Abstract
Chronic kidney disease (CKD) patients require dialysis to manage the progressive complications of uraemia. Yet, many physicians and patients do not recognize that dialysis initiation, although often necessary, subjects patients to substantial risk for cardiovascular (CV) death. While most recognize CV mortality risk approximately doubles with CKD the new data presented here show that this risk spikes to >20 times higher than the US population average at the initiation of chronic renal replacement therapy, and this elevated CV risk continues through the first 4 months of dialysis. Moreover, this peak reflects how dialysis itself changes the pathophysiology of CV disease and transforms its presentation, progression, and prognosis. This article reviews how dialysis initiation modifies the interpretation of circulating biomarkers, alters the accuracy of CV imaging, and worsens prognosis. We advocate a multidisciplinary approach and outline the issues practitioners should consider to optimize CV care for this unique and vulnerable population during a perilous passage.
Keywords: End-stage renal disease, Sudden death, Haemodialysis initiation
Graphical abstract
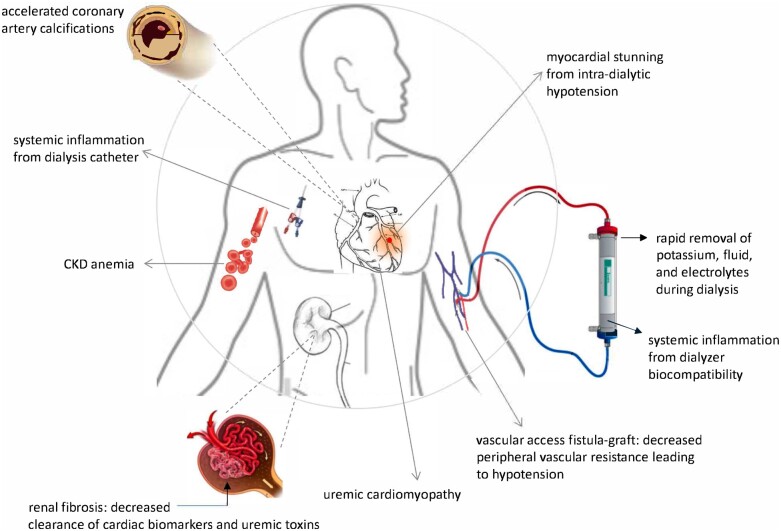
The initiation of dialysis superimposes new CV challenges on the substrate of chronic kidney disease (CKD). These factors include increased myocardial oxygen demand, for example, due to access-associated increase in cardiac output, and hypotension due to volume shifts during dialysis. Intra-dialytic hypotension can also decrease oxygen supply to the myocardium, particularly a lower diastolic pressure head for coronary artery perfusion augmented by arteriosclerosis affecting both the intimal and medial layers of conduit arteries. Hypotension superimposed on macro- and micro-vascular disease can lead to the myocardial stunning documented during and immediately post-dialysis. In addition, dialysis involves inflammatory insults from innate immune activation due to contact with the dialysis membrane and catheters. Rapid fluid and electrolyte shifts during dialysis can predispose towards arrhythmia, contributing to the augmented sudden death that follows the initiation of dialysis. All of these new factors related to dialysis initiation superimpose upon the chronic anaemia, the uraemic cardiomyopathy, and uraemic toxins associated with CKD.
Introduction
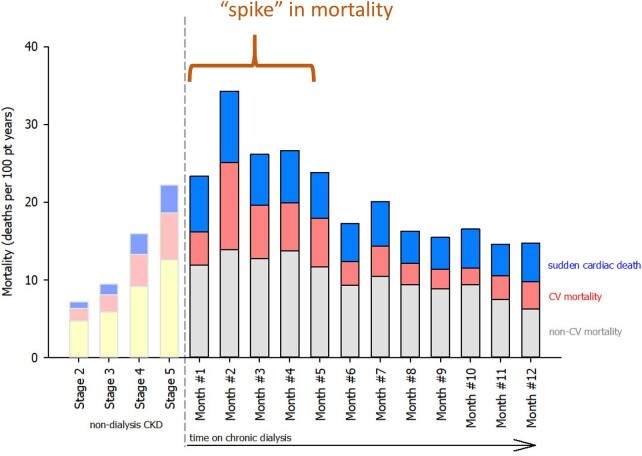
Worldwide, more than 700 000 patients will start chronic dialysis this year, and this number is expected to double in the next 20 years.1 Both the European Society of Cardiology and American Heart Association Guidelines recognize chronic kidney disease (CKD) as a marker of heart disease, but neither articulates that starting dialysis for end-stage kidney disease (ESKD) may be a period of high risk because the cardiovascular (CV) death rate unexpectedly surges in the first 4 months of dialysis therapy, as demonstrated by the new data presented here (Figure 1).3–5
Figure 1.
Standardized mortality rates in the chronic kidney disease and dialysis population, Medicare 5% CKD sample (2011–2014). Our analysis of a 5% random sample of Medicare claims highlights ‘the spike’ in cardiovascular mortality that occurs when dialysis starts that extends into the first 4 months of chronic dialysis. Direct standardized rates for age, gender, and race were calculated as per methods outlined by Ahmad et al.2 See Supplementary material online for details regarding the generation of this figure.
Nephrologists only recently recognized this spike in CV mortality because traditional studies followed patients after Day 90 when patients become Medicare eligible. Other practitioners who co-manage these vulnerable patients may not share this awareness of ‘the spike’ mandating its broader recognition. Starting dialysis entails a major shift in the pathophysiology of disease that changes the clinical CV manifestations of heart disease that underlies this peak in CV mortality.4 , 6
This review article summarizes how this pathophysiological shift changes the presentation, diagnosis, and management of CV disease the moment CKD patients start dialysis.
We advocate a multi-disciplinary approach to mitigate the spike in CV mortality precipitated by starting chronic dialysis, concordant with a recent Kidney Disease Improving Global Outcomes (KDIGO) Controversies Conference.7
Epidemiology surrounding the start of dialysis
Almost 60% of the US population will develop CKD (Table 1) with consequently enhanced CV mortality.8 , 9 The lifetime risk of ESKD is 3.6%, which is higher than death from suicide, automobile accidents, firearms, or cancer of either breast, colon, or prostate.8 , 10 , 11
Table 1.
Chronic kidney disease classification and grade
| CKD grade | Classification | Glomerular filtration rate (mL/min) |
|---|---|---|
| Stage 1–2 | Mild | 60–89 |
| Stage 3 | Moderate | 30–59 |
| Stage 4 | Severe | 15–29 |
| Stage 5 | Kidney failure | <15 |
| Dialysis | End-stage renal failure | Dialysis |
CV mortality during the first 4 months following the start of chronic dialysis exceeds by 20-fold that of age-matched individuals in the general US population.3 , 4 , 6 , 12 , 13 We present new data in Figure 1 illustrating this critical spike in mortality when dialysis begins where the combined rate of CV death and sudden cardiac death (SCD) is 14 events per 100 patient-years during these first 4 months. CV/SCD mortality peaks during Month 2 of dialysis, yielding an annualized rate of 20.4 deaths per 100 patient-years, then subsequently plateaus to a steady state of 7 deaths per 100 patient-years at Month 8. The combined CV and SCD death risk in the first 4 months is remarkably 50% higher than Stage 5 CKD, an equally comorbid population who are not yet on dialysis but quickly approaching ESKD.
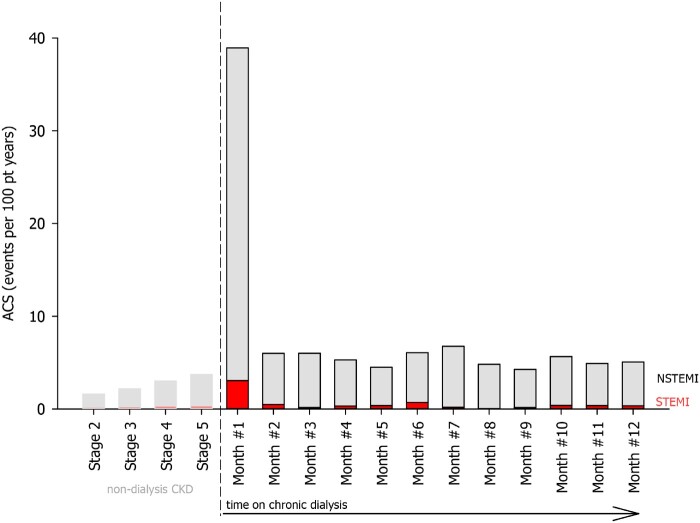
Dialysis initiation changes the causes of CV death. Ischaemic heart disease causes the majority of deaths in CKD. Once dialysis starts, SCD risk doubles and becomes the most common cause of death, suggesting a shift in mechanisms (Figure 1).14 The rate of SCD exceeds by 3.5-fold that of pre-dialysis CKD 4 patients. Myocardial infarction (MI) also peaks during this period (Figure 2). The incidence of MI is 0.27 events per 100 patient-years in the general population, ranges from 1 to 2 events per 100 patient-years in mild CKD, increases to >3 events per 100 patient-years on dialysis, and ‘spikes’ to 34 events per 100 patient-years in the first month of chronic renal replacement therapy, which is >11 times higher than pre-dialysis CKD 4 patients (Figure 2).15 , 16 Stroke rates parallel those of MI possibly from repeated cerebral hypoperfusion, anticoagulation, and inflammation from dialysis.17 The incident stroke and transient ischaemic attack rate in the year before dialysis of 0.25 event per 100 patient-months increases 7-fold on dialysis initiation and then declines in the following month.17
Figure 2.
