.K. Structural alterations of wogonin significantly reduce the inhibitory activity against COX-2 catalyzed PGE2Production from LPS-Induced RAW 264.7 cells. Biomol. Ther. 2009;17(4):418–421. [Google Scholar]
- 93.Mufti Arjmand R., E.B.a.C.S.D XIAP: cell death regulation meets copper homeostasis. Arch. Biochem. Biophys. 2007;(July 15) doi: 10.1016/j.abb.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Letelier M.E. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 2005;151(2):71–82. doi: 10.1016/j.cbi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Pamp K. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic. Res. 2005;39(1):31–40. doi: 10.1080/10715760400023671. [DOI] [PubMed] [Google Scholar]
- 96.Hegde M.L. Specific Inhibition of NEIL-initiated repair of oxidized base damage in human genome by copper and iron: potential etiological linkage to neurodegenerative diseases. J. Biol. Chem. 2010;285(37):28812–28825. doi: 10.1074/jbc.M110.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaier E.D., Eipper B.A., Mains R.E. Copper signaling in the mammalian nervous system: synaptic effects. J. Neurosci. Res. 2013;91(1):2–19. doi: 10.1002/jnr.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michael Aschner K.M.E., Dorman David C. Manganese dosimetry: species differences and implications for neurotoxicity. Crit. Rev. Toxicol. 2005 doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- 99.Shiek S.S.J., Ahmed W.S. Metallomic profiling and linkage map analysis of early Parkinson’s Disease: a new insight to aluminum marker for the possible diagnosis. PLoS One. 2010;(June 22) doi: 10.1371/journal.pone.0011252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herrero Hernandez E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006;27(3):333–339. doi: 10.1016/j.neuro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Guilarte T.R. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem. 2008;105(5):1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bowman A.B., Aschner M. Considerations on manganese (Mn) treatments for in vitro studies. Neurotoxicology. 2014;41:141–142. doi: 10.1016/j.neuro.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reaney S.H., Bench G., Smith D.R. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol. Sci. 2006;93(1):114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- 104.Robison G. X-ray fluorescence imaging: a new tool for studying manganese neurotoxicity. PLoS One. 2012;7(11):e48899. doi: 10.1371/journal.pone.0048899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robisona Gregory, T.Z Sherleen Fub, Jiangb Wendy, Fulpera Rachael, Raul, Barreac d., Zhengb Wei, Pushkara Yulia. X-ray fluorescence imaging of the hippocampal formation after manganese exposure. Metallomics. 2013 doi: 10.1039/c3mt00133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar K.K. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci. Rep. 2014;4:6801. doi: 10.1038/srep06801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buettner Garry R., C.F.N Min Wang, Rodgers V.G.J., Schafer Freya Q. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med. 2006 doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.WoŸniak-Celmer Edyta, S.a.O.d.a.J.C Theoretical models of catalytic domains of protein phosphatases 1 and 2A with Zn2+ and Mn2+ metal dications and putative bioligands in their catalytic centers. Acta bio chimica polonica. 2001;3(March) [PubMed] [Google Scholar]
- 109.Quadri M. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 2012;90(3):467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stamelou M. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov. Disord. 2012;27(10):1317–1322. doi: 10.1002/mds.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zecca L. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. 2004;101(26):9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Troadec Jean-Denis, M.M, Darios FreÂdeÂric, A.H, Ruberg* *Merle, Colpaert Francis, Michel Patrick P. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001 doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 113.Garcia S.J. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol. Sci. 2006;92(2):516–525. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- 114.Anderson J.G., Cooney P.T., Erikson K.M. Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol. Sci. 2007;95(1):188–195. doi: 10.1093/toxsci/kfl130. [DOI] [PubMed] [Google Scholar]
