Abstract
Aging is associated with functional and metabolic decline and is a risk factor for all noncommunicable diseases. Even though mice are routinely used for modeling human aging and aging-related conditions, no comprehensive assessment to date has been conducted on normative mouse aging. To address this gap, the Study of Longitudinal Aging in Mice (SLAM) was designed and implemented by the National Institute on Aging (NIA/NIH) as the mouse counterpart to the Baltimore Longitudinal Study of Aging (BLSA). In this manuscript, we describe the premise, study design, methodologies, and technologies currently employed in SLAM. We also discuss current and future study directions. In this large population mouse study, inbred C57BL/6J and outbred UM-HET3 mice of both sexes are longitudinally evaluated for functional, phenotypic, and biological health, and collection of biospecimens is conducted throughout their life span. Within the longitudinal cohorts, a cross-sectional arm of the study has also been implemented for the well-controlled collection of tissues to generate a biorepository. SLAM and studies stemming from SLAM seek to identify and characterize phenotypic and biological predictors of mouse aging and age-associated conditions, examine the degrees of functional and biomolecular variability that occur within inbred and genetically heterogeneous mouse populations with age, and assess whether these changes are consistent with alterations observed in human aging in BLSA. The findings from these studies will be critical for evaluating the utility of mouse models for studying different aspects of aging, both in terms of interpreting prior findings and designing and implementing future studies.
Keywords: Aging, Biomarkers, Longitudinal, Mice, Preclinical
Rationale for Study Inception
Aging as a Global Public Health Problem
Increasing human life expectancy is leading to an unprecedented growth of elderly populations around the globe. As people worldwide are living longer, there is an associated exponential growth in the burden of age-related diseases (1). Recent studies indicate that life-span extension in the absence of improvements in quality of life and health is counterproductive (2). To tackle this issue, the World Health Organization (WHO) released the “Global strategy and action plan on ageing and health” in 2015, in which 5 strategic objectives were put forth and aimed at achieving healthy aging for all (3). A thorough understanding of the aging process at the organism, organ, and cellular levels is required in order to achieve this goal (4). Aging itself is characterized by the accumulation of unrepaired damage at the cellular level that leads to a decline in cognitive, physical, and organismal functions, and increased burden of diseases that will ultimately cause death (5). Aging is also associated with progressive comorbidities such as frailty (6) and reduced gait speed (7) that play an important role in age-related functional decline in older adults. Targeting molecular pathways associated with aging has, therefore, been proposed as an effective strategy to prevent and treat chronic conditions (8) and has been the principle behind the larger research framework established based on the geroscience hypothesis (9). However, the successful clinical translation of interventions targeting aging is directly dependent on having a comprehensive understanding of the normative aging process (10).
Longitudinal Studies on Aging in Humans
Given the gradual loss of optimal physiological and biological functions with advancing age, emphasis has been placed on studying normative aging in nondiseased individuals (11). Longitudinal studies of aging collect a plethora of health measures throughout the life span of the same individuals (12). Data generated from these studies help identify trajectories associated with aging (functional, phenotypic, and biological metrics of aging) in a given population, leading to a greater understanding of the link between aging and age-associated chronic diseases (13,14). Given the high heterogeneity seen within human populations, cross-sectional sampling cannot accurately capture the variations that occur in different parameters throughout life. There are multiple ongoing human longitudinal aging studies that carry specific strengths and limitations (15). Amongst these, the Baltimore Longitudinal Study of Aging (BLSA) is one of the world’s oldest studies on healthy normative aging in humans (16). Since its inception by Dr. Nathan Shock in 1958, BLSA has provided valuable insight on age-associated metabolic dysfunction (17) and hematological changes (18), as well as declines in gait speed (19) and cognition (20). Furthermore, an extensive repository of longitudinally collected biological samples such as blood and urine allow further assessment of gradual changes associated with the aging process. In addition to providing numerous assessments of individual phenotypes, the measurement of multiple metrics has allowed the identification of biomarker signatures associated with aging (21,22). Such predictors can potentially help better define aging and outcomes associated with aging (23,24), and will allow researchers to study aging over a shorter time frame, rather than the entire life span where mortality is the primary endpoint.
Life expectancy at birth in the United States is about 74–86 years for males and 80–89 years for females (25), making it extremely challenging to assess the entire human life course for research purposes. In addition to being lengthy, financially cumbersome, and resource intensive, human aging studies also have major limitations related to restricted biological sampling and complications involved with intervention testing. Because of these issues, animals have been used as models of human aging and disease. Current ongoing longitudinal aging and intervention studies in nonhuman primates have shed light on important aspects of primate aging biology (26). On the other hand, mice have a shorter generation time (~5–7 weeks from birth to reproductive age), a relatively short life span (~2.5–3 years), and are convenient to handle and house, which have made them an attractive mammalian model organism in aging research (27,28).
The Need for Longitudinal Aging Studies in Mice
Research using mouse models has greatly contributed to the progress made on understanding human diseases (29,30). Within aging research, mice have been used to identify and characterize some of the key drivers of health and survival, and have served as a preclinical model to test life-span-extending compounds in various studies, including the NIA-sponsored Interventions Testing Program (ITP) (31–33). However, recent work has demonstrated that genetic heterogeneity, as well as other factors like sex and age of administration, can dramatically impact the effectiveness of antiaging interventions, including caloric restriction (CR) (34). Furthermore, most of the proposed metrics of aging originated from cross-sectional studies and are based on associations with chronological age that often fail to accurately predict mortality. Thus, a true model of functional or phenotypic normative aging domains is lacking, highlighting the need to prioritize these types of studies within preclinical models of geroscience research (35). Additionally, the ITP has recently demonstrated that even when considerable care is taken to standardize conditions across study sites, including husbandry, diet, and mouse strain, variability in life span between test sites remains (36). Aging is a dynamic process that occurs gradually over time with the rate of aging changing at specific times of the life span. Yet, in mice chronological, normative aging is still poorly characterized and understood. Although power calculations are necessary and standard practice for preclinical and clinical studies, large comprehensive resources for such calculations in mouse aging studies are currently inadequate and rely on many assumptions (37). The inherent biological differences between mice and humans are an important consideration for any research utilizing mouse models. However, the use of mice as reliable models of human aging has been greatly hindered by the lack of a comprehensive analysis of normal aging across sex and strains (38,39). Therein, translationally relevant preclinical biomarkers have been identified as a key area of investigation for the development of successful antiaging therapeutics (40). Although similarities in core biological processes between mice and humans are widely accepted (41), key differences can lead to limited clinical success (42). In general, studies of aging and disease in mice and other model organisms presume a degree of consistency between different species. While most studies of specific diseases are preceded by a thorough assessment of the suitability of mice for a given study, this type of vigorous characterization of aging in mice has been lacking.
