Abstract
Nampt consists of iNampt and eNampt, might contribute to modulating obesity-related malignancies and impairing response to chemotherapy in a range of cancers. This study explored the role of Nampt and adiposity in the progression and response to neo-adjuvant chemotherapy of esophageal squamous cell carcinoma (ESCC). Patients with ESCC were treated with 2 cycles of neo-adjuvant chemotherapy, then evaluated for surgery. Tumor regression grading (TRG) and prognosis of these patients were collected. Anthropometry was well utilized. Serum eNampt was determined by enzyme-linked immunosorbent assay, iNampt expression in tissues were assessed by PCR, western blot and immunohistochemistry. eNampt in sera elevated significantly in these over-weight or obese patients, and was positively associated with body mass index (BMI), waist circumference, visceral fat area (VFA), subcutaneous fat area (SFA) and total fat area (TFA) (P<0.05). iNampt expression in the mRNA and protein levels were up-regulated in ESCC compared to their adjacent non-tumor specimens (P<0.05). iNampt protein staining revealed mainly in the cytoplasm and nuclei, while it was not related to serum eNampt, BMI, waist circumference, VFA, SFA and TFA (P>0.05). Pre-treatment iNampt, BMI, SFA, TFA and age significantly correlated with neo-adjuvant chemotherapy response, and iNampt expression and age were independent predictors (P<0.05). Pre-treatment iNampt, ypT, ypN, ypTNM stage and TRG were associated with the survival of ESCCs, and ypN stage and TRG were independent prognostic factors (P<0.05). In conclusion, iNampt impaired ESCC response to neo-adjuvant chemotherapy independent of eNampt, targeting iNampt to increase ESCC response to neo-adjuvant chemotherapy would improve the prognosis of ESCCs.
Keywords: Esophageal squamous cell carcinoma, nampt, neo-adjuvant chemotherapy, chemotherapy response, obesity, tumor regression grading
Introduction
ESCC is more predominant in Asia, Africa and South America [1], and acts as one of the primary cause of cancer-related death in China [2]. ESCC is highly malignant, aggressive and invasive, and has poor therapeutic effect. Although early detections, surgical techniques and multimodality treatment have advanced in recent years, most of patients with ESCC are still diagnosed in the middle or advanced stage, and the general outcome remains very poor with 5-year survival rates about 10% [3]. Multimodality treatment which consists of operation and non-operative treatment such as chemotherapy, radiotherapy and immunotherapy, has become the mainstream therapy. Neo-adjuvant chemoradiotherapy plus esophagectomy for esophageal cancer is recommended according to the CROSS clinical trial result [4], but preoperative chemotherapy combined with surgery is also a standard treatment for esophageal cancer with clinical stage II/III [5], especially in Chinese Han population.
With the current global obesity epidemic, a great quantity of patients diagnosed with cancer are overweight or obese, and obesity appears to connect with about 20% of cancers such as endometrial, cardia gastric, colorectal, postmenopausal breast, prostate and renal [6]. Allowing for the current obesity pandemic, there is an urgent need to figure out the mechanisms underlying obesity-mediated cancer progression. The correlation between obesity and ESCC is very complex. Obesity paradox in cancer is currently existing, that obesity might boost cancer development, but not correlate negatively with the outcomes of these patients, especially for ESCC [7,8], but the mainstream of opinions reveal that obesity, especially abdominal obesity might be related to an elevated risk for ESCC [9]. Obesity reduces response to chemotherapy in a range of cancers including breast cancer [10], pancreatic cancer [11], prostate cancer [12] and special state of gastric cancer [13]. The mechanisms underlying the negative effects of obesity on therapeutic response seem likely to be multifactorial [14]. The dysfunctional angiogenesis and inflammation exhibited in adipose tissue in obesity state [15], adipose stromal cell [12], an obesogenic microenvironment [10] and adipocytes [16] may enhance tumor progression and resistance to chemotherapy.
Nicotinamide phosphoribosyltransferase (Nampt) is composed of extracellular and intracellular forms, known as eNampt and iNampt, respectively. eNampt, as an active protein in the extracellular space, is first identified as pre-B-cell colony-enhancing factor (PBEF) boosting early B cell proliferation [17], then considered to be secreted preferentially by visceral adipose tissue and termed as visfatin [18]. Up to date, eNampt is believed to be not only secreted from pre-B cells, but also produced by most of other cell types such as adipocytes, immune cells, brain cells and cancer cells [19]. These malignant cells include breast cancer, hepatoma, colorectal cancer, melanoma, neuroblastoma, glioma, prostate cancer and cervical cancer. eNampt in serum goes up with the augment of obesity, plays multiple roles in physiological and pathological processes, and may act as an important mechanistic link in the complex network of cytokines making a positive impact on obesity-associated tumor progression. eNampt is demonstrated to play a pivotal role in mediating cancer cell metabolism, accelerating epithelial-to-mesenchymal transition and constructing the local tumor microenvironment [19]. iNampt is a pleiotropic protein functioning as key enzyme, catalyzes the rate-limiting process in the NAD+ biosynthesis pathway from nicotinamide. eNampt, a typical cytokine, also possesses the function similar with iNampt. Hence, both eNampt and iNampt increase intracellular NAD+ which is an essential coenzyme for various physiological processes including DNA repair, energy metabolism, cell growth and cell death. Plenty of these signal pathways are typically dysregulated in cancer cells, making NAD+ as an intriguing target for cancer therapeutics [20,21]. eNampt confers resistance to chemotherapy in human colorectal cancer, non-small cell lung cancer, melanoma and breast cancer [22-25], iNampt is also associated with chemotherapy resistance in melanoma, and colon cancer through Sirt1 and PARP [24,26]. This knowledge could be used to increase the efficacy of Nampt inhibitors and chemotherapy. Collectively, Nampt plays an important role and has drawn wide attention in the field of cancer. However, up to date, data regarding the role of Nampt and the correlation between Nampt and chemotherapy response in ESCCs is relatively limited. To explore the role of Nampt in the progression and chemotherapy response of ESCC, serum eNampt, hematologic profile and iNampt expression in biopsy specimens from ESCCs before the initial treatment, the final CT image before operation, and tumor regression grading in the pathology of these resected tumor tissues were assessed.