- 115.Aschner M. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11(4):252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tjalkens R.B. Manganese suppresses ATP-dependent intercellular calcium waves in astrocyte networks through alteration of mitochondrial and endoplasmic reticulum calcium dynamics. Brain Res. 2006;1113(1):210–219. doi: 10.1016/j.brainres.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 117.Moreno J.A. Manganese-induced NF-kappaB activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol. Sci. 2011;122(1):121–133. doi: 10.1093/toxsci/kfr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sengupta Amitabha, Mense Sarah M., Lan Changgui, Zhou Mei, Mauro Rory E., Lisa G.B.K.ellerman, Volsky David J., Louis Elan D., Graziano Joseph H., Zhang L. Gene expression profiling of human primary astrocytes exposed to manganese chloride indicates selective effects on several. Neurotoxicology. 2007;(May) doi: 10.1016/j.neuro.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu B. Inhibition of calpain prevents manganese-induced cell injury and alpha-synuclein oligomerization in organotypic brain slice cultures. PLoS One. 2015;10(3):e0119205. doi: 10.1371/journal.pone.0119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uversky V.N., Li J., Fink A.L. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J. Biol. Chem. 2001;276(14):10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 121.Lin X. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gitler A.D. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 2009;41(3):308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gartel A.L., Kandel E.S. miRNAs: little known mediators of oncogenesis. Semin. Cancer Biol. 2008;18(2):103–110. doi: 10.1016/j.semcancer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 124.Junn E. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kegel Kimberly B., M.K, Sapp Ellen, McIntyre Charmian, Castaño JoséG., Aronin Neil, DiFiglia M. Huntingtin expression stimulates endosomal–lysosomal activity, endosome tubulation, and autophagy. J. Neurosci. 2000;(October 1) doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Opoka W. Development and validation of an anodic stripping voltammetric method for determination of Zn(2+) ions in brain microdialysate samples. Biol. Trace Elem. Res. 2011;142(3):671–682. doi: 10.1007/s12011-010-8790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morris D.R., Levenson C.W. Ion channels and zinc: mechanisms of neurotoxicity and neurodegeneration. J. Toxicol. 2012;2012:785647. doi: 10.1155/2012/785647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamasaki S. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007;177(4):637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawahara M., Tanaka K.I., Kato-Negishi M. Zinc, carnosine, and neurodegenerative diseases. Nutrients. 2018;10(2) doi: 10.3390/nu10020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du K. Decreased circulating zinc levels in Parkinson’s disease: a meta-analysis study. Sci. Rep. 2017;7(1):3902. doi: 10.1038/s41598-017-04252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Remelli M. Interaction of divalent cations with peptide fragments from Parkinson’s disease genes. Dalton Trans. 2013;42(17):5964–5974. doi: 10.1039/c2dt32222f. [DOI] [PubMed] [Google Scholar]
- 132.Sheline C.T. Serum or target deprivation-induced neuronal death causes oxidative neuronal accumulation of Zn2+ and loss of NAD+ Eur. J. Neurosci. 2010;32(6):894–904. doi: 10.1111/j.1460-9568.2010.07372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manzerra P. Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2001;98(20):11055–11061. doi: 10.1073/pnas.191353598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aizenman Elias, A.K.S, Hartnett Karen A., Dineley *KirkE., McLaughlin Beth Ann, Reynolds a.I.J. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 2000 doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 135.Xu L., Pu J. Alpha-synuclein in parkinson’s disease: from pathogenetic dysfunction to potential clinical application. Parkinsons Dis. 2016;2016:1720621. doi: 10.1155/2016/1720621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 137.McNaught K.S. Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 2003;179(1):38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 138.Kumar V. Alpha-synuclein aggregation, Ubiquitin proteasome system impairment, and l-Dopa response in zinc-induced Parkinsonism: resemblance to sporadic Parkinson’s disease. Mol. Cell. Biochem. 2017;444(1-2):149–160. doi: 10.1007/s11010-017-3239-y. [DOI] [PubMed] [Google Scholar]
- 139.Yang W. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum. Mol. Genet. 2007;16(23):2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- 140.Wei Y.-H., Lee H.-C. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biolog Med. 2013 doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 141.Wu Z. Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol. Aging. 2012;33(1):199. doi: 10.1016/j.neurobiolaging.2010.06.018. e1-12. [DOI] [PubMed] [Google Scholar]
- 142.Johri A., Beal M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012;342(3):619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kumar V., Gill K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 144.Johnson V.J., Kim S.H., Sharma R.P. Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol. Sci. 2005;83(2):329–339. doi: 10.1093/toxsci/kfi028. [DOI] [PubMed] [Google Scholar]