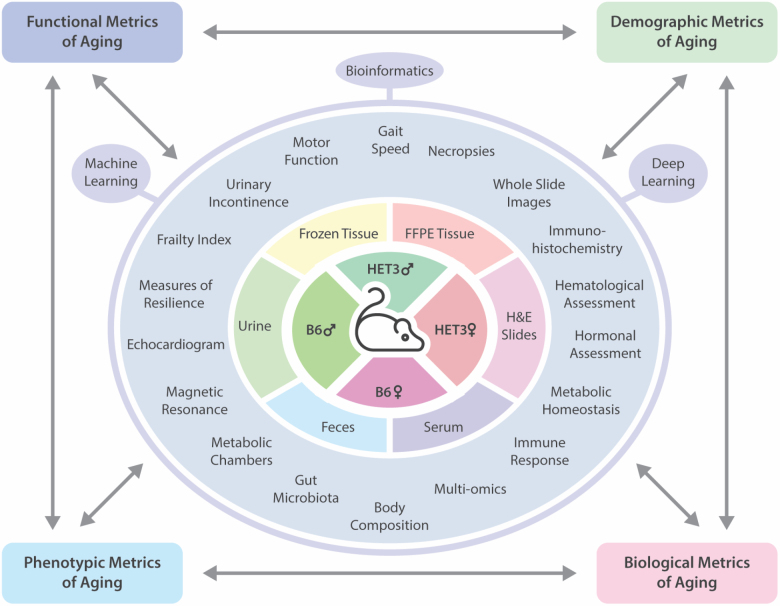
A comprehensive assessment of normative aging in the mouse is needed in order to fill a gap in knowledge regarding the strengths and limitations of mice as a preclinical research tool for the study of the aging process. Therefore, the Study of Longitudinal Aging in Mice (SLAM) was implemented in order to collect repeated measures on functional, phenotypic, and biological changes over time, and to identify biomarkers that predict morbidity and mortality across 2 different strains of mice of both sexes (Figure 1). One of the primary goals of the SLAM study is to determine whether the trajectories of change driving the aging phenotypes in mice differ from those in humans in BLSA, and to identify factors that are reliable predictors and/or drivers of aging across species. These characterizations will allow a better understanding of how to best utilize mice as a robust preclinical tool for human aging and age-related diseases. The added advantage of SLAM and BLSA both being conducted at the same site uniquely positions the NIA IRP to effectively execute this translational gerontology study to its full capacity. In the following sections, we describe the study design (strains, diet, timeline, and type of measurements), and implementation strategy (methods, biorepository/database setup, and data analysis) currently employed in SLAM, as well as discussing its future directions.
Figure 1.
Identification and characterization of longitudinal aging biomarkers in mice.
Study Description
Animals and Diet
Two strains of mice—C57BL/6J and HET3—of both sexes were chosen for the study. Both an inbred and an outbred strain were employed in order to account for genetic heterogeneity as well as strain-specific pathologies. Inbred mice have long been preferred over outbred animals due to the assumption that they may present less phenotypic variability (43), although this notion has been debated (44). C57BL/6J mice are one of the most commonly used strains in biomedical research and their inclusion within SLAM will allow comparison to previous and parallel studies that provide an incredible wealth of information on how dietary alterations, genetic modifications, and other interventions impact aging and age-related diseases. However, as prior work has clearly demonstrated the important role that genetic heterogeneity can have on aging and antiaging interventions (34,45), this study also includes parallel cohorts of HET-3 mice, the same strain of mice used in the ITP. By using both strains, we will be able to directly assess the relative importance of genetic heterogeneity versus further variability caused by epigenetic or environmental differences in driving normal aging. Sex-specific differences in aging phenotypes have been noted in previous studies (46), where males and females show differences in behavior and health span, as well as in mortality patterns (47,48). SLAM will allow to comprehensively examine aging in both strains and sexes, leading to an unparalleled understanding of the changes that constitute normal aging in mice, and how these vary between different groups of animals.
Mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 8 weeks of age. At The Jackson Laboratory, mice consume LabDiet which consists of 22% protein, 17% fat, and 61% carbohydrates. To account for logistical considerations, shipments were divided into different cohorts of 200 mice each, spaced 3 months apart. Currently, 2800 mice (700 mice per sex and strain) have been enrolled into the study. Upon enrollment, mice are acclimatized for 1 month at the NIA Biomedical Research Center (Baltimore, MD) during which time they are fed a standard chow diet (Envigo 2018SX; Envigo, Indianapolis, IN), which contains approximately 24% protein, 18% fat, and 58% carbohydrate. To conform with ITP studies, at 3 months of age, mice are switched to the open source NIH-31 diet consisting of 24% protein, 14% fat, and 62% carbohydrate (3.0 kcal/g [Envigo]). All procedures are conducted as per guidelines established by the Animal Care and Use Committee (ACUC) of the NIA IRP under protocol number TGB-458–2021.