Standardized acute coronary syndrome rates in the chronic kidney disease and dialysis population, Medicare 5% CKD sample (2011–2014). Our analysis of a 5% random sample of Medicare claims shows the rate of myocardial infarction, while is already heightened in chronic kidney disease, and peaks right after dialysis starts. Direct standardized rates for age, gender, and race were calculated as per methods outlined by Ahmad et al.2 See supplementary material for details regarding the generation of this figure.
Compared to the general population, both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) increase in CKD. While STEMI rates remain flat around 0.2 events per 100 patient-years for all levels of CKD, NSTEMI rates increase as glomerular filtration rate (GFR) decreases, suggesting that the presentation, mechanisms, and diagnosis of acute coronary syndrome (ACS) change during the progression of renal dysfunction.18 Moreover, kidney disease impairs the clearance of biomarkers of cardiac injury e.g. troponin, perhaps heightening the detection of NSTEMI.19
Presentation: detection of cardiovascular disease in patients starting dialysis
ESKD complicates the presentation of heart disease for patients starting dialysis by impairing the performance of biomarker assays, decreasing the sensitivity of non-invasive testing, and increasing the risk of procedural complication. Dialysis patients with coronary artery disease (CAD) may not exhibit typical symptoms due to coexistent diabetes, uraemic neuropathy, and limited exercise capacity. Overall, these challenges may hinder accurate diagnoses, delay vital therapies, and complicate testing for these patients at the very time of greatest need for optimal CV care.
Imaging modalities
Over 50% of patients starting dialysis have angiographically obstructive CAD, with multi-vessel involvement in 25–40%.20 , 21 Dobutamine stress ECHO (DSE) predicts severe CAD in dialysis patients (Table 2) even though ∼50% of patients starting dialysis have left ventricular (LV) hypertrophy (LVH) that blunts the detection of wall-motion abnormalities.22 , 23 Myocardial perfusion imaging (MPI) has lower sensitivity in dialysis patients than in the general population due to reduced coronary vasodilatory response and balanced ischaemia because of multi-vessel involvement (Table 2).24–26 Frequent baseline electrocardiogram (ECG) abnormalities and inadequate functional capacity limit the predictive ability of exercise electrocardiography (sensitivity = 35%, specificity = 60%) in the dialysis population.27 Only two small dialysis studies evaluate the performance of coronary artery calcification (CAC) scoring for detecting CAD: Rosario et al. reported a sensitivity = 0.64, specificity = 0.65.24 , 28 Winther et al.29 reported a sensitivity = 0.67, specificity = 0.77 (27) Similarly, data on coronary computed tomography angiography is sparse: 93% sensitivity and 63% specificity in 138 advanced CKD patients who underwent concurrent coronary arteriography.
Table 2.
Performance characteristics of fifth-generation hs-TNI assay, dobutamine stress ECHO, and myocardial perfusion scintigraphy CAD in the dialysis and general population
| Sensitivity |
specificity |
|||
|---|---|---|---|---|
| Dialysis population | General population | Dialysis population | General population | |
| Fifth-generation hs-TNI (27) | 0.41 | 0.93 | 1.00 | 0.94 |
| Dobutamine stress ECHO (35) | 0.79 | 0.85 | 0.89 | 0.77 |
| Myocardial perfusion scintigraphy (35) | 0.74 | 0.87 | 0.70 | 0.64 |
DSE, MPI, or CAC testing in patients starting dialysis can effectively risk stratify subjects for future CAD events.30 However, results from ISCHEMIA-CKD indicate that advanced CKD and dialysis patients with a positive non-invasive stress test do not benefit from angiography/revascularization additional to optimal medical therapy; moreover, angiography/revascularization causes harm where the risk of stroke was 3.76 higher than the medication alone group.31 A surprising 31.1% of angiograms showed no obstructive lesions, suggesting that epicardial coronary artery stenoses alone do not account for excess mortality in end-stage renal disease. Thus, these invasive procedures generally should be reserved for severe cases where patients become refractory to maximal medical management, have decompensated heart failure, with recent ACS, or have an LV ejection fraction < 35% who were excluded from the ISCHEMIA-CKD trial.
For severe and refractory symptomatic patients approaching dialysis [estimated GFR (eGFR) < 30 mL/min], a positive DSE or MPI can present a clinical dilemma. In the ISCHEMIA-CKD trial, angiogram +/- revascularization resulted in 7.9% risk for contrast nephropathy and 48% risk increase in death and dialysis initiation when compared to medical treatment alone. The risk of dialysis initiation after coronary artery bypass grafting (CABG) was 11–12.5%. Thus, invasive management can provoke contrast-induced nephropathy and CABG can hasten dialysis start and premature exposure to the CV spike. Risk-scoring tools for contrast-induced acute kidney injury and dialysis can inform decisions about the risk vs. benefit of invasive procedures (Table 3).32
Table 3.
Risk prediction of contrast-induced nephropathy after percutaneous coronary intervention32
| Risk factor | Scoring |
|---|---|
Hypotension
|
5 points |
| Use of intra-aortic balloon pump | 5 points |
Heart failure:
|
5 points |
| Elderly: >75 years old | 4 points |
| Anaemia: haematocrit <39% for men or <36% for women | 3 points |
| Diabetes | 3 points |
| Volume of contrast medium | 1 point per 100 cc |
|
Serum creatinine level >1.5 mg/dL (133 μmol/L) OR Estimated GFR <60 mL/min/1.73 m2 body surface area |
4 points 2 points for GFR = 40–60 mL/min 4 points for GFR = 20–39 mL/min 6 points for GFR < 20 mL/min |
| Total score | Risk of contrast-induced nephropathya | Risk of dialysis |
|---|---|---|
| ≤5 | 7.5% | 0.04% |
| 6–10 | 14% | 0.12% |
| 11–16 | 26.1% | 1.09% |
| ≥16 | 57.3% | 12.6% |
>25% or >5 mg/dL (44 umol/L) increase in serum creatinine.
Circulating biomarkers
Troponin concentrations increase immediately after haemodialysis (HD), suggesting that the procedure itself can induce haemoconcentration and/or cardiac injury from rapid fluid removal and coronary hypoperfusion in a population with predisposing obstructive or microvascular CAD33 , 34; furthermore, both cardiac troponin T and cardiac troponin I fragments depend on renal clearance, which may contribute to raised serum troponin concentrations due to myocardial injury.19 These mechanisms may also contribute to the ‘spike’ in MI, NSTEMI, and stroke following dialysis initiation (Figure 2).17
Newer fifth-generation troponin testing (hs-TNI) detects levels 100-fold lower than traditional assays, which complicates the diagnostic interpretation of a positive troponin for MI in dialysis patients.35 Asymptomatic dialysis patients already have elevated hs-TNI (>99th percentile values) from increased ventricular pressure, coronary microvascular dysfunction, anaemia, hypotension, and myocardial uraemic toxicity with impaired renal hs-TNI clearance.34 , 36 These mechanisms decrease the test specificity for ACS in this population.37 , 38 (Table 2). As such, The 2018 Universal Definition of MI39 requires a > 20% increase in serial hs-TNI values in symptomatic dialysis patients with EKG changes for ACS, although this strategy has not undergone rigorous testing in trials.40–44
Patients starting dialysis have high proBNP and B-type natriuretic peptide (BNP) levels due to reduced renal clearance, prevalent LVH, and fluid retention from advanced CKD. ProBNP (more than BNP) are cleared by the kidney and dialysis, further increasing inter- and intra-patient variability,45 , 46 such that establishing cut-off values for these biomarkers remains difficult, limiting their clinical utility in dialysis patients.47
Pathophysiology: the mechanisms of cardiovascular disease shift when dialysis starts
Myocardial stunning
Haemodialysis is associated with intra-dialytic myocardial stunning.48 In 70 HD patients, echocardiograms performed before, during, and after HD determined the direct effect of dialysis on cardiac function. Surprisingly, 64% of patients had new or worsening regional wall motion abnormalities (RWMA) during dialysis, indicating that the procedure can precipitate regional ischaemia. Furthermore, such RWMA associated with death or a major CV event 1 year later.48 In a follow-up study, 12 patients underwent intradialytic cardiac magnetic resonance scanning, which showed immediate worsening of LV function (cardiac and stroke volume index), peak systolic circumferential/longitudinal strain, and decreasing myocardial perfusion during HD.49 These studies link HD to cardiac dysfunction.