- 145.VanDuyn N. The metal transporter SMF-3/DMT-1 mediates aluminum-induced dopamine neuron degeneration. J. Neurochem. 2013;124(1):147–157. doi: 10.1111/jnc.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campbell A. Pro-inflammatory effects of aluminum in human glioblastoma cells. Brain Res. 2002;933(1):60–65. doi: 10.1016/s0006-8993(02)02305-3. [DOI] [PubMed] [Google Scholar]
- 147.Ritz B. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010;67(5):600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michael J., Hurley B.B., Gentleman Steve M., Dexter David T. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain. 2013;(April 12) doi: 10.1093/brain/awt134. [DOI] [PubMed] [Google Scholar]
- 149.Chan C.S., Gertler T.S., Surmeier D.J. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32(5):249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Caraveo Gabriela, Auluck Pavan K., Whitesella Luke. Calcineurin determines toxic versus beneficial responses to α-synuclein. PNAS. 2014;(August 13) doi: 10.1073/pnas.1413201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Angelova Plamena R., M.H.R.L, Horrocks Mathew H. Calcium is a key factor in α-synuclein induced neurotoxicity. J. Cell. Sci. 2016 doi: 10.1242/jcs.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Betzer C. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018;19(5) doi: 10.15252/embr.201744617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lashuel H.A. Α-synuclein, especially the parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 2002;322(5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 154.Dargusch Richard, D.P, Tan Shirlee, Liu Yuanbin, Schuber David. The role of Bax in glutamate-induced nerve cell death. J. Neurochem. 2000;(August 31) doi: 10.1046/j.1471-4159.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 155.Dupont G., Combettes L. Fine tuning of cytosolic Ca (2+) oscillations. F1000Res. 2016;5 doi: 10.12688/f1000research.8438.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Surmeier D.J., Schumacker P.T. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J. Biol. Chem. 2013;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Verma M. Mitochondrial calcium dysregulation contributes to dendrite degeneration mediated by PD/LBD-Associated LRRK2 mutants. J. Neurosci. 2017;37(46):11151–11165. doi: 10.1523/JNEUROSCI.3791-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Needleman H. Lead poisoning. Annu. Rev. Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 159.Gurer H., Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000;29(10):927–945. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 160.Adhikari A. 5-Aminolevulinate and 4, 5-dioxovalerate ions decrease GABA(A) receptor density in neuronal cells, synaptosomes and rat brain. Brain Res. 2006;1093(1):95–104. doi: 10.1016/j.brainres.2006.03.103. [DOI] [PubMed] [Google Scholar]
- 161.Aykin-Burns N. Oxidative effects of lead in young and adult Fisher 344 rats. Arch. Environ. Contam. Toxicol. 2003;44(3):417–420. doi: 10.1007/s00244-002-2023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lahiri D.K., Maloney B., Zawia N.H. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry. 2009;14(11):992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yokoo Edna M., J.G.V, Grattan Lynn, Schmidt S.érgio Luís, Silbergeld I.Pa.E.K. Low level methylmercury exposure affects neuropsychological function in adults. BioMed Central. 2003;(4 June) doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mutter J., Naumann J., Guethlin C. Comments on the article "the toxicology of mercury and its chemical compounds" by Clarkson and Magos (2006) Crit. Rev. Toxicol. 2007;37(6):537–549. doi: 10.1080/10408440701385770. discussion 551-552. [DOI] [PubMed] [Google Scholar]
- 165.Cariccio V.L. Mercury involvement in neuronal damage and in neurodegenerative diseases. Biol. Trace Elem. Res. 2019;187(2):341–356. doi: 10.1007/s12011-018-1380-4. [DOI] [PubMed] [Google Scholar]
- 166.Xu1 Fenglian, Farkas1 Svetlana. Mercury-induced toxicity of rat cortical neurons is mediated through N-methyl-D-Aspartate receptors. Mol. Brain. 2012 doi: 10.1186/1756-6606-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huel G. Hair mercury negatively correlates with calcium pump activity in human term newborns and their mothers at delivery. Environ. Health Perspect. 2008;116(2):263–267. doi: 10.1289/ehp.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farina M., Rocha J.B., Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89(15-16):555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bjorklund G. Iron and other metals in the pathogenesis of Parkinson’s disease: toxic effects and possible detoxification. J. Inorg. Biochem. 2019;199:110717. doi: 10.1016/j.jinorgbio.2019.110717. [DOI] [PubMed] [Google Scholar]