Timeline of the Study and Longitudinal/ Cross-sectional Measurements
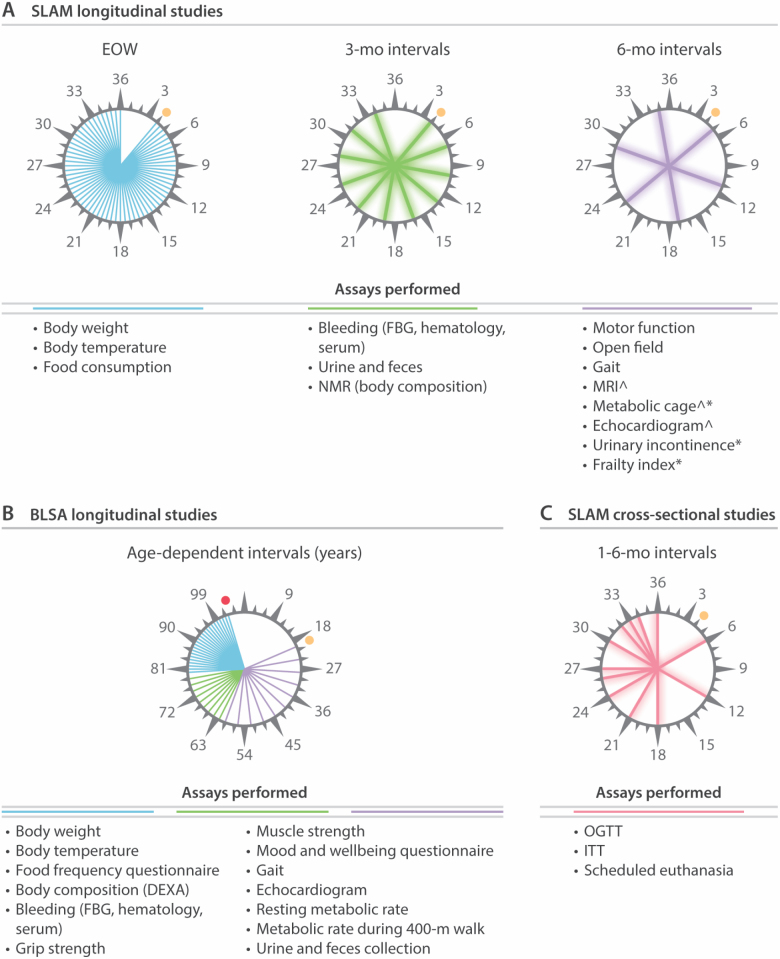
Conveniently and noninvasively obtained functional, phenotypic, demographic, and biological biomarkers of aging can provide a proxy for health and survival (49). While such markers have been previously examined in humans, very limited measurements have been performed in mice (50). In SLAM, measurement and sampling are conducted primarily in a longitudinal manner with a cross-sectional arm included for tissue collection. Measurements are collected from 3 months until 30–36 months of age at predefined intervals as per previously established protocols (Figure 2) (51). Longitudinal testing conducted in SLAM includes for all animals, assessment of body weight, food consumption, and body temperature every 2 weeks; evaluation of fasting blood glucose, body composition, and hematological measurements (ADVIA120, Siemens Healthineers), and collection of blood, serum, urine, and feces every 3 months; measurements of gait speed (MotoRater, TSE), mouse frailty index, motor function (rotarod, grip strength, cage top, and wire hang), and mouse behavior (open field, urine incontinence) every 6 months. Additionally, measurements of indirect calorimetry (Comprehensive Lab Animal Monitoring System, Columbus Instruments), echocardiography, and magnetic resonance imaging (7-Tesla MRI, Brucker) are performed every 6 months in a subset of animals. A detailed listing of the longitudinal tests is depicted in Figure 2A and Supplementary Table 2. These time intervals were selected to correspond with the longitudinal measurements performed in participants of the BLSA in which longitudinal testing is performed in healthy volunteers every 4 years from 20 to 60 years of age, every 2 years from age 60 to 79, and yearly in participants over 80 years old (Figure 2B).
Figure 2.
Study design for SLAM and corresponding measurements performed in BLSA. (A) Schematic representation of the longitudinal measurements performed in SLAM and the frequency of testing. *The frequency of these tests was increased after 15 months of age. ^Selected animals only. (B) Schematic representation of the BLSA equivalents to measurements performed in SLAM and the frequency with which these were performed. (C) Schematic representation of the timing and measurements performed in the cross-sectional arm of SLAM. FBG = fasting blood glucose; NMR = nuclear magnetic resonance; MRI = magnetic resonance imaging; OGTT = oral glucose tolerance test; ITT = insulin tolerance test; DEXA = dual-energy X-ray absorptiometry. Orange circles indicate the initiation of studies. Red circle indicates the oldest participant of BLSA (103 years).
State-of-the-art technology and protocols routinely used by the Translational Gerontology Branch (TGB) of the NIA Intramural Research Program for mouse phenotyping have been optimized for longitudinal data acquisition during this study. Functional and phenotypic characterization carried out in the SLAM study are not conducted in a specified order, as factors such as time point, availability of personnel, scheduling of equipment, and potential conflicts with other parallel testing have to be considered. While the order of testing may vary based on these considerations, extreme care has been taken to ensure that the tests are spaced out and performed as not to cause undue stress on the animals or impact subsequent measurements. In cases where testing of younger and older cohorts of mice overlapped, the older cohort is given priority. In some cases, especially in older mice, the health status of the mouse also needs to be considered where stressful testing could further deteriorate their health. However, efforts are made not to prospectively eliminate sick mice so as to not introduce selection bias in our tests. In order to directly compare the aging process between mice and humans, SLAM tests were chosen based on tests that are conducted in BLSA. Results from BLSA have shown that body composition modifies with age (52), as a result of changes in food consumption and metabolic parameters during aging (53). Hematologic changes are much explored in aging studies, and an increase in white blood cells, a marker of inflammation related to cancer, cerebrovascular, and cardiovascular mortality, was observed in BLSA (54). Longitudinally measured sarcopenia, frailty, risk to fall, and fatigability are considered to be hallmarks of human phenotypic and functional aging (55,56). Thus, far these parameters have not been examined within a longitudinal study of aging in mice. Noninvasive measurements such as gait and grip strength can be useful predictors of human aging (57). Gait studies in mice are uncommon but our previous cross-sectional studies showed that some gait parameters (ie, speed decline) change with age similar to humans, while others (ie, step length) differ between species (58). No longitudinal investigation of gait alterations in mice has ever been carried out, thus it is unclear whether the close association observed in humans between alterations in gait speed and mortality risk is comparable in mice. Behavioral changes like urine incontinence have been identified as markers for aging; however, few studies have explored this marker in a longitudinal setting with mice (59). The approach utilized in SLAM will allow us to longitudinally track functional, phenotypic, and biological changes that occur with normative aging in mice and to evaluate the predictive power of these alterations for overall mortality at different stages of life.