Other mechanisms also contribute to the excess CV death with dialysis initiation. HD rapidly removes fluid, potassium, calcium, bicarbonate, and magnesium from the intravascular space. Electrolyte shifts can trigger arrhythmias and drive excess SCD peri-dialysis. Excessive ultrafiltration decreases intravascular volume, reducing coronary perfusion, while the extracorporeal circuit subsumes approximately one unit of blood, reducing the already impaired oxygen-carrying capacity caused by the chronic anaemia commonly encountered in ESKD. Further, the creation of an arterio-venous shunt for dialysis access also reduces total peripheral resistance and diastolic pressure that drives coronary perfusion, and increases cardiac output, increasing myocardial oxygen demand.50 , 51 The high prevalence of obstructive coronary plaques further impedes coronary blood flow.52 In non-CKD patients, cardiac injury doubles and CV mortality increases 30% with a diastolic blood pressure (DBP) <60 mmHg relative to 80–89 mmHg.53 Twenty percent of dialysis patients have a pre-dialysis DBP < 65 and an additional 17% of patients will become hypotensive during HD.54 , 55 Indeed, low DBP or intra-dialytic hypotension are associated with increased CV mortality.54–56 Thus, aggressive blood pressure (BP) targets (e.g. 120/70 mmHg) derived from landmark non-dialysis trials such as SPRINT may not apply during dialysis.57 In fact, a dialysis randomized controlled trial (RCT) showed the opposite: lowering pre-dialysis BP to 143/74 increased CV events by 18% when compared to a pre-dialysis BP of 156/82 mmHg.58 These data collectively suggest that BP targets differ in dialysis, and highlight the pitfalls of extrapolating results of general outcome trials to patients starting dialysis.
Inflammation
Systemic inflammation links strongly to atherosclerosis and subsequent CV events in both the general and dialysis population.59 , 60 Thirty to sixty percent of US and European dialysis patients have C-reactive protein (CRP) levels > 3 mg/L, a level designated as high risk.61 , 62 Exceeding this cut-off point is associated with a doubling of CV mortality risk in the general population.63 Contact of blood with the dialysis membrane can trigger innate immune activation. A single HD session recirculates 100 litres of patient whole blood against 1.2 m2 of polymer fibre membrane and 200 litres of pre-mixed dialysate. Indeed, in vitro and in vivo studies suggest the large interface of human blood against an engineered membrane incites an inflammatory reaction through immune cell stimulation, complement activation, and cytokine generation.64–67 Similarly, peritoneal dialysis patients expose their abdominal cavity to 8–16 L of dialysate daily that contains plasticizers, which incite a direct inflammatory response.68 Dialysate from poorly processed municipal water can contain bacterial products including endotoxins that can cross the dialysis membrane into the patient’s bloodstream to activate monocytes that induce inflammation.68 In addition, 80% of patients in the USA initiate chronic dialysis using a catheter as their vascular access.69 Multiple studies show a statistically significant association between elevated CRP levels and catheter use in dialysis patients.70 Both anti-inflammatory and LDL-lowering effects of statins might decrease the risk of coronary events in CKD patients, but we lack evidence of their efficacy once patients start dialysis. In the Study of Heart and Renal Protection (SHARP), simvastatin with ezetimibe decreased MIs, strokes, and arterial revascularization procedures in CKD, but not in dialysis patients.71 Two separate dialysis trials also demonstrated the lack of improved CV and ACS outcomes with LDL lowering.72–74 The failure of statins to improve outcomes in ESKD in SHARP and other trials may reflect the distinct pathophysiology of CV events and/or the advanced state of arterial disease in this population. While MIs account for many CKD deaths, statins do not directly prevent arrythmias, which cause much mortality in dialysis patients.75
Anti-inflammatory therapies to prevent CV complications in the dialysis population remains in its infancy. Moderate CKD patients randomized to an antibody that neutralizes the pro-inflammatory cytokine interleukin-1β, canakinumab, significantly reduced their risk of major adverse CV events by 18% compared to placebo.76 The beneficial effect was most pronounced [hazard ratio (HR) = 0.68] in those whose hsCRP fell to <2 mg/L on canakinumab. These data support further trials of anti-inflammatory treatments in patients starting dialysis.
Vascular calcification
Coronary and other arterial calcification may contribute to CV disease and are highly prevalent in patients starting dialysis. While only 10.1% of the Multi-Ethnic Study of Atherosclerosis population has CAC, more than 70% of patients starting dialysis will have CAC.77–79 In dialysis patients, CAC is associated with increased mortality and diastolic dysfunction by echocardiography.80 , 81 Calciphylaxis occurs almost exclusively in the dialysis population when microvascular calcification of the dermo-hypodermic arterioles leads to necrotic skin lesion.82 The 6-month survival of patients with calciphylaxis is ∼50%, mostly due to sepsis.83
The genesis of CAC may differ in dialysis patients. While autopsy studies show similar coronary plaque burden in dialysis and non-CKD patients who die of cardiac death, atheromata of dialysis patients harbour markedly more calcium.84 The extensive arterial calcifications in ESKD extend to intra-myocardial arterioles.85 Aortic calcification causes arterial stiffness that increases pulse pressure, reduces DBP, and thus decreases coronary perfusion.86 Aortic stiffness also raises LV afterload, which can increase LV mass, an independent predictor of death, in > 70% of those starting dialysis.87 These abnormalities may predispose to heart failure with preserved systolic function in patients with CKD and on dialysis.
The complex pathogenesis of uraemic vascular calcification includes systemic inflammation and disordered mineral metabolism.88–90 Arterial smooth muscle cells can acquire osteoblastic functions that promote arterial calcification in CKD due to hyperphosphataemia, oxidative stress, and activity of the osteoblastic transcription factor RUNX2.91 , 92 Furthermore, ESKD patients have low concentrations of calcification inhibitors such as fetuin-A and matrix gla-protein, favouring calcification.93–95 FGF23, which increases markedly in patients undergoing dialysis, can induce LVH in uraemic rats.96 , 97 For these reasons, normalizing calcium, phosphorus, PTH, and FGF23 merit consideration as interventions to limit CV events in those starting dialysis.
Impaired autonomic modulation
Finally, several heart rate variability studies provide evidence of impaired cardiac autonomic modulation in dialysis patients.98 This autonomic imbalance may predispose to lethal arrythmias.99
Prevention: decreasing cardiovascular burden as dialysis starts
Reducing CV risk in patients starting dialysis merits a combined approach. Nephrologists optimize dialysate prescription, dialysis temperature, and ultrafiltration rate to remove excess fluid and normalize electrolytes while minimizing hypotension and coronary hypoperfusion during dialysis.100 , 101 Peritoneal dialysis (PD) places fewer haemodynamic demands on the heart. According to observational studies, PD may mitigate the ‘cardiovascular spike’; however, it remains inconclusive if HD vs. PD improves long-term survival.102–104 The only attempted randomized controlled trial of HD vs. PD halted because of inadequate enrolment, as most patients clearly prefer one modality.105 Frequent, shorter dialysis (6 times per week) can control BP, phosphorus, and improve LV function over conventional protocols (3 times per week)106 , 107; however, these RCTs lack adequate power for mortality and did not enrol patients just starting dialysis.
CV disease management during dialysis overlaps with outpatient care, mandating an integrated, multidisciplinary approach. Dialysis initiation should signal the care team to shift management targets strategies to align with the altered mechanisms of disease. As CV clinical trials typically exclude those with advanced CKD and dialysis, we lack conclusive data to inform diabetes, BP, cardiomyopathy, and arrhythmia management of dialysis.108 We must therefore base practice on the pathophysiology of disease with extrapolation from small validation trials in dialysis patients or on trials done in the non-dialysis population. The safety of such extrapolation remains unproved, and all members of the care team should acknowledge these limitations.