- 170.Fernandes Azevedo B. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012;2012:949048. doi: 10.1155/2012/949048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bjorklund G. Metals and parkinson’s disease: mechanisms and biochemical processes. Curr. Med. Chem. 2018;25(19):2198–2214. doi: 10.2174/0929867325666171129124616. [DOI] [PubMed] [Google Scholar]
- 172.Hsu Y.C. Association between history of dental amalgam fillings and risk of parkinson’s disease: a population-based retrospective cohort study in Taiwan. PLoS One. 2016;11(12):e0166552. doi: 10.1371/journal.pone.0166552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Palacios N. A prospective analysis of airborne metal exposures and risk of Parkinson disease in the nurses’ health study cohort. Environ. Health Perspect. 2014;122(9):933–938. doi: 10.1289/ehp.1307218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Teixeira F.B. Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int. J. Environ. Res. Public Health. 2014;11(9):9171–9185. doi: 10.3390/ijerph110909171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Manfroi C.B. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol. Sci. 2004;81(1):172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- 176.Simone1 U.D. Human Co-culture model of neurons and astrocytes to test acute cytotoxicity of neurotoxic compounds. Int. J. Toxicol. 2017 doi: 10.1177/1091581817739428. [DOI] [PubMed] [Google Scholar]
- 177.Amonpatumrat S. L-glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J. Pharmacol. Sci. 2008;108(3):280–289. doi: 10.1254/jphs.08118fp. [DOI] [PubMed] [Google Scholar]
- 178.Shanker G. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Brain Res. Mol. Brain Res. 2004;128(1):48–57. doi: 10.1016/j.molbrainres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 179.Venkatesan R.S., Sadiq A.M. Effect of morin-5’-sulfonic acid sodium salt on the expression of apoptosis related proteins caspase 3, Bax and Bcl 2 due to the mercury induced oxidative stress in albino rats. Biomed. Pharmacother. 2017;85:202–208. doi: 10.1016/j.biopha.2016.09.090. [DOI] [PubMed] [Google Scholar]
- 180.Coopera Joel F., Kusnecov Alexander W. Methylmercuric chloride induces activation of neuronal stress circuitry and alters exploratory behavior in the mouse. Neuroscience. 2007;(September 21) doi: 10.1016/j.neuroscience.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dardiotis E. H. Pylori and Parkinson’s disease: meta-analyses including clinical severity. Clin. Neurol. Neurosurg. 2018;175:16–24. doi: 10.1016/j.clineuro.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 182.Freire C., Koifman S. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology. 2012;33(5):947–971. doi: 10.1016/j.neuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 183.Hernandez A.F. Application of novel technologies and mechanistic data for risk assessment under the real-life risk simulation (RLRS) approach. Food Chem. Toxicol. 2020;137:111123. doi: 10.1016/j.fct.2020.111123. [DOI] [PubMed] [Google Scholar]
- 184.Abdollahi Mohammad, A.R, Shadnia Shahin, Nikfar Shekoufeh, Rezaie A. Pesticides and oxidative stress: a review. Med. Sci. Monit. 2004 [PubMed] [Google Scholar]
- 185.Tsatsakis A.M. Hormetic Neurobehavioral effects of low dose toxic chemical mixtures in real-life risk simulation (RLRS) in rats. Food Chem. Toxicol. 2019;125:141–149. doi: 10.1016/j.fct.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 186.Yan D. Pesticide exposure and risk of Parkinson’s disease: dose-response meta-analysis of observational studies. Regul. Toxicol. Pharmacol. 2018;96:57–63. doi: 10.1016/j.yrtph.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 187.Ahmed H. Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed. Pharmacother. 2017;90:638–649. doi: 10.1016/j.biopha.2017.03.100. [DOI] [PubMed] [Google Scholar]
- 188.Delamarre A., Meissner W.G. Epidemiology, environmental risk factors and genetics of Parkinson’s disease. Presse Med. 2017;46(2 Pt 1):175–181. doi: 10.1016/j.lpm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 189.Brouwer M. Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ. Int. 2017;107:100–110. doi: 10.1016/j.envint.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 190.Liu C. A scientometric analysis and visualization of research on parkinson’s disease associated with pesticide exposure. Front. Public Health. 2020;8:91. doi: 10.3389/fpubh.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abe M., Bonini N.M. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol. 2013;23(1):30–36. doi: 10.1016/j.tcb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goodall E.F. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front. Cell. Neurosci. 2013;7:178. doi: 10.3389/fncel.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cloutier F. MicroRNAs as potential circulating biomarkers for amyotrophic lateral sclerosis. J. Mol. Neurosci. 2015;56(1):102–112. doi: 10.1007/s12031-014-0471-8. [DOI] [PubMed] [Google Scholar]