Additionally, flash frozen and formalin-fixed paraffin-embedded (FFPE) organs, collected cross-sectionally at selected time points, will allow us to further evaluate how these alterations may drive age-related changes in specific organs. Prior to planned euthanasia, animals designated for cross-sectional tissue collection undergo glucose tolerance tests (GTT) and insulin tolerance tests (ITT) to assess metabolic function. In future cohorts, other measures of resilience will also be performed. Animal health is checked twice daily, and death is recorded for each animal. Moribund animals are euthanized if any of the following events are observed—loss of righting ability, failure to eat or drink, excessive weight loss (>20% within 1 week), severe inner ear infection as evidenced by a head tilt and refractory to treatment, tumors exceeding Animal Research Advisory Committee (ARAC) guidelines, or other illness or injury determined by the attending veterinarian to cause chronic pain or suffering. Due to the importance of understanding the cause of death or subclinical age-associated diseases, all mice found dead or moribund and euthanized are immediately formalin fixed and examined for a full necropsy and histopathological analyses by the Division of Veterinary Resources (DVR/NIH, Bethesda, MD).
Implementation Strategy
Biorepository Establishment
SLAM generates over 1000 samples every month and therefore, processing, organization, and storage of samples are critical. All cross-sectionally and longitudinally collected biological specimens (ie serum, whole blood, urine, feces, tissue samples) are labeled with mouse unique identification number, cohort number, age at collection, and date of collection, then stored under optimal conditions for downstream analyses. Longitudinal blood samples are collected by submandibular bleeds at approximately 2 pm, following a 6-hour fast. Measurement of blood glucose levels is performed immediately after collection and hematologic measures (ADVIA) are assessed in samples kept on ice within 2 hours of collection. Serum samples are separated by centrifugation at 14 000 rpm for 15 minutes. Urine and feces are collected in the morning in fed mice. Serum, whole blood, urine, and feces are stored at −80 °C. These samples will be used for assessment of various endpoints—such as hormones, cytokines, metabolites, and proteins. The blood biomarkers that have been found to be associated with better survival outcomes in humans (60) have not been examined in depth thus far in mice, another area of research that SLAM will be able to address with the longitudinally and cross-sectionally collected samples.
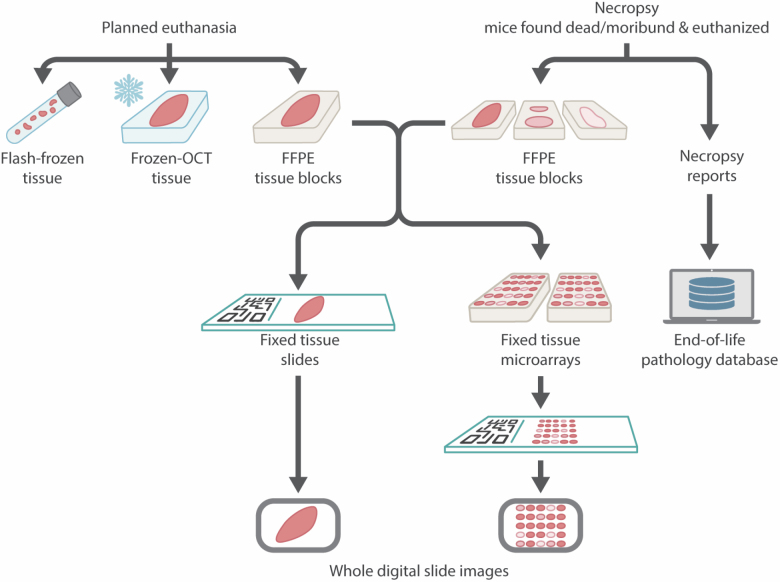
The SLAM tissue biorepository strategy is illustrated in Figure 3. At different time points throughout the study, a subset of mice is euthanized for tissue collection to establish a biorepository of both macroscopically normal flash frozen tissues and FFPE tissue blocks with the corresponding hematoxylin and eosin (H&E) stained slides (Supplementary Tables 3 and 4). Cross-sectional euthanasia is initially carried out every 6 months, then every 1–3 months as mice reach middle-age and late-life stages (Figure 2C). All euthanasia for cross-sectional sample collection are carried out in the morning between 8 am and 12 pm in fed animals. Mice that show macroscopic signs of disease (eg, neoplasms) are eliminated from sample collection for biochemistry. This becomes an important factor to consider especially in older cohorts of mice where pathological conditions are more prevalent. For flash frozen biospecimens, mice are euthanized by cervical dislocation, followed by cardiac puncture for blood collection and perfusion with cold phosphate-buffered saline (PBS; 1×) to avoid alterations to biochemical parameters that can occur during anesthesia. Organs (listed in Supplementary Table 3) are then removed and processed separately, flash frozen in liquid nitrogen, and stored at −80 °C. For collection of fixed tissues, selected mice are euthanized with injectable anesthetic overdose (ketamine/xylazine) or anesthetic inhalant overdose with isoflurane, followed by perfusion with cold PBS and fixation. Formalin-fixed tissues are sampled and trimmed at the TGB according to ad hoc modified protocols (61–65) and then processed for histology (paraffin embedding and H&E staining) at The Oncology Tissue Service at Johns Hopkins University (Baltimore, MD). Additional histochemical (eg, Masson’s trichrome and periodic acid-Schiff) and immunohistochemical staining (eg, neoplastic lesions immunophenotyping) are being performed for diagnostic and research purposes as well. Whole slide images are acquired with a high-resolution Zeiss Axio Scan Z1 digital slide scanner (Zeiss, Oberkochen, Germany). As per the goals of the NIA Geropathology Research Network (66), this will lead to the establishment of the first comprehensive murine geropathology whole slide digital library, integrated with pathology reports and a full spectrum of bioclinical annotations acquired during life and postmortem.
Figure 3.
SLAM tissue biorepository strategy.
While the collection and inventory of the specimens is ongoing, we are establishing a comprehensive, user-friendly database and a SLAM website. All the biological samples and whole slides images will be made available via special requests for research studies aimed at exploring age-associated changes in specific tissues using the website. Requests will be prioritized following review by senior members of the TGB of the NIA based on the overall strength of the proposals and their congruence with the mission and goals of the NIA. All researchers receiving samples from SLAM will also be required to adhere to the same transparency and data availability standards established for SLAM. Based on funding, availability of resources and services, a formal introduction of the SLAM website and biorepository to the research community will be made in the near future.