Blood pressure management
During the first 4 months of dialysis, fluctuating BP presents a management challenge.109 Sodium restriction and dialysis fluid removal offer first-line therapies for hypertension at this juncture.110 , 111 Most patients have excess volume at dialysis initiation; however, ultrafiltration in the first months of dialysis will reduce BP and permit reduction of hypertensive medications. Nephrologists often advise omission of BP medication before a session to achieve a pre-dialysis BP = 150–159/80–99 that reduces intra-dialytic hypotension and vascular access thrombosis58 although pre- and post-dialysis BP correlate minimally with CV mortality.112 In contrast, a 20/10 mmHg increase in inter-dialytic BP taken at home or in the doctor’s office is associated with a significant 50% increase in CV mortality.113 A meta-analysis of dialysis subgroups from RCTs reports that a 4–5/2–3 mmHg lowering of BP with anti-hypertensive medication in dialysis patients reduces both CV events and mortality by 29%.114 No dialysis RCTs have evaluated BP targets, but extrapolation from general population studies suggests that dialysis patients could target a home or outpatient-measured BP of 120–135/60–80 mmHg in between HD treatments.115
Anti-hypertenisve medications
Some, albeit limited, RCTs have evaluated classes of anti-hypertensive drugs in this context. Beta-blockers might seem a logical first choice, as arrhythmia may contribute to death in dialysis initiation. One trial randomized 200 hypertensive dialysis patients to atenolol vs. lisinopril and stopped early as the incidence of MI, stroke, chronic heart failure (CHF), and CV death increased two-fold in those allocated to lisinopril.112 Even though a meta-analysis of five RCTs of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) vs. placebo reported significant reductions in LV mass index, these findings have not consistently translated into improved outcomes.116 Four small trials randomized dialysis patients to an ACEI/ARB or placebo. Two of these trials showed CV benefit with ACEI/ARB while two other trials did not, such that the efficacy of renin–angiotensin–aldosterone system (RAAS) inhibition remains unclear in ESKD.110 Amlodipine (vs. placebo) in dialysis patients reduces mortality, non-fatal stroke, and coronary/peripheral revascularization by 47% when compared to placebo in one small trial (n = 251).117 Finally, 253 non-heart failure dialysis patients randomly received 25 mg spironolactone or placebo. Those treated with spironolactone had 58% less cardiocerebrovascular death, sudden death, and cardiac arrest, but no statistically significant difference in the incidence of hyperkalaemia.118 Another RCT found that spironolactone (25 mg/day) significantly decreased CV/cerebral hospitalization and death by 60% and all-cause mortality by 65% in HD when compared to placebo (n = 157), while serious hyperkalaemia occurred in only 1.9% of patients on the drug.119 Given the small size of these trials and their inclusion of subjects without a history of hyperkalaemia, the use of spironolactone in those starting dialysis requires caution pending the results from two ongoing larger trials examining the safety and efficacy of mineralocorticoid antagonists in dialysis.120 , 121
In addition to class of drug, whether dialysis removes the prescribed drug requires consideration. A propensity-matched observational study reported 40% higher mortality in dialysis patients prescribed dialysable beta-blockers (atenolol, metoprolol) when compared to non-dialysable beta-blockers (propranolol, carvedilol).122 ARBs are not cleared by the kidney or removed by dialysis and thus do not require dosing adjustments, which simplifies their prescribing in dialysis. The use of an ACEI can present more challenges, as each drug has a different renal and dialysis clearance properties (see Table 4). Dialysis does not remove amlodipine, diltiazem, or doxazocin. Dialysable medications should be avoided or taken after dialysis to avoid their removal from the bloodstream.
Table 4.
Cardiovascular medications dosing and clearance for patients starting dialysis123–125
| Medication | Drug clearance |
Comments | |
|---|---|---|---|
| Kidneys | Dialysis | ||
| Anticoagulant and antiplatelet | |||
| Enoxaparin | Yes | No | 20–30 mg SQ for DVT prophylaxis likely acceptable; avoid otherwise |
| Apixaban | Yes | Negligible | PK modelled dose of 2.5 mg b.i.d.; but no data to support safety and efficacy of drug; prescribe drug at own discretion |
| Dabigatran | Yes | Yes | Contraindicated; drug bio-accumulation can cause bleeding |
| Edoxaban | Yes | Negligible | Not recommended; drug bio-accumulation can cause bleeding |
| Rivaroxaban | Yes | No | PK models suggest 15 mg qd; but no data to support safety and efficacy of drug; prescribe drug at own discretion |
| Fondaparinux | Yes | Unknown | Not indicated |
| Eptifibatide | Yes | Yes | Contraindicated; drug bio-accumulation can cause bleeding |
| Prasugrel | Yes | No | No dosage adjustment |
| Ticagrelor | No | No | No dosage adjustment |
| Dalteparin | Yes | No | 5000 units prophylaxis ×7 days likely safe; monitor anti-Xa levels for treatment level dosing |
| Aspirin | Yes | Yes | Administer after HD on dialysis days |
| Clopidogrel | Yes | No | |
| Cangrelor | Yes | Unknown | No dose adjustment |
| Diabetic drugs | |||
| Insulin | Yes | No | Decrease initial dose by 50% |
| Glipizide | No | Unknown | No dose reduction needed in dialysis |
| Repaglinide | No | unknown | No dose reduction needed in dialysis |
| Metformin | Yes | Yes | Contraindicated; causes lactic acidosis |
| Thiazolidinediones (rosiglitazone, pioglitazone) | No | No | Not recommended; risk of oedema and CHF in anuric dialysis patients |
| Diabetic drugs: GLP-1 inhibitors | |||
| Exenatide | Yes | Unknown | Not recommended: severe GI side effects, many reports of AKI126 |
| Liraglutide | No | No127 | Not recommended; no safety and efficacy data |
| Dulaglitide | No | Unknown | Not recommended; no safety and efficacy data, increased risk AKI128 |
| All SGLT2 inhibitors | Yes | Unknown | Contraindicated |
| Diabetic drugs: DPP-4 inhibitors | |||
| Sitaglipin | Yes | Minimal | 25 mg qd |
| Saxaglipin | Yes | Yes | 2.5 mg qd, post-dialysis |
| Linagliptin | No | No | No dose adjustment |
| Aglopliptin | Yes | No | 6.25 mg qd |
| B-blockers | |||
| Atenolol | Yes | Yes | Administer after HD, reduce starting dose ∼50% |
| Metoprolol | No | Yes | Administer after HD |
| Carvdedilol, | No | No | |
| Labetalol | No | No | |
| Propranolol | No | No | No dose adjustment, but use with caution as weak active metabolite cleared by kidney |
| Bisoprolol | yes | no | Initial: 2.5 mg daily; increase cautiously |
| Other anti-hypertensive drugs | |||
| ACEI | Yes/No | Yes/No | Renal and dialysis clearance vary by ACEI type; Use ARB instead as all ARB require no dose adjustment thus simpler to dose |
| ARB | No | No | |
| Amlodipine | No | No | |
| Clonidine | Yes | No | Decrease starting dose but no mcg recommendations provided |
| Doxazocin | No | No | |
| Isosorbide mononitrate | Yes | Yes | Dose after dialysis |
| Spironolactone | Yes | Unknown | 25 mg qd |
| Anti-arrhythmic | |||
| Procainamide (Ia) | Yes | Yes | Monitor procainamide/N-acetylprocainamide (NAPA) concentrations; supplementation may be necessary |
| Quinidine (Ia) | Yes | Yes | No dosage adjustment provided in manufacturer’s labelling. Use with caution. Some clinicians advocate 75% normal dose after HD |
| Disopyramide (Ia) | Yes | No | Reduce dose; monitor drug levels and ECG; increased risk of torsades do pointes |
| Lidocaine (Ib) | No | No | |
| Mexiletine (Ib) | No | Yes | |
| Propafenone (Ic) | No | No | Accumulation of active metabolites in ESKD; no dose recommendation on label but some recommend 50% dose reduction |
| Flecainide (Ic) | Yes | No | Reduce dose 50%; plasma levels to guide dosing |
| Sotalol (III) | Yes | Yes | Contraindicated |
| Dofetilide (III) | Yes | No | Contraindicated |
| Amiodarone (III) | No | No | Safest anti-arrhythmic to use in ESKD |
| Digoxin | Yes | No | Keep serum level ≤1 if not avoid; rapid shifts in potassium during haemodialysis may precipitate arrhythmias through hypokalaemia2 |
For dialysis patients with reduced ejection fraction (EF) heart failure, small ESKD trials validate RCT findings in the general population. Carvedilol (up to 25 mg BID) significantly improved LV EF by 27%, CV mortality by 78%, and survival by 49% when compared to placebo (n = 114) in dialysis patients with an EF < 35%.129 , 130 Similarly, two small placebo-controlled spironolactone (25 mg/day) trials discussed above indicate promise for mineralocorticoid antagonism; however, we await confirmatory results from an ongoing larger trial120 conducted in dialysis patients with an EF < 40%. While RAAS dialysis trials in the non-heart failure cohorts described above were inconclusive, ACEI/ARB therapy may have efficacy when dialysis patients have coexisting heart failure. In a double-blind trial of 332 HD patients with an EF < 40% on ACEI randomly receiving add-on telmisartan (40–80 mg/day) vs. placebo, the ARB significantly reduced CV mortality (HR = 0.42) and CHF hospitalization (HR = 0.38) without causing excess hyperkalaemia.131
Medication reconciliation
The start of dialysis should prompt a careful review of a patient’s medications. Some CV medications depend on renal clearance, necessitating dose reduction or discontinuation, as renal function is minimal at dialysis initiation. Additionally, dialysis clears some medications, which may require a supplementary dose given after dialysis to maintain therapeutic drug levels (see Table 4). The use of antiarrhythmic drugs proves challenging in this population, as these drugs are often dialysable and cleared by the kidney, which can lead to fluctuations in the drug levels. Additionally, many anti-arrhythmics have a narrow therapeutic window, and toxicity can precipitate lethal arrhythmias in a population that is already prone to SCD.132 Dose reduction, plasma level testing and ECG monitoring may enhance the use of these drugs in dialysis patients.132 Amiodarone merits consideration in these patients due to its predictable elimination/clearance unaffected by dialysis.132 Forty percent of dialysis patients have a prolonged QTc interval, and QTc intervals increase after HD, likely from the rapid removal of potassium, magnesium, and calcium.133 Clinicians should consider omitting QT-prolonging medications before patients initiate dialysis, especially if the baseline ECG shows a long QT interval.