- 194.Goh S.Y. Role of MicroRNAs in Parkinson’s disease. Int. J. Mol. Sci. 2019;20(22) doi: 10.3390/ijms20225649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leggio L. microRNAs in Parkinson’s disease: from pathogenesis to novel diagnostic and therapeutic approaches. Int. J. Mol. Sci. 2017;18(12) doi: 10.3390/ijms18122698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dinis-Oliveira R.J. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 197.Zhang X.F., Thompson M., Xu Y.H. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab. Invest. 2016;96(5):496–507. doi: 10.1038/labinvest.2015.161. [DOI] [PubMed] [Google Scholar]
- 198.Tangamornsuksan W. Paraquat exposure and Parkinson’s disease: a systematic review and meta-analysis. Arch. Environ. Occup. Health. 2019;74(5):225–238. doi: 10.1080/19338244.2018.1492894. [DOI] [PubMed] [Google Scholar]
- 199.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Narasimhan M. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014;228(3):179–191. doi: 10.1016/j.toxlet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gui Ya Xing, H.L, Zhang Li Shan, Lv Wen, Hu Xing Yue. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;(September 30) doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fragkouli A., Doxakis E. miR-7 and miR-153 protect neurons against MPP(+)-induced cell death via upregulation of mTOR pathway. Front. Cell. Neurosci. 2014;8:182. doi: 10.3389/fncel.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang Q. Paraquat and MPTP alter microRNA expression profiles, and downregulated expression of miR-17-5p contributes to PQ-induced dopaminergic neurodegeneration. J. Appl. Toxicol. 2018;38(5):665–677. doi: 10.1002/jat.3571. [DOI] [PubMed] [Google Scholar]
- 204.Cushing L. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Margis R., Margis R., Rieder C.R. Identification of blood microRNAs associated to Parkinsonis disease. J. Biotechnol. 2011;152(3):96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 206.Ding H. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Parkinsonism Relat. Disord. 2016;22:68–73. doi: 10.1016/j.parkreldis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 207.Dardiotis E. Organochlorine pesticide levels in Greek patients with Parkinson’s disease. Toxicol. Rep. 2020;7:596–601. doi: 10.1016/j.toxrep.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Burgos K. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS One. 2014;9(5):e94839. doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tolosa E. MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol. Aging. 2018;69:283–291. doi: 10.1016/j.neurobiolaging.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 210.Kim W. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging. 2014;35(7):1712–1721. doi: 10.1016/j.neurobiolaging.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aloizou A.-M. Parkinson’s disease and pesticides: are microRNAs the missing link? Sci. Total Environ. 2020;744:140591. doi: 10.1016/j.scitotenv.2020.140591. [DOI] [PubMed] [Google Scholar]
- 212.Vale C. The organochlorine pesticides γ-hexachlorocyclohexane (lindane), α-endosulfan and dieldrin differentially interact with GABAA and glycine-gated chloride channels in primary cultures of cerebellar granule cells. Neuroscience. 2003;117(2):397–403. doi: 10.1016/s0306-4522(02)00875-8. [DOI] [PubMed] [Google Scholar]
- 213.Kalinina T.S. Expression of the miR-190 family is increased under DDT exposure in vivo and in vitro. Mol. Biol. Rep. 2018;45(6):1937–1945. doi: 10.1007/s11033-018-4343-0. [DOI] [PubMed] [Google Scholar]
- 214.Seong K.M., Coates B.S., Pittendrigh B.R. Impacts of sub-lethal DDT exposures on microRNA and putative target transcript expression in DDT resistant and susceptible Drosophila melanogaster strains. Front. Genet. 2019;10:45. doi: 10.3389/fgene.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Docea A.O. CYP polymorphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol. Rep. 2017;4:335–341. doi: 10.1016/j.toxrep.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Johnson M.E., Bobrovskaya L. An update on the rotenone models of Parkinson’s disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology. 2015;46:101–116. doi: 10.1016/j.neuro.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 217.Tanner C.M. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Horst C.H. Signature of aberrantly expressed microRNAs in the striatum of rotenone-induced parkinsonian rats. Neurochem. Res. 2018;43(11):2132–2140. doi: 10.1007/s11064-018-2638-0. [DOI] [PubMed] [Google Scholar]