Data Management and Data Analysis
All data are acquired on predesigned templates that contain information pertaining to date of test, age, animal unique identification number, cohort number, and name of operator. Test results and measurements from SLAM are recorded electronically in a standardized format with hard copies of paper datasheets scanned and filed for duplication. All data are currently entered and stored in Microsoft Excel and processed using the programming language R for statistical analysis and graphical representation. SLAM data are organized in a database that is secure, reproducible, and scalable. An open source version control software called GIT is placed for source control, and folders used for data storage are routinely duplicated to avoid any unforeseen data loss. Additional data storage and computing power are available through NIA intramural research program cloud computing resources. Data analysis is conducted on an ongoing basis by a team of data scientists to evaluate potential study adjustments and also address relevant and complex questions emerging in the field. With the size, diversity, and complexity of the data obtained through SLAM, traditional analytical methods have serious limitations and therefore, machine learning approaches (eg image and video-based deep learning) and advanced statistical methods are being applied to data in order to efficiently analyze the data, estimate biological age, and explore novel associations for further hypothesis generation/testing. The cohort-based approach utilized in this work lends itself to this type of analysis, due to the ready availability of distinct groups of animals for training and validation purposes. All data collected in SLAM will be published in a timely manner and made available to the broader research community through publicly accessible databases upon publication. Links to these databases will be provided through the SLAM website.
Study Management and Logistics
Two of the major challenges associated with establishing and maintaining a study of this caliber are cost and personnel. This study is entirely funded through the Intramural Research Program of the NIA/NIH. A management plan is in place to assure that each mouse is tested at the appropriate timepoint by the assigned personnel following the correct standard operating procedure. The workload is divided between a team of highly skilled and uniformly trained laboratory personnel, animal care specialists, research fellows, and staff scientists. Regular meetings are held to discuss study updates, training, and SLAM data. During the 2020 SARS-CoV-2 pandemic, some disruptions to repeated testing were experienced due to unavailability of personnel but with the continued enrollment of future cohorts, these effects will be mitigated.
Limitations and Improvements
During this ongoing study, we have made several important observations that are pertinent to limitations in mouse aging research. Blood pressure, respiration, and bone density were initially measured within SLAM, but had to be halted due to time, personnel, and space constraints and the additional stress imposed on the animals during the procedures. As we have now collected an abundance of data in unstressed mice, we are currently working to reincorporate some of these measurements into future cohorts as well as adding additional measurements, including assessment of myography, auditory function and gut leakage, implantation of wireless telemetry devices, and functional measures of resilience (ie, response to vaccine, cold challenge). Continual and repeated testing the mice has brought to our attention some important aspects of commonly used tests regarding their feasibility, validity, and translatability in longitudinal studies. For example, rotarod testing was found to improve with age when performed longitudinally, in contrast to what has been shown in cross-sectional studies (67). While this has been observed in other studies (68), the scale at which SLAM is conducted allows us to conclusively demonstrate these improvements. Furthermore, most of mouse behavioral and phenotypic tests have been developed based on young mice and thus, the effect of aging on such tests remain largely unknown (69). Additionally, some of these tests are used in genetic models of specific diseases such as type 2 diabetes, Alzheimer’s disease, heart disease, and the analysis and results of these measurements may be substantially different under conditions of normal mouse aging. Echocardiograms in humans can be used to assess the extent of atherosclerosis and complications arising from this condition. However, in normal mouse aging atherosclerotic plaque formation does not occur due to differences in circulating lipids between mice and humans (70). As such, the analysis of these tests must focus on other parameters known to be related to cardiac aging in mice. The current protocols for some measurements of motor function (ie, wire hang, cage top) include time cutoffs that we have discovered are far below the actual capacity of both young and aged animals, resulting in ~90% of animals achieving the maximum score. We have thus modified our protocols to assess the maximum capacity for animals to perform these tasks and found that for many individuals, this far exceeds the standard cutoffs that are recommended. Such observations will be published as a way to alert the mouse aging research community about caveats to consider. On a similar note, performing tests in older mice comes with the added risk of adverse outcomes during the procedure, so additional precautions and care had to be taken when handling and testing older mice. Similarly, our initial results indicate that some measurements were more applicable for older mice compared to young mice (eg. frailty index) and therefore, testing frequencies have been adjusted accordingly for later cohorts. It is also important to stress how essential planning and organization for sample collection and storage is during a large, long-term study such as SLAM. Designated storage space and uniformity in labeling/annotation are critical for maintaining continuity and efficiently locating samples for follow-up analyses, and this is an area that we continue to improve on as the study matures beyond its initial phase.