Hyperkalaemia
Hyperkalaemia is prevalent in patients starting dialysis and is associated with increased risk of SCD.134 , 135 Hyperkalaemia refractory to dietary restriction and dialysis removal can be treated with sodium polystyrene or newer agents that remove potassium by exchanging cations for potassium in the distal colon.136
Anticoagulation
The practice of anticoagulation in dialysis persists in a state of clinical equipoise. About 30% of HD patient have atrial fibrillation at the start of dialysis; yet, it remains unclear if the benefits of warfarin outweigh the risks of accelerating bleeding, CAC, and calciphylaxis in dialysis.137 Direct oral anticoagulants do not aggravate arterial calcification, but these drugs depend on renal clearance to variable extents, such that drug accumulation can enhance bleeding risk.138 The US FDA label does make pharmacokinetically-based dose recommendation for the use of rivaroxaban and apixaban in dialysis even though ESKD patients were excluded from all phase 3 clinical trials for atrial fibrillation and venous thromboembolism prophylaxis.139 , 140 The risk of bleeding was 20% higher in apixaban vs. warfarin (statistically non-significant) in an RCT of dialysis patients with atrial fibrillation; however, the trial was underpowered and terminated early because of slow enrolment.141
Implantable cardioverter-defibrillator
Given the high risk of SCD and frequent cardiomyopathy, CV implantable cardioverter-defibrillator (ICD) use is prevalent (10%) in the dialysis population.142 ICDs may have reduced efficacy in dialysis patients compared to non-dialysis patients.143 , 144 More study is needed on the risk–benefit of ICD therapy once patients start dialysis. Concurrent comorbid conditions, the dialysis access, and repeated cannulation raise the risk of device infection.145 Additionally, ICD infection-related mortality in dialysis remains significantly higher than in the general population even after prompt device extraction.146–149
Anaemia
Erythropoietin and IV iron can treat the anaemia of CKD to increase haemoglobin to 10-12 g/dL to attempt to limit cardiac complications and regress LV mass,150 yet raising haemoglobin levels beyond 12 g/dL is associated with CV harm.151 Ongoing trials for Hypoxia Inducible Factors (HIF) inhibitors may demonstrate that these drugs can raise haemoglobin without the CV risks seen with EPO.152
Diabetes management
Unfortunately, we lack trial evidence to inform diabetes management in patients starting dialysis, and the efficacy and safety of different anti-diabetic agents. Because of this information gap, HD patients are typically switched to insulin, although some physicians will elect to use an oral agent. Among the sulphonylurea/meglitinide drugs, glipizide, and repaglinide depend least on renal clearance.153 , 154 Metformin (lactic acidosis), thiazolidinediones (oedema and CHF), GLP-1 agonists, and SGLT2 inhibitors are all not recommended in dialysis. FDA labels do support DDP-4 inhibitor prescription in dialysis. A small dialysis trial (n = 129) showed that sitagliptin is equivalent to glipizide at lowering Hba1c with less severe hypoglycaemia, but larger studies are needed.123 Given the reduction in CV events recently demonstrated with SGLT2 inhibitors and GLP-1 agonists, we urgently need more information about the utility and safety of these classes of agents in the dialysis setting.
Finally, providers should ensure that CKD patients have nephrology care by the time their eGFR falls below 30 mL/min for vascular access and pre-emptive transplantation planning.124 The best outcome and quality of life for patients with progressive CKD is pre-emptive kidney transplantation, when clinical circumstances permit.125 Fistula or graft vascular access should be placed for HD because it associated with better cardiac outcomes than those using a catheter.6 There is evidence that brachial plexus block is superior to general and local anaesthesia in fistula placement because it improves haemodynamics and fistula maturation.126 If urgent, new-generation multilaminated grafts can be placed that can be used 24–72 h after implantation.127 Altered dialysis initiation practices to preserve residual renal function, such as peritoneal dialysis and starting with twice weekly dialysis, are under study.128
Conclusion
Dialysis initiation encompasses the period of highest CV risk in the spectrum of CKD, entailing a 20-fold mortality excess over age-matched controls. This ‘cardiovascular spike’ coincides with the start of dialysis, which shifts the mechanism of disease to alter the manifestations, response, and outcomes of CV disease in this vulnerable population. This critical period affords an opportunity for clinicians to heighten their vigilance for CV complications while ongoing research attempts to address the many gaps in our knowledge regarding optimum management of this perilous point in the patient’s journey with CKD. A cooperative approach between the nephrology and CV care teams assumes even greater importance during this critical passage.
Search strategy
Search strategy and selection criteria: Data for this seminar were identified from PubMed, MEDLINE, and references from relevant articles using the search terms ‘incident dialysis’, ‘cardiovascular’, ‘dialysis initiation’, and ‘dialysis early mortality’. Only articles published in English after 1980 were included.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
Standard analytic file data provided by USRDS Data Coordinating Center.
Funding
P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund.
Conflict of interest: P.L. is an unpaid consultant to, or involved in, clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion, Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Merck, Novartis, Pfizer, Sanofi-Regeneron. P.L. is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. P.L. laboratory has received research funding in the last 2 years from Novartis. P.L. is on the Board of Directors of XBiotech, Inc. P.L. has a financial interest in Xbiotech, a company developing therapeutic human antibodies. P.L. interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Supplementary Material
Contributor Information
Kevin Chan, National Institute of Diabetes and Digestive and Kidney Disease, Division of Kidney, Urology, and Hematology, 6707 Democracy Blvd, Bethesda, MD 20892-5458, USA.
Sharon M Moe, Division of Nephrology, Indiana University School of Medicine, 950 W. Walnut Street R2-202, Indianapolis, IN 46202, USA.
Rajiv Saran, Division of Nephrology, Department of Internal Medicine, University of Michigan, 1500 E Medical Center Dr # 31, Ann Arbor, MI 48109, USA.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 77 Ave. Louis Pasteur, NRB-741-G, Boston, MA 02115, USA.
References
- 1. Wetmore JB, Collins AJ. Meeting the World's need for maintenance dialysis. J Am Soc Nephrol 2015;26:2601–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age Standardization of Rates: a New WHO Standard (GPE Discussion Paper Series: No. 31). 2000, 1–14.