Material and methods
Patients and tissues/sera
ESCC patients with clinical stage T2-3NxM0 from Chinese Han population who were performed neo-adjuvant chemotherapy plus surgery (n = 60) from the First Affiliated Hospital of Xi’an Jiaotong University from Sept 2013 to Sept 2015 were collected. Tumor samples from endoscopic biopsy and patients’ sera before the first cycle of neo-adjuvant chemotherapy, and resected tumor specimens after operation from them were obtained. These patients consisted of 43 males and 17 females, with the median age 63 year-old (ranged from 33 to 76 year-old). The study was with the approval of the Institutional Review Board of Xi’an Jiaotong University, and all of these patients knew the study and signed informed consent forms. Clinical stage T2-3 of patients had been identified by endoscopic ultrasound (EUS), then were treated with neo-adjuvant chemotherapy. After 2 cycles of paclitaxel (50 mg/m2 on Day 1) combined with carboplatin (AUC 2 on Day 1) regimen chemotherapy, all the patients were assessed for operation because of the suitable tumor stage or at the request of patients. These patients were grouped by gender, age, tumor location, differentiation, tumor size and clinical (c-) T stage by the time of diagnosis. These ESCC patients were further treated with constant surveillance, chemotherapy only or combined with radiotherapy after operation according to NCCN guidelines (version 2013) and followed up over a five-year period. Disease-specific survival of patients with ESCC was collected. Another small panel of patients with surgery only (n = 5) from the First Affiliated Hospital of Xi’an Jiaotong University were also collected, resected tumor samples and their adjacent non-tumor tissues from them were obtained.
Assessment of chemotherapy response
TRG was utilized to assess chemotherapy response of ESCC in the pathology according to the College of American Pathologists Cancer Protocols (CAP-TRG) according to NCCN guideline in the study. The detailed information is as follows: Tumor Regression Score (TRS) = 0, no malignant cells with viability (complete response); TRS = 1, a small amount or small groups of malignant cells exist (moderate response); TRS = 2, Residual cancer outgrown by fibrosis (minimal response); TRS = 3, Minimal or no malignant cells are killed (poor response). Response evaluation criteria in solid tumors RECIST 1.1 was also adopted to evaluate ESCC response to neo-adjuvant chemotherapy in the image, and the grade of response consists of complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The detailed are as follows: CR, all target lesions disappear; PR, at least a 30% decrease; PD, at least a 20% increase in the sum of diameters of target lesions; SD, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the baseline sum diameters while on study [27].
Body composition analysis
Anthropomorphic data were well measured by the time of first cycle of neo-adjuvant chemotherapy by a single researcher. Waist circumference was determined, patients with a waist circumference exceeding 94 cm in men and 80 cm in women were considered to be central obesity [28]. Weight and height were also assessed, and BMI was calculated as weight/height2, and patients were divided into lean (BMI<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2) according to World Health Organization criterion. VFA and SFA were determined from cross-sectional transverse images of computed tomography scanning at the L3-4 disc space [29]. Patients with VFA larger than 130 cm2 were considered to be visceral obesity. TFA equaled to VFA plus SFA.
Serum analysis
eNampt in sera of these patients with ESCC was estimated with enzyme-linked immunosorbent assay (ELISA) kits (Linco Research, Inc., USA) according to the manufacturers’ protocols. Biotinylated, monoclonal anti-eNampt antibody and HRP-conjugated streptavidin was successively utilized in the experimental procedure as previously reported [28], and the OD of each well was collected at 450 nm.
Real-time PCR
Total RNA from tissues was extracted from tissues using the TRIzol reagent (Invitrogen, San Diego, CA), then reverse-transcribed to cDNA with the help of a cDNA Synthesis Kit (Takara Biotechnology, Dalian, China) following the manufacturers’ instructions. mRNA levels of target genes were determined by real-time PCR (DNA Engine Opticon 2; Bio-Rad, Hercules, CA, USA) utilizing SYBR Green PCR Master Mix (Takara Biotechnology) and specific intron-spanning human primers according to the manufacturer’s instructions. Values were determined as the mean of triplicate measurements and levels were normalized to β-actin mRNA expression. Nampt primer adopted in the reaction system was as follows: forward: 5’-AAGAGACTGCTGGCATAGGA-3’, reverse: 5’-ACCACAGATACAGGCACTGA-3’.