Current and Future Directions
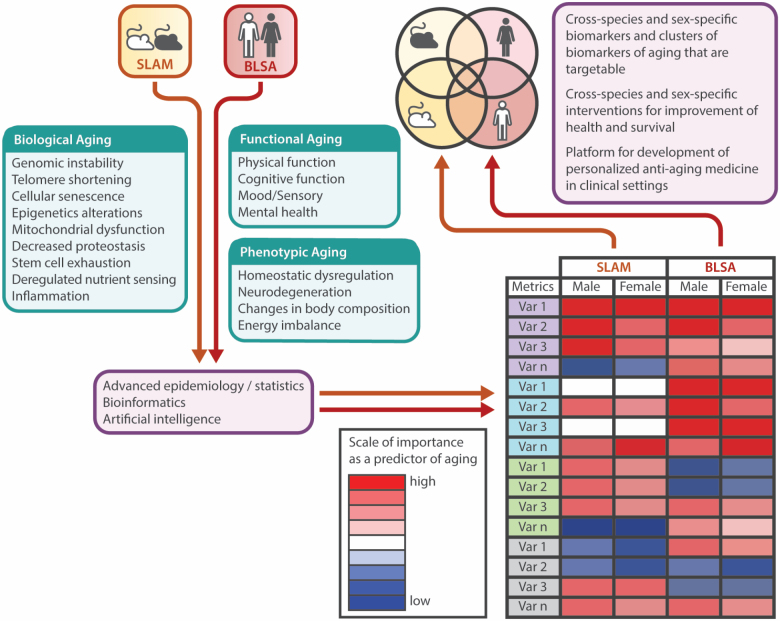
SLAM is an ongoing study that is continuously evolving based on key observations made within the study and on new developments in the field of aging research. SLAM data are being used to explore, compare, and validate mouse aging indices against observations from human aging studies (BLSA) that are built on the Geroscience hypothesis—such as pillars of aging, inflammation, metabolism, and resilience (71,72). Our initial efforts have been focused on building a framework to enable us to identify metrics of aging that could capture the hierarchical and temporal declines, and relationships between phenotypic, functional, and biological aging based on 4 domains: body composition, energy regulation and homeostasis, motor function, and onset of behavioral and neurological deficits (73). Using this framework and quantifying the phenotypic and biological changes associated with advancing age in mice will enhance our understanding of how these measures translate to a similar framework developed in BLSA (Figure 4). Using longitudinal data from SLAM and BLSA, we propose to characterize both shared and distinct aspects of mouse and human aging biology. To evaluate different parameters based on their importance as a predictor of aging, we will explore a number of critical indices and sub-indices for each species: functional aging (ie, physical function, cognitive function, mood/sensory, mental health), phenotypic aging (ie, body composition, energetics, homeostatic mechanisms), and biological aging (ie, genomic instability, telomere shortening, cellular senescence, epigenetic alterations, mitochondrial dysfunction, decreased proteostasis, stem cell exhaustion, deregulated nutrient sensing, inflammation). Parallel and simultaneous clustering of diverse variables will be achieved by advanced statistical methods such as bioinformatics and artificial intelligence applications. We surmise that there will be large overlaps between the species, but these hypotheses need to be thoroughly tested, and initial data from these studies indicate that some key aging characteristics are differentially regulated with age between mice and humans.
Figure 4.
Translational gerontology framework to compare biomarkers of aging between mice and humans. BLSA and SLAM data will be leveraged to identify biomarkers and clusters of biomarkers associated with aging by examining individual variables related to functional, phenotypic, and biological aging. The effect of sex/strain/race will be also be explored.
To help further evaluate whether factors driving organismal aging are modifiable, we have initiated a longitudinal intervention study that builds on the baseline SLAM study, where small molecules and dietary interventions are being tested as effective means to prevent and/or mitigate aging and age-associated phenotypes. The intervention arm of SLAM will be performed under the same format as the original SLAM study. As such, it will include all of functional, behavioral, phenotypic, and biological analyses conducted during SLAM. By using the same study design to first identify biomarkers of aging and then test how those biomarkers are modulated by a given intervention, more accurate and reproducible estimates of the efficacy of the intervention can be made. This will allow us to build off the important work being done with ITP, where very few longitudinal assessments are performed. Indeed, initial efforts will be focused on known antiaging interventions that have been established to be effective by the ITP and other programs. We anticipate that this will allow us and others to more efficiently and effectively test novel antiaging interventions. The use of both sexes in these follow-up studies will allow us to explore the factors that underlie sex-specific intervention successes observed in prior life-span studies (74). Ultimately, the full wealth of SLAM data will be used to identify aging biomarker signatures that can be used to evaluate the potential efficacy of various nutritional, pharmacological, or genetic modulators, without examining the full mouse life span. Initial studies conducted as part of this larger secondary SLAM study will examine high probability candidates for life-span extension, including but not limited to metformin, rapamycin and CR, with the specific goal of examining how such interventions modify biomarkers of aging in mice.
Overall, the SLAM study will provide a wealth of data on normal mouse aging, as well biological samples that can be utilized by the broader research community. All of these resources will be made available to the broader research community through the SLAM website (Figure 5). As part of this initiative, the identification of aging biomarkers will not only foster progress in the field but will also allow stratification of mouse cohorts (based on strain and sex), and provide early diagnostic information useful for validation within other mouse longevity projects such as the NIA ITP (36). In addition to this, harnessing the size and power of this dataset will allow us to explore a number of specific questions that are of critical importance for improving our understanding of how different factors impact aging. For example, as highlighted earlier, sex differences in aging phenotypes have been noted in previous studies (47). It is well known that a strong sexual dimorphism in lipid deposition exists in both mice and humans, with female mice accumulating a much higher amount of subcutaneous fat than males, who store most of their lipids in the visceral compartment. Recent work has demonstrated that fat mass plays a critical role in rodent life span and is one of the best predictors of how well an animal will respond to CR (34). Body composition data acquired from NMR and MRI in SLAM will allow us to directly assess whether alterations in fat mass differentially affect life span in male and female mice and evaluate how differences in specific fat depots contribute to these effects. Similarly, metabolic rate changes with age and depends on many factors, like body composition, body temperature, and physical function. Human studies have shown that low resting metabolic rate is related to optimal aging (53). SLAM can help unravel the mechanisms that underlie such observations, and by direct comparison to data acquired from BLSA, the translatability of mice as a model of human metabolic function and aging can be investigated.
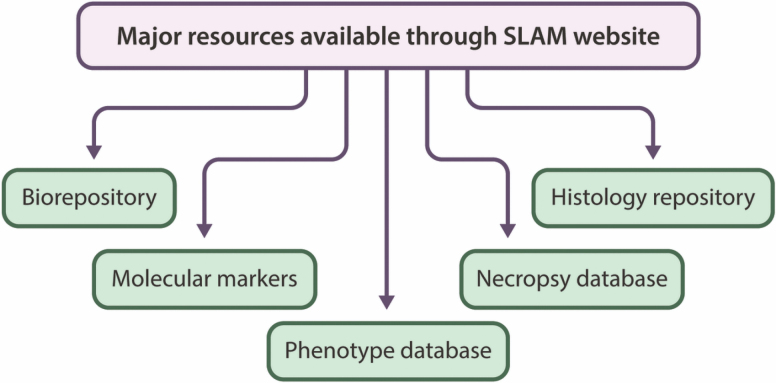
Figure 5.
Expected major resources that will be available through SLAM.