- 3. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 4. Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 2011;6:2642–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hazara AM, Bhandari S. Early mortality rates after commencement of maintenance hemodialysis: a systematic review and meta-analysis. Ther Apher Dial 2020;24:275–284. [DOI] [PubMed] [Google Scholar]
- 6. Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007;2:89–99. [DOI] [PubMed] [Google Scholar]
- 7. Turakhia MP, Blankestijn PJ, Carrero J-J, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits-Filho R, Reinecke H, Shroff GR, Zareba W, Cheung M, Wheeler DC, Winkelmayer WC, Wanner C, Amann K, Banerjee D, Bansal N, Boriani G, Bunch J, Chan CT, Charytan DM, Conen D, Friedman AN, Genovesi S, Holden RM, House AA, Jadoul M, Jardine AG, Johnson DW, Jun M, Labriola L, Mark PB, McCullough PA, Nolin TD, Potpara TS, Pun PH, Ribeiro ALP, Rossignol P, Shen JI, Sood MM, Tsukamoto Y, Wang AY-M, Weir MR, Wetmore JB, Wranicz JK, Yamasaki H; Conference Participants. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018;39:2314–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grams ME, Chow EKH, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 2013;62:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bansal N, Katz R, Robinson-Cohen C, Odden MC, Dalrymple L, Shlipak MG, Sarnak MJ, Siscovick DS, Zelnick L, Psaty BM, Kestenbaum B, Correa A, Afkarian M, Young B, de Boer IH. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol 2016;98104:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Safety Council. Odds of dying. https://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/?gclid=EAIaIQobChMIpKWz8YCv3AIVhI2zCh2ASAZ2EAMYASAAEgKwZPD_BwEdata-details (22 November 2019).
- 11.National Cancer Society. Lifetime risk of developing or dying from cancer. https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html (22 November 2019).
- 12. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for disease control and prevention: National Center for Health Statistics. https://www.cdc.gov/nchs/fastats/heart-disease.htm (22 November 2019).
- 14. Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int 2002;62:1799–1805. [DOI] [PubMed] [Google Scholar]
- 16. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 17. Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 2013;24:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Acute coronary syndrome rates by eGFR category by CKD stage 2007. https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q628&Strat=CKD+Stage&Year=2007#refreshPosition (22 November 2019).
- 19. Diris JHC, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation 2004;109:23–25. [DOI] [PubMed] [Google Scholar]
- 20. Joki N, Hase H, Nakamura R, Yamaguchi T. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant 1997;12:718–723. [DOI] [PubMed] [Google Scholar]
- 21. Ohtake T, Kobayashi S, Moriya H, Negishi K, Okamoto K, Maesato K, Saito S. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol 2005;16:1141–1148. [DOI] [PubMed] [Google Scholar]
- 22. Yuda S, Khoury V, Marwick TH. Influence of wall stress and left ventricular geometry on the accuracy of dobutamine stress echocardiography. J Am Coll Cardiol 2002;40:1311–1319. [DOI] [PubMed] [Google Scholar]
- 23. Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int 2005;67:217–226. [DOI] [PubMed] [Google Scholar]
- 24. Wang LW, Fahim MA, Hayen A, Mitchell RL, Baines L, Lord S, Craig JC, Webster AC. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst Rev 2011;12:CD008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J 2004;147:1017–1023. [DOI] [PubMed] [Google Scholar]
- 26. Dilsizian V, Gewirtz H, Marwick TH, Kwong RY, Raggi P, Al-Mallah MH, Herzog CA. Cardiac imaging for coronary heart disease risk stratification in chronic kidney disease. JACC Cardiovasc Imaging 2020;S1936-878X(20)30531-3. [DOI] [PubMed] [Google Scholar]
- 27. Sharma R, Pellerin D, Gaze DC, Gregson H, Streather CP, Collinson PO, Brecker SJD. Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant 2005;20:2207–2214. [DOI] [PubMed] [Google Scholar]
- 28. Rosário MA, de Lima JJ, Parga JR, Avila LF, Gowdak LH, Lemos PA, Rochitte CE. Coronary calcium score as predictor of stenosis and events in pretransplant renal chronic failure. Arq Bras Cardiol 2010;94:252–243. 252–60, 239–47. [DOI] [PubMed] [Google Scholar]
- 29. Winther S, Svensson M, Jorgensen HS, Bouchelouche K, Gormsen LC, Pedersen BB, Holm NR, Botker HE, Ivarsen P, Bottcher M. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging 2015;8:553–562. [DOI] [PubMed] [Google Scholar]
- 30. Hakeem A, Bhatti S, Chang SM. Screening and risk stratification of coronary artery disease in end-stage renal disease. JACC Cardiovasc Imaging 2014;7:715–728. [DOI] [PubMed] [Google Scholar]
- 31. Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, Berger JS, Mathew RO, Bockeria O, Broderick S, Pracon R, Herzog CA, Huang Z, Stone GW, Boden WE, Newman JD, Ali ZA, Mark DB, Spertus JA, Alexander KP, Chaitman BR, Chertow GM, Hochman JS. Management of coronary disease in patients with advanced kidney disease. N Engl J Med 2020;382:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 33. Wayand D, Baum HRG, SchäTzle G, SchäRf J, Neumeier D. Cardiac troponin T and I in end-stage renal failure. Clin Chem 2000;46:1345–1350. [PubMed] [Google Scholar]
- 34. van der Linden N, Cornelis T, Kimenai DM, Klinkenberg LJJ, Hilderink JM, Luck S, Litjens EJR, Peeters F, Streng AS, Breidthardt T, van Loon LJC, Bekers O, Kooman JP, Westermark PO, Mueller C, Meex SJR. Origin of cardiac troponin T elevations in chronic kidney disease. Circulation 2017;136:1073–1075. [DOI] [PubMed] [Google Scholar]
- 35. Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, Plein S, Mueller C, Haaf P. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017;12:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 37. Parikh RH, Seliger SL, DeFilippi CR. Use and interpretation of high sensitivity cardiac troponins in patients with chronic kidney disease with and without acute myocardial infarction. Clin Biochem 2015;48:247–253. [DOI] [PubMed] [Google Scholar]
- 38. Gunsolus I, Sandoval Y, Smith SW, Sexter A, Schulz K, Herzog CA, Apple FS. Renal dysfunction influences the diagnostic and prognostic performance of high-sensitivity cardiac troponin I. J Am Soc Nephrol 2018;29:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 40. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HDJoint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionKatus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035.22923432 [Google Scholar]
- 41. Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Puelacher C, Sabti Z, Rubini Gimenez M, Tschirky S, Du Fay de Lavallaz J, Kozhuharov N, Sazgary L, Mueller D, Breidthardt T, Strebel I, Flores Widmer D, Shrestha S, Miro O, Martin-Sanchez FJ, Morawiec B, Parenica J, Geigy N, Keller DI, Rentsch K, von Eckardstein A, Osswald S, Reichlin T, Mueller C. 0/1-Hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation 2018;137:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kraus D, von Jeinsen B, Tzikas S, Palapies L, Zeller T, Bickel C, Fette G, Lackner KJ, Drechsler C, Neumann JT, Baldus S, Blankenberg S, Munzel T, Wanner C, Zeiher AM, Keller T. Cardiac troponins for the diagnosis of acute myocardial infarction in chronic kidney disease. J Am Heart Assoc 2018;7:e008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller-Hodges E, Anand A, Shah ASV, Chapman AR, Gallacher P, Lee KK, Farrah T, Halbesma N, Blackmur JP, Newby DE, Mills NL, Dhaun N. High-sensitivity cardiac troponin and the risk stratification of patients with renal impairment presenting with suspected acute coronary syndrome. Circulation 2018;137:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. deFilippi CR, Herzog CA. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin Chem 2017;63:59–65. [DOI] [PubMed] [Google Scholar]
- 45. Isakova T, Wahl P, Vargas GS, Gutirrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu C-Y, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011;79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, Bellanuova I, Tripepi R, Seminara G, Parlongo S, Stancanelli B, Bonanno G, Fatuzzo P, Rapisarda F, Belluardo P, Signorelli SS, Heublein DM, Lainchbury JG, Leskinen HK, Bailey KR, Redfield MM, Burnett JC. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc 2001;76:1111–1119. [DOI] [PubMed] [Google Scholar]
- 47. Mueller C, Laule-Kilian K, Scholer A, Nusbaumer C, Zeller T, Staub D, Perruchoud AP. B-type natriuretic peptide for acute dyspnea in patients with kidney disease: insights from a randomized comparison. Kidney Int 2005;67:278–284. [DOI] [PubMed] [Google Scholar]
- 48. Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009;4:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buchanan C, Mohammed A, Cox E, Köhler K, Canaud B, Taal MW, Selby NM, Francis S, McIntyre CW. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol 2017;28:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coskun I, Colkesen Y, Altay H, Ozkan U, Demirturk OS, Gulcan O, Guvener M. Hemodynamic effects of left upper extremity arteriovenous fistula on ipsilateral internal mammary coronary artery bypass graft. Thorac Cardiovasc Surg 2013;61:663–667. [DOI] [PubMed] [Google Scholar]
- 51. MacRae JM, Levin A, Belenkie I. Editorials: the cardiovascular effects of arteriovenous fistulas in chronic kidney disease: a cause for concern? Semin Dial 2006;19:349–352. [DOI] [PubMed] [Google Scholar]
- 52. Ramanathan T, Skinner H. Coronary blood flow. Continuing Educ Anaesth Crit Care Pain 2005;5:61–64. [Google Scholar]
- 53. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac eventsimplications for blood pressure control. J Am Coll Cardiol 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, Maddux FW, Diaz-Buxo JA. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 2014;18:415–422. [DOI] [PubMed] [Google Scholar]
- 55. Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int 2002;62:1784–1790. [DOI] [PubMed] [Google Scholar]
- 56. Iseki K, Miyasato F, Tokuyama K, Nishime K, Uehara H, Shiohira Y, Sunagawa H, Yoshihara K, Yoshi S, Toma S, Kowatari T, Wake T, Oura T, Fukiyama K. Low diastolic blood pressure, hypoalbuminemia, and risk of death in a cohort of chronic hemodialysis patients. Kidney Int 1997;51:1212–1217. [DOI] [PubMed] [Google Scholar]
- 57., Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; Sprint Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miskulin DC, Gassman J, Schrader R, Gul A, Jhamb M, Ploth DW, Negrea L, Kwong RY, Levey AS, Singh AK, Harford A, Paine S, Kendrick C, Rahman M, Zager P. BP in dialysis: results of a pilot study. J Am Soc Nephrol 2018;29:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 60. Wanner C, Zimmermann J, Schwedler S, Metzger T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl 2002;61:S99–S102. [DOI] [PubMed] [Google Scholar]
- 61. Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000;35:469–476. [DOI] [PubMed] [Google Scholar]
- 62. Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int 1998;54:627–636. [DOI] [PubMed] [Google Scholar]
- 63. Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int 2005;68:766–772. [DOI] [PubMed] [Google Scholar]
- 64. Honkanen E, Gronhagen-Riska C, Teppo AM, Maury CP, Meri S. Acute-phase proteins during hemodialysis: correlations with serum interleukin-1 beta levels and different dialysis membranes. Nephron 1991;57:283–287. [DOI] [PubMed] [Google Scholar]
- 65. Bingel M, Lonnemann G, Koch KM, Dinarello CA, Shaldon S. Plasma interleukin-1 activity during hemodialysis: the influence of dialysis membranes. Nephron 1988;50:273–276. [DOI] [PubMed] [Google Scholar]
- 66. Pertosa G, Tarantino EA, Gesualdo L, Montinaro V, Schena FP. C5b-9 generation and cytokine production in hemodialyzed patients. Kidney Int Suppl 1993;41:S221–S225. [PubMed] [Google Scholar]
- 67. Canivet E, Lavaud S, Wong T, Guenounou M, Willemin JC, Potron G, Chanard J. Cuprophane but not synthetic membrane induces increases in serum tumor necrosis factor-alpha levels during hemodialysis. Am J Kidney Dis 1994;23:41–46. [DOI] [PubMed] [Google Scholar]
- 68. Ga KAYSEN. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol 2001;12:1549–1557. [DOI] [PubMed] [Google Scholar]
- 69.System USRD. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. In. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 70. Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC. Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int 2009;76:1063–1069. [DOI] [PubMed] [Google Scholar]
- 71. Baigent C, Landry M. Study of heart and renal protection (SHARP). Kidney Int Suppl 2003;63:S207–S210. [DOI] [PubMed] [Google Scholar]
- 72. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 74. Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 75. Strippoli GFM, Craig JC. Sunset for statins after AURORA? N Engl J Med 2009;360:1455–1457. [DOI] [PubMed] [Google Scholar]
- 76. Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin-1beta by Canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]
- 77. Okwuosa TM, Greenland P, Ning H, Liu K, Bild DE, Burke GL, Eng J, Lloyd-Jones DM. Distribution of coronary artery calcium scores by framingham 10-year risk strata in the MESA (multi-ethnic study of atherosclerosis): potential implications for coronary risk assessment. J Am Coll Cardiol 2011;57:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 1996;27:394–401. [DOI] [PubMed] [Google Scholar]
- 79. Chertow GM, Burke SK, Raggi PA. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62:245–252. [DOI] [PubMed] [Google Scholar]
- 80. Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J 2001;141:375–382. [DOI] [PubMed] [Google Scholar]
- 81. Anaya P, Blomquist G, Davenport D, Monier-Faugere M-C, Sorrell V, Malluche H. Coronary artery calcification in CKD-5D patients is tied to adverse cardiac function and increased mortality. Clin Nephrol 2016;86:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med 2018;378:1704–1714. [DOI] [PubMed] [Google Scholar]
- 83. McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, Bridges AD, Claus PL, Davis MD, Dillon JJ, El-Zoghby ZM, Hickson LJ, Kumar R, McBane RD, McCarthy-Fruin KA, McEvoy MT, Pittelkow MR, Wetter DA, Williams AW. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc 2016;91:1384–1394. [DOI] [PubMed] [Google Scholar]
- 84. Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000;15:218–223. [DOI] [PubMed] [Google Scholar]
- 85. Moe SM. Calcium as a cardiovascular toxin in CKD-MBD. Bone 2017;100:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N. Vascular calcification in chronic kidney disease. Am J Kidney Dis 2004;43:572–579. [DOI] [PubMed] [Google Scholar]
- 87. Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995;47:186–192. [DOI] [PubMed] [Google Scholar]
- 88. Yung L-M, Sánchez-Duffhues G, ten Dijke P, Yu PB. Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells. Cardiovasc Res 2015;108:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem 2010;110:935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Heine GH, Nangaku M, Fliser D. Calcium and phosphate impact cardiovascular risk. Eur Heart J 2013;34:1112–1121. [DOI] [PubMed] [Google Scholar]
- 91. Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 2008;19:213–216. [DOI] [PubMed] [Google Scholar]
- 92. Ketteler M. Fetuin-A and extraosseous calcification in uremia. Curr Opin Nephrol Hypertens 2005;14:337–342. [DOI] [PubMed] [Google Scholar]
- 93. Hermans MMH, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW, Leunissen KML, Krediet RT, Dekker FWa. Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 2007;72:202–207. [DOI] [PubMed] [Google Scholar]
- 94. Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aoun M, Makki M, Azar H, Matta H, Chelala DN. High dephosphorylated-uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol 2017;18:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drüeke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 2015;132:27–39. [DOI] [PubMed] [Google Scholar]
- 98. Poulikakos D, Banerjee D, Malik M. Risk of sudden cardiac death in chronic kidney disease. J Cardiovasc Electrophysiol 2014;25:222–231. [DOI] [PubMed] [Google Scholar]
- 99. Poulikakos D, Hnatkova K, Skampardoni S, Green D, Kalra P, Malik M. Sudden cardiac death in dialysis: arrhythmic mechanisms and the value of non-invasive electrophysiology. Front Physiol 2019;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015;26:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 2011;79:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Inrig JK, Toto RD. Differential outcomes between dialysis modalities: purely a reflection of selection bias? J Am Soc Nephrol 2011;22:989–990. [DOI] [PubMed] [Google Scholar]
- 103. Liu X, Huang R, Wu H, Wu J, Wang J, Yu X, Yang X. Patient characteristics and risk factors of early and late death in incident peritoneal dialysis patients. Sci Rep 2016;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jung HY, Choi H, Choi JY, Cho JH, Park SH, Kim CD, Ryu DR, Kim YL; ESRD Registry Committee of the Korean Society of Nephrology. Dialysis modality-related disparities in sudden cardiac death: hemodialysis versus peritoneal dialysis. Kidney Res Clin Pract 2019;38:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Korevaar JC, Feith GW, Dekker FWa. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003;64:2222–2228. [DOI] [PubMed] [Google Scholar]
- 106. Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis. J Am Med Assoc 2007;298:1291–1299. [DOI] [PubMed] [Google Scholar]
- 107. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010;363:2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rossignol P, Agarwal R, Canaud B, Charney A, Chatellier G, Craig JC, Cushman WC, Gansevoort RT, Fellström B, Garza D, Guzman N, Holtkamp FA, London GM, Massy ZA, Mebazaa A, Mol PGM, Pfeffer MA, Rosenberg Y, Ruilope LM, Seltzer J, Shah AM, Shah S, Singh B, Stefánsson BV, Stockbridge N, Stough WG, Thygesen K, Walsh M, Wanner C, Warnock DG, Wilcox CS, Wittes J, Pitt B, Thompson A, Zannad F. Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur Heart J 2019;40:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rohrscheib MR, Myers OB, Servilla KS, Adams CD, Miskulin D, Bedrick EJ, Hunt WC, Lindsey DE, Gabaldon D, Zager PG. Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol 2008;3:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Georgianos PI, Agarwal R. Pharmacotherapy of hypertension in chronic dialysis patients. Clin J Am Soc Nephrol 2016;11:2062–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension 2009;53:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant 2014;29:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2007;2:1228–1234. [DOI] [PubMed] [Google Scholar]
- 114. Heerspink H, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 2009;373:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Turner JM, Peixoto AJ. Blood pressure targets for hemodialysis patients. Kidney Int 2017;92:816–823. [DOI] [PubMed] [Google Scholar]
- 116. Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR; for the Alberta Kidney Disease Network. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol 2010;5:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant 2008;23:3605–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin C, Zhang Q, Zhang H, Lin A. Long-term effects of low-dose spironolactone on chronic dialysis patients: a randomized placebo-controlled study. J Clin Hypertens 2016;18:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, Shio N. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 2014;63:528–536. [DOI] [PubMed] [Google Scholar]
- 120. Rossingol P. ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST). 2013. https://clinicaltrials.gov/ct2/show/NCT01848639 (22 November 2019).