Western blot
Protein from tumor and non-tumor tissues were isolated with RIPA buffer, and the concentration of them were determined by BCA method (Pierce). 25 μg/lane protein was separated by SDS-PAGE and then transferred onto PVDF membranes from Millipore. Western blot analysis was then carried out utilizing monoclonal, anti-iNampt antibody (dilution 1:200, Santa Cruz Biotechnology, CA) or monoclonal, anti-β-actin antibody (dilution 1:200, Santa Cruz Biotechnology). These blots were well developed with chemiluminescence substrate solution (Pierce), then with exposure to X-ray film [28].
Immunohistochemistry (IHC)
IHC was performed on target specimens which had been made for paraformaldehyde-fixed paraffin sections. Monoclonal, anti-iNampt antibody purchased from Santa Cruz Biotechnology (dilution 1:50) was well utilized in theses IHC with streptavidin peroxidase (SP) conjugated method. IHC was carried out and the staining results for iNampt protein were semi-quantitatively estimated as previously reported [28], and tissue sections with no less than 4 in the total score were termed as iNampt-positive staining. All of histological analysis were conducted by three independent pathologists.
Statistical analysis
Values were exhibited as the mean ± standard deviation (SD). These continuous variables were compared by adopting unpaired t tests, and categorical variables were estimated utilizing χ2 test. Spearman or Pearson correlation coefficients were used to estimate the correlations between variables. Survival analysis of these target ESCCs was conducted by Kalplan-Meier and Cox’s proportional hazards regression method. That P<0.05 was termed as statistically significant.
Results
Response to neo-adjuvant chemotherapy of ESCC
Chemotherapy response was well assessed in the pathology from resected tumor specimens by CAP-TRG, and in the image by RECIST 1.1 on the 22th day after the start of the 2nd cycle of neo-adjuvant chemotherapy. These changes were shown as follows. In the pathology, 4 of 60 ESCC patients revealed complete response (TRS = 0), 13 patients reached moderate response (TRS = 1), 22 patients got minimal response (TRS = 2), and 21 patents attained poor response (TRS = 3, Figure 1). In the image, changes of tumors were also evaluated after 2 cycles of neo-adjuvant chemotherapy, and 3.3% (2/60) patients with ESCC revealed CR, 58.3% (35/60) of them reached PR, 36.7% (22/60) of them got SD, and 1.7% (1/60) attained PD (Figures 2 and 3). 4 patients got complete response in the pathology, however only 2 patients with CR in the image were found, but the difference had no significance, this indicated that pathological TRG might be better to reveal response to chemotherapy of ESCC.
Figure 1.
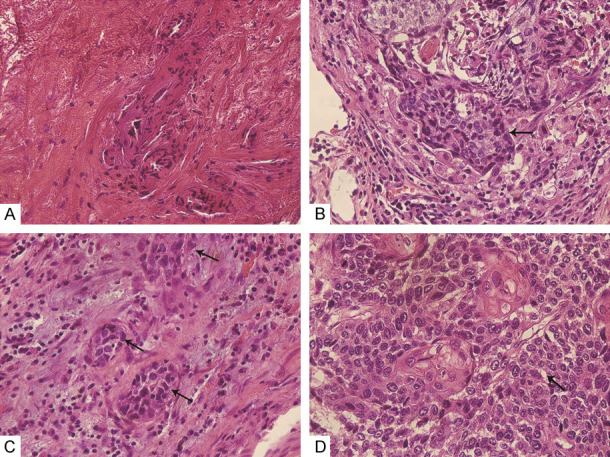
Response to chemotherapy of ESCC assessed in the pathology by CAP-TRG following 2 cycles of neo-adjuvant chemotherapy, and H&E stained sections were demonstrated at ×400 magnification. (A) TRS = 0, (B) TRS = 1, (C) TRS = 2, and (D) TRS = 3.
Figure 2.
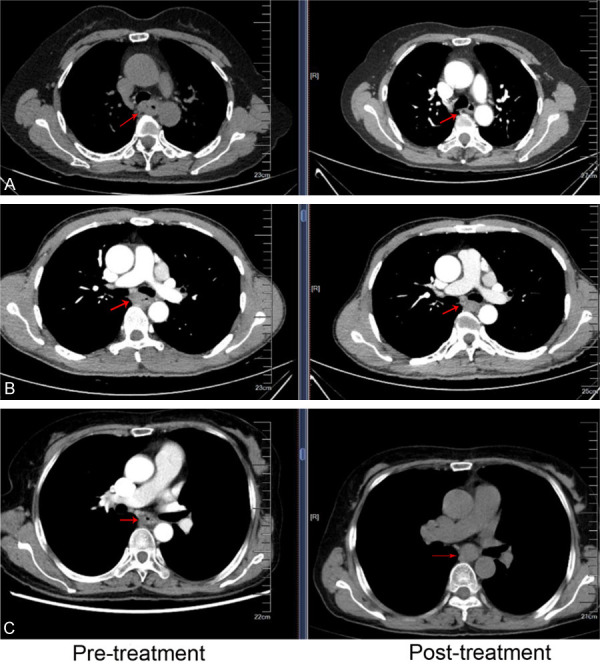
Response to neo-adjuvant chemotherapy of ESCC evaluated in the image by RECIST 1.1 following 2 cycles of chemotherapy. (A) CR, (B) PR, and (C) SD.