Summary
Here, we have highlighted the goals, significance, study design, and key current methods of SLAM—a longitudinal study that was originally established to address the absence of robust datasets on repeated measures of physiological, functional parameters, and aging phenotypes on large populations of mice in the context of normal aging. Longitudinal assessments of metabolic, hematological, functional, behavioral, and biological parameters are collected from 2 strains of mice of both sexes for a total of 2800 animals. This study will provide invaluable insight into normative mouse aging and establish a platform suitable for subsequent testing of interventions aimed at delaying the progression of phenotypic and biological aging. We foresee that SLAM will be used as a gold standard for longitudinal mouse aging, with specific uses as a power calculation tool, for aging biomarker identification, and as a system for aging data/ biospecimen sharing. The identification of aging biomarkers from phenotypic, biochemical, and -omics data and their parallel validation in an epidemiological study like BLSA, shall provide key information for ultimately improving health outcomes in the elderly.
Supplementary Material
Acknowledgments
The authors would like to especially acknowledge all past and present TGB personnel involved in the SLAM study, specifically animal technicians Dawn Boyer, Dawn Nines, Sarah Eckroth, Sharon Ensor, and Erica Matifas. The authors also wish to thank the Division of Veterinary Research (DVR) of the NIH. The Comparative Medicine Section of the NIH/NIA provided invaluable personnel support without which this study is not possible. We wish to also thank Marc Raley (NIA/NIDA Visual Media Services) for figure design.
The complete list of current and past investigators involved in SLAM is provided in Supplementary Table 1.
Funding
This work was supported by the Intramural Research Program of the NIA/NIH.
Conflict of Interest
None declared.
Author Contributions
All authors contributed to the development and implementation of various aspects of this study. All authors listed by individual name contributed to either writing the original draft or reviewing and editing of the manuscript.
References
- 1. Olshansky SJ. From lifespan to healthspan. J Am Med Assoc. 2018;320:1323–1324. doi: 10.1001/jama.2018.12621 [DOI] [PubMed] [Google Scholar]
- 2. Rivera-Tavarez CE. Can we increase our health span? Phys Med Rehabil Clin N Am. 2017;28:681–692. doi: 10.1016/j.pmr.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Beard JR, Officer A, de Carvalho IA, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serrano M. Understanding aging. N Engl J Med. 2017;376:1083–1085. doi: 10.1056/NEJMcibr1615878 [DOI] [PubMed] [Google Scholar]
- 5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hou P, Xue HP, Mao XE, Li YN, Wu LF, Liu YB. Inflammation markers are associated with frailty in elderly patients with coronary heart disease. Aging (Albany NY). 2018;10:2636–2645. doi: 10.18632/aging.101575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. J Am Med Assoc. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barzilai N, Cuervo AM, Austad S. Aging as a biological target for prevention and therapy. J Am Med Assoc. 2018;320:1321–1322. doi: 10.1001/jama.2018.9562 [DOI] [PubMed] [Google Scholar]
- 9. Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman JC, Milman S, Hashmi SK, et al. Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci. 2016;71:1424–1434. doi: 10.1093/gerona/glw149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702 [DOI] [PubMed] [Google Scholar]
- 12. Martin FC, Romero Ortuño R. Longitudinal studies of ageing: from insights to impacts: commentary to accompany themed collection on longitudinal studies. Age Ageing. 2019;48:481–485. doi: 10.1093/ageing/afz028 [DOI] [PubMed] [Google Scholar]
- 13. Fozard JL, Metter EJ, Brant LJ. Next steps in describing aging and disease in longitudinal studies. J Gerontol. 1990;45:P116–P127. doi: 10.1093/geronj/45.4.p116 [DOI] [PubMed] [Google Scholar]
- 14. Shock NW. Criteria for the design of research studies on chronic disease. J Chronic Dis. 1955;1:224–226. doi: 10.1016/0021-9681(55)90211-3 [DOI] [PubMed] [Google Scholar]
- 15. Stanziano DC, Whitehurst M, Graham P, Roos BA. A review of selected longitudinal studies on aging: past findings and future directions. J Am Geriatr Soc. 2010;58(Suppl. 2):S292–S297. doi: 10.1111/j.1532-5415.2010.02936.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shock NW. A New research building for gerontology. Gerontologist. 1964;4:185–189. doi: 10.1093/geront/4.4.185 [DOI] [PubMed] [Google Scholar]
- 17. Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res. 2018;123:886–904. doi: 10.1161/CIRCRESAHA.118.312806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bender BS, Nagel JE, Adler WH, Andres R. Absolute peripheral blood lymphocyte count and subsequent mortality of elderly men. The Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 1986;34:649–654. doi: 10.1111/j.1532-5415.1986.tb04906.x [DOI] [PubMed] [Google Scholar]
- 19. Ko SU, Simonsick EM, Husson LM, Ferrucci L. Sex-specific gait patterns of older adults with knee osteoarthritis: results from the Baltimore Longitudinal Study of Aging. Curr Gerontol Geriatr Res. 2011;2011:175763. doi: 10.1155/2011/175763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varma VR, Oommen AM, Varma S, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. 2018;15:e1002482. doi: 10.1371/journal.pmed.1002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63:1416–1419. doi: 10.1093/gerona/63.12.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo PL, Schrack JA, Shardell MD, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287:373–394. doi: 10.1111/joim.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Margolick JB, Ferrucci L. Accelerating aging research: how can we measure the rate of biologic aging? Exp Gerontol. 2015;64:78–80. doi: 10.1016/j.exger.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19:e13080. doi: 10.1111/acel.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001–2014. J Am Med Assoc. 2016;315:1750–1766. doi: 10.1001/jama.2016.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattison JA, Colman RJ, Beasley TM, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- 28. Folgueras AR, Freitas-Rodríguez S, Velasco G, López-Otín C. Mouse models to disentangle the hallmarks of human aging. Circ Res. 2018;123:905–924. doi: 10.1161/CIRCRESAHA.118.312204 [DOI] [PubMed] [Google Scholar]
- 29. Kleinert M, Clemmensen C, Hofmann SM, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14:140–162. doi: 10.1038/nrendo.2017.161 [DOI] [PubMed] [Google Scholar]
- 30. Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192 [DOI] [PubMed] [Google Scholar]
- 31. Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815 [DOI] [PubMed] [Google Scholar]