- 121. Wilkinson J, Pohl K. Aldosterone bloCkade for Health Improvement EValuation in End-stage Renal Disease (ACHIEVE). 2017. https://clinicaltrials.gov/ct2/show/NCT03020303 (22 November 2019).
- 122. Weir MA, Dixon SN, Fleet JL, Roberts MA, Hackam DG, Oliver MJ, Suri RS, Quinn RR, Ozair S, Beyea MM, Kitchlu A, Garg AX. Blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol 2015;26:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Arjona Ferreira JC, Corry D, Mogensen CE, Sloan L, Xu L, Golm GT, Gonzalez EJ, Davies MJ, Kaufman KD, Goldstein BJ. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013;61:579–587. [DOI] [PubMed] [Google Scholar]
- 124. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 125. Sayin B, Colak T, Tutal E, Sezer S. Comparison of preemptive kidney transplant recipients with nonpreemptive kidney recipients in single center: 5 years of follow-up. Int J Nephrol Renovasc Dis 2013;6:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gao C, Weng C, He C, Xu J, Yu L. Comparison of regional and local anesthesia for arteriovenous fistula creation in end-stage renal disease: a systematic review and meta-analysis. BMC Anesthesiol 2020;20:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lawson JH, Niklason LE, Roy-Chaudhury P. Challenges and novel therapies for vascular access in haemodialysis. Nat Rev Nephrol 2020;16:586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Deira J, Suárez MA, López F, García-Cabrera E, Gascón A, Torregrosa E, García GE, Huertas J, de la Flor JC, Puello S, Gómez-Raja J, Grande J, Lerma JL, Corradino C, Musso C, Ramos M, Martín J, Basile C, Casino FG. IHDIP: a controlled randomized trial to assess the security and effectiveness of the incremental hemodialysis in incident patients. BMC Nephrol 2019;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cice G, Ferrara L, Di Benedetto A, Russo PE, Marinelli G, Pavese F, Iacono A. Dilated cardiomyopathy in dialysis patients–beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol 2001;37:407–411. [DOI] [PubMed] [Google Scholar]
- 130. Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol 2003;41:1438–1444. [DOI] [PubMed] [Google Scholar]
- 131. Cice G, Di Benedetto A, D'Isa S, D'Andrea A, Marcelli D, Gatti E, Calabrò R. Effects of telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure: a double-blind, placebo-controlled trial. J Am Coll Cardiol 2010;56:1701–1708. [DOI] [PubMed] [Google Scholar]
- 132. Chow T, Galvin J, McGovern B. Antiarrhythmic drug therapy in patients with renal failure, liver failure, and congestive heart failure. Heart Dis 1999;1:98–107. [PubMed] [Google Scholar]
- 133. Covic A, Diaconita M, Gusbeth-Tatomir P, Covic M, Botezan A, Ungureanu G, Goldsmith DJ. Haemodialysis increases QT c interval but not QT c dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant 2002;17:2170–2177. [DOI] [PubMed] [Google Scholar]
- 134. Huang CW, Lee MJ, Lee PT, Hsu CY, Huang WC, Chen CL, Chou KJ, Fang HC. Low potassium dialysate as a protective factor of sudden cardiac death in hemodialysis patients with hyperkalemia. PLoS One 2015;10:e0139886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME; CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 2018;39:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension 2015;66:731–738. [DOI] [PubMed] [Google Scholar]
- 137. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CWA. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 138. Chan KE, Giugliano RP, Patel MR, Abramson S, Jardine M, Zhao S, Perkovic V, Maddux FW, Piccini JP. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J Am Coll Cardiol 2016;67:2888–2899. [DOI] [PubMed] [Google Scholar]
- 139.US Food and Drug Administration. XARELTO (rivaroxaban) tablets, for oral use: initial U.S. Approval: 2011. In: Pharmaceuticals J (ed). Belgium; 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202439s017lbl.pdf.
- 140.US Food and Drug Administration. ELIQUIS (apixaban) tablets for oral use Initial U.S. Approval: 2012. In: Squibb B-M, (ed). New York; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf.
- 141. Pokorney SD. RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation (RENAL-AF)—Presentation Slides. In: AHA 2019 Annual Scientific Sessions. 2019.
- 142. Nakhoul GN, Schold JD, Arrigain S, Harb SC, Jolly S, Wilkoff BL, Nally JV, Navaneethan SD. Implantable cardioverter-defibrillators in patients with CKD: a propensity-matched mortality analysis. Clin J Am Soc Nephrol 2015;10:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pun PH, Hellkamp AS, Sanders GD, Middleton JP, Hammill SC, Al-Khalidi HR, Curtis LH, Fonarow GC, Al-Khatib SM. Primary prevention implantable cardioverter defibrillators in end-stage kidney disease patients on dialysis: a matched cohort study. Nephrol Dial Transplant 2015;30:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Khattak A, Charytan DM. Shades of Grey: the conundrum of implantable defibrillators in individuals with advanced CKD. Clin J Am Soc Nephrol 2015;10:1107–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Saad TF, Ahmed W, Davis K, Jurkovitz C. Cardiovascular implantable electronic devices in hemodialysis patients: prevalence and implications for arteriovenous hemodialysis access interventions. Semin Dial 2015;28:94–100. [DOI] [PubMed] [Google Scholar]
- 146. Guha A, Maddox WR, Colombo R, Nahman NS, Kintziger KW, Waller JL, Diamond M, Murphy M, Kheda M, Litwin SE, Sorrentino RA. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm 2015;12:2395–2401. [DOI] [PubMed] [Google Scholar]
- 147. Opelami O, Sakhuja A, Liu X, Tang WHW, Schold JD, Navaneethan SD. Outcomes of infected cardiovascular implantable devices in dialysis patients. Am J Nephrol 2014;40:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lin Y-S, Chen T-H, Lin M-S, Chen DY, Mao C-T, Hsu J-T, Chen H-C, Chen M-C. Impact of chronic kidney disease on short-term cardiac implantable electronic device related infection: a nationwide population-based cohort study. Medicine 2016;95:e2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hickson LJ, Gooden JY, Le KY, Baddour LM, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Sohail MR. Clinical presentation and outcomes of cardiovascular implantable electronic device infections in hemodialysis patients. Am J Kidney Dis 2014;64:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta-analysis. Clin J Am Soc Nephrol 2009;4:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJV, Parfrey P, Parving H-h, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–2032. [DOI] [PubMed] [Google Scholar]
- 152. Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, Chan DTM, Leong R, Poole L, Zhong M, Saikali KG, Franco M, Hemmerich S, Yu KHP, Neff TB. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol 2016;27:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Daugirdas JT, Ing TS, Blake PG. Handbook of Dialysis. Philadelphia, PA: Wolters Kluwer Health, 2015. [Google Scholar]
- 154. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005;45:S1–153. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Standard analytic file data provided by USRDS Data Coordinating Center.