Figure 3.
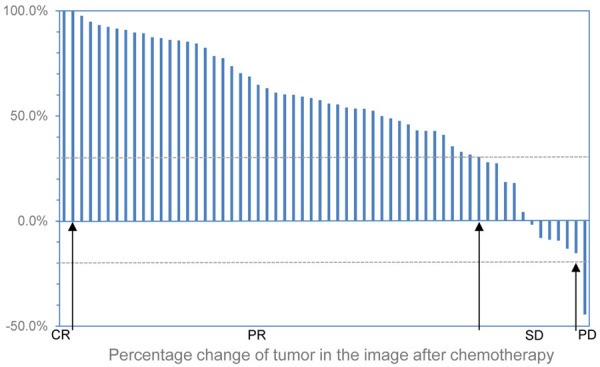
Percentage change of tumor in the image of all the target patients after 2 cycles of neo-adjuvant chemotherapy. Taking as reference the baseline sum diameters, CR (n = 2), all target lesions disappeared; PR (n = 35), at least a 30% decrease in the sum of diameters of target lesions; PD (n = 1), at least a 20% increase; SD (n = 22), neither reached PR nor attained PD.
Serum eNampt before the first cycle of chemotherapy
Serum concentration of eNampt was determined adopting ELISA and the correlation between eNampt and clinical characteristics measured for obesity status was also assessed. The average level of eNampt in sera was significantly elevated in these ESCC patients who were termed as obese by BMI (8.44 ± 0.83 versus 6.82 ± 1.68 ng/ml for BMI 25 kg/m2 or higher versus less than 25 kg/m2, P = 0.001), waist circumference (8.01 ± 1.29 versus 6.79 ± 1.67 ng/ml, P = 0.003), and VFA (8.14 ± 0.93 versus 6.89 ± 1.57 ng/ml, P = 0.009). Circulating eNampt of patients with ESCC was correlated with BMI (r = 0.43, P = 0.001), waist circumference (r = 0.44, P<0.001), VFA (r = 0.46, P<0.001), SFA (r = 0.44, P<0.001) and TFA (r = 0.50, P<0.001). Therefore, TFA was the most relevant factor associating with eNampt level in sera, but was not an independent affecting factor (P = 0.154). Circulating eNampt did not correlate with gender, age, tumor differentiation, tumor size, tumor location and cT stage (P>0.05). These result revealed that eNampt might originate from a variety of cells including ESCC cells, but was not secreted mainly from ESCC cells.
iNampt expression in ESCC and clinicopathological features
Analysis of iNampt expression in ESCC and their adjacent non-tumor tissues from another small panel of patients was conducted in the mRNA and protein levels. Compared to their adjacent non-tumor tissues, ESCC had elevated iNampt expression in the mRNA and protein levels regardless of the context of obesity status (P<0.05, Figure 4). iNampt expression in ESCC tissues from endoscopic biopsy of those target patients by the time of first diagnosis was determined semi-qualitatively by IHC analysis. Immunoreactivity for iNampt protein was revealed mainly in the cytoplasm and nuclei (Figure 5). iNampt expression in these tumors were not related to gender, age of patients and tumor differentiation, tumor size, tumor location and cT stage (P>0.05, Table 1). iNampt level in those tumor specimens from target ESCCs was also estimated focusing on metabolic characteristics, but they were also not related to BMI, Waist circumference, VFA, SFA, TFA and serum eNampt level (P>0.05, Table 2).
Figure 4.
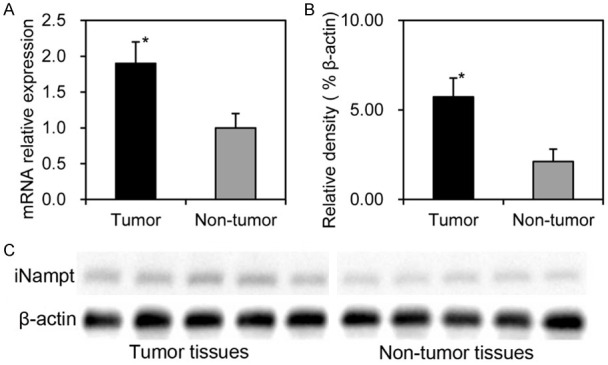
iNampt expression in ESCC and their adjacent non-tumor tissues (n = 5) was shown, mRNA expression was measured by means of qRT-PCR using β-actin as internal control (A), protein expression was determined by Western blot (B and C). Compared to these adjacent non-tumor, ESCC had elevated iNampt expression in the mRNA level (1.9 ± 0.3 versus 1.0 ± 0.2) and protein level (5.73 ± 1.03% versus 2.12 ± 0.67%). *; P<0.05.
Figure 5.
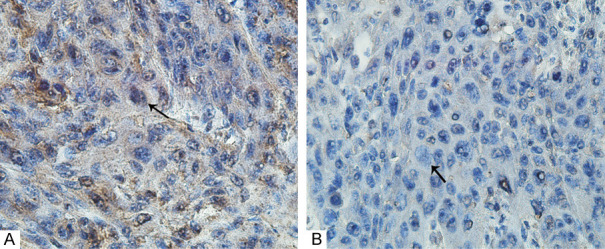
iNampt protein expression in these tumors from endoscopic biopsy was evaluated, and the sections were demonstrated at ×400 magnification. (A) positive staining and (B) negative staining.
Table 1.
Relationship between clinicopathological features and iNampt expression in ESCC before chemotherapy
| Clinicopathological features | Categories | iNampt expression | χ2 | P | |
|---|---|---|---|---|---|
|
| |||||
| - | + | ||||
| Gender | Male | 20 | 23 | 2.84 | 0.092 |
| Female | 12 | 5 | |||
| Age (year-old) | <60 | 11 | 11 | 0.16 | 0.694 |
| ≥60 | 21 | 17 | |||
| Tumor differentiation | G1-2 | 26 | 26 | 1.74 | 0.187 |
| G3 | 6 | 2 | |||
| Tumor size (cm) | <6 | 17 | 11 | 1.15 | 0.284 |
| ≥6 | 15 | 17 | |||
| Tumor location | U/M | 28 | 22 | 0.86 | 0.355 |
| L | 4 | 6 | |||
| cT stage | T2 | 9 | 5 | 0.88 | 0.348 |
| T3 | 23 | 23 | |||
| eNampt | Above the median | 17 | 14 | 0.06 | 0.809 |
| Below the median | 15 | 14 | |||
Table 2.
Correlations between iNampt expression in ESCC and measures of obesity
| Measures of obesity | iNampt expression | |
|---|---|---|
|
| ||
| r | P | |
| Body mass index | -0.05 | 0.712 |
| Waist circumference | -0.12 | 0.353 |
| Visceral fat area | -0.07 | 0.608 |
| Superficial fat area | -0.17 | 0.208 |
| Total fat area | -0.13 | 0.319 |
eNampt in sera, iNampt in tumor, obesity status before neo-adjuvant chemotherapy and response to chemotherapy
CAP-TRG in tumor regression grading systems was utilized for assessment of chemotherapy response by NCCN guideline. Univariate analysis showed that pre-treatment BMI, SFA, TFA, iNampt and age of patients were significantly associated with TRG (P<0.05, Table 3), then were predictors of response to neo-adjuvant chemotherapy. Only iNampt (OR = 2.00, P = 0.004) and age (OR = 0.88, P = 0.018) were independent predictors of neo-adjuvant chemotherapy response of target ESCCs according to multivariate analysis, and patients with elevated expression of iNampt in pre-treatment ESCC tissues, or younger in the age had poorer response to chemotherapy.
Table 3.
Correlations between clinicopathological features, eNampt in sera, iNampt in tumors and obesity status with chemotherapy response in the pathology
| Clinicopathological features | CAP-TRG | χ2 or t | P | |
|---|---|---|---|---|
|
| ||||
| 0-1 | 2-3 | |||
| Gender | 1.93 | 0.165 | ||
| Male | 10 | 33 | ||
| Female | 7 | 10 | ||
| Tumor differentiation | 0.38 | 0.537 | ||
| G1-2 | 14 | 38 | ||
| G3 | 3 | 5 | ||
| Tumor location | 1.99 | 0.159 | ||
| U/M | 16 | 34 | ||
| L | 1 | 9 | ||
| cT stage | 0.49 | 0.484 | ||
| T2 | 5 | 9 | ||
| T3 | 12 | 34 | ||
| Tumor size (cm) | 6.15 ± 2.41 | 6.82 ± 2.83 | -0.861 | 0.393 |
| Age (year-old) | 64.94 ± 7.62 | 59.95 ± 8.70 | 2.068 | 0.043* |
| eNampt (ng/ml) | 7.78 ± 1.75 | 7.01 ± 1.60 | 1.646 | 0.105 |
| BMI (kg/m2) | 24.04 ± 3.64 | 21.88 ± 2.81 | 2.466 | 0.017* |
| Waist circumference (cm) | 87.92 ± 9.58 | 82.86 ± 8.72 | 1.969 | 0.054 |
| VFA (cm2) | 113.71 ± 43.02 | 88.33 ± 50.23 | 1.832 | 0.072 |
| SFA (cm2) | 121.76 ± 59.69 | 85.19 ± 48.62 | 2.460 | 0.017* |
| TFA (cm2) | 235.47 ± 92.80 | 173.51 ± 88.82 | 2.405 | 0.019* |
| iNampt | 1.94 ± 1.64 | 3.98 ± 2.22 | -3.423 | 0.001* |
Statistically significant.
eNampt in sera, iNampt in tumor before neo-adjuvant chemotherapy, TRG, ypTNM stage and survival
On univariate regression analysis, ypT (T0-1 versus T2-3), ypN (negative versus positive), ypTNM stage (I versus II&III), iNampt (negative versus positive staining) and TRG (TRS 0-1 versus 2-3) correlated to the survival of ESCCs (P<0.05), but pre-treatment eNampt was not correlated with the prognosis of these ESCC patients (P>0.05, Table 4). Tumor-specific survival was significantly reduced in those patients with iNampt-positive staining tumors than that with iNampt-negative staining tumors (36.82 ± 2.48 versus 57.71 ± 3.92 months, P<0.001, Figure 6). iNampt-positive tumors were correlated with death from ESCC compared with iNampt-negative tumors (HR = 3.50, P<0.001), and TRS 2-3 was also related to death from ESCC compared with TRS 0-1 (HR = 3.84, P<0.001), and lymph node positive metastasis after neo-adjuvant chemotherapy was correlated with death from ESCC compared with negative lymph node metastasis (HR = 3.79, P<0.001). On the basis of multivariate analysis, both ypN stage and TRG were independently correlated with the survival of ESCCs (P<0.05).
Table 4.
Cox regression analysis of factors associated with death from ESCC
| Features | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| eNampt | ||||
| Below the median | 1 | 0.915 | ||
| Above the median | 0.92 (0.50-1.67) | |||
| iNampt | ||||
| Negative | 1 | <0.001* | 0.54 (0.27-1.07) | 0.079 |
| Positive | 3.50 (1.86-6.56) | 1 | ||
| ypT category | ||||
| T0-1 | 1 | 0.001* | 0.64 (0.24-1.72) | 0.374 |
| T2-3 | 3.82 (1.75-8.36) | 1 | ||
| ypN category | ||||
| Negative | 1 | <0.001* | 0.27 (0.12-0.62) | 0.002* |
| Positive | 3.79 (2.02-7.09) | 1 | ||
| ypTNM stage | ||||
| I | 1 | <0.001* | 0.45 (0.16-1.26) | 0.129 |
| II&III | 6.19 (2.82-13.61) | 1 | ||
| CAP-TRG | ||||
| 0-1 | 1 | <0.001* | 0.18 (0.06-0.55) | 0.002* |
| 2-3 | 3.84 (2.05-7.17) | 1 | ||
Statistically significant.
Figure 6.
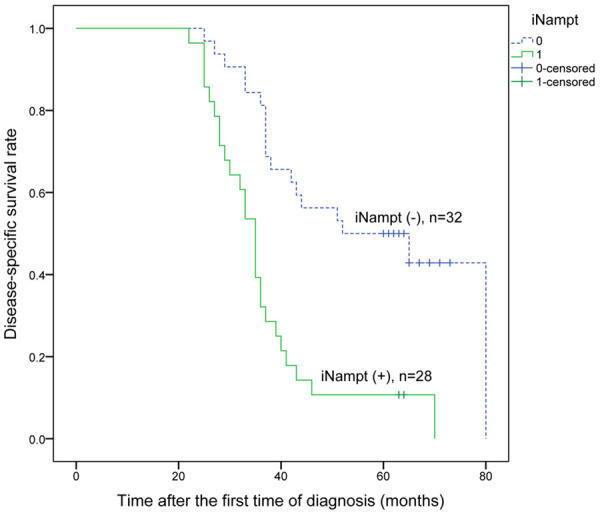
Kaplan-Meier analysis of disease-specific survival of ESCCs with 2 cycles of neo-adjuvant chemotherapy following surgery relative to iNampt expression.
Discussion
Obesity raised the morbidity of multiple malignancies according to previous clinical studies [6], prompted a number of cancers progression, and reduced response to chemotherapy [12,16,30]. Certainly, different results from multiple reports were existed. A study demonstrated that there may be no significant correlation between overweight or obesity and the outcomes of low-risk GTN [31]. Another study from New Zealand population revealed that obesity was not significantly associated with the survival of breast cancer, and there were small associations between obesity and loco-regional or metastatic recurrence rates, but without significance [32]. In male patients with metastatic melanoma who were treated with targeted or immune therapy, obese patients had better outcomes in the progression-free survival and overall survival than those patients with normal BMI [33]. The results of this study demonstrated that TRS 0-1 in the TRG was independently associated with the improved survival of target ESCCs, and higher grade in SFA, TFA and BMI were predictors of better response to neo-adjuvant chemotherapy (TRS 0-1) in ESCC according to univariate analysis, that was consistent with part of the above studies.
Obesity reduced response to chemotherapy in a range of cancers including breast, pancreatic, prostate and special state of gastric cancer [10-13], but the results of this study found that there was no correlation between obesity and worse response to neo-adjuvant chemotherapy. Obesity has a variety of impacts on target cells including endocrine effect from adipokines. These potent adipokines produced mainly by pre-adipocytes and mature adipocytes of white adipose tissue, such as leptin, adiponectin and resistin are implicated in cell growth, proliferation, cell cycle control, apoptosis and angiogenesis [34], and could accelerate tumor progression and metastasis. eNampt with biological potential, also functions as a paracrine and autocrine cytokine, triggering a wide range of intracellular signaling pathways with varying temporal dynamics, especially for cancer cells [19]. eNampt may boost tumor progression in plenty of cancers, such as breast cancer [35] and prostate cancer [36]. Furthermore, the level of circulating eNampt was correlated with tumor progression of malignant astrocytomas, colorectal, ovarian and gastric cancer [19]. However, no significant association between ESCC progression and eNampt was found in this study. eNampt in sera of target patients with ESCC was significantly related to these markers of obesity including BMI, waist circumference and TFA indicating that adipose tissue could produce eNampt and secret it into the sera, but eNampt provided by adipose tissue and others could not change the expression of iNampt in ESCC tissues, and no correlation was found between them.
iNampt closely connected with the progression of malignant tumors including esophagogastric junction adenocarcinoma [28], and was associated with increasing the expression of genes related to chemotherapy resistance [26]. iNampt was also over-expressed in the ESCC specimens and related to worse response to chemotherapy in this study, but iNampt was not associated with clinicophathological characteristics such as cT stage and tumor size. It was very interesting that iNampt expression in ESCC tissues was significantly lower than that in esophagogastric junction adenocarcinoma with iNampt expression being related to tumor invasive depth, lymph nodes metastasis and TNM stage [28], that was not shown in this study. Therefore, the different results might be attributed to the different connection between the tumors and obesity, and ESCC was lowly correlated with obesity. Another interesting result in this study was that iNampt expression seemed to be gender-dependent (P = 0.092), and further analysis showed that only age (OR = 0.88, P = 0.018) was an independent predictor of neo-adjuvant chemotherapy response according to multivariate analysis, but was not associated with the survival of ESCCs. That the median disease-specific survival was significantly longer in these patients with iNampt-negative tumors than that with iNampt-positive tumors was consistent with the previous studies [28]. ypT, ypN, ypTNM stage, pre-treatment iNampt expression in tumor tissues and TRG correlated with the survival of ESCCs, and both ypN stage and TRG were independent prognostic factors.
In conclusion, pre-treatment iNampt expression in tumor tissues impaired esophageal squamous cell carcinoma response to neo-adjuvant chemotherapy independent of circulating eNampt, but was not an independent prognostic factor. Moreover, ypN stage and TRG were independently associated with the survival of ESCCs. Therefore, targeting iNampt to increase response to neo-adjuvant chemotherapy would improve the prognosis of ESCCs, and that need further analysis in future.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 81703079).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210–215. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Toxopeus E, van der Schaaf M, van Lanschot J, Lagergren J, Lagergren P, van der Gaast A, Wijnhoven B. Outcome of patients treated within and outside a randomized clinical trial on neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: extrapolation of a randomized clinical trial (CROSS) Ann Surg Oncol. 2018;25:2441–2448. doi: 10.1245/s10434-018-6554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohda M, Kuwano H. Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg. 2017;23:1–11. doi: 10.5761/atcs.ra.16-00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Zuo C, Liu G, Che P, Li G, Li X, Chen H. Cumulative evidence for the relationship between body mass index and the risk of esophageal cancer: an updated meta-analysis with evidence from 25 observational studies. J Gastroenterol Hepatol. 2020;35:730–743. doi: 10.1111/jgh.14917. [DOI] [PubMed] [Google Scholar]
- 8.Trestini I, Carbognin L, Bonaiuto C, Tortora G, Bria E. The obesity paradox in cancer: clinical insights and perspectives. Eat Weight Disord. 2018;23:185–193. doi: 10.1007/s40519-018-0489-y. [DOI] [PubMed] [Google Scholar]
- 9.Cho JH, Shin CM, Han KD, Yoon H, Park YS, Kim N, Lee DH. Abdominal obesity increases risk for esophageal cancer: a nationwide population-based cohort study of South Korea. J Gastroenterol. 2020;55:307–316. doi: 10.1007/s00535-019-01648-9. [DOI] [PubMed] [Google Scholar]
- 10.Mendez-Hernandez A, Gallegos-Arreola MP, Moreno-Macias H, Espinosa Fematt J, Perez-Morales R. LEP rs7799039, LEPR rs1137101, and ADIPOQ rs2241766 and 1501299 polymorphisms are associated with obesity and chemotherapy response in mexican women with breast cancer. Clin Breast Cancer. 2017;17:453–462. doi: 10.1016/j.clbc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Cascetta P, Cavaliere A, Piro G, Torroni L, Santoro R, Tortora G, Melisi D, Carbone C. Pancreatic cancer and obesity: molecular mechanisms of cell transformation and chemoresistance. Int J Mol Sci. 2018;19:3331. doi: 10.3390/ijms19113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su F, Ahn S, Saha A, DiGiovanni J, Kolonin MG. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene. 2019;38:1979–1988. doi: 10.1038/s41388-018-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmela C, Velho S, Agostinho L, Branco F, Santos M, Santos MP, Oliveira MH, Strecht J, Maio R, Cravo M, Baracos VE. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J Gastric Cancer. 2017;17:74–87. doi: 10.5230/jgc.2017.17.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014;96:458–463. doi: 10.1038/clpt.2014.136. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D, Incio J, Shankaraiah RC, Jain RK. Obesity and cancer: an angiogenic and inflammatory link. Microcirculation. 2016;23:191–206. doi: 10.1111/micc.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehuede C, Li X, Dauvillier S, Vaysse C, Franchet C, Clement E, Esteve D, Longue M, Chaltiel L, Le Gonidec S, Lazar I, Geneste A, Dumontet C, Valet P, Nieto L, Fallone F, Muller C. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP) Breast Cancer Res. 2019;21:7. doi: 10.1186/s13058-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Q, Li L, Li R, Yang M, Liu H, Nowicki MJ, Zong H, Xu J, Yang G. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Ann Med. 2009;41:311–320. doi: 10.1080/07853890902729760. [DOI] [PubMed] [Google Scholar]
- 19.Grolla AA, Travelli C, Genazzani AA, Sethi JK. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br J Pharmacol. 2016;173:2182–2194. doi: 10.1111/bph.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy BE, Sharif T, Martell E, Dai C, Kim Y, Lee PW, Gujar SA. NAD(+) salvage pathway in cancer metabolism and therapy. Pharmacol Res. 2016;114:274–283. doi: 10.1016/j.phrs.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Liu J, Park J, Rai P, Zhai RG. Subcellular compartmentalization of NAD(+) and its role in cancer: a sereNADe of metabolic melodies. Pharmacol Ther. 2019;200:27–41. doi: 10.1016/j.pharmthera.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan X, Zhao J, Zhang R. Visfatin mediates doxorubicin resistance in human colorectal cancer cells via up regulation of multidrug resistance 1 (MDR1) Cancer Chemother Pharmacol. 2017;80:395–403. doi: 10.1007/s00280-017-3365-y. [DOI] [PubMed] [Google Scholar]
- 23.Cao Z, Liang N, Yang H, Li S. Visfatin mediates doxorubicin resistance in human non-small-cell lung cancer via Akt-mediated up-regulation of ABCC1. Cell Prolif. 2017;50:e12366. doi: 10.1111/cpr.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohanna M, Cerezo M, Nottet N, Bille K, Didier R, Beranger G, Mograbi B, Rocchi S, Yvan-Charvet L, Ballotti R, Bertolotto C. Pivotal role of NAMPT in the switch of melanoma cells toward an invasive and drug-resistant phenotype. Genes Dev. 2018;32:448–461. doi: 10.1101/gad.305854.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge X, Zhao Y, Dong L, Seng J, Zhang X, Dou D. NAMPT regulates PKM2 nuclear location through 14-3-3zeta: conferring resistance to tamoxifen in breast cancer. J Cell Physiol. 2019;234:23409–23420. doi: 10.1002/jcp.28910. [DOI] [PubMed] [Google Scholar]
- 26.Lucena-Cacace A, Otero-Albiol D, Jimenez-Garcia MP, Munoz-Galvan S, Carnero A. NAMPT is a potent oncogene in colon cancer progression that modulates cancer stem cell properties and resistance to therapy through Sirt1 and PARP. Clin Cancer Res. 2018;24:1202–1215. doi: 10.1158/1078-0432.CCR-17-2575. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Bai E, Zhang Y, Jia Z, He S, Fu J. Role of nampt and visceral adiposity in esophagogastric junction adenocarcinoma. J Immunol Res. 2017;2017:3970605. doi: 10.1155/2017/3970605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donohoe CL, Doyle SL, McGarrigle S, Cathcart MC, Daly E, O’Grady A, Lysaght J, Pidgeon GP, Reynolds JV. Role of the insulin-like growth factor 1 axis and visceral adiposity in oesophageal adenocarcinoma. Br J Surg. 2012;99:387–396. doi: 10.1002/bjs.8658. [DOI] [PubMed] [Google Scholar]
- 30.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maesta I, Horowitz NS, Goldstein DP, Bernstein MR, Ramirez LA, Moulder J, Berkowitz RS. Response to chemotherapy in overweight/obese patients with low-risk gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2015;25:734–740. doi: 10.1097/IGC.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 32.Elwood JM, Tin Tin S, Kuper-Hommel M, Lawrenson R, Campbell I. Obesity and breast cancer outcomes in chemotherapy patients in New Zealand - a population-based cohort study. BMC Cancer. 2018;18:76. doi: 10.1186/s12885-017-3971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 35.Huang JY, Wang YY, Lo S, Tseng LM, Chen DR, Wu YC, Hou MF, Yuan SF. Visfatin mediates malignant behaviors through adipose-derived stem cells intermediary in breast cancer. Cancers (Basel) 2019;12:29. doi: 10.3390/cancers12010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel ST, Mistry T, Brown JE, Digby JE, Adya R, Desai KM, Randeva HS. A novel role for the adipokine visfatin/pre-B cell colony-enhancing factor 1 in prostate carcinogenesis. Peptides. 2010;31:51–57. doi: 10.1016/j.peptides.2009.10.001. [DOI] [PubMed] [Google Scholar]