- 33. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J Gerontol A Biol Sci Med Sci. 2016;71:1395–1406. doi: 10.1093/gerona/glw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ackert-Bicknell CL, Anderson LC, Sheehan S, et al. Aging research using mouse models. Curr Protoc Mouse Biol. 2015;5:95–133. doi: 10.1002/9780470942390.mo140195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4:2. doi: 10.1186/1747-5341-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016;2016:170–176. doi: 10.1093/emph/eow014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burd CE, Gill MS, Niedernhofer LJ, et al. Barriers to the preclinical development of therapeutics that target aging mechanisms. J Gerontol A Biol Sci Med Sci. 2016;71:1388–1394. doi: 10.1093/gerona/glw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breschi A, Gingeras TR, Guigó R. Comparative transcriptomics in human and mouse. Nat Rev Genet. 2017;18:425–440. doi: 10.1038/nrg.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burns TC, Verfaillie CM. From mice to mind: strategies and progress in translating neuroregeneration. Eur J Pharmacol. 2015;759:90–100. doi: 10.1016/j.ejphar.2015.03.041 [DOI] [PubMed] [Google Scholar]
- 43. Festing MF. Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research. ILAR J. 2014;55:399–404. doi: 10.1093/ilar/ilu036 [DOI] [PubMed] [Google Scholar]
- 44. Tuttle AH, Philip VM, Chesler EJ, Mogil JS. Comparing phenotypic variation between inbred and outbred mice. Nat Methods. 2018;15:994–996. doi: 10.1038/s41592-018-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vaanholt LM, Magee V, Speakman JR. Factors predicting individual variability in diet-induced weight loss in MF1 mice. Obesity (Silver Spring). 2012;20:285–294. doi: 10.1038/oby.2011.279 [DOI] [PubMed] [Google Scholar]
- 46. Fischer KE, Riddle NC. Sex differences in aging: genomic instability. J Gerontol A Biol Sci Med Sci. 2018;73:166–174. doi: 10.1093/gerona/glx105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Austad SN. Sex differences in health and aging: a dialog between the brain and gonad? Geroscience. 2019;41:267–273. doi: 10.1007/s11357-019-00081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrucci L, Levine ME, Kuo PL, Simonsick EM. Time and the metrics of aging. Circ Res. 2018;123:740–744. doi: 10.1161/CIRCRESAHA.118.312816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Richardson A, Fischer KE, Speakman JR, et al. Measures of healthspan as indices of aging in mice—a recommendation. J Gerontol A Biol Sci Med Sci. 2016;71:427–430. doi: 10.1093/gerona/glv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bellantuono I, de Cabo R, Ehninger D, et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc. 2020;15:540–574. doi: 10.1038/s41596-019-0256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fabbri E, Chiles Shaffer N, Gonzalez-Freire M, et al. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle. 2017;8:490–499. doi: 10.1002/jcsm.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:667–672. doi: 10.1111/jgs.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ruggiero C, Metter EJ, Cherubini A, et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007;49:1841–1850. doi: 10.1016/j.jacc.2007.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chiles Shaffer N, Fabbri E, Ferrucci L, Shardell M, Simonsick EM, Studenski S. Muscle quality, strength, and lower extremity physical performance in the Baltimore Longitudinal Study of Aging. J Frailty Aging. 2017;6:183–187. doi: 10.14283/jfa.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bair WN, Petr M, Alfaras I, et al. Of aging mice and men: gait speed decline is a translatable trait, with species-specific underlying properties. J Gerontol A Biol Sci Med Sci. 2019;74:1413–1416. doi: 10.1093/gerona/glz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Birder LA, Kullmann AF, Chapple CR. The aging bladder insights from animal models. Asian J Urol. 2018;5:135–140. doi: 10.1016/j.ajur.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roberts JA, Varma VR, Huang CW, et al. Blood metabolite signature of metabolic syndrome implicates alterations in amino acid metabolism: findings from the Baltimore Longitudinal Study of Aging (BLSA) and the Tsuruoka Metabolomics Cohort Study (TMCS). Int J Mol Sci. 2020;21:1249. doi: 10.3390/ijms21041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pettan-Brewer C, Treuting PM. Practical pathology of aging mice. Pathobiol Aging Age Relat Dis. 2011;1. doi: 10.3402/pba.v1i0.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Slaoui M, Bauchet AL, Fiette L. Tissue sampling and processing for histopathology evaluation. Methods Mol Biol. 2017;1641:101–114. doi: 10.1007/978-1-4939-7172-5_4 [DOI] [PubMed] [Google Scholar]
- 63. Ruehl-Fehlert C, Kittel B, Morawietz G, et al. ; RITA Group; NACAD Group . Revised guides for organ sampling and trimming in rats and mice–part 1. Exp Toxicol Pathol. 2003;55:91–106. [PubMed] [Google Scholar]
- 64. Kittel B, Ruehl-Fehlert C, Morawietz G, et al. ; RITA Group; NACAD Group . Revised guides for organ sampling and trimming in rats and mice—Part 2. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol. 2004;55:413–431. doi: 10.1078/0940-2993-00349 [DOI] [PubMed] [Google Scholar]
- 65. Morawietz G, Ruehl-Fehlert C, Kittel B, et al. ; RITA Group; NACAD Group . Revised guides for organ sampling and trimming in rats and mice–Part 3. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol. 2004;55:433–449. doi: 10.1078/0940-2993-00350 [DOI] [PubMed] [Google Scholar]
- 66. Ladiges W, Ikeno Y, Niedernhofer L, et al. The Geropathology Research Network: an interdisciplinary approach for integrating pathology into research on aging. J Gerontol A Biol Sci Med Sci. 2016;71:431–434. doi: 10.1093/gerona/glv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barreto G, Huang TT, Giffard RG. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. J Neurosurg Anesthesiol. 2010;22:214–219. doi: 10.1097/ANA.0b013e3181d56c98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11. doi: 10.1186/s13041-016-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee YT, Lin HY, Chan YW, et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 2017;16:12. doi: 10.1186/s12944-016-0402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hurria A, Carpenter CR, McFarland F, et al. Models and studies of aging: executive summary of a report from the U13 Conference Series. J Am Geriatr Soc. 2019;67:428–433. doi: 10.1111/jgs.15788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc. 2019;67:1559–1564. doi: 10.1111/jgs.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gonzalez-Freire M, Diaz-Ruiz A, Hauser D, et al. The road ahead for health and lifespan interventions. Ageing Res Rev. 2020;59:101037. doi: 10.1016/j.arr.2020.101037